WO2017126607A1 - 化学強化ガラスおよび化学強化用ガラス - Google Patents
化学強化ガラスおよび化学強化用ガラス Download PDFInfo
- Publication number
- WO2017126607A1 WO2017126607A1 PCT/JP2017/001755 JP2017001755W WO2017126607A1 WO 2017126607 A1 WO2017126607 A1 WO 2017126607A1 JP 2017001755 W JP2017001755 W JP 2017001755W WO 2017126607 A1 WO2017126607 A1 WO 2017126607A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- glass
- mpa
- chemically strengthened
- value
- less
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C3/00—Glass compositions
- C03C3/04—Glass compositions containing silica
- C03C3/076—Glass compositions containing silica with 40% to 90% silica, by weight
- C03C3/083—Glass compositions containing silica with 40% to 90% silica, by weight containing aluminium oxide or an iron compound
- C03C3/085—Glass compositions containing silica with 40% to 90% silica, by weight containing aluminium oxide or an iron compound containing an oxide of a divalent metal
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C21/00—Treatment of glass, not in the form of fibres or filaments, by diffusing ions or metals in the surface
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C21/00—Treatment of glass, not in the form of fibres or filaments, by diffusing ions or metals in the surface
- C03C21/001—Treatment of glass, not in the form of fibres or filaments, by diffusing ions or metals in the surface in liquid phase, e.g. molten salts, solutions
- C03C21/002—Treatment of glass, not in the form of fibres or filaments, by diffusing ions or metals in the surface in liquid phase, e.g. molten salts, solutions to perform ion-exchange between alkali ions
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C3/00—Glass compositions
- C03C3/04—Glass compositions containing silica
- C03C3/076—Glass compositions containing silica with 40% to 90% silica, by weight
- C03C3/083—Glass compositions containing silica with 40% to 90% silica, by weight containing aluminium oxide or an iron compound
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C3/00—Glass compositions
- C03C3/04—Glass compositions containing silica
- C03C3/076—Glass compositions containing silica with 40% to 90% silica, by weight
- C03C3/083—Glass compositions containing silica with 40% to 90% silica, by weight containing aluminium oxide or an iron compound
- C03C3/085—Glass compositions containing silica with 40% to 90% silica, by weight containing aluminium oxide or an iron compound containing an oxide of a divalent metal
- C03C3/087—Glass compositions containing silica with 40% to 90% silica, by weight containing aluminium oxide or an iron compound containing an oxide of a divalent metal containing calcium oxide, e.g. common sheet or container glass
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C3/00—Glass compositions
- C03C3/04—Glass compositions containing silica
- C03C3/076—Glass compositions containing silica with 40% to 90% silica, by weight
- C03C3/089—Glass compositions containing silica with 40% to 90% silica, by weight containing boron
- C03C3/091—Glass compositions containing silica with 40% to 90% silica, by weight containing boron containing aluminium
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C3/00—Glass compositions
- C03C3/04—Glass compositions containing silica
- C03C3/076—Glass compositions containing silica with 40% to 90% silica, by weight
- C03C3/089—Glass compositions containing silica with 40% to 90% silica, by weight containing boron
- C03C3/091—Glass compositions containing silica with 40% to 90% silica, by weight containing boron containing aluminium
- C03C3/093—Glass compositions containing silica with 40% to 90% silica, by weight containing boron containing aluminium containing zinc or zirconium
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C3/00—Glass compositions
- C03C3/04—Glass compositions containing silica
- C03C3/076—Glass compositions containing silica with 40% to 90% silica, by weight
- C03C3/097—Glass compositions containing silica with 40% to 90% silica, by weight containing phosphorus, niobium or tantalum
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C4/00—Compositions for glass with special properties
- C03C4/18—Compositions for glass with special properties for ion-sensitive glass
Definitions
- the present invention relates to chemically tempered glass.
- cover glasses made of chemically strengthened glass have been used in order to enhance the protection and aesthetics of display devices of mobile devices such as mobile phones, smartphones, personal digital assistants (PDAs), and tablet terminals.
- mobile devices such as mobile phones, smartphones, personal digital assistants (PDAs), and tablet terminals.
- PDAs personal digital assistants
- Patent Document 1 discloses a formula (10) indicating an allowable limit of internal tensile stress of tempered glass, and chemicals with less scattering of fragments even if the strength of chemically tempered glass is increased by adjusting CT ′ below. It was said that tempered glass was obtained.
- the internal tensile stress CT ′ described in Patent Document 1 is derived from the following formula (11) using measured values of CS and DOL ′.
- CT ′ ⁇ ⁇ 38.7 ⁇ ln (t) +48.2 (10)
- CS ⁇ DOL ′ (t ⁇ 2 ⁇ DOL ′) ⁇ CT ′ (11)
- DOL ′ corresponds to the depth of the ion exchange layer.
- Patent Document 1 sometimes lacks the strength of chemically strengthened glass. This is because the influence of the glass composition is not fully taken into account, the above formula for obtaining CT ′ assumes that the stress profile is linearly approximated, and the point at which the stress is zero is equal to the ion diffusion layer depth It is thought that the cause is.
- the present invention improves these problems and provides a chemically strengthened glass with higher strength.
- the first aspect of the present invention is a chemically strengthened glass having a surface compressive stress (CS) of 300 MPa or more, and a compressive stress value (CS 90 ) at a depth of 90 ⁇ m from the glass surface is 25 MPa or more, or glass.
- the compressive stress value (CS 100 ) at a depth of 100 ⁇ m from the surface is 15 MPa or more, Each of SiO 2 , Al 2 O 3 , B 2 O 3 , P 2 O 5 , Li 2 O, Na 2 O, K 2 O, MgO, CaO, SrO, BaO and ZrO 2 in the mother composition of the chemically strengthened glass.
- X SiO 2 ⁇ 329 + Al 2 O 3 ⁇ 786 + B 2 O 3 ⁇ 627 + P 2 O 5 ⁇ ( ⁇ 941) + Li 2 O ⁇ 927 + Na 2 O ⁇ 47.5 + K 2 O ⁇ ( ⁇ 371) + MgO ⁇ 1230 + CaO ⁇ 1154 + SrO ⁇ 733 + ZrO 2 ⁇ 51.8
- the first aspect of the present invention is a chemically strengthened glass having a surface compressive stress (CS) of 300 MPa or more, and a compressive stress value (CS 90 ) at a depth of 90 ⁇ m from the glass surface is 25 MPa or more, or glass.
- the compressive stress value (CS 100 ) at a depth of 100 ⁇ m from the surface is 15 MPa or more, Each of SiO 2 , Al 2 O 3 , B 2 O 3 , P 2 O 5 , Li 2 O, Na 2 O, K 2 O, MgO, CaO, SrO, BaO and ZrO 2 in the mother composition of the chemically strengthened glass.
- Chemically tempered glass having a Z value of 20000 or more calculated based on the following formula using the content of the component in terms of mole percentage based on the oxide may be used.
- Z SiO 2 ⁇ 237 + Al 2 O 3 ⁇ 524 + B 2 O 3 ⁇ 228 + P 2 O 5 ⁇ ( ⁇ 756) + Li 2 O ⁇ 538 + Na 2 O ⁇ 44.2 + K 2 O ⁇ ( ⁇ 387) + MgO ⁇ 660 + CaO ⁇ 569 + SrO ⁇ 291 + ZrO 2 ⁇ 510
- the chemically tempered glass of the first aspect is preferably a plate having a thickness t of 2 mm or less.
- the second aspect of the present invention is a chemically strengthened glass having a surface compressive stress (CS) of 300 MPa or more and satisfying the following formulas (1) and (2).
- StL (t) ⁇ a ⁇ t + 7000 (unit: MPa ⁇ ⁇ m) (1) a ⁇ 30000 (unit: MPa ⁇ ⁇ m / mm) (2) (Here, t is the plate thickness (mm), and StL (t) is the value of St Limit when the plate thickness is t.)
- the chemically tempered glass of the second aspect preferably satisfies a ⁇ 35000.
- the second aspect may be a chemically strengthened glass having a surface compressive stress (CS) of 300 MPa or more and satisfying the following formulas (3), (4), and (5).
- CS surface compressive stress
- the chemically tempered glass of the second aspect is preferably a plate having a thickness t of 2 mm or less.
- the compressive stress value (CS 90 ) at a depth of 90 ⁇ m from the glass surface is 25 MPa or more, or the compressive stress value (CS 100 ) at a depth of 100 ⁇ m from the glass surface. Is preferably 15 MPa or more.
- an average crack height by a sand drop test described later is 250 mm or more, a number of crushing by an indenter press-in test described later is 30 or less, and a sheet thickness t is 0.4-2 mm.
- a chemically strengthened glass having a surface compressive stress (CS) of 300 MPa or more and a depth (DOL) of the compressive stress layer of 100 ⁇ m or more.
- the product (CS 100 ⁇ t 2 ) of the compressive stress value at a depth of 100 ⁇ m from the glass surface and the square of the plate thickness t (mm) (CS 100 ⁇ t 2 ) is 5 MPa ⁇ mm 2 or more. preferable.
- the area Sc (MPa ⁇ ⁇ m) of the compressive stress layer is preferably 30000 MPa ⁇ ⁇ m or more.
- Chemically tempered glass of the present invention is preferably a depth d h in areas of one-half the size of the surface compressive stress of the internal compressive stress (CS) is 8 ⁇ m or more.
- Chemically tempered glass of the present invention it is preferable to position d M compressive stress is maximum in the range of 5 ⁇ m from the glass surface.
- the depth (DOL) of the compressive stress layer is preferably 110 ⁇ m or more.
- ⁇ CS DOL-20 (unit: MPa / ⁇ m) calculated by the following formula using the compressive stress value CS DOL-20 at a depth of 20 ⁇ m on the glass surface side from DOL is 0. .4 or more is preferable.
- ⁇ CS DOL-20 CS DOL-20 / 20
- ⁇ CS 100-90 (CS 90 -CS 100 ) / (100-90)
- the fracture toughness value (K1c) of the glass having the matrix composition of the chemically strengthened glass is 0.7 MPa ⁇ m 1/2 or more.
- the area St (MPa ⁇ ⁇ m) of the internal tensile layer is preferably StL (t) (MPa ⁇ ⁇ m) or less.
- t is the plate thickness (mm)
- StL (t) is the value of St Limit when the plate thickness is t.
- the internal tensile layer stress CT (MPa) is preferably CTL (t) (MPa) or less.
- t is the plate thickness (mm)
- CTL (t) is the value of CT Limit at the plate thickness t.
- the matrix composition of the chemically tempered glass is expressed in terms of mole percentage on the basis of oxide, and SiO 2 is 50 to 80%, Al 2 O 3 is 1 to 30%, and B 2 O 3 is 0. ⁇ 6%, P 2 O 5 0 ⁇ 6%, Li 2 O 0 ⁇ 20%, Na 2 O 0 ⁇ 8%, K 2 O 0 ⁇ 10%, MgO 0 ⁇ 20%, CaO It is preferable to contain 0-20%, SrO 0-20%, BaO 0-15%, ZnO 0-10%, TiO 2 0-5%, and ZrO 2 0-8%.
- the present invention is expressed in terms of mole percentage based on oxides, and SiO 2 is 63 to 80%, Al 2 O 3 is 7 to 30%, B 2 O 3 is 0 to 5%, and P 2 O 5 is 0 to 4%, Li 2 O 5-15%, Na 2 O 4-8%, K 2 O 0-2%, MgO 3-10%, CaO 0-5%, SrO 0-20% the BaO 0 ⁇ 15% of ZnO 0 ⁇ 10% of TiO 2 0 ⁇ 1% of ZrO 2 containing 0-8% Does not contain Ta 2 O 5 , Gd 2 O 3 , As 2 O 3 , Sb 2 O 3 , Mole percentages based on oxides of each component of SiO 2 , Al 2 O 3 , B 2 O 3 , P 2 O 5 , Li 2 O, Na 2 O, K 2 O, MgO, CaO, SrO, BaO and ZrO 2.
- X SiO 2 ⁇ 329 + Al 2 O 3 ⁇ 786 + B 2 O 3 ⁇ 627 + P 2 O 5 ⁇ ( ⁇ 941) + Li 2 O ⁇ 927 + Na 2 O ⁇ 47.5 + K 2 O ⁇ ( ⁇ 371) + MgO ⁇ 1230 + CaO ⁇ 1154 + SrO ⁇ 733 + ZrO 2 ⁇ 51.8
- the content of ZrO 2 in terms of oxide-based mole percentage is 1.2% or less. Further, it is preferable that the K 2 O content by mole percentage based on oxides is 0.5% or more. Further, it is preferable content of B 2 O 3 by mole percentage based on oxides is 1% or less. Further, it is preferable that the content of Al 2 O 3 by mole percentage based on oxides is not more than 11%. Further, the devitrification temperature T is preferably equal to or lower than the temperature T4 at which the viscosity becomes 10 4 dPa ⁇ s.
- the present invention provides a high-strength chemically strengthened glass in which scattering of fragments due to breakage is suppressed.
- FIG. 1 is a conceptual diagram showing a stress profile of chemically strengthened glass
- (a) is a view showing an example of a stress profile of chemically strengthened glass
- (b) is an enlarged left half of the stress profile of (a).
- It is a figure and (c) is a figure which shows the depth of the position where the compressive stress in each of profile A and B becomes the maximum.
- FIG. 2 is a schematic diagram showing how a sample for measuring the surface compressive stress (CS) of chemically strengthened glass is produced, (a) shows a sample before polishing, and (b) shows a thin piece after polishing. The sample is shown.
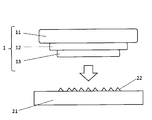
- FIG. 3 is a schematic diagram showing a test method for a drop-on-sand test.
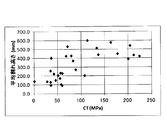
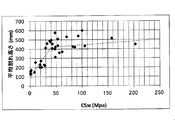
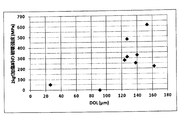
- FIG. 4 is a graph plotting the relationship between DOL and average crack height of chemically strengthened glass or glass.
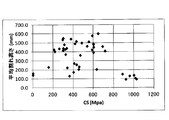
- FIG. 5 is a graph plotting the relationship between CT and average crack height of chemically strengthened glass or glass.
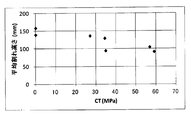
- FIG. 6 is a graph plotting the relationship between CT and average crack height of chemically strengthened glass.
- FIG. 7 is a graph plotting the relationship between the surface compressive stress value CS and the average crack height of chemically strengthened glass or glass.
- FIG. 8 is a graph plotting the relationship between the compressive stress value CS 90 and the average crack height of chemically strengthened glass or glass.
- FIG. 9 is a graph plotting the relationship between the compressive stress value CS 100 and the average crack height of chemically strengthened glass or glass.
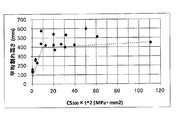
- FIG. 10 is a graph plotting the relationship between the product of the compressive stress value CS 100 and the square of the sheet thickness t (CS 100 ⁇ t 2 ) and the average crack height of chemically strengthened glass or glass.
- FIG. 11 is a graph showing test results of a four-point bending test for chemically strengthened glass.
- FIG. 12 is a graph plotting the relationship between CS and bending strength for chemically strengthened glass.
- FIG. 13 is a graph plotting the relationship between DOL and bending strength for chemically strengthened glass.
- FIG. 14 is a graph showing a stress profile of virtual chemically strengthened glass.
- FIG. 15 shows measurement examples of St Limit and CT Limit
- (a) is a graph showing the relationship between the area St of the internal tensile stress layer and the number of fractures, and (b) is surrounded by a dotted line in (a).
- (C) is a graph showing the relationship between the internal tensile stress CT and the number of fractures, and (d) is an enlarged view of a portion surrounded by a dotted line in (c).
- FIG. 16 is an explanatory diagram of a sample used for fracture toughness measurement by the DCDC method.
- FIG. 17 is a diagram showing a K1-v curve showing the relationship between the stress intensity factor K1 and the crack growth rate v used for the measurement of fracture toughness value by the DCDC method.
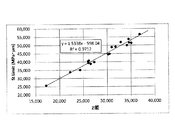
- FIG. 18 is a graph plotting the relationship between St Limit and X value for chemically strengthened glass.
- FIG. 19 is a graph plotting the relationship between St Limit and Z value for chemically strengthened glass.
- FIG. 20 is a graph plotting the relationship between St Limit and Young's modulus for chemically strengthened glass.
- FIG. 21 is a graph plotting the relationship between the X value and the Z value for chemically strengthened glass.
- FIG. 22 is a graph obtained by plotting ST Limit of chemically strengthened glass against the plate thickness t.
- FIG. 23 is a graph in which the CT limit of chemically strengthened glass is plotted against the thickness t.
- the surface compressive stress (CS) is 300 MPa or more, and the compressive stress value (CS 90 ) of a portion having a depth of 90 ⁇ m from the glass surface is 25 MPa or more, or a depth of 100 ⁇ m from the glass surface.
- This is a chemically strengthened glass having a partial compressive stress value (CS 100 ) of 15 MPa or more.
- the chemically strengthened glass of the first aspect has a compressive stress layer formed on the surface by chemical strengthening treatment (ion exchange treatment).
- the surface of the glass is ion-exchanged to form a surface layer in which compressive stress remains.
- an alkali metal ion typically Li ion or Na ion
- an alkali ion having a larger ionic radius Typically, Na ions or K ions are substituted for Li ions, and K ions are substituted for Na ions.
- the surface compressive stress (CS) of the chemically strengthened glass is 300 MPa or more.
- CS surface compressive stress
- the CS of the chemically strengthened glass is preferably 350 MPa or more, more preferably 400 MPa or more, and further preferably 450 MPa or more.
- the upper limit of CS of chemically tempered glass is not particularly limited, but if CS is too large, there will be a greater risk of splattering if it breaks. From this viewpoint, it is, for example, 2000 MPa or less, preferably 1500 MPa or less, more preferably 1000 MPa or less, and further preferably 800 MPa or less.
- CS of chemically strengthened glass can be appropriately adjusted by adjusting chemical strengthening conditions, glass composition, and the like.
- CS of chemically tempered glass of the first aspect the value CS F and CS A by the following two kinds of measurement methods are defined as follows. The same applies to the compressive stress value (CS x ) at the depth of x ⁇ m from the glass surface.
- CS F is a value determined by the accessory program FsmV of measured surface stress meter with a surface stress meter FSM-6000 of Orihara Seisakusho.
- CS A is a value measured by the following procedure using a birefringence imaging system Abrio-IM manufactured by Tokyo Instruments Inc.
- a cross section of chemically tempered glass having a size of 10 mm ⁇ 10 mm or more and a thickness of about 0.2 to 2 mm is polished in a range of 150 to 250 ⁇ m and thinned.
- grinding to a target thickness of about 50 ⁇ m with a # 1000 diamond electrodeposition grindstone then grinding to a target thickness of about 10 ⁇ m using a # 2000 diamond electrodeposition grindstone, and finally mirroring with cerium oxide The target thickness.
- F ⁇ / (C ⁇ t ′)
- Formula (A) Wherein (A), F represents a stress (MPa), [delta] is a phase difference (retardation) (nm), C is a photoelastic constant (nm cm -1 MPa), t ' is the sample thickness (cm).
- the inventors of the present invention are excellent in chemically strengthened glass (hereinafter also referred to as high-DOL glass) having a DOL of a predetermined value or more and a compressive stress value at a predetermined depth inside the compressive stress layer of a predetermined value or more. It has been found that it has resistance to falling on sand. Moreover, it has been found that such high DOL glass has high resistance to falling on sand even when CT is relatively large. From the above viewpoint, in the first aspect, the compressive stress value (CS 90 ) of the portion of the chemically strengthened glass having a depth of 90 ⁇ m from the glass surface is preferably 25 MPa or more, and more preferably 30 MPa or more. preferable.
- the compression stress value of the depth of the portion of 100 ⁇ m from the glass surface (CS 100) is not less than 15 MPa, and more preferably not less than 20 MPa.
- the product CS 100 ⁇ t 2 of the compressive stress value at a depth of 100 ⁇ m from the glass surface and the square of the plate thickness t (mm) is 5 MPa ⁇ mm 2 or more. Preferably there is.
- CS 90 When CS 90 is 25 MPa or more, it can have sufficient resistance against breakage caused by scratches caused by collision with sharp corners such as sand that can collide with chemically strengthened glass in a practical situation, that is, Excellent resistance to falling on sand. Further, the present inventors have found that a chemically strengthened glass having a CS 90 of 25 MPa or more can provide a chemically strengthened glass having a high resistance to dropping on sand even if CT is relatively large.
- CS 90 is more preferably 30 MPa or more, further preferably 35 MPa or more, still more preferably 40 MPa or more, particularly preferably 45 MPa or more, and most preferably 50 MPa or more.
- the upper limit of CS 90 is not particularly limited, but from the viewpoint of safety at the time of destruction, it is, for example, 250 MPa or less, preferably 200 MPa or less, more preferably 150 MPa or less, particularly preferably. 100 MPa or less, and most preferably 75 MPa or less.
- CS 100 is more preferably 20 MPa or more, further preferably 23 MPa or more, still more preferably 26 MPa or more, particularly preferably 30 MPa or more, and most preferably 33 MPa or more.
