WO2018221669A1 - 電解質組成物及び二次電池 - Google Patents
電解質組成物及び二次電池 Download PDFInfo
- Publication number
- WO2018221669A1 WO2018221669A1 PCT/JP2018/021007 JP2018021007W WO2018221669A1 WO 2018221669 A1 WO2018221669 A1 WO 2018221669A1 JP 2018021007 W JP2018021007 W JP 2018021007W WO 2018221669 A1 WO2018221669 A1 WO 2018221669A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- electrolyte
- group
- positive electrode
- negative electrode
- electrolyte composition
- Prior art date
Links
- 239000003792 electrolyte Substances 0.000 title claims abstract description 185
- 239000000203 mixture Substances 0.000 title claims abstract description 124
- 239000002245 particle Substances 0.000 claims abstract description 110
- 239000002608 ionic liquid Substances 0.000 claims abstract description 60
- 150000003839 salts Chemical class 0.000 claims abstract description 45
- 229920000642 polymer Polymers 0.000 claims abstract description 34
- 230000005661 hydrophobic surface Effects 0.000 claims abstract description 20
- 229910003002 lithium salt Inorganic materials 0.000 claims abstract description 12
- 159000000002 lithium salts Chemical class 0.000 claims abstract description 9
- 159000000007 calcium salts Chemical class 0.000 claims abstract description 7
- 159000000003 magnesium salts Chemical class 0.000 claims abstract description 7
- 159000000000 sodium salts Chemical class 0.000 claims abstract description 7
- -1 pyrrolidinium cation Chemical class 0.000 claims description 35
- 229910004298 SiO 2 Inorganic materials 0.000 claims description 22
- 150000001450 anions Chemical class 0.000 claims description 12
- BLRPTPMANUNPDV-UHFFFAOYSA-N Silane Chemical compound [SiH4] BLRPTPMANUNPDV-UHFFFAOYSA-N 0.000 claims description 11
- 229910000077 silane Inorganic materials 0.000 claims description 11
- BQCIDUSAKPWEOX-UHFFFAOYSA-N 1,1-Difluoroethene Chemical compound FC(F)=C BQCIDUSAKPWEOX-UHFFFAOYSA-N 0.000 claims description 9
- 150000001768 cations Chemical class 0.000 claims description 9
- HCDGVLDPFQMKDK-UHFFFAOYSA-N hexafluoropropylene Chemical group FC(F)=C(F)C(F)(F)F HCDGVLDPFQMKDK-UHFFFAOYSA-N 0.000 claims description 8
- 239000002210 silicon-based material Substances 0.000 claims description 8
- 125000003647 acryloyl group Chemical group O=C([*])C([H])=C([H])[H] 0.000 claims description 7
- PYVHTIWHNXTVPF-UHFFFAOYSA-N F.F.F.F.C=C Chemical compound F.F.F.F.C=C PYVHTIWHNXTVPF-UHFFFAOYSA-N 0.000 claims description 5
- ODINCKMPIJJUCX-UHFFFAOYSA-N calcium oxide Inorganic materials [Ca]=O ODINCKMPIJJUCX-UHFFFAOYSA-N 0.000 claims description 5
- KPUWHANPEXNPJT-UHFFFAOYSA-N disiloxane Chemical class [SiH3]O[SiH3] KPUWHANPEXNPJT-UHFFFAOYSA-N 0.000 claims description 5
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 claims description 5
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 claims description 4
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 claims description 4
- RAXXELZNTBOGNW-UHFFFAOYSA-O Imidazolium Chemical compound C1=C[NH+]=CN1 RAXXELZNTBOGNW-UHFFFAOYSA-O 0.000 claims description 4
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 claims description 4
- NQRYJNQNLNOLGT-UHFFFAOYSA-O Piperidinium(1+) Chemical compound C1CC[NH2+]CC1 NQRYJNQNLNOLGT-UHFFFAOYSA-O 0.000 claims description 4
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 claims description 4
- 125000003277 amino group Chemical group 0.000 claims description 4
- 125000003700 epoxy group Chemical group 0.000 claims description 4
- SUPCQIBBMFXVTL-UHFFFAOYSA-N ethyl 2-methylprop-2-enoate Chemical compound CCOC(=O)C(C)=C SUPCQIBBMFXVTL-UHFFFAOYSA-N 0.000 claims description 4
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 claims description 4
- 239000011976 maleic acid Substances 0.000 claims description 4
- JUJWROOIHBZHMG-UHFFFAOYSA-O pyridinium Chemical compound C1=CC=[NH+]C=C1 JUJWROOIHBZHMG-UHFFFAOYSA-O 0.000 claims description 4
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 claims description 4
- 229910018072 Al 2 O 3 Inorganic materials 0.000 claims description 3
- 229910002706 AlOOH Inorganic materials 0.000 claims description 3
- 229910010413 TiO 2 Inorganic materials 0.000 claims description 3
- GEIAQOFPUVMAGM-UHFFFAOYSA-N ZrO Inorganic materials [Zr]=O GEIAQOFPUVMAGM-UHFFFAOYSA-N 0.000 claims description 3
- 125000005462 imide group Chemical group 0.000 claims 1
- 239000000463 material Substances 0.000 description 34
- 229910052744 lithium Inorganic materials 0.000 description 22
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 20
- 239000011777 magnesium Chemical group 0.000 description 20
- 238000000034 method Methods 0.000 description 20
- 239000011575 calcium Substances 0.000 description 19
- VZSRBBMJRBPUNF-UHFFFAOYSA-N 2-(2,3-dihydro-1H-inden-2-ylamino)-N-[3-oxo-3-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)propyl]pyrimidine-5-carboxamide Chemical compound C1C(CC2=CC=CC=C12)NC1=NC=C(C=N1)C(=O)NCCC(N1CC2=C(CC1)NN=N2)=O VZSRBBMJRBPUNF-UHFFFAOYSA-N 0.000 description 18
- 238000004519 manufacturing process Methods 0.000 description 18
- 239000007774 positive electrode material Substances 0.000 description 17
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 16
- 239000002612 dispersion medium Substances 0.000 description 15
- 239000011164 primary particle Substances 0.000 description 15
- 125000000217 alkyl group Chemical group 0.000 description 14
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 13
- 125000004432 carbon atom Chemical group C* 0.000 description 13
- 229910052751 metal Inorganic materials 0.000 description 12
- 239000002184 metal Substances 0.000 description 12
- 239000007773 negative electrode material Substances 0.000 description 12
- 239000011245 gel electrolyte Substances 0.000 description 11
- 239000002002 slurry Substances 0.000 description 11
- 229910052782 aluminium Inorganic materials 0.000 description 10
- 239000011230 binding agent Substances 0.000 description 10
- 239000006258 conductive agent Substances 0.000 description 10
- 230000000052 comparative effect Effects 0.000 description 9
- 150000001875 compounds Chemical class 0.000 description 9
- PXHVJJICTQNCMI-UHFFFAOYSA-N nickel Substances [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 9
- 239000004810 polytetrafluoroethylene Substances 0.000 description 9
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 9
- 229910002012 Aerosil® Inorganic materials 0.000 description 8
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 8
- 230000001681 protective effect Effects 0.000 description 8
- 239000011734 sodium Substances 0.000 description 8
- 238000011156 evaluation Methods 0.000 description 7
- 239000010408 film Substances 0.000 description 7
- 239000011888 foil Substances 0.000 description 7
- 239000010439 graphite Substances 0.000 description 7
- 229910002804 graphite Inorganic materials 0.000 description 7
- 229910021437 lithium-transition metal oxide Inorganic materials 0.000 description 7
- 238000002360 preparation method Methods 0.000 description 7
- JCVQKRGIASEUKR-UHFFFAOYSA-N triethoxy(phenyl)silane Chemical compound CCO[Si](OCC)(OCC)C1=CC=CC=C1 JCVQKRGIASEUKR-UHFFFAOYSA-N 0.000 description 7
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 6
- 229910045601 alloy Inorganic materials 0.000 description 6
- 239000000956 alloy Substances 0.000 description 6
- 229920001577 copolymer Polymers 0.000 description 6
- FFUAGWLWBBFQJT-UHFFFAOYSA-N hexamethyldisilazane Chemical compound C[Si](C)(C)N[Si](C)(C)C FFUAGWLWBBFQJT-UHFFFAOYSA-N 0.000 description 6
- 239000011347 resin Substances 0.000 description 6
- 229920005989 resin Polymers 0.000 description 6
- 239000000758 substrate Substances 0.000 description 6
- 239000010936 titanium Substances 0.000 description 6
- 229910020808 NaBF Inorganic materials 0.000 description 5
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 5
- 125000004183 alkoxy alkyl group Chemical group 0.000 description 5
- 238000007599 discharging Methods 0.000 description 5
- 239000008151 electrolyte solution Substances 0.000 description 5
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 5
- 229910052759 nickel Inorganic materials 0.000 description 5
- 239000010935 stainless steel Substances 0.000 description 5
- 229910001220 stainless steel Inorganic materials 0.000 description 5
- 229910052719 titanium Inorganic materials 0.000 description 5
- 229910020366 ClO 4 Inorganic materials 0.000 description 4
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 4
- 229910013075 LiBF Inorganic materials 0.000 description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- 125000000129 anionic group Chemical group 0.000 description 4
- 239000003575 carbonaceous material Substances 0.000 description 4
- 229910052802 copper Inorganic materials 0.000 description 4
- 239000010949 copper Substances 0.000 description 4
- 239000000835 fiber Substances 0.000 description 4
- 150000003949 imides Chemical class 0.000 description 4
- 239000011572 manganese Substances 0.000 description 4
- 125000001624 naphthyl group Chemical group 0.000 description 4
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 4
- 229920000139 polyethylene terephthalate Polymers 0.000 description 4
- 239000005020 polyethylene terephthalate Substances 0.000 description 4
- 239000011163 secondary particle Substances 0.000 description 4
- 238000003860 storage Methods 0.000 description 4
- 239000012756 surface treatment agent Substances 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 229920000049 Carbon (fiber) Polymers 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 239000006230 acetylene black Substances 0.000 description 3
- 239000004917 carbon fiber Substances 0.000 description 3
- 239000011248 coating agent Substances 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 3
- 229910052809 inorganic oxide Inorganic materials 0.000 description 3
- XEEYBQQBJWHFJM-UHFFFAOYSA-N iron Substances [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 3
- 229910052749 magnesium Inorganic materials 0.000 description 3
- 229910052748 manganese Inorganic materials 0.000 description 3
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 3
- 239000011259 mixed solution Substances 0.000 description 3
- 230000003647 oxidation Effects 0.000 description 3
- 238000007254 oxidation reaction Methods 0.000 description 3
- 150000004756 silanes Chemical class 0.000 description 3
- 229910052710 silicon Inorganic materials 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 229910052723 transition metal Inorganic materials 0.000 description 3
- 150000003624 transition metals Chemical class 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- RNFJDJUURJAICM-UHFFFAOYSA-N 2,2,4,4,6,6-hexaphenoxy-1,3,5-triaza-2$l^{5},4$l^{5},6$l^{5}-triphosphacyclohexa-1,3,5-triene Chemical compound N=1P(OC=2C=CC=CC=2)(OC=2C=CC=CC=2)=NP(OC=2C=CC=CC=2)(OC=2C=CC=CC=2)=NP=1(OC=1C=CC=CC=1)OC1=CC=CC=C1 RNFJDJUURJAICM-UHFFFAOYSA-N 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- MKYBYDHXWVHEJW-UHFFFAOYSA-N N-[1-oxo-1-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)propan-2-yl]-2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidine-5-carboxamide Chemical compound O=C(C(C)NC(=O)C=1C=NC(=NC=1)NCC1=CC(=CC=C1)OC(F)(F)F)N1CC2=C(CC1)NN=N2 MKYBYDHXWVHEJW-UHFFFAOYSA-N 0.000 description 2
- 239000004743 Polypropylene Substances 0.000 description 2
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- 239000012300 argon atmosphere Substances 0.000 description 2
- 238000009835 boiling Methods 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 239000006229 carbon black Substances 0.000 description 2
- 239000011362 coarse particle Substances 0.000 description 2
- 150000001923 cyclic compounds Chemical class 0.000 description 2
- QHGJSLXSVXVKHZ-UHFFFAOYSA-N dilithium;dioxido(dioxo)manganese Chemical compound [Li+].[Li+].[O-][Mn]([O-])(=O)=O QHGJSLXSVXVKHZ-UHFFFAOYSA-N 0.000 description 2
- 238000010494 dissociation reaction Methods 0.000 description 2
- 230000005593 dissociations Effects 0.000 description 2
- 238000004090 dissolution Methods 0.000 description 2
- 239000003063 flame retardant Substances 0.000 description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- 239000005001 laminate film Substances 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 239000004014 plasticizer Substances 0.000 description 2
- 229920001155 polypropylene Polymers 0.000 description 2
- 229920002545 silicone oil Polymers 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 229910000319 transition metal phosphate Inorganic materials 0.000 description 2
- HQYALQRYBUJWDH-UHFFFAOYSA-N trimethoxy(propyl)silane Chemical compound CCC[Si](OC)(OC)OC HQYALQRYBUJWDH-UHFFFAOYSA-N 0.000 description 2
- 229910052726 zirconium Inorganic materials 0.000 description 2
- WYTZZXDRDKSJID-UHFFFAOYSA-N (3-aminopropyl)triethoxysilane Chemical compound CCO[Si](OCC)(OCC)CCCN WYTZZXDRDKSJID-UHFFFAOYSA-N 0.000 description 1
- ZXMGHDIOOHOAAE-UHFFFAOYSA-N 1,1,1-trifluoro-n-(trifluoromethylsulfonyl)methanesulfonamide Chemical compound FC(F)(F)S(=O)(=O)NS(=O)(=O)C(F)(F)F ZXMGHDIOOHOAAE-UHFFFAOYSA-N 0.000 description 1
