WO2011021597A1 - キノリン誘導体含有医薬組成物 - Google Patents
キノリン誘導体含有医薬組成物 Download PDFInfo
- Publication number
- WO2011021597A1 WO2011021597A1 PCT/JP2010/063804 JP2010063804W WO2011021597A1 WO 2011021597 A1 WO2011021597 A1 WO 2011021597A1 JP 2010063804 W JP2010063804 W JP 2010063804W WO 2011021597 A1 WO2011021597 A1 WO 2011021597A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- composition according
- group
- examples
- salt
- hydrogen atom
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1611—Inorganic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/02—Inorganic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/36—Polysaccharides; Derivatives thereof, e.g. gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1629—Organic macromolecular compounds
- A61K9/1652—Polysaccharides, e.g. alginate, cellulose derivatives; Cyclodextrin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4841—Filling excipients; Inactive ingredients
- A61K9/485—Inorganic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4841—Filling excipients; Inactive ingredients
- A61K9/4866—Organic macromolecular compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D215/00—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems
- C07D215/02—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom
- C07D215/16—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D215/48—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen
Definitions
- the present invention relates to a pharmaceutical composition containing a quinoline derivative useful as a medicine. More specifically, the present invention relates to a pharmaceutical composition having improved elution of a quinoline derivative or a pharmacologically acceptable salt thereof or a solvate thereof.
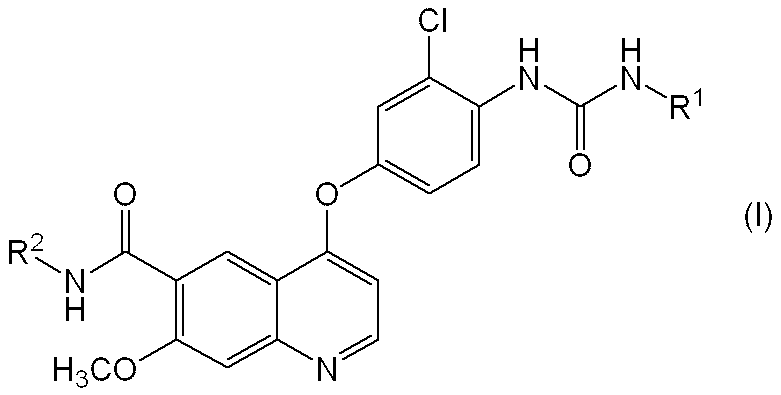
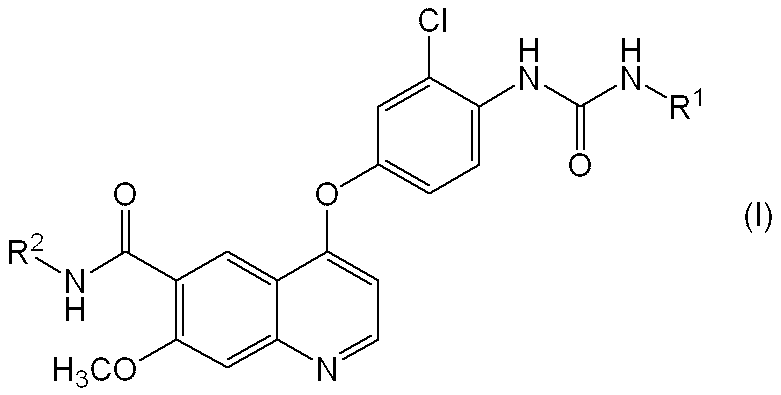
- a quinoline derivative represented by the following formula (I) or a pharmacologically acceptable salt thereof or a solvate thereof has a potent angiogenesis inhibitory action (Patent Document 1).
- c-Kit kinase inhibitory action Patent Document 2
- R 1 represents a hydrogen atom, a C 1-6 alkyl group or a C 3-8 cycloalkyl group
- R 2 represents a hydrogen atom or a methoxy group.
- the quinoline derivative (I) is decomposed under humidified and warm storage conditions when it is used as a pharmaceutical composition. Further, when the pharmaceutical composition absorbs moisture, the surface of the composition may gel, and the elution of the quinoline derivative (I), which is a medicinal component, may be delayed.
- the quinoline derivative (I) and (1) a 5% (W / W) aqueous solution or suspension having a pH of 8 or higher, and / or (2) silicic acid or A preparation containing the salt or a solvate thereof has been developed (Patent Document 3).
- an object of the present invention is to obtain a pharmaceutical composition which is excellent in the dissolution property of the quinoline derivative (I) and is stable even after long-term storage.
- a pharmaceutical composition comprising a compound represented by the following formula (I) or a pharmacologically acceptable salt thereof or a solvate thereof, and (2) a basic substance.
- R 1 represents a hydrogen atom, a C 1-6 alkyl group or a C 3-8 cycloalkyl group
- R 2 represents a hydrogen atom or a methoxy group.
- composition according to [2], wherein the salt is an alkaline earth metal salt.
- the alkaline earth metal salt is a magnesium salt or a calcium salt.
- the disintegrant is carmellose sodium, carmellose calcium, carboxymethyl starch sodium, croscarmellose sodium, low-substituted hydroxypropylcellulose, or crospovidone.
- the pharmacologically acceptable salt is hydrochloride, hydrobromide, p-toluenesulfonate, sulfate, methanesulfonate or ethanesulfonate [1] to [10] The composition as described in any one of these.
- the compound represented by the formula (I) is 4- (3-chloro-4- (cyclopropylaminocarbonyl) aminophenoxy) -7-methoxy-6-quinolinecarboxamide methanesulfonate [1] The composition according to any one of [11] to [11].
- the pharmaceutical composition of the present invention is excellent in the dissolution of the quinoline derivative (I), which is the main drug, and is excellent in absorbability to the living body. In addition, it is a pharmaceutical composition that is stable even after long-term storage.
- FIG. 3 is a diagram showing the dissolution pattern of Compound A in the preparations obtained in Examples 4 to 6 and Comparative Example 1.
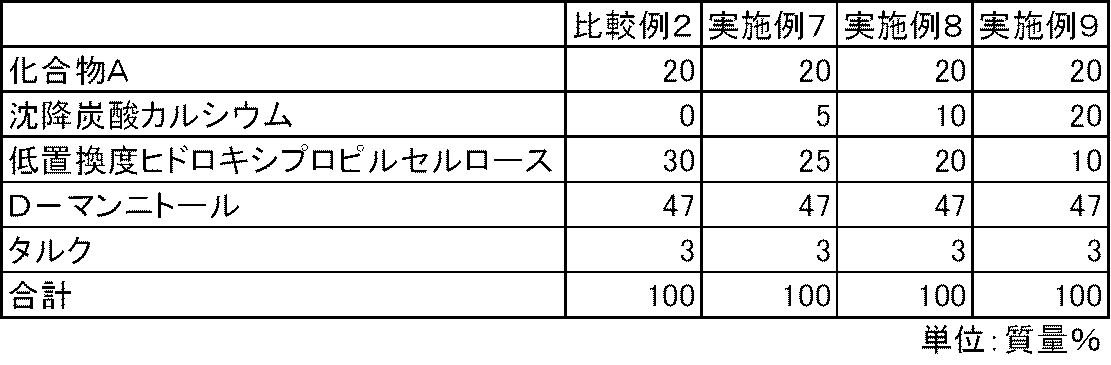
- FIG. 3 is a diagram showing the elution pattern of Compound A in the preparations obtained in Examples 7 to 9 and Comparative Example 2.
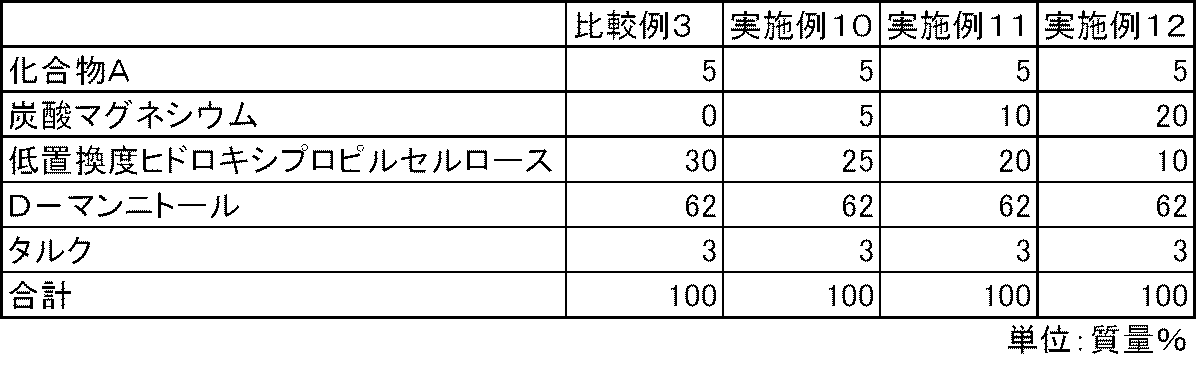
- FIG. 3 is a diagram showing the elution pattern of Compound A in the preparations obtained in Examples 10 to 12 and Comparative Example 3.
- FIG. 3 is a diagram showing the elution pattern of Compound A in the preparations obtained in Examples 13 to 15 and Comparative Example 4.
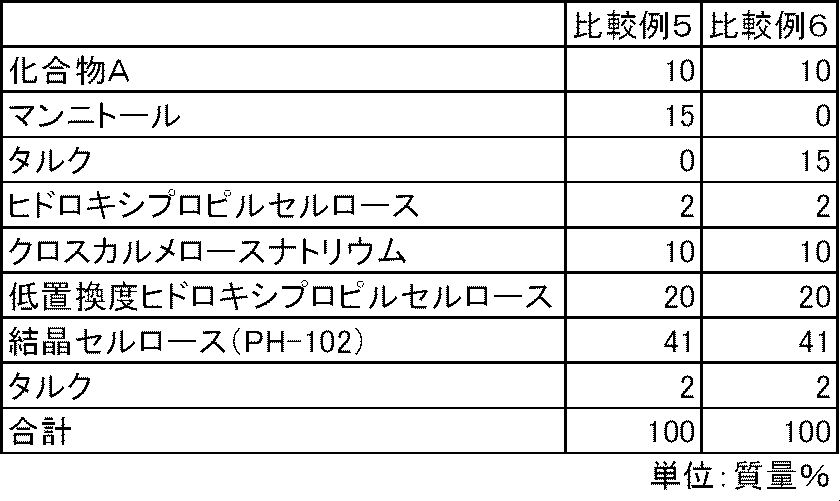
- FIG. 6 is a graph showing the elution pattern of Compound A in the preparations obtained in Examples 16 to 17 and Comparative Example 5.
- FIG. 3 is a diagram showing the dissolution pattern of Compound A in the preparations obtained in Examples 4 to 6 and Comparative Example 1.
- FIG. 3 is a diagram showing the elution pattern of Compound A in the preparations obtained in Examples 7 to 9 and Comparative Example 2.
- FIG. 3 is a diagram showing the elution
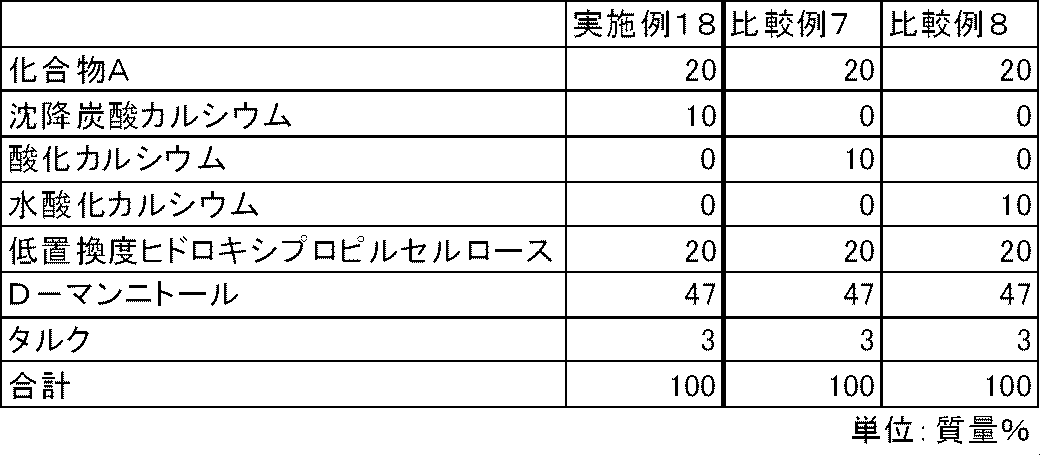
- FIG. 6 is a diagram showing the elution pattern of Compound A in the preparations obtained in Example 18 and Comparative Examples 7 to 8.
- FIG. 3 is a diagram showing the elution pattern of Compound A in the preparations obtained in Example 19 and Comparative Examples 9 to 10.
- the pharmaceutical composition means a composition containing the quinoline derivative (I) and a basic substance as essential components.
- the mixing ratio of the quinoline derivative (I) and the basic substance is not particularly limited, but is usually 1: 0.5 to 50, preferably 1: 1 to 25, and more preferably 1: 2 to 12.5.
- the blending ratio of the quinoline derivative (I) to the total mass of the pharmaceutical composition (excluding capsules) is usually 0.25 to 50% by mass, preferably 0.5 to 25% by mass, and more preferably 1 to 12. 5% by mass.
- the blending ratio of the basic substance to the total mass of the pharmaceutical composition is usually 1 to 60% by mass, preferably 5 to 50% by mass, and more preferably 10 to 40% by mass.
- the basic substance which concerns on this invention should just be mix
- the dosage form of the pharmaceutical composition specifically means solid preparations such as granules, fine granules, tablets and capsules. Preferred are fine granules, granules and capsules filled with these fine granules and granules.
- the quinoline derivative (I) is a compound disclosed in International Publication No. 2002/32872 pamphlet.
- Preferred quinoline derivatives (I) are 4- (3-fluoro-4- (cyclopropylaminocarbonyl) aminophenoxy) -7- (2-methoxyethoxy) -6-quinolinecarboxamide, 4- (3-chloro-4- (cyclopropylaminocarbonyl) aminophenoxy) -7-methoxy-6-quinolinecarboxamide, 4- (3-chloro-4- (cyclopropylaminocarbonyl) aminophenoxy) -7- (2-methoxyethoxy) -6-quinolinecarboxamide, 4- (3-chloro-4- (cyclopropylaminocarbonyl) aminophenoxy) -7- (2-hydroxyethoxy) -6-quinolinecarboxamide, 4- (3-chloro-4- (cyclopropylaminocarbonyl) aminophenoxy) -7-((2S) -2,3-dihydroxypropyl
- More preferred quinoline derivative (I) is 4- (3-chloro-4- (methylaminocarbonyl) aminophenoxy) -7-methoxy-6-quinolinecarboxamide, 4- (3-chloro-4- (ethylaminocarbonyl) aminophenoxy) -7-methoxy-6-quinolinecarboxamide, 4- (3-chloro-4- (cyclopropylaminocarbonyl) aminophenoxy) -7-methoxy-6-quinolinecarboxamide, N6-methoxy-4- (3-chloro-4-(((cyclopropylamino) carbonyl) amino) phenoxy) -7-methoxy-6-quinolinecarboxamide, and N6-methoxy-4- (3-chloro-4 -(((Ethylamino) carbonyl) amino) phenoxy) -7-methoxy-6-quinolinecarboxamide, a quinoline derivative selected from the group consisting of pharmaceutically acceptable salts or solvates thereof. .
- Particularly preferred quinoline derivatives (I) are: 4- (3-Chloro-4- (cyclopropylaminocarbonyl) aminophenoxy) -7-methoxy-6-quinolinecarboxamide or a pharmaceutically acceptable salt thereof or a solvate thereof.
- the pharmacologically acceptable salt means hydrochloride, hydrobromide, p-toluenesulfonate, sulfate, methanesulfonate or ethanesulfonate. Preferred is methanesulfonate.
