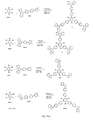
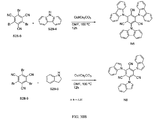
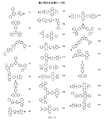
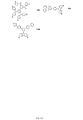
JP2017518281A - 有機発光ダイオード材料 - Google Patents
有機発光ダイオード材料 Download PDFInfo
- Publication number
- JP2017518281A JP2017518281A JP2016567427A JP2016567427A JP2017518281A JP 2017518281 A JP2017518281 A JP 2017518281A JP 2016567427 A JP2016567427 A JP 2016567427A JP 2016567427 A JP2016567427 A JP 2016567427A JP 2017518281 A JP2017518281 A JP 2017518281A
- Authority
- JP
- Japan
- Prior art keywords
- compound
- structural formulas
- exemplary embodiments
- independently
- present
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
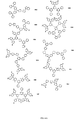
- 0 *[n]1c(ccc(-c(cc2)ccc2-[n]2c3ccccc3c3c2cccc3)c2)c2c2cc(-c(cc3)ccc3N[C@](C=CC=C3)C3=C(C=CC=C3)C3=C)ccc12 Chemical compound *[n]1c(ccc(-c(cc2)ccc2-[n]2c3ccccc3c3c2cccc3)c2)c2c2cc(-c(cc3)ccc3N[C@](C=CC=C3)C3=C(C=CC=C3)C3=C)ccc12 0.000 description 28
- VKFWHTYHLBFBPG-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c(cc2)ccc2-[n]2c(ccc(-[n]3c4ccccc4c4c3cccc4)c3)c3c3ccccc23)c1 Chemical compound c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c(cc2)ccc2-[n]2c(ccc(-[n]3c4ccccc4c4c3cccc4)c3)c3c3ccccc23)c1 VKFWHTYHLBFBPG-UHFFFAOYSA-N 0.000 description 3
- CEWVZHDJGNPLJF-UHFFFAOYSA-N Cc(cc(cc1C)-c2nc(-c3ccccc3)nc(-c3ccccc3)n2)c1-[n]1c2ccccc2c2c1cccc2 Chemical compound Cc(cc(cc1C)-c2nc(-c3ccccc3)nc(-c3ccccc3)n2)c1-[n]1c2ccccc2c2c1cccc2 CEWVZHDJGNPLJF-UHFFFAOYSA-N 0.000 description 2
- FKZDHTRZEYOOGJ-UHFFFAOYSA-N C(C1)C=CC=C1C(N=C(C1C=CC=CC1)N1)=NC1N(C(C1C2)=Cc3c2c(cccc2)c2[n]3-c2ccccc2)c2c1cccc2 Chemical compound C(C1)C=CC=C1C(N=C(C1C=CC=CC1)N1)=NC1N(C(C1C2)=Cc3c2c(cccc2)c2[n]3-c2ccccc2)c2c1cccc2 FKZDHTRZEYOOGJ-UHFFFAOYSA-N 0.000 description 1
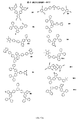
- IGVUPWLKRJUAIN-UHFFFAOYSA-N C(C1C(C=C2)=Nc(cc3)c1cc3-c(cc1)ccc1N(c1ccccc1)c1ccccc1)=C2c(cc1)ccc1N(c1ccccc1)c1ccccc1 Chemical compound C(C1C(C=C2)=Nc(cc3)c1cc3-c(cc1)ccc1N(c1ccccc1)c1ccccc1)=C2c(cc1)ccc1N(c1ccccc1)c1ccccc1 IGVUPWLKRJUAIN-UHFFFAOYSA-N 0.000 description 1
- PXGPVOPPZMPDRL-ASXRURRBSA-N C(C1C=C(C(C2)C(N3C(CC4)=CC=C4c4cc(-[n]5c(C6=CC=CCC6C6=CCCC=C66)c6nc5)ccc4)=CC=C2/C(/c2ccccc2)=C/C2C=CC=CC2)C3=CC1)/C(/c1ccccc1)=C/C1=CC=CCC1 Chemical compound C(C1C=C(C(C2)C(N3C(CC4)=CC=C4c4cc(-[n]5c(C6=CC=CCC6C6=CCCC=C66)c6nc5)ccc4)=CC=C2/C(/c2ccccc2)=C/C2C=CC=CC2)C3=CC1)/C(/c1ccccc1)=C/C1=CC=CCC1 PXGPVOPPZMPDRL-ASXRURRBSA-N 0.000 description 1
- JMUOOSYGFDBACA-UHFFFAOYSA-N C1C=CC(N(C2=CC=CCC2)C(C=C2C3=CC=CCC33)=CCC2N3C(C=C2)=CCC2C(C2)=CC(c3ccccc3)=CC2c2ccccc2)=CC1 Chemical compound C1C=CC(N(C2=CC=CCC2)C(C=C2C3=CC=CCC33)=CCC2N3C(C=C2)=CCC2C(C2)=CC(c3ccccc3)=CC2c2ccccc2)=CC1 JMUOOSYGFDBACA-UHFFFAOYSA-N 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N C1CCCCC1 Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
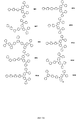
- ONDZTJMYLQROPY-UHFFFAOYSA-N CC(C1)CC(c(cc2)ccc2N2c3ccccc3C(C)(C3CC3)C3C=CC=CC23)=CC1Br Chemical compound CC(C1)CC(c(cc2)ccc2N2c3ccccc3C(C)(C3CC3)C3C=CC=CC23)=CC1Br ONDZTJMYLQROPY-UHFFFAOYSA-N 0.000 description 1
- GAWABHDWVJKRQM-UHFFFAOYSA-N CC(C1=CC=CCC1)(c1ccccc1)C1=CCC(C(Cc(cc2)ccc2-c(c(C2CC2)c(c(I)c2F)F)c2F)c(cc2)c3cc2N(C2=CC=CC4C2C4)c2ccccc2)C3=C1 Chemical compound CC(C1=CC=CCC1)(c1ccccc1)C1=CCC(C(Cc(cc2)ccc2-c(c(C2CC2)c(c(I)c2F)F)c2F)c(cc2)c3cc2N(C2=CC=CC4C2C4)c2ccccc2)C3=C1 GAWABHDWVJKRQM-UHFFFAOYSA-N 0.000 description 1
- ZBMPEYFYKBQRQI-UHFFFAOYSA-N CC(C1C=CC=CC11)(c2ccccc2N1c(cc1)ccc1-c1cc(-c(cc2)cc3c2[o]cn3)cc(-c(cc2)cc3c2[o]cn3)c1)N=C=C Chemical compound CC(C1C=CC=CC11)(c2ccccc2N1c(cc1)ccc1-c1cc(-c(cc2)cc3c2[o]cn3)cc(-c(cc2)cc3c2[o]cn3)c1)N=C=C ZBMPEYFYKBQRQI-UHFFFAOYSA-N 0.000 description 1
- AHDSYMVAUJZCOP-UHFFFAOYSA-N CC1(C)OB(c(cc2)ccc2-[n]2c(cccc3)c3c3ccccc23)OC1(C)C Chemical compound CC1(C)OB(c(cc2)ccc2-[n]2c(cccc3)c3c3ccccc23)OC1(C)C AHDSYMVAUJZCOP-UHFFFAOYSA-N 0.000 description 1
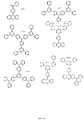
- MMTDHZWCHYIEFJ-UHFFFAOYSA-N CC1(C)c(cccc2)c2N(c(cc2)ccc2-c2ccc3[nH]c(ccc(-c(cc4)ccc4N(c4ccccc4C4(C)C)c5c4cccc5)c4)c4c3c2)c2c1cccc2 Chemical compound CC1(C)c(cccc2)c2N(c(cc2)ccc2-c2ccc3[nH]c(ccc(-c(cc4)ccc4N(c4ccccc4C4(C)C)c5c4cccc5)c4)c4c3c2)c2c1cccc2 MMTDHZWCHYIEFJ-UHFFFAOYSA-N 0.000 description 1
- JSEQNGYLWKBMJI-UHFFFAOYSA-N CC1(C)c2ccccc2Nc2c1cccc2 Chemical compound CC1(C)c2ccccc2Nc2c1cccc2 JSEQNGYLWKBMJI-UHFFFAOYSA-N 0.000 description 1
- FNPGJPKNKADBGK-UHFFFAOYSA-N Cc(cc1)cc(C)c1-c1ccccc1 Chemical compound Cc(cc1)cc(C)c1-c1ccccc1 FNPGJPKNKADBGK-UHFFFAOYSA-N 0.000 description 1
- SQNZJJAZBFDUTD-UHFFFAOYSA-N Cc1cc(C)c(C)cc1C Chemical compound Cc1cc(C)c(C)cc1C SQNZJJAZBFDUTD-UHFFFAOYSA-N 0.000 description 1
- AUHZEENZYGFFBQ-UHFFFAOYSA-N Cc1cc(C)cc(C)c1 Chemical compound Cc1cc(C)cc(C)c1 AUHZEENZYGFFBQ-UHFFFAOYSA-N 0.000 description 1
- FEJJYLIDLAQVCY-UHFFFAOYSA-N FC(CC(CC1F)C(CC2)=CCC2N(c(c(C2=C3)c4)ccc4N(c4ccccc4)c4ccccc4)C2=CCC3N(c2ccccc2)c2ccccc2)C1F Chemical compound FC(CC(CC1F)C(CC2)=CCC2N(c(c(C2=C3)c4)ccc4N(c4ccccc4)c4ccccc4)C2=CCC3N(c2ccccc2)c2ccccc2)C1F FEJJYLIDLAQVCY-UHFFFAOYSA-N 0.000 description 1
