KR20180098655A - 탈아스탈트화된 오일로부터의 브라이트 스톡의 제조 - Google Patents
탈아스탈트화된 오일로부터의 브라이트 스톡의 제조 Download PDFInfo
- Publication number
- KR20180098655A KR20180098655A KR1020187021933A KR20187021933A KR20180098655A KR 20180098655 A KR20180098655 A KR 20180098655A KR 1020187021933 A KR1020187021933 A KR 1020187021933A KR 20187021933 A KR20187021933 A KR 20187021933A KR 20180098655 A KR20180098655 A KR 20180098655A
- Authority
- KR
- South Korea
- Prior art keywords
- less
- molecules
- composition
- base stock
- lubricant
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 238000002360 preparation method Methods 0.000 title description 3
- 239000000203 mixture Substances 0.000 claims abstract description 201
- 239000000314 lubricant Substances 0.000 claims abstract description 156
- 238000000034 method Methods 0.000 claims description 80
- 125000004432 carbon atom Chemical group C* 0.000 claims description 60
- 239000000654 additive Substances 0.000 claims description 48
- 229920006395 saturated elastomer Polymers 0.000 claims description 40
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 claims description 34
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 34
- 229910052717 sulfur Inorganic materials 0.000 claims description 34
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 33
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 33
- 239000011593 sulfur Substances 0.000 claims description 33
- 238000000434 field desorption mass spectrometry Methods 0.000 claims description 24
- 238000004821 distillation Methods 0.000 claims description 23
- 230000000996 additive effect Effects 0.000 claims description 12
- 229910021385 hard carbon Inorganic materials 0.000 claims 1
- 239000002904 solvent Substances 0.000 abstract description 163
- 230000015572 biosynthetic process Effects 0.000 abstract description 14
- 239000002585 base Substances 0.000 description 196
- 239000003921 oil Substances 0.000 description 177
- 229940060184 oil ingredients Drugs 0.000 description 149
- 239000003054 catalyst Substances 0.000 description 130
- 238000009835 boiling Methods 0.000 description 82
- 238000004517 catalytic hydrocracking Methods 0.000 description 75
- 229910052751 metal Inorganic materials 0.000 description 71
- 239000002184 metal Substances 0.000 description 71
- -1 C 5 alkane Chemical class 0.000 description 66
- 239000000047 product Substances 0.000 description 61
- 238000005984 hydrogenation reaction Methods 0.000 description 56
- 230000002378 acidificating effect Effects 0.000 description 54
- 230000008569 process Effects 0.000 description 52
- 238000006243 chemical reaction Methods 0.000 description 50
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 46
- 230000003197 catalytic effect Effects 0.000 description 42
- 239000003599 detergent Substances 0.000 description 42
- 239000002270 dispersing agent Substances 0.000 description 42
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 41
- 125000003118 aryl group Chemical group 0.000 description 40
- 239000007789 gas Substances 0.000 description 40
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 description 38
- 229930195733 hydrocarbon Natural products 0.000 description 36
- 239000010457 zeolite Substances 0.000 description 36
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 35
- 229910052739 hydrogen Inorganic materials 0.000 description 34
- 239000001257 hydrogen Substances 0.000 description 34
- OFBQJSOFQDEBGM-UHFFFAOYSA-N Pentane Chemical compound CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 31
- 229910021536 Zeolite Inorganic materials 0.000 description 31
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 31
- 150000002430 hydrocarbons Chemical class 0.000 description 31
- 239000010687 lubricating oil Substances 0.000 description 30
- 239000000446 fuel Substances 0.000 description 29
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 27
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 26
- 229910052757 nitrogen Inorganic materials 0.000 description 25
- 239000003963 antioxidant agent Substances 0.000 description 24
- 238000012545 processing Methods 0.000 description 24
- 238000011282 treatment Methods 0.000 description 23
- 150000001335 aliphatic alkanes Chemical class 0.000 description 22
- 229910052799 carbon Inorganic materials 0.000 description 21
- 239000011230 binding agent Substances 0.000 description 20
- 239000000377 silicon dioxide Substances 0.000 description 20
- 239000001993 wax Substances 0.000 description 20
- 229920000642 polymer Polymers 0.000 description 19
- 239000001294 propane Substances 0.000 description 19
- SNOOUWRIMMFWNE-UHFFFAOYSA-M sodium;6-[(3,4,5-trimethoxybenzoyl)amino]hexanoate Chemical compound [Na+].COC1=CC(C(=O)NCCCCCC([O-])=O)=CC(OC)=C1OC SNOOUWRIMMFWNE-UHFFFAOYSA-M 0.000 description 19
- 230000007935 neutral effect Effects 0.000 description 17
- 229910000510 noble metal Inorganic materials 0.000 description 17
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 15
- 125000001183 hydrocarbyl group Chemical group 0.000 description 15
- 238000004519 manufacturing process Methods 0.000 description 15
- 239000000463 material Substances 0.000 description 15
- 239000003607 modifier Substances 0.000 description 15
- 150000001875 compounds Chemical class 0.000 description 14
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 description 14
- 230000036961 partial effect Effects 0.000 description 14
- 230000002829 reductive effect Effects 0.000 description 14
- 238000004252 FT/ICR mass spectrometry Methods 0.000 description 13
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerol Natural products OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 13
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 13
- 230000000052 comparative effect Effects 0.000 description 13
- 150000002989 phenols Chemical class 0.000 description 13
- 229920005862 polyol Chemical class 0.000 description 13
- 238000009826 distribution Methods 0.000 description 12
- 239000002808 molecular sieve Substances 0.000 description 12
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 12
- 238000000638 solvent extraction Methods 0.000 description 12
- 239000004215 Carbon black (E152) Substances 0.000 description 11
- 150000001412 amines Chemical class 0.000 description 11
- 235000014113 dietary fatty acids Nutrition 0.000 description 11
- POULHZVOKOAJMA-UHFFFAOYSA-N dodecanoic acid Chemical compound CCCCCCCCCCCC(O)=O POULHZVOKOAJMA-UHFFFAOYSA-N 0.000 description 11
- 150000002148 esters Chemical class 0.000 description 11
- 239000000194 fatty acid Substances 0.000 description 11
- 229930195729 fatty acid Natural products 0.000 description 11
- 229920000058 polyacrylate Polymers 0.000 description 11
- 229920000193 polymethacrylate Polymers 0.000 description 11
- 239000000523 sample Substances 0.000 description 10
- 238000000926 separation method Methods 0.000 description 10
- 238000001644 13C nuclear magnetic resonance spectroscopy Methods 0.000 description 9
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 9
- 239000002199 base oil Substances 0.000 description 9
- 239000001273 butane Substances 0.000 description 9
- 239000010779 crude oil Substances 0.000 description 9
- 238000000354 decomposition reaction Methods 0.000 description 9
- 238000009472 formulation Methods 0.000 description 9
- 150000002739 metals Chemical class 0.000 description 9
- 229910052750 molybdenum Inorganic materials 0.000 description 9
- 239000010705 motor oil Substances 0.000 description 9
- 229910052759 nickel Inorganic materials 0.000 description 9
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 8
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 8
- 125000000217 alkyl group Chemical group 0.000 description 8
- 239000000356 contaminant Substances 0.000 description 8
- 239000003112 inhibitor Substances 0.000 description 8
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 description 8
- 239000007788 liquid Substances 0.000 description 8
- 239000011777 magnesium Substances 0.000 description 8
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 8
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 8
- 238000012360 testing method Methods 0.000 description 8
- 239000008186 active pharmaceutical agent Substances 0.000 description 7
- 230000003078 antioxidant effect Effects 0.000 description 7
- 150000004982 aromatic amines Chemical class 0.000 description 7
- 239000011575 calcium Substances 0.000 description 7
- 238000001816 cooling Methods 0.000 description 7
- 229910052749 magnesium Inorganic materials 0.000 description 7
- 239000011733 molybdenum Substances 0.000 description 7
- 239000002530 phenolic antioxidant Substances 0.000 description 7
- 229910052697 platinum Inorganic materials 0.000 description 7
- 150000003077 polyols Chemical class 0.000 description 7
- 239000011148 porous material Substances 0.000 description 7
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 6
- FALRKNHUBBKYCC-UHFFFAOYSA-N 2-(chloromethyl)pyridine-3-carbonitrile Chemical class ClCC1=NC=CC=C1C#N FALRKNHUBBKYCC-UHFFFAOYSA-N 0.000 description 6
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 6
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 6
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 6
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 6
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 6
- 125000001931 aliphatic group Chemical group 0.000 description 6
- 150000001342 alkaline earth metals Chemical class 0.000 description 6
- 229910052791 calcium Inorganic materials 0.000 description 6
- ZMRQTIAUOLVKOX-UHFFFAOYSA-L calcium;diphenoxide Chemical compound [Ca+2].[O-]C1=CC=CC=C1.[O-]C1=CC=CC=C1 ZMRQTIAUOLVKOX-UHFFFAOYSA-L 0.000 description 6
- 239000003795 chemical substances by application Substances 0.000 description 6
- 230000000694 effects Effects 0.000 description 6
- 229910052742 iron Inorganic materials 0.000 description 6
- NNPPMTNAJDCUHE-UHFFFAOYSA-N isobutane Chemical compound CC(C)C NNPPMTNAJDCUHE-UHFFFAOYSA-N 0.000 description 6
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 6
- 230000003647 oxidation Effects 0.000 description 6
- 238000007254 oxidation reaction Methods 0.000 description 6
- 239000012188 paraffin wax Substances 0.000 description 6
- 229920000768 polyamine Polymers 0.000 description 6
- 239000011435 rock Substances 0.000 description 6
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 6
- 150000003839 salts Chemical class 0.000 description 6
- 229940014800 succinic anhydride Drugs 0.000 description 6
- KZNICNPSHKQLFF-UHFFFAOYSA-N succinimide Chemical compound O=C1CCC(=O)N1 KZNICNPSHKQLFF-UHFFFAOYSA-N 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 5
- JXLHNMVSKXFWAO-UHFFFAOYSA-N azane;7-fluoro-2,1,3-benzoxadiazole-4-sulfonic acid Chemical compound N.OS(=O)(=O)C1=CC=C(F)C2=NON=C12 JXLHNMVSKXFWAO-UHFFFAOYSA-N 0.000 description 5
- 238000012512 characterization method Methods 0.000 description 5
- 238000000605 extraction Methods 0.000 description 5
- 239000012530 fluid Substances 0.000 description 5
- 238000005194 fractionation Methods 0.000 description 5
- 239000000295 fuel oil Substances 0.000 description 5
- 239000012208 gear oil Substances 0.000 description 5
- 238000002156 mixing Methods 0.000 description 5
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 229910052720 vanadium Inorganic materials 0.000 description 5
- LEONUFNNVUYDNQ-UHFFFAOYSA-N vanadium atom Chemical compound [V] LEONUFNNVUYDNQ-UHFFFAOYSA-N 0.000 description 5
- 239000004034 viscosity adjusting agent Substances 0.000 description 5
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 4
