JP2012149259A - 複合体および複合膜 - Google Patents
複合体および複合膜 Download PDFInfo
- Publication number
- JP2012149259A JP2012149259A JP2012034502A JP2012034502A JP2012149259A JP 2012149259 A JP2012149259 A JP 2012149259A JP 2012034502 A JP2012034502 A JP 2012034502A JP 2012034502 A JP2012034502 A JP 2012034502A JP 2012149259 A JP2012149259 A JP 2012149259A
- Authority
- JP
- Japan
- Prior art keywords
- polymer
- composite
- membrane
- mixture
- silicate
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000012528 membrane Substances 0.000 title claims abstract description 65
- 239000002131 composite material Substances 0.000 title claims abstract description 48
- 229920000642 polymer Polymers 0.000 claims abstract description 51
- BPQQTUXANYXVAA-UHFFFAOYSA-N Orthosilicate Chemical compound [O-][Si]([O-])([O-])[O-] BPQQTUXANYXVAA-UHFFFAOYSA-N 0.000 claims abstract description 49
- 239000000203 mixture Substances 0.000 claims abstract description 26
- 238000005341 cation exchange Methods 0.000 claims abstract description 20
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 claims abstract description 18
- 229920000554 ionomer Polymers 0.000 claims abstract description 16
- 238000005349 anion exchange Methods 0.000 claims abstract description 14
- 239000000446 fuel Substances 0.000 claims abstract description 12
- 238000000926 separation method Methods 0.000 claims abstract description 8
- 238000000502 dialysis Methods 0.000 claims abstract description 7
- 238000002156 mixing Methods 0.000 claims abstract description 5
- 238000009792 diffusion process Methods 0.000 claims abstract description 4
- 238000000605 extraction Methods 0.000 claims abstract 3
- 238000001471 micro-filtration Methods 0.000 claims abstract 3
- 230000003204 osmotic effect Effects 0.000 claims abstract 3
- 238000005373 pervaporation Methods 0.000 claims abstract 3
- 238000000108 ultra-filtration Methods 0.000 claims abstract 3
- 239000004020 conductor Substances 0.000 claims abstract 2
- GUJOJGAPFQRJSV-UHFFFAOYSA-N dialuminum;dioxosilane;oxygen(2-);hydrate Chemical compound O.[O-2].[O-2].[O-2].[Al+3].[Al+3].O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O GUJOJGAPFQRJSV-UHFFFAOYSA-N 0.000 claims description 22
- 238000000034 method Methods 0.000 claims description 19
- 125000003118 aryl group Chemical group 0.000 claims description 18
- 229910052901 montmorillonite Inorganic materials 0.000 claims description 17
- 239000002253 acid Substances 0.000 claims description 16
- 239000000725 suspension Substances 0.000 claims description 13
- 239000002734 clay mineral Substances 0.000 claims description 10
- 238000004519 manufacturing process Methods 0.000 claims description 10
- 229920002492 poly(sulfone) Polymers 0.000 claims description 9
- 125000000217 alkyl group Chemical group 0.000 claims description 8
- 230000008569 process Effects 0.000 claims description 8
- 150000004760 silicates Chemical class 0.000 claims description 8
- 239000002904 solvent Substances 0.000 claims description 7
- 238000006243 chemical reaction Methods 0.000 claims description 6
- 238000005342 ion exchange Methods 0.000 claims description 6
- 239000004696 Poly ether ether ketone Substances 0.000 claims description 5
- 229910052731 fluorine Inorganic materials 0.000 claims description 5
- 229920002530 polyetherether ketone Polymers 0.000 claims description 5
- 239000002243 precursor Substances 0.000 claims description 5
- 239000000126 substance Substances 0.000 claims description 5
- RWRDLPDLKQPQOW-UHFFFAOYSA-N tetrahydropyrrole Natural products C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 claims description 5
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 claims description 4
- 229920005601 base polymer Polymers 0.000 claims description 4
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical group O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 claims description 4
- 229910052794 bromium Inorganic materials 0.000 claims description 4
- 229910052801 chlorine Inorganic materials 0.000 claims description 4
- 238000004132 cross linking Methods 0.000 claims description 4
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical group O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 claims description 4
- 150000007530 organic bases Chemical class 0.000 claims description 4
- 229910021536 Zeolite Inorganic materials 0.000 claims description 3
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 3
- 230000015556 catabolic process Effects 0.000 claims description 3
- 239000003054 catalyst Substances 0.000 claims description 3
- 238000006731 degradation reaction Methods 0.000 claims description 3
- 229910052739 hydrogen Inorganic materials 0.000 claims description 3
- 230000000813 microbial effect Effects 0.000 claims description 3
- 239000000243 solution Substances 0.000 claims description 3
- 125000001425 triazolyl group Chemical group 0.000 claims description 3
- 239000010457 zeolite Substances 0.000 claims description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-O Imidazolium Chemical compound C1=C[NH+]=CN1 RAXXELZNTBOGNW-UHFFFAOYSA-O 0.000 claims description 2
- 101100537948 Mus musculus Trir gene Proteins 0.000 claims description 2
- 239000004695 Polyether sulfone Substances 0.000 claims description 2
- WTKZEGDFNFYCGP-UHFFFAOYSA-O Pyrazolium Chemical compound C1=CN[NH+]=C1 WTKZEGDFNFYCGP-UHFFFAOYSA-O 0.000 claims description 2
- 238000005260 corrosion Methods 0.000 claims description 2
- 230000007797 corrosion Effects 0.000 claims description 2
- 230000001590 oxidative effect Effects 0.000 claims description 2
- 229920006393 polyether sulfone Polymers 0.000 claims description 2
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 claims description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-O pyridinium Chemical compound C1=CC=[NH+]C=C1 JUJWROOIHBZHMG-UHFFFAOYSA-O 0.000 claims description 2
- 239000005267 main chain polymer Substances 0.000 claims 10
- 229920000123 polythiophene Polymers 0.000 claims 5
- IOJUPLGTWVMSFF-UHFFFAOYSA-N benzothiazole Chemical compound C1=CC=C2SC=NC2=C1 IOJUPLGTWVMSFF-UHFFFAOYSA-N 0.000 claims 4
- 229920000767 polyaniline Polymers 0.000 claims 4
- 229920000128 polypyrrole Polymers 0.000 claims 4
- 229920002554 vinyl polymer Polymers 0.000 claims 4
- 125000001072 heteroaryl group Chemical group 0.000 claims 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 claims 3
- BAXOFTOLAUCFNW-UHFFFAOYSA-N 1H-indazole Chemical compound C1=CC=C2C=NNC2=C1 BAXOFTOLAUCFNW-UHFFFAOYSA-N 0.000 claims 2
- UJOBWOGCFQCDNV-UHFFFAOYSA-N 9H-carbazole Chemical compound C1=CC=C2C3=CC=CC=C3NC2=C1 UJOBWOGCFQCDNV-UHFFFAOYSA-N 0.000 claims 2
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 claims 2
- SIKJAQJRHWYJAI-UHFFFAOYSA-N Indole Chemical compound C1=CC=C2NC=CC2=C1 SIKJAQJRHWYJAI-UHFFFAOYSA-N 0.000 claims 2
- ZCQWOFVYLHDMMC-UHFFFAOYSA-N Oxazole Chemical compound C1=COC=N1 ZCQWOFVYLHDMMC-UHFFFAOYSA-N 0.000 claims 2
- 239000004734 Polyphenylene sulfide Substances 0.000 claims 2
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical compound C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 claims 2
- KAESVJOAVNADME-UHFFFAOYSA-N Pyrrole Chemical compound C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 claims 2
- 229920003295 Radel® Polymers 0.000 claims 2
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 claims 2
- 229920004695 VICTREX™ PEEK Polymers 0.000 claims 2
- JYIBXUUINYLWLR-UHFFFAOYSA-N aluminum;calcium;potassium;silicon;sodium;trihydrate Chemical compound O.O.O.[Na].[Al].[Si].[K].[Ca] JYIBXUUINYLWLR-UHFFFAOYSA-N 0.000 claims 2
- QRUDEWIWKLJBPS-UHFFFAOYSA-N benzotriazole Chemical compound C1=CC=C2N[N][N]C2=C1 QRUDEWIWKLJBPS-UHFFFAOYSA-N 0.000 claims 2
- 229910001603 clinoptilolite Inorganic materials 0.000 claims 2
- 125000002883 imidazolyl group Chemical group 0.000 claims 2
- 238000001728 nano-filtration Methods 0.000 claims 2
- 229920000069 polyphenylene sulfide Polymers 0.000 claims 2
- 125000003226 pyrazolyl group Chemical group 0.000 claims 2
