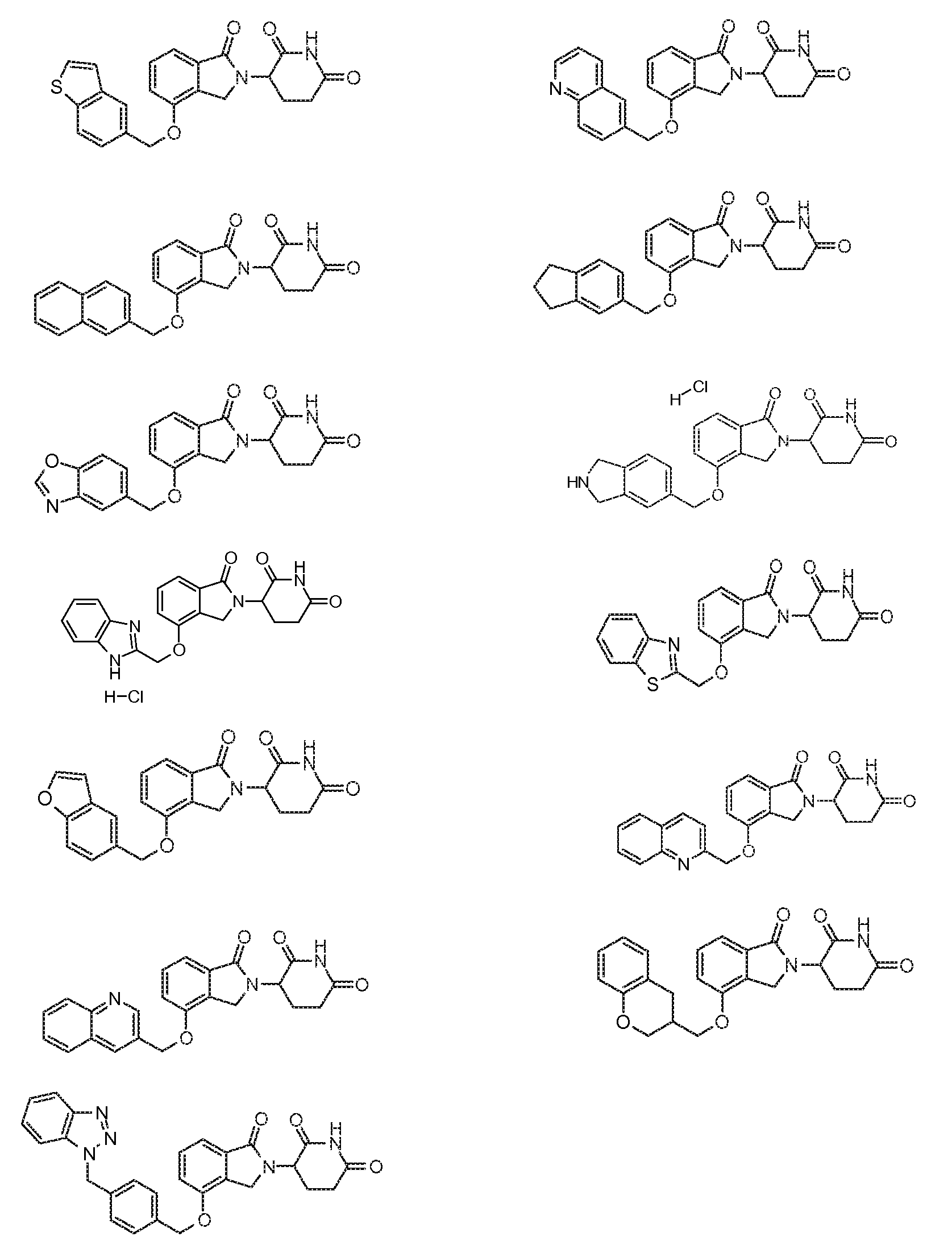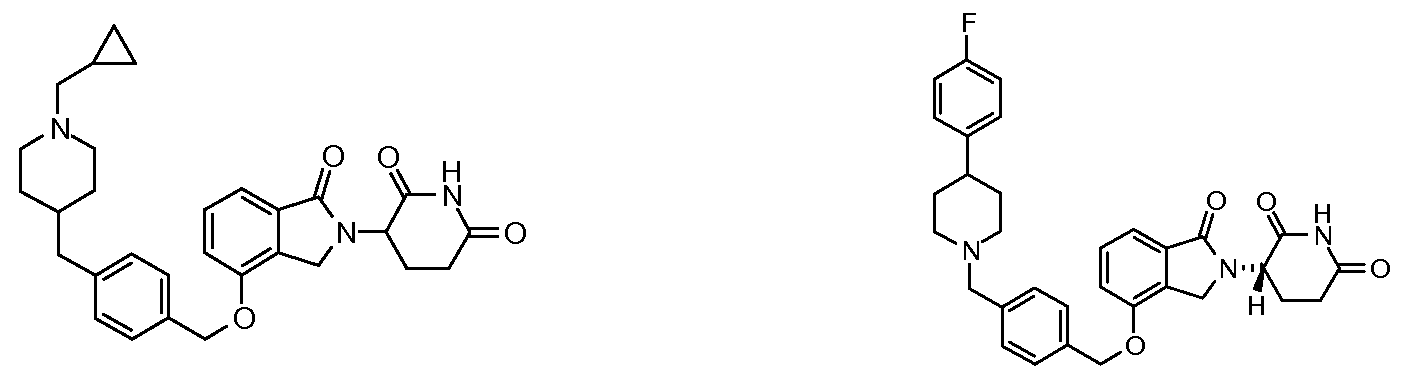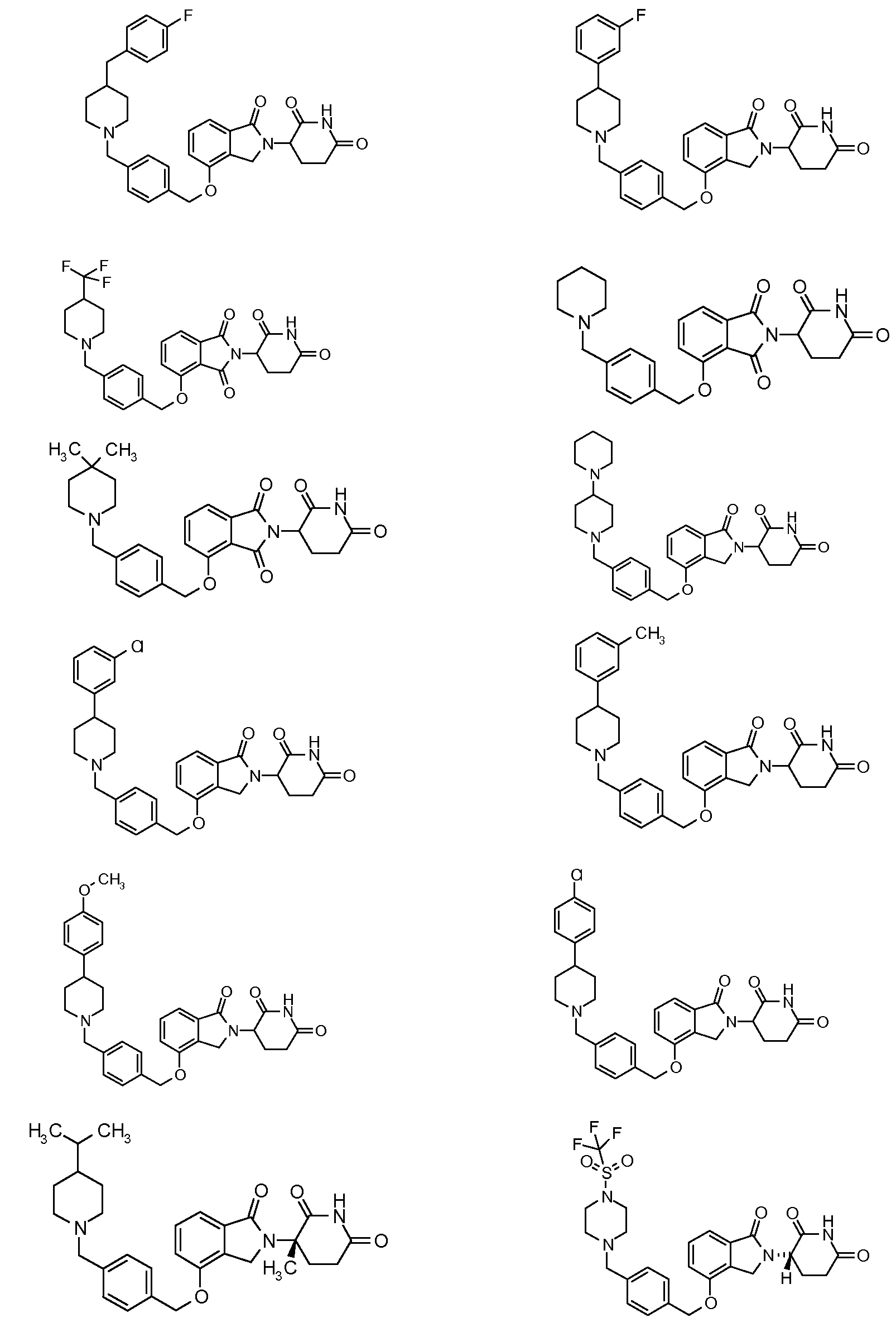RU2012138709A - Производные арилметокси изоиндолина и композиции, включающие их, и способы их применения - Google Patents
Производные арилметокси изоиндолина и композиции, включающие их, и способы их применения Download PDFInfo
- Publication number
- RU2012138709A RU2012138709A RU2012138709/04A RU2012138709A RU2012138709A RU 2012138709 A RU2012138709 A RU 2012138709A RU 2012138709/04 A RU2012138709/04 A RU 2012138709/04A RU 2012138709 A RU2012138709 A RU 2012138709A RU 2012138709 A RU2012138709 A RU 2012138709A
- Authority
- RU
- Russia
- Prior art keywords
- alkyl
- substituted
- halogens
- aryl
- heteroaryl
- Prior art date
Links
- -1 THEM Chemical compound 0.000 title claims 2
- GWVMLCQWXVFZCN-UHFFFAOYSA-N isoindoline Chemical compound C1=CC=C2CNCC2=C1 GWVMLCQWXVFZCN-UHFFFAOYSA-N 0.000 title 1
- 239000000203 mixture Substances 0.000 title 1
- 229910052736 halogen Inorganic materials 0.000 claims abstract 36
- 150000002367 halogens Chemical class 0.000 claims abstract 29
- 125000000217 alkyl group Chemical group 0.000 claims abstract 27
- 150000001875 compounds Chemical class 0.000 claims abstract 21
- 125000000041 C6-C10 aryl group Chemical group 0.000 claims abstract 13
- 125000001072 heteroaryl group Chemical group 0.000 claims abstract 12
- 150000003839 salts Chemical class 0.000 claims abstract 12
- 239000012453 solvate Substances 0.000 claims abstract 12
- 125000003545 alkoxy group Chemical group 0.000 claims abstract 10
- 125000000623 heterocyclic group Chemical group 0.000 claims abstract 9
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims abstract 9
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims abstract 9
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims abstract 6
- 125000004093 cyano group Chemical group *C#N 0.000 claims abstract 6
- 125000001424 substituent group Chemical group 0.000 claims abstract 6
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical group [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 claims abstract 5
- 229910052805 deuterium Inorganic materials 0.000 claims abstract 5
- 125000003118 aryl group Chemical group 0.000 claims abstract 3
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 claims 15
- 229910052739 hydrogen Inorganic materials 0.000 claims 10
- 239000001257 hydrogen Substances 0.000 claims 10
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims 8
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims 7
- 208000035475 disorder Diseases 0.000 claims 6
- 125000004191 (C1-C6) alkoxy group Chemical group 0.000 claims 4
- FCEHBMOGCRZNNI-UHFFFAOYSA-N 1-benzothiophene Chemical compound C1=CC=C2SC=CC2=C1 FCEHBMOGCRZNNI-UHFFFAOYSA-N 0.000 claims 4
- 230000006735 deficit Effects 0.000 claims 4
- 125000004043 oxo group Chemical group O=* 0.000 claims 4
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 claims 3
- IANQTJSKSUMEQM-UHFFFAOYSA-N 1-benzofuran Chemical compound C1=CC=C2OC=CC2=C1 IANQTJSKSUMEQM-UHFFFAOYSA-N 0.000 claims 2
- HBEDSQVIWPRPAY-UHFFFAOYSA-N 2,3-dihydrobenzofuran Chemical compound C1=CC=C2OCCC2=C1 HBEDSQVIWPRPAY-UHFFFAOYSA-N 0.000 claims 2
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 claims 2
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 claims 2
- IOJUPLGTWVMSFF-UHFFFAOYSA-N benzothiazole Chemical compound C1=CC=C2SC=NC2=C1 IOJUPLGTWVMSFF-UHFFFAOYSA-N 0.000 claims 2
- 125000002619 bicyclic group Chemical group 0.000 claims 2
- 201000010099 disease Diseases 0.000 claims 2
- 150000002431 hydrogen Chemical class 0.000 claims 2
- PQNFLJBBNBOBRQ-UHFFFAOYSA-N indane Chemical compound C1=CC=C2CCCC2=C1 PQNFLJBBNBOBRQ-UHFFFAOYSA-N 0.000 claims 2
- AWJUIBRHMBBTKR-UHFFFAOYSA-N isoquinoline Chemical compound C1=NC=CC2=CC=CC=C21 AWJUIBRHMBBTKR-UHFFFAOYSA-N 0.000 claims 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims 2
- 201000001320 Atherosclerosis Diseases 0.000 claims 1
- XCNFEEZOOUZSFZ-UHFFFAOYSA-N C1=CC=C2CNCC2=C1.C1=CC=C2OC=NC2=C1 Chemical compound C1=CC=C2CNCC2=C1.C1=CC=C2OC=NC2=C1 XCNFEEZOOUZSFZ-UHFFFAOYSA-N 0.000 claims 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 claims 1
- 208000035473 Communicable disease Diseases 0.000 claims 1
- 206010061598 Immunodeficiency Diseases 0.000 claims 1
- 208000029462 Immunodeficiency disease Diseases 0.000 claims 1