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 4
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 4
- CPLXHLVBOLITMK-UHFFFAOYSA-N Magnesium oxide Chemical compound [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 4
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 4
- XQVWYOYUZDUNRW-UHFFFAOYSA-N N-Phenyl-1-naphthylamine Chemical compound C=1C=CC2=CC=CC=C2C=1NC1=CC=CC=C1 XQVWYOYUZDUNRW-UHFFFAOYSA-N 0.000 description 4
- 239000005642 Oleic acid Substances 0.000 description 4
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 4
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 4
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 4
- 150000001298 alcohols Chemical class 0.000 description 4
- 239000002518 antifoaming agent Substances 0.000 description 4
- 150000001491 aromatic compounds Chemical class 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 238000004061 bleaching Methods 0.000 description 4
- 150000001735 carboxylic acids Chemical class 0.000 description 4
- 229920001577 copolymer Polymers 0.000 description 4
- 238000007324 demetalation reaction Methods 0.000 description 4
- 230000000994 depressogenic effect Effects 0.000 description 4
- 238000003795 desorption Methods 0.000 description 4
- 239000003085 diluting agent Substances 0.000 description 4
- 150000004665 fatty acids Chemical class 0.000 description 4
- HYBBIBNJHNGZAN-UHFFFAOYSA-N furfural Chemical compound O=CC1=CC=CO1 HYBBIBNJHNGZAN-UHFFFAOYSA-N 0.000 description 4
- 238000010438 heat treatment Methods 0.000 description 4
- ZSIAUFGUXNUGDI-UHFFFAOYSA-N hexan-1-ol Chemical compound CCCCCCO ZSIAUFGUXNUGDI-UHFFFAOYSA-N 0.000 description 4
- 230000007062 hydrolysis Effects 0.000 description 4
- 238000006460 hydrolysis reaction Methods 0.000 description 4
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 4
- 229910052987 metal hydride Inorganic materials 0.000 description 4
- 150000004681 metal hydrides Chemical class 0.000 description 4
- 229910052763 palladium Inorganic materials 0.000 description 4
- 238000001228 spectrum Methods 0.000 description 4
- KDYFGRWQOYBRFD-UHFFFAOYSA-N succinic acid Chemical class OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 4
- RZRNAYUHWVFMIP-KTKRTIGZSA-N 1-oleoylglycerol Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(O)CO RZRNAYUHWVFMIP-KTKRTIGZSA-N 0.000 description 3
- XDOFQFKRPWOURC-UHFFFAOYSA-N 16-methylheptadecanoic acid Chemical compound CC(C)CCCCCCCCCCCCCCC(O)=O XDOFQFKRPWOURC-UHFFFAOYSA-N 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- MQHWFIOJQSCFNM-UHFFFAOYSA-L Magnesium salicylate Chemical class [Mg+2].OC1=CC=CC=C1C([O-])=O.OC1=CC=CC=C1C([O-])=O MQHWFIOJQSCFNM-UHFFFAOYSA-L 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 3
- 229910003294 NiMo Inorganic materials 0.000 description 3
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 3
- 229920002367 Polyisobutene Polymers 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 239000004480 active ingredient Substances 0.000 description 3
- 229910052783 alkali metal Inorganic materials 0.000 description 3
- 150000001340 alkali metals Chemical class 0.000 description 3
- 150000001408 amides Chemical class 0.000 description 3
- 239000010426 asphalt Substances 0.000 description 3
- 239000006227 byproduct Substances 0.000 description 3
- AVVIDTZRJBSXML-UHFFFAOYSA-L calcium;2-carboxyphenolate;dihydrate Chemical compound O.O.[Ca+2].OC1=CC=CC=C1C([O-])=O.OC1=CC=CC=C1C([O-])=O AVVIDTZRJBSXML-UHFFFAOYSA-L 0.000 description 3
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 3
- 239000012876 carrier material Substances 0.000 description 3
- 238000002485 combustion reaction Methods 0.000 description 3
- 239000007859 condensation product Substances 0.000 description 3
- 238000006482 condensation reaction Methods 0.000 description 3
- 238000005336 cracking Methods 0.000 description 3
- 230000007423 decrease Effects 0.000 description 3
- DMBHHRLKUKUOEG-UHFFFAOYSA-N diphenylamine Chemical compound C=1C=CC=CC=1NC1=CC=CC=C1 DMBHHRLKUKUOEG-UHFFFAOYSA-N 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 238000001125 extrusion Methods 0.000 description 3
- YQEMORVAKMFKLG-UHFFFAOYSA-N glycerine monostearate Natural products CCCCCCCCCCCCCCCCCC(=O)OC(CO)CO YQEMORVAKMFKLG-UHFFFAOYSA-N 0.000 description 3
- RZRNAYUHWVFMIP-HXUWFJFHSA-N glycerol monolinoleate Natural products CCCCCCCCC=CCCCCCCCC(=O)OC[C@H](O)CO RZRNAYUHWVFMIP-HXUWFJFHSA-N 0.000 description 3
- SVUQHVRAGMNPLW-UHFFFAOYSA-N glycerol monostearate Natural products CCCCCCCCCCCCCCCCC(=O)OCC(O)CO SVUQHVRAGMNPLW-UHFFFAOYSA-N 0.000 description 3
- 230000005484 gravity Effects 0.000 description 3
- 230000002706 hydrostatic effect Effects 0.000 description 3
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 3
- 230000002401 inhibitory effect Effects 0.000 description 3
- 239000001282 iso-butane Substances 0.000 description 3
- 229910044991 metal oxide Inorganic materials 0.000 description 3
- 150000004706 metal oxides Chemical class 0.000 description 3
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 3
- 230000000737 periodic effect Effects 0.000 description 3
- 229910052698 phosphorus Inorganic materials 0.000 description 3
- 239000011574 phosphorus Substances 0.000 description 3
- PDEDQSAFHNADLV-UHFFFAOYSA-M potassium;disodium;dinitrate;nitrite Chemical compound [Na+].[Na+].[K+].[O-]N=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O PDEDQSAFHNADLV-UHFFFAOYSA-M 0.000 description 3
- 238000000197 pyrolysis Methods 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 229960004889 salicylic acid Drugs 0.000 description 3
- 230000002000 scavenging effect Effects 0.000 description 3
- 150000003333 secondary alcohols Chemical class 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 150000003900 succinic acid esters Chemical class 0.000 description 3
- RINCXYDBBGOEEQ-UHFFFAOYSA-N succinic anhydride Chemical class O=C1CCC(=O)O1 RINCXYDBBGOEEQ-UHFFFAOYSA-N 0.000 description 3
- 229960002317 succinimide Drugs 0.000 description 3
- 150000003871 sulfonates Chemical class 0.000 description 3
- 229910052723 transition metal Inorganic materials 0.000 description 3
- WMYJOZQKDZZHAC-UHFFFAOYSA-H trizinc;dioxido-sulfanylidene-sulfido-$l^{5}-phosphane Chemical class [Zn+2].[Zn+2].[Zn+2].[O-]P([O-])([S-])=S.[O-]P([O-])([S-])=S WMYJOZQKDZZHAC-UHFFFAOYSA-H 0.000 description 3
- 229910052721 tungsten Inorganic materials 0.000 description 3
- 239000011701 zinc Substances 0.000 description 3
- QHZLMUACJMDIAE-UHFFFAOYSA-N 1-monopalmitoylglycerol Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(O)CO QHZLMUACJMDIAE-UHFFFAOYSA-N 0.000 description 2
- NFIDBGJMFKNGGQ-UHFFFAOYSA-N 2-(2-methylpropyl)phenol Chemical compound CC(C)CC1=CC=CC=C1O NFIDBGJMFKNGGQ-UHFFFAOYSA-N 0.000 description 2
- IHQZONJYGAQKGK-UHFFFAOYSA-N 2-tert-butyl-4-dodecylphenol Chemical compound CCCCCCCCCCCCC1=CC=C(O)C(C(C)(C)C)=C1 IHQZONJYGAQKGK-UHFFFAOYSA-N 0.000 description 2
- XCIGNJPXXAPZDP-UHFFFAOYSA-N 2-tert-butyl-4-heptylphenol Chemical compound CCCCCCCC1=CC=C(O)C(C(C)(C)C)=C1 XCIGNJPXXAPZDP-UHFFFAOYSA-N 0.000 description 2
- ZXENURKTAAQNOU-UHFFFAOYSA-N 2-tert-butyl-4-octylphenol Chemical compound CCCCCCCCC1=CC=C(O)C(C(C)(C)C)=C1 ZXENURKTAAQNOU-UHFFFAOYSA-N 0.000 description 2
- KAKZBPTYRLMSJV-UHFFFAOYSA-N Butadiene Chemical compound C=CC=C KAKZBPTYRLMSJV-UHFFFAOYSA-N 0.000 description 2
- IRIAEXORFWYRCZ-UHFFFAOYSA-N Butylbenzyl phthalate Chemical compound CCCCOC(=O)C1=CC=CC=C1C(=O)OCC1=CC=CC=C1 IRIAEXORFWYRCZ-UHFFFAOYSA-N 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- 229920000089 Cyclic olefin copolymer Polymers 0.000 description 2
- OTMSDBZUPAUEDD-UHFFFAOYSA-N Ethane Chemical compound CC OTMSDBZUPAUEDD-UHFFFAOYSA-N 0.000 description 2
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 2
- 239000005977 Ethylene Substances 0.000 description 2
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 2
- VQTUBCCKSQIDNK-UHFFFAOYSA-N Isobutene Chemical compound CC(C)=C VQTUBCCKSQIDNK-UHFFFAOYSA-N 0.000 description 2
- RRHGJUQNOFWUDK-UHFFFAOYSA-N Isoprene Chemical compound CC(=C)C=C RRHGJUQNOFWUDK-UHFFFAOYSA-N 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- AMQJEAYHLZJPGS-UHFFFAOYSA-N N-Pentanol Chemical compound CCCCCO AMQJEAYHLZJPGS-UHFFFAOYSA-N 0.000 description 2
- 238000005481 NMR spectroscopy Methods 0.000 description 2
- 102220500397 Neutral and basic amino acid transport protein rBAT_M41T_mutation Human genes 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 2
- 150000001336 alkenes Chemical class 0.000 description 2
- 125000002877 alkyl aryl group Chemical group 0.000 description 2
- 150000005215 alkyl ethers Chemical class 0.000 description 2
- 125000002947 alkylene group Chemical group 0.000 description 2
- 125000000129 anionic group Chemical group 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 239000010953 base metal Substances 0.000 description 2
- 229910052728 basic metal Inorganic materials 0.000 description 2
- 150000001721 carbon Chemical group 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 150000001793 charged compounds Chemical group 0.000 description 2
- 239000007795 chemical reaction product Substances 0.000 description 2
- 239000003086 colorant Substances 0.000 description 2
- 239000012141 concentrate Substances 0.000 description 2
- 238000012937 correction Methods 0.000 description 2
- 238000005260 corrosion Methods 0.000 description 2
- 230000007797 corrosion Effects 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 239000013530 defoamer Substances 0.000 description 2
- 238000006297 dehydration reaction Methods 0.000 description 2
- 238000005115 demineralization Methods 0.000 description 2
- 230000002328 demineralizing effect Effects 0.000 description 2
- 238000000151 deposition Methods 0.000 description 2
- 230000008021 deposition Effects 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 239000002283 diesel fuel Substances 0.000 description 2
- 238000000113 differential scanning calorimetry Methods 0.000 description 2
- 230000008030 elimination Effects 0.000 description 2
- 238000003379 elimination reaction Methods 0.000 description 2
- 125000005842 heteroatom Chemical group 0.000 description 2
- IPCSVZSSVZVIGE-UHFFFAOYSA-N hexadecanoic acid Chemical compound CCCCCCCCCCCCCCCC(O)=O IPCSVZSSVZVIGE-UHFFFAOYSA-N 0.000 description 2
- NAQMVNRVTILPCV-UHFFFAOYSA-N hexane-1,6-diamine Chemical compound NCCCCCCN NAQMVNRVTILPCV-UHFFFAOYSA-N 0.000 description 2
- 230000036571 hydration Effects 0.000 description 2
- 238000006703 hydration reaction Methods 0.000 description 2
- 239000012535 impurity Substances 0.000 description 2
- 230000010354 integration Effects 0.000 description 2
- 239000003350 kerosene Substances 0.000 description 2
- 239000003879 lubricant additive Substances 0.000 description 2
- 230000001050 lubricating effect Effects 0.000 description 2
- 239000000395 magnesium oxide Substances 0.000 description 2
- 229940072082 magnesium salicylate Drugs 0.000 description 2
- 238000002844 melting Methods 0.000 description 2
- 230000008018 melting Effects 0.000 description 2
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 150000002902 organometallic compounds Chemical class 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- WXZMFSXDPGVJKK-UHFFFAOYSA-N pentaerythritol Chemical class OCC(CO)(CO)CO WXZMFSXDPGVJKK-UHFFFAOYSA-N 0.000 description 2
- 235000021317 phosphate Nutrition 0.000 description 2
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 2
- 229920001195 polyisoprene Polymers 0.000 description 2
- 229920001451 polypropylene glycol Polymers 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- 150000003138 primary alcohols Chemical class 0.000 description 2