- YQFWGCSKGJMGHE-UHFFFAOYSA-N 1-methyl-1-propylpyrrolidin-1-ium Chemical compound CCC[N+]1(C)CCCC1 YQFWGCSKGJMGHE-UHFFFAOYSA-N 0.000 description 1
- DOYKFSOCSXVQAN-UHFFFAOYSA-N 3-[diethoxy(methyl)silyl]propyl 2-methylprop-2-enoate Chemical compound CCO[Si](C)(OCC)CCCOC(=O)C(C)=C DOYKFSOCSXVQAN-UHFFFAOYSA-N 0.000 description 1
- LZMNXXQIQIHFGC-UHFFFAOYSA-N 3-[dimethoxy(methyl)silyl]propyl 2-methylprop-2-enoate Chemical compound CO[Si](C)(OC)CCCOC(=O)C(C)=C LZMNXXQIQIHFGC-UHFFFAOYSA-N 0.000 description 1
- OKWYEBJNFREPEV-UHFFFAOYSA-N 3-[dimethoxy(phenylmethoxy)silyl]propan-1-amine Chemical compound NCCC[Si](OC)(OC)OCC1=CC=CC=C1 OKWYEBJNFREPEV-UHFFFAOYSA-N 0.000 description 1
- URDOJQUSEUXVRP-UHFFFAOYSA-N 3-triethoxysilylpropyl 2-methylprop-2-enoate Chemical compound CCO[Si](OCC)(OCC)CCCOC(=O)C(C)=C URDOJQUSEUXVRP-UHFFFAOYSA-N 0.000 description 1
- XDLMVUHYZWKMMD-UHFFFAOYSA-N 3-trimethoxysilylpropyl 2-methylprop-2-enoate Chemical compound CO[Si](OC)(OC)CCCOC(=O)C(C)=C XDLMVUHYZWKMMD-UHFFFAOYSA-N 0.000 description 1
- 238000004438 BET method Methods 0.000 description 1
- KRHYYFGTRYWZRS-UHFFFAOYSA-M Fluoride anion Chemical compound [F-] KRHYYFGTRYWZRS-UHFFFAOYSA-M 0.000 description 1
- 208000029422 Hypernatremia Diseases 0.000 description 1
- 229910000733 Li alloy Inorganic materials 0.000 description 1
- 229910004984 Li(Co1/3Ni1/3Mn1/3)O2 Inorganic materials 0.000 description 1
- 229910013063 LiBF 4 Inorganic materials 0.000 description 1
- 229910013684 LiClO 4 Inorganic materials 0.000 description 1
- 229910010707 LiFePO 4 Inorganic materials 0.000 description 1
- 229910015645 LiMn Inorganic materials 0.000 description 1
- 229910013716 LiNi Inorganic materials 0.000 description 1
- 229910014411 LiNi1/2Mn1/2O2 Inorganic materials 0.000 description 1
- 229910013870 LiPF 6 Inorganic materials 0.000 description 1
- PWHULOQIROXLJO-UHFFFAOYSA-N Manganese Chemical compound [Mn] PWHULOQIROXLJO-UHFFFAOYSA-N 0.000 description 1
- NIPNSKYNPDTRPC-UHFFFAOYSA-N N-[2-oxo-2-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)ethyl]-2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidine-5-carboxamide Chemical compound O=C(CNC(=O)C=1C=NC(=NC=1)NCC1=CC(=CC=C1)OC(F)(F)F)N1CC2=C(CC1)NN=N2 NIPNSKYNPDTRPC-UHFFFAOYSA-N 0.000 description 1
- AFCARXCZXQIEQB-UHFFFAOYSA-N N-[3-oxo-3-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)propyl]-2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidine-5-carboxamide Chemical compound O=C(CCNC(=O)C=1C=NC(=NC=1)NCC1=CC(=CC=C1)OC(F)(F)F)N1CC2=C(CC1)NN=N2 AFCARXCZXQIEQB-UHFFFAOYSA-N 0.000 description 1
- 239000002033 PVDF binder Substances 0.000 description 1
- 239000004695 Polyether sulfone Substances 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000004642 Polyimide Substances 0.000 description 1
- 229910018286 SbF 6 Inorganic materials 0.000 description 1
- BOTDANWDWHJENH-UHFFFAOYSA-N Tetraethyl orthosilicate Chemical compound CCO[Si](OCC)(OCC)OCC BOTDANWDWHJENH-UHFFFAOYSA-N 0.000 description 1
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 229920000800 acrylic rubber Polymers 0.000 description 1
- 238000005054 agglomeration Methods 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 229910003481 amorphous carbon Inorganic materials 0.000 description 1
- 150000001449 anionic compounds Chemical class 0.000 description 1
- 229910021383 artificial graphite Inorganic materials 0.000 description 1
- 229910052788 barium Inorganic materials 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 229910052796 boron Inorganic materials 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000002041 carbon nanotube Substances 0.000 description 1
- 229910021393 carbon nanotube Inorganic materials 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 239000006231 channel black Substances 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 238000002485 combustion reaction Methods 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000000748 compression moulding Methods 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 150000004696 coordination complex Chemical class 0.000 description 1
- 239000011889 copper foil Substances 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 230000002542 deteriorative effect Effects 0.000 description 1
- BXHHZLMBMOBPEH-UHFFFAOYSA-N diethyl-(2-methoxyethyl)-methylazanium Chemical compound CC[N+](C)(CC)CCOC BXHHZLMBMOBPEH-UHFFFAOYSA-N 0.000 description 1
- URSLCTBXQMKCFE-UHFFFAOYSA-N dihydrogenborate Chemical compound OB(O)[O-] URSLCTBXQMKCFE-UHFFFAOYSA-N 0.000 description 1
- JJQZDUKDJDQPMQ-UHFFFAOYSA-N dimethoxy(dimethyl)silane Chemical compound CO[Si](C)(C)OC JJQZDUKDJDQPMQ-UHFFFAOYSA-N 0.000 description 1
- AHUXYBVKTIBBJW-UHFFFAOYSA-N dimethoxy(diphenyl)silane Chemical compound C=1C=CC=CC=1[Si](OC)(OC)C1=CC=CC=C1 AHUXYBVKTIBBJW-UHFFFAOYSA-N 0.000 description 1
- WHGNXNCOTZPEEK-UHFFFAOYSA-N dimethoxy-methyl-[3-(oxiran-2-ylmethoxy)propyl]silane Chemical compound CO[Si](C)(OC)CCCOCC1CO1 WHGNXNCOTZPEEK-UHFFFAOYSA-N 0.000 description 1
- 125000000118 dimethyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 238000007598 dipping method Methods 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 238000007606 doctor blade method Methods 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- CWAFVXWRGIEBPL-UHFFFAOYSA-N ethoxysilane Chemical compound CCO[SiH3] CWAFVXWRGIEBPL-UHFFFAOYSA-N 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 239000006232 furnace black Substances 0.000 description 1
- 229910052732 germanium Inorganic materials 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- CZWLNMOIEMTDJY-UHFFFAOYSA-N hexyl(trimethoxy)silane Chemical compound CCCCCC[Si](OC)(OC)OC CZWLNMOIEMTDJY-UHFFFAOYSA-N 0.000 description 1
- 230000005660 hydrophilic surface Effects 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 229910001412 inorganic anion Inorganic materials 0.000 description 1
- 239000002198 insoluble material Substances 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 229920003049 isoprene rubber Polymers 0.000 description 1
- 239000003273 ketjen black Substances 0.000 description 1
- 238000010030 laminating Methods 0.000 description 1
- 238000003475 lamination Methods 0.000 description 1
- 239000006233 lamp black Substances 0.000 description 1
- 229910052746 lanthanum Inorganic materials 0.000 description 1
- 238000007561 laser diffraction method Methods 0.000 description 1
- 239000011344 liquid material Substances 0.000 description 1
- 239000001989 lithium alloy Substances 0.000 description 1
- 229910003473 lithium bis(trifluoromethanesulfonyl)imide Inorganic materials 0.000 description 1
- QSZMZKBZAYQGRS-UHFFFAOYSA-N lithium;bis(trifluoromethylsulfonyl)azanide Chemical compound [Li+].FC(F)(F)S(=O)(=O)[N-]S(=O)(=O)C(F)(F)F QSZMZKBZAYQGRS-UHFFFAOYSA-N 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 150000002736 metal compounds Chemical class 0.000 description 1
- 239000006262 metallic foam Substances 0.000 description 1
- BFXIKLCIZHOAAZ-UHFFFAOYSA-N methyltrimethoxysilane Chemical compound CO[Si](C)(OC)OC BFXIKLCIZHOAAZ-UHFFFAOYSA-N 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 239000004570 mortar (masonry) Substances 0.000 description 1
- PHQOGHDTIVQXHL-UHFFFAOYSA-N n'-(3-trimethoxysilylpropyl)ethane-1,2-diamine Chemical compound CO[Si](OC)(OC)CCCNCCN PHQOGHDTIVQXHL-UHFFFAOYSA-N 0.000 description 1
- MQWFLKHKWJMCEN-UHFFFAOYSA-N n'-[3-[dimethoxy(methyl)silyl]propyl]ethane-1,2-diamine Chemical compound CO[Si](C)(OC)CCCNCCN MQWFLKHKWJMCEN-UHFFFAOYSA-N 0.000 description 1
- KTQDYGVEEFGIIL-UHFFFAOYSA-N n-fluorosulfonylsulfamoyl fluoride Chemical compound FS(=O)(=O)NS(F)(=O)=O KTQDYGVEEFGIIL-UHFFFAOYSA-N 0.000 description 1
- 229910021382 natural graphite Inorganic materials 0.000 description 1
- 229910052758 niobium Inorganic materials 0.000 description 1
- 150000004767 nitrides Chemical class 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 125000004433 nitrogen atom Chemical group N* 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 150000002891 organic anions Chemical class 0.000 description 1
- 229920000620 organic polymer Polymers 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 125000004437 phosphorous atom Chemical group 0.000 description 1
- 229910052698 phosphorus Chemical group 0.000 description 1
- 229920001643 poly(ether ketone) Polymers 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 229920006393 polyether sulfone Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920001721 polyimide Polymers 0.000 description 1
- 239000005518 polymer electrolyte Substances 0.000 description 1
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 239000005060 rubber Substances 0.000 description 1
- 238000001878 scanning electron micrograph Methods 0.000 description 1
- 238000000790 scattering method Methods 0.000 description 1
- 239000000565 sealant Substances 0.000 description 1
- 238000007873 sieving Methods 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- SUKJFIGYRHOWBL-UHFFFAOYSA-N sodium hypochlorite Chemical compound [Na+].Cl[O-] SUKJFIGYRHOWBL-UHFFFAOYSA-N 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 229910052712 strontium Inorganic materials 0.000 description 1
- 229920003048 styrene butadiene rubber Polymers 0.000 description 1
- 238000004381 surface treatment Methods 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 239000006234 thermal black Substances 0.000 description 1
- 239000010409 thin film Substances 0.000 description 1
- CPUDPFPXCZDNGI-UHFFFAOYSA-N triethoxy(methyl)silane Chemical compound CCO[Si](C)(OCC)OCC CPUDPFPXCZDNGI-UHFFFAOYSA-N 0.000 description 1
- NBXZNTLFQLUFES-UHFFFAOYSA-N triethoxy(propyl)silane Chemical compound CCC[Si](OCC)(OCC)OCC NBXZNTLFQLUFES-UHFFFAOYSA-N 0.000 description 1
- JXUKBNICSRJFAP-UHFFFAOYSA-N triethoxy-[3-(oxiran-2-ylmethoxy)propyl]silane Chemical compound CCO[Si](OCC)(OCC)CCCOCC1CO1 JXUKBNICSRJFAP-UHFFFAOYSA-N 0.000 description 1
- ZNOCGWVLWPVKAO-UHFFFAOYSA-N trimethoxy(phenyl)silane Chemical compound CO[Si](OC)(OC)C1=CC=CC=C1 ZNOCGWVLWPVKAO-UHFFFAOYSA-N 0.000 description 1
- DQZNLOXENNXVAD-UHFFFAOYSA-N trimethoxy-[2-(7-oxabicyclo[4.1.0]heptan-4-yl)ethyl]silane Chemical compound C1C(CC[Si](OC)(OC)OC)CCC2OC21 DQZNLOXENNXVAD-UHFFFAOYSA-N 0.000 description 1
- BPSIOYPQMFLKFR-UHFFFAOYSA-N trimethoxy-[3-(oxiran-2-ylmethoxy)propyl]silane Chemical compound CO[Si](OC)(OC)CCCOCC1CO1 BPSIOYPQMFLKFR-UHFFFAOYSA-N 0.000 description 1
- 229910052720 vanadium Inorganic materials 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0564—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of organic materials only
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/34—Silicon-containing compounds
- C08K3/36—Silica
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K9/00—Use of pretreated ingredients
- C08K9/04—Ingredients treated with organic substances
- C08K9/06—Ingredients treated with organic substances with silicon-containing compounds
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
- H01M10/0525—Rocking-chair batteries, i.e. batteries with lithium insertion or intercalation in both electrodes; Lithium-ion batteries
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0564—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of organic materials only
- H01M10/0565—Polymeric materials, e.g. gel-type or solid-type
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0564—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of organic materials only
- H01M10/0566—Liquid materials
- H01M10/0568—Liquid materials characterised by the solutes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0564—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of organic materials only
- H01M10/0566—Liquid materials
- H01M10/0569—Liquid materials characterised by the solvents
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/058—Construction or manufacture
- H01M10/0585—Construction or manufacture of accumulators having only flat construction elements, i.e. flat positive electrodes, flat negative electrodes and flat separators
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/054—Accumulators with insertion or intercalation of metals other than lithium, e.g. with magnesium or aluminium
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M2300/00—Electrolytes
- H01M2300/0017—Non-aqueous electrolytes
- H01M2300/0025—Organic electrolyte
- H01M2300/0045—Room temperature molten salts comprising at least one organic ion
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M2300/00—Electrolytes
- H01M2300/0017—Non-aqueous electrolytes
- H01M2300/0065—Solid electrolytes
- H01M2300/0082—Organic polymers
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M2300/00—Electrolytes
- H01M2300/0088—Composites
- H01M2300/0091—Composites in the form of mixtures
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P70/00—Climate change mitigation technologies in the production process for final industrial or consumer products
- Y02P70/50—Manufacturing or production processes characterised by the final manufactured product
Definitions
- the present invention relates to an electrolyte composition and a secondary battery.
