WO2015093411A1 - リチウムイオン電池用電極、リチウムイオン電池及びリチウムイオン電池用電極の製造方法 - Google Patents
リチウムイオン電池用電極、リチウムイオン電池及びリチウムイオン電池用電極の製造方法 Download PDFInfo
- Publication number
- WO2015093411A1 WO2015093411A1 PCT/JP2014/083027 JP2014083027W WO2015093411A1 WO 2015093411 A1 WO2015093411 A1 WO 2015093411A1 JP 2014083027 W JP2014083027 W JP 2014083027W WO 2015093411 A1 WO2015093411 A1 WO 2015093411A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- electrode
- lithium ion
- active material
- ion battery
- conductive
- Prior art date
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/13—Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof
- H01M4/131—Electrodes based on mixed oxides or hydroxides, or on mixtures of oxides or hydroxides, e.g. LiCoOx
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
- H01M10/0525—Rocking-chair batteries, i.e. batteries with lithium insertion or intercalation in both electrodes; Lithium-ion batteries
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/04—Processes of manufacture in general
- H01M4/0402—Methods of deposition of the material
- H01M4/0404—Methods of deposition of the material by coating on electrode collectors
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/04—Processes of manufacture in general
- H01M4/0402—Methods of deposition of the material
- H01M4/0416—Methods of deposition of the material involving impregnation with a solution, dispersion, paste or dry powder
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/04—Processes of manufacture in general
- H01M4/043—Processes of manufacture in general involving compressing or compaction
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/04—Processes of manufacture in general
- H01M4/0471—Processes of manufacture in general involving thermal treatment, e.g. firing, sintering, backing particulate active material, thermal decomposition, pyrolysis
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/13—Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/13—Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof
- H01M4/133—Electrodes based on carbonaceous material, e.g. graphite-intercalation compounds or CFx
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/13—Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof
- H01M4/139—Processes of manufacture
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/13—Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof
- H01M4/139—Processes of manufacture
- H01M4/1391—Processes of manufacture of electrodes based on mixed oxides or hydroxides, or on mixtures of oxides or hydroxides, e.g. LiCoOx
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/13—Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof
- H01M4/139—Processes of manufacture
- H01M4/1393—Processes of manufacture of electrodes based on carbonaceous material, e.g. graphite-intercalation compounds or CFx
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/483—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides for non-aqueous cells
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/485—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of mixed oxides or hydroxides for inserting or intercalating light metals, e.g. LiTi2O4 or LiTi2OxFy
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/58—Selection of substances as active materials, active masses, active liquids of inorganic compounds other than oxides or hydroxides, e.g. sulfides, selenides, tellurides, halogenides or LiCoFy; of polyanionic structures, e.g. phosphates, silicates or borates
- H01M4/583—Carbonaceous material, e.g. graphite-intercalation compounds or CFx
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/62—Selection of inactive substances as ingredients for active masses, e.g. binders, fillers
- H01M4/624—Electric conductive fillers
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/62—Selection of inactive substances as ingredients for active masses, e.g. binders, fillers
- H01M4/624—Electric conductive fillers
- H01M4/625—Carbon or graphite
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/62—Selection of inactive substances as ingredients for active masses, e.g. binders, fillers
- H01M4/624—Electric conductive fillers
- H01M4/626—Metals
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/64—Carriers or collectors
- H01M4/66—Selection of materials
- H01M4/661—Metal or alloys, e.g. alloy coatings
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/64—Carriers or collectors
- H01M4/66—Selection of materials
- H01M4/663—Selection of materials containing carbon or carbonaceous materials as conductive part, e.g. graphite, carbon fibres
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/64—Carriers or collectors
- H01M4/66—Selection of materials
- H01M4/665—Composites
- H01M4/667—Composites in the form of layers, e.g. coatings
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/64—Carriers or collectors
- H01M4/70—Carriers or collectors characterised by shape or form
- H01M4/80—Porous plates, e.g. sintered carriers
- H01M4/806—Nonwoven fibrous fabric containing only fibres
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/64—Carriers or collectors
- H01M4/70—Carriers or collectors characterised by shape or form
- H01M4/80—Porous plates, e.g. sintered carriers
- H01M4/808—Foamed, spongy materials
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M50/00—Constructional details or processes of manufacture of the non-active parts of electrochemical cells other than fuel cells, e.g. hybrid cells
- H01M50/40—Separators; Membranes; Diaphragms; Spacing elements inside cells
- H01M50/46—Separators, membranes or diaphragms characterised by their combination with electrodes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M2004/021—Physical characteristics, e.g. porosity, surface area
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/362—Composites
- H01M4/366—Composites as layered products
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P70/00—Climate change mitigation technologies in the production process for final industrial or consumer products
- Y02P70/50—Manufacturing or production processes characterised by the final manufactured product
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02T—CLIMATE CHANGE MITIGATION TECHNOLOGIES RELATED TO TRANSPORTATION
- Y02T10/00—Road transport of goods or passengers
- Y02T10/60—Other road transportation technologies with climate change mitigation effect
- Y02T10/70—Energy storage systems for electromobility, e.g. batteries
Definitions
- the present invention relates to a lithium ion battery electrode, a lithium ion battery, and a method for manufacturing a lithium ion battery electrode.
- a lithium ion secondary battery is formed by applying a positive electrode or a negative electrode active material or the like to a positive electrode or negative electrode current collector using a binder.
- a positive electrode active material or the like is applied to one surface of the current collector using a binder and a positive electrode layer is applied to the opposite surface, and a negative electrode active material or the like is applied to the opposite surface using a binder.
- a bipolar electrode having a negative electrode layer is formed (see, for example, Patent Document 1).
- Patent Document 1 a paste for electrode formation is applied with a thickness of about 25 ⁇ m.
- Patent Document 2 discloses a means for increasing the energy density of a battery by increasing the film thickness of an electrode, thereby reducing the relative proportion of the current collector and the separator.
- the thickness of the electrode can be increased, the relative ratio of the current collector and the separator can be reduced, which is considered effective for increasing the capacity of the battery. .
- the proportion of the active material that is far from the current collector increases.
- the electron conductivity of the active material itself is not high, it is considered that the electrons are not smoothly moved from the active material that is far from the current collector to the current collector. Therefore, if the thickness of the electrode is simply increased, the proportion of the active material that is not effectively used due to poor electronic conductivity will increase even if the amount of the active material increases. As a result, there arises a problem that the battery cannot be increased in capacity despite the increase in the thickness of the electrode.
- the inventors of the present invention formed a conductive path that electrically connects the thickness direction of the electrodes, and electrons generated from the active material passed through the conductive path to collect the current collector. As a result, the inventors have found that an electrode having excellent electron conductivity can be obtained even when the thickness of the electrode is increased.
- the present invention is an electrode for a lithium ion battery comprising a first main surface disposed on the separator side of a lithium ion battery and a second main surface disposed on the current collector side, wherein the thickness of the electrode
- the conductive member (A) and a plurality of active material particles (B) made of an electron conductive material are provided between the first main surface and the second main surface, and the conductive member (B) has a thickness of 150 to 5000 ⁇ m.
- At least a part of A) forms a conductive path that electrically connects the first main surface to the second main surface, and the conductive path includes the active material particles ( B) an electrode for a lithium ion battery in contact with; a lithium ion battery using the electrode for a lithium ion battery of the present invention as a negative electrode and / or a positive electrode; Including member (A), A step (P1) of preparing a structure (Z) having a void in the first main surface and a second main surface, and slurry (X) containing the active material particles (B).
- Step (P2) of applying to the first main surface or the second main surface of (Z) and filling the voids in the structure (Z) with the active material particles (B) by applying pressure or reduced pressure A process for producing an electrode for a lithium ion battery comprising the step (P3) of: a method for producing an electrode for a lithium ion battery of the present invention, wherein the conductive member (A) and the active material particles (B) ) Containing slurry (Y) on the film (E) and pressurizing or depressurizing the active material particles (B) and the conductive member (A) into the film (E).
- a process for producing an electrode for a lithium ion battery comprising the step (Q2) of fixing on the surface;
- a method for producing an ion battery battery wherein a slurry (Y) containing the conductive member (A) and the active material particles (B) is applied onto a current collector to form a slurry layer on the current collector Step (T1), placing a separator on the slurry layer, and absorbing the liquid from the upper surface side of the separator, and bringing the active material particles (B) and the conductive member (A) into the current collector
- a method of manufacturing an electrode for a lithium ion battery comprising a step (T2) of fixing between the separators; and a first main surface disposed on the separator side of the lithium ion battery, and disposed on the current collector side
- a conductive member (A) made of an electron conductive material, a large number of active material particles (B), and a resin (between the first main surface and the second main surface).
- a method for producing an electrode for a lithium ion battery comprising:
- the electrode for a lithium ion battery of the present invention includes a conductive member made of an electron conductive material between the first main surface and the second main surface of the electrode, and the conductive member extends from the first main surface to the second main surface. Therefore, the electrons generated from the active material can flow to the current collector through the conductive path. Therefore, even when the thickness of the electrode is increased to 150 to 5000 ⁇ m and the amount of the active material is relatively increased, electrons generated from the active material far from the current collector smoothly reach the current collector. Reach. Therefore, the electrode for a lithium ion battery is excellent in electronic conductivity and suitable for increasing the capacity of the lithium ion battery.
- FIG. 1 is a cross-sectional view schematically showing an example of the structure of a lithium ion battery including the lithium ion battery electrode of the present invention as a positive electrode and a negative electrode.
- FIG. 2 is a cross-sectional view schematically showing only the positive electrode of the lithium ion battery shown in FIG.
- FIG. 3 is a cross-sectional view schematically showing another example of the lithium ion battery electrode of the present invention.
- FIG. 4 is a cross-sectional view schematically showing another example of the lithium ion battery electrode of the present invention.
- FIG. 5 is a cross-sectional view schematically showing another example of the lithium ion battery electrode of the present invention.
- FIG. 1 is a cross-sectional view schematically showing an example of the structure of a lithium ion battery including the lithium ion battery electrode of the present invention as a positive electrode and a negative electrode.
- FIG. 2 is a cross-sectional view schematically showing only the positive electrode of the lithium ion battery shown
- FIGS. 7A and 7B are process diagrams schematically showing a process of filling active material particles into voids in the structure.
- 8A and 8B are process diagrams schematically showing a process of fixing the active material particles and the conductive member on the film.
- FIG. 9A, FIG. 9B, and FIG. 9C are process diagrams schematically showing a process of fixing the active material particles and the conductive member between the current collector and the separator.
- FIG. 10A and FIG. 10B are process diagrams schematically showing a process of fixing the active material particles and the conductive member with a resin.
- the electrode for a lithium ion battery of the present invention is an electrode for a lithium ion battery comprising a first main surface disposed on the separator side of the lithium ion battery and a second main surface disposed on the current collector side,
- the electrode has a thickness of 150 to 5000 ⁇ m,
- a conductive member (A) made of an electron conductive material and a large number of active material particles (B) At least a part of the conductive member (A) forms a conductive path that electrically connects the first main surface to the second main surface, and the conductive path is formed around the conductive path. It is in contact with the active material particles (B).
- Examples of the lithium ion battery electrode of the present invention include an example in which the conductive member (A) is a conductive fiber constituting a part of a nonwoven fabric, an example of a conductive fiber constituting a part of a woven fabric or a knitted fabric, Examples are conductive fibers that exist discretely between the first main surface and the second main surface, and examples are conductive resins that constitute part of the foamed resin.
- the conductive member (A) is a conductive fiber constituting a part of the nonwoven fabric will be described with reference to the drawings.
- FIG. 1 is a cross-sectional view schematically showing an example of the structure of a lithium ion battery including the lithium ion battery electrode of the present invention as a positive electrode and a negative electrode.
- a lithium ion battery 1 shown in FIG. 1 includes a positive electrode 10 and a negative electrode 20, and a separator 30 is provided between the positive electrode 10 and the negative electrode 20.
- a current collector 40 is provided on the surface of the positive electrode 10 opposite to the separator 30, and a current collector 50 is provided on the surface of the negative electrode 20 opposite to the separator 30.
- a laminated structure is formed in the order of current collector 40-positive electrode 10-separator 30-negative electrode 20-current collector 50, so that the lithium ion battery 1 is formed as a whole.
- the lithium ion battery electrode of the present invention is a concept that does not include a separator and a current collector, and the positive electrode 10 and the negative electrode 20 shown in FIG. 1 are both lithium ion battery electrodes of the present invention.
- the positive electrode 10 is a sheet-like electrode having a predetermined thickness t1, and includes a first main surface 11 disposed on the separator 30 side and a second main surface 12 disposed on the current collector 40 side.
- the positive electrode 10 includes positive electrode active material particles 14.
- the negative electrode 20 is also a sheet-like electrode having a predetermined thickness t2, and includes a first main surface 21 disposed on the separator 30 side and a second main surface 22 disposed on the current collector 50 side. Yes.
- the negative electrode 20 includes negative electrode active material particles 24.
- the thickness t1 of the positive electrode 10 and the thickness t2 of the negative electrode 20 are 150 to 5000 ⁇ m, respectively. When the electrode is thick in this way, a large amount of active material can be contained in the battery, and the lithium ion battery has a high capacity.
- the thickness t1 of the positive electrode for a lithium ion battery of the present invention is preferably 150 to 1500 ⁇ m, more preferably 200 to 950 ⁇ m, and further preferably 250 to 900 ⁇ m.
- the thickness t2 of the negative electrode for a lithium ion battery of the present invention is preferably 150 to 1500 ⁇ m, more preferably 200 to 950 ⁇ m, and further preferably 250 to 900 ⁇ m.
- Such a lithium ion battery using the electrode for a lithium ion battery of the present invention for the negative electrode and / or the positive electrode is the lithium ion battery of the present invention.
- FIG. 2 is a cross-sectional view schematically showing only the positive electrode of the lithium ion battery shown in FIG.
- the positive electrode 10 includes the first main surface 11 and the second main surface 12 as described above. And between the 1st main surface 11 and the 2nd main surface 12, the electroconductive fiber 13 as a conductive member (A) and the positive electrode active material particle 14 as an active material particle (B) are contained.
- the conductive member (A) is the conductive fiber 13 constituting a part of the nonwoven fabric. Since there are many voids in the nonwoven fabric, a lithium ion battery electrode can be formed by filling the voids with active material particles. The filling of the active material particles into the voids will be described in detail in the item of the method for producing an electrode for a lithium ion battery of the present invention.
- one end of some of the fibers reaches the first main surface 11, and the other end reaches the second main surface 12. Accordingly, at least a part of the conductive fiber 13 forms a conductive path that electrically connects the first main surface 11 to the second main surface 12.
- a large number of conductive fibers 13 are entangled between the first main surface 11 and the second main surface 12, but a plurality of conductive fibers 13 are in contact with each other from the first main surface 11. Even when the second main surface 12 is continuously connected, it can be said that the conductive fibers form a conductive path that electrically connects the first main surface to the second main surface.
- the fiber shown as the conductive fiber 13a is an example in which one conductive fiber is a conductive path, and the two fibers shown as the conductive fiber 13b are in contact with two conductive fibers to be a conductive path. This is an example.
- Examples of conductive fibers include carbon fibers such as PAN-based carbon fibers and pitch-based carbon fibers, conductive fibers obtained by uniformly dispersing highly conductive metal and graphite in synthetic fibers, and metals such as stainless steel. Examples thereof include fiberized metal fibers, conductive fibers in which the surface of organic fiber is coated with metal, and conductive fibers in which the surface of organic fiber is coated with a resin containing a conductive substance. Among these conductive fibers, carbon fibers are preferable.
- the electrical conductivity when the conductive member (A) is a conductive fiber is preferably 50 mS / cm or more, and more preferably 80 to 500 mS / cm.
- the electrical conductivity of the conductive fiber is determined by measuring the volume resistivity according to JIS R 7609 "Carbon Fiber-Determination of Volume Resistivity" and taking the reciprocal of the volume resistivity. If the electrical conductivity of the conductive fiber is 50 mS / cm or more, the resistance when the conductive fiber electrically connecting the first main surface to the second main surface is formed by the conductive fiber is small, and current collection This is preferable because electrons move more smoothly from an active material that is far from the body.
- the average fiber diameter of the conductive fibers is preferably 0.1 to 20 ⁇ m, and more preferably 0.5 to 2.0 ⁇ m.
- the fiber diameter of the conductive fiber is measured by SEM observation.
- the average fiber diameter of the conductive fibers is measured for each of the 10 fibers existing in the 30 ⁇ m square field, and the diameter near the center is measured for each of the three fields.
- the average diameter of the total 30 fibers is measured. Value.
- the fiber length of the conductive fiber is not particularly limited, but the total of the fiber length of the conductive fiber per unit volume of the electrode is preferably 10,000 to 50,000,000 cm / cm 3 , More preferably, it is 20,000 to 50,000,000 cm / cm 3 , and still more preferably 1,000,000 to 10,000,000 cm / cm 3 .
- the total per unit volume of the electrode of the fiber length of the conductive fiber is derived from the following formula.
- Total per unit volume of electrode of conductive fiber length [(Average fiber length of conductive fibers) ⁇ (weight of conductive fibers used per unit area of electrode) / (specific gravity of conductive fibers)] / [(unit area of electrode) ⁇ (electrode thickness)]
- the average fiber length of the conductive fibers is measured by SEM observation. The length of 10 arbitrary fibers existing in a 30 ⁇ m square visual field is measured, this measurement is performed for three visual fields, and the measured value of the average fiber length of conductive fibers is obtained with the average value of the total length of 30 fibers.
- the positive electrode active material particles 14 are active material particles filled in the voids of the nonwoven fabric.
- positive electrode active material particles composite oxides of lithium and transition metals (for example, LiCoO 2 , LiNiO 2 , LiMnO 2 and LiMn 2 O 4 ), transition metal oxides (for example, MnO 2 and V 2 O 5 ), transition metals And sulfides (eg, MoS 2 and TiS 2 ) and conductive polymers (eg, polyaniline, polyvinylidene fluoride, polypyrrole, polythiophene, polyacetylene, poly-p-phenylene, and polycarbazole).
- lithium and transition metals for example, LiCoO 2 , LiNiO 2 , LiMnO 2 and LiMn 2 O 4
- transition metal oxides for example, MnO 2 and V 2 O 5
- transition metals And sulfides eg, MoS 2 and TiS 2
- conductive polymers eg, polyaniline
- the active material particles (B) are preferably coated active material particles in which at least a part of the surface is coated with a coating agent containing a coating resin and a conductive additive.
- a coating agent containing a coating resin and a conductive additive.
- the coating agent contains a coating resin, and when the periphery of the positive electrode active material particles is coated with the coating agent, the volume change of the electrode is alleviated and the expansion of the electrode can be suppressed.
- the coating resin examples include vinyl resin, urethane resin, polyester resin, polyamide resin, epoxy resin, polyimide resin, silicone resin, phenol resin, melamine resin, urea resin, aniline resin, ionomer resin, polycarbonate, and the like.
- vinyl resin, urethane resin, polyester resin or polyamide resin is preferable.
- the conductive path formed by the conductive fibers 13 is in contact with the positive electrode active material particles 14 around the conductive path.
- the conductive path is made of a conductive member that is an electron conductive material, electrons can smoothly reach the current collector.
- the active material particles are coated active material particles, even when the coating agent is in contact with the conductive path, it can be considered that the conductive path is in contact with the active material particle.
- the movement of electrons has been described by taking as an example the case where the electrons generated from the positive electrode active material particles reach the current collector.
- the electrons flowing from the current collector toward the positive electrode active material particles are similarly conductive paths.
- the cathode active material particles can be smoothly reached through. That is, the same effect can be obtained during charging and discharging.
- the positive electrode 10 may further include a conductive additive 16.
- a conductive support agent it selects from the material which has electroconductivity. Specifically, metals [aluminum, stainless steel (SUS), silver, gold, copper, titanium, etc.], carbon [graphite and carbon black (acetylene black, ketjen black, furnace black, channel black, thermal lamp black, etc.), etc. , And mixtures thereof, but are not limited thereto.
- These conductive assistants may be used alone or in combination of two or more.
- these alloys or metal oxides may be used.
- conductive aids may be those obtained by coating a conductive material (a metal material among the conductive aid materials described above) around the particle ceramic material or resin material with plating or the like.