- 238000001223 reverse osmosis Methods 0.000 claims 2
- UGUHFDPGDQDVGX-UHFFFAOYSA-N 1,2,3-thiadiazole Chemical compound C1=CSN=N1 UGUHFDPGDQDVGX-UHFFFAOYSA-N 0.000 claims 1
- YGTAZGSLCXNBQL-UHFFFAOYSA-N 1,2,4-thiadiazole Chemical compound C=1N=CSN=1 YGTAZGSLCXNBQL-UHFFFAOYSA-N 0.000 claims 1
- NWUYHJFMYQTDRP-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;1-ethenyl-2-ethylbenzene;styrene Chemical compound C=CC1=CC=CC=C1.CCC1=CC=CC=C1C=C.C=CC1=CC=CC=C1C=C NWUYHJFMYQTDRP-UHFFFAOYSA-N 0.000 claims 1
- BCMCBBGGLRIHSE-UHFFFAOYSA-N 1,3-benzoxazole Chemical compound C1=CC=C2OC=NC2=C1 BCMCBBGGLRIHSE-UHFFFAOYSA-N 0.000 claims 1
- OSSNTDFYBPYIEC-UHFFFAOYSA-N 1-ethenylimidazole Chemical compound C=CN1C=CN=C1 OSSNTDFYBPYIEC-UHFFFAOYSA-N 0.000 claims 1
- HYZJCKYKOHLVJF-UHFFFAOYSA-N 1H-benzimidazole Chemical compound C1=CC=C2NC=NC2=C1 HYZJCKYKOHLVJF-UHFFFAOYSA-N 0.000 claims 1
- UYWWLYCGNNCLKE-UHFFFAOYSA-N 2-pyridin-4-yl-1h-benzimidazole Chemical compound N=1C2=CC=CC=C2NC=1C1=CC=NC=C1 UYWWLYCGNNCLKE-UHFFFAOYSA-N 0.000 claims 1
- VHMICKWLTGFITH-UHFFFAOYSA-N 2H-isoindole Chemical compound C1=CC=CC2=CNC=C21 VHMICKWLTGFITH-UHFFFAOYSA-N 0.000 claims 1
- MCGBIXXDQFWVDW-UHFFFAOYSA-N 4,5-dihydro-1h-pyrazole Chemical compound C1CC=NN1 MCGBIXXDQFWVDW-UHFFFAOYSA-N 0.000 claims 1
- NSPMIYGKQJPBQR-UHFFFAOYSA-N 4H-1,2,4-triazole Chemical compound C=1N=CNN=1 NSPMIYGKQJPBQR-UHFFFAOYSA-N 0.000 claims 1
- ROFVEXUMMXZLPA-UHFFFAOYSA-N Bipyridyl Chemical group N1=CC=CC=C1C1=CC=CC=N1 ROFVEXUMMXZLPA-UHFFFAOYSA-N 0.000 claims 1
- WRYCSMQKUKOKBP-UHFFFAOYSA-N Imidazolidine Chemical compound C1CNCN1 WRYCSMQKUKOKBP-UHFFFAOYSA-N 0.000 claims 1
- 241000047703 Nonion Species 0.000 claims 1
- 229920012266 Poly(ether sulfone) PES Polymers 0.000 claims 1
- 239000004642 Polyimide Substances 0.000 claims 1
- 229920000491 Polyphenylsulfone Polymers 0.000 claims 1
- 125000002947 alkylene group Chemical group 0.000 claims 1
- 239000003957 anion exchange resin Substances 0.000 claims 1
- JUPQTSLXMOCDHR-UHFFFAOYSA-N benzene-1,4-diol;bis(4-fluorophenyl)methanone Chemical compound OC1=CC=C(O)C=C1.C1=CC(F)=CC=C1C(=O)C1=CC=C(F)C=C1 JUPQTSLXMOCDHR-UHFFFAOYSA-N 0.000 claims 1
- 239000003729 cation exchange resin Substances 0.000 claims 1
- 238000001704 evaporation Methods 0.000 claims 1
- 239000011888 foil Substances 0.000 claims 1
- 239000003205 fragrance Substances 0.000 claims 1
- 150000002244 furazanes Chemical class 0.000 claims 1
- PZOUSPYUWWUPPK-UHFFFAOYSA-N indole Natural products CC1=CC=CC2=C1C=CN2 PZOUSPYUWWUPPK-UHFFFAOYSA-N 0.000 claims 1
- RKJUIXBNRJVNHR-UHFFFAOYSA-N indolenine Natural products C1=CC=C2CC=NC2=C1 RKJUIXBNRJVNHR-UHFFFAOYSA-N 0.000 claims 1
- ZLTPDFXIESTBQG-UHFFFAOYSA-N isothiazole Chemical compound C=1C=NSC=1 ZLTPDFXIESTBQG-UHFFFAOYSA-N 0.000 claims 1
- CTAPFRYPJLPFDF-UHFFFAOYSA-N isoxazole Chemical compound C=1C=NOC=1 CTAPFRYPJLPFDF-UHFFFAOYSA-N 0.000 claims 1
- WCPAKWJPBJAGKN-UHFFFAOYSA-N oxadiazole Chemical compound C1=CON=N1 WCPAKWJPBJAGKN-UHFFFAOYSA-N 0.000 claims 1
- XKJCHHZQLQNZHY-UHFFFAOYSA-N phthalimide Chemical compound C1=CC=C2C(=O)NC(=O)C2=C1 XKJCHHZQLQNZHY-UHFFFAOYSA-N 0.000 claims 1
- 229910052615 phyllosilicate Inorganic materials 0.000 claims 1
- 229920005649 polyetherethersulfone Polymers 0.000 claims 1
- 229920001721 polyimide Polymers 0.000 claims 1
- 125000004076 pyridyl group Chemical group 0.000 claims 1
- 229910052645 tectosilicate Inorganic materials 0.000 claims 1
- 150000003536 tetrazoles Chemical class 0.000 claims 1
- 150000003852 triazoles Chemical class 0.000 claims 1
- 229910052649 zeolite group Inorganic materials 0.000 claims 1
- 230000003197 catalytic effect Effects 0.000 abstract description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 17
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 14
- 239000002585 base Substances 0.000 description 13
- -1 polyphenylene Polymers 0.000 description 13
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 9
- 239000003795 chemical substances by application Substances 0.000 description 9
- 125000000524 functional group Chemical group 0.000 description 8
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 7
- 210000004027 cell Anatomy 0.000 description 6
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 5
- 150000001768 cations Chemical class 0.000 description 5
- 239000011148 porous material Substances 0.000 description 5
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 4
- 241000233866 Fungi Species 0.000 description 4
- 239000002841 Lewis acid Substances 0.000 description 4
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 4
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 4
- 239000011521 glass Substances 0.000 description 4
- 150000003839 salts Chemical class 0.000 description 4
- 125000000542 sulfonic acid group Chemical group 0.000 description 4
- XTHPWXDJESJLNJ-UHFFFAOYSA-N sulfurochloridic acid Chemical class OS(Cl)(=O)=O XTHPWXDJESJLNJ-UHFFFAOYSA-N 0.000 description 4
- 150000007513 acids Chemical class 0.000 description 3
- 125000003277 amino group Chemical group 0.000 description 3
- 150000001450 anions Chemical class 0.000 description 3
- 239000000440 bentonite Substances 0.000 description 3
- 229910000278 bentonite Inorganic materials 0.000 description 3
- 238000005868 electrolysis reaction Methods 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 239000010410 layer Substances 0.000 description 3
- 150000007517 lewis acids Chemical class 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 229910021645 metal ion Inorganic materials 0.000 description 3
- 150000007522 mineralic acids Chemical class 0.000 description 3
- 230000035699 permeability Effects 0.000 description 3
- 239000000377 silicon dioxide Substances 0.000 description 3
- IIACRCGMVDHOTQ-UHFFFAOYSA-N sulfamic acid Chemical compound NS(O)(=O)=O IIACRCGMVDHOTQ-UHFFFAOYSA-N 0.000 description 3
- 229920005992 thermoplastic resin Polymers 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 description 2
- 229920005372 Plexiglas® Polymers 0.000 description 2
- 239000004693 Polybenzimidazole Substances 0.000 description 2
- 239000003513 alkali Substances 0.000 description 2
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 2
- 125000004432 carbon atom Chemical group C* 0.000 description 2
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 2
- 239000011737 fluorine Substances 0.000 description 2
- 239000007789 gas Substances 0.000 description 2
- KWLMIXQRALPRBC-UHFFFAOYSA-L hectorite Chemical compound [Li+].[OH-].[OH-].[Na+].[Mg+2].O1[Si]2([O-])O[Si]1([O-])O[Si]([O-])(O1)O[Si]1([O-])O2 KWLMIXQRALPRBC-UHFFFAOYSA-L 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 229910044991 metal oxide Inorganic materials 0.000 description 2
- 150000004706 metal oxides Chemical class 0.000 description 2
- 230000001035 methylating effect Effects 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 150000003013 phosphoric acid derivatives Chemical group 0.000 description 2
- 229920002480 polybenzimidazole Polymers 0.000 description 2
- 229920002717 polyvinylpyridine Polymers 0.000 description 2
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 2
- 235000012239 silicon dioxide Nutrition 0.000 description 2
- 150000003384 small molecules Chemical class 0.000 description 2
- 125000006850 spacer group Chemical group 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 239000000375 suspending agent Substances 0.000 description 2
- 229910052902 vermiculite Inorganic materials 0.000 description 2
- 239000010455 vermiculite Substances 0.000 description 2
- 235000019354 vermiculite Nutrition 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- KXDHJXZQYSOELW-UHFFFAOYSA-N Carbamic acid Chemical compound NC(O)=O KXDHJXZQYSOELW-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 239000004971 Cross linker Substances 0.000 description 1