- 206010028980 Neoplasm Diseases 0.000 claims 1
- 208000002193 Pain Diseases 0.000 claims 1
- 208000030852 Parasitic disease Diseases 0.000 claims 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 claims 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 claims 1
- 230000033115 angiogenesis Effects 0.000 claims 1
- 239000010425 asbestos Substances 0.000 claims 1
- RFRXIWQYSOIBDI-UHFFFAOYSA-N benzarone Chemical compound CCC=1OC2=CC=CC=C2C=1C(=O)C1=CC=C(O)C=C1 RFRXIWQYSOIBDI-UHFFFAOYSA-N 0.000 claims 1
- QRUDEWIWKLJBPS-UHFFFAOYSA-N benzotriazole Chemical compound C1=CC=C2N[N][N]C2=C1 QRUDEWIWKLJBPS-UHFFFAOYSA-N 0.000 claims 1
- 201000011510 cancer Diseases 0.000 claims 1
- 229910052801 chlorine Inorganic materials 0.000 claims 1
- 239000000460 chlorine Substances 0.000 claims 1
- 125000001309 chloro group Chemical group Cl* 0.000 claims 1
- VZWXIQHBIQLMPN-UHFFFAOYSA-N chromane Chemical compound C1=CC=C2CCCOC2=C1 VZWXIQHBIQLMPN-UHFFFAOYSA-N 0.000 claims 1
- 230000004064 dysfunction Effects 0.000 claims 1
- 208000034737 hemoglobinopathy Diseases 0.000 claims 1
- 125000005842 heteroatom Chemical group 0.000 claims 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims 1
- UTCSSFWDNNEEBH-UHFFFAOYSA-N imidazo[1,2-a]pyridine Chemical compound C1=CC=CC2=NC=CN21 UTCSSFWDNNEEBH-UHFFFAOYSA-N 0.000 claims 1
- 230000007813 immunodeficiency Effects 0.000 claims 1
- 208000015181 infectious disease Diseases 0.000 claims 1
- 208000018337 inherited hemoglobinopathy Diseases 0.000 claims 1
- 125000000904 isoindolyl group Chemical group C=1(NC=C2C=CC=CC12)* 0.000 claims 1
- 208000002780 macular degeneration Diseases 0.000 claims 1
- 239000008194 pharmaceutical composition Substances 0.000 claims 1
- 230000002685 pulmonary effect Effects 0.000 claims 1
- 229910052895 riebeckite Inorganic materials 0.000 claims 1
- 208000017520 skin disease Diseases 0.000 claims 1
- 208000011580 syndromic disease Diseases 0.000 claims 1
- 125000005843 halogen group Chemical group 0.000 abstract 7
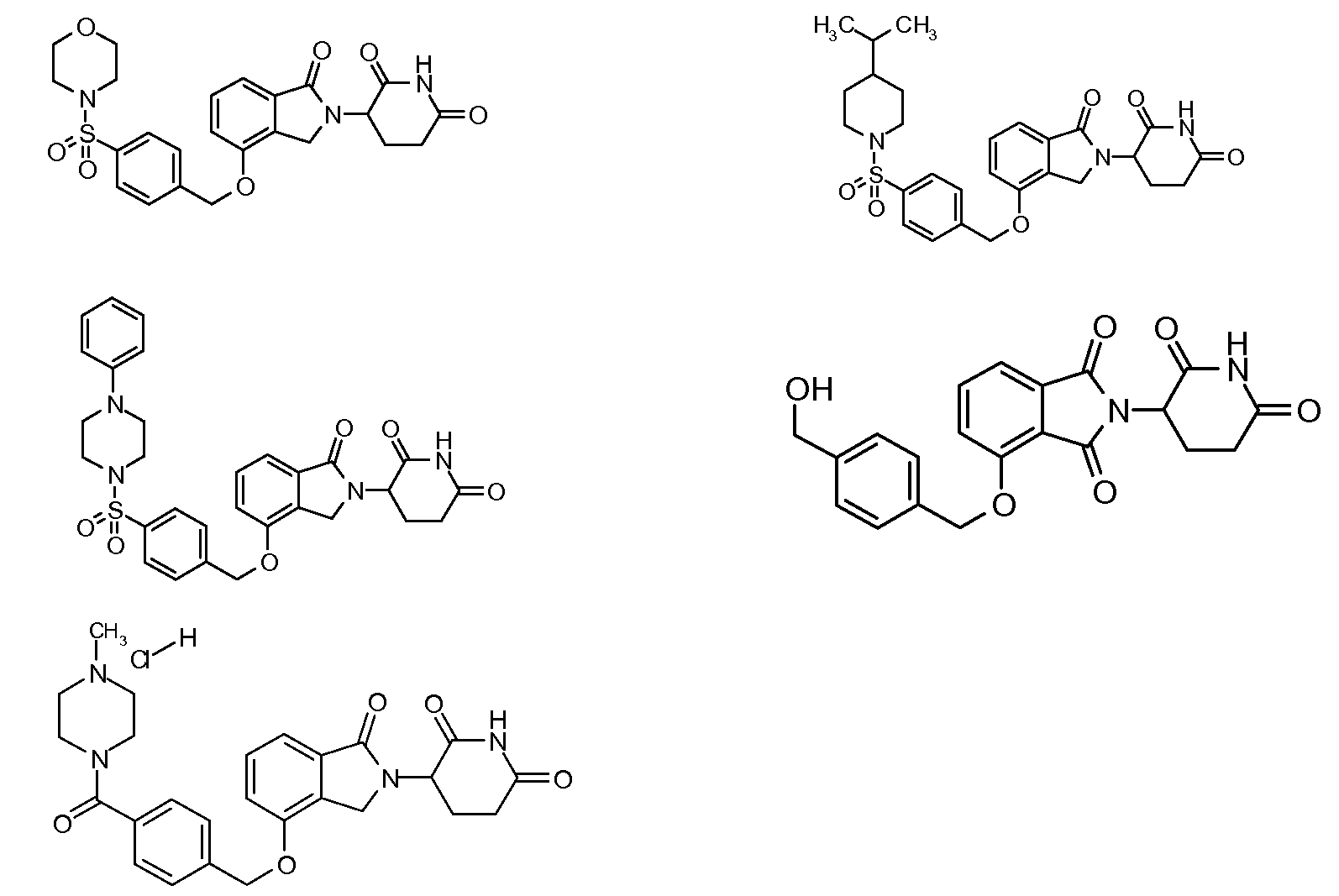
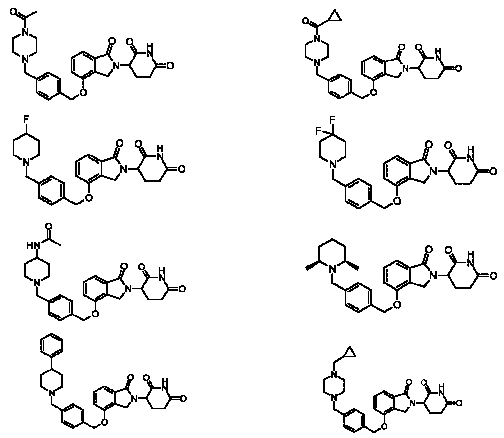
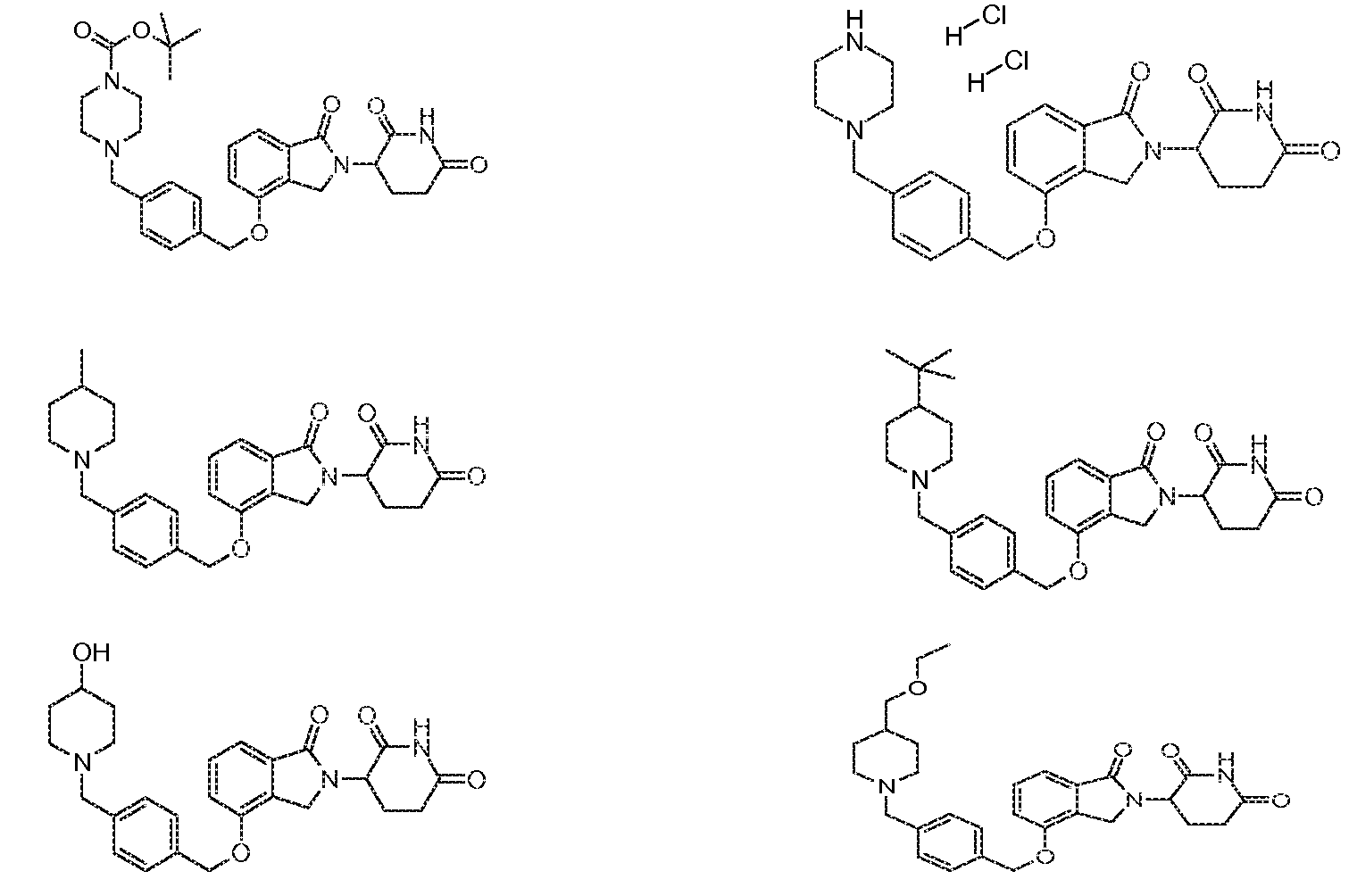
- 0 *c1ccc(C2CCN(Cc3ccc(COc(cccc4C(N5C(CCC(N6)=O)C6=O)=O)c4C5=O)cc3)CC2)cc1 Chemical compound *c1ccc(C2CCN(Cc3ccc(COc(cccc4C(N5C(CCC(N6)=O)C6=O)=O)c4C5=O)cc3)CC2)cc1 0.000 description 1
- GMSCQHYOSCXXAD-SWGQDTFXSA-N C/C=C/C(CN1CCN(Cc2ccc(COc3cccc(C(N4C(CCC(N5)=O)C5=O)=O)c3C4=O)cc2)CC1)(F)F Chemical compound C/C=C/C(CN1CCN(Cc2ccc(COc3cccc(C(N4C(CCC(N5)=O)C5=O)=O)c3C4=O)cc2)CC1)(F)F GMSCQHYOSCXXAD-SWGQDTFXSA-N 0.000 description 1
- XTJUZPPQDVYOMW-UHFFFAOYSA-N C=C(CCC1N(Cc2c3cccc2OCc2ccc(CN4Cc5nc(C(F)(F)F)n[n]5CC4)cc2)C3=O)NC1=O Chemical compound C=C(CCC1N(Cc2c3cccc2OCc2ccc(CN4Cc5nc(C(F)(F)F)n[n]5CC4)cc2)C3=O)NC1=O XTJUZPPQDVYOMW-UHFFFAOYSA-N 0.000 description 1
- LRPQMEZMKLJOFG-JPEXTOSHSA-O CC(C)C1CCN(CC(/C=C(/COc2cccc3c2CN(C(CCC(N2)=O)C2=O)C3=O)\[OH2+])=N)CC1 Chemical compound CC(C)C1CCN(CC(/C=C(/COc2cccc3c2CN(C(CCC(N2)=O)C2=O)C3=O)\[OH2+])=N)CC1 LRPQMEZMKLJOFG-JPEXTOSHSA-O 0.000 description 1
- RCQPABPAAWOZEY-UHFFFAOYSA-N COc1ccc(CN(Cc2ccc(COc3cccc4c3CN(C(CCC(N3)=O)C3=O)C4=O)cc2)CC2)c2c1 Chemical compound COc1ccc(CN(Cc2ccc(COc3cccc4c3CN(C(CCC(N3)=O)C3=O)C4=O)cc2)CC2)c2c1 RCQPABPAAWOZEY-UHFFFAOYSA-N 0.000 description 1
- MWXFIOTZIJCNPG-UHFFFAOYSA-N C[n]1c2ccccc2nc1Cc1ccc(COc2c(CN(C(CCC(N3)=O)C3=O)C3=O)c3ccc2)cc1 Chemical compound C[n]1c2ccccc2nc1Cc1ccc(COc2c(CN(C(CCC(N3)=O)C3=O)C3=O)c3ccc2)cc1 MWXFIOTZIJCNPG-UHFFFAOYSA-N 0.000 description 1
- MHMOWQGDFFJBRE-UHFFFAOYSA-N Cc1nc(-c2ccccc2)c[n]1Cc1ccc(COc2cccc3c2CN(C(CCC(N2)=O)C2=O)C3=O)cc1 Chemical compound Cc1nc(-c2ccccc2)c[n]1Cc1ccc(COc2cccc3c2CN(C(CCC(N2)=O)C2=O)C3=O)cc1 MHMOWQGDFFJBRE-UHFFFAOYSA-N 0.000 description 1
- LOBRHDDWENFJFE-MHZLTWQESA-N N#Cc1cc(CN(Cc2ccc(COc3c(CN([C@@H](CCC(N4)=O)C4=O)C4=O)c4ccc3)cc2)CC2)c2cc1 Chemical compound N#Cc1cc(CN(Cc2ccc(COc3c(CN([C@@H](CCC(N4)=O)C4=O)C4=O)c4ccc3)cc2)CC2)c2cc1 LOBRHDDWENFJFE-MHZLTWQESA-N 0.000 description 1
- JWBFFGBRVWZARF-UHFFFAOYSA-N O=C(c1c(C2)c(OCc(cc3)c[n]4c3nc(CN3CCOCC3)c4)ccc1)N2C(CCC(N1)=O)C1=O Chemical compound O=C(c1c(C2)c(OCc(cc3)c[n]4c3nc(CN3CCOCC3)c4)ccc1)N2C(CCC(N1)=O)C1=O JWBFFGBRVWZARF-UHFFFAOYSA-N 0.000 description 1
- VQDRNQBRRSNVOR-UHFFFAOYSA-N O=C(c1c(C2)c(OCc3ccc(CN(CC4)CCN4c(c(F)c4)ccc4F)cc3)ccc1)N2C(CCC(N1)=O)C1=O Chemical compound O=C(c1c(C2)c(OCc3ccc(CN(CC4)CCN4c(c(F)c4)ccc4F)cc3)ccc1)N2C(CCC(N1)=O)C1=O VQDRNQBRRSNVOR-UHFFFAOYSA-N 0.000 description 1