- the solvate means a hydrate, a dimethyl sulfoxide solvate or an acetic acid solvate.
- the quinoline derivative (I) is a salt of 4- (3-chloro-4- (cyclopropylaminocarbonyl) aminophenoxy) -7-methoxy-6-quinolinecarboxamide disclosed in WO 2005/063713 or It is preferably a crystal of the solvate.
- a particularly preferred quinoline derivative (I) is a C-type crystal of methanesulfonate of 4- (3-chloro-4- (cyclopropylaminocarbonyl) aminophenoxy) -7-methoxy-6-quinolinecarboxamide.
- the quinoline derivative (I) is useful as a prophylactic / therapeutic agent for various tumors and an anti-metastasis agent for tumors.
- effective tumors include thyroid cancer, non-small cell lung cancer, melanoma, laryngeal cancer, esophageal cancer, stomach cancer, colon cancer, hepatocellular carcinoma, renal cell carcinoma, pancreatic cancer, bladder cancer, breast cancer, uterine cancer,
- Examples include ovarian cancer, prostate cancer, testicular cancer, gastrointestinal stromal tumor, sarcoma, osteosarcoma, hemangioma, malignant lymphoma, myeloid leukemia, neuroma, glioma and the like.
- the basic substance means a basic inorganic salt.
- basic inorganic salts include beryllium carbonate, magnesium carbonate, calcium carbonate, strontium carbonate, barium carbonate, potassium carbonate, calcium hydrogen phosphate, titanium oxide and the like.
- it is an alkaline earth metal salt of carbonic acid, more preferably magnesium carbonate or calcium carbonate.
- a disintegrant may be further added to the pharmaceutical composition according to the present invention.
- the disintegrant include corn starch, partially pregelatinized starch, hydroxypropyl starch, carmellose, carmellose sodium, carmellose calcium, carboxymethyl starch sodium, croscarmellose sodium, low-substituted hydroxypropylcellulose, crospovidone and the like. it can. Preferred is croscarmellose sodium, low-substituted hydroxypropylcellulose, or crospovidone.
- the pharmaceutical composition according to the present invention can be produced by a known method such as the method described in the General Formulation of the 15th revised Japanese Pharmacopoeia.
- the quinoline derivative (I) in the case of granules, stirring granulation, extrusion granulation, rolling granulation, fluidized bed by adding excipients, binders, disintegrants, solvents and the like to the quinoline derivative (I) as necessary. It can be produced by granulation, spray granulation and the like. While spraying water or a binder solution such as sucrose, hydroxypropylcellulose, hydroxypropylmethylcellulose, etc. onto the core material such as purified sucrose spherical granules, lactose / crystalline cellulose spherical granules, sucrose / starch spherical granules, granular crystalline cellulose, etc.
- a binder solution such as sucrose, hydroxypropylcellulose, hydroxypropylmethylcellulose, etc.
- the core material such as purified sucrose spherical granules, lactose / crystalline cellulose spherical
- a spraying agent containing an additive such as quinoline derivative (I) and corn starch, crystalline cellulose, hydroxypropylcellulose, methylcellulose, polyvinylpyrrolidone may be coated. Furthermore, you may sizing and grind
- Tablets are prepared by adding tablets, binders, disintegrants, lubricants, antioxidants, corrigents, colorants, fragrances, and the like to the granules thus prepared as necessary. It can also be. Further, a necessary excipient may be added to the quinoline derivative (I) and directly compressed into tablets. In addition, quinoline derivative (I) added and mixed with excipients such as lactose, sucrose, glucose, starch, microcrystalline cellulose, licorice powder, mannitol, calcium phosphate, calcium sulfate, etc. It can also be filled.
- excipient examples include lactose, sucrose, glucose, fructose, starch, potato starch, corn starch, wheat starch, rice starch, crystalline cellulose, microcrystalline cellulose, licorice powder, mannitol, erythritol, maltitol, sorbitol, trehalose. , Anhydrous silicic acid, calcium silicate, sodium bicarbonate, calcium phosphate, anhydrous calcium phosphate, calcium sulfate and the like.
- binder examples include gelatin, starch, gum arabic, tragacanth, carboxymethylcellulose, hydroxypropylcellulose, hydroxypropylmethylcellulose, polyvinylpyrrolidone, methylcellulose, partially pregelatinized starch, pregelatinized starch, polyvinyl alcohol, sodium alginate, pullulan, Examples thereof include glycerin.
- disintegrant examples include corn starch, partially pregelatinized starch, hydroxypropyl starch, carmellose, carmellose sodium, carmellose calcium, sodium carboxymethyl starch, croscarmellose sodium, low-substituted hydroxypropylcellulose, crospovidone, etc. Can do.
- lubricant examples include magnesium stearate, stearic acid, calcium stearate, sodium stearyl fumarate, talc, macrogol and the like.
- antioxidants examples include sodium ascorbate, L-cysteine, sodium sulfite, tocopherol, soybean lecithin and the like.
- flavoring agent examples include citric acid, ascorbic acid, tartaric acid, malic acid, aspartame, acesulfame potassium, thaumatin, sodium saccharin, glycyrrhizin dipotassium, sodium glutamate, 5′-sodium inosinate, and 5′-sodium guanylate. Can be mentioned.
- colorant examples include titanium oxide, iron sesquioxide, yellow iron sesquioxide, cochineal, carmine, riboflavin, edible yellow No. 5, and edible blue No. 2.
- fragrances examples include lemon oil, orange oil, menthol, brackish oil, borneol, and vanilla flavor.
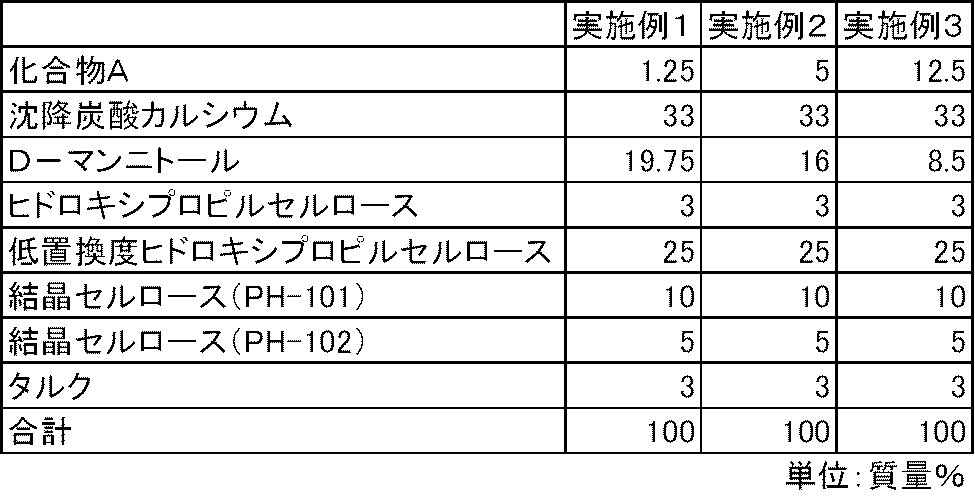
- Examples 1 to 3 C-form crystals of 4- (3-chloro-4- (cyclopropylaminocarbonyl) aminophenoxy) -7-methoxy-6-quinolinecarboxamide methanesulfonate hereinafter referred to as Compound A
- D-mannitol Product Name: Mannitol, Merck, Precipitated Calcium Carbonate (Product Name: Whiten F, Calcium Shiraishi), Hydroxypropylcellulose (HPC-L, Nippon Soda), Low-substituted hydroxypropylcellulose (Product Name: L-HPC (LH) -21), Shin-Etsu Chemical Co., Ltd.) and crystalline cellulose (trade name: Theolas PH-101, Asahi Kasei) according to the prescription ratios in Table 1, a high-speed agitation granulator (device name: FM-VG-10, manufactured by POWREC) Used to perform wet granulation with purified water as solvent.
- the granules having a moisture content of less than 2% were dried and sized using a pulverizing and sizing device (device name: Power Mill P-04S, Showa Giken Co., Ltd.) so that the granule diameter was less than 1 mm. .
- a pulverizing and sizing device device name: Power Mill P-04S, Showa Giken Co., Ltd.
- crystalline cellulose trade name: Theolas PH-102, Asahi Kasei
- talc trade name: Hyfiller 17, Iwai Kagaku
- 100 mg of the obtained granules were filled into a No. 4 hard capsule to produce a capsule containing Compound A.
- Test example 1 For the capsules of Examples 4 to 9 and Comparative Examples 1 and 2, the dissolution property of Compound A was determined according to the dissolution test method (paddle method, test solution: first solution) described in the 15th revision Japanese Pharmacopoeia. investigated. As a result, the elution of Compound A was insufficient in the capsules of Comparative Examples 1 and 2 containing no calcium carbonate. On the other hand, in the capsules of Examples 4 to 9 containing calcium carbonate, the elution of Compound A was good (FIGS. 1 and 2).
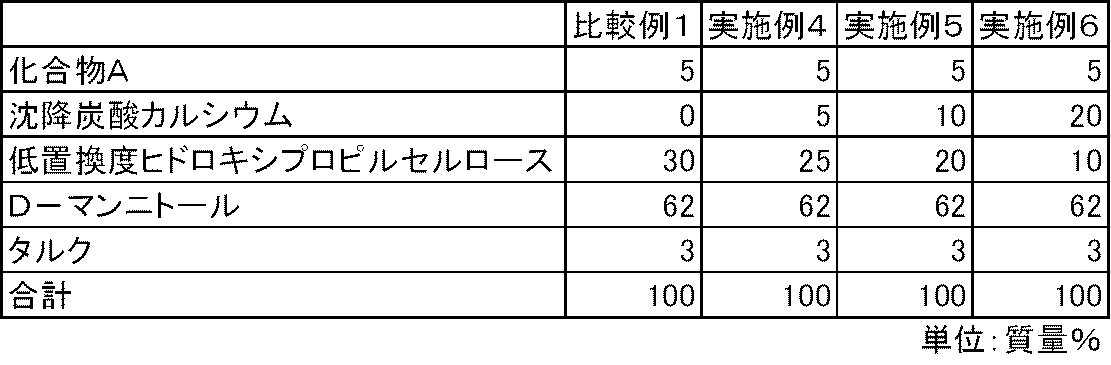
- Examples 10 to 15 and Comparative Examples 3 to 4 Compound A, magnesium carbonate (Kyowa Chemical Industry), low-substituted hydroxypropylcellulose, D-mannitol and talc were mixed thoroughly using a mortar and pestle according to the formulation ratios in Tables 4 and 5. 100 mg of the resulting mixture was filled into No. 3 hard capsules to produce capsules of Examples 10 to 15. In the same manner, capsules of Comparative Examples 3 to 4 containing no magnesium carbonate were produced.
- Test example 2 In the same manner as in Test Example 1, the elution of Compound A was examined for the capsules of Examples 10 to 15 and Comparative Examples 3 to 4. In the capsules of Comparative Examples 3 to 4 containing no magnesium carbonate, the elution of Compound A was insufficient. On the other hand, in the capsules of Examples 10 to 15 containing magnesium carbonate, the elution of Compound A was good (FIGS. 3 and 4).
- Examples 16 to 17 and Comparative Examples 5 to 6 Purified water is added to Compound A, precipitated calcium carbonate or magnesium carbonate, hydroxypropyl cellulose, croscarmellose sodium (trade name: Ac-di-sol, Asahi Kasei), granulated using a mortar and pestle, and then dried. The granulated particles were sized so that the granule diameter was less than 1 mm. Thereafter, crystalline cellulose (trade name: Theolas PH-102, Asahi Kasei), low-substituted hydroxypropylcellulose, and talc (trade name: High Filler 17, Iwai Chemical) are added to the sized granules according to the formulation ratio in Table 6. And mixed well.
- crystalline cellulose trade name: Theolas PH-102, Asahi Kasei
- talc trade name: High Filler 17, Iwai Chemical
- capsules of Comparative Examples 5 to 6 containing no precipitated calcium carbonate and magnesium carbonate and containing mannitol or talc instead were prepared according to the formulation ratios in Table 7.
- Test example 3 In the same manner as in Test Example 1, the dissolution properties of Compound A were examined for the capsules of Examples 16 to 17 and Comparative Example 5. In the capsule of Comparative Example 5 containing no calcium carbonate or magnesium carbonate, the elution of Compound A was insufficient. On the other hand, in the capsules of Examples 16 to 17 containing calcium carbonate or magnesium carbonate, the elution of compound A was good (FIG. 5).
- Test example 4 The capsules of Examples 16 to 17 and Comparative Example 6 were stored in an open system for 1 week in an environment of a temperature of 60 ° C. and a relative humidity of 75%. In the capsule of Comparative Example 6 in which calcium carbonate and magnesium carbonate were not blended, degradation products increased. On the other hand, in the capsules of Examples 16 to 17 containing calcium carbonate or magnesium carbonate, no increase in degradation products was observed (Table 8).
- Test Example 5 In the same manner as in Test Example 1, the dissolution properties of Compound A were examined for the capsules of Examples 18 to 19 and Comparative Examples 7 to 10. As a result, in the capsules of Comparative Examples 7 to 10 containing calcium oxide, calcium hydroxide, magnesium oxide or magnesium hydroxide, the elution of compound A was insufficient. On the other hand, in the capsules of Examples 18 to 19 containing calcium carbonate or magnesium carbonate, the elution of Compound A was good (FIGS. 6 and 7).
