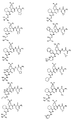
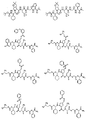
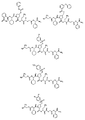
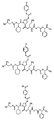
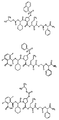
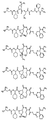
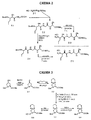
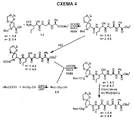
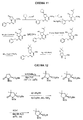
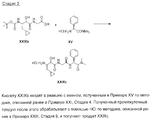
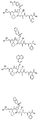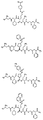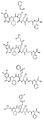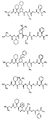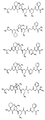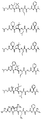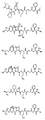RU2404189C9 - Новые пептиды как ингибиторы ns3-серинпротеазы вируса гепатита c - Google Patents
Новые пептиды как ингибиторы ns3-серинпротеазы вируса гепатита c Download PDFInfo
- Publication number
- RU2404189C9 RU2404189C9 RU2004125279/04A RU2004125279A RU2404189C9 RU 2404189 C9 RU2404189 C9 RU 2404189C9 RU 2004125279/04 A RU2004125279/04 A RU 2004125279/04A RU 2004125279 A RU2004125279 A RU 2004125279A RU 2404189 C9 RU2404189 C9 RU 2404189C9
- Authority
- RU
- Russia
- Prior art keywords
- group
- alkyl
- compound
- chr
- aryl
- Prior art date
Links
- 241000711549 Hepacivirus C Species 0.000 title claims abstract 19
- 108091005804 Peptidases Proteins 0.000 title claims abstract 11
- 239000004365 Protease Substances 0.000 title claims abstract 11
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 title claims abstract 11
- 108090000765 processed proteins & peptides Proteins 0.000 title claims abstract 7
- 102000004196 processed proteins & peptides Human genes 0.000 title claims 4
- 239000003112 inhibitor Substances 0.000 title 1
- 150000001875 compounds Chemical class 0.000 claims abstract 68
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract 13
- 239000003814 drug Substances 0.000 claims abstract 6
- 201000010099 disease Diseases 0.000 claims abstract 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract 4
- 229940079593 drug Drugs 0.000 claims abstract 2
- 230000000694 effects Effects 0.000 claims abstract 2
- 125000000217 alkyl group Chemical group 0.000 claims 35
- 125000003118 aryl group Chemical group 0.000 claims 24
- 229910052739 hydrogen Inorganic materials 0.000 claims 21
- 125000000753 cycloalkyl group Chemical group 0.000 claims 20
- -1 aryl heteroaryl Chemical group 0.000 claims 17
- 125000001424 substituent group Chemical group 0.000 claims 14
- 229910005965 SO 2 Inorganic materials 0.000 claims 12
- 125000004432 carbon atom Chemical group C* 0.000 claims 11
- 125000003710 aryl alkyl group Chemical group 0.000 claims 10
- 125000002837 carbocyclic group Chemical group 0.000 claims 10
- CYRMSUTZVYGINF-UHFFFAOYSA-N trichlorofluoromethane Chemical compound FC(Cl)(Cl)Cl CYRMSUTZVYGINF-UHFFFAOYSA-N 0.000 claims 10
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims 9
- 125000002877 alkyl aryl group Chemical group 0.000 claims 8
- 125000001072 heteroaryl group Chemical group 0.000 claims 8
- 125000003545 alkoxy group Chemical group 0.000 claims 7
- 229910052736 halogen Inorganic materials 0.000 claims 7
- 150000002367 halogens Chemical class 0.000 claims 7
- 229910052760 oxygen Inorganic materials 0.000 claims 7
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 claims 6
- 125000004122 cyclic group Chemical group 0.000 claims 6
- 239000012634 fragment Substances 0.000 claims 6
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims 6
- 229940079322 interferon Drugs 0.000 claims 6
- 150000003839 salts Chemical class 0.000 claims 6
- 229910052717 sulfur Inorganic materials 0.000 claims 6
- 102000014150 Interferons Human genes 0.000 claims 5
- 108010050904 Interferons Proteins 0.000 claims 5
- 125000003342 alkenyl group Chemical group 0.000 claims 5
- 125000005213 alkyl heteroaryl group Chemical group 0.000 claims 5
- 125000000304 alkynyl group Chemical group 0.000 claims 5
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims 5
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 claims 4
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 claims 4
- 229910052799 carbon Inorganic materials 0.000 claims 4
- PXBRQCKWGAHEHS-UHFFFAOYSA-N dichlorodifluoromethane Chemical compound FC(F)(Cl)Cl PXBRQCKWGAHEHS-UHFFFAOYSA-N 0.000 claims 4
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims 4
- 125000004446 heteroarylalkyl group Chemical group 0.000 claims 4
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims 4
- 125000004433 nitrogen atom Chemical group N* 0.000 claims 4
- 125000000962 organic group Chemical group 0.000 claims 4
- 125000004430 oxygen atom Chemical group O* 0.000 claims 4
- 125000004434 sulfur atom Chemical group 0.000 claims 4
- 125000000008 (C1-C10) alkyl group Chemical group 0.000 claims 3
- 125000006552 (C3-C8) cycloalkyl group Chemical group 0.000 claims 3
- 239000003443 antiviral agent Substances 0.000 claims 3
- 125000006297 carbonyl amino group Chemical group [H]N([*:2])C([*:1])=O 0.000 claims 3
- 125000002843 carboxylic acid group Chemical group 0.000 claims 3
- 229910052731 fluorine Inorganic materials 0.000 claims 3
- 125000001160 methoxycarbonyl group Chemical group [H]C([H])([H])OC(*)=O 0.000 claims 3
- 229920001184 polypeptide Polymers 0.000 claims 3
- 125000006432 1-methyl cyclopropyl group Chemical group [H]C([H])([H])C1(*)C([H])([H])C1([H])[H] 0.000 claims 2
- 125000000094 2-phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])* 0.000 claims 2
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical group NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 claims 2
- 102000006992 Interferon-alpha Human genes 0.000 claims 2
- 108010047761 Interferon-alpha Proteins 0.000 claims 2
- 125000003368 amide group Chemical group 0.000 claims 2
- 125000006615 aromatic heterocyclic group Chemical group 0.000 claims 2
- 125000004104 aryloxy group Chemical group 0.000 claims 2
- 125000004429 atom Chemical group 0.000 claims 2
- 125000001246 bromo group Chemical group Br* 0.000 claims 2
- 125000001309 chloro group Chemical group Cl* 0.000 claims 2
- 239000003937 drug carrier Substances 0.000 claims 2
- 125000001153 fluoro group Chemical group F* 0.000 claims 2
- 125000000623 heterocyclic group Chemical group 0.000 claims 2
- 125000000592 heterocycloalkyl group Chemical group 0.000 claims 2
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 claims 2