- RDGCXWADESRGEL-UHFFFAOYSA-N O=C(c1c(C2)c(OCc3ccc(CN4Cc5cccnc5CC4)cc3)ccc1)N2C(CCC(N1)=O)C1=O Chemical compound O=C(c1c(C2)c(OCc3ccc(CN4Cc5cccnc5CC4)cc3)ccc1)N2C(CCC(N1)=O)C1=O RDGCXWADESRGEL-UHFFFAOYSA-N 0.000 description 1
- AKLXWWIHIKYEDM-UHFFFAOYSA-N O=C(c1c(C2)c(OCc3ccc(Cc4nc5ccccc5[nH]4)cc3)ccc1)N2C(CCC(N1)=O)C1=O Chemical compound O=C(c1c(C2)c(OCc3ccc(Cc4nc5ccccc5[nH]4)cc3)ccc1)N2C(CCC(N1)=O)C1=O AKLXWWIHIKYEDM-UHFFFAOYSA-N 0.000 description 1
- PCAXXQAWHMAPGW-UHFFFAOYSA-N O=C(c1c(C2)c(OCc3ncc(CN4CCOCC4)cc3)ccc1)N2C(CCC(N1)=O)C1=O Chemical compound O=C(c1c(C2)c(OCc3ncc(CN4CCOCC4)cc3)ccc1)N2C(CCC(N1)=O)C1=O PCAXXQAWHMAPGW-UHFFFAOYSA-N 0.000 description 1
- FCGBMECEBNWUQE-UHFFFAOYSA-N O=C(c1c2c(OCc3ccc(CN(CC4)CCC4c(c(F)c4)ccc4F)cc3)ccc1)N(C(CCC(N1)=O)C1=O)C2=O Chemical compound O=C(c1c2c(OCc3ccc(CN(CC4)CCC4c(c(F)c4)ccc4F)cc3)ccc1)N(C(CCC(N1)=O)C1=O)C2=O FCGBMECEBNWUQE-UHFFFAOYSA-N 0.000 description 1
- XSDNPQYKFJIYJX-UHFFFAOYSA-N O=C(c1c2c(OCc3ccc(CN(CC4)CCN4S(C(F)(F)F)(=O)=O)cc3)ccc1)N(C(CCC(N1)=O)C1=O)C2=O Chemical compound O=C(c1c2c(OCc3ccc(CN(CC4)CCN4S(C(F)(F)F)(=O)=O)cc3)ccc1)N(C(CCC(N1)=O)C1=O)C2=O XSDNPQYKFJIYJX-UHFFFAOYSA-N 0.000 description 1
- RDEVIVPUSNFQHP-OFFJZUQKSA-N O=C(c1cccc(OCc2ccc(CN(C3)C[C@@H]4[C@H]3CCCC4)cc2)c1C1)N1C(CCC(N1)=O)C1=O Chemical compound O=C(c1cccc(OCc2ccc(CN(C3)C[C@@H]4[C@H]3CCCC4)cc2)c1C1)N1C(CCC(N1)=O)C1=O RDEVIVPUSNFQHP-OFFJZUQKSA-N 0.000 description 1
- SWBSLYQICWZGOQ-UHFFFAOYSA-N [O-]C1C(Cc2ccc(COc3cccc4c3CN(C(CCC(N3)=O)C3=O)C4=O)cc2)=CCCOCC1 Chemical compound [O-]C1C(Cc2ccc(COc3cccc4c3CN(C(CCC(N3)=O)C3=O)C4=O)cc2)=CCCOCC1 SWBSLYQICWZGOQ-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4412—Non condensed pyridines; Hydrogenated derivatives thereof having oxo groups directly attached to the heterocyclic ring
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/20—Hypnotics; Sedatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P39/00—General protective or antinoxious agents
- A61P39/02—Antidotes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/04—Antihaemorrhagics; Procoagulants; Haemostatic agents; Antifibrinolytic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/14—Vasoprotectives; Antihaemorrhoidals; Drugs for varicose therapy; Capillary stabilisers
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D407/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen atoms as the only ring hetero atoms, not provided for by group C07D405/00
- C07D407/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen atoms as the only ring hetero atoms, not provided for by group C07D405/00 containing two hetero rings
- C07D407/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen atoms as the only ring hetero atoms, not provided for by group C07D405/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D407/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen atoms as the only ring hetero atoms, not provided for by group C07D405/00
- C07D407/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen atoms as the only ring hetero atoms, not provided for by group C07D405/00 containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D491/00—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00
- C07D491/02—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00 in which the condensed system contains two hetero rings
- C07D491/10—Spiro-condensed systems
- C07D491/107—Spiro-condensed systems with only one oxygen atom as ring hetero atom in the oxygen-containing ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D493/00—Heterocyclic compounds containing oxygen atoms as the only ring hetero atoms in the condensed system
- C07D493/02—Heterocyclic compounds containing oxygen atoms as the only ring hetero atoms in the condensed system in which the condensed system contains two hetero rings
- C07D493/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D495/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms
- C07D495/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms in which the condensed system contains two hetero rings
- C07D495/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D498/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D498/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms in which the condensed system contains two hetero rings
- C07D498/08—Bridged systems
