EP0755052B1 - Medium für optische Aufzeichnung - Google Patents
Medium für optische Aufzeichnung Download PDFInfo
- Publication number
- EP0755052B1 EP0755052B1 EP96111774A EP96111774A EP0755052B1 EP 0755052 B1 EP0755052 B1 EP 0755052B1 EP 96111774 A EP96111774 A EP 96111774A EP 96111774 A EP96111774 A EP 96111774A EP 0755052 B1 EP0755052 B1 EP 0755052B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- group
- carbon atoms
- wavelength
- layer
- recording medium
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 0 CC1SC(N=Nc(c(*)c2*)c(*)c(*)c2N)=NN1 Chemical compound CC1SC(N=Nc(c(*)c2*)c(*)c(*)c2N)=NN1 0.000 description 5
Images
Classifications
-
- G—PHYSICS
- G11—INFORMATION STORAGE
- G11B—INFORMATION STORAGE BASED ON RELATIVE MOVEMENT BETWEEN RECORD CARRIER AND TRANSDUCER
- G11B7/00—Recording or reproducing by optical means, e.g. recording using a thermal beam of optical radiation by modifying optical properties or the physical structure, reproducing using an optical beam at lower power by sensing optical properties; Record carriers therefor
- G11B7/24—Record carriers characterised by shape, structure or physical properties, or by the selection of the material
- G11B7/241—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material
- G11B7/242—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material of recording layers
- G11B7/244—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material of recording layers comprising organic materials only
- G11B7/246—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material of recording layers comprising organic materials only containing dyes
- G11B7/247—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material of recording layers comprising organic materials only containing dyes methine or polymethine dyes
- G11B7/2472—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material of recording layers comprising organic materials only containing dyes methine or polymethine dyes cyanine
-
- G—PHYSICS
- G11—INFORMATION STORAGE
- G11B—INFORMATION STORAGE BASED ON RELATIVE MOVEMENT BETWEEN RECORD CARRIER AND TRANSDUCER
- G11B7/00—Recording or reproducing by optical means, e.g. recording using a thermal beam of optical radiation by modifying optical properties or the physical structure, reproducing using an optical beam at lower power by sensing optical properties; Record carriers therefor
- G11B7/24—Record carriers characterised by shape, structure or physical properties, or by the selection of the material
- G11B7/241—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material
- G11B7/242—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material of recording layers
- G11B7/244—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material of recording layers comprising organic materials only
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B45/00—Complex metal compounds of azo dyes
- C09B45/34—Preparation from o-monohydroxy azo compounds having in the o'-position an atom or functional group other than hydroxyl, alkoxy, carboxyl, amino or keto groups
-
- G—PHYSICS
- G11—INFORMATION STORAGE
- G11B—INFORMATION STORAGE BASED ON RELATIVE MOVEMENT BETWEEN RECORD CARRIER AND TRANSDUCER
- G11B7/00—Recording or reproducing by optical means, e.g. recording using a thermal beam of optical radiation by modifying optical properties or the physical structure, reproducing using an optical beam at lower power by sensing optical properties; Record carriers therefor
- G11B7/24—Record carriers characterised by shape, structure or physical properties, or by the selection of the material
- G11B7/241—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material
- G11B7/242—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material of recording layers
- G11B7/244—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material of recording layers comprising organic materials only
- G11B7/246—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material of recording layers comprising organic materials only containing dyes
- G11B7/248—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material of recording layers comprising organic materials only containing dyes porphines; azaporphines, e.g. phthalocyanines
-
- G—PHYSICS
- G11—INFORMATION STORAGE
- G11B—INFORMATION STORAGE BASED ON RELATIVE MOVEMENT BETWEEN RECORD CARRIER AND TRANSDUCER
- G11B7/00—Recording or reproducing by optical means, e.g. recording using a thermal beam of optical radiation by modifying optical properties or the physical structure, reproducing using an optical beam at lower power by sensing optical properties; Record carriers therefor
- G11B7/24—Record carriers characterised by shape, structure or physical properties, or by the selection of the material
- G11B7/241—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material
- G11B7/242—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material of recording layers
- G11B7/244—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material of recording layers comprising organic materials only
- G11B7/249—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material of recording layers comprising organic materials only containing organometallic compounds
-
- G—PHYSICS
- G11—INFORMATION STORAGE
- G11B—INFORMATION STORAGE BASED ON RELATIVE MOVEMENT BETWEEN RECORD CARRIER AND TRANSDUCER
- G11B7/00—Recording or reproducing by optical means, e.g. recording using a thermal beam of optical radiation by modifying optical properties or the physical structure, reproducing using an optical beam at lower power by sensing optical properties; Record carriers therefor
- G11B7/24—Record carriers characterised by shape, structure or physical properties, or by the selection of the material
- G11B7/241—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material
- G11B7/252—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material of layers other than recording layers
- G11B7/257—Record carriers characterised by shape, structure or physical properties, or by the selection of the material characterised by the selection of the material of layers other than recording layers of layers having properties involved in recording or reproduction, e.g. optical interference layers or sensitising layers or dielectric layers, which are protecting the recording layers
Definitions
- the present invention relates to an optical recording medium, specifically to an optical recording medium which is capable of recording and reproducing in wavelengths of plural lasers.
- CD-R (CD-Recordable) has been developed and proposed as a write-once type optical recording medium which meets compact disk (hereinafter abbreviated as CD) standards (for example, Nikkei Electronics, No. 465, p. 107, January 23, 1989 issue, and OPTICAL DATA STORAGE DIGEST SERIES vol. 1, p. 45, 1989).
- CD compact disk
- This CD-R is laminated with a recording layer, a reflective layer and a protective layer in this order on a transparent resin substrate, and irradiation of a laser beam to the above recording layer at a high power causes physical or chemical change on the recording layer and allows information to be recorded thereon in the form of pits.
- the pits thus formed are irradiated with a laser beam at a low power to detect a change in a reflectance, whereby the information recorded in the pits can be reproduced.
- Such an optical recording medium uses a near infrared semiconductor laser having a wavelength falling in a range of 770 to 830 nm for recording and reproducing and meets CD standards such as a red book and an orange book, and therefore is characterized by that it is usable for CD players and CD-ROM players.
- optical recording media having such interchangeability that recording/reproducing or reproducing is possible with a red laser and recording/reproducing or reproducing is possible as well with a conventional near infrared semiconductor laser having a wavelength of 780 nm.
- CD and CD-ROM media have less dependence on wavelength in terms of a reflectance and can readily be reproduced with players for high density-media.
- conventional CD-R media use dyes in a recording layer, the optical characteristics depend greatly on wavelengths, and as a result, the reflectances of the CD-R media change to a large extent depending on the wavelengths.
- the reflectances to light beam having a wavelength of about 780 nm come up to 65 % or more
- the reflectances to red laser having a wavelength selected from a range of 620 to 690 nm are as small as about 10 % and the modulation degrees are small as well because a dye used in a recording layer has a large absorptivity and a small refractive index.
- a function-separating recording layer in which a dye layer having a high reflectance and causing no change of the state by heat energy of a laser beam and an organic substance layer having a light absorbing power are laminated in order is proposed in Japanese Patent Application Laid-Open No. 58-112794 (1983) as an example of an optical recording medium provided with two-layered dye.
- a medium in which a cyanine dye or merocyanine dye having a high reflectance and an organic light absorbing layer are laminated is proposed in Japanese Patent Application Laid-Open No. 60-239948 (1985).
- Dye recording layers having different optical constants are proposed in Japanese Patent Application Laid-Open No. 63-153192 (1988).
- a medium in which two kinds of organic dyes having different transmittance and absorptance to a laser beam having a certain wavelength are laminated is proposed in Japanese Patent Application Laid-Open No. 1-110193 (1989). Further, a recording layer comprising an organic two-layer having different transmittance or melting points to a laser beam having a certain wavelength is proposed in Japanese Patent Application Laid-Open No. 4-330649 (1992).
- these proposals are confined to improvement in a reflectance, optical degradation, high sensitivity recording and an error generating rate to a laser beam having a certain wavelength, and they are not optical recording media which are usable for recording and reproducing with plural kinds of laser beams.
- An object of the present invention is to solve the problems described above, that is, to provide an optical recording medium which can be recorded/reproduced or reproduced with light beam having a wavelength selected from wavelengths of 620 to 690 nm and which can be recorded/reproduced or reproduced based on the CD standards as well with light beam having a wavelength selected from conventional wavelengths of 770 to 830 nm and has good recording characteristics.
- the present invention relates to:
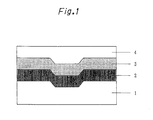
- Fig. 1 is a cross-sectional structural view of the optical recording medium, wherein;
- the optical recording medium of the present invention has a structure in which a dye layer is formed on a substrate and a reflective layer is provided thereon. Further, in order to protect the reflective layer, a protective layer may be provided on the reflective layer, or two sheets of media may be stuck together.