- KRHYYFGTRYWZRS-UHFFFAOYSA-M Fluoride anion Chemical compound [F-] KRHYYFGTRYWZRS-UHFFFAOYSA-M 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- HBTLFSOYVRZLDA-UHFFFAOYSA-N NOS(Cl)(=O)=O Chemical compound NOS(Cl)(=O)=O HBTLFSOYVRZLDA-UHFFFAOYSA-N 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical class OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical group [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 229920000265 Polyparaphenylene Polymers 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 229910005948 SO2Cl Inorganic materials 0.000 description 1
- 239000004113 Sepiolite Substances 0.000 description 1
- 229910004298 SiO 2 Inorganic materials 0.000 description 1
- 229910004283 SiO 4 Inorganic materials 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical group [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 150000001242 acetic acid derivatives Chemical class 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 239000012670 alkaline solution Substances 0.000 description 1
- HPTYUNKZVDYXLP-UHFFFAOYSA-N aluminum;trihydroxy(trihydroxysilyloxy)silane;hydrate Chemical compound O.[Al].[Al].O[Si](O)(O)O[Si](O)(O)O HPTYUNKZVDYXLP-UHFFFAOYSA-N 0.000 description 1
- 229910001588 amesite Inorganic materials 0.000 description 1
- 150000001412 amines Chemical group 0.000 description 1
- 239000000010 aprotic solvent Substances 0.000 description 1
- 239000012736 aqueous medium Substances 0.000 description 1
- 235000019568 aromas Nutrition 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical group [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 230000001588 bifunctional effect Effects 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 238000009395 breeding Methods 0.000 description 1
- 230000001488 breeding effect Effects 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- VNSBYDPZHCQWNB-UHFFFAOYSA-N calcium;aluminum;dioxido(oxo)silane;sodium;hydrate Chemical compound O.[Na].[Al].[Ca+2].[O-][Si]([O-])=O VNSBYDPZHCQWNB-UHFFFAOYSA-N 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 150000007942 carboxylates Chemical class 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 229910010293 ceramic material Inorganic materials 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 238000002485 combustion reaction Methods 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 239000002322 conducting polymer Substances 0.000 description 1
- 229920001940 conductive polymer Polymers 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- FPAFDBFIGPHWGO-UHFFFAOYSA-N dioxosilane;oxomagnesium;hydrate Chemical compound O.[Mg]=O.[Mg]=O.[Mg]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O FPAFDBFIGPHWGO-UHFFFAOYSA-N 0.000 description 1
- 238000000909 electrodialysis Methods 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 150000002357 guanidines Chemical class 0.000 description 1
- 229940083094 guanine derivative acting on arteriolar smooth muscle Drugs 0.000 description 1
- 229910052621 halloysite Inorganic materials 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 229910000271 hectorite Inorganic materials 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 125000001183 hydrocarbyl group Chemical class 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 229910052900 illite Inorganic materials 0.000 description 1
- 238000001566 impedance spectroscopy Methods 0.000 description 1
- 239000011229 interlayer Substances 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229910000000 metal hydroxide Inorganic materials 0.000 description 1
- 150000004692 metal hydroxides Chemical class 0.000 description 1
- 239000010445 mica Substances 0.000 description 1
- 229910052618 mica group Inorganic materials 0.000 description 1
- 239000002114 nanocomposite Substances 0.000 description 1
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 1
- 150000002829 nitrogen Chemical class 0.000 description 1
- 125000004433 nitrogen atom Chemical group N* 0.000 description 1
- VGIBGUSAECPPNB-UHFFFAOYSA-L nonaaluminum;magnesium;tripotassium;1,3-dioxido-2,4,5-trioxa-1,3-disilabicyclo[1.1.1]pentane;iron(2+);oxygen(2-);fluoride;hydroxide Chemical compound [OH-].[O-2].[O-2].[O-2].[O-2].[O-2].[F-].[Mg+2].[Al+3].[Al+3].[Al+3].[Al+3].[Al+3].[Al+3].[Al+3].[Al+3].[Al+3].[K+].[K+].[K+].[Fe+2].O1[Si]2([O-])O[Si]1([O-])O2.O1[Si]2([O-])O[Si]1([O-])O2.O1[Si]2([O-])O[Si]1([O-])O2.O1[Si]2([O-])O[Si]1([O-])O2.O1[Si]2([O-])O[Si]1([O-])O2.O1[Si]2([O-])O[Si]1([O-])O2.O1[Si]2([O-])O[Si]1([O-])O2 VGIBGUSAECPPNB-UHFFFAOYSA-L 0.000 description 1
- 229910000273 nontronite Inorganic materials 0.000 description 1
- 150000004010 onium ions Chemical class 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 238000006053 organic reaction Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-O oxonium Chemical compound [OH3+] XLYOFNOQVPJJNP-UHFFFAOYSA-O 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052625 palygorskite Inorganic materials 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- AQSJGOWTSHOLKH-UHFFFAOYSA-N phosphite(3-) Chemical class [O-]P([O-])[O-] AQSJGOWTSHOLKH-UHFFFAOYSA-N 0.000 description 1
- XYFCBTPGUUZFHI-UHFFFAOYSA-O phosphonium Chemical compound [PH4+] XYFCBTPGUUZFHI-UHFFFAOYSA-O 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 230000005588 protonation Effects 0.000 description 1
- 238000000197 pyrolysis Methods 0.000 description 1
- 230000036647 reaction Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 229910000275 saponite Inorganic materials 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 229930195734 saturated hydrocarbon Natural products 0.000 description 1
- 238000004062 sedimentation Methods 0.000 description 1
- 229910052624 sepiolite Inorganic materials 0.000 description 1
- 235000019355 sepiolite Nutrition 0.000 description 1
- 229910021647 smectite Inorganic materials 0.000 description 1
- 239000002689 soil Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000011343 solid material Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 125000000020 sulfo group Chemical group O=S(=O)([*])O[H] 0.000 description 1
- 238000006277 sulfonation reaction Methods 0.000 description 1
- 125000001273 sulfonato group Chemical class [O-]S(*)(=O)=O 0.000 description 1
- 229910052717 sulfur Chemical group 0.000 description 1
- 239000011593 sulfur Chemical group 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 229920001169 thermoplastic Polymers 0.000 description 1
- 239000004416 thermosoftening plastic Substances 0.000 description 1
- 229930195735 unsaturated hydrocarbon Natural products 0.000 description 1
- 238000004065 wastewater treatment Methods 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D69/00—Semi-permeable membranes for separation processes or apparatus characterised by their form, structure or properties; Manufacturing processes specially adapted therefor
- B01D69/12—Composite membranes; Ultra-thin membranes
- B01D69/125—In situ manufacturing by polymerisation, polycondensation, cross-linking or chemical reaction
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D69/00—Semi-permeable membranes for separation processes or apparatus characterised by their form, structure or properties; Manufacturing processes specially adapted therefor
- B01D69/14—Dynamic membranes
- B01D69/141—Heterogeneous membranes, e.g. containing dispersed material; Mixed matrix membranes
- B01D69/1411—Heterogeneous membranes, e.g. containing dispersed material; Mixed matrix membranes containing dispersed material in a continuous matrix
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D71/00—Semi-permeable membranes for separation processes or apparatus characterised by the material; Manufacturing processes specially adapted therefor
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D71/00—Semi-permeable membranes for separation processes or apparatus characterised by the material; Manufacturing processes specially adapted therefor
- B01D71/02—Inorganic material
- B01D71/024—Oxides
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D71/00—Semi-permeable membranes for separation processes or apparatus characterised by the material; Manufacturing processes specially adapted therefor
- B01D71/06—Organic material
- B01D71/58—Other polymers having nitrogen in the main chain, with or without oxygen or carbon only
