KR20190058509A - 융합 단백질을 이용하는 t-세포 수용체 리프로그래밍을 위한 조성물 및 방법 - Google Patents
융합 단백질을 이용하는 t-세포 수용체 리프로그래밍을 위한 조성물 및 방법 Download PDFInfo
- Publication number
- KR20190058509A KR20190058509A KR1020197009508A KR20197009508A KR20190058509A KR 20190058509 A KR20190058509 A KR 20190058509A KR 1020197009508 A KR1020197009508 A KR 1020197009508A KR 20197009508 A KR20197009508 A KR 20197009508A KR 20190058509 A KR20190058509 A KR 20190058509A
- Authority
- KR
- South Korea
- Prior art keywords
- tcr
- domain
- tfp
- cell
- cells
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
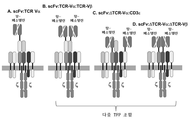
- 108091008874 T cell receptors Proteins 0.000 title claims abstract description 354
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 title claims abstract description 354
- 238000000034 method Methods 0.000 title claims abstract description 172
- 108020001507 fusion proteins Proteins 0.000 title claims abstract description 25
- 102000037865 fusion proteins Human genes 0.000 title claims abstract description 25
- 239000000203 mixture Substances 0.000 title description 37
- 230000008672 reprogramming Effects 0.000 title 1
- 210000001744 T-lymphocyte Anatomy 0.000 claims abstract description 284
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 120
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 85
- 201000011510 cancer Diseases 0.000 claims abstract description 80
- 201000010099 disease Diseases 0.000 claims abstract description 69
- 210000004027 cell Anatomy 0.000 claims description 400
- 230000027455 binding Effects 0.000 claims description 172
- 150000007523 nucleic acids Chemical class 0.000 claims description 161
- 241000282414 Homo sapiens Species 0.000 claims description 148
- 102000039446 nucleic acids Human genes 0.000 claims description 143
- 108020004707 nucleic acids Proteins 0.000 claims description 143
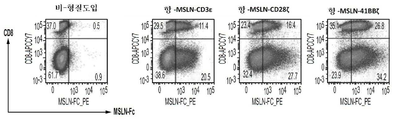
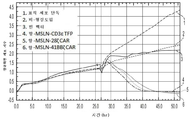
- 108090000015 Mesothelin Proteins 0.000 claims description 132
- 102000003735 Mesothelin Human genes 0.000 claims description 131
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 120
- 239000013598 vector Substances 0.000 claims description 117
- 239000000427 antigen Substances 0.000 claims description 116
- 108091007433 antigens Proteins 0.000 claims description 116
- 102000036639 antigens Human genes 0.000 claims description 116
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 112
- 229920001184 polypeptide Polymers 0.000 claims description 104
- 108090000623 proteins and genes Proteins 0.000 claims description 102
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 90
- 230000014509 gene expression Effects 0.000 claims description 79
- 102000004169 proteins and genes Human genes 0.000 claims description 63
- 230000004068 intracellular signaling Effects 0.000 claims description 60
- 102000008394 Immunoglobulin Fragments Human genes 0.000 claims description 56
- 108010021625 Immunoglobulin Fragments Proteins 0.000 claims description 55
- 108020004414 DNA Proteins 0.000 claims description 52
- 238000000338 in vitro Methods 0.000 claims description 47
- 241000124008 Mammalia Species 0.000 claims description 44
- 102100034922 T-cell surface glycoprotein CD8 alpha chain Human genes 0.000 claims description 44
- 102100036011 T-cell surface glycoprotein CD4 Human genes 0.000 claims description 42
- 230000003834 intracellular effect Effects 0.000 claims description 41
- 230000002401 inhibitory effect Effects 0.000 claims description 40
- 125000003729 nucleotide group Chemical group 0.000 claims description 40
- 239000003795 chemical substances by application Substances 0.000 claims description 39
- 108010019670 Chimeric Antigen Receptors Proteins 0.000 claims description 38
- 108020004999 messenger RNA Proteins 0.000 claims description 37
- 108091028043 Nucleic acid sequence Proteins 0.000 claims description 35
- 239000002773 nucleotide Substances 0.000 claims description 33
- 150000001413 amino acids Chemical class 0.000 claims description 31
- 239000012634 fragment Substances 0.000 claims description 31
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 claims description 30
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 claims description 30
- 230000011664 signaling Effects 0.000 claims description 30
- -1 LIGHT Proteins 0.000 claims description 27
- 230000000694 effects Effects 0.000 claims description 27
- 230000004048 modification Effects 0.000 claims description 22
- 238000012986 modification Methods 0.000 claims description 22
- 230000004936 stimulating effect Effects 0.000 claims description 22
- 230000004913 activation Effects 0.000 claims description 20
- 206010033128 Ovarian cancer Diseases 0.000 claims description 18
- 102000004127 Cytokines Human genes 0.000 claims description 17
- 108090000695 Cytokines Proteins 0.000 claims description 17
- 239000013612 plasmid Substances 0.000 claims description 17
- 230000019491 signal transduction Effects 0.000 claims description 16
- 230000000638 stimulation Effects 0.000 claims description 16
- 241000713666 Lentivirus Species 0.000 claims description 14
- 108091036407 Polyadenylation Proteins 0.000 claims description 14
- 239000003446 ligand Substances 0.000 claims description 14
- 206010027406 Mesothelioma Diseases 0.000 claims description 13
- 239000012528 membrane Substances 0.000 claims description 13
- 206010061535 Ovarian neoplasm Diseases 0.000 claims description 12
- 208000005718 Stomach Neoplasms Diseases 0.000 claims description 12
- 206010017758 gastric cancer Diseases 0.000 claims description 12
- 201000011549 stomach cancer Diseases 0.000 claims description 12
- 108020005345 3' Untranslated Regions Proteins 0.000 claims description 11
- 102100027207 CD27 antigen Human genes 0.000 claims description 11
- 101000914511 Homo sapiens CD27 antigen Proteins 0.000 claims description 11
- 206010052747 Adenocarcinoma pancreas Diseases 0.000 claims description 10
- 101000809875 Homo sapiens TYRO protein tyrosine kinase-binding protein Proteins 0.000 claims description 10
- 102100038717 TYRO protein tyrosine kinase-binding protein Human genes 0.000 claims description 10
- 102100022153 Tumor necrosis factor receptor superfamily member 4 Human genes 0.000 claims description 10
- 201000002094 pancreatic adenocarcinoma Diseases 0.000 claims description 10
- 108091032973 (ribonucleotides)n+m Proteins 0.000 claims description 9
- 102100038080 B-cell receptor CD22 Human genes 0.000 claims description 9
- 101000884305 Homo sapiens B-cell receptor CD22 Proteins 0.000 claims description 9
- ZUBDGKVDJUIMQQ-UBFCDGJISA-N endothelin-1 Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(O)=O)NC(=O)[C@H]1NC(=O)[C@H](CC=2C=CC=CC=2)NC(=O)[C@@H](CC=2C=CC(O)=CC=2)NC(=O)[C@H](C(C)C)NC(=O)[C@H]2CSSC[C@@H](C(N[C@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N2)=O)NC(=O)[C@@H](CO)NC(=O)[C@H](N)CSSC1)C1=CNC=N1 ZUBDGKVDJUIMQQ-UBFCDGJISA-N 0.000 claims description 9
- 230000035515 penetration Effects 0.000 claims description 9
- 108050009340 Endothelin Proteins 0.000 claims description 8
- 102000002045 Endothelin Human genes 0.000 claims description 8
- 101000917839 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-B Proteins 0.000 claims description 8
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 claims description 8
- 102100029185 Low affinity immunoglobulin gamma Fc region receptor III-B Human genes 0.000 claims description 8
- 206010058467 Lung neoplasm malignant Diseases 0.000 claims description 8
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 8
- 230000005809 anti-tumor immunity Effects 0.000 claims description 8
- 201000005202 lung cancer Diseases 0.000 claims description 8
- 208000020816 lung neoplasm Diseases 0.000 claims description 8
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 8
- 238000004519 manufacturing process Methods 0.000 claims description 8
- 201000002528 pancreatic cancer Diseases 0.000 claims description 8
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 8
- 230000002062 proliferating effect Effects 0.000 claims description 8
- 230000001177 retroviral effect Effects 0.000 claims description 8
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 claims description 8
- 206010006187 Breast cancer Diseases 0.000 claims description 7
- 101000917858 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 claims description 7
- 108010073807 IgG Receptors Proteins 0.000 claims description 7
- 102000009490 IgG Receptors Human genes 0.000 claims description 7
- 239000003814 drug Substances 0.000 claims description 7
- 102000005962 receptors Human genes 0.000 claims description 7
- 108020003175 receptors Proteins 0.000 claims description 7
- 102100029360 Hematopoietic cell signal transducer Human genes 0.000 claims description 6
- 102100026122 High affinity immunoglobulin gamma Fc receptor I Human genes 0.000 claims description 6
- 101000990188 Homo sapiens Hematopoietic cell signal transducer Proteins 0.000 claims description 6
- 101000913074 Homo sapiens High affinity immunoglobulin gamma Fc receptor I Proteins 0.000 claims description 6
- 101000851376 Homo sapiens Tumor necrosis factor receptor superfamily member 8 Proteins 0.000 claims description 6
- 102100025390 Integrin beta-2 Human genes 0.000 claims description 6
- 108091093037 Peptide nucleic acid Proteins 0.000 claims description 6
- 108010003723 Single-Domain Antibodies Proteins 0.000 claims description 6
- 102100025237 T-cell surface antigen CD2 Human genes 0.000 claims description 6
- 101710165473 Tumor necrosis factor receptor superfamily member 4 Proteins 0.000 claims description 6
- 102100036857 Tumor necrosis factor receptor superfamily member 8 Human genes 0.000 claims description 6
- 241000701161 unidentified adenovirus Species 0.000 claims description 6
- 230000003612 virological effect Effects 0.000 claims description 6
- 102100025466 Carcinoembryonic antigen-related cell adhesion molecule 3 Human genes 0.000 claims description 5
- 101000914337 Homo sapiens Carcinoembryonic antigen-related cell adhesion molecule 3 Proteins 0.000 claims description 5
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 claims description 5
- 101000934346 Homo sapiens T-cell surface antigen CD2 Proteins 0.000 claims description 5
- 101000934341 Homo sapiens T-cell surface glycoprotein CD5 Proteins 0.000 claims description 5
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 claims description 5
- 102100025244 T-cell surface glycoprotein CD5 Human genes 0.000 claims description 5
- 208000036832 Adenocarcinoma of ovary Diseases 0.000 claims description 4
- 102000006306 Antigen Receptors Human genes 0.000 claims description 4
- 108010083359 Antigen Receptors Proteins 0.000 claims description 4
- 208000003174 Brain Neoplasms Diseases 0.000 claims description 4
- 208000026310 Breast neoplasm Diseases 0.000 claims description 4
- 206010008342 Cervix carcinoma Diseases 0.000 claims description 4
- 206010009944 Colon cancer Diseases 0.000 claims description 4
- 206010061328 Ovarian epithelial cancer Diseases 0.000 claims description 4
- 206010060862 Prostate cancer Diseases 0.000 claims description 4
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 4
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 claims description 4
- 230000000735 allogeneic effect Effects 0.000 claims description 4
- 201000010881 cervical cancer Diseases 0.000 claims description 4
- 208000029742 colonic neoplasm Diseases 0.000 claims description 4
- 201000006585 gastric adenocarcinoma Diseases 0.000 claims description 4
- 208000013371 ovarian adenocarcinoma Diseases 0.000 claims description 4
- 201000006588 ovary adenocarcinoma Diseases 0.000 claims description 4
- 150000004044 tetrasaccharides Chemical class 0.000 claims description 4
- 229960000549 4-dimethylaminophenol Drugs 0.000 claims description 3
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-dimethylaminopyridine Substances CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 claims description 3
- 208000036764 Adenocarcinoma of the esophagus Diseases 0.000 claims description 3
- 101000935040 Homo sapiens Integrin beta-2 Proteins 0.000 claims description 3
- 101000917826 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor II-a Proteins 0.000 claims description 3
- 101000917824 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor II-b Proteins 0.000 claims description 3
- 108010073816 IgE Receptors Proteins 0.000 claims description 3
- 102000009438 IgE Receptors Human genes 0.000 claims description 3
- 108010064593 Intercellular Adhesion Molecule-1 Proteins 0.000 claims description 3
- 102100037877 Intercellular adhesion molecule 1 Human genes 0.000 claims description 3
- 102100029204 Low affinity immunoglobulin gamma Fc region receptor II-a Human genes 0.000 claims description 3
- 108010064548 Lymphocyte Function-Associated Antigen-1 Proteins 0.000 claims description 3
- 108010085220 Multiprotein Complexes Proteins 0.000 claims description 3
- 102000007474 Multiprotein Complexes Human genes 0.000 claims description 3
- 206010030137 Oesophageal adenocarcinoma Diseases 0.000 claims description 3
- 201000008274 breast adenocarcinoma Diseases 0.000 claims description 3
- 230000002357 endometrial effect Effects 0.000 claims description 3
- 208000028653 esophageal adenocarcinoma Diseases 0.000 claims description 3
- 210000004475 gamma-delta t lymphocyte Anatomy 0.000 claims description 3
- 201000007270 liver cancer Diseases 0.000 claims description 3
- 208000014018 liver neoplasm Diseases 0.000 claims description 3
- YACKEPLHDIMKIO-UHFFFAOYSA-N methylphosphonic acid Chemical class CP(O)(O)=O YACKEPLHDIMKIO-UHFFFAOYSA-N 0.000 claims description 3
- 208000016632 ovarian clear cell cancer Diseases 0.000 claims description 3
- 201000003707 ovarian clear cell carcinoma Diseases 0.000 claims description 3
- 230000002463 transducing effect Effects 0.000 claims description 3
- 208000000461 Esophageal Neoplasms Diseases 0.000 claims description 2
- 101000878602 Homo sapiens Immunoglobulin alpha Fc receptor Proteins 0.000 claims description 2
- 101001042104 Homo sapiens Inducible T-cell costimulator Proteins 0.000 claims description 2
- 101000878605 Homo sapiens Low affinity immunoglobulin epsilon Fc receptor Proteins 0.000 claims description 2
- 102100038005 Immunoglobulin alpha Fc receptor Human genes 0.000 claims description 2
- 102100021317 Inducible T-cell costimulator Human genes 0.000 claims description 2
- 102100038007 Low affinity immunoglobulin epsilon Fc receptor Human genes 0.000 claims description 2
- 102100029193 Low affinity immunoglobulin gamma Fc region receptor III-A Human genes 0.000 claims description 2
- 206010030155 Oesophageal carcinoma Diseases 0.000 claims description 2
- 102100040678 Programmed cell death protein 1 Human genes 0.000 claims description 2
- 208000006265 Renal cell carcinoma Diseases 0.000 claims description 2
- 206010039491 Sarcoma Diseases 0.000 claims description 2
- 102000012740 beta Adrenergic Receptors Human genes 0.000 claims description 2
- 108010079452 beta Adrenergic Receptors Proteins 0.000 claims description 2
- 201000004101 esophageal cancer Diseases 0.000 claims description 2
- 201000008819 extrahepatic bile duct carcinoma Diseases 0.000 claims description 2
- 208000000649 small cell carcinoma Diseases 0.000 claims description 2
- 201000010897 colon adenocarcinoma Diseases 0.000 claims 1
- 208000018463 endometrial serous adenocarcinoma Diseases 0.000 claims 1
- 210000000581 natural killer T-cell Anatomy 0.000 claims 1