- the pharmaceutical composition of the present invention is useful as a medicament for the prevention and treatment of tumors because it is excellent in elution and stability of the quinoline derivative.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Inorganic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oncology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Quinoline Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
Description
すなわち、本発明は、以下の[1]~[12]を提供する。
[1](1)下記式(I)で示される化合物もしくはその薬理学的に許容される塩またはそれらの溶媒和物、および(2)塩基性物質を含む医薬組成物。
[2]塩基性物質が炭酸塩である[1]に記載の組成物。
[3]塩がアルカリ土類金属塩である[2]に記載の組成物。
[4]アルカリ土類金属塩がマグネシウム塩またはカルシウム塩である[3]に記載の組成物。
[5]崩壊剤を含む[1]~[4]のいずれか一項に記載の組成物。
[6]崩壊剤がカルメロースナトリウム、カルメロースカルシウム、カルボキシメチルスターチナトリウム、クロスカルメロースナトリウム、低置換度ヒドロキシプロピルセルロースまたはクロスポビドンである[5]に記載の組成物。
[7]R1が水素原子、メチル基、エチル基、n-プロピル基またはシクロプロピル基である[1]~[6]のいずれか一項に記載の組成物。
[8]R1がシクロプロピル基である[1]~[7]のいずれか一項に記載の組成物。
[9]R2が水素原子、メトキシ基またはエトキシ基である請求項1~8のいずれか一項に記載の組成物。
[10]R2が水素原子である[1]~[9]のいずれか一項に記載の組成物。
[11]薬理学的に許容される塩が、塩酸塩、臭化水素酸塩、p-トルエンスルホン酸塩、硫酸塩、メタンスルホン酸塩またはエタンスルホン酸塩である[1]~[10]のいずれか一項に記載の組成物。
[12]式(I)で示される化合物が4-(3-クロロ-4-(シクロプロピルアミノカルボニル)アミノフェノキシ)-7-メトキシ-6-キノリンカルボキシアミドのメタンスルホン酸塩である[1]~[11]のいずれか一項に記載の組成物。
4-(3-フルオロ-4-(シクロプロピルアミノカルボニル)アミノフェノキシ)-7-(2-メトキシエトキシ)-6-キノリンカルボキサミド、
4-(3-クロロ-4-(シクロプロピルアミノカルボニル)アミノフェノキシ)-7-メトキシ-6-キノリンカルボキサミド、
4-(3-クロロ-4-(シクロプロピルアミノカルボニル)アミノフェノキシ)-7-(2-メトキシエトキシ)-6-キノリンカルボキサミド、
4-(3-クロロ-4-(シクロプロピルアミノカルボニル)アミノフェノキシ)-7-(2-ヒドロキシエトキシ)-6-キノリンカルボキサミド、
4-(3-クロロ-4-(シクロプロピルアミノカルボニル)アミノフェノキシ)-7-((2S)-2,3-ジヒドロキシプロピル)オキシ-6-キノリンカルボキサミド、
4-(3-クロロ-4-(メチルアミノカルボニル)アミノフェノキシ)-7-メトキシ-6-キノリンカルボキサミド、
4-(3-クロロ-4-(エチルアミノカルボニル)アミノフェノキシ)-7-メトキシ-6-キノリンカルボキサミド、
N6-メトキシ-4-(3-クロロ-4-(((エチルアミノ)カルボニル)アミノ)フェノキシ)-7-メトキシ-6-キノリンカルボキサミド、
4-(3-クロロ-4-(シクロプロピルアミノカルボニル)アミノフェノキシ)-7-(2-エトキシエトキシ)-6-キノリンカルボキサミド、
4-(4-((シクロプロピルアミノ)カルボニル)アミノフェノキシ)-7-(2-メトキシエトキシ)-6-キノリンカルボキサミド、
N-(2-フルオロ-4-〔(6-カルバモイル-7-メトキシ-4-キノリル)オキシ〕フェニル)-N’-シクロプロピルウレア、
N6-(2-ヒドロキシエチル)-4-(3-クロロ-4-(((シクロプロピルアミノ)カルボニル)アミノ)フェノキシ)-7-メトキシ-6-キノリンカルボキサミド、
4-(3-クロロ-4-(1-プロピルアミノカルボニル)アミノフェノキシ)-7-メトキシ-6-キノリンカルボキサミド、
4-(3-クロロ-4-(cis-2-フルオロ-シクロプロピルアミノカルボニル)アミノフェノキシ)-7-メトキシ-6-キノリンカルボキサミド、
N6-メチル-4-(3-クロロ-4-(((シクロプロピルアミノ)カルボニル)アミノ)フェノキシ)-7-(2-メトキシエトキシ)-6-キノリンカルボキサミドおよび
N6-メチル-4-(3-クロロ-4-(((エチルアミノ)カルボニル)アミノ)フェノキシ)-7-メトキシ-6-キノリンカルボキサミド
からなる群から選ばれるキノリン誘導体、もしくはその薬理学的に許容される塩またはそれらの溶媒和物である。
4-(3-クロロ-4-(メチルアミノカルボニル)アミノフェノキシ)-7-メトキシ-6-キノリンカルボキシアミド、
4-(3-クロロ-4-(エチルアミノカルボニル)アミノフェノキシ)-7-メトキシ-6-キノリンカルボキシアミド、
4-(3-クロロ-4-(シクロプロピルアミノカルボニル)アミノフェノキシ)-7-メトキシ-6-キノリンカルボキシアミド、
N6-メトキシ-4-(3-クロロ-4-(((シクロプロピルアミノ)カルボニル)アミノ)フェノキシ)-7-メトキシ-6-キノリンカルボキシアミド、および
N6-メトキシ-4-(3-クロロ-4-(((エチルアミノ)カルボニル)アミノ)フェノキシ)-7-メトキシ-6-キノリンカルボキシアミド
からなる群から選ばれるキノリン誘導体、もしくはその薬理学的に許容される塩またはそれらの溶媒和物である。
4-(3-クロロ-4-(シクロプロピルアミノカルボニル)アミノフェノキシ)-7-メトキシ-6-キノリンカルボキシアミド、もしくはその薬理学的に許容される塩またはそれらの溶媒和物である。
4-(3-クロロ-4-(シクロプロピルアミノカルボニル)アミノフェノキシ)-7-メトキシ-6-キノリンカルボキシアミドのメタンスルホン酸塩のC型結晶(以下、化合物Aと称する)、D-マンニトール(商品名:マンニトール、メルク)、沈降炭酸カルシウム(商品名:ホワイトンF、白石カルシウム)、ヒドロキシプロピルセルロース(HPC-L、日本曹達)、低置換度ヒドロキシプロピルセルロース(商品名:L-HPC(LH-21)、信越化学工業)および結晶セルロース(商品名:セオラスPH-101、旭化成)を表1の処方割合に従って、高速攪拌造粒装置(装置名:FM-VG-10、パウレック社製)を使用して、精製水を溶媒とする湿式造粒を実施した。さらに乾燥して、水分含有量2%未満とした顆粒を、粉砕整粒装置(装置名:パワーミルP-04S、昭和技研社製)を使用して、顆粒径1mm未満となるように整粒した。その後、整粒した顆粒に、表1の処方割合に従って、結晶セルロース(商品名:セオラスPH-102、旭化成)およびタルク(商品名:ハイフィラー17、岩井化学)を添加し、タンブラー型混合機(商品名:10L/20L交換型タンブラー混合機、東洋パッキング社製)を使用して十分に混合した。得られた顆粒100mgを4号のハードカプセルに充填し、化合物Aを含むカプセル剤を製造した。
化合物A、沈降炭酸カルシウム、低置換度ヒドロキシプロピルセルロース、D-マンニトールおよびタルクを表2および表3の処方割合に従って、乳鉢と乳棒を使用して十分に混合した。得られた混合物100mgを3号のハードカプセルに充填し、実施例4~9のカプセル剤を製造した。また、同様の方法で、沈降炭酸カルシウムを含まない比較例1~2のカプセル剤を製造した。
実施例4~9および比較例1~2のカプセル剤について、第十五改正日本薬局方に記載されている溶出試験法(パドル法、試験液:第1液)に従って、化合物Aの溶出性を検討した。その結果、炭酸カルシウムを配合しない比較例1~2のカプセル剤では、化合物Aの溶出は不十分であった。一方、炭酸カルシウムを配合した実施例4~9のカプセル剤では、化合物Aの溶出は良好であった(図1および図2)。
化合物A、炭酸マグネシウム(協和化学工業)、低置換度ヒドロキシプロピルセルロース、D-マンニトールおよびタルクを表4および表5の処方割合に従って、乳鉢と乳棒を使用して十分に混合した。得られた混合物100mgを3号のハードカプセルに充填し、実施例10~15のカプセル剤を製造した。また、同様の方法で、炭酸マグネシウムを含まない比較例3~4のカプセル剤を製造した。
試験例1と同様の方法で、実施例10~15および比較例3~4のカプセル剤について、化合物Aの溶出性を検討した。炭酸マグネシウムを配合しない比較例3~4のカプセル剤では、化合物Aの溶出は不十分であった。一方、炭酸マグネシウムを配合した実施例10~15のカプセル剤では、化合物Aの溶出は良好であった(図3および図4)。
化合物A、沈降炭酸カルシウムまたは炭酸マグネシウム、ヒドロキシプロピルセルロース、クロスカルメロースナトリウム(商品名:Ac-di-sol、旭化成)に精製水を加えて、乳鉢と乳棒を使用して造粒した後、乾燥した顆粒を顆粒径1mm未満となるように整粒した。その後、整粒した顆粒に、表6の処方割合に従って、結晶セルロース(商品名:セオラスPH-102、旭化成)、低置換度ヒドロキシプロピルセルロースおよびタルク(商品名:ハイフィラー17、岩井化学)を添加し、十分に混合した。得られた混合物100mgを4号のハードカプセルに充填し、実施例16~17のカプセル剤を製造した。また、同様に表7の処方割合に従って、沈降炭酸カルシウムおよび炭酸マグネシウムを含まず、代わりにマンニトールまたはタルクを含む比較例5~6のカプセル剤を製造した。
試験例1と同様の方法で、実施例16~17および比較例5のカプセル剤について、化合物Aの溶出性を検討した。炭酸カルシウムおよび炭酸マグネシウムを配合しない比較例5のカプセル剤では、化合物Aの溶出は不十分であった。一方、炭酸カルシウムまたは炭酸マグネシウムを配合した実施例16~17のカプセル剤では、化合物Aの溶出は良好であった(図5)。
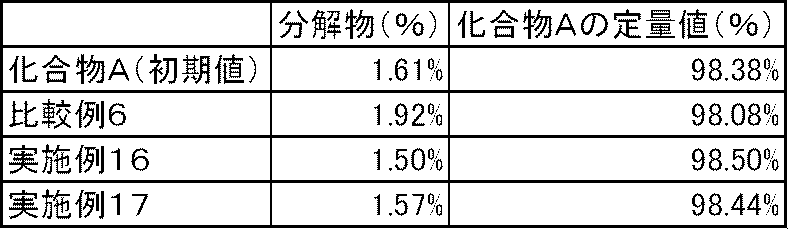
実施例16~17および比較例6のカプセル剤について、温度60℃、相対湿度75%の環境に開放系で1週間保存した後、分解物の生成を高速液体クロマトグラフで測定した。炭酸カルシウムおよび炭酸マグネシウムを配合しない比較例6のカプセル剤では、分解物が増加した。一方、炭酸カルシウムまたは炭酸マグネシウムを配合した実施例16~17のカプセル剤では、分解物の増加は認められなかった(表8)。
実施例4~9、比較例1~2と同様の方法で、表9および10の処方に従って各成分を混合した。得られた混合物100mgを3号のハードカプセルに充填し、実施例18~19、比較例7~10のカプセル剤を製造した。
試験例1と同様の方法で、実施例18~19、比較例7~10のカプセル剤について、化合物Aの溶出性を検討した。その結果、酸化カルシウム、水酸化カルシウム、酸化マグネシウムまたは水酸化マグネシウムを配合した比較例7~10のカプセルでは、化合物Aの溶出は不十分であった。一方、炭酸カルシウムまたは炭酸マグネシウムを配合した実施例18~19のカプセルでは、化合物Aの溶出は良好であった(図6および図7)。
Claims (12)
- 塩基性物質が炭酸塩である請求項1に記載の組成物。
- 塩がアルカリ土類金属塩である請求項2に記載の組成物。
- アルカリ土類金属塩がマグネシウム塩またはカルシウム塩である請求項3に記載の組成物。
- 崩壊剤を含む請求項1~4のいずれか一項に記載の組成物。
- 崩壊剤がカルメロースナトリウム、カルメロースカルシウム、カルボキシメチルスターチナトリウム、クロスカルメロースナトリウム、低置換度ヒドロキシプロピルセルロースまたはクロスポビドンである請求項5に記載の組成物。
- R1が水素原子、メチル基、エチル基、n-プロピル基またはシクロプロピル基である請求項1~6のいずれか一項に記載の組成物。
- R1がシクロプロピル基である請求項1~7のいずれか一項に記載の組成物。
- R2が水素原子、メトキシ基またはエトキシ基である請求項1~8のいずれか一項に記載の組成物。
- R2が水素原子である請求項1~9のいずれか一項に記載の組成物。
- 薬理学的に許容される塩が、塩酸塩、臭化水素酸塩、p-トルエンスルホン酸塩、硫酸塩、メタンスルホン酸塩またはエタンスルホン酸塩である請求項1~10のいずれか一項に記載の組成物。
- 式(I)で示される化合物が4-(3-クロロ-4-(シクロプロピルアミノカルボニル)アミノフェノキシ)-7-メトキシ-6-キノリンカルボキシアミドのメタンスルホン酸塩である請求項1~11のいずれか一項に記載の組成物。
Priority Applications (29)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PL10809938T PL2468281T3 (pl) | 2009-08-19 | 2010-08-16 | Kompozycja farmaceutyczna zawierająca pochodną chinoliny |
| DK10809938.3T DK2468281T3 (en) | 2009-08-19 | 2010-08-16 | Quinolinderivatholdig pharmaceutical composition |
| BR112012003592A BR112012003592B8 (pt) | 2009-08-19 | 2010-08-16 | composição farmacêutica contendo derivado de quinolina |
| CN2010800305086A CN102470133B (zh) | 2009-08-19 | 2010-08-16 | 含有喹啉衍生物的药物组合物 |
| KR1020127003846A KR101496395B1 (ko) | 2009-08-19 | 2010-08-16 | 퀴놀린 유도체 함유 의약 조성물 |
| RU2012103471A RU2548673C3 (ru) | 2009-08-19 | 2010-08-16 | Фармацевтическая композиция, содержащая производное хинолина |
| HRP20160283TT HRP20160283T1 (hr) | 2009-08-19 | 2010-08-16 | Farmaceutski pripravak koji sadrži derivat kinolina |
| MEP-2016-45A ME02359B (me) | 2009-08-19 | 2010-08-16 | Farmaceutska kompozicija koja sadrži derivate hinolina |
| NZ598291A NZ598291A (en) | 2009-08-19 | 2010-08-16 | Quinoline derivative-containing pharmaceutical composition |
| MX2012002011A MX2012002011A (es) | 2009-08-19 | 2010-08-16 | Composicion farmaceutica que contiene derivado de quinolina. |
| SG2011086022A SG178009A1 (en) | 2009-08-19 | 2010-08-16 | Quinoline derivative-containing pharmaceutical composition |
| US13/322,961 US20120077842A1 (en) | 2009-08-19 | 2010-08-16 | Quinoline derivative-containing pharmaceutical composition |
| HK12110288.6A HK1169599B (en) | 2009-08-19 | 2010-08-16 | Quinoline derivative-containing pharmaceutical composition |
| UAA201203132A UA105671C2 (uk) | 2009-08-19 | 2010-08-16 | Фармацевтична композиція, яка містить похідне хіноліну |
| RS20160176A RS54686B1 (sr) | 2009-08-19 | 2010-08-16 | Farmaceutska kompozicija koja sadrži derivate hinolina |
| JP2011527665A JP5048871B2 (ja) | 2009-08-19 | 2010-08-16 | キノリン誘導体含有医薬組成物 |
| MX2014010594A MX344927B (es) | 2009-08-19 | 2010-08-16 | Composición farmacéutica que contiene derivado de quinolina. |
| HK12108351.2A HK1167607B (en) | 2009-08-19 | 2010-08-16 | Quinoline derivative-containing pharmaceutical composition |
| SI201031141A SI2468281T1 (sl) | 2009-08-19 | 2010-08-16 | Farmacevtski sestavki, ki vsebujejo derivate kinolina |
| ES10809938.3T ES2564797T3 (es) | 2009-08-19 | 2010-08-16 | Composición farmacéutica con contenido en un derivado de quinolina |
| CA2771403A CA2771403C (en) | 2009-08-19 | 2010-08-16 | Quinoline derivative-containing pharmaceutical composition |
| AU2010285740A AU2010285740C1 (en) | 2009-08-19 | 2010-08-16 | Quinoline derivative-containing pharmaceutical composition |
| EP10809938.3A EP2468281B1 (en) | 2009-08-19 | 2010-08-16 | Quinoline derivative-containing pharmaceutical composition |
| ZA2011/08697A ZA201108697B (en) | 2009-08-19 | 2011-11-25 | Quinoline derivatives-containing pharmaceutical composition |
| IL217197A IL217197A (en) | 2009-08-19 | 2011-12-25 | A pharmaceutical preparation containing a quinoline derivative |
| MA34683A MA33581B1 (fr) | 2009-08-19 | 2012-03-12 | Composition pharmaceutique contenant un dérivé de quinoléine |
| US13/923,858 US20130296365A1 (en) | 2009-08-19 | 2013-06-21 | Quinoline derivative-containing pharmaceutical composition |
| SM201600077T SMT201600077B (it) | 2009-08-19 | 2016-03-17 | Composizione farmaceutica contenente un derivato della chinolina |
| US17/228,025 US12508313B2 (en) | 2009-08-19 | 2021-04-12 | Quinoline derivative-containing pharmaceutical composition |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2009-190145 | 2009-08-19 | ||
| JP2009190145 | 2009-08-19 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/322,961 A-371-Of-International US20120077842A1 (en) | 2009-08-19 | 2010-08-16 | Quinoline derivative-containing pharmaceutical composition |
| US13/923,858 Continuation US20130296365A1 (en) | 2009-08-19 | 2013-06-21 | Quinoline derivative-containing pharmaceutical composition |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011021597A1 true WO2011021597A1 (ja) | 2011-02-24 |
Family
ID=43607048
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2010/063804 Ceased WO2011021597A1 (ja) | 2009-08-19 | 2010-08-16 | キノリン誘導体含有医薬組成物 |
Country Status (32)
| Country | Link |
|---|---|
| US (3) | US20120077842A1 (ja) |
| EP (1) | EP2468281B1 (ja) |
| JP (1) | JP5048871B2 (ja) |
| KR (1) | KR101496395B1 (ja) |
| CN (1) | CN102470133B (ja) |
| AU (1) | AU2010285740C1 (ja) |
| BR (1) | BR112012003592B8 (ja) |
| CA (1) | CA2771403C (ja) |
| CL (1) | CL2012000412A1 (ja) |
| CO (1) | CO6440512A2 (ja) |
| CY (1) | CY1117481T1 (ja) |
| DK (1) | DK2468281T3 (ja) |
| ES (1) | ES2564797T3 (ja) |
| HR (1) | HRP20160283T1 (ja) |
| HU (1) | HUE026957T2 (ja) |
| IL (1) | IL217197A (ja) |
| MA (1) | MA33581B1 (ja) |
| ME (1) | ME02359B (ja) |
| MX (2) | MX344927B (ja) |
| MY (1) | MY162940A (ja) |
| NZ (1) | NZ598291A (ja) |
| PE (1) | PE20121030A1 (ja) |
| PL (1) | PL2468281T3 (ja) |
| RS (1) | RS54686B1 (ja) |
| RU (1) | RU2548673C3 (ja) |
| SG (1) | SG178009A1 (ja) |
| SI (1) | SI2468281T1 (ja) |
| SM (1) | SMT201600077B (ja) |
| TH (1) | TH121482A (ja) |
| UA (1) | UA105671C2 (ja) |
| WO (1) | WO2011021597A1 (ja) |
| ZA (1) | ZA201108697B (ja) |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016136745A1 (ja) * | 2015-02-25 | 2016-09-01 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | キノリン誘導体の苦味抑制方法 |
| WO2017186197A1 (en) | 2016-04-27 | 2017-11-02 | Zentiva, K.S. | Salts of lenvatinib |
| US10259791B2 (en) | 2014-08-28 | 2019-04-16 | Eisai R&D Management Co., Ltd. | High-purity quinoline derivative and method for manufacturing same |
| US10517861B2 (en) | 2013-05-14 | 2019-12-31 | Eisai R&D Management Co., Ltd. | Biomarkers for predicting and assessing responsiveness of endometrial cancer subjects to lenvatinib compounds |