- PBDKQFUZWRMMBW-UHFFFAOYSA-N N#CC(C(C(CC1)=CC=C1C(CC1)=CC=C1[n](cc1)c2c1cccc2)C(C#N)=C1c2ccc(C3C=CC([n](cc4)c5c4cccc5)=CC3)cc2)C(C(CC2)=CC=C2C2C=CC(N(CC3)C4=C3C=CCC4)=CC2)=C1C#N Chemical compound N#CC(C(C(CC1)=CC=C1C(CC1)=CC=C1[n](cc1)c2c1cccc2)C(C#N)=C1c2ccc(C3C=CC([n](cc4)c5c4cccc5)=CC3)cc2)C(C(CC2)=CC=C2C2C=CC(N(CC3)C4=C3C=CCC4)=CC2)=C1C#N PBDKQFUZWRMMBW-UHFFFAOYSA-N 0.000 description 1
- GFKSXYJMPBUNCS-UHFFFAOYSA-N N#CC1=C(c(cc2)ccc2-c2c(C(CC=C3)C4=CCCC=C4)c3cnc2)C(C#N)=C2c(cc3)ccc3C3=CCCc(cc4)c3[n]4-c(cc3)ccc3[N]#CC2C1C(C1C=C2)C1C=C2c1c(CCC(C=C2)c3ccccc3)c2cnc1 Chemical compound N#CC1=C(c(cc2)ccc2-c2c(C(CC=C3)C4=CCCC=C4)c3cnc2)C(C#N)=C2c(cc3)ccc3C3=CCCc(cc4)c3[n]4-c(cc3)ccc3[N]#CC2C1C(C1C=C2)C1C=C2c1c(CCC(C=C2)c3ccccc3)c2cnc1 GFKSXYJMPBUNCS-UHFFFAOYSA-N 0.000 description 1
- BWLGBWXLFZJVJR-UHFFFAOYSA-N [BH+]c1ccc2[o]cnc2c1 Chemical compound [BH+]c1ccc2[o]cnc2c1 BWLGBWXLFZJVJR-UHFFFAOYSA-N 0.000 description 1
- SPPONIYAQHCYOB-UHFFFAOYSA-N c(cc1)ccc1N(c1ccccc1)c(cc1)cc(Oc2c3)c1Nc2ccc3N(c1ccccc1)c1ccccc1 Chemical compound c(cc1)ccc1N(c1ccccc1)c(cc1)cc(Oc2c3)c1Nc2ccc3N(c1ccccc1)c1ccccc1 SPPONIYAQHCYOB-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C225/00—Compounds containing amino groups and doubly—bound oxygen atoms bound to the same carbon skeleton, at least one of the doubly—bound oxygen atoms not being part of a —CHO group, e.g. amino ketones
- C07C225/22—Compounds containing amino groups and doubly—bound oxygen atoms bound to the same carbon skeleton, at least one of the doubly—bound oxygen atoms not being part of a —CHO group, e.g. amino ketones having amino groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/56—Ring systems containing three or more rings
- C07D209/80—[b, c]- or [b, d]-condensed
- C07D209/82—Carbazoles; Hydrogenated carbazoles
- C07D209/86—Carbazoles; Hydrogenated carbazoles with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to carbon atoms of the ring system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/56—Ring systems containing three or more rings
- C07D209/80—[b, c]- or [b, d]-condensed
- C07D209/82—Carbazoles; Hydrogenated carbazoles
- C07D209/88—Carbazoles; Hydrogenated carbazoles with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to carbon atoms of the ring system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D251/00—Heterocyclic compounds containing 1,3,5-triazine rings
- C07D251/02—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings
- C07D251/12—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings having three double bonds between ring members or between ring members and non-ring members
- C07D251/14—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings having three double bonds between ring members or between ring members and non-ring members with hydrogen or carbon atoms directly attached to at least one ring carbon atom
- C07D251/24—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings having three double bonds between ring members or between ring members and non-ring members with hydrogen or carbon atoms directly attached to at least one ring carbon atom to three ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D265/00—Heterocyclic compounds containing six-membered rings having one nitrogen atom and one oxygen atom as the only ring hetero atoms
- C07D265/28—1,4-Oxazines; Hydrogenated 1,4-oxazines
- C07D265/34—1,4-Oxazines; Hydrogenated 1,4-oxazines condensed with carbocyclic rings
- C07D265/38—[b, e]-condensed with two six-membered rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/10—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a carbon chain containing aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/10—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a carbon chain containing aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/10—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings linked by a carbon chain containing aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
- H10K85/636—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine comprising heteroaromatic hydrocarbons as substituents on the nitrogen atom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/654—Aromatic compounds comprising a hetero atom comprising only nitrogen as heteroatom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6572—Polycyclic condensed heteroaromatic hydrocarbons comprising only nitrogen in the heteroaromatic polycondensed ring system, e.g. phenanthroline or carbazole
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6576—Polycyclic condensed heteroaromatic hydrocarbons comprising only sulfur in the heteroaromatic polycondensed ring system, e.g. benzothiophene
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1007—Non-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1011—Condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1014—Carbocyclic compounds bridged by heteroatoms, e.g. N, P, Si or B
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1022—Heterocyclic compounds bridged by heteroatoms, e.g. N, P, Si or B
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1029—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1029—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom
- C09K2211/1033—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom with oxygen
