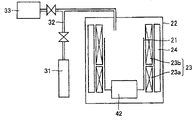
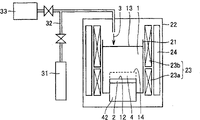
CN100550303C - 第ⅲ族氮化物晶体及其制备方法 - Google Patents
第ⅲ族氮化物晶体及其制备方法 Download PDFInfo
- Publication number
- CN100550303C CN100550303C CNB2004100896735A CN200410089673A CN100550303C CN 100550303 C CN100550303 C CN 100550303C CN B2004100896735 A CNB2004100896735 A CN B2004100896735A CN 200410089673 A CN200410089673 A CN 200410089673A CN 100550303 C CN100550303 C CN 100550303C
- Authority
- CN
- China
- Prior art keywords
- group iii
- melt
- iii nitride
- crystal
- nitrogen
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000013078 crystal Substances 0.000 title claims abstract description 409
- 150000004767 nitrides Chemical class 0.000 title claims abstract description 199
- 238000002360 preparation method Methods 0.000 title claims abstract description 35
- 239000000155 melt Substances 0.000 claims abstract description 176
- 238000006243 chemical reaction Methods 0.000 claims abstract description 82
- 239000000126 substance Substances 0.000 claims abstract description 77
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 claims abstract description 74
- 238000000034 method Methods 0.000 claims abstract description 74
- 239000003054 catalyst Substances 0.000 claims abstract description 37
- 229910052757 nitrogen Inorganic materials 0.000 claims abstract description 30
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 claims description 84
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 30
- 239000001301 oxygen Substances 0.000 claims description 30
- 229910052760 oxygen Inorganic materials 0.000 claims description 30
- 239000000758 substrate Substances 0.000 claims description 28
- 238000010438 heat treatment Methods 0.000 claims description 25
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 claims description 21
- 239000010703 silicon Substances 0.000 claims description 21
- 229910052710 silicon Inorganic materials 0.000 claims description 21
- 239000002019 doping agent Substances 0.000 claims description 19
- 238000004519 manufacturing process Methods 0.000 claims description 16
- 239000007789 gas Substances 0.000 claims description 13
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 12
- 229910002804 graphite Inorganic materials 0.000 claims description 12
- 239000010439 graphite Substances 0.000 claims description 12
- 230000001678 irradiating effect Effects 0.000 claims description 7
- 239000007788 liquid Substances 0.000 claims description 7
- 150000001875 compounds Chemical class 0.000 claims description 6
- 230000008569 process Effects 0.000 claims description 6
- 230000003647 oxidation Effects 0.000 claims description 5
- 238000007254 oxidation reaction Methods 0.000 claims description 5
- -1 nitride compound Chemical class 0.000 claims description 2
- 238000002203 pretreatment Methods 0.000 claims 1
- 239000011734 sodium Substances 0.000 description 36
- 238000001816 cooling Methods 0.000 description 17