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Heart & Thoracic Surgery (AREA)
- Diabetes (AREA)
- Hematology (AREA)
- Cardiology (AREA)
- Pain & Pain Management (AREA)
- Vascular Medicine (AREA)
- Physical Education & Sports Medicine (AREA)
- Tropical Medicine & Parasitology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Rheumatology (AREA)
- Virology (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Epidemiology (AREA)
- Urology & Nephrology (AREA)
- Pulmonology (AREA)
- Ophthalmology & Optometry (AREA)
- Anesthesiology (AREA)
- AIDS & HIV (AREA)
- Toxicology (AREA)
- Dermatology (AREA)
Abstract
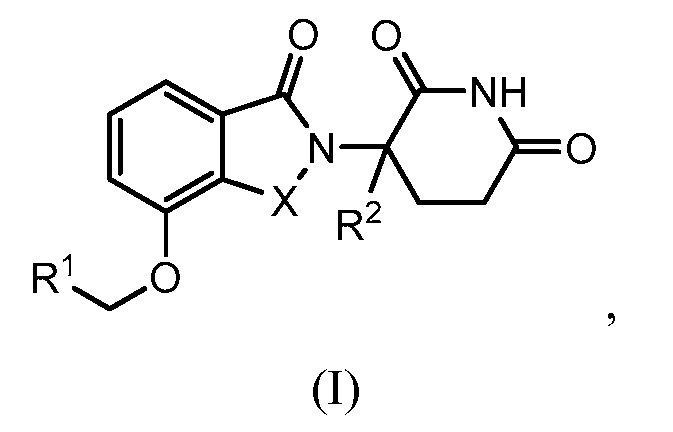
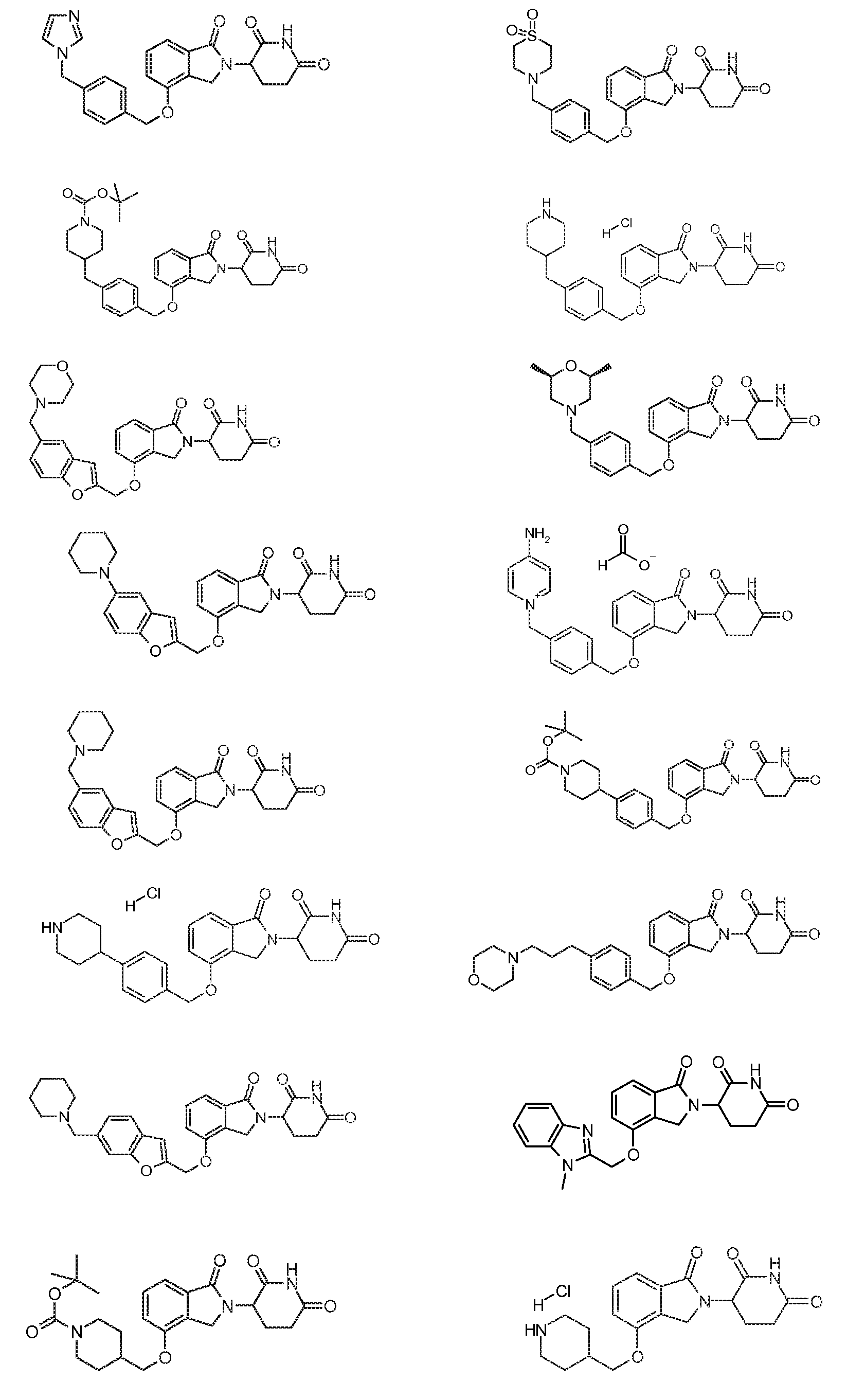
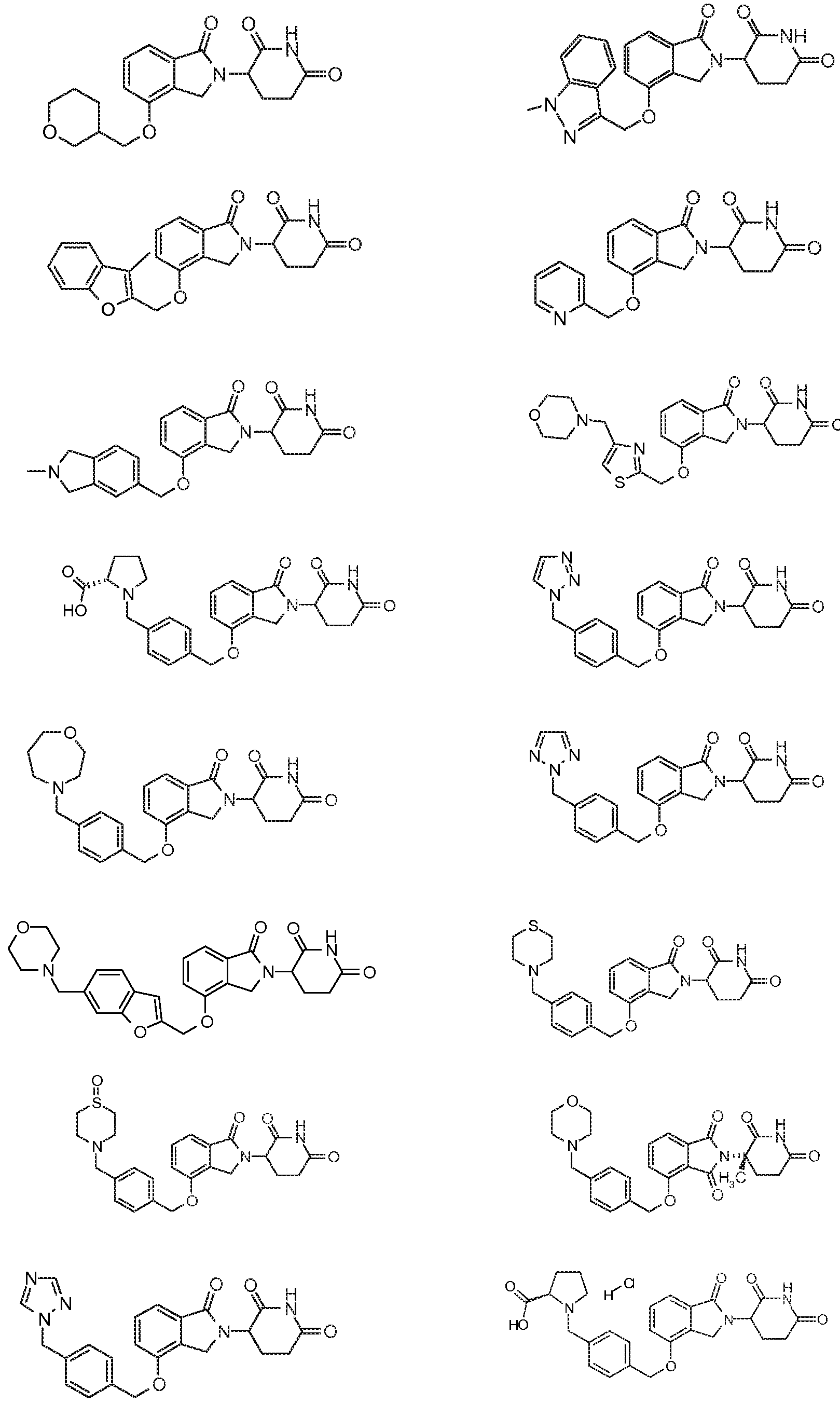
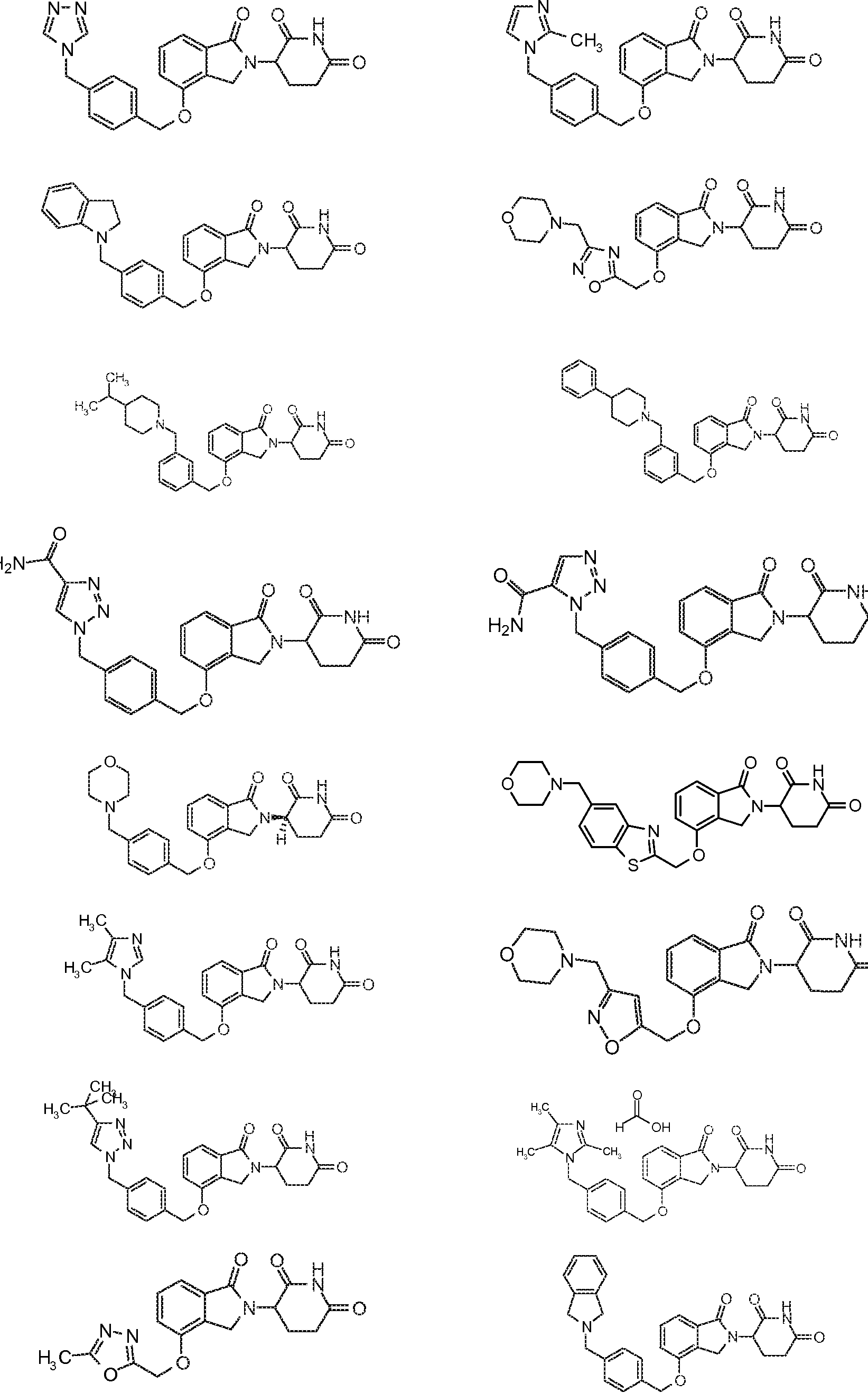
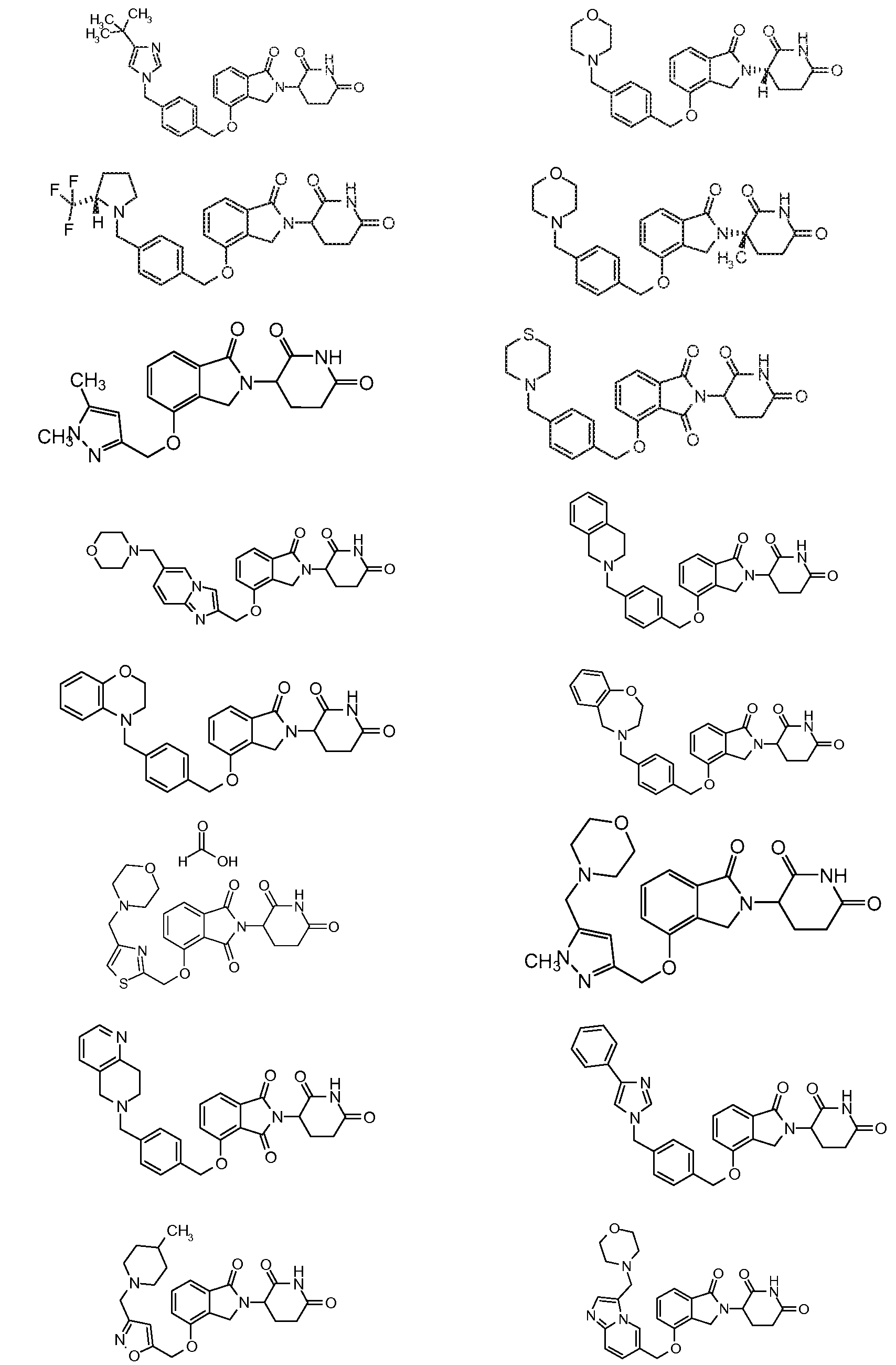
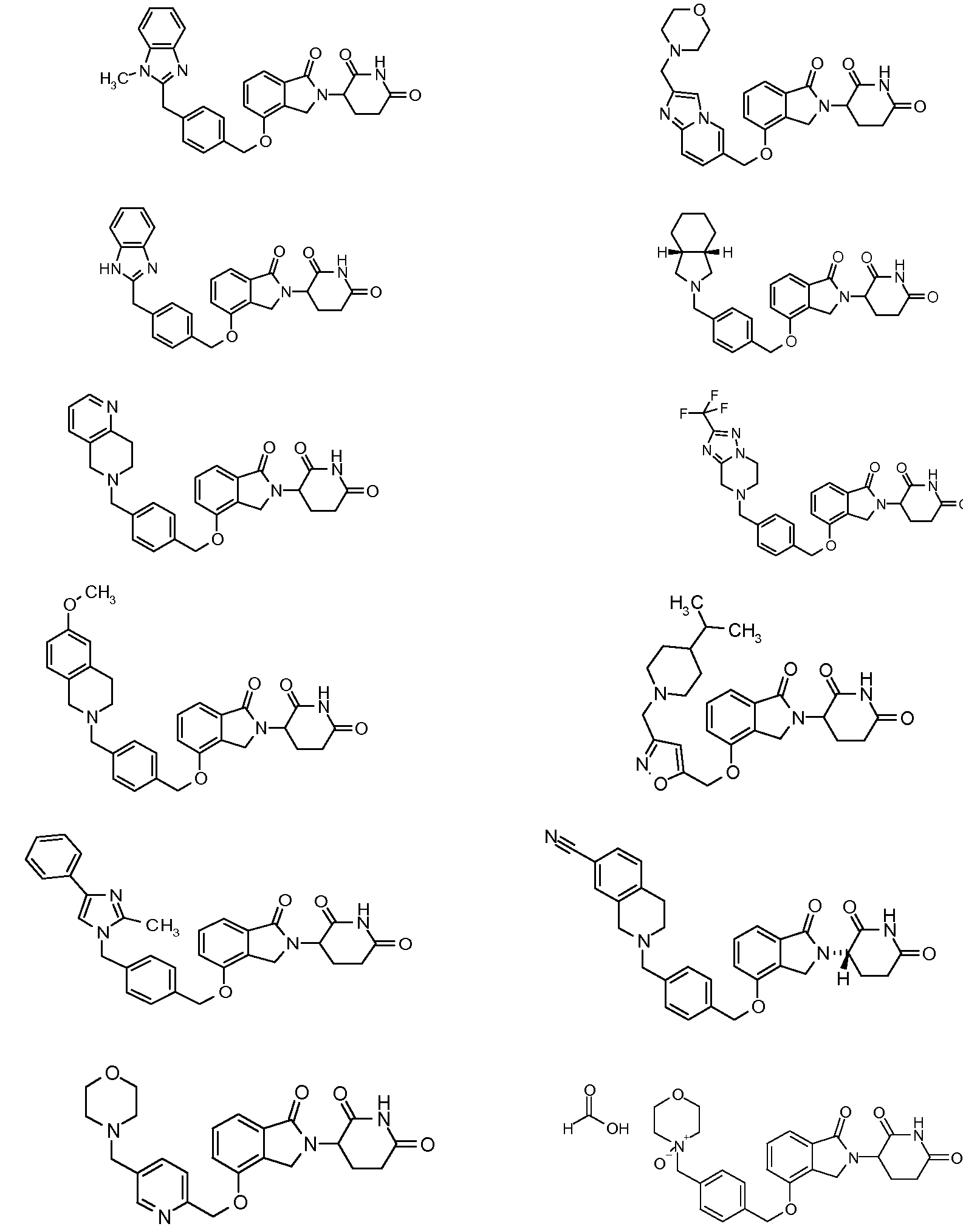
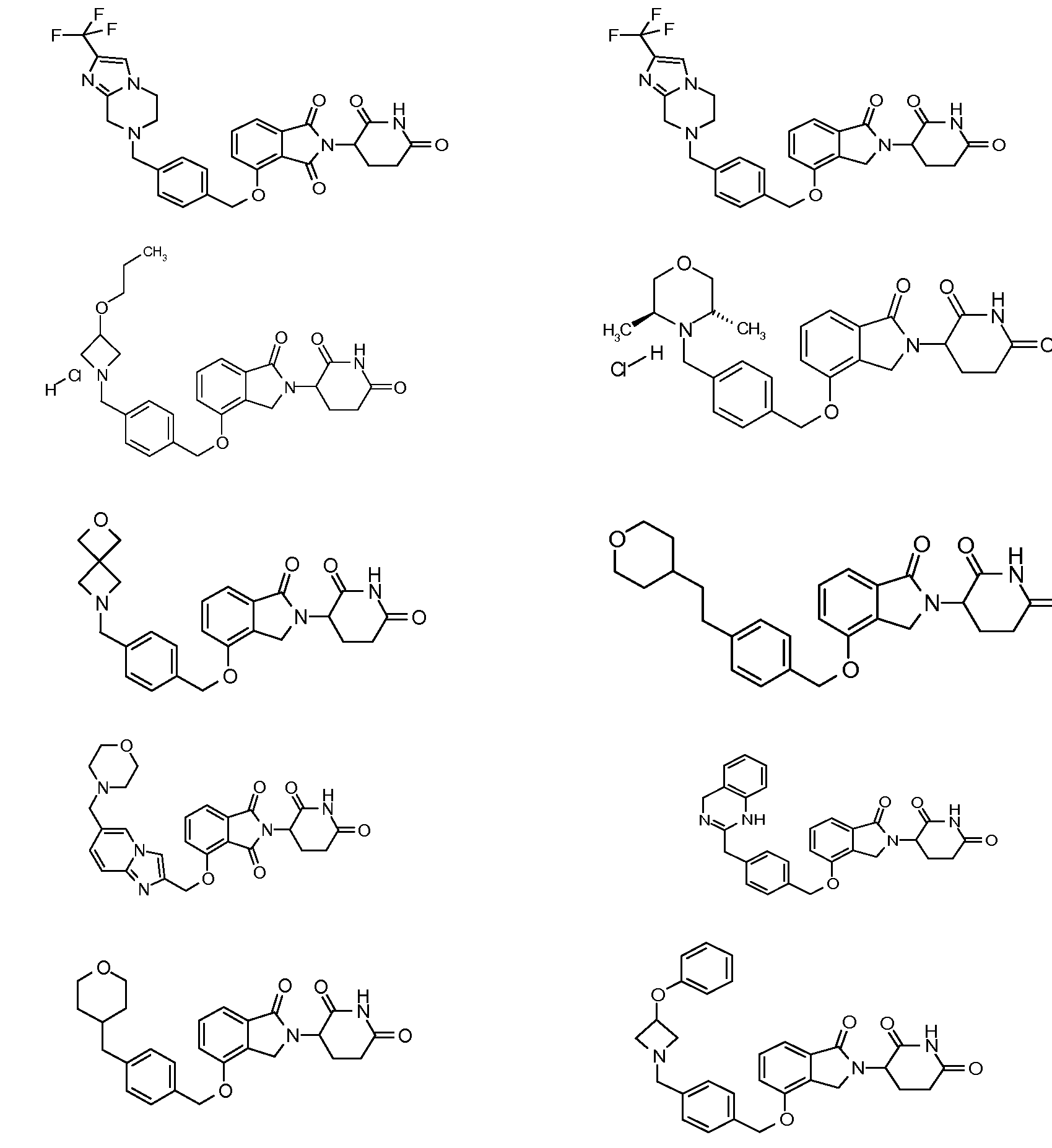
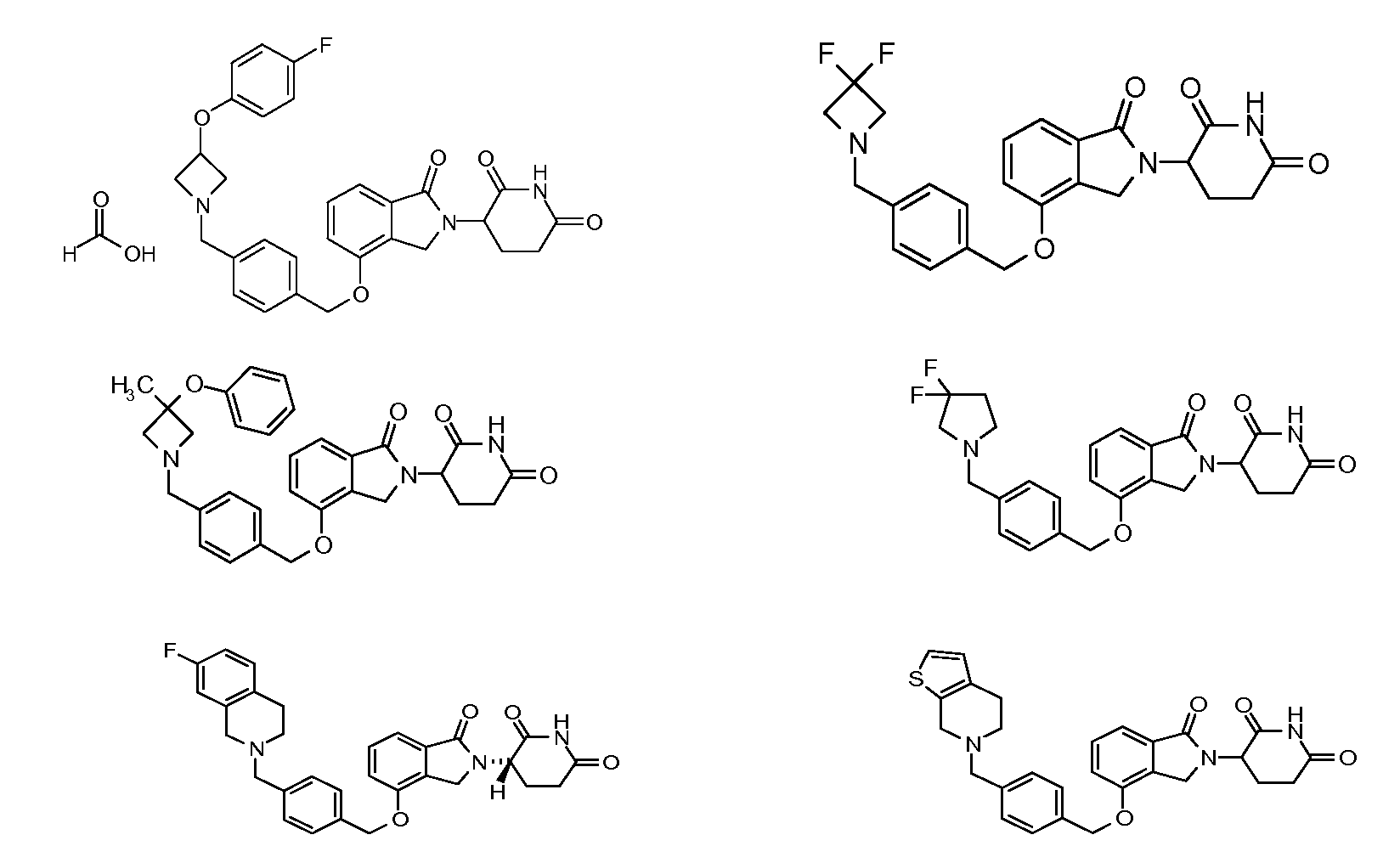
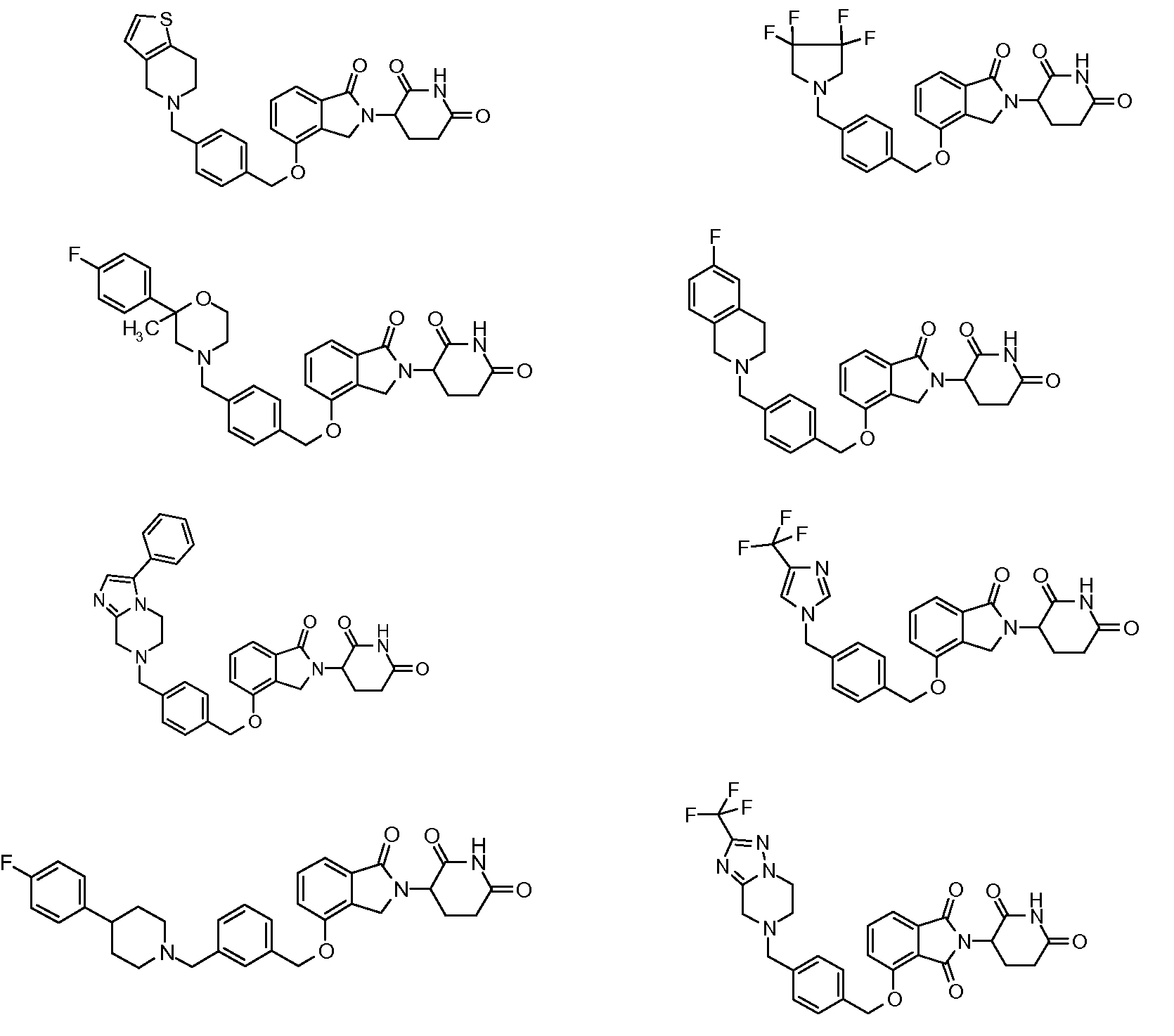
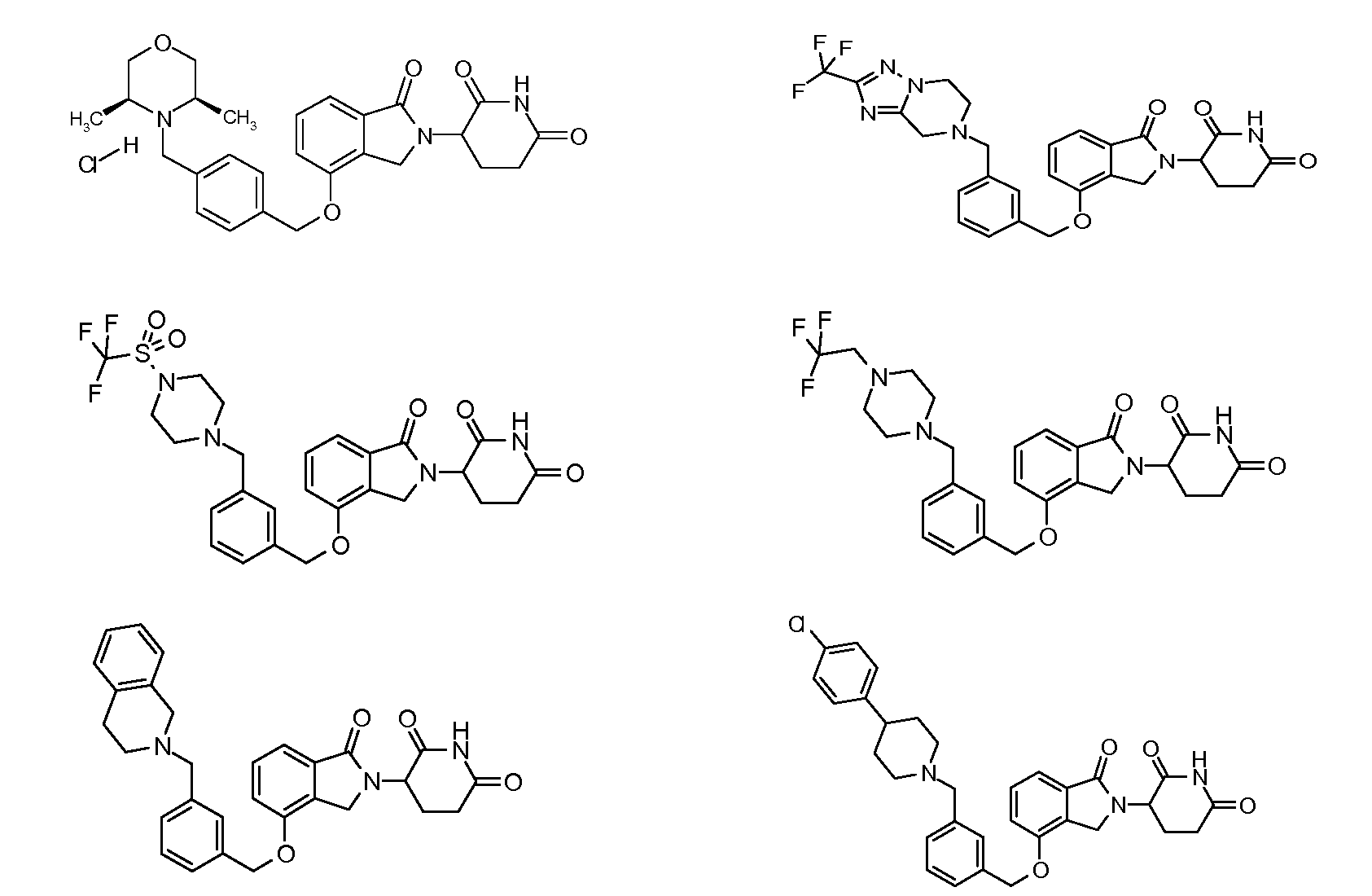
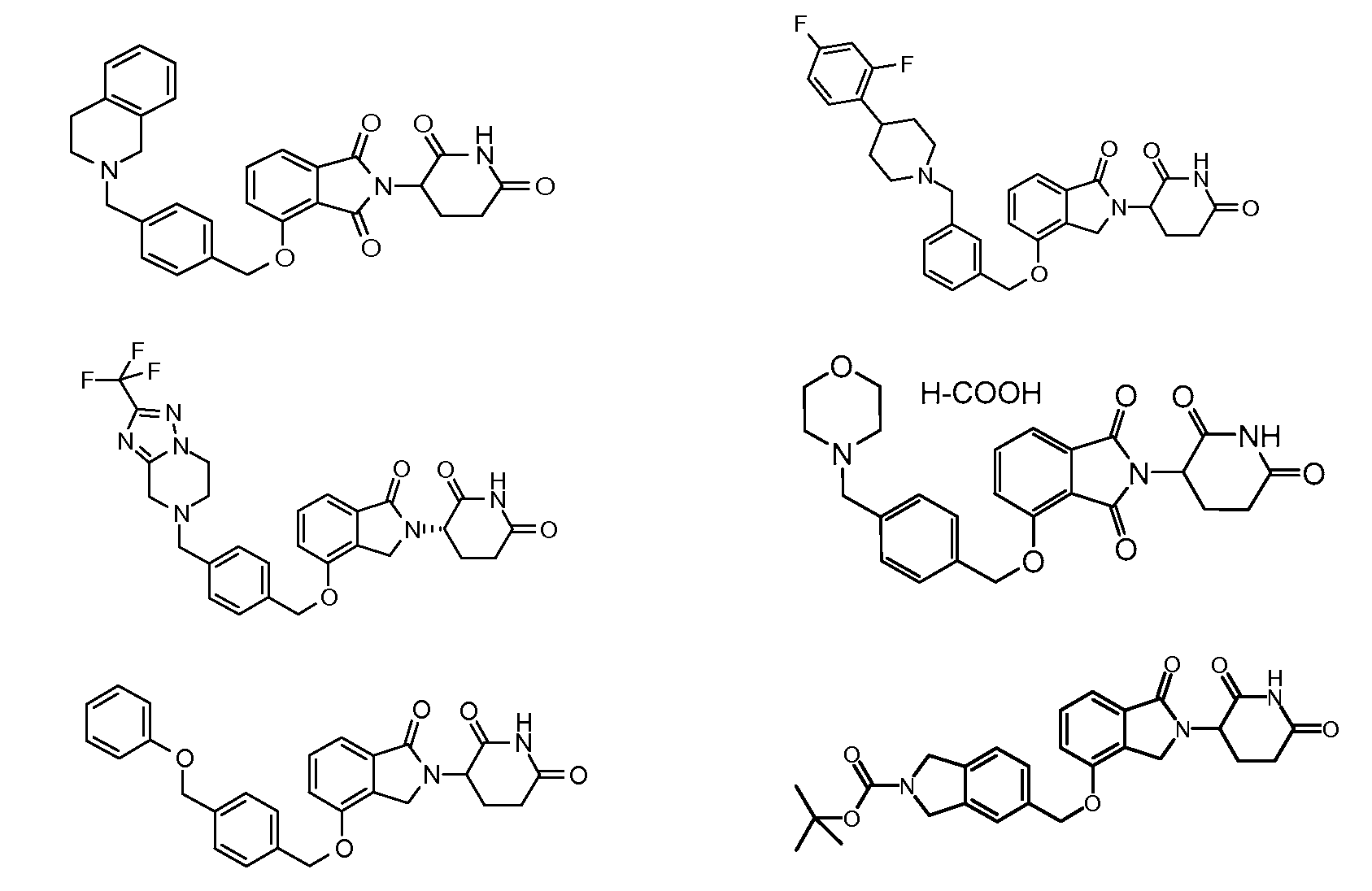
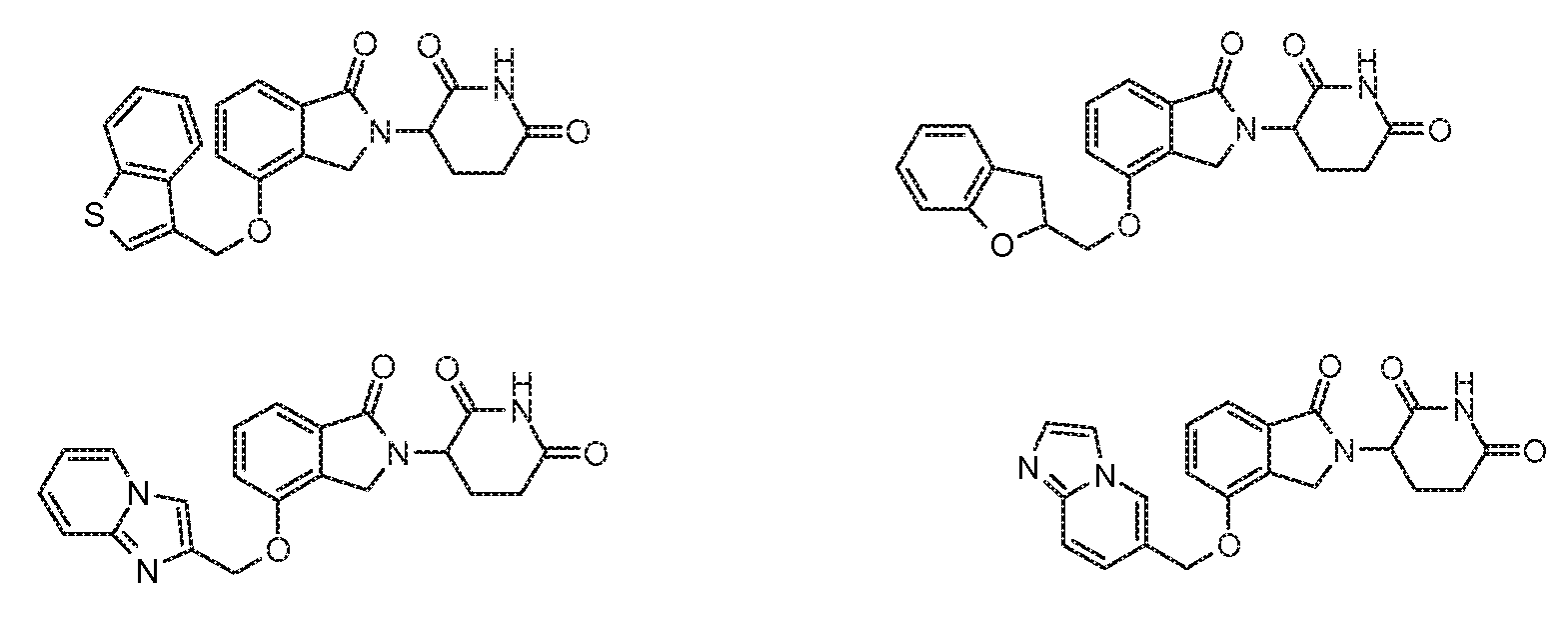
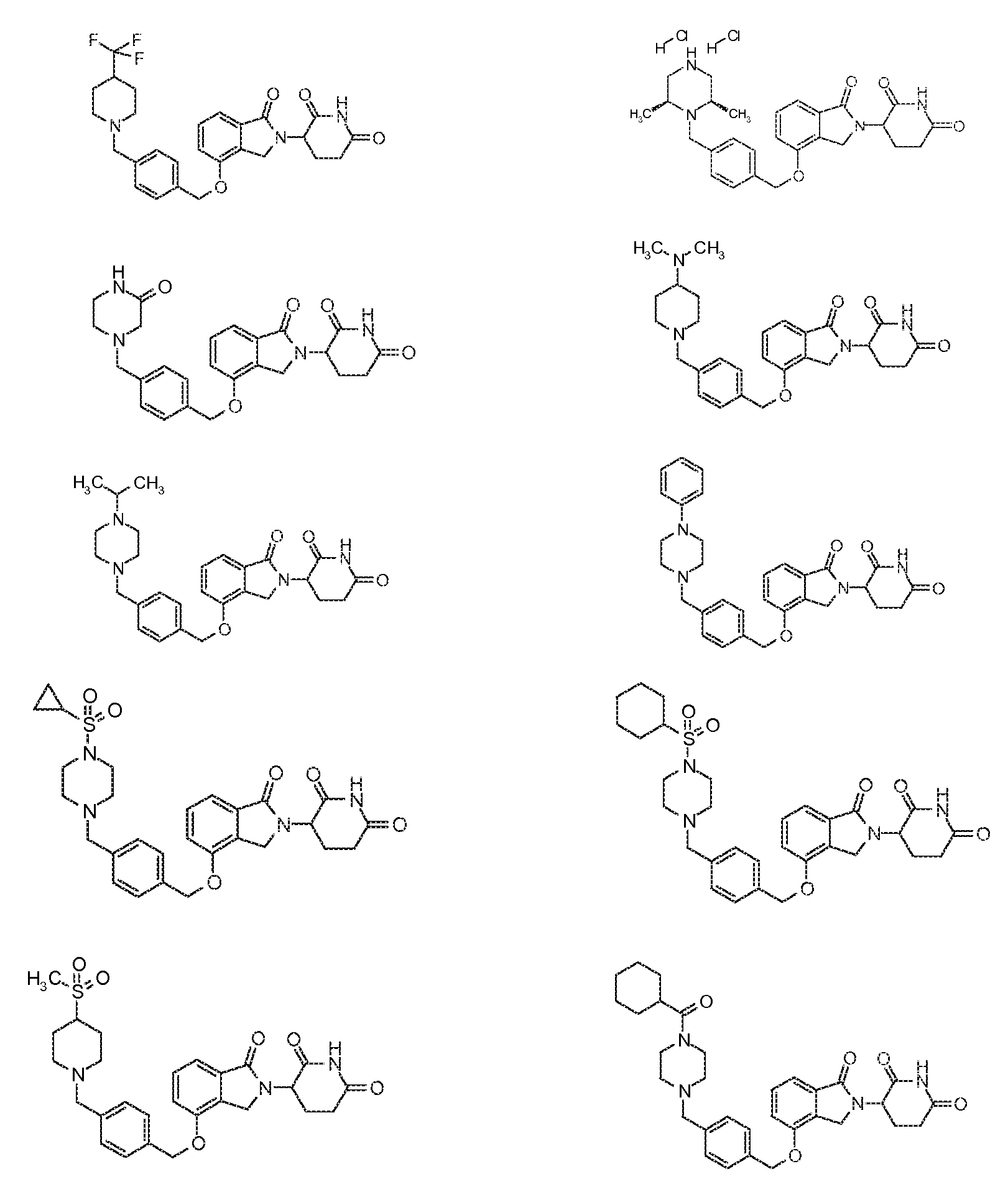
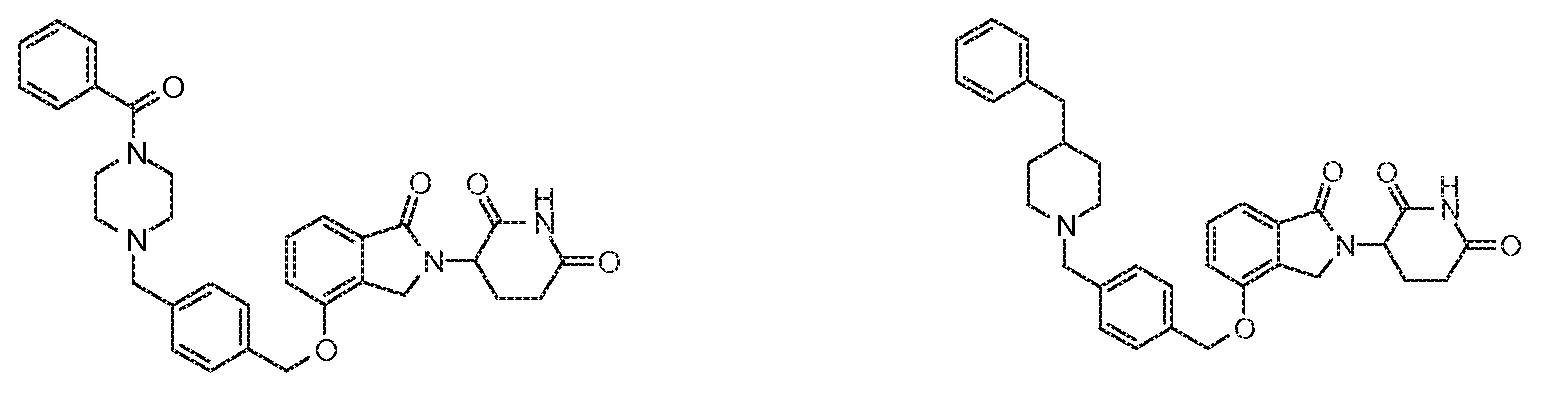
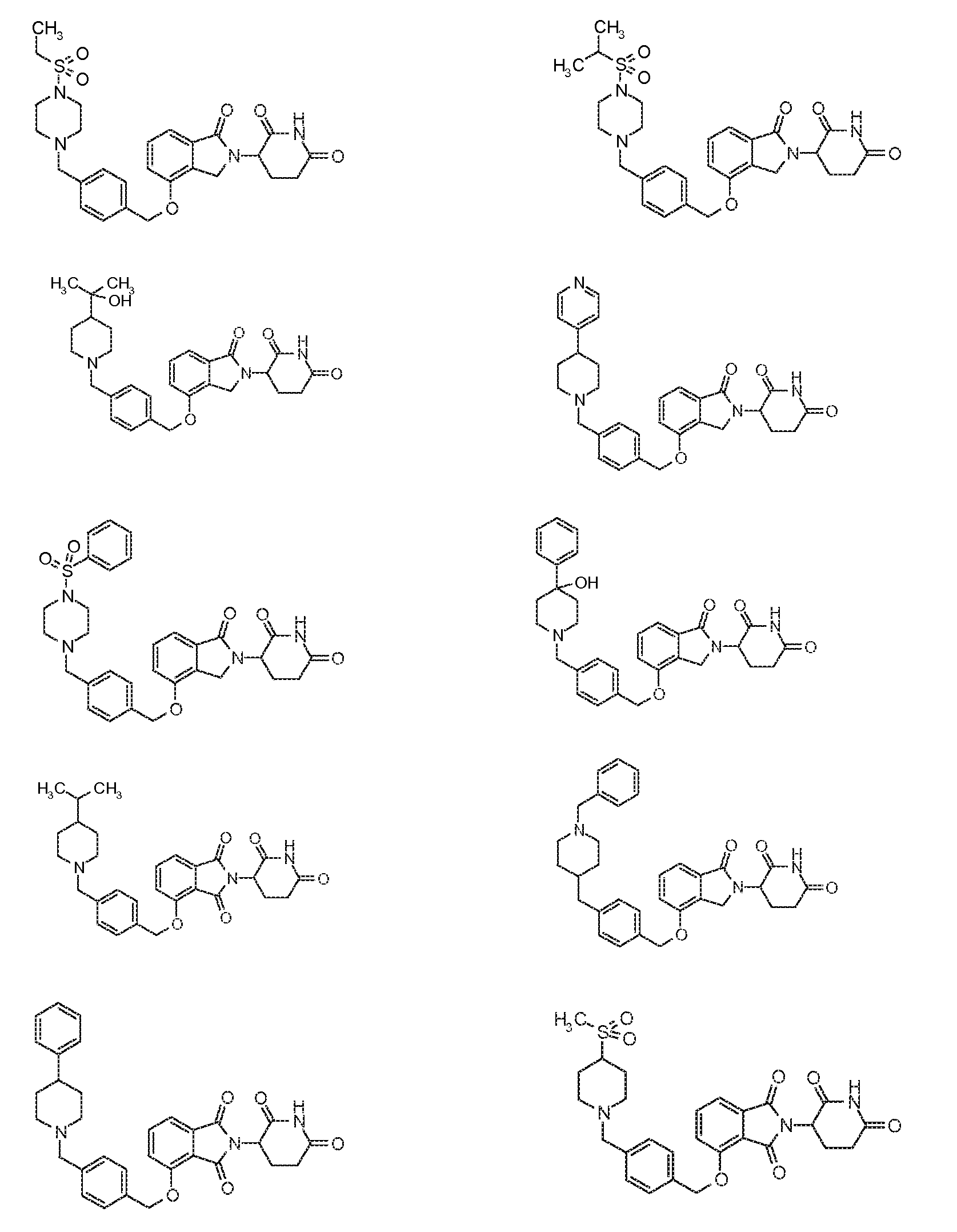
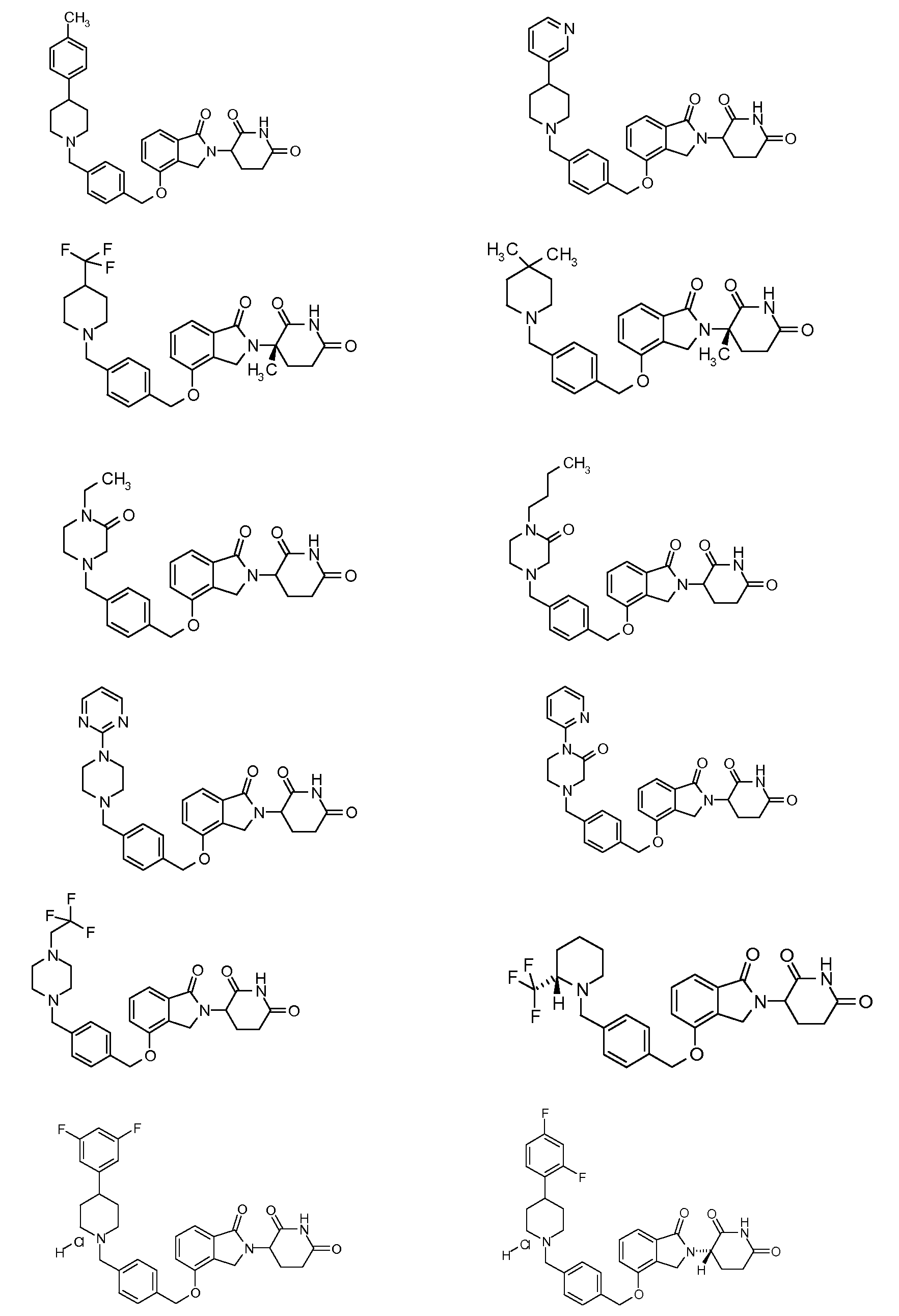
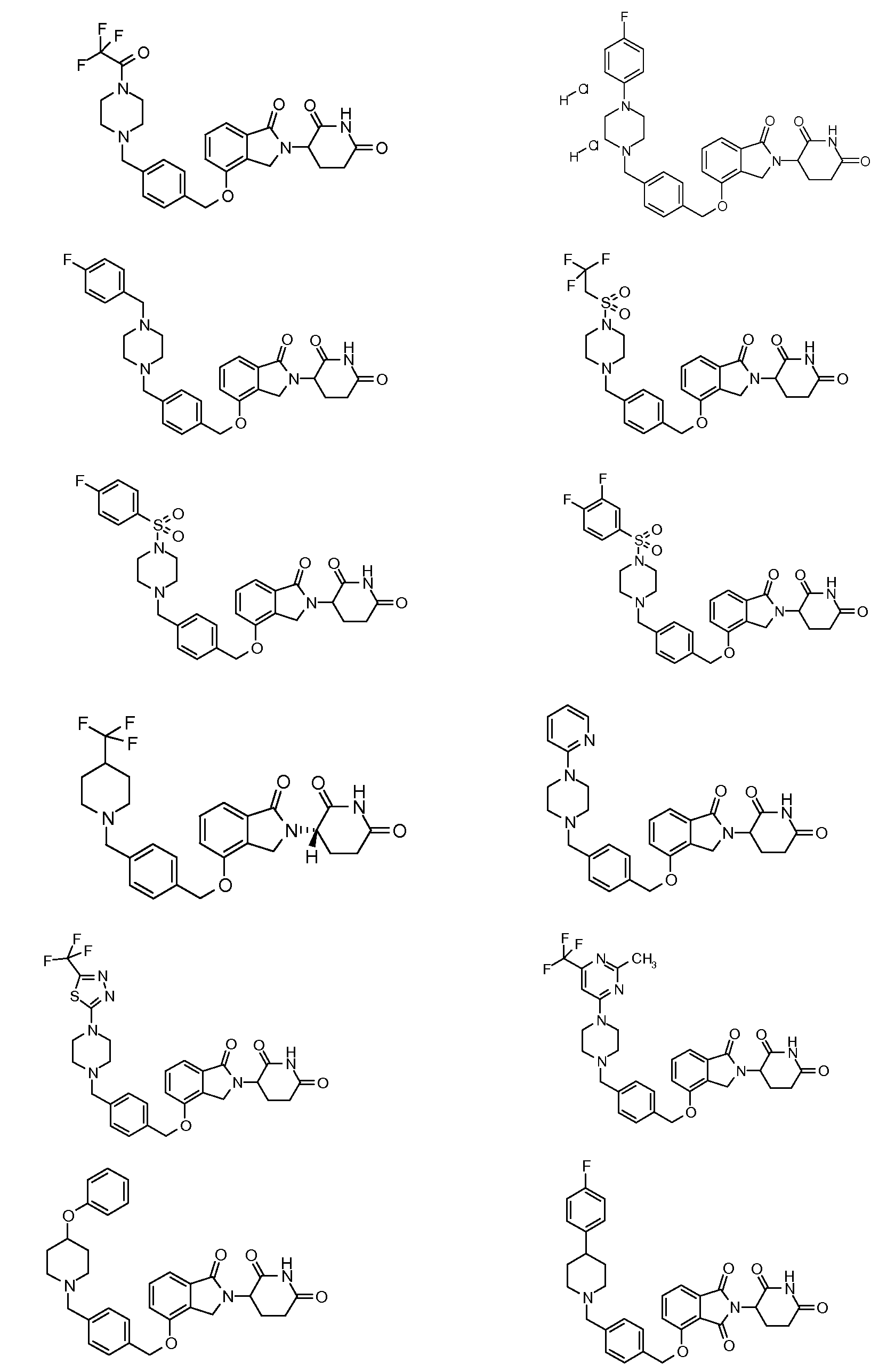
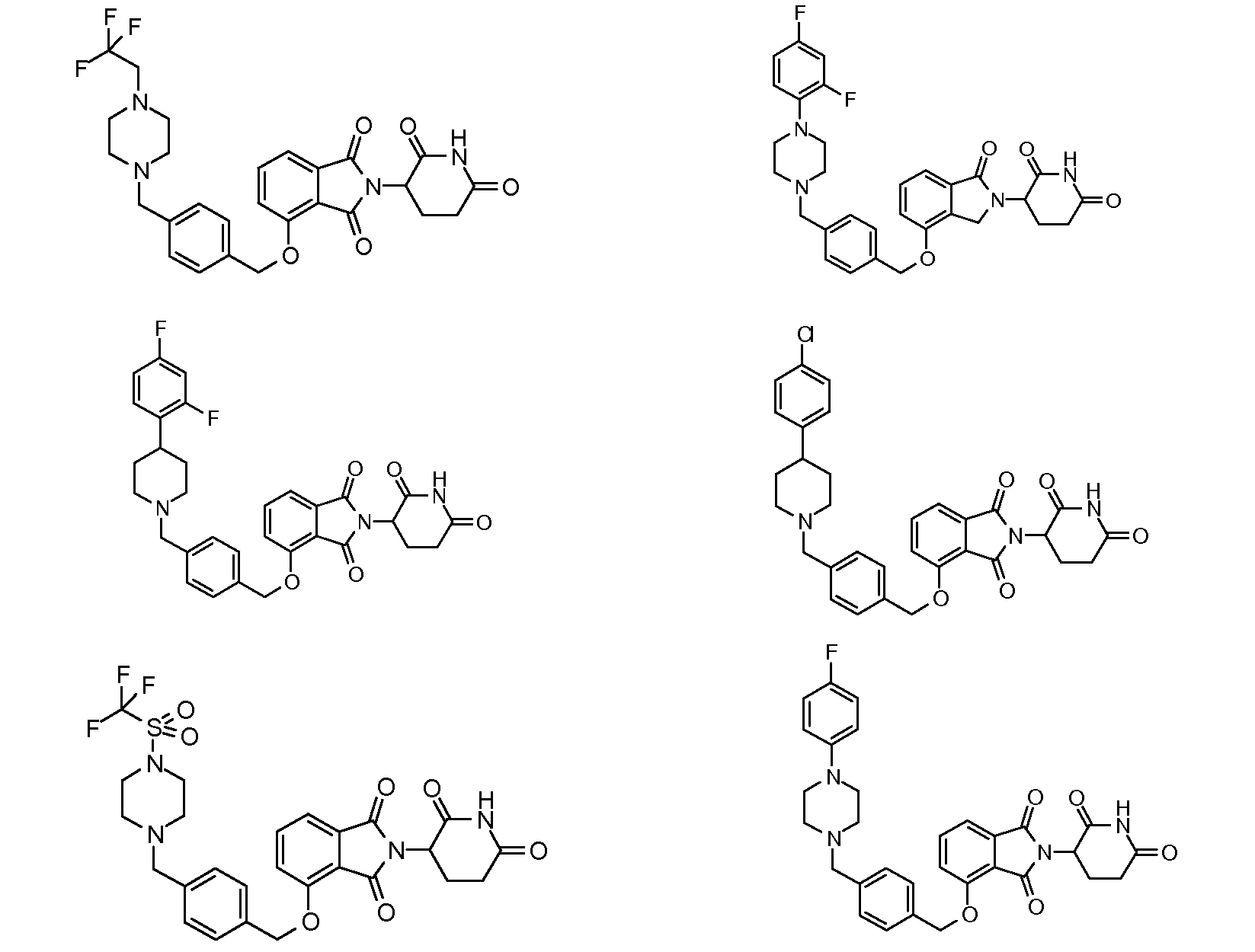
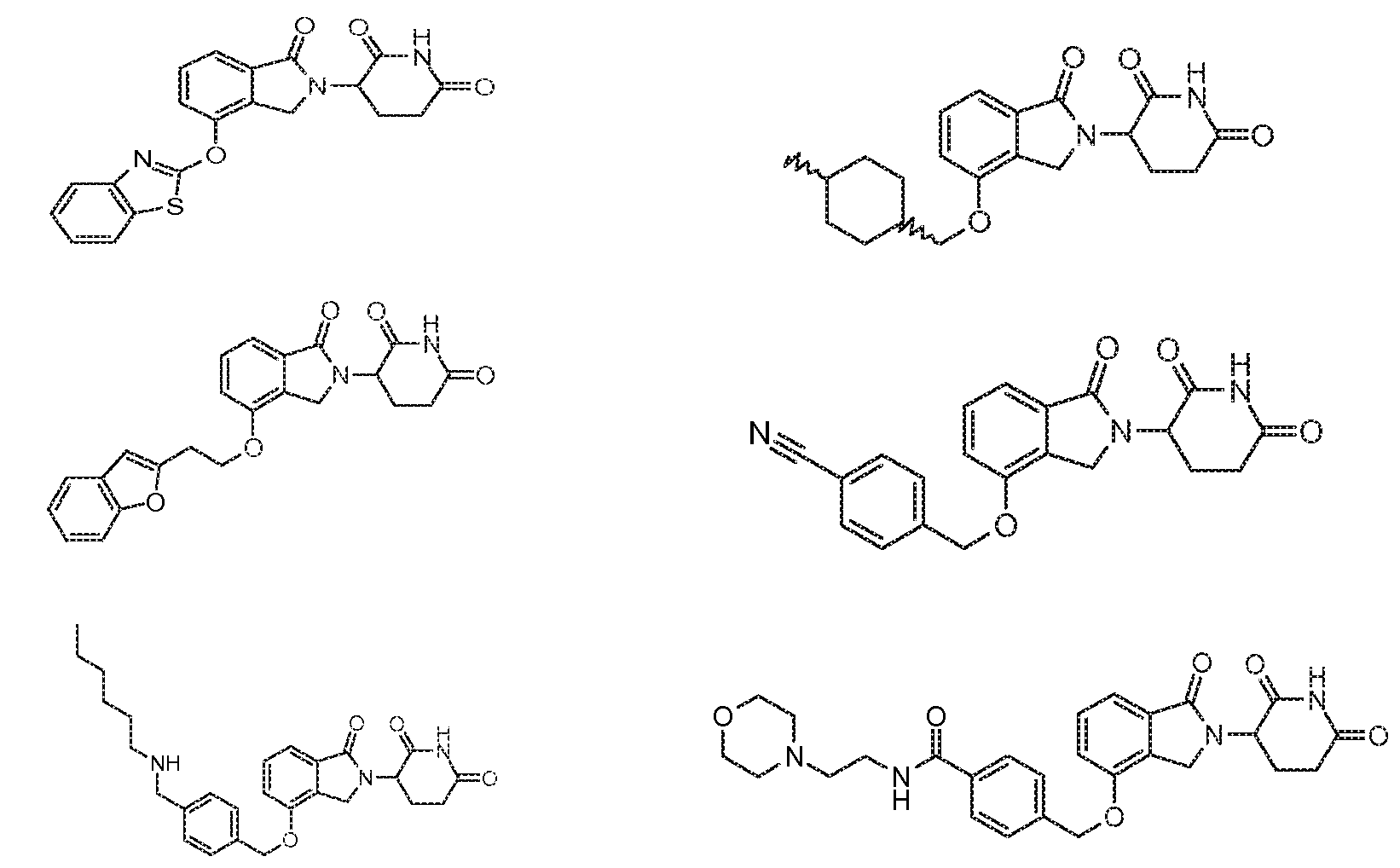
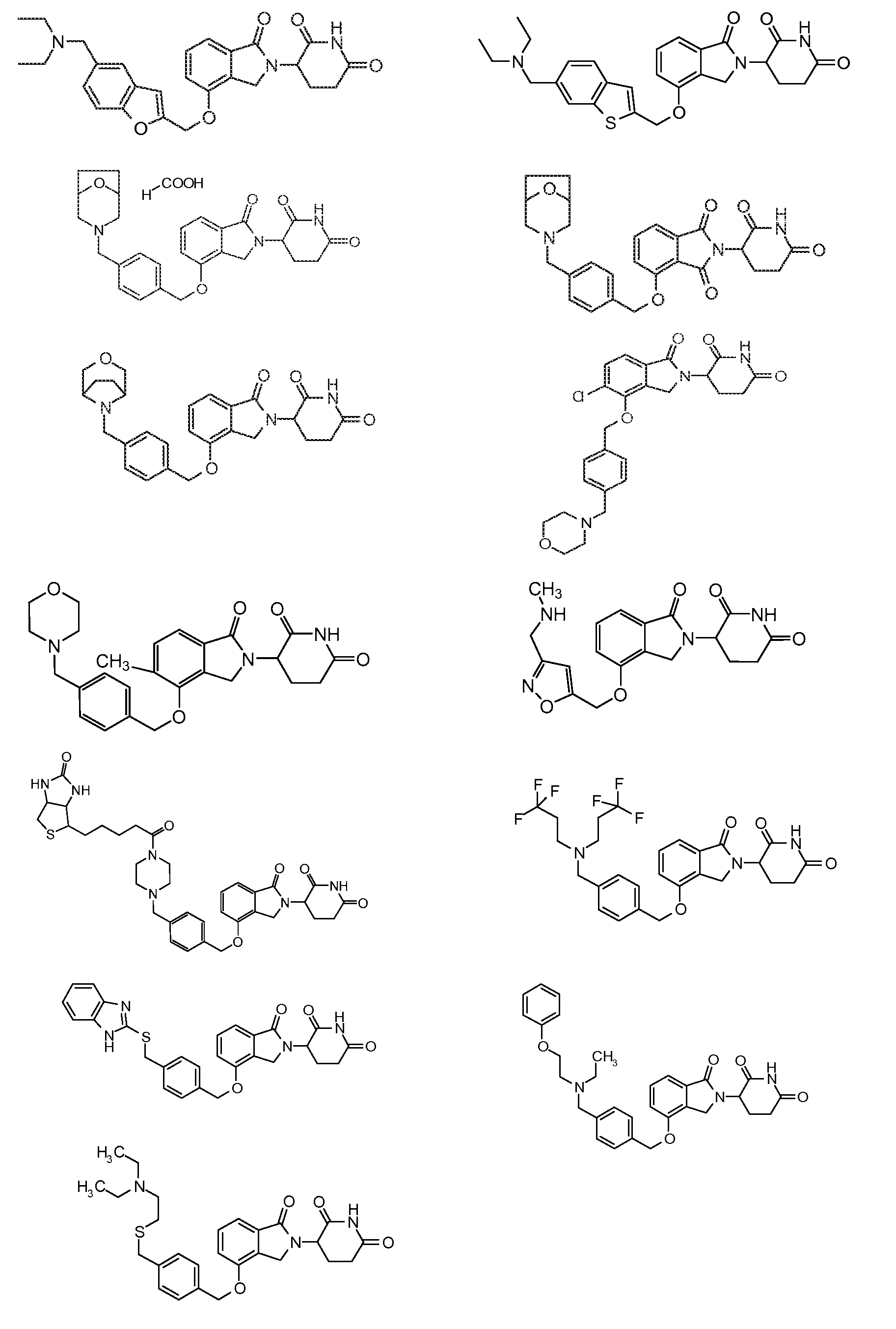
1. Соединение формулы (I):или его фармацевтически приемлемая соль, сольват или стереоизомер, в которой:X обозначает C=O илиCH;Rобозначает -Y-R;Rобозначает H или(C-C)алкил;Y обозначает 6-10-членный арил, гетероарил или гетероцикл, каждый из которых может быть замещен одним или более галогенами; или связь;Rобозначает -(CH)-арил, -O-(CH)-арил или -(CH)-O-арил, в которых арил может быть замещен одним или более из следующих заместителей:(C-C)алкил, который сам может быть замещен одним или более галогенами; (C-C)алкокси, который сам замещен одним или более галогенами; оксо; амино; карбоксил; циано; гидроксил; галоген; дейтерий; 6-10-членный арил или гетероарил, в случае необходимости замещенный одним или более (C-C)алкилами, (C-C)алкокси или галогенами; -CONH; или -COO-(C-C)алкил, причем алкил может быть замещен одним или более галогенами;-(CH)-гетероцикл, -O-(CH)-гетероцикл или -(CH)-O-гетероцикл, причем гетероцикл может быть замещен одним или более из следующих заместителей: (C-C)алкил, сам в случае необходимости замещенный одним или более галогенами; (C-C)алкокси, сам замещенный одним или более галогенами; оксо; амино; карбоксил; циано; гидроксил; галоген; дейтерий; 6-10-членный арил или гетероарил, в случае необходимости замещенный одним или более (C-C)алкилами, (C-C)алкокси или галогенами; -CONH; или -COO-(C-C)алкил, причем алкил может быть замещен одним или более галогенами; или-(CH)-гетероарил, -O-(CH)-гетероарил или -(CH)-O-гетероарил, причем гетероарил может быть замещен одним или более из следующих заместителей: (C-C)алкил, сам в случае необходимости замещенный одним или более галогенами;(C-C)алкокси, сам замещенный одним или более галогенами; оксо; амино; карбоксил; циано; гидроксил; галоген; д
Claims (20)
1. Соединение формулы (I):
или его фармацевтически приемлемая соль, сольват или стереоизомер, в которой:
X обозначает C=O или CH2;
R1 обозначает -Y-R3;
R2 обозначает H или (C1-C6)алкил;
Y обозначает 6-10-членный арил, гетероарил или гетероцикл, каждый из которых может быть замещен одним или более галогенами; или связь;
R3 обозначает -(CH2)n-арил, -O-(CH2)n-арил или -(CH2)n-O-арил, в которых арил может быть замещен одним или более из следующих заместителей: (C1-C6)алкил, который сам может быть замещен одним или более галогенами; (C1-C6)алкокси, который сам замещен одним или более галогенами; оксо; амино; карбоксил; циано; гидроксил; галоген; дейтерий; 6-10-членный арил или гетероарил, в случае необходимости замещенный одним или более (C1-C6)алкилами, (C1-C6)алкокси или галогенами; -CONH2; или -COO-(C1-C6)алкил, причем алкил может быть замещен одним или более галогенами;
-(CH2)n-гетероцикл, -O-(CH2)n-гетероцикл или -(CH2)n-O-гетероцикл, причем гетероцикл может быть замещен одним или более из следующих заместителей: (C1-C6)алкил, сам в случае необходимости замещенный одним или более галогенами; (C1-C6)алкокси, сам замещенный одним или более галогенами; оксо; амино; карбоксил; циано; гидроксил; галоген; дейтерий; 6-10-членный арил или гетероарил, в случае необходимости замещенный одним или более (C1-C6)алкилами, (C1-C6)алкокси или галогенами; -CONH2; или -COO-(C1-C6)алкил, причем алкил может быть замещен одним или более галогенами; или