- 238000011282 treatment Methods 0.000 abstract description 51
- 102000017420 CD3 protein, epsilon/gamma/delta subunit Human genes 0.000 description 116
- 108050005493 CD3 protein, epsilon/gamma/delta subunit Proteins 0.000 description 116
- 125000005647 linker group Chemical group 0.000 description 95
- 206010057190 Respiratory tract infections Diseases 0.000 description 59
- 102000053602 DNA Human genes 0.000 description 50
- 235000018102 proteins Nutrition 0.000 description 49
- 229920002477 rna polymer Polymers 0.000 description 47
- 235000001014 amino acid Nutrition 0.000 description 34
- 101000946843 Homo sapiens T-cell surface glycoprotein CD8 alpha chain Proteins 0.000 description 33
- 101000716102 Homo sapiens T-cell surface glycoprotein CD4 Proteins 0.000 description 31
- 229940024606 amino acid Drugs 0.000 description 30
- 150000002632 lipids Chemical class 0.000 description 26
- 238000013518 transcription Methods 0.000 description 22
- 230000035897 transcription Effects 0.000 description 22
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 20
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 20
- 238000003752 polymerase chain reaction Methods 0.000 description 20
- 108060003951 Immunoglobulin Proteins 0.000 description 18
- 102000018358 immunoglobulin Human genes 0.000 description 18
- 102000040430 polynucleotide Human genes 0.000 description 18
- 108091033319 polynucleotide Proteins 0.000 description 18
- 239000002157 polynucleotide Substances 0.000 description 18
- 241000699670 Mus sp. Species 0.000 description 17
- 239000000047 product Substances 0.000 description 17
- 238000006467 substitution reaction Methods 0.000 description 17
- 108091026898 Leader sequence (mRNA) Proteins 0.000 description 16
- 125000000539 amino acid group Chemical group 0.000 description 16
- 239000011324 bead Substances 0.000 description 16
- 208000035475 disorder Diseases 0.000 description 15
- 210000004881 tumor cell Anatomy 0.000 description 15
- 101100519207 Mus musculus Pdcd1 gene Proteins 0.000 description 14
- 238000003556 assay Methods 0.000 description 14
- 230000006870 function Effects 0.000 description 14
- 238000002347 injection Methods 0.000 description 14
- 239000007924 injection Substances 0.000 description 14
- 241001465754 Metazoa Species 0.000 description 13
- 239000002502 liposome Substances 0.000 description 13
- 210000001519 tissue Anatomy 0.000 description 13
- 238000013519 translation Methods 0.000 description 13
- 101000576802 Homo sapiens Mesothelin Proteins 0.000 description 12
- 210000004369 blood Anatomy 0.000 description 12
- 239000008280 blood Substances 0.000 description 12
- 239000002245 particle Substances 0.000 description 12
- 230000001086 cytosolic effect Effects 0.000 description 11
- 239000012636 effector Substances 0.000 description 11
- 230000001976 improved effect Effects 0.000 description 11
- 238000001727 in vivo Methods 0.000 description 11
- 230000000977 initiatory effect Effects 0.000 description 11
- 210000004379 membrane Anatomy 0.000 description 11
- 241000700605 Viruses Species 0.000 description 10
- 239000013604 expression vector Substances 0.000 description 10
- 230000004044 response Effects 0.000 description 10
- 230000001225 therapeutic effect Effects 0.000 description 10
- 238000002560 therapeutic procedure Methods 0.000 description 10
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 9
- 108010074708 B7-H1 Antigen Proteins 0.000 description 9
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 9
- 102100024216 Programmed cell death 1 ligand 1 Human genes 0.000 description 9
- 208000009956 adenocarcinoma Diseases 0.000 description 9
- 239000000306 component Substances 0.000 description 9
- 150000001875 compounds Chemical class 0.000 description 9
- 239000000463 material Substances 0.000 description 9
- 238000000926 separation method Methods 0.000 description 9
- 238000001890 transfection Methods 0.000 description 9
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 8
- 241000699666 Mus <mouse, genus> Species 0.000 description 8
- 108700008625 Reporter Genes Proteins 0.000 description 8
- 108091023045 Untranslated Region Proteins 0.000 description 8
- 230000000295 complement effect Effects 0.000 description 8
- 230000000875 corresponding effect Effects 0.000 description 8
- 230000028993 immune response Effects 0.000 description 8
- 239000003112 inhibitor Substances 0.000 description 8
- 238000009126 molecular therapy Methods 0.000 description 8
- 230000008569 process Effects 0.000 description 8
- 230000001105 regulatory effect Effects 0.000 description 8
- 238000012546 transfer Methods 0.000 description 8
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 7
- 101000851370 Homo sapiens Tumor necrosis factor receptor superfamily member 9 Proteins 0.000 description 7
- 102100036856 Tumor necrosis factor receptor superfamily member 9 Human genes 0.000 description 7
- 230000009286 beneficial effect Effects 0.000 description 7
- 230000008901 benefit Effects 0.000 description 7
- 230000015572 biosynthetic process Effects 0.000 description 7
- 239000005090 green fluorescent protein Substances 0.000 description 7
- 238000011534 incubation Methods 0.000 description 7
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 7
- 239000008194 pharmaceutical composition Substances 0.000 description 7
- 230000035755 proliferation Effects 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- 208000024891 symptom Diseases 0.000 description 7
- 239000013603 viral vector Substances 0.000 description 7
- 102100029822 B- and T-lymphocyte attenuator Human genes 0.000 description 6
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 6
- 102100039498 Cytotoxic T-lymphocyte protein 4 Human genes 0.000 description 6
- 108090000626 DNA-directed RNA polymerases Proteins 0.000 description 6
- 102000004163 DNA-directed RNA polymerases Human genes 0.000 description 6
- 239000004471 Glycine Substances 0.000 description 6
- 101000864344 Homo sapiens B- and T-lymphocyte attenuator Proteins 0.000 description 6
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 6
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 6
- 101000946860 Homo sapiens T-cell surface glycoprotein CD3 epsilon chain Proteins 0.000 description 6
- 102100027268 Interferon-stimulated gene 20 kDa protein Human genes 0.000 description 6
- 108010002350 Interleukin-2 Proteins 0.000 description 6
- 102000000588 Interleukin-2 Human genes 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 241000283984 Rodentia Species 0.000 description 6
- 102100035794 T-cell surface glycoprotein CD3 epsilon chain Human genes 0.000 description 6
- 238000004422 calculation algorithm Methods 0.000 description 6
- 230000007423 decrease Effects 0.000 description 6
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 6
- 238000000684 flow cytometry Methods 0.000 description 6
- 230000001965 increasing effect Effects 0.000 description 6
- 230000001404 mediated effect Effects 0.000 description 6
- 210000005259 peripheral blood Anatomy 0.000 description 6
- 239000011886 peripheral blood Substances 0.000 description 6
- 239000002953 phosphate buffered saline Substances 0.000 description 6
- 230000008488 polyadenylation Effects 0.000 description 6
- 239000000523 sample Substances 0.000 description 6
- 238000003786 synthesis reaction Methods 0.000 description 6
- 206010014733 Endometrial cancer Diseases 0.000 description 5
- 206010014759 Endometrial neoplasm Diseases 0.000 description 5
- 102000001398 Granzyme Human genes 0.000 description 5
- 108060005986 Granzyme Proteins 0.000 description 5
- 102100034458 Hepatitis A virus cellular receptor 2 Human genes 0.000 description 5
- 101000889276 Homo sapiens Cytotoxic T-lymphocyte protein 4 Proteins 0.000 description 5
- 101001068133 Homo sapiens Hepatitis A virus cellular receptor 2 Proteins 0.000 description 5
- 101001137987 Homo sapiens Lymphocyte activation gene 3 protein Proteins 0.000 description 5
- 108020004684 Internal Ribosome Entry Sites Proteins 0.000 description 5
- 102000017578 LAG3 Human genes 0.000 description 5
- 108091036066 Three prime untranslated region Proteins 0.000 description 5
- 238000004458 analytical method Methods 0.000 description 5
- 210000003719 b-lymphocyte Anatomy 0.000 description 5
- 238000002659 cell therapy Methods 0.000 description 5
- 238000010367 cloning Methods 0.000 description 5
- 239000002299 complementary DNA Substances 0.000 description 5
- 238000012217 deletion Methods 0.000 description 5
- 230000037430 deletion Effects 0.000 description 5
- 230000003053 immunization Effects 0.000 description 5
- 238000002649 immunization Methods 0.000 description 5
- 230000003993 interaction Effects 0.000 description 5
- 210000004698 lymphocyte Anatomy 0.000 description 5
- 230000036210 malignancy Effects 0.000 description 5
- 229920000642 polymer Polymers 0.000 description 5
- 241000894007 species Species 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- 241001430294 unidentified retrovirus Species 0.000 description 5
- 238000011144 upstream manufacturing Methods 0.000 description 5
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 208000023275 Autoimmune disease Diseases 0.000 description 4
- 102100024263 CD160 antigen Human genes 0.000 description 4
- 102100037904 CD9 antigen Human genes 0.000 description 4
- 108091026890 Coding region Proteins 0.000 description 4
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 4
- 108010036949 Cyclosporine Proteins 0.000 description 4
- 102100025137 Early activation antigen CD69 Human genes 0.000 description 4
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 4
- BCCRXDTUTZHDEU-VKHMYHEASA-N Gly-Ser Chemical compound NCC(=O)N[C@@H](CO)C(O)=O BCCRXDTUTZHDEU-VKHMYHEASA-N 0.000 description 4
- 208000032672 Histiocytosis haematophagic Diseases 0.000 description 4
- 101000761938 Homo sapiens CD160 antigen Proteins 0.000 description 4
- 101000934374 Homo sapiens Early activation antigen CD69 Proteins 0.000 description 4
- 101000777628 Homo sapiens Leukocyte antigen CD37 Proteins 0.000 description 4
- 101001138062 Homo sapiens Leukocyte-associated immunoglobulin-like receptor 1 Proteins 0.000 description 4
- 101000934338 Homo sapiens Myeloid cell surface antigen CD33 Proteins 0.000 description 4
- 101000831007 Homo sapiens T-cell immunoreceptor with Ig and ITIM domains Proteins 0.000 description 4
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 4
- 241000725303 Human immunodeficiency virus Species 0.000 description 4
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 4
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 4
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 4
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 4
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 4
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 4
- 102100031586 Leukocyte antigen CD37 Human genes 0.000 description 4
- 102100020943 Leukocyte-associated immunoglobulin-like receptor 1 Human genes 0.000 description 4
- 102100025243 Myeloid cell surface antigen CD33 Human genes 0.000 description 4
- 108010038807 Oligopeptides Proteins 0.000 description 4
- 102000015636 Oligopeptides Human genes 0.000 description 4
- 108700026244 Open Reading Frames Proteins 0.000 description 4
- 108020004511 Recombinant DNA Proteins 0.000 description 4
- 102100024834 T-cell immunoreceptor with Ig and ITIM domains Human genes 0.000 description 4
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 description 4
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 description 4
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 4
- 239000004473 Threonine Substances 0.000 description 4
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 4
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 4
- 238000007792 addition Methods 0.000 description 4
- 230000000259 anti-tumor effect Effects 0.000 description 4
- 238000013459 approach Methods 0.000 description 4
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 4
- 239000000872 buffer Substances 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- 229960001265 ciclosporin Drugs 0.000 description 4
- 229930182912 cyclosporin Natural products 0.000 description 4
- 238000004090 dissolution Methods 0.000 description 4
- 238000004520 electroporation Methods 0.000 description 4
- 238000005516 engineering process Methods 0.000 description 4
- 238000007710 freezing Methods 0.000 description 4
- 230000008014 freezing Effects 0.000 description 4
- 230000004927 fusion Effects 0.000 description 4
- 238000001476 gene delivery Methods 0.000 description 4
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 4
- 230000003463 hyperproliferative effect Effects 0.000 description 4
- 210000000987 immune system Anatomy 0.000 description 4
- 230000001939 inductive effect Effects 0.000 description 4
- 230000005764 inhibitory process Effects 0.000 description 4
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 4
- 229960000310 isoleucine Drugs 0.000 description 4
- FZWBNHMXJMCXLU-BLAUPYHCSA-N isomaltotriose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O)O1 FZWBNHMXJMCXLU-BLAUPYHCSA-N 0.000 description 4
- 230000002147 killing effect Effects 0.000 description 4
- 208000032839 leukemia Diseases 0.000 description 4
- 210000000265 leukocyte Anatomy 0.000 description 4
- 229920002521 macromolecule Polymers 0.000 description 4
- HPNSFSBZBAHARI-UHFFFAOYSA-N micophenolic acid Natural products OC1=C(CC=C(C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-UHFFFAOYSA-N 0.000 description 4
- 230000035772 mutation Effects 0.000 description 4
- HPNSFSBZBAHARI-RUDMXATFSA-N mycophenolic acid Chemical compound OC1=C(C\C=C(/C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-RUDMXATFSA-N 0.000 description 4
- 230000002688 persistence Effects 0.000 description 4
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 4
- 239000013641 positive control Substances 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 description 4
- 230000002103 transcriptional effect Effects 0.000 description 4
- 102000035160 transmembrane proteins Human genes 0.000 description 4
- 108091005703 transmembrane proteins Proteins 0.000 description 4
- 239000004474 valine Substances 0.000 description 4
- 238000005406 washing Methods 0.000 description 4
- 108020004705 Codon Proteins 0.000 description 3
- 241000701022 Cytomegalovirus Species 0.000 description 3
- 241000702421 Dependoparvovirus Species 0.000 description 3
- 101100118093 Drosophila melanogaster eEF1alpha2 gene Proteins 0.000 description 3
- 238000002965 ELISA Methods 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- 101000738354 Homo sapiens CD9 antigen Proteins 0.000 description 3
- 101000666896 Homo sapiens V-type immunoglobulin domain-containing suppressor of T-cell activation Proteins 0.000 description 3
- 108091006905 Human Serum Albumin Proteins 0.000 description 3
- 102000008100 Human Serum Albumin Human genes 0.000 description 3
- 206010020751 Hypersensitivity Diseases 0.000 description 3
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 3
- 239000000232 Lipid Bilayer Substances 0.000 description 3
- 108060001084 Luciferase Proteins 0.000 description 3
- 239000005089 Luciferase Substances 0.000 description 3
- 208000006994 Precancerous Conditions Diseases 0.000 description 3
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 3
- 108700019146 Transgenes Proteins 0.000 description 3
- 102100038282 V-type immunoglobulin domain-containing suppressor of T-cell activation Human genes 0.000 description 3