| US11369623B2 (en) | 2015-06-16 | 2022-06-28 | Prism Pharma Co., Ltd. | Anticancer combination of a CBP/catenin inhibitor and an immune checkpoint inhibitor |
| US11547705B2 (en) | 2015-03-04 | 2023-01-10 | Merck Sharp & Dohme Llc | Combination of a PD-1 antagonist and a VEGF-R/FGFR/RET tyrosine kinase inhibitor for treating cancer |
| US12220398B2 (en) | 2015-08-20 | 2025-02-11 | Eisai R&D Management Co., Ltd. | Tumor therapeutic agent |
| US12226409B2 (en) | 2017-05-16 | 2025-02-18 | Eisai R&D Management Co., Ltd. | Treatment of hepatocellular carcinoma |
| US12303505B2 (en) | 2017-02-08 | 2025-05-20 | Eisai R&D Management Co., Ltd. | Tumor-treating pharmaceutical composition |
| US12508313B2 (en) | 2009-08-19 | 2025-12-30 | Eisai R&D Management Co., Ltd. | Quinoline derivative-containing pharmaceutical composition |
Families Citing this family (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8969379B2 (en) | 2004-09-17 | 2015-03-03 | Eisai R&D Management Co., Ltd. | Pharmaceutical compositions of 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7=methoxy-6-quinolinecarboxide |
| JP4989476B2 (ja) | 2005-08-02 | 2012-08-01 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | 血管新生阻害物質の効果を検定する方法 |
| JP5209966B2 (ja) * | 2005-09-01 | 2013-06-12 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | 崩壊性の改善された医薬組成物の製造方法 |
| WO2007052849A1 (ja) | 2005-11-07 | 2007-05-10 | Eisai R & D Management Co., Ltd. | 血管新生阻害物質とc-kitキナーゼ阻害物質との併用 |
| JP5190361B2 (ja) * | 2006-05-18 | 2013-04-24 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | 甲状腺癌に対する抗腫瘍剤 |
| CN101511793B (zh) * | 2006-08-28 | 2011-08-03 | 卫材R&D管理有限公司 | 针对未分化型胃癌的抗肿瘤剂 |
| WO2008093855A1 (ja) * | 2007-01-29 | 2008-08-07 | Eisai R & D Management Co., Ltd. | 未分化型胃癌治療用組成物 |
| EP2218712B1 (en) * | 2007-11-09 | 2015-07-01 | Eisai R&D Management Co., Ltd. | Combination of anti-angiogenic substance and anti-tumor platinum complex |
| BR112012032462A2 (pt) | 2010-06-25 | 2016-11-08 | Eisai R&D Man Co Ltd | agente antitumoral empregando compostos que, em combinação, têm efeito inibidor de quinase. |
| US8962650B2 (en) | 2011-04-18 | 2015-02-24 | Eisai R&D Management Co., Ltd. | Therapeutic agent for tumor |
| ES2705950T3 (es) | 2011-06-03 | 2019-03-27 | Eisai R&D Man Co Ltd | Biomarcadores para predecir y valorar la capacidad de respuesta de sujetos con cáncer de tiroides y de riñón a compuestos de lenvatinib |
| CN104755463A (zh) | 2012-12-21 | 2015-07-01 | 卫材R&D管理有限公司 | 非晶态形式的喹啉衍生物及其生产方法 |
| CN106139156B (zh) * | 2014-11-14 | 2019-01-29 | 江苏恒瑞医药股份有限公司 | 一种含有喹啉衍生物或其盐的药物组合物 |
| CN106075456A (zh) * | 2015-04-27 | 2016-11-09 | 南京圣和药业股份有限公司 | 一种含乐伐替尼的药物组合物及其应用 |
| EP3287444A4 (en) * | 2015-05-21 | 2018-09-12 | Crystal Pharmatech Co., Ltd. | New crystal form of lenvatinib methanesulfonate salt and preparation method thereof |
| WO2017028660A1 (zh) * | 2015-08-17 | 2017-02-23 | 江苏恒瑞医药股份有限公司 | 一种含有喹啉衍生物或其盐的药物组合物 |
| RU2606592C1 (ru) * | 2015-10-07 | 2017-01-10 | Открытое Акционерное Общество "Татхимфармпрепараты" | Фармацевтическая композиция, содержащая кальциевую соль розувастатина (варианты) |
| PL3384901T3 (pl) | 2017-04-04 | 2025-01-13 | Synthon B.V. | Kompozycja farmaceutyczna zawierająca mesylan lenwatynibu |
| US10583133B2 (en) | 2018-03-12 | 2020-03-10 | Shilpa Medicare Limited | Pharmaceutical compositions of lenvatinib |
| CN110404079B (zh) * | 2018-04-27 | 2023-01-24 | 北京睿创康泰医药研究院有限公司 | 一种不含碳酸盐、低基因毒性杂质含量的喹啉衍生物或其盐的药物组合物 |
| AU2019352722A1 (en) | 2018-10-04 | 2021-04-01 | Synthon B.V. | Crystalline forms and processes of lenvatinib besylate |
| EP3632436B1 (en) | 2018-10-04 | 2022-04-20 | Synthon B.V. | Pharmaceutical composition comprising lenvatinib salts |
| CN113087666B (zh) * | 2020-01-09 | 2021-12-14 | 南京正大天晴制药有限公司 | 无定形喹啉甲酰胺衍生物的制备方法 |
| WO2021185006A1 (zh) * | 2020-03-18 | 2021-09-23 | 上海博志研新药物技术有限公司 | 一种仑伐替尼药物组合物、其制备方法及应用 |
| EP4147689A1 (en) | 2021-09-13 | 2023-03-15 | Lotus Pharmaceutical Co., Ltd. | Lenvatinib formulation |
| CN114306271B (zh) * | 2021-11-24 | 2023-04-07 | 石药集团中奇制药技术(石家庄)有限公司 | 一种仑伐替尼组合物 |
| CN115671074B (zh) * | 2022-11-15 | 2024-06-04 | 郑州德迈药业有限公司 | 一种甲磺酸仑伐替尼制剂及其制备方法 |
| EP4424303A1 (en) | 2023-02-28 | 2024-09-04 | Stada Arzneimittel Ag | Lenvatinib composition with improved bioavailability |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002032872A1 (fr) * | 2000-10-20 | 2002-04-25 | Eisai Co., Ltd. | Composes a noyau aromatique azote |
| JP2004155773A (ja) * | 2002-10-16 | 2004-06-03 | Takeda Chem Ind Ltd | 安定な固形製剤 |
| WO2004080462A1 (ja) * | 2003-03-10 | 2004-09-23 | Eisai Co., Ltd. | c-Kitキナーゼ阻害剤 |
| WO2005063713A1 (ja) * | 2003-12-25 | 2005-07-14 | Eisai Co., Ltd. | 4−(3−クロロ−4−(シクロプロピルアミノカルボニル)アミノフェノキシ)−7−メトキシ−6−キノリンカルボキサミドの塩またはその溶媒和物の結晶およびそれらの製造方法 |
| WO2006030826A1 (ja) * | 2004-09-17 | 2006-03-23 | Eisai R & D Management Co., Ltd. | 医薬組成物 |
Family Cites Families (324)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CU22545A1 (es) | 1994-11-18 | 1999-03-31 | Centro Inmunologia Molecular | Obtención de un anticuerpo quimérico y humanizado contra el receptor del factor de crecimiento epidérmico para uso diagnóstico y terapéutico |
| GB1458148A (en) * | 1974-04-19 | 1976-12-08 | Wyeth John & Brother Ltd | Carbocyclic-fused ring quinoline derivatives |
| JPS57123267A (en) | 1981-01-23 | 1982-07-31 | Kansai Paint Co Ltd | Thermosetting paint composition |
| SE8103843L (sv) | 1981-06-18 | 1982-12-19 | Astra Laekemedel Ab | Farmaceutisk mixtur |
| JPS59101423A (ja) | 1982-12-02 | 1984-06-12 | Takada Seiyaku Kk | 新規なニフエジピン固形製剤 |
| US4526988A (en) | 1983-03-10 | 1985-07-02 | Eli Lilly And Company | Difluoro antivirals and intermediate therefor |
| ATE85080T1 (de) | 1984-02-17 | 1993-02-15 | Genentech Inc | Menschlicher transformationswachstumsfaktor und vorlaeufer oder fragment hiervon, zellen, dna, vektoren und verfahren zu ihrer herstellung, zusammensetzungen und produkte, die diese enthalten, sowie davon abgeleitete antikoerper und diagnostizierverfahren. |
| US4582789A (en) | 1984-03-21 | 1986-04-15 | Cetus Corporation | Process for labeling nucleic acids using psoralen derivatives |
| DE8411409U1 (de) | 1984-04-11 | 1984-08-30 | Dr.-Ing. Walter Frohn-Betriebe, 8000 München | Entgasungsventil fuer lager- und/oder transportbehaelter |
| US4563417A (en) | 1984-08-31 | 1986-01-07 | Miles Laboratories, Inc. | Nucleic acid hybridization assay employing antibodies to intercalation complexes |
| EP0183858B1 (de) | 1984-11-22 | 1988-09-14 | Holsten-Brauerei AG | Bier und Verfahren zu dessen Herstellung |
| DE3587500T2 (de) | 1984-12-04 | 1993-12-16 | Lilly Co Eli | Tumorbehandlung bei Säugetieren. |
| JPS62168137A (ja) | 1985-12-20 | 1987-07-24 | Fuji Photo Film Co Ltd | ハロゲン化銀カラ−写真感光材料およびその処理方法 |
| JPH07106295B2 (ja) | 1986-07-22 | 1995-11-15 | エーザイ株式会社 | 調湿剤 |
| US4743450A (en) | 1987-02-24 | 1988-05-10 | Warner-Lambert Company | Stabilized compositions |
| CA1339136C (en) | 1987-07-01 | 1997-07-29 | Sailesh Amilal Varia | Amorphous form of aztreonam |
| US5009894A (en) * | 1988-03-07 | 1991-04-23 | Baker Cummins Pharmaceuticals, Inc. | Arrangement for and method of administering a pharmaceutical preparation |
| AU4128089A (en) | 1988-09-15 | 1990-03-22 | Rorer International (Overseas) Inc. | Monoclonal antibodies specific to human epidermal growth factor receptor and therapeutic methods employing same |
| US5143854A (en) | 1989-06-07 | 1992-09-01 | Affymax Technologies N.V. | Large scale photolithographic solid phase synthesis of polypeptides and receptor binding screening thereof |
| US4983615A (en) | 1989-06-28 | 1991-01-08 | Hoechst-Roussel Pharmaceuticals Inc. | Heteroarylamino- and heteroaryloxypyridinamine compounds which are useful in treating skin disorders |
| US5180818A (en) | 1990-03-21 | 1993-01-19 | The University Of Colorado Foundation, Inc. | Site specific cleavage of single-stranded dna |
| US5210015A (en) | 1990-08-06 | 1993-05-11 | Hoffman-La Roche Inc. | Homogeneous assay system using the nuclease activity of a nucleic acid polymerase |
| DE69132843T2 (de) | 1990-12-06 | 2002-09-12 | Affymetrix, Inc. (N.D.Ges.D.Staates Delaware) | Identifizierung von Nukleinsäuren in Proben |
| GB9105677D0 (en) | 1991-03-19 | 1991-05-01 | Ici Plc | Heterocyclic compounds |
| US5367057A (en) | 1991-04-02 | 1994-11-22 | The Trustees Of Princeton University | Tyrosine kinase receptor flk-2 and fragments thereof |
| US5721237A (en) | 1991-05-10 | 1998-02-24 | Rhone-Poulenc Rorer Pharmaceuticals Inc. | Protein tyrosine kinase aryl and heteroaryl quinazoline compounds having selective inhibition of HER-2 autophosphorylation properties |
| US5710158A (en) | 1991-05-10 | 1998-01-20 | Rhone-Poulenc Rorer Pharmaceuticals Inc. | Aryl and heteroaryl quinazoline compounds which inhibit EGF and/or PDGF receptor tyrosine kinase |
| CA2102780C (en) | 1991-05-10 | 2007-01-09 | Alfred P. Spada | Bis mono-and bicyclic aryl and heteroaryl compounds which inhibit egf and/or pdgf receptor tyrosine kinase |
| JPH04341454A (ja) | 1991-05-16 | 1992-11-27 | Canon Inc | シート収納装置 |
| US5750376A (en) | 1991-07-08 | 1998-05-12 | Neurospheres Holdings Ltd. | In vitro growth and proliferation of genetically modified multipotent neural stem cells and their progeny |
| JPH05194259A (ja) | 1991-08-30 | 1993-08-03 | Mitsubishi Kasei Corp | 抗消化性潰瘍剤 |
| CA2137275A1 (en) | 1992-06-03 | 1993-12-09 | Richard L. Eckert | Bandage for continuous application of biologicals |
| TW271400B (ja) | 1992-07-30 | 1996-03-01 | Pfizer | |
| GB9221220D0 (en) | 1992-10-09 | 1992-11-25 | Sandoz Ag | Organic componds |
| JPH06153952A (ja) | 1992-11-26 | 1994-06-03 | Nobuaki Tamamaki | 微量未知二重鎖dna分子の増幅、標識を行うための前処理方法 |
| GB9323290D0 (en) | 1992-12-10 | 1994-01-05 | Zeneca Ltd | Quinazoline derivatives |
| SG45369A1 (en) | 1993-01-19 | 1998-10-16 | Warner Lambert Co | Stable oral ci-981 formulation and process of preparing same |
| US6027880A (en) | 1995-08-02 | 2000-02-22 | Affymetrix, Inc. | Arrays of nucleic acid probes and methods of using the same for detecting cystic fibrosis |
| JPH07176103A (ja) | 1993-12-20 | 1995-07-14 | Canon Inc | 光磁気記録再生システムならびにこれに用いる磁気ヘッド及び光磁気記録媒体 |
| GB9326136D0 (en) | 1993-12-22 | 1994-02-23 | Erba Carlo Spa | Biologically active 3-substituted oxindole derivatives useful as anti-angiogenic agents |
| IL112249A (en) | 1994-01-25 | 2001-11-25 | Warner Lambert Co | Pharmaceutical compositions containing di and tricyclic pyrimidine derivatives for inhibiting tyrosine kinases of the epidermal growth factor receptor family and some new such compounds |
| US6811779B2 (en) | 1994-02-10 | 2004-11-02 | Imclone Systems Incorporated | Methods for reducing tumor growth with VEGF receptor antibody combined with radiation and chemotherapy |
| JP3660391B2 (ja) | 1994-05-27 | 2005-06-15 | 株式会社東芝 | 半導体装置の製造方法 |
| JPH0848078A (ja) | 1994-08-05 | 1996-02-20 | Nippon Paper Ind Co Ltd | 感熱記録体 |
| GB9510757D0 (en) | 1994-09-19 | 1995-07-19 | Wellcome Found | Therapeuticaly active compounds |
| US5656454A (en) | 1994-10-04 | 1997-08-12 | President And Fellows Of Harvard College | Endothelial cell-specific enhancer |
| IL115256A0 (en) | 1994-11-14 | 1995-12-31 | Warner Lambert Co | 6-Aryl pyrido (2,3-d) pyrimidines and naphthyridines and their use |
| JPH08176138A (ja) | 1994-12-19 | 1996-07-09 | Mercian Corp | イソクマリン誘導体 |
| US5948438A (en) | 1995-01-09 | 1999-09-07 | Edward Mendell Co., Inc. | Pharmaceutical formulations having improved disintegration and/or absorptivity |
| US5658374A (en) | 1995-02-28 | 1997-08-19 | Buckman Laboratories International, Inc. | Aqueous lecithin-based release aids and methods of using the same |