-(CH2)n-гетероарил, -O-(CH2)n-гетероарил или -(CH2)n-O-гетероарил, причем гетероарил может быть замещен одним или более из следующих заместителей: (C1-C6)алкил, сам в случае необходимости замещенный одним или более галогенами; (C1-C6)алкокси, сам замещенный одним или более галогенами; оксо; амино; карбоксил; циано; гидроксил; галоген; дейтерий; 6-10-членный арил или гетероарил, в случае необходимости замещенный одним или более (C1-C6)алкилами, (C1-C6)алкокси или галогенами; -CONH2; или -COO-(C1-C6)алкил, причем алкил может быть замещен одним или более галогенами; и
n=0, 1, 2 или 3.
2. Соединение по п.1, в котором X обозначает CH2.
3. Соединение по п.1, в котором Y обозначает фенил и R3 обозначает (CH2)n-гетероцикл.
4. Соединение по п.1, в котором Y обозначает гетероарил и R3 обозначает (CH2)n-гетероцикл.
5. Соединение по п.1, в котором Y обозначает связь.
6. Соединение по п.1, в котором Y обозначает связь и R3 обозначает (CH2)n-гетероцикл или -(CH2)n-гетероарил.
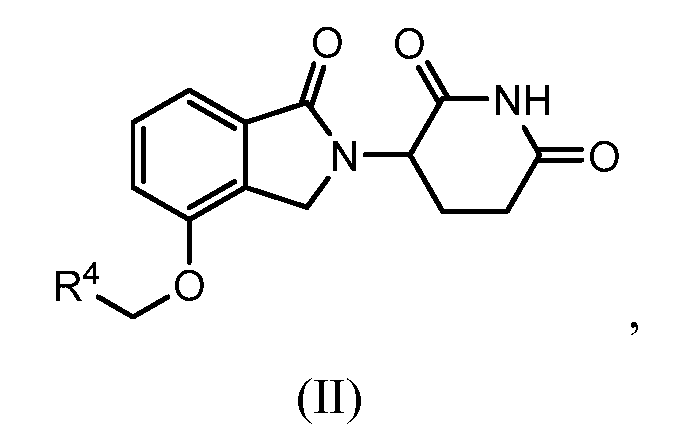
8. Соединение формулы (II):
или его фармацевтически приемлемая соль, сольват или стереоизомер, в которой:
R4 обозначает незамещенное 9-10-членное бициклическое кольцо, представляющее собой бензотиазол, хинолин, изохинолин, нафталин, 2,3-дигидро-1H-инден, бензо[d][1,2,3]триазол, имидазо[1,2-а]пиридин, бензофуран, 2,3-дигидробензофуран, бензотиофен, бензо[d]оксазол изоиндолин или хроман;
при условии, что, если бициклическое кольцо представляет собой бензофуран или бензотиофен, то кольцо не связано с изоиндольным кольцом через положение 2.
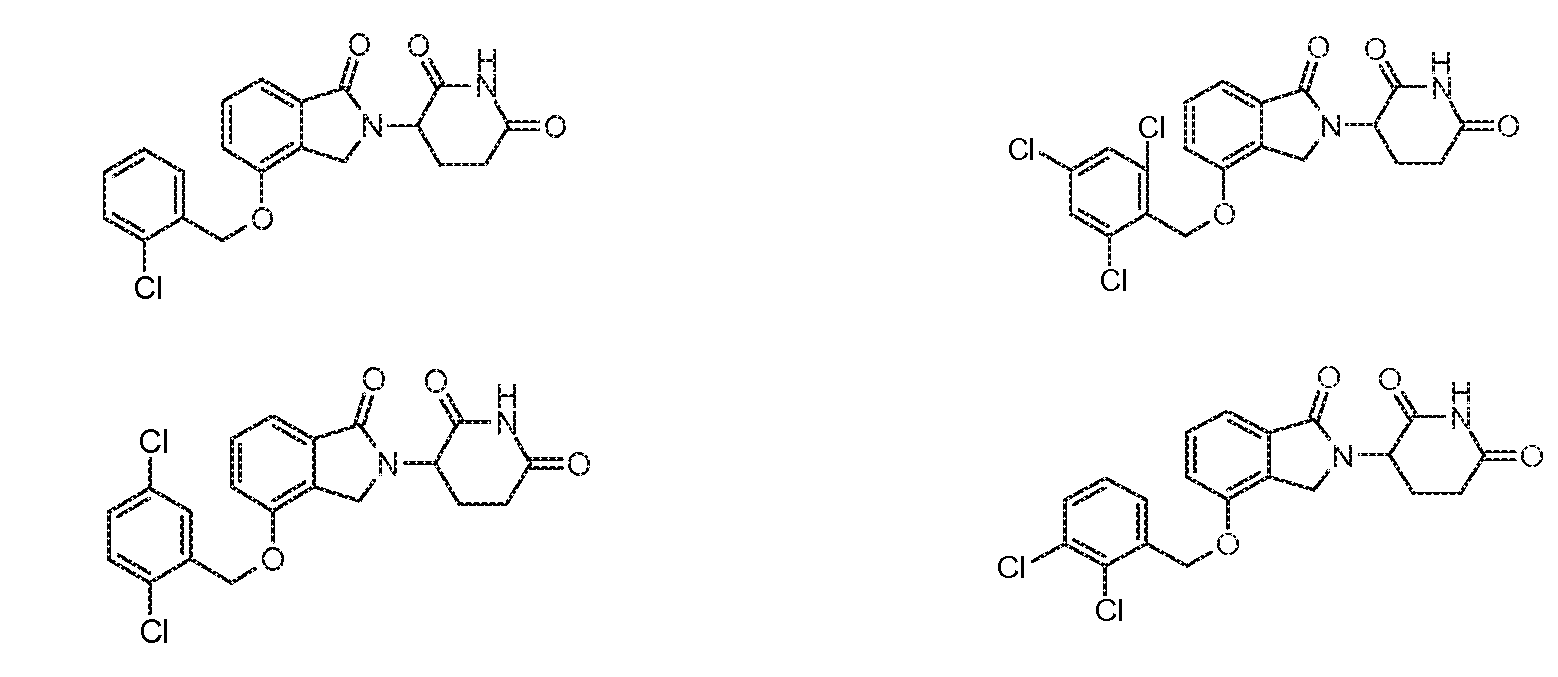
10. Соединение формулы (III):
или его фармацевтически приемлемая соль, сольват или стереоизомер, в которой:
X обозначает CH2 или C=O;
R5, R6 и R7 обозначают каждый независимо водород, галоген, нитро, карбамоил, амино, -SO2R8, -CONR9R10, -(C1-C6)алкил или -(C1-C6)алкокси, причем указанный алкил или алкокси может быть замещен одним или более галогенами, амино, гидроксилами или NR9R10;
R8 обозначает (C1-C6)алкил, в случае необходимости замещенный (C1-C6)алкилом или (C6-C10)арилом; амино, в случае необходимости замещенный (C1-C6)алкилом или (C6-C10)арилом; или 6-10-членный гетероцикл, в случае необходимости замещенный (C1-C6)алкилом или (C6-C10)арилом;
R9 и R10 обозначают каждый независимо водород, 6-10-членный арил, -COO-(C1-C6)алкил, -(C0-C6)алкил-CHO, -(C0-C6)алкил-COOH, -(C0-C6)алкил-NR9'R10', -(C0-C6)алкил-(5-10-членный гетероцикл), -(C1-C6)алкил-ОН, -(C1-C6)алкил-О-(C1-C6)алкил, (C1-C6)алкил или (C3-C6)циклоалкил; или
R9 и R10 вместе могут образовывать в случае необходимости замещенное 5-6-членное кольцо, содержащее один или более гетероатомов; и
R9' и R10' обозначают каждым независимо водород или (C1-C6)алкил;
при условии, что все R5-R7 не могут быть водородом; и
при условии, что, если один из R5-R7 обозначает водород и остальные два из R5-R7 оба являются хлором, тогда два атома хлора не могут быть в положениях 3 и 4 фенильного кольца.
11. Соединение по п.10, в котором один из R5-R7 обозначает водород и остальные два из R5-R7 обозначают каждый независимо галоген, (C1-C6)алкокси или (C1-C6)алкил.
12. Соединение по п.10, в котором два из R5-R7 обозначают водород и оставшийся из R5-R7 обозначает галоген, (C1-C6)алкокси или (C1-C6)алкил.
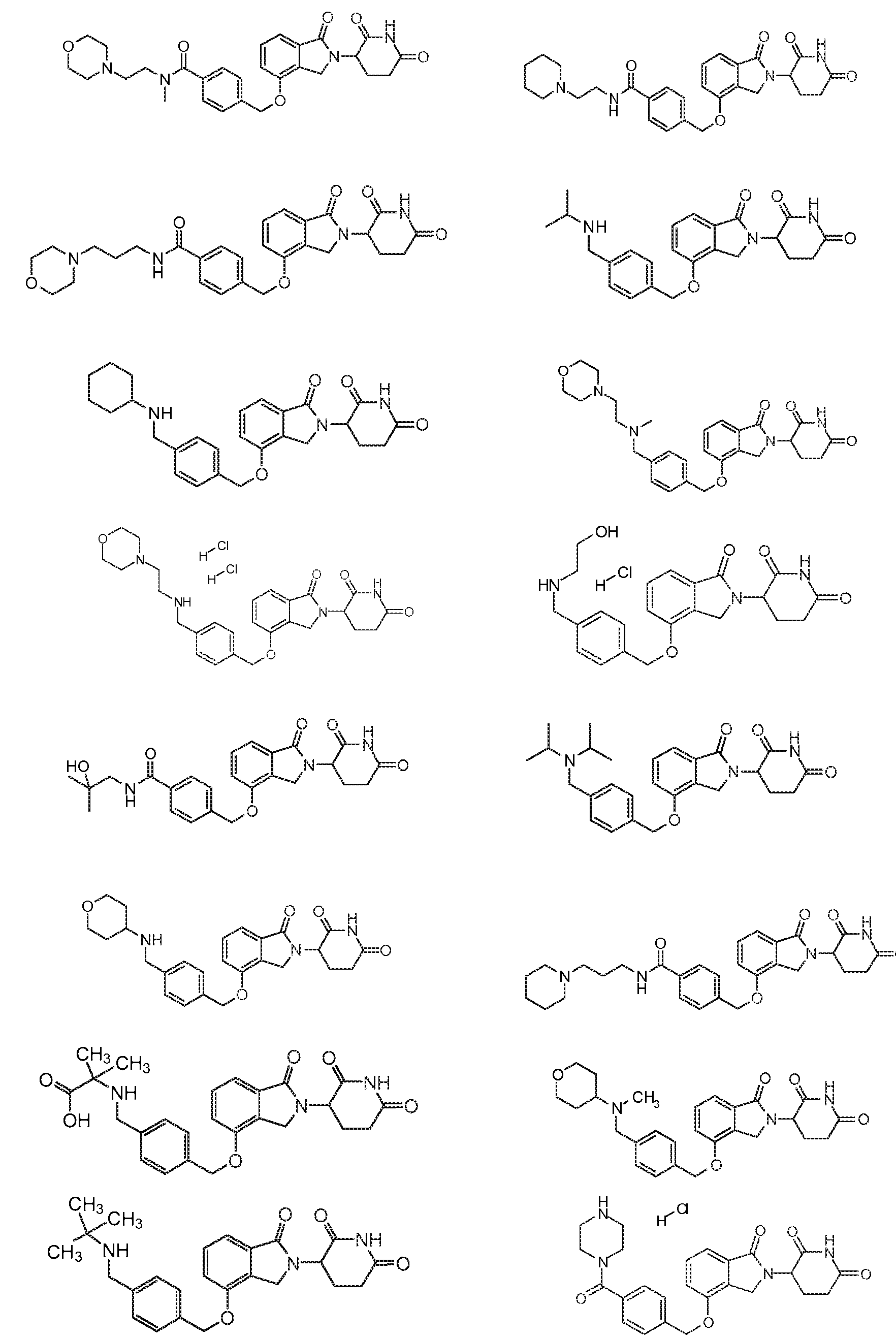
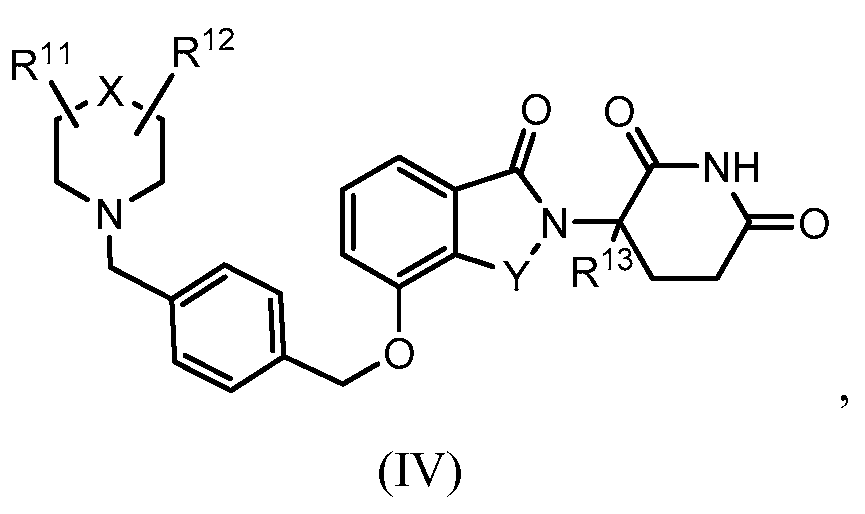
14. Соединение формулы (IV):
или его фармацевтически приемлемая соль, сольват или стереоизомер, в которой:
X обозначает N или C;
Y обозначает CH2 или C=O;
R11 и R12 обозначают каждый независимо водород, -(C1-C6)алкил, -(C1-C6)алкил-(C3-C6)циклоалкил, -(C1-C6)алкокси, -(C6-C10)арил, -CO(C1-C6)алкил, -CO(C3-C6)циклоалкил, -CO(C6-C10)арил, -COO(C1-C6)алкил, галоген, гидроксил, оксо, 3-10-членный гетероцикл, 6-10-членный гетероарил, -NHCO(C1-C6)алкил, -(CH2)n-фенил, -SO2(C1-C6)алкил, -SO2(C3-C6)циклоалкил, -SO2(C6-C10)арил или -NR14R15, причем алкильная, арильная или гетероарильная часть каждой из групп может быть замещенной одним или более галогенами, гидроксилами или -(C1-C6)алкокси;
R13 обозначает водород или -(C1-C6)алкил;
R14 и R15 обозначают каждый независимо водород или -(C1-C6)алкил; и
n=0, 1, 2 или 3.
