WO2009113399A1 - 研磨パッド - Google Patents
研磨パッド Download PDFInfo
- Publication number
- WO2009113399A1 WO2009113399A1 PCT/JP2009/053481 JP2009053481W WO2009113399A1 WO 2009113399 A1 WO2009113399 A1 WO 2009113399A1 JP 2009053481 W JP2009053481 W JP 2009053481W WO 2009113399 A1 WO2009113399 A1 WO 2009113399A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- polishing
- polyurethane foam
- layer
- isocyanate
- polishing pad
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B24—GRINDING; POLISHING
- B24B—MACHINES, DEVICES, OR PROCESSES FOR GRINDING OR POLISHING; DRESSING OR CONDITIONING OF ABRADING SURFACES; FEEDING OF GRINDING, POLISHING, OR LAPPING AGENTS
- B24B37/00—Lapping machines or devices; Accessories
- B24B37/11—Lapping tools
- B24B37/20—Lapping pads for working plane surfaces
- B24B37/24—Lapping pads for working plane surfaces characterised by the composition or properties of the pad materials
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/30—Low-molecular-weight compounds
- C08G18/32—Polyhydroxy compounds; Polyamines; Hydroxyamines
- C08G18/3203—Polyhydroxy compounds
- C08G18/3206—Polyhydroxy compounds aliphatic
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/40—High-molecular-weight compounds
- C08G18/4009—Two or more macromolecular compounds not provided for in one single group of groups C08G18/42 - C08G18/64
- C08G18/4018—Mixtures of compounds of group C08G18/42 with compounds of group C08G18/48
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/40—High-molecular-weight compounds
- C08G18/42—Polycondensates having carboxylic or carbonic ester groups in the main chain
- C08G18/4266—Polycondensates having carboxylic or carbonic ester groups in the main chain prepared from hydroxycarboxylic acids and/or lactones
- C08G18/4269—Lactones
- C08G18/4277—Caprolactone and/or substituted caprolactone
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/40—High-molecular-weight compounds
- C08G18/48—Polyethers
- C08G18/4854—Polyethers containing oxyalkylene groups having four carbon atoms in the alkylene group
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/65—Low-molecular-weight compounds having active hydrogen with high-molecular-weight compounds having active hydrogen
- C08G18/66—Compounds of groups C08G18/42, C08G18/48, or C08G18/52
- C08G18/6603—Compounds of groups C08G18/42, C08G18/48, or C08G18/52 with compounds of group C08G18/32 or polyamines of C08G18/38
- C08G18/6607—Compounds of groups C08G18/42, C08G18/48, or C08G18/52 with compounds of group C08G18/32 or polyamines of C08G18/38 with compounds of group C08G18/3203
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/65—Low-molecular-weight compounds having active hydrogen with high-molecular-weight compounds having active hydrogen
- C08G18/66—Compounds of groups C08G18/42, C08G18/48, or C08G18/52
- C08G18/6603—Compounds of groups C08G18/42, C08G18/48, or C08G18/52 with compounds of group C08G18/32 or polyamines of C08G18/38
- C08G18/6607—Compounds of groups C08G18/42, C08G18/48, or C08G18/52 with compounds of group C08G18/32 or polyamines of C08G18/38 with compounds of group C08G18/3203
- C08G18/6611—Compounds of groups C08G18/42, C08G18/48, or C08G18/52 with compounds of group C08G18/32 or polyamines of C08G18/38 with compounds of group C08G18/3203 having at least three hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/65—Low-molecular-weight compounds having active hydrogen with high-molecular-weight compounds having active hydrogen
- C08G18/66—Compounds of groups C08G18/42, C08G18/48, or C08G18/52
- C08G18/6633—Compounds of group C08G18/42
- C08G18/6637—Compounds of group C08G18/42 with compounds of group C08G18/32 or polyamines of C08G18/38
- C08G18/664—Compounds of group C08G18/42 with compounds of group C08G18/32 or polyamines of C08G18/38 with compounds of group C08G18/3203
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/65—Low-molecular-weight compounds having active hydrogen with high-molecular-weight compounds having active hydrogen
- C08G18/66—Compounds of groups C08G18/42, C08G18/48, or C08G18/52
- C08G18/6633—Compounds of group C08G18/42
- C08G18/6637—Compounds of group C08G18/42 with compounds of group C08G18/32 or polyamines of C08G18/38
- C08G18/664—Compounds of group C08G18/42 with compounds of group C08G18/32 or polyamines of C08G18/38 with compounds of group C08G18/3203
- C08G18/6644—Compounds of group C08G18/42 with compounds of group C08G18/32 or polyamines of C08G18/38 with compounds of group C08G18/3203 having at least three hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/65—Low-molecular-weight compounds having active hydrogen with high-molecular-weight compounds having active hydrogen
- C08G18/66—Compounds of groups C08G18/42, C08G18/48, or C08G18/52
- C08G18/6666—Compounds of group C08G18/48 or C08G18/52
- C08G18/667—Compounds of group C08G18/48 or C08G18/52 with compounds of group C08G18/32 or polyamines of C08G18/38
- C08G18/6674—Compounds of group C08G18/48 or C08G18/52 with compounds of group C08G18/32 or polyamines of C08G18/38 with compounds of group C08G18/3203
- C08G18/6677—Compounds of group C08G18/48 or C08G18/52 with compounds of group C08G18/32 or polyamines of C08G18/38 with compounds of group C08G18/3203 having at least three hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/70—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the isocyanates or isothiocyanates used
- C08G18/72—Polyisocyanates or polyisothiocyanates
- C08G18/77—Polyisocyanates or polyisothiocyanates having heteroatoms in addition to the isocyanate or isothiocyanate nitrogen and oxygen or sulfur
- C08G18/78—Nitrogen
- C08G18/79—Nitrogen characterised by the polyisocyanates used, these having groups formed by oligomerisation of isocyanates or isothiocyanates
- C08G18/797—Nitrogen characterised by the polyisocyanates used, these having groups formed by oligomerisation of isocyanates or isothiocyanates containing carbodiimide and/or uretone-imine groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2101/00—Manufacture of cellular products
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L83/00—Compositions of macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing silicon with or without sulfur, nitrogen, oxygen or carbon only; Compositions of derivatives of such polymers
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/24—Structurally defined web or sheet [e.g., overall dimension, etc.]
- Y10T428/24273—Structurally defined web or sheet [e.g., overall dimension, etc.] including aperture
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/249921—Web or sheet containing structurally defined element or component
- Y10T428/249953—Composite having voids in a component [e.g., porous, cellular, etc.]
- Y10T428/249975—Void shape specified [e.g., crushed, flat, round, etc.]
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/249921—Web or sheet containing structurally defined element or component
- Y10T428/249953—Composite having voids in a component [e.g., porous, cellular, etc.]
- Y10T428/249978—Voids specified as micro
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/249921—Web or sheet containing structurally defined element or component
- Y10T428/249953—Composite having voids in a component [e.g., porous, cellular, etc.]
- Y10T428/249978—Voids specified as micro
- Y10T428/249979—Specified thickness of void-containing component [absolute or relative] or numerical cell dimension
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/31504—Composite [nonstructural laminate]
- Y10T428/31551—Of polyamidoester [polyurethane, polyisocyanate, polycarbamate, etc.]
Definitions
- the present invention relates to a polishing pad (for rough polishing or finish polishing) used for polishing surfaces of optical materials such as lenses and reflection mirrors, silicon wafers, glass substrates for hard disks, and aluminum substrates.
- the polishing pad of the present invention is suitably used as a polishing pad for finishing.
- mirror polishing of semiconductor wafers such as silicon wafers, lenses, and glass substrates mainly involves rough polishing for the purpose of adjusting flatness and in-plane uniformity, improvement of surface roughness, and removal of scratches. There is intended finish polishing.
- the finish polishing is usually performed by attaching a suede-like artificial leather made of soft urethane foam on a rotatable surface plate and supplying an abrasive containing colloidal silica to an alkali-based aqueous solution.
- Patent Document 1 a suede-like artificial leather made of soft urethane foam on a rotatable surface plate and supplying an abrasive containing colloidal silica to an alkali-based aqueous solution.
- polishing pads used for finish polishing In addition to the above, the following have been proposed as polishing pads used for finish polishing.
- a suede-like finish polishing pad consisting of a nap layer in which polyurethane foam is formed with a number of elongated fine holes (nap) formed in the thickness direction using a foaming agent and a base fabric that reinforces the nap layer has been proposed.
- Patent Document 2 A suede-like finish polishing pad consisting of a nap layer in which polyurethane foam is formed with a number of elongated fine holes (nap) formed in the thickness direction using a foaming agent and a base fabric that reinforces the nap layer has been proposed.
- the surface layer having a thickness of 0.2 to 2.0 mm and an elastic compressibility of 50 to 4% is laminated on the back side of the surface layer, and the thickness is 0.2 to 2 mm.
- the intermediate support layer has an elastic compressibility of 2 to 0.1%, and is laminated on the back side of the intermediate support layer.
- the thickness is 0.15 to 2.0 mm, and the elastic compressibility is 50 to 4%.
- a polishing cloth having a back surface layer is proposed (Patent Document 3).
- Patent Document 4 a polishing cloth for finish polishing that is suede-like and has a surface roughness of 5 ⁇ m or less in arithmetic mean roughness (Ra) has been proposed.
- a polishing cloth for finishing polishing comprising a base material part and a surface layer (nap layer) formed on the base material part, wherein the surface layer contains a polyvinyl halide or a vinyl halide copolymer.
- Patent Document 5 has been proposed.
- Patent Document 6 an abrasive cloth obtained by impregnating a substrate with a resin solution and then drying by heating.
- Patent Document 7 a polishing cloth obtained by applying a resin solution containing a polyvinyl halide or a vinyl halide copolymer to a base material and coagulating it wet, and then heat-treating has been proposed.
- the wet curing method is a method in which a urethane resin solution in which a urethane resin is dissolved in a water-soluble organic solvent such as dimethylformamide is applied onto a substrate, which is treated in water and wet solidified to form a porous silver surface layer.
- the surface layer (nap layer) is formed by grinding the surface of the silver surface layer after washing and drying.
- a polishing pad for finishing having a substantially spherical hole having an average diameter of 1 to 30 ⁇ m is manufactured by a wet curing method.
- the conventional polishing pad has a problem that the durability is low and the flattening characteristics are gradually deteriorated because the bubbles have an elongated structure or the mechanical strength of the surface layer material itself is low. Further, the conventional polishing pad has a problem that the polishing rate is difficult to stabilize.
- An object of the present invention is to provide a polishing pad having excellent durability and excellent polishing rate stability.
- the present invention provides a polishing pad in which a polishing layer is provided on a base material layer, wherein the polishing layer is made of a thermosetting polyurethane foam containing substantially spherical open cells having an opening, and the thermosetting
- the polyurethane foam contains an isocyanate component and an active hydrogen group-containing compound as raw material components, the isocyanate component contains 90% by weight or more of diphenylmethane diisocyanate and / or a modified product thereof, and the active hydrogen group-containing compound is: 60 to 98% by weight of polycaprolactone polyol, 15 to 40% by weight of a compound having 3 functional groups that react with isocyanate groups, and an isocyanate group concentration of 10 to 10 with respect to the total amount of the isocyanate component and the active hydrogen group-containing compound.
- polishing layer has a compressibility A in a dry state and a compressibility in a wet state.
- Polishing pad absolute value of the rate of change [ ⁇ (B-A) / A ⁇ ⁇ 100] is equal to or is 100 or less, about.
- the conventional polishing pad for finishing has a long and slender structure or the mechanical strength of the material of the polishing layer itself is low. Therefore, when pressure is repeatedly applied to the polishing layer, “sagging” occurs, resulting in poor durability. It is considered to be.
- the durability of the polishing layer can be improved by forming the polishing layer with a thermosetting polyurethane foam containing substantially spherical open cells having openings. Therefore, when the polishing pad of the present invention is used, the planarization characteristics can be maintained high for a long time.
- the substantially spherical shape means a spherical shape and an elliptical shape.
- Oval and spherical bubbles are those having a major axis L to minor axis S ratio (L / S) of 5 or less, preferably 3 or less, more preferably 1.5 or less.
- thermosetting polyurethane foam formed from the raw material components and containing substantially spherical open cells having openings has moderate hydrophilicity, and the slurry penetrates quickly into the thermosetting polyurethane foam.
- the polishing layer of the present invention made of polyurethane foam is characterized by a short time until the polishing rate is stabilized (dummy polishing time is short).
- the absolute value of the change rate [ ⁇ (BA) / A ⁇ ⁇ 100] of the compression rate A in the dry state and the compression rate B in the wet state is 100 or less, And the wet compressibility is small, and the compressibility is unlikely to decrease even when wet, so that the dummy polishing time can be shortened.
- the content of diphenylmethane diisocyanate and / or a modified product thereof in the isocyanate component is less than 90% by weight, the cohesive force of the isocyanate component that forms the hard domain of the polyurethane resin is reduced, and it becomes difficult to form an open cell structure.
- the polishing rate decreases because the hardness of the polishing layer decreases. Since the hardness of the polishing layer becomes too high, the polishing rate decreases, and scratches are likely to occur on the object to be polished.
- thermosetting polyurethane foam preferably has a weight change rate of 10% or more after being immersed in water for 24 hours.
- weight change rate is less than 10%, the familiarity with the slurry is deteriorated, so that the polishing rate tends to decrease.
- the polishing layer is preferably self-adhering to the base material layer. Thereby, it can prevent effectively that a grinding
- the thermosetting polyurethane foam preferably has an open cell structure with an average cell diameter of 40 to 100 ⁇ m and an average opening diameter of 5 to 30 ⁇ m.
- the present invention relates to a semiconductor device manufacturing method including a step of polishing a surface of a semiconductor wafer using the polishing pad.
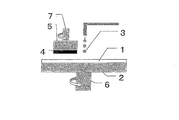
- Polishing pad 2 Polishing surface plate 3: Abrasive (slurry) 4: Polishing target (semiconductor wafer, lens, glass plate) 5: Support base (polishing head) 6, 7: Rotating shaft
- the polishing pad of the present invention includes a polishing layer made of a thermosetting polyurethane foam (hereinafter referred to as polyurethane foam) containing substantially spherical open cells having openings, and a base material layer.
- the thermosetting polyurethane foam contains an isocyanate component and an active hydrogen group-containing compound as raw material components, and the isocyanate component contains diphenylmethane diisocyanate and / or a modified product thereof in an amount of 90% by weight or more.
- the hydrogen group-containing compound contains 60 to 98% by weight of polycaprolactone polyol and 15 to 40% by weight of a compound having 3 functional groups that react with isocyanate groups, and is based on the total amount of the isocyanate component and the active hydrogen group-containing compound.
- the isocyanate group concentration is 10 to 15% by weight.
- Polyurethane resin is excellent in abrasion resistance, and it is possible to easily obtain polymers having desired physical properties by changing the raw material composition. Also, it is easy to form almost spherical fine bubbles by mechanical foaming (including mechanical flossing). Since it can be formed, it is a preferable material for forming the polishing layer.
- the polyurethane resin is composed of an isocyanate component and an active hydrogen group-containing compound (high molecular weight polyol, low molecular weight polyol, alcohol amine, chain extender, etc.).
- diphenylmethane diisocyanate and / or a modified product thereof as an isocyanate component.
- diphenylmethane diisocyanate examples include 2,2'-diphenylmethane diisocyanate, 2,4'-diphenylmethane diisocyanate, and 4,4'-diphenylmethane diisocyanate.
- modified MDI examples include carbodiimide-modified MDI, urethane-modified MDI, allophanate-modified MDI, and burette-modified MDI.
- the number of isocyanate groups in diphenylmethane diisocyanate and / or a modified product thereof is preferably 2 to 2.1.
- Diphenylmethane diisocyanate and / or a modified product thereof needs to be used in an amount of 90% by weight or more, preferably 98% by weight or more in the total isocyanate component.
- aromatic diisocyanates such as 2,4-toluene diisocyanate, 2,6-toluene diisocyanate, 1,5-naphthalene diisocyanate, p-phenylene diisocyanate, m-phenylene diisocyanate, p-xylylene diisocyanate, m-xylylene diisocyanate, Aliphatic diisocyanates such as ethylene diisocyanate, 2,2,4-trimethylhexamethylene diisocyanate, 1,6-hexamethylene diisocyanate, 1,4-cyclohexane diisocyanate, 4,4'-dicyclohexylmethane diisocyanate, isophorone diisocyanate, norbornane diisocyanate And alicyclic diisocyanates such as These may be used alone or in combination of two or more.
- polycaprolactone polyol As the high molecular weight polyol.
- the polycaprolactone polyol include polycaprolactone diol, polycaprolactone triol, and polycaprolactone tetraol. These may be used alone or in combination of two or more.
- polycaprolactone diol and polycaprolactone triol it is preferable to use polycaprolactone diol and polycaprolactone triol.
- polycaprolactone diol is preferably used 0.2 to 10 times by weight with respect to polycaprolactone triol.
- the polycaprolactone polyol needs to be used in an amount of 60 to 98% by weight, preferably 65 to 90% by weight, in the total active hydrogen group-containing compound.
- the number average molecular weight of the polycaprolactone polyol is not particularly limited, but is preferably 500 to 2000, more preferably 500 to 1500 from the viewpoint of the elastic properties of the resulting polyurethane. If the number average molecular weight is less than 500, a polyurethane using the number average molecular weight does not have sufficient elastic properties and tends to be a brittle polymer. Therefore, the polishing layer made of this polyurethane foam becomes too hard, and scratches are likely to occur on the surface of the object to be polished. On the other hand, when the number average molecular weight exceeds 2000, polyurethane using the same becomes too soft. Therefore, the durability of the polishing layer made of this polyurethane foam tends to deteriorate.
- Examples of other high molecular weight polyols include those usually used in the technical field of polyurethane.
- a polyether polyol typified by polytetramethylene ether glycol, polyethylene glycol, etc.
- a polyester polyol typified by polybutylene adipate a polyester polycarbonate exemplified by a reaction product of a polyester glycol such as polycaprolactone and an alkylene carbonate, etc.
- Polyester polycarbonate polyol obtained by reacting polyol and ethylene carbonate with polyhydric alcohol and then reacting the obtained reaction mixture with organic dicarboxylic acid, polycarbonate polyol obtained by transesterification reaction between polyhydroxyl compound and aryl carbonate, polymer particles
- polymer particles examples thereof include a polymer polyol which is a dispersed polyether polyol. These may be used alone or in combination of two or more.
- a compound having 3 functional groups that react with an isocyanate group examples include high molecular weight polyols having 3 functional groups such as polycaprolactone triol; low molecular weight triols such as trimethylolpropane, glycerin, triethanolamine, and 1,2,6-hexanetriol; monoethanolamine, diethanolamine, monopropanol Examples include alcohol amines such as amines. These may be used alone or in combination of two or more. In particular, polycaprolactone triol and trimethylolpropane are preferably used.
- the compound having 3 functional groups needs to be used in an amount of 15 to 40% by weight, preferably 25 to 40% by weight, in the total active hydrogen group-containing compound.
- an amount of the compound having 3 functional groups it is possible to suppress the swelling in the wet state and the generation of scratches due to the increased hardness of the polishing layer. Moreover, it becomes easy to form an open cell structure.
- a low molecular weight component having a hydroxyl value or an amine value of 1000 to 2000 mgKOH / g is more preferably 1000 to 1500 mgKOH / g.
- the hydroxyl value or the amine value is less than 1000 mgKOH / g, the effect of improving the formation of open cells tends to be insufficient.
- the hydroxyl value or amine value exceeds 2000 mgKOH / g, scratches tend to occur on the wafer surface.
- diethylene glycol, 1,2-propylene glycol, 1,3-butanediol, and 1,4-butanediol are preferably used.
- the low molecular weight component is preferably used in an amount of 2 to 15% by weight, preferably 2 to 12% by weight in the total active hydrogen group-containing compound.
- the cell membrane is easily broken and not only is easy to form open cells, but also the mechanical properties of the polyurethane foam are improved.
- a chain extender is used for curing the isocyanate-terminated prepolymer.
- the chain extender is an organic compound having at least two active hydrogen groups, and examples of the active hydrogen group include a hydroxyl group, a primary or secondary amino group, and a thiol group (SH).
- the isocyanate group concentration with respect to the total amount of the isocyanate component and the active hydrogen group-containing compound needs to be 10 to 15% by weight, preferably 12 to 13% by weight.
- the ratio of the isocyanate component and the active hydrogen group-containing compound can be variously changed depending on the molecular weight of each and the desired physical properties of the polyurethane foam.
- the number of isocyanate groups in the isocyanate component relative to the total number of active hydrogen groups (hydroxyl group + amino group) in the active hydrogen group-containing compound is preferably 0.80 to 1.20. More preferably, it is 0.99 to 1.15. When the number of isocyanate groups is outside the above range, curing failure occurs and the required specific gravity, hardness, compression ratio, etc. tend not to be obtained.
- the polyurethane resin can be produced by applying a known urethanization technique such as a melting method or a solution method, but is preferably produced by a melting method in consideration of cost, working environment, and the like.
- Polyurethane resin can be produced by either the prepolymer method or the one-shot method.
- an isocyanate-terminated prepolymer having a molecular weight of about 800 to 5000 is preferable because it has excellent processability and physical properties.
- the first component containing the isocyanate group-containing compound and the second component containing the active hydrogen group-containing compound are mixed and cured.
- the isocyanate-terminated prepolymer becomes an isocyanate group-containing compound
- the chain extender becomes an active hydrogen group-containing compound.
- the isocyanate component is an isocyanate group-containing compound
- the chain extender and the polyol component are active hydrogen group-containing compounds.
- the polyurethane foam which is a material for forming the polishing layer of the present invention, can be produced by a mechanical foaming method (including a mechanical floss method) using a silicon surfactant.
- a mechanical foaming method using a silicon surfactant which is a polyalkylsiloxane or a copolymer of an alkylsiloxane and a polyetheralkylsiloxane is preferred.
- silicon-based surfactants include SH-192 and L-5340 (manufactured by Toray Dow Corning Silicone), B-8443 (manufactured by Goldschmidt), and the like.
- the silicon surfactant is preferably added in an amount of 2 to 10% by weight, more preferably 3 to 6% by weight, in the polyurethane foam.
- stabilizers such as antioxidants, lubricants, pigments, fillers, antistatic agents, and other additives may be added.
- the first component obtained by adding a silicon surfactant to an isocyanate-terminated prepolymer obtained by reacting an isocyanate component and a high molecular weight polyol is mechanically stirred in the presence of a non-reactive gas, and the non-reactive gas is removed. Disperse as fine bubbles to obtain a cell dispersion. Then, a second component containing a chain extender is added to the cell dispersion and mixed to prepare a cell-dispersed urethane composition.
- a catalyst may be appropriately added to the second component.
- a silicon-based surfactant was added to at least one of the first component containing the isocyanate component (or isocyanate-terminated prepolymer) and the second component containing the active hydrogen group-containing compound, and the silicon-based surfactant was added.
- the components are mechanically stirred in the presence of a non-reactive gas, and the non-reactive gas is dispersed as fine bubbles to obtain a bubble dispersion. Then, the remaining components are added to the cell dispersion and mixed to prepare a cell-dispersed urethane composition.
- a silicon-based surfactant is added to at least one of the first component containing the isocyanate component (or isocyanate-terminated prepolymer) and the second component containing the active hydrogen group-containing compound, and the first component and the second component are added. Is mechanically stirred in the presence of a non-reactive gas, and the non-reactive gas is dispersed as fine bubbles to prepare a cell-dispersed urethane composition.
- the cell-dispersed urethane composition may be prepared by a mechanical floss method.
- the mechanical floss method is a method in which raw material components are put into a mixing chamber of a mixing head and a non-reactive gas is mixed and mixed and stirred by a mixer such as an Oaks mixer to make the non-reactive gas into a fine bubble state in the raw material mixture. It is a method of dispersing in.
- the mechanical floss method is a preferable method because the density of the polyurethane foam can be easily adjusted by adjusting the amount of the non-reactive gas mixed therein. Moreover, since the polyurethane foam which has a substantially spherical fine cell can be continuously shape
- non-reactive gas used to form the fine bubbles non-flammable gases are preferable, and specific examples include nitrogen, oxygen, carbon dioxide, rare gases such as helium and argon, and mixed gases thereof. In view of cost, it is most preferable to use air that has been dried to remove moisture.
- a stirring device for dispersing the non-reactive gas in the form of fine bubbles a known stirring device can be used without any particular limitation. Specifically, a homogenizer, a dissolver, a two-axis planetary mixer (planetary mixer), a mechanical A floss foaming machine etc. are illustrated.
- the shape of the stirring blade of the stirring device is not particularly limited, but it is preferable to use a whipper type stirring blade because fine bubbles can be obtained.
- the rotational speed of the stirring blade is preferably 500 to 2000 rpm, more preferably 800 to 1500 rpm. The stirring time is appropriately adjusted according to the target density.
- the stirring for preparing the cell dispersion in the foaming step and the stirring for mixing the first component and the second component use different stirring devices.
- the agitation in the mixing step may not be agitation that forms bubbles, and it is preferable to use an agitation device that does not involve large bubbles.
- a planetary mixer is suitable. There is no problem even if the same stirring device is used as the stirring device for the foaming step for preparing the bubble dispersion and the mixing step for mixing each component, and the stirring conditions such as adjusting the rotation speed of the stirring blades are adjusted as necessary. It is also suitable to use after adjustment.
- the cell-dispersed urethane composition prepared by the above method is applied onto the base material layer, and the cell-dispersed urethane composition is cured to form a polyurethane foam (polishing layer) directly on the base material layer.
- the substrate layer is not particularly limited.
- plastic films such as polypropylene, polyethylene, polyester, polyamide, and polyvinyl chloride, polymer resin foams such as polyurethane foam and polyethylene foam, rubber properties such as butadiene rubber and isoprene rubber.
- polymer resin foams such as plastic films such as polypropylene, polyethylene, polyester, polyamide, and polyvinyl chloride, polyurethane foam, and polyethylene foam.
- the base material layer preferably has a hardness equivalent to that of the polyurethane foam or harder in order to impart toughness to the polishing pad.
- the thickness of the base material layer is not particularly limited, but is preferably 20 to 1000 ⁇ m, more preferably 50 to 1000 ⁇ m from the viewpoint of strength, flexibility and the like. 800 ⁇ m.
- a roll coater such as gravure, kiss, or comma
- a die coater such as slot or phanten
- a squeeze coater or a curtain coater
- Any method may be used as long as a uniform coating film can be formed on the base material layer.
- Heating and post-curing the polyurethane foam that has reacted until the cell-dispersed urethane composition is applied to the base material layer and no longer flows is effective in improving the physical properties of the polyurethane foam and is extremely suitable.
- Post-cure is preferably performed at 30 to 80 ° C. for 10 minutes to 6 hours, and it is preferably performed at normal pressure because the bubble shape becomes stable.
- a known catalyst for promoting a polyurethane reaction such as a tertiary amine may be used.
- the type and addition amount of the catalyst are selected in consideration of the flow time for coating on the substrate layer after the mixing step of each component.
- the polyurethane foam may be produced by a batch method in which each component is weighed and put into a container and mechanically stirred, and each component and a non-reactive gas are continuously supplied to a stirring device and mechanically stirred. Further, a continuous production method in which a cell-dispersed urethane composition is sent out to produce a molded product may be used.
- the method for uniformly adjusting the thickness of the polyurethane foam is not particularly limited, and examples thereof include a method of buffing with an abrasive, a method of pressing with a press plate, and a method of slicing with a slicer.
- pressing it is preferable to make the thickness of the cell-dispersed urethane composition as close as possible to the thickness of the intended polishing layer.
- the thickness of the cell dispersed urethane composition is adjusted to 80 to 100% of the thickness of the intended polishing layer.
- the cell-dispersed urethane composition prepared by the above method is applied on the base material layer, and a release sheet is laminated on the cell-dispersed urethane composition. Thereafter, the polyurethane foam may be formed by curing the cell-dispersed urethane composition while making the thickness uniform by a pressing means.
- the cell-dispersed urethane composition prepared by the above method is applied onto a release sheet, and a base material layer is laminated on the cell-dispersed urethane composition. Thereafter, the polyurethane foam may be formed by curing the cell-dispersed urethane composition while making the thickness uniform by a pressing means.
- the material for forming the release sheet is not particularly limited, and examples thereof include general resin and paper.
- the release sheet preferably has a small dimensional change due to heat.
- the surface of the release sheet may be subjected to a release treatment.
- the pressing means for making the thickness of the sandwich sheet composed of the base material layer, the cell-dispersed urethane composition (cell-dispersed urethane layer), and the release sheet is not particularly limited.
- the thickness may be constant by a coater roll, a nip roll, or the like.
- the reacted polyurethane foam is heated until it does not flow, and post-cure to form a polishing layer.
- Post cure conditions are the same as described above.
- the release sheet on the upper surface side or the lower surface side of the polyurethane foam is peeled to obtain a polishing pad.
- the skin layer is formed on the polyurethane foam
- the skin layer is removed by buffing or the like.
- the polyurethane foam is formed by the mechanical foaming method as described above, the variation in bubbles is smaller on the lower surface side than on the upper surface side of the polyurethane foam. Therefore, when the release sheet on the lower surface side is peeled off and the lower surface side of the polyurethane foam is used as the polishing surface, the polishing surface has a small variation in bubbles, and the stability of the polishing rate is further improved.
- the polyurethane foam may not be formed directly on the base material layer, but may be bonded to the base material layer using a double-sided tape after forming the polishing layer.
- the shape of the polishing pad of the present invention is not particularly limited, and may be a long shape of about several meters in length or a round shape having a diameter of several tens of centimeters.
- the average cell diameter of the polyurethane foam is preferably 40 to 100 ⁇ m, more preferably 60 to 80 ⁇ m.
- the average opening diameter of the polyurethane foam is preferably 5 to 30 ⁇ m, more preferably 20 to 30 ⁇ m.
- the specific gravity of the polyurethane foam is preferably 0.2 to 0.6, more preferably 0.3 to 0.5.
- the specific gravity is less than 0.2, the durability of the polishing layer tends to decrease.
- it is larger than 0.6, the material needs to have a low crosslinking density in order to obtain a certain elastic modulus. In that case, the permanent set increases and the durability tends to deteriorate.
- the hardness of the polyurethane foam is preferably 10 to 95 degrees, more preferably 40 to 90 degrees as measured by an Asker C hardness meter.
- Asker C hardness is less than 10 degrees, the durability of the polishing layer tends to decrease, or the surface smoothness of the polished material after polishing tends to deteriorate.
- it exceeds 95 degrees scratches are likely to occur on the surface of the material to be polished.
- thermosetting polyurethane foam preferably has a weight change rate of 10% or more after being immersed in water for 24 hours, more preferably 12 to 30%.
- the polishing layer made of the thermosetting polyurethane foam has an absolute value of a change rate [ ⁇ (BA) / A ⁇ ⁇ 100] of the compressibility A in the dry state and the compressibility B in the wet state is 100 or less. , Preferably 60 or less.
- the surface of the polishing layer may have an uneven structure for holding and renewing the slurry.
- the polishing layer made of foam has many openings on the polishing surface and has the function of holding and updating the slurry.
- the slurry can be held and updated more efficiently. It can be performed well, and destruction of the polishing object due to adsorption with the polishing object can be prevented.
- the concavo-convex structure is not particularly limited as long as it is a shape that holds and renews the slurry.
- an XY lattice groove for example, an XY lattice groove, a concentric circular groove, a through hole, a non-penetrating hole, a polygonal column, a cylinder, a spiral groove, Examples include eccentric circular grooves, radial grooves, and combinations of these grooves.
- these uneven structures are generally regular, but in order to make the slurry retention and renewability desirable, the groove pitch, groove width, groove depth, etc. should be changed for each range. Is also possible.
- the method for producing the concavo-convex structure is not particularly limited.
- a method of machine cutting using a jig such as a tool of a predetermined size, pouring a resin into a mold having a predetermined surface shape, and curing.
- a method of producing a resin by pressing a method of producing using photolithography, a method of producing using a printing technique, a carbon dioxide laser, etc.
- Examples include a manufacturing method using laser light.
- the thickness of the polishing layer is not particularly limited, but is usually about 0.2 to 2 mm, preferably 0.5 to 1.5 mm.
- the polishing pad of the present invention may be provided with a double-sided tape on the surface to be bonded to the platen.
- the semiconductor device is manufactured through a process of polishing the surface of the semiconductor wafer using the polishing pad.
- a semiconductor wafer is generally a laminate of a wiring metal and an oxide film on a silicon wafer.
- the method and apparatus for polishing the semiconductor wafer are not particularly limited.
- a polishing surface plate 2 that supports the polishing pad 1
- a support table (polishing head) 5 that supports the semiconductor wafer 4
- This is performed using a backing material for performing uniform pressurization and a polishing apparatus equipped with a polishing agent 3 supply mechanism.
- the polishing pad 1 is attached to the polishing surface plate 2 by attaching it with a double-sided tape, for example.
- the polishing surface plate 2 and the support base 5 are disposed so that the polishing pad 1 and the semiconductor wafer 4 supported on each of the polishing surface plate 2 and the support table 5 face each other, and are provided with rotating shafts 6 and 7 respectively. Further, a pressurizing mechanism for pressing the semiconductor wafer 4 against the polishing pad 1 is provided on the support base 5 side. In polishing, the semiconductor wafer 4 is pressed against the polishing pad 1 while rotating the polishing surface plate 2 and the support base 5, and polishing is performed while supplying slurry.
- the flow rate of the slurry, the polishing load, the polishing platen rotation speed, and the wafer rotation speed are not particularly limited and are appropriately adjusted.
- a semiconductor device is manufactured by dicing, bonding, packaging, or the like.
- the semiconductor device is used for an arithmetic processing device, a memory, and the like.
- a glass substrate for a lens or hard disk can be finished and polished by the same method as described above.
- thermosetting polyurethane foam (Measurement of weight change rate of thermosetting polyurethane foam)
- the produced thermosetting polyurethane foam was cut into a size of 5 mm ⁇ 5 mm ⁇ thickness 1 mm to obtain a sample.
- the sample was dried in an oven at 70 ° C. for 24 hours, and the weight (W1) after drying was measured.
- the dried sample was immersed in pure water at 23 ° C. for 24 hours, and then the sample taken out from the water was put in a sealed container, and the condition of a rotational speed of 10,000 rpm / min was used using a centrifuge (KUBOTA-6800, manufactured by Kubota Seisakusho). To remove the water. Thereafter, the weight (W2) of the sample was measured.
- thermosetting polyurethane foam cut into a circle having a diameter of 7 mm (thickness: 1 mm) was allowed to stand for 40 hours in an environment of a temperature of 23 ° C. and a humidity of 50%.
- the compression ratio A was measured under the following conditions using a thermal analysis measuring instrument TMA (manufactured by SEICO INSTRUMENTS, SS6000).
- Compression rate A (%) ⁇ (T1-T2) / T1 ⁇ ⁇ 100
- T1 When a stress of 29.4 kPa (300 g / cm 2 ) was applied to the sample from an unloaded state and held for 60 seconds, the sample thickness T2: a stress of 176.4 kPa (1800 g / cm 2 ) further from the state of T1 1.
- Thickness of the sample when it is loaded and held for 60 seconds Measurement of compressibility in a wet state A thermosetting polyurethane foam cut into a circular shape (thickness 1 mm) having a diameter of 7 mm is immersed in pure water at 23 ° C. for 24 hours, then taken out from the water, and the compressibility B is obtained in the same manner as described above. It was measured. 3.
- the polishing rate was measured every 10 minutes while polishing the workpiece under the following conditions using the prepared polishing pad, and the time taken to reach the steady value from the initial value was defined as the dummy polishing time. Further, the average polishing rate after reaching a steady value was measured.
- polishing machine MAT, BC-15 Pressure: 100 g / cm 2 Head speed: 50 rpm Surface plate speed: 40 rpm
- Slurry Cerium oxide (SHOROX F-3, Showa Denko) / Water (100 g / L) Slurry supply amount: 100 mL / min Workpiece: ⁇ 76 0.8t B270 (manufactured by SCHOTT DES AG)
- the initial polishing rate stability (%) obtained from the following formula from the average polishing rate from the (n-5) th sheet to the nth sheet and the maximum polishing speed and the minimum polishing speed of the five consecutive polishing speeds ) was 10% or less, and was determined to be a steady value.
- Initial polishing rate stability (%) ⁇ (maximum polishing rate ⁇ minimum polishing rate) / average polishing rate ⁇ ⁇ 100
- Example 1 In a container, 38 parts by weight of polytetramethylene ether glycol (PTMG650) having a number average molecular weight of 650, 25 parts by weight of polycaprolactone diol (manufactured by Daicel Chemical Industries, Plaxel 205, hydroxyl value: 208 mgKOH / g, functional group number 2), polycaprolactone Triol (Daicel Chemical Industries, Plaxel 305, hydroxyl value: 305 mg KOH / g, functional group number 3) 35 parts by weight, trimethylolpropane (hydroxyl value: 1128 mg KOH / g, functional group number 3) 2 parts by weight, silicon-based surfactant 10 parts by weight (manufactured by Goldschmidt, B8443) and 0.1 part by weight of a catalyst (manufactured by Kao, No.
- PTMG650 polytetramethylene ether glycol
- Plaxel 205 hydroxyl value: 208 mgKOH /
- the prepared cell-dispersed urethane composition was applied onto a release-treated release sheet (Toyobo, polyethylene terephthalate, thickness: 0.1 mm) to form a cell-dispersed urethane layer. And the base material layer (polyethylene terephthalate, thickness: 0.2 mm) was covered on this cell dispersion
- the cell-dispersed urethane layer is made 1.5 mm thick with a nip roll, and then cured at 70 ° C. for 3 hours to form a polyurethane foam (open cell structure, average cell diameter: 68.5 ⁇ m, average opening diameter: 24 ⁇ m, specific gravity: 0 .47, C hardness: 50 degrees).
- the release sheet under the polyurethane foam was peeled off.
- the surface of the polyurethane foam is sliced using a band saw type slicer (manufactured by Fecken) to a thickness of 0.6 mm, and the thickness accuracy is adjusted by buffing to obtain a 0.5 mm thick polishing layer. Formed.
- the polyurethane foam had substantially spherical open cells.
- a double-sided tape double tack tape, manufactured by Sekisui Chemical Co., Ltd. was bonded to the surface of the base material layer using a laminator to prepare a polishing pad.
- Examples 2 and 3 and Comparative Examples 1 and 2 A polishing pad was prepared in the same manner as in Example 1 except that the blending ratio shown in Table 1 was adopted.
- the compounds in Table 1 are as follows.
- PTMG1000 polytetramethylene ether glycol with a number average molecular weight of 1000
- PTMG650 polytetramethylene ether glycol with a number average molecular weight of 650
- Plaxel 210N polycaprolactone diol (manufactured by Daicel Chemical Industries, hydroxyl value: 110 mgKOH / g, functional group number 2 )
- Plaxel 205 polycaprolactone diol (manufactured by Daicel Chemical Industries, hydroxyl value: 208 mgKOH / g, number of functional groups 2)
- Plaxel 305 Polycaprolactone triol (manufactured by Daicel Chemical Industries, hydroxyl value: 305 mg KOH / g, functional group number 3)
- DEG Diethylene
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Finish Polishing, Edge Sharpening, And Grinding By Specific Grinding Devices (AREA)
- Polyurethanes Or Polyureas (AREA)
- Mechanical Treatment Of Semiconductor (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2009801061033A CN101952943B (zh) | 2008-03-12 | 2009-02-26 | 研磨垫 |
| KR1020107015876A KR101455218B1 (ko) | 2008-03-12 | 2009-02-26 | 연마 패드 |
| US12/864,819 US8476328B2 (en) | 2008-03-12 | 2009-02-26 | Polishing pad |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2008063034A JP4593643B2 (ja) | 2008-03-12 | 2008-03-12 | 研磨パッド |
| JP2008-063034 | 2008-03-12 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2009113399A1 true WO2009113399A1 (ja) | 2009-09-17 |
Family
ID=41065068
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2009/053481 Ceased WO2009113399A1 (ja) | 2008-03-12 | 2009-02-26 | 研磨パッド |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US8476328B2 (enExample) |
| JP (1) | JP4593643B2 (enExample) |
| KR (1) | KR101455218B1 (enExample) |
| CN (1) | CN101952943B (enExample) |
| TW (1) | TWI431026B (enExample) |
| WO (1) | WO2009113399A1 (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20130012105A1 (en) * | 2010-03-31 | 2013-01-10 | Toyo Tire & Rubber Co., Ltd. | Polishing pad and production method therefor, and production method for semiconductor device |
| US20130035021A1 (en) * | 2010-03-26 | 2013-02-07 | Toyo Tire & Rubber Co., Ltd. | Polishing pad, manufacturing method therefor, and method for manufacturing a semiconductor device |
| US9079289B2 (en) | 2011-09-22 | 2015-07-14 | Toyo Tire & Rubber Co., Ltd. | Polishing pad |
| CN113319734A (zh) * | 2021-07-06 | 2021-08-31 | 北京半导体专用设备研究所(中国电子科技集团公司第四十五研究所) | 化学抛光装置及其方法 |
Families Citing this family (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100960585B1 (ko) * | 2005-07-15 | 2010-06-03 | 도요 고무 고교 가부시키가이샤 | 적층 시트 및 그 제조 방법 |
| JP4884726B2 (ja) * | 2005-08-30 | 2012-02-29 | 東洋ゴム工業株式会社 | 積層研磨パッドの製造方法 |
| US20100009611A1 (en) * | 2006-09-08 | 2010-01-14 | Toyo Tire & Rubber Co., Ltd. | Method for manufacturing a polishing pad |
| KR101181885B1 (ko) * | 2006-09-08 | 2012-09-11 | 도요 고무 고교 가부시키가이샤 | 연마 패드 |
| MY157714A (en) | 2007-01-15 | 2016-07-15 | Rohm & Haas Elect Mat | Polishing pad and a method for manufacturing the same |
| JP4593643B2 (ja) * | 2008-03-12 | 2010-12-08 | 東洋ゴム工業株式会社 | 研磨パッド |
| JP5484145B2 (ja) * | 2010-03-24 | 2014-05-07 | 東洋ゴム工業株式会社 | 研磨パッド |
| JP5839162B2 (ja) * | 2010-07-12 | 2016-01-06 | Jsr株式会社 | 化学機械研磨パッドおよび化学機械研磨方法 |
| KR101356402B1 (ko) * | 2010-11-30 | 2014-01-28 | 한국타이어 주식회사 | 폴리우레탄 폼 및 이를 포함하는 공기입 타이어 |
| JP5687118B2 (ja) * | 2011-04-15 | 2015-03-18 | 富士紡ホールディングス株式会社 | 研磨パッド及びその製造方法 |
| JP5853408B2 (ja) * | 2011-05-09 | 2016-02-09 | 旭硝子株式会社 | 磁気記録媒体用ガラス基板の製造方法および磁気記録媒体用ガラス基板 |
| JP5759888B2 (ja) * | 2011-12-28 | 2015-08-05 | 東洋ゴム工業株式会社 | 研磨パッド |
| US9144880B2 (en) | 2012-11-01 | 2015-09-29 | Rohm And Haas Electronic Materials Cmp Holdings, Inc. | Soft and conditionable chemical mechanical polishing pad |
| US9238295B2 (en) | 2013-05-31 | 2016-01-19 | Rohm And Haas Electronic Materials Cmp Holdings, Inc. | Soft and conditionable chemical mechanical window polishing pad |
| US9233451B2 (en) | 2013-05-31 | 2016-01-12 | Rohm And Haas Electronic Materials Cmp Holdings, Inc. | Soft and conditionable chemical mechanical polishing pad stack |
| US9238296B2 (en) | 2013-05-31 | 2016-01-19 | Rohm And Haas Electronic Materials Cmp Holdings, Inc. | Multilayer chemical mechanical polishing pad stack with soft and conditionable polishing layer |
| US20150306731A1 (en) | 2014-04-25 | 2015-10-29 | Rohm And Haas Electronic Materials Cmp Holdings, Inc. | Chemical mechanical polishing pad |
| JP2016014637A (ja) * | 2014-07-03 | 2016-01-28 | 東洋ゴム工業株式会社 | クッションパッドの変形を検出するシステムおよびその製造方法 |
| CN104385122A (zh) * | 2014-11-04 | 2015-03-04 | 无锡市华明化工有限公司 | 一种具有散热结构的研磨机 |
| US9484212B1 (en) | 2015-10-30 | 2016-11-01 | Rohm And Haas Electronic Materials Cmp Holdings, Inc. | Chemical mechanical polishing method |
| US10208154B2 (en) | 2016-11-30 | 2019-02-19 | Rohm And Haas Electronic Materials Cmp Holdings, Inc. | Formulations for chemical mechanical polishing pads and CMP pads made therewith |
| JP6919579B2 (ja) * | 2018-01-17 | 2021-08-18 | 株式会社Sumco | 貼り合わせウェーハの製造方法、貼り合わせウェーハ |
| JP7141230B2 (ja) * | 2018-03-30 | 2022-09-22 | 富士紡ホールディングス株式会社 | 研磨パッド及びその製造方法 |
| CN109015341B (zh) * | 2018-08-03 | 2020-08-11 | 成都时代立夫科技有限公司 | 一种基于多孔氧化铈的cmp抛光层及其制备方法 |
| JP7562994B2 (ja) * | 2020-06-08 | 2024-10-08 | 株式会社Sumco | ウェーハ外周部の研磨装置 |
| CN114702912B (zh) * | 2022-03-18 | 2023-10-17 | 安徽禾臣新材料有限公司 | 一种玻璃显示屏抛光用吸附垫及其制备方法 |
| CN115873207B (zh) * | 2023-02-17 | 2023-06-27 | 山东一诺威聚氨酯股份有限公司 | 高性能cmp聚氨酯抛光垫及其制备方法 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006035367A (ja) * | 2004-07-27 | 2006-02-09 | Toray Ind Inc | 研磨パッドおよび研磨装置 |
| JP2007307700A (ja) * | 2006-04-19 | 2007-11-29 | Toyo Tire & Rubber Co Ltd | 研磨パッドの製造方法 |
| WO2008026451A1 (fr) * | 2006-08-28 | 2008-03-06 | Toyo Tire & Rubber Co., Ltd. | Tampon de polissage |
Family Cites Families (111)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3049463A (en) * | 1959-09-09 | 1962-08-14 | Dennison Mfg Co | Decorated foam and method of making the same |
| BE636018A (enExample) * | 1962-08-13 | 1900-01-01 | ||
| US4216177A (en) * | 1979-05-16 | 1980-08-05 | Rogers Corporation | Polyurethane foam product and process of manufacture thereof from thermosetting frothed mixture |
| JPS6042431A (ja) | 1983-08-19 | 1985-03-06 | Mitui Toatsu Chem Inc | ポリウレタンフォームシート類及びその多層体の熱加工方法 |
| JPS61187657A (ja) | 1985-02-15 | 1986-08-21 | Wako Pure Chem Ind Ltd | 新規試薬による螢光検出方法 |
| US4762902A (en) * | 1985-12-16 | 1988-08-09 | The B. F. Goodrich Company | Electron curable polyurethanes |
| JP2734007B2 (ja) | 1988-10-07 | 1998-03-30 | ソニー株式会社 | 研磨装置および研磨方法 |
| JP2977884B2 (ja) | 1990-10-19 | 1999-11-15 | 大日本印刷株式会社 | 研磨テープの製造方法 |
| JP2552954B2 (ja) | 1990-11-29 | 1996-11-13 | 東京シート株式会社 | ウレタンフォーム成形品の製造方法 |
| JPH05329852A (ja) | 1992-05-29 | 1993-12-14 | Fuji Kobunshi Kk | 発泡ポリウレタン成型物の製造方法 |
| JP3024373B2 (ja) | 1992-07-07 | 2000-03-21 | 信越半導体株式会社 | シート状弾性発泡体及びウェーハ研磨加工用治具 |
| JP3549219B2 (ja) | 1993-03-15 | 2004-08-04 | セーレン株式会社 | フォーム複合体の製造法 |
| US5554686A (en) * | 1993-08-20 | 1996-09-10 | Minnesota Mining And Manufacturing Company | Room temperature curable silane-terminated polyurethane dispersions |
| EP0692507A1 (en) * | 1994-07-11 | 1996-01-17 | Basf Corporation | Flexible open-cell polyurethane foam |
| DE19506671C2 (de) * | 1995-02-25 | 1999-11-18 | Basf Ag | Verfahren zur Herstellung von Polyurethan-Schaumstoffen |
| US6099954A (en) * | 1995-04-24 | 2000-08-08 | Rodel Holdings, Inc. | Polishing material and method of polishing a surface |
| JPH10329005A (ja) | 1997-06-03 | 1998-12-15 | Toshiba Corp | 研磨布及び研磨装置 |
| JPH11207758A (ja) | 1998-01-28 | 1999-08-03 | Hitachi Chem Co Ltd | 化粧面を有する繊維強化ポリウレタンフォームおよびその製造方法 |
| JP2000246620A (ja) | 1999-03-03 | 2000-09-12 | Okamoto Machine Tool Works Ltd | ウエハ研磨用パッド |
| JP2001062703A (ja) | 1999-08-27 | 2001-03-13 | Asahi Chem Ind Co Ltd | 多孔性樹脂窓付き研磨パッド |
| JP2003516872A (ja) * | 1999-12-14 | 2003-05-20 | ロデール ホールディングス インコーポレイテッド | 高分子又は高分子複合材研磨パッドの製造方法 |
| WO2001096434A1 (en) | 2000-06-13 | 2001-12-20 | Toyo Tire & Rubber Co., Ltd. | Process for producing polyurethane foam, polyurethane foam, and abrasive sheet |
| US6656019B1 (en) * | 2000-06-29 | 2003-12-02 | International Business Machines Corporation | Grooved polishing pads and methods of use |
| US6803495B2 (en) * | 2000-06-28 | 2004-10-12 | World Properties, Inc. | Polyurethane foam composition and method of manufacture thereof |
| PL356275A1 (en) * | 2000-07-28 | 2004-06-28 | Woodbridge Foam Corporation | Foamed isocyanate-based polymer having improved hardness properties and process for production thereof |
| JP2002060452A (ja) | 2000-08-10 | 2002-02-26 | Toho Chem Ind Co Ltd | 吸音・制振材用ポリウレタンフォームの製造方法 |
| WO2002043921A1 (en) * | 2000-12-01 | 2002-06-06 | Toyo Boseki Kabushiki Kaisha | Polishing pad, method of manufacturing the polishing pad, and cushion layer for polishing pad |
| US6572463B1 (en) * | 2000-12-27 | 2003-06-03 | Lam Research Corp. | Methods for making reinforced wafer polishing pads utilizing direct casting and apparatuses implementing the same |
| US6561889B1 (en) * | 2000-12-27 | 2003-05-13 | Lam Research Corporation | Methods for making reinforced wafer polishing pads and apparatuses implementing the same |
| WO2002051587A1 (en) | 2000-12-27 | 2002-07-04 | Lam Research Corporation | Methods for making reinforced wafer polshing pads and apparatuses implementing the same |
| US6420448B1 (en) * | 2001-01-18 | 2002-07-16 | Foamex Lp | Energy absorbing foams |
| JP2002217149A (ja) * | 2001-01-19 | 2002-08-02 | Shin Etsu Handotai Co Ltd | ウエーハの研磨装置及び研磨方法 |
| JP2002226608A (ja) | 2001-02-01 | 2002-08-14 | Toyo Tire & Rubber Co Ltd | 研磨パッド用ポリウレタン発泡体の製造方法及びポリウレタン発泡体 |
| JP3455187B2 (ja) * | 2001-02-01 | 2003-10-14 | 東洋ゴム工業株式会社 | 研磨パッド用ポリウレタン発泡体の製造装置 |
| JP2002264912A (ja) | 2001-03-12 | 2002-09-18 | Shibazaki Seisakusho Ltd | 内容液充填方法および閉止装置入り飲料 |
| JP2002307293A (ja) | 2001-04-09 | 2002-10-23 | Rodel Nitta Co | 研磨クロス |
| US7378454B2 (en) * | 2001-04-09 | 2008-05-27 | Toyo Tire & Rubber Co., Ltd. | Polyurethane composition and polishing pad |
| JP4659273B2 (ja) | 2001-05-31 | 2011-03-30 | ニッタ・ハース株式会社 | 被研磨物保持用のバッキング材の製造方法 |
| JP2003037089A (ja) | 2001-07-26 | 2003-02-07 | Shin Etsu Handotai Co Ltd | ウェーハの研磨方法 |
| JP2003053657A (ja) | 2001-08-10 | 2003-02-26 | Ebara Corp | 研磨面構成部材及び該研磨面構成部材を用いた研磨装置 |
| JP2003062748A (ja) * | 2001-08-24 | 2003-03-05 | Inoac Corp | 研磨用パッド |
| JP2003100681A (ja) | 2001-09-20 | 2003-04-04 | Memc Japan Ltd | 仕上げ研磨パッド |
| TWI222390B (en) | 2001-11-13 | 2004-10-21 | Toyo Boseki | Polishing pad and its production method |
| JP3455208B2 (ja) | 2001-11-13 | 2003-10-14 | 東洋紡績株式会社 | 半導体ウエハ研磨パッド、半導体ウエハの研磨方法、研磨パッド用研磨シート、及び研磨シート用発泡体ブロック |
| KR100877383B1 (ko) * | 2001-11-13 | 2009-01-07 | 도요 고무 고교 가부시키가이샤 | 연마 패드 및 그 제조 방법 |
| JP3570681B2 (ja) | 2001-12-10 | 2004-09-29 | 東洋ゴム工業株式会社 | 研磨パッド |
| JP2003209079A (ja) | 2002-01-15 | 2003-07-25 | Sumitomo Bakelite Co Ltd | 多孔性プラスチック粒子研磨パッド |
| JP2003220550A (ja) | 2002-01-24 | 2003-08-05 | Sumitomo Bakelite Co Ltd | 研磨用パッドおよびその製造方法 |
| JP3774202B2 (ja) | 2002-04-03 | 2006-05-10 | 三洋化成工業株式会社 | 軟質ポリウレタンフォームの製造方法 |
| JP4086531B2 (ja) | 2002-04-16 | 2008-05-14 | 株式会社イノアックコーポレーション | クッション体 |
| JP2004025407A (ja) | 2002-06-27 | 2004-01-29 | Jsr Corp | 化学機械研磨用研磨パッド |
| JP2004042189A (ja) | 2002-07-11 | 2004-02-12 | Inoac Corp | 研磨用パッド |
| US20040024719A1 (en) * | 2002-07-31 | 2004-02-05 | Eytan Adar | System and method for scoring messages within a system for harvesting community kowledge |
| JP2004087647A (ja) * | 2002-08-26 | 2004-03-18 | Nihon Micro Coating Co Ltd | 研磨パッド及び方法 |
| JP2004119657A (ja) | 2002-09-26 | 2004-04-15 | Toray Ind Inc | 研磨パッド、研磨装置、およびそれを用いた研磨方法 |
| JP2004169038A (ja) | 2002-11-06 | 2004-06-17 | Kimimasa Asano | ポリウレタン・ポリウレア系均一研磨シート材 |
| CN1318469C (zh) | 2002-11-18 | 2007-05-30 | 东省A&T株式会社 | 具有微孔的聚氨酯泡沫的制备方法和由此获得的抛光垫 |
| JP2005001083A (ja) | 2003-06-13 | 2005-01-06 | Sumitomo Bakelite Co Ltd | 研磨用積層体および研磨方法 |
| WO2004054779A1 (ja) | 2002-11-25 | 2004-07-01 | Sumitomo Bakelite Company Limited | 研磨用独立発泡体の製造方法、研磨用発泡シート、研磨用積層体と研磨方法、研磨用積層体の製造方法、および溝付き研磨パッド |
| JP4078643B2 (ja) | 2002-12-10 | 2008-04-23 | 東洋ゴム工業株式会社 | 研磨パッドの製造方法、研磨パッド、及び半導体デバイスの製造方法 |
| JP4233319B2 (ja) | 2002-12-12 | 2009-03-04 | 東洋ゴム工業株式会社 | 研磨パッドの製造方法及び研磨パッド |
| US7066801B2 (en) | 2003-02-21 | 2006-06-27 | Dow Global Technologies, Inc. | Method of manufacturing a fixed abrasive material |
| JP4532077B2 (ja) | 2003-03-27 | 2010-08-25 | ニッタ・ハース株式会社 | 仕上げ研磨用研磨布 |
| JP2004335713A (ja) | 2003-05-07 | 2004-11-25 | Rodel Nitta Co | 仕上げ研磨用研磨布 |
| JP2004337992A (ja) | 2003-05-13 | 2004-12-02 | Disco Abrasive Syst Ltd | 固定砥粒研磨パッド,及び固定砥粒研磨パッドを使用したシリコンウェハの研磨方法 |
| JP4373152B2 (ja) | 2003-07-17 | 2009-11-25 | 東レコーテックス株式会社 | 研磨シート |
| JP2005035367A (ja) * | 2003-07-18 | 2005-02-10 | Toyota Motor Corp | ブレーキ制御装置 |
| JP4189963B2 (ja) | 2003-08-21 | 2008-12-03 | 東洋ゴム工業株式会社 | 研磨パッド |
| US7074115B2 (en) * | 2003-10-09 | 2006-07-11 | Rohm And Haas Electronic Materials Cmp Holdings, Inc. | Polishing pad |
| JP2005131720A (ja) | 2003-10-29 | 2005-05-26 | Toray Ind Inc | 研磨パッドの製造方法 |
| JP4555559B2 (ja) * | 2003-11-25 | 2010-10-06 | 富士紡ホールディングス株式会社 | 研磨布及び研磨布の製造方法 |
| CN1946539A (zh) | 2003-12-05 | 2007-04-11 | 弗罗伊登伯格无纺公司 | 用于连续成形一种用作半导体抛光垫片的均一片材的工艺和设备 |
| US20050171224A1 (en) * | 2004-02-03 | 2005-08-04 | Kulp Mary J. | Polyurethane polishing pad |
| KR100817233B1 (ko) * | 2004-03-11 | 2008-03-27 | 도요 고무 고교 가부시키가이샤 | 연마 패드 및 반도체 디바이스의 제조 방법 |
| JP2005330621A (ja) | 2004-05-20 | 2005-12-02 | Nitta Haas Inc | 研磨布の製造方法 |
| JP2006075914A (ja) | 2004-09-07 | 2006-03-23 | Nitta Haas Inc | 研磨布 |
| US20060089095A1 (en) * | 2004-10-27 | 2006-04-27 | Swisher Robert G | Polyurethane urea polishing pad |
| KR101107044B1 (ko) * | 2004-12-10 | 2012-01-25 | 도요 고무 고교 가부시키가이샤 | 연마 패드 및 연마 패드의 제조 방법 |
| US7261625B2 (en) * | 2005-02-07 | 2007-08-28 | Inoac Corporation | Polishing pad |
| JP4862189B2 (ja) | 2005-02-14 | 2012-01-25 | 日本発條株式会社 | 研磨パッド用クッション材 |
| JP2006231429A (ja) | 2005-02-22 | 2006-09-07 | Inoac Corp | 研磨パッドおよびその製造方法 |
| KR100909605B1 (ko) * | 2005-03-08 | 2009-07-27 | 도요 고무 고교 가부시키가이샤 | 연마 패드 및 그 제조 방법 |
| JP2006255828A (ja) | 2005-03-17 | 2006-09-28 | Nitta Haas Inc | 研磨布およびその製造方法 |
| JP4526987B2 (ja) | 2005-03-22 | 2010-08-18 | 株式会社イノアックコーポレーション | 研磨用バフ材の製造方法 |
| JP4832789B2 (ja) | 2005-04-19 | 2011-12-07 | 富士紡ホールディングス株式会社 | 研磨布 |
| JP2006334745A (ja) | 2005-06-03 | 2006-12-14 | Inoac Corp | 研磨用吸着パッド及びその製造方法 |
| JP2006339570A (ja) | 2005-06-06 | 2006-12-14 | Toray Ind Inc | 研磨パッドおよび研磨装置 |
| JP5308611B2 (ja) * | 2005-06-07 | 2013-10-09 | 日東電工株式会社 | 粘着剤組成物および粘着シート類 |
| KR100960585B1 (ko) * | 2005-07-15 | 2010-06-03 | 도요 고무 고교 가부시키가이샤 | 적층 시트 및 그 제조 방법 |
| JP4884726B2 (ja) * | 2005-08-30 | 2012-02-29 | 東洋ゴム工業株式会社 | 積層研磨パッドの製造方法 |
| JP2007112032A (ja) | 2005-10-21 | 2007-05-10 | Pilot Corporation | 開閉蓋付の筆記具 |
| JP5031236B2 (ja) * | 2006-01-10 | 2012-09-19 | 東洋ゴム工業株式会社 | 研磨パッド |
| TW200800489A (en) * | 2006-04-19 | 2008-01-01 | Toyo Tire & Amp Rubber Co Ltd | Method for manufacturing polishing pad |
| JP2007283712A (ja) | 2006-04-19 | 2007-11-01 | Toyo Tire & Rubber Co Ltd | 溝付き長尺研磨パッドの製造方法 |
| WO2007123168A1 (ja) | 2006-04-19 | 2007-11-01 | Toyo Tire & Rubber Co., Ltd. | 研磨パッドの製造方法 |
| JP2007307639A (ja) * | 2006-05-17 | 2007-11-29 | Toyo Tire & Rubber Co Ltd | 研磨パッド |
| JP5110677B2 (ja) * | 2006-05-17 | 2012-12-26 | 東洋ゴム工業株式会社 | 研磨パッド |
| US7445847B2 (en) * | 2006-05-25 | 2008-11-04 | Rohm And Haas Electronic Materials Cmp Holdings, Inc. | Chemical mechanical polishing pad |
| JP5315632B2 (ja) * | 2006-06-29 | 2013-10-16 | 住友化学株式会社 | ウレタン樹脂で被覆されてなる生物活性物質含有の被覆粒状物 |
| US20100009611A1 (en) * | 2006-09-08 | 2010-01-14 | Toyo Tire & Rubber Co., Ltd. | Method for manufacturing a polishing pad |
| KR101181885B1 (ko) * | 2006-09-08 | 2012-09-11 | 도요 고무 고교 가부시키가이샤 | 연마 패드 |
| US7438636B2 (en) * | 2006-12-21 | 2008-10-21 | Rohm And Haas Electronic Materials Cmp Holdings, Inc. | Chemical mechanical polishing pad |
| JP4943139B2 (ja) | 2006-12-25 | 2012-05-30 | 株式会社イノアックコーポレーション | ポリエステル系ポリウレタン発泡体 |
| MY157714A (en) * | 2007-01-15 | 2016-07-15 | Rohm & Haas Elect Mat | Polishing pad and a method for manufacturing the same |
| US7569268B2 (en) * | 2007-01-29 | 2009-08-04 | Rohm And Haas Electronic Materials Cmp Holdings, Inc. | Chemical mechanical polishing pad |
| JP4954762B2 (ja) * | 2007-03-27 | 2012-06-20 | 東洋ゴム工業株式会社 | ポリウレタン発泡体の製造方法 |
| JP5078000B2 (ja) * | 2007-03-28 | 2012-11-21 | 東洋ゴム工業株式会社 | 研磨パッド |
| JP4971028B2 (ja) * | 2007-05-16 | 2012-07-11 | 東洋ゴム工業株式会社 | 研磨パッドの製造方法 |
| JP4943233B2 (ja) * | 2007-05-31 | 2012-05-30 | 東洋ゴム工業株式会社 | 研磨パッドの製造方法 |
| JP4593643B2 (ja) * | 2008-03-12 | 2010-12-08 | 東洋ゴム工業株式会社 | 研磨パッド |
| JP5393434B2 (ja) * | 2008-12-26 | 2014-01-22 | 東洋ゴム工業株式会社 | 研磨パッド及びその製造方法 |
-
2008
- 2008-03-12 JP JP2008063034A patent/JP4593643B2/ja active Active
-
2009
- 2009-02-26 CN CN2009801061033A patent/CN101952943B/zh active Active
- 2009-02-26 US US12/864,819 patent/US8476328B2/en active Active
- 2009-02-26 KR KR1020107015876A patent/KR101455218B1/ko active Active
- 2009-02-26 WO PCT/JP2009/053481 patent/WO2009113399A1/ja not_active Ceased
- 2009-02-27 TW TW98106388A patent/TWI431026B/zh active
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006035367A (ja) * | 2004-07-27 | 2006-02-09 | Toray Ind Inc | 研磨パッドおよび研磨装置 |
| JP2007307700A (ja) * | 2006-04-19 | 2007-11-29 | Toyo Tire & Rubber Co Ltd | 研磨パッドの製造方法 |
| WO2008026451A1 (fr) * | 2006-08-28 | 2008-03-06 | Toyo Tire & Rubber Co., Ltd. | Tampon de polissage |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20130035021A1 (en) * | 2010-03-26 | 2013-02-07 | Toyo Tire & Rubber Co., Ltd. | Polishing pad, manufacturing method therefor, and method for manufacturing a semiconductor device |
| US9181386B2 (en) * | 2010-03-26 | 2015-11-10 | Toyo Tire & Rubber Co., Ltd. | Polishing pad, manufacturing method therefor, and method for manufacturing a semiconductor device |
| US20130012105A1 (en) * | 2010-03-31 | 2013-01-10 | Toyo Tire & Rubber Co., Ltd. | Polishing pad and production method therefor, and production method for semiconductor device |
| US9018273B2 (en) * | 2010-03-31 | 2015-04-28 | Toyo Tire & Rubber Co., Ltd. | Polishing pad and production method therefor, and production method for semiconductor device |
| US9079289B2 (en) | 2011-09-22 | 2015-07-14 | Toyo Tire & Rubber Co., Ltd. | Polishing pad |
| CN113319734A (zh) * | 2021-07-06 | 2021-08-31 | 北京半导体专用设备研究所(中国电子科技集团公司第四十五研究所) | 化学抛光装置及其方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2009218500A (ja) | 2009-09-24 |
| TWI431026B (zh) | 2014-03-21 |
| CN101952943A (zh) | 2011-01-19 |
| JP4593643B2 (ja) | 2010-12-08 |
| TW200942557A (en) | 2009-10-16 |
| KR20100131419A (ko) | 2010-12-15 |
| CN101952943B (zh) | 2012-07-11 |
| US8476328B2 (en) | 2013-07-02 |
| US20100317263A1 (en) | 2010-12-16 |
| KR101455218B1 (ko) | 2014-10-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4593643B2 (ja) | 研磨パッド | |
| JP5248152B2 (ja) | 研磨パッド | |
| JP5393434B2 (ja) | 研磨パッド及びその製造方法 | |
| KR101181885B1 (ko) | 연마 패드 | |
| JP4261586B2 (ja) | 研磨パッドの製造方法 | |
| JP4986129B2 (ja) | 研磨パッド | |
| JP5306677B2 (ja) | 研磨パッド | |
| JP5528169B2 (ja) | 研磨パッドおよびその製造方法、ならびに半導体デバイスの製造方法 | |
| JP5377909B2 (ja) | 研磨パッド及びその製造方法 | |
| JP5230227B2 (ja) | 研磨パッド | |
| JP5393040B2 (ja) | 研磨パッド | |
| JP4237800B2 (ja) | 研磨パッド | |
| JP4465376B2 (ja) | 研磨パッドの製造方法 | |
| JP2009214220A (ja) | 研磨パッド | |
| JP5184200B2 (ja) | 研磨パッド | |
| JP4465368B2 (ja) | 研磨パッド | |
| JP5132369B2 (ja) | 研磨パッド | |
| JP5465578B2 (ja) | 研磨パッドおよびその製造方法、ならびに半導体デバイスの製造方法 | |
| JP5738729B2 (ja) | 研磨パッド |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 200980106103.3 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 09721088 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20107015876 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12864819 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 09721088 Country of ref document: EP Kind code of ref document: A1 |