WO2004018587A1 - 有機エレクトロルミネッセンス素子及びアントラセン誘導体 - Google Patents
有機エレクトロルミネッセンス素子及びアントラセン誘導体 Download PDFInfo
- Publication number
- WO2004018587A1 WO2004018587A1 PCT/JP2003/010402 JP0310402W WO2004018587A1 WO 2004018587 A1 WO2004018587 A1 WO 2004018587A1 JP 0310402 W JP0310402 W JP 0310402W WO 2004018587 A1 WO2004018587 A1 WO 2004018587A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- substituted
- unsubstituted
- carbon atoms
- organic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C13/00—Cyclic hydrocarbons containing rings other than, or in addition to, six-membered aromatic rings
- C07C13/28—Polycyclic hydrocarbons or acyclic hydrocarbon derivatives thereof
- C07C13/32—Polycyclic hydrocarbons or acyclic hydrocarbon derivatives thereof with condensed rings
- C07C13/62—Polycyclic hydrocarbons or acyclic hydrocarbon derivatives thereof with condensed rings with more than three condensed rings
- C07C13/66—Polycyclic hydrocarbons or acyclic hydrocarbon derivatives thereof with condensed rings with more than three condensed rings the condensed ring system contains only four rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C15/00—Cyclic hydrocarbons containing only six-membered aromatic rings as cyclic parts
- C07C15/20—Polycyclic condensed hydrocarbons
- C07C15/27—Polycyclic condensed hydrocarbons containing three rings
- C07C15/28—Anthracenes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C43/00—Ethers; Compounds having groups, groups or groups
- C07C43/02—Ethers
- C07C43/20—Ethers having an ether-oxygen atom bound to a carbon atom of a six-membered aromatic ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C43/00—Ethers; Compounds having groups, groups or groups
- C07C43/02—Ethers
- C07C43/257—Ethers having an ether-oxygen atom bound to carbon atoms both belonging to six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- H—ELECTRICITY
- H05—ELECTRIC TECHNIQUES NOT OTHERWISE PROVIDED FOR
- H05B—ELECTRIC HEATING; ELECTRIC LIGHT SOURCES NOT OTHERWISE PROVIDED FOR; CIRCUIT ARRANGEMENTS FOR ELECTRIC LIGHT SOURCES, IN GENERAL
- H05B33/00—Electroluminescent light sources
- H05B33/12—Light sources with substantially two-dimensional radiating surfaces
- H05B33/14—Light sources with substantially two-dimensional radiating surfaces characterised by the chemical or physical composition or the arrangement of the electroluminescent material, or by the simultaneous addition of the electroluminescent material in or onto the light source
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/615—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/615—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene
- H10K85/622—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene containing four rings, e.g. pyrene
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/615—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene
- H10K85/626—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene containing more than one polycyclic condensed aromatic rings, e.g. bis-anthracene
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/654—Aromatic compounds comprising a hetero atom comprising only nitrogen as heteroatom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6572—Polycyclic condensed heteroaromatic hydrocarbons comprising only nitrogen in the heteroaromatic polycondensed ring system, e.g. phenanthroline or carbazole
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2603/00—Systems containing at least three condensed rings
- C07C2603/02—Ortho- or ortho- and peri-condensed systems
- C07C2603/04—Ortho- or ortho- and peri-condensed systems containing three rings
- C07C2603/22—Ortho- or ortho- and peri-condensed systems containing three rings containing only six-membered rings
- C07C2603/24—Anthracenes; Hydrogenated anthracenes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2603/00—Systems containing at least three condensed rings
- C07C2603/02—Ortho- or ortho- and peri-condensed systems
- C07C2603/04—Ortho- or ortho- and peri-condensed systems containing three rings
- C07C2603/22—Ortho- or ortho- and peri-condensed systems containing three rings containing only six-membered rings
- C07C2603/26—Phenanthrenes; Hydrogenated phenanthrenes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2603/00—Systems containing at least three condensed rings
- C07C2603/02—Ortho- or ortho- and peri-condensed systems
- C07C2603/40—Ortho- or ortho- and peri-condensed systems containing four condensed rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2603/00—Systems containing at least three condensed rings
- C07C2603/02—Ortho- or ortho- and peri-condensed systems
- C07C2603/40—Ortho- or ortho- and peri-condensed systems containing four condensed rings
- C07C2603/42—Ortho- or ortho- and peri-condensed systems containing four condensed rings containing only six-membered rings
- C07C2603/50—Pyrenes; Hydrogenated pyrenes
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1007—Non-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1011—Condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1014—Carbocyclic compounds bridged by heteroatoms, e.g. N, P, Si or B
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K2102/00—Constructional details relating to the organic devices covered by this subclass
- H10K2102/10—Transparent electrodes, e.g. using graphene
- H10K2102/101—Transparent electrodes, e.g. using graphene comprising transparent conductive oxides [TCO]
- H10K2102/103—Transparent electrodes, e.g. using graphene comprising transparent conductive oxides [TCO] comprising indium oxides, e.g. ITO
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/80—Constructional details
- H10K50/805—Electrodes
- H10K50/81—Anodes
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/80—Constructional details
- H10K50/805—Electrodes
- H10K50/82—Cathodes
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/30—Coordination compounds
- H10K85/321—Metal complexes comprising a group IIIA element, e.g. Tris (8-hydroxyquinoline) gallium [Gaq3]
- H10K85/324—Metal complexes comprising a group IIIA element, e.g. Tris (8-hydroxyquinoline) gallium [Gaq3] comprising aluminium, e.g. Alq3
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S428/00—Stock material or miscellaneous articles
- Y10S428/917—Electroluminescent
Definitions
- the present invention relates to an organic electroluminescent device and an anthracene derivative, and more particularly, to an organic electroluminescent device having high luminous efficiency and long life.
- the present invention relates to an anthracene derivative that achieves this.
- E organic electroluminescent device
- a fluorescent substance is generated by the recombination energy of holes injected from an anode and electrons injected from a cathode by applying an electric field. It is a self-luminous element using the principle of emitting light.
- C. Tang, Kodak's C. W. Tang et al. Report on low-voltage driven organic EL devices with stacked devices (CW Tang, SA Vansly ke, Applied Physics Letters, 51 rolls) , Pp. 913, pp. 1987), research on organic EL devices using organic materials as constituent materials has been actively conducted. Tang et al.
- the element structure of the organic EL element includes a hole transport (injection) layer, a two-layer electron transport / emission layer, or a hole transport (injection) layer, an emission layer, and an electron transport (injection) layer
- the three-layer type is well known.
- the device structure and formation method are devised.
- a light emitting material such as a chelate complex such as tris (8-quinolinolate) aluminum complex, a coumarin derivative, a tetraphenylbutadiene derivative, a bisstyrylarylene derivative, and an oxaziazole derivative are known.
- a chelate complex such as tris (8-quinolinolate) aluminum complex
- a coumarin derivative such as tris (8-quinolinolate) aluminum complex
- a coumarin derivative such as a tetraphenylbutadiene derivative
- a bisstyrylarylene derivative such as an oxaziazole derivative
- Japanese Patent Application Laid-Open No. H08-012600 discloses a device using a fluoranthracene derivative as a light emitting material. Although such anthracene derivatives are used as blue light-emitting materials, it has been desired to extend the life of the device. Further, an element material having a fluoranthene group at the 9,10 positions of anthracene is disclosed in Japanese Patent Application Laid-Open No. 2001-257704. Such anthracene derivatives are also used as a blue light-emitting material, but there has been a demand for an improvement in device life. Further, Japanese Patent Application Laid-Open No. 2000-187,776 discloses that various anthracene derivatives are used as a hole transport material. However, its synthesis has not been actually performed, and its evaluation as a luminescent material ⁇ I ⁇ has not yet been made. Disclosure of the invention
- the present invention has been made to solve the above problems, and has as its object to provide an organic EL device having high luminous efficiency and a long life, and an anthracene derivative realizing the same.
- the present inventors have conducted intensive studies in order to achieve the above object, and have found that a compound having an anthracene structure having an asymmetric specific structure represented by the following general formula (1) or (2) is converted to an organic compound.
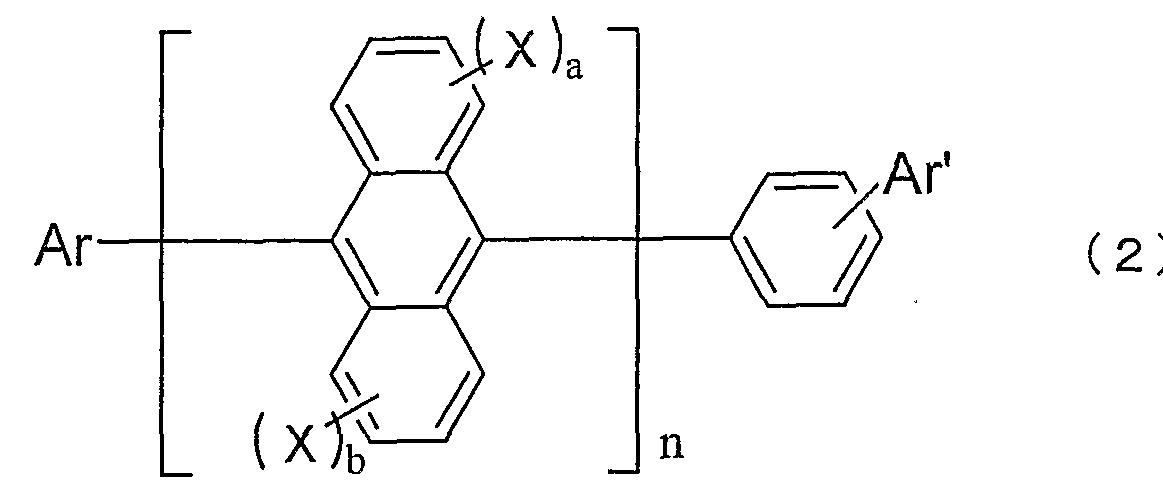
- the inventors have found that an organic EL device having a high luminous efficiency and a long lifetime can be obtained when used as a luminescent material for an EL device, and have completed the present invention. That is, the present invention provides an organic EL device in which one or more organic thin-film layers including at least a light-emitting layer are sandwiched between a cathode and an anode. At least one layer provides an organic EL device containing the anthracene derivative represented by the following general formula (1) or (2) alone or as a component of a mixture.
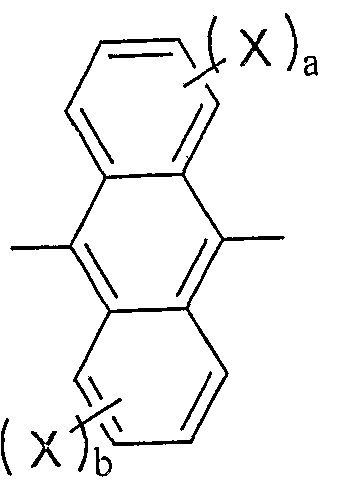
- Ar is a substituted or unsubstituted fused aromatic group having 10 to 50 nuclear carbon atoms.
- a r ′ is a substituted or unsubstituted aromatic group having 6 to 50 nuclear carbon atoms.
- X is a substituted or unsubstituted aromatic group having 6 to 50 nuclear carbon atoms, a substituted or unsubstituted aromatic heterocyclic group having 5 to 50 nuclear atoms, a substituted or unsubstituted carbon number of 1 to 5 Alkyl group having 0, substituted or unsubstituted alkoxy group having 1 to 50 carbon atoms, substituted or unsubstituted aralkyl group having 6 to 50 carbon atoms, substituted or unsubstituted having 5 to 50 carbon atoms Aryloxy group, substituted or unsubstituted aryloxy group having 5 to 50 carbon atoms, substituted or unsubstituted alkoxycarbonyl group having 1 to 50 carbon atoms, carboxyl group, halogen atom, cyano group, nitro group, hydroxyl Group.
- a, b and c are each an integer of 0-4.
- n! Is an integer of ⁇ 3. If n is 2 or more,
- anthracene derivative represented by the following general formula (2).
- Ar is a substituted or unsubstituted fused aromatic group having 10 to 50 nuclear carbon atoms.
- a r ′ is a substituted or unsubstituted aromatic group having 6 to 50 nuclear carbon atoms.
- X is a substituted or unsubstituted aromatic group having 6 to 50 nuclear carbon atoms, a substituted or unsubstituted aromatic heterocyclic group having 5 to 50 nuclear atoms, a substituted or unsubstituted carbon number of 1 to 5 Alkyl group having 0, substituted or unsubstituted alkoxy group having 1 to 50 carbon atoms, substituted or unsubstituted aralkyl group having 6 to 50 carbon atoms, substituted or unsubstituted aralkyl group having 5 to 50 carbon atoms.
- Aryloxy group substituted or unsubstituted aryloxy group having 5 to 50 nuclear atoms, substituted or unsubstituted alkoxycarbonyl group having 1 to 50 carbon atoms, carboxyl group, halogen atom, cyano group, toro group, hydroxyl Group.
- a and b are each an integer of 0-4.
- n is an integer of 1-3. If n is 2 or more,
- the organic EL device of the present invention is an organic EL device in which one or more organic thin film layers including at least a light-emitting layer are sandwiched between a cathode and an anode, wherein at least one of the organic thin film layers has the general structure described above.
- the anthracene derivative represented by the formula (1) is contained alone or as a component of a mixture.
- Ar is a substituted or unsubstituted condensed aromatic group having 10 to 50 nuclear carbon atoms.
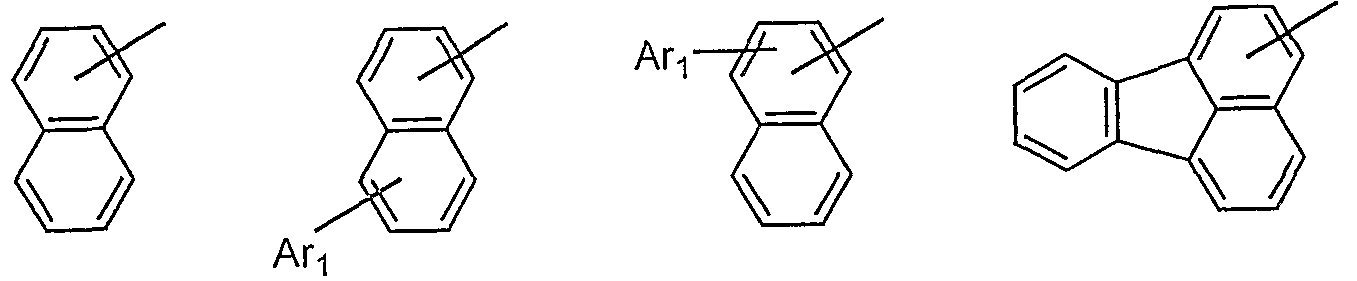
- Examples of the condensed aromatic group include 1-naphthyl group, 2-naphthyl group, 1-anthryl group, 2-anthryl group, 9-anthryl group, 1-phananthryl group, 2-pentanthryl group, 3-pentanthryl group, 4 1-phenanthryl group, 9-phenanthryl group, 1-naphthacenyl group, 2-naphthacenyl group, 9-naphthacenyl group, 1-pyrenyl group, 2-pyrenyl group, 4-pyrenyl group, 3-methyl-2-naphthyl group, 4-methyl Examples thereof include 11-naphthyl group and 4-methyl-11-anthryl group.
- (A r is a substituted or unsubstituted aromatic group having 6 to 50 nuclear carbon atoms.).
- a r is, for example, a phenyl group, a 1-naphthyl group, a 2-naphthyl group, a 1-anthryl group, a 2-anthryl group, a 9-anthryl group, a 1-phenanthryl group, a 2-pentanthryl group, a 3-pentanthryl group , 4-phenanthryl group, 9-1 Phenanthryl group, 1-naphthacenyl group, 2-naphthacenyl group, 9-naphthacenyl group, 1-pyrenyl group, 2-pyrenyl group, 4-pyrenyl group, 2-biphenylyl group, 3-biphenylyl group, 4-biphenyl Ruyl group, p-Ethylphenyl 4-yl group, p-Terphenyl-3-yl group, p-Terphenyl-2-yl group, m-Ethylphenyl 4-yl group,
- a r ′ is a substituted or unsubstituted aromatic group having 6 to 50 nuclear carbon atoms.
- the aromatic group include phenyl, 1-naphthyl, 2-naphthyl, 1-anthryl, 2-anthryl, 9-anthryl, 1-phenanthryl, 2-phenanthryl, and 3-phenanthryl.
- X is a substituted or unsubstituted aromatic group having 6 to 50 nuclear carbon atoms, a substituted or unsubstituted aromatic heterocyclic group having 5 to 50 nuclear atoms, Substituted alkyl group having 1 to 50 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 50 carbon atoms, substituted or unsubstituted aralkyl group having 6 to 50 carbon atoms, substituted or unsubstituted nucleus Aryloxy group having 5 to 50 atoms, substituted or unsubstituted arylthio group having 5 to 50 atoms, substituted or unsubstituted carboxyl group having 1 to 50 carbon atoms, halogen atom, cyano Group, nitro group and hydroxyl group.
- Examples of the substituted or unsubstituted aromatic group for X include phenyl, 1-naphthyl, 2-naphthyl, 1-anthryl, 2-anthryl, 9-anthryl, 1-phenanthryl, 2-phenanthryl group, 3-phenanthryl group, 4-phenanthryl group, 9-phenanthryl group, 1-naphthacenyl group, 2-naphthacenyl group, 9-naphthacenyl group, 1-pyrenyl group, 2-pyrenyl group, 4-pyrenyl group, 2-biphenylyl group, 3-biphenylyl group, 4-biphenylyl group, p-phenyl-4-yl group, p-phenyl-1-yl 3-pyl group, p-fuyl-2-yl group Leu-2-yl group, m-terphenyl-4-yl group, m-terphenyl-3-yl group, m-
- Examples of the substituted or unsubstituted aromatic heterocyclic group for X include: 1 monopyrrolyl group, 2-pyrrolyl group, 3_pyrrolyl group, pyrazur group, 2-pyridinyl group, 3-pyrrolyl group Pyridinyl group, 4-pyridinyl group, 1-indolyl group, 2—indolyl group, 3—indolyl group, 4 _indolyl group, 5—indolyl group, 6—indolyl group, 7—indolyl group, 1—isoindolyl group, 2— Isoindolyl, 3-isoindolyl, 4-isoindolyl, 5-isoindolyl, 6-isoindolyl, 7-isoindolyl, 2-furyl, 3-furyl, 2-benzofuranyl, 3-benzofuranyl, 4 Benzofuranyl, 5-benzofuranyl, 6-benzo
- 8-phenanthroline_5-yl group 2,8-phenanthroline-1-6-yl group, 2,8-phenanthroline-1 7-yl group, 2,8-phenanthroline-1-9-yl group, 2,8-phenanthroline 1 10-yl group, 2,7-phenanthroline-1-yl group,, 7-phenanthroline-1-3-yl group, 2,7-phenanthroline-4-yl group, 2,7-phenanthroline-1 5-y 2,7-phenanthroline-18-yl group, 2,7-phenanthroline-18-yl group, 2,7-phenanthroline-19-yl group, 2,7-phenanthroline-1 10-yl group Phenyl, 1-phenazinyl, 2-phenazulyl, 1-phenothiazinyl, 2-phenothiazidinyl, 3-phenothiazinyl, 4-phenothiazinyl, 10-phenothiazinyl, 1-phenyl ⁇ noxazinyl group, 2-punoxazinyl group
- Examples of the substituted or unsubstituted alkyl group in X include methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, s-butyl group, isobutyl group, t-butyl group, n-pentyl group, n —Hexyl group, n-heptyl group, n-octyl group, hydroxymethyl group, 1-hydroxyethyl group, 2-hydroxyethyl group, 2-hydroxyisobutyl group, 1,2-dihydroxyethyl group, 1,3-dihydroxyisopropyl group, 2,3-dihydroxy-t-butyl group, 1,2,3-trihydroxypropyl group, chloromethyl group, 1-chloroethyl group, 2-chloroethyl group, 2 _ Isobutyl isobutyl, 1,2-dichloroethyl, 1,3-dichloroisopropyl
- a substituted or unsubstituted alkoxy group is a group represented by OY.