- the upper limit of CS 100 is not particularly limited, it is, for example, 200 MPa or less, preferably 150 MPa or less, more preferably 100 MPa or less, and particularly preferably 75 MPa or less, from the viewpoint of safety at breakage. Most preferably, it is 50 MPa or less.
- CS 100 ⁇ t 2 is preferably 5 MPa ⁇ mm 2 or more, more preferably 7 MPa ⁇ mm 2 or more, further preferably 10 MPa ⁇ mm 2 or more, and particularly preferably 15 MPa ⁇ mm 2 or more. Most preferably, it is 20 MPa ⁇ mm 2 or more.
- CS 100 ⁇ t 2 is particularly limited, from the viewpoint of safety at break, for example at 120 MPa ⁇ mm 2 or less, preferably not more 100 MPa ⁇ mm 2 or less, more preferably 80MPa ⁇ Mm 2 or less, particularly preferably 60 MPa ⁇ mm 2 or less, and most preferably 40 MPa ⁇ mm 2 or less.
- the depth d h (see FIG. 1B) where the internal compressive stress is half the surface compressive stress (CS) is 8 ⁇ m or more. Preferably there is.
- d h is at 8 ⁇ m or more, resistance is improved with respect to strength reduction of the flexural strength at the scratching.
- d h is preferably 8 ⁇ m or more, more preferably 10 ⁇ m or more, further preferably 12 ⁇ m or more, and particularly preferably 15 ⁇ m or more.
- d h it is not particularly restricted upper limit of d h, from the viewpoint of safety at break is, for example, 70 ⁇ m or less, preferably 60 ⁇ m or less, more preferably 50 ⁇ m or less, more preferably 40 ⁇ m or less And particularly preferably 30 ⁇ m or less.
- the depth d M (see FIG. 1 (c)) where the compressive stress is maximized is in the range of 10 ⁇ m or less from the glass surface. If d M is located deeper than 10 ⁇ m from the glass surface, the effect of the bending strength improvement by the chemical strengthening treatment is not sufficiently obtained, which may lead to a bending strength decreases.
- d M is preferably 10 ⁇ m or less, more preferably 8 ⁇ m or less, more preferably 5 ⁇ m or less.
- the DOL is preferably 100 ⁇ m or more.
- the DOL is 100 ⁇ m or more, it can have sufficient resistance against breakage caused by scratches caused by collision with a sharp object such as sand that can collide with chemically strengthened glass in a practical scene.
- the DOL is more preferably 110 ⁇ m or more, further preferably 120 ⁇ m or more, and particularly preferably 130 ⁇ m or more.
- the upper limit of DOL is not particularly limited, but from the viewpoint of safety at the time of destruction, it is, for example, 200 ⁇ m or less, preferably 180 ⁇ m or less, more preferably 160 ⁇ m or less, and particularly preferably 150 ⁇ m. It is as follows.
- the DOL can be adjusted as appropriate by adjusting chemical strengthening conditions, glass composition, and the like.
- ⁇ CS DOL-20 (unit: MPa / ⁇ m) calculated by the following formula using the compressive stress value CS DOL-20 at a depth of 20 ⁇ m from the DOL on the glass surface side is 0.4.
- the above is preferable.
- ⁇ CS DOL-20 CS DOL-20 / 20
- ⁇ CS DOL-20 is more preferably 0.5 or more, 0.6 or more, 0.7 or more, 0.8 or more, 0.9 or more, 1.0 or more, 1.2 or more step by step. 1.4 or more, 1.5 or more.
- the upper limit of ⁇ CS DOL-20 is not particularly limited, but from the viewpoint of crushing safety, it is, for example, 4.0 or less, preferably 3.0 or less, more preferably 2.0 or less, More preferably, it is 1.7 or less, typically 1.6 or less.
- ⁇ CS 100-90 (unit: MPa / ⁇ m) calculated by the following formula using CS 90 and CS 100 is preferably 0.4 or more.
- ⁇ CS 100-90 (CS 90 -CS 100 ) / (100-90)
- DerutaCS 100-90 is more preferably less stepwise, of 0.5 or more, 0.6 or more, 0.7 or more, 0.8 or more, 0.9 or more, 1.0 or more, 1.2 or more 1.4 or more, 1.5 or more.
- the upper limit of ⁇ CS 100-90 is not particularly limited, but from the viewpoint of crushing safety, it is, for example, 4.0 or less, preferably 3.0 or less, more preferably 2.0 or less, More preferably, it is 1.7 or less, typically 1.6 or less.
- the DOL of the chemically strengthened glass in the first embodiment is the depth from the glass surface where the stress becomes zero in the stress profile, and is measured by a surface stress meter FSM-6000 manufactured by Orihara Seisakusho Co., Ltd. Is the value analyzed by.
- the measurement can be performed using a thinned sample as shown in FIG. 2B using a birefringence imaging system Abrio-IM manufactured by Tokyo Instruments Inc.
- the area Sc (MPa ⁇ ⁇ m) of the compressive stress layer is preferably 30000 MPa ⁇ ⁇ m or more.
- the area Sc (MPa ⁇ ⁇ m) of the compressive stress layer is 30000 MPa ⁇ ⁇ m or more, by introducing larger CS and DOL, sharp objects such as sand that can collide with chemically strengthened glass in practical situations It is possible to obtain chemically strengthened glass having sufficient resistance against breakage caused by scratches caused by collision with the glass.
- Sc is more preferably 32000 MPa ⁇ ⁇ m or more, and in the following, stepwise is 34000 MPa ⁇ ⁇ m or more, 36000 MPa ⁇ ⁇ m or more, 38000 MPa ⁇ ⁇ m or more, 40000 MPa ⁇ ⁇ m or more, 42000 MPa ⁇ ⁇ m or more, 44000 MPa ⁇ ⁇ m or more, 44000 MPa ⁇ ⁇ m or more. Further preferred.
- Sc of chemically tempered glass of the first aspect is the value Sc F and Sc A by the following two kinds of measurement methods are defined as follows.
- Sc F is a value calculated using a value measured by a surface stress meter FSM-6000 manufactured by Orihara Seisakusho Co., Ltd. and analyzed by the attached program FsmV
- Sc A is a method similar to the CS A measurement described above. It is a value obtained by measurement using a certain birefringence imaging system Abrio-IM and a thinned sample.
- the area St (MPa ⁇ ⁇ m) of the internal tensile layer of the chemically strengthened glass in the first aspect is defined as follows by values St F and St A obtained by the following two types of measurement methods.
- St F is a value calculated using a value measured by a surface stress meter FSM-6000 manufactured by Orihara Seisakusho Co., Ltd. and analyzed by an attached program FsmV
- St A is a method similar to the above-mentioned CS A measurement. It is a value obtained by measurement using a certain birefringence imaging system Abrio-IM and a thinned sample.
- a stress profile is created by two methods, St F or St A is calculated, and St can be obtained.
- Fig. 1 (a) shows a conceptual diagram of Sc and St. Sc and St are theoretically equal values, and it is preferable to calculate such that 0.95 ⁇ Sc / St ⁇ 1.05.
- the mother composition of chemically strengthened glass is the composition of glass before chemical strengthening (hereinafter also referred to as chemically strengthened glass).
- the part (henceforth a tensile-stress part) which has the tensile stress of chemically strengthened glass is a part which is not ion-exchanged.
- the tensile-stress part of chemically strengthened glass has the same composition as the glass before chemical strengthening.
- the composition of the tensile stress portion can be regarded as the matrix composition.
- the preferable aspect of the mother composition of chemically strengthened glass is mentioned later.
- the inventors of the present invention have a good correlation between the X value and the Z value calculated based on the above formulas and the number of fragments (the number of fractures) generated at the time of breaking (crushing) the chemically strengthened glass, and the larger the X value and the Z value are. It was experimentally found that the number of pieces to be crushed at the time of breaking the glass tends to be reduced.
- the X value is preferably 30000 MPa ⁇ ⁇ m or more, and stepwise below. 32,000 MPa ⁇ ⁇ m or more, 34000 MPa ⁇ ⁇ m or more, 36000 MPa ⁇ ⁇ m or more, 38000 MPa ⁇ ⁇ m or more, 40000 MPa ⁇ ⁇ m or more, 42000 MPa ⁇ ⁇ m or more, 44000 MPa ⁇ ⁇ m or more, 45000 MPa ⁇ ⁇ m or more, 46000 MPa ⁇ ⁇ m or more preferable.
- the Z value is preferably 20000 MPa ⁇ ⁇ m or more.
- stepwise 22000 MPa ⁇ ⁇ m or more, 24000 MPa ⁇ ⁇ m or more, 26000 MPa ⁇ ⁇ m or more, 28000 MPa ⁇ ⁇ m or more, 29000 MPa ⁇ ⁇ m or more.
- 30000 MPa ⁇ ⁇ m or more is more preferable.
- X value and Z value can be adjusted by the amount of each component in the mother composition of chemically strengthened glass.
- the matrix composition of the chemically strengthened glass is not particularly limited, but the chemical strengthening treatment that gives the above-described chemical strengthening characteristics to the glass after chemical strengthening is applicable, and the value of X May be selected as appropriate so that the glass composition is 30000 or more and / or the value of Z is 20000 or more.
- the Y value calculated based on the following formula correlates with the number of fragments (the number of fractures) generated when the chemically strengthened glass is broken (crushed), and the larger the Y value, the smaller the number of fractures when the glass is broken.
- the Y value is 0.7 or more in the chemically tempered glass of the first aspect from the viewpoint of reducing the number of crushed and higher safety even when the glass breaks. Is preferably 0.75 or more, more preferably 0.77 or more, particularly preferably 0.80 or more, and most preferably 0.82 or more.
- the devitrification temperature T is preferably a temperature T4 or less at which the viscosity becomes 10 4 dPa ⁇ s. This is because when the devitrification temperature T is higher than T4, quality deterioration due to devitrification is likely to occur during glass plate forming by the float method or the like.
- the chemically strengthened glass of the first aspect is plate-shaped (glass plate)
- its thickness (t) is not particularly limited, but in order to increase the effect of chemical strengthening, for example, 2 mm Or less, preferably 1.5 mm or less, more preferably 1 mm or less, further preferably 0.9 mm or less, particularly preferably 0.8 mm or less, and most preferably 0.7 mm or less.
- the plate thickness is, for example, 0.1 mm or more, preferably 0.2 mm or more, more preferably 0.4 mm or more, from the viewpoint of obtaining a sufficient strength improvement effect by the chemical strengthening treatment. More preferably, it is 0.5 mm or more.
- the chemically strengthened glass of the first aspect may have a shape other than a plate shape depending on a product to be used, an application, and the like.
- the glass plate may have an edge shape etc. from which the outer periphery thickness differs.
- the said glass plate has two main surfaces and the end surface which forms plate thickness adjacent to these, and the two main surfaces may form the flat surface mutually parallel.
- the form of the glass plate is not limited to this.
- the two main surfaces may not be parallel to each other, and all or a part of one or both of the two main surfaces may be a curved surface.
- the glass plate may be, for example, a flat glass plate without warpage or a curved glass plate having a curved surface.
- security is obtained.
- a mobile device such as a smartphone
- a collision object having a collision part with a small angle hereinafter also referred to as an acute angle object
- sand a collision object having a collision part with a small angle
- sand a collision object having a collision part with a small angle
- sand a collision object having a collision part with a small angle
- sand such as sand
- the chemically strengthened glass according to the first embodiment is also excellent in resistance to breakage (drop resistance on sand) caused by scratches caused by collision with sharp objects such as sand that can collide in a practical scene.
- ⁇ Second aspect> It continues and demonstrates the chemically strengthened glass which concerns on a 2nd aspect.
- One of the chemically strengthened glasses of the second aspect is a chemically strengthened glass having a surface compressive stress (CS) of 300 MPa or more and satisfying the following formulas (1) and (2).
- StL (t) is a value obtained by the following measurement.
- a glass of 25 mm ⁇ 25 mm ⁇ plate thickness t (mm) is subjected to a chemical strengthening treatment under various chemical strengthening treatment conditions so that the internal tensile stress area (St; unit MPa ⁇ ⁇ m) is changed.
- a chemically strengthened glass having a tensile stress area (St; unit MPa ⁇ ⁇ m) is produced.
- each of these chemically strengthened glasses was broken by an indenter press-in test in which a load of 3 to 10 kgf was held for 15 seconds, and the pieces of chemically strengthened glass after breakage were broken.
- the number (number of crushing) of each is measured.
- Stn value which is the St value of the maximum number of fractures n of less than 10 and the Stm value of the minimum number of fractures m of more than 10
- StL (t) value is defined by the equation.
- StL (t) value Stn + (10 ⁇ n) ⁇ (Stm ⁇ Stn) / (mn)
- a region of 25 mm ⁇ 25 mm is displayed in the chemically strengthened glass, and the above StL (t) measurement is performed in the region.
- StL (t) depends on the plate thickness t (mm) and a, and a is a parameter depending on the glass composition. StL (t) changes linearly with respect to t, and its slope can be described by a parameter a that changes with composition. In addition, by setting the value of a to 30000 MPa ⁇ ⁇ m / mm or more, even when larger CS and DOL are introduced, it is possible to obtain a highly safe crushing mode with less crushing number.
- the value of a is more preferably 32000 MPa ⁇ ⁇ m / mm or more, and in the following steps, 34000 MPa ⁇ ⁇ m / mm or more, 36000 MPa ⁇ ⁇ m / mm or more, 38000 MPa ⁇ ⁇ m / mm or more, 40000 MPa ⁇ ⁇ m / mm or more. It is more preferably 42,000 MPa ⁇ ⁇ m / mm or more, 44000 MPa ⁇ ⁇ m / mm or more, 46000 MPa ⁇ ⁇ m / mm or more, 48000 MPa ⁇ ⁇ m / mm or more, 50000 MPa ⁇ ⁇ m / mm or more.
- the value of a is preferably 53000 MPa ⁇ ⁇ m / mm or less.
- One of the chemically strengthened glasses of the second aspect is a chemically strengthened glass having a surface compressive stress (CS) of 300 MPa or more and satisfying the following formulas (3), (4) and (5).
- CS surface compressive stress
- CTL (t) is a value obtained by the following measurement. Specifically, a glass of 25 mm ⁇ 25 mm ⁇ sheet thickness t (mm) is subjected to chemical strengthening treatment under various chemical strengthening treatment conditions so that the internal tensile stress CT (unit: MPa) changes, A chemically tempered glass having an internal tensile stress CT (unit: MPa) of 1 is prepared. Then, using a diamond indenter having a facing indenter angle of 60 degrees, each of these chemically strengthened glasses was broken by an indenter press-in test in which a load of 3 to 10 kgf was held for 15 seconds, and the pieces of chemically strengthened glass after breakage were broken. The number (number of crushing) of each is measured.
- CT Limit value CTL (t) when the plate thickness is t (mm).
- CTn value that is the CT value of the maximum number of fractures n that is less than 10
- CTm value that is the CT value of the minimum number of fractures m that is greater than 10
- CTL (t) value CTn + (10 ⁇ n) ⁇ (CTm ⁇ CTn) / (mn)
- CTL (t) value CTn + (10 ⁇ n) ⁇ (CTm ⁇ CTn) / (mn)
- CTL (t) depends on the plate thickness t (mm), b, and c, and b and c are parameters that depend on the glass composition. CTL (t) decreases with increasing t and can be described using natural logarithm as shown in Equation (3). According to this embodiment, by setting the values of b and c to 14 MPa or more and 48.4 MPa or more, respectively, even when a larger CS and DOL are introduced than before, the number of crushing is less and the crushing mode is high in safety. It can be.
- the value of b is more preferably 14 MPa or more.
- stepwise 15 MPa or more, 16 MPa or more, 17 MPa or more, 18 MPa or more, 19 MPa or more, 20 MPa or more, 21 MPa or more, 22 MPa or more, 23 MPa or more, 24 MPa or more, 25 MPa.
- it is preferably 26 MPa or more, 27 MPa or more, 28 MPa or more, 29 MPa or more, or 30 MPa or more.
- the value of c is more preferably 48.4 MPa or more.
- stepwise 49 MPa or more, 50 MPa or more, 51 MPa or more, 52 MPa or more, 53 MPa or more, 54 MPa or more, 55 MPa or more, 56 MPa or more, 57 MPa or more, 58 MPa or more.
- CTL (t) is preferably smaller than ⁇ 35 ⁇ ln (t) +75.
- the St value and the CT value are the values St F and CT F measured by the surface stress meter FSM-6000 manufactured by Orihara Seisakusho and analyzed by the attached program FsmV, or the birefringence imaging system Abrio-IM and the thinned sample.
- CT F is equal to the analyzed values CT_CV at FsmV, is different from the CT 'obtained by the following equation (11).
- DOL ′ (t ⁇ 2 ⁇ DOL ′) ⁇ CT ′ (11)
- DOL ′ corresponds to the depth of the ion exchange layer.
- CT ′ approximates the stress profile linearly and assumes that the point at which the stress becomes zero is equal to the ion diffusion layer depth, so it is estimated to be larger than the actual internal tensile stress. This is not suitable as an index of internal tensile stress in this embodiment.
- the chemically strengthened glass of the second aspect has a compressive stress layer formed on the surface by chemical strengthening treatment (ion exchange treatment).
- the chemically strengthened glass of the second aspect has a surface compressive stress (CS) of 300 MPa or more.
- CS surface compressive stress
- the reason for limitation of CS and the preferable numerical range in the chemically strengthened glass of the second aspect are the same as those of the first aspect.
- CS 90, CS 100 and CS 100 ⁇ accompanying the preferable range and it t 2 in the chemical strengthened glass according to the second embodiment is the same as the first embodiment.
- the compressive stress value (CS 90 ) at a depth of 90 ⁇ m from the glass surface is 25 MPa or more, or the compressive stress value (CS 100 ) at a depth of 100 ⁇ m from the glass surface is 15 MPa or more, it is practical. It can have sufficient resistance against damage caused by scratches caused by collision with sand and other sharp objects that can collide with chemically strengthened glass in a typical situation, that is, chemical strengthening with excellent resistance to falling on sand Can with glass.
- preferable numerical ranges and technical effects associated therewith of d h and d M in the chemical strengthened glass according to the second embodiment is the same as the first embodiment.
- preferable numerical range of DOL in the chemically strengthened glass of the second aspect and the technical effect associated therewith are the same as those of the first aspect.
- preferred numerical ranges of Sc and St and the technical effects associated therewith in the chemically strengthened glass of the second aspect are the same as those of the first aspect.
- the chemically strengthened glass of the second aspect is a plate having a plate thickness t of 2 mm or less.
- the preferred numerical range of the plate thickness t and the technical effect associated therewith in the chemically strengthened glass of the second aspect are the same as in the first aspect.
- the chemically strengthened glass of the second aspect can take various shapes other than the plate shape, similarly to the chemically strengthened glass of the first aspect.
- the average crack height by the sand drop test under the following conditions is 250 mm or more, The number of crushing by indentation test under the following conditions is 30 or less, The plate thickness t is 0.4-2 mm, The surface compressive stress (CS) is 300 MPa or more, and The depth (DOL) of a compressive-stress layer is related with the chemically strengthened glass which is 100 micrometers or more.
- the average crack height of the chemically tempered glass in the third aspect by a drop-on-sand test is 250 mm or more, preferably 300 mm or more, more preferably 350 mm or more from the viewpoint of having excellent drop-to-sand resistance.
- the average crack height of the chemically strengthened glass in the third aspect is measured by a drop-on-sand test under the following conditions.
- Sand drop test conditions Chemically tempered glass (50 mm x 50 mm x plate thickness t (mm)) is bonded to a hard nylon mock board (50 mm x 50 mm, weight: 54 g) via a double-sided sponge tape (50 mm x 50 mm x thickness 3 mm) and measured. Prepare a sample. Next, on a SUS plate having a size of 15 cm ⁇ 15 cm, 1 g of silica sand (No. 5 silica sand made by Takefori Co., Ltd.) is evenly spread, and the prepared measurement sample is placed with chemically tempered glass facing down. It is dropped from a predetermined height (falling height) onto the surface of the SUS plate coated with silica sand.
- silica sand No. 5 silica sand made by Takefori Co., Ltd.
- the drop test is carried out by starting from a drop height of 10 mm and increasing the height by 10 mm.
- the height at which the chemically strengthened glass is broken is defined as the crack height (unit: mm).
- the drop test is carried out five times or more for each example, and the average value of the crack height in the drop test is defined as the average crack height (unit: mm).
- the number of crushing by the indentation test of the chemically strengthened glass in the third aspect is 30 or less, preferably 20 from the viewpoint that even if it breaks (crushing), it becomes safer (crushing). Or less, more preferably 10 or less, still more preferably 5 or less, and particularly preferably 2 or less.
- the number of fractures of the chemically strengthened glass in the third aspect is measured by an indenter press-in test under the following conditions.