- 150000003873 salicylate salts Chemical class 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 229920006132 styrene block copolymer Polymers 0.000 description 2
- 239000001384 succinic acid Substances 0.000 description 2
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 2
- 238000010998 test method Methods 0.000 description 2
- 150000003624 transition metals Chemical class 0.000 description 2
- 150000003626 triacylglycerols Chemical class 0.000 description 2
- CYRMSUTZVYGINF-UHFFFAOYSA-N trichlorofluoromethane Chemical compound FC(Cl)(Cl)Cl CYRMSUTZVYGINF-UHFFFAOYSA-N 0.000 description 2
- PVNIQBQSYATKKL-UHFFFAOYSA-N tripalmitin Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(OC(=O)CCCCCCCCCCCCCCC)COC(=O)CCCCCCCCCCCCCCC PVNIQBQSYATKKL-UHFFFAOYSA-N 0.000 description 2
- DCXXMTOCNZCJGO-UHFFFAOYSA-N tristearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(OC(=O)CCCCCCCCCCCCCCCCC)COC(=O)CCCCCCCCCCCCCCCCC DCXXMTOCNZCJGO-UHFFFAOYSA-N 0.000 description 2
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 2
- 239000010937 tungsten Substances 0.000 description 2
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 2
- 229910052725 zinc Inorganic materials 0.000 description 2
- SXYOAESUCSYJNZ-UHFFFAOYSA-L zinc;bis(6-methylheptoxy)-sulfanylidene-sulfido-$l^{5}-phosphane Chemical compound [Zn+2].CC(C)CCCCCOP([S-])(=S)OCCCCCC(C)C.CC(C)CCCCCOP([S-])(=S)OCCCCCC(C)C SXYOAESUCSYJNZ-UHFFFAOYSA-L 0.000 description 2
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical compound OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 1
- 125000006702 (C1-C18) alkyl group Chemical group 0.000 description 1
- 125000000923 (C1-C30) alkyl group Chemical group 0.000 description 1
- XTFIVUDBNACUBN-UHFFFAOYSA-N 1,3,5-trinitro-1,3,5-triazinane Chemical compound [O-][N+](=O)N1CN([N+]([O-])=O)CN([N+]([O-])=O)C1 XTFIVUDBNACUBN-UHFFFAOYSA-N 0.000 description 1
- GFAZGHREJPXDMH-UHFFFAOYSA-N 1,3-dipalmitoylglycerol Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(O)COC(=O)CCCCCCCCCCCCCCC GFAZGHREJPXDMH-UHFFFAOYSA-N 0.000 description 1
- HBXWUCXDUUJDRB-UHFFFAOYSA-N 1-octadecoxyoctadecane Chemical compound CCCCCCCCCCCCCCCCCCOCCCCCCCCCCCCCCCCCC HBXWUCXDUUJDRB-UHFFFAOYSA-N 0.000 description 1
- WJFKNYWRSNBZNX-UHFFFAOYSA-N 10H-phenothiazine Chemical compound C1=CC=C2NC3=CC=CC=C3SC2=C1 WJFKNYWRSNBZNX-UHFFFAOYSA-N 0.000 description 1
- DKCPKDPYUFEZCP-UHFFFAOYSA-N 2,6-di-tert-butylphenol Chemical compound CC(C)(C)C1=CC=CC(C(C)(C)C)=C1O DKCPKDPYUFEZCP-UHFFFAOYSA-N 0.000 description 1
- SZATXRHXOOLEFV-UHFFFAOYSA-N 2,6-ditert-butyl-4-dodecylphenol Chemical compound CCCCCCCCCCCCC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 SZATXRHXOOLEFV-UHFFFAOYSA-N 0.000 description 1
- OEHMRECZRLQSRD-UHFFFAOYSA-N 2,6-ditert-butyl-4-heptylphenol Chemical compound CCCCCCCC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 OEHMRECZRLQSRD-UHFFFAOYSA-N 0.000 description 1
- RRKBRXPIJHVKIC-UHFFFAOYSA-N 2-(2-ethylhexyl)phenol Chemical compound CCCCC(CC)CC1=CC=CC=C1O RRKBRXPIJHVKIC-UHFFFAOYSA-N 0.000 description 1
- UTXPMECBRCEYCI-UHFFFAOYSA-N 2-[2-[2-[2-(4-nonylphenoxy)ethoxy]ethoxy]ethoxy]ethanol Chemical compound CCCCCCCCCC1=CC=C(OCCOCCOCCOCCO)C=C1 UTXPMECBRCEYCI-UHFFFAOYSA-N 0.000 description 1
- CYEJMVLDXAUOPN-UHFFFAOYSA-N 2-dodecylphenol Chemical compound CCCCCCCCCCCCC1=CC=CC=C1O CYEJMVLDXAUOPN-UHFFFAOYSA-N 0.000 description 1
- ROGIWVXWXZRRMZ-UHFFFAOYSA-N 2-methylbuta-1,3-diene;styrene Chemical compound CC(=C)C=C.C=CC1=CC=CC=C1 ROGIWVXWXZRRMZ-UHFFFAOYSA-N 0.000 description 1
- LIPXCSZFXJTFSK-UHFFFAOYSA-N 2-tert-butyl-4-dodecyl-6-methylphenol Chemical compound CCCCCCCCCCCCC1=CC(C)=C(O)C(C(C)(C)C)=C1 LIPXCSZFXJTFSK-UHFFFAOYSA-N 0.000 description 1
- PMRDUCIMVOFYBX-UHFFFAOYSA-N 2-tert-butyl-4-heptyl-6-methylphenol Chemical compound CCCCCCCC1=CC(C)=C(O)C(C(C)(C)C)=C1 PMRDUCIMVOFYBX-UHFFFAOYSA-N 0.000 description 1
- ZXABMDQSAABDMG-UHFFFAOYSA-N 3-ethenoxyprop-1-ene Chemical compound C=CCOC=C ZXABMDQSAABDMG-UHFFFAOYSA-N 0.000 description 1
- VPWNQTHUCYMVMZ-UHFFFAOYSA-N 4,4'-sulfonyldiphenol Chemical class C1=CC(O)=CC=C1S(=O)(=O)C1=CC=C(O)C=C1 VPWNQTHUCYMVMZ-UHFFFAOYSA-N 0.000 description 1
- WVYWICLMDOOCFB-UHFFFAOYSA-N 4-methyl-2-pentanol Chemical compound CC(C)CC(C)O WVYWICLMDOOCFB-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N Acrylic acid Chemical compound OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 229910018072 Al 2 O 3 Inorganic materials 0.000 description 1
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 1
- 229930185605 Bisphenol Natural products 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 1
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- 239000005069 Extreme pressure additive Substances 0.000 description 1
- 208000033830 Hot Flashes Diseases 0.000 description 1
- 206010060800 Hot flush Diseases 0.000 description 1
- 239000005909 Kieselgur Substances 0.000 description 1
- 238000006612 Kolbe reaction Methods 0.000 description 1
- 239000005639 Lauric acid Substances 0.000 description 1
- OYHQOLUKZRVURQ-HZJYTTRNSA-N Linoleic acid Chemical compound CCCCC\C=C/C\C=C/CCCCCCCC(O)=O OYHQOLUKZRVURQ-HZJYTTRNSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-M Methacrylate Chemical compound CC(=C)C([O-])=O CERQOIWHTDAKMF-UHFFFAOYSA-M 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 1
- NTIZESTWPVYFNL-UHFFFAOYSA-N Methyl isobutyl ketone Chemical compound CC(C)CC(C)=O NTIZESTWPVYFNL-UHFFFAOYSA-N 0.000 description 1
- UIHCLUNTQKBZGK-UHFFFAOYSA-N Methyl isobutyl ketone Natural products CCC(C)C(C)=O UIHCLUNTQKBZGK-UHFFFAOYSA-N 0.000 description 1
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 1
- IGFHQQFPSIBGKE-UHFFFAOYSA-N Nonylphenol Natural products CCCCCCCCCC1=CC=C(O)C=C1 IGFHQQFPSIBGKE-UHFFFAOYSA-N 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- QAPVYZRWKDXNDK-UHFFFAOYSA-N P,P-Dioctyldiphenylamine Chemical compound C1=CC(CCCCCCCC)=CC=C1NC1=CC=C(CCCCCCCC)C=C1 QAPVYZRWKDXNDK-UHFFFAOYSA-N 0.000 description 1
- 235000021314 Palmitic acid Nutrition 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000004820 Pressure-sensitive adhesive Substances 0.000 description 1
- 239000004614 Process Aid Substances 0.000 description 1
- 229910004298 SiO 2 Inorganic materials 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- 239000002174 Styrene-butadiene Substances 0.000 description 1
- ZJCCRDAZUWHFQH-UHFFFAOYSA-N Trimethylolpropane Chemical compound CCC(CO)(CO)CO ZJCCRDAZUWHFQH-UHFFFAOYSA-N 0.000 description 1
- BAECOWNUKCLBPZ-HIUWNOOHSA-N Triolein Natural products O([C@H](OCC(=O)CCCCCCC/C=C\CCCCCCCC)COC(=O)CCCCCCC/C=C\CCCCCCCC)C(=O)CCCCCCC/C=C\CCCCCCCC BAECOWNUKCLBPZ-HIUWNOOHSA-N 0.000 description 1
- PHYFQTYBJUILEZ-UHFFFAOYSA-N Trioleoylglycerol Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC(OC(=O)CCCCCCCC=CCCCCCCCC)COC(=O)CCCCCCCC=CCCCCCCCC PHYFQTYBJUILEZ-UHFFFAOYSA-N 0.000 description 1
- 229920004923 Triton X-15 Chemical group 0.000 description 1
- 229920004895 Triton X-35 Chemical group 0.000 description 1
- QZYDAIMOJUSSFT-UHFFFAOYSA-N [Co].[Ni].[Mo] Chemical compound [Co].[Ni].[Mo] QZYDAIMOJUSSFT-UHFFFAOYSA-N 0.000 description 1
- XYRMLECORMNZEY-UHFFFAOYSA-B [Mo+4].[Mo+4].[Mo+4].[O-]P([O-])([S-])=S.[O-]P([O-])([S-])=S.[O-]P([O-])([S-])=S.[O-]P([O-])([S-])=S Chemical compound [Mo+4].[Mo+4].[Mo+4].[O-]P([O-])([S-])=S.[O-]P([O-])([S-])=S.[O-]P([O-])([S-])=S.[O-]P([O-])([S-])=S XYRMLECORMNZEY-UHFFFAOYSA-B 0.000 description 1
- 238000005299 abrasion Methods 0.000 description 1
- 238000005903 acid hydrolysis reaction Methods 0.000 description 1
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 1
- 239000011149 active material Substances 0.000 description 1
- WNLRTRBMVRJNCN-UHFFFAOYSA-N adipic acid Chemical class OC(=O)CCCCC(O)=O WNLRTRBMVRJNCN-UHFFFAOYSA-N 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 125000003158 alcohol group Chemical group 0.000 description 1
- 150000007933 aliphatic carboxylic acids Chemical class 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 229910001860 alkaline earth metal hydroxide Inorganic materials 0.000 description 1
- 229910000287 alkaline earth metal oxide Inorganic materials 0.000 description 1
- 125000003342 alkenyl group Chemical group 0.000 description 1
- 125000004450 alkenylene group Chemical group 0.000 description 1
- 229910000323 aluminium silicate Inorganic materials 0.000 description 1
- 239000011959 amorphous silica alumina Substances 0.000 description 1
- 239000012491 analyte Substances 0.000 description 1
- 150000001448 anilines Chemical class 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 239000002519 antifouling agent Substances 0.000 description 1
- 239000013556 antirust agent Substances 0.000 description 1
- 239000012223 aqueous fraction Substances 0.000 description 1
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 1
- 239000010692 aromatic oil Substances 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- 229910052788 barium Inorganic materials 0.000 description 1
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 1
- 150000003819 basic metal compounds Chemical class 0.000 description 1
- 229910001570 bauxite Inorganic materials 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- 125000002619 bicyclic group Chemical group 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 1
- 239000004327 boric acid Substances 0.000 description 1
- 229910052796 boron Inorganic materials 0.000 description 1
- 150000001639 boron compounds Chemical class 0.000 description 1
- MTAZNLWOLGHBHU-UHFFFAOYSA-N butadiene-styrene rubber Chemical compound C=CC=C.C=CC1=CC=CC=C1 MTAZNLWOLGHBHU-UHFFFAOYSA-N 0.000 description 1
- BTANRVKWQNVYAZ-UHFFFAOYSA-N butan-2-ol Chemical compound CCC(C)O BTANRVKWQNVYAZ-UHFFFAOYSA-N 0.000 description 1
- VBIGULIJWJPALH-UHFFFAOYSA-L calcium;2-carboxyphenolate Chemical class [Ca+2].OC1=CC=CC=C1C([O-])=O.OC1=CC=CC=C1C([O-])=O VBIGULIJWJPALH-UHFFFAOYSA-L 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 150000007942 carboxylates Chemical class 0.000 description 1
- 238000010538 cationic polymerization reaction Methods 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- MJSNUBOCVAKFIJ-LNTINUHCSA-N chromium;(z)-4-oxoniumylidenepent-2-en-2-olate Chemical compound [Cr].C\C(O)=C\C(C)=O.C\C(O)=C\C(C)=O.C\C(O)=C\C(C)=O MJSNUBOCVAKFIJ-LNTINUHCSA-N 0.000 description 1
- 239000012459 cleaning agent Substances 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- WHDPTDWLEKQKKX-UHFFFAOYSA-N cobalt molybdenum Chemical compound [Co].[Co].[Mo] WHDPTDWLEKQKKX-UHFFFAOYSA-N 0.000 description 1
- 238000004939 coking Methods 0.000 description 1
- 230000009918 complex formation Effects 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 239000002178 crystalline material Substances 0.000 description 1
- 150000005676 cyclic carbonates Chemical class 0.000 description 1
- 230000009849 deactivation Effects 0.000 description 1
- 125000002704 decyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 238000007872 degassing Methods 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 229910003460 diamond Inorganic materials 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 150000005690 diesters Chemical class 0.000 description 1
- 125000005028 dihydroxyaryl group Chemical group 0.000 description 1
- 239000012470 diluted sample Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 239000004205 dimethyl polysiloxane Substances 0.000 description 1
- 150000002009 diols Chemical class 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 239000000806 elastomer Substances 0.000 description 1
- 230000005684 electric field Effects 0.000 description 1
