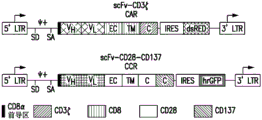
CN112458057A - 用于免疫疗法的组合物和方法 - Google Patents
用于免疫疗法的组合物和方法 Download PDFInfo
- Publication number
- CN112458057A CN112458057A CN202011103486.3A CN202011103486A CN112458057A CN 112458057 A CN112458057 A CN 112458057A CN 202011103486 A CN202011103486 A CN 202011103486A CN 112458057 A CN112458057 A CN 112458057A
- Authority
- CN
- China
- Prior art keywords
- antigen
- cells
- cell
- receptor
- tumor
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000000034 method Methods 0.000 title claims abstract description 108
- 239000000203 mixture Substances 0.000 title description 36
- 238000009169 immunotherapy Methods 0.000 title description 6
- 210000004027 cell Anatomy 0.000 claims abstract description 332
- 239000000427 antigen Substances 0.000 claims abstract description 307
- 108091007433 antigens Proteins 0.000 claims abstract description 307
- 102000036639 antigens Human genes 0.000 claims abstract description 307
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 241
- 210000001744 T-lymphocyte Anatomy 0.000 claims abstract description 143
- 108091008034 costimulatory receptors Proteins 0.000 claims abstract description 101
- 108020003175 receptors Proteins 0.000 claims abstract description 89
- 230000028993 immune response Effects 0.000 claims abstract description 48
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 claims abstract description 29
- 210000003289 regulatory T cell Anatomy 0.000 claims abstract description 9
- 102000005962 receptors Human genes 0.000 claims description 88
- 108010019670 Chimeric Antigen Receptors Proteins 0.000 claims description 87
- 102100041003 Glutamate carboxypeptidase 2 Human genes 0.000 claims description 72
- 101000892862 Homo sapiens Glutamate carboxypeptidase 2 Proteins 0.000 claims description 72
- 102100036735 Prostate stem cell antigen Human genes 0.000 claims description 61
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 claims description 48
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 claims description 48
- 241000701022 Cytomegalovirus Species 0.000 claims description 46
- 239000013598 vector Substances 0.000 claims description 45
- -1 EGP-40 Proteins 0.000 claims description 41
- 239000003446 ligand Substances 0.000 claims description 39
- 150000007523 nucleic acids Chemical class 0.000 claims description 36
- 102100025475 Carcinoembryonic antigen-related cell adhesion molecule 5 Human genes 0.000 claims description 30
- 102000039446 nucleic acids Human genes 0.000 claims description 29
- 108020004707 nucleic acids Proteins 0.000 claims description 29
- 108090000623 proteins and genes Proteins 0.000 claims description 29
- 108091008874 T cell receptors Proteins 0.000 claims description 28
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 claims description 28
- 210000004881 tumor cell Anatomy 0.000 claims description 28
- 102000004169 proteins and genes Human genes 0.000 claims description 27
- 102000003735 Mesothelin Human genes 0.000 claims description 26
- 108090000015 Mesothelin Proteins 0.000 claims description 26
- 238000011282 treatment Methods 0.000 claims description 26
- 108010022366 Carcinoembryonic Antigen Proteins 0.000 claims description 25
- 102100032912 CD44 antigen Human genes 0.000 claims description 22
- 101000868273 Homo sapiens CD44 antigen Proteins 0.000 claims description 22
- 101000610551 Homo sapiens Prominin-1 Proteins 0.000 claims description 22
- 102100040120 Prominin-1 Human genes 0.000 claims description 22
- 108010066687 Epithelial Cell Adhesion Molecule Proteins 0.000 claims description 21
- 102000018651 Epithelial Cell Adhesion Molecule Human genes 0.000 claims description 21
- 230000004913 activation Effects 0.000 claims description 21
- 230000001717 pathogenic effect Effects 0.000 claims description 21
- 244000052769 pathogen Species 0.000 claims description 20
- 206010060862 Prostate cancer Diseases 0.000 claims description 19
- 229920001481 poly(stearyl methacrylate) Polymers 0.000 claims description 19
- 101000851370 Homo sapiens Tumor necrosis factor receptor superfamily member 9 Proteins 0.000 claims description 18
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 18
- 102100036856 Tumor necrosis factor receptor superfamily member 9 Human genes 0.000 claims description 18
- 102100025570 Cancer/testis antigen 1 Human genes 0.000 claims description 17
- 101000856237 Homo sapiens Cancer/testis antigen 1 Proteins 0.000 claims description 17
- 108010053099 Vascular Endothelial Growth Factor Receptor-2 Proteins 0.000 claims description 17
- 102100033177 Vascular endothelial growth factor receptor 2 Human genes 0.000 claims description 17
- 230000011664 signaling Effects 0.000 claims description 16
- 101001109501 Homo sapiens NKG2-D type II integral membrane protein Proteins 0.000 claims description 15
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 claims description 15
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 claims description 15
- 102100022680 NKG2-D type II integral membrane protein Human genes 0.000 claims description 15
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 claims description 15
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 claims description 15
- 101000994365 Homo sapiens Integrin alpha-6 Proteins 0.000 claims description 14
- 101000581981 Homo sapiens Neural cell adhesion molecule 1 Proteins 0.000 claims description 14
- 102100032816 Integrin alpha-6 Human genes 0.000 claims description 14
- 102000003729 Neprilysin Human genes 0.000 claims description 14
- 108090000028 Neprilysin Proteins 0.000 claims description 14
- 102100027347 Neural cell adhesion molecule 1 Human genes 0.000 claims description 14
- 230000004068 intracellular signaling Effects 0.000 claims description 14
- 239000000126 substance Substances 0.000 claims description 14
- 102100031940 Epithelial cell adhesion molecule Human genes 0.000 claims description 13
- 101000920667 Homo sapiens Epithelial cell adhesion molecule Proteins 0.000 claims description 13
- 229940014144 folate Drugs 0.000 claims description 13
- OVBPIULPVIDEAO-LBPRGKRZSA-N folic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-LBPRGKRZSA-N 0.000 claims description 13
- 235000019152 folic acid Nutrition 0.000 claims description 13
- 239000011724 folic acid Substances 0.000 claims description 13
- 102000040430 polynucleotide Human genes 0.000 claims description 13
- 108091033319 polynucleotide Proteins 0.000 claims description 13
- 239000002157 polynucleotide Substances 0.000 claims description 13
- 230000001177 retroviral effect Effects 0.000 claims description 13
- 210000004698 lymphocyte Anatomy 0.000 claims description 12
- 101001133056 Homo sapiens Mucin-1 Proteins 0.000 claims description 11
- 102100034256 Mucin-1 Human genes 0.000 claims description 11
- 206010006187 Breast cancer Diseases 0.000 claims description 10
- 101000874179 Homo sapiens Syndecan-1 Proteins 0.000 claims description 10
- 102100035721 Syndecan-1 Human genes 0.000 claims description 10
- 208000026310 Breast neoplasm Diseases 0.000 claims description 9
- 230000001939 inductive effect Effects 0.000 claims description 9
- 239000008194 pharmaceutical composition Substances 0.000 claims description 9
- 108700012439 CA9 Proteins 0.000 claims description 8
- 102100024423 Carbonic anhydrase 9 Human genes 0.000 claims description 8
- 108010009685 Cholinergic Receptors Proteins 0.000 claims description 8
- 206010033128 Ovarian cancer Diseases 0.000 claims description 8
- 206010035226 Plasma cell myeloma Diseases 0.000 claims description 8
- 102000034337 acetylcholine receptors Human genes 0.000 claims description 8
- 230000003213 activating effect Effects 0.000 claims description 8
- 230000001605 fetal effect Effects 0.000 claims description 8
- 102100031585 ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 Human genes 0.000 claims description 7
- 102100038080 B-cell receptor CD22 Human genes 0.000 claims description 7
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 claims description 7
- 102100030595 HLA class II histocompatibility antigen gamma chain Human genes 0.000 claims description 7
- 102100031573 Hematopoietic progenitor cell antigen CD34 Human genes 0.000 claims description 7
- 101000777636 Homo sapiens ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 Proteins 0.000 claims description 7
- 101000884305 Homo sapiens B-cell receptor CD22 Proteins 0.000 claims description 7
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 claims description 7
- 101001082627 Homo sapiens HLA class II histocompatibility antigen gamma chain Proteins 0.000 claims description 7
- 101000777663 Homo sapiens Hematopoietic progenitor cell antigen CD34 Proteins 0.000 claims description 7
- 101001078143 Homo sapiens Integrin alpha-IIb Proteins 0.000 claims description 7
- 101000934338 Homo sapiens Myeloid cell surface antigen CD33 Proteins 0.000 claims description 7
- 101000914496 Homo sapiens T-cell antigen CD7 Proteins 0.000 claims description 7
- 101000851376 Homo sapiens Tumor necrosis factor receptor superfamily member 8 Proteins 0.000 claims description 7
- 102100025306 Integrin alpha-IIb Human genes 0.000 claims description 7
- 208000034578 Multiple myelomas Diseases 0.000 claims description 7
- 102100025243 Myeloid cell surface antigen CD33 Human genes 0.000 claims description 7
- 206010061535 Ovarian neoplasm Diseases 0.000 claims description 7
- 102100027208 T-cell antigen CD7 Human genes 0.000 claims description 7
- 102100036857 Tumor necrosis factor receptor superfamily member 8 Human genes 0.000 claims description 7
- 230000030833 cell death Effects 0.000 claims description 7
- 230000001461 cytolytic effect Effects 0.000 claims description 7
- 208000004736 B-Cell Leukemia Diseases 0.000 claims description 6
- 102100022153 Tumor necrosis factor receptor superfamily member 4 Human genes 0.000 claims description 6
- 101710165473 Tumor necrosis factor receptor superfamily member 4 Proteins 0.000 claims description 6
- 210000001778 pluripotent stem cell Anatomy 0.000 claims description 6
- 239000013603 viral vector Substances 0.000 claims description 6
- 102100026423 Adhesion G protein-coupled receptor E5 Human genes 0.000 claims description 5
- 102100027207 CD27 antigen Human genes 0.000 claims description 5
- 102100032937 CD40 ligand Human genes 0.000 claims description 5
- 102000016289 Cell Adhesion Molecules Human genes 0.000 claims description 5
- 108010067225 Cell Adhesion Molecules Proteins 0.000 claims description 5
- 101000718243 Homo sapiens Adhesion G protein-coupled receptor E5 Proteins 0.000 claims description 5
- 101000914511 Homo sapiens CD27 antigen Proteins 0.000 claims description 5
- 101000868215 Homo sapiens CD40 ligand Proteins 0.000 claims description 5
- 230000000139 costimulatory effect Effects 0.000 claims description 5
- 238000010494 dissociation reaction Methods 0.000 claims description 5
- 230000005593 dissociations Effects 0.000 claims description 5
- 230000002265 prevention Effects 0.000 claims description 5
- 238000004519 manufacturing process Methods 0.000 claims description 4
- 101150047061 tag-72 gene Proteins 0.000 claims description 4
- 239000003814 drug Substances 0.000 claims description 3
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 3
- 230000003834 intracellular effect Effects 0.000 claims 20
- 101001136592 Homo sapiens Prostate stem cell antigen Proteins 0.000 claims 10
- 101000914324 Homo sapiens Carcinoembryonic antigen-related cell adhesion molecule 5 Proteins 0.000 claims 5
- 101000914321 Homo sapiens Carcinoembryonic antigen-related cell adhesion molecule 7 Proteins 0.000 claims 5
- 101000617725 Homo sapiens Pregnancy-specific beta-1-glycoprotein 2 Proteins 0.000 claims 5
- 101001103039 Homo sapiens Inactive tyrosine-protein kinase transmembrane receptor ROR1 Proteins 0.000 claims 3
- 101001103036 Homo sapiens Nuclear receptor ROR-alpha Proteins 0.000 claims 3
- 102100039615 Inactive tyrosine-protein kinase transmembrane receptor ROR1 Human genes 0.000 claims 3
- 102000010451 Folate receptor alpha Human genes 0.000 claims 2
- 108050001931 Folate receptor alpha Proteins 0.000 claims 2
- 230000009826 neoplastic cell growth Effects 0.000 abstract description 50
- 230000001965 increasing effect Effects 0.000 abstract description 11
- 230000007170 pathology Effects 0.000 abstract description 4
- 101710120463 Prostate stem cell antigen Proteins 0.000 description 51
- 108090000765 processed proteins & peptides Proteins 0.000 description 50
- 229920001184 polypeptide Polymers 0.000 description 46
- 102000004196 processed proteins & peptides Human genes 0.000 description 46
- 241000699670 Mus sp. Species 0.000 description 34
- 241000282414 Homo sapiens Species 0.000 description 30
- 230000014509 gene expression Effects 0.000 description 27
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 26
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 26
- 235000018102 proteins Nutrition 0.000 description 25
- 241000194017 Streptococcus Species 0.000 description 23
- 241000700605 Viruses Species 0.000 description 23
- 230000000694 effects Effects 0.000 description 19
- 208000015181 infectious disease Diseases 0.000 description 19
- 210000001519 tissue Anatomy 0.000 description 19
- 201000010099 disease Diseases 0.000 description 18
- 230000008029 eradication Effects 0.000 description 18
- 239000012634 fragment Substances 0.000 description 17
- 230000008685 targeting Effects 0.000 description 16
- 102000004127 Cytokines Human genes 0.000 description 15
- 108090000695 Cytokines Proteins 0.000 description 15
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 14
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 14
- 150000001413 amino acids Chemical group 0.000 description 14
- 239000011780 sodium chloride Substances 0.000 description 13
- 230000003612 virological effect Effects 0.000 description 12
- 241000880493 Leptailurus serval Species 0.000 description 11
- 230000006044 T cell activation Effects 0.000 description 11
- 238000002474 experimental method Methods 0.000 description 11
- 238000002347 injection Methods 0.000 description 11
- 239000007924 injection Substances 0.000 description 11
- 239000001509 sodium citrate Substances 0.000 description 11
- 230000009885 systemic effect Effects 0.000 description 11
- 230000001225 therapeutic effect Effects 0.000 description 11
- 238000010361 transduction Methods 0.000 description 11
- 230000026683 transduction Effects 0.000 description 11
- HRXKRNGNAMMEHJ-UHFFFAOYSA-K trisodium citrate Chemical compound [Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O HRXKRNGNAMMEHJ-UHFFFAOYSA-K 0.000 description 11
- 229940038773 trisodium citrate Drugs 0.000 description 11
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 10
- SOEGEPHNZOISMT-BYPYZUCNSA-N Gly-Ser-Gly Chemical compound NCC(=O)N[C@@H](CO)C(=O)NCC(O)=O SOEGEPHNZOISMT-BYPYZUCNSA-N 0.000 description 10
- 229940024606 amino acid Drugs 0.000 description 10
- 230000001404 mediated effect Effects 0.000 description 10
- 201000009030 Carcinoma Diseases 0.000 description 9
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 9
- 101001039269 Rattus norvegicus Glycine N-methyltransferase Proteins 0.000 description 9
- 235000001014 amino acid Nutrition 0.000 description 9
- 238000013459 approach Methods 0.000 description 9
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Chemical compound NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 9
- 108010001064 glycyl-glycyl-glycyl-glycine Proteins 0.000 description 9
- 238000009396 hybridization Methods 0.000 description 9
- 230000007774 longterm Effects 0.000 description 9
- 230000035755 proliferation Effects 0.000 description 9
- 230000000638 stimulation Effects 0.000 description 9
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 8
- SITLTJHOQZFJGG-UHFFFAOYSA-N N-L-alpha-glutamyl-L-valine Natural products CC(C)C(C(O)=O)NC(=O)C(N)CCC(O)=O SITLTJHOQZFJGG-UHFFFAOYSA-N 0.000 description 8
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 8
- 208000035475 disorder Diseases 0.000 description 8
- 230000006870 function Effects 0.000 description 8
- 108010089804 glycyl-threonine Proteins 0.000 description 8
- 210000000822 natural killer cell Anatomy 0.000 description 8
- 210000000056 organ Anatomy 0.000 description 8
- 230000004936 stimulating effect Effects 0.000 description 8
- 238000005406 washing Methods 0.000 description 8
- 102000006306 Antigen Receptors Human genes 0.000 description 7
- 108010083359 Antigen Receptors Proteins 0.000 description 7
- 102000017420 CD3 protein, epsilon/gamma/delta subunit Human genes 0.000 description 7
- 108050005493 CD3 protein, epsilon/gamma/delta subunit Proteins 0.000 description 7
- 241000711573 Coronaviridae Species 0.000 description 7
- 101000934341 Homo sapiens T-cell surface glycoprotein CD5 Proteins 0.000 description 7
- 102100025244 T-cell surface glycoprotein CD5 Human genes 0.000 description 7
- 230000000890 antigenic effect Effects 0.000 description 7
- 230000009089 cytolysis Effects 0.000 description 7
- 210000002865 immune cell Anatomy 0.000 description 7
- 238000000338 in vitro Methods 0.000 description 7
- 238000001727 in vivo Methods 0.000 description 7
- 208000023958 prostate neoplasm Diseases 0.000 description 7
- 230000004044 response Effects 0.000 description 7
- 208000024891 symptom Diseases 0.000 description 7
- 206010061598 Immunodeficiency Diseases 0.000 description 6
- 241001465754 Metazoa Species 0.000 description 6
- 108091028043 Nucleic acid sequence Proteins 0.000 description 6
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 6
- 239000000654 additive Substances 0.000 description 6
- 238000004458 analytical method Methods 0.000 description 6
- 108010068380 arginylarginine Proteins 0.000 description 6
- 201000011510 cancer Diseases 0.000 description 6
- 230000001413 cellular effect Effects 0.000 description 6
- 108700010039 chimeric receptor Proteins 0.000 description 6
- 108010050848 glycylleucine Proteins 0.000 description 6
- 239000005090 green fluorescent protein Substances 0.000 description 6
- 238000004020 luminiscence type Methods 0.000 description 6
- 230000004048 modification Effects 0.000 description 6
- 238000012986 modification Methods 0.000 description 6
- 230000036515 potency Effects 0.000 description 6
- 230000009257 reactivity Effects 0.000 description 6
- 230000002829 reductive effect Effects 0.000 description 6
- 241000894007 species Species 0.000 description 6
- 208000035473 Communicable disease Diseases 0.000 description 5
- 108020004414 DNA Proteins 0.000 description 5
- 101000946843 Homo sapiens T-cell surface glycoprotein CD8 alpha chain Proteins 0.000 description 5
- 101000655352 Homo sapiens Telomerase reverse transcriptase Proteins 0.000 description 5
- 241000701044 Human gammaherpesvirus 4 Species 0.000 description 5
- 241000725303 Human immunodeficiency virus Species 0.000 description 5
- KAFOIVJDVSZUMD-UHFFFAOYSA-N Leu-Gln-Gln Natural products CC(C)CC(N)C(=O)NC(CCC(N)=O)C(=O)NC(CCC(N)=O)C(O)=O KAFOIVJDVSZUMD-UHFFFAOYSA-N 0.000 description 5
- 206010027476 Metastases Diseases 0.000 description 5
- HJEBZBMOTCQYDN-ACZMJKKPSA-N Ser-Glu-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O HJEBZBMOTCQYDN-ACZMJKKPSA-N 0.000 description 5
- 244000057717 Streptococcus lactis Species 0.000 description 5
- 235000014897 Streptococcus lactis Nutrition 0.000 description 5
- 230000006052 T cell proliferation Effects 0.000 description 5
- 102100034922 T-cell surface glycoprotein CD8 alpha chain Human genes 0.000 description 5
- VRUFCJZQDACGLH-UVOCVTCTSA-N Thr-Leu-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VRUFCJZQDACGLH-UVOCVTCTSA-N 0.000 description 5
- UPODKYBYUBTWSV-BZSNNMDCSA-N Tyr-Phe-Cys Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CS)C(O)=O)C1=CC=C(O)C=C1 UPODKYBYUBTWSV-BZSNNMDCSA-N 0.000 description 5
- 108010081404 acein-2 Proteins 0.000 description 5
- 208000009956 adenocarcinoma Diseases 0.000 description 5
- 108010077245 asparaginyl-proline Proteins 0.000 description 5
- 238000003556 assay Methods 0.000 description 5
- 230000001086 cytosolic effect Effects 0.000 description 5
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 description 5
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 5
- 239000007788 liquid Substances 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 201000001441 melanoma Diseases 0.000 description 5
- 230000009401 metastasis Effects 0.000 description 5
- 210000005259 peripheral blood Anatomy 0.000 description 5
- 239000011886 peripheral blood Substances 0.000 description 5
- 210000002307 prostate Anatomy 0.000 description 5
- 230000001105 regulatory effect Effects 0.000 description 5
- 210000000130 stem cell Anatomy 0.000 description 5
- 230000009385 viral infection Effects 0.000 description 5
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 4
- 241000709661 Enterovirus Species 0.000 description 4
- JXFLPKSDLDEOQK-JHEQGTHGSA-N Gln-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCC(N)=O JXFLPKSDLDEOQK-JHEQGTHGSA-N 0.000 description 4
- UQJNXZSSGQIPIQ-FBCQKBJTSA-N Gly-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)CN UQJNXZSSGQIPIQ-FBCQKBJTSA-N 0.000 description 4
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 4
- 108010065920 Insulin Lispro Proteins 0.000 description 4
- 108010002350 Interleukin-2 Proteins 0.000 description 4
- YBAFDPFAUTYYRW-UHFFFAOYSA-N N-L-alpha-glutamyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCC(O)=O YBAFDPFAUTYYRW-UHFFFAOYSA-N 0.000 description 4
- 206010029260 Neuroblastoma Diseases 0.000 description 4
- LNLNHXIQPGKRJQ-SRVKXCTJSA-N Pro-Arg-Arg Chemical compound NC(N)=NCCC[C@@H](C(O)=O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCCN1 LNLNHXIQPGKRJQ-SRVKXCTJSA-N 0.000 description 4
- 241000711931 Rhabdoviridae Species 0.000 description 4
- 206010039491 Sarcoma Diseases 0.000 description 4
- HBZBPFLJNDXRAY-FXQIFTODSA-N Ser-Ala-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O HBZBPFLJNDXRAY-FXQIFTODSA-N 0.000 description 4
- WBAJDGWKRIHOAC-GVXVVHGQSA-N Val-Lys-Gln Chemical compound [H]N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O WBAJDGWKRIHOAC-GVXVVHGQSA-N 0.000 description 4
- 208000036142 Viral infection Diseases 0.000 description 4
- SRHNADOZAAWYLV-XLMUYGLTSA-N alpha-L-Fucp-(1->2)-beta-D-Galp-(1->4)-[alpha-L-Fucp-(1->3)]-beta-D-GlcpNAc Chemical compound O[C@H]1[C@H](O)[C@H](O)[C@H](C)O[C@H]1O[C@H]1[C@H](O[C@H]2[C@@H]([C@@H](NC(C)=O)[C@H](O)O[C@@H]2CO)O[C@H]2[C@H]([C@H](O)[C@H](O)[C@H](C)O2)O)O[C@H](CO)[C@H](O)[C@@H]1O SRHNADOZAAWYLV-XLMUYGLTSA-N 0.000 description 4
- 210000003719 b-lymphocyte Anatomy 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 210000004369 blood Anatomy 0.000 description 4
- 239000008280 blood Substances 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- 239000011651 chromium Substances 0.000 description 4
- 238000001514 detection method Methods 0.000 description 4
- 230000009977 dual effect Effects 0.000 description 4
- 210000001671 embryonic stem cell Anatomy 0.000 description 4
- 108010063718 gamma-glutamylaspartic acid Proteins 0.000 description 4
- 108010010147 glycylglutamine Proteins 0.000 description 4
- 108010015792 glycyllysine Proteins 0.000 description 4
- 208000026278 immune system disease Diseases 0.000 description 4
- 238000001802 infusion Methods 0.000 description 4
- 208000032839 leukemia Diseases 0.000 description 4
- 238000010369 molecular cloning Methods 0.000 description 4
- 230000001575 pathological effect Effects 0.000 description 4
- 230000002093 peripheral effect Effects 0.000 description 4
- 230000003389 potentiating effect Effects 0.000 description 4
- 108010004914 prolylarginine Proteins 0.000 description 4
- 150000003839 salts Chemical class 0.000 description 4
- 230000028327 secretion Effects 0.000 description 4
- 238000000926 separation method Methods 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 238000007920 subcutaneous administration Methods 0.000 description 4
- 238000006467 substitution reaction Methods 0.000 description 4
- 239000006228 supernatant Substances 0.000 description 4
- 238000012360 testing method Methods 0.000 description 4
- 238000002560 therapeutic procedure Methods 0.000 description 4
- 108010061238 threonyl-glycine Proteins 0.000 description 4
- 108010020532 tyrosyl-proline Proteins 0.000 description 4
- 108010073969 valyllysine Proteins 0.000 description 4
- 239000003981 vehicle Substances 0.000 description 4
- 238000001262 western blot Methods 0.000 description 4
- HKZAAJSTFUZYTO-LURJTMIESA-N (2s)-2-[[2-[[2-[[2-[(2-aminoacetyl)amino]acetyl]amino]acetyl]amino]acetyl]amino]-3-hydroxypropanoic acid Chemical compound NCC(=O)NCC(=O)NCC(=O)NCC(=O)N[C@@H](CO)C(O)=O HKZAAJSTFUZYTO-LURJTMIESA-N 0.000 description 3
- YYAVDNKUWLAFCV-ACZMJKKPSA-N Ala-Ser-Gln Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O YYAVDNKUWLAFCV-ACZMJKKPSA-N 0.000 description 3
- XKXAZPSREVUCRT-BPNCWPANSA-N Ala-Tyr-Met Chemical compound CSCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@H](C)N)CC1=CC=C(O)C=C1 XKXAZPSREVUCRT-BPNCWPANSA-N 0.000 description 3
- HQIZDMIGUJOSNI-IUCAKERBSA-N Arg-Gly-Arg Chemical compound N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O HQIZDMIGUJOSNI-IUCAKERBSA-N 0.000 description 3
- DIIGDGJKTMLQQW-IHRRRGAJSA-N Arg-Lys-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCN=C(N)N)N DIIGDGJKTMLQQW-IHRRRGAJSA-N 0.000 description 3
- 108010051330 Arg-Pro-Gly-Pro Proteins 0.000 description 3
- VITDJIPIJZAVGC-VEVYYDQMSA-N Asn-Met-Thr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VITDJIPIJZAVGC-VEVYYDQMSA-N 0.000 description 3
- USNJAPJZSGTTPX-XVSYOHENSA-N Asp-Phe-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O USNJAPJZSGTTPX-XVSYOHENSA-N 0.000 description 3
- 208000023275 Autoimmune disease Diseases 0.000 description 3
- 241000894006 Bacteria Species 0.000 description 3
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 3
- 206010009944 Colon cancer Diseases 0.000 description 3
- 206010061818 Disease progression Diseases 0.000 description 3
- 241000194032 Enterococcus faecalis Species 0.000 description 3
- 206010014967 Ependymoma Diseases 0.000 description 3
- RBWKVOSARCFSQQ-FXQIFTODSA-N Gln-Gln-Ser Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O RBWKVOSARCFSQQ-FXQIFTODSA-N 0.000 description 3
- KVQOVQVGVKDZNW-GUBZILKMSA-N Gln-Ser-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)N)N KVQOVQVGVKDZNW-GUBZILKMSA-N 0.000 description 3
- SYZZMPFLOLSMHL-XHNCKOQMSA-N Gln-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)N)N)C(=O)O SYZZMPFLOLSMHL-XHNCKOQMSA-N 0.000 description 3
- WPJDPEOQUIXXOY-AVGNSLFASA-N Gln-Tyr-Asn Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)O WPJDPEOQUIXXOY-AVGNSLFASA-N 0.000 description 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 3
- BYYNJRSNDARRBX-YFKPBYRVSA-N Gly-Gln-Gly Chemical compound NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O BYYNJRSNDARRBX-YFKPBYRVSA-N 0.000 description 3
- GLACUWHUYFBSPJ-FJXKBIBVSA-N Gly-Pro-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)CN GLACUWHUYFBSPJ-FJXKBIBVSA-N 0.000 description 3
- YGHSQRJSHKYUJY-SCZZXKLOSA-N Gly-Val-Pro Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)CN YGHSQRJSHKYUJY-SCZZXKLOSA-N 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- AFPFGFUGETYOSY-HGNGGELXSA-N His-Ala-Glu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O AFPFGFUGETYOSY-HGNGGELXSA-N 0.000 description 3
- APDIECQNNDGFPD-PYJNHQTQSA-N Ile-His-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](C(C)C)C(=O)O)N APDIECQNNDGFPD-PYJNHQTQSA-N 0.000 description 3
- 108060003951 Immunoglobulin Proteins 0.000 description 3
- ZRLUISBDKUWAIZ-CIUDSAMLSA-N Leu-Ala-Asp Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC(O)=O ZRLUISBDKUWAIZ-CIUDSAMLSA-N 0.000 description 3
- LJBVRCDPWOJOEK-PPCPHDFISA-N Leu-Thr-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O LJBVRCDPWOJOEK-PPCPHDFISA-N 0.000 description 3
- WUHBLPVELFTPQK-KKUMJFAQSA-N Leu-Tyr-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(O)=O WUHBLPVELFTPQK-KKUMJFAQSA-N 0.000 description 3
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 3
- 102000004083 Lymphotoxin-alpha Human genes 0.000 description 3
- 108090000542 Lymphotoxin-alpha Proteins 0.000 description 3
- UWKNTTJNVSYXPC-CIUDSAMLSA-N Lys-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN UWKNTTJNVSYXPC-CIUDSAMLSA-N 0.000 description 3
- CENKQZWVYMLRAX-ULQDDVLXSA-N Lys-Phe-Met Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCSC)C(O)=O CENKQZWVYMLRAX-ULQDDVLXSA-N 0.000 description 3
- 241000588650 Neisseria meningitidis Species 0.000 description 3
- 108010012255 Neural Cell Adhesion Molecule L1 Proteins 0.000 description 3
- 102100024964 Neural cell adhesion molecule L1 Human genes 0.000 description 3
- 201000010133 Oligodendroglioma Diseases 0.000 description 3
- SBVPYBFMIGDIDX-SRVKXCTJSA-N Pro-Pro-Pro Chemical compound OC(=O)[C@@H]1CCCN1C(=O)[C@H]1N(C(=O)[C@H]2NCCC2)CCC1 SBVPYBFMIGDIDX-SRVKXCTJSA-N 0.000 description 3
- YMTLKLXDFCSCNX-BYPYZUCNSA-N Ser-Gly-Gly Chemical compound OC[C@H](N)C(=O)NCC(=O)NCC(O)=O YMTLKLXDFCSCNX-BYPYZUCNSA-N 0.000 description 3
- MUJQWSAWLLRJCE-KATARQTJSA-N Ser-Leu-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MUJQWSAWLLRJCE-KATARQTJSA-N 0.000 description 3
- XQJCEKXQUJQNNK-ZLUOBGJFSA-N Ser-Ser-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O XQJCEKXQUJQNNK-ZLUOBGJFSA-N 0.000 description 3
- PZVGOVRNGKEFCB-KKHAAJSZSA-N Thr-Asn-Val Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](C(C)C)C(=O)O)N)O PZVGOVRNGKEFCB-KKHAAJSZSA-N 0.000 description 3
- NJGMALCNYAMYCB-JRQIVUDYSA-N Thr-Tyr-Asn Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(O)=O NJGMALCNYAMYCB-JRQIVUDYSA-N 0.000 description 3
- KIMOCKLJBXHFIN-YLVFBTJISA-N Trp-Ile-Gly Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(O)=O)=CNC2=C1 KIMOCKLJBXHFIN-YLVFBTJISA-N 0.000 description 3
- 102100036922 Tumor necrosis factor ligand superfamily member 13B Human genes 0.000 description 3
- 102100032101 Tumor necrosis factor ligand superfamily member 9 Human genes 0.000 description 3
- QUILOGWWLXMSAT-IHRRRGAJSA-N Tyr-Gln-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O QUILOGWWLXMSAT-IHRRRGAJSA-N 0.000 description 3
- BSCBBPKDVOZICB-KKUMJFAQSA-N Tyr-Leu-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O BSCBBPKDVOZICB-KKUMJFAQSA-N 0.000 description 3
- NUQZCPSZHGIYTA-HKUYNNGSSA-N Tyr-Trp-Gly Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CC3=CC=C(C=C3)O)N NUQZCPSZHGIYTA-HKUYNNGSSA-N 0.000 description 3