- Indenter press-fit test conditions Using a diamond indenter with an indenter angle of 60 degrees facing the chemically tempered glass of 25 mm x 25 mm x plate thickness t (mm), a chemical strengthening is performed by an indentation press-in test that holds a load of 3 to 10 kgf for 15 seconds. The glass is broken, and the number of fractures of the chemically strengthened glass after the breakage is measured. When chemically tempered glass having a size larger than 25 mm ⁇ 25 mm is used, a 25 mm ⁇ 25 mm region is displayed in the chemically tempered glass, and an indenter press-in test and the number of fractures are measured in the region. When the chemically tempered glass has a curved surface shape, a projected area of 25 mm ⁇ 25 mm is displayed on the curved surface of the chemically tempered glass, and the indenter press-in test and the number of crushing are measured within the region.
- the chemically strengthened glass of the third aspect is plate-shaped (glass plate), and the thickness (t) thereof is, for example, 2 mm or less from the viewpoint of enabling remarkable strength improvement by chemical strengthening, Preferably it is 1.5 mm or less, More preferably, it is 1 mm or less, More preferably, it is 0.9 mm or less, Especially preferably, it is 0.8 mm or less, Most preferably, it is 0.7 mm or less.
- board thickness is 0.3 mm or more from a viewpoint of obtaining the effect of sufficient intensity
- the chemically strengthened glass of the third aspect has a surface compressive stress (CS) of 300 MPa or more.
- CS surface compressive stress
- the reason for limitation of CS and the preferable numerical range in the chemically strengthened glass of the third aspect are the same as those of the first aspect.
- the DOL in the chemically strengthened glass of the third aspect has sufficient resistance against breakage caused by scratches caused by collision with sharp objects such as sand that can collide with the chemically strengthened glass in a practical situation. From this point of view, it is 100 ⁇ m or more.
- the DOL is more preferably 110 ⁇ m or more, further preferably 120 ⁇ m or more, and particularly preferably 130 ⁇ m or more.
- the preferable numerical ranges of CS 90 , CS 100 and CS 100 ⁇ t 2 in the chemically strengthened glass of the third aspect and the technical effects associated therewith are the same as those of the first aspect.
- preferable numerical ranges and technical effects associated therewith of d h and d M in the chemical strengthened glass of the third aspect is similar to the first embodiment.
- preferable numerical ranges of Sc and St in the chemically strengthened glass of the third aspect and the technical effects associated therewith are the same as those of the first aspect.
- the chemically tempered glass according to the third aspect is a chemically tempered glass having a low safety and high safety even if CT or St is large.
- the glass composition of the chemically strengthened glass may be referred to as a mother composition of the chemically strengthened glass.
- the portion having the tensile stress of the chemically strengthened glass (hereinafter also referred to as the tensile stress portion) is a portion that is not ion-exchanged. It has the same composition as the glass before chemical strengthening. In that case, the composition of the tensile stress portion of the chemically strengthened glass can be regarded as the mother composition of the chemically strengthened glass.
- composition of the glass can be simply determined by semi-quantitative analysis by a fluorescent X-ray method, but more accurately, it can be measured by a wet analysis method such as ICP emission analysis.
- content of each component shall be represented by the molar percentage display of an oxide basis.
- the composition for the chemically strengthened glass of the present invention (the mother composition of the chemically strengthened glass of the present invention)
- SiO 2 is 50 to 80%
- Al 2 O 3 is 1 to 30%
- B 2 O 3 is 0. ⁇ 5%
- P 2 O 5 0-4% Li 2 O 3-20%, Na 2 O 0-8%
- K 2 O 0-10% MgO 3-20%
- CaO It preferably contains 0-20%, SrO 0-20%, BaO 0-15%, ZnO 0-10%, TiO 2 0-1% and ZrO 2 0-8%.
- Z SiO 2 ⁇ 237 + Al 2 O 3 ⁇ 524 + B 2 O 3 ⁇ 228 + P 2 O 5 ⁇ (-756) + Li 2 O ⁇ 538 + Na 2 O ⁇ 44.2 + K 2 O ⁇ (-387) + MgO ⁇ 660 + CaO ⁇ 569 + SrO ⁇
- the value of Z calculated based on 291 + ZrO 2 ⁇ 510 is preferably 20000 or more.
- SiO 2 is a component constituting the skeleton of glass. Further, a component to increase chemical durability, and a component to reduce the occurrence of cracks when scratched (indentation) on the glass surface, it is preferable that the content of SiO 2 is 50% or more. More preferably, the content of SiO 2 is 54% or more, 58% or more, 60% or more, 63% or more, 66% or more, 68% or more in a stepwise manner. On the other hand, if the content of SiO 2 exceeds 80%, the meltability is remarkably lowered. The content of SiO 2 is 80% or less, more preferably 78% or less, still more preferably 76% or less, particularly preferably 74% or less, and most preferably 72% or less.
- Al 2 O 3 is a component that improves the friability of chemically strengthened glass.
- the high crushability of the glass means that the number of fragments when the glass is broken is small. Highly friable glass is safe because it is difficult for fragments to scatter when broken.
- the content of Al 2 O 3 is 1% or more. It is preferable.
- Al 2 O 3 is a component that increases the Tg of the glass and is also a component that increases the Young's modulus.
- the content of Al 2 O 3 is more preferably 3% or more, 5% or more, 7% or more, 8% or more, 9% or more, 10% or more, 11% or more, or 12% or more step by step. 13% or more.
- the content of Al 2 O 3 is more than 30%, the acid resistance of the glass is lowered or the devitrification temperature is increased. Further, the viscosity of the glass increases and the meltability decreases.
- the content of Al 2 O 3 is preferably 30% or less, more preferably 25% or less, still more preferably 20% or less, particularly preferably 18% or less, and most preferably 15% or less.
- Al 2 O 3 content is preferably not more than 11%, or less, in stages, 10%, 9% or less, 8% or less, 7% or less Is preferred.
- B 2 O 3 is a component that improves the chipping resistance of the chemically strengthened glass or chemically strengthened glass and improves the meltability.
- B 2 O 3 is not essential, but the content in the case of containing B 2 O 3 is preferably 0.5% or more, more preferably 1% or more, further preferably, in order to improve the meltability. 2% or more.
- the content of B 2 O 3 exceeds 5%, striae are generated at the time of melting and the quality of the glass for chemical strengthening tends to deteriorate, so 5% or less is preferable.
- the content of B 2 O 3 is more preferably 4% or less, still more preferably 3% or less, and particularly preferably 1% or less. It is preferably not contained in order to increase acid resistance.
- P 2 O 5 is a component that improves ion exchange performance and chipping resistance.
- P 2 O 5 may not be contained, but the content in the case of containing P 2 O 5 is preferably 0.5% or more, more preferably 1% or more, and further preferably 2% or more. is there.
- the content of P 2 O 5 exceeds 4%, the crushed property of the chemically strengthened glass is lowered, and the acid resistance is remarkably lowered.
- the content of P 2 O 5 is preferably 4% or less, more preferably 3% or less, still more preferably 2% or less, and particularly preferably 1% or less. It is preferably not contained in order to increase acid resistance.
- Li 2 O is a component that forms surface compressive stress by ion exchange and is a component that improves the friability of chemically strengthened glass.
- the content of Li 2 O is preferably 3% or more, more preferably 4%. More preferably, it is 5% or more, particularly preferably 6% or more, and typically 7% or more.
- the content of Li 2 O exceeds 20%, the acid resistance of the glass is significantly reduced.
- the Li 2 O content is preferably 20% or less, more preferably 18% or less, still more preferably 16% or less, particularly preferably 15% or less, and most preferably 13% or less.
- the magnitude of compressive stress is such that the Li 2 O content exceeds 3%. Decreases, and it becomes difficult for CS 90 to achieve 30 MPa or more.
- the content of Li 2 O is preferably 3% or less, more preferably 2% or less, further preferably 1% or less, particularly preferably 0.5% or less, and most preferably Li 2.
- O is not substantially contained. In the present specification, “substantially does not contain” means that it is not contained except for inevitable impurities contained in raw materials and the like, that is, it is not intentionally contained. Specifically, it indicates that the content in the glass composition is less than 0.1 mol%.
- Na 2 O is a component that forms a surface compressive stress layer by ion exchange and improves the meltability of the glass.
- Li ion on the glass surface is exchanged with Na ion and chemical strengthening treatment is performed such that the CS 90 is 30 MPa or more
- Na 2 O may not be contained, but when the melting property of glass is important. You may contain.
- the content when Na 2 O is contained is preferably 1% or more.
- the content of Na 2 O is more preferably 2% or more, and further preferably 3% or more.
- the content of Na 2 O exceeds 8%, the surface compressive stress formed by ion exchange is remarkably reduced.
- the content of Na 2 O is preferably 8% or less, more preferably 7% or less, still more preferably 6% or less, particularly preferably 5% or less, and most preferably 4% or less.
- Na is essential, and its content is 5% or more.
- the Na 2 O content is preferably 5% or more, more preferably 7% or more, still more preferably 9% or more, particularly preferably 11% or more, and most preferably 12% or more.
- the content of Na 2 O exceeds 20%, the acid resistance of the glass is significantly reduced.
- the content of Na 2 O is preferably 20% or less, more preferably 18% or less, further preferably 16% or less, particularly preferably 15% or less, and most preferably 14% or less.
- the content of Na 2 O is preferably 10 % Or less, more preferably 9% or less, further preferably 7% or less, particularly preferably 6% or less, and most preferably 5% or less.
- the content of Na 2 O is preferably 2% or more, more preferably 3% or more, and further preferably 4% or more.
- K 2 O may be included to improve ion exchange performance.
- the content is preferably 0.5% or more, more preferably 1% or more, still more preferably 2% or more, and particularly preferably 3% or more.
- the content of K 2 O is more than 10%, the friability of the chemically strengthened glass is lowered, so the content of K 2 O is preferably 10% or less.
- the content of K 2 O is more preferably 8% or less, further preferably 6% or less, particularly preferably 4% or less, and most preferably 2% or less.
- MgO is a component that increases the surface compressive stress of chemically strengthened glass, and is a component that improves crushability, and is preferably contained.
- the content is preferably 3% or more, and more preferably 4% or more, 5% or more, 6% or more, 7% or more, or 8% or more stepwise.
- the content of MgO exceeds 20%, the glass for chemical strengthening tends to devitrify when melted.
- the content of MgO is preferably 20% or less, more preferably 18% or less, 15% or less, 14% or less, 13% or less, 12% or less, 11% or less, 10% or less stepwise. .
- CaO is a component that improves the meltability of the chemically strengthened glass, and is a component that improves the crushability of the chemically strengthened glass, and may be contained.
- the content is preferably 0.5% or more, more preferably 1% or more, further preferably 2% or more, particularly preferably 3% or more, and most preferably 5% or more. is there.
- the content of CaO exceeds 20%, the ion exchange performance is remarkably deteriorated, so 20% or less is preferable.
- the content of CaO is more preferably 14% or less, and still more preferably 10% or less, 8% or less, 6% or less, 3% or less, 1% or less stepwise.
- SrO is a component that improves the meltability of the chemically strengthened glass, and is a component that improves the crushability of the chemically strengthened glass, and may be contained.
- the content is preferably 0.5% or more, more preferably 1% or more, further preferably 2% or more, particularly preferably 3% or more, and most preferably 5% or more. is there.
- the SrO content exceeds 20%, the ion exchange performance is remarkably lowered, so 20% or less is preferable.
- the content of the SrO content is more preferably 14% or less, and further preferably 10% or less, 8% or less, 6% or less, 3% or less, or 1% or less stepwise.
- BaO is a component that improves the meltability of the chemically strengthened glass, and is a component that improves the crushability of the chemically strengthened glass, and may be contained.
- the content is preferably 0.5% or more, more preferably 1% or more, still more preferably 2% or more, particularly preferably 3% or more, and most preferably 5% or more. is there.
- the content of BaO exceeds 15%, the ion exchange performance is significantly lowered.
- the content of BaO is preferably 15% or less, more preferably 10% or less, 8% or less, 6% or less, 3% or less, 1% or less.
- ZnO is a component that improves the meltability of the glass and may be contained. Content in the case of containing ZnO becomes like this. Preferably it is 0.25% or more, More preferably, it is 0.5% or more. On the other hand, when the ZnO content exceeds 10%, the weather resistance of the glass is significantly lowered.
- the content of ZnO is preferably 10% or less, more preferably 7% or less, further preferably 5% or less, particularly preferably 2% or less, and most preferably 1% or less.
- TiO 2 is a component that improves the crushability of chemically strengthened glass, and may be contained.
- the content of the case of containing the TiO 2 is preferably 0.1% or more, more preferably 0.15% or more, further preferably 0.2% or more.
- the content of TiO 2 is preferably 1% or less, more preferably 0.5% or less, and still more preferably 0.25% or less.
- ZrO 2 is a component that increases the surface compressive stress due to ion exchange, has the effect of improving the crushability of the glass for chemical strengthening, and may be contained.
- the content is preferably 0.5% or more, more preferably 1% or more.
- the content of ZrO 2 is easily devitrified when melted is 8 percent, the quality of the chemically tempered glass may be lowered.
- the content of ZrO 2 is preferably 8% or less, more preferably 6% or less, further preferably 4% or less, particularly preferably 2% or less, and most preferably 1.2% or less. .
- Y 2 O 3 , La 2 O 3 , and Nb 2 O 5 are components that improve the crushability of the chemically strengthened glass, and may be contained.
- the content is preferably 0.5% or more, more preferably 1% or more, still more preferably 1.5% or more, particularly preferably 2% or more, most preferably Preferably it is 2.5% or more.
- the contents of Y 2 O 3 , La 2 O 3 , and Nb 2 O 5 are each over 8%, the glass tends to be devitrified at the time of melting, and the quality of the chemically strengthened glass may be deteriorated.
- Y 2 O 3 , La 2 O 3 and Nb 2 O 5 are each preferably 8% or less, more preferably 6% or less, still more preferably 5% or less, and particularly preferably 4%. Or less, most preferably 3% or less.
- Ta 2 O 5 and Gd 2 O 3 may be contained in a small amount in order to improve the crushability of chemically strengthened glass. However, the refractive index and the reflectance are increased, so 1% or less is preferable, and 0.5% or less. Is more preferable, and it is still more preferable not to contain.
- coloring and using glass you may add a coloring component in the range which does not inhibit achievement of a desired chemical strengthening characteristic.
- the coloring component include Co 3 O 4 , MnO 2 , Fe 2 O 3 , NiO, CuO, Cr 2 O 3 , V 2 O 5 , Bi 2 O 3 , SeO 2 , TiO 2 , CeO 2 , and Er 2.
- O 3 , Nd 2 O 3 and the like are preferable.
- the content of the coloring component is preferably in a range of 7% or less in total in terms of oxide-based mole percentage. If it exceeds 7%, the glass tends to be devitrified, which is not desirable. This content is preferably 5% or less, more preferably 3% or less, and even more preferably 1% or less. When giving priority to the visible light transmittance of glass, it is preferable that these components are not substantially contained.
- SO 3 As a fining agent for melting the glass, SO 3 , chloride, fluoride and the like may be appropriately contained. It is preferable not to contain As 2 O 3 . When containing Sb 2 O 3 content of preferably 0.3% or less, more preferably 0.1% or less, and most preferably not contained.
- the chemically strengthened glass of the present invention can impart antibacterial properties by having silver ions on the surface.
- the glass for chemical strengthening of the present invention preferably has a fracture toughness value (K1c) of 0.7 MPa ⁇ m 1/2 or more, more preferably 0.75 MPa ⁇ m 1/2 or more, and 0 further preferably .77MPa ⁇ m 1/2 or more, particularly preferably at 0.80 MPa ⁇ m 1/2 or more, and most preferably 0.82 MPa ⁇ m 1/2 or more.
- K1c fracture toughness value
- the fracture toughness value (K1c) in the present specification is a fracture toughness value obtained by measuring a K1-v curve by a DCDC method described in detail in Examples described later.
- the area St (MPa ⁇ ⁇ m) of the internal tensile layer is preferably not more than StL (t) (MPa ⁇ ⁇ m). If St is equal to or less than StL (t), the number of pieces to be crushed is reduced even if it is actually broken.
- the internal tensile stress CT is preferably CTL (t) (MPa) or less. If CT is equal to or less than CTL (t), the number of fractures is reduced even if the CT is actually destroyed.
- the Young's modulus of the chemically strengthened glass is 70 GPa or more, the compressive stress value (CS 0 ) at the outermost surface of the chemically strengthened glass, and the compressive stress value at a depth of 1 ⁇ m from the glass surface ( The difference from CS 1 ) is preferably 50 MPa or less. This is preferable because warpage hardly occurs when the glass surface is polished after the chemical strengthening treatment.
- the Young's modulus of the glass for chemical strengthening is more preferably 74 GPa or more, particularly preferably 78 GPa or more, and further preferably 82 GPa or more.
- the upper limit of the Young's modulus is not particularly limited, but is, for example, 90 GPa or less, preferably 88 GPa or less.
- the Young's modulus can be measured by, for example, an ultrasonic pulse method.
- CS 0 and CS 1 are preferably 50 MPa or less, more preferably 40 MPa or less, and further preferably 30 MPa or less.
- CS 0 is preferably 300 MPa or more, more preferably 350 MPa or more, and further preferably 400 MPa or more.
- the upper limit of CS 0 is not particularly limited, but is, for example, 1200 MPa or less, preferably 1000 MPa or less, and more preferably 800 MPa or less.
- CS 1 is preferably not less than 250 MPa, more preferably at least 300 MPa, more preferably at least 350 MPa.
- the upper limit of CS 1 is not particularly limited, but is, for example, 1150 MPa or less, preferably 1100 MPa or less, and more preferably 1050 MPa or less.
- the chemically strengthened glass of the present invention can be produced, for example, as follows.
- the glass for chemical strengthening treatment is preferably the glass for chemical strengthening of the present invention.
- Glass subjected to the chemical strengthening treatment can be produced by a usual method. For example, the raw material of each component of glass is prepared and heated and melted in a glass melting furnace. Thereafter, the glass is homogenized by a known method, formed into a desired shape such as a glass plate, and slowly cooled.
- Examples of the glass plate forming method include a float method, a press method, a fusion method, and a downdraw method.
- a float method suitable for mass production is preferable.
- continuous molding methods other than the float method, that is, the fusion method and the downdraw method are also preferable.
- the molded glass is ground and polished as necessary to form a glass substrate.
- the glass substrate is cut into a predetermined shape and size, or when the glass substrate is chamfered, if the glass substrate is cut or chamfered before the chemical strengthening process described later, the subsequent chemical strengthening process is performed. Is preferable because a compressive stress layer is also formed on the end face.
- the chemically strengthened glass of the present invention can be produced by subjecting the obtained glass plate to chemical strengthening treatment, followed by washing and drying.
- the chemical strengthening treatment can be performed by a conventionally known method.
- the glass plate is brought into contact with a melt of a metal salt (for example, potassium nitrate) containing a metal ion (typically, K ions) having a large ionic radius by dipping or the like.
- a metal salt for example, potassium nitrate
- K ions typically, K ions
- Small ion radius metal ions typically Na or Li ions
- the chemical strengthening treatment is not particularly limited. For example, by immersing the glass plate in a molten salt such as potassium nitrate heated to 360 to 600 ° C. for 0.1 to 500 hours. It can be carried out.
- the heating temperature of the molten salt is preferably 375 to 500 ° C.
- the immersion time of the glass plate in the molten salt is preferably 0.3 to 200 hours.
- Examples of molten salts for performing chemical strengthening treatment include nitrates, sulfates, carbonates, and chlorides.
- examples of the nitrate include lithium nitrate, sodium nitrate, potassium nitrate, cesium nitrate, and silver nitrate.
- examples of the sulfate include lithium sulfate, sodium sulfate, potassium sulfate, cesium sulfate, and silver sulfate.
- Examples of the carbonate include lithium carbonate, sodium carbonate, and potassium carbonate.
- Examples of the chloride include lithium chloride, sodium chloride, potassium chloride, cesium chloride, silver chloride and the like. These molten salts may be used alone or in combination of two or more.
- the treatment conditions for the chemical strengthening treatment are not particularly limited, and the characteristics and composition of the glass, the type of the molten salt, and the surface compressive stress (CS) and compression desired for the chemically strengthened glass finally obtained.
- Appropriate conditions may be selected in consideration of chemical strengthening characteristics such as stress layer depth (DOL).
- the chemical strengthening treatment may be performed only once, or multiple times of chemical strengthening treatment (multi-stage strengthening) may be performed under two or more different conditions.
- multi-stage strengthening may be performed under two or more different conditions.
- the first-stage chemical strengthening process after performing the chemical strengthening process under the condition that the CS is relatively low, the second-stage chemical strengthening process is performed under the condition that the CS is relatively high.
- the tempering process is performed, the internal tensile stress area (St) can be suppressed while increasing the CS on the outermost surface of the chemically strengthened glass, and as a result, the internal tensile stress (CT) can be suppressed to a low level.
- the chemically tempered glass of the present invention is particularly useful as a cover glass used for mobile devices such as mobile phones, smartphones, personal digital assistants (PDAs), and tablet terminals.
- mobile devices such as mobile phones, smartphones, personal digital assistants (PDAs), and tablet terminals.
- non-portable products such as televisions (TVs), personal computers (PCs), cover glass for display devices such as touch panels, wall surfaces of elevators, wall surfaces of buildings such as houses and buildings (full display), and construction of window glass, etc.
- TVs televisions
- PCs personal computers
- cover glass for display devices such as touch panels, wall surfaces of elevators, wall surfaces of buildings such as houses and buildings (full display), and construction of window glass, etc.