- 239000003974 emollient agent Substances 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 125000004185 ester group Chemical group 0.000 description 1
- 238000007046 ethoxylation reaction Methods 0.000 description 1
- 150000002191 fatty alcohols Chemical class 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- 238000007730 finishing process Methods 0.000 description 1
- 238000003682 fluorination reaction Methods 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- VZCYOOQTPOCHFL-OWOJBTEDSA-L fumarate(2-) Chemical class [O-]C(=O)\C=C\C([O-])=O VZCYOOQTPOCHFL-OWOJBTEDSA-L 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 238000004817 gas chromatography Methods 0.000 description 1
- 238000002309 gasification Methods 0.000 description 1
- 239000003349 gelling agent Substances 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- UHUSDOQQWJGJQS-UHFFFAOYSA-N glycerol 1,2-dioctadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(CO)OC(=O)CCCCCCCCCCCCCCCCC UHUSDOQQWJGJQS-UHFFFAOYSA-N 0.000 description 1
- JEJLGIQLPYYGEE-UHFFFAOYSA-N glycerol dipalmitate Natural products CCCCCCCCCCCCCCCC(=O)OCC(CO)OC(=O)CCCCCCCCCCCCCCC JEJLGIQLPYYGEE-UHFFFAOYSA-N 0.000 description 1
- 150000002314 glycerols Chemical class 0.000 description 1
- 230000026030 halogenation Effects 0.000 description 1
- 238000005658 halogenation reaction Methods 0.000 description 1
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 150000004678 hydrides Chemical class 0.000 description 1
- 125000000743 hydrocarbylene group Chemical group 0.000 description 1
- 238000007327 hydrogenolysis reaction Methods 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 238000000752 ionisation method Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 238000006317 isomerization reaction Methods 0.000 description 1
- 238000004898 kneading Methods 0.000 description 1
- 229940049918 linoleate Drugs 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 238000001819 mass spectrum Methods 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000000155 melt Substances 0.000 description 1
- 239000013335 mesoporous material Substances 0.000 description 1
- 150000002736 metal compounds Chemical class 0.000 description 1
- 239000006078 metal deactivator Substances 0.000 description 1
- 229910000000 metal hydroxide Inorganic materials 0.000 description 1
- 150000004692 metal hydroxides Chemical class 0.000 description 1
- 229910052976 metal sulfide Inorganic materials 0.000 description 1
- 238000001465 metallisation Methods 0.000 description 1
- 239000004200 microcrystalline wax Substances 0.000 description 1
- 235000019808 microcrystalline wax Nutrition 0.000 description 1
- DDTIGTPWGISMKL-UHFFFAOYSA-N molybdenum nickel Chemical compound [Ni].[Mo] DDTIGTPWGISMKL-UHFFFAOYSA-N 0.000 description 1
- KHYKFSXXGRUKRE-UHFFFAOYSA-J molybdenum(4+) tetracarbamodithioate Chemical compound C(N)([S-])=S.[Mo+4].C(N)([S-])=S.C(N)([S-])=S.C(N)([S-])=S KHYKFSXXGRUKRE-UHFFFAOYSA-J 0.000 description 1
- 229940105132 myristate Drugs 0.000 description 1
- 125000001421 myristyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- BQLZCNHPJNMDIO-UHFFFAOYSA-N n-(4-octylphenyl)naphthalen-1-amine Chemical compound C1=CC(CCCCCCCC)=CC=C1NC1=CC=CC2=CC=CC=C12 BQLZCNHPJNMDIO-UHFFFAOYSA-N 0.000 description 1
- WQEPLUUGTLDZJY-UHFFFAOYSA-N n-Pentadecanoic acid Natural products CCCCCCCCCCCCCCC(O)=O WQEPLUUGTLDZJY-UHFFFAOYSA-N 0.000 description 1
- SNWVRVDHQRBBFG-UHFFFAOYSA-N n-phenyl-n-(2,4,4-trimethylpentan-2-yl)naphthalen-1-amine Chemical compound C=1C=CC2=CC=CC=C2C=1N(C(C)(C)CC(C)(C)C)C1=CC=CC=C1 SNWVRVDHQRBBFG-UHFFFAOYSA-N 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- MOWMLACGTDMJRV-UHFFFAOYSA-N nickel tungsten Chemical compound [Ni].[W] MOWMLACGTDMJRV-UHFFFAOYSA-N 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 239000002736 nonionic surfactant Substances 0.000 description 1
- 229910052755 nonmetal Inorganic materials 0.000 description 1
- 229920000847 nonoxynol Polymers 0.000 description 1
- 125000001400 nonyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- SNQQPOLDUKLAAF-UHFFFAOYSA-N nonylphenol Chemical compound CCCCCCCCCC1=CC=CC=C1O SNQQPOLDUKLAAF-UHFFFAOYSA-N 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 229940049964 oleate Drugs 0.000 description 1
- 125000001117 oleyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])/C([H])=C([H])\C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 229920000620 organic polymer Polymers 0.000 description 1
- 125000002524 organometallic group Chemical group 0.000 description 1
- 238000010525 oxidative degradation reaction Methods 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 150000004707 phenolate Chemical class 0.000 description 1
- 229950000688 phenothiazine Drugs 0.000 description 1
- OJMIONKXNSYLSR-UHFFFAOYSA-N phosphorous acid Chemical class OP(O)O OJMIONKXNSYLSR-UHFFFAOYSA-N 0.000 description 1
- 239000002798 polar solvent Substances 0.000 description 1
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920001522 polyglycol ester Polymers 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 239000010970 precious metal Substances 0.000 description 1
- QQONPFPTGQHPMA-UHFFFAOYSA-N propylene Natural products CC=C QQONPFPTGQHPMA-UHFFFAOYSA-N 0.000 description 1
- 125000004805 propylene group Chemical group [H]C([H])([H])C([H])([*:1])C([H])([H])[*:2] 0.000 description 1
- 238000000425 proton nuclear magnetic resonance spectrum Methods 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 150000005839 radical cations Chemical class 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 239000000565 sealant Substances 0.000 description 1
- 239000013049 sediment Substances 0.000 description 1
- 229920005573 silicon-containing polymer Polymers 0.000 description 1
- 229920002545 silicone oil Polymers 0.000 description 1
- 239000010802 sludge Substances 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- BPILDHPJSYVNAF-UHFFFAOYSA-M sodium;diiodomethanesulfonate Chemical compound [Na+].[O-]S(=O)(=O)C(I)I BPILDHPJSYVNAF-UHFFFAOYSA-M 0.000 description 1
- 239000011877 solvent mixture Substances 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 125000004079 stearyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 239000011115 styrene butadiene Substances 0.000 description 1
- 229920003048 styrene butadiene rubber Polymers 0.000 description 1
- 150000003440 styrenes Chemical class 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- TUNFSRHWOTWDNC-UHFFFAOYSA-N tetradecanoic acid Chemical compound CCCCCCCCCCCCCC(O)=O TUNFSRHWOTWDNC-UHFFFAOYSA-N 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- PHYFQTYBJUILEZ-IUPFWZBJSA-N triolein Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(OC(=O)CCCCCCC\C=C/CCCCCCCC)COC(=O)CCCCCCC\C=C/CCCCCCCC PHYFQTYBJUILEZ-IUPFWZBJSA-N 0.000 description 1
- 150000004072 triols Chemical class 0.000 description 1
- 238000005199 ultracentrifugation Methods 0.000 description 1
- 238000005292 vacuum distillation Methods 0.000 description 1
- 229920001567 vinyl ester resin Polymers 0.000 description 1
- 239000007762 w/o emulsion Substances 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M101/00—Lubricating compositions characterised by the base-material being a mineral or fatty oil
- C10M101/02—Petroleum fractions
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G67/00—Treatment of hydrocarbon oils by at least one hydrotreatment process and at least one process for refining in the absence of hydrogen only
- C10G67/02—Treatment of hydrocarbon oils by at least one hydrotreatment process and at least one process for refining in the absence of hydrogen only plural serial stages only
- C10G67/04—Treatment of hydrocarbon oils by at least one hydrotreatment process and at least one process for refining in the absence of hydrogen only plural serial stages only including solvent extraction as the refining step in the absence of hydrogen
- C10G67/0454—Solvent desasphalting
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G67/00—Treatment of hydrocarbon oils by at least one hydrotreatment process and at least one process for refining in the absence of hydrogen only
- C10G67/02—Treatment of hydrocarbon oils by at least one hydrotreatment process and at least one process for refining in the absence of hydrogen only plural serial stages only
- C10G67/04—Treatment of hydrocarbon oils by at least one hydrotreatment process and at least one process for refining in the absence of hydrogen only plural serial stages only including solvent extraction as the refining step in the absence of hydrogen
- C10G67/0454—Solvent desasphalting
- C10G67/0463—The hydrotreatment being a hydrorefining
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2300/00—Aspects relating to hydrocarbon processing covered by groups C10G1/00 - C10G99/00
- C10G2300/10—Feedstock materials
- C10G2300/1077—Vacuum residues
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2400/00—Products obtained by processes covered by groups C10G9/00 - C10G69/14
- C10G2400/10—Lubricating oil
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2203/00—Organic non-macromolecular hydrocarbon compounds and hydrocarbon fractions as ingredients in lubricant compositions
- C10M2203/10—Petroleum or coal fractions, e.g. tars, solvents, bitumen
- C10M2203/1006—Petroleum or coal fractions, e.g. tars, solvents, bitumen used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2020/00—Specified physical or chemical properties or characteristics, i.e. function, of component of lubricating compositions
- C10N2020/01—Physico-chemical properties
- C10N2020/011—Cloud point
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2020/00—Specified physical or chemical properties or characteristics, i.e. function, of component of lubricating compositions
- C10N2020/01—Physico-chemical properties
- C10N2020/015—Distillation range
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2020/00—Specified physical or chemical properties or characteristics, i.e. function, of component of lubricating compositions
- C10N2020/01—Physico-chemical properties
- C10N2020/02—Viscosity; Viscosity index
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2020/00—Specified physical or chemical properties or characteristics, i.e. function, of component of lubricating compositions
- C10N2020/01—Physico-chemical properties
- C10N2020/065—Saturated Compounds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2030/00—Specified physical or chemical properties which is improved by the additive characterising the lubricating composition, e.g. multifunctional additives
- C10N2030/40—Low content or no content compositions
- C10N2030/43—Sulfur free or low sulfur content compositions
-
- C10N2220/022—
-
- C10N2220/031—
-
- C10N2230/43—
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Production Of Liquid Hydrocarbon Mixture For Refining Petroleum (AREA)
- Lubricants (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
Description
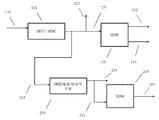
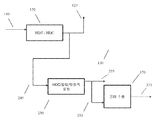
도 2는 탈아스팔트화된 오일을 가공하여 윤활유 베이스 스톡을 형성하기 위한 구성의 또 다른 예를 개략적으로 도시한다.
도 3은 탈아스팔트화된 오일을 가공하여 윤활유 베이스 스톡을 형성하기 위한 구성의 또 다른 예를 개략적으로 도시한다.
도 4는 다양한 수준의 수첨 처리 가혹도에서 펜탄 탈아스팔트화된 오일을 처리한 결과를 나타낸다.
도 5는 산성(sour) 수첨 분해와 비-산성(sweet) 수첨분해의 다양한 조합 구성으로 탈아스팔트화된 오일을 처리한 결과를 도시한다.
도 6은 탈아스팔트화된 오일을 가공하여 윤활유 베이스 스톡을 형성하기 위한 구성의 일례를 개략적으로 도시한다.
도 7은 다양한 프로판 탈아스팔트화된 공급물 및 기준 베이스 스톡으로부터 제조된 윤활유 베이스 스톡의 특성을 도시한다.
도 8은 다양한 부탄 탈아스팔트화된 공급물로부터 제조된 윤활유 베이스 스톡의 특성을 도시한다.
도 9는 그룹 I 및 그룹 II 브라이트 스톡을 사용하여 형성된 제형화된 윤활유의 특성을 도시한다.
도 10은 그룹 I 및 그룹 II 브라이트 스톡을 사용하여 형성된 제형화된 윤활유의 특성을 도시한다.
도 11은 그룹 I 및 그룹 II 브라이트 스톡을 사용하여 형성된 제형화된 윤활유의 특성을 도시한다.
도 12는 다양한 펜탄 탈아스팔트화된 공급물로부터 제조된 윤활유 베이스 스톡의 특성을 도시한다.
도 13은 다양한 펜탄 탈아스팔트화된 공급물로부터 제조된 윤활유 베이스 스톡의 특성을 도시한다.
| 화합물 |
대략적인 중량% (유용함) |
대략적인 중량% (바람직함) |
| 분산제 세정제 |
0.1-20 0.1-20 |
0.1-8 0.1-8 |
| 마찰 개질제 | 0.01-5 | 0.01-1.5 |
| 산화방지제 | 0.1-5 | 0.1-1.5 |
| 유동점 강하제(PPD) | 0.0-5 | 0.01-1.5 |
| 소포제 | 0.001-3 | 0.001-0.15 |
| 점도 지수 개질제 (순수 중합체 기준) |
0.0-8 | 0.1-6 |
| 내마모제 | 0.1-2 | 0.5-1 |
| 억제제 및 방청제 | 0.01-5 | 0.01-1.5 |
| API 도 | 14.0 |
| 황 (중량%) | 2.8 |
| 질소 (wppm) | 2653 |
| Ni (wppm) | 9.5 |
| V (wppm) | 14.0 |
| CCR (중량%) | 8.3 |
| 왁스 (중량%) | 3.9 |
| GCD 증류 (중량%) | (℃) |
| 5% | 480 |
| 10% | 505 |
| 30% | 558 |
| 50% | 597 |
| 70% | 641 |
| 90% | 712 |
| 생성물 분획 | |
| VI | 104.4 |
| KV @100℃ | 26.6 |
| KV @40℃ | 337 |
| 유동점 (℃) | -28 |
| 운점 (℃) | 8.4 |
| 전환율 (510℃에 대한 중량%) | 49 |
| 생성물 분획 | |
| VI | 104.4 |
| KV @100℃ | 25.7 |
| KV @40℃ | 321 |
| 유동점 (℃) | -27 |
| 운점 (℃) | -7.1 |
| API 도 | 13.7 |
| 황 (중량%) | 3.6 |
| 질소 (wppm) | 2099 |
| Ni (wppm) | 5.2 |
| V (wppm) | 14.0 |
| CCR (중량%) | 8.1 |
| 왁스 (중량%) | 4.2 |
| GCD 증류 (중량%) | (℃) |
| 5% | 422 |
| 10% | 465 |
| 30% | 541 |
| 50% | 584 |
| 70% | n/a |
| 90% | 652 |
| 생성물 분획 | 560℃+ | 라피네이트 | 추출물 |
| CDW 유출물 | (수율 92.2%) | ||
| API | 30.0 | 30.2 | 27.6 |
| VI | 104.2 | 105.2 | 89 |
| KV @100℃ | 29.8 | 30.3 | 29.9 |
| KV @40℃ | 401 | 405 | 412 |
| 유동점 (℃) | -21 | -30 | |
| 운점 (℃) | 7.8 | -24 |
| 생성물 분획 | 과소-탈랍 | 고-가혹도 |
| VI | 106.6 | 106.4 |
| KV @100℃ | 37.6 | 30.5 |
| KV @40℃ | 551 | 396 |
| 유동점 (℃) | -24 | -24 |
| 운점 (℃) | 8.6 | 4.9 |
| 잔유 (566℃+) | 잔유 A | 잔유 B |
| API 도 (도) | 5.4 | 4.4 |
| 비중 (15℃) (g/cc) | 1.0336 | 1.0412 |
| 전체 황 (중량%) | 4.56 | 5.03 |
| 니켈 (wppm) | 43.7 | 48.7 |
| 바나듐 (wppm) | 114 | 119 |
| TAN (mg KOH/g) | 0.314 | 0.174 |
| 전체 질소 (wppm) | 4760 | 4370 |
| 염기성 질소 (wppm) | 1210 | 1370 |
| 탄소 잔사 (중량%) | 24.4 | 25.8 |
| n-헵단 불용물 (중량%) | 7.68 | 8.83 |
| 왁스 (전체 - DSC) (중량%) | 1.4 | 1.32 |
| KV @ 100℃ (cSt) | 5920 | 11200 |
| KV @ 135℃ (cSt) | 619 | 988 |
| C3 DAO | C4 DAO | C5 DAO | |
| API 도(도) | 22.4 | 12.9 | 12.6 |
| 비중 (15℃) (g/cc) | 0.9138 | 0.9782 | 0.9808 |
| 전체 황 (중량%) | 2.01 | 3.82 | 3.56 |
| 니켈 (wppm) | < 0.1 | 5.2 | 5.3 |
| 바나듐 (wppm) | < 0.1 | 15.6 | 17.4 |
| 전체 질소 (wppm) | 504 | 2116 | 1933 |
| 염기성 질소 (wppm) | 203 | <N/A> | 478 |
| 탄소 잔사 (중량%) | 1.6 | 8.3 | 11.0 |
| KV @ 100℃ (cSt) | 33.3 | 124 | 172 |
| VI | 96 | 61 | <N/A> |
| 심디스트(SimDist) (ASTM D2887) ℃ |
|||
| 5 중량% | 509 | 490 | 527 |
| 10 중량% | 528 | 515 | 546 |
| 30 중량% | 566 | 568 | 588 |
| 50 중량% | 593 | 608 | 619 |
| 70 중량% | 623 | 657 | 664 |
| 90 중량% | 675 | <N/A> | <N/A> |
| 95 중량% | 701 | <N/A> | <N/A> |
Claims (15)
- 900℉(482℃) 이상의 T10 증류점; 80 이상의 점도 지수; 90 중량% 이상(또는 95 중량% 이상)의 포화물 함량; 300 wppm 미만의 황 함량; 100℃에서 14 cSt 이상의 동점도(kinematic viscosity); 40℃에서 320 cSt 이상(또는 340 cSt 이상, 또는 350 cSt 이상)의 동점도; 및 조성물의 100개의 탄소 원자 당 1.7개 이상(또는 1.8개 이상 또는 1.9개 이상)의 말단/펜던트 프로필 기와 말단/펜던트 에틸 기의 합을 포함하는 윤활유 베이스 스톡 조성물.
- 제 1 항에 있어서,
상기 말단/펜던트 프로필 기의 총 수가 조성물의 100개의 탄소 원자 당 0.85개 초과이거나, 또는 상기 말단/펜던트 에틸기의 총 수가 조성물의 100개의 탄소 원자 당 0.85개 초과이거나, 또는 이들의 조합인, 윤활유 베이스 스톡 조성물. - 제 1 항 또는 제 2 항에 있어서,
a) 상기 윤활유 베이스 스톡 조성물이 -6℃ 이하의 유동점을 갖거나;
b) 상기 윤활제 베이스 스톡 조성물이 -2℃ 이하의 운점을 갖거나;
c) 상기 윤활유 베이스 스톡 조성물이 25℃ 이하의 운점과 유동점의 차이를 포함하거나; 또는
d) a)와 b), a)와 c), b)와 c), 또는 a)와 b)와 c)의 조합인,
윤활유 베이스 스톡 조성물. - 제 1 항 내지 제 3 항 중 어느 한 항에 있어서,
상기 윤활유 베이스 스톡 조성물이 0.060 이상의 엡실론 탄소 원자에 대한 말단/펜던트 프로필 기의 비를 갖거나; 또는 상기 윤활유 베이스 스톡 조성물이 0.060 이상의 엡실론 탄소 원자에 대한 말단/펜던트 에틸 기의 비를 갖거나; 또는 이들의 조합인, 윤활유 베이스 스톡 조성물. - 제 1 항 내지 제 4 항 중 어느 한 항에 있어서,
상기 윤활유 베이스 스톡 조성물이 0.10 이상의 엡실론 탄소 원자에 대한 말단/펜던트 프로필 기와 말단/펜던트 에틸 기의 합의 비를 갖는, 윤활유 베이스 스톡 조성물. - 제 1 항 내지 제 5 항 중 어느 한 항에 있어서,
상기 윤활유 베이스 스톡 조성물이 1.5 이상의 탁도(turbidity) 및 0℃ 이하의 운점을 갖거나, 또는 상기 윤활유 베이스 스톡 조성물이 2.0 이상의 탁도를 갖거나, 또는 상기 윤활유 베이스 스톡 조성물이 4.0 이하의 탁도를 갖거나, 또는 이들의 조합인, 윤활유 베이스 스톡 조성물. - 제 1 항 내지 제 6 항 중 어느 한 항에 있어서,
상기 윤활유 베이스 스톡 조성물이 1000℉(538℃) 이상 또는 1050℉(566℃) 이상의 T50 증류점을 포함하거나, 또는 1150℉(621℃) 이상 또는 1200℉(649℃) 이상의 T90 증류점을 포함하거나, 또는 이들의 조합인, 윤활유 베이스 스톡 조성물. - 제 1 항 내지 제 7 항 중 어느 한 항에 있어서,
상기 윤활유 베이스 스톡 조성물이 (FDMS에 의해 결정시) 100개의 분자 당 2개의 포화 고리를 포함하는 분자 17개 이상 및 100개의 분자 당 3개의 포화 고리를 포함하는 분자 20개 이상을 포함하는, 윤활유 베이스 스톡 조성물. - 제 1 항 내지 제 8 항 중 어느 한 항에 있어서,
상기 윤활유 베이스 스톡 조성물이 0.1 중량% 이하의 콘라드손 탄소 잔사(Conradson Carbon Residue) 함량을 포함하거나, 또는 상기 점도 지수가 90 이상이거나, 또는 100℃에서의 동점도가 20 cSt 이상이거나, 또는 40℃에서의 동점도가 340 cSt 이상이거나, 또는 이들의 조합인, 윤활유 베이스 스톡 조성물. - 제 1 항 내지 제 9 항 중 어느 한 항에 있어서,
상기 윤활유 베이스 스톡 조성물이 (FDMS에 의해 결정시 또는 FTICR-MS에 의해 결정시) 100개의 분자 당 6개의 포화 고리를 포함하는 분자 7개 미만을 포함하거나, 100개의 분자 당 6개 이상의 포화 고리를 포함하는 분자 16개 미만을 포함하거나, 또는 2개의 포화 고리를 포함하는 분자에 대한 6개 이상의 포화 고리를 포함하는 분자의 비가 0.8 이하이거나, 또는 이들의 조합인, 윤활유 베이스 스톡 조성물. - 제 1 항 내지 제 10 항 중 어느 한 항에 있어서,
상기 윤활유 베이스 스톡 조성물이 조성물 중의 100개의 탄소 원자 당 14.5개 미만의 엡실론 탄소 원자를 포함하는, 윤활유 베이스 스톡 조성물. - 제 1 항 내지 제 11 항 중 어느 한 항에 있어서,
상기 윤활유 베이스 스톡 조성물이 (FTICR-MS에 의해 결정시) 100개의 분자 당 6개의 포화 고리를 포함하는 분자 7개 미만, 100개의 분자 당 7개의 포화 고리를 포함하는 분자 4개 미만, 100개의 분자 당 8개의 포화 고리를 포함하는 분자 2개 미만, 및 100개의 분자 당 9개의 포화 고리를 포함하는 분자 1개 미만을 포함하는, 윤활유 베이스 스톡 조성물. - 제 1 항 내지 제 12 항 중 어느 한 항에 있어서,
i) 상기 윤활유 베이스 스톡 조성물이 (FDMS에 의해 결정시) 100개의 분자 당 2개의 포화 고리를 포함하는 분자 20개 이상 및 100개의 분자 당 3개의 포화 고리를 포함하는 분자 22개 이상을 포함하고, 이때 상기 윤활유 베이스 스톡 조성물은 임의적으로 0.02 중량% 이하의 콘라드손 탄소 잔사 함량을 포함하거나;
ii) 상기 윤활유 베이스 스톡 조성물이 (FDMS에 의해 결정시) 100개의 분자 당 6개의 포화 고리를 포함하는 분자 7개 미만을 포함하고, 이때 상기 윤활유 베이스 스톡 조성물은 임의적으로 0.1 중량% 이하의 콘라드손 탄소 잔사 함량을 포함하거나; 또는
iii) i) 및 ii)의 조합인, 윤활유 베이스 스톡 조성물. - 제 1 항 내지 제 13 항 중 어느 한 항에 있어서,
상기 윤활유 베이스 스톡 조성물이, (FTICR-MS에 의해 결정시) 100개의 분자 당 6개 이상의 포화 고리를 포함하는 분자 16개 미만을 포함하거나, 4 내지 6개의 포화 고리를 포함하는 분자에 대한 1 내지 3개의 포화 고리를 포함하는 분자의 비가 1.1 이상이거나, 또는 이들의 조합인, 윤활유 베이스 스톡 조성물. - 제 1 항 내지 제 14 항 중 어느 한 항의 윤활유 베이스 스톡 조성물 및 1종 이상의 첨가제를 포함하는 제형화된 윤활제.