- 241000700618 Vaccinia virus Species 0.000 description 3
- CVUDMNSZAIZFAE-UHFFFAOYSA-N Val-Arg-Pro Natural products NC(N)=NCCCC(NC(=O)C(N)C(C)C)C(=O)N1CCCC1C(O)=O CVUDMNSZAIZFAE-UHFFFAOYSA-N 0.000 description 3
- ILMVQSHENUZYIZ-JYJNAYRXSA-N Val-Met-Tyr Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N ILMVQSHENUZYIZ-JYJNAYRXSA-N 0.000 description 3
- 108010076324 alanyl-glycyl-glycine Proteins 0.000 description 3
- 108010041407 alanylaspartic acid Proteins 0.000 description 3
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 3
- 125000000539 amino acid group Chemical group 0.000 description 3
- 230000006023 anti-tumor response Effects 0.000 description 3
- 108010069205 aspartyl-phenylalanine Proteins 0.000 description 3
- 230000009286 beneficial effect Effects 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 3
- 210000000481 breast Anatomy 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 238000002659 cell therapy Methods 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 229910052804 chromium Inorganic materials 0.000 description 3
- 230000000295 complement effect Effects 0.000 description 3
- 108010060199 cysteinylproline Proteins 0.000 description 3
- 231100000433 cytotoxic Toxicity 0.000 description 3
- 238000012217 deletion Methods 0.000 description 3
- 230000037430 deletion Effects 0.000 description 3
- 230000005750 disease progression Effects 0.000 description 3
- 239000012636 effector Substances 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 239000003623 enhancer Substances 0.000 description 3
- 230000002708 enhancing effect Effects 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 238000000684 flow cytometry Methods 0.000 description 3
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 230000004927 fusion Effects 0.000 description 3
- 238000012239 gene modification Methods 0.000 description 3
- 108010078144 glutaminyl-glycine Proteins 0.000 description 3
- 108010057083 glutamyl-aspartyl-leucine Proteins 0.000 description 3
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 3
- 108010050475 glycyl-leucyl-tyrosine Proteins 0.000 description 3
- 108010079413 glycyl-prolyl-glutamic acid Proteins 0.000 description 3
- 102000018358 immunoglobulin Human genes 0.000 description 3
- 238000002955 isolation Methods 0.000 description 3
- 108010044374 isoleucyl-tyrosine Proteins 0.000 description 3
- 108010034529 leucyl-lysine Proteins 0.000 description 3
- 210000001165 lymph node Anatomy 0.000 description 3
- 108010003700 lysyl aspartic acid Proteins 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 108010051242 phenylalanylserine Proteins 0.000 description 3
- 230000026731 phosphorylation Effects 0.000 description 3
- 238000006366 phosphorylation reaction Methods 0.000 description 3
- 108010090894 prolylleucine Proteins 0.000 description 3
- 238000001959 radiotherapy Methods 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 230000004043 responsiveness Effects 0.000 description 3
- 230000002441 reversible effect Effects 0.000 description 3
- 239000000523 sample Substances 0.000 description 3
- 230000019491 signal transduction Effects 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 239000002562 thickening agent Substances 0.000 description 3
- 210000001541 thymus gland Anatomy 0.000 description 3
- 238000012546 transfer Methods 0.000 description 3
- 108010044292 tryptophyltyrosine Proteins 0.000 description 3
- 210000003171 tumor-infiltrating lymphocyte Anatomy 0.000 description 3
- 241001529453 unidentified herpesvirus Species 0.000 description 3
- 241000712461 unidentified influenza virus Species 0.000 description 3
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 2
- 108010082808 4-1BB Ligand Proteins 0.000 description 2
- 241000186041 Actinomyces israelii Species 0.000 description 2
- 206010000830 Acute leukaemia Diseases 0.000 description 2
- RLMISHABBKUNFO-WHFBIAKZSA-N Ala-Ala-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)NCC(O)=O RLMISHABBKUNFO-WHFBIAKZSA-N 0.000 description 2
- SIGTYDNEPYEXGK-ZANVPECISA-N Ala-Gly-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)CNC(=O)[C@@H](N)C)C(O)=O)=CNC2=C1 SIGTYDNEPYEXGK-ZANVPECISA-N 0.000 description 2
- CKLDHDOIYBVUNP-KBIXCLLPSA-N Ala-Ile-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(O)=O CKLDHDOIYBVUNP-KBIXCLLPSA-N 0.000 description 2
- OLVCTPPSXNRGKV-GUBZILKMSA-N Ala-Pro-Pro Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 OLVCTPPSXNRGKV-GUBZILKMSA-N 0.000 description 2
- WQKAQKZRDIZYNV-VZFHVOOUSA-N Ala-Ser-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WQKAQKZRDIZYNV-VZFHVOOUSA-N 0.000 description 2
- IOFVWPYSRSCWHI-JXUBOQSCSA-N Ala-Thr-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](C)N IOFVWPYSRSCWHI-JXUBOQSCSA-N 0.000 description 2
- MTDDMSUUXNQMKK-BPNCWPANSA-N Ala-Tyr-Arg Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N MTDDMSUUXNQMKK-BPNCWPANSA-N 0.000 description 2
- QRIYOHQJRDHFKF-UWJYBYFXSA-N Ala-Tyr-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)C)CC1=CC=C(O)C=C1 QRIYOHQJRDHFKF-UWJYBYFXSA-N 0.000 description 2
- 241000712892 Arenaviridae Species 0.000 description 2
- UXJCMQFPDWCHKX-DCAQKATOSA-N Arg-Arg-Glu Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(O)=O)C(O)=O UXJCMQFPDWCHKX-DCAQKATOSA-N 0.000 description 2
- IASNWHAGGYTEKX-IUCAKERBSA-N Arg-Arg-Gly Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)NCC(O)=O IASNWHAGGYTEKX-IUCAKERBSA-N 0.000 description 2
- TTXYKSADPSNOIF-IHRRRGAJSA-N Arg-Asp-Phe Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O TTXYKSADPSNOIF-IHRRRGAJSA-N 0.000 description 2
- CVKOQHYVDVYJSI-QTKMDUPCSA-N Arg-His-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CCCN=C(N)N)N)O CVKOQHYVDVYJSI-QTKMDUPCSA-N 0.000 description 2
- GXXWTNKNFFKTJB-NAKRPEOUSA-N Arg-Ile-Ser Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O GXXWTNKNFFKTJB-NAKRPEOUSA-N 0.000 description 2
- SSZGOKWBHLOCHK-DCAQKATOSA-N Arg-Lys-Asn Chemical compound NC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCCN=C(N)N SSZGOKWBHLOCHK-DCAQKATOSA-N 0.000 description 2
- PRLPSDIHSRITSF-UNQGMJICSA-N Arg-Phe-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PRLPSDIHSRITSF-UNQGMJICSA-N 0.000 description 2
- HGKHPCFTRQDHCU-IUCAKERBSA-N Arg-Pro-Gly Chemical compound NC(N)=NCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O HGKHPCFTRQDHCU-IUCAKERBSA-N 0.000 description 2
- VUGWHBXPMAHEGZ-SRVKXCTJSA-N Arg-Pro-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCN=C(N)N VUGWHBXPMAHEGZ-SRVKXCTJSA-N 0.000 description 2
- QCTOLCVIGRLMQS-HRCADAONSA-N Arg-Tyr-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CCCN=C(N)N)N)C(=O)O QCTOLCVIGRLMQS-HRCADAONSA-N 0.000 description 2
- WOZDCBHUGJVJPL-AVGNSLFASA-N Arg-Val-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N WOZDCBHUGJVJPL-AVGNSLFASA-N 0.000 description 2
- CPTXATAOUQJQRO-GUBZILKMSA-N Arg-Val-Ser Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O CPTXATAOUQJQRO-GUBZILKMSA-N 0.000 description 2
- JREOBWLIZLXRIS-GUBZILKMSA-N Asn-Glu-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O JREOBWLIZLXRIS-GUBZILKMSA-N 0.000 description 2
- PTSDPWIHOYMRGR-UGYAYLCHSA-N Asn-Ile-Asn Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(O)=O PTSDPWIHOYMRGR-UGYAYLCHSA-N 0.000 description 2
- LSJQOMAZIKQMTJ-SRVKXCTJSA-N Asn-Phe-Asp Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(O)=O)C(O)=O LSJQOMAZIKQMTJ-SRVKXCTJSA-N 0.000 description 2
- LTDGPJKGJDIBQD-LAEOZQHASA-N Asn-Val-Gln Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O LTDGPJKGJDIBQD-LAEOZQHASA-N 0.000 description 2
- ZAESWDKAMDVHLL-RCOVLWMOSA-N Asn-Val-Gly Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O ZAESWDKAMDVHLL-RCOVLWMOSA-N 0.000 description 2
- PBVLJOIPOGUQQP-CIUDSAMLSA-N Asp-Ala-Leu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O PBVLJOIPOGUQQP-CIUDSAMLSA-N 0.000 description 2
- MRQQMVZUHXUPEV-IHRRRGAJSA-N Asp-Arg-Phe Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O MRQQMVZUHXUPEV-IHRRRGAJSA-N 0.000 description 2
- CJUKAWUWBZCTDQ-SRVKXCTJSA-N Asp-Leu-Lys Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O CJUKAWUWBZCTDQ-SRVKXCTJSA-N 0.000 description 2
- LIVXPXUVXFRWNY-CIUDSAMLSA-N Asp-Lys-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O LIVXPXUVXFRWNY-CIUDSAMLSA-N 0.000 description 2
- UZFHNLYQWMGUHU-DCAQKATOSA-N Asp-Lys-Arg Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O UZFHNLYQWMGUHU-DCAQKATOSA-N 0.000 description 2
- AHWRSSLYSGLBGD-CIUDSAMLSA-N Asp-Pro-Glu Chemical compound OC(=O)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O AHWRSSLYSGLBGD-CIUDSAMLSA-N 0.000 description 2
- WMLFFCRUSPNENW-ZLUOBGJFSA-N Asp-Ser-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O WMLFFCRUSPNENW-ZLUOBGJFSA-N 0.000 description 2
- JDDYEZGPYBBPBN-JRQIVUDYSA-N Asp-Thr-Tyr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O JDDYEZGPYBBPBN-JRQIVUDYSA-N 0.000 description 2
- GIKOVDMXBAFXDF-NHCYSSNCSA-N Asp-Val-Leu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O GIKOVDMXBAFXDF-NHCYSSNCSA-N 0.000 description 2
- 206010003571 Astrocytoma Diseases 0.000 description 2
- 206010004146 Basal cell carcinoma Diseases 0.000 description 2
- 241000702628 Birnaviridae Species 0.000 description 2
- 206010005003 Bladder cancer Diseases 0.000 description 2
- 102100025221 CD70 antigen Human genes 0.000 description 2
- 208000005243 Chondrosarcoma Diseases 0.000 description 2
- 201000009047 Chordoma Diseases 0.000 description 2
- 208000006332 Choriocarcinoma Diseases 0.000 description 2
- 241000193449 Clostridium tetani Species 0.000 description 2
- 241000186227 Corynebacterium diphtheriae Species 0.000 description 2
- 241000709687 Coxsackievirus Species 0.000 description 2
- MXZYQNJCBVJHSR-KATARQTJSA-N Cys-Lys-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CS)N)O MXZYQNJCBVJHSR-KATARQTJSA-N 0.000 description 2
- 241000991587 Enterovirus C Species 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- 208000006168 Ewing Sarcoma Diseases 0.000 description 2
- 201000008808 Fibrosarcoma Diseases 0.000 description 2
- 241000711950 Filoviridae Species 0.000 description 2
- 241000710831 Flavivirus Species 0.000 description 2
- 208000032612 Glial tumor Diseases 0.000 description 2
- 206010018338 Glioma Diseases 0.000 description 2
- TWHDOEYLXXQYOZ-FXQIFTODSA-N Gln-Asn-Gln Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N TWHDOEYLXXQYOZ-FXQIFTODSA-N 0.000 description 2
- NVEASDQHBRZPSU-BQBZGAKWSA-N Gln-Gln-Gly Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O NVEASDQHBRZPSU-BQBZGAKWSA-N 0.000 description 2
- DCWNCMRZIZSZBL-KKUMJFAQSA-N Gln-Pro-Tyr Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CCC(=O)N)N)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O DCWNCMRZIZSZBL-KKUMJFAQSA-N 0.000 description 2
- WOMUDRVDJMHTCV-DCAQKATOSA-N Glu-Arg-Arg Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O WOMUDRVDJMHTCV-DCAQKATOSA-N 0.000 description 2
- BUAKRRKDHSSIKK-IHRRRGAJSA-N Glu-Glu-Tyr Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 BUAKRRKDHSSIKK-IHRRRGAJSA-N 0.000 description 2
- ITBHUUMCJJQUSC-LAEOZQHASA-N Glu-Ile-Gly Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(O)=O ITBHUUMCJJQUSC-LAEOZQHASA-N 0.000 description 2
- VSRCAOIHMGCIJK-SRVKXCTJSA-N Glu-Leu-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O VSRCAOIHMGCIJK-SRVKXCTJSA-N 0.000 description 2
- VMKCPNBBPGGQBJ-GUBZILKMSA-N Glu-Leu-Asn Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)O)N VMKCPNBBPGGQBJ-GUBZILKMSA-N 0.000 description 2
- GJBUAAAIZSRCDC-GVXVVHGQSA-N Glu-Leu-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O GJBUAAAIZSRCDC-GVXVVHGQSA-N 0.000 description 2
- BKMOHWJHXQLFEX-IRIUXVKKSA-N Glu-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](CCC(=O)O)N)O BKMOHWJHXQLFEX-IRIUXVKKSA-N 0.000 description 2
- UGVQELHRNUDMAA-BYPYZUCNSA-N Gly-Ala-Gly Chemical compound [NH3+]CC(=O)N[C@@H](C)C(=O)NCC([O-])=O UGVQELHRNUDMAA-BYPYZUCNSA-N 0.000 description 2
- IWAXHBCACVWNHT-BQBZGAKWSA-N Gly-Asp-Arg Chemical compound NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N IWAXHBCACVWNHT-BQBZGAKWSA-N 0.000 description 2
- QITBQGJOXQYMOA-ZETCQYMHSA-N Gly-Gly-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)CNC(=O)CN QITBQGJOXQYMOA-ZETCQYMHSA-N 0.000 description 2
- AFWYPMDMDYCKMD-KBPBESRZSA-N Gly-Leu-Tyr Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 AFWYPMDMDYCKMD-KBPBESRZSA-N 0.000 description 2
- PDUHNKAFQXQNLH-ZETCQYMHSA-N Gly-Lys-Gly Chemical compound NCCCC[C@H](NC(=O)CN)C(=O)NCC(O)=O PDUHNKAFQXQNLH-ZETCQYMHSA-N 0.000 description 2
- JJGBXTYGTKWGAT-YUMQZZPRSA-N Gly-Pro-Glu Chemical compound NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O JJGBXTYGTKWGAT-YUMQZZPRSA-N 0.000 description 2
- OHUKZZYSJBKFRR-WHFBIAKZSA-N Gly-Ser-Asp Chemical compound [H]NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O OHUKZZYSJBKFRR-WHFBIAKZSA-N 0.000 description 2
- HUFUVTYGPOUCBN-MBLNEYKQSA-N Gly-Thr-Ile Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O HUFUVTYGPOUCBN-MBLNEYKQSA-N 0.000 description 2
- FFALDIDGPLUDKV-ZDLURKLDSA-N Gly-Thr-Ser Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O FFALDIDGPLUDKV-ZDLURKLDSA-N 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- JBCLFWXMTIKCCB-UHFFFAOYSA-N H-Gly-Phe-OH Natural products NCC(=O)NC(C(O)=O)CC1=CC=CC=C1 JBCLFWXMTIKCCB-UHFFFAOYSA-N 0.000 description 2
- 241000606768 Haemophilus influenzae Species 0.000 description 2
- 208000001258 Hemangiosarcoma Diseases 0.000 description 2
- 241000700739 Hepadnaviridae Species 0.000 description 2
- 241000700721 Hepatitis B virus Species 0.000 description 2
- 241000709721 Hepatovirus A Species 0.000 description 2
- 241000700586 Herpesviridae Species 0.000 description 2
- LSQHWKPPOFDHHZ-YUMQZZPRSA-N His-Asp-Gly Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)NCC(=O)O)N LSQHWKPPOFDHHZ-YUMQZZPRSA-N 0.000 description 2
- LJUIEESLIAZSFR-SRVKXCTJSA-N His-Leu-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC1=CN=CN1)N LJUIEESLIAZSFR-SRVKXCTJSA-N 0.000 description 2
- NKRWVZQTPXPNRZ-SRVKXCTJSA-N His-Met-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC1=CN=CN1 NKRWVZQTPXPNRZ-SRVKXCTJSA-N 0.000 description 2
- 208000017604 Hodgkin disease Diseases 0.000 description 2
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 2
- 241000701085 Human alphaherpesvirus 3 Species 0.000 description 2
- AWTDTFXPVCTHAK-BJDJZHNGSA-N Ile-Cys-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCCN)C(=O)O)N AWTDTFXPVCTHAK-BJDJZHNGSA-N 0.000 description 2
- LPXHYGGZJOCAFR-MNXVOIDGSA-N Ile-Glu-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CC(C)C)C(=O)O)N LPXHYGGZJOCAFR-MNXVOIDGSA-N 0.000 description 2
- GTSAALPQZASLPW-KJYZGMDISA-N Ile-His-Trp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)O)N GTSAALPQZASLPW-KJYZGMDISA-N 0.000 description 2
- DMSVBUWGDLYNLC-IAVJCBSLSA-N Ile-Ile-Phe Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 DMSVBUWGDLYNLC-IAVJCBSLSA-N 0.000 description 2
- FBGXMKUWQFPHFB-JBDRJPRFSA-N Ile-Ser-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N FBGXMKUWQFPHFB-JBDRJPRFSA-N 0.000 description 2
- JERJIYYCOGBAIJ-OBAATPRFSA-N Ile-Tyr-Trp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)O)N JERJIYYCOGBAIJ-OBAATPRFSA-N 0.000 description 2
- 108010002386 Interleukin-3 Proteins 0.000 description 2
- 102000015696 Interleukins Human genes 0.000 description 2
- 108010063738 Interleukins Proteins 0.000 description 2
- 108020004684 Internal Ribosome Entry Sites Proteins 0.000 description 2
- 241000701377 Iridoviridae Species 0.000 description 2
- 241000588915 Klebsiella aerogenes Species 0.000 description 2
- 241000588747 Klebsiella pneumoniae Species 0.000 description 2
- IBMVEYRWAWIOTN-UHFFFAOYSA-N L-Leucyl-L-Arginyl-L-Proline Natural products CC(C)CC(N)C(=O)NC(CCCN=C(N)N)C(=O)N1CCCC1C(O)=O IBMVEYRWAWIOTN-UHFFFAOYSA-N 0.000 description 2
- SITWEMZOJNKJCH-UHFFFAOYSA-N L-alanine-L-arginine Natural products CC(N)C(=O)NC(C(O)=O)CCCNC(N)=N SITWEMZOJNKJCH-UHFFFAOYSA-N 0.000 description 2
- TYYLDKGBCJGJGW-UHFFFAOYSA-N L-tryptophan-L-tyrosine Natural products C=1NC2=CC=CC=C2C=1CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 TYYLDKGBCJGJGW-UHFFFAOYSA-N 0.000 description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 2
- 208000018142 Leiomyosarcoma Diseases 0.000 description 2
- WNGVUZWBXZKQES-YUMQZZPRSA-N Leu-Ala-Gly Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)NCC(O)=O WNGVUZWBXZKQES-YUMQZZPRSA-N 0.000 description 2
- IBMVEYRWAWIOTN-RWMBFGLXSA-N Leu-Arg-Pro Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@@H]1C(O)=O IBMVEYRWAWIOTN-RWMBFGLXSA-N 0.000 description 2
- KAFOIVJDVSZUMD-DCAQKATOSA-N Leu-Gln-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O KAFOIVJDVSZUMD-DCAQKATOSA-N 0.000 description 2
- BABSVXFGKFLIGW-UWVGGRQHSA-N Leu-Gly-Arg Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCNC(N)=N BABSVXFGKFLIGW-UWVGGRQHSA-N 0.000 description 2
- OYQUOLRTJHWVSQ-SRVKXCTJSA-N Leu-His-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CO)C(O)=O OYQUOLRTJHWVSQ-SRVKXCTJSA-N 0.000 description 2
- NRFGTHFONZYFNY-MGHWNKPDSA-N Leu-Ile-Tyr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 NRFGTHFONZYFNY-MGHWNKPDSA-N 0.000 description 2
- ODRREERHVHMIPT-OEAJRASXSA-N Leu-Thr-Phe Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 ODRREERHVHMIPT-OEAJRASXSA-N 0.000 description 2
- VHTIZYYHIUHMCA-JYJNAYRXSA-N Leu-Tyr-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(O)=O VHTIZYYHIUHMCA-JYJNAYRXSA-N 0.000 description 2
- YQFZRHYZLARWDY-IHRRRGAJSA-N Leu-Val-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCCCN YQFZRHYZLARWDY-IHRRRGAJSA-N 0.000 description 2
- 108060001084 Luciferase Proteins 0.000 description 2
- 239000005089 Luciferase Substances 0.000 description 2
- 206010025323 Lymphomas Diseases 0.000 description 2
- GAOJCVKPIGHTGO-UWVGGRQHSA-N Lys-Arg-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O GAOJCVKPIGHTGO-UWVGGRQHSA-N 0.000 description 2
- LMVOVCYVZBBWQB-SRVKXCTJSA-N Lys-Asp-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCCCN LMVOVCYVZBBWQB-SRVKXCTJSA-N 0.000 description 2
- KSFQPRLZAUXXPT-GARJFASQSA-N Lys-Cys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CS)NC(=O)[C@H](CCCCN)N)C(=O)O KSFQPRLZAUXXPT-GARJFASQSA-N 0.000 description 2
- IMAKMJCBYCSMHM-AVGNSLFASA-N Lys-Glu-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CCCCN IMAKMJCBYCSMHM-AVGNSLFASA-N 0.000 description 2
- NKKFVJRLCCUJNA-QWRGUYRKSA-N Lys-Gly-Lys Chemical compound NCCCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCCN NKKFVJRLCCUJNA-QWRGUYRKSA-N 0.000 description 2
- AIRZWUMAHCDDHR-KKUMJFAQSA-N Lys-Leu-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O AIRZWUMAHCDDHR-KKUMJFAQSA-N 0.000 description 2
- ZJSZPXISKMDJKQ-JYJNAYRXSA-N Lys-Phe-Glu Chemical compound NCCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CCC(O)=O)C(O)=O)CC1=CC=CC=C1 ZJSZPXISKMDJKQ-JYJNAYRXSA-N 0.000 description 2
- PDIDTSZKKFEDMB-UWVGGRQHSA-N Lys-Pro-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O PDIDTSZKKFEDMB-UWVGGRQHSA-N 0.000 description 2
- GHKXHCMRAUYLBS-CIUDSAMLSA-N Lys-Ser-Asn Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O GHKXHCMRAUYLBS-CIUDSAMLSA-N 0.000 description 2
- IOQWIOPSKJOEKI-SRVKXCTJSA-N Lys-Ser-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O IOQWIOPSKJOEKI-SRVKXCTJSA-N 0.000 description 2
- DIBZLYZXTSVGLN-CIUDSAMLSA-N Lys-Ser-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O DIBZLYZXTSVGLN-CIUDSAMLSA-N 0.000 description 2
- 241000829100 Macaca mulatta polyomavirus 1 Species 0.000 description 2
- 241000712079 Measles morbillivirus Species 0.000 description 2
- 208000007054 Medullary Carcinoma Diseases 0.000 description 2
- 208000000172 Medulloblastoma Diseases 0.000 description 2
- 206010027406 Mesothelioma Diseases 0.000 description 2
- ONGCSGVHCSAATF-CIUDSAMLSA-N Met-Ala-Glu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCC(O)=O ONGCSGVHCSAATF-CIUDSAMLSA-N 0.000 description 2
- 241000700601 Moniliformis Species 0.000 description 2
- 241000711386 Mumps virus Species 0.000 description 2
- 241000186359 Mycobacterium Species 0.000 description 2
- 241000186367 Mycobacterium avium Species 0.000 description 2
- 241000187484 Mycobacterium gordonae Species 0.000 description 2
- 241000186363 Mycobacterium kansasii Species 0.000 description 2
- XZFYRXDAULDNFX-UHFFFAOYSA-N N-L-cysteinyl-L-phenylalanine Natural products SCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 XZFYRXDAULDNFX-UHFFFAOYSA-N 0.000 description 2
- 108010025020 Nerve Growth Factor Proteins 0.000 description 2
- 102000015336 Nerve Growth Factor Human genes 0.000 description 2
- 241000712464 Orthomyxoviridae Species 0.000 description 2
- 241000702244 Orthoreovirus Species 0.000 description 2
- 241000711504 Paramyxoviridae Species 0.000 description 2
- 208000002606 Paramyxoviridae Infections Diseases 0.000 description 2
- 241000606860 Pasteurella Species 0.000 description 2
- MMJJFXWMCMJMQA-STQMWFEESA-N Phe-Pro-Gly Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)NCC(O)=O)C1=CC=CC=C1 MMJJFXWMCMJMQA-STQMWFEESA-N 0.000 description 2
- ZYNBEWGJFXTBDU-ACRUOGEOSA-N Phe-Tyr-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](CC2=CC=CC=C2)N ZYNBEWGJFXTBDU-ACRUOGEOSA-N 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 241000709664 Picornaviridae Species 0.000 description 2
- 208000007641 Pinealoma Diseases 0.000 description 2
- 208000007452 Plasmacytoma Diseases 0.000 description 2
- 241000700625 Poxviridae Species 0.000 description 2
- CGBYDGAJHSOGFQ-LPEHRKFASA-N Pro-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@@H]2CCCN2 CGBYDGAJHSOGFQ-LPEHRKFASA-N 0.000 description 2
- WWAQEUOYCYMGHB-FXQIFTODSA-N Pro-Asn-Asn Chemical compound NC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H]1CCCN1 WWAQEUOYCYMGHB-FXQIFTODSA-N 0.000 description 2
- UPJGUQPLYWTISV-GUBZILKMSA-N Pro-Gln-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O UPJGUQPLYWTISV-GUBZILKMSA-N 0.000 description 2
- JMVQDLDPDBXAAX-YUMQZZPRSA-N Pro-Gly-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1 JMVQDLDPDBXAAX-YUMQZZPRSA-N 0.000 description 2
- YYARMJSFDLIDFS-FKBYEOEOSA-N Pro-Phe-Trp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O YYARMJSFDLIDFS-FKBYEOEOSA-N 0.000 description 2
- SXJOPONICMGFCR-DCAQKATOSA-N Pro-Ser-Lys Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)O SXJOPONICMGFCR-DCAQKATOSA-N 0.000 description 2
- 241000589516 Pseudomonas Species 0.000 description 2
- 108020004511 Recombinant DNA Proteins 0.000 description 2
- 208000006265 Renal cell carcinoma Diseases 0.000 description 2
- 241000702247 Reoviridae Species 0.000 description 2
- 241000725643 Respiratory syncytial virus Species 0.000 description 2
- 201000000582 Retinoblastoma Diseases 0.000 description 2
- 241000606701 Rickettsia Species 0.000 description 2
- 241000607142 Salmonella Species 0.000 description 2
- 201000010208 Seminoma Diseases 0.000 description 2
- WDXYVIIVDIDOSX-DCAQKATOSA-N Ser-Arg-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CO)CCCN=C(N)N WDXYVIIVDIDOSX-DCAQKATOSA-N 0.000 description 2
- QPFJSHSJFIYDJZ-GHCJXIJMSA-N Ser-Asp-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CO QPFJSHSJFIYDJZ-GHCJXIJMSA-N 0.000 description 2
- OLIJLNWFEQEFDM-SRVKXCTJSA-N Ser-Asp-Phe Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 OLIJLNWFEQEFDM-SRVKXCTJSA-N 0.000 description 2
- KMWFXJCGRXBQAC-CIUDSAMLSA-N Ser-Cys-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CO)N KMWFXJCGRXBQAC-CIUDSAMLSA-N 0.000 description 2
- SFTZWNJFZYOLBD-ZDLURKLDSA-N Ser-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO SFTZWNJFZYOLBD-ZDLURKLDSA-N 0.000 description 2
- OQPNSDWGAMFJNU-QWRGUYRKSA-N Ser-Gly-Tyr Chemical compound OC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 OQPNSDWGAMFJNU-QWRGUYRKSA-N 0.000 description 2
- XXXAXOWMBOKTRN-XPUUQOCRSA-N Ser-Gly-Val Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O XXXAXOWMBOKTRN-XPUUQOCRSA-N 0.000 description 2
- QGAHMVHBORDHDC-YUMQZZPRSA-N Ser-His-Gly Chemical compound OC[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CN=CN1 QGAHMVHBORDHDC-YUMQZZPRSA-N 0.000 description 2
- NNFMANHDYSVNIO-DCAQKATOSA-N Ser-Lys-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O NNFMANHDYSVNIO-DCAQKATOSA-N 0.000 description 2
- FKYWFUYPVKLJLP-DCAQKATOSA-N Ser-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CO FKYWFUYPVKLJLP-DCAQKATOSA-N 0.000 description 2
- SRSPTFBENMJHMR-WHFBIAKZSA-N Ser-Ser-Gly Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SRSPTFBENMJHMR-WHFBIAKZSA-N 0.000 description 2
- XJDMUQCLVSCRSJ-VZFHVOOUSA-N Ser-Thr-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O XJDMUQCLVSCRSJ-VZFHVOOUSA-N 0.000 description 2
- SNXUIBACCONSOH-BWBBJGPYSA-N Ser-Thr-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CO)C(O)=O SNXUIBACCONSOH-BWBBJGPYSA-N 0.000 description 2
- KIEIJCFVGZCUAS-MELADBBJSA-N Ser-Tyr-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CO)N)C(=O)O KIEIJCFVGZCUAS-MELADBBJSA-N 0.000 description 2
- HSWXBJCBYSWBPT-GUBZILKMSA-N Ser-Val-Val Chemical compound CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CO)C(C)C)C(O)=O HSWXBJCBYSWBPT-GUBZILKMSA-N 0.000 description 2
- 208000021712 Soft tissue sarcoma Diseases 0.000 description 2
- 241000191967 Staphylococcus aureus Species 0.000 description 2
- 241000193998 Streptococcus pneumoniae Species 0.000 description 2
- 241000187747 Streptomyces Species 0.000 description 2
- 230000024932 T cell mediated immunity Effects 0.000 description 2
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 description 2
- 102000046283 TNF-Related Apoptosis-Inducing Ligand Human genes 0.000 description 2
- DJDSEDOKJTZBAR-ZDLURKLDSA-N Thr-Gly-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O DJDSEDOKJTZBAR-ZDLURKLDSA-N 0.000 description 2
- SCSVNSNWUTYSFO-WDCWCFNPSA-N Thr-Lys-Glu Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O SCSVNSNWUTYSFO-WDCWCFNPSA-N 0.000 description 2
- XNTVWRJTUIOGQO-RHYQMDGZSA-N Thr-Met-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)C(O)=O XNTVWRJTUIOGQO-RHYQMDGZSA-N 0.000 description 2
- MXNAOGFNFNKUPD-JHYOHUSXSA-N Thr-Phe-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MXNAOGFNFNKUPD-JHYOHUSXSA-N 0.000 description 2
- STUAPCLEDMKXKL-LKXGYXEUSA-N Thr-Ser-Asn Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O STUAPCLEDMKXKL-LKXGYXEUSA-N 0.000 description 2
- XHWCDRUPDNSDAZ-XKBZYTNZSA-N Thr-Ser-Glu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N)O XHWCDRUPDNSDAZ-XKBZYTNZSA-N 0.000 description 2
- SGAOHNPSEPVAFP-ZDLURKLDSA-N Thr-Ser-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SGAOHNPSEPVAFP-ZDLURKLDSA-N 0.000 description 2
- IEZVHOULSUULHD-XGEHTFHBSA-N Thr-Ser-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O IEZVHOULSUULHD-XGEHTFHBSA-N 0.000 description 2
- COYHRQWNJDJCNA-NUJDXYNKSA-N Thr-Thr-Thr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O COYHRQWNJDJCNA-NUJDXYNKSA-N 0.000 description 2
- KZTLZZQTJMCGIP-ZJDVBMNYSA-N Thr-Val-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KZTLZZQTJMCGIP-ZJDVBMNYSA-N 0.000 description 2
- 241000710924 Togaviridae Species 0.000 description 2
- 241000589884 Treponema pallidum Species 0.000 description 2
- MBLJBGZWLHTJBH-SZMVWBNQSA-N Trp-Val-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 MBLJBGZWLHTJBH-SZMVWBNQSA-N 0.000 description 2
- UOXPLPBMEPLZBW-WDSOQIARSA-N Trp-Val-Lys Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(O)=O)=CNC2=C1 UOXPLPBMEPLZBW-WDSOQIARSA-N 0.000 description 2
- 101710097160 Tumor necrosis factor ligand superfamily member 10 Proteins 0.000 description 2
- SMLCYZYQFRTLCO-UWJYBYFXSA-N Tyr-Cys-Ala Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(O)=O SMLCYZYQFRTLCO-UWJYBYFXSA-N 0.000 description 2
- KYPMKDGKAYQCHO-RYUDHWBXSA-N Tyr-Met Chemical compound CSCC[C@@H](C(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 KYPMKDGKAYQCHO-RYUDHWBXSA-N 0.000 description 2
- UDNYEPLJTRDMEJ-RCOVLWMOSA-N Val-Asn-Gly Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)NCC(=O)O)N UDNYEPLJTRDMEJ-RCOVLWMOSA-N 0.000 description 2
- BMGOFDMKDVVGJG-NHCYSSNCSA-N Val-Asp-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N BMGOFDMKDVVGJG-NHCYSSNCSA-N 0.000 description 2
- XKVXSCHXGJOQND-ZOBUZTSGSA-N Val-Asp-Trp Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)N XKVXSCHXGJOQND-ZOBUZTSGSA-N 0.000 description 2
- VFOHXOLPLACADK-GVXVVHGQSA-N Val-Gln-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](C(C)C)N VFOHXOLPLACADK-GVXVVHGQSA-N 0.000 description 2
- DJEVQCWNMQOABE-RCOVLWMOSA-N Val-Gly-Asp Chemical compound CC(C)[C@@H](C(=O)NCC(=O)N[C@@H](CC(=O)O)C(=O)O)N DJEVQCWNMQOABE-RCOVLWMOSA-N 0.000 description 2
- KZKMBGXCNLPYKD-YEPSODPASA-N Val-Gly-Thr Chemical compound CC(C)[C@H](N)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O KZKMBGXCNLPYKD-YEPSODPASA-N 0.000 description 2
- QPPZEDOTPZOSEC-RCWTZXSCSA-N Val-Met-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](C(C)C)N)O QPPZEDOTPZOSEC-RCWTZXSCSA-N 0.000 description 2
- PGQUDQYHWICSAB-NAKRPEOUSA-N Val-Ser-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](C(C)C)N PGQUDQYHWICSAB-NAKRPEOUSA-N 0.000 description 2
- PZTZYZUTCPZWJH-FXQIFTODSA-N Val-Ser-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)O)N PZTZYZUTCPZWJH-FXQIFTODSA-N 0.000 description 2
- HTONZBWRYUKUKC-RCWTZXSCSA-N Val-Thr-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O HTONZBWRYUKUKC-RCWTZXSCSA-N 0.000 description 2
- 208000014070 Vestibular schwannoma Diseases 0.000 description 2
- 208000033559 Waldenström macroglobulinemia Diseases 0.000 description 2
- 208000008383 Wilms tumor Diseases 0.000 description 2
- 208000004064 acoustic neuroma Diseases 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- 238000004220 aggregation Methods 0.000 description 2
- 108010086434 alanyl-seryl-glycine Proteins 0.000 description 2
- 230000000735 allogeneic effect Effects 0.000 description 2
- 230000002424 anti-apoptotic effect Effects 0.000 description 2
- 230000000118 anti-neoplastic effect Effects 0.000 description 2
- 210000000612 antigen-presenting cell Anatomy 0.000 description 2
- 108010013835 arginine glutamate Proteins 0.000 description 2
- 108010062796 arginyllysine Proteins 0.000 description 2
- 108010060035 arginylproline Proteins 0.000 description 2
- 108010047857 aspartylglycine Proteins 0.000 description 2
- 108010092854 aspartyllysine Proteins 0.000 description 2
- 108010068265 aspartyltyrosine Proteins 0.000 description 2
- 244000309743 astrovirus Species 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- 230000033228 biological regulation Effects 0.000 description 2
- 230000005540 biological transmission Effects 0.000 description 2
- 210000000988 bone and bone Anatomy 0.000 description 2