- lithium secondary batteries have been attracting attention as power sources for electric vehicle batteries, power storage batteries, and the like because of their high energy density.
- lithium secondary batteries as batteries for electric vehicles include zero-emission electric vehicles that are not equipped with engines, hybrid electric vehicles that are equipped with both engines and secondary batteries, and plug-in hybrids that are charged directly from the power system. It is used in electric vehicles such as electric vehicles.
- lithium secondary batteries as power storage batteries are used in stationary power storage systems that supply power stored in advance in an emergency when the power system is shut off.
- lithium secondary battery having a higher energy density is required and has been developed.
- lithium secondary batteries for electric vehicles require high safety in addition to high input / output characteristics and high energy density, more advanced technology for ensuring safety is required.
- the gel electrolyte has an ionic conductivity equivalent to that of the electrolyte solution used in the conventional lithium secondary battery, so that it is released without deteriorating the battery performance by changing the electrolyte solution to the gel electrolyte.
- Patent Document 1 discloses a gel electrolyte layer containing a plasticizer containing a lithium salt, a matrix polymer in which the plasticizer is dispersed, and a fibrous insoluble material.
- the fibrous insoluble matter contained in the gel electrolyte in an amount of 0.1 wt% to 50 wt% has a ratio of fiber length to fiber diameter of 10 to 3000, fiber length of 10 ⁇ m to 1 cm, and fiber diameter.
- Patent Document 2 discloses a gel electrolyte and a gel electrolyte battery.
- the gel electrolyte layer is formed by swelling a matrix polymer with an electrolytic solution and contains a large amount of a low viscosity solvent having a low boiling point.
- a gel electrolyte containing a large amount of a low-boiling low-viscosity solvent By using a gel electrolyte containing a large amount of a low-boiling low-viscosity solvent, a gel electrolyte battery excellent in temperature characteristics, current characteristics, capacity, and charge / discharge characteristics at low temperatures is provided.
- the electrical conductivity of the conventional gel electrolyte as described above is insufficient.
- the discharge characteristics of the secondary battery may be remarkably deteriorated.
- the main object of the present invention is to provide an electrolyte composition capable of producing a secondary battery having excellent discharge characteristics.
- the first aspect of the present invention is at least one selected from the group consisting of one or more polymers, oxide particles having a hydrophobic surface, lithium salt, sodium salt, calcium salt, and magnesium salt.
- An electrolyte composition comprising an electrolyte salt of the above and an ionic liquid.
- the oxide particles are preferably surface-treated with a silicon-containing compound.
- the silicon-containing compound is preferably at least one selected from the group consisting of alkoxysilane, epoxy group-containing silane, amino group-containing silane, (meth) acryloyl group-containing silane, silazane, and siloxane.
- the oxide particles are preferably at least one selected from the group consisting of SiO 2 , Al 2 O 3 , AlOOH, MgO, CaO, ZrO 2 , TiO 2 , Li 7 La 3 Zr 2 O 12 , and BaTiO 3 . Particles.
- the ionic liquid preferably contains at least one selected from the group consisting of a chain quaternary onium cation, a piperidinium cation, a pyrrolidinium cation, a pyridinium cation, and an imidazolium cation as a cation component.
- the ionic liquid preferably contains at least one anion component represented by the following general formula (A) as an anion component.
- A (SO 2 C m F 2m + 1 ) (SO 2 C n F 2n + 1 ) ⁇ (A)
- M and n each independently represents an integer of 0 to 5.
- the polymer preferably has a first structural unit selected from the group consisting of ethylene tetrafluoride and vinylidene fluoride.
- the polymer is preferably a structural unit constituting the polymer, the first structural unit, and a second structural unit selected from the group consisting of hexafluoropropylene, acrylic acid, maleic acid, ethyl methacrylate, and methyl methacrylate. , Is included.
- the electrolyte salt is preferably an imide lithium salt.
- a second aspect of the present invention is a secondary battery including a positive electrode, a negative electrode, and an electrolyte layer made of an electrolyte composition provided between the positive electrode and the negative electrode.
- an electrolyte composition capable of producing a secondary battery having excellent discharge characteristics can be provided.
- the secondary battery using such an electrolyte composition can be provided.
- FIG. 1 is a perspective view showing a secondary battery according to a first embodiment. It is a disassembled perspective view which shows one Embodiment of the electrode group in the secondary battery shown in FIG.
- FIG. 2 is a schematic cross-sectional view showing an embodiment of an electrode group in the secondary battery shown in FIG. 1.
- A) is a schematic cross section which shows the electrolyte sheet which concerns on one Embodiment
- (b) is a schematic cross section which shows the electrolyte sheet which concerns on other embodiment.
- a numerical range indicated by using “to” indicates a range including the numerical values described before and after “to” as the minimum value and the maximum value, respectively.
- the upper limit value or the lower limit value described in one numerical range may be replaced with the upper limit value or the lower limit value described in another stepwise description.
- the upper limit value or the lower limit value of the numerical range may be replaced with the values shown in the examples.
- FIG. 1 is a perspective view showing the secondary battery according to the first embodiment.
- the secondary battery 1 includes an electrode group 2 including a positive electrode, a negative electrode, and an electrolyte layer, and a bag-shaped battery outer package 3 that houses the electrode group 2.
- a positive electrode current collecting tab 4 and a negative electrode current collecting tab 5 are provided on the positive electrode and the negative electrode, respectively.
- the positive electrode current collecting tab 4 and the negative electrode current collecting tab 5 protrude from the inside of the battery outer package 3 to the outside so that the positive electrode and the negative electrode can be electrically connected to the outside of the secondary battery 1, respectively.
- the battery outer package 3 may be formed of, for example, a laminate film.
- the laminate film may be, for example, a laminated film in which a resin film such as a polyethylene terephthalate (PET) film, a metal foil such as aluminum, copper, and stainless steel, and a sealant layer such as polypropylene are laminated in this order.
- PET polyethylene terephthalate
- metal foil such as aluminum, copper, and stainless steel
- a sealant layer such as polypropylene
- FIG. 2 is an exploded perspective view showing an embodiment of the electrode group 2 in the secondary battery 1 shown in FIG.
- FIG. 3 is a schematic cross-sectional view showing an embodiment of the electrode group 2 in the secondary battery 1 shown in FIG.
- the electrode group 2 ⁇ / b> A includes a positive electrode 6, an electrolyte layer 7, and a negative electrode 8 in this order.
- the positive electrode 6 includes a positive electrode current collector 9 and a positive electrode mixture layer 10 provided on the positive electrode current collector 9.
- the positive electrode current collector 9 is provided with a positive electrode current collector tab 4.
- the negative electrode 8 includes a negative electrode current collector 11 and a negative electrode mixture layer 12 provided on the negative electrode current collector 11.
- the negative electrode current collector 11 is provided with a negative electrode current collector tab 5.
- the positive electrode current collector 9 may be formed of aluminum, stainless steel, titanium, or the like. Specifically, the positive electrode current collector 9 may be, for example, an aluminum perforated foil having a hole diameter of 0.1 to 10 mm, an expanded metal, a foamed metal plate, or the like. In addition to the above, the positive electrode current collector 9 may be formed of any material as long as it does not cause changes such as dissolution and oxidation during use of the battery, and its shape, manufacturing method, etc. Not limited.
- the thickness of the positive electrode current collector 9 may be 10 ⁇ m or more and 100 ⁇ m or less, and is preferably 10 ⁇ m or more and 50 ⁇ m or less from the viewpoint of reducing the volume of the entire positive electrode, and the positive electrode current collector 9 has a small curvature when forming a battery. From the viewpoint of turning, it is more preferably 10 ⁇ m or more and 20 ⁇ m or less.
- the positive electrode mixture layer 10 contains a positive electrode active material, a conductive agent, and a binder.
- the positive electrode active material may be a lithium transition metal compound such as a lithium transition metal oxide or a lithium transition metal phosphate.
- the lithium transition metal oxide may be, for example, lithium manganate, lithium nickelate, lithium cobaltate, or the like.
- Lithium transition metal oxide is a part of transition metals such as Mn, Ni, Co, etc. contained in lithium manganate, lithium nickelate, lithium cobaltate, etc., one or more other transition metals or Mg Or a lithium transition metal oxide substituted with a metal element (typical element) such as Al. That is, the lithium transition metal oxide may be a compound represented by LiM 1 O 2 or LiM 1 O 4 (M 1 includes at least one transition metal).
- lithium transition metal oxides are Li (Co 1/3 Ni 1/3 Mn 1/3 ) O 2 , LiNi 1/2 Mn 1/2 O 2 , LiNi 1/2 Mn 3/2 O. it may be 4 or the like.
- the lithium transition metal oxide is preferably a compound represented by the following formula (1).
- Lithium transition metal phosphates are LiFePO 4 , LiMnPO 4 , LiMn x M 3 1-x PO 4 (0.3 ⁇ x ⁇ 1, M 3 is Fe, Ni, Co, Ti, Cu, Zn, Mg, and Or at least one element selected from the group consisting of Zr).
- the positive electrode active material may be primary particles that are not granulated, or may be secondary particles that are granulated.
- the particle diameter of the positive electrode active material is adjusted to be equal to or less than the thickness of the positive electrode mixture layer 10.
- the coarse particles are removed in advance by sieving classification, wind classification, etc.
- a positive electrode active material having a diameter is selected.
- the average particle diameter of the positive electrode active material is preferably 0.1 ⁇ m or more, more preferably 1 ⁇ m or more, and preferably 30 ⁇ m or less, more preferably 25 ⁇ m or less.
- the average particle diameter of the positive electrode active material is the particle diameter (D50) when the ratio (volume fraction) to the volume of the entire positive electrode active material is 50%.
- the average particle diameter (D50) of the positive electrode active material is obtained by measuring a suspension obtained by suspending the positive electrode active material in water by a laser scattering method using a laser scattering particle size measuring device (for example, Microtrac). It is obtained by.
- the content of the positive electrode active material may be 70% by mass or more, 80% by mass or more, or 85% by mass or more based on the total amount of the positive electrode mixture layer.
- the content of the positive electrode active material may be 95% by mass or less, 92% by mass or less, or 90% by mass or less based on the total amount of the positive electrode mixture layer.
- the conductive agent is not particularly limited, and may be a carbon material such as graphite, acetylene black, carbon black, carbon fiber, or carbon nanotube.
- the conductive agent may be a mixture of two or more carbon materials described above.
- the content of the conductive agent may be 0.1% by mass or more, 1% by mass or more, or 3% by mass or more based on the total amount of the positive electrode mixture layer.
- the content of the conductive agent is preferably 15% by mass or less, more preferably, based on the total amount of the positive electrode mixture layer, from the viewpoint of suppressing the increase in the volume of the positive electrode 6 and the accompanying decrease in the energy density of the secondary battery 1. It is 10 mass% or less, More preferably, it is 8 mass% or less.
- the binder is not limited as long as it does not decompose on the surface of the positive electrode 6, but selected from the group consisting of ethylene tetrafluoride, vinylidene fluoride, hexafluoropropylene, acrylic acid, maleic acid, ethyl methacrylate, and methyl methacrylate.
- the polymer may contain at least one selected from the group consisting of a monomer, a rubber such as styrene-butadiene rubber, isoprene rubber, and acrylic rubber.
- the binder is preferably a copolymer containing ethylene tetrafluoride and vinylidene fluoride as structural units.
- the content of the binder may be 0.5% by mass or more, 1% by mass or more, or 3% by mass or more based on the total amount of the positive electrode mixture layer.
- the content of the binder may be 20% by mass or less, 15% by mass or less, or 10% by mass or less based on the total amount of the positive electrode mixture layer.
- the positive electrode mixture layer 10 may further contain an ionic liquid.
- the content of the ionic liquid contained in the positive electrode mixture layer 10 is preferably 3% by mass or more, more preferably 5% by mass or more, and still more preferably 10% by mass or more based on the total amount of the positive electrode mixture layer.
- the content of the ionic liquid contained in the positive electrode mixture layer 10 is preferably 30% by mass or less, more preferably 25% by mass or less, and still more preferably 20% by mass or less, based on the total amount of the positive electrode mixture layer.