- the dye layer comprises a two-layer structure of a recording layer and an optical interference layer
- a laminating order of the recording layer and the optical interference layer may be reversed, and other layers may be present between the substrate and the dye layer or the optical interference layer or between the recording layer or the optical interference layer and the reflective layer.
- the materials for the substrate may be any one as long as it transmits rays having wavelengths for recording and reproducing. Used are, for example, polymer such as polycarbonate resins, vinyl chloride resins, acrylic resins such as polymethylmethacrylate, polystyrene resins and epoxy resins and inorganic materials such as glass. These materials for the substrate are molded into discoid substrates by injection molding. If necessary, guide grooves and pits for showing recording positions are formed on the surface of the substrate in some cases. Such guide grooves and pits are provided preferably in molding the substrate. They can be provided as well by forming a UV-cured resin layer on the substrate. Usually, when the optical recording medium is used for CD, it is a disc having a thickness of about 1.2 mm and a diameter of about 80 to 120 mm, and it has a hole having a diameter of about 15 mm in the center thereof.
- the dye layer is provided on the substrate, and the dye layer of the present invention contains a compound represented by Formula (1) or a metal complex thereof having a maximum absorption wavelength ( ⁇ max) falling in a range of less than 630 nm. Above all, preferred is the compound having a large refractive index and a small absorbance at wavelengths of 620 to 830 nm.
- ⁇ 1 in the optical interference layer containing a metal complex of the azo compound as a main component is preferably 0.15 or less
- an attenuation coefficient at ⁇ 2 is preferably 0.2 or less.
- the azo compound or the metal complex thereof used in the present invention is the azo compound represented by Formula (1) described above or the metal complex thereof.
- a halogen atom includes fluorine, chlorine, bromine and iodine, preferably fluorine, chlorine and bromine.
- An alkyl group is an unsubstitued or substitued alkyl group having 1 to 15 carbon atoms in total.
- the unsubstituted alkyl group includes a linear, branched or cyclic alkyl group.
- a substituent of the substituted alkyl group includes a halogen atom, an alkoxyl group, an alkoxyalkoxy group, an alkoxyalkoxyalkoxy group, an alkoxycarbonyl group, an alkoxycarbonyloxy group, an alkoxyalkoxycarbonyloxy group, a hydroxyl group, a hydroxyalkoxy group, a hydroxyalkoxyalkoxy group, a cyano group, an acyloxy group, an acyloxyalkoxy group, an acyloxyalkoxyalkoxy group, a sulfonic acid group, an alkylcarbonylamino group, an alkylsulfoamino group, a sulfonamide group,
- a linear or branched alkyl group is an alkyl group having 1 to 15 carbon atoms and includes, considering processability by coating on polycarbonate, acryl, epoxy or polyolefin substrates, methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, t-butyl, n-pentyl, isopentyl, 2-methylbutyl, 1-methylbutyl, neopentyl, 1,2-dimethylpropyl, 1,1-dimethylpropyl, cyclopentyl, n-hexyl, 4-methylpentyl, 3-methylpentyl, 2-methylpentyl, 1-methylpentyl, 3,3-dimethylbutyl, 2,3-dimethylbutyl, 1,3-dimethylbutyl, 2,2-dimethylbutyl, 1,2-dimethylbutyl, 1,1-dimethylbutyl, 3-ethylbutyl
- An alkoxyalkyl group includes groups having 2 to 15 carbon atoms, such as methoxymethyl, ethoxymethyl, propoxymethyl, butoxymethyl, methoxyethyl, ethoxyethyl, propoxyethyl, butoxyethyl, n-hexyloxyethyl, 4-methylpentyloxyethyl, 1,3-dimethylbutoxyethyl, 2-ethylhexyloxyethyl, n-octyloxyethyl, 3,5,5-trimethylhexyloxyethyl, 2-methyl-1-isopropylpropoxyethyl, 3-methyl-1-isopropylbutoxyethyl, 2-ethoxy-l-methylethyl, 3-methoxybutyl, 3,3,3-trifluoropropoxyethyl and 3,3,3-trichloropropoxyethyl.
- alkoxyalkoxyalkyl group examples include -methoxyethoxyethyl, ethoxyethoxyethyl, propoxyethoxyethyl, butoxyethoxyethyl, hexyloxyethoxyethyl, 1,2-dimethylpropoxyethoxyethyl, 3-methyl-1-isobutylbutoxyethoxyethyl, 2-methoxy-1-methylethoxyethyl, 2-butoxy-1-methylethoxyethyl, 2-(2'-ethoxy-1'-methylethoxy)-1-methylethyl, 3,3,3-trifluoropropoxyethoxyethyl and 3,3,3-trichloropropoxyethoxyethyl.
- alkoxyalkoxyalkoxyalkyl group examples include methoxyethoxyethoxyethyl, ethoxyethoxyethoxyethyl, butoxyethoxyethoxyethyl, 2,2,2-trifluoroethoxyethoxyethoxyethyl and 2,2,2-trichloroethoxyethoxyethoxyethyl.
- alkoxycarbonylalkyl group examples include methoxycarbonylmethyl, ethoxycarbonylmethyl, butoxycarbonylmethyl, methoxycarbonyethyl, ethoxycarbonylethyl, butoxycarbonylethyl, 2,2,3,3-tetrafluoropropoxycarbonylmethyl and 2,2,3,3-tetrachloropropoxycarbonylmethyl.
- alkoxycarbonyloxyalkyl group examples include methoxycarbonyloxyethyl, ethoxycarbonyloxyethyl, butoxycarbonyloxyethyl, 2,2,2-trifluoroethoxycarbonyloxyethyl and 2,2,2-trichloroethoxycarbonyloxyethyl.
- alkoxyalkoxycarbonyloxyalkyl group includes methoxyethoxycarbonyloxyethyl, ethoxyethoxycarbonyloxyethyl, butoxyethoxycarbonyloxyethyl, 2,2,2-trifluoroethoxyethoxycarbonyloxyethyl and 2,2,2-trichloroethoxyethoxycarbonyloxyethyl.
- hydroxyalkyl group examples include 2-hydroxyethyl, 4-hydroxybutyl, 2-hydroxy-3-methoxypropyl, 2-hydroxy-3-chloropropyl, 2-hydroxy-3-ethoxypropyl, 3-butoxy-2-hydroxypropyl, 2-hydroxy-3-phenoxypropyl, 2-hydroxypropyl and 2-hydroxybutyl.
- hydroxyalkoxyalkyl group examples include hydroxyethoxyethyl, 2-(2'-hydroxy-1'-methylethoxy)-1-methylethyl, 2-(3'-fluoro-2'-hydroxypropoxy)ethyl and 2-(3'-chloro-2'-hydroxypropoxy)ethyl.
- hydroxyalkoxyalkoxyalkyl group examples include hydroxyethoxyethoxyethyl, [2'-(2"-hydroxy-1"-methylethoxy)-1'-methylethoxy]ethoxyethyl, [2'-( 2"-fluoro-1"-hydroxyethoxy)-1'-methylethoxy]ethoxyethyl and [2'-(2"-chloro-1"-hydroxyethoxy)-1'-methylethoxy]ethoxyethyl.
- cyanoalkyl group examples include 2-cyanoethyl, 2-cyanopropyl, 2-cyanobutyl, 4-cyanobutyl, 2-cyano-3-methoxypropyl, 2-cyano-3-chloropropyl, 2-cyano-3-ethoxypropyl, 3-butoxy-2-cyanopropyl and 2-cyano-3-phenoxypropyl.
- an acyloxyalkyl group examples include acetoxyethyl, propionyloxyethyl, butyryloxyethyl, valeryloxyethyl, 1-ethylpentylcarbonyloxyethyl, 2,4,4-trimethylpentylcarbonyloxyethyl, 3-fluoro-butyryloxyethyl and 3-chlorobutyryloxyethyl.
- an acyloxyalkoxyalkyl group examples include acetoxyethoxyethyl, propionyloxyethoxyethyl, valeryloxyethoxyethyl, 1-ethylpentylcarbonyloxyethoxyethyl, 2,4,4-trimethylpentylcarbonyloxyethoxyethyl, 2-fluoroprpionyloxyethoxyethyl and 2-chloropropionyloxyethoxyethyl.
- an acyloxyalkoxyalkoxyalkyl group include acetoxyethoxyethoxyethyl, propionyloxyethoxyethoxyethyl,valeryloxyethoxyethoxyethyl, 1-ethylpentylcarbonyloxyethoxyethoxyethyl, 2,4,4-trimethylpentylcarbonyloxyethoxyethoxyethyl, 2-fluoropropionyloxyethoxyethoxyethyl and 2-chloropropionyloxyethoxyethoxyethyl.
- halogenated alkyl group examples include chloromethyl, chloroethyl, 2,2,2-trifluoroethyl, trifluoromethyl, bromomethyl and iodomethyl.