- B01D71/62—Polycondensates having nitrogen-containing heterocyclic rings in the main chain
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D71/00—Semi-permeable membranes for separation processes or apparatus characterised by the material; Manufacturing processes specially adapted therefor
- B01D71/06—Organic material
- B01D71/66—Polymers having sulfur in the main chain, with or without nitrogen, oxygen or carbon only
- B01D71/68—Polysulfones; Polyethersulfones
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J21/00—Catalysts comprising the elements, oxides, or hydroxides of magnesium, boron, aluminium, carbon, silicon, titanium, zirconium, or hafnium
- B01J21/16—Clays or other mineral silicates
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J31/00—Catalysts comprising hydrides, coordination complexes or organic compounds
- B01J31/02—Catalysts comprising hydrides, coordination complexes or organic compounds containing organic compounds or metal hydrides
- B01J31/06—Catalysts comprising hydrides, coordination complexes or organic compounds containing organic compounds or metal hydrides containing polymers
- B01J31/08—Ion-exchange resins
- B01J31/10—Ion-exchange resins sulfonated
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J31/00—Catalysts comprising hydrides, coordination complexes or organic compounds
- B01J31/16—Catalysts comprising hydrides, coordination complexes or organic compounds containing coordination complexes
- B01J31/1616—Coordination complexes, e.g. organometallic complexes, immobilised on an inorganic support, e.g. ship-in-a-bottle type catalysts
- B01J31/1625—Coordination complexes, e.g. organometallic complexes, immobilised on an inorganic support, e.g. ship-in-a-bottle type catalysts immobilised by covalent linkages, i.e. pendant complexes with optional linking groups
- B01J31/1633—Coordination complexes, e.g. organometallic complexes, immobilised on an inorganic support, e.g. ship-in-a-bottle type catalysts immobilised by covalent linkages, i.e. pendant complexes with optional linking groups covalent linkages via silicon containing groups
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/50—Catalysts, in general, characterised by their form or physical properties characterised by their shape or configuration
- B01J35/58—Fabrics or filaments
- B01J35/59—Membranes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B60—VEHICLES IN GENERAL
- B60T—VEHICLE BRAKE CONTROL SYSTEMS OR PARTS THEREOF; BRAKE CONTROL SYSTEMS OR PARTS THEREOF, IN GENERAL; ARRANGEMENT OF BRAKING ELEMENTS ON VEHICLES IN GENERAL; PORTABLE DEVICES FOR PREVENTING UNWANTED MOVEMENT OF VEHICLES; VEHICLE MODIFICATIONS TO FACILITATE COOLING OF BRAKES
- B60T1/00—Arrangements of braking elements, i.e. of those parts where braking effect occurs specially for vehicles
- B60T1/02—Arrangements of braking elements, i.e. of those parts where braking effect occurs specially for vehicles acting by retarding wheels
- B60T1/06—Arrangements of braking elements, i.e. of those parts where braking effect occurs specially for vehicles acting by retarding wheels acting otherwise than on tread, e.g. employing rim, drum, disc, or transmission or on double wheels
- B60T1/065—Arrangements of braking elements, i.e. of those parts where braking effect occurs specially for vehicles acting by retarding wheels acting otherwise than on tread, e.g. employing rim, drum, disc, or transmission or on double wheels employing disc
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J5/00—Manufacture of articles or shaped materials containing macromolecular substances
- C08J5/20—Manufacture of shaped structures of ion-exchange resins
- C08J5/22—Films, membranes or diaphragms
- C08J5/2206—Films, membranes or diaphragms based on organic and/or inorganic macromolecular compounds
- C08J5/2275—Heterogeneous membranes
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16D—COUPLINGS FOR TRANSMITTING ROTATION; CLUTCHES; BRAKES
- F16D55/00—Brakes with substantially-radial braking surfaces pressed together in axial direction, e.g. disc brakes
- F16D55/24—Brakes with substantially-radial braking surfaces pressed together in axial direction, e.g. disc brakes with a plurality of axially-movable discs, lamellae, or pads, pressed from one side towards an axially-located member
- F16D55/26—Brakes with substantially-radial braking surfaces pressed together in axial direction, e.g. disc brakes with a plurality of axially-movable discs, lamellae, or pads, pressed from one side towards an axially-located member without self-tightening action
- F16D55/36—Brakes with a plurality of rotating discs all lying side by side
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16D—COUPLINGS FOR TRANSMITTING ROTATION; CLUTCHES; BRAKES
- F16D55/00—Brakes with substantially-radial braking surfaces pressed together in axial direction, e.g. disc brakes
- F16D55/24—Brakes with substantially-radial braking surfaces pressed together in axial direction, e.g. disc brakes with a plurality of axially-movable discs, lamellae, or pads, pressed from one side towards an axially-located member
- F16D55/26—Brakes with substantially-radial braking surfaces pressed together in axial direction, e.g. disc brakes with a plurality of axially-movable discs, lamellae, or pads, pressed from one side towards an axially-located member without self-tightening action
- F16D55/36—Brakes with a plurality of rotating discs all lying side by side
- F16D55/40—Brakes with a plurality of rotating discs all lying side by side actuated by a fluid-pressure device arranged in or one the brake
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16D—COUPLINGS FOR TRANSMITTING ROTATION; CLUTCHES; BRAKES
- F16D65/00—Parts or details
- F16D65/02—Braking members; Mounting thereof
- F16D65/04—Bands, shoes or pads; Pivots or supporting members therefor
- F16D65/092—Bands, shoes or pads; Pivots or supporting members therefor for axially-engaging brakes, e.g. disc brakes
- F16D65/095—Pivots or supporting members therefor
- F16D65/097—Resilient means interposed between pads and supporting members or other brake parts
- F16D65/0973—Resilient means interposed between pads and supporting members or other brake parts not subjected to brake forces
- F16D65/0974—Resilient means interposed between pads and supporting members or other brake parts not subjected to brake forces acting on or in the vicinity of the pad rim in a direction substantially transverse to the brake disc axis
- F16D65/0977—Springs made from sheet metal
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16D—COUPLINGS FOR TRANSMITTING ROTATION; CLUTCHES; BRAKES
- F16D65/00—Parts or details
- F16D65/02—Braking members; Mounting thereof
- F16D65/12—Discs; Drums for disc brakes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/02—Details
- H01M8/0289—Means for holding the electrolyte
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/1016—Fuel cells with solid electrolytes characterised by the electrolyte material
- H01M8/1018—Polymeric electrolyte materials
- H01M8/102—Polymeric electrolyte materials characterised by the chemical structure of the main chain of the ion-conducting polymer
- H01M8/1023—Polymeric electrolyte materials characterised by the chemical structure of the main chain of the ion-conducting polymer having only carbon, e.g. polyarylenes, polystyrenes or polybutadiene-styrenes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/1016—Fuel cells with solid electrolytes characterised by the electrolyte material
- H01M8/1018—Polymeric electrolyte materials
- H01M8/102—Polymeric electrolyte materials characterised by the chemical structure of the main chain of the ion-conducting polymer
- H01M8/103—Polymeric electrolyte materials characterised by the chemical structure of the main chain of the ion-conducting polymer having nitrogen, e.g. sulfonated polybenzimidazoles [S-PBI], polybenzimidazoles with phosphoric acid, sulfonated polyamides [S-PA] or sulfonated polyphosphazenes [S-PPh]