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1044—Heterocyclic compounds characterised by ligands containing two nitrogen atoms as heteroatoms
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1044—Heterocyclic compounds characterised by ligands containing two nitrogen atoms as heteroatoms
- C09K2211/1048—Heterocyclic compounds characterised by ligands containing two nitrogen atoms as heteroatoms with oxygen
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1059—Heterocyclic compounds characterised by ligands containing three nitrogen atoms as heteroatoms
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1059—Heterocyclic compounds characterised by ligands containing three nitrogen atoms as heteroatoms
- C09K2211/1062—Heterocyclic compounds characterised by ligands containing three nitrogen atoms as heteroatoms with oxygen
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1074—Heterocyclic compounds characterised by ligands containing more than three nitrogen atoms as heteroatoms
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1074—Heterocyclic compounds characterised by ligands containing more than three nitrogen atoms as heteroatoms
- C09K2211/1081—Heterocyclic compounds characterised by ligands containing more than three nitrogen atoms as heteroatoms with sulfur
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1092—Heterocyclic compounds characterised by ligands containing sulfur as the only heteroatom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K2101/00—Properties of the organic materials covered by group H10K85/00
- H10K2101/10—Triplet emission
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K2101/00—Properties of the organic materials covered by group H10K85/00
- H10K2101/20—Delayed fluorescence emission
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Electroluminescent Light Sources (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Quinoline Compounds (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Nitrogen And Oxygen As The Only Ring Hetero Atoms (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Plural Heterocyclic Compounds (AREA)
- Nitrogen And Oxygen Or Sulfur-Condensed Heterocyclic Ring Systems (AREA)
- Indole Compounds (AREA)
Applications Claiming Priority (25)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201461996836P | 2014-05-14 | 2014-05-14 | |
| US61/996,836 | 2014-05-14 | ||
| US201461997579P | 2014-06-05 | 2014-06-05 | |
| US61/997,579 | 2014-06-05 | ||
| US201462028045P | 2014-07-23 | 2014-07-23 | |
| US62/028,045 | 2014-07-23 | ||
| US201462033869P | 2014-08-06 | 2014-08-06 | |
| US62/033,869 | 2014-08-06 | ||
| US201462048497P | 2014-09-10 | 2014-09-10 | |
| US62/048,497 | 2014-09-10 | ||
| US201462061369P | 2014-10-08 | 2014-10-08 | |
| US201462061460P | 2014-10-08 | 2014-10-08 | |
| US62/061,460 | 2014-10-08 | ||
| US62/061,369 | 2014-10-08 | ||
| US201462075490P | 2014-11-05 | 2014-11-05 | |
| US62/075,490 | 2014-11-05 | ||
| US201462093097P | 2014-12-17 | 2014-12-17 | |
| US62/093,097 | 2014-12-17 | ||
| US201562117045P | 2015-02-17 | 2015-02-17 | |
| US62/117,045 | 2015-02-17 | ||
| US201562139336P | 2015-03-27 | 2015-03-27 | |
| US62/139,336 | 2015-03-27 | ||
| US201562155764P | 2015-05-01 | 2015-05-01 | |
| US62/155,764 | 2015-05-01 | ||
| PCT/US2015/030598 WO2015175678A1 (en) | 2014-05-14 | 2015-05-13 | Organic light-emitting diode materials |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2017518281A true JP2017518281A (ja) | 2017-07-06 |
| JP2017518281A5 JP2017518281A5 (enExample) | 2018-06-21 |
Family
ID=53284535
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2016567427A Pending JP2017518281A (ja) | 2014-05-14 | 2015-05-13 | 有機発光ダイオード材料 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20170244049A1 (enExample) |
| EP (1) | EP3143100A1 (enExample) |
| JP (1) | JP2017518281A (enExample) |
| KR (1) | KR20170005853A (enExample) |
| CN (1) | CN106661001A (enExample) |
| WO (1) | WO2015175678A1 (enExample) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016082207A (ja) * | 2014-10-22 | 2016-05-16 | 三星ディスプレイ株式會社Samsung Display Co.,Ltd. | 有機エレクトロルミネッセンス素子用材料及びそれを用いた有機エレクトロルミネッセンス素子 |
| JP2017054972A (ja) * | 2015-09-10 | 2017-03-16 | コニカミノルタ株式会社 | 有機エレクトロルミネッセンス素子、表示装置、照明装置、π共役系化合物、及び発光性薄膜 |
| JP2018035129A (ja) * | 2015-10-27 | 2018-03-08 | 国立大学法人山形大学 | ピリミジン誘導体、それよりなる発光材料及びそれを用いた有機el素子 |
| JP2018113364A (ja) * | 2017-01-12 | 2018-07-19 | 日本放送協会 | 有機エレクトロルミネッセンス素子 |
| CN109942551A (zh) * | 2017-11-08 | 2019-06-28 | 辛诺拉有限公司 | 有机分子,特别是其在光电设备中的用途 |
| JP2020172483A (ja) * | 2019-04-05 | 2020-10-22 | 三星ディスプレイ株式會社Samsung Display Co.,Ltd. | 有機電界発光素子及び有機電界発光素子用化合物 |
| JP2021524867A (ja) * | 2018-09-27 | 2021-09-16 | 武漢尚賽光電科技有限公司Wuhan Sunshine Optoelectronics Tech Co Ltd | 1,2,4−チアジアゾール系化合物及びその製造方法と使用 |
| JP2021533580A (ja) * | 2018-08-03 | 2021-12-02 | ▲広▼▲東▼聚▲華▼印刷▲顯▼示技▲術▼有限公司 | 電子輸送材料及びその使用 |
| JP2022008036A (ja) * | 2020-04-03 | 2022-01-13 | 株式会社半導体エネルギー研究所 | アリールアミン化合物、正孔輸送層用材料、正孔注入層用材料、発光デバイス、発光装置、電子機器および照明装置 |
Families Citing this family (147)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TWI637944B (zh) * | 2013-11-28 | 2018-10-11 | 九州有機光材股份有限公司 | 發光材料、有機發光元件及化合物 |
| US10934248B2 (en) | 2013-11-28 | 2021-03-02 | Kyulux, Inc. | Light-emitting material, organic light-emitting device, and compound |
