EP1506203B1 - Synthese von epothilonen, zwischenprodukte dafür, analoga und deren verwendungen - Google Patents
Synthese von epothilonen, zwischenprodukte dafür, analoga und deren verwendungen Download PDFInfo
- Publication number
- EP1506203B1 EP1506203B1 EP03793304A EP03793304A EP1506203B1 EP 1506203 B1 EP1506203 B1 EP 1506203B1 EP 03793304 A EP03793304 A EP 03793304A EP 03793304 A EP03793304 A EP 03793304A EP 1506203 B1 EP1506203 B1 EP 1506203B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- compound
- alkyl
- aliphatic
- dehydro
- tumor
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 0 C[C@@](CC=CC(*)CC[C@](C(C)=CC1=CSC(C*)C1)OC(CC(***)C1(C)C)O)C(*)[C@@](C)C1=O Chemical compound C[C@@](CC=CC(*)CC[C@](C(C)=CC1=CSC(C*)C1)OC(CC(***)C1(C)C)O)C(*)[C@@](C)C1=O 0.000 description 7
- HLCDYZXJTJMIHX-PZTGSQHCSA-N CC(C)S/C=C(/C=C(\C)/[C@H](C/C=C(/C)\C/C=C/[C@H](C)[C@@H]([C@@H](C)C(C(C)(C)[C@H](C1)O)=O)O)OC1=O)\N Chemical compound CC(C)S/C=C(/C=C(\C)/[C@H](C/C=C(/C)\C/C=C/[C@H](C)[C@@H]([C@@H](C)C(C(C)(C)[C@H](C1)O)=O)O)OC1=O)\N HLCDYZXJTJMIHX-PZTGSQHCSA-N 0.000 description 1
- OQZPGZFKZZFIOO-VOTSOKGWSA-N CC/C=C(\CC=C)/C(F)(F)F Chemical compound CC/C=C(\CC=C)/C(F)(F)F OQZPGZFKZZFIOO-VOTSOKGWSA-N 0.000 description 1
- IJUVDLIGWVERJJ-UHFFFAOYSA-N CCCC(CC=C)C(F)(F)F Chemical compound CCCC(CC=C)C(F)(F)F IJUVDLIGWVERJJ-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/06—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings linked by a carbon chain containing only aliphatic carbon atoms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/06—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings linked by a carbon chain containing only aliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D419/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen, oxygen, and sulfur atoms as the only ring hetero atoms
- C07D419/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen, oxygen, and sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D419/06—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen, oxygen, and sulfur atoms as the only ring hetero atoms containing two hetero rings linked by a carbon chain containing only aliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D493/00—Heterocyclic compounds containing oxygen atoms as the only ring hetero atoms in the condensed system
- C07D493/02—Heterocyclic compounds containing oxygen atoms as the only ring hetero atoms in the condensed system in which the condensed system contains two hetero rings
- C07D493/04—Ortho-condensed systems
Definitions
- Epothilones A and B ( 2a and 2b , Scheme 1) are naturally occurring cytotoxic macrolides that were isolated from a cellulose degrading mycobacterium, Sorangium cellulosum (Höfle et al. Angew. Chem., Int. Ed Engl. 1996, 35, 1567 and J. Antibiot. 1996 , 49, 560, Despite their vastly different structures, epothilones A and B share the same mechanism of action as paclitaxel (Taxol ® ) which involves growth inhibition of tumor cells by tubulin polymerization and stabilization of microtubule assemblies (Bollag et al. Cancer Res. 1995, 55, 2325.
- Taxol ® is far from an ideal drug. Its marginal water solubility necessitates recourse to formulation vehicles such as Cremophores that pose their own risks and management issues (Essayan et al. J. Allergy Clin. Immunol. 1996, 97, 42. Moreover, Taxol ® is vulnerable to deactivation through multiple drug resistance (MDR) (Giannakakou et al. J. Biol. Chem. 1997, 272, 17118. However, it has also been demonstrated that epothilones A and B retain remarkable potency against MDR tumor cells (Kowalski et al. Mol. Biol. Cell.
- MDR multiple drug resistance
- epothilone B 2b , EpoB, in Scheme 1
- epothilone B 2b , EpoB, in Scheme 1
- epothilone B 2b , EpoB, in Scheme 1
- epothilone B 2b , EpoB, in Scheme 1
- it unfortunately possesses, at least in xenograft mice, a worrisomely narrow therapeutic index (Su et al. Angew. Chem. Int. Ed Engl. 1997, 36 ,1093; Harris et al. J. Org. Chem. 1999 , 64, 8434.
- protecting group it is meant that a particular functional moiety, e.g., O, S, or N, is temporarily blocked so that a reaction can be carried out selectively at another reactive site in a multifunctional compound.
- a protecting group reacts selectively in good yield to give a protected substrate that is stable to the projected reactions; the protecting group must be selectively removed in good yield by readily available, preferably nontoxic reagents that do not attack the other funcational groups; the protecting group forms an easily separable derivative (more preferably without the generation of new stereogenic centers); and the protecting group has a minimum of additional functionality to avoid further sites of reaction.
- oxygen, sulfur, nitrogen and carbon protecting groups may be utilized.
- Exemplary protecting groups are detailed herein, however, it will be appreciated that the present invention is not intended to be limited to these protecting groups; rather, a variety of additional equivalent protecting groups can be readily identified using the above criteria and utilized in the method of the present invention. Additionally, a variety of protecting groups are described in "Protective Groups in Organic Synthesis" Third Ed. Greene, T.W. and Wuts, P.G., Eds., John Wiley & Sons, New York: 1999.
- the compounds, as described herein, may be substituted with any number of substituents or functional moieties.
- substituted whether preceded by the term “optionally” or not, and substituents contained in formulas of this invention, refer to the replacement of hydrogen radicals in a given structure with the radical of a specified substituent.
- substituents When more than one position in any given structure may be substituted with more than one substituent selected from a specified group, the substituent may be either the same or different at every position.
- Combinations of substituents and variables envisioned by this invention are preferably those that result in the formation of stable compounds useful in the treatment, for example of proliferative disorders, including, but not limited to cancer.
- stable preferably refers to compounds which possess stability sufficient to allow manufacture and which maintain the integrity of the compound for a sufficient period of time to be detected and preferably for a sufficient period of time to be useful for the purposes detailed herein.
- aliphatic includes both saturated and unsaturated, straight chain (i.e., unbranched), branched, cyclic, or polycyclic aliphatic hydrocarbons, which are optionally substituted with one or more functional groups.
- aliphatic is intended herein to include, but is not limited to, alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, and cycloalkynyl moieties.
- alkyl includes straight, branched and cyclic alkyl groups.
- alkyl alkenyl
- alkynyl alkynyl
- lower alkyl is used to indicate those alkyl groups (cyclic, acyclic, substituted, unsubstituted, branched or unbranched) having 1-6 carbon atoms.
- the alkyl, alkenyl and alkynyl groups employed in the invention contain 1-20 aliphatic carbon atoms. In certain other embodiments, the alkyl, alkenyl, and alkynyl groups employed in the invention contain 1-10 aliphatic carbon atoms. In yet other embodiments, the alkyl, alkenyl, and alkynyl groups employed in the invention contain 1-8 aliphatic carbon atoms. In still other embodiments, the alkyl, alkenyl, and alkynyl groups employed in the invention contain 1-6 aliphatic carbon atoms. In yet other embodiments, the alkyl, alkenyl, and alkynyl groups employed in the invention contain 1-4 carbon atoms.
- Illustrative aliphatic groups thus include, but are not limited to, for example, methyl, ethyl, n-propyl, isopropyl, cyclopropyl, -CH 2 -cyclopropyl, allyl, n-butyl, sec-butyl, isobutyl, tert-butyl, cyclobutyl, -CH 2 -cyclobutyl, n-pentyl, sec-pentyl, isopentyl, tert-pentyl, cyclopentyl, -CH 2 -cyclopentyl, n-hexyl, sec-hexyl, cyclohexyl, -CH 2 -cyclohexyl moieties and the like, which again, may bear one or more substituents.
- Alkenyl groups include, but are not limited to, for example, ethenyl, propenyl, butenyl, 1-methyl-2-buten-1-yl, and the like.
- Representative alkynyl groups include, but are not limited to, ethynyl, 2-propynyl (propargyl), 1-propynyl and the like.
- alkoxy refers to an alkyl group, as previously defined, attached to the parent molecular moiety through an oxygen atom or through a sulfur atom.
- the alkyl group contains 1-20 alipahtic carbon atoms.
- the alkyl group contains 1-10 aliphatic carbon atoms.
- the alkyl, alkenyl, and alkynyl groups employed in the invention contain 1-8 aliphatic carbon atoms.
- the alkyl group contains 1-6 aliphatic carbon atoms.
- the alkyl group contains 1-4 aliphatic carbon atoms.
- alkoxy examples include but are not limited to, methoxy, ethoxy, propoxy, isopropoxy, n-butoxy, tert-butoxy, neopentoxy and n-hexoxy.
- thioalkyl examples include, but are not limited to, methylthio, ethylthio, propylthio, isopropylthio, n-butylthio, and the like.
- alkylamino refers to a group having the structure -NHR' wherein R' is alkyl, as defined herein.
- the alkyl group contains 1-20 aliphatic carbon atoms.
- the alkyl group contains 1-10 aliphatic carbon atoms.
- the alkyl, alkenyl, and alkynyl groups employed in the invention contain 1-8 aliphatic carbon atoms.
- the alkyl group contains 1-6 aliphatic carbon atoms.
- the alkyl group contains 1-4 aliphatic carbon atoms.
- alkylamino include, but are not limited to, methylamino, ethylamino, iso-propylamino and the like.
- substituents of the above-described aliphatic (and other) moieties of compounds of the invention include, but are not limited to aliphatic; heteroaliphatic; aryl; heteroaryl; arylalkyl; heteroarylalkyl; alkoxy; aryloxy; heteroalkoxy; heteroaryloxy; alkylthio; arylthio; heteroalkylthio; heteroarylthio; F; Cl; Br; I; -OH; -NO 2 ; -CN; -CF 3 ; -CH 2 CF 3 ; -CHCl 2 ; -CH 2 OH; -CH 2 CH 2 OH; -CH 2 NH 2 ; - CH 2 SO 2 CH 3 ; -C(O)R x ; -CO 2 (R x ); -CON(R x ) 2 ; -OC(O)R x ; -OCO 2 R x ; -OCON(R x )
- aryl and heteroaryl refer to stable mono- or polycyclic, heterocyclic, polycyclic, and polyheterocyclic unsaturated moieties having preferably 3-14 carbon atoms, each of which may be substituted or unsubstituted.
- Substituents include, but are not limited to, any of the previously mentioned substitutents, i.e., the substituents recited for aliphatic moieties, or for other moieties as disclosed herein, resulting in the formation of a stable compound.
- aryl refers to a mono- or bicyclic carbocyclic ring system having one or two aromatic rings including, but not limited to, phenyl, naphthyl, tetrahydronaphthyl, indanyl, indenyl and the like.
- heteroaryl refers to a cyclic aromatic radical having from five to ten ring atoms of which one ring atom is selected from S, O and N; zero, one or two ring atoms are additional heteroatoms independently selected from S, O and N; and the remaining ring atoms are carbon, the radical being joined to the rest of the molecule via any of the ring atoms, such as, for example, pyridyl, pyrazinyl, pyrimidinyl, pyrrolyl, pyrazolyl, imidazolyl, thiazolyl, oxazolyl, isooxazolyl, thiadiazolyl,oxadiazolyl, thiophenyl, furanyl, quinolinyl, isoquinolinyl, and the like.
- aryl and heteroaryl groups can be unsubstituted or substituted, wherein substitution includes replacement of one, two or three of the hydrogen atoms thereon independently with any one or more of the following moieties including, but not limited to: aliphatic; heteroaliphatic; aryl; heteroaryl; arylalkyl; heteroarylalkyl; alkoxy; aryloxy; heteroalkoxy; heteroaryloxy; alkylthio; arylthio; heteroalkylthio; heteroarylthio; F; Cl; Br; I; -OH; -NO 2 ; -CN; -CF 3 ; -CH 2 CF 3 ; -CHCl 2 ; -CH 2 OH; -CH 2 CH 2 OH; -CH 2 NH 2 ; -CH 2 SO 2 CH 3 ; -C(O)R x ; -CO 2 (R x ); -CON(R
- cycloalkyl refers specifically to groups having three to seven, preferably three to ten carbon atoms. Suitable cycloalkyls include, but are not limited to cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl and the like, which, as in the case of other aliphatic, heteroaliphatic or hetercyclic moieties, may optionally be substituted with substituents including, but not limited to aliphatic; heteroaliphatic; aryl; heteroaryl; arylalkyl; heteroarylalkyl; alkoxy; aryloxy; heteroalkoxy; heteroaryloxy; alkylthio; arylthio; heteroalkylthio; heteroarylthio; F; Cl; Br, I; -OH; -NO 2 ; -CN; -CF 3 ; -CH 2 CF 3 ; -CHCl 2 ;
- heteroaliphatic refers to aliphatic moieties that contain one or more oxygen, sulfur, nitrogen, phosphorus or silicon atoms, e.g., in place of carbon atoms. Heteroaliphatic moieties may be branched, unbranched, cyclic or acyclic and include saturated and unsaturated heterocycles such as morpholino, pyrrolidinyl, etc.
- heteroaliphatic moieties are substituted by independent replacement of one or more of the hydrogen atoms thereon with one or more moieties including, but not limited to aliphatic; heteroaliphatic; aryl; heteroaryl; arylalkyl; heteroarylalkyl; alkoxy; aryloxy; heteroalkoxy; heteroaryloxy; alkylthio; arylthio; heteroalkylthio; heteroarylthio; F; Cl; Br; I; -OH; -NO 2 ; -CN; -CF 3 ; -CH 2 CF 3 ; -CHCl 2 ; -CH 2 OH; -CH 2 CH 2 OH; -CH 2 NH 2 ; -CH 2 SO 2 CH 3 ; -C(O)R x ; -CO 2 (R x ); - CON(R x ) 2 ; -OC(O)R x ; -OCO 2 R x ; -
- haloalkyl denotes an alkyl group, as defined above, having one, two, or three halogen atoms attached thereto and is exemplified by such groups as chloromethyl, bromoethyl, trifluoromethyl, and the like.
- heterocycloalkyl refers to a non-aromatic 5-, 6-, or 7- membered ring or a polycyclic group, including, but not limited to a bi- or tri-cyclic group comprising fused six-membered rings having between one and three heteroatoms independently selected from oxygen, sulfur and nitrogen, wherein (i) each 5-membered ring has 0 to 1 double bonds and each 6-membered ring has 0 to 2 double bonds, (ii) the nitrogen and sulfur heteroatoms may be optionally be oxidized, (iii) the nitrogen heteroatom may optionally be quaternized, and (iv) any of the above heterocyclic rings may be fused to a benzene ring.
- heterocycles include, but are not limited to, pyrrolidinyl, pyrazolinyl, pyrazolidinyl, imidazolinyl, imidazolidinyl, piperidinyl, piperazinyl, oxazolidinyl, isoxazolidinyl, morpholinyl, thiazolidinyl, isothiazolidinyl, and tetrahydrofuryl.
- a "substituted heterocycloalkyl or heterocycle” group refers to a heterocycloalkyl or heterocycle group, as defined above, substituted by the independent replacement of one, two or three of the hydrogen atoms thereon with but are not limited to aliphatic; heteroaliphatic; aryl; heteroaryl; arylalkyl; heteroarylalkyl; alkoxy; aryloxy; heteroalkoxy; heteroaryloxy; alkylthio; arylthio; heteroalkylthio; heteroarylthio; F; Cl; Br; I; -OH; -NO 2 ; -CN; -CF 3 ; -CH 2 CF 3 ; -CHCl 2 ; -CH 2 OH; -CH 2 CH 2 OH; -CH 2 NH 2 ; -CH 2 SO 2 CH 3 ; -C(O)R x ; -CO 2 (R x );
- label As used herein, the term “labeled” is intended to mean that a compound has at least one element, isotope or chemical compound attached to enable the detection of the compound.