- 239000010410 layer Substances 0.000 description 16
- 229910001873 dinitrogen Inorganic materials 0.000 description 14
- 239000000463 material Substances 0.000 description 14
- 238000010586 diagram Methods 0.000 description 10
- 229910052594 sapphire Inorganic materials 0.000 description 9
- 239000010980 sapphire Substances 0.000 description 9
- 238000004090 dissolution Methods 0.000 description 8
- 229910052783 alkali metal Inorganic materials 0.000 description 5
- 230000000052 comparative effect Effects 0.000 description 5
- 210000002381 plasma Anatomy 0.000 description 5
- 239000000843 powder Substances 0.000 description 5
- 150000001340 alkali metals Chemical class 0.000 description 4
- 239000012535 impurity Substances 0.000 description 4
- 230000001737 promoting effect Effects 0.000 description 4
- 229910052723 transition metal Inorganic materials 0.000 description 4
- 150000003624 transition metals Chemical class 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 3
- 229910052782 aluminium Inorganic materials 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 238000009413 insulation Methods 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- KKCBUQHMOMHUOY-UHFFFAOYSA-N sodium oxide Chemical compound [O-2].[Na+].[Na+] KKCBUQHMOMHUOY-UHFFFAOYSA-N 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 238000002834 transmittance Methods 0.000 description 3
- 229910018084 Al-Fe Inorganic materials 0.000 description 2
- 229910018192 Al—Fe Inorganic materials 0.000 description 2
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- 238000002441 X-ray diffraction Methods 0.000 description 2
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 2
- 229910052804 chromium Inorganic materials 0.000 description 2
- 238000009792 diffusion process Methods 0.000 description 2
- 238000007716 flux method Methods 0.000 description 2
- 229910052733 gallium Inorganic materials 0.000 description 2
- 239000011261 inert gas Substances 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- 229910052748 manganese Inorganic materials 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 238000001004 secondary ion mass spectrometry Methods 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 239000010935 stainless steel Substances 0.000 description 2
- 229910001220 stainless steel Inorganic materials 0.000 description 2
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 238000009529 body temperature measurement Methods 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 238000000151 deposition Methods 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 229910001026 inconel Inorganic materials 0.000 description 1
- 229910052738 indium Inorganic materials 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 239000010453 quartz Substances 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- 229910001948 sodium oxide Inorganic materials 0.000 description 1
- 239000002344 surface layer Substances 0.000 description 1
- 239000010409 thin film Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C30—CRYSTAL GROWTH