- sulfonalkyl group examples include sulfonmethyl, sulfonethyl and sulfonpropyl.
- alkylcarbonylaminoalkyl group examples include methylcarbonylaminoethyl, ethylcarbonylaminoethyl, propylcarbonylaminoethyl, cyclohexylcarbonylaminoethyl and succiniminoethyl.
- alkylsulfoaminoalkyl group examples include methylsulfoaminoethyl, ethylsulfoaminoethyl and propylsulfoaminoethyl.
- sulfonamidealkyl group examples include sulfonamidemethyl, sulfonamidethyl and sulfonamidepropyl.
- alkylaminoalkyl group examples include N-methylaminomethyl, N,N-dimethylaminomethyl, N,N-diethylaminomethyl, N,N-dipropylaminomethyl and N,N-dibutylaminomethyl.
- aminoalkyl group examples include aminomethyl, aminoethyl and aminopropyl.
- alkylsulfonalkyl group examples include methylsulfonmethyl, ethylsulfonmethyl, butylsulfonmethyl, methylsulfonethyl, ethylsulfonethyl, butylsulfonethyl, 2,2,3,3-tetrafluoropropylsulfonmethyl and 2,2,3,3-tetrachloropropylsulfonmethyl.
- alkoxyl groups having the same substituents as the alkyl groups given above have, and include preferably lower alkoxyl groups such as methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy, isobutoxy, sec-butoxy, t-butoxy, n-pentyloxy, isopentyloxy, neopentyloxy and 2-methylbutoxy.
- aryl groups having the same substituents as the alkyl groups given above have, and include preferably phenyl, nitrophenyl, cyanophenyl, hydroxyphenyl, methylphenyl, trifluoromethylphenyl, naphthyl, nitronaphthyl, cyanonaphthyl, hydroxynaphthyl, methylnaphthyl and trifluoromethylnaphthyl.
- acyl groups having the same substituents as the alkyl groups given above have, and include preferably formyl, acetyl, ethylcarbonyl, n-propylcarbonyl, isopropylcarbonyl, n-butylcarbonyl, isobutylcarbonyl, sec-butylcarbonyl, t-butylcarbonyl, n-pentylcarbonyl, isopentylcarbonyl, neopentylcarbonyl, 2-methylbutylcarbonyl and nitrobenzylcarbonyl.
- alkylcarboxyl groups having the same substituents as the alkyl groups given above have, and include preferably lower alkylcarboxyl groups such as methylcarboxyl, ethylcarboxyl, n-propylcarboxyl, isopropylcarboxyl, n-butylcarboxyl, isobutylcarboxyl, sec-butylcarboxyl, t-butylcarboxyl, n-pentylcarboxyl, isopentylcarboxyl, neopentylcarboxyl and 2-methylbutylcarboxyl.
- lower alkylcarboxyl groups such as methylcarboxyl, ethylcarboxyl, n-propylcarboxyl, isopropylcarboxyl, n-butylcarboxyl, isobutylcarboxyl, sec-butylcarboxyl, t-butylcarboxy
- a substituted or unsubstituted aralkyl group are aralkyl groups having the same substituents as the alkyl groups given above have, and include preferably benzyl, nitrobenzyl, cyanobenzyl, hydroxybenzyl, methylbenzyl, trifluoromethylbenzyl, naphthylmethyl, nitronaphthylmethyl, cyanonaphthylmethyl, hydroxynaphthylmethyl, methylnaphthylmethyl and trifluoromethylnaphthylmethyl.
- alkylcarbonylamino groups having the same substituents as the alkyl groups given above have, and include preferably lower alkylcarbonylamino groups such as acetylamino, ethylcarbonylamino, n-propylcarbonylamino, isopropylcarbonylamino, n-butylcarbonylamino, isobutylcarbonylamino, sec-butylcarbonylamino, t-butylcarbonylamino, n-pentylcarbonylamino, isopentylcarbonylamino, neopentylcarbonylamino, 2-methylbutylcarbonylamino, cyclohexylcarbonylamino and succinimino.
- lower alkylcarbonylamino groups such as acetylamino, ethylcarbonylamino, n-propylcarbonylamino, isopropylcarbonylamino, n-
- alkylsulfoamino groups having the same substituents as the alkyl groups given above have, and include preferably lower alkylsulfoamino groups such as methylsulfoamino, ethylsulfoamino, n-propylsulfoamino, isopropylsulfoamino, n-butylsulfoamino, isobutylsulfoamino, sec-butylsulfoamino, t-butylsulfoamino, n-pentylsulfoamino, isopentylsulfoamino, neopentylsulfoamino, 2-methylbutylsulfoamino and cyclohexylsulfoamino.
- lower alkylsulfoamino groups such as methylsulfoamino, ethylsulf
- alkylamino groups having the same substituents as the alkyl groups given above have, and include preferably lower alkylamino groups such as N-methylamino, N,N-dimethylamino, N,N-diethylamino, N,N-dipropylamino and N,N-dibutylamino.
- alkylsulfonyl groups having the same substituents as the alkyl groups given above have, and include preferably lower alkylsulfonyl groups such as methylsulfonyl, ethylsulfonyl, n-propylsulfonyl, isopropylsulfonyl, n-butylsulfonyl, isobutylsulfonyl, sec-butylsulfonyl, t-butylsulfonyl, n-pentylsulfonyl, isopentylsulfonyl, neopentylsulfonyl, 2-methylbutylsulfonyl, 2-hydroxyethylsulfonyl and 2-cyanoethylsulfonyl.
- lower alkylsulfonyl groups such as methylsulfonyl, ethylsulfonyl,
- alkenyl groups having the same substituents as the alkenyl groups given above have, and include preferably lower alkenyl groups such as propenyl, 1-butenyl, isobutenyl, 1-pentenyl, 2-pentenyl, 2-methyl-1-butenyl, 3-methyl-1-butenyl, 2-methyl-2-butenyl, 2,2-dicyanovinyl, 2-cyano-2-methylcarboxylvinyl and 2-cyano-2-methylsulfonvinyl.
- alkylthio groups having the same substituents as the alkyl groups given above have, and include preferably lower alkylthio groups such as methylthio, ethylthio, n-propylthio, isopropylthio, n-butylthio, isobutylthio, sec-butylthio, t-butylthio, n-pentylsthio, isopentylthio, neopentylthio, 2-methylbutylthio and methylcarboxylethylthio.
- lower alkylthio groups such as methylthio, ethylthio, n-propylthio, isopropylthio, n-butylthio, isobutylthio, sec-butylthio, t-butylthio, n-pentylsthio, isopentylthio, neopentylthio, 2-methyl
- alkylazomethine groups having the same substituents as the alkyl groups given above have, and include preferably lower alkylazomethine groups such as methylazomethine, ethylazomethine, n-propylazomethine, isopropylazomethine, n-butylazomethine, isobutylazomethine, sec-butylazomethine, t-butylazomethine, n-pentylazomethine, isopentylazomethine, neopentylazomethine, 2-methylbutylazomethine and hydroxyethylazomethine.
- lower alkylazomethine groups such as methylazomethine, ethylazomethine, n-propylazomethine, isopropylazomethine, n-butylazomethine, isobutylazomethine, sec-butylazomethin
- alkylaminosulfonyl groups having the same substituents as the alkyl groups given above have, and include preferably lower alkylaminosulfonyl groups such as N-methylaminosulfonyl, N-ethylaminosulfonyl, N-(n-propyl)aminosulfonyl, N-(isopropyl)aminosulfonyl, N-(n-butyl)aminosulfonyl, N-(isobutyl)aminosulfonyl, N-(secbutyl)aminosulfonyl, N-(t-butyl)aminosulfonyl, N-(n-pentyl)aminosulfonyl, N-(isopentyl)aminosulfonyl, N-(neopentyl)aminosulfonyl, N-(2-methyl
- the compound of the present invention represented by Formula (1) can be produced in the following manner by known methods. That is, an amine component represented by the following Formula (4) is turned into an azo component, which is added to a solution of a coupling component represented by the following Formula (5) to carry out a coupling reaction, whereby the azo compound represented by Formula (1) is obtained: wherein R 1 to R 7 , X and Y are synonymous with those described in Formula (1).
- the metal complex of the azo compound described above can be produced by known methods, for example, a method described in Furukawa, Analytica Chimica Acta 140 (1982), 281 to 289.
- Preferred as a metal for forming the metal complex of the azo compound are, for example, metals such as nickel, cobalt, iron, ruthenium, rhodium, palladium, copper, osmium, iridium, platinum, zinc and magnesium, and more preferred are nickel, cobalt, copper, palladium, iron and zinc.
- the metal complexes of the azo compounds may be used alone or in a mixture of the plural compounds.