| US5624937A (en) | 1995-03-02 | 1997-04-29 | Eli Lilly And Company | Chemical compounds as inhibitors of amyloid beta protein production |
| EP0817775B1 (en) | 1995-03-30 | 2001-09-12 | Pfizer Inc. | Quinazoline derivatives |
| GB9508538D0 (en) | 1995-04-27 | 1995-06-14 | Zeneca Ltd | Quinazoline derivatives |
| US5880141A (en) | 1995-06-07 | 1999-03-09 | Sugen, Inc. | Benzylidene-Z-indoline compounds for the treatment of disease |
| EP0831829B1 (en) | 1995-06-07 | 2003-08-20 | Pfizer Inc. | Heterocyclic ring-fused pyrimidine derivatives |
| JPH0923885A (ja) | 1995-07-12 | 1997-01-28 | Dai Ichi Seiyaku Co Ltd | 遺伝子発現ライブラリー及びその製造法 |
| GB9514265D0 (en) | 1995-07-13 | 1995-09-13 | Wellcome Found | Hetrocyclic compounds |
| GB9520822D0 (en) | 1995-10-11 | 1995-12-13 | Wellcome Found | Therapeutically active compounds |
| AR004010A1 (es) | 1995-10-11 | 1998-09-30 | Glaxo Group Ltd | Compuestos heterociclicos |
| US6346398B1 (en) | 1995-10-26 | 2002-02-12 | Ribozyme Pharmaceuticals, Inc. | Method and reagent for the treatment of diseases or conditions related to levels of vascular endothelial growth factor receptor |
| JP4009681B2 (ja) | 1995-11-07 | 2007-11-21 | キリンファーマ株式会社 | 血小板由来成長因子受容体自己リン酸化を阻害するキノリン誘導体ならびにキナゾリン誘導体およびそれらを含有する薬学的組成物 |
| US5849759A (en) | 1995-12-08 | 1998-12-15 | Berlex Laboratories, Inc. | Naphthyl-substituted benzimidazole derivatives as anti-coagulants |
| GB9604361D0 (en) | 1996-02-29 | 1996-05-01 | Pharmacia Spa | 4-Substituted pyrrolopyrimidine compounds as tyrosine kinase inhibitors |
| JPH09234074A (ja) | 1996-03-04 | 1997-09-09 | Sumitomo Electric Ind Ltd | アダプター二本鎖dna及びそれを用いたdna増幅方法 |
| DE69729583T2 (de) | 1996-04-17 | 2005-06-09 | Bristol-Myers Squibb Pharma Co. | N-(amidinophenyl)-n'-(subst.)-3h-2,4-benzodiazepin-3-on derivative als faktor xa inhibitoren |
| US6057100A (en) | 1996-06-07 | 2000-05-02 | Eos Biotechnology, Inc. | Oligonucleotide arrays |
| CA2258093A1 (en) | 1996-06-28 | 1998-01-08 | Mark E. Duggan | Fibrinogen receptor antagonists |
| HRP970371A2 (en) | 1996-07-13 | 1998-08-31 | Kathryn Jane Smith | Heterocyclic compounds |
| ES2186908T3 (es) | 1996-07-13 | 2003-05-16 | Glaxo Group Ltd | Compuestos heterociciclos condensados como inhibidores de pproteina-tirosina-quinasas. |
| US6207669B1 (en) | 1996-07-13 | 2001-03-27 | Glaxo Wellcome Inc. | Bicyclic heteroaromatic compounds as protein tyrosine kinase inhibitors |
| CA2263479A1 (en) | 1996-09-25 | 1998-04-02 | Zeneca Limited | Quinoline derivatives inhibiting the effect of growth factors such as vegf |
| WO1998014437A1 (en) | 1996-09-30 | 1998-04-09 | Nihon Nohyaku Co., Ltd. | 1,2,3-thiadiazole derivatives and salts thereof, disease controlling agents for agricultural and horticultural use, and method for the use thereof |
| EP0837063A1 (en) | 1996-10-17 | 1998-04-22 | Pfizer Inc. | 4-Aminoquinazoline derivatives |
| IL129825A0 (en) | 1996-11-27 | 2000-02-29 | Pfizer | Fused bicyclic pyrimidine derivatives |
| WO1998032436A1 (en) | 1997-01-29 | 1998-07-30 | Eli Lilly And Company | Treatment for premenstrual dysphoric disorder |
| CO4950519A1 (es) | 1997-02-13 | 2000-09-01 | Novartis Ag | Ftalazinas, preparaciones farmaceuticas que las comprenden y proceso para su preparacion |
| ATE345339T1 (de) | 1997-02-19 | 2006-12-15 | Berlex Lab | N-heterocyclische derivate als nos inhibitoren |
| US6090556A (en) | 1997-04-07 | 2000-07-18 | Japan Science & Technology Corporation | Method for quantitatively determining the expression of a gene |
| WO1998050346A2 (en) | 1997-04-18 | 1998-11-12 | Smithkline Beecham Plc | Acetamide and urea derivatives, process for their preparation and their use in the treatment of cns disorders |
| CA2290520C (en) | 1997-05-23 | 2009-01-27 | Bayer Corporation | Inhibition of p38 kinase activity by aryl ureas |
| JP2002503248A (ja) | 1997-06-10 | 2002-01-29 | シントン・ベスローテン・フェンノートシャップ | 4−フェニルピペリジン化合物 |
| US6093742A (en) | 1997-06-27 | 2000-07-25 | Vertex Pharmaceuticals, Inc. | Inhibitors of p38 |
| WO1999001738A2 (en) | 1997-06-30 | 1999-01-14 | University Of Maryland, Baltimore | Heparin binding-epidermal growth factor in the diagnosis of interstitial cystitis |
| CN1261794A (zh) | 1997-07-01 | 2000-08-02 | 辉瑞产品公司 | 增溶的舍曲林组合物 |
| BE1011251A3 (fr) | 1997-07-03 | 1999-06-01 | Ucb Sa | Compositions pharmaceutiques administrables par voie orale, comprenant une substance active et une cyclodextrine. |
| CO4940418A1 (es) | 1997-07-18 | 2000-07-24 | Novartis Ag | Modificacion de cristal de un derivado de n-fenil-2- pirimidinamina, procesos para su fabricacion y su uso |
| JP3765918B2 (ja) | 1997-11-10 | 2006-04-12 | パイオニア株式会社 | 発光ディスプレイ及びその駆動方法 |
| JP4194678B2 (ja) | 1997-11-28 | 2008-12-10 | キリンファーマ株式会社 | キノリン誘導体およびそれを含む医薬組成物 |
| CA2315646C (en) | 1997-12-22 | 2010-02-09 | Bayer Corporation | Inhibition of raf kinase using symmetrical and unsymmetrical substituted diphenyl ureas |
| TR200002618T2 (tr) | 1997-12-22 | 2001-04-20 | Bayer Corporation | Sübstitüe edilmiş heterosiklik üreler kullanılarak raf kinazın inhibe edilmesi |
| CA2315720A1 (en) | 1997-12-22 | 1999-07-01 | Bayer Corporation | Inhibition of p38 kinase activity using substituted heterocyclic ureas |
| ATE346600T1 (de) | 1997-12-22 | 2006-12-15 | Bayer Pharmaceuticals Corp | Inhibierung der p38 kinase-aktivität durch die verwendung von aryl- und heteroarylsubstituierten harnstoffen |
| GB9800575D0 (en) | 1998-01-12 | 1998-03-11 | Glaxo Group Ltd | Heterocyclic compounds |
| RS49779B (sr) | 1998-01-12 | 2008-06-05 | Glaxo Group Limited, | Biciklična heteroaromatična jedinjenja kao inhibitori protein tirozin kinaze |
| TR200002447T2 (tr) | 1998-02-25 | 2000-11-21 | Genetics Institute, Inc. | Fosfolinaz enzimlerinin inhibitörleri |
| JPH11322596A (ja) | 1998-05-12 | 1999-11-24 | Shionogi & Co Ltd | 白金錯体および環状リン酸エステルアミドを含有する抗癌剤 |
| UA60365C2 (uk) | 1998-06-04 | 2003-10-15 | Пфайзер Продактс Інк. | Похідні ізотіазолу, спосіб їх одержання, фармацевтична композиція та спосіб лікування гіперпроліферативного захворювання у ссавця |
| EP2272840B1 (en) | 1998-06-17 | 2012-08-22 | Eisai R&D Management Co., Ltd. | Intermediate compound for the preparation of halichondrin analogs |
| US6653341B1 (en) | 1998-06-17 | 2003-11-25 | Eisai Co., Ltd. | Methods and compositions for use in treating cancer |
| US8097648B2 (en) | 1998-06-17 | 2012-01-17 | Eisai R&D Management Co., Ltd. | Methods and compositions for use in treating cancer |
| NZ512189A (en) | 1998-11-19 | 2003-10-31 | Warner Lambert Co | N-[4-(3-chloro-4-fluoro-phenylamino)-7-(3-morpholin-4- yl-propoxy)-quinazolin-6-yl]-acrylamide useful as an irreversible inhibitor of tyrosine kinases |
| TWI230618B (en) | 1998-12-15 | 2005-04-11 | Gilead Sciences Inc | Pharmaceutical compositions of 9-[2-[[bis[(pivaloyloxy)methyl]phosphono]methoxy]ethyl]adenine and tablets or capsules containing the same |
| EP1140840B1 (en) | 1999-01-13 | 2006-03-22 | Bayer Pharmaceuticals Corp. | -g(v)-carboxyaryl substituted diphenyl ureas as raf kinase inhibitors |
| UA73492C2 (en) | 1999-01-19 | 2005-08-15 | Aromatic heterocyclic compounds as antiinflammatory agents | |
| NZ513006A (en) | 1999-01-22 | 2003-10-31 | Kirin Brewery | Quinoline derivatives and quinazoline derivatives |
| UA71945C2 (en) | 1999-01-27 | 2005-01-17 | Pfizer Prod Inc | Substituted bicyclic derivatives being used as anticancer agents |
| JP3270834B2 (ja) | 1999-01-27 | 2002-04-02 | ファイザー・プロダクツ・インク | 抗がん剤として有用なヘテロ芳香族二環式誘導体 |
| SK288138B6 (sk) | 1999-02-10 | 2013-11-04 | Astrazeneca Ab | Quinazoline derivatives as angiogenesis inhibitors |
| GB9904103D0 (en) | 1999-02-24 | 1999-04-14 | Zeneca Ltd | Quinoline derivatives |
| JP2000328080A (ja) | 1999-03-12 | 2000-11-28 | Shin Etsu Chem Co Ltd | シートベルト用低摩擦化処理剤 |
| RS49836B (sr) | 1999-03-31 | 2008-08-07 | Pfizer Products Inc., | Postupci i intermedijeri za dobijanje anti-kancernih jedinjenja |
| BR0010017A (pt) | 1999-04-28 | 2002-06-11 | Univ Texas | Composições e processos para o tratamento de câncer por inibição seletiva de vegf |
| WO2000071097A1 (fr) | 1999-05-20 | 2000-11-30 | Takeda Chemical Industries, Ltd. | Composition contenant du sel d'acide ascorbique |
| JP4304357B2 (ja) | 1999-05-24 | 2009-07-29 | 独立行政法人理化学研究所 | 完全長cDNAライブラリーの作成法 |
| PE20010306A1 (es) | 1999-07-02 | 2001-03-29 | Agouron Pharma | Compuestos de indazol y composiciones farmaceuticas que los contienen utiles para la inhibicion de proteina kinasa |
| AU6762400A (en) | 1999-08-12 | 2001-03-13 | Cor Therapeutics, Inc. | Inhibitors of factor xa |
| GT200000158A (es) | 1999-09-28 | 2002-03-16 | Piridinas y piridacinas sustituidas con actividad de inhibicion de angiogenesis. | |
| UA75054C2 (uk) | 1999-10-13 | 2006-03-15 | Бьорінгер Інгельхайм Фарма Гмбх & Ко. Кг | Заміщені в положенні 6 індолінони, їх одержання та їх застосування як лікарського засобу |
| JP2001131071A (ja) | 1999-10-29 | 2001-05-15 | Meiji Seika Kaisha Ltd | 非晶質および非晶質を含有する医薬組成物 |
| WO2001032926A2 (en) | 1999-11-01 | 2001-05-10 | Curagen Corporation | Differentially expressed genes involved in angiogenesis, the polypeptides encoded thereby, and methods of using the same |
| CA2389360C (en) | 1999-11-16 | 2008-06-03 | Steffen Breitfelder | Urea derivatives as anti-inflammatory agents |
| UA75055C2 (uk) | 1999-11-30 | 2006-03-15 | Пфайзер Продактс Інк. | Похідні бензоімідазолу, що використовуються як антипроліферативний засіб, фармацевтична композиція на їх основі |
| MXPA02006263A (es) | 1999-12-22 | 2004-02-26 | Sugen Inc | Metodos de modulacion de la funcion de la cinasa de tirosina c-kit de la proteina con compuestos de indolinona. |
| ATE289311T1 (de) | 1999-12-24 | 2005-03-15 | Kyowa Hakko Kogyo Kk | Kondensierte purinderivate |
| HK1049839A1 (en) | 1999-12-24 | 2003-05-30 | Kirin Pharma Kabushiki Kaisha | Quinoline and quinazoline derivatives and drugs containing the same |
| ATE369359T1 (de) | 2000-02-15 | 2007-08-15 | Sugen Inc | Pyrrol substituierte indolin-2-on protein kinase inhibitoren |
| JP3657203B2 (ja) | 2000-04-21 | 2005-06-08 | エーザイ株式会社 | 銅クロロフィリン塩含有液剤組成物 |
| EP1287029A2 (en) | 2000-06-09 | 2003-03-05 | Corixa Corporation | Compositions and methods for the therapy and diagnosis of colon cancer |
| AU2001277621A1 (en) | 2000-08-09 | 2002-03-04 | Astrazeneca Ab | Antiangiogenic bicyclic derivatives |
| TWI283575B (en) | 2000-10-31 | 2007-07-11 | Eisai Co Ltd | Medicinal compositions for concomitant use as anticancer agent |
| WO2002041882A2 (en) | 2000-11-22 | 2002-05-30 | Novartis Ag | Combination comprising an agent decreasing vegf activity and an agent decreasing egf activity |
| JP2004517080A (ja) | 2000-11-29 | 2004-06-10 | グラクソ グループ リミテッド | Tie−2および/またはvegfr−2の阻害剤として有用なベンゾイミダゾール誘導体 |
| EP1373569A4 (en) | 2001-03-02 | 2006-02-08 | Univ Pittsburgh | PCR METHOD |
| EP1490362A2 (en) | 2001-03-08 | 2004-12-29 | Millennium Pharmaceuticals, Inc. | (homo)piperazine substituted quinolines for inhibiting the phosphorylation of kinases |
| IL157898A0 (en) | 2001-04-06 | 2004-03-28 | Wyeth Corp | Antineoplastic combinations such as rapamycin together with gemcitabine or fluorouracil |
| WO2002085926A2 (de) | 2001-04-19 | 2002-10-31 | GESELLSCHAFT FüR BIOTECHNOLOGISCHE FORSCHUNG MBH (GBF) | Verfahren zur herstellung stabiler, regenerierbarer antikörper-arrays |
| JP3602513B2 (ja) | 2001-04-27 | 2004-12-15 | 麒麟麦酒株式会社 | アゾリル基を有するキノリン誘導体およびキナゾリン誘導体 |
| US6821987B2 (en) | 2001-04-27 | 2004-11-23 | Kirin Beer Kabushiki Kaisha | Quinoline derivatives and quinazoline derivatives having azolyl group |
| JP2003026576A (ja) | 2001-05-09 | 2003-01-29 | Eisai Co Ltd | 味覚改善製剤 |