15. Соединение по п.14, в котором X обозначает N.
16. Соединение по п.14, в котором Y обозначает CH2.
19. Фармацевтическая композиция, включающая соединение по любому из пп.1-18 или его фармацевтически приемлемую соль, сольват или стереоизомер.
20. Способ лечения, контроля или профилактики заболевания или нарушения, включающий введение пациенту соединения по любому из пп.1-18 или его фармацевтически приемлемой соли, сольвата или стереоизомера, причем заболевание или нарушение представляет собой рак, нарушения, связанные с ангиогенезом, боль, дегенерацию желтого пятна или связанный синдром, кожное заболевание, легочное нарушение, связанное с асбестом нарушение, паразитарное заболевание, иммунодефицит, нарушение ЦНС, повреждение ЦНС, атеросклероз или связанное нарушение, дисфункциональный сон или связанное нарушение, инфекционное заболевание, гемоглобинопатию или связанное нарушение или связанное с TNFα нарушение.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US30361810P | 2010-02-11 | 2010-02-11 | |
| US61/303,618 | 2010-02-11 | ||
| PCT/US2011/024269 WO2011100380A1 (en) | 2010-02-11 | 2011-02-10 | Arylmethoxy isoindoline derivatives and compositions comprising and methods of using the same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2012138709A true RU2012138709A (ru) | 2014-03-20 |
| RU2567753C2 RU2567753C2 (ru) | 2015-11-10 |
Family
ID=43708752
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2012138709/04A RU2567753C2 (ru) | 2010-02-11 | 2011-02-10 | Производные арилметокси изоиндолина и композиции, включающие их, и способы их применения |
Country Status (33)
| Country | Link |
|---|---|
| US (8) | US8518972B2 (ru) |
| EP (6) | EP4289838A3 (ru) |
| JP (5) | JP2013519675A (ru) |
| KR (2) | KR101931468B1 (ru) |
| CN (2) | CN102822165B (ru) |
| AR (1) | AR081058A1 (ru) |
| AU (1) | AU2011215877C1 (ru) |
| CA (1) | CA2787823C (ru) |
| CO (1) | CO6571916A2 (ru) |
| CR (1) | CR20120414A (ru) |
| CY (3) | CY1119177T1 (ru) |
| DK (3) | DK3202461T3 (ru) |
| EC (1) | ECSP12012098A (ru) |
| ES (5) | ES2713482T3 (ru) |
| HR (3) | HRP20171078T1 (ru) |
| HU (3) | HUE042011T2 (ru) |
| IL (2) | IL220992A (ru) |
| LT (3) | LT2536706T (ru) |
| ME (2) | ME02766B (ru) |
| MX (3) | MX337169B (ru) |
| NI (1) | NI201200132A (ru) |
| NZ (3) | NZ601289A (ru) |
| PH (2) | PH12012501607A1 (ru) |
| PL (3) | PL3202460T3 (ru) |
| PT (3) | PT3202460T (ru) |
| RS (3) | RS56232B1 (ru) |
| RU (1) | RU2567753C2 (ru) |
| SG (3) | SG10202012179RA (ru) |
| SI (3) | SI3202460T1 (ru) |
| SM (3) | SMT201900420T1 (ru) |
| TR (1) | TR201903027T4 (ru) |
| UA (2) | UA115220C2 (ru) |
| WO (1) | WO2011100380A1 (ru) |
Cited By (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2695521C2 (ru) * | 2014-10-30 | 2019-07-23 | Канпу Биофармасьютикалс, Лтд. | Производное изоиндолина, промежуточный продукт, способ получения, фармацевтическая композиция и ее применение |
| US10584101B2 (en) | 2016-10-11 | 2020-03-10 | Arvinas, Inc. | Compounds and methods for the targeted degradation of androgen receptor |
| US10604506B2 (en) | 2017-01-26 | 2020-03-31 | Arvinas Operations, Inc. | Modulators of estrogen receptor proteolysis and associated methods of use |
| US10647698B2 (en) | 2016-12-01 | 2020-05-12 | Arvinas Operations, Inc. | Tetrahydronaphthalene and tetrahydroisoquinoline derivatives as estrogen receptor degraders |
| US10723717B2 (en) | 2016-12-23 | 2020-07-28 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of rapidly accelerated fibrosarcoma polypeptides |
| US10772962B2 (en) | 2015-08-19 | 2020-09-15 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of bromodomain-containing proteins |
| US10806737B2 (en) | 2016-12-23 | 2020-10-20 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of fetal liver kinase polypeptides |
| RU2738833C2 (ru) * | 2014-04-14 | 2020-12-17 | Арвинас, Оперэйшнз, Инк. | Имидные модуляторы протеолиза и способы их применения |
| US10946017B2 (en) | 2015-06-05 | 2021-03-16 | Arvinas Operations, Inc. | Tank-binding kinase-1 PROTACs and associated methods of use |
| US10994015B2 (en) | 2016-12-23 | 2021-05-04 | Arvinas Operations, Inc. | EGFR proteolysis targeting chimeric molecules and associated methods of use |
| US11065231B2 (en) | 2017-11-17 | 2021-07-20 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of interleukin-1 receptor- associated kinase 4 polypeptides |
| US11161841B2 (en) | 2018-04-04 | 2021-11-02 | Arvinas Operations, Inc. | Modulators of proteolysis and associated methods of use |
| US11173211B2 (en) | 2016-12-23 | 2021-11-16 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of rapidly accelerated Fibrosarcoma polypeptides |
| US11191741B2 (en) | 2016-12-24 | 2021-12-07 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of enhancer of zeste homolog 2 polypeptide |
| US11352351B2 (en) | 2015-01-20 | 2022-06-07 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of androgen receptor |
| US11427548B2 (en) | 2015-01-20 | 2022-08-30 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of androgen receptor |
| US11458123B2 (en) | 2016-11-01 | 2022-10-04 | Arvinas Operations, Inc. | Tau-protein targeting PROTACs and associated methods of use |
| RU2781643C2 (ru) * | 2018-01-25 | 2022-10-17 | Фудзимото Ко., Лтд. | Производное тиофена и его применение |
| US11707452B2 (en) | 2018-08-20 | 2023-07-25 | Arvinas Operations, Inc. | Modulators of alpha-synuclein proteolysis and associated methods of use |
| US11883393B2 (en) | 2019-12-19 | 2024-01-30 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of androgen receptor |
| US11912699B2 (en) | 2019-07-17 | 2024-02-27 | Arvinas Operations, Inc. | Tau-protein targeting compounds and associated |
| US11957759B1 (en) | 2022-09-07 | 2024-04-16 | Arvinas Operations, Inc. | Rapidly accelerated fibrosarcoma (RAF) degrading compounds and associated methods of use |
| US11986532B2 (en) | 2021-04-16 | 2024-05-21 | Arvinas Operations, Inc. | Modulators of BCL6 proteolysis and associated methods of use |
| US12043612B2 (en) | 2020-05-09 | 2024-07-23 | Arvinas Operations, Inc. | Methods of manufacturing a bifunctional compound, ultrapure forms of the bifunctional compound, and dosage forms comprising the same |
| US12162859B2 (en) | 2020-09-14 | 2024-12-10 | Arvinas Operations, Inc. | Crystalline and amorphous forms of a compound for the targeted degradation of estrogen receptor |
| US12180193B2 (en) | 2020-08-28 | 2024-12-31 | Arvinas Operations, Inc. | Accelerating fibrosarcoma protein degrading compounds and associated methods of use |
| US12208095B2 (en) | 2019-08-26 | 2025-01-28 | Arvinas Operations, Inc. | Methods of treating breast cancer with tetrahydronaphthalene derivatives as estrogen receptor degraders |
| US12239711B2 (en) | 2014-04-14 | 2025-03-04 | Arvinas Operations, Inc. | Cereblon ligands and bifunctional compounds comprising the same |
| US12310975B2 (en) | 2019-10-17 | 2025-05-27 | Arvinas Operations, Inc. | Modulators of BCL6 proteolysis and associated methods of use |
| US12441708B2 (en) | 2017-01-31 | 2025-10-14 | Arvinas Operations, Inc. | Cereblon ligands and bifunctional compounds comprising the same |
| US12448399B2 (en) | 2023-01-26 | 2025-10-21 | Arvinas Operations, Inc. | Cereblon-based KRAS degrading PROTACs and uses related thereto |
| US12496301B2 (en) | 2023-12-08 | 2025-12-16 | Arvinas Operations, Inc. | Use of androgen receptor degrader for the treatment of spinal and bulbar muscular atrophy |
Families Citing this family (126)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5645816B2 (ja) | 2009-05-25 | 2014-12-24 | 国立大学法人東京工業大学 | 中枢神経細胞の増殖及び分化に係る中核因子を含む医薬組成物 |
| RS56232B1 (sr) | 2010-02-11 | 2017-11-30 | Celgene Corp | Derivati arilmetoksi izoindolina i kompozicije koje ih obuhvataju i postupci njihove primene |
| US9090585B2 (en) | 2011-03-28 | 2015-07-28 | Deuterx, Llc | 2,6-dioxo-3-deutero-piperdin-3-yl-isoindoline compounds |
| EP2702410A2 (en) | 2011-04-29 | 2014-03-05 | Celgene Corporation | Methods for the treatment of cancer and inflammatory diseases using cereblon as a predictor |
| MX358517B (es) | 2012-06-29 | 2018-08-24 | Celgene Corp | Métodos para determinar eficacia de fármacos usando proteínas asociadas a cereblon. |
| EA029485B1 (ru) | 2012-08-09 | 2018-04-30 | Селджин Корпорейшн | Способы лечения рака, выбранного из лимфомы, лейкоза, миеломы, глиобластомы, рака ободочной и прямой кишки и печеночно-клеточной карциномы, с использованием 3-(4-((4-(морфолинометил)бензил)окси)-1-оксоизоиндолин-2-ил)пиперидин-2,6-диона |
| ES2885769T3 (es) | 2012-08-09 | 2021-12-15 | Celgene Corp | Una forma sólida de clorhidrato de (s)-3-(4-((4-morpholinometil)bencil)oxi)-1-oxoisoindolin-2-il)piperidina-2,6-diona |
| EP3741372A1 (en) * | 2012-08-09 | 2020-11-25 | Celgene Corporation | (s)-3-[4-(4-morphlin-4-ylmethylbenzyloxy)-1- oxo-1,3-dihydro-isoindo-2-yl]piperidine-2,6-dione for use in the treatment of immune-related and inflammatory diseases |
| US20150038511A1 (en) * | 2012-08-09 | 2015-02-05 | Celgene Corporation | Treatment of immune-related and inflammatory diseases |
| US20140343058A1 (en) * | 2012-08-09 | 2014-11-20 | Celgene Corporation | Treatment of systemic lupus erythematosus |
| BR112015002183A2 (pt) * | 2012-08-09 | 2017-07-04 | Celgene Corp | processos para a preparação de (s) -3- (4 - ((4- (morfolinometil) benzil) oxi) -1-oxoisoindolin-2-il) -piperidina-2,6-diona e formas farmaceuticamente aceitáveis da mesma |
| US9587281B2 (en) | 2012-08-14 | 2017-03-07 | Celgene Corporation | Cereblon isoforms and their use as biomarkers for therapeutic treatment |
| WO2014039960A1 (en) * | 2012-09-10 | 2014-03-13 | Celgene Corporation | Methods for the treatment of locally advanced breast cancer |
| AU2013204922B2 (en) | 2012-12-20 | 2015-05-14 | Celgene Corporation | Chimeric antigen receptors |
| CA2935495C (en) | 2013-01-14 | 2021-04-20 | Deuterx, Llc | 3-(5-substituted-4-oxoquinazolin-3(4h)-yl)-3-deutero-piperidine-2,6-dione derivatives |
| WO2014116573A1 (en) | 2013-01-22 | 2014-07-31 | Celgene Corporation | Processes for the preparation of isotopologues of 3-(4-((4-(morpholinomethyl)benzyl)oxy)-1-oxoisoindolin-2-yl)piperidine-2,6-dione and pharmaceutically acceptable salts thereof |
| AU2014236597A1 (en) | 2013-03-14 | 2015-09-24 | Deuterx, Llc | 3-(substituted-4-oxo-quinazolin-3(4H)-yl)-3-deutero-piperidine-2,6-dione derivatives |
| CA2907397C (en) | 2013-03-15 | 2022-11-22 | Anthrogenesis Corporation | Modified t lymphocytes |
| WO2014172429A1 (en) | 2013-04-17 | 2014-10-23 | Signal Pharmaceuticals, Llc | Combination therapy comprising a tor kinase inhibitor and an imid compound for treating cancer |
| UA117141C2 (uk) * | 2013-10-08 | 2018-06-25 | Селджин Корпорейшн | Склади (s)-3-(4-((4-(морфолінометил)бензил)оксі)-1-оксоізоіндолін-2-іл)піперидин-2,6-діону |
| MX386337B (es) | 2013-12-06 | 2025-03-18 | Celgene Corp | Métodos para determinar la eficacia del fármaco para el tratamiento de linfoma difuso de células b grandes, mieloma múltiple, y cánceres mieloides. |
| ES2969532T3 (es) | 2014-05-19 | 2024-05-21 | Celgene Corp | 3-(4-((4-(Morfolinometil-bencil)oxi)-1-oxoisoindolin-2-il)piperidin-2,6-diona para el tratamiento de lupus eritematoso sistémico |
| JP6640126B2 (ja) | 2014-06-27 | 2020-02-05 | セルジーン コーポレイション | セレブロン及び他のe3ユビキチンリガーゼの立体構造の変化を誘導するための組成物及び方法 |
| US10316030B2 (en) | 2014-08-07 | 2019-06-11 | Calithera Biosciences, Inc. | Crystal forms of glutaminase inhibitors |
| EP3925609B1 (en) | 2014-08-22 | 2025-07-30 | Celgene Corporation | Methods of treating multiple myeloma with immunomodulatory compounds in combination with antibodies |
| EP3653628B1 (en) * | 2015-07-21 | 2022-09-14 | ImmunoGen, Inc. | Methods of preparing cytotoxic benzodiazepine derivatives |
| US9809603B1 (en) | 2015-08-18 | 2017-11-07 | Deuterx, Llc | Deuterium-enriched isoindolinonyl-piperidinonyl conjugates and oxoquinazolin-3(4H)-yl-piperidinonyl conjugates and methods of treating medical disorders using same |
| AU2016332236B2 (en) * | 2015-09-29 | 2019-04-11 | Kangpu Biopharmaceuticals, Ltd. | Pharmaceutical composition and application thereof |
| US10830762B2 (en) | 2015-12-28 | 2020-11-10 | Celgene Corporation | Compositions and methods for inducing conformational changes in cereblon and other E3 ubiquitin ligases |
| CA3020281A1 (en) * | 2016-04-06 | 2017-10-12 | The Regents Of The University Of Michigan | Monofunctional intermediates for ligand-dependent target protein degradation |
| UA123168C2 (uk) * | 2016-04-12 | 2021-02-24 | Дзе Ріджентс Оф Дзе Юніверсіті Оф Мічіган | Деструктори білка вет |
| TWI794171B (zh) | 2016-05-11 | 2023-03-01 | 美商滬亞生物國際有限公司 | Hdac抑制劑與pd-l1抑制劑之組合治療 |
| TWI808055B (zh) | 2016-05-11 | 2023-07-11 | 美商滬亞生物國際有限公司 | Hdac 抑制劑與 pd-1 抑制劑之組合治療 |
| CA3041840A1 (en) | 2016-10-28 | 2018-05-03 | Icahn School Of Medicine At Mount Sinai | Compositions and methods for treating ezh2-mediated cancer |
| EP4295918A3 (en) | 2016-11-02 | 2024-03-20 | Bristol-Myers Squibb Company | Bispecific antibody against bcma and cd3 and an immunological drug for combined use in treating multiple myeloma |
| EP3551185A4 (en) | 2016-12-08 | 2021-07-14 | Icahn School of Medicine at Mount Sinai | COMPOSITIONS AND METHODS OF TREATMENT OF CDK4 / 6 MEDIATED CANCER |
| AU2017376704B2 (en) | 2016-12-16 | 2021-08-05 | Kangpu Biopharmaceuticals, Ltd. | Composition, application thereof and treatment method |
| WO2018140671A1 (en) | 2017-01-27 | 2018-08-02 | Celgene Corporation | 3-(1-oxo-4-((4-((3-oxomorpholino) methyl)benzyl)oxy)isoindolin-2-yl)piperidine-2,6-dione and isotopologues thereof |
| JP7534849B2 (ja) | 2017-02-03 | 2024-08-15 | セルジーン コーポレイション | セレブロンに対する小分子の親和性の測定方法 |
| US20200000776A1 (en) | 2017-02-13 | 2020-01-02 | Kangpu Biopharmaceuticals, Ltd. | Combination treating prostate cancer, pharmaceutical composition and treatment method |
| CN108929307A (zh) | 2017-05-22 | 2018-12-04 | 苏州偶领生物医药有限公司 | 一类异吲哚酮-酰亚胺环-1,3-二酮-2-烯化合物、其组合物和用途 |
| SG11202000143PA (en) * | 2017-07-10 | 2020-02-27 | Celgene Corp | Antiproliferative compounds and methods of use thereof |
| CN118638025A (zh) | 2017-08-21 | 2024-09-13 | 细胞基因公司 | 制备(s)-4,5-二氨基-5-氧代戊酸叔丁酯的工艺 |
| US10513515B2 (en) | 2017-08-25 | 2019-12-24 | Biotheryx, Inc. | Ether compounds and uses thereof |
| IL273428B2 (en) | 2017-09-22 | 2023-09-01 | Jubilant Epipad LLC | Heterocyclic compounds as pad inhibitors |
| CN111225915B (zh) | 2017-10-18 | 2023-03-07 | 朱比兰特埃皮帕德有限公司 | 作为pad抑制剂的咪唑并吡啶化合物 |
| BR112020008851A2 (pt) | 2017-11-06 | 2020-10-20 | Jubilant Prodel LLC | composto da fórmula i, processo de preparação de compostos da fórmula i, composição farmacêutica, método para o tratamento e/ou prevenção de várias doenças, uso, método para o tratamento de câncer, método de tratamento de câncer e método para o tratamento e/ou prevenção de câncer e doenças infecciosas |
| JP7368369B2 (ja) | 2017-11-24 | 2023-10-24 | ジュビラント・エピスクライブ・エルエルシー | Prmt5阻害剤としてのヘテロ環式化合物 |
| IL315310A (en) | 2017-12-26 | 2024-10-01 | Kymera Therapeutics Inc | Irak degraders and uses thereof |
| US11299485B2 (en) | 2018-01-25 | 2022-04-12 | Fujimoto Co., Ltd. | Thiophene derivative and use thereof |
| EP3743066A4 (en) | 2018-01-26 | 2021-09-08 | Yale University | IMIDE-BASED PROTEOLYSIS MODULATORS AND RELATED METHODS OF USE |
| WO2019164891A1 (en) | 2018-02-21 | 2019-08-29 | Celgene Corporation | Bcma-binding antibodies and uses thereof |
| US11472799B2 (en) | 2018-03-06 | 2022-10-18 | Icahn School Of Medicine At Mount Sinai | Serine threonine kinase (AKT) degradation / disruption compounds and methods of use |
| JP7279063B6 (ja) | 2018-03-13 | 2024-02-15 | ジュビラント プローデル エルエルシー | Pd1/pd-l1相互作用/活性化の阻害剤としての二環式化合物 |
| US11358952B2 (en) * | 2018-04-23 | 2022-06-14 | Celgene Corporation | Substituted 4-aminoisoindoline-1,3-dione compounds, compositions thereof, and methods of treatment therewith |
| CA3104298A1 (en) | 2018-06-21 | 2019-12-26 | Icahn School Of Medicine At Mount Sinai | Wd40 repeat domain protein 5 (wdr5) degradation / disruption compounds and methods of use |
| AU2019294414B2 (en) | 2018-06-29 | 2023-06-29 | F. Hoffmann-La Roche Ag | Compounds |
| WO2020010227A1 (en) | 2018-07-06 | 2020-01-09 | Kymera Therapeutics, Inc. | Protein degraders and uses thereof |
| PL3820573T3 (pl) * | 2018-07-10 | 2024-02-19 | Novartis Ag | Pochodne 3-(5-hydroksy-1-oksoizoindolin-2-ylo)piperydyno-2,6-dionu i ich zastosowanie w leczeniu chorób zależnych od palca cynkowego z rodziny ikaros 2 (ikzf2) |
| AR116109A1 (es) | 2018-07-10 | 2021-03-31 | Novartis Ag | Derivados de 3-(5-amino-1-oxoisoindolin-2-il)piperidina-2,6-diona y usos de los mismos |
| EP3830093A1 (en) | 2018-07-27 | 2021-06-09 | Biotheryx, Inc. | Bifunctional compounds as cdk modulators |
| CN112689627B (zh) * | 2018-09-07 | 2022-03-29 | 南京明德新药研发有限公司 | 三环取代哌啶二酮类化合物 |
| EP3862348A4 (en) * | 2018-09-30 | 2022-06-22 | Shanghai Institute of Materia Medica, Chinese Academy of Sciences | ISOINDOLINE COMPOUND, PROCESS OF MANUFACTURE, PHARMACEUTICAL COMPOSITION AND USE THEREOF |
| US11529339B2 (en) | 2018-10-01 | 2022-12-20 | Celgene Corporation | Combination therapy for the treatment of cancer |
| US12263190B2 (en) | 2018-11-08 | 2025-04-01 | Juno Therapeutics, Inc. | Methods and combinations for treatment and T cell modulation |
| JP7623943B2 (ja) | 2018-11-30 | 2025-01-29 | カイメラ セラピューティクス, インコーポレイテッド | Irak分解剤およびそれらの使用 |
| WO2020118098A1 (en) * | 2018-12-05 | 2020-06-11 | Vividion Therapeutics, Inc. | Substituted isoindolinones as modulators of cereblon-mediated neo-substrate recruitment |
| AU2019392231B2 (en) * | 2018-12-06 | 2022-10-20 | Shanghai Institute Of Materia Medica, Chinese Academy Of Sciences | Isoindoline compound, and preparation method, pharmaceutical composition, and application of isoindoline compound |
| BR112021013435A2 (pt) * | 2019-01-09 | 2021-10-19 | Celgene Corporation | Formas sólidas que compreendem (s)-4-(4-(4-(((2-(2,6-dioxopiperidin-3-il)-1-oxoisoindolin-4-il)oxi)metil)benzil)piperazin-1-il)-3-fluorobenzonitrila e sais do mesmo, e composições que compreendem e métodos de uso do mesmo |
| CN113597301A (zh) * | 2019-01-09 | 2021-11-02 | 细胞基因公司 | 包含(s)-4-(4-(4-(((2-(2,6-二氧代哌啶-3-基)-1-氧代异吲哚啉-4-基)氧基)甲基)苄基)哌嗪-1-基)-3-氟苄腈的药物组合物以及使用它的方法 |
| EA202191903A1 (ru) | 2019-01-09 | 2021-11-12 | Селджин Корпорейшн | Антипролиферативные соединения и вторые активные агенты для комбинированного применения |
| AR119715A1 (es) | 2019-04-12 | 2022-01-05 | Celgene Corp | Métodos para tratar linfoma no hodgkin con el uso de 2-(2,6-dioxopiperidin-3-il)-4-((2-fluoro-4-((3-morfolinoazetidin-1-il)metil)bencil)amino)isoindolin-1,3-diona |
| CN114423463B (zh) | 2019-05-06 | 2025-09-26 | 西奈山伊坎医学院 | 作为hpk1的降解剂的异双功能化合物 |
| EP4048666A1 (en) * | 2019-10-21 | 2022-08-31 | Celgene Corporation | Solid forms comprising (s)-2-(2,6-dioxopiperidin-3-yl)-4-((2-fluoro-4-((3-morpholinoazetidin-1-yl)methyl)benzyl)amino)isoindoline-1,3-dione and salts thereof, and compositions comprising the same and their use |
| CN115697387A (zh) | 2019-11-05 | 2023-02-03 | 细胞基因公司 | 抗bcma嵌合抗原受体的用途 |
| BR112022007548A2 (pt) | 2019-11-07 | 2022-07-12 | Juno Therapeutics Inc | Combinação de uma terapia de célula t e (s)-3-[4-(4-morfolin-4-ilmetil-benzilóxi)-1-oxo-1,3-di-hidro-isoindol-2-il]-piperidina-2,6-diona |
