WO2012060204A1 - インクジェット光造形法における、光造形品形成用モデル材、光造形品の光造形時の形状支持用サポート材および光造形品の製造方法 - Google Patents
インクジェット光造形法における、光造形品形成用モデル材、光造形品の光造形時の形状支持用サポート材および光造形品の製造方法 Download PDFInfo
- Publication number
- WO2012060204A1 WO2012060204A1 PCT/JP2011/073778 JP2011073778W WO2012060204A1 WO 2012060204 A1 WO2012060204 A1 WO 2012060204A1 JP 2011073778 W JP2011073778 W JP 2011073778W WO 2012060204 A1 WO2012060204 A1 WO 2012060204A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- support material
- model material
- water
- article
- model
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C67/00—Shaping techniques not covered by groups B29C39/00 - B29C65/00, B29C70/00 or B29C73/00
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C64/00—Additive manufacturing, i.e. manufacturing of three-dimensional [3D] objects by additive deposition, additive agglomeration or additive layering, e.g. by 3D printing, stereolithography or selective laser sintering
- B29C64/30—Auxiliary operations or equipment
- B29C64/35—Cleaning
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C64/00—Additive manufacturing, i.e. manufacturing of three-dimensional [3D] objects by additive deposition, additive agglomeration or additive layering, e.g. by 3D printing, stereolithography or selective laser sintering
- B29C64/10—Processes of additive manufacturing
- B29C64/106—Processes of additive manufacturing using only liquids or viscous materials, e.g. depositing a continuous bead of viscous material
- B29C64/112—Processes of additive manufacturing using only liquids or viscous materials, e.g. depositing a continuous bead of viscous material using individual droplets, e.g. from jetting heads
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C64/00—Additive manufacturing, i.e. manufacturing of three-dimensional [3D] objects by additive deposition, additive agglomeration or additive layering, e.g. by 3D printing, stereolithography or selective laser sintering
- B29C64/40—Structures for supporting 3D objects during manufacture and intended to be sacrificed after completion thereof
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B33—ADDITIVE MANUFACTURING TECHNOLOGY
- B33Y—ADDITIVE MANUFACTURING, i.e. MANUFACTURING OF THREE-DIMENSIONAL [3-D] OBJECTS BY ADDITIVE DEPOSITION, ADDITIVE AGGLOMERATION OR ADDITIVE LAYERING, e.g. BY 3-D PRINTING, STEREOLITHOGRAPHY OR SELECTIVE LASER SINTERING
- B33Y10/00—Processes of additive manufacturing
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B33—ADDITIVE MANUFACTURING TECHNOLOGY
- B33Y—ADDITIVE MANUFACTURING, i.e. MANUFACTURING OF THREE-DIMENSIONAL [3-D] OBJECTS BY ADDITIVE DEPOSITION, ADDITIVE AGGLOMERATION OR ADDITIVE LAYERING, e.g. BY 3-D PRINTING, STEREOLITHOGRAPHY OR SELECTIVE LASER SINTERING
- B33Y70/00—Materials specially adapted for additive manufacturing
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/10—Esters
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F290/00—Macromolecular compounds obtained by polymerising monomers on to polymers modified by introduction of aliphatic unsaturated end or side groups
- C08F290/02—Macromolecular compounds obtained by polymerising monomers on to polymers modified by introduction of aliphatic unsaturated end or side groups on to polymers modified by introduction of unsaturated end groups
- C08F290/06—Polymers provided for in subclass C08G
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F290/00—Macromolecular compounds obtained by polymerising monomers on to polymers modified by introduction of aliphatic unsaturated end or side groups
- C08F290/02—Macromolecular compounds obtained by polymerising monomers on to polymers modified by introduction of aliphatic unsaturated end or side groups on to polymers modified by introduction of unsaturated end groups
- C08F290/06—Polymers provided for in subclass C08G
- C08F290/067—Polyurethanes; Polyureas
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L33/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
- C08L33/24—Homopolymers or copolymers of amides or imides
- C08L33/26—Homopolymers or copolymers of acrylamide or methacrylamide
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L75/00—Compositions of polyureas or polyurethanes; Compositions of derivatives of such polymers
- C08L75/04—Polyurethanes
- C08L75/14—Polyurethanes having carbon-to-carbon unsaturated bonds
- C08L75/16—Polyurethanes having carbon-to-carbon unsaturated bonds having terminal carbon-to-carbon unsaturated bonds
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D11/00—Inks
- C09D11/02—Printing inks
- C09D11/10—Printing inks based on artificial resins
- C09D11/101—Inks specially adapted for printing processes involving curing by wave energy or particle radiation, e.g. with UV-curing following the printing
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D11/00—Inks
- C09D11/30—Inkjet printing inks
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29K—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES B29B, B29C OR B29D, RELATING TO MOULDING MATERIALS OR TO MATERIALS FOR MOULDS, REINFORCEMENTS, FILLERS OR PREFORMED PARTS, e.g. INSERTS
- B29K2033/00—Use of polymers of unsaturated acids or derivatives thereof as moulding material
- B29K2033/04—Polymers of esters
- B29K2033/08—Polymers of acrylic acid esters, e.g. PMA, i.e. polymethylacrylate
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29K—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES B29B, B29C OR B29D, RELATING TO MOULDING MATERIALS OR TO MATERIALS FOR MOULDS, REINFORCEMENTS, FILLERS OR PREFORMED PARTS, e.g. INSERTS
- B29K2033/00—Use of polymers of unsaturated acids or derivatives thereof as moulding material
- B29K2033/26—Polymers of acrylamide or methacrylamide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29K—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES B29B, B29C OR B29D, RELATING TO MOULDING MATERIALS OR TO MATERIALS FOR MOULDS, REINFORCEMENTS, FILLERS OR PREFORMED PARTS, e.g. INSERTS
- B29K2105/00—Condition, form or state of moulded material or of the material to be shaped
- B29K2105/0005—Condition, form or state of moulded material or of the material to be shaped containing compounding ingredients
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29K—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES B29B, B29C OR B29D, RELATING TO MOULDING MATERIALS OR TO MATERIALS FOR MOULDS, REINFORCEMENTS, FILLERS OR PREFORMED PARTS, e.g. INSERTS
- B29K2105/00—Condition, form or state of moulded material or of the material to be shaped
- B29K2105/0005—Condition, form or state of moulded material or of the material to be shaped containing compounding ingredients
- B29K2105/0047—Agents changing thermal characteristics
- B29K2105/005—Heat sensitisers or absorbers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29K—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES B29B, B29C OR B29D, RELATING TO MOULDING MATERIALS OR TO MATERIALS FOR MOULDS, REINFORCEMENTS, FILLERS OR PREFORMED PARTS, e.g. INSERTS
- B29K2995/00—Properties of moulding materials, reinforcements, fillers, preformed parts or moulds
- B29K2995/0012—Properties of moulding materials, reinforcements, fillers, preformed parts or moulds having particular thermal properties
- B29K2995/0017—Heat stable
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29K—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES B29B, B29C OR B29D, RELATING TO MOULDING MATERIALS OR TO MATERIALS FOR MOULDS, REINFORCEMENTS, FILLERS OR PREFORMED PARTS, e.g. INSERTS
- B29K2995/00—Properties of moulding materials, reinforcements, fillers, preformed parts or moulds
- B29K2995/0037—Other properties
- B29K2995/007—Hardness
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29K—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES B29B, B29C OR B29D, RELATING TO MOULDING MATERIALS OR TO MATERIALS FOR MOULDS, REINFORCEMENTS, FILLERS OR PREFORMED PARTS, e.g. INSERTS
- B29K2995/00—Properties of moulding materials, reinforcements, fillers, preformed parts or moulds
- B29K2995/0037—Other properties
- B29K2995/0081—Tear strength
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29K—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES B29B, B29C OR B29D, RELATING TO MOULDING MATERIALS OR TO MATERIALS FOR MOULDS, REINFORCEMENTS, FILLERS OR PREFORMED PARTS, e.g. INSERTS
- B29K2995/00—Properties of moulding materials, reinforcements, fillers, preformed parts or moulds
- B29K2995/0037—Other properties
- B29K2995/0094—Geometrical properties
- B29K2995/0096—Dimensional stability
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B33—ADDITIVE MANUFACTURING TECHNOLOGY
- B33Y—ADDITIVE MANUFACTURING, i.e. MANUFACTURING OF THREE-DIMENSIONAL [3-D] OBJECTS BY ADDITIVE DEPOSITION, ADDITIVE AGGLOMERATION OR ADDITIVE LAYERING, e.g. BY 3-D PRINTING, STEREOLITHOGRAPHY OR SELECTIVE LASER SINTERING
- B33Y70/00—Materials specially adapted for additive manufacturing
- B33Y70/10—Composites of different types of material, e.g. mixtures of ceramics and polymers or mixtures of metals and biomaterials
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B33—ADDITIVE MANUFACTURING TECHNOLOGY
- B33Y—ADDITIVE MANUFACTURING, i.e. MANUFACTURING OF THREE-DIMENSIONAL [3-D] OBJECTS BY ADDITIVE DEPOSITION, ADDITIVE AGGLOMERATION OR ADDITIVE LAYERING, e.g. BY 3-D PRINTING, STEREOLITHOGRAPHY OR SELECTIVE LASER SINTERING
- B33Y80/00—Products made by additive manufacturing
Definitions
- the present invention relates to a model material for forming an optical modeling product, a support material for shape support at the time of optical modeling of an optical modeling article, a two-component photocurable resin composition for inkjet optical modeling, and the composition
- the present invention relates to a photofabricated article obtained by photocuring a photocurable composition and a method of producing a photoshaped product using the composition.
- Japanese Patent Application Laid-Open No. 1-204915 JP-A-8-59760 Japanese Patent Application Laid-Open No. 9-169827 Unexamined-Japanese-Patent No. 9-316111 JP 2001-310918 A JP 2004-59601 A JP 2002-67174 A Japanese Patent Application Laid-Open No. 2002-178412 Japanese Patent Application Laid-Open No. 2004-255839 EP1274551 B1 EP1741545A2 Unexamined-Japanese-Patent No. 2010-155889 JP, 2010-155926, A
- the method of photofabrication by irradiating a laser beam, an ultraviolet ray, etc. is generally a laser beam, an ultraviolet ray, etc. from the upper side or the lower side of the liquid surface while raising and lowering the liquid surface of the liquid photocurable resin.
- a dedicated dark room because the liquid photocurable resin is cured even by light from the outside, etc.
- the support material in the methods disclosed in Patent Documents 10 and 11, since the support material basically becomes a cross-linked gel that forms a bond with the model material, it takes time to remove the support material. And it is difficult to remove details. With regard to the support material disclosed in Patent Document 12, basically it is difficult to simultaneously achieve the support power and the water solubility, and it takes a long time to remove the support material after the model material is cured, When the concentration of the acrylamide group having an acryloyl group is reduced or the amount of the chain transfer agent is increased, there is a problem that the support power can not be maintained. Furthermore, in the prior art described in the above-mentioned known documents, when the model material and the support material are discharged by the ink jet method, the two are mixed during curing until they are mixed. The problem of expansion and deformation etc. was not considered at all.
- a model material for forming a photofabricated article in an inkjet photofabrication method comprising a curable resin component having a weighted average value of 9.0 to 10.3 of SP value; a water-soluble monofunctional ethylenically unsaturated resin
- Shape-supporting support material comprising a combination of a model material for forming an optical shaped article by inkjet optical shaping and a support material for supporting the shape of the optical shaped article during optical shaping,
- the model material is the model material as described above, and the support material
- the model material, the support material, the two-component photocurable resin composition, the photofabricated article formed using the composition, and the method for producing the same of the present invention have the following effects.
- (1) There is very little swelling deformation due to water or moisture absorption during photocuring and after curing of the model material.
- the photocured material of the support material is excellent in water solubility and is easy to remove after photofabrication.
- (3) In the two-part photocurable resin composition, the support material is not compatible with the model material, and the photofabricated product has excellent mechanical properties.
- An optically formed article formed using the two-part photocurable resin composition is excellent in formation accuracy.
- the method for producing the photofabricated article is excellent in productivity.
- Schematic of inkjet three-dimensional modeling system Side view of a schematic view showing the configuration of a three-dimensional modeling apparatus
- Plan view of a schematic view showing the configuration of a three-dimensional modeling apparatus Schematic view of one of each printer head from below
- Schematic of material supply system that supplies model material and support material for each printer head
- Schematic showing the process of creating a three-dimensional object by operating the three-dimensional forming apparatus (A) is a schematic view showing a model including a support material whose molding is completed, and (B) is a schematic view showing a model where a support material is removed from a model including a support material whose molding is complete
- the model material for forming an optical shaping article in the inkjet optical shaping method of the present invention is characterized in that it is a model material containing a curable resin component having a weighted average value of 9.0 to 10.3 of SP value.
- the weighted average value (hereinafter sometimes simply referred to as the SP value) of the SP value of the curable resin component of the model material of the present invention is 9.0 to 10.3, preferably 9.2 to 10 .0.
- the SP value is more than 10.3, if it is immersed in water or washed with water jet in order to remove the cured product of the support material described later, the cured product of the model material swells and deforms with water, and it deforms even if it is dried When the cured product of the model material is left standing, it absorbs moisture and is easily deformed. If the SP value is less than 9.0, the cured product becomes brittle and the toughness is lowered.
- the SP value of the curable resin component of the model material can be adjusted to the above range by selecting the types and contents of the later-described curable resin components (A) to (C) constituting the model material. .
- SP means a solubility parameter (solubility parameter), which serves as a measure of the mutual solubility of substances, and it is known that the smaller the difference in SP value between substances, the larger the mutual solubility.
- the SP value of the copolymer or blend is calculated by the method proposed by Fedors et al. Described in the following document. In this method, the SP value of the copolymer or blend is assumed to satisfy the addition rule, and in the copolymer, the SP value of the constituent monomer, or in the blend, the SP value of the constituent component Proportional allocation in%) is calculated as a weighted average of SP values.
- the curable resin component includes a monofunctional ethylenically unsaturated monomer (A), a polyfunctional ethylenically unsaturated monomer not containing a urethane group (B), and a urethane group-containing ethylenically unsaturated group. It contains a saturated monomer (C) and a photopolymerization initiator (D).
- the model material is designed such that the weighted average value of the curable resin components in the model material, that is, the SP values of (A) to (C), is 9.0 to 10.3.
- the monofunctional ethylenically unsaturated monomer (A) one ethylenically unsaturated group [(meth) acryloyl group, N-vinyl group etc.]
- the compound is not particularly limited as long as it has a compound, but from the viewpoint of decreasing the SP value, a hydrophobic monofunctional ethylenically unsaturated monomer (A1) (SP value is 10 or less) is preferable. .
- Examples of (A1) include linear or branched alkyl (meth) acrylates [compounds having 4 to 30 carbon atoms (hereinafter abbreviated as C), such as methyl (meth) acrylate, ethyl (meth) acrylate, isobutyl (meth) acrylate, lauryl (Meth) acrylate, stearyl (meth) acrylate, isostearyl (meth) acrylate and t-butyl (meth) acrylate]; alicyclic containing (meth) acrylate [compound of C6 to 20, such as cyclohexyl (meth) acrylate, 4- t-Cyclohexyl (meth) acrylate, isobornyl (meth) acrylate and dicyclopentanyl (meth) acrylate]; heterocycle-containing (meth) acrylate [compound of C5 to 20, such as tetrahydrofurfuryl (meth)
- homopolymers are more preferable from the viewpoint of improvement of shaping accuracy to endure molding temperature (50 to 90 ° C.) at curing of the model material and heat resistance at the time of use of the optical shaped article itself Having a high glass transition temperature (hereinafter abbreviated as Tg) of 50 ° C.
- the body (A2) can be contained.
- water-soluble means that the solubility (25 ° C.) in water is 1 (g / water 100 g) or more.
- (A2) C5 to 15 hydroxyl group-containing (meth) acrylate [hydroxyethyl (meth) acrylate, hydroxypropyl (meth) acrylate, 4-hydroxybutyl (meth) acrylate etc.]; number average molecular weight [abbreviated below as Mn .
- the measurement is by gel permeation chromatography (GPC).
- PEG polyethylene glycol
- PPG polypropylene glycol
- C3-15 (meth) acrylamide derivatives [(meth) acrylamides, N-methyl (meth) acrylamides, N-ethyl (Meth) acrylamide, N-propyl (meth) acrylamide, N-butyl (meth) acrylamide, N, N'-dimethyl (meth) acrylamide, N, N'-diethyl (meth) acrylamide, N-hye Rokishiechiru (meth) acrylamide
- the content of (A2) is usually 10% or less based on the weight of the model material, preferably 5% or less, more preferably 3% or less, from the viewpoint of reducing the water swelling rate of the photocured material of the model material described later. Most preferably, it is 0%.
- the monofunctional ethylenically unsaturated monomers (A) may be used alone or in combination of two or more as needed.
- the polyfunctional ethylenically unsaturated monomer (B) having no urethane group does not have a urethane group, and two or more of them.
- the compound is not particularly limited as long as it is a compound having the above ethylenically unsaturated group.
- (B) is not particularly limited as long as it has two or more (preferably 2 to 3) ethylenic unsaturated groups in the molecule, but it is preferable from the viewpoint of reducing the SP value.
- Examples of (B1) include linear or branched alkylene glycol di (meth) acrylates [C10-25 compounds such as 1,6-hexanediol di (meth) acrylate, neopentyl glycol di (meth) acrylate, 1,9 -Nonanediol di (meth) acrylate, 3-methyl-1,5-pentanediol di (meth) acrylate, 2-n-butyl-2-ethyl-1,3-propanediol di (meth) acrylate], alicyclic And di-containing (meth) acrylates [C10-30 compounds such as dimethylol tricyclodecane di (meth) acrylate].
- C10-25 compounds such as 1,6-hexanediol di (meth) acrylate, neopentyl glycol di (meth) acrylate, 1,9 -Nonanediol di (meth) acrylate, 3-
- homopolymers are more preferable from the viewpoint of improving shaping accuracy to endure molding temperature (50 to 90 ° C.) at the time of curing of the model material and from the viewpoint of heat resistance at the time of use Having a high glass transition temperature (50 ° C.
- the polyfunctional ethylenically unsaturated monomer (B) having no urethane group may be used singly or in combination of two or more as needed.
- the urethane group-containing ethylenically unsaturated monomer (C) has one or more ethylenically unsaturated groups and contains a urethane group. It is a monomer.
- Examples of (C) include those formed of a compound (a) having a hydroxyl group and a (meth) acryloyl group and a polyisocyanate (b), and from the viewpoint of reducing the SP value, hydrophobicity (SP (SP) is preferred. The value is 10.9 or less) (C1).
- (A) includes compounds of C 5 or more and Mn 5,000 or less, for example, the following, and a mixture of two or more of them.
- (A4) reaction product of (meth) acrylic acid and epoxide (C8 to 30) 3-phenoxy-2-hydroxypropyl (meth) acrylate, 3-biphenoxy-2-hydroxypropyl (meth) acrylate;
- (a5 ) Reaction product of (meth) acrylic acid and trifunctional or higher functional polyol (molecular weight 92 or more and Mn 5,000 or less) Glycerin mono- and di (meth) acrylate, trimethylolpropane mono- and di (meth) acrylate, Pentaerythritol mono-, di- and tri (meth) acrylates, ditrimethylolpropane mono-, di- and tri (meth) acrylates, dipentaerythritol mono-, di-, tri-, tetra- and penta (meth) acrylates, And their AO adducts (additional mole number 1 to 10 ) And the like; among these (a), preferred from the
- Poly (di, tri or higher) isocyanates (b) are compounds 6 to 20 of aromatic polyisocyanates [C (excluding C in NCO groups, the same shall apply hereinafter), such as 2,4- and / or 2, 6-tolylene diisocyanate (TDI), 4,4'- and / or 2,4'-diphenylmethane diisocyanate (MDI), aliphatic polyisocyanate [compound of C 2-18, such as hexamethylene diisocyanate (HDI)], fat Cyclic polyisocyanates [C4-45 compounds such as isophorone diisocyanate (IPDI), 2,4- and / or 2,6-methylcyclohexane diisocyanate (hydrogenated TDI), dicyclohexylmethane-4,4'-diisocyanate (hydrogenated) MDI)], araliphatic polyisocyanates [C8-15 Compounds such as m- and / or p- xy
- (f) When producing the urethane group-containing ethylenically unsaturated monomer (C), from the viewpoint of the toughness and elongation of the cured product, other components other than (a) which have a hydroxyl group and do not have an unsaturated group (F) may be contained as a reaction component.
- examples of (f) include polyhydric alcohols (ethylene glycol, propylene glycol, glycerin, polyalkylene glycol and the like) and monovalent alcohols (methanol, ethanol and the like), each having a C1 or more and Mn of 3,000 or less.
- monohydric alcohols are preferable from the viewpoint of the impact resistance of the cured product.
- the lower limit of Mn of (C) is preferably 500, more preferably 700, from the viewpoint of the impact resistance of the cured product, and the upper limit is preferably 5,000, more preferably from the viewpoint of the handleability of the composition and the shaping accuracy of the cured product. It is 2,000.
- the functional group number of the ethylenically unsaturated group (C) is preferably 1 to 20, more preferably 1 to 3 from the viewpoint of hardness and impact resistance of the cured product.
- photopolymerization initiator (D) As the photopolymerization initiator (D), benzoin compounds [C14-18 compounds such as benzoin, benzoin methyl ether, benzoin ethyl ether, benzoin propyl ether, benzoin isobutyl ether]; acetophenone compounds [C8-18 compounds such as acetophenone, 2,2-diethoxy-2-phenylacetophenone, 2,2-diethoxy-2-phenylacetophenone, 1,1-dichloroacetophenone, 2-hydroxy-2-methyl-phenylpropane- 1-one, diethoxyacetophenone, 1-hydroxycyclohexyl phenyl ketone, 2-methyl-1- [4- (methylthio) phenyl] -2-morpholinopropan-1-one]; anthraquinone compound [C14-19 Compounds such as 2-ethylanthraquinone, 2-t-butylanthraquinone, 2-chloroan
- acetophenone compounds and phosphine oxides are preferred, more preferably 1-hydroxycyclohexyl phenyl ketone, 2-methyl-1- [4 from the viewpoint of light resistance that the cured product hardly yellows.
- Particularly preferred is 1-hydroxycyclohexyl phenyl ketone, 2,4,6-trimethyl benzoyl diphenyl phosphine oxide.
- the content (% by weight) of each of (A) to (D) in the model material is preferably 50 to 90%, more preferably 55 to 50%, from the viewpoint of improvement of the Tg of the photocured product and brittleness.
- (B) is preferably 3 to 25%, more preferably 4 to 20%, from the viewpoint of mechanical strength and brittleness of the photocured product;
- (C) is preferably from the viewpoint of toughness and hardness of the photocured product 5 to 35%, more preferably 8 to 30%;
- (D) is preferably 0.1 to 10%, more preferably 0.3 to 8% in view of the photocuring speed and mechanical properties of the photocured product .
- additives (E) In the model material, other additives (E) can be contained, if necessary, in a range that does not inhibit the effects of the present invention.
- (E) includes a polymerization inhibitor, a surfactant, a colorant, an antioxidant, a chain transfer agent, a filler, and the like, and can be selected variously according to the purpose. One kind of single use or two Any combination of species or more may be used.
- the molding temperature is about 50 to 90 ° C. From the viewpoint of improving the stability of the monomer, it is preferable to add a polymerization inhibitor.
- polymerization inhibitors examples include phenol compounds [hydroquinone, hydroquinone monomethyl ether, 2,6-di-t-butyl-p-cresol, 2,2-methylene-bis- (4-methyl-6-t-butylphenol), 1 1,1,3-Tris- (2-methyl-4-hydroxy-5-t-butylphenyl) butane etc.], sulfur compounds [dilaurylthiodipropionate etc.], phosphorus compounds [triphenyl phosphite etc.], amines Compounds [phenothiazine etc.] and the like can be mentioned.
- the amount of the polymerization inhibitor used is usually 5% or less based on the total weight of (A) to (D), and preferably 0.1 to 3% from the viewpoint of monomer stability and polymerization rate.
- the surfactant may have a molecular weight of 264 or more and Mn 5,000 or less, for example, PEG type nonionic surfactant [ethylene oxide (hereinafter abbreviated as EO) of 1 to 40 moles of nonylphenol, adduct of 1 to 40 moles of stearic acid EO, etc.
- PEG type nonionic surfactant [ethylene oxide (hereinafter abbreviated as EO) of 1 to 40 moles of nonylphenol, adduct of 1 to 40 moles of stearic acid EO, etc.
- Polyhydric alcohol type nonionic surfactants (Sorbitan palmitic acid monoester, sorbitan stearic acid monoester, sorbitan stearic acid triester, etc.), fluorine-containing surfactants (perfluoroalkyl EO 1 to 50 molar adducts, perfluoro) Examples thereof include alkyl carboxylates, perfluoroalkyl betaines, etc., modified silicone oils [polyether modified silicone oils, (meth) acrylate modified silicone oils, etc.].
- the amount of surfactant used is usually 3% or less based on the total weight of (A) to (D), and preferably 0.1 to 2% from the viewpoint of addition effect and physical properties of the photocured product.
- Colorants include pigments and / or dyes.
- Pigments include organic and inorganic pigments, for example the following. (1) Azo pigments Insoluble monoazo pigments (toluidine red, permanent carmine FB, fast yellow G etc.), insoluble disazo pigments (disazo yellow AAA, disazo orange PMP etc.), azo lake (soluble azo pigment) (lake red C, brilliant carmine 6B Etc.), condensed azo pigments, chelate azo pigments etc .; (2) polycyclic pigments phthalocyanine blue, indanthrone blue, quinacridone red, dioxazine violet etc .; (3) dyed lake basic dye (such as Victoria Pure Blue BO lake) Acid dyes (alkali blue toner etc.); (4) Others azine pigments (aniline black etc.), daylight fluorescent pigments, nitroso pigments, nitro pigments, natural pigments etc.
- Azo pigments Insoluble monoazo pigments (toluidine red, permanent carmine FB, fast yellow G
- Examples of the inorganic pigment include the following (1) and (2).
- Metal oxides iron oxide, chromium oxide, titanium oxide etc.
- carbon black carbon black
- the amount of the colorant used is usually 2% or less based on the total weight of (A) to (D), and preferably 0.1 to 1% from the viewpoint of the coloring effect and the physical properties of the photocured product.
- antioxidants examples include phenol compounds [monocyclic phenol (such as 2,6-di-t-butyl-p-cresol), bisphenol (such as 2,2′-methylenebis (4-methyl-6-t-butylphenol), etc.] , Polycyclic phenols [1,3,5-trimethyl-2,4,6-tris (3,5-di-t-butyl-4-hydroxybenzyl) benzene etc.], sulfur compounds (dilauryl 3,3 ' Thiodipropionate and the like), phosphorus compounds (triphenyl phosphite and the like), amine compounds (octylated diphenylamine and the like) and the like.
- the amount of the antioxidant used is usually 3% or less based on the total weight of (A) to (D), and preferably 0.1 to 2% from the viewpoint of the antioxidant effect and the physical properties of the photocured product.
- hydrocarbons such as aromatic hydrocarbons (toluene, xylene etc.) and unsaturated aliphatic hydrocarbons (1-butene, 1-nonene etc.)]; halogenated hydrocarbons C1-24 compounds, such as dichloromethane, carbon tetrachloride); alcohols (C1-24 compounds, such as methanol, 1-butanol); thiols (C1-24 compounds, such as ethyl thiol, 1-octylthiol); C3-24 compounds, such as acetone, methyl ethyl ketone); aldehydes (C2-18 compounds, such as 2-methyl-2-propyl aldehyde, 1-pentyl aldehyde); phenols (C6 to 36 compounds, such as phenol, m-, p- and o-cresol); quinones (C6-24 compounds, eg hydroquino) ); Compound of amine (C6-24 compounds such as aromatic hydrocarbons (
- the amount of the chain transfer agent used is usually 10% or less based on the total weight of (A) to (D), preferably from the viewpoint of the polymerizability of the monomer and the compatibility of the monomer with the chain transfer agent. It is 0.05 to 5%.
- metal powder aluminum powder, copper powder, etc.
- metal oxide alumina, silica, talc, mica, clay, etc.
- metal hydroxide aluminum hydroxide, etc.
- metal salt calcium carbonate, silica, etc.
- Calcium acid etc. fibers [inorganic fibers (carbon fibers, glass fibers, asbestos etc.), organic fibers (cotton, nylon, acrylic, rayon fibers etc.) etc], microballoons (glass, shirasu, phenol resin etc.), carbons (Carbon black, graphite, coal powder, etc.), metal sulfide (molybdenum disulfide, etc.), organic powder (wood powder, etc.), etc.
- fibers [inorganic fibers (carbon fibers, glass fibers, asbestos etc.), organic fibers (cotton, nylon, acrylic, rayon fibers etc.) etc], microballoons (glass, shirasu, phenol resin etc.), carbons (Carbon black, graph
- the amount of the filler to be used is usually 30% or less based on the total weight of (A) to (D), and preferably 3 to 20% from the viewpoint of the filling effect, the inkjet dischargeable viscosity, and the physical properties of the photocured product.
- the total amount of (E) used is usually 30% or less based on the total weight of (A) to (D), and preferably 0.05 to 20% from the viewpoint of addition effect and physical properties of the photocured product.
- Water-soluble component in model material The content of the water-soluble component in the model material is preferably 10% by weight or less, more preferably 5% by weight or less from the viewpoint of preventing water-swelling deformation and moisture absorption deformation of the photocured product. It is.
- water-soluble component refers to a component having a solubility in water as defined above of 1 (g / water 100 g) or more, and the components (A) to (D) constituting the model material and This indicates the solubility of (E).
- the Tg of the photo-cured product of the model material is the heat resistance of the photo-cured product and the photo-processed product.
- the temperature is 50 to 120 ° C., more preferably 55 to 110 ° C., and particularly preferably 60 to 100 ° C. from the viewpoint of reducing the warpage of the product.
- the Tg of the photocured product is a value evaluated by the method described later. The Tg can be adjusted to the above range by selecting the type and content of each of the components (A) to (D) constituting the model material.
- the water swelling rate (% by weight) of the light cured product of the model material is preferably 1% or less, more preferably 0.7% or less, particularly from the viewpoint of optical shaping accuracy. Preferably it is 0.5% or less.
- the water swelling ratio of the photocured product is a value evaluated by the method described later. The water swelling ratio can be adjusted to the above range by selecting the type and content of each of the components (A) to (D) constituting the model material.
- the water swelling deformation (mm) of the photocured material of the model material is preferably 2 mm or less, more preferably 1 mm or less, particularly preferably 0.5 mm, from the viewpoint of optical shaping accuracy. It is below.
- water swelling deformation is a value evaluated by the below-mentioned method.
- the water swelling deformation can be adjusted to the above range by selecting the type and content of each of the components (A) to (D) constituting the model material.
- the model material of the present invention is a two-component photocurable resin composition formed by combining a model material for forming an optical shaped article by an inkjet optical shaping method and a support material for supporting the shape of the optical shaped article at the time of optical shaping. It is used as a model material.
- a well-known support material can be used as a support material used together in a two-pack type photocurable resin composition, the ease of removal after photofabrication by the excellent water solubility of a light-cured product, and a model material It is preferable to use the support material of the present invention described below from the viewpoint of the excellent shaping accuracy and mechanical properties of the photoformed product due to the incompatibility.
- the support material in the present invention includes a water-soluble monofunctional ethylenically unsaturated monomer (F), an alkylene oxide adduct containing an oxypropylene group and / or water (G), and a photopolymerization initiator (D) ).
- F water-soluble monofunctional ethylenically unsaturated monomer
- G alkylene oxide adduct containing an oxypropylene group and / or water
- D photopolymerization initiator
- Water-soluble monofunctional ethylenically unsaturated monomer (F) is a support to rapidly dissolve the cured product of the support material in water after photofabrication. It is used as a component of wood.
- the water-soluble polyfunctional ethylenically unsaturated monomer (H) in (F) may be by-produced in the production process of (F), but its content (% by weight) is Preferably, it is 1% or less, more preferably 0.5% or less, particularly preferably 0% from the viewpoint of water solubility of the homopolymer after polymerization of (F).
- hydroxyl group-containing (meth) acrylate [hydroxyethyl (meth) acrylate, hydroxypropyl (meth) acrylate and 4-hydroxybutyl (meth) acrylate etc.]; hydroxyl group containing Mn 200 to 1,000 (Meth) acrylate [polyethylene glycol mono (meth) acrylate, monoalkoxy (C1-4) polyethylene glycol mono (meth) acrylate, polypropylene glycol mono (meth) acrylate, monoalkoxy (C1-4) polypropylene glycol mono (meth) acrylate And mono (meth) acrylates of PEG-PPG block polymers, etc.]; C3-15 (meth) acrylamide derivatives [(meth) acrylamide, N-methyl (meth) acrylamide, N-ethyl T) Acrylamide, N-Propylmetha) Acrylamide, N-Butylmetha) Acrylamide, N, N'-Dimethyl
- water-soluble polyfunctional ethylenically unsaturated monomer (H) examples include polyethylene glycol di (meth) acrylate, polypropylene glycol di (meth) acrylate, di (meth) acrylate of PEG-PPG block polymer, and the like. .
- acrylate and acrylamide derivatives from the viewpoint of photoreactivity, more preferred are hydroxyethyl acrylate, hydroxypropyl acrylate and 4-hydroxybutyl acrylate, acrylamide, acryloyl morpholine, N-methyl acrylamide, N-ethyl acrylamide, N-propyl acrylamide, N-butyl acrylamide, N, N'-dimethyl acrylamide, N, N'-diethyl acrylamide, N-hydroxyethyl-, N-hydroxypropyl- and N-hydroxybutyl acrylamide
- acryloyl morpholine and N-hydroxyethyl acrylamide are preferable from the viewpoint of hypoallergenicity to the human body.
- (G) is an alkylene oxide adduct containing an oxypropylene group.
- (G) at least propylene oxide alone or propylene oxide and other alkylene oxide are added to an active hydrogen compound.
- the active hydrogen compound include monohydric to tetrahydric alcohols and amine compounds. Among these, preferred are dihydric alcohols and water.
- (G) a number average compatible with (F) before curing and incompatible with the photocured product of (F) from the viewpoint of achieving both the support power of the cured product of the support material and the solubility in water.
- the number average molecular weight of polyoxypropylene glycol is preferably 200 to 3,000, and more preferably 400 to 2,000.
- (G) water is preferred from the viewpoint of preventing compatibility or mixing of the model material and the support material before curing.
- (G) is water
- the model material and the support material before curing do not become compatible or mixed, so the model material resulting from the compatibility and mixing problem of the model material and the support material before curing It is possible to completely solve the problems of the deterioration of physical properties and the problem of swelling and deformation of the mixed parts of the respective cured products of and the support material.
- the photopolymerization initiator used for the support material is basically the same as the above-mentioned photopolymerization initiator (D) in the model material, but when (G) is water, Water soluble photoinitiators are used.
- (D) examples of the water-soluble photopolymerization initiator include 1- [4- (2-hydroxyethoxy) phenyl] -2-methyl-1-propan-1-one, etc. There is no particular limitation if it is.
- Each content ratio (% by weight) of (F), (G), and (D) in the support material is a viewpoint that the photocured product of the support material exerts a supporting power as a solid and the photocured product From the viewpoint of the solubility in water, preferably 3 to 45%, more preferably 3 to 43%, particularly preferably 4 to 40%;
- (G) is the solubility and support power of the photocured product in water
- Preferably 50 to 95%, more preferably 53 to 93%, particularly preferably 55 to 90% from the viewpoint of (D) is preferable from the viewpoint of the photocurability of the support material and the solubility of the photocured product in water Is preferably 0.1 to 10%, more preferably 0.3 to 8%, particularly preferably 0.5 to 6%.
- the support material other additives (E) can be contained, if necessary, in a range that does not inhibit the effects of the present invention.
- the same one as other additives (E) in the model material can be used.
- (E) contains a polymerization inhibitor, a coloring agent, an antioxidant, a chain transfer agent, a filler, etc., and can be variously selected according to the purpose, and one kind of single use or a combination of two or more kinds It may be either.
- the use amount (%) of each (E) based on the total weight of (F), (G) and (D) is the amount of each (E) based on the total weight of (A) to (D) in the model material.
- the water solubility of the cured product of the support material is the water dissolution time measured by the method described later (it is necessary for the cured product to be completely dissolved after being immersed in water Time) can be evaluated.
- the water dissolution time is usually 24 hours or less, preferably 0.1 to 20 hours, more preferably 0.1 to 12 hours from the viewpoint of support power and formation accuracy.
- the water dissolution time can be adjusted to the above range by selecting the type and amount of use of the components (F), (G) and (D) constituting the support material.
- the support power in the present invention is the ability of the cured product of the support material to support the cured product of the model material, and the durometer of the cured product of the support material measured by the method described later. It can express with hardness (unit: HDA).
- the support power is preferably 17 to 35, more preferably 20 to 30, from the viewpoint of the shaping accuracy of the light-shaped article and the solubility of the cured product of the support material in water.
- the support force can be adjusted to the above range by selecting the type and amount of use of the components (F), (G) and (D) constituting the support material.
- the support material of the present invention is a two-component photocurable resin composition formed by combining a model material for forming an optical shaped article by an inkjet optical shaping method and a support material for supporting the shape of the optical shaped article at the time of optical shaping. It is used as a support material.
- known model materials can be used as model materials to be used in combination, but there is little swelling deformation due to water or moisture absorption during light curing and after curing, and non-supporting material It is preferable to use the above-mentioned model material of the present invention from the viewpoint of the excellent shaping accuracy and mechanical properties of the light shaping product by the compatibility.
- the light-shaped article of the present invention is usually produced by the following procedure using a light-forming apparatus described later.
- (1) Production of Two-Component Photocurable Resin Composition A two-component photocurable resin composition using the model material of the present invention and the support material of the present invention will be described.
- (1-1) Production of Model Material The curable resin components (A) to (D) of the model material and other additives (E) added as needed are uniformly mixed using a mixing and stirring apparatus etc. The resin composition of the material is manufactured.
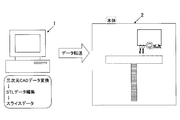
- FIG. 1 is a schematic view of an inkjet three-dimensional modeling system. As shown in FIG. 1, this system is composed of 1 such as a personal computer and a three-dimensional modeling apparatus 2 connected to the 1. 1 receives input of three-dimensional CAD data of an object to be formed, converts the input CAD data as data for three-dimensional formation, for example, to STL (abbreviation of Stereo Lithography) data, and further, this three-dimensional Data of each layer (layer) sliced in the Z direction is generated from the STL data.
- 1 such as a personal computer and a three-dimensional modeling apparatus 2 connected to the 1. 1 receives input of three-dimensional CAD data of an object to be formed, converts the input CAD data as data for three-dimensional formation, for example, to STL (abbreviation of Stereo Lithography) data, and further, this three-dimensional Data of each layer (layer) sliced in the Z direction is generated from the STL data.
- STL abbreviation of Stereo Lithography
- data of a support material for supporting the model material at the time of formation is also generated.
- the software installed in 1 By arranging the support material around the X and Y directions of the lower position model material, the support material is automatically provided so as to support the overhang portion from below.
- 1 also has the function of performing positioning and orientation determination in the X, Y and Z directions of three-dimensional data for modeling within the modeling space of the three-dimensional modeling apparatus 2 using STL data.
- STL data There is. More specifically, while displaying a virtual three-dimensional space in which a modeling space on a modeling table held by the three-dimensional modeling apparatus 2 is three-dimensionally expressed on one screen, at an initial setting position of this space, The three-dimensional STL data of the object to be modeled is displayed, and the three-dimensional STL data of the object to be modeled is held on the screen using a pointing device such as a mouse or a cursor. The desired position and orientation can be determined relative to the build space.
- the three-dimensional modeling apparatus 2 receives data of each layer (layer) sliced in the Z direction described above from one at a time or in units of each layer, and based on the data of each layer, the three-dimensional modeling apparatus 2 has A two-dimensional printer head is scanned in a main scanning direction (X direction) and a sub scanning direction (Y direction), and a support material is discharged from a printer material discharge printer head from a model material and a support material discharge printer head. To form each layer.
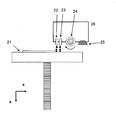
- the three-dimensional modeling apparatus 2 includes a modeling table 21 movable in the Z direction, a printer head 22 for model material for discharging a model material on the modeling table, and a printing head 22 on the modeling table.
- the above-described printer heads 22 and 23, the roller 24 for smoothing, and the UV light source 25 are positioned and mounted on the printer head module 26, and the main scanning direction above the modeling table 21 by a driving unit (not shown) It is integrally driven in the (X direction) and the sub scanning direction (Y direction).
- FIG. 4 is a schematic view of one of the printer heads 22 and 23 as viewed from below. As shown in FIG. 4, each of the printer heads 22 and 23 is provided at a predetermined interval in the sub scanning direction (Y direction) on the surface facing the modeling table, for discharging the model material and the support material. It has a plurality of orifices.
- the roller 24 when the model material and the support material are discharged from the printer heads 22 and 23 at least on the modeling table in the main scanning direction (forward path) moving from the left to the right, the roller 24 is the right end in the main scanning direction described above.
- the roller rotates in the direction of the arrow shown in the figure, that is, in the clockwise direction.
- the printer head module 26 is preferably controlled to rotate in the same direction as the scanning direction when the roller 24 acts.
- the UV light source 25 extends along the sub-scanning direction, and its length desirably has a length including at least all the orifices provided in each printer head.
- the UV light source 25 it is preferable to use a UV lamp or an LED that is generally used to cure a photocurable resin.
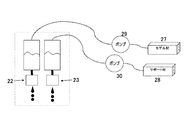
- FIG. 5 is a schematic view of a material supply system for supplying model materials and support materials to the respective printer heads 22 and 23.
- Cartridges 27 and 28 of a model material and a support material are respectively connected to the respective printer heads 22 and 23, and supply pumps 29 and 30 are provided along the connection path, respectively.
- Each of the cartridges 27 and 28 is replaceable when there is no model material or support material inside.
- each slice data in the 1 to Z direction is sent to the three-dimensional modeling apparatus 2.
- the three-dimensional modeling apparatus 2 reciprocates the printer head module 26 in the main scanning direction, and during the reciprocation, the printer head 22 and model material and support material at appropriate positions based on the received slice data.
- Each layer corresponding to each slice data is stacked on the forming table by controlling the discharge from the step S23. At least model material is discharged from the printer head 22 to an appropriate position in each layer, and if necessary, the support material is also discharged from the printer head 23 to an appropriate position to form each layer.
- the roller 24 has already been applied on the forming table 21 in the process from the left to the left), the model material and the support are removed. While in contact with the surface of the material, it keeps rotating in the above-mentioned rotational direction. Then, a layer positioned on the uppermost surface formed on the modeling table 21 by irradiating the ultraviolet light from the UV light source 25 mounted on the printer head module 26 to the surface smoothed by the roller 24.
- each layer is formed of at least a model material, and a support material is added as needed. Accordingly, each layer is formed by discharging the model material and the support material from each of the printer heads 22 and 23 to form the layer positioned on the top surface of the modeling table 21, smoothing the surface of the layer by the roller 24, and smoothing The curing of the layer by irradiation of ultraviolet light to the layer located on the top surface of the shaping table 21 is performed, and by repeating these steps, a three-dimensional model is to be shaped.
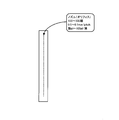
- FIG. 6 is a schematic view showing the process of creating a three-dimensional structure by operating the three-dimensional structure forming apparatus 2.
- the part M shown in the figure is the part on which each layer is laminated by the model material
- the part S is the part on which the layers are laminated by the support material.
- the model material of the portion of M is approximately S-shaped from the lower side to the upper side in the Z direction, in order to support the left and right curved portions of the model material shown in the figure, It is shaped so that the support material is provided in the part of.
- FIG. 7A is a schematic view showing a model including the support material completed in this way
- FIG. 7B is a diagram showing the support material removed from the model including the support material completed. Is a schematic view showing a model that has been As shown in FIG. 7A, at the time when the three-dimensional modeling apparatus 2 completes the formation of the three-dimensional model, a support material for supporting the model material being formed is integral with this model. Is formed. For this reason, since this support material is made of a water-soluble material, for example, by immersing in water, it is possible to obtain a model consisting of only a model material as shown in FIG. 7B.
- parts mean parts by weight and% means weight%.
- Examples 1-1 to 1-7 and Comparative Examples 1-1 to 1-5 Uniform mixing was carried out using the composition (parts) shown in Table 1 to obtain model materials of Examples and Comparative Examples. Each model material was evaluated by the below-mentioned ⁇ evaluation item 1>. The results are shown in Table 1.
- Comparative Example 1-1 is the above-mentioned Patent Document 1 (Japanese Patent Application Laid-Open No. 1-204915); Comparative Examples 1-2 and 1-3 are Patent Document 13 (Japanese Patent Application Laid-Open No. 2010-155926); Comparative Example In 1-4, the model materials described in Patent Document 10 (EP1274551 B1) were used.
- Examples 2-1 to 2-13 and Comparative Examples 2-1 to 2-6 The support materials of Examples and Comparative Examples were obtained by uniformly mixing the composition (parts) shown in Table 2. Each support material was evaluated by the below-mentioned ⁇ evaluation item 2>. The results are shown in Table 2.
- Comparative Examples 2-1 and 2-2 the supports described in Patent Document 10 (EP1274551B1); and in Comparative Examples 2-3 and 2-4, Patent Documents 12 (Japanese Patent Application Laid-Open No. 2010-155889). The material was used.
- Examples 3-1 to 3-4 and Comparative Examples 3-1 to 3-3 As shown in Table 3, the relationship between the model material / support material was evaluated by ⁇ Evaluation Item 3> described later. The results are shown in Table 3.
- the combination of the model material / support material in the comparative example is a combination of the ones described in the patent documents 12 and 13 described in the comparative examples 3-1 and 3-2, and the patent document 10 is a combination of those described in 10.
- A-1 Isobornyl acrylate [trade name "light acrylate IBXA, manufactured by Kyoeisha Chemical Co., Ltd., average number of functional groups 1]
- A-2 acryloyl morpholine (trade name” ACMO ", manufactured by Kojin Co., Ltd., Average functional group number 1]
- A-3 2-hydroxy-3-phenoxypropyl acrylate [trade name "epoxy ester M-600A”, manufactured by Kyoeisha Chemical Co., Ltd., average functional group number 1]
- A-4 phenoxyethyl acrylate [commercial item Name “SR-339”, Sartmar Co., Ltd., average functionality 1]
- A-5 1-adamantyl acrylate [trade name “1-AdA”, Osaka Organic Chemical Industry Co., Ltd., average functionality 1]
- a -6 Stearyl acrylate [trade name "STA”, manufactured by Osaka Organic Chemical Industry Co., Ltd., having an
- B-1 Dicyclopentadimethylol diacrylate [trade name "Light Acrylate DCP-A, manufactured by Kyoeisha Chemical Co., Ltd., average functional group number 2]
- B-2 Acrylic acid of PO 2 molar adduct of bisphenol A diglycidyl ether Acid adduct [trade name “epoxy ester 3002A”, manufactured by Kyoeisha Chemical Co., Ltd., average number of functional groups 2]
- B-3 trimethylolpropane triacrylate [trade name “SR-351”, manufactured by Sartomer Co., Ltd., average functionality Radical number 3]
- B-4 1, 6-hexanediol diacrylate [trade name "Light Acrylate 1, 6 HX-A", manufactured by Kyoeisha Chemical Co., Ltd., average functional group number 2]
- C-3 Urethane acrylate oligomer [trade name “Photomer 6010”, Cogniss Co., Ltd. product, average number of functional groups 2]
- D-1 1,3,5-trimethyl benzoyl diphenyl phosphine oxide [trade name “Lucillin TPO”, BASF ( Co., Ltd.]
- D-2 1-hydroxycyclohexyl phenyl ketone [trade name "IRGACURE 184", manufactured by Ciba Specialty Chemicals Co., Ltd.]
- D-3 2-methyl-1- [4- (methylthio) phenyl] -2 -Morpholinopropan-1-one (trade name "IRGACURE 907", manufactured by Ciba Specialty Chemicals Co., Ltd.)
- D-4 1- [4- (2-hydroxyethoxy) phenyl] -2-methyl-1-propane- 1-ON [trade name "IRGACURE 2959”, manufactured by Ciba Specialty Chemicals Inc.]
- E-1 Polyether modified polydimethyl siloxane [trade name "BYK 307", manufactured by Bick Chemie Japan Ltd.]
- E-2 Hydroquinone monomethyl ether [manufactured by Wako Pure Chemical Industries, Ltd.]
- E-3 Carbon black [Products Name "MHI Black # 220", Made in Gokuh Pigment Co., Ltd.]
- F-1 N-hydroxyethyl acrylamide [trade name "HEAA”, Kojin Co., Ltd., average functionality 1]
- F-2 Acryloyl morpholine [trade name "ACMO”, Kojin Co., average Functional group number 1]
- F-3 Polyethylene glycol monoacrylate (Mn about 336) [trade name "BisomerPEA 6", Cognis KK-made, average functional group number 1]
- G-1 PPG (Mn about 400) [brand name "San Nicks PP-400, Sanyo Chemical Industries, Ltd.
- G-2 PPG (Mn about 1,000) [trade name "Sannicks PP-1000” Sanyo Chemical Industries, Ltd.
- G-3 Water G-4: Polyoxyethylene polyoxypropylene glycol (Mn about 2,000) [trade name "Nypol PE-61", manufactured by Sanyo Chemical Industries, Ltd.]
- G-5 Polyoxypropylene Glyceryl ether (Mn about 1,500) [trade name "Sannicks GP-1500” manufactured by Sanyo Chemical Industries, Ltd.]
- G'-1 propylene glycol [manufactured by Wako Pure Chemical Industries, Ltd.]
- G'-2 PEG (Mn about 400) [trade name "PEG-400", manufactured by Sanyo Chemical Industries, Ltd.]
- E-4 2,4-diphenyl-4-methyl-pentene [manufactured by Wako Pure Chemical Industries, Ltd.]
- E-5 diphosphorous acid [manufactured by Wako Pure Chemical Industries, Ltd.]
- E-6 phenothiazine [sum Optical Pure Chemical Industries, Ltd.]
- H-1 Polyethylene glycol diacrylate (Mn about 600) [trade name "SR-610", manufactured by Sartomer Co., Ltd., average functionality 2]
- H-2 polyethylene glycol diacrylate (Mn 1,000) [trade name " SR-740A ", Sartmar Co., Ltd., average functionality 3]
- the cured product was released from the glass plate and cut into a shape of 5 mm wide and 50 mm long with a cutter to obtain a test piece of a molded product.
- the performance of the test piece was evaluated by the following method. An evaluation result is shown by the average value of five test pieces.
- ⁇ Evaluation item 1> Glass transition point (Tg) (° C.)
- a dynamic viscoelasticity measurement (DMA) device [Model No. “Rheogel-E4000”, manufactured by UBM Co., Ltd.], using the DMA method, tensile by the DMA method Mode, measured at 10 Hz.
- Silicone rubber stopper [trade name "silicone rubber stopper No. 1", made by TERRAOKA, upper diameter 16 mm, lower diameter 12 mm, height 19 mm] processed and central portion longitudinally
- the hole has a diameter of 4 mm penetrating through it, and this is placed on the slide glass so that the hole faces up, the support material is poured into the hole and filled, and ultraviolet light is 1,000 mJ / cm 2 with an ultraviolet irradiation device. Irradiation gave a cured product.
- the cured product was immersed in 100 ml of deionized water in a beaker together with a silicone rubber stopper.
- Supportability is the ability of the cured product of the support material to support the cured product of the model material, and was evaluated by type A durometer hardness according to JIS K7215. Evaluation samples were prepared as follows, and hardness measurement was performed at a thickness of about 5 mm. A spacer of 5 mm in thickness is disposed on four sides of the upper surface of a glass plate [trade name “GLASSPLATE”, manufactured by As One Corp., 200 mm ⁇ 200 mm ⁇ 5 mm thickness], and divided into 3 cm ⁇ 3 cm squares. After casting the resin composition of Table 1 into the square, another similar glass plate is placed to prevent air from entering. (If air gets in, tilt the glass plate and pull it out).
- the whole measuring cylinder was covered with aluminum foil so as not to receive light, and the state after standing for 24 hours was observed, and evaluation was made according to the following criteria from the viewpoint of the separation of the model material and the support material.
- ⁇ Evaluation criteria> ⁇ : good separation (the model material and the support material are completely separated at the interface) ⁇ : the separation property is sufficiently recognized (the model material and the support material are almost separated at the interface) ⁇ : Separability is observed but insufficient (the model material and the support material are separated, but the interface becomes cloudy and compatible state) ⁇ : There is no separability. (The model material and the support material become totally turbid and compatible)
- ⁇ The surface is slightly sticky. (A small amount of cured support material remains on the contact surface of the support material.)
- X The surface swells in the form of gel. (A cured product of many support agents remains on the contact surface of the support material. A mixture of model material and support material.)
- the molded product obtained by curing the resin composition (Examples 1-1 to 1-7) of the model material of the present invention is the resin composition of the comparative model material (Comparative Example 1-1 to Compared with moldings obtained by curing 1-5), swelling deformation due to water and deformation at the time of drying are less, so that not only can the shaping accuracy in the optical shaping of the model material be improved, but also after curing of the model material It can be seen that the moisture absorption and deformation of the photofabricated object can also be suppressed, which is more excellent.
- the cured product obtained by curing the resin composition (Examples 2-1 to 2-13) of the support material of the present invention is the resin composition of the comparative support material (Comparative Examples 2-1 to 2). It is understood that the water solubility and the supporting power are more excellent in comparison with the cured product obtained by curing 2-6). Further, from the results of Table 3, the resin composition of the model material of the present invention (Examples 1-1, 1-6) is the resin composition of the comparison model material (Comparative Examples 1-2, 1-4).
- the resin composition of the support material is not compatible with or mixed with the resin composition of the support material, there is hardly any mixing portion between the model material and the support material until curing, which causes swelling deformation of the cured product, deterioration of physical properties, and shaping It is possible to suppress the deviation of the accuracy and the like, and it is understood that it is more excellent.
- the model material constituting the two-component photocurable resin composition for inkjet photofabrication of the present invention has very little swelling or moisture absorption deformation due to water or moisture absorption during photocuring and after curing, and the light of the support material
- the cured product is excellent in water solubility, easy to remove after photofabrication, and the resulting photofabricated product is excellent in modeling accuracy and mechanical properties, so the two-component photocurable resin composition has a three-dimensional model. It can be suitably used as a material and is extremely useful.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Polymers & Plastics (AREA)
- Mechanical Engineering (AREA)
- Optics & Photonics (AREA)
- Physics & Mathematics (AREA)
- Life Sciences & Earth Sciences (AREA)
- Wood Science & Technology (AREA)
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020137013811A KR101884038B1 (ko) | 2010-11-01 | 2011-10-17 | 잉크젯 광조형법에서의, 광조형품 형성용 모델재, 광조형품의 광조형시의 형상 지지용 서포트재 및 광조형품의 제조 방법 |
| CN201180052940.XA CN103189187B (zh) | 2010-11-01 | 2011-10-17 | 在喷墨三维印刷法中用于形成光加工模型的造型材料、在光加工时用于支持光加工模型的形状的支持材料和光加工模型的制造方法 |
| EP11837855.3A EP2636511B1 (en) | 2010-11-01 | 2011-10-17 | Modeling material for forming photoshaped article by ink-jet photoshaping method, support material for shape supporting during formation of photoshaped article by the photoshaping method, and process for producing photoshaped article by the photoshaping method |
| US13/868,157 US9556346B2 (en) | 2010-11-01 | 2013-04-23 | Modeling material for forming photofabrication model in ink-jet three dimensional printing, supporting material for supporting the shape of photofabrication model on photofabrication and production method of photofabrication model |
| US15/164,905 US9790382B2 (en) | 2010-11-01 | 2016-05-26 | Modeling material for forming photofabrication model in ink-jet three dimensional printing, supporting material for supporting the shape of photofabrication model on photofabrication and production method of photofabrication model |
| US15/164,906 US9796863B2 (en) | 2010-11-01 | 2016-05-26 | Modeling material for forming photofabrication model in ink-jet three dimensional printing, supporting material for supporting the shape of photofabrication model on photofabrication and production method of photofabrication model |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010244909 | 2010-11-01 | ||
| JP2010-244909 | 2010-11-01 | ||
| JP2011-208389 | 2011-09-26 | ||
| JP2011208389A JP5890990B2 (ja) | 2010-11-01 | 2011-09-26 | インクジェット光造形法における、光造形品形成用モデル材、光造形品の光造形時の形状支持用サポート材および光造形品の製造方法 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/868,157 Continuation US9556346B2 (en) | 2010-11-01 | 2013-04-23 | Modeling material for forming photofabrication model in ink-jet three dimensional printing, supporting material for supporting the shape of photofabrication model on photofabrication and production method of photofabrication model |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012060204A1 true WO2012060204A1 (ja) | 2012-05-10 |
Family
ID=46024322
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/073778 Ceased WO2012060204A1 (ja) | 2010-11-01 | 2011-10-17 | インクジェット光造形法における、光造形品形成用モデル材、光造形品の光造形時の形状支持用サポート材および光造形品の製造方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (3) | US9556346B2 (enExample) |
| EP (1) | EP2636511B1 (enExample) |
| JP (1) | JP5890990B2 (enExample) |
| KR (1) | KR101884038B1 (enExample) |
| CN (3) | CN105058791B (enExample) |
| WO (1) | WO2012060204A1 (enExample) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20140265033A1 (en) * | 2013-03-15 | 2014-09-18 | Michelin Recherche Et Technique S.A. | Method for improved tire mold manufacturing |
| WO2015056614A1 (ja) * | 2013-10-15 | 2015-04-23 | コニカミノルタ株式会社 | 三次元造形用インクジェットインク組成物および三次元造形物の製造方法 |
| CN106915084A (zh) * | 2017-03-07 | 2017-07-04 | 杭州杭景模型有限公司 | 3d打印机及其打印平台 |
| CN108117799A (zh) * | 2017-12-31 | 2018-06-05 | 中山市威傲联复合材料有限公司 | 一种室内led显示屏间隙遮蔽油墨及其制备方法 |
| WO2020017615A1 (ja) | 2018-07-18 | 2020-01-23 | Kjケミカルズ株式会社 | 三次元造形サポート材用活性エネルギー線硬化性樹脂組成物とインク |
| CN113386359A (zh) * | 2015-08-14 | 2021-09-14 | 斯特拉塔西斯公司 | 支撑材料制剂及制造三维模型物体的方法 |
| US20220134640A1 (en) * | 2019-07-19 | 2022-05-05 | Stratasys Ltd. | Additive manufacturing of three-dimensional objects containing a transparent material |
Families Citing this family (149)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5691155B2 (ja) * | 2009-11-10 | 2015-04-01 | ソニー株式会社 | 立体造形物の造形方法及び造形装置 |
| JP5890990B2 (ja) * | 2010-11-01 | 2016-03-22 | 株式会社キーエンス | インクジェット光造形法における、光造形品形成用モデル材、光造形品の光造形時の形状支持用サポート材および光造形品の製造方法 |
| WO2014068579A1 (en) * | 2012-11-05 | 2014-05-08 | Yehoshua Sheinman | System and method for direct inkjet printing of 3d objects |
| EP2969465B1 (en) | 2013-03-14 | 2019-05-01 | Stratasys Ltd. | Polymer based molds and methods of manufacturing there of |
| EP3045483B1 (en) * | 2013-09-11 | 2018-04-04 | Sanyo Chemical Industries, Ltd. | Active energy ray curable resin composition, and cured product |
| WO2015049873A1 (ja) * | 2013-10-03 | 2015-04-09 | コニカミノルタ株式会社 | インク組成物および画像又は三次元造形物形成方法 |
| JP6162565B2 (ja) * | 2013-10-04 | 2017-07-12 | 株式会社ミマキエンジニアリング | 三次元造形装置及び三次元造形対象物の成形方法 |
| JP2015077754A (ja) * | 2013-10-18 | 2015-04-23 | ローランドディー.ジー.株式会社 | 三次元造形装置 |
| GB2521386A (en) | 2013-12-18 | 2015-06-24 | Ibm | Improvements in 3D printing |
| JP2015131398A (ja) * | 2014-01-09 | 2015-07-23 | セイコーエプソン株式会社 | 三次元造形物の製造方法、三次元造形物製造装置、インクセットおよび三次元造形物 |
| JP6387614B2 (ja) * | 2014-01-09 | 2018-09-12 | セイコーエプソン株式会社 | 三次元造形物の製造方法およびインクセット |
| JP6273849B2 (ja) | 2014-01-15 | 2018-02-07 | セイコーエプソン株式会社 | 三次元造形物の製造方法、三次元造形物製造装置およびインクセット |
| JP6367560B2 (ja) * | 2014-01-20 | 2018-08-01 | ローランドディー.ジー.株式会社 | 三次元造形装置および三次元造形方法 |
| JP6405634B2 (ja) * | 2014-01-28 | 2018-10-17 | セイコーエプソン株式会社 | 三次元造形物の製造方法、三次元造形物製造装置および三次元造形物 |
| US20170173865A1 (en) | 2014-02-10 | 2017-06-22 | Stratasys Ltd. | Composition and method for additive manufacturing of an object |
| JP6085578B2 (ja) * | 2014-03-11 | 2017-02-22 | 住友重機械工業株式会社 | 膜形成方法及び膜形成装置 |
| EP3116961A1 (en) * | 2014-03-11 | 2017-01-18 | 3D Systems, Incorporated | Inks for 3d printing |
| JP6399084B2 (ja) * | 2014-03-14 | 2018-10-03 | コニカミノルタ株式会社 | 3d造形用光硬化性組成物および3d造形物の製造方法 |
| JP6081401B2 (ja) * | 2014-03-25 | 2017-02-15 | アイ−スクウェアード・ゲゼルシャフト・ミット・ベシュレンクテル・ハフツング | サポート部形成用の光硬化性樹脂組成物 |
| JP6454977B2 (ja) * | 2014-03-26 | 2019-01-23 | セイコーエプソン株式会社 | 三次元造形物製造装置 |
| JP2015189007A (ja) | 2014-03-27 | 2015-11-02 | セイコーエプソン株式会社 | 造形物の製造方法 |
| JP6405721B2 (ja) * | 2014-06-04 | 2018-10-17 | 東洋インキScホールディングス株式会社 | 光学立体造形用硬化性材料および立体造形物 |
| JP6269333B2 (ja) * | 2014-06-09 | 2018-01-31 | コニカミノルタ株式会社 | 3d造形用モデル材組成物、3d造形用インクセットおよび3d造形物の製造方法 |
| JP6269339B2 (ja) * | 2014-06-17 | 2018-01-31 | コニカミノルタ株式会社 | 3d造形用組成液、3d造形用インクセットおよび3d造形物の製造方法 |
| JP6586284B2 (ja) * | 2014-06-20 | 2019-10-02 | 株式会社キーエンス | インクジェット光造形法における光造形品形成用モデル材及び光造形品の製造方法 |
| JP2016020489A (ja) * | 2014-06-20 | 2016-02-04 | 三洋化成工業株式会社 | 活性エネルギー線硬化性樹脂組成物 |
| EP2974850A1 (de) * | 2014-07-17 | 2016-01-20 | Marabu GmbH & Co. KG | Verdruckbares Baumaterial für 3D-Druck |
| FR3024659B1 (fr) | 2014-08-05 | 2016-09-30 | Oreal | Procede de fabrication additive d'un objet tridimensionnel comprenant ou constituant une composition cosmetique par projection directe utilisant un materiau photoactivable, et appareil associe |
| US9694542B2 (en) * | 2014-09-12 | 2017-07-04 | Ricoh Company, Ltd. | Method and apparatus for molding three-dimensional object and molding data generation method for three-dimensional object |
| EP3235630B1 (en) | 2014-12-16 | 2020-08-19 | FUJIFILM Corporation | Actinic-ray-curable inkjet ink composition for 3d printing, three-dimensional modeling method, and actinic-ray-curable inkjet ink set for 3d printing |
| JP6451288B2 (ja) * | 2014-12-17 | 2019-01-16 | コニカミノルタ株式会社 | モデル材インク、インクセットおよび3d造形物を製造する方法 |
| JP6451287B2 (ja) * | 2014-12-17 | 2019-01-16 | コニカミノルタ株式会社 | 3d造形物の製造方法、モデル材インクおよびインクセット |
| TWI592760B (zh) * | 2014-12-30 | 2017-07-21 | 羅門哈斯電子材料韓國有限公司 | 與經外塗佈之光致抗蝕劑一起使用之塗層組合物 |
| JP6578660B2 (ja) * | 2015-01-22 | 2019-09-25 | 富士ゼロックス株式会社 | 三次元造形用支持材、三次元造形用組成物セット、および三次元造形装置、三次元造形物の製造方法 |
| WO2016118151A1 (en) * | 2015-01-23 | 2016-07-28 | Hewlett-Packard Development Company, L.P. | Susceptor materials for 3d printing using microwave processing |
| EP3251818B1 (en) * | 2015-01-26 | 2019-12-11 | KJ Chemicals Corporation | Active energy ray-curable resin composition for three-dimensional model supporting material |
| US10286598B2 (en) | 2015-02-06 | 2019-05-14 | Kj Chemicals Corporation | Method for manufacturing three-dimensional molded product and three-dimensional molded product using the same |
| CN107405838A (zh) * | 2015-03-03 | 2017-11-28 | 巴斯夫欧洲公司 | 使用两种预支撑材料生产三维结构的方法 |
| MX2017011244A (es) * | 2015-03-03 | 2017-11-01 | Basf Se | Metodo para producir una estructura tridimensional mediante impresion 3d. |
| WO2016143559A1 (ja) * | 2015-03-10 | 2016-09-15 | コニカミノルタ株式会社 | 立体造形用インク組成物、インクセットおよび立体造形物の製造方法 |
| KR20170129181A (ko) * | 2015-03-11 | 2017-11-24 | 스트라타시스 엘티디. | 지지체 재료 조성물 및 이를 이용한 적층 제조 방법 |
| KR102307599B1 (ko) * | 2015-03-16 | 2021-10-05 | 엘지전자 주식회사 | 3차원 인쇄 장치 |
| KR102523252B1 (ko) | 2015-03-19 | 2023-04-20 | 다우 글로벌 테크놀로지스 엘엘씨 | 광조절된 라디칼 중합을 사용하는 적층 가공 방법 |
| CN104801703B (zh) * | 2015-03-26 | 2016-08-24 | 成都新柯力化工科技有限公司 | 一种用于三维打印的柔性金属粉及其制备方法和应用方法 |
| US20160279881A1 (en) * | 2015-03-27 | 2016-09-29 | Seiko Epson Corporation | Three-dimensional modeling apparatus |
| JP6777414B2 (ja) * | 2015-03-31 | 2020-10-28 | 三洋化成工業株式会社 | 活性エネルギー線硬化性樹脂組成物 |
| JP6543498B2 (ja) * | 2015-04-02 | 2019-07-10 | 株式会社キーエンス | インクジェット光造形法における光造形品の製造方法 |
| KR101644017B1 (ko) * | 2015-04-10 | 2016-07-29 | 제주대학교 산학협력단 | 3차원 프린팅 구조물의 지지 구조 적층 시스템 및 이를 이용한 3차원 프린팅 방법 |
| US9751263B2 (en) * | 2015-04-20 | 2017-09-05 | Xerox Corporation | Injection molding to finish parts printed with a three-dimensional object printer |
| WO2016199611A1 (ja) | 2015-06-08 | 2016-12-15 | 富士フイルム株式会社 | 3次元印刷用活性光線硬化型インクジェットインクセット、3次元印刷方法、及び、3次元印刷システム |
| JP6493875B2 (ja) * | 2015-06-08 | 2019-04-03 | 富士フイルム株式会社 | 3次元造形物の形成方法 |
| GR1009027B (el) * | 2015-06-10 | 2017-05-09 | Κωνσταντινος Σωζοντος Αντωνιου | Διαδικασια απομακρυνσης υποστηριξεων κατα τη δημιουργια τρισδιαστατων μοντελων με τη μεθοδο του φωτοπολυμερισμου με τη χρηση δυο ή περισσοτερων ρητινων |
| JP6485239B2 (ja) * | 2015-06-12 | 2019-03-20 | 富士ゼロックス株式会社 | 造形装置 |
| KR101680334B1 (ko) * | 2015-06-15 | 2016-11-29 | 주식회사 퓨쳐캐스트 | 3차원 프린팅 방식을 이용한 금형 제작방법 |
| US10882245B2 (en) * | 2015-07-06 | 2021-01-05 | Ricoh Company, Ltd. | Method of manufacturing three-dimensional object, liquid set for manufacturing three-dimensional object, device for manufacturing three-dimensional object, and gel object |
| JP6724303B2 (ja) * | 2015-07-22 | 2020-07-15 | 株式会社リコー | 立体造形物の製造方法 |
| JP6724304B2 (ja) * | 2015-07-22 | 2020-07-15 | 株式会社リコー | 立体造形物の製造方法 |
| KR102037558B1 (ko) | 2015-07-20 | 2019-10-28 | 주식회사 엘지화학 | 3d 프린팅 지지체용 잉크 조성물 및 이를 이용한 3d 프린팅 제조방법 |
| JP6679234B2 (ja) * | 2015-07-29 | 2020-04-15 | マクセルホールディングス株式会社 | モデル材用樹脂組成物、サポート材用樹脂組成物、光造形品、および、光造形品の製造方法 |
| JP6720483B2 (ja) * | 2015-07-31 | 2020-07-08 | 富士ゼロックス株式会社 | 三次元造形用組成物セット、及び三次元造形物の製造方法 |
| JP6710506B2 (ja) | 2015-08-21 | 2020-06-17 | 株式会社ミマキエンジニアリング | インクジェット造形方法 |
| JP6574980B2 (ja) * | 2015-09-01 | 2019-09-18 | Kjケミカルズ株式会社 | モデル材用活性エネルギー線硬化性樹脂組成物 |
| JP2017052177A (ja) * | 2015-09-09 | 2017-03-16 | 富士ゼロックス株式会社 | 三次元造形物の製造方法、三次元造形用支持材、三次元造形用支持材カートリッジ、及び三次元造形用組成物セット |
| JP6796069B2 (ja) * | 2015-09-15 | 2020-12-02 | マクセルホールディングス株式会社 | 光造形用インクセット、および、光造形品の製造方法 |
| CN108025486B (zh) * | 2015-09-15 | 2021-05-04 | 麦克赛尔控股株式会社 | 模型材用树脂组合物、光造型用油墨组及光造型品的制造方法 |
| EP3147707B1 (de) * | 2015-09-25 | 2019-04-24 | Ivoclar Vivadent AG | Keramik- und glaskeramikschlicker für die stereolithography |
| FR3042195B1 (fr) | 2015-10-09 | 2017-11-24 | Arkema France | Oligomere ionique et composition polymerisable le contenant pour materiaux hydro-fragmentables a usage provisoire |
| JP6808439B2 (ja) * | 2015-10-30 | 2021-01-06 | キヤノン株式会社 | 立体物の製造方法および立体物の製造に用いる除去液 |
| JP6669985B2 (ja) | 2015-11-12 | 2020-03-18 | セイコーエプソン株式会社 | 三次元造形物の製造方法 |
| CN105440200B (zh) * | 2015-12-15 | 2017-08-22 | 中山职业技术学院 | 一种有机‑无机杂化3d打印材料及其制备方法 |
| EP3402644A4 (en) | 2016-01-15 | 2019-08-14 | Stratasys Ltd. | BRUSH FORMULATION IN THE WATER AND METHOD FOR THE GENERATIVE MANUFACTURE THEREWITH |
| JP6672874B2 (ja) * | 2016-02-22 | 2020-03-25 | 富士ゼロックス株式会社 | 三次元造形用支持材、三次元造形用支持材カートリッジ、三次元造形用組成物セット、三次元造形装置、及び三次元造形物の製造方法 |
| KR101942244B1 (ko) | 2016-02-26 | 2019-01-28 | 주식회사 엘지화학 | 3d 프린팅 지지체용 잉크 조성물 및 이를 이용한 3d 프린팅 제조방법 |
| JP6812116B2 (ja) * | 2016-03-14 | 2021-01-13 | マクセルホールディングス株式会社 | モデル材用樹脂組成物、および、光造形品の製造方法 |
| JP6819671B2 (ja) * | 2016-03-18 | 2021-01-27 | 株式会社リコー | 活性エネルギー線硬化型組成物、立体造形物の製造方法、及び立体造形物の製造装置 |
| JP6926395B2 (ja) * | 2016-03-28 | 2021-08-25 | 東洋インキScホールディングス株式会社 | 重合性組成物 |
| KR102125596B1 (ko) | 2016-04-08 | 2020-06-22 | 주식회사 엘지화학 | 3d 프린팅 지지체용 잉크 조성물 |
| JP6680887B2 (ja) | 2016-04-15 | 2020-04-15 | ヒューレット−パッカード デベロップメント カンパニー エル.ピー.Hewlett‐Packard Development Company, L.P. | 複合粒状構築材料 |
| JP6903283B2 (ja) * | 2016-05-13 | 2021-07-14 | 株式会社リコー | 活性エネルギー線硬化型組成物、硬化物、組成物収容容器、像形成装置、及び像形成方法 |
| JP6891474B2 (ja) * | 2016-06-13 | 2021-06-18 | 株式会社リコー | 形状支持用液体、及び立体造形物の製造方法 |
| US11078374B2 (en) | 2016-06-13 | 2021-08-03 | Ricoh Company, Ltd. | Active-energy-ray-curable liquid composition, three-dimensional object forming material set, method for producing three-dimensional object, and three-dimensional object producing apparatus |
| JP6870275B2 (ja) * | 2016-10-27 | 2021-05-12 | 株式会社リコー | 立体造形用支持材、立体造形物の製造方法、及び立体造形物の製造装置 |
| JP6938860B2 (ja) * | 2016-06-13 | 2021-09-22 | 株式会社リコー | 形状支持用液体、及び立体造形物の製造方法 |
| CN109312177B (zh) * | 2016-06-13 | 2022-03-29 | 株式会社理光 | 活性能量射线固化性液体组合物、三维物体形成材料套组、三维物体制备方法和三维物体制备设备 |
| CN109328201A (zh) * | 2016-06-22 | 2019-02-12 | 麦克赛尔控股株式会社 | 模塑材料用树脂组合物和光造型品的制造方法 |
| JP6848223B2 (ja) | 2016-06-28 | 2021-03-24 | 株式会社リコー | 活性エネルギー線硬化型液体組成物、立体造形物の製造方法、立体造形物の製造装置 |
| WO2018005501A1 (en) | 2016-06-30 | 2018-01-04 | 3M Innovative Properties Company | Printable compositions including highly viscous components and methods of creating 3d articles therefrom |
| KR102261399B1 (ko) | 2016-06-30 | 2021-06-07 | 신에쓰 가가꾸 고교 가부시끼가이샤 | 자외선 경화성 실리콘 조성물 및 그의 경화물 |
| JP6819106B2 (ja) * | 2016-07-12 | 2021-01-27 | 株式会社リコー | 活性エネルギー線硬化型組成物、インク、収容容器、2次元又は3次元の像形成装置、2次元又は3次元の像形成方法、及び、塗工物 |
| EP3508335B1 (en) * | 2016-09-01 | 2023-02-01 | Nippon Shokubai Co., Ltd. | Photocurable support material composition for inkjet 3d printers, ink for inkjet 3d printers, cartridge for inkjet 3d printers, method for producing support material and method for producing optically shaped article |
| US11607844B2 (en) | 2016-10-04 | 2023-03-21 | Tokyo Printing Ink Mfg. Co., Ltd. | Treatment agent for additive manufacturing apparatus |
| JP6774288B2 (ja) * | 2016-10-04 | 2020-10-21 | 共栄社化学株式会社 | 活性線硬化性樹脂組成物 |
| JP7701780B2 (ja) | 2016-10-28 | 2025-07-02 | ストラタシス,インコーポレイテッド | 熱硬化性組成物及びそれからの三次元物体の形成 |
| CN106738874B (zh) * | 2016-11-24 | 2019-01-29 | 南京航空航天大学 | 一种快速去除3d打印支撑的方法 |
| JP6381614B2 (ja) * | 2016-11-29 | 2018-08-29 | マクセルホールディングス株式会社 | サポート材組成物 |
| EP3560683A4 (en) * | 2016-12-22 | 2020-08-26 | Maxell Holdings, Ltd. | INK SET FOR OPTICALLY SHAPED AND OPTICALLY SHAPED OBJECT PRODUCTION PROCESS USING IT |
| JPWO2018142485A1 (ja) * | 2017-01-31 | 2019-11-14 | マクセルホールディングス株式会社 | 光造形用インクセット、光造形品、及び、光造形品の製造方法 |
| JP2018122499A (ja) * | 2017-01-31 | 2018-08-09 | マクセルホールディングス株式会社 | 光造形用インクセット、光造形品、及び、光造形品の製造方法 |
| US20200407581A1 (en) * | 2017-01-31 | 2020-12-31 | Maxell Holdings, Ltd. | Optical shaping ink set, optically shaped article, and method for producing optically shaped article |
| CN110023058B (zh) * | 2017-02-06 | 2021-08-10 | 惠普发展公司,有限责任合伙企业 | 3d打印 |
| US10781228B2 (en) * | 2017-02-06 | 2020-09-22 | Hewlett-Packard Development Company, L.P. | Fusing agent including a metal bis(dithiolene) complex |
| US11945152B2 (en) | 2017-03-06 | 2024-04-02 | Maxell, Ltd. | Model material ink set, support material composition, ink set, three-dimensional shaped object, and method for manufacturing three-dimensional shaped object |
| JP2018154092A (ja) * | 2017-03-21 | 2018-10-04 | 株式会社リコー | 立体造形物の製造方法、及び立体造形物のデータの作成方法 |
| JP6880912B2 (ja) * | 2017-03-28 | 2021-06-02 | 株式会社リコー | 立体造形用液体セット、立体造形物の製造方法、及び立体造形装置 |
| JP6930176B2 (ja) | 2017-03-29 | 2021-09-01 | 株式会社リコー | 立体造形用組成物のセット、立体造形物の製造方法、及び立体造形装置 |
| ES2920055T3 (es) | 2017-04-25 | 2022-08-01 | Basf Se | Una composición utilizada en un sistema de impresión 3D y su aplicación |
| CN114750408A (zh) * | 2017-05-15 | 2022-07-15 | 霍洛公司 | 粘性膜三维打印系统和方法 |
| EP3415314A1 (en) * | 2017-06-15 | 2018-12-19 | Mimaki Engineering Co., Ltd. | Building apparatus and building method |
| JP6815940B2 (ja) * | 2017-06-15 | 2021-01-20 | 株式会社ミマキエンジニアリング | 造形装置及び造形方法 |
| EP4574852A3 (en) * | 2017-06-30 | 2025-10-15 | Align Technology, Inc. | 3d printed composites from a single resin by patterned light exposures |
| CN111148483B (zh) | 2017-07-28 | 2022-11-15 | 斯特拉塔西斯公司 | 制造具有血管特性的物体的方法和系统 |
| DK3658352T3 (da) * | 2017-07-28 | 2024-01-02 | Stratasys Ltd | Additive fremstillingsprocesser, der anvender et materiale med et blødt kropsvævs egenskaber |
| EP4289607B1 (en) | 2017-07-28 | 2024-10-30 | Stratasys Ltd. | Method and system for fabricating object featuring properties of a hard tissue |
| JP7165186B2 (ja) * | 2017-07-28 | 2022-11-02 | ストラタシス リミテッド | 軟らかい材料からなる三次元物体の付加製造において使用可能な配合物 |
| CN111344328B (zh) * | 2017-11-09 | 2023-05-30 | 科思创德国股份有限公司 | 制造物件的方法和可自由基交联树脂在增材制造法中的用途 |
| US10899088B2 (en) | 2017-11-17 | 2021-01-26 | Matsuura Machinery Corporation | Support and method of shaping workpiece and support |
| WO2019129560A1 (en) | 2017-12-29 | 2019-07-04 | Basf Se | A composition to produce support sub-structures for 3d photopolymer jetting |
| EP3732044A1 (en) * | 2017-12-31 | 2020-11-04 | Stratasys Ltd. | Modeling material formulations usable in additive manufacturing of three-dimensional objects at low temperatures |
| IL275770B2 (en) | 2017-12-31 | 2024-07-01 | Stratasys Ltd | Support material formulations usable in additive manufacturing of three-dimensional objects at low temperatures |
| JP7052397B2 (ja) * | 2018-02-14 | 2022-04-12 | 株式会社リコー | 立体造形物用組成物、立体造形物の製造装置、及び立体造形物の製造方法 |
| JP7112207B2 (ja) | 2018-02-20 | 2022-08-03 | 株式会社ミマキエンジニアリング | 三次元造形物 |
| CN111615447B (zh) | 2018-03-02 | 2023-04-04 | 株式会社日本触媒 | 支撑材料及光造型物的制造法、喷墨三维打印机用光固化性支撑材料用组合物、墨水、墨盒 |
| EP3753957B1 (en) * | 2018-03-30 | 2023-09-06 | Mitsui Chemicals, Inc. | Photolithographic curable composition, evaporative pattern, and method for producing three dimensional shaped article |
| WO2020015905A1 (en) | 2018-07-18 | 2020-01-23 | Arkema France | Articles prepared using curable compositions based on polymerizable ionic species |
| WO2020028232A1 (en) * | 2018-08-01 | 2020-02-06 | Carbon, Inc. | Production of low density products by additive manufacturing |
| JP6962290B2 (ja) | 2018-08-02 | 2021-11-05 | 信越化学工業株式会社 | 光造形用紫外線硬化型シリコーン組成物およびその硬化物 |
| CN109232791B (zh) * | 2018-08-20 | 2020-06-16 | 珠海赛纳打印科技股份有限公司 | 一种3d打印用光固化非透明材料及其制备方法、3d打印制品及3d打印机 |
| JP7180684B2 (ja) | 2018-09-20 | 2022-11-30 | 信越化学工業株式会社 | 紫外線硬化性シリコーン組成物及びその硬化物 |
| JP7541011B2 (ja) | 2018-09-24 | 2024-08-27 | ビーエーエスエフ ソシエタス・ヨーロピア | 3d印刷において使用するための光硬化性組成物 |
| WO2020095846A1 (ja) * | 2018-11-07 | 2020-05-14 | ナガセケムテックス株式会社 | 光硬化性樹脂組成物および樹脂硬化物 |
| JP2020117603A (ja) * | 2019-01-22 | 2020-08-06 | 株式会社リコー | 活性エネルギー線硬化型組成物、活性エネルギー線硬化型インクジェットインク、組成物収容容器、インクジェット吐出装置および硬化物 |
| JP2020151958A (ja) * | 2019-03-20 | 2020-09-24 | 株式会社リコー | 立体造形物の製造装置、立体造形物の製造方法、及び立体造形プログラム |
| WO2020189654A1 (en) * | 2019-03-18 | 2020-09-24 | Ricoh Company, Ltd. | Three-dimensional object forming apparatus, three-dimensional object forming method, and program |
| JP7451967B2 (ja) * | 2019-11-28 | 2024-03-19 | 株式会社リコー | 活性エネルギー線急速硬化型組成物、造形方法、及び造形装置 |
| JP2021084410A (ja) * | 2019-11-29 | 2021-06-03 | 株式会社リコー | 立体造形用組成物セット、立体造形物、立体造形物の製造方法、2次元又は3次元の像形成装置及び2次元又は3次元の像形成方法 |
| JP7463718B2 (ja) * | 2019-12-25 | 2024-04-09 | Dic株式会社 | 硬化性樹脂組成物、硬化物及び立体造形物 |
| WO2022051521A1 (en) * | 2020-09-03 | 2022-03-10 | Basf Se | Reactive polyurethane elastomer |
| US20230407132A1 (en) * | 2020-10-28 | 2023-12-21 | Covestro (Netherlands) B.V. | (meth)acrylate-functional radiation curable compositions for additive fabrication |
| CN112831022B (zh) * | 2020-12-31 | 2022-07-15 | 中电保力(北京)科技有限公司 | 一种手办模型材料及其制备方法 |
| US20240117097A1 (en) * | 2021-01-29 | 2024-04-11 | Nagase Chemtex Corporation | Resin composition for three-dimensional photoshaping |
| KR20230164045A (ko) * | 2021-04-02 | 2023-12-01 | 디아이씨 가부시끼가이샤 | 광 조형용 경화성 수지 조성물, 경화물 및 입체 조형물 |
| JP7342910B2 (ja) | 2021-04-23 | 2023-09-12 | 信越化学工業株式会社 | 光造形用紫外線硬化性シリコーン組成物、その硬化物および硬化物の製造方法 |
| WO2023084517A1 (en) | 2021-11-12 | 2023-05-19 | Polyfos 3D Ltd | Vat polymerization process |
| JP7757746B2 (ja) * | 2021-12-06 | 2025-10-22 | セイコーエプソン株式会社 | 放射線硬化型インクジェット組成物及びインクジェット記録装置 |
| JP7124948B1 (ja) | 2021-12-15 | 2022-08-24 | 株式会社リコー | 活性エネルギー線硬化型組成物、活性エネルギー線硬化型インク組成物、インクジェット用インク組成物、組成物収容容器、2次元又は3次元の像形成装置、2次元又は3次元の像形成方法、硬化物、加飾体、積層体、フレキシブルデバイス用部材、及びフレキシブルデバイス |
| US20230391935A1 (en) * | 2022-06-06 | 2023-12-07 | Toyota Motor Engineering & Manufacturing North America, Inc. | Resins for digital light processing 3d printing |
| CN116237516B (zh) * | 2022-12-19 | 2024-12-27 | 深圳奇遇科技有限公司 | 用于光固化3d打印的金属浆料及其制备方法、应用 |
| WO2024214654A1 (ja) * | 2023-04-12 | 2024-10-17 | 三井化学株式会社 | 複合樹脂硬化物の製造方法、硬化性樹脂組成物、インク、インクセット、および、硬化物 |
| JP2025002169A (ja) | 2023-06-22 | 2025-01-09 | 信越化学工業株式会社 | 室温硬化性オルガノポリシロキサン組成物を用いた3次元(3d)成形品を製造する方法及び該方法で製造した3次元(3d)成形品 |
Citations (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH01204915A (ja) | 1988-01-27 | 1989-08-17 | Desoto Inc | 光学的立体造形用樹脂組成物 |
| JPH0859760A (ja) | 1994-08-18 | 1996-03-05 | Japan Synthetic Rubber Co Ltd | 光学的立体造形用樹脂組成物 |
| JPH09169827A (ja) | 1995-10-20 | 1997-06-30 | Teijin Seiki Co Ltd | 光硬化性樹脂組成物 |
| JPH09316111A (ja) | 1996-05-30 | 1997-12-09 | Japan Synthetic Rubber Co Ltd | 光硬化性樹脂組成物および樹脂製型の製造方法 |
| JP2001310918A (ja) | 2000-04-26 | 2001-11-06 | Mitsubishi Rayon Co Ltd | 光造形用硬化性組成物および成形品 |
| JP2002067174A (ja) | 2000-08-30 | 2002-03-05 | Minolta Co Ltd | データ処理装置及び方法、並びに三次元造形装置及び方法 |
| JP2002178412A (ja) | 2000-12-14 | 2002-06-26 | Sanyo Electric Co Ltd | 光造形装置及び光造形品の制作方法 |
| JP2004059601A (ja) | 2002-07-24 | 2004-02-26 | Mitsubishi Rayon Co Ltd | 光学的立体造形用樹脂組成物、及び立体造形物 |
| JP2004255839A (ja) | 2003-02-28 | 2004-09-16 | Hitachi Printing Solutions Ltd | インクジェット方式の三次元造形装置及びその造形法 |
| JP2005508404A (ja) * | 2001-10-03 | 2005-03-31 | スリーディー システムズ インコーポレーテッド | 相変化支持材料組成物 |
| EP1274551B1 (en) | 2000-03-13 | 2006-10-04 | Objet Geometries Ltd. | Three dimensional printing method and model obtained |
| EP1741545A2 (en) | 2000-03-13 | 2007-01-10 | Objet Geometries Ltd. | Compositions and methods for use in three dimensional model printing |
| JP2008507619A (ja) * | 2004-07-26 | 2008-03-13 | ストラタシス・インコーポレイテッド | 三次元造形用の可溶性材料および工程 |
| WO2008146685A1 (ja) * | 2007-05-23 | 2008-12-04 | Showa Denko K.K. | エーテル結合を有する反応性ウレタン化合物、硬化性組成物および硬化物 |
| JP2010155889A (ja) | 2008-12-26 | 2010-07-15 | Jsr Corp | 光硬化性液状樹脂組成物およびインクジェット光造形法による支持体の製造方法 |
| JP2010155926A (ja) | 2008-12-26 | 2010-07-15 | Jsr Corp | 光硬化性液状樹脂組成物およびインクジェット光造形法による立体造形物の製造方法 |
| JP2011020412A (ja) * | 2009-07-17 | 2011-02-03 | Altech Co Ltd | 三次元造型方法 |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4228049A (en) * | 1978-11-13 | 1980-10-14 | Desoto, Inc. | Aqueous coatings based on copolymers with a mixture of unsaturated esters of polymeric polyol and bisphenol-ethylene oxide adduct |
| JP3205487B2 (ja) | 1995-07-04 | 2001-09-04 | ローレルバンクマシン株式会社 | 硬貨処理機 |
| US7300619B2 (en) * | 2000-03-13 | 2007-11-27 | Objet Geometries Ltd. | Compositions and methods for use in three dimensional model printing |
| GB0212062D0 (en) * | 2002-05-24 | 2002-07-03 | Vantico Ag | Jetable compositions |
| WO2005021248A1 (ja) * | 2003-08-27 | 2005-03-10 | Fuji Photo Film Co., Ltd. | 三次元造形物の製造方法 |
| JP2008146685A (ja) | 2005-03-30 | 2008-06-26 | Pioneer Electronic Corp | 光ピックアップ装置及び情報記録再生装置 |
| JP2007131789A (ja) * | 2005-11-11 | 2007-05-31 | Fujifilm Corp | 着色分散液、インク組成物、それを用いた画像形成方法、及び記録物 |
| EP2664442B1 (en) | 2006-12-08 | 2018-02-14 | 3D Systems Incorporated | Three dimensional printing material system |
| JP5606817B2 (ja) * | 2010-07-27 | 2014-10-15 | 富士フイルム株式会社 | 活性放射線硬化型インクジェット用インク組成物、印刷物、印刷物成形体、及び印刷物の製造方法 |
| KR101446091B1 (ko) * | 2010-10-27 | 2014-10-06 | 파일2파트, 인코포레이티드 | 3차원 물체 제작 방법 및 장치 |
| JP5890990B2 (ja) | 2010-11-01 | 2016-03-22 | 株式会社キーエンス | インクジェット光造形法における、光造形品形成用モデル材、光造形品の光造形時の形状支持用サポート材および光造形品の製造方法 |
-
2011
- 2011-09-26 JP JP2011208389A patent/JP5890990B2/ja active Active
- 2011-10-17 WO PCT/JP2011/073778 patent/WO2012060204A1/ja not_active Ceased
- 2011-10-17 CN CN201510462996.2A patent/CN105058791B/zh active Active
- 2011-10-17 KR KR1020137013811A patent/KR101884038B1/ko active Active
- 2011-10-17 EP EP11837855.3A patent/EP2636511B1/en active Active
- 2011-10-17 CN CN201510463347.4A patent/CN105111380B/zh not_active Expired - Fee Related
- 2011-10-17 CN CN201180052940.XA patent/CN103189187B/zh active Active
-
2013
- 2013-04-23 US US13/868,157 patent/US9556346B2/en active Active
-
2016
- 2016-05-26 US US15/164,906 patent/US9796863B2/en active Active
- 2016-05-26 US US15/164,905 patent/US9790382B2/en not_active Expired - Fee Related
Patent Citations (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH01204915A (ja) | 1988-01-27 | 1989-08-17 | Desoto Inc | 光学的立体造形用樹脂組成物 |
| JPH0859760A (ja) | 1994-08-18 | 1996-03-05 | Japan Synthetic Rubber Co Ltd | 光学的立体造形用樹脂組成物 |
| JPH09169827A (ja) | 1995-10-20 | 1997-06-30 | Teijin Seiki Co Ltd | 光硬化性樹脂組成物 |
| JPH09316111A (ja) | 1996-05-30 | 1997-12-09 | Japan Synthetic Rubber Co Ltd | 光硬化性樹脂組成物および樹脂製型の製造方法 |
| EP1741545A2 (en) | 2000-03-13 | 2007-01-10 | Objet Geometries Ltd. | Compositions and methods for use in three dimensional model printing |
| EP1274551B1 (en) | 2000-03-13 | 2006-10-04 | Objet Geometries Ltd. | Three dimensional printing method and model obtained |
| JP2001310918A (ja) | 2000-04-26 | 2001-11-06 | Mitsubishi Rayon Co Ltd | 光造形用硬化性組成物および成形品 |
| JP2002067174A (ja) | 2000-08-30 | 2002-03-05 | Minolta Co Ltd | データ処理装置及び方法、並びに三次元造形装置及び方法 |
| JP2002178412A (ja) | 2000-12-14 | 2002-06-26 | Sanyo Electric Co Ltd | 光造形装置及び光造形品の制作方法 |
| JP2005508404A (ja) * | 2001-10-03 | 2005-03-31 | スリーディー システムズ インコーポレーテッド | 相変化支持材料組成物 |
| JP2004059601A (ja) | 2002-07-24 | 2004-02-26 | Mitsubishi Rayon Co Ltd | 光学的立体造形用樹脂組成物、及び立体造形物 |
| JP2004255839A (ja) | 2003-02-28 | 2004-09-16 | Hitachi Printing Solutions Ltd | インクジェット方式の三次元造形装置及びその造形法 |
| JP2008507619A (ja) * | 2004-07-26 | 2008-03-13 | ストラタシス・インコーポレイテッド | 三次元造形用の可溶性材料および工程 |
| WO2008146685A1 (ja) * | 2007-05-23 | 2008-12-04 | Showa Denko K.K. | エーテル結合を有する反応性ウレタン化合物、硬化性組成物および硬化物 |
| JP2010155889A (ja) | 2008-12-26 | 2010-07-15 | Jsr Corp | 光硬化性液状樹脂組成物およびインクジェット光造形法による支持体の製造方法 |
| JP2010155926A (ja) | 2008-12-26 | 2010-07-15 | Jsr Corp | 光硬化性液状樹脂組成物およびインクジェット光造形法による立体造形物の製造方法 |
| JP2011020412A (ja) * | 2009-07-17 | 2011-02-03 | Altech Co Ltd | 三次元造型方法 |
Non-Patent Citations (2)
| Title |
|---|
| ROBERT F. FEDORS, POLYMER ENGINEERING AND SCIENCE, vol. 14, no. 2, February 1974 (1974-02-01), pages 147 - 154 |
| See also references of EP2636511A4 |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20140265033A1 (en) * | 2013-03-15 | 2014-09-18 | Michelin Recherche Et Technique S.A. | Method for improved tire mold manufacturing |
| WO2015056614A1 (ja) * | 2013-10-15 | 2015-04-23 | コニカミノルタ株式会社 | 三次元造形用インクジェットインク組成物および三次元造形物の製造方法 |
| JPWO2015056614A1 (ja) * | 2013-10-15 | 2017-03-09 | コニカミノルタ株式会社 | 三次元造形用インクジェットインク組成物および三次元造形物の製造方法 |
| CN113386359B (zh) * | 2015-08-14 | 2023-08-29 | 斯特拉塔西斯公司 | 支撑材料制剂及制造三维模型物体的方法 |
| US12233606B2 (en) | 2015-08-14 | 2025-02-25 | Stratasys Ltd. | Support material formulation and additive manufacturing processes employing same |
| CN113386359A (zh) * | 2015-08-14 | 2021-09-14 | 斯特拉塔西斯公司 | 支撑材料制剂及制造三维模型物体的方法 |
| US11850802B2 (en) | 2015-08-14 | 2023-12-26 | Stratasys Ltd. | Support material formulation and additive manufacturing processes employing same |
| CN106915084A (zh) * | 2017-03-07 | 2017-07-04 | 杭州杭景模型有限公司 | 3d打印机及其打印平台 |
| CN106915084B (zh) * | 2017-03-07 | 2019-04-02 | 杭州杭景模型有限公司 | 3d打印机及其打印平台 |
| CN108117799A (zh) * | 2017-12-31 | 2018-06-05 | 中山市威傲联复合材料有限公司 | 一种室内led显示屏间隙遮蔽油墨及其制备方法 |
| WO2020017615A1 (ja) | 2018-07-18 | 2020-01-23 | Kjケミカルズ株式会社 | 三次元造形サポート材用活性エネルギー線硬化性樹脂組成物とインク |
| US11826963B2 (en) | 2018-07-18 | 2023-11-28 | Kj Chemicals Corporation | Active energy ray-curable resin composition and ink for three-dimensional molding support materials |
| KR20210033937A (ko) | 2018-07-18 | 2021-03-29 | 케이제이 케미칼즈 가부시키가이샤 | 3 차원 조형 서포트재용 활성 에너지선 경화성 수지 조성물과 잉크 |
| US20220134640A1 (en) * | 2019-07-19 | 2022-05-05 | Stratasys Ltd. | Additive manufacturing of three-dimensional objects containing a transparent material |
| US12290980B2 (en) * | 2019-07-19 | 2025-05-06 | Stratasys Ltd. | Additive manufacturing of three-dimensional objects containing a transparent material |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2012111226A (ja) | 2012-06-14 |
| EP2636511B1 (en) | 2019-02-20 |
| CN103189187A (zh) | 2013-07-03 |
| CN105111380A (zh) | 2015-12-02 |
| CN105058791B (zh) | 2017-08-18 |
| KR101884038B1 (ko) | 2018-08-29 |
| CN103189187B (zh) | 2015-09-02 |
| KR20130141561A (ko) | 2013-12-26 |
| EP2636511A4 (en) | 2018-01-03 |
| US20160263826A1 (en) | 2016-09-15 |
| CN105111380B (zh) | 2018-10-16 |
| US20130234370A1 (en) | 2013-09-12 |
| CN105058791A (zh) | 2015-11-18 |
| US9556346B2 (en) | 2017-01-31 |
| US9790382B2 (en) | 2017-10-17 |
| JP5890990B2 (ja) | 2016-03-22 |
| US9796863B2 (en) | 2017-10-24 |
| US20160264796A1 (en) | 2016-09-15 |
| EP2636511A1 (en) | 2013-09-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6366085B2 (ja) | インクジェット光造形装置を用いた三次元造形方法に適用されるサポート材及びモデル材とサポート材の組み合わせ | |
| JP5890990B2 (ja) | インクジェット光造形法における、光造形品形成用モデル材、光造形品の光造形時の形状支持用サポート材および光造形品の製造方法 | |
| JP6586284B2 (ja) | インクジェット光造形法における光造形品形成用モデル材及び光造形品の製造方法 | |
| JP6423793B2 (ja) | 活性エネルギー線硬化性樹脂組成物及び硬化物 | |
| US20200407581A1 (en) | Optical shaping ink set, optically shaped article, and method for producing optically shaped article | |
| CN105820552B (zh) | 三维造型用支持材料、三维造型用组合物组、三维造型装置及制备三维成型体的方法 | |
| WO2019230136A1 (ja) | 光造形用インクセット | |
| JP2024060129A (ja) | モデル材用樹脂組成物 | |
| JP2019172785A (ja) | 活性エネルギー線硬化性組成物及び硬化物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11837855 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2011837855 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20137013811 Country of ref document: KR Kind code of ref document: A |