- An electrolyte salt may be dissolved in the ionic liquid contained in the positive electrode mixture layer 10.
- an electrolyte salt used in an electrolyte composition described later can be used.
- the thickness of the positive electrode mixture layer 10 is a thickness that is equal to or greater than the average particle diameter of the positive electrode active material, specifically, 10 ⁇ m or more, 15 ⁇ m or more, or 20 ⁇ m or more. Good.
- the thickness of the positive electrode mixture layer 10 may be 100 ⁇ m or less, 80 ⁇ m or less, or 70 ⁇ m or less.
- the negative electrode current collector 11 may be a metal such as aluminum, copper, nickel, stainless steel, or an alloy thereof. Since the negative electrode current collector 11 is light and has a high weight energy density, it is preferably aluminum or an alloy thereof. The negative electrode current collector 11 is preferably copper from the viewpoint of ease of processing into a thin film and cost.
- the thickness of the negative electrode current collector 11 may be 10 ⁇ m or more and 100 ⁇ m or less, and is preferably 10 ⁇ m or more and 50 ⁇ m or less from the viewpoint of reducing the volume of the entire negative electrode, and the negative electrode current collector 11 has a small curvature when forming a battery. From the viewpoint of turning, it is more preferably 10 ⁇ m or more and 20 ⁇ m or less.
- the negative electrode mixture layer 12 contains a negative electrode active material and a binder.
- the negative electrode active material those commonly used in the field of energy devices can be used.
- the negative electrode active material include metal lithium, lithium titanate (Li 4 Ti 5 O 12 ), a lithium alloy or other metal compound, a carbon material, a metal complex, and an organic polymer compound.
- the negative electrode active material may be one of these alone or a mixture of two or more.
- Carbon materials include natural graphite (flaky graphite, etc.), graphite such as artificial graphite, amorphous carbon, carbon fiber, acetylene black, ketjen black, channel black, furnace black, lamp black, thermal black And carbon black.
- the negative electrode active material is silicon, tin, or a compound containing these elements (oxide, nitride, alloy with other metals) from the viewpoint of obtaining a larger theoretical capacity (for example, 500 to 1500 Ah / kg). May be.
- the average particle diameter (D 50 ) of the negative electrode active material is preferably 1 ⁇ m or more from the viewpoint of obtaining a well-balanced negative electrode that suppresses an increase in irreversible capacity associated with a decrease in particle diameter and has enhanced electrolyte salt retention ability. More preferably, it is 5 ⁇ m or more, more preferably 10 ⁇ m or more, preferably 50 ⁇ m or less, more preferably 40 ⁇ m or less, and further preferably 30 ⁇ m or less.
- the average particle diameter (D 50 ) of the negative electrode active material is measured by the same method as the average particle diameter (D 50 ) of the positive electrode active material described above.
- the content of the negative electrode active material may be 60% by mass or more, 65% by mass or more, or 70% by mass or more based on the total amount of the negative electrode mixture layer.
- the content of the negative electrode active material may be 99% by mass or less, 95% by mass or less, or 90% by mass or less based on the total amount of the negative electrode mixture layer.
- the binder and its content may be the same as the binder and its content in the positive electrode mixture layer 10 described above.
- the negative electrode mixture layer 12 may further contain a conductive agent from the viewpoint of further reducing the resistance of the negative electrode 8.
- the conductive agent and its content may be the same as the conductive agent and its content in the positive electrode mixture layer 10 described above.
- the negative electrode mixture layer 12 may further contain an ionic liquid.
- the content of the ionic liquid contained in the negative electrode mixture layer 12 is preferably 3% by mass or more, more preferably 5% by mass or more, and still more preferably 10% by mass or more based on the total amount of the negative electrode mixture layer.
- the content of the ionic liquid contained in the negative electrode mixture layer 12 is preferably 30% by mass or less, more preferably 25% by mass or less, and still more preferably 20% by mass or less, based on the total amount of the negative electrode mixture layer.
- an electrolyte salt similar to the electrolyte salt that can be used for the positive electrode mixture layer 10 described above may be dissolved.
- the thickness of the negative electrode mixture layer 12 may be 10 ⁇ m or more, 15 ⁇ m or more, or 20 ⁇ m or more.
- the thickness of the negative electrode mixture layer 12 may be 100 ⁇ m or less, 80 ⁇ m or less, or 70 ⁇ m or less.
- the electrolyte layer 7 is formed, for example, by producing an electrolyte sheet using an electrolyte composition.
- the electrolyte composition includes one or more polymers, oxide particles, at least one electrolyte salt selected from the group consisting of a lithium salt, a sodium salt, a calcium salt, and a magnesium salt, an ionic liquid, Containing.
- the polymer preferably has a first structural unit selected from the group consisting of ethylene tetrafluoride and vinylidene fluoride.
- the structural unit constituting the polymer includes the first structural unit and a second structural unit selected from the group consisting of hexafluoropropylene, acrylic acid, maleic acid, ethyl methacrylate, and methyl methacrylate. It may be. That is, the first structural unit and the second structural unit may be included in one kind of polymer to form a copolymer, and each of the first structural unit and the second structural unit may be included in another polymer and have the first structural unit. And at least two types of polymers, the second polymer having the second structural unit.
- the polymer may be polytetrafluoroethylene, polyvinylidene fluoride, a copolymer of vinylidene fluoride and hexafluoropropylene, or the like.
- the content of the polymer is preferably 3% by mass or more based on the total amount of the electrolyte composition (electrolyte layer).
- the content of the polymer is preferably 50% by mass or less, more preferably 40% by mass or less, based on the total amount of the electrolyte composition.
- the content of the polymer is preferably 3 to 50% by mass or 3 to 40% by mass based on the total amount of the electrolyte composition.
- the polymer according to the present embodiment has excellent affinity with the ionic liquid contained in the electrolyte composition, the polymer in the ionic liquid is retained. Thereby, the leakage of the ionic liquid when a load is applied to the electrolyte composition is suppressed.
- the oxide particles may be, for example, inorganic oxide particles.
- the inorganic oxide is an inorganic oxide containing, for example, Li, Mg, Al, Si, Ca, Ti, Zr, La, Na, K, Ba, Sr, V, Nb, B, Ge and the like as constituent elements. Good.
- the oxide particles are preferably at least one selected from the group consisting of SiO 2 , Al 2 O 3 , AlOOH, MgO, CaO, ZrO 2 , TiO 2 , Li 7 La 3 Zr 2 O 12 , and BaTiO 3 . Particles. Since the oxide particles have polarity, it is possible to promote dissociation of the electrolyte in the electrolyte layer 7 and improve battery characteristics.
- the oxide particles have a hydrophobic surface.
- the oxide particles usually have a hydroxyl group on the surface and tend to be hydrophilic.
- the oxide particles having a hydrophobic surface have fewer hydroxyl groups on the surface than the oxide particles having no hydrophobic surface. Therefore, when oxide particles having a hydrophobic surface are used, an ionic liquid contained in the electrolyte composition (for example, ions having an anionic component of N (SO 2 F) 2 ⁇ , N (SO 2 CF 3 ) 2 ⁇ , etc.) Since the (liquid) is hydrophobic, it is expected that the affinity between the oxide particles and the ionic liquid is improved. For this reason, it is considered that the liquid retention of the ionic liquid in the electrolyte layer is further improved, and as a result, the ionic conductivity is further improved.
- an ionic liquid contained in the electrolyte composition for example, ions having an anionic component of N (SO 2 F) 2 ⁇ , N (SO 2 CF 3 )
- the oxide particles having a hydrophobic surface can be obtained by, for example, treating hydrophilic oxide particles with a surface treatment agent capable of imparting a hydrophobic surface. That is, the oxide particles having a hydrophobic surface may be oxide particles surface-treated with a surface treatment agent capable of imparting a hydrophobic surface. Examples of the surface treatment agent include silicon-containing compounds.
- the oxide particles having a hydrophobic surface may be oxide particles surface-treated with a silicon-containing compound. That is, the oxide particle having a hydrophobic surface may be one in which the surface of the oxide particle and the silicon atom of the silicon-containing compound are bonded via an oxygen atom.
- the silicon-containing compound as the surface treatment agent is preferably at least one selected from the group consisting of alkoxysilane, epoxy group-containing silane, amino group-containing silane, (meth) acryloyl group-containing silane, silazane, and siloxane.
- Alkoxysilanes are methyltrimethoxysilane, dimethyldimethoxysilane, phenyltrimethoxysilane, phenyltriethoxysilane, dimethoxydiphenylsilane, n-propyltrimethoxysilane, hexyltrimethoxysilane, tetraethoxysilane, methyltriethoxysilane, dimethyldi It may be ethoxysilane, n-propyltriethoxysilane or the like.
- Epoxy group-containing silanes are 2- (3,4-epoxycyclohexyl) ethyltrimethoxysilane, 3-glycidoxypropylmethyldimethoxysilane, 3-glycidoxypropyltrimethoxysilane, 3-glycidoxypropylmethyldiethoxy. Silane, 3-glycidoxypropyltriethoxysilane and the like may be used.
- Amino group-containing silanes are N-2- (aminoethyl) -3-aminopropylmethyldimethoxysilane, N-2- (aminoethyl) -3-aminopropyltrimethoxysilane, 3-aminopropyltriethoxysilane, N- It may be phenyl-3-aminopropyltrimethoxysilane.
- (Meth) acryloyl group-containing silanes include 3-methacryloyloxypropylmethyldimethoxysilane, 3-methacryloyloxypropyltrimethoxysilane, 3-methacryloyloxypropylmethyldiethoxysilane, 3-methacryloyloxypropyltriethoxysilane, 3-acryloyloxy It may be propyltrimethoxysilane or the like.
- the (meth) acryloyl group means an acryloyl group or a methacryloyl group corresponding thereto.
- Silazane may be hexamethyldisilazane or the like.
- the siloxane may be a silicone oil such as dimethylsiloxane.
- One or both of these terminals may have a reactive functional group (such as a carboxyl group).
- oxide particles having a hydrophobic surface those produced by a known method may be used, or commercially available products may be used as they are.
- oxide particles are classified into primary particles (particles that do not constitute secondary particles) integrally forming a single particle and a plurality of primary particles as judged from an apparent geometric form. Secondary particles formed by agglomeration.
- the specific surface area of the oxide particles may be, for example, 2 to 380 m 2 / g.
- the specific surface area is 2 to 380 m 2 / g, the obtained secondary battery tends to have excellent discharge characteristics.
- the specific surface area of the oxide particles may be 5 m 2 / g or more, 10 m 2 / g or more, 15 m 2 / g or more, 20 m 2 / g or more, or 30 m 2 / g or more.
- the specific surface area of the oxide particles is 350 m 2 / g or less, 300 m 2 / g or less, 250 m 2 / g or less, 200 m 2 /. g or less, 180 m 2 / g or less, 150 m 2 / g or less, 130 m 2 / g or less, 100 m 2 / g or less, 80 m 2 / g or less, or 60 m 2 / g or less.
- the specific surface area of the oxide particles means the specific surface area of the whole oxide particles including primary particles and secondary particles, and is measured by the BET method.
- the average primary particle size of the oxide particles is preferably 0.005 ⁇ m (5 nm) or more, more preferably 0.01 ⁇ m (10 nm) or more, and still more preferably, from the viewpoint of further improving the electrical conductivity. Is 0.015 ⁇ m (15 nm) or more. From the viewpoint of making the electrolyte layer 7 thin, the average primary particle size of the oxide particles is preferably 1 ⁇ m or less, more preferably 0.1 ⁇ m or less, and even more preferably 0.05 ⁇ m or less.
- the average primary particle diameter of the oxide particles is preferably 0.005 to 1 ⁇ m, 0.01 to 0 from the viewpoint of thinning the electrolyte composition and suppressing the protrusion of the oxide particles from the surface of the electrolyte composition. .1 ⁇ m, or 0.015 to 0.05 ⁇ m.
- the average primary particle size of the oxide particles can be measured by observing the oxide particles with a transmission electron microscope or the like.
- the average particle diameter of the oxide particles is preferably 0.005 ⁇ m or more, more preferably 0.01 ⁇ m or more, and further preferably 0.03 ⁇ m or more.
- the average particle diameter of the oxide particles is preferably 5 ⁇ m or less, more preferably 3 ⁇ m or less, and even more preferably 1 ⁇ m or less.
- the average particle diameter of the oxide particles is measured by a laser diffraction method, and corresponds to a particle diameter at which the volume accumulation is 50% when the volume accumulation particle size distribution curve is drawn from the small particle diameter side.
- the shape of the oxide particles may be, for example, a block shape or a substantially spherical shape.
- the aspect ratio of the oxide particles is preferably 10 or less, more preferably 5 or less, and even more preferably 2 or less, from the viewpoint of facilitating thinning of the electrolyte layer 7.
- the aspect ratio is calculated from the scanning electron micrograph of the oxide particles.
- the length of the particles in the long axis direction (maximum length of the particles) and the length of the particles in the short axis direction (minimum length of the particles) Defined as the ratio of The length of the particles is obtained by statistically calculating the above photograph using commercially available image processing software (for example, image analysis software manufactured by Asahi Kasei Engineering Co., Ltd., Image A (registered trademark)).
- the content of the oxide particles is preferably 5% by mass or more, more preferably 10% by mass or more, still more preferably 15% by mass or more, and particularly preferably 20% by mass or more, based on the total amount of the electrolyte composition (electrolyte layer). Moreover, it is preferably 60% by mass or less, more preferably 50% by mass or less, and still more preferably 40% by mass or less.