- 239000000654 additive Substances 0.000 description 3
- 230000000996 additive effect Effects 0.000 description 3
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 3
- 235000004279 alanine Nutrition 0.000 description 3
- 229960000548 alemtuzumab Drugs 0.000 description 3
- 230000000172 allergic effect Effects 0.000 description 3
- 108010087408 alpha-beta T-Cell Antigen Receptors Proteins 0.000 description 3
- 238000010171 animal model Methods 0.000 description 3
- 230000000890 antigenic effect Effects 0.000 description 3
- 208000006673 asthma Diseases 0.000 description 3
- 208000010668 atopic eczema Diseases 0.000 description 3
- 230000001580 bacterial effect Effects 0.000 description 3
- 210000001185 bone marrow Anatomy 0.000 description 3
- 238000002619 cancer immunotherapy Methods 0.000 description 3
- 230000015556 catabolic process Effects 0.000 description 3
- 230000010261 cell growth Effects 0.000 description 3
- 210000000170 cell membrane Anatomy 0.000 description 3
- 238000002512 chemotherapy Methods 0.000 description 3
- 238000003776 cleavage reaction Methods 0.000 description 3
- 235000018417 cysteine Nutrition 0.000 description 3
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 3
- 206010052015 cytokine release syndrome Diseases 0.000 description 3
- 230000001461 cytolytic effect Effects 0.000 description 3
- 238000006731 degradation reaction Methods 0.000 description 3
- 210000004443 dendritic cell Anatomy 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- 239000008121 dextrose Substances 0.000 description 3
- 229940088598 enzyme Drugs 0.000 description 3
- IJJVMEJXYNJXOJ-UHFFFAOYSA-N fluquinconazole Chemical compound C=1C=C(Cl)C=C(Cl)C=1N1C(=O)C2=CC(F)=CC=C2N=C1N1C=NC=N1 IJJVMEJXYNJXOJ-UHFFFAOYSA-N 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 238000001415 gene therapy Methods 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 239000003018 immunosuppressive agent Substances 0.000 description 3
- 229940125721 immunosuppressive agent Drugs 0.000 description 3
- 208000015181 infectious disease Diseases 0.000 description 3
- 208000027866 inflammatory disease Diseases 0.000 description 3
- 230000031146 intracellular signal transduction Effects 0.000 description 3
- 230000007774 longterm Effects 0.000 description 3
- 206010025135 lupus erythematosus Diseases 0.000 description 3
- FVVLHONNBARESJ-NTOWJWGLSA-H magnesium;potassium;trisodium;(2r,3s,4r,5r)-2,3,4,5,6-pentahydroxyhexanoate;acetate;tetrachloride;nonahydrate Chemical compound O.O.O.O.O.O.O.O.O.[Na+].[Na+].[Na+].[Mg+2].[Cl-].[Cl-].[Cl-].[Cl-].[K+].CC([O-])=O.OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O FVVLHONNBARESJ-NTOWJWGLSA-H 0.000 description 3
- 230000003211 malignant effect Effects 0.000 description 3
- 239000003550 marker Substances 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 238000001565 modulated differential scanning calorimetry Methods 0.000 description 3
- 238000010369 molecular cloning Methods 0.000 description 3
- 238000002703 mutagenesis Methods 0.000 description 3
- 231100000350 mutagenesis Toxicity 0.000 description 3
- 210000004985 myeloid-derived suppressor cell Anatomy 0.000 description 3
- 230000002611 ovarian Effects 0.000 description 3
- 230000036961 partial effect Effects 0.000 description 3
- 230000002441 reversible effect Effects 0.000 description 3
- 230000007017 scission Effects 0.000 description 3
- 230000028327 secretion Effects 0.000 description 3
- 230000009870 specific binding Effects 0.000 description 3
- 150000003431 steroids Chemical class 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 239000006228 supernatant Substances 0.000 description 3
- 230000004083 survival effect Effects 0.000 description 3
- 229940124597 therapeutic agent Drugs 0.000 description 3
- 230000009261 transgenic effect Effects 0.000 description 3
- 230000001052 transient effect Effects 0.000 description 3
- 238000002054 transplantation Methods 0.000 description 3
- 239000003981 vehicle Substances 0.000 description 3
- 230000003442 weekly effect Effects 0.000 description 3
- 238000001262 western blot Methods 0.000 description 3
- GFFGJBXGBJISGV-UHFFFAOYSA-N Adenine Chemical compound NC1=NC=NC2=C1N=CN2 GFFGJBXGBJISGV-UHFFFAOYSA-N 0.000 description 2
- 208000010507 Adenocarcinoma of Lung Diseases 0.000 description 2
- 206010001197 Adenocarcinoma of the cervix Diseases 0.000 description 2
- 208000034246 Adenocarcinoma of the cervix uteri Diseases 0.000 description 2
- 239000004475 Arginine Substances 0.000 description 2
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- 102100038078 CD276 antigen Human genes 0.000 description 2
- 101150013553 CD40 gene Proteins 0.000 description 2
- 102100035793 CD83 antigen Human genes 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 2
- 201000009030 Carcinoma Diseases 0.000 description 2
- 241000282693 Cercopithecidae Species 0.000 description 2
- 108091007741 Chimeric antigen receptor T cells Proteins 0.000 description 2
- 108091033380 Coding strand Proteins 0.000 description 2
- 206010052360 Colorectal adenocarcinoma Diseases 0.000 description 2
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 2
- 229920002307 Dextran Polymers 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 241000588724 Escherichia coli Species 0.000 description 2
- 108060002716 Exonuclease Proteins 0.000 description 2
- 108090000331 Firefly luciferases Proteins 0.000 description 2
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 2
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 2
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 2
- NYHBQMYGNKIUIF-UUOKFMHZSA-N Guanosine Chemical compound C1=NC=2C(=O)NC(N)=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O NYHBQMYGNKIUIF-UUOKFMHZSA-N 0.000 description 2
- 208000036066 Hemophagocytic Lymphohistiocytosis Diseases 0.000 description 2
- 101000946856 Homo sapiens CD83 antigen Proteins 0.000 description 2
- 101000623901 Homo sapiens Mucin-16 Proteins 0.000 description 2
- 101000801234 Homo sapiens Tumor necrosis factor receptor superfamily member 18 Proteins 0.000 description 2
- 206010061598 Immunodeficiency Diseases 0.000 description 2
- 108010002386 Interleukin-3 Proteins 0.000 description 2
- 102000004889 Interleukin-6 Human genes 0.000 description 2
- 108090001005 Interleukin-6 Proteins 0.000 description 2
- 108091092195 Intron Proteins 0.000 description 2
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 2
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 2
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 2
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 2
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 2
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 2
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 2
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 2
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 2
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 2
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 2
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 2
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 2
- 239000004472 Lysine Substances 0.000 description 2
- 102100026964 M1-specific T cell receptor beta chain Human genes 0.000 description 2
- 208000004987 Macrophage activation syndrome Diseases 0.000 description 2
- 102100023123 Mucin-16 Human genes 0.000 description 2
- 101710124239 Poly(A) polymerase Proteins 0.000 description 2
- 102000015623 Polynucleotide Adenylyltransferase Human genes 0.000 description 2
- 108010024055 Polynucleotide adenylyltransferase Proteins 0.000 description 2
- RJKFOVLPORLFTN-LEKSSAKUSA-N Progesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)C)[C@@]1(C)CC2 RJKFOVLPORLFTN-LEKSSAKUSA-N 0.000 description 2
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 2
- 241000589517 Pseudomonas aeruginosa Species 0.000 description 2
- 108091034057 RNA (poly(A)) Proteins 0.000 description 2
- 239000012979 RPMI medium Substances 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 2
- 108091027967 Small hairpin RNA Proteins 0.000 description 2
- 241000191967 Staphylococcus aureus Species 0.000 description 2
- 201000005010 Streptococcus pneumonia Diseases 0.000 description 2
- 241000193998 Streptococcus pneumoniae Species 0.000 description 2
- 241000193996 Streptococcus pyogenes Species 0.000 description 2
- 230000006044 T cell activation Effects 0.000 description 2
- 230000006052 T cell proliferation Effects 0.000 description 2
- 210000000662 T-lymphocyte subset Anatomy 0.000 description 2
- 108700012920 TNF Proteins 0.000 description 2
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 2
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 2
- 108060008683 Tumor Necrosis Factor Receptor Proteins 0.000 description 2
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 2
- 102100033728 Tumor necrosis factor receptor superfamily member 18 Human genes 0.000 description 2
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 description 2
- DRTQHJPVMGBUCF-XVFCMESISA-N Uridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-XVFCMESISA-N 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 150000003838 adenosines Chemical class 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 208000026935 allergic disease Diseases 0.000 description 2
- 238000009175 antibody therapy Methods 0.000 description 2
- 239000003963 antioxidant agent Substances 0.000 description 2
- 230000006907 apoptotic process Effects 0.000 description 2
- 239000012736 aqueous medium Substances 0.000 description 2
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 2
- 125000003118 aryl group Chemical group 0.000 description 2
- 235000009582 asparagine Nutrition 0.000 description 2
- 229960001230 asparagine Drugs 0.000 description 2
- 235000003704 aspartic acid Nutrition 0.000 description 2
- LMEKQMALGUDUQG-UHFFFAOYSA-N azathioprine Chemical compound CN1C=NC([N+]([O-])=O)=C1SC1=NC=NC2=C1NC=N2 LMEKQMALGUDUQG-UHFFFAOYSA-N 0.000 description 2
- 229960002170 azathioprine Drugs 0.000 description 2
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 239000012472 biological sample Substances 0.000 description 2
- 230000005540 biological transmission Effects 0.000 description 2
- 210000001772 blood platelet Anatomy 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 239000001506 calcium phosphate Substances 0.000 description 2
- 229910000389 calcium phosphate Inorganic materials 0.000 description 2
- 235000011010 calcium phosphates Nutrition 0.000 description 2
- 235000012730 carminic acid Nutrition 0.000 description 2
- 239000004106 carminic acid Substances 0.000 description 2
- 230000020411 cell activation Effects 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 201000006662 cervical adenocarcinoma Diseases 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 210000000349 chromosome Anatomy 0.000 description 2
- 229940080423 cochineal Drugs 0.000 description 2
- 239000000084 colloidal system Substances 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- 239000000356 contaminant Substances 0.000 description 2
- 230000016396 cytokine production Effects 0.000 description 2
- 210000000805 cytoplasm Anatomy 0.000 description 2
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical compound NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 2
- 230000003013 cytotoxicity Effects 0.000 description 2
- 231100000135 cytotoxicity Toxicity 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 229940119744 dextran 40 Drugs 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 239000000539 dimer Substances 0.000 description 2
- 230000006806 disease prevention Effects 0.000 description 2
- 210000003743 erythrocyte Anatomy 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 102000013165 exonuclease Human genes 0.000 description 2
- 239000013613 expression plasmid Substances 0.000 description 2
- 230000002496 gastric effect Effects 0.000 description 2
- 235000013922 glutamic acid Nutrition 0.000 description 2
- 239000004220 glutamic acid Substances 0.000 description 2
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 2
- 210000003714 granulocyte Anatomy 0.000 description 2
- 239000001963 growth medium Substances 0.000 description 2
- 210000002443 helper t lymphocyte Anatomy 0.000 description 2
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 2
- 208000014752 hemophagocytic syndrome Diseases 0.000 description 2
- 230000009610 hypersensitivity Effects 0.000 description 2
- 239000012642 immune effector Substances 0.000 description 2
- 230000002163 immunogen Effects 0.000 description 2
- 229940121354 immunomodulator Drugs 0.000 description 2
- 230000010354 integration Effects 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 238000010253 intravenous injection Methods 0.000 description 2
- 229960005386 ipilimumab Drugs 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 238000001638 lipofection Methods 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 238000001325 log-rank test Methods 0.000 description 2
- 201000005249 lung adenocarcinoma Diseases 0.000 description 2
- 210000001165 lymph node Anatomy 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 229930182817 methionine Natural products 0.000 description 2
- 229960000485 methotrexate Drugs 0.000 description 2
- 210000005087 mononuclear cell Anatomy 0.000 description 2
- 229940014456 mycophenolate Drugs 0.000 description 2
- 229960000951 mycophenolic acid Drugs 0.000 description 2
- 239000013642 negative control Substances 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 210000004940 nucleus Anatomy 0.000 description 2
- 108010079892 phosphoglycerol kinase Proteins 0.000 description 2
- 150000003904 phospholipids Chemical class 0.000 description 2
- 208000024356 pleural disease Diseases 0.000 description 2
- 230000000770 proinflammatory effect Effects 0.000 description 2
- 230000000069 prophylactic effect Effects 0.000 description 2
- 230000005855 radiation Effects 0.000 description 2
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 description 2
- 230000010076 replication Effects 0.000 description 2
- 230000001850 reproductive effect Effects 0.000 description 2
- 229960003452 romidepsin Drugs 0.000 description 2
- OHRURASPPZQGQM-GCCNXGTGSA-N romidepsin Chemical compound O1C(=O)[C@H](C(C)C)NC(=O)C(=C/C)/NC(=O)[C@H]2CSSCC\C=C\[C@@H]1CC(=O)N[C@H](C(C)C)C(=O)N2 OHRURASPPZQGQM-GCCNXGTGSA-N 0.000 description 2
- 238000010187 selection method Methods 0.000 description 2
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 2
- BNRNXUUZRGQAQC-UHFFFAOYSA-N sildenafil Chemical compound CCCC1=NN(C)C(C(N2)=O)=C1N=C2C(C(=CC=1)OCC)=CC=1S(=O)(=O)N1CCN(C)CC1 BNRNXUUZRGQAQC-UHFFFAOYSA-N 0.000 description 2
- 229960002930 sirolimus Drugs 0.000 description 2
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 description 2
- 239000004055 small Interfering RNA Substances 0.000 description 2
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 2
- 210000000952 spleen Anatomy 0.000 description 2
- 230000002269 spontaneous effect Effects 0.000 description 2
- 206010041823 squamous cell carcinoma Diseases 0.000 description 2
- 238000011301 standard therapy Methods 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 230000010474 transient expression Effects 0.000 description 2
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 2
- 102000003298 tumor necrosis factor receptor Human genes 0.000 description 2