- materials such as construction materials, table tops, interiors of automobiles, airplanes, etc., and cover glasses thereof, and for applications such as a casing having a curved surface shape that is not a plate shape by bending or molding.
- the glass plates were formed so as to have respective glass compositions represented by mole percentages based on oxides shown in the table.
- Example S-1 to S-6, S-13 to S-23, and S-30 to S-33 the glass plates were formed so as to have respective glass compositions represented by mole percentages based on oxides shown in the table.
- Example S-1 to S-6, S-13 to S-23, and S-30 to S-33 the glass plates were formed so as to have respective glass compositions represented by mole percentages based on oxides shown in the table.
- the table was produced in a float kiln.
- Commonly used glass raw materials such as oxides, hydroxides, carbonates or nitrates are appropriately selected and melted in a melting kiln, and formed to a plate thickness of 1.1 to 1.3 mm by a float process.
- the obtained plate glass was cut and ground, and finally both surfaces were processed into mirror surfaces to obtain plate glass having a length of 50 mm ⁇ width 50 mm ⁇ plate thickness
- the glass compositions are expressed in terms of mole percentages based on the oxides shown in the table.
- a glass plate was prepared by melting a platinum crucible. Commonly used glass materials such as oxides, hydroxides, carbonates or nitrates were appropriately selected and weighed so as to give 1000 g of glass. Next, the mixed raw materials were put into a platinum crucible, put into a resistance heating electric furnace at 1500 to 1700 ° C., melted for about 3 hours, defoamed and homogenized. The obtained molten glass was poured into a mold material, held at a temperature of glass transition point + 50 ° C.
- the obtained glass block was cut and ground, and finally both surfaces were processed into mirror surfaces to obtain a plate glass having a length of 50 mm, a width of 50 mm, and a plate thickness t (mm).
- the plate thickness t (mm) is shown in the table.
- a chemically strengthened glass was obtained by subjecting each glass of Examples S-1 to S-13, S-15 to S-29, and S-31 to S-53 to a chemical strengthening treatment.
- the chemical strengthening treatment conditions for each glass are shown in the table.
- the glasses of Examples S-14 and S-30 were not subjected to chemical strengthening treatment.
- surface compressive stress CS (unit: MPa), compressive stress layer thickness DOL (unit: ⁇ m), internal tensile stress CT ( (Unit: MPa), compressive stress value CS x (unit: MPa) at a depth of x ⁇ m from the glass surface, compressive stress value at a depth of x ⁇ m from the glass surface and the square of the thickness t (mm)
- the product CS x ⁇ t 2 (unit: MPa ⁇ mm 2 ), the depth d h (unit: ⁇ m) from the glass surface at which the compressive stress value is one half of the surface compressive stress, is the surface made by Orihara Seisakusho.
- CS was measured with a surface stress meter FSM-6000 manufactured by Orihara Seisakusho Co., and the above-mentioned Abrio-IM and a method using a thin sample was measured DOL, CT, the CS x, CS x ⁇ t 2 , d h. These results are shown in the table.
- the Sc value (unit: MPa ⁇ ⁇ m), ⁇ CS 100-90 (unit: MPa / ⁇ m), CS DOL-20 (unit: MPa), ⁇ CS DOL-20 (unit: MPa / MPa) ( ⁇ m) is also shown.
- Examples S-1 to S-53 For each of Examples S-1 to S-53, X and Z values were calculated based on the glass composition.
- the chemically tempered glasses of Examples S-1 to S-13, S-15 to S-29, and S-31 to S-53 have the same glass composition before chemical tempering (the mother composition of chemically tempered glass). Based on this, X and Z values were calculated. These results are shown in the table.
- ⁇ Devitrification temperature T> The glass before chemical strengthening was pulverized, classified using a sieve of 4 mm mesh and 2 mm mesh, washed with pure water, and dried to obtain cullet. 2-5g cullet placed on a platinum plate and kept in an electric furnace maintained at a constant temperature for 17 hours, taken out into the air at room temperature, cooled, and then repeatedly observed for the presence of devitrification with a polarizing microscope.
- the devitrification temperature T was estimated. The results are shown in Table 1.
- the description that the devitrification temperature T is T1 to T2 means that there is devitrification at T1, and no devitrification at T2.
- FIG. 3 is a schematic diagram showing a test method for a drop-on-sand test.
- chemically tempered glass is also described as “glass”.
- a hard nylon mock plate 11 50 mm ⁇ 50 mm ⁇ thickness 18 mm, weight: 54 g
- glass 13 50 mm ⁇ 50 mm ⁇ plate thickness t (mm)
- sponge double-sided tape 12 # 2310 manufactured by Sekisui Chemical Co., Ltd.
- 50 mm ⁇ 50 mm ⁇ thickness 3 mm to obtain a measurement sample 1 (total weight: 61 g).
- 1 g of silica sand 22 No.
- FIG. 4 shows a graph plotting the relationship between the DOL (unit: ⁇ m) and the average crack height (unit: mm) of the chemically strengthened glass or glass of Examples S-1 to S-35.
- FIG. 5 shows a graph plotting the relationship between the CT (unit: MPa) and the average crack height (unit: mm) of the chemically strengthened glass or glass of Examples S-1 to S-35.
- FIG. 6 shows the relationship between the CT (unit: MPa) and the average crack height (unit: mm) of the glass having a DOL of less than 50 ⁇ m among the chemically strengthened glasses of Examples S-1 to S-35. The plotted graph is shown.
- FIG. 7 is a graph plotting the relationship between the surface compressive stress value CS (unit: MPa) and the average crack height (unit: mm) of the chemically strengthened glass or glass of Examples S-1 to S-35.
- FIG. 8 shows the compressive stress value CS 90 (unit: MPa) and average crack height (unit: unit) of the chemically tempered glass or glass of Examples S-1 to S-35 at a depth of 90 ⁇ m from the glass surface. mm) is plotted.
- FIG. 9 shows the compressive stress value CS 100 (unit: MPa) and the average crack height (unit) of the chemically tempered glass or glass of Examples S-1 to S-35 at a depth of 100 ⁇ m from the glass surface. : Mm) is plotted.
- FIG. 10 shows that the average crack height is highly correlated with CS 100 ⁇ t 2 .
- the average crack height as the CS 100 ⁇ t 2 is more than 5 MPa ⁇ mm 2 becomes more about 300 mm, it can be seen that achieved a significant improvement in strength.
- Example S-1 ⁇ Four-point bending test after wounding or unscratched>
- a glass plate having the same glass composition as in Example S-1 and a thickness of 1.1 to 1.3 mm was produced by the float process under the same conditions as in Example S-1.
- the obtained plate glass was cut and ground, and finally processed into a double-sided mirror surface to obtain a plate glass having a length of 5 mm ⁇ width of 40 mm ⁇ thickness of 1.0 mm.
- chemical strengthening treatment was performed under the chemical strengthening conditions shown in the columns of Examples 4PB-1 to 4PB-6 in Table 10 to produce the chemically strengthened glasses of Examples 4PB-1 to 4PB-6.
- Example S-7 a glass block having the same glass composition as in Example S-7 was produced by melting a platinum crucible under the same conditions as in Example S-7. The obtained glass block was cut and ground, and finally both surfaces were processed into mirror surfaces to obtain a plate glass having a length of 5 mm ⁇ width of 40 mm ⁇ thickness of 0.8 mm. Thereafter, chemical tempering treatment was performed under the chemical tempering conditions shown in the columns of Examples 4PB-7 to 4PB-9 in Table 10 below to produce the chemically tempered glasses of Examples 4PB-7 to 4PB-9.
- the strengthening temperature (unit: ° C.) in Table 10 is the temperature of the molten salt during the chemical strengthening treatment.
- the salt concentration, the ratio of KNO 3 in weight of the molten salt used in the chemical strengthening treatment (KNO 3 / KNO 3 + Na 2 O) ⁇ 100 ( unit:%) indicating the.
- the tempering time represents the immersion time (unit: time) of the glass in the molten salt.
- the surface compressive stress (CS, unit: MPa) and the thickness of the compressive stress layer (DOL, unit: ⁇ m) were measured using a surface stress meter FSM manufactured by Orihara Seisakusho. Measured with -6000 and accompanying program FsmV. Further, based on the obtained CS and DOL, the internal tensile stress (CT, unit: MPa) was calculated. These results are shown in Table 10 and Table 11.
- Example 4 By pressing a diamond indenter (face-to-face indenter angle: 110 °) with a load of 0.5 Kgf, 1 Kgf, 1.5 Kgf or 2 Kgf for 15 seconds against each chemically strengthened glass of PB-1 to 4PB-9, The glass surface was damaged. Next, a four-point bending test was performed under conditions of a lower span of 30 mm, an upper span of 10 mm, and a crosshead speed of 0.5 mm / min, and the fracture stress (MPa) under each scratch condition was measured. Table 11 and FIG. 11 show the fracture stress values (bending strength, unit: MPa) when a four-point bending test is performed when uninjured and at each indenter press-fitting load. In FIG. 11, (a) to (i) represent test results for the chemically strengthened glasses of Examples 4PB-1 to 4PB-9, respectively.
- Fig. 12 shows a plot of the relationship between fracture strength and CS when uninjured. From FIG. 12, it can be seen that when CS is 300 MPa or more, the fracture strength when not damaged can be 350 MPa or more. When the smartphone or tablet PC is dropped, a tensile stress is generated on the cover glass surface, and the magnitude reaches about 350 MPa. For this reason, it is desirable that CS is 300 MPa or more.
- FIG. 13 shows a plot of the relationship between the breaking strength and DOL of Examples 4PB-1 to 4PB-9 when 2 kgf was injured.
- Chemically strengthened glass having a DOL of 100 ⁇ m or more has a fracture strength of 200 MPa or more even after 2 kgf of damage by a diamond indenter (face-to-face indenter angle: 110 °), and even after damage under a higher load. It shows high fracture strength and higher reliability as a cover glass even when damaged.
- DOL is preferably 100 ⁇ m or more, more preferably 110 ⁇ m or more, further preferably 120 ⁇ m or more, and particularly preferably 130 ⁇ m or more.
- FIG. 14 shows a stress profile of a virtual chemically strengthened glass having a plate thickness of 1 mm.
- Table 12 shows CS, DOL, CT, Sc, and St of each profile.
- the reinforcement profile shown in FIG. 14 and Table 12 is created by the following equation.
- F (x) ⁇ + ERFC ( ⁇ ⁇ x) ⁇ CT Note that x is the depth from the glass surface, and the function ERFC (c) is a complementary error function.
- the values of the constants ⁇ and ⁇ are shown in Table 12.
- the chemically strengthened glass having these profiles is expected to achieve high strength against the drop-on-sand test and end face bending. It is expected that the higher the CS value and the chemically strengthened glass into which higher CS 90 and CS 100 are introduced, the higher the strength, and from Table 12, the Sc value of the chemically strengthened glass of the present invention is about 30000 MPa ⁇ ⁇ m or more. I understand that. At this time, the St value is equal to the Sc value as described above. In the unlikely event that breakage occurs, it is desirable that the glass breaks safely. For this purpose, it is desirable that the St Limit value described later be a larger value.
- the internal tensile stress area (St; unit MPa ⁇ ⁇ m) in which the number of fractures is 10 is represented by the St Limit value
- the internal tensile stress CT (unit: MPa) in which the number of fractures is 10 is represented by CT.
- Limit value When the number of fractures exceeds 10, use the Stn value which is the St value of the maximum number of fractures n of less than 10 and the Stm value of the minimum number of fractures m of more than 10 and The St Limit value was defined by the formula.
- StLimit value Stn + (10 ⁇ n) ⁇ (Stm ⁇ Stn) / (mn)
- CTn value that is the CT value of the maximum number of fractures n of less than 10
- CTm value that is the CT value of the minimum number of fractures m of more than 10
- the CT Limit value was defined by the following equation.
- CTLimit value CTn + (10 ⁇ n) ⁇ (CTm ⁇ CTn) / (mn)
- CTF is a value equal to the value CT_CV analyzed by FsmV.
- FIG. 15 and Table 13 show measurement examples when t is 1 mm.
- FIG. 15 shows measurement examples of St Limit and CT Limit.
- (A) is a graph showing the relationship between the area St (MPa ⁇ ⁇ m) of the internal tensile stress layer and the number of fractures when the plate thickness (t) is 1 mm.
- (b) is an enlarged view of a portion surrounded by a dotted line in (a).
- (c) is a graph showing the relationship between the internal tensile stress CT (MPa) and the number of fractures when the plate thickness (t) is 1 mm, and (d) is the portion surrounded by the dotted line in (c). It is an enlarged view.
- StL10 in (b) and CTL10 in (d) indicate the internal tensile stress area (St; unit MPa ⁇ ⁇ m) and the internal tensile stress (CT; unit MPa) when the number of fractures is 10, respectively.
- a glass with a higher St Limit value or CT Limit value is a glass with improved crushability.
- St Limit value and the CT Limit value are indicators for expressing the degree of friability, and do not prescribe the allowable limit of the crushing mode.
- the St limit value was determined in the same manner as described above. Tables 14-15 show.
- Tables 14 to 15 show the Young's modulus E (unit: GPa) and the fracture toughness value K1c (unit: MPa ⁇ m 1/2 ) measured by the DCDC method for the glass before chemical strengthening.
- the Young's modulus E was measured by an ultrasonic pulse method (JIS R1602). Further, the fracture toughness value was determined by the DCDC method with reference to the method described in MY He, MR Turner and AG Evans, Acta Metall. Mater. 43 (1995) 3453.
- the relationship between the stress intensity factor K1 (unit: MPa ⁇ m 1/2 ) and the crack growth rate v (unit: m / s) as shown in FIG. 17 is shown using Tensilon UTA-5kN manufactured by the company.
- the K1-v curve was measured, and the obtained Region III data was regressed and extrapolated by a linear equation, and the stress intensity factor K1 of 0.1 m / s was taken as the fracture toughness value K1c.
- X SiO 2 ⁇ 329 + Al 2 O 3 ⁇ 786 + B 2 O 3 ⁇ 627 + P 2 O 5 ⁇ ( ⁇ 941) + Li 2 O ⁇ 927 + Na 2 O ⁇ 47.5 + K 2 O ⁇ ( ⁇ 371) + MgO ⁇ 1230 + CaO ⁇ 1154 + SrO ⁇ 733 + ZrO 2 ⁇ 51.8
- Y SiO 2 ⁇ 0.00884 + Al 2 O 3 ⁇ 0.0120 + B 2 O 3 ⁇ (-0.00373) + P 2 O 5 ⁇ 0.000681 + Li 2 O ⁇ 0.00735 + Na 2 O ⁇ (-0.00234) + K 2 O ⁇ ( ⁇ 0.00608) + MgO ⁇ 0.0105 + CaO ⁇ 0.00789 + SrO
- FIG. 18 is a graph plotting the relationship between the St limit and the Z value when the thickness t is 1 mm
- FIG. 19 is a graph plotting the relationship between the St limit and the Young's modulus when the thickness t is 1 mm.
- a graph plotting the relationship is shown in FIG. 20, and a graph plotting the relationship between the X value and the Z value is shown in FIG.
- the X value and the Z value correlate with St Limit at 1 mm with high accuracy, and are parameters that express the crushability at the time of fracture of chemically strengthened glass with high accuracy. I understand that. It was also found that St Limit increases as the X value and Z value increase. Here, as the St Limit of the chemically strengthened glass is larger, even if the chemically strengthened glass breaks, it represents a safer breakage with a smaller number of fractures. For example, in the case of chemically strengthened glass having an X value and a Z value of 30000 or more and 20000 or more, St Limit is larger than 30000 MPa, for example, high strength of 1 mm where Sc or St is 30000 MPa or more as described above. Even in the case of chemically strengthened glass, it can be said that a glass with higher safety can be realized because the number of fractures when the glass is broken is sufficiently small.
- Glasses were produced in the following manner so that each glass composition represented by the oxide-based molar percentage shown in Examples 2-1 to 2-53 in Tables 16 to 20 was used. Commonly used glass materials such as oxides, hydroxides, carbonates or nitrates were appropriately selected and weighed so as to give 1000 g of glass. Next, the mixed raw materials were put into a platinum crucible, put into a resistance heating electric furnace at 1500 to 1700 ° C., melted for about 3 hours, defoamed and homogenized. The obtained molten glass was poured into a mold material, held at a temperature of glass transition point + 50 ° C. for 1 hour, and then cooled to room temperature at a rate of 0.5 ° C./min to obtain a glass block. The obtained glass block was cut, ground, and polished and subjected to the following measurements.
- Commonly used glass materials such as oxides, hydroxides, carbonates or nitrates were appropriately selected and weighed so as to give 1000 g of glass.
- Density measurement was performed by a submerged weighing method (a method for measuring density and specific gravity of JISZ8807 solid).
- the linear expansion coefficient ⁇ and the glass transition point Tg were measured according to the method of JIS R3102 “Testing method for average linear expansion coefficient of glass”.
- Young's modulus E, synthesis rate G, and Poisson's ratio were measured by the ultrasonic pulse method (JIS R1602). Examples 2-1 to 2-53 show the X value, Y value, and Z value.
- the devitrification temperature T was estimated, and the temperature T4 at which the viscosity was 10 4 dPa ⁇ s was measured. These results are shown in Tables 16-20.
- Example 2-51 is an example described in US Patent Application Publication No. 2015/0259244.
- Example 2-1, 2-3 to 2-50, 2-52 the X value was 30000 or more, and even when larger CS and DOL were introduced, the number of fractures when breaking the glass was sufficiently small. This is an example in which highly safe glass can be realized.
- the X value is 30000 or less.
- the Z value was 20000 or more, and even when larger CS and DOL were introduced, the number of fractures when breaking the glass was sufficiently small. This is an example in which highly safe glass can be realized.
- Example 2-2 and Example 2-51 the Z value is 20000 or less.
- Chemically strengthened glass having various internal tensile stress areas (St; unit MPa ⁇ ⁇ m) or internal tensile stress CT (unit: MPa) was prepared by performing chemical strengthening treatment under chemical strengthening treatment conditions. Then, using a diamond indenter having an indenter angle of 60 degrees, an indenter press-in test in which a load of 3 kgf is maintained for 15 seconds, each of these chemically strengthened glasses is broken, and the number of pieces of broken glass (fracture) Number) was measured respectively.
- the internal tensile stress area (St; unit MPa ⁇ ⁇ m) in which the number of fractures is 10 is represented by the St Limit value
- the internal tensile stress CT (unit: MPa) in which the number of fractures is 10 is represented by CT.
- Limit value When the number of fractures exceeds 10, use the Stn value which is the St value of the maximum number of fractures n of less than 10 and the Stm value of the minimum number of fractures m of more than 10 and The St Limit value was defined by the formula.
- StLimit value Stn + (10 ⁇ n) ⁇ (Stm ⁇ Stn) / (mn)
- CTn value that is the CT value of the maximum number of fractures n of less than 10
- CTm value that is the CT value of the minimum number of fractures m of more than 10
- the CT Limit value was defined by the following equation.
- CTLimit value CTn + (10 ⁇ n) ⁇ (CTm ⁇ CTn) / (mn)
- CTF is a value equal to the value CT_CV analyzed by FsmV.
- Tables 21 and 22 show the values of St Limit and CT Limit regarding the chemically tempered glass and sheet thickness of Examples CT-5, CT-16, CT-17, and CT-26.
- 22 and 23 are diagrams in which ST Limit and CT Limit of each chemically strengthened glass of Examples CT-5, CT-16, CT-17, and CT-26 are plotted against the plate thickness t (mm), respectively. Show.
- St Limit tends to increase linearly with respect to the plate thickness and is approximately expressed by the following equation.
- St (a, t) a ⁇ t + 7000 (unit: MPa ⁇ ⁇ m)
- a in the above formula varies with chemically strengthened glass.
- ST Limit is larger in each plate thickness, and even if larger CS and DOL are introduced, it can be used as a chemically strengthened glass having a smaller number of crushing.
- CT Limit tends to decrease with increasing plate thickness and is approximately expressed by the following equation.