| WO2021105335A1 (en) | 2019-11-27 | 2021-06-03 | Captor Therapeutics S.A. | Piperidine-2, 6-dione derivatives which bind to cereblon, and methods of use thereof |
| AU2020392427B2 (en) | 2019-11-27 | 2024-03-07 | Captor Therapeutics S.A. | Piperidine-2, 6-dione derivatives which bind to cereblon, and methods of use thereof |
| EP4069245A1 (en) | 2019-12-02 | 2022-10-12 | Celgene Corporation | Therapy for the treatment of cancer |
| ES2986594T3 (es) * | 2019-12-12 | 2024-11-12 | Accutar Biotechnology Inc | Nuevos derivados de cromano que tienen actividad de degradación del receptor de estrógeno y usos de los mismos |
| KR20220145325A (ko) * | 2019-12-17 | 2022-10-28 | 카이메라 쎄라퓨틱스 인코포레이티드 | Irak 분해제 및 이의 용도 |
| CN114828959B (zh) | 2019-12-18 | 2024-04-02 | 诺华股份有限公司 | 3-(5-甲氧基-1-氧代异吲哚啉-2-基)哌啶-2,6-二酮衍生物及其用途 |
| CN116234803A (zh) | 2019-12-23 | 2023-06-06 | 冰洲石生物科技公司 | 用于治疗癌症的雌激素受体降解剂和细胞周期蛋白依赖性激酶抑制剂的组合 |
| CN115038694A (zh) * | 2020-01-20 | 2022-09-09 | 康朴生物医药技术(上海)有限公司 | 一种异吲哚啉衍生物、其药物组合物及应用 |
| US20230096517A1 (en) * | 2020-02-25 | 2023-03-30 | Shanghaitech University | Glutarimide skeleton-based compounds and application thereof |
| WO2021222150A2 (en) | 2020-04-28 | 2021-11-04 | Anwita Biosciences, Inc. | Interleukin-2 polypeptides and fusion proteins thereof, and their pharmaceutical compositions and therapeutic applications |
| CN115803027A (zh) | 2020-04-30 | 2023-03-14 | 百时美施贵宝公司 | 治疗细胞因子相关的不良事件的方法 |
| US12103924B2 (en) | 2020-06-01 | 2024-10-01 | Icahn School Of Medicine At Mount Sinai | Mitogen-activated protein kinase kinase (MEK) degradation compounds and methods of use |
| IL299293A (en) | 2020-06-25 | 2023-02-01 | Celgene Corp | Cancer treatment methods with combined treatments |
| CN113896711A (zh) * | 2020-07-06 | 2022-01-07 | 北京诺诚健华医药科技有限公司 | 杂环类免疫调节剂 |
| CN115916768B (zh) * | 2020-07-20 | 2024-12-24 | 江苏恒瑞医药股份有限公司 | 含硫异吲哚啉类衍生物、其制备方法及其在医药上的应用 |
| CA3186919A1 (en) | 2020-08-03 | 2022-02-10 | Sylvain Cottens | Low molecular weight protein degraders and their applications |
| WO2022051495A1 (en) * | 2020-09-02 | 2022-03-10 | Ann And Robert H. Lurie Children's Hospital Of Chicago | Methods and compositions for the treatment of pulmonary hypertension and cancer |
| CA3191282A1 (en) | 2020-09-11 | 2022-03-17 | William Pierceall | Combination therapy for cancer |
| WO2022066580A1 (en) * | 2020-09-23 | 2022-03-31 | Kinnate Biopharma Inc. | Raf degrading compounds |
| CN116234553A (zh) | 2020-10-02 | 2023-06-06 | 新基公司 | 治疗系统性红斑狼疮的方法和作为对疗法临床敏感性的预测指标的生物标志物的用途 |
| EP4241278A2 (en) | 2020-11-04 | 2023-09-13 | Celgene Corporation | Car t cell therapy in patients who have had prior anti-cancer alkylator therapy |
| CN112174976A (zh) * | 2020-11-19 | 2021-01-05 | 江西中医药大学 | 从水芹菜中分离的二苯并呋喃型木脂素及其方法和在抗痛风性关节炎上的用途 |
| WO2022146151A1 (en) | 2020-12-30 | 2022-07-07 | Captor Therapeutics S.A. | Novel compounds which bind to cereblon, and methods of use thereof |
| AR124547A1 (es) | 2020-12-30 | 2023-04-05 | Kymera Therapeutics Inc | Degradadores de irak y sus usos |
| TW202241872A (zh) * | 2021-01-05 | 2022-11-01 | 大陸商江蘇恆瑞醫藥股份有限公司 | 稠雜環基取代的環己二醯亞胺衍生物、其製備方法及其在醫藥上的應用 |
| CA3208313A1 (en) | 2021-01-13 | 2022-07-21 | Monte Rosa Therapeutics Ag | Isoindolinone compounds |
| US11773103B2 (en) | 2021-02-15 | 2023-10-03 | Kymera Therapeutics, Inc. | IRAK4 degraders and uses thereof |
| MX2023009527A (es) | 2021-02-15 | 2023-08-24 | Kymera Therapeutics Inc | Degradadores de la cinasa 4 asociada al receptor de interleucina 1 (irak4) y usos de los mismos. |
| UY39671A (es) | 2021-03-15 | 2022-10-31 | Novartis Ag | Derivados de pirazolopiridina y sus usos. |
| CA3211149A1 (en) * | 2021-03-31 | 2022-10-06 | Phillip Martin Cowley | Pharmaceutical compound |
| WO2022255890A1 (en) | 2021-06-01 | 2022-12-08 | Captor Therapeutics S.A. | Compounds which bind to cereblon, and use thereof |
| WO2022255889A1 (en) | 2021-06-01 | 2022-12-08 | Captor Therapeutics S.A. | Compounds which bind to cereblon, and use thereof |
| CA3215410A1 (en) | 2021-06-03 | 2022-12-08 | Novartis Ag | 3-(1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives and medical uses thereof |
| CN115504963A (zh) * | 2021-06-22 | 2022-12-23 | 苏州开拓药业股份有限公司 | 一种c-Myc蛋白降解剂 |
| WO2023025136A1 (zh) * | 2021-08-27 | 2023-03-02 | 杭州格博生物医药有限公司 | 异吲哚啉酮化合物及其用途 |
| EP4422635A4 (en) | 2021-10-29 | 2025-11-26 | Kymera Therapeutics Inc | IRAQ4 DEGRADATION AGENTS AND THEIR SYNTHESIS |
| CN118510766A (zh) | 2022-01-19 | 2024-08-16 | 江苏恒瑞医药股份有限公司 | 一种含硫异吲哚啉类衍生物的晶型 |
| CA3243560A1 (en) | 2022-01-31 | 2023-08-03 | Kymera Therapeutics, Inc. | Iraqi Degradation Agents and Their Uses |
| WO2023220655A1 (en) | 2022-05-11 | 2023-11-16 | Celgene Corporation | Methods to overcome drug resistance by re-sensitizing cancer cells to treatment with a prior therapy via treatment with a t cell therapy |
| EP4522183A2 (en) | 2022-05-11 | 2025-03-19 | Celgene Corporation | Methods and uses related to t cell therapy and production of same |
| CN115160211B (zh) * | 2022-06-23 | 2023-11-03 | 温州大学 | 一种异吲哚啉酮类化合物的绿色合成方法 |
| CN117285524A (zh) * | 2022-06-24 | 2023-12-26 | 中国科学院上海药物研究所 | 一类取代的4-氨基异吲哚啉类化合物、其制备方法、药物组合物及应用 |
| WO2024064646A1 (en) | 2022-09-20 | 2024-03-28 | Celgene Corporation | Salts and solid forms of (s)- or racemic 3-(4-((4-(morpholinomethyl)benzyl)oxy)-1-oxoisoindolin-2-yl)piperidine-2,6-dione and methods of using the same |
| WO2024097905A1 (en) | 2022-11-02 | 2024-05-10 | Celgene Corporation | Methods of treatment with t cell therapy and immunomodulatory agent maintenance therapy |
| WO2024104823A1 (en) | 2022-11-16 | 2024-05-23 | Basf Se | New substituted tetrahydrobenzoxazepine |
| WO2024167423A1 (en) | 2023-02-07 | 2024-08-15 | Captor Therapeutics S.A. | Gspt1 degrader compounds |
| WO2025042742A1 (en) | 2023-08-18 | 2025-02-27 | Bristol-Myers Squibb Company | Compositions comprising antibodies that bind bcma and cd3 and methods of treatment |
| WO2025076472A1 (en) | 2023-10-06 | 2025-04-10 | Juno Therapeutics, Inc. | Combination therapies with a cell therapy expressing a gprc5d-targeting car and related methods and uses |
| TW202532072A (zh) | 2023-12-08 | 2025-08-16 | 美商西建公司 | 治療多發性骨髓瘤之療法 |
| WO2025195464A1 (zh) * | 2024-03-21 | 2025-09-25 | 上海惠康济民生物医药技术有限公司 | 取代的异吲哚啉类化合物、制备方法、药物组合物及应用 |
Family Cites Families (110)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3536809A (en) | 1969-02-17 | 1970-10-27 | Alza Corp | Medication method |
| US3598123A (en) | 1969-04-01 | 1971-08-10 | Alza Corp | Bandage for administering drugs |
| US3845770A (en) | 1972-06-05 | 1974-11-05 | Alza Corp | Osmatic dispensing device for releasing beneficial agent |
| US3916899A (en) | 1973-04-25 | 1975-11-04 | Alza Corp | Osmotic dispensing device with maximum and minimum sizes for the passageway |
| US4008719A (en) | 1976-02-02 | 1977-02-22 | Alza Corporation | Osmotic system having laminar arrangement for programming delivery of active agent |
| US4421865A (en) * | 1981-06-08 | 1983-12-20 | Standard Oil Company (Sohio) | Selective hydrogen-deuterium interchange using ion exchange resins |
| US5001116A (en) | 1982-12-20 | 1991-03-19 | The Children's Medical Center Corporation | Inhibition of angiogenesis |
| US4994443A (en) | 1982-12-20 | 1991-02-19 | The Children's Medical Center Corporation | Inhibition of angiogenesis |
| KR890002631B1 (ko) | 1984-10-04 | 1989-07-21 | 몬산토 캄파니 | 생물학적으로 활성인 소마토트로핀을 지속적으로 유리하는 조성물 |
| IE58110B1 (en) | 1984-10-30 | 1993-07-14 | Elan Corp Plc | Controlled release powder and process for its preparation |
| US5149820A (en) * | 1987-03-11 | 1992-09-22 | Norsk Hydro A.S. | Deuterated compounds |
| US5073543A (en) | 1988-07-21 | 1991-12-17 | G. D. Searle & Co. | Controlled release formulations of trophic factors in ganglioside-lipsome vehicle |
| IT1229203B (it) | 1989-03-22 | 1991-07-25 | Bioresearch Spa | Impiego di acido 5 metiltetraidrofolico, di acido 5 formiltetraidrofolico e dei loro sali farmaceuticamente accettabili per la preparazione di composizioni farmaceutiche in forma a rilascio controllato attive nella terapia dei disturbi mentali organici e composizioni farmaceutiche relative. |
| PH30995A (en) | 1989-07-07 | 1997-12-23 | Novartis Inc | Sustained release formulations of water soluble peptides. |
| US5120548A (en) | 1989-11-07 | 1992-06-09 | Merck & Co., Inc. | Swelling modulated polymeric drug delivery device |
| KR0166088B1 (ko) | 1990-01-23 | 1999-01-15 | . | 수용해도가 증가된 시클로덱스트린 유도체 및 이의 용도 |
| US5733566A (en) | 1990-05-15 | 1998-03-31 | Alkermes Controlled Therapeutics Inc. Ii | Controlled release of antiparasitic agents in animals |
| WO1992014455A1 (en) * | 1991-02-14 | 1992-09-03 | The Rockefeller University | METHOD FOR CONTROLLING ABNORMAL CONCENTRATION TNF α IN HUMAN TISSUES |
| US5580578A (en) | 1992-01-27 | 1996-12-03 | Euro-Celtique, S.A. | Controlled release formulations coated with aqueous dispersions of acrylic polymers |
| EP0664128A4 (en) * | 1992-10-07 | 1997-12-17 | Sumitomo Pharma | PHARMACEUTICAL COMPOSITION USED TO INHIBIT THE PRODUCTION OF TUMOR NECROSIS FACTORS. |
| TW333456B (en) | 1992-12-07 | 1998-06-11 | Takeda Pharm Ind Co Ltd | A pharmaceutical composition of sustained-release preparation the invention relates to a pharmaceutical composition of sustained-release preparation which comprises a physiologically active peptide. |
| US5591767A (en) | 1993-01-25 | 1997-01-07 | Pharmetrix Corporation | Liquid reservoir transdermal patch for the administration of ketorolac |
| US6087324A (en) | 1993-06-24 | 2000-07-11 | Takeda Chemical Industries, Ltd. | Sustained-release preparation |
| WO1995003009A1 (en) | 1993-07-22 | 1995-02-02 | Oculex Pharmaceuticals, Inc. | Method of treatment of macular degeneration |
| US5770589A (en) | 1993-07-27 | 1998-06-23 | The University Of Sydney | Treatment of macular degeneration |
| IT1270594B (it) | 1994-07-07 | 1997-05-07 | Recordati Chem Pharm | Composizione farmaceutica a rilascio controllato di moguisteina in sospensione liquida |
| IT1274549B (it) | 1995-05-23 | 1997-07-17 | Indena Spa | Uso di flavanolignani per la preparazione di medicamenti ad attivita' antiproliferativa nei tumori dell'utero,dell'ovaio e del seno |
| CA2224381A1 (en) | 1995-06-27 | 1997-01-16 | Takeda Chemical Industries, Ltd. | Method of producing sustained-release preparation |
| TW448055B (en) | 1995-09-04 | 2001-08-01 | Takeda Chemical Industries Ltd | Method of production of sustained-release preparation |
| JP2909418B2 (ja) | 1995-09-18 | 1999-06-23 | 株式会社資生堂 | 薬物の遅延放出型マイクロスフイア |
| US5980945A (en) | 1996-01-16 | 1999-11-09 | Societe De Conseils De Recherches Et D'applications Scientifique S.A. | Sustained release drug formulations |
| US5800819A (en) | 1996-01-25 | 1998-09-01 | National Institute For Pharmaceutical Research And Development Federal Ministry Of Science And Technology | Piper guineense, pterocarpus osun, eugenia caryophyllata, and sorghum bicolor extracts for treating sickle cell disease |
| US6264970B1 (en) | 1996-06-26 | 2001-07-24 | Takeda Chemical Industries, Ltd. | Sustained-release preparation |
| US5635517B1 (en) | 1996-07-24 | 1999-06-29 | Celgene Corp | Method of reducing TNFalpha levels with amino substituted 2-(2,6-dioxopiperidin-3-YL)-1-oxo-and 1,3-dioxoisoindolines |
| DE69717831T3 (de) | 1996-07-24 | 2018-08-30 | Celgene Corp. | Substituierte 2-(2,6-dioxopiperidin-3-yl)-phthalimide und -1-oxoisoindoline und verfahren zur reduzierung des tnf-alpha-spiegels |
| HU228769B1 (en) | 1996-07-24 | 2013-05-28 | Celgene Corp | Substituted 2(2,6-dioxopiperidin-3-yl)phthalimides and -1-oxoisoindolines and their use for production of pharmaceutical compositions for mammals to reduce the level of tnf-alpha |
| US6281230B1 (en) | 1996-07-24 | 2001-08-28 | Celgene Corporation | Isoindolines, method of use, and pharmaceutical compositions |
| US6419961B1 (en) | 1996-08-29 | 2002-07-16 | Takeda Chemical Industries, Ltd. | Sustained release microcapsules of a bioactive substance and a biodegradable polymer |
| CA2217134A1 (en) | 1996-10-09 | 1998-04-09 | Sumitomo Pharmaceuticals Co., Ltd. | Sustained release formulation |
| CA2219698C (en) | 1996-10-31 | 2007-09-04 | Takeda Chemical Industries, Ltd. | Sustained-release preparation |
| KR20000057693A (ko) | 1996-12-20 | 2000-09-25 | 다케다 야쿠힌 고교 가부시키가이샤 | 지효성 제제의 제조 방법 |
| US5891474A (en) | 1997-01-29 | 1999-04-06 | Poli Industria Chimica, S.P.A. | Time-specific controlled release dosage formulations and method of preparing same |
| CZ299253B6 (cs) * | 1998-03-16 | 2008-05-28 | Celgene Corporation | Isoindolinový derivát, jeho použití pro výrobu léciva a farmaceutická kompozice tento derivát obsahující |
| US6613358B2 (en) | 1998-03-18 | 2003-09-02 | Theodore W. Randolph | Sustained-release composition including amorphous polymer |
| US6015803A (en) | 1998-05-04 | 2000-01-18 | Wirostko; Emil | Antibiotic treatment of age-related macular degeneration |
| KR19990085365A (ko) | 1998-05-16 | 1999-12-06 | 허영섭 | 지속적으로 약물 조절방출이 가능한 생분해성 고분자 미립구 및그 제조방법 |
| US6225348B1 (en) | 1998-08-20 | 2001-05-01 | Alfred W. Paulsen | Method of treating macular degeneration with a prostaglandin derivative |
| US6001368A (en) | 1998-09-03 | 1999-12-14 | Protein Technologies International, Inc. | Method for inhibiting or reducing the risk of macular degeneration |
| US6248363B1 (en) | 1999-11-23 | 2001-06-19 | Lipocine, Inc. | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions |
| US7182953B2 (en) | 1999-12-15 | 2007-02-27 | Celgene Corporation | Methods and compositions for the prevention and treatment of atherosclerosis restenosis and related disorders |
| DE60117486T2 (de) | 2000-08-23 | 2006-11-16 | General Electric Co. | Spritzgegossene Keramik-Metallhalogenidbogenröhre mit einem nicht-konischen Ende |
| US6458810B1 (en) * | 2000-11-14 | 2002-10-01 | George Muller | Pharmaceutically active isoindoline derivatives |
| US20030045552A1 (en) * | 2000-12-27 | 2003-03-06 | Robarge Michael J. | Isoindole-imide compounds, compositions, and uses thereof |
| AU2002343220A1 (en) | 2001-11-09 | 2003-05-19 | Matsushita Electric Industrial Co., Ltd. | Moving picture coding method and apparatus |
| US7968569B2 (en) | 2002-05-17 | 2011-06-28 | Celgene Corporation | Methods for treatment of multiple myeloma using 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione |
| US7393862B2 (en) | 2002-05-17 | 2008-07-01 | Celgene Corporation | Method using 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione for treatment of certain leukemias |
| US7323479B2 (en) | 2002-05-17 | 2008-01-29 | Celgene Corporation | Methods for treatment and management of brain cancer using 1-oxo-2-(2,6-dioxopiperidin-3-yl)-4-methylisoindoline |
| CA2672000C (en) | 2002-05-17 | 2011-11-29 | Celgene Corporation | Methods and compositions using 3-(4-amino-1-oxo-1,3-dihydroisoindol-2-yl)-piperidine-2,6-dione for treatment and management of lymphoma |
| US7189740B2 (en) | 2002-10-15 | 2007-03-13 | Celgene Corporation | Methods of using 3-(4-amino-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione for the treatment and management of myelodysplastic syndromes |
| US20050203142A1 (en) | 2002-10-24 | 2005-09-15 | Zeldis Jerome B. | Methods of using and compositions comprising immunomodulatory compounds for treatment, modification and management of pain |
| US20040091455A1 (en) | 2002-10-31 | 2004-05-13 | Zeldis Jerome B. | Methods of using and compositions comprising immunomodulatory compounds for treatment and management of macular degeneration |
| US7563810B2 (en) | 2002-11-06 | 2009-07-21 | Celgene Corporation | Methods of using 3-(4-amino-1-oxo-1,3-dihydroisoindol-2-yl)-piperidine-2,6-dione for the treatment and management of myeloproliferative diseases |
| UA83504C2 (en) | 2003-09-04 | 2008-07-25 | Селджин Корпорейшн | Polymorphic forms of 3-(4-amino-1-oxo-1,3 dihydro-isoindol-2-yl)-piperidine-2,6-dione |
| SG133603A1 (en) | 2003-09-17 | 2007-07-30 | Us Gov Health & Human Serv | Thalidomide analogs as tnf-alpha modulators |
| US20050100529A1 (en) | 2003-11-06 | 2005-05-12 | Zeldis Jerome B. | Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of asbestos-related diseases and disorders |
| KR20120039065A (ko) | 2003-12-02 | 2012-04-24 | 셀진 코포레이션 | 혈색소병증 및 빈혈의 치료 및 관리를 위한 방법및 조성물 |
| US20050143344A1 (en) | 2003-12-30 | 2005-06-30 | Zeldis Jerome B. | Methods and compositions using immunomodulatory compounds for the treatment and management of central nervous system disorders or diseases |
| BRPI0509019A (pt) | 2004-03-22 | 2007-08-07 | Celgene Corp | métodos para tratar, prevenir ou controlar um distúrbio ou doença de pele, para tratar, prevenir ou controlar ceratose senil e para tratar ou controlar ceratose, composição farmacêutica, forma de dosagem unitária individual, e, kit |
| US20050222209A1 (en) | 2004-04-01 | 2005-10-06 | Zeldis Jerome B | Methods and compositions for the treatment, prevention or management of dysfunctional sleep and dysfunctional sleep associated with disease |
| MXPA06012278A (es) | 2004-04-23 | 2007-01-31 | Celgene Corp | Metodos de uso y composiciones que comprenden compuestos inmunomoduladores para el tratamiento y manejo de la hipertension pulmonar. |
| CA2565447A1 (en) | 2004-05-05 | 2005-12-01 | Celgene Corporation | Method of using and compositions comprising immunomodulatory compounds for the treatment and management of myeloproliferative diseases |
| EP1778676B1 (en) * | 2004-07-16 | 2010-08-25 | Schering Corporation | Hydantoin derivatives for the treatment of inflammatory disorders |
| US7244759B2 (en) * | 2004-07-28 | 2007-07-17 | Celgene Corporation | Isoindoline compounds and methods of making and using the same |
| US7405237B2 (en) | 2004-07-28 | 2008-07-29 | Celgene Corporation | Isoindoline compounds and methods of their use |
| ES2438725T3 (es) * | 2004-09-03 | 2014-01-20 | Celgene Corporation | Procedimientos para la preparación de 2-(2,6-dioxopiperidin-3-il)-1-oxoisoindolinas sustituidas |
| CN101098694A (zh) | 2004-11-12 | 2008-01-02 | 细胞基因公司 | 使用免疫调节化合物治疗和控制寄生性疾病的方法和组合物 |
| BRPI0518282A2 (pt) | 2004-11-23 | 2008-11-11 | Celgene Corp | uso de uma quantidade terapeuticamente ou profilaticamente efetiva de um composto imunomodulatàrio |
| WO2006060507A2 (en) | 2004-12-01 | 2006-06-08 | Celgene Corporation | Compositions comprising immunomodulatory compounds and the use thereof for the treatment of immunodeficiency disorders |
| CA2612612C (en) | 2005-06-30 | 2014-03-11 | Celgene Corporation | Processes for the preparation of 4-amino-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione compounds |
| MX2008002765A (es) * | 2005-08-31 | 2008-04-07 | Celgene Corp | Compuestos de isoindol-imida y composiciones que la comprenden y metodos para usar los mismos. |
| TWI398252B (zh) | 2006-05-26 | 2013-06-11 | Novartis Ag | 吡咯并嘧啶化合物及其用途 |
| RS51725B (sr) | 2006-09-15 | 2011-10-31 | Celgene Corporation | Jedinjenja n-metilaminometil izoindola i kompozicije koje ih sadrže i upotreba istih |
| CA2665778A1 (en) | 2006-10-19 | 2008-05-15 | Celgene Corporation | Methods and compositions using immunomodulatory compounds for the treatment and management of spirochete and other obligate intracellular bacterial diseases |
| CN103641814A (zh) * | 2007-03-20 | 2014-03-19 | 细胞基因公司 | 4’-o-取代的异吲哚啉衍生物和包含它的组合物及使用方法 |
| RS56232B1 (sr) | 2010-02-11 | 2017-11-30 | Celgene Corp | Derivati arilmetoksi izoindolina i kompozicije koje ih obuhvataju i postupci njihove primene |
| WO2012027065A2 (en) | 2010-08-27 | 2012-03-01 | Celgene Corporation | Combination therapy for treatment of disease |
| EP2640189A4 (en) | 2010-11-18 | 2014-03-19 | Deuteria Pharmaceuticals Inc | 3-deutero-pomalidomide |
| WO2012079022A1 (en) | 2010-12-10 | 2012-06-14 | Concert Pharmaceuticals, Inc. | Substituted dioxopiperidinyl phthalimide derivatives |
| US9090585B2 (en) | 2011-03-28 | 2015-07-28 | Deuterx, Llc | 2,6-dioxo-3-deutero-piperdin-3-yl-isoindoline compounds |
| MX358517B (es) | 2012-06-29 | 2018-08-24 | Celgene Corp | Métodos para determinar eficacia de fármacos usando proteínas asociadas a cereblon. |
| EA029485B1 (ru) | 2012-08-09 | 2018-04-30 | Селджин Корпорейшн | Способы лечения рака, выбранного из лимфомы, лейкоза, миеломы, глиобластомы, рака ободочной и прямой кишки и печеночно-клеточной карциномы, с использованием 3-(4-((4-(морфолинометил)бензил)окси)-1-оксоизоиндолин-2-ил)пиперидин-2,6-диона |
| EP3741372A1 (en) | 2012-08-09 | 2020-11-25 | Celgene Corporation | (s)-3-[4-(4-morphlin-4-ylmethylbenzyloxy)-1- oxo-1,3-dihydro-isoindo-2-yl]piperidine-2,6-dione for use in the treatment of immune-related and inflammatory diseases |
| US20140343058A1 (en) | 2012-08-09 | 2014-11-20 | Celgene Corporation | Treatment of systemic lupus erythematosus |
| BR112015002183A2 (pt) | 2012-08-09 | 2017-07-04 | Celgene Corp | processos para a preparação de (s) -3- (4 - ((4- (morfolinometil) benzil) oxi) -1-oxoisoindolin-2-il) -piperidina-2,6-diona e formas farmaceuticamente aceitáveis da mesma |
| US20150038511A1 (en) | 2012-08-09 | 2015-02-05 | Celgene Corporation | Treatment of immune-related and inflammatory diseases |
| ES2885769T3 (es) | 2012-08-09 | 2021-12-15 | Celgene Corp | Una forma sólida de clorhidrato de (s)-3-(4-((4-morpholinometil)bencil)oxi)-1-oxoisoindolin-2-il)piperidina-2,6-diona |
| US9587281B2 (en) | 2012-08-14 | 2017-03-07 | Celgene Corporation | Cereblon isoforms and their use as biomarkers for therapeutic treatment |
| WO2014039960A1 (en) | 2012-09-10 | 2014-03-13 | Celgene Corporation | Methods for the treatment of locally advanced breast cancer |
| WO2014116573A1 (en) | 2013-01-22 | 2014-07-31 | Celgene Corporation | Processes for the preparation of isotopologues of 3-(4-((4-(morpholinomethyl)benzyl)oxy)-1-oxoisoindolin-2-yl)piperidine-2,6-dione and pharmaceutically acceptable salts thereof |
| WO2014151180A1 (en) | 2013-03-14 | 2014-09-25 | Celgene Corporation | Treatment of psoriatic arthritis using apremilast |
| WO2014172429A1 (en) | 2013-04-17 | 2014-10-23 | Signal Pharmaceuticals, Llc | Combination therapy comprising a tor kinase inhibitor and an imid compound for treating cancer |
| UA117141C2 (uk) | 2013-10-08 | 2018-06-25 | Селджин Корпорейшн | Склади (s)-3-(4-((4-(морфолінометил)бензил)оксі)-1-оксоізоіндолін-2-іл)піперидин-2,6-діону |
| MX386337B (es) | 2013-12-06 | 2025-03-18 | Celgene Corp | Métodos para determinar la eficacia del fármaco para el tratamiento de linfoma difuso de células b grandes, mieloma múltiple, y cánceres mieloides. |
| US9415049B2 (en) | 2013-12-20 | 2016-08-16 | Celgene Avilomics Research, Inc. | Heteroaryl compounds and uses thereof |
| ES2969532T3 (es) | 2014-05-19 | 2024-05-21 | Celgene Corp | 3-(4-((4-(Morfolinometil-bencil)oxi)-1-oxoisoindolin-2-il)piperidin-2,6-diona para el tratamiento de lupus eritematoso sistémico |
| JP6640126B2 (ja) | 2014-06-27 | 2020-02-05 | セルジーン コーポレイション | セレブロン及び他のe3ユビキチンリガーゼの立体構造の変化を誘導するための組成物及び方法 |
| EP3188745A1 (en) | 2014-08-15 | 2017-07-12 | Celgene Corporation | Dosage titration of apremilast for the treatment of diseases ameliorated by pde4 inhibition |
| US20170242014A1 (en) | 2014-10-13 | 2017-08-24 | Celgene Corporaton | Methods for treating solid tumors and the use of biomarkers as a predictor of clinical sensitivity to immunomodulatory therapies |
| ES2812877T3 (es) | 2014-10-30 | 2021-03-18 | Kangpu Biopharmaceuticals Ltd | Derivado de isoindolina, producto intermedio, método de preparación, composición farmacéutica y uso del mismo |
| US9717745B2 (en) | 2015-03-19 | 2017-08-01 | Zhejiang DTRM Biopharma Co. Ltd. | Pharmaceutical compositions and their use for treatment of cancer and autoimmune diseases |
-
2011
- 2011-02-10 RS RS20170700A patent/RS56232B1/sr unknown
- 2011-02-10 CN CN201180018485.1A patent/CN102822165B/zh active Active
- 2011-02-10 PT PT17157170T patent/PT3202460T/pt unknown
- 2011-02-10 UA UAA201210643A patent/UA115220C2/uk unknown
- 2011-02-10 NZ NZ601289A patent/NZ601289A/en unknown
- 2011-02-10 SG SG10202012179RA patent/SG10202012179RA/en unknown
- 2011-02-10 ES ES17157178T patent/ES2713482T3/es active Active
- 2011-02-10 SM SM20190420T patent/SMT201900420T1/it unknown
- 2011-02-10 DK DK17157178.9T patent/DK3202461T3/en active
- 2011-02-10 PL PL17157170T patent/PL3202460T3/pl unknown
- 2011-02-10 DK DK11704156.6T patent/DK2536706T3/en active
- 2011-02-10 MX MX2013011071A patent/MX337169B/es unknown
- 2011-02-10 HR HRP20171078TT patent/HRP20171078T1/hr unknown
- 2011-02-10 EP EP23192393.9A patent/EP4289838A3/en active Pending
- 2011-02-10 NZ NZ700054A patent/NZ700054A/en unknown
- 2011-02-10 JP JP2012552978A patent/JP2013519675A/ja not_active Withdrawn
- 2011-02-10 LT LTEP11704156.6T patent/LT2536706T/lt unknown
- 2011-02-10 PH PH1/2012/501607A patent/PH12012501607A1/en unknown
- 2011-02-10 ES ES11704156.6T patent/ES2638517T3/es active Active
- 2011-02-10 LT LTEP17157178.9T patent/LT3202461T/lt unknown
- 2011-02-10 EP EP17157170.6A patent/EP3202460B1/en active Active
- 2011-02-10 CN CN201510039358.XA patent/CN104693193B/zh active Active
- 2011-02-10 ES ES16166423T patent/ES2730763T3/es active Active
- 2011-02-10 KR KR1020177036343A patent/KR101931468B1/ko active Active
- 2011-02-10 SM SM20170360T patent/SMT201700360T1/it unknown
- 2011-02-10 SG SG10201501062SA patent/SG10201501062SA/en unknown
- 2011-02-10 EP EP11704156.6A patent/EP2536706B1/en active Active
- 2011-02-10 RU RU2012138709/04A patent/RU2567753C2/ru active
- 2011-02-10 SI SI201131754T patent/SI3202460T1/sl unknown
- 2011-02-10 PL PL11704156T patent/PL2536706T3/pl unknown
- 2011-02-10 EP EP17157178.9A patent/EP3202461B1/en active Active
- 2011-02-10 HU HUE17157178A patent/HUE042011T2/hu unknown
- 2011-02-10 NZ NZ717149A patent/NZ717149A/en unknown
- 2011-02-10 PT PT17157178T patent/PT3202461T/pt unknown
- 2011-02-10 MX MX2016001880A patent/MX367522B/es unknown
- 2011-02-10 RS RSP20190965 patent/RS59275B1/sr unknown
- 2011-02-10 HU HUE17157170 patent/HUE044652T2/hu unknown
- 2011-02-10 DK DK17157170.6T patent/DK3202460T3/da active
- 2011-02-10 ME MEP-2017-136A patent/ME02766B/me unknown
- 2011-02-10 KR KR1020127023615A patent/KR101812356B1/ko active Active
- 2011-02-10 TR TR2019/03027T patent/TR201903027T4/tr unknown
- 2011-02-10 EP EP19178064.2A patent/EP3599236B1/en active Active
- 2011-02-10 ES ES19178064T patent/ES2956743T3/es active Active
- 2011-02-10 SM SM20190155T patent/SMT201900155T1/it unknown
- 2011-02-10 AU AU2011215877A patent/AU2011215877C1/en active Active
- 2011-02-10 UA UAA201601384A patent/UA114856C2/uk unknown
- 2011-02-10 SG SG2012059259A patent/SG183257A1/en unknown
- 2011-02-10 SI SI201131680T patent/SI3202461T1/sl unknown
- 2011-02-10 HU HUE11704156A patent/HUE033009T2/en unknown
- 2011-02-10 US US13/025,105 patent/US8518972B2/en active Active
- 2011-02-10 WO PCT/US2011/024269 patent/WO2011100380A1/en not_active Ceased
- 2011-02-10 SI SI201131241T patent/SI2536706T1/sl unknown
- 2011-02-10 PL PL17157178T patent/PL3202461T3/pl unknown
- 2011-02-10 LT LTEP17157170.6T patent/LT3202460T/lt unknown
- 2011-02-10 MX MX2012009237A patent/MX2012009237A/es active IP Right Grant
- 2011-02-10 PT PT117041566T patent/PT2536706T/pt unknown
- 2011-02-10 ES ES17157170T patent/ES2738776T3/es active Active
- 2011-02-10 CA CA2787823A patent/CA2787823C/en active Active
- 2011-02-10 EP EP16166423.0A patent/EP3106460B1/en active Active
- 2011-02-10 ME MEP-2019-195A patent/ME03441B/me unknown
- 2011-02-10 RS RS20190249A patent/RS58523B1/sr unknown
- 2011-02-11 AR ARP110100436A patent/AR081058A1/es active IP Right Grant
-
2012
- 2012-07-17 IL IL220992A patent/IL220992A/en active IP Right Grant
- 2012-08-09 NI NI201200132A patent/NI201200132A/es unknown
- 2012-08-09 EC ECSP12012098 patent/ECSP12012098A/es unknown
- 2012-08-09 CR CR20120414A patent/CR20120414A/es unknown
- 2012-08-10 CO CO12135141A patent/CO6571916A2/es active IP Right Grant
-
2013
- 2013-07-26 US US13/952,386 patent/US9309219B2/en active Active
-
2014
- 2014-05-14 PH PH12014501082A patent/PH12014501082B1/en unknown
-
2016
- 2016-01-14 JP JP2016004951A patent/JP6215976B2/ja active Active
- 2016-02-10 US US15/040,971 patent/US9828361B2/en active Active
- 2016-02-10 US US15/040,980 patent/US9822094B2/en active Active
- 2016-04-13 JP JP2016080097A patent/JP2016172746A/ja not_active Withdrawn
- 2016-09-02 JP JP2016171451A patent/JP6270944B2/ja active Active
- 2016-11-08 IL IL248843A patent/IL248843B/en active IP Right Grant
-
2017
- 2017-06-27 JP JP2017125219A patent/JP2017200944A/ja active Pending
- 2017-07-18 CY CY20171100759T patent/CY1119177T1/el unknown
- 2017-10-17 US US15/786,334 patent/US10189814B2/en active Active
-
2018
- 2018-11-19 US US16/195,550 patent/US10669257B2/en active Active
-
2019
- 2019-02-22 CY CY20191100227T patent/CY1121729T1/el unknown
- 2019-02-26 HR HRP20190368TT patent/HRP20190368T1/hr unknown
- 2019-07-19 HR HRP20191312TT patent/HRP20191312T1/hr unknown
- 2019-08-02 CY CY20191100823T patent/CY1121858T1/el unknown
-
2020
- 2020-03-31 US US16/836,832 patent/US11414399B2/en active Active
-
2022
- 2022-07-18 US US17/867,294 patent/US20220411402A1/en active Pending
Cited By (53)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2738833C2 (ru) * | 2014-04-14 | 2020-12-17 | Арвинас, Оперэйшнз, Инк. | Имидные модуляторы протеолиза и способы их применения |
| US12239711B2 (en) | 2014-04-14 | 2025-03-04 | Arvinas Operations, Inc. | Cereblon ligands and bifunctional compounds comprising the same |
| RU2738833C9 (ru) * | 2014-04-14 | 2022-02-28 | Арвинас, Оперэйшнз, Инк. | Имидные модуляторы протеолиза и способы их применения |
| RU2695521C9 (ru) * | 2014-10-30 | 2019-08-19 | Канпу Биофармасьютикалс, Лтд. | Производное изоиндолина, промежуточный продукт, способ получения, фармацевтическая композиция и ее применение |
| RU2695521C2 (ru) * | 2014-10-30 | 2019-07-23 | Канпу Биофармасьютикалс, Лтд. | Производное изоиндолина, промежуточный продукт, способ получения, фармацевтическая композиция и ее применение |
| US11427548B2 (en) | 2015-01-20 | 2022-08-30 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of androgen receptor |
| US12312316B2 (en) | 2015-01-20 | 2025-05-27 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of androgen receptor |
| US11352351B2 (en) | 2015-01-20 | 2022-06-07 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of androgen receptor |
| US10946017B2 (en) | 2015-06-05 | 2021-03-16 | Arvinas Operations, Inc. | Tank-binding kinase-1 PROTACs and associated methods of use |
| US10772962B2 (en) | 2015-08-19 | 2020-09-15 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of bromodomain-containing proteins |
| US11554171B2 (en) | 2015-08-19 | 2023-01-17 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of bromodomain-containing proteins |
| US12171831B2 (en) | 2015-08-19 | 2024-12-24 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of bromodomain- containing proteins |
| US10844021B2 (en) | 2016-10-11 | 2020-11-24 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of androgen receptor |
| US12077509B2 (en) | 2016-10-11 | 2024-09-03 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of androgen receptor |
| US11964945B2 (en) | 2016-10-11 | 2024-04-23 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of androgen receptor |
| US11952347B2 (en) | 2016-10-11 | 2024-04-09 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of androgen receptor |
| US11236051B2 (en) | 2016-10-11 | 2022-02-01 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of androgen receptor |
| US10584101B2 (en) | 2016-10-11 | 2020-03-10 | Arvinas, Inc. | Compounds and methods for the targeted degradation of androgen receptor |
| US11458123B2 (en) | 2016-11-01 | 2022-10-04 | Arvinas Operations, Inc. | Tau-protein targeting PROTACs and associated methods of use |
| US12172981B2 (en) | 2016-12-01 | 2024-12-24 | Arvinas Operations, Inc. | Tetrahydronaphthalene and tetrahydroisoquinoline derivatives as estrogen receptor degraders |
| US11104666B2 (en) | 2016-12-01 | 2021-08-31 | Arvinas Operations, Inc. | Tetrahydronaphthalene and tetrahydroisoquinoline derivatives as estrogen receptor degraders |
| US10899742B1 (en) | 2016-12-01 | 2021-01-26 | Arvinas Operations, Inc. | Tetrahydronaphthalene and tetrahydroisoquinoline derivatives as estrogen receptor degraders |
| US10647698B2 (en) | 2016-12-01 | 2020-05-12 | Arvinas Operations, Inc. | Tetrahydronaphthalene and tetrahydroisoquinoline derivatives as estrogen receptor degraders |
| US11597720B2 (en) | 2016-12-01 | 2023-03-07 | Arvinas Operations, Inc. | Tetrahydronaphthalene and tetrahydroisoquinoline derivatives as estrogen receptor degraders |
| US10994015B2 (en) | 2016-12-23 | 2021-05-04 | Arvinas Operations, Inc. | EGFR proteolysis targeting chimeric molecules and associated methods of use |
| US10723717B2 (en) | 2016-12-23 | 2020-07-28 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of rapidly accelerated fibrosarcoma polypeptides |
| US10806737B2 (en) | 2016-12-23 | 2020-10-20 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of fetal liver kinase polypeptides |
| US11986531B2 (en) | 2016-12-23 | 2024-05-21 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of rapidly accelerated fibrosarcoma polypeptides |
| US11173211B2 (en) | 2016-12-23 | 2021-11-16 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of rapidly accelerated Fibrosarcoma polypeptides |
| US11191741B2 (en) | 2016-12-24 | 2021-12-07 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of enhancer of zeste homolog 2 polypeptide |
| US11857519B2 (en) | 2016-12-24 | 2024-01-02 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of enhancer of zeste homolog 2 polypeptide |
| US12275716B2 (en) | 2017-01-26 | 2025-04-15 | Arvinas Operations, Inc. | Modulators of estrogen receptor proteolysis and associated methods of use |
| US10604506B2 (en) | 2017-01-26 | 2020-03-31 | Arvinas Operations, Inc. | Modulators of estrogen receptor proteolysis and associated methods of use |
| US11384063B2 (en) | 2017-01-26 | 2022-07-12 | Arvinas Operations, Inc. | Modulators of estrogen receptor proteolysis and associated methods of use |
| US12441708B2 (en) | 2017-01-31 | 2025-10-14 | Arvinas Operations, Inc. | Cereblon ligands and bifunctional compounds comprising the same |
| US11065231B2 (en) | 2017-11-17 | 2021-07-20 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of interleukin-1 receptor- associated kinase 4 polypeptides |
| US12036209B2 (en) | 2017-11-17 | 2024-07-16 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of Interleukin-1 receptor-associated kinase 4 polypeptides |
| RU2781643C2 (ru) * | 2018-01-25 | 2022-10-17 | Фудзимото Ко., Лтд. | Производное тиофена и его применение |
| US11161841B2 (en) | 2018-04-04 | 2021-11-02 | Arvinas Operations, Inc. | Modulators of proteolysis and associated methods of use |
| US11707452B2 (en) | 2018-08-20 | 2023-07-25 | Arvinas Operations, Inc. | Modulators of alpha-synuclein proteolysis and associated methods of use |
| US11912699B2 (en) | 2019-07-17 | 2024-02-27 | Arvinas Operations, Inc. | Tau-protein targeting compounds and associated |
| US12208095B2 (en) | 2019-08-26 | 2025-01-28 | Arvinas Operations, Inc. | Methods of treating breast cancer with tetrahydronaphthalene derivatives as estrogen receptor degraders |
| US12310975B2 (en) | 2019-10-17 | 2025-05-27 | Arvinas Operations, Inc. | Modulators of BCL6 proteolysis and associated methods of use |
| US12427144B2 (en) | 2019-12-19 | 2025-09-30 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of androgen receptor |
| US11883393B2 (en) | 2019-12-19 | 2024-01-30 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of androgen receptor |
| US12043612B2 (en) | 2020-05-09 | 2024-07-23 | Arvinas Operations, Inc. | Methods of manufacturing a bifunctional compound, ultrapure forms of the bifunctional compound, and dosage forms comprising the same |
| US12180193B2 (en) | 2020-08-28 | 2024-12-31 | Arvinas Operations, Inc. | Accelerating fibrosarcoma protein degrading compounds and associated methods of use |
| US12162859B2 (en) | 2020-09-14 | 2024-12-10 | Arvinas Operations, Inc. | Crystalline and amorphous forms of a compound for the targeted degradation of estrogen receptor |
| US11986532B2 (en) | 2021-04-16 | 2024-05-21 | Arvinas Operations, Inc. | Modulators of BCL6 proteolysis and associated methods of use |
| US11957759B1 (en) | 2022-09-07 | 2024-04-16 | Arvinas Operations, Inc. | Rapidly accelerated fibrosarcoma (RAF) degrading compounds and associated methods of use |
| US12156916B2 (en) | 2022-09-07 | 2024-12-03 | Arvinas Operations, Inc. | Rapid accelerated fibrosarcoma (RAF) degrading compounds and associated methods of use |
| US12448399B2 (en) | 2023-01-26 | 2025-10-21 | Arvinas Operations, Inc. | Cereblon-based KRAS degrading PROTACs and uses related thereto |
| US12496301B2 (en) | 2023-12-08 | 2025-12-16 | Arvinas Operations, Inc. | Use of androgen receptor degrader for the treatment of spinal and bulbar muscular atrophy |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2012138709A (ru) | Производные арилметокси изоиндолина и композиции, включающие их, и способы их применения | |
| JP2013520443A5 (ru) | ||
| RU2016108987A (ru) | Замещенные бензоазепины в качестве модуляторов toll-подобного рецептора | |
| RU2010119645A (ru) | Замещенные гетероциклом пиперазинодигидротиенопиримидины | |
| RU2015120478A (ru) | Пери-карбинолы | |
| RU2016137181A (ru) | Противофиброзные пиридиноны | |
| JP2013507425A5 (ru) | ||
| RU2019138177A (ru) | Производные 2-аминохинолина | |
| RU2012121630A (ru) | АЛЛОСТЕРИЧЕСКИЕ СРЕДСТВА ПОТЕНЦИРОВАНИЯ mGluR4 КОМПОЗИЦИИ И СПОСОБЫ ЛЕЧЕНИЯ НЕВРОЛОГИЧЕСКИХ ДИСФУНКЦИЙ | |
| RU2016102036A (ru) | Производное хинолина | |
| JP2012529486A5 (ru) | ||
| AR093532A1 (es) | Compuestos y composiciones para el tratamiento de enfermedades parasitarias | |
| JP2013512903A5 (ru) | ||
| JPWO2016104617A1 (ja) | キノリン誘導体 | |
| JP2012524055A5 (ru) | ||
| KR20030046509A (ko) | 인지 장애의 치료를 위한 아세틸콜린에스테라제 저해제 및지에이비에이에이 역 작용물질의 조합 용도 | |
| RU2012112050A (ru) | Производные дигидроптеридинона, способ их получения и фармацевтическое применение | |
| JPWO2020081999A5 (ru) | ||
| RU2017115809A (ru) | Комбинированная терапия ингибиторами с-с хемокинового рецептора 9 (ccr9) и антителами, блокирующими альфа4бета7-интегрин | |
| RU2014144883A (ru) | Новые производные фенил-тетрагидроизохинолина | |
| RU2014120477A (ru) | Производные пиримидин-4-она и их применение в лечении, облегчении или профилактике вирусного заболевания | |
| JP2012522766A5 (ru) | ||
| RU2018142988A (ru) | Производные пиридиндикарбоксамида в качестве ингибиторов бромодомена | |
| JP2018529737A5 (ru) | ||
| JP2019528279A5 (ru) |