- the electrolyte salt is at least one selected from the group consisting of a lithium salt, a sodium salt, a calcium salt, and a magnesium salt.
- the electrolyte salt is a compound used to exchange cations between the positive electrode 6 and the negative electrode 8.
- the above electrolyte salt is preferable in that it has a low degree of dissociation at a low temperature and easily diffuses in an ionic liquid, and does not thermally decompose at a high temperature, so that the environmental temperature at which the secondary battery can be used is wide.
- the electrolyte salt may be an electrolyte salt used in a fluorine ion battery.
- the anion component of the electrolyte salt includes halide ions (I ⁇ , Cl ⁇ , Br ⁇ etc.), SCN ⁇ , BF 4 ⁇ , BF 3 (CF 3 ) ⁇ , BF 3 (C 2 F 5 ) ⁇ , PF 6 ⁇ .
- the anion component of the electrolyte salt is preferably an anion represented by the formula (A) exemplified by anion components of the ionic liquid described later such as N (SO 2 F) 2 ⁇ , N (SO 2 CF 3 ) 2 —, etc.
- the component is PF 6 ⁇ , BF 4 ⁇ , B (O 2 C 2 O 2 ) 2 ⁇ , or ClO 4 ⁇ .
- [FSI] ⁇ N (SO 2 F) 2 ⁇ , bis (fluorosulfonyl) imide anion [TFSI] ⁇ : N (SO 2 CF 3 ) 2 ⁇ , bis (trifluoromethanesulfonyl) imide anion [BOB] ⁇ : B (O 2 C 2 O 2 ) 2 ⁇ , bisoxalate borate anion [f3C] ⁇ : C (SO 2 F) 3 ⁇ , tris (fluorosulfonyl) carbanion
- Lithium salts include LiPF 6 , LiBF 4 , Li [FSI], Li [TFSI], Li [f 3 C], Li [BOB], LiClO 4 , LiBF 3 (CF 3 ), LiBF 3 (C 2 F 5 ), LiBF 3 (C 3 F 7 ), LiBF 3 (C 4 F 9 ), LiC (SO 2 CF 3 ) 3 , CF 3 SO 2 OLi, CF 3 COOLi, and R′COOLi (R ′ has 1 to 4 carbon atoms) An alkyl group, a phenyl group, or a naphthyl group).
- Sodium salts include NaPF 6 , NaBF 4 , Na [FSI], Na [TFSI], Na [f 3 C], Na [BOB], NaClO 4 , NaBF 3 (CF 3 ), NaBF 3 (C 2 F 5 ), NaBF 3 (C 3 F 7 ), NaBF 3 (C 4 F 9 ), NaC (SO 2 CF 3 ) 3 , CF 3 SO 2 ONa, CF 3 COONa, and R′COONa (R ′ has 1 to 4 carbon atoms) An alkyl group, a phenyl group, or a naphthyl group).
- the calcium salts are Ca (PF 6 ) 2 , Ca (BF 4 ) 2 , Ca [FSI] 2 , Ca [TFSI] 2 , Ca [f3C] 2 , Ca [BOB] 2 , Ca (ClO 4 ) 2 , Ca [BF 3 (CF 3 )] 2 , Ca [BF 3 (C 2 F 5 )] 2 , Ca [BF 3 (C 3 F 7 )] 2 , Ca [BF 3 (C 4 F 9 )] 2 , Ca [C (SO 2 CF 3) 3] 2, (CF 3 SO 2 O) 2 Ca, (CF 3 COO) 2 Ca, and (R'COO) 2 Ca (R 'is alkyl having 1 to 4 carbon atoms A group, a phenyl group, or a naphthyl group).
- Magnesium salts are Mg (PF 6 ) 2 , Mg (BF 4 ) 2 , Mg [FSI] 2 , Mg [TFSI] 2 , Mg [f 3 C] 2 , Mg [BOB] 2 , Na (ClO 4 ) 2 , Mg [BF 3 (CF 3 )] 2 , Mg [BF 3 (C 2 F 5 )] 2 , Mg [BF 3 (C 3 F 7 )] 2 , Mg [BF 3 (C 4 F 9 )] 2 , Mg [C (SO 2 CF 3 ) 3 ] 2 , (CF 3 SO 3 ) 2 Mg, (CF 3 COO) 2 Mg, and (R′COO) 2 Mg (R ′ is an alkyl group having 1 to 4 carbon atoms. , A phenyl group, or a naphthyl group).
- the electrolyte salt is preferably one selected from the group consisting of an imide lithium salt, an imide sodium salt, an imide calcium salt, and an imide magnesium salt, and more preferably an imide lithium salt.
- the imide-based lithium salt may be Li [TFSI], Li [FSI], or the like.
- the imide-based sodium salt may be Na [TFSI], Na [FSI] or the like.
- the imide-based calcium salt may be Ca [TFSI] 2 , Ca [FSI] 2 or the like.
- the imide-based magnesium salt may be Mg [TFSI] 2 , Mg [FSI] 2 or the like.
- the ionic liquid contains the following anion component and cation component. Note that the ionic liquid in the present embodiment is a liquid material at ⁇ 20 ° C. or higher.
- the anion component of the ionic liquid is not particularly limited, but is an anion of a halogen such as Cl ⁇ , Br ⁇ and I ⁇ , an inorganic anion such as BF 4 ⁇ and N (SO 2 F) 2 — , B (C 6 H 5 ) 4 ⁇ , CH 3 SO 2 O ⁇ , CF 3 SO 2 O ⁇ , N (SO 2 C 4 F 9 ) 2 ⁇ , N (SO 2 CF 3 ) 2 ⁇ , N (SO 2 C 2 F 5 ) 2 ⁇ Or an organic anion.
- a halogen such as Cl ⁇ , Br ⁇ and I ⁇
- an inorganic anion such as BF 4 ⁇ and N (SO 2 F) 2 — , B (C 6 H 5 ) 4 ⁇ , CH 3 SO 2 O ⁇ , CF 3 SO 2 O ⁇ , N (SO 2 C 4 F 9 ) 2 ⁇ , N (SO 2 CF 3 ) 2 ⁇
- the anionic component of the ionic liquid preferably contains at least one anionic component represented by the following general formula (A). N (SO 2 C m F 2m + 1 ) (SO 2 C n F 2n + 1 ) ⁇ (A)
- M and n each independently represents an integer of 0 to 5.
- m and n may be the same as or different from each other, and are preferably the same as each other.
- Examples of the anion component represented by the formula (A) include N (SO 2 C 4 F 9 ) 2 ⁇ , N (SO 2 F) 2 ⁇ , N (SO 2 CF 3 ) 2 ⁇ , N (SO 2 C 2 F 5 ) 2 — .
- the anionic component of the ionic liquid is more preferably N (SO 2 C 4 F 9 ) 2 ⁇ , CF 3 SO from the viewpoint of further improving the ionic conductivity with a relatively low viscosity and further improving the charge / discharge characteristics.
- the cation component of the ionic liquid is not particularly limited, but is preferably at least one selected from the group consisting of a chain quaternary onium cation, a piperidinium cation, a pyrrolidinium cation, a pyridinium cation, and an imidazolium cation.
- the chain quaternary onium cation is, for example, a compound represented by the following general formula (2).
- R 1 to R 4 each independently represents a chain alkyl group having 1 to 20 carbon atoms, or a chain alkoxyalkyl group represented by R—O— (CH 2 ) n —.
- R represents a methyl group or an ethyl group, and n represents an integer of 1 to 4
- X represents a nitrogen atom or a phosphorus atom.
- the number of carbon atoms of the alkyl group represented by R 1 to R 4 is preferably 1 to 20, more preferably 1 to 10, and still more preferably 1 to 5.
- the piperidinium cation is, for example, a nitrogen-containing six-membered cyclic compound represented by the following general formula (3).
- R 5 and R 6 are each independently an alkyl group having 1 to 20 carbon atoms or an alkoxyalkyl group represented by R—O— (CH 2 ) n — (R is methyl And n represents an integer of 1 to 4.
- the number of carbon atoms of the alkyl group represented by R 5 and R 6 is preferably 1 to 20, more preferably 1 to 10, and still more preferably 1 to 5.
- the pyrrolidinium cation is, for example, a five-membered cyclic compound represented by the following general formula (4).
- R 7 and R 8 are each independently an alkyl group having 1 to 20 carbon atoms, or an alkoxyalkyl group represented by R—O— (CH 2 ) n — (R is methyl And n represents an integer of 1 to 4.
- the carbon number of the alkyl group represented by R 7 and R 8 is preferably 1-20, more preferably 1-10, and still more preferably 1-5.
- a pyridinium cation is a compound shown, for example by General formula (5).
- R 9 to R 13 each independently represents an alkyl group having 1 to 20 carbon atoms, an alkoxyalkyl group represented by R—O— (CH 2 ) n — (R represents a methyl group) Or an ethyl group, and n represents an integer of 1 to 4), or a hydrogen atom.
- the number of carbon atoms of the alkyl group represented by R 9 to R 13 is preferably 1 to 20, more preferably 1 to 10, and still more preferably 1 to 5.
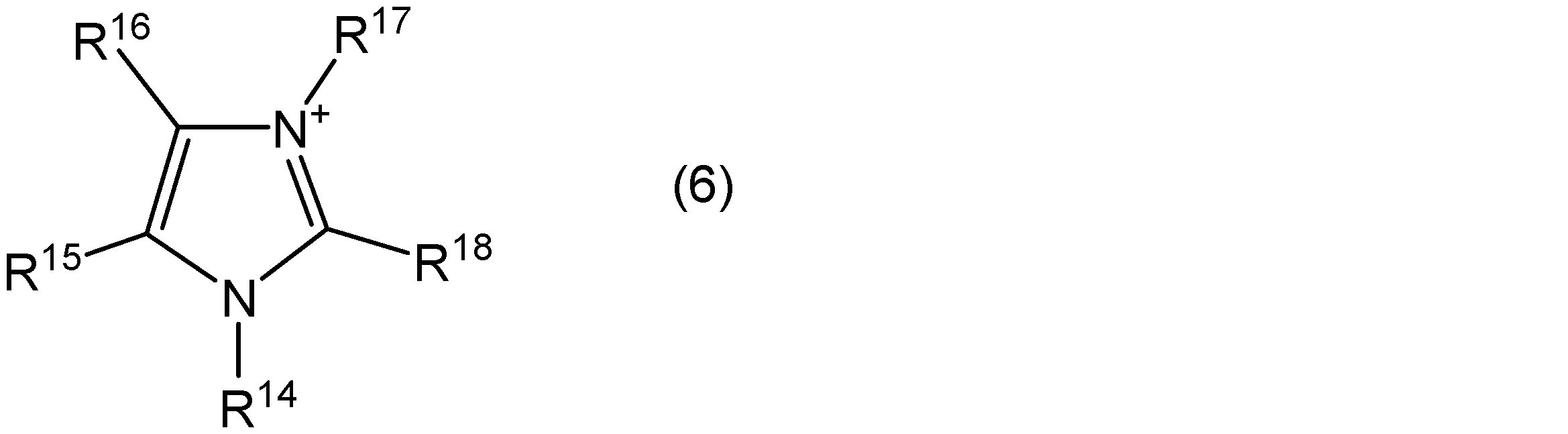
- the imidazolium cation is, for example, a compound represented by the general formula (6).
- R 14 to R 18 are each independently an alkyl group having 1 to 20 carbon atoms, an alkoxyalkyl group represented by R—O— (CH 2 ) n — (R is a methyl group) Or an ethyl group, and n represents an integer of 1 to 4), or a hydrogen atom.
- the number of carbon atoms of the alkyl group represented by R 14 to R 18 is preferably 1 to 20, more preferably 1 to 10, and still more preferably 1 to 5.
- the total content of the electrolyte salt and the ionic liquid is 10% by mass or more based on the total amount of the electrolyte composition (electrolyte layer) from the viewpoint of suitably producing the electrolyte layer. It may be 80 mass% or less.
- the content of the ionic liquid is preferably 20% by mass or more, more preferably 30% by mass or more, based on the total amount of the electrolyte composition, from the viewpoint of enabling charging and discharging of the lithium secondary battery at a high load factor. is there.
- the molar concentration of the ionic liquid in which the electrolyte salt is dissolved is preferably 0.5 mol / L or more, more preferably 0. 0.7 mol / L or more, more preferably 1.0 mol / L or more, preferably 2.0 mol / L or less, more preferably 1.8 mol / L or less, still more preferably 1.6 mol / L or less.
- the thickness of the electrolyte layer 7 is preferably 5 ⁇ m or more, more preferably 10 ⁇ m or more, from the viewpoint of increasing the electrical conductivity and improving the strength. From the viewpoint of suppressing the resistance of the electrolyte layer 7, the thickness of the electrolyte layer 7 is preferably 200 ⁇ m or less, more preferably 150 ⁇ m or less, still more preferably 100 ⁇ m or less, and particularly preferably 50 ⁇ m or less.
- the manufacturing method of the secondary battery 1 mentioned above includes the first step of forming the positive electrode mixture layer 10 on the positive electrode current collector 9 to obtain the positive electrode 6, and the negative electrode mixture on the negative electrode current collector 11. A second step of forming the layer 12 to obtain the negative electrode 8 and a third step of providing the electrolyte layer 7 between the positive electrode 6 and the negative electrode 8 are provided.
- the positive electrode 6 is obtained by, for example, dispersing a material used for the positive electrode mixture layer in a dispersion medium using a kneader, a disperser or the like to obtain a slurry-like positive electrode mixture, and then the positive electrode mixture. Is applied onto the positive electrode current collector 9 by a doctor blade method, a dipping method, a spray method or the like, and then the dispersion medium is volatilized. After volatilizing the dispersion medium, a compression molding step using a roll press may be provided as necessary.
- the positive electrode mixture layer 10 may be formed as a positive electrode mixture layer having a multilayer structure by performing the above-described steps from application of the positive electrode mixture to volatilization of the dispersion medium a plurality of times.
- the dispersion medium used in the first step may be water, 1-methyl-2-pyrrolidone (hereinafter also referred to as NMP), or the like.
- the dispersion medium is a compound other than the ionic liquid described above.
- the method of forming the negative electrode mixture layer 12 on the negative electrode current collector 11 may be the same method as in the first step described above.
- the electrolyte layer 7 is formed by producing an electrolyte sheet using the electrolyte composition.
- FIG. 4A is a schematic cross-sectional view showing an electrolyte sheet according to an embodiment. As shown in FIG. 4A, the electrolyte sheet 13 ⁇ / b> A includes a base material 14 and an electrolyte layer 7 provided on the base material 14.
- the electrolyte sheet 13A is produced, for example, by dispersing a material used for the electrolyte layer 7 in a dispersion medium to obtain a slurry, and applying the slurry onto the base material 14 and then volatilizing the dispersion medium.
- the dispersion medium is preferably water, NMP, toluene or the like.
- the substrate 14 is not limited as long as it has heat resistance that can withstand the heating when the dispersion medium is volatilized, does not react with the electrolyte composition, and does not swell with the electrolyte composition. May be formed.
- the base material 14 may be a film made of a resin (general-purpose engineer plastic) such as polyethylene terephthalate, polytetrafluoroethylene, polyimide, polyethersulfone, or polyetherketone.
- the substrate 14 only needs to have a heat-resistant temperature that can withstand the processing temperature for volatilizing the dispersion medium in the process of manufacturing the electrolyte layer.
- the heat-resistant temperature is a lower temperature of the softening point (temperature at which plastic deformation starts) or the melting point of the base material 14.
- the heat-resistant temperature of the base material 14 is preferably 50 ° C. or higher, more preferably 100 ° C. or higher, further preferably 150 ° C. or higher, from the viewpoint of adaptability with the ionic liquid used for the electrolyte layer 7. It may be 400 ° C. or lower. If the base material which has said heat-resistant temperature is used, the above dispersion media (NMP, toluene, etc.) can be used conveniently.
- the thickness of the base material 14 is preferably as thin as possible while maintaining the strength that can withstand the tensile force of the coating apparatus.
- the thickness of the substrate 14 is preferably 5 ⁇ m or more, more preferably 10 ⁇ m or more, from the viewpoint of securing strength when the electrolyte composition is applied to the substrate 14 while reducing the volume of the entire electrolyte sheet 13A.
- it is 25 micrometers or more, Preferably it is 100 micrometers or less, More preferably, it is 50 micrometers or less, More preferably, it is 40 micrometers or less.
- the electrolyte sheet can be continuously produced while being wound into a roll.
- the electrolyte layer 7 may be damaged by the surface of the electrolyte layer 7 coming into contact with the back surface of the substrate 14 and a part of the electrolyte layer 7 sticking to the substrate 14.
- the electrolyte sheet may be provided with a protective material on the side opposite to the base material 14 of the electrolyte layer 7 as another embodiment.
- FIG. 4B is a schematic cross-sectional view showing an electrolyte sheet according to another embodiment. As shown in FIG. 4B, the electrolyte sheet 13 ⁇ / b> B further includes a protective material 15 on the opposite side of the electrolyte layer 7 from the base material 14.
- the protective material 15 may be any material that can be easily peeled off from the electrolyte layer 7, and is preferably a nonpolar resin film such as polyethylene, polypropylene, or polytetrafluoroethylene. When a nonpolar resin film is used, the electrolyte layer 7 and the protective material 15 do not stick to each other, and the protective material 15 can be easily peeled off.
- a nonpolar resin film such as polyethylene, polypropylene, or polytetrafluoroethylene.
- the thickness of the protective material 15 is preferably 5 ⁇ m or more, more preferably 10 ⁇ m, and preferably 100 ⁇ m or less, more preferably 50 ⁇ m or less, from the viewpoint of ensuring strength while reducing the volume of the entire electrolyte sheet 13B. Preferably it is 30 micrometers or less.
- the heat resistant temperature of the protective material 15 is preferably ⁇ 30 ° C. or higher, more preferably 0 ° C. or higher, and preferably 100 ° C. from the viewpoint of suppressing deterioration in a low temperature environment and suppressing softening in a high temperature environment. Below, more preferably 50 ° C. or less.
- the protective material 15 it is not necessary to increase the heat-resistant temperature because the volatilization step of the dispersion medium described above is not essential.
- the method of providing the electrolyte layer 7 between the positive electrode 6 and the negative electrode 8 using the electrolyte sheet 13A is, for example, by peeling the base material 14 from the electrolyte sheet 13A and laminating the positive electrode 6, the electrolyte layer 7 and the negative electrode 8 by lamination.
- the secondary battery 1 is obtained by stacking.
- the electrolyte layer 7 is positioned on the positive electrode mixture layer 10 side of the positive electrode 6 and on the negative electrode mixture layer 12 side of the negative electrode 8, that is, the positive electrode current collector 9, the positive electrode mixture layer 10, and the electrolyte layer 7.
- the negative electrode mixture layer 12 and the negative electrode current collector 11 are laminated in this order.
- the electrolyte layer 7 kneads the material used for the electrolyte layer 7, and sandwiches the obtained kneaded material between resin sheets such as polytetrafluoroethylene (PTFE), It is formed by producing an electrolyte sheet by pressing with a roll press or the like.
- resin sheets such as polytetrafluoroethylene (PTFE)
- FIG. 5 is a schematic cross-sectional view showing an embodiment of an electrode group in the secondary battery according to the second embodiment.
- the secondary battery in the second embodiment is different from the secondary battery in the first embodiment in that the electrode group 2 ⁇ / b> B includes a bipolar electrode 16. That is, the electrode group 2B includes the positive electrode 6, the first electrolyte layer 7, the bipolar electrode 16, the second electrolyte layer 7, and the negative electrode 8 in this order.
- the bipolar electrode 16 includes a bipolar electrode current collector 17, a positive electrode mixture layer 10 provided on a surface (positive electrode surface) on the negative electrode 8 side of the bipolar electrode current collector 17, and a positive electrode 6 side of the bipolar electrode current collector 17.
- Negative electrode mixture layer 12 provided on the surface (negative electrode surface).
- the positive electrode surface may be preferably formed of a material excellent in oxidation resistance, and may be formed of aluminum, stainless steel, titanium, or the like.
- the negative electrode surface of the bipolar electrode current collector 17 using graphite or an alloy as the negative electrode active material may be formed of a material that does not form an alloy with lithium, specifically, stainless steel, nickel, iron, titanium, or the like. It may be formed.
- the bipolar electrode current collector 17 may be a clad material in which different metal foils are laminated.
- the bipolar electrode current collector 17 may be a single metal foil.
- the bipolar electrode current collector 17 as a single metal foil may be an aluminum perforated foil having a hole diameter of 0.1 to 10 mm, an expanded metal, a metal foam plate, or the like.
- the bipolar electrode current collector 17 may be formed of any material as long as it does not cause changes such as dissolution and oxidation during use of the battery, and its shape, manufacturing method, etc. Is not limited.
- the thickness of the bipolar electrode current collector 17 may be 10 ⁇ m or more and 100 ⁇ m or less, and is preferably 10 ⁇ m or more and 50 ⁇ m or less from the viewpoint of reducing the volume of the entire positive electrode. More preferably, the thickness is 10 ⁇ m or more and 20 ⁇ m or less.
- the manufacturing method of the secondary battery includes a first step of forming the positive electrode mixture layer 10 on the positive electrode current collector 9 to obtain the positive electrode 6, and the negative electrode mixture layer on the negative electrode current collector 11.
- a positive electrode mixture layer 10 is formed on one surface of the bipolar electrode current collector 17, and a negative electrode mixture layer 12 is formed on the other surface of the bipolar electrode current collector 17. 16 and a fourth step of providing the electrolyte layer 7 between the positive electrode 6 and the bipolar electrode 16 and between the negative electrode 8 and the bipolar electrode 16.
- the first step and the second step may be the same method as the first step and the second step in the first embodiment.
- the method of forming the positive electrode mixture layer 10 on one surface of the bipolar electrode current collector 17 may be the same method as the first step in the first embodiment.
- the method of forming the negative electrode mixture layer 12 on the other surface of the bipolar electrode current collector 17 may be the same method as the second step in the first embodiment.
- the electrolyte layer 7 is formed by producing an electrolyte sheet using an electrolyte composition.
- the manufacturing method of the electrolyte sheet may be the same method as the manufacturing method of the electrolyte sheets 13A and 13B in the first embodiment.
- the method of providing the electrolyte layer 7 between the negative electrode 8 and the bipolar electrode 16 may be the same method as the method of providing the electrolyte layer 7 between the positive electrode 6 and the bipolar electrode 16 described above. .
- PVDF-HFP hexafluoropropylene
- silazane hexamethyldisilazane
- NMP was further added to adjust the viscosity, and this slurry was applied onto a polyethylene terephthalate base material (product name: Teonex R-Q51, manufactured by Teijin DuPont Films, Inc., thickness 38 ⁇ m) using an applicator. .
- the applied slurry was heated and dried at 80 ° C. for 1 hour to volatilize the dispersion medium and obtain an electrolyte sheet.
- the obtained electrolyte sheet was punched into ⁇ 16 mm to form an electrolyte layer.
- acetylene black conductive agent, average particle size 48 nm, product name: HS-100, Denka Co., Ltd.
- This positive electrode mixture slurry is applied on a current collector (aluminum foil having a thickness of 20 ⁇ m) at a coating amount of 147 g / m 2 and dried at 80 ° C., whereby a positive electrode with a mixture density of 2.9 g / cm 3 is obtained. A mixture layer was formed. This was punched to 15 mm to make a positive electrode.
- This negative electrode mixture slurry was applied on a current collector (copper foil having a thickness of 10 ⁇ m) at a coating amount of 68 g / m 2 and dried at 80 ° C., whereby a negative electrode having a mixture density of 1.9 g / cm 3 . A mixture layer was formed. This was punched to ⁇ 16 mm to form a negative electrode.
- a coin-type battery for evaluation was produced using the positive electrode, the electrolyte layer, and the negative electrode.
- the positive electrode, the electrolyte layer, and the negative electrode were stacked in this order and placed in a CR2032-type coin cell container, and then the upper part of the battery container was crimped and sealed through an insulating gasket.
- Example 1-2 In the production of the electrolyte layer, instead of the SiO 2 particles used in Example 1-1, as the oxide particles having a hydrophobic surface, SiO 2 particles “RY50” (surface-treated with siloxane (dimethyl silicone oil)) (product) A coin-type battery was produced in the same manner as in Example 1-1 except that name: AEROSIL RY50, manufactured by Nippon Aerosil Co., Ltd., specific surface area: 30 m 2 / g, average primary particle size: about 40 nm was used.
- name: AEROSIL RY50 manufactured by Nippon Aerosil Co., Ltd., specific surface area: 30 m 2 / g, average primary particle size: about 40 nm was used.
- Example 1-3 In the preparation of the electrolyte layer, as an oxide particle having a hydrophobic surface, in place of the SiO 2 particles used in Example 1-1, (meth) SiO 2 particles "RM50" surface treated with acryloyl group-containing silane (A coin-type battery was produced in the same manner as in Example 1-1 except that product name: AEROSIL RM50, manufactured by Nippon Aerosil Co., Ltd., specific surface area: 50 m 2 / g, average primary particle size: about 40 nm. .
- Example 1-4 In the production of the electrolyte layer, an ionic liquid in which an electrolyte salt was dissolved was replaced with 1.5 mol / L instead of 1.5 mol / L / Li [TFSI] / [DEME] [TFSI] used in Example 1-1.
- a coin-type battery was fabricated in the same manner as in Example 1-1 except that / Li [FSI] / [Py13] [FSI] was used.
- a coin-type battery was produced in the same manner as in Example 1-4, except that the change was made.
- Example 1-6 In the production of the electrolyte layer, instead of the SiO 2 particles used in Example 1-1, as the oxide particles having a hydrophobic surface, SiO 2 particles “RX200” surface-treated with silazane (hexamethyldisilazane) ( product name: AEROSIL RX200, Nippon Aerosil Co., Ltd., specific surface area: 140 m 2 / g, average primary particle diameter: except for using about 12 nm), in the same manner as in example 1-1 to prepare a coin battery .
- silazane hexamethyldisilazane
- a coin-type battery was fabricated in the same manner as in Example 1-6, except for changing to.
- SiO 2 particles surface-treated with phenyltriethoxysilane were prepared by a wet method.
- An aqueous acetic acid solution adjusted to pH 4 and ethanol were mixed at a mass ratio of 9: 1 to prepare a mixed solution.
- the non-surface-treated hydrophilic SiO 2 particles used in Comparative Example 1-1 were added and stirred for 10 minutes.
- phenyltriethoxysilane product name: KBE-103, manufactured by Shin-Etsu Chemical Co., Ltd.
- KBE-103 manufactured by Shin-Etsu Chemical Co., Ltd.
- the mixed solution was filtered under reduced pressure, and the obtained powder (solid) was dried at 100 ° C. and pulverized to obtain SiO 2 particles surface-treated with phenyltriethoxysilane.
- the obtained SiO 2 particles had a specific surface area of 395 m 2 / g and an average primary particle size of about 7 nm.
- the mixture obtained by distillation and polytetrafluoroethylene were mixed at a mass ratio (mixture: polytetrafluoroethylene) 95: 5 and kneaded for 30 minutes or more using a mortar to obtain an electrolyte composition.
- the obtained electrolyte composition was sandwiched between two polytetrafluoroethylene (PTFE) sheets and pressed with a roll press to obtain an electrolyte sheet with a thickness of 50 ⁇ m. This electrolyte sheet was punched into ⁇ 16 mm to form an electrolyte layer.
- PTFE polytetrafluoroethylene
- Example 1-1 ⁇ Production of evaluation coin cell battery and evaluation of discharge characteristics> A coin-type battery was produced in the same manner as in Example 1-1 except that the obtained electrolyte layer was used, and the same evaluation as in Example 1-1 was performed. The results are shown in Table 2.
- Comparative Example 2-1 In the preparation of the electrolyte layer, hydrophilic SiO 2 particles not subjected to surface treatment used in Comparative Example 1-1 were used instead of SiO 2 particles surface-treated with phenyltriethoxysilane used in Example 2-1. A coin-type battery of Comparative Example 2-1 was produced in the same manner as in Example 2-1, except that the same evaluation as in Example 1-1 was performed. The results are shown in Table 2.
- the secondary batteries using the electrolyte compositions of Examples 1-1 to 1-7 containing oxide particles having a hydrophobic surface contain oxide particles having no hydrophobic surface.
- the discharge characteristics were excellent.
- the secondary battery using the electrolyte composition of Example 2-1 is similar to Comparative Example 2-1.
- the discharge characteristics were excellent. From these results, it was confirmed that the electrolyte composition of the present invention can produce a secondary battery having excellent discharge characteristics.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- Physics & Mathematics (AREA)
- General Physics & Mathematics (AREA)
- Dispersion Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Materials Engineering (AREA)
- Secondary Cells (AREA)
- Battery Electrode And Active Subsutance (AREA)
Abstract
Description
N(SO2CmF2m+1)(SO2CnF2n+1)- (A)
[m及びnは、それぞれ独立に0~5の整数を表す。]
図1は、第1実施形態に係る二次電池を示す斜視図である。図1に示すように、二次電池1は、正極、負極、及び電解質層から構成される電極群2と、電極群2を収容する袋状の電池外装体3と、を備えている。正極及び負極には、それぞれ正極集電タブ4及び負極集電タブ5が設けられている。正極集電タブ4及び負極集電タブ5は、それぞれ正極及び負極が二次電池1の外部と電気的に接続可能なように、電池外装体3の内部から外部へ突き出している。
LiaNibCocM2 dO2+e (1)
[式(1)中、M2は、Al、Mn、Mg、及びCaからなる群より選ばれる少なくとも1種であり、a、b、c、d、及びeは、それぞれ0.2≦a≦1.2、0.5≦b≦0.9、0.1≦c≦0.4、0≦d≦0.2、-0.2≦e≦0.2、かつb+c+d=1を満たす数である。]
[FSI]-:N(SO2F)2 -、ビス(フルオロスルホニル)イミドアニオン
[TFSI]-:N(SO2CF3)2 -、ビス(トリフルオロメタンスルホニル)イミドアニオン
[BOB]-:B(O2C2O2)2 -、ビスオキサレートボラートアニオン
[f3C]-:C(SO2F)3 -、トリス(フルオロスルホニル)カルボアニオン
N(SO2CmF2m+1)(SO2CnF2n+1)- (A)
次に、第2実施形態に係る二次電池について説明する。図5は、第2実施形態に係る二次電池における電極群の一実施形態を示す模式断面図である。図5に示すように、第2実施形態における二次電池が第1実施形態における二次電池と異なる点は、電極群2Bが、バイポーラ電極16を備えている点である。すなわち、電極群2Bは、正極6と、第1の電解質層7と、バイポーラ電極16と、第2の電解質層7と、負極8と、をこの順に備えている。
<電解質層の作製>
乾燥アルゴン雰囲気下で乾燥したリチウムビス(トリフルオロメタンスルホニル)イミド(Li[TFSI])を電解質塩として用い、イオン液体であるN,N-ジエチル-N-メチル-N-(2-メトキシエチル)アンモニウム ビス(トリフルオロメタンスルホニル)イミド([DEME][TFSI])に、電解質塩を1.5mol/Lの濃度で溶解させた(以下、電解質塩を溶解させたイオン液体の組成を表す際に、「電解質塩の濃度/電解質塩の種類/イオン液体の種類」と表記することがある。)。次に、ポリマとしてのフッ化ビニリデンとヘキサフルオロプロピレンとのコポリマ(PVDF-HFP)と、疎水性表面を有する酸化物粒子としてのシラザン(ヘキサメチルジシラザン)で表面処理されたSiO2粒子「RX50」(製品名:AEROSIL RX50、日本アエロジル株式会社製、比表面積:35m2/g、平均一次粒径:約40nm)と、を混合した後、分散媒としてのN-メチル-2-ピロリドン(NMP)を添加し、スラリを作製した。このスラリに、上述の電解質塩を溶解させたイオン液体(1.5mol/L/Li[TFSI]/[DEME][TFSI])を更に添加し、混合することによって、電解質組成物のスラリを得た。このとき、電解質組成物におけるポリマと酸化物粒子と電解質塩を溶解させたイオン液体との質量比は、ポリマ:酸化物粒子:電解質塩を溶解させたイオン液体=34:23:43であった。その後、NMPを更に加えて粘度を調節し、このスラリをポリエチレンテレフタレート製の基材(製品名:テオネックスR-Q51、帝人デュポンフィルム株式会社製、厚さ38μm)上にアプリケータを用いて塗布した。塗布したスラリを80℃で1時間加熱乾燥することにより、分散媒を揮発させて、電解質シートを得た。得られた電解質シートをφ16mmに打ち抜き、電解質層とした。
層状型リチウム・ニッケル・マンガン・コバルト複合酸化物(正極活物質)78.5質量部、アセチレンブラック(導電剤、平均粒径48nm、製品名:HS-100、デンカ株式会社)5質量部、フッ化ビニリデンとヘキサフルオロプロピレンとのコポリマ溶液(結着剤、固形分12質量%)2.5質量部、及び電解質塩を溶解させたイオン液体(1.5mol/L/Li[FSI]/[Py13][FSI](N-メチル-N-プロピルピロリジニウム ビス(フルオロスルホニル)イミド))14質量部を混合して正極合剤スラリを調製した。この正極合剤スラリを集電体(厚さ20μmのアルミニウム箔)上に塗工量147g/m2で塗工し、80℃で乾燥させることにより、合剤密度2.9g/cm3の正極合剤層を形成した。これをφ15mmに打ち抜き、正極とした。
黒鉛1(負極活物質、日立化成株式会社製)78質量部、黒鉛2(負極活物質、日本黒鉛工業株式会社製)2.4質量部、炭素繊維(導電剤、製品名:VGCF-H、昭和電工株式会社)0.6質量部、フッ化ビニリデンとヘキサフルオロプロピレンとのコポリマ溶液(結着剤、固形分12質量%)5質量部、及び電解質塩を溶解させたイオン液体(1.5mol/L/Li[FSI]/[Py13][FSI])14質量部を混合して負極合剤スラリを調製した。この負極合剤スラリを集電体(厚さ10μmの銅箔)上に塗工量68g/m2で塗工し、80℃で乾燥させることにより、合剤密度1.9g/cm3の負極合剤層を形成した。これをφ16mmに打ち抜き、負極とした。
正極、電解質層、負極を用いて評価用コイン型電池を作製した。正極、電解質層、負極をこの順に重ね、CR2032型のコインセル容器内に配置した後、絶縁性のガスケットを介して電池容器上部をかしめて密閉した。
電解質層の作製において、疎水性表面を有する酸化物粒子として、実施例1-1で用いたSiO2粒子に代えて、シロキサン(ジメチルシリコーンオイル)で表面処理されたSiO2粒子「RY50」(製品名:AEROSIL RY50、日本アエロジル株式会社製、比表面積:30m2/g、平均一次粒径:約40nm)を用いた以外は、実施例1-1と同様にして、コイン型電池を作製した。
電解質層の作製において、疎水性表面を有する酸化物粒子として、実施例1-1で用いたSiO2粒子に代えて、(メタ)アクリロイル基含有シランで表面処理されたSiO2粒子「RM50」(製品名:AEROSIL RM50、日本アエロジル株式会社製、比表面積:50m2/g、平均一次粒径:約40nm)を用いた以外は、実施例1-1と同様にして、コイン型電池を作製した。
電解質層の作製において、電解質塩を溶解させたイオン液体として、実施例1-1で用いた1.5mol/L/Li[TFSI]/[DEME][TFSI]に代えて、1.5mol/L/Li[FSI]/[Py13][FSI]を用いた以外は、実施例1-1と同様にして、コイン型電池を作製した。
電解質層の作製において、電解質組成物におけるポリマと酸化物粒子と電解質塩を溶解させたイオン液体との質量比を、ポリマ:酸化物粒子:電解質塩を溶解させたイオン液体=30:20:50に変更した以外は、実施例1-4と同様にして、コイン型電池を作製した。
電解質層の作製において、疎水性表面を有する酸化物粒子として、実施例1-1で用いたSiO2粒子に代えて、シラザン(ヘキサメチルジシラザン)で表面処理されたSiO2粒子「RX200」(製品名:AEROSIL RX200、日本アエロジル株式会社製、比表面積:140m2/g、平均一次粒径:約12nm)を用いた以外は、実施例1-1と同様にして、コイン型電池を作製した。
電解質層の作製において、電解質組成物におけるポリマと酸化物粒子と電解質塩を溶解させたイオン液体との質量比を、ポリマ:酸化物粒子:電解質塩を溶解させたイオン液体=30:20:50に変更した以外は、実施例1-6と同様にして、コイン型電池を作製した。
電解質層の作製において、実施例1-1で用いたSiO2粒子に代えて、表面処理されていない親水性SiO2粒子「S5130」(製品名:S5130、SIGMA-ALDRICH社製、比表面積:395m2/g、平均一次粒径:約7nm)を用い、ポリマと酸化物粒子と電解質塩を溶解させたイオン液体との質量比を22:22:56に変更した以外は、実施例1-1と同様にして、コイン型電池を作製した。
得られた実施例1-1~1-7及び比較例1-1のコイン型電池の25℃での放電容量を、充放電装置(東洋システム株式会社製)を用いて、以下の充放電条件に基づき測定した。
(1)終止電圧4.2V、0.05Cで定電流定電圧(CCCV)充電を行った後、0.05Cで終止電圧2.7Vまで定電流(CC)放電するサイクルを3サイクル行い、放電容量を求めた。なお、Cとは「電流値(A)/電池容量(Ah)」を意味する。
(2)次いで、終止電圧4.2V、0.1Cで定電流定電圧(CCCV)充電を行った後、0.5Cで終止電圧2.7Vまで定電流(CC)放電するサイクルを1サイクル行い、放電容量を求めた。
放電特性(%)=(2)で得られた放電容量/(1)の3サイクル目で得られた放電容量×100
<フェニルトリエトキシシランで表面処理されたSiO2粒子の作製>
フェニルトリエトキシシランで表面処理されたSiO2粒子を湿式法にて作製した。pH4に調整した酢酸水溶液とエタノールとを質量比9:1で混合し、混合溶液を調製した。この混合溶液に、比較例1-1で用いた表面処理されていない親水性SiO2粒子を加えて、10分間撹拌した。次いで、フェニルトリエトキシシラン(製品名:KBE-103、信越化学株式会社製)を親水性SiO2粒子に対して1質量%となるように添加し、更に10分間撹拌した。混合溶液を減圧濾過し、得られた粉末(固体)を100℃で乾燥し、粉砕することによって、フェニルトリエトキシシランで表面処理されたSiO2粒子を得た。得られたSiO2粒子の比表面積は395m2/g、平均一次粒径は約7nmであった。
乾燥アルゴン雰囲気下で乾燥したLi[TFSI]を電解質塩として用い、イオン液体である[DEME][TFSI]に、電解質塩を1.5mol/Lの濃度で溶解させた。得られた電解質塩を溶解させたイオン液体と上述で作製したフェニルトリエトキシシランで表面処理されたSiO2粒子とを、体積比(電解質塩を溶解させたイオン液体:SiO2)80:20で、メタノール中で30分以上撹拌しながら混合した。その後、エバポレータを用いて60℃で蒸留した。蒸留により得られた混合物と、ポリ四フッ化エチレンを質量比(混合物:ポリ四フッ化エチレン)95:5で混合し、乳鉢を用いて30分以上混練し、電解質組成物を得た。このとき、電解質組成物におけるポリマと酸化物粒子と電解質塩を溶解させたイオン液体との質量比は、ポリマ:酸化物粒子:電解質塩を溶解させたイオン液体=5:40:55であった。得られた電解質組成物を2枚のポリ四フッ化エチレン(PTFE)シート間に挟み、ロールプレス機でプレスすることにより、厚さ50μmの電解質シートを得た。この電解質シートをφ16mmに打ち抜き、電解質層とした。
得られた電解質層を用いた以外は、実施例1-1と同様にして、コイン型電池を作製し、実施例1-1と同様の評価を行った。結果を表2に示す。
電解質層の作製において、実施例2-1で用いたフェニルトリエトキシシランで表面処理されたSiO2粒子に代えて、比較例1-1で用いた表面処理されていない親水性SiO2粒子を用いた以外は、実施例2-1と同様にして、比較例2-1のコイン型電池を作製し、実施例1-1と同様の評価を行った。結果を表2に示す。
Claims (10)
- 1種又は2種以上のポリマと、
疎水性表面を有する酸化物粒子と、
リチウム塩、ナトリウム塩、カルシウム塩、及びマグネシウム塩からなる群より選ばれる少なくとも1種の電解質塩と、
イオン液体と、
を含有する、電解質組成物。 - 前記酸化物粒子がケイ素含有化合物で表面処理されている、請求項1に記載の電解質組成物。
- 前記ケイ素含有化合物が、アルコキシシラン、エポキシ基含有シラン、アミノ基含有シラン、(メタ)アクリロイル基含有シラン、シラザン、及びシロキサンからなる群より選ばれる少なくとも1種である、請求項2に記載の電解質組成物。
- 前記酸化物粒子が、SiO2、Al2O3、AlOOH、MgO、CaO、ZrO2、TiO2、Li7La3Zr2O12、及びBaTiO3からなる群より選ばれる少なくとも1種の粒子である、請求項1~3のいずれか一項に記載の電解質組成物。
- 前記イオン液体が、カチオン成分として、鎖状四級オニウムカチオン、ピペリジニウムカチオン、ピロリジニウムカチオン、ピリジニウムカチオン、及びイミダゾリウムカチオンからなる群より選ばれる少なくとも1種を含有する、請求項1~4のいずれか一項に記載の電解質組成物。
- 前記イオン液体が、アニオン成分として、下記一般式(A)で表されるアニオン成分の少なくとも1種を含有する、請求項1~5のいずれか一項に記載の電解質組成物。
N(SO2CmF2m+1)(SO2CnF2n+1)- (A)
[m及びnは、それぞれ独立に0~5の整数を表す。] - 前記ポリマが、四フッ化エチレン及びフッ化ビニリデンからなる群より選ばれる第1の構造単位を有する、請求項1~6のいずれか一項に記載の電解質組成物。
- 前記ポリマを構成する構造単位の中に、前記第1の構造単位と、ヘキサフルオロプロピレン、アクリル酸、マレイン酸、エチルメタクリレート、及びメチルメタクリレートからなる群より選ばれる第2の構造単位と、が含まれる、請求項7に記載の電解質組成物。
- 前記電解質塩が、イミド系リチウム塩である、請求項1~8のいずれか一項に記載の電解質組成物。
- 正極と、
負極と、
前記正極及び前記負極の間に設けられた、請求項1~9のいずれか一項に記載の電解質組成物からなる電解質層と、
を備える、二次電池。
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US16/617,104 US11923505B2 (en) | 2017-06-01 | 2018-05-31 | Electrolyte composition and rechargeable battery |
| KR1020197037578A KR102655290B1 (ko) | 2017-06-01 | 2018-05-31 | 전해질 조성물 및 이차 전지 |
| EP18809800.8A EP3633779A4 (en) | 2017-06-01 | 2018-05-31 | ELECTROLYTE COMPOSITION AND RECHARGEABLE BATTERY |
| CN201880035455.3A CN110679027A (zh) | 2017-06-01 | 2018-05-31 | 电解质组合物和二次电池 |
| CN202410663385.3A CN118472388A (zh) | 2017-06-01 | 2018-05-31 | 电解质组合物和二次电池 |
| JP2019521306A JP7442911B2 (ja) | 2017-06-01 | 2018-05-31 | 電解質組成物、二次電池、及び電解質シートの製造方法 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2017109371 | 2017-06-01 | ||
| JP2017-109371 | 2017-06-01 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018221669A1 true WO2018221669A1 (ja) | 2018-12-06 |
Family
ID=64454805
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2018/021007 WO2018221669A1 (ja) | 2017-06-01 | 2018-05-31 | 電解質組成物及び二次電池 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US11923505B2 (ja) |
| EP (1) | EP3633779A4 (ja) |
| JP (1) | JP7442911B2 (ja) |
| KR (1) | KR102655290B1 (ja) |
| CN (2) | CN118472388A (ja) |
| WO (1) | WO2018221669A1 (ja) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2020004343A1 (ja) * | 2018-06-26 | 2020-01-02 | 日立化成株式会社 | 二次電池及びその製造方法 |
| WO2021001970A1 (ja) * | 2019-07-03 | 2021-01-07 | 昭和電工マテリアルズ株式会社 | 電解質シート及び二次電池 |
| WO2021038862A1 (ja) * | 2019-08-30 | 2021-03-04 | 昭和電工マテリアルズ株式会社 | 電解質シート及びその製造方法、並びに二次電池 |
| JP2022025869A (ja) * | 2020-07-30 | 2022-02-10 | 株式会社豊田自動織機 | 蓄電セル |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113851709B (zh) * | 2021-10-13 | 2023-03-17 | 上海电气集团股份有限公司 | 固态电解质及其制备方法和应用 |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2000164254A (ja) | 1998-11-26 | 2000-06-16 | Sony Corp | ゲル状電解質及びゲル状電解質電池 |
| JP2001229966A (ja) * | 2000-02-10 | 2001-08-24 | Mitsui Chemicals Inc | ゲル状電解質およびリチウム電池 |
| JP2003157719A (ja) * | 2001-11-22 | 2003-05-30 | Hitachi Maxell Ltd | 常温溶融塩型固体電解質およびそれを用いた全固体電気化学素子 |
| JP2007141467A (ja) | 2005-11-14 | 2007-06-07 | Sony Corp | ゲル電解質およびゲル電解質電池 |
| JP2007280948A (ja) * | 2006-03-17 | 2007-10-25 | Nippon Synthetic Chem Ind Co Ltd:The | 電解質およびそれを用いたリチウム二次電池 |
| JP2008130229A (ja) * | 2006-11-16 | 2008-06-05 | National Institute Of Advanced Industrial & Technology | リチウム二次電池 |
| JP2010198757A (ja) * | 2009-02-23 | 2010-09-09 | Sony Corp | 非水電解質組成物及び非水電解質二次電池 |
| JP2017059432A (ja) * | 2015-09-17 | 2017-03-23 | 株式会社日立製作所 | 擬似固体電解質およびそれを用いた全固体リチウム二次電池 |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH07282850A (ja) * | 1994-04-12 | 1995-10-27 | Yuasa Corp | 電 池 |
| AU2003292624A1 (en) * | 2002-12-27 | 2004-07-29 | Nippon Aerosil Co., Ltd. | Inorganic oxide powder for use in gel electrolyte of battery and gel composition containing inorganic oxide powder |
| US20100239916A1 (en) * | 2003-08-08 | 2010-09-23 | Max-Planck-Gesellschaft Zur Forderung Der Wissenschaften E.V. | Non-aqueous electrolyte and a battery, a supercapacitor, an electrochromic device and a solar cell including such an electrolyte |
| KR101943647B1 (ko) * | 2009-02-23 | 2019-01-29 | 가부시키가이샤 무라타 세이사쿠쇼 | 비수 전해질 조성물, 비수 전해질 이차 전지 및 비수 전해질 이차 전지의 제조 방법 |
| JP5375418B2 (ja) * | 2009-08-04 | 2013-12-25 | コニカミノルタ株式会社 | 二次電池用電解質組成物および二次電池 |
| JP5381636B2 (ja) * | 2009-11-18 | 2014-01-08 | コニカミノルタ株式会社 | 電池用固体電解質およびリチウムイオン二次電池 |
| JP2011134459A (ja) | 2009-12-22 | 2011-07-07 | Konica Minolta Holdings Inc | 電解質組成物、二次電池、および化合物 |
| DE102012101670A1 (de) * | 2012-02-29 | 2013-08-29 | Jacobs University Bremen Ggmbh | Leitsalz für Lithium-basierte Energiespeicher |
| JP6685951B2 (ja) * | 2017-02-21 | 2020-04-22 | 株式会社東芝 | 二次電池、複合電解質、電池パック及び車両 |
-
2018
- 2018-05-31 US US16/617,104 patent/US11923505B2/en active Active
- 2018-05-31 JP JP2019521306A patent/JP7442911B2/ja active Active
- 2018-05-31 WO PCT/JP2018/021007 patent/WO2018221669A1/ja active Application Filing
- 2018-05-31 CN CN202410663385.3A patent/CN118472388A/zh active Pending
- 2018-05-31 KR KR1020197037578A patent/KR102655290B1/ko active IP Right Grant
- 2018-05-31 CN CN201880035455.3A patent/CN110679027A/zh active Pending
- 2018-05-31 EP EP18809800.8A patent/EP3633779A4/en active Pending
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2000164254A (ja) | 1998-11-26 | 2000-06-16 | Sony Corp | ゲル状電解質及びゲル状電解質電池 |
| JP2001229966A (ja) * | 2000-02-10 | 2001-08-24 | Mitsui Chemicals Inc | ゲル状電解質およびリチウム電池 |
| JP2003157719A (ja) * | 2001-11-22 | 2003-05-30 | Hitachi Maxell Ltd | 常温溶融塩型固体電解質およびそれを用いた全固体電気化学素子 |
| JP2007141467A (ja) | 2005-11-14 | 2007-06-07 | Sony Corp | ゲル電解質およびゲル電解質電池 |
| JP2007280948A (ja) * | 2006-03-17 | 2007-10-25 | Nippon Synthetic Chem Ind Co Ltd:The | 電解質およびそれを用いたリチウム二次電池 |
| JP2008130229A (ja) * | 2006-11-16 | 2008-06-05 | National Institute Of Advanced Industrial & Technology | リチウム二次電池 |
| JP2010198757A (ja) * | 2009-02-23 | 2010-09-09 | Sony Corp | 非水電解質組成物及び非水電解質二次電池 |
| JP2017059432A (ja) * | 2015-09-17 | 2017-03-23 | 株式会社日立製作所 | 擬似固体電解質およびそれを用いた全固体リチウム二次電池 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3633779A4 |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2020004343A1 (ja) * | 2018-06-26 | 2020-01-02 | 日立化成株式会社 | 二次電池及びその製造方法 |
| JPWO2020004343A1 (ja) * | 2018-06-26 | 2021-07-15 | 昭和電工マテリアルズ株式会社 | 二次電池及びその製造方法 |
| JP7546910B2 (ja) | 2018-06-26 | 2024-09-09 | エルジー エナジー ソリューション リミテッド | 二次電池及びその製造方法 |
| WO2021001970A1 (ja) * | 2019-07-03 | 2021-01-07 | 昭和電工マテリアルズ株式会社 | 電解質シート及び二次電池 |
| WO2021038862A1 (ja) * | 2019-08-30 | 2021-03-04 | 昭和電工マテリアルズ株式会社 | 電解質シート及びその製造方法、並びに二次電池 |
| JP2022025869A (ja) * | 2020-07-30 | 2022-02-10 | 株式会社豊田自動織機 | 蓄電セル |
| JP7532985B2 (ja) | 2020-07-30 | 2024-08-14 | 株式会社豊田自動織機 | 蓄電セル |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3633779A1 (en) | 2020-04-08 |
| JPWO2018221669A1 (ja) | 2020-04-02 |
| KR102655290B1 (ko) | 2024-04-04 |
| KR20200014333A (ko) | 2020-02-10 |
| US20200259214A1 (en) | 2020-08-13 |
| US11923505B2 (en) | 2024-03-05 |
| CN110679027A (zh) | 2020-01-10 |
| EP3633779A4 (en) | 2021-01-13 |
| JP7442911B2 (ja) | 2024-03-05 |
| CN118472388A (zh) | 2024-08-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7442911B2 (ja) | 電解質組成物、二次電池、及び電解質シートの製造方法 | |
| JP6562184B2 (ja) | 電解質組成物、二次電池、及び電解質シートの製造方法 | |
| JP7423120B2 (ja) | 電解質スラリー組成物、電解質シートの製造方法、及び二次電池の製造方法 | |
| WO2018220800A1 (ja) | 電解質組成物、二次電池、及び電解質シートの製造方法 | |
| JP2018206561A (ja) | 二次電池 | |
| WO2018221668A1 (ja) | 電解質組成物及び二次電池 | |
| JP7438207B2 (ja) | 電池用スラリ組成物、並びに、電極、電解質シート、及び電池部材の製造方法 | |
| WO2020017439A1 (ja) | 電解質シートの製造方法及び二次電池の製造方法 | |
| JP7416426B2 (ja) | 二次電池用電池部材の製造方法 | |
| JP7446657B2 (ja) | 二次電池用電極、二次電池用電解質層及び二次電池 | |
| CN108735972B (zh) | 二次电池用电池构件的制造方法 | |
| JP2020113527A (ja) | 電解質スラリ組成物及びその製造方法、並びに、電解質シート及びその製造方法 | |
| WO2021001970A1 (ja) | 電解質シート及び二次電池 | |
| JP7438605B2 (ja) | 二次電池用電池部材の製造方法 | |
| JP2020205146A (ja) | 電解質シート及びその製造方法、並びに二次電池 | |
| JP2020136223A (ja) | リチウム二次電池 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18809800 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2019521306 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20197037578 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2018809800 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2018809800 Country of ref document: EP Effective date: 20200102 |