- the conductive auxiliary agent 16 may be contained in the coating agent 15, and the conductive auxiliary agent 16 may be in contact with the positive electrode active material particles 14. When the conductive auxiliary agent is contained in the coating agent or in contact with the positive electrode active material particles, the electron conductivity from the positive electrode active material particles to the conductive path can be further increased.
- the lithium ion battery electrode of the present invention is a negative electrode
- the same configuration can be adopted except that the negative electrode active material particles are used as the active material particles (B) instead of the positive electrode active material particles.
- Negative electrode active material particles include graphite, non-graphitizable carbon, amorphous carbon, polymer compound fired bodies (for example, those obtained by firing and carbonizing phenol resin, furan resin, etc.), cokes (for example, pitch coke, needle coke).
- carbon fibers carbon fibers, conductive polymers (eg polyacetylene and polypyrrole), tin, silicon, and metal alloys (eg lithium-tin alloys, lithium-silicon alloys, lithium-aluminum alloys and lithium-aluminum-manganese) Alloys), composite oxides of lithium and transition metals (for example, Li 4 Ti 5 O 12 ), and the like.
- conductive polymers eg polyacetylene and polypyrrole
- metal alloys eg lithium-tin alloys, lithium-silicon alloys, lithium-aluminum alloys and lithium-aluminum-manganese
- composite oxides of lithium and transition metals for example, Li 4 Ti 5 O 12
- the negative electrode since the conductive path is in contact with the negative electrode active material particles around the conductive path, as in the case of the positive electrode, electrons generated from the negative electrode active material particles immediately reach the conductive path and flow through the conductive path. Smoothly reaches the current collector. Also
- FIG. 3 is a cross-sectional view schematically showing another example of the lithium ion battery electrode of the present invention.
- the conductive member (A) is a conductive fiber 113 constituting a part of the fabric.
- the fabric is composed of warp yarns 113a and weft yarns 113b made of conductive fibers.
- the electrode (positive electrode) 110 in the form shown in FIG. 3 has the same structure as the positive electrode shown in FIG. 2 except that the cloth-like fiber structure corresponding to the nonwoven fabric in FIG. 2 is a woven fabric.
- the weaving method of the woven fabric is not particularly limited, and a woven fabric woven with a plain weave, a twill weave, a satin weave, a pile weave, or the like can be used. Further, a knitted fabric made of conductive fibers may be used instead of the woven fabric.
- the method of knitting the knitted fabric is not particularly limited, and a knitted fabric knitted by a flat knitting, a vertical knitting, a circular knitting or the like can be used.
- lithium ion battery electrodes are formed by filling the voids with active material particles. be able to.
- the conductive fibers 113 At least some of the fibers reach the first main surface 111, and the other part reaches the second main surface 112. Therefore, at least a part of the conductive fiber 113 forms a conductive path that electrically connects the first main surface 111 to the second main surface 112. Since other configurations such as a preferable type of conductive fiber and a type of active material are the same as those of the lithium ion battery electrode shown in FIG. 2, detailed description thereof is omitted. Moreover, it can also be set as the negative electrode for lithium ion batteries of this invention by making an active material particle (B) into a negative electrode active material particle.
- FIG. 4 is a cross-sectional view schematically showing another example of the lithium ion battery electrode of the present invention.
- the conductive member (A) is a conductive fiber 213 that exists discretely between the first main surface 211 and the second main surface 212.
- the conductive fiber 213 is not a part of a structure made of conductive fibers such as the nonwoven fabric, woven fabric, or knitted fabric shown in FIGS.
- the method for manufacturing the electrode shown in FIG. 4 will be described in detail later.
- This electrode is manufactured using a slurry containing conductive fibers and active material particles, and the conductive fibers are dispersed in the active material particles. However, it should not be said that the active material particles are filled in the voids between the fibers.
- the fiber shown as the conductive fiber 213a is an example in which one conductive fiber is a conductive path, and the two fibers shown as the conductive fiber 213b are conductive when the two conductive fibers come into contact with each other.
- This is an example of a passage. Since other configurations such as a preferable type of conductive fiber and a type of active material are the same as those of the lithium ion battery electrode shown in FIG. 2, detailed description thereof is omitted.
- it can also be set as the negative electrode for lithium ion batteries of this invention by making an active material particle (B) into a negative electrode active material particle.
- the electrode for a lithium ion battery of the present invention in the form shown in FIG. 4 is loose enough that the conductive fibers and the active material particles (B) as the conductive member (A) are fixed on the film (E) and do not flow. The shape may be maintained.
- the film (E) is made of a highly conductive material (conductive material)
- the film (E) can be used in place of the current collector, and even if the current collector is in contact with the film (E), it is conductive. It is preferable because sex is not inhibited.
- the membrane (E) is not shown in FIG. A method for producing an electrode for a lithium ion battery in which conductive fibers as the conductive member (A) and active material particles (B) are fixed on the film (E) will be described in detail later.
- FIG. 5 is a cross-sectional view schematically showing another example of the lithium ion battery electrode of the present invention.
- the electrode (positive electrode) 210 ′ shown in FIG. 5 is different in that the conductive fibers 213 as the conductive member (A) and the positive electrode active material particles 14 as the active material particles (B) are fixed by the resin 214.
- the other configuration is the same as that of the electrode 210 having the configuration shown in FIG.
- Resin (F) includes vinyl resin, urethane resin, polyester resin, polyamide resin and the like.
- FIG. 6 is a cross-sectional view schematically showing another example of the lithium ion battery electrode of the present invention.
- the conductive member (A) is a conductive resin 313 that constitutes a part of the foamed resin. Since many voids exist in the foamed resin, an electrode for a lithium ion battery can be formed by filling the voids with active material particles.
- the resin subjected to the conductive treatment examples include a resin provided with conductivity by forming a conductive thin film on the surface of the resin, a resin provided with conductivity by mixing a conductive filler such as metal or carbon fiber inside the resin, and the like. Can be mentioned.
- the resin itself may be a conductive polymer, or a resin in which conductivity is further imparted to the conductive polymer. Examples of the method for forming a conductive thin film on the surface of the resin include metal plating, vapor deposition, and sputtering.
- the conductive resin 313 is continuous from the first main surface 311 to the second main surface 312, and the conductive resin 313 is from the first main surface 311 to the second main surface 312. Are formed to electrically connect the two.
- a resin foam is preferable, and examples thereof include polyurethane foam, polystyrene foam, polyethylene foam, and polypropylene foam.
- a foamed resin obtained by plating the surface of the polyurethane foam with a metal such as nickel is preferable.
- the electrical conductivity of the foamed resin containing the conductive resin may be 100 mS / cm or more. It is preferably 150 to 500 mS / cm.
- the electrical conductivity of the foamed resin is determined by the four-terminal method.
- the electrical conductivity of the foamed resin including the conductive resin is 100 mS / cm or more, a conductive path that electrically connects the first main surface to the second main surface is formed by the conductive fiber. This is preferable because the electron resistance from the active material is small and the movement of electrons from the active material that is far from the current collector is performed more smoothly.
- the preferred active material particle form is the same as the form of the lithium ion battery electrode shown in FIG. Moreover, it can also be set as the negative electrode for lithium ion batteries of this invention by making an active material particle (B) into a negative electrode active material particle.
- the volume ratio of the conductive member (A) is preferably 0.1 to 15 vol% based on the volume of the electrode. More preferably, it is 1 to 6 vol%. That is, the volume occupied by the conductive member (A) in the electrode is preferably relatively small.
- the fact that the volume occupied by the conductive member (A) is small means that a large number of active material particles (B) are filled in the voids not occupied by the conductive member (A). By filling (B), a high capacity lithium ion battery electrode is obtained.
- the term “multiple active material particles” is not a word meaning to specifically define the number of active materials present in the electrode, but the active material particles include the first main surface and the second main material. It means that there exist as many as the space between the main surfaces can be filled.
- the volume ratio of the active material particles (B) is preferably 30 to 80 vol%, and preferably 45 to 60 vol%, based on the volume of the electrode. More preferred. By increasing the proportion of the active material particles (B), a high capacity lithium ion battery electrode is obtained.
- the ratio (V A / V B ) of the volume VA occupied by the conductive member (A) to the volume V B occupied by the active material particles ( B ) is 0.00125-0. 0.5 is preferable, and 0.03 to 0.35 is more preferable. Since the volume occupied by the conductive member (A) is small and most of the conductive material (A) is the active material particles (B), a high capacity lithium ion battery electrode is obtained.
- the volume of the conductive member (A) and the volume of the active material particles (B) are measured by the following method.
- the conductive member (A) and the active material particle (B) were dried and the weight [w (g)] per 1 cm 2 of electrode mixed with the conductive material (A) and the film thickness [t (cm)] of the electrode were measured.
- V A (w ⁇ WA / dA) / (t ⁇ 1) ⁇ 100
- V B (w ⁇ WB / dB) / (t ⁇ 1) ⁇ 100
- One aspect of the method for producing an electrode for a lithium ion battery of the present invention is a method for producing an electrode for a lithium ion battery of the present invention, comprising the conductive member (A), having a void therein, The step (P1) of preparing the structure (Z) having one main surface and the second main surface, and the slurry (X) containing the active material particles (B) are converted into the first of the structure (Z). A step (P2) of applying to the main surface or the second main surface, and a step (P3) of filling the voids in the structure (Z) with the active material particles (B) by applying pressure or reduced pressure. It is characterized by including.
- the manufacturing method of the above aspect is suitable for manufacturing the electrode for a lithium ion battery of the aspect described with reference to FIG. 2, FIG. 3 or FIG.
- a structure (Z) including a conductive member (A) and having a void therein and having a first main surface and a second main surface is prepared (step P1).
- the structure (Z) has a large number of voids.
- the void in the present specification means a space having an open portion in which a material (conductive fiber or conductive resin) constituting the structure is present. The boundary of the gap is not clear, and the gaps are connected. Therefore, the term “multiple voids” is not a word meaning that the number of voids is defined by counting the number of voids existing in the structure (Z), and the active material particles are not present in the structure (Z).
- the volume occupied by the voids for filling is large, which means that there is a space that can be filled with many active material particles.
- a non-woven fabric containing a conductive member (A) made of conductive fiber a woven fabric or a knitted fabric containing a conductive member (A) made of conductive fiber, or a conductive member made of conductive resin
- a foamed resin containing (A) a foamed resin containing (A).
- the details of the nonwoven fabric, the woven fabric, the knitted fabric and the foamed resin are the same as those described on the page of the lithium ion battery electrode of the present invention, and thus detailed description thereof is omitted.
- 7 (a) and 7 (b) are process diagrams schematically showing a process of filling active material particles into voids in the structure. The example using a nonwoven fabric as a structure is shown.
- the slurry (X) containing the active material particles (B) is applied to the first main surface or the second main surface of the structure (Z) (step P2).
- active material particle (B) what was demonstrated by description of the electrode for lithium ion batteries of this invention can be used, and coated active material particle can be used conveniently.
- the coated active material particles are, for example, dropped in a resin solution containing a lithium ion battery active material coating resin over 1 to 90 minutes in a state where lithium ion battery active material particles are put in a universal mixer and stirred at 30 to 500 rpm.
- the mixture can be obtained by mixing, further mixing a conductive additive, raising the temperature to 50 to 200 ° C.
- the slurry containing the active material particles (B) is preferably a solvent slurry (X1) containing the solvent (C) or an electrolyte slurry (X2) containing the electrolyte (D).
- the solvent (C) include water, 1-methyl-2-pyrrolidone (N-methylpyrrolidone), methyl ethyl ketone, dimethylformamide, dimethylacetamide, N, N-dimethylaminopropylamine and tetrahydrofuran.
- electrolyte solution (D) the electrolyte solution containing electrolyte and nonaqueous solvent used for manufacture of a lithium ion battery can be used.
- electrolyte those used in ordinary electrolytic solutions can be used.
- lithium salts of inorganic acids such as LiPF 6 , LiBF 4 , LiSbF 6 , LiAsF 6 and LiClO 4 , LiN (CF 3 SO 2 ) 2 , LiN (C 2 F 5 SO 2 ) 2, and lithium salts of organic acids such as LiC (CF 3 SO 2 ) 3 .
- LiPF 6 lithium salts of inorganic acids
- LiBF 4 LiSbF 6 , LiAsF 6 and LiClO 4
- LiN (CF 3 SO 2 ) 2 LiN (C 2 F 5 SO 2 ) 2
- lithium salts of organic acids such as LiC (CF 3 SO 2 ) 3 .
- preferred from the viewpoints of cell output and charge-discharge cycle characteristics is LiPF 6.
- non-aqueous solvent those used in ordinary electrolytic solutions can be used, for example, lactone compounds, cyclic or chain carbonates, chain carboxylates, cyclic or chain ethers, phosphates, nitriles. Compounds, amide compounds, sulfones, sulfolanes and the like and mixtures thereof can be used.
- a non-aqueous solvent may be used individually by 1 type, and may use 2 or more types together.
- lactone compounds, cyclic carbonates, chain carbonates and phosphates are preferred from the viewpoint of battery output and charge / discharge cycle characteristics, and more preferred are lactone compounds, cyclic carbonates and chains.
- a carbonic acid ester is more preferable, and a mixed liquid of a cyclic carbonate and a chain carbonate is more preferable.
- Particularly preferred is a mixed solution of ethylene carbonate (EC) and diethyl carbonate (DEC).
- the slurry (X) is prepared by dispersing and slurrying the active material particles (B) and, if necessary, the conductive additive and the binder at a concentration of 10 to 60% by weight based on the weight of the solvent or electrolyte. It is preferable to do.
- a conductive support agent what was demonstrated by description of the electrode for lithium ion batteries of this invention can be used.
- the binder include high molecular compounds such as starch, polyvinylidene fluoride, polyvinyl alcohol, carboxymethyl cellulose, polyvinyl pyrrolidone, tetrafluoroethylene, styrene-butadiene rubber, polyethylene, and polypropylene.
- the slurry containing the active material particles (B) can be applied to the first main surface or the second main surface of the structure (Z) using an arbitrary coating device such as a bar coater or a brush.
- FIG. 7A schematically shows a state in which the slurry is applied on the second main surface of the nonwoven fabric as the structure, and the slurry containing the positive electrode active material particles 14 on the second main surface 62 of the nonwoven fabric 60. Is applied.
- the active material particles (B) are filled in the voids in the structure (Z) by applying pressure or reduced pressure (step P3).
- a method of pressurizing operation a method of pressing using a press machine from the slurry application surface can be mentioned.
- a method for the decompression operation a method in which a filter paper or a mesh is applied to the surface on which the slurry is not applied to the structure, and suction is performed by a vacuum pump. Since there are voids in the structure (Z), the active material particles (B) can be filled in the voids in the structure (X) by a pressure or reduced pressure operation.
- FIG. 7A shows an arrow indicating the direction in which the pressure is applied from above the slurry application surface, and an arrow indicating the direction in which the pressure is reduced from below the filter paper 70.
- FIG. 7B shows an electrode 10 for a lithium ion battery in which the active material particles (B) are filled in the voids in the structure (Z).
- the lithium ion battery electrode shown in FIG. 7B is the same as the lithium ion battery electrode 10 shown in FIG.
- the slurry containing the active material particles (B) is the solvent slurry (X1) containing the solvent (C)
- the voids in the structure (Z) are the active material particles (B) and the electrolyte solution (D).
- This is a preferable configuration as an electrode for a lithium ion battery.
- impurities other than the electrolytic solution are not mixed as a liquid component in the electrode for a lithium ion battery.
- the active material particles are structured by the above-described steps.
- a lithium ion battery electrode can be manufactured by filling the inside void.
- Another aspect of the method for producing an electrode for a lithium ion battery of the present invention is a method for producing an electrode for a lithium ion battery of the present invention, which is a slurry containing the conductive member (A) and the active material particles (B) ( Applying (Y) on the film (E) (Q1); And a step (Q2) of fixing the active material particles (B) and the conductive member (A) on the film (E) by applying pressure or reduced pressure.
- the manufacturing method of the above aspect is suitable for manufacturing the electrode for a lithium ion battery of the aspect described with reference to FIG. In particular, it is more suitable for producing a positive electrode of a lithium ion battery.
- FIGS. 8A and 8B are process diagrams schematically showing a process of fixing the active material particles and the conductive member on the film.
- a slurry (Y) containing a conductive member (A) and active material particles (B) is applied on the film (E) (step Q1).
- the slurry (Y) include those obtained by further adding conductive fibers as the conductive member (A) to the slurry (X) described above and dispersing the conductive fibers in the slurry.
- the conductive fiber the conductive fiber described on the page of the lithium ion battery electrode of the present invention can be used, but the shape of the conductive fiber is an independent shape of each fiber. Preferably, it does not have a three-dimensional structure such as a nonwoven fabric, a woven fabric, or a knitted fabric. When each of the conductive fibers is independent, the conductive fibers are dispersed in the slurry.
- the slurry (Y) is preferably an electrolyte slurry (Y1) containing an electrolyte (D).
- the electrolytic solution (D) the same one as the electrolytic solution (D) in the above-described electrolytic solution slurry (X2) can be used.
- the slurry (Y) may be a solvent slurry containing the solvent (C).
- the membrane (E) those capable of separating the active material particles and the conductive member, the electrolytic solution and the solvent in the subsequent pressurization or decompression step are preferable.
- the film (E) is made of a highly conductive material (conductive material)
- the film (E) can be used instead of the current collector, and the current collector and the film (E) are brought into contact with each other. Is preferable because the conductivity is not hindered.
- a material having an electric conductivity of 100 mS / cm or more can be preferably used. Examples of materials having such characteristics include filter papers, metal meshes and the like in which conductive fibers such as carbon fibers are blended.
- a metal mesh it is preferable to use a stainless steel mesh, for example, a SUS316 twilled woven wire mesh (manufactured by Sunnet Kogyo) and the like.
- the mesh opening of the metal mesh is preferably set so that the active material particles and the conductive member do not pass through, for example, a 2300 mesh mesh is preferably used.
- the slurry (Y) can be applied onto the film (E) using an arbitrary coating apparatus such as a bar coater or a brush.
- FIG. 8A schematically shows a state in which the slurry is applied on the membrane, and the slurry containing the active material particles 14 and the conductive fibers 213 is applied on the filter paper 470 as the membrane.
- step Q2 the active material particles (B) and the conductive member (A) are fixed on the film (E) by applying pressure or reduced pressure (step Q2).
- step Q3 the same method as in the above-described step (P3) can be used.
- the electrolytic solution or solvent is removed from the slurry (Y) by pressurizing or depressurizing, and the conductive member (A ) And the active material particles (B) are fixed on the film (E), and the shape is maintained so as not to flow.
- FIG. 8B shows an electrode 210 in which conductive fibers 213 as active members (A) and active material particles 14 are fixed on a filter paper 470.
- the film (E) When the film (E) is made of a conductive material in the electrode 210, the film (E) can be used as a current collector, and the current collector and the film (E) are brought into contact with each other as a current collector. It can also function. That is, in the electrode 210, the second main surface 212 can be defined as a portion where the conductive fiber 213 as the conductive member (A) is in contact with the filter paper 470. When the film (E) is a material having no conductivity, the film (E) may be disposed on the separator side. Moreover, it is good also considering a film
- the membrane (E) is a membrane that does not transmit the active material particles (B) but transmits the electrolyte solution (D).
- the pressing step (Q3) is a step for increasing the density of the active material particles (B) by further increasing the pressure difference as compared with the pressurization or reduced pressure in the step (Q2).
- the pressing step (Q3) includes both an aspect in which pressurization is applied when the step (Q2) is reduced pressure and an aspect in which the pressurization pressure is further increased when the step (Q2) is pressurized. It is.
- a step (Q4) of transferring the lithium ion battery electrode fixed on the membrane (E) to the main surface of the current collector or the separator is performed, and the first main surface of the lithium ion battery electrode is the separator. It is preferable to form a lithium ion battery electrode disposed on the main surface, or to form a lithium ion battery electrode in which the second main surface of the lithium ion battery electrode is disposed on the main surface of the current collector.
- the main surface [first main surface 211 in FIG. 8B] on the opposite side to the film (E) of the electrode for the lithium ion battery fixed on the film (E) is collected. It is preferable that the transfer is performed in contact with the main surface of the body or the separator.
- the film (E) is made of a conductive material and the film (E) is used instead of the current collector, it is preferable that the main surface on the opposite side of the film (E) is brought into contact with the main surface of the separator for transfer. .