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/1016—Fuel cells with solid electrolytes characterised by the electrolyte material
- H01M8/1018—Polymeric electrolyte materials
- H01M8/102—Polymeric electrolyte materials characterised by the chemical structure of the main chain of the ion-conducting polymer
- H01M8/1032—Polymeric electrolyte materials characterised by the chemical structure of the main chain of the ion-conducting polymer having sulfur, e.g. sulfonated-polyethersulfones [S-PES]
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/1016—Fuel cells with solid electrolytes characterised by the electrolyte material
- H01M8/1018—Polymeric electrolyte materials
- H01M8/1041—Polymer electrolyte composites, mixtures or blends
- H01M8/1046—Mixtures of at least one polymer and at least one additive
- H01M8/1051—Non-ion-conducting additives, e.g. stabilisers, SiO2 or ZrO2
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/1016—Fuel cells with solid electrolytes characterised by the electrolyte material
- H01M8/1018—Polymeric electrolyte materials
- H01M8/1069—Polymeric electrolyte materials characterised by the manufacturing processes
- H01M8/1081—Polymeric electrolyte materials characterised by the manufacturing processes starting from solutions, dispersions or slurries exclusively of polymers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2325/00—Details relating to properties of membranes
- B01D2325/18—Membrane materials having mixed charged functional groups
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2325/00—Details relating to properties of membranes
- B01D2325/26—Electrical properties
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2383/00—Characterised by the use of macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing silicon with or without sulfur, nitrogen, oxygen, or carbon only; Derivatives of such polymers
- C08J2383/02—Polysilicates
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16D—COUPLINGS FOR TRANSMITTING ROTATION; CLUTCHES; BRAKES
- F16D55/00—Brakes with substantially-radial braking surfaces pressed together in axial direction, e.g. disc brakes
- F16D2055/0004—Parts or details of disc brakes
- F16D2055/0008—Brake supports
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16D—COUPLINGS FOR TRANSMITTING ROTATION; CLUTCHES; BRAKES
- F16D55/00—Brakes with substantially-radial braking surfaces pressed together in axial direction, e.g. disc brakes
- F16D2055/0004—Parts or details of disc brakes
- F16D2055/0062—Partly lined, i.e. braking surface extending over only a part of the disc circumference
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16D—COUPLINGS FOR TRANSMITTING ROTATION; CLUTCHES; BRAKES
- F16D55/00—Brakes with substantially-radial braking surfaces pressed together in axial direction, e.g. disc brakes
- F16D2055/0075—Constructional features of axially engaged brakes
- F16D2055/0091—Plural actuators arranged side by side on the same side of the rotor
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16D—COUPLINGS FOR TRANSMITTING ROTATION; CLUTCHES; BRAKES
- F16D65/00—Parts or details
- F16D65/02—Braking members; Mounting thereof
- F16D2065/13—Parts or details of discs or drums
- F16D2065/1304—Structure
- F16D2065/1316—Structure radially segmented
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16D—COUPLINGS FOR TRANSMITTING ROTATION; CLUTCHES; BRAKES
- F16D65/00—Parts or details
- F16D65/02—Braking members; Mounting thereof
- F16D2065/13—Parts or details of discs or drums
- F16D2065/134—Connection
- F16D2065/1348—Connection resilient
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16D—COUPLINGS FOR TRANSMITTING ROTATION; CLUTCHES; BRAKES
- F16D65/00—Parts or details
- F16D65/02—Braking members; Mounting thereof
- F16D2065/13—Parts or details of discs or drums
- F16D2065/134—Connection
- F16D2065/1356—Connection interlocking
- F16D2065/1368—Connection interlocking with relative movement both radially and axially
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16D—COUPLINGS FOR TRANSMITTING ROTATION; CLUTCHES; BRAKES
- F16D65/00—Parts or details
- F16D65/02—Braking members; Mounting thereof
- F16D2065/13—Parts or details of discs or drums
- F16D2065/134—Connection
- F16D2065/1392—Connection elements
- F16D2065/1396—Ancillary resilient elements, e.g. anti-rattle or retraction springs
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16D—COUPLINGS FOR TRANSMITTING ROTATION; CLUTCHES; BRAKES
- F16D2121/00—Type of actuator operation force
- F16D2121/18—Electric or magnetic
- F16D2121/20—Electric or magnetic using electromagnets
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/50—Fuel cells
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P70/00—Climate change mitigation technologies in the production process for final industrial or consumer products
- Y02P70/50—Manufacturing or production processes characterised by the final manufactured product
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/249921—Web or sheet containing structurally defined element or component
- Y10T428/249953—Composite having voids in a component [e.g., porous, cellular, etc.]
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/249921—Web or sheet containing structurally defined element or component
- Y10T428/249953—Composite having voids in a component [e.g., porous, cellular, etc.]
- Y10T428/249967—Inorganic matrix in void-containing component
- Y10T428/249969—Of silicon-containing material [e.g., glass, etc.]
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Organic Chemistry (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Sustainable Development (AREA)
- Sustainable Energy (AREA)
- Materials Engineering (AREA)
- General Engineering & Computer Science (AREA)
- Inorganic Chemistry (AREA)
- Dispersion Chemistry (AREA)
- Crystallography & Structural Chemistry (AREA)
- Mechanical Engineering (AREA)
- Polymers & Plastics (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Composite Materials (AREA)
- Transportation (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Separation Using Semi-Permeable Membranes (AREA)
- Manufacture Of Macromolecular Shaped Articles (AREA)
- Silicates, Zeolites, And Molecular Sieves (AREA)
- Conductive Materials (AREA)
- Fuel Cell (AREA)
- Laminated Bodies (AREA)
Abstract
【解決手段】アイオノマーおよび無機の、場合によっては官能化された層状ケイ酸塩からなる複合体または複合膜に関しここで、アイオノマーは、(a)陽イオン交換ポリマー、(b)陰イオン交換ポリマー、(c)陽イオン交換基および陰イオン交換基の両方をポリマー鎖上に有するポリマー、(d)混合比が(a)100%乃至(b)100%である(a)と(b)との混合物、とすることができる。また、複合体/複合膜の以下の適用に関し、100℃より高い温度における膜燃料電池(H2燃料電池PEFC、直接メタノール燃料電池DMFC)のプロトン伝導体、透析、拡散透析、ガス分離、浸透気化、浸透抽出、精密ろ過および限外ろ過等の(電気)膜分離法、触媒膜反応装置の触媒膜。
【選択図】図1
Description
・250℃まで水和水を保有した状態にある。
・さらに金属陽イオンおよび金属酸化物をこれらの物質中に貯蔵することが可能であり、これにより内部プロトン伝導性が次の一般系のように引き起こされる。
Mn+(H2O)−>(M−OH)(n−1)++H+ [有機反応におけるゼオライト、粘土およびヘテロポリ酸( Zeolite, clay and hereropoly acid in organic reactions)、Y.イズミ、K.ウラベ、M.オナカ、1992年、ワインハイム、VCH出版、26頁]。
・ルイス酸空孔を示す層状ケイ酸塩を、塩基ポリマーの塩基基と酸−塩基相互作用によりインターカレートする(interkalieren)ことができる[プラスチックナノ複合材、シンポジウム:発明から革新へ、1998年5月6日ケルンにおける化学工業基金シンポジウム用出版物、(Kunststoffnanokomposite, Symposium:Von der Invention zur Innovation, Publikation zum Symposium des Fonds der Chemishen Industrie am 6. Mai 1998 in Koln)]。この性質により、ある種の層状ケイ酸塩/ポリマー複合材が既に合成されている。したがって、Muhlhauptらがモンモリロナイトとポリプロピレン、モンモリロナイトとポリアミド、およびモンモリロナイトとプレキシガラスの複合材を作っている。これらの複合材により、例えばプレキシガラスはモンモリロナイトとの混合物によって不燃性となる。混合された層状ケイ酸塩が燃焼により発生した熱分解ガスに対するバリアとなるからである。
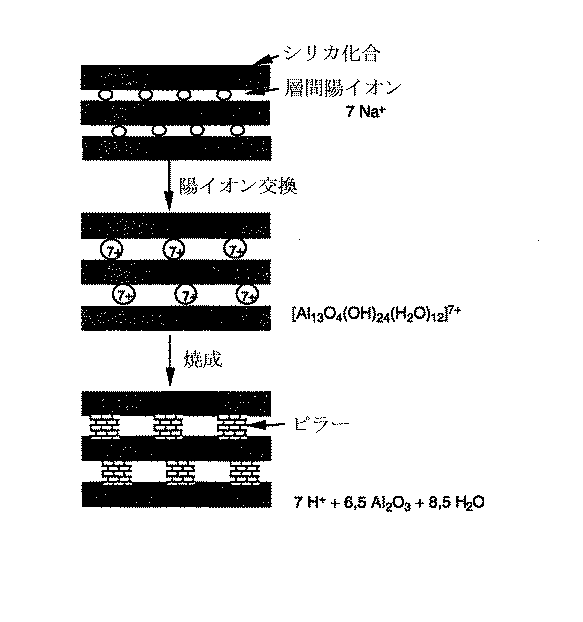
層状ケイ酸塩の下には、SiO4四面体が2次元の無限の架橋で結び付けられた一般的なケイ酸塩が見られる。(陰イオンに対する実験式は(Si2O5 2−)nである。)個々の層はその間にある陽イオンにより互いに結合している。その際、天然に存在する層状ケイ酸塩中では陽イオンの大部分はNa、K、Mg、Alおよび/またはCaである。
R1、R2、R3、R4は、互いに異なる水素または直鎖、分岐鎖、飽和もしくは不飽和の、1乃至40個、好ましくは1乃至20個の炭素原子を有する炭化水素であり、場合によっては少なくとも1つの官能基を有するか、または2つの残基が互いに結合しており、好ましくは5乃至10個の炭素原子を有し、特には1つまたは複数の窒素原子を有する複素環式残基であり、Xはリンまたは窒素であり、Yは酸素または硫黄であり、nは1乃至5、好ましくは1乃至3のいずれかの整数であり、かつZは1つの陰イオンである。
好適な官能基はヒドロキシル、ニトロまたはスルホ基であり、ここでカルボキシルおよびスルホン酸基が特に好ましい。同様に、スルホ塩化物およびカルボン酸塩化物が特に好ましい。
本発明はさらに複合膜の製造方法に関する。以下にプロトン伝導性の高いプロトン導電性化合物の製造の一例を説明する。