- DIGQNXIGRZPYDK-WKSCXVIASA-N (2R)-6-amino-2-[[2-[[(2S)-2-[[2-[[(2R)-2-[[(2S)-2-[[(2R,3S)-2-[[2-[[(2S)-2-[[2-[[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2S,3S)-2-[[(2R)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[2-[[(2S)-2-[[(2R)-2-[[2-[[2-[[2-[(2-amino-1-hydroxyethylidene)amino]-3-carboxy-1-hydroxypropylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1-hydroxyethylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxyethylidene]amino]-1-hydroxypropylidene]amino]-1,3-dihydroxypropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxybutylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1-hydroxypropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxyethylidene]amino]-1,5-dihydroxy-5-iminopentylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxybutylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxyethylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1-hydroxyethylidene]amino]hexanoic acid Chemical compound C[C@@H]([C@@H](C(=N[C@@H](CS)C(=N[C@@H](C)C(=N[C@@H](CO)C(=NCC(=N[C@@H](CCC(=N)O)C(=NC(CS)C(=N[C@H]([C@H](C)O)C(=N[C@H](CS)C(=N[C@H](CO)C(=NCC(=N[C@H](CS)C(=NCC(=N[C@H](CCCCN)C(=O)O)O)O)O)O)O)O)O)O)O)O)O)O)O)N=C([C@H](CS)N=C([C@H](CO)N=C([C@H](CO)N=C([C@H](C)N=C(CN=C([C@H](CO)N=C([C@H](CS)N=C(CN=C(C(CS)N=C(C(CC(=O)O)N=C(CN)O)O)O)O)O)O)O)O)O)O)O)O DIGQNXIGRZPYDK-WKSCXVIASA-N 0.000 description 1
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 1
- VGONTNSXDCQUGY-RRKCRQDMSA-N 2'-deoxyinosine Chemical group C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC2=O)=C2N=C1 VGONTNSXDCQUGY-RRKCRQDMSA-N 0.000 description 1
- BGFTWECWAICPDG-UHFFFAOYSA-N 2-[bis(4-chlorophenyl)methyl]-4-n-[3-[bis(4-chlorophenyl)methyl]-4-(dimethylamino)phenyl]-1-n,1-n-dimethylbenzene-1,4-diamine Chemical compound C1=C(C(C=2C=CC(Cl)=CC=2)C=2C=CC(Cl)=CC=2)C(N(C)C)=CC=C1NC(C=1)=CC=C(N(C)C)C=1C(C=1C=CC(Cl)=CC=1)C1=CC=C(Cl)C=C1 BGFTWECWAICPDG-UHFFFAOYSA-N 0.000 description 1
- IOJUJUOXKXMJNF-UHFFFAOYSA-N 2-acetyloxybenzoic acid [3-(nitrooxymethyl)phenyl] ester Chemical compound CC(=O)OC1=CC=CC=C1C(=O)OC1=CC=CC(CO[N+]([O-])=O)=C1 IOJUJUOXKXMJNF-UHFFFAOYSA-N 0.000 description 1
- GOJUJUVQIVIZAV-UHFFFAOYSA-N 2-amino-4,6-dichloropyrimidine-5-carbaldehyde Chemical group NC1=NC(Cl)=C(C=O)C(Cl)=N1 GOJUJUVQIVIZAV-UHFFFAOYSA-N 0.000 description 1
- 108010082808 4-1BB Ligand Proteins 0.000 description 1
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 1
- 101710134681 40 kDa protein Proteins 0.000 description 1
- OGHAROSJZRTIOK-FCIPNVEPSA-O 7-methylguanosine Chemical compound C1=2N=C(N)NC(=O)C=2[N+](C)=CN1[C@@H]1O[C@@H](CO)[C@H](O)[C@H]1O OGHAROSJZRTIOK-FCIPNVEPSA-O 0.000 description 1
- 108020005176 AU Rich Elements Proteins 0.000 description 1
- 102000007469 Actins Human genes 0.000 description 1
- 108010085238 Actins Proteins 0.000 description 1
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 description 1
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 description 1
- 229930024421 Adenine Natural products 0.000 description 1
- 241000321096 Adenoides Species 0.000 description 1
- 241000588813 Alcaligenes faecalis Species 0.000 description 1
- 239000012117 Alexa Fluor 700 Substances 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 108700028369 Alleles Proteins 0.000 description 1
- 206010003445 Ascites Diseases 0.000 description 1
- 241000714230 Avian leukemia virus Species 0.000 description 1
- 102000006942 B-Cell Maturation Antigen Human genes 0.000 description 1
- 108010008014 B-Cell Maturation Antigen Proteins 0.000 description 1
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 1
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 description 1
- 208000035143 Bacterial infection Diseases 0.000 description 1
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 1
- 102100026189 Beta-galactosidase Human genes 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 1
- 108700012434 CCL3 Proteins 0.000 description 1
- 108091033409 CRISPR Proteins 0.000 description 1
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 1
- 229940045513 CTLA4 antagonist Drugs 0.000 description 1
- 241000282836 Camelus dromedarius Species 0.000 description 1
- 102100025570 Cancer/testis antigen 1 Human genes 0.000 description 1
- 241000222122 Candida albicans Species 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 102000000844 Cell Surface Receptors Human genes 0.000 description 1
- 108010001857 Cell Surface Receptors Proteins 0.000 description 1
- 102000000013 Chemokine CCL3 Human genes 0.000 description 1
- 102000001326 Chemokine CCL4 Human genes 0.000 description 1
- 108010055165 Chemokine CCL4 Proteins 0.000 description 1
- 108010035563 Chloramphenicol O-acetyltransferase Proteins 0.000 description 1
- 108020004638 Circular DNA Proteins 0.000 description 1
- 108091062157 Cis-regulatory element Proteins 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 108020004635 Complementary DNA Proteins 0.000 description 1
- 102000004420 Creatine Kinase Human genes 0.000 description 1
- 108010042126 Creatine kinase Proteins 0.000 description 1
- MIKUYHXYGGJMLM-GIMIYPNGSA-N Crotonoside Natural products C1=NC2=C(N)NC(=O)N=C2N1[C@H]1O[C@@H](CO)[C@H](O)[C@@H]1O MIKUYHXYGGJMLM-GIMIYPNGSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- NYHBQMYGNKIUIF-UHFFFAOYSA-N D-guanosine Natural products C1=2NC(N)=NC(=O)C=2N=CN1C1OC(CO)C(O)C1O NYHBQMYGNKIUIF-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-QTVWNMPRSA-N D-mannopyranose Chemical compound OC[C@H]1OC(O)[C@@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-QTVWNMPRSA-N 0.000 description 1
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 1
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 1
- GZDFHIJNHHMENY-UHFFFAOYSA-N Dimethyl dicarbonate Chemical compound COC(=O)OC(=O)OC GZDFHIJNHHMENY-UHFFFAOYSA-N 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 102100031780 Endonuclease Human genes 0.000 description 1
- 108010042407 Endonucleases Proteins 0.000 description 1
- 241000701867 Enterobacteria phage T7 Species 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical class C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 241000206602 Eukaryota Species 0.000 description 1
- 108700024394 Exon Proteins 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 229920001917 Ficoll Polymers 0.000 description 1
- 102000001390 Fructose-Bisphosphate Aldolase Human genes 0.000 description 1
- 108010068561 Fructose-Bisphosphate Aldolase Proteins 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 1
- 108700039691 Genetic Promoter Regions Proteins 0.000 description 1
- 102000004457 Granulocyte-Macrophage Colony-Stimulating Factor Human genes 0.000 description 1
- 102000025850 HLA-A2 Antigen Human genes 0.000 description 1
- 108010074032 HLA-A2 Antigen Proteins 0.000 description 1
- 102000006354 HLA-DR Antigens Human genes 0.000 description 1
- 108010058597 HLA-DR Antigens Proteins 0.000 description 1
- 241000606768 Haemophilus influenzae Species 0.000 description 1
- 102100031573 Hematopoietic progenitor cell antigen CD34 Human genes 0.000 description 1
- 102000001554 Hemoglobins Human genes 0.000 description 1
- 108010054147 Hemoglobins Proteins 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 description 1
- 101000856237 Homo sapiens Cancer/testis antigen 1 Proteins 0.000 description 1
- 101000777663 Homo sapiens Hematopoietic progenitor cell antigen CD34 Proteins 0.000 description 1
- 101001046686 Homo sapiens Integrin alpha-M Proteins 0.000 description 1
- 101000971538 Homo sapiens Killer cell lectin-like receptor subfamily F member 1 Proteins 0.000 description 1
- 101000946889 Homo sapiens Monocyte differentiation antigen CD14 Proteins 0.000 description 1
- 101000633784 Homo sapiens SLAM family member 7 Proteins 0.000 description 1
- 101000634853 Homo sapiens T cell receptor alpha chain constant Proteins 0.000 description 1
- 101000611183 Homo sapiens Tumor necrosis factor Proteins 0.000 description 1
- 101000795169 Homo sapiens Tumor necrosis factor receptor superfamily member 13C Proteins 0.000 description 1
- 108090000144 Human Proteins Proteins 0.000 description 1
- 102000003839 Human Proteins Human genes 0.000 description 1
- 229920001612 Hydroxyethyl starch Polymers 0.000 description 1
- 208000001953 Hypotension Diseases 0.000 description 1
- 206010021143 Hypoxia Diseases 0.000 description 1
- 101150102264 IE gene Proteins 0.000 description 1
- 229940124790 IL-6 inhibitor Drugs 0.000 description 1
- 102000009786 Immunoglobulin Constant Regions Human genes 0.000 description 1
- 108010009817 Immunoglobulin Constant Regions Proteins 0.000 description 1
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 1
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- 206010062016 Immunosuppression Diseases 0.000 description 1
- 102100022338 Integrin alpha-M Human genes 0.000 description 1
- 108010002352 Interleukin-1 Proteins 0.000 description 1
- 102000003814 Interleukin-10 Human genes 0.000 description 1
- 108090000174 Interleukin-10 Proteins 0.000 description 1
- 108050003558 Interleukin-17 Proteins 0.000 description 1
- 102000013691 Interleukin-17 Human genes 0.000 description 1
- 102000000646 Interleukin-3 Human genes 0.000 description 1
- 108090000978 Interleukin-4 Proteins 0.000 description 1
- 102000004388 Interleukin-4 Human genes 0.000 description 1
- 108090001007 Interleukin-8 Proteins 0.000 description 1
- 102000004890 Interleukin-8 Human genes 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- 102100021458 Killer cell lectin-like receptor subfamily F member 1 Human genes 0.000 description 1
- 241000254158 Lampyridae Species 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 108700018351 Major Histocompatibility Complex Proteins 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 108010061593 Member 14 Tumor Necrosis Factor Receptors Proteins 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 201000009906 Meningitis Diseases 0.000 description 1
- 102100025096 Mesothelin Human genes 0.000 description 1
- 102000003792 Metallothionein Human genes 0.000 description 1
- 108090000157 Metallothionein Proteins 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- 206010027480 Metastatic malignant melanoma Diseases 0.000 description 1
- 102100035877 Monocyte differentiation antigen CD14 Human genes 0.000 description 1
- 241000713333 Mouse mammary tumor virus Species 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 241000204031 Mycoplasma Species 0.000 description 1
- 102000003505 Myosin Human genes 0.000 description 1
- 108060008487 Myosin Proteins 0.000 description 1
- 108091008877 NK cell receptors Proteins 0.000 description 1
- 102000010648 Natural Killer Cell Receptors Human genes 0.000 description 1
- 206010028813 Nausea Diseases 0.000 description 1
- 241000588653 Neisseria Species 0.000 description 1
- 238000000636 Northern blotting Methods 0.000 description 1
- 108091005461 Nucleic proteins Proteins 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 240000007019 Oxalis corniculata Species 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 208000030852 Parasitic disease Diseases 0.000 description 1
- KHGNFPUMBJSZSM-UHFFFAOYSA-N Perforine Natural products COC1=C2CCC(O)C(CCC(C)(C)O)(OC)C2=NC2=C1C=CO2 KHGNFPUMBJSZSM-UHFFFAOYSA-N 0.000 description 1
- 229940123333 Phosphodiesterase 5 inhibitor Drugs 0.000 description 1
- 208000002151 Pleural effusion Diseases 0.000 description 1
- 108010039918 Polylysine Proteins 0.000 description 1
- 102100037935 Polyubiquitin-C Human genes 0.000 description 1
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 101710089372 Programmed cell death protein 1 Proteins 0.000 description 1
- 102000003923 Protein Kinase C Human genes 0.000 description 1
- 108090000315 Protein Kinase C Proteins 0.000 description 1
- 102000016971 Proto-Oncogene Proteins c-kit Human genes 0.000 description 1
- 108010014608 Proto-Oncogene Proteins c-kit Proteins 0.000 description 1
- 108020005161 RNA Caps Proteins 0.000 description 1
- 108010065868 RNA polymerase SP6 Proteins 0.000 description 1
- 101000916532 Rattus norvegicus Zinc finger and BTB domain-containing protein 38 Proteins 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- 241000712907 Retroviridae Species 0.000 description 1
- 241000714474 Rous sarcoma virus Species 0.000 description 1
- 102100029198 SLAM family member 7 Human genes 0.000 description 1
- 238000012300 Sequence Analysis Methods 0.000 description 1
- 108010074687 Signaling Lymphocytic Activation Molecule Family Member 1 Proteins 0.000 description 1
- 102000008115 Signaling Lymphocytic Activation Molecule Family Member 1 Human genes 0.000 description 1
- 241000700584 Simplexvirus Species 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- 108020004459 Small interfering RNA Proteins 0.000 description 1
- 238000002105 Southern blotting Methods 0.000 description 1
- 208000013128 Squamous cell carcinoma of pancreas Diseases 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 102100029452 T cell receptor alpha chain constant Human genes 0.000 description 1
- 230000005867 T cell response Effects 0.000 description 1
- 101710137500 T7 RNA polymerase Proteins 0.000 description 1
- 239000004098 Tetracycline Substances 0.000 description 1
- 102000006601 Thymidine Kinase Human genes 0.000 description 1
- 108020004440 Thymidine kinase Proteins 0.000 description 1
- 208000024770 Thyroid neoplasm Diseases 0.000 description 1
- 102100040247 Tumor necrosis factor Human genes 0.000 description 1
- 102100032101 Tumor necrosis factor ligand superfamily member 9 Human genes 0.000 description 1
- 102100029690 Tumor necrosis factor receptor superfamily member 13C Human genes 0.000 description 1
- 102100028785 Tumor necrosis factor receptor superfamily member 14 Human genes 0.000 description 1
- 108010056354 Ubiquitin C Proteins 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- QYSXJUFSXHHAJI-XFEUOLMDSA-N Vitamin D3 Natural products C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C/C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-XFEUOLMDSA-N 0.000 description 1
- 108010017070 Zinc Finger Nucleases Proteins 0.000 description 1
- ATBOMIWRCZXYSZ-XZBBILGWSA-N [1-[2,3-dihydroxypropoxy(hydroxy)phosphoryl]oxy-3-hexadecanoyloxypropan-2-yl] (9e,12e)-octadeca-9,12-dienoate Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCC\C=C\C\C=C\CCCCC ATBOMIWRCZXYSZ-XZBBILGWSA-N 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 238000011374 additional therapy Methods 0.000 description 1
- 229960000643 adenine Drugs 0.000 description 1
- 210000002534 adenoid Anatomy 0.000 description 1
- 229960005305 adenosine Drugs 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 230000004520 agglutination Effects 0.000 description 1
- 229940005347 alcaligenes faecalis Drugs 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 150000001323 aldoses Chemical class 0.000 description 1
- 150000001338 aliphatic hydrocarbons Chemical class 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- 125000000266 alpha-aminoacyl group Chemical group 0.000 description 1
- 102000006707 alpha-beta T-Cell Antigen Receptors Human genes 0.000 description 1
- AWUCVROLDVIAJX-UHFFFAOYSA-N alpha-glycerophosphate Natural products OCC(O)COP(O)(O)=O AWUCVROLDVIAJX-UHFFFAOYSA-N 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 150000001414 amino alcohols Chemical class 0.000 description 1
- 238000000137 annealing Methods 0.000 description 1
- 230000001093 anti-cancer Effects 0.000 description 1
- 230000003141 anti-fusion Effects 0.000 description 1
- 230000005875 antibody response Effects 0.000 description 1
- 210000000628 antibody-producing cell Anatomy 0.000 description 1
- 210000000612 antigen-presenting cell Anatomy 0.000 description 1