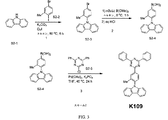
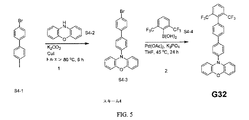
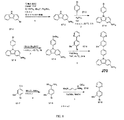
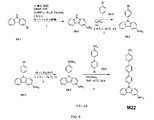
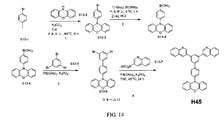
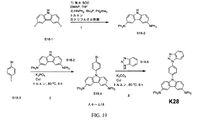
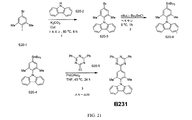
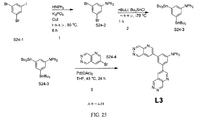
| US9972795B2 (en) * | 2014-05-14 | 2018-05-15 | Presidents And Fellows Of Harvard College | Organic light-emitting diode materials |
| CN113793905A (zh) * | 2014-09-26 | 2021-12-14 | Udc 爱尔兰有限责任公司 | 具有高效率的荧光有机发光元件 |
| EP3218368B1 (de) * | 2014-11-11 | 2020-11-25 | Merck Patent GmbH | Materialien für organische elektrolumineszenzvorrichtungen |
| EP3032605B1 (en) * | 2014-12-08 | 2019-08-21 | LG Display Co., Ltd. | Organic light emitting display device |
| WO2016111196A1 (ja) * | 2015-01-08 | 2016-07-14 | 国立大学法人九州大学 | 化合物、混合物、発光層、有機発光素子およびアシストドーパント |
| JP6764671B2 (ja) * | 2015-04-14 | 2020-10-07 | 株式会社半導体エネルギー研究所 | 複素環化合物、発光素子、発光装置、電子機器、および照明装置 |
| CN110957430B (zh) * | 2015-04-24 | 2022-07-08 | 株式会社Lg化学 | 有机发光器件 |
| GB201507340D0 (en) * | 2015-04-29 | 2015-06-10 | Univ St Andrews | Light emitting devices and compounds |
| WO2016208240A1 (ja) * | 2015-06-23 | 2016-12-29 | 株式会社カネカ | 有機el材料およびそれを用いた有機el素子 |
| KR102548610B1 (ko) * | 2015-07-17 | 2023-06-27 | 다우 글로벌 테크놀로지스 엘엘씨 | 전계발광 디바이스용 발광 성분으로서의 n-아릴-하이드로아크리딘 |
| GB201513037D0 (en) | 2015-07-23 | 2015-09-09 | Merck Patent Gmbh | Phenyl-derived compound for use in organic electronic devices |
| US11201291B2 (en) * | 2015-07-30 | 2021-12-14 | Sichuan Knowledge Express Institute For Innovative Technologies Co., Ltd | Organic molecules having two non-conjugated bridges between a donor and an acceptor for effective thermally activated delayed fluorescence for use in optoelectronic devices |
| US11730009B2 (en) | 2015-09-24 | 2023-08-15 | Lg Chem, Ltd. | Compound and organic light-emitting element comprising same |
| KR102572294B1 (ko) * | 2015-09-25 | 2023-08-30 | 덕산네오룩스 주식회사 | 유기전기 소자용 화합물, 이를 이용한 유기전기소자 및 그 전자 장치 |
| JP6705148B2 (ja) * | 2015-10-15 | 2020-06-03 | コニカミノルタ株式会社 | π共役系化合物、有機エレクトロルミネッセンス素子材料、発光材料、発光性薄膜、有機エレクトロルミネッセンス素子、表示装置及び照明装置 |
| CN105294670B (zh) | 2015-11-20 | 2019-07-09 | 上海天马有机发光显示技术有限公司 | 有机电致发光化合物及其有机光电装置 |
| TWI594476B (zh) * | 2015-12-11 | 2017-08-01 | 友達光電股份有限公司 | 螢光有機發光材料及有機電激發光裝置 |
| CN105567217B (zh) * | 2015-12-15 | 2017-12-01 | 华南理工大学 | 一种多刺激‑响应有机小分子发光材料及制备与应用 |
| WO2017106634A1 (en) | 2015-12-17 | 2017-06-22 | Incyte Corporation | N-phenyl-pyridine-2-carboxamide derivatives and their use as pd-1/pd-l1 protein/protein interaction modulators |
| TWI850624B (zh) | 2015-12-22 | 2024-08-01 | 美商英塞特公司 | 作為免疫調節劑之雜環化合物 |
| DE102016212614B4 (de) * | 2015-12-25 | 2021-05-20 | Shanghai Tianma AM-OLED Co., Ltd. | Stickstoffhaltige heterocyclische Verbindung, organische fotoelektrische Vorrichtung und Verfahren zum Herstellen einer organischen fotoelektrischen Vorrichtung |
| CN105399696B (zh) * | 2015-12-25 | 2019-12-24 | 上海天马有机发光显示技术有限公司 | 有机电致发光化合物及其有机光电装置 |
| CN105418486A (zh) | 2015-12-25 | 2016-03-23 | 上海天马有机发光显示技术有限公司 | 有机电致发光化合物及其有机光电装置 |
| KR102659372B1 (ko) * | 2016-03-04 | 2024-04-22 | 주식회사 동진쎄미켐 | 신규 화합물 및 이를 포함하는 유기발광소자 |
| CN107056770A (zh) * | 2016-04-25 | 2017-08-18 | 中节能万润股份有限公司 | 一种以含氮五元杂环为核心的化合物及其在有机电致发光器件上的应用 |
| CN106279203B (zh) * | 2016-04-25 | 2018-08-14 | 中节能万润股份有限公司 | 一种含酮和氮杂环的化合物及其在有机电致发光器件上的应用 |
| CN107057682A (zh) * | 2016-04-25 | 2017-08-18 | 中节能万润股份有限公司 | 一种以吖啶酮为核心的化合物及其在有机电致发光器件上的应用 |
| KR102501267B1 (ko) * | 2016-04-26 | 2023-02-21 | 덕산네오룩스 주식회사 | 지연 형광 재료 및 이를 이용한 유기전기소자 및 그 전자 장치 |
| JP7081898B2 (ja) * | 2016-04-28 | 2022-06-07 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | 有機エレクトロルミネッセンス素子、表示装置及び照明装置 |
| JP7253646B2 (ja) * | 2016-04-28 | 2023-04-06 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | π共役系化合物、有機エレクトロルミネッセンス素子材料、発光材料、電荷輸送材料、発光性薄膜、有機エレクトロルミネッセンス素子、表示装置及び照明装置 |
| KR102651014B1 (ko) * | 2016-05-26 | 2024-03-26 | 삼성디스플레이 주식회사 | 함질소 화합물 및 이를 포함하는 유기 전계 발광 소자 |
| TW201808902A (zh) * | 2016-05-26 | 2018-03-16 | 美商英塞特公司 | 作為免疫調節劑之雜環化合物 |
| CN106045977B (zh) * | 2016-05-31 | 2019-03-15 | 太原理工大学 | 一种基于咔唑和1,2,4-三氮唑的双极性蓝色磷光主体材料 |
| CN106083825A (zh) * | 2016-06-07 | 2016-11-09 | 石家庄诚志永华显示材料有限公司 | 吡嗪衍生物及其在有机电致发光器件中的应用 |
| PT3472167T (pt) | 2016-06-20 | 2022-11-11 | Incyte Corp | Compostos heterocíclicos como imunomoduladores |
| DE102016112377B4 (de) * | 2016-07-06 | 2020-06-04 | Cynora Gmbh | Organische Moleküle, insbesondere zur Verwendung in organischen optoelektronischen Vorrichtungen |
| WO2018008718A1 (ja) * | 2016-07-07 | 2018-01-11 | 保土谷化学工業株式会社 | ベンゾアゾール環構造を有する化合物および有機エレクトロルミネッセンス素子 |
| US20180016260A1 (en) | 2016-07-14 | 2018-01-18 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| KR102631944B1 (ko) * | 2016-08-03 | 2024-02-02 | 삼성디스플레이 주식회사 | 방향족 화합물 및 이를 포함하는 유기 전계 발광 소자 |
| CN106467533A (zh) * | 2016-08-08 | 2017-03-01 | 江苏三月光电科技有限公司 | 一种以吖啶酮为核心的化合物及其应用 |
| KR102207689B1 (ko) | 2016-08-24 | 2021-01-25 | 시노라 게엠베하 | 유기 분자, 특히 유기 광전자 디바이스에 사용하기 위한 유기 분자 |
| JP6648082B2 (ja) * | 2016-08-25 | 2020-02-14 | サイノラ ゲゼルシャフト ミット ベシュレンクテル ハフツング | 特に有機光電子デバイスに使用するための有機分子 |
