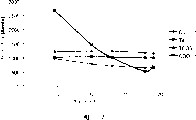CN1305984C - 低k介电材料的化学机械抛光方法 - Google Patents
低k介电材料的化学机械抛光方法 Download PDFInfo
- Publication number
- CN1305984C CN1305984C CNB038131722A CN03813172A CN1305984C CN 1305984 C CN1305984 C CN 1305984C CN B038131722 A CNB038131722 A CN B038131722A CN 03813172 A CN03813172 A CN 03813172A CN 1305984 C CN1305984 C CN 1305984C
- Authority
- CN
- China
- Prior art keywords
- nonionic surfactant
- alkyl
- polyoxyethylene
- amphiphilic nonionic
- polishing
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 238000005498 polishing Methods 0.000 title claims abstract description 121
- 238000000034 method Methods 0.000 title claims description 32
- 239000000126 substance Substances 0.000 title description 16
- 239000003989 dielectric material Substances 0.000 title description 7
- 239000002736 nonionic surfactant Substances 0.000 claims abstract description 57
- 239000000758 substrate Substances 0.000 claims abstract description 46
- 239000007788 liquid Substances 0.000 claims abstract description 16
- -1 polyoxyethylene Polymers 0.000 claims description 73
- 239000000203 mixture Substances 0.000 claims description 69
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims description 50
- 229920003171 Poly (ethylene oxide) Polymers 0.000 claims description 35
- 239000000377 silicon dioxide Substances 0.000 claims description 22
- 150000001875 compounds Chemical class 0.000 claims description 20
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 claims description 19
- 229910052715 tantalum Inorganic materials 0.000 claims description 19
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 claims description 19
- 235000012239 silicon dioxide Nutrition 0.000 claims description 17
- 229910052751 metal Inorganic materials 0.000 claims description 16
- 239000002184 metal Substances 0.000 claims description 16
- 229960001866 silicon dioxide Drugs 0.000 claims description 16
- 239000004698 Polyethylene Substances 0.000 claims description 12
- 239000002253 acid Substances 0.000 claims description 12
- 229920006395 saturated elastomer Polymers 0.000 claims description 12
- 125000000217 alkyl group Chemical class 0.000 claims description 11
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 claims description 9
- 229910052802 copper Inorganic materials 0.000 claims description 9
- 239000010949 copper Substances 0.000 claims description 9
- 150000002148 esters Chemical class 0.000 claims description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 7
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 6
- 229910052799 carbon Inorganic materials 0.000 claims description 6
- 229920000642 polymer Polymers 0.000 claims description 6
- 150000004706 metal oxides Chemical class 0.000 claims description 5
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 claims description 5
- 239000002245 particle Substances 0.000 claims description 5
- 229920001451 polypropylene glycol Polymers 0.000 claims description 5
- 229910052814 silicon oxide Inorganic materials 0.000 claims description 5
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 claims description 4
- 239000004205 dimethyl polysiloxane Substances 0.000 claims description 4
- 229910044991 metal oxide Inorganic materials 0.000 claims description 4
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 claims description 4
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 claims description 4
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 claims description 3
- 125000005037 alkyl phenyl group Chemical group 0.000 claims description 3
- 239000011324 bead Substances 0.000 claims description 3
- ILLHQJIJCRNRCJ-UHFFFAOYSA-N dec-1-yne Chemical compound CCCCCCCCC#C ILLHQJIJCRNRCJ-UHFFFAOYSA-N 0.000 claims description 3
- 229920000573 polyethylene Polymers 0.000 claims description 3
- 229920001296 polysiloxane Polymers 0.000 claims description 3
- 239000000600 sorbitol Substances 0.000 claims description 3
- FFJCNSLCJOQHKM-CLFAGFIQSA-N (z)-1-[(z)-octadec-9-enoxy]octadec-9-ene Chemical compound CCCCCCCC\C=C/CCCCCCCCOCCCCCCCC\C=C/CCCCCCCC FFJCNSLCJOQHKM-CLFAGFIQSA-N 0.000 claims description 2
- 229920002701 Polyoxyl 40 Stearate Polymers 0.000 claims description 2
- KJTLSVCANCCWHF-UHFFFAOYSA-N Ruthenium Chemical compound [Ru] KJTLSVCANCCWHF-UHFFFAOYSA-N 0.000 claims description 2
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 claims description 2
- 229920013701 VORANOL™ Polymers 0.000 claims description 2
- 150000003973 alkyl amines Chemical class 0.000 claims description 2
- 239000004411 aluminium Substances 0.000 claims description 2
- 229910052782 aluminium Inorganic materials 0.000 claims description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 2
- 229920001400 block copolymer Polymers 0.000 claims description 2
- 238000007334 copolymerization reaction Methods 0.000 claims description 2
- OCDXZFSOHJRGIL-UHFFFAOYSA-N cyclohexyloxycyclohexane Polymers C1CCCCC1OC1CCCCC1 OCDXZFSOHJRGIL-UHFFFAOYSA-N 0.000 claims description 2
- 239000011521 glass Substances 0.000 claims description 2
- 229910052741 iridium Inorganic materials 0.000 claims description 2
- GKOZUEZYRPOHIO-UHFFFAOYSA-N iridium atom Chemical compound [Ir] GKOZUEZYRPOHIO-UHFFFAOYSA-N 0.000 claims description 2
- 229910052759 nickel Inorganic materials 0.000 claims description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 2
- 229910052697 platinum Inorganic materials 0.000 claims description 2
- 229920000259 polyoxyethylene lauryl ether Polymers 0.000 claims description 2
- 229940099429 polyoxyl 40 stearate Drugs 0.000 claims description 2
- 229910052707 ruthenium Inorganic materials 0.000 claims description 2
- 125000004079 stearyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 2
- 229910052719 titanium Inorganic materials 0.000 claims description 2
- 239000010936 titanium Substances 0.000 claims description 2
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 claims description 2
- 229910052721 tungsten Inorganic materials 0.000 claims description 2
- 239000010937 tungsten Substances 0.000 claims description 2
- 229910052703 rhodium Inorganic materials 0.000 claims 1
- 239000010948 rhodium Substances 0.000 claims 1
- MHOVAHRLVXNVSD-UHFFFAOYSA-N rhodium atom Chemical compound [Rh] MHOVAHRLVXNVSD-UHFFFAOYSA-N 0.000 claims 1
- 238000007517 polishing process Methods 0.000 abstract description 3
- 239000003795 chemical substances by application Substances 0.000 description 78
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 12
- 239000000463 material Substances 0.000 description 12
- 229910052710 silicon Inorganic materials 0.000 description 12
- 239000010703 silicon Substances 0.000 description 12
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 11
- 150000002500 ions Chemical class 0.000 description 10
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 9
- 230000000694 effects Effects 0.000 description 9
- 150000003839 salts Chemical class 0.000 description 9
- 238000005260 corrosion Methods 0.000 description 7
- 230000007797 corrosion Effects 0.000 description 7
- 239000003112 inhibitor Substances 0.000 description 7
- 239000002002 slurry Substances 0.000 description 7
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 6
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 6
- 239000008139 complexing agent Substances 0.000 description 6
- 239000003352 sequestering agent Substances 0.000 description 6
- CUNWUEBNSZSNRX-RKGWDQTMSA-N (2r,3r,4r,5s)-hexane-1,2,3,4,5,6-hexol;(z)-octadec-9-enoic acid Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O CUNWUEBNSZSNRX-RKGWDQTMSA-N 0.000 description 5
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 5
- IYFATESGLOUGBX-YVNJGZBMSA-N Sorbitan monopalmitate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O IYFATESGLOUGBX-YVNJGZBMSA-N 0.000 description 5
- 239000004147 Sorbitan trioleate Substances 0.000 description 5
- PRXRUNOAOLTIEF-ADSICKODSA-N Sorbitan trioleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@@H](OC(=O)CCCCCCC\C=C/CCCCCCCC)[C@H]1OC[C@H](O)[C@H]1OC(=O)CCCCCCC\C=C/CCCCCCCC PRXRUNOAOLTIEF-ADSICKODSA-N 0.000 description 5
- 239000006185 dispersion Substances 0.000 description 5
- 230000002209 hydrophobic effect Effects 0.000 description 5
- 230000003647 oxidation Effects 0.000 description 5
- 238000007254 oxidation reaction Methods 0.000 description 5
- 239000002210 silicon-based material Substances 0.000 description 5
- 235000019337 sorbitan trioleate Nutrition 0.000 description 5
- 229960000391 sorbitan trioleate Drugs 0.000 description 5
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical compound OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 4
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 4
- LWZFANDGMFTDAV-BURFUSLBSA-N [(2r)-2-[(2r,3r,4s)-3,4-dihydroxyoxolan-2-yl]-2-hydroxyethyl] dodecanoate Chemical compound CCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O LWZFANDGMFTDAV-BURFUSLBSA-N 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- QRUDEWIWKLJBPS-UHFFFAOYSA-N benzotriazole Chemical compound C1=CC=C2N[N][N]C2=C1 QRUDEWIWKLJBPS-UHFFFAOYSA-N 0.000 description 4