- a phthalocyanine compound contained in the dye layer is the phthalocyanine compound represented by Formula (2) having an absorption maximum in a wavelength range of 680 to 900 nm and having preferably a large refractive index and a small absorbance in a wavelength range of 770 to 830 nm.
- the optical constants (refractive index n and attenuation coefficient k) required for the recording layer containing the phthalocyanine compound are 1.8 or more for n and 0.04 to 0.20 for k at the wavelength ⁇ 1 of the laser beams, and 1.1 or more for n and 0.04 to 0.6 for k at the wavelength ⁇ 2 .
- n is a smaller value than the above range at the wavelength ⁇ 1 , it is difficult to obtain the reflectance and the modulation degree which satisfy the CD standards.
- the value of n smaller than the above range also at the wavelength ⁇ 2 makes it difficult to obtain the reflectance needed for reading signals accurately.
- the value of k exceeding 0.20 at the wavelength ⁇ 1 lowers the reflectance and makes it difficult to satisfy the CD standards, and the value of k less than 0.04 makes recording impossible.
- the value of k exceeding 0.6 at the wavelength ⁇ 2 not only increases the absorbance too much and does not provide the reflectance needed for reproduction but also makes the signals to be liable to change by reproducing light. Accordingly, it is not suited to practical use.
- k has to be 0.04 or more.
- the hydrocarbon group includes a saturated hydrocarbon group such as methyl, ethyl, butyl, pentyl, hexyl, heptyl, octyl, nonyl, decyl, dodecyl, cyclohexyl and dimethylcyclohexyl, and unsaturated hydrocarbon group such as ethenyl, butenyl, hexenyl, octenyl, dodecenyl, butynyl, heptynyl, phenyl, methylphenyl, butylphenyl and hexylphenyl.
- These hydrocarbon groups may be linear, branched or cyclic and may be substituted with halogen, an amino group, a cyano group, an ether group or hydroxyl group.
- a halogen atom includes fluorine, chlorine, bromine and iodine.
- substitution positions of the substituents of Y 1 to Y 8 and A 1 to A 4 bonded to the benzene rings constituting a phthalocyanine ring shall not specifically be restricted, and the kind and number of the substituents may be different for the four benzene rings in the molecule.
- the divalent metal atom represented by M includes Cu, Zn, Fe, Co, Ni, Ru, Pd, Pt, Mg, Ti, Be, Ca, Ba, Pb and Cd
- one-substituted trivalent metal includes Al-Cl, Al-Br, Ga-Cl, Ga-Br, In-Cl, In-Br, Ti-Cl, Ti-Br, Al-C 6 H 5 , Al-C 6 H 4 (CH 3 ), In-C 10 H 7 , Mn(OH), Mn(OC 6 H 5 ), Mn(OSi(CH 3 ) 3 ), FeCl and RuCl
- two-substituted tetravalent metal includes CrCl 2 , SiCl 2 , CeBr 2 , SnCl 2 , TiCl 2 , Mn(OH) 2 , Sn(OH) 2 , TiR 2 , CrR 2 , SiR 2 , SnR 2 , GeR 2 (R represents an alkyl group
- the concrete examples of the above phthalocyanine compound include the following compounds (Formulas (a) to (c)). To be detailed, given are the compounds described in Japanese Patent Application Laid-Open No. 3-62878 (1991), Japanese Patent Application Laid-Open No. 3-141582 (1991) and Japanese Patent Application Laid-Open No. 3-215466 (1991), and they can be synthesized by the methods described in these publications.
- phthalocyanine compounds may be used alone or in a mixture of the plural compounds.
- a method for providing the dye layer in the present invention includes, for example, dipping, spraying, spin coating, casting, sputtering, chemical deposition and vacuum deposition, and the spin coating is preferred since it is simple.
- a coating solution prepared by dissolving or dispersing the azo compound represented by Formula (1) or the metal complex thereof and/or the phthalocyanine compound represented by Formula (2) (hereinafter referred to as the dye) in a range of 0.05 to 30 weight %, preferably 0.5 to 20 weight % is used.
- a solvent which does not damage the substrate is preferably selected.
- the solvent includes, for example, alcohol series solvents such as methanol, ethanol, isopropanol, octafluoropentanol, allyl alcohol, methyl cellosolve, ethyl cellosolve and tetrafluoropropanol, aliphatic or alicyclic hydrocarbon series solvents such as hexane, heptane, octane, decane, cyclohexane, methylcyclohexane, ethylcyclohexane and dimethylcyclohexane, aromatic hydrocarbon series solvents such as toluene, xylene and benzene, halogenated hydrocarbon solvents such as carbon tetrachloride, chloroform, tetrachloroethane and dibromoethane, ether solvents such as diethyl ether, dibutyl ether, diisopropyl ether and dioxane, ket
- the dye layer comprises a two-layer structure of the recording layer and the optical interference layer
- the phthalocyanine compound used in the recording layer and the azo compound or the metal complex thereof used in the optical interference layer each dissolved in different polar solvents are used, and each is preferably not dissolved in the other solvent.
- the azo compound or the metal complex (dye) thereof used in the optical interference layer is dissolved in high polar solvents, for example, alcohol solvents and water, and coated, and the phthalocyanine compound (dye) used in the recording layer is dissolved in a low polar solvent, for example, aliphatic or alicyclic hydrocarbon solvents, aromatic hydrocarbon solvents, carbon tetrachloride and ether solvents, and coated.
- solvents for coating and forming the respective dye layers are preferably an aliphatic or alicyclic hydrocarbon solvent and a mixed solvent thereof with other solvents, and an alcohol solvent and a mixed solvent thereof with other solvents.
- a combination of the dyes soluble in the respective solvents is particularly desired.
- a film thickness of the dye layer shall not specifically be restricted and is preferably 50 to 300 nm.
- the film thickness of the dye layer smaller than 50 nm not only makes recording impossible or causes distortion on recording signals because of large heat diffusion, but also decreases signal amplitudes. As a result the CD standards are not satisfied. Meanwhile, the film thickness larger than 300 nm lowers the reflectance to deteriorate the reproducing signal characteristics.
- an average film thickness of the optical interference layer satisfies the following equation at the wavelengths of ⁇ 1 and ⁇ 2 and preferably is a thickness at which the reflectance becomes larger at the respective laser wavelengths: 70 ⁇ ni ⁇ di ⁇ 300 wherein ni is a refractive index of the optical interference layer, and di is a film thickness of the optical interference layer.
- ni ⁇ di is less than 70
- the reflectance to light having the wavelength ⁇ 2 is less than 15 %, and the modulation degree decreases as well.
- ni ⁇ di exceeds 300
- the reflectance to light having the wavelength ⁇ 1 is less than 65 %.
- additives such as a quencher, a thermal decomposition promotor of dyes, a UV absorber and an adhesive may be blended with the dyes described above, or groups having such effects may be introduced as substituents into the dyes.
- Preferred as the quencher are metal complexes based on acetyl acetonates, bisdithio- ⁇ -diketones, bisdithiols such as bisphenyldithiol, thiocatechols, salicylaldehyde oximes and thiobisphenolates. Further, amines are suitable as well.
- the thermal decomposition promoter shall not specifically be restricted as long as promotion of thermal decomposition of the dyes can be confirmed by means of thermogravimetric analysis (TG analysis), and includes metal compounds such as metalic antiknocking agents, metallocene compounds and acetylacetonato metal complexes.
- TG analysis thermogravimetric analysis
- binders, leveling agents, and defoaming agents may be used as well in combination, if necessary.
- the preferred binder includes polyvinyl alcohol, polyvinyl pyrrolidone, nittrocellulose, cellulose acetate, ketone resins, acrylic resins, polystyrene resins, urethane resins, polyvinyl butyral, polycarbonate, polyolefins, and the like.
- dyes other than those described above may be added as well in order to improve recording characteristics.
- the examples of the dyes include pentamethinecyanine dyes, heptamethinecyanine dyes, squalillium dyes, naphthoquinone dyes, azo dyes, naphthalocyanine dyes, phthalocyanine dyes and anthraquinone dyes.
- a blending ratio of these dyes falls in a level of 0.1 to 10 %.
- a layer comprising inorganic substances or polymers may be provided on the substrate in order to improve the solvent resistance of the substrate, reflectance and the recording sensitivity.
- the content of the azo compound represented by Formula (1) or the metal complex thereof and/or the phthalocyanine compound represented by Formula (2) in the dye layer is 30 % or more, preferably 60 % or more. It may be substantially 100 %.
- a reflective layer having a thickness of preferably 50 to 300 nm is formed on the dye layer described above.
- Substances providing sufficiently high reflectances at wavelengths of reproducing lights for example, metals such as Au, Al, Ag, Cu, Ti, Cr, Ni, Pt, Ta, Cr and Pd can be used as a material for the reflective layer alone or in the form of alloys.
- Au and Al have the high reflectances and therefore are suitable to the material for the reflective layer.
- the following ones may be contained.