| DE102016115854B3 (de) * | 2016-08-25 | 2018-01-25 | Cynora Gmbh | Organische Moleküle, insbesondere zur Verwendung in organischen optoelektronischen Vorrichtungen |
| JP6986737B2 (ja) * | 2016-08-30 | 2021-12-22 | 国立大学法人山形大学 | 新規イソニコチノニトリル誘導体、及びそれを用いた有機el素子 |
| CN107880027B (zh) * | 2016-09-30 | 2021-03-02 | 中节能万润股份有限公司 | 一种以三嗪为核心的化合物及其在有机电致发光器件上的应用 |
| DE102016120373B3 (de) * | 2016-10-25 | 2017-08-24 | Cynora Gmbh | Organische Moleküle, insbesondere zur Verwendung in organischen optoelektronischen Vorrichtungen |
| WO2018077492A1 (de) * | 2016-10-25 | 2018-05-03 | Cynora Gmbh | Organische moleküle, insbesondere zur verwendung in organischen optoelektronischen vorrichtungen |
| KR101970863B1 (ko) * | 2016-10-31 | 2019-04-19 | 성균관대학교산학협력단 | 지연형광 재료 및 이를 포함하는 유기 발광장치 |
| KR102147502B1 (ko) * | 2016-11-01 | 2020-08-24 | 시노라 게엠베하 | 유기 분자, 특히 유기 광전자 디바이스에 사용하기 위한 유기 분자 |
| US10897016B2 (en) * | 2016-11-14 | 2021-01-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10833276B2 (en) | 2016-11-21 | 2020-11-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102685454B1 (ko) * | 2016-12-07 | 2024-07-16 | 솔루스첨단소재 주식회사 | 유기 화합물 및 이를 이용한 유기 전계 발광 소자 |
| CN106699659B (zh) * | 2016-12-14 | 2018-12-18 | 中节能万润股份有限公司 | 一种n-苯基吖啶酮类有机电致发光材料及其制备方法和应用 |
| JP7101678B2 (ja) | 2016-12-22 | 2022-07-15 | インサイト・コーポレイション | 免疫調節剤としての複素環式化合物 |
| TW201835049A (zh) | 2016-12-22 | 2018-10-01 | 美商英塞特公司 | 作為免疫調節劑之雜環化合物 |
| JP2018111751A (ja) * | 2017-01-10 | 2018-07-19 | 国立大学法人九州大学 | 発光材料、化合物および有機発光素子 |
| US11456426B2 (en) | 2017-02-07 | 2022-09-27 | Samsung Display Co., Ltd. | Organic molecules for use in organic optoelectronic devices |
| DE102017103542B3 (de) * | 2017-02-21 | 2018-03-29 | Cynora Gmbh | Organische Moleküle, insbesondere zur Verwendung in optoelektronischen Vorrichtungen |
| KR102852285B1 (ko) | 2017-02-27 | 2025-08-28 | 삼성전자주식회사 | 축합환 화합물 및 이를 포함한 유기 발광 소자 |
| JP2020113557A (ja) * | 2017-03-28 | 2020-07-27 | 保土谷化学工業株式会社 | アザカルバゾール構造を有する化合物および有機エレクトロルミネッセンス素子 |
| CN106966955A (zh) * | 2017-04-21 | 2017-07-21 | 瑞声光电科技(常州)有限公司 | 联苯化合物及发光器件 |
| US10790455B2 (en) | 2017-05-18 | 2020-09-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102072208B1 (ko) * | 2017-05-31 | 2020-01-31 | 삼성에스디아이 주식회사 | 유기 광전자 소자용 조성물, 유기 광전자 소자 및 표시 장치 |
| DE102017112435B4 (de) * | 2017-06-06 | 2019-01-17 | Cynora Gmbh | Organische Moleküle, insbesondere zur Verwendung in optoelektronischen Vorrichtungen |
| JP6900514B2 (ja) * | 2017-06-06 | 2021-07-07 | サイノラ ゲゼルシャフト ミット ベシュレンクテル ハフツング | 有機分子、特に光電子デバイスに用いる有機分子 |
| CN109111433A (zh) * | 2017-06-22 | 2019-01-01 | 北京鼎材科技有限公司 | 一种有机电致发光化合物及其用途和有机电致发光器件 |
| EP3421452A1 (en) * | 2017-06-27 | 2019-01-02 | Cynora Gmbh | Carbazole derivatives and their use in optoelectronic devices |
| DE102017114250B3 (de) | 2017-06-27 | 2018-09-13 | Cynora Gmbh | Organische Moleküle, insbesondere zur Verwendung in optoelektronischen Vorrichtungen |
| DE102017114345B4 (de) | 2017-06-28 | 2019-01-17 | Cynora Gmbh | Organische Moleküle, insbesondere zur Verwendung in organischen optoelektronischen Vorrichtungen |
| DE102017114372B3 (de) | 2017-06-28 | 2018-10-11 | Cynora Gmbh | Organische moleküle, insbesondere zur verwendung in optoelektronischen vorrichtungen |
| KR102577731B1 (ko) * | 2017-06-30 | 2023-09-14 | 솔루스첨단소재 주식회사 | 유기 화합물 및 이를 포함하는 유기 전계 발광 소자 |
| CN107417690B (zh) * | 2017-09-07 | 2019-01-29 | 南方科技大学 | 一种不对称催化合成吡咯吲哚啉的方法 |
| DE102017122471B3 (de) * | 2017-09-27 | 2019-01-24 | Cynora Gmbh | Organische moleküle, insbesondere zur verwendung in optoelektronischen vorrichtungen |
| CN109574909A (zh) * | 2017-09-29 | 2019-04-05 | 江苏三月光电科技有限公司 | 一种以酮类结构为核心的有机化合物及其在oled器件中的应用 |
| EP3697782B1 (en) * | 2017-10-19 | 2021-09-01 | cynora GmbH | Organic molecules for use in optoelectronic devices |
| US11444250B2 (en) * | 2017-12-05 | 2022-09-13 | Kyulux, Inc. | Composition of matter for use in organic light-emitting diodes |
| CN108129381A (zh) * | 2017-12-14 | 2018-06-08 | 江苏第二师范学院 | 紫色延迟荧光材料及其制备方法、晶型和应用 |
| KR102148199B1 (ko) * | 2017-12-19 | 2020-08-26 | 재단법인대구경북과학기술원 | 전자수송용 유기반도체 소재 |
| KR102536247B1 (ko) * | 2017-12-22 | 2023-05-25 | 삼성디스플레이 주식회사 | 헤테로고리 화합물 및 이를 포함한 유기 발광 소자 |
| CN111278829B (zh) * | 2018-01-04 | 2023-01-17 | 株式会社Lg化学 | 化合物和包含其的有机发光器件 |
| CN108191853B (zh) * | 2018-01-10 | 2020-08-07 | 北京鼎材科技有限公司 | 一种有机电致发光材料与器件 |
| CN108178767B (zh) * | 2018-01-19 | 2020-09-22 | 华南理工大学 | 一种基于吡嗪受体单元的有机小分子发光材料及其制备方法和应用 |
| CN110066227A (zh) * | 2018-01-24 | 2019-07-30 | 北京鼎材科技有限公司 | 有机电致发光材料及发光器件 |
| KR102054489B1 (ko) * | 2018-03-06 | 2019-12-10 | 한남대학교 산학협력단 | 청색 인광 호스트 화합물 및 이의 제조방법 |
| RS66699B1 (sr) | 2018-03-30 | 2025-05-30 | Incyte Corp | Heterociklična jedinjenja kao imunomodulatori |
| KR102703716B1 (ko) | 2018-04-09 | 2024-09-06 | 삼성전자주식회사 | 축합환 화합물 및 이를 포함한 유기 발광 소자 |
| KR20190121418A (ko) | 2018-04-17 | 2019-10-28 | 삼성디스플레이 주식회사 | 유기 전계 발광 소자 및 유기 전계 발광 소자용 함질소 화합물 |
| TW202425987A (zh) | 2018-05-11 | 2024-07-01 | 美商英塞特公司 | 作為免疫調節劑之雜環化合物 |
| CN108658980A (zh) * | 2018-05-18 | 2018-10-16 | 长春海谱润斯科技有限公司 | 一种含有邻菲啰啉的芳胺类化合物及其有机电致发光器件 |
| WO2019218360A1 (zh) * | 2018-05-18 | 2019-11-21 | 深圳市柔宇科技有限公司 | 聚集诱导蓝光材料及其制备方法、显示装置 |
| KR102617841B1 (ko) * | 2018-05-29 | 2023-12-26 | 덕산네오룩스 주식회사 | 유기전기소자용 화합물, 이를 이용한 유기전기소자 및 그 전자 장치 |
| US11339143B2 (en) | 2018-06-26 | 2022-05-24 | Samsung Electronics Co., Ltd. | Condensed cyclic compound and organic light-emitting device including the same |
| CN108864068B (zh) * | 2018-07-27 | 2021-12-28 | 武汉天马微电子有限公司 | 一种化合物以及有机发光显示装置 |
| CN109627233A (zh) * | 2018-07-27 | 2019-04-16 | 华南理工大学 | 一种基于咔唑衍生物取代的氮杂环分子的有机光电材料及其制备方法和应用 |
| CN108929322A (zh) * | 2018-08-12 | 2018-12-04 | 瑞声科技(南京)有限公司 | 一种含有氮杂咔唑单元的化合物及其应用 |
| CN108912148A (zh) * | 2018-08-12 | 2018-11-30 | 瑞声科技(南京)有限公司 | 一种含有氮杂咔唑-咪唑单元的化合物及其应用 |
| EP3613744B1 (en) * | 2018-08-21 | 2024-03-20 | Samsung Display Co., Ltd. | Organic molecules for optoelectronic devices |
| US12098143B2 (en) | 2018-08-29 | 2024-09-24 | Samsung Display Co., Ltd. | Organic molecules for optoelectronic devices |
| US11485706B2 (en) | 2018-09-11 | 2022-11-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN109096279B (zh) * | 2018-09-28 | 2020-11-03 | 武汉天马微电子有限公司 | 氮杂环化合物、显示面板以及显示装置 |
| CN109096252B (zh) * | 2018-09-29 | 2020-09-22 | 湘潭大学 | 基于2,10’-联吖啶衍生物的有机热活性延迟荧光材料及其应用 |
| CN109593079A (zh) * | 2018-11-15 | 2019-04-09 | 华南理工大学 | 含吡啶基团的通用型双极主体材料及其制备与在有机发光二极管中的应用 |
| CN109734608B (zh) * | 2018-11-29 | 2022-04-08 | 宇瑞(上海)化学有限公司 | 一种有机化合物及其使用该化合物的有机电致器件 |
| KR102329345B1 (ko) * | 2018-12-06 | 2021-11-19 | 한국생산기술연구원 | 벤조페논 작용기 함유 화합물, 상기 화합물의 광경화물을 포함한 유기물층을 구비한 유기전자소자 |
| CN109503481B (zh) * | 2018-12-17 | 2020-06-02 | 武汉华星光电半导体显示技术有限公司 | 热活化延迟荧光化合物及其制备方法与有机电致发光二极管器件 |
| CN111349040B (zh) * | 2018-12-21 | 2023-02-03 | 陕西师范大学 | 有机室温磷光和白光发光材料及其制备方法 |
| CN111377908A (zh) * | 2018-12-27 | 2020-07-07 | 北京鼎材科技有限公司 | 一种热活化延迟荧光化合物及其应用 |
| KR102753926B1 (ko) * | 2019-03-11 | 2025-01-15 | 삼성디스플레이 주식회사 | 헤테로시클릭 화합물 및 이를 포함한 유기 발광 소자 |
| CN109942601B (zh) * | 2019-03-18 | 2021-09-21 | 深圳大学 | 一种荧光材料、制备方法及应用 |
| CN109913205B (zh) * | 2019-03-18 | 2021-12-07 | 深圳大学 | 一种荧光材料、制备方法及应用 |
| CN110003115B (zh) * | 2019-03-21 | 2022-09-20 | 北京大学深圳研究生院 | 一种蓝色有机发光材料、发光器件及制备方法 |
| CN111747933B (zh) * | 2019-03-29 | 2022-03-01 | 吉林省元合电子材料有限公司 | 一种取代的1,3,5-三嗪化合物、组合物及其应用 |
| CN111747937B (zh) * | 2019-03-29 | 2022-01-14 | 吉林省元合电子材料有限公司 | 一种1,3,5-三嗪类化合物、组合物及其应用 |
| CN111808076A (zh) * | 2019-04-12 | 2020-10-23 | 冠能光电材料(深圳)有限责任公司 | 一种电子传输空穴阻挡有机材料及其在薄膜发光二极管应用 |
| CN110078754A (zh) * | 2019-05-27 | 2019-08-02 | 上海天马有机发光显示技术有限公司 | 化合物、显示面板以及显示装置 |
| CN110143960B (zh) * | 2019-05-30 | 2021-06-01 | 武汉华星光电半导体显示技术有限公司 | 绿光热活化延迟荧光材料及其制备方法、有机电致发光器件 |
| KR102801363B1 (ko) * | 2019-07-17 | 2025-05-07 | 삼성디스플레이 주식회사 | 유기 전계 발광 소자 및 유기 전계 발광 소자용 다환 화합물 |
| CN110305121A (zh) * | 2019-07-25 | 2019-10-08 | 硕明(常州)光源科技有限公司 | 一种n-酰基咔唑类化合物及其制备方法和应用 |
| CN112430217B (zh) * | 2019-08-26 | 2024-03-19 | 北京鼎材科技有限公司 | 一种化合物及其应用 |
| JP7559059B2 (ja) | 2019-09-30 | 2024-10-01 | インサイト・コーポレイション | 免疫調節剤としてのピリド[3,2-d]ピリミジン化合物 |
| CN110642842B (zh) * | 2019-09-30 | 2023-03-24 | 武汉天马微电子有限公司 | 化合物、显示面板以及显示装置 |
| KR102516811B1 (ko) | 2019-10-23 | 2023-03-31 | 삼성에스디아이 주식회사 | 유기 광전자 소자용 화합물, 유기 광전자 소자용 조성물, 유기 광전자 소자 및 표시 장치 |
| CN110804047A (zh) | 2019-11-06 | 2020-02-18 | 武汉华星光电半导体显示技术有限公司 | 热活化延迟荧光材料及其制备方法 |
| PH12022551136A1 (en) | 2019-11-11 | 2023-10-09 | Incyte Corp | Salts and crystalline forms of a pd-1/pd-l1 inhibitor |
| CN112851565B (zh) * | 2019-11-27 | 2023-02-10 | 杭州师范大学 | 一种室温磷光性能的有机发光材料及其制备方法和应用 |
| CN111171010A (zh) * | 2020-01-13 | 2020-05-19 | 北京大学深圳研究生院 | 一种阴极电刺激响应材料及其制备方法 |
| CN111484515B (zh) * | 2020-04-21 | 2023-04-14 | 濮阳惠成电子材料股份有限公司 | 一种均三嗪硼酸频那醇酯的合成方法 |
| CN111662286B (zh) * | 2020-05-19 | 2021-08-24 | 浙江虹舞科技有限公司 | 一种含有吡啶并三氮唑及衍生物受体结构单元的可见光延迟荧光材料及应用 |
| CN111620817B (zh) * | 2020-06-04 | 2022-09-27 | 常州大学 | 新型蓝色热活性延迟荧光材料及其应用 |
| CN113782697B (zh) * | 2020-06-09 | 2025-08-26 | 常州强力电子新材料股份有限公司 | 有机电致发光元件 |
| CN111747938B (zh) * | 2020-07-03 | 2021-04-27 | 长春海谱润斯科技股份有限公司 | 一种芳胺化合物及其有机电致发光器件 |
| KR102517277B1 (ko) * | 2020-09-24 | 2023-04-04 | (주)랩토 | 시아노기가 치환된 아릴 또는 헤테로아릴 유도체 및 이를 포함한 유기전계발광소자 |
| CN112174959B (zh) * | 2020-11-04 | 2022-11-18 | 浙江虹舞科技有限公司 | 一类基于1,6-萘啶受体结构单元的有机发光材料及其应用 |
| TW202233616A (zh) | 2020-11-06 | 2022-09-01 | 美商英塞特公司 | 用於製備pd-1/pd-l1抑制劑以及其鹽及結晶形式之方法 |
| US11780836B2 (en) | 2020-11-06 | 2023-10-10 | Incyte Corporation | Process of preparing a PD-1/PD-L1 inhibitor |
| TW202233615A (zh) | 2020-11-06 | 2022-09-01 | 美商英塞特公司 | Pd—1/pd—l1抑制劑之結晶形式 |
| CN113429388A (zh) * | 2021-06-28 | 2021-09-24 | 常州大学 | 基于三氟甲基吡啶衍生物受体的蓝色热活性延迟荧光材料及其应用 |
| CN113480410A (zh) * | 2021-07-07 | 2021-10-08 | 南京伊派森化学科技有限公司 | 一种2,4-二溴苯甲醇的合成方法 |
| CN113831343B (zh) * | 2021-07-23 | 2023-09-05 | 安徽秀朗新材料科技有限公司 | 一种基于咪唑并吡嗪受体材料的热活性延迟荧光材料、制备方法及其应用 |
| WO2023085860A1 (ko) * | 2021-11-12 | 2023-05-19 | 주식회사 엘지화학 | 화합물 및 이를 포함하는 유기 발광 소자 |
| CN116813502A (zh) * | 2022-03-18 | 2023-09-29 | 中国石油化工股份有限公司 | 一种高水平偶极取向的四苯基苯类衍生物及其制备方法与应用 |
| KR102885090B1 (ko) * | 2022-03-29 | 2025-11-13 | 주식회사 엘지화학 | 화합물 및 이를 포함하는 유기 발광 소자 |
| CN115160217B (zh) * | 2022-08-04 | 2023-09-22 | 山东达因海洋生物制药股份有限公司 | 一种吡仑帕奈的制备方法、合成中间体及降解杂质的制备方法 |
| CN115974861A (zh) * | 2022-12-26 | 2023-04-18 | 苏州奥为光电新材料有限公司 | 一种三嗪衍生物的热激活延迟材料及其制备方法和应用 |
| CN116355226A (zh) * | 2023-02-22 | 2023-06-30 | 闽都创新实验室 | 一种有机磷光材料及其制备方法和应用 |
Citations (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH02178670A (ja) * | 1988-12-29 | 1990-07-11 | Canon Inc | 電子写真感光体 |
| JPH02183259A (ja) * | 1989-01-10 | 1990-07-17 | Canon Inc | 電子写真感光体 |