- Y include a methyl group, an ethyl group, a propyl group, an isopropyl group, an n-butyl group, an s-butyl group, and an isobutyl group.
- substituted or unsubstituted aralkyl groups include benzyl, 1-phenylethyl, 2-phenylethyl, 1-phenylisopropyl, 2-phenylisopropyl, phenyl-t-butyl, ⁇ -naphthylmethyl Group, 1- ⁇ -naphthylethyl group, 2- ⁇ -naphthylethyl group, 1- ⁇ -naphthylisopropyl group, 1- ⁇ -naphthylisopropyl group, 3-naphthylmethyl group, I- ⁇ -naphthylethyl group, 2-) 8-naphthylethyl group, 11-naphthylisopropyl group, 2_ ⁇ -naphthylisopropyl group, 1_pyrrolylmethyl group, 2- (1-pyrrolyl) ethyl group, ⁇ -methylbenzyl group
- a substituted or unsubstituted aryloxy group is represented as 1 0 Y, and examples of ⁇ , Phenyl, 1-naphthyl, 2-naphthyl, 1 anthryl, 2-anthryl, 9 anthryl, 1 phenanthryl, 2-phenanthryl, 3-phenylanthryl, 4-phenylanthryl, 9 1-phenanthryl group, 1-naphthacenyl group, 2-naphthacenyl group, 9-naphthacenyl group, 1-pyrenyl group, 2 _pyrenyl group, 4-pyrenyl group, 2-biphenylyl group, 3-biphenylyl group, 4-1 Biphenylyl group, p-terphenyl-1-yl group, p-nitrophenyl-3-yl group, p-terphenyl-2-yl group, m-terphenyl-4-yl group, m-nitrophenyl-3-yl Group
- a substituted or unsubstituted arylthio group is represented by one SY ", and examples of Y" include a phenyl group, a 1-naphthyl group, a 2-naphthyl group, a 1-anthryl group and a 21-anthryl group , 9-anthryl, 1-phananthryl, 2-phenanthryl, 3-phenanthryl, 4-fananthryl, 9-fananthryl, 1-naphthacenyl, 2-naphthacenyl, 9-naphthacenyl, 1-pyrenyl, 2—pi Renyl, 4-pyrenyl, 2-biphenyl, 3-biphenyl, 4-biphenyl, p-phenyl 4-yl, p-phenyl 3-yl Group, p-vinyl 1-yl, m-vinyl 4-yl, m-vinyl 3-yl, m-vinyl 2-yl,
- a substituted or unsubstituted alkoxycarbonyl group is represented by C 0 0 Z, and examples of Z include methyl, ethyl, propyl, isopropyl, n-butyl, s-butyl, and isobutyl.
- Examples of the divalent group forming a ring include a tetramethylene group, a pentamethylene group, a hexamethylene group, a diphenylmethane 1,2′-diyl group, a diphenylethane 1,3,3,1-diyl group, and a diphenyl group. And propane-1,4,1-diyl group.
- halogen atom examples include fluorine, chlorine, bromine and iodine.
- a, b and c are each an integer of 0 to 4.
- n is an integer of 1-3. When n is 2 or more, the groups in [] may be the same or different.
- Examples of the substituent in the groups represented by Ar, Ar ′ and X include a halogen atom, a hydroxyl group, a nitro group, a cyano group, an alkyl group, an aryl group, and a cycloalkyl group.
- Specific examples of the anthracene derivative represented by the general formula (1) of the present invention are shown below, but are not limited to these exemplified compounds.
- Me represents a methyl group and Bu represents a butyl group.
- the anthracene derivative represented by the above general formula (2) of the present invention is a novel compound among those included in the above general formula (1).
- Ar is a substituted or unsubstituted condensed aromatic group having 10 to 50 nuclear carbon atoms.
- a r ′ is a substituted or unsubstituted aromatic group having 6 to 50 nuclear carbon atoms.
- the general formula (2) represents a substituted or unsubstituted aromatic group having 6 to 50 nuclear carbon atoms, a substituted or unsubstituted aromatic heterocyclic group having 5 to 50 nuclear atoms, a substituted or unsubstituted Alkyl group having 1 to 50 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 50 carbon atoms, substituted or unsubstituted aralkyl group having 6 to 50 carbon atoms, number of substituted or unsubstituted nuclear atoms 5-50 aryloxy group, substituted or unsubstituted nuclear atom number 5-50 arylthio group, substituted or unsubstituted alkoxycarbonyl group having 1-50 carbon atom, carboxyl group, halogen atom , A cyano group, a nitro group, and a hydroxyl group. ,
- the substituents in the groups represented by Ar, Ar ′ and X include a halogen atom, a hydroxyl group, a nitro group, a cyano group, an alkyl group, an aryl group, a cycloalkyl group, an alkoxy group, and an aromatic heterocyclic ring.
- a halogen atom a hydroxyl group, a nitro group, a cyano group, an alkyl group, an aryl group, a cycloalkyl group, an alkoxy group, and an aromatic heterocyclic ring.
- a and b are each an integer of 0 to 4, and preferably 0 to 1.
- n is an integer of 1-3. When n is 2 or more, the groups in [] may be the same or different.
- anthracene derivative represented by the general formula (2) of the present invention include: Among the specific examples of the general formula U), (AN1) to (AN4), (AN7) to (AN14), (AN17) to (AN24), (AN27) to (AN36) ), (AN39) to (AN4.3) and (AN46) to (AN48), but are not limited to these exemplified compounds.
- anthracene derivative represented by the general formula (2) of the present invention is preferably used as a material for an organic EL device.
- the anthracene derivative of the general formula (1) or (2) used in the organic EL device of the present invention is commercially available arylporic acid or arylporonic acid synthesized by a known method or a derivative thereof, and halogen.
- the compound can be synthesized by appropriately combining a Suzuki ripple reaction, a halogenation reaction, and a boration reaction. The synthesis scheme is shown below.
- R 1 , R 2 hydroxyl group or
- R 1 and R 2 combine to form a ring
- the reaction is usually carried out under normal pressure under an inert atmosphere of nitrogen, argon, helium or the like, but may be carried out under pressurized conditions if necessary.
- the reaction temperature is in the range of 15 to 300 ° C, particularly preferably 30 to 200 ° C.
- reaction solvent examples include water, aromatic hydrocarbons such as benzene, toluene, and xylene; ethers such as 1,2-dimethoxetane, getyl ether, methyl tert-butyl ether, tetrahydrofuran, and dioxane; pentane; Saturated hydrocarbons such as xane, heptane, octane and cyclohexane, dichloromethane, dichloromethane, carbon tetrachloride, halogens such as carbon tetrachloride, 1,2-dichloroethane, 1,1,1-trichloroethane, acetonitrile, benzone Nitriles such as nitriles, esters such as ethyl acetate, methyl acetate and butyl acetate, amides such as N, N-dimethylformamide, N, N-dimethylacetamide and N-methyl
- toluene, 1,2, -dimethoxetane, dioxane, and water are preferred.
- the amount of the solvent to be used is generally 3 to 50 times by weight, preferably 4 to 20 times by weight, relative to arylpolyacid or a derivative thereof.
- Examples of the base used for the reaction include sodium carbonate, potassium carbonate, sodium hydroxide hydroxide, sodium hydrogen carbonate, sodium hydrogen carbonate, magnesium carbonate, lithium carbonate, potassium fluoride, cesium fluoride, and chloride. Cesium, cesium bromide, cesium carbonate, potassium phosphate, sodium methoxide, potassium t-butoxide, sodium t-butoxide, lithium t-butoxide, and the like are preferable, and sodium carbonate is preferable.
- the amount of these bases to be used is generally 0.7 to 10 molar equivalents, preferably 0.9 to 6 molar equivalents, relative to arylpoic acid or a derivative thereof.
- Examples of the catalyst used in the reaction include tetrakis (triphenylphosphine) palladium, dichlorobis (triphenylphosphine) palladium, dichloro [bis (diphenylphosphino) ethane] palladium, and dichloro [bis (diphenylphosphino) phenyl] Pan] Palladium catalyst such as palladium, dichloro [bis (diphenylphosphino) butane] palladium, dichloro [bis (diphenylphosphino) phene] palladium, tetrakis (triphenylphosphine) nigel, dichlorobis (trif Nickel, dichloro [bis (diphenylphosphino) ethane] Nickel, dichloro [bis (diphenylphosphino) phenyl.
- Nickel catalysts such as ronon] nickel, dichloro [bis (diphenylphosphino) butane] nigel, and dichloro [bis (diphenylphosphino) phenoctene] nickel, and the like, preferably tetrakis (triphenyl) Nylphosphine) is palladium.
- the amount of these catalysts to be used is generally 0.01 to 1 molar equivalent, preferably 0.01 to 0.1 molar equivalent, relative to the halogenated anthracene derivative.
- halogen in the halogenated anthracene derivative examples include an iodine atom, a bromine atom, a chlorine atom and the like, and preferably an iodine atom and a bromine atom.
- the halogenating agent in the halogenation reaction is not particularly limited.
- N-halogenated succinic acid imide is preferably used.
- the amount of the halogenating agent to be used is generally 8 to 10 molar equivalents, preferably 1 to 5 molar equivalents, relative to the anthracene derivative.
- the reaction is usually performed in an inert solvent under an inert atmosphere such as nitrogen, argon, and helium.
- inert solvent examples include N, N-dimethylformamide, N, N-dimethylacetamide, N-methylpyrrolidone, dimethylsulfoxide, carbon tetrachloride, cyclobenzene, dichlorobenzene, nitrobenzene, Examples thereof include toluene, xylene methyl sorb, ethyl sorb, water and the like, and preferred are N, N-dimethylformamide and N-methylpyrrolidone.
- solvent Is usually 3 to 50 times by weight, preferably 5 to 20 times by weight, based on the anthracene derivative.
- the reaction temperature is usually carried out at 0 ° (: ⁇ 200 ° C, preferably 20 °; 1.1.20 ° C).
- the boration reaction is carried out by a known method (edited by The Chemical Society of Japan, Experimental Chemistry Lecture, 4th Edition, Vol. 24, pages 61 to 90, page 0 (3 ⁇ 46111., ⁇ 01.60, 7508 '(1995), etc.)).
- a reaction via a lithiation of a halogenated anthracene derivative or a Grignard reaction the reaction is usually performed in an inert atmosphere of nitrogen, argon, helium, etc., and the reaction solvent is inert.
- Solvents are used, for example, saturated hydrocarbons such as pentane, hexane, heptane, octane and cyclohexane, 1,2-dimethyloxetane, getyl ether, methyl-t-butylether, tetrahydrofuran, dioxane, etc.
- Aromatic hydrocarbons such as ethers, benzene, toluene, and xylene can be used singly or as a mixed solvent, preferably dimethyl ether.
- the amount of the solvent is usually 3 to 50 times by weight, preferably 4 to 20 times by weight of the halogenated anthracene derivative.
- the lithifying agent examples include alkyl metal reagents such as n-butyllithium, t-butyllithium, phenyllithium, and methyllithium, and amide bases such as lithium diisopropylamide and lithium pistrimethylsilylamide. And preferably n-butyllithium.
- the Grignard reagent can be prepared by reacting a halogenated anthracene derivative with metal magnesium.
- the trialkyl borate as a borate oxidizing agent include trimethyl borate, triethyl borate, triisopropyl borate, tributyl borate and the like, and preferably trimethyl borate and triisopropyl borate. .
- the amounts of the lithiating agent and the metallic magnesium are usually 1 to 10 molar equivalents, preferably 1 to 2 molar equivalents, respectively, based on the halogenated anthracene derivative, and the amount of the trialkyl borate used is It is usually 1 to 10 molar equivalents, preferably 1 to 5 molar equivalents, based on the anthracene derivative.
- the reaction temperature is usually 100- PT / JP2003 / 010402
- the temperature is 50 ° C, preferably 75 to 10 ° C.
- the light emitting layer contains the anthracene derivative represented by the general formula (1) or (2) as a main component.
- the light emitting layer further contains an arylamine compound and / or a styrylamine compound.
- styrylamine compound a compound represented by the following general formula (A) is preferable.
- Ar 2 is Fuweniru group, Bifuweniru group, Tafuweniru group, a stilbene group, a group selected from distyryl Rua aryl group, Ar 3 and Arufaganma 4 is a hydrogen atom or carbon atoms each 6-20 An aromatic group, and Ar 2 , Ar 3 and Ar 4 may be substituted, p is an integer of 1 to 4. More preferably, at least one of Ar 3 or Ar 4 is substituted with a styryl group. ing. )
- examples of the aromatic group having 6 to 20 carbon atoms include a phenyl group, a naphthyl group, an anthranyl group, a phenanthryl group, and a terphenyl group.
- arylamine compound a compound represented by the following general formula (B) is preferable.
- a r 5 ⁇ A r 7 is a substituted or unsubstituted Ariru group with carbon number. 5 to 4 0.
- Q is an integer from 1 to 4.
- the aryl group having a nuclear carbon number of 5 to 40 includes, for example, a phenyl group, a naphthyl group, an anthranyl group, a phenanthryl group, a pyrenyl group, a coronyl group, a biphenyl group, a terphenyl group, a pyrrolyl group, Furanyl group, thiophenyl group, benzothiophenyl group, oxaziazolyl group, diphenylanthranyl group, indril group, carbazolyl group, pyridyl group, benzoquinolyl group, fluoranthenyl group, acenaphthofluoranthyl group, stilbene group, etc.
- substituents of this aryl group include alkyl groups having 1 to 6 carbon atoms (ethyl group, methyl group, i-propyl group, n-propyl group, s-butyl group, t-butyl group).
- Anode / organic semiconductor layer / luminescent layer / adhesion improving layer / cathode (8) Anode / hole injection layer / hole transport layer / emission layer / electron injection layer / cathode
- the configuration (8) is usually preferably used, but is not limited to these.
- This organic EL device is usually manufactured on a light-transmitting substrate.
- the light-transmitting substrate is a substrate that supports the organic EL element.
- the light-transmitting substrate preferably has a light transmittance of 50% or more in the visible region of 400 to 700 nm, and a more smooth substrate. Preferably, it is used.
- a translucent substrate for example, a glass plate, a synthetic resin plate or the like is suitably used.
- the glass plate include a plate formed of soda-lime glass, norm-strontium-containing glass, lead glass, aluminosilicate glass, borate glass, normium borate glass, and quartz.
- the synthetic resin plate include plates of polycarbonate resin, acryl resin, polyethylene terephthalate resin, polyether sulfide resin, and polysulfone resin.
- anode those using a metal, an alloy, an electrically conductive compound or a mixture thereof having a large work function (4 eV or more) as an electrode material are preferably used.
- an electrode material metals such as Au, Cu l, I TO (I Nji ⁇ mucin O sulfoxide), Sn0 2, Z nO, include conductive material such as I n-Z n-0 Can be
- a thin film can be formed from these electrode substances by a method such as a vapor deposition method or a sputtering method.
- This anode is used for the light emitting layer When light emitted from the anode is taken out from the anode, it is desirable that the anode has characteristics such that the transmittance of the anode with respect to the light emission is greater than 10%.
- the sheet resistance of the anode is preferably several hundreds ⁇ / port or less. Further, the thickness of the anode depends on the material, but is usually selected in the range of 10 nm to 1 ⁇ , preferably 10 to 200 nm.
- a metal having a low work function (4 eV or less), an alloy, an electrically conductive compound, or a mixture thereof is used as an electrode material.
- electrodes materials include sodium, sodium ⁇ mu potassium alloy, Maguneshiu arm, lithium, magnesium 'silver alloy, aluminum / aluminum oxide, A1 / L i 2 ⁇ , A 1 / L i 0 2 , A1 / LiF, aluminum-lithium alloy, aluminum, rare earth metal, etc.
- This cathode can be manufactured by forming a thin film from these electrode substances by a method such as vapor deposition or sputtering.
- the transmittance of the cathode with respect to the emitted light be greater than 10%.
- the sheet resistance as the cathode is preferably several hundred ⁇ / port or less, and the film thickness is usually 1 ′ Onm Onl, preferably 50 ⁇ 200 nm.
- a chalcogenide layer, a metal halide layer or a metal oxide layer (hereinafter, referred to as a surface layer) is formed on at least one surface of the pair of electrodes thus manufactured. It is preferable to arrange. Specifically, a chalcogenide (including oxide) layer of a metal such as gay or aluminum is provided on the anode surface on the light emitting layer side, and a metal halide layer or metal oxide layer is provided on the cathode surface on the light emitting layer side. It is good to arrange. Thereby, the driving can be stabilized.
- the chalcogenide include, for example, SiOx (1 ⁇ X ⁇ 2), A1Ox (1 ⁇ X ⁇ l.5), SiON, SiAlON, and the like. , for example, L i F, MgF 2, CaF 2, etc. fluorides of rare earth metals. properly preferred, as the metal oxide, for example, C s 2 0, L iz 0 , Mg 0, S r 0402
- a mixed region of an electron transfer compound and a reducing dopant or a hole transfer compound and an oxidizable dopant is formed on at least one surface of the pair of electrodes manufactured as described above. It is also preferable to arrange a mixed region. By doing so, the electron transfer compound is reduced and becomes an anion, so that the mixed region can more easily inject and transfer electrons to the light emitting layer.