- 229940098773 bovine serum albumin Drugs 0.000 description 2
- 208000003362 bronchogenic carcinoma Diseases 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 230000006037 cell lysis Effects 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- 208000006990 cholangiocarcinoma Diseases 0.000 description 2
- 208000024207 chronic leukemia Diseases 0.000 description 2
- 230000004186 co-expression Effects 0.000 description 2
- 239000002299 complementary DNA Substances 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 238000012258 culturing Methods 0.000 description 2
- 208000002445 cystadenocarcinoma Diseases 0.000 description 2
- 238000011266 cytolytic assay Methods 0.000 description 2
- 231100000599 cytotoxic agent Toxicity 0.000 description 2
- 230000001472 cytotoxic effect Effects 0.000 description 2
- 238000002784 cytotoxicity assay Methods 0.000 description 2
- 231100000263 cytotoxicity test Toxicity 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 231100000517 death Toxicity 0.000 description 2
- 210000004443 dendritic cell Anatomy 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 239000008121 dextrose Substances 0.000 description 2
- 230000004069 differentiation Effects 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 238000004520 electroporation Methods 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 229940092559 enterobacter aerogenes Drugs 0.000 description 2
- 239000012894 fetal calf serum Substances 0.000 description 2
- 230000005714 functional activity Effects 0.000 description 2
- 238000002825 functional assay Methods 0.000 description 2
- 108010006664 gamma-glutamyl-glycyl-glycine Proteins 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 238000001476 gene delivery Methods 0.000 description 2
- 238000001415 gene therapy Methods 0.000 description 2
- 230000005017 genetic modification Effects 0.000 description 2
- 235000013617 genetically modified food Nutrition 0.000 description 2
- 208000005017 glioblastoma Diseases 0.000 description 2
- 108010079547 glutamylmethionine Proteins 0.000 description 2
- 230000013595 glycosylation Effects 0.000 description 2
- 238000006206 glycosylation reaction Methods 0.000 description 2
- XBGGUPMXALFZOT-UHFFFAOYSA-N glycyl-L-tyrosine hemihydrate Natural products NCC(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 XBGGUPMXALFZOT-UHFFFAOYSA-N 0.000 description 2
- 108010020688 glycylhistidine Proteins 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- 229940047650 haemophilus influenzae Drugs 0.000 description 2
- 208000025750 heavy chain disease Diseases 0.000 description 2
- 201000002222 hemangioblastoma Diseases 0.000 description 2
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 2
- 108010092114 histidylphenylalanine Proteins 0.000 description 2
- 210000004408 hybridoma Anatomy 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 238000002513 implantation Methods 0.000 description 2
- 230000001976 improved effect Effects 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 230000002779 inactivation Effects 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 230000000977 initiatory effect Effects 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 229940047122 interleukins Drugs 0.000 description 2
- 210000000936 intestine Anatomy 0.000 description 2
- 238000010253 intravenous injection Methods 0.000 description 2
- 230000009545 invasion Effects 0.000 description 2
- 108010078274 isoleucylvaline Proteins 0.000 description 2
- 231100000636 lethal dose Toxicity 0.000 description 2
- 108010073472 leucyl-prolyl-proline Proteins 0.000 description 2
- 210000000265 leukocyte Anatomy 0.000 description 2
- 230000021633 leukocyte mediated immunity Effects 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 206010024627 liposarcoma Diseases 0.000 description 2
- 239000012669 liquid formulation Substances 0.000 description 2
- 210000004185 liver Anatomy 0.000 description 2
- 201000005202 lung cancer Diseases 0.000 description 2
- 208000020816 lung neoplasm Diseases 0.000 description 2
- 108010064235 lysylglycine Proteins 0.000 description 2
- 108010017391 lysylvaline Proteins 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 206010027191 meningioma Diseases 0.000 description 2
- 108020004999 messenger RNA Proteins 0.000 description 2
- 208000010658 metastatic prostate carcinoma Diseases 0.000 description 2
- 108010056582 methionylglutamic acid Proteins 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- 238000013508 migration Methods 0.000 description 2
- 238000010172 mouse model Methods 0.000 description 2
- 230000035772 mutation Effects 0.000 description 2
- 208000001611 myxosarcoma Diseases 0.000 description 2
- 230000001613 neoplastic effect Effects 0.000 description 2
- 229940053128 nerve growth factor Drugs 0.000 description 2
- 201000008968 osteosarcoma Diseases 0.000 description 2
- 208000008443 pancreatic carcinoma Diseases 0.000 description 2
- 208000004019 papillary adenocarcinoma Diseases 0.000 description 2
- 201000010198 papillary carcinoma Diseases 0.000 description 2
- 108010083476 phenylalanyltryptophan Proteins 0.000 description 2
- 239000002953 phosphate buffered saline Substances 0.000 description 2
- 230000001766 physiological effect Effects 0.000 description 2
- 239000002504 physiological saline solution Substances 0.000 description 2
- 208000024724 pineal body neoplasm Diseases 0.000 description 2
- 201000004123 pineal gland cancer Diseases 0.000 description 2
- 230000004481 post-translational protein modification Effects 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 230000002035 prolonged effect Effects 0.000 description 2
- 108010014614 prolyl-glycyl-proline Proteins 0.000 description 2
- 108010093296 prolyl-prolyl-alanine Proteins 0.000 description 2
- 108010070643 prolylglutamic acid Proteins 0.000 description 2
- 230000000069 prophylactic effect Effects 0.000 description 2
- 238000011321 prophylaxis Methods 0.000 description 2
- XJMOSONTPMZWPB-UHFFFAOYSA-M propidium iodide Chemical compound [I-].[I-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CCC[N+](C)(CC)CC)=C1C1=CC=CC=C1 XJMOSONTPMZWPB-UHFFFAOYSA-M 0.000 description 2
- 230000020978 protein processing Effects 0.000 description 2
- 201000009410 rhabdomyosarcoma Diseases 0.000 description 2
- 201000008407 sebaceous adenocarcinoma Diseases 0.000 description 2
- 108010069117 seryl-lysyl-aspartic acid Proteins 0.000 description 2
- 108010026333 seryl-proline Proteins 0.000 description 2
- 208000000587 small cell lung carcinoma Diseases 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 206010041823 squamous cell carcinoma Diseases 0.000 description 2
- 229940031000 streptococcus pneumoniae Drugs 0.000 description 2
- 238000010254 subcutaneous injection Methods 0.000 description 2
- 239000007929 subcutaneous injection Substances 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 206010042863 synovial sarcoma Diseases 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 230000002381 testicular Effects 0.000 description 2
- 230000002463 transducing effect Effects 0.000 description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 2
- 241000701161 unidentified adenovirus Species 0.000 description 2
- 238000011870 unpaired t-test Methods 0.000 description 2
- 210000000626 ureter Anatomy 0.000 description 2
- 210000003708 urethra Anatomy 0.000 description 2
- 210000003932 urinary bladder Anatomy 0.000 description 2
- 206010046766 uterine cancer Diseases 0.000 description 2
- 208000012991 uterine carcinoma Diseases 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 230000003442 weekly effect Effects 0.000 description 2
- DIGQNXIGRZPYDK-WKSCXVIASA-N (2R)-6-amino-2-[[2-[[(2S)-2-[[2-[[(2R)-2-[[(2S)-2-[[(2R,3S)-2-[[2-[[(2S)-2-[[2-[[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2S,3S)-2-[[(2R)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[2-[[(2S)-2-[[(2R)-2-[[2-[[2-[[2-[(2-amino-1-hydroxyethylidene)amino]-3-carboxy-1-hydroxypropylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1-hydroxyethylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxyethylidene]amino]-1-hydroxypropylidene]amino]-1,3-dihydroxypropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxybutylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1-hydroxypropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxyethylidene]amino]-1,5-dihydroxy-5-iminopentylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxybutylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxyethylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1-hydroxyethylidene]amino]hexanoic acid Chemical compound C[C@@H]([C@@H](C(=N[C@@H](CS)C(=N[C@@H](C)C(=N[C@@H](CO)C(=NCC(=N[C@@H](CCC(=N)O)C(=NC(CS)C(=N[C@H]([C@H](C)O)C(=N[C@H](CS)C(=N[C@H](CO)C(=NCC(=N[C@H](CS)C(=NCC(=N[C@H](CCCCN)C(=O)O)O)O)O)O)O)O)O)O)O)O)O)O)O)N=C([C@H](CS)N=C([C@H](CO)N=C([C@H](CO)N=C([C@H](C)N=C(CN=C([C@H](CO)N=C([C@H](CS)N=C(CN=C(C(CS)N=C(C(CC(=O)O)N=C(CN)O)O)O)O)O)O)O)O)O)O)O)O DIGQNXIGRZPYDK-WKSCXVIASA-N 0.000 description 1
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 1
- RJBDSRWGVYNDHL-XNJNKMBASA-N (2S,4R,5S,6S)-2-[(2S,3R,4R,5S,6R)-5-[(2S,3R,4R,5R,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-2-[(2R,3S,4R,5R,6R)-4,5-dihydroxy-2-(hydroxymethyl)-6-[(E,2R,3S)-3-hydroxy-2-(octadecanoylamino)octadec-4-enoxy]oxan-3-yl]oxy-3-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-5-amino-6-[(1S,2R)-2-[(2S,4R,5S,6S)-5-amino-2-carboxy-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxan-2-yl]oxy-1,3-dihydroxypropyl]-4-hydroxyoxane-2-carboxylic acid Chemical compound CCCCCCCCCCCCCCCCCC(=O)N[C@H](CO[C@@H]1O[C@H](CO)[C@@H](O[C@@H]2O[C@H](CO)[C@H](O[C@@H]3O[C@H](CO)[C@H](O)[C@H](O)[C@H]3NC(C)=O)[C@H](O[C@@]3(C[C@@H](O)[C@H](N)[C@H](O3)[C@H](O)[C@@H](CO)O[C@@]3(C[C@@H](O)[C@H](N)[C@H](O3)[C@H](O)[C@H](O)CO)C(O)=O)C(O)=O)[C@H]2O)[C@H](O)[C@H]1O)[C@@H](O)\C=C\CCCCCCCCCCCCC RJBDSRWGVYNDHL-XNJNKMBASA-N 0.000 description 1
- QMOQBVOBWVNSNO-UHFFFAOYSA-N 2-[[2-[[2-[(2-azaniumylacetyl)amino]acetyl]amino]acetyl]amino]acetate Chemical compound NCC(=O)NCC(=O)NCC(=O)NCC(O)=O QMOQBVOBWVNSNO-UHFFFAOYSA-N 0.000 description 1
- BFSVOASYOCHEOV-UHFFFAOYSA-N 2-diethylaminoethanol Chemical compound CCN(CC)CCO BFSVOASYOCHEOV-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N Acrylic acid Chemical compound OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 102000005869 Activating Transcription Factors Human genes 0.000 description 1
- 108010005254 Activating Transcription Factors Proteins 0.000 description 1
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 description 1
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 description 1
- 206010000871 Acute monocytic leukaemia Diseases 0.000 description 1
- 208000031261 Acute myeloid leukaemia Diseases 0.000 description 1
- 206010000890 Acute myelomonocytic leukaemia Diseases 0.000 description 1
- 206010048998 Acute phase reaction Diseases 0.000 description 1
- 208000036762 Acute promyelocytic leukaemia Diseases 0.000 description 1
- 208000010507 Adenocarcinoma of Lung Diseases 0.000 description 1
- 206010001197 Adenocarcinoma of the cervix Diseases 0.000 description 1
- 208000034246 Adenocarcinoma of the cervix uteri Diseases 0.000 description 1
- 208000003200 Adenoma Diseases 0.000 description 1
- 206010001233 Adenoma benign Diseases 0.000 description 1
- 241000701242 Adenoviridae Species 0.000 description 1
- 241000701386 African swine fever virus Species 0.000 description 1
- CXRCVCURMBFFOL-FXQIFTODSA-N Ala-Ala-Pro Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O CXRCVCURMBFFOL-FXQIFTODSA-N 0.000 description 1
- LWUWMHIOBPTZBA-DCAQKATOSA-N Ala-Arg-Lys Chemical compound NC(=N)NCCC[C@H](NC(=O)[C@@H](N)C)C(=O)N[C@@H](CCCCN)C(O)=O LWUWMHIOBPTZBA-DCAQKATOSA-N 0.000 description 1
- LSLIRHLIUDVNBN-CIUDSAMLSA-N Ala-Asp-Lys Chemical compound C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCCCN LSLIRHLIUDVNBN-CIUDSAMLSA-N 0.000 description 1
- KUDREHRZRIVKHS-UWJYBYFXSA-N Ala-Asp-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O KUDREHRZRIVKHS-UWJYBYFXSA-N 0.000 description 1
- DAEFQZCYZKRTLR-ZLUOBGJFSA-N Ala-Cys-Asp Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(O)=O)C(O)=O DAEFQZCYZKRTLR-ZLUOBGJFSA-N 0.000 description 1
- FUSPCLTUKXQREV-ACZMJKKPSA-N Ala-Glu-Ala Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O FUSPCLTUKXQREV-ACZMJKKPSA-N 0.000 description 1
- IXTPACPAXIOCRG-ACZMJKKPSA-N Ala-Glu-Cys Chemical compound C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CS)C(=O)O)N IXTPACPAXIOCRG-ACZMJKKPSA-N 0.000 description 1
- PAIHPOGPJVUFJY-WDSKDSINSA-N Ala-Glu-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O PAIHPOGPJVUFJY-WDSKDSINSA-N 0.000 description 1
- OMMDTNGURYRDAC-NRPADANISA-N Ala-Glu-Val Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O OMMDTNGURYRDAC-NRPADANISA-N 0.000 description 1
- LJFNNUBZSZCZFN-WHFBIAKZSA-N Ala-Gly-Cys Chemical compound N[C@@H](C)C(=O)NCC(=O)N[C@@H](CS)C(=O)O LJFNNUBZSZCZFN-WHFBIAKZSA-N 0.000 description 1
- VGPWRRFOPXVGOH-BYPYZUCNSA-N Ala-Gly-Gly Chemical compound C[C@H](N)C(=O)NCC(=O)NCC(O)=O VGPWRRFOPXVGOH-BYPYZUCNSA-N 0.000 description 1
- OBVSBEYOMDWLRJ-BFHQHQDPSA-N Ala-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@H](C)N OBVSBEYOMDWLRJ-BFHQHQDPSA-N 0.000 description 1
- TZDNWXDLYFIFPT-BJDJZHNGSA-N Ala-Ile-Leu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(O)=O TZDNWXDLYFIFPT-BJDJZHNGSA-N 0.000 description 1
- SUMYEVXWCAYLLJ-GUBZILKMSA-N Ala-Leu-Gln Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O SUMYEVXWCAYLLJ-GUBZILKMSA-N 0.000 description 1
- DPNZTBKGAUAZQU-DLOVCJGASA-N Ala-Leu-His Chemical compound C[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N DPNZTBKGAUAZQU-DLOVCJGASA-N 0.000 description 1
- OYJCVIGKMXUVKB-GARJFASQSA-N Ala-Leu-Pro Chemical compound C[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N OYJCVIGKMXUVKB-GARJFASQSA-N 0.000 description 1
- CHFFHQUVXHEGBY-GARJFASQSA-N Ala-Lys-Pro Chemical compound C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@@H]1C(=O)O)N CHFFHQUVXHEGBY-GARJFASQSA-N 0.000 description 1
- WEZNQZHACPSMEF-QEJZJMRPSA-N Ala-Phe-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)C)CC1=CC=CC=C1 WEZNQZHACPSMEF-QEJZJMRPSA-N 0.000 description 1
- IPZQNYYAYVRKKK-FXQIFTODSA-N Ala-Pro-Ala Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O IPZQNYYAYVRKKK-FXQIFTODSA-N 0.000 description 1
- ADSGHMXEAZJJNF-DCAQKATOSA-N Ala-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N ADSGHMXEAZJJNF-DCAQKATOSA-N 0.000 description 1
- FFZJHQODAYHGPO-KZVJFYERSA-N Ala-Pro-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N FFZJHQODAYHGPO-KZVJFYERSA-N 0.000 description 1
- RTZCUEHYUQZIDE-WHFBIAKZSA-N Ala-Ser-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O RTZCUEHYUQZIDE-WHFBIAKZSA-N 0.000 description 1
- NZGRHTKZFSVPAN-BIIVOSGPSA-N Ala-Ser-Pro Chemical compound C[C@@H](C(=O)N[C@@H](CO)C(=O)N1CCC[C@@H]1C(=O)O)N NZGRHTKZFSVPAN-BIIVOSGPSA-N 0.000 description 1
- SAHQGRZIQVEJPF-JXUBOQSCSA-N Ala-Thr-Lys Chemical compound C[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CCCCN SAHQGRZIQVEJPF-JXUBOQSCSA-N 0.000 description 1
- QDGMZAOSMNGBLP-MRFFXTKBSA-N Ala-Trp-Tyr Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CC3=CC=C(C=C3)O)C(=O)O)N QDGMZAOSMNGBLP-MRFFXTKBSA-N 0.000 description 1
- BHFOJPDOQPWJRN-XDTLVQLUSA-N Ala-Tyr-Gln Chemical compound C[C@H](N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCC(N)=O)C(O)=O BHFOJPDOQPWJRN-XDTLVQLUSA-N 0.000 description 1
- CLOMBHBBUKAUBP-LSJOCFKGSA-N Ala-Val-His Chemical compound C[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N CLOMBHBBUKAUBP-LSJOCFKGSA-N 0.000 description 1
- REWSWYIDQIELBE-FXQIFTODSA-N Ala-Val-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O REWSWYIDQIELBE-FXQIFTODSA-N 0.000 description 1
- 201000003076 Angiosarcoma Diseases 0.000 description 1
- 108010032595 Antibody Binding Sites Proteins 0.000 description 1
- GIVATXIGCXFQQA-FXQIFTODSA-N Arg-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCN=C(N)N GIVATXIGCXFQQA-FXQIFTODSA-N 0.000 description 1
- XPSGESXVBSQZPL-SRVKXCTJSA-N Arg-Arg-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O XPSGESXVBSQZPL-SRVKXCTJSA-N 0.000 description 1
- DPXDVGDLWJYZBH-GUBZILKMSA-N Arg-Asn-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O DPXDVGDLWJYZBH-GUBZILKMSA-N 0.000 description 1
- RVDVDRUZWZIBJQ-CIUDSAMLSA-N Arg-Asn-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O RVDVDRUZWZIBJQ-CIUDSAMLSA-N 0.000 description 1
- IIABBYGHLYWVOS-FXQIFTODSA-N Arg-Asn-Ser Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O IIABBYGHLYWVOS-FXQIFTODSA-N 0.000 description 1
- MFAMTAVAFBPXDC-LPEHRKFASA-N Arg-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)C(=O)O MFAMTAVAFBPXDC-LPEHRKFASA-N 0.000 description 1
- VXXHDZKEQNGXNU-QXEWZRGKSA-N Arg-Asp-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CCCN=C(N)N VXXHDZKEQNGXNU-QXEWZRGKSA-N 0.000 description 1
- SKTGPBFTMNLIHQ-KKUMJFAQSA-N Arg-Glu-Phe Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O SKTGPBFTMNLIHQ-KKUMJFAQSA-N 0.000 description 1
- UFBURHXMKFQVLM-CIUDSAMLSA-N Arg-Glu-Ser Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O UFBURHXMKFQVLM-CIUDSAMLSA-N 0.000 description 1
- AQPVUEJJARLJHB-BQBZGAKWSA-N Arg-Gly-Ala Chemical compound OC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](N)CCCN=C(N)N AQPVUEJJARLJHB-BQBZGAKWSA-N 0.000 description 1
- OQCWXQJLCDPRHV-UWVGGRQHSA-N Arg-Gly-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC(C)C)C(O)=O OQCWXQJLCDPRHV-UWVGGRQHSA-N 0.000 description 1
- YQGZIRIYGHNSQO-ZPFDUUQYSA-N Arg-Ile-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N YQGZIRIYGHNSQO-ZPFDUUQYSA-N 0.000 description 1
- LVMUGODRNHFGRA-AVGNSLFASA-N Arg-Leu-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O LVMUGODRNHFGRA-AVGNSLFASA-N 0.000 description 1
- YBZMTKUDWXZLIX-UWVGGRQHSA-N Arg-Leu-Gly Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O YBZMTKUDWXZLIX-UWVGGRQHSA-N 0.000 description 1
- BTJVOUQWFXABOI-IHRRRGAJSA-N Arg-Lys-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCCNC(N)=N BTJVOUQWFXABOI-IHRRRGAJSA-N 0.000 description 1
- RIQBRKVTFBWEDY-RHYQMDGZSA-N Arg-Lys-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O RIQBRKVTFBWEDY-RHYQMDGZSA-N 0.000 description 1
- LXMKTIZAGIBQRX-HRCADAONSA-N Arg-Phe-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)NC(=O)[C@H](CCCN=C(N)N)N)C(=O)O LXMKTIZAGIBQRX-HRCADAONSA-N 0.000 description 1
- AOHKLEBWKMKITA-IHRRRGAJSA-N Arg-Phe-Ser Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N AOHKLEBWKMKITA-IHRRRGAJSA-N 0.000 description 1
- BSYKSCBTTQKOJG-GUBZILKMSA-N Arg-Pro-Ala Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O BSYKSCBTTQKOJG-GUBZILKMSA-N 0.000 description 1
- ATABBWFGOHKROJ-GUBZILKMSA-N Arg-Pro-Ser Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O ATABBWFGOHKROJ-GUBZILKMSA-N 0.000 description 1
- KXOPYFNQLVUOAQ-FXQIFTODSA-N Arg-Ser-Ala Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O KXOPYFNQLVUOAQ-FXQIFTODSA-N 0.000 description 1
- VENMDXUVHSKEIN-GUBZILKMSA-N Arg-Ser-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O VENMDXUVHSKEIN-GUBZILKMSA-N 0.000 description 1
- KMFPQTITXUKJOV-DCAQKATOSA-N Arg-Ser-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O KMFPQTITXUKJOV-DCAQKATOSA-N 0.000 description 1
- JOTRDIXZHNQYGP-DCAQKATOSA-N Arg-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCCN=C(N)N)N JOTRDIXZHNQYGP-DCAQKATOSA-N 0.000 description 1
- OQPAZKMGCWPERI-GUBZILKMSA-N Arg-Ser-Val Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O OQPAZKMGCWPERI-GUBZILKMSA-N 0.000 description 1
- AIFHRTPABBBHKU-RCWTZXSCSA-N Arg-Thr-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O AIFHRTPABBBHKU-RCWTZXSCSA-N 0.000 description 1
- YNSUUAOAFCVINY-OSUNSFLBSA-N Arg-Thr-Ile Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O YNSUUAOAFCVINY-OSUNSFLBSA-N 0.000 description 1
- UVTGNSWSRSCPLP-UHFFFAOYSA-N Arg-Tyr Natural products NC(CCNC(=N)N)C(=O)NC(Cc1ccc(O)cc1)C(=O)O UVTGNSWSRSCPLP-UHFFFAOYSA-N 0.000 description 1
- FTMRPIVPSDVGCC-GUBZILKMSA-N Arg-Val-Cys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N FTMRPIVPSDVGCC-GUBZILKMSA-N 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- YNDLOUMBVDVALC-ZLUOBGJFSA-N Asn-Ala-Ala Chemical compound C[C@@H](C(=O)N[C@@H](C)C(=O)O)NC(=O)[C@H](CC(=O)N)N YNDLOUMBVDVALC-ZLUOBGJFSA-N 0.000 description 1
- CMLGVVWQQHUXOZ-GHCJXIJMSA-N Asn-Ala-Ile Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O CMLGVVWQQHUXOZ-GHCJXIJMSA-N 0.000 description 1
- HAJWYALLJIATCX-FXQIFTODSA-N Asn-Asn-Arg Chemical compound C(C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC(=O)N)N)CN=C(N)N HAJWYALLJIATCX-FXQIFTODSA-N 0.000 description 1
- DAPLJWATMAXPPZ-CIUDSAMLSA-N Asn-Asn-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CC(N)=O DAPLJWATMAXPPZ-CIUDSAMLSA-N 0.000 description 1
- KXFCBAHYSLJCCY-ZLUOBGJFSA-N Asn-Asn-Ser Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O KXFCBAHYSLJCCY-ZLUOBGJFSA-N 0.000 description 1
- SPIPSJXLZVTXJL-ZLUOBGJFSA-N Asn-Cys-Ser Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(O)=O SPIPSJXLZVTXJL-ZLUOBGJFSA-N 0.000 description 1
- UPALZCBCKAMGIY-PEFMBERDSA-N Asn-Gln-Ile Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O UPALZCBCKAMGIY-PEFMBERDSA-N 0.000 description 1
- GNKVBRYFXYWXAB-WDSKDSINSA-N Asn-Glu-Gly Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O GNKVBRYFXYWXAB-WDSKDSINSA-N 0.000 description 1
- OLGCWMNDJTWQAG-GUBZILKMSA-N Asn-Glu-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC(N)=O OLGCWMNDJTWQAG-GUBZILKMSA-N 0.000 description 1
- OLVIPTLKNSAYRJ-YUMQZZPRSA-N Asn-Gly-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CC(=O)N)N OLVIPTLKNSAYRJ-YUMQZZPRSA-N 0.000 description 1
- RAQMSGVCGSJKCL-FOHZUACHSA-N Asn-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC(N)=O RAQMSGVCGSJKCL-FOHZUACHSA-N 0.000 description 1
- WIDVAWAQBRAKTI-YUMQZZPRSA-N Asn-Leu-Gly Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O WIDVAWAQBRAKTI-YUMQZZPRSA-N 0.000 description 1
- JEEFEQCRXKPQHC-KKUMJFAQSA-N Asn-Leu-Phe Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O JEEFEQCRXKPQHC-KKUMJFAQSA-N 0.000 description 1
- RAUPFUCUDBQYHE-AVGNSLFASA-N Asn-Phe-Glu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(O)=O)C(O)=O RAUPFUCUDBQYHE-AVGNSLFASA-N 0.000 description 1
- MVXJBVVLACEGCG-PCBIJLKTSA-N Asn-Phe-Ile Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O MVXJBVVLACEGCG-PCBIJLKTSA-N 0.000 description 1
- JTXVXGXTRXMOFJ-FXQIFTODSA-N Asn-Pro-Asn Chemical compound NC(=O)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(O)=O JTXVXGXTRXMOFJ-FXQIFTODSA-N 0.000 description 1
- IDUUACUJKUXKKD-VEVYYDQMSA-N Asn-Pro-Thr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(O)=O IDUUACUJKUXKKD-VEVYYDQMSA-N 0.000 description 1
- MKJBPDLENBUHQU-CIUDSAMLSA-N Asn-Ser-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O MKJBPDLENBUHQU-CIUDSAMLSA-N 0.000 description 1
- MYTHOBCLNIOFBL-SRVKXCTJSA-N Asn-Ser-Tyr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O MYTHOBCLNIOFBL-SRVKXCTJSA-N 0.000 description 1
- UXHYOWXTJLBEPG-GSSVUCPTSA-N Asn-Thr-Thr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O UXHYOWXTJLBEPG-GSSVUCPTSA-N 0.000 description 1
- RTFXPCYMDYBZNQ-SRVKXCTJSA-N Asn-Tyr-Asn Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(O)=O RTFXPCYMDYBZNQ-SRVKXCTJSA-N 0.000 description 1
- DPWDPEVGACCWTC-SRVKXCTJSA-N Asn-Tyr-Ser Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(O)=O DPWDPEVGACCWTC-SRVKXCTJSA-N 0.000 description 1
- XEGZSHSPQNDNRH-JRQIVUDYSA-N Asn-Tyr-Thr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XEGZSHSPQNDNRH-JRQIVUDYSA-N 0.000 description 1
- XPGVTUBABLRGHY-BIIVOSGPSA-N Asp-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC(=O)O)N XPGVTUBABLRGHY-BIIVOSGPSA-N 0.000 description 1
- XYBJLTKSGFBLCS-QXEWZRGKSA-N Asp-Arg-Val Chemical compound NC(N)=NCCC[C@@H](C(=O)N[C@@H](C(C)C)C(O)=O)NC(=O)[C@@H](N)CC(O)=O XYBJLTKSGFBLCS-QXEWZRGKSA-N 0.000 description 1
- YNQIDCRRTWGHJD-ZLUOBGJFSA-N Asp-Asn-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CC(O)=O YNQIDCRRTWGHJD-ZLUOBGJFSA-N 0.000 description 1
- UGKZHCBLMLSANF-CIUDSAMLSA-N Asp-Asn-Leu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O UGKZHCBLMLSANF-CIUDSAMLSA-N 0.000 description 1
- AAIUGNSRQDGCDC-ZLUOBGJFSA-N Asp-Cys-Cys Chemical compound C([C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CS)C(=O)O)N)C(=O)O AAIUGNSRQDGCDC-ZLUOBGJFSA-N 0.000 description 1
- DZQKLNLLWFQONU-LKXGYXEUSA-N Asp-Cys-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CC(=O)O)N)O DZQKLNLLWFQONU-LKXGYXEUSA-N 0.000 description 1
- WBDWQKRLTVCDSY-WHFBIAKZSA-N Asp-Gly-Asp Chemical compound OC(=O)C[C@H](N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(O)=O WBDWQKRLTVCDSY-WHFBIAKZSA-N 0.000 description 1
- BIVYLQMZPHDUIH-WHFBIAKZSA-N Asp-Gly-Cys Chemical compound C([C@@H](C(=O)NCC(=O)N[C@@H](CS)C(=O)O)N)C(=O)O BIVYLQMZPHDUIH-WHFBIAKZSA-N 0.000 description 1
- PSLSTUMPZILTAH-BYULHYEWSA-N Asp-Gly-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC(O)=O PSLSTUMPZILTAH-BYULHYEWSA-N 0.000 description 1
- QCVXMEHGFUMKCO-YUMQZZPRSA-N Asp-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC(O)=O QCVXMEHGFUMKCO-YUMQZZPRSA-N 0.000 description 1
- PZXPWHFYZXTFBI-YUMQZZPRSA-N Asp-Gly-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC(O)=O PZXPWHFYZXTFBI-YUMQZZPRSA-N 0.000 description 1
- KYQNAIMCTRZLNP-QSFUFRPTSA-N Asp-Ile-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C(C)C)C(O)=O KYQNAIMCTRZLNP-QSFUFRPTSA-N 0.000 description 1
- JNNVNVRBYUJYGS-CIUDSAMLSA-N Asp-Leu-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O JNNVNVRBYUJYGS-CIUDSAMLSA-N 0.000 description 1
- UMHUHHJMEXNSIV-CIUDSAMLSA-N Asp-Leu-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC(O)=O UMHUHHJMEXNSIV-CIUDSAMLSA-N 0.000 description 1
- FQHBAQLBIXLWAG-DCAQKATOSA-N Asp-Lys-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(=O)O)N FQHBAQLBIXLWAG-DCAQKATOSA-N 0.000 description 1
- GYWQGGUCMDCUJE-DLOVCJGASA-N Asp-Phe-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C)C(O)=O GYWQGGUCMDCUJE-DLOVCJGASA-N 0.000 description 1
- IDDMGSKZQDEDGA-SRVKXCTJSA-N Asp-Phe-Asn Chemical compound OC(=O)C[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CC(N)=O)C(O)=O)CC1=CC=CC=C1 IDDMGSKZQDEDGA-SRVKXCTJSA-N 0.000 description 1
- KRQFMDNIUOVRIF-KKUMJFAQSA-N Asp-Phe-His Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)NC(=O)[C@H](CC(=O)O)N KRQFMDNIUOVRIF-KKUMJFAQSA-N 0.000 description 1
- PWAIZUBWHRHYKS-MELADBBJSA-N Asp-Phe-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)NC(=O)[C@H](CC(=O)O)N)C(=O)O PWAIZUBWHRHYKS-MELADBBJSA-N 0.000 description 1
- NONWUQAWAANERO-BZSNNMDCSA-N Asp-Phe-Tyr Chemical compound C([C@H](NC(=O)[C@H](CC(O)=O)N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=CC=C1 NONWUQAWAANERO-BZSNNMDCSA-N 0.000 description 1
- DWOSGXZMLQNDBN-FXQIFTODSA-N Asp-Pro-Cys Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CC(=O)O)N)C(=O)N[C@@H](CS)C(=O)O DWOSGXZMLQNDBN-FXQIFTODSA-N 0.000 description 1
- GHAHOJDCBRXAKC-IHPCNDPISA-N Asp-Trp-Tyr Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC3=CC=C(C=C3)O)C(=O)O)NC(=O)[C@H](CC(=O)O)N GHAHOJDCBRXAKC-IHPCNDPISA-N 0.000 description 1
- HTSSXFASOUSJQG-IHPCNDPISA-N Asp-Tyr-Trp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O HTSSXFASOUSJQG-IHPCNDPISA-N 0.000 description 1
- XWKPSMRPIKKDDU-RCOVLWMOSA-N Asp-Val-Gly Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O XWKPSMRPIKKDDU-RCOVLWMOSA-N 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 1
- 241000972773 Aulopiformes Species 0.000 description 1
- 108010028006 B-Cell Activating Factor Proteins 0.000 description 1
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 1
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 description 1
- 241000193830 Bacillus <bacterium> Species 0.000 description 1
- 241000193738 Bacillus anthracis Species 0.000 description 1
- 208000035143 Bacterial infection Diseases 0.000 description 1
- 241000606125 Bacteroides Species 0.000 description 1
- 241001148536 Bacteroides sp. Species 0.000 description 1
- 206010004593 Bile duct cancer Diseases 0.000 description 1
- 241000589968 Borrelia Species 0.000 description 1
- 241000589969 Borreliella burgdorferi Species 0.000 description 1
- 241000701822 Bovine papillomavirus Species 0.000 description 1
- 208000003174 Brain Neoplasms Diseases 0.000 description 1
- 206010006417 Bronchial carcinoma Diseases 0.000 description 1
- 108010046080 CD27 Ligand Proteins 0.000 description 1
- 210000001239 CD8-positive, alpha-beta cytotoxic T lymphocyte Anatomy 0.000 description 1
- 241001286462 Caio Species 0.000 description 1
- 241000589876 Campylobacter Species 0.000 description 1
- 241000589994 Campylobacter sp. Species 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 108090000565 Capsid Proteins Proteins 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 208000010667 Carcinoma of liver and intrahepatic biliary tract Diseases 0.000 description 1
- 206010057248 Cell death Diseases 0.000 description 1
- 102100023321 Ceruloplasmin Human genes 0.000 description 1
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 description 1
- 241001533384 Circovirus Species 0.000 description 1
- 241001503987 Clematis vitalba Species 0.000 description 1
- 241000193403 Clostridium Species 0.000 description 1
- 241000193468 Clostridium perfringens Species 0.000 description 1
- 108020004705 Codon Proteins 0.000 description 1
- 108010071942 Colony-Stimulating Factors Proteins 0.000 description 1
- 102000007644 Colony-Stimulating Factors Human genes 0.000 description 1
- 241000186216 Corynebacterium Species 0.000 description 1
- 241000186249 Corynebacterium sp. Species 0.000 description 1
- 208000009798 Craniopharyngioma Diseases 0.000 description 1
- 102000001189 Cyclic Peptides Human genes 0.000 description 1
- 108010069514 Cyclic Peptides Proteins 0.000 description 1
- 241000252233 Cyprinus carpio Species 0.000 description 1
- BUIYOWKUSCTBRE-CIUDSAMLSA-N Cys-Arg-Gln Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O BUIYOWKUSCTBRE-CIUDSAMLSA-N 0.000 description 1
- JTNKVWLMDHIUOG-IHRRRGAJSA-N Cys-Arg-Phe Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O JTNKVWLMDHIUOG-IHRRRGAJSA-N 0.000 description 1
- QLCPDGRAEJSYQM-LPEHRKFASA-N Cys-Arg-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CS)N)C(=O)O QLCPDGRAEJSYQM-LPEHRKFASA-N 0.000 description 1
- VZKXOWRNJDEGLZ-WHFBIAKZSA-N Cys-Asp-Gly Chemical compound SC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(O)=O VZKXOWRNJDEGLZ-WHFBIAKZSA-N 0.000 description 1
- WDQXKVCQXRNOSI-GHCJXIJMSA-N Cys-Asp-Ile Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O WDQXKVCQXRNOSI-GHCJXIJMSA-N 0.000 description 1
- BPHKULHWEIUDOB-FXQIFTODSA-N Cys-Gln-Gln Chemical compound SC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O BPHKULHWEIUDOB-FXQIFTODSA-N 0.000 description 1
- KABHAOSDMIYXTR-GUBZILKMSA-N Cys-Glu-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CS)N KABHAOSDMIYXTR-GUBZILKMSA-N 0.000 description 1
- GDNWBSFSHJVXKL-GUBZILKMSA-N Cys-Lys-Gln Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O GDNWBSFSHJVXKL-GUBZILKMSA-N 0.000 description 1
- CIVXDCMSSFGWAL-YUMQZZPRSA-N Cys-Lys-Gly Chemical compound C(CCN)C[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CS)N CIVXDCMSSFGWAL-YUMQZZPRSA-N 0.000 description 1
- UIKLEGZPIOXFHJ-DLOVCJGASA-N Cys-Phe-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C)C(O)=O UIKLEGZPIOXFHJ-DLOVCJGASA-N 0.000 description 1
- SWJYSDXMTPMBHO-FXQIFTODSA-N Cys-Pro-Ser Chemical compound [H]N[C@@H](CS)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O SWJYSDXMTPMBHO-FXQIFTODSA-N 0.000 description 1
- BCWIFCLVCRAIQK-ZLUOBGJFSA-N Cys-Ser-Cys Chemical compound C([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CS)N)O BCWIFCLVCRAIQK-ZLUOBGJFSA-N 0.000 description 1
- ABLQPNMKLMFDQU-BIIVOSGPSA-N Cys-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CS)N)C(=O)O ABLQPNMKLMFDQU-BIIVOSGPSA-N 0.000 description 1
- KZZYVYWSXMFYEC-DCAQKATOSA-N Cys-Val-Leu Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O KZZYVYWSXMFYEC-DCAQKATOSA-N 0.000 description 1
- ALTQTAKGRFLRLR-GUBZILKMSA-N Cys-Val-Val Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H](CS)N ALTQTAKGRFLRLR-GUBZILKMSA-N 0.000 description 1
- 150000008574 D-amino acids Chemical class 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 241001115402 Ebolavirus Species 0.000 description 1
- 241001466953 Echovirus Species 0.000 description 1
- 241000588914 Enterobacter Species 0.000 description 1
- 241000194033 Enterococcus Species 0.000 description 1
- 241001495410 Enterococcus sp. Species 0.000 description 1
- 208000000832 Equine Encephalomyelitis Diseases 0.000 description 1
- 241000186810 Erysipelothrix rhusiopathiae Species 0.000 description 1
- 208000031637 Erythroblastic Acute Leukemia Diseases 0.000 description 1
- 208000036566 Erythroleukaemia Diseases 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 102000015212 Fas Ligand Protein Human genes 0.000 description 1
- 108010039471 Fas Ligand Protein Proteins 0.000 description 1
- 108010067306 Fibronectins Proteins 0.000 description 1
- 102000016359 Fibronectins Human genes 0.000 description 1
- 229920001917 Ficoll Polymers 0.000 description 1
- 108090000331 Firefly luciferases Proteins 0.000 description 1
- 206010017533 Fungal infection Diseases 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 241001288226 Fusibacter Species 0.000 description 1
- 241000605986 Fusobacterium nucleatum Species 0.000 description 1
- 241001663880 Gammaretrovirus Species 0.000 description 1
- 208000005577 Gastroenteritis Diseases 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 208000034826 Genetic Predisposition to Disease Diseases 0.000 description 1
- 241000713813 Gibbon ape leukemia virus Species 0.000 description 1
- NNQHEEQNPQYPGL-FXQIFTODSA-N Gln-Ala-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(O)=O NNQHEEQNPQYPGL-FXQIFTODSA-N 0.000 description 1
- ZFADFBPRMSBPOT-KKUMJFAQSA-N Gln-Arg-Phe Chemical compound N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(O)=O ZFADFBPRMSBPOT-KKUMJFAQSA-N 0.000 description 1
- XOKGKOQWADCLFQ-GARJFASQSA-N Gln-Arg-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(=O)N)N)C(=O)O XOKGKOQWADCLFQ-GARJFASQSA-N 0.000 description 1
- AAOBFSKXAVIORT-GUBZILKMSA-N Gln-Asn-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O AAOBFSKXAVIORT-GUBZILKMSA-N 0.000 description 1
- LMPBBFWHCRURJD-LAEOZQHASA-N Gln-Asn-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCC(=O)N)N LMPBBFWHCRURJD-LAEOZQHASA-N 0.000 description 1
- KVXVVDFOZNYYKZ-DCAQKATOSA-N Gln-Gln-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O KVXVVDFOZNYYKZ-DCAQKATOSA-N 0.000 description 1
- MADFVRSKEIEZHZ-DCAQKATOSA-N Gln-Gln-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCC(=O)N)N MADFVRSKEIEZHZ-DCAQKATOSA-N 0.000 description 1
- SNLOOPZHAQDMJG-CIUDSAMLSA-N Gln-Glu-Glu Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O SNLOOPZHAQDMJG-CIUDSAMLSA-N 0.000 description 1
- KCJJFESQRXGTGC-BQBZGAKWSA-N Gln-Glu-Gly Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O KCJJFESQRXGTGC-BQBZGAKWSA-N 0.000 description 1
- CLPQUWHBWXFJOX-BQBZGAKWSA-N Gln-Gly-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(O)=O CLPQUWHBWXFJOX-BQBZGAKWSA-N 0.000 description 1
- FGYPOQPQTUNESW-IUCAKERBSA-N Gln-Gly-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CCC(=O)N)N FGYPOQPQTUNESW-IUCAKERBSA-N 0.000 description 1
- RGAOLBZBLOJUTP-GRLWGSQLSA-N Gln-Ile-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)O)NC(=O)[C@H](CCC(=O)N)N RGAOLBZBLOJUTP-GRLWGSQLSA-N 0.000 description 1
- JKGHMESJHRTHIC-SIUGBPQLSA-N Gln-Ile-Tyr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)NC(=O)[C@H](CCC(=O)N)N JKGHMESJHRTHIC-SIUGBPQLSA-N 0.000 description 1
- YPMDZWPZFOZYFG-GUBZILKMSA-N Gln-Leu-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YPMDZWPZFOZYFG-GUBZILKMSA-N 0.000 description 1
- HSHCEAUPUPJPTE-JYJNAYRXSA-N Gln-Leu-Tyr Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)NC(=O)[C@H](CCC(=O)N)N HSHCEAUPUPJPTE-JYJNAYRXSA-N 0.000 description 1
- TWIAMTNJOMRDAK-GUBZILKMSA-N Gln-Lys-Asp Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(O)=O TWIAMTNJOMRDAK-GUBZILKMSA-N 0.000 description 1
- ILKYYKRAULNYMS-JYJNAYRXSA-N Gln-Lys-Phe Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O ILKYYKRAULNYMS-JYJNAYRXSA-N 0.000 description 1
- XZLLTYBONVKGLO-SDDRHHMPSA-N Gln-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(=O)N)N)C(=O)O XZLLTYBONVKGLO-SDDRHHMPSA-N 0.000 description 1
- SFAFZYYMAWOCIC-KKUMJFAQSA-N Gln-Phe-Arg Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N SFAFZYYMAWOCIC-KKUMJFAQSA-N 0.000 description 1
- DRNMNLKUUKKPIA-HTUGSXCWSA-N Gln-Phe-Thr Chemical compound C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CCC(N)=O)C(O)=O DRNMNLKUUKKPIA-HTUGSXCWSA-N 0.000 description 1
- XQDGOJPVMSWZSO-SRVKXCTJSA-N Gln-Pro-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(=O)N)N XQDGOJPVMSWZSO-SRVKXCTJSA-N 0.000 description 1
- RWQCWSGOOOEGPB-FXQIFTODSA-N Gln-Ser-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(O)=O RWQCWSGOOOEGPB-FXQIFTODSA-N 0.000 description 1
- KPNWAJMEMRCLAL-GUBZILKMSA-N Gln-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)N)N KPNWAJMEMRCLAL-GUBZILKMSA-N 0.000 description 1
- DYVMTEWCGAVKSE-HJGDQZAQSA-N Gln-Thr-Arg Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)O DYVMTEWCGAVKSE-HJGDQZAQSA-N 0.000 description 1
- VOUSELYGTNGEPB-NUMRIWBASA-N Gln-Thr-Asp Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O VOUSELYGTNGEPB-NUMRIWBASA-N 0.000 description 1
- STHSGOZLFLFGSS-SUSMZKCASA-N Gln-Thr-Thr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O STHSGOZLFLFGSS-SUSMZKCASA-N 0.000 description 1
- KGNSGRRALVIRGR-QWRGUYRKSA-N Gln-Tyr Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 KGNSGRRALVIRGR-QWRGUYRKSA-N 0.000 description 1
- CSMHMEATMDCQNY-DZKIICNBSA-N Gln-Val-Tyr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O CSMHMEATMDCQNY-DZKIICNBSA-N 0.000 description 1
- UTKICHUQEQBDGC-ACZMJKKPSA-N Glu-Ala-Cys Chemical compound C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCC(=O)O)N UTKICHUQEQBDGC-ACZMJKKPSA-N 0.000 description 1
- OGMQXTXGLDNBSS-FXQIFTODSA-N Glu-Ala-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(O)=O OGMQXTXGLDNBSS-FXQIFTODSA-N 0.000 description 1
- CKRUHITYRFNUKW-WDSKDSINSA-N Glu-Asn-Gly Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O CKRUHITYRFNUKW-WDSKDSINSA-N 0.000 description 1
- HJIFPJUEOGZWRI-GUBZILKMSA-N Glu-Asp-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)O)N HJIFPJUEOGZWRI-GUBZILKMSA-N 0.000 description 1
- SAEBUDRWKUXLOM-ACZMJKKPSA-N Glu-Cys-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@@H](N)CCC(O)=O SAEBUDRWKUXLOM-ACZMJKKPSA-N 0.000 description 1
- KVBPDJIFRQUQFY-ACZMJKKPSA-N Glu-Cys-Ser Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(O)=O KVBPDJIFRQUQFY-ACZMJKKPSA-N 0.000 description 1
- CLROYXHHUZELFX-FXQIFTODSA-N Glu-Gln-Asp Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O CLROYXHHUZELFX-FXQIFTODSA-N 0.000 description 1
- NKLRYVLERDYDBI-FXQIFTODSA-N Glu-Glu-Asp Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O NKLRYVLERDYDBI-FXQIFTODSA-N 0.000 description 1
- BUZMZDDKFCSKOT-CIUDSAMLSA-N Glu-Glu-Glu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O BUZMZDDKFCSKOT-CIUDSAMLSA-N 0.000 description 1
- SJPMNHCEWPTRBR-BQBZGAKWSA-N Glu-Glu-Gly Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O SJPMNHCEWPTRBR-BQBZGAKWSA-N 0.000 description 1
- MUSGDMDGNGXULI-DCAQKATOSA-N Glu-Glu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O MUSGDMDGNGXULI-DCAQKATOSA-N 0.000 description 1
- KUTPGXNAAOQSPD-LPEHRKFASA-N Glu-Glu-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCC(=O)O)N)C(=O)O KUTPGXNAAOQSPD-LPEHRKFASA-N 0.000 description 1
- OAGVHWYIBZMWLA-YFKPBYRVSA-N Glu-Gly-Gly Chemical compound OC(=O)CC[C@H](N)C(=O)NCC(=O)NCC(O)=O OAGVHWYIBZMWLA-YFKPBYRVSA-N 0.000 description 1
- DVLZZEPUNFEUBW-AVGNSLFASA-N Glu-His-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CCC(=O)O)N DVLZZEPUNFEUBW-AVGNSLFASA-N 0.000 description 1
- BIHMNDPWRUROFZ-JYJNAYRXSA-N Glu-His-Phe Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O BIHMNDPWRUROFZ-JYJNAYRXSA-N 0.000 description 1
- XTZDZAXYPDISRR-MNXVOIDGSA-N Glu-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N XTZDZAXYPDISRR-MNXVOIDGSA-N 0.000 description 1
- GRHXUHCFENOCOS-ZPFDUUQYSA-N Glu-Ile-Met Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](CCC(=O)O)N GRHXUHCFENOCOS-ZPFDUUQYSA-N 0.000 description 1
- GXMXPCXXKVWOSM-KQXIARHKSA-N Glu-Ile-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)O)N GXMXPCXXKVWOSM-KQXIARHKSA-N 0.000 description 1
- HVYWQYLBVXMXSV-GUBZILKMSA-N Glu-Leu-Ala Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O HVYWQYLBVXMXSV-GUBZILKMSA-N 0.000 description 1
- DNPCBMNFQVTHMA-DCAQKATOSA-N Glu-Leu-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O DNPCBMNFQVTHMA-DCAQKATOSA-N 0.000 description 1
- IOUQWHIEQYQVFD-JYJNAYRXSA-N Glu-Leu-Tyr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O IOUQWHIEQYQVFD-JYJNAYRXSA-N 0.000 description 1
- CUPSDFQZTVVTSK-GUBZILKMSA-N Glu-Lys-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCC(O)=O CUPSDFQZTVVTSK-GUBZILKMSA-N 0.000 description 1
- QMOSCLNJVKSHHU-YUMQZZPRSA-N Glu-Met-Gly Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(=O)NCC(O)=O QMOSCLNJVKSHHU-YUMQZZPRSA-N 0.000 description 1
- CQAHWYDHKUWYIX-YUMQZZPRSA-N Glu-Pro-Gly Chemical compound OC(=O)CC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O CQAHWYDHKUWYIX-YUMQZZPRSA-N 0.000 description 1
- PAZQYODKOZHXGA-SRVKXCTJSA-N Glu-Pro-His Chemical compound N[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O PAZQYODKOZHXGA-SRVKXCTJSA-N 0.000 description 1
- WIKMTDVSCUJIPJ-CIUDSAMLSA-N Glu-Ser-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCCN=C(N)N WIKMTDVSCUJIPJ-CIUDSAMLSA-N 0.000 description 1
- VNCNWQPIQYAMAK-ACZMJKKPSA-N Glu-Ser-Ser Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O VNCNWQPIQYAMAK-ACZMJKKPSA-N 0.000 description 1
- DMYACXMQUABZIQ-NRPADANISA-N Glu-Ser-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O DMYACXMQUABZIQ-NRPADANISA-N 0.000 description 1
- GPSHCSTUYOQPAI-JHEQGTHGSA-N Glu-Thr-Gly Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O GPSHCSTUYOQPAI-JHEQGTHGSA-N 0.000 description 1
- JVZLZVJTIXVIHK-SXNHZJKMSA-N Glu-Trp-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)NC(=O)[C@H](CCC(=O)O)N JVZLZVJTIXVIHK-SXNHZJKMSA-N 0.000 description 1
- HHSKZJZWQFPSKN-AVGNSLFASA-N Glu-Tyr-Asp Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(O)=O)C(O)=O HHSKZJZWQFPSKN-AVGNSLFASA-N 0.000 description 1
- MFYLRRCYBBJYPI-JYJNAYRXSA-N Glu-Tyr-Lys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N)O MFYLRRCYBBJYPI-JYJNAYRXSA-N 0.000 description 1
- YQPFCZVKMUVZIN-AUTRQRHGSA-N Glu-Val-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O YQPFCZVKMUVZIN-AUTRQRHGSA-N 0.000 description 1
- ZYRXTRTUCAVNBQ-GVXVVHGQSA-N Glu-Val-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N ZYRXTRTUCAVNBQ-GVXVVHGQSA-N 0.000 description 1
- MFVQGXGQRIXBPK-WDSKDSINSA-N Gly-Ala-Glu Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O MFVQGXGQRIXBPK-WDSKDSINSA-N 0.000 description 1
- JRDYDYXZKFNNRQ-XPUUQOCRSA-N Gly-Ala-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)CN JRDYDYXZKFNNRQ-XPUUQOCRSA-N 0.000 description 1
- BGVYNAQWHSTTSP-BYULHYEWSA-N Gly-Asn-Ile Chemical compound [H]NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O BGVYNAQWHSTTSP-BYULHYEWSA-N 0.000 description 1
- MHHUEAIBJZWDBH-YUMQZZPRSA-N Gly-Asp-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)CN MHHUEAIBJZWDBH-YUMQZZPRSA-N 0.000 description 1
- PMNHJLASAAWELO-FOHZUACHSA-N Gly-Asp-Thr Chemical compound [H]NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PMNHJLASAAWELO-FOHZUACHSA-N 0.000 description 1
- XXGQRGQPGFYECI-WDSKDSINSA-N Gly-Cys-Glu Chemical compound NCC(=O)N[C@@H](CS)C(=O)N[C@H](C(O)=O)CCC(O)=O XXGQRGQPGFYECI-WDSKDSINSA-N 0.000 description 1
- LGQZOQRDEUIZJY-YUMQZZPRSA-N Gly-Cys-Lys Chemical compound NCCCC[C@H](NC(=O)[C@H](CS)NC(=O)CN)C(O)=O LGQZOQRDEUIZJY-YUMQZZPRSA-N 0.000 description 1
- CQZDZKRHFWJXDF-WDSKDSINSA-N Gly-Gln-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CN CQZDZKRHFWJXDF-WDSKDSINSA-N 0.000 description 1
- DTRUBYPMMVPQPD-YUMQZZPRSA-N Gly-Gln-Arg Chemical compound [H]NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O DTRUBYPMMVPQPD-YUMQZZPRSA-N 0.000 description 1
- KTSZUNRRYXPZTK-BQBZGAKWSA-N Gly-Gln-Glu Chemical compound NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O KTSZUNRRYXPZTK-BQBZGAKWSA-N 0.000 description 1
- BPQYBFAXRGMGGY-LAEOZQHASA-N Gly-Gln-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)CN BPQYBFAXRGMGGY-LAEOZQHASA-N 0.000 description 1
- GNPVTZJUUBPZKW-WDSKDSINSA-N Gly-Gln-Ser Chemical compound [H]NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O GNPVTZJUUBPZKW-WDSKDSINSA-N 0.000 description 1
- DHDOADIPGZTAHT-YUMQZZPRSA-N Gly-Glu-Arg Chemical compound NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N DHDOADIPGZTAHT-YUMQZZPRSA-N 0.000 description 1
- YYPFZVIXAVDHIK-IUCAKERBSA-N Gly-Glu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CN YYPFZVIXAVDHIK-IUCAKERBSA-N 0.000 description 1
- LHRXAHLCRMQBGJ-RYUDHWBXSA-N Gly-Glu-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)CN LHRXAHLCRMQBGJ-RYUDHWBXSA-N 0.000 description 1
- MBOAPAXLTUSMQI-JHEQGTHGSA-N Gly-Glu-Thr Chemical compound [H]NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MBOAPAXLTUSMQI-JHEQGTHGSA-N 0.000 description 1
- FSPVILZGHUJOHS-QWRGUYRKSA-N Gly-His-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CC1=CNC=N1 FSPVILZGHUJOHS-QWRGUYRKSA-N 0.000 description 1
- YTSVAIMKVLZUDU-YUMQZZPRSA-N Gly-Leu-Asp Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O YTSVAIMKVLZUDU-YUMQZZPRSA-N 0.000 description 1
- CCBIBMKQNXHNIN-ZETCQYMHSA-N Gly-Leu-Gly Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O CCBIBMKQNXHNIN-ZETCQYMHSA-N 0.000 description 1
- NNCSJUBVFBDDLC-YUMQZZPRSA-N Gly-Leu-Ser Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O NNCSJUBVFBDDLC-YUMQZZPRSA-N 0.000 description 1
- LHYJCVCQPWRMKZ-WEDXCCLWSA-N Gly-Leu-Thr Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O LHYJCVCQPWRMKZ-WEDXCCLWSA-N 0.000 description 1
- WDEHMRNSGHVNOH-VHSXEESVSA-N Gly-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)CN)C(=O)O WDEHMRNSGHVNOH-VHSXEESVSA-N 0.000 description 1
- NTBOEZICHOSJEE-YUMQZZPRSA-N Gly-Lys-Ser Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O NTBOEZICHOSJEE-YUMQZZPRSA-N 0.000 description 1
- IBYOLNARKHMLBG-WHOFXGATSA-N Gly-Phe-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CC1=CC=CC=C1 IBYOLNARKHMLBG-WHOFXGATSA-N 0.000 description 1
- IEGFSKKANYKBDU-QWHCGFSZSA-N Gly-Phe-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)NC(=O)CN)C(=O)O IEGFSKKANYKBDU-QWHCGFSZSA-N 0.000 description 1
- WCORRBXVISTKQL-WHFBIAKZSA-N Gly-Ser-Ser Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O WCORRBXVISTKQL-WHFBIAKZSA-N 0.000 description 1
- FKESCSGWBPUTPN-FOHZUACHSA-N Gly-Thr-Asn Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(O)=O FKESCSGWBPUTPN-FOHZUACHSA-N 0.000 description 1
- NVTPVQLIZCOJFK-FOHZUACHSA-N Gly-Thr-Asp Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O NVTPVQLIZCOJFK-FOHZUACHSA-N 0.000 description 1
- BXDLTKLPPKBVEL-FJXKBIBVSA-N Gly-Thr-Met Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCSC)C(O)=O BXDLTKLPPKBVEL-FJXKBIBVSA-N 0.000 description 1
- UIQGJYUEQDOODF-KWQFWETISA-N Gly-Tyr-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)CN)CC1=CC=C(O)C=C1 UIQGJYUEQDOODF-KWQFWETISA-N 0.000 description 1
- GBYYQVBXFVDJPJ-WLTAIBSBSA-N Gly-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)CN)O GBYYQVBXFVDJPJ-WLTAIBSBSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 1
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 1
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 1
- 206010061192 Haemorrhagic fever Diseases 0.000 description 1
- 241000150562 Hantaan orthohantavirus Species 0.000 description 1
- 241000589989 Helicobacter Species 0.000 description 1
- 241000590002 Helicobacter pylori Species 0.000 description 1
- 206010073069 Hepatic cancer Diseases 0.000 description 1
- 208000005331 Hepatitis D Diseases 0.000 description 1
- 208000037262 Hepatitis delta Diseases 0.000 description 1
- 206010019786 Hepatitis non-A non-B Diseases 0.000 description 1
- BIAKMWKJMQLZOJ-ZKWXMUAHSA-N His-Ala-Ala Chemical compound C[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1cnc[nH]1)C(O)=O BIAKMWKJMQLZOJ-ZKWXMUAHSA-N 0.000 description 1
- MDBYBTWRMOAJAY-NHCYSSNCSA-N His-Asn-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC1=CN=CN1)N MDBYBTWRMOAJAY-NHCYSSNCSA-N 0.000 description 1
- VLPMGIJPAWENQB-SRVKXCTJSA-N His-Cys-Leu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O VLPMGIJPAWENQB-SRVKXCTJSA-N 0.000 description 1
- VYMGAXSNYUFVCK-GUBZILKMSA-N His-Gln-Asn Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC(=O)N)C(=O)O)N VYMGAXSNYUFVCK-GUBZILKMSA-N 0.000 description 1
- HYWZHNUGAYVEEW-KKUMJFAQSA-N His-Phe-Ser Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CC2=CN=CN2)N HYWZHNUGAYVEEW-KKUMJFAQSA-N 0.000 description 1
- IAYPZSHNZQHQNO-KKUMJFAQSA-N His-Ser-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC2=CN=CN2)N IAYPZSHNZQHQNO-KKUMJFAQSA-N 0.000 description 1
- GIRSNERMXCMDBO-GARJFASQSA-N His-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CC2=CN=CN2)N)C(=O)O GIRSNERMXCMDBO-GARJFASQSA-N 0.000 description 1
- FCPSGEVYIVXPPO-QTKMDUPCSA-N His-Thr-Arg Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O FCPSGEVYIVXPPO-QTKMDUPCSA-N 0.000 description 1
- 101000934356 Homo sapiens CD70 antigen Proteins 0.000 description 1
- 101001109508 Homo sapiens NKG2-A/NKG2-B type II integral membrane protein Proteins 0.000 description 1
- 101000904196 Homo sapiens Pancreatic secretory granule membrane major glycoprotein GP2 Proteins 0.000 description 1
- 101000638251 Homo sapiens Tumor necrosis factor ligand superfamily member 9 Proteins 0.000 description 1
- 241001207270 Human enterovirus Species 0.000 description 1
- 241000713772 Human immunodeficiency virus 1 Species 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- HDOYNXLPTRQLAD-JBDRJPRFSA-N Ile-Ala-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)O)N HDOYNXLPTRQLAD-JBDRJPRFSA-N 0.000 description 1
- HLYBGMZJVDHJEO-CYDGBPFRSA-N Ile-Arg-Arg Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N HLYBGMZJVDHJEO-CYDGBPFRSA-N 0.000 description 1
- SJIGTGZVQGLMGG-NAKRPEOUSA-N Ile-Cys-Arg Chemical compound N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)O SJIGTGZVQGLMGG-NAKRPEOUSA-N 0.000 description 1
- KBHYLOIVRVBBEB-JBDRJPRFSA-N Ile-Cys-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CS)C(=O)O)N KBHYLOIVRVBBEB-JBDRJPRFSA-N 0.000 description 1
- PPSQSIDMOVPKPI-BJDJZHNGSA-N Ile-Cys-Leu Chemical compound N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(=O)O PPSQSIDMOVPKPI-BJDJZHNGSA-N 0.000 description 1
- DURWCDDDAWVPOP-JBDRJPRFSA-N Ile-Cys-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)O)N DURWCDDDAWVPOP-JBDRJPRFSA-N 0.000 description 1
- LWWILHPVAKKLQS-QXEWZRGKSA-N Ile-Gly-Met Chemical compound CC[C@H](C)[C@@H](C(=O)NCC(=O)N[C@@H](CCSC)C(=O)O)N LWWILHPVAKKLQS-QXEWZRGKSA-N 0.000 description 1
- AFERFBZLVUFWRA-HTFCKZLJSA-N Ile-Ile-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CS)C(=O)O)N AFERFBZLVUFWRA-HTFCKZLJSA-N 0.000 description 1
- HUWYGQOISIJNMK-SIGLWIIPSA-N Ile-Ile-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N HUWYGQOISIJNMK-SIGLWIIPSA-N 0.000 description 1
- KLBVGHCGHUNHEA-BJDJZHNGSA-N Ile-Leu-Ala Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)O)N KLBVGHCGHUNHEA-BJDJZHNGSA-N 0.000 description 1
- GAZGFPOZOLEYAJ-YTFOTSKYSA-N Ile-Leu-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)O)N GAZGFPOZOLEYAJ-YTFOTSKYSA-N 0.000 description 1
- PHRWFSFCNJPWRO-PPCPHDFISA-N Ile-Leu-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N PHRWFSFCNJPWRO-PPCPHDFISA-N 0.000 description 1
- FFJQAEYLAQMGDL-MGHWNKPDSA-N Ile-Lys-Tyr Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 FFJQAEYLAQMGDL-MGHWNKPDSA-N 0.000 description 1
- UOPBQSJRBONRON-STECZYCISA-N Ile-Met-Tyr Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CCSC)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 UOPBQSJRBONRON-STECZYCISA-N 0.000 description 1
- LRAUKBMYHHNADU-DKIMLUQUSA-N Ile-Phe-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)[C@@H](C)CC)CC1=CC=CC=C1 LRAUKBMYHHNADU-DKIMLUQUSA-N 0.000 description 1
- XQLGNKLSPYCRMZ-HJWJTTGWSA-N Ile-Phe-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C(C)C)C(=O)O)N XQLGNKLSPYCRMZ-HJWJTTGWSA-N 0.000 description 1
- PELCGFMHLZXWBQ-BJDJZHNGSA-N Ile-Ser-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)O)N PELCGFMHLZXWBQ-BJDJZHNGSA-N 0.000 description 1
- PXKACEXYLPBMAD-JBDRJPRFSA-N Ile-Ser-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)O)N PXKACEXYLPBMAD-JBDRJPRFSA-N 0.000 description 1
- SAEWJTCJQVZQNZ-IUKAMOBKSA-N Ile-Thr-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N SAEWJTCJQVZQNZ-IUKAMOBKSA-N 0.000 description 1
- REXAUQBGSGDEJY-IGISWZIWSA-N Ile-Tyr-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)O)N REXAUQBGSGDEJY-IGISWZIWSA-N 0.000 description 1
- DZMWFIRHFFVBHS-ZEWNOJEFSA-N Ile-Tyr-Phe Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=CC=C2)C(=O)O)N DZMWFIRHFFVBHS-ZEWNOJEFSA-N 0.000 description 1
- 208000029462 Immunodeficiency disease Diseases 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- 108010002352 Interleukin-1 Proteins 0.000 description 1
- 102000000589 Interleukin-1 Human genes 0.000 description 1
- 108090000177 Interleukin-11 Proteins 0.000 description 1
- 102000013462 Interleukin-12 Human genes 0.000 description 1
- 108010065805 Interleukin-12 Proteins 0.000 description 1
- 102000007482 Interleukin-13 Receptor alpha2 Subunit Human genes 0.000 description 1
- 108010085418 Interleukin-13 Receptor alpha2 Subunit Proteins 0.000 description 1
- 102000003812 Interleukin-15 Human genes 0.000 description 1
- 108090000172 Interleukin-15 Proteins 0.000 description 1
- 102100030703 Interleukin-22 Human genes 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- 208000005016 Intestinal Neoplasms Diseases 0.000 description 1
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- 150000008575 L-amino acids Chemical class 0.000 description 1
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- LHSGPCFBGJHPCY-UHFFFAOYSA-N L-leucine-L-tyrosine Natural products CC(C)CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 LHSGPCFBGJHPCY-UHFFFAOYSA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 1
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 1
- 206010023825 Laryngeal cancer Diseases 0.000 description 1
- 241000589248 Legionella Species 0.000 description 1
- 241000589242 Legionella pneumophila Species 0.000 description 1
- 208000007764 Legionnaires' Disease Diseases 0.000 description 1
- 241000713666 Lentivirus Species 0.000 description 1
- 241000589902 Leptospira Species 0.000 description 1
- MDVZJYGNAGLPGJ-KKUMJFAQSA-N Leu-Asn-Phe Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 MDVZJYGNAGLPGJ-KKUMJFAQSA-N 0.000 description 1
- YKNBJXOJTURHCU-DCAQKATOSA-N Leu-Asp-Arg Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N YKNBJXOJTURHCU-DCAQKATOSA-N 0.000 description 1
- ULXYQAJWJGLCNR-YUMQZZPRSA-N Leu-Asp-Gly Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(O)=O ULXYQAJWJGLCNR-YUMQZZPRSA-N 0.000 description 1
- DLCOFDAHNMMQPP-SRVKXCTJSA-N Leu-Asp-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O DLCOFDAHNMMQPP-SRVKXCTJSA-N 0.000 description 1
- MYGQXVYRZMKRDB-SRVKXCTJSA-N Leu-Asp-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCCCN MYGQXVYRZMKRDB-SRVKXCTJSA-N 0.000 description 1
- JQSXWJXBASFONF-KKUMJFAQSA-N Leu-Asp-Phe Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O JQSXWJXBASFONF-KKUMJFAQSA-N 0.000 description 1
- MMEDVBWCMGRKKC-GARJFASQSA-N Leu-Asp-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N MMEDVBWCMGRKKC-GARJFASQSA-N 0.000 description 1
- CLVUXCBGKUECIT-HJGDQZAQSA-N Leu-Asp-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O CLVUXCBGKUECIT-HJGDQZAQSA-N 0.000 description 1
- FQZPTCNSNPWHLJ-AVGNSLFASA-N Leu-Gln-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(O)=O FQZPTCNSNPWHLJ-AVGNSLFASA-N 0.000 description 1
- WIDZHJTYKYBLSR-DCAQKATOSA-N Leu-Glu-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O WIDZHJTYKYBLSR-DCAQKATOSA-N 0.000 description 1
- HPBCTWSUJOGJSH-MNXVOIDGSA-N Leu-Glu-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O HPBCTWSUJOGJSH-MNXVOIDGSA-N 0.000 description 1
- FEHQLKKBVJHSEC-SZMVWBNQSA-N Leu-Glu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC(C)C)C(O)=O)=CNC2=C1 FEHQLKKBVJHSEC-SZMVWBNQSA-N 0.000 description 1
- FMEICTQWUKNAGC-YUMQZZPRSA-N Leu-Gly-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O FMEICTQWUKNAGC-YUMQZZPRSA-N 0.000 description 1
- PBGDOSARRIJMEV-DLOVCJGASA-N Leu-His-Ala Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](C)C(O)=O PBGDOSARRIJMEV-DLOVCJGASA-N 0.000 description 1
- KVOFSTUWVSQMDK-KKUMJFAQSA-N Leu-His-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(C)C)CC1=CN=CN1 KVOFSTUWVSQMDK-KKUMJFAQSA-N 0.000 description 1
- IFMPDNRWZZEZSL-SRVKXCTJSA-N Leu-Leu-Cys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(O)=O IFMPDNRWZZEZSL-SRVKXCTJSA-N 0.000 description 1
- KYIIALJHAOIAHF-KKUMJFAQSA-N Leu-Leu-His Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 KYIIALJHAOIAHF-KKUMJFAQSA-N 0.000 description 1
- XVZCXCTYGHPNEM-UHFFFAOYSA-N Leu-Leu-Pro Natural products CC(C)CC(N)C(=O)NC(CC(C)C)C(=O)N1CCCC1C(O)=O XVZCXCTYGHPNEM-UHFFFAOYSA-N 0.000 description 1
- RXGLHDWAZQECBI-SRVKXCTJSA-N Leu-Leu-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O RXGLHDWAZQECBI-SRVKXCTJSA-N 0.000 description 1
- UCNNZELZXFXXJQ-BZSNNMDCSA-N Leu-Leu-Tyr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 UCNNZELZXFXXJQ-BZSNNMDCSA-N 0.000 description 1
- ONPJGOIVICHWBW-BZSNNMDCSA-N Leu-Lys-Tyr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 ONPJGOIVICHWBW-BZSNNMDCSA-N 0.000 description 1
- KQFZKDITNUEVFJ-JYJNAYRXSA-N Leu-Phe-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(C)C)CC1=CC=CC=C1 KQFZKDITNUEVFJ-JYJNAYRXSA-N 0.000 description 1
- SYRTUBLKWNDSDK-DKIMLUQUSA-N Leu-Phe-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O SYRTUBLKWNDSDK-DKIMLUQUSA-N 0.000 description 1
- DRWMRVFCKKXHCH-BZSNNMDCSA-N Leu-Phe-Leu Chemical compound CC(C)C[C@H]([NH3+])C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C([O-])=O)CC1=CC=CC=C1 DRWMRVFCKKXHCH-BZSNNMDCSA-N 0.000 description 1
- DPURXCQCHSQPAN-AVGNSLFASA-N Leu-Pro-Pro Chemical compound CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 DPURXCQCHSQPAN-AVGNSLFASA-N 0.000 description 1
- CHJKEDSZNSONPS-DCAQKATOSA-N Leu-Pro-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O CHJKEDSZNSONPS-DCAQKATOSA-N 0.000 description 1
- ADJWHHZETYAAAX-SRVKXCTJSA-N Leu-Ser-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N ADJWHHZETYAAAX-SRVKXCTJSA-N 0.000 description 1
- XOWMDXHFSBCAKQ-SRVKXCTJSA-N Leu-Ser-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC(C)C XOWMDXHFSBCAKQ-SRVKXCTJSA-N 0.000 description 1
- SBANPBVRHYIMRR-UHFFFAOYSA-N Leu-Ser-Pro Natural products CC(C)CC(N)C(=O)NC(CO)C(=O)N1CCCC1C(O)=O SBANPBVRHYIMRR-UHFFFAOYSA-N 0.000 description 1
- PPGBXYKMUMHFBF-KATARQTJSA-N Leu-Ser-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PPGBXYKMUMHFBF-KATARQTJSA-N 0.000 description 1
- HWMQRQIFVGEAPH-XIRDDKMYSA-N Leu-Ser-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(C)C)C(O)=O)=CNC2=C1 HWMQRQIFVGEAPH-XIRDDKMYSA-N 0.000 description 1
- QWWPYKKLXWOITQ-VOAKCMCISA-N Leu-Thr-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC(C)C QWWPYKKLXWOITQ-VOAKCMCISA-N 0.000 description 1
- URHJPNHRQMQGOZ-RHYQMDGZSA-N Leu-Thr-Met Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCSC)C(O)=O URHJPNHRQMQGOZ-RHYQMDGZSA-N 0.000 description 1
- ILDSIMPXNFWKLH-KATARQTJSA-N Leu-Thr-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O ILDSIMPXNFWKLH-KATARQTJSA-N 0.000 description 1
- HGLKOTPFWOMPOB-MEYUZBJRSA-N Leu-Thr-Tyr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 HGLKOTPFWOMPOB-MEYUZBJRSA-N 0.000 description 1
- ARNIBBOXIAWUOP-MGHWNKPDSA-N Leu-Tyr-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O ARNIBBOXIAWUOP-MGHWNKPDSA-N 0.000 description 1
- JGKHAFUAPZCCDU-BZSNNMDCSA-N Leu-Tyr-Leu Chemical compound CC(C)C[C@H]([NH3+])C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C([O-])=O)CC1=CC=C(O)C=C1 JGKHAFUAPZCCDU-BZSNNMDCSA-N 0.000 description 1
- FBNPMTNBFFAMMH-AVGNSLFASA-N Leu-Val-Arg Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N FBNPMTNBFFAMMH-AVGNSLFASA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 241000186779 Listeria monocytogenes Species 0.000 description 1
- 208000016604 Lyme disease Diseases 0.000 description 1
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 1
- 102000003959 Lymphotoxin-beta Human genes 0.000 description 1
- 108090000362 Lymphotoxin-beta Proteins 0.000 description 1
- KCXUCYYZNZFGLL-SRVKXCTJSA-N Lys-Ala-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O KCXUCYYZNZFGLL-SRVKXCTJSA-N 0.000 description 1
- NCTDKZKNBDZDOL-GARJFASQSA-N Lys-Asn-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCCCN)N)C(=O)O NCTDKZKNBDZDOL-GARJFASQSA-N 0.000 description 1
- IWWMPCPLFXFBAF-SRVKXCTJSA-N Lys-Asp-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O IWWMPCPLFXFBAF-SRVKXCTJSA-N 0.000 description 1
- MLLKLNYPZRDIQG-GUBZILKMSA-N Lys-Cys-Gln Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N MLLKLNYPZRDIQG-GUBZILKMSA-N 0.000 description 1
- MQMIRLVJXQNTRJ-SDDRHHMPSA-N Lys-Gln-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCCCN)N)C(=O)O MQMIRLVJXQNTRJ-SDDRHHMPSA-N 0.000 description 1
- LLSUNJYOSCOOEB-GUBZILKMSA-N Lys-Glu-Asp Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O LLSUNJYOSCOOEB-GUBZILKMSA-N 0.000 description 1
- XNKDCYABMBBEKN-IUCAKERBSA-N Lys-Gly-Gln Chemical compound NCCCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCC(N)=O XNKDCYABMBBEKN-IUCAKERBSA-N 0.000 description 1
- LCMWVZLBCUVDAZ-IUCAKERBSA-N Lys-Gly-Glu Chemical compound [NH3+]CCCC[C@H]([NH3+])C(=O)NCC(=O)N[C@H](C([O-])=O)CCC([O-])=O LCMWVZLBCUVDAZ-IUCAKERBSA-N 0.000 description 1
- UETQMSASAVBGJY-QWRGUYRKSA-N Lys-Gly-His Chemical compound NCCCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC1=CNC=N1 UETQMSASAVBGJY-QWRGUYRKSA-N 0.000 description 1
- NNKLKUUGESXCBS-KBPBESRZSA-N Lys-Gly-Tyr Chemical compound [H]N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O NNKLKUUGESXCBS-KBPBESRZSA-N 0.000 description 1
- OWRUUFUVXFREBD-KKUMJFAQSA-N Lys-His-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(O)=O OWRUUFUVXFREBD-KKUMJFAQSA-N 0.000 description 1
- QBEPTBMRQALPEV-MNXVOIDGSA-N Lys-Ile-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CCCCN QBEPTBMRQALPEV-MNXVOIDGSA-N 0.000 description 1
- ZXFRGTAIIZHNHG-AJNGGQMLSA-N Lys-Ile-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)O)NC(=O)[C@H](CCCCN)N ZXFRGTAIIZHNHG-AJNGGQMLSA-N 0.000 description 1
- PINHPJWGVBKQII-SRVKXCTJSA-N Lys-Leu-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCCCN)N PINHPJWGVBKQII-SRVKXCTJSA-N 0.000 description 1
- HVAUKHLDSDDROB-KKUMJFAQSA-N Lys-Lys-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O HVAUKHLDSDDROB-KKUMJFAQSA-N 0.000 description 1
- QQPSCXKFDSORFT-IHRRRGAJSA-N Lys-Lys-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCCCN QQPSCXKFDSORFT-IHRRRGAJSA-N 0.000 description 1
- KFSALEZVQJYHCE-AVGNSLFASA-N Lys-Met-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCCCN)N KFSALEZVQJYHCE-AVGNSLFASA-N 0.000 description 1
- AZOFEHCPMBRNFD-BZSNNMDCSA-N Lys-Phe-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CCCCN)C(O)=O)CC1=CC=CC=C1 AZOFEHCPMBRNFD-BZSNNMDCSA-N 0.000 description 1
- LUAJJLPHUXPQLH-KKUMJFAQSA-N Lys-Phe-Ser Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCCCN)N LUAJJLPHUXPQLH-KKUMJFAQSA-N 0.000 description 1
- LUTDBHBIHHREDC-IHRRRGAJSA-N Lys-Pro-Lys Chemical compound NCCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O LUTDBHBIHHREDC-IHRRRGAJSA-N 0.000 description 1
- JHNOXVASMSXSNB-WEDXCCLWSA-N Lys-Thr-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O JHNOXVASMSXSNB-WEDXCCLWSA-N 0.000 description 1
- RMOKGALPSPOYKE-KATARQTJSA-N Lys-Thr-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O RMOKGALPSPOYKE-KATARQTJSA-N 0.000 description 1
- XABXVVSWUVCZST-GVXVVHGQSA-N Lys-Val-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCCN XABXVVSWUVCZST-GVXVVHGQSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- QXEVZBXTDTVPCP-GMOBBJLQSA-N Met-Asn-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCSC)N QXEVZBXTDTVPCP-GMOBBJLQSA-N 0.000 description 1
- QWTGQXGNNMIUCW-BPUTZDHNSA-N Met-Asn-Trp Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O QWTGQXGNNMIUCW-BPUTZDHNSA-N 0.000 description 1
- PTYVBBNIAQWUFV-DCAQKATOSA-N Met-Cys-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CCSC)N PTYVBBNIAQWUFV-DCAQKATOSA-N 0.000 description 1
- YLLWCSDBVGZLOW-CIUDSAMLSA-N Met-Gln-Ala Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O YLLWCSDBVGZLOW-CIUDSAMLSA-N 0.000 description 1
- VOOINLQYUZOREH-SRVKXCTJSA-N Met-Gln-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCSC)N VOOINLQYUZOREH-SRVKXCTJSA-N 0.000 description 1
- KQBJYJXPZBNEIK-DCAQKATOSA-N Met-Glu-Arg Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CCCNC(N)=N KQBJYJXPZBNEIK-DCAQKATOSA-N 0.000 description 1
- SJDQOYTYNGZZJX-SRVKXCTJSA-N Met-Glu-Leu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O SJDQOYTYNGZZJX-SRVKXCTJSA-N 0.000 description 1
- XKJUFUPCHARJKX-UWVGGRQHSA-N Met-Gly-His Chemical compound CSCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC1=CNC=N1 XKJUFUPCHARJKX-UWVGGRQHSA-N 0.000 description 1
- AXHNAGAYRGCDLG-UWVGGRQHSA-N Met-Lys-Gly Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O AXHNAGAYRGCDLG-UWVGGRQHSA-N 0.000 description 1
- DJJBHQHOZLUBCN-WDSOQIARSA-N Met-Lys-Trp Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O DJJBHQHOZLUBCN-WDSOQIARSA-N 0.000 description 1
- LXCSZPUQKMTXNW-BQBZGAKWSA-N Met-Ser-Gly Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O LXCSZPUQKMTXNW-BQBZGAKWSA-N 0.000 description 1
- DBMLDOWSVHMQQN-XGEHTFHBSA-N Met-Ser-Thr Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O DBMLDOWSVHMQQN-XGEHTFHBSA-N 0.000 description 1
- SPSSJSICDYYTQN-HJGDQZAQSA-N Met-Thr-Gln Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCC(N)=O SPSSJSICDYYTQN-HJGDQZAQSA-N 0.000 description 1
- YGNUDKAPJARTEM-GUBZILKMSA-N Met-Val-Ala Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O YGNUDKAPJARTEM-GUBZILKMSA-N 0.000 description 1
- 102000003792 Metallothionein Human genes 0.000 description 1
- 108090000157 Metallothionein Proteins 0.000 description 1
- 206010027457 Metastases to liver Diseases 0.000 description 1
- 208000035489 Monocytic Acute Leukemia Diseases 0.000 description 1
- 108010008707 Mucin-1 Proteins 0.000 description 1
- 102000007298 Mucin-1 Human genes 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 241000186364 Mycobacterium intracellulare Species 0.000 description 1
- 241000187479 Mycobacterium tuberculosis Species 0.000 description 1
- 208000031888 Mycoses Diseases 0.000 description 1
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 description 1
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 description 1
- 208000033835 Myelomonocytic Acute Leukemia Diseases 0.000 description 1
- KZNQNBZMBZJQJO-UHFFFAOYSA-N N-glycyl-L-proline Natural products NCC(=O)N1CCCC1C(O)=O KZNQNBZMBZJQJO-UHFFFAOYSA-N 0.000 description 1
- AJHCSUXXECOXOY-UHFFFAOYSA-N N-glycyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)CN)C(O)=O)=CNC2=C1 AJHCSUXXECOXOY-UHFFFAOYSA-N 0.000 description 1
- 108010002311 N-glycylglutamic acid Proteins 0.000 description 1
- 102100022682 NKG2-A/NKG2-B type II integral membrane protein Human genes 0.000 description 1
- 241000588653 Neisseria Species 0.000 description 1
- 241000588652 Neisseria gonorrhoeae Species 0.000 description 1
- 206010061309 Neoplasm progression Diseases 0.000 description 1
- 208000009277 Neuroectodermal Tumors Diseases 0.000 description 1
- 101710163270 Nuclease Proteins 0.000 description 1
- 108091005461 Nucleic proteins Proteins 0.000 description 1
- 102000004473 OX40 Ligand Human genes 0.000 description 1
- 108010042215 OX40 Ligand Proteins 0.000 description 1
- 208000001388 Opportunistic Infections Diseases 0.000 description 1
- 241000702259 Orbivirus Species 0.000 description 1
- 241000150218 Orthonairovirus Species 0.000 description 1
- 208000007571 Ovarian Epithelial Carcinoma Diseases 0.000 description 1
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 1
- 102100024019 Pancreatic secretory granule membrane major glycoprotein GP2 Human genes 0.000 description 1
- 241001631646 Papillomaviridae Species 0.000 description 1
- 208000030852 Parasitic disease Diseases 0.000 description 1
- 241000701945 Parvoviridae Species 0.000 description 1
- 241000606856 Pasteurella multocida Species 0.000 description 1
- 102000002508 Peptide Elongation Factors Human genes 0.000 description 1
- 108010068204 Peptide Elongation Factors Proteins 0.000 description 1
- 241000150350 Peribunyaviridae Species 0.000 description 1
- BJEYSVHMGIJORT-NHCYSSNCSA-N Phe-Ala-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=CC=C1 BJEYSVHMGIJORT-NHCYSSNCSA-N 0.000 description 1
- HCTXJGRYAACKOB-SRVKXCTJSA-N Phe-Asn-Asp Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N HCTXJGRYAACKOB-SRVKXCTJSA-N 0.000 description 1
- UUWCIPUVJJIEEP-SRVKXCTJSA-N Phe-Asn-Cys Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CS)C(=O)O)N UUWCIPUVJJIEEP-SRVKXCTJSA-N 0.000 description 1
- IUVYJBMTHARMIP-PCBIJLKTSA-N Phe-Asp-Ile Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O IUVYJBMTHARMIP-PCBIJLKTSA-N 0.000 description 1
- CUMXHKAOHNWRFQ-BZSNNMDCSA-N Phe-Asp-Tyr Chemical compound C([C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=CC=C1 CUMXHKAOHNWRFQ-BZSNNMDCSA-N 0.000 description 1
- ZBYHVSHBZYHQBW-SRVKXCTJSA-N Phe-Cys-Asp Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(=O)O)C(=O)O)N ZBYHVSHBZYHQBW-SRVKXCTJSA-N 0.000 description 1
- KAGCQPSEVAETCA-JYJNAYRXSA-N Phe-Gln-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CC1=CC=CC=C1)N KAGCQPSEVAETCA-JYJNAYRXSA-N 0.000 description 1
- IDUCUXTUHHIQIP-SOUVJXGZSA-N Phe-Gln-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CC2=CC=CC=C2)N)C(=O)O IDUCUXTUHHIQIP-SOUVJXGZSA-N 0.000 description 1
- JJHVFCUWLSKADD-ONGXEEELSA-N Phe-Gly-Ala Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)N[C@@H](C)C(O)=O JJHVFCUWLSKADD-ONGXEEELSA-N 0.000 description 1
- QPVFUAUFEBPIPT-CDMKHQONSA-N Phe-Gly-Thr Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O QPVFUAUFEBPIPT-CDMKHQONSA-N 0.000 description 1
- OSBADCBXAMSPQD-YESZJQIVSA-N Phe-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC2=CC=CC=C2)N OSBADCBXAMSPQD-YESZJQIVSA-N 0.000 description 1
- IEOHQGFKHXUALJ-JYJNAYRXSA-N Phe-Met-Arg Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O IEOHQGFKHXUALJ-JYJNAYRXSA-N 0.000 description 1
- WEDZFLRYSIDIRX-IHRRRGAJSA-N Phe-Ser-Arg Chemical compound NC(=N)NCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=CC=C1 WEDZFLRYSIDIRX-IHRRRGAJSA-N 0.000 description 1
- HBXAOEBRGLCLIW-AVGNSLFASA-N Phe-Ser-Gln Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N HBXAOEBRGLCLIW-AVGNSLFASA-N 0.000 description 1
- ILGCZYGFYQLSDZ-KKUMJFAQSA-N Phe-Ser-His Chemical compound N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O ILGCZYGFYQLSDZ-KKUMJFAQSA-N 0.000 description 1
- GMWNQSGWWGKTSF-LFSVMHDDSA-N Phe-Thr-Ala Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O GMWNQSGWWGKTSF-LFSVMHDDSA-N 0.000 description 1
- VGTJSEYTVMAASM-RPTUDFQQSA-N Phe-Thr-Tyr Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O VGTJSEYTVMAASM-RPTUDFQQSA-N 0.000 description 1
- BTAIJUBAGLVFKQ-BVSLBCMMSA-N Phe-Trp-Val Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](C(C)C)C(O)=O)C1=CC=CC=C1 BTAIJUBAGLVFKQ-BVSLBCMMSA-N 0.000 description 1
- FRMKIPSIZSFTTE-HJOGWXRNSA-N Phe-Tyr-Phe Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O FRMKIPSIZSFTTE-HJOGWXRNSA-N 0.000 description 1
- 241000713137 Phlebovirus Species 0.000 description 1
- 108010047620 Phytohemagglutinins Proteins 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 241001505332 Polyomavirus sp. Species 0.000 description 1
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 1
- VXCHGLYSIOOZIS-GUBZILKMSA-N Pro-Ala-Arg Chemical compound NC(N)=NCCC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1 VXCHGLYSIOOZIS-GUBZILKMSA-N 0.000 description 1
- SSSFPISOZOLQNP-GUBZILKMSA-N Pro-Arg-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(O)=O SSSFPISOZOLQNP-GUBZILKMSA-N 0.000 description 1
- OLHDPZMYUSBGDE-GUBZILKMSA-N Pro-Arg-Cys Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CS)C(=O)O OLHDPZMYUSBGDE-GUBZILKMSA-N 0.000 description 1
- ICTZKEXYDDZZFP-SRVKXCTJSA-N Pro-Arg-Pro Chemical compound N([C@@H](CCCN=C(N)N)C(=O)N1[C@@H](CCC1)C(O)=O)C(=O)[C@@H]1CCCN1 ICTZKEXYDDZZFP-SRVKXCTJSA-N 0.000 description 1
- JARJPEMLQAWNBR-GUBZILKMSA-N Pro-Asp-Arg Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O JARJPEMLQAWNBR-GUBZILKMSA-N 0.000 description 1
- LSIWVWRUTKPXDS-DCAQKATOSA-N Pro-Gln-Arg Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O LSIWVWRUTKPXDS-DCAQKATOSA-N 0.000 description 1
- LHALYDBUDCWMDY-CIUDSAMLSA-N Pro-Glu-Ala Chemical compound C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1)C(O)=O LHALYDBUDCWMDY-CIUDSAMLSA-N 0.000 description 1
- FRKBNXCFJBPJOL-GUBZILKMSA-N Pro-Glu-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O FRKBNXCFJBPJOL-GUBZILKMSA-N 0.000 description 1
- UEHYFUCOGHWASA-HJGDQZAQSA-N Pro-Glu-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1 UEHYFUCOGHWASA-HJGDQZAQSA-N 0.000 description 1
- CLNJSLSHKJECME-BQBZGAKWSA-N Pro-Gly-Ala Chemical compound OC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H]1CCCN1 CLNJSLSHKJECME-BQBZGAKWSA-N 0.000 description 1
- ULIWFCCJIOEHMU-BQBZGAKWSA-N Pro-Gly-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1 ULIWFCCJIOEHMU-BQBZGAKWSA-N 0.000 description 1
- XQSREVQDGCPFRJ-STQMWFEESA-N Pro-Gly-Phe Chemical compound [H]N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O XQSREVQDGCPFRJ-STQMWFEESA-N 0.000 description 1
- AFXCXDQNRXTSBD-FJXKBIBVSA-N Pro-Gly-Thr Chemical compound [H]N1CCC[C@H]1C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O AFXCXDQNRXTSBD-FJXKBIBVSA-N 0.000 description 1
- KLSOMAFWRISSNI-OSUNSFLBSA-N Pro-Ile-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H]1CCCN1 KLSOMAFWRISSNI-OSUNSFLBSA-N 0.000 description 1
- BRJGUPWVFXKBQI-XUXIUFHCSA-N Pro-Leu-Ile Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O BRJGUPWVFXKBQI-XUXIUFHCSA-N 0.000 description 1
- VTFXTWDFPTWNJY-RHYQMDGZSA-N Pro-Leu-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VTFXTWDFPTWNJY-RHYQMDGZSA-N 0.000 description 1
- RMODQFBNDDENCP-IHRRRGAJSA-N Pro-Lys-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O RMODQFBNDDENCP-IHRRRGAJSA-N 0.000 description 1
- SMFQZMGHCODUPQ-ULQDDVLXSA-N Pro-Lys-Phe Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O SMFQZMGHCODUPQ-ULQDDVLXSA-N 0.000 description 1
- ULWBBFKQBDNGOY-RWMBFGLXSA-N Pro-Lys-Pro Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCCCN)C(=O)N2CCC[C@@H]2C(=O)O ULWBBFKQBDNGOY-RWMBFGLXSA-N 0.000 description 1
- DSGSTPRKNYHGCL-JYJNAYRXSA-N Pro-Phe-Met Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCSC)C(O)=O DSGSTPRKNYHGCL-JYJNAYRXSA-N 0.000 description 1
- KDBHVPXBQADZKY-GUBZILKMSA-N Pro-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 KDBHVPXBQADZKY-GUBZILKMSA-N 0.000 description 1
- JLMZKEQFMVORMA-SRVKXCTJSA-N Pro-Pro-Arg Chemical compound NC(N)=NCCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 JLMZKEQFMVORMA-SRVKXCTJSA-N 0.000 description 1
- GFHOSBYCLACKEK-GUBZILKMSA-N Pro-Pro-Asn Chemical compound [H]N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(O)=O GFHOSBYCLACKEK-GUBZILKMSA-N 0.000 description 1
- RCYUBVHMVUHEBM-RCWTZXSCSA-N Pro-Pro-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(O)=O RCYUBVHMVUHEBM-RCWTZXSCSA-N 0.000 description 1
- ITUDDXVFGFEKPD-NAKRPEOUSA-N Pro-Ser-Ile Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O ITUDDXVFGFEKPD-NAKRPEOUSA-N 0.000 description 1
- LNICFEXCAHIJOR-DCAQKATOSA-N Pro-Ser-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O LNICFEXCAHIJOR-DCAQKATOSA-N 0.000 description 1
- AIOWVDNPESPXRB-YTWAJWBKSA-N Pro-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@@H]2CCCN2)O AIOWVDNPESPXRB-YTWAJWBKSA-N 0.000 description 1
- VBZXFFYOBDLLFE-HSHDSVGOSA-N Pro-Trp-Thr Chemical compound N([C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H]([C@H](O)C)C(O)=O)C(=O)[C@@H]1CCCN1 VBZXFFYOBDLLFE-HSHDSVGOSA-N 0.000 description 1
- JXVXYRZQIUPYSA-NHCYSSNCSA-N Pro-Val-Gln Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O JXVXYRZQIUPYSA-NHCYSSNCSA-N 0.000 description 1
- 208000033826 Promyelocytic Acute Leukemia Diseases 0.000 description 1
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 1
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 1
- 241000125945 Protoparvovirus Species 0.000 description 1
- 208000010362 Protozoan Infections Diseases 0.000 description 1
- 108010079005 RDV peptide Proteins 0.000 description 1
- 241000711798 Rabies lyssavirus Species 0.000 description 1
- 241000712907 Retroviridae Species 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 241000702670 Rotavirus Species 0.000 description 1
- FIXILCYTSAUERA-FXQIFTODSA-N Ser-Ala-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O FIXILCYTSAUERA-FXQIFTODSA-N 0.000 description 1
- LVVBAKCGXXUHFO-ZLUOBGJFSA-N Ser-Ala-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(O)=O LVVBAKCGXXUHFO-ZLUOBGJFSA-N 0.000 description 1
- SRTCFKGBYBZRHA-ACZMJKKPSA-N Ser-Ala-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O SRTCFKGBYBZRHA-ACZMJKKPSA-N 0.000 description 1
- BTKUIVBNGBFTTP-WHFBIAKZSA-N Ser-Ala-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)NCC(O)=O BTKUIVBNGBFTTP-WHFBIAKZSA-N 0.000 description 1
- HRNQLKCLPVKZNE-CIUDSAMLSA-N Ser-Ala-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O HRNQLKCLPVKZNE-CIUDSAMLSA-N 0.000 description 1
- JPIDMRXXNMIVKY-VZFHVOOUSA-N Ser-Ala-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JPIDMRXXNMIVKY-VZFHVOOUSA-N 0.000 description 1
- QGMLKFGTGXWAHF-IHRRRGAJSA-N Ser-Arg-Phe Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O QGMLKFGTGXWAHF-IHRRRGAJSA-N 0.000 description 1
- HBOABDXGTMMDSE-GUBZILKMSA-N Ser-Arg-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(O)=O HBOABDXGTMMDSE-GUBZILKMSA-N 0.000 description 1
- UCXDHBORXLVBNC-ZLUOBGJFSA-N Ser-Asn-Cys Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(O)=O UCXDHBORXLVBNC-ZLUOBGJFSA-N 0.000 description 1
- RDFQNDHEHVSONI-ZLUOBGJFSA-N Ser-Asn-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O RDFQNDHEHVSONI-ZLUOBGJFSA-N 0.000 description 1
- FTVRVZNYIYWJGB-ACZMJKKPSA-N Ser-Asp-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O FTVRVZNYIYWJGB-ACZMJKKPSA-N 0.000 description 1
- HEQPKICPPDOSIN-SRVKXCTJSA-N Ser-Asp-Tyr Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 HEQPKICPPDOSIN-SRVKXCTJSA-N 0.000 description 1
- RNFKSBPHLTZHLU-WHFBIAKZSA-N Ser-Cys-Gly Chemical compound C([C@@H](C(=O)N[C@@H](CS)C(=O)NCC(=O)O)N)O RNFKSBPHLTZHLU-WHFBIAKZSA-N 0.000 description 1
- CDVFZMOFNJPUDD-ACZMJKKPSA-N Ser-Gln-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O CDVFZMOFNJPUDD-ACZMJKKPSA-N 0.000 description 1
- BQWCDDAISCPDQV-XHNCKOQMSA-N Ser-Gln-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CO)N)C(=O)O BQWCDDAISCPDQV-XHNCKOQMSA-N 0.000 description 1
- UQFYNFTYDHUIMI-WHFBIAKZSA-N Ser-Gly-Ala Chemical compound OC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](N)CO UQFYNFTYDHUIMI-WHFBIAKZSA-N 0.000 description 1
- SVWQEIRZHHNBIO-WHFBIAKZSA-N Ser-Gly-Cys Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CS)C(O)=O SVWQEIRZHHNBIO-WHFBIAKZSA-N 0.000 description 1
- GZFAWAQTEYDKII-YUMQZZPRSA-N Ser-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO GZFAWAQTEYDKII-YUMQZZPRSA-N 0.000 description 1
- WSTIOCFMWXNOCX-YUMQZZPRSA-N Ser-Gly-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CO)N WSTIOCFMWXNOCX-YUMQZZPRSA-N 0.000 description 1
- KDGARKCAKHBEDB-NKWVEPMBSA-N Ser-Gly-Pro Chemical compound C1C[C@@H](N(C1)C(=O)CNC(=O)[C@H](CO)N)C(=O)O KDGARKCAKHBEDB-NKWVEPMBSA-N 0.000 description 1
- UIGMAMGZOJVTDN-WHFBIAKZSA-N Ser-Gly-Ser Chemical compound OC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O UIGMAMGZOJVTDN-WHFBIAKZSA-N 0.000 description 1
- CLKKNZQUQMZDGD-SRVKXCTJSA-N Ser-His-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CO)CC1=CN=CN1 CLKKNZQUQMZDGD-SRVKXCTJSA-N 0.000 description 1
- JIPVNVNKXJLFJF-BJDJZHNGSA-N Ser-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CO)N JIPVNVNKXJLFJF-BJDJZHNGSA-N 0.000 description 1
- DOSZISJPMCYEHT-NAKRPEOUSA-N Ser-Ile-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C(C)C)C(O)=O DOSZISJPMCYEHT-NAKRPEOUSA-N 0.000 description 1
- GJFYFGOEWLDQGW-GUBZILKMSA-N Ser-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CO)N GJFYFGOEWLDQGW-GUBZILKMSA-N 0.000 description 1
- IUXGJEIKJBYKOO-SRVKXCTJSA-N Ser-Leu-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CO)N IUXGJEIKJBYKOO-SRVKXCTJSA-N 0.000 description 1
- XUDRHBPSPAPDJP-SRVKXCTJSA-N Ser-Lys-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CO XUDRHBPSPAPDJP-SRVKXCTJSA-N 0.000 description 1
- AXVNLRQLPLSIPQ-FXQIFTODSA-N Ser-Met-Cys Chemical compound CSCC[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CO)N AXVNLRQLPLSIPQ-FXQIFTODSA-N 0.000 description 1
- XKFJENWJGHMDLI-QWRGUYRKSA-N Ser-Phe-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)NCC(O)=O XKFJENWJGHMDLI-QWRGUYRKSA-N 0.000 description 1
- RRVFEDGUXSYWOW-BZSNNMDCSA-N Ser-Phe-Phe Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O RRVFEDGUXSYWOW-BZSNNMDCSA-N 0.000 description 1
- MQUZANJDFOQOBX-SRVKXCTJSA-N Ser-Phe-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(O)=O MQUZANJDFOQOBX-SRVKXCTJSA-N 0.000 description 1
- ADJDNJCSPNFFPI-FXQIFTODSA-N Ser-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CO ADJDNJCSPNFFPI-FXQIFTODSA-N 0.000 description 1
- NMZXJDSKEGFDLJ-DCAQKATOSA-N Ser-Pro-Lys Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CO)N)C(=O)N[C@@H](CCCCN)C(=O)O NMZXJDSKEGFDLJ-DCAQKATOSA-N 0.000 description 1
- OVQZAFXWIWNYKA-GUBZILKMSA-N Ser-Pro-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO)N OVQZAFXWIWNYKA-GUBZILKMSA-N 0.000 description 1
- AZWNCEBQZXELEZ-FXQIFTODSA-N Ser-Pro-Ser Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O AZWNCEBQZXELEZ-FXQIFTODSA-N 0.000 description 1
- VGQVAVQWKJLIRM-FXQIFTODSA-N Ser-Ser-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O VGQVAVQWKJLIRM-FXQIFTODSA-N 0.000 description 1
- FVFUOQIYDPAIJR-XIRDDKMYSA-N Ser-Trp-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)NC(=O)[C@H](CO)N FVFUOQIYDPAIJR-XIRDDKMYSA-N 0.000 description 1
- BIWBTRRBHIEVAH-IHPCNDPISA-N Ser-Tyr-Trp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O BIWBTRRBHIEVAH-IHPCNDPISA-N 0.000 description 1
- IAOHCSQDQDWRQU-GUBZILKMSA-N Ser-Val-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O IAOHCSQDQDWRQU-GUBZILKMSA-N 0.000 description 1
- PCMZJFMUYWIERL-ZKWXMUAHSA-N Ser-Val-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O PCMZJFMUYWIERL-ZKWXMUAHSA-N 0.000 description 1
- BEBVVQPDSHHWQL-NRPADANISA-N Ser-Val-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O BEBVVQPDSHHWQL-NRPADANISA-N 0.000 description 1
- JZRYFUGREMECBH-XPUUQOCRSA-N Ser-Val-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O JZRYFUGREMECBH-XPUUQOCRSA-N 0.000 description 1
- YEDSOSIKVUMIJE-DCAQKATOSA-N Ser-Val-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O YEDSOSIKVUMIJE-DCAQKATOSA-N 0.000 description 1
- LGIMRDKGABDMBN-DCAQKATOSA-N Ser-Val-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CO)N LGIMRDKGABDMBN-DCAQKATOSA-N 0.000 description 1
- SIEBDTCABMZCLF-XGEHTFHBSA-N Ser-Val-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O SIEBDTCABMZCLF-XGEHTFHBSA-N 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 241000700584 Simplexvirus Species 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- 206010041067 Small cell lung cancer Diseases 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- 208000034254 Squamous cell carcinoma of the cervix uteri Diseases 0.000 description 1
- 241000191940 Staphylococcus Species 0.000 description 1
- 208000005718 Stomach Neoplasms Diseases 0.000 description 1
- 241000193996 Streptococcus pyogenes Species 0.000 description 1
- 229940100514 Syk tyrosine kinase inhibitor Drugs 0.000 description 1
- 230000020385 T cell costimulation Effects 0.000 description 1
- 230000037453 T cell priming Effects 0.000 description 1
- 101000588258 Taenia solium Paramyosin Proteins 0.000 description 1
- 208000024313 Testicular Neoplasms Diseases 0.000 description 1
- 206010057644 Testis cancer Diseases 0.000 description 1
- NJEMRSFGDNECGF-GCJQMDKQSA-N Thr-Ala-Asp Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC(O)=O NJEMRSFGDNECGF-GCJQMDKQSA-N 0.000 description 1
- CAJFZCICSVBOJK-SHGPDSBTSA-N Thr-Ala-Thr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O CAJFZCICSVBOJK-SHGPDSBTSA-N 0.000 description 1
- DGDCHPCRMWEOJR-FQPOAREZSA-N Thr-Ala-Tyr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 DGDCHPCRMWEOJR-FQPOAREZSA-N 0.000 description 1
- XSLXHSYIVPGEER-KZVJFYERSA-N Thr-Ala-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O XSLXHSYIVPGEER-KZVJFYERSA-N 0.000 description 1
- XYEXCEPTALHNEV-RCWTZXSCSA-N Thr-Arg-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O XYEXCEPTALHNEV-RCWTZXSCSA-N 0.000 description 1
- PKXHGEXFMIZSER-QTKMDUPCSA-N Thr-Arg-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N)O PKXHGEXFMIZSER-QTKMDUPCSA-N 0.000 description 1
- NAXBBCLCEOTAIG-RHYQMDGZSA-N Thr-Arg-Lys Chemical compound NC(N)=NCCC[C@H](NC(=O)[C@@H](N)[C@H](O)C)C(=O)N[C@@H](CCCCN)C(O)=O NAXBBCLCEOTAIG-RHYQMDGZSA-N 0.000 description 1
- JVTHIXKSVYEWNI-JRQIVUDYSA-N Thr-Asn-Tyr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O JVTHIXKSVYEWNI-JRQIVUDYSA-N 0.000 description 1
- MFEBUIFJVPNZLO-OLHMAJIHSA-N Thr-Asp-Asn Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O MFEBUIFJVPNZLO-OLHMAJIHSA-N 0.000 description 1
- KRPKYGOFYUNIGM-XVSYOHENSA-N Thr-Asp-Phe Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N)O KRPKYGOFYUNIGM-XVSYOHENSA-N 0.000 description 1
- KZUJCMPVNXOBAF-LKXGYXEUSA-N Thr-Cys-Asp Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(O)=O)C(O)=O KZUJCMPVNXOBAF-LKXGYXEUSA-N 0.000 description 1
- YAAPRMFURSENOZ-KATARQTJSA-N Thr-Cys-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCCN)C(=O)O)N)O YAAPRMFURSENOZ-KATARQTJSA-N 0.000 description 1
- LIXBDERDAGNVAV-XKBZYTNZSA-N Thr-Gln-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O LIXBDERDAGNVAV-XKBZYTNZSA-N 0.000 description 1
- LKEKWDJCJSPXNI-IRIUXVKKSA-N Thr-Glu-Tyr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 LKEKWDJCJSPXNI-IRIUXVKKSA-N 0.000 description 1
- XFTYVCHLARBHBQ-FOHZUACHSA-N Thr-Gly-Asn Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O XFTYVCHLARBHBQ-FOHZUACHSA-N 0.000 description 1
- JKGGPMOUIAAJAA-YEPSODPASA-N Thr-Gly-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O JKGGPMOUIAAJAA-YEPSODPASA-N 0.000 description 1
- XOWKUMFHEZLKLT-CIQUZCHMSA-N Thr-Ile-Ala Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(O)=O XOWKUMFHEZLKLT-CIQUZCHMSA-N 0.000 description 1
- JRAUIKJSEAKTGD-TUBUOCAGSA-N Thr-Ile-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H]([C@@H](C)O)N JRAUIKJSEAKTGD-TUBUOCAGSA-N 0.000 description 1
- FQPDRTDDEZXCEC-SVSWQMSJSA-N Thr-Ile-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O FQPDRTDDEZXCEC-SVSWQMSJSA-N 0.000 description 1
- FLPZMPOZGYPBEN-PPCPHDFISA-N Thr-Leu-Ile Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O FLPZMPOZGYPBEN-PPCPHDFISA-N 0.000 description 1
- YOOAQCZYZHGUAZ-KATARQTJSA-N Thr-Leu-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YOOAQCZYZHGUAZ-KATARQTJSA-N 0.000 description 1
- ZSPQUTWLWGWTPS-HJGDQZAQSA-N Thr-Lys-Asp Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(O)=O ZSPQUTWLWGWTPS-HJGDQZAQSA-N 0.000 description 1
- CJXURNZYNHCYFD-WDCWCFNPSA-N Thr-Lys-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N)O CJXURNZYNHCYFD-WDCWCFNPSA-N 0.000 description 1
- SPVHQURZJCUDQC-VOAKCMCISA-N Thr-Lys-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O SPVHQURZJCUDQC-VOAKCMCISA-N 0.000 description 1
- MGJLBZFUXUGMML-VOAKCMCISA-N Thr-Lys-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)O)N)O MGJLBZFUXUGMML-VOAKCMCISA-N 0.000 description 1
- KZURUCDWKDEAFZ-XVSYOHENSA-N Thr-Phe-Asn Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(=O)N)C(=O)O)N)O KZURUCDWKDEAFZ-XVSYOHENSA-N 0.000 description 1
- WNQJTLATMXYSEL-OEAJRASXSA-N Thr-Phe-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(C)C)C(O)=O WNQJTLATMXYSEL-OEAJRASXSA-N 0.000 description 1
- NWECYMJLJGCBOD-UNQGMJICSA-N Thr-Phe-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C(C)C)C(O)=O NWECYMJLJGCBOD-UNQGMJICSA-N 0.000 description 1
- MROIJTGJGIDEEJ-RCWTZXSCSA-N Thr-Pro-Pro Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 MROIJTGJGIDEEJ-RCWTZXSCSA-N 0.000 description 1
- AHERARIZBPOMNU-KATARQTJSA-N Thr-Ser-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O AHERARIZBPOMNU-KATARQTJSA-N 0.000 description 1
- AAZOYLQUEQRUMZ-GSSVUCPTSA-N Thr-Thr-Asn Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC(N)=O AAZOYLQUEQRUMZ-GSSVUCPTSA-N 0.000 description 1
- MFMGPEKYBXFIRF-SUSMZKCASA-N Thr-Thr-Gln Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(O)=O MFMGPEKYBXFIRF-SUSMZKCASA-N 0.000 description 1
- LECUEEHKUFYOOV-ZJDVBMNYSA-N Thr-Thr-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@@H](N)[C@@H](C)O LECUEEHKUFYOOV-ZJDVBMNYSA-N 0.000 description 1
- BEZTUFWTPVOROW-KJEVXHAQSA-N Thr-Tyr-Arg Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N)O BEZTUFWTPVOROW-KJEVXHAQSA-N 0.000 description 1
- BZTSQFWJNJYZSX-JRQIVUDYSA-N Thr-Tyr-Asp Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(O)=O)C(O)=O BZTSQFWJNJYZSX-JRQIVUDYSA-N 0.000 description 1
- REJRKTOJTCPDPO-IRIUXVKKSA-N Thr-Tyr-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(O)=O)C(O)=O REJRKTOJTCPDPO-IRIUXVKKSA-N 0.000 description 1
- FYBFTPLPAXZBOY-KKHAAJSZSA-N Thr-Val-Asp Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O FYBFTPLPAXZBOY-KKHAAJSZSA-N 0.000 description 1
- MNYNCKZAEIAONY-XGEHTFHBSA-N Thr-Val-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O MNYNCKZAEIAONY-XGEHTFHBSA-N 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 239000004473 Threonine Substances 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 101800000385 Transmembrane protein Proteins 0.000 description 1
- 102000008579 Transposases Human genes 0.000 description 1
- 108010020764 Transposases Proteins 0.000 description 1
- 241000589886 Treponema Species 0.000 description 1
- 229920004890 Triton X-100 Polymers 0.000 description 1
- 239000013504 Triton X-100 Substances 0.000 description 1
- MJBBMTOGSOSAKJ-HJXMPXNTSA-N Trp-Ala-Ile Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O MJBBMTOGSOSAKJ-HJXMPXNTSA-N 0.000 description 1
- VZBWRZGNEPBRDE-HZUKXOBISA-N Trp-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC2=CNC3=CC=CC=C32)N VZBWRZGNEPBRDE-HZUKXOBISA-N 0.000 description 1
- AVYVKJMBNLPWRX-WFBYXXMGSA-N Trp-Ala-Ser Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O)=CNC2=C1 AVYVKJMBNLPWRX-WFBYXXMGSA-N 0.000 description 1
- ADBFWLXCCKIXBQ-XIRDDKMYSA-N Trp-Asn-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)N ADBFWLXCCKIXBQ-XIRDDKMYSA-N 0.000 description 1
- UTQBQJNSNXJNIH-IHPCNDPISA-N Trp-Asn-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC2=CNC3=CC=CC=C32)N UTQBQJNSNXJNIH-IHPCNDPISA-N 0.000 description 1
- BXKWZPXTTSCOMX-AQZXSJQPSA-N Trp-Asn-Thr Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O BXKWZPXTTSCOMX-AQZXSJQPSA-N 0.000 description 1
- RCMHSGRBJCMFLR-BPUTZDHNSA-N Trp-Met-Asn Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(O)=O)=CNC2=C1 RCMHSGRBJCMFLR-BPUTZDHNSA-N 0.000 description 1
- IVBJBFSWJDNQFW-XIRDDKMYSA-N Trp-Pro-Glu Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O IVBJBFSWJDNQFW-XIRDDKMYSA-N 0.000 description 1
- 102000012883 Tumor Necrosis Factor Ligand Superfamily Member 14 Human genes 0.000 description 1
- 108010065158 Tumor Necrosis Factor Ligand Superfamily Member 14 Proteins 0.000 description 1
- 108060008683 Tumor Necrosis Factor Receptor Proteins 0.000 description 1
- 102100026890 Tumor necrosis factor ligand superfamily member 4 Human genes 0.000 description 1
- 102100032100 Tumor necrosis factor ligand superfamily member 8 Human genes 0.000 description 1
- 108091005956 Type II transmembrane proteins Proteins 0.000 description 1
- XGEUYEOEZYFHRL-KKXDTOCCSA-N Tyr-Ala-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=C(O)C=C1 XGEUYEOEZYFHRL-KKXDTOCCSA-N 0.000 description 1
- OOEUVMFKKZYSRX-LEWSCRJBSA-N Tyr-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC2=CC=C(C=C2)O)N OOEUVMFKKZYSRX-LEWSCRJBSA-N 0.000 description 1
- IIJWXEUNETVJPV-IHRRRGAJSA-N Tyr-Arg-Ser Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CO)C(=O)O)N)O IIJWXEUNETVJPV-IHRRRGAJSA-N 0.000 description 1
- CKKFTIQYURNSEI-IHRRRGAJSA-N Tyr-Asn-Arg Chemical compound NC(N)=NCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 CKKFTIQYURNSEI-IHRRRGAJSA-N 0.000 description 1
- NGALWFGCOMHUSN-AVGNSLFASA-N Tyr-Gln-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O NGALWFGCOMHUSN-AVGNSLFASA-N 0.000 description 1
- RYSNTWVRSLCAJZ-RYUDHWBXSA-N Tyr-Gln-Gly Chemical compound OC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 RYSNTWVRSLCAJZ-RYUDHWBXSA-N 0.000 description 1
- CKHQKYHIZCRTAP-SOUVJXGZSA-N Tyr-Gln-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CC2=CC=C(C=C2)O)N)C(=O)O CKHQKYHIZCRTAP-SOUVJXGZSA-N 0.000 description 1
- CTDPLKMBVALCGN-JSGCOSHPSA-N Tyr-Gly-Val Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O CTDPLKMBVALCGN-JSGCOSHPSA-N 0.000 description 1
- LQGDFDYGDQEMGA-PXDAIIFMSA-N Tyr-Ile-Trp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)[C@H](CC3=CC=C(C=C3)O)N LQGDFDYGDQEMGA-PXDAIIFMSA-N 0.000 description 1
- KHCSOLAHNLOXJR-BZSNNMDCSA-N Tyr-Leu-Leu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O KHCSOLAHNLOXJR-BZSNNMDCSA-N 0.000 description 1
- GITNQBVCEQBDQC-KKUMJFAQSA-N Tyr-Lys-Asn Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O GITNQBVCEQBDQC-KKUMJFAQSA-N 0.000 description 1
- SZEIFUXUTBBQFQ-STQMWFEESA-N Tyr-Pro-Gly Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O SZEIFUXUTBBQFQ-STQMWFEESA-N 0.000 description 1
- VPEFOFYNHBWFNQ-UFYCRDLUSA-N Tyr-Pro-Tyr Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 VPEFOFYNHBWFNQ-UFYCRDLUSA-N 0.000 description 1
- VYQQQIRHIFALGE-UWJYBYFXSA-N Tyr-Ser-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 VYQQQIRHIFALGE-UWJYBYFXSA-N 0.000 description 1
- QFXVAFIHVWXXBJ-AVGNSLFASA-N Tyr-Ser-Glu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(O)=O QFXVAFIHVWXXBJ-AVGNSLFASA-N 0.000 description 1
- MDXLPNRXCFOBTL-BZSNNMDCSA-N Tyr-Ser-Tyr Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O MDXLPNRXCFOBTL-BZSNNMDCSA-N 0.000 description 1
- WQOHKVRQDLNDIL-YJRXYDGGSA-N Tyr-Thr-Ser Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O WQOHKVRQDLNDIL-YJRXYDGGSA-N 0.000 description 1
- HMPMGPISLMLHSI-JBACZVJFSA-N Tyr-Trp-Gln Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC3=CC=C(C=C3)O)N HMPMGPISLMLHSI-JBACZVJFSA-N 0.000 description 1
- ANHVRCNNGJMJNG-BZSNNMDCSA-N Tyr-Tyr-Cys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)N[C@@H](CS)C(=O)O)N)O ANHVRCNNGJMJNG-BZSNNMDCSA-N 0.000 description 1
- AEOFMCAKYIQQFY-YDHLFZDLSA-N Tyr-Val-Asn Chemical compound NC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 AEOFMCAKYIQQFY-YDHLFZDLSA-N 0.000 description 1
- 229910052770 Uranium Inorganic materials 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- SLLKXDSRVAOREO-KZVJFYERSA-N Val-Ala-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](C)NC(=O)[C@H](C(C)C)N)O SLLKXDSRVAOREO-KZVJFYERSA-N 0.000 description 1
- VJOWWOGRNXRQMF-UVBJJODRSA-N Val-Ala-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)C(C)C)C(O)=O)=CNC2=C1 VJOWWOGRNXRQMF-UVBJJODRSA-N 0.000 description 1
- CVUDMNSZAIZFAE-TUAOUCFPSA-N Val-Arg-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@@H]1C(=O)O)N CVUDMNSZAIZFAE-TUAOUCFPSA-N 0.000 description 1
- GXAZTLJYINLMJL-LAEOZQHASA-N Val-Asn-Gln Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N GXAZTLJYINLMJL-LAEOZQHASA-N 0.000 description 1
- LIQJSDDOULTANC-QSFUFRPTSA-N Val-Asn-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](C(C)C)N LIQJSDDOULTANC-QSFUFRPTSA-N 0.000 description 1
- LNYOXPDEIZJDEI-NHCYSSNCSA-N Val-Asn-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](C(C)C)N LNYOXPDEIZJDEI-NHCYSSNCSA-N 0.000 description 1
- FPCIBLUVDNXPJO-XPUUQOCRSA-N Val-Cys-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CS)C(=O)NCC(O)=O FPCIBLUVDNXPJO-XPUUQOCRSA-N 0.000 description 1
- XJFXZQKJQGYFMM-GUBZILKMSA-N Val-Cys-Val Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(=O)O)N XJFXZQKJQGYFMM-GUBZILKMSA-N 0.000 description 1
- AGKDVLSDNSTLFA-UMNHJUIQSA-N Val-Gln-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N1CCC[C@@H]1C(=O)O)N AGKDVLSDNSTLFA-UMNHJUIQSA-N 0.000 description 1
- PWRITNSESKQTPW-NRPADANISA-N Val-Gln-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CO)C(=O)O)N PWRITNSESKQTPW-NRPADANISA-N 0.000 description 1
- ROLGIBMFNMZANA-GVXVVHGQSA-N Val-Glu-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](C(C)C)N ROLGIBMFNMZANA-GVXVVHGQSA-N 0.000 description 1
- VHRLUTIMTDOVCG-PEDHHIEDSA-N Val-Ile-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)O)NC(=O)[C@H](C(C)C)N VHRLUTIMTDOVCG-PEDHHIEDSA-N 0.000 description 1
- RWOGENDAOGMHLX-DCAQKATOSA-N Val-Lys-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C(C)C)N RWOGENDAOGMHLX-DCAQKATOSA-N 0.000 description 1
- IJGPOONOTBNTFS-GVXVVHGQSA-N Val-Lys-Glu Chemical compound [H]N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O IJGPOONOTBNTFS-GVXVVHGQSA-N 0.000 description 1
- DIOSYUIWOQCXNR-ONGXEEELSA-N Val-Lys-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O DIOSYUIWOQCXNR-ONGXEEELSA-N 0.000 description 1
- QRVPEKJBBRYISE-XUXIUFHCSA-N Val-Lys-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C(C)C)N QRVPEKJBBRYISE-XUXIUFHCSA-N 0.000 description 1
- ZRSZTKTVPNSUNA-IHRRRGAJSA-N Val-Lys-Leu Chemical compound CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)C(C)C)C(O)=O ZRSZTKTVPNSUNA-IHRRRGAJSA-N 0.000 description 1
- JAKHAONCJJZVHT-DCAQKATOSA-N Val-Lys-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)O)N JAKHAONCJJZVHT-DCAQKATOSA-N 0.000 description 1
- VNGKMNPAENRGDC-JYJNAYRXSA-N Val-Phe-Arg Chemical compound NC(N)=NCCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)CC1=CC=CC=C1 VNGKMNPAENRGDC-JYJNAYRXSA-N 0.000 description 1
- QSPOLEBZTMESFY-SRVKXCTJSA-N Val-Pro-Val Chemical compound CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(O)=O QSPOLEBZTMESFY-SRVKXCTJSA-N 0.000 description 1
- RYHUIHUOYRNNIE-NRPADANISA-N Val-Ser-Gln Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N RYHUIHUOYRNNIE-NRPADANISA-N 0.000 description 1
- KRAHMIJVUPUOTQ-DCAQKATOSA-N Val-Ser-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N KRAHMIJVUPUOTQ-DCAQKATOSA-N 0.000 description 1
- GBIUHAYJGWVNLN-AEJSXWLSSA-N Val-Ser-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N1CCC[C@@H]1C(=O)O)N GBIUHAYJGWVNLN-AEJSXWLSSA-N 0.000 description 1
- GBIUHAYJGWVNLN-UHFFFAOYSA-N Val-Ser-Pro Natural products CC(C)C(N)C(=O)NC(CO)C(=O)N1CCCC1C(O)=O GBIUHAYJGWVNLN-UHFFFAOYSA-N 0.000 description 1
- GUIYPEKUEMQBIK-JSGCOSHPSA-N Val-Tyr-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCC(O)=O GUIYPEKUEMQBIK-JSGCOSHPSA-N 0.000 description 1
- JXWGBRRVTRAZQA-ULQDDVLXSA-N Val-Tyr-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](C(C)C)N JXWGBRRVTRAZQA-ULQDDVLXSA-N 0.000 description 1
- PGBMPFKFKXYROZ-UFYCRDLUSA-N Val-Tyr-Phe Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=CC=C2)C(=O)O)N PGBMPFKFKXYROZ-UFYCRDLUSA-N 0.000 description 1
- ZLNYBMWGPOKSLW-LSJOCFKGSA-N Val-Val-Asp Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O ZLNYBMWGPOKSLW-LSJOCFKGSA-N 0.000 description 1
- AOILQMZPNLUXCM-AVGNSLFASA-N Val-Val-Lys Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCCCN AOILQMZPNLUXCM-AVGNSLFASA-N 0.000 description 1
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 1
- 241000700647 Variola virus Species 0.000 description 1
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 description 1
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 1
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 208000016025 Waldenstroem macroglobulinemia Diseases 0.000 description 1
- 108010017070 Zinc Finger Nucleases Proteins 0.000 description 1
- 239000003070 absorption delaying agent Substances 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 230000021736 acetylation Effects 0.000 description 1
- 238000006640 acetylation reaction Methods 0.000 description 1
- 208000017733 acquired polycythemia vera Diseases 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 208000021841 acute erythroid leukemia Diseases 0.000 description 1
- 208000011912 acute myelomonocytic leukemia M4 Diseases 0.000 description 1
- 230000004658 acute-phase response Effects 0.000 description 1
- 210000005006 adaptive immune system Anatomy 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 108700010877 adenoviridae proteins Proteins 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 238000001042 affinity chromatography Methods 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 108010005233 alanylglutamic acid Proteins 0.000 description 1
- 108010044940 alanylglutamine Proteins 0.000 description 1
- 108010087924 alanylproline Proteins 0.000 description 1
- 238000011316 allogeneic transplantation Methods 0.000 description 1
- 239000003708 ampul Substances 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000000845 anti-microbial effect Effects 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 230000030741 antigen processing and presentation Effects 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 230000009118 appropriate response Effects 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 108010043240 arginyl-leucyl-glycine Proteins 0.000 description 1
- 108010018691 arginyl-threonyl-arginine Proteins 0.000 description 1
- 238000000149 argon plasma sintering Methods 0.000 description 1
- 235000009582 asparagine Nutrition 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 108010038633 aspartylglutamate Proteins 0.000 description 1
- 210000001130 astrocyte Anatomy 0.000 description 1
- 229940065181 bacillus anthracis Drugs 0.000 description 1
- 208000022362 bacterial infectious disease Diseases 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 201000007180 bile duct carcinoma Diseases 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 239000012472 biological sample Substances 0.000 description 1
- 238000005415 bioluminescence Methods 0.000 description 1
- 230000029918 bioluminescence Effects 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 201000001531 bladder carcinoma Diseases 0.000 description 1
- 201000006598 bladder squamous cell carcinoma Diseases 0.000 description 1
- 201000000053 blastoma Diseases 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 1
- 239000004327 boric acid Substances 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 201000008274 breast adenocarcinoma Diseases 0.000 description 1
- 201000008275 breast carcinoma Diseases 0.000 description 1
- 201000010983 breast ductal carcinoma Diseases 0.000 description 1
- 201000003714 breast lobular carcinoma Diseases 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 229960001631 carbomer Drugs 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 230000021523 carboxylation Effects 0.000 description 1
- 238000006473 carboxylation reaction Methods 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 229940105329 carboxymethylcellulose Drugs 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 239000013592 cell lysate Substances 0.000 description 1
- 239000002771 cell marker Substances 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 238000001516 cell proliferation assay Methods 0.000 description 1
- 230000033383 cell-cell recognition Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 201000006662 cervical adenocarcinoma Diseases 0.000 description 1
- 208000019065 cervical carcinoma Diseases 0.000 description 1
- 201000006612 cervical squamous cell carcinoma Diseases 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 238000001311 chemical methods and process Methods 0.000 description 1
- 238000007385 chemical modification Methods 0.000 description 1
- 239000012707 chemical precursor Substances 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 238000003501 co-culture Methods 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 229940047120 colony stimulating factors Drugs 0.000 description 1
- 238000002591 computed tomography Methods 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- QTCANKDTWWSCMR-UHFFFAOYSA-N costic aldehyde Natural products C1CCC(=C)C2CC(C(=C)C=O)CCC21C QTCANKDTWWSCMR-UHFFFAOYSA-N 0.000 description 1
- 230000004940 costimulation Effects 0.000 description 1
- 238000009109 curative therapy Methods 0.000 description 1
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 230000016396 cytokine production Effects 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 230000007402 cytotoxic response Effects 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 239000002619 cytotoxin Substances 0.000 description 1
- 230000005860 defense response to virus Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000000432 density-gradient centrifugation Methods 0.000 description 1
- 238000001212 derivatisation Methods 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- UGMCXQCYOVCMTB-UHFFFAOYSA-K dihydroxy(stearato)aluminium Chemical compound CCCCCCCCCCCCCCCCCC(=O)O[Al](O)O UGMCXQCYOVCMTB-UHFFFAOYSA-K 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 239000002612 dispersion medium Substances 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 201000008184 embryoma Diseases 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 239000012645 endogenous antigen Substances 0.000 description 1
- 208000037828 epithelial carcinoma Diseases 0.000 description 1
- 230000000925 erythroid effect Effects 0.000 description 1
- 210000003238 esophagus Anatomy 0.000 description 1
- JTTUGDHMPCOYDR-UHFFFAOYSA-N ethane;methyl hydrogen sulfate Chemical compound CC.COS(O)(=O)=O JTTUGDHMPCOYDR-UHFFFAOYSA-N 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 208000021045 exocrine pancreatic carcinoma Diseases 0.000 description 1
- 210000004700 fetal blood Anatomy 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 230000005021 gait Effects 0.000 description 1
- 210000000232 gallbladder Anatomy 0.000 description 1
- 229940044627 gamma-interferon Drugs 0.000 description 1
- 150000002270 gangliosides Chemical class 0.000 description 1
- 206010017758 gastric cancer Diseases 0.000 description 1
- 238000001502 gel electrophoresis Methods 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 230000007614 genetic variation Effects 0.000 description 1
- 210000004907 gland Anatomy 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 230000002518 glial effect Effects 0.000 description 1
- 230000001434 glomerular Effects 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 235000004554 glutamine Nutrition 0.000 description 1
- 108010040856 glutamyl-cysteinyl-alanine Proteins 0.000 description 1
- VPZXBVLAVMBEQI-UHFFFAOYSA-N glycyl-DL-alpha-alanine Natural products OC(=O)C(C)NC(=O)CN VPZXBVLAVMBEQI-UHFFFAOYSA-N 0.000 description 1
- 108010087823 glycyltyrosine Proteins 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 210000002216 heart Anatomy 0.000 description 1
- 229940037467 helicobacter pylori Drugs 0.000 description 1
- 201000005787 hematologic cancer Diseases 0.000 description 1
- 208000024200 hematopoietic and lymphoid system neoplasm Diseases 0.000 description 1
- 208000006454 hepatitis Diseases 0.000 description 1
- 231100000283 hepatitis Toxicity 0.000 description 1
- 208000029570 hepatitis D virus infection Diseases 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 108010025306 histidylleucine Proteins 0.000 description 1
- 108010018006 histidylserine Proteins 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 238000001794 hormone therapy Methods 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 125000001165 hydrophobic group Chemical group 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 229940071676 hydroxypropylcellulose Drugs 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 230000008004 immune attack Effects 0.000 description 1
- 230000006058 immune tolerance Effects 0.000 description 1
- 230000003053 immunization Effects 0.000 description 1
- 238000002649 immunization Methods 0.000 description 1
- 230000007813 immunodeficiency Effects 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 230000016784 immunoglobulin production Effects 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 230000036512 infertility Effects 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 230000015788 innate immune response Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 229940047124 interferons Drugs 0.000 description 1
- 230000004073 interleukin-2 production Effects 0.000 description 1
- 108010074108 interleukin-21 Proteins 0.000 description 1
- 201000002313 intestinal cancer Diseases 0.000 description 1
- 230000031146 intracellular signal transduction Effects 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 230000002601 intratumoral effect Effects 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 201000010985 invasive ductal carcinoma Diseases 0.000 description 1
- ISTFUJWTQAMRGA-UHFFFAOYSA-N iso-beta-costal Natural products C1C(C(=C)C=O)CCC2(C)CCCC(C)=C21 ISTFUJWTQAMRGA-UHFFFAOYSA-N 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 108010027338 isoleucylcysteine Proteins 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 210000000244 kidney pelvis Anatomy 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 206010023841 laryngeal neoplasm Diseases 0.000 description 1
- 229940115932 legionella pneumophila Drugs 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 108010047926 leucyl-lysyl-tyrosine Proteins 0.000 description 1
- 108010091871 leucylmethionine Proteins 0.000 description 1
- 108010012058 leucyltyrosine Proteins 0.000 description 1
- 238000001638 lipofection Methods 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 239000008297 liquid dosage form Substances 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 201000002250 liver carcinoma Diseases 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 201000005296 lung carcinoma Diseases 0.000 description 1
- 208000037829 lymphangioendotheliosarcoma Diseases 0.000 description 1
- 208000012804 lymphangiosarcoma Diseases 0.000 description 1
- 230000001926 lymphatic effect Effects 0.000 description 1
- 230000002934 lysing effect Effects 0.000 description 1
- 108010044348 lysyl-glutamyl-aspartic acid Proteins 0.000 description 1
- 108010057952 lysyl-phenylalanyl-lysine Proteins 0.000 description 1
- 108010043322 lysyl-tryptophyl-alpha-lysine Proteins 0.000 description 1
- 108010054155 lysyllysine Proteins 0.000 description 1
- 108010038320 lysylphenylalanine Proteins 0.000 description 1
- 230000002101 lytic effect Effects 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 238000007885 magnetic separation Methods 0.000 description 1
- 230000036210 malignancy Effects 0.000 description 1
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 1
- 210000001161 mammalian embryo Anatomy 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 208000023356 medullary thyroid gland carcinoma Diseases 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 230000001394 metastastic effect Effects 0.000 description 1
- 206010061289 metastatic neoplasm Diseases 0.000 description 1
- 108010085203 methionylmethionine Proteins 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 238000000520 microinjection Methods 0.000 description 1
- 210000000110 microvilli Anatomy 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 201000000050 myeloid neoplasm Diseases 0.000 description 1
- 210000000581 natural killer T-cell Anatomy 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 208000007538 neurilemmoma Diseases 0.000 description 1
- 210000004882 non-tumor cell Anatomy 0.000 description 1
- 230000009701 normal cell proliferation Effects 0.000 description 1
- 238000002515 oligonucleotide synthesis Methods 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 230000002611 ovarian Effects 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 210000003101 oviduct Anatomy 0.000 description 1
- 239000006179 pH buffering agent Substances 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 238000002559 palpation Methods 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 201000008129 pancreatic ductal adenocarcinoma Diseases 0.000 description 1
- 238000004091 panning Methods 0.000 description 1
- 239000000123 paper Substances 0.000 description 1
- 244000045947 parasite Species 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 238000005192 partition Methods 0.000 description 1
- 229940051027 pasteurella multocida Drugs 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 229960003742 phenol Drugs 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 108010018625 phenylalanylarginine Proteins 0.000 description 1
- 108010073025 phenylalanylphenylalanine Proteins 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 230000001885 phytohemagglutinin Effects 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 210000004180 plasmocyte Anatomy 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 238000002264 polyacrylamide gel electrophoresis Methods 0.000 description 1
- 208000037244 polycythemia vera Diseases 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920000656 polylysine Polymers 0.000 description 1
- 238000003752 polymerase chain reaction Methods 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000003449 preventive effect Effects 0.000 description 1
- 230000037452 priming Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 238000004393 prognosis Methods 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 230000009696 proliferative response Effects 0.000 description 1
- 108010077112 prolyl-proline Proteins 0.000 description 1
- 108010031719 prolyl-serine Proteins 0.000 description 1
- 229940021993 prophylactic vaccine Drugs 0.000 description 1
- 201000005825 prostate adenocarcinoma Diseases 0.000 description 1
- 201000001514 prostate carcinoma Diseases 0.000 description 1
- 210000001938 protoplast Anatomy 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 238000002708 random mutagenesis Methods 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 238000004064 recycling Methods 0.000 description 1
- WPPDXAHGCGPUPK-UHFFFAOYSA-N red 2 Chemical compound C1=CC=CC=C1C(C1=CC=CC=C11)=C(C=2C=3C4=CC=C5C6=CC=C7C8=C(C=9C=CC=CC=9)C9=CC=CC=C9C(C=9C=CC=CC=9)=C8C8=CC=C(C6=C87)C(C=35)=CC=2)C4=C1C1=CC=CC=C1 WPPDXAHGCGPUPK-UHFFFAOYSA-N 0.000 description 1
- 108010054624 red fluorescent protein Proteins 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 108010056030 retronectin Proteins 0.000 description 1
- 238000010839 reverse transcription Methods 0.000 description 1
- 210000003705 ribosome Anatomy 0.000 description 1
- 201000005404 rubella Diseases 0.000 description 1
- 235000019515 salmon Nutrition 0.000 description 1
- 206010039667 schwannoma Diseases 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 238000010187 selection method Methods 0.000 description 1
- 108010048397 seryl-lysyl-leucine Proteins 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 210000002027 skeletal muscle Anatomy 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 201000000849 skin cancer Diseases 0.000 description 1
- HELHAJAZNSDZJO-OLXYHTOASA-L sodium L-tartrate Chemical compound [Na+].[Na+].[O-]C(=O)[C@H](O)[C@@H](O)C([O-])=O HELHAJAZNSDZJO-OLXYHTOASA-L 0.000 description 1
- 229910001415 sodium ion Inorganic materials 0.000 description 1
- 239000001433 sodium tartrate Substances 0.000 description 1
- 229960002167 sodium tartrate Drugs 0.000 description 1
- 235000011004 sodium tartrates Nutrition 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000008247 solid mixture Substances 0.000 description 1
- 210000001082 somatic cell Anatomy 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 230000009295 sperm incapacitation Effects 0.000 description 1
- 210000000278 spinal cord Anatomy 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 108010005652 splenotritin Proteins 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 201000011549 stomach cancer Diseases 0.000 description 1
- 238000013517 stratification Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 210000004243 sweat Anatomy 0.000 description 1
- 201000010965 sweat gland carcinoma Diseases 0.000 description 1
- 210000000225 synapse Anatomy 0.000 description 1
- 238000002626 targeted therapy Methods 0.000 description 1
- 201000003120 testicular cancer Diseases 0.000 description 1
- 210000001550 testis Anatomy 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 229940021747 therapeutic vaccine Drugs 0.000 description 1
- 210000000115 thoracic cavity Anatomy 0.000 description 1
- 108010031491 threonyl-lysyl-glutamic acid Proteins 0.000 description 1
- 210000001685 thyroid gland Anatomy 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 210000003437 trachea Anatomy 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 230000010474 transient expression Effects 0.000 description 1
- 238000011277 treatment modality Methods 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 108010080629 tryptophan-leucine Proteins 0.000 description 1
- 108010038745 tryptophylglycine Proteins 0.000 description 1
- 201000008827 tuberculosis Diseases 0.000 description 1
- 210000005239 tubule Anatomy 0.000 description 1
- 210000005102 tumor initiating cell Anatomy 0.000 description 1
- 102000003298 tumor necrosis factor receptor Human genes 0.000 description 1
- 230000005751 tumor progression Effects 0.000 description 1
- 108010071635 tyrosyl-prolyl-arginine Proteins 0.000 description 1
- 241000724775 unclassified viruses Species 0.000 description 1
- 241001430294 unidentified retrovirus Species 0.000 description 1
- 230000003827 upregulation Effects 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- 208000010570 urinary bladder carcinoma Diseases 0.000 description 1
- 210000004291 uterus Anatomy 0.000 description 1
- 210000001215 vagina Anatomy 0.000 description 1
- 239000004474 valine Substances 0.000 description 1
- 210000005166 vasculature Anatomy 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 108010000998 wheylin-2 peptide Proteins 0.000 description 1
- 239000000230 xanthan gum Substances 0.000 description 1
- 229920001285 xanthan gum Polymers 0.000 description 1
- 235000010493 xanthan gum Nutrition 0.000 description 1
- 229940082509 xanthan gum Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/39558—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against tumor tissues, cells, antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001102—Receptors, cell surface antigens or cell surface determinants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001102—Receptors, cell surface antigens or cell surface determinants
- A61K39/001103—Receptors for growth factors
- A61K39/001106—Her-2/neu/ErbB2, Her-3/ErbB3 or Her 4/ErbB4
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001102—Receptors, cell surface antigens or cell surface determinants
- A61K39/001103—Receptors for growth factors
- A61K39/001109—Vascular endothelial growth factor receptors [VEGFR]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001102—Receptors, cell surface antigens or cell surface determinants
- A61K39/001111—Immunoglobulin superfamily
- A61K39/001113—CD22, BL-CAM, siglec-2 or sialic acid- binding Ig-related lectin 2
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001102—Receptors, cell surface antigens or cell surface determinants
- A61K39/001111—Immunoglobulin superfamily
- A61K39/001114—CD74, Ii, MHC class II invariant chain or MHC class II gamma chain
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001102—Receptors, cell surface antigens or cell surface determinants
- A61K39/001116—Receptors for cytokines
- A61K39/001117—Receptors for tumor necrosis factors [TNF], e.g. lymphotoxin receptor [LTR] or CD30
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001102—Receptors, cell surface antigens or cell surface determinants
- A61K39/001116—Receptors for cytokines
- A61K39/001119—Receptors for interleukins [IL]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001102—Receptors, cell surface antigens or cell surface determinants
- A61K39/001124—CD20
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001102—Receptors, cell surface antigens or cell surface determinants
- A61K39/001128—CD44 not IgG
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001102—Receptors, cell surface antigens or cell surface determinants
- A61K39/001129—Molecules with a "CD" designation not provided for elsewhere
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001152—Transcription factors, e.g. SOX or c-MYC
- A61K39/001153—Wilms tumor 1 [WT1]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001154—Enzymes
- A61K39/001157—Telomerase or TERT [telomerase reverse transcriptase]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001166—Adhesion molecules, e.g. NRCAM, EpCAM or cadherins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001166—Adhesion molecules, e.g. NRCAM, EpCAM or cadherins
- A61K39/001168—Mesothelin [MSLN]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001169—Tumor associated carbohydrates
- A61K39/00117—Mucins, e.g. MUC-1
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001169—Tumor associated carbohydrates
- A61K39/001171—Gangliosides, e.g. GM2, GD2 or GD3
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/00118—Cancer antigens from embryonic or fetal origin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/00118—Cancer antigens from embryonic or fetal origin
- A61K39/001182—Carcinoembryonic antigen [CEA]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001184—Cancer testis antigens, e.g. SSX, BAGE, GAGE or SAGE
- A61K39/001186—MAGE
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001184—Cancer testis antigens, e.g. SSX, BAGE, GAGE or SAGE
- A61K39/001188—NY-ESO
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001193—Prostate associated antigens e.g. Prostate stem cell antigen [PSCA]; Prostate carcinoma tumor antigen [PCTA]; PAP or PSGR
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001193—Prostate associated antigens e.g. Prostate stem cell antigen [PSCA]; Prostate carcinoma tumor antigen [PCTA]; PAP or PSGR
- A61K39/001195—Prostate specific membrane antigen [PSMA]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/44—Antibodies bound to carriers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/11—T-cells, e.g. tumour infiltrating lymphocytes [TIL] or regulatory T [Treg] cells; Lymphokine-activated killer [LAK] cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4274—Prostate associated antigens e.g. Prostate stem cell antigen [PSCA]; Prostate carcinoma tumor antigen [PCTA]; Prostatic acid phosphatase [PAP]; Prostate-specific G-protein-coupled receptor [PSGR]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4274—Prostate associated antigens e.g. Prostate stem cell antigen [PSCA]; Prostate carcinoma tumor antigen [PCTA]; Prostatic acid phosphatase [PAP]; Prostate-specific G-protein-coupled receptor [PSGR]
- A61K40/4276—Prostate specific membrane antigen [PSMA]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/08—Drugs for disorders of the urinary system of the prostate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/08—Drugs for genital or sexual disorders; Contraceptives for gonadal disorders or for enhancing fertility, e.g. inducers of ovulation or of spermatogenesis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/14—Drugs for genital or sexual disorders; Contraceptives for lactation disorders, e.g. galactorrhoea
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70517—CD8
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70521—CD28, CD152
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70578—NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2809—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against the T-cell receptor (TcR)-CD3 complex
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
- C07K16/3069—Reproductive system, e.g. ovaria, uterus, testes, prostate
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0636—T lymphocytes
- C12N5/0638—Cytotoxic T lymphocytes [CTL] or lymphokine activated killer cells [LAK]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/24—Hydrolases (3) acting on glycosyl compounds (3.2)
- C12N9/2402—Hydrolases (3) acting on glycosyl compounds (3.2) hydrolysing O- and S- glycosyl compounds (3.2.1)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/7051—T-cell receptor (TcR)-CD3 complex
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/31—Immunoglobulins specific features characterized by aspects of specificity or valency multispecific
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/622—Single chain antibody (scFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/74—Inducing cell proliferation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/03—Fusion polypeptide containing a localisation/targetting motif containing a transmembrane segment
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/33—Fusion polypeptide fusions for targeting to specific cell types, e.g. tissue specific targeting, targeting of a bacterial subspecies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/70—Fusion polypeptide containing domain for protein-protein interaction
- C07K2319/74—Fusion polypeptide containing domain for protein-protein interaction containing a fusion for binding to a cell surface receptor
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2510/00—Genetically modified cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2999/00—Further aspects of viruses or vectors not covered by groups C12N2710/00 - C12N2796/00 or C12N2800/00
- C12N2999/002—Adverse teaching
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Microbiology (AREA)
- Pharmacology & Pharmacy (AREA)
- Mycology (AREA)
- Oncology (AREA)
- Organic Chemistry (AREA)
- Cell Biology (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Zoology (AREA)
- Engineering & Computer Science (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biomedical Technology (AREA)
- Gastroenterology & Hepatology (AREA)
- Toxicology (AREA)
- Wood Science & Technology (AREA)
- Developmental Biology & Embryology (AREA)
- Reproductive Health (AREA)
- Biotechnology (AREA)
- Gynecology & Obstetrics (AREA)
- Pregnancy & Childbirth (AREA)
- General Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Hematology (AREA)
- Vascular Medicine (AREA)
- Endocrinology (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261709072P | 2012-10-02 | 2012-10-02 | |
| US61/709,072 | 2012-10-02 | ||
| CN201380063036.8A CN104853766A (zh) | 2012-10-02 | 2013-10-02 | 用于免疫疗法的组合物和方法 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201380063036.8A Division CN104853766A (zh) | 2012-10-02 | 2013-10-02 | 用于免疫疗法的组合物和方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN112458057A true CN112458057A (zh) | 2021-03-09 |
Family
ID=50435409
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201380063036.8A Pending CN104853766A (zh) | 2012-10-02 | 2013-10-02 | 用于免疫疗法的组合物和方法 |
| CN202011102703.7A Pending CN112430580A (zh) | 2012-10-02 | 2013-10-02 | 用于免疫疗法的组合物和方法 |
| CN202011103486.3A Pending CN112458057A (zh) | 2012-10-02 | 2013-10-02 | 用于免疫疗法的组合物和方法 |
Family Applications Before (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201380063036.8A Pending CN104853766A (zh) | 2012-10-02 | 2013-10-02 | 用于免疫疗法的组合物和方法 |
| CN202011102703.7A Pending CN112430580A (zh) | 2012-10-02 | 2013-10-02 | 用于免疫疗法的组合物和方法 |
Country Status (16)
| Country | Link |
|---|---|
| US (4) | US10654928B2 (OSRAM) |
| EP (2) | EP3597215A1 (OSRAM) |
| JP (5) | JP6441802B2 (OSRAM) |
| KR (1) | KR102198058B1 (OSRAM) |
| CN (3) | CN104853766A (OSRAM) |
| AU (3) | AU2013327136A1 (OSRAM) |
| CA (1) | CA2886859C (OSRAM) |
| ES (1) | ES2743738T3 (OSRAM) |
| IL (2) | IL238047B (OSRAM) |
| MX (1) | MX370148B (OSRAM) |
| NZ (2) | NZ746914A (OSRAM) |
| PH (2) | PH12015500747B1 (OSRAM) |
| RU (1) | RU2729401C2 (OSRAM) |
| SG (1) | SG11201502598SA (OSRAM) |
| WO (1) | WO2014055668A1 (OSRAM) |
| ZA (1) | ZA201502880B (OSRAM) |
Families Citing this family (355)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9783591B2 (en) | 2012-02-22 | 2017-10-10 | The Trustees Of The University Of Pennsylvania | Use of the CD2 signaling domain in second-generation chimeric antigen receptors |
| CN104853766A (zh) | 2012-10-02 | 2015-08-19 | 纪念斯隆-凯特琳癌症中心 | 用于免疫疗法的组合物和方法 |
| US10117896B2 (en) | 2012-10-05 | 2018-11-06 | The Trustees Of The University Of Pennsylvania | Use of a trans-signaling approach in chimeric antigen receptors |
| ES2949648T3 (es) | 2012-12-20 | 2023-10-02 | Purdue Research Foundation | Células T que expresan un receptor antigénico quimérico como terapia contra el cáncer |
| AU2013204922B2 (en) | 2012-12-20 | 2015-05-14 | Celgene Corporation | Chimeric antigen receptors |
| SG11201505858VA (en) | 2013-01-28 | 2015-09-29 | St Jude Childrens Res Hospital | A chimeric receptor with nkg2d specificity for use in cell therapy against cancer and infectious disease |
| EP2953475B1 (en) * | 2013-02-06 | 2019-07-03 | Celgene Corporation | Modified t lymphocytes having improved specificity |
| MX378463B (es) | 2013-02-26 | 2025-03-10 | Memorial Sloan Kettering Cancer Center | Composiciones y usos de las mismas para inmunoterapia. |
| HK1220205A1 (zh) | 2013-03-15 | 2017-04-28 | Celgene Corporation | 修饰的t淋巴细胞 |
| CA3185368A1 (en) * | 2013-04-03 | 2014-10-09 | Memorial Sloan-Kettering Cancer Center | Effective generation of tumor-targeted t cells derived from pluripotent stem cells |
| ES2792849T3 (es) * | 2014-02-10 | 2020-11-12 | Univ Emory | Expresión de polipéptido químico con receptores de linfocitos variables en células inmunes y usos para tratar el cáncer |
| KR102463529B1 (ko) | 2014-04-10 | 2022-11-07 | 시애틀 칠드런즈 호스피탈 디/비/에이 시애틀 칠드런즈 리서치 인스티튜트 | 약물 관련 트랜스진 발현 |
| CA2946312A1 (en) | 2014-04-23 | 2015-10-29 | Juno Therapeutics, Inc. | Methods for isolating, culturing, and genetically engineering immune cell populations for adoptive therapy |
| KR102161927B1 (ko) | 2014-04-25 | 2020-10-06 | 블루버드 바이오, 인코포레이티드. | 양자 세포 치료제를 제조하는 개선된 방법 |
| MX382565B (es) | 2014-04-25 | 2025-03-13 | 2Seventy Bio Inc | Receptores de antigenos quimericos del promotor mnd. |
| EP3805371A1 (en) | 2014-05-15 | 2021-04-14 | National University of Singapore | Modified natural killer cells and uses thereof |
| ES2846811T3 (es) * | 2014-06-06 | 2021-07-29 | Bluebird Bio Inc | Composiciones de células T mejoradas |
| EP4166148A1 (en) | 2014-06-06 | 2023-04-19 | Memorial Sloan-Kettering Cancer Center | Mesothelin-targeted chimeric antigen receptors and uses thereof |
| WO2016011210A2 (en) | 2014-07-15 | 2016-01-21 | Juno Therapeutics, Inc. | Engineered cells for adoptive cell therapy |
| EP3722316A1 (en) | 2014-07-21 | 2020-10-14 | Novartis AG | Treatment of cancer using a cd33 chimeric antigen receptor |
| JP7054622B2 (ja) | 2014-07-21 | 2022-04-14 | ノバルティス アーゲー | ヒト化抗bcmaキメラ抗原受容体を使用した癌の処置 |
| SG10201900455YA (en) | 2014-07-24 | 2019-02-27 | Bluebird Bio Inc | Bcma chimeric antigen receptors |
| HRP20201153T1 (hr) | 2014-08-08 | 2021-01-22 | The Board Of Trustees Of The Leland Stanford Junior University | Pd-1 sredstva visokog afiniteta i načini uporabe |
| TWI805109B (zh) | 2014-08-28 | 2023-06-11 | 美商奇諾治療有限公司 | 對cd19具專一性之抗體及嵌合抗原受體 |
| ES2688035T3 (es) * | 2014-08-29 | 2018-10-30 | Gemoab Monoclonals Gmbh | Receptor de antígeno universal que expresa células inmunes para direccionamiento de antígenos múltiples diversos, procedimiento para fabricación del mismo y utilización del mismo para tratamiento de cáncer, infecciones y enfermedades autoinmunes |
| IL293714A (en) | 2014-10-20 | 2022-08-01 | Juno Therapeutics Inc | Methods and compositions for dosing in adoptive cell therapy |
| AU2015338984A1 (en) * | 2014-10-31 | 2017-04-27 | The Trustees Of The University Of Pennsylvania | Methods and compositions for modified T cells |
| EP3215601B1 (en) | 2014-11-05 | 2020-05-27 | Juno Therapeutics, Inc. | Methods for transduction and cell processing |
| KR20250067192A (ko) | 2014-12-03 | 2025-05-14 | 주노 쎄러퓨티크스 인코퍼레이티드 | 입양 세포 치료를 위한 방법 및 조성물 |
| WO2016094304A2 (en) | 2014-12-12 | 2016-06-16 | Bluebird Bio, Inc. | Bcma chimeric antigen receptors |
| CA3224507A1 (en) * | 2014-12-24 | 2016-06-30 | Autolus Limited | Cell co-expressing chimeric antigen receptors binding cd19 and cd22 |
| MA41346A (fr) | 2015-01-12 | 2017-11-21 | Juno Therapeutics Inc | Eléments régulateurs post-transcriptionnels d'hépatite modifiée |
| MX2017009254A (es) | 2015-01-16 | 2017-10-12 | Juno Therapeutics Inc | Anticuerpos y receptores de antigeno quimerico especificos para ror1. |
| CN114058582A (zh) | 2015-01-26 | 2022-02-18 | 菲特治疗公司 | 用于诱导造血细胞分化的方法和组合物 |
| US20170151281A1 (en) | 2015-02-19 | 2017-06-01 | Batu Biologics, Inc. | Chimeric antigen receptor dendritic cell (car-dc) for treatment of cancer |
| AU2016222887B2 (en) * | 2015-02-24 | 2022-07-14 | The Regents Of The University Of California | Binding-triggered transcriptional switches and methods of use thereof |
| KR102632082B1 (ko) | 2015-02-27 | 2024-02-02 | 아이셀 진 테라퓨틱스 엘엘씨 | 혈액 악성종양을 표적으로 하는 키메라 항원 수용체(car), 조성물 및 이의 용도 |
| EP3277727B1 (en) * | 2015-04-02 | 2021-11-03 | Memorial Sloan Kettering Cancer Center | Tnfrsf14/ hvem proteins and methods of use thereof |
| JP6961490B2 (ja) | 2015-04-08 | 2021-11-05 | ノバルティス アーゲー | Cd20療法、cd22療法、およびcd19キメラ抗原受容体(car)発現細胞との併用療法 |
| AU2016248090A1 (en) * | 2015-04-15 | 2017-11-02 | Prospect CharterCare RWMC, LLC d/b/a Roger Williams Medical Center | Hepatic arterial infusion of CAR-T cells |
| WO2016166568A1 (en) | 2015-04-16 | 2016-10-20 | Juno Therapeutics Gmbh | Methods, kits and apparatus for expanding a population of cells |
| WO2016168595A1 (en) | 2015-04-17 | 2016-10-20 | Barrett David Maxwell | Methods for improving the efficacy and expansion of chimeric antigen receptor-expressing cells |
| EP3286225B1 (en) | 2015-04-23 | 2020-07-01 | Baylor College of Medicine | Cd5 chimeric antigen receptor for adoptive t cell therapy |
| RU2017140778A (ru) | 2015-04-25 | 2019-05-27 | Дзе Дженерал Хоспитал Корпорейшн | Комбинированная терапия антифугетактическим средством и противораковым средством, и композиции для лечения рака |
| IL303905A (en) | 2015-05-18 | 2023-08-01 | Tcr2 Therapeutics Inc | Compositions and methods for TCR programming using fusion proteins |
| AU2016271147B2 (en) | 2015-05-29 | 2022-09-08 | Juno Therapeutics, Inc. | Composition and methods for regulating inhibitory interactions in genetically engineered cells |
| EP4424326A3 (en) | 2015-06-10 | 2024-11-13 | ImmunityBio, Inc. | Modified nk-92 cells for treating cancer |
| CN115058395B (zh) | 2015-06-25 | 2025-07-18 | 美商生物细胞基因治疗有限公司 | 嵌合抗原受体(car)、组合物及其使用方法 |
| US11173179B2 (en) | 2015-06-25 | 2021-11-16 | Icell Gene Therapeutics Llc | Chimeric antigen receptor (CAR) targeting multiple antigens, compositions and methods of use thereof |
| WO2017222593A1 (en) | 2016-06-24 | 2017-12-28 | Icell Gene Therapeutics Llc | Chimeric antigen receptors (cars), compositions and methods thereof |
| MA42895A (fr) | 2015-07-15 | 2018-05-23 | Juno Therapeutics Inc | Cellules modifiées pour thérapie cellulaire adoptive |
| AR105433A1 (es) * | 2015-07-21 | 2017-10-04 | Novartis Ag | Métodos para mejorar la eficacia y expansión de las células inmunes |
| EP3328994A4 (en) * | 2015-07-31 | 2019-04-17 | Memorial Sloan-Kettering Cancer Center | CD56-Faced Antigen-Binding Proteins and Uses Thereof |
| GB201513540D0 (en) * | 2015-07-31 | 2015-09-16 | King S College London | Therapeutic agents |
| JP7162530B2 (ja) | 2015-08-07 | 2022-10-28 | シアトル チルドレンズ ホスピタル (ディービーエイ シアトル チルドレンズ リサーチ インスティテュート) | 固形腫瘍を標的とする二重特異性car t細胞 |
| CN105087495B (zh) * | 2015-08-21 | 2016-04-27 | 深圳市茵冠生物科技有限公司 | 双嵌合抗原受体修饰的t淋巴细胞及其制备方法 |
| US11242375B2 (en) | 2015-09-04 | 2022-02-08 | Memorial Sloan Kettering Cancer Center | Immune cell compositions and methods of use |
| WO2017049208A1 (en) | 2015-09-18 | 2017-03-23 | The General Hospital Corporation Dba Massachusetts General Hospital | Localized delivery of anti-fugetactic agent for treatment of cancer |
| CN108289954B (zh) | 2015-09-24 | 2022-05-31 | 阿布维特罗有限责任公司 | Hiv抗体组合物和使用方法 |
| CN105924529B (zh) * | 2015-10-13 | 2019-08-06 | 中国人民解放军总医院 | 嵌合抗原受体及其基因和重组表达载体、car138-nkt细胞及其制备方法和应用 |
| MA45488A (fr) | 2015-10-22 | 2018-08-29 | Juno Therapeutics Gmbh | Procédés, kits et appareil de culture de cellules |
| MA45489A (fr) | 2015-10-22 | 2018-08-29 | Juno Therapeutics Gmbh | Procédés de culture de cellules, kits et appareil associés |
| AU2016341527B2 (en) | 2015-10-22 | 2023-04-27 | Juno Therapeutics Gmbh | Methods, kits, agents and apparatuses for transduction |
| MX2018004721A (es) | 2015-10-23 | 2018-07-06 | Eureka Therapeutics Inc | Construcciones quimericas anticuerpo/receptor de celulas t y usos de las mismas. |
| CN115806940A (zh) | 2015-11-04 | 2023-03-17 | 菲特治疗公司 | 多能细胞的基因组工程改造 |
| EP3371301A4 (en) | 2015-11-04 | 2019-06-26 | Fate Therapeutics, Inc. | METHOD AND COMPOSITIONS FOR INDUCING THE DIFFERENTIATION OF HEMATOPOIETIC CELLS |
| MA44314A (fr) | 2015-11-05 | 2018-09-12 | Juno Therapeutics Inc | Récepteurs chimériques contenant des domaines induisant traf, et compositions et méthodes associées |
| WO2017079703A1 (en) | 2015-11-05 | 2017-05-11 | Juno Therapeutics, Inc. | Vectors and genetically engineered immune cells expressing metabolic pathway modulators and uses in adoptive cell therapy |
| EP4212547A1 (en) | 2015-12-03 | 2023-07-19 | Juno Therapeutics, Inc. | Modified chimeric receptors and related compositions and methods |
| CA3007258A1 (en) | 2015-12-03 | 2017-06-08 | Mark L. Bonyhadi | Compositions and methods for reducing immune responses against cell therapies |
| HK1257295A1 (zh) | 2015-12-04 | 2019-10-18 | Novartis Ag | 用於免疫肿瘤学的组合物和方法 |
| EP4012415A3 (en) | 2015-12-04 | 2022-12-07 | Juno Therapeutics, Inc. | Methods and compositions related to toxicity associated with cell therapy |
| US11479755B2 (en) | 2015-12-07 | 2022-10-25 | 2Seventy Bio, Inc. | T cell compositions |
| WO2017112877A1 (en) * | 2015-12-22 | 2017-06-29 | Icell Gene Therapeutics, Llc | Chimeric antigen receptors and enhancement of anti-tumor activity |
| US11571469B2 (en) | 2016-01-07 | 2023-02-07 | Mayo Foundation For Medical Education And Research | Methods of treating cancer with interferon wherein the cancer cells are HLA negative or have reduced HLA expression |
| MA43758A (fr) | 2016-03-16 | 2018-11-28 | Yuan Ji | Procédés pour déterminer le dosage d'un agent thérapeutique et traitements associés |
| WO2017161212A1 (en) | 2016-03-16 | 2017-09-21 | Juno Therapeutics, Inc. | Methods for adaptive design of a treatment regimen and related treatments |
| US11518814B2 (en) | 2016-03-22 | 2022-12-06 | Seattle Children's Hospital | Early intervention methods to prevent or ameliorate toxicity |
| CN109195990A (zh) | 2016-03-30 | 2019-01-11 | Musc研究发展基金会 | 通过靶向糖蛋白a重复优势蛋白(garp)治疗和诊断癌症以及单独或联合提供有效免疫疗法的方法 |
| JP7282521B2 (ja) | 2016-04-08 | 2023-05-29 | パーデュー・リサーチ・ファウンデイション | Car t細胞療法のための方法および組成物 |
| MX2018013445A (es) | 2016-05-06 | 2019-09-09 | Juno Therapeutics Inc | Celulas diseñadas geneticamente y metodos para obtener las mismas. |
| WO2017193059A1 (en) | 2016-05-06 | 2017-11-09 | The Regents Of The University Of California | Systems and methods for targeting cancer cells |
| SG11201809542XA (en) * | 2016-05-25 | 2018-12-28 | Council Queensland Inst Medical Res | Methods of immunotherapy |
| MX388294B (es) | 2016-05-27 | 2025-03-19 | Aadigen Llc | Peptidos y nanoparticulas para suministro intracelular de moleculas editoras de genoma. |
| EP3463401A4 (en) | 2016-06-03 | 2020-01-22 | Memorial Sloan Kettering Cancer Center | ADOPTIVE CELL THERAPIES AS EARLY TREATMENT OPTIONS |
| MA45341A (fr) | 2016-06-06 | 2019-04-10 | Hutchinson Fred Cancer Res | Procédés de traitement de malignités de lymphocytes b au moyen d'une thérapie cellulaire adoptive |
| WO2018005559A1 (en) | 2016-06-27 | 2018-01-04 | Juno Therapeutics, Inc. | Method of identifying peptide epitopes, molecules that bind such epitopes and related uses |
| MA45491A (fr) | 2016-06-27 | 2019-05-01 | Juno Therapeutics Inc | Épitopes à restriction cmh-e, molécules de liaison et procédés et utilisations associés |
| CN109844099B (zh) * | 2016-07-25 | 2024-01-02 | 美国政府(由卫生和人类服务部的部长所代表) | 产生经修饰的自然杀伤细胞的方法及使用方法 |
| CA3031955A1 (en) | 2016-07-29 | 2018-02-01 | Juno Therapeutics, Inc. | Immunomodulatory polypeptides and related compositions and methods |
| KR20230107408A (ko) | 2016-07-29 | 2023-07-14 | 주노 쎄러퓨티크스 인코퍼레이티드 | 항-cd19 항체에 대한 항-이디오타입 항체 |
| WO2018023094A1 (en) | 2016-07-29 | 2018-02-01 | Juno Therapeutics, Inc. | Methods for assessing the presence or absence of replication competent virus |
| JP7109789B2 (ja) | 2016-08-02 | 2022-08-01 | ティーシーアール2 セラピューティクス インク. | 融合タンパク質を使用したtcrの再プログラム化のための組成物及び方法 |
| EP3494132A4 (en) * | 2016-08-03 | 2020-03-18 | Washington University | Gene editing of car-t cells for the treatment of t cell malignancies with chimeric antigen receptors |
| CA3000514A1 (en) * | 2016-08-04 | 2018-02-08 | Memorial Sloan-Kettering Cancer Center | Cancer antigen targets and uses thereof |
| US11401332B2 (en) | 2016-08-23 | 2022-08-02 | The Regents Of The University Of California | Proteolytically cleavable chimeric polypeptides and methods of use thereof |
| AU2017319151B2 (en) | 2016-08-30 | 2024-01-11 | Memorial Sloan Kettering Cancer Center | Immune cell compositions and methods of use for treating viral and other infections |
| WO2018049420A1 (en) | 2016-09-12 | 2018-03-15 | Juno Therapeutics, Inc. | Perfusion bioreactor bag assemblies |
| EP4353319A3 (en) | 2016-09-28 | 2024-06-05 | Atossa Therapeutics, Inc. | Methods of adoptive cell therapy |
| KR20190062505A (ko) | 2016-10-03 | 2019-06-05 | 주노 쎄러퓨티크스 인코퍼레이티드 | Hpv-특이적 결합 분자 |
| ES2875959T3 (es) | 2016-10-07 | 2021-11-11 | Tcr2 Therapeutics Inc | Composiciones y métodos para reprogramación de receptores de linfocitos T mediante el uso de proteínas de fusión |
| MA46649A (fr) | 2016-10-13 | 2019-08-21 | Juno Therapeutics Inc | Méthodes et compositions d'immunothérapie impliquant des modulateurs de la voie métabolique du tryptophane |
| CN110139669A (zh) | 2016-11-03 | 2019-08-16 | 朱诺治疗学股份有限公司 | T细胞疗法和btk抑制剂的组合疗法 |
| MA46716A (fr) | 2016-11-03 | 2019-09-11 | Juno Therapeutics Inc | Polythérapie de thérapie cellulaire et d'inhibiteur de la microglie |
| CN110022906A (zh) | 2016-11-04 | 2019-07-16 | 蓝鸟生物公司 | 抗bcma car t细胞组合物 |
| AU2017363311A1 (en) | 2016-11-22 | 2019-06-13 | TCR2 Therapeutics Inc. | Compositions and methods for TCR reprogramming using fusion proteins |
| AU2017367695A1 (en) | 2016-12-02 | 2019-06-13 | Juno Therapeutics, Inc. | Engineered B cells and related compositions and methods |
| MA46963A (fr) | 2016-12-03 | 2019-10-09 | Juno Therapeutics Inc | Méthodes pour déterminer le dosage de céllules car-t |
| AU2017368332A1 (en) | 2016-12-03 | 2019-06-13 | Juno Therapeutics, Inc. | Methods for modulation of CAR-T cells |
| CN110545826A (zh) | 2016-12-03 | 2019-12-06 | 朱诺治疗学股份有限公司 | 用于与激酶抑制剂组合使用治疗性t细胞的方法和组合物 |
| US20190350978A1 (en) | 2016-12-05 | 2019-11-21 | Juno Therapeutics, Inc. | Production of engineered cells for adoptive cell therapy |
| CN110291200B (zh) | 2016-12-12 | 2024-05-14 | 西雅图儿童医院(Dba西雅图儿童研究所) | 对哺乳动物细胞中转基因表达的药剂配体诱导具有增大的灵敏度的嵌合转录因子变异体 |
| EP3336107A1 (en) * | 2016-12-15 | 2018-06-20 | Miltenyi Biotec GmbH | Immune cells expressing an antigen binding receptor and a chimeric costimulatory receptor |
| CN106749677B (zh) * | 2017-01-04 | 2020-06-12 | 上海交通大学医学院附属瑞金医院 | 一种靶向mll白血病的双特异性嵌合抗原受体基因及其应用 |
| CN110691792A (zh) | 2017-01-10 | 2020-01-14 | 朱诺治疗学股份有限公司 | 细胞疗法的表观遗传学分析及相关方法 |
| CN110418802A (zh) | 2017-01-20 | 2019-11-05 | 朱诺治疗学有限公司 | 细胞表面缀合物及相关的细胞组合物和方法 |
| MX2019009552A (es) | 2017-02-17 | 2019-10-02 | Hutchinson Fred Cancer Res | Terapias de combinacion para el tratamiento de canceres relacionados con el antigeno de maduracion de celulas b (bcma) y trastornos autoinmunitarios. |
| ES2988795T3 (es) | 2017-02-27 | 2024-11-21 | Juno Therapeutics Inc | Composiciones, artículos de fabricación y métodos relacionados con la dosificación en terapia celular |
| US11850262B2 (en) | 2017-02-28 | 2023-12-26 | Purdue Research Foundation | Compositions and methods for CAR T cell therapy |
| AU2018231190B2 (en) | 2017-03-08 | 2023-05-25 | Memorial Sloan Kettering Cancer Center | Immune cell compositions and methods of use |
| AU2018234640C9 (en) | 2017-03-14 | 2024-10-03 | Juno Therapeutics, Inc. | Methods for cryogenic storage |
| KR20230166145A (ko) | 2017-03-15 | 2023-12-06 | 옥스포드 바이오메디카(유케이) 리미티드 | 방법 |
| EP3601561A2 (en) | 2017-03-22 | 2020-02-05 | Novartis AG | Compositions and methods for immunooncology |
| EP3600395A4 (en) | 2017-03-23 | 2021-05-05 | The General Hospital Corporation | BLOCKING OF CXCR4 / CXCR7 AND TREATMENT OF HUMAN PAPILLOMAVIRUS-ASSOCIATED DISEASE |
| SG11201908337VA (en) | 2017-03-27 | 2019-10-30 | Nat Univ Singapore | Stimulatory cell lines for ex vivo expansion and activation of natural killer cells |
| CA3056439A1 (en) | 2017-03-27 | 2018-10-04 | National University Of Singapore | Truncated nkg2d chimeric receptors and uses thereof in natural killer cell immunotherapy |
| KR102009040B1 (ko) | 2017-04-05 | 2019-08-08 | 한국생명공학연구원 | Nk 세포 활성화 융합 단백질, nk 세포 및 이들을 포함하는 약제학적 조성물 |
| AU2018250336B2 (en) | 2017-04-07 | 2025-02-20 | Juno Therapeutics, Inc. | Engineered cells expressing prostate-specific membrane antigen (PSMA) or a modified form thereof and related methods |
| MA54103A (fr) | 2017-04-14 | 2021-09-15 | Juno Therapeutics Inc | Procédés d'évaluation de la glycosylation de surface cellulaire |
| EA201992469A1 (ru) | 2017-04-18 | 2020-05-27 | Фуджифилм Селльюлар Дайнамикс, Инк. | Антигенспецифические иммунные эффекторные клетки |
| AU2018258045B2 (en) * | 2017-04-26 | 2024-02-29 | Eureka Therapeutics, Inc. | Chimeric antibody/T-cell receptor constructs and uses thereof |
| EP4647493A2 (en) | 2017-04-27 | 2025-11-12 | Juno Therapeutics, Inc. | Oligomeric particle reagents and methods of use thereof |
| DK3618842T3 (da) | 2017-05-01 | 2024-01-02 | Juno Therapeutics Inc | Kombination af en celleterapi og en immunomodulerende forbindelse |
| WO2018223098A1 (en) | 2017-06-02 | 2018-12-06 | Juno Therapeutics, Inc. | Articles of manufacture and methods related to toxicity associated with cell therapy |
| WO2018223101A1 (en) | 2017-06-02 | 2018-12-06 | Juno Therapeutics, Inc. | Articles of manufacture and methods for treatment using adoptive cell therapy |
| EP3538645B1 (en) | 2017-06-20 | 2021-01-20 | Institut Curie | Immune cells defective for suv39h1 |
| CN118853581A (zh) | 2017-06-21 | 2024-10-29 | 美商生物细胞基因治疗有限公司 | 嵌合抗原受体(CARs)、组合物及其使用方法 |
| AU2018288863A1 (en) | 2017-06-22 | 2020-01-30 | Board Of Regents, The University Of Texas System | Methods for producing regulatory immune cells and uses thereof |
| CA3067602A1 (en) | 2017-06-29 | 2019-01-03 | Juno Therapeutics, Inc. | Mouse model for assessing toxicities associated with immunotherapies |
| AU2018306307B2 (en) | 2017-07-25 | 2025-04-17 | Board Of Regents, The University Of Texas System | Enhanced chimeric antigen receptors and use thereof |
| US20230242654A1 (en) | 2017-07-29 | 2023-08-03 | Juno Therapeutics, Inc. | Reagents for expanding cells expressing recombinant receptors |
| CN107326014B (zh) * | 2017-07-31 | 2019-09-24 | 时力生物科技(北京)有限公司 | 一种双特异性嵌合抗原受体修饰的t淋巴细胞及其制备方法和应用 |
| ES2959953T3 (es) | 2017-08-09 | 2024-02-29 | Juno Therapeutics Inc | Métodos para producir composiciones celulares genéticamente modificadas y composiciones relacionadas |
| MX2020001491A (es) | 2017-08-09 | 2020-08-06 | Juno Therapeutics Inc | Metodos y composiciones para preparar celulas geneticamente modificadas. |
| US20210071258A1 (en) | 2017-09-01 | 2021-03-11 | Juno Therapeutics, Inc. | Gene expression and assessment of risk of developing toxicity following cell therapy |
| EP3679370A1 (en) | 2017-09-07 | 2020-07-15 | Juno Therapeutics, Inc. | Methods of identifying cellular attributes related to outcomes associated with cell therapy |
| CN109517820B (zh) | 2017-09-20 | 2021-09-24 | 北京宇繁生物科技有限公司 | 一种靶向HPK1的gRNA以及HPK1基因编辑方法 |
| EP3692063A1 (en) | 2017-10-03 | 2020-08-12 | Juno Therapeutics, Inc. | Hpv-specific binding molecules |
| KR102823603B1 (ko) | 2017-10-12 | 2025-06-23 | 더 보드 오브 리젠츠 오브 더 유니버시티 오브 텍사스 시스템 | 면역요법을 위한 t 세포 수용체 |
| KR20200074997A (ko) | 2017-11-01 | 2020-06-25 | 주노 쎄러퓨티크스 인코퍼레이티드 | B-세포 성숙 항원에 특이적인 항체 및 키메릭 항원 수용체 |
| EP3704229B1 (en) | 2017-11-01 | 2023-12-20 | Juno Therapeutics, Inc. | Process for producing a t cell composition |
| US11564946B2 (en) | 2017-11-01 | 2023-01-31 | Juno Therapeutics, Inc. | Methods associated with tumor burden for assessing response to a cell therapy |
| WO2019089982A1 (en) | 2017-11-01 | 2019-05-09 | Juno Therapeutics, Inc. | Method of assessing activity of recombinant antigen receptors |
| PL3703750T3 (pl) | 2017-11-01 | 2025-04-07 | Juno Therapeutics, Inc. | Chimeryczne receptory antygenowe specyficzne dla antygenu dojrzewania komórek b i kodujące polinukleotydy |
| BR112020008478A2 (pt) | 2017-11-01 | 2020-10-20 | Editas Medicine, Inc. | métodos, composições e componentes para edição de crispr-cas9 de tgfbr2 em células t para imunota-rapia |
| US12031975B2 (en) | 2017-11-01 | 2024-07-09 | Juno Therapeutics, Inc. | Methods of assessing or monitoring a response to a cell therapy |
| SG11202003688PA (en) | 2017-11-01 | 2020-05-28 | Juno Therapeutics Inc | Process for generating therapeutic compositions of engineered cells |
| CN111542545A (zh) | 2017-11-03 | 2020-08-14 | 索伦托治疗有限公司 | Cd38定向嵌合抗原受体构建体 |
| KR20200079312A (ko) | 2017-11-06 | 2020-07-02 | 에디타스 메디신, 인코포레이티드 | 면역요법을 위한 t 세포 내 cblb의 crispr-cas9 편집 방법, 조성물 및 성분 |
| CA3080395A1 (en) | 2017-11-06 | 2019-05-09 | Juno Therapeutics, Inc. | Combination of a cell therapy and a gamma secretase inhibitor |
| CN111556789A (zh) | 2017-11-10 | 2020-08-18 | 朱诺治疗学股份有限公司 | 封闭系统低温器皿 |
| CN111655270A (zh) * | 2017-11-14 | 2020-09-11 | 纪念斯隆-凯特琳癌症中心 | 分泌il-36的免疫应答细胞及其用途 |
| EP3716980A1 (en) | 2017-12-01 | 2020-10-07 | Juno Therapeutics, Inc. | Methods for dosing and for modulation of genetically engineered cells |
| EP3720882A4 (en) | 2017-12-05 | 2021-10-27 | The Medical Research, Infrastructure and Health Services Fund of the Tel Aviv Medical Center | T LYMPHOCYTES INCLUDING TWO DIFFERENT ANTIGENIC CHEMERICAL RECEPTORS AND THEIR USES |
| CN111587254B (zh) | 2017-12-05 | 2025-02-25 | 特拉维夫医疗中心医学研究基础设施及卫生服务基金 | 包含抗cd38和抗cd138嵌合抗原受体的t-细胞及其用途 |
| KR102853341B1 (ko) | 2017-12-08 | 2025-09-02 | 주노 쎄러퓨티크스 인코퍼레이티드 | 조작된 t 세포의 조성물을 제조하는 방법 |
| CA3084446A1 (en) | 2017-12-08 | 2019-06-13 | Juno Therapeutics, Inc. | Phenotypic markers for cell therapy and related methods |
| SG11202005228YA (en) | 2017-12-08 | 2020-07-29 | Juno Therapeutics Inc | Serum-free media formulation for culturing cells and methods of use thereof |
| CN112204048A (zh) | 2017-12-15 | 2021-01-08 | 朱诺治疗学股份有限公司 | 抗cct5结合分子及其使用方法 |
| CN109988242A (zh) * | 2018-01-02 | 2019-07-09 | 武汉波睿达生物科技有限公司 | 联合嵌合抗原受体、表达载体、慢病毒及t细胞 |
| WO2019134036A1 (en) | 2018-01-03 | 2019-07-11 | Qu Biologics Inc. | Innate targeting of adoptive cellular therapies |
| WO2019139972A1 (en) | 2018-01-09 | 2019-07-18 | Board Of Regents, The University Of Texas System | T cell receptors for immunotherapy |
| JP2021511784A (ja) * | 2018-01-19 | 2021-05-13 | カースゲン セラピューティクス カンパニー リミテッドCarsgen Therapeutics Co., Ltd. | SynNotch受容体によるIL12の発現調節 |
| BR112020014913A2 (pt) | 2018-01-22 | 2020-12-08 | Seattle Children's Hospital (dba Seattle Children's Research Institute) | Métodos para uso de células t car |
| CN111971059A (zh) | 2018-01-31 | 2020-11-20 | 细胞基因公司 | 使用过继细胞疗法和检查点抑制剂的组合疗法 |
| JP2021511802A (ja) | 2018-01-31 | 2021-05-13 | ジュノー セラピューティクス インコーポレイテッド | 複製可能ウイルスの存在または非存在を評価するための方法および試薬 |
| CN108314739B (zh) * | 2018-02-05 | 2020-07-14 | 深圳市默赛尔生物医学科技发展有限公司 | 多信号嵌合抗原受体及其表达基因、其修饰的nk细胞及应用 |
| SG11202007156QA (en) | 2018-02-09 | 2020-08-28 | Nat Univ Singapore | Activating chimeric receptors and uses thereof in natural killer cell immunotherapy |