- 239000003443 antiviral agent Substances 0.000 description 1
- 230000001640 apoptogenic effect Effects 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000000823 artificial membrane Substances 0.000 description 1
- 230000002238 attenuated effect Effects 0.000 description 1
- 230000001363 autoimmune Effects 0.000 description 1
- 208000022362 bacterial infectious disease Diseases 0.000 description 1
- 108010028263 bacteriophage T3 RNA polymerase Proteins 0.000 description 1
- 108010005774 beta-Galactosidase Proteins 0.000 description 1
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 1
- DRTQHJPVMGBUCF-PSQAKQOGSA-N beta-L-uridine Natural products O[C@H]1[C@@H](O)[C@H](CO)O[C@@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-PSQAKQOGSA-N 0.000 description 1
- 239000003012 bilayer membrane Substances 0.000 description 1
- 238000005842 biochemical reaction Methods 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 230000008827 biological function Effects 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 210000000601 blood cell Anatomy 0.000 description 1
- 210000002798 bone marrow cell Anatomy 0.000 description 1
- 239000012888 bovine serum Substances 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- 229960005520 bryostatin Drugs 0.000 description 1
- MJQUEDHRCUIRLF-TVIXENOKSA-N bryostatin 1 Chemical compound C([C@@H]1CC(/[C@@H]([C@@](C(C)(C)/C=C/2)(O)O1)OC(=O)/C=C/C=C/CCC)=C\C(=O)OC)[C@H]([C@@H](C)O)OC(=O)C[C@H](O)C[C@@H](O1)C[C@H](OC(C)=O)C(C)(C)[C@]1(O)C[C@@H]1C\C(=C\C(=O)OC)C[C@H]\2O1 MJQUEDHRCUIRLF-TVIXENOKSA-N 0.000 description 1
- MUIWQCKLQMOUAT-AKUNNTHJSA-N bryostatin 20 Natural products COC(=O)C=C1C[C@@]2(C)C[C@]3(O)O[C@](C)(C[C@@H](O)CC(=O)O[C@](C)(C[C@@]4(C)O[C@](O)(CC5=CC(=O)O[C@]45C)C(C)(C)C=C[C@@](C)(C1)O2)[C@@H](C)O)C[C@H](OC(=O)C(C)(C)C)C3(C)C MUIWQCKLQMOUAT-AKUNNTHJSA-N 0.000 description 1
- 239000007975 buffered saline Substances 0.000 description 1
- 230000000981 bystander Effects 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 229940095731 candida albicans Drugs 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 210000001715 carotid artery Anatomy 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 230000021164 cell adhesion Effects 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 239000002458 cell surface marker Substances 0.000 description 1
- 229940030156 cell vaccine Drugs 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000005754 cellular signaling Effects 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 125000003636 chemical group Chemical group 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 238000009104 chemotherapy regimen Methods 0.000 description 1
- 208000018805 childhood acute lymphoblastic leukemia Diseases 0.000 description 1
- 201000011633 childhood acute lymphocytic leukemia Diseases 0.000 description 1
- 201000002687 childhood acute myeloid leukemia Diseases 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 239000013599 cloning vector Substances 0.000 description 1
- 238000012761 co-transfection Methods 0.000 description 1
- 206010009887 colitis Diseases 0.000 description 1
- 238000001246 colloidal dispersion Methods 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 238000013329 compounding Methods 0.000 description 1
- 238000004590 computer program Methods 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 230000003750 conditioning effect Effects 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229940111134 coxibs Drugs 0.000 description 1
- 238000005138 cryopreservation Methods 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 239000003255 cyclooxygenase 2 inhibitor Substances 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 102000003675 cytokine receptors Human genes 0.000 description 1
- 108010057085 cytokine receptors Proteins 0.000 description 1
- 229940104302 cytosine Drugs 0.000 description 1
- 230000003436 cytoskeletal effect Effects 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 238000004925 denaturation Methods 0.000 description 1
- 230000036425 denaturation Effects 0.000 description 1
- 238000009795 derivation Methods 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- BPHQZTVXXXJVHI-UHFFFAOYSA-N dimyristoyl phosphatidylglycerol Chemical compound CCCCCCCCCCCCCC(=O)OCC(COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCCCCCCCC BPHQZTVXXXJVHI-UHFFFAOYSA-N 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 231100000371 dose-limiting toxicity Toxicity 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 241001493065 dsRNA viruses Species 0.000 description 1
- 210000003162 effector t lymphocyte Anatomy 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 201000003908 endometrial adenocarcinoma Diseases 0.000 description 1
- 208000029382 endometrium adenocarcinoma Diseases 0.000 description 1
- 239000002158 endotoxin Substances 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 230000008029 eradication Effects 0.000 description 1
- 229940023064 escherichia coli Drugs 0.000 description 1
- 239000003797 essential amino acid Substances 0.000 description 1
- 235000020776 essential amino acid Nutrition 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 238000013401 experimental design Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000010195 expression analysis Methods 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 210000004700 fetal blood Anatomy 0.000 description 1
- 229960000390 fludarabine Drugs 0.000 description 1
- GIUYCYHIANZCFB-FJFJXFQQSA-N fludarabine phosphate Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O GIUYCYHIANZCFB-FJFJXFQQSA-N 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 125000001153 fluoro group Chemical group F* 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 239000012595 freezing medium Substances 0.000 description 1
- 238000010230 functional analysis Methods 0.000 description 1
- 238000002825 functional assay Methods 0.000 description 1
- 238000007306 functionalization reaction Methods 0.000 description 1
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 1
- 229960005277 gemcitabine Drugs 0.000 description 1
- 238000012215 gene cloning Methods 0.000 description 1
- 238000012239 gene modification Methods 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 230000005017 genetic modification Effects 0.000 description 1
- 235000013617 genetically modified food Nutrition 0.000 description 1
- 210000004602 germ cell Anatomy 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 1
- 108010033706 glycylserine Proteins 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- UYTPUPDQBNUYGX-UHFFFAOYSA-N guanine Chemical class O=C1NC(N)=NC2=C1N=CN2 UYTPUPDQBNUYGX-UHFFFAOYSA-N 0.000 description 1
- 229940029575 guanosine Drugs 0.000 description 1
- 230000003394 haemopoietic effect Effects 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 210000003494 hepatocyte Anatomy 0.000 description 1
- 229940064366 hespan Drugs 0.000 description 1
- 208000021760 high fever Diseases 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 230000036543 hypotension Effects 0.000 description 1
- 230000007954 hypoxia Effects 0.000 description 1
- 230000008004 immune attack Effects 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 230000016784 immunoglobulin production Effects 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 230000001506 immunosuppresive effect Effects 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 229910052738 indium Inorganic materials 0.000 description 1
- 231100000405 induce cancer Toxicity 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 102000006495 integrins Human genes 0.000 description 1
- 108010044426 integrins Proteins 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 230000002601 intratumoral effect Effects 0.000 description 1
- 206010073095 invasive ductal breast carcinoma Diseases 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 238000005304 joining Methods 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 210000003292 kidney cell Anatomy 0.000 description 1
- 239000010410 layer Substances 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 238000004020 luminiscence type Methods 0.000 description 1
- 210000004324 lymphatic system Anatomy 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 208000006178 malignant mesothelioma Diseases 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 210000005033 mesothelial cell Anatomy 0.000 description 1
- 208000021039 metastatic melanoma Diseases 0.000 description 1
- 238000000520 microinjection Methods 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 230000000877 morphologic effect Effects 0.000 description 1
- 239000012452 mother liquor Substances 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 201000010879 mucinous adenocarcinoma Diseases 0.000 description 1
- 208000022669 mucinous neoplasm Diseases 0.000 description 1
- 210000000066 myeloid cell Anatomy 0.000 description 1
- 239000002088 nanocapsule Substances 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 229960005027 natalizumab Drugs 0.000 description 1
- 210000000822 natural killer cell Anatomy 0.000 description 1
- 230000008693 nausea Effects 0.000 description 1
- 108010069768 negative elongation factor Proteins 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 239000007764 o/w emulsion Substances 0.000 description 1
- 229920002113 octoxynol Polymers 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 210000000277 pancreatic duct Anatomy 0.000 description 1
- 201000006691 pancreatic squamous cell carcinoma Diseases 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 229930192851 perforin Natural products 0.000 description 1
- 210000003516 pericardium Anatomy 0.000 description 1
- 210000005105 peripheral blood lymphocyte Anatomy 0.000 description 1
- 210000004303 peritoneum Anatomy 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 239000002590 phosphodiesterase V inhibitor Substances 0.000 description 1
- 238000000053 physical method Methods 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 229940081858 plasmalyte a Drugs 0.000 description 1
- 210000004224 pleura Anatomy 0.000 description 1
- 229920000656 polylysine Polymers 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 210000004986 primary T-cell Anatomy 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 239000000186 progesterone Substances 0.000 description 1
- 229960003387 progesterone Drugs 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 238000013102 re-test Methods 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000000306 recurrent effect Effects 0.000 description 1
- 238000005057 refrigeration Methods 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 230000022532 regulation of transcription, DNA-dependent Effects 0.000 description 1
- 210000003289 regulatory T cell Anatomy 0.000 description 1
- 230000003362 replicative effect Effects 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 229930002330 retinoic acid Natural products 0.000 description 1
- 238000003757 reverse transcription PCR Methods 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 206010039073 rheumatoid arthritis Diseases 0.000 description 1
- 210000003705 ribosome Anatomy 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 229960003310 sildenafil Drugs 0.000 description 1
- 238000004088 simulation Methods 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 201000000849 skin cancer Diseases 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 238000011476 stem cell transplantation Methods 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 206010042863 synovial sarcoma Diseases 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 229960000835 tadalafil Drugs 0.000 description 1
- IEHKWSGCTWLXFU-IIBYNOLFSA-N tadalafil Chemical compound C1=C2OCOC2=CC([C@@H]2C3=C([C]4C=CC=CC4=N3)C[C@H]3N2C(=O)CN(C3=O)C)=C1 IEHKWSGCTWLXFU-IIBYNOLFSA-N 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 229960002180 tetracycline Drugs 0.000 description 1
- 235000019364 tetracycline Nutrition 0.000 description 1
- 150000003522 tetracyclines Chemical class 0.000 description 1
- 238000010257 thawing Methods 0.000 description 1
- 230000002992 thymic effect Effects 0.000 description 1
- 229940104230 thymidine Drugs 0.000 description 1
- 201000002510 thyroid cancer Diseases 0.000 description 1
- 108010078373 tisagenlecleucel Proteins 0.000 description 1
- 229960003989 tocilizumab Drugs 0.000 description 1
- 230000005026 transcription initiation Effects 0.000 description 1
- 230000005030 transcription termination Effects 0.000 description 1
- 238000010361 transduction Methods 0.000 description 1
- 230000026683 transduction Effects 0.000 description 1
- 238000003146 transient transfection Methods 0.000 description 1
- 229950007217 tremelimumab Drugs 0.000 description 1
- 230000005740 tumor formation Effects 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- 230000004222 uncontrolled growth Effects 0.000 description 1
- 241001529453 unidentified herpesvirus Species 0.000 description 1
- 241001515965 unidentified phage Species 0.000 description 1
- DRTQHJPVMGBUCF-UHFFFAOYSA-N uracil arabinoside Natural products OC1C(O)C(CO)OC1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-UHFFFAOYSA-N 0.000 description 1
- 229940045145 uridine Drugs 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- 229960005486 vaccine Drugs 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 210000002845 virion Anatomy 0.000 description 1
- 230000004304 visual acuity Effects 0.000 description 1
- 238000011179 visual inspection Methods 0.000 description 1
- QYSXJUFSXHHAJI-YRZJJWOYSA-N vitamin D3 Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C\C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-YRZJJWOYSA-N 0.000 description 1
- 235000005282 vitamin D3 Nutrition 0.000 description 1
- 239000011647 vitamin D3 Substances 0.000 description 1
- 229940021056 vitamin d3 Drugs 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 238000012447 xenograft mouse model Methods 0.000 description 1
- 229940055760 yervoy Drugs 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/11—T-cells, e.g. tumour infiltrating lymphocytes [TIL] or regulatory T [Treg] cells; Lymphokine-activated killer [LAK] cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/30—Cellular immunotherapy characterised by the recombinant expression of specific molecules in the cells of the immune system
- A61K40/31—Chimeric antigen receptors [CAR]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4202—Receptors, cell surface antigens or cell surface determinants
- A61K40/4224—Molecules with a "CD" designation not provided for elsewhere
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4231—Cytokines
- A61K40/4234—Interleukins [IL]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4231—Cytokines
- A61K40/4235—Interferons [IFN]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4254—Adhesion molecules, e.g. NRCAM, EpCAM or cadherins
- A61K40/4255—Mesothelin [MSLN]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
- C07K14/4701—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals not used
- C07K14/4748—Tumour specific antigens; Tumour rejection antigen precursors [TRAP], e.g. MAGE
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0636—T lymphocytes
- C12N5/0638—Cytotoxic T lymphocytes [CTL] or lymphokine activated killer cells [LAK]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0646—Natural killers cells [NK], NKT cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/57—Medicinal preparations containing antigens or antibodies characterised by the type of response, e.g. Th1, Th2