- 239000012964 benzotriazole Substances 0.000 description 4
- 229910000420 cerium oxide Inorganic materials 0.000 description 4
- 239000013530 defoamer Substances 0.000 description 4
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 4
- BMMGVYCKOGBVEV-UHFFFAOYSA-N oxo(oxoceriooxy)cerium Chemical compound [Ce]=O.O=[Ce]=O BMMGVYCKOGBVEV-UHFFFAOYSA-N 0.000 description 4
- 230000001737 promoting effect Effects 0.000 description 4
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 3
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical group C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 229910002651 NO3 Inorganic materials 0.000 description 3
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 3
- 229910004298 SiO 2 Inorganic materials 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 3
- 239000000654 additive Substances 0.000 description 3
- 229920001577 copolymer Polymers 0.000 description 3
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 3
- 239000004065 semiconductor Substances 0.000 description 3
- 150000003852 triazoles Chemical class 0.000 description 3
- GLDQAMYCGOIJDV-UHFFFAOYSA-N 2,3-dihydroxybenzoic acid Chemical compound OC(=O)C1=CC=CC(O)=C1O GLDQAMYCGOIJDV-UHFFFAOYSA-N 0.000 description 2
- NECRQCBKTGZNMH-UHFFFAOYSA-N 3,5-dimethylhex-1-yn-3-ol Chemical compound CC(C)CC(C)(O)C#C NECRQCBKTGZNMH-UHFFFAOYSA-N 0.000 description 2
- ZVZFHCZCIBYFMZ-UHFFFAOYSA-N 6-methylheptoxybenzene Chemical compound CC(C)CCCCCOC1=CC=CC=C1 ZVZFHCZCIBYFMZ-UHFFFAOYSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 2
- KFSLWBXXFJQRDL-UHFFFAOYSA-N Peracetic acid Chemical compound CC(=O)OO KFSLWBXXFJQRDL-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- DLRVVLDZNNYCBX-UHFFFAOYSA-N Polydextrose Polymers OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(O)O1 DLRVVLDZNNYCBX-UHFFFAOYSA-N 0.000 description 2
- 239000004642 Polyimide Substances 0.000 description 2
- 229920001213 Polysorbate 20 Polymers 0.000 description 2
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- HVUMOYIDDBPOLL-XWVZOOPGSA-N Sorbitan monostearate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O HVUMOYIDDBPOLL-XWVZOOPGSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 239000006061 abrasive grain Substances 0.000 description 2
- 230000000996 additive effect Effects 0.000 description 2
- 150000001408 amides Chemical class 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 239000008365 aqueous carrier Substances 0.000 description 2
- 150000003851 azoles Chemical class 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- XTEGARKTQYYJKE-UHFFFAOYSA-N chloric acid Chemical compound OCl(=O)=O XTEGARKTQYYJKE-UHFFFAOYSA-N 0.000 description 2
- 229940005991 chloric acid Drugs 0.000 description 2
- 230000003750 conditioning effect Effects 0.000 description 2
- 238000013016 damping Methods 0.000 description 2
- 239000010432 diamond Substances 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 238000005189 flocculation Methods 0.000 description 2
- 230000016615 flocculation Effects 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- LNTHITQWFMADLM-UHFFFAOYSA-N gallic acid Chemical compound OC(=O)C1=CC(O)=C(O)C(O)=C1 LNTHITQWFMADLM-UHFFFAOYSA-N 0.000 description 2
- 125000000623 heterocyclic group Chemical group 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- MOYKHGMNXAOIAT-JGWLITMVSA-N isosorbide dinitrate Chemical compound [O-][N+](=O)O[C@H]1CO[C@@H]2[C@H](O[N+](=O)[O-])CO[C@@H]21 MOYKHGMNXAOIAT-JGWLITMVSA-N 0.000 description 2
- 229960000201 isosorbide dinitrate Drugs 0.000 description 2
- 150000002894 organic compounds Chemical class 0.000 description 2
- 229920000620 organic polymer Polymers 0.000 description 2
- 239000007800 oxidant agent Substances 0.000 description 2
- 125000006353 oxyethylene group Chemical group 0.000 description 2
- LLYCMZGLHLKPPU-UHFFFAOYSA-M perbromate Chemical compound [O-]Br(=O)(=O)=O LLYCMZGLHLKPPU-UHFFFAOYSA-M 0.000 description 2
- VLTRZXGMWDSKGL-UHFFFAOYSA-M perchlorate Inorganic materials [O-]Cl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-M 0.000 description 2
- VLTRZXGMWDSKGL-UHFFFAOYSA-N perchloric acid Chemical compound OCl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-N 0.000 description 2
- KHIWWQKSHDUIBK-UHFFFAOYSA-N periodic acid Chemical compound OI(=O)(=O)=O KHIWWQKSHDUIBK-UHFFFAOYSA-N 0.000 description 2
- 125000000864 peroxy group Chemical group O(O*)* 0.000 description 2
- JRKICGRDRMAZLK-UHFFFAOYSA-L persulfate group Chemical group S(=O)(=O)([O-])OOS(=O)(=O)[O-] JRKICGRDRMAZLK-UHFFFAOYSA-L 0.000 description 2
- XNGIFLGASWRNHJ-UHFFFAOYSA-L phthalate(2-) Chemical compound [O-]C(=O)C1=CC=CC=C1C([O-])=O XNGIFLGASWRNHJ-UHFFFAOYSA-L 0.000 description 2
- XNGIFLGASWRNHJ-UHFFFAOYSA-N phthalic acid Chemical compound OC(=O)C1=CC=CC=C1C(O)=O XNGIFLGASWRNHJ-UHFFFAOYSA-N 0.000 description 2
- 229920000768 polyamine Polymers 0.000 description 2
- 229920001721 polyimide Polymers 0.000 description 2
- 229920000193 polymethacrylate Polymers 0.000 description 2
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 2
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 2
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 2
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 2
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 2
- 239000004810 polytetrafluoroethylene Substances 0.000 description 2
- 235000011118 potassium hydroxide Nutrition 0.000 description 2
- 238000001556 precipitation Methods 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- WGTYBPLFGIVFAS-UHFFFAOYSA-M tetramethylammonium hydroxide Chemical compound [OH-].C[N+](C)(C)C WGTYBPLFGIVFAS-UHFFFAOYSA-M 0.000 description 2
- XOLBLPGZBRYERU-UHFFFAOYSA-N tin dioxide Chemical compound O=[Sn]=O XOLBLPGZBRYERU-UHFFFAOYSA-N 0.000 description 2
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 2
- HOVAGTYPODGVJG-UVSYOFPXSA-N (3s,5r)-2-(hydroxymethyl)-6-methoxyoxane-3,4,5-triol Chemical compound COC1OC(CO)[C@@H](O)C(O)[C@H]1O HOVAGTYPODGVJG-UVSYOFPXSA-N 0.000 description 1
- QGLWBTPVKHMVHM-KTKRTIGZSA-N (z)-octadec-9-en-1-amine Chemical compound CCCCCCCC\C=C/CCCCCCCCN QGLWBTPVKHMVHM-KTKRTIGZSA-N 0.000 description 1
- 150000000177 1,2,3-triazoles Chemical class 0.000 description 1
- 150000000178 1,2,4-triazoles Chemical class 0.000 description 1
- TUSDEZXZIZRFGC-UHFFFAOYSA-N 1-O-galloyl-3,6-(R)-HHDP-beta-D-glucose Natural products OC1C(O2)COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC1C(O)C2OC(=O)C1=CC(O)=C(O)C(O)=C1 TUSDEZXZIZRFGC-UHFFFAOYSA-N 0.000 description 1
- 239000004160 Ammonium persulphate Substances 0.000 description 1
- 235000017060 Arachis glabrata Nutrition 0.000 description 1
- 241001553178 Arachis glabrata Species 0.000 description 1
- 235000010777 Arachis hypogaea Nutrition 0.000 description 1
- 235000018262 Arachis monticola Nutrition 0.000 description 1
- OMPJBNCRMGITSC-UHFFFAOYSA-N Benzoylperoxide Chemical compound C=1C=CC=CC=1C(=O)OOC(=O)C1=CC=CC=C1 OMPJBNCRMGITSC-UHFFFAOYSA-N 0.000 description 1
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 229920005682 EO-PO block copolymer Polymers 0.000 description 1
- 239000001263 FEMA 3042 Substances 0.000 description 1
- 229910001111 Fine metal Inorganic materials 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- REYJJPSVUYRZGE-UHFFFAOYSA-N Octadecylamine Chemical compound CCCCCCCCCCCCCCCCCCN REYJJPSVUYRZGE-UHFFFAOYSA-N 0.000 description 1
- 241000233855 Orchidaceae Species 0.000 description 1
- LRBQNJMCXXYXIU-PPKXGCFTSA-N Penta-digallate-beta-D-glucose Natural products OC1=C(O)C(O)=CC(C(=O)OC=2C(=C(O)C=C(C=2)C(=O)OC[C@@H]2[C@H]([C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)O2)OC(=O)C=2C=C(OC(=O)C=3C=C(O)C(O)=C(O)C=3)C(O)=C(O)C=2)O)=C1 LRBQNJMCXXYXIU-PPKXGCFTSA-N 0.000 description 1
- 235000008331 Pinus X rigitaeda Nutrition 0.000 description 1
- 235000011613 Pinus brutia Nutrition 0.000 description 1
- 241000018646 Pinus brutia Species 0.000 description 1
- 229920002582 Polyethylene Glycol 600 Polymers 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 229920000292 Polyquinoline Polymers 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical compound CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 description 1
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 1
- XSTXAVWGXDQKEL-UHFFFAOYSA-N Trichloroethylene Chemical compound ClC=C(Cl)Cl XSTXAVWGXDQKEL-UHFFFAOYSA-N 0.000 description 1
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 description 1
- IJCWFDPJFXGQBN-RYNSOKOISA-N [(2R)-2-[(2R,3R,4S)-4-hydroxy-3-octadecanoyloxyoxolan-2-yl]-2-octadecanoyloxyethyl] octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@@H](OC(=O)CCCCCCCCCCCCCCCCC)[C@H]1OC[C@H](O)[C@H]1OC(=O)CCCCCCCCCCCCCCCCC IJCWFDPJFXGQBN-RYNSOKOISA-N 0.000 description 1
- BQODPTQLXVVEJG-UHFFFAOYSA-N [O].C=C Chemical compound [O].C=C BQODPTQLXVVEJG-UHFFFAOYSA-N 0.000 description 1
- 238000005299 abrasion Methods 0.000 description 1
- 239000003082 abrasive agent Substances 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- ZUQAPLKKNAQJAU-UHFFFAOYSA-N acetylenediol Chemical compound OC#CO ZUQAPLKKNAQJAU-UHFFFAOYSA-N 0.000 description 1