- a substance containing Au as a main component is suitable since the reflective layer having a high reflectance can readily be obtained.
- main component means a component having a content of 50 % or more.
- a multilayer film may be formed for the reflective layer with materials other than metals by laminating a thin film having a low refractive index and a thin film having a high refractive index after the other.
- Methods for forming the reflective layer include, for example, sputtering, ion plating, chemical deposition and vacuum deposition. Further, a known inorganic or organic intermediate layer and an adhesive layer may be provided on the substrate or under the reflective layer in order to improve the reflectance and the recording characteristics.
- a protective layer is formed on the reflective layer in order to protect the dye layer and the reflective layer. Two sheets of the media may be stuck together.
- the organic materials include thermoplastic resins, thermosetting resins, electron beam-curing resins, and UV-curing resins.
- the inorganic materials include SiO 2 , SiN 4 , MgF 2 , and SnO 2 .
- the protective layer can be formed by applying a coating solution prepared by dissolving a thermoplastic resin or a thermosetting resin in a suitable solvent, and drying it. A UV curing resin is applied as it is or as a solution prepared by dissolving it in a suitable solvent, and it is irradiated with UV rays to cure, whereby the protective layer can be formed.
- Acrylate resins such as, for example, urethane acrylates, epoxy acrylates and polyester acrylates can be used as the UV-curing resin. These materials may be used alone or in a mixture, and they may be used not only in a single layer film but also in a multilayer film.
- Coating methods such as spin coating and casting, and methods such as sputtering and chemical deposition are used as a method for forming the protective layer as is the case with the recording layer.
- the spin coating is preferred.
- the film thickness of the protective layer falls in a range of 0.1 to 100 ⁇ m. In the present invention, however, it is 3 to 30 ⁇ m, preferably 5 to 20 ⁇ m.
- printing such as labeling can be applied on the protective layer.
- a film of a UV-curing resin or an inorganic film may be formed on a specular side of the substrate in order to protect the surface and prevent dusts from sticking thereon.
- the optical recording medium of the present invention thus obtained can record and reproduce by focusing laser beams on the dye layer.
- Signals used in recording include, for example, EMF modulation signals used for CD.
- the medium of the present invention can be recorded and reproduced with laser beams having a wavelength of about 780 nm since the reflectance of 65 % or more to light having a wavelength selected from 770 to 830 nm can be obtained. Further, recorded information can be reproduced with commercial CD and CD-ROM players. The characteristics of the signals reproduced satisfy the orange book standards which are the standards of CD-R.
- the medium of the present invention provides a reflectance of 15 % or more to light having a wavelength falling in a range of 620 to 690 nm and therefore can be reproduced with an optical disc player for high density recording media loaded with a laser having a wavelength selected from arrange of 620 to 690 nm.
- Light used for players for high density recording media in the next age has a wavelength falling in a range of 620 to 690 nm.
- Lasers having wavelengths falling in this range include dye lasers, which have wavelengths to be selected in a wide range of a visible ray region, and a helium-neon laser having a wavelength of 633 nm.
- semiconductor lasers which are put into practical use have a wavelength of, for example, 635 nm, 650 nm or about 680 nm.
- the medium of the present invention can be recorded as well with light having a wavelength selected from a range of 620 to 690 nm.
- the azo compound (1-19) of 0.2 g shown in Table-1 out of the azo compounds represented by Formula (1) and the phthalocyanine compound of 0.02 g represented by the chemical formula (a-11) described previously were dissolved in diacetone alcohol (produced by Tokyo Kasei K.K.) of 10 ml to prepare a dye solution.
- diacetone alcohol produced by Tokyo Kasei K.K.
- Used as a substrate was a disc (made of polycarbonate resin) having a diameter of 120 mm and a thickness of 1.2 mm with a spiral pre-groove (track pitch: 1.6 ⁇ m).
- the dye solution was spin-coated on this substrate at a revolution of 1500 rpm and dried at 70°C for 2 hours to form a recording layer.
- Au was sputtered on this recording layer with a sputtering equipment (CDI-900: manufactured by Balzers Co., Ltd.) to form a reflective layer having a thickness of 100 nm.
- Sputtering was carried out at a gas pressure of 1.33 Pa (1.0 x 10 -2 Torr).
- a UV-curing resin SD-17 (manufactured by Dainippon Ink Chemical Ind. Co., Ltd.) was spin-coated on the reflective layer and then irradiated with UV rays to form a protective layer having a thickness of 6 ⁇ m.
- this recorded sample was reproduced and evaluated with a commercial CD player having a reproducing wavelength of 780 nm to find that the sample showed good recording characteristics.
- Optical recording media were produced in the same manner as described in Example 1, except that the azo compounds described in Table-1 and the phthalocyanine compounds represented by the chemical formulas (a-10 to 11) described previously were used in combination.
- Example 2 Recording was carried out on the optical recording media thus obtained in the same manner as that in Example 1 at a linear velocity of 5.6 m/s and a laser power of 10 mW with the optical disc evaluation apparatus DDU-1000 (manufactured by Pulstec Ind. Co., Ltd.) loaded with the red semiconductor laser head having a wavelength of 680 nm and the EFM encoder (manufactured by Kenwood Co., Ltd.). After recording, the same measurements as those in Example 1 were carried out to find that the good recording characteristics were shown in any cases.
- a solution prepared by dissolving 0.8 g of the phthalocyanine compound represented by the chemical formula (b-7) described previously in dimethylcyclohexane (produced by Tokyo Kasei K.K.) of 40 ml was used to form a recording layer by spin-coating.
- the azo compound (1-31) described in Table-1 was used to form an interference layer on this recording layer on the same conditions as those in Example 1, whereby an optical recording medium comprising two layers of the interference layer and the recording layer was prepared.
- the signals were reproduced with an evaluation apparatus loaded with a red semiconductor laser head having a wavelength of 680 nm to find that such good recording characteristics as the reflectance of 31 % were shown.
- Optical recording media were produced in the same manner as that described in Example 29, except that the azo compounds described in Table-1 and the phthalocyanine compounds represented by the chemical formulas (b-1 to c-10) described previously were used in combination.
- the signals were reproduced with the evaluation apparatus loaded with the red semiconductor laser head having a wavelength of 680 nm to find that such good recording characteristics as the reflectances of 30 % or more were shown in the respective cases.
- the signals were reproduced with the evaluation apparatus loaded with the red semiconductor laser head having a wavelength of 680 nm to find that such good recording characteristics as the reflectance of 30 % were shown.
- An optical recording medium was produced in the same manner as that described in Example 47, except that the azo compound (1-39) described in Table-1 and the phthalocyanine compound (b-7) represented by the chemical formula described previously were used in combination.
- the signals were reproduced with the evaluation apparatus loaded with the red semiconductor laser head having a wavelength of 680 nm to find that such good recording characteristics as the reflectance of 30 % were shown.
- the metal complex (1-93) of the azo compound of 0.2 g shown in Table-1 out of the metal complexes of the azo compounds represented by Formula (1) and 0.02 g of the phthalocyanine compound (a-10) represented by the chemical formula described previously were dissolved in 2,2,3,3-tetrafluoro-1-propanol of 10 ml to prepare a dye solution.
- Used as a substrate was a disc having a diameter of 120 mm and a thickness of 1.2 mm, which was made of polycarbonate resin and had a spiral pre-groove (track pitch: 1.6 ⁇ m).
- the dye solution was spin-coated on this substrate at a revolution of 1500 rpm and dried at 70°C for 2 hours to form a recording layer.
- Au was sputtered on this recording layer with the sputtering equipment (CDI-900: manufactured by Balzers Co., Ltd.) to form a reflective layer having a thickness of 100 nm.
- Sputtering was carried out at a gas pressure of 1.33 Pa (1.0 x 10 -2 Torr).
- the UV-curing resin SD-17 manufactured by Dainippon Ink Chemical Ind. Co., Ltd.
- EFM signals were recorded on this optical recording medium at a linear velocity of 2.8 m/s and a laser power of 8 mW with a writer (CDD-521: manufactured by Philiphs Co., Ltd.).
- the signals were reproduced with a commercial CD player (YAMAHA CDX-1050; laser wavelength: 786 nm) to determine the reflectance, the error rate and the modulation degree. As a result, the distortion of the reproducing wave form was small, and therefore the good values satisfying the orange book standards were shown.
- the signals recorded on this medium were reproduced with the optical disc evaluation apparatus (DDU-1000: manufactured by Pulstec Ind.
- the signals recorded on the medium with the above drive (DDU-1000) having a wavelength of 680 nm were reproduced with the commercial CD player (YAMAHA CDX-1050; laser wavelength: 786 nm) to determine the reflectance, the error rate and the I3/Itop.
- the good values satisfying the orange book standards were shown in any cases.