| KR102828999B1 (ko) | 2018-02-11 | 2025-07-04 | 메모리얼 슬로안 케터링 캔서 센터 | 비-hla 제한된 t 세포 수용체 및 이의 용도 |
| EP3752601A4 (en) | 2018-02-15 | 2022-03-23 | Memorial Sloan-Kettering Cancer Center | FOXP3-TARGETING AGENT COMPOSITIONS AND METHODS OF USE FOR ADOPTIVE CELL THERAPY |
| CA3091674A1 (en) | 2018-02-23 | 2019-08-29 | Endocyte, Inc. | Sequencing method for car t cell therapy |
| US20210046159A1 (en) | 2018-03-09 | 2021-02-18 | Ospedale San Raffaele S.R.L. | Il-1 antagonist and toxicity induced by cell therapy |
| WO2019170147A1 (zh) | 2018-03-09 | 2019-09-12 | 科济生物医药(上海)有限公司 | 用于治疗肿瘤的方法和组合物 |
| EP3774919A4 (en) * | 2018-03-30 | 2021-12-29 | Eureka Therapeutics, Inc. | Constructs targeting cd22 and uses thereof |
| SG11202008976YA (en) | 2018-04-02 | 2020-10-29 | Nat Univ Singapore | Neutralization of human cytokines with membrane-bound anti-cytokine non-signaling binders expressed in immune cells |
| WO2019195491A1 (en) | 2018-04-05 | 2019-10-10 | Juno Therapeutics, Inc. | T cells expressing a recombinant receptor, related polynucleotides and methods |
| KR102764123B1 (ko) | 2018-04-05 | 2025-02-05 | 주노 쎄러퓨티크스 인코퍼레이티드 | Τ 세포 수용체 및 이를 발현하는 조작된 세포 |
| JP7589047B2 (ja) | 2018-04-05 | 2024-11-25 | ジュノー セラピューティクス インコーポレイテッド | 組換え受容体を発現する細胞の作製方法および関連組成物 |
| EP3781177A4 (en) | 2018-04-19 | 2022-03-02 | Baylor College of Medicine | REPROGRAMMING OF CD4 T CELLS INTO CYTOTOXIC CD8 CELLS BY FORCED EXPRESSION OF CD8AB AND CLASS 1 RESTRICTED T CELL RECEPTORS |
| CN108864288A (zh) * | 2018-04-26 | 2018-11-23 | 上海怡豪生物科技有限公司 | 乳腺癌的双靶点car-t治疗载体及其构建方法和应用 |
| BR112020022185A2 (pt) | 2018-05-03 | 2021-02-02 | Juno Therapeutics Inc | terapia de combinação de uma terapia de células t do receptor de antígeno quimérico (car) e um inibidor de quinase |
| TWI890660B (zh) | 2018-06-13 | 2025-07-21 | 瑞士商諾華公司 | Bcma 嵌合抗原受體及其用途 |
| SG11202012342WA (en) * | 2018-06-18 | 2021-01-28 | Eureka Therapeutics Inc | Constructs targeting prostate-specific membrane antigen (psma) and uses thereof |
| CN110615843B (zh) * | 2018-06-20 | 2023-05-09 | 上海隆耀生物科技有限公司 | 一种包含第三信号受体的嵌合抗原受体及其应用 |
| CN112930199B (zh) | 2018-07-24 | 2024-08-13 | 恺兴生命科技(上海)有限公司 | 免疫效应细胞治疗肿瘤的方法 |
| SG11202101204TA (en) | 2018-08-09 | 2021-03-30 | Juno Therapeutics Inc | Processes for generating engineered cells and compositions thereof |
| KR20210059715A (ko) | 2018-08-09 | 2021-05-25 | 주노 쎄러퓨티크스 인코퍼레이티드 | 통합된 핵산 평가 방법 |
| KR102839381B1 (ko) | 2018-08-29 | 2025-07-28 | 내셔널 유니버시티 오브 싱가포르 | 유전적으로 변형된 면역 세포의 생존 및 확장을 특이적으로 자극하는 방법 |
| BR112021003631A2 (pt) * | 2018-09-05 | 2021-05-18 | Glaxosmithkline Intellectual Property Development Limited | modificação de célula t |
| EA202190693A1 (ru) | 2018-09-11 | 2021-07-27 | Джуно Терапьютикс, Инк. | Способы анализа масс-спектрометрии композиций модифицированных клеток |
| IL281413B2 (en) | 2018-09-19 | 2024-09-01 | Fujifilm Cellular Dynamics Inc | Protein l for activation and expansion of chimeric antigen receptor-modified immune cells |
| AU2019370705A1 (en) | 2018-10-31 | 2021-05-27 | Juno Therapeutics Gmbh | Methods for selection and stimulation of cells and apparatus for same |
| PE20211058A1 (es) | 2018-11-01 | 2021-06-07 | Juno Therapeutics Inc | Receptores de antigenos quimericos especificos para el miembro d del grupo 5 de la clase c del receptor acoplado a proteina g (gprc5d) |
| CA3117419A1 (en) | 2018-11-01 | 2020-05-07 | Juno Therapeutics, Inc. | Methods for treatment using chimeric antigen receptors specific for b-cell maturation antigen |
| EP3877054B1 (en) | 2018-11-06 | 2023-11-01 | Juno Therapeutics, Inc. | Process for producing genetically engineered t cells |
| AU2019377854A1 (en) | 2018-11-08 | 2021-05-27 | Juno Therapeutics, Inc. | Methods and combinations for treatment and T cell modulation |
| KR20210104713A (ko) | 2018-11-16 | 2021-08-25 | 주노 쎄러퓨티크스 인코퍼레이티드 | B 세포 악성 종양 치료를 위한 조작된 t 세포 투약 방법 |
| WO2020106621A1 (en) | 2018-11-19 | 2020-05-28 | Board Of Regents, The University Of Texas System | A modular, polycistronic vector for car and tcr transduction |
| BR112021010297A2 (pt) | 2018-11-28 | 2021-08-24 | Board Of Regents, The University Of Texas System | Edição de genomas multiplexadores de células imunes para aumentar a funcionalidade e resistência ao ambiente supressivo |
| MX2021006393A (es) | 2018-11-29 | 2021-10-13 | Univ Texas | Metodos para expansion ex vivo de celulas exterminadoras naturales y uso de las mismas. |
| EP3886894B1 (en) | 2018-11-30 | 2024-03-13 | Juno Therapeutics, Inc. | Methods for dosing and treatment of b cell malignancies in adoptive cell therapy |
| WO2020113194A2 (en) | 2018-11-30 | 2020-06-04 | Juno Therapeutics, Inc. | Methods for treatment using adoptive cell therapy |
| WO2020160050A1 (en) | 2019-01-29 | 2020-08-06 | Juno Therapeutics, Inc. | Antibodies and chimeric antigen receptors specific for receptor tyrosine kinase like orphan receptor 1 (ror1) |
| KR20220030205A (ko) * | 2019-02-08 | 2022-03-10 | 디앤에이 투포인토 인크. | 면역 세포의 트랜스포존 기반 변형 |
| AU2020226401A1 (en) * | 2019-02-18 | 2021-10-14 | Memorial Sloan-Kettering Cancer Center | Combinations of multiple chimeric antigen receptors for immunotherapy |
| GB201902277D0 (en) | 2019-02-19 | 2019-04-03 | King S College London | Therapeutic agents |
| AU2020232691B2 (en) | 2019-03-05 | 2023-06-29 | Nkarta, Inc. | CD19-directed chimeric antigen receptors and uses thereof in immunotherapy |
| WO2020198128A1 (en) * | 2019-03-22 | 2020-10-01 | The Regents Of The University Of California | Switchable chimeric antigen receptor-engineered human natural killer cells |
| AU2020272074A1 (en) * | 2019-04-12 | 2021-11-25 | The Trustees Of The University Of Pennsylvania | Compositions and Methods Comprising a High Affinity Chimeric Antigen Receptor (CAR) with Cross-Reactivity to Clinically-Relevant EGFR Mutated Proteins |
| JP7711945B2 (ja) | 2019-04-30 | 2025-07-23 | センティ バイオサイエンシズ インコーポレイテッド | キメラ受容体及びその使用方法 |
| CN114025788A (zh) | 2019-05-01 | 2022-02-08 | 朱诺治疗学股份有限公司 | 从经修饰的tgfbr2基因座表达重组受体的细胞、相关多核苷酸和方法 |
| MX2021013223A (es) | 2019-05-01 | 2022-02-17 | Juno Therapeutics Inc | Celulas que expresan un receptor quimerico de un locus cd247 modificado, polinucleotidos relacionados y metodos. |
| CA3139965A1 (en) | 2019-06-07 | 2020-12-10 | Ivie AIFUWA | Automated t cell culture |
| KR20220034782A (ko) | 2019-06-12 | 2022-03-18 | 주노 쎄러퓨티크스 인코퍼레이티드 | 세포 매개 세포 독성 요법 및 친생존 bcl2 패밀리 단백질 억제제의 병용 요법 |
| AU2020318781A1 (en) | 2019-07-23 | 2022-02-10 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Immune cells defective for SUV39H1 |
| US20220298222A1 (en) | 2019-08-22 | 2022-09-22 | Juno Therapeutics, Inc. | Combination therapy of a t cell therapy and an enhancer of zeste homolog 2 (ezh2) inhibitor and related methods |
| US20220298223A1 (en) * | 2019-08-28 | 2022-09-22 | King's College London | B CELL TARGETED PARALLEL CAR (pCAR) THERAPEUTIC AGENTS |
| CN114600172A (zh) | 2019-08-30 | 2022-06-07 | 朱诺治疗学股份有限公司 | 用于将细胞分类的机器学习方法 |
| BR112022003790A2 (pt) | 2019-09-02 | 2022-05-31 | Inst Nat Sante Rech Med | Método para selecionar um peptídeo neoantigênico tumoral, peptídeos neoantigênicos tumorais, peptídeo neoantigênico tumoral isolado, população de células dendríticas autólogas, vacina ou composição imunogênica, anticorpos, método de produção de um anticorpo, receptor de células t, polinucleotídeo, vetor, célula imune, célula t, peptídeo neoantigênico e população de células imunes |
| US20220401539A1 (en) | 2019-10-22 | 2022-12-22 | Institut Curie | Immunotherapy Targeting Tumor Neoantigenic Peptides |
| AU2020377043A1 (en) | 2019-10-30 | 2022-06-02 | Juno Therapeutics Gmbh | Cell selection and/or stimulation devices and methods of use |
| EP4055383A1 (en) | 2019-11-05 | 2022-09-14 | Juno Therapeutics, Inc. | Methods of determining attributes of therapeutic t cell compositions |
| JP2022554353A (ja) | 2019-11-07 | 2022-12-28 | ジュノー セラピューティクス インコーポレイテッド | T細胞療法と(s)-3-[4-(4-モルホリン-4-イルメチル-ベンジルオキシ)-1-オキソ-1,3-ジヒドロ-イソインドール-2-イル]-ピペリジン-2,6-ジオンとの併用 |
| BR112022009679A2 (pt) | 2019-11-26 | 2022-08-09 | Novartis Ag | Receptores de antígeno quimérico cd19 e cd22 e usos dos mesmos |
| US20230192869A1 (en) | 2019-12-06 | 2023-06-22 | Juno Therapeutics, Inc. | Anti-idiotypic antibodies to gprc5d-targeted binding domains and related compositions and methods |
| JP2023504740A (ja) | 2019-12-06 | 2023-02-06 | ジュノー セラピューティクス インコーポレイテッド | Bcma標的結合ドメインに対する抗イディオタイプ抗体ならびに関連する組成物および方法 |
| WO2021113770A1 (en) | 2019-12-06 | 2021-06-10 | Juno Therapeutics, Inc. | Methods related to toxicity and response associated with cell therapy for treating b cell malignancies |
| US10980836B1 (en) | 2019-12-11 | 2021-04-20 | Myeloid Therapeutics, Inc. | Therapeutic cell compositions and methods of manufacturing and use thereof |
| MX2022009041A (es) | 2020-01-24 | 2022-10-07 | Juno Therapeutics Inc | Metodos de dosificacion y tratamiento de linfoma folicular y linfoma de zona marginal en terapia celular adoptiva. |
| EP4097218A1 (en) | 2020-01-28 | 2022-12-07 | Juno Therapeutics, Inc. | Methods for t cell transduction |
| CN115768443A (zh) | 2020-02-12 | 2023-03-07 | 朱诺治疗学股份有限公司 | Cd19定向嵌合抗原受体t细胞组合物和方法及其用途 |
| BR112022015968A2 (pt) | 2020-02-12 | 2022-10-11 | Juno Therapeutics Inc | Composições de células t do receptor de antígeno quimérico direcionado a bcma e métodos e usos das mesmas |
| CA3168337A1 (en) | 2020-02-17 | 2021-08-26 | Marie-Andree Forget | Methods for expansion of tumor infiltrating lymphocytes and use thereof |
| CN115087460A (zh) * | 2020-02-17 | 2022-09-20 | 美天施生物科技有限两合公司 | 用于提供具有针对肿瘤微环境细胞的嵌合抗原受体(car)的个性化细胞的方法 |
| WO2021207689A2 (en) | 2020-04-10 | 2021-10-14 | Juno Therapeutics, Inc. | Methods and uses related to cell therapy engineered with a chimeric antigen receptor targeting b-cell maturation antigen |
| WO2021222330A2 (en) | 2020-04-28 | 2021-11-04 | Juno Therapeutics, Inc. | Combination of bcma-directed t cell therapy and an immunomodulatory compound |
| WO2021228999A1 (en) | 2020-05-12 | 2021-11-18 | Institut Curie | Neoantigenic epitopes associated with sf3b1 mutations |
| KR20230022868A (ko) | 2020-05-13 | 2023-02-16 | 주노 쎄러퓨티크스 인코퍼레이티드 | 재조합 수용체를 발현하는 공여자-배치 세포의 제조 방법 |
| KR20230024283A (ko) | 2020-05-13 | 2023-02-20 | 주노 쎄러퓨티크스 인코퍼레이티드 | 임상 반응과 관련된 특징을 식별하는 방법 및 이의 용도 |
| CN115803084A (zh) | 2020-05-21 | 2023-03-14 | 得克萨斯大学体系董事会 | 具有vgll1特异性的t细胞受体和其用途 |
| US20240216508A1 (en) | 2020-06-26 | 2024-07-04 | Juno Therapeutics Gmbh | Engineered t cells conditionally expressing a recombinant receptor, related polynucleotides and methods |
| CN116348485A (zh) | 2020-07-17 | 2023-06-27 | 英研生物(英国)有限公司 | 提供用于过继性细胞疗法的靶向共刺激的受体 |
| JP2023535371A (ja) | 2020-07-17 | 2023-08-17 | シミュレックス インコーポレイテッド | 免疫抑制シグナル伝達をリダイレクトするためのキメラMyD88受容体ならびに関連する組成物および方法 |
| CA3190266A1 (en) | 2020-07-30 | 2022-02-03 | Institut Curie | Immune cells defective for socs1 |
| WO2022029660A1 (en) | 2020-08-05 | 2022-02-10 | Juno Therapeutics, Inc. | Anti-idiotypic antibodies to ror1-targeted binding domains and related compositions and methods |
| KR20230054391A (ko) | 2020-08-21 | 2023-04-24 | 12343096 캐나다 인코포레이티드 | 모듈러 조립 수용체 및 그의 용도 |
| MX2023004598A (es) | 2020-10-23 | 2023-06-29 | Asher Biotherapeutics Inc | Fusiones con moléculas de unión al antígeno cd8 para modular la función de las células inmunitarias. |
| CA3197423A1 (en) | 2020-11-04 | 2022-05-12 | Daniel Getts | Engineered chimeric fusion protein compositions and methods of use thereof |
| EP4240756A1 (en) | 2020-11-04 | 2023-09-13 | Juno Therapeutics, Inc. | Cells expressing a chimeric receptor from a modified invariant cd3 immunoglobulin superfamily chain locus and related polynucleotides and methods |
| US20230051406A1 (en) | 2020-11-13 | 2023-02-16 | Catamaran Bio, Inc. | Genetically modified natural killer cells and methods of use thereof |
| WO2022115611A1 (en) | 2020-11-25 | 2022-06-02 | Catamaran Bio, Inc. | Cellular therapeutics engineered with signal modulators and methods of use thereof |
| TW202237827A (zh) | 2020-12-03 | 2022-10-01 | 美商世紀治療股份有限公司 | 經基因工程改造之細胞及其用途 |
| US11661459B2 (en) | 2020-12-03 | 2023-05-30 | Century Therapeutics, Inc. | Artificial cell death polypeptide for chimeric antigen receptor and uses thereof |
| WO2022133030A1 (en) | 2020-12-16 | 2022-06-23 | Juno Therapeutics, Inc. | Combination therapy of a cell therapy and a bcl2 inhibitor |
| WO2022133169A1 (en) | 2020-12-18 | 2022-06-23 | Century Therapeutics, Inc. | Chimeric antigen receptor systems with adaptable receptor specificity |
| KR20230151513A (ko) | 2021-01-11 | 2023-11-01 | 사나 바이오테크놀로지, 인크. | Cd8 표적 바이러스 벡터의 용도 |
| WO2022187406A1 (en) | 2021-03-03 | 2022-09-09 | Juno Therapeutics, Inc. | Combination of a t cell therapy and a dgk inhibitor |
| MX2023010641A (es) | 2021-03-11 | 2024-02-07 | Mnemo Therapeutics | Peptidos neoantigénicos tumorales y usos de los mismos. |
| KR20230172630A (ko) | 2021-03-11 | 2023-12-22 | 므네모 테라퓨틱스 | 종양 네오 항원성 펩타이드 |
| KR20240006721A (ko) | 2021-03-11 | 2024-01-15 | 엥스띠뛰 퀴리 | 막 형질 전환 네오 안티젠 펩타이드 |
| EP4308133A4 (en) | 2021-03-17 | 2025-01-22 | Myeloid Therapeutics, Inc. | MANIPULATED CHIMERIC FUSION PROTEIN COMPOSITIONS AND METHODS OF USE THEREOF |
| JP2024511418A (ja) | 2021-03-22 | 2024-03-13 | ジュノー セラピューティクス インコーポレイテッド | 治療用細胞組成物の効力を決定する方法 |
| AU2022244229A1 (en) | 2021-03-22 | 2023-09-14 | Juno Therapeutics, Inc. | Method to assess potency of viral vector particles |
| JP2024513054A (ja) | 2021-03-29 | 2024-03-21 | ジュノー セラピューティクス インコーポレイテッド | リンパ腫の治療のためのcar t細胞療法および免疫調節化合物の組合せ |
| US20240181052A1 (en) | 2021-03-29 | 2024-06-06 | Juno Therapeutics, Inc. | Methods for dosing and treatment with a combination of a checkpoint inhibitor therapy and a car t cell therapy |
| CN118201618A (zh) | 2021-04-16 | 2024-06-14 | 细胞基因公司 | 与bcma定向t细胞疗法的组合疗法 |
| IL307598A (en) | 2021-04-16 | 2023-12-01 | Juno Therapeutics Inc | T-cell therapy in patients who have had a previous stem cell transplant |
| JP2024514942A (ja) | 2021-04-22 | 2024-04-03 | ベイラー カレッジ オブ メディスン | 殺夾雑活性を低下させた免疫細胞を工学的に作製する方法 |
| CN117916256A (zh) | 2021-05-06 | 2024-04-19 | 朱诺治疗学有限公司 | 用于刺激和转导t细胞的方法 |
| US20240269282A1 (en) | 2021-05-10 | 2024-08-15 | Institut Curie | Methods for the Treatment of Cancer, Inflammatory Diseases and Autoimmune Diseases |
| IL308445A (en) | 2021-05-11 | 2024-01-01 | Myeloid Therapeutics Inc | Methods and compositions for genomic integration |
| EP4346912A1 (en) | 2021-05-25 | 2024-04-10 | Institut Curie | Myeloid cells overexpressing bcl2 |
| TW202306997A (zh) | 2021-06-16 | 2023-02-16 | 英商英斯特生物科技有限公司 | 用於在過繼細胞療法中提供標靶共刺激之受體 |
| WO2023288278A1 (en) | 2021-07-16 | 2023-01-19 | Instil Bio (Uk) Limited | Chimeric molecules providing targeted costimulation for adoptive cell therapy |
| IL310550A (en) | 2021-08-04 | 2024-03-01 | Univ Colorado Regents | LAT-activating chimeric antigen receptor T cells and methods of using them |
| WO2023015217A1 (en) | 2021-08-04 | 2023-02-09 | Sana Biotechnology, Inc. | Use of cd4-targeted viral vectors |
| CN117836405A (zh) * | 2021-08-13 | 2024-04-05 | 先声创新公司 | 三维培养多能干细胞产生造血干细胞 |
| WO2023081715A1 (en) | 2021-11-03 | 2023-05-11 | Viracta Therapeutics, Inc. | Combination of car t-cell therapy with btk inhibitors and methods of use thereof |
| EP4426339A1 (en) | 2021-11-03 | 2024-09-11 | Celgene Corporation | Chimeric antigen receptors specific for b-cell maturation antigen for use in treating myeloma |
| WO2023081900A1 (en) | 2021-11-08 | 2023-05-11 | Juno Therapeutics, Inc. | Engineered t cells expressing a recombinant t cell receptor (tcr) and related systems and methods |
| US20250354167A1 (en) | 2021-12-09 | 2025-11-20 | Zygosity Limited | Vector |
| EP4448775A1 (en) | 2021-12-17 | 2024-10-23 | Sana Biotechnology, Inc. | Modified paramyxoviridae attachment glycoproteins |
| WO2023109901A1 (en) | 2021-12-17 | 2023-06-22 | Shanghai Henlius Biotech, Inc. | Anti-ox40 antibodies and methods of use |
| KR20240116755A (ko) | 2021-12-17 | 2024-07-30 | 상하이 헨리우스 바이오테크, 인크. | 항-ox40 항체, 다중 특이적 항체 및 이의 사용 방법 |
| US20250059239A1 (en) | 2021-12-17 | 2025-02-20 | Sana Biotechnology, Inc. | Modified paramyxoviridae fusion glycoproteins |
| EP4456910A1 (en) | 2021-12-28 | 2024-11-06 | Mnemo Therapeutics | Immune cells with inactivated suv39h1 and modified tcr |
| JP2025504882A (ja) | 2022-01-21 | 2025-02-19 | ムネモ・セラピューティクス | Rnaによるsuv39h1発現の調節 |
| JP2025504002A (ja) | 2022-01-28 | 2025-02-06 | ジュノー セラピューティクス インコーポレイテッド | 細胞組成物を製造する方法 |
| EP4472646A1 (en) | 2022-02-01 | 2024-12-11 | Sana Biotechnology, Inc. | Cd3-targeted lentiviral vectors and uses thereof |
| AU2023223404A1 (en) | 2022-02-22 | 2024-08-29 | Juno Therapeutics, Inc. | Proteinase 3 (pr3) chimeric autoantibody receptor t cells and related methods and uses |
| AU2023236826A1 (en) | 2022-03-18 | 2024-09-26 | The Regents Of The University Of Colorado, A Body Corporate | Genetically engineered t-cell co-receptors and methods of use thereof |
| US20250302930A1 (en) | 2022-03-24 | 2025-10-02 | Institut Curie | Immunotherapy targeting tumor transposable element derived neoantigenic peptides in glioblastoma |
| WO2023179740A1 (en) | 2022-03-25 | 2023-09-28 | Shanghai Henlius Biotech , Inc. | Anti-msln antibodies and methods of use |
| WO2023187024A1 (en) | 2022-03-31 | 2023-10-05 | Institut Curie | Modified rela protein for inducing interferon expression and engineered immune cells with improved interferon expression |
| WO2023193015A1 (en) | 2022-04-01 | 2023-10-05 | Sana Biotechnology, Inc. | Cytokine receptor agonist and viral vector combination therapies |
| WO2023196921A1 (en) | 2022-04-06 | 2023-10-12 | The Regents Of The University Of Colorado, A Body Corporate | Granzyme expressing t cells and methods of use |
| US20250304913A1 (en) | 2022-04-06 | 2025-10-02 | The Regents Of The University Of Colorado, A Body Corporate | Chimeric antigen receptor t cells and methods of use thereof |
| EP4514382A1 (en) | 2022-04-28 | 2025-03-05 | Musc Foundation for Research Development | Chimeric antigen receptor modified regulatory t cells for treating cancer |
| WO2023213969A1 (en) | 2022-05-05 | 2023-11-09 | Juno Therapeutics Gmbh | Viral-binding protein and related reagents, articles, and methods of use |
| WO2023220641A2 (en) | 2022-05-11 | 2023-11-16 | Juno Therapeutics, Inc. | Methods and uses related to t cell therapy and production of same |
| US20250302954A1 (en) | 2022-05-11 | 2025-10-02 | Celgene Corporation | Methods to overcome drug resistance by re-sensitizing cancer cells to treatment with a prior therapy via treatment with a t cell therapy |
| WO2023222617A1 (en) | 2022-05-16 | 2023-11-23 | Miltenyi Biotec B.V. & Co. KG | Endogenous signaling molecule activating chimeric antigen receptors and methods of generation thereof |
| EP4279085A1 (en) | 2022-05-20 | 2023-11-22 | Mnemo Therapeutics | Compositions and methods for treating a refractory or relapsed cancer or a chronic infectious disease |
| WO2023230581A1 (en) | 2022-05-25 | 2023-11-30 | Celgene Corporation | Methods of manufacturing t cell therapies |
| WO2023230548A1 (en) | 2022-05-25 | 2023-11-30 | Celgene Corporation | Method for predicting response to a t cell therapy |
| CN115058387B (zh) * | 2022-06-11 | 2023-12-01 | 重庆医科大学 | 一种人胚胎干细胞与前列腺癌细胞共培养制备抗前列腺癌药物的方法 |
| KR20250029137A (ko) | 2022-06-22 | 2025-03-04 | 주노 쎄러퓨티크스 인코퍼레이티드 | Cd19-표적화된 car t 세포의 2차 요법을 위한 치료 방법 |
| EP4547230A1 (en) | 2022-06-29 | 2025-05-07 | Juno Therapeutics, Inc. | Lipid nanoparticles for delivery of nucleic acids |
| WO2024031091A2 (en) | 2022-08-05 | 2024-02-08 | Juno Therapeutics, Inc. | Chimeric antigen receptors specific for gprc5d and bcma |
| AU2023329484A1 (en) | 2022-08-26 | 2025-02-20 | Juno Therapeutics, Inc. | Antibodies and chimeric antigen receptors specific for delta-like ligand 3 (dll3) |
| JP2025531850A (ja) | 2022-09-08 | 2025-09-25 | ジュノー セラピューティクス インコーポレイテッド | T細胞療法および継続的または間欠的dgk阻害剤投薬の組合せ |
| CN117736335A (zh) * | 2022-09-20 | 2024-03-22 | 深圳先进技术研究院 | 靶向间皮素和nkg2d配体的双靶向car-t细胞及其应用 |
| WO2024062138A1 (en) | 2022-09-23 | 2024-03-28 | Mnemo Therapeutics | Immune cells comprising a modified suv39h1 gene |
| WO2024081820A1 (en) | 2022-10-13 | 2024-04-18 | Sana Biotechnology, Inc. | Viral particles targeting hematopoietic stem cells |
| EP4611798A1 (en) | 2022-11-02 | 2025-09-10 | Celgene Corporation | Methods of treatment with t cell therapy and immunomodulatory agent maintenance therapy |
| WO2024100604A1 (en) | 2022-11-09 | 2024-05-16 | Juno Therapeutics Gmbh | Methods for manufacturing engineered immune cells |
| WO2024102948A1 (en) | 2022-11-11 | 2024-05-16 | Celgene Corporation | Fc receptor-homolog 5 (fcrh5) specific binding molecules and bispecific t-cell engaging antibodies including same and related methods |
| KR20250121074A (ko) | 2022-12-09 | 2025-08-11 | 주노 쎄러퓨티크스 인코퍼레이티드 | 홀로그래픽 이미징을 사용하여 세포 표현형을 예측하기 위한 기계 학습 방법 |
| AR131320A1 (es) | 2022-12-13 | 2025-03-05 | Juno Therapeutics Inc | Receptores de antígenos quiméricos específicos para baff-r y cd19 y métodos y usos de los mismos |
| TW202430215A (zh) | 2022-12-14 | 2024-08-01 | 美商旗艦先鋒創新有限責任(Vii)公司 | 用於將治療劑遞送至骨之組成物和方法 |
| CN121002052A (zh) | 2023-02-03 | 2025-11-21 | C3S2 有限公司 | 用于非病毒生产工程化免疫细胞的方法 |
| TW202502363A (zh) | 2023-02-28 | 2025-01-16 | 美商奇諾治療有限公司 | 用於治療全身性自體免疫疾病之細胞療法 |
| US12257304B2 (en) | 2023-03-03 | 2025-03-25 | Arsenal Biosciences, Inc. | Systems targeting PSMA and CA9 |
| WO2024220574A1 (en) | 2023-04-18 | 2024-10-24 | Sana Biotechnology, Inc. | Universal protein g fusogens and adapter systems thereof and related lipid particles and uses |
| WO2024220588A1 (en) | 2023-04-18 | 2024-10-24 | Juno Therapeutics, Inc. | Cytotoxicity assay for assessing potency of therapeutic cell compositions |
| WO2024220560A1 (en) | 2023-04-18 | 2024-10-24 | Sana Biotechnology, Inc. | Engineered protein g fusogens and related lipid particles and methods thereof |
| WO2024220598A2 (en) | 2023-04-18 | 2024-10-24 | Sana Biotechnology, Inc. | Lentiviral vectors with two or more genomes |
| WO2024226858A1 (en) | 2023-04-26 | 2024-10-31 | Juno Therapeutics, Inc. | Methods for viral vector manufacturing |
| WO2024243365A2 (en) | 2023-05-23 | 2024-11-28 | Juno Therapeutics, Inc. | Activation markers of t cells and method for assessing t cell activation |
| WO2025054202A1 (en) | 2023-09-05 | 2025-03-13 | Sana Biotechnology, Inc. | Method of screening a sample comprising a transgene with a unique barcode |
| EP4520334A1 (en) | 2023-09-07 | 2025-03-12 | Mnemo Therapeutics | Methods and compositions for improving immune response |
| WO2025052001A1 (en) | 2023-09-07 | 2025-03-13 | Mnemo Therapeutics | Methods and compositions for improving immune response |
| WO2025059362A1 (en) | 2023-09-13 | 2025-03-20 | Juno Therapeutics, Inc. | Combination therapies with a cell therapy expressing a gprc5d-targeting car and related methods and uses |
| WO2025076472A1 (en) | 2023-10-06 | 2025-04-10 | Juno Therapeutics, Inc. | Combination therapies with a cell therapy expressing a gprc5d-targeting car and related methods and uses |
| WO2025097055A2 (en) | 2023-11-02 | 2025-05-08 | The Broad Institute, Inc. | Compositions and methods of use of t cells in immunotherapy |
| WO2025096975A1 (en) | 2023-11-02 | 2025-05-08 | The Regents Of The University Of Colorado, A Body Corporate | Compositions and methods of enhancing immune cell therapies by runx2 modulation |
| WO2025128434A1 (en) | 2023-12-14 | 2025-06-19 | The Regents Of The University Of Colorado, A Body Corporate | Compositions and methods for the treatment of ven/aza resistant acute myeloid leukemia |
| WO2025147545A1 (en) | 2024-01-03 | 2025-07-10 | Juno Therapeutics, Inc. | Lipid nanoparticles for delivery of nucleic acids and related methods and uses |
| WO2025151838A1 (en) | 2024-01-12 | 2025-07-17 | Sana Biotechnology, Inc. | Safety switches to control in vitro and in vivo proliferation of cell therapy products |
| WO2025163107A1 (en) | 2024-02-01 | 2025-08-07 | Institut Gustave Roussy | Immune cells defective for znf217 and uses thereof |
| WO2025184421A1 (en) | 2024-02-28 | 2025-09-04 | Juno Therapeutics, Inc. | Chimeric antigen receptors and antibodies specific for delta-like ligand 3 (dll3) and related methods |
| WO2025212519A1 (en) | 2024-04-01 | 2025-10-09 | Moonlight Bio, Inc. | Dll3 binding proteins and uses thereof |
| US20250345431A1 (en) | 2024-05-10 | 2025-11-13 | Juno Therapeutics, Inc. | Genetically engineered t cells expressing a cd19 chimeric antigen receptor (car) and uses thereof for allogeneic cell therapy |
| WO2025250011A1 (en) | 2024-05-29 | 2025-12-04 | Stichting Het Nederlands Kanker Instituut-Antoni van Leeuwenhoek Ziekenhuis | Treatment for cancer |
| WO2025259108A1 (en) | 2024-06-11 | 2025-12-18 | Prinses Máxima Centrum Voor Kinderoncologie B.V. | Brain organoid |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101119745A (zh) * | 2002-08-20 | 2008-02-06 | 热尼特里克斯有限责任公司 | 凝集素组合物和调节对抗原免疫应答的方法 |
| WO2008121420A1 (en) * | 2007-03-30 | 2008-10-09 | Memorial Sloan-Kettering Cancer Center | Constitutive expression of costimulatory ligands on adoptively transferred t lymphocytes |
| WO2012109624A2 (en) * | 2011-02-11 | 2012-08-16 | Zyngenia, Inc. | Monovalent and multivalent multispecific complexes and uses thereof |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5399346A (en) | 1989-06-14 | 1995-03-21 | The United States Of America As Represented By The Department Of Health And Human Services | Gene therapy |
| US5712149A (en) | 1995-02-03 | 1998-01-27 | Cell Genesys, Inc. | Chimeric receptor molecules for delivery of co-stimulatory signals |
| GB9605515D0 (en) | 1996-03-15 | 1996-05-15 | Medical Res Council | Improvements in or relating to cell stimulation |
| US7446190B2 (en) * | 2002-05-28 | 2008-11-04 | Sloan-Kettering Institute For Cancer Research | Nucleic acids encoding chimeric T cell receptors |
| US20050129671A1 (en) | 2003-03-11 | 2005-06-16 | City Of Hope | Mammalian antigen-presenting T cells and bi-specific T cells |
| US20050113564A1 (en) * | 2003-11-05 | 2005-05-26 | St. Jude Children's Research Hospital | Chimeric receptors with 4-1BB stimulatory signaling domain |
| US8315214B2 (en) | 2007-05-18 | 2012-11-20 | Research In Motion Limited | Method and system for discontinuous reception de-synchronization detection |
| GB201108236D0 (en) * | 2011-05-17 | 2011-06-29 | Ucl Business Plc | Method |
| US10981969B2 (en) * | 2011-07-29 | 2021-04-20 | The Trustees Of The University Of Pennsylvania | Switch costimulatory receptors |
| CN104853766A (zh) * | 2012-10-02 | 2015-08-19 | 纪念斯隆-凯特琳癌症中心 | 用于免疫疗法的组合物和方法 |
| US10117896B2 (en) * | 2012-10-05 | 2018-11-06 | The Trustees Of The University Of Pennsylvania | Use of a trans-signaling approach in chimeric antigen receptors |
| CA2888332C (en) | 2012-11-09 | 2021-01-19 | Dsm Ip Assets B.V. | Process for enzymatic hydrolysis of lignocellulosic material and fermentation of sugars |
| EP2953475B1 (en) | 2013-02-06 | 2019-07-03 | Celgene Corporation | Modified t lymphocytes having improved specificity |
| US9745368B2 (en) | 2013-03-15 | 2017-08-29 | The Trustees Of The University Of Pennsylvania | Targeting cytotoxic cells with chimeric receptors for adoptive immunotherapy |
-
2013
- 2013-10-02 CN CN201380063036.8A patent/CN104853766A/zh active Pending
- 2013-10-02 MX MX2015004287A patent/MX370148B/es active IP Right Grant
- 2013-10-02 SG SG11201502598SA patent/SG11201502598SA/en unknown
- 2013-10-02 KR KR1020157011160A patent/KR102198058B1/ko active Active
- 2013-10-02 NZ NZ746914A patent/NZ746914A/en unknown
- 2013-10-02 CA CA2886859A patent/CA2886859C/en active Active
- 2013-10-02 JP JP2015534828A patent/JP6441802B2/ja active Active
- 2013-10-02 EP EP19179456.9A patent/EP3597215A1/en active Pending
- 2013-10-02 ES ES13844468T patent/ES2743738T3/es active Active
- 2013-10-02 WO PCT/US2013/063097 patent/WO2014055668A1/en not_active Ceased
- 2013-10-02 RU RU2015116901A patent/RU2729401C2/ru active
- 2013-10-02 CN CN202011102703.7A patent/CN112430580A/zh active Pending
- 2013-10-02 NZ NZ706541A patent/NZ706541A/en unknown
- 2013-10-02 EP EP13844468.2A patent/EP2903637B1/en active Active
- 2013-10-02 AU AU2013327136A patent/AU2013327136A1/en not_active Abandoned
- 2013-10-02 CN CN202011103486.3A patent/CN112458057A/zh active Pending
-
2015
- 2015-03-30 IL IL238047A patent/IL238047B/en active IP Right Grant
- 2015-04-01 US US14/676,255 patent/US10654928B2/en active Active
- 2015-04-06 PH PH12015500747A patent/PH12015500747B1/en unknown
- 2015-04-28 ZA ZA201502880A patent/ZA201502880B/en unknown
-
2018
- 2018-03-15 JP JP2018047734A patent/JP2018108111A/ja not_active Withdrawn
- 2018-06-15 AU AU2018204297A patent/AU2018204297B2/en active Active
-
2019
- 2019-10-25 PH PH12019502424A patent/PH12019502424A1/en unknown
-
2020
- 2020-02-07 IL IL272539A patent/IL272539B/en active IP Right Grant
- 2020-02-17 JP JP2020024521A patent/JP2020096625A/ja not_active Withdrawn
- 2020-02-17 JP JP2020024520A patent/JP6963051B2/ja active Active
- 2020-04-13 US US16/847,059 patent/US11712469B2/en active Active
- 2020-12-24 AU AU2020294287A patent/AU2020294287B2/en active Active
-
2021
- 2021-12-01 JP JP2021195401A patent/JP2022024161A/ja not_active Withdrawn
-
2023
- 2023-06-13 US US18/333,753 patent/US12263220B2/en active Active
-
2025
- 2025-02-26 US US19/063,828 patent/US20250186585A1/en active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101119745A (zh) * | 2002-08-20 | 2008-02-06 | 热尼特里克斯有限责任公司 | 凝集素组合物和调节对抗原免疫应答的方法 |
| WO2008121420A1 (en) * | 2007-03-30 | 2008-10-09 | Memorial Sloan-Kettering Cancer Center | Constitutive expression of costimulatory ligands on adoptively transferred t lymphocytes |
| WO2012109624A2 (en) * | 2011-02-11 | 2012-08-16 | Zyngenia, Inc. | Monovalent and multivalent multispecific complexes and uses thereof |
Non-Patent Citations (1)
| Title |
|---|
| CLAUDIA M KOWOLIK ET AL.: "CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells", CANCER RES * |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US12263220B2 (en) | Compositions and methods for immunotherapy | |
| JP7367104B2 (ja) | 免疫療法のための組成物および方法 | |
| JP2022186837A (ja) | 免疫治療のための組成物および方法 | |
| AU2014225788B2 (en) | Engager cells for immunotherapy | |
| JP2022066290A (ja) | Fc受容体様5を標的とするキメラ抗原受容体およびその使用 | |
| RU2832516C2 (ru) | Композиции и способы для иммунотерапии | |
| HK40021640A (en) | Compositions and methods for immunotherapy | |
| HK1208631B (en) | Compositions and methods for immunotherapy |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| WD01 | Invention patent application deemed withdrawn after publication |
Application publication date: 20210309 |
|
| WD01 | Invention patent application deemed withdrawn after publication |