- C30B—SINGLE-CRYSTAL GROWTH; UNIDIRECTIONAL SOLIDIFICATION OF EUTECTIC MATERIAL OR UNIDIRECTIONAL DEMIXING OF EUTECTOID MATERIAL; REFINING BY ZONE-MELTING OF MATERIAL; PRODUCTION OF A HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; SINGLE CRYSTALS OR HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; AFTER-TREATMENT OF SINGLE CRYSTALS OR A HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; APPARATUS THEREFOR
- C30B29/00—Single crystals or homogeneous polycrystalline material with defined structure characterised by the material or by their shape
- C30B29/10—Inorganic compounds or compositions
- C30B29/38—Nitrides
-
- C—CHEMISTRY; METALLURGY
- C30—CRYSTAL GROWTH
- C30B—SINGLE-CRYSTAL GROWTH; UNIDIRECTIONAL SOLIDIFICATION OF EUTECTIC MATERIAL OR UNIDIRECTIONAL DEMIXING OF EUTECTOID MATERIAL; REFINING BY ZONE-MELTING OF MATERIAL; PRODUCTION OF A HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; SINGLE CRYSTALS OR HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; AFTER-TREATMENT OF SINGLE CRYSTALS OR A HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; APPARATUS THEREFOR
- C30B9/00—Single-crystal growth from melt solutions using molten solvents
-
- C—CHEMISTRY; METALLURGY
- C30—CRYSTAL GROWTH
- C30B—SINGLE-CRYSTAL GROWTH; UNIDIRECTIONAL SOLIDIFICATION OF EUTECTIC MATERIAL OR UNIDIRECTIONAL DEMIXING OF EUTECTOID MATERIAL; REFINING BY ZONE-MELTING OF MATERIAL; PRODUCTION OF A HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; SINGLE CRYSTALS OR HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; AFTER-TREATMENT OF SINGLE CRYSTALS OR A HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; APPARATUS THEREFOR
- C30B29/00—Single crystals or homogeneous polycrystalline material with defined structure characterised by the material or by their shape
- C30B29/10—Inorganic compounds or compositions
- C30B29/40—AIIIBV compounds wherein A is B, Al, Ga, In or Tl and B is N, P, As, Sb or Bi
- C30B29/403—AIII-nitrides
-
- C—CHEMISTRY; METALLURGY
- C30—CRYSTAL GROWTH
- C30B—SINGLE-CRYSTAL GROWTH; UNIDIRECTIONAL SOLIDIFICATION OF EUTECTIC MATERIAL OR UNIDIRECTIONAL DEMIXING OF EUTECTOID MATERIAL; REFINING BY ZONE-MELTING OF MATERIAL; PRODUCTION OF A HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; SINGLE CRYSTALS OR HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; AFTER-TREATMENT OF SINGLE CRYSTALS OR A HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; APPARATUS THEREFOR
- C30B29/00—Single crystals or homogeneous polycrystalline material with defined structure characterised by the material or by their shape
- C30B29/10—Inorganic compounds or compositions
- C30B29/40—AIIIBV compounds wherein A is B, Al, Ga, In or Tl and B is N, P, As, Sb or Bi
- C30B29/403—AIII-nitrides
- C30B29/406—Gallium nitride
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Crystallography & Structural Chemistry (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Crystals, And After-Treatments Of Crystals (AREA)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2003373025 | 2003-10-31 | ||