- membrane (E) it is preferable to perform the process of peeling a film
- Another aspect of the method for producing an electrode for a lithium ion battery of the present invention is a method for producing an electrode for a lithium ion battery of the present invention, which is a slurry containing the conductive member (A) and the active material particles (B) ( Y) is applied onto the current collector to form a slurry layer on the current collector (T1); A separator is placed on the slurry layer, and liquid is absorbed from the upper surface side of the separator to fix the active material particles (B) and the conductive member (A) between the current collector and the separator. Including a step (T2).
- the manufacturing method according to the above aspect is the lithium ion battery electrode according to the aspect described with reference to FIG. 4, that is, the electrode in which the conductive member is a conductive fiber discretely existing between the first main surface and the second main surface. Suitable for manufacturing. In particular, it is more suitable to manufacture the negative electrode of a lithium ion battery.
- FIG. 9A, FIG. 9B, and FIG. 9C are process diagrams schematically showing the process of fixing the active material particles and the conductive member between the current collector and the separator.
- a slurry (Y) containing a conductive member (A) and active material particles (B) is applied on a current collector to form a slurry layer (step T1).
- the current collector include aluminum, copper, aluminum, titanium, stainless steel, nickel, baked carbon, conductive polymer, and conductive glass.
- the slurry (Y) the same slurry as the slurry (Y) described with reference to FIG. 8 can be used, and conductive fibers as the conductive member (A) are further added to the slurry (X) to add the slurry to the slurry.
- distributed the conductive fiber is mentioned.
- the slurry (Y) is preferably an electrolyte slurry (Y1) containing the electrolyte (D).
- the electrolytic solution (D) the same one as the electrolytic solution (D) in the above-described electrolytic solution slurry (X2) can be used.
- the slurry (Y) may be a solvent slurry containing the
- the slurry (Y) can be applied onto the current collector using an arbitrary coating apparatus such as a bar coater or a brush.
- FIG. 9A schematically shows a state in which the slurry layer 225 is formed by applying the slurry onto the current collector 50, and the negative electrode active material particles 24 and the conductive fibers 223 are formed on the current collector 50.
- a slurry layer 225 is formed.
- the periphery of the negative electrode active material particles 24 is covered with a coating agent 25, and the conductive auxiliary agent 26 is included in the slurry.
- the conductive fiber 223, the coating agent 25, and the conductive auxiliary agent 26 are the same as the conductive fiber 213, the coating agent 15, and the conductive tablet 16 described in detail in the description of the lithium ion battery electrode (positive electrode) of the present invention. is there.
- the negative electrode active material particles 24 are the same as the negative electrode active material particles whose details are described in the description of the electrode for a lithium ion battery of the present invention.
- the separator is placed on the slurry layer, and liquid is absorbed from the upper surface side of the separator to fix the active material particles (B) and the conductive member (A) between the current collector and the separator (step) T2).
- the separator 30 is placed on the slurry layer 225. Then, liquid is absorbed from the upper surface side of the separator 30.
- an aramid separator manufactured by Japan Vilene Co., Ltd.
- a polyethylene a microporous film made of a polypropylene film, a multilayer film of a porous polyethylene film and polypropylene, a polyester fiber, an aramid fiber, a non-woven fabric made of glass fiber, and the like, and Those having ceramic fine particles such as silica, alumina and titania attached to the surface thereof can be mentioned.
- the liquid absorption may be performed by sucking the liquid that has been pressed from the upper surface side or the lower surface side of the separator and leached out from the upper surface of the separator, or by sucking the liquid by reducing the pressure from the upper surface side of the separator. May be performed. Furthermore, liquid absorption from the upper surface side of the separator may be performed by placing a liquid-absorbing material on the upper surface of the separator. As the liquid-absorbing material, a liquid-absorbing cloth such as towel, paper, liquid-absorbing resin, or the like can be used.
- the electrolyte or solvent is removed from the slurry (Y) by liquid absorption, and the conductive fibers and the active material particles (B) as the conductive member (A) are fixed between the current collector and the separator and do not flow.
- the shape is maintained loosely.
- the method of pressurization is not particularly limited, it can be carried out by various methods. For example, a method using a known press machine and a method of applying pressure by placing a heavy object or the like as a weight may be mentioned, and the pressurization may be performed while vibrating with an ultrasonic vibrator or the like. Pressure when pressurized from the upper side or the lower side of the separator is preferably 0.8 ⁇ 41kg / cm 2, more preferably 0.9 ⁇ 10kg / cm 2. When the pressure is within this range, the conductive path inside the electrode can be satisfactorily formed, which is preferable because the battery can have a higher capacity.
- FIG. 9C shows an electrode 220 in which conductive fibers 223 and active material particles 24 as the conductive member (A) are fixed between the current collector 50 and the separator 30.
- the electrode 220 the first main surface 221 of the electrode is in contact with the separator 30, and the second main surface 222 of the electrode is in contact with the current collector 50.
- the electrode is manufactured in a state where the electrode is sandwiched between the separator and the current collector. Therefore, it is not necessary to separately perform a step of disposing a separator and a current collector on both sides of the electrode, and this is preferable because the number of electrodes in a preferable form as a bipolar electrode can be obtained with a small number of steps.
- Another aspect of the method for producing an electrode for a lithium ion battery of the present invention includes a first main surface disposed on the separator side of the lithium ion battery, and a second main surface disposed on the current collector side, and A conductive member (A) made of an electron conductive material, a large number of active material particles (B), and a resin (F) are included between the first main surface and the second main surface, and the conductive member (A) At least a part forms a conductive path that electrically connects the first main surface to the second main surface, and the conductive path is connected to the active material particles (B) around the conductive path.
- a method of manufacturing a contact electrode for a lithium ion battery The conductive member (A), the active material particles (B), and the electrode composition containing the resin (F) are heated and pressed, so that the conductive member (A) and the active material are made of the resin (F). It includes a step (R1) of fixing the particles (B).
- the manufacturing method of the above embodiment is suitable for manufacturing the lithium ion battery electrode of the embodiment described with reference to FIG.
- FIG. 10A and FIG. 10B are process diagrams schematically showing a process of fixing the active material particles and the conductive member with a resin.
- an electrode composition containing a conductive member (A), active material particles (B) and a resin (F) is prepared.
- the conductive member (A) as in the case of the conductive member (A) preferably used in the method for producing an electrode for a lithium ion battery according to the embodiment described with reference to FIGS. It is preferable to use conductive fibers having an independent shape.
- the active material particles (B) the same active material particles (B) used in the method for producing an electrode for a lithium ion battery of another embodiment can be used.
- the resin (F) it is preferable to use vinyl resin, urethane resin, polyester resin, polyamide resin or the like. These resins are preferable in terms of moldability.
- the resin (F) may be present in the form of a resin solution dissolved in a solvent, or may be present in a solid form such as pellets that are fluidized by heating.
- the coating resin contained in the coating agent may be a resin (F).
- the conductive member (A) and the active material particles (B) may be dispersed in the resin solution.
- the resin (F) even when the resin (F) is present in a solid form, the resin (F), the conductive member (A), and the active material particles (B) may be dispersed without being unevenly distributed at a specific portion. preferable.
- the electroconductive member (A) and active material particle (B) are fixed with resin (F) by heat-pressing the prepared composition for electrodes (process R1).
- the method of hot pressing is not particularly limited, but as shown in FIG. 10 (a), an electrode composition comprising positive electrode active material particles 14, conductive fibers 213, and resin 214 on a plate 570 such as a metal plate.
- coating and heat-pressing from the upper surface is mentioned.
- coating of the composition for electrodes can be performed using arbitrary coating apparatuses, such as a bar coater and a brush.
- a heat press can be performed using a normal heat press apparatus.
- the resin (F) is a resin for coating the coated active material particles
- the conductive member (A) and the coated active material particles are applied to the plate and heated and pressed.
- (Coating) Active material particles are fixed.
- the active material particles fixed by the coating resin may be coated active material particles that are still coated with the coating resin, or may be active material particles that have been peeled off.
- the conditions for the hot press may be determined as appropriate depending on the curing conditions of the resin used, and are not particularly limited. It is preferable to heat press under conditions. In the case of vinyl resin, it is preferable to heat press under conditions of 80 to 180 ° C., 0.01 to 5 MPa, and 5 to 300 seconds. As shown in FIG. 10B, an electrode 210 ′ in which the conductive fibers 213 and the positive electrode active material particles 14 are fixed with a resin 214 can be manufactured by hot pressing.
- the lithium ion battery using the electrode for a lithium ion battery of the present invention can be obtained by combining a counter electrode and storing it in a cell container together with a separator, injecting an electrolytic solution, and sealing the cell container.
- a positive electrode is formed on one surface of the current collector, and a negative electrode is formed on the other surface to produce a bipolar electrode.
- the bipolar electrode is laminated with a separator and stored in a cell container. It can also be obtained by pouring and sealing the cell container.
- the electrode for lithium ion batteries of this invention for any one of a positive electrode and a negative electrode, and it is good also considering a positive electrode and a negative electrode as a lithium ion battery electrode of this invention as a lithium ion battery.
- separators polyethylene, a microporous film made of polypropylene film, a multilayer film of porous polyethylene film and polypropylene, non-woven fabric made of polyester fiber, aramid fiber, glass fiber, etc., and silica, alumina, titania etc. on their surface And those having ceramic fine particles attached thereto.
- the electrolytic solution As the electrolytic solution, the electrolytic solution described above as the electrolytic solution (D) can be used.
- an initiator solution prepared by dissolving 0.583 parts of 2,2′-azobis (2,4-dimethylvaleronitrile) in 26 parts of ethyl acetate was continuously added using a dropping funnel over 2 hours. Furthermore, the polymerization was continued for 4 hours at the boiling point. After removing the solvent to obtain 582 parts of resin, 1,360 parts of isopropanol was added to obtain a coating resin solution comprising a vinyl resin having a resin concentration of 30% by weight.
- LiCoO 2 powder [Nippon Chemical Industry Co., Ltd. cell seed C-5H] 100 parts by weight, 100 parts by weight of water and 1200 parts by weight of ⁇ 3 mm alumina balls were put in a pot mill container and ground for 20 minutes, and LiCoO having an average particle size of 2.3 ⁇ m. 100 parts by weight of 2 powders were obtained.
- Non-graphitizable carbon Carbotron (registered trademark) PS (F) manufactured by Kureha Battery Materials Japan Co., Ltd.] 100 parts by weight, water 200 parts by weight and ⁇ 0.1 mm zirconia ball 1000 parts by weight are put in a pot mill container. For 15 minutes to obtain 100 parts by weight of non-graphitizable carbon having an average particle size of 2.5 ⁇ m.
- ⁇ Preparation of coated negative electrode active material particles (B-3)> Resin for coating in a state where 90 parts by weight of non-graphitizable carbon [Carbotron (registered trademark) PS (F) manufactured by Kureha Battery Materials Japan Co., Ltd.] is put in a universal mixer and stirred at room temperature and 150 rpm. The solution (resin solid content concentration of 30% by weight) was added dropwise and mixed over 60 minutes so that the resin solid content was 5 parts by weight, and stirred for another 30 minutes. Next, 5 parts by weight of acetylene black [Denka Black (registered trademark) manufactured by Denki Kagaku Kogyo Co., Ltd.] was mixed in three portions with stirring, and the mixture was heated to 70 ° C. with stirring for 30 minutes. The pressure was reduced to 01 MPa and held for 30 minutes. By the above operation, coated negative electrode active material particles (B-3) were obtained.
- Non-graphitizable carbon Carbotron (registered trademark) PS (F) manufactured by
- the coated negative electrode active material particles (B-4) were obtained in the same manner as in the production method (B-3) except that the amount was changed to 90 parts by weight of non-graphitizable carbon having a thickness of 0.5 ⁇ m.
- Carbon fiber (C) is, Eiichi Yasuda, Asao Oya, Shinya Komura, Shigeki Tomonoh, Takashi Nishizawa, Shinsuke Nagata, Takashi Akatsu, CARBON, 50,2012,1432-1434 and Eiichi Yasuda, Takashi Akatsu, Yasuhiro Tanabe, Kazumasa Nakamura, Yasuto Hoshikawa, Naoya Miyajima, TANSO, 255, 2012, pages 254 to 265 were used as a reference for production.
- a resin composition was prepared by melt-kneading using a single screw extruder. The resin composition was melt-extruded and spun at 390 ° C. The spun resin composition was placed in an electric furnace and held at 270 ° C. for 3 hours under a nitrogen atmosphere to stabilize the carbon precursor. Next, the electric furnace was heated to 500 ° C. over 1 hour and held at 500 ° C.
- the electric furnace was heated up to 1000 ° C. over 2 hours and held at 1000 ° C. for 30 minutes, and the remaining stabilized carbon precursor was used as a conductive fiber.
- 90 parts by weight of the obtained conductive fiber, 500 parts by weight of water and 1000 parts by weight of zirconia balls having a diameter of 0.1 mm were placed in a pot mill container and pulverized for 5 minutes.
- the zirconia balls were classified and dried at 100 ° C. to obtain conductive carbon fibers (C). From the result of measurement by SEM, the average fiber diameter was 0.9 ⁇ m, and the average fiber length was 25 ⁇ m.
- LiPF 6 was dissolved at a rate of 1 mol / L in a mixed solvent of ethylene carbonate (EC) and diethyl carbonate (DEC) (volume ratio 1: 1) to prepare an electrolytic solution for a lithium ion battery.
- EC ethylene carbonate
- DEC diethyl carbonate
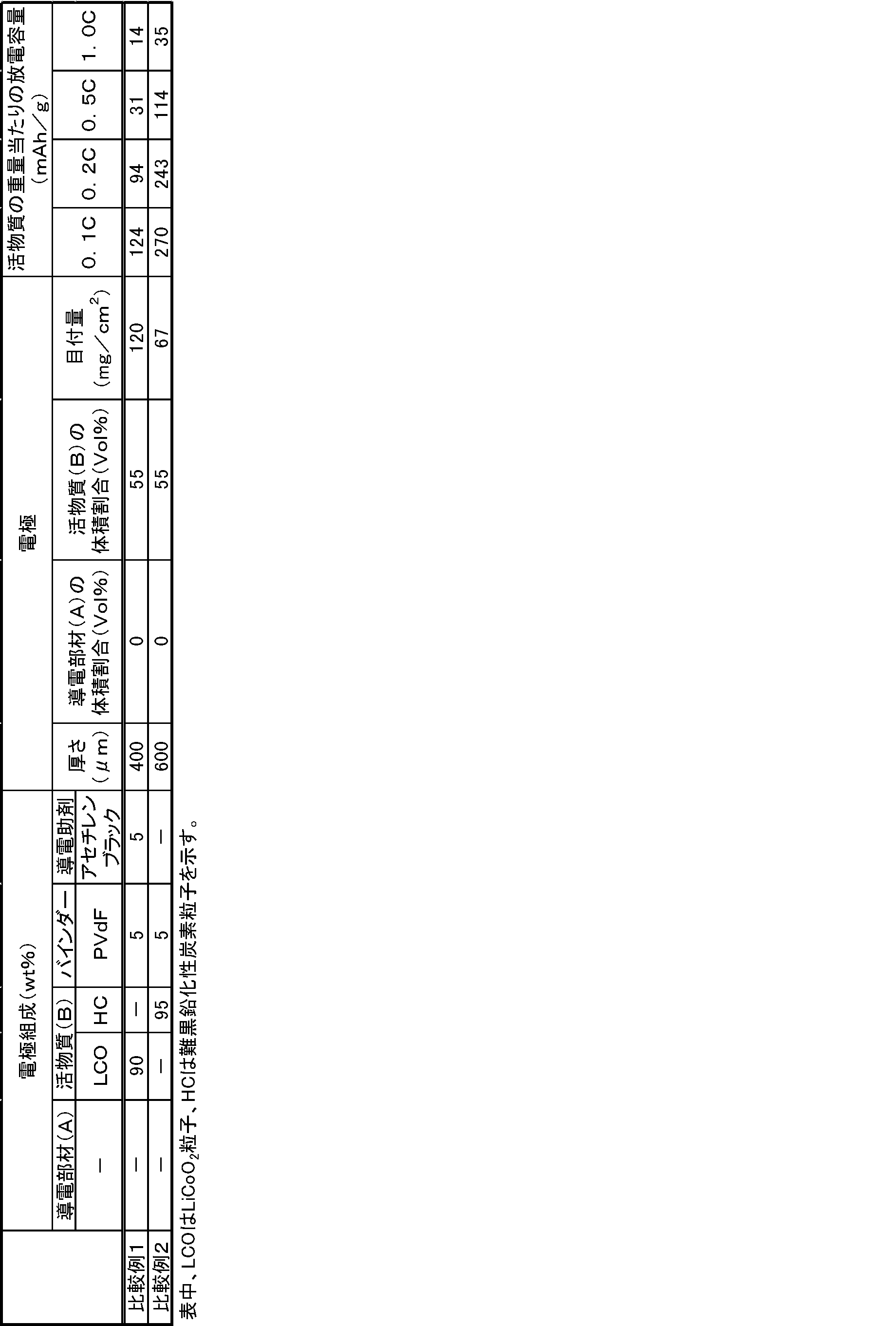
- Example 1 A urethane foam [Seiren Co., Ltd. Sui-70-5005: thickness 450 ⁇ m: electrical conductivity 300 mS / cm] prepared by conducting nickel plating was prepared.
- the urethane foam is a structure (Z) that includes a conductive resin as the conductive member (A), has a large number of voids, and includes a first main surface and a second main surface.
- LiCoO 2 powder cell seed C-8G manufactured by Nippon Chemical Industry Co., Ltd.
- acetylene black [Denka Black (registered trademark) manufactured by Denki Kagaku Kogyo Co., Ltd.] 4.75 Part by weight was mixed with an N-methylpyrrolidone (hereinafter referred to as NMP) solution containing 4.75 parts by weight of polyvinylidene fluoride (manufactured by Sigma Aldrich) to prepare a solvent slurry.
- NMP N-methylpyrrolidone
- Example 2 The coated positive electrode active material particles (B-1) were mixed with the electrolytic solution to prepare an electrolytic solution slurry.
- An electrolyte solution slurry having an amount of 95 parts by weight of the coated positive electrode active material particles was applied to one main surface of the urethane foam with respect to 5 parts by weight of the urethane foam similar to Example 1,
- a positive electrode for a lithium ion battery was prepared by applying pressure from the top of the coated surface at a pressure of 1.5 kg / cm 2 to fill the voids in the urethane foam with coated positive electrode active material particles.
- Example 3 90 parts by weight of the coated positive electrode active material particles (B-1) and 5 parts by weight of acetylene black [Denka Black (registered trademark) manufactured by Denki Kagaku Kogyo Co., Ltd.] were mixed with the electrolyte to prepare an electrolyte slurry. .
- An amount of electrolyte slurry in which the weight of components other than the electrolyte in the electrolyte slurry is 95 parts by weight is applied to one main surface of the urethane foam with respect to 5 parts by weight of the urethane foam similar to Example 1.
- the positive electrode for a lithium ion battery was produced by applying pressure from the surface of the electrolyte slurry applied at a pressure of 1.5 kg / cm 2 to fill the voids in the urethane foam with the coated positive electrode active material particles.
- Table 1 shows the electrode composition, thickness, volume ratio of the conductive member (A) and the active material (B), and the basis weight of the electrodes of the positive electrodes for lithium ion batteries produced in Examples 1 to 3.
- Example 4 A carbon fiber non-woven fabric [Osaka Gas Chemical Co., Ltd. Donakabo Paper S-253: thickness 650 ⁇ m: electric conductivity 400 mS / cm] was prepared.
- the non-woven fabric is a structure (Z) including a conductive member (A) made of conductive fibers, having a large number of voids, and having a first main surface and a second main surface.
- the non-woven fabric is referred to as non-woven fabric A.
- LiCoO 2 powder manufactured by Nippon Chemical Industry Co., Ltd., Cellseed C-8G
- acetylene black Denka Black (registered trademark) by Denki Kagaku Kogyo Co., Ltd.
- a solvent slurry (C) was prepared by mixing with NMP solution containing 5 parts by weight. The solvent slurry in an amount such that the weight of the components other than NMP in the solvent slurry is 98 parts by weight with respect to 2 parts by weight of the nonwoven fabric A is applied to one main surface of the nonwoven fabric A, and from above the coating surface of the solvent slurry.
- the positive electrode active material particles were filled in the voids in the nonwoven fabric A by applying a pressure of 2.0 kg / cm 2 . Then, it dried at 80 degreeC and the normal pressure for 120 minutes, the solvent was distilled off, then, it dried under reduced pressure at 80 degreeC for 8 hours, and the positive electrode for lithium ion batteries was produced.
- Example 5 An electrolyte slurry similar to that in Example 2 was prepared. An electrolyte slurry in an amount such that the weight of the coated positive electrode active material particles (B-1) is 98 parts by weight with respect to 2 parts by weight of the same nonwoven fabric A as in Example 4 was applied to one main surface of the nonwoven fabric A. A positive electrode for a lithium ion battery was prepared by applying pressure at a pressure of 1.5 kg / cm 2 from above the coating surface of the electrolyte slurry to fill the voids in the nonwoven fabric A with the coated positive electrode active material particles.
- Example 6 A carbon fiber non-woven fabric [Osaka Gas Chemical Co., Ltd. Donakabo Paper S-259P: thickness 500 ⁇ m: electric conductivity 500 mS / cm] was prepared.
- the non-woven fabric is a structure (Z) including a conductive member (A) made of conductive fibers, having a large number of voids, and having a first main surface and a second main surface.
- the non-woven fabric is referred to as non-woven fabric B.
- a positive electrode for a lithium ion battery was produced in the same manner as in Example 4 except that the nonwoven fabric A was changed to the nonwoven fabric B in Example 4.
- Example 7 A positive electrode for a lithium ion battery was produced in the same manner as in Example 5 except that the nonwoven fabric A was changed to the nonwoven fabric B in Example 5.
- Table 2 shows the electrode composition, thickness, volume ratio of the conductive member (A) and the active material (B), and the basis weight of the electrodes of the positive electrodes for lithium ion batteries produced in Examples 4 to 7.
- Carbon fiber [Donakabo Chop S-231 manufactured by Osaka Gas Chemical Co., Ltd .: average fiber length 3300 ⁇ m, average fiber diameter 13 ⁇ m: electrical conductivity 200 mS / cm] was prepared as a conductive member (A).
- the carbon fiber is referred to as carbon fiber A.
- a stainless steel mesh [SUS316 twill woven 2300 mesh manufactured by Sunnet Kogyo Co., Ltd.] is prepared, and an electrolytic solution slurry is applied to the stainless steel mesh, followed by suction filtration (reduced pressure), thereby providing a coated positive electrode. Active material particles and carbon fibers were fixed on a stainless steel mesh to produce a positive electrode for a lithium ion battery.
- Example 9 With respect to the positive electrode for lithium ion batteries produced in Example 8, the electrolyte slurry was further pressurized at a pressure of 1.5 kg / cm 2 to produce a positive electrode for lithium ion batteries.
- Carbon fiber [Donakabo Mild S-243: average fiber length: 500 ⁇ m, average fiber diameter: 13 ⁇ m: electrical conductivity: 200 mS / cm] manufactured by Osaka Gas Chemical Co., Ltd. was prepared as a conductive member (A).
- the carbon fiber is referred to as carbon fiber B.
- Example 8 Prepare the same stainless steel mesh as in Example 8 as the membrane (E), apply the electrolyte slurry to the stainless steel mesh, suction filter (depressurize), and pressurize with a pressure of 1.5 kg / cm 2
- the coated positive electrode active material particles and the carbon fibers were fixed on a stainless steel mesh to produce a positive electrode for a lithium ion battery.
- Examples 11 and 12 A positive electrode for a lithium ion battery was produced in the same manner as in Example 10 except that the thickness of the electrode to be produced was reduced by making the amount of the electrolyte slurry applied in Example 10 smaller than that in Example 10.
- Example 13 a positive electrode for a lithium ion battery was prepared by peeling off the electrode fixed on the stainless steel mesh.
- Example 14> A mixed powder prepared by dry-mixing 1.75 parts by weight of the above carbon fiber B and 98.25 parts by weight of the coated positive electrode active material particles (B-1) is prepared, spread on a metal plate (iron plate), and mixed using an applicator. The powder was made uniform, and then heated and pressed at 180 ° C., 1.5 MPa for 1 minute to produce an electrode for a lithium ion battery in which carbon fibers and (coated) positive electrode active material particles were fixed with a coating resin. The electrode was used for evaluation of discharge capacity after peeling from the iron plate.
- Example 15> The carbon fiber (C) (average fiber length 25 ⁇ m, average fiber diameter 0.9 ⁇ m: electrical conductivity 30 mS / cm) produced in the above ⁇ Preparation of carbon fiber (C)> was prepared as a conductive member (A).
- the carbon fiber is referred to as carbon fiber C.
- Example 8 Prepare the same stainless steel mesh as in Example 8 as the membrane (E), apply the electrolyte slurry to the stainless steel mesh, suction filter (depressurize), and pressurize with a pressure of 1.5 kg / cm 2 By fixing the coated positive electrode active material particles and the carbon fiber on the stainless steel mesh, the electrode was peeled off to produce a positive electrode for a lithium ion battery.
- Examples 16 and 17 The ratio of the carbon fibers C and the coated positive electrode active material particles (B-2) in Example 15 was changed as shown in Table 3, and the thickness of the electrode was adjusted by changing the amount of electrolyte slurry to be applied.
- a positive electrode for a lithium ion battery was produced in the same manner as in Example 15.
- Example 15 a force of pressurizing with a pressurizing pressure 1.5 kg / cm 2, Example 18, 4.0 kg / cm 2, Example 19, except that was changed to 35 kg / cm 2, the Example 15 In the same manner, a positive electrode for a lithium ion battery was produced.
- Examples 20 to 23 The carbon fiber C and the coated positive electrode active material particles (B-2) in Example 15 were used as shown in Table 3, and the thickness of the electrode was adjusted by changing the amount of the electrolyte slurry to be applied. In the same manner as in Example 15, a positive electrode for a lithium ion battery was produced.
- Table 3 shows the total per unit volume [shown as “total fiber length (cm / cm 3 ) of the conductive member (A)]” and the basis weight of the electrode.
- the thickness of the positive electrode for a lithium ion battery does not include the thickness of the film (E) in Examples 8 to 13 and 15 to 23 and the thickness of the iron plate in Example 14.
- Example 24 The urethane foam similar to Example 1 was prepared as a structure (Z). Separately, 80.75 parts by weight of non-graphitizable carbon (Carbotron (registered trademark) PS (F) manufactured by Kureha Battery Materials Japan Co., Ltd.) as negative electrode active material particles was added to polyvinylidene fluoride (Sigma Aldrich). (Manufactured) A solvent slurry was prepared by mixing with 4.25 parts by weight of NMP solution.
- non-graphitizable carbon Carbotron (registered trademark) PS (F) manufactured by Kureha Battery Materials Japan Co., Ltd.
- Polyvinylidene fluoride Sigma Aldrich
- An amount of solvent slurry in which the weight of components other than NMP in the solvent slurry is 85 parts by weight with respect to 15 parts by weight of the urethane foam is applied to one main surface of the urethane foam,
- the pressure was applied at a pressure of 2.0 kg / cm 2 to fill the voids in the urethane foam with the negative electrode active material particles.
- the solvent was removed by drying at 80 ° C. for 120 minutes under normal pressure, and then dried under reduced pressure at 80 ° C. for 8 hours to prepare a negative electrode for a lithium ion battery.
- Non-graphitizable carbon as negative electrode active material particles [Carbotron (registered trademark) PS (F)] manufactured by Kureha Battery Materials Japan Ltd., 76.5 parts by weight, acetylene black [manufactured by Denki Kagaku Kogyo Co., Ltd.] 4.25 parts by weight of Denka Black (registered trademark) was mixed with an NMP solution containing 4.25 parts by weight of polyvinylidene fluoride (manufactured by Sigma Aldrich) to prepare a solvent slurry.
- An amount of solvent slurry in which the weight of components other than NMP in the solvent slurry is 85 parts by weight with respect to 15 parts by weight of the urethane foam is applied to one main surface of the urethane foam,
- the pressure was applied at a pressure of 2.0 kg / cm 2 to fill the voids in the urethane foam with the negative electrode active material particles.
- the solvent was removed by drying at 80 ° C. for 120 minutes under normal pressure, and then dried under reduced pressure at 80 ° C. for 8 hours to prepare a negative electrode for a lithium ion battery.
- Example 26 The coated negative electrode active material particles (B-3) were mixed with the electrolytic solution to prepare an electrolytic solution slurry.
- the coated negative electrode active material particles were filled in the voids in the urethane foam by applying a pressure of 1.5 kg / cm 2 from the top of the coated surface to prepare a negative electrode for a lithium ion battery.
- Example 27 80 parts by weight of the coated negative electrode active material particles (B-3) and 5 parts by weight of acetylene black [Denka Black (registered trademark) manufactured by Denki Kagaku Kogyo Co., Ltd.] were mixed with the electrolyte to prepare an electrolyte slurry. .
- an electrolytic solution slurry in an amount that the weight of components other than the electrolytic solution in the electrolytic slurry is 85 parts by weight is applied to one main surface of the urethane foam.
- the negative electrode for a lithium ion battery was prepared by applying pressure at a pressure of 1.5 kg / cm 2 from above the application surface of the electrolyte slurry and filling the voids in the urethane foam with the coated negative electrode active material particles.
- Table 4 shows the electrode composition, thickness, volume ratio of the conductive member (A) and the active material (B), and the basis weight of the electrodes of the negative electrodes for lithium ion batteries prepared in Examples 24 to 27.
- Example 28 A carbon fiber B similar to that in Example 10 was prepared as the conductive member (A). 4.2 parts by weight of the above carbon fiber B and 95.8 parts by weight of non-graphitizable carbon as negative electrode active material particles similar to those in Example 24 were mixed with the above electrolytic solution to prepare an electrolytic solution slurry.
- Example 29 and 30 A negative electrode for a lithium ion battery was produced in the same manner as in Example 28 except that the thickness of the electrode to be produced was reduced by making the amount of the electrolyte slurry applied in Example 28 smaller than that in Example 28.
- Example 31 In Example 28, in place of 95.8 parts by weight of the non-graphitizable carbon as the negative electrode active material particles, 95.8 parts by weight of the coated negative electrode active material particles (B-3) were used. Similarly, a negative electrode for a lithium ion battery was produced.
- Examples 32 and 33> A negative electrode for a lithium ion battery was produced in the same manner as in Example 31 except that the thickness of the electrode to be produced was reduced by making the amount of the electrolyte slurry applied in Example 31 smaller than that in Example 31.
- Example 34 A carbon fiber C similar to that in Example 15 was prepared as the conductive member (A). In Example 28, 4.2 parts by weight of the carbon fiber C is used instead of the carbon fiber B as the conductive member (A), and 95.8 parts by weight of the non-graphitizable carbon as the negative electrode active material particles is used. A negative electrode for a lithium ion battery was produced in the same manner as in Example 28 except that 95.8 parts by weight of the coated negative electrode active material particles (B-3) was used.
- Example 34 instead of the coated negative electrode active material particles (B-3), the coated negative electrode active material particles (B-4) were used, and the ratio of the carbon fiber C to the coated negative electrode active material particles (B-4) A negative electrode for a lithium ion battery was produced in the same manner as in Example 34, except that was changed as shown in Table 5.
- Examples 38 to 40> The carbon fiber C and the coated negative electrode active material particles (B-4) in Example 35 were used as shown in Table 5, and the thickness of the electrode was adjusted by changing the amount of the electrolyte slurry to be applied. In the same manner as in Example 35, a negative electrode for a lithium ion battery was produced.
- Example 41 In Example 35, the member for applying the prepared electrolyte slurry was changed from an aramid separator to a copper foil having a thickness of 20 ⁇ m as a current collector, and after applying the electrolyte slurry to the copper foil, the aramid separator was placed. By applying pressure from the upper surface of the separator at 1.5 kg / cm 2 , the liquid that had oozed out from the upper surface of the separator was absorbed, and a negative electrode for a lithium ion battery including a current collector was produced.
- Non-graphitizable carbon [Carbotron (registered trademark) PS (F)] manufactured by Kureha Battery Materials Japan Co., Ltd.] as negative electrode active material particles, 5 parts by weight of polyvinylidene fluoride (manufactured by Sigma-Aldrich)
- a solvent slurry was prepared by mixing with an NMP solution containing parts. The solvent slurry is applied to one side of a 20 ⁇ m thick copper foil in the air using a wire bar, dried at 80 ° C./3 hours at normal pressure, and then vacuum dried at 80 ° C./8 hours to evaporate the solvent.
- a negative electrode for a lithium ion battery of Comparative Example 2 was produced.
- Table 6 shows the electrode composition and thickness of the positive and negative electrodes for lithium ion batteries prepared in Comparative Examples 1 and 2, the volume ratio of the conductive member (A) and the active material (B), and the basis weight of the electrode.
- the negative electrode produced in any of Examples 24 to 41 and Comparative Example 2 was punched out to 17 mm ⁇ , and arranged at both ends in the 2032 type coin cell together with the positive electrode made of 17 mm ⁇ Li metal.
- As the current collector on the negative electrode side a 20 ⁇ m thick copper foil was used, and in the negative electrodes of Examples 28 to 40 using an aramid separator, the aramid separator was disposed on the separator side (positive electrode side). Further, since the negative electrode of Example 41 was integrated with the current collector and the separator, a copper foil as a current collector was not used separately, and an aramid separator was disposed on the separator side (positive electrode side).
- the lithium ion battery electrode according to each example even when the electrode thickness is increased, the lithium ion battery electrode is excellent in electronic conductivity, although the electrode thickness is large. Thus, it was found that the discharge capacity per weight of the active material was high. It can be used as an electrode for a lithium ion battery having an excellent discharge capacity per unit area.
- the electrode for a lithium ion battery obtained by the present invention is particularly useful as an electrode for a bipolar secondary battery and a lithium ion secondary battery used for a mobile phone, a personal computer, a hybrid vehicle, and an electric vehicle.
- Lithium ion battery 1 Lithium ion battery electrode (positive electrode) 10, 110, 210, 210 ', 310 First main surface of positive electrode 11, 111, 211, 311 Second main surface of positive electrode 12, 112, 212, 312 Conductive fibers constituting part of the nonwoven fabric 13, 13a, 13b Cathode active material particles 14 Coating agent 15, 25 Conductive aid 16, 26 Lithium ion battery electrode (negative electrode) 20, 220 First main surface 21 221 of the negative electrode Second main surface 22, 222 of negative electrode Negative electrode active material particles 24 Separator 30 Current collector 40, 50 Nonwoven fabric (structure) 60 Nonwoven fabric second main surface 62 Filter paper 70, 470 Conductive fiber constituting part of fabric 113 Warp yarn 113a Weft 113b Conductive fibers 213, 213a, 213b, and 223 that exist discretely between the first main surface and the second main surface Resin 214 Slurry layer 225 Conductive resin 313 Board 570
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Composite Materials (AREA)
- Inorganic Chemistry (AREA)
- Dispersion Chemistry (AREA)
- Battery Electrode And Active Subsutance (AREA)
Abstract
Description
しかしながら、双極型電極において電極の厚さを厚くすると、集電体からの距離が遠い活物質の割合が増加する。ここで、活物質自体の電子伝導性は高くないため、集電体からの距離が遠い活物質から集電体までの電子の移動はスムーズに行われないと考えられる。そのため、電極の厚さを単に厚くしただけでは活物質の量が増えたとしても電子伝導性が悪く有効に利用されない活物質の割合が増えてしまうことになる。結果として、電極の厚さを厚くしたにもかかわらず電池の高容量化が達成できないという問題が生じてしまう。
本発明のリチウムイオン電池用電極は、リチウムイオン電池のセパレータ側に配置される第1主面と、集電体側に配置される第2主面とを備えたリチウムイオン電池用電極であって、
上記電極の厚さは150~5000μmであり、
上記第1主面と上記第2主面の間に、電子伝導性材料からなる導電部材(A)及び多数の活物質粒子(B)を含み、
上記導電部材(A)の少なくとも一部は、上記第1主面から上記第2主面までを電気的に接続する導電通路を形成しており、上記導電通路は、上記導電通路の周囲の上記活物質粒子(B)と接していることを特徴とする。
まず、本発明のリチウムイオン電池用電極のうち、導電部材(A)が不織布の一部を構成する導電性繊維である例について図面を使用して説明する。
図1に示すリチウムイオン電池1は、正極10と負極20を備えており、正極10と負極20の間にはセパレータ30が設けられている。
正極10のセパレータ30と反対の面には集電体40が、負極20のセパレータ30と反対の面には集電体50が設けられている。上記構造をまとめると、集電体40-正極10-セパレータ30-負極20-集電体50の順に積層構造が形成され、全体としてリチウムイオン電池1となっている。
正極10は所定の厚さt1を有するシート状の電極であり、セパレータ30側に配置される第1主面11及び集電体40側に配置される第2主面12を備えている。正極10には正極活物質粒子14が含まれている。
同様に、負極20も所定の厚さt2を有するシート状の電極であり、セパレータ30側に配置される第1主面21及び集電体50側に配置される第2主面22を備えている。負極20には負極活物質粒子24が含まれている。
正極10の厚さt1及び負極20の厚さt2は、それぞれ150~5000μmであり、このように電極が厚いと、電池内に多くの活物質を含ませることができ、リチウムイオン電池を高容量化することができる。
本発明のリチウムイオン電池用正極の厚さt1は好ましくは150~1500μmであり、より好ましくは200~950μmであり、さらに好ましくは250~900μmである。
本発明のリチウムイオン電池用負極の厚さt2は好ましくは150~1500μmであり、より好ましくは200~950μmであり、さらに好ましくは250~900μmである。
このような、本発明のリチウムイオン電池用電極を負極及び/又は正極に用いたリチウムイオン電池は本発明のリチウムイオン電池である。
図2は、図1に示すリチウムイオン電池の正極のみを模式的に示す断面図である。
正極10は、上述したように第1主面11と第2主面12を備えている。そして、第1主面11と第2主面12の間には、導電部材(A)としての導電性繊維13及び活物質粒子(B)としての正極活物質粒子14が含まれている。
また、第1主面11と第2主面12の間には多数の導電性繊維13が絡み合って存在しているが、複数本の導電性繊維13が接触していて第1主面11から第2主面12までを連続的に繋いでいる場合も、導電性繊維が第1主面から第2主面までを電気的に接続する導電通路を形成しているといえる。
図2には、第1主面11から第2主面12までを電気的に接続している導電通路に相当する導電性繊維13の例を示している。導電性繊維13aとして示す繊維は1本の導電性繊維が導電通路となっている例であり、導電性繊維13bとして示す2本の繊維は2本の導電性繊維が接触して導電通路となっている例である。
導電性繊維の電気伝導度が50mS/cm以上であると、導電性繊維により第1主面から第2主面までを電気的に接続する導電通路を形成させた際の抵抗が小さく、集電体からの距離が遠い活物質からの電子の移動がよりスムーズに行われるため好ましい。
導電性繊維の繊維径は、SEM観察し測定する。導電性繊維の平均繊維径は30μm角視野中に存在する任意の繊維10本についてそれぞれ中央付近の直径を測定し、この測定を三視野について行い、合計30本の繊維の径の平均値をもって測定値とする。
導電性繊維の繊維長の電極の単位体積あたりの合計は、以下の式より導かれる。
(導電性繊維の繊維長の電極の単位体積あたりの合計)=
[(導電性繊維の平均繊維長)×(電極の単位面積あたりに使用した導電性繊維の重量)/(導電性繊維の比重)]/[(電極の単位面積)×(電極厚さ)]
なお、導電性繊維の平均繊維長は、SEM観察し測定する。30μm角視野中に存在する任意の繊維10本についてその長さを測定し、この測定を三視野について行い、合計30本の繊維の長さの平均値をもって導電性繊維の平均繊維長の測定値とする。
図2に示す形態では、正極活物質粒子14の周囲が被覆剤15で被覆されている。被覆剤は被覆用樹脂を含んでおり、正極活物質粒子の周囲が被覆剤で被覆されていると、電極の体積変化が緩和され、電極の膨脹を抑制することができる。被覆用樹脂の例としては、ビニル樹脂、ウレタン樹脂、ポリエステル樹脂、ポリアミド樹脂、エポキシ樹脂、ポリイミド樹脂、シリコーン樹脂、フェノール樹脂、メラミン樹脂、ユリア樹脂、アニリン樹脂、アイオノマー樹脂、ポリカーボネート等が挙げられる。これらの中ではビニル樹脂、ウレタン樹脂、ポリエステル樹脂又はポリアミド樹脂が好ましい。
なお、活物質粒子が被覆活物質粒子である場合、被覆剤と導電通路が接している場合も、導電通路が活物質粒子と接しているとみなすことができる。
導電助剤としては、導電性を有する材料から選択される。
具体的には、金属[アルミニウム、ステンレス(SUS)、銀、金、銅及びチタン等]、カーボン[グラファイト及びカーボンブラック(アセチレンブラック、ケッチェンブラック、ファーネスブラック、チャンネルブラック、サーマルランプブラック等)等]、及びこれらの混合物等が挙げられるが、これらに限定されるわけではない。
これらの導電助剤は1種単独で用いられてもよいし、2種以上併用してもよい。また、これらの合金又は金属酸化物が用いられてもよい。電気的安定性の観点から、好ましくはアルミニウム、ステンレス、カーボン、銀、金、銅、チタン及びこれらの混合物であり、より好ましくは銀、金、アルミニウム、ステンレス及びカーボンであり、さらに好ましくはカーボンである。またこれらの導電助剤とは、粒子系セラミック材料や樹脂材料の周りに導電性材料(上記した導電助剤の材料のうち金属のもの)をめっき等でコーティングしたものでもよい。
また、導電助剤16は、被覆剤15の中に含まれていてもよく、導電助剤16が正極活物質粒子14と接していてもよい。導電助剤が被覆剤の中に含まれていたり、正極活物質粒子と接していたりすると、正極活物質粒子から導電通路に達するまでの電子伝導性をさらに高めることができる。
負極活物質粒子としては、黒鉛、難黒鉛化性炭素、アモルファス炭素、高分子化合物焼成体(例えばフェノール樹脂及びフラン樹脂等を焼成し炭素化したもの等)、コークス類(例えばピッチコークス、ニードルコークス及び石油コークス等)、炭素繊維、導電性高分子(例えばポリアセチレン及びポリピロール等)、スズ、シリコン、及び金属合金(例えばリチウム-スズ合金、リチウム-シリコン合金、リチウム-アルミニウム合金及びリチウム-アルミニウム-マンガン合金等)、リチウムと遷移金属との複合酸化物(例えばLi4Ti5O12等)等が挙げられる。
負極においても、導電通路が導電通路の周囲の負極活物質粒子と接しているので、正極の場合と同様に、負極活物質粒子から発生した電子がすぐに導電通路に達し、導電通路を流れてスムーズに集電体にまで達する。また、集電体から負極活物質粒子に向かって流れる電子もスムーズに負極活物質にまで達することができる。
図3に示す形態の電極(正極)110では、導電部材(A)が織物の一部を構成する導電性繊維113である。織物は導電性繊維からなる縦糸113a及び横糸113bから構成されている。図3に示す形態の電極(正極)110は、図2における不織布に対応する布状の繊維構造が織物であるほかは、図2に示す正極と同様の構造を有している。
織物の織り方は特に限定されるものではなく、平織り、綾織り、朱子織り、パイル織り等で織られた織物が使用可能である。
また、織物に代えて導電性繊維からなる編物を用いてもよい。
編物の編み方は特に限定されるものではなく、横編、縦編、丸編等で編まれた編物が使用可能である。
好ましい導電性繊維の種類、活物質の種類等のその他の構成は図2に示すリチウムイオン電池用電極の形態と同様であるため、その詳細な説明を省略する。
また、活物質粒子(B)を負極活物質粒子とすることによって本発明のリチウムイオン電池用負極とすることもできる。
図4に示す形態の電極(正極)210では、導電部材(A)は第1主面211と第2主面212の間に離散して存在する導電性繊維213である。
導電性繊維213は、図2及び図3に示した不織布、織物又は編物のような導電性繊維からなる構造体の一部ではない。図4に示す形態の電極の製造方法については後で詳しく説明するが、この電極は導電性繊維と活物質粒子を含むスラリーを用いて製造されており、活物質粒子中に導電性繊維が離散して存在する形態といえ、繊維間の空隙に活物質粒子が充填されたというべきものではない。
図4において導電性繊維213aとして示す繊維は1本の導電性繊維が導電通路となっている例であり、導電性繊維213bとして示す2本の繊維は2本の導電性繊維が接触して導電通路となっている例である。
好ましい導電性繊維の種類、活物質の種類等のその他の構成は図2に示すリチウムイオン電池用電極の形態と同様であるため、その詳細な説明を省略する。また、活物質粒子(B)を負極活物質粒子とすることによって本発明のリチウムイオン電池用負極とすることもできる。
図5は、本発明のリチウムイオン電池用電極の別の形態の例を模式的に示す断面図である。図5に示す形態の電極(正極)210´は、導電部材(A)としての導電性繊維213と活物質粒子(B)としての正極活物質粒子14が樹脂214によって固定されている点で異なるが、その他は図4に示す形態の電極210と同様の構成である。
図6に示す形態の電極(正極)310では、導電部材(A)は発泡樹脂の一部を構成する導電化された樹脂313である。発泡樹脂には多くの空隙が存在するため、その空隙に活物質粒子を充填させることによってリチウムイオン電池用電極を形成させることができる。
樹脂の表面に導体の薄膜を形成する方法としては、金属めっき処理、蒸着処理、スパッタリング処理等が挙げられる。
特に、ポリウレタンフォームの表面をニッケル等の金属でめっき処理してなる発泡樹脂であることが好ましい。
発泡樹脂の電気伝導度は、四端子法によって求められる。
導電化処理された樹脂を含む発泡樹脂の電気伝導度が100mS/cm以上であると、導電性繊維により第1主面から第2主面までを電気的に接続する導電通路を形成させた際の電気抵抗が小さく、集電体からの距離が遠い活物質からの電子の移動がよりスムーズに行われるため好ましい。
なお、本明細書における「多数の活物質粒子」とは、電極中に存在する活物質の数を具体的に規定することを意味する文言ではなく、活物質粒子が第1主面と第2主面の間の空隙を充填することのできる数だけ存在していることを意味している。
また、本発明のリチウムイオン電池用電極においては、電極の体積を基準として、活物質粒子(B)の占める体積の割合が30~80vol%であることが好ましく、45~60vol%であることがより好ましい。活物質粒子(B)の割合が多くなることによって、高容量のリチウムイオン電池用電極となる。
また、本発明のリチウムイオン電池用電極においては、導電部材(A)の占める体積VAの活物質粒子(B)の占める体積VBに対する比率(VA/VB)が0.00125~0.5であることが好ましく、0.03~0.35であることがより好ましい。
導電部材(A)の占める体積が少なく大部分が活物質粒子(B)であることによって、高容量のリチウムイオン電池用電極となる。
なお、導電部材(A)の体積及び活物質粒子(B)の体積は、以下の方法により測定する。
電解液等を乾燥させ導電部材(A)及び活物質粒子(B)が混合した電極1cm2当たりの重量[w(g)]並びに電極の膜厚[t(cm)]を測定し、導電部材(A)の真比重[dA(g/cm3)]、活物質粒子(B)の真比重[dB(g/cm3)]並びに導電部材(A)及び活物質粒子(B)の本発明の電極を構成する材料の合計重量に対する仕込み割合(WA、WB)から算出する。
VA=(w×WA/dA)/(t×1)×100
VB=(w×WB/dB)/(t×1)×100
本発明のリチウムイオン電池用電極の製造方法の一の態様は、本発明のリチウムイオン電池用電極の製造方法であって、上記導電部材(A)を含み、その中に空隙を有し、第1主面と第2主面を備えた構造体(Z)を準備する工程(P1)と、上記活物質粒子(B)を含むスラリー(X)を、上記構造体(Z)の上記第1主面又は上記第2主面に塗布する工程(P2)と、加圧又は減圧して上記活物質粒子(B)を上記構造体(Z)中の上記空隙に充填する工程(P3)とを含むことを特徴とする。
構造体(Z)は多数の空隙を有している。本明細書における空隙とは、構造体を構成する材料(導電性繊維や導電化処理された樹脂)が周囲に存在しており開放部分を有する空間を意味する。空隙の境界は明確ではなく、空隙同士は繋がっている。そのため、「多数の空隙」とは、構造体(Z)に存在する空隙の数を数えることにより空隙の数を規定することを意味する文言ではなく、構造体(Z)内に活物質粒子が充填されるための空隙が占める体積が大きく、多数の活物質粒子を充填し得る空間が存在していることを意味している。
構造体(Z)としては、導電性繊維からなる導電部材(A)を含む不織布、導電性繊維からなる導電部材(A)を含む織物若しくは編物、又は、導電化処理された樹脂からなる導電部材(A)を含む発泡樹脂を用いることが好ましい。不織布、織物、編物及び発泡樹脂の詳細については本発明のリチウムイオン電池用電極の頁で説明したものと同様であるのでその詳細な説明を省略する。
活物質粒子(B)としては、本発明のリチウムイオン電池用電極の説明で説明したものを用いることができ、被覆活物質粒子を好適に用いることができる。被覆活物質粒子は、例えば、リチウムイオン電池活物質粒子を万能混合機に入れて30~500rpmで撹拌した状態で、リチウムイオン電池活物質被覆用樹脂を含む樹脂溶液を1~90分かけて滴下混合し、さらに導電助剤を混合し、撹拌したまま50~200℃に昇温し、0.007~0.04MPaまで減圧した後に10~150分保持することにより得ることができる。
活物質粒子(B)を含むスラリーは、溶剤(C)を含む溶剤スラリー(X1)であるか、電解液(D)を含む電解液スラリー(X2)であることが好ましい。
溶剤(C)としては、水、1-メチル-2-ピロリドン(N-メチルピロリドン)、メチルエチルケトン、ジメチルホルムアミド、ジメチルアセトアミド、N,N-ジメチルアミノプロピルアミン及びテトラヒドロフラン等が挙げられる。
また、電解液(D)としては、リチウムイオン電池の製造に用いられる、電解質及び非水溶媒を含有する電解液を使用することができる。
電解質としては、通常の電解液に用いられているもの等が使用でき、例えば、LiPF6、LiBF4、LiSbF6、LiAsF6及びLiClO4等の無機酸のリチウム塩、LiN(CF3SO2)2、LiN(C2F5SO2)2及びLiC(CF3SO2)3等の有機酸のリチウム塩等が挙げられる。これらの内、電池出力及び充放電サイクル特性の観点から好ましいのはLiPF6である。
非水溶媒は1種を単独で用いてもよいし、2種以上を併用してもよい。
導電助剤としては本発明のリチウムイオン電池用電極の説明で説明したものを用いることができる。
結着剤としてはデンプン、ポリフッ化ビニリデン、ポリビニルアルコール、カルボキシメチルセルロース、ポリビニルピロリドン、テトラフルオロエチレン、スチレン-ブタジエンゴム、ポリエチレン及びポリプロピレン等の高分子化合物が挙げられる。
図7(a)には構造体としての不織布の第2主面上にスラリーを塗布した様子を模式的に示しており、不織布60の第2主面62に、正極活物質粒子14を含むスラリーが塗布されている。
加圧操作の方法としては、スラリーの塗布面の上からプレス機を用いてプレスする方法が挙げられる。また、減圧操作の方法としては、構造体にスラリーが塗布されていない側の面に濾紙やメッシュ等を当てて、真空ポンプにより吸引する方法が挙げられる。
構造体(Z)には空隙があるため、加圧又は減圧操作により活物質粒子(B)を構造体(X)中の空隙に充填することができる。
図7(a)にはスラリーの塗布面の上から加圧する向きを示す矢印、及び、濾紙70の下から減圧する向きを示す矢印を示している。また、図7(b)には活物質粒子(B)を構造体(Z)中の空隙に充填されてなるリチウムイオン電池用電極10を示している。図7(b)に示すリチウムイオン電池用電極は図2に示すリチウムイオン電池用電極10と同様である。
また、溶剤スラリーを用いる場合と異なり、リチウムイオン電池用電極内の液体成分として電解液以外の不純物が混入しない点でも好ましい。
加圧又は減圧して、上記活物質粒子(B)と上記導電部材(A)を上記膜(E)上に定着する工程(Q2)とを含むことを特徴とする。
スラリー(Y)としては、上述したスラリー(X)にさらに導電部材(A)としての導電性繊維を加えてスラリー中に導電性繊維を分散させたものが挙げられる。
導電性繊維としては、本発明のリチウムイオン電池用電極の頁で説明した導電性繊維を用いることができるが、導電性繊維の形状は、繊維の1本1本が独立した形状となっていることが好ましく、不織布、織物、編物といった立体構造を有していないことが好ましい。導電性繊維の1本1本が独立していると、スラリー中で分散された状態となる。
このような特性を有する材料の例としては、炭素繊維等の導電性繊維を配合した濾紙、金属メッシュ等が挙げられる。
金属メッシュとしては、ステンレス製メッシュを用いることが好ましく、例えばSUS316製の綾畳織金網(サンネット工業製)等が挙げられる。金属メッシュの目開きは、活物質粒子及び導電部材が通過しない程度とすることが好ましく、例えば2300メッシュのものを用いることが好ましい。
図8(a)には膜上にスラリーを塗布した様子を模式的に示しており、膜としての濾紙470上に、活物質粒子14と導電性繊維213を含むスラリーが塗布されている。
加圧操作、減圧操作の方法としては、上述した工程(P3)と同様の方法を用いることができ、加圧又は減圧によりスラリー(Y)から電解液又は溶剤が除去されて、導電部材(A)としての導電性繊維と活物質粒子(B)が膜(E)の上に定着されて、流動しない程度に緩くその形状が維持された状態となる。
図8(b)には、導電部材(A)としての導電性繊維213と活物質粒子14が濾紙470上で定着されてなる電極210を示している。
電極210において膜(E)が導電性材料からなるとき、膜(E)は集電体として使用することができ、また、集電体と膜(E)を接触させて一つの集電体として機能させることもできる。すなわち、電極210において第2主面212は導電部材(A)としての導電性繊維213が濾紙470と接触する部分として定めることができる。
膜(E)が導電性を有さない材料であるときは、膜(E)をセパレータ側に配置するようにするとよい。また、膜(E)をセパレータとしてもよい。導電性を有さない材料からなる膜の例としては、アラミドセパレータ(日本バイリーン株式会社製)等が挙げられる。
プレス工程(Q3)は、工程(Q2)における加圧又は減圧よりも、さらに圧力差を大きくして活物質粒子(B)の密度を向上させる工程である。プレス工程(Q3)は、工程(Q2)が減圧である場合に加圧を加えるという態様と、工程(Q2)が加圧である場合に加圧する圧力をさらに高くするという態様の両方を含む概念である。
上記スラリー層の上にセパレータを載置して、セパレータの上面側から吸液して、上記活物質粒子(B)と上記導電部材(A)を上記集電体と上記セパレータの間に定着する工程(T2)とを含むことを特徴とする。
集電体としては、アルミ、銅、アルミニウム、チタン、ステンレス鋼、ニッケル、焼成炭素、導電性高分子及び導電性ガラス等が挙げられる。
スラリー(Y)としては、図8を用いて説明したスラリー(Y)と同様のスラリーを用いることができ、スラリー(X)にさらに導電部材(A)としての導電性繊維を加えてスラリー中に導電性繊維を分散させたものが挙げられる。
スラリー(Y)は、電解液(D)を含む電解液スラリー(Y1)であることが好ましい。電解液(D)としては上述した電解液スラリー(X2)における電解液(D)と同様のものを用いることができる。また、スラリー(Y)は溶剤(C)を含む溶剤スラリーであってもよい。
図9(a)には集電体50上にスラリーを塗布してスラリー層225を形成した様子を模式的に示しており、集電体50上に、負極活物質粒子24と導電性繊維223を含むスラリーが塗布されており、スラリー層225が形成されている。
図9(a)に示す形態では、負極活物質粒子24の周囲が被覆剤25で被覆されており、スラリーには導電助剤26が含まれている。
導電性繊維223、被覆剤25及び導電助剤26については本発明のリチウムイオン電池用電極(正極)の説明でその詳細を説明した導電性繊維213、被覆剤15、導電錠剤16とそれぞれ同様である。
また、負極活物質粒子24も、本発明のリチウムイオン電池用電極の説明でその詳細を説明した負極活物質粒子と同様である。
セパレータとしては、アラミドセパレータ(日本バイリーン株式会社製)、ポリエチレン、ポリプロピレン製フィルムの微多孔膜、多孔性のポリエチレンフィルムとポリプロピレンとの多層フィルム、ポリエステル繊維、アラミド繊維、ガラス繊維等からなる不織布、及びそれらの表面にシリカ、アルミナ、チタニア等のセラミック微粒子を付着させたもの等が挙げられる。
吸液性材料としては、タオル等の吸液性布、紙、吸液性樹脂等を使用することができる。
吸液によりスラリー(Y)から電解液又は溶剤が除去されて、導電部材(A)としての導電性繊維と活物質粒子(B)が集電体とセパレータの間に定着されて、流動しない程度に緩くその形状が維持された状態となる。
加圧の方法は特に限定されないが、種々の方法で実施できる。たとえば、公知のプレス機を用いる方法及び重量物等を重りとして載置して加圧する方法が挙げられ、加圧は超音波振動機等で加振しながら行っても良い。セパレータの上面側又は下面側から加圧する場合の圧力は、0.8~41kg/cm2が好ましく、0.9~10kg/cm2がより好ましい。圧力がこの範囲にあると電極内部の導電通路を良好に形成することができるので電池をより高容量化でき好ましい。
電極220においては、電極の第1主面221がセパレータ30と接しており、電極の第2主面222が集電体50と接している。
このようなリチウムイオン電池用電極の製造方法であると、電極がセパレータと集電体で挟まれた状態で製造される。そのため、電極の両側にセパレータと集電体を配置する工程を別途行う必要がなく、双極型電極として好ましい形態の電極が少ない工程で得られるため好ましい。
上記導電部材(A)、上記活物質粒子(B)及び上記樹脂(F)を含む電極用組成物を、加熱プレスすることにより、上記樹脂(F)によって上記導電部材(A)及び上記活物質粒子(B)を固定する工程(R1)を含むことを特徴とする。
導電部材(A)としては、図8(a)及び図8(b)を用いて説明した態様のリチウムイオン電池用電極の製造方法で好ましく用いられる導電部材(A)と同様に、繊維の1本1本が独立した形状の導電性繊維を用いることが好ましい。
活物質粒子(B)としては、他の態様のリチウムイオン電池用電極の製造方法で用いる活物質粒子(B)と同様のものを用いることができる。
電極用組成物中において、樹脂(F)は溶剤に溶解された樹脂溶液の形態で存在していてもよいし、加熱によって流動化するペレット等、固体の形で存在していてもよい。
また、活物質粒子(B)が被覆活物質粒子である場合には、被覆剤に含まれる被覆用樹脂が樹脂(F)であってもよい。
加熱プレスの方法は特に限定されるものではないが、図10(a)に示すように金属板等の板570に、正極活物質粒子14、導電性繊維213、樹脂214を含む電極用組成物を塗布し、上面から加熱プレスする方法が挙げられる。
電極用組成物の塗布は、バーコーター、刷毛等の任意の塗工装置を用いて行うことができる。また、加熱プレスは通常の加熱プレス装置を用いて行うことができる。
また、樹脂(F)が被覆活物質粒子の被覆用樹脂である場合、導電部材(A)と被覆活物質粒子を板に塗布して加熱プレスすると、加熱によって溶融した被覆用樹脂により導電部材と(被覆)活物質粒子が固定される。
被覆用樹脂により固定される活物質粒子は、被覆用樹脂で被覆されたままの被覆活物質粒子であってもよく、被覆が剥がれた活物質粒子であってもよい。
ビニル樹脂の場合、80~180℃、0.01~5MPa、5~300秒の条件で加熱プレスすることが好ましい。
加熱プレスにより、図10(b)に示すように、導電性繊維213と正極活物質粒子14が樹脂214で固定されてなる電極210´を製造することができる。
また、集電体の一方の面に正極を形成し、もう一方の面に負極を形成して双極型電極を作製し、双極型電極をセパレータと積層してセル容器に収納し、電解液を注入し、セル容器を密封することでも得られる。
また、正極、負極のいずれか一方に本発明のリチウムイオン電池用電極を用いてもよく、正極、負極を共に本発明のリチウムイオン電池用電極としてリチウムイオン電池としてもよい。
撹拌機、温度計、還流冷却管、滴下ロート及び窒素ガス導入管を付した4つ口フラスコに、酢酸エチル83部とメタノール17部とを仕込み68℃に昇温した。次いで、メタクリル酸242.8部、メチルメタクリレート97.1部、2-エチルヘキシルメタクリレート242.8部、酢酸エチル52.1部及びメタノール10.7部を配合したモノマー配合液と、2,2’-アゾビス(2,4-ジメチルバレロニトリル)0.263部を酢酸エチル34.2部に溶解した開始剤溶液とを4つ口フラスコ内に窒素を吹き込みながら、撹拌下、滴下ロートで4時間かけて連続的に滴下してラジカル重合を行った。滴下終了後、2,2’-アゾビス(2,4-ジメチルバレロニトリル)0.583部を酢酸エチル26部に溶解した開始剤溶液を滴下ロートを用いて2時間かけて連続的に追加した。さらに、沸点で重合を4時間継続した。溶媒を除去し、樹脂582部を得た後、イソプロパノールを1,360部加えて、樹脂濃度30重量%のビニル樹脂からなる被覆用樹脂溶液を得た。
LiCoO2粉末[日本化学工業(株)製 セルシードC-5H]100重量部、水100重量部とΦ3mmアルミナボール1200重量部をポットミル容器に入れ、20分間粉砕し、平均粒子径2.3μmのLiCoO2粉末100重量部を得た。
難黒鉛化性炭素[(株)クレハ・バッテリー・マテリアルズ・ジャパン製 カーボトロン(登録商標)PS(F)]100重量部、水200重量部とΦ0.1mmジルコニアボール1000重量部をポットミル容器に入れ、15分間粉砕し、平均粒子径2.5μmの難黒鉛化性炭素100重量部を得た。
LiCoO2粉末[日本化学工業(株)製 セルシードC-8G]96重量部を万能混合機に入れ、室温、150rpmで撹拌した状態で、被覆用樹脂溶液(樹脂固形分濃度30重量%)を樹脂固形分として2重量部になるように60分かけて滴下混合し、さらに30分撹拌した。
次いで、撹拌した状態でアセチレンブラック[電気化学工業(株)製 デンカブラック(登録商標)]2重量部を3回に分けて混合し、30分撹拌したままで70℃に昇温し、100mmHgまで減圧し30分保持した。上記操作により被覆正極活物質粒子(B-1)を得た。
LiCoO2粉末[日本化学工業(株)製 セルシードC-8G]96重量部を、上記した<正極活物質粒子の粉砕>で得た平均粒子径2.3μmのLiCoO2粉末に変更する以外は、(B-1)の作製方法と同様の操作をして、被覆正極活物質粒子(B-2)を得た。
難黒鉛化性炭素[(株)クレハ・バッテリー・マテリアルズ・ジャパン製 カーボトロン(登録商標)PS(F)]90重量部を万能混合機に入れ、室温、150rpmで撹拌した状態で、被覆用樹脂溶液(樹脂固形分濃度30重量%)を樹脂固形分として5重量部になるように60分かけて滴下混合し、さらに30分撹拌した。
次いで、撹拌した状態でアセチレンブラック[電気化学工業(株)製 デンカブラック(登録商標)]5重量部を3回に分けて混合し、30分撹拌したままで70℃に昇温し、0.01MPaまで減圧し30分保持した。上記操作により被覆負極活物質粒子(B-3)を得た。
難黒鉛化性炭素[(株)クレハ・バッテリー・マテリアルズ・ジャパン製 カーボトロン(登録商標)PS(F)]90重量部を、上記した<負極活物質粒子の粉砕>で得た平均粒子径2.5μmの難黒鉛化性炭素90重量部に変更する以外は、(B-3)の作製方法と同様の操作をして、被覆負極活物質粒子(B-4)を得た。
炭素繊維(C)は、Eiichi Yasuda,Asao Oya,Shinya Komura,Shigeki Tomonoh,Takashi Nishizawa,Shinsuke Nagata,Takashi Akatsu、CARBON、50、2012、1432-1434及びEiichi Yasuda,Takashi Akatsu,Yasuhiro Tanabe,Kazumasa Nakamura,Yasuto Hoshikawa,Naoya Miyajima、TANSO、255、2012、254~265頁の製造方法を参考にして製造した。
炭素前駆体として合成メソフェーズピッチAR・MPH[三菱ガス化学(株)製]10重量部とポリメチルペンテンTPX RT18[三井化学(株)製]90重量部を、バレル温度310℃、窒素雰囲気下で一軸押出機を用いて溶融混練し、樹脂組成物を調製した。
上記樹脂組成物を390℃で溶融押出し紡糸した。紡糸した樹脂組成物を電気炉に入れ、窒素雰囲気下270℃で3時間保持し炭素前駆体を安定化させた。ついで、電気炉を1時間かけて500℃まで昇温し、500℃で1時間保持し、ポリメチルペンテンを分解除去した。電気炉を2時間かけて1000℃まで昇温し1000℃で30分間保持し、残った安定化させた炭素前駆体を導電性繊維とした。
得られた導電性繊維90重量部、水500重量部とΦ0.1mmのジルコニアボール1000重量部をポットミル容器に入れ5分間粉砕した。ジルコニアボールを分級後、100℃で乾燥し、導電性の炭素繊維(C)を得た。
SEMでの測定結果より、平均繊維径は、0.9μm、平均繊維長は、25μmであった。
エチレンカーボネート(EC)とジエチルカーボネート(DEC)の混合溶媒(体積比率1:1)に、LiPF6を1mol/Lの割合で溶解させてリチウムイオン電池用電解液を作製した。
ニッケルメッキにより導電化処理されたウレタンフォーム[セーレン(株)製 Sui-70-5005:厚さ450μm:電気伝導度300mS/cm]を準備した。上記ウレタンフォームは導電部材(A)として導電化処理された樹脂を含み、多数の空隙を有し、第1主面と第2主面を備えた構造体(Z)である。
別途、正極活物質粒子としてのLiCoO2粉末[日本化学工業(株)製 セルシードC-8G]85.5重量部、アセチレンブラック[電気化学工業(株)製 デンカブラック(登録商標)]4.75重量部を、ポリフッ化ビニリデン(シグマアルドリッチ社製)4.75重量部を含むN-メチルピロリドン(以下、NMP)溶液と混合して溶剤スラリーを作製した。
上記ウレタンフォーム5重量部に対し、溶剤スラリー中のNMP以外の成分の重量が95重量部となる量の溶剤スラリーを、上記ウレタンフォームの一方の主面に塗布し、溶剤スラリーの塗布面の上から加圧圧力2.0kg/cm2で加圧して正極活物質粒子をウレタンフォーム内の空隙に充填した。その後、80℃、120分常圧乾燥して溶剤を留去して、その後80℃、8時間減圧乾燥してリチウムイオン電池用正極を作製した。
上記被覆正極活物質粒子(B-1)を上記電解液と混合して、電解液スラリーを作製した。
実施例1と同様のウレタンフォーム5重量部に対し、被覆正極活物質粒子の重量が95重量部となる量の電解液スラリーを、上記ウレタンフォームの一方の主面に塗布し、電解液スラリーの塗布面の上から加圧圧力1.5kg/cm2で加圧して被覆正極活物質粒子をウレタンフォーム内の空隙に充填してリチウムイオン電池用正極を作製した。
上記被覆正極活物質粒子(B-1)90重量部及びアセチレンブラック[電気化学工業(株)製 デンカブラック(登録商標)]5重量部を上記電解液と混合して、電解液スラリーを作製した。
実施例1と同様のウレタンフォーム5重量部に対し、電解液スラリー中の電解液以外の成分の重量が95重量部となる量の電解液スラリーを、上記ウレタンフォームの一方の主面に塗布し、電解液スラリーの塗布面の上から加圧圧力1.5kg/cm2で加圧して被覆正極活物質粒子をウレタンフォーム内の空隙に充填してリチウムイオン電池用正極を作製した。
炭素繊維製不織布[大阪ガスケミカル(株)製 ドナカーボ・ペーパー S-253:厚さ650μm:電気伝導度400mS/cm]を準備した。上記不織布は導電性繊維からなる導電部材(A)を含み、多数の空隙を有し、第1主面と第2主面を備えた構造体(Z)である。以下、上記不織布を不織布Aとする。
別途、LiCoO2粉末[日本化学工業(株)製 セルシードC-8G]88重量部及びアセチレンブラック[電気化学工業(株)製 デンカブラック(登録商標)]5重量部を、ポリフッ化ビニリデン(シグマアルドリッチ社製)5重量部を含むNMP溶液と混合して溶剤スラリー(C)を作製した。
上記不織布A2重量部に対し、溶剤スラリー中のNMP以外の成分の重量が98重量部となる量の溶剤スラリーを、上記不織布Aの一方の主面に塗布し、溶剤スラリーの塗布面の上から加圧圧力2.0kg/cm2で加圧して正極活物質粒子を不織布A内の空隙に充填した。その後、80℃、120分常圧乾燥して溶剤を留去して、その後80℃、8時間減圧乾燥してリチウムイオン電池用正極を作製した。
実施例2と同様の電解液スラリーを作製した。
実施例4と同様の不織布A2重量部に対し、被覆正極活物質粒子(B-1)の重量が98重量部となる量の電解液スラリーを、上記不織布Aの一方の主面に塗布し、電解液スラリーの塗布面の上から加圧圧力1.5kg/cm2で加圧して被覆正極活物質粒子を不織布A内の空隙に充填してリチウムイオン電池用正極を作製した。
炭素繊維製不織布[大阪ガスケミカル(株)製 ドナカーボ・ペーパー S-259P:厚さ500μm:電気伝導度500mS/cm]を準備した。上記不織布は導電性繊維からなる導電部材(A)を含み、多数の空隙を有し、第1主面と第2主面を備えた構造体(Z)である。以下、上記不織布を不織布Bとする。
実施例4において不織布Aを不織布Bに変更したほかは実施例4と同様にしてリチウムイオン電池用正極を作製した。
実施例5において不織布Aを不織布Bに変更したほかは実施例5と同様にしてリチウムイオン電池用正極を作製した。
炭素繊維[大阪ガスケミカル(株)製 ドナカーボ・チョップ S-231:平均繊維長3300μm、平均繊維径13μm:電気伝導度200mS/cm]を導電部材(A)として準備した。以下、上記炭素繊維を炭素繊維Aとする。
上記炭素繊維A1.75重量部及び被覆正極活物質粒子(B-1)98.25重量部を上記電解液と混合して、電解液スラリーを作製した。
膜(E)としてステンレス製メッシュ[サンネット工業(株)製 SUS316綾畳織2300メッシュ]を準備し、上記ステンレス製メッシュに電解液スラリーを塗布し、吸引濾過(減圧)することにより、被覆正極活物質粒子と炭素繊維をステンレス製メッシュ上に定着させてリチウムイオン電池用正極を作製した。
実施例8で作製したリチウムイオン電池用正極に対し、電解液スラリーをさらに加圧圧力1.5kg/cm2で加圧してリチウムイオン電池用正極を作製した。
炭素繊維[大阪ガスケミカル(株)製 ドナカーボ・ミルド S-243:平均繊維長500μm、平均繊維径13μm:電気伝導度200mS/cm]を導電部材(A)として準備した。以下、上記炭素繊維を炭素繊維Bとする。
上記炭素繊維B1.75重量部及び被覆正極活物質粒子(B-1)98.25重量部を上記電解液と混合して、電解液スラリーを作製した。
膜(E)として実施例8と同様のステンレス製メッシュを準備し、上記ステンレス製メッシュに電解液スラリーを塗布し、吸引濾過(減圧)するとともに加圧圧力1.5kg/cm2で加圧することにより、被覆正極活物質粒子と炭素繊維をステンレス製メッシュ上に定着させてリチウムイオン電池用正極を作製した。
実施例10において塗布する電解液スラリーの量を実施例10よりも少なくすることにより、作製する電極の厚さを薄くした他は実施例10と同様にしてリチウムイオン電池用正極を作製した。
実施例12において、ステンレス製メッシュ上に定着させた電極を剥離したリチウムイオン電池用正極を作製した。
上記炭素繊維B1.75重量部及び被覆正極活物質粒子(B-1)98.25重量部を乾式混合した混合粉末を作製し、金属板(鉄板)の上に散布し、アプリケーターを用いて混合粉末を均一にならした後、180℃、1.5MPa、1分加熱プレスすることにより炭素繊維及び(被覆)正極活物質粒子が被覆用樹脂で固定されてなるリチウムイオン電池用電極を作製した。
上記電極は鉄板から剥離したのちに放電容量の評価に用いた。
上記<炭素繊維(C)の作製>で製造した炭素繊維(C)(平均繊維長25μm、平均繊維径0.9μm:電気伝導度30mS/cm)を導電部材(A)として準備した。以下、上記炭素繊維を炭素繊維Cとする。
上記炭素繊維C1重量部及び被覆正極活物質粒子(B-2)99重量部を上記電解液と混合して、電解液スラリーを作製した。
膜(E)として実施例8と同様のステンレス製メッシュを準備し、上記ステンレス製メッシュに電解液スラリーを塗布し、吸引濾過(減圧)するとともに加圧圧力1.5kg/cm2で加圧することにより、被覆正極活物質粒子と炭素繊維をステンレス製メッシュ上に定着させた後、電極を剥離してリチウムイオン電池用正極を作製した。
実施例15における炭素繊維Cと被覆正極活物質粒子(B-2)の割合を表3に示すように変更し、塗布する電解液スラリーの量を変更して電極の厚さを調整した他は実施例15と同様にしてリチウムイオン電池用正極を作製した。
実施例15において、加圧圧力1.5kg/cm2で加圧する力を、実施例18は、4.0kg/cm2、実施例19は、35kg/cm2に変更した他は、実施例15と同様にしてリチウムイオン電池用正極を作製した。
実施例15における炭素繊維Cと被覆正極活物質粒子(B-2)を表3に示すように使用し、塗布する電解液スラリーの量を変更して電極の厚さを調整した他は、実施例15と同様にしてリチウムイオン電池用正極を作製した。
なお、リチウムイオン電池用正極の厚さには、実施例8~13及び15~23における膜(E)の厚さ及び実施例14における鉄板の厚さは含まない。
構造体(Z)として実施例1と同様のウレタンフォームを準備した。
別途、負極活物質粒子としての難黒鉛化性炭素[(株)クレハ・バッテリー・マテリアルズ・ジャパン製 カーボトロン(登録商標)PS(F)]80.75重量部を、ポリフッ化ビニリデン(シグマアルドリッチ社製)4.25重量部を含むNMP溶液と混合して溶剤スラリーを作製した。
上記ウレタンフォーム15重量部に対し、溶剤スラリー中のNMP以外の成分の重量が85重量部となる量の溶剤スラリーを、上記ウレタンフォームの一方の主面に塗布し、溶剤スラリーの塗布面の上から加圧圧力2.0kg/cm2で加圧して負極活物質粒子をウレタンフォーム内の空隙に充填した。その後、80℃、120分常圧乾燥して溶剤を留去して、その後80℃、8時間減圧乾燥してリチウムイオン電池用負極を作製した。
負極活物質粒子としての難黒鉛化性炭素[(株)クレハ・バッテリー・マテリアルズ・ジャパン製 カーボトロン(登録商標)PS(F)]76.5重量部、アセチレンブラック[電気化学工業(株)製 デンカブラック(登録商標)]4.25重量部を、ポリフッ化ビニリデン(シグマアルドリッチ社製)4.25重量部を含むNMP溶液と混合して溶剤スラリーを作製した。
上記ウレタンフォーム15重量部に対し、溶剤スラリー中のNMP以外の成分の重量が85重量部となる量の溶剤スラリーを、上記ウレタンフォームの一方の主面に塗布し、溶剤スラリーの塗布面の上から加圧圧力2.0kg/cm2で加圧して負極活物質粒子をウレタンフォーム内の空隙に充填した。その後、80℃、120分常圧乾燥して溶剤を留去して、その後80℃、8時間減圧乾燥してリチウムイオン電池用負極を作製した。
上記被覆負極活物質粒子(B-3)を上記電解液と混合して、電解液スラリーを作製した。
実施例24と同様のウレタンフォーム15重量部に対し、被覆負極活物質粒子の重量が85重量部となる量の電解液スラリーを、上記ウレタンフォームの一方の主面に塗布し、電解液スラリーの塗布面の上から加圧圧力1.5kg/cm2で加圧して被覆負極活物質粒子をウレタンフォーム内の空隙に充填してリチウムイオン電池用負極を作製した。
上記被覆負極活物質粒子(B-3)80重量部及びアセチレンブラック[電気化学工業(株)製 デンカブラック(登録商標)]5重量部を上記電解液と混合して、電解液スラリーを作製した。
実施例24と同様のウレタンフォーム5重量部に対し、電解液スラリー中の電解液以外の成分の重量が85重量部となる量の電解液スラリーを、上記ウレタンフォームの一方の主面に塗布し、電解液スラリーの塗布面の上から加圧圧力1.5kg/cm2で加圧して被覆負極活物質粒子をウレタンフォーム内の空隙に充填してリチウムイオン電池用負極を作製した。
導電部材(A)として実施例10と同様の炭素繊維Bを準備した。
上記炭素繊維B4.2重量部及び実施例24と同様の負極活物質粒子としての難黒鉛化性炭素95.8重量部を上記電解液と混合して、電解液スラリーを作製した。
膜(E)としてアラミドセパレータ(日本バイリーン株式会社製)を準備し、上記アラミドセパレータに電解液スラリーを塗布し、吸引濾過(減圧)するとともに加圧圧力1.5kg/cm2で加圧することにより、負極活物質粒子と炭素繊維をアラミドセパレータ上に定着させてリチウムイオン電池用負極を作製した。
実施例28において塗布する電解液スラリーの量を実施例28よりも少なくすることにより、作製する電極の厚さを薄くした他は実施例28と同様にしてリチウムイオン電池用負極を作製した。
実施例28において、負極活物質粒子としての難黒鉛化性炭素95.8重量部に代えて、上記被覆負極活物質粒子(B-3)95.8重量部を用いたほかは実施例28と同様にして、リチウムイオン電池用負極を作製した。
実施例31において塗布する電解液スラリーの量を実施例31よりも少なくすることにより、作製する電極の厚さを薄くした他は実施例31と同様にしてリチウムイオン電池用負極を作製した。
導電部材(A)として実施例15と同様の炭素繊維Cを準備した。
実施例28において、導電部材(A)として上記炭素繊維Bに代えて上記炭素繊維C4.2重量部を用い、負極活物質粒子としての難黒鉛化性炭素95.8重量部に代えて、上記被覆負極活物質粒子(B-3)95.8重量部を用いたほかは実施例28と同様にして、リチウムイオン電池用負極を作製した。
実施例34において、上記被覆負極活物質粒子(B-3)に代えて、上記被覆負極活物質粒子(B-4)を用い、炭素繊維Cと被覆負極活物質粒子(B-4)の割合を表5に示すように変更した他は実施例34と同様にしてリチウムイオン電池用負極を作製した。
実施例35における炭素繊維Cと被覆負極活物質粒子(B-4)を表5に示すように使用し、塗布する電解液スラリーの量を変更して電極の厚さを調整した他は、実施例35と同様にしてリチウムイオン電池用負極を作製した。
実施例35において、作製した電解液スラリーを塗布する部材をアラミドセパレータから集電体である厚さ20μmの銅箔に変更し、銅箔に電解液スラリーを塗布した後、アラミドセパレータを載置してセパレータの上面から1.5kg/cm2で加圧することでセパレーターの上面から浸みだした液を吸液して、集電体を含むリチウムイオン電池用負極を作製した。
正極活物質粒子としてのLiCoO2粉末[日本化学工業(株)製 セルシードC-8G]90重量部、アセチレンブラック[電気化学工業(株)製 デンカブラック(登録商標)]5重量部を、ポリフッ化ビニリデン(シグマアルドリッチ社製)5重量部を含むNMP溶液と混合して溶剤スラリーを作製した。
上記溶剤スラリーを、大気中でワイヤーバーを用いて厚さ20μmのアルミニウム電解箔上の片面に塗布し、100℃で15分間乾燥させて比較例1のリチウムイオン電池用正極を作製した。
負極活物質粒子としての難黒鉛化性炭素[(株)クレハ・バッテリー・マテリアルズ・ジャパン製 カーボトロン(登録商標)PS(F)]95重量部を、ポリフッ化ビニリデン(シグマアルドリッチ社製)5重量部を含むNMP溶液と混合して溶剤スラリーを作製した。
上記溶剤スラリーを、大気中でワイヤーバーを用いて厚さ20μmの銅箔上の片面に塗布し、常圧で80℃/3時間乾燥後、真空乾燥を80℃/8時間行って溶媒を蒸発させて比較例2のリチウムイオン電池用負極を作製した。
実施例1~23及び比較例1のいずれかで作製した正極を、17mmφに打ち抜き、17mmφのLi金属からなる負極と共に2032型コインセル内の両端に配置した。
正極側の集電体としては厚さ20μmのアルミニウム電解箔を用い、ステンレス製メッシュ上に定着させた実施例8~12の正極においては、ステンレス製メッシュを集電体側に配置した。
電極間にセパレータ(セルガード3501)を2枚挿入し、リチウムイオン電池用セルを作製した。セルに上記電解液を注液密封し、以下の方法で放電容量(mAh)を測定し、活物質の重量で除して活物質の重量当たりの放電容量(mAh/g)として評価した。
実施例24~41及び比較例2のいずれかで作製した負極を、17mmφに打ち抜き、17mmφのLi金属からなる正極と共に2032型コインセル内の両端に配置した。
負極側の集電体としては厚さ20μmの銅箔を用い、アラミドセパレータを用いた実施例28~40の負極においては、アラミドセパレータをセパレータ側(正極側)に配置した。
また、実施例41の負極は集電体及びセパレータと一体化しているので、集電体としての銅箔を別途使用せず、アラミドセパレータをセパレータ側(正極側)に配置した。
電極間にセパレータ(セルガード3501)を2枚挿入し、リチウムイオン電池用セルを作製した。セルに上記電解液を注液密封し、以下の方法で放電容量(mAh)を測定し、活物質の重量で除して活物質の重量当たりの放電容量(mAh/g)として評価した。
室温下、充放電測定装置「バッテリーアナライザー1470型」[東陽テクニカ(株)製]を用いて、0.1C、0.2C、0.5C又は1.0Cの電流で負極評価用の場合電圧2.5Vまで、正極評価用の場合4.3Vまで充電し、10分間の休止後、0.1C、0.2C、0.5C又は1.0Cの電流で負極評価用の場合電圧10mVまで、正極評価用の場合2.7Vまで放電して電池容量を測定した。
各実施例及び各比較例における活物質の重量当たりの放電容量(mAh/g)を表1~6に示した。
リチウムイオン電池用電極(正極) 10、110、210、210´、310
正極の第1主面 11、111、211、311
正極の第2主面 12、112、212、312
不織布の一部を構成する導電性繊維 13、13a、13b
正極活物質粒子 14
被覆剤 15、25
導電助剤 16、26
リチウムイオン電池用電極(負極) 20、220
負極の第1主面 21、221
負極の第2主面 22、222
負極活物質粒子 24
セパレータ 30
集電体 40、50
不織布(構造体) 60
不織布の第2主面 62
濾紙 70、470
織物の一部を構成する導電性繊維 113
縦糸 113a
横糸 113b
第1主面と第2主面の間に離散して存在する導電性繊維 213、213a、213b、223
樹脂 214
スラリー層 225
導電化された樹脂 313
板 570
Claims (24)
- リチウムイオン電池のセパレータ側に配置される第1主面と、集電体側に配置される第2主面とを備えたリチウムイオン電池用電極であって、
前記電極の厚さは150~5000μmであり、
前記第1主面と前記第2主面の間に、電子伝導性材料からなる導電部材(A)及び多数の活物質粒子(B)を含み、
前記導電部材(A)の少なくとも一部は、前記第1主面から前記第2主面までを電気的に接続する導電通路を形成しており、前記導電通路は、前記導電通路の周囲の前記活物質粒子(B)と接していることを特徴とするリチウムイオン電池用電極。 - 前記導電部材(A)は、不織布の一部を構成する導電性繊維、織物若しくは編物の一部を構成する導電性繊維、又は、前記第1主面と前記第2主面の間に離散して存在する導電性繊維であり、
前記導電性繊維の電気伝導度は50mS/cm以上である請求項1に記載のリチウムイオン電池用電極。 - 前記導電部材(A)である導電性繊維の平均繊維径が0.1~20μmである請求項1又は2に記載のリチウムイオン電池用電極。
- 前記導電部材(A)である導電性繊維の繊維長の電極の単位体積あたりの合計が10,000~50,000,000cm/cm3である請求項1~3のいずれかに記載のリチウムイオン電池用電極。
- 前記導電部材(A)は、発泡樹脂の一部を構成する導電化処理された樹脂であり、
前記導電化処理された樹脂を含む発泡樹脂の電気伝導度は100mS/cm以上である請求項1に記載のリチウムイオン電池用電極。 - 前記電極の体積を基準として、前記導電部材(A)の占める体積の割合が0.1~15vol%である請求項1~5のいずれかに記載のリチウムイオン電池用電極。
- 前記電極の体積を基準として、前記活物質粒子(B)の占める体積の割合が30~80vol%である請求項1~6のいずれかに記載のリチウムイオン電池用電極。
- 前記電極中において、前記導電部材(A)の占める体積VAの前記活物質粒子(B)の占める体積VBに対する比率(VA/VB)が0.00125~0.5である請求項1~7のいずれかに記載のリチウムイオン電池用電極。
- 前記活物質粒子(B)が、表面の少なくとも一部が被覆用樹脂及び導電助剤を含む被覆剤で被覆されてなる被覆活物質粒子である請求項1~8のいずれかに記載のリチウムイオン電池用電極。
- 請求項1~9のいずれかに記載のリチウムイオン電池用電極を負極及び/又は正極に用いたリチウムイオン電池。
- 請求項1~9のいずれかに記載のリチウムイオン電池用電極の製造方法であって、
前記導電部材(A)を含み、その中に空隙を有し、第1主面と第2主面を備えた構造体(Z)を準備する工程(P1)と、
前記活物質粒子(B)を含むスラリー(X)を、前記構造体(Z)の前記第1主面又は前記第2主面に塗布する工程(P2)と、
加圧又は減圧して前記活物質粒子(B)を前記構造体(Z)中の前記空隙に充填する工程(P3)とを含むことを特徴とするリチウムイオン電池用電極の製造方法。 - 前記スラリー(X)は、溶剤(C)を含む溶剤スラリー(X1)であり、前記工程(P3)の後に、溶剤(C)を留去する工程(P4)を含む請求項11に記載のリチウムイオン電池用電極の製造方法。
- 前記スラリー(X)は、電解液(D)を含む電解液スラリー(X2)であり、
前記工程(P2)において、前記活物質粒子(B)及び前記電解液(D)を前記空隙に充填する請求項11に記載のリチウムイオン電池用電極の製造方法。 - 前記構造体(Z)は、導電性繊維からなる導電部材(A)を含む不織布、導電性繊維からなる導電部材(A)を含む織物若しくは編物、又は、導電化処理された樹脂からなる導電部材(A)を含む発泡樹脂である請求項11~13のいずれかに記載のリチウムイオン電池用電極の製造方法。
- 請求項1~9のいずれかに記載のリチウムイオン電池用電極の製造方法であって、
前記導電部材(A)及び前記活物質粒子(B)を含むスラリー(Y)を、膜(E)上に塗布する工程(Q1)と、
加圧又は減圧して、前記活物質粒子(B)と前記導電部材(A)を前記膜(E)上に定着する工程(Q2)とを含むことを特徴とするリチウムイオン電池用電極の製造方法。 - 前記スラリー(Y)は、電解液(D)を含む電解液スラリー(Y1)であり、
前記膜(E)が前記活物質粒子(B)を透過させず前記電解液(D)を透過させる膜であり、
前記工程(Q2)において、加圧又は減圧して前記電解液(D)を前記膜(E)を透過させて除去する請求項15に記載のリチウムイオン電池用電極の製造方法。 - 前記工程(Q2)の後、スラリー(Y)をさらに強い圧力で加圧するプレス工程(Q3)を行う請求項15又は16に記載のリチウムイオン電池用電極の製造方法。
- 前記膜(E)の電気伝導度は100mS/cm以上である請求項15~17のいずれかに記載のリチウムイオン電池用電極の製造方法。
- 前記膜(E)上に定着された前記リチウムイオン電池用電極を、集電体又はセパレータの主面に転写する工程(Q4)を行って、リチウムイオン電池用電極の第1主面がセパレータの主面に配置されたリチウムイオン電池用電極を形成する、又は、リチウムイオン電池用電極の第2主面が集電体の主面に配置されたリチウムイオン電池用電極を形成する、請求項15~17のいずれかに記載のリチウムイオン電池用電極の製造方法。
- 請求項1~9のいずれかに記載のリチウムイオン電池用電極の製造方法であって、
前記導電部材(A)及び前記活物質粒子(B)を含むスラリー(Y)を、集電体上に塗布して集電体上にスラリー層を形成する工程(T1)と、
前記スラリー層の上にセパレータを載置して、セパレータの上面側から吸液して、前記活物質粒子(B)と前記導電部材(A)を前記集電体と前記セパレータの間に定着する工程(T2)とを含むことを特徴とするリチウムイオン電池用電極の製造方法。 - 前記スラリー(Y)は、電解液(D)を含む電解液スラリー(Y1)である請求項20に記載のリチウムイオン電池用電極の製造方法。
- 前記セパレータの上面に吸液性材料を置いて前記セパレータの上面側からの吸液を行う請求項20又は21に記載のリチウムイオン電池用電極の製造方法。
- リチウムイオン電池のセパレータ側に配置される第1主面と、集電体側に配置される第2主面とを備え、かつ、前記第1主面と前記第2主面の間に、電子伝導性材料からなる導電部材(A)、多数の活物質粒子(B)及び樹脂(F)を含み、前記導電部材(A)の少なくとも一部は、前記第1主面から前記第2主面までを電気的に接続する導電通路を形成しており、前記導電通路は、前記導電通路の周囲の前記活物質粒子(B)と接しているリチウムイオン電池用電極の製造方法であって、
前記導電部材(A)、前記活物質粒子(B)及び前記樹脂(F)を含む電極用組成物を、加熱プレスすることにより、前記樹脂(F)によって前記導電部材(A)及び前記活物質粒子(B)を固定する工程(R1)を含むことを特徴とするリチウムイオン電池用電極の製造方法。 - 前記導電部材(A)は導電性繊維である請求項15~23のいずれかに記載のリチウムイオン電池用電極の製造方法。
Priority Applications (9)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP14871738.2A EP3086387B1 (en) | 2013-12-20 | 2014-12-12 | Electrode for lithium-ion cell, lithium-ion cell, and method for manufacturing electrode for lithium-ion cell |
| KR1020187016756A KR102065867B1 (ko) | 2013-12-20 | 2014-12-12 | 리튬 이온 전지용 전극, 리튬 이온 전지 및 리튬 이온 전지용 전극의 제조 방법 |
| KR1020167015990A KR20160086923A (ko) | 2013-12-20 | 2014-12-12 | 리튬 이온 전지용 전극, 리튬 이온 전지 및 리튬 이온 전지용 전극의 제조 방법 |
| US15/105,655 US10276858B2 (en) | 2013-12-20 | 2014-12-12 | Electrode for lithium-ion cell, lithium-ion cell, and method for manufacturing electrode for lithium-ion cell |
| CN201480069667.5A CN105849942B (zh) | 2013-12-20 | 2014-12-12 | 锂离子电池用电极、锂离子电池和锂离子电池用电极的制造方法 |
| JP2015553514A JP6199993B2 (ja) | 2013-12-20 | 2014-12-12 | リチウムイオン電池用電極、リチウムイオン電池及びリチウムイオン電池用電極の製造方法 |
| US15/951,647 US10727476B2 (en) | 2013-12-20 | 2018-04-12 | Electrode for lithium-ion cell, lithium-ion cell, and method for manufacturing electrode for lithium-ion cell |
| US16/900,382 US11322732B2 (en) | 2013-12-20 | 2020-06-12 | Electrode for lithium-ion cell, lithium-ion cell, and method for manufacturing electrode for lithium-ion cell |
| US16/900,351 US11233229B2 (en) | 2013-12-20 | 2020-06-12 | Electrode for lithium-ion cell, lithium-ion cell, and method for manufacturing electrode for lithium-ion cell |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013-264023 | 2013-12-20 | ||
| JP2013264023 | 2013-12-20 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US15/105,655 A-371-Of-International US10276858B2 (en) | 2013-12-20 | 2014-12-12 | Electrode for lithium-ion cell, lithium-ion cell, and method for manufacturing electrode for lithium-ion cell |
| US15/951,647 Division US10727476B2 (en) | 2013-12-20 | 2018-04-12 | Electrode for lithium-ion cell, lithium-ion cell, and method for manufacturing electrode for lithium-ion cell |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015093411A1 true WO2015093411A1 (ja) | 2015-06-25 |
Family
ID=53402757
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/083027 WO2015093411A1 (ja) | 2013-12-20 | 2014-12-12 | リチウムイオン電池用電極、リチウムイオン電池及びリチウムイオン電池用電極の製造方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (4) | US10276858B2 (ja) |
| EP (1) | EP3086387B1 (ja) |
| JP (1) | JP6199993B2 (ja) |
| KR (2) | KR20160086923A (ja) |
| CN (1) | CN105849942B (ja) |
| WO (1) | WO2015093411A1 (ja) |
Cited By (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017108620A1 (de) * | 2015-12-21 | 2017-06-29 | Bayerische Motoren Werke Aktiengesellschaft | Elektrode und galvanische zelle mit einem formkörper aus kohlenstoffschaum und verfahren zu deren herstellung |
| JP2017147222A (ja) * | 2016-02-12 | 2017-08-24 | 三洋化成工業株式会社 | リチウムイオン電池 |
| WO2017219235A1 (en) * | 2016-06-21 | 2017-12-28 | GM Global Technology Operations LLC | Lithium-ion battery with wire-containing electrodes |
| JP2018045902A (ja) * | 2016-09-15 | 2018-03-22 | 三洋化成工業株式会社 | リチウムイオン電池及びその製造方法 |
| WO2018055956A1 (ja) * | 2016-09-26 | 2018-03-29 | 日産自動車株式会社 | 非水電解質二次電池用負極 |
| WO2018055955A1 (ja) * | 2016-09-26 | 2018-03-29 | 日産自動車株式会社 | 非水電解質二次電池用正極 |
| JP2018055968A (ja) * | 2016-09-29 | 2018-04-05 | 三洋化成工業株式会社 | リチウムイオン電池及びその製造方法 |
| WO2018110655A1 (ja) * | 2016-12-15 | 2018-06-21 | 日産自動車株式会社 | 二次電池用電極及び二次電池 |
| JP2018530121A (ja) * | 2015-10-02 | 2018-10-11 | ナノテク インスツルメンツ インク | 超高エネルギー密度を有するリチウム電池の製造方法 |
| WO2018194164A1 (ja) * | 2017-04-21 | 2018-10-25 | 三洋化成工業株式会社 | リチウムイオン電極用粘着剤、リチウムイオン電池用電極及びリチウムイオン電池用電極の製造方法 |
| JP2018181802A (ja) * | 2017-04-21 | 2018-11-15 | 三洋化成工業株式会社 | 電極活物質成形体の製造方法及びリチウムイオン電池の製造方法 |
| JP2019046765A (ja) * | 2017-09-07 | 2019-03-22 | 三洋化成工業株式会社 | リチウムイオン電池用電極の製造方法及びリチウムイオン電池用電極の製造装置 |
| JP2019537221A (ja) * | 2017-05-15 | 2019-12-19 | エルジー・ケム・リミテッド | 全固体電池用電極及びその製造方法 |
| JP2020087863A (ja) * | 2018-11-30 | 2020-06-04 | 日産自動車株式会社 | 電池用電極の製造方法および電池用電極の製造装置 |
| JP2020126814A (ja) * | 2019-02-06 | 2020-08-20 | トヨタ自動車株式会社 | 二次電池 |
| WO2020196591A1 (ja) * | 2019-03-28 | 2020-10-01 | Apb株式会社 | リチウムイオン電池用部材の製造方法 |
| WO2020250892A1 (ja) * | 2019-06-13 | 2020-12-17 | 昭和電工マテリアルズ株式会社 | 二次電池 |
| US10957908B2 (en) | 2015-03-27 | 2021-03-23 | Nissan Motor Co., Ltd. | Electrode for lithium ion battery, lithium ion battery, and method for producing electrode for lithium ion battery |
| US11211596B2 (en) | 2017-04-21 | 2021-12-28 | Sanyo Chemical Industries, Ltd. | Method for manufacturing electrode active material molding for lithium-ion battery and method for manufacturing lithium-ion battery |
| WO2022024605A1 (ja) * | 2020-07-31 | 2022-02-03 | パナソニックIpマネジメント株式会社 | 二次電池用正極 |
| US11522242B2 (en) | 2016-03-10 | 2022-12-06 | Nissan Motor Co., Ltd. | Battery pack |
Families Citing this family (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20180090749A1 (en) * | 2016-09-29 | 2018-03-29 | GM Global Technology Operations LLC | Making channeled electrodes for batteries and capacitors |
| CN106784624B (zh) * | 2016-12-16 | 2019-11-05 | 山东精工电子科技有限公司 | 一种锂离子电池正极片及其制备方法 |
| US12080843B2 (en) * | 2017-11-16 | 2024-09-03 | Apple Inc. | Battery cell with multiple separator layers that include adhesive and ceramic material |
| JP6927012B2 (ja) * | 2017-12-15 | 2021-08-25 | トヨタ自動車株式会社 | 蓄電デバイス用電極の製造方法、蓄電デバイス用電極および蓄電デバイス |
| KR102535891B1 (ko) * | 2018-03-08 | 2023-05-23 | 주식회사 아모그린텍 | 플렉서블 배터리, 이의 제조방법 및 이를 포함하는 보조배터리 |
| US20190296335A1 (en) * | 2018-03-23 | 2019-09-26 | EnPower, Inc. | Electrochemical cells having improved ionic conductivity |
| US10991942B2 (en) | 2018-03-23 | 2021-04-27 | EnPower, Inc. | Electrochemical cells having one or more multilayer electrodes |
| US20190308116A1 (en) * | 2018-04-10 | 2019-10-10 | Craig Alan Brodersen | Solvent based cannabinoid extraction process with improved efficiency, safety, quality, which yields a homogenous and pasteurized product |
| US11870037B2 (en) | 2018-04-10 | 2024-01-09 | Apple Inc. | Porous ceramic separator materials and formation processes |
| KR102417774B1 (ko) | 2018-04-20 | 2022-07-05 | 삼성에스디아이 주식회사 | 리튬 이차 전지용 음극 및 이를 포함하는 리튬 이차 전지 |
| KR102417773B1 (ko) | 2018-04-27 | 2022-07-05 | 삼성에스디아이 주식회사 | 리튬 이차 전지용 음극 및 이를 포함하는 리튬 이차 전지 |
| JP7525984B2 (ja) * | 2018-05-30 | 2024-07-31 | 三洋化成工業株式会社 | 電極活物質層の製造方法、リチウムイオン電池用電極の製造方法及びリチウムイオン電池の製造方法 |
| DE102018114804A1 (de) * | 2018-06-20 | 2019-12-24 | Audi Aktiengesellschaft | Batterie |
| WO2020112969A1 (en) * | 2018-11-30 | 2020-06-04 | Ionic Materials, Inc. | Batteries and electrodes with coated active materials |
| JP7206978B2 (ja) * | 2019-02-06 | 2023-01-18 | トヨタ自動車株式会社 | 全固体電池およびその製造方法 |
| JP7281934B2 (ja) * | 2019-03-25 | 2023-05-26 | 三洋化成工業株式会社 | リチウムイオン電池 |
| US11335908B2 (en) * | 2019-07-30 | 2022-05-17 | International Business Machines Corporation | Rechargeable metal halide battery |
| US10998553B1 (en) | 2019-10-31 | 2021-05-04 | EnPower, Inc. | Electrochemical cell with integrated ceramic separator |
| WO2021222648A1 (en) * | 2020-04-29 | 2021-11-04 | Nextech Batteries, Inc. | Method for attaching a conductive tab to an electrode and assembly therein |
| JP2024519898A (ja) * | 2021-05-20 | 2024-05-21 | アリゾナ ボード オブ リージェンツ オン ビハーフ オブ アリゾナ ステート ユニバーシティ | γ-アルミナセパレーターにより有効化された急速充電準固体状態Li金属バッテリー |
| US11594784B2 (en) | 2021-07-28 | 2023-02-28 | EnPower, Inc. | Integrated fibrous separator |
| CN116417575B (zh) * | 2023-06-09 | 2024-01-26 | 深圳海辰储能控制技术有限公司 | 多层复合极片、储能装置及制备方法 |
Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH06338330A (ja) * | 1993-05-28 | 1994-12-06 | Shin Kobe Electric Mach Co Ltd | 高分子固体電解質電池及びその製造方法 |
| JPH09204936A (ja) | 1996-01-26 | 1997-08-05 | Toray Ind Inc | 電 池 |
| JPH117981A (ja) * | 1997-06-16 | 1999-01-12 | Nec Corp | 二次電池 |
| JP2006318759A (ja) * | 2005-05-12 | 2006-11-24 | Sony Corp | 電池 |
| WO2007145015A1 (ja) * | 2006-06-16 | 2007-12-21 | Sharp Kabushiki Kaisha | 正極、その製造方法及びその正極を用いたリチウム二次電池 |
| JP2009146580A (ja) * | 2007-12-11 | 2009-07-02 | Osaka Gas Chem Kk | リチウムイオン二次電池用負極活物質シート及びその製造方法 |
| JP2010009905A (ja) * | 2008-06-26 | 2010-01-14 | Sumitomo Electric Ind Ltd | リチウム系二次電池用正極の集電体並びにそれを備えた正極及び電池 |
| JP2011086480A (ja) | 2009-10-15 | 2011-04-28 | Toray Ind Inc | リチウムイオン電池電極用バインダー、それを用いたリチウムイオン電池電極用ペーストおよびリチウムイオン電池電極の製造方法 |
| JP2011210666A (ja) * | 2010-03-30 | 2011-10-20 | Mitsubishi Chemicals Corp | 非水系二次電池電極用の樹脂被覆活物質 |
| WO2012165422A1 (ja) * | 2011-05-31 | 2012-12-06 | 日本ゼオン株式会社 | リチウム二次電池正極用複合粒子、リチウム二次電池正極用複合粒子の製造方法、リチウム二次電池用正極の製造方法、リチウム二次電池用正極、及びリチウム二次電池 |
| JP2013196956A (ja) * | 2012-03-21 | 2013-09-30 | Toppan Printing Co Ltd | 非水電解液二次電池用電極、その製造方法及びそれを備えた非水電解液二次電池 |
| JP2013538002A (ja) * | 2010-09-22 | 2013-10-07 | コミッサリア ア レネルジー アトミーク エ オ ゼネルジ ザルタナテイヴ | リチウム電池の電極およびその製造方法 |
| JP2013218895A (ja) * | 2012-04-09 | 2013-10-24 | Toyota Motor Corp | 電極とその製造方法ならびにその電極を備える非水電解質二次電池 |
| JP2013246945A (ja) * | 2012-05-25 | 2013-12-09 | Uacj Corp | 多孔質金属集電体を用いた電極の製造方法 |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH10284055A (ja) * | 1997-02-04 | 1998-10-23 | Mitsubishi Electric Corp | リチウムイオン二次電池用電極およびそれを用いたリチウムイオン二次電池 |
| US20050048369A1 (en) * | 2003-08-28 | 2005-03-03 | Matsushita Electric Industrial Co., Ltd. | Negative electrode for non-aqueous electrolyte secondary battery, production method thereof and non-aqueous electrolyte secondary battery |
| JP4182060B2 (ja) * | 2005-01-17 | 2008-11-19 | シャープ株式会社 | リチウム二次電池 |
| US8405957B2 (en) | 2005-12-08 | 2013-03-26 | Hitachi Maxell, Ltd. | Separator for electrochemical device and method for producing the same, and electrochemical device and method for producing the same |
| CN100539262C (zh) * | 2006-12-31 | 2009-09-09 | 比亚迪股份有限公司 | 一种包覆锂离子二次电池负极活性物质的方法 |
| KR100918050B1 (ko) * | 2007-10-02 | 2009-09-18 | 삼성에스디아이 주식회사 | 리튬 이차 전지용 음극 활물질, 이를 포함하는 리튬 이차전지용 음극, 및 리튬 이차 전지 |
| KR101025278B1 (ko) * | 2008-05-29 | 2011-03-29 | 삼성에스디아이 주식회사 | 음극활물질, 이를 구비하는 음극 및 리튬이차전지 |
| JP4835881B2 (ja) * | 2009-03-31 | 2011-12-14 | 宇部興産株式会社 | リチウムイオン電池用電極およびその製造方法 |
| FI123479B (fi) * | 2009-06-10 | 2013-05-31 | Enfucell Ltd | Ohutparisto |
| US20110070495A1 (en) * | 2009-09-23 | 2011-03-24 | Alliance For Sustainable Energy, Llc | Method of fabricating electrodes including high-capacity, binder-free anodes for lithium-ion batteries |
| EP2698851B1 (en) | 2011-04-13 | 2018-12-12 | Sei Corporation | Electrode material for lithium secondary battery and lithium secondary battery |
| US9070932B2 (en) * | 2011-10-11 | 2015-06-30 | Massachusetts Institute Of Technology | Carbon electrodes |
| CN103187591B (zh) * | 2011-12-28 | 2015-11-25 | 清华大学 | 锂离子电池的制备方法 |
| KR20130133624A (ko) * | 2012-05-29 | 2013-12-09 | (주)오렌지파워 | 리튬 이차 전지용 전극 및 이를 포함하는 리튬 이차 전지 |
| US8993159B2 (en) * | 2012-12-13 | 2015-03-31 | 24M Technologies, Inc. | Semi-solid electrodes having high rate capability |
| JP2015011776A (ja) * | 2013-06-26 | 2015-01-19 | 株式会社東芝 | 二次電池電極およびリチウムイオン二次電池 |
-
2014
- 2014-12-12 KR KR1020167015990A patent/KR20160086923A/ko not_active Application Discontinuation
- 2014-12-12 EP EP14871738.2A patent/EP3086387B1/en active Active
- 2014-12-12 US US15/105,655 patent/US10276858B2/en active Active
- 2014-12-12 CN CN201480069667.5A patent/CN105849942B/zh active Active
- 2014-12-12 JP JP2015553514A patent/JP6199993B2/ja active Active
- 2014-12-12 KR KR1020187016756A patent/KR102065867B1/ko active IP Right Grant
- 2014-12-12 WO PCT/JP2014/083027 patent/WO2015093411A1/ja active Application Filing
-
2018
- 2018-04-12 US US15/951,647 patent/US10727476B2/en active Active
-
2020
- 2020-06-12 US US16/900,382 patent/US11322732B2/en active Active
- 2020-06-12 US US16/900,351 patent/US11233229B2/en active Active
Patent Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH06338330A (ja) * | 1993-05-28 | 1994-12-06 | Shin Kobe Electric Mach Co Ltd | 高分子固体電解質電池及びその製造方法 |
| JPH09204936A (ja) | 1996-01-26 | 1997-08-05 | Toray Ind Inc | 電 池 |
| JPH117981A (ja) * | 1997-06-16 | 1999-01-12 | Nec Corp | 二次電池 |
| JP2006318759A (ja) * | 2005-05-12 | 2006-11-24 | Sony Corp | 電池 |
| WO2007145015A1 (ja) * | 2006-06-16 | 2007-12-21 | Sharp Kabushiki Kaisha | 正極、その製造方法及びその正極を用いたリチウム二次電池 |
| JP2009146580A (ja) * | 2007-12-11 | 2009-07-02 | Osaka Gas Chem Kk | リチウムイオン二次電池用負極活物質シート及びその製造方法 |
| JP2010009905A (ja) * | 2008-06-26 | 2010-01-14 | Sumitomo Electric Ind Ltd | リチウム系二次電池用正極の集電体並びにそれを備えた正極及び電池 |
| JP2011086480A (ja) | 2009-10-15 | 2011-04-28 | Toray Ind Inc | リチウムイオン電池電極用バインダー、それを用いたリチウムイオン電池電極用ペーストおよびリチウムイオン電池電極の製造方法 |
| JP2011210666A (ja) * | 2010-03-30 | 2011-10-20 | Mitsubishi Chemicals Corp | 非水系二次電池電極用の樹脂被覆活物質 |
| JP2013538002A (ja) * | 2010-09-22 | 2013-10-07 | コミッサリア ア レネルジー アトミーク エ オ ゼネルジ ザルタナテイヴ | リチウム電池の電極およびその製造方法 |
| WO2012165422A1 (ja) * | 2011-05-31 | 2012-12-06 | 日本ゼオン株式会社 | リチウム二次電池正極用複合粒子、リチウム二次電池正極用複合粒子の製造方法、リチウム二次電池用正極の製造方法、リチウム二次電池用正極、及びリチウム二次電池 |
| JP2013196956A (ja) * | 2012-03-21 | 2013-09-30 | Toppan Printing Co Ltd | 非水電解液二次電池用電極、その製造方法及びそれを備えた非水電解液二次電池 |
| JP2013218895A (ja) * | 2012-04-09 | 2013-10-24 | Toyota Motor Corp | 電極とその製造方法ならびにその電極を備える非水電解質二次電池 |
| JP2013246945A (ja) * | 2012-05-25 | 2013-12-09 | Uacj Corp | 多孔質金属集電体を用いた電極の製造方法 |
Non-Patent Citations (3)
| Title |
|---|
| EIICHI YASUDA; ASAO OYA; SHINYA KOMURA; SHIGEKI TOMONOH; TAKASHI NISHIZAWA; SHINSUKE NAGATA; TAKASHI AKATSU, CARBON, vol. 50, 2012, pages 1432 - 1434 |
| EIICHI YASUDA; TAKASHI AKATSU; YASUHIRO TANABE; KAZUMASA NAKAMURA; YASUTO HOSHIKAWA; NAOYA MIYAJIMA, TANSO, vol. 255, 2012, pages 254 - 265 |
| See also references of EP3086387A4 |
Cited By (39)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10957908B2 (en) | 2015-03-27 | 2021-03-23 | Nissan Motor Co., Ltd. | Electrode for lithium ion battery, lithium ion battery, and method for producing electrode for lithium ion battery |
| JP7233926B2 (ja) | 2015-10-02 | 2023-03-07 | ナノテク インスツルメンツ インク | 超高エネルギー密度を有するリチウム電池の製造方法 |
| JP2018530121A (ja) * | 2015-10-02 | 2018-10-11 | ナノテク インスツルメンツ インク | 超高エネルギー密度を有するリチウム電池の製造方法 |
| CN108292734A (zh) * | 2015-12-21 | 2018-07-17 | 宝马股份公司 | 具有由碳泡沫制成的成形体的电极和原电池及其制造方法 |
| WO2017108620A1 (de) * | 2015-12-21 | 2017-06-29 | Bayerische Motoren Werke Aktiengesellschaft | Elektrode und galvanische zelle mit einem formkörper aus kohlenstoffschaum und verfahren zu deren herstellung |
| JP2017147222A (ja) * | 2016-02-12 | 2017-08-24 | 三洋化成工業株式会社 | リチウムイオン電池 |
| US11522242B2 (en) | 2016-03-10 | 2022-12-06 | Nissan Motor Co., Ltd. | Battery pack |
| WO2017219235A1 (en) * | 2016-06-21 | 2017-12-28 | GM Global Technology Operations LLC | Lithium-ion battery with wire-containing electrodes |
| CN109314223A (zh) * | 2016-06-21 | 2019-02-05 | 通用汽车环球科技运作有限责任公司 | 具有含线电极的锂离子电池组 |
| JP2018045902A (ja) * | 2016-09-15 | 2018-03-22 | 三洋化成工業株式会社 | リチウムイオン電池及びその製造方法 |
| JP2018055836A (ja) * | 2016-09-26 | 2018-04-05 | 日産自動車株式会社 | 非水電解質二次電池用正極 |
| EP3518325A4 (en) * | 2016-09-26 | 2019-08-21 | Nissan Motor Co., Ltd. | NEGATIVE ELECTRODE OF A SECONDARY BATTERY WITH A WATER-FREE ELECTROLYTE |
| US11302915B2 (en) | 2016-09-26 | 2022-04-12 | Nissan Motor Co., Ltd. | Negative electrode for non-aqueous electrolyte secondary battery |
| WO2018055956A1 (ja) * | 2016-09-26 | 2018-03-29 | 日産自動車株式会社 | 非水電解質二次電池用負極 |
| WO2018055955A1 (ja) * | 2016-09-26 | 2018-03-29 | 日産自動車株式会社 | 非水電解質二次電池用正極 |
| JP2018055841A (ja) * | 2016-09-26 | 2018-04-05 | 日産自動車株式会社 | 非水電解質二次電池用負極 |
| EP3518324A4 (en) * | 2016-09-26 | 2019-08-21 | Nissan Motor Co., Ltd. | POSITIVE ELECTRODE FOR SECONDARY BATTERY WITH A WATER-FREE ELECTROLYTE |
| JP2018055968A (ja) * | 2016-09-29 | 2018-04-05 | 三洋化成工業株式会社 | リチウムイオン電池及びその製造方法 |
| WO2018110655A1 (ja) * | 2016-12-15 | 2018-06-21 | 日産自動車株式会社 | 二次電池用電極及び二次電池 |
| US10790535B2 (en) | 2016-12-15 | 2020-09-29 | Nissan Motor Co., Ltd. | Electrode for secondary cell, and secondary cell |
| JP2018181802A (ja) * | 2017-04-21 | 2018-11-15 | 三洋化成工業株式会社 | 電極活物質成形体の製造方法及びリチウムイオン電池の製造方法 |
| JPWO2018194164A1 (ja) * | 2017-04-21 | 2020-06-25 | 三洋化成工業株式会社 | リチウムイオン電極用粘着剤、リチウムイオン電池用電極及びリチウムイオン電池用電極の製造方法 |
| JP7160796B2 (ja) | 2017-04-21 | 2022-10-25 | 三洋化成工業株式会社 | リチウムイオン電極用粘着剤、リチウムイオン電池用電極及びリチウムイオン電池用電極の製造方法 |
| US11430973B2 (en) | 2017-04-21 | 2022-08-30 | Sanyo Chemical Industries, Ltd. | Adhesive for lithium-ion electrode, electrode for lithium-ion battery and method for manufacturing electrode for lithium-ion battery |
| WO2018194164A1 (ja) * | 2017-04-21 | 2018-10-25 | 三洋化成工業株式会社 | リチウムイオン電極用粘着剤、リチウムイオン電池用電極及びリチウムイオン電池用電極の製造方法 |
| US11211596B2 (en) | 2017-04-21 | 2021-12-28 | Sanyo Chemical Industries, Ltd. | Method for manufacturing electrode active material molding for lithium-ion battery and method for manufacturing lithium-ion battery |
| JP2019537221A (ja) * | 2017-05-15 | 2019-12-19 | エルジー・ケム・リミテッド | 全固体電池用電極及びその製造方法 |
| US10910630B2 (en) | 2017-05-15 | 2021-02-02 | Lg Chem, Ltd. | Electrode for all solid type battery and method for manufacturing the same |
| JP2019046765A (ja) * | 2017-09-07 | 2019-03-22 | 三洋化成工業株式会社 | リチウムイオン電池用電極の製造方法及びリチウムイオン電池用電極の製造装置 |
| JP7097283B2 (ja) | 2018-11-30 | 2022-07-07 | 日産自動車株式会社 | 電池用電極の製造方法および電池用電極の製造装置 |
| JP2020087863A (ja) * | 2018-11-30 | 2020-06-04 | 日産自動車株式会社 | 電池用電極の製造方法および電池用電極の製造装置 |
| JP2020126814A (ja) * | 2019-02-06 | 2020-08-20 | トヨタ自動車株式会社 | 二次電池 |
| JP7189041B2 (ja) | 2019-02-06 | 2022-12-13 | トヨタ自動車株式会社 | 二次電池 |
| JP2020166937A (ja) * | 2019-03-28 | 2020-10-08 | 三洋化成工業株式会社 | リチウムイオン電池用部材の製造方法 |
| US11309537B2 (en) | 2019-03-28 | 2022-04-19 | Apb Corporation | Production method for lithium-ion battery member |
| WO2020196591A1 (ja) * | 2019-03-28 | 2020-10-01 | Apb株式会社 | リチウムイオン電池用部材の製造方法 |
| WO2020250394A1 (ja) * | 2019-06-13 | 2020-12-17 | 昭和電工マテリアルズ株式会社 | 二次電池 |
| WO2020250892A1 (ja) * | 2019-06-13 | 2020-12-17 | 昭和電工マテリアルズ株式会社 | 二次電池 |
| WO2022024605A1 (ja) * | 2020-07-31 | 2022-02-03 | パナソニックIpマネジメント株式会社 | 二次電池用正極 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20180301691A1 (en) | 2018-10-18 |
| US11322732B2 (en) | 2022-05-03 |
| US20200358078A1 (en) | 2020-11-12 |
| CN105849942A (zh) | 2016-08-10 |
| US20170033350A1 (en) | 2017-02-02 |
| KR20160086923A (ko) | 2016-07-20 |
| US10276858B2 (en) | 2019-04-30 |
| KR102065867B1 (ko) | 2020-01-13 |
| JPWO2015093411A1 (ja) | 2017-03-16 |
| KR20180071389A (ko) | 2018-06-27 |
| EP3086387A1 (en) | 2016-10-26 |
| EP3086387A4 (en) | 2016-12-28 |
| US11233229B2 (en) | 2022-01-25 |
| US20200358077A1 (en) | 2020-11-12 |
| EP3086387B1 (en) | 2019-05-08 |
| CN105849942B (zh) | 2019-07-16 |
| JP6199993B2 (ja) | 2017-09-20 |
| US10727476B2 (en) | 2020-07-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6199993B2 (ja) | リチウムイオン電池用電極、リチウムイオン電池及びリチウムイオン電池用電極の製造方法 | |
| JP6716288B2 (ja) | リチウムイオン電池用電極、リチウムイオン電池及びリチウムイオン電池用電極の製造方法 | |
| KR101778482B1 (ko) | 리튬 이온 2차 전지용 부극 재료 및 그 제조 방법 및 이것을 사용한 리튬 이온 2차 전지용 부극 및 리튬 이온 2차 전지 | |
| US20060154147A1 (en) | Carbon material for electrode and method for producing same, battery electrode and method for producing same, and battery and method for producing same | |
| KR20120091178A (ko) | 탄소 섬유제 부직포, 탄소 섬유, 및 그 제조 방법, 전극, 전지, 및 필터 | |
| KR20130094366A (ko) | 음극 활물질 및 이를 포함하는 리튬 전지 | |
| CN113892201A (zh) | 负极活性物质、负极以及二次电池 | |
| JP2017062992A (ja) | リチウムイオン二次電池用負極材、リチウムイオン二次電池用負極材スラリー、リチウムイオン二次電池用負極、及びリチウムイオン二次電池 | |
| JP2017062991A (ja) | リチウムイオン二次電池用負極材、リチウムイオン二次電池用負極材スラリー、リチウムイオン二次電池用負極、及びリチウムイオン二次電池 | |
| JP7226431B2 (ja) | リチウムイオン二次電池用負極材、リチウムイオン二次電池用負極材の製造方法、リチウムイオン二次電池用負極材スラリー、リチウムイオン二次電池用負極、及びリチウムイオン二次電池 | |
| JP6939880B2 (ja) | リチウムイオン二次電池用負極材、リチウムイオン二次電池用負極、及びリチウムイオン二次電池 | |
| JP7226558B2 (ja) | リチウムイオン二次電池用負極材の製造方法及びリチウムイオン二次電池の製造方法 | |
| JP3951219B2 (ja) | リチウム二次電池用負極及びその製造法並びにリチウム二次電池 | |
| JP2021036508A (ja) | 活物質複合体、電極、蓄電デバイス及び活物質複合体の製造方法 | |
| TW201024219A (en) | Method for manufacturing nano carbon fibers | |
| JP5853293B2 (ja) | リチウム二次電池用負極 | |
| JP2021036509A (ja) | 活物質複合体の製造装置及び活物質複合体の製造方法 | |
| KR20240077784A (ko) | 복합 고체 전해질, 이의 제조방법 및 이를 포함하는 전고체 전지 | |
| CN111971828A (zh) | 锂离子二次电池用负极材的制造方法和锂离子二次电池的制造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14871738 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| ENP | Entry into the national phase |
Ref document number: 2015553514 Country of ref document: JP Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 20167015990 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15105655 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REEP | Request for entry into the european phase |
Ref document number: 2014871738 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2014871738 Country of ref document: EP |