- Optical recording media were produced in the same manner as that described in Example 49, except that the metal complexes of the azo compounds described in Table-1 out of the metal complexes of the azo compounds represented by Formula (1), and the phthalocyanine compounds represented by the chemical formulas (a-10 to 11) described previously were used in combination (shown in Table 3).
- the metal complex (1-89) of the azo compound of 0.2 g shown in Table-1 out of the metal complexes of the azo compounds represented by Formula (1) was dissolved in 2,2,3,3-tetrafluoro-1-propanol of 10 ml to prepare a dye solution 1.
- Used as a substrate was a disc having a diameter of 120 mm and a thickness of 1.2 mm, which was made of polycarbonate resin and had a spiral pre-groove (170 nm thick, 0.5 ⁇ m wide, track pitch: 1.6 ⁇ m).
- the dye solution 1 was spin-coated on this substrate at a revolution of 1500 rpm and dried at 70°C for 3 hours to form a dye optical interference layer.
- the refractive index was 1.9 and the attenuation coefficient was 0.05 at 780 nm; the refractive index was 2.1 and the attenuation coefficient was 0.04 at 680 nm; and the refractive index was 2.5 and the attenuation coefficient was 0.10 at 635 nm.
- the products (ni ⁇ di) of the refractive indices and the film thickness at 780, 680 and 635 nm were 152, 168 and 200, respectively.
- a dye solution 2 prepared by dissolving 0.25 g of the phthalocyanine compound (c-2) represented by the chemical formula described previously in 1,2-dimethylcyclohexane of 10 ml was spin-coated at a revolution of 1600 rpm and dried at 70°C for 2 hours to form a recording layer.
- the refractive index was 2.2 and the attenuation coefficient was 0.08 at 780 nm; the refractive index was 1.2 and the attenuation coefficient was 0.49 at 680 nm; and the refractive index was 1.2 and the attenuation coefficient was 0.34 at 635 nm.
- EFM signals were recorded on this optical recording medium at a linear velocity of 2.8 m/s and a laser power of 8 mW with the writer (CDD-521: manufactured by Philiphs Co., Ltd.).
- the signals were reproduced with the commercial CD player (YAMAHA CDX-1050; laser wavelength: 786 nm) to determine the reflectance, the error rate and the modulation degree.
- the distortion of the reproducing wave form was small, and therefore the good values satisfying the orange book standards were shown.
- the signals recorded on this medium were reproduced with the optical disc evaluation apparatus (DDU-1000: manufactured by Pulstec Ind. Co., Ltd.) loaded with the red semiconductor laser head having wavelengths of 680 nm and 635 nm to determine the reflectance, the error rate and the modulation degree (I3/Itop) of the shortest pit. The good values were shown in any cases.
- the signals recorded on the medium with the above drive (DDU-1000) having a wavelength of 680 nm were reproduced with the commercial CD player (YAMAHA CDX-1050; laser wavelength: 786 nm) to determine the reflectance, the error rate and the I3/Itop.
- the good values satisfying the orange book standards were shown in any cases.
- Optical recording media each having a recording layer formed on an optical interference layer were produced in the same manner as that described in Example 55, except that the metal complexes of the azo compounds described in Table-1 out of the metal complexes of the azo compounds represented by Formula (1), and the phthalocyanine compounds represented by the chemical formulas (b-3 and c-2) described previously were used in combination (shown in Table-3).
- the media thus produced was recorded at 780 nm and 680 nm and reproduced at 786 nm, 680 nm and 635 nm in the same manners as those in Example 55. As a result, the good recording characteristics were shown in any of the above wavelengths.
- An optical recording medium was prepared in the same manner as that described in Example 55, except that the coating order was reversed and that the phthalocyanine compound (c-2) represented by the chemical formula described previously was applied for a recording layer and the metal complex (1-89) of the azo compound described in Table-1 was applied thereon for an optical interference layer.
- the medium thus prepared was recorded and reproduced in the same manners as those in Example 55. As a result, the good recording characteristics were shown in any of the wavelengths.
- Optical recording media each having an optical interference layer formed on a recording layer were prepared and evaluated in the same manners as those described in Example 59, except that the metal complexes of the azo compounds described in Table-1 and the phthalocyanine compounds represented by the chemical formulas (a-6 to c-2) described previously were used in combination.
- Table-3 Described in Table-3 are the combinations of the dyes, the optical constants [refractive index (n) and attenuation coefficient (k)] of the recording layers and the optical interference layers at 780, 680 and 635 nm, the film thicknesses of the optical interference layers and the ni ⁇ di values.
- All the media had large modulation degrees and small error rates and jitters in a recording mode of high to low at the respective wavelengths of 780, 680 and 635 nm, and therefore very good reproductions could be made.
- An optical recording medium was prepared in the same manner as that in Example 55, except that the thickness of the optical interference layer comprising the azo compound was changed to 35 nm.
- the products (ni ⁇ di) of the refractive indices and the film thickness of the optical interference layer at 780, 680 and 635 nm were 67, 74 and 88, respectively.
- the medium thus prepared was evaluated in the same manner as that described in Example 55 to find that the reproducing signal wave forms were distorted at the respective wavelengths of 680 and 635 nm, and the reflectance was low.
- An optical recording medium was prepared in the same manner as that described in Example 59, except that the thickness of the optical interference layer comprising the azo compound was changed to 160 nm.
- the products (ni ⁇ di) of the refractive indices and the film thickness of the optical interference layer at 780, 680 and 635 nm were 304, 336 and 400, respectively.
- the medium thus prepared was evaluated in the same manner as that in Example 59 to find that the reproducing signal wave forms were distorted at the respective wavelengths of 786, 680 and 635 nm, and the reflectance was low as well.
- An optical recording medium was prepared in the same manner as that described in Example 49, except that only the metal complex (1-93) of the azo compound described in Table-1 was used in the dye layer.
- the medium thus prepared was evaluated in the same manner as that in Example 49 to find that recording could not be carried out at 780 nm and the sensitivity was low at 680 nm, which therefore made fine recording impossible.
- An optical recording medium was prepared in the same manner as that described in Example 49, except that only the phthalocyanine compound (a-10) represented by the chemical formula described previously was used in the dye layer.