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562271543P | 2015-12-28 | 2015-12-28 | |
| US62/271,543 | 2015-12-28 | ||
| US15/390,943 | 2016-12-27 | ||
| US15/390,943 US10590360B2 (en) | 2015-12-28 | 2016-12-27 | Bright stock production from deasphalted oil |
| PCT/US2016/068806 WO2017117178A1 (en) | 2015-12-28 | 2016-12-28 | Bright stock production from deasphalted oil |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20180098655A true KR20180098655A (ko) | 2018-09-04 |
| KR102762110B1 KR102762110B1 (ko) | 2025-02-03 |
Family
ID=57799912
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020187021933A Active KR102762110B1 (ko) | 2015-12-28 | 2016-12-28 | 탈아스탈트화된 오일로부터의 브라이트 스톡의 제조 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US10590360B2 (ko) |
| EP (1) | EP3397730B1 (ko) |
| JP (2) | JP6997721B2 (ko) |
| KR (1) | KR102762110B1 (ko) |
| CN (1) | CN108473887B (ko) |
| AU (1) | AU2016381604A1 (ko) |
| SG (1) | SG11201804665WA (ko) |
| WO (1) | WO2017117178A1 (ko) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10590360B2 (en) * | 2015-12-28 | 2020-03-17 | Exxonmobil Research And Engineering Company | Bright stock production from deasphalted oil |
| US20170183576A1 (en) | 2015-12-28 | 2017-06-29 | Exxonmobil Research And Engineering Company | Bright stock and heavy neutral production from resid deasphalting |
| CN108127083A (zh) * | 2017-12-23 | 2018-06-08 | 安徽鑫宏机械有限公司 | 一种精密铸件砂型模壳的脱蜡回收熔模工艺 |
| CN108929764B (zh) * | 2018-08-28 | 2021-11-02 | 广东山之风环保科技有限公司 | 一种半合成不锈钢切削液及其制备方法 |
| US11485920B2 (en) * | 2020-05-22 | 2022-11-01 | ExxonMobil Technology and Engineering Company | Ultra low sulfur marine fuel compositions |
| WO2021252143A1 (en) * | 2020-06-09 | 2021-12-16 | Exxonmobil Research And Engineering Company | Lubricants having improved low temperature, oxidation and deposit control performance |
| CN115667469B (zh) * | 2020-06-09 | 2025-10-28 | 埃克森美孚技术与工程公司 | 具有改善的氧化和沉积物控制性能的润滑剂 |
| CN115386395B (zh) * | 2021-05-20 | 2024-06-04 | 国家能源投资集团有限责任公司 | 降低费托合成油浊点的方法及络合剂与络合剂的应用 |
| CN121152865A (zh) * | 2023-05-01 | 2025-12-16 | 埃克森美孚技术与工程公司 | 高性能齿轮油和相关方法 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR870005068A (ko) * | 1985-11-01 | 1987-06-04 | 에드워드 에취. 바란스 | 윤활류의 제조 공정 |
| US6191078B1 (en) * | 1999-09-21 | 2001-02-20 | Exxonmobil Research And Engineering Company | Part-synthetic, aviation piston engine lubricant |
| US20050000857A1 (en) * | 2002-10-30 | 2005-01-06 | Eric Benazzi | Flexible process for the production of oil bases and middle distillates with a converting pretreatment stage followed by a catalytic dewaxing stage |
| US20050051463A1 (en) * | 2003-09-09 | 2005-03-10 | Chevron U.S.A. Inc. | Production of high quality lubricant bright stock |
Family Cites Families (204)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1815022A (en) | 1930-05-03 | 1931-07-14 | Standard Oil Dev Co | Hydrocarbon oil and process for manufacturing the same |
| US1948296A (en) | 1930-07-07 | 1934-02-20 | Union Oil Co | Method for producing asphalt |
| US1988712A (en) | 1931-08-04 | 1935-01-22 | Union Oil Co | Process for production of lubricating oil |
| US2015748A (en) | 1933-06-30 | 1935-10-01 | Standard Oil Dev Co | Method for producing pour inhibitors |
| US2100993A (en) | 1934-12-14 | 1937-11-30 | Rohm & Haas | Process for preparing esters and products |
| US2191498A (en) | 1935-11-27 | 1940-02-27 | Socony Vacuum Oil Co Inc | Mineral oil composition and method of making |
| US2213798A (en) | 1938-06-18 | 1940-09-03 | Texas Co | Removal of asphalt from hydrocarbon oil |
| US2387501A (en) | 1944-04-04 | 1945-10-23 | Du Pont | Hydrocarbon oil |
| US2655479A (en) | 1949-01-03 | 1953-10-13 | Standard Oil Dev Co | Polyester pour depressants |
| US2721878A (en) | 1951-08-18 | 1955-10-25 | Exxon Research Engineering Co | Strong acid as a polymerization modifier in the production of liquid polymers |
| US2721877A (en) | 1951-08-22 | 1955-10-25 | Exxon Research Engineering Co | Lubricating oil additives and a process for their preparation |
| US2666746A (en) | 1952-08-11 | 1954-01-19 | Standard Oil Dev Co | Lubricating oil composition |
| US3036003A (en) | 1957-08-07 | 1962-05-22 | Sinclair Research Inc | Lubricating oil composition |
| US2940920A (en) | 1959-02-19 | 1960-06-14 | Kerr Mc Gee Oil Ind Inc | Separation of asphalt-type bituminous materials |
| DE1248643B (de) | 1959-03-30 | 1967-08-31 | The Lubrizol Corporation, Cleveland, Ohio (V. St. A.) | Verfahren zur Herstellung von öllöslichen aeylierten Aminen |
| US3444170A (en) | 1959-03-30 | 1969-05-13 | Lubrizol Corp | Process which comprises reacting a carboxylic intermediate with an amine |
| US3215707A (en) | 1960-06-07 | 1965-11-02 | Lubrizol Corp | Lubricant |
| US3200107A (en) | 1961-06-12 | 1965-08-10 | Lubrizol Corp | Process for preparing acylated amine-cs2 compositions and products |
| US3087936A (en) | 1961-08-18 | 1963-04-30 | Lubrizol Corp | Reaction product of an aliphatic olefinpolymer-succinic acid producing compound with an amine and reacting the resulting product with a boron compound |
| US3329658A (en) | 1962-05-14 | 1967-07-04 | Monsanto Co | Dispersency oil additives |
| US3449250A (en) | 1962-05-14 | 1969-06-10 | Monsanto Co | Dispersency oil additives |
| NL296139A (ko) | 1963-08-02 | |||
| NL296536A (ko) | 1963-08-12 | |||
| US3322670A (en) | 1963-08-26 | 1967-05-30 | Standard Oil Co | Detergent-dispersant lubricant additive having anti-rust and anti-wear properties |
| US3250715A (en) | 1964-02-04 | 1966-05-10 | Lubrizol Corp | Terpolymer product and lubricating composition containing it |
| US3287254A (en) | 1964-06-03 | 1966-11-22 | Chevron Res | Residual oil conversion process |
| US3316177A (en) | 1964-12-07 | 1967-04-25 | Lubrizol Corp | Functional fluid containing a sludge inhibiting detergent comprising the polyamine salt of the reaction product of maleic anhydride and an oxidized interpolymer of propylene and ethylene |
| NL145565B (nl) | 1965-01-28 | 1975-04-15 | Shell Int Research | Werkwijze ter bereiding van een smeermiddelcompositie. |
| US3574576A (en) | 1965-08-23 | 1971-04-13 | Chevron Res | Distillate fuel compositions having a hydrocarbon substituted alkylene polyamine |
| US3704308A (en) | 1965-10-22 | 1972-11-28 | Standard Oil Co | Boron-containing high molecular weight mannich condensation |
| US3697574A (en) | 1965-10-22 | 1972-10-10 | Standard Oil Co | Boron derivatives of high molecular weight mannich condensation products |
| US3798165A (en) | 1965-10-22 | 1974-03-19 | Standard Oil Co | Lubricating oils containing high molecular weight mannich condensation products |
| US3751365A (en) | 1965-10-22 | 1973-08-07 | Standard Oil Co | Concentrates and crankcase oils comprising oil solutions of boron containing high molecular weight mannich reaction condensation products |
| US3756953A (en) | 1965-10-22 | 1973-09-04 | Standard Oil Co | Vatives of high molecular weight mannich reaction condensation concentrate and crankcase oils comprising oil solutions of boron deri |
| US3272746A (en) | 1965-11-22 | 1966-09-13 | Lubrizol Corp | Lubricating composition containing an acylated nitrogen compound |
| US3413347A (en) | 1966-01-26 | 1968-11-26 | Ethyl Corp | Mannich reaction products of high molecular weight alkyl phenols, aldehydes and polyaminopolyalkyleneamines |
| US3822209A (en) | 1966-02-01 | 1974-07-02 | Ethyl Corp | Lubricant additives |
| GB1174593A (en) | 1966-05-02 | 1969-12-17 | Ruberoid Co Ltd | Bituminous Sheeting |
| GB1216198A (en) | 1967-02-02 | 1970-12-16 | Gulf Research Development Co | Improved process for the production of lubricating oil |
| US3519565A (en) | 1967-09-19 | 1970-07-07 | Lubrizol Corp | Oil-soluble interpolymers of n-vinylthiopyrrolidones |
| US3703536A (en) | 1967-11-24 | 1972-11-21 | Standard Oil Co | Preparation of oil-soluble boron derivatives of an alkylene polyamine-substituted phenol-formaldehyde addition product |
| US3541012A (en) | 1968-04-15 | 1970-11-17 | Lubrizol Corp | Lubricants and fuels containing improved acylated nitrogen additives |
| GB1244435A (en) | 1968-06-18 | 1971-09-02 | Lubrizol Corp | Oil-soluble graft polymers derived from degraded ethylene-propylene interpolymers |
| GB1282887A (en) | 1968-07-03 | 1972-07-26 | Lubrizol Corp | Acylation of nitrogen-containing products |
| DE1930607A1 (de) | 1968-07-03 | 1970-01-29 | Sun Oil Co | Verfahren zur Herstellung von Schmieroel mit hohem Viskositaetsindex |
| US3726882A (en) | 1968-11-08 | 1973-04-10 | Standard Oil Co | Ashless oil additives |
| US3725480A (en) | 1968-11-08 | 1973-04-03 | Standard Oil Co | Ashless oil additives |
| US3702300A (en) | 1968-12-20 | 1972-11-07 | Lubrizol Corp | Lubricant containing nitrogen-containing ester |
| US3454607A (en) | 1969-02-10 | 1969-07-08 | Lubrizol Corp | High molecular weight carboxylic compositions |
| US3595791A (en) | 1969-03-11 | 1971-07-27 | Lubrizol Corp | Basic,sulfurized salicylates and method for their preparation |
| US3652616A (en) | 1969-08-14 | 1972-03-28 | Standard Oil Co | Additives for fuels and lubricants |
| US3627675A (en) | 1969-10-16 | 1971-12-14 | Foster Wheeler Corp | Solvent deasphalting with two light hydrocarbon solvents |
| US3632511A (en) | 1969-11-10 | 1972-01-04 | Lubrizol Corp | Acylated nitrogen-containing compositions processes for their preparationand lubricants and fuels containing the same |
| FR2194767B1 (ko) | 1972-08-04 | 1975-03-07 | Shell France | |
| US3803039A (en) | 1970-07-13 | 1974-04-09 | Standard Oil Co | Oil solution of aliphatic acid derivatives of high molecular weight mannich condensation product |
| US3765911A (en) | 1970-10-27 | 1973-10-16 | London Oil Refining Co | Processing of rubber and the like, and to processing compositions therefor |
| US3804763A (en) | 1971-07-01 | 1974-04-16 | Lubrizol Corp | Dispersant compositions |
| US3787374A (en) | 1971-09-07 | 1974-01-22 | Lubrizol Corp | Process for preparing high molecular weight carboxylic compositions |
| US3755433A (en) | 1971-12-16 | 1973-08-28 | Texaco Inc | Ashless lubricating oil dispersant |
| CA1003778A (en) | 1972-04-06 | 1977-01-18 | Peter Ladeur | Hydrocarbon conversion process |
| US4100082A (en) | 1976-01-28 | 1978-07-11 | The Lubrizol Corporation | Lubricants containing amino phenol-detergent/dispersant combinations |
| US4454059A (en) | 1976-11-12 | 1984-06-12 | The Lubrizol Corporation | Nitrogenous dispersants, lubricants and concentrates containing said nitrogenous dispersants |
| BR7800984A (pt) | 1977-02-25 | 1979-01-02 | Lubrizol Corp | Composicao lubrificante;e concentrado para formulacao de composicoes lubrificantes |
| US4125459A (en) | 1977-03-28 | 1978-11-14 | Kerr-Mcgee Refining Corporation | Hydrocarbon solvent treatment of bituminous materials |
| US4234435A (en) | 1979-02-23 | 1980-11-18 | The Lubrizol Corporation | Novel carboxylic acid acylating agents, derivatives thereof, concentrate and lubricant compositions containing the same, and processes for their preparation |
| US4426305A (en) | 1981-03-23 | 1984-01-17 | Edwin Cooper, Inc. | Lubricating compositions containing boronated nitrogen-containing dispersants |
| NL8202827A (nl) | 1982-07-13 | 1984-02-01 | Shell Int Research | Werkwijze voor de bereiding van asfaltenenarme koolwaterstofmengsels. |
| FR2579985B1 (ko) | 1985-04-05 | 1988-07-15 | Inst Francais Du Petrole | |
| US4686028A (en) | 1985-04-05 | 1987-08-11 | Driesen Roger P Van | Upgrading of high boiling hydrocarbons |
| US4767551A (en) | 1985-12-02 | 1988-08-30 | Amoco Corporation | Metal-containing lubricant compositions |
| FR2598716B1 (fr) | 1986-05-15 | 1988-10-21 | Total France | Procede de desasphaltage d'une charge hydrocarbonee lourde |
| US4798684A (en) | 1987-06-09 | 1989-01-17 | The Lubrizol Corporation | Nitrogen containing anti-oxidant compositions |
| US5124025A (en) | 1989-07-18 | 1992-06-23 | Amoco Corporation | Process for deasphalting resid, recovering oils, removing fines from decanted oil and apparatus therefor |
| US4982051A (en) | 1990-01-18 | 1991-01-01 | Texaco Inc. | Separation of furfural/middle distillate streams |
| US5366648A (en) | 1990-02-23 | 1994-11-22 | The Lubrizol Corporation | Functional fluids useful at high temperatures |
| US5084197A (en) | 1990-09-21 | 1992-01-28 | The Lubrizol Corporation | Antiemulsion/antifoam agent for use in oils |
| US5358627A (en) | 1992-01-31 | 1994-10-25 | Union Oil Company Of California | Hydroprocessing for producing lubricating oil base stocks |
| US5302279A (en) | 1992-12-23 | 1994-04-12 | Mobil Oil Corporation | Lubricant production by hydroisomerization of solvent extracted feedstocks |
| AU719520B2 (en) | 1995-09-19 | 2000-05-11 | Lubrizol Corporation, The | Additive compositions for lubricants and functional fluids |
| US5976353A (en) | 1996-06-28 | 1999-11-02 | Exxon Research And Engineering Co | Raffinate hydroconversion process (JHT-9601) |
| US5871634A (en) | 1996-12-10 | 1999-02-16 | Exxon Research And Engineering Company | Process for blending potentially incompatible petroleum oils |
| JP3866380B2 (ja) | 1997-06-30 | 2007-01-10 | 出光興産株式会社 | ディーゼル燃料油組成物 |
| US7513989B1 (en) | 1997-07-15 | 2009-04-07 | Exxonmobil Research And Engineering Company | Hydrocracking process using bulk group VIII/Group VIB catalysts |
| CN1105165C (zh) | 1997-11-28 | 2003-04-09 | 英菲诺姆美国公司 | 润滑油组合物 |
| FR2777290B1 (fr) | 1998-04-09 | 2000-05-12 | Inst Francais Du Petrole | Procede d'amelioration de l'indice de cetane d'une coupe gasoil |
| WO2000006670A1 (en) | 1998-07-29 | 2000-02-10 | Texaco Development Corporation | Integration of solvent deasphalting and gasification |
| US6461497B1 (en) | 1998-09-01 | 2002-10-08 | Atlantic Richfield Company | Reformulated reduced pollution diesel fuel |
| WO2000042123A1 (en) | 1999-01-11 | 2000-07-20 | Texaco Development Corporation | Integration of solvent deasphalting, gasification, and hydrotreating |
| US7261805B2 (en) | 1999-02-24 | 2007-08-28 | Exxonmobil Research And Engineering Company | Process for catalytic dewaxing and catalytic cracking of hydrocarbon streams |
| JP3999912B2 (ja) | 1999-07-06 | 2007-10-31 | 新日本石油株式会社 | A重油組成物 |
| JP3999911B2 (ja) | 1999-07-06 | 2007-10-31 | 新日本石油株式会社 | A重油組成物 |
| US20020005374A1 (en) | 2000-02-15 | 2002-01-17 | Bearden Roby | Heavy feed upgrading based on solvent deasphalting followed by slurry hydroprocessing of asphalt from solvent deasphalting (fcb-0009) |
| US7029571B1 (en) | 2000-02-16 | 2006-04-18 | Indian Oil Corporation Limited | Multi stage selective catalytic cracking process and a system for producing high yield of middle distillate products from heavy hydrocarbon feedstocks |
| US6533925B1 (en) | 2000-08-22 | 2003-03-18 | Texaco Development Corporation | Asphalt and resin production to integration of solvent deasphalting and gasification |
| US6323164B1 (en) | 2000-11-01 | 2001-11-27 | Ethyl Corporation | Dispersant (meth) acrylate copolymers having excellent low temperature properties |
| DK1425365T3 (da) | 2001-09-07 | 2014-01-20 | Shell Int Research | Dieselbrændstof og fremgangsmåde til fremstilling og anvendelse deraf |
| US20030191032A1 (en) | 2002-01-31 | 2003-10-09 | Deckman Douglas E. | Mixed TBN detergents and lubricating oil compositions containing such detergents |
| FR2836150B1 (fr) | 2002-02-15 | 2004-04-09 | Inst Francais Du Petrole | Procede d'amelioration de coupes gazoles aromatiques et naphteno-aromatiques |
| JP4268373B2 (ja) | 2002-05-31 | 2009-05-27 | 新日本石油株式会社 | 軽油組成物(2) |
| JP4152127B2 (ja) | 2002-05-31 | 2008-09-17 | 新日本石油株式会社 | 軽油組成物(1) |
| JP4072396B2 (ja) | 2002-08-07 | 2008-04-09 | 新日本石油株式会社 | 軽油組成物 |
| JP2004067906A (ja) | 2002-08-07 | 2004-03-04 | Nippon Oil Corp | 軽油組成物及びその製造方法 |
| US7144497B2 (en) | 2002-11-20 | 2006-12-05 | Chevron U.S.A. Inc. | Blending of low viscosity Fischer-Tropsch base oils with conventional base oils to produce high quality lubricating base oils |
| JP4639290B2 (ja) | 2002-12-20 | 2011-02-23 | エニ、ソシエタ、ペル、アチオニ | 重質粗油及び蒸留残渣のような重質原料油の転化方法 |
| SE522918E (sv) | 2003-02-27 | 2012-11-06 | Eco Par Ab | Ett nytt alternativt bränsle för dieselmotorer med låga emissioner och hög energitäthet |
| JP4567947B2 (ja) | 2003-03-07 | 2010-10-27 | Jx日鉱日石エネルギー株式会社 | 軽油組成物 |
| JP2004269685A (ja) | 2003-03-07 | 2004-09-30 | Nippon Oil Corp | 軽油組成物及びその製造方法 |
| JP4567948B2 (ja) | 2003-03-07 | 2010-10-27 | Jx日鉱日石エネルギー株式会社 | 軽油組成物およびその製造方法 |
| JP4575646B2 (ja) | 2003-03-07 | 2010-11-04 | Jx日鉱日石エネルギー株式会社 | 軽油組成物 |
| US7141157B2 (en) | 2003-03-11 | 2006-11-28 | Chevron U.S.A. Inc. | Blending of low viscosity Fischer-Tropsch base oils and Fischer-Tropsch derived bottoms or bright stock |
| WO2004090078A1 (en) | 2003-04-11 | 2004-10-21 | Sasol Technology (Pty) Ltd | Low sulphur diesel fuel and aviation turbine fuel |
| US20040209082A1 (en) | 2003-04-17 | 2004-10-21 | Lee Willy W. | Process of Coating Tacky and Soft Polymer Pellets |
| US7053254B2 (en) | 2003-11-07 | 2006-05-30 | Chevron U.S.A, Inc. | Process for improving the lubricating properties of base oils using a Fischer-Tropsch derived bottoms |
| EP1720961B1 (en) | 2004-03-02 | 2014-12-10 | Shell Internationale Research Maatschappij B.V. | Process to continuously prepare two or more base oil grades and middle distillates |
| JP4620381B2 (ja) | 2004-06-02 | 2011-01-26 | 出光興産株式会社 | 軽油組成物 |
| JP4643966B2 (ja) | 2004-10-01 | 2011-03-02 | Jx日鉱日石エネルギー株式会社 | 水素化精製軽油の製造方法、水素化精製軽油及び軽油組成物 |
| JP4482469B2 (ja) | 2004-10-12 | 2010-06-16 | コスモ石油株式会社 | 軽油組成物の製造方法 |
| JP4482470B2 (ja) | 2004-10-12 | 2010-06-16 | コスモ石油株式会社 | 軽油組成物の製造方法 |
| US20060101712A1 (en) | 2004-11-15 | 2006-05-18 | Burnett Don E | Small off-road engine green fuel |
| US7279090B2 (en) | 2004-12-06 | 2007-10-09 | Institut Francais Du Pe'trole | Integrated SDA and ebullated-bed process |
| JP4563216B2 (ja) | 2005-02-25 | 2010-10-13 | コスモ石油株式会社 | 灯油組成物 |
| JP4593376B2 (ja) | 2005-06-08 | 2010-12-08 | コスモ石油株式会社 | ディーゼルエンジン用燃料油組成物 |
| WO2007003623A1 (en) * | 2005-07-01 | 2007-01-11 | Shell Internationale Research Maatschappij B.V. | Process to prepare a blended brightstock |
| JP2007009159A (ja) | 2005-07-04 | 2007-01-18 | Nippon Oil Corp | 水素化精製軽油の製造方法、水素化精製軽油及び軽油組成物 |
| JP5166686B2 (ja) | 2005-09-16 | 2013-03-21 | コスモ石油株式会社 | 灯油組成物 |
| AR059751A1 (es) | 2006-03-10 | 2008-04-23 | Shell Int Research | Composiciones de combustible diesel |
| US7582591B2 (en) * | 2006-04-07 | 2009-09-01 | Chevron U.S.A. Inc. | Gear lubricant with low Brookfield ratio |
| JP5052874B2 (ja) | 2006-12-05 | 2012-10-17 | コスモ石油株式会社 | ディーゼルエンジン用燃料油組成物 |
| JP5052876B2 (ja) | 2006-12-05 | 2012-10-17 | コスモ石油株式会社 | ディーゼルエンジン用燃料油組成物 |
| JP5052875B2 (ja) | 2006-12-05 | 2012-10-17 | コスモ石油株式会社 | ディーゼルエンジン用燃料油組成物 |
| FR2910487B1 (fr) | 2006-12-21 | 2010-09-03 | Inst Francais Du Petrole | Procede de conversion de residus incluant 2 desasphaltages en serie |
| JP5144316B2 (ja) | 2007-03-15 | 2013-02-13 | コスモ石油株式会社 | 灯油組成物 |
| SG183713A1 (en) * | 2007-08-13 | 2012-09-27 | Shell Int Research | Lubricating base oil blend |
| US8048833B2 (en) | 2007-08-17 | 2011-11-01 | Exxonmobil Research And Engineering Company | Catalytic antioxidants |
| US7964090B2 (en) | 2008-05-28 | 2011-06-21 | Kellogg Brown & Root Llc | Integrated solvent deasphalting and gasification |
| JP5043754B2 (ja) | 2008-06-04 | 2012-10-10 | コスモ石油株式会社 | ディーゼルエンジン用燃料油組成物 |
| JP5205641B2 (ja) | 2008-06-04 | 2013-06-05 | コスモ石油株式会社 | ディーゼルエンジン用燃料油組成物 |
| JP5205639B2 (ja) | 2008-06-04 | 2013-06-05 | コスモ石油株式会社 | ディーゼルエンジン用燃料油組成物及びディーゼルエンジン用燃料油組成物の製造方法 |
| JP5205640B2 (ja) | 2008-06-04 | 2013-06-05 | コスモ石油株式会社 | ディーゼルエンジン用燃料油組成物の製造方法 |
| KR100934331B1 (ko) | 2008-06-17 | 2009-12-29 | 에스케이루브리컨츠 주식회사 | 고급 나프텐계 베이스 오일의 제조방법 |
| US8361309B2 (en) | 2008-06-19 | 2013-01-29 | Chevron U.S.A. Inc. | Diesel composition and method of making the same |
| US20090313890A1 (en) | 2008-06-19 | 2009-12-24 | Chevron U.S.A. Inc. | Diesel composition and method of making the same |
| JP4994327B2 (ja) | 2008-08-08 | 2012-08-08 | Jx日鉱日石エネルギー株式会社 | 灯油組成物及びその製造方法 |
| US8932454B2 (en) | 2008-09-18 | 2015-01-13 | Exxonmobile Research And Engineering Co. | Mesoporous Y hydrocracking catalyst and associated hydrocracking processes |
| US8361434B2 (en) | 2008-09-18 | 2013-01-29 | Exxonmobil Research And Engineering Company | Extra mesoporous Y zeolite |
| WO2010039294A1 (en) | 2008-10-01 | 2010-04-08 | Chevron U.S.A. Inc. | A process to make a 110 neutral base oil with improved properties |
| FR2937047B1 (fr) | 2008-10-10 | 2012-07-27 | Nyco Sa | Utilisation d'un additif a base d'oligomeres pour stabiliser une composition lubrifiante pour chaine de convoyage |
| EP2346963B1 (en) | 2008-10-22 | 2018-11-21 | Chevron U.S.A., Inc. | A high energy distillate fuel composition |
| EP2199371A1 (en) | 2008-12-15 | 2010-06-23 | Total Raffinage Marketing | Process for aromatic hydrogenation and cetane value increase of middle distillate feedstocks |
| US8394255B2 (en) | 2008-12-31 | 2013-03-12 | Exxonmobil Research And Engineering Company | Integrated hydrocracking and dewaxing of hydrocarbons |
| US8366908B2 (en) | 2008-12-31 | 2013-02-05 | Exxonmobil Research And Engineering Company | Sour service hydroprocessing for lubricant base oil production |
| FR2943070B1 (fr) | 2009-03-12 | 2012-12-21 | Total Raffinage Marketing | Fluide hydrocarbone hydrodeparaffine utilise dans la fabrication de fluides industriels, agricoles ou a usage domestique |
| JP2010215723A (ja) | 2009-03-13 | 2010-09-30 | Idemitsu Kosan Co Ltd | 軽油基材の製造方法 |
| JP5361499B2 (ja) | 2009-04-01 | 2013-12-04 | Jx日鉱日石エネルギー株式会社 | 改質器付き予混合圧縮着火エンジン用燃料油組成物 |
| JP2010241875A (ja) | 2009-04-01 | 2010-10-28 | Japan Energy Corp | 改質器付きディーゼルエンジン用燃料油組成物 |
| JP2010241869A (ja) | 2009-04-01 | 2010-10-28 | Japan Energy Corp | 改質器付きディーゼルエンジン用燃料油組成物 |
| US8658030B2 (en) | 2009-09-30 | 2014-02-25 | General Electric Company | Method for deasphalting and extracting hydrocarbon oils |
| JP5518454B2 (ja) | 2009-12-11 | 2014-06-11 | Jx日鉱日石エネルギー株式会社 | ディーゼルハイブリッド用燃料組成物 |
| JP5467890B2 (ja) | 2010-02-15 | 2014-04-09 | Jx日鉱日石エネルギー株式会社 | 改質器付き予混合圧縮着火エンジン用燃料油の製造方法 |
| JP5520101B2 (ja) | 2010-03-05 | 2014-06-11 | Jx日鉱日石エネルギー株式会社 | 軽油組成物 |
| JP5520114B2 (ja) | 2010-03-31 | 2014-06-11 | Jx日鉱日石エネルギー株式会社 | 軽油組成物 |
| JP5520115B2 (ja) | 2010-03-31 | 2014-06-11 | Jx日鉱日石エネルギー株式会社 | 軽油組成物 |
| JP5128633B2 (ja) | 2010-04-22 | 2013-01-23 | コスモ石油株式会社 | 灯油組成物 |
| JP5128632B2 (ja) | 2010-04-22 | 2013-01-23 | コスモ石油株式会社 | 灯油組成物 |
| JP5128631B2 (ja) | 2010-04-22 | 2013-01-23 | コスモ石油株式会社 | ディーゼルエンジン用燃料油組成物 |
| KR101796782B1 (ko) | 2010-05-07 | 2017-11-13 | 에스케이이노베이션 주식회사 | 고급 납센계 윤활기유 및 중질 윤활기유를 병산 제조하는 방법 |
| KR101779605B1 (ko) * | 2010-06-04 | 2017-09-19 | 에스케이이노베이션 주식회사 | 감압증류된 탈아스팔트유를 이용한 윤활기유 제조방법 |
| US8992764B2 (en) | 2010-06-29 | 2015-03-31 | Exxonmobil Research And Engineering Company | Integrated hydrocracking and dewaxing of hydrocarbons |
| US8617383B2 (en) | 2010-06-29 | 2013-12-31 | Exxonmobil Research And Engineering Company | Integrated hydrocracking and dewaxing of hydrocarbons |
| US9487723B2 (en) | 2010-06-29 | 2016-11-08 | Exxonmobil Research And Engineering Company | High viscosity high quality group II lube base stocks |
| US8475648B2 (en) | 2010-06-29 | 2013-07-02 | Chevron U.S.A. Inc. | Catalytic processes and systems for base oil production from heavy feedstock |
| US20120000829A1 (en) | 2010-06-30 | 2012-01-05 | Exxonmobil Research And Engineering Company | Process for the preparation of group ii and group iii lube base oils |
| JP2012021085A (ja) | 2010-07-15 | 2012-02-02 | Showa Shell Sekiyu Kk | 軽油燃料組成物 |
| US8557106B2 (en) | 2010-09-30 | 2013-10-15 | Exxonmobil Research And Engineering Company | Hydrocracking process selective for improved distillate and improved lube yield and properties |
| US8222471B2 (en) * | 2010-12-13 | 2012-07-17 | Chevron U.S.A. Inc. | Process for making a high viscosity base oil with an improved viscosity index |
| US9418828B2 (en) | 2010-12-16 | 2016-08-16 | Exxonmobil Research And Engineering Company | Characterization of petroleum saturates |
| US8778171B2 (en) | 2011-07-27 | 2014-07-15 | Exxonmobil Research And Engineering Company | Hydrocracking catalysts containing stabilized aggregates of small crystallites of zeolite Y associated hydrocarbon conversion processes |
| JP5615215B2 (ja) | 2011-03-22 | 2014-10-29 | Jx日鉱日石エネルギー株式会社 | 軽油組成物及びその製造方法 |
| US9200218B2 (en) | 2011-03-31 | 2015-12-01 | Exxonmobil Research And Engineering Company | Fuels hydrocracking with dewaxing of fuel products |
| WO2013012661A1 (en) | 2011-07-20 | 2013-01-24 | Exxonmobil Research And Engineering Company | Production of lubricating oil basestocks |
| WO2013015883A1 (en) | 2011-07-27 | 2013-01-31 | Saudi Arabian Oil Company | Production of synthesis gas from solvent deasphalting process bottoms in a membrane wall gasification reactor |
| US8993495B2 (en) | 2011-12-02 | 2015-03-31 | Exxonmobil Research And Engineering Company | Upgrading deasphalting residue to high performance asphalt |
| US9074139B2 (en) | 2011-12-07 | 2015-07-07 | IFP Energies Nouvelles | Process for coal conversion comprising at least one step of liquefaction for the manufacture of aromatics |
| US9005380B2 (en) | 2012-03-23 | 2015-04-14 | Johann Haltermann Limited | High performance liquid rocket propellant |
| CN104245902B (zh) * | 2012-04-04 | 2017-06-27 | 国际壳牌研究有限公司 | 制备渣油基油的方法 |
| JP5312646B2 (ja) | 2012-07-11 | 2013-10-09 | コスモ石油株式会社 | ディーゼルエンジン用燃料油組成物 |
| JP5328973B2 (ja) | 2012-11-26 | 2013-10-30 | コスモ石油株式会社 | ディーゼルエンジン用燃料油組成物 |
| FR2999190B1 (fr) | 2012-12-10 | 2015-08-14 | Total Raffinage Marketing | Procede d'obtention de solvants hydrocarbones de temperature d'ebullition superieure a 300°c et de point d'ecoulement inferieur ou egal a -25°c |
| SG10201704490XA (en) | 2012-12-27 | 2017-07-28 | Jx Nippon Oil & Energy Corp | System lubricant composition for crosshead diesel engines |
| US9359565B2 (en) | 2013-01-16 | 2016-06-07 | Exxonmobil Research And Engineering Company | Field enhanced separation of hydrocarbon fractions |
| US8999901B2 (en) | 2013-03-12 | 2015-04-07 | Exxonmobil Research And Engineering Company | Lubricant base stocks with improved filterability |
| US9284500B2 (en) * | 2013-03-14 | 2016-03-15 | Exxonmobil Research And Engineering Company | Production of base oils from petrolatum |
| EP2970806A1 (en) | 2013-03-14 | 2016-01-20 | ExxonMobil Research and Engineering Company | High viscosity high quality group ii lube base stocks |
| JP6026940B2 (ja) * | 2013-03-29 | 2016-11-16 | Jxエネルギー株式会社 | 潤滑油基油及びその製造方法 |
| KR101566581B1 (ko) | 2013-04-22 | 2015-11-05 | 에스케이이노베이션 주식회사 | 중간 유분의 용매 추출을 통한 친환경 경유 및 납센계 기유의 병산 방법 |
| US9605218B2 (en) | 2013-06-20 | 2017-03-28 | Exxonmobil Research And Engineering Company | Integrated hydrocracking and slurry hydroconversion of heavy oils |
| SG11201603359UA (en) | 2013-12-03 | 2016-05-30 | Exxonmobil Res & Eng Co | Hydrocracking of gas oils with increased distillate yield |
| JP6181538B2 (ja) | 2013-12-11 | 2017-08-16 | 出光興産株式会社 | 燃料油基材、及びその製造方法並びに燃料油組成物 |
| US9719034B2 (en) | 2013-12-23 | 2017-08-01 | Exxonmobil Research And Engineering Company | Co-production of lubricants and distillate fuels |
| CN105316036B (zh) * | 2014-06-16 | 2019-10-25 | 中国石油化工股份有限公司 | 一种生产超高粘度指数润滑油基础油的方法 |
| JP6294169B2 (ja) | 2014-06-24 | 2018-03-14 | 出光興産株式会社 | 灯油組成物および灯油組成物の製造方法 |
| CN104725222A (zh) * | 2015-01-28 | 2015-06-24 | 黄小平 | 一种冷冻机油基础油、制备方法、用该基础油制备的冷冻机油组合物及其制备方法 |
| US20170183576A1 (en) | 2015-12-28 | 2017-06-29 | Exxonmobil Research And Engineering Company | Bright stock and heavy neutral production from resid deasphalting |
| US10590360B2 (en) * | 2015-12-28 | 2020-03-17 | Exxonmobil Research And Engineering Company | Bright stock production from deasphalted oil |
-
2016
- 2016-12-27 US US15/390,943 patent/US10590360B2/en active Active
- 2016-12-28 WO PCT/US2016/068806 patent/WO2017117178A1/en not_active Ceased
- 2016-12-28 CN CN201680076738.3A patent/CN108473887B/zh active Active
- 2016-12-28 SG SG11201804665WA patent/SG11201804665WA/en unknown
- 2016-12-28 KR KR1020187021933A patent/KR102762110B1/ko active Active
- 2016-12-28 EP EP16826637.7A patent/EP3397730B1/en active Active
- 2016-12-28 AU AU2016381604A patent/AU2016381604A1/en not_active Abandoned
- 2016-12-28 JP JP2018553055A patent/JP6997721B2/ja active Active
-
2021
- 2021-11-05 JP JP2021181294A patent/JP2022025132A/ja active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR870005068A (ko) * | 1985-11-01 | 1987-06-04 | 에드워드 에취. 바란스 | 윤활류의 제조 공정 |
| US6191078B1 (en) * | 1999-09-21 | 2001-02-20 | Exxonmobil Research And Engineering Company | Part-synthetic, aviation piston engine lubricant |
| US20050000857A1 (en) * | 2002-10-30 | 2005-01-06 | Eric Benazzi | Flexible process for the production of oil bases and middle distillates with a converting pretreatment stage followed by a catalytic dewaxing stage |
| US20050051463A1 (en) * | 2003-09-09 | 2005-03-10 | Chevron U.S.A. Inc. | Production of high quality lubricant bright stock |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2022025132A (ja) | 2022-02-09 |
| US20170211005A1 (en) | 2017-07-27 |
| AU2016381604A1 (en) | 2018-07-19 |
| SG11201804665WA (en) | 2018-07-30 |
| CN108473887A (zh) | 2018-08-31 |
| WO2017117178A1 (en) | 2017-07-06 |
| CN108473887B (zh) | 2021-08-17 |
| EP3397730A1 (en) | 2018-11-07 |
| JP2019501274A (ja) | 2019-01-17 |
| EP3397730B1 (en) | 2020-08-26 |
| KR102762110B1 (ko) | 2025-02-03 |
| JP6997721B2 (ja) | 2022-02-04 |
| US10590360B2 (en) | 2020-03-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR102762110B1 (ko) | 탈아스탈트화된 오일로부터의 브라이트 스톡의 제조 | |
| KR102909503B1 (ko) | 고유한 분기 구조를 보여주는 탄화수소 혼합물 | |
| US10287516B2 (en) | Block processing configurations for base stock production from deasphalted oil | |
| US20170183578A1 (en) | Bright stock production from low severity resid deasphalting | |
| EP4162013B1 (en) | Lubricants having improved low temperature, oxidation and deposit control performance | |
| US11767489B2 (en) | Fluids for electric vehicles | |
| EP3374472B1 (en) | High viscosity base stock compositions | |
| US10865352B2 (en) | Removal of polynuclear aromatics from severely hydrotreated base stocks | |
| US11352579B2 (en) | Group III base stocks and lubricant compositions | |
| EP3708639B1 (en) | Method of manufacturing a mineral base oil having high viscosity index and improved volatility | |
| EP4162014B1 (en) | Lubricants having improved oxidation and deposit control performance | |
| JP5390743B2 (ja) | 熱処理油組成物 | |
| US20190194571A1 (en) | Lubricant compositions having improved low temperature performance | |
| US12234424B2 (en) | Hydrocarbon compositions useful as lubricants for improved oxidation stability |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20180727 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20211217 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20231221 Patent event code: PE09021S01D |
|
| E90F | Notification of reason for final refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Final Notice of Reason for Refusal Patent event date: 20240826 Patent event code: PE09021S02D |
|
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
Patent event code: PE07011S01D Comment text: Decision to Grant Registration Patent event date: 20241030 |
|
| GRNT | Written decision to grant | ||
| PR0701 | Registration of establishment |
Comment text: Registration of Establishment Patent event date: 20250124 Patent event code: PR07011E01D |
|
| PR1002 | Payment of registration fee |
Payment date: 20250124 End annual number: 3 Start annual number: 1 |
|
| PG1601 | Publication of registration |