| JP2009021335A (ja) * | 2007-07-11 | 2009-01-29 | Konica Minolta Holdings Inc | 有機エレクトロルミネッセンス素子、表示装置及び照明装置 |
| WO2009072587A1 (en) * | 2007-12-03 | 2009-06-11 | Semiconductor Energy Laboratory Co., Ltd. | Carbazole derivative, and light-emitting element, light-emitting device, and electronic device using carbazole derivative |
| WO2011013783A1 (ja) * | 2009-07-31 | 2011-02-03 | 富士フイルム株式会社 | 有機電界発光素子 |
| JP2011509247A (ja) * | 2007-12-28 | 2011-03-24 | ユニバーサル ディスプレイ コーポレイション | リン光発光ダイオード中の、カルバゾールを含む物質 |
| JP2011071474A (ja) * | 2009-07-31 | 2011-04-07 | Fujifilm Corp | 電荷輸送材料及び有機電界発光素子 |
| JP2012036164A (ja) * | 2010-06-17 | 2012-02-23 | ▲いく▼▲雷▼光電科技股▲分▼有限公司 | 有機エレクトロルミネッセンス装置に用いられる化合物及び有機エレクトロルミネッセンス装置 |
| US20120086329A1 (en) * | 2010-10-08 | 2012-04-12 | Universal Disolay Corporation | Novel 3, 9-Linked Oliogocarbazole-Based Hosts, Containing DBT and DBF Fragments, Separated by Aromatic Spacers |
| JP2013016717A (ja) * | 2011-07-06 | 2013-01-24 | Konica Minolta Holdings Inc | 有機エレクトロルミネッセンス素子、表示装置及び照明装置 |
| JP2013145791A (ja) * | 2012-01-13 | 2013-07-25 | ▲いく▼▲雷▼光電科技股▲分▼有限公司 | カルバゾール誘導体とそれを用いた有機エレクトロルミネセンス装置及びその製造方法 |
| US20130292659A1 (en) * | 2010-12-08 | 2013-11-07 | Hyung-Sun Kim | Compound for organic optoelectronic device, organic light emitting diode including the same, and display device including the organic light emitting diode |
| WO2013165192A1 (en) * | 2012-05-02 | 2013-11-07 | Rohm And Haas Electronic Materials Korea Ltd. | Novel organic electroluminescence compounds and organic electroluminescence device containing the same |
| US20140001449A1 (en) * | 2010-08-26 | 2014-01-02 | Solvay Sa | N-phenyl triscarbazole |
| WO2014014310A1 (en) * | 2012-07-20 | 2014-01-23 | Rohm And Haas Electronic Materials Korea Ltd. | A novel combination of a host compound and a dopant compound and an organic electroluminescence device comprising the same |
| WO2014133121A1 (ja) * | 2013-03-01 | 2014-09-04 | 国立大学法人九州大学 | 化合物、発光材料および有機発光素子 |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6830828B2 (en) * | 1998-09-14 | 2004-12-14 | The Trustees Of Princeton University | Organometallic complexes as phosphorescent emitters in organic LEDs |
| KR100957288B1 (ko) * | 2002-03-15 | 2010-05-12 | 이데미쓰 고산 가부시키가이샤 | 유기 전기발광 소자용 재료 및 이를 이용한 유기 전기발광소자 |
| JP4103491B2 (ja) * | 2002-08-07 | 2008-06-18 | コニカミノルタホールディングス株式会社 | 有機エレクトロルミネッセンス素子及び表示装置 |
| DE112011103904B4 (de) * | 2010-11-24 | 2022-12-15 | Merck Patent Gmbh | Materialien für organische Elektrolumineszenzvorrichtungen |
| KR102029108B1 (ko) * | 2011-05-13 | 2019-10-07 | 이데미쓰 고산 가부시키가이샤 | 유기 el 다색 발광 장치 |
| EP2787549A4 (en) * | 2011-12-02 | 2015-09-23 | Univ Kyushu Nat Univ Corp | ORGANIC LIGHT-EMITTING COMPONENT AND DELAYED FLUORESCENCE MATERIAL AND COMPOUND USED THEREFROM |
| US9209411B2 (en) * | 2012-12-07 | 2015-12-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
-
2015
- 2015-05-13 WO PCT/US2015/030598 patent/WO2015175678A1/en not_active Ceased
- 2015-05-13 US US15/310,234 patent/US20170244049A1/en not_active Abandoned
- 2015-05-13 EP EP15727132.1A patent/EP3143100A1/en not_active Withdrawn
- 2015-05-13 KR KR1020167034925A patent/KR20170005853A/ko not_active Withdrawn
- 2015-05-13 JP JP2016567427A patent/JP2017518281A/ja active Pending
- 2015-05-13 CN CN201580038816.6A patent/CN106661001A/zh active Pending
Patent Citations (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH02178670A (ja) * | 1988-12-29 | 1990-07-11 | Canon Inc | 電子写真感光体 |
| JPH02183259A (ja) * | 1989-01-10 | 1990-07-17 | Canon Inc | 電子写真感光体 |
| JP2009021335A (ja) * | 2007-07-11 | 2009-01-29 | Konica Minolta Holdings Inc | 有機エレクトロルミネッセンス素子、表示装置及び照明装置 |
| WO2009072587A1 (en) * | 2007-12-03 | 2009-06-11 | Semiconductor Energy Laboratory Co., Ltd. | Carbazole derivative, and light-emitting element, light-emitting device, and electronic device using carbazole derivative |
| JP2011509247A (ja) * | 2007-12-28 | 2011-03-24 | ユニバーサル ディスプレイ コーポレイション | リン光発光ダイオード中の、カルバゾールを含む物質 |
| WO2011013783A1 (ja) * | 2009-07-31 | 2011-02-03 | 富士フイルム株式会社 | 有機電界発光素子 |
| JP2011071474A (ja) * | 2009-07-31 | 2011-04-07 | Fujifilm Corp | 電荷輸送材料及び有機電界発光素子 |
| JP2012036164A (ja) * | 2010-06-17 | 2012-02-23 | ▲いく▼▲雷▼光電科技股▲分▼有限公司 | 有機エレクトロルミネッセンス装置に用いられる化合物及び有機エレクトロルミネッセンス装置 |
| US20140001449A1 (en) * | 2010-08-26 | 2014-01-02 | Solvay Sa | N-phenyl triscarbazole |
| US20120086329A1 (en) * | 2010-10-08 | 2012-04-12 | Universal Disolay Corporation | Novel 3, 9-Linked Oliogocarbazole-Based Hosts, Containing DBT and DBF Fragments, Separated by Aromatic Spacers |
| US20130292659A1 (en) * | 2010-12-08 | 2013-11-07 | Hyung-Sun Kim | Compound for organic optoelectronic device, organic light emitting diode including the same, and display device including the organic light emitting diode |
| JP2013016717A (ja) * | 2011-07-06 | 2013-01-24 | Konica Minolta Holdings Inc | 有機エレクトロルミネッセンス素子、表示装置及び照明装置 |
| JP2013145791A (ja) * | 2012-01-13 | 2013-07-25 | ▲いく▼▲雷▼光電科技股▲分▼有限公司 | カルバゾール誘導体とそれを用いた有機エレクトロルミネセンス装置及びその製造方法 |
| WO2013165192A1 (en) * | 2012-05-02 | 2013-11-07 | Rohm And Haas Electronic Materials Korea Ltd. | Novel organic electroluminescence compounds and organic electroluminescence device containing the same |
| WO2014014310A1 (en) * | 2012-07-20 | 2014-01-23 | Rohm And Haas Electronic Materials Korea Ltd. | A novel combination of a host compound and a dopant compound and an organic electroluminescence device comprising the same |
| WO2014133121A1 (ja) * | 2013-03-01 | 2014-09-04 | 国立大学法人九州大学 | 化合物、発光材料および有機発光素子 |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016082207A (ja) * | 2014-10-22 | 2016-05-16 | 三星ディスプレイ株式會社Samsung Display Co.,Ltd. | 有機エレクトロルミネッセンス素子用材料及びそれを用いた有機エレクトロルミネッセンス素子 |
| JP2017054972A (ja) * | 2015-09-10 | 2017-03-16 | コニカミノルタ株式会社 | 有機エレクトロルミネッセンス素子、表示装置、照明装置、π共役系化合物、及び発光性薄膜 |
| JP2018035129A (ja) * | 2015-10-27 | 2018-03-08 | 国立大学法人山形大学 | ピリミジン誘導体、それよりなる発光材料及びそれを用いた有機el素子 |
| JP2018113364A (ja) * | 2017-01-12 | 2018-07-19 | 日本放送協会 | 有機エレクトロルミネッセンス素子 |
| CN109942551A (zh) * | 2017-11-08 | 2019-06-28 | 辛诺拉有限公司 | 有机分子,特别是其在光电设备中的用途 |
| US12312338B2 (en) | 2017-11-08 | 2025-05-27 | Samsung Display Co., Ltd. | Organic molecules for use in optoelectronic devices |
| CN109942551B (zh) * | 2017-11-08 | 2022-12-30 | 三星显示有限公司 | 有机分子,特别是其在光电设备中的用途 |
| JP7116258B2 (ja) | 2018-08-03 | 2022-08-09 | ▲広▼▲東▼聚▲華▼印刷▲顯▼示技▲術▼有限公司 | 電子輸送材料及びその使用 |
| JP2021533580A (ja) * | 2018-08-03 | 2021-12-02 | ▲広▼▲東▼聚▲華▼印刷▲顯▼示技▲術▼有限公司 | 電子輸送材料及びその使用 |
| US11778906B2 (en) | 2018-08-03 | 2023-10-03 | Guangdong Juhua Printed Display Technology Co. Ltd | Electron transport material and application thereof |
| JP2021524867A (ja) * | 2018-09-27 | 2021-09-16 | 武漢尚賽光電科技有限公司Wuhan Sunshine Optoelectronics Tech Co Ltd | 1,2,4−チアジアゾール系化合物及びその製造方法と使用 |
| JP7553009B2 (ja) | 2019-04-05 | 2024-09-18 | 三星ディスプレイ株式會社 | 有機電界発光素子及び有機電界発光素子用化合物 |
| JP2020172483A (ja) * | 2019-04-05 | 2020-10-22 | 三星ディスプレイ株式會社Samsung Display Co.,Ltd. | 有機電界発光素子及び有機電界発光素子用化合物 |
| JP2022008036A (ja) * | 2020-04-03 | 2022-01-13 | 株式会社半導体エネルギー研究所 | アリールアミン化合物、正孔輸送層用材料、正孔注入層用材料、発光デバイス、発光装置、電子機器および照明装置 |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20170005853A (ko) | 2017-01-16 |
| WO2015175678A1 (en) | 2015-11-19 |
| CN106661001A (zh) | 2017-05-10 |
| US20170244049A1 (en) | 2017-08-24 |
| CN106661001A8 (zh) | 2017-07-07 |
| EP3143100A1 (en) | 2017-03-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2017518281A (ja) | 有機発光ダイオード材料 | |
| JP7735484B2 (ja) | 発光素子、発光装置、照明装置および電子機器 | |
| Oda et al. | Multiple resonance effect-induced sky-blue thermally activated delayed fluorescence with a narrow emission band | |
| WO2017011531A2 (en) | Organic light-emitting diode materials | |
| Zhao et al. | Cyclometalated platinum complexes with aggregation-induced phosphorescence emission behavior and highly efficient electroluminescent ability | |
| JP6374329B2 (ja) | 有機エレクトロルミネッセンス素子、有機エレクトロルミネッセンス素子用材料、および電子機器 | |
| TW201739751A (zh) | 延遲螢光有機電場發光元件 | |
| Ma et al. | Rational utilization of intramolecular hydrogen bonds to achieve blue TADF with EQEs of nearly 30% and single emissive layer All-TADF WOLED | |
| TW202144375A (zh) | 發光元件 | |
| Lee et al. | Simple molecular-engineering approach for enhancing orientation and outcoupling efficiency of thermally activated delayed fluorescent emitters without red-shifting emission | |
| KR20120039470A (ko) | 발광층용 재료 및 이것을 사용한 유기 전계 발광 소자 | |
| Chen et al. | Ultrapure blue phosphorescent organic light-emitting diodes employing a twisted Pt (II) complex | |
| WO2017176841A1 (en) | Compounds for organic light emitting diode materials | |
| Li et al. | Selectively modulating triplet exciton formation in host materials for highly efficient blue electrophosphorescence | |
| Chen et al. | Ratiometric tuning of luminescence: interplay between the locally excited and interligand charge-transfer states in pyrazolate-based boron compounds | |
| KR20250006322A (ko) | 화합물, 유기 일렉트로루미네센스 소자용 재료, 유기 일렉트로루미네센스 소자 및 전자 기기 | |
| Fu et al. | An efficient and weak efficiency-roll-off near-infrared (NIR) polymer light-emitting diode (PLED) based on a PVK-supported Zn 2+–Yb 3+-containing metallopolymer | |
| JP7269602B2 (ja) | 多環芳香族化合物およびその多量体 | |
| KR102341609B1 (ko) | 유기 일렉트로루미네센스 소자 및 전자 기기 | |
| Lee et al. | Silicon-based carbazole and oxadiazole hybrid as a bipolar host material for phosphorescent organic light-emitting diodes | |
| KR20230156781A (ko) | 유기 일렉트로루미네센스 소자 및 전자 기기 | |
| Istiqomah et al. | Impact of π-Expanded Boron-Carbonyl Hybrid Acceptors on TADF Properties: Controlling Local Triplet Excited States and Unusual Emission Tuning | |
| Viet et al. | Synthesis and characterization of europium (III), terbium (III) complexes and their mixture for making white light emission powder | |
| CN115594656A (zh) | 电致发光装置用的主体材料 | |
| Pei et al. | Theoretical Study and Design for Thermally Activated Delayed Fluorescence Emitters with Through-Space Charge Transfer from an Acridine Derivative Donor to an O-Bridged Triphenylboron Boroxy Acceptor |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180509 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20180509 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20181227 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20190118 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20190409 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190617 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20190719 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20200302 |