| US6812341B1 (en) | 2001-05-11 | 2004-11-02 | Ambion, Inc. | High efficiency mRNA isolation methods and compositions |
| BR0209647A (pt) | 2001-05-16 | 2004-07-27 | Novartis Ag | Combinação que compreende n-{5-[4-(4-metil-piperazino-metil)-benzoilamido]-2-metil fenil}-4-(3-piridil)-2-pirimidina-amina e um agente quimioterapêutico |
| DE60233736D1 (de) | 2001-06-22 | 2009-10-29 | Kirin Pharma K K | Chinolinderivat und chinazolinderivat, die die selbstphosphorylierung des hepatocytus-proliferator-rezeptors hemmen, und diese enthaltende medizinische zusammensetzung |
| US20030013208A1 (en) | 2001-07-13 | 2003-01-16 | Milagen, Inc. | Information enhanced antibody arrays |
| GB0117144D0 (en) | 2001-07-13 | 2001-09-05 | Glaxo Group Ltd | Process |
| JP4824213B2 (ja) | 2001-07-16 | 2011-11-30 | 日本メナード化粧品株式会社 | キトサン含有錠剤 |
| GB0119467D0 (en) | 2001-08-09 | 2001-10-03 | Smithkline Beecham Plc | Novel compound |
| US7063946B2 (en) | 2001-09-10 | 2006-06-20 | Meso Scale Technologies, Llc. | Methods, reagents, kits and apparatus for protein function analysis |
| EP1427379B1 (en) | 2001-09-20 | 2008-08-13 | AB Science | Use of potent, selective and non toxic c-kit inhibitors for treating interstitial cystitis |
| US20040266779A1 (en) | 2001-09-27 | 2004-12-30 | Anderson Kenneth C. | Use of c-kit inhibitors for the treatment of myeloma |
| US6765012B2 (en) | 2001-09-27 | 2004-07-20 | Allergan, Inc. | 3-(Arylamino)methylene-1,3-dihydro-2H-indol-2-ones as kinase inhibitors |
| EP1435959A2 (en) | 2001-10-09 | 2004-07-14 | University of Cincinnati | Inhibitors of the egf receptor for the treatment of thyroid cancer |
| US7521053B2 (en) | 2001-10-11 | 2009-04-21 | Amgen Inc. | Angiopoietin-2 specific binding agents |
| US7658924B2 (en) | 2001-10-11 | 2010-02-09 | Amgen Inc. | Angiopoietin-2 specific binding agents |
| US7495104B2 (en) | 2001-10-17 | 2009-02-24 | Kirin Beer Kabushiki Kaisha | Quinoline or quinazoline derivatives inhibiting auto-phosphorylation of fibroblast growth factor receptors |
| AR037438A1 (es) | 2001-11-27 | 2004-11-10 | Wyeth Corp | 3-cianoquinolinas como inhibidores de egf-r y her2 quinasas, un proceso para su preparacion, composiciones farmaceuticas y el uso de dichos compuestos para la fabricacion de medicamentos |
| GB0201508D0 (en) | 2002-01-23 | 2002-03-13 | Novartis Ag | Organic compounds |
| WO2003074045A1 (en) | 2002-03-05 | 2003-09-12 | Eisai Co., Ltd. | Antitumor agent comprising combination of sulfonamide-containing heterocyclic compound with angiogenesis inhibitor |
| AU2003220058B2 (en) | 2002-03-12 | 2008-05-01 | Toyama Chemical Company Limited | Palatable oral suspension and method |
| WO2003079020A2 (en) | 2002-03-20 | 2003-09-25 | Dana-Farber Cancer Institute Inc. | Methods and compositions for the identification, assessment, and therapy of small cell lung cancer |
| JPWO2003093238A1 (ja) | 2002-05-01 | 2005-09-08 | 麒麟麦酒株式会社 | マクロファージコロニー刺激因子受容体自己リン酸化を阻害するキノリン誘導体およびキナゾリン誘導体 |
| UA77303C2 (en) | 2002-06-14 | 2006-11-15 | Pfizer | Derivatives of thienopyridines substituted by benzocondensed heteroarylamide useful as therapeutic agents, pharmaceutical compositions and methods for their use |
| WO2004006862A2 (en) | 2002-07-16 | 2004-01-22 | Children's Medical Center Corporation | A method for the modulation of angiogenesis |
| US7169936B2 (en) | 2002-07-23 | 2007-01-30 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Indolinone derivatives substituted in the 6-position, their preparation and their use as medicaments |
| US7252976B2 (en) | 2002-08-28 | 2007-08-07 | Board Of Regents The University Of Texas System | Quantitative RT-PCR to AC133 to diagnose cancer and monitor angiogenic activity in a cell sample |
| BR0313871A (pt) | 2002-08-30 | 2005-07-19 | Eisai Co Ltd | Derivados aromáticos contendo nitrogênio |
| EP1551378A4 (en) | 2002-10-09 | 2006-09-06 | Kosan Biosciences Inc | EPO D + 5-FU / GEMCITABIN |
| GB0223380D0 (en) | 2002-10-09 | 2002-11-13 | Astrazeneca Ab | Combination therapy |
| EP2596792A1 (en) | 2002-10-16 | 2013-05-29 | Takeda Pharmaceutical Company Limited | Stable solid preparations |
| BR0315547A (pt) * | 2002-10-21 | 2005-09-20 | Warner Lambert Co | Derivados de quinolina como antagonistas de crth2 |
| EP1566379A4 (en) | 2002-10-29 | 2005-11-09 | Kirin Brewery | CHINOLINE DERIVATIVES AND CHINAZOLINE DERIVATIVES AS INHIBITORS OF FLT3 AUTOPHOSPHORYLATION AND THE MEDICAL COMPOSITIONS CONTAINING THEREOF |
| DE10250711A1 (de) | 2002-10-31 | 2004-05-19 | Degussa Ag | Pharmazeutische und kosmetische Zubereitungen |
| MXPA05004919A (es) | 2002-11-06 | 2005-08-18 | Cyclacel Ltd | Composicion farmaceutica que comprende un inhibidor cdk y gemcitabina. |
| GB0226434D0 (en) | 2002-11-13 | 2002-12-18 | Astrazeneca Ab | Combination product |
| ITSV20020056A1 (it) | 2002-11-14 | 2004-05-15 | Alstom Transp Spa | Dispositivo e metodo di verifica di motori software logici di comando di impianti ferroviari, in particolare di impianti di stazione |
| AR042042A1 (es) | 2002-11-15 | 2005-06-08 | Sugen Inc | Administracion combinada de una indolinona con un agente quimioterapeutico para trastornos de proliferacion celular |
| CA2511970C (en) | 2003-01-14 | 2012-06-26 | Cytokinetics, Inc. | Urea derivatives useful in the treatment of heart failure |
| JP3581361B1 (ja) | 2003-02-17 | 2004-10-27 | 株式会社脳機能研究所 | 脳活動測定装置 |
| CA2517886A1 (en) | 2003-03-05 | 2004-09-16 | Celgene Corporation | Diphenylethylene compounds and uses thereof |
| MXPA05009751A (es) | 2003-03-14 | 2005-10-26 | Taisho Pharmaceutical Co Ltd | Anticuerpo monoclonal e hibridoma que lo produce. |
| RU2366655C2 (ru) | 2003-03-14 | 2009-09-10 | Оно Фармасьютикал Ко., Лтд. | Азотсодержащие гетероциклические производные и лекарственные средства, содержащие их в качестве активного ингредиента |
| HRP20050867A2 (en) | 2003-04-02 | 2005-12-31 | Pliva - Istra�ivanje i razvoj d.o.o. | Pharmaceutical compositions having reduced bitter taste |
| US20070117842A1 (en) | 2003-04-22 | 2007-05-24 | Itaru Arimoto | Polymorph of 4-[3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6- quinolinecarboxamide and a process for the preparation of the same |
| KR100503949B1 (ko) | 2003-04-28 | 2005-07-26 | 주식회사유한양행 | 염산 온단세트론의 쓴맛을 효과적으로 은폐한 경구용 구강속붕해정 조성물 |
| US7107104B2 (en) | 2003-05-30 | 2006-09-12 | Medtronic, Inc. | Implantable cortical neural lead and method |
| JP2005008534A (ja) | 2003-06-17 | 2005-01-13 | Soc De Conseils De Recherches & D'applications Scientifiques (Scras) | 抗癌剤及び癌の治療方法 |
| AU2004255022B2 (en) | 2003-07-10 | 2007-08-23 | Astrazeneca Ab | Use of the quinazoline derivative ZD6474 combined with platinum compounds and optionally ionising radiation in the treatment of diseases associated with angiogenesis and/or increased vascular permeability |
| CN100508976C (zh) | 2003-07-24 | 2009-07-08 | 史密丝克莱恩比彻姆公司 | 口腔溶解薄膜 |
| EP1653934B1 (en) | 2003-08-15 | 2008-05-14 | AB Science | Use of c-kit inhibitors for treating type ii diabetes |
| US7485658B2 (en) | 2003-08-21 | 2009-02-03 | Osi Pharmaceuticals, Inc. | N-substituted pyrazolyl-amidyl-benzimidazolyl c-Kit inhibitors |
| RU2006108791A (ru) | 2003-08-21 | 2006-07-27 | Оси Фармасьютикалз, Инк. (Us) | N-замещенные пиразолиламидилбензимидазолилы в качестве с-kit ингибиторов |
| EP1664021A1 (en) | 2003-08-21 | 2006-06-07 | OSI Pharmaceuticals, Inc. | N-substituted benzimidazolyl c-kit inhibitors |
| US7312243B1 (en) | 2003-08-29 | 2007-12-25 | Jay Pravda | Materials and methods for treatment of gastrointestinal disorders |
| JP2007505938A (ja) | 2003-09-23 | 2007-03-15 | ノバルティス アクチエンゲゼルシャフト | Vegf受容体阻害剤と化学療法剤の組み合わせ |
| PL2392565T3 (pl) | 2003-09-26 | 2014-08-29 | Exelixis Inc | Modulatory c-Met i sposoby stosowania |
| US7683172B2 (en) | 2003-11-11 | 2010-03-23 | Eisai R&D Management Co., Ltd. | Urea derivative and process for preparing the same |
| KR20060110307A (ko) | 2003-11-28 | 2006-10-24 | 노파르티스 아게 | 단백질 키나아제 의존성 질환의 치료에서의 디아릴 우레아유도체 |
| US6984403B2 (en) | 2003-12-04 | 2006-01-10 | Pfizer Inc. | Azithromycin dosage forms with reduced side effects |
| BRPI0417302A (pt) | 2003-12-05 | 2007-03-06 | Compound Therapeutics Inc | inibidores de receptores de fator de crescimento endotelial vascular do tipo 2 |
| WO2005070891A2 (en) | 2004-01-23 | 2005-08-04 | Amgen Inc | Compounds and methods of use |
| NZ547517A (en) | 2004-02-27 | 2009-04-30 | Eisai R&D Man Co Ltd | Novel pyridine and pyrimidine derivatives for hepatocyte growth and tumour inihibition |
| KR20050091462A (ko) | 2004-03-12 | 2005-09-15 | 한국과학기술연구원 | 푸로피리미딘 화합물 및 이를 포함하는 ddr2 티로신키나아제 활성 저해제 |
| US20050244493A1 (en) | 2004-04-30 | 2005-11-03 | Withiam Michael C | Rapidly disintegrating tablets comprising calcium carbonate |
| MXPA06011958A (es) | 2004-05-21 | 2006-12-15 | Chiron Corp | Derivados de quinolina sustituidos como inhibidores de cinesina mitotica. |
| CN1960732A (zh) | 2004-06-03 | 2007-05-09 | 霍夫曼-拉罗奇有限公司 | 用吉西他滨和egfr-抑制剂治疗 |
| AU2005265027A1 (en) | 2004-06-18 | 2006-01-26 | The Government Of The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Methods for the identification and use of compounds suitable for the treatment of drug resistant cancer cells |
| US20050288521A1 (en) | 2004-06-29 | 2005-12-29 | Phytogen Life Sciences Inc. | Semi-synthetic conversion of paclitaxel to docetaxel |
| WO2006030941A1 (ja) | 2004-09-13 | 2006-03-23 | Eisai R & D Management Co., Ltd. | スルホンアミド含有化合物の血管新生阻害物質との併用 |
| US8772269B2 (en) | 2004-09-13 | 2014-07-08 | Eisai R&D Management Co., Ltd. | Use of sulfonamide-including compounds in combination with angiogenesis inhibitors |
| US7306807B2 (en) | 2004-09-13 | 2007-12-11 | Wyeth | Hemorrhagic feline calicivirus, calicivirus vaccine and method for preventing calicivirus infection or disease |
| CA2581375A1 (en) | 2004-09-27 | 2006-04-06 | Kosan Biosciences Incorporated | Specific kinase inhibitors |
| RU2404992C2 (ru) | 2004-10-19 | 2010-11-27 | Эмджен Инк. | Ангиопоэтин-2-специфические связывающие агенты |
| EP1827445A2 (en) | 2004-11-22 | 2007-09-05 | King Pharmaceuticals Research and Development Inc. | Enhancing treatment of cancer and hif-1 mediated disoders with adenosine a3 receptor antagonists |
| JP4773456B2 (ja) | 2004-11-23 | 2011-09-14 | ドン ファ ファーマシューティカル カンパニー リミテッド | 生体利用率を向上させた経口用製剤 |
| EP1824843A2 (en) | 2004-12-07 | 2007-08-29 | Locus Pharmaceuticals, Inc. | Inhibitors of protein kinases |
| PL1838733T3 (pl) | 2004-12-21 | 2012-02-29 | Medimmune Ltd | Przeciwciała skierowane przeciwko angiopoetynie-2 i ich zastosowania |
| US20060198885A1 (en) | 2005-02-22 | 2006-09-07 | Sun Pharmaceutical Industries Ltd. | Oral pharmaceutical composition |
| JP5106098B2 (ja) | 2005-02-28 | 2012-12-26 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | スルホンアミド化合物の抗癌剤との新規併用 |
| PT1859793E (pt) | 2005-02-28 | 2011-07-05 | Eisai R&D Man Co Ltd | Uso combinado inovador de um composto de sulfonamida no tratamento oncológico |
| EP2161336B2 (en) | 2005-05-09 | 2017-03-29 | ONO Pharmaceutical Co., Ltd. | Human monoclonal antibodies to programmed death 1(PD-1) and methods for treating cancer using anti-PD-1 antibodies alone or in combination with other immunotherapeutics |
| RU2404774C2 (ru) | 2005-05-17 | 2010-11-27 | Актелион Фармасьютиклз Лтд | Диспергируемые таблетки бозентана |
| CA2608733A1 (en) | 2005-05-17 | 2007-02-01 | Plexxikon, Inc. | Pyrrol (2,3-b) pyridine derivatives protein kinase inhibitors |
| EP2395004B1 (en) | 2005-06-22 | 2016-01-20 | Plexxikon Inc. | Pyrrolo [2,3-b]pyridine derivatives as protein kinase inhibitors |
| WO2006137474A1 (ja) | 2005-06-23 | 2006-12-28 | Eisai R & D Management Co., Ltd. | 4-(3-クロロ-4-(シクロプロピルアミノカルボニル)アミノフェノキシ)-7-メトキシ-6-キノリンカルボキサミドの塩のアモルファスおよびその製造方法 |
| US7550483B2 (en) * | 2005-06-23 | 2009-06-23 | Eisai R&D Management Co., Ltd. | Amorphous salt of 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide and process for preparing the same |
| WO2007000347A2 (en) | 2005-06-29 | 2007-01-04 | Roselli, Patrizia | Agonistic and antagonistic peptide mimetics of the vegf alpha-helix binding region for use in therapy |
| AU2006230974C1 (en) | 2005-07-11 | 2012-02-02 | Takeda Pharma A/S | Benzimidazole formulation |
| US8101799B2 (en) | 2005-07-21 | 2012-01-24 | Ardea Biosciences | Derivatives of N-(arylamino) sulfonamides as inhibitors of MEK |
| US20080219977A1 (en) | 2005-07-27 | 2008-09-11 | Isaiah Josh Fidler | Combinations Comprising Gemcitabine and Tyrosine Kinase Inhibitors for the Treatment of Pancreatic Cancer |
| JP5066446B2 (ja) | 2005-08-01 | 2012-11-07 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | 血管新生阻害物質の効果を予測する方法 |
| JP4989476B2 (ja) | 2005-08-02 | 2012-08-01 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | 血管新生阻害物質の効果を検定する方法 |
| KR100950737B1 (ko) | 2005-08-24 | 2010-03-31 | 에자이 알앤드디 매니지먼트 가부시키가이샤 | 신규 피리딘 유도체 및 피리미딘 유도체(3) |
| JP5209966B2 (ja) | 2005-09-01 | 2013-06-12 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | 崩壊性の改善された医薬組成物の製造方法 |
| WO2007052849A1 (ja) | 2005-11-07 | 2007-05-10 | Eisai R & D Management Co., Ltd. | 血管新生阻害物質とc-kitキナーゼ阻害物質との併用 |
| WO2007061130A1 (ja) | 2005-11-22 | 2007-05-31 | Eisai R & D Management Co., Ltd. | 多発性骨髄腫に対する抗腫瘍剤 |
| AR059066A1 (es) * | 2006-01-27 | 2008-03-12 | Amgen Inc | Combinaciones del inhibidor de la angiopoyetina -2 (ang2) y el inhibidor del factor de crecimiento endotelial vascular (vegf) |
| JP5058150B2 (ja) | 2006-02-22 | 2012-10-24 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | 安定化医薬組成物 |
| WO2007109057A2 (en) | 2006-03-16 | 2007-09-27 | Novartis Ag | Solid dosage form containing a taste masked active agent |
| KR100728926B1 (ko) | 2006-03-20 | 2007-06-15 | 삼성전자주식회사 | 3축 힌지 구조를 갖는 휴대용 전자기기 |
| JP5190361B2 (ja) | 2006-05-18 | 2013-04-24 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | 甲状腺癌に対する抗腫瘍剤 |
| CA2654243A1 (en) | 2006-06-22 | 2007-12-27 | Solvay Pharmaceuticals B.V. | Oral pharmaceutical composition of a poorly water-soluble active substance |
| ES2375284T3 (es) | 2006-08-23 | 2012-02-28 | Eisai R&D Management Co., Ltd. | Sal de un derivado de fenoxipiridina, o cristal de la misma, y procedimiento de producción de la misma. |
| CN101511793B (zh) | 2006-08-28 | 2011-08-03 | 卫材R&D管理有限公司 | 针对未分化型胃癌的抗肿瘤剂 |
| BRPI0716555A2 (pt) | 2006-09-07 | 2013-09-24 | Astrazeneca Ab | mÉtodos para prever a probabilidade de que um paciente É um candidato para tratamento com uma droga de ret responderÁ ao citado tratamento, e para tratar um paciente, iniciador direto mutante de arms, e, kit diagnàstico |
| WO2008045566A1 (en) | 2006-10-12 | 2008-04-17 | Ptc Therapeutics, Inc. | Methods for dosing an orally active 1,2,4-oxadiazole for nonsense mutation suppression therapy |
| JP2009184925A (ja) | 2006-11-02 | 2009-08-20 | Dai Ichi Seiyaku Co Ltd | 5−(1h−1,2,3−トリアゾール−4−イル)−1h−ピラゾール誘導体 |
| AU2008206045A1 (en) | 2007-01-19 | 2008-07-24 | Ardea Biosciences, Inc. | Inhibitors of MEK |
| EP2116246A1 (en) | 2007-01-19 | 2009-11-11 | Eisai R&D Management Co., Ltd. | Composition for treatment of pancreatic cancer |
| WO2008093855A1 (ja) | 2007-01-29 | 2008-08-07 | Eisai R & D Management Co., Ltd. | 未分化型胃癌治療用組成物 |
| JP2008214249A (ja) | 2007-03-02 | 2008-09-18 | Takeda Chem Ind Ltd | 製剤における溶出改善方法および溶出性の改善された製剤 |
| CN101622015A (zh) | 2007-03-05 | 2010-01-06 | 协和发酵麒麟株式会社 | 药物组合物 |
| CA2680161A1 (en) | 2007-03-05 | 2008-09-12 | Kyowa Hakko Kirin Co., Ltd. | Pharmaceutical composition |
| US7807172B2 (en) | 2007-06-13 | 2010-10-05 | University Of Washington | Methods and compositions for detecting thyroglobulin in a biological sample |
| PE20090368A1 (es) | 2007-06-19 | 2009-04-28 | Boehringer Ingelheim Int | Anticuerpos anti-igf |
| CA2694646C (en) | 2007-07-30 | 2017-09-05 | Ardea Biosciences, Inc. | Combinations of mek inhibitors and raf kinase inhibitors and uses thereof |
| EP2218712B1 (en) | 2007-11-09 | 2015-07-01 | Eisai R&D Management Co., Ltd. | Combination of anti-angiogenic substance and anti-tumor platinum complex |
| JP2009132660A (ja) | 2007-11-30 | 2009-06-18 | Eisai R & D Management Co Ltd | 食道癌治療用組成物 |
| JP5399926B2 (ja) | 2008-01-29 | 2014-01-29 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | 血管阻害物質とタキサンとの併用 |
| GB2456907A (en) | 2008-01-30 | 2009-08-05 | Astrazeneca Ab | Method for determining subsequent VEGFR2 inhibitor therapy comprising measuring baseline VEGF level. |
| EP3028743A3 (en) * | 2008-03-05 | 2017-01-25 | Vicus Therapeutics, LLC | Compositions for mucositis and oncology therapies |
| US8044240B2 (en) | 2008-03-06 | 2011-10-25 | Ardea Biosciences Inc. | Polymorphic form of N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxyphenyl)-1-(2,3-dihydroxypropyl)cyclopropane-1-sulfonamide and uses thereof |
| EP2262837A4 (en) | 2008-03-12 | 2011-04-06 | Merck Sharp & Dohme | PD-1 BINDING PROTEINS |
| MX2010011314A (es) | 2008-04-14 | 2010-11-12 | Ardea Biosciences Inc | Composiciones y metodos de preparacion y uso de las mismas. |
| JP2009263298A (ja) | 2008-04-28 | 2009-11-12 | Ss Pharmaceut Co Ltd | 不快な味を隠ぺいした経口組成物 |
| US8637554B2 (en) | 2008-05-07 | 2014-01-28 | The Trustees Of The University Of Pennsylvania | Methods for treating thyroid cancer |
| EP2288383A1 (en) | 2008-05-14 | 2011-03-02 | Amgen, Inc | Combinations vegf(r) inhibitors and hepatocyte growth factor (c-met) inhibitors for the treatment of cancer |
| WO2009150255A2 (en) | 2008-06-13 | 2009-12-17 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | Markers for predicting response and survival in anti-egfr treated patients |
| CN102089007B (zh) | 2008-07-11 | 2013-05-15 | 诺瓦提斯公司 | (a)磷酸肌醇3-激酶抑制剂和(b)ras/raf/mek通路调节剂的组合产品 |
| WO2010048304A2 (en) | 2008-10-21 | 2010-04-29 | Bayer Healthcare Llc | Identification of signature genes associated with hepatocellular carcinoma |
| WO2010086964A1 (ja) | 2009-01-28 | 2010-08-05 | 株式会社 静岡カフェイン工業所 | がん治療のための併用療法 |
| US20120164148A1 (en) | 2009-08-07 | 2012-06-28 | The Wistar Institute | Compositions Containing JARID1B Inhibitors and Methods for Treating Cancer |
| WO2011021597A1 (ja) | 2009-08-19 | 2011-02-24 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | キノリン誘導体含有医薬組成物 |
| WO2011022335A1 (en) | 2009-08-21 | 2011-02-24 | Mount Sinai School Of Medicine Of New York University | Methods of using cd44 fusion proteins to treat cancer |
| EP2293071A1 (en) | 2009-09-07 | 2011-03-09 | Universität Zu Köln | Biomarker for colorectal cancer |
| BR112012032462A2 (pt) | 2010-06-25 | 2016-11-08 | Eisai R&D Man Co Ltd | agente antitumoral empregando compostos que, em combinação, têm efeito inibidor de quinase. |
| WO2012008563A1 (ja) | 2010-07-16 | 2012-01-19 | 協和発酵キリン株式会社 | 含窒素芳香族複素環誘導体 |
| EP3156800A1 (en) | 2010-07-19 | 2017-04-19 | F. Hoffmann-La Roche AG | Blood plasma biomarkers for bevacizumab combination therapies for treatment of breast cancer |
| SG187119A1 (en) | 2010-07-19 | 2013-02-28 | Hoffmann La Roche | Method to identify a patient with an increased likelihood of responding to an anti-cancer therapy |
| MX339427B (es) | 2010-07-19 | 2016-05-25 | Hoffmann La Roche | Biomarcadores de plasma de sangre para terapias de combinacion con bevacizumab para tratamiento de cancer pancreatico. |
| BR112012033162A2 (pt) | 2010-07-19 | 2016-10-25 | Hoffmann La Roche | método de identificação de pacientes, método de previsão da capacidade de reação de pacientes, método de determinação da probabilidade de um paciente com câncer exibir benefícios de terapia anticâncer, método de otimização da eficácia terapêutica, método de tratamento de câncer, kit e conjunto de compostos |
| WO2012019300A1 (en) | 2010-08-10 | 2012-02-16 | Siu K W Michael | Endometrial cancer biomarkers and methods of identifying and using same |
| JP5259880B2 (ja) | 2010-09-01 | 2013-08-07 | 興和株式会社 | 経口剤 |
| US20120077837A1 (en) | 2010-09-24 | 2012-03-29 | Eisai R&D Management Co., Ltd. | Anti-tumor agent |
| RU2568258C2 (ru) | 2011-02-28 | 2015-11-20 | Саншайн Лейк Фарма Ко., Лтд | Замещенные соединения хинолина и способы их использования |
| WO2012119095A1 (en) | 2011-03-02 | 2012-09-07 | Board Of Regents, The University Of Texas System | Fus1/tusc2 therapies |
| JP6322413B2 (ja) | 2011-03-10 | 2018-05-09 | プロヴェクタス ファーマテック,インク. | 改善されたがん治療のための局所および全身性免疫修飾療法の組み合わせ |
| DK2691112T3 (en) | 2011-03-31 | 2018-07-30 | Merck Sharp & Dohme | STABLE FORMULATIONS OF ANTIBODIES AGAINST HUMAN PD (PROGRAMMED DEATH) 1- RECEPTOR AND RELATED TREATMENTS |
| US8962650B2 (en) | 2011-04-18 | 2015-02-24 | Eisai R&D Management Co., Ltd. | Therapeutic agent for tumor |
| WO2012154935A1 (en) | 2011-05-12 | 2012-11-15 | Eisai R&D Management Co., Ltd. | Biomarkers that are predictive of responsiveness or non-responsiveness to treatment with lenvatinib or a pharmaceutically acceptable salt thereof |
| WO2012157672A1 (ja) | 2011-05-17 | 2012-11-22 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | 血管新生阻害剤の効果を予測する方法 |
| ES2705950T3 (es) | 2011-06-03 | 2019-03-27 | Eisai R&D Man Co Ltd | Biomarcadores para predecir y valorar la capacidad de respuesta de sujetos con cáncer de tiroides y de riñón a compuestos de lenvatinib |
| TR201820873T4 (tr) | 2011-08-01 | 2019-01-21 | Hoffmann La Roche | Pd-1 ekseni bağlayıcı antagonistler ve mek inhibitörlerinin kullanıldığı kanser tedavisine yönelik yöntemler. |
| AU2013201121A1 (en) | 2011-09-20 | 2013-04-04 | Vical Incorporated | Synergistic anti-tumor efficacy using alloantigen combination immunotherapy |
| JP5941558B2 (ja) | 2012-02-28 | 2016-06-29 | 株式会社ソウル製薬Seoul Pharma. Co., Ltd. | シルデナフィルを有効成分として含有し、且つ、苦味の隠蔽された高含量速溶フィルム |
| AR090263A1 (es) | 2012-03-08 | 2014-10-29 | Hoffmann La Roche | Terapia combinada de anticuerpos contra el csf-1r humano y las utilizaciones de la misma |
| KR20240135058A (ko) | 2012-05-15 | 2024-09-10 | 브리스톨-마이어스 스큅 컴퍼니 | Pd-1/pd-l1 신호전달을 방해하는 것에 의한 암 면역요법 |
| US8992915B2 (en) | 2012-05-16 | 2015-03-31 | Boehringer Ingelheim International Gmbh | Combination of CD37 antibodies with ICE |
| HRP20171788T1 (hr) | 2012-10-02 | 2017-12-29 | Bristol-Myers Squibb Company | Kombinacija anti-kir antitijela i anti-pd-1 antitijela u liječenju raka |
| EP2928464A1 (en) | 2012-12-04 | 2015-10-14 | Eisai R&D Management Co., Ltd. | Use of eribulin in the treatment of breast cancer |
| US10980804B2 (en) | 2013-01-18 | 2021-04-20 | Foundation Medicine, Inc. | Methods of treating cholangiocarcinoma |
| MX374488B (es) | 2013-03-15 | 2025-03-06 | Genentech Inc | Biomarcadores y su uso en el tratamiento de condiciones relacionadas con pd-1 y pd-l1. |
| JP6411379B2 (ja) | 2013-05-14 | 2018-10-24 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | レンバチニブ化合物に対する子宮内膜がん対象の応答性を予測及び評価するためのバイオマーカー |
| CA2915005C (en) | 2013-06-26 | 2021-12-28 | Eisai R&D Management Co., Ltd. | Use of eribulin and lenvatinib as combination therapy for treatment of cancer |
| US9174998B2 (en) | 2013-12-25 | 2015-11-03 | Eisai R&D Management Co., Ltd. | (6S,9aS)-N-benzyl-6-[(4-hydroxyphenyl)methyl]-4,7-dioxo-8-({6-[3-(piperazin-1-yl)azetidin-1-yl]pyridin-2-yl}methyl)-2-(prop-2-en-1-yl)-octahydro-1H-pyrazino[2,1-c][1,2,4]triazine-1-carboxamide compound |
| JOP20200094A1 (ar) | 2014-01-24 | 2017-06-16 | Dana Farber Cancer Inst Inc | جزيئات جسم مضاد لـ pd-1 واستخداماتها |