- the medium thus prepared was evaluated in the same manner as that in Example 49 to find that the wave form was distorted and the reflectances at 635 and 680 nm were low.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Thermal Transfer Or Thermal Recording In General (AREA)
- Optical Record Carriers And Manufacture Thereof (AREA)
Claims (10)
- Optisches Aufzeichnungsmedium, umfassend zumindest eine Farbschicht, eine reflektierende Schicht und eine auf einem Substrat angeordnete Schutzschicht, worin eine Azoverbindung der folgenden Formel (1) oder ein Metallkomplex hiervon mit einem Absorptionsmaximum bei einer Wellenlänge im Bereich von 450 bis 630 nm und eine Phthalocyaninverbindung der folgenden Formel (2) mit einem Absorptionsmaximum bei einer Wellenlänge im Bereich von 680 bis 900 nm in der Farbschicht enthalten sind: worinR1 und R2 unabhängig ein Wasserstoffatom, eine Alkylgruppe mit 1 bis 15 Kohlenstoffatomen, eine Arylgruppe mit 6 bis 21 Kohlenstoffatomen, eine Aralkylgruppe mit 7 bis 22 Kohlenstoffatomen oder eine Alkenylgruppe mit 2 bis 16 Kohlenstoffatomen bedeuten;R3, R4, R5 und R6 unabhängig ein Wasserstoffatom, ein Halogenatom, eine Hydroxylgruppe, eine Carboxylgruppe, eine Sulfonsäuregruppe, eine Sulfonamidgruppe, eine Aminogruppe, eine Alkylgruppe mit 1 bis 15 Kohlenstoffatomen, eine Alkoxygruppe mit 1 bis 15 Kohlenstoffatomen, eine Arylgruppe mit 6 bis 21 Kohlenstoffatomen, eine Acylgruppe mit 1 bis 15 Kohlenstoffatomen, eine Alkylcarboxylgruppe mit 2 bis 16 Kohlenstoffatomen, eine Aralkylgruppe mit 2 bis 22 Kohlenstoffatomen, eine Alkylcarbonylaminogruppe mit 2 bis 16 Kohlenstoffatomen,eine Alkylsulfaminogruppe mit 1 bis 15 Kohlenstoffatomen, eine Alkylaminogruppe mit 1 bis 15 Kohlenstoffatomen, eine Alkylsulfonylgruppe mit 1 bis 15 Kohlenstoffatomen oder eine Alkenylgruppe mit 2 bis 16 Kohlenstoffatomen wiedergeben;R1 und R4, R2 und R6 und R1 und R2 Ringe bilden können;R7 ein Wasserstoffatom, ein Halogenatom, eine Hydroxylgruppe, eine Carboxylgruppe, eine Sulfonsäuregruppe, eine Sulfonamidgruppe, eine Aminogruppe, eine Alkylgruppe mit 1 bis 15 Kohlenstoffatomen, eine Alkoxygruppe mit 1 bis 15 Kohlenstoffatomen, eine Arylgruppe mit 6 bis 21 Kohlenstoffatomen, eine Acylgruppe mit 1 bis 15 Kohlenstoffatomen, eine Alkylcarboxylgruppe mit 2 bis 16 Kohlenstoffatomen, eine Aralkylgruppe mit 7 bis 22 Kohlenstoffatomen, eine Alkylcarbonylaminogruppe mit 2 bis 16 Kohlenstoffatomen, eine Alkylsulfaminogruppe mit 1 bis 15 Kohlenstoffatomen, eine Alkylaminogruppe mit 1 bis 15 Kohlenstoffatomen, eine Alkylsulfonylgruppe mit 1 bis 15 Kohlenstoffatomen, eine Alkenylgruppe mit 2 bis 16 Kohlenstoffatomen, eine Cyanogruppe, eine Nitrogruppe, eine Mercaptogruppe, eine Thiocyanogruppe, eine Chlorsulfonsäuregruppe, eine Alkylthiogruppe mit 1 bis 15 Kohlenstoffatomen, eine Alkylazomethingruppe mit 2 bis 16 Kohlenstoffatomen oder eine Alkylaminosulfonylgruppe mit 1 bis 15 Kohlenstoffatomen bedeutet;X ein Schwefelatom oder N-R8 ist, worin R8 ein Wasserstoffatom, eine Alkylgruppe mit 1 bis 15 Kohlenstoffatomen, eine Arylgruppe mit 6 bis 21 Kohlenstoffatomen, eine Aralkylgruppe mit 7 bis 22 Kohlenstoffatomen oder eine Alkenylgruppe mit 2 bis 16 Kohlenstoffatomen darstellt;Y für ein Stickstoffatom oder C-R9 steht, worin R9 synonym ist mit den für R7 angegebenen Bedeutungen; mit der Maßgabe, daß, wenn X für ein Schwefelatom steht, Y ein Stickstoffatom bedeutet und, wenn X für N-R8 steht, Y für C-R9 steht; worin Y1, Y2, Y3, Y4, Y5, Y6, Y7 und Y8 unabhängig ein Wasserstoffatom, eine Kohlenwasserstoffgruppe mit 1 bis 20 Kohlenstoffatomen, eine Alkoxygruppe mit 1 bis 20 Kohlenstoffatomen oder eine Alkylthiogruppe mit 1 bis 20 Kohlenstoffatomen wiedergeben; in den jeweiligen Kombination von Y1 und Y2, Y3 und Y4, Y5 und Y6 und Y7 und Y8 diese unter Bildung von Ringen kombiniert sein können, wenn sie zueinander benachbart vorliegen;A1, A2, A3 und A4 unabhängig ein Halogenatom oder eine Nitrogruppe darstellen;l1, l2, l3 und l4 eine ganze Zahl von 0 bis 3 bedeuten;m1, m2, m3 und m4 für eine ganze Zahl von 0 bis 3 stehen;undM für zwei Wasserstoffatome, ein zweiwertiges Metallatom, ein dreiwertiges oder vierwertiges, substituiertes Metallatom oder ein Oxymetall steht.
- Optisches Aufzeichnungsmedium gemäß Anspruch 1, worin die in der Farbschicht enthaltene Verbindung mit einem Absorptionsmaximum bei einer Wellenlänge im Bereich von 450 bis 630 nm ein Metallkomplex einer Azoverbindung der folgenden Formel (3) ist, worin R1, R2, R4, R5 und R6 synonym sind mit den für Formel (1) angegebenen Bedeutungen; R10 eine Hydroxylgruppe oder eine Carboxylgruppe bedeutet; und R11 für ein Wasserstoffatom oder ein Halogenatom steht.
- Optisches Aufzeichnungsmedium gemäß Anspruch 1,worin die Farbschicht eine einschichtige Struktur umfaßt, die eine Mischung der Azoverbindung der Formel (1) oder des Metallkomplexes hiervon mit einem Absorptionsmaximum bei einer Wellenlänge im Bereich von 450 bis 630 nm und der Phthalocyaninverbindung der Formel (2) mit einem Absorptionsmaximum bei einer Wellenlänge im Bereich von 680 bis 900 nm enthält.
- Optisches Aufzeichnungsmedium gemäß Anspruch 1,worin die Farbschicht eine zweischichtige Struktur einer optischen Interferenzschicht, enthaltend die Azoverbindung der Formel (1) oder den Metallkomplex hiervon mit einem Absorptionsmaximum bei einer Wellenlänge im Bereich von 450 bis 630 nm,und einer Aufzeichnungsschicht, enthaltend die Phthalocyaninverbindung der Formel (2) mit einem Absorptionsmaximum bei einer Wellenlänge im Bereich von 680 bis 900 nm, umfaßt.
- Optisches Aufzeichnungsmedium gemäß Anspruch 4,worin eine Gleichung von 70≤ni x di≤300 sich auf Licht mit einer Wellenlänge, die für die Aufzeichnung und Reproduktion verwendet wird, bezieht, worin ni einen realen Teil eines komplexen Brechungsindex bedeutet und di eine Schichtdicke in der optischen Interferenzschicht wiedergibt.
- Optisches Aufzeichnungsmedium gemäß einem der Ansprüche 1 bis 5, das befähigt ist zur Aufzeichnung und/oder Reproduktion mit einem Laserstrahl mit einer Wellenlänge λ1, ausgewählt unter Wellenlängen im Bereich von 770 bis 830 nm, und das befähigt ist zur Aufzeichnung und/oder Reproduktion ebenfalls mit einem Laserstrahl mit einer Wellenlänge λ2, ausgewählt unter Wellenlängen im Bereich von 620 bis 690 nm.
- Optisches Aufzeichnungsmedium gemäß Anspruch 6, das befähigt ist zur Reproduktion mit einem Laserstrahl mit der Wellenlänge λ2.
- Optisches Aufzeichnungsmedium gemäß Anspruch 6 oder 7, worin der Laserstrahl mit der Wellenlänge λ1 ein Reflexionsvermögen von 65% oder mehr aufweist und der Laserstrahl mit der Wellenlänge λ2 ein Reflexionsvermögen von 15% oder mehr aufweist, die durch das Substrat gemessen werden.
- Optisches Aufzeichnungsmedium gemäß Anspruch 8,worin der Laserstrahl mit der Wellenlänge λ2 ein Reflexionsvermögen von 20% oder mehr aufweist, das durch das Substrat gemessen wird.
- Metallkomplex einer Azoverbindung der Formel (4) worinR1 und R2 unabhängig ein Wasserstoffatom, eine Alkylgruppe mit 1 bis 15 Kohlenstoffatomen, eine Arylgruppe mit 6 bis 21 Kohlenstoffatomen, eine Aralkylgruppe mit 7 bis 22 Kohlenstoffatomen oder eine Alkenylgruppe mit 2 bis 16 Kohlenstoffatomen bedeuten;R11 ein Wasserstoffatom oder ein Halogenatom wiedergibt; Me für Nickel, Kobalt, Kupfer, Palladium, Eisen und Zink steht.