・複合体は、100℃よりはるかに高い温度で非常に高いイオン導電性を示す。特に、この温度域においても複合体のプロトン伝導性は非常に良好である。これは、一方では粘土鉱物の保水特性に、他方では粘土鉱物の固有プロトン導電性に起因するものである。プロトン導電性が良好であることにより、この複合体を前記の温度域で燃料電池膜に適用することができる。
・空孔中でポリマー分子およびゼオライト等の粘土鉱物が相互に作用することができるので、空孔をケイ酸塩に設けることにより複合膜の化学的、機械的および熱的安定性が著しく向上する。特に、アイオノマー混合物を含有する塩基性ポリマーおよび塩基性ポリマー成分を、ケイ酸塩のルイス酸空孔中の塩基性基の相互作用によりインターカレートすることが可能である。これにより酸性ケイ酸塩と塩基性ポリマー鎖との間のイオン的架橋が形成され、これは系のpHに依存せず、特に複合膜が強酸または強塩基媒質中に置かれた場合、機械的、化学的および熱的安定性の向上に寄与する。
・層状−/網状ケイ酸塩の型。
・複合体中のケイ酸塩の重量割合。
・ケイ酸塩の空孔中へのスペーサー分子(Spacermolekulen)および二官能分子の意図的導入。この際、スペーサー分子と透過分子との相互作用の型および強度は、膜の露出している官能基と透過分子の官能基の型によって決まる。ベントナイト表面上のアルカリベントナイトに対する交換において、例えばアミノスルホン酸またはアミノカルボン酸とアミン官能物(Aminfunktion)を組み合わせる。第2官能基は、ポリマーとの反応またはプロトン移送電気膜法に使用可能である。
・本発明に好適な粘土鉱物を構成するシリカ化合(silicatischen)ルイス酸の触媒特性を本発明に好適な複合物にも用いることができる。
1.スルホン化ポリエーテルエーテルケトン(スルホン化率70%)を5重量%のモンモリロナイトとDMAc中に溶解し、溶剤を蒸発させて厚さ50μmの膜を得る。この膜を菌類で汚染した水性培地に入れる。菌類による分解は検出されない。モンモリロナイトの入っていないコントロールでは、非常に繁殖が起こり、かつ分解される。
b)2.aと同じ混合物に8重量%の活性化モンモリロナイトをさらに加え、2.aと同様にして膜を得る。比抵抗は27.7[Ω×cm]である。
Claims (24)
- 酸および/または有機塩基と層状/網状ケイ酸塩とを含有するプロトン導電性複合体であって、酸−塩基複合物の割合が1乃至99重量%であり、層状/網状ケイ酸塩の割合が99乃至1重量%であることを特徴とする複合体。
- 複合体および複合体混合膜の製造方法であって、アイオノマー溶液またはアイオノマー前駆体の溶液を層状もしくは網状ケイ酸塩、または両者の混合物と混合し、得られた懸濁液から溶剤を蒸発させることを特徴とする方法。ここで、アイオノマーは、(a)陽イオン交換ポリマー(陽イオン交換基−SO3H、−COOH、−PO3H2を有し、該ポリマーを前記陽イオン交換基の何れか、または前記陽イオン交換基の混合物のみで修飾可能なもの)。ここで、該ポリマーは架橋を形成しないかまたは共有的に架橋を形成し得る。ここで、ポリマー骨格鎖は、ビニルポリマー、アリール主鎖ポリマー、ポリチアゾール、ポリピラゾール、ポリピロール、ポリアニリン、ポリチオフェンまたはこれらの任意の混合物とすることができる。
(b)陰イオン交換ポリマー(陰イオン交換基−NR3 +(R=H、アルキル、アリール)、ピリジニウム PyrR+、イミダゾリウム ImR+、ピラゾリウム PyrazR+、トリアゾリウム TriR+および他の有機塩基芳香および/または非芳香族基(R=H、アルキル、アリール)を有し、該ポリマーを前記陰イオン交換基の何れか、または前記陰イオン交換基の混合物のみで修飾可能なもの)。ここで、該ポリマーは架橋を形成しないかまたは共有的に架橋を形成し得る。ここで、ポリマー骨格鎖は、ビニルポリマー、アリール主鎖ポリマー、ポリチアゾール、ポリピラゾール、ポリピロール、ポリアニリン、ポリチオフェンまたはこれらの任意の混合物とすることができる。
(c)ポリマー鎖上に(a)の陰イオン交換基と(b)の陽イオン交換基との両方を有するポリマー。ここで、ポリマー骨格鎖は、ビニルポリマー、アリール主鎖ポリマー、ポリチアゾール、ポリピラゾール、ポリピロール、ポリアニリン、ポリチオフェンまたはこれらの任意の混合物とすることができる。
(d)(a)と(b)の混合物であって、混合比が(a)100%から(b)100%にまで達することができる。ここで、該混合物はイオン的架橋に加えて、さらに共有的に架橋を形成している。ここで、ポリマー骨格鎖は、ビニルポリマー、アリール主鎖ポリマー、ポリチアゾール、ポリピラゾール、ポリピロール、ポリアニリン、ポリチオフェンまたはこれらの任意の混合物とすることができる。
ここで、アイオノマーの前駆体としては、(a)陽イオン交換樹脂の前駆体。
(a1)CoHal−、CONR2−またはCOOR−基(R=H、アルキル、アリールであり、Hal=F、Cl、Br、I)を有するポリマー。
(a2)SO2Hal−、SO2NR2−またはSO2OR−基(R=H、アルキル、アリールであり、Hal=F、Cl、Br、I)を有するポリマー。
(a3)PO3Hal2−、PO3(NR2)2−またはPO3(OR)2−基(R=H、アルキル、アリールであり、Hal=F、Cl、Br、I)を有するポリマー。
(b)陰イオン交換樹脂の前駆体(NR2−基(R=H、アルキル、アリール)、ピリジル Pyr、イミダゾイル Im、ピラゾリル Pyraz、トリアゾリル Triおよび/または他の有機塩基芳香および/または非芳香基を有する)。ここで、無機成分は、層状ケイ酸塩もしくは網状ケイ酸塩またはこれらの任意の混合物とすることができる。 - a)層状ケイ酸塩(フィロケイ酸塩)群の中で、一般にベントナイト群、特にはモンモリロナイト/バイデライト系、さらにはモンモリロナイトが好適であることを特徴とする請求項1記載の方法。
b)ピラー化(pillartierte)層状ケイ酸塩を用いることを特徴とする請求項1記載の方法。 - a)網状ケイ酸塩(テクトケイ酸塩)群の中で、一般にゼオライト群、特にはクリノプチロライトが好適であることを特徴とする請求項1記載の方法。
b)ピラー化網状ケイ酸塩を用いることを特徴とする請求項1記載の方法。 - 天然層状ケイ酸塩および合成層状ケイ酸塩の両者を用いることを特徴とする、請求項1乃至4の何れか1項に記載の方法。
- 塩基成分がイミダゾール、ビニルイミダゾール、ピラゾール、オキサゾール、カルバゾール、インドール、イソインドール、デヒドロオキサゾール、イソオキサゾール、チアゾール、ベンゾチアゾール、イソチアゾール、ベンゾイミダゾール、イミダゾリジン、インダゾール、4,5−ジヒドロピラゾール、1,2,3−オキサジアゾ−ル、フラザン、1,2,3−チアジアゾール、1,2,4−チアジアゾール、1,2,3−ベンゾトリアゾール、1,2,4−トリアゾール、テトラゾール、ピロール、アニリン、ピロリジンまたはピラゾール基を有することを特徴とする請求項1記載の方法。
- アイオノマーとして酸−塩基混合物(d)が、ならびに合成および天然起源の両方の粘土鉱物モンモリロナイトが好適であり、かつそれが官能化されている事を特徴とする請求項2記載の方法。
- アイオノマーとして酸−塩基混合物(d)が、およびゼオライトとしてクリノプチロライトが好適であることを特徴とする請求項2記載の方法。
- 酸ポリマーのポリマー骨格鎖を、アリール主鎖ポリマー群より選択したことを特徴とする、請求項1乃至8のいずれか1項に記載の方法。該アリール主鎖ポリマーの取り得る構造は次式の通りである。
− ポリエーテルスルホン PSU Udel(登録商標)([R1−R5−R2−R6−R2−R5]n;R2:x=1、R4=H)
− ポリエーテルスルホン PES VICTREX(登録商標)([R2−R6−R2−R5]n;R2:x=1、R4=H)
− ポリフェニルスルホン RADEL R(登録商標)([(R2)2−R5−R2−R6−R2]n;R2:x=2、R4=H)
− ポリエーテルエーテルスルホン RADEL A(登録商標)([R5−R2−R5−R2−R6]n−[R5−R2−R6−R2]m;R2:x=1、R4=H、n/m=0.18)
− ポリフェニレンスルフィド PPS([R2−R8]n;R2:x=1、R4=H)
− ポリフェリレンオキシド PPO([R2−R5]n;R4=CH3)
である。 - 塩基ポリマーのポリマー骨格鎖をアリール主鎖ポリマー群〔化1〕またはヘトアリール(Hetaryl)主鎖ポリマーから選択したことを特徴とする、請求項1または2記載の方法。ヘトアリール主鎖ポリマーの取り得る構造は次式の通りである。
本発明に好適なヘトアリールポリマーとしては、以下のものが考えられる。
− ポリイミダゾール、ポリベンズイミダゾール− ポリピラゾール、ポリベンズピラゾール− ポリオキサゾール、ポリベンズオキサゾール− ポリチアゾール、ポリベンズチアゾール− ポリチオフェン、ポリベンズチオフェン− ポリピリジン− ポリイミド - 酸−塩基混合物において、請求項9〔化1〕記載の酸ポリマーを請求項10〔化2〕および請求項6記載の塩基ポリマーと組み合わせることを特徴とする、請求項1乃至10に記載の方法。
- 非イオン伝導性複合体および複合体混合膜であって、請求項1乃至11において20乃至98重量%の基ポリマーおよび2乃至80重量%の層状/網状ケイ酸塩を膜用途および膜分離法に用いることにより入手可能な非イオン伝導性複合体および複合体混合膜。
- −40℃乃至200℃の温度で膜燃料電池(H2燃料電池または直接メタノール燃料電池)に組み込んだ、請求項1乃至11のいずれか1項に記載の複合体および複合体混合膜。
- 透析、拡散透析、ガス分離、浸透気化、浸透抽出、精密ろ過、限外ろ過、ナノろ過および逆浸透等の(電気)膜分離法に複合体および複合体混合膜を組み込んだことを特徴とする、請求項1乃至12のいずれか1項に記載の方法。
- 触媒膜として、または膜反応装置に組み込んだことを特徴とする、請求項1乃至12のいずれか1項に記載の複合体および複合体混合膜。
- 平面構造体、特には膜、箔、電極の被覆のための、請求項1乃至12のいずれか1項に記載の複合体。
- 有機成分および本発明に好適なケイ酸塩成分を−40℃乃至300℃の温度において溶剤中で、または場合によっては溶剤無しで互いに接触させることを特徴とする、請求項1に記載の複合体の製造方法。
- 400℃まで温度安定性を有する、請求項1乃至17のいずれか1項に記載のプロトン伝導体を含有する複合体。
- 請求項2、6、9、10および11に記載の対応する混合物または単独の成分であって、溶液または懸濁液または溶剤フリーであるケイ酸塩成分と共に存在する混合物または成分で膜が被覆されていることを特徴とする、請求項16記載の複合膜または複合体の製造。
- 請求項2、6、9、10および11の溶液または懸濁液または溶剤フリーであるケイ酸塩成分自体もしくは成分の混合物で被覆したことを特徴とする、請求項16記載の複合膜または複合体の製造。
- 膜ならびに透析、拡散透析、ガス分離、浸透気化、浸透抽出、精密ろ過、限外ろ過、ナノろ過および逆浸透等の(電気)膜分離法に組込み、かつ微生物分解または酸化腐食に対して安定であることを特徴とする複合体の製造。
- 複合体から製造した膜の選択透過性を変えるための請求項1乃至21のいずれか1項に記載の複合体の製造。
- 無機成分を少なくとも2つの異なる塩基成分と混合することを特徴とする、請求項1乃至22のいずれか1項に記載の複合体の製造。ここで、塩基成分を高分子または低分子とすることができる。
- 型を共有する任意の型に前記複合体を組込むことを特徴とする方法。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19919881A DE19919881A1 (de) | 1999-04-30 | 1999-04-30 | Organisch-Anorganische Komposites und Kompositmembranen aus Ionomeren oder Ionomerblends und aus Schicht- oder Gerätsilicaten |
| DE19919881.0 | 1999-04-30 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2001501354A Division JP2003501516A (ja) | 1999-04-30 | 2000-05-02 | 複合体および複合膜 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014037390A Division JP2014167113A (ja) | 1999-04-30 | 2014-02-27 | 複合体の製造方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| JP2012149259A true JP2012149259A (ja) | 2012-08-09 |
Family
ID=7906530
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2001501354A Pending JP2003501516A (ja) | 1999-04-30 | 2000-05-02 | 複合体および複合膜 |
| JP2012034502A Pending JP2012149259A (ja) | 1999-04-30 | 2012-02-20 | 複合体および複合膜 |
| JP2014037390A Pending JP2014167113A (ja) | 1999-04-30 | 2014-02-27 | 複合体の製造方法 |
| JP2017044440A Pending JP2017125205A (ja) | 1999-04-30 | 2017-03-08 | 複合体の製造方法 |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2001501354A Pending JP2003501516A (ja) | 1999-04-30 | 2000-05-02 | 複合体および複合膜 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014037390A Pending JP2014167113A (ja) | 1999-04-30 | 2014-02-27 | 複合体の製造方法 |
| JP2017044440A Pending JP2017125205A (ja) | 1999-04-30 | 2017-03-08 | 複合体の製造方法 |
Country Status (14)
| Country | Link |
|---|---|
| US (4) | US7049020B2 (ja) |
| EP (2) | EP1870429A3 (ja) |
| JP (4) | JP2003501516A (ja) |
| KR (1) | KR100858131B1 (ja) |
| CN (1) | CN1320038C (ja) |
| AT (1) | ATE368703T1 (ja) |
| AU (1) | AU780722B2 (ja) |
| BR (1) | BR0010171B1 (ja) |
| CA (1) | CA2369703C (ja) |
| DE (2) | DE19919881A1 (ja) |
| ES (1) | ES2293901T3 (ja) |
| IL (2) | IL146209A0 (ja) |
| WO (1) | WO2000074827A2 (ja) |
| ZA (1) | ZA200109819B (ja) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014167113A (ja) * | 1999-04-30 | 2014-09-11 | Thomas Haering | 複合体の製造方法 |
| JP2018505234A (ja) * | 2014-12-04 | 2018-02-22 | エルジー・ケム・リミテッド | 高分子電解質膜 |
| US10312542B2 (en) | 2014-12-04 | 2019-06-04 | Lg Chem, Ltd. | Halogenated compound, polymer comprising same, and polymer electrolyte membrane comprising same |
| US10407521B2 (en) | 2014-12-04 | 2019-09-10 | Lg Chem, Ltd. | Polymer and polymer electrolyte membrane comprising same |
| US10483576B2 (en) | 2014-12-04 | 2019-11-19 | Lg Chem, Ltd. | Polymer electrolyte membrane |
Families Citing this family (77)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE10021106A1 (de) * | 2000-05-02 | 2001-11-08 | Univ Stuttgart | Polymere Membranen |
| DE10024575A1 (de) * | 2000-11-02 | 2001-11-22 | Univ Stuttgart | Kovalent vernetzte Polymere und Polymermembranen via Sulfinatalkylierung |
| NL1017412C2 (nl) * | 2001-02-21 | 2002-08-22 | Tno | Werkwijze voor het tegen biologische aangroei beschermen van oppervlakken. |
| US20040137296A1 (en) * | 2001-05-18 | 2004-07-15 | Werner Schunk | Fuel cell |
| DE10295737B4 (de) * | 2001-05-21 | 2018-05-09 | Thomas Häring | Kovalent vernetzter Komposit, kovalente vernetzte Kompositmembran, Verfahren zu deren Herstellung und Verwendung der Membranen |
| EP1284518A1 (en) * | 2001-08-16 | 2003-02-19 | Samsung Electronics Co., Ltd. | Reinforced composite ionic conductive polymer membrane and fuel cell adopting the same |
| KR100407793B1 (ko) * | 2001-09-04 | 2003-12-01 | 한국과학기술연구원 | 분리능이 있는 수소 이온 교환 복합막, 복합 용액, 그제조방법 및 이를 포함하는 연료전지 |
| DE10209774A1 (de) * | 2002-02-28 | 2004-07-29 | Universität Stuttgart - Institut für Chemische Verfahrenstechnik | Composites und Compositemembranen |
| EP1429408A4 (en) * | 2002-03-25 | 2007-10-31 | Matsushita Electric Industrial Co Ltd | Assembly of an electrolytic membrane / elecrode for a fuel cell and process for its production |
| US20040018410A1 (en) * | 2002-06-10 | 2004-01-29 | Hongli Dai | Additive for direct methanol fuel cells |
| KR100477897B1 (ko) * | 2002-07-10 | 2005-03-18 | 광주과학기술원 | 음이온 교환기로서 피리디늄 또는 그 유도체를 도입한 음이온 교환막과 그 제조방법 및 상기 막을 포함하는 장치 |
| JP3878521B2 (ja) * | 2002-07-18 | 2007-02-07 | 本田技研工業株式会社 | プロトン伝導性高分子固体電解質およびその製造方法 |
| US6630265B1 (en) * | 2002-08-13 | 2003-10-07 | Hoku Scientific, Inc. | Composite electrolyte for fuel cells |
| EP1555707A4 (en) * | 2002-10-22 | 2008-07-02 | Yasuaki Takeuchi | BLUE SEA MINIATURE AND FUEL CELL WITH INTERCALING COMPLEX DAF R AS FIXED ELECTROLYTE MEMBRANE |
| CN100561784C (zh) * | 2002-10-22 | 2009-11-18 | Lg化学株式会社 | 利用层状硅酸盐矿物和夹层化合物的固体电解质膜的质子交换膜燃料电池 |
| JP4045918B2 (ja) * | 2002-10-23 | 2008-02-13 | トヨタ自動車株式会社 | プロトン伝導膜及びその製造方法 |
| KR100481591B1 (ko) * | 2002-11-13 | 2005-04-08 | 주식회사 협진아이엔씨 | 연료전지용 고분자 나노복합막, 그의 제조방법 및 이를이용한 연료전지 |
| RU2230400C1 (ru) * | 2002-11-18 | 2004-06-10 | Закрытое акционерное общество "Индепендент Пауэр Технолоджис" "ИПТ" | Спиртово-воздушный топливный элемент |
| JP4107116B2 (ja) | 2003-03-14 | 2008-06-25 | トヨタ自動車株式会社 | プロトン伝導性材料、プロトン伝導性材料膜、及び燃料電池 |
| FR2853306B1 (fr) * | 2003-04-02 | 2005-06-10 | Commissariat Energie Atomique | Materiau composite a base de borophosphosilicate pouvant notamment constituer une menbrane electrolytique et procede de fabrication. |
| CN100416708C (zh) * | 2003-04-25 | 2008-09-03 | 积水化学工业株式会社 | 质子传导膜,制造该膜的方法和使用该膜的燃料电池 |
| US6962959B2 (en) | 2003-08-28 | 2005-11-08 | Hoku Scientific, Inc. | Composite electrolyte with crosslinking agents |
| KR100528345B1 (ko) | 2003-08-29 | 2005-11-15 | 삼성에스디아이 주식회사 | 고분자 나노 복합막 및 이를 채용한 연료 전지 |
| DE102004001974A1 (de) * | 2004-01-13 | 2005-08-04 | Basf Ag | Komposit-Membran |
| WO2005090480A1 (ja) * | 2004-03-23 | 2005-09-29 | Mitsubishi Gas Chemical Co., Inc. | 固体高分子電解質、固体高分子ゲル膜、固体高分子電解質膜、および燃料電池 |
| US20090004548A1 (en) * | 2004-04-08 | 2009-01-01 | Toagosei Co., Ltd. | Electrolyte Membrane, Method for Producing Membrane Electrode Assembly, and Fuel Cell |
| FR2869032B1 (fr) * | 2004-04-15 | 2006-06-02 | Commissariat Energie Atomique | Procede de preparation de particules d'argile conductrices de protons et materiau comprenant de telles particules |
| US7604746B2 (en) * | 2004-04-27 | 2009-10-20 | Mcmaster University | Pervaporation composite membranes |
| JP4716706B2 (ja) * | 2004-10-20 | 2011-07-06 | 日産自動車株式会社 | プロトン伝導性コンポジット型電解質膜及びその製造方法 |
| JP2006290723A (ja) * | 2004-11-17 | 2006-10-26 | Cci Corp | 層間化合物及びその製造方法並びに複合材料 |
| JP4936673B2 (ja) * | 2005-02-10 | 2012-05-23 | 株式会社東芝 | 高分子電解質膜および直接メタノール型燃料電池 |
| CN100478384C (zh) * | 2005-02-22 | 2009-04-15 | 中国科学院化学研究所 | 一种功能化聚烯烃/蒙脱土纳米复合材料及其制备方法 |
| JP4925091B2 (ja) * | 2005-02-25 | 2012-04-25 | 日産自動車株式会社 | プロトン伝導性コンポジット型電解質膜及びその製造方法 |
| JP4611072B2 (ja) * | 2005-03-25 | 2011-01-12 | シーシーアイ株式会社 | 層間化合物の製造方法 |
| JP4747647B2 (ja) * | 2005-04-12 | 2011-08-17 | 株式会社カネカ | 高分子電解質膜およびその製造方法 |
| CN100402134C (zh) * | 2005-04-26 | 2008-07-16 | 哈尔滨工业大学 | 一种聚合物/蒙脱土纳米复合亲水膜的制备方法 |
| US20060258875A1 (en) * | 2005-05-10 | 2006-11-16 | Clementine Reyes | Methods for manufacturing supported nanocatalysts and methods for using supported nanocatalysts |
| KR100696521B1 (ko) * | 2005-05-25 | 2007-03-19 | 삼성에스디아이 주식회사 | 수소 이온 전도성 무기물, 이를 포함한 고분자 나노 복합막및 이를 채용한 연료 전지 |
| KR100708713B1 (ko) | 2005-09-24 | 2007-04-17 | 삼성에스디아이 주식회사 | 나노복합체, 나노복합 전해질막 및 이를 이용한 연료전지 |
| DE102005056564B4 (de) * | 2005-11-25 | 2009-11-12 | Gkss-Forschungszentrum Geesthacht Gmbh | Polymerelektrolytmembran mit Koordinationspolymer, Verfahren zu seiner Herstellung sowie Verwendung in einer Brennstoffzelle |
| KR100791780B1 (ko) * | 2005-12-31 | 2008-01-03 | 성균관대학교산학협력단 | 연료전지용 산/염기/무기물 고분자 전해질 복합막 및 이의제조 방법 |
| US8097229B2 (en) * | 2006-01-17 | 2012-01-17 | Headwaters Technology Innovation, Llc | Methods for manufacturing functionalized inorganic oxides and polymers incorporating same |
| KR100846478B1 (ko) | 2006-05-16 | 2008-07-17 | 삼성에스디아이 주식회사 | 담지 촉매, 그 제조방법 및 이를 이용한 연료전지 |
| CN100386365C (zh) * | 2006-05-30 | 2008-05-07 | 武汉理工大学 | 无机矿物-质子传导树脂插层复合质子交换膜及其制备方法 |
| KR100803199B1 (ko) * | 2006-09-18 | 2008-02-14 | 삼성에스디아이 주식회사 | 나노복합체 이온 착물을 이용한 전해질막 및 이를 채용한연료전지 |
| ES2613960T3 (es) | 2007-08-25 | 2017-05-29 | De Montfort University | Agente antimicrobiano y/o catalizador para reacciones químicas |
| JP2009256654A (ja) * | 2008-03-27 | 2009-11-05 | Sumitomo Chemical Co Ltd | 高分子電解質組成物 |
| DK2376215T3 (en) * | 2008-12-11 | 2019-01-28 | Univ California | FILTER CONE |
| RU2411070C1 (ru) * | 2009-08-18 | 2011-02-10 | Государственное образовательное учреждение высшего профессионального образования "Кубанский государственный университет" (ГОУ ВПО КубГУ) | Композиционная ионообменная мембрана |
| US8703831B2 (en) | 2009-08-26 | 2014-04-22 | Evoqua Water Technologies Pte. Ltd. | Ion exchange membranes |
| CN101698137B (zh) * | 2009-10-29 | 2011-06-29 | 浙江大学 | 聚电解质络合物/膨润土杂化渗透汽化膜的制备方法 |
| CN101804305B (zh) * | 2010-04-30 | 2013-05-15 | 北京碧水源膜科技有限公司 | 可导电增强管状多孔体复合膜及其制备方法与它们在污水除磷中的应用 |
| CN103237600B (zh) | 2010-10-15 | 2016-07-13 | 伊沃夸水处理技术有限责任公司 | 阴离子交换膜及制造方法 |
| CA2814701C (en) | 2010-10-15 | 2018-11-06 | Siemens Industry, Inc. | Process for making a monomer solution for making cation exchange membranes |
| DE102011076590A1 (de) | 2011-05-27 | 2012-11-29 | Wacker Chemie Ag | Polymerfilme auf der Basis von Polyazolen |
| CN103566780B (zh) * | 2012-07-27 | 2015-06-10 | 清华大学 | 一种氟取代聚芳醚复合阴离子电解质膜的制备方法 |
| CN102863636B (zh) * | 2012-09-19 | 2014-07-23 | 清华大学 | 一种原位聚合法制备含氟聚芳醚复合阴离子交换膜的方法 |
| US20150315042A1 (en) | 2012-10-04 | 2015-11-05 | Evoqua Water Technologies Llc | High-Performance Anion Exchange Membranes and Methods of Making Same |
| ES2816948T3 (es) | 2012-10-11 | 2021-04-06 | Evoqua Water Tech Llc | Membranas de intercambio iónico revestidas |
| PT2906608T (pt) | 2012-10-12 | 2024-10-22 | Univ California | Membranas de polianilina, utilizações, e respetivos métodos |
| EP2724773A1 (en) * | 2012-10-25 | 2014-04-30 | Nederlandse Organisatie voor toegepast -natuurwetenschappelijk onderzoek TNO | Nanosieve composite membrane |
| CA2912407C (en) | 2013-05-15 | 2023-04-18 | The Regents Of The University Of California | Polyaniline membranes formed by phase inversion for forward osmosis applications |
| US9302995B2 (en) | 2013-06-10 | 2016-04-05 | The United States Of America, As Represented By The Secretary Of The Navy | Electrically conducting oligo(pyrazoles) |
| JP6674383B2 (ja) | 2014-04-08 | 2020-04-01 | ザ リージェンツ オブ ザ ユニバーシティ オブ カリフォルニア | ポリアニリン系耐塩素性親水性濾過膜 |
| WO2016097855A1 (en) * | 2014-12-17 | 2016-06-23 | King Abdullah University Of Science And Technology | Xylene isomerization |
| CN105148749B (zh) * | 2015-08-20 | 2018-04-10 | 中国科学技术大学 | 一种扩散渗析膜及其制备方法 |
| CN105226302B (zh) * | 2015-09-24 | 2017-07-25 | 北京化工大学 | 外加电场辅助聚苯并咪唑和活性白土复合膜及其制备方法 |
| CN109517384A (zh) * | 2017-09-20 | 2019-03-26 | 四川东邦碳纤维材料有限公司 | 一种电池框体用材料及由其制备的电池框体 |
| CN109666158B (zh) * | 2017-10-17 | 2023-04-07 | 厦门逍扬运动科技有限公司 | 一种杂化动态聚合物及其应用 |
| US11028265B2 (en) | 2017-12-14 | 2021-06-08 | The Government Of The United States Of America, As Represented By The Secretary Of The Navy | Electrically conducting poly(pyrazoles) |
| CN109758917B (zh) * | 2018-09-18 | 2021-07-02 | 张伟 | 一种一二价阳离子选择性离子交换膜的制备方法 |
| WO2020068932A1 (en) | 2018-09-25 | 2020-04-02 | Evoqua Water Technologies Llc | Monovalent selective cation exchange membrane |
| CN111137902B (zh) * | 2018-11-05 | 2022-06-07 | 清华大学 | H-Si-O体系材料、负极活性材料及其制备方法、电化学电池负极材料及电化学电池 |
| CN111162301B (zh) * | 2018-11-07 | 2021-08-20 | 清华大学 | 改性质子交换膜及其制备方法、粘结剂、燃料电池及水电解装置 |
| CN109768321A (zh) * | 2019-03-22 | 2019-05-17 | 广州大学 | 一种基于铝柱撑黏土的锂电池固态电解质及其制备方法 |
| CN113750822B (zh) * | 2021-09-28 | 2023-06-30 | 太原理工大学 | 基于聚苯胺插层改性酸活化蒙脱土的混合基质复合膜的制备方法及应用 |
| CN114220984B (zh) * | 2022-02-21 | 2022-05-20 | 长沙理工大学 | Speek/改性膨润土复合离子交换膜及其制备方法 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH01138237A (ja) * | 1987-08-04 | 1989-05-31 | Kao Corp | 共役系高分子−陽イオン交換体複合膜およびその製造法 |
| JPH0693114A (ja) * | 1992-06-13 | 1994-04-05 | Hoechst Ag | 高分子電解質膜およびその製造方法 |
| JPH0948856A (ja) * | 1995-08-04 | 1997-02-18 | Mitsubishi Chem Corp | 結晶性熱可塑性樹脂組成物の製造方法 |
| JPH09251857A (ja) * | 1996-03-15 | 1997-09-22 | Agency Of Ind Science & Technol | 固体イオン導電体 |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB980185A (en) * | 1960-08-26 | 1965-01-13 | American Cyanamid Co | Ion-exchange compositions |
| JPS5935355B2 (ja) * | 1978-02-18 | 1984-08-28 | 京セラミタ株式会社 | 電気感応記録体用導電性組成物 |
| US4557856A (en) * | 1978-02-18 | 1985-12-10 | Mita Industrial Co., Ltd. | Electrically conductive composition for electro-responsive recording materials |
| JPS54110495A (en) * | 1978-02-20 | 1979-08-29 | Mita Industrial Co Ltd | Conductive composition |
| JPS55131033A (en) * | 1979-03-30 | 1980-10-11 | Mitsubishi Petrochem Co Ltd | Resin composition for wrapping material |
| DE3407149A1 (de) * | 1984-02-28 | 1985-08-29 | Basf Ag, 6700 Ludwigshafen | Membrane aus organischen polymeren, die kristalline traegerverbindungen enthalten, deren herstellung und verwendung |
| ES2052648T3 (es) * | 1987-08-04 | 1994-07-16 | Kao Corp | Una membrana compuesta intercambiadora de cationes de polimeros conjugado y su proceso de preparacion. |
| JP3037547B2 (ja) * | 1993-09-03 | 2000-04-24 | 三菱レイヨン株式会社 | 導電性組成物、導電体及びその形成方法 |
| US5795496A (en) * | 1995-11-22 | 1998-08-18 | California Institute Of Technology | Polymer material for electrolytic membranes in fuel cells |
| JPH09295810A (ja) * | 1996-02-27 | 1997-11-18 | Du Pont Kk | 複合材料およびその製造方法ならびに複合材料含有樹脂組成物およびその製造方法 |
| US5658460A (en) * | 1996-05-07 | 1997-08-19 | The Dow Chemical Company | Use of inorganic ammonium cation salts to maintain the flux and salt rejection characteristics of reverse osmosis and nanofiltration membranes during drying |
| DE19632285A1 (de) * | 1996-08-09 | 1998-02-19 | Hoechst Ag | Protonenleiter mit einer Temperaturbeständigkeit in einem weiten Bereich und guten Protonenleitfähigkeiten |
| GB9708365D0 (en) * | 1997-04-25 | 1997-06-18 | Johnson Matthey Plc | Proton conducting membranes |
| US6495209B1 (en) * | 1998-02-20 | 2002-12-17 | Lynntech, Inc. | Process of making a composite membrane |
| DE19817376A1 (de) * | 1998-04-18 | 1999-10-21 | Univ Stuttgart Lehrstuhl Und I | Säure-Base-Polymerblends und ihre Verwendung in Membranprozessen |
| US6908604B2 (en) * | 1999-05-17 | 2005-06-21 | Exxonmobil Chemical Patents Inc. | Macrostructures of porous inorganic material and process for their preparation |
| DE19919881A1 (de) | 1999-04-30 | 2000-11-02 | Univ Stuttgart | Organisch-Anorganische Komposites und Kompositmembranen aus Ionomeren oder Ionomerblends und aus Schicht- oder Gerätsilicaten |
| US6610770B1 (en) * | 1999-10-04 | 2003-08-26 | Elementis Specialties, Inc. | Organoclay/polymer compositions with flame retardant properties |
| US6982303B2 (en) * | 2000-05-19 | 2006-01-03 | Jochen Kerres | Covalently cross-linked polymers and polymer membranes via sulfinate alkylation |
| US6403721B1 (en) * | 2000-09-29 | 2002-06-11 | Solvay Engineered Polymers | Engineered polyolefin materials with enhanced surface durability |
-
1999
- 1999-04-30 DE DE19919881A patent/DE19919881A1/de not_active Ceased
-
2000
- 2000-05-02 EP EP07015005A patent/EP1870429A3/de not_active Withdrawn
- 2000-05-02 CN CNB00809599XA patent/CN1320038C/zh not_active Expired - Fee Related
- 2000-05-02 ES ES00934975T patent/ES2293901T3/es not_active Expired - Lifetime
- 2000-05-02 DE DE50014531T patent/DE50014531D1/de not_active Expired - Lifetime
- 2000-05-02 BR BRPI0010171-0A patent/BR0010171B1/pt not_active IP Right Cessation
- 2000-05-02 AT AT00934975T patent/ATE368703T1/de active
- 2000-05-02 KR KR1020017013909A patent/KR100858131B1/ko not_active Expired - Fee Related
- 2000-05-02 WO PCT/EP2000/003910 patent/WO2000074827A2/de not_active Ceased
- 2000-05-02 EP EP00934975A patent/EP1177247B1/de not_active Expired - Lifetime
- 2000-05-02 IL IL14620900A patent/IL146209A0/xx unknown
- 2000-05-02 AU AU50635/00A patent/AU780722B2/en not_active Ceased
- 2000-05-02 CA CA002369703A patent/CA2369703C/en not_active Expired - Fee Related
- 2000-05-02 JP JP2001501354A patent/JP2003501516A/ja active Pending
-
2001
- 2001-10-28 IL IL146209A patent/IL146209A/en not_active IP Right Cessation
- 2001-10-30 US US09/984,564 patent/US7049020B2/en not_active Expired - Lifetime
- 2001-11-29 ZA ZA200109819A patent/ZA200109819B/xx unknown
-
2006
- 2006-02-06 US US11/348,870 patent/US7674505B2/en not_active Expired - Fee Related
-
2009
- 2009-10-21 US US12/603,017 patent/US8110517B2/en not_active Expired - Fee Related
-
2012
- 2012-02-06 US US13/367,038 patent/US9675939B2/en not_active Expired - Fee Related
- 2012-02-20 JP JP2012034502A patent/JP2012149259A/ja active Pending
-
2014
- 2014-02-27 JP JP2014037390A patent/JP2014167113A/ja active Pending
-
2017
- 2017-03-08 JP JP2017044440A patent/JP2017125205A/ja active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH01138237A (ja) * | 1987-08-04 | 1989-05-31 | Kao Corp | 共役系高分子−陽イオン交換体複合膜およびその製造法 |
| JPH0693114A (ja) * | 1992-06-13 | 1994-04-05 | Hoechst Ag | 高分子電解質膜およびその製造方法 |
| JPH0948856A (ja) * | 1995-08-04 | 1997-02-18 | Mitsubishi Chem Corp | 結晶性熱可塑性樹脂組成物の製造方法 |
| JPH09251857A (ja) * | 1996-03-15 | 1997-09-22 | Agency Of Ind Science & Technol | 固体イオン導電体 |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014167113A (ja) * | 1999-04-30 | 2014-09-11 | Thomas Haering | 複合体の製造方法 |
| JP2018505234A (ja) * | 2014-12-04 | 2018-02-22 | エルジー・ケム・リミテッド | 高分子電解質膜 |
| US10312542B2 (en) | 2014-12-04 | 2019-06-04 | Lg Chem, Ltd. | Halogenated compound, polymer comprising same, and polymer electrolyte membrane comprising same |
| US10361447B2 (en) | 2014-12-04 | 2019-07-23 | Lg Chem, Ltd. | Polymer and polymer electrolyte membrane comprising same |
| US10411283B2 (en) | 2014-12-04 | 2019-09-10 | Lg Chem, Ltd. | Polymer electrolyte membrane |
| US10407521B2 (en) | 2014-12-04 | 2019-09-10 | Lg Chem, Ltd. | Polymer and polymer electrolyte membrane comprising same |
| US10446864B2 (en) | 2014-12-04 | 2019-10-15 | Lg Chem, Ltd. | Polymer and polymer electrolyte membrane comprising same |
| US10483576B2 (en) | 2014-12-04 | 2019-11-19 | Lg Chem, Ltd. | Polymer electrolyte membrane |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2017125205A (ja) | 複合体の製造方法 | |
| US6982303B2 (en) | Covalently cross-linked polymers and polymer membranes via sulfinate alkylation | |
| JP3688201B2 (ja) | エンジニアリングイオノマーブレンド及びエンジニアリングイオノマーブレンド膜 | |
| US9126147B2 (en) | Modification of drawn film | |
| CN100354344C (zh) | 通过亚磺酸盐烷基化得到的共价键交联聚合物和聚合物膜 | |
| JP2004530020A5 (ja) | ||
| JP2012162722A (ja) | スルフィナートアルキル化を介した共有結合架橋ポリマーおよびポリマー膜 | |
| Chakrabarty et al. | Chitosan based membranes for separation, pervaporation and fuel cell applications: Recent developments |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130827 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20131127 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20131202 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140227 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140729 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20141029 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20141104 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20141114 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20141119 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20150428 |