- CT (b, c, t) ⁇ b ⁇ ln (t) + c (unit: MPa)
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Geochemistry & Mineralogy (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Ceramic Engineering (AREA)
- Glass Compositions (AREA)
- Surface Treatment Of Glass (AREA)
- Surface Treatment Of Glass Fibres Or Filaments (AREA)
Abstract
Description
CT’≦-38.7×ln(t)+48.2 (10)
CS×DOL’=(t-2×DOL’)×CT’ (11)
ここで、DOL’はイオン交換層の深さに相当する。
前記化学強化ガラスの母組成におけるSiO2、Al2O3、B2O3、P2O5、Li2O、Na2O、K2O、MgO、CaO、SrO、BaO及びZrO2の各成分の酸化物基準のモル百分率表示による含有量を用いて、下記式に基づき算出されるXの値が30000以上である化学強化ガラスである。
X=SiO2×329+Al2O3×786+B2O3×627+P2O5×(-941)+Li2O×927+Na2O×47.5+K2O×(-371)+MgO×1230+CaO×1154+SrO×733+ZrO2×51.8
前記化学強化ガラスの母組成におけるSiO2、Al2O3、B2O3、P2O5、Li2O、Na2O、K2O、MgO、CaO、SrO、BaO及びZrO2の各成分の酸化物基準のモル百分率表示による含有量を用いて、下記式に基づき算出されるZの値が20000以上である化学強化ガラスでもよい。
Z=SiO2×237+Al2O3×524+B2O3×228+P2O5×(-756)+Li2O×538+Na2O×44.2+K2O×(-387)+MgO×660+CaO×569+SrO×291+ZrO2×510
StL(t)≧a×t+7000 (単位:MPa・μm) (1)
a≧30000 (単位:MPa・μm/mm) (2)
(ここで、tは板厚(mm)であり、StL(t)は板厚tのときのSt Limitの値である。)
CTL(t)≧-b×ln(t)+c (単位:MPa) (3)
b≧14 (単位:MPa) (4)
c≧48.4 (単位:MPa) (5)
(ここで、tは板厚(mm)であり、CTL(t)は板厚tのときのCT Limitの値である。)
ΔCSDOL-20=CSDOL-20/20
ΔCS100-90=(CS90-CS100)/(100-90)
(ここで、tは板厚(mm)であり、StL(t)は板厚tのときのSt Limitの値である。)
(ここで、tは板厚(mm)であり、CTL(t)は板厚tのときのCT Limitの値である。)
Ta2O5、Gd2O3、As2O3、Sb2O3を含有せず、
SiO2、Al2O3、B2O3、P2O5、Li2O、Na2O、K2O、MgO、CaO、SrO、BaO及びZrO2の各成分の酸化物基準のモル百分率表示による含有量を用いて、下記式に基づき算出されるXの値が30000以上である化学強化用ガラスにも関する。
X=SiO2×329+Al2O3×786+B2O3×627+P2O5×(-941)+Li2O×927+Na2O×47.5+K2O×(-371)+MgO×1230+CaO×1154+SrO×733+ZrO2×51.8
また、酸化物基準のモル百分率表示によるK2Oの含有量が0.5%以上であることが好ましい。
また、酸化物基準のモル百分率表示によるB2O3の含有量が1%以下であることが好ましい。
また、酸化物基準のモル百分率表示によるAl2O3の含有量が11%以下であることが好ましい。
また、失透温度Tが、粘度が104dPa・sとなる温度T4以下であることが好ましい。
まず、第1の態様に係る化学強化ガラスについて説明する。
本態様は、前記化学強化ガラスの母組成におけるSiO2、Al2O3、B2O3、P2O5、Li2O、Na2O、K2O、MgO、CaO、SrO、BaO及びZrO2の各成分の酸化物基準のモル百分率表示による含有量を用いて、下記式に基づき算出されるXの値が30000以上、及び/又は、下記式に基づき算出されるZの値が20000以上である。
X=SiO2×329+Al2O3×786+B2O3×627+P2O5×(-941)+Li2O×927+Na2O×47.5+K2O×(-371)+MgO×1230+CaO×1154+SrO×733+ZrO2×51.8
Z=SiO2×237+Al2O3×524+B2O3×228+P2O5×(-756)+Li2O×538+Na2O×44.2+K2O×(-387)+MgO×660+CaO×569+SrO×291+ZrO2×510
CS=CSF=1.28×CSA
F=δ/(C×t’)・・・式(A)
式(A)中、Fは応力(MPa)、δは位相差(リタデーション)(nm)、Cは光弾性定数(nm cm-1MPa)、t’はサンプルの厚さ(cm)を示す。
以上の観点から、第1の態様においては、化学強化ガラスの、ガラス表面から90μmの深さの部分の圧縮応力値(CS90)が25MPa以上であることが好ましく、30MPa以上であることがより好ましい。また、化学強化ガラスの、ガラス表面から100μmの深さの部分の圧縮応力値(CS100)が15MPa以上であることが好ましく、20MPa以上であることがより好ましい。また、第1の態様の化学強化ガラスにおいては、ガラス表面から100μmの深さの部分の圧縮応力値と板厚t(mm)の二乗との積CS100×t2が5MPa・mm2以上であることが好ましい。
ΔCSDOL-20=CSDOL-20/20
ΔCSDOL-20を0.4以上とすることにより、鋭角物で加傷された後の曲げ強度(加傷後曲げ強度)を高くすることができる。ΔCSDOL-20は、より好ましくは、以下、段階的に、0.5以上、0.6以上、0.7以上、0.8以上、0.9以上、1.0以上、1.2以上、1.4以上、1.5以上である。一方、ΔCSDOL-20の上限は特に限定されるものではないが、破砕の安全性の観点からは、例えば4.0以下であり、好ましくは3.0以下、より好ましくは2.0以下、さらに好ましくは1.7以下、典型的には1.6以下である。
ΔCS100-90=(CS90-CS100)/(100-90)
ΔCS100-90を0.4以上とすることにより、鋭角物で加傷された後の曲げ強度(加傷後曲げ強度)を高くすることができる。ΔCS100-90は、より好ましくは、以下、段階的に、0.5以上、0.6以上、0.7以上、0.8以上、0.9以上、1.0以上、1.2以上、1.4以上、1.5以上である。一方、ΔCS100-90の上限は特に限定されるものではないが、破砕の安全性の観点からは、例えば4.0以下であり、好ましくは3.0以下、より好ましくは2.0以下、さらに好ましくは1.7以下、典型的には1.6以下である。
Sc=ScF=1.515×ScA
ここで、ScFは折原製作所社製の表面応力計FSM-6000により測定され付属プログラムFsmVにより解析される値を用いて算出した値であり、ScAは前述のCSA測定と同様の手法である、複屈折イメージングシステムAbrio-IMおよび薄片化サンプルを用いた測定により得られる値である。
St=StF=1.515×StA
ここで、StFは折原製作所社製の表面応力計FSM-6000により測定され付属プログラムFsmVにより解析される値を用いて算出した値であり、StAは前述のCSA測定と同様の手法である、複屈折イメージングシステムAbrio-IMおよび薄片化サンプルを用いた測定により得られる値である。上記と同様に二手法により応力プロファイルを作成し、StFもしくはStAを算出し、Stを得ることができる。
なお、化学強化ガラスの母組成とは、化学強化前のガラス(以下、化学強化用ガラスともいう)の組成である。ここで、化学強化ガラスの引張応力を有する部分(以下、引張応力部分ともいう)はイオン交換されていない部分である。そして、化学強化ガラスの厚みが十分大きい場合には、化学強化ガラスの引張応力部分は、化学強化前のガラスと同じ組成を有している。その場合には、引張応力部分の組成を母組成とみなすことができる。また、化学強化ガラスの母組成の好ましい態様については後述する。
X=SiO2×329+Al2O3×786+B2O3×627+P2O5×(-941)+Li2O×927+Na2O×47.5+K2O×(-371)+MgO×1230+CaO×1154+SrO×733+ZrO2×51.8
Z=SiO2×237+Al2O3×524+B2O3×228+P2O5×(-756)+Li2O×538+Na2O×44.2+K2O×(-387)+MgO×660+CaO×569+SrO×291+ZrO2×510
Y=SiO2×0.00884+Al2O3×0.0120+B2O3×(-0.00373)+P2O5×0.000681+Li2O×0.00735+Na2O×(-0.00234)+K2O×(-0.00608)+MgO×0.0105+CaO×0.00789+SrO×0.00752+BaO×0.00472+ZrO2×0.0202
たとえば、スマートフォン等のモバイル機器は、誤って落下した際に、砂などの、角度の小さい衝突部分を有する衝突物(以下、鋭角物ともいう)に衝突し、カバーガラスとしての化学強化ガラスが破損してしまう機会が比較的高いため、鋭角物に衝突した場合でも破損しにくい化学強化ガラスが求められている。
第1の態様に係る化学強化ガラスは、実用的な場面において衝突しうる砂等の鋭角物との衝突によって生じる傷に起因する破壊に対する耐性(砂上落下耐性)にも優れる。
つづいて、第2の態様に係る化学強化ガラスについて説明する。
第2の態様の化学強化ガラスの一つは、表面圧縮応力(CS)が300MPa以上であり、かつ、下記式(1)及び(2)を満たす化学強化ガラスである。
StL(t)≧a×t+7000 (単位:MPa・μm) (1)
a≧30000 (単位:MPa・μm/mm)(2)
(ここで、tは板厚(mm)であり、StL(t)は板厚tのときのSt Limitの値である。)
StL(t)値 = Stn+(10-n)×(Stm-Stn)/(m-n)
25mm×25mmより大きなサイズの化学強化ガラスを用いるときは、化学強化ガラス内に25mm×25mmの領域を表示し、その領域内で上記のStL(t)測定を行う。
CTL(t)≧-b×ln(t)+c (単位:MPa) (3)
b≧14 (単位:MPa) (4)
c≧48.4 (単位:MPa) (5)
(ここで、tは板厚(mm)であり、CTL(t)は板厚tのときのCT Limitの値である。)
CTL(t)値 = CTn+(10-n)×(CTm-CTn)/(m-n)
25mm×25mmより大きなサイズの化学強化ガラスを用いるときは、化学強化ガラス内に25mm×25mmの領域を表示し、その領域内で上記のCTL(t)測定を行う。
St=StF=1.515×StA
CT=CTF=1.28×CTA
ここで、CTFはFsmVにて解析される値CT_CVと等しい値であり、下記式(11)で求められるCT’とは異なるものである。
CS×DOL’=(t-2×DOL’)×CT’ (11)
ここで、DOL’はイオン交換層の深さに相当する。CT’を求める上記式は、応力プロファイルを線形で近似しており、また、応力がゼロとなる点をイオン拡散層深さと等しいと仮定している為、実際の内部引張応力よりも大きく見積もってしまうという問題があり、本実施形態における内部引張応力の指標としては不適である。
また、第2の態様の化学強化ガラスにおけるdh及びdMの好ましい数値範囲及びそれに付随する技術的効果は、第1の態様と同様である。
また、第2の態様の化学強化ガラスにおけるDOLの好ましい数値範囲及びそれに付随する技術的効果は、第1の態様と同様である。
さらに、第2の態様の化学強化ガラスにおけるSc及びStの好ましい数値範囲及びそれに付随する技術的効果は、第1の態様と同様である。
また、第2の態様の化学強化ガラスは、第1の態様の化学強化ガラスと同様に、板状以外の各種形状をとりうる。
つづいて、第3の態様に係る化学強化ガラスについて説明する。
下記条件での圧子圧入試験による破砕数が30個以下であり、
板厚tが0.4~2mmであり、
表面圧縮応力(CS)が300MPa以上であり、かつ、
圧縮応力層の深さ(DOL)が100μm以上である化学強化ガラスに関する。
硬質ナイロン製のモック板(50mm×50mm、重量:54g)に化学強化ガラス(50mm×50mm×板厚t(mm))をスポンジ両面テープ(50mm×50mm×厚み3mm)を介して貼り合わせ、測定試料を作製する。次に、15cm×15cmのサイズのSUS板上に、1gのけい砂(竹折社製5号けい砂)を均一となるようにまき、作製した測定試料を、化学強化ガラスを下にして、けい砂がまかれたSUS板の表面に所定の高さ(落下高さ)から落下させる。落下試験は、落下高さ:10mmから開始して、10mmずつ高さを上げて実施し、化学強化ガラスが割れた高さを割れ高さ(単位mm)とする。落下試験は各例について5回以上実施し、落下試験での割れ高さの平均値を、平均割れ高さ(単位:mm)とする。
25mm×25mm×板厚t(mm)の化学強化ガラスに対して、対面角の圧子角度60度を有するダイヤモンド圧子を用いて、3~10kgfの荷重を15秒間保持する圧子圧入試験により、化学強化ガラスを破壊させて、破壊後の化学強化ガラスの破砕数を計測する。25mm×25mmより大きなサイズの化学強化ガラスを用いるときは、化学強化ガラス内に25mm×25mmの領域を表示し、その領域内で圧子圧入試験および破砕数の計測を行う。化学強化ガラスが曲面形状を持つときは、投影面積で25mm×25mmのサイズを化学強化ガラスの曲面上に表示させ、その領域内で圧子圧入試験および破砕数の計測を行う。
また、第3の態様の化学強化ガラスにおけるdh及びdMの好ましい数値範囲及びそれに付随する技術的効果は、第1の態様と同様である。
さらに、第3の態様の化学強化ガラスにおけるSc及びStの好ましい数値範囲及びそれに付随する技術的効果も、第1の態様と同様である。
つづいて、本発明の化学強化用ガラスについて説明する。
化学強化ガラスの厚みが十分大きい場合には、化学強化ガラスの引張応力を有する部分(以下、引張応力部分ともいう)は、イオン交換されていない部分であるから、化学強化ガラスの引張応力部分は、化学強化前のガラスと同じ組成を有している。その場合は、化学強化ガラスの、引張応力部分の組成を化学強化ガラスの母組成とみなすことができる。
なお、各成分の含有量は、特に断りのない限り、酸化物基準のモル百分率表示で表すものとする。
たとえば、SiO2を63~80%、Al2O3を7~30%、B2O3を0~5%、P2O5を0~4%、Li2Oを5~15%、Na2Oを4~8%、K2Oを0~2%、MgOを3~10%、CaOを0~5%、SrOを0~20%、BaOを0~15%、ZnOを0~10%、TiO2を0~1%、ZrO2を0~8%を含有し、Ta2O5、Gd2O3、As2O3、Sb2O3を含有しないガラスが挙げられる。
本化学強化用ガラスは、X=SiO2×329+Al2O3×786+B2O3×627+P2O5×(-941)+Li2O×927+Na2O×47.5+K2O×(-371)+MgO×1230+CaO×1154+SrO×733+ZrO2×51.8に基づき算出されるXの値が30000以上であることが好ましい。
また、Z=SiO2×237+Al2O3×524+B2O3×228+P2O5×(-756)+Li2O×538+Na2O×44.2+K2O×(-387)+MgO×660+CaO×569+SrO×291+ZrO2×510に基づき算出されるZの値が20000以上であることが好ましい。
ガラス表面のLiイオンをNaイオンに交換し、上記CS90が30MPa以上になるような化学強化処理を行う場合、Li2Oの含有量は、好ましくは3%以上であり、より好ましくは4%以上、さらに好ましくは5%以上、特に好ましくは6%以上、典型的には7%以上である。一方、Li2Oの含有量が20%超ではガラスの耐酸性が著しく低下する。Li2Oの含有量は、20%以下であることが好ましく、より好ましくは18%以下、さらに好ましくは16%以下、特に好ましくは15%以下、最も好ましくは13%以下である。
一方、ガラス表面のNaイオンをKイオンに交換し、上記CS90が30MPa以上になるような化学強化処理を行う場合、Li2Oの含有量が3%超であると、圧縮応力の大きさが低下し、CS90が30MPa以上を達成することが難しくなる。この場合、Li2Oの含有量は、3%以下であることが好ましく、より好ましくは2%以下、さらに好ましくは1%以下、特に好ましくは0.5%以下であり、最も好ましくはLi2Oを実質的に含有しない。
なお、本明細書において「実質的に含有しない」とは、原材料等に含まれる不可避の不純物を除いて含有しない、すなわち、意図的に含有させたものではないことを意味する。具体的には、ガラス組成中の含有量が、0.1モル%未満であることを指す。
ガラス表面のLiイオンをNaイオンに交換し、上記CS90が30MPa以上になるような化学強化処理を行う場合、Na2Oは含有しなくてもよいが、ガラスの溶融性を重視する場合は含有してもよい。Na2Oを含有させる場合の含有量は1%以上であると好ましい。Na2Oの含有量は、より好ましくは2%以上、さらに好ましくは3%以上である。一方、Na2Oの含有量が8%超ではイオン交換により形成される表面圧縮応力が著しく低下する。Na2Oの含有量は、好ましくは8%以下であり、より好ましくは7%以下、さらに好ましくは6%以下、特に好ましくは5%以下、最も好ましくは4%以下である。
一方、ガラス表面のNaイオンをKイオンに交換し、上記CS90が30MPa以上になるような化学強化処理を行う場合にはNaは必須であり、その含有量は5%以上である。Na2Oの含有量は、好ましくは5%以上であり、より好ましくは7%以上、さらに好ましくは9%以上、特に好ましくは11%以上、最も好ましくは12%以上である。一方、Na2Oの含有量が20%超ではガラスの耐酸性が著しく低下する。Na2Oの含有量は、好ましくは20%以下であり、より好ましくは18%以下、さらに好ましくは16%以下、特に好ましくは15%以下、最も好ましくは14%以下である。
硝酸カリウムと硝酸ナトリウムの混合溶融塩に浸漬する等の方法により、ガラス表面のLiイオンとNaイオン、NaイオンとKイオンを同時にイオン交換する場合には、Na2Oの含有量は、好ましくは10%以下であり、より好ましくは9%以下、さらに好ましくは7%以下、特に好ましくは6%以下、最も好ましくは5%以下である。また、Na2Oの含有量は、好ましくは2%以上、より好ましくは3%以上、さらに好ましくは4%以上である。
Ta2O5、Gd2O3は、化学強化ガラスの破砕性を改善するために少量含有してもよいが、屈折率や反射率が高くなるので1%以下が好ましく、0.5%以下がより好ましく、含有しないことがさらに好ましい。
表1~9に示される例S-1~S-13、S-15~S-29及びS-31~S-53の各化学強化ガラスと、例S-14及びS-30のガラスを、以下のようにして作製した。
なお、例S-14及びS-30のガラスについては、化学強化処理は行わなかった。
また、いくつかの例については、Sc値(単位:MPa・μm)、ΔCS100-90(単位:MPa/μm)、CSDOL-20(単位:MPa)、ΔCSDOL-20(単位:MPa/μm)をあわせて示す。
化学強化前のガラスを粉砕し、4mmメッシュと2mmメッシュの篩を用いて分級し、純水で洗浄した後、乾燥してカレットを得た。2~5gのカレットを白金皿に載せて一定温度に保った電気炉中で17時間保持し、室温の大気中に取り出して冷却した後、偏光顕微鏡で失透の有無を観察する操作を繰り返して、失透温度Tを見積もった。その結果を表1中に示す。ここで、失透温度TがT1~T2の記載は、T1で失透有、T2で失透なしを意味する。
化学強化前のガラスについて、回転粘度計(ASTM C 965-96に準ずる)により粘度が104dPa・sとなる温度T4を測定した。結果を表中に示す。なお、*を付している数値は、計算値である。
つづいて、例S-1~S-13、S-15~S-29及びS-31~S-45の各化学強化ガラス及び例S-14、S-30のガラスについて、以下の試験方法により砂上落下試験を行い、平均割れ高さ(単位:mm)を測定した。
まず、硬質ナイロン製のモック板11(50mm×50mm×厚み18mm、重量:54g)にガラス13(50mm×50mm×板厚t(mm))をスポンジ両面テープ12(積水化学社製の#2310、50mm×50mm×厚み3mm)を介して貼り合わせ、測定試料1(総重量:61g)を作製した。次に、15cm×15cmのサイズのSUS板21上に、1gのけい砂22(竹折社製5号けい砂)を均一となるようにまき、作製した測定試料1を、ガラス13を下にして、けい砂22がまかれたSUS板21の表面に所定の高さ(落下高さ)から落下させた。落下試験は、落下高さ:10mmから開始して、10mmずつ高さを上げて実施し、ガラス13が割れた高さを割れ高さ(単位mm)とした。落下試験は各例について5~10回実施し、落下試験での割れ高さの平均値を、平均割れ高さ(単位:mm)とした。これらの結果を表中に示す。
図5に、例S-1~S-35の化学強化ガラスまたはガラスのCT(単位:MPa)と平均割れ高さ(単位:mm)との関係をプロットしたグラフを示す。
また、図6に例S-1~S-35の化学強化ガラスのうち、DOLが50μm未満の例について、ガラスのCT(単位:MPa)と平均割れ高さ(単位:mm)との関係をプロットしたグラフを示す。
図7に、例S-1~S-35の化学強化ガラスまたはガラスの、表面圧縮応力値CS(単位:MPa)と平均割れ高さ(単位:mm)との関係をプロットしたグラフを示す。また、図8に、例S-1~S-35の化学強化ガラスまたはガラスの、ガラス表面から90μmの深さの部分の圧縮応力値CS90(単位:MPa)と平均割れ高さ(単位:mm)との関係をプロットしたグラフを示す。さらに、図9に、例S-1~S-35の化学強化ガラスまたはガラスの、ガラス表面から100μmの深さの部分の圧縮応力値CS100(単位:MPa)、と平均割れ高さ(単位:mm)との関係をプロットしたグラフを示す。
図10に、例S-1~S-35の化学強化ガラスまたはガラスの、ガラス表面から100μmの深さの部分の圧縮応力値CS100(単位:MPa)と板厚t(mm)の二乗との積(CS100×t2)(単位:MPa・mm2)と平均割れ高さ(単位:mm)との関係をプロットしたグラフを示す。
図7~9より、平均割れ高さはCSとの相関性が小さく、内部の圧縮応力CS90、CS100との相関性が高いことが分かる。CS90、CS100がそれぞれ、30MPa、20MPaを超えると平均割れ高さが300mm程度以上となり、大幅な強度向上を達成できることが分かる。
図10より、平均割れ高さはCS100×t2との相関性が高いことが分かる。CS100×t2が5MPa・mm2を超えると平均割れ高さが300mm程度以上となり、大幅な強度向上を達成できることが分かる。
25mm×25mm×板厚t(mm)のサイズを有する例S-19及び例S-36~S-53の化学強化ガラスに対して、対面角の圧子角度60度を有するダイヤモンド圧子を用いて、3~10kgfの荷重を15秒間保持する圧子圧入試験により、化学強化ガラスを破壊させて、破壊後の化学強化ガラスの破砕数を計測した。これらの結果を表4及び表7~9に示す。
例S-1と同じガラス組成を有し、厚み1.1~1.3mmのガラス板を、例S-1と同じ条件でフロート法により作製した。得られた板ガラスを切断、研削し、最後に両面鏡面に加工して、縦5mm×横40mm×厚み1.0mmの板状ガラスを得た。その後、表10の例4PB-1~4PB-6の欄に示される各化学強化条件で化学強化処理を行って例4PB-1~4PB-6の各化学強化ガラスを作製した。
また、例S-7と同じガラス組成を有するガラスブロックを、例S-7と同じ条件で白金るつぼ溶融により作製した。得られたガラスブロックを切断、研削し、最後に両面を鏡面に加工して、縦5mm×横40mm×厚み0.8mmの板状ガラスを得た。その後、下記表10の例4PB-7~4PB-9の欄に示される各化学強化条件で化学強化処理を行って、例4PB-7~4PB-9の各化学強化ガラスを作製した。
なお、表10中の強化温度(単位:℃)とは、化学強化処理の際の溶融塩の温度である。また、塩濃度とは、化学強化処理の際に使用した溶融塩中の重量基準でのKNO3の割合=(KNO3/KNO3+Na2O)×100(単位:%)を示す。また、強化時間とは、溶融塩中へのガラスの浸漬時間(単位:時間)を表す。
F(x)=α+ERFC(β×x)-CT
なお、xはガラス表面からの深さ、関数ERFC(c)は相補誤差関数である。定数α、βの値は表12に示してある。
ガラス組成と化学強化ガラスの破砕性の関係を評価するため、種々の化学強化条件により種々のSt値をもった化学強化ガラスを作製し、破壊時の破砕数とSt値の関係を調査した。具体的には、25mm×25mm×厚みt(mm)のガラスに対して、内部引張応力面積(St;単位MPa・μm)が変化するように種々の化学強化処理条件で化学強化処理を行って、種々の内部引張応力面積(St;単位MPa・μm)を有する化学強化ガラスを作製した。そして、破砕数が10個となった内部引張応力面積(St;単位MPa・μm)を、St Limit値、また、破砕数が10個となった内部引張り応力CT(単位:MPa)を、CT Limit値、と規定した。破砕数が10個をまたぐ場合、10個未満となる最大破砕数n個のSt値であるStn値と、10個超となる最小破砕数m個のSt値であるStm値を用いて、下式によってSt Limit値を規定した。
StLimit値 = Stn+(10-n)×(Stm-Stn)/(m-n)
また、破砕数が10個をまたぐ場合、10個未満となる最大破砕数n個のCT値であるCTn値と、10個超となる最小破砕数m個のCT値であるCTm値を用いて、下式によってCT Limit値を規定した。
CTLimit値 = CTn+(10-n)×(CTm-CTn)/(m-n)
St=StF=1.515×StA
CT=CTF=1.28×CTA
ここで、CTFはFsmVにて解析される値CT_CVと等しい値である。
なお、ヤング率Eは、超音波パルス法(JIS R1602)により測定した。
また、破壊靭性値は、M.Y. He, M.R. Turner and A.G. Evans, Acta Metall. Mater. 43 (1995) 3453.に記載の方法を参考に、DCDC法により、図16に示される形状のサンプルおよびオリエンテック社製のテンシロンUTA-5kNを用いて、図17に示されるような、応力拡大係数K1(単位:MPa・m1/2)とクラック進展速度v(単位:m/s)との関係を示すK1-v曲線を測定し、得られたRegionIIIのデータを一次式で回帰、外挿し、0.1m/sの応力拡大係数K1を破壊靭性値K1cとした。
X=SiO2×329+Al2O3×786+B2O3×627+P2O5×(-941)+Li2O×927+Na2O×47.5+K2O×(-371)+MgO×1230+CaO×1154+SrO×733+ZrO2×51.8