- labels fall into three classes: a) isotopic labels, which may be radioactive or heavy isotopes, including, but not limited to, 2 H, 3 H, 32 P, 35 S, 67 Ga, 99m Tc(Tc-99m), 111 In, 123 I, 125 I, 169 Yb and 186 Re; b) immune labels, which may be antibodies or antigens; and c) colored or fluorescent dyes.
- the labels may be incorporated into the compound at any position that does not interfere with the biological activity or characteristic of the compound that is being detected.
- photoaffinity labeling is utilized for the direct elucidation of interinolecular interactions in biological systems (e.g., to probe the epothilone binding site in a tubulin dimer).
- a variety of known photophores can be employed, most relying on photoconversion of diazo compounds, azides, or diazirines to nitrenes or carbenes ( See , Bayley, H., Photogenerated Reagents in Biochemistry and Molecular Biology (1983), Elsevier, Amsterdam.).
- the photoaffinity labels employed are o-, m- and p-azidobenzoyls, substituted with one or more halogen moieties, including, but not limited to 4-azido-2,3,5,6-tetrafluorobenzoic acid.
- Polymer refers to a composition comprising chains that may be open, closed, linear, branched or cross-linked of repeating units (monomers) that may be the same or different. It will be appreciated that in certain embodiments the term polymer refers to biopolymers, which, as used herein, is intended to refer to polymeric materials found in nature or based upon those materials found in nature, including, but not limited to nucleic acids, peptides, and mimetics thereof. In certain other embodiments, the term polymer refers to synthetic polymers, such as biodegradable polymers or other polymeric materials. It will be appreciated that polymeric solid supports are also encompassed by the polymers of the present invention.
- solid support is meant to include, but is not limited to, pellets, disks, capillaries, hollow fibers, needles, pins, solid fibers, cellulose beads, pore-glass beads, silica gels, polystyrene beads optionally cross-linked with divinylbenzene, grafted co-poly beads, poly-acrylamide beads, latex beads, dimethylacrylamide beads optionally crosslinked with N-N'-bis-acryloylethylenediamine, and glass particles coated with a hydrophobic polymer.
- An exemplary solid support is a Tentagel amino resin, a composite of 1) a polystyrene bead crosslinked with divinylbenzene and 2) PEG (polyethylene glycol).
- Tentagel is a particularly useful solid support because it provides a versatile support for use in on-bead or off-bead assays, and it also undergoes excellent swelling in solvents ranging from toluene to water.
- the present invention provides novel synthetic methodologies enabling access to macrocycles having a broad range of biological and pharmacological activity, as well as novel compounds with such activity, novel therapeutic compositions, and methods of using these compounds and compositions.
- the inventive compounds are useful in the treatment of cancer.
- Certain compounds of the invention exhibit cytotoxic or growth inhibitory effects on cancer cells lines, exhibit an ability to polymerize tubulin and stabilize microtubule assemblies, and/or lead to shrinkage or diappearance of tumors in cancer cell xenograft models.
- the compounds may have reduced or minimal side effects including toxicity to vital organs, nausea, vomiting, diarrhea, allopecia, weight loss, weight gain, liver toxicity, skin disorders, etc.
- the compounds may also be easier to formulate due to increased water solubility, decreased toxicity, increased therapeutic range, increased efficacy, etc.
- the compounds of the invention include compounds of the general formula as further defined below:
- X is O. In other embodiments, X is NH. In other embodiments, X is CH 2 .
- R 2 is a substituted or unsubstituted thiazole. In certain embodiments, R 2 is 2-methyl-thiazo-4-yl. In other embodiments, R 2 is 2-hydroxylmethyl-thiazo-4-yl. In yet other embodiments, R 2 is 2-aminomethyl-thiazo-4-yl. In other embodiments, R 2 is 2-thiolmethyl-thiazo-4-yl.
- R 2 is a substituted or unsubstituted oxazole. In certain embodiments, R 2 is 2-methyl-oxazo-4-yl. In other embodiments, R 2 is 2-hydroxylmethyl-oxazo-4-yl. In yet other embodiments, R 2 is 2-aminomethyl-oxazo-4-yl. In other embodiments, R 2 is 2-thiolmethyl-oxazo-4-yl.
- R B is hydrogen, methyl, ethyl, -CF 3 , -CH 2 F, -CF 2 H. In certain embodiments, R B is methyl. In yet other embodiments, R B is -CF 3 . In certain embodiments, R B is hydrogen. In other embodiments, R B is ethyl.
- Certain preferred compounds include, for example:
- inventive compounds and pharmaceutical compositions thereof may be in the form of an individual enantiomer, diastereomer or geometric isomer, or may be in the form of a mixture of stereoisomers.
- the compounds of the invention are enantiopure compounds.
- a mixtures of stereoisomers or diastereomers are provided.
- the invention encompasses the compounds as individual isomers substantially free of other isomers and alternatively, as mixtures of various isomers, e.g., racemic mixtures of stereoisomers. Additionally, the invention encompasses both (Z) and (E) double bond isomers unless otherwise specifically designated.
- the double bond at the C12-C13 position is in the cis or Z configuration. In some embodiments, the double bond at the C9-C10 position is in the trans or E configuration. In still other embodiments, the double bond at the C12-C13 position is in the cis or Z configuration, and the double bond at the C9-C10 position is in the trans or E configuration.
- the invention also encompasses tautomers of specific compounds as described above.
- the present invention provides pharmaceutically acceptable derivatives of the inventive compounds, and methods of treating a subject using these compounds, pharmaceutical compositions thereof, or either of these in combination with one or more additional therapeutic agents.
- pharmaceutically acceptable derivative denotes any pharmaceutically acceptable salt, ester, or salt of such ester, of such compound, or any other adduct or derivative which, upon administration to a patient, is capable of providing (directly or indirectly) a compound as otherwise described herein, or a metabolite or residue thereof.
- Pharmaceutically acceptable derivatives thus include among others pro-drugs.
- a prodrug is a derivative of a compound, usually with significantly reduced pharmacological activity, which contains an additional moiety that is susceptible to removal in vivo yielding the parent molecule as the pharmacologically active species.
- An example of a pro-drug is an ester that is cleaved in vivo to yield a compound of interest.
- Pro-drugs of a variety of compounds, and materials and methods for derivatizing the parent compounds to create the pro-drugs Are known and may be adapted to the present invention. Certain exemplary pharmaceutical compositions and pharmaceutically acceptable derivatives will be discussed in more detail herein below.
- the present invention provides an efficient and modular route for the synthesis of epothilones, desoxyepothilones and analogues thereof.
- synthesis of certain exemplary compounds is described in the Exemplification herein, it will be appreciated that this methodology is generally applicable to the generation of analogues and conjugates as discussed above for each of the classes and subclasses described herein.
- the 9,10-dehydroepothilone compounds of the present invention may be prepared in a variety of ways using synthetic methodologies useful in the synthesis of epothilones.
- the compounds are prepared using a convergent synthetic route.
- the epothilone may be synthesized by preparing two or three intermediates which are brought together to yield the desired compound.
- one of the intermediates is an acyl portion containing carbons 1-9, and another intermediate contains carbons 10-15 and may also contain the thiazole side chain. These two roughly equal portions of the epothilone may be brought together first using an esterification reaction between C-1 and an oxygen off C-15.
- the macrocycle may then be closed using a carbon-carbon coupling reaction such as a Suzuki coupling or ring closing metathesis reaction.
- a carbon-carbon coupling reaction such as a Suzuki coupling or ring closing metathesis reaction.
- the final ring closing step is accomplished using a ring closing metathesis reaction to form the 9,10-double bond and close the macrocycle.
- the ring closing metathesis reaction is accomplished using an organometallic catalyst such as the Grubbs catalyst as shown in Scheme 8 below.
- the 9,10-double bond is reduced or oxidized, or the 9,10-double bond may bo further functionalized to prepare additional epothilone derivatives.
- the final ring closing step is accomplished using a ring closing metathesis reaction to form the 12,13-double bond and close the macrocycle.
- the 12,13-double bond is reduced or oxidized.
- a macroaldolization or macrolactonization reaction is used to form the macrocycle.
- This invention also provides a pharmaceutical preparation comprising at least one of the compounds as described above and herein, or a pharmaceutically acceptable derivative thereof, which compounds are capable of inhibiting the growth of or killing cancer cells, and, in certain embodiments of special interest are capable of inhibiting the growth of or killing multidrug resistant cancer cells.
- the pharmaceutical preparation also comprises as solubilizing or emulsifying agent such as Cremophor (polyoxyl 35 castor oil) or Solutol (polyethylene glycol 660 12-hydroxystreaiate).
- compositions comprising any one of the compounds as described herein, and optionally comprise a pharmaceutically acceptable carrier.
- these compositions optionally further comprise one or more additional therapeutic agents.
- the additional therapeutic agent is an anticancer agent, as discussed in more detail herein.
- a pharmaceutically acceptable derivative includes, but is not limited to, pharmaceutically acceptable salts, esters, salts of such esters, or any other adduct or derivative which upon administration to a patient in need is capable of providing, directly or indirectly, a compound as otherwise described herein, or a metabolite or residue thereof, e.g., a prodrug.
- the term "pharmaceutically acceptable salt” refers to those salts which are, within the scope of sound medical judgement, suitable for use in contact with the tissues of humans and lower animals without undue toxicity, irritation, allergic response and the like, and are commensurate with a reasonable benefit/risk ratio.
- Pharmaceutically acceptable salts are well known in the art. For example, S. M. Berge, et al. describe pharmaceutically acceptable salts in detail in J. Pharmaceutical Sciences, 66: 1-19 (1977), incorporated herein by reference.
- the salts can be prepared in situ during the final isolation and purification of the compounds of the invention, or separately by reacting the free base function with a suitable organic acid.
- Examples of pharmaceutically acceptable, nontoxic acid addition salts are salts of an amino group formed with inorganic acids such as hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid and perchloric acid or with organic acids such as acetic acid, oxalic acid, maleic acid, tartaric acid, citric acid, succinic acid or malonic acid or by using other methods used in the art such as ion exchange.
- inorganic acids such as hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid and perchloric acid
- organic acids such as acetic acid, oxalic acid, maleic acid, tartaric acid, citric acid, succinic acid or malonic acid or by using other methods used in the art such as ion exchange.
- salts include adipate, alginate, ascorbate, aspartate, benzenesulfonate, benzoate, bisulfate, borate, butyrate, camphorate, camphorsulfonate, citrate, cyclopentanepropionate, digluconate, dodecylsulfate, ethanesulfonate, formate, fumarate, glucoheptonate, glycerophosphate, gluconate, hernisulfate, heptanoate, hexanoate, hydroiodide, 2-hydroxy-ethanesulfonate, lactobionate, lactate, laurate, lauryl sulfate, malate, maleate, malonate, methanesulfonate, 2-naphthalenesulfonate, nicotinate, nitrate, oleate, oxalate, palmitate, pamoate, pectinate,
- alkali or alkaline earth metal salts include sodium, lithium, potassium, calcium, magnesium, and the like.
- Further pharmaceutically acceptable salts include, when appropriate, nontoxic ammonium, quaternary ammonium, and amine cations formed using counterions such as halide, hydroxide, carboxylate, sulfate, phosphate, nitrate, loweralkyl sulfonate and aryl sulfonate.
- ester refers to esters which hydrolyze in vivo and include those that break down readily in the human body to leave the parent compound or a salt thereof.
- Suitable ester groups include, for example, those derived from pharmaceutically acceptable aliphatic carboxylic acids, particularly alkanoic, alkenoic, cycloalkanoic and alkanedioic acids, in which each alkyl or alkenyl moiety advantageously has not more than 6 carbon atoms.
- esters include formates, acetates, propionates, butyrates, acrylates and ethylsuccinates.
- prodrugs refers to those prodrugs of the compounds of the present invention which are, within the scope of sound medical judgment, suitable for use in contact with the tissues of humans and lower animals with undue toxicity, irritation, allergic response, and the like, commensurate with a reasonable benefit/risk ratio, and effective for their intended use, as well as the zwitterionic forms, where possible, of the compounds of the invention.
- prodrug refers to compounds that are rapidly transformed in vivo to yield the parent compound of the above formula, for example by hydrolysis in blood. A thorough discussion is provided in T. Higuchi and V. Stella, Pro-drugs as Novel Delivery Systems, Vol. 14 of the A.C.S. Symposium Series, and in Edward B. Roche, ed., Bioreversible Carriers in Drug Design, American Pharmaceutical Association and Pergamon Press, 1987, both of which are incorporated herein by reference.
- the pharmaceutical compositions of the present invention additionally comprise a pharmaceutically acceptable carrier, which, as used herein, includes any and all solvents, diluents, or other liquid vehicle, dispersion or suspension aids, surface active agents, isotonic agents, thickening or emulsifying agents, preservatives, solid binders, lubricants and the like, as suited to the particular dosage form desired.
- a pharmaceutically acceptable carrier includes any and all solvents, diluents, or other liquid vehicle, dispersion or suspension aids, surface active agents, isotonic agents, thickening or emulsifying agents, preservatives, solid binders, lubricants and the like, as suited to the particular dosage form desired.
- Remington's Pharmaceutical Sciences, Fifteenth Edition, E. W. Martin (Mack Publishing Co., Easton, Pa., 1975) discloses various carriers used in formulating pharmaceutical compositions and known techniques for the preparation thereof.
- any conventional carrier medium is incompatible with the anti-cancer compounds of the invention, such as by producing any undesirable biological effect or otherwise interacting in a deleterious manner with any other component(s) of the pharmaceutical composition, its use is contemplated to be within the scope of this invention.
- materials which can serve as pharmaceutically acceptable carriers include, but are not limited to, sugars such as lactose, glucose and sucrose; starches such as corn starch and potato starch; cellulose and its derivatives such as sodium carboxymethyl cellulose, ethyl cellulose and cellulose acetate; powdered tragacanth; malt; gelatin; talc; Cremophor; Solutol; excipients such as cocoa butter and suppository waxes; oils such as peanut oil, cottonseed oil; safflower oil; sesame oil; olive oil; corn oil and soybean oil; glycols; such a propylene glycol; esters such as ethyl oleate and ethyl laurate; agar; buffering agents such as magnesium hydroxide and aluminum hydroxide; alginic acid; pyrogen-free water; isotonic saline; Ringer's solution; ethyl alcohol, and phosphate buffer solutions, as well as other
- the invention further provides a method for inhibiting tumor growth and/or tumor metastasis.
- the invention provides a compound of the invention for treating cancers by inhibiting tumor growth and/or tumor metastasis for tumors multidrug resistant cancer cells.
- the compound or a pharmaceutically acceptable derivative thereof is administered to a subject in a therapeutically effective amount (including, but not limited to a human or animal) in need of it.
- the therapeutically effective amount is an amount sufficient to kill or inhibit the growth of multidrug resistant cancer cell lines.
- the inventive compounds are useful for the treatment of solid tumors.
- the compounds and pharmaceutical compositions of the present invention may be used in treating or preventing any disease or conditions including proliferative diseases (e.g., cancer), autoimmune diseases (e.g., rheumatoid arthritis), and infections (e.g., bacterial, fungal, etc. ).
- the compounds and pharmaceutical compositions may be administered to animals, preferably mammals ( e.g., domesticated animals, cats, dogs, mice, rats), and more preferably humans. Any method of administration may be used to deliver the compound of pharmaceutical compositions to the animal. In certain embodiments, the compound or pharmaceutical composition is administered parenterally.
- tumor cells are killed, or their growth is inhibited by contacting the tumor cells with an inventive compound or composition, as described herein.
- a therapeutically effective amount of an inventive compound, or a pharmaceutical composition comprising an inventive compound can be administered to a subject in need thereof, in such amounts and for such time as is necessary to achieve the desired result.
- a "therapeutically effective amount" of the inventive compound or pharmaceutical composition is that amount effective for killing or inhibiting the growth of tumor cells.
- the compounds and compositions, according to the method of the present invention may be administered using any amount and any route of administration effective for killing or inhibiting the growth of tumor cells.
- the expression “amount effective to kill or inhibit the growth of tumor cells”, as used herein, refers to a sufficient amount of agent to kill or inhibit the growth of tumor cells.