- the hole transfer compound is oxidized and becomes a cation, so that the mixed region can more easily inject and transfer holes to the light emitting layer.
- Preferred oxidizing dopants include various Lewis acids and axceptor compounds.
- Preferred reducing dopants include Al-metals, Al-metal compounds, Al-earth metals, rare earth metals and their compounds.
- the light emitting layer comprises:
- Injection function A function that can inject holes from the anode or hole injection layer when an electric field is applied, and can inject electrons from the cathode or electron injection layer.
- Light-emitting function Provides a field for recombination of electrons and holes, and has the function of connecting this to light emission.
- the light emitting layer is particularly preferably a molecular deposition film.
- the molecular deposition film is a thin film formed by deposition from a material compound in a gas phase or a film formed by solidification from a material compound in a solution state or a liquid phase. Films can be distinguished from thin films (molecule accumulation films) formed by the LB method by differences in the cohesive structure and higher-order structure, and the resulting functional differences.
- the light emitting layer can be formed.
- the light-emitting layer may contain a known light-emitting material other than the light-emitting material of the present invention, if desired, as long as the object of the present invention is not impaired.
- a light emitting layer containing another known light emitting material may be laminated on the light emitting layer containing.
- the hole injection / transport layer is a layer that assists hole injection into the light-emitting layer and transports it to the light-emitting region. It has a high hole mobility and usually has an ionization energy of 5.5 eV or less. small. Electric field application of such materials are preferred which can transport holes to the emitting layer at a lower electric field intensity as the hole injecting and transporting layer, further hole mobility of, for example, 1 0 4 ⁇ 1 0 s V / cm sometimes, those at least 1 0- 6 cm 2 / V 's are preferred.
- any of materials conventionally used as a charge transporting material for holes in a photoconductive material and known materials used in a hole injection layer of an organic EL device can be used. You can select and use one.
- the hole injecting / transporting material may be formed into a thin film by a known method such as a vacuum evaporation method, a spin coating method, a casting method, and an LB method.
- the thickness of the hole injection / transport layer is not particularly limited, but is usually 5 nm to 5 m.
- the electron injection layer and the transport layer are layers that help inject electrons into the light emitting layer and transport the electrons to the light emitting region.
- the electron mobility is high, and the adhesion improving layer is formed in the electron injection layer.
- This is a layer made of a material having good adhesion to the cathode.
- a metal complex of 8-hydroxyquinoline or a derivative thereof is preferable.
- Specific examples of the metal complex of 8-hydroxyquinoline or a derivative thereof include a metal chelate oxinoide compound containing a chelate of quinoxin (generally 8-quinolinol or 8-hydroxyquinoline), for example, tris (8-quinolinol) al Minimum can be used as the electron injection material.
- an insulating thin film layer is placed between the pair of electrodes. You may enter.
- Examples of the material used for the insulating layer include aluminum oxide, lithium fluoride, lithium oxide, cesium fluoride, cesium oxide, magnesium oxide, magnesium fluoride, calcium oxide, calcium fluoride, aluminum nitride, titanium nitride, and acid.
- Examples include silicon oxide, germanium oxide, silicon nitride, boron nitride, molybdenum oxide, ruthenium oxide, and vanadium oxide. These mixtures and laminates may be used.
- an anode, a light emitting layer, a hole injection layer if necessary, and a necessary (electron injection layer are formed by the above materials and methods.
- the cathode may be formed, and the organic EL device can be manufactured in the reverse order from the cathode to the anode.
- an organic EL device having a configuration in which an anode, a hole injection layer, a light emitting layer, a Z electron injection layer, and a cathode are sequentially provided on a light-transmitting substrate will be described.
- a thin film made of an anode material is formed on a suitable translucent substrate by a vapor deposition method or a sputtering method so as to have a thickness of 1 m or less, preferably 10 to 200 nm.
- a hole injection layer is provided on the anode.
- the hole injection layer can be formed by vacuum deposition, spin coating, casting, LB, etc. as described above, but a uniform film is easily obtained and pinholes are generated. It is preferable to form the film by a vacuum deposition method from the viewpoint of difficulty.
- the deposition conditions vary depending on the compound to be used (the material of the hole injection layer), the crystal structure and the recombination structure of the target hole injection layer, and the like.
- a light emitting layer is provided on the hole injection layer.
- This light-emitting layer is also formed using the light-emitting material according to the present invention by vacuum evaporation, sputtering, spin coating, or casting. Although it can be formed by reducing the thickness of the light-emitting material by such a method as above, it is preferable to form the light-emitting material by a vacuum evaporation method from the viewpoint that a uniform film is easily obtained and pinholes are hardly generated.
- the deposition conditions vary depending on the compound used, but can be generally selected from the same condition range as the formation of the hole injection layer.
- the thickness is preferably in the range of 10 to 40 nm.
- an electron injection layer is provided on the light emitting layer.
- the film is formed by a vacuum evaporation method because it is necessary to obtain a uniform film.
- the deposition conditions can be selected from the same condition ranges as for the hole injection layer and the light emitting layer.
- the cathode is laminated to obtain an organic EL device.
- the cathode is made of metal, and can be formed by vapor deposition or sputtering. However, in order to protect the underlying organic layer from damage during film formation, a vacuum deposition method is preferable. In the production of the organic EL device described above, it is preferable to produce the anode to the cathode consistently by a single evacuation.
- the anode When a DC voltage is applied to this organic EL device, the anode can be made to have a polarity and the cathode can be made to have a single polarity, and a voltage of 3 to 40 V can be applied to emit light. Even if a voltage is applied in the opposite polarity, no current flows and no light emission occurs. Further, when an AC voltage is applied, uniform light emission is observed only when the anode has ten polarities and the cathode has one polarity. In this case, the waveform of the applied AC may be arbitrary.
- poronic acid triisopropyl ester manufactured by Tokyo Kasei
- 2N hydrochloric acid 20N hydrochloric acid at 40 ° C or less at 10 ° C or less!
- 10 milliliters of toluene were added. This was separated, dried over sodium sulfate, concentrated under reduced pressure, hexane was added, and the precipitated crystals were collected by filtration.
- Nil fluoranthene 5.13 g, tetrakis (triphenylphosphine) palladium (0) 0.33 g (manufactured by Tokyo Kasei Co., Ltd.), 1,2-dimethoxetane 60 milliliter (manufactured by Hiroshima Wako) and sodium carbonate 4.55 g (Hiroshima Wako) was dissolved in 21 milliliters of water, and the mixture was heated and stirred for 24 hours while refluxing. After the reaction, the mixture was cooled to room temperature, and the precipitated crystals were collected by filtration. This compound was purified by column chromatography to obtain 3.3 g of a pale yellow solid.
- Mouthphenyl) naphthalene 4.05 g, tetrakis (triphenylphosphine) palladium (0) 0.33 g (manufactured by Tokyo Kasei), 1,2-dimethoxyxetane 60 milliliter (manufactured by Hiroshima Wako) and sodium carbonate
- a solution prepared by dissolving 4.55 g (manufactured by Hiroshima Wako) in 21 milliliters of water was added thereto, and the mixture was heated and stirred for 24 hours while refluxing. After the reaction, the reaction mixture was cooled to room temperature, and the precipitated crystals were collected by filtration. This compound was purified by column chromatography to obtain 3.6 g of a pale yellow solid.
- Example 5 (manufacture of organic EL device)
- TPD2332 film 25 mm ⁇ 75 mm ⁇ 1.1 mm thick glass substrate with IT ⁇ transparent electrode (manufactured by Geomatic) was subjected to ultrasonic cleaning in isopropyl alcohol for 5 minutes, and then UV ozone cleaning for 30 minutes.
- the glass substrate with the transparent electrode lines after washing is mounted on a substrate holder of a vacuum deposition apparatus.
- the transparent electrode lines are formed on the surface on the side where the transparent electrode lines are formed so as to cover the transparent electrodes.
- N'-bis (N, N, diphenyl 4-aminophenyl) 1 N, N-diphenyl 4, 4, 4, diamino-11, 1-biphenyl film hereinafter referred to as TPD2332 film
- This TPD232 film functions as a hole injection layer. Subsequently, the following N, N, N,, N'-tetra (4-biphenyl) diaminobiphenylene film (hereinafter referred to as TBDB film) with a film thickness of 20 nm was formed on the -TPD2332 film. . This film functions as a hole transport layer. Further, a compound (AN 8) having a thickness of 4 O nm as a light emitting material was deposited on the TBDB film by vapor deposition.
- This film functions as a light emitting layer.
- An 108q film having a thickness of 1011111 was formed on this film.
- a reducing dopant Li Li source: manufactured by SAES Getter One
- Alq Alq
- Alq Alq: Li film (thickness 1 Onm) is formed as an electron injection layer (cathode).
- a metal A1 was vapor-deposited on the Alq: Li film to form a metal cathode, thereby manufacturing an organic EL device.
- the luminous efficiency and the initial luminance were measured with a 100 nit device to determine the half life of the device under a normal use environment. Table 1 shows the results.
- An organic EL device was manufactured in the same manner as in Example 5 except that the compounds described in Table 1 were used instead of AN8 as the luminescent material, and the luminous efficiency and the initial luminance were set to 10 OOnit in a normal operating environment. The half-life below was measured. Table 1 shows the results.
- Example 9 manufactured of organic EL element).
- Example 5 an organic EL device was manufactured in the same manner except that the following aromatic amine D2 was used in place of the amine compound D1 having a styryl group, and the luminous efficiency and the initial luminance were set to lOOunit. The half-life was measured under normal use environment. Table 1 shows the results.
- Example 1 was repeated except that 1- (4-butamorphinyl) naphthalene was used instead of 2- (4-promophenyl) naphthalene, to obtain 7.8 g of a pale yellow solid.
- Example 12 (Synthesis of Compound (AN49)) Example 1 was repeated, except that 2- (3-bromounyl) naphthalene was used instead of 2- (4-bromophenyl) naphthalene, to obtain 6.9 g of a pale yellow solid.
- An organic EL device was manufactured in the same manner as in Example 5 except that the compounds described in Table 1 were used instead of AN8 as the luminescent material, and the luminous efficiency and the initial luminance were set to 10 OOnit in a normal operating environment. The half-life below was measured. The results are shown in Table 1. Comparative Example 1 (manufacture of organic EL device)
- An organic EL device was manufactured in the same manner as in Example 5, except that an 1 was used instead of AN 8 as the luminescent material, and the luminous efficiency and the initial luminance were 1000 ⁇ under a normal use environment. The half-life was measured. Table 1 shows the results.
- An organic EL device was manufactured in the same manner as in Example 5 except that an2 was used instead of AN8 as the light-emitting material, and the luminous efficiency and the initial luminance were reduced by half in a normal operating environment with 100 nm. The life was measured. Table 1 shows the results.
- the organic EL devices of Examples 5 to 9 and 13 to 15 had high luminous efficiency and extremely long life.
- the organic EL devices of Comparative Examples 1 and 2 had low luminous efficiency and short life.
- the organic EL device of the present invention and the organic EL device using the anthracene derivative of the present invention have high luminous efficiency and long life. Therefore, it is useful as an organic EL device that is expected to be used for a long time.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Electroluminescent Light Sources (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Luminescent Compositions (AREA)
Abstract
Description
Claims
Priority Applications (10)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT03792695T ATE452954T1 (de) | 2002-08-23 | 2003-08-18 | Organische elektrolumineszenzvorrichtung und anthracenderivat |
| EP10184035.3A EP2261302B2 (en) | 2002-08-23 | 2003-08-18 | Organic electroluminescence device and anthracene derivative |
| JP2004530558A JP4041816B2 (ja) | 2002-08-23 | 2003-08-18 | 有機エレクトロルミネッセンス素子及びアントラセン誘導体 |
| EP03792695.3A EP1553154B2 (en) | 2002-08-23 | 2003-08-18 | Organic electroluminescence device and anthracene derivative |
| DE60330696T DE60330696D1 (de) | 2002-08-23 | 2003-08-18 | Organische elektrolumineszenzvorrichtung und anthracenderivat |
| US10/524,825 US7839074B2 (en) | 2002-08-23 | 2003-08-18 | Organic electroluminescence device and anthracene derivative |
| US12/902,452 US8318324B2 (en) | 2002-08-23 | 2010-10-12 | Organic electroluminescence device and anthracene derivative |
| US13/482,031 US8785006B2 (en) | 2002-08-23 | 2012-05-29 | Organic electroluminescence device and anthracene derivative |
| US14/301,865 US9583716B2 (en) | 2002-08-23 | 2014-06-11 | Organic electroluminescence device and anthracene derivative |