- UNRQTHVKJQUDDF-UHFFFAOYSA-N acetylpyruvic acid Chemical compound CC(=O)CC(=O)C(O)=O UNRQTHVKJQUDDF-UHFFFAOYSA-N 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 150000001414 amino alcohols Chemical class 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- ROOXNKNUYICQNP-UHFFFAOYSA-N ammonium persulfate Chemical compound [NH4+].[NH4+].[O-]S(=O)(=O)OOS([O-])(=O)=O ROOXNKNUYICQNP-UHFFFAOYSA-N 0.000 description 1
- 235000019395 ammonium persulphate Nutrition 0.000 description 1
- 150000003863 ammonium salts Chemical class 0.000 description 1
- 239000012296 anti-solvent Substances 0.000 description 1
- 229920006187 aquazol Polymers 0.000 description 1
- 239000012861 aquazol Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 125000000732 arylene group Chemical group 0.000 description 1
- 210000002469 basement membrane Anatomy 0.000 description 1
- UMIVXZPTRXBADB-UHFFFAOYSA-N benzocyclobutene Chemical compound C1=CC=C2CCC2=C1 UMIVXZPTRXBADB-UHFFFAOYSA-N 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical class OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- 235000019400 benzoyl peroxide Nutrition 0.000 description 1
- 229910002056 binary alloy Inorganic materials 0.000 description 1
- SXDBWCPKPHAZSM-UHFFFAOYSA-M bromate Inorganic materials [O-]Br(=O)=O SXDBWCPKPHAZSM-UHFFFAOYSA-M 0.000 description 1
- SXDBWCPKPHAZSM-UHFFFAOYSA-N bromic acid Chemical compound OBr(=O)=O SXDBWCPKPHAZSM-UHFFFAOYSA-N 0.000 description 1
- 150000001728 carbonyl compounds Chemical class 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 125000002843 carboxylic acid group Chemical group 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 238000003486 chemical etching Methods 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 229910052681 coesite Inorganic materials 0.000 description 1
- 239000008119 colloidal silica Substances 0.000 description 1
- 229910052906 cristobalite Inorganic materials 0.000 description 1
- CFBGXYDUODCMNS-UHFFFAOYSA-N cyclobutene Chemical compound C1CC=C1 CFBGXYDUODCMNS-UHFFFAOYSA-N 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- LSXWFXONGKSEMY-UHFFFAOYSA-N di-tert-butyl peroxide Chemical compound CC(C)(C)OOC(C)(C)C LSXWFXONGKSEMY-UHFFFAOYSA-N 0.000 description 1
- 150000004985 diamines Chemical class 0.000 description 1
- 150000005690 diesters Chemical class 0.000 description 1
- QLBHNVFOQLIYTH-UHFFFAOYSA-L dipotassium;2-[2-[bis(carboxymethyl)amino]ethyl-(carboxylatomethyl)amino]acetate Chemical compound [K+].[K+].OC(=O)CN(CC([O-])=O)CCN(CC(O)=O)CC([O-])=O QLBHNVFOQLIYTH-UHFFFAOYSA-L 0.000 description 1
- JZBWUTVDIDNCMW-UHFFFAOYSA-L dipotassium;oxido sulfate Chemical compound [K+].[K+].[O-]OS([O-])(=O)=O JZBWUTVDIDNCMW-UHFFFAOYSA-L 0.000 description 1
- JRBPAEWTRLWTQC-UHFFFAOYSA-N dodecylamine Chemical compound CCCCCCCCCCCCN JRBPAEWTRLWTQC-UHFFFAOYSA-N 0.000 description 1
- 229940009662 edetate Drugs 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- 238000007046 ethoxylation reaction Methods 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- NBVXSUQYWXRMNV-UHFFFAOYSA-N fluoromethane Chemical compound FC NBVXSUQYWXRMNV-UHFFFAOYSA-N 0.000 description 1
- WBJINCZRORDGAQ-UHFFFAOYSA-N formic acid ethyl ester Natural products CCOC=O WBJINCZRORDGAQ-UHFFFAOYSA-N 0.000 description 1
- 230000000855 fungicidal effect Effects 0.000 description 1
- 239000000417 fungicide Substances 0.000 description 1
- 229940074391 gallic acid Drugs 0.000 description 1
- 235000004515 gallic acid Nutrition 0.000 description 1
- LRBQNJMCXXYXIU-QWKBTXIPSA-N gallotannic acid Chemical compound OC1=C(O)C(O)=CC(C(=O)OC=2C(=C(O)C=C(C=2)C(=O)OC[C@H]2[C@@H]([C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)O2)OC(=O)C=2C=C(OC(=O)C=3C=C(O)C(O)=C(O)C=3)C(O)=C(O)C=2)O)=C1 LRBQNJMCXXYXIU-QWKBTXIPSA-N 0.000 description 1
- 229910052732 germanium Inorganic materials 0.000 description 1
- GNPVGFCGXDBREM-UHFFFAOYSA-N germanium atom Chemical compound [Ge] GNPVGFCGXDBREM-UHFFFAOYSA-N 0.000 description 1
- YBMRDBCBODYGJE-UHFFFAOYSA-N germanium oxide Inorganic materials O=[Ge]=O YBMRDBCBODYGJE-UHFFFAOYSA-N 0.000 description 1
- 229940050410 gluconate Drugs 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 150000002443 hydroxylamines Chemical class 0.000 description 1
- 150000002484 inorganic compounds Chemical class 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 239000011147 inorganic material Substances 0.000 description 1
- 229910017053 inorganic salt Inorganic materials 0.000 description 1
- 239000011810 insulating material Substances 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- ICIWUVCWSCSTAQ-UHFFFAOYSA-M iodate Chemical compound [O-]I(=O)=O ICIWUVCWSCSTAQ-UHFFFAOYSA-M 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical class CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 1
- 239000000395 magnesium oxide Substances 0.000 description 1
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 1
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical compound [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 description 1
- 229940049920 malate Drugs 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N malic acid Chemical compound OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- HOVAGTYPODGVJG-UHFFFAOYSA-N methyl beta-galactoside Natural products COC1OC(CO)C(O)C(O)C1O HOVAGTYPODGVJG-UHFFFAOYSA-N 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 230000006855 networking Effects 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 125000004433 nitrogen atom Chemical group N* 0.000 description 1
- JPMIIZHYYWMHDT-UHFFFAOYSA-N octhilinone Chemical compound CCCCCCCCN1SC=CC1=O JPMIIZHYYWMHDT-UHFFFAOYSA-N 0.000 description 1
- 229920002114 octoxynol-9 Polymers 0.000 description 1
- 150000002888 oleic acid derivatives Chemical class 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 239000006259 organic additive Substances 0.000 description 1
- 125000000962 organic group Chemical group 0.000 description 1
- 239000011368 organic material Substances 0.000 description 1
- 239000012285 osmium tetroxide Substances 0.000 description 1
- 229910000489 osmium tetroxide Inorganic materials 0.000 description 1
- PVADDRMAFCOOPC-UHFFFAOYSA-N oxogermanium Chemical compound [Ge]=O PVADDRMAFCOOPC-UHFFFAOYSA-N 0.000 description 1
- 238000010979 pH adjustment Methods 0.000 description 1
- 235000020232 peanut Nutrition 0.000 description 1
- LLYCMZGLHLKPPU-UHFFFAOYSA-N perbromic acid Chemical compound OBr(=O)(=O)=O LLYCMZGLHLKPPU-UHFFFAOYSA-N 0.000 description 1
- 229920001983 poloxamer Polymers 0.000 description 1
- 229920000052 poly(p-xylylene) Polymers 0.000 description 1
- 239000001259 polydextrose Substances 0.000 description 1
- 229940035035 polydextrose Drugs 0.000 description 1
- 235000013856 polydextrose Nutrition 0.000 description 1
- 239000002861 polymer material Substances 0.000 description 1
- 229920006389 polyphenyl polymer Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Substances [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 1
- 235000015320 potassium carbonate Nutrition 0.000 description 1
- IWZKICVEHNUQTL-UHFFFAOYSA-M potassium hydrogen phthalate Chemical compound [K+].OC(=O)C1=CC=CC=C1C([O-])=O IWZKICVEHNUQTL-UHFFFAOYSA-M 0.000 description 1
- 229940093916 potassium phosphate Drugs 0.000 description 1
- 229910000160 potassium phosphate Inorganic materials 0.000 description 1
- 235000011009 potassium phosphates Nutrition 0.000 description 1
- WQGWDDDVZFFDIG-UHFFFAOYSA-N pyrogallol Chemical compound OC1=CC=CC(O)=C1O WQGWDDDVZFFDIG-UHFFFAOYSA-N 0.000 description 1
- 229910001404 rare earth metal oxide Inorganic materials 0.000 description 1
- 238000005245 sintering Methods 0.000 description 1
- 235000017550 sodium carbonate Nutrition 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- PFUVRDFDKPNGAV-UHFFFAOYSA-N sodium peroxide Chemical compound [Na+].[Na+].[O-][O-] PFUVRDFDKPNGAV-UHFFFAOYSA-N 0.000 description 1
- MWNQXXOSWHCCOZ-UHFFFAOYSA-L sodium;oxido carbonate Chemical compound [Na+].[O-]OC([O-])=O MWNQXXOSWHCCOZ-UHFFFAOYSA-L 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 239000001570 sorbitan monopalmitate Substances 0.000 description 1
- 235000011071 sorbitan monopalmitate Nutrition 0.000 description 1
- 229940031953 sorbitan monopalmitate Drugs 0.000 description 1
- 239000001587 sorbitan monostearate Substances 0.000 description 1
- 235000011076 sorbitan monostearate Nutrition 0.000 description 1
- 229940035048 sorbitan monostearate Drugs 0.000 description 1
- 229960005078 sorbitan sesquioleate Drugs 0.000 description 1
- 239000001589 sorbitan tristearate Substances 0.000 description 1
- 235000011078 sorbitan tristearate Nutrition 0.000 description 1
- 229960004129 sorbitan tristearate Drugs 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 229910052682 stishovite Inorganic materials 0.000 description 1
- 238000005728 strengthening Methods 0.000 description 1
- 229940086735 succinate Drugs 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 235000015523 tannic acid Nutrition 0.000 description 1
- 229940033123 tannic acid Drugs 0.000 description 1