- 125000002346 iodo group Chemical group I* 0.000 claims 2
- 125000000468 ketone group Chemical group 0.000 claims 2
- 238000004519 manufacturing process Methods 0.000 claims 2
- 238000000034 method Methods 0.000 claims 2
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims 2
- 125000004458 methylaminocarbonyl group Chemical group [H]N(C(*)=O)C([H])([H])[H] 0.000 claims 2
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 claims 2
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 claims 2
- 229910052757 nitrogen Inorganic materials 0.000 claims 2
- 125000004076 pyridyl group Chemical group 0.000 claims 2
- 229920006395 saturated elastomer Polymers 0.000 claims 2
- 229930195734 saturated hydrocarbon Natural products 0.000 claims 2
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 claims 2
- 125000001544 thienyl group Chemical group 0.000 claims 2
- KXDHJXZQYSOELW-UHFFFAOYSA-N Carbamic acid Chemical group NC(O)=O KXDHJXZQYSOELW-UHFFFAOYSA-N 0.000 claims 1
- 208000005176 Hepatitis C Diseases 0.000 claims 1
- IWUCXVSUMQZMFG-AFCXAGJDSA-N Ribavirin Chemical group N1=C(C(=O)N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 IWUCXVSUMQZMFG-AFCXAGJDSA-N 0.000 claims 1
- 125000005248 alkyl aryloxy group Chemical group 0.000 claims 1
- 125000004422 alkyl sulphonamide group Chemical group 0.000 claims 1
- 150000001356 alkyl thiols Chemical group 0.000 claims 1
- 150000001408 amides Chemical group 0.000 claims 1
- 125000003277 amino group Chemical group 0.000 claims 1
- 125000005128 aryl amino alkyl group Chemical group 0.000 claims 1
- 125000004421 aryl sulphonamide group Chemical group 0.000 claims 1
- 229910052794 bromium Inorganic materials 0.000 claims 1
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 claims 1
- 125000005392 carboxamide group Chemical group NC(=O)* 0.000 claims 1
- 229910052801 chlorine Inorganic materials 0.000 claims 1
- 125000004093 cyano group Chemical group *C#N 0.000 claims 1
- 125000004186 cyclopropylmethyl group Chemical group [H]C([H])(*)C1([H])C([H])([H])C1([H])[H] 0.000 claims 1
- 125000001188 haloalkyl group Chemical group 0.000 claims 1
- 125000004404 heteroalkyl group Chemical group 0.000 claims 1
- 125000005553 heteroaryloxy group Chemical group 0.000 claims 1
- 125000005842 heteroatom Chemical group 0.000 claims 1
- 239000000203 mixture Substances 0.000 claims 1
- 229960000329 ribavirin Drugs 0.000 claims 1
- HZCAHMRRMINHDJ-DBRKOABJSA-N ribavirin Natural products O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1N=CN=C1 HZCAHMRRMINHDJ-DBRKOABJSA-N 0.000 claims 1
- 239000012453 solvate Substances 0.000 claims 1
- 238000007920 subcutaneous administration Methods 0.000 claims 1
- 208000024891 symptom Diseases 0.000 claims 1
- 125000004213 tert-butoxy group Chemical group [H]C([H])([H])C(O*)(C([H])([H])[H])C([H])([H])[H] 0.000 claims 1
- 239000000126 substance Substances 0.000 abstract 1
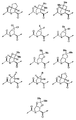
- 0 CC(C)C1CC(*)CCC1 Chemical compound CC(C)C1CC(*)CCC1 0.000 description 29
- BVLGUNJHOBNHLD-UHFFFAOYSA-N C1C[U]CNC1 Chemical compound C1C[U]CNC1 BVLGUNJHOBNHLD-UHFFFAOYSA-N 0.000 description 1
- CHXLCHDHIIEQMX-UHFFFAOYSA-N CC(C(CC1(C2)OCCO1)N2C(C)=O)=O Chemical compound CC(C(CC1(C2)OCCO1)N2C(C)=O)=O CHXLCHDHIIEQMX-UHFFFAOYSA-N 0.000 description 1
- CCTIVQTZDUHJQD-DOYHFWSASA-N CC(C)(C)NC(N[C@@H](C1CCCCC1)C(N(C[C@@H]1C(C)(C)[C@@H]11)C1C(NC(CC1CC1)C(C(NCC(NC(C(N(C)C)=O)c1ccccc1)=O)=O)=O)=O)=O)=O Chemical compound CC(C)(C)NC(N[C@@H](C1CCCCC1)C(N(C[C@@H]1C(C)(C)[C@@H]11)C1C(NC(CC1CC1)C(C(NCC(NC(C(N(C)C)=O)c1ccccc1)=O)=O)=O)=O)=O)=O CCTIVQTZDUHJQD-DOYHFWSASA-N 0.000 description 1
- UZIQVNCFQWEPJD-AZPOEJFESA-N CC(C)(C)OC(NC(C(C)(C)O)C(N(C[C@@H]1C(C)(C)[C@@H]11)C1C(NC(CC1CCC1)C(C(NCC(NC(C(N(C)C)=O)c1ccccc1)=O)=O)=O)=O)=O)=O Chemical compound CC(C)(C)OC(NC(C(C)(C)O)C(N(C[C@@H]1C(C)(C)[C@@H]11)C1C(NC(CC1CCC1)C(C(NCC(NC(C(N(C)C)=O)c1ccccc1)=O)=O)=O)=O)=O)=O UZIQVNCFQWEPJD-AZPOEJFESA-N 0.000 description 1
- RXOSRMFZQJALHX-DOYHFWSASA-N CC(C)(C)OC(N[C@@H](C1CCCCC1)C(N(C[C@@H]1C(C)(C)[C@@H]11)C1C(NC(CC1CC1)C(C(NCC(NC(C(N(C)C)=O)c1ccccc1)=O)=O)=O)=O)=O)=O Chemical compound CC(C)(C)OC(N[C@@H](C1CCCCC1)C(N(C[C@@H]1C(C)(C)[C@@H]11)C1C(NC(CC1CC1)C(C(NCC(NC(C(N(C)C)=O)c1ccccc1)=O)=O)=O)=O)=O)=O RXOSRMFZQJALHX-DOYHFWSASA-N 0.000 description 1
- CXHFZIYUWOJVMX-PYLXUTCPSA-N CC(C)(C)OC(N[C@@H](C1CCCCC1)C(N(C[C@@H]1OC(C)(C)C[C@@H]11)[C@@H]1C(NC(CC1CC1)C(C(NCC(N[C@H](C(N(C)C)=O)c(cc1)ccc1Br)=O)=O)=O)=O)=O)=O Chemical compound CC(C)(C)OC(N[C@@H](C1CCCCC1)C(N(C[C@@H]1OC(C)(C)C[C@@H]11)[C@@H]1C(NC(CC1CC1)C(C(NCC(N[C@H](C(N(C)C)=O)c(cc1)ccc1Br)=O)=O)=O)=O)=O)=O CXHFZIYUWOJVMX-PYLXUTCPSA-N 0.000 description 1
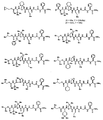
- LNAQSWUSQQVQME-UHFFFAOYSA-N CC(C)(C1CC2(C)CC1)C2OC Chemical compound CC(C)(C1CC2(C)CC1)C2OC LNAQSWUSQQVQME-UHFFFAOYSA-N 0.000 description 1
- UGPWKCHVLNHYJH-UHFFFAOYSA-N CC(C)C(CC1)C(C(NC(CC2CC2)C(C(NCC(OC(C)(C)c2ccccc2)=O)=O)=O)=O)N1C(C(C(C)(C)C)NC(NC(C)(C)C)=O)=O Chemical compound CC(C)C(CC1)C(C(NC(CC2CC2)C(C(NCC(OC(C)(C)c2ccccc2)=O)=O)=O)=O)N1C(C(C(C)(C)C)NC(NC(C)(C)C)=O)=O UGPWKCHVLNHYJH-UHFFFAOYSA-N 0.000 description 1
- LOQDBMDPAIZJNU-UHFFFAOYSA-N CC(C)C(Cc1ccccc1)C(C)(C)C Chemical compound CC(C)C(Cc1ccccc1)C(C)(C)C LOQDBMDPAIZJNU-UHFFFAOYSA-N 0.000 description 1
- DZICCLVVNVURFW-UHFFFAOYSA-N CC(C)C1(C)CCOCC1 Chemical compound CC(C)C1(C)CCOCC1 DZICCLVVNVURFW-UHFFFAOYSA-N 0.000 description 1
- YQPSXMHHSGZLTH-UHFFFAOYSA-N CC(C)C1CCC(C)(C)CC1 Chemical compound CC(C)C1CCC(C)(C)CC1 YQPSXMHHSGZLTH-UHFFFAOYSA-N 0.000 description 1
- GGAPZWPHDVGGGP-KIYNQFGBSA-N CC(C)CC(C[C@H]1C(C)=O)CN1C(C)=O Chemical compound CC(C)CC(C[C@H]1C(C)=O)CN1C(C)=O GGAPZWPHDVGGGP-KIYNQFGBSA-N 0.000 description 1
- ORHQRPWTYKZJFR-UHFFFAOYSA-N CC(C)CC1(CC(O)=O)CCCCC1 Chemical compound CC(C)CC1(CC(O)=O)CCCCC1 ORHQRPWTYKZJFR-UHFFFAOYSA-N 0.000 description 1
- GBEBMXGNSZYQLY-PGFJXMCPSA-N CC(C)NC(NC(C(C)(C)C)C(N(C[C@@H]1C(C)(C)[C@@H]11)C1C(NC(CC1CCC1)C(C(N)=O)=O)=O)=O)=O Chemical compound CC(C)NC(NC(C(C)(C)C)C(N(C[C@@H]1C(C)(C)[C@@H]11)C1C(NC(CC1CCC1)C(C(N)=O)=O)=O)=O)=O GBEBMXGNSZYQLY-PGFJXMCPSA-N 0.000 description 1
- PKSDZIPFONBHEK-GUFYVYCGSA-N CC(C)[C@@H](CC1)[C@@H](C(NC(CCC2CC2)C(C(N)=O)=O)=O)N1C(C(C(C)(C)C)NC(NC(C)(C)C)=O)=O Chemical compound CC(C)[C@@H](CC1)[C@@H](C(NC(CCC2CC2)C(C(N)=O)=O)=O)N1C(C(C(C)(C)C)NC(NC(C)(C)C)=O)=O PKSDZIPFONBHEK-GUFYVYCGSA-N 0.000 description 1
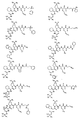
- DFIOPCWYHVACRI-UHFFFAOYSA-N CC(CC1)CC1OI Chemical compound CC(CC1)CC1OI DFIOPCWYHVACRI-UHFFFAOYSA-N 0.000 description 1
- QCZDTWZBWVUFET-UHFFFAOYSA-N CC(CCC1)C1OI Chemical compound CC(CCC1)C1OI QCZDTWZBWVUFET-UHFFFAOYSA-N 0.000 description 1
- SRWVPLHVSALKIO-UHFFFAOYSA-N CC(NC(C)(C)C)I Chemical compound CC(NC(C)(C)C)I SRWVPLHVSALKIO-UHFFFAOYSA-N 0.000 description 1
- WCOULYCKPPOATA-KXUCPTDWSA-N CC([C@H]([C@@H]1[C@H](C2)COC1)N2C(C)=O)=O Chemical compound CC([C@H]([C@@H]1[C@H](C2)COC1)N2C(C)=O)=O WCOULYCKPPOATA-KXUCPTDWSA-N 0.000 description 1
- WPCAPFUWDSGBMR-FNZOIDNWSA-N CCCC(C(C(CCCC(/C=C/C(C(O)=O)c1ccccc1)=O)=O)=O)NC[C@H](CC[C@@H]1c2ccccc2)N1C([C@H](C1CCCCC1)NC(OCC(C)C)=O)=O Chemical compound CCCC(C(C(CCCC(/C=C/C(C(O)=O)c1ccccc1)=O)=O)=O)NC[C@H](CC[C@@H]1c2ccccc2)N1C([C@H](C1CCCCC1)NC(OCC(C)C)=O)=O WPCAPFUWDSGBMR-FNZOIDNWSA-N 0.000 description 1
- XHOWKUDQAWMNDW-YDJKICJBSA-N CCCC(C(C(NCC(NC(CC(N(C)C)=O)c1ccccc1)=O)=O)=O)NC(C(C[C@H](C1)C(C)C)N1C([C@H](C1CCCCC1)NC(OC(C)(C)C)=O)=O)=O Chemical compound CCCC(C(C(NCC(NC(CC(N(C)C)=O)c1ccccc1)=O)=O)=O)NC(C(C[C@H](C1)C(C)C)N1C([C@H](C1CCCCC1)NC(OC(C)(C)C)=O)=O)=O XHOWKUDQAWMNDW-YDJKICJBSA-N 0.000 description 1
- SUZVQRCIEDPLAU-GFCCVEGCSA-N CCCCC[C@H](CC(C)C)NCC Chemical compound CCCCC[C@H](CC(C)C)NCC SUZVQRCIEDPLAU-GFCCVEGCSA-N 0.000 description 1
- RNIJSZGJNGTOTB-UHFFFAOYSA-N CNC1C(CC2)CC2C1 Chemical compound CNC1C(CC2)CC2C1 RNIJSZGJNGTOTB-UHFFFAOYSA-N 0.000 description 1
- UUSXHTWHKQBMTA-LZRTYROISA-N C[C@@H](C(CCC1(C)CC1)C1)[C@@H](C(C)=O)N1C(C)=O Chemical compound C[C@@H](C(CCC1(C)CC1)C1)[C@@H](C(C)=O)N1C(C)=O UUSXHTWHKQBMTA-LZRTYROISA-N 0.000 description 1
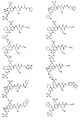
- GPSDUZXPYCFOSQ-UHFFFAOYSA-N Cc1cc(C(O)=O)ccc1 Chemical compound Cc1cc(C(O)=O)ccc1 GPSDUZXPYCFOSQ-UHFFFAOYSA-N 0.000 description 1
- WSMQKESQZFQMFW-UHFFFAOYSA-N Cc1n[nH]c(C(O)=O)c1 Chemical compound Cc1n[nH]c(C(O)=O)c1 WSMQKESQZFQMFW-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/04—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing only normal peptide links
- C07K5/06—Dipeptides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/04—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing only normal peptide links
- C07K5/08—Tripeptides
- C07K5/0802—Tripeptides with the first amino acid being neutral
- C07K5/0804—Tripeptides with the first amino acid being neutral and aliphatic
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/02—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing at least one abnormal peptide link
- C07K5/0202—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing at least one abnormal peptide link containing the structure -NH-X-X-C(=0)-, X being an optionally substituted carbon atom or a heteroatom, e.g. beta-amino acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/04—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing only normal peptide links
- C07K5/08—Tripeptides
- C07K5/0802—Tripeptides with the first amino acid being neutral
- C07K5/0812—Tripeptides with the first amino acid being neutral and aromatic or cycloaliphatic
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/04—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing only normal peptide links
- C07K5/08—Tripeptides
- C07K5/0827—Tripeptides containing heteroatoms different from O, S, or N
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K7/00—Peptides having 5 to 20 amino acids in a fully defined sequence; Derivatives thereof
- C07K7/02—Linear peptides containing at least one abnormal peptide link
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Genetics & Genomics (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Virology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Crystallography & Structural Chemistry (AREA)
- Oncology (AREA)
- Engineering & Computer Science (AREA)
- Communicable Diseases (AREA)
- Gastroenterology & Hepatology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/052,386 US7244721B2 (en) | 2000-07-21 | 2002-01-18 | Peptides as NS3-serine protease inhibitors of hepatitis C virus |
| US10/052,386 | 2002-01-18 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| RU2004125279A RU2004125279A (ru) | 2006-01-10 |
| RU2404189C2 RU2404189C2 (ru) | 2010-11-20 |
| RU2404189C9 true RU2404189C9 (ru) | 2011-05-10 |
Family
ID=27609106
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2004125279/04A RU2404189C9 (ru) | 2002-01-18 | 2003-01-16 | Новые пептиды как ингибиторы ns3-серинпротеазы вируса гепатита c |
Country Status (28)
| Country | Link |
|---|---|
| US (2) | US7244721B2 (enExample) |
| EP (1) | EP1481000B1 (enExample) |
| JP (1) | JP4563033B2 (enExample) |
| KR (1) | KR101020355B1 (enExample) |
| CN (2) | CN1633446A (enExample) |
| AR (1) | AR038183A1 (enExample) |
| AT (1) | ATE469914T1 (enExample) |
| AU (1) | AU2009210423B2 (enExample) |
| BR (1) | BR0306931A (enExample) |
| CA (1) | CA2473032A1 (enExample) |
| CO (1) | CO5650168A2 (enExample) |
| CY (1) | CY1111225T1 (enExample) |
| DE (1) | DE60332814D1 (enExample) |
| DK (1) | DK1481000T3 (enExample) |
| EC (1) | ECSP045191A (enExample) |
| IL (1) | IL162815A0 (enExample) |
| MX (1) | MXPA04006934A (enExample) |
| MY (1) | MY140710A (enExample) |
| NO (1) | NO20042792L (enExample) |
| NZ (1) | NZ571073A (enExample) |
| PE (1) | PE20030852A1 (enExample) |
| PL (1) | PL372747A1 (enExample) |
| PT (1) | PT1481000E (enExample) |
| RU (1) | RU2404189C9 (enExample) |
| SI (1) | SI1481000T1 (enExample) |
| TW (1) | TWI334872B (enExample) |
| WO (1) | WO2003062265A2 (enExample) |
| ZA (1) | ZA200405304B (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2625793C2 (ru) * | 2011-12-23 | 2017-07-19 | Ипсен Мэньюфэкчеринг Айэлэнд Лимитед | Способ синтеза терапевтических пептидов |
| RU2794746C1 (ru) * | 2022-04-15 | 2023-04-24 | Общество С Ограниченной Ответственностью "Промомед Рус" | Способ получения (1r,2s,5s)-n-[(1s)-1-циано-2-[(3s)-2-оксопирролидин-3-ил]этил]-3-[(2s)-3,3-диметил-2-[(2,2,2-трифторацетил)амино]бутаноил]-6,6-диметил-3-азабицикло[3.1.0]гексан-2-карбоксамида |
Families Citing this family (162)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1133649C (zh) | 1996-10-18 | 2004-01-07 | 沃泰克斯药物股份有限公司 | 丝氨酸蛋白酶、特别是丙型肝炎病毒ns3蛋白酶的抑制剂 |
| SI1385870T1 (sl) | 2000-07-21 | 2010-08-31 | Schering Corp | Peptidi kot NS3-serin proteazni inhibitorji virusa hepatitisa C |
| SV2003000617A (es) | 2000-08-31 | 2003-01-13 | Lilly Co Eli | Inhibidores de la proteasa peptidomimetica ref. x-14912m |
| JP2003007697A (ja) * | 2001-06-21 | 2003-01-10 | Hitachi Kokusai Electric Inc | 半導体装置の製造方法、基板処理方法および基板処理装置 |
| MY140680A (en) | 2002-05-20 | 2010-01-15 | Bristol Myers Squibb Co | Hepatitis c virus inhibitors |
| MXPA05013751A (es) | 2003-06-17 | 2006-03-08 | Schering Corp | Procedimiento e intermediarios para la preparacion de 3-(amino)-3-ciclobutilmetil-2-hidroxi-propionamida o sus sales. |
| AR044694A1 (es) * | 2003-06-17 | 2005-09-21 | Schering Corp | Proceso y compuestos intermedios para la preparacion de (1r, 2s,5s) - 3 azabiciclo [3,1,0] hexano-2- carboxamida, n- [3- amino-1- (ciclobutilmetil) - 2, 3 - dioxopropil] -3- [ (2s) - 2 - [[ [ 1,1- dimetiletil] amino] carbonilamino] -3,3-dimetil -1- oxobutil]-6,6 dimetilo |
| SG180022A1 (en) * | 2003-06-17 | 2012-05-30 | Schering Corp | Process and intermediates for the preparation of (1r,2s,5s)-6,6-dimethyl-3-azabicyclo[3,1,0]hexane-2-carboxylates or salts thereof |
| CN1867579A (zh) * | 2003-08-26 | 2006-11-22 | 先灵公司 | 丙肝病毒的新的肽模拟物ns3-丝氨酸蛋白酶抑制剂 |
| MY148123A (en) | 2003-09-05 | 2013-02-28 | Vertex Pharma | Inhibitors of serine proteases, particularly hcv ns3-ns4a protease |
| US20110150835A1 (en) * | 2003-09-26 | 2011-06-23 | Schering Corporation | Macrocyclic Inhibitors of Hepatitis C Virus NS3 Serine Protease |
| MXPA06003455A (es) * | 2003-09-26 | 2006-05-31 | Schering Corp | Inhibidores macrociclicos de la serina proteasa ns3 del virus de la hepatitis c. |
| US7491794B2 (en) * | 2003-10-14 | 2009-02-17 | Intermune, Inc. | Macrocyclic compounds as inhibitors of viral replication |
| EP1742913A1 (en) | 2003-12-11 | 2007-01-17 | Schering Corporation | Inhibitors of hepatitis c virus ns3/ns4a serine protease |
| DK1713823T3 (da) | 2004-01-30 | 2010-03-08 | Medivir Ab | HCV NS-3 serinproteaseinhibitorer |
| CN1938332B (zh) * | 2004-02-04 | 2011-10-19 | 沃泰克斯药物股份有限公司 | 丝氨酸蛋白酶、特别是hcv ns3-ns4a蛋白酶的抑制剂 |
| SG184700A1 (en) | 2004-02-20 | 2012-10-30 | Boehringer Ingelheim Int | Viral polymerase inhibitors |
| ATE434621T1 (de) * | 2004-02-27 | 2009-07-15 | Schering Corp | Neue verbindungen als inhibitoren der ns3- serinprotease des hepatitis-c-virus |
| CA2557301A1 (en) * | 2004-02-27 | 2005-09-15 | Schering Corporation | Cyclobutenedione groups-containing compounds as inhibitors of hepatitis c virus ns3 serine protease |
| US7192957B2 (en) * | 2004-02-27 | 2007-03-20 | Schering Corporation | Compounds as inhibitors of hepatitis C virus NS3 serine protease |
| RU2006134000A (ru) | 2004-02-27 | 2008-04-10 | Шеринг Корпорейшн (US) | Новые кетоамиды с циклическим p4s, действующие как ингибиторы сериновой протеазы ns3 ируса гепатита с |
| US7342041B2 (en) | 2004-02-27 | 2008-03-11 | Schering Corporation | 3,4-(cyclopentyl)-fused proline compounds as inhibitors of hepatitis C virus NS3 serine protease |
| CA2557495C (en) * | 2004-02-27 | 2014-04-15 | Schering Corporation | Sulfur compounds as inhibitors of hepatitis c virus ns3 serine protease |
| WO2005085275A1 (en) * | 2004-02-27 | 2005-09-15 | Schering Corporation | Inhibitors of hepatitis c virus ns3 protease |
| US7816326B2 (en) * | 2004-02-27 | 2010-10-19 | Schering Corporation | Sulfur compounds as inhibitors of hepatitis C virus NS3 serine protease |
| AR049635A1 (es) * | 2004-05-06 | 2006-08-23 | Schering Corp | (1r,2s,5s)-n-((1s)-3-amino-1-(ciclobutilmetil)-2,3-dioxopropil)-3-((2s)-2-((((1,1-dimetiletil)amino)carbonil)amino)-3,3-dimetil-1-oxobutil)-6,6-dimetil-3-azabiciclo(3.1.0)hexan-2-carboxamida como inhibidor de la ns3/ns4a serina proteasa del virus de la hepatitis c |
| US7399749B2 (en) * | 2004-05-20 | 2008-07-15 | Schering Corporation | Substituted prolines as inhibitors of hepatitis C virus NS3 serine protease |
| GB0426661D0 (en) * | 2004-12-06 | 2005-01-05 | Isis Innovation | Pyrrolidine compounds |
| US7994221B2 (en) | 2004-12-06 | 2011-08-09 | Siga Technologies, Inc. | Sulfonyl semicarbazides, carbonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arenaviruses |
| US8642596B2 (en) | 2004-12-06 | 2014-02-04 | Siga Technologies, Inc. | Sulfonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arena viruses |
| US8410149B2 (en) | 2004-12-06 | 2013-04-02 | Siga Technologies Inc. | Sulfonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arenaviruses |
| US20060281689A1 (en) * | 2005-06-02 | 2006-12-14 | Schering Corporation | Method for modulating activity of HCV protease through use of a novel HCV protease inhibitor to reduce duration of treatment period |
| ES2572980T3 (es) | 2005-06-02 | 2016-06-03 | Merck Sharp & Dohme Corp. | Combinación de inhibidores de la proteasa del VHC con un tensioactivo |
| WO2006130666A2 (en) * | 2005-06-02 | 2006-12-07 | Schering Corporation | Medicaments and methods combining a hcv protease inhibitor and an akr competitor |
| EP1898941A2 (en) * | 2005-06-02 | 2008-03-19 | Schering Corporation | Controlled-release formulation useful for treating disorders associated with hepatitis c virus |
| US20060276405A1 (en) * | 2005-06-02 | 2006-12-07 | Schering Corporation | Methods for treating hepatitis C |
| WO2006130687A2 (en) * | 2005-06-02 | 2006-12-07 | Schering Corporation | Liver/plasma concentration ratio for dosing hepatitis c virus protease inhibitor |
| US20060275366A1 (en) * | 2005-06-02 | 2006-12-07 | Schering Corporation | Controlled-release formulation |
| US20060276407A1 (en) * | 2005-06-02 | 2006-12-07 | Schering Corporation | Methods of treating hepatitis C virus |
| US8119602B2 (en) | 2005-06-02 | 2012-02-21 | Schering Corporation | Administration of HCV protease inhibitors in combination with food to improve bioavailability |
| WO2006130552A2 (en) * | 2005-06-02 | 2006-12-07 | Schering Corporation | Methods of treating hepatitis c virus |
| US7608592B2 (en) * | 2005-06-30 | 2009-10-27 | Virobay, Inc. | HCV inhibitors |
| GEP20105124B (en) * | 2005-07-25 | 2010-11-25 | Array Biopharma Inc | Novel macrocyclic inhibitors of hepatitis c virus replication |
| PE20070211A1 (es) | 2005-07-29 | 2007-05-12 | Medivir Ab | Compuestos macrociclicos como inhibidores del virus de hepatitis c |
| US8399615B2 (en) * | 2005-08-19 | 2013-03-19 | Vertex Pharmaceuticals Incorporated | Processes and intermediates |
| DE602006013492D1 (de) * | 2005-08-19 | 2010-05-20 | Vertex Pharma | Verfahren und zwischenprodukte |
| US7964624B1 (en) | 2005-08-26 | 2011-06-21 | Vertex Pharmaceuticals Incorporated | Inhibitors of serine proteases |
| AR055395A1 (es) | 2005-08-26 | 2007-08-22 | Vertex Pharma | Compuestos inhibidores de la actividad de la serina proteasa ns3-ns4a del virus de la hepatitis c |
| BRPI0617274A2 (pt) | 2005-10-11 | 2011-07-19 | Intermune Inc | compostos e métodos para a inibição de replicação viral de hepatite c |
| AR056805A1 (es) * | 2005-11-14 | 2007-10-24 | Schering Corp | Un proceso para oxidacion para la preparcion de n- (3- amino-1- ( ciclobutilmetil) -2,3- dioxopropil ) -3-(n-(( ter-butilamino) carbonil )-3- metil- l- valil) -6,6- dimetil-3- azabiciclo (3.1.0) hexano -2- carboxamida y compuestos ralacionados |
| WO2007075790A1 (en) * | 2005-12-22 | 2007-07-05 | Schering Corporation | Process for the preparation of 6, 6-dimethyl-3-azabicyclo-[3.1.0]-hexane compounds and enantiomeric salts thereof |
| CN101336229B (zh) | 2005-12-23 | 2012-06-13 | 西兰岛药物有限公司 | 改性的拟赖氨酸化合物 |
| US7816348B2 (en) | 2006-02-03 | 2010-10-19 | Boehringer Ingelheim International Gmbh | Viral polymerase inhibitors |
| US8039475B2 (en) * | 2006-02-27 | 2011-10-18 | Vertex Pharmaceuticals Incorporated | Co-crystals and pharmaceutical compositions comprising the same |
| KR20080112303A (ko) | 2006-03-16 | 2008-12-24 | 버텍스 파마슈티칼스 인코포레이티드 | 중수소화 c형 간염 프로테아제 억제제 |
| KR101059593B1 (ko) * | 2006-04-11 | 2011-08-25 | 노파르티스 아게 | Hcv/hiv 억제제 및 이들의 용도 |
| WO2007120595A2 (en) * | 2006-04-11 | 2007-10-25 | Novartis Ag | Amines for the treatment of hcv |
| WO2007137080A2 (en) | 2006-05-23 | 2007-11-29 | Irm Llc | Compounds and compositions as channel activating protease inhibitors |
| RU2008152171A (ru) * | 2006-07-05 | 2010-08-10 | Интермьюн, Инк. (Us) | Новые ингибиторы вирусной репликации гепатита с |
| WO2008008776A2 (en) | 2006-07-11 | 2008-01-17 | Bristol-Myers Squibb Company | Hepatitis c virus inhibitors |
| US8329159B2 (en) * | 2006-08-11 | 2012-12-11 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US7659270B2 (en) * | 2006-08-11 | 2010-02-09 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US7759495B2 (en) * | 2006-08-11 | 2010-07-20 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| CN101506167A (zh) | 2006-08-17 | 2009-08-12 | 贝林格尔.英格海姆国际有限公司 | 病毒聚合酶抑制剂 |
| US8343477B2 (en) | 2006-11-01 | 2013-01-01 | Bristol-Myers Squibb Company | Inhibitors of hepatitis C virus |
| US7772180B2 (en) | 2006-11-09 | 2010-08-10 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US8003604B2 (en) | 2006-11-16 | 2011-08-23 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US7888464B2 (en) * | 2006-11-16 | 2011-02-15 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US7763584B2 (en) | 2006-11-16 | 2010-07-27 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| CA2673111A1 (en) * | 2006-12-07 | 2008-06-19 | Schering Corporation | Ph sensitive matrix formulation |
| US20080188545A1 (en) | 2006-12-21 | 2008-08-07 | Alimardanov Asaf R | Synthesis of pyrrolidine compounds |
| WO2008074035A1 (en) | 2006-12-27 | 2008-06-19 | Abbott Laboratories | Hcv protease inhibitors and uses thereof |
| BRPI0807483A2 (pt) * | 2007-02-09 | 2014-05-13 | Irm Llc | Compostos e composições como inibidores de protease de ativação de canal |
| PT2114924E (pt) | 2007-02-27 | 2012-04-03 | Vertex Pharma | Co-cristais e composições farmacêuticas que compreendem os mesmos |
| EP2134717A2 (en) | 2007-02-27 | 2009-12-23 | Vertex Pharmceuticals Incorporated | Inhibitors of serine proteases |
| WO2009008913A2 (en) * | 2007-03-23 | 2009-01-15 | Schering Corporation | P1-nonepimerizable ketoamide inhibitors of hcv ns3 protease |
| WO2008137779A2 (en) * | 2007-05-03 | 2008-11-13 | Intermune, Inc. | Novel macrocyclic inhibitors of hepatitis c virus replication |
| MX2009012093A (es) * | 2007-05-10 | 2010-01-25 | Intermune Inc | Nuevos peptidos inhibidores de la replicacion del virus de la hepatitis c. |
| EP2164847B1 (en) | 2007-07-03 | 2011-09-14 | Actelion Pharmaceuticals Ltd. | 3-aza-bicyclo[3.3.0]octane compounds |
| KR20100046047A (ko) | 2007-07-27 | 2010-05-04 | 액테리온 파마슈티칼 리미티드 | 2-아자-비시클로[3.3.0]옥탄 유도체 |
| US8242140B2 (en) | 2007-08-03 | 2012-08-14 | Boehringer Ingelheim International Gmbh | Viral polymerase inhibitors |
| JP5443360B2 (ja) | 2007-08-30 | 2014-03-19 | バーテックス ファーマシューティカルズ インコーポレイテッド | 共結晶体およびそれを含む医薬組成物 |
| JP5095824B2 (ja) | 2007-10-10 | 2012-12-12 | ノバルティス アーゲー | スピロピロリジン類およびhcvおよびhiv感染に対するその使用 |
| BRPI0821342A2 (pt) | 2007-12-19 | 2019-09-24 | Boehringer Ingelheim Int | inibidores da polimerase viral |
| JP5203468B2 (ja) * | 2007-12-21 | 2013-06-05 | メルク・シャープ・アンド・ドーム・コーポレーション | 3−アミノ−3−シクロブチルメチル−2−ヒドロキシプロピオンアミドまたはその塩の合成のための方法 |
| US8202996B2 (en) | 2007-12-21 | 2012-06-19 | Bristol-Myers Squibb Company | Crystalline forms of N-(tert-butoxycarbonyl)-3-methyl-L-valyl-(4R)-4-((7-chloro-4-methoxy-1-isoquinolinyl)oxy)-N- ((1R,2S)-1-((cyclopropylsulfonyl)carbamoyl)-2-vinylcyclopropyl)-L-prolinamide |
| MX2010010871A (es) | 2008-04-01 | 2010-10-26 | Astellas Pharma Inc | Compuesto de indolinona. |
| CN102046622A (zh) * | 2008-04-15 | 2011-05-04 | 因特蒙公司 | 丙型肝炎病毒复制的新颖大环抑制剂 |
| US8163921B2 (en) | 2008-04-16 | 2012-04-24 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US8044023B2 (en) | 2008-05-29 | 2011-10-25 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US7964560B2 (en) | 2008-05-29 | 2011-06-21 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| ES2535736T3 (es) | 2008-06-10 | 2015-05-14 | Novartis Ag | Derivados de pirazina como bloqueadores de los canales de sodio epitelial |
| CN102816106A (zh) | 2008-06-24 | 2012-12-12 | 默沙东公司 | 用于制备基本上立体异构纯的稠合二环脯氨酸化合物的生物催化方法 |
| EP2303858A1 (en) * | 2008-07-03 | 2011-04-06 | Vertex Pharmceuticals Incorporated | Preparation of protected alpha-keto beta-amino esters and amides |
| US8188137B2 (en) | 2008-08-15 | 2012-05-29 | Avila Therapeutics, Inc. | HCV protease inhibitors and uses thereof |
| US8207341B2 (en) | 2008-09-04 | 2012-06-26 | Bristol-Myers Squibb Company | Process or synthesizing substituted isoquinolines |
| US8044087B2 (en) | 2008-09-29 | 2011-10-25 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US8563505B2 (en) | 2008-09-29 | 2013-10-22 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US8283310B2 (en) | 2008-12-15 | 2012-10-09 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| CN102271699A (zh) | 2009-01-07 | 2011-12-07 | 西尼克斯公司 | 用于治疗hcv和hiv感染的环孢菌素衍生物 |
| AR075584A1 (es) * | 2009-02-27 | 2011-04-20 | Intermune Inc | COMPOSICIONES TERAPEUTICAS QUE COMPRENDEN beta-D-2'-DESOXI-2'-FLUORO-2'-C-METILCITIDINA Y UN DERIVADO DE ACIDO ISOINDOL CARBOXILICO Y SUS USOS. COMPUESTO. |
| US20110182850A1 (en) * | 2009-04-10 | 2011-07-28 | Trixi Brandl | Organic compounds and their uses |
| US8512690B2 (en) | 2009-04-10 | 2013-08-20 | Novartis Ag | Derivatised proline containing peptide compounds as protease inhibitors |
| WO2010138889A1 (en) | 2009-05-28 | 2010-12-02 | Concert Pharmaceuticals, Inc. | Peptides for the treatment of hcv infections |
| US8980920B2 (en) | 2009-05-29 | 2015-03-17 | Merck Sharp & Dohme Corp. | Antiviral compounds of three linked aryl moieties to treat diseases such as hepatitis C |
| AR077139A1 (es) | 2009-06-23 | 2011-08-03 | Gilead Sciences Inc | Composiciones farmaceuticas utiles para tratar el vch |
| EP2477980B1 (en) | 2009-09-15 | 2016-06-08 | Taigen Biotechnology Co., Ltd. | Hcv protease inhibitors |
| CN105001302A (zh) * | 2009-09-28 | 2015-10-28 | 英特穆恩公司 | C型肝炎病毒复制的环肽抑制剂 |
| EP2503881B1 (en) | 2009-11-25 | 2015-05-13 | Merck Sharp & Dohme Corp. | Fused tricyclic compounds and derivatives thereof useful for the treatment of viral diseases |
| US20130156731A1 (en) | 2009-12-22 | 2013-06-20 | Kevin X. Chen | Fused tricyclic compounds and methods of use thereof for the treatment of viral diseas |
| EP2539334A1 (en) | 2010-02-25 | 2013-01-02 | Vereniging Voor Christelijk Hoger Onderwijs, Wetenschappelijk Onderzoek En Patiëntenzorg | Process for the preparation of -acyloxy -formamido amides |
| US8609635B2 (en) | 2010-03-09 | 2013-12-17 | Merck Sharp & Dohme Corp. | Fused tricyclic silyl compounds and methods of use thereof for the treatment of viral diseases |
| AU2011286276A1 (en) | 2010-07-26 | 2013-01-24 | Merck Sharp & Dohme Corp. | Substituted biphenylene compounds and methods of use thereof for the treatment of viral diseases |
| CA2812779A1 (en) | 2010-09-29 | 2012-04-19 | Merck Sharp & Dohme Corp. | Fused tetracycle derivatives and methods of use thereof for the treatment of viral diseases |
| CN102010425B (zh) * | 2010-11-04 | 2013-04-10 | 山东大学 | 1,4-二硫-7-氮杂螺[4,4]壬烷-8-羧酸衍生物类组蛋白去乙酰化酶抑制剂及其应用 |
| EA201391519A1 (ru) | 2011-04-13 | 2014-03-31 | Мерк Шарп И Доум Корп. | 2'-замещенные нуклеозидные производные и способы их применения для лечения вирусных заболеваний |
| WO2012142075A1 (en) | 2011-04-13 | 2012-10-18 | Merck Sharp & Dohme Corp. | 2'-azido substituted nucleoside derivatives and methods of use thereof for the treatment of viral diseases |
| US8957203B2 (en) | 2011-05-05 | 2015-02-17 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US8691757B2 (en) | 2011-06-15 | 2014-04-08 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US8822679B2 (en) * | 2011-06-24 | 2014-09-02 | California Institute Of Technology | Quaternary heteroatom containing compounds |
| US20130267699A1 (en) | 2011-06-24 | 2013-10-10 | California Institute Of Technology | Quaternary heteroatom containing compounds |
| WO2013033900A1 (en) | 2011-09-08 | 2013-03-14 | Merck Sharp & Dohme Corp. | Tetracyclic heterocycle compounds and methods of use thereof for the treatment of viral diseases |
| WO2013033899A1 (en) | 2011-09-08 | 2013-03-14 | Merck Sharp & Dohme Corp. | Substituted benzofuran compounds and methods of use thereof for the treatment of viral diseases |
| WO2013033901A1 (en) | 2011-09-08 | 2013-03-14 | Merck Sharp & Dohme Corp. | Heterocyclic-substituted benzofuran derivatives and methods of use thereof for the treatment of viral diseases |
| WO2013039876A1 (en) | 2011-09-14 | 2013-03-21 | Merck Sharp & Dohme Corp. | Silyl-containing heterocyclic compounds and methods of use thereof for the treatment of viral diseases |
| PT107924A (pt) | 2011-10-21 | 2014-12-03 | Abbvie Inc | Tratamento de combinação de daa (eg. com abt-072 ou abt-333) para utilização no tratamento de hcv |
| US8466159B2 (en) | 2011-10-21 | 2013-06-18 | Abbvie Inc. | Methods for treating HCV |
| ES2572329B1 (es) | 2011-10-21 | 2017-08-24 | Abbvie Inc. | Combinación de al menos dos agentes antivirales de acción directa y ribavirina pero no interferón, para uso en el tratamientodel vhc |
| US8492386B2 (en) | 2011-10-21 | 2013-07-23 | Abbvie Inc. | Methods for treating HCV |
| US20130123325A1 (en) | 2011-11-14 | 2013-05-16 | Sven Ruf | Use of boceprevir and related compounds in atherosclerosis, heart failure, renal diseases, liver diseases or inflammatory diseases |
| WO2013178682A2 (en) | 2012-05-30 | 2013-12-05 | Chemo Ibérica, S.A. | Multicomponent process for the preparation of bicyclic compounds |
| WO2014053533A1 (en) | 2012-10-05 | 2014-04-10 | Sanofi | Use of substituted 3-heteroaroylamino-propionic acid derivatives as pharmaceuticals for prevention/treatment of atrial fibrillation |
| WO2014062196A1 (en) | 2012-10-19 | 2014-04-24 | Bristol-Myers Squibb Company | Hepatitis c virus inhibitors |
| WO2014071007A1 (en) | 2012-11-02 | 2014-05-08 | Bristol-Myers Squibb Company | Hepatitis c virus inhibitors |
| US9643999B2 (en) | 2012-11-02 | 2017-05-09 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| EP2914613B1 (en) | 2012-11-02 | 2017-11-22 | Bristol-Myers Squibb Company | Hepatitis c virus inhibitors |
| WO2014070974A1 (en) | 2012-11-05 | 2014-05-08 | Bristol-Myers Squibb Company | Hepatitis c virus inhibitors |
| EP2964664B1 (en) | 2013-03-07 | 2017-01-11 | Bristol-Myers Squibb Company | Hepatitis c virus inhibitors |
| EP3184095A1 (en) | 2013-05-23 | 2017-06-28 | IP Gesellschaft für Management mbH | Administration units comprising polymorph 1 of 2-(2-methylamino-pyrimidin-4-yl]-1h-indole-5-carboxylic acid [(s)-1-carbamoyl-2-(phenyl-pyrimidin-2-yl-amino)-ethyl]-amide |
| CN103387510B (zh) * | 2013-08-08 | 2015-09-09 | 苏州永健生物医药有限公司 | 一种β-氨基-alpha-羟基环丁基丁酰胺盐酸盐的合成方法 |
| EP3063140A4 (en) | 2013-10-30 | 2017-11-08 | Merck Sharp & Dohme Corp. | Pseudopolymorphs of an hcv ns5a inhibitor and uses thereof |
| TWI659019B (zh) | 2014-02-28 | 2019-05-11 | 日商帝人製藥股份有限公司 | 吡唑醯胺衍生物 |
| US10421696B2 (en) | 2014-12-18 | 2019-09-24 | California Institute Of Technology | Enantioselective synthesis of α-quaternary mannich adducts by palladium-catalyzed allylic alkylation |
| EP3274342B1 (en) | 2015-03-27 | 2022-05-04 | California Institute of Technology | Asymmetric catalytic decarboxylative alkyl alkylation using low catalyst concentrations and a robust precatalyst |
| WO2017156239A1 (en) | 2016-03-11 | 2017-09-14 | California Institute Of Technology | Compositions and methods for acylating lactams |
| CA3022119A1 (en) | 2016-04-28 | 2017-11-02 | Emory University | Alkyne containing nucleotide and nucleoside therapeutic compositions and uses related thereto |
| WO2017222915A1 (en) | 2016-06-21 | 2017-12-28 | Inception 4, Inc. | Heterocyclic prolinamide derivatives |
| EP3472151A4 (en) | 2016-06-21 | 2020-03-04 | Orion Ophthalmology LLC | CARBOCYCLIC PROLINAMIDE DERIVATIVES |
| BR112018076766A2 (pt) | 2016-06-21 | 2019-04-02 | Orion Ophthalmology LLC | derivados de prolinamida alifática |
| US11324799B2 (en) | 2017-05-05 | 2022-05-10 | Zealand Pharma A/S | Gap junction intercellular communication modulators and their use for the treatment of diabetic eye disease |
| EP3480190B1 (en) | 2017-11-01 | 2023-01-04 | California Institute of Technology | Methods for enantioselective allylic alkylation of esters, lactones, and lactams with unactivated allylic alcohols |
| EP3699173A1 (en) | 2018-10-18 | 2020-08-26 | California Institute of Technology | Gem-disubstituted pyrrolidines, piperazines, and diazepanes, and compositions and methods of making the same |
| WO2021226546A1 (en) * | 2020-05-08 | 2021-11-11 | The Board Of Trustees Of The Leland Stanford Junior University | Protease inhibitors for treatment or prevention of coronavirus disease |
| US11351149B2 (en) | 2020-09-03 | 2022-06-07 | Pfizer Inc. | Nitrile-containing antiviral compounds |
| CA3213008A1 (en) * | 2021-04-01 | 2022-10-06 | Miles Stuart Congreve | Sars-cov-2 mpro inhibitor compounds |
| EP4366831A4 (en) | 2021-07-09 | 2025-06-11 | Aligos Therapeutics, Inc. | ANTI-VIRAL COMPOUNDS |
| CN114149415A (zh) * | 2021-07-26 | 2022-03-08 | 中国药科大学 | 一种拟肽类化合物及其衍生物、制备方法、药物组合物和应用 |
| CN117836272B (zh) * | 2021-08-02 | 2025-02-28 | 北京华益健康药物研究中心 | 用于治疗或预防冠状病毒感染的3cl蛋白酶小分子抑制剂及其用途 |
| US12065428B2 (en) | 2021-09-17 | 2024-08-20 | Aligos Therapeutics, Inc. | Anti-viral compounds |
| WO2023133174A1 (en) * | 2022-01-07 | 2023-07-13 | Merck Sharp & Dohme Llc | Protease inhibitors for treating or preventing coronavirus infection |
| WO2024151465A1 (en) * | 2023-01-09 | 2024-07-18 | Merck Sharp & Dohme Llc | Protease inhibitors for treating or preventing coronavirus infection |
| WO2025201636A1 (en) | 2023-03-31 | 2025-10-02 | Syngenta Crop Protection Ag | Pesticidally-active 2,2-dihalocyclopropyl compounds |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU93036735A (ru) * | 1993-07-16 | 1996-04-27 | Малое предприятие "Рохат" | Пептидные антигены вируса гепатита с, их производные и способ получения диагностикума на их основе |
| WO2001040262A1 (en) * | 1999-12-03 | 2001-06-07 | Bristol-Myers Squibb Pharma Company | Alpha-ketoamide inhibitors of hepatitis c virus ns3 protease |
Family Cites Families (39)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA1341029C (en) | 1985-02-04 | 2000-06-20 | Michael Kolb | Peptidase inhibitors |
| US5496927A (en) * | 1985-02-04 | 1996-03-05 | Merrell Pharmaceuticals Inc. | Peptidase inhibitors |
| HU216017B (hu) | 1987-11-18 | 1999-04-28 | Chiron Corp. | Eljárás HCV-1 polipeptidek, HCV-1 polinukleotidok, rekombináns vektorok és gazdasejtek, valamint immunesszé kit, hepatitis C vírusfertőzés elleni vakcinák, a fertőzés kimutatására szolgáló diagnosztikumok előállítására, és immunvizsgálati és vírustenyészt |
| ZA897514B (en) | 1988-10-07 | 1990-06-27 | Merrell Dow Pharma | Novel peptidase inhibitors |
| ATE132182T1 (de) | 1989-02-01 | 1996-01-15 | Asahi Glass Co Ltd | Azeotrope oder azeotropähnliche zusammensetzung auf der basis von chlorfluorkohlenwasserstoffen |
| US5359138A (en) * | 1989-04-15 | 1994-10-25 | Zaidan Hojin Biseibutsu Kagaku Kenkyu Kai | Poststatin and related compounds or salts thereof |
| WO1990012805A1 (fr) * | 1989-04-15 | 1990-11-01 | Zaidan Hojin Biseibutsu Kagaku Kenkyu Kai | Postostatine et son compose connexe, ou leurs sels |
| DK0527788T3 (da) | 1990-04-04 | 2004-09-06 | Chiron Corp | Hepatitis C virus protease |
| JP2804817B2 (ja) | 1990-04-13 | 1998-09-30 | 財団法人微生物化学研究会 | 3―アミノ―2―オキソ脂肪酸誘導体の製造法 |
| JPH04149166A (ja) | 1990-10-12 | 1992-05-22 | Nippon Kayaku Co Ltd | 新規ケト酸アミド誘導体 |
| JPH06504061A (ja) | 1990-12-28 | 1994-05-12 | コーテックス ファーマシューティカルズ インコーポレイテッド | 神経変性の治療および予防におけるカルパイン阻害剤の使用 |
| WO1993015193A1 (en) * | 1992-01-31 | 1993-08-05 | Abbott Laboratories | Mammalian expression systems for hcv proteins |
| CA2138124A1 (en) | 1992-06-24 | 1994-01-06 | David D. Eveleth, Jr. | Use of calpain inhibitors in the inhibition and treatment of medical conditions associated with increased calpain activity |
| US5514694A (en) * | 1992-09-21 | 1996-05-07 | Georgia Tech Research Corp | Peptidyl ketoamides |
| US5414018A (en) * | 1993-09-24 | 1995-05-09 | G. D. Searle & Co. | Alkylaminoalkyl-terminated sulfide/sulfonyl-containing propargyl amino-diol compounds for treatment of hypertension |
| US5843450A (en) * | 1994-02-14 | 1998-12-01 | Abbott Laboratories | Hepatitis GB Virus synthetic peptides and uses thereof |
| IT1272179B (it) * | 1994-02-23 | 1997-06-16 | Angeletti P Ist Richerche Bio | Metodologia per riprodurre in vitro l'attivita' proteolitica della proteasi ns3 del virus hcv. |
| US5843752A (en) * | 1995-05-12 | 1998-12-01 | Schering Corporation | Soluble active hepatitis C virus protease |
| US5919765A (en) | 1995-06-07 | 1999-07-06 | Cor Therapeutics, Inc. | Inhibitors of factor XA |
| GB9517022D0 (en) | 1995-08-19 | 1995-10-25 | Glaxo Group Ltd | Medicaments |
| US5763576A (en) * | 1995-10-06 | 1998-06-09 | Georgia Tech Research Corp. | Tetrapeptide α-ketoamides |
| IL120310A (en) | 1996-03-01 | 2002-02-10 | Akzo Nobel Nv | Serine protease inhibitors and pharmaceuticals containing them |
| US5633388A (en) * | 1996-03-29 | 1997-05-27 | Viropharma Incorporated | Compounds, compositions and methods for treatment of hepatitis C |
| IT1285158B1 (it) | 1996-09-17 | 1998-06-03 | Angeletti P Ist Richerche Bio | Polipeptidi solubili con l'attivita' di serino-proteasi di ns3 del virus dell'epatite c, e procedimento per la loro preparazione e il |
| JP2000506933A (ja) | 1996-09-24 | 2000-06-06 | ザ、プロクター、エンド、ギャンブル、カンパニー | タンパク質分解酵素、ペプチドアルデヒドおよびホウ酸源を含有した液体洗剤 |
| US5922757A (en) | 1996-09-30 | 1999-07-13 | The Regents Of The University Of California | Treatment and prevention of hepatic disorders |
| CN1133649C (zh) * | 1996-10-18 | 2004-01-07 | 沃泰克斯药物股份有限公司 | 丝氨酸蛋白酶、特别是丙型肝炎病毒ns3蛋白酶的抑制剂 |
| WO1998029435A1 (en) | 1996-12-27 | 1998-07-09 | Boehringer Ingelheim (Canada) Ltd. | Peptidomimetic inhibitors of the human cytomegalovirus protease |
| WO1998037180A2 (en) | 1997-02-22 | 1998-08-27 | Abbott Laboratories | Hcv fusion protease and polynucleotide encoding same |
| US6143715A (en) | 1997-08-11 | 2000-11-07 | Boehringer Ingelheim (Canada) Ltd. | Hepatitis C inhibitor peptide analogues |
| EP1003775B1 (en) | 1997-08-11 | 2005-03-16 | Boehringer Ingelheim (Canada) Ltd. | Hepatitis c inhibitor peptides |
| GB9809664D0 (en) | 1998-05-06 | 1998-07-01 | Hoffmann La Roche | a-Ketoamide derivatives |
| GB9812523D0 (en) | 1998-06-10 | 1998-08-05 | Angeletti P Ist Richerche Bio | Peptide inhibitors of hepatitis c virus ns3 protease |
| US6576613B1 (en) * | 1998-07-24 | 2003-06-10 | Corvas International, Inc. | Title inhibitors of urokinase |
| JP2000256396A (ja) | 1999-03-03 | 2000-09-19 | Dainippon Pharmaceut Co Ltd | 複素環式化合物およびその中間体ならびにエラスターゼ阻害剤 |
| ATE297946T1 (de) | 2000-04-03 | 2005-07-15 | Vertex Pharma | Inhibitoren von serin proteasen, speziell der hepatitis-c-virus ns3-protease |
| HK1047947A1 (zh) * | 2000-04-05 | 2003-03-14 | Schering Corporation | 包含n-环状p2部分的丙型肝炎病毒的大环ns3-丝氨酸蛋白酶抑制剂 |
| SI1385870T1 (sl) * | 2000-07-21 | 2010-08-31 | Schering Corp | Peptidi kot NS3-serin proteazni inhibitorji virusa hepatitisa C |
| SV2003000617A (es) | 2000-08-31 | 2003-01-13 | Lilly Co Eli | Inhibidores de la proteasa peptidomimetica ref. x-14912m |
-
2002
- 2002-01-18 US US10/052,386 patent/US7244721B2/en not_active Expired - Lifetime
-
2003
- 2003-01-16 CN CNA038059339A patent/CN1633446A/zh active Pending
- 2003-01-16 DE DE60332814T patent/DE60332814D1/de not_active Expired - Lifetime
- 2003-01-16 CN CN201010143451A patent/CN101792483A/zh active Pending
- 2003-01-16 SI SI200331853T patent/SI1481000T1/sl unknown
- 2003-01-16 BR BR0306931-1A patent/BR0306931A/pt not_active IP Right Cessation
- 2003-01-16 MX MXPA04006934A patent/MXPA04006934A/es active IP Right Grant
- 2003-01-16 RU RU2004125279/04A patent/RU2404189C9/ru not_active IP Right Cessation
- 2003-01-16 PL PL03372747A patent/PL372747A1/xx not_active IP Right Cessation
- 2003-01-16 KR KR1020047011022A patent/KR101020355B1/ko not_active Expired - Fee Related
- 2003-01-16 AT AT03731956T patent/ATE469914T1/de active
- 2003-01-16 CA CA002473032A patent/CA2473032A1/en not_active Abandoned
- 2003-01-16 TW TW092100868A patent/TWI334872B/zh not_active IP Right Cessation
- 2003-01-16 EP EP03731956A patent/EP1481000B1/en not_active Expired - Lifetime
- 2003-01-16 PT PT03731956T patent/PT1481000E/pt unknown
- 2003-01-16 DK DK03731956.3T patent/DK1481000T3/da active
- 2003-01-16 PE PE2003000049A patent/PE20030852A1/es not_active Application Discontinuation
- 2003-01-16 NZ NZ571073A patent/NZ571073A/en not_active IP Right Cessation
- 2003-01-16 IL IL16281503A patent/IL162815A0/xx unknown
- 2003-01-16 JP JP2003562142A patent/JP4563033B2/ja not_active Expired - Fee Related
- 2003-01-16 MY MYPI20030137A patent/MY140710A/en unknown
- 2003-01-16 WO PCT/US2003/001430 patent/WO2003062265A2/en not_active Ceased
- 2003-01-17 AR ARP030100131A patent/AR038183A1/es unknown
-
2004
- 2004-07-02 NO NO20042792A patent/NO20042792L/no not_active Application Discontinuation
- 2004-07-02 ZA ZA200405304A patent/ZA200405304B/en unknown
- 2004-07-15 EC EC2004005191A patent/ECSP045191A/es unknown
- 2004-07-16 CO CO04068146A patent/CO5650168A2/es not_active Application Discontinuation
-
2007
- 2007-03-06 US US11/714,457 patent/US7592316B2/en not_active Expired - Lifetime
-
2009
- 2009-08-21 AU AU2009210423A patent/AU2009210423B2/en not_active Ceased
-
2010
- 2010-09-01 CY CY20101100797T patent/CY1111225T1/el unknown
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU93036735A (ru) * | 1993-07-16 | 1996-04-27 | Малое предприятие "Рохат" | Пептидные антигены вируса гепатита с, их производные и способ получения диагностикума на их основе |
| WO2001040262A1 (en) * | 1999-12-03 | 2001-06-07 | Bristol-Myers Squibb Pharma Company | Alpha-ketoamide inhibitors of hepatitis c virus ns3 protease |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2625793C2 (ru) * | 2011-12-23 | 2017-07-19 | Ипсен Мэньюфэкчеринг Айэлэнд Лимитед | Способ синтеза терапевтических пептидов |
| RU2794746C1 (ru) * | 2022-04-15 | 2023-04-24 | Общество С Ограниченной Ответственностью "Промомед Рус" | Способ получения (1r,2s,5s)-n-[(1s)-1-циано-2-[(3s)-2-оксопирролидин-3-ил]этил]-3-[(2s)-3,3-диметил-2-[(2,2,2-трифторацетил)амино]бутаноил]-6,6-диметил-3-азабицикло[3.1.0]гексан-2-карбоксамида |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2404189C9 (ru) | Новые пептиды как ингибиторы ns3-серинпротеазы вируса гепатита c | |
| RU2003105217A (ru) | Новые пептиды, как ингибиторы ns3-серинпротеазы вируса гепатита с | |
| RU2006134005A (ru) | Новые соединения, действующие как ингибиторы сериновой протеазы ns3 вируса гепатита с | |
| JP2004504404A5 (enExample) | ||
| RU2006134003A (ru) | Серосодержащие соединения, действующие как ингибиторы сериновой протеазы ns3 вируса гепатита с | |
| RU2006134002A (ru) | Новые соединения, действующие как ингибиторы сериновой протеазы ns3 вируса гепатита с | |
| RU2355700C9 (ru) | Новые пептиды как ингибиторы ns3-серинпротеазы вируса гепатита с | |
| RU2006134000A (ru) | Новые кетоамиды с циклическим p4s, действующие как ингибиторы сериновой протеазы ns3 ируса гепатита с | |
| RU2006124594A (ru) | Ингибиторы сериновой протеазы ns3/ns4а вируса гепатита с | |
| JP2007525510A5 (enExample) | ||
| JP2007535496A5 (enExample) | ||
| JP2005504042A5 (enExample) | ||
| JP2007535491A5 (enExample) | ||
| JP2004513881A5 (enExample) | ||
| JP2005524628A5 (enExample) | ||
| JP2007525514A5 (enExample) | ||
| RU2003105221A (ru) | Новые пептиды, как ингибиторы ns3-серинпротеазы вируса гепатита с | |
| JP2007525512A5 (enExample) | ||
| ATE447573T1 (de) | Chinoxalinyl-makrocyclische hepatitis c serin protease-hemmer | |
| EP2341065A3 (en) | Inhibitors of serine proteases, particularly HCV NS3-NS4a protease | |
| JP2007515405A5 (enExample) | ||
| JP2007513971A5 (enExample) | ||
| RU2002123350A (ru) | Дипептиднитрильные ингибиторы катепсина К | |
| EP1958956A3 (en) | Peptidomimetic protease inhibitors | |
| AR029150A1 (es) | Un derivado de piperidina, su empleo, un procedimiento para prepararlo y una composicin farmaceutica que lo comprende. |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| TH4A | Reissue of patent specification | ||
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20130117 |