| JP2003373025 | 2003-10-31 | ||
| JP2004195666 | 2004-07-01 | ||
| JP2004195666A JP4534631B2 (ja) | 2003-10-31 | 2004-07-01 | Iii族窒化物結晶の製造方法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN1612295A CN1612295A (zh) | 2005-05-04 |
| CN100550303C true CN100550303C (zh) | 2009-10-14 |
Family
ID=34467811
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNB2004100896735A Expired - Fee Related CN100550303C (zh) | 2003-10-31 | 2004-10-29 | 第ⅲ族氮化物晶体及其制备方法 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20050098090A1 (enExample) |
| EP (2) | EP1538241B1 (enExample) |
| JP (1) | JP4534631B2 (enExample) |
| KR (5) | KR20050041994A (enExample) |
| CN (1) | CN100550303C (enExample) |
| DE (1) | DE602004018452D1 (enExample) |
| TW (1) | TWI399796B (enExample) |
Families Citing this family (47)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005298269A (ja) * | 2004-04-12 | 2005-10-27 | Sumitomo Electric Ind Ltd | Iii族窒化物結晶基板およびその製造方法ならびにiii族窒化物半導体デバイス |
| EP1930294A4 (en) * | 2005-08-24 | 2012-12-26 | Mitsubishi Chem Corp | METHOD FOR PRODUCING GROUP 13 METAL NITRIDE CRYSTAL, METHOD FOR PRODUCING A SEMICONDUCTOR DEVICE, AND SOLUTION AND MELT USED THEREOF |
| JP4788524B2 (ja) * | 2005-08-24 | 2011-10-05 | 三菱化学株式会社 | 第13族金属窒化物結晶の製造方法およびこれらの製造方法に用いる溶液と融液 |
| EP1775356A3 (en) | 2005-10-14 | 2009-12-16 | Ricoh Company, Ltd. | Crystal growth apparatus and manufacturing method of group III nitride crystal |
| JP4863264B2 (ja) * | 2006-03-17 | 2012-01-25 | 豊田合成株式会社 | 半導体結晶の製造方法 |
| WO2007057892A2 (en) * | 2005-11-17 | 2007-05-24 | Mosaic Crystals Ltd. | Gan crystal sheet |
| WO2007108338A1 (ja) | 2006-03-23 | 2007-09-27 | Ngk Insulators, Ltd. | 窒化物単結晶の製造方法および装置 |
| CN101405438B (zh) | 2006-03-23 | 2012-06-27 | 日本碍子株式会社 | 氮化物单晶的制造装置 |
| JP5187848B2 (ja) | 2006-03-23 | 2013-04-24 | 日本碍子株式会社 | 単結晶の製造方法 |
| JP2007254201A (ja) * | 2006-03-23 | 2007-10-04 | Ngk Insulators Ltd | 単結晶の製造方法 |
| JP4827107B2 (ja) | 2006-03-24 | 2011-11-30 | 日本碍子株式会社 | 窒化物単結晶の製造方法 |
| JP4936310B2 (ja) * | 2006-04-07 | 2012-05-23 | 豊田合成株式会社 | Iii族窒化物系化合物半導体の製造装置 |
| JP2007284267A (ja) * | 2006-04-13 | 2007-11-01 | Sumitomo Electric Ind Ltd | GaN結晶の製造方法 |
| JP4720672B2 (ja) * | 2006-08-14 | 2011-07-13 | 住友金属工業株式会社 | 窒化アルミニウム単結晶の製造方法 |
| JPWO2008117571A1 (ja) * | 2007-03-26 | 2010-07-15 | 日本碍子株式会社 | 窒化物単結晶の製造方法 |
| JP5235864B2 (ja) | 2007-03-27 | 2013-07-10 | 日本碍子株式会社 | 窒化物単結晶の製造方法 |
| JP4881496B2 (ja) * | 2007-04-24 | 2012-02-22 | 豊田合成株式会社 | Iii族窒化物系化合物半導体の製造方法 |
| US8361222B2 (en) | 2007-04-24 | 2013-01-29 | Toyoda Gosei Co., Ltd. | Method for producing group III nitride-based compound semiconductor |
| US9281438B2 (en) * | 2007-09-28 | 2016-03-08 | Ricoh Company, Ltd. | Process for producing group III element nitride crystal and apparatus for producing group III element nitride crystal |
| JP4941448B2 (ja) * | 2007-10-26 | 2012-05-30 | 豊田合成株式会社 | Iii族窒化物半導体製造装置 |
| JP2009114035A (ja) * | 2007-11-08 | 2009-05-28 | Toyoda Gosei Co Ltd | Iii族窒化物半導体製造装置および製造方法 |
| JP5560528B2 (ja) * | 2008-01-28 | 2014-07-30 | 住友電気工業株式会社 | Iii族窒化物単結晶インゴットの製造方法、及びiii族窒化物単結晶基板の製造方法 |
| JP5229792B2 (ja) * | 2008-03-25 | 2013-07-03 | 国立大学法人大阪大学 | Iii族元素窒化物結晶の製造方法およびそれにより得られるiii族元素窒化物結晶 |
| JP4886722B2 (ja) * | 2008-03-25 | 2012-02-29 | 日本碍子株式会社 | 窒化物単結晶の製造方法 |
| JP5056688B2 (ja) * | 2008-09-15 | 2012-10-24 | 豊田合成株式会社 | Iii族窒化物半導体結晶の製造方法 |
| JP5310257B2 (ja) * | 2009-05-21 | 2013-10-09 | 株式会社リコー | 窒化物結晶製造方法 |
| JP2010077022A (ja) * | 2009-11-30 | 2010-04-08 | Sumitomo Electric Ind Ltd | Iii族窒化物結晶基板およびその製造方法ならびにiii族窒化物半導体デバイス |
| JP5729182B2 (ja) | 2010-08-31 | 2015-06-03 | 株式会社リコー | n型III族窒化物単結晶の製造方法、n型III族窒化物単結晶および結晶基板 |
| CN102618920A (zh) * | 2012-04-25 | 2012-08-01 | 浙江华友电子有限公司 | 一种单晶炉熔料过程中的热能控制方法 |
| JP5522204B2 (ja) * | 2012-06-14 | 2014-06-18 | 豊田合成株式会社 | Iii族窒化物半導体結晶の製造方法 |
| CN103603031A (zh) * | 2013-12-06 | 2014-02-26 | 北京大学东莞光电研究院 | 一种通过调控釜体内部流场制备高质量单晶体材料的方法 |
| CN106460228B (zh) * | 2014-03-03 | 2019-04-26 | 国立大学法人大阪大学 | Iii族氮化物结晶的制造方法及iii族氮化物结晶制造装置 |
| JP6534030B2 (ja) * | 2014-08-28 | 2019-06-26 | 国立大学法人名古屋大学 | AlN単結晶の作製方法 |
| CN104878451B (zh) * | 2015-06-16 | 2017-07-28 | 北京大学东莞光电研究院 | 一种氮化物单晶生长装置 |
| CN111052415B (zh) | 2017-08-24 | 2023-02-28 | 日本碍子株式会社 | 13族元素氮化物层、自立基板以及功能元件 |
| WO2019038892A1 (ja) | 2017-08-24 | 2019-02-28 | 日本碍子株式会社 | 13族元素窒化物層、自立基板および機能素子 |
| WO2019039189A1 (ja) * | 2017-08-24 | 2019-02-28 | 日本碍子株式会社 | 13族元素窒化物層、自立基板および機能素子 |
| JP6851486B2 (ja) * | 2017-08-24 | 2021-03-31 | 日本碍子株式会社 | 13族元素窒化物層、自立基板および機能素子 |
| US11309455B2 (en) | 2017-08-24 | 2022-04-19 | Ngk Insulators, Ltd. | Group 13 element nitride layer, free-standing substrate and functional element |
| WO2019039208A1 (ja) * | 2017-08-24 | 2019-02-28 | 日本碍子株式会社 | 13族元素窒化物層、自立基板および機能素子 |
| CN111052413B (zh) * | 2017-08-24 | 2023-08-15 | 日本碍子株式会社 | 13族元素氮化物层、自立基板以及功能元件 |
| JP6851485B2 (ja) * | 2017-08-24 | 2021-03-31 | 日本碍子株式会社 | 13族元素窒化物層、自立基板および機能素子 |
| JP7051094B2 (ja) * | 2018-05-01 | 2022-04-11 | 国立大学法人東北大学 | 窒化アルミニウム結晶の製造方法 |
| CN109706524A (zh) * | 2019-03-07 | 2019-05-03 | 中国电子科技集团公司第四十六研究所 | 一种降低氮化镓单晶氧原子浓度的方法 |
| JP7063293B2 (ja) * | 2019-03-18 | 2022-05-09 | 豊田合成株式会社 | Iii族窒化物半導体の製造方法 |
| US11280024B2 (en) * | 2019-03-18 | 2022-03-22 | Toyoda Gosei Co., Ltd. | Method for producing a group III nitride semiconductor by controlling the oxygen concentration of the furnace internal atmosphere |
| JP7147644B2 (ja) * | 2019-03-18 | 2022-10-05 | 豊田合成株式会社 | Iii族窒化物半導体の製造方法 |
Family Cites Families (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4190630A (en) * | 1978-01-03 | 1980-02-26 | Vsesojuzny Nauchno-Isslekovatelsky Institut Monokristallov Stsintillyatsionnykh Materialov I Osobo Chistykh Khimicheskikh Veschestv | Apparatus for pulling single crystals from melt |
| JPH07277897A (ja) * | 1994-04-04 | 1995-10-24 | Katsutoshi Yoneya | 窒化アルミニウム単結晶の合成方法 |
| US5868837A (en) | 1997-01-17 | 1999-02-09 | Cornell Research Foundation, Inc. | Low temperature method of preparing GaN single crystals |
| US6270569B1 (en) * | 1997-06-11 | 2001-08-07 | Hitachi Cable Ltd. | Method of fabricating nitride crystal, mixture, liquid phase growth method, nitride crystal, nitride crystal powders, and vapor phase growth method |
| TW428331B (en) * | 1998-05-28 | 2001-04-01 | Sumitomo Electric Industries | Gallium nitride single crystal substrate and method of producing the same |
| US6562124B1 (en) * | 1999-06-02 | 2003-05-13 | Technologies And Devices International, Inc. | Method of manufacturing GaN ingots |
| US6592663B1 (en) * | 1999-06-09 | 2003-07-15 | Ricoh Company Ltd. | Production of a GaN bulk crystal substrate and a semiconductor device formed on a GaN bulk crystal substrate |
| JP4011828B2 (ja) | 1999-06-09 | 2007-11-21 | 株式会社リコー | Iii族窒化物結晶の結晶成長方法及びiii族窒化物結晶の製造方法 |
| JP3929657B2 (ja) | 1999-09-29 | 2007-06-13 | 株式会社リコー | 結晶成長方法およびiii族窒化物結晶の製造方法 |
| JP4094780B2 (ja) | 1999-08-24 | 2008-06-04 | 株式会社リコー | 結晶成長方法および結晶成長装置並びにiii族窒化物結晶の製造方法および結晶製造装置 |
| JP2001128587A (ja) | 1999-11-05 | 2001-05-15 | Kochi Prefecture | 付着性水産生物飼育装置およびこれを用いた飼育方法 |
| JP2001338887A (ja) * | 2000-05-26 | 2001-12-07 | Sumitomo Electric Ind Ltd | Iii−v族窒化物系半導体の成長方法及び成長装置 |
| US20020148402A1 (en) * | 2001-04-13 | 2002-10-17 | Sindo Kou | Growing of homogeneous crystals by bottom solid feeding |
| US7001457B2 (en) * | 2001-05-01 | 2006-02-21 | Ricoh Company, Ltd. | Crystal growth method, crystal growth apparatus, group-III nitride crystal and group-III nitride semiconductor device |
| PL219109B1 (pl) * | 2001-06-06 | 2015-03-31 | Ammono Spółka Z Ograniczoną Odpowiedzialnością | Sposób otrzymywania objętościowego monokrystalicznego azotku zawierającego gal oraz urządzenie do otrzymywania objętościowego monokrystalicznego azotku zawierającego gal |
| US6488767B1 (en) * | 2001-06-08 | 2002-12-03 | Advanced Technology Materials, Inc. | High surface quality GaN wafer and method of fabricating same |
| JP2003137698A (ja) * | 2001-10-26 | 2003-05-14 | Ulvac Japan Ltd | Iii−v族半導体材料 |
| US6949140B2 (en) * | 2001-12-05 | 2005-09-27 | Ricoh Company, Ltd. | Crystal growth method, crystal growth apparatus, group-III nitride crystal and group-III nitride semiconductor device |
| US7097707B2 (en) * | 2001-12-31 | 2006-08-29 | Cree, Inc. | GaN boule grown from liquid melt using GaN seed wafers |
| US7063741B2 (en) * | 2002-03-27 | 2006-06-20 | General Electric Company | High pressure high temperature growth of crystalline group III metal nitrides |
| JP4216612B2 (ja) * | 2003-01-29 | 2009-01-28 | 株式会社リコー | Iii族窒化物結晶の製造方法 |
| JP4248276B2 (ja) * | 2003-03-17 | 2009-04-02 | 株式会社リコー | Iii族窒化物の結晶製造方法 |
-
2004
- 2004-07-01 JP JP2004195666A patent/JP4534631B2/ja not_active Expired - Fee Related
- 2004-10-01 EP EP04023416A patent/EP1538241B1/en not_active Expired - Lifetime
- 2004-10-01 DE DE602004018452T patent/DE602004018452D1/de not_active Expired - Lifetime
- 2004-10-01 EP EP08003737A patent/EP1942211B1/en not_active Expired - Lifetime
- 2004-10-06 TW TW093130254A patent/TWI399796B/zh not_active IP Right Cessation
- 2004-10-29 CN CNB2004100896735A patent/CN100550303C/zh not_active Expired - Fee Related
- 2004-10-30 KR KR1020040087631A patent/KR20050041994A/ko not_active Ceased
- 2004-11-01 US US10/904,249 patent/US20050098090A1/en not_active Abandoned
-
2011
- 2011-03-14 KR KR1020110022579A patent/KR101122327B1/ko not_active Expired - Fee Related
- 2011-03-14 KR KR1020110022578A patent/KR101187999B1/ko not_active Expired - Fee Related
- 2011-03-14 KR KR1020110022580A patent/KR101117364B1/ko not_active Expired - Fee Related
- 2011-03-14 KR KR1020110022581A patent/KR101075931B1/ko not_active Expired - Fee Related
Non-Patent Citations (1)
| Title |
|---|
| Conditions for seeded growth of GaN crystals bythe Na flux method. Masato Aoki, Hisanori Yamane, MasahikoShimada,SeijiSarayama, Francis J. DiSalvo.Materials Letters,Vol.56 No.5. 2002 * |
Also Published As
| Publication number | Publication date |
|---|---|
| TW200515488A (en) | 2005-05-01 |
| EP1538241A3 (en) | 2005-06-22 |
| KR101075931B1 (ko) | 2011-10-21 |
| CN1612295A (zh) | 2005-05-04 |
| KR101117364B1 (ko) | 2012-03-07 |
| KR20050041994A (ko) | 2005-05-04 |
| KR20110043560A (ko) | 2011-04-27 |
| KR101187999B1 (ko) | 2012-10-08 |
| EP1538241A2 (en) | 2005-06-08 |
| EP1942211A1 (en) | 2008-07-09 |
| JP2005154254A (ja) | 2005-06-16 |
| JP4534631B2 (ja) | 2010-09-01 |
| EP1538241B1 (en) | 2008-12-17 |
| TWI399796B (zh) | 2013-06-21 |
| EP1942211B1 (en) | 2011-09-14 |
| KR20110043563A (ko) | 2011-04-27 |
| US20050098090A1 (en) | 2005-05-12 |
| KR20110043562A (ko) | 2011-04-27 |
| KR20110043561A (ko) | 2011-04-27 |
| DE602004018452D1 (de) | 2009-01-29 |
| KR101122327B1 (ko) | 2012-03-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN100550303C (zh) | 第ⅲ族氮化物晶体及其制备方法 | |
| US8461071B2 (en) | Polycrystalline group III metal nitride with getter and method of making | |
| CN101586253B (zh) | N型ⅲ族氮化物基化合物半导体及其制造方法 | |
| CN102383181B (zh) | 制备n-型Ⅲ族氮化物单晶的方法、所述单晶、和晶体基板 | |
| WO2010060034A1 (en) | METHODS FOR PRODUCING GaN NUTRIENT FOR AMMONOTHERMAL GROWTH | |
| US7294199B2 (en) | Nitride single crystal and producing method thereof | |
| CN104047054A (zh) | 低碳的iii族元素氮化物晶体 | |
| JP5428706B2 (ja) | SiC単結晶の製造方法 | |
| CN113818085B (zh) | 助熔剂法均匀化生长氮化物单晶的系统及方法 | |
| JP5299213B2 (ja) | Iii族窒化物結晶の製造方法 | |
| WO2018047844A1 (ja) | 窒化ガリウム積層体の製造方法 | |
| CN101243011B (zh) | 第13族金属氮化物结晶的制造方法、半导体器件的制造方法和这些制造方法中使用的溶液和熔融液 | |
| JP2004231467A (ja) | 窒化物単結晶およびその製造方法 | |
| EP1612300B1 (en) | Method for producing a single crystal of AlN. | |
| CN119553363A (zh) | 一种环境友好型的气相生长iii族氮化物晶体的方法 | |
| JP2014152066A (ja) | 窒化ガリウム単結晶の成長方法 | |
| CN101346309A (zh) | 多晶硅的制造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| CF01 | Termination of patent right due to non-payment of annual fee | ||
| CF01 | Termination of patent right due to non-payment of annual fee |
Granted publication date: 20091014 Termination date: 20171029 |