- A61K2039/572—Medicinal preparations containing antigens or antibodies characterised by the type of response, e.g. Th1, Th2 cytotoxic response
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/58—Medicinal preparations containing antigens or antibodies raising an immune response against a target which is not the antigen used for immunisation
- A61K2039/585—Medicinal preparations containing antigens or antibodies raising an immune response against a target which is not the antigen used for immunisation wherein the target is cancer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/60—Medicinal preparations containing antigens or antibodies characteristics by the carrier linked to the antigen
- A61K2039/6006—Cells
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/7051—T-cell receptor (TcR)-CD3 complex
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/569—Single domain, e.g. dAb, sdAb, VHH, VNAR or nanobody®
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/02—Fusion polypeptide containing a localisation/targetting motif containing a signal sequence
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/03—Fusion polypeptide containing a localisation/targetting motif containing a transmembrane segment
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/035—Fusion polypeptide containing a localisation/targetting motif containing a signal for targeting to the external surface of a cell, e.g. to the outer membrane of Gram negative bacteria, GPI- anchored eukaryote proteins
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/33—Fusion polypeptide fusions for targeting to specific cell types, e.g. tissue specific targeting, targeting of a bacterial subspecies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/70—Fusion polypeptide containing domain for protein-protein interaction
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Biomedical Technology (AREA)
- Epidemiology (AREA)
- Zoology (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- Wood Science & Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Medicinal Chemistry (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Cell Biology (AREA)
- General Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Hematology (AREA)
- Gastroenterology & Hepatology (AREA)
- Toxicology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Peptides Or Proteins (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662405551P | 2016-10-07 | 2016-10-07 | |
| US62/405,551 | 2016-10-07 | ||
| US201762510108P | 2017-05-23 | 2017-05-23 | |
| US62/510,108 | 2017-05-23 | ||
| PCT/US2017/055628 WO2018067993A1 (en) | 2016-10-07 | 2017-10-06 | Compositions and methods for t-cell receptors reprogramming using fusion proteins |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20190058509A true KR20190058509A (ko) | 2019-05-29 |
Family
ID=60302443
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197009508A Ceased KR20190058509A (ko) | 2016-10-07 | 2017-10-06 | 융합 단백질을 이용하는 t-세포 수용체 리프로그래밍을 위한 조성물 및 방법 |
Country Status (18)
| Country | Link |
|---|---|
| US (4) | US10208285B2 (enExample) |
| EP (2) | EP3445787B1 (enExample) |
| JP (2) | JP7217970B2 (enExample) |
| KR (1) | KR20190058509A (enExample) |
| CN (2) | CN109689686B (enExample) |
| AU (1) | AU2017341048A1 (enExample) |
| BR (1) | BR112019007100A2 (enExample) |
| CA (1) | CA3036745A1 (enExample) |
| DK (1) | DK3445787T3 (enExample) |
| ES (1) | ES2875959T3 (enExample) |
| GB (1) | GB2564823B8 (enExample) |
| HU (1) | HUE054080T2 (enExample) |
| IL (3) | IL292551B2 (enExample) |
| MX (1) | MX2019003899A (enExample) |
| PL (1) | PL3445787T3 (enExample) |
| PT (1) | PT3445787T (enExample) |
| TW (2) | TW202332690A (enExample) |
| WO (1) | WO2018067993A1 (enExample) |
Families Citing this family (67)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DK3298033T4 (da) | 2015-05-18 | 2023-10-02 | Tcr2 Therapeutics Inc | Sammensætninger og medicinske anvendelser til tcr-reprogrammering under anvendelse af fusionsproteiner |
| US11623958B2 (en) | 2016-05-20 | 2023-04-11 | Harpoon Therapeutics, Inc. | Single chain variable fragment CD3 binding proteins |
| IL263102B2 (en) | 2016-05-20 | 2023-11-01 | Harpoon Therapeutics Inc | A serum albumin-binding protein with a single site |
| JP7109789B2 (ja) | 2016-08-02 | 2022-08-01 | ティーシーアール2 セラピューティクス インク. | 融合タンパク質を使用したtcrの再プログラム化のための組成物及び方法 |
| CA3036745A1 (en) | 2016-10-07 | 2018-04-12 | TCR2 Therapeutics Inc. | Compositions and methods for t-cell receptors reprogramming using fusion proteins |
| CA3044593A1 (en) | 2016-11-22 | 2018-05-31 | TCR2 Therapeutics Inc. | Compositions and methods for tcr reprogramming using fusion proteins |
| AU2017382902A1 (en) * | 2016-12-21 | 2019-07-18 | TCR2 Therapeutics Inc. | Engineered T cells for the treatment of cancer |
| WO2018160754A2 (en) | 2017-02-28 | 2018-09-07 | Harpoon Therapeutics, Inc. | Inducible monovalent antigen binding protein |
| IL310182A (en) | 2017-04-26 | 2024-03-01 | Eureka Therapeutics Inc ׂ A Delaware Corp | Structures of chimeric antibody/T-cell receptor and uses thereof |
| MX420947B (es) | 2017-04-26 | 2025-02-10 | Eureka Therapeutics Inc | Constructos que reconocen específicamente glipicano 3 y usos de estos. |
| KR102376863B1 (ko) * | 2017-05-12 | 2022-03-21 | 하푼 테라퓨틱스, 인크. | 메소텔린 결합 단백질 |
| US10730954B2 (en) * | 2017-05-12 | 2020-08-04 | Harpoon Therapeutics, Inc. | MSLN targeting trispecific proteins and methods of use |
| EP3638295A1 (en) * | 2017-06-13 | 2020-04-22 | TCR2 Therapeutics Inc. | Compositions and methods for tcr reprogramming using fusion proteins |
| IL315737A (en) | 2017-10-13 | 2024-11-01 | Harpoon Therapeutics Inc | B-cell maturation antigen-binding proteins |
| CN111630070B (zh) | 2017-10-13 | 2024-07-30 | 哈普恩治疗公司 | 三特异性蛋白质及使用方法 |
| BR112020016138A2 (pt) * | 2018-02-11 | 2020-12-15 | Memorial Sloan-Kettering Cancer Center | Receptores de células t não restritos ao hla e usos dos mesmos |
| AU2019222560B2 (en) | 2018-02-17 | 2025-07-03 | Flagship Pioneering Innovations V, Inc. | Compositions and methods for membrane protein delivery |
| WO2019222275A2 (en) * | 2018-05-14 | 2019-11-21 | TCR2 Therapeutics Inc. | Compositions and methods for tcr reprogramming using inducible fusion proteins |
| AU2019271138A1 (en) | 2018-05-14 | 2021-01-07 | Harpoon Therapeutics, Inc. | Binding moiety for conditional activation of immunoglobulin molecules |
| WO2020011247A1 (en) * | 2018-07-13 | 2020-01-16 | Nanjing Legend Biotech Co., Ltd. | Co-receptor systems for treating infectious diseases |
| US12240915B2 (en) | 2018-08-30 | 2025-03-04 | Innovative Cellular Therapeutics Holdings, Ltd. | Chimeric antigen receptor cells for treating solid tumor |
| TW202026006A (zh) * | 2018-08-30 | 2020-07-16 | 美商Tcr2療法股份有限公司 | 使用融合蛋白進行tcr再程式化之組成物及方法 |
| CN109097396B (zh) * | 2018-09-10 | 2022-10-11 | 上海细胞治疗集团有限公司 | 一种制备靶向间皮素的car-t细胞的方法 |
| WO2020061482A1 (en) | 2018-09-21 | 2020-03-26 | Harpoon Therapeutics, Inc. | Egfr binding proteins and methods of use |
| US10815311B2 (en) | 2018-09-25 | 2020-10-27 | Harpoon Therapeutics, Inc. | DLL3 binding proteins and methods of use |
| WO2020123649A1 (en) * | 2018-12-11 | 2020-06-18 | Lonza Walkersville, Inc. | Bedside automated cell engineering system and methods |
| US20200289568A1 (en) * | 2019-03-11 | 2020-09-17 | The Regents Of The University Of California | Deterministic mechanoporation for cell engineering |
| CA3134511A1 (en) * | 2019-03-22 | 2020-10-01 | TCR2 Therapeutics Inc. | Compositions and methods for tcr reprogramming using fusion proteins |
| EP3715368A1 (en) | 2019-03-28 | 2020-09-30 | Albert-Ludwigs-Universität Freiburg | Chimeric antigen receptors, vectors coding for such receptors and their use in the modification of t cells |
| WO2020201527A1 (en) | 2019-04-04 | 2020-10-08 | Umc Utrecht Holding B.V. | Modified immune receptor constructs |
| JP2022530037A (ja) * | 2019-04-22 | 2022-06-27 | ティーシーアール2 セラピューティクス インク. | 融合タンパク質を使用するtcr再プログラミングのための組成物及び方法 |
| AU2020276117A1 (en) * | 2019-05-16 | 2021-12-09 | Memorial Sloan-Kettering Cancer Center | Mesothelin cars and uses thereof |
| CN114729383A (zh) | 2019-07-02 | 2022-07-08 | 弗莱德哈钦森癌症研究中心 | 重组ad35载体及相关基因疗法改进 |
| AU2020316429B2 (en) * | 2019-07-23 | 2022-01-06 | Wen Yang | Composition and method for adoptive immunotherapy |
| WO2021030153A2 (en) * | 2019-08-09 | 2021-02-18 | A2 Biotherapeutics, Inc. | Engineered t cell receptors and uses thereof |
| US20210060069A1 (en) * | 2019-08-23 | 2021-03-04 | Innovative Cellular Therapeutics Holdings, Ltd. | Coupled redirected cells and uses thereof |
| BR112022004458A2 (pt) * | 2019-09-12 | 2022-05-31 | Tcr2 Therapeutics Inc | Composições e métodos para reprogramação de tcr usando proteínas de fusão |
| CN112500492B (zh) * | 2019-09-13 | 2023-08-04 | 中国科学院分子细胞科学卓越创新中心 | 一种嵌合抗原受体及其用途 |
| EP3795583A1 (en) * | 2019-09-19 | 2021-03-24 | Ecole Polytechnique Federale de Lausanne (EPFL) | Il10/fc fusion proteins useful as enhancers of immunotherapies |
| US20220389075A1 (en) | 2019-11-12 | 2022-12-08 | A2 Biotherapeutics, Inc. | Engineered t cell receptors and uses thereof |
| CN110903401A (zh) * | 2019-11-20 | 2020-03-24 | 浙江大学 | 一种靶向cd19的二代嵌合抗原受体及其表达载体与应用 |
| WO2021133959A2 (en) * | 2019-12-24 | 2021-07-01 | TCR2 Therapeutics Inc. | Compositions and methods for gamma delta tcr reprogramming using fusion proteins |
| AU2021205501A1 (en) * | 2020-01-10 | 2022-08-18 | TCR2 Therapeutics Inc. | Compositions and methods for autoimmunity regulation |
| US12076343B2 (en) | 2020-02-19 | 2024-09-03 | Innovative Cellular Therapeutics Holdings, Ltd. | Engineered safety in cell therapy |
| WO2021168303A1 (en) | 2020-02-21 | 2021-08-26 | Harpoon Therapeutics, Inc. | Flt3 binding proteins and methods of use |
| WO2022020720A1 (en) * | 2020-07-24 | 2022-01-27 | TCR2 Therapeutics Inc. | Compositions and methods for treating cancer |
| JP2023549780A (ja) * | 2020-11-04 | 2023-11-29 | ジュノー セラピューティクス インコーポレイテッド | 改変されたインバリアントcd3免疫グロブリンスーパーファミリー鎖遺伝子座からキメラ受容体を発現する細胞ならびに関連するポリヌクレオチドおよび方法 |
| WO2022099119A1 (en) * | 2020-11-09 | 2022-05-12 | A2 Biotherapeutics, Inc. | T-cell receptor substitutions in transmembrane domain |
| AU2021410788A1 (en) * | 2020-12-23 | 2023-08-03 | TCR2 Therapeutics Inc. | Compositions and methods for tcr reprogramming using fusion proteins |
| EP4301755A1 (en) | 2021-03-03 | 2024-01-10 | Juno Therapeutics, Inc. | Combination of a t cell therapy and a dgk inhibitor |
| EP4334341A2 (en) | 2021-05-06 | 2024-03-13 | Juno Therapeutics GmbH | Methods for stimulating and transducing t cells |
| WO2023010436A1 (zh) * | 2021-08-05 | 2023-02-09 | 卡瑞济(北京)生命科技有限公司 | Tcr表达构建体以及其制备方法和用途 |
| PE20241173A1 (es) | 2021-10-14 | 2024-05-28 | Arsenal Biosciences Inc | Celulas inmunitarias que tienen arnhc coexpresados y sistemas de compuerta logica |
| WO2023081767A1 (en) | 2021-11-05 | 2023-05-11 | Precision Biosciences, Inc. | Methods for immunotherapy |
| WO2023086379A2 (en) * | 2021-11-10 | 2023-05-19 | TCR2 Therapeutics Inc. | Compositions and methods for tcr reprogramming using fusion proteins |
| WO2023091910A1 (en) | 2021-11-16 | 2023-05-25 | Precision Biosciences, Inc. | Methods for cancer immunotherapy |
| WO2023108150A1 (en) | 2021-12-10 | 2023-06-15 | Precision Biosciences, Inc. | Methods for cancer immunotherapy |
| JP2025501272A (ja) | 2021-12-28 | 2025-01-17 | ムネモ・セラピューティクス | 不活性化されたsuv39h1及び改変tcrを有する免疫細胞 |
| US20250115916A1 (en) | 2022-01-21 | 2025-04-10 | Mnemo Therapeutics | Modulation of suv39h1 expression by rnas |
| WO2023200761A2 (en) * | 2022-04-11 | 2023-10-19 | IN8bio, Inc. | Ipsc-based gamma-delta t-cells, compositions and methods of use thereof |
| WO2023213969A1 (en) | 2022-05-05 | 2023-11-09 | Juno Therapeutics Gmbh | Viral-binding protein and related reagents, articles, and methods of use |
| EP4279085A1 (en) | 2022-05-20 | 2023-11-22 | Mnemo Therapeutics | Compositions and methods for treating a refractory or relapsed cancer or a chronic infectious disease |
| CN120152717A (zh) | 2022-09-08 | 2025-06-13 | 朱诺治疗学股份有限公司 | T细胞疗法和连续或间歇dgk抑制剂给药的组合 |
| WO2024062138A1 (en) | 2022-09-23 | 2024-03-28 | Mnemo Therapeutics | Immune cells comprising a modified suv39h1 gene |
| WO2024100604A1 (en) | 2022-11-09 | 2024-05-16 | Juno Therapeutics Gmbh | Methods for manufacturing engineered immune cells |
| CN121002052A (zh) | 2023-02-03 | 2025-11-21 | C3S2 有限公司 | 用于非病毒生产工程化免疫细胞的方法 |
| WO2025101820A1 (en) | 2023-11-08 | 2025-05-15 | Fred Hutchinson Cancer Center | Compositions and methods for cellular immunotherapy |
Family Cites Families (232)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR901228A (fr) | 1943-01-16 | 1945-07-20 | Deutsche Edelstahlwerke Ag | Système d'aimant à entrefer annulaire |
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US5225539A (en) | 1986-03-27 | 1993-07-06 | Medical Research Council | Recombinant altered antibodies and methods of making altered antibodies |
| US6548640B1 (en) | 1986-03-27 | 2003-04-15 | Btg International Limited | Altered antibodies |
| GB8607679D0 (en) | 1986-03-27 | 1986-04-30 | Winter G P | Recombinant dna product |
| US6534055B1 (en) | 1988-11-23 | 2003-03-18 | Genetics Institute, Inc. | Methods for selectively stimulating proliferation of T cells |
| US5858358A (en) | 1992-04-07 | 1999-01-12 | The United States Of America As Represented By The Secretary Of The Navy | Methods for selectively stimulating proliferation of T cells |
| US6905680B2 (en) | 1988-11-23 | 2005-06-14 | Genetics Institute, Inc. | Methods of treating HIV infected subjects |
| US6352694B1 (en) | 1994-06-03 | 2002-03-05 | Genetics Institute, Inc. | Methods for inducing a population of T cells to proliferate using agents which recognize TCR/CD3 and ligands which stimulate an accessory molecule on the surface of the T cells |
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5703055A (en) | 1989-03-21 | 1997-12-30 | Wisconsin Alumni Research Foundation | Generation of antibodies through lipid mediated DNA delivery |
| US5399346A (en) | 1989-06-14 | 1995-03-21 | The United States Of America As Represented By The Department Of Health And Human Services | Gene therapy |
| US5585362A (en) | 1989-08-22 | 1996-12-17 | The Regents Of The University Of Michigan | Adenovirus vectors for gene therapy |
| GB8928874D0 (en) | 1989-12-21 | 1990-02-28 | Celltech Ltd | Humanised antibodies |
| DE69233482T2 (de) | 1991-05-17 | 2006-01-12 | Merck & Co., Inc. | Verfahren zur Verminderung der Immunogenität der variablen Antikörperdomänen |
| US5199942A (en) | 1991-06-07 | 1993-04-06 | Immunex Corporation | Method for improving autologous transplantation |
| DE122004000008I1 (de) | 1991-06-14 | 2005-06-09 | Genentech Inc | Humanisierter Heregulin Antikörper. |
| US5565332A (en) | 1991-09-23 | 1996-10-15 | Medical Research Council | Production of chimeric antibodies - a combinatorial approach |
| GB9125768D0 (en) | 1991-12-04 | 1992-02-05 | Hale Geoffrey | Therapeutic method |
| EP1291360A1 (en) | 1991-12-13 | 2003-03-12 | Xoma Corporation | Methods and materials for preparation of modified antibody variable domains and therapeutic uses thereof |
| GB9203459D0 (en) | 1992-02-19 | 1992-04-08 | Scotgen Ltd | Antibodies with germ-line variable regions |
| IL104570A0 (en) | 1992-03-18 | 1993-05-13 | Yeda Res & Dev | Chimeric genes and cells transformed therewith |
| US5350674A (en) | 1992-09-04 | 1994-09-27 | Becton, Dickinson And Company | Intrinsic factor - horse peroxidase conjugates and a method for increasing the stability thereof |