- the exact amount required will vary from subject to subject, depending on the species, age, and general condition of the subject, the severity of the infection, the particular anticancer agent, its mode of administration, and the like.
- the anticancer compounds of the invention are preferably formulated in dosage unit form for ease of administration and uniformity of dosage.
- dosage unit form refers to a physically discrete unit of anticancer agent appropriate for the patient to be treated. It will be understood, however, that the total daily usage of the compounds and compositions of the present invention will be decided by the attending physician within the scope of sound medical judgment.
- the specific therapeutically effective dose level for any particular patient or organism will depend upon a variety of factors including the disorder being treated and the severity of the disorder; the activity of the specific compound employed; the specific composition employed; the age, body weight, general health, sex and diet of the patient; the time of administration, route of administration, and rate of excretion of the specific compound employed; the duration of the treatment; drugs used in combination or coincidental with the specific compound employed; and like factors well known in the medical arts.
- the pharmaceutical compositions of this invention can be administered to humans and other animals orally, rectally, parenterally, intracisternally, intravaginally, intraperitoneally, topically (as by powders, ointments, or drops), bucally, as an oral or nasal spray, or the like, depending on the severity of the infection being treated.
- the inventive compounds as described herein are formulated by conjugating with water soluble chelators, or water soluble polymers such as polyethylene glycol as poly (1-glutamic acid), or poly (1-aspartic acid), as described in U.S. Patent 5,977,163.
- the compounds of the invention may be administered orally or parenterally at dosage levels sufficient to deliver from about 0.001 mg/kg to about 100 mg/kg, from about 0.01 mg/kg to about 50 mg/kg, preferably from about 0.1 mg/kg to about 40 mg/kg, preferably from about 0.5 mg/kg to about 30 mg/kg, from about 0.01 mg/kg to about 10 mg/kg, from about 0.1 mg/kg to about 10 mg/kg, and more preferably from about 1 mg/kg to about 25 mg/kg, of subject body weight per day, one or more times a day, to obtain the desired therapeutic effect.
- the desired dosage may delivered as delivered every other day, every third day, every week, every two weeks, every three weeks, or every four weeks. In certain embodiments, the desired dosage may be delivered using multiple administrations ( e.g. , two, three, four, five, six, seven, eight, nine, or ten administrations).
- Liquid dosage forms for oral administration include, but are not limited to, pharmaceutically acceptable emulsions, microemulsions, solutions, suspensions, syrups and elixirs.
- the liquid dosage forms may contain inert diluents commonly used in the art such as, for example, water or other solvents, solubilizing agents and emulsifiers such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol, 1,3-butylene glycol, dimethylformamide, oils (in particular, cottonseed, groundnut, corn, germ, olive, castor, and sesame oils), glycerol, tetrahydrofurfuryl alcohol, polyethylene glycols and fatty acid esters of sorbitan, and mixtures thereof.
- the oral compositions can also include adjuvants such as, for example, water or other solvents, solubil
- sterile injectable aqueous or oleaginous suspensions may be formulated according to the known art using suitable dispersing or wetting agents and suspending agents.
- the sterile injectable preparation may also be a sterile injectable solution, suspension or emulsion in a nontoxic parenterally acceptable diluent or solvent, for example, as a solution in 1,3-butanediol.
- acceptable vehicles and solvents that may be employed are water, Ringer's solution, U.S.P. and isotonic sodium chloride solution.
- sterile, fixed oils are conventionally employed as a solvent or suspending medium.
- any bland fixed oil can be employed including synthetic mono- or diglycerides.
- fatty acids such as oleic acid are used in the preparation ofinjectables.
- the injectable formulations can be sterilized, for example, by filtration through a bacterial-retaining filter, or by incorporating sterilizing agents in the form of sterile solid compositions which can be dissolved or dispersed in sterile water or other sterile injectable medium prior to use.
- the rate of drug release can be controlled.
- biodegradable polymers include poly(orthoesters) and poly(anhydrides).
- Depot injectable formulations are also prepared by entrapping the drug in liposomes or microemulsions which are compatible with body tissues.
- compositions for rectal or vaginal administration are preferably suppositories which can be prepared by mixing the compounds of this invention with suitable nonirritating excipients or carriers such as cocoa butter, polyethylene glycol or a suppository wax which are solid at ambient temperature but liquid at body temperature and therefore melt in the rectum or vaginal cavity and release the active compound.
- suitable nonirritating excipients or carriers such as cocoa butter, polyethylene glycol or a suppository wax which are solid at ambient temperature but liquid at body temperature and therefore melt in the rectum or vaginal cavity and release the active compound.
- Solid dosage forms for oral administration include capsules, tablets, pills, powders, and granules.
- the active compound is mixed with at least one inert, pharmaceutically acceptable excipient or carrier such as sodium citrate or dicalcium phosphate and/or a) fillers or extenders such as starches, lactose, sucrose, glucose, mannitol, and silicic acid, b) binders such as, for example, carboxymethylcellulose, alginates, gelatin, polyvinylpyrrolidinone, sucrose, and acacia, c) humectants such as glycerol, d) disintegrating agents such as agar--agar, calcium carbonate, potato or tapioca starch, alginic acid, certain silicates, and sodium carbonate, e) solution retarding agents such as paraffin, f) absorption accelerators such as quaternary ammonium compounds, g) wetting agents such as, for example, cetyl alcohol and g
- Solid compositions of a similar type may also be employed as fillers in soft and hard-filled gelatin capsules using such excipients as lactose or milk sugar as well as high molecular weight polyethylene glycols and the like.
- the solid dosage forms of tablets, dragees, capsules, pills, and granules can be prepared with coatings and shells such as enteric coatings and other coatings well known in the pharmaceutical formulating art. They may optionally contain opacifying agents and can also be of a composition that they release the active ingredient(s) only, or preferentially, in a certain part of the intestinal tract, optionally, in a delayed manner. Examples of embedding compositions which can be used include polymeric substances and waxes. Solid compositions of a similar type may also be employed as fillers in soft and hard-filled gelatin capsules using such excipients as lactose or milk sugar as well as high molecular weight polethylene glycols and the like.
- the active compounds can also be in micro-encapsulated form with one or more excipients as noted above.
- the solid dosage forms of tablets, dragees, capsules, pills, and granules can be prepared with coatings and shells such as enteric coatings, release controlling coatings and other coatings well known in the pharmaceutical formulating art.
- the active compound may be admixed with at least one inert diluent such as sucrose, lactose or starch.
- Such dosage forms may also comprise, as is normal practice, additional substances other than inert diluents, e.g., tableting lubricants and other tableting aids such a magnesium stearate and microcrystalline cellulose.
- the dosage forms may also comprise buffering agents. They may optionally contain opacifying agents and can also be of a composition that they release the active ingredient(s) only, or preferentially, in a certain part of the intestinal tract, optionally, in a delayed manner.
- buffering agents include polymeric substances and waxes.
- Dosage forms for topical or transdermal administration of a compound of this invention include ointments, pastes, creams, lotions, gels, powders, solutions, sprays, inhalants or patches.
- the active component is admixed under sterile conditions with a pharmaceutically acceptable carrier and any needed preservatives or buffers as may be required.
- Ophthalmic formulation, ear drops, and eye drops are also contemplated as being within the scope of this invention.
- the present invention contemplates the use of transdermal patches, which have the added advantage of providing controlled delivery of a compound to the body.
- Such dosage forms can be made by dissolving or dispensing the compound in the proper medium.
- Absorption enhancers can also be used to increase the flux of the compound across the skin. The rate can be controlled by either providing a rate controlling membrane or by dispersing the compound in a polymer matrix or gel.
- the compounds of the present invention are useful as anticancer agents, and thus may be useful in the treatment of cancer, by effecting tumor cell death or inhibiting the growth of tumor cells.
- inventive anticancer agents are useful in the treatment of cancers and other proliferative disorders, including, but not limited to breast cancer, brain cancer, skin cancer, cervical cancer, colon and rectal cancer, leukemia, lung cancer, melanoma, multiple myeloma, non-Hodgkin's lymphoma, ovarian cancer, pancreatic cancer, prostate cancer, and gastric cancer, to name a few.
- the inventive anticancer agents are active against leukemia cells and melanoma cells, and thus are useful for the treatment of leukemias (e.g., myeloid, lymphocytic, promyelocytic, myelocytic and lymphoblastic leukemias, whether acute or chromic forms) and malignant melanomas.
- the inventive anticancer agents are active against solid tumors and also kill and/or inhibit the growth of multidrug resistant cells (MDR cells).
- MDR cells multidrug resistant cells
- the inventive anticancer agents are active against cancers which are resistant to other known anti-neoplastic agents or which have been found not to respond clinically to other known anti-neoplastic agents.
- the inventive anticancer agents are active against cancer which are resistant to other anti-neoplastic microtubule-stabilizing agents (e.g. , paclitaxel).
- the compounds and pharmaceutical compositions of the present invention can be employed in combination therapies, that is, the compounds and pharmaceutical compositions can be administered concurrently with, prior to, or subsequent to, one or more other desired therapeutics or medical procedures.
- the particular combination of therapies (therapeutics or procedures) to employ in a combination regimen will take into account compatibility of the desired therapeutics and/or procedures and the desired therapeutic effect to be achieved.
- the therapies employed may achieve a desired effect for the same disorder (for example, an inventive compound may be administered concurrently with another anticancer agent), or they may achieve different effects (e.g., control of any adverse effects).
- a pharmaceutical pack or kit comprising one or more containers filled with one or more of the ingredients of the pharmaceutical compositions of the invention, can, include an additional approved therapeutic agent for use as a combination therapy.
- an additional approved therapeutic agent for use as a combination therapy can be a notice in the form prescribed by a governmental agency regulating the manufacture, use or sale of pharmaceutical products, which notice reflects approval by the agency of manufacture, use or sale for human administration.
- This Example describes the synthesis of trans -9,10-dehydro-12,13-desoxyepothilone B, 26-trifluoro- trans- 9,10-dehydro-12,13-desoxyepothilone B, 26-trifluoro-12,13-desoxyepothilone B, and 12,13-desoxyepothilone B and biological testing of these compounds.
- Fluorinated derivatives of epothilones were prepared and tested given the enhanced pharmacokinetics and chemotherapeutic indices of other medicinal agents with fluorine substitutions (Ojima, I.; Inoue, T.; Chakravarty, S.; J. Fluorine Chem. 1999, 97 ; Newman, R. A.; Yang, J.; Finlay, M. R. V.; Cabral, F., Vourloumis, D.; Stephens, L. C.; Troncoso, P.; Wu, X.; Logothetis, C. J.; Nicolaou, K. C.; Navone, N. M. Cancer Chemother. Pharmacol. 2001, 48, 319-326).
- Synthetic 19 was evaluated as to its cytotoxic activity. As shown in Table 1-1 below, direct comparison of the previously reported [17]ddEpoB (23) with 27-F 3 -[17]ddEpoB (19) indicated that the new perfluorinated compound possessed a comparably high cytotoxic potency. Table 1-1.
- CCRF-CEM is a human T-cell acute lymphoblastic leukemia cell line.
- the CCRF-CEM/ VBL100 , CCRF-CEM/ VM1 and CCRF- CEM/ Taxol cell lines all overexpress P-glycoprotein and display a multidrug resistance phenotype to MDR associated oncolytics (Ojima, I.; Inoue, T.; Chakravarty, S.; J. Fluorine Chem. 1999 , 97 ; Newman, R. A.; Yang, J.; Finlay, M. R. V.; Cabral, F., Vourloumis, D.; Stephens, L. C.; Troncoso, P.; Wu, X.; Logothetis, C. J.; Nicolaou, K. C.; Navone, N. M. Cancer Chemother. Pharmacol. 2001, 48 , 319-326).
- CCRF-CEM is a human T-cell acute lymphoblastic leukemia cell line.
- CCRF-CEM/ VBL100 , CCRF-CEM/ VM1 and CCRF- CEM/ Taxol cell lines all overexpress P-glycoprotein and display a multidrug resistance phenotype to MDR associated oncolytics (Ptié, G.; Thibonnet, J.; Abarbri, M.; Duchêne, A.; Parrain, J. Synlett 1998, 839; incorporated herein by reference).
- Epo 3 (28) is the first 12,13-desoxyepothilone compound that possessrd substantially improved cytotoxicity relative to that of dEpoB (1). Table 1-3.
- CCRF-CEM In vitro Cytotoxicities (IC 50 ) with Tumor Cell Lines a Compound CCRF-CEM(C) C/VBL 100 C/Taxol ( ⁇ M) ( ⁇ M) ( ⁇ M) Epo 1 (1, dEpoB) 0.0036 0.016 0.0046 Epo 2 (2) 0.0041 0.080 0.018 Epo 3 (28) 0.0009 0.0042 0.0012 Epo 4 (29) 0.0035 0.0210 0.0057 a XTT assay following 72 h inhibition.
- CCRF-CEM is a human T-cell acute lymphoblastic leukemia cell line.
- the CCRF-CEM/VBL 100 cell line is resistant to vinblastine and CCRF-CEM/Taxol to taxol.
- Epothilone 28 demonstrated a markedly improved potency in inhibiting on the growth of implanted tumors, relative to dEpoB (see Figure 10).
- the improved potency and plasma stability allows very substantial reduction of drug dosing (an order of magnitude) in the context of xenografts of 28 (Epo 3).
- compound 28 enjoys major pharmaceutical advantages relative to dEpoB (1). This allowed for the reduction of the dosing levels for 28 relative to 1 in xenograft experiments to be reduced by an order of magnitude.
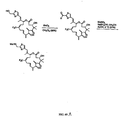
- Epoxidation of 28 with 2,2'-dimethydioxirane (DMDO) proceeded with high chemoselectively at the more substituted C12-C13 olefin to give an 87% yield of a 1:2.6 ratio of the ( E )-9,10-dehydroepothilone B (49) and its diastereomer (50) .
- the stereochemistry of the epoxides was determined by selective diimide reduction of the C9-C10 double bonds. Examination of the spectral properties of these products revealed the minor product (49) to be dEpoB.
- ( E )-9,10-dchydroepothilone B (51) was evaluated against a variety of cell types to determine their antitumor potential. As shown in Table 1-4, ( E )-9,10-dehydroepothiloneB (49) exhibits high cytotoxic activity against a variety of sensitive and resistant tumor cell lines. Direct comparison of 49 and EpoB (51) indicates this new analog possesses nearly 3-fold more potency than EpoB (51) making it one of the most potent epothilone analogs reported to date. Interestingly, ⁇ -epoxide series (50, 52) displayed a much lower activity than EpoB (51) . The graph below shows the findings for in vivo studies of compound 49 . Table 1-4.
- CCRF-CEM In vitro cytotoxicities (IC 50 ) with tumor cell lines a compound CCRF-CEM CCRF-CEM/VBL CCRF-CEM/Taxol 1 (dEpoB) 0.0036 0.016 0.0046 28 0.0009 0.0042 0.0012 51 (EpoB). 0.00062 0.0037 0.0011 49 0.00023 0.00032 0.00042 50 0.0134 0.0959 0.0802 52 0.083 0.4519 0.1507 a XTT assay following 72 h inhibition.
- CCRF-CEM is a human T-cell acute lymphoblastic leukemia cell line.
- the CCRF-CEM/VBL and CCRF-CEM/Taxol cell lines all overexpress P-glycoprotein and display a multidrug resistance phenotype to MDR-associated oncolytics.
- Infrared spectra were obtained on a Perkin-Elmer FT-IR model 1600 spectrometer. Optical rotations were obtained on a JASCO model DIP-370 digital polarimeter at 22 ⁇ 2 °C.
- Analytical thin-layer chromatography was performed on E. Merck silica gel 60 F254 plates. Compounds which were not UV active were visualized by dipping the plates in a ceric ammonium molybdate or para -anisaldehyde solution and heating. Silica gel chromatography was performed using the indicated solvent on Davisil ® (grade 1740, type 60A, 170-400 mesh) silica gel.
- TES triethylsilyl
- TBS Dimethyltertbutylsilyl
- EDCI 1-ethyl-3-(3-dimethylaminopropyl)carbodiimiae
- HF-PY hydrogen fluoride in pyridine
- DMAP 4- N, N -dimethylaminopyridine
- DCM dichloromethane
- DMF N, N- dimethylformamide
- THF tetrahydrofuran.