- 229920002258 tannic acid Polymers 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 229910002058 ternary alloy Inorganic materials 0.000 description 1
- DZLFLBLQUQXARW-UHFFFAOYSA-N tetrabutylammonium Chemical compound CCCC[N+](CCCC)(CCCC)CCCC DZLFLBLQUQXARW-UHFFFAOYSA-N 0.000 description 1
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 1
- 229910000314 transition metal oxide Inorganic materials 0.000 description 1
- 229920000428 triblock copolymer Polymers 0.000 description 1
- 229910052905 tridymite Inorganic materials 0.000 description 1
- BYGOPQKDHGXNCD-UHFFFAOYSA-N tripotassium;iron(3+);hexacyanide Chemical compound [K+].[K+].[K+].[Fe+3].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-] BYGOPQKDHGXNCD-UHFFFAOYSA-N 0.000 description 1
- GPRLSGONYQIRFK-MNYXATJNSA-N triton Chemical compound [3H+] GPRLSGONYQIRFK-MNYXATJNSA-N 0.000 description 1
- 125000006839 xylylene group Chemical group 0.000 description 1
- 229910052726 zirconium Inorganic materials 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L21/00—Processes or apparatus adapted for the manufacture or treatment of semiconductor or solid state devices or of parts thereof
- H01L21/02—Manufacture or treatment of semiconductor devices or of parts thereof
- H01L21/04—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer
- H01L21/18—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer the devices having semiconductor bodies comprising elements of Group IV of the Periodic Table or AIIIBV compounds with or without impurities, e.g. doping materials
- H01L21/30—Treatment of semiconductor bodies using processes or apparatus not provided for in groups H01L21/20 - H01L21/26
- H01L21/31—Treatment of semiconductor bodies using processes or apparatus not provided for in groups H01L21/20 - H01L21/26 to form insulating layers thereon, e.g. for masking or by using photolithographic techniques; After treatment of these layers; Selection of materials for these layers
- H01L21/3205—Deposition of non-insulating-, e.g. conductive- or resistive-, layers on insulating layers; After-treatment of these layers
- H01L21/321—After treatment
- H01L21/32115—Planarisation
- H01L21/3212—Planarisation by chemical mechanical polishing [CMP]
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B24—GRINDING; POLISHING
- B24D—TOOLS FOR GRINDING, BUFFING OR SHARPENING
- B24D3/00—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09G—POLISHING COMPOSITIONS; SKI WAXES
- C09G1/00—Polishing compositions
- C09G1/02—Polishing compositions containing abrasives or grinding agents
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K3/00—Materials not provided for elsewhere
- C09K3/14—Anti-slip materials; Abrasives
- C09K3/1454—Abrasive powders, suspensions and pastes for polishing
- C09K3/1463—Aqueous liquid suspensions
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/66—Non-ionic compounds
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L21/00—Processes or apparatus adapted for the manufacture or treatment of semiconductor or solid state devices or of parts thereof
- H01L21/02—Manufacture or treatment of semiconductor devices or of parts thereof
- H01L21/04—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer
- H01L21/18—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer the devices having semiconductor bodies comprising elements of Group IV of the Periodic Table or AIIIBV compounds with or without impurities, e.g. doping materials
- H01L21/30—Treatment of semiconductor bodies using processes or apparatus not provided for in groups H01L21/20 - H01L21/26
- H01L21/302—Treatment of semiconductor bodies using processes or apparatus not provided for in groups H01L21/20 - H01L21/26 to change their surface-physical characteristics or shape, e.g. etching, polishing, cutting
- H01L21/304—Mechanical treatment, e.g. grinding, polishing, cutting
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L21/00—Processes or apparatus adapted for the manufacture or treatment of semiconductor or solid state devices or of parts thereof
- H01L21/02—Manufacture or treatment of semiconductor devices or of parts thereof
- H01L21/04—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer
- H01L21/18—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer the devices having semiconductor bodies comprising elements of Group IV of the Periodic Table or AIIIBV compounds with or without impurities, e.g. doping materials
- H01L21/30—Treatment of semiconductor bodies using processes or apparatus not provided for in groups H01L21/20 - H01L21/26
- H01L21/302—Treatment of semiconductor bodies using processes or apparatus not provided for in groups H01L21/20 - H01L21/26 to change their surface-physical characteristics or shape, e.g. etching, polishing, cutting
- H01L21/306—Chemical or electrical treatment, e.g. electrolytic etching
- H01L21/30625—With simultaneous mechanical treatment, e.g. mechanico-chemical polishing
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L21/00—Processes or apparatus adapted for the manufacture or treatment of semiconductor or solid state devices or of parts thereof
- H01L21/02—Manufacture or treatment of semiconductor devices or of parts thereof
- H01L21/04—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer
- H01L21/18—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer the devices having semiconductor bodies comprising elements of Group IV of the Periodic Table or AIIIBV compounds with or without impurities, e.g. doping materials
- H01L21/30—Treatment of semiconductor bodies using processes or apparatus not provided for in groups H01L21/20 - H01L21/26
- H01L21/31—Treatment of semiconductor bodies using processes or apparatus not provided for in groups H01L21/20 - H01L21/26 to form insulating layers thereon, e.g. for masking or by using photolithographic techniques; After treatment of these layers; Selection of materials for these layers
- H01L21/3105—After-treatment
- H01L21/31051—Planarisation of the insulating layers
- H01L21/31053—Planarisation of the insulating layers involving a dielectric removal step
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L21/00—Processes or apparatus adapted for the manufacture or treatment of semiconductor or solid state devices or of parts thereof
- H01L21/02—Manufacture or treatment of semiconductor devices or of parts thereof
- H01L21/02104—Forming layers
- H01L21/02107—Forming insulating materials on a substrate
- H01L21/02109—Forming insulating materials on a substrate characterised by the type of layer, e.g. type of material, porous/non-porous, pre-cursors, mixtures or laminates
- H01L21/02112—Forming insulating materials on a substrate characterised by the type of layer, e.g. type of material, porous/non-porous, pre-cursors, mixtures or laminates characterised by the material of the layer
- H01L21/02123—Forming insulating materials on a substrate characterised by the type of layer, e.g. type of material, porous/non-porous, pre-cursors, mixtures or laminates characterised by the material of the layer the material containing silicon
- H01L21/02126—Forming insulating materials on a substrate characterised by the type of layer, e.g. type of material, porous/non-porous, pre-cursors, mixtures or laminates characterised by the material of the layer the material containing silicon the material containing Si, O, and at least one of H, N, C, F, or other non-metal elements, e.g. SiOC, SiOC:H or SiONC
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L21/00—Processes or apparatus adapted for the manufacture or treatment of semiconductor or solid state devices or of parts thereof
- H01L21/02—Manufacture or treatment of semiconductor devices or of parts thereof
- H01L21/04—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer
- H01L21/18—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer the devices having semiconductor bodies comprising elements of Group IV of the Periodic Table or AIIIBV compounds with or without impurities, e.g. doping materials
- H01L21/30—Treatment of semiconductor bodies using processes or apparatus not provided for in groups H01L21/20 - H01L21/26
- H01L21/31—Treatment of semiconductor bodies using processes or apparatus not provided for in groups H01L21/20 - H01L21/26 to form insulating layers thereon, e.g. for masking or by using photolithographic techniques; After treatment of these layers; Selection of materials for these layers
- H01L21/312—Organic layers, e.g. photoresist
Landscapes
- Engineering & Computer Science (AREA)
- Physics & Mathematics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- General Physics & Mathematics (AREA)
- Manufacturing & Machinery (AREA)
- Computer Hardware Design (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Power Engineering (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Mechanical Treatment Of Semiconductor (AREA)
- Finish Polishing, Edge Sharpening, And Grinding By Specific Grinding Devices (AREA)
Abstract
本发明提供一种抛光含低k介电层的基板的方法,其包括(i)以包括(a)研磨剂、抛光垫或其组合,(b)两亲型非离子界面活性剂及(c)液体载体的化学机械抛光体系接触基板;及(ii)研磨至少一部分的基板以抛光基板。
Description
发明所属的技术领域
本发明关于供抛光低k介电材料的化学机械抛光组合物。
背景技术
平面化或抛光基板表面的组合物及方法为本技术领域熟知。抛光组合物(亦称为抛光淤浆)典型地含有在水溶液中研磨材料且以浸透抛光组合物的抛光垫接触表面涂布至表面上。典型的研磨材料包括二氧化硅、氧化铈、氧化铝、氧化锆及氧化锡。例如美国专利第5,527,423号叙述化学机械抛光金属层的方法,其以包括在含水介质中的高纯度微细氧化金属颗粒的抛光淤浆接触表面。抛光淤浆典型地与抛光垫(如抛光布或抛光盘)一同使用。适当的抛光垫揭示在美国专利第6,062,968、6,117,000及6,126,532号中,其揭示使用具有开室多孔网路的烧结聚胺基甲酸乙酯抛光垫;以及美国专利第5,489,233号,其揭示使用具有表面纹理或图案的固态抛光垫。另一选择为研磨材料混入抛光垫中。美国专利第5,958,794号揭示固定研磨剂的抛光垫。
硅基金属间介电层的抛光组合物在半导体工业中已特别良好地发展,硅基介电质的抛光及研磨的化学及机械本性已有相当好的了解。然而,硅基介电材料的一个问题为其介电常数相当高(约为3.9或更高,视如残留水分含量的因素而定)。结果,导电层间的电容亦较高,这同样也限制了电流可操作的速度(频率)。开发减少电容的策略包括(1)混入低电阻值的金属(如铜),及(2)以比二氧化硅低介电常数的绝缘材料提供电隔离。这类低介电常数材料典型地包括有机聚合材料、无机与无机多孔介电材料、及掺混或复合有机与无机材料(可为多孔或无孔的)。低介电常数材料混入半导体结构中同时在半导体晶片加工中仍能利用传统化学机械抛光(CMP)体系抛光得到的介电材料的表面。
含低介电常数材料的基板的几种化学机械抛光组合物为习知的。例如,美国专利第6,043,155号揭示无机及有机绝缘膜的氧化铈基淤浆。美国专利第6,046,112号揭示抛光包括氧化锆及氢氧化四甲基铵或氢氧化四丁基铵的低介电材料的抛光组合物。美国专利第6,270,395号揭示包括研磨剂及氧化剂的低介电材料的抛光组合物。
界面活性剂一般用于化学机械抛光组合物中作为分散剂或絮凝剂。例如,美国专利第6,270,393号揭示包括氧化铝、无机盐、水溶性螯合剂及据宣称作为研磨剂分散剂的界面活性剂的研磨淤浆。美国专利第6,313,039号揭示包括研磨剂、羟基胺化合物、氧化剂及任选的据宣称改变欲抛光基板上表面电荷的界面活性剂的抛光组合物。美国专利第6,348,076号揭示包括界面活性剂(特别是阴离子界面活性剂)的金属层CMP的抛光组合物。美国公开专利申请案2001/0005009 A1揭示包括作为分散剂的界面活性剂(包括阴离子、阳离子、两性及非离子界面活性剂)的抛光组合物。美国公开专利申请案2001/0008828 A1揭示铜及隔离膜的含水抛光组合物,其包括研磨剂、有机酸、杂环化合物、氧化剂及任选的界面活性剂。美国公开专利申请案2001/0013507A1揭示抛光低介电常数无机聚合物层的方法,此层包括氧化锆研磨剂及非离子、阴离子、阳离子或两性界面活性剂(据宣称起安定抛光淤浆对抗沉淀、絮凝及分解作用)。WO 01/32794 A1揭示包括有机添加剂的CMP的钽隔离淤浆,添加剂可为任何界面活性剂,其据宣称与氧化硅或铜基板的表面形成键并抑制氧化硅沉淀及铜污染的形成。EP 810 302 B1揭示包括脱水山梨醇脂肪酸酯及脱水山梨醇酯肪酸酯聚氧乙烯衍生物作腐蚀抑制剂的抛光组合物。EP 1088 869 A1揭示包括研磨颗粒及HLB值为6或更低的两亲界面活性剂的CMP含水分散物。EP 1 148 538 A1揭示包括氧化铈研磨剂及据宣称作为分散剂的界面活性剂(如阴离子、非离子、阳离子或两性的)的抛光组合物。
虽然在化学机械抛光组合物中界面活性剂的使用已熟知,没有现有技术文献认定非离子界面活性剂比其他形式的界面活性剂(如阴离子、阳离子及两性界面活性剂)有特别的效果。已发现非离子界面活性剂可提供在基板层移除速率中改良的选择性。本发明的这些及其他优点,以及额外的发明特征由在此提供的发明说明变得明显。
发明概要
本发明提供一种抛光基板的方法,包括(i)以包括(a)研磨剂、抛光垫或其组合,(b)两亲型非离子界面活性剂及(c)液体载体的化学机械抛光体系接触包括介电层的基板;以及(ii)研磨至少一部分的基板以抛光介电层,其中该介电层的介电常数为3.5或更低。
附图简单说明
图1为表示界面活性剂浓度及低k介电材料的移除速率间关系的图。
图2为表示界面活性剂的HLB值及钽(Ta)、二氧化硅(TEOS)及低k介电材料(CDO)的移除速率间关系的图。
发明详细说明
本发明是涉及一种抛光基板的方法,包括(i)以包括(a)磨擦物、抛光垫或其组合,(b)两亲型非离子界面活性剂及(c)液体载体的化学机械抛光体系接触基板,及(ii)研磨至少一部分基板以抛光基板。
在此叙述的化学机械抛光体系包括研磨剂、抛光垫或两者皆有。CMP体系同时包括研磨剂及抛光垫优选。研磨剂可为任何适当的形式(如;研磨颗粒)。研磨剂可固定在抛光垫上及/或可为颗粒形式并悬浮在液体载体中。抛光垫可为任何适当抛光垫。研磨剂(当存在并悬浮在液体载体中时)及两亲型非离子界面活性剂,以及任何悬浮在液体载体中的其他组分,形成CMP体系的抛光组合物。
研磨剂可为任何适当研磨剂(如金属氧化物)。例如,研磨剂可为选自由氧化铝、氧化硅、氧化钛、氧化铈、氧化锆、氧化锗、氧化镁、其共形成产物及其组合组成的群的金属氧化物研磨剂。研磨剂亦可为聚合物颗粒或涂布颗粒。典型地,研磨剂是选自由氧化铝、氧化硅、其共形成产物、涂布的氧化金属颗粒、聚合物颗粒及其组合组成的群。研磨剂为氧化硅优选。抛光体系典型地包括0.1重量%至20重量%(如0.5重量%至15重量%,或1重量%至10重量%)研磨剂,这以液体载体及溶解或悬浮在其中的任何化合物的重量为基础。
两亲型非离子界面活性剂为具亲水部分及疏水部分的界面活性剂。为了本发明的目的,两亲型非离子界面活性剂定义为具有头基及尾基者。头基为界面活性剂的疏水部分,而尾基为界面活性剂的亲水部分。可使用任何适当的头基及任何适当的尾基。两亲型非离子界面活性剂可包括头基及尾基的任何适当组合。例如,两亲型非离子界面活性剂可只包括一个头基结合一个尾基;或在一些具体实施方案中,可包括多个(如二或更多)头基及/或多个(如二或更多)尾基。两亲型非离子界面活性剂为水溶性优选。
头基可为基本上是疏水性的任何适当基。例如,适当头基包括聚硅氧烷、四C1-C4烷基癸炔、饱和或部分未饱和C6-30烷基、聚氧化丙烯、C6-12烷基苯基或环己基、聚乙烯或其混合物。饱和或部分未饱和C6-30烷基任选可由例如短链(C1-5)烷基、C6-30芳香基、短链(C1-5)碳氟化合物、羟基、卤素、羧酸、酯、胺、酰胺、二醇及其类似物的官能基取代。当头基团为饱和或部分未饱和C6-30烷基时,以亲水基取代的程度极低(如小于3,或少于2个亲水基)优选。头基团不以亲水基(如羟基及羧酸基)取代更优选。
尾基可为基本上是亲水基的任何适当基团。例如,适当尾基团包括那些包含聚氧乙烯基(具有4或更多(如6或更多或8或更多)氧化乙烯重复单元优选)、脱水山梨醇基、高取代饱和或部分未饱和C6-30烷基或其混合物(如聚氧乙烯脱水山梨醇)者。高度取代饱和或部分未饱和C6-30烷基以亲水官能机(例如羟基)取代优选。
两亲型非离子界面活性剂可为包括四烷基癸炔头基团及氧乙烯尾基团的炔二醇界面活性剂,如2,4,7,9-四甲基-5-癸炔-4,7-二醇乙氧基化物。两亲型非离子界面活性剂亦可选自由聚氧乙烯烷基醚及聚氧乙烯烷基酸酯形成的群,其中烷基为饱和或部分未饱和的C6-30烷基且任选为支链的。例如,两亲型非离子界面活性剂可为聚氧乙烯月桂基醚、聚氧乙烯十六烷基醚、聚氧乙烯硬脂基醚、聚氧乙烯油基醚、聚氧乙烯单月桂酸酯、聚氧乙烯单硬脂酸酯、聚氧乙烯二硬脂酸酯或聚氧乙烯单油酸酯。相似地,两亲型非离子界面活性剂可为聚氧乙烯烷基苯基醚或聚氧乙烯烷基环己基醚,其中烷基为饱和或部分未饱和的C6-30烷基且可任选为支链的,如聚氧乙烯辛基苯基醚或聚氧乙烯壬基苯基醚。
两亲型非离子界面活性剂亦可为脱水山梨醇烷基酸酯或聚氧乙烯脱水山梨醇烷基酸酯,其中烷基为饱和或部分未饱和的C6-30烷基且可任选为支链的。例如,两亲型非离子界面活性剂可为脱水山梨醇单月桂酸酯、脱水山梨醇单油酸酯、脱水山梨醇单棕榈酸酯、脱水山梨醇单硬脂酸酯、脱水山梨醇倍半油酸酯、脱水山梨醇三油酸酯或脱水山梨醇三硬脂酸酯,以及聚氧乙烯脱水山梨醇单月桂酸酯、聚氧乙烯脱水山梨醇单棕榈酸酯、聚氧乙烯脱水山梨醇单硬脂酸酯、聚氧乙烯脱水山梨醇三硬脂酸酯、聚氧乙烯脱水山梨醇单油酸酯、聚氧乙烯脱水山梨醇三油酸酯或聚氧乙烯脱水山梨醇四油酸酯。
两亲型非离子界面活性剂可为包括聚二甲基硅氧烷及聚氧乙烯、聚氧乙烯及聚氧丙烯或聚氧乙烯及聚乙烯的嵌段或接枝共聚合物。两亲型非离子界面介面活性剂亦可为聚氧乙烯烷基胺(如聚氧乙烯月桂胺、聚氧乙烯硬脂胺、聚氧乙烯油胺)、乙氧基化酰胺、乙氧基化烷基烷醇酰胺、烷基聚葡萄糖(如汉高(Henkel)的界面活性剂普拉塔兰(Plantaren)或烷基葡萄糖的乙氧化酯或二酯(如阿梅柯尔(A merchol)的PEG-120甲基葡萄糖二油酸酯及其类似物)。
优选的两亲型非离子界面活性剂包括聚氧乙烯脱水山梨醇烷基酸酯(如聚氧乙烯脱水山梨醇单月桂酸酯、聚氧乙烯单棕榈酸酯、聚氧乙烯脱水山梨醇倍半油酸酯及聚氧乙烯脱水山梨醇三油酸酯)、烷基苯基聚氧乙烯(如罗尼-普朗克(Rhone-Poulenc)的界面活性剂埃格波尔(Igepal))及乙炔二醇基界面活性剂(如Air Products的界面活性剂苏尔费诺尔(Surfynol))。
抛光体系典型地包括0.002wt%或更多两亲型非离子界面活性剂,这以液体载体及溶解或悬浮在其中的任何化合物的重量为基础。抛光体系包括0.005重量%至1.0重量%(如0.01重量%至0.5重量%)的两亲型非离子界面活性剂优选(以液体载体及溶解或悬浮在其中的任何化合物的重量为基础)。两亲型非离子界面活性剂的量部分取决于界面活性剂的类型。例如,当两亲型非离子界面活性剂为氧化乙烯及氧化丙烯的共聚合物(如BASF的界面活性剂普罗尼克(Pluronic)L101或普-尼克31R1)时,其量为0.05重量%或更少(如0.02重量%或更少,或0.01重量%或更少)优选。当界面活性剂为脱水山梨醇脂肪酸酯(如脱水山梨醇单月桂酸酯、脱水山梨醇单棕榈酸酯、脱水山梨醇倍半油酸酯、脱水山梨醇三油酸酯),其量为0.01重量%或更多(如0.02重量%或更多,或0.05重量%或更多)优选。
两亲型非离子界面活性剂典型地具有亲水-亲油平衡(HLB)值为7或更大(如10或更大,或12或更大)。HLB值表示在水中界面活性剂的溶解度且如此与界面活性剂的亲水部分的重量%量(如氧化乙烯的重量%量)有关。在一些情况中,含氧化乙烯基的非离子界面活性剂的HLB值大约等于氧化乙烯基的重量%量除5。低HLB值表示亲油界面活性剂(即具有小数目的亲水基),而高HLB值指示亲水界面活性剂(具有大数目的亲水基)。
选择用于本发明化学机械抛光体系的两亲型非离子界面活性剂的类型部分取决于欲抛光基板的的类型。例如,当低k介电层为掺杂碳的二氧化硅材料时,两亲型非离子界面活性剂的类型取决于掺杂碳水平。典型掺杂碳二氧化硅(CDO)低k介电材料具有SiwCxOyHz的化学式,此处x约为(0.10-0.25)y。当x等于零时,材料与未掺杂的二氧化硅相同,其与两亲型非离子界面活性剂几乎没有交互作用。当二氧化硅材料以有机基团改变(即x>0)时,基板表面变得格外增加疏水性。尽管不想结合理论,认为掺杂的二氧化硅的疏水性质驱使非离子两性界面活性剂吸附在表面上。在低水平的掺杂碳下,合宜的疏水头基团较大以更完整地覆盖CDO层的表面。例如,在低水平的掺杂碳下,头基可为聚丙烯或聚氧丙烯。随掺杂水平增加,疏水头基的大小可较小。
亲水尾基的长度对控制低k介电材料表面上的抛光环境是重要的。若亲水尾基太小,立体隔离不足以防止化学浸蚀及/或因磨耗而移除基板表面。藉选择具有长、庞大亲水尾基的两亲型非离子界面活性剂,厚立体障碍可在低k介电材料的表面上产生藉以实质上减少低k介电层移除的速率。这类两亲型非离子界面活性剂将具有反映高亲水尾基的重量%量的高HLB值。
液体载体用于协助涂布研磨剂(当存在并悬浮在液体载体中时)、两亲型非离子界面活性剂及任何任选添加剂至欲抛光(如平面化)的适当基板表面上。液体载体典型地为含水载体且可为单独的水,可包括水及适当的水可溶混溶剂,或可为乳液。适当水可溶混溶剂包括醇如甲醇、乙醇等。含水载体由水组成优选,以去离子水更优选。
抛光组合物可具有任何适当pH。典型地,抛光组合物的pH为6或更高(如7或更高,或8或更高)及pH为12或更低(如11或更低)。
在此叙述的抛光体系可用于抛光(如平面化)基板。基板包括介电常数为3.5或更少(如3或更少,或1至3)的低k介电层。例如,介电层可包括有机改质硅玻璃如掺杂碳的二氧化硅(CDO)或有机聚合物膜如选自由聚酰亚胺、氟化聚酰亚胺、聚亚芳香基及聚亚芳基醚(如陶氏化学(Dow Chemical)的SiLKTM、联合信号(Allied Signal)的法拉尔(FLARETM)及舒马赫尔(Schumacher)的韦洛斯(VELOXTM)、聚苯并环丁烯、二乙烯基硅氧烷双苯并环丁烯(DVS-BCB)、聚四氟乙烯(PTFE)、聚硅氧烷、聚亚萘基醚、聚喹啉、paralynes(Parylene AF4、脂肪族四氟化聚对亚二甲苯基)、其共聚合物及其组合组成的群的聚合物。低k介电层包括掺杂碳二氧化硅。
基板任选还包括介电层(如二氧化硅)及/或金属层。金属层可包括任何适当金属。例如,金属层可包括铜、钽、钛、钨、铝、镍、铂、钌、铱、锗、其合金(如其二元合金及其三元合金)及其组合。金属层包括铜及/或钽优选。两亲型非离子界面活性剂抑制低k介电层的移除速率而不实质上影响存在基板表面上任何其他层(如氧化层、金属层)的移除速率。
在此叙述的抛光体系任选还包括氧化剂。氧化剂可为任何适当氧化剂。适当氧化剂包括无机及有机过化合物、溴酸盐、硝酸盐、氯酸盐、铬酸盐、碘酸盐、铁及铜盐(如硝酸盐、硫酸盐、EDTA及柠檬酸盐)、稀土及过渡金属氧化物(如四氧化饿)、铁氰酸钾、二铬酸钾、碘酸及其类似物。过化合物(如霍雷简明化学辞典(Hawley′s Condensed Chemical Dictionary)所定义)为含至少一个过氧基(-O-O-)的化合物或含在其最高氧化态的元素的化合物。含至少一个过氧基化合物的实例包括但不限于过氧化氢及其加合物如氢过氧化物及过碳酸盐、有机过氧化物如过氧化二苯甲酰、过乙酸及二叔丁基过氧化物、单过硫酸盐(SO5 2-)、二过硫酸盐(S2O8 2-)及过氧化钠。含在最高氧化态的元素的化合物的实例包括但不限于过碘酸、过碘酸盐、过溴酸、过溴酸盐、过氯酸、过氯酸盐、过硼酸、过硼酸盐及过锰酸盐。二级氧化剂为过氧化氢、单过硫酸钾(亦称为过氧单硫酸钾且可购自杜邦标示化学式为2KHSO5·KHSO4·K2SO4(FW614.78)之欧克沙(Oxone)氧化剂)、过硫酸铵或其组合优选。
在此叙述的抛光体系任选还包括络合剂或螯合剂。络合剂或螯合剂为增强欲移除基板的移除速率的任何适当化学添加剂。适当螯合剂或络合剂可包括例如羰基化合物(如乙酰丙酮酸盐及其类似物)、简单羧酸盐(如乙酸盐、芳香羧酸盐及其类似物)、含一或多个羟基的羧酸盐(如乙醇酸盐、乳酸盐、葡萄糖酸盐、五倍子酸及其盐及其类似物)、二、三及多羧酸盐(如草酸盐、邻苯二甲酸盐、柠檬酸盐、琥珀酸盐、酒石酸盐、苹果酸盐、依地酸盐(如EDTA二钾、其混合物及其类似物)、含一或多个磺酸基及/或膦基及其类似物。适当螯合剂或络合剂亦可包括例如二醇、三醇或多醇(如乙二醇、焦儿茶酸、焦五倍子酸、丹宁酸及其类似物)及含胺化合物(如氨、氨基酸、氨基醇、二胺、三胺及多胺及其类似物)。络合剂为羧酸盐优选,以草酸盐更优选。螯合剂或络合剂的选择将取决于在以抛光组合物抛光基板的当中欲移除基板层的种类。
将会理解许多前面提及的化合物可为盐(即金属盐、铵盐或其类似物)、酸或部分盐的形式存在。例如,柠檬酸盐包括柠檬酸、以及其单、二及三盐,邻苯二甲酸盐包括邻苯二甲酸,以及其单盐(如邻苯二甲酸氢钾)及二盐,过氯酸盐包括相应的酸(即过氯酸)、以及其盐。此外,某些化合物或试剂可有多种功能。例如,一些化合物可同时作为螯合剂及氧化剂(如某些铁的硝酸盐及其类似物)。
在此叙述的抛光体系任选还包括腐蚀抑制剂。腐蚀抑制剂(即膜形成剂)可为任何适当的腐蚀抑制剂。典型地,腐蚀抑制剂为含有含杂原子官能基的有机化合物。例如,腐蚀抑制剂为具至少一个5或6员杂环作活性官能基的杂环有机化合物,其中该杂环含至少一个氮原子,例如吡咯化合物。腐蚀抑制剂为三唑优选,以1,2,4三唑、1,2,3三唑或苯三唑更优选。
在此叙述的抛光体系任选还包括一或多种化合物,如pH调整剂、调节剂或缓冲液及其类似物。适当pH调整剂、调节剂或缓冲液可包括例如氢氧化钠、碳酸钠、氢氧化钾、碳酸钾、硫酸、氢氯酸、硝酸、磷酸、柠檬酸、磷酸钾、其混合物及其类似物。
在此叙述的抛光体系任选还包括一或多种组分,如消泡剂及杀虫剂。消泡剂及杀虫剂可分别为任何适当的消泡剂及杀霉菌剂。消泡剂优选聚二甲基硅氧烷聚合物。杀虫剂优选卡松(Kathon)886杀虫剂(罗姆及哈斯(Rohm and Hass))。
在此叙述的抛光体系特别适合与化学机械抛光(CMP)仪器结合使用。典型地,仪器包括滚筒,其在使用时运动且具有由轨道、线性或圆周运动产生的速度;抛光垫,其在使用时与滚筒接触并随之移动;及藉接触并相对欲接触欲抛光基板的抛光垫表面移动支撑欲抛光基板的载体。抛光基板在基板接触相对基板移动的抛光垫时发生,典型地在其间用本发明抛光组合物,使至少一部分的基板研磨而抛光基板。CMP仪器可为任何适当的CMP仪器,许多皆为本技术领域已知。
下面实施例进一步描述本发明,当然不应构成任何对发明范围的限制。
实例1
本实施例描述两亲型非离子界面活性剂对低k介电常数材料的基板移除选择性与铜、钽及二氧化硅材料的移除速率比较的优点。
含钽(Ta)、二氧化硅(SiO2)或掺杂碳二氧化硅(CDO)的相似空白晶片基板以不同抛光组合物(抛光组合物1A-1I)抛光。每一抛光组合物含7重量%的胶态二氧化硅(120nm至150nm平均颗粒直径)、0.02重量%的苯三唑、0.30重量%的乙酸、3重量%的过氧化氢及各种浓度的不同界面活性剂,且pH为8。抛光组合物1A(比较的)含1000ppm的阴离子界面活性剂,聚甲基丙烯酸铵(汉普郡化学(Hampshire Chemical)的达可德(Daxad)32界面活性剂)。抛光组合物1B(比较的)含阳离子的聚乙烯胺(BASF的卢柏索尔(Lupasol)SKA界面活性剂)。抛光组合物1C-1E(本发明)分别含非离子界面活性剂(特别是EO/PO嵌段共聚合物界面活性剂),普罗尼克(Puronic)31R1界面活性剂(PO/EO/PO,10%聚氧乙烯,MW=3250,BASF)、普罗尼克L101界面活性剂(EO/PO/EO,10%聚氧乙烯,MW=3800,HLB=1,BASF),具有氧化乙烯-氧化丙烯共聚物侧链的聚二甲基硅氧烷(硅维(Silwet)7001界面活性剂,HLB 13-17,OSI特用品)及聚(2-乙基2-唑啉)(阿奎索尔(Aquazol)50界面活性剂,MW=50K,聚合物化学创新(Polymer Chemistry Innovations)。抛光组合物1F-1I(本发明)含脱水山梨醇酯界面活性剂,分别为脱水山梨醇单月桂酸酯、脱水山梨醇单棕榈酸酯、脱水山梨醇倍半油酸酯及脱水山梨醇三油酸酯。
每一抛光组合物1A-1I测定对基板的钽、二氧化硅及掺杂碳二氧化硅层的移除速率。表1总结每一抛光组合物在界面活性剂存在下移除速率(RR)的减少百分比(%Drop)。
表1
| 抛光组合物 | 界面活性剂 | 量(ppm) | %DropTa RR | %DropSiO2 RR | %DropCDO RR |
| 1A(比较) | 聚甲基丙烯酸铵(阴离子) | 1000 | -16.1 | -24.2 | -18.4 |
| 1B(比较) | 聚乙烯胺(阳离子) | 100500 | 7.061.5 | -8.064.0 | ---55.1 |
| 1C(本发明) | EO/PO/EO(普罗尼克L101) | 1001000 | 7.743.2 | -5.455.5 | 44.567.8 |
| 1D(本发明) | EO/PO/EO(普罗尼克L101) | 100500 | 10.240.5 | -2.115.9 | 53.966.2 |
| 1E(本发明) | EO/PO聚二甲基硅氧烷(硅维7001) | 1001000 | 036 | -10.138.3 | 40.174.7 |
| 1F(本发明) | 脱水山梨醇单月桂酸酯 | 100500 | 9.43.1 | 15.015.3 | 40.748.7 |
| 1G(本发明) | 脱水山梨醇单棕榈酸酯 | 500 | 8.1 | 0.9 | 25.4 |
| 1H(本发明) | 脱水山梨醇倍半油酸酯 | 500 | 8.7 | 5.5 | 30.5 |
| 1I(本发明) | 山梨酸三油酸酯 | 500 | 10.6 | 4.9 | 28.6 |
总结在表1的结果证实两亲型非离子界面活性剂的存在可减少介电常数为3.5或更低的介电层(如CDO基板层)的移除速率,同时留下其他基板材料(如金属及/或氧化物层)的移除速率实质上未改变。
实例2
本实施例描述两亲型非离子界面活性剂在低k介电常数材料的基板移除速率上的效果与界面活性剂的浓度的关系。
含钽(Ta)、二氧化硅(PETEOS)或黑钻石(Black Diamond)低k介电(Applied Materials)材料的相似空白晶片基板以不同的抛光组合物(抛光组合物2A-2E)抛光。抛光组合物2A(对照)含12重量%的胶态氧化硅、0.01重量%的苯并三唑、0.3重量%的乙酸、3重量%的过氧化氢且无界面活性剂而pH为10(以KOH调整)。抛光组合物2B-2E(本发明)除了分别含50、100、200及400ppm的聚氧乙烯(40)壬基苯基醚(埃格波尔(Igepal)CO-890界面活性剂,罗尼-普朗克)外其他与抛光组合物2A相同。每一抛光组合物测定钽、PETOS及低k介电材料的移除速率(RR)。结果总结在表2及图1中。
表2 移除速率与界面活性剂浓度的关系
| 抛光组合物 | 界面活性剂 | 界面活性剂浓度(ppm) | Ta RR(/分钟) | PETEOSRR(/分钟) | 低k介电材料RR(/分钟) |
| 2A(对照) | 无 | 0 | 1013 | 1082 | 2600 |
| 2B(本发明) | 埃格波尔CO-890 | 50 | 1019 | 1034 | 786 |
| 2C(本发明) | 埃格波尔CO-890 | 100 | 1005 | 1052 | 503 |
| 2D(本发明) | 埃格波尔CO-890 | 200 | 972 | 1006 | 375 |
| 2E(本发明) | 埃格波尔CO-890 | 400 | 912 | 964 | 328 |
总结在表2中的结果证实两亲型非离子界面活性剂的存在对低k介电材料的移除速率有显著的效果,但对钽及PETEOS层的移除速率上只有微小的效果。
实例3
本实施例描述两亲型非离子界面活性剂在低k介电常数材料的基板移除速率上的效果与界面活性剂的HLB值的关系。
含钽(Ta)、二氧化硅(TEOS)或掺杂碳二氧化硅(CDO)的相似空白晶片基板以不同的抛光组合物(抛光组合物3A-3E)抛光。每一抛光组合物含12重量%的胶态氧化硅、0.10重量%的苯并三唑、0.3重量%的乙酸、3重量%的过氧化氢及200ppm的界面活性剂而pH为10。抛光组合物3A-3E(本发明)分别含HLB为4.6的聚氧乙烯(2)异辛基苯基醚(埃格波尔CO-210界面活性剂,罗尼-普朗克)、HLB为10的聚氧乙烯(5)异辛基苯基醚(埃格波尔CO-520界面活性剂,罗尼-普朗克)、HLB为13的聚氧乙烯(9)壬基苯基醚(埃格波尔CO-630界面活性剂,罗尼-普朗克)、HLB为17.8的聚氧乙烯(40)壬基苯基醚(埃格波尔CO-890界面活性剂,罗尼-普朗克)及HLB为19的聚氧乙烯(100)壬基苯基醚(埃格波尔CO-990界面活性剂,罗尼-普朗克)。每一抛光组合物测定对Ta、TEOS及CDO层的移除速率(RR)。结果总结在表3及图2中。
表3 移除速率与HLB的关系
| 抛光组合物 | 界面活性剂(200ppm) | HLB | Ta RR(/分钟) | TEOS RR(/分钟) | CDO RR(/分钟) |
| 3A | 埃格波尔CO-210 | 4.6 | 1033 | 1219 | 2667 |
| 3B | 埃格波尔CO-520 | 10 | 1051 | 1237 | 1459 |
| 3C | 埃格波尔CO-630 | 13 | 1031 | 1237 | 1003 |
| 3D | 埃格波尔CO-890 | 17.8 | 1004 | 1184 | 529 |
| 3E | 埃格波尔CO-990 | 19 | 1000 | 1164 | 689 |
表3中的结果证实低k介电材料的移除速率随界面活性剂的HLB值增加而减少,同时其他基板层(如金属及氧化物层)的移除速率实质上不受影响。
实例4
本实施例描述含一个头基及两个尾基的两亲型非离子界面活性剂对低k介电常数材料移除的基板移除选择性与钽及二氧化硅材料的移除速率比较的优点。
含钽、二氧化硅及掺杂碳二氧化硅(CDO)的相似基板以不同抛光组合物(抛光组合物4A-4F)抛光。每一抛光组合物含7重量%的胶态氧化硅及0.02重量%的苯并三唑且pH为8。抛光组合物4A(对照)不合界面活性剂。抛光组合物4B-4E(本发明)分别含75、150、300及1000ppm的HLB值为17的2,4,7,9四甲基5-癸炔-4,7-二醇乙氧基化物(30)(苏尔费诺尔(Surfynol)485界面活性剂,Air Products)。每一与抛光组合物4B-4E使用的两亲型非离子界面活性剂含一个头基及二个尾基。抛光组合物4F(比较)含200ppm的HLB为4的2,4,7,9-四甲基-5-癸炔-4,7-二醇(苏尔费诺尔104PA界面活性剂,Air Products)。
每一抛光组合物4A-4F测定对基板的钽、二氧化硅及掺杂碳二氧化硅层的移除速率。表4总结每一抛光组合物在界面活性剂存在下移除速率(RR)的减少百分比(%Drop)。
表4
| 抛光组合物 | 界面活性剂 | 头基 | 尾基 | %DropTa RR | %DropSiO2 RR | %DropCDO RR |
| 4A(对照) | 无 | --- | --- | 0 | 0 | 0 |
| 4B(本发明) | 苏尔费诺尔485(75ppm) | Me4癸炔 | 双(EO)15 | -0.4 | -12.9 | 59.1 |
| 4C(本发明) | 苏尔费诺尔485(150ppm) | Me4癸炔 | 双(EO)15 | 2.4 | -8.5 | 71.7 |
| 4D(本发明) | 苏尔费诺尔485(300ppm) | Me4癸炔 | 双(EO)15 | 4.5 | 0.0 | 63.0 |
| 4E(本发明) | 苏尔费诺尔485(1000ppm) | Me4癸炔 | 双(EO)15 | 19.2 | 7.5 | 70.4 |
| 4F(比较) | 苏尔费诺尔104P(1000ppm) | Me4癸炔 | 无 | 11.8 | 4.0 | 21.4 |
表4中总结的结果证实具一个四甲基癸炔头基及多个聚氧乙烯尾基的炔二醇界面活性剂对低k介电材料的移除速率有意想不到的效果而实质上不影响其他金属或氧化物层的移除速率。
实例5
此实施例描述两亲型非离子界面活性剂对低k介电常数材料移除的基板移除选择性与钽及二氧化硅材料的移除速率比较的优点。
含钽、二氧化硅及掺杂碳二氧化硅(CDO)的相似空白晶片基板以不同抛光组合物(抛光组合物5A-5K)抛光。每一抛光组合物含12重量%的研磨剂、0.02重量%的苯并三唑及167ppm的界面活性剂且pH为10。抛光组合物5A(比较)含分子量为600(EO14)的聚乙二醇。抛光组合物5B-5F(本发明)每一个皆含聚氧乙烯及聚氧丙烯的三嵌段共聚合物(EO/PO/EO),具体地为EO20-PO70-EO20、EO1-PO17-EO1、EO13-PO30-EO13、EO76-PO29-EO76及EO11-PO16-EO11。抛光组合物5G-5I(本发明)每一个皆含聚乙烯及聚氧乙烯的嵌段共聚合物,具体地分别为PE12-EO4、PE8-EO10及PE8-EO41。抛光组合物5J及5K(本发明)分别含辛基苯基聚氧乙烯及辛基环己基聚氧乙烯(特力顿(Triton)X-100及特力顿X-100R界面活性剂,Union Caride)。
每一抛光组合物测定的钽、二氧化硅及CDO空白晶片移除速率及不同层的移除速率(RR)的减少百分比(%Drop)总结在表5中。
表5
| 抛光组合物 | 界面活性剂 | 头基 | 尾基 | %DropTa RR | %DropSiO2 RR | %DropCDO RR |
| 5A(比较) | PEG 600 | 无 | (EO)14 | 3.6 | 9.7 | 12.4 |
| 5B(本发明) | EO/PO/EO | (PO)70 | 双(EO)20 | 36.1 | 42.6 | 69.4 |
| 5C(本发明) | EO/PO/EO | (PO)17 | 双(EO)1 | 6.9 | 10.7 | 53.4 |
| 5D(本发明) | EO/PO/EO | (PO)30 | 双(EO)13 | 0.9 | 1.7 | 75.6 |
| 5E(本发明) | EO/PO/EO | (PO)29 | 双(EO)76 | 9.1 | 7.0 | 41.2 |
| 5F(本发明) | EO/PO/EO | (PO)16 | 双(EO)11 | -0.1 | -1.9 | 60.9 |
| 5G(本发明) | PE/PEG870 | (PE)12 | (EO)4 | 0.9 | 7.6 | 2.1 |
| 5H(本发明) | PE/PEG920 | (PE)8 | (EO)10 | 0.9 | 6.4 | 38.8 |
| 5I(本发明) | PE/PEGMP89C | (PE)8 | (EO)41 | 6.9 | 13.0 | 69.5 |
| 5J(本发明) | 特力顿X-100 | 辛基苯基 | (EO)9 | 0.8 | 0.9 | 54.0 |
| 5K(本发明) | 特力顿X-100R | 辛基环己基 | (EO)9 | 8.5 | 10.3 | 54.1 |
总结在表5中的结果证实抛光体系中两亲型非离子界面活性剂存在减少低k介电材料的移除速率而实质上不影响其他基板层的移除速率。
Claims (27)
1.一种抛光基板的方法,包括:
(i)以含有下列组分的化学机械抛光体系接触包括介电层的基板:
(a)研磨剂、抛光垫或其组合;
(b)两亲型非离子界面活性剂,及;
(c)液体载体,及
(ii)研磨至少一部分的该基板以抛光该介电层;其中该介电层的介电常数为3.5或更低。
2.权利要求1的方法,其中该两亲型非离子界面活性剂的量为0.002重量%-1.0重量%。
3.权利要求1的方法,其中该两亲型非离子界面活性剂的HLB为7或更高。
4.权利要求1的方法,其中该两亲型非离子界面活性剂包括头基及尾基。
5.权利要求4的方法,其中该尾基包括具有4或更多氧化乙烯重复单元的聚氧乙烯、脱水山梨醇或其混合物。
6.权利要求4的方法,其中该头基包括聚硅氧烷、四C1-4烷基癸炔、饱和或部分未饱和C6-30烷基、聚氧丙烯、C6-12烷基苯基、C6-12烷基环己基、聚乙烯或其混合物。
7.权利要求6的方法,其中该两亲型非离子界面活性剂为2,4,7,9-四甲基-5-癸炔-4,7-二醇乙氧基化物。
8.权利要求5的方法,其中该两亲型非离子界面活性剂是选自聚氧乙烯烷基醚及聚氧乙烯烷基酸酯,其中该烷基为饱和或部分未饱和的C6-30烷基且任选为支链的。
9.权利要求8的方法,其中该两亲型非离子界面活性剂为聚氧乙烯月桂基醚、聚氧乙烯十六烷基醚、聚氧乙烯硬脂基醚、聚氧乙烯油基醚、聚氧乙烯单月桂酸酯、聚氧乙烯单硬脂酸酯、聚氧乙烯二硬脂酸酯、聚氧乙烯单油酸酯或其组合。
10.权利要求8的方法,其中该两亲型非离子界面活性剂为聚氧乙烯烷基苯基醚或聚氧乙烯烷基环己基醚,其中该烷基为饱和或部分未饱和的C6-30烷基且可任选为支链的。
11.权利要求5的方法,其中该两亲型非离子界面活性剂为聚氧乙烯脱水山梨醇烷基酸酯,其中该烷基为饱和或部分未饱和的C6-30烷基且可任选为支链的。
12.权利要求5的方法,其中该两亲型非离子界面活性剂为含聚二甲基硅氧烷及聚氧乙烯的嵌段或接枝共聚合物。
13.权利要求5的方法,其中该两亲型非离子界面活性剂为含聚氧乙烯及聚氧丙烯或聚氧乙烯及聚乙烯的嵌段共聚合物。
14.权利要求5的方法,其中该两亲型非离子界面活性剂是选自由聚氧乙烯烷基胺、乙氧基化烷基烷醇酰胺及其组合组成的群。
15.权利要求1的方法,其中该体系的pH为6或更高。
16.权利要求1的方法,其中该体系包括研磨剂。
17.权利要求11的方法,其中该研磨剂是选自由氧化铝、氧化硅、其共形成的产品、经涂覆的金属氧化物颗粒、聚合物颗粒及其组合组成的群。
18.权利要求11的方法,其中该研磨剂悬浮在该液体载体中。
19.权利要求11的方法,其中该体系还包括抛光垫。
20.权利要求1的方法,其中该液体载体包括水。
21.权利要求1的方法,其中该抛光体系包括,以该液体载体及溶解或悬浮在其中的任何化合物的重量为基础,0.005重量%或更多的两亲型非离子界面活性剂。
22.权利要求16的方法,其中该抛光体系包括,以该液体载体及溶解或悬浮在其中的任何化合物的重量为基础,0.005重量%至0.05重量%的两亲型非离子界面活性剂。
23.权利要求1的方法,其中该介电层为有机改质硅玻璃。
24.权利要求18的方法,其中该介电层为掺杂碳的二氧化硅。
25.权利要求1的方法,其中该基板还包括金属层。
26.权利要求25的方法,其中该金属层包括铜、钽、钛、钨、铝、镍、铂、钌、铱或铑。
27.权利要求26的方法,其中该金属层包括铜或钽。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/165,100 | 2002-06-07 | ||
| US10/165,100 US6974777B2 (en) | 2002-06-07 | 2002-06-07 | CMP compositions for low-k dielectric materials |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN1659249A CN1659249A (zh) | 2005-08-24 |
| CN1305984C true CN1305984C (zh) | 2007-03-21 |
Family
ID=29710360
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNB038131722A Expired - Lifetime CN1305984C (zh) | 2002-06-07 | 2003-05-26 | 低k介电材料的化学机械抛光方法 |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US6974777B2 (zh) |
| EP (1) | EP1534795B1 (zh) |
| JP (1) | JP4773091B2 (zh) |
| KR (1) | KR100729331B1 (zh) |
| CN (1) | CN1305984C (zh) |
| AT (1) | ATE334176T1 (zh) |
| AU (1) | AU2003274812A1 (zh) |
| DE (1) | DE60307111T2 (zh) |
| SG (1) | SG108491A1 (zh) |
| TW (1) | TWI227728B (zh) |
| WO (1) | WO2003104343A2 (zh) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102399494A (zh) * | 2010-09-10 | 2012-04-04 | 安集微电子(上海)有限公司 | 一种化学机械抛光液 |
| TWI588248B (zh) * | 2013-10-10 | 2017-06-21 | 卡博特微電子公司 | 用於拋光基材之溼式氧化鈰組合物、及其相關方法 |
Families Citing this family (93)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5759917A (en) * | 1996-12-30 | 1998-06-02 | Cabot Corporation | Composition for oxide CMP |
| US7348300B2 (en) * | 1999-05-04 | 2008-03-25 | Air Products And Chemicals, Inc. | Acetylenic diol ethylene oxide/propylene oxide adducts and processes for their manufacture |
| US7208049B2 (en) * | 2003-10-20 | 2007-04-24 | Air Products And Chemicals, Inc. | Process solutions containing surfactants used as post-chemical mechanical planarization treatment |
| US6936543B2 (en) * | 2002-06-07 | 2005-08-30 | Cabot Microelectronics Corporation | CMP method utilizing amphiphilic nonionic surfactants |
| US6905974B2 (en) * | 2002-08-08 | 2005-06-14 | Micron Technology, Inc. | Methods using a peroxide-generating compound to remove group VIII metal-containing residue |
| US7077975B2 (en) * | 2002-08-08 | 2006-07-18 | Micron Technology, Inc. | Methods and compositions for removing group VIII metal-containing materials from surfaces |
| TWI256971B (en) * | 2002-08-09 | 2006-06-21 | Hitachi Chemical Co Ltd | CMP abrasive and method for polishing substrate |
| CN100370587C (zh) * | 2002-09-06 | 2008-02-20 | 旭硝子株式会社 | 绝缘膜研磨剂组合物及半导体集成电路的制造方法 |
| US20040144038A1 (en) * | 2002-12-09 | 2004-07-29 | Junaid Ahmed Siddiqui | Composition and associated method for oxide chemical mechanical planarization |
| US6893476B2 (en) * | 2002-12-09 | 2005-05-17 | Dupont Air Products Nanomaterials Llc | Composition and associated methods for chemical mechanical planarization having high selectivity for metal removal |
| US7553345B2 (en) * | 2002-12-26 | 2009-06-30 | Kao Corporation | Polishing composition |
| US20050097825A1 (en) * | 2003-11-06 | 2005-05-12 | Jinru Bian | Compositions and methods for a barrier removal |
| US20050121969A1 (en) * | 2003-12-04 | 2005-06-09 | Ismail Emesh | Lubricant for wafer polishing using a fixed abrasive pad |
| JP4801326B2 (ja) * | 2004-05-06 | 2011-10-26 | 三井化学株式会社 | 研磨用スラリー |
| JP2006016438A (ja) * | 2004-06-30 | 2006-01-19 | Dongwoo Fine-Chem Co Ltd | 電子部品洗浄液 |
| US7161247B2 (en) * | 2004-07-28 | 2007-01-09 | Cabot Microelectronics Corporation | Polishing composition for noble metals |
| US6979252B1 (en) | 2004-08-10 | 2005-12-27 | Dupont Air Products Nanomaterials Llc | Low defectivity product slurry for CMP and associated production method |
| PL4008949T3 (pl) * | 2004-09-29 | 2024-04-29 | Signify Holding B.V. | Urządzenie oświetleniowe |
| US7563383B2 (en) * | 2004-10-12 | 2009-07-21 | Cabot Mircroelectronics Corporation | CMP composition with a polymer additive for polishing noble metals |
| US7524347B2 (en) * | 2004-10-28 | 2009-04-28 | Cabot Microelectronics Corporation | CMP composition comprising surfactant |
| US7790618B2 (en) * | 2004-12-22 | 2010-09-07 | Rohm And Haas Electronic Materials Cmp Holdings, Inc. | Selective slurry for chemical mechanical polishing |
| US7208325B2 (en) * | 2005-01-18 | 2007-04-24 | Applied Materials, Inc. | Refreshing wafers having low-k dielectric materials |
| JP4740622B2 (ja) * | 2005-03-25 | 2011-08-03 | 株式会社トッパンTdkレーベル | 化学的機械的研磨スラリー |
| US20070039926A1 (en) * | 2005-08-17 | 2007-02-22 | Cabot Microelectronics Corporation | Abrasive-free polishing system |
| US20070077865A1 (en) * | 2005-10-04 | 2007-04-05 | Cabot Microelectronics Corporation | Method for controlling polysilicon removal |
| US7265055B2 (en) * | 2005-10-26 | 2007-09-04 | Cabot Microelectronics Corporation | CMP of copper/ruthenium substrates |
| EP1956642A4 (en) * | 2005-11-11 | 2011-04-06 | Hitachi Chemical Co Ltd | POLISHING AGENT FOR SILICONE OXIDE, LIQUID ADDITIVE AND POLISHING PROCESS |
| KR100643632B1 (ko) * | 2005-12-23 | 2006-11-10 | 제일모직주식회사 | 실리콘 웨이퍼 연마용 슬러리 조성물 및 이를 이용한연마방법 |
| JP2007184395A (ja) * | 2006-01-06 | 2007-07-19 | Fujifilm Corp | 金属用研磨液 |
| JP2007214155A (ja) * | 2006-02-07 | 2007-08-23 | Fujifilm Corp | バリア用研磨液及び化学的機械的研磨方法 |
| US7442323B2 (en) * | 2006-06-02 | 2008-10-28 | E. I. Du Pont De Nemours And Company | Potassium monopersulfate solutions |
| US7678700B2 (en) * | 2006-09-05 | 2010-03-16 | Cabot Microelectronics Corporation | Silicon carbide polishing method utilizing water-soluble oxidizers |
| US9129907B2 (en) * | 2006-09-08 | 2015-09-08 | Cabot Microelectronics Corporation | Onium-containing CMP compositions and methods of use thereof |
| CN101153206A (zh) * | 2006-09-29 | 2008-04-02 | 安集微电子(上海)有限公司 | 用于抛光多晶硅的化学机械抛光液 |
| US20080105652A1 (en) * | 2006-11-02 | 2008-05-08 | Cabot Microelectronics Corporation | CMP of copper/ruthenium/tantalum substrates |
| US7456107B2 (en) * | 2006-11-09 | 2008-11-25 | Cabot Microelectronics Corporation | Compositions and methods for CMP of low-k-dielectric materials |
| JP2008130988A (ja) * | 2006-11-24 | 2008-06-05 | Fujimi Inc | 研磨用組成物及び研磨方法 |
| US20080149591A1 (en) * | 2006-12-21 | 2008-06-26 | Junaid Ahmed Siddiqui | Method and slurry for reducing corrosion on tungsten during chemical mechanical polishing |
| US20080148652A1 (en) * | 2006-12-21 | 2008-06-26 | Junaid Ahmed Siddiqui | Compositions for chemical mechanical planarization of copper |
| US20080149884A1 (en) * | 2006-12-21 | 2008-06-26 | Junaid Ahmed Siddiqui | Method and slurry for tuning low-k versus copper removal rates during chemical mechanical polishing |
| US20080148649A1 (en) * | 2006-12-21 | 2008-06-26 | Zhendong Liu | Ruthenium-barrier polishing slurry |
| JP5371207B2 (ja) * | 2007-06-08 | 2013-12-18 | 富士フイルム株式会社 | 研磨液及び研磨方法 |
| US8008202B2 (en) * | 2007-08-01 | 2011-08-30 | Cabot Microelectronics Corporation | Ruthenium CMP compositions and methods |
| US7915071B2 (en) * | 2007-08-30 | 2011-03-29 | Dupont Air Products Nanomaterials, Llc | Method for chemical mechanical planarization of chalcogenide materials |
| US20090124173A1 (en) * | 2007-11-09 | 2009-05-14 | Cabot Microelectronics Corporation | Compositions and methods for ruthenium and tantalum barrier cmp |
| JP2009164186A (ja) * | 2007-12-28 | 2009-07-23 | Fujimi Inc | 研磨用組成物 |
| JP5220428B2 (ja) * | 2008-02-01 | 2013-06-26 | 株式会社フジミインコーポレーテッド | 研磨用組成物を用いた研磨方法 |
| CN101946309A (zh) * | 2008-02-18 | 2011-01-12 | Jsr株式会社 | 化学机械研磨用水系分散体以及化学机械研磨方法 |
| KR101202720B1 (ko) * | 2008-02-29 | 2012-11-19 | 주식회사 엘지화학 | 화학적 기계적 연마용 수계 슬러리 조성물 및 화학적 기계적 연마 방법 |
| US8058183B2 (en) * | 2008-06-23 | 2011-11-15 | Applied Materials, Inc. | Restoring low dielectric constant film properties |
| JP5467804B2 (ja) * | 2008-07-11 | 2014-04-09 | 富士フイルム株式会社 | 窒化ケイ素用研磨液及び研磨方法 |
| KR101094662B1 (ko) * | 2008-07-24 | 2011-12-20 | 솔브레인 주식회사 | 폴리실리콘 연마정지제를 함유하는 화학 기계적 연마조성물 |
| US8247327B2 (en) * | 2008-07-30 | 2012-08-21 | Cabot Microelectronics Corporation | Methods and compositions for polishing silicon-containing substrates |
| CN102150242B (zh) * | 2008-09-08 | 2013-05-15 | 三菱瓦斯化学株式会社 | 铜布线表面保护液及半导体电路元件的制造方法 |
| US20100081279A1 (en) * | 2008-09-30 | 2010-04-01 | Dupont Air Products Nanomaterials Llc | Method for Forming Through-base Wafer Vias in Fabrication of Stacked Devices |
| JP5362319B2 (ja) * | 2008-10-21 | 2013-12-11 | 花王株式会社 | 研磨液組成物 |
| US8506661B2 (en) * | 2008-10-24 | 2013-08-13 | Air Products & Chemicals, Inc. | Polishing slurry for copper films |
| CN104845532B (zh) | 2009-06-22 | 2017-10-03 | 嘉柏微电子材料股份公司 | 化学机械抛光组合物以及用于抑制多晶硅移除速率的方法 |
| CN102640275B (zh) * | 2009-11-30 | 2015-12-02 | 巴斯夫欧洲公司 | 从衬底去除本体材料层的方法以及适于该方法的化学机械抛光剂 |
| US8916473B2 (en) | 2009-12-14 | 2014-12-23 | Air Products And Chemicals, Inc. | Method for forming through-base wafer vias for fabrication of stacked devices |
| JP5926959B2 (ja) * | 2009-12-22 | 2016-05-25 | 石原産業株式会社 | チタン酸リチウム、該チタン酸リチウムの製造方法、該製造方法に用いるスラリー、該チタン酸リチウムを含む電極活物質及び該電極活物質を用いたリチウム二次電池 |
| JP5587620B2 (ja) * | 2010-01-25 | 2014-09-10 | 株式会社フジミインコーポレーテッド | 研磨用組成物及びそれを用いた研磨方法 |
| JP5492603B2 (ja) * | 2010-03-02 | 2014-05-14 | 株式会社フジミインコーポレーテッド | 研磨用組成物及びそれを用いた研磨方法 |
| JP6101421B2 (ja) * | 2010-08-16 | 2017-03-22 | インテグリス・インコーポレーテッド | 銅または銅合金用エッチング液 |
| TWI565770B (zh) * | 2010-10-07 | 2017-01-11 | 巴斯夫歐洲公司 | 水性研磨組成物及用來化學機械研磨具有經圖案化或未經圖案化低k介電層之基板之方法 |
| US9070632B2 (en) | 2010-10-07 | 2015-06-30 | Basf Se | Aqueous polishing composition and process for chemically mechanically polishing substrates having patterned or unpatterned low-k dielectric layers |
| CN103249790A (zh) | 2010-12-10 | 2013-08-14 | 巴斯夫欧洲公司 | 用于化学机械抛光包含氧化硅电介质和多晶硅膜的基底的含水抛光组合物和方法 |
| KR20140012660A (ko) | 2011-03-11 | 2014-02-03 | 바스프 에스이 | 베이스 웨이퍼 관통 비아들을 형성하는 방법 |
| EP2568024A1 (en) | 2011-09-07 | 2013-03-13 | Basf Se | A chemical mechanical polishing (cmp) composition comprising a glycoside |
| JP6125507B2 (ja) | 2011-09-07 | 2017-05-10 | ビーエーエスエフ ソシエタス・ヨーロピアBasf Se | グリコシドを含む化学機械研磨(cmp)組成物 |
| TWI456013B (zh) * | 2012-04-10 | 2014-10-11 | Uwiz Technology Co Ltd | 研磨液組成物 |
| US9633863B2 (en) | 2012-07-11 | 2017-04-25 | Cabot Microelectronics Corporation | Compositions and methods for selective polishing of silicon nitride materials |
| US20140054266A1 (en) * | 2012-08-24 | 2014-02-27 | Wiechang Jin | Compositions and methods for selective polishing of platinum and ruthenium materials |
| EP2952550A4 (en) * | 2013-02-01 | 2016-09-28 | Fujimi Inc | SURFACE LENS POLISHING COMPOSITION |
| US9284472B2 (en) | 2013-08-09 | 2016-03-15 | Fujimi Incorporated | SiCN and SiN polishing slurries and polishing methods using the same |
| US20170243752A1 (en) * | 2014-08-29 | 2017-08-24 | Fujimi Incorporated | Polishing composition and method for producing polishing composition |
| KR102380774B1 (ko) | 2014-11-14 | 2022-04-04 | 삼성전자주식회사 | 슬러리 화합물 이를 이용한 반도체 소자의 제조 방법 |
| BR112017011562A2 (pt) * | 2014-12-05 | 2018-01-02 | 3M Innovative Properties Co | composição abrasiva |
| CN105619267B (zh) * | 2016-03-01 | 2018-02-02 | 中国科学院微电子研究所 | 一种抛光粒子的设计方法、抛光粒子及研磨液 |
| US10711159B2 (en) * | 2016-12-30 | 2020-07-14 | Fujifilm Electronic Materials U.S.A., Inc. | Polishing compositions |
| US10181408B2 (en) | 2017-01-31 | 2019-01-15 | Rohm And Haas Electronic Materials Cmp Holdings, Inc. | Chemical mechanical polishing method for tungsten using polyglycols and polyglycol derivatives |
| KR20190074597A (ko) | 2017-12-20 | 2019-06-28 | 주식회사 케이씨텍 | Sti 공정용 연마 슬러리 조성물 |
| JP7316797B2 (ja) * | 2018-09-04 | 2023-07-28 | 株式会社フジミインコーポレーテッド | 研磨用組成物および研磨システム |
| US11260420B2 (en) * | 2018-10-17 | 2022-03-01 | Portland State University | Nanowires with magnetic coatings and methods for making and using |
| KR102279324B1 (ko) * | 2018-12-21 | 2021-07-21 | 주식회사 케이씨텍 | 연마 슬러리 조성물 |
| US11001733B2 (en) * | 2019-03-29 | 2021-05-11 | Fujimi Incorporated | Compositions for polishing cobalt and low-K material surfaces |
| KR102676957B1 (ko) * | 2019-04-02 | 2024-06-19 | 가부시끼가이샤 레조낙 | 연마액, 연마액 세트, 연마 방법 및 결함 억제 방법 |
| US10787592B1 (en) * | 2019-05-16 | 2020-09-29 | Rohm And Haas Electronic Materials Cmp Holdings, I | Chemical mechanical polishing compositions and methods having enhanced defect inhibition and selectively polishing silicon nitride over silicon dioxide in an acid environment |
| JP7340969B2 (ja) | 2019-06-28 | 2023-09-08 | 東京応化工業株式会社 | シリコンエッチング液、シリコンエッチング方法、及びシリコンフィン構造体の製造方法 |
| KR20220065996A (ko) * | 2019-09-24 | 2022-05-23 | 후지필름 일렉트로닉 머티리얼스 유.에스.에이., 아이엔씨. | 연마 조성물 및 이의 사용 방법 |
| KR102637819B1 (ko) * | 2020-03-31 | 2024-02-16 | 삼성에스디아이 주식회사 | 텅스텐 패턴 웨이퍼 연마용 cmp 슬러리 조성물 및 이를 이용한 텅스텐 패턴 웨이퍼의 연마 방법 |
| KR102708256B1 (ko) * | 2021-11-29 | 2024-09-24 | 주식회사 케이씨텍 | Cmp 슬러리 조성물 |
| WO2023186762A1 (en) | 2022-03-31 | 2023-10-05 | Basf Se | Compositions and methods for tungsten etching inhibition |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1289811A (zh) * | 1999-09-27 | 2001-04-04 | 不二见美国股份有限公司 | 抛光组合物 |
| CN1323027A (zh) * | 2000-04-28 | 2001-11-21 | 三井金属矿业株式会社 | 磁记录介质用玻璃基板的制造方法 |
| CN1334849A (zh) * | 1998-09-24 | 2002-02-06 | 联合讯号公司 | 用于低介电常数材料的氧化抛光淤浆 |
Family Cites Families (48)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4752628A (en) * | 1987-05-15 | 1988-06-21 | Nalco Chemical Company | Concentrated lapping slurries |
| US4867757A (en) * | 1988-09-09 | 1989-09-19 | Nalco Chemical Company | Lapping slurry compositions with improved lap rate |
| US5352277A (en) * | 1988-12-12 | 1994-10-04 | E. I. Du Pont De Nemours & Company | Final polishing composition |
| US5123958A (en) * | 1990-05-25 | 1992-06-23 | Wiand Ronald C | Polishing composition and method |
| AU648704B2 (en) * | 1991-11-25 | 1994-04-28 | National Starch And Chemical Investment Holding Corporation | Method of extruding starch under low moisture conditions using feed starch having coarse particle size |
| TW274625B (zh) * | 1994-09-30 | 1996-04-21 | Hitachi Seisakusyo Kk | |
| US5527423A (en) * | 1994-10-06 | 1996-06-18 | Cabot Corporation | Chemical mechanical polishing slurry for metal layers |
| US5860848A (en) * | 1995-06-01 | 1999-01-19 | Rodel, Inc. | Polishing silicon wafers with improved polishing slurries |
| US5958794A (en) * | 1995-09-22 | 1999-09-28 | Minnesota Mining And Manufacturing Company | Method of modifying an exposed surface of a semiconductor wafer |
| KR100336598B1 (ko) * | 1996-02-07 | 2002-05-16 | 이사오 우치가사키 | 산화 세륨 연마제 제조용 산화 세륨 입자 |
| EP0810302B1 (en) | 1996-05-30 | 2001-08-01 | Nalco Chemical Company | Use of a mixture of surfactants for corrosion inhibiting |
| EP0852615B1 (en) * | 1996-07-25 | 2005-12-14 | DuPont Air Products NanoMaterials L.L.C. | Chemical mechanical polishing composition and process |
| US6132637A (en) * | 1996-09-27 | 2000-10-17 | Rodel Holdings, Inc. | Composition and method for polishing a composite of silica and silicon nitride |
| US5876490A (en) * | 1996-12-09 | 1999-03-02 | International Business Machines Corporatin | Polish process and slurry for planarization |
| US6153525A (en) * | 1997-03-13 | 2000-11-28 | Alliedsignal Inc. | Methods for chemical mechanical polish of organic polymer dielectric films |
| CN1258241A (zh) * | 1997-04-18 | 2000-06-28 | 卡伯特公司 | 用于半导体底物的抛光垫 |
| US6126532A (en) * | 1997-04-18 | 2000-10-03 | Cabot Corporation | Polishing pads for a semiconductor substrate |
| US6099604A (en) * | 1997-08-21 | 2000-08-08 | Micron Technology, Inc. | Slurry with chelating agent for chemical-mechanical polishing of a semiconductor wafer and methods related thereto |
| JP3371775B2 (ja) * | 1997-10-31 | 2003-01-27 | 株式会社日立製作所 | 研磨方法 |
| US6063306A (en) * | 1998-06-26 | 2000-05-16 | Cabot Corporation | Chemical mechanical polishing slurry useful for copper/tantalum substrate |
| US6117000A (en) * | 1998-07-10 | 2000-09-12 | Cabot Corporation | Polishing pad for a semiconductor substrate |
| TW455626B (en) * | 1998-07-23 | 2001-09-21 | Eternal Chemical Co Ltd | Chemical mechanical abrasive composition for use in semiconductor processing |
| JP2000109816A (ja) * | 1998-10-05 | 2000-04-18 | Okamoto Machine Tool Works Ltd | 研磨剤スラリ−の調製方法 |
| SG78405A1 (en) * | 1998-11-17 | 2001-02-20 | Fujimi Inc | Polishing composition and rinsing composition |
| US6046112A (en) * | 1998-12-14 | 2000-04-04 | Taiwan Semiconductor Manufacturing Company | Chemical mechanical polishing slurry |
| KR100475976B1 (ko) * | 1998-12-25 | 2005-03-15 | 히다치 가세고교 가부시끼가이샤 | Cmp 연마제, cmp 연마제용 첨가액 및 기판의 연마방법 |
| KR100472882B1 (ko) * | 1999-01-18 | 2005-03-07 | 가부시끼가이샤 도시바 | 수계 분산체, 이를 이용한 화학 기계 연마용 수계 분산체조성물, 웨이퍼 표면의 연마 방법 및 반도체 장치의 제조방법 |
| US6426295B1 (en) * | 1999-02-16 | 2002-07-30 | Micron Technology, Inc. | Reduction of surface roughness during chemical mechanical planarization(CMP) |
| KR20010111261A (ko) | 1999-02-18 | 2001-12-17 | 갤반 마틴 | 유전율이 낮은 중합체 층의 cmp법 |
| US20010013507A1 (en) * | 1999-02-18 | 2001-08-16 | Hosali Sharath D. | Method for CMP of low dielectric constant polymer layers |
| US6375693B1 (en) * | 1999-05-07 | 2002-04-23 | International Business Machines Corporation | Chemical-mechanical planarization of barriers or liners for copper metallurgy |
| JP2001015462A (ja) * | 1999-06-30 | 2001-01-19 | Toshiba Corp | スラリー、cmp法および半導体装置の製造方法 |
| US20010054706A1 (en) * | 1999-07-19 | 2001-12-27 | Joseph A. Levert | Compositions and processes for spin etch planarization |
| US6376381B1 (en) * | 1999-08-31 | 2002-04-23 | Micron Technology, Inc. | Planarizing solutions, planarizing machines, and methods for mechanical and/or chemical-mechanical planarization of microelectronic substrate assemblies |
| TW499471B (en) * | 1999-09-01 | 2002-08-21 | Eternal Chemical Co Ltd | Chemical mechanical/abrasive composition for semiconductor processing |
| JP4075247B2 (ja) * | 1999-09-30 | 2008-04-16 | Jsr株式会社 | 化学機械研磨用水系分散体 |
| US6348076B1 (en) * | 1999-10-08 | 2002-02-19 | International Business Machines Corporation | Slurry for mechanical polishing (CMP) of metals and use thereof |
| US6503418B2 (en) | 1999-11-04 | 2003-01-07 | Advanced Micro Devices, Inc. | Ta barrier slurry containing an organic additive |
| JP3490038B2 (ja) * | 1999-12-28 | 2004-01-26 | Necエレクトロニクス株式会社 | 金属配線形成方法 |
| JP3841995B2 (ja) * | 1999-12-28 | 2006-11-08 | Necエレクトロニクス株式会社 | 化学的機械的研磨用スラリー |
| TW572980B (en) * | 2000-01-12 | 2004-01-21 | Jsr Corp | Aqueous dispersion for chemical mechanical polishing and chemical mechanical polishing process |
| JP4001219B2 (ja) * | 2000-10-12 | 2007-10-31 | Jsr株式会社 | 化学機械研磨用水系分散体及び化学機械研磨方法 |
| JP2001269859A (ja) * | 2000-03-27 | 2001-10-02 | Jsr Corp | 化学機械研磨用水系分散体 |
| JP4253141B2 (ja) * | 2000-08-21 | 2009-04-08 | 株式会社東芝 | 化学機械研磨用スラリおよび半導体装置の製造方法 |
| KR100481651B1 (ko) * | 2000-08-21 | 2005-04-08 | 가부시끼가이샤 도시바 | 화학 기계 연마용 슬러리 및 반도체 장치의 제조 방법 |
| JP3768401B2 (ja) * | 2000-11-24 | 2006-04-19 | Necエレクトロニクス株式会社 | 化学的機械的研磨用スラリー |
| JP2002161267A (ja) * | 2000-11-27 | 2002-06-04 | Hitachi Chem Co Ltd | 白金族金属用研磨液及びそれを用いた研磨方法 |
| JP4009986B2 (ja) * | 2000-11-29 | 2007-11-21 | 株式会社フジミインコーポレーテッド | 研磨用組成物、およびそれを用いてメモリーハードディスクを研磨する研磨方法 |
-
2002
- 2002-06-07 US US10/165,100 patent/US6974777B2/en not_active Expired - Lifetime
-
2003
- 2003-05-26 JP JP2004511407A patent/JP4773091B2/ja not_active Expired - Fee Related
- 2003-05-26 AU AU2003274812A patent/AU2003274812A1/en not_active Abandoned
- 2003-05-26 SG SG2004071809A patent/SG108491A1/en unknown
- 2003-05-26 WO PCT/IB2003/002266 patent/WO2003104343A2/en active IP Right Grant
- 2003-05-26 KR KR1020047019821A patent/KR100729331B1/ko active IP Right Grant
- 2003-05-26 AT AT03740859T patent/ATE334176T1/de not_active IP Right Cessation
- 2003-05-26 CN CNB038131722A patent/CN1305984C/zh not_active Expired - Lifetime
- 2003-05-26 EP EP03740859A patent/EP1534795B1/en not_active Expired - Lifetime
- 2003-05-26 DE DE60307111T patent/DE60307111T2/de not_active Expired - Lifetime
- 2003-06-06 TW TW092115419A patent/TWI227728B/zh not_active IP Right Cessation
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1334849A (zh) * | 1998-09-24 | 2002-02-06 | 联合讯号公司 | 用于低介电常数材料的氧化抛光淤浆 |
| CN1289811A (zh) * | 1999-09-27 | 2001-04-04 | 不二见美国股份有限公司 | 抛光组合物 |
| CN1323027A (zh) * | 2000-04-28 | 2001-11-21 | 三井金属矿业株式会社 | 磁记录介质用玻璃基板的制造方法 |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102399494A (zh) * | 2010-09-10 | 2012-04-04 | 安集微电子(上海)有限公司 | 一种化学机械抛光液 |
| CN102399494B (zh) * | 2010-09-10 | 2014-12-31 | 安集微电子(上海)有限公司 | 一种化学机械抛光液 |
| TWI588248B (zh) * | 2013-10-10 | 2017-06-21 | 卡博特微電子公司 | 用於拋光基材之溼式氧化鈰組合物、及其相關方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| DE60307111D1 (de) | 2006-09-07 |
| EP1534795B1 (en) | 2006-07-26 |
| EP1534795A2 (en) | 2005-06-01 |
| TWI227728B (en) | 2005-02-11 |
| US6974777B2 (en) | 2005-12-13 |
| AU2003274812A1 (en) | 2003-12-22 |
| CN1659249A (zh) | 2005-08-24 |
| AU2003274812A8 (en) | 2003-12-22 |
| TW200401819A (en) | 2004-02-01 |
| SG108491A1 (en) | 2007-01-31 |
| KR20050005543A (ko) | 2005-01-13 |
| WO2003104343A2 (en) | 2003-12-18 |
| JP2005529485A (ja) | 2005-09-29 |
| US20030228762A1 (en) | 2003-12-11 |
| WO2003104343A3 (en) | 2004-02-26 |
| JP4773091B2 (ja) | 2011-09-14 |
| KR100729331B1 (ko) | 2007-06-15 |
| ATE334176T1 (de) | 2006-08-15 |
| DE60307111T2 (de) | 2006-11-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1305984C (zh) | 低k介电材料的化学机械抛光方法 | |
| JP5964795B2 (ja) | 両親媒性非イオン性界面活性剤を利用したcmp法 | |
| JP5468903B2 (ja) | Low−k誘電体材料のcmp用組成物及び方法 | |
| JP4782673B2 (ja) | Cmp用被覆金属酸化物粒子 | |
| KR101477360B1 (ko) | 폴리규소 제거 속도를 억제하기 위한 cmp 조성물 및 방법 | |
| CN1753962A (zh) | 可调控的去除阻隔物的抛光浆料 | |
| TW201412908A (zh) | 用於氮化矽材料之選擇性拋光之組合物及方法 | |
| JP3849091B2 (ja) | 化学機械研磨用水系分散体 | |
| EP1535979A2 (en) | Compositions and methods for chemical mechanical polishing silica and silicon nitride | |
| JP6999714B2 (ja) | シャロートレンチアイソレーション化学機械研磨スラリー | |
| KR102544607B1 (ko) | 화학적 기계적 연마 슬러리 조성물 및 이를 이용한 반도체 소자의 제조방법 | |
| JP2016003275A (ja) | タングステン系材料用研磨剤、研磨剤用貯蔵液、及び研磨方法 | |
| TW202303732A (zh) | 研磨用組合物、研磨方法及研磨完成基板的製造方法 | |
| CN118562394A (zh) | 化学机械抛光组合物及抛光方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| CP01 | Change in the name or title of a patent holder |
Address after: Illinois, USA Patentee after: CMC Materials Co.,Ltd. Address before: Illinois, USA Patentee before: CABOT MICROELECTRONICS Corp. |
|
| CP01 | Change in the name or title of a patent holder | ||
| CX01 | Expiry of patent term |
Granted publication date: 20070321 |
|
| CX01 | Expiry of patent term |