| US5639641A (en) | 1992-09-09 | 1997-06-17 | Immunogen Inc. | Resurfacing of rodent antibodies |
| US7175843B2 (en) | 1994-06-03 | 2007-02-13 | Genetics Institute, Llc | Methods for selectively stimulating proliferation of T cells |
| US5731168A (en) | 1995-03-01 | 1998-03-24 | Genentech, Inc. | Method for making heteromultimeric polypeptides |
| US6692964B1 (en) | 1995-05-04 | 2004-02-17 | The United States Of America As Represented By The Secretary Of The Navy | Methods for transfecting T cells |
| US7067318B2 (en) | 1995-06-07 | 2006-06-27 | The Regents Of The University Of Michigan | Methods for transfecting T cells |
| GB9809658D0 (en) | 1998-05-06 | 1998-07-01 | Celltech Therapeutics Ltd | Biological products |
| US7081518B1 (en) | 1999-05-27 | 2006-07-25 | The United States Of America As Represented By The Department Of Health And Human Services | Anti-mesothelin antibodies having high binding affinity |
| HK1047109A1 (zh) | 1999-10-15 | 2003-02-07 | University Of Massachusetts | 作为指定基因干预工具的rna干预轨迹基因 |
| US6326193B1 (en) | 1999-11-05 | 2001-12-04 | Cambria Biosciences, Llc | Insect control agent |
| US6797514B2 (en) | 2000-02-24 | 2004-09-28 | Xcyte Therapies, Inc. | Simultaneous stimulation and concentration of cells |
| KR20030032922A (ko) | 2000-02-24 | 2003-04-26 | 싸이트 테라피스 인코포레이티드 | 세포의 동시 자극 및 농축 |
| US7572631B2 (en) | 2000-02-24 | 2009-08-11 | Invitrogen Corporation | Activation and expansion of T cells |
| US6867041B2 (en) | 2000-02-24 | 2005-03-15 | Xcyte Therapies, Inc. | Simultaneous stimulation and concentration of cells |
| WO2001096584A2 (en) | 2000-06-12 | 2001-12-20 | Akkadix Corporation | Materials and methods for the control of nematodes |
| AU2001271589A1 (en) | 2000-06-30 | 2002-01-14 | Zymogenetics Inc. | Mammalian secreted proteins |
| CN1294148C (zh) | 2001-04-11 | 2007-01-10 | 中国科学院遗传与发育生物学研究所 | 环状单链三特异抗体 |
| EP1474451A2 (en) | 2002-02-13 | 2004-11-10 | Micromet AG | De-immunized (poly)peptide constructs |
| DE10244457A1 (de) | 2002-09-24 | 2004-04-01 | Johannes-Gutenberg-Universität Mainz | Verfahren zur rationalen Mutagenese von alpha/beta T-Zell Rezeptoren und entsprechend mutierte MDM2-Protein spezifische alpha/beta T-Zell Rezeptoren |
| NZ540196A (en) | 2002-11-08 | 2008-09-26 | Ablynx Nv | Camelidae antibodies against imminoglobulin E and use thereof for the treatment of allergic disorders |
| US20050129671A1 (en) | 2003-03-11 | 2005-06-16 | City Of Hope | Mammalian antigen-presenting T cells and bi-specific T cells |
| US7251470B2 (en) | 2003-06-25 | 2007-07-31 | Nokia Corporation | Emergency response system with personal emergency device |
| AU2004255216B2 (en) | 2003-07-01 | 2010-08-19 | Immunomedics, Inc. | Multivalent carriers of bi-specific antibodies |
| US7902338B2 (en) | 2003-07-31 | 2011-03-08 | Immunomedics, Inc. | Anti-CD19 antibodies |
| WO2005042743A2 (en) | 2003-08-18 | 2005-05-12 | Medimmune, Inc. | Humanization of antibodies |
| CA2537055A1 (en) | 2003-08-22 | 2005-04-21 | Medimmune, Inc. | Humanization of antibodies |
| US8076459B2 (en) | 2003-10-16 | 2011-12-13 | Micromet Ag | Multispecfic deimmunized CD3-binders |
| US20130266551A1 (en) | 2003-11-05 | 2013-10-10 | St. Jude Children's Research Hospital, Inc. | Chimeric receptors with 4-1bb stimulatory signaling domain |
| EP1786918A4 (en) | 2004-07-17 | 2009-02-11 | Imclone Systems Inc | NEW BISPECIFIC ANTIBODY TETRAVALENT |
| WO2006018833A2 (en) | 2004-08-16 | 2006-02-23 | Tadiran Spectralink Ltd. | A wearable device, system and method for measuring a pulse while a user is in motion |
| US9707302B2 (en) | 2013-07-23 | 2017-07-18 | Immunomedics, Inc. | Combining anti-HLA-DR or anti-Trop-2 antibodies with microtubule inhibitors, PARP inhibitors, bruton kinase inhibitors or phosphoinositide 3-kinase inhibitors significantly improves therapeutic outcome in cancer |
| AU2006223301B2 (en) | 2005-03-10 | 2010-11-04 | Eisai, Inc. | Anti-mesothelin antibodies |
| WO2006124641A2 (en) | 2005-05-12 | 2006-11-23 | The Government Of The United States, As Represented By The Secretary Of Health And Human Services, National Institutes Of Health | Anti-mesothelin antibodies useful for immunological assays |
| EP2500352A1 (en) | 2005-08-19 | 2012-09-19 | Abbott Laboratories | Dual variable domain immunoglobulin and uses thereof |
| AT503861B1 (de) | 2006-07-05 | 2008-06-15 | F Star Biotech Forsch & Entw | Verfahren zur manipulation von t-zell-rezeptoren |
| JP5840837B2 (ja) | 2007-03-30 | 2016-01-06 | メモリアル スローン−ケタリング キャンサー センター | 養子移入tリンパ球における副刺激リガンドの構成性発現 |
| MX2010005603A (es) | 2007-11-26 | 2010-08-02 | Bayer Schering Pharma Ag | Anticuerpos antimesotelina y usos de los mismos. |
| EP2225275A4 (en) | 2007-11-28 | 2013-04-03 | Medimmune Llc | PROTEIN FORMULATION |
| KR20090092900A (ko) | 2008-02-28 | 2009-09-02 | 신준호 | 어린이 및 노약자 안전보호시스템 |
| US20100122358A1 (en) | 2008-06-06 | 2010-05-13 | Crescendo Biologics Limited | H-Chain-only antibodies |
| US20090322513A1 (en) | 2008-06-27 | 2009-12-31 | Franklin Dun-Jen Hwang | Medical emergency alert system and method |
| WO2010025177A1 (en) | 2008-08-26 | 2010-03-04 | City Of Hope | Method and compositions for enhanced anti-tumor effector functioning of t cells |
| AU2009299791B2 (en) | 2008-10-01 | 2016-02-25 | Amgen Research (Munich) Gmbh | Cross-species-specific PSCAxCD3, CD19xCD3, C-METxCD3, EndosialinxCD3, EpCAMxC D3, IGF-1RxCD3 or FAPalpha xCD3 bispecific single chain antibody |
| HUE028175T2 (en) | 2008-11-07 | 2016-12-28 | Amgen Res Munich Gmbh | Treatment of acute lymphoblastic leukemia |
| NZ594985A (en) | 2009-03-10 | 2013-07-26 | Biogen Idec Inc | Anti-bcma (b-cell maturation antigen, cd269, tnfrsf17) antibodies |
| EP2421899B1 (en) | 2009-04-23 | 2016-06-08 | The United States of America, as represented by The Secretary, Department of Health and Human Services | Anti-human ror1 antibodies |
| EP2258719A1 (en) | 2009-05-19 | 2010-12-08 | Max-Delbrück-Centrum für Molekulare Medizin (MDC) | Multiple target T cell receptor |
| EP3260092A1 (en) | 2009-10-23 | 2017-12-27 | Nexisvision, Inc. | Corneal denervation for treatment of ocular pain |
| WO2011059836A2 (en) | 2009-10-29 | 2011-05-19 | Trustees Of Dartmouth College | T cell receptor-deficient t cell compositions |
| PT2361936T (pt) | 2010-02-25 | 2016-07-15 | Affimed Gmbh | Molécula que se liga a antigénio e utilizações desta |
| PL2552959T3 (pl) | 2010-03-26 | 2017-08-31 | Memorial Sloan-Kettering Cancer Center | Przeciwciała przeciwko MUC16 i metody stosowania |
| WO2012008860A2 (en) | 2010-07-16 | 2012-01-19 | Auckland Uniservices Limited | Bacterial nitroreductase enzymes and methods relating thereto |
| EP3467101A3 (en) | 2010-09-08 | 2019-06-19 | Baylor College of Medicine | Immunotherapy of brain tumor using geneticially engineered gd2-specific t cells |
| HUE048639T2 (hu) | 2010-10-27 | 2020-08-28 | Amgen Res Munich Gmbh | Eljárás DLBCL kezelésére |
| NZ609967A (en) | 2010-10-27 | 2015-04-24 | Baylor College Medicine | Chimeric cd27 receptors for redirecting t cells to cd70-positive malignancies |
| CA2818992C (en) | 2010-12-01 | 2021-05-04 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Chimeric rabbit/human ror1 antibodies |
| PH12013501201A1 (en) | 2010-12-09 | 2013-07-29 | Univ Pennsylvania | Use of chimeric antigen receptor-modified t cells to treat cancer |
| GB201020995D0 (en) | 2010-12-10 | 2011-01-26 | Bioinvent Int Ab | Biological materials and uses thereof |
| EP3296321B1 (en) | 2010-12-20 | 2020-07-15 | F. Hoffmann-La Roche AG | Anti-mesothelin antibodies and immunoconjugates |
| WO2012097313A2 (en) | 2011-01-14 | 2012-07-19 | The Regents Of The University Of California | Therapeutic antibodies against ror-1 protein and methods for use of same |
| AU2012207356A1 (en) | 2011-01-18 | 2013-08-01 | The Trustees Of The University Of Pennsylvania | Compositions and methods for treating cancer |
| JP5955871B2 (ja) | 2011-03-17 | 2016-07-20 | ミルテニイ バイオテック ゲゼルシャフト ミット ベシュレンクテル ハフツング | Tcrアルファ/ベータを枯渇させた細胞調製物 |
| PL2694549T3 (pl) | 2011-04-08 | 2019-01-31 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Chimeryczne receptory antygenowe anty-wariant III receptora czynnika wzrostu naskórka i ich zastosowanie do leczenia raka |
| UA117901C2 (uk) | 2011-07-06 | 2018-10-25 | Ґенмаб Б.В. | Спосіб посилення ефекторної функції вихідного поліпептиду, його варіанти та їх застосування |
| WO2013040557A2 (en) | 2011-09-16 | 2013-03-21 | The Trustees Of The University Of Pennsylvania | Rna engineered t cells for the treatment of cancer |
| US9272002B2 (en) | 2011-10-28 | 2016-03-01 | The Trustees Of The University Of Pennsylvania | Fully human, anti-mesothelin specific chimeric immune receptor for redirected mesothelin-expressing cell targeting |
| WO2013067492A1 (en) | 2011-11-03 | 2013-05-10 | The Trustees Of The University Of Pennsylvania | Isolated b7-h4 specific compositions and methods of use thereof |
| US20140322216A1 (en) | 2011-11-08 | 2014-10-30 | The Trustees Of The University Of Pennsylvania | Glypican-3-specific antibody and uses thereof |
| TWI679212B (zh) | 2011-11-15 | 2019-12-11 | 美商安進股份有限公司 | 針對bcma之e3以及cd3的結合分子 |
| EP2814846B1 (en) | 2012-02-13 | 2020-01-08 | Seattle Children's Hospital d/b/a Seattle Children's Research Institute | Bispecific chimeric antigen receptors and therapeutic uses thereof |
| CN104159909A (zh) | 2012-02-22 | 2014-11-19 | 宾夕法尼亚大学董事会 | 产生用于癌症治疗的t细胞持续性群体的组合物和方法 |
| ES2959443T3 (es) | 2012-02-22 | 2024-02-26 | Univ Pennsylvania | Uso del dominio de señalización de CD2 en receptores de antígenos quiméricos de segunda generación |
| EP2828290B1 (en) | 2012-03-23 | 2018-08-15 | The United States of America, represented by the Secretary, Department of Health and Human Services | Anti-mesothelin chimeric antigen receptors |
| KR102086874B1 (ko) | 2012-04-11 | 2020-03-10 | 더 유나이티드 스테이츠 오브 어메리카, 애즈 리프리젠티드 바이 더 세크러테리, 디파트먼트 오브 헬쓰 앤드 휴먼 서비씨즈 | B-세포 성숙 항원을 표적화하는 키메라 항원 수용체 |
| US20130280220A1 (en) | 2012-04-20 | 2013-10-24 | Nabil Ahmed | Chimeric antigen receptor for bispecific activation and targeting of t lymphocytes |
| CN111676196A (zh) | 2012-05-25 | 2020-09-18 | 塞勒克提斯公司 | 工程化异体和免疫抑制耐受性t细胞的方法 |
| EP3584256A1 (en) | 2012-07-13 | 2019-12-25 | The Trustees Of The University Of Pennsylvania | Methods of assessing the suitability of transduced t cells for administration |
| CA2876730A1 (en) | 2012-07-13 | 2014-01-16 | The Trustees Of The University Of Pennsylvania | Enhancing activity of car t cells by co-introducing a bispecific antibody |
| WO2014011993A2 (en) | 2012-07-13 | 2014-01-16 | The Trustees Of The University Of Pennsylvania | Epitope spreading associated with car t-cells |
| IL293944A (en) | 2012-08-20 | 2022-08-01 | Hutchinson Fred Cancer Res | Method and preparations for cellular immunotherapy |
| EP2900695B1 (en) * | 2012-09-27 | 2018-01-17 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Mesothelin antibodies and methods for eliciting potent antitumor activity |
| AR092745A1 (es) | 2012-10-01 | 2015-04-29 | Univ Pennsylvania | Composiciones que comprenden un dominio de union anti-fap y metodos para hacer blanco en celulas estromales para el tratamiento del cancer |
| KR102198058B1 (ko) | 2012-10-02 | 2021-01-06 | 메모리얼 슬로안 케터링 캔서 센터 | 면역치료용 조성물 및 방법 |
| CN104271602B (zh) | 2012-11-21 | 2020-08-21 | 武汉友芝友生物制药有限公司 | 双特异性抗体 |
| US8697359B1 (en) | 2012-12-12 | 2014-04-15 | The Broad Institute, Inc. | CRISPR-Cas systems and methods for altering expression of gene products |
| US20150329640A1 (en) | 2012-12-20 | 2015-11-19 | Bluebird Bio, Inc. | Chimeric antigen receptors and immune cells targeting b cell malignancies |
| EP2953974B1 (en) | 2013-02-05 | 2017-12-20 | EngMab Sàrl | Bispecific antibodies against cd3epsilon and bcma |
| KR102417657B1 (ko) | 2013-02-06 | 2022-07-07 | 안트로제네시스 코포레이션 | 개선된 특이성을 갖는 변경된 t 림프구 |
| KR102332790B1 (ko) | 2013-02-15 | 2021-12-01 | 더 리젠츠 오브 더 유니버시티 오브 캘리포니아 | 키메라 항원 수용체 및 이의 이용 방법 |
| EP3744736A1 (en) | 2013-02-20 | 2020-12-02 | Novartis AG | Effective targeting of primary human leukemia using anti-cd123 chimeric antigen receptor engineered t cells |
| CN105143270B (zh) | 2013-02-26 | 2019-11-12 | 罗切格利卡特公司 | 双特异性t细胞活化抗原结合分子 |
| KR102363191B1 (ko) | 2013-02-26 | 2022-02-17 | 메모리얼 슬로안 케터링 캔서 센터 | 면역치료용 조성물 및 방법 |
| US9393257B2 (en) | 2013-03-01 | 2016-07-19 | Regents Of The University Of Minnesota | TALEN-based gene correction |
| EP2964675B1 (en) | 2013-03-05 | 2018-06-20 | Baylor College Of Medicine | Engager cells for immunotherapy |
| US10150800B2 (en) | 2013-03-15 | 2018-12-11 | Zyngenia, Inc. | EGFR-binding modular recognition domains |
| CA2904265C (en) | 2013-03-15 | 2023-08-08 | Victor D. FEDOROV | Compositions and methods for immunotherapy |
| CN105518018B (zh) | 2013-03-15 | 2020-04-03 | 细胞基因公司 | 修饰的t淋巴细胞 |
| AR095374A1 (es) | 2013-03-15 | 2015-10-14 | Amgen Res Munich Gmbh | Moléculas de unión para bcma y cd3 |
| US9446105B2 (en) | 2013-03-15 | 2016-09-20 | The Trustees Of The University Of Pennsylvania | Chimeric antigen receptor specific for folate receptor β |
| UY35468A (es) | 2013-03-16 | 2014-10-31 | Novartis Ag | Tratamiento de cáncer utilizando un receptor quimérico de antígeno anti-cd19 |
| CA3185368A1 (en) | 2013-04-03 | 2014-10-09 | Memorial Sloan-Kettering Cancer Center | Effective generation of tumor-targeted t cells derived from pluripotent stem cells |
| US11311575B2 (en) | 2013-05-13 | 2022-04-26 | Cellectis | Methods for engineering highly active T cell for immunotherapy |
| RU2727447C2 (ru) | 2013-05-13 | 2020-07-21 | Селлектис | Cd19-специфический химерный антигенный рецептор и его применения |
| EP3004168A4 (en) | 2013-05-24 | 2017-03-01 | Board of Regents, The University of Texas System | Chimeric antigen receptor-targeting monoclonal antibodies |
| EP3309248B1 (en) | 2013-05-29 | 2021-06-09 | Cellectis | Methods for engineering t cells for immunotherapy by using rna-guided cas nuclease system |
| ES2883131T3 (es) | 2013-05-29 | 2021-12-07 | Cellectis | Métodos para la modificación de células T para inmunoterapia utilizando el sistema de nucleasa CAS guiado por ARN |
| AU2014287011A1 (en) | 2013-07-12 | 2016-02-25 | Zymeworks Inc. | Bispecific CD3 and CD19 antigen binding constructs |
| US9879218B2 (en) | 2013-08-01 | 2018-01-30 | Fermentalg | Methods for the production of diatom biomass |
| EP3027755B1 (en) | 2013-08-02 | 2019-10-09 | The Regents of The University of California | Engineering antiviral t cell immunity through stem cells and chimeric antigen receptors |
| GB201317929D0 (en) | 2013-10-10 | 2013-11-27 | Ucl Business Plc | Chimeric antigen receptor |
| CN105829349B (zh) | 2013-10-15 | 2023-02-03 | 斯克利普斯研究所 | 肽嵌合抗原受体t细胞开关和其用途 |
| US20150238631A1 (en) | 2013-10-15 | 2015-08-27 | The California Institute For Biomedical Research | Chimeric antigen receptor t cell switches and uses thereof |
| EP4026909A1 (en) | 2013-12-19 | 2022-07-13 | Novartis AG | Human mesothelin chimeric antigen receptors and uses thereof |
| PT3083671T (pt) | 2013-12-20 | 2020-12-24 | Hutchinson Fred Cancer Res | Moléculas efetoras quiméricas etiquetadas e recetores destas |
| EP3087101B1 (en) | 2013-12-20 | 2024-06-05 | Novartis AG | Regulatable chimeric antigen receptor |
| JP2017504601A (ja) | 2013-12-20 | 2017-02-09 | セレクティスCellectis | 免疫療法のためにマルチインプットシグナル感受性t細胞を操作する方法 |
| DK3089994T3 (da) | 2013-12-30 | 2022-07-04 | Epimab Biotherapeutics Inc | Fabs-in-tandem-immunglobulin og anvendelser deraf |
| AU2015206040B2 (en) | 2014-01-14 | 2018-11-01 | Cellectis | Chimeric antigen receptor using antigen recognition domains derived from cartilaginous fish |