- Ketone 48 was azeotroped with benzene (5 mL x 2) and then dried under high vacuum for 0.5 h.
- benzene 5 mL x 2
- t -BuOK 2.4 mL of a 1.0 M solution in THF, 2.43 mmol
- ketone 48 (1.10 g, 1.62 mmol) in THF (13 mL) over 10 min, and the resulting mixture was allowed to warm to -20°C over 2 h.
- the reaction was quenched with sat. aqueous NH 4 Cl (15 mL) and extracted with EtOAc (50 mL x 3). The combined organic layers were washed with brine (20 mL), dried over Na 2 SO 4 and concentrated.
- Ketone 48 was azeotroped with benzene (5 mL x 2) and then dried under high vacuum for 0.5 h.
- benzene 5 mL x 2
- t- BuOK 2.2 mL of a 1.0 M solution in THF, 2.20 mmol
- Example 2 Alternative synthetic strategies for synthesizing intermediates of epothilones
- Ester 84 A mixture of alcohol 83 (16.71 g, 0.07386 mol) and pyridine (15.0 mL, 2.5 equiv) was cooled to -10°C and treated with thionyl chloride (11.3 mL, 2.1 equiv) slowly over 11 min. The resulting mixture was warmed to 55 °C and stirred for 12 h. The reaction mixture was cooled to -5 °C, quenched with water (200 mL) and extracted with CH 2 Cl 2 (2 x 200 mL, 2 x 150 mL). Combined organics were washed with saturated NaHCO 3 (2 x 200 mL), and brine (200 mL), dried (MgSO 4 ), and concentrated in vacuo.
- ⁇ -Hydroxyoxazolidinone 88 To a cooled (-78 °C) of TES protected 4-Benzyl-3-hydroxy acetyl-oxazolidin-2-one 7 (16.28 g, 1.92 equiv) in THF (160 mL) was added a solution of LHMDS (42.0 mL, 1.73 equiv) in THF (1.0 M) dropwise over 51 min and the resulting mixture was stirred at -78 °C for 35 min. The reaction mixture was treated with a solution of iodide 86 (6.69 g, 24.2 mmol) in THF (10 mL) and the resulting mixture was allowed to warm to room temperature slowly overnight.
- reaction mixture was concentrated in vacuo to remove HOAc, quenched with saturated NaHCO 3 (400 mL), and extracted with EtOAc (3 x 200 mL). Combined organics were dried (MgSO 4 ), and concentrated in vacuo.
- reaction mixture was cooled to 0 °C, quenched by slow addition of 1N aq. Tartaric acid (100 mL), stirred at room temperature for 25 min, and extracted with EtOAc (3 x 200 mL). Combined organics were dried (MgSO 4 ), and concentrated in vacuo.
- ⁇ -Hydroxyketone 90 To a cooled (0 °C) solution of ⁇ -hydroxyamide 89 (4.87 g, 18.2 mmol) in THF (150 mL) was added a solution of MeMgBr (75 mL, 12 equiv) in ether (3.0 M). After 5 min, the reaction mixture was quenched with saturated NH 4 Cl (250 mL), and extracted with EtOAc (5 x 200 mL). Combined organics were dried (MgSO 4 ), and concentrated in vacuo.
- Example 10 Epothilone Analogs that ablate Xenograft Tumors to a Non-Relapsable State
- fully synthetic 28 has not fully met the stringent standards of highly favorable effective therapeutic index and elimination of tumors to a non- relapsing state.
- dEpoB (30 mg/kg), paclitaxel (20 mg/kg) and F 3 -deH-dEpoB ( 29 , 20 and 30 mg/kg) against human mammary carcinoma MX-1 xenografts in terms of tumor disappearance and relapse were closely studied, and the results are shown in Table 10-1.
- Each dose group consisted of four or more nude mice.
- Body weight refers to total body weight minus tumor weight. All three compounds achieved tumor disappearance. On day 10 after suspension of treatment, 5/10 (dEpoB), 2/7 (Paclitaxel); and 0/4 (compound 29 ) mice relapsed.
- agent 29 completely suppressed the growth of the human mammary carcinoma MX-1 xenografts, shrank the tumors and made them disappear for as long as 64 days is impressive. Moreover, following the cures accomplished by 29 , (20 mg/kg or 30 mg/kg Q2Dx6, i.v.-6hr infusion, Table 1, above) the body weight of the xenografts returned to pretreatment control level within 12-18 days after suspension of treatment. This finding suggests the lack of vital organ damage. At a curative low dosage of 10 mg/kg, Q2DX12 (Figure 59B), the maximal body weight decrease was only 12%, with a body weight gain of 6% during the last three doses. The body weight recovered to the pretreatment control level only three days after cessation of treatment. Table 1 above shows that the animals could survive body weight losses of as much as 27%. The therapeutic safety margin realized herein is remarkably broad for a curative cancer therapeutic agent.
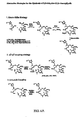
- stereocenters 6, 7, and 8 are derived from the trivially available ketone 30 and aldehyde 31.
- the corresponding aldehyde was condensed with t-butyl acetate to afforded an aldol like product. Since this condensation is not diastereomerically-controlled, a remedial measure was necessary and achieved. Oxidation of this 1:1 mixture of C3 epimers, afforded ketone 69. Following a highly successful Noyori reduction (Noyori et al. J. Am. Chem. Soc. 1987, 109, 5856; incorporated herein by reference) under the conditions shown, alcohol 70 was in hand.
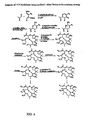
- Gram quantities of structurally novel epothilones have been prepared by total syntheses in the setting of an academic scale laboratory. Reagents and conditions: (a) EDCI, DMAP, CH 2 Cl 2 , 25, 0 °C to rt, 86% from t -butyl ester; (b) Grubb's catalyst, toluene, 110 °C, 20 min, 71 %; (c) (i) KHMDS, 101, THF, - 78 °C to -20 °C, 70%; (ii) HF-pyridine, THF, 98%.
- Infrared spectra were obtained on a Perkin-Elmer FT-IR model 1600 spectrometer. Optical rotations were obtained on a JASCO model DIP-370 digital polarimeter. Analytical thin-layer chromatography was performed on E. Merck silica gel 60 F254 plates. Compounds which were not UV active were visualized by dipping the plates in para -anisaldehyde solution and heating. Preparative thin layer chromatography was performed using the indicated solvent on Whatman ® (LK6F Silica gel 60A) TLC plate.
- Paclitaxel (Taxol ® ) and vinblastine sulfate (VBL) were purchased from Sigma. All these compounds were dissolved in dimethylsulfoxide for the in vitro assays, (except VBL in saline).
- all epothilones and paclitaxel were dissolved in Cremophor/ethanol (1:1) vehicle and then diluted with saline for iv infusion for 6 hrs via tail vein using a custom-designed mini-catheter (T.-C. Chou et al. Proc. Natl. Acad. Sci. USA. 2001, 98, 8113-8118; T.-C. Chou et al. Proc. Natl. Acad. Sci. U. S. A. 1998, 95, 15798-15802).
- the CCRF-CEM human lymphoblastic leukemia cells were obtained from Dr. William Beck of the University of Illinois, Chicago.
- Human mammary carcinoma (MX-1) and human lung carcinoma cells (A549) were obtained from American Type Culture Collection (ATCC, Rockville, MD).
- the paclitaxel-resistant A549/taxol cells (44-fold resistance) were developed with the method as described above (T.-C. Chou et al. Proc. Natl. Acad. Sci. USA. 2001, 98, 8113-8118).
- Athymic nude mice bearing the nu/nu gene were obtained from NCI, Frederick, MD and used for all human tumor xenografts. Male nude mice 6 weeks or older weighing 20-22 g or more were used. Drugs were administered via the tail vein for 6 hours by i.v. infusion using a home-made infusion mini-catheter and containment tube (T.-C. Chou et al. Proc. Natl. Acad. Sci. USA. 2001, 98, 8113-8118).
- a programmable Harvard PHD2000 syringe pump with multitrack was used for i.v. infusion.
- a typical 6 hrs infusion volume for each drug in Cremophor/ethanol (1:1) was 100 ml in 2.0 ml of saline.
- Tumor volume was assessed by measuring length x width x height (or width) by using a caliper.
- the body weight refers to total weight minus the weight of the tumor. All animal studies conducted in accordance with the guidelines for the National Institute of Health Guide for the Care and Use of Animals and the protocol approved by the Memorial Sloan-Kettering Cancer Center's Institutional Animal Care and Use Committee.
- Cytotoxicity Assays In preparation for in vitro cytotoxicity assays, cells were cultured at an initial density 2-5 x 10 4 cells per milliliter. They were maintained in a 5% CO 2 -humidified atmosphere at 37°C in RPMI medium 1640 (GIBCO/BRL) containing penicillin (100 units/mL), streptomycin (100 ⁇ g/mL, GIBCO/BRL), and 5% heat-inactivated FBS.
- RPMI medium 1640 containing penicillin (100 units/mL), streptomycin (100 ⁇ g/mL, GIBCO/BRL), and 5% heat-inactivated FBS.
- cytotoxicity of the drug was determined in 96-well microtiter plates by using the sulforhodamine B method (P. Skehan et al. J. Natl. Cancer. Inst. 1990 , 82 , 1107-1112).
- cytotoxicity was measured, in duplicate, by using the 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-5-carboxanilide)-2H-terazodium hydroxide (XTT), microculture method (D. A. Scudiero et al. Cancer Res. 1988, 48 , 4827-4833).
- the Agilent autosampler set at 37°C, picked up 20 ⁇ l of the sample, loaded it onto the cartridge, washed it with water, then the Prospekt-2 diverted the mobile phase stream through the extraction cartridge onto the analytical column, Reliance Stable Bond C8 4x80 mm with guard column (MacMod, Chadds Ford, PA) and the eluent was monitored at 250 nm.
- the mobile phase consisted of 53 or 65% acetonitrile/0.1% formic acid at 0.4 ml/min, so that the retention time of the compound of interest was about 6 minutes.
- Sample preparation involved the addition of equal volumes of plasma to PBS for a total volume of 300-400 ⁇ l, filtered, and the addition of 0.5-2 ⁇ l of the substrate (20 mM) to achieve about 30-50 mAU at 250 nm in the HPLC analysis.
- 20 ⁇ l (400 ⁇ g) or S9 fraction was mixed with 280 ⁇ l of PBS then proceeded as above. The sampling period was controlled by the autosampler and peak area data were collected to compare the rate of disappearance of the parent compound.
- a typical experiment involves culturing cells (e.g. , CCRF-CEM) at an initial density of 2-5x10 4 cells per ml. They are maintained in a 5% CO 2 -humidified atmosphere at 37°C in RPMI medium 1640 (GIBCO/BRL) containing penicillin (100 units/ml), streptomycin (100 ⁇ g/ml) (GIBCO/BRL), and 5% heat-inactivated fetal bovine serum.
- RPMI medium 1640 RPMI medium 1640
- penicillin 100 units/ml
- streptomycin 100 ⁇ g/ml
- fetal bovine serum fetal bovine serum
- cytotoxicitiy is measured by using the 2,-3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5 carboxanilide)-2 H terazodium hydroxide (XTT)-microculture tetrazonium method in duplicate in 96-well microtiter plates.
- XTT terazodium hydroxide
- the absorbance of each well is measured with a microplate reader (EL-340, Bio-Tek, Burlington, VT). Each run entails six or seven concentrations of the tested drugs. Dose-effect relationship data are analyzed with the median-effect plot.
- CCRF-CEM human T cells acute lymphoblastic leukemic cells, its teniposide-resistant subline (CCRF-CEM/VM 1 ) and vinblastine-resistant subline (CCRF-CEM/VBL 100 ) are obtained from W.T. Beck (University of Illinois, Chicago, II).
- certain of the inventive compounds demonstrated activity in CCRF-CEM cell lines and CCRF-CEM cell lines resistant to Taxol.
- Certain of these compounds exhibit IC 50 s in the range of 0.0015 to about 0.120 for CCRF-CEM cell lines.
- Certain other compounds exhibit IC 50 s in the range of 0.0015 to about 10.5.
- Certain of these compounds also exhibit IC 50 s in the range of 0.011 to about 0.80 for CCRF-CEM/Taxol resistant cell lines and certain other compounds exhibit IC 50 s in the range of about 0.011 to about 13.0 ⁇ M.
- 26F-EpoD exhibits activities in the range of 0.0015 ⁇ M for CCRF-CEM cell lines and in the range of 0.011 ⁇ M for CCRF-CEM/Taxol resistant cell lines (Figure 11).
- Athymic nude mice bearing the nu/nu gene are typically used for tumor xenografts.
- Outbred, Swiss-background mice were obtained from Charles River Laboratories. Male mice 8 weeks or older weighing 22 g and up were used for most experiments.
- the drug was administered via the tail vein for 6 hr.-i.v. infusion. Each individual mouse was confined in a perforated Falcon polypropylene tube restrainer for drug administration. Tumor volume was assessed by measuring length x width x height (or width) using a caliper.
- the programmable Harvard PHD2000 syringe pump (Harvard Apparatus) with multi-track was used for i.v. infusion.
- mice were euthanized when tumors reached ⁇ 10% of their total body weight.
- 9,10-dehydro-EpoB was tested in nude mice bearing human mammary carcinoma MX-1.
- 9,10-dehydro-EpoB was formulated as follows: 9,10-dehydro-EpoB was dissolved in ethanol and Cremophor was added (1:1) at a concentration of 20 mg/ml. This solution was diluted with saline for i.v. infusion. The diluted solution was used for i.v. infusion within one hour. Tumor size and body weight were then measured using dosages of 10 mg/kg, 20 mg/kg, and 30 mg/kg over 15 days.
- Tumor size and body weight were also measured using a dosage regimen of 0.4 mg/kg Q3Dx2, 0.5 mg/kg Q3Dx2, and 0.6 mg/kg Q3Dx5 (see Figures 33, 34, 55 and 56). The every third day dosing regimen was used to reduce toxicity.
- Other therapeutic studies on 9,10-dehydro-Epo B are shown in Figures 70 and 71 (CCRF-CEM/Taxol Q3Dx5) and in Figures 23 and 24 (HCT-116, Q2Dx7).
- 9,10-dehydro-12,13-desoxyepothilone B (iso-490 epothilorie), is three times more efficacious than dEpoB.
- 9,10-dehydro-12,13-desxoyepothilone D has been shown to arrest tumor growth after two to three infusions of 10 mg/kg or 20 mg/kg, each of which was administered every other day.
- Better results in mice were obtained using a dose of 30 mg/kg of 9,10-dehydro-12,13-desoxyepothilone B using two 6 hour infusion iv every other day.
- 9,10-dehydro-12,13-desoxyepothilone B shows decreased toxicity as compared to other epothilones, greater potency in arresting tumor growth, and greater serum stability.
- 9,10-dehydro-Epo B when administered every three days 9-11 times, 6 hour iv infusion, at 0.4-0.6 mg/kg, led to shrinkage and disappearance of the tumor in nude mice with implanted human mammary carcinoma MX-1 xenografts ( Figures 68 and 69). Administration every other day for 8 doses led to tumor growth suppression but no shrinkage of the tumor.
- 9,10-dehydro-Epo B was administered every other day for 9 doses, the implanted tumor continued to shrink moderately from the second to the eighth day, but body weight recovered very slowly from 76% to 82% of the control during the same period. On the tenth day, one-fourth of tumor was gone.
- 9,10-dehydro-EpoB When a dosage of 0.6 mg/kg of 9,10-dehydro-EpoB was administered Q2Wx6, 6 hour infusion, to nude mice with HCT-116 xenografts, four out of four mice died of toxicity within three days after the sixth dosage. 9,10-dehydro-EpoB abolished tumor growth against CCRF-CEM/Taxol using 0.6 mg/kg, Q3Dx5,x2 schedule ( Figures 70 and 71).
- 26-trifluoro-9,10-dehydro-12,13-desoxy-epothilone B (F 3 -deH-dEpoB) as shown in the Figures is curative at 20 mg/kg and 30 mg/kg, Q2Dx6, 6 hour infusions, in a nude mouse model implanted with human mammary carcinoma MX-1 xenografts. The data also suggests that 30 mg/kg Q2Dx6 is approximately the maximal tolerated dose.
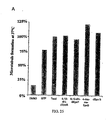
- Figure 59 summarizes the effect of 26-F 3 -9,10-deH-dEpo B (and other epothilones) against MX-1 xenograft, A. at low dose; B. against large tumor; against A549 lung carcinoma xenograft, C; and against Taxol resistant lung carcinoma A549/Taxol xenograft, D.
- Figure 61 lists in vitro potency of C-21 modified epothilones against CCRF-CEM, CCRF-CEM/VBL and CCRF-CEM/Taxol.
- Figure 62 shows therapeutic effect of 26-F 3 -9,10-deH-dEpoB (15mg/kg and 30mg/kg) and Taxol (20mg/kg) Q2Dx8, 6 hour i.v. infusion against human T-cell lymphoblastic leukemia CCRF-CEM xenograft. Similar body weight decreases were observed in all three groups of treatment ( Figure 63).
- 9,10-dehydro-EpoB the most potent epothilone known in vitro, although highly efficacious, showed a narrow therapeutic safety margin in vivo.
Landscapes
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Oxygen Or Sulfur (AREA)
- Plural Heterocyclic Compounds (AREA)
- Saccharide Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicinal Preparation (AREA)
- Low-Molecular Organic Synthesis Reactions Using Catalysts (AREA)
Claims (26)
- Verbindung der Formel:
- Verbindung nach Anspruch 1, wobei R5 Wasserstoff ist; und R6 ist Wasserstoff.
- Verbindung nach Anspruch 1 oder Anspruch 2, wobei RB Methyl ist.
- Verbindung nach Anspruch 1 oder Anspruch 2, wobei RB Wasserstoff ist.
- Verbindung nach Anspruch 1 oder Anspruch 2, wobei RB -CF3 ist
- Verbindung nach einem der Ansprüche 1 bis 5, wobei R1 Methyl ist.
- Verbindung nach einem der Ansprüche 1 bis 6, wobei R2ist, wobei R8 unabhängig Wasserstoff, Halogen, -OR9, -SR9, -N(R9)2, -CY3, - CHY2, -CH2Y ist, wobei Y F, Br, Cl, I, ORB', NHRB', N(RB')2, oder SRB', -(CV2)nOR9, - (CV2)nN(R9)2, -(CV2)nSR9, -(C=O)R9, -O(C=O)R9, -(C=O)OR9, -O(C=O)OR9;-NH(C=O)R9, -NH(C=O)OR9, -(C=O)NHR9, oder ein zyklischer oder azyklischer, linearer oder verzweigter C1-20Aliphat, C1-20Heteroaliphat, C3-14Aryl, C3-14Heteroaryl, C3-14ArylC1-20Alkyl, oder C3-14HeteroarylC1-20Alkyl-Anteil ist, der gegebenenfalls mit einem oder mehreren Auftreten von Halogen, -OR9, -SR9, -N(R9)2, -(CV2)nOR9, - (CV2)nN(R9)2, -(CV2)nSR9, -(C=O)R9, -O(C=O)R9, -(C=O)OR9, -O(C=O)OR9;-NH(C=O)R9, -NH(C=O)OR9, -(C=O)NHR9, oder einem zyklischen oder azyklischen, linearen oder verzweigten, gesättigten oder ungesättigten C1-20Aliphatic, C1-20 Heteroaliphat, C3-14Aryl, C3-14Heteroaryl, C3-14ArylC1-20Alkyl, oder C3-14HeteroarylC1-20 Alkyl-Anteil substituiert ist,wobei jedes Auftreten von R9 unabhängig Wasserstoff; eine Schutzgruppe; ein zyklischer oder azyklischer, linearer oder verzweigter, substituierter oder unsubstituierter C1-20Aliphat, C1-20Heteroaliphat, C3-14Aryl oder C3-14Heteroaryl-Anteil ist;wobei jedes Auftreten von V unabhängig Wasserstoff, Halogen, Hydroxyl, Thio, Amino, C1-20Alkylamino, oder geschütztes Hydroxyl, Thio oder Amino ist; jedes Auftreten von t unabhängig 0, 1 oder 2 ist; und jedes Auftreten von n unabhängig 0-10 ist; und pharmazeutisch akzeptable Derivate davon.
- Verbindung nach einem der Ansprüche 7 bis 9, wobei R8 Methyl ist.
- Verbindung nach einem der Ansprüche 7 bis 9, wobei R8 -CH2OH ist.
- Verbindung nach einem der Ansprüche 1 bis 11, wobei X O ist.
- Verbindung nach einem der Ansprüche 1 bis 11, wobei X NH ist.
- Eine isolierte Verbindung nach einem der Ansprüche 1 bis 13.
- Eine isolierte Verbindung nach Anspruch 15.
- Eine isolierte Verbindung nach Anspruch 17.
- Pharmazeutische Zusammensetzung, welche eine Verbindung nach einem der Ansprüche 1 bis 18 und einen pharmazeutisch akzeptablen Exzipienten aufweist.
- Pharmazeutische Zusammensetzung nach Anspruch 19, welche weiters CREMOPHOR aufweist.
- Pharmazeutische Zusammensetzung nach Anspruch 19 oder Anspruch 20, welche weiters Ethanol aufweist.
- Verbindung nach einem der Ansprüche 1 bis 18 oder eine pharmazeutische Zusammensetzung nach einem der Ansprüche 19 bis 21 für die Verwendung in der Medizin.
- Verbindung oder eine pharmazeutische Zusammensetzung nach Anspruch 22 zur Verwendung bei der Verhinderung und/oder Behandlung von Krebs.
- Verwendung einer Verbindung nach einem der Ansprüche 1 bis 18 bei der Herstellung eines Medikamentes für die Verhinderung und/oder Behandlung von Krebs.
- Verwendung nach Anspruch 24 zum Inhibieren des Tumorwachstums und/oder Tumormetastasen.
- Verwendung nach Anspruch 24 oder 25 zum Behandeln von Krebs, einschließlich gegen Arzneimittel mehrfach-resistenter Krebszellen.
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP06026750A EP1767535B1 (de) | 2002-08-23 | 2003-08-22 | Synthese von Epothilonen, Zwischenprodukte davon, Analoga und ihre Verwendung |
| SI200330738T SI1506203T1 (sl) | 2002-08-23 | 2003-08-22 | Sinteza epotilonov, intermediati za to, analogi in njihove uporabe |
| EP09177388A EP2186811A1 (de) | 2002-08-23 | 2003-08-22 | Synthese von Epothilonen, Zwischenprodukte davon, Analoga und ihre Verwendung |
Applications Claiming Priority (15)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US40582302P | 2002-08-23 | 2002-08-23 | |
| US405823P | 2002-08-23 | ||
| US40858902P | 2002-09-06 | 2002-09-06 | |
| US408589P | 2002-09-06 | ||
| US42312902P | 2002-11-01 | 2002-11-01 | |
| US423129P | 2002-11-01 | ||
| US45615903P | 2003-03-20 | 2003-03-20 | |
| US456159P | 2003-03-20 | ||
| US402004 | 2003-03-28 | ||
| US10/402,004 US6921769B2 (en) | 2002-08-23 | 2003-03-28 | Synthesis of epothilones, intermediates thereto and analogues thereof |
| US10/435,408 US7649006B2 (en) | 2002-08-23 | 2003-05-09 | Synthesis of epothilones, intermediates thereto and analogues thereof |
| US435408 | 2003-05-09 | ||
| US49674103P | 2003-08-21 | 2003-08-21 | |
| US496741P | 2003-08-21 | ||
| PCT/US2003/026367 WO2004018478A2 (en) | 2002-08-23 | 2003-08-22 | Synthesis of epothilones, intermediates thereto, analogues and uses thereof |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP06026750A Division EP1767535B1 (de) | 2002-08-23 | 2003-08-22 | Synthese von Epothilonen, Zwischenprodukte davon, Analoga und ihre Verwendung |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP1506203A2 EP1506203A2 (de) | 2005-02-16 |
| EP1506203B1 true EP1506203B1 (de) | 2007-01-03 |
Family
ID=31950966
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP09177388A Withdrawn EP2186811A1 (de) | 2002-08-23 | 2003-08-22 | Synthese von Epothilonen, Zwischenprodukte davon, Analoga und ihre Verwendung |
| EP06026750A Expired - Lifetime EP1767535B1 (de) | 2002-08-23 | 2003-08-22 | Synthese von Epothilonen, Zwischenprodukte davon, Analoga und ihre Verwendung |
| EP03793304A Expired - Lifetime EP1506203B1 (de) | 2002-08-23 | 2003-08-22 | Synthese von epothilonen, zwischenprodukte dafür, analoga und deren verwendungen |
Family Applications Before (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP09177388A Withdrawn EP2186811A1 (de) | 2002-08-23 | 2003-08-22 | Synthese von Epothilonen, Zwischenprodukte davon, Analoga und ihre Verwendung |
| EP06026750A Expired - Lifetime EP1767535B1 (de) | 2002-08-23 | 2003-08-22 | Synthese von Epothilonen, Zwischenprodukte davon, Analoga und ihre Verwendung |
Country Status (18)
| Country | Link |
|---|---|
| US (1) | US7875638B2 (de) |
| EP (3) | EP2186811A1 (de) |
| JP (2) | JP4791183B2 (de) |
| KR (1) | KR101173510B1 (de) |
| CN (2) | CN102532120A (de) |
| AT (2) | ATE450534T1 (de) |
| AU (1) | AU2003260002B2 (de) |
| CA (1) | CA2496477C (de) |
| DE (2) | DE60310916T2 (de) |
| DK (2) | DK1767535T3 (de) |
| ES (2) | ES2281692T3 (de) |
| IL (1) | IL167046A (de) |
| MX (1) | MXPA05002113A (de) |
| NZ (1) | NZ538522A (de) |
| PH (1) | PH12012501351A1 (de) |
| PT (2) | PT1767535E (de) |
| SI (1) | SI1767535T1 (de) |
| WO (1) | WO2004018478A2 (de) |
Families Citing this family (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7649006B2 (en) | 2002-08-23 | 2010-01-19 | Sloan-Kettering Institute For Cancer Research | Synthesis of epothilones, intermediates thereto and analogues thereof |
| US7384964B2 (en) * | 2002-08-23 | 2008-06-10 | Sloan-Kettering Institute For Cancer Research | Synthesis of epothilones, intermediates thereto, analogues and uses thereof |
| WO2004018478A2 (en) * | 2002-08-23 | 2004-03-04 | Sloan-Kettering Institute For Cancer Research | Synthesis of epothilones, intermediates thereto, analogues and uses thereof |
| CN100553634C (zh) * | 2002-10-11 | 2009-10-28 | 达纳-法伯癌症研究公司 | 用于治疗多发性骨髓瘤的埃坡霉素衍生物 |
| ES2336911T3 (es) * | 2002-11-07 | 2010-04-19 | Kosan Biosciences, Inc. | Trans-9,10-dehidroepotilonas c y d, sus analogos y procedimientos de preparacion. |
| EP1670487A4 (de) * | 2003-10-09 | 2008-05-21 | Kosan Biosciences Inc | Therapeutische formulierungen |
| WO2006047575A2 (en) | 2004-10-25 | 2006-05-04 | Dekk-Tec Inc. | Salts of isophosphoramide mustard and analogs thereof as anti-tumor agents |
| US7238816B2 (en) * | 2005-04-21 | 2007-07-03 | Hoffmann-La Roche Inc. | Preparation of epothilone derivatives |
| WO2008098138A1 (en) | 2007-02-07 | 2008-08-14 | The Government Of The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Synthetic macrocyclic compounds for treating cancer |
| PT2155682E (pt) | 2007-04-06 | 2015-10-15 | Ziopharm Oncology Inc | Sais de mostarda de isofosforamida e análogos da mesma |
| US7955824B2 (en) * | 2007-05-11 | 2011-06-07 | Kosan Biosciences Incorporated | Methods of making epothilones |
| WO2009112077A1 (en) * | 2008-03-13 | 2009-09-17 | F. Hoffmann-La Roche Ag | Process for the preparation of epothilone precursor compounds |
| NZ590995A (en) | 2008-07-31 | 2012-12-21 | Ziopharm Oncology Inc | Oral formulations of salts of isophosphoramide mustard and its analogs and their use in the treatment of a condition characterised by abnormal cell growth or differentiation (such as cancer) |
| US8361992B2 (en) | 2009-02-24 | 2013-01-29 | Dekk-Tec, Inc. | Complexes of 4-hydroperoxy ifosfamide as anti-tumor agents |
| WO2010138686A1 (en) | 2009-05-29 | 2010-12-02 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Mdri-inverse agents |
| CN101747326B (zh) * | 2010-01-12 | 2012-07-25 | 山东大学 | 18元大环内酯类埃博霉素化合物及其应用 |
| US9492405B2 (en) | 2010-03-10 | 2016-11-15 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Use of fenoterol and fenoterol analogues in the treatment of glioblastomas and astrocytomas |
| WO2013009475A1 (en) | 2011-07-11 | 2013-01-17 | The United States Of America, As Represented By The Secretary, Department Of Health & Human Services | Photosensitizing antibody-phuorophore conjugates |
| WO2012033601A1 (en) | 2010-08-20 | 2012-03-15 | The United States Of America, As Represented By The Secretary, Department Of Health & Human Services | Thiosemicarbazones with mdr1 - inverse activity |
| EP2854855B1 (de) | 2012-05-25 | 2016-04-27 | The United States of America, as represented by The Secretary, Department of Health and Human Services | Verfahren zur regulierung von mit der cannabinoidrezeptoraktivität assoziierten erkrankungen |
| MX2016003744A (es) | 2013-10-11 | 2016-08-11 | Us Health | Anticuerpos tem8 y su uso. |
| JP6796058B2 (ja) | 2014-08-08 | 2020-12-02 | ザ ユナイテッド ステイツ オブ アメリカ, アズ リプレゼンテッド バイ ザ セクレタリー, デパートメント オブ ヘルス アンド ヒューマン サービシーズ | インビトロおよびインビボにおける標的の光制御除去 |
| MX2018005236A (es) | 2015-10-27 | 2018-08-15 | Hoffmann La Roche | Macrociclos peptidicos contra acinetobacter baumannii. |
| WO2019099615A1 (en) | 2017-11-17 | 2019-05-23 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Use of copeptin to modulate the immune response and hematopoiesis, and stimulate bone growth |
| US11505573B2 (en) | 2018-03-28 | 2022-11-22 | Hoffmann-La Roche Inc. | Peptide macrocycles against Acinetobacter baumannii |
| EP3774912A1 (de) | 2018-04-10 | 2021-02-17 | The United States of America, as represented by the Secretary, Department of Health and Human Services | Kombination von nah-infrarot-fotoimmunotherapie gegen krebszellen und wirtsimmunaktivierung |
| US11819532B2 (en) | 2018-04-23 | 2023-11-21 | Hoffmann-La Roche Inc. | Peptide macrocycles against Acinetobacter baumannii |
| MX2022000174A (es) | 2019-07-02 | 2022-05-20 | Us Health | Anticuerpos monoclonales que se enlazan a egfrviii y sus usos. |
| WO2021154705A1 (en) | 2020-01-27 | 2021-08-05 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Rab13 and net1 antisense oligonucleotides to treat metastatic cancer |
| AU2022213415A1 (en) | 2021-01-29 | 2023-08-03 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Near infrared photoimmunotherapy (nir-pit) combination therapy to treat cancer |
| KR20240004764A (ko) | 2021-04-30 | 2024-01-11 | 칼리버 임뮤노쎄라퓨틱스, 인크. | 변형된 mhc 발현을 위한 종양용해성 바이러스 |
| WO2024006965A1 (en) | 2022-06-30 | 2024-01-04 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Cd25-specific antibodies and uses thereof |
Family Cites Families (227)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4605661A (en) * | 1984-06-18 | 1986-08-12 | Eli Lilly And Company | Aromastase inhibiting α,α-diarylimidazole-4(5)-propionitriles, α,α-diarylimidazole-4(5)-propionamides, and 4(5)-(2,2-diarylethyl)imidazoles |
| EP0225292A3 (de) | 1985-12-06 | 1988-11-30 | Ciba-Geigy Ag | Einige N-substituierte Buttersäureamidderivate |
| DE3811574A1 (de) * | 1988-03-31 | 1989-10-19 | Schering Ag | N-substituierte imidazole, verfahren zu ihrer herstellung sowie ihre verwendung in arzneimitteln |
| DE4138042C2 (de) | 1991-11-19 | 1993-10-14 | Biotechnolog Forschung Gmbh | Epothilone, deren Herstellungsverfahren sowie diese Verbindungen enthaltende Mittel |
| US20030203976A1 (en) * | 1993-07-19 | 2003-10-30 | William L. Hunter | Anti-angiogenic compositions and methods of use |
| DK0797988T3 (da) * | 1993-07-19 | 2009-05-11 | Univ British Columbia | Anti-angiogene præparater og fremgangsmåder til anvendelse deraf |
| US5994341A (en) | 1993-07-19 | 1999-11-30 | Angiogenesis Technologies, Inc. | Anti-angiogenic Compositions and methods for the treatment of arthritis |
| ES2206607T3 (es) | 1995-11-17 | 2004-05-16 | Gesellschaft Fur Biotechnologische Forschung Mbh (Gbf) | Derivados de epotilones, preparacion y utilizacion. |
| DE19607702A1 (de) | 1996-02-29 | 1997-09-04 | Biotechnolog Forschung Gmbh | Heterozyklische Verbindungen, Herstellungsverfahren und Mittel |
| US6441025B2 (en) * | 1996-03-12 | 2002-08-27 | Pg-Txl Company, L.P. | Water soluble paclitaxel derivatives |
| NZ332234A (en) | 1996-03-12 | 2000-06-23 | Pg Txl Company Lp | Water soluble paclitaxel prodrugs formed by conjugating paclitaxel or docetaxel with a polyglutamic acid polymer and use for treating cancer |
| US20040106985A1 (en) | 1996-04-26 | 2004-06-03 | Jang G. David | Intravascular stent |
| US5917084A (en) | 1996-07-03 | 1999-06-29 | Millennium Pharmaceuticals, Inc. | Antifungal agents |
| DE19645361A1 (de) | 1996-08-30 | 1998-04-30 | Ciba Geigy Ag | Zwischenprodukte innerhalb der Totalsynthese von Epothilon A und B, Teil II |
| DE19645362A1 (de) | 1996-10-28 | 1998-04-30 | Ciba Geigy Ag | Verfahren zur Herstellung von Epothilon A und B und Derivaten |
| DE19636343C1 (de) | 1996-08-30 | 1997-10-23 | Schering Ag | Zwischenprodukte innerhalb der Totalsynthese von Epothilon A und B |
| US5969145A (en) * | 1996-08-30 | 1999-10-19 | Novartis Ag | Process for the production of epothilones and intermediate products within the process |
| DE19638870B4 (de) | 1996-09-23 | 2009-05-14 | Helmholtz-Zentrum für Infektionsforschung GmbH | Tubulysine, Verfahren zu ihrer Gewinnung und sie enthaltende Mittel |
| DE59712968D1 (de) * | 1996-11-18 | 2008-10-30 | Biotechnolog Forschung Gmbh | Epothilone E und F |
| US6867305B2 (en) * | 1996-12-03 | 2005-03-15 | Sloan-Kettering Institute For Cancer Research | Synthesis of epothilones, intermediates thereto and analogues thereof |
| US6204388B1 (en) | 1996-12-03 | 2001-03-20 | Sloan-Kettering Institute For Cancer Research | Synthesis of epothilones, intermediates thereto and analogues thereof |
| AU756699B2 (en) * | 1996-12-03 | 2003-01-23 | Sloan-Kettering Institute For Cancer Research | Synthesis of epothilones, intermediates thereto, analogues and uses thereof |
| US6660758B1 (en) * | 1996-12-13 | 2003-12-09 | The Scripps Research Institute | Epothilone analogs |
| US6380394B1 (en) * | 1996-12-13 | 2002-04-30 | The Scripps Research Institute | Epothilone analogs |
| DE19701758A1 (de) | 1997-01-20 | 1998-07-23 | Wessjohann Ludgar A Dr | Epothilone-Synthesebausteine |
| PT975638E (pt) | 1997-02-25 | 2002-12-31 | Biotechnolog Forschung Mbh Gbf | Epothilons modificados nas cadeias laterais |
| US20020086812A1 (en) | 1997-03-04 | 2002-07-04 | Schweinfest Clifford W. | Methods and compositions for diagnosis and treatment of cancer |
| DE19713970B4 (de) | 1997-04-04 | 2006-08-31 | R&D-Biopharmaceuticals Gmbh | Epothilone-Synthesebausteine II - Prenylderivate |
| EP0975622B1 (de) | 1997-04-18 | 2002-10-09 | Studiengesellschaft Kohle mbH | Selektive olefinmetathese von bi- oder polyfunktionellen substraten in komprimiertem kohlendioxid als reaktionsmedium |
| DE19849464A1 (de) | 1997-04-30 | 2000-04-27 | Schering Ag | Wirkstofffreisetzende Stents, Verfahren zu ihrer Herstellung und ihre Verwendung zur Restenoseprophylaxe |
| US6117659A (en) | 1997-04-30 | 2000-09-12 | Kosan Biosciences, Inc. | Recombinant narbonolide polyketide synthase |
| DE19720312A1 (de) | 1997-05-15 | 1998-11-19 | Hoechst Ag | Zubereitung mit erhöhter in vivo Verträglichkeit |
| DE19821954A1 (de) | 1997-05-15 | 1998-11-19 | Biotechnolog Forschung Gmbh | Verfahren zur Herstellung eines Epothilon-Derivats |
| US20030049841A1 (en) * | 1997-06-16 | 2003-03-13 | Short Jay M. | High throughput or capillary-based screening for a bioactivity or biomolecule |
| DE19726627A1 (de) | 1997-06-17 | 1998-12-24 | Schering Ag | Zwischenprodukte, Verfahren zu ihrer Herstellung und ihre Verwendung zur Herstellung von Epothilon |
| US6605599B1 (en) * | 1997-07-08 | 2003-08-12 | Bristol-Myers Squibb Company | Epothilone derivatives |
| EP1001951B1 (de) | 1997-07-16 | 2002-09-25 | Schering Aktiengesellschaft | Thiazolderivate, verfahren zur herstellung und verwendung |
| DE19735574A1 (de) | 1997-08-09 | 1999-02-11 | Schering Ag | Neue [C1(Carboxa)-C6]-Fragmente, Verfahren zu ihrer Herstellung und ihre Verwendung zur Synthese von Epothilon und Epothilonderivaten |
| DE19735575A1 (de) | 1997-08-09 | 1999-02-11 | Schering Ag | Neue (C13-C15)-Fragmente, Verfahren zur Herstellung und ihre Verwendung zur Synthese von Epothilon und Epothilonderivaten |
| DE19813821A1 (de) | 1998-03-20 | 1999-09-23 | Schering Ag | Verfahren zur Herstellung von C1-C6-Bausteinen zur Totalsynthese von Epothilon und Epothilon-Derivaten |
| DE19749717A1 (de) | 1997-10-31 | 1999-05-06 | Schering Ag | Neue C1-C6-Bausteine zur Totalsynthese neuer Epothilon-Derivate sowie Verfahren zur Herstellung dieser Bausteine |
| CA2299608A1 (en) | 1997-08-09 | 1999-02-18 | Schering Aktiengesellschaft | New epothilone derivatives, method for producing same and their pharmaceutical use |
| DE19751200A1 (de) | 1997-11-13 | 1999-05-20 | Schering Ag | Neue Epothilon-Derivate, Verfahren zu deren Herstellung und ihre pharmazeutische Verwendung |
| DE19735578A1 (de) | 1997-08-09 | 1999-02-11 | Schering Ag | Neue (C1-C6)-Fragmente, Verfahren zur Herstellung und ihre Verwendung zur Synthese von Epothilon und Epothilonderivaten |
| DE19744135C1 (de) | 1997-09-29 | 1999-03-25 | Schering Ag | Beschichtete medizinische Implantate, Verfahren zu ihrer Herstellung und ihre Verwendung zur Restenoseprophylaxe |
| US6365749B1 (en) * | 1997-12-04 | 2002-04-02 | Bristol-Myers Squibb Company | Process for the preparation of ring-opened epothilone intermediates which are useful for the preparation of epothilone analogs |
| US6320045B1 (en) | 1997-12-04 | 2001-11-20 | Bristol-Myers Squibb Company | Process for the reduction of oxiranyl epothilones to olefinic epothilones |
| US6096757A (en) | 1998-12-21 | 2000-08-01 | Schering Corporation | Method for treating proliferative diseases |
| US6280999B1 (en) | 1998-01-23 | 2001-08-28 | Kosan Bioscience | Sorangium polyketide synthases and encoding DNA therefor |
| US6090601A (en) | 1998-01-23 | 2000-07-18 | Kosan Bioscience | Sorangium polyketide synthase |
| PL201329B1 (pl) | 1998-02-05 | 2009-03-31 | Novartis Ag | Preparat farmaceutyczny w postaci koncentratu infuzyjnego |
| US6683100B2 (en) * | 1999-01-19 | 2004-01-27 | Novartis Ag | Organic compounds |
| DE19804673A1 (de) * | 1998-02-06 | 1999-08-12 | Studiengesellschaft Kohle Mbh | Verfahren zur Darstellung makrocyclischer Produkte durch ringschliessende Diin-Metathese |
| US6194181B1 (en) * | 1998-02-19 | 2001-02-27 | Novartis Ag | Fermentative preparation process for and crystal forms of cytostatics |
| JP2002504540A (ja) | 1998-02-25 | 2002-02-12 | スローン−ケッタリング インスティトゥート フォア キャンサー リサーチ | エポチロンの合成、その中間体およびそのアナログ |
| US6302838B1 (en) | 1998-02-25 | 2001-10-16 | Novartis Ag | Cancer treatment with epothilones |
| FR2775187B1 (fr) * | 1998-02-25 | 2003-02-21 | Novartis Ag | Utilisation de l'epothilone b pour la fabrication d'une preparation pharmaceutique antiproliferative et d'une composition comprenant l'epothilone b comme agent antiproliferatif in vivo |
| US6380395B1 (en) * | 1998-04-21 | 2002-04-30 | Bristol-Myers Squibb Company | 12, 13-cyclopropane epothilone derivatives |
| US6498257B1 (en) | 1998-04-21 | 2002-12-24 | Bristol-Myers Squibb Company | 2,3-olefinic epothilone derivatives |
| US6407103B2 (en) | 1998-04-21 | 2002-06-18 | Bristol-Myers Squibb Pharma Company | Indeno [1,2-c] pyrazol-4-ones and their uses |
| JPH11322611A (ja) * | 1998-05-14 | 1999-11-24 | Sankyo Co Ltd | 抗ウイルス剤 |
| GB9810659D0 (en) * | 1998-05-18 | 1998-07-15 | Ciba Geigy Ag | Organic compounds |
| US6121029A (en) | 1998-06-18 | 2000-09-19 | Novartis Ag | Genes for the biosynthesis of epothilones |
| DE19826988A1 (de) | 1998-06-18 | 1999-12-23 | Biotechnolog Forschung Gmbh | Epothilon-Nebenkomponenten |
| DE19923001A1 (de) | 1999-05-13 | 2000-11-16 | Schering Ag | Epothilon-Derivate, Verfahren zu deren Herstellung, Zwischenprodukte und ihre pharmazeutische Verwendung |
| DE19830060A1 (de) | 1998-06-30 | 2000-02-10 | Schering Ag | Epothilon-Derivate, Verfahren zu deren Herstellung, Zwischenprodukte und ihre pharmazeutische Verwendung |
| WO2000001838A2 (en) * | 1998-07-02 | 2000-01-13 | The Board Of Regents Of The Leland Stanford Junior University | Methods for making polyketides |
| US20030199535A1 (en) | 1998-07-30 | 2003-10-23 | Claudio Cavazza | Method for preventing and/or treating peripheral neuropathies induced by the administration of an anticancer agent |
| KR20010085877A (ko) * | 1998-10-02 | 2001-09-07 | 추후제출 | 폴리케타이드 합성 효소와 그 재조합 dna 구성물 |
| DE19846493A1 (de) | 1998-10-09 | 2000-04-13 | Biotechnolog Forschung Gmbh | DNA-Sequenzen für die enzymatische Synthese von Polyketid- oder Heteropolyketidverbindungen |
| DE19848306A1 (de) | 1998-10-14 | 2000-04-20 | Schering Ag | Verfahren zur Herstellung von Epothilon B und Derivaten sowie Zwischenprodukte für dieses Verfahren |
| WO2000026349A2 (en) | 1998-10-29 | 2000-05-11 | Kosan Biosciences, Inc. | Recombinant oleandolide polyketide synthase |
| US6303767B1 (en) | 1998-11-05 | 2001-10-16 | Kosan Biosciences, Inc. | Nucleic acids encoding narbonolide polyketide synthase enzymes from streptomyces narbonensis |
| KR100716272B1 (ko) | 1998-11-20 | 2007-05-09 | 코산 바이오사이언시즈, 인코포레이티드 | 에포틸론 및 에포틸론 유도체의 생산을 위한 재조합 방법 및 물질 |
| US6410301B1 (en) | 1998-11-20 | 2002-06-25 | Kosan Biosciences, Inc. | Myxococcus host cells for the production of epothilones |
| PT1140944E (pt) | 1998-12-22 | 2004-01-30 | Novartis Pharma Gmbh | Derivados de epotilona e sua utilizacao como agentes antitumorais |
| US6780620B1 (en) | 1998-12-23 | 2004-08-24 | Bristol-Myers Squibb Company | Microbial transformation method for the preparation of an epothilone |
| US6419692B1 (en) | 1999-02-03 | 2002-07-16 | Scimed Life Systems, Inc. | Surface protection method for stents and balloon catheters for drug delivery |
| US6596875B2 (en) | 2000-02-07 | 2003-07-22 | James David White | Method for synthesizing epothilones and epothilone analogs |
| AU776511B2 (en) | 1999-02-18 | 2004-09-09 | Baylor University | Compositions and methods for use in targeting vascular destruction |
| DE19908767A1 (de) | 1999-02-18 | 2000-10-19 | Schering Ag | Neue Epothilon-Derivate, Verfahren zu deren Herstellung und ihre pharmazeutische Verwendung |
| DE19908765A1 (de) | 1999-02-18 | 2000-08-24 | Schering Ag | 16-Halogen-Epothilon-Derivate, Verfahren zu deren Herstellung und ihre pharmazeutische Verwendung |
| SK11852001A3 (sk) * | 1999-02-18 | 2002-04-04 | Schering Aktiengesellschaft | Deriváty epotiólonu, spôsoby ich výroby a ich farmaceutické použitie |
| DE19908763A1 (de) | 1999-02-18 | 2000-08-24 | Schering Ag | Neue Epothilon-Derivate, Verfahren zu deren Herstellung und ihre pharmazeutische Verwendung |
| DE19908760A1 (de) | 1999-02-18 | 2000-08-24 | Schering Ag | Neue Epothilon-Derivate, Verfahren zu deren Herstellung und ihre pharmazeutische Verwendung |
| DE19954230A1 (de) | 1999-11-04 | 2001-11-15 | Schering Ag | 16-Halogen-Epothilon-Derivate, Verfahren zu deren Herstellung und ihre pharmazeutische Verwendung |
| US6406722B1 (en) | 1999-02-18 | 2002-06-18 | Robert G. Gallaher | Method of treating viral infections and lesions with taxane compounds |
| DE19930111A1 (de) | 1999-07-01 | 2001-01-04 | Biotechnolog Forschung Gmbh | C-21 Modifizierte Epothilone |
| DE19907588A1 (de) | 1999-02-22 | 2000-08-24 | Biotechnolog Forschung Gmbh | C-21 Modifizierte Epothilone |
| GEP20033067B (en) | 1999-02-22 | 2003-09-25 | Bristol Myers Squibb Co | C-21 Modified Epothilones |
| US20020058286A1 (en) | 1999-02-24 | 2002-05-16 | Danishefsky Samuel J. | Synthesis of epothilones, intermediates thereto and analogues thereof |
| US6291684B1 (en) | 1999-03-29 | 2001-09-18 | Bristol-Myers Squibb Company | Process for the preparation of aziridinyl epothilones from oxiranyl epothilones |
| US6211412B1 (en) | 1999-03-29 | 2001-04-03 | The University Of Kansas | Synthesis of epothilones |
| US6603015B2 (en) | 1999-03-29 | 2003-08-05 | University Of Kansas | Synthesis of epothilones |
| DE60013347T2 (de) | 1999-04-06 | 2005-09-08 | Seiko Epson Corp. | Tintenzusammensetzung enthaltend einen Kupferkomplex-Farbstoff |
| CA2369303A1 (en) | 1999-04-14 | 2000-10-19 | Arthur B. Pardee | Method and composition for the treatment of cancer |
| RU2312860C2 (ru) * | 1999-04-15 | 2007-12-20 | Бристол-Маерс Сквибб Компани | Циклические ингибиторы протеинтирозинкиназ |
| US7125875B2 (en) * | 1999-04-15 | 2006-10-24 | Bristol-Myers Squibb Company | Cyclic protein tyrosine kinase inhibitors |
| DE10015836A1 (de) | 2000-03-27 | 2001-10-11 | Schering Ag | 6-Alkenyl- und 6-Alkinyl-Epothilon-Derivate, Verfahren zu deren Herstellung sowie ihre Verwendung in pharmazeutischen Präparaten |
| DE19954228A1 (de) | 1999-11-04 | 2001-09-13 | Schering Ag | 6-Alkenyl-und 6-Alkinyl-Epothilon-Derivate, Verfahren zu deren Herstellung sowie ihre Verwendung in pharmazeutischen Präparaten |
| JP4707240B2 (ja) * | 1999-05-05 | 2011-06-22 | アベンティス・フアーマ・リミテッド | 細胞接着調節剤としての尿素 |
| KR100621185B1 (ko) | 1999-09-09 | 2006-09-06 | 삼성전자주식회사 | 디디씨 모니터의 동작 기록형 마이크로컴퓨터의 제어방법 |
| US6569867B2 (en) | 1999-10-01 | 2003-05-27 | Kosan Biosciences, Inc. | Polyketide derivatives |
| US20020147197A1 (en) | 1999-10-08 | 2002-10-10 | Newman Michael J. | Methods and compositions for enhancing pharmaceutical treatments |
| US20030054977A1 (en) * | 1999-10-12 | 2003-03-20 | Cell Therapeutics, Inc. | Manufacture of polyglutamate-therapeutic agent conjugates |
| US20020045220A1 (en) * | 1999-10-13 | 2002-04-18 | Chaitan Khosla | Biosynthesis of polyketide synthase substrates |
| CN1086389C (zh) * | 1999-11-12 | 2002-06-19 | 中国科学院上海有机化学研究所 | 异埃坡霉素及合成方法 |
| US20030036177A1 (en) * | 2002-08-13 | 2003-02-20 | Joachim Strohhacker | Single colonies of myxobacteria cells |
| AU2001243372A1 (en) | 2000-03-01 | 2001-09-12 | Sloan-Kettering Institute For Cancer Research | Synthesis of epothilones, intermediates thereto and analogues thereof |
| US6518421B1 (en) * | 2000-03-20 | 2003-02-11 | Bristol-Myers Squibb Company | Process for the preparation of epothilone analogs |
| US6593115B2 (en) * | 2000-03-24 | 2003-07-15 | Bristol-Myers Squibb Co. | Preparation of epothilone intermediates |
| TWI310684B (en) * | 2000-03-27 | 2009-06-11 | Bristol Myers Squibb Co | Synergistic pharmaceutical kits for treating cancer |
| DE10020517A1 (de) * | 2000-04-19 | 2001-10-25 | Schering Ag | Neue Epothilon-Derivate, Verfahren zu deren Herstellung und ihre pharmazeutische Verwendung |
| DE10020899A1 (de) | 2000-04-20 | 2001-10-25 | Schering Ag | 9-Oxa-Epothilon-Derivate, Verfahren zu deren Herstellung sowie ihre Verwendung in pharmazeutischen Präparaten |
| US6589968B2 (en) | 2001-02-13 | 2003-07-08 | Kosan Biosciences, Inc. | Epothilone compounds and methods for making and using the same |
| US6998256B2 (en) * | 2000-04-28 | 2006-02-14 | Kosan Biosciences, Inc. | Methods of obtaining epothilone D using crystallization and /or by the culture of cells in the presence of methyl oleate |
| US6489314B1 (en) | 2001-04-03 | 2002-12-03 | Kosan Biosciences, Inc. | Epothilone derivatives and methods for making and using the same |
| AU2001261121A1 (en) * | 2000-05-02 | 2001-11-12 | Kosan Biosciences, Inc. | Overproduction hosts for biosynthesis of polyketides |
| WO2001092255A2 (en) * | 2000-05-26 | 2001-12-06 | Kosan Biosciences, Inc. | Epothilone derivatives and methods for making and using the same |
| AU7902501A (en) | 2000-07-25 | 2002-02-05 | Kosan Biosciences Inc | Fermentation process for epothilones |
| AU2001283275A1 (en) | 2000-08-09 | 2002-02-18 | Kosan Biosciences, Inc. | Bio-intermediates for use in the chemical synthesis of polyketides |
| UA75365C2 (en) * | 2000-08-16 | 2006-04-17 | Bristol Myers Squibb Co | Epothilone analog polymorph modifications, a method for obtaining thereof (variants), a pharmaceutical composition based thereon |
| CA2418479C (en) * | 2000-08-18 | 2007-12-18 | The Board Of Trustees Of The University Of Illinois | Prodrugs of betulinic acid derivatives for the treatment of cancer |
| US7766956B2 (en) | 2000-09-22 | 2010-08-03 | Boston Scientific Scimed, Inc. | Intravascular stent and assembly |
| EP1414384A4 (de) * | 2000-10-13 | 2005-07-27 | Univ Mississippi | Synthese von epothilonen und verwandteln analoga |
| DE10051136A1 (de) | 2000-10-16 | 2002-04-18 | Ludger A Wessjohann | Epothilone-Synthesebausteine III und Verfahren zur Herstellung von Epothilon B, D und Epothilonderivaten |
| DE50111753D1 (de) * | 2000-10-16 | 2007-02-08 | R & D Biopharmaceuticals | Epothilon-synthesebausteine i: unsymmetrisch substituierte acyloine und acyloinderivate, verfahren zu deren herstellung sowie deren verwendung zur herstellung von epothilonen und epothilonderivaten |
| EP1387677A2 (de) | 2000-11-07 | 2004-02-11 | Dana-Farber Cancer Institute, Inc. | Verfahren zur behandlung von hämatologischen tumoren und krebs mit beta lapachone |
| US7070797B2 (en) * | 2000-11-07 | 2006-07-04 | Dana Farber Cancer Institute, Inc. | Method of treating hematologic tumors and cancers |
| WO2002042432A2 (en) * | 2000-11-22 | 2002-05-30 | Novartis Ag | Epothilone resistant cell lines |
| GB0029895D0 (en) * | 2000-12-07 | 2001-01-24 | Novartis Ag | Organic compounds |
| NZ526871A (en) | 2001-01-25 | 2006-01-27 | Bristol Myers Squibb Co | Pharmaceutical dosage forms of epothilones for oral administration |
| SK288098B6 (sk) | 2001-01-25 | 2013-07-02 | Bristol-Myers Squibb Company | Methods of administering epothilone analogs for the treatment of cancer |
| KR20030071853A (ko) | 2001-01-25 | 2003-09-06 | 브리스톨-마이어스스퀴브컴파니 | 에포틸론 유사체를 함유한 비경구용 제제 |
| US20030004338A1 (en) * | 2001-02-01 | 2003-01-02 | Li Wen Sen | Process for the preparation of epothilone analogs |
| US6893859B2 (en) * | 2001-02-13 | 2005-05-17 | Kosan Biosciences, Inc. | Epothilone derivatives and methods for making and using the same |
| RU2003128311A (ru) * | 2001-02-20 | 2005-03-10 | Бристол-Маерс Сквибб Компани (Us) | Способ лечения резистентных опухолей с применением аналогов эпотилона |
| EE200300397A (et) * | 2001-02-20 | 2003-12-15 | Bristol-Myers Squibb Company | Epotilooni derivaadid refraktaarsete kasvajate raviks |
| IL157312A0 (en) * | 2001-02-27 | 2004-02-19 | Biotechnolog Forschung Gmbh | Processes for the preparation of epothilone derivatives and compounds produced thereby |
| WO2002067941A2 (en) | 2001-02-27 | 2002-09-06 | Novartis Ag | Combination comprising a signal transduction inhibitor and an epothilone derivative |
| WO2002072085A1 (en) * | 2001-03-14 | 2002-09-19 | Bristol-Myers Squibb Company | Combination of epothilone analogs and chemotherapeutic agents for the treatment of proliferative diseases |
| WO2002074042A2 (en) | 2001-03-19 | 2002-09-26 | Novartis Ag | Combinations comprising an antidiarrheal agent and an epothilone or an epothilone derivative |
| US20020169125A1 (en) | 2001-03-21 | 2002-11-14 | Cell Therapeutics, Inc. | Recombinant production of polyanionic polymers and uses thereof |
| US6825166B2 (en) | 2001-03-23 | 2004-11-30 | Tapestry Pharmaceuticals, Inc. | Molecular conjugates for use in treatment of cancer |
| US7081454B2 (en) * | 2001-03-28 | 2006-07-25 | Bristol-Myers Squibb Co. | Tyrosine kinase inhibitors |
| US6900214B2 (en) | 2001-03-29 | 2005-05-31 | Bristol-Myers Squibb Company | Cyano-substituted dihydropyrimidine compounds and their use to treat diseases |
| US20030003048A1 (en) | 2001-04-26 | 2003-01-02 | Chun Li | Diagnostic imaging compositions, their methods of synthesis and use |
| US6906188B2 (en) * | 2001-04-30 | 2005-06-14 | State Of Oregon Acting By And Through The State Board Of Higher Education On Behalf Of Oregon State University | Method for synthesizing epothilones and epothilone analogs |
| US20030023082A1 (en) * | 2001-05-15 | 2003-01-30 | Gary Ashley | Epothilone derivatives and methods for making and using the same |
| MXPA03010909A (es) | 2001-06-01 | 2004-02-17 | Bristol Myers Squibb Co | Derivados de epotilona. |
| JP4779298B2 (ja) | 2001-06-25 | 2011-09-28 | 味の素株式会社 | 抗腫瘍剤 |
| US6933385B2 (en) | 2001-08-03 | 2005-08-23 | Schering Ag | Protected 3,5-dihydroxy-2,2-dimethyl-valeroamides for the synthesis of epothilones and derivatives and process for the production and the use |
| DE10138347A1 (de) | 2001-08-03 | 2003-02-27 | Schering Ag | Geschützte 3,5-Dihydroxy-2,2-dimethyl-valeronitrile für die Synthese von Epothilonen- und Derivaten und Verfahren zur Herstellung |
| US6872715B2 (en) * | 2001-08-06 | 2005-03-29 | Kosan Biosciences, Inc. | Benzoquinone ansamycins |
| PT1420780E (pt) * | 2001-08-23 | 2008-12-29 | Novartis Ag | Análogos ciclopropilados e ciclobutilados da epotilona |
| HUP0402341A3 (en) | 2001-08-31 | 2005-11-28 | Bristol Myers Squibb Company P | Compositions containing thiazol-2-ylamines for the treatment of cancer |
| US20030176368A1 (en) | 2001-09-06 | 2003-09-18 | Danishefsky Samuel J. | Synthesis of epothilones, intermediates thereto and analogues thereof |
| ES2276950T3 (es) | 2001-09-06 | 2007-07-01 | Schering Corporation | Inhibidores de la 17beta-hidroxiesteroide deshidrogenasa de tipo 3 para el tratamiento de enfermedades dependientes de androgenos. |
| WO2003029195A1 (fr) | 2001-09-28 | 2003-04-10 | Sumika Fine Chemicals Co., Ltd. | Intermediaires pour l'elaboration d'un derive de l'epothilone, et leur procede de production |
| TW200300350A (en) | 2001-11-14 | 2003-06-01 | Bristol Myers Squibb Co | C-5 modified indazolylpyrrolotriazines |
| WO2003045324A2 (en) | 2001-11-26 | 2003-06-05 | Kosan Biosciences, Inc. | 14-methyl-epothilones |
| JP2005515201A (ja) * | 2001-12-03 | 2005-05-26 | シェーリング コーポレイション | 癌の処置におけるfptインヒビターおよび少なくとも2つの抗腫瘍性剤の使用 |
| DE10164592A1 (de) | 2001-12-21 | 2003-07-03 | Schering Ag | C1-C6-Epothilon-Fragmente und Verfahren für die Herstellung von C1-C6-Fragmenten von Epothilonen und deren Derivaten |
| US6884608B2 (en) | 2001-12-26 | 2005-04-26 | Bristol-Myers Squibb Company | Compositions and methods for hydroxylating epothilones |
| TW200303202A (en) | 2002-02-15 | 2003-09-01 | Bristol Myers Squibb Co | Method of preparation of 21-amino epothilone derivatives |
| AU2003212457A1 (en) | 2002-03-01 | 2003-09-16 | University Of Notre Dame | Derivatives of epothilone b and d and synthesis thereof |
| EP1340498A1 (de) | 2002-03-01 | 2003-09-03 | Schering Aktiengesellschaft | Verwendung von Epothilonen zur Behandlung von mit proliferativen Prozessen assoziierten Gehirnerkrankungen |
| AU2003216491A1 (en) | 2002-03-07 | 2003-09-22 | The Board Of Trustees Of The University Of Illinois | Microtubule stabilizing compounds |
| US6719540B2 (en) * | 2002-03-12 | 2004-04-13 | Bristol-Myers Squibb Company | C3-cyano epothilone derivatives |
| WO2003077903A1 (en) | 2002-03-12 | 2003-09-25 | Bristol-Myers Squibb Company | C12-cyano epothilone derivatives |
| US6900208B2 (en) * | 2002-03-28 | 2005-05-31 | Bristol Myers Squibb Company | Pyrrolopyridazine compounds and methods of use thereof for the treatment of proliferative disorders |
| TW200400191A (en) * | 2002-05-15 | 2004-01-01 | Bristol Myers Squibb Co | Pharmaceutical compositions and methods of using C-21 modified epothilone derivatives |
| JP2006514681A (ja) * | 2002-05-20 | 2006-05-11 | コーザン バイオサイエンシス インコーポレイテッド | エポチロンdの投与方法 |
| AU2003243561A1 (en) * | 2002-06-14 | 2003-12-31 | Bristol-Myers Squibb Company | Combination of epothilone analogs and chemotherapeutic agents for the treatment of proliferative diseases |
| TW200401638A (en) * | 2002-06-20 | 2004-02-01 | Bristol Myers Squibb Co | Heterocyclic inhibitors of kinases |
| DE10232094A1 (de) | 2002-07-15 | 2004-02-05 | GESELLSCHAFT FüR BIOTECHNOLOGISCHE FORSCHUNG MBH (GBF) | 5-Thiaepothilone und 15-disubstituierte Epothilone |
| TWI329112B (en) * | 2002-07-19 | 2010-08-21 | Bristol Myers Squibb Co | Novel inhibitors of kinases |
| WO2004012735A2 (en) | 2002-07-31 | 2004-02-12 | Schering Ag | New effector conjugates, process for their production and their pharmaceutical use |
| US6951859B2 (en) * | 2002-08-02 | 2005-10-04 | Bristol-Myers Squibb Company | Pyrrolotriazine kinase inhibitors |
| PT1546152E (pt) | 2002-08-02 | 2008-03-12 | Scripps Research Inst | Derivados de epotilona |
| AU2003260723A1 (en) | 2002-08-17 | 2004-03-03 | The Queens Universlty Of Belfast | Use of vinca alkaloyds, taxane, cryptophycine, ephitoline or eleutherobine for treating alzheimer |
| WO2004018635A2 (en) | 2002-08-21 | 2004-03-04 | Kosan Biosciences, Inc. | Myxococcus xanthus bacteriophage mx9 transformation and integration system |
| US20040126379A1 (en) | 2002-08-21 | 2004-07-01 | Boehringer Ingelheim International Gmbh | Compositions and methods for treating cancer using cytotoxic CD44 antibody immunoconjugates and chemotherapeutic agents |
| EP1391213A1 (de) | 2002-08-21 | 2004-02-25 | Boehringer Ingelheim International GmbH | Zusammensetzungen und Verfahren zur Behandlung von Krebs mit einem Maytansinoid-CD44-Antikörper Immunokonjugat und chemotherapeutische Mittel |
| US7649006B2 (en) * | 2002-08-23 | 2010-01-19 | Sloan-Kettering Institute For Cancer Research | Synthesis of epothilones, intermediates thereto and analogues thereof |
| US6921769B2 (en) * | 2002-08-23 | 2005-07-26 | Sloan-Kettering Institute For Cancer Research | Synthesis of epothilones, intermediates thereto and analogues thereof |
| WO2004018478A2 (en) | 2002-08-23 | 2004-03-04 | Sloan-Kettering Institute For Cancer Research | Synthesis of epothilones, intermediates thereto, analogues and uses thereof |
| WO2004017943A2 (en) | 2002-08-23 | 2004-03-04 | Medigene Oncology Gmbh | Non-vesicular cationic lipid formulations |
| KR20050057139A (ko) | 2002-09-04 | 2005-06-16 | 쉐링 코포레이션 | 사이클린 의존성 키나제 억제제로서의 피라졸로피리미딘 |
| US20040043089A1 (en) | 2002-09-04 | 2004-03-04 | Rabie A-Bakr M | Buguzhi agent and composition and methods of preparing and administering the same |
| KR20050033659A (ko) | 2002-09-04 | 2005-04-12 | 쉐링 코포레이션 | 사이클린 의존성 키나제 억제제로서의 피라졸로[1,5-a]피리미딘 화합물 |
| EP1545533A1 (de) | 2002-09-04 | 2005-06-29 | Schering Corporation | Pyrazolopyrimidine als hemmstoffe cyclin-abhängiger kinasen |
| MXPA05002573A (es) | 2002-09-04 | 2005-09-08 | Schering Corp | Pirazolopirimidinas como inhibidores de cinasa dependientes de ciclina. |
| GB0221312D0 (en) | 2002-09-13 | 2002-10-23 | Novartis Ag | Organic compounds |
| US20040115319A1 (en) | 2002-09-16 | 2004-06-17 | Agcert International, Llc | Food-borne pathogen and spoilage detection device and method |
| JP4845379B2 (ja) | 2002-09-19 | 2011-12-28 | シェーリング コーポレイション | サイクリン依存性キナーゼインヒビターとしてのイミダゾピリジン |
| ATE382048T1 (de) | 2002-09-19 | 2008-01-15 | Schering Corp | Pyrazolopyrimdine als hemmstoffe cyclin abhängiger kinasen |
| DE10244847A1 (de) | 2002-09-20 | 2004-04-01 | Ulrich Prof. Dr. Speck | Medizinische Vorrichtung zur Arzneimittelabgabe |
| CN1694706A (zh) | 2002-09-23 | 2005-11-09 | 先灵公司 | 用作依赖细胞周期蛋白的激酶抑制剂的新的咪唑并吡嗪 |
| WO2004026254A2 (en) | 2002-09-23 | 2004-04-01 | Bristol-Myers Squibb Company | Methods for the preparation, isolation and purification of epothilone b, and x-ray crystal structures of epothilone b |
| EP1543008B1 (de) | 2002-09-23 | 2007-11-07 | Schering Corporation | Imidazopyrazine als cdk-inhibitoren |
| US20040058899A1 (en) * | 2002-09-25 | 2004-03-25 | Klimko Peter G. | Use of epothilones and analogs in conjunction with ophthalmic surgery |
| US7232826B2 (en) | 2002-09-30 | 2007-06-19 | Bristol-Myers Squibb Company | Tyrosine kinase inhibitors |
| TW200501960A (en) | 2002-10-02 | 2005-01-16 | Bristol Myers Squibb Co | Synergistic kits and compositions for treating cancer |
| US7091193B2 (en) | 2002-10-09 | 2006-08-15 | Kosan Biosciences Incorporated | Therapeutic formulations |
| MXPA05003706A (es) | 2002-10-09 | 2005-07-01 | Kosan Biosciences Inc | Epo d+5-fu/gemcitabina. |
| CN100553634C (zh) | 2002-10-11 | 2009-10-28 | 达纳-法伯癌症研究公司 | 用于治疗多发性骨髓瘤的埃坡霉素衍生物 |
| AU2003267751A1 (en) | 2002-10-15 | 2004-05-04 | Board Of Supervisors Of Louisiana State Universityand Agricultural And Mechanical College | Use of epothilone derivatives for the treatment of hyperparathyroidism |
| AU2003287526A1 (en) | 2002-11-06 | 2004-06-03 | Protein-stabilized liposomal formulations of pharmaceutical agents | |
| ES2336911T3 (es) | 2002-11-07 | 2010-04-19 | Kosan Biosciences, Inc. | Trans-9,10-dehidroepotilonas c y d, sus analogos y procedimientos de preparacion. |
| EP1585751A4 (de) | 2002-11-14 | 2008-08-13 | Celmed Oncology Usa Inc | Peptiddeformylase-aktivierte prodrugs |
| ES2330517T3 (es) | 2002-11-28 | 2009-12-11 | Wolfgang Richter | Derivados de tia-epotilona para el tratamiento del cancer. |
| US8088605B2 (en) | 2002-12-04 | 2012-01-03 | Technologies Biolactics Inc. | Exopolysaccharides delivery system for active molecules |
| DE10256982A1 (de) | 2002-12-05 | 2004-06-24 | Schering Ag | Neue Effektor-Konjugate, Verfahren zu Ihrer Herstellung und Ihre Pharmazeutische Verwendung |
| JP2006510626A (ja) | 2002-12-05 | 2006-03-30 | シエーリング アクチエンゲゼルシャフト | 増殖疾患の処理における部位特異的供給のためのエポチロン類似体 |
| EP1575454A4 (de) | 2002-12-09 | 2006-11-29 | Medtronic Vascular Inc | Modularer stent mit polymerbrücken an modularen kontaktstellen |
| KR20050095826A (ko) | 2002-12-09 | 2005-10-04 | 아메리칸 바이오사이언스, 인크. | 약리학적 물질의 조성물 및 그 전달방법 |
| BR0317150A (pt) | 2002-12-09 | 2005-11-01 | Novartis Ag | Estabilizadores de microtúbulos em stents para o tratamento de estenose |
| AU2003303058A1 (en) | 2002-12-12 | 2004-07-09 | Conforma Therapeutics Corporation | Cytotoxins and diagnostic imaging agents comprising hsp90 ligands |
| WO2004054622A1 (en) | 2002-12-13 | 2004-07-01 | Immunomedics, Inc. | Immunoconjugates with an intracellularly-cleavable linkage |
| TW200420565A (en) | 2002-12-13 | 2004-10-16 | Bristol Myers Squibb Co | C-6 modified indazolylpyrrolotriazines |
| GB0230024D0 (en) | 2002-12-23 | 2003-01-29 | Novartis Ag | Organic compounds |
| US7189716B2 (en) | 2003-01-03 | 2007-03-13 | Bristol-Myers Squibb Company | Tyrosine kinase inhibitors |
| AU2003219947A1 (en) | 2003-02-26 | 2004-09-28 | Bristol-Myers Squibb Company | Compositions and methods for hydroxylating epothilones |
| GB0305928D0 (en) | 2003-03-14 | 2003-04-23 | Novartis Ag | Organic compounds |
| GB0306907D0 (en) | 2003-03-26 | 2003-04-30 | Angiogene Pharm Ltd | Boireductively-activated prodrugs |
| JP2006523235A (ja) | 2003-03-28 | 2006-10-12 | コーザン バイオサイエンシス インコーポレイテッド | 再狭窄を予防するための装置、方法及び組成物 |
-
2003
- 2003-08-22 WO PCT/US2003/026367 patent/WO2004018478A2/en active IP Right Grant
- 2003-08-22 ES ES03793304T patent/ES2281692T3/es not_active Expired - Lifetime
- 2003-08-22 CN CN2011103358062A patent/CN102532120A/zh active Pending
- 2003-08-22 EP EP09177388A patent/EP2186811A1/de not_active Withdrawn
- 2003-08-22 SI SI200331750T patent/SI1767535T1/sl unknown
- 2003-08-22 NZ NZ538522A patent/NZ538522A/en not_active IP Right Cessation
- 2003-08-22 DE DE60310916T patent/DE60310916T2/de not_active Expired - Lifetime
- 2003-08-22 DK DK06026750.7T patent/DK1767535T3/da active
- 2003-08-22 PT PT06026750T patent/PT1767535E/pt unknown
- 2003-08-22 AT AT06026750T patent/ATE450534T1/de active
- 2003-08-22 AT AT03793304T patent/ATE350383T1/de active
- 2003-08-22 DE DE60330407T patent/DE60330407D1/de not_active Expired - Lifetime
- 2003-08-22 ES ES06026750T patent/ES2336937T3/es not_active Expired - Lifetime
- 2003-08-22 KR KR1020057003043A patent/KR101173510B1/ko not_active IP Right Cessation
- 2003-08-22 MX MXPA05002113A patent/MXPA05002113A/es active IP Right Grant
- 2003-08-22 AU AU2003260002A patent/AU2003260002B2/en not_active Ceased
- 2003-08-22 EP EP06026750A patent/EP1767535B1/de not_active Expired - Lifetime
- 2003-08-22 PT PT03793304T patent/PT1506203E/pt unknown
- 2003-08-22 CN CNA038225611A patent/CN1759115A/zh active Pending
- 2003-08-22 DK DK03793304T patent/DK1506203T3/da active
- 2003-08-22 JP JP2005501774A patent/JP4791183B2/ja not_active Expired - Fee Related
- 2003-08-22 CA CA2496477A patent/CA2496477C/en not_active Expired - Fee Related
- 2003-08-22 EP EP03793304A patent/EP1506203B1/de not_active Expired - Lifetime
-
2005
- 2005-02-22 IL IL167046A patent/IL167046A/en not_active IP Right Cessation
-
2008
- 2008-06-09 US US12/135,823 patent/US7875638B2/en not_active Expired - Fee Related
-
2011
- 2011-04-20 JP JP2011094302A patent/JP2011195589A/ja active Pending
-
2012
- 2012-06-29 PH PH12012501351A patent/PH12012501351A1/en unknown
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1506203B1 (de) | Synthese von epothilonen, zwischenprodukte dafür, analoga und deren verwendungen | |
| US8513429B2 (en) | Synthesis of epothilones, intermediates thereto and analogues thereof | |
| US20050192440A1 (en) | Method for synthesizing epothilones and epothilone analogs | |
| EP1722791A2 (de) | Synthese von epothilonen, zwischenprodukte dafür, analoga und deren verwendungen | |
| US20030176368A1 (en) | Synthesis of epothilones, intermediates thereto and analogues thereof | |
| US6921769B2 (en) | Synthesis of epothilones, intermediates thereto and analogues thereof | |
| RU2462463C2 (ru) | Синтез эпотилонов, их промежуточных продуктов, аналогов и их применения | |
| US20070203346A1 (en) | Method for synthesizing epothilones and epothilone analogs | |
| ZA200502337B (en) | Synthesis of epothilones, intermediates thereto, analogues and uses thereof | |
| AU2011202835A1 (en) | Synthesis of epothilones, intermediates thereto, analogues and uses thereof | |
| MXPA06009792A (en) | Synthesis of epothilones, intermediates thereto, analogues and uses thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20040719 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL LT LV MK |
|
| 17Q | First examination report despatched |
Effective date: 20050127 |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: CHO, YOUNG, SHIN Inventor name: CHOU, TING-CHAO Inventor name: YOSHIMURA, FUMIHIKO Inventor name: DONG, HUAJIN Inventor name: RIVKIN, ALEXEY Inventor name: DANISHEFSKY, SAMUEL, J. Inventor name: GABARDA ORTEGA, ANA ESTHER |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL LT LV MK |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REF | Corresponds to: |
Ref document number: 60310916 Country of ref document: DE Date of ref document: 20070215 Kind code of ref document: P |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20070404 |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: TRGR |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: NV Representative=s name: KIRKER & CIE S.A. Ref country code: PT Ref legal event code: SC4A Free format text: AVAILABILITY OF NATIONAL TRANSLATION Effective date: 20070323 |
|
| REG | Reference to a national code |
Ref country code: HU Ref legal event code: AG4A Ref document number: E001245 Country of ref document: HU |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: T3 |
|
| REG | Reference to a national code |
Ref country code: GR Ref legal event code: EP Ref document number: 20070401069 Country of ref document: GR |
|
| ET | Fr: translation filed | ||
| RIN2 | Information on inventor provided after grant (corrected) |
Inventor name: DANISHEFSKY, SAMUEL, J. Inventor name: CHO, YOUNG, SHIN Inventor name: CHOU, TING-CHAO Inventor name: RIVKIN, ALEXEY Inventor name: DONG, HUAJIN Inventor name: GABARDA ORTEGA, ANA ESTHER Inventor name: YOSHIMURA, FUMIHIKO |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2281692 Country of ref document: ES Kind code of ref document: T3 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20071005 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20070103 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20070103 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: MC Payment date: 20150626 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: LU Payment date: 20150813 Year of fee payment: 13 Ref country code: NL Payment date: 20150809 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SK Payment date: 20150810 Year of fee payment: 13 Ref country code: CH Payment date: 20150811 Year of fee payment: 13 Ref country code: DK Payment date: 20150811 Year of fee payment: 13 Ref country code: GB Payment date: 20150819 Year of fee payment: 13 Ref country code: EE Payment date: 20150729 Year of fee payment: 13 Ref country code: FI Payment date: 20150810 Year of fee payment: 13 Ref country code: DE Payment date: 20150818 Year of fee payment: 13 Ref country code: IE Payment date: 20150810 Year of fee payment: 13 Ref country code: ES Payment date: 20150713 Year of fee payment: 13 Ref country code: PT Payment date: 20150820 Year of fee payment: 13 Ref country code: CZ Payment date: 20150806 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20150629 Year of fee payment: 13 Ref country code: GR Payment date: 20150722 Year of fee payment: 13 Ref country code: SE Payment date: 20150811 Year of fee payment: 13 Ref country code: AT Payment date: 20150727 Year of fee payment: 13 Ref country code: SI Payment date: 20150708 Year of fee payment: 13 Ref country code: HU Payment date: 20150709 Year of fee payment: 13 Ref country code: TR Payment date: 20150730 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20150827 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20150811 Year of fee payment: 13 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160831 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 60310916 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MM9D Effective date: 20160822 |
|
| REG | Reference to a national code |
Ref country code: EE Ref legal event code: MM4A Ref document number: E001242 Country of ref document: EE Effective date: 20160831 |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: EBP Effective date: 20160831 |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: EUG |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160831 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MM Effective date: 20160901 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MM01 Ref document number: 350383 Country of ref document: AT Kind code of ref document: T Effective date: 20160822 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20160822 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160831 Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160823 Ref country code: FI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160822 Ref country code: GR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170309 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160831 Ref country code: EE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160831 Ref country code: HU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160823 |
|
| REG | Reference to a national code |
Ref country code: SK Ref legal event code: MM4A Ref document number: E 1788 Country of ref document: SK Effective date: 20160822 |
|
| REG | Reference to a national code |
Ref country code: GR Ref legal event code: ML Ref document number: 20070401069 Country of ref document: GR Effective date: 20170309 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20170428 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170222 Ref country code: SK Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160822 Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160822 Ref country code: CZ Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160822 Ref country code: SI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160823 |
|
| REG | Reference to a national code |
Ref country code: SI Ref legal event code: KO00 Effective date: 20170418 Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160901 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160831 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160822 Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170301 Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160831 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160822 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160822 Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160822 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160823 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20181128 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160822 |