| US15/405,028 US10217943B2 (en) | 2002-08-23 | 2017-01-12 | Organic electroluminescence device and anthracene derivative |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2002-243545 | 2002-08-23 | ||
| JP2002243545 | 2002-08-23 |
Related Child Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10524825 A-371-Of-International | 2003-08-18 | ||
| US10/524,825 A-371-Of-International US7839074B2 (en) | 2002-08-23 | 2003-08-18 | Organic electroluminescence device and anthracene derivative |
| US12/902,452 Continuation US8318324B2 (en) | 2002-08-23 | 2010-10-12 | Organic electroluminescence device and anthracene derivative |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2004018587A1 true WO2004018587A1 (ja) | 2004-03-04 |
Family
ID=31944101
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2003/010402 Ceased WO2004018587A1 (ja) | 2002-08-23 | 2003-08-18 | 有機エレクトロルミネッセンス素子及びアントラセン誘導体 |
Country Status (9)
| Country | Link |
|---|---|
| US (5) | US7839074B2 (ja) |
| EP (3) | EP2261302B2 (ja) |
| JP (3) | JP4041816B2 (ja) |
| KR (2) | KR100924462B1 (ja) |
| CN (2) | CN100505963C (ja) |
| AT (2) | ATE555182T1 (ja) |
| DE (1) | DE60330696D1 (ja) |
| TW (1) | TWI284485B (ja) |
| WO (1) | WO2004018587A1 (ja) |
Cited By (125)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005091686A1 (ja) * | 2004-03-19 | 2005-09-29 | Chisso Corporation | 有機電界発光素子 |
| WO2005110950A1 (en) * | 2004-04-29 | 2005-11-24 | Eastman Kodak Company | Synthesis of unsymmetric anthracene compounds |
| WO2005121057A1 (ja) * | 2004-06-09 | 2005-12-22 | Idemitsu Kosan Co., Ltd. | アントラセン誘導体及びそれを利用した有機エレクトロルミネッセンス素子 |
| JP2006045503A (ja) * | 2004-07-09 | 2006-02-16 | Chisso Corp | 発光材料およびこれを用いた有機電界発光素子 |
| WO2006049860A1 (en) * | 2004-10-29 | 2006-05-11 | Eastman Kodak Company | Organic element for electroluminescent devices |
| US7056601B2 (en) | 2003-10-24 | 2006-06-06 | Eastman Kodak Company | OLED device with asymmetric host |
| WO2006097208A1 (de) * | 2005-03-16 | 2006-09-21 | Merck Patent Gmbh | Neue materialien für organische elektrolumineszenzvorrichtungen |
| WO2006103916A1 (ja) * | 2005-03-25 | 2006-10-05 | Idemitsu Kosan Co., Ltd. | 有機エレクトロルミネッセンス素子 |
| WO2006104044A1 (ja) * | 2005-03-28 | 2006-10-05 | Idemitsu Kosan Co., Ltd. | アントリルアリーレン誘導体、有機エレクトロルミネッセンス素子用材料、及びそれを用いた有機エレクトロルミネッセンス素子 |
| WO2006108498A1 (de) * | 2005-04-12 | 2006-10-19 | Merck Patent Gmbh | Organische elektrolumineszenzvorrichtungen |
| JP2007027779A (ja) * | 2002-12-24 | 2007-02-01 | Lg Electronics Inc | 有機電界発光デバイス |
| WO2007021117A1 (en) * | 2005-08-16 | 2007-02-22 | Gracel Display Inc. | Green electroluminescent compounds and organic electroluminescent device using the same |
| WO2007022845A1 (de) | 2005-08-26 | 2007-03-01 | Merck Patent Gmbh | Neue materialien für organische elektrolumineszenzvorrichtungen |
| WO2007029410A1 (ja) * | 2005-09-08 | 2007-03-15 | Idemitsu Kosan Co., Ltd. | ポリアリールアミンを用いた有機エレクトロルミネッセンス素子 |
| JP2007524745A (ja) * | 2004-02-17 | 2007-08-30 | イーストマン コダック カンパニー | 各種ドーパントを含むアントラセン誘導体ホスト |
| JP2007238500A (ja) * | 2006-03-08 | 2007-09-20 | Mitsui Chemicals Inc | アントラセン化合物および該化合物を含有する有機電界発光素子 |
| WO2007110129A1 (de) | 2006-03-24 | 2007-10-04 | Merck Patent Gmbh | Neue materialien für organische elektrolumineszenzvorrichtungen |
| WO2007123256A1 (en) * | 2006-04-20 | 2007-11-01 | Canon Kabushiki Kaisha | Compound and organic light emitting device |
| WO2008006449A1 (de) | 2006-07-11 | 2008-01-17 | Merck Patent Gmbh | Neue materialien für organische elektrolumineszenzvorrichtungen |
| US7326371B2 (en) | 2004-03-25 | 2008-02-05 | Eastman Kodak Company | Electroluminescent device with anthracene derivative host |
| JP2008504247A (ja) * | 2004-06-26 | 2008-02-14 | メルク パテント ゲーエムベーハー | 有機電子デバイスのための化合物 |
| WO2008056722A1 (en) | 2006-11-09 | 2008-05-15 | Idemitsu Kosan Co., Ltd. | Organic el material-containing solution, method for forming thin film of organic el material, thin film of organic el material, and organic el device |
| JP2008521857A (ja) * | 2004-12-01 | 2008-06-26 | メルク パテント ゲーエムベーハー | 有機電子デバイスのための化合物 |
| WO2008082178A1 (en) * | 2006-12-29 | 2008-07-10 | Doosan Corporation | Diaryl trianthracene derivatives and organic light emitting layer or diode comprising the same |
| WO2008105472A1 (ja) | 2007-02-28 | 2008-09-04 | Idemitsu Kosan Co., Ltd. | 有機el材料含有溶液、有機el薄膜形成方法、有機el薄膜を含む有機el素子および有機elディスプレイパネル製造方法 |
| WO2008143229A1 (ja) | 2007-05-21 | 2008-11-27 | Idemitsu Kosan Co., Ltd. | アントラセン誘導体及びそれを利用した有機エレクトロルミネッセンス素子 |
| DE102007024850A1 (de) | 2007-05-29 | 2008-12-04 | Merck Patent Gmbh | Neue Materialien für organische Elektrolumineszenzvorrichtungen |
| JP2009518831A (ja) * | 2005-12-08 | 2009-05-07 | メルク パテント ゲーエムベーハー | 有機エレクトロルミネセンス素子 |
| WO2009102054A1 (ja) * | 2008-02-15 | 2009-08-20 | Idemitsu Kosan Co., Ltd. | 有機発光媒体および有機el素子 |
| DE102008008953A1 (de) | 2008-02-13 | 2009-08-20 | Merck Patent Gmbh | Neue Materialien für organische Elektrolumineszenzvorrichtungen |
| DE102008018670A1 (de) | 2008-04-14 | 2009-10-15 | Merck Patent Gmbh | Neue Materialien für organische Elektrolumineszenzvorrichtungen |
| WO2010013676A1 (ja) * | 2008-07-28 | 2010-02-04 | 出光興産株式会社 | 有機発光媒体及び有機el素子 |
| DE102008054141A1 (de) | 2008-10-31 | 2010-05-06 | Merck Patent Gmbh | Neue Materialien für organische Elektrolumineszenzvorrichtungen |
| US7763761B2 (en) | 2004-05-27 | 2010-07-27 | Idemitsu Kosan Co., Ltd. | Asymmetric pyrene derivative and organic electroluminescence device employing the same |
| WO2010083869A2 (de) | 2009-01-23 | 2010-07-29 | Merck Patent Gmbh | Materialien für organische elektrolumineszenzvorrichtungen |
| DE102009009277A1 (de) | 2009-02-17 | 2010-08-19 | Merck Patent Gmbh | Organische elektronische Vorrichtung |
| JP2010209144A (ja) * | 2009-03-06 | 2010-09-24 | Mitsubishi Chemicals Corp | 有機電界発光素子材料、有機電界発光素子用組成物、有機電界発光素子、有機elディスプレイおよび有機el照明 |
| WO2010149259A2 (en) | 2009-06-22 | 2010-12-29 | Merck Patent Gmbh | Conducting formulation |
| WO2011012212A1 (de) | 2009-07-27 | 2011-02-03 | Merck Patent Gmbh | Neue materialien für organische elektrolumineszenzvorrichtungen |
| US7887931B2 (en) | 2003-10-24 | 2011-02-15 | Global Oled Technology Llc | Electroluminescent device with anthracene derivative host |
| WO2011035836A1 (de) | 2009-09-23 | 2011-03-31 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2011054442A2 (de) | 2009-11-06 | 2011-05-12 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| DE102009052428A1 (de) | 2009-11-10 | 2011-05-12 | Merck Patent Gmbh | Verbindung für elektronische Vorrichtungen |
| DE102009033371A1 (de) | 2009-07-16 | 2011-05-12 | Merck Patent Gmbh | Materialien für elektronische Vorrichtungen |
| WO2011074253A1 (ja) | 2009-12-16 | 2011-06-23 | 出光興産株式会社 | 有機発光媒体 |
| WO2011074254A1 (ja) | 2009-12-16 | 2011-06-23 | 出光興産株式会社 | 有機発光媒体 |
| WO2011076380A1 (en) | 2009-12-23 | 2011-06-30 | Merck Patent Gmbh | Composition for the preparation of organic electronic (oe) devices |
| WO2011076324A1 (en) | 2009-12-23 | 2011-06-30 | Merck Patent Gmbh | Compositions comprising organic semiconducting compounds |
| DE102010005697A1 (de) | 2010-01-25 | 2011-07-28 | Merck Patent GmbH, 64293 | Verbindungen für elektronische Vorrichtungen |
| EP2355198A1 (en) | 2006-05-08 | 2011-08-10 | Global OLED Technology LLC | OLED electron-injecting layer |
| DE102010009903A1 (de) | 2010-03-02 | 2011-09-08 | Merck Patent Gmbh | Verbindungen für elektronische Vorrichtungen |
| DE102010013068A1 (de) | 2010-03-26 | 2011-09-29 | Merck Patent Gmbh | Verbindungen für elektronische Vorrichtungen |
| WO2011128035A1 (en) | 2010-04-12 | 2011-10-20 | Merck Patent Gmbh | Composition and method for preparation of organic electronic devices |
| WO2011128034A1 (en) | 2010-04-12 | 2011-10-20 | Merck Patent Gmbh | Composition having improved performance |
| WO2011147523A1 (en) | 2010-05-27 | 2011-12-01 | Merck Patent Gmbh | Formulation and method for preparation of organic electronic devices |
| DE102010024335A1 (de) | 2010-06-18 | 2011-12-22 | Merck Patent Gmbh | Verbindungen für elektronische Vorrichtungen |
| DE102010024542A1 (de) | 2010-06-22 | 2011-12-22 | Merck Patent Gmbh | Materialien für elektronische Vorrichtungen |
| WO2012016630A1 (de) | 2010-08-05 | 2012-02-09 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2012045384A1 (de) | 2010-10-09 | 2012-04-12 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2012048780A1 (de) | 2010-10-15 | 2012-04-19 | Merck Patent Gmbh | Verbindungen für elektronische vorrichtungen |
| DE102010054316A1 (de) | 2010-12-13 | 2012-06-14 | Merck Patent Gmbh | Substituierte Tetraarylbenzole |
| WO2012095143A1 (de) | 2011-01-13 | 2012-07-19 | Merck Patent Gmbh | Verbindungen für organische elektrolumineszenzvorrichtungen |
| DE102011011104A1 (de) | 2011-02-12 | 2012-08-16 | Merck Patent Gmbh | Substituierte Dibenzonaphtacene |
| WO2012110182A1 (de) | 2011-02-17 | 2012-08-23 | Merck Patent Gmbh | Verbindungen für elektronische vorrichtungen |
| US8263973B2 (en) | 2008-12-19 | 2012-09-11 | E I Du Pont De Nemours And Company | Anthracene compounds for luminescent applications |
| US8273468B2 (en) | 2007-06-01 | 2012-09-25 | E I Du Pont De Nemours And Company | Green luminescent materials |
| WO2012139692A1 (de) | 2011-04-13 | 2012-10-18 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2012139693A1 (de) | 2011-04-13 | 2012-10-18 | Merck Patent Gmbh | Verbindungen für elektronische vorrichtungen |
| WO2012144176A1 (ja) | 2011-04-18 | 2012-10-26 | 出光興産株式会社 | ピレン誘導体、有機発光媒体、及びこれらを含む有機エレクトロルミネッセンス素子 |
| WO2012143079A1 (de) | 2011-04-18 | 2012-10-26 | Merck Patent Gmbh | Verbindungen für elektronische vorrichtungen |
| WO2012149992A1 (de) | 2011-05-04 | 2012-11-08 | Merck Patent Gmbh | Vorrichtung zur aufbewahrung von frischwaren |
| WO2012169635A1 (ja) * | 2011-06-10 | 2012-12-13 | 国立大学法人名古屋大学 | アリール基で置換された多環性芳香族化合物の製造方法 |
| US8431245B2 (en) | 2009-09-29 | 2013-04-30 | E. I. Du Pont De Nemours And Company | Deuterated compounds for luminescent applications |
| US8431250B2 (en) | 2009-04-24 | 2013-04-30 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative, and organic electroluminescent element comprising the same |
| WO2013060418A1 (en) | 2011-10-27 | 2013-05-02 | Merck Patent Gmbh | Materials for electronic devices |
| WO2013072740A2 (ja) | 2011-11-15 | 2013-05-23 | ユーディーシー アイルランド リミテッド | 電荷輸送材料、有機電界発光素子及び該素子を用いたことを特徴とする発光装置、表示装置または照明装置 |
| KR101273057B1 (ko) * | 2008-02-21 | 2013-06-10 | 주식회사 엘지화학 | 신규한 안트라센 유도체 및 이를 이용한 유기 전자 소자 |
| DE102013200942A1 (de) | 2012-01-23 | 2013-07-25 | Udc Ireland Ltd. | Organisches Elektrolumineszenz-Element, Ladungstransportmaterial für organisches Elektrolumineszenz-Element, und Licht-emittierende Vorrichtung, Anzeigevorrichtung und Beleuchtungsvorrichtung, die jeweils das Element verwenden |
| US8497495B2 (en) | 2009-04-03 | 2013-07-30 | E I Du Pont De Nemours And Company | Electroactive materials |
| WO2013120577A1 (en) | 2012-02-14 | 2013-08-22 | Merck Patent Gmbh | Spirobifluorene compounds for organic electroluminescent devices |
| US8531100B2 (en) | 2008-12-22 | 2013-09-10 | E I Du Pont De Nemours And Company | Deuterated compounds for luminescent applications |
| US8604247B2 (en) | 2007-06-01 | 2013-12-10 | E I Du Pont De Nemours And Company | Chrysenes for deep blue luminescent applications |
| US8617720B2 (en) | 2009-12-21 | 2013-12-31 | E I Du Pont De Nemours And Company | Electroactive composition and electronic device made with the composition |
| US8623520B2 (en) | 2007-11-21 | 2014-01-07 | Idemitsu Kosan Co., Ltd. | Fused aromatic derivative and organic electroluminescence device using the same |
| KR101350524B1 (ko) * | 2007-11-21 | 2014-01-16 | 주식회사 엘지화학 | 신규한 안트라센 유도체 및 이를 이용한 유기 전자 소자 |
| EP2713416A1 (en) * | 2004-07-08 | 2014-04-02 | Junji Kido | Organic devices, organic electroluminescent devices and organic solar cells |
| JP2014082479A (ja) * | 2012-09-27 | 2014-05-08 | Jnc Corp | 有機電界発光素子 |
| US8759818B2 (en) | 2009-02-27 | 2014-06-24 | E I Du Pont De Nemours And Company | Deuterated compounds for electronic applications |
| US8795847B2 (en) | 2005-12-08 | 2014-08-05 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US8883324B2 (en) | 2005-01-05 | 2014-11-11 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative and organic electroluminescent device using same |
| US8968883B2 (en) | 2009-08-13 | 2015-03-03 | E I Du Pont De Nemours And Company | Chrysene derivative materials |
| WO2015064560A1 (ja) * | 2013-10-29 | 2015-05-07 | Jnc株式会社 | アントラセン系化合物、発光層用材料、これを用いた有機電界発光素子、表示装置および照明装置 |
| US9040170B2 (en) | 2004-09-20 | 2015-05-26 | Global Oled Technology Llc | Electroluminescent device with quinazoline complex emitter |
| WO2015082037A1 (en) | 2013-12-06 | 2015-06-11 | Merck Patent Gmbh | Compositions containing a polymeric binder which comprises acrylic and/or methacrylic acid ester units |
| WO2015165563A1 (de) | 2014-04-30 | 2015-11-05 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| US9214636B2 (en) | 2006-02-28 | 2015-12-15 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device |
| WO2015192941A1 (de) | 2014-06-18 | 2015-12-23 | Merck Patent Gmbh | Zusammensetzungen für elektronische vorrichtungen |
| US9293716B2 (en) | 2010-12-20 | 2016-03-22 | Ei Du Pont De Nemours And Company | Compositions for electronic applications |
| US9331285B2 (en) | 2009-12-16 | 2016-05-03 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative and organic electroluminescent element using same |
| WO2016120007A1 (en) | 2015-01-30 | 2016-08-04 | Merck Patent Gmbh | Formulations with a low particle content |
| US9496506B2 (en) | 2009-10-29 | 2016-11-15 | E I Du Pont De Nemours And Company | Deuterated compounds for electronic applications |
| WO2017008883A1 (en) | 2015-07-15 | 2017-01-19 | Merck Patent Gmbh | Composition comprising organic semiconducting compounds |
| US9640773B2 (en) | 2011-09-16 | 2017-05-02 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative and organic electroluminescence element using same |
| US9666826B2 (en) | 2005-11-30 | 2017-05-30 | Global Oled Technology Llc | Electroluminescent device including an anthracene derivative |
| WO2018189134A1 (de) | 2017-04-13 | 2018-10-18 | Merck Patent Gmbh | Zusammensetzung für organische elektronische vorrichtungen |
| WO2019007823A1 (en) | 2017-07-03 | 2019-01-10 | Merck Patent Gmbh | LOW-PHENOL IMPURITY FORMULATIONS |
| US10435350B2 (en) | 2014-09-19 | 2019-10-08 | Idemitsu Kosan Co., Ltd. | Organic electroluminecence device |
| WO2020039708A1 (ja) | 2018-08-23 | 2020-02-27 | 国立大学法人九州大学 | 有機エレクトロルミネッセンス素子 |
| EP3647393A1 (de) | 2013-07-30 | 2020-05-06 | Merck Patent GmbH | Materialien für elektronische vorrichtungen |
| EP3712229A1 (de) | 2013-07-30 | 2020-09-23 | Merck Patent GmbH | Materialien für elektronische vorrichtungen |
| US11094886B2 (en) | 2019-09-13 | 2021-08-17 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent element and electronic device |
| US11094888B2 (en) * | 2006-06-22 | 2021-08-17 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent device using aryl amine derivative containing heterocycle |
| KR20220063233A (ko) | 2019-09-13 | 2022-05-17 | 이데미쓰 고산 가부시키가이샤 | 유기 일렉트로루미네센스 소자 및 전자 기기 |
| KR20220062618A (ko) | 2019-09-13 | 2022-05-17 | 이데미쓰 고산 가부시키가이샤 | 유기 일렉트로루미네센스 소자 및 전자 기기 |
| KR20220066099A (ko) | 2019-09-13 | 2022-05-23 | 이데미쓰 고산 가부시키가이샤 | 유기 일렉트로루미네센스 소자 및 전자 기기 |
| KR20220069028A (ko) | 2019-09-13 | 2022-05-26 | 이데미쓰 고산 가부시키가이샤 | 유기 일렉트로루미네센스 소자 및 전자 기기 |
| KR20220069030A (ko) | 2019-09-13 | 2022-05-26 | 이데미쓰 고산 가부시키가이샤 | 유기 일렉트로루미네센스 소자 및 전자 기기 |
| CN114716295A (zh) * | 2021-01-04 | 2022-07-08 | 浙江光昊光电科技有限公司 | 一种稠环化合物及其在有机电子器件的应用 |
| KR20220104237A (ko) | 2019-11-26 | 2022-07-26 | 이데미쓰 고산 가부시키가이샤 | 화합물, 유기 일렉트로루미네센스 소자 및 전자 기기 |
| KR20220119596A (ko) | 2019-12-27 | 2022-08-30 | 이데미쓰 고산 가부시키가이샤 | 유기 일렉트로루미네센스 소자 및 전자 기기 |
| KR20220141780A (ko) | 2020-02-14 | 2022-10-20 | 이데미쓰 고산 가부시키가이샤 | 유기 일렉트로루미네센스 소자 및 전자 기기 |
| US11512039B2 (en) | 2016-11-23 | 2022-11-29 | Guangzhou Chinaray Optoelectronic Materials Ltd. | Aromatic amine derivatives, preparation methods therefor, and uses thereof |
| US11518723B2 (en) | 2016-11-23 | 2022-12-06 | Guangzhou Chinaray Optoelectronic Materials Ltd. | Fused ring compound, high polymer, mixture, composition and organic electronic component |
| WO2023063289A1 (ja) | 2021-10-14 | 2023-04-20 | 出光興産株式会社 | 有機固体アップコンバージョン材料 |
| US11723266B2 (en) | 2019-09-13 | 2023-08-08 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent element and electronic device |
Families Citing this family (187)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7541099B2 (en) * | 2004-05-21 | 2009-06-02 | Semiconductor Energy Laboratory Co., Ltd. | Anthracene derivative and light emitting element and light emitting device using the same |
| DE102004031000A1 (de) * | 2004-06-26 | 2006-01-12 | Covion Organic Semiconductors Gmbh | Organische Elektrolumineszenzvorrichtungen |
| US20060019116A1 (en) * | 2004-07-22 | 2006-01-26 | Eastman Kodak Company | White electroluminescent device with anthracene derivative host |
| JP4536730B2 (ja) * | 2004-09-02 | 2010-09-01 | エルジー・ケム・リミテッド | アントラセン誘導体及びこれを発光物質として用いた有機発光素子 |
| US7646010B2 (en) * | 2004-11-26 | 2010-01-12 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element, light-emitting device, and electronic device |
| KR100696505B1 (ko) * | 2005-03-31 | 2007-03-19 | 삼성에스디아이 주식회사 | 유기 전계 발광 소자 및 그 제조방법 |
| TWI361018B (en) * | 2005-04-18 | 2012-03-21 | Sony Corp | Display device and a method of manufacturing the s |
| US7622619B2 (en) | 2005-07-20 | 2009-11-24 | Lg Display Co., Ltd. | Synthesis process |
| US8766023B2 (en) | 2005-07-20 | 2014-07-01 | Lg Display Co., Ltd. | Synthesis process |
| KR100828173B1 (ko) * | 2005-11-22 | 2008-05-08 | (주)그라쎌 | 유기 발광 화합물 및 이를 발광재료로 채용하고 있는 표시소자 |
| DE102005058558A1 (de) * | 2005-12-08 | 2007-06-14 | Merck Patent Gmbh | Organische Elektrolumineszenzvorrichtungen |
| KR100872692B1 (ko) * | 2006-03-06 | 2008-12-10 | 주식회사 엘지화학 | 신규한 안트라센 유도체 및 이를 이용한 유기 전자 소자 |
| KR100887870B1 (ko) * | 2006-04-12 | 2009-03-06 | 주식회사 엘지화학 | 신규한 안트라센 유도체, 이의 제조방법 및 이를 이용한유기전자소자 |
| WO2007145979A2 (en) * | 2006-06-05 | 2007-12-21 | E. I. Du Pont De Nemours And Company | Liquid composition for deposition of organic active materials in the field of oled printing |
| US20080160342A1 (en) * | 2006-12-29 | 2008-07-03 | Hong Meng | Host compositions for luminescent materials |
| KR20090128427A (ko) * | 2007-02-28 | 2009-12-15 | 이데미쓰 고산 가부시키가이샤 | 유기 el 소자 |
| JP5465825B2 (ja) * | 2007-03-26 | 2014-04-09 | 出光興産株式会社 | 半導体装置、半導体装置の製造方法及び表示装置 |
| JP5401449B2 (ja) * | 2007-06-01 | 2014-01-29 | イー・アイ・デュポン・ドウ・ヌムール・アンド・カンパニー | 緑色発光用途のためのクリセン類 |
| KR101612131B1 (ko) * | 2007-06-20 | 2016-04-12 | 이데미쓰 고산 가부시키가이샤 | 다환계 환집합 화합물 및 그것을 이용한 유기 전기 발광 소자 |
| KR100935356B1 (ko) * | 2007-11-19 | 2010-01-06 | 다우어드밴스드디스플레이머티리얼 유한회사 | 녹색 발광 화합물 및 이를 발광재료로서 채용하고 있는유기 전기 발광 소자 |
| JPWO2009066666A1 (ja) * | 2007-11-20 | 2011-04-07 | 出光興産株式会社 | 高分子化合物及びそれを用いた有機エレクトロルミネッセンス素子 |
| WO2009066809A1 (en) * | 2007-11-22 | 2009-05-28 | Gracel Display Inc. | Blue electroluminescent compounds with high efficiency and display device using the same |
| CN101918511A (zh) * | 2007-11-23 | 2010-12-15 | 葛来西雅帝史派有限公司 | 发光化合物和使用该化合物的电致发光器件 |
| KR100940938B1 (ko) * | 2007-12-04 | 2010-02-08 | 다우어드밴스드디스플레이머티리얼 유한회사 | 신규한 유기 발광 화합물 및 이를 발광재료로서 채용하고있는 유기 전기 발광 소자 |
| KR100987822B1 (ko) * | 2007-12-17 | 2010-10-13 | (주)씨에스엘쏠라 | 유기 발광 화합물 및 이를 구비한 유기 발광 소자 |
| US8174185B2 (en) * | 2007-12-21 | 2012-05-08 | E I Du Pont De Nemours And Company | Charge transport materials for luminescent applications |
| KR100991416B1 (ko) * | 2007-12-31 | 2010-11-03 | 다우어드밴스드디스플레이머티리얼 유한회사 | 유기 발광 화합물 및 이를 포함하는 유기 발광 소자 |
| KR100974562B1 (ko) * | 2007-12-31 | 2010-08-06 | 다우어드밴스드디스플레이머티리얼 유한회사 | 신규한 유기 발광 화합물 및 이를 발광재료로서 채용하고있는 유기 발광 소자 |
| KR101001384B1 (ko) * | 2008-02-29 | 2010-12-14 | 다우어드밴스드디스플레이머티리얼 유한회사 | 신규한 유기 발광 화합물 및 이를 발광재료로서 채용하고있는 유기 전기 발광 소자 |
| KR100901887B1 (ko) * | 2008-03-14 | 2009-06-09 | (주)그라쎌 | 신규한 유기 발광 화합물 및 이를 채용하고 있는 유기 발광소자 |
| KR20140056245A (ko) | 2008-03-19 | 2014-05-09 | 이데미쓰 고산 가부시키가이샤 | 안트라센 유도체, 발광 재료 및 유기 전기발광 소자 |
| KR100989815B1 (ko) * | 2008-03-20 | 2010-10-29 | 다우어드밴스드디스플레이머티리얼 유한회사 | 신규한 유기 발광 화합물 및 이를 발광재료로서 채용하고있는 유기 발광 소자 |
| KR100946411B1 (ko) * | 2008-03-28 | 2010-03-09 | 다우어드밴스드디스플레이머티리얼 유한회사 | 신규한 유기 발광 화합물 및 이를 발광재료로서 채용하고있는 유기 발광 소자 |
| KR20090105495A (ko) * | 2008-04-02 | 2009-10-07 | (주)그라쎌 | 신규한 유기 발광 화합물 및 이를 발광재료로서 채용하고있는 유기 전기 발광 소자 |
| KR100910150B1 (ko) * | 2008-04-02 | 2009-08-03 | (주)그라쎌 | 신규한 유기 발광 화합물 및 이를 발광재료로서 채용하고있는 유기 발광 소자 |
| KR101495547B1 (ko) * | 2008-04-17 | 2015-02-25 | 롬엔드하스전자재료코리아유한회사 | 신규한 전자 재료용 화합물 및 이를 포함하는 유기 전자소자 |
| KR20090111915A (ko) * | 2008-04-23 | 2009-10-28 | (주)그라쎌 | 신규한 유기 발광 화합물 및 이를 발광재료로서 채용하고있는 유기 발광 소자 |
| KR100984341B1 (ko) | 2008-05-09 | 2010-09-30 | (주)씨에스엘쏠라 | 유기 발광 소자 및 이에 사용되는 유기 발광 화합물 |
| KR100974125B1 (ko) | 2008-05-14 | 2010-08-04 | 주식회사 두산 | 비대칭 안트라센 유도체의 제조 및 이를 이용한 유기전계발광 소자 |
| KR20100000772A (ko) * | 2008-06-25 | 2010-01-06 | 다우어드밴스드디스플레이머티리얼 유한회사 | 신규한 유기 발광 화합물 및 이를 발광재료로서 채용하고있는 유기 발광 소자 |
| EP2145936A3 (en) * | 2008-07-14 | 2010-03-17 | Gracel Display Inc. | Fluorene and pyrene derivatives and organic electroluminescent device using the same |
| KR20100041043A (ko) * | 2008-10-13 | 2010-04-22 | 다우어드밴스드디스플레이머티리얼 유한회사 | 신규한 유기 발광 화합물 및 이를 발광재료로서 채용하고 있는 유기 발광 소자 |
| US8039129B2 (en) * | 2009-04-06 | 2011-10-18 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device and material for organic electroluminescence device |
| WO2011015265A2 (en) | 2009-08-04 | 2011-02-10 | Merck Patent Gmbh | Electronic devices comprising multi cyclic hydrocarbons |
| KR101097313B1 (ko) * | 2009-08-10 | 2011-12-23 | 삼성모바일디스플레이주식회사 | 유기 발광 소자 |
| KR101193182B1 (ko) | 2009-09-02 | 2012-10-19 | 삼성디스플레이 주식회사 | 유기 발광 소자 |
| KR101683786B1 (ko) * | 2009-09-09 | 2016-12-09 | 에스에프씨 주식회사 | 유기전계발광소자 |
| WO2011076326A1 (en) | 2009-12-22 | 2011-06-30 | Merck Patent Gmbh | Electroluminescent functional surfactants |
| EP2517278B1 (en) | 2009-12-22 | 2019-07-17 | Merck Patent GmbH | Electroluminescent formulations |
| WO2011076323A1 (en) | 2009-12-22 | 2011-06-30 | Merck Patent Gmbh | Formulations comprising phase-separated functional materials |
| DE102010009193B4 (de) | 2010-02-24 | 2022-05-19 | MERCK Patent Gesellschaft mit beschränkter Haftung | Zusammensetzung enthaltend Fluor-Fluor Assoziate, Verfahren zu deren Herstellung, deren Verwendung sowie organische elektronische Vorrichtung diese enthaltend |
| KR101757016B1 (ko) | 2010-03-11 | 2017-07-11 | 메르크 파텐트 게엠베하 | 방사성 섬유 |
| EP2544765A1 (en) | 2010-03-11 | 2013-01-16 | Merck Patent GmbH | Fibers in therapy and cosmetics |
| KR101191644B1 (ko) | 2010-05-18 | 2012-10-17 | 삼성디스플레이 주식회사 | 유기 재료와 이를 이용한 유기 발광 장치 |
| US10190043B2 (en) * | 2010-05-27 | 2019-01-29 | Merck Patent Gmbh | Compositions comprising quantum dots |
| US9093656B2 (en) | 2010-07-26 | 2015-07-28 | Merck Patent Gmbh | Quantum dots and hosts |
| US20130226268A1 (en) | 2010-07-26 | 2013-08-29 | Merck Patent Gmbh | Nanocrystals in devices |
| JP5786578B2 (ja) * | 2010-10-15 | 2015-09-30 | Jnc株式会社 | 発光層用材料およびこれを用いた有機電界発光素子 |
| WO2012110178A1 (en) | 2011-02-14 | 2012-08-23 | Merck Patent Gmbh | Device and method for treatment of cells and cell tissue |
| US9923152B2 (en) | 2011-03-24 | 2018-03-20 | Merck Patent Gmbh | Organic ionic functional materials |
| TW201245408A (en) * | 2011-04-08 | 2012-11-16 | Du Pont | Electronic device |
| JP6223961B2 (ja) | 2011-05-12 | 2017-11-01 | メルク パテント ゲーエムベーハー | 有機イオン性機能材料、組成物および電子素子 |
| KR101885697B1 (ko) | 2011-06-28 | 2018-08-07 | 삼성디스플레이 주식회사 | 헤테로고리 화합물, 이를 포함한 유기 전계 발광 소자 및 상기 유기 전계 발광 소자를 포함하는 평판 표시 장치 |
| EP2810315A1 (en) | 2012-01-30 | 2014-12-10 | Merck Patent GmbH | Nanocrystals on fibers |
| KR101989059B1 (ko) | 2012-08-22 | 2019-06-14 | 삼성디스플레이 주식회사 | 캐스케이드형 화합물 및 이를 포함한 유기 발광 소자 |
| US9312500B2 (en) | 2012-08-31 | 2016-04-12 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device |
| EP2915199B1 (de) | 2012-10-31 | 2021-03-31 | Merck Patent GmbH | Elektronische vorrichtung |
| WO2014072017A1 (de) | 2012-11-12 | 2014-05-15 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| US8962160B2 (en) | 2012-12-26 | 2015-02-24 | Feng-Wen Yen | Material for organic electroluminescent device |
| WO2014106524A2 (de) | 2013-01-03 | 2014-07-10 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| EP2941470B1 (de) | 2013-01-03 | 2018-09-05 | Merck Patent GmbH | Elektronische vorrichtung |
| US9048437B2 (en) | 2013-01-29 | 2015-06-02 | Luminescence Technology Corporation | Organic compound for organic electroluminescent device |
| KR102050484B1 (ko) | 2013-03-04 | 2019-12-02 | 삼성디스플레이 주식회사 | 안트라센 유도체 및 이를 포함하는 유기전계발광소자 |
| KR102107106B1 (ko) | 2013-05-09 | 2020-05-07 | 삼성디스플레이 주식회사 | 스티릴계 화합물 및 이를 포함한 유기 발광 소자 |
| KR102104637B1 (ko) * | 2013-06-28 | 2020-04-27 | 삼성디스플레이 주식회사 | 유기전계발광소자 |
| KR102269131B1 (ko) | 2013-07-01 | 2021-06-25 | 삼성디스플레이 주식회사 | 화합물 및 이를 포함한 유기 발광 소자 |
| US10062850B2 (en) | 2013-12-12 | 2018-08-28 | Samsung Display Co., Ltd. | Amine-based compounds and organic light-emitting devices comprising the same |
| KR20150105584A (ko) * | 2014-03-07 | 2015-09-17 | 삼성디스플레이 주식회사 | 화합물 및 이를 포함한 유기 발광 소자 |
| KR20150132795A (ko) | 2014-05-16 | 2015-11-26 | 삼성디스플레이 주식회사 | 유기 발광 소자 |
| KR102327086B1 (ko) | 2014-06-11 | 2021-11-17 | 삼성디스플레이 주식회사 | 유기 발광 소자 |
| WO2016034262A1 (de) | 2014-09-05 | 2016-03-10 | Merck Patent Gmbh | Formulierungen und elektronische vorrichtungen |
| WO2016107663A1 (de) | 2014-12-30 | 2016-07-07 | Merck Patent Gmbh | Formulierungen und elektronische vorrichtungen |
| KR102343145B1 (ko) | 2015-01-12 | 2021-12-27 | 삼성디스플레이 주식회사 | 축합환 화합물 및 이를 포함한 유기 발광 소자 |
| KR102316684B1 (ko) | 2015-01-21 | 2021-10-26 | 삼성디스플레이 주식회사 | 유기 발광 소자 |
| KR102316682B1 (ko) | 2015-01-21 | 2021-10-26 | 삼성디스플레이 주식회사 | 유기 발광 소자 |
| KR102316683B1 (ko) | 2015-01-21 | 2021-10-26 | 삼성디스플레이 주식회사 | 유기 발광 소자 |
| EP3250658B1 (en) | 2015-01-30 | 2019-06-19 | Merck Patent GmbH | Materials for electronic devices |
| KR102331598B1 (ko) | 2015-03-23 | 2021-11-26 | 삼성디스플레이 주식회사 | 유기 발광 소자 및 이를 포함하는 유기 발광 표시 장치 |
| KR102570137B1 (ko) | 2015-03-30 | 2023-08-23 | 메르크 파텐트 게엠베하 | 실록산 용매를 포함하는 유기 기능성 재료의 제형 |
| US10312449B2 (en) | 2015-05-27 | 2019-06-04 | Samsung Display Co., Ltd. | Organic light-emitting device |
| US10367147B2 (en) | 2015-05-27 | 2019-07-30 | Samsung Display Co., Ltd. | Organic light-emitting device |
| KR101826427B1 (ko) * | 2015-06-05 | 2018-02-06 | 주식회사 엘지화학 | 이중 스피로형 유기 화합물 및 이를 포함하는 유기 전자 소자 |
| WO2016198141A1 (en) | 2015-06-12 | 2016-12-15 | Merck Patent Gmbh | Esters containing non-aromatic cycles as solvents for oled formulations |
| US11046884B2 (en) | 2015-08-28 | 2021-06-29 | Merck Patent Gmbh | Formulation of an organic functional material comprising an epoxy group containing solvent |
| JP7016535B2 (ja) | 2015-10-26 | 2022-02-07 | オーティーアイ ルミオニクス インコーポレーテッド | パターン化されたコーティングを含む表面およびデバイス上のコーティングをパターン化する方法 |
| CN107709330B (zh) * | 2015-11-17 | 2020-03-27 | 株式会社Lg化学 | 螺环化合物和包含其的有机发光元件 |
| KR102511544B1 (ko) | 2015-11-20 | 2023-03-17 | 삼성디스플레이 주식회사 | 광전 소자 및 이의 제조 방법 |
| CN108368361A (zh) | 2015-12-10 | 2018-08-03 | 默克专利有限公司 | 含有包含非芳族环的酮的制剂 |
| WO2017102048A1 (en) | 2015-12-15 | 2017-06-22 | Merck Patent Gmbh | Esters containing aromatic groups as solvents for organic electronic formulations |
| US11407916B2 (en) | 2015-12-16 | 2022-08-09 | Merck Patent Gmbh | Formulations containing a mixture of at least two different solvents |
| KR102723604B1 (ko) | 2015-12-16 | 2024-10-29 | 메르크 파텐트 게엠베하 | 고체 용매를 함유하는 제형 |
| US11107999B2 (en) | 2016-01-05 | 2021-08-31 | Samsung Display Co., Ltd. | Condensed cyclic compound and an organic light-emitting device including the same |
| EP3411455B1 (de) | 2016-02-05 | 2020-10-21 | Merck Patent GmbH | Materialien für elektronische vorrichtungen |
| WO2017140404A1 (en) | 2016-02-17 | 2017-08-24 | Merck Patent Gmbh | Formulation of an organic functional material |
| DE102016003104A1 (de) | 2016-03-15 | 2017-09-21 | Merck Patent Gmbh | Behälter umfassend eine Formulierung enthaltend mindestens einen organischen Halbleiter |
| KR102467109B1 (ko) | 2016-05-11 | 2022-11-14 | 메르크 파텐트 게엠베하 | 전기화학 전지용 조성물 |
| WO2017216129A1 (en) | 2016-06-16 | 2017-12-21 | Merck Patent Gmbh | Formulation of an organic functional material |
| CN109312183A (zh) | 2016-06-17 | 2019-02-05 | 默克专利有限公司 | 有机功能材料的制剂 |
| TW201815998A (zh) | 2016-06-28 | 2018-05-01 | 德商麥克專利有限公司 | 有機功能材料之調配物 |
| CN109563402B (zh) | 2016-08-04 | 2022-07-15 | 默克专利有限公司 | 有机功能材料的制剂 |
| TWI764942B (zh) | 2016-10-10 | 2022-05-21 | 德商麥克專利有限公司 | 電子裝置 |
| DE102017008794A1 (de) | 2016-10-17 | 2018-04-19 | Merck Patent Gmbh | Materialien zur Verwendung in elektronischen Vorrichtungen |
| EP3532565B1 (en) | 2016-10-31 | 2021-04-21 | Merck Patent GmbH | Formulation of an organic functional material |
| US10950792B2 (en) | 2016-10-31 | 2021-03-16 | Merck Patent Gmbh | Formulation of an organic functional material |
| EP3535240B1 (de) | 2016-11-02 | 2022-11-16 | Merck Patent GmbH | Materialien für elektronische vorrichtungen |
| CN110088112A (zh) | 2016-11-08 | 2019-08-02 | 默克专利有限公司 | 用于电子器件的化合物 |
| TWI756292B (zh) | 2016-11-14 | 2022-03-01 | 德商麥克專利有限公司 | 具有受體基團與供體基團之化合物 |
| TW201833118A (zh) | 2016-11-22 | 2018-09-16 | 德商麥克專利有限公司 | 用於電子裝置之材料 |
| KR102472751B1 (ko) | 2016-12-06 | 2022-11-30 | 메르크 파텐트 게엠베하 | 전자 디바이스의 제조 방법 |
| KR102486614B1 (ko) | 2016-12-13 | 2023-01-09 | 메르크 파텐트 게엠베하 | 유기 기능성 재료의 제형 |
| KR102463125B1 (ko) | 2016-12-22 | 2022-11-04 | 메르크 파텐트 게엠베하 | 전자 디바이스용 재료 |
| WO2018114883A1 (de) | 2016-12-22 | 2018-06-28 | Merck Patent Gmbh | Mischungen umfassend mindestens zwei organisch funktionelle verbindungen |
| TWI763772B (zh) | 2017-01-30 | 2022-05-11 | 德商麥克專利有限公司 | 電子裝置之有機元件的形成方法 |
| TWI791481B (zh) | 2017-01-30 | 2023-02-11 | 德商麥克專利有限公司 | 形成有機電致發光(el)元件之方法 |
| CN110291064B (zh) | 2017-02-02 | 2023-04-28 | 默克专利有限公司 | 用于电子器件的材料 |
| KR102557516B1 (ko) | 2017-03-02 | 2023-07-20 | 메르크 파텐트 게엠베하 | 유기 전자 디바이스용 재료 |
| WO2018178136A1 (en) | 2017-03-31 | 2018-10-04 | Merck Patent Gmbh | Printing method for an organic light emitting diode (oled) |
| KR102632027B1 (ko) | 2017-04-10 | 2024-01-31 | 메르크 파텐트 게엠베하 | 유기 기능성 재료의 제형 |
| EP3615542B1 (de) | 2017-04-25 | 2023-08-23 | Merck Patent GmbH | Verbindungen für elektronische vorrichtungen |
| KR20250036969A (ko) | 2017-04-26 | 2025-03-14 | 오티아이 루미오닉스 인크. | 표면의 코팅을 패턴화하는 방법 및 패턴화된 코팅을 포함하는 장치 |
| WO2018202603A1 (en) | 2017-05-03 | 2018-11-08 | Merck Patent Gmbh | Formulation of an organic functional material |
| TW201920343A (zh) | 2017-06-21 | 2019-06-01 | 德商麥克專利有限公司 | 電子裝置用材料 |
| US11767299B2 (en) | 2017-06-23 | 2023-09-26 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| EP3645766A1 (en) | 2017-06-26 | 2020-05-06 | Merck Patent GmbH | Homogeneous mixtures |
| US20200136045A1 (en) | 2017-06-28 | 2020-04-30 | Merck Patent Gmbh | Materials for electronic devices |
| KR102698061B1 (ko) | 2017-07-18 | 2024-08-22 | 메르크 파텐트 게엠베하 | 유기 기능성 재료의 제형 |
| KR102516056B1 (ko) * | 2017-07-21 | 2023-03-31 | 삼성디스플레이 주식회사 | 축합환 화합물 및 이를 포함한 유기 발광 소자 |
| EP4603562A3 (en) | 2017-07-28 | 2025-11-26 | Merck Patent GmbH | Spirobifluorene derivatives for use in electronic devices |
| JP7250773B2 (ja) | 2017-09-08 | 2023-04-03 | メルク パテント ゲーエムベーハー | 電子デバイス用材料 |
| KR20250035612A (ko) | 2017-11-23 | 2025-03-12 | 메르크 파텐트 게엠베하 | 전자 디바이스용 재료 |
| JP7634988B2 (ja) | 2017-12-15 | 2025-02-25 | メルク パテント ゲーエムベーハー | 有機エレクトロルミネッセントデバイスに使用するための置換芳香族アミン |
| KR102666621B1 (ko) | 2017-12-15 | 2024-05-16 | 메르크 파텐트 게엠베하 | 유기 기능성 재료의 제형 |
| JP7402800B2 (ja) | 2017-12-20 | 2023-12-21 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | ヘテロ芳香族化合物 |
| US11751415B2 (en) | 2018-02-02 | 2023-09-05 | Oti Lumionics Inc. | Materials for forming a nucleation-inhibiting coating and devices incorporating same |
| CN111712551A (zh) | 2018-02-26 | 2020-09-25 | 默克专利有限公司 | 有机功能材料的制剂 |
| KR20200132912A (ko) | 2018-03-16 | 2020-11-25 | 메르크 파텐트 게엠베하 | 유기 전계발광 디바이스용 재료 |
| KR20210022046A (ko) | 2018-06-15 | 2021-03-02 | 메르크 파텐트 게엠베하 | 유기 기능성 재료의 포뮬레이션 |
| KR102721590B1 (ko) * | 2018-08-17 | 2024-10-23 | 엘지디스플레이 주식회사 | 유기전계발광소자 |
| WO2020064582A1 (de) | 2018-09-24 | 2020-04-02 | Merck Patent Gmbh | Verfahren zur herstellung von granulat |
| CN112930606A (zh) | 2018-11-06 | 2021-06-08 | 默克专利有限公司 | 用于形成电子器件的有机元件的方法 |
| KR102094830B1 (ko) * | 2018-11-30 | 2020-03-30 | 에스에프씨 주식회사 | 다환 방향족 유도체 화합물 및 이를 이용한 유기발광소자 |
| US11985891B2 (en) | 2018-11-30 | 2024-05-14 | Sfc Co., Ltd. | Polycyclic aromatic compounds and organic electroluminescent devices using the same |
| US20220127286A1 (en) | 2019-03-04 | 2022-04-28 | Merck Patent Gmbh | Ligands for nano-sized materials |
| WO2020178804A1 (en) | 2019-03-07 | 2020-09-10 | Oti Lumionics Inc. | Materials for forming a nucleation-inhibiting coating and devices incorporating same |
| CN113950630A (zh) | 2019-04-18 | 2022-01-18 | Oti照明公司 | 用于形成成核抑制涂层的材料和结合所述成核抑制涂层的装置 |
| US12069938B2 (en) | 2019-05-08 | 2024-08-20 | Oti Lumionics Inc. | Materials for forming a nucleation-inhibiting coating and devices incorporating same |
| EP4139971A1 (en) | 2020-04-21 | 2023-03-01 | Merck Patent GmbH | Emulsions comprising organic functional materials |
| EP4139408A1 (en) | 2020-04-21 | 2023-03-01 | Merck Patent GmbH | Formulation of an organic functional material |
| KR20230028465A (ko) | 2020-06-23 | 2023-02-28 | 메르크 파텐트 게엠베하 | 혼합물의 제조 방법 |
| WO2022123431A1 (en) | 2020-12-07 | 2022-06-16 | Oti Lumionics Inc. | Patterning a conductive deposited layer using a nucleation inhibiting coating and an underlying metallic coating |
| CN116635491A (zh) | 2020-12-08 | 2023-08-22 | 默克专利有限公司 | 油墨体系和用于喷墨印刷的方法 |
| WO2022223675A1 (en) | 2021-04-23 | 2022-10-27 | Merck Patent Gmbh | Formulation of an organic functional material |
| KR20240012506A (ko) | 2021-05-21 | 2024-01-29 | 메르크 파텐트 게엠베하 | 적어도 하나의 기능성 물질의 연속 정제 방법 및 적어도 하나의 기능성 물질의 연속 정제를 위한 디바이스 |
| CN117730638A (zh) | 2021-08-02 | 2024-03-19 | 默克专利有限公司 | 通过组合油墨进行的印刷方法 |
| EP4410074B1 (de) | 2021-09-28 | 2025-05-21 | Merck Patent GmbH | Materialien für elektronische vorrichtungen |
| CN118056486A (zh) | 2021-09-28 | 2024-05-17 | 默克专利有限公司 | 用于电子器件的材料 |
| KR20240075872A (ko) | 2021-09-28 | 2024-05-29 | 메르크 파텐트 게엠베하 | 전자 디바이스용 재료 |
| EP4410071A1 (de) | 2021-09-28 | 2024-08-07 | Merck Patent GmbH | Materialien für elektronische vorrichtungen |
| TW202349760A (zh) | 2021-10-05 | 2023-12-16 | 德商麥克專利有限公司 | 電子裝置之有機元件的形成方法 |
| EP4437814A1 (de) | 2021-11-25 | 2024-10-02 | Merck Patent GmbH | Materialien für elektronische vorrichtungen |
| WO2023117837A1 (de) | 2021-12-21 | 2023-06-29 | Merck Patent Gmbh | Verfahren zur herstellung von deuterierten organischen verbindungen |
| EP4479385A1 (de) | 2022-02-14 | 2024-12-25 | Merck Patent GmbH | Materialien für elektronische vorrichtungen |
| KR20250011205A (ko) | 2022-05-18 | 2025-01-21 | 메르크 파텐트 게엠베하 | 중수소화 유기 화합물의 제조를 위한 방법 |
| TW202411366A (zh) | 2022-06-07 | 2024-03-16 | 德商麥克專利有限公司 | 藉由組合油墨來印刷電子裝置功能層之方法 |
| WO2024013004A1 (de) | 2022-07-11 | 2024-01-18 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| TW202440819A (zh) | 2022-12-16 | 2024-10-16 | 德商麥克專利有限公司 | 有機功能性材料之調配物 |
| WO2024133048A1 (en) | 2022-12-20 | 2024-06-27 | Merck Patent Gmbh | Method for preparing deuterated aromatic compounds |
| EP4665715A1 (en) | 2023-02-17 | 2025-12-24 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| WO2024218109A1 (de) | 2023-04-20 | 2024-10-24 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2024240725A1 (de) | 2023-05-25 | 2024-11-28 | Merck Patent Gmbh | Tris[1,2,4]triazolo[1,5-a:1',5'-c:1'',5''-e][1,3,5]triazin-derivate zur verwendung in organischen elektrolumineszenzvorrichtungen |
| WO2025012253A1 (en) | 2023-07-12 | 2025-01-16 | Merck Patent Gmbh | Materials for electronic devices |
| WO2025021855A1 (de) | 2023-07-27 | 2025-01-30 | Merck Patent Gmbh | Materialien für organische lichtemittierende vorrichtungen und organische sensoren |
| WO2025032039A1 (en) | 2023-08-07 | 2025-02-13 | Merck Patent Gmbh | Process for the preparation of an electronic device |
| WO2025132547A1 (en) | 2023-12-21 | 2025-06-26 | Merck Patent Gmbh | Mechanochemical method for deuterating organic compounds |
| WO2025132551A1 (de) | 2023-12-22 | 2025-06-26 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2025196145A1 (en) | 2024-03-22 | 2025-09-25 | Merck Patent Gmbh | Materials for organic light emitting devices |
| WO2025210013A1 (de) | 2024-04-04 | 2025-10-09 | Merck Patent Gmbh | Verbindungen für elektronische vorrichtungen, insbesondere verbindungen für oleds |
Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH10294179A (ja) * | 1997-04-21 | 1998-11-04 | Mitsubishi Chem Corp | 有機電界発光素子及び蛍光材料 |
| JPH113782A (ja) * | 1997-06-12 | 1999-01-06 | Toppan Printing Co Ltd | 有機薄膜el素子 |
| EP0952200A2 (en) | 1998-03-20 | 1999-10-27 | Eastman Kodak Company | Organic electroluminescent elements for stable blue electroluminescent devices |
| EP1009044A2 (en) * | 1998-12-09 | 2000-06-14 | Eastman Kodak Company | Electroluminescent device with anthracene derivatives hole transport layer |
| JP2001223082A (ja) * | 2000-02-08 | 2001-08-17 | Toray Ind Inc | 発光素子 |
| EP1167488A1 (en) | 1999-09-21 | 2002-01-02 | Idemitsu Kosan Company Limited | Organic electroluminescence and organic luminous medium |
| WO2002014244A1 (en) * | 2000-08-10 | 2002-02-21 | Mitsui Chemicals, Inc. | Hydrocarbon compound, material for organic electroluminescent element and organic electroluminescent element |
| US20020048688A1 (en) | 2000-03-30 | 2002-04-25 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device and organic light emitting medium |
| WO2002038524A1 (en) * | 2000-11-08 | 2002-05-16 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent element |
| JP2002329580A (ja) * | 2001-02-22 | 2002-11-15 | Canon Inc | 有機発光素子 |
Family Cites Families (45)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4769292A (en) * | 1987-03-02 | 1988-09-06 | Eastman Kodak Company | Electroluminescent device with modified thin film luminescent zone |
| JP2814435B2 (ja) | 1987-03-02 | 1998-10-22 | イーストマン・コダック・カンパニー | 改良薄膜発光帯をもつ電場発光デバイス |
| JP2897138B2 (ja) | 1989-06-30 | 1999-05-31 | 株式会社リコー | 電界発光素子 |
| JP3200889B2 (ja) | 1991-10-23 | 2001-08-20 | ソニー株式会社 | 画像の振動補正装置 |
| JPH07138561A (ja) | 1993-11-17 | 1995-05-30 | Idemitsu Kosan Co Ltd | 有機エレクトロルミネッセンス素子 |
| JP3816969B2 (ja) | 1994-04-26 | 2006-08-30 | Tdk株式会社 | 有機el素子 |
| EP0681019B1 (en) † | 1994-04-26 | 1999-09-01 | TDK Corporation | Phenylanthracene derivative and organic EL element |
| JP3506281B2 (ja) | 1995-01-26 | 2004-03-15 | 出光興産株式会社 | 有機エレクトロルミネッセンス素子 |
| JP3724833B2 (ja) | 1995-03-06 | 2005-12-07 | 出光興産株式会社 | 有機エレクトロルミネッセンス素子 |
| WO1998008360A1 (en) | 1996-08-19 | 1998-02-26 | Tdk Corporation | Organic electroluminescent device |
| US6582837B1 (en) * | 1997-07-14 | 2003-06-24 | Nec Corporation | Organic electroluminescence device |
| US5935721A (en) * | 1998-03-20 | 1999-08-10 | Eastman Kodak Company | Organic electroluminescent elements for stable electroluminescent |
| JP3357857B2 (ja) * | 1998-07-24 | 2002-12-16 | 出光興産株式会社 | 有機エレクトロルミネッセンス素子及びその製造方法 |
| US6361886B2 (en) * | 1998-12-09 | 2002-03-26 | Eastman Kodak Company | Electroluminescent device with improved hole transport layer |
| JP4429438B2 (ja) | 1999-01-19 | 2010-03-10 | 出光興産株式会社 | アミノ化合物及びそれを用いた有機エレクトロルミネッセンス素子 |
| JP3838816B2 (ja) | 1999-06-03 | 2006-10-25 | Tdk株式会社 | 有機el素子用化合物および有機el素子 |
| JP2001052870A (ja) * | 1999-06-03 | 2001-02-23 | Tdk Corp | 有機el素子 |
| JP2001097897A (ja) | 1999-09-30 | 2001-04-10 | Idemitsu Kosan Co Ltd | 有機化合物及びそれを用いた有機エレクトロルミネッセンス素子 |
| JP3970495B2 (ja) * | 2000-01-11 | 2007-09-05 | Tdk株式会社 | 有機el素子 |
| JP4274667B2 (ja) | 2000-03-10 | 2009-06-10 | 三井化学株式会社 | 炭化水素化合物および有機電界発光素子 |
| DE60140720D1 (de) * | 2000-03-29 | 2010-01-21 | Idemitsu Kosan Co | Anthracenderivate und organische elektrolumineszente vorrichtung die unter verwendung dieser derivate hergestellt ist |
| JP4996794B2 (ja) * | 2000-08-10 | 2012-08-08 | 三井化学株式会社 | 炭化水素化合物、有機電界発光素子用材料および有機電界発光素子 |
| JP2002124385A (ja) * | 2000-10-19 | 2002-04-26 | Idemitsu Kosan Co Ltd | 有機エレクトロルミネッセンス素子 |
| US6998487B2 (en) * | 2001-04-27 | 2006-02-14 | Lg Chem, Ltd. | Double-spiro organic compounds and organic electroluminescent devices using the same |
| JP4220696B2 (ja) | 2001-10-16 | 2009-02-04 | 三井化学株式会社 | 炭化水素化合物、有機電界発光素子用材料および有機電界発光素子 |
| JP4080213B2 (ja) * | 2002-02-01 | 2008-04-23 | 三井化学株式会社 | 有機電界発光素子 |
| JP2003261472A (ja) * | 2002-03-07 | 2003-09-16 | Mitsui Chemicals Inc | 有機電界発光素子および新規炭化水素化合物 |
| JP4299028B2 (ja) * | 2002-03-11 | 2009-07-22 | Tdk株式会社 | 有機el素子 |
| JP4646494B2 (ja) * | 2002-04-11 | 2011-03-09 | 出光興産株式会社 | 新規含窒素複素環誘導体及びそれを用いた有機エレクトロルミネッセンス素子 |
| JP4170655B2 (ja) † | 2002-04-17 | 2008-10-22 | 出光興産株式会社 | 新規芳香族化合物及びそれを利用した有機エレクトロルミネッセンス素子 |
| US7164155B2 (en) | 2002-05-15 | 2007-01-16 | Semiconductor Energy Laboratory Co., Ltd. | Light emitting device |
| JP4364551B2 (ja) * | 2002-05-15 | 2009-11-18 | 株式会社半導体エネルギー研究所 | 発光装置の作製方法 |
| JP2004042485A (ja) * | 2002-07-12 | 2004-02-12 | Mitsui Chemicals Inc | 光記録媒体及び炭化水素化合物 |
| CN101068041B (zh) | 2002-07-19 | 2010-08-18 | 出光兴产株式会社 | 有机电致发光装置和有机发光介质 |
| US7169482B2 (en) † | 2002-07-26 | 2007-01-30 | Lg.Philips Lcd Co., Ltd. | Display device with anthracene and triazine derivatives |
| US20040018380A1 (en) | 2002-07-26 | 2004-01-29 | Xerox Corporation | Display device with anthracene and triazine derivatives |
| JP4025136B2 (ja) * | 2002-07-31 | 2007-12-19 | 出光興産株式会社 | アントラセン誘導体、有機エレクトロルミネッセンス素子用発光材料及び有機エレクトロルミネッセンス素子 |
| JP4025137B2 (ja) * | 2002-08-02 | 2007-12-19 | 出光興産株式会社 | アントラセン誘導体及びそれを利用した有機エレクトロルミネッセンス素子 |
| JP2004079421A (ja) * | 2002-08-21 | 2004-03-11 | Tdk Corp | 有機el素子 |
| WO2004063159A1 (ja) † | 2003-01-10 | 2004-07-29 | Idemitsu Kosan Co., Ltd. | 含窒素複素環誘導体及びそれを用いた有機エレクトロルミネッセンス素子 |
| US7887931B2 (en) † | 2003-10-24 | 2011-02-15 | Global Oled Technology Llc | Electroluminescent device with anthracene derivative host |
| EP1627891A1 (en) † | 2004-08-11 | 2006-02-22 | Covion Organic Semiconductors GmbH | Polymers for use in organic electroluminescent devices |
| KR100670254B1 (ko) † | 2004-12-20 | 2007-01-16 | 삼성에스디아이 주식회사 | 트리아릴아민계 화합물 및 이를 이용한 유기 전계 발광 소자 |
| DE102005058558A1 (de) † | 2005-12-08 | 2007-06-14 | Merck Patent Gmbh | Organische Elektrolumineszenzvorrichtungen |
| DE102007024850A1 (de) † | 2007-05-29 | 2008-12-04 | Merck Patent Gmbh | Neue Materialien für organische Elektrolumineszenzvorrichtungen |
-
2003
- 2003-08-18 US US10/524,825 patent/US7839074B2/en not_active Expired - Lifetime
- 2003-08-18 CN CNB038198886A patent/CN100505963C/zh not_active Expired - Lifetime
- 2003-08-18 EP EP10184035.3A patent/EP2261302B2/en not_active Expired - Lifetime
- 2003-08-18 AT AT09175111T patent/ATE555182T1/de active
- 2003-08-18 JP JP2004530558A patent/JP4041816B2/ja not_active Expired - Lifetime
- 2003-08-18 KR KR1020057003056A patent/KR100924462B1/ko not_active Expired - Lifetime
- 2003-08-18 CN CN2009101408869A patent/CN101628847B/zh not_active Expired - Lifetime
- 2003-08-18 KR KR1020097005383A patent/KR100946476B1/ko not_active Expired - Lifetime
- 2003-08-18 DE DE60330696T patent/DE60330696D1/de not_active Expired - Lifetime
- 2003-08-18 EP EP09175111.5A patent/EP2145937B2/en not_active Expired - Lifetime
- 2003-08-18 AT AT03792695T patent/ATE452954T1/de not_active IP Right Cessation
- 2003-08-18 TW TW092122650A patent/TWI284485B/zh not_active IP Right Cessation
- 2003-08-18 EP EP03792695.3A patent/EP1553154B2/en not_active Expired - Lifetime
- 2003-08-18 WO PCT/JP2003/010402 patent/WO2004018587A1/ja not_active Ceased
-
2007
- 2007-08-27 JP JP2007219766A patent/JP4448872B2/ja not_active Expired - Lifetime
-
2008
- 2008-04-16 JP JP2008106614A patent/JP4864931B2/ja not_active Expired - Lifetime
-
2010
- 2010-10-12 US US12/902,452 patent/US8318324B2/en not_active Expired - Fee Related
-
2012
- 2012-05-29 US US13/482,031 patent/US8785006B2/en not_active Expired - Lifetime
-
2014
- 2014-06-11 US US14/301,865 patent/US9583716B2/en not_active Expired - Fee Related
-
2017
- 2017-01-12 US US15/405,028 patent/US10217943B2/en not_active Expired - Lifetime
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH10294179A (ja) * | 1997-04-21 | 1998-11-04 | Mitsubishi Chem Corp | 有機電界発光素子及び蛍光材料 |
| JPH113782A (ja) * | 1997-06-12 | 1999-01-06 | Toppan Printing Co Ltd | 有機薄膜el素子 |
| EP0952200A2 (en) | 1998-03-20 | 1999-10-27 | Eastman Kodak Company | Organic electroluminescent elements for stable blue electroluminescent devices |
| EP1009044A2 (en) * | 1998-12-09 | 2000-06-14 | Eastman Kodak Company | Electroluminescent device with anthracene derivatives hole transport layer |
| EP1167488A1 (en) | 1999-09-21 | 2002-01-02 | Idemitsu Kosan Company Limited | Organic electroluminescence and organic luminous medium |
| JP2001223082A (ja) * | 2000-02-08 | 2001-08-17 | Toray Ind Inc | 発光素子 |
| US20020048688A1 (en) | 2000-03-30 | 2002-04-25 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device and organic light emitting medium |
| WO2002014244A1 (en) * | 2000-08-10 | 2002-02-21 | Mitsui Chemicals, Inc. | Hydrocarbon compound, material for organic electroluminescent element and organic electroluminescent element |
| EP1221434A1 (en) | 2000-08-10 | 2002-07-10 | Mitsui Chemicals, Inc. | Hydrocarbon compound, material for organic electroluminescent element and organic electroluminescent element |
| WO2002038524A1 (en) * | 2000-11-08 | 2002-05-16 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent element |
| JP2002329580A (ja) * | 2001-02-22 | 2002-11-15 | Canon Inc | 有機発光素子 |
Cited By (203)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8436344B2 (en) | 2002-12-24 | 2013-05-07 | Lg Display Co., Ltd. | Organic electroluminescent device |
| US7700201B2 (en) | 2002-12-24 | 2010-04-20 | Lg Display Co., Ltd. | Organic electroluminescent device |
| JP2007027779A (ja) * | 2002-12-24 | 2007-02-01 | Lg Electronics Inc | 有機電界発光デバイス |
| US7887931B2 (en) | 2003-10-24 | 2011-02-15 | Global Oled Technology Llc | Electroluminescent device with anthracene derivative host |
| US7056601B2 (en) | 2003-10-24 | 2006-06-06 | Eastman Kodak Company | OLED device with asymmetric host |
| JP2007524745A (ja) * | 2004-02-17 | 2007-08-30 | イーストマン コダック カンパニー | 各種ドーパントを含むアントラセン誘導体ホスト |
| JP2012248852A (ja) * | 2004-02-17 | 2012-12-13 | Global Oled Technology Llc | 各種ドーパントを含むアントラセン誘導体ホスト |
| JPWO2005091686A1 (ja) * | 2004-03-19 | 2008-02-07 | チッソ株式会社 | 有機電界発光素子 |
| WO2005091686A1 (ja) * | 2004-03-19 | 2005-09-29 | Chisso Corporation | 有機電界発光素子 |
| JP2012104842A (ja) * | 2004-03-25 | 2012-05-31 | Global Oled Technology Llc | アントラセン誘導体ホストを有するエレクトロルミネッセンス・デバイス |
| US7326371B2 (en) | 2004-03-25 | 2008-02-05 | Eastman Kodak Company | Electroluminescent device with anthracene derivative host |
| WO2005110950A1 (en) * | 2004-04-29 | 2005-11-24 | Eastman Kodak Company | Synthesis of unsymmetric anthracene compounds |
| US7763761B2 (en) | 2004-05-27 | 2010-07-27 | Idemitsu Kosan Co., Ltd. | Asymmetric pyrene derivative and organic electroluminescence device employing the same |
| US8318995B2 (en) | 2004-05-27 | 2012-11-27 | Idemitsu Kosan Co., Ltd. | Asymmetric pyrene derivative and organic electroluminescence device employing the same |
| US7504526B2 (en) | 2004-06-09 | 2009-03-17 | Idemitsu Kosan Co., Ltd. | Anthracene derivative and organic electroluminescence device employing the same |
| WO2005121057A1 (ja) * | 2004-06-09 | 2005-12-22 | Idemitsu Kosan Co., Ltd. | アントラセン誘導体及びそれを利用した有機エレクトロルミネッセンス素子 |
| JP2008504247A (ja) * | 2004-06-26 | 2008-02-14 | メルク パテント ゲーエムベーハー | 有機電子デバイスのための化合物 |
| US9653688B2 (en) | 2004-07-08 | 2017-05-16 | Junji Kido | Organic devices, organic electroluminescent devices and organic solar cells |
| US9466799B2 (en) | 2004-07-08 | 2016-10-11 | Junji Kido | Organic devices, organic electroluminescent devices and organic solar cells |
| EP2713416A1 (en) * | 2004-07-08 | 2014-04-02 | Junji Kido | Organic devices, organic electroluminescent devices and organic solar cells |
| JP2006045503A (ja) * | 2004-07-09 | 2006-02-16 | Chisso Corp | 発光材料およびこれを用いた有機電界発光素子 |
| US9040170B2 (en) | 2004-09-20 | 2015-05-26 | Global Oled Technology Llc | Electroluminescent device with quinazoline complex emitter |
| US7544425B2 (en) | 2004-10-29 | 2009-06-09 | Eastman Kodak Company | Organic element for electroluminescent devices |
| WO2006049860A1 (en) * | 2004-10-29 | 2006-05-11 | Eastman Kodak Company | Organic element for electroluminescent devices |
| JP2008521857A (ja) * | 2004-12-01 | 2008-06-26 | メルク パテント ゲーエムベーハー | 有機電子デバイスのための化合物 |
| US8883324B2 (en) | 2005-01-05 | 2014-11-11 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative and organic electroluminescent device using same |
| JP2008538350A (ja) * | 2005-03-16 | 2008-10-23 | メルク パテント ゲーエムベーハー | 有機エレクトロルミネセンスデバイスのための新規材料 |
| CN101142294B (zh) * | 2005-03-16 | 2011-12-07 | 默克专利有限公司 | 用于有机电致发光器件的新颖材料 |
| WO2006097208A1 (de) * | 2005-03-16 | 2006-09-21 | Merck Patent Gmbh | Neue materialien für organische elektrolumineszenzvorrichtungen |
| US8124249B2 (en) | 2005-03-16 | 2012-02-28 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2006103916A1 (ja) * | 2005-03-25 | 2006-10-05 | Idemitsu Kosan Co., Ltd. | 有機エレクトロルミネッセンス素子 |
| US7985491B2 (en) | 2005-03-28 | 2011-07-26 | Idemitsu Kosan Co., Ltd. | Anthrylarylene derivative, material for organic electroluminescence device and organic electroluminescence device using same |
| JPWO2006104044A1 (ja) * | 2005-03-28 | 2008-09-04 | 出光興産株式会社 | アントリルアリーレン誘導体、有機エレクトロルミネッセンス素子用材料、及びそれを用いた有機エレクトロルミネッセンス素子 |
| WO2006104044A1 (ja) * | 2005-03-28 | 2006-10-05 | Idemitsu Kosan Co., Ltd. | アントリルアリーレン誘導体、有機エレクトロルミネッセンス素子用材料、及びそれを用いた有機エレクトロルミネッセンス素子 |
| WO2006108498A1 (de) * | 2005-04-12 | 2006-10-19 | Merck Patent Gmbh | Organische elektrolumineszenzvorrichtungen |
| CN101243157B (zh) * | 2005-08-16 | 2012-09-05 | 葛来西雅帝史派有限公司 | 绿色电致发光化合物和使用该化合物的有机电致发光器件 |
| WO2007021117A1 (en) * | 2005-08-16 | 2007-02-22 | Gracel Display Inc. | Green electroluminescent compounds and organic electroluminescent device using the same |
| KR100788254B1 (ko) * | 2005-08-16 | 2007-12-27 | (주)그라쎌 | 녹색 발광 화합물 및 이를 발광재료로서 채용하고 있는발광소자 |
| EP3211058A1 (de) | 2005-08-26 | 2017-08-30 | Merck Patent GmbH | Neue materialien für organische elektrolumineszenzvorrichtungen |
| WO2007022845A1 (de) | 2005-08-26 | 2007-03-01 | Merck Patent Gmbh | Neue materialien für organische elektrolumineszenzvorrichtungen |
| WO2007029410A1 (ja) * | 2005-09-08 | 2007-03-15 | Idemitsu Kosan Co., Ltd. | ポリアリールアミンを用いた有機エレクトロルミネッセンス素子 |
| US9666826B2 (en) | 2005-11-30 | 2017-05-30 | Global Oled Technology Llc | Electroluminescent device including an anthracene derivative |
| US9017825B2 (en) | 2005-12-08 | 2015-04-28 | Merck Patent Gmbh | Anthracene derivatives and their use in organic electroluminescent devices |
| US8795847B2 (en) | 2005-12-08 | 2014-08-05 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| JP2009518342A (ja) * | 2005-12-08 | 2009-05-07 | メルク パテント ゲーエムベーハー | 有機エレクトロルミネセンス素子のための新規材料 |
| JP2009518831A (ja) * | 2005-12-08 | 2009-05-07 | メルク パテント ゲーエムベーハー | 有機エレクトロルミネセンス素子 |
| US8785001B2 (en) | 2005-12-08 | 2014-07-22 | Merck Patent Gmbh | Organic electroluminescent devices |
| US9214636B2 (en) | 2006-02-28 | 2015-12-15 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device |
| JP2007238500A (ja) * | 2006-03-08 | 2007-09-20 | Mitsui Chemicals Inc | アントラセン化合物および該化合物を含有する有機電界発光素子 |
| US8999521B2 (en) | 2006-03-24 | 2015-04-07 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2007110129A1 (de) | 2006-03-24 | 2007-10-04 | Merck Patent Gmbh | Neue materialien für organische elektrolumineszenzvorrichtungen |
| US8026664B2 (en) | 2006-04-20 | 2011-09-27 | Canon Kabushiki Kaisha | Compound and organic light emitting device |
| WO2007123256A1 (en) * | 2006-04-20 | 2007-11-01 | Canon Kabushiki Kaisha | Compound and organic light emitting device |
| EP2355198A1 (en) | 2006-05-08 | 2011-08-10 | Global OLED Technology LLC | OLED electron-injecting layer |
| US11678571B2 (en) | 2006-06-22 | 2023-06-13 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent device using aryl amine derivative containing heterocycle |
| US11152574B2 (en) | 2006-06-22 | 2021-10-19 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent device using aryl amine derivative containing heterocycle |
| US11094888B2 (en) * | 2006-06-22 | 2021-08-17 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent device using aryl amine derivative containing heterocycle |
| WO2008006449A1 (de) | 2006-07-11 | 2008-01-17 | Merck Patent Gmbh | Neue materialien für organische elektrolumineszenzvorrichtungen |
| WO2008056722A1 (en) | 2006-11-09 | 2008-05-15 | Idemitsu Kosan Co., Ltd. | Organic el material-containing solution, method for forming thin film of organic el material, thin film of organic el material, and organic el device |
| WO2008082178A1 (en) * | 2006-12-29 | 2008-07-10 | Doosan Corporation | Diaryl trianthracene derivatives and organic light emitting layer or diode comprising the same |
| US9290691B2 (en) | 2007-02-28 | 2016-03-22 | Idemitsu Kosan Co., Ltd. | Organic el material-containing solution, method for forming organic el thin film, organic el device comprising organic el thin film, and method for manufacturing organic el display panel |
| JP5320282B2 (ja) * | 2007-02-28 | 2013-10-23 | 出光興産株式会社 | 有機el材料含有溶液、有機el薄膜形成方法、有機el薄膜を含む有機el素子および有機elディスプレイパネル製造方法 |
| WO2008105472A1 (ja) | 2007-02-28 | 2008-09-04 | Idemitsu Kosan Co., Ltd. | 有機el材料含有溶液、有機el薄膜形成方法、有機el薄膜を含む有機el素子および有機elディスプレイパネル製造方法 |
| WO2008143229A1 (ja) | 2007-05-21 | 2008-11-27 | Idemitsu Kosan Co., Ltd. | アントラセン誘導体及びそれを利用した有機エレクトロルミネッセンス素子 |
| DE102007024850A1 (de) | 2007-05-29 | 2008-12-04 | Merck Patent Gmbh | Neue Materialien für organische Elektrolumineszenzvorrichtungen |
| US8273468B2 (en) | 2007-06-01 | 2012-09-25 | E I Du Pont De Nemours And Company | Green luminescent materials |
| US8604247B2 (en) | 2007-06-01 | 2013-12-10 | E I Du Pont De Nemours And Company | Chrysenes for deep blue luminescent applications |
| KR101350524B1 (ko) * | 2007-11-21 | 2014-01-16 | 주식회사 엘지화학 | 신규한 안트라센 유도체 및 이를 이용한 유기 전자 소자 |
| JP5399920B2 (ja) * | 2007-11-21 | 2014-01-29 | 出光興産株式会社 | 縮合芳香族誘導体及びそれを用いた有機エレクトロルミネッセンス素子 |
| US8623520B2 (en) | 2007-11-21 | 2014-01-07 | Idemitsu Kosan Co., Ltd. | Fused aromatic derivative and organic electroluminescence device using the same |
| US10153450B2 (en) | 2007-11-21 | 2018-12-11 | Idemitsu Kosan Co., Ltd. | Fused aromatic derivative and organic electroluminescence device using the same |
| US8993123B2 (en) | 2008-02-13 | 2015-03-31 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102008008953B4 (de) | 2008-02-13 | 2019-05-09 | Merck Patent Gmbh | Neue Materialien für organische Elektrolumineszenzvorrichtungen |
| DE102008008953A1 (de) | 2008-02-13 | 2009-08-20 | Merck Patent Gmbh | Neue Materialien für organische Elektrolumineszenzvorrichtungen |
| US8450727B2 (en) | 2008-02-15 | 2013-05-28 | Idemitsu Kosan Co., Ltd. | Organic luminescent medium and organic EL device |
| US9024301B2 (en) | 2008-02-15 | 2015-05-05 | Idemitsu Kosan Co., Ltd. | Organic luminescent medium and organic EL device |
| WO2009102054A1 (ja) * | 2008-02-15 | 2009-08-20 | Idemitsu Kosan Co., Ltd. | 有機発光媒体および有機el素子 |
| KR101273057B1 (ko) * | 2008-02-21 | 2013-06-10 | 주식회사 엘지화학 | 신규한 안트라센 유도체 및 이를 이용한 유기 전자 소자 |
| DE102008018670A1 (de) | 2008-04-14 | 2009-10-15 | Merck Patent Gmbh | Neue Materialien für organische Elektrolumineszenzvorrichtungen |
| JPWO2010013676A1 (ja) * | 2008-07-28 | 2012-01-12 | 出光興産株式会社 | 有機発光媒体及び有機el素子 |
| WO2010013676A1 (ja) * | 2008-07-28 | 2010-02-04 | 出光興産株式会社 | 有機発光媒体及び有機el素子 |
| DE102008054141A1 (de) | 2008-10-31 | 2010-05-06 | Merck Patent Gmbh | Neue Materialien für organische Elektrolumineszenzvorrichtungen |
| US8263973B2 (en) | 2008-12-19 | 2012-09-11 | E I Du Pont De Nemours And Company | Anthracene compounds for luminescent applications |
| US8531100B2 (en) | 2008-12-22 | 2013-09-10 | E I Du Pont De Nemours And Company | Deuterated compounds for luminescent applications |
| DE102009005746A1 (de) | 2009-01-23 | 2010-07-29 | Merck Patent Gmbh | Materialien für organische Elektrolumineszenzvorrichtungen |
| WO2010083869A2 (de) | 2009-01-23 | 2010-07-29 | Merck Patent Gmbh | Materialien für organische elektrolumineszenzvorrichtungen |
| US9006503B2 (en) | 2009-01-23 | 2015-04-14 | Merck Patent Gmbh | Organic electroluminescence devices containing substituted benzo[C]phenanthrenes |
| US8710284B2 (en) | 2009-01-23 | 2014-04-29 | Merck Patent Gmbh | Materials for organic electroluminescent devices containing substituted 10-benzo[c]phenanthrenes |
| DE102009009277A1 (de) | 2009-02-17 | 2010-08-19 | Merck Patent Gmbh | Organische elektronische Vorrichtung |
| DE102009009277B4 (de) | 2009-02-17 | 2023-12-07 | Merck Patent Gmbh | Organische elektronische Vorrichtung, Verfahren zu deren Herstellung und Verwendung von Verbindungen |
| WO2010094378A1 (de) | 2009-02-17 | 2010-08-26 | Merck Patent Gmbh | Organische elektronische vorrichtung |
| US9066410B2 (en) | 2009-02-17 | 2015-06-23 | Merck Patent Gmbh | Organic electronic device |
| US8890131B2 (en) | 2009-02-27 | 2014-11-18 | E I Du Pont De Nemours And Company | Deuterated compounds for electronic applications |
| US8759818B2 (en) | 2009-02-27 | 2014-06-24 | E I Du Pont De Nemours And Company | Deuterated compounds for electronic applications |
| JP2010209144A (ja) * | 2009-03-06 | 2010-09-24 | Mitsubishi Chemicals Corp | 有機電界発光素子材料、有機電界発光素子用組成物、有機電界発光素子、有機elディスプレイおよび有機el照明 |
| US8497495B2 (en) | 2009-04-03 | 2013-07-30 | E I Du Pont De Nemours And Company | Electroactive materials |
| US8431250B2 (en) | 2009-04-24 | 2013-04-30 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative, and organic electroluminescent element comprising the same |
| US9741938B2 (en) | 2009-04-24 | 2017-08-22 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative, and organic electroluminescent element comprising the same |
| US10686137B2 (en) | 2009-04-24 | 2020-06-16 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative, and organic electroluminescent element comprising the same |
| US10263191B2 (en) | 2009-04-24 | 2019-04-16 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative, and organic electroluminescent element comprising the same |
| US9166179B2 (en) | 2009-04-24 | 2015-10-20 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative, and organic electroluminescent element comprising the same |
| US9466800B2 (en) | 2009-04-24 | 2016-10-11 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative, and organic electroluminescent element comprising the same |
| US11024806B2 (en) | 2009-04-24 | 2021-06-01 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative, and organic electroluminescent element comprising the same |
| WO2010149259A2 (en) | 2009-06-22 | 2010-12-29 | Merck Patent Gmbh | Conducting formulation |
| DE102009033371A1 (de) | 2009-07-16 | 2011-05-12 | Merck Patent Gmbh | Materialien für elektronische Vorrichtungen |
| DE102009034625A1 (de) | 2009-07-27 | 2011-02-03 | Merck Patent Gmbh | Neue Materialien für organische Elektrolumineszenzvorrichtungen |
| WO2011012212A1 (de) | 2009-07-27 | 2011-02-03 | Merck Patent Gmbh | Neue materialien für organische elektrolumineszenzvorrichtungen |
| US8968883B2 (en) | 2009-08-13 | 2015-03-03 | E I Du Pont De Nemours And Company | Chrysene derivative materials |
| WO2011035836A1 (de) | 2009-09-23 | 2011-03-31 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| US8431245B2 (en) | 2009-09-29 | 2013-04-30 | E. I. Du Pont De Nemours And Company | Deuterated compounds for luminescent applications |
| US9496506B2 (en) | 2009-10-29 | 2016-11-15 | E I Du Pont De Nemours And Company | Deuterated compounds for electronic applications |
| WO2011054442A2 (de) | 2009-11-06 | 2011-05-12 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| DE102009053191A1 (de) | 2009-11-06 | 2011-05-12 | Merck Patent Gmbh | Materialien für elektronische Vorrichtungen |
| DE102009052428A1 (de) | 2009-11-10 | 2011-05-12 | Merck Patent Gmbh | Verbindung für elektronische Vorrichtungen |
| WO2011057701A1 (de) | 2009-11-10 | 2011-05-19 | Merck Patent Gmbh | Organische verbindungen für elektroluminiszenz vorrichtungen |
| US9923146B2 (en) | 2009-12-16 | 2018-03-20 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative and organic electroluminescent element using same |
| WO2011074253A1 (ja) | 2009-12-16 | 2011-06-23 | 出光興産株式会社 | 有機発光媒体 |
| US9331285B2 (en) | 2009-12-16 | 2016-05-03 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative and organic electroluminescent element using same |
| WO2011074254A1 (ja) | 2009-12-16 | 2011-06-23 | 出光興産株式会社 | 有機発光媒体 |
| US8617720B2 (en) | 2009-12-21 | 2013-12-31 | E I Du Pont De Nemours And Company | Electroactive composition and electronic device made with the composition |
| WO2011076380A1 (en) | 2009-12-23 | 2011-06-30 | Merck Patent Gmbh | Composition for the preparation of organic electronic (oe) devices |
| WO2011076325A1 (en) | 2009-12-23 | 2011-06-30 | Merck Patent Gmbh | Compositions comprising polymeric binders |
| EP2725632A1 (en) | 2009-12-23 | 2014-04-30 | Merck Patent GmbH | Use of compositions comprising polymeric inert binders for the fabricaiton of light-emitting diode |
| WO2011076324A1 (en) | 2009-12-23 | 2011-06-30 | Merck Patent Gmbh | Compositions comprising organic semiconducting compounds |
| WO2011088877A1 (de) | 2010-01-25 | 2011-07-28 | Merck Patent Gmbh | Verbindungen für elektronische vorrichtungen |
| DE102010005697A1 (de) | 2010-01-25 | 2011-07-28 | Merck Patent GmbH, 64293 | Verbindungen für elektronische Vorrichtungen |
| WO2011107186A2 (de) | 2010-03-02 | 2011-09-09 | Merck Patent Gmbh | Verbindungen für elektronische vorrichtungen |
| DE102010009903A1 (de) | 2010-03-02 | 2011-09-08 | Merck Patent Gmbh | Verbindungen für elektronische Vorrichtungen |
| DE102010013068A1 (de) | 2010-03-26 | 2011-09-29 | Merck Patent Gmbh | Verbindungen für elektronische Vorrichtungen |
| WO2011116869A1 (de) | 2010-03-26 | 2011-09-29 | Merck Patent Gmbh | Verbindungen für elektronische vorrichtungen |
| WO2011128035A1 (en) | 2010-04-12 | 2011-10-20 | Merck Patent Gmbh | Composition and method for preparation of organic electronic devices |
| WO2011128034A1 (en) | 2010-04-12 | 2011-10-20 | Merck Patent Gmbh | Composition having improved performance |
| WO2011147523A1 (en) | 2010-05-27 | 2011-12-01 | Merck Patent Gmbh | Formulation and method for preparation of organic electronic devices |
| DE102010024335A1 (de) | 2010-06-18 | 2011-12-22 | Merck Patent Gmbh | Verbindungen für elektronische Vorrichtungen |
| WO2011157346A1 (de) | 2010-06-18 | 2011-12-22 | Merck Patent Gmbh | Verbindungen für elektronische vorrichtungen |
| DE102010024542A1 (de) | 2010-06-22 | 2011-12-22 | Merck Patent Gmbh | Materialien für elektronische Vorrichtungen |
| WO2011160757A1 (de) | 2010-06-22 | 2011-12-29 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2012016630A1 (de) | 2010-08-05 | 2012-02-09 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| DE102010033548A1 (de) | 2010-08-05 | 2012-02-09 | Merck Patent Gmbh | Materialien für elektronische Vorrichtungen |
| WO2012045384A1 (de) | 2010-10-09 | 2012-04-12 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| DE102010048074A1 (de) | 2010-10-09 | 2012-04-12 | Merck Patent Gmbh | Materialien für elektronische Vorrichtungen |
| WO2012048780A1 (de) | 2010-10-15 | 2012-04-19 | Merck Patent Gmbh | Verbindungen für elektronische vorrichtungen |
| DE102010048607A1 (de) | 2010-10-15 | 2012-04-19 | Merck Patent Gmbh | Verbindungen für elektronische Vorrichtungen |
| DE102010054316A1 (de) | 2010-12-13 | 2012-06-14 | Merck Patent Gmbh | Substituierte Tetraarylbenzole |
| WO2012079678A1 (de) | 2010-12-13 | 2012-06-21 | Merck Patent Gmbh | Substituierte tetraarylbenzole |
| US9293716B2 (en) | 2010-12-20 | 2016-03-22 | Ei Du Pont De Nemours And Company | Compositions for electronic applications |
| WO2012095143A1 (de) | 2011-01-13 | 2012-07-19 | Merck Patent Gmbh | Verbindungen für organische elektrolumineszenzvorrichtungen |
| DE102011011104A1 (de) | 2011-02-12 | 2012-08-16 | Merck Patent Gmbh | Substituierte Dibenzonaphtacene |
| WO2012107163A1 (de) | 2011-02-12 | 2012-08-16 | Merck Patent Gmbh | Substituierte dibenzonaphtacene |
| DE102011011539A1 (de) | 2011-02-17 | 2012-08-23 | Merck Patent Gmbh | Verbindungen für elektronische Vorrichtungen |
| WO2012110182A1 (de) | 2011-02-17 | 2012-08-23 | Merck Patent Gmbh | Verbindungen für elektronische vorrichtungen |
| WO2012139692A1 (de) | 2011-04-13 | 2012-10-18 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2012139693A1 (de) | 2011-04-13 | 2012-10-18 | Merck Patent Gmbh | Verbindungen für elektronische vorrichtungen |
| WO2012144176A1 (ja) | 2011-04-18 | 2012-10-26 | 出光興産株式会社 | ピレン誘導体、有機発光媒体、及びこれらを含む有機エレクトロルミネッセンス素子 |
| US10964891B2 (en) | 2011-04-18 | 2021-03-30 | Idemitsu Kosan Co., Ltd. | Pyrene derivative, organic light-emitting medium, and organic electroluminescent element containing pyrene derivative or organic light- emitting medium |
| WO2012143079A1 (de) | 2011-04-18 | 2012-10-26 | Merck Patent Gmbh | Verbindungen für elektronische vorrichtungen |
| US10014476B2 (en) | 2011-04-18 | 2018-07-03 | Idemitsu Kosan Co., Ltd. | Pyrene derivative, organic light-emitting medium, and organic electroluminescent element containing pyrene derivative or organic light-emitting medium |
| WO2012149992A1 (de) | 2011-05-04 | 2012-11-08 | Merck Patent Gmbh | Vorrichtung zur aufbewahrung von frischwaren |
| US9056822B2 (en) | 2011-06-10 | 2015-06-16 | National University Corporation Nagoya University | Method for producing polycyclic aromatic compound substituted by aryl group |
| WO2012169635A1 (ja) * | 2011-06-10 | 2012-12-13 | 国立大学法人名古屋大学 | アリール基で置換された多環性芳香族化合物の製造方法 |
| US9640773B2 (en) | 2011-09-16 | 2017-05-02 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative and organic electroluminescence element using same |
| WO2013060418A1 (en) | 2011-10-27 | 2013-05-02 | Merck Patent Gmbh | Materials for electronic devices |
| WO2013072740A2 (ja) | 2011-11-15 | 2013-05-23 | ユーディーシー アイルランド リミテッド | 電荷輸送材料、有機電界発光素子及び該素子を用いたことを特徴とする発光装置、表示装置または照明装置 |
| DE102013200942B4 (de) | 2012-01-23 | 2023-07-27 | Udc Ireland Ltd. | Organisches Elektrolumineszenz-Element, Ladungstransportmaterial für organisches Elektrolumineszenz-Element, und Licht-emittierende Vorrichtung, Anzeigevorrichtung und Beleuchtungsvorrichtung, die jeweils das Element umfassen |
| DE102013200942A1 (de) | 2012-01-23 | 2013-07-25 | Udc Ireland Ltd. | Organisches Elektrolumineszenz-Element, Ladungstransportmaterial für organisches Elektrolumineszenz-Element, und Licht-emittierende Vorrichtung, Anzeigevorrichtung und Beleuchtungsvorrichtung, die jeweils das Element verwenden |
| WO2013120577A1 (en) | 2012-02-14 | 2013-08-22 | Merck Patent Gmbh | Spirobifluorene compounds for organic electroluminescent devices |
| EP3235892A1 (en) | 2012-02-14 | 2017-10-25 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| EP3101088A1 (en) | 2012-02-14 | 2016-12-07 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| JP2014082479A (ja) * | 2012-09-27 | 2014-05-08 | Jnc Corp | 有機電界発光素子 |
| EP3647393A1 (de) | 2013-07-30 | 2020-05-06 | Merck Patent GmbH | Materialien für elektronische vorrichtungen |
| EP3712229A1 (de) | 2013-07-30 | 2020-09-23 | Merck Patent GmbH | Materialien für elektronische vorrichtungen |
| WO2015064560A1 (ja) * | 2013-10-29 | 2015-05-07 | Jnc株式会社 | アントラセン系化合物、発光層用材料、これを用いた有機電界発光素子、表示装置および照明装置 |
| WO2015082037A1 (en) | 2013-12-06 | 2015-06-11 | Merck Patent Gmbh | Compositions containing a polymeric binder which comprises acrylic and/or methacrylic acid ester units |
| WO2015165563A1 (de) | 2014-04-30 | 2015-11-05 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| EP3533794A2 (de) | 2014-04-30 | 2019-09-04 | Merck Patent GmbH | Materialien für elektronische vorrichtungen |
| DE102014008722A1 (de) | 2014-06-18 | 2015-12-24 | Merck Patent Gmbh | Zusammensetzungen für elektronische Vorrichtungen |
| WO2015192941A1 (de) | 2014-06-18 | 2015-12-23 | Merck Patent Gmbh | Zusammensetzungen für elektronische vorrichtungen |
| DE102014008722B4 (de) | 2014-06-18 | 2024-08-22 | Merck Patent Gmbh | Zusammensetzungen für elektronische Vorrichtungen, Formulierung diese enthaltend, Verwendung der Zusammensetzung, Verwendung der Formulierung sowie organische elektronische Vorrichtung enthaltend die Zusammensetzung |
| US10435350B2 (en) | 2014-09-19 | 2019-10-08 | Idemitsu Kosan Co., Ltd. | Organic electroluminecence device |
| WO2016120007A1 (en) | 2015-01-30 | 2016-08-04 | Merck Patent Gmbh | Formulations with a low particle content |
| WO2017008883A1 (en) | 2015-07-15 | 2017-01-19 | Merck Patent Gmbh | Composition comprising organic semiconducting compounds |
| US11512039B2 (en) | 2016-11-23 | 2022-11-29 | Guangzhou Chinaray Optoelectronic Materials Ltd. | Aromatic amine derivatives, preparation methods therefor, and uses thereof |
| US11518723B2 (en) | 2016-11-23 | 2022-12-06 | Guangzhou Chinaray Optoelectronic Materials Ltd. | Fused ring compound, high polymer, mixture, composition and organic electronic component |
| WO2018189134A1 (de) | 2017-04-13 | 2018-10-18 | Merck Patent Gmbh | Zusammensetzung für organische elektronische vorrichtungen |
| US11778907B2 (en) | 2017-04-13 | 2023-10-03 | Merck Patent Gmbh | Composition for organic electronic devices |
| WO2019007823A1 (en) | 2017-07-03 | 2019-01-10 | Merck Patent Gmbh | LOW-PHENOL IMPURITY FORMULATIONS |
| WO2020039708A1 (ja) | 2018-08-23 | 2020-02-27 | 国立大学法人九州大学 | 有機エレクトロルミネッセンス素子 |
| US11094886B2 (en) | 2019-09-13 | 2021-08-17 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent element and electronic device |
| KR20220066099A (ko) | 2019-09-13 | 2022-05-23 | 이데미쓰 고산 가부시키가이샤 | 유기 일렉트로루미네센스 소자 및 전자 기기 |
| KR20250172907A (ko) | 2019-09-13 | 2025-12-09 | 이데미쓰 고산 가부시키가이샤 | 유기 일렉트로루미네센스 소자 및 전자 기기 |
| KR20250171429A (ko) | 2019-09-13 | 2025-12-08 | 이데미쓰 고산 가부시키가이샤 | 유기 일렉트로루미네센스 소자 및 전자 기기 |
| KR20220063233A (ko) | 2019-09-13 | 2022-05-17 | 이데미쓰 고산 가부시키가이샤 | 유기 일렉트로루미네센스 소자 및 전자 기기 |
| KR20220062618A (ko) | 2019-09-13 | 2022-05-17 | 이데미쓰 고산 가부시키가이샤 | 유기 일렉트로루미네센스 소자 및 전자 기기 |
| US11839148B2 (en) | 2019-09-13 | 2023-12-05 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent element and electronic device |
| KR20220069030A (ko) | 2019-09-13 | 2022-05-26 | 이데미쓰 고산 가부시키가이샤 | 유기 일렉트로루미네센스 소자 및 전자 기기 |
| KR20220069028A (ko) | 2019-09-13 | 2022-05-26 | 이데미쓰 고산 가부시키가이샤 | 유기 일렉트로루미네센스 소자 및 전자 기기 |
| US11723266B2 (en) | 2019-09-13 | 2023-08-08 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent element and electronic device |
| KR20220104237A (ko) | 2019-11-26 | 2022-07-26 | 이데미쓰 고산 가부시키가이샤 | 화합물, 유기 일렉트로루미네센스 소자 및 전자 기기 |
| KR20220119596A (ko) | 2019-12-27 | 2022-08-30 | 이데미쓰 고산 가부시키가이샤 | 유기 일렉트로루미네센스 소자 및 전자 기기 |
| US11552259B1 (en) | 2020-02-14 | 2023-01-10 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent element and electronic device |
| KR20220141780A (ko) | 2020-02-14 | 2022-10-20 | 이데미쓰 고산 가부시키가이샤 | 유기 일렉트로루미네센스 소자 및 전자 기기 |
| CN114716295A (zh) * | 2021-01-04 | 2022-07-08 | 浙江光昊光电科技有限公司 | 一种稠环化合物及其在有机电子器件的应用 |
| WO2023063289A1 (ja) | 2021-10-14 | 2023-04-20 | 出光興産株式会社 | 有機固体アップコンバージョン材料 |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101325410B1 (ko) | 유기 전기 발광 소자용 발광 재료, 이를 이용한 유기 전기 발광 소자 및 유기 전기 발광 소자용 재료 | |
| KR100924462B1 (ko) | 유기 전기발광 소자 및 안트라센 유도체 | |
| KR101156241B1 (ko) | 비대칭 모노 안트라센 유도체, 유기 전기 발광 소자용 재료및 이를 이용한 유기 전기 발광 소자 | |
| JP4025136B2 (ja) | アントラセン誘導体、有機エレクトロルミネッセンス素子用発光材料及び有機エレクトロルミネッセンス素子 | |
| JP6318273B2 (ja) | 含窒素複素環誘導体及びそれを用いた有機エレクトロルミネッセンス素子 | |
| WO2003087023A1 (en) | Novel aromatic compound and organic electroluminescnet element containing the same | |
| KR20050025195A (ko) | 안트라센 유도체 및 이를 이용한 유기 전기발광 소자 | |
| KR20070029107A (ko) | 안트라센 유도체 및 그것을 이용한 유기 전기발광 소자 | |
| JPWO2006104044A1 (ja) | アントリルアリーレン誘導体、有機エレクトロルミネッセンス素子用材料、及びそれを用いた有機エレクトロルミネッセンス素子 | |
| JPWO2006085434A1 (ja) | ビスアントラセン誘導体及びそれを利用した有機エレクトロルミネッセンス素子 | |
| KR101296029B1 (ko) | 바이페닐 유도체, 유기 전기발광 소자용 재료, 및 그것을이용한 유기 전기발광 소자 | |
| WO2009084544A1 (ja) | 含窒素複素環誘導体及びそれを用いた有機エレクトロルミネッセンス素子 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): CN IN JP KR US |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LU MC NL PT RO SE SI SK TR |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2004530558 Country of ref document: JP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2003792695 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2006043858 Country of ref document: US Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 10524825 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 20038198886 Country of ref document: CN Ref document number: 228/CHENP/2005 Country of ref document: IN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020057003056 Country of ref document: KR |
|
| WWP | Wipo information: published in national office |
Ref document number: 1020057003056 Country of ref document: KR |
|
| WWP | Wipo information: published in national office |
Ref document number: 2003792695 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 10524825 Country of ref document: US |