Y=SiO2×0.00884+Al2O3×0.0120+B2O3×(-0.00373)+P2O5×0.000681+Li2O×0.00735+Na2O×(-0.00234)+K2O×(-0.00608)+MgO×0.0105+CaO×0.00789+SrO×0.00752+BaO×0.00472+ZrO2×0.0202
Z=SiO2×237+Al2O3×524+B2O3×228+P2O5×(-756)+Li2O×538+Na2O×44.2+K2O×(-387)+MgO×660+CaO×569+SrO×291+ZrO2×510
線膨張係数αおよびガラス転移点Tg測定はJISR3102『ガラスの平均線膨張係数の試験方法』の方法に準じて測定した。
ヤング率Eおよび合成率Gおよびポアソン比測定は超音波パルス法(JIS R1602)により測定した。
また、例2-1~2-53についてX値、Y値及びZ値を示す。
また、上記同様に、失透温度Tを見積もるとともに、粘度が104dPa・sとなる温度T4を測定した。
これらの結果を表16~20に示す。
例2-1、2-3~2-50、2-52についてはZ値が20000以上であり、より大きなCS、DOLを導入したときにおいても、ガラスの破壊時の破砕数が十分に少ないより安全性の高いガラスが実現できる例である。一方、例2-2、例2-51においてはZ値が20000以下である。
ガラス板厚と化学強化ガラスの破砕性の関係を評価するため、種々の組成および化学強化条件により種々のSt値、CT値をもった化学強化ガラスを作製し、破壊時の板厚、破砕数、St値およびCT値の関係を調査した。具体的には、25mm×25mm×厚みt(mm)のガラスに対して、内部引張応力面積(St;単位MPa・μm)、もしくは内部引張り応力CT(単位:MPa)が変化するように種々の化学強化処理条件で化学強化処理を行って、種々の内部引張応力面積(St;単位MPa・μm)もしくは内部引っ張り応力CT(単位:MPa)を有する化学強化ガラスを作製した。そして、対面角の圧子角度60度を有するダイヤモンド圧子を用いて、3kgfの荷重を15秒間保持する圧子圧入試験により、これら化学強化ガラスをそれぞれ破壊させて、破壊後のガラスの破片の数(破砕数)をそれぞれ計測した。そして、破砕数が10個となった内部引張応力面積(St;単位MPa・μm)を、St Limit値、また、破砕数が10個となった内部引張り応力CT(単位:MPa)を、CT Limit値、と規定した。破砕数が10個をまたぐ場合、10個未満となる最大破砕数n個のSt値であるStn値と、10個超となる最小破砕数m個のSt値であるStm値を用いて、下式によってSt Limit値を規定した。
StLimit値 = Stn+(10-n)×(Stm-Stn)/(m-n)
また、破砕数が10個をまたぐ場合、10個未満となる最大破砕数n個のCT値であるCTn値と、10個超となる最小破砕数m個のCT値であるCTm値を用いて、下式によってCT Limit値を規定した。
CTLimit値 = CTn+(10-n)×(CTm-CTn)/(m-n)
St=StF=1.515×StA
CT=CTF=1.28×CTA
ここで、CTFはFsmVにて解析される値CT_CVと等しい値である。
St(a,t)= a×t+7000 (単位:MPa・μm)
CT(b、c、t)= -b×ln(t)+c (単位:MPa)
なお、本出願は、2016年1月21日付けで出願された日本特許出願(特願2016-010002)及び2016年10月18日付けで出願された日本特許出願(特願2016-204745)に基づいており、その全体が引用により援用される。
11 モック板
12 スポンジ両面テープ
13 ガラス
21 SUS板
22 けい砂
Claims (24)
- 表面圧縮応力(CS)が300MPa以上の化学強化ガラスであって、
ガラス表面から90μmの深さの部分の圧縮応力値(CS90)が25MPa以上、又は、ガラス表面から100μmの深さの部分の圧縮応力値(CS100)が15MPa以上であり、
前記化学強化ガラスの母組成におけるSiO2、Al2O3、B2O3、P2O5、Li2O、Na2O、K2O、MgO、CaO、SrO、BaO及びZrO2の各成分の酸化物基準のモル百分率表示による含有量を用いて、下記式に基づき算出されるXの値が30000以上である化学強化ガラス。
X=SiO2×329+Al2O3×786+B2O3×627+P2O5×(-941)+Li2O×927+Na2O×47.5+K2O×(-371)+MgO×1230+CaO×1154+SrO×733+ZrO2×51.8 - 表面圧縮応力(CS)が300MPa以上の化学強化ガラスであって、
ガラス表面から90μmの深さの部分の圧縮応力値(CS90)が25MPa以上、又は、ガラス表面から100μmの深さの部分の圧縮応力値(CS100)が15MPa以上であり、
前記化学強化ガラスの母組成におけるSiO2、Al2O3、B2O3、P2O5、Li2O、Na2O、K2O、MgO、CaO、SrO、BaO及びZrO2の各成分の酸化物基準のモル百分率表示による含有量を用いて、下記式に基づき算出されるZの値が20000以上である化学強化ガラス。
Z=SiO2×237+Al2O3×524+B2O3×228+P2O5×(-756)+Li2O×538+Na2O×44.2+K2O×(-387)+MgO×660+CaO×569+SrO×291+ZrO2×510 - 板厚tが2mm以下の板状である請求項1または2のいずれかに記載の化学強化ガラス。
- 表面圧縮応力(CS)が300MPa以上であり、かつ、
下記式(1)及び(2)を満たす化学強化ガラス。
StL(t)≧a×t+7000(単位:MPa・μm) (1)
a≧30000 (単位:MPa・μm/mm)(2)
(ここで、tは板厚(mm)であり、StL(t)は板厚tのときのSt Limitの値である。) - a≧35000である請求項4に記載の化学強化ガラス。
- 表面圧縮応力(CS)が300MPa以上であり、かつ、
下記式(3)、(4)及び(5)を満たす化学強化ガラス。
CTL(t)≧-b×ln(t)+c (単位:MPa) (3)
b≧14 (単位:MPa) (4)
c≧48.4 (単位:MPa) (5)
(ここで、tは板厚(mm)であり、CTL(t)は板厚tのときのCT Limitの値である。) - 前記板厚tが2mm以下の板状である請求項4~6のいずれか1項に記載の化学強化ガラス。
- ガラス表面から90μmの深さの部分の圧縮応力値(CS90)が25MPa以上、又は、ガラス表面から100μmの深さの部分の圧縮応力値(CS100)が15MPa以上である請求項4~7のいずれか1項に記載の化学強化ガラス。
- 下記条件での砂上落下試験による平均割れ高さが250mm以上であり、
下記条件での圧子圧入試験による破砕数が30個以下であり、
板厚tが0.4~2mmであり、
表面圧縮応力(CS)が300MPa以上であり、かつ、
圧縮応力層の深さ(DOL)が100μm以上である化学強化ガラス。
砂上落下試験条件:
硬質ナイロン製のモック板(50mm×50mm、重量:54g)に化学強化ガラス(50mm×50mm×板厚t(mm))をスポンジ両面テープ(50mm×50mm×厚み3mm)を介して貼り合わせ、測定試料を作製する。次に、15cm×15cmのサイズのSUS板上に、1gのけい砂(竹折社製5号けい砂)を均一となるようにまき、作製した測定試料を、化学強化ガラスを下にして、けい砂がまかれたSUS板の表面に所定の高さ(落下高さ)から落下させる。落下試験は、落下高さ:10mmから開始して、10mmずつ高さを上げて実施し、化学強化ガラスが割れた高さを割れ高さ(単位mm)とする。落下試験は各例について5回以上実施し、落下試験での割れ高さの平均値を、平均割れ高さ(単位:mm)とする。
圧子圧入試験条件:
25mm×25mm×板厚t(mm)の化学強化ガラスに対して、対面角の圧子角度60度を有するダイヤモンド圧子を用いて、3~10kgfの荷重を15秒間保持する圧子圧入試験により、化学強化ガラスを破壊させて、破壊後の化学強化ガラスの破砕数を計測する。25mm×25mmより大きなサイズの化学強化ガラスを用いるときは、化学強化ガラス内に25mm×25mmの領域を表示し、その領域内で圧子圧入試験および破砕数の計測を行う。化学強化ガラスが曲面形状を持つときは、投影面積で25mm×25mmのサイズを化学強化ガラスの曲面上に表示させ、その領域内で圧子圧入試験および破砕数の計測を行う。 - ガラス表面から100μmの深さの部分の圧縮応力値と板厚t(mm)の二乗との積(CS100×t2)が5MPa・mm2以上である請求項1~9のいずれか1項に記載の化学強化ガラス。
- 圧縮応力層の面積Sc(MPa・μm)が30000MPa・μm以上である請求項1~10のいずれか1項に記載の化学強化ガラス。
- 内部の圧縮応力の大きさが表面圧縮応力(CS)の2分の1になる部分の深さdhが8μm以上である請求項1~11のいずれか1項に記載の化学強化ガラス。
- 圧縮応力が最大となる位置dMがガラス表面から5μmの範囲にある請求項1~12のいずれか1項に記載の化学強化ガラス。
- 圧縮応力層の深さ(DOL)が110μm以上である請求項1~13のいずれか1項に記載の化学強化ガラス。
- 前記化学強化ガラスの母組成を有するガラスの破壊靱性値(K1c)が0.7MPa・m1/2以上である請求項1~14のいずれか1項に記載の化学強化ガラス。
- 内部引張層の面積St(MPa・μm)が、StL(t)(MPa・μm)以下である請求項1~15のいずれか1項に記載の化学強化ガラス。
(ここで、tは板厚(mm)であり、StL(t)は板厚tのときのSt Limitの値である。) - 内部引張層応力CT(MPa)が、CTL(t)(MPa)以下である請求項1~16のいずれか1項に記載の化学強化ガラス。
(ここで、tは板厚(mm)であり、CTL(t)は板厚tのときのCT Limitの値である。) - 前記化学強化ガラスの母組成が、酸化物基準のモル百分率表示で、SiO2を50~80%、Al2O3を1~30%、B2O3を0~5%、P2O5を0~4%、Li2Oを0~20%、Na2Oを0~8%、K2Oを0~10%、MgOを3~20%、CaOを0~20%、SrOを0~20%、BaOを0~15%、ZnOを0~10%、TiO2を0~1%、ZrO2を0~8%を含有する請求項1~17のいずれか1項に記載の化学強化ガラス。
- 酸化物基準のモル百分率表示で、SiO2を63~80%、Al2O3を7~30%、B2O3を0~5%、P2O5を0~4%、Li2Oを5~15%、Na2Oを4~8%、K2Oを0~2%、MgOを3~10%、CaOを0~5%、SrOを0~20%、BaOを0~15%、ZnOを0~10%、TiO2を0~1%、ZrO2を0~8%を含有し、
Ta2O5、Gd2O3、As2O3、Sb2O3を含有せず、
SiO2、Al2O3、B2O3、P2O5、Li2O、Na2O、K2O、MgO、CaO、SrO、BaO及びZrO2の各成分の酸化物基準のモル百分率表示による含有量を用いて、下記式に基づき算出されるXの値が30000以上である化学強化用ガラス。
X=SiO2×329+Al2O3×786+B2O3×627+P2O5×(-941)+Li2O×927+Na2O×47.5+K2O×(-371)+MgO×1230+CaO×1154+SrO×733+ZrO2×51.8 - 酸化物基準のモル百分率表示によるZrO2の含有量が1.2%以下である請求項19に記載の化学強化用ガラス。
- 酸化物基準のモル百分率表示によるK2Oの含有量が0.5%以上である請求項19または20に記載の化学強化用ガラス。
- 酸化物基準のモル百分率表示によるB2O3の含有量が1%以下である請求項19~21のいずれか1項に記載の化学強化用ガラス。
- 酸化物基準のモル百分率表示によるAl2O3の含有量が11%以下である請求項19~22のいずれか1項に記載の化学強化用ガラス。
- 失透温度Tが、粘度が104dPa・sとなる温度T4以下である、請求項19~23のいずれか1項に記載の化学強化用ガラス。
Priority Applications (29)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202211233035.0A CN115572077A (zh) | 2016-01-21 | 2017-01-19 | 化学强化玻璃以及化学强化用玻璃 |
| KR1020207026654A KR102292273B1 (ko) | 2016-01-21 | 2017-01-19 | 화학 강화 유리 및 화학 강화용 유리 |
| CN202211232239.2A CN115572076A (zh) | 2016-01-21 | 2017-01-19 | 化学强化玻璃以及化学强化用玻璃 |
| KR1020197015480A KR102161536B1 (ko) | 2016-01-21 | 2017-01-19 | 화학 강화 유리 및 화학 강화용 유리 |
| KR1020207015390A KR102126835B1 (ko) | 2016-01-21 | 2017-01-19 | 화학 강화 유리 및 화학 강화용 유리 |
| CN202211233164.XA CN115650602B (zh) | 2016-01-21 | 2017-01-19 | 化学强化玻璃以及化学强化用玻璃 |
| CN202211232234.XA CN115650601B (zh) | 2016-01-21 | 2017-01-19 | 化学强化玻璃以及化学强化用玻璃 |
| CN202111534127.8A CN114349368B (zh) | 2016-01-21 | 2017-01-19 | 化学强化玻璃以及化学强化用玻璃 |
| CN202110986861.1A CN113754276B (zh) | 2016-01-21 | 2017-01-19 | 化学强化玻璃以及化学强化用玻璃 |
| KR1020197017823A KR102073143B1 (ko) | 2016-01-21 | 2017-01-19 | 화학 강화 유리 및 화학 강화용 유리 |
| KR1020197008706A KR102027956B1 (ko) | 2016-01-21 | 2017-01-19 | 화학 강화 유리 및 화학 강화용 유리 |
| DE112017000454.3T DE112017000454T5 (de) | 2016-01-21 | 2017-01-19 | Chemisch gehärtetes Glas und Glas zum chemischen Härten |
| CN202011142412.0A CN113024108B (zh) | 2016-01-21 | 2017-01-19 | 化学强化玻璃以及化学强化用玻璃 |
| CN202111532739.3A CN114315133B (zh) | 2016-01-21 | 2017-01-19 | 化学强化玻璃以及化学强化用玻璃 |
| KR1020187030257A KR102121414B1 (ko) | 2016-01-21 | 2017-01-19 | 화학 강화 유리 및 화학 강화용 유리 |
| KR1020217025788A KR102569434B1 (ko) | 2016-01-21 | 2017-01-19 | 화학 강화 유리 및 화학 강화용 유리 |
| CN201780007591.7A CN108473370B (zh) | 2016-01-21 | 2017-01-19 | 化学强化玻璃以及化学强化用玻璃 |
| KR1020177016471A KR101927014B1 (ko) | 2016-01-21 | 2017-01-19 | 화학 강화 유리 및 화학 강화용 유리 |
| KR1020197035146A KR102074835B1 (ko) | 2016-01-21 | 2017-01-19 | 화학 강화 유리 및 화학 강화용 유리 |
| CN201910486194.3A CN110255892B (zh) | 2016-01-21 | 2017-01-19 | 化学强化玻璃以及化学强化用玻璃 |
| JP2017562079A JP6327407B2 (ja) | 2016-01-21 | 2017-01-19 | 化学強化ガラスおよび化学強化用ガラス |
| CN202211232235.4A CN115677235A (zh) | 2016-01-21 | 2017-01-19 | 化学强化玻璃以及化学强化用玻璃 |
| KR1020237027967A KR102630405B1 (ko) | 2016-01-21 | 2017-01-19 | 화학 강화 유리 및 화학 강화용 유리 |
| KR1020187014737A KR102024126B1 (ko) | 2016-01-21 | 2017-01-19 | 화학 강화 유리 및 화학 강화용 유리 |
| US15/908,227 US10384974B2 (en) | 2016-01-21 | 2018-02-28 | Chemically strengthened glass, and glass for chemical strengthening |
| US15/920,009 US10370287B2 (en) | 2016-01-21 | 2018-03-13 | Chemically strengthened glass, and glass for chemical strengthening |
| US16/033,787 US10472272B2 (en) | 2016-01-21 | 2018-07-12 | Chemically strengthened glass, and glass for chemical strengthening |
| US16/291,407 US20190194057A1 (en) | 2016-01-21 | 2019-03-04 | Chemically strengthened glass, and glass for chemical strengthening |
| US17/024,109 US11767252B2 (en) | 2016-01-21 | 2020-09-17 | Chemically strengthened glass, and glass for chemical strengthening |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016-010002 | 2016-01-21 | ||
| JP2016010002 | 2016-01-21 | ||
| JP2016-204745 | 2016-10-18 | ||
| JP2016204745 | 2016-10-18 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US15/908,227 Continuation US10384974B2 (en) | 2016-01-21 | 2018-02-28 | Chemically strengthened glass, and glass for chemical strengthening |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017126607A1 true WO2017126607A1 (ja) | 2017-07-27 |
Family
ID=59362484
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2017/001755 WO2017126607A1 (ja) | 2016-01-21 | 2017-01-19 | 化学強化ガラスおよび化学強化用ガラス |
Country Status (7)
| Country | Link |
|---|---|
| US (5) | US10384974B2 (ja) |
| JP (8) | JP6327407B2 (ja) |
| KR (11) | KR102126835B1 (ja) |
| CN (16) | CN114349368B (ja) |
| DE (3) | DE202017007276U1 (ja) |
| TW (7) | TWI644879B (ja) |
| WO (1) | WO2017126607A1 (ja) |
Cited By (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018074335A1 (ja) * | 2016-10-18 | 2018-04-26 | 旭硝子株式会社 | 化学強化用ガラス、化学強化ガラスおよび化学強化ガラスの製造方法 |
| CN108147657A (zh) * | 2017-12-29 | 2018-06-12 | 深圳市东丽华科技有限公司 | 一种素玻璃、强化玻璃及制备方法 |
| JP2018104285A (ja) * | 2016-01-21 | 2018-07-05 | 旭硝子株式会社 | 化学強化ガラスおよび化学強化用ガラス |
| WO2019069567A1 (ja) * | 2017-10-02 | 2019-04-11 | 日本電気硝子株式会社 | 強化ガラス板及び強化ガラス板付デバイス |
| WO2019080190A1 (zh) * | 2017-10-26 | 2019-05-02 | 中国南玻集团股份有限公司 | 铝硅酸盐玻璃及其制备方法、电子设备 |
| JP2019116417A (ja) * | 2017-12-26 | 2019-07-18 | 日本電気硝子株式会社 | カバーガラス |
| JP2019147727A (ja) * | 2018-02-28 | 2019-09-05 | 日本電気硝子株式会社 | 強化ガラス及び強化用ガラス |
| WO2019194110A1 (ja) * | 2018-04-04 | 2019-10-10 | Agc株式会社 | 化学強化用ガラス |
| JPWO2018159386A1 (ja) * | 2017-02-28 | 2020-01-16 | 日本電気硝子株式会社 | アルミノケイ酸塩ガラス |
| WO2020075708A1 (ja) * | 2018-10-09 | 2020-04-16 | 日本電気硝子株式会社 | 強化ガラスおよび強化ガラスの製造方法 |
| CN111099823A (zh) * | 2018-10-25 | 2020-05-05 | 深圳市东丽华科技有限公司 | 素玻璃及玻璃器件 |
| WO2020121889A1 (ja) * | 2018-12-11 | 2020-06-18 | Agc株式会社 | ガラス、化学強化ガラスおよびそれを含む電子機器 |
| JPWO2019131528A1 (ja) * | 2017-12-26 | 2020-11-19 | 日本電気硝子株式会社 | カバーガラス |
| JP2020200238A (ja) * | 2017-09-04 | 2020-12-17 | ショット アクチエンゲゼルシャフトSchott AG | 可撓性および/または折畳み可能な物品ならびに可撓性および/または折畳み可能な物品の手配方法 |
| JP2021031317A (ja) * | 2019-08-20 | 2021-03-01 | Agc株式会社 | リチウムアルミノシリケートガラスの製造方法、およびフロートガラス板 |
| JP2021075426A (ja) * | 2019-11-11 | 2021-05-20 | 石塚硝子株式会社 | 化学強化用アルミノボロシケートガラス及び化学強化ガラス品 |
| US11365149B2 (en) | 2016-01-21 | 2022-06-21 | AGC Inc. | Chemically strengthened glass and method for manufacturing chemically strengthened glass |
| US11401198B2 (en) * | 2016-12-30 | 2022-08-02 | Tunghsu Group Co., Ltd. | Silicate product and strengthening method thereof |
| JP2022177042A (ja) * | 2018-02-28 | 2022-11-30 | 日本電気硝子株式会社 | 強化ガラス及び強化用ガラス |
| US11621480B2 (en) | 2020-06-12 | 2023-04-04 | AGC Inc. | Protective member and communication terminal device including the same |
| WO2023243574A1 (ja) * | 2022-06-15 | 2023-12-21 | Agc株式会社 | 化学強化用ガラス及びガラス |
| CN117865465A (zh) * | 2023-06-29 | 2024-04-12 | 重庆鑫景特种玻璃有限公司 | 一种着色化学强化玻璃及其制法和应用 |
| JP7537298B2 (ja) | 2021-02-10 | 2024-08-21 | Agc株式会社 | 強化ガラス板、および強化ガラス板の製造方法 |
| US12122709B2 (en) | 2018-10-09 | 2024-10-22 | Nippon Electric Glass Co., Ltd. | Reinforced glass and method for producing reinforced glass |
Families Citing this family (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TWI762083B (zh) * | 2015-09-17 | 2022-04-21 | 美商康寧公司 | 特性量測經離子交換之含鋰化學強化玻璃的方法 |
| US20230416144A1 (en) * | 2017-10-17 | 2023-12-28 | PGBC Intellectual Holdings, LLC | Chemically-strengthened thin glass substrates new paradigms for modified curvature and methods of manufacture |
| CN108129020B (zh) * | 2017-12-13 | 2019-06-07 | 东旭科技集团有限公司 | 一种玻璃用组合物、铝硅酸盐玻璃及其制备方法和应用 |
| KR102564323B1 (ko) | 2018-02-05 | 2023-08-08 | 에이지씨 가부시키가이샤 | 화학 강화용 유리 |
| EP3802451B1 (en) * | 2018-06-08 | 2024-02-14 | Corning Incorporated | Fracture resistant stress profiles in glasses |
| CN108585480B (zh) * | 2018-07-10 | 2021-05-04 | 科立视材料科技有限公司 | 一种二步法化学强化碱铝硅酸玻璃组合物及其制备方法 |
| US11130705B2 (en) * | 2018-09-11 | 2021-09-28 | Corning Incorporated | Glass-based articles with improved fracture resistance |
| CA3117986A1 (en) | 2018-11-26 | 2020-06-04 | Owens Corning Intellectual Capital, Llc | High performance fiberglass composition with improved specific modulus |
| KR20210096140A (ko) | 2018-11-26 | 2021-08-04 | 오웬스 코닝 인텔렉츄얼 캐피탈 엘엘씨 | 향상된 탄성 계수를 갖는 고성능 섬유 유리 조성물 |
| WO2020121888A1 (ja) * | 2018-12-11 | 2020-06-18 | Agc株式会社 | 化学強化ガラス板、並びに化学強化ガラスを含むカバーガラス及び電子機器 |
| KR102130995B1 (ko) * | 2018-12-27 | 2020-07-09 | (주)유티아이 | 광학 필터용 글라스 기판의 강도 개선 방법 및 이에 의한 강화 글라스 기반 광학 필터 |
| CN113302167B (zh) * | 2019-01-18 | 2023-08-22 | Agc株式会社 | 化学强化玻璃及其制造方法 |
| JP7242891B2 (ja) * | 2019-03-15 | 2023-03-20 | コーニング インコーポレイテッド | 化学的耐久性のアルミノケイ酸塩ガラス組成物およびそれから形成されたガラス物品 |
| CN111847872B (zh) * | 2019-04-30 | 2022-06-14 | 重庆鑫景特种玻璃有限公司 | 一种可用于化学强化的低介电常数的玻璃、强化玻璃 |
| CN110040982B (zh) * | 2019-05-14 | 2021-08-27 | 重庆鑫景特种玻璃有限公司 | 具有复合应力优势的化学强化玻璃及其制备方法与应用 |
| WO2020246274A1 (ja) * | 2019-06-03 | 2020-12-10 | Agc株式会社 | ガラス、化学強化ガラスおよびその製造方法 |
| CN114007994B (zh) * | 2019-06-26 | 2023-09-12 | Agc株式会社 | 化学强化玻璃的制造方法和化学强化玻璃 |
| WO2021041031A1 (en) | 2019-08-30 | 2021-03-04 | Corning Incorporated | Scratch resistant glass and method of making |
| KR102052687B1 (ko) * | 2019-09-02 | 2019-12-05 | 이준우 | 습식 이온 교환에 의한 방화유리 강화 방법 |
| KR102052688B1 (ko) * | 2019-09-02 | 2019-12-05 | 이준우 | 건식 이온 교환에 의한 방화유리 강화 방법 |
| RU2726812C1 (ru) * | 2019-09-25 | 2020-07-15 | Российская Федерация, От Имени Которой Выступает Министерство Промышленности И Торговли Российской Федерации | Стекло, упрочняемое ионным обменом |
| WO2021065562A1 (ja) | 2019-09-30 | 2021-04-08 | 国立研究開発法人産業技術総合研究所 | ガラスおよびその製造方法 |
| WO2021091761A1 (en) | 2019-11-04 | 2021-05-14 | Corning Incorporated | Stress profiles of highly frangible glasses |
| CN114901604A (zh) * | 2019-11-12 | 2022-08-12 | 康宁股份有限公司 | 高cte、高uv透射率和高杨氏模量玻璃 |
| KR20210077854A (ko) | 2019-12-17 | 2021-06-28 | 삼성디스플레이 주식회사 | 유리 제품 및 그 제조 방법 |
| KR20210127268A (ko) | 2020-04-13 | 2021-10-22 | 삼성디스플레이 주식회사 | 유리 제품 및 이를 포함하는 디스플레이 장치 |
| JP7484369B2 (ja) * | 2020-04-17 | 2024-05-16 | Agc株式会社 | アルミノシリケートガラス及びその製造方法 |
| CN113716850A (zh) * | 2020-05-12 | 2021-11-30 | 福耀玻璃工业集团股份有限公司 | 使超薄高铝盖板玻璃具有安全破碎行为的方法 |
| EP3909923A1 (en) | 2020-05-15 | 2021-11-17 | Corning Incorporated | Strengthened glass articles and methods of forming the same |
| US11827559B2 (en) | 2020-06-23 | 2023-11-28 | Corning Incorporated | Frangible glass articles and methods of making the same |
| CN115776973A (zh) * | 2020-07-10 | 2023-03-10 | Agc株式会社 | 玻璃和化学强化玻璃 |
| CN112592056A (zh) * | 2020-10-30 | 2021-04-02 | 重庆鑫景特种玻璃有限公司 | 具有低变化幅度的张应力区的安全强化玻璃及制法和应用 |
| CN112441740A (zh) * | 2020-12-22 | 2021-03-05 | 中国洛阳浮法玻璃集团有限责任公司 | 一种高透高强度超薄中铝玻璃的制备方法 |
| CN116023025B (zh) * | 2023-01-19 | 2024-06-28 | 清远南玻节能新材料有限公司 | 用于离子交换的铝硼硅酸盐玻璃及其制备方法和应用 |
| CN117865464A (zh) * | 2023-06-29 | 2024-04-12 | 重庆鑫景特种玻璃有限公司 | 一种着色化学强化玻璃及其制备方法和应用 |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2000516903A (ja) * | 1997-04-11 | 2000-12-19 | サン―ゴバン ビトラージュ | ガラス組成物及び化学強化されるガラスで製造された基材 |
| JP2001302278A (ja) * | 2000-02-17 | 2001-10-31 | Hoya Corp | 陰極線管用ガラス、陰極線管用ガラスパネル、及び陰極線管、並びにそれらの製造方法 |
| JP2002507538A (ja) * | 1998-03-20 | 2002-03-12 | ピルキントン パブリック リミテッド カンパニー | 化学的に強化した無ホウ素フロートガラス組成物 |
| JP2002174810A (ja) * | 2000-12-08 | 2002-06-21 | Hoya Corp | ディスプレイ用ガラス基板及びその製造方法並びにこれを用いたディスプレイ |
| JP2006083045A (ja) * | 2004-09-17 | 2006-03-30 | Hitachi Ltd | ガラス部材 |
| JP2012232882A (ja) * | 2011-04-18 | 2012-11-29 | Asahi Glass Co Ltd | 化学強化ガラスの製造方法および化学強化用ガラス |
| JP2013520388A (ja) * | 2010-02-26 | 2013-06-06 | ショット アクチエンゲゼルシャフト | 化学強化ガラス |
| JP2013536155A (ja) * | 2010-08-26 | 2013-09-19 | コーニング インコーポレイテッド | ガラスを強化する二段階法 |
| JP2013542159A (ja) * | 2010-09-13 | 2013-11-21 | サン−ゴバン グラス フランス | ガラス板 |
Family Cites Families (89)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR1596961A (ja) * | 1968-04-19 | 1970-06-22 | ||
| JPS50732A (ja) | 1973-05-02 | 1975-01-07 | ||
| US4156755A (en) | 1978-04-19 | 1979-05-29 | Ppg Industries, Inc. | Lithium containing ion exchange strengthened glass |
| JPS60180936A (ja) * | 1984-02-27 | 1985-09-14 | Nippon Electric Glass Co Ltd | 高強度耐熱ガラス製品の製造方法 |
| US4549894A (en) * | 1984-06-06 | 1985-10-29 | Corning Glass Works | Ultraviolet absorbing photochromic glass of low silver content |
| JPS61101434A (ja) * | 1984-10-23 | 1986-05-20 | Nippon Sheet Glass Co Ltd | 透明結晶化ガラス |
| US5674790A (en) * | 1995-12-15 | 1997-10-07 | Corning Incorporated | Strengthening glass by ion exchange |
| CN1207086A (zh) * | 1996-09-04 | 1999-02-03 | 保谷株式会社 | 信息记录介质基片用玻璃及玻璃基片 |
| JP3412804B2 (ja) | 1996-12-26 | 2003-06-03 | Hoya株式会社 | 情報記録媒体用基板 |
| US5972460A (en) | 1996-12-26 | 1999-10-26 | Hoya Corporation | Information recording medium |
| JP3959588B2 (ja) | 1999-05-13 | 2007-08-15 | 日本板硝子株式会社 | 情報記録媒体用ガラス基板、情報記録媒体用ガラス基板の製造方法及び情報記録媒体 |
| SG99350A1 (en) * | 2000-02-17 | 2003-10-27 | Hoya Corp | Glass for cathode-ray tube, strengthened glass, method for the production thereof and use thereof |
| JP3995902B2 (ja) * | 2001-05-31 | 2007-10-24 | Hoya株式会社 | 情報記録媒体用ガラス基板及びそれを用いた磁気情報記録媒体 |
| JP2004131314A (ja) | 2002-10-09 | 2004-04-30 | Asahi Glass Co Ltd | 透明導電膜付き化学強化ガラス基板、およびその製造方法 |
| US7727917B2 (en) | 2003-10-24 | 2010-06-01 | Schott Ag | Lithia-alumina-silica containing glass compositions and glasses suitable for chemical tempering and articles made using the chemically tempered glass |
| US7566673B2 (en) | 2003-10-31 | 2009-07-28 | Konica Minolta Opto, Inc. | Glass substrate for an information recording medium and information recording medium employing it |
| JP5146897B2 (ja) * | 2004-04-05 | 2013-02-20 | 日本電気硝子株式会社 | 照明用ガラス |
| DE102004022629B9 (de) * | 2004-05-07 | 2008-09-04 | Schott Ag | Gefloatetes Lithium-Aluminosilikat-Flachglas mit hoher Temperaturbeständigkeit, das chemisch und thermisch vorspannbar ist und dessen Verwendung |
| US20060006300A1 (en) * | 2004-07-09 | 2006-01-12 | Reason William B | Golf accessory attachment device |
| JP4218839B2 (ja) | 2005-05-18 | 2009-02-04 | Hoya株式会社 | 情報記録媒体用ガラス基板及びそれを用いた磁気情報記録媒体 |
| US8304078B2 (en) * | 2005-09-12 | 2012-11-06 | Saxon Glass Technologies, Inc. | Chemically strengthened lithium aluminosilicate glass having high strength effective to resist fracture upon flexing |
| JP2007099557A (ja) * | 2005-10-04 | 2007-04-19 | Nippon Electric Glass Co Ltd | 強化ガラス物品およびその製造方法 |
| JP5605736B2 (ja) | 2006-05-25 | 2014-10-15 | 日本電気硝子株式会社 | 強化ガラス及びその製造方法 |
| MY152135A (en) | 2006-06-08 | 2014-08-15 | Hoya Corp | Glass for use in substrate for information recording medium, substrate for information recording medium and information recording medium, and their manufacturing method |
| KR20090027259A (ko) * | 2006-10-10 | 2009-03-16 | 니폰 덴키 가라스 가부시키가이샤 | 강화 유리 기판 |
| CN101679105B (zh) * | 2007-06-07 | 2015-06-17 | 日本电气硝子株式会社 | 强化玻璃基板及其制造方法 |
| JP5743125B2 (ja) * | 2007-09-27 | 2015-07-01 | 日本電気硝子株式会社 | 強化ガラス及び強化ガラス基板 |
| CN101977860B (zh) * | 2008-03-19 | 2013-08-21 | Hoya株式会社 | 磁记录介质基板用玻璃、磁记录介质基板、磁记录介质和它们的制造方法 |
| CN103043900A (zh) * | 2008-08-08 | 2013-04-17 | 康宁股份有限公司 | 强化的玻璃制品及其制造方法 |
| JP5429684B2 (ja) * | 2008-11-11 | 2014-02-26 | 日本電気硝子株式会社 | 強化ガラス基板及びその製造方法 |
| JP5622069B2 (ja) | 2009-01-21 | 2014-11-12 | 日本電気硝子株式会社 | 強化ガラス、強化用ガラス及び強化ガラスの製造方法 |
| US8802581B2 (en) | 2009-08-21 | 2014-08-12 | Corning Incorporated | Zircon compatible glasses for down draw |
| JP2011105587A (ja) * | 2009-10-22 | 2011-06-02 | Nippon Sheet Glass Co Ltd | 鱗片状ガラス及びその製造方法 |
| JP2011111364A (ja) * | 2009-11-27 | 2011-06-09 | Ohara Inc | 陽極接合用ガラス |
| JP5483262B2 (ja) * | 2009-12-04 | 2014-05-07 | 日本電気硝子株式会社 | 合わせガラス |
| JP5110074B2 (ja) | 2009-12-28 | 2012-12-26 | 住友電気工業株式会社 | 結晶の製造方法および発光素子の製造方法 |
| DE102010009585B4 (de) | 2010-02-26 | 2012-04-19 | Schott Ag | Lithium-Aluminosilicatglas mit hohen E-Modul, Verfahren zu dessen Herstellung und Verwendung |
| CN102167507B (zh) | 2010-02-26 | 2016-03-16 | 肖特玻璃科技(苏州)有限公司 | 用于3d紧密模压的薄锂铝硅玻璃 |
| JP5051329B2 (ja) * | 2010-05-19 | 2012-10-17 | 旭硝子株式会社 | 化学強化用ガラスおよびディスプレイ装置用ガラス板 |
| US8759238B2 (en) * | 2010-05-27 | 2014-06-24 | Corning Incorporated | Ion exchangeable glasses |
| US8778820B2 (en) | 2010-05-27 | 2014-07-15 | Corning Incorporated | Glasses having low softening temperatures and high toughness |
| US9540278B2 (en) * | 2010-05-27 | 2017-01-10 | Corning Incorporated | Ion exchangeable glasses |
| JP2010202514A (ja) | 2010-06-10 | 2010-09-16 | Hoya Corp | 携帯型液晶ディスプレイ用のガラス基板及びその製造方法並びにこれを用いた携帯型液晶ディスプレイ |
| JP2012020921A (ja) * | 2010-06-18 | 2012-02-02 | Asahi Glass Co Ltd | ディスプレイ装置用のガラスおよびガラス板 |
| CN103097315B (zh) * | 2010-09-27 | 2015-10-14 | 旭硝子株式会社 | 化学强化用玻璃、化学强化玻璃及显示装置用玻璃板 |
| JP5720499B2 (ja) * | 2010-10-26 | 2015-05-20 | 旭硝子株式会社 | 基板用ガラスおよびガラス基板 |
| US9346703B2 (en) | 2010-11-30 | 2016-05-24 | Corning Incorporated | Ion exchangable glass with deep compressive layer and high damage threshold |
| JP5834793B2 (ja) * | 2010-12-24 | 2015-12-24 | 旭硝子株式会社 | 化学強化ガラスの製造方法 |
| JP5896338B2 (ja) * | 2011-01-18 | 2016-03-30 | 日本電気硝子株式会社 | 強化用ガラスの製造方法及び強化ガラス板の製造方法 |
| CN102690059B (zh) | 2011-03-23 | 2016-08-03 | 肖特玻璃科技(苏州)有限公司 | 用于化学钢化的铝硅酸盐玻璃和玻璃陶瓷 |
| CN102709723B (zh) * | 2011-03-28 | 2015-05-13 | 泰科电子(上海)有限公司 | Usb连接器 |
| EP2692706B1 (en) * | 2011-03-31 | 2016-03-30 | Nippon Sheet Glass Company, Limited | Glass composition suitable for chemical strengthening and chemically strengthened glass article |
| JP2012250861A (ja) * | 2011-05-31 | 2012-12-20 | Asahi Glass Co Ltd | 化学強化ガラス板 |
| JP5751036B2 (ja) * | 2011-06-09 | 2015-07-22 | 旭硝子株式会社 | 強化ガラス及びその製造方法、該強化ガラスの表面応力測定方法 |
| US9783452B2 (en) | 2011-07-01 | 2017-10-10 | Corning Incorporated | Ion-exchanged glass of high surface compression and shallow depth of layer with high resistance to radial crack formation from vickers indentation |
| TWI591039B (zh) | 2011-07-01 | 2017-07-11 | 康寧公司 | 具高壓縮應力的離子可交換玻璃 |
| JP5737043B2 (ja) * | 2011-07-29 | 2015-06-17 | 旭硝子株式会社 | 基板用ガラスおよびガラス基板 |
| US10280112B2 (en) | 2011-08-19 | 2019-05-07 | Corning Incorporated | Ion exchanged glass with high resistance to sharp contact failure and articles made therefrom |
| JP5724779B2 (ja) * | 2011-09-13 | 2015-05-27 | 旭硝子株式会社 | 化学強化ガラスの強度測定方法、化学強化ガラスの割れ再現方法及び化学強化ガラスの製造方法 |
| EP3342759B1 (en) | 2011-11-16 | 2021-08-25 | Corning Incorporated | Ion exchangeable glass with high crack initiation threshold |
| KR101930681B1 (ko) * | 2011-11-18 | 2018-12-18 | 에이지씨 가부시키가이샤 | 화학 강화용 유리 |
| CN103999140B (zh) * | 2011-12-16 | 2015-12-23 | 旭硝子株式会社 | 显示器用保护玻璃、显示器用保护玻璃的制造方法 |
| CN103249121A (zh) | 2012-02-13 | 2013-08-14 | 电信科学技术研究院 | 一种机器型通信终端的触发控制方法、装置及系统 |
| US9359251B2 (en) * | 2012-02-29 | 2016-06-07 | Corning Incorporated | Ion exchanged glasses via non-error function compressive stress profiles |
| CN102690057B (zh) * | 2012-04-10 | 2015-09-16 | 东旭集团有限公司 | 一种触摸屏盖板玻璃用的玻璃 |
| US9156725B2 (en) * | 2012-05-30 | 2015-10-13 | Corning Incorporated | Down-drawable chemically strengthened glass for information storage devices |
| JP6168288B2 (ja) * | 2012-06-13 | 2017-07-26 | 日本電気硝子株式会社 | 強化ガラス及び強化ガラス板 |
| US9139469B2 (en) | 2012-07-17 | 2015-09-22 | Corning Incorporated | Ion exchangeable Li-containing glass compositions for 3-D forming |
| KR102159763B1 (ko) * | 2012-08-17 | 2020-09-25 | 코닝 인코포레이티드 | 초-박형 강화 유리 |
| JPWO2014045809A1 (ja) * | 2012-09-20 | 2016-08-18 | 旭硝子株式会社 | 化学強化ガラスの製造方法 |
| US20140154661A1 (en) * | 2012-11-30 | 2014-06-05 | Corning Incorporated | Durable glass articles for use as writable erasable marker boards |
| WO2014166250A1 (en) * | 2013-04-10 | 2014-10-16 | Schott Glass Technologies (Suzhou) Co. Ltd. | Chemically toughened glass |
| CN105189385A (zh) * | 2013-04-25 | 2015-12-23 | 旭硝子株式会社 | 化学强化用玻璃板及其制造方法 |
| US11079309B2 (en) | 2013-07-26 | 2021-08-03 | Corning Incorporated | Strengthened glass articles having improved survivability |
| JP5668828B1 (ja) * | 2013-11-22 | 2015-02-12 | 旭硝子株式会社 | 化学強化ガラス板 |
| JP6135773B2 (ja) * | 2013-12-13 | 2017-05-31 | 旭硝子株式会社 | 化学強化用ガラスおよび化学強化ガラス並びに化学強化ガラスの製造方法 |
| DE102013114225B4 (de) * | 2013-12-17 | 2017-03-16 | Schott Ag | Chemisch vorspannbares Glas und daraus hergestelltes Glaselement |
| FR3015507B1 (fr) * | 2013-12-20 | 2016-12-30 | Seb Sa | Compositions aqueuses pour primaires de revetements antiadhesifs et leur procede de preparation |
| US9517968B2 (en) * | 2014-02-24 | 2016-12-13 | Corning Incorporated | Strengthened glass with deep depth of compression |
| EP3110331B1 (en) | 2014-02-26 | 2018-10-10 | Brainlab AG | Tracking soft tissue in medical images |
| JPWO2015166891A1 (ja) * | 2014-04-30 | 2017-04-20 | 旭硝子株式会社 | ガラス |
| JP6386801B2 (ja) * | 2014-06-10 | 2018-09-05 | Agcセラミックス株式会社 | アルミナ溶融鋳造耐火物とその製造方法 |
| JP6390203B2 (ja) | 2014-06-24 | 2018-09-19 | リコーイメージング株式会社 | 撮像装置及びノイズ補正方法 |
| JP5900560B2 (ja) | 2014-09-01 | 2016-04-06 | 旭硝子株式会社 | 基板用ガラスおよびガラス基板 |
| CN206580739U (zh) | 2014-10-08 | 2017-10-24 | 康宁股份有限公司 | 玻璃基制品 |
| CN106746741B (zh) * | 2014-12-23 | 2020-04-10 | 深圳南玻应用技术有限公司 | 铝硅酸盐玻璃、铝硅酸盐玻璃的强化方法及强化玻璃 |
| US10579106B2 (en) * | 2015-07-21 | 2020-03-03 | Corning Incorporated | Glass articles exhibiting improved fracture performance |
| CN114349368B (zh) | 2016-01-21 | 2022-11-25 | Agc株式会社 | 化学强化玻璃以及化学强化用玻璃 |
| CN110217983B (zh) * | 2016-01-21 | 2022-09-06 | Agc株式会社 | 化学强化玻璃以及化学强化玻璃的制造方法 |
-
2017
- 2017-01-19 CN CN202111534127.8A patent/CN114349368B/zh active Active
- 2017-01-19 CN CN202110986741.1A patent/CN113800783A/zh active Pending
- 2017-01-19 CN CN202211233035.0A patent/CN115572077A/zh active Pending
- 2017-01-19 KR KR1020207015390A patent/KR102126835B1/ko active IP Right Grant
- 2017-01-19 CN CN201810952965.9A patent/CN109133670B/zh active Active
- 2017-01-19 CN CN202011142412.0A patent/CN113024108B/zh active Active
- 2017-01-19 DE DE202017007276.9U patent/DE202017007276U1/de active Active
- 2017-01-19 CN CN202211232234.XA patent/CN115650601B/zh active Active
- 2017-01-19 KR KR1020197017823A patent/KR102073143B1/ko active IP Right Grant
- 2017-01-19 CN CN202211232235.4A patent/CN115677235A/zh active Pending
- 2017-01-19 CN CN201910528904.4A patent/CN110330228A/zh active Pending
- 2017-01-19 KR KR1020217025788A patent/KR102569434B1/ko active IP Right Grant
- 2017-01-19 KR KR1020197035146A patent/KR102074835B1/ko active IP Right Grant
- 2017-01-19 CN CN202110986861.1A patent/CN113754276B/zh active Active
- 2017-01-19 KR KR1020207026654A patent/KR102292273B1/ko active IP Right Grant
- 2017-01-19 DE DE202017007305.6U patent/DE202017007305U1/de active Active
- 2017-01-19 KR KR1020177016471A patent/KR101927014B1/ko active IP Right Grant
- 2017-01-19 CN CN201811180740.2A patent/CN109455926B/zh active Active
- 2017-01-19 CN CN202111532739.3A patent/CN114315133B/zh active Active
- 2017-01-19 KR KR1020187014737A patent/KR102024126B1/ko active IP Right Grant
- 2017-01-19 KR KR1020197008706A patent/KR102027956B1/ko active IP Right Grant
- 2017-01-19 CN CN201811234810.8A patent/CN109320098B/zh active Active
- 2017-01-19 CN CN202211232239.2A patent/CN115572076A/zh active Pending
- 2017-01-19 KR KR1020237027967A patent/KR102630405B1/ko active IP Right Grant
- 2017-01-19 CN CN201910486194.3A patent/CN110255892B/zh active Active
- 2017-01-19 DE DE112017000454.3T patent/DE112017000454T5/de active Pending
- 2017-01-19 KR KR1020187030257A patent/KR102121414B1/ko active IP Right Grant
- 2017-01-19 CN CN201780007591.7A patent/CN108473370B/zh active Active
- 2017-01-19 KR KR1020197015480A patent/KR102161536B1/ko active IP Right Grant
- 2017-01-19 JP JP2017562079A patent/JP6327407B2/ja active Active
- 2017-01-19 WO PCT/JP2017/001755 patent/WO2017126607A1/ja active Application Filing
- 2017-01-19 CN CN202211233164.XA patent/CN115650602B/zh active Active
- 2017-01-20 TW TW107111929A patent/TWI644879B/zh active
- 2017-01-20 TW TW111122553A patent/TWI788269B/zh active
- 2017-01-20 TW TW107137003A patent/TWI678351B/zh active
- 2017-01-20 TW TW106102257A patent/TWI770002B/zh active
- 2017-01-20 TW TW108119486A patent/TWI759603B/zh active
- 2017-01-20 TW TW111105932A patent/TWI790922B/zh active
- 2017-01-20 TW TW108121125A patent/TWI689477B/zh active
- 2017-12-01 JP JP2017231614A patent/JP6394776B2/ja active Active
-
2018
- 2018-02-28 US US15/908,227 patent/US10384974B2/en active Active
- 2018-03-13 US US15/920,009 patent/US10370287B2/en active Active
- 2018-03-22 JP JP2018055141A patent/JP6424978B2/ja active Active
- 2018-07-12 US US16/033,787 patent/US10472272B2/en active Active
- 2018-10-25 JP JP2018200912A patent/JP6583511B2/ja active Active
-
2019
- 2019-03-04 US US16/291,407 patent/US20190194057A1/en not_active Abandoned
- 2019-06-12 JP JP2019109487A patent/JP6583583B2/ja active Active
- 2019-09-05 JP JP2019162190A patent/JP7059993B2/ja active Active
-
2020
- 2020-09-17 US US17/024,109 patent/US11767252B2/en active Active
-
2022
- 2022-04-08 JP JP2022064554A patent/JP2022082778A/ja active Pending
-
2024
- 2024-04-30 JP JP2024073865A patent/JP2024096285A/ja active Pending
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2000516903A (ja) * | 1997-04-11 | 2000-12-19 | サン―ゴバン ビトラージュ | ガラス組成物及び化学強化されるガラスで製造された基材 |
| JP2002507538A (ja) * | 1998-03-20 | 2002-03-12 | ピルキントン パブリック リミテッド カンパニー | 化学的に強化した無ホウ素フロートガラス組成物 |
| JP2001302278A (ja) * | 2000-02-17 | 2001-10-31 | Hoya Corp | 陰極線管用ガラス、陰極線管用ガラスパネル、及び陰極線管、並びにそれらの製造方法 |
| JP2002174810A (ja) * | 2000-12-08 | 2002-06-21 | Hoya Corp | ディスプレイ用ガラス基板及びその製造方法並びにこれを用いたディスプレイ |
| JP2006083045A (ja) * | 2004-09-17 | 2006-03-30 | Hitachi Ltd | ガラス部材 |
| JP2013520388A (ja) * | 2010-02-26 | 2013-06-06 | ショット アクチエンゲゼルシャフト | 化学強化ガラス |
| JP2013536155A (ja) * | 2010-08-26 | 2013-09-19 | コーニング インコーポレイテッド | ガラスを強化する二段階法 |
| JP2013542159A (ja) * | 2010-09-13 | 2013-11-21 | サン−ゴバン グラス フランス | ガラス板 |
| JP2012232882A (ja) * | 2011-04-18 | 2012-11-29 | Asahi Glass Co Ltd | 化学強化ガラスの製造方法および化学強化用ガラス |
Non-Patent Citations (1)
| Title |
|---|
| AKIO MAKISHIMA ET AL.: "Glass Materials Design System: VitrES", FUJITSU, vol. 44, no. 6, 10 November 1993 (1993-11-10), pages 560 - 565, ISSN: 0016-2515 * |
Cited By (55)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10370287B2 (en) | 2016-01-21 | 2019-08-06 | AGC Inc. | Chemically strengthened glass, and glass for chemical strengthening |
| US11390560B2 (en) | 2016-01-21 | 2022-07-19 | AGC Inc. | Chemically strengthened glass and method for manufacturing chemically strengthened glass |
| JP2018104285A (ja) * | 2016-01-21 | 2018-07-05 | 旭硝子株式会社 | 化学強化ガラスおよび化学強化用ガラス |
| US11365149B2 (en) | 2016-01-21 | 2022-06-21 | AGC Inc. | Chemically strengthened glass and method for manufacturing chemically strengthened glass |
| US11767252B2 (en) | 2016-01-21 | 2023-09-26 | AGC Inc. | Chemically strengthened glass, and glass for chemical strengthening |
| US10384974B2 (en) | 2016-01-21 | 2019-08-20 | AGC Inc. | Chemically strengthened glass, and glass for chemical strengthening |
| WO2018074335A1 (ja) * | 2016-10-18 | 2018-04-26 | 旭硝子株式会社 | 化学強化用ガラス、化学強化ガラスおよび化学強化ガラスの製造方法 |
| US11535548B2 (en) | 2016-10-18 | 2022-12-27 | AGC Inc. | Glass for chemical strengthening, chemically strengthened glass and method for manufacturing chemically strengthened glass |
| JPWO2018074335A1 (ja) * | 2016-10-18 | 2019-08-08 | Agc株式会社 | 化学強化用ガラス、化学強化ガラスおよび化学強化ガラスの製造方法 |
| JP7040456B2 (ja) | 2016-10-18 | 2022-03-23 | Agc株式会社 | 化学強化用ガラス、化学強化ガラスおよび化学強化ガラスの製造方法 |
| US11401198B2 (en) * | 2016-12-30 | 2022-08-02 | Tunghsu Group Co., Ltd. | Silicate product and strengthening method thereof |
| JPWO2018159386A1 (ja) * | 2017-02-28 | 2020-01-16 | 日本電気硝子株式会社 | アルミノケイ酸塩ガラス |
| JP7339605B2 (ja) | 2017-02-28 | 2023-09-06 | 日本電気硝子株式会社 | アルミノケイ酸塩ガラス |
| JP2020200238A (ja) * | 2017-09-04 | 2020-12-17 | ショット アクチエンゲゼルシャフトSchott AG | 可撓性および/または折畳み可能な物品ならびに可撓性および/または折畳み可能な物品の手配方法 |
| WO2019069567A1 (ja) * | 2017-10-02 | 2019-04-11 | 日本電気硝子株式会社 | 強化ガラス板及び強化ガラス板付デバイス |
| JP2019064875A (ja) * | 2017-10-02 | 2019-04-25 | 日本電気硝子株式会社 | 強化ガラス板及び強化ガラス板付デバイス |
| WO2019080190A1 (zh) * | 2017-10-26 | 2019-05-02 | 中国南玻集团股份有限公司 | 铝硅酸盐玻璃及其制备方法、电子设备 |
| JPWO2019131528A1 (ja) * | 2017-12-26 | 2020-11-19 | 日本電気硝子株式会社 | カバーガラス |
| JP7276667B2 (ja) | 2017-12-26 | 2023-05-18 | 日本電気硝子株式会社 | カバーガラス |
| JP2019116417A (ja) * | 2017-12-26 | 2019-07-18 | 日本電気硝子株式会社 | カバーガラス |
| JP7303482B2 (ja) | 2017-12-26 | 2023-07-05 | 日本電気硝子株式会社 | カバーガラス |
| US12122708B2 (en) | 2017-12-29 | 2024-10-22 | Chongqing Aureavia Hi-Tech Glass Co., Ltd. | Mother glass, reinforced glass and preparation method |
| WO2019127818A1 (zh) * | 2017-12-29 | 2019-07-04 | 深圳市东丽华科技有限公司 | 一种素玻璃、强化玻璃及制备方法 |
| CN108147657A (zh) * | 2017-12-29 | 2018-06-12 | 深圳市东丽华科技有限公司 | 一种素玻璃、强化玻璃及制备方法 |
| CN108147657B (zh) * | 2017-12-29 | 2020-11-03 | 重庆鑫景特种玻璃有限公司 | 一种素玻璃、强化玻璃及制备方法 |
| CN111819159A (zh) * | 2018-02-28 | 2020-10-23 | 日本电气硝子株式会社 | 强化玻璃及强化用玻璃 |
| JP2022177042A (ja) * | 2018-02-28 | 2022-11-30 | 日本電気硝子株式会社 | 強化ガラス及び強化用ガラス |
| JP2019147727A (ja) * | 2018-02-28 | 2019-09-05 | 日本電気硝子株式会社 | 強化ガラス及び強化用ガラス |
| WO2019167550A1 (ja) * | 2018-02-28 | 2019-09-06 | 日本電気硝子株式会社 | 強化ガラス及び強化用ガラス |
| JP7134397B2 (ja) | 2018-02-28 | 2022-09-12 | 日本電気硝子株式会社 | 強化ガラス及び強化用ガラス |
| CN111819159B (zh) * | 2018-02-28 | 2023-01-10 | 日本电气硝子株式会社 | 强化玻璃及强化用玻璃 |
| JP7328629B2 (ja) | 2018-02-28 | 2023-08-17 | 日本電気硝子株式会社 | 強化ガラス及び強化用ガラス |
| WO2019194110A1 (ja) * | 2018-04-04 | 2019-10-10 | Agc株式会社 | 化学強化用ガラス |
| US12024464B2 (en) | 2018-04-04 | 2024-07-02 | AGC Inc. | Glass for chemical strengthening |
| JPWO2019194110A1 (ja) * | 2018-04-04 | 2021-04-01 | Agc株式会社 | 化学強化用ガラス |
| KR102644011B1 (ko) * | 2018-04-04 | 2024-03-07 | 에이지씨 가부시키가이샤 | 화학 강화용 유리 |
| KR20200139156A (ko) * | 2018-04-04 | 2020-12-11 | 에이지씨 가부시키가이샤 | 화학 강화용 유리 |
| CN111954647A (zh) * | 2018-04-04 | 2020-11-17 | Agc株式会社 | 化学强化用玻璃 |
| JP7248020B2 (ja) | 2018-04-04 | 2023-03-29 | Agc株式会社 | 化学強化用ガラス |
| JPWO2020075708A1 (ja) * | 2018-10-09 | 2021-09-02 | 日本電気硝子株式会社 | 強化ガラスおよび強化ガラスの製造方法 |
| JP7367691B2 (ja) | 2018-10-09 | 2023-10-24 | 日本電気硝子株式会社 | 強化ガラスおよび強化ガラスの製造方法 |
| WO2020075708A1 (ja) * | 2018-10-09 | 2020-04-16 | 日本電気硝子株式会社 | 強化ガラスおよび強化ガラスの製造方法 |
| US12122709B2 (en) | 2018-10-09 | 2024-10-22 | Nippon Electric Glass Co., Ltd. | Reinforced glass and method for producing reinforced glass |
| CN111099823B (zh) * | 2018-10-25 | 2022-04-12 | 重庆鑫景特种玻璃有限公司 | 基于素玻璃化学强化后的玻璃及玻璃器件 |
| CN111099823A (zh) * | 2018-10-25 | 2020-05-05 | 深圳市东丽华科技有限公司 | 素玻璃及玻璃器件 |
| WO2020121889A1 (ja) * | 2018-12-11 | 2020-06-18 | Agc株式会社 | ガラス、化学強化ガラスおよびそれを含む電子機器 |
| JPWO2020121889A1 (ja) * | 2018-12-11 | 2021-10-28 | Agc株式会社 | ガラス、化学強化ガラスおよびそれを含む電子機器 |
| JP7310830B2 (ja) | 2018-12-11 | 2023-07-19 | Agc株式会社 | ガラス、化学強化ガラスおよびそれを含む電子機器 |
| JP2021031317A (ja) * | 2019-08-20 | 2021-03-01 | Agc株式会社 | リチウムアルミノシリケートガラスの製造方法、およびフロートガラス板 |
| JP7234856B2 (ja) | 2019-08-20 | 2023-03-08 | Agc株式会社 | リチウムアルミノシリケートガラスの製造方法、およびフロートガラス板 |
| JP2021075426A (ja) * | 2019-11-11 | 2021-05-20 | 石塚硝子株式会社 | 化学強化用アルミノボロシケートガラス及び化学強化ガラス品 |
| US11621480B2 (en) | 2020-06-12 | 2023-04-04 | AGC Inc. | Protective member and communication terminal device including the same |
| JP7537298B2 (ja) | 2021-02-10 | 2024-08-21 | Agc株式会社 | 強化ガラス板、および強化ガラス板の製造方法 |
| WO2023243574A1 (ja) * | 2022-06-15 | 2023-12-21 | Agc株式会社 | 化学強化用ガラス及びガラス |
| CN117865465A (zh) * | 2023-06-29 | 2024-04-12 | 重庆鑫景特种玻璃有限公司 | 一种着色化学强化玻璃及其制法和应用 |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6583583B2 (ja) | 化学強化ガラスおよび化学強化用ガラス | |
| WO2017126605A1 (ja) | 化学強化ガラス及び化学強化ガラスの製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| ENP | Entry into the national phase |
Ref document number: 20177016471 Country of ref document: KR Kind code of ref document: A |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17741484 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2017562079 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 112017000454 Country of ref document: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 17741484 Country of ref document: EP Kind code of ref document: A1 |