| EP3102237B1 (en) | 2014-02-04 | 2020-12-02 | Incyte Corporation | Combination of a pd-1 antagonist and an ido1 inhibitor for treating cancer |
| EP3122354B1 (en) | 2014-03-28 | 2022-06-15 | Universita' degli Studi di Genova | Tyrosine kinase inhibitors for use in a method of treating cancer in association with a reduced caloric intake |
| DK3524595T3 (da) | 2014-08-28 | 2022-09-19 | Eisai R&D Man Co Ltd | Quinolinderivat af høj renhed og fremgangsmåde til fremstilling deraf |
| WO2016140717A1 (en) | 2015-03-04 | 2016-09-09 | Merck Sharp & Dohme Corp. | Combination of a pd-1 antagonist and a vegfr/fgfr/ret tyrosine kinase inhibitor for treating cancer |
| EP3287444A4 (en) | 2015-05-21 | 2018-09-12 | Crystal Pharmatech Co., Ltd. | New crystal form of lenvatinib methanesulfonate salt and preparation method thereof |
| US11078278B2 (en) | 2015-05-29 | 2021-08-03 | Bristol-Myers Squibb Company | Treatment of renal cell carcinoma |
| US11369623B2 (en) | 2015-06-16 | 2022-06-28 | Prism Pharma Co., Ltd. | Anticancer combination of a CBP/catenin inhibitor and an immune checkpoint inhibitor |
| US10259817B2 (en) | 2015-06-23 | 2019-04-16 | Eisai R&D Management Co., Ltd. | Crystal (6S,9aS)-N-benzyl-8-({6-[3-(4-ethylpiperazin-1-yl)azetidin-1-yl]pyridin-2-yl}methyl)-6-(2-fluoro-4-hydroxybenzyl)-4,7-dioxo-2-(prop-2-en-1-yl)hexahydro-2H-pyrazino[2,1-c][1,2,4]triazine-1(6H)-carboxamide |
| US20200375975A1 (en) | 2016-04-15 | 2020-12-03 | Eisai R&D Management Co., Ltd. | Treatment of Renal Cell Carcinoma with Lenvatinib and Everolimus |
| CN107305202B (zh) | 2016-04-22 | 2020-04-17 | 北京睿创康泰医药研究院有限公司 | 分析甲磺酸乐伐替尼及其制剂杂质的hplc方法及杂质作参比标准的用途 |
| WO2018147275A1 (ja) | 2017-02-08 | 2018-08-16 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | 腫瘍治療用医薬組成物 |
| PL3384901T3 (pl) | 2017-04-04 | 2025-01-13 | Synthon B.V. | Kompozycja farmaceutyczna zawierająca mesylan lenwatynibu |
| CN110494423B (zh) | 2017-04-25 | 2022-04-26 | 苏州科睿思制药有限公司 | 乐伐替尼甲磺酸盐的新晶型及其制备方法 |
| CN109988112A (zh) | 2017-12-29 | 2019-07-09 | 四川科伦药物研究院有限公司 | 仑伐替尼甲磺酸盐的晶型及其制备方法 |
| US10583133B2 (en) | 2018-03-12 | 2020-03-10 | Shilpa Medicare Limited | Pharmaceutical compositions of lenvatinib |
| WO2019228485A1 (zh) | 2018-06-01 | 2019-12-05 | 成都苑东生物制药股份有限公司 | 一种甲磺酸乐伐替尼新晶型及其制备方法 |
| CN110818634B (zh) | 2018-08-13 | 2021-11-30 | 上海新礼泰药业有限公司 | 甲磺酸乐伐替尼的精制方法 |
| CN110903239A (zh) | 2018-09-18 | 2020-03-24 | 苏州科睿思制药有限公司 | 乐伐替尼甲磺酸盐的新晶型及其制备方法 |
| CN110563644A (zh) | 2019-10-30 | 2019-12-13 | 北京赛思源生物医药技术有限公司 | 一种仑伐替尼甲磺酸盐的新晶型 |
| EP4147689A1 (en) | 2021-09-13 | 2023-03-15 | Lotus Pharmaceutical Co., Ltd. | Lenvatinib formulation |
-
2010
- 2010-08-16 WO PCT/JP2010/063804 patent/WO2011021597A1/ja not_active Ceased
- 2010-08-16 MY MYPI2011700172A patent/MY162940A/en unknown
- 2010-08-16 NZ NZ598291A patent/NZ598291A/xx unknown
- 2010-08-16 JP JP2011527665A patent/JP5048871B2/ja active Active
- 2010-08-16 CA CA2771403A patent/CA2771403C/en active Active
- 2010-08-16 UA UAA201203132A patent/UA105671C2/uk unknown
- 2010-08-16 RS RS20160176A patent/RS54686B1/sr unknown
- 2010-08-16 PL PL10809938T patent/PL2468281T3/pl unknown
- 2010-08-16 ME MEP-2016-45A patent/ME02359B/me unknown
- 2010-08-16 US US13/322,961 patent/US20120077842A1/en not_active Abandoned
- 2010-08-16 AU AU2010285740A patent/AU2010285740C1/en active Active
- 2010-08-16 BR BR112012003592A patent/BR112012003592B8/pt active IP Right Grant
- 2010-08-16 TH TH1001000221A patent/TH121482A/th unknown
- 2010-08-16 HU HUE10809938A patent/HUE026957T2/en unknown
- 2010-08-16 EP EP10809938.3A patent/EP2468281B1/en active Active
- 2010-08-16 SI SI201031141A patent/SI2468281T1/sl unknown
- 2010-08-16 PE PE2011002081A patent/PE20121030A1/es active IP Right Grant
- 2010-08-16 HR HRP20160283TT patent/HRP20160283T1/hr unknown
- 2010-08-16 KR KR1020127003846A patent/KR101496395B1/ko active Active
- 2010-08-16 MX MX2014010594A patent/MX344927B/es unknown
- 2010-08-16 SG SG2011086022A patent/SG178009A1/en unknown
- 2010-08-16 ES ES10809938.3T patent/ES2564797T3/es active Active
- 2010-08-16 RU RU2012103471A patent/RU2548673C3/ru active Protection Beyond IP Right Term
- 2010-08-16 MX MX2012002011A patent/MX2012002011A/es unknown
- 2010-08-16 CN CN2010800305086A patent/CN102470133B/zh not_active Ceased
- 2010-08-16 DK DK10809938.3T patent/DK2468281T3/en active
-
2011
- 2011-11-25 ZA ZA2011/08697A patent/ZA201108697B/en unknown
- 2011-12-25 IL IL217197A patent/IL217197A/en active IP Right Grant
-
2012
- 2012-02-09 CO CO12022608A patent/CO6440512A2/es not_active Application Discontinuation
- 2012-02-16 CL CL2012000412A patent/CL2012000412A1/es unknown
- 2012-03-12 MA MA34683A patent/MA33581B1/fr unknown
-
2013
- 2013-06-21 US US13/923,858 patent/US20130296365A1/en not_active Abandoned
-
2016
- 2016-03-17 SM SM201600077T patent/SMT201600077B/xx unknown
- 2016-04-05 CY CY20161100273T patent/CY1117481T1/el unknown
-
2021
- 2021-04-12 US US17/228,025 patent/US12508313B2/en active Active
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002032872A1 (fr) * | 2000-10-20 | 2002-04-25 | Eisai Co., Ltd. | Composes a noyau aromatique azote |
| JP2004155773A (ja) * | 2002-10-16 | 2004-06-03 | Takeda Chem Ind Ltd | 安定な固形製剤 |
| WO2004080462A1 (ja) * | 2003-03-10 | 2004-09-23 | Eisai Co., Ltd. | c-Kitキナーゼ阻害剤 |
| WO2005063713A1 (ja) * | 2003-12-25 | 2005-07-14 | Eisai Co., Ltd. | 4−(3−クロロ−4−(シクロプロピルアミノカルボニル)アミノフェノキシ)−7−メトキシ−6−キノリンカルボキサミドの塩またはその溶媒和物の結晶およびそれらの製造方法 |
| WO2006030826A1 (ja) * | 2004-09-17 | 2006-03-23 | Eisai R & D Management Co., Ltd. | 医薬組成物 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP2468281A4 * |
Cited By (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12508313B2 (en) | 2009-08-19 | 2025-12-30 | Eisai R&D Management Co., Ltd. | Quinoline derivative-containing pharmaceutical composition |
| US10517861B2 (en) | 2013-05-14 | 2019-12-31 | Eisai R&D Management Co., Ltd. | Biomarkers for predicting and assessing responsiveness of endometrial cancer subjects to lenvatinib compounds |
| EP3825305A1 (en) | 2014-08-28 | 2021-05-26 | Eisai R&D Management Co., Ltd. | Process for preparing lenvatinib |
| EP4089076A1 (en) | 2014-08-28 | 2022-11-16 | Eisai R&D Management Co., Ltd. | High-purity quinoline derivative and method for manufacturing same |
| US11186547B2 (en) | 2014-08-28 | 2021-11-30 | Eisai R&D Management Co., Ltd. | High-purity quinoline derivative and method for manufacturing same |
| US10259791B2 (en) | 2014-08-28 | 2019-04-16 | Eisai R&D Management Co., Ltd. | High-purity quinoline derivative and method for manufacturing same |
| EP3524595A1 (en) | 2014-08-28 | 2019-08-14 | Eisai R&D Management Co., Ltd. | High-purity quinoline derivative and method for manufacturing same |
| US10407393B2 (en) | 2014-08-28 | 2019-09-10 | Eisai R&D Management Co., Ltd. | High-purity quinoline derivative and method for manufacturing same |
| US10822307B2 (en) | 2014-08-28 | 2020-11-03 | Eisai R&D Management Co., Ltd. | High-purity quinoline derivative and method for manufacturing same |
| AU2016224583B2 (en) * | 2015-02-25 | 2021-06-03 | Eisai R&D Management Co., Ltd. | Method for suppressing bitterness of quinoline derivative |
| KR102763349B1 (ko) * | 2015-02-25 | 2025-02-07 | 에자이 알앤드디 매니지먼트 가부시키가이샤 | 퀴놀린 유도체의 고미 억제 방법 |
| US11090386B2 (en) | 2015-02-25 | 2021-08-17 | Eisai R&D Management Co., Ltd. | Method for suppressing bitterness of quinoline derivative |
| JPWO2016136745A1 (ja) * | 2015-02-25 | 2017-11-30 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | キノリン誘導体の苦味抑制方法 |
| WO2016136745A1 (ja) * | 2015-02-25 | 2016-09-01 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | キノリン誘導体の苦味抑制方法 |
| KR20170122734A (ko) * | 2015-02-25 | 2017-11-06 | 에자이 알앤드디 매니지먼트 가부시키가이샤 | 퀴놀린 유도체의 고미 억제 방법 |
| EP3263106B1 (en) | 2015-02-25 | 2023-10-25 | Eisai R&D Management Co., Ltd. | Method for suppressing bitterness of quinoline derivative |
| US11547705B2 (en) | 2015-03-04 | 2023-01-10 | Merck Sharp & Dohme Llc | Combination of a PD-1 antagonist and a VEGF-R/FGFR/RET tyrosine kinase inhibitor for treating cancer |
| US12083112B2 (en) | 2015-03-04 | 2024-09-10 | Eisai R&D Management Co., Ltd. | Combination of a PD-1 antagonist and a VEGFR/FGFR/RET tyrosine kinase inhibitor for treating cancer |
| US11369623B2 (en) | 2015-06-16 | 2022-06-28 | Prism Pharma Co., Ltd. | Anticancer combination of a CBP/catenin inhibitor and an immune checkpoint inhibitor |
| US12220398B2 (en) | 2015-08-20 | 2025-02-11 | Eisai R&D Management Co., Ltd. | Tumor therapeutic agent |
| WO2017186197A1 (en) | 2016-04-27 | 2017-11-02 | Zentiva, K.S. | Salts of lenvatinib |
| US12303505B2 (en) | 2017-02-08 | 2025-05-20 | Eisai R&D Management Co., Ltd. | Tumor-treating pharmaceutical composition |
| US12226409B2 (en) | 2017-05-16 | 2025-02-18 | Eisai R&D Management Co., Ltd. | Treatment of hepatocellular carcinoma |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US12508313B2 (en) | Quinoline derivative-containing pharmaceutical composition | |
| EP3606511B1 (en) | Pharmaceutical composition comprising lenvatinib mesylate | |
| WO2012043709A1 (ja) | 難溶性薬物の溶解性改善製剤 | |
| EP3860606B1 (en) | Pharmaceutical composition comprising lenvatinib esylate or tosylate | |
| TWI501950B (zh) | 含喹啉衍生物的藥學組成物 | |
| EP4424303A1 (en) | Lenvatinib composition with improved bioavailability | |
| JP7370126B2 (ja) | エルロチニブを有効成分とする医薬錠剤 | |
| JP6336078B2 (ja) | 医薬組成物 | |
| HK1167607B (en) | Quinoline derivative-containing pharmaceutical composition | |
| HK1169599B (en) | Quinoline derivative-containing pharmaceutical composition | |
| JP2019073488A (ja) | アプレピタントを有効成分とする医薬錠剤 | |
| WO2023238929A1 (ja) | ピミテスピブを含有する医薬組成物 | |
| JP6945377B2 (ja) | エルロチニブを有効成分とする医薬錠剤及びその製造方法 | |
| KR20220088362A (ko) | 1-(5-(2,4-다이플루오로페닐)-1-((3-플루오로페닐)술포닐)-4-메톡시-1h-피롤-3-일)-n-메틸메탄아민을 포함하는 신규한 경구투여용 제제 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201080030508.6 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10809938 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2011527665 Country of ref document: JP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2010285740 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13322961 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2010809938 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 002081-2011 Country of ref document: PE |
|
| ENP | Entry into the national phase |
Ref document number: 2010285740 Country of ref document: AU Date of ref document: 20100816 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: DZP2012000036 Country of ref document: DZ |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12022608 Country of ref document: CO |
|
| ENP | Entry into the national phase |
Ref document number: 20127003846 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012000412 Country of ref document: CL Ref document number: 2771403 Country of ref document: CA Ref document number: MX/A/2012/002011 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1201000221 Country of ref document: TH |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2371/CHENP/2012 Country of ref document: IN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: A201203132 Country of ref document: UA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012103471 Country of ref document: RU |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112012003592 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112012003592 Country of ref document: BR Kind code of ref document: A2 Effective date: 20120216 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: P-2016/0176 Country of ref document: RS |