Applications Claiming Priority (12)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP18401395 | 1995-07-20 | ||
| JP18401395 | 1995-07-20 | ||
| JP184013/95 | 1995-07-20 | ||
| JP19662495 | 1995-08-01 | ||
| JP19662495 | 1995-08-01 | ||
| JP196624/95 | 1995-08-01 | ||
| JP220527/95 | 1995-08-29 | ||
| JP22052795 | 1995-08-29 | ||
| JP22052795 | 1995-08-29 | ||
| JP27995395 | 1995-10-27 | ||
| JP279953/95 | 1995-10-27 | ||
| JP27995395 | 1995-10-27 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0755052A2 EP0755052A2 (de) | 1997-01-22 |
| EP0755052A3 EP0755052A3 (de) | 1997-02-12 |
| EP0755052B1 true EP0755052B1 (de) | 1999-11-17 |
Family
ID=27475142
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP96111774A Expired - Lifetime EP0755052B1 (de) | 1995-07-20 | 1996-07-22 | Medium für optische Aufzeichnung |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP0755052B1 (de) |
| KR (1) | KR100201210B1 (de) |
| DE (1) | DE69605157T2 (de) |
| TW (1) | TW340864B (de) |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3486709B2 (ja) * | 1996-06-21 | 2004-01-13 | 株式会社リコー | 光記録媒体 |
| DE69730398T2 (de) * | 1996-11-20 | 2005-09-08 | Mitsubishi Chemical Corp. | Sulfonamide und Verfahren zu ihrer Herstellung, Metall-chelates hergestellt aus den Sulfonamiden, und optisches Aufzeichnungsmaterial hergestellt aus den Metall-chelaten |
| DE69735460T2 (de) * | 1996-12-18 | 2006-11-16 | Mitsubishi Kagaku Media Co. Ltd. | Optische Aufzeichnungsplatte |
| JPH10297097A (ja) * | 1997-04-28 | 1998-11-10 | Ricoh Co Ltd | 光記録媒体 |
| KR20010012368A (ko) | 1997-05-08 | 2001-02-15 | 마츠시타 덴끼 산교 가부시키가이샤 | 광기록매체 및 그것을 사용한 정보의 기록재생방법 |
| KR100316772B1 (ko) | 1998-04-09 | 2002-01-16 | 윤종용 | 광기록 매체 |
| JP3685368B2 (ja) | 1998-08-18 | 2005-08-17 | 株式会社リコー | 光記録媒体 |
| US20030006516A1 (en) * | 2001-03-28 | 2003-01-09 | Horst Berneth | Optical data storage medium containing a heterocyclic azo dye as the light-absorbing compound in the information layer |
| US7572517B2 (en) | 2002-07-08 | 2009-08-11 | Target Technology Company, Llc | Reflective or semi-reflective metal alloy coatings |
| WO2004102552A2 (en) * | 2003-05-16 | 2004-11-25 | Koninklijke Philips Electronics N.V. | Optical data storage medium for write once recording |
| JP4778300B2 (ja) | 2004-12-15 | 2011-09-21 | 株式会社リコー | 追記型光記録媒体 |
| JP2009070515A (ja) * | 2007-09-14 | 2009-04-02 | Mitsubishi Chemicals Corp | 光記録媒体 |
| CN108017650A (zh) * | 2017-03-08 | 2018-05-11 | 先尼科化工(上海)有限公司 | 一种用于滤光片的酞菁化合物及其制备方法和应用方法 |
Family Cites Families (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0023386A1 (de) * | 1979-07-26 | 1981-02-04 | Imperial Chemical Industries Plc | Monoazodispersionsfarbstoffe, ihre Herstellung und ihre Verwendung bei synthetischen Textilmaterialien |
| JPS58112794A (ja) | 1981-12-28 | 1983-07-05 | Ricoh Co Ltd | 光学的情報記録媒体 |
| JPS60239948A (ja) | 1984-05-15 | 1985-11-28 | Ricoh Co Ltd | 光情報記録媒体 |
| JPS6230090A (ja) | 1984-12-28 | 1987-02-09 | Kuraray Co Ltd | 光情報記録媒体 |
| DE3577491D1 (de) * | 1984-12-28 | 1990-06-07 | Kuraray Co | Optisches informationsaufzeichnungsmedium. |
| JPH0771869B2 (ja) | 1986-06-30 | 1995-08-02 | 株式会社クラレ | 光学記録媒体 |
| JPH0771867B2 (ja) | 1986-06-30 | 1995-08-02 | 株式会社クラレ | 光情報記録媒体 |
| JPH0771868B2 (ja) | 1986-06-30 | 1995-08-02 | 株式会社クラレ | 光記録媒体 |
| JPS63153192A (ja) | 1986-08-11 | 1988-06-25 | Sumitomo Chem Co Ltd | 光情報記録媒体 |
| JPH01110193A (ja) | 1987-10-23 | 1989-04-26 | Mitsui Toatsu Chem Inc | 光記録媒体 |
| JPH0756019B2 (ja) | 1988-12-15 | 1995-06-14 | 三井東圧化学株式会社 | 近赤外線吸収剤及びそれを用いた表示・記録材料 |
| JP2859702B2 (ja) | 1989-09-25 | 1999-02-24 | 三井化学株式会社 | フタロニトリル化合物 |
| JPH03141582A (ja) | 1989-10-25 | 1991-06-17 | Nissin Electric Co Ltd | エレクトロルミネッセンス素子の製造方法 |
| WO1991014740A1 (fr) | 1990-03-19 | 1991-10-03 | Mitsubishi Kasei Corporation | Compose de chelation metallique et support d'enregistrement optique comprenant celui-ci |
| JP2870952B2 (ja) | 1990-03-19 | 1999-03-17 | 三菱化学株式会社 | 光学記録体 |
| US5298608A (en) * | 1990-05-17 | 1994-03-29 | Mitsubishi Kasei Corporation | Metal chelate of a monoazo compound and optical recording medium using it |
| KR0160944B1 (ko) | 1990-05-25 | 1998-12-15 | 에또오 다께또시 | 색소 배합 조성물 및 광학 기록 매체 |
| JP2890687B2 (ja) | 1990-06-13 | 1999-05-17 | 三菱化学株式会社 | アゾ金属キレート色素および該色素を用いた光学記録媒体 |
| JPH04330649A (ja) | 1991-02-13 | 1992-11-18 | Ricoh Co Ltd | 光情報記録媒体 |
| JP3168469B2 (ja) | 1991-04-05 | 2001-05-21 | 三菱化学株式会社 | 光学記録媒体 |
| JP3019469B2 (ja) | 1991-06-10 | 2000-03-13 | 三菱化学株式会社 | 光記録媒体 |
| JP3175279B2 (ja) | 1992-04-02 | 2001-06-11 | 三菱化学株式会社 | 金属キレート化合物および該化合物を用いた光学記録媒体 |
| JPH0819341B2 (ja) | 1992-06-02 | 1996-02-28 | 三菱化学株式会社 | 金属キレート化合物及び該化合物を用いた光学記録媒体 |
| EP0572973B1 (de) * | 1992-06-02 | 1998-08-19 | Mitsubishi Chemical Corporation | Metallchelat sowie optisches Aufzeichnungsmedium, das diese Verbindung verwendet |
| JPH06107149A (ja) | 1992-09-29 | 1994-04-19 | Aisin Seiki Co Ltd | ブレーキ液圧制御装置 |
| WO1995002848A1 (en) * | 1993-07-12 | 1995-01-26 | Minnesota Mining And Manufacturing Company | Multifunctional azo compounds and polymers derived therefrom for nonlinear optics |
-
1996
- 1996-07-11 TW TW085108498A patent/TW340864B/zh active
- 1996-07-20 KR KR1019960029445A patent/KR100201210B1/ko not_active IP Right Cessation
- 1996-07-22 DE DE69605157T patent/DE69605157T2/de not_active Expired - Fee Related
- 1996-07-22 EP EP96111774A patent/EP0755052B1/de not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| DE69605157D1 (de) | 1999-12-23 |
| EP0755052A2 (de) | 1997-01-22 |
| EP0755052A3 (de) | 1997-02-12 |
| KR970007897A (ko) | 1997-02-21 |
| KR100201210B1 (ko) | 1999-06-15 |
| TW340864B (en) | 1998-09-21 |
| DE69605157T2 (de) | 2000-08-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0676751B1 (de) | Optisches Aufzeichnungsmedium | |
| KR100295371B1 (ko) | 광기록매체 | |
| DE60027325T2 (de) | Optisches Aufzeichnungsmedium und neue Azaporphyrinverbindung | |
| EP0755052B1 (de) | Medium für optische Aufzeichnung | |
| JP2000108513A (ja) | 光記録媒体 | |
| JPH1058828A (ja) | 光記録媒体 | |
| JP3614586B2 (ja) | ジピロメテン金属キレート化合物及びこれを用いた光記録媒体 | |
| US5672462A (en) | Optical recording medium | |
| JP3820037B2 (ja) | 光記録媒体 | |
| KR20000011773A (ko) | 광정보 기록 매체 | |
| US5658707A (en) | Optical recording media | |
| US5145963A (en) | Optical information recording medium and nickel dithiolate complex compounds employed in the same | |
| JPH08156408A (ja) | 光記録媒体 | |
| US5013634A (en) | Optical information recording medium and nickel complex compounds employed in the same | |
| JPH0899467A (ja) | 光記録媒体 | |
| JPH08108625A (ja) | 光記録媒体 | |
| JPH1011799A (ja) | 光記録媒体 | |
| JPH09175031A (ja) | 光記録媒体 | |
| JP2000222772A (ja) | 光記録媒体 | |
| JPH0940659A (ja) | 光記録媒体 | |
| JP3704230B2 (ja) | 光記録媒体 | |
| JP3705879B2 (ja) | 光記録媒体 | |
| JPH09193546A (ja) | 光記録媒体 | |
| JPH08108624A (ja) | 光記録媒体 | |
| JP3654398B2 (ja) | 光記録媒体 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| 17P | Request for examination filed |
Effective date: 19960722 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): DE FR GB NL |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): DE FR GB NL |
|
| 17Q | First examination report despatched |
Effective date: 19970701 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: MITSUI CHEMICALS, INC. |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): DE FR GB NL |
|
| REF | Corresponds to: |
Ref document number: 69605157 Country of ref document: DE Date of ref document: 19991223 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20020709 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20020717 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20020730 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20020731 Year of fee payment: 7 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20030722 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040201 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040203 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20030722 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040331 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 20040201 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |