EP0329822A2 - Procédé d'amplification d'acides nucléiques - Google Patents
Procédé d'amplification d'acides nucléiques Download PDFInfo
- Publication number
- EP0329822A2 EP0329822A2 EP88113948A EP88113948A EP0329822A2 EP 0329822 A2 EP0329822 A2 EP 0329822A2 EP 88113948 A EP88113948 A EP 88113948A EP 88113948 A EP88113948 A EP 88113948A EP 0329822 A2 EP0329822 A2 EP 0329822A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- sequence
- primer
- dna
- template
- nucleic acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/70—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving virus or bacteriophage
- C12Q1/701—Specific hybridization probes
- C12Q1/702—Specific hybridization probes for retroviruses
- C12Q1/703—Viruses associated with AIDS
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6844—Nucleic acid amplification reactions
- C12Q1/6865—Promoter-based amplification, e.g. nucleic acid sequence amplification [NASBA], self-sustained sequence replication [3SR] or transcription-based amplification system [TAS]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6888—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for detection or identification of organisms
- C12Q1/689—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for detection or identification of organisms for bacteria
Definitions
- This invention relates to a process for amplifying a specific nucleic acid sequence.
- the phrase "specific nucleic acid sequence” means a single stranded or double stranded nucleic acid which one wishes to amplify; "sample” means a mixture containing nucleic acids; "sufficiently complementary” means that two nucleic acids, a primer and a template, are capable of specific interaction which allows efficient, primer-dependent and template-directed synthesis of DNA, under given conditions of ionic strength and temperature.
- nucleic acid probes are highly specific, it is preferable in some situations to probe the nucleic acid sequence itself rather than the protein produced by the nucleic acid sequence.
- a diagnostic method based solely on protein detection would be unreliable for determining the presence of infectious particles of hepatitis B virus, due to the presence of significant levels of non-infectious antigen particles which lack the DNA genome.
- the various subtypes of human papilloma virus found in either pre-cancerous or benign cervical tumors can be distinguished only by the use of nucleic acid probe hybridization.
- the microbiology of AIDS makes it certain that an assay based on the presence of AIDS specific nucleic acid sequence would be superior as a diagnostic.
- One method to amplify is to 'grow out' the sample, that is, to arrange conditions so that the living biological material present in the sample can replicate itself. Replication increases the quantity of nucleic acid sequences to detectable levels.
- food samples In the food industry, for example, in order to test processed food for the food-poisoning bacteria Salmonella, food samples must be incubated for a number of days to increase the quantity of nucleic acids. In clinical samples, pathogens must also be allowed to increase their number by growing out over some considerable time.
- United States Patent No. 4,683,195 issued on July 28, 1987 to Cetus Corporation and United States Patent No. 4,683,202 issued on July 28, 1987 to Cetus Corporation are each directed to a process for amplifying a target nucleic acid sequence contained in a sample.
- United States Patent No. 4,683,195 relates to a process in which a sample suspected of containing a target nucleic acid sequence is treated with oligonucleotide primers such that a primer extension product is synthesized which in turn serves as a template, resulting in amplification of the target nucleic acid sequence.
- the primer extension product is separated from the template in the preferred embodiment using heat denaturation.
- 4,683,202 relates to a process for amplifying a target nucleic acid sequence having two separate complementary strands.
- the process includes treating the strands with primers to synthesize extension products, separating the primer extension products from the templates, and in turn using the primer extension products as templates.
- each cycle of the amplification process takes place by the synthesis from a first template, of a second template, the second template in turn is used to synthesize the first template. This procedure is repeated, thus, each cycle of the amplification process is based on the synthesis of one product from one substrate.
- This invention relates to an amplification process which is expedient and requires less participation and fewer manipulations by the user of the process than conventional amplification processes.
- the amplification takes place at a relatively constant ambient temperature.
- each cycle of the process generates a plurality of copies of product from one substrate.
- the amplification process of this invention may be used to increase the quantity of a specific nucleic acid thus circumventing the copy number problem. Hence, probe assays may be more readily used.
- the amplification process could also be used to increase the purity of a specific nucleic acid sequence as a substitute for conventional cloning methodology.
- a process for amplifying a specific nucleic acid sequence is used.
- the process involves:
- a sequence of the first or the second primer is sufficiently complementary to a sequence of the specific nucleic acid sequence and a sequence of the first or the second primer is sufficiently homologous to a sequence of the specific nucleic acid sequence.
- a 3′ end of the first primer is oriented towards a 3′ end of the second primer on complementary strands.
- the second primer of DNA has a sequence at its 3′ end which is sufficiently complementary to the DNA sequence of the second template.
- the second primer has at its 5′ end an antisense sequence of a promoter and an antisense sequence of a transcription initiation site for a RNA polymerase.
- the third DNA sequence covalently attached to the second template is complementary to the DNA sequence at the 5′ end of the second primer.
- a process for amplifying a specific nucleic acid sequence involves combining a first primer, a second primer, ribonuclease H, a RNA-directed DNA polymerase, a DNA-directed DNA polymerase, a RNA polymerase, ribonucleoside triphosphates and deoxyribonucleoside triphosphates with a sample.
- the first primer of DNA has a sequence which is sufficiently complementary to a first template of RNA.
- the second primer of DNA has a sequence which is sufficiently complementary to a second template of DNA, and an antisense sequence of a promoter and an antisense sequence of a transcription initiation site which are recognized as substrate by the RNA polymerase.
- a sequence of the first primer or the second primer is sufficiently complementary to a sequence of the specific nucleic acid sequence and a sequence of the first primer or the second primer is sufficiently homologous to a sequence of the specific nucleic acid.
- a 3′ end of the first primer is oriented towards a 3′ end of the second primer on complementary strands.
- a process for amplifying a specific nucleic acid sequence involves adding a first primer, a second primer, avian myoblastosis viral polymerase, E. coli ribonuclease H, bacteriophage T7 RNA polymerase, ribonucleoside triphosphates and deoxyribonucleoside triphosphates to a sample.
- the first primer of DNA has a sequence which is sufficiently complementary to a first template of RNA.
- the second primer of DNA has a sequence which is sufficiently complementary to a second template of DNA, and an antisense sequence of a promoter and an antisense sequence of a transcription initiation site which are recognized as substrate by T7 RNA polymerase.
- a sequence of the first primer or the second primer is sufficiently complementary to a sequence of the specific nucleic acid sequence and a sequence of the first primer or the second primer is sufficiently homologous to a sequence of the specific nucleic acid sequence.
- a 3′ end of the first primer is oriented towards a 3′ end of the second primer on complementary strands.
- This invention relates to a process for amplifying a specific nucleic acid sequence.
- the amplification involves the alternate synthesis of DNA and RNA and is generally illustrated in Figure 1.
- single-stranded RNA is converted to single-stranded DNA which in turn is converted to a functional template for the synthesis of a plurality of copies of the original single-stranded RNA.
- a first primer and a second primer are used in the amplification process.
- a sequence of the first primer or the second primer is sufficiently complementary to a sequence of the specific nucleic acid sequence and a sequence of the first or the second primer is sufficiently homologous to a sequence of the specific nucleic acid sequence.
- both the first primer and second primer are sufficiently complementary and sufficiently homologous to a sequence of the specific nucleic acid sequence, for example, if the specific nucleic acid sequence is double stranded DNA.
- the RNA is converted to single-stranded DNA by hybridizing an oligonucleotide primer (the first primer) to the RNA (the first template) and synthesizing a complementary strand of DNA from the first primer, (the first DNA sequence) by using a RNA-directed DNA polymerase.
- the resulting single-stranded DNA (the second template) is separated from the first template by, for example, hydrolysis of the first template and by using a ribonuclease which is specific for RNA-DNA hybrids (for example, ribonuclease H).
- the second template is converted to a form which is capable of RNA synthesis by hybridizing a synthetic oligonucleotide (the second primer), which contains at its 3′ end a sequence which is sufficiently complementary to the 3′ end of the second template and toward its 5′ end a sequence containing the antisense strand of a promoter and antisense sequence of a transcription initiation site, and by synthesizing a second DNA sequence covalently attached to the 3′ end of the second primer using the second template as a template and synthesizing a third DNA sequence covalently attached to the 3′ end of the second template using the second primer as a template, using DNA-directed DNA polymerase.
- the second primer synthetic oligonucleotide
- the resulting functional derivative of the second template which is a third template, is used for the synthesis of a plurality of copies of RNA, the first template, by using a RNA polymerase which is specific for the promoter and transcription initiation site defined by the second primer.
- Each newly synthesized first template can be converted to further copies of the second template and the third template by repeating the cycle. In addition, repetition of the cycle does not require participation or manipulation by the user.
- the amplification process commences with the addition of a suitable template nucleic acid to the appropriate enzymes, primers, and cofactors under the appropriate reaction conditions.
- This template nucleic acid is in a form which is capable of homogenous and continuous amplification and can function as an intermediate in the cycle set forth in Figure 1.
- the amplification process involves the net consumption of precursors (primers, ribonucleoside triphosphates and deoxyribonucleoside triphosphates) and the net accumulation of products (RNA and DNA).
- precursors primary, ribonucleoside triphosphates and deoxyribonucleoside triphosphates
- RNA and DNA the net accumulation of products
- the processes of RNA and DNA synthesis will proceed asynchronously until sufficient levels of nucleic acids have been synthesized to allow detection.
- the amplification process may be monitored by, for example, the synthesis of a labeled product from a labeled precursor.
- the first primer is an oligodeoxyribonucleotide which has at its 3′ end a sequence which is sufficiently complementary to the 3′ end of the first template.
- the sequence at the 3′ end of the first primer has a particular length and base composition to allow specific and efficient synthesis of the first DNA sequence, under the given conditions of ionic strength and temperature.
- the first primer may be sufficiently complementary to a region internal to the 3′ end of the first template in the first cycle. In subsequent cycles, the 5′ end of the first primer would be complementary to the 3′ end of the first template. It is contemplated that the first primer may be composed partially or completely of nucleotides or nucleotide analogs other than the natural deoxyribonucleotides.
- the 5′ end of the first primer may contain sequences which are not complementary to the first template in the first cycle.
- the non-complementary sequences may be complementary to a nucleic acid which can be immobilized, or to which can be bound a useful non-nucleic acid component, such as a reporter to facilitate detection.
- the non-complementary sequences may include an antisense sequence of a promoter and an antisense sequence of a transcription initiation site, which could be used for the synthesis of RNA. This RNA would be complementary to the first template and could be used as an intermediate in another amplification cycle.
- the second primer is an oligodeoxyribonucleotide which contains at its 3′ end a sequence which is sufficiently complementary to the 3′ end of the second template.
- the second primer has a particular length and base composition to allow specific and efficient synthesis of the second and third DNA sequences, under the given conditions of ionic strength and temperature.
- the second primer contains the antisense sequence of a functional promoter and the antisense sequence of a transcription initiation site. This sequence, when used as a template for synthesis of the third DNA sequence, contains sufficient information to allow specific and efficient binding of a RNA polymerase and initiation of transcription at the desired site.
- the promoter sequence may be derived from the antisense strand of a functional promoter.
- the transcription initiation site may be derived from the 5′ terminal sequence of a natural RNA transcript.
- the 5′-terminal sequence of the second primer is AATTCTAATACGACTCACTATAGGGAG. This sequence contains the antisense sequence of the promoter and the antisense sequence of the transcription initiation site for T7 RNA polymerase.
- the transcription initiation site and promoter for another phage RNA polymerase may be used.
- sequences which are unrelated to the promoter function may be included at the 5′ end of the second primer or between the transcription initiation site and the sequence at the 3′ end which hybridizes to the second template.
- the second primer may be composed partially or completely of nucleotides or nucleotide analogs other than natural deoxyribonucleotides.
- Each enzyme or enzyme preparation should be free of deleterious deoxyribonuclease (“DNase”) activities, such as the 5′ or 3′ exonuclease activities which are often associated with certain DNA polymerases and single-strand or double-strand specific exonuclease or endonucleases.
- DNase deoxyribonuclease
- Each enzyme or enzyme preparation should be free of deleterious ribonuclease (“RNase”) activities, with the exception of the preferred addition of a ribonuclease activity which is specific for hybrids of RNA and DNA (for example, ribonuclease H).
- RNase deleterious ribonuclease
- each enzyme should be reasonably active under the common reaction conditions which are used for the other enzymatic processes, and non-enzymatic processes, such as hybridizing oligonucleotide primers to the RNA or DNA templates.
- RNA-directed RNA polymerase may be used. It should be understood that if alternative RNA polymerases are used, then the necessary changes to the promoter and initiation sequences of the second primer should be made according to the template specificity of the particular RNA polymerase.
- RNA-directed DNA polymerase which is used in this invention may be any enzyme capable of synthesizing DNA from an oligodeoxyribonucleotide primer and a RNA template.
- this enzyme may contain activities for DNA-directed DNA polymerase and RNase H.
- the avian myoblastosis viral polymerase (“AMV reverse transcriptase") is used.
- the RNA-directed DNA polymerase could be from another retrovirus, such as Maloney murine leukemia virus.
- RNA-directed DNA polymerases could be used.
- the DNA-directed DNA polymerase which is used in this invention may be any enzyme capable of synthesizing DNA from an oligodeoxyribonucleotide primer and a DNA template. This enzyme should not contain either 5′- or 3′- exonuclease activities, which are associated with many types of DNA polymerase. In the preferred embodiment, the AMV reverse transcriptase is used. However, other DNA-directed DNA polymerases which naturally lack the 5′- or 3′- exonuclease activities could be used. These could include certain eukaryotic DNA polymerases, such as, DNA polymerase ⁇ or ⁇ those DNA polymerases which could be isolated from a mammalian tissue, such as calf thymus.
- DNA polymerase could be made useful by removing the undesirable exonuclease activities either by alteration of the DNA polymerase gene followed by expression of the altered polymerase in a suitable host cell, or by chemical modification of the DNA polymerase protein.
- Altered versions of DNA polymerase could be made from the Klenow fragment of E. coli DNA polymerase I or the bacteriophage T7 DNA polymerase. It should be understood that such alternative DNA-directed DNA polymerase activities are added to supplement the activity contributed by the RNA-directed DNA polymerase, since in the preferred embodiment, both RNA-directed and DNA-directed DNA polymerase activities are supplied by the same enzyme.
- the RNase H which could be used in this invention may be any enzyme capable of hydrolyzing a RNA which is annealed to a complementary DNA. This enzyme should not be capable of hydrolyzing single or double-stranded RNA or any DNA.
- the E. coli RNase H is used.
- other RNase H enzymes could be used, such as calf thymus RNase H. Since RNase H is an intrinsic activity of AMV reverse transcriptase, the E. coli RNase H will be supplemented in the preferred embodiment by the RNase H of AMV reverse transcriptase.
- any other enzyme capable of separating the second template from the first template could be used.
- the abovementioned enzymes and primers are mixed together in a reaction vessel which contains the necessary buffers and cofactors for both DNA and RNA synthesis.
- the ionic conditions and reaction temperature should be compatible with specific hybridization of the primers to the DNA and RNA templates as is known to those skilled in the art.
- the reaction mixture should be free of such agents which would interfere with the amplification process, specifically substances which could greatly inhibit the activity of the enzymes, interfere with the hybridizing of primers and templates, or degrade non-productively the nucleic acid intermediates and products.
- a labeled precursor may be added to the reaction mixture.
- Amplification is determined by quantitive or qualitative analysis of labeled products, which can be separated from the labeled precursor by using methods known in the art.
- a labeled precursor may be a ribonucleoside triphosphate for detecting RNA synthesis, or a deoxynucleoside triphosphate or an oligonucleotide primer for detecting DNA synthesis.
- the type of label may be a radioisotope or a useful chemical group, such as biotin, a chromophore, a fluorophore, or a hapten which could bind to an antibody, or possibly a protein or an enzyme.
- the labeled products may be separated from the labeled precursors on the basis of solubility, charge, or size.
- the labeled DNA or RNA may be hybridized to a nucleic acid which contains a complementary sequence and which can be immobilized.
- the products of the amplification process may be bound to an immobilized support, hybridized to a nucleic acid probe containing a complementary sequence, and separated from the unhybridized nucleic acid probe which remains in solution.
- the products, DNA or RNA may be bound directly to a solid support by any stable interaction, such as hydrophobic, electrostatic, or covalent interaction.
- the products may contain certain chemical groups, for example, biotin, which may be incorporated into the products during the amplification process to allow binding to an immobilized protein, for example, avidin or streptavidin.
- the products may be hybridized to a nucleic acid which contains a complementary sequence and which can be immobilized.
- the nucleic acid probe would contain a complementary sequence which forms a sufficiently stable interaction with a product of the amplification process to allow binding under the conditions of hybridization and sustained binding under the conditions used for removal of the unhybridized nucleic acid probe.
- the complementary sequence would be derived from that part of the specific nucleic acid sequence which is between the sequences of the first primer and the second primer.
- the nucleic acid probe may be a single-stranded DNA or RNA, or a double-stranded DNA or RNA which can be made single-stranded, or an oligonucleotide which can be composed of deoxyribonucleotides and/or ribonucleotides.
- the nucleic acid probe may contain a chemical group which could covalently bind to a product DNA or RNA under the appropriate conditions.
- the nucleic acid probe may be labeled with a radioisotope or a useful chemical group, such as biotin, a chromophore, a fluorophore, or a hapten which could bind to an antibody.
- the nucleic acid probe could be conjugated to a protein or enzyme, for example, a phosphatase or a peroxidase.
- the nucleic acid probe may contain sequences which would allow in vitro replication of the probe.
- the products of the amplification process may be analyzed by methods which are typically used for nucleic acids that have been enriched by molecular cloning techniques.
- the synthesis of a specific DNA sequence may be detected by digestion of the synthesized DNA with a restriction endonuclease, followed by electrophoretic separation and detection using methods known in the art.
- the sequence of amplified RNA may be determined by DNA synthesis using a RNA-directed DNA polymerase, the first primer, and dideoxynucleoside triphosphates (Stoflet et al., 1988).
- the sequence of the amplified third template may be determined by RNA synthesis using the DNA-directed RNA polymerase used in the amplification process, and 3′-deoxyribonucleoside triphosphates (Axelrod & Kramer, 1985).
- the amplified RNA may encode a polypeptide which could be translated, in vitro . The polypeptide product of the in vitro translation could be analyzed by using an antibody.
- a sample suspected of containing or known to contain the specific nucleic acid sequence is added to the reaction mixture in the form of a template nucleic acid which is capable of homogeneous and continuous amplification and may be any intermediate in the cycle set forth in Figure 1.
- the template nucleic acid may be a single-stranded RNA which contains at its 5′ end a sequence which is sufficiently homologous to that which is at the 3′ end of the second primer, and contains a sequence which is sufficiently complementary to the first primer.
- a template nucleic acid of this form would function as a first template in the amplification process.
- the template nucleic acid may be a single-stranded DNA which contains at its 3′ end a sequence which is sufficiently complementary to at least the 3′ end of the second primer, and contains a sequence which is sufficiently homologous to that which is at the 3′ end of the first primer.
- a template nucleic acid of this form would function as a second template in the amplification process.
- the template nucleic acid may be a double-stranded DNA, one strand of which contains at its 5′ end the entire sequence of the second primer and contains a sequence which is sufficiently complementary to the first primer. The double-stranded DNA functions as a third template in the amplification process.
- a template nucleic acid which could function as a first template could be a naturally occurring RNA or a RNA fragment which could be generated from a larger RNA molecule by using site specific hydrolysis methods known in the art (Shibahara et al., 1987).
- a template nucleic acid which could function as a second template could be generated from a single-stranded DNA or RNA to which has been hybridized an oligonucleotide which is capable of blocking DNA synthesis.
- This blocking oligonucleotide may contain a chemical group, which could covalently bind to the template, under the appropriate conditions.
- DNA synthesis from this blocked template using the first primer could result in a synthesized DNA with the same 3′ end as the second template.
- the original template is RNA, then the resulting DNA-RNA hybrid may be used directly as a template nucleic acid. If the original template is DNA, then the resulting copy of the second template could then be separated from the original template by using chemical or thermal denaturation methods.
- This single-stranded DNA could be converted to a transcriptionally functional double-stranded DNA by hybridizing the first primer to the single-stranded DNA, and by synthesizing a DNA sequence which is covalently attached to the first primer and complementary to the single-stranded DNA.
- Oligonucleotides were synthesized using an Applied Biosystems 380A DNA synthesizer. Columns, phosphoramidites, and reagents used for oligonucleotide synthesis were obtained from Applied Biosystems, Inc. through Technical Marketing Associates. Oligonucleotides were purified by polyacrylamide gel electrophoresis followed by DEAE cellulose chromatography. The radioisotope [ ⁇ -32p] UTP (800 Ci/mmol) was from Amersham. Enzymes for digesting and ligating DNA were purchased from New England Biolabs, and used according to the supplier's recommendations. Preparations containing the large fragment of DNA polymerase 1 (Klenow) were also purchased from New England Biolabs.
- E. coli transformants were grown on YT medium (Miller, 1972) containing 50 ug/ml ampicillin. Plasmid DNA was purified by a rapid boiling method (Holmes and Quigley, 1981). DNA fragments and vectors used for all constructions were separated by electrophoresis on low melting point agarose, and purified from the molten agarose by phenol extraction and ethanol precipitation (Maniatis et al., 1982). Plasmid DNA was sequenced using a modification (Hattori et al., 1985) of the dideoxy method (Sanger et al., 1977). Reactions were run using the -20 universal primer (New England Biolabs).
- Example 1 Design and synthesis of oligonucleotides for a gag test system
- a synthetic DNA sequence ( Figure 2A) was designed to include an EcoRI site, a T7 phage promoter, a sequence required for initiation of transcription by T7 RNA polymerase and a 19 bp hybridization region (hybridization region 1).
- the 47 b antisense strand oligonucleotide (T7H1.GAG) involved in the cloning of these elements also serves as the first primer.
- Hybridization region 2 lies 53 bp away from hybridization region 1 and is 20 bp in length.
- the primer made to this region (H2.GAG) is a 20 b oligonucleotide duplicate of the sense strand and is not used for cloning.
- gag test DNA could be transcribed by T7 RNA polymerase to produce a RNA fragment (first template) useful as an amplification intermediate involved in three of the steps.
- T7H1.GAG and H2.GAG serve as primers in the test system.
- the standard 50 ul reaction mixture used to amplify RNA transcribed from the gag test oligonucleotides contained 0.34 uM T7H1.GAG, 0.34 uM H2.GAG, 50 mM TrisHCl (pH 8.45), 6 mM MgCl2, 40 mM KCl, 10 mM DTT, 0.5 mM NTP, 1 mM dNTP, 40 units RNasin, 20 units T7 RNA polymerase, 20 units reverse transcriptase, 0.8 units RNase H and 10 - 20 uCi [ ⁇ -32p] UTP.
- the reactions contained amounts of template (N2.GAG) varying from 1 ng to 1 fg. One reaction contained no template.
- RNA amplification from N2.GAG after 3h Reaction Template RNA Synthesized (ug) Fold amplification 1 1 ng 3.5 3.5 x 103 2 100 pg 4.4 4.4 x 104 3 10 pg 4.1 4.1 x 105 4 1 pg 3.0 3.0 x 106 5 100 fg 2.7 2.7 x 107 6 10 fg 1.9 1.9 x 108 7 1 fg 0.78 7.8 x 108 8 - 0.046 -
- Example 6 Use of DNA restriction fragment as template.
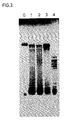
- RNA which was synthesized after a reaction time of 3h was analyzed by PAGE ( Figure 6, lanes 1-6, 11 and 12, numbered the same as the reactions).
- the major band representing a RNA of about 100 b was present in reactions (lanes) 1-6 but absent in the no template reactions (lanes 11 and 12).
- the RNA in lane 0 was a standard, which was prepared following the teaching of Example 3. There was no apparent qualitative difference in the synthesized RNA either with (lanes 2, 4 and 6) or without (lanes 1, 3, and 5) the additional of 1 ug of MspI-degested calf thymus DNA.
- Example 8 Use of ribosomal RNA as a template; amplification of internal sequences
- Example 9 Use of ribosomal RNA as a template; amplification of 5′-terminal sequences.
- Two primers are used for amplifying RNA sequences which are homologous to a part of E.Coli 16S rRNA.
- One of these primers RIB12.PR (TTACTCACCCGTCCGCC) is complementary to 16S rRNA.
- the other T7H1RIB5.PR (AATTCTAATACGACTCACTATAGGGAGAAATTGAAGAGTTTGATCAT) is complementary to the 3′end of the DNA synthesized by using RIB12.PR as a primer and 16S rRNA as a template.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ZA896476A ZA896476B (en) | 1988-08-26 | 1989-08-24 | Nucleic acid amplification process |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CA000559709A CA1340807C (fr) | 1988-02-24 | 1988-02-24 | Procede d'amplification d'une sequence d'acide nucleique |
| CA559709 | 1988-02-24 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0329822A2 true EP0329822A2 (fr) | 1989-08-30 |
| EP0329822A3 EP0329822A3 (en) | 1990-08-01 |
| EP0329822B1 EP0329822B1 (fr) | 1994-06-08 |
Family
ID=4137506
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP88113948A Expired - Lifetime EP0329822B1 (fr) | 1988-02-24 | 1988-08-26 | Procédé d'amplification d'acides nucléiques |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US5409818A (fr) |
| EP (1) | EP0329822B1 (fr) |
| KR (2) | KR960015744B1 (fr) |
| AT (1) | ATE106948T1 (fr) |
| CA (1) | CA1340807C (fr) |
| DE (1) | DE3850093T2 (fr) |
| ES (1) | ES2053648T3 (fr) |
| WO (1) | WO1991002814A1 (fr) |
Cited By (179)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0479376A1 (fr) | 1990-10-05 | 1992-04-08 | Akzo Nobel N.V. | Peptides agissants immunochimiquemeur avec des anticorps contre le virus d'hépatite non-A, non-B |
| WO1992008800A1 (fr) * | 1990-11-13 | 1992-05-29 | Siska Diagnostics, Inc. | Amplification d'acides nucleiques par replication bi-enzymatique auto-entretenue de sequences |
| WO1993015228A1 (fr) * | 1992-01-29 | 1993-08-05 | Hitachi Chemical Co., Ltd. | Support d'immobilisation de polynucleotide |
| EP0566198A3 (fr) * | 1992-04-13 | 1994-08-03 | Enichem Spa | |
| ES2056028A1 (es) * | 1993-02-18 | 1994-09-16 | Inia | Procedimiento para la seleccion de oligonucleotidos para la amplificacion especifica de genomas altamente variables. |
| WO1995003430A1 (fr) * | 1993-07-23 | 1995-02-02 | Gen-Probe Incorporated | Procede d'amelioration de l'amplification de l'acide nucleique |
| WO1995027721A1 (fr) * | 1994-04-07 | 1995-10-19 | Akzo Nobel N.V. | Compositions lyophilisees comprenant de l'arn |
| EP0688873A2 (fr) | 1994-06-23 | 1995-12-27 | Bayer Ag | Test DNA rapide pour la détection de staphylococcus aureus résistant à la chinolone dans du matériel clinique |
| WO1996006191A3 (fr) * | 1994-08-18 | 1996-03-14 | Akzo Nobel Nv | Amorces et sondes destinees a l'amplification et a la detection de l'acide nucleique du cytomegalovirus |
| EP0710724A2 (fr) | 1994-10-06 | 1996-05-08 | Akzo Nobel N.V. | Antigènes de Toxoplasma gondii |
| EP0713922A1 (fr) | 1994-10-28 | 1996-05-29 | BIO MERIEUX, Société anonyme | Oligonucléotide utilisable comme amorce dans une méthode d'amplification basée sur une réplication avec déplacement de brin |
| US5554516A (en) * | 1992-05-06 | 1996-09-10 | Gen-Probe Incorporated | Nucleic acid sequence amplification method, composition and kit |
| EP0778352A1 (fr) * | 1988-12-16 | 1997-06-11 | Siska Diagnostics, Inc. | Essai par réplication auto-entretenue de séquences |
| US5643730A (en) * | 1991-09-23 | 1997-07-01 | Pfizer Inc. | Process for detecting specific mRNA and DNA in cells |
| WO1998006736A1 (fr) | 1996-08-14 | 1998-02-19 | Life Technologies, Inc. | Compositions stables pour l'amplification et le sequençage d'acides nucleiques |
| EP0731174A3 (fr) * | 1989-07-11 | 1998-03-25 | Gen-Probe Incorporated | Méthodes pour l'amplification de séquences d'acide nucléique |
| US5766849A (en) * | 1989-07-11 | 1998-06-16 | Gen-Probe Incorporated | Methods of amplifying nucleic acids using promoter-containing primer sequence |
| US5789169A (en) * | 1994-11-03 | 1998-08-04 | Regents Of The University Of California | Methods for the diagnosis of glaucoma |
| EP0803578A3 (fr) * | 1996-04-26 | 1999-01-13 | Toyo Boseki Kabushiki Kaisha | Coffret pour l'amplification et la détection des acides nucléiques du cytomegalovirus (CMV) utilisant une séquence d'acide nucléique ss2.7 |
| WO1999046400A1 (fr) | 1998-03-13 | 1999-09-16 | Life Technologies, Inc. | Compositions et procedes d'augmentation de la synthese des molecules d'acide nucleique |
| US6025133A (en) * | 1996-12-30 | 2000-02-15 | Gen-Probe Incorporated | Promoter-sequestered oligonucleoside and method of use |
| US6031150A (en) * | 1994-04-13 | 2000-02-29 | Baylor College Of Medicine | Sulfonylurea receptor trangenic rodents |
| US6132954A (en) * | 1996-08-20 | 2000-10-17 | Baylor College Of Medicine | Methods of screening for agents that delay a cell cycle and compositions comprising era and an analogue of wild-type era |
| WO2000073764A2 (fr) | 1999-06-01 | 2000-12-07 | Baylor College Of Medicine | Compositions et methodes pour l'utilisation therapeutique d'une sequence associee au gene atonal dans le traitement de la surdite, de l'osteoarthrite et d'une proliferation cellulaire anormale |
| US6171788B1 (en) | 1997-01-28 | 2001-01-09 | The Regents Of The University Of California | Methods for the diagnosis, prognosis and treatment of glaucoma and related disorders |
| US6225097B1 (en) | 1997-09-17 | 2001-05-01 | Toyota Jidosha Kabushiki Kaisha | Decaprenyl diphosphate synthetase gene |
| US6248867B1 (en) | 1994-11-03 | 2001-06-19 | Univ California | Trabecular meshwork induced glucocorticoid response (TIGR) fusion protein |
| US6261773B1 (en) | 1998-03-17 | 2001-07-17 | Toyo Boseki Kabushiki Kaisha | Reagent for nucleic acid amplification and process for nucleic acid amplification |
| WO2001064959A1 (fr) * | 2000-03-02 | 2001-09-07 | Akzo Nobel N.V. | Detection d'arn du virus de l'hepatite b |
| WO2001066705A1 (fr) * | 2000-03-07 | 2001-09-13 | Akzo Nobel N.V. | Mutants d'arn polymerases presentant une stabilite thermique amelioree |
| US6306588B1 (en) | 1997-02-07 | 2001-10-23 | Invitrogen Corporation | Polymerases for analyzing or typing polymorphic nucleic acid fragments and uses thereof |
| EP0776376B1 (fr) * | 1994-07-15 | 2001-11-21 | Akzo Nobel N.V. | Utilisation d'arn polymerase pour ameliorer un procede d'amplification d'acide nucleique |
| WO2002004511A2 (fr) | 2000-07-10 | 2002-01-17 | Board Of Regents, The University Of Texas System | Genes du chromosome 3p21.3 constituant des suppresseurs de tumeurs |
| EP1229130A2 (fr) * | 2000-12-04 | 2002-08-07 | PrimaGen B.V. | Tests basés sur les acides nucléiques des organelles cellulaires endosymbiontiques et les composés identifiables |
| US6475724B1 (en) | 1997-01-28 | 2002-11-05 | The Regents Of The University Of California | Nucleic acids, kits, and methods for the diagnosis, prognosis and treatment of glaucoma and related disorders |
| WO2002095002A2 (fr) | 2001-05-22 | 2002-11-28 | University Of Chicago | Arn polymerase dependant de l'adn a brin unique de virion n4 |
| WO2003053220A2 (fr) | 2001-12-17 | 2003-07-03 | Corixa Corporation | Compositions et procedes applicables a la therapie et au diagnostic pour la maladie intestinale inflammatoire |
| US6589734B1 (en) | 1989-07-11 | 2003-07-08 | Gen-Probe Incorporated | Detection of HIV |
| EP0525882B1 (fr) * | 1991-08-02 | 2004-01-14 | bioMerieux B.V. | Quantification d'acides nucléiques |
| WO2004062599A2 (fr) | 2003-01-06 | 2004-07-29 | Corixa Corporation | Composes d'aminoalkyle glucosaminide phosphate et leur utilisation |
| US6808883B1 (en) | 1999-04-15 | 2004-10-26 | Bayer Ag | Automatable rapid test for detection of cancer, based on telomerase (hTC) mRNA with specific primers and probes |
| US6830902B1 (en) | 1999-07-02 | 2004-12-14 | Invitrogen Corporation | Compositions and methods for enhanced sensitivity and specificity of nucleic acid synthesis |
| WO2005019409A2 (fr) | 2002-07-15 | 2005-03-03 | Board Of Regents, The University Of Texas System | Criblage combinatoire de banques de proteines par expression periplasmique |
| US6905858B2 (en) | 1997-01-02 | 2005-06-14 | Invitrogen Corporation | Nucleic acid-free thermostable enzymes and methods of production thereof |
| WO2005112544A2 (fr) | 2004-02-19 | 2005-12-01 | The Governors Of The University Of Alberta | Polymorphismes du promoteur de la leptine et utilisations correspondantes |
| USRE38960E1 (en) * | 1994-03-16 | 2006-01-31 | Gen-Probe Incorporated | Isothermal strand displacement nucleic acid amplification |
| US7026117B2 (en) | 1990-02-16 | 2006-04-11 | Orchid Biosciences, Inc. | Method for determining specific nucleotide variations |
| US7029861B1 (en) | 1998-09-15 | 2006-04-18 | Board Of Regents, The University Of Texas System | LPS-response gene compositions and methods |
| US7049102B1 (en) | 1989-09-22 | 2006-05-23 | Board Of Trustees Of Leland Stanford University | Multi-gene expression profile |
| US7105318B2 (en) | 1997-11-04 | 2006-09-12 | Roche Diagnostics Gmbh | Specific and sensitive nucleic acid detection method |
| US7138511B1 (en) | 1997-01-28 | 2006-11-21 | The Regents Of The University Of California | Nucleic acids, kits and methods for the diagnosis, prognosis and treatment of glaucoma and related disorders |
| WO2006122354A1 (fr) | 2005-05-17 | 2006-11-23 | Ozgene Pty Ltd | Systeme de clonage sequentiel |
| US7141420B2 (en) | 1997-02-27 | 2006-11-28 | University Of Utah Research Foundation | Nucleic acid and amino acid sequences for ATP-binding cassette transporter and methods of screening for agents that modify ATP-binding cassette transporter |
| EP1734134A2 (fr) | 1999-02-09 | 2006-12-20 | Laboratory Corporation of America Holdings, Inc. | Nouveau gène spécifique à la prostate servant au diagnostic, au prognostic et au traitment du cancer de la prostate |
| WO2007016275A2 (fr) | 2005-08-02 | 2007-02-08 | Focus Diagnostics, Inc. | Procedes et compositions pour detecter le virus bk |
| EP1801220A2 (fr) | 1997-12-18 | 2007-06-27 | Monsanto Technology, LLC | Plantes transgéniques résistant aux insectes et procédés permettant d'améliorer l'activité de delta-toxine contre des insectes |
| US7306804B2 (en) | 1998-11-17 | 2007-12-11 | Board Of Regents, The University Of Texas System | HIV-specific T-cell induction |
| US7342111B2 (en) | 1999-04-30 | 2008-03-11 | University Of Florida Research Foundation, Inc. | Adeno-associated virus-delivered ribozyme compositions and methods of use |
| EP1908851A2 (fr) | 2001-09-19 | 2008-04-09 | Intergenetics Incorporated | Analyse génétique pour la stratification du risque de cancer |
| WO2008067547A2 (fr) | 2006-11-30 | 2008-06-05 | Research Development Foundation | Bibliothèques d'immunoglobulines améliorées |
| WO2008092866A1 (fr) | 2007-01-29 | 2008-08-07 | Scientific Institute Of Public Health (Iph) | Détection d'événements dans des plantes transgéniques |
| EP1975231A1 (fr) | 2002-01-22 | 2008-10-01 | Corixa Corporation | Compositions et procédés de détection, diagnostic et de thérapie de malignancies hématologiques |
| EP1978098A2 (fr) | 1999-12-10 | 2008-10-08 | Invitrogen Corporation | Utilisation de sites à recombinaison multiples avec une spécificité unique de clonage recombinatoire |
| US7449292B2 (en) | 2003-09-12 | 2008-11-11 | University Of Cincinnati | Methods for predicting relative efficacy of a beta blocker therapy based on a B1-adrenergic receptor polymorphism |
| WO2008137475A2 (fr) | 2007-05-01 | 2008-11-13 | Research Development Foundation | Banques d'immunoglobulines fc |
| EP1997889A2 (fr) | 2003-01-29 | 2008-12-03 | 454 Corporation | Procédé de préparation de bibliothèques ADN à simple brin |
| US7494788B2 (en) | 2005-07-11 | 2009-02-24 | Molecular Kinetics, Inc. | Entropic bristle domain sequences and their use in recombinant protein production |
| US7501252B2 (en) | 1992-01-10 | 2009-03-10 | Life Technologies Corporation | Use of predetermined nucleotides having altered base pairing characteristics in the amplification of nucleic acid molecules |
| EP2044948A1 (fr) | 2002-08-12 | 2009-04-08 | Jennerex Biotherapeutics ULC | Procédés et compositions concernant des poxvirus et le cancer |
| US7537886B1 (en) | 1999-06-22 | 2009-05-26 | Life Technologies Corporation | Primers and methods for the detection and discrimination of nucleic acids |
| US7585512B1 (en) | 1990-05-08 | 2009-09-08 | Thomas Jefferson University | Composition and method of using tumor cells |
| EP2105502A1 (fr) | 2000-12-12 | 2009-09-30 | Corixa Corporation | Composés et procédés de thérapie et de diagnostic du cancer du poumon |
| EP2105496A1 (fr) | 2000-12-08 | 2009-09-30 | Life Technologies Corporation | Méthodes et compositions destinées à la synthèse de molécules d'acide nucléique à l'aide de sites de reconnaissance multiples |
| EP2135960A1 (fr) | 2001-09-19 | 2009-12-23 | Intergenetics Incorporated | Analyse génétique pour la stratification du risque de cancer par détermination du profile allelique des génes VDR-ApaI et CYP11b2 |
| EP2184289A1 (fr) | 1997-04-22 | 2010-05-12 | Life Technologies Corporation | Procédés de production de transcriptases inverses ASLV composées de plusieurs subunités |
| EP2192128A2 (fr) | 2000-04-21 | 2010-06-02 | Corixa Corporation | Composés et méthodes pour le traitement et le diagnostique des infections de Chlamydia |
| EP2246444A1 (fr) | 2004-09-14 | 2010-11-03 | The Regents of the University of Colorado, A Body Corporate | Procédé de traitement faisant intervenir du bucindolol fondé sur le ciblage génétique |
| EP2270034A2 (fr) | 2004-06-03 | 2011-01-05 | Athlomics Pty Ltd | Agents et procédé permettant le diagnostic de stress |
| WO2011008530A2 (fr) | 2009-06-29 | 2011-01-20 | Luminex Corporation | Amorces chimériques avec conformations en épingle à cheveux et procédés d'utilisation de celles-ci |
| EP2295551A1 (fr) | 2000-05-26 | 2011-03-16 | Life Technologies Corporation | Transcriptases inverses thermostables et utilisations |
| EP2295569A1 (fr) | 2003-07-25 | 2011-03-16 | Ambion, Inc. | Procédés et compositions pour isoler des petites molécules de RNA |
| EP2299267A2 (fr) | 2005-11-16 | 2011-03-23 | Novartis AG | Biomarqueurs pour un traitement par des anticorps anti-NogoA de lésions de la moelle épinière |
| EP2298891A1 (fr) | 2003-07-25 | 2011-03-23 | Ambion, Inc. | Procédés et compositions pour isoler des petites molécules d'ARN |
| EP2319941A2 (fr) | 2005-10-21 | 2011-05-11 | GeneNews Inc. | Procédé et appareil pour corréler des niveaux de produits biomarqueurs avec une maladie |
| EP2325303A2 (fr) | 2002-09-13 | 2011-05-25 | Life Technologies Corporation | Transcriptases inverses thermostables et leurs utilisations |
| EP2336367A1 (fr) | 1997-08-08 | 2011-06-22 | bioMerieux B.V. | Séquences nucléotidiques pouvant être utilisées comme amorces et comme sondes au cours de l'amplification et la détection de tous les sous-types du VIH-1 |
| WO2011079273A2 (fr) | 2009-12-23 | 2011-06-30 | Arca Biopharma, Inc. | Procédés et compositions pour maladies et affections cardiovasculaires |
| EP2365079A1 (fr) | 2005-03-05 | 2011-09-14 | Seegene, Inc. | Procédés utilisant un oligonucléotide à double spécificité et oligonucléotide à double spécificité |
| EP2365078A1 (fr) | 2005-03-05 | 2011-09-14 | Seegene, Inc. | Procédés utilisant un oligonucléotide à double spécificité et oligonucléotide à double spécificité |
| EP2366712A2 (fr) | 1997-01-30 | 2011-09-21 | The Board Of Regents, The University Of Texas System | Suppresseur de tumeur désigné par TS10Q23.3 |
| EP2368868A1 (fr) | 2005-10-28 | 2011-09-28 | Praecis Pharmaceuticals Inc. | Procédé d'identification de composés intéressants utilisant des bibliothèques codées |
| WO2011127933A1 (fr) | 2010-04-16 | 2011-10-20 | Nuevolution A/S | Complexes bifonctionnels et procédés de fabrication et d'utilisation de tels complexes |
| WO2011133933A2 (fr) | 2010-04-23 | 2011-10-27 | University Of Florida Research Foundation, Inc. | Compositions de guanylate cyclase raav et méthodes de traitement de l'amaurose-1 congénitale de leber (lca1) |
| WO2011139721A1 (fr) | 2010-04-27 | 2011-11-10 | The Regents Of The University Of California | Biomarqueurs de cancer et leurs procédés d'utilisation |
| WO2011145777A1 (fr) | 2010-05-20 | 2011-11-24 | 광주과학기술원 | Composition pharmaceutique comprenant un inhibiteur de hif-2α comme ingrédient actif pour la prévention ou le traitement de l'arthrite |
| WO2011145795A1 (fr) | 2010-05-20 | 2011-11-24 | 광주과학기술원 | Souris transgénique hif-2α spécifique du cartilage comme modèle animal pour l'arthrite, et son utilisation |
| EP2390352A1 (fr) | 2003-03-18 | 2011-11-30 | Quantum Genetics Ireland Limited | Systemes et procedes pour accroitre la production de proteines et de lait chez des bovins laitiers |
| US8137911B2 (en) | 2001-05-22 | 2012-03-20 | Cellscript, Inc. | Preparation and use of single-stranded transcription substrates for synthesis of transcription products corresponding to target sequences |
| EP2442109A1 (fr) | 2006-07-14 | 2012-04-18 | The Regents of the University of California | Biomarqueurs du cancer et procédé d'utilisation correspondant |
| EP2455489A2 (fr) | 2006-05-25 | 2012-05-23 | Monsanto Technology LLC | Procédé pour identifier des locus de traits quantitatifs résistants aux maladies dans le soja et compositions correspondantes |
| WO2012090073A2 (fr) | 2010-12-30 | 2012-07-05 | The Netherlands Cancer Institute | Procédés et compositions pour prédire la sensibilité à la chimiothérapie |
| US8222023B2 (en) | 2006-03-15 | 2012-07-17 | Micronics, Inc. | Integrated nucleic acid assays |
| EP2476761A2 (fr) | 2005-07-07 | 2012-07-18 | Athlomics Pty Ltd | Gènes de marqueur de polynucléotide et leur expression pour le diagnostic de l'endotoxine |
| WO2012109133A1 (fr) | 2011-02-07 | 2012-08-16 | Research Development Foundation | Polypeptides fc modifiés d'immunoglobuline |
| WO2012120377A2 (fr) | 2011-03-08 | 2012-09-13 | King Abdullah University Of Science And Technology | Biomarqueur moléculaire destiné à la détection précoce du cancer des ovaires |
| WO2012138783A2 (fr) | 2011-04-04 | 2012-10-11 | Netherlands Cancer Institute | Procédés et compositions pour prédire une résistance à un traitement anti-cancéreux |
| WO2012138789A2 (fr) | 2011-04-04 | 2012-10-11 | Netherlands Cancer Institute | Procédés et compositions pour prédire une résistance à un traitement anti-cancéreux |
| WO2012149038A1 (fr) | 2011-04-25 | 2012-11-01 | Advanced Bioscience Laboratories, Inc. | Protéines tronquées d'enveloppe (env) du vih, procédés et compositions associés à celles-ci |
| WO2012158238A2 (fr) | 2011-02-28 | 2012-11-22 | University Of Iowa Research Foundation | Changements d'hormone antimullérienne dans la grossesse et prédiction d'évolution indésirable de grossesse et du sexe |
| WO2013045505A1 (fr) | 2011-09-28 | 2013-04-04 | Novartis Ag | Biomarqueurs pour traitement combiné par des agents sraa |
| WO2013071233A1 (fr) | 2011-11-10 | 2013-05-16 | The United States Of America, As Represented By The Secretary, Department Of Health & Human Services | Procédés de détection d'agents infectieux et nouveau virus détecté par ces procédés |
| WO2013074676A2 (fr) | 2011-11-14 | 2013-05-23 | The General Hospital Corporation | Dosages et procédés pour la sélection d'un schéma thérapeutique pour un sujet atteint d'une dépression |
| US8460867B2 (en) | 2001-12-10 | 2013-06-11 | Novartis Ag | Methods of treating psychosis and schizophrenia based on polymorphisms in the CNTF gene |
| WO2013093629A2 (fr) | 2011-12-20 | 2013-06-27 | Netherlands Cancer Institute | Vaccins modulaires, procédés et compositions qui y sont liés |
| WO2013096798A2 (fr) | 2011-12-22 | 2013-06-27 | Ibis Biosciences, Inc. | Amplification d'une séquence provenant d'un acide ribonucléique |
| EP2623605A1 (fr) | 2007-04-09 | 2013-08-07 | University of Florida Research Foundation, Inc. | Compositions de vecteur RAAV comprenant des protéines de capside à tyrosine modifié et procédés d'utilisation |
| WO2013116698A2 (fr) | 2012-02-02 | 2013-08-08 | Invenra, Inc. | Crible haut débit pour des polypeptides biologiquement actifs |
| WO2013144249A1 (fr) | 2012-03-29 | 2013-10-03 | Novartis Ag | Produit pharmaceutique à des fins diagnostiques |
| US8568730B2 (en) | 2009-04-22 | 2013-10-29 | Indiana University Research & Technology Corporation | Compositions for use in the treatment of chronic obstructive pulmonary diseases and asthma |
| US8614062B1 (en) | 2009-07-24 | 2013-12-24 | University Of South Florida | RNA-based system and method to differentiate seafood |
| US8722866B2 (en) | 2001-08-01 | 2014-05-13 | The United States Of America, As Represented By The Department Of Veterans Affairs | Isoform-selective inhibitors and activators of PDE3 cyclic nucleotide phosphodiesterases |
| US8735066B2 (en) | 1997-01-30 | 2014-05-27 | Board Of Regents, The University Of Texas System | Tumor suppressor designated TS10Q23.3 |
| EP2740805A1 (fr) | 2012-12-07 | 2014-06-11 | SuppreMol GmbH | Stratification et traitement de patients du purpura thrombocytopénique idiopathique |
| US8841432B2 (en) | 2011-04-21 | 2014-09-23 | Snu R&Db Foundation | Shuttle vectors for mycobacteria-escherichia coli and uses thereof |
| WO2014148817A1 (fr) | 2013-03-21 | 2014-09-25 | 주식회사 현일바이오 | Procédé de détection sélective pour mycobacterium tuberculosis et des mycobactéries non tuberculeuses et kit utilisant celui-ci |
| US8859467B2 (en) | 2009-07-17 | 2014-10-14 | Bioatla, Llc | Simultaneous, integrated selection and evolution of antibody/protein performance and expression in production hosts |
| WO2014171698A1 (fr) | 2013-04-18 | 2014-10-23 | 사회복지법인 삼성생명공익재단 | Méthode de diagnostic de la dystrophie myotonique de type 1 |
| WO2014183023A1 (fr) | 2013-05-09 | 2014-11-13 | Trustees Of Boston University | Utilisation de la plexine-a4 comme biomarqueur et cible thérapeutique pour la maladie d'alzheimer |
| EP2839837A1 (fr) | 2006-09-15 | 2015-02-25 | Ottawa Hospital Research Institute | Rhabdovirus Oncolytique Farmington |
| WO2015105888A1 (fr) | 2014-01-07 | 2015-07-16 | Bioatla, Llc | Orthologues ciblant les protéines |
| WO2015112767A2 (fr) | 2014-01-22 | 2015-07-30 | Life Technologies Corporation | Nouvelles rétrotranscriptases pour une utilisation dans la synthèse d'acide nucléique à haute température |
| EP2913402A1 (fr) | 2010-07-16 | 2015-09-02 | Tocagen Inc. | Détection de rétrovirus |
| EP2940027A1 (fr) | 2004-07-08 | 2015-11-04 | Corixa Corporation | Composes d'iaminoalkylglucosaminide phosphate et leur utilisation |
| WO2015191916A1 (fr) | 2014-06-11 | 2015-12-17 | Micronics, Inc. | Cartouches microfluidiques et appareil à témoins de dosage intégrés pour l'analyse d'acides nucléiques |
| WO2016086227A2 (fr) | 2014-11-26 | 2016-06-02 | The Regents Of The University Of California | Compositions thérapeutiques comprenant des facteurs de transcription et leurs procédés de préparation et d'utilisation |
| US9409983B2 (en) | 2009-07-23 | 2016-08-09 | The Board Of Trustess Of The University Of Illinois | Methods and compositions involving PBEF inhibitors for lung inflammation conditions and diseases |
| EP3056566A1 (fr) | 2005-01-28 | 2016-08-17 | Life Technologies Corporation | Inhibiteurs multi-composants de polymerases d'acides nucleiques et methodes associees |
| WO2016130516A1 (fr) | 2015-02-09 | 2016-08-18 | Research Development Foundation | Polypeptides ayant un domaine fc d'immunoglobuline modifié manifestant une activation du complément améliorée |
| EP3093341A1 (fr) | 2000-05-26 | 2016-11-16 | Life Technologies Corporation | Transcriptases inverses thermostables et utilisations |
| US9540670B2 (en) | 2011-10-06 | 2017-01-10 | bioMérieux | Mutated T7 RNA Polymerases |
| WO2017015075A1 (fr) | 2015-07-17 | 2017-01-26 | President And Fellows Of Harvard College | Procédés d'amplification de séquences d'acides nucléiques |
| WO2017040380A2 (fr) | 2015-08-28 | 2017-03-09 | Research Development Foundation | Variants de fc d'anticorps modifiés |
| EP3249054A1 (fr) | 2012-12-20 | 2017-11-29 | Biomatrica, INC. | Formulations et procédés pour stabiliser des réactifs pcr |
| WO2017214068A1 (fr) | 2016-06-05 | 2017-12-14 | Berg Llc | Systèmes et procédés de stratification de patient et identification de biomarqueurs potentiels |
| US9895692B2 (en) | 2010-01-29 | 2018-02-20 | Micronics, Inc. | Sample-to-answer microfluidic cartridge |
| US9896664B2 (en) | 2009-12-10 | 2018-02-20 | Turnstone Limited Partnership | Oncolytic rhabdovirus |
| US9919047B2 (en) | 2011-01-04 | 2018-03-20 | Sillajen, Inc. | Generation of antibodies to tumor antigens and generation of tumor specific complement dependent cytotoxicity by administration of oncolytic vaccinia virus |
| WO2018128516A1 (fr) | 2017-01-09 | 2018-07-12 | 연세대학교 산학협력단 | Composition pharmaceutique pour un traitement de précancer buccal et procédé de prédiction ou de diagnostic d'un précancer buccal ou d'un cancer buccal |
| WO2018140606A1 (fr) | 2017-01-26 | 2018-08-02 | Oklahoma Medical Research Foundation | Biomarqueurs de l'activité, de l'intensité et de l'éruption de la maladie du lupus érythémateux disséminé |
| US10040853B2 (en) | 2011-09-09 | 2018-08-07 | Fred Hutchinson Cancer Research Center | Methods and compositions involving NKG2D inhibitors and cancer |
| US10065186B2 (en) | 2012-12-21 | 2018-09-04 | Micronics, Inc. | Fluidic circuits and related manufacturing methods |
| US10087440B2 (en) | 2013-05-07 | 2018-10-02 | Micronics, Inc. | Device for preparation and analysis of nucleic acids |
| US10125373B2 (en) | 2013-01-22 | 2018-11-13 | Arizona Board Of Regents On Behalf Of Arizona State University | Geminiviral vector for expression of rituximab |
| WO2018219997A1 (fr) | 2017-05-30 | 2018-12-06 | Roche Diagnostics Gmbh | Catalyseurs pour inverser des produits d'addition de formaldéhyde et des réticulations |
| US10172879B2 (en) | 2015-01-21 | 2019-01-08 | D.R.Nano Co., Ltd | Nanocomplexes for co-delivering a drug and siRNA and uses thereof |
| WO2019017680A2 (fr) | 2017-07-19 | 2019-01-24 | 국민대학교 산학협력단 | Micro-arn utilisé en tant que biomarqueur de la maladie de parkinson et kit diagnostique le mettant en oeuvre |
| US10190153B2 (en) | 2013-05-07 | 2019-01-29 | Micronics, Inc. | Methods for preparation of nucleic acid-containing samples using clay minerals and alkaline solutions |
| US10363293B2 (en) | 2013-02-21 | 2019-07-30 | Turnstone Limited Partnership | Vaccine composition |
| US10386377B2 (en) | 2013-05-07 | 2019-08-20 | Micronics, Inc. | Microfluidic devices and methods for performing serum separation and blood cross-matching |
| US10393739B2 (en) | 2013-10-03 | 2019-08-27 | Oklahoma Medical Research Foundation | Biomarkers for systemic lupus erythematosus disease activity, and intensity and flare |
| US10436713B2 (en) | 2012-12-21 | 2019-10-08 | Micronics, Inc. | Portable fluorescence detection system and microassay cartridge |
| US10518262B2 (en) | 2012-12-21 | 2019-12-31 | Perkinelmer Health Sciences, Inc. | Low elasticity films for microfluidic use |
| WO2020089841A1 (fr) | 2018-10-31 | 2020-05-07 | Garcia Joe G N | Biomarqueurs et méthodes d'utilisation de ces biomarqueurs pour détecter une lésion pulmonaire radio-induite |
| US10772951B2 (en) | 2011-06-08 | 2020-09-15 | Children's Hospital Of Eastern Ontario Research Institute Inc. | Compositions and methods for glioblastoma treatment |
| WO2020223576A1 (fr) | 2019-04-30 | 2020-11-05 | Chondrial Therapeutics, Inc. | Marqueurs sensibles à la frataxine pour déterminer l'efficacité d'une thérapie de remplacement de frataxine |
| WO2021246820A1 (fr) | 2020-06-05 | 2021-12-09 | 주식회사 씨젠 | Kit de transport d'échantillon pour la détection d'agents pathogènes respiratoires et procédés de détection d'agents pathogènes respiratoires à l'aide de celui-ci |
| WO2022047359A1 (fr) | 2020-08-31 | 2022-03-03 | Berg Llc | Biomarqueurs protéiques du cancer du pancréas |
| US11339421B2 (en) | 2016-02-23 | 2022-05-24 | Industry Foundation Of Chonnam National University | Leukemia diagnostic kit targeting prohibitin gene and diagnostic method using same |
| US11447772B2 (en) | 2017-07-19 | 2022-09-20 | Kookmin University Industry Academy Cooperation Foundation | miRNA as biomarker for Parkinson's disease and diagnostic kit using same |
| WO2022216841A1 (fr) | 2021-04-06 | 2022-10-13 | Berg Llc | Marqueurs protéiques pour un cancer du sein positif au récepteur des œstrogènes (er) de type luminal a (la) et de type luminal b1 (lb1) |
| WO2022216846A1 (fr) | 2021-04-06 | 2022-10-13 | Berg Llc | Marqueurs protéiques pour le cancer du sein du type positif aux récepteurs des oestrogènes (er) et du type négatif aux récepteurs des oestrogènes (er) |
| WO2022265965A1 (fr) | 2021-06-14 | 2022-12-22 | 10X Genomics, Inc. | Variants de la transcriptase inverse pour une performance améliorée |
| US11543411B2 (en) | 2014-12-05 | 2023-01-03 | Prelude Corporation | DCIS recurrence and invasive breast cancer |
| EP4242329A2 (fr) | 2014-12-08 | 2023-09-13 | Berg LLC | Utilisation de marqueurs comprenant de la filamine a dans le diagnostic et le traitement du cancer de la prostate |
| WO2023196937A1 (fr) | 2022-04-06 | 2023-10-12 | Larimar Therapeutics, Inc. | Marqueurs sensibles à la frataxine pour surveiller une thérapie de remplacement de frataxine |
| EP4275684A1 (fr) | 2018-05-25 | 2023-11-15 | Arca Biopharma, Inc. | Procédés et compositions impliquant du bucindolol pour le traitement de la fibrillation auriculaire |
| US11821900B2 (en) | 2018-09-14 | 2023-11-21 | Prelude Corporation | Method of selection for treatment of subjects at risk of invasive breast cancer |
| WO2023230531A1 (fr) | 2022-05-24 | 2023-11-30 | Lunglife Ai, Inc. | Procédés de détection de cellules génétiquement anormales circulantes |
| WO2023239940A1 (fr) | 2022-06-10 | 2023-12-14 | Research Development Foundation | Variants de fc igg1 sélectifs de fcriib modifiés et leurs utilisations |
Families Citing this family (534)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA1340807C (fr) * | 1988-02-24 | 1999-11-02 | Lawrence T. Malek | Procede d'amplification d'une sequence d'acide nucleique |
| JP2650159B2 (ja) * | 1988-02-24 | 1997-09-03 | アクゾ・ノベル・エヌ・ベー | 核酸増幅方法 |
| US5130238A (en) * | 1988-06-24 | 1992-07-14 | Cangene Corporation | Enhanced nucleic acid amplification process |
| AU633036B2 (en) * | 1988-12-09 | 1993-01-21 | Chemicon International, Inc. | Amplified dna assay |
| IE66597B1 (en) * | 1989-05-10 | 1996-01-24 | Akzo Nv | Method for the synthesis of ribonucleic acid (RNA) |
| CA2020958C (fr) * | 1989-07-11 | 2005-01-11 | Daniel L. Kacian | Methodes d'amplification de sequences d'acide nucleique |
| WO1991004340A1 (fr) * | 1989-09-20 | 1991-04-04 | Cambridge Biotech Corporation | Amplification isothermique in vitro de l'acide nucleique |
| US5545522A (en) | 1989-09-22 | 1996-08-13 | Van Gelder; Russell N. | Process for amplifying a target polynucleotide sequence using a single primer-promoter complex |
| IE65771B1 (en) * | 1990-01-25 | 1995-11-15 | Zeneca Ltd | Amplification of nucleotide sequences using vectorette units |
| WO1991013994A1 (fr) * | 1990-03-13 | 1991-09-19 | Commonwealth Scientific And Industrial Research Organisation | Expression de genes |
| US5194370A (en) * | 1990-05-16 | 1993-03-16 | Life Technologies, Inc. | Promoter ligation activated transcription amplification of nucleic acid sequences |
| ATE176002T1 (de) * | 1990-07-24 | 1999-02-15 | Hoffmann La Roche | Verringerung von nicht spezifischer amplifikation während einer (in vitro) nukleinsäure amplifikation unter verwendung von modifizierten nukleinsäure basen |
| NZ240079A (en) * | 1990-10-09 | 1993-07-27 | Boehringer Mannheim Gmbh | Method for the detection of a nucleic acid or part thereof |
| RU2017821C1 (ru) * | 1990-10-10 | 1994-08-15 | Анатолий Михайлович Онищенко | Способ амплификации днк и устройство для его осуществления |
| US5518900A (en) * | 1993-01-15 | 1996-05-21 | Molecular Tool, Inc. | Method for generating single-stranded DNA molecules |
| WO1992018521A1 (fr) * | 1991-04-10 | 1992-10-29 | Life Technologies, Inc. | Procede d'amplification et de modification d'une sequence d'arn |
| WO1992020820A1 (fr) * | 1991-05-15 | 1992-11-26 | Vanderbilt University | Methode de determination du potentiel metastatique des cellules tumorales |
| EP0517361A1 (fr) * | 1991-05-30 | 1992-12-09 | Amoco Corporation | Méthode de détection et identification d'organismes pathogènes en utilisant des séquences cibles comme détecteurs |
| US5169766A (en) * | 1991-06-14 | 1992-12-08 | Life Technologies, Inc. | Amplification of nucleic acid molecules |
| US5137814A (en) * | 1991-06-14 | 1992-08-11 | Life Technologies, Inc. | Use of exo-sample nucleotides in gene cloning |
| US5229283A (en) * | 1991-06-14 | 1993-07-20 | Life Technologies, Inc. | Use of exo-sample nucleotides in gene cloning |
| DE4129653A1 (de) * | 1991-09-06 | 1993-03-11 | Boehringer Mannheim Gmbh | Verfahren zum nachweis aehnlicher nukleinsaeuren |
| DE4132133A1 (de) * | 1991-09-26 | 1993-04-01 | Boehringer Mannheim Gmbh | Verfahren zur spezifischen herstellung von ribonukleinsaeuren |
| DE4213029A1 (de) * | 1991-09-26 | 1993-04-01 | Boehringer Mannheim Gmbh | Verfahren zur spezifischen vervielfaeltigung von nukleins aeuresequenzen |
| US5981179A (en) * | 1991-11-14 | 1999-11-09 | Digene Diagnostics, Inc. | Continuous amplification reaction |
| US6100099A (en) | 1994-09-06 | 2000-08-08 | Abbott Laboratories | Test strip having a diagonal array of capture spots |
| WO1993023560A1 (fr) * | 1992-05-11 | 1993-11-25 | Schuepbach Joerg | Procede de mise en evidence de la transcriptase reverse |
| US5843640A (en) * | 1992-06-19 | 1998-12-01 | Northwestern University | Method of simultaneously detecting amplified nucleic acid sequences and cellular antigens in cells |
| US5580971A (en) * | 1992-07-28 | 1996-12-03 | Hitachi Chemical Company, Ltd. | Fungal detection system based on rRNA probes |
| JPH07509361A (ja) * | 1992-07-28 | 1995-10-19 | 日立化成工業株式会社 | 遺伝子検出システム |
| US6261808B1 (en) * | 1992-08-04 | 2001-07-17 | Replicon, Inc. | Amplification of nucleic acid molecules via circular replicons |
| US5614389A (en) * | 1992-08-04 | 1997-03-25 | Replicon, Inc. | Methods for the isothermal amplification of nucleic acid molecules |
| WO1994003624A1 (fr) * | 1992-08-04 | 1994-02-17 | Auerbach Jeffrey I | Procedes d'amplification isothermique de molecules d'acide nucleique |
| US5733733A (en) * | 1992-08-04 | 1998-03-31 | Replicon, Inc. | Methods for the isothermal amplification of nucleic acid molecules |
| US5834202A (en) * | 1992-08-04 | 1998-11-10 | Replicon, Inc. | Methods for the isothermal amplification of nucleic acid molecules |
| ZA936015B (en) * | 1992-08-24 | 1994-03-10 | Akzo Nv | Elimination of false negatives in nuleic acid detection. |
| ZA936016B (en) * | 1992-08-24 | 1994-03-10 | Akzo Nv | Method for nucleic acid amplification |
| DE4236708A1 (de) * | 1992-10-30 | 1994-05-05 | Bayer Ag | Spezifische Gensonden und Verfahren zur Diagnostik von Candida albicans |
| DE4302459A1 (de) * | 1993-01-29 | 1994-08-04 | Bayer Ag | Sulfocumarinhaltige Nukleotide und deren Verwendung in Nachweisverfahren für Nukleinsäuren |
| DE4344742A1 (de) * | 1993-06-09 | 1994-12-15 | Boehringer Mannheim Gmbh | Verfahren zur Immobilisierung von Nukleinsäuren |
| FR2708288B1 (fr) | 1993-07-26 | 1995-09-01 | Bio Merieux | Procédé d'amplification d'acides nucléiques par transcription utilisant le déplacement, réactifs et nécessaire pour la mise en Óoeuvre de ce procédé. |
| GB2284209A (en) * | 1993-11-25 | 1995-05-31 | Ole Buchardt | Nucleic acid analogue-induced transcription of RNA from a double-stranded DNA template |
| WO1995015399A1 (fr) | 1993-12-01 | 1995-06-08 | Toyo Boseki Kabushiki Kaisha | Procede d'amplification et de detection d'une sequence nucleotidique au moyen d'enzymes thermostables |
| WO2000033244A2 (fr) * | 1998-11-27 | 2000-06-08 | Synaptics (Uk) Limited | Capteur de position |
| US6010847A (en) | 1994-08-18 | 2000-01-04 | Akzo Nobel N.V. | Oligonucleotides that can be used in the amplification and detection of CMV nucleic acid |
| US5656427A (en) * | 1994-08-29 | 1997-08-12 | Gen-Probe Incorporated | Nucleic acid hybridization assay probes, helper probes and amplification oligonucleotides targeted to Mycoplasma pneumoniae nucleic acid |
| FR2724934B1 (fr) * | 1994-09-26 | 1997-01-24 | Bio Merieux | Oligonucleotide chimere et son utilisation dans l'obtention de transcrits d'un acide nucleique |
| US5849879A (en) * | 1994-11-03 | 1998-12-15 | The Regents Of The University Of California | Methods for the diagnosis of glaucoma |
| US5654141A (en) * | 1994-11-18 | 1997-08-05 | Thomas Jefferson University | Amplification based detection of bacterial infection |
| US6001610A (en) * | 1994-11-23 | 1999-12-14 | Roche Diagnostics, Gmbh | Method for the particularly sensitive detection of nucleic acids |
| DE4441626A1 (de) * | 1994-11-23 | 1996-05-30 | Boehringer Mannheim Gmbh | Verfahren zum besonders sensitiven Nachweis von Nukleinsäuren |
| US5665545A (en) * | 1994-11-28 | 1997-09-09 | Akzo Nobel N.V. | Terminal repeat amplification method |
| EP0799888B1 (fr) * | 1994-12-09 | 2005-05-18 | Wakunaga Seiyaku Kabushiki Kaisha | Procede d'inhibition de l'hybridation non specifique dans une extension d'amorce |
| CA2139070C (fr) * | 1994-12-23 | 2010-03-30 | Burton W. Blais | Methode pour ameliorer l'aptitude a la detection d'essais d'acide nucleique utilisant une reaction en chaine de polymerase |
| US5925518A (en) * | 1995-05-19 | 1999-07-20 | Akzo Nobel N.V. | Nucleic acid primers for amplification of a mycobacteria RNA template |
| US5856096A (en) * | 1995-09-20 | 1999-01-05 | Ctrc Research Foundation | Rapid and sensitive assays for detecting and distinguishing between processive and non-processive telomerase activities |
| US5747255A (en) * | 1995-09-29 | 1998-05-05 | Lynx Therapeutics, Inc. | Polynucleotide detection by isothermal amplification using cleavable oligonucleotides |
| US5854033A (en) | 1995-11-21 | 1998-12-29 | Yale University | Rolling circle replication reporter systems |
| JP3974941B2 (ja) | 1995-11-21 | 2007-09-12 | イェール ユニバーシティ | 単分子セグメントの増幅および検出 |
| US6048688A (en) * | 1996-11-12 | 2000-04-11 | Kimberly-Clark Corporation | Method for detection of Pseudomonas aeruginosa using polymerase chain reaction |
| US7195871B2 (en) * | 1996-01-24 | 2007-03-27 | Third Wave Technologies, Inc | Methods and compositions for detecting target sequences |
| US20080160524A1 (en) * | 1996-01-24 | 2008-07-03 | Third Wave Technologies, Inc. | Methods and Compositions for Detecting Target Sequences |
| EP0914462A4 (fr) * | 1996-03-18 | 2002-05-22 | Molecular Biology Resources | Amplification de sequences d'acides nucleiques cibles |
| US6759217B2 (en) | 1996-03-26 | 2004-07-06 | Oncomedx, Inc. | Method enabling use of extracellular RNA extracted from plasma or serum to detect, monitor or evaluate cancer |
| CA2250118C (fr) | 1996-03-26 | 2009-09-29 | Michael S. Kopreski | Procede permettant d'employer de l'arn extracellulaire extrait de plasma ou de serum a la detection, a la surveillance ou a l'evaluation d'un cancer |
| US7785842B2 (en) * | 1996-03-26 | 2010-08-31 | Oncomedx, Inc. | Comparative analysis of extracellular RNA species |
| US8043835B1 (en) | 1996-03-26 | 2011-10-25 | Oncomedx, Inc. | Methods for detecting and monitoring cancer using extracellular RNA |
| US5712127A (en) * | 1996-04-29 | 1998-01-27 | Genescape Inc. | Subtractive amplification |
| US6436638B1 (en) | 1996-05-09 | 2002-08-20 | Metropolitan Water District Of Southern California | Cryptosporidium detection method |
| US5770368A (en) * | 1996-05-09 | 1998-06-23 | Metropolitan Water District Of Southern California | Cryptosporidium detection method |
| US5876992A (en) | 1996-07-03 | 1999-03-02 | Molecular Biology Resources, Inc. | Method and formulation for stabilization of enzymes |
| US6117635A (en) * | 1996-07-16 | 2000-09-12 | Intergen Company | Nucleic acid amplification oligonucleotides with molecular energy transfer labels and methods based thereon |
| US5866336A (en) * | 1996-07-16 | 1999-02-02 | Oncor, Inc. | Nucleic acid amplification oligonucleotides with molecular energy transfer labels and methods based thereon |
| JPH1028585A (ja) * | 1996-07-16 | 1998-02-03 | Toyobo Co Ltd | 耐熱性リボヌクレアーゼhを用いる核酸増幅法 |
| US6537783B1 (en) | 1996-08-02 | 2003-03-25 | Bio Merieux | Prefunctionalized nucleotide and process for amplifying a sequence using a prefunctionalized nucleotide |
| WO1998008981A1 (fr) * | 1996-08-30 | 1998-03-05 | Life Technologies, Inc. | PROCEDES D'IDENTIFICATION ET D'ISOLEMENT DE SEQUENCES DE NUCLEOTIDES SPECIFIQUES DANS L'ADNc ET L'ADN GENOMIQUE |
| US6043283A (en) * | 1996-09-20 | 2000-03-28 | Baylor College Of Medicine | Tyramine compounds and their neuronal effects |
| US6071493A (en) | 1996-09-20 | 2000-06-06 | Baylor College Of Medicine | Method of screening for an agent that inhibits mononuclear phagocyte-plaque component complex formation |
| US6096273A (en) * | 1996-11-05 | 2000-08-01 | Clinical Micro Sensors | Electrodes linked via conductive oligomers to nucleic acids |
| US7381525B1 (en) * | 1997-03-07 | 2008-06-03 | Clinical Micro Sensors, Inc. | AC/DC voltage apparatus for detection of nucleic acids |
| AU746549B2 (en) | 1996-11-20 | 2002-05-02 | Becton Dickinson & Company | Microfabricated isothermal nucleic acid amplification devices and methods |
| US20040142327A1 (en) * | 1996-11-22 | 2004-07-22 | Jhy-Jhu Lin | Methods for identifying and isolating DNA polymorphisms or genetic markers |
| JP2001524808A (ja) | 1996-12-10 | 2001-12-04 | ジーントレイス・システムズ・インコーポレイテッド | 放出可能な不揮発性の質量標識分子 |
| US8440396B2 (en) * | 1997-03-14 | 2013-05-14 | Oncomedx, Inc. | Method enabling use of extracellular RNA extracted from plasma or serum to detect, monitor or evaluate cancer |
| US20070009934A1 (en) * | 1997-03-14 | 2007-01-11 | Kopreski Michael S | Method enabling use of extracellular RNA extracted from plasma or serum to detect, monitor or evaluate cancer |
| US6204024B1 (en) | 1997-09-12 | 2001-03-20 | Akzo Nobel N.V. | CCR5 RNA transcription based amplification assay |
| US6461817B1 (en) | 1997-09-12 | 2002-10-08 | The Public Health Research Institute Of The City Of New York | Non-competitive co-amplification methods |
| ZA989950B (en) * | 1997-11-17 | 1999-05-04 | Akzo Nobel Nv | Transcription based amplification of double stranded DNA targets |
| US5968732A (en) * | 1997-12-31 | 1999-10-19 | Akzo Nobel, N.V. | Isothermal transcription based assay for the detection and genotyping of dengue virus |
| US6093542A (en) * | 1998-01-09 | 2000-07-25 | Akzo Nobel N.V. | Isothermal transcription based amplification assay for the detection and quantitation of macrophage derived chemokine RNA |
| US6121023A (en) * | 1998-01-22 | 2000-09-19 | Akzo Nobel N.V. | Isothermal transcription based assay for the detection and quantification of the chemokine rantes |
| US6673914B1 (en) | 1998-01-22 | 2004-01-06 | John Wayne Cancer Institute | Human tumor-associated gene |
| US6686150B1 (en) | 1998-01-27 | 2004-02-03 | Clinical Micro Sensors, Inc. | Amplification of nucleic acids with electronic detection |
| JP2002500897A (ja) | 1998-01-27 | 2002-01-15 | クリニカル・マイクロ・センサーズ・インコーポレイテッド | 電子的核酸検出の増幅 |
| USH1825H (en) * | 1998-01-30 | 1999-12-07 | Akzo Nobel N.V. | Isothermal transcription based assay for the detection of HTLV I and HTLV II RNA |
| US6365346B1 (en) * | 1998-02-18 | 2002-04-02 | Dade Behring Inc. | Quantitative determination of nucleic acid amplification products |
| ATE429517T1 (de) * | 1998-03-04 | 2009-05-15 | Biomerieux Bv | Vervielfältigung und nachweis von ebv barf1 zur diagnose von nasopharyngealen karzinom |
| US20050244954A1 (en) * | 1998-06-23 | 2005-11-03 | Blackburn Gary F | Binding acceleration techniques for the detection of analytes |
| JP4438110B2 (ja) * | 1998-07-01 | 2010-03-24 | 東ソー株式会社 | 標的核酸の定量方法 |
| US6316229B1 (en) | 1998-07-20 | 2001-11-13 | Yale University | Single molecule analysis target-mediated ligation of bipartite primers |
| CA2342838A1 (fr) | 1998-09-15 | 2000-03-23 | Yale University | Clonage moleculaire a amplification selon le modele du cercle roulant |
| CA2342837A1 (fr) * | 1998-09-15 | 2000-03-23 | Yale University | Long segment terminal artificiel a repetitions |
| US8163524B2 (en) * | 1998-09-22 | 2012-04-24 | Oncomedx, Inc. | Comparative analysis of extracellular RNA species |
| US20060204989A1 (en) * | 1998-09-22 | 2006-09-14 | Kopreski Michael S | Comparative analysis of extracellular RNA species |
| US20090233276A1 (en) * | 1998-09-22 | 2009-09-17 | Oncomedx, Inc. | Method Enabling the Use of Extracellular Ribonucleic Acid (RNA) Extracted from Plasma or Serum to Detect, Monitor or Evaluate Cancer or Premalignant Conditions |
| US6743906B1 (en) | 1998-10-02 | 2004-06-01 | Board Of Regents, The University Of Texas | PPP2R1B is a tumor suppressor |
| US6153411A (en) | 1998-10-30 | 2000-11-28 | American Water Works Company, Inc. | Methods and kits for detection of Cryptosporidium parvum using immunomagnetic separation and amplification |
| EP1829856A3 (fr) * | 1998-11-12 | 2009-02-25 | Invitrogen Corporation | Réactifs de transfection |
| US6187566B1 (en) | 1999-03-09 | 2001-02-13 | Applied Gene Technologies, Inc. | Method of labeling a nucleic acid amplicon with simultaneous contamination prevention |
| WO2000056916A2 (fr) * | 1999-03-18 | 2000-09-28 | Exiqon A/S | Detection de mutations dans des genes par des amorces de lna specifiques |
| US6630333B1 (en) * | 1999-03-23 | 2003-10-07 | Invitrogen Corporation | Substantially pure reverse transriptases and methods of production thereof |
| US6303305B1 (en) | 1999-03-30 | 2001-10-16 | Roche Diagnostics, Gmbh | Method for quantification of an analyte |
| US6582906B1 (en) | 1999-04-05 | 2003-06-24 | Affymetrix, Inc. | Proportional amplification of nucleic acids |
| US6235479B1 (en) | 1999-04-13 | 2001-05-22 | Bio Merieux, Inc. | Methods and devices for performing analysis of a nucleic acid sample |
| EP1923471B1 (fr) | 1999-04-20 | 2012-12-19 | Illumina, Inc. | Détection de réactions d'acide nucléique sur les réseaux à perle |
| US20030207295A1 (en) * | 1999-04-20 | 2003-11-06 | Kevin Gunderson | Detection of nucleic acid reactions on bead arrays |
| US20060275782A1 (en) * | 1999-04-20 | 2006-12-07 | Illumina, Inc. | Detection of nucleic acid reactions on bead arrays |
| WO2000068411A1 (fr) * | 1999-05-12 | 2000-11-16 | Invitrogen Corporation | Compositions et methodes d'amelioration de la sensibilite et de la specificite d'une synthese d'acide nucleique |
| US8080380B2 (en) * | 1999-05-21 | 2011-12-20 | Illumina, Inc. | Use of microfluidic systems in the detection of target analytes using microsphere arrays |
| NZ515673A (en) * | 1999-05-21 | 2004-03-26 | Invitrogen Corp | Compositions and methods for labeling of nucleic acid molecules |
| US8481268B2 (en) | 1999-05-21 | 2013-07-09 | Illumina, Inc. | Use of microfluidic systems in the detection of target analytes using microsphere arrays |
| US6495320B1 (en) | 1999-07-21 | 2002-12-17 | Affymetrix, Inc. | Even length proportional amplification of nucleic acids |
| CA2380258C (fr) | 1999-07-26 | 2008-07-15 | Clinical Micro Sensors, Inc. | Determination de sequences d'acides nucleiques par detection electronique |
| US6864050B2 (en) | 1999-07-30 | 2005-03-08 | Affymetrix, Inc. | Single-phase amplification of nucleic acids |
| US6242188B1 (en) | 1999-07-30 | 2001-06-05 | Applied Gene Technologies, Inc. | Sample processing to release nucleic acids for direct detection |
| US6379930B1 (en) | 1999-07-30 | 2002-04-30 | Applied Gene Technologies, Inc. | Stabilization of nucleic acid amplification cocktails |
| US6692918B2 (en) * | 1999-09-13 | 2004-02-17 | Nugen Technologies, Inc. | Methods and compositions for linear isothermal amplification of polynucleotide sequences |
| US6958225B2 (en) | 1999-10-27 | 2005-10-25 | Affymetrix, Inc. | Complexity management of genomic DNA |
| US6582938B1 (en) | 2001-05-11 | 2003-06-24 | Affymetrix, Inc. | Amplification of nucleic acids |
| US6794138B1 (en) | 1999-12-16 | 2004-09-21 | Affymetrix, Inc. | Methods of small sample amplification |
| US7582420B2 (en) | 2001-07-12 | 2009-09-01 | Illumina, Inc. | Multiplex nucleic acid reactions |
| US20050214825A1 (en) * | 2000-02-07 | 2005-09-29 | John Stuelpnagel | Multiplex sample analysis on universal arrays |
| US7955794B2 (en) * | 2000-09-21 | 2011-06-07 | Illumina, Inc. | Multiplex nucleic acid reactions |
| US8076063B2 (en) * | 2000-02-07 | 2011-12-13 | Illumina, Inc. | Multiplexed methylation detection methods |
| US6291187B1 (en) | 2000-05-12 | 2001-09-18 | Molecular Staging, Inc. | Poly-primed amplification of nucleic acid sequences |
| EP1158055A1 (fr) | 2000-05-26 | 2001-11-28 | Xu Qi University of Teaxs Laboratoire de Leucémie Chen | Méthode pour le diagnostic de cancers |
| US20060166227A1 (en) * | 2000-06-20 | 2006-07-27 | Stephen Kingsmore | Protein expression profiling |
| AU2001271595A1 (en) * | 2000-06-26 | 2002-01-08 | Nugen Technologies, Inc. | Methods and compositions for transcription-based nucleic acid amplification |
| US7846733B2 (en) * | 2000-06-26 | 2010-12-07 | Nugen Technologies, Inc. | Methods and compositions for transcription-based nucleic acid amplification |
| US6323009B1 (en) * | 2000-06-28 | 2001-11-27 | Molecular Staging, Inc. | Multiply-primed amplification of nucleic acid sequences |
| AU7172201A (en) | 2000-06-30 | 2002-01-14 | Molecular Staging Inc | Signal amplification with lollipop probes |
| JP2002142765A (ja) * | 2000-07-14 | 2002-05-21 | Tosoh Corp | 新規ゲノム解析法 |
| US7198924B2 (en) | 2000-12-11 | 2007-04-03 | Invitrogen Corporation | Methods and compositions for synthesis of nucleic acid molecules using multiple recognition sites |
| WO2002020852A1 (fr) | 2000-09-01 | 2002-03-14 | Gen-Probe Incorporated | Amplification de sequences vih-1 pour la detection de sequences associees aux mutations de la resistance aux medicaments |
| EP1366192B8 (fr) * | 2000-10-24 | 2008-10-29 | The Board of Trustees of the Leland Stanford Junior University | Caracterisation multiplex directe d'adn genomique |
| JP2005503755A (ja) * | 2000-12-13 | 2005-02-10 | ニューゲン テクノロジーズ, インコーポレイテッド | 核酸配列の複数コピーを作製するための方法および組成物、ならびにそれらを検出する方法 |
| US6794141B2 (en) * | 2000-12-22 | 2004-09-21 | Arcturus Bioscience, Inc. | Nucleic acid amplification |
| US20040185455A1 (en) * | 2000-12-26 | 2004-09-23 | Masamitsu Shimada | Method of detecting pathogenic microorganism |
| US6893847B2 (en) * | 2001-01-17 | 2005-05-17 | Tosoh Corporation | Oligonucleotide for detecting Salmonella and method of detecting Salmonella |
| JPWO2002062993A1 (ja) * | 2001-02-06 | 2004-06-10 | タカラバイオ株式会社 | 増幅核酸及びその固定化物 |
| KR100809949B1 (ko) * | 2001-02-15 | 2008-03-06 | 다카라 바이오 가부시키가이샤 | 염기 치환의 검출 방법 |
| US6620586B2 (en) * | 2001-02-20 | 2003-09-16 | Applied Gene Technologies, Inc. | Methods and compositions for analyzing nucleic acids |
| EP1381697B1 (fr) * | 2001-02-27 | 2009-03-18 | Virco Bvba | Amplification de sonde circulaire (asc) de molecules d'acide nucleique circularisees |
| CA2416963C (fr) * | 2001-03-09 | 2012-01-10 | Nugen Technologies, Inc. | Procedes et compositions permettant l'amplification de sequences d'arn |
| US6573051B2 (en) * | 2001-03-09 | 2003-06-03 | Molecular Staging, Inc. | Open circle probes with intramolecular stem structures |
| WO2002072773A2 (fr) * | 2001-03-09 | 2002-09-19 | Nugen Technologies, Inc. | Methodes et compositions pour amplification de sequences d'arn |
| US20030092157A1 (en) * | 2001-03-16 | 2003-05-15 | Hayden Michael R. | Compositions, screening systems and methods for modulating HDL cholesterol and triglyceride levels |
| US6596489B2 (en) | 2001-03-30 | 2003-07-22 | Applied Gene Technologies | Methods and compositions for analyzing nucleotide sequence mismatches using RNase H |
| AU2003303395A1 (en) * | 2001-05-22 | 2004-07-22 | Dahl, Gary, A. | Target-dependent transcription using deletion mutants of n4 rna polymerase |
| CA2448484A1 (fr) * | 2001-05-25 | 2002-12-05 | Xenon Genetics, Inc. | Procedes de diagnostic de maladies cardio-vasculaires, faibles niveaux de cholesterol ldl, et niveaux eleves de triglyceride |
| CN100379861C (zh) * | 2001-06-12 | 2008-04-09 | 宝生物工程株式会社 | 核酸扩增或检测用试剂的稳定化方法和保存方法 |
| BR0210610A (pt) * | 2001-06-22 | 2005-04-19 | Marshfield Clinic | Métodos e oligonucleotìdeos para detecção de salmonella sp., e.coli 0157:h7 e listeria monocitogenes |
| DE60229958D1 (de) * | 2001-06-28 | 2009-01-02 | Mountain View Pharmaceuticals | Polymerstabilisierte proteinasen |
| US9261460B2 (en) | 2002-03-12 | 2016-02-16 | Enzo Life Sciences, Inc. | Real-time nucleic acid detection processes and compositions |
| US9777312B2 (en) * | 2001-06-30 | 2017-10-03 | Enzo Life Sciences, Inc. | Dual polarity analysis of nucleic acids |
| US20040161741A1 (en) | 2001-06-30 | 2004-08-19 | Elazar Rabani | Novel compositions and processes for analyte detection, quantification and amplification |
| WO2003006677A2 (fr) | 2001-07-12 | 2003-01-23 | Illumina, Inc. | Reactions multiplex d'acides nucleiques |
| US7297778B2 (en) | 2001-07-25 | 2007-11-20 | Affymetrix, Inc. | Complexity management of genomic DNA |
| DK1421215T3 (da) * | 2001-07-25 | 2011-06-27 | Oncomedx Inc | Fremgangsmåder til evaluering af patologiske tilstande under anvendelse af ekstracellulært RNA |
| US6872529B2 (en) * | 2001-07-25 | 2005-03-29 | Affymetrix, Inc. | Complexity management of genomic DNA |
| TWI335938B (en) * | 2001-08-15 | 2011-01-11 | Rna replication and amplification | |
| KR20040028991A (ko) * | 2001-08-20 | 2004-04-03 | 다카라 바이오 가부시키가이샤 | 핵산 증폭 방법 |
| WO2003020979A1 (fr) * | 2001-08-31 | 2003-03-13 | Rosetta Inpharmactis Llc. | Procedes de preparation d'echantillons d'acide nucleique utiles pour cribler des reseaux d'adn |
| US20030165859A1 (en) | 2001-10-23 | 2003-09-04 | Invitrogen Corporation | Primers and methods for the detection and discrimination of nucleic acids |
| US20030104454A1 (en) * | 2001-11-05 | 2003-06-05 | Kopreski Michael S. | Method for detection of DNA methyltransferase RNA in plasma and serum |
| US20100159464A1 (en) * | 2001-11-05 | 2010-06-24 | Oncomedx, Inc. | Method for Detection of DNA Methyltransferase RNA in Plasma and Serum |
| DE60228995D1 (de) * | 2001-12-19 | 2008-10-30 | Affymetrix Inc | Arrayplatten und verfahren zur herstellung von arrayplatten |
| WO2003064679A2 (fr) * | 2002-01-30 | 2003-08-07 | Id Biomedical Corporation | Procedes pour detecter des microorganismes resistant a la vancomycine et composition pour la mise en oeuvre dudit procede |
| US7553619B2 (en) * | 2002-02-08 | 2009-06-30 | Qiagen Gmbh | Detection method using dissociated rolling circle amplification |
| US7354742B2 (en) | 2002-02-22 | 2008-04-08 | Ortho-Mcneil Pharmaceutical, Inc. | Method for generating amplified RNA |
| US9353405B2 (en) | 2002-03-12 | 2016-05-31 | Enzo Life Sciences, Inc. | Optimized real time nucleic acid detection processes |
| US20060246434A1 (en) * | 2002-03-15 | 2006-11-02 | Erlander Mark G | Nucleic acid amplification |
| WO2004011665A2 (fr) * | 2002-05-17 | 2004-02-05 | Nugen Technologies, Inc. | Procedes de fragmentation, d'etiquetage et d'immobilisation d'acides nucleiques |
| US20030219754A1 (en) * | 2002-05-23 | 2003-11-27 | Oleksy Jerome E. | Fluorescence polarization detection of nucleic acids |
| US20030219755A1 (en) * | 2002-05-24 | 2003-11-27 | Nanibhushan Dattagupta | Compositions and methods for performing hybridization assays using target enhanced signal amplification (TESA) |
| US20040219516A1 (en) * | 2002-07-18 | 2004-11-04 | Invitrogen Corporation | Viral vectors containing recombination sites |
| US20040011650A1 (en) * | 2002-07-22 | 2004-01-22 | Frederic Zenhausern | Method and apparatus for manipulating polarizable analytes via dielectrophoresis |
| EP1546392A4 (fr) * | 2002-08-02 | 2006-05-24 | Stratatech Corp | Detection d'adn specifique d'espece |
| WO2004013290A2 (fr) * | 2002-08-05 | 2004-02-12 | Invitrogen Corporation | Compositions et procedes applicables a la biologie moleculaire |
| EP1539209A2 (fr) * | 2002-09-05 | 2005-06-15 | Invitrogen Corporation | Compositions et procedes de synthese d'acides nucleiques |
| AU2002951411A0 (en) | 2002-09-16 | 2002-09-26 | The University Of Sydney | Genotype screen |
| JP2006500030A (ja) | 2002-09-20 | 2006-01-05 | イェール ユニバーシティ | リボスイッチ、その使用方法、ならびにリボスイッチとともに用いるための組成物 |
| US20040259105A1 (en) * | 2002-10-03 | 2004-12-23 | Jian-Bing Fan | Multiplex nucleic acid analysis using archived or fixed samples |
| US20040219565A1 (en) | 2002-10-21 | 2004-11-04 | Sakari Kauppinen | Oligonucleotides useful for detecting and analyzing nucleic acids of interest |
| US7074561B2 (en) * | 2002-10-22 | 2006-07-11 | Biomerieux, Inc. | Isothermal amplification based assay for the detection and quantitation of alpha-fetoprotein mRNA |
| US20040096829A1 (en) * | 2002-11-14 | 2004-05-20 | Allaire Normand E. | Absolute quantitation of nucleic acids by RT-PCR |
| AU2003297557B2 (en) * | 2002-11-21 | 2009-02-26 | Cellscript, Inc. | Methods for using primers that encode one strand of a double-stranded promoter |
| CN1230531C (zh) * | 2002-12-09 | 2005-12-07 | 清华大学 | 从样品中分离细胞粒子的方法 |
| CN1223680C (zh) | 2002-12-10 | 2005-10-19 | 清华大学 | 扩增靶细胞或病毒的核酸的方法 |
| EP1573009B1 (fr) | 2002-12-18 | 2011-09-21 | Third Wave Technologies, Inc. | Detection de petits acides nucleiques |
| US7955795B2 (en) * | 2003-06-06 | 2011-06-07 | Qiagen Gmbh | Method of whole genome amplification with reduced artifact production |
| US9487823B2 (en) | 2002-12-20 | 2016-11-08 | Qiagen Gmbh | Nucleic acid amplification |
| US6977153B2 (en) * | 2002-12-31 | 2005-12-20 | Qiagen Gmbh | Rolling circle amplification of RNA |
| US7297780B2 (en) | 2003-01-06 | 2007-11-20 | Third Wave Technologies, Inc. | Reactive functional groups for preparation of modified nucleic acid |
| US6852494B2 (en) * | 2003-01-10 | 2005-02-08 | Linden Technologies, Inc. | Nucleic acid amplification |
| WO2004083806A2 (fr) | 2003-01-22 | 2004-09-30 | University Of South Florida | Appareil a genocapteur autonome et procedes d'utilisation de celui-ci |
| US6943768B2 (en) | 2003-02-21 | 2005-09-13 | Xtellus Inc. | Thermal control system for liquid crystal cell |
| US20060192960A1 (en) * | 2003-03-24 | 2006-08-31 | Rencs Erik V | Polarization detection |
| US8043834B2 (en) | 2003-03-31 | 2011-10-25 | Qiagen Gmbh | Universal reagents for rolling circle amplification and methods of use |
| JP4782668B2 (ja) | 2003-03-31 | 2011-09-28 | エフ.ホフマン−ラ ロシュ アーゲー | 日本脳炎ウィルス血清グループのメンバーを包含する一定のフラビウィルスを検出するための組成物及び方法 |
| US20040265870A1 (en) * | 2003-04-09 | 2004-12-30 | Invitrogen Corporation | Methods of synthesizing and labeling nucleic acid molecules |
| WO2004092418A2 (fr) * | 2003-04-14 | 2004-10-28 | Nugen Technologies, Inc. | Amplification globale effectuee avec une amorce composite amorcee de maniere aleatoire |
| CA2528572C (fr) | 2003-06-10 | 2020-08-25 | The Trustees Of Boston University | Analyse de l'expression genetique des cellules epitheliales de voies aeriennes pour diagnostiquer un cancer du poumon |
| EP2216415B2 (fr) * | 2003-08-01 | 2017-01-04 | Life Technologies Corporation | Compositions et procédés de préparation de courtes molécules d'ARN et d'autres acides nucléiques |
| JP4790621B2 (ja) | 2003-11-26 | 2011-10-12 | アドバンディーエックス, インコーポレイテッド | 特定のStaphylococcus種の分析のためのペプチド核酸プローブ |
| DE602004027538D1 (de) | 2003-12-01 | 2010-07-15 | Life Technologies Corp | Rekombinationsstellen enthaltende nukleinsäuremoleküle und verfahren zur verwendung davon |
| US20050208538A1 (en) | 2003-12-29 | 2005-09-22 | Nurith Kurn | Methods for analysis of nucleic acid methylation status and methods for fragmentation, labeling and immobilization of nucleic acids |
| WO2005076908A2 (fr) * | 2004-02-04 | 2005-08-25 | Eppendorf Ag | Amplification d'acide nucleique ameliore de proteine antigel |
| EP1564306B1 (fr) | 2004-02-17 | 2013-08-07 | Affymetrix, Inc. | Procédé de fragmentation et de marquage d'ADN |
| EP1574583A1 (fr) * | 2004-03-10 | 2005-09-14 | Roche Diagnostics GmbH | Procédé pour isoler des bactéries des échantillons biologiques |
| US7462451B2 (en) * | 2004-04-26 | 2008-12-09 | Third Wave Technologies, Inc. | Compositions for modifying nucleic acids |
| ES2560433T3 (es) | 2004-04-26 | 2016-02-19 | Wako Pure Chemical Industries, Ltd. | Cebadores para la detección del bacilo de la tuberculosis y procedimiento de detección del bacilo de la tuberculosis humana con los mismos |
| CN103884698B (zh) | 2004-06-07 | 2017-04-12 | 先锋生物科技股份有限公司 | 用于微流体器件的光学透镜系统和方法 |
| US7776530B2 (en) * | 2004-06-29 | 2010-08-17 | Wallac Oy | Integrated nucleic acid analysis |
| US20090269737A1 (en) * | 2004-06-29 | 2009-10-29 | Wallac Oy | Integrated non-homogeneous nucleic acid amplification and detection |
| EP1767655A4 (fr) | 2004-07-13 | 2007-08-01 | Takeda Pharmaceutical | Méthode de contrôle des fonctions d'une cellule |
| WO2006026388A2 (fr) | 2004-08-27 | 2006-03-09 | Gen-Probe Incorporated | Techniques d'amplification d'acide nucleique avec une seule amorce |
| US7713697B2 (en) * | 2004-08-27 | 2010-05-11 | Gen-Probe Incorporated | Methods and kits for amplifying DNA |
| US20060073506A1 (en) | 2004-09-17 | 2006-04-06 | Affymetrix, Inc. | Methods for identifying biological samples |
| US20060068430A1 (en) * | 2004-09-20 | 2006-03-30 | Sigma-Aldrich Co. | Purification of biomolecules from contaminating intact nucleic acids |
| US20060073511A1 (en) | 2004-10-05 | 2006-04-06 | Affymetrix, Inc. | Methods for amplifying and analyzing nucleic acids |
| US7682782B2 (en) | 2004-10-29 | 2010-03-23 | Affymetrix, Inc. | System, method, and product for multiple wavelength detection using single source excitation |
| EP1652580A1 (fr) | 2004-10-29 | 2006-05-03 | Affymetrix, Inc. | Microréseaux à haut débit, ensembles réseaux et procédé de leurs fabrications |
| CA2587670A1 (fr) | 2004-11-01 | 2006-05-11 | George Mason University | Compositions et methodes pour le diagnostic de troubles du colon |
| JP2008522588A (ja) * | 2004-12-06 | 2008-07-03 | バイオヴェリス コーポレイション | 炭疽菌(Bacillusanthracis)を検出するための方法及び組成物 |
| EP1819828A2 (fr) * | 2004-12-11 | 2007-08-22 | CytoGenix, Inc. | Biosynthese acellulaire d'acide nucleique de haute qualite et utilisations correspondantes |
| EP1841879A4 (fr) | 2005-01-25 | 2009-05-27 | Population Genetics Technologi | Amplification isotherme d'adn |
| DK2529619T3 (en) | 2005-02-17 | 2016-01-11 | Biogen Ma Inc | Treatment of neurological disorders |
| CN101128604A (zh) | 2005-02-28 | 2008-02-20 | 生物探索公司 | 用于进行涉及核酸分子的直接酶反应的方法 |
| PL1863519T3 (pl) | 2005-03-31 | 2014-03-31 | Massachusetts Gen Hospital | Modulowanie aktywności HGF/HGFR do leczenia obrzęku wskutek niedrożności naczyń chłonnych |
| US20060223071A1 (en) * | 2005-04-01 | 2006-10-05 | Wisniewski Michele E | Methods, compositions, and kits for detecting nucleic acids in a single vessel |
| EP1863908B1 (fr) | 2005-04-01 | 2010-11-17 | Qiagen GmbH | Transcription inverse et amplification d'arn avec degradation simultanee d'adn |
| CA2605158A1 (fr) | 2005-04-14 | 2006-10-26 | The Trustees Of Boston University | Diagnostic des troubles pulmonaires mettant en oeuvre la prediction de categories |
| US20060240442A1 (en) * | 2005-04-20 | 2006-10-26 | Vevea Dirk N | Methods and oligonucleotides for the detection of Salmonella SP., E coli 0157:H7, and Listeria monocytogenes |
| US7550264B2 (en) | 2005-06-10 | 2009-06-23 | Datascope Investment Corporation | Methods and kits for sense RNA synthesis |
| US7838646B2 (en) | 2005-08-18 | 2010-11-23 | Quest Diagnostics Investments Incorporated | Cystic fibrosis transmembrane conductance regulator gene mutations |
| EP1929046B1 (fr) | 2005-09-07 | 2012-07-11 | Nugen Technologies, Inc. | Procedure d'amplication d'acide nucleique amelioree |
| US8980246B2 (en) | 2005-09-07 | 2015-03-17 | Sillajen Biotherapeutics, Inc. | Oncolytic vaccinia virus cancer therapy |
| EP1762627A1 (fr) | 2005-09-09 | 2007-03-14 | Qiagen GmbH | Procédé pour l'activation d'acides nucléiques pour effectuer une réaction d'une polymérase |
| WO2007044671A2 (fr) * | 2005-10-06 | 2007-04-19 | Lucigen Corporation | Polymerases virales thermostables, et leurs methodes d'utilisation |
| US8609829B2 (en) * | 2005-10-17 | 2013-12-17 | Gen-Probe Incorporated | Compositions and methods to detect Legionella pneumophila nucleic acid |
| US7422857B2 (en) | 2005-10-28 | 2008-09-09 | University Of South Florida | Detection of polyketide synthetase gene expression in Karenia brevis |
| US20070207455A1 (en) * | 2005-11-17 | 2007-09-06 | Third Wave Technologies, Inc. | Compositions and methods for detecting an HCV-1 subtype |
| US7981606B2 (en) * | 2005-12-21 | 2011-07-19 | Roche Molecular Systems, Inc. | Control for nucleic acid testing |
| JP2009524411A (ja) | 2005-12-21 | 2009-07-02 | イェール ユニバーシティー | リボスイッチの調節に関連した方法および組成物 |
| WO2008018904A2 (fr) | 2006-01-18 | 2008-02-14 | Argos Therapeutics, Inc. | Systèmes et procédés destinés au traitement d'échantillons dans un réceptacle fermé, et dispositifs associés |
| WO2007087262A2 (fr) * | 2006-01-23 | 2007-08-02 | Population Genetics Technologies Ltd. | Amplification sélective du génome |
| US20090176878A1 (en) * | 2007-10-05 | 2009-07-09 | Washington University In St. Louis | Genetic polymorphisms and substance dependence |
| US20110143344A1 (en) * | 2006-03-01 | 2011-06-16 | The Washington University | Genetic polymorphisms and substance dependence |
| AU2007223788B2 (en) | 2006-03-09 | 2012-11-29 | The Trustees Of Boston University | Diagnostic and prognostic methods for lung disorders using gene expression profiles from nose epithelial cells |
| US7947441B2 (en) * | 2006-04-14 | 2011-05-24 | University Of South Florida | Molecular detection and quantification of Enterococci |
| EP2522751B1 (fr) | 2006-06-01 | 2018-11-21 | Third Wave Technologies, Inc. | Détection d'acides nucléiques à l'aide d'un essai INVADER amélioré |
| US11001881B2 (en) | 2006-08-24 | 2021-05-11 | California Institute Of Technology | Methods for detecting analytes |
| EP2050828A3 (fr) | 2006-06-12 | 2009-08-26 | Oncomethylome Sciences S.A. | Marqueurs de méthylation destinés à une détection et un pronostic précoces de cancers du colon |
| EP2038429B1 (fr) * | 2006-06-30 | 2013-08-21 | Nugen Technologies, Inc. | Procédés de fragmentation et de marquage d'acides nucléiques |
| US11525156B2 (en) | 2006-07-28 | 2022-12-13 | California Institute Of Technology | Multiplex Q-PCR arrays |
| US8048626B2 (en) | 2006-07-28 | 2011-11-01 | California Institute Of Technology | Multiplex Q-PCR arrays |
| CA2660286A1 (fr) | 2006-08-09 | 2008-02-21 | Homestead Clinical Corporation | Proteines specifiques d'organes et procedes d'utilisation |
| US11560588B2 (en) | 2006-08-24 | 2023-01-24 | California Institute Of Technology | Multiplex Q-PCR arrays |
| EP1914303A1 (fr) * | 2006-10-09 | 2008-04-23 | Qiagen GmbH | ADN polymerases de Thermus eggertssonii |
| US9845494B2 (en) | 2006-10-18 | 2017-12-19 | Affymetrix, Inc. | Enzymatic methods for genotyping on arrays |
| US8026065B2 (en) | 2006-11-02 | 2011-09-27 | Yale University | Assessment of oocyte competence |
| WO2008079972A2 (fr) | 2006-12-20 | 2008-07-03 | Bayer Healthcare Llc | Composé d'hydroxy méthyle phényle pyrazolyle urée utilisé pour traiter un cancer |
| US7794937B2 (en) | 2006-12-22 | 2010-09-14 | Quest Diagnostics Investments Incorporated | Cystic fibrosis transmembrane conductance regulator gene mutations |
| CA2673772A1 (fr) * | 2007-01-17 | 2008-07-24 | Meridian Bioscience, Inc. | Reactifs stables et necessaires intervenant dans l'amplification isotherme induite par la boucle (lamp) |
| GB0701253D0 (en) | 2007-01-23 | 2007-02-28 | Diagnostics For The Real World | Nucleic acid amplification and testing |
| EP1956097A1 (fr) | 2007-02-06 | 2008-08-13 | bioMerieux B.V. | Procédé de discrimination des polymorphismes mononucléotidiques (SNPs) |
| US8183359B2 (en) * | 2007-03-01 | 2012-05-22 | Gen-Probe Incorporated | Kits for amplifying DNA |
| JP2010520750A (ja) * | 2007-03-01 | 2010-06-17 | ジェン−プロウブ インコーポレイテッド | Dnaを増幅するための方法およびキット |
| EP1970440A1 (fr) * | 2007-03-06 | 2008-09-17 | Qiagen GmbH | Stabilisation de polymérase par des détergents ioniques |
| WO2009006438A2 (fr) | 2007-06-29 | 2009-01-08 | Epicentre Technologies Corporation | Adn de copie et arn sens |
| GB0713183D0 (en) * | 2007-07-06 | 2007-08-15 | King S College London | Method |
| KR101376359B1 (ko) * | 2007-08-01 | 2014-03-27 | 다나-파버 캔서 인스티튜트 인크. | 표적 서열 강화 |
| CA2694832A1 (fr) | 2007-08-06 | 2009-02-12 | Orion Genomics Llc | Nouveaux polymorphismes nucleotidiques uniques et combinaisons de nouveaux polymorphismes connus permettant de determiner l'expression specifique d'allele du gene igf2 |
| EP2183394B1 (fr) * | 2007-09-07 | 2012-12-12 | Third Wave Technologies, Inc. | Procédés et applications pour la quantification d'une cible |
| CA2639416C (fr) | 2007-09-11 | 2019-12-31 | F. Hoffmann-La Roche Ag | Test de diagnostic pour la susceptibilite aux inhibiteurs de la b-raf kinase |
| US20090136942A1 (en) * | 2007-09-18 | 2009-05-28 | Oncomedx, Inc. | Analysis of Extracellular RNA |
| EP2217720A4 (fr) | 2007-11-01 | 2010-12-08 | Univ Iowa Res Found | Analyse de lieu de rca pour estimer la sensibilité à l'amd et au mpgnii |
| EP2071034A1 (fr) | 2007-12-12 | 2009-06-17 | bioMérieux | Procédé pour le traitement d'une solution en vue d'éliminer un acide ribonucléique après amplification |
| JP5503552B2 (ja) | 2007-12-21 | 2014-05-28 | バイオメリュー・エスエイ | メチシリン耐性黄色ブドウ球菌の検出 |
| US8034568B2 (en) * | 2008-02-12 | 2011-10-11 | Nugen Technologies, Inc. | Isothermal nucleic acid amplification methods and compositions |
| CA2715774A1 (fr) | 2008-02-19 | 2009-08-27 | Oncomethylome Sciences Sa | Detection et pronostic de cancer du poumon |
| JP5651022B2 (ja) * | 2008-03-15 | 2015-01-07 | ホロジック,インコーポレーテッド | 増幅反応中の核酸分子を分析するための組成物及び方法 |
| EP2626435B1 (fr) | 2008-03-21 | 2016-06-01 | MDxHealth S.A. | Détection et pronostic du cancer des cervicales |
| WO2009117698A2 (fr) | 2008-03-21 | 2009-09-24 | Nugen Technologies, Inc. | Procédés d'amplification d'arn en présence d'adn |
| EP2113574A1 (fr) | 2008-04-28 | 2009-11-04 | Biotype AG | Substances et procédés pour un dosage de profilage à base d'ADN |
| JP2009268665A (ja) * | 2008-05-07 | 2009-11-19 | Canon Inc | 吸入装置 |
| WO2010011506A2 (fr) * | 2008-07-23 | 2010-01-28 | The Washington University | Facteurs de risque et cible thérapeutique pour des troubles neurodégénératifs |
| GB0814570D0 (en) | 2008-08-08 | 2008-09-17 | Diagnostics For The Real World | Isolation of nucleic acid |
| WO2010028014A1 (fr) * | 2008-09-03 | 2010-03-11 | Abbott Laboratories | Dosages et kits pour déterminer un tropisme du vih-1 |
| US9127312B2 (en) | 2011-02-09 | 2015-09-08 | Bio-Rad Laboratories, Inc. | Analysis of nucleic acids |
| CA2679750A1 (fr) | 2008-09-23 | 2010-03-23 | Nexus Dx, Inc. | Methodes de detection d'acides nucleiques dans un echantillon |
| EP2172563A1 (fr) | 2008-09-24 | 2010-04-07 | bioMérieux S.A. | Procédé pour diminuer la dépendance envers la variation de séquence d'une cible d'acide nucléique dans une analyse d'hybridation de diagnostic |
| PL2963709T3 (pl) | 2008-10-24 | 2017-11-30 | Epicentre Technologies Corporation | Kompozycje końców transpozonu i sposoby modyfikacji kwasów nukleinowych |
| US9080211B2 (en) | 2008-10-24 | 2015-07-14 | Epicentre Technologies Corporation | Transposon end compositions and methods for modifying nucleic acids |
| CA3161998A1 (en) | 2009-02-11 | 2010-08-19 | Caris Mpi, Inc. | Molecular profiling of tumors |
| ES2544265T3 (es) | 2009-02-11 | 2015-08-28 | Orion Genomics, Llc | Combinaciones de polimorfismos para determinar la expresión específica de alelo de IGF2 |
| WO2010124257A2 (fr) * | 2009-04-24 | 2010-10-28 | Colby Pharmaceutical Company | Procédés et coffrets pour déterminer les taux de radicaux libres d'oxygène (ofr) dans des tissus animaux et humains en tant que marqueur de pronostic pour le cancer et d'autres pathophysiologies |
| WO2010126913A1 (fr) | 2009-04-27 | 2010-11-04 | Gen-Probe Incorporated | Procédés et coffrets pour une utilisation pour l'amplification sélective de séquences cibles |
| BRPI1011432A2 (pt) | 2009-05-08 | 2019-09-24 | Novartis Ag | ensaios genericos para deteccao de virus influenza |
| WO2011032040A1 (fr) | 2009-09-10 | 2011-03-17 | Centrillion Technology Holding Corporation | Procédés de séquençage ciblé |
| US10174368B2 (en) | 2009-09-10 | 2019-01-08 | Centrillion Technology Holdings Corporation | Methods and systems for sequencing long nucleic acids |
| WO2011032088A1 (fr) | 2009-09-11 | 2011-03-17 | Arca Biopharma, Inc. | Polymorphismes dans le gène pde3a |
| HUE038708T2 (hu) | 2009-09-14 | 2018-11-28 | Sillajen Biotherapeutics Inc | Onkolitikus vaccinia vírus kombinációs rákterápia |
| WO2011036173A1 (fr) | 2009-09-24 | 2011-03-31 | Oncomethylome Sciences S.A. | Détection et pronostic du cancer du col de l'utérus |
| US20160186266A1 (en) | 2009-10-27 | 2016-06-30 | Carislife Sciences, Inc. | Molecular profiling for personalized medicine |
| KR20170098985A (ko) | 2009-11-19 | 2017-08-30 | 솔리스 바이오다인 | 폴리펩티드 안정성 및 활성을 증가시키는 조성물 및 관련된 방법 |
| JP2013511991A (ja) * | 2009-11-25 | 2013-04-11 | クアンタライフ, インコーポレイテッド | 遺伝子材料を検出する方法および組成物 |
| CN102648295B (zh) | 2009-12-07 | 2017-08-08 | 伊鲁米那股份有限公司 | 用于多重基因分型的多样品索引 |
| US8501122B2 (en) | 2009-12-08 | 2013-08-06 | Affymetrix, Inc. | Manufacturing and processing polymer arrays |
| US8835358B2 (en) | 2009-12-15 | 2014-09-16 | Cellular Research, Inc. | Digital counting of individual molecules by stochastic attachment of diverse labels |
| WO2011084772A2 (fr) | 2009-12-21 | 2011-07-14 | Northwestern University | Troubles alléliques causés par des mutations dans trpv4 |
| US20110151457A1 (en) | 2009-12-22 | 2011-06-23 | Elitech Holding B.V. | Hypertheromostable endonuclease iv substrate probe |
| US20110165568A1 (en) | 2009-12-31 | 2011-07-07 | Life Technologies Corporation | Sequences of e.coli 055:h7 genome |
| EP2545189B1 (fr) | 2010-03-08 | 2018-01-10 | Dana-Farber Cancer Institute, Inc. | Enrichissement d'une pcr froide complète doté d'une séquence de blocage de référence |
| WO2011128096A1 (fr) | 2010-04-16 | 2011-10-20 | Roche Diagnostics Gmbh | Marqueurs de polymorphisme destinés à prédire la réponse à un traitement par un médicament anticorps monoclonal inhibant les récepteurs de l'interleukine-6 |
| CA2796744A1 (fr) | 2010-04-17 | 2011-10-20 | Bayer Healthcare Llc | Metabolites synthetiques d'omega-carboxyaryldiphenylurees fluorosubstituees pour le traitement et la prevention de maladies et d'etats |
| JP5454338B2 (ja) | 2010-04-28 | 2014-03-26 | 株式会社島津製作所 | 液滴操作によるリアルタイム核酸増幅法 |
| CA2798123C (fr) | 2010-05-05 | 2020-06-23 | The Governing Council Of The University Of Toronto | Procede de traitement d'echantillons seches utilisant un dispositif microfluidique numerique |
| US20130143214A1 (en) | 2010-06-04 | 2013-06-06 | Chronix Biomedical | Prostate cancer associated circulating nucleic acid biomarkers |
| WO2012018638A2 (fr) | 2010-07-26 | 2012-02-09 | Biomatrica, Inc. | Compositions de stabilisation d'adn, d'arn, de protéines dans le sang et d'autres échantillons biologiques lors du transport et du stockage à températures ambiantes |
| EP2598661B1 (fr) | 2010-07-26 | 2017-09-27 | Biomatrica, INC. | Compositions de stabilisation d'adn, d'arn, de protéines salivaires et d'autres échantillons biologiques lors du transport et du stockage à températures ambiantes |
| US20130261003A1 (en) | 2010-08-06 | 2013-10-03 | Ariosa Diagnostics, In. | Ligation-based detection of genetic variants |
| US11203786B2 (en) | 2010-08-06 | 2021-12-21 | Ariosa Diagnostics, Inc. | Detection of target nucleic acids using hybridization |
| US20130040375A1 (en) | 2011-08-08 | 2013-02-14 | Tandem Diagnotics, Inc. | Assay systems for genetic analysis |
| US8822663B2 (en) | 2010-08-06 | 2014-09-02 | Moderna Therapeutics, Inc. | Engineered nucleic acids and methods of use thereof |
| US8700338B2 (en) | 2011-01-25 | 2014-04-15 | Ariosa Diagnosis, Inc. | Risk calculation for evaluation of fetal aneuploidy |
| US10167508B2 (en) | 2010-08-06 | 2019-01-01 | Ariosa Diagnostics, Inc. | Detection of genetic abnormalities |
| US10533223B2 (en) | 2010-08-06 | 2020-01-14 | Ariosa Diagnostics, Inc. | Detection of target nucleic acids using hybridization |
| US20140342940A1 (en) | 2011-01-25 | 2014-11-20 | Ariosa Diagnostics, Inc. | Detection of Target Nucleic Acids using Hybridization |
| US20120034603A1 (en) | 2010-08-06 | 2012-02-09 | Tandem Diagnostics, Inc. | Ligation-based detection of genetic variants |
| US11031095B2 (en) | 2010-08-06 | 2021-06-08 | Ariosa Diagnostics, Inc. | Assay systems for determination of fetal copy number variation |
| EP2614163B1 (fr) | 2010-09-07 | 2016-01-06 | Novartis AG | Tests génériques pour la détection de réovirus de mammifères |
| WO2012040619A2 (fr) | 2010-09-24 | 2012-03-29 | Massachusetts Eye And Ear Infirmary | Méthodes et compositions pour le pronostic et/ou la détection d'une dégénérescence maculaire liée à l'âge |
| PT3590949T (pt) | 2010-10-01 | 2022-08-02 | Modernatx Inc | Ácidos ribonucleicos contendo n1-metilpseudouracilos e suas utilizações |
| US20150218639A1 (en) | 2014-01-17 | 2015-08-06 | Northwestern University | Biomarkers predictive of predisposition to depression and response to treatment |
| US20150225792A1 (en) | 2014-01-17 | 2015-08-13 | Northwestern University | Compositions and methods for identifying depressive disorders |
| US10233501B2 (en) | 2010-10-19 | 2019-03-19 | Northwestern University | Biomarkers predictive of predisposition to depression and response to treatment |
| US10093981B2 (en) | 2010-10-19 | 2018-10-09 | Northwestern University | Compositions and methods for identifying depressive disorders |
| WO2012086243A1 (fr) | 2010-12-21 | 2012-06-28 | 株式会社 島津製作所 | Dispositif et procédé de traitement d'un composant cible dans un tube |
| US10131947B2 (en) | 2011-01-25 | 2018-11-20 | Ariosa Diagnostics, Inc. | Noninvasive detection of fetal aneuploidy in egg donor pregnancies |
| US8756020B2 (en) | 2011-01-25 | 2014-06-17 | Ariosa Diagnostics, Inc. | Enhanced risk probabilities using biomolecule estimations |
| US9994897B2 (en) | 2013-03-08 | 2018-06-12 | Ariosa Diagnostics, Inc. | Non-invasive fetal sex determination |
| WO2012103031A2 (fr) | 2011-01-25 | 2012-08-02 | Ariosa Diagnostics, Inc. | Détection d'anomalies génétiques |
| US11270781B2 (en) | 2011-01-25 | 2022-03-08 | Ariosa Diagnostics, Inc. | Statistical analysis for non-invasive sex chromosome aneuploidy determination |
| CN110129436A (zh) | 2011-02-02 | 2019-08-16 | 精密科学公司 | Dna甲基化的数字序列分析 |
| CA2827200A1 (fr) | 2011-02-24 | 2012-08-30 | Hill's Pet Nutrition, Inc. | Compositions et procedes pour diagnostiquer et traiter des troubles renaux chez un felin |
| EP2678452B1 (fr) | 2011-02-25 | 2016-02-24 | Novartis AG | Témoin positif interne exogène pour détection de virus |
| WO2012118745A1 (fr) | 2011-02-28 | 2012-09-07 | Arnold Oliphant | Systèmes de dosage pour détection d'aneuploïdie et détermination du sexe |
| EP2691541B1 (fr) | 2011-03-31 | 2017-10-18 | Dana-Farber Cancer Institute, Inc. | Procédés pour éléver le niveau de séquences mutées dans un mélange de séquences sauvages et mutées |
| US8710200B2 (en) | 2011-03-31 | 2014-04-29 | Moderna Therapeutics, Inc. | Engineered nucleic acids encoding a modified erythropoietin and their expression |
| US20120252682A1 (en) | 2011-04-01 | 2012-10-04 | Maples Corporate Services Limited | Methods and systems for sequencing nucleic acids |
| US8993341B2 (en) | 2011-05-12 | 2015-03-31 | Exact Sciences Corporation | Removal of PCR inhibitors |
| US8808990B2 (en) | 2011-05-12 | 2014-08-19 | Exact Sciences Corporation | Serial isolation of multiple DNA targets from stool |
| CN103649298A (zh) | 2011-05-12 | 2014-03-19 | 精密科学公司 | 核酸的分离 |
| EP2721175B1 (fr) | 2011-06-15 | 2015-09-16 | Hill's Pet Nutrition, Inc. | Compositions et méthodes de diagnostic et de surveillance de l'hyperthyroïdie chez un félin |
| EP2721170A2 (fr) | 2011-06-17 | 2014-04-23 | Life Technologies Corporation | Compositions et procédés de détection d'espèces et souches de cronobacter spp. et de cronobacter |
| CA2839896A1 (fr) | 2011-06-21 | 2012-12-27 | Alnylam Pharmaceuticals, Inc. | Dosages et procedes de determination de l'activite d'un agent therapeutique chez un sujet |
| WO2013024175A2 (fr) | 2011-08-17 | 2013-02-21 | Technische Universität München | Moyens et méthodes de diagnostic pour le diabète de type 2 |
| DK2748332T3 (en) | 2011-08-24 | 2018-07-16 | Oxoid Ltd | COMPOSITIONS AND METHODS FOR DETECTING MULTIPLE MICROORGANISMS |
| ES2589455T3 (es) | 2011-08-31 | 2016-11-14 | F. Hoffmann-La Roche Ag | Método para predecir el riesgo de hipertensión asociado a la terapia antiangiogénesis |
| KR20140064923A (ko) | 2011-08-31 | 2014-05-28 | 에프. 호프만-라 로슈 아게 | 혈관신생 억제제에 대한 반응성 |
| US8712697B2 (en) | 2011-09-07 | 2014-04-29 | Ariosa Diagnostics, Inc. | Determination of copy number variations using binomial probability calculations |
| US9464124B2 (en) | 2011-09-12 | 2016-10-11 | Moderna Therapeutics, Inc. | Engineered nucleic acids and methods of use thereof |
| WO2013043715A1 (fr) | 2011-09-19 | 2013-03-28 | Genentech, Inc. | Polythérapies comportant des antagonistes de c-met et de b-raf |
| SG11201401196WA (en) | 2011-10-03 | 2014-05-29 | Moderna Therapeutics Inc | Modified nucleosides, nucleotides, and nucleic acids, and uses thereof |
| SG10201510189WA (en) | 2011-10-19 | 2016-01-28 | Nugen Technologies Inc | Compositions And Methods For Directional Nucleic Acid Amplification And Sequencing |
| WO2013066641A1 (fr) | 2011-10-21 | 2013-05-10 | Chronix Biomedical | Biomarqueurs des acides nucléiques circulants associés au cancer colorectal |
| AU2012342682A1 (en) | 2011-11-23 | 2014-05-22 | F. Hoffmann-La Roche Ag | Responsiveness to angiogenesis inhibitors |
| US8748097B1 (en) | 2011-12-02 | 2014-06-10 | President And Fellows Of Harvard College | Identification of agents for treating calcium disorders and uses thereof |
| AU2012352180A1 (en) | 2011-12-16 | 2014-07-31 | Moderna Therapeutics, Inc. | Modified nucleoside, nucleotide, and nucleic acid compositions |
| WO2013095935A1 (fr) | 2011-12-19 | 2013-06-27 | Hill's Pet Nutrition, Inc. | Compositions et procédés pour le diagnostic et le traitement de l'hyperthyroïdisme dans des animaux de compagnie |
| JP5807542B2 (ja) | 2011-12-22 | 2015-11-10 | 株式会社島津製作所 | 対象成分を操作するためのチップデバイス及びそれを用いた方法 |
| US9115394B2 (en) | 2011-12-22 | 2015-08-25 | Roche Molecular Systems, Inc. | Methods and reagents for reducing non-specific amplification |
| CA2858284A1 (fr) | 2011-12-23 | 2013-06-27 | Biomerieux | Detection de souches variantes meca de staphylococcus aureus resistant a la methycilline |
| EP2798089B1 (fr) | 2011-12-30 | 2018-05-23 | Bio-rad Laboratories, Inc. | Procédés et compositions pour la mise en uvre de réactions d'amplification d'acide nucléique |
| EP3578697B1 (fr) | 2012-01-26 | 2024-03-06 | Tecan Genomics, Inc. | Compositions et procédés d'enrichissement de séquence d'acide nucléique ciblé et de génération de bibliothèques à haute efficacité |
| GB2501611B (en) | 2012-02-21 | 2015-03-04 | Cytonics Corp | Alpha-2-macroglobulin compositions and therapeutic uses |
| US10941396B2 (en) | 2012-02-27 | 2021-03-09 | Becton, Dickinson And Company | Compositions and kits for molecular counting |
| US11177020B2 (en) | 2012-02-27 | 2021-11-16 | The University Of North Carolina At Chapel Hill | Methods and uses for molecular tags |
| US9572897B2 (en) | 2012-04-02 | 2017-02-21 | Modernatx, Inc. | Modified polynucleotides for the production of cytoplasmic and cytoskeletal proteins |
| EP3392347A1 (fr) | 2012-04-02 | 2018-10-24 | Life Technologies Corporation | Compositions et procédés pour la détection de mycobacterium avium paratuberculosis |
| US10501512B2 (en) | 2012-04-02 | 2019-12-10 | Modernatx, Inc. | Modified polynucleotides |
| EP3505176A1 (fr) | 2012-04-02 | 2019-07-03 | Moderna Therapeutics, Inc. | Polynucléotides modifiés pour la production de protéines sécrétées |
| US9283287B2 (en) | 2012-04-02 | 2016-03-15 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of nuclear proteins |
| CN114854832A (zh) | 2012-04-19 | 2022-08-05 | 生命技术公司 | 核酸扩增 |
| EP3095879B1 (fr) | 2012-04-19 | 2018-09-19 | Life Technologies Corporation | Amplification d'acides nucléiques |
| US9133490B2 (en) | 2012-05-16 | 2015-09-15 | Transgenomic, Inc. | Step-up method for COLD-PCR enrichment |
| US10289800B2 (en) | 2012-05-21 | 2019-05-14 | Ariosa Diagnostics, Inc. | Processes for calculating phased fetal genomic sequences |
| CN109082462B (zh) | 2012-05-21 | 2022-10-28 | 斯克利普斯研究所 | 样品制备方法 |
| AU2013205064B2 (en) | 2012-06-04 | 2015-07-30 | Gen-Probe Incorporated | Compositions and Methods for Amplifying and Characterizing HCV Nucleic Acid |
| JP6181751B2 (ja) | 2012-06-18 | 2017-08-16 | ニューゲン テクノロジーズ, インコーポレイテッド | 望まれない核酸配列のネガティブ選択のための組成物および方法 |
| US20140005061A1 (en) | 2012-06-29 | 2014-01-02 | Life Technologies Corporation | Compositions and methods for detection of multiple microorganisms |
| US20150011396A1 (en) | 2012-07-09 | 2015-01-08 | Benjamin G. Schroeder | Methods for creating directional bisulfite-converted nucleic acid libraries for next generation sequencing |
| JP2015522293A (ja) | 2012-07-19 | 2015-08-06 | アリオサ ダイアグノスティックス インコーポレイテッドAriosa Diagnostics,Inc. | 多重化連続ライゲーションに基づく遺伝子変異体の検出 |
| WO2014015217A1 (fr) | 2012-07-19 | 2014-01-23 | Vertex Pharmaceuticals Incorporated | Biomarqueurs pour patients infectés par le hcv |
| EP2895620B1 (fr) | 2012-09-11 | 2017-08-02 | Life Technologies Corporation | Amplification d'acides nucléiques |
| WO2014043143A1 (fr) | 2012-09-11 | 2014-03-20 | Life Technologies Corporation | Amplification d'acides nucléiques |
| US9212392B2 (en) | 2012-09-25 | 2015-12-15 | Exact Sciences Corporation | Normalization of polymerase activity |
| JP6144355B2 (ja) | 2012-11-26 | 2017-06-07 | モデルナティエックス インコーポレイテッドModernaTX,Inc. | 化学修飾mRNA |
| AU2013202805B2 (en) | 2013-03-14 | 2015-07-16 | Gen-Probe Incorporated | System and method for extending the capabilities of a diagnostic analyzer |
| AU2014244608B2 (en) | 2013-03-14 | 2017-09-14 | Exact Sciences Corporation | Detecting neoplasm |
| US9255265B2 (en) | 2013-03-15 | 2016-02-09 | Illumina, Inc. | Methods for producing stranded cDNA libraries |
| US8980864B2 (en) | 2013-03-15 | 2015-03-17 | Moderna Therapeutics, Inc. | Compositions and methods of altering cholesterol levels |
| EP2971130A4 (fr) | 2013-03-15 | 2016-10-05 | Nugen Technologies Inc | Séquençage séquentiel |
| GB201308313D0 (en) | 2013-05-09 | 2013-06-19 | Medical Res Council | Assay Method |
| DK3004388T4 (da) | 2013-05-29 | 2023-08-28 | Chronix Biomedical | Påvisning og kvantificering af cellefrit dna fra donor i kredsløbet hos organtransplantatmodtagere |
| AU2014278730B2 (en) | 2013-06-13 | 2020-12-10 | F. Hoffmann-La Roche Ag | Statistical analysis for non-invasive sex chromosome aneuploidy determination |
| WO2015013465A2 (fr) | 2013-07-25 | 2015-01-29 | Dch Molecular Diagnostics, Inc. | Méthodes et compositions de détection d'une contamination bactérienne |
| EP3156797B1 (fr) | 2013-08-08 | 2019-06-12 | Institut Pasteur | Corrélation de l'activité de maladie avec expansions clonales de cellules t cd8+ spécifiques 16 du papillomavirus humain chez des patients victimes d'un lichen plan buccal érosif sévère |
| DK3039158T3 (en) | 2013-08-28 | 2019-03-04 | Becton Dickinson Co | MASSIVE PARALLEL SINGLE CELL CELL ANALYSIS |
| AU2014312249A1 (en) | 2013-08-28 | 2016-03-24 | Cytonics Corporation | Systems, compositions, and methods for transplantation and treating conditions |
| CA2924669C (fr) | 2013-09-20 | 2023-03-21 | The Regents Of The University Of Michigan | Compositions et methodes pour l'analyse de la radiosensibilite |
| US10023626B2 (en) | 2013-09-30 | 2018-07-17 | Modernatx, Inc. | Polynucleotides encoding immune modulating polypeptides |
| WO2015051214A1 (fr) | 2013-10-03 | 2015-04-09 | Moderna Therapeutics, Inc. | Polynucléotides codant pour un récepteur de lipoprotéines de faible densité |
| US10253358B2 (en) | 2013-11-04 | 2019-04-09 | Exact Sciences Development Company, Llc | Multiple-control calibrators for DNA quantitation |
| US9546399B2 (en) | 2013-11-13 | 2017-01-17 | Nugen Technologies, Inc. | Compositions and methods for identification of a duplicate sequencing read |
| CN105940115B (zh) | 2013-11-15 | 2021-07-06 | 巴斯德研究所 | 恶性疟原虫青蒿素耐药性的分子标记物 |
| WO2015089438A1 (fr) | 2013-12-13 | 2015-06-18 | Northwestern University | Biomarqueurs pour les états de stress post-traumatique |
| WO2015095689A1 (fr) | 2013-12-19 | 2015-06-25 | Exact Sciences Corporation | Molécules témoins d'acides nucléiques de synthèse |
| US20170009288A1 (en) | 2014-02-03 | 2017-01-12 | Thermo Fisher Scientific Baltics Uab | Method for controlled dna fragmentation |
| US9745614B2 (en) | 2014-02-28 | 2017-08-29 | Nugen Technologies, Inc. | Reduced representation bisulfite sequencing with diversity adaptors |
| ES2812753T3 (es) | 2014-03-31 | 2021-03-18 | Mayo Found Medical Education & Res | Detección de neoplasma colorectal |
| CA2943405A1 (fr) | 2014-03-31 | 2015-10-08 | Debiopharm International Sa | Fusions de fgfr |
| CN113826612B (zh) | 2014-06-10 | 2022-11-22 | 生物马特里卡公司 | 在环境温度下稳定凝血细胞 |
| CN106572974B (zh) | 2014-07-15 | 2021-04-23 | 生命技术公司 | 用于将分子有效递送到细胞的具有脂质聚集体的组合物和方法 |
| US10364458B2 (en) | 2014-07-16 | 2019-07-30 | Tangen Biosciences, Inc. | Isothermal methods for amplifying nucleic acid samples |
| EP3177740B1 (fr) | 2014-08-06 | 2021-01-13 | Nugen Technologies, Inc. | Mesures numériques à partir de séquençage ciblé |
| US10450562B2 (en) | 2014-09-09 | 2019-10-22 | Igenomx International Genomics Corporation | Methods and compositions for rapid nucleic acid library preparation |
| WO2016073353A1 (fr) | 2014-11-03 | 2016-05-12 | Tangen Biosciences, Inc. | Appareil et procédé pour la capture et la rupture de cellules, de spores ou de virus |
| JP7231326B2 (ja) | 2014-11-10 | 2023-03-01 | ジェネンテック, インコーポレイテッド | Il-33媒介性障害のための治療及び診断方法 |
| US10465249B2 (en) | 2014-12-12 | 2019-11-05 | Exact Sciences Development Company, Llc | Method of characterizing ZDHHC1 DNA |
| US10011878B2 (en) | 2014-12-12 | 2018-07-03 | Exact Sciences Development Company | Compositions and methods for performing methylation detection assays |
| US9708647B2 (en) | 2015-03-23 | 2017-07-18 | Insilixa, Inc. | Multiplexed analysis of nucleic acid hybridization thermodynamics using integrated arrays |
| CN115927612A (zh) | 2015-03-27 | 2023-04-07 | 精密科学公司 | 检测食管疾病 |
| EP3286338B1 (fr) | 2015-04-24 | 2023-11-01 | Atila Biosystems Incorporated | Amplification avec des amorces de composition nucléotidique limitée |
| WO2016174130A1 (fr) | 2015-04-28 | 2016-11-03 | Université De Strasbourg | Modèle de culture de cellules humaines sur la base de signatures génétiques cliniques |
| CN108026494A (zh) | 2015-06-05 | 2018-05-11 | 米罗库鲁斯公司 | 限制蒸发和表面结垢的空气基质数字微流控装置和方法 |
| EP3303548A4 (fr) | 2015-06-05 | 2019-01-02 | Miroculus Inc. | Gestion de l'évaporation dans des dispositifs microfluidiques numériques |
| EP3307908B1 (fr) | 2015-06-09 | 2019-09-11 | Life Technologies Corporation | Procédés pour le marquage moléculaire |
| US9789087B2 (en) | 2015-08-03 | 2017-10-17 | Thomas Jefferson University | PAR4 inhibitor therapy for patients with PAR4 polymorphism |
| TWI710642B (zh) | 2015-08-17 | 2020-11-21 | 美商庫拉腫瘤技術股份有限公司 | 以法呢基轉移酶(farnesyltransferase)抑制劑治療癌症病患之方法 |
| US9499861B1 (en) | 2015-09-10 | 2016-11-22 | Insilixa, Inc. | Methods and systems for multiplex quantitative nucleic acid amplification |
| WO2017070096A1 (fr) | 2015-10-18 | 2017-04-27 | Affymetrix, Inc. | Génotypage multiallèles de polymorphismes mononucléotidiques et d'insertions-délétions |
| KR101651817B1 (ko) | 2015-10-28 | 2016-08-29 | 대한민국 | Ngs 라이브러리 제작용 프라이머 세트 및 이를 이용한 ngs 라이브러리 제작방법 및 키트 |
| CA3002196A1 (fr) | 2015-10-30 | 2017-05-04 | Exact Sciences Development Company, Llc | Detection par amplification multiplexee et isolement et detection d'adn issu de plasma |
| AU2016368265B2 (en) | 2015-12-08 | 2021-10-28 | Biomatrica, Inc. | Reduction of erythrocyte sedimentation rate |
| WO2017100283A1 (fr) | 2015-12-09 | 2017-06-15 | Life Technologies Corporation | Détection et détermination quantitative de molécules d'acide nucléique associées à une surface |
| US10626383B2 (en) | 2016-01-15 | 2020-04-21 | Thermo Fisher Scientific Baltics Uab | Thermophilic DNA polymerase mutants |
| WO2017155858A1 (fr) | 2016-03-07 | 2017-09-14 | Insilixa, Inc. | Identification de séquence d'acide nucléique à l'aide d'une extension de base unique cyclique en phase solide |
| WO2017184968A1 (fr) | 2016-04-22 | 2017-10-26 | Kura Oncology, Inc. | Méthodes de sélection de patients atteints de cancer pour le traitement avec des inhibiteurs de farnésyltransférase |
| US20170321286A1 (en) | 2016-05-05 | 2017-11-09 | Exact Sciences Corporation | Detection of lung neoplasia by amplification of rna sequences |
| CN116064796A (zh) | 2016-05-05 | 2023-05-05 | 精密科学公司 | 通过分析甲基化dna来检测肺肿瘤 |
| CA2932910A1 (fr) | 2016-06-14 | 2017-12-14 | Entos Pharmaceuticals Inc. | Methodes de diagnostic et de traitement de cancer metastatique |
| CN110291402B (zh) | 2016-06-27 | 2023-09-01 | 朱诺治疗学股份有限公司 | 鉴定肽表位的方法、结合此类表位的分子和相关用途 |
| MA45491A (fr) | 2016-06-27 | 2019-05-01 | Juno Therapeutics Inc | Épitopes à restriction cmh-e, molécules de liaison et procédés et utilisations associés |
| AU2017299597B2 (en) | 2016-07-19 | 2023-08-24 | Exact Sciences Corporation | Methylated control DNA |
| EP3488003B1 (fr) | 2016-07-19 | 2023-10-25 | Exact Sciences Corporation | Molécules témoins d'acides nucléiques provenant d'organismes non humains |
| WO2018029532A1 (fr) | 2016-08-10 | 2018-02-15 | Institut Pasteur | Procédés et réactifs utilisés pour la détection du paludisme à plasmodium falciparum résistant à la pipéraquine |
| US10596572B2 (en) | 2016-08-22 | 2020-03-24 | Miroculus Inc. | Feedback system for parallel droplet control in a digital microfluidic device |
| EP4293128A3 (fr) | 2016-09-02 | 2024-03-20 | Mayo Foundation for Medical Education and Research | Détection d'un carcinome hépatocellulaire |
| WO2018071522A1 (fr) | 2016-10-11 | 2018-04-19 | Life Technologies Corporation | Amplification rapide d'acides nucléiques |
| EP3534885B1 (fr) | 2016-11-03 | 2021-01-20 | Kura Oncology, Inc. | Inhibiteurs de farnésyltransférase pour utilisation dans le traitement de cancer |
| WO2018111835A1 (fr) | 2016-12-12 | 2018-06-21 | Dana-Farber Cancer Institute, Inc. | Compositions et procédés pour le codage par code-barres moléculaire de molécules d'adn avant l'enrichissement des mutations et/ou la détection des mutations |
| JP2020515815A (ja) | 2016-12-28 | 2020-05-28 | ミロキュラス インコーポレイテッド | デジタルマイクロ流体デバイスおよび方法 |
| WO2018129368A2 (fr) | 2017-01-06 | 2018-07-12 | Editas Medicine, Inc. | Procédés d'évaluation de la coupure par les nucléases |
| JP7289264B2 (ja) | 2017-01-27 | 2023-06-09 | エグザクト サイエンシーズ コーポレーション | メチル化dnaの分析による結腸腫瘍の検出 |
| CN114796218A (zh) | 2017-02-21 | 2022-07-29 | 库拉肿瘤学公司 | 使用法尼基转移酶抑制剂治疗癌症的方法 |
| US9956215B1 (en) | 2017-02-21 | 2018-05-01 | Kura Oncology, Inc. | Methods of treating cancer with farnesyltransferase inhibitors |
| US11285486B2 (en) | 2017-03-24 | 2022-03-29 | Gen-Probe Incorporated | Cover assembly and related methods of use |
| WO2018187476A1 (fr) | 2017-04-04 | 2018-10-11 | Miroculus Inc. | Appareils microfluidiques numériques et procédés de manipulation et de traitement de gouttelettes encapsulées |
| US10914729B2 (en) | 2017-05-22 | 2021-02-09 | The Trustees Of Princeton University | Methods for detecting protein binding sequences and tagging nucleic acids |
| CN110914448A (zh) | 2017-06-02 | 2020-03-24 | 昂飞股份有限公司 | 使用差异性标记的等位基因特异性探针分析混合样品的基于阵列的方法 |
| US11441174B2 (en) | 2017-06-02 | 2022-09-13 | Affymetrix, Inc. | Array-based methods for analysing mixed samples using differently labelled allele-specific probes |
| WO2018231818A1 (fr) | 2017-06-16 | 2018-12-20 | Life Technologies Corporation | Acides nucléiques de régulation, et compositions, kits et utilisations de ces derniers |
| WO2019002178A1 (fr) | 2017-06-26 | 2019-01-03 | Thermo Fisher Scientific Baltics Uab | Mutants d'adn polymérases thermophiles |
| CA3155871A1 (en) | 2017-07-10 | 2019-01-17 | Gen-Probe Incorporated | Analytical systems and methods for nucleic acid amplification using sample assigning parameters |
| US11174511B2 (en) | 2017-07-24 | 2021-11-16 | Dana-Farber Cancer Institute, Inc. | Methods and compositions for selecting and amplifying DNA targets in a single reaction mixture |
| US11413617B2 (en) | 2017-07-24 | 2022-08-16 | Miroculus Inc. | Digital microfluidics systems and methods with integrated plasma collection device |
| US10806730B2 (en) | 2017-08-07 | 2020-10-20 | Kura Oncology, Inc. | Methods of treating cancer with farnesyltransferase inhibitors |
| KR20200051601A (ko) | 2017-08-07 | 2020-05-13 | 쿠라 온콜로지, 인크. | 파르네실전달효소 억제제를 이용하여 암을 치료하는 방법 |
| CN115582155A (zh) | 2017-09-01 | 2023-01-10 | 米罗库鲁斯公司 | 数字微流控设备及其使用方法 |
| US11099202B2 (en) | 2017-10-20 | 2021-08-24 | Tecan Genomics, Inc. | Reagent delivery system |
| WO2019089982A1 (fr) | 2017-11-01 | 2019-05-09 | Juno Therapeutics, Inc. | Procédé d'évaluation de l'activité de récepteurs antigéniques de recombinaison |
| MX2020004507A (es) | 2017-11-09 | 2020-08-13 | Alnylam Pharmaceuticals Inc | Ensayos y métodos para determinar la expresión del gen de quimiotaxina 2 derivada de leucocitos (lect2). |
| US10648025B2 (en) | 2017-12-13 | 2020-05-12 | Exact Sciences Development Company, Llc | Multiplex amplification detection assay II |
| WO2019130347A1 (fr) | 2017-12-26 | 2019-07-04 | Sree Chitra Tirunal Institute For Medical Sciences And Technology | Amorces pour l'amplification isotherme du gène mpt64 de mycobacterium tuberculosis et processus associé |
| AU2019212931A1 (en) | 2018-01-29 | 2020-08-27 | Gen-Probe Incorporated | Analytical systems and methods |
| MA51741A (fr) | 2018-02-09 | 2021-05-19 | Hoffmann La Roche | Procédés thérapeutiques et de diagnostic pour des maladies inflammatoires médiées par des mastocytes |
| EP3775272A4 (fr) | 2018-04-02 | 2021-12-29 | Progenity, Inc. | Procédés, systèmes et compositions de comptage de molécules d'acide nucléique |
| CA3105684A1 (fr) | 2018-07-10 | 2020-01-16 | Gen-Probe Incorporated | Procedes et systemes de detection et de quantification d'acides nucleiques |
| US11408030B2 (en) | 2018-09-10 | 2022-08-09 | Andy Madrid | Test for detecting Alzheimer's disease |
| SG11202104296TA (en) | 2018-11-01 | 2021-05-28 | Kura Oncology Inc | Methods of treating cancer with farnesyltransferase inhibitors |
| CA3117768A1 (fr) | 2018-11-28 | 2020-06-04 | Keygene N.V. | Enrichissement cible par protection d'endonuclease |
| IL311084A (en) | 2018-11-30 | 2024-04-01 | Caris Mpi Inc | New generation molecular characterization |
| WO2020142347A2 (fr) | 2018-12-31 | 2020-07-09 | Gen-Probe Incorporated | Systèmes et procédés de remplissage de cartouches à puits multiples avec des réactifs solides |
| EP3927840A1 (fr) | 2019-02-21 | 2021-12-29 | Keygene N.V. | Génotypage de polyploïdes |
| US20220142983A1 (en) | 2019-03-01 | 2022-05-12 | Kura Oncology, Inc. | Methods of treating cancer with farnesyltransferase inhibitors |
| CN113924041A (zh) | 2019-03-14 | 2022-01-11 | 因斯利克萨公司 | 基于时间门控的荧光检测的方法和系统 |
| SG11202110472WA (en) | 2019-03-29 | 2021-10-28 | Kura Oncology Inc | Methods of treating squamous cell carcinomas with farnesyltransferase inhibitors |
| TW202102218A (zh) | 2019-04-01 | 2021-01-16 | 美商庫拉腫瘤技術股份有限公司 | 以法呢基(farnesyl)轉移酶抑制劑治療癌症的方法 |
| EP3947718A4 (fr) | 2019-04-02 | 2022-12-21 | Enumera Molecular, Inc. | Procédés, systèmes et compositions de comptage de molécules d'acide nucléique |
| EP3953041A4 (fr) | 2019-04-08 | 2023-01-25 | Miroculus Inc. | Appareils microfluidiques numériques à cartouches multiples et procédés d'utilisation |
| BR112021020779A8 (pt) | 2019-04-17 | 2022-01-25 | Igenomix S L | Métodos aperfeiçoados para diagnóstico precoce de leiomiomas e leiomiossarcomas uterinos |
| US20220305001A1 (en) | 2019-05-02 | 2022-09-29 | Kura Oncology, Inc. | Methods of treating acute myeloid leukemia with farnesyltransferase inhibitors |
| CA3176699A1 (fr) | 2019-05-03 | 2020-11-12 | Gen-Probe Incorporated | Systeme de transport de receptacle pour systeme analytique |
| US11524298B2 (en) | 2019-07-25 | 2022-12-13 | Miroculus Inc. | Digital microfluidics devices and methods of use thereof |
| EP4051811A4 (fr) | 2019-10-31 | 2023-12-06 | Mayo Foundation for Medical Education and Research | Détection du cancer de l'ovaire |
| EP4069865A4 (fr) | 2019-12-02 | 2023-12-20 | Caris MPI, Inc. | Prédicteur de réponse au platine dans une approche pan-cancer |
| WO2021116371A1 (fr) | 2019-12-12 | 2021-06-17 | Keygene N.V. | Manipulation d'acide nucléique semi-solide |
| AU2020407850A1 (en) | 2019-12-20 | 2022-06-30 | Keygene N.V. | NGS library preparation using covalently closed nucleic acid molecule ends |
| US20230110203A1 (en) | 2020-03-13 | 2023-04-13 | Medimmune Limited | Therapeutic methods for the treatment of subjects with risk alelles in il33 |
| US11761031B2 (en) | 2020-05-28 | 2023-09-19 | The Hong Kong University Of Science And Technology | Method for real time monitoring of nucleic acid amplicons mediated by loop oligonucleotide probes |
| CA3188230A1 (fr) | 2020-08-19 | 2022-02-24 | John B. Kisiel | Detection de lymphome non hodgkinien |
| JP2023543424A (ja) | 2020-09-21 | 2023-10-16 | ビオラ・セラピューティクス・インコーポレイテッド | 無細胞dnaを単離するための組成物および方法 |
| JP2023543602A (ja) | 2020-10-06 | 2023-10-17 | キージーン ナムローゼ フェンノートシャップ | 標的化された配列付加 |
| US20230393163A1 (en) | 2020-10-21 | 2023-12-07 | Gen-Probe Incorporated | Fluid container management system |
| US20240002904A1 (en) | 2020-11-24 | 2024-01-04 | Keygene N.V. | Targeted enrichment using nanopore selective sequencing |
| EP4251750A1 (fr) | 2020-11-25 | 2023-10-04 | Koninklijke Nederlandse Akademie van Wetenschappen | Profilage ribosomique dans des cellules individuelles |
| EP4320443A1 (fr) | 2021-04-06 | 2024-02-14 | BPGbio, Inc. | Marqueurs protéiques pour le pronostic de la progression du cancer du sein |
| WO2023011660A1 (fr) | 2021-08-06 | 2023-02-09 | Sansure Biotech Inc. | Compositions pour liquéfier un échantillon biologique visqueux, produit combiné, agents liquéfiants, kits associés et procédés et application associés |
| US11857961B2 (en) | 2022-01-12 | 2024-01-02 | Miroculus Inc. | Sequencing by synthesis using mechanical compression |
| WO2023240201A1 (fr) | 2022-06-08 | 2023-12-14 | Larimar Therapeutics, Inc. | Marqueurs sensibles à la frataxine pour surveiller la progression et le traitement du syndrome de leigh |
| WO2024015331A1 (fr) | 2022-07-12 | 2024-01-18 | Genentech, Inc. | Méthodes thérapeutiques et diagnostiques pour la sclérose en plaques |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1988010315A1 (fr) * | 1987-06-19 | 1988-12-29 | Siska Diagnostics, Inc. | Systemes d'amplification/detection d'acides nucleiques a base de transcription |
| EP0310229A1 (fr) * | 1987-07-31 | 1989-04-05 | The Board Of Trustees Of The Leland Stanford Junior University | Amplification sélective de séquences de cible oligonucléotide |
| WO1989006700A1 (fr) * | 1988-01-21 | 1989-07-27 | Genentech, Inc. | Amplification et detection de sequences d'acides nucleiques |
| EP0201184B1 (fr) * | 1985-03-28 | 1992-12-16 | F. Hoffmann-La Roche Ag | Procédé pour l'amplification des séquences d'acide nucléique |
| EP0200362B1 (fr) * | 1985-03-28 | 1993-01-20 | F. Hoffmann-La Roche Ag | Procédé pour l'amplification, la détection et/ou le clonage des séquences d'acide nucléique |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4683195A (en) * | 1986-01-30 | 1987-07-28 | Cetus Corporation | Process for amplifying, detecting, and/or-cloning nucleic acid sequences |
| US4800159A (en) * | 1986-02-07 | 1989-01-24 | Cetus Corporation | Process for amplifying, detecting, and/or cloning nucleic acid sequences |
| JP2517241B2 (ja) * | 1986-08-19 | 1996-07-24 | 郁男 山科 | 遺伝子 |
| EP0272098A3 (fr) * | 1986-12-15 | 1990-06-06 | City Of Hope National Medical Center | Procédé d'amplification et de détection de séquences ARN |
| DE3726934A1 (de) * | 1987-08-13 | 1989-02-23 | Merck Patent Gmbh | Verfahren zum nachweis von nukleinsaeure-sequenzen |
| CA1340807C (fr) * | 1988-02-24 | 1999-11-02 | Lawrence T. Malek | Procede d'amplification d'une sequence d'acide nucleique |
| AU4829690A (en) * | 1988-12-16 | 1990-07-10 | Siska Diagnostics, Inc. | Self-sustained, sequence replication system |
-
1988
- 1988-02-24 CA CA000559709A patent/CA1340807C/fr not_active Expired - Lifetime
- 1988-06-24 US US07/211,384 patent/US5409818A/en not_active Expired - Lifetime
- 1988-08-26 DE DE3850093T patent/DE3850093T2/de not_active Expired - Lifetime
- 1988-08-26 EP EP88113948A patent/EP0329822B1/fr not_active Expired - Lifetime
- 1988-08-26 ES ES88113948T patent/ES2053648T3/es not_active Expired - Lifetime
- 1988-08-26 AT AT88113948T patent/ATE106948T1/de not_active IP Right Cessation
-
1989
- 1989-02-23 KR KR1019890002142A patent/KR960015744B1/ko not_active IP Right Cessation
- 1989-08-19 KR KR1019910700397A patent/KR920702866A/ko not_active Application Discontinuation
- 1989-08-19 WO PCT/EP1989/000981 patent/WO1991002814A1/fr active Application Filing
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0201184B1 (fr) * | 1985-03-28 | 1992-12-16 | F. Hoffmann-La Roche Ag | Procédé pour l'amplification des séquences d'acide nucléique |
| EP0200362B1 (fr) * | 1985-03-28 | 1993-01-20 | F. Hoffmann-La Roche Ag | Procédé pour l'amplification, la détection et/ou le clonage des séquences d'acide nucléique |
| WO1988010315A1 (fr) * | 1987-06-19 | 1988-12-29 | Siska Diagnostics, Inc. | Systemes d'amplification/detection d'acides nucleiques a base de transcription |
| EP0310229A1 (fr) * | 1987-07-31 | 1989-04-05 | The Board Of Trustees Of The Leland Stanford Junior University | Amplification sélective de séquences de cible oligonucléotide |
| WO1989006700A1 (fr) * | 1988-01-21 | 1989-07-27 | Genentech, Inc. | Amplification et detection de sequences d'acides nucleiques |
Non-Patent Citations (3)
| Title |
|---|
| Biochemicals for Molecular Biology, Boehringer Mannheim 1987, pp. 107-108 * |
| Enzyme Nomenclature, Academic Press, 1984, pp. 302-303 * |
| Science 239, 491, 1988 * |
Cited By (249)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0778352A1 (fr) * | 1988-12-16 | 1997-06-11 | Siska Diagnostics, Inc. | Essai par réplication auto-entretenue de séquences |
| US7083922B2 (en) | 1989-07-11 | 2006-08-01 | Gen-Probe Incorporated | Detection of HIV |
| US6589734B1 (en) | 1989-07-11 | 2003-07-08 | Gen-Probe Incorporated | Detection of HIV |
| US5766849A (en) * | 1989-07-11 | 1998-06-16 | Gen-Probe Incorporated | Methods of amplifying nucleic acids using promoter-containing primer sequence |
| EP0731174A3 (fr) * | 1989-07-11 | 1998-03-25 | Gen-Probe Incorporated | Méthodes pour l'amplification de séquences d'acide nucléique |
| US7049102B1 (en) | 1989-09-22 | 2006-05-23 | Board Of Trustees Of Leland Stanford University | Multi-gene expression profile |
| US7132235B2 (en) | 1990-02-16 | 2006-11-07 | Orchid Cellmark Inc. | Reagent kit for determining specific nucleotide variations |
| US7026117B2 (en) | 1990-02-16 | 2006-04-11 | Orchid Biosciences, Inc. | Method for determining specific nucleotide variations |
| US7585512B1 (en) | 1990-05-08 | 2009-09-08 | Thomas Jefferson University | Composition and method of using tumor cells |
| EP0479376A1 (fr) | 1990-10-05 | 1992-04-08 | Akzo Nobel N.V. | Peptides agissants immunochimiquemeur avec des anticorps contre le virus d'hépatite non-A, non-B |
| WO1992008800A1 (fr) * | 1990-11-13 | 1992-05-29 | Siska Diagnostics, Inc. | Amplification d'acides nucleiques par replication bi-enzymatique auto-entretenue de sequences |
| EP0525882B1 (fr) * | 1991-08-02 | 2004-01-14 | bioMerieux B.V. | Quantification d'acides nucléiques |
| US5643730A (en) * | 1991-09-23 | 1997-07-01 | Pfizer Inc. | Process for detecting specific mRNA and DNA in cells |
| US7501252B2 (en) | 1992-01-10 | 2009-03-10 | Life Technologies Corporation | Use of predetermined nucleotides having altered base pairing characteristics in the amplification of nucleic acid molecules |
| WO1993015228A1 (fr) * | 1992-01-29 | 1993-08-05 | Hitachi Chemical Co., Ltd. | Support d'immobilisation de polynucleotide |
| EP0566198A3 (fr) * | 1992-04-13 | 1994-08-03 | Enichem Spa | |
| US5554516A (en) * | 1992-05-06 | 1996-09-10 | Gen-Probe Incorporated | Nucleic acid sequence amplification method, composition and kit |
| US5888729A (en) * | 1992-05-06 | 1999-03-30 | Gen-Probe Incorporated | Oligonucleotide probes and methods for detecting Streptococcus pneumoniae |
| ES2056028A1 (es) * | 1993-02-18 | 1994-09-16 | Inia | Procedimiento para la seleccion de oligonucleotidos para la amplificacion especifica de genomas altamente variables. |
| EP0656425A1 (fr) * | 1993-07-23 | 1995-06-07 | Gen-Probe Incorporated | Procédé pour améliorer l'amplification des acides nucléiques |
| WO1995003430A1 (fr) * | 1993-07-23 | 1995-02-02 | Gen-Probe Incorporated | Procede d'amelioration de l'amplification de l'acide nucleique |
| USRE39007E1 (en) * | 1994-03-16 | 2006-03-07 | Gen-Probe Incorporated | Isothermal strand displacement nucleic acid amplification |
| USRE38960E1 (en) * | 1994-03-16 | 2006-01-31 | Gen-Probe Incorporated | Isothermal strand displacement nucleic acid amplification |
| WO1995027721A1 (fr) * | 1994-04-07 | 1995-10-19 | Akzo Nobel N.V. | Compositions lyophilisees comprenant de l'arn |
| US6031150A (en) * | 1994-04-13 | 2000-02-29 | Baylor College Of Medicine | Sulfonylurea receptor trangenic rodents |
| US6054313A (en) * | 1994-04-13 | 2000-04-25 | Baylor College Of Medicine | Nucleic acid and amino acid sequences for mammalian sulfonylurea receptor |
| EP0688873A2 (fr) | 1994-06-23 | 1995-12-27 | Bayer Ag | Test DNA rapide pour la détection de staphylococcus aureus résistant à la chinolone dans du matériel clinique |
| EP0776376B1 (fr) * | 1994-07-15 | 2001-11-21 | Akzo Nobel N.V. | Utilisation d'arn polymerase pour ameliorer un procede d'amplification d'acide nucleique |
| WO1996006191A3 (fr) * | 1994-08-18 | 1996-03-14 | Akzo Nobel Nv | Amorces et sondes destinees a l'amplification et a la detection de l'acide nucleique du cytomegalovirus |
| EP0710724A2 (fr) | 1994-10-06 | 1996-05-08 | Akzo Nobel N.V. | Antigènes de Toxoplasma gondii |
| EP0713922A1 (fr) | 1994-10-28 | 1996-05-29 | BIO MERIEUX, Société anonyme | Oligonucléotide utilisable comme amorce dans une méthode d'amplification basée sur une réplication avec déplacement de brin |
| US5789169A (en) * | 1994-11-03 | 1998-08-04 | Regents Of The University Of California | Methods for the diagnosis of glaucoma |
| US6248867B1 (en) | 1994-11-03 | 2001-06-19 | Univ California | Trabecular meshwork induced glucocorticoid response (TIGR) fusion protein |
| US6150161A (en) * | 1994-11-03 | 2000-11-21 | The Regents Of The University Of California | Methods for the diagnosis of glaucoma |
| EP0803578A3 (fr) * | 1996-04-26 | 1999-01-13 | Toyo Boseki Kabushiki Kaisha | Coffret pour l'amplification et la détection des acides nucléiques du cytomegalovirus (CMV) utilisant une séquence d'acide nucléique ss2.7 |
| WO1998006736A1 (fr) | 1996-08-14 | 1998-02-19 | Life Technologies, Inc. | Compositions stables pour l'amplification et le sequençage d'acides nucleiques |
| EP2264045A1 (fr) | 1996-08-14 | 2010-12-22 | Life Technologies Corporation | Composition pour l'amplification et pour la séquenciation de l'ADN |
| US6132954A (en) * | 1996-08-20 | 2000-10-17 | Baylor College Of Medicine | Methods of screening for agents that delay a cell cycle and compositions comprising era and an analogue of wild-type era |
| US6025133A (en) * | 1996-12-30 | 2000-02-15 | Gen-Probe Incorporated | Promoter-sequestered oligonucleoside and method of use |
| US6905858B2 (en) | 1997-01-02 | 2005-06-14 | Invitrogen Corporation | Nucleic acid-free thermostable enzymes and methods of production thereof |
| US8062848B2 (en) | 1997-01-02 | 2011-11-22 | Life Technologies Corporation | Nucleic acid-free thermostable enzymes and methods of production thereof |
| US6475724B1 (en) | 1997-01-28 | 2002-11-05 | The Regents Of The University Of California | Nucleic acids, kits, and methods for the diagnosis, prognosis and treatment of glaucoma and related disorders |
| US6171788B1 (en) | 1997-01-28 | 2001-01-09 | The Regents Of The University Of California | Methods for the diagnosis, prognosis and treatment of glaucoma and related disorders |
| US7138511B1 (en) | 1997-01-28 | 2006-11-21 | The Regents Of The University Of California | Nucleic acids, kits and methods for the diagnosis, prognosis and treatment of glaucoma and related disorders |
| US8735066B2 (en) | 1997-01-30 | 2014-05-27 | Board Of Regents, The University Of Texas System | Tumor suppressor designated TS10Q23.3 |
| EP2366712A2 (fr) | 1997-01-30 | 2011-09-21 | The Board Of Regents, The University Of Texas System | Suppresseur de tumeur désigné par TS10Q23.3 |
| US6306588B1 (en) | 1997-02-07 | 2001-10-23 | Invitrogen Corporation | Polymerases for analyzing or typing polymorphic nucleic acid fragments and uses thereof |
| US7501237B2 (en) | 1997-02-07 | 2009-03-10 | Life Technologies Corporation | Polymerases for analyzing or typing polymorphic nucleic acid fragments and uses thereof |
| US7192579B2 (en) | 1997-02-27 | 2007-03-20 | Baylor College Of Medicine | Methods of gene therapy using nucleic acid sequences for ATP-binding cassette transporter |
| US7189511B2 (en) | 1997-02-27 | 2007-03-13 | Baylor College Of Medicine | Methods of screening and diagnostics using ATP-binding cassette transporter |
| US7141420B2 (en) | 1997-02-27 | 2006-11-28 | University Of Utah Research Foundation | Nucleic acid and amino acid sequences for ATP-binding cassette transporter and methods of screening for agents that modify ATP-binding cassette transporter |
| US8129353B2 (en) | 1997-02-27 | 2012-03-06 | Baylor College Of Medicine | Methods of gene therapy using nucleic acid sequences for ATP-binding cassette transporter |
| EP2184289A1 (fr) | 1997-04-22 | 2010-05-12 | Life Technologies Corporation | Procédés de production de transcriptases inverses ASLV composées de plusieurs subunités |
| EP2251347A2 (fr) | 1997-04-22 | 2010-11-17 | Life Technologies Corporation | Procédés de production de transcriptases inverses ASLV composées de plusieurs sous-unités |
| EP2336367A1 (fr) | 1997-08-08 | 2011-06-22 | bioMerieux B.V. | Séquences nucléotidiques pouvant être utilisées comme amorces et comme sondes au cours de l'amplification et la détection de tous les sous-types du VIH-1 |
| US6413761B2 (en) | 1997-09-17 | 2002-07-02 | Toyota Jidosha Kabushiki Kaisha | Decaprenyl diphosphate synthetase gene |
| US6410280B1 (en) | 1997-09-17 | 2002-06-25 | Toyota Jidosha Kabushiki Kaisha | Decaprenyl diphosphate synthetase gene |
| US6225097B1 (en) | 1997-09-17 | 2001-05-01 | Toyota Jidosha Kabushiki Kaisha | Decaprenyl diphosphate synthetase gene |
| US7105318B2 (en) | 1997-11-04 | 2006-09-12 | Roche Diagnostics Gmbh | Specific and sensitive nucleic acid detection method |
| EP1801220A2 (fr) | 1997-12-18 | 2007-06-27 | Monsanto Technology, LLC | Plantes transgéniques résistant aux insectes et procédés permettant d'améliorer l'activité de delta-toxine contre des insectes |
| WO1999046400A1 (fr) | 1998-03-13 | 1999-09-16 | Life Technologies, Inc. | Compositions et procedes d'augmentation de la synthese des molecules d'acide nucleique |
| US6261773B1 (en) | 1998-03-17 | 2001-07-17 | Toyo Boseki Kabushiki Kaisha | Reagent for nucleic acid amplification and process for nucleic acid amplification |
| US7029861B1 (en) | 1998-09-15 | 2006-04-18 | Board Of Regents, The University Of Texas System | LPS-response gene compositions and methods |
| US7306804B2 (en) | 1998-11-17 | 2007-12-11 | Board Of Regents, The University Of Texas System | HIV-specific T-cell induction |
| EP1734134A2 (fr) | 1999-02-09 | 2006-12-20 | Laboratory Corporation of America Holdings, Inc. | Nouveau gène spécifique à la prostate servant au diagnostic, au prognostic et au traitment du cancer de la prostate |
| US7993830B2 (en) | 1999-02-09 | 2011-08-09 | Laboratory Corporation Of America Holdings | Prostate-specific gene for diagnosis, prognosis and management of prostate cancer |
| US6808883B1 (en) | 1999-04-15 | 2004-10-26 | Bayer Ag | Automatable rapid test for detection of cancer, based on telomerase (hTC) mRNA with specific primers and probes |
| US7342111B2 (en) | 1999-04-30 | 2008-03-11 | University Of Florida Research Foundation, Inc. | Adeno-associated virus-delivered ribozyme compositions and methods of use |
| WO2000073764A2 (fr) | 1999-06-01 | 2000-12-07 | Baylor College Of Medicine | Compositions et methodes pour l'utilisation therapeutique d'une sequence associee au gene atonal dans le traitement de la surdite, de l'osteoarthrite et d'une proliferation cellulaire anormale |
| EP2325198A1 (fr) | 1999-06-01 | 2011-05-25 | Baylor College Of Medicine | Compositions et procédés pour l'utilisation thérapeutique d'une séquence à association atonale |
| US7537886B1 (en) | 1999-06-22 | 2009-05-26 | Life Technologies Corporation | Primers and methods for the detection and discrimination of nucleic acids |
| US8043816B2 (en) | 1999-07-02 | 2011-10-25 | Life Technologies Corporation | Compositions and methods for temperature-dependent nucleic acid synthesis |
| US6830902B1 (en) | 1999-07-02 | 2004-12-14 | Invitrogen Corporation | Compositions and methods for enhanced sensitivity and specificity of nucleic acid synthesis |
| EP1978098A2 (fr) | 1999-12-10 | 2008-10-08 | Invitrogen Corporation | Utilisation de sites à recombinaison multiples avec une spécificité unique de clonage recombinatoire |
| EP2210948A2 (fr) | 1999-12-10 | 2010-07-28 | Life Technologies Corporation | Utilisation de sites à recombinaison multiples avec une spécificité unique de clonage recombinatoire |
| WO2001064959A1 (fr) * | 2000-03-02 | 2001-09-07 | Akzo Nobel N.V. | Detection d'arn du virus de l'hepatite b |
| WO2001066705A1 (fr) * | 2000-03-07 | 2001-09-13 | Akzo Nobel N.V. | Mutants d'arn polymerases presentant une stabilite thermique amelioree |
| US7507567B2 (en) | 2000-03-07 | 2009-03-24 | Biomerieus B.V. | RNA polymerase mutants with increased thermostability |
| EP2192128A2 (fr) | 2000-04-21 | 2010-06-02 | Corixa Corporation | Composés et méthodes pour le traitement et le diagnostique des infections de Chlamydia |
| EP3093341A1 (fr) | 2000-05-26 | 2016-11-16 | Life Technologies Corporation | Transcriptases inverses thermostables et utilisations |
| EP2295551A1 (fr) | 2000-05-26 | 2011-03-16 | Life Technologies Corporation | Transcriptases inverses thermostables et utilisations |
| WO2002004511A2 (fr) | 2000-07-10 | 2002-01-17 | Board Of Regents, The University Of Texas System | Genes du chromosome 3p21.3 constituant des suppresseurs de tumeurs |
| CN1322143C (zh) * | 2000-12-04 | 2007-06-20 | 普里马根公司 | 检测线粒体核酸与核基因核酸比率确定药物活性和/或副作用的方法 |
| WO2002046470A3 (fr) * | 2000-12-04 | 2004-01-15 | Primagen Holding Bv | Analyse d'organites cellulaires endosymbiontes et composes pouvant etre identifies par cette analyse |
| EP1229130A2 (fr) * | 2000-12-04 | 2002-08-07 | PrimaGen B.V. | Tests basés sur les acides nucléiques des organelles cellulaires endosymbiontiques et les composés identifiables |
| EP1229130A3 (fr) * | 2000-12-04 | 2003-10-22 | PrimaGen Holding B.V. | Tests basés sur les acides nucléiques des organelles cellulaires endosymbiontiques et les composés identifiables |
| EP2105496A1 (fr) | 2000-12-08 | 2009-09-30 | Life Technologies Corporation | Méthodes et compositions destinées à la synthèse de molécules d'acide nucléique à l'aide de sites de reconnaissance multiples |
| EP2105502A1 (fr) | 2000-12-12 | 2009-09-30 | Corixa Corporation | Composés et procédés de thérapie et de diagnostic du cancer du poumon |
| US8137911B2 (en) | 2001-05-22 | 2012-03-20 | Cellscript, Inc. | Preparation and use of single-stranded transcription substrates for synthesis of transcription products corresponding to target sequences |
| WO2002095002A2 (fr) | 2001-05-22 | 2002-11-28 | University Of Chicago | Arn polymerase dependant de l'adn a brin unique de virion n4 |
| US8722866B2 (en) | 2001-08-01 | 2014-05-13 | The United States Of America, As Represented By The Department Of Veterans Affairs | Isoform-selective inhibitors and activators of PDE3 cyclic nucleotide phosphodiesterases |
| EP1908851A2 (fr) | 2001-09-19 | 2008-04-09 | Intergenetics Incorporated | Analyse génétique pour la stratification du risque de cancer |
| EP2135960A1 (fr) | 2001-09-19 | 2009-12-23 | Intergenetics Incorporated | Analyse génétique pour la stratification du risque de cancer par détermination du profile allelique des génes VDR-ApaI et CYP11b2 |
| US8460867B2 (en) | 2001-12-10 | 2013-06-11 | Novartis Ag | Methods of treating psychosis and schizophrenia based on polymorphisms in the CNTF gene |
| WO2003053220A2 (fr) | 2001-12-17 | 2003-07-03 | Corixa Corporation | Compositions et procedes applicables a la therapie et au diagnostic pour la maladie intestinale inflammatoire |
| EP1975231A1 (fr) | 2002-01-22 | 2008-10-01 | Corixa Corporation | Compositions et procédés de détection, diagnostic et de thérapie de malignancies hématologiques |
| WO2005019409A2 (fr) | 2002-07-15 | 2005-03-03 | Board Of Regents, The University Of Texas System | Criblage combinatoire de banques de proteines par expression periplasmique |
| EP2044948A1 (fr) | 2002-08-12 | 2009-04-08 | Jennerex Biotherapeutics ULC | Procédés et compositions concernant des poxvirus et le cancer |
| EP2269618A1 (fr) | 2002-08-12 | 2011-01-05 | Jennerex Biotherapeutics ULC | Virus de vaccinia oncolytique pour usage en combinaison aven une chémiothérapie pour le traitement du cancer |
| EP2269619A1 (fr) | 2002-08-12 | 2011-01-05 | Jennerex Biotherapeutics ULC | Procédés et compositions concernant des poxvirus et le cancer |
| EP2325303A2 (fr) | 2002-09-13 | 2011-05-25 | Life Technologies Corporation | Transcriptases inverses thermostables et leurs utilisations |
| EP2940028A1 (fr) | 2003-01-06 | 2015-11-04 | Corixa Corporation | Composes d'aminoalkyle glucosaminide phosphate et leur utilisation |
| WO2004062599A2 (fr) | 2003-01-06 | 2004-07-29 | Corixa Corporation | Composes d'aminoalkyle glucosaminide phosphate et leur utilisation |
| EP2145955A2 (fr) | 2003-01-29 | 2010-01-20 | 454 Corporation | Amplification d'acides nucléiques par emulsion de billes |
| EP2261372A2 (fr) | 2003-01-29 | 2010-12-15 | 454 Life Sciences Corporation | Procédé d'amplification et de séquençage d'acides nucléiques |
| US8748102B2 (en) | 2003-01-29 | 2014-06-10 | 454 Life Sciences Corporation | Bead emulsion nucleic acid amplification |
| EP1997889A2 (fr) | 2003-01-29 | 2008-12-03 | 454 Corporation | Procédé de préparation de bibliothèques ADN à simple brin |
| US8012690B2 (en) | 2003-01-29 | 2011-09-06 | 454 Life Sciences Corporation | Bead emulsion nucleic acid amplification |
| EP2159285A2 (fr) | 2003-01-29 | 2010-03-03 | 454 Life Sciences Corporation | Procédés d'amplification et de séquençage d'acides nucléiques |
| EP2390352A1 (fr) | 2003-03-18 | 2011-11-30 | Quantum Genetics Ireland Limited | Systemes et procedes pour accroitre la production de proteines et de lait chez des bovins laitiers |
| EP2298891A1 (fr) | 2003-07-25 | 2011-03-23 | Ambion, Inc. | Procédés et compositions pour isoler des petites molécules d'ARN |
| EP3366771A1 (fr) | 2003-07-25 | 2018-08-29 | Life Technologies Corporation | Procédé pour isoler des petites molécules d'arn |
| EP2902490A1 (fr) | 2003-07-25 | 2015-08-05 | Life Technologies Corporation | Procédé pour isoler des petites molécules d'ARN |
| EP2295569A1 (fr) | 2003-07-25 | 2011-03-16 | Ambion, Inc. | Procédés et compositions pour isoler des petites molécules de RNA |
| US7449292B2 (en) | 2003-09-12 | 2008-11-11 | University Of Cincinnati | Methods for predicting relative efficacy of a beta blocker therapy based on a B1-adrenergic receptor polymorphism |
| WO2005112544A2 (fr) | 2004-02-19 | 2005-12-01 | The Governors Of The University Of Alberta | Polymorphismes du promoteur de la leptine et utilisations correspondantes |
| EP2527446A1 (fr) | 2004-06-03 | 2012-11-28 | Athlomics Pty Ltd | Agents et procédés pour diagnostiquer le stress |
| EP2527447A1 (fr) | 2004-06-03 | 2012-11-28 | Athlomics Pty Ltd | Agents et procédés pour diagnostiquer le stress |
| EP2270034A2 (fr) | 2004-06-03 | 2011-01-05 | Athlomics Pty Ltd | Agents et procédé permettant le diagnostic de stress |
| EP2940027A1 (fr) | 2004-07-08 | 2015-11-04 | Corixa Corporation | Composes d'iaminoalkylglucosaminide phosphate et leur utilisation |
| US8916603B2 (en) | 2004-09-14 | 2014-12-23 | The Regents Of The University Of Colorado, A Body Corporate | Methods for treatment with bucindolol based on genetic targeting |
| US8093286B2 (en) | 2004-09-14 | 2012-01-10 | The Regents Of The University Of Colorado, A Body Corporate | Methods for treatment with bucindolol based on genetic targeting |
| EP2246444A1 (fr) | 2004-09-14 | 2010-11-03 | The Regents of the University of Colorado, A Body Corporate | Procédé de traitement faisant intervenir du bucindolol fondé sur le ciblage génétique |
| US8080578B2 (en) | 2004-09-14 | 2011-12-20 | The Regents Of The University Of Colorado, A Body Corporate | Methods for treatment with bucindolol based on genetic targeting |
| US9505846B2 (en) | 2005-01-28 | 2016-11-29 | Life Technologies Corporation | Multi-component inhibitors of nucleic acid polymerases |
| EP3056566A1 (fr) | 2005-01-28 | 2016-08-17 | Life Technologies Corporation | Inhibiteurs multi-composants de polymerases d'acides nucleiques et methodes associees |
| EP2365079A1 (fr) | 2005-03-05 | 2011-09-14 | Seegene, Inc. | Procédés utilisant un oligonucléotide à double spécificité et oligonucléotide à double spécificité |
| EP2365078A1 (fr) | 2005-03-05 | 2011-09-14 | Seegene, Inc. | Procédés utilisant un oligonucléotide à double spécificité et oligonucléotide à double spécificité |
| WO2006122354A1 (fr) | 2005-05-17 | 2006-11-23 | Ozgene Pty Ltd | Systeme de clonage sequentiel |
| EP2476761A2 (fr) | 2005-07-07 | 2012-07-18 | Athlomics Pty Ltd | Gènes de marqueur de polynucléotide et leur expression pour le diagnostic de l'endotoxine |
| US7494788B2 (en) | 2005-07-11 | 2009-02-24 | Molecular Kinetics, Inc. | Entropic bristle domain sequences and their use in recombinant protein production |
| EP2662456A1 (fr) | 2005-08-02 | 2013-11-13 | Focus Diagnostics, Inc. | Procédés et compositions pour détecter le virus BK |
| WO2007016275A2 (fr) | 2005-08-02 | 2007-02-08 | Focus Diagnostics, Inc. | Procedes et compositions pour detecter le virus bk |
| EP2319941A2 (fr) | 2005-10-21 | 2011-05-11 | GeneNews Inc. | Procédé et appareil pour corréler des niveaux de produits biomarqueurs avec une maladie |
| EP2368868A1 (fr) | 2005-10-28 | 2011-09-28 | Praecis Pharmaceuticals Inc. | Procédé d'identification de composés intéressants utilisant des bibliothèques codées |
| EP2299267A2 (fr) | 2005-11-16 | 2011-03-23 | Novartis AG | Biomarqueurs pour un traitement par des anticorps anti-NogoA de lésions de la moelle épinière |
| US8222023B2 (en) | 2006-03-15 | 2012-07-17 | Micronics, Inc. | Integrated nucleic acid assays |
| US8772017B2 (en) | 2006-03-15 | 2014-07-08 | Micronics, Inc. | Integrated nucleic acid assays |
| EP2455491A2 (fr) | 2006-05-25 | 2012-05-23 | Monsanto Technology LLC | Procédé pour identifier des locus de traits quantitatifs résistants aux maladies dans le soja et compositions correspondantes |
| EP2455490A2 (fr) | 2006-05-25 | 2012-05-23 | Monsanto Technology LLC | Procédé pour identifier des locus de traits quantitatifs résistants aux maladies dans le soja et compositions correspondantes |
| EP2455489A2 (fr) | 2006-05-25 | 2012-05-23 | Monsanto Technology LLC | Procédé pour identifier des locus de traits quantitatifs résistants aux maladies dans le soja et compositions correspondantes |
| EP2442108A1 (fr) | 2006-07-14 | 2012-04-18 | The Regents of the University of California | Biomarqueurs du cancer et procédé d'utilisation correspondant |
| EP2450710A2 (fr) | 2006-07-14 | 2012-05-09 | The Regents of the University of California | Biomarqueurs du cancer et procédé d'utilisation correspondant |
| EP2442109A1 (fr) | 2006-07-14 | 2012-04-18 | The Regents of the University of California | Biomarqueurs du cancer et procédé d'utilisation correspondant |
| EP3796002A1 (fr) | 2006-07-14 | 2021-03-24 | The Regents of The University of California | Biomarqueurs du cancer et leurs procédés d'utilisation |
| EP2839837A1 (fr) | 2006-09-15 | 2015-02-25 | Ottawa Hospital Research Institute | Rhabdovirus Oncolytique Farmington |
| WO2008067547A2 (fr) | 2006-11-30 | 2008-06-05 | Research Development Foundation | Bibliothèques d'immunoglobulines améliorées |
| WO2008092866A1 (fr) | 2007-01-29 | 2008-08-07 | Scientific Institute Of Public Health (Iph) | Détection d'événements dans des plantes transgéniques |
| EP2623605A1 (fr) | 2007-04-09 | 2013-08-07 | University of Florida Research Foundation, Inc. | Compositions de vecteur RAAV comprenant des protéines de capside à tyrosine modifié et procédés d'utilisation |
| EP3492596A1 (fr) | 2007-04-09 | 2019-06-05 | University of Florida Research Foundation, Inc. | Compositions de vecteur raav comprenant des protéines de capside à tyrosine modifié et procédés d'utilisation |
| WO2008137475A2 (fr) | 2007-05-01 | 2008-11-13 | Research Development Foundation | Banques d'immunoglobulines fc |
| US8568730B2 (en) | 2009-04-22 | 2013-10-29 | Indiana University Research & Technology Corporation | Compositions for use in the treatment of chronic obstructive pulmonary diseases and asthma |
| EP2789689A1 (fr) | 2009-06-29 | 2014-10-15 | Luminex Corporation | Amorces chimères avec des conformations en épingle à cheveux et leurs procédés d'utilisation |
| WO2011008530A2 (fr) | 2009-06-29 | 2011-01-20 | Luminex Corporation | Amorces chimériques avec conformations en épingle à cheveux et procédés d'utilisation de celles-ci |
| EP3636759A1 (fr) | 2009-07-17 | 2020-04-15 | BioAtla LLC | Sélection simultanée et intégrée et évolution des performances d'anticorps/de protéines et de l'expression dans des hôtes de production |
| US10047357B2 (en) | 2009-07-17 | 2018-08-14 | Bioatla, Llc | Simultaneous, integrated selection and evolution of antibody/protein performance and expression in production hosts |
| EP3042957A1 (fr) | 2009-07-17 | 2016-07-13 | Bioatla LLC | Evolution et sélection simultanée of intégrée d'anticorps/performance de protéines et expression dans des hôtes de production |
| EP2907873A1 (fr) | 2009-07-17 | 2015-08-19 | Bioatla LLC | Évolution et sélection simultanée et intégrée d'anticorps/performance de protéines et expression dans des hôtes de production |
| US8859467B2 (en) | 2009-07-17 | 2014-10-14 | Bioatla, Llc | Simultaneous, integrated selection and evolution of antibody/protein performance and expression in production hosts |
| US10920216B2 (en) | 2009-07-17 | 2021-02-16 | Bioatla, Inc. | Simultaneous, integrated selection and evolution of antibody/protein performance and expression in production hosts |
| US10106788B2 (en) | 2009-07-17 | 2018-10-23 | Bioatla, Llc | Simultaneous, integrated selection and evolution of antibody/protein performance and expression in production hosts |
| US9409983B2 (en) | 2009-07-23 | 2016-08-09 | The Board Of Trustess Of The University Of Illinois | Methods and compositions involving PBEF inhibitors for lung inflammation conditions and diseases |
| US8614062B1 (en) | 2009-07-24 | 2013-12-24 | University Of South Florida | RNA-based system and method to differentiate seafood |
| US9896664B2 (en) | 2009-12-10 | 2018-02-20 | Turnstone Limited Partnership | Oncolytic rhabdovirus |
| WO2011079273A2 (fr) | 2009-12-23 | 2011-06-30 | Arca Biopharma, Inc. | Procédés et compositions pour maladies et affections cardiovasculaires |
| US9895692B2 (en) | 2010-01-29 | 2018-02-20 | Micronics, Inc. | Sample-to-answer microfluidic cartridge |
| WO2011127933A1 (fr) | 2010-04-16 | 2011-10-20 | Nuevolution A/S | Complexes bifonctionnels et procédés de fabrication et d'utilisation de tels complexes |
| EP3540059A1 (fr) | 2010-04-16 | 2019-09-18 | Nuevolution A/S | Complexes bifonctionnels et procédés de fabrication et d'utilisation de tels complexes |
| WO2011133933A2 (fr) | 2010-04-23 | 2011-10-27 | University Of Florida Research Foundation, Inc. | Compositions de guanylate cyclase raav et méthodes de traitement de l'amaurose-1 congénitale de leber (lca1) |
| EP4056700A1 (fr) | 2010-04-23 | 2022-09-14 | University of Florida Research Foundation, Inc. | Compositions raav-guanylate cyclase et procédés de traitement de l'amaurose-1 congénitale de leber (lca1) |
| EP3486320A1 (fr) | 2010-04-23 | 2019-05-22 | University of Florida Research Foundation, Inc. | Compositions raav-guanylate cyclase et procédés de traitement de l'amaurose-1 congénitale de leber (lca1) |
| WO2011139721A1 (fr) | 2010-04-27 | 2011-11-10 | The Regents Of The University Of California | Biomarqueurs de cancer et leurs procédés d'utilisation |
| EP3508854A1 (fr) | 2010-04-27 | 2019-07-10 | The Regents of The University of California | Biomarqueurs du cancer et leurs procédés d'utilisation |
| WO2011145777A1 (fr) | 2010-05-20 | 2011-11-24 | 광주과학기술원 | Composition pharmaceutique comprenant un inhibiteur de hif-2α comme ingrédient actif pour la prévention ou le traitement de l'arthrite |
| WO2011145795A1 (fr) | 2010-05-20 | 2011-11-24 | 광주과학기술원 | Souris transgénique hif-2α spécifique du cartilage comme modèle animal pour l'arthrite, et son utilisation |
| EP2913402A1 (fr) | 2010-07-16 | 2015-09-02 | Tocagen Inc. | Détection de rétrovirus |
| WO2012090073A2 (fr) | 2010-12-30 | 2012-07-05 | The Netherlands Cancer Institute | Procédés et compositions pour prédire la sensibilité à la chimiothérapie |
| US9919047B2 (en) | 2011-01-04 | 2018-03-20 | Sillajen, Inc. | Generation of antibodies to tumor antigens and generation of tumor specific complement dependent cytotoxicity by administration of oncolytic vaccinia virus |
| WO2012109133A1 (fr) | 2011-02-07 | 2012-08-16 | Research Development Foundation | Polypeptides fc modifiés d'immunoglobuline |
| WO2012158238A2 (fr) | 2011-02-28 | 2012-11-22 | University Of Iowa Research Foundation | Changements d'hormone antimullérienne dans la grossesse et prédiction d'évolution indésirable de grossesse et du sexe |
| WO2012120377A2 (fr) | 2011-03-08 | 2012-09-13 | King Abdullah University Of Science And Technology | Biomarqueur moléculaire destiné à la détection précoce du cancer des ovaires |
| US9057107B2 (en) | 2011-03-08 | 2015-06-16 | King Abdullah University Of Science And Technology | Molecular biomarker set for early detection of ovarian cancer |
| US9914974B2 (en) | 2011-03-08 | 2018-03-13 | King Abdullah University Of Science And Technology | Molecular biomarker set for early detection of ovarian cancer |
| WO2012138789A2 (fr) | 2011-04-04 | 2012-10-11 | Netherlands Cancer Institute | Procédés et compositions pour prédire une résistance à un traitement anti-cancéreux |
| WO2012138783A2 (fr) | 2011-04-04 | 2012-10-11 | Netherlands Cancer Institute | Procédés et compositions pour prédire une résistance à un traitement anti-cancéreux |
| US8841432B2 (en) | 2011-04-21 | 2014-09-23 | Snu R&Db Foundation | Shuttle vectors for mycobacteria-escherichia coli and uses thereof |
| WO2012149038A1 (fr) | 2011-04-25 | 2012-11-01 | Advanced Bioscience Laboratories, Inc. | Protéines tronquées d'enveloppe (env) du vih, procédés et compositions associés à celles-ci |
| US10772951B2 (en) | 2011-06-08 | 2020-09-15 | Children's Hospital Of Eastern Ontario Research Institute Inc. | Compositions and methods for glioblastoma treatment |
| US11654192B2 (en) | 2011-06-08 | 2023-05-23 | Children's Hospital Of Eastern Ontario Research Institute Inc. | Compositions and methods for glioblastoma treatment |
| US10040853B2 (en) | 2011-09-09 | 2018-08-07 | Fred Hutchinson Cancer Research Center | Methods and compositions involving NKG2D inhibitors and cancer |
| WO2013045505A1 (fr) | 2011-09-28 | 2013-04-04 | Novartis Ag | Biomarqueurs pour traitement combiné par des agents sraa |
| US9540670B2 (en) | 2011-10-06 | 2017-01-10 | bioMérieux | Mutated T7 RNA Polymerases |
| WO2013071233A1 (fr) | 2011-11-10 | 2013-05-16 | The United States Of America, As Represented By The Secretary, Department Of Health & Human Services | Procédés de détection d'agents infectieux et nouveau virus détecté par ces procédés |
| WO2013074676A2 (fr) | 2011-11-14 | 2013-05-23 | The General Hospital Corporation | Dosages et procédés pour la sélection d'un schéma thérapeutique pour un sujet atteint d'une dépression |
| WO2013093629A2 (fr) | 2011-12-20 | 2013-06-27 | Netherlands Cancer Institute | Vaccins modulaires, procédés et compositions qui y sont liés |
| WO2013096798A2 (fr) | 2011-12-22 | 2013-06-27 | Ibis Biosciences, Inc. | Amplification d'une séquence provenant d'un acide ribonucléique |
| WO2013116698A2 (fr) | 2012-02-02 | 2013-08-08 | Invenra, Inc. | Crible haut débit pour des polypeptides biologiquement actifs |
| WO2013144249A1 (fr) | 2012-03-29 | 2013-10-03 | Novartis Ag | Produit pharmaceutique à des fins diagnostiques |
| EP3524691A1 (fr) | 2012-12-07 | 2019-08-14 | SuppreMol GmbH | Stratification et traitement de patients du purpura thrombocytopénique idiopathique |
| EP2740805A1 (fr) | 2012-12-07 | 2014-06-11 | SuppreMol GmbH | Stratification et traitement de patients du purpura thrombocytopénique idiopathique |
| EP3249054A1 (fr) | 2012-12-20 | 2017-11-29 | Biomatrica, INC. | Formulations et procédés pour stabiliser des réactifs pcr |
| US10518262B2 (en) | 2012-12-21 | 2019-12-31 | Perkinelmer Health Sciences, Inc. | Low elasticity films for microfluidic use |
| US11181105B2 (en) | 2012-12-21 | 2021-11-23 | Perkinelmer Health Sciences, Inc. | Low elasticity films for microfluidic use |
| US10065186B2 (en) | 2012-12-21 | 2018-09-04 | Micronics, Inc. | Fluidic circuits and related manufacturing methods |
| US10436713B2 (en) | 2012-12-21 | 2019-10-08 | Micronics, Inc. | Portable fluorescence detection system and microassay cartridge |
| US10125373B2 (en) | 2013-01-22 | 2018-11-13 | Arizona Board Of Regents On Behalf Of Arizona State University | Geminiviral vector for expression of rituximab |
| US10660947B2 (en) | 2013-02-21 | 2020-05-26 | Turnstone Limited Partnership | Vaccine composition |
| US10363293B2 (en) | 2013-02-21 | 2019-07-30 | Turnstone Limited Partnership | Vaccine composition |
| US10646557B2 (en) | 2013-02-21 | 2020-05-12 | Turnstone Limited Partnership | Vaccine composition |
| WO2014148817A1 (fr) | 2013-03-21 | 2014-09-25 | 주식회사 현일바이오 | Procédé de détection sélective pour mycobacterium tuberculosis et des mycobactéries non tuberculeuses et kit utilisant celui-ci |
| WO2014171698A1 (fr) | 2013-04-18 | 2014-10-23 | 사회복지법인 삼성생명공익재단 | Méthode de diagnostic de la dystrophie myotonique de type 1 |
| US10087440B2 (en) | 2013-05-07 | 2018-10-02 | Micronics, Inc. | Device for preparation and analysis of nucleic acids |
| US10190153B2 (en) | 2013-05-07 | 2019-01-29 | Micronics, Inc. | Methods for preparation of nucleic acid-containing samples using clay minerals and alkaline solutions |
| US11016108B2 (en) | 2013-05-07 | 2021-05-25 | Perkinelmer Health Sciences, Inc. | Microfluidic devices and methods for performing serum separation and blood cross-matching |
| US10386377B2 (en) | 2013-05-07 | 2019-08-20 | Micronics, Inc. | Microfluidic devices and methods for performing serum separation and blood cross-matching |
| WO2014183023A1 (fr) | 2013-05-09 | 2014-11-13 | Trustees Of Boston University | Utilisation de la plexine-a4 comme biomarqueur et cible thérapeutique pour la maladie d'alzheimer |
| US11585810B2 (en) | 2013-10-03 | 2023-02-21 | Oklahoma Medical Research Foundation | Biomarkers for systemic lupus erythematosus disease activity, and intensity and flare |
| US10393739B2 (en) | 2013-10-03 | 2019-08-27 | Oklahoma Medical Research Foundation | Biomarkers for systemic lupus erythematosus disease activity, and intensity and flare |
| WO2015105888A1 (fr) | 2014-01-07 | 2015-07-16 | Bioatla, Llc | Orthologues ciblant les protéines |
| EP3447493A1 (fr) | 2014-01-07 | 2019-02-27 | Bioatla, LLC | Orthologues ciblant les protéines |
| US11293929B2 (en) | 2014-01-07 | 2022-04-05 | Bioatla, Inc. | Proteins targeting orthologs |
| WO2015112767A2 (fr) | 2014-01-22 | 2015-07-30 | Life Technologies Corporation | Nouvelles rétrotranscriptases pour une utilisation dans la synthèse d'acide nucléique à haute température |
| WO2015191916A1 (fr) | 2014-06-11 | 2015-12-17 | Micronics, Inc. | Cartouches microfluidiques et appareil à témoins de dosage intégrés pour l'analyse d'acides nucléiques |
| WO2016086227A2 (fr) | 2014-11-26 | 2016-06-02 | The Regents Of The University Of California | Compositions thérapeutiques comprenant des facteurs de transcription et leurs procédés de préparation et d'utilisation |
| US11543411B2 (en) | 2014-12-05 | 2023-01-03 | Prelude Corporation | DCIS recurrence and invasive breast cancer |
| EP4242329A2 (fr) | 2014-12-08 | 2023-09-13 | Berg LLC | Utilisation de marqueurs comprenant de la filamine a dans le diagnostic et le traitement du cancer de la prostate |
| US10172879B2 (en) | 2015-01-21 | 2019-01-08 | D.R.Nano Co., Ltd | Nanocomplexes for co-delivering a drug and siRNA and uses thereof |
| WO2016130516A1 (fr) | 2015-02-09 | 2016-08-18 | Research Development Foundation | Polypeptides ayant un domaine fc d'immunoglobuline modifié manifestant une activation du complément améliorée |
| WO2017015075A1 (fr) | 2015-07-17 | 2017-01-26 | President And Fellows Of Harvard College | Procédés d'amplification de séquences d'acides nucléiques |
| WO2017040380A2 (fr) | 2015-08-28 | 2017-03-09 | Research Development Foundation | Variants de fc d'anticorps modifiés |
| US11339421B2 (en) | 2016-02-23 | 2022-05-24 | Industry Foundation Of Chonnam National University | Leukemia diagnostic kit targeting prohibitin gene and diagnostic method using same |
| WO2017214068A1 (fr) | 2016-06-05 | 2017-12-14 | Berg Llc | Systèmes et procédés de stratification de patient et identification de biomarqueurs potentiels |
| WO2018128516A1 (fr) | 2017-01-09 | 2018-07-12 | 연세대학교 산학협력단 | Composition pharmaceutique pour un traitement de précancer buccal et procédé de prédiction ou de diagnostic d'un précancer buccal ou d'un cancer buccal |
| WO2018140606A1 (fr) | 2017-01-26 | 2018-08-02 | Oklahoma Medical Research Foundation | Biomarqueurs de l'activité, de l'intensité et de l'éruption de la maladie du lupus érythémateux disséminé |
| WO2018219997A1 (fr) | 2017-05-30 | 2018-12-06 | Roche Diagnostics Gmbh | Catalyseurs pour inverser des produits d'addition de formaldéhyde et des réticulations |
| WO2019017680A2 (fr) | 2017-07-19 | 2019-01-24 | 국민대학교 산학협력단 | Micro-arn utilisé en tant que biomarqueur de la maladie de parkinson et kit diagnostique le mettant en oeuvre |
| US11447772B2 (en) | 2017-07-19 | 2022-09-20 | Kookmin University Industry Academy Cooperation Foundation | miRNA as biomarker for Parkinson's disease and diagnostic kit using same |
| EP4275684A1 (fr) | 2018-05-25 | 2023-11-15 | Arca Biopharma, Inc. | Procédés et compositions impliquant du bucindolol pour le traitement de la fibrillation auriculaire |
| US11821900B2 (en) | 2018-09-14 | 2023-11-21 | Prelude Corporation | Method of selection for treatment of subjects at risk of invasive breast cancer |
| WO2020089841A1 (fr) | 2018-10-31 | 2020-05-07 | Garcia Joe G N | Biomarqueurs et méthodes d'utilisation de ces biomarqueurs pour détecter une lésion pulmonaire radio-induite |
| WO2020223576A1 (fr) | 2019-04-30 | 2020-11-05 | Chondrial Therapeutics, Inc. | Marqueurs sensibles à la frataxine pour déterminer l'efficacité d'une thérapie de remplacement de frataxine |
| WO2021246820A1 (fr) | 2020-06-05 | 2021-12-09 | 주식회사 씨젠 | Kit de transport d'échantillon pour la détection d'agents pathogènes respiratoires et procédés de détection d'agents pathogènes respiratoires à l'aide de celui-ci |
| WO2022047359A1 (fr) | 2020-08-31 | 2022-03-03 | Berg Llc | Biomarqueurs protéiques du cancer du pancréas |
| WO2022216846A1 (fr) | 2021-04-06 | 2022-10-13 | Berg Llc | Marqueurs protéiques pour le cancer du sein du type positif aux récepteurs des oestrogènes (er) et du type négatif aux récepteurs des oestrogènes (er) |
| WO2022216841A1 (fr) | 2021-04-06 | 2022-10-13 | Berg Llc | Marqueurs protéiques pour un cancer du sein positif au récepteur des œstrogènes (er) de type luminal a (la) et de type luminal b1 (lb1) |
| WO2022265965A1 (fr) | 2021-06-14 | 2022-12-22 | 10X Genomics, Inc. | Variants de la transcriptase inverse pour une performance améliorée |
| WO2023196937A1 (fr) | 2022-04-06 | 2023-10-12 | Larimar Therapeutics, Inc. | Marqueurs sensibles à la frataxine pour surveiller une thérapie de remplacement de frataxine |
| WO2023230531A1 (fr) | 2022-05-24 | 2023-11-30 | Lunglife Ai, Inc. | Procédés de détection de cellules génétiquement anormales circulantes |
| WO2023239940A1 (fr) | 2022-06-10 | 2023-12-14 | Research Development Foundation | Variants de fc igg1 sélectifs de fcriib modifiés et leurs utilisations |
Also Published As
| Publication number | Publication date |
|---|---|
| WO1991002814A1 (fr) | 1991-03-07 |
| DE3850093T2 (de) | 1994-11-03 |
| KR890013184A (ko) | 1989-09-21 |
| ES2053648T3 (es) | 1994-08-01 |
| CA1340807C (fr) | 1999-11-02 |
| EP0329822B1 (fr) | 1994-06-08 |
| US5409818A (en) | 1995-04-25 |
| KR960015744B1 (ko) | 1996-11-20 |
| KR920702866A (ko) | 1992-10-28 |
| EP0329822A3 (en) | 1990-08-01 |
| ATE106948T1 (de) | 1994-06-15 |
| DE3850093D1 (de) | 1994-07-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0329822B1 (fr) | Procédé d'amplification d'acides nucléiques | |
| US6063603A (en) | Nucleic acid amplification process | |
| EP0487628B1 (fr) | Procede d'amplification ameliore d'acides nucleiques | |
| US5466586A (en) | Method for the synthesis of ribonucleic acid (RNA) | |
| KR100392551B1 (ko) | 핵산 증폭방법을 개선시키기 위한 rna폴리머라제의 용도 | |
| US5437990A (en) | Selective amplification of target polynucleotide sequences | |
| US5169766A (en) | Amplification of nucleic acid molecules | |
| US5665545A (en) | Terminal repeat amplification method | |
| JP2843586B2 (ja) | 転写に基づいた核酸増幅/検出系 | |
| FI99143C (fi) | Itseään ylläpitävä sekvenssin replikaatiosysteemi | |
| US5869251A (en) | Use of primers containing nucleotides having altered base pairing characteristics in the amplification of nucleic acid molecules | |
| US6063604A (en) | Target nucleic acid sequence amplification | |
| JPH02503054A (ja) | 核酸配列の増幅および検出 | |
| KR20010041371A (ko) | 핵산의 비특이적 증폭방법 | |
| IE892698A1 (en) | Nucleic acid amplification process | |
| CA2223527A1 (fr) | Amplification lineaire liee d'acides nucleiques | |
| JPH0576399A (ja) | イン ビトロで複製可能な核酸の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AT BE CH DE ES FR GB GR IT LI LU NL SE |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): AT BE CH DE ES FR GB GR IT LI LU NL SE |
|
| 17P | Request for examination filed |
Effective date: 19901127 |
|
| 17Q | First examination report despatched |
Effective date: 19910502 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE ES FR GB GR IT LI LU NL SE |
|
| REF | Corresponds to: |
Ref document number: 106948 Country of ref document: AT Date of ref document: 19940615 Kind code of ref document: T |
|
| REF | Corresponds to: |
Ref document number: 3850093 Country of ref document: DE Date of ref document: 19940714 |
|
| RAP2 | Party data changed (patent owner data changed or rights of a patent transferred) |
Owner name: CANGENE CORPORATION |
|
| ET | Fr: translation filed | ||
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2053648 Country of ref document: ES Kind code of ref document: T3 |
|
| REG | Reference to a national code |
Ref country code: GR Ref legal event code: FG4A Free format text: 3012159 |
|
| ITF | It: translation for a ep patent filed |
Owner name: SAIC BREVETTI S.R.L. |
|
| EAL | Se: european patent in force in sweden |
Ref document number: 88113948.9 |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| 26 | Opposition filed |
Opponent name: GEN- PROBE INC. Effective date: 19950308 |
|
| NLR1 | Nl: opposition has been filed with the epo |
Opponent name: GEN-PROBE INC. |
|
| PLBF | Reply of patent proprietor to notice(s) of opposition |
Free format text: ORIGINAL CODE: EPIDOS OBSO |
|
| PLBL | Opposition procedure terminated |
Free format text: ORIGINAL CODE: EPIDOS OPPC |
|
| PLBM | Termination of opposition procedure: date of legal effect published |
Free format text: ORIGINAL CODE: 0009276 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: OPPOSITION PROCEDURE CLOSED |
|
| 27C | Opposition proceedings terminated |
Effective date: 19970807 |
|
| NLR2 | Nl: decision of opposition | ||
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: LU Payment date: 20070809 Year of fee payment: 20 Ref country code: DE Payment date: 20070809 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20070824 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 20070718 Year of fee payment: 20 Ref country code: CH Payment date: 20070813 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20070810 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20070816 Year of fee payment: 20 Ref country code: BE Payment date: 20070911 Year of fee payment: 20 Ref country code: SE Payment date: 20070717 Year of fee payment: 20 Ref country code: NL Payment date: 20070719 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20070831 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GR Payment date: 20070730 Year of fee payment: 20 |
|
| BE20 | Be: patent expired |
Owner name: *CANGENE CORP. Effective date: 20080826 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 Expiry date: 20080825 |
|
| EUG | Se: european patent has lapsed | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20080826 |
|
| NLV7 | Nl: ceased due to reaching the maximum lifetime of a patent |
Effective date: 20080826 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20080827 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20080825 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20080827 |