| US11028143B2 (en) | 2014-01-21 | 2021-06-08 | Novartis Ag | Enhanced antigen presenting ability of RNA CAR T cells by co-introduction of costimulatory molecules |
| TWI681969B (zh) | 2014-01-23 | 2020-01-11 | 美商再生元醫藥公司 | 針對pd-1的人類抗體 |
| EP3097123A1 (en) | 2014-01-24 | 2016-11-30 | The United States of America, as represented by The Secretary, Department of Health and Human Services | Anti-ny-br-1 polypeptides, proteins, and chimeric antigen receptors |
| RS66199B1 (sr) | 2014-02-04 | 2024-12-31 | The United States Of America As Represented By The Secretary Department Of Health And Human Services | Metode za proizvodnju autolognih t ćelija koje su korisne za lečenje b ćelijskih maligniteta i drugih karcinoma i njihove kompozicije |
| KR102480433B1 (ko) * | 2014-02-07 | 2022-12-21 | 맥마스터 유니버시티 | 3기능성 t 세포-항원 커플러 및 이의 제조 방법 및 용도 |
| MX369513B (es) | 2014-02-14 | 2019-11-11 | Univ Texas | Receptores de antígenos quiméricos y métodos de realización. |
| CA2937711C (en) | 2014-02-14 | 2020-10-20 | Cellectis | Cells for immunotherapy engineered for targeting antigen present both on immune cells and pathological cells |
| US10196608B2 (en) | 2014-02-21 | 2019-02-05 | Cellectis | Method for in situ inhibition of regulatory T cells |
| CN106029098A (zh) | 2014-02-25 | 2016-10-12 | 免疫医疗公司 | 人源化rfb4抗cd22抗体 |
| KR101605421B1 (ko) | 2014-03-05 | 2016-03-23 | 국립암센터 | B 세포 림프종 세포를 특이적으로 인지하는 단일클론항체 및 이의 용도 |
| EP3811970A1 (en) | 2014-03-15 | 2021-04-28 | Novartis AG | Regulatable chimeric antigen receptor |
| EP3593812A3 (en) | 2014-03-15 | 2020-05-27 | Novartis AG | Treatment of cancer using chimeric antigen receptor |
| DK3126381T4 (da) | 2014-04-01 | 2022-04-04 | Biontech Cell&Gene Therapies Gmbh | Claudin-6-specifikke immunoreceptorer og t-celleepitoper |
| JP6698546B2 (ja) | 2014-04-14 | 2020-05-27 | セレクティスCellectis | 癌免疫療法のためのbcma(cd269)特異的キメラ抗原受容体 |
| RS60544B1 (sr) | 2014-04-25 | 2020-08-31 | Bluebird Bio Inc | Poboljšani metodi za proizvodnju adoptivnih ćelijskih terapija |
| CN107074929B (zh) | 2014-05-02 | 2021-09-03 | 宾夕法尼亚大学董事会 | 嵌合自身抗体受体t细胞的组合物和方法 |
| WO2015177349A1 (en) | 2014-05-23 | 2015-11-26 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Anti- egfr conformational single domain antibodies and uses thereof |
| CN106535925A (zh) | 2014-05-23 | 2017-03-22 | 佛罗里达大学研究基金会有限公司 | 基于car的免疫治疗 |
| JP2017518053A (ja) | 2014-06-06 | 2017-07-06 | メモリアル スローン−ケタリング キャンサー センター | メソセリン標的化キメラ抗原受容体およびその使用 |
| KR20170032406A (ko) | 2014-07-15 | 2017-03-22 | 주노 쎄러퓨티크스 인코퍼레이티드 | 입양 세포 치료를 위한 조작된 세포 |
| JP7054622B2 (ja) | 2014-07-21 | 2022-04-14 | ノバルティス アーゲー | ヒト化抗bcmaキメラ抗原受容体を使用した癌の処置 |
| EP3172231B1 (en) | 2014-07-24 | 2021-05-05 | Bluebird Bio, Inc. | Bcma chimeric antigen receptors |
| MX367787B (es) | 2014-07-29 | 2019-09-06 | Cellectis | Receptores de antígenos quimericos ror1 (ntrkr1) específicos para inmunoterapia del cáncer. |
| EP2982692A1 (en) | 2014-08-04 | 2016-02-10 | EngMab AG | Bispecific antibodies against CD3epsilon and BCMA |
| US9994817B2 (en) | 2014-08-07 | 2018-06-12 | Northwestern University | Use of ligands for the programmed cell death receptor conjugated to solid supports for cultivating human regulatory T cells |
| WO2016019969A1 (en) | 2014-08-08 | 2016-02-11 | Ludwig-Maximilians-Universität München | Subcutaneously administered bispecific antibodies for use in the treatment of cancer |
| ES2819451T3 (es) | 2014-08-08 | 2021-04-16 | Univ Leland Stanford Junior | Agentes PD-1 de alta afinidad y procedimientos de uso |
| JP2017524365A (ja) | 2014-08-12 | 2017-08-31 | アントフロゲネシス コーポレーション | リンパ節のb細胞領域、皮膚、または腸管にホーミングするように操作されたcar−t細胞 |
| ES3034398T3 (en) | 2014-08-28 | 2025-08-18 | Bioatla Inc | Conditionally active chimeric antigen receptors for modified t-cells |
| GB201415347D0 (en) | 2014-08-29 | 2014-10-15 | Ucl Business Plc | Signalling system |
| WO2016036678A1 (en) | 2014-09-02 | 2016-03-10 | Medimmune, Llc | Formulations of bispecific antibodies |
| JP2017527310A (ja) | 2014-09-09 | 2017-09-21 | ユーナム・セラピューティクスUnum Therapeutics | キメラ受容体および免疫療法におけるその使用 |
| AU2015317351B2 (en) | 2014-09-19 | 2020-07-02 | City Of Hope | Costimulatory chimeric antigen receptor T cells targeting IL3Ra2 |
| WO2016054520A2 (en) | 2014-10-03 | 2016-04-07 | The California Institute For Biomedical Research | Engineered cell surface proteins and uses thereof |
| CN107109368A (zh) | 2014-10-07 | 2017-08-29 | 塞勒克提斯公司 | 用于调节car诱导的免疫细胞活性的方法 |
| SG11201703203RA (en) | 2014-10-20 | 2017-05-30 | Juno Therapeutics Inc | Methods and compositions for dosing in adoptive cell therapy |
| HK1243333A1 (zh) | 2014-10-31 | 2018-07-13 | The Trustees Of The University Of Pennsylvania | 用於修饰的t细胞的方法和组合物 |
| US10688166B2 (en) | 2014-11-03 | 2020-06-23 | Cerus Corporation | Compositions and methods for improved car-T cell therapies |
| HUE050264T2 (hu) | 2014-11-05 | 2020-11-30 | Juno Therapeutics Inc | Eljárások transzdukálásra és sejtfeldolgozásra |
| PL3221357T3 (pl) | 2014-11-20 | 2020-11-02 | F. Hoffmann-La Roche Ag | Wspólne łańcuchy lekkie i sposoby zastosowania |
| EP3023437A1 (en) | 2014-11-20 | 2016-05-25 | EngMab AG | Bispecific antibodies against CD3epsilon and BCMA |
| EP3025719B1 (en) | 2014-11-26 | 2018-09-26 | Miltenyi Biotec GmbH | Combination immunotherapy of antigen-recognizing receptors and hematopoietic cells for the treatment of diseases |
| EP3029067A1 (en) | 2014-12-01 | 2016-06-08 | Deutsches Krebsforschungszentrum | Use of blocking-reagents for reducing unspecific T cell-activation |
| EP3029068A1 (en) | 2014-12-03 | 2016-06-08 | EngMab AG | Bispecific antibodies against CD3epsilon and BCMA for use in the treatment of diseases |
| JP7068820B2 (ja) | 2014-12-03 | 2022-05-17 | ジュノー セラピューティクス インコーポレイテッド | 養子細胞療法のための方法および組成物 |
| US20180334490A1 (en) | 2014-12-03 | 2018-11-22 | Qilong H. Wu | Methods for b cell preconditioning in car therapy |
| EP3227432B1 (en) | 2014-12-05 | 2023-10-11 | Memorial Sloan Kettering Cancer Center | Chimeric antigen receptors targeting b-cell maturation antigen and uses thereof |
| KR102701479B1 (ko) | 2014-12-05 | 2024-09-03 | 메모리얼 슬로안 케터링 캔서 센터 | B-세포 성숙화 항원을 표적화하는 항체 및 사용 방법 |
| EP3227339B1 (en) | 2014-12-05 | 2021-11-10 | Memorial Sloan-Kettering Cancer Center | Chimeric antigen receptors targeting g-protein coupled receptor and uses thereof |
| EP3233095A2 (en) | 2014-12-17 | 2017-10-25 | Cellectis | INHIBITORY CHIMERIC ANTIGEN RECEPTOR (iCAR OR N-CAR) EXPRESSING NON-T CELL TRANSDUCTION DOMAIN |
| EP3037170A1 (en) | 2014-12-27 | 2016-06-29 | Miltenyi Biotec GmbH | Multisort cell separation method |
| WO2016109410A2 (en) | 2014-12-29 | 2016-07-07 | Novartis Ag | Methods of making chimeric antigen receptor-expressing cells |
| JP2018503399A (ja) | 2015-01-14 | 2018-02-08 | コンパス セラピューティクス リミテッド ライアビリティ カンパニー | 多特異性免疫調節抗原結合構築物 |
| US11459390B2 (en) | 2015-01-16 | 2022-10-04 | Novartis Ag | Phosphoglycerate kinase 1 (PGK) promoters and methods of use for expressing chimeric antigen receptor |
| TWI718118B (zh) | 2015-01-16 | 2021-02-11 | 美商奇諾治療有限公司 | 針對ror1之特異性抗體及嵌合抗原受體 |
| GB201501175D0 (en) | 2015-01-23 | 2015-03-11 | Univ Oslo Hf | A universal T-cell for personalised medicine |
| EP3250611B1 (en) | 2015-01-26 | 2021-04-21 | The University of Chicago | Car t-cells recognizing cancer-specific il 13r-alpha2 |
| US11161907B2 (en) | 2015-02-02 | 2021-11-02 | Novartis Ag | Car-expressing cells against multiple tumor antigens and uses thereof |
| AU2016215110A1 (en) | 2015-02-05 | 2017-09-21 | Stc.Unm | Anti-pre-BCR antagonists and methods |
| AU2016214978B2 (en) | 2015-02-05 | 2021-12-09 | The University Of Queensland | Targeting constructs for delivery of payloads |
| US20160228547A1 (en) | 2015-02-06 | 2016-08-11 | Batu Biologics, Inc. | Chimeric antigen receptor targeting of tumor endothelium |
| EP3256496B1 (en) | 2015-02-12 | 2020-12-30 | University Health Network | Chimeric antigen receptors |
| US20160237407A1 (en) | 2015-02-17 | 2016-08-18 | Batu Biologics, Inc. | Universal donor chimeric antigen receptor cells |
| JP6858128B2 (ja) | 2015-02-18 | 2021-04-14 | エンリヴェックス セラピューティクス リミテッド | 癌治療のための免疫療法とサイトカイン制御療法との組み合わせ |
| WO2016138034A1 (en) | 2015-02-24 | 2016-09-01 | The Regents Of The University Of California | Binding-triggered transcriptional switches and methods of use thereof |
| EP3089761B1 (en) | 2015-03-02 | 2019-06-12 | Innovative Cellular Therapeutics Co., Ltd. | Reducing immune tolerance induced by pd l1 |
| GB201504840D0 (en) | 2015-03-23 | 2015-05-06 | Ucl Business Plc | Chimeric antigen receptor |
| US11261232B2 (en) | 2015-04-02 | 2022-03-01 | Memorial Sloan Kettering Cancer Center | TNFRSF14 / HVEM proteins and methods of use thereof |
| DK3298033T4 (da) | 2015-05-18 | 2023-10-02 | Tcr2 Therapeutics Inc | Sammensætninger og medicinske anvendelser til tcr-reprogrammering under anvendelse af fusionsproteiner |
| CA2989949A1 (en) | 2015-06-19 | 2016-12-22 | Sebastian KOBOLD | Pd-1-cd28 fusion proteins and their use in medicine |
| TWI796283B (zh) | 2015-07-31 | 2023-03-21 | 德商安美基研究(慕尼黑)公司 | Msln及cd3抗體構築體 |
| JP6816133B2 (ja) | 2015-10-05 | 2021-01-20 | プレシジョン バイオサイエンシズ,インク. | 改変ヒトt細胞受容体アルファ定常領域遺伝子を含む遺伝子改変細胞 |
| WO2017112741A1 (en) | 2015-12-22 | 2017-06-29 | Novartis Ag | Mesothelin chimeric antigen receptor (car) and antibody against pd-l1 inhibitor for combined use in anticancer therapy |
| AU2017229687A1 (en) | 2016-03-08 | 2018-09-20 | Takeda Pharmaceutical Company Limited | Inducible binding proteins and methods of use |
| CN109328074A (zh) | 2016-04-01 | 2019-02-12 | 凯德药业股份有限公司 | 嵌合抗原和t细胞受体及使用的方法 |
| EP4371570A3 (en) | 2016-06-08 | 2024-07-17 | Xencor, Inc. | Treatment of igg4-related diseases with anti-cd19 antibodies crossbinding to cd32b |
| JP2019528051A (ja) | 2016-07-21 | 2019-10-10 | ディヴェロップメント センター フォー バイオテクノロジー | 修飾抗原結合Fab断片及びこれを含む抗原結合分子 |
| SG11201900677SA (en) | 2016-07-28 | 2019-02-27 | Novartis Ag | Combination therapies of chimeric antigen receptors adn pd-1 inhibitors |
| JP7109789B2 (ja) | 2016-08-02 | 2022-08-01 | ティーシーアール2 セラピューティクス インク. | 融合タンパク質を使用したtcrの再プログラム化のための組成物及び方法 |
| CA3036745A1 (en) | 2016-10-07 | 2018-04-12 | TCR2 Therapeutics Inc. | Compositions and methods for t-cell receptors reprogramming using fusion proteins |
| CA3044593A1 (en) | 2016-11-22 | 2018-05-31 | TCR2 Therapeutics Inc. | Compositions and methods for tcr reprogramming using fusion proteins |
| AU2017382902A1 (en) | 2016-12-21 | 2019-07-18 | TCR2 Therapeutics Inc. | Engineered T cells for the treatment of cancer |
| WO2018165619A1 (en) | 2017-03-09 | 2018-09-13 | Cytomx Therapeutics, Inc. | Cd147 antibodies, activatable cd147 antibodies, and methods of making and use thereof |
| IL310182A (en) | 2017-04-26 | 2024-03-01 | Eureka Therapeutics Inc ׂ A Delaware Corp | Structures of chimeric antibody/T-cell receptor and uses thereof |
| KR102376863B1 (ko) | 2017-05-12 | 2022-03-21 | 하푼 테라퓨틱스, 인크. | 메소텔린 결합 단백질 |
| US10730954B2 (en) | 2017-05-12 | 2020-08-04 | Harpoon Therapeutics, Inc. | MSLN targeting trispecific proteins and methods of use |
| EP3638295A1 (en) | 2017-06-13 | 2020-04-22 | TCR2 Therapeutics Inc. | Compositions and methods for tcr reprogramming using fusion proteins |
| WO2019173693A1 (en) | 2018-03-09 | 2019-09-12 | TCR2 Therapeutics Inc. | Compositions and methods for tcr reprogramming using fusion proteins |
| WO2019222275A2 (en) | 2018-05-14 | 2019-11-21 | TCR2 Therapeutics Inc. | Compositions and methods for tcr reprogramming using inducible fusion proteins |
| JP2021530503A (ja) | 2018-07-26 | 2021-11-11 | ティーシーアール2 セラピューティクス インク. | 標的特異的融合タンパク質を使用してtcrをリプログラミングするための組成物及び方法 |
| TW202026006A (zh) | 2018-08-30 | 2020-07-16 | 美商Tcr2療法股份有限公司 | 使用融合蛋白進行tcr再程式化之組成物及方法 |
| WO2020061526A1 (en) | 2018-09-21 | 2020-03-26 | Harpoon Therapeutics, Inc. | Conditionally activated target-binding molecules |
-
2017
- 2017-10-06 CA CA3036745A patent/CA3036745A1/en active Pending
- 2017-10-06 MX MX2019003899A patent/MX2019003899A/es unknown
- 2017-10-06 IL IL292551A patent/IL292551B2/en unknown
- 2017-10-06 JP JP2019513450A patent/JP7217970B2/ja active Active
- 2017-10-06 EP EP17797464.9A patent/EP3445787B1/en active Active
- 2017-10-06 GB GB1818962.1A patent/GB2564823B8/en not_active Expired - Fee Related
- 2017-10-06 EP EP20211103.5A patent/EP3848392A1/en not_active Withdrawn
- 2017-10-06 IL IL302917A patent/IL302917A/en unknown
- 2017-10-06 AU AU2017341048A patent/AU2017341048A1/en not_active Abandoned
- 2017-10-06 PT PT177974649T patent/PT3445787T/pt unknown
- 2017-10-06 PL PL17797464T patent/PL3445787T3/pl unknown
- 2017-10-06 BR BR112019007100A patent/BR112019007100A2/pt not_active Application Discontinuation
- 2017-10-06 HU HUE17797464A patent/HUE054080T2/hu unknown
- 2017-10-06 DK DK17797464.9T patent/DK3445787T3/da active
- 2017-10-06 CN CN201780039623.1A patent/CN109689686B/zh not_active Expired - Fee Related
- 2017-10-06 CN CN202010760989.1A patent/CN112280796A/zh active Pending
- 2017-10-06 KR KR1020197009508A patent/KR20190058509A/ko not_active Ceased
- 2017-10-06 ES ES17797464T patent/ES2875959T3/es active Active
- 2017-10-06 WO PCT/US2017/055628 patent/WO2018067993A1/en not_active Ceased
- 2017-10-11 TW TW111149290A patent/TW202332690A/zh unknown
- 2017-10-11 TW TW106134703A patent/TWI790213B/zh not_active IP Right Cessation
-
2018
- 2018-02-05 US US15/888,897 patent/US10208285B2/en active Active
- 2018-12-17 US US16/222,846 patent/US11377638B2/en active Active
-
2019
- 2019-04-02 IL IL265766A patent/IL265766B/en unknown
-
2020
- 2020-08-10 US US16/989,606 patent/US11085021B2/en not_active Expired - Fee Related
-
2022
- 2022-05-26 US US17/825,861 patent/US20220298478A1/en not_active Abandoned
-
2023
- 2023-01-17 JP JP2023005276A patent/JP2023052446A/ja active Pending
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN109689686B (zh) | 使用融合蛋白进行t细胞受体重编程的组合物和方法 | |
| JP7524249B2 (ja) | 抗癌治療における組み合わせ使用のためのメソテリンキメラ抗原受容体(car)およびpd-l1阻害剤に対する抗体 | |
| US20240398913A1 (en) | Methods for improving the efficacy and expansion of chimeric antigen receptor-expressing cells | |
| AU2021203514B2 (en) | Treatment of cancer using GFR alpha-4 chimeric antigen receptor | |
| JP7189860B2 (ja) | ヒト化抗EGFRvIIIキメラ抗原受容体を用いたがんの処置 | |
| AU2017250304B2 (en) | Compositions and methods for selective protein expression | |
| KR102594343B1 (ko) | Cd33 키메라 항원 수용체를 사용한 암의 치료 | |
| RU2724999C2 (ru) | Химерный антигенный рецептор (car) против cd123 для использования в лечении злокачественных опухолей | |
| CA2999070A1 (en) | Car t cell therapies with enhanced efficacy | |
| WO2014130635A1 (en) | Effective targeting of primary human leukemia using anti-cd123 chimeric antigen receptor engineered t cells | |
| KR20210069048A (ko) | 융합 단백질을 이용하는 tcr 재프로그래밍을 위한 조성물 및 방법 | |
| RU2795467C2 (ru) | Композиции и способы для избирательной экспрессии белка | |
| HK40096534A (en) | Compositions and methods for selective protein expression | |
| HK1261086B (en) | Compositions and methods for selective expression of chimeric antigen receptors | |
| HK1261086A1 (en) | Compositions and methods for selective expression of chimeric antigen receptors |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20190402 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20201005 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20220805 Patent event code: PE09021S01D |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20230329 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20230920 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20230329 Comment text: Notification of reason for refusal Patent event code: PE06011S01I Patent event date: 20220805 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |