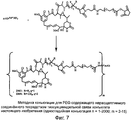
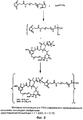
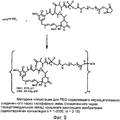
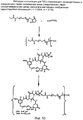
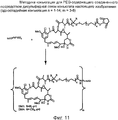
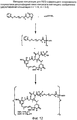
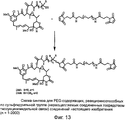
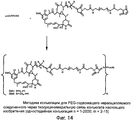
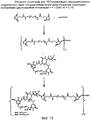
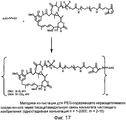
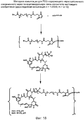
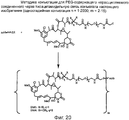
RU2487877C2 - Высокоэффективные конъюгаты и гидрофильные сшивающие агенты (линкеры) - Google Patents
Высокоэффективные конъюгаты и гидрофильные сшивающие агенты (линкеры) Download PDFInfo
- Publication number
- RU2487877C2 RU2487877C2 RU2010148740/04A RU2010148740A RU2487877C2 RU 2487877 C2 RU2487877 C2 RU 2487877C2 RU 2010148740/04 A RU2010148740/04 A RU 2010148740/04A RU 2010148740 A RU2010148740 A RU 2010148740A RU 2487877 C2 RU2487877 C2 RU 2487877C2
- Authority
- RU
- Russia
- Prior art keywords
- antibody
- cells
- carbon atoms
- chain
- cell
- Prior art date
Links
- 239000003431 cross linking reagent Substances 0.000 title 1
- 230000003389 potentiating effect Effects 0.000 title 1
- -1 2-nitrophenyl Chemical group 0.000 claims abstract 13
- 125000004432 carbon atom Chemical group C* 0.000 claims abstract 12
- 125000001931 aliphatic group Chemical group 0.000 claims abstract 9
- 206010028980 Neoplasm Diseases 0.000 claims abstract 6
- 239000011230 binding agent Substances 0.000 claims abstract 6
- 150000001875 compounds Chemical class 0.000 claims abstract 6
- 208000023275 Autoimmune disease Diseases 0.000 claims abstract 3
- 208000030852 Parasitic disease Diseases 0.000 claims abstract 3
- 206010052779 Transplant rejections Diseases 0.000 claims abstract 3
- 208000036142 Viral infection Diseases 0.000 claims abstract 3
- 125000003342 alkenyl group Chemical group 0.000 claims abstract 3
- 125000000217 alkyl group Chemical group 0.000 claims abstract 3
- 125000000304 alkynyl group Chemical group 0.000 claims abstract 3
- 125000006165 cyclic alkyl group Chemical group 0.000 claims abstract 3
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract 3
- 230000009385 viral infection Effects 0.000 claims abstract 3
- 125000001917 2,4-dinitrophenyl group Chemical group [H]C1=C([H])C(=C([H])C(=C1*)[N+]([O-])=O)[N+]([O-])=O 0.000 claims abstract 2
- 231100000433 cytotoxic Toxicity 0.000 claims abstract 2
- 230000001472 cytotoxic effect Effects 0.000 claims abstract 2
- 208000024908 graft versus host disease Diseases 0.000 claims abstract 2
- 125000000636 p-nitrophenyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1*)[N+]([O-])=O 0.000 claims abstract 2
- 210000004027 cell Anatomy 0.000 claims 27
- 102000006495 integrins Human genes 0.000 claims 6
- 108010044426 integrins Proteins 0.000 claims 6
- 239000012634 fragment Substances 0.000 claims 5
- 239000003795 chemical substances by application Substances 0.000 claims 4
- 238000000034 method Methods 0.000 claims 4
- 102100031585 ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 Human genes 0.000 claims 3
- 102100038080 B-cell receptor CD22 Human genes 0.000 claims 3
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 claims 3
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 claims 3
- 102100025012 Dipeptidyl peptidase 4 Human genes 0.000 claims 3
- 102000018651 Epithelial Cell Adhesion Molecule Human genes 0.000 claims 3
- 108010066687 Epithelial Cell Adhesion Molecule Proteins 0.000 claims 3
- 101000777636 Homo sapiens ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 Proteins 0.000 claims 3
- 101000884305 Homo sapiens B-cell receptor CD22 Proteins 0.000 claims 3
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 claims 3
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 claims 3
- 101000908391 Homo sapiens Dipeptidyl peptidase 4 Proteins 0.000 claims 3
- 239000000427 antigen Substances 0.000 claims 3
- 102000036639 antigens Human genes 0.000 claims 3
- 108091007433 antigens Proteins 0.000 claims 3
- 101150013553 CD40 gene Proteins 0.000 claims 2
- 102100032912 CD44 antigen Human genes 0.000 claims 2
- 102100025221 CD70 antigen Human genes 0.000 claims 2
- 102100037241 Endoglin Human genes 0.000 claims 2
- 102000050554 Eph Family Receptors Human genes 0.000 claims 2
- 108091008815 Eph receptors Proteins 0.000 claims 2
- 102000051096 EphA2 Receptor Human genes 0.000 claims 2
- 108010055196 EphA2 Receptor Proteins 0.000 claims 2
- 101000868273 Homo sapiens CD44 antigen Proteins 0.000 claims 2
- 101000934356 Homo sapiens CD70 antigen Proteins 0.000 claims 2
- 101000881679 Homo sapiens Endoglin Proteins 0.000 claims 2
- 101000935040 Homo sapiens Integrin beta-2 Proteins 0.000 claims 2
- 101000777628 Homo sapiens Leukocyte antigen CD37 Proteins 0.000 claims 2
- 101001133056 Homo sapiens Mucin-1 Proteins 0.000 claims 2
- 101000623901 Homo sapiens Mucin-16 Proteins 0.000 claims 2
- 101000934338 Homo sapiens Myeloid cell surface antigen CD33 Proteins 0.000 claims 2
- 101000581981 Homo sapiens Neural cell adhesion molecule 1 Proteins 0.000 claims 2
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 claims 2
- 101000874179 Homo sapiens Syndecan-1 Proteins 0.000 claims 2
- 101000610605 Homo sapiens Tumor necrosis factor receptor superfamily member 10A Proteins 0.000 claims 2
- 101000851376 Homo sapiens Tumor necrosis factor receptor superfamily member 8 Proteins 0.000 claims 2
- 102100025390 Integrin beta-2 Human genes 0.000 claims 2
- 102100031586 Leukocyte antigen CD37 Human genes 0.000 claims 2
- 102000003735 Mesothelin Human genes 0.000 claims 2
- 108090000015 Mesothelin Proteins 0.000 claims 2
- 102100034256 Mucin-1 Human genes 0.000 claims 2
- 102100023123 Mucin-16 Human genes 0.000 claims 2
- 101100369076 Mus musculus Tdgf1 gene Proteins 0.000 claims 2
- 102100025243 Myeloid cell surface antigen CD33 Human genes 0.000 claims 2
- 102100027347 Neural cell adhesion molecule 1 Human genes 0.000 claims 2
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 claims 2
- 102100029986 Receptor tyrosine-protein kinase erbB-3 Human genes 0.000 claims 2
- 101710100969 Receptor tyrosine-protein kinase erbB-3 Proteins 0.000 claims 2
- 102100035721 Syndecan-1 Human genes 0.000 claims 2
- 102100040113 Tumor necrosis factor receptor superfamily member 10A Human genes 0.000 claims 2
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 claims 2
- 102100036857 Tumor necrosis factor receptor superfamily member 8 Human genes 0.000 claims 2
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 claims 2
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 claims 2
- 108010087914 epidermal growth factor receptor VIII Proteins 0.000 claims 2
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 claims 2
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 claims 2
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 claims 2
- 210000004881 tumor cell Anatomy 0.000 claims 2
- 206010006187 Breast cancer Diseases 0.000 claims 1
- 208000026310 Breast neoplasm Diseases 0.000 claims 1
- 206010009944 Colon cancer Diseases 0.000 claims 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 claims 1
- 102000001301 EGF receptor Human genes 0.000 claims 1
- 108060006698 EGF receptor Proteins 0.000 claims 1
- 102100035139 Folate receptor alpha Human genes 0.000 claims 1
- 101001023230 Homo sapiens Folate receptor alpha Proteins 0.000 claims 1
- 206010033128 Ovarian cancer Diseases 0.000 claims 1
- 206010061535 Ovarian neoplasm Diseases 0.000 claims 1
- 206010060862 Prostate cancer Diseases 0.000 claims 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims 1
- 101100537553 Rattus norvegicus Tnfrsf8 gene Proteins 0.000 claims 1
- 206010041067 Small cell lung cancer Diseases 0.000 claims 1
- 102000004584 Somatomedin Receptors Human genes 0.000 claims 1
- 108010017622 Somatomedin Receptors Proteins 0.000 claims 1
- 208000005718 Stomach Neoplasms Diseases 0.000 claims 1
- 210000001744 T-lymphocyte Anatomy 0.000 claims 1
- 208000024313 Testicular Neoplasms Diseases 0.000 claims 1
- 206010057644 Testis cancer Diseases 0.000 claims 1
- 241000700605 Viruses Species 0.000 claims 1
- 230000001363 autoimmune Effects 0.000 claims 1
- 210000003719 b-lymphocyte Anatomy 0.000 claims 1
- 229950002903 bivatuzumab Drugs 0.000 claims 1
- 210000004369 blood Anatomy 0.000 claims 1
- 239000008280 blood Substances 0.000 claims 1
- 210000000988 bone and bone Anatomy 0.000 claims 1
- 210000004556 brain Anatomy 0.000 claims 1
- 210000000481 breast Anatomy 0.000 claims 1
- 210000001072 colon Anatomy 0.000 claims 1
- 239000003085 diluting agent Substances 0.000 claims 1
- 239000003937 drug carrier Substances 0.000 claims 1
- 102000006815 folate receptor Human genes 0.000 claims 1
- 108020005243 folate receptor Proteins 0.000 claims 1
- 206010017758 gastric cancer Diseases 0.000 claims 1
- 210000003734 kidney Anatomy 0.000 claims 1
- 210000004072 lung Anatomy 0.000 claims 1
- 210000004324 lymphatic system Anatomy 0.000 claims 1
- 210000002752 melanocyte Anatomy 0.000 claims 1
- 244000005700 microbiome Species 0.000 claims 1
- 210000000066 myeloid cell Anatomy 0.000 claims 1
- 210000000056 organ Anatomy 0.000 claims 1
- 210000001672 ovary Anatomy 0.000 claims 1
- 210000000496 pancreas Anatomy 0.000 claims 1
- 244000045947 parasite Species 0.000 claims 1
- 238000007911 parenteral administration Methods 0.000 claims 1
- 229960002087 pertuzumab Drugs 0.000 claims 1
- 239000000546 pharmaceutical excipient Substances 0.000 claims 1
- 210000002381 plasma Anatomy 0.000 claims 1
- 210000002307 prostate Anatomy 0.000 claims 1
- 229960004641 rituximab Drugs 0.000 claims 1
- 150000003839 salts Chemical class 0.000 claims 1
- 229950008684 sibrotuzumab Drugs 0.000 claims 1
- 208000000587 small cell lung carcinoma Diseases 0.000 claims 1
- 239000012453 solvate Substances 0.000 claims 1
- 206010041823 squamous cell carcinoma Diseases 0.000 claims 1
- 208000017572 squamous cell neoplasm Diseases 0.000 claims 1
- 201000011549 stomach cancer Diseases 0.000 claims 1
- 201000003120 testicular cancer Diseases 0.000 claims 1
- 238000012360 testing method Methods 0.000 claims 1
- 210000001550 testis Anatomy 0.000 claims 1
- 229960000575 trastuzumab Drugs 0.000 claims 1
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 abstract 2
- 208000009329 Graft vs Host Disease Diseases 0.000 abstract 1
- 239000003814 drug Substances 0.000 abstract 1
- 230000000694 effects Effects 0.000 abstract 1
- 239000000126 substance Substances 0.000 abstract 1
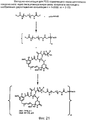
- 0 CC(CO*(C)CCNC(CCN(C(CC1CSC(*)(*)*C*(C)C(N(C)C(C)C(O[C@](CC(N(C)c(cc(CC(C)=CC=CC(C(C2)(N3)O)OC)cc4OC)c4Cl)=O)C4(C)OC4C(C)C2OC3=O)=O)=O)=O)C1=O)=O)NC(CCN(C(C=C1)=O)C1=O)=O Chemical compound CC(CO*(C)CCNC(CCN(C(CC1CSC(*)(*)*C*(C)C(N(C)C(C)C(O[C@](CC(N(C)c(cc(CC(C)=CC=CC(C(C2)(N3)O)OC)cc4OC)c4Cl)=O)C4(C)OC4C(C)C2OC3=O)=O)=O)=O)C1=O)=O)NC(CCN(C(C=C1)=O)C1=O)=O 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/5375—1,4-Oxazines, e.g. morpholine
- A61K31/5386—1,4-Oxazines, e.g. morpholine spiro-condensed or forming part of bridged ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/537—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines spiro-condensed or forming part of bridged ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/54—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/56—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule
- A61K47/59—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes
- A61K47/60—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes the organic macromolecular compound being a polyoxyalkylene oligomer, polymer or dendrimer, e.g. PEG, PPG, PEO or polyglycerol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/68033—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug being a maytansine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6843—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a material from animals or humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6849—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a receptor, a cell surface antigen or a cell surface determinant
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6851—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a determinant of a tumour cell
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6889—Conjugates wherein the antibody being the modifying agent and wherein the linker, binder or spacer confers particular properties to the conjugates, e.g. peptidic enzyme-labile linkers or acid-labile linkers, providing for an acid-labile immuno conjugate wherein the drug may be released from its antibody conjugated part in an acidic, e.g. tumoural or environment
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D498/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D498/12—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms in which the condensed system contains three hetero rings
- C07D498/18—Bridged systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6851—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a determinant of a tumour cell
- A61K47/6863—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a determinant of a tumour cell the tumour determinant being from stomach or intestines cancer cell
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6891—Pre-targeting systems involving an antibody for targeting specific cells
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Veterinary Medicine (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Organic Chemistry (AREA)
- Epidemiology (AREA)
- Cell Biology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Zoology (AREA)
- Oncology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Virology (AREA)
- Communicable Diseases (AREA)
- Transplantation (AREA)
- Mycology (AREA)
- Microbiology (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Nitrogen And Oxygen Or Sulfur-Condensed Heterocyclic Ring Systems (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US4928908P | 2008-04-30 | 2008-04-30 | |
| US61/049,289 | 2008-04-30 | ||
| PCT/US2009/042259 WO2009134976A1 (en) | 2008-04-30 | 2009-04-30 | Potent conjugates and hydrophilic linkers |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2010148740A RU2010148740A (ru) | 2012-06-10 |
| RU2487877C2 true RU2487877C2 (ru) | 2013-07-20 |
Family
ID=41255418
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2010148740/04A RU2487877C2 (ru) | 2008-04-30 | 2009-04-30 | Высокоэффективные конъюгаты и гидрофильные сшивающие агенты (линкеры) |
Country Status (16)
| Country | Link |
|---|---|
| US (3) | US9150649B2 (cg-RX-API-DMAC7.html) |
| EP (1) | EP2276506A4 (cg-RX-API-DMAC7.html) |
| JP (2) | JP2011523628A (cg-RX-API-DMAC7.html) |
| KR (1) | KR20100137585A (cg-RX-API-DMAC7.html) |
| CN (2) | CN102083461B (cg-RX-API-DMAC7.html) |
| AU (1) | AU2009243009B2 (cg-RX-API-DMAC7.html) |
| BR (1) | BRPI0911442A2 (cg-RX-API-DMAC7.html) |
| CA (1) | CA2722109A1 (cg-RX-API-DMAC7.html) |
| IL (1) | IL208937A0 (cg-RX-API-DMAC7.html) |
| MX (1) | MX2010011808A (cg-RX-API-DMAC7.html) |
| NZ (1) | NZ588851A (cg-RX-API-DMAC7.html) |
| RU (1) | RU2487877C2 (cg-RX-API-DMAC7.html) |
| SG (1) | SG189817A1 (cg-RX-API-DMAC7.html) |
| UA (1) | UA108598C2 (cg-RX-API-DMAC7.html) |
| WO (2) | WO2009134952A2 (cg-RX-API-DMAC7.html) |
| ZA (1) | ZA201007806B (cg-RX-API-DMAC7.html) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2653443C2 (ru) * | 2016-07-15 | 2018-05-08 | Закрытое Акционерное Общество "Биокад" | Биспецифичные анти-her2/анти-her3 антитела |
| RU2754369C2 (ru) * | 2016-03-02 | 2021-09-01 | Эйсай Ар Энд Ди Менеджмент Ко., Лтд. | Конъюгаты антитело-лекарственное средство на основе эрибулина и способы применения |
| RU2844753C2 (ru) * | 2016-03-02 | 2025-08-05 | Эйсай Ар Энд Ди Менеджмент Ко., Лтд. | Конъюгаты антитело-лекарственное средство на основе эрибулина и способы применения |
Families Citing this family (181)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7276497B2 (en) * | 2003-05-20 | 2007-10-02 | Immunogen Inc. | Cytotoxic agents comprising new maytansinoids |
| EP1789391B1 (en) | 2004-07-23 | 2017-06-28 | Endocyte, Inc. | Bivalent linkers and conjugates thereof |
| WO2007024536A2 (en) | 2005-08-24 | 2007-03-01 | Immunogen, Inc. | Process for preparing maytansinoid antibody conjugates |
| CA2680535C (en) | 2007-03-14 | 2016-09-20 | Endocyte, Inc. | Binding ligand linked drug delivery conjugates of tubulysins |
| BRPI0809435A2 (pt) | 2007-03-27 | 2014-09-09 | Sea Lane Biotechnologies, Llc | Constructos e bibliotecas que compreendem sequências de cadeia leve substituida de anticorpos |
| US9877965B2 (en) | 2007-06-25 | 2018-01-30 | Endocyte, Inc. | Vitamin receptor drug delivery conjugates for treating inflammation |
| CA2690943A1 (en) | 2007-06-25 | 2008-12-31 | Endocyte, Inc. | Conjugates containing hydrophilic spacer linkers |
| SG189817A1 (en) | 2008-04-30 | 2013-05-31 | Immunogen Inc | Potent conjugates and hydrophilic linkers |
| KR20230003298A (ko) | 2008-04-30 | 2023-01-05 | 이뮤노젠 아이엔씨 | 가교제 및 그 용도 |
| CN102482345A (zh) | 2009-05-13 | 2012-05-30 | 航道生物技术有限责任公司 | 针对流感病毒的中和分子 |
| SG176068A1 (en) * | 2009-06-03 | 2011-12-29 | Immunogen Inc | Conjugation methods |
| AU2015201534B2 (en) * | 2009-06-03 | 2016-11-24 | Immunogen, Inc. | Conjugation methods |
| FR2947269B1 (fr) | 2009-06-29 | 2013-01-18 | Sanofi Aventis | Nouveaux composes anticancereux |
| FR2949469A1 (fr) * | 2009-08-25 | 2011-03-04 | Sanofi Aventis | Derives anticancereux, leur preparation et leur application en therapeutique |
| AU2010292172A1 (en) | 2009-09-09 | 2012-05-03 | Centrose, Llc | Extracellular targeted drug conjugates |
| TW201117814A (en) * | 2009-10-02 | 2011-06-01 | Sanofi Aventis | New maytansinoids and the use of said maytansinoids to prepare conjugates with an antibody |
| EP2486023A4 (en) * | 2009-10-06 | 2014-05-07 | Immunogen Inc | EFFICIENT CONJUGATES AND HYDROPHILIC BINDER |
| RS55738B1 (sr) | 2010-02-24 | 2017-07-31 | Immunogen Inc | Imunokonjugati protiv folatnog receptora 1 i njihove upotrebe |
| EP3620467A1 (en) | 2010-03-12 | 2020-03-11 | Debiopharm International SA | Cd37-binding molecules and immunoconjugates thereof |
| FR2963007B1 (fr) | 2010-07-26 | 2013-04-05 | Sanofi Aventis | Derives anticancereux, leur preparation et leur application therapeutique |
| WO2012019024A2 (en) | 2010-08-04 | 2012-02-09 | Immunogen, Inc. | Her3-binding molecules and immunoconjugates thereof |
| TW201302793A (zh) | 2010-09-03 | 2013-01-16 | Glaxo Group Ltd | 新穎之抗原結合蛋白 |
| EA201390575A1 (ru) | 2010-10-29 | 2014-01-30 | Иммьюноджен, Инк. | Неантагонистические egfr-связывающие молекулы и их иммуноконъюгаты |
| MX2013004761A (es) | 2010-10-29 | 2013-08-27 | Immunogen Inc | Nuevas moleculas de union al receptor del factor de crecimiento epidermico (egfr) e inmunoconjugados de estas. |
| MX2013005046A (es) * | 2010-11-03 | 2013-12-12 | Immunogen Inc | Agentes citotoxicos que comprenden nuevos derivados de ansamitocina. |
| CN103313990B (zh) * | 2010-11-17 | 2016-07-20 | 基因泰克公司 | 丙氨酰美登醇抗体偶联物 |
| CN103260406B (zh) | 2010-12-09 | 2016-10-19 | 伊缪诺金公司 | 制备带电荷交联剂的方法 |
| DK2675480T3 (en) | 2011-02-15 | 2019-04-15 | Immunogen Inc | PROCEDURES FOR PREPARING CONJUGATES |
| WO2012122514A1 (en) * | 2011-03-09 | 2012-09-13 | Centrose, Llc | Extracellular targeted drug conjugates |
| MY171008A (en) | 2011-03-29 | 2019-09-23 | Immunogen Inc | Preparation of maytansinoid antibody conjugates by a one-step process |
| CN103717262B (zh) | 2011-03-29 | 2017-09-15 | 伊缪诺金公司 | 通过一步法制备类美登素抗体缀合物 |
| BR112013025415B1 (pt) | 2011-04-01 | 2021-03-16 | Immunogen, Inc | uso de um imunoconjugado antirreceptor de folato 1 (folr1) |
| WO2012138749A1 (en) * | 2011-04-04 | 2012-10-11 | Immunogen, Inc. | Methods for decreasing ocular toxicity of antibody drug conjugates |
| ME03071B (me) | 2011-04-21 | 2019-01-20 | Bristol Myers Squibb Co | Polipeptidi antitela koji antagonizuju cd40 |
| US8815226B2 (en) | 2011-06-10 | 2014-08-26 | Mersana Therapeutics, Inc. | Protein-polymer-drug conjugates |
| TWI597065B (zh) | 2011-06-10 | 2017-09-01 | 梅爾莎納醫療公司 | 蛋白質-聚合物-藥物共軛體 |
| US10300140B2 (en) | 2011-07-28 | 2019-05-28 | I2 Pharmaceuticals, Inc. | Sur-binding proteins against ERBB3 |
| MX2014006087A (es) | 2011-11-21 | 2014-08-01 | Immunogen Inc | Metodo de tratamiento de tumores que son resistentes a terapias contra el receptor del factor de crecimiento epidermico (egfr) con agentes citotoxicos conjugados con anticuerpos dirigidos contra el receptor del factor de crecimiento epidermico. |
| WO2013096828A1 (en) | 2011-12-22 | 2013-06-27 | Sea Lane Biotechnologies, Llc | Surrogate binding proteins |
| RU2624731C2 (ru) * | 2012-01-27 | 2017-07-06 | Ф. Хоффманн-Ля Рош Аг | Конъюгаты антагонистов интегрина для нацеленной доставки к клеткам, экспрессирующим vla-4 |
| US10080805B2 (en) | 2012-02-24 | 2018-09-25 | Purdue Research Foundation | Cholecystokinin B receptor targeting for imaging and therapy |
| US20140080175A1 (en) | 2012-03-29 | 2014-03-20 | Endocyte, Inc. | Processes for preparing tubulysin derivatives and conjugates thereof |
| MX362514B (es) | 2012-08-31 | 2019-01-22 | Immunogen Inc | Kits y ensayos de diagnostico para la deteccion del receptor 1 de folato. |
| MX359599B (es) | 2012-10-04 | 2018-09-12 | Immunogen Inc | Uso de una membrana de pvdf para purificar conjugados de agentes de unión celular y agentes citotóxicos. |
| US20150306242A1 (en) * | 2012-10-04 | 2015-10-29 | Immunogen, Inc. | Process for preparing stable antibody maytansinoid conjugates |
| IN2015DN04147A (cg-RX-API-DMAC7.html) | 2012-10-16 | 2015-10-16 | Endocyte Inc | |
| NO2789793T3 (cg-RX-API-DMAC7.html) * | 2012-10-24 | 2018-01-27 | ||
| IN2015DN02349A (cg-RX-API-DMAC7.html) * | 2012-10-24 | 2015-08-28 | Polytherics Ltd | |
| EP2922818B1 (en) | 2012-11-24 | 2018-09-05 | Hangzhou Dac Biotech Co., Ltd | Hydrophilic linkers and their uses for conjugation of drugs to cell binding molecules |
| WO2014089177A2 (en) | 2012-12-04 | 2014-06-12 | Massachusetts Institute Of Technology | Compounds, conjugates and compositions of epipolythiodiketopiperazines and polythiodiketopiperazines |
| CN103288957B (zh) | 2012-12-21 | 2015-01-28 | 百奥泰生物科技(广州)有限公司 | 一种抑制肿瘤生长的抗体药物衍生物及其制备方法和用途 |
| CN104650113A (zh) * | 2012-12-21 | 2015-05-27 | 百奥泰生物科技(广州)有限公司 | 类美登素衍生物及其制备方法和用途 |
| US9968673B2 (en) * | 2013-02-26 | 2018-05-15 | Commissariat á l'ènergie atomique et aux ènergies alternatives | Immunogenic composition in emulsion form comprising two dispersed phases, one comprising an antigen and the other comprising an immunostimulating agent |
| FR3002454B1 (fr) * | 2013-02-26 | 2015-04-10 | Commissariat Energie Atomique | Composition immunogene sous forme d'emulsion |
| WO2014134483A2 (en) * | 2013-02-28 | 2014-09-04 | Immunogen, Inc. | Conjugates comprising cell-binding agents and cytotoxic agents |
| CN110143999B (zh) | 2013-03-15 | 2023-12-05 | 酵活英属哥伦比亚省公司 | 具细胞毒性和抗有丝分裂的化合物以及其使用方法 |
| MY183572A (en) | 2013-03-15 | 2021-02-26 | Regeneron Pharma | Biologically active molecules, conjugates thereof, and therapeutic uses |
| MX370829B (es) * | 2013-05-02 | 2020-01-08 | Hoffmann La Roche | Uso de un anticuerpo para cd20 afucosilado con un conjugado de anticuerpo para cd22-fármaco. |
| US10208125B2 (en) | 2013-07-15 | 2019-02-19 | University of Pittsburgh—of the Commonwealth System of Higher Education | Anti-mucin 1 binding agents and uses thereof |
| TWI641620B (zh) * | 2013-08-21 | 2018-11-21 | 再生元醫藥公司 | 抗-prlr抗體及其用途 |
| CN105530942B (zh) | 2013-08-26 | 2019-10-11 | 瑞泽恩制药公司 | 一种包含大环内酯类非对映体的药物组合物、其制备方法和用途 |
| PL3038650T3 (pl) | 2013-08-30 | 2021-11-22 | Immunogen, Inc. | Przeciwciała i testy do wykrywania receptora folianu 1 |
| SG10201801779XA (en) | 2013-09-05 | 2018-04-27 | Jackson Lab | Compositions for rna-chromatin interaction analysis and uses thereof |
| IL298044B2 (en) | 2013-10-08 | 2024-11-01 | Immunogen Inc | Dosing regimens of anti-FOLR1 immunoconjugate |
| CN105813655B (zh) | 2013-10-11 | 2022-03-15 | 阿萨纳生物科技有限责任公司 | 蛋白-聚合物-药物缀合物 |
| CN105979970B (zh) | 2013-10-11 | 2019-09-10 | 梅尔莎纳医疗公司 | 蛋白质-聚合物-药物共轭物 |
| CN103483357B (zh) * | 2013-10-12 | 2015-11-18 | 齐鲁制药有限公司 | 一种抗体-美登素偶联物的中间体新晶型及其制备方法 |
| WO2015081857A1 (en) * | 2013-12-02 | 2015-06-11 | Hong Kong Baptist University | Anticancer maytansinoids with two fused macrocyclic rings |
| JP6560681B2 (ja) | 2013-12-27 | 2019-08-14 | ザイムワークス インコーポレイティド | 薬物結合体のためのスルホンアミド含有連結系 |
| AU2015210578B2 (en) * | 2014-01-29 | 2020-04-16 | Jiangsu Hengrui Medicine Co., Ltd. | Ligand-cytotoxic drug conjugate, preparation method therefor, and uses thereof |
| MY178160A (en) | 2014-03-11 | 2020-10-06 | Regeneron Pharma | Anti-egfrviii antibodies and uses thereof |
| JP6805425B2 (ja) | 2014-03-24 | 2020-12-23 | ザ・トラスティーズ・オブ・コランビア・ユニバーシティー・イン・ザ・シティー・オブ・ニューヨーク | タグ付けされたヌクレオチドを製造するための化学的方法 |
| EP3138568B1 (en) * | 2014-04-29 | 2019-04-17 | Genequantum Healthcare (Suzhou) Co., Ltd. | New stable antibody-drug conjugate, preparation method therefor, and use thereof |
| CA2953371C (en) | 2014-06-30 | 2021-08-24 | Tarveda Therapeutics, Inc. | Targeted conjugates and particles and formulations thereof |
| DK3160513T3 (da) | 2014-06-30 | 2020-04-06 | Glykos Finland Oy | Saccharidderivat af en toksisk payload og antistofkonjugater deraf |
| CA2958882A1 (en) | 2014-09-02 | 2016-03-10 | Immunogen, Inc. | Methods for formulating antibody drug conjugate compositions |
| WO2016036804A1 (en) | 2014-09-03 | 2016-03-10 | Immunogen, Inc. | Cytotoxic benzodiazepine derivatives |
| EP3188733B1 (en) * | 2014-09-03 | 2019-11-06 | ImmunoGen, Inc. | Conjugates comprising cell-binding agents and cytotoxic agents |
| SG11201701455SA (en) | 2014-09-03 | 2017-03-30 | Immunogen Inc | Cytotoxic benzodiazepine derivatives |
| KR102494557B1 (ko) | 2014-09-17 | 2023-02-02 | 자임워크스 비씨 인코포레이티드 | 세포독성 및 항유사분열성 화합물, 그리고 이를 이용하는 방법 |
| MX386328B (es) | 2015-03-27 | 2025-03-18 | Regeneron Pharma | Derivados de maitansinoide, conjugados de los mismos, y metodos de uso. |
| SG10202110908WA (en) | 2015-05-04 | 2021-11-29 | Cytomx Therapeutics Inc | Anti-cd166 antibodies, activatable anti-cd166 antibodies, and methods of use thereof |
| US10449258B2 (en) | 2015-06-09 | 2019-10-22 | Xdcexplorer (Shanghai) Co., Ltd. | Antibody drug conjugate, intermediate, preparation method, pharmaceutical composition and uses thereof |
| SI3313845T1 (sl) | 2015-06-29 | 2021-01-29 | Immunogen, Inc. | Konjugati spremenjenih protiteles cisteina |
| JP6700321B2 (ja) * | 2015-07-04 | 2020-05-27 | ハンジョウ ディーエーシー バイオテック シーオー.,エルティディ.Hangzhou Dac Biotech Co.,Ltd. | 細胞結合分子の特異的共役体 |
| NZ737726A (en) | 2015-07-06 | 2023-03-31 | Regeneron Pharma | Multispecific antigen-binding molecules and uses thereof |
| AU2016297087B2 (en) | 2015-07-21 | 2021-02-18 | Immunogen, Inc | Methods of preparing cytotoxic benzodiazepine derivatives |
| US10509035B2 (en) | 2015-08-07 | 2019-12-17 | Gamamabs Pharma Sa | Antibodies, antibody drug conjugates and methods of use |
| KR102700777B1 (ko) | 2015-09-17 | 2024-08-29 | 이뮤노젠 아이엔씨 | 항-folr1 면역접합체를 포함하는 치료제 조합 |
| CA3001712A1 (en) | 2015-10-28 | 2017-05-04 | Tarveda Therapeutics, Inc. | Sstr-targeted conjugates and particles and formulations thereof |
| CN108495619A (zh) | 2015-11-10 | 2018-09-04 | 儿研所儿童医学中心 | 棘霉素制剂及其制备方法和使用方法 |
| PT3380525T (pt) | 2015-11-25 | 2024-02-05 | Immunogen Inc | Formulações farmacêuticas e métodos que as utilizam |
| CN108602890A (zh) | 2015-12-11 | 2018-09-28 | 瑞泽恩制药公司 | 用于减少或预防对egfr和/或erbb3阻滞剂具有抗性的肿瘤生长的方法 |
| MX390630B (es) | 2016-01-25 | 2025-03-21 | Regeneron Pharma | Derivados de maitansinoide, conjugados de los mismos y metodos de uso. |
| US11603346B2 (en) | 2016-03-07 | 2023-03-14 | Hanmi Pharm. Co., Ltd. | Polyethylene glycol derivative and use thereof |
| EP3448891A1 (en) | 2016-04-28 | 2019-03-06 | Regeneron Pharmaceuticals, Inc. | Methods of making multispecific antigen-binding molecules |
| US10918627B2 (en) | 2016-05-11 | 2021-02-16 | Massachusetts Institute Of Technology | Convergent and enantioselective total synthesis of Communesin analogs |
| CN109963870B (zh) | 2016-06-08 | 2023-07-28 | 艾伯维公司 | 抗b7-h3抗体和抗体药物偶联物 |
| JP2019524687A (ja) | 2016-07-01 | 2019-09-05 | グラクソスミスクライン、インテレクチュアル、プロパティー、ディベロップメント、リミテッドGlaxosmithkline Intellectual Property Development Limited | 抗体薬物複合体およびこれを用いた治療方法 |
| US10772972B2 (en) | 2016-09-23 | 2020-09-15 | Regeneron Pharmaceuticals, Inc. | Anti-STEAP2 antibody drug conjugates, and compositions and uses thereof |
| HRP20231281T1 (hr) | 2016-09-23 | 2024-02-02 | Regeneron Pharmaceuticals, Inc. | Bispecifična anti-muc16-cd3 antitijela i konjugati anti-muc16 s lijekom |
| KR20190074310A (ko) | 2016-11-08 | 2019-06-27 | 리제너론 파마슈티칼스 인코포레이티드 | 스테로이드 및 이의 단백질-접합체 |
| TWI782930B (zh) | 2016-11-16 | 2022-11-11 | 美商再生元醫藥公司 | 抗met抗體,結合met之雙特異性抗原結合分子及其使用方法 |
| MA46887A (fr) | 2016-11-23 | 2019-10-02 | Immunogen Inc | Sulfonation sélective de dérivés de benzodiazépine |
| IL266917B2 (en) | 2016-11-29 | 2023-10-01 | Regeneron Pharma | Anti-human prolactin receptor (prlr) antibody-drug conjugates and use thereof in combination therapy of prlr positive breast cancer |
| EP3554544A4 (en) | 2016-12-16 | 2020-07-29 | Bluefin Biomedicine, Inc. | ANTI-PROTEIN 1 ANTIBODY CONTAINING AN ANTI-CUB DOMAIN (CDCP1), ANTIBODY-DRUG CONJUGATES AND THEIR METHODS OF USE |
| LT3558391T (lt) | 2016-12-23 | 2022-07-11 | Immunogen, Inc. | Imunokonjugatai, kurių taikinys yra adam9, ir jų naudojimo būdai |
| WO2018129029A1 (en) | 2017-01-04 | 2018-07-12 | Immunogen, Inc. | Met antibodies and immunoconjugates and uses thereof |
| US20200061031A1 (en) | 2017-02-28 | 2020-02-27 | Kinki University | Method for treating egfr-tki-resistant non-small cell lung cancer by administration of anti-her3 antibody-drug conjugate |
| KR102681168B1 (ko) | 2017-02-28 | 2024-07-04 | 이뮤노젠 아이엔씨 | 자기희생적 펩티드 링커 및 이들의 접합체를 갖는 메이탄시노이드 유도체 |
| EP3381474A1 (en) * | 2017-03-27 | 2018-10-03 | Alfasigma S.p.A. | Histone deacetylase inhibitors-based antibody drug conjugates (adcs) and use in therapy |
| WO2018181059A1 (ja) | 2017-03-30 | 2018-10-04 | 日油株式会社 | ヘテロ二官能性単分散ポリエチレングリコール及びそれを用いた複合体 |
| WO2018195243A1 (en) | 2017-04-20 | 2018-10-25 | Immunogen, Inc. | Cytotoxic benzodiazepine derivatives and conjugates thereof |
| WO2018209239A1 (en) | 2017-05-11 | 2018-11-15 | Massachusetts Institute Of Technology | Potent agelastatin derivatives as modulators for cancer invasion and metastasis |
| JP7364471B2 (ja) | 2017-05-18 | 2023-10-18 | レゲネロン ファーマシューティカルス,インコーポレーテッド | シクロデキストリンタンパク質薬物コンジュゲート |
| CN111065622A (zh) | 2017-05-18 | 2020-04-24 | 里珍纳龙药品有限公司 | 双八氢菲羧酰胺类化合物及其蛋白质偶联物 |
| JP7348844B2 (ja) | 2017-06-07 | 2023-09-21 | リジェネロン・ファーマシューティカルズ・インコーポレイテッド | 内部移行酵素のための組成物および方法 |
| CA3067311A1 (en) * | 2017-06-20 | 2018-12-27 | Sorrento Therapeutics, Inc. | Cd38 antibody drug conjugate |
| US10640508B2 (en) | 2017-10-13 | 2020-05-05 | Massachusetts Institute Of Technology | Diazene directed modular synthesis of compounds with quaternary carbon centers |
| WO2019094395A2 (en) | 2017-11-07 | 2019-05-16 | Regeneron Pharmaceuticals, Inc. | Hydrophilic linkers for antibody drug conjugates |
| CN109771658B (zh) * | 2017-11-14 | 2021-12-10 | 博瑞生物医药(苏州)股份有限公司 | 靶向多臂偶联物 |
| WO2019133652A1 (en) | 2017-12-28 | 2019-07-04 | Immunogen, Inc. | Benzodiazepine derivatives |
| AU2019205542B2 (en) | 2018-01-08 | 2025-10-23 | Regeneron Pharmaceuticals, Inc. | Steroids and antibody-conjugates thereof |
| CA3087993A1 (en) * | 2018-01-12 | 2019-07-18 | Immunogen, Inc. | Methods for antibody drug conjugation, purification, and formulation |
| AU2019262953B2 (en) | 2018-04-30 | 2025-12-04 | Regeneron Pharmaceuticals, Inc. | Antibodies, and bispecific antigen-binding molecules that bind HER2 and/or APLP2, conjugates, and uses thereof |
| SG11202010909RA (en) | 2018-05-09 | 2020-12-30 | Regeneron Pharma | Anti-msr1 antibodies and methods of use thereof |
| US12252544B2 (en) | 2018-05-17 | 2025-03-18 | Regeneron Pharmaceuticals, Inc. | Anti-CD63 antibodies, conjugates, and uses thereof |
| US20210275685A1 (en) | 2018-06-26 | 2021-09-09 | Immunogen, Inc. | Immunoconjugates targeting adam9 and methods of use thereof |
| WO2020014306A1 (en) | 2018-07-10 | 2020-01-16 | Immunogen, Inc. | Met antibodies and immunoconjugates and uses thereof |
| TW202024133A (zh) | 2018-09-20 | 2020-07-01 | 日商第一三共股份有限公司 | 利用投予抗her3抗體-藥物結合物之her3突變癌之治療 |
| CN109350748B (zh) * | 2018-10-24 | 2021-06-11 | 沈阳药科大学 | 氧化还原双敏感键桥连小分子前药及其自组装纳米粒 |
| TWI897855B (zh) | 2018-10-26 | 2025-09-21 | 美商免疫遺傳股份有限公司 | E p C A M 抗體、可活化抗體及免疫偶聯物以及其用途 |
| MA53455B1 (fr) | 2018-11-20 | 2022-11-30 | Regeneron Pharma | Dérivés de bis-octahydrophénanthrène carboxamide et leurs conjugués protéiques destinés à être utilisés en tant qu'agonistes de lxr |
| SG11202106658XA (en) | 2018-12-21 | 2021-07-29 | Regeneron Pharma | Rifamycin analogs and antibody-drug conjugates thereof |
| EA202191769A1 (ru) | 2018-12-21 | 2021-12-08 | Регенерон Фармасьютикалз, Инк. | Тубулизины и конъюгаты белок-тубулизин |
| KR20250156850A (ko) | 2019-01-08 | 2025-11-03 | 리제너론 파마슈티칼스 인코포레이티드 | 흔적이 없는 링커 및 이의 단백질-접합체 |
| MX2021010114A (es) | 2019-02-21 | 2021-12-10 | Regeneron Pharma | Métodos para tratar el cáncer ocular mediante el uso de anticuerpos anti-met y moléculas de unión a antígeno bispecíficas que se unen a met. |
| JP7682798B2 (ja) | 2019-03-20 | 2025-05-26 | ザ リージェンツ オブ ザ ユニバーシティ オブ カリフォルニア | クローディン6抗体及び薬物複合体 |
| JP7682797B2 (ja) | 2019-03-20 | 2025-05-26 | ザ リージェンツ オブ ザ ユニバーシティ オブ カリフォルニア | クローディン6二重特異性抗体 |
| TW202102506A (zh) | 2019-03-29 | 2021-01-16 | 美商伊繆諾金公司 | 苯二氮平衍生物 |
| EP4316524A3 (en) | 2019-04-26 | 2024-04-24 | ImmunoGen, Inc. | Camptothecin derivatives |
| US11535634B2 (en) | 2019-06-05 | 2022-12-27 | Massachusetts Institute Of Technology | Compounds, conjugates, and compositions of epipolythiodiketopiperazines and polythiodiketopiperazines and uses thereof |
| PE20220485A1 (es) | 2019-07-10 | 2022-04-04 | Cybrexa 3 Inc | Conjugados peptidicos de agentes dirigidos a microtubulos como terapeuticos |
| PE20220563A1 (es) | 2019-07-10 | 2022-04-13 | Cybrexa 2 Inc | Conjugados peptidicos de citotoxinas como terapeuticos |
| MX2022002886A (es) | 2019-09-16 | 2022-04-06 | Regeneron Pharma | Proteinas de union met radiomarcadas para la obtencion de imagenes por inmuno-pet. |
| US11814428B2 (en) | 2019-09-19 | 2023-11-14 | Regeneron Pharmaceuticals, Inc. | Anti-PTCRA antibody-drug conjugates and uses thereof |
| CN115348874A (zh) | 2020-01-24 | 2022-11-15 | 里珍纳龙药品有限公司 | 蛋白质-抗病毒化合物偶联物 |
| BR112022017064A2 (pt) | 2020-02-25 | 2022-11-16 | Mediboston Inc | Derivados da camptotecina e seus conjugados |
| KR20220148200A (ko) | 2020-02-28 | 2022-11-04 | 리제너론 파마슈티칼스 인코포레이티드 | Her2에 결합하는 이중특이적 항원 결합 분자 및 이의 사용 방법 |
| US11701427B2 (en) | 2020-04-16 | 2023-07-18 | Regeneron Pharmaceuticals, Inc. | Diels-alder conjugation methods |
| EP4171653A2 (en) | 2020-06-24 | 2023-05-03 | Regeneron Pharmaceuticals, Inc. | Tubulysins and protein-tubulysin conjugates |
| EP4178624A2 (en) | 2020-07-07 | 2023-05-17 | Bionecure Therapeutics, Inc. | Maytansinoids as adc payloads and their use for the treatment of cancer |
| AU2021308190A1 (en) | 2020-07-13 | 2023-02-02 | Regeneron Pharmaceuticals, Inc. | Camptothecin analogs conjugated to a glutamine residue in a protein, and their use |
| BR112023000711A2 (pt) | 2020-07-17 | 2023-01-31 | Daiichi Sankyo Co Ltd | Método para produzir uma composição de conjugado de anticorpo-fármaco, e, composição de conjugado de anticorpo-fármaco |
| KR20230070337A (ko) | 2020-09-14 | 2023-05-22 | 리제너론 파마슈티칼스 인코포레이티드 | Glp1 펩티도미메틱을 포함하는 항체-약물 접합체 및 이의 용도 |
| CN116406379A (zh) | 2020-10-22 | 2023-07-07 | 瑞泽恩制药公司 | 抗fgfr2抗体和其使用方法 |
| AR124681A1 (es) | 2021-01-20 | 2023-04-26 | Abbvie Inc | Conjugados anticuerpo-fármaco anti-egfr |
| US12030888B2 (en) | 2021-02-24 | 2024-07-09 | Massachusetts Institute Of Technology | Himastatin derivatives, and processes of preparation thereof, and uses thereof |
| US20220378929A1 (en) | 2021-02-25 | 2022-12-01 | MediBoston Limted | Anti-her2 antibody-drug conjugates and uses thereof |
| EP4304658A4 (en) | 2021-03-08 | 2025-03-12 | Genequantum Healthcare (Suzhou) Co., Ltd. | Antibody-immunagonist conjugate and applications thereof |
| WO2022218331A1 (en) | 2021-04-14 | 2022-10-20 | Genequantum Healthcare (Suzhou) Co., Ltd. | Linkers, conjugates and applications thereof |
| JP2024529960A (ja) | 2021-07-28 | 2024-08-14 | レゲネロン ファーマシューティカルス,インコーポレーテッド | タンパク質-抗ウイルス化合物コンジュゲート |
| JP2024534101A (ja) * | 2021-08-20 | 2024-09-18 | プサン ナショナル ユニバーシティ インダストリー - ユニバーシティ コーポレイション ファンデーション | オブジェクトが固定された膨潤性高分子の製造方法 |
| US12377157B2 (en) | 2021-12-15 | 2025-08-05 | The Administrators Of The Tulane Educational Fund | Conjugates, their compositions, and their related methods |
| WO2023129518A1 (en) | 2021-12-29 | 2023-07-06 | Regeneron Pharmaceuticals, Inc. | Tubulysins and protein-tubulysin conjugates |
| MX2024008640A (es) | 2022-01-12 | 2024-07-24 | Regeneron Pharma | Analogos de camptotecina conjugados a un residuo de glutamina en una proteina y su uso. |
| MX2024008361A (es) | 2022-01-14 | 2024-09-10 | Regeneron Pharma | Derivados de verrucarina a y conjugados anticuerpo-fármaco de los mismos. |
| JP2025512735A (ja) | 2022-03-11 | 2025-04-22 | リジェネロン ファーマシューティカルズ,インク. | Glp1ペプチド模倣体を含む抗glp1r抗体係留型薬物コンジュゲートおよびその使用 |
| CN120239705A (zh) | 2022-07-21 | 2025-07-01 | 萤火虫生物股份有限公司 | 糖皮质激素受体激动剂和其缀合物 |
| US20240108744A1 (en) | 2022-07-27 | 2024-04-04 | Mediboston Limited | Auristatin derivatives and conjugates thereof |
| KR20250128394A (ko) | 2022-11-30 | 2025-08-27 | 리제너론 파마슈티칼스 인코포레이티드 | Tlr7 작용제 및 이의 항체-약물-접합체 |
| AU2023407365A1 (en) | 2022-12-21 | 2025-07-31 | Regeneron Pharmaceuticals, Inc. | Prodrugs of topoisomerase i inhibitor for adc conjugations and methods of use thereof |
| CN121001751A (zh) | 2023-02-09 | 2025-11-21 | 里珍纳龙药品有限公司 | 借助逆电子需求狄尔斯-阿尔德反应的抗体-药物缀合物 |
| WO2024220628A1 (en) * | 2023-04-18 | 2024-10-24 | The Regents Of The University Of Colorado, A Body Corporate | Target for combatting the foreign body response to implantable biomaterials |
| WO2024229105A1 (en) | 2023-05-02 | 2024-11-07 | Regeneron Pharmaceuticals, Inc. | Anti-human m-cadherin (cdh15) antibodies, conjugates, and uses thereof for delivery of genetic payloads to muscle cells |
| CN121219016A (zh) | 2023-06-02 | 2025-12-26 | 第一三共株式会社 | 抗-her3抗体-药物缀合物和rasg12c抑制剂的组合 |
| WO2025014533A1 (en) | 2023-07-10 | 2025-01-16 | Regeneron Pharmaceuticals, Inc. | Anti-human cacng1 antibody-drug conjugates and uses thereof |
| WO2025094146A1 (en) | 2023-11-02 | 2025-05-08 | Immunogen Switzerland Gmbh | Vasconstrictors for reducing ocular toxicity of antibody-maytansinoid conjugates |
| WO2025096921A1 (en) | 2023-11-03 | 2025-05-08 | Regeneron Pharmaceuticals, Inc. | Peptide acids as a glp1r agonist and antibody-drug conjugates thereof |
| WO2025117727A1 (en) | 2023-11-29 | 2025-06-05 | Regeneron Pharmaceuticals, Inc. | Analogs of quinoxaline/quinoline cytotoxins, linker- payloads, protein-drug conjugates, and uses thereof |
| CN118580194A (zh) * | 2024-05-21 | 2024-09-03 | 南方科技大学 | 一种双功能分子探针的制备方法 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030220245A1 (en) * | 2000-06-02 | 2003-11-27 | Hubbell Jeffrey A | Conjugate addition reactions for the controlled delivery of pharmaceutical active compounds |
| US20050169933A1 (en) * | 2003-10-10 | 2005-08-04 | Immunogen, Inc. | Method of targeting specific cell populations using cell-binding agent maytansinoid conjugates linked via a non-cleavable linker, said conjugates, and methods of making said conjugates |
| US20060127352A1 (en) * | 1999-02-01 | 2006-06-15 | Eidgenossische Technische Hochschule Zurich | Conjugate addition reactions for the controlled delivery of pharmaceutically active compounds |
| RU2404810C2 (ru) * | 2004-06-01 | 2010-11-27 | Дженентек, Инк. | Конъюгаты антитело-лекарственное средство и способы |
Family Cites Families (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7157458B2 (en) * | 2001-04-17 | 2007-01-02 | Cryolife, Inc. | Bifunctional energy-reversible acyl-compositions |
| WO2002087497A2 (en) * | 2001-04-26 | 2002-11-07 | Board Of Regents, The University Of Texas System | Therapeutic agent/ligand conjugate compositions, their methods of synthesis and use |
| US6441163B1 (en) | 2001-05-31 | 2002-08-27 | Immunogen, Inc. | Methods for preparation of cytotoxic conjugates of maytansinoids and cell binding agents |
| US6716821B2 (en) | 2001-12-21 | 2004-04-06 | Immunogen Inc. | Cytotoxic agents bearing a reactive polyethylene glycol moiety, cytotoxic conjugates comprising polyethylene glycol linking groups, and methods of making and using the same |
| US8877901B2 (en) * | 2002-12-13 | 2014-11-04 | Immunomedics, Inc. | Camptothecin-binding moiety conjugates |
| US6596757B1 (en) * | 2002-05-14 | 2003-07-22 | Immunogen Inc. | Cytotoxic agents comprising polyethylene glycol-containing taxanes and their therapeutic use |
| US7276497B2 (en) | 2003-05-20 | 2007-10-02 | Immunogen Inc. | Cytotoxic agents comprising new maytansinoids |
| AU2004240541B2 (en) | 2003-05-20 | 2009-08-20 | Immunogen, Inc. | Improved cytotoxic agents comprising new maytansinoids |
| US9005625B2 (en) * | 2003-07-25 | 2015-04-14 | Novo Nordisk A/S | Antibody toxin conjugates |
| ZA200601182B (en) * | 2003-10-10 | 2007-04-25 | Immunogen Inc | Method of targeting specific cell populations using cell-binding agent maytansinoid conjugates linked via a non-cleavable linker, said conjugates, and methods of making said conjugates |
| CN104447992A (zh) | 2004-09-23 | 2015-03-25 | 健泰科生物技术公司 | 半胱氨酸改造的抗体和偶联物 |
| JP2008518023A (ja) * | 2004-10-27 | 2008-05-29 | メディミューン,インコーポレーテッド | 同族抗原に対する親和性を改変することによる抗体特異性の調節 |
| EP1853322B1 (en) * | 2005-02-11 | 2014-06-25 | ImmunoGen, Inc. | Process for preparing maytansinoid antibody conjugates |
| US20090226465A1 (en) * | 2005-10-31 | 2009-09-10 | Jackson David Y | Macrocyclic depsipeptide antibody-drug conjugates and methods |
| PT2032606E (pt) * | 2006-05-30 | 2014-02-05 | Genentech Inc | Anticorpos e imunoconjugados e respetivas utilizações |
| US20080311040A1 (en) * | 2007-03-06 | 2008-12-18 | Flagship Ventures | METHODS AND COMPOSITIONS FOR IMPROVED THERAPEUTIC EFFECTS WITH siRNA |
| EP2176295B1 (en) * | 2007-07-16 | 2014-11-19 | Genentech, Inc. | Humanized anti-cd79b antibodies and immunoconjugates and methods of use |
| SG189817A1 (en) | 2008-04-30 | 2013-05-31 | Immunogen Inc | Potent conjugates and hydrophilic linkers |
-
2009
- 2009-04-30 SG SG2013033188A patent/SG189817A1/en unknown
- 2009-04-30 NZ NZ588851A patent/NZ588851A/xx not_active IP Right Cessation
- 2009-04-30 BR BRPI0911442-4A patent/BRPI0911442A2/pt not_active IP Right Cessation
- 2009-04-30 UA UAA201014270A patent/UA108598C2/ru unknown
- 2009-04-30 CA CA2722109A patent/CA2722109A1/en not_active Abandoned
- 2009-04-30 CN CN200980125295.2A patent/CN102083461B/zh not_active Expired - Fee Related
- 2009-04-30 EP EP09739778.0A patent/EP2276506A4/en not_active Withdrawn
- 2009-04-30 MX MX2010011808A patent/MX2010011808A/es active IP Right Grant
- 2009-04-30 KR KR1020107026963A patent/KR20100137585A/ko not_active Abandoned
- 2009-04-30 JP JP2011507633A patent/JP2011523628A/ja not_active Withdrawn
- 2009-04-30 CN CN201410407711.0A patent/CN104258413A/zh active Pending
- 2009-04-30 WO PCT/US2009/042203 patent/WO2009134952A2/en not_active Ceased
- 2009-04-30 RU RU2010148740/04A patent/RU2487877C2/ru not_active IP Right Cessation
- 2009-04-30 WO PCT/US2009/042259 patent/WO2009134976A1/en not_active Ceased
- 2009-04-30 AU AU2009243009A patent/AU2009243009B2/en not_active Ceased
- 2009-10-06 US US12/574,466 patent/US9150649B2/en not_active Expired - Fee Related
-
2010
- 2010-10-26 IL IL208937A patent/IL208937A0/en unknown
- 2010-11-01 ZA ZA2010/07806A patent/ZA201007806B/en unknown
-
2012
- 2012-05-17 US US13/473,974 patent/US20120226026A1/en not_active Abandoned
-
2015
- 2015-04-24 JP JP2015089052A patent/JP5980989B2/ja not_active Expired - Fee Related
- 2015-08-25 US US14/835,236 patent/US20160114052A1/en not_active Abandoned
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060127352A1 (en) * | 1999-02-01 | 2006-06-15 | Eidgenossische Technische Hochschule Zurich | Conjugate addition reactions for the controlled delivery of pharmaceutically active compounds |
| US20030220245A1 (en) * | 2000-06-02 | 2003-11-27 | Hubbell Jeffrey A | Conjugate addition reactions for the controlled delivery of pharmaceutical active compounds |
| US20050169933A1 (en) * | 2003-10-10 | 2005-08-04 | Immunogen, Inc. | Method of targeting specific cell populations using cell-binding agent maytansinoid conjugates linked via a non-cleavable linker, said conjugates, and methods of making said conjugates |
| RU2404810C2 (ru) * | 2004-06-01 | 2010-11-27 | Дженентек, Инк. | Конъюгаты антитело-лекарственное средство и способы |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2754369C2 (ru) * | 2016-03-02 | 2021-09-01 | Эйсай Ар Энд Ди Менеджмент Ко., Лтд. | Конъюгаты антитело-лекарственное средство на основе эрибулина и способы применения |
| RU2844753C2 (ru) * | 2016-03-02 | 2025-08-05 | Эйсай Ар Энд Ди Менеджмент Ко., Лтд. | Конъюгаты антитело-лекарственное средство на основе эрибулина и способы применения |
| RU2653443C2 (ru) * | 2016-07-15 | 2018-05-08 | Закрытое Акционерное Общество "Биокад" | Биспецифичные анти-her2/анти-her3 антитела |
Also Published As
| Publication number | Publication date |
|---|---|
| CN102083461A (zh) | 2011-06-01 |
| ZA201007806B (en) | 2013-01-30 |
| RU2010148740A (ru) | 2012-06-10 |
| US20100129314A1 (en) | 2010-05-27 |
| CN104258413A (zh) | 2015-01-07 |
| JP2011523628A (ja) | 2011-08-18 |
| WO2009134952A3 (en) | 2010-01-07 |
| AU2009243009A1 (en) | 2009-11-05 |
| JP2015163622A (ja) | 2015-09-10 |
| MX2010011808A (es) | 2011-03-04 |
| KR20100137585A (ko) | 2010-12-30 |
| AU2009243009B2 (en) | 2014-09-11 |
| EP2276506A4 (en) | 2014-05-07 |
| IL208937A0 (en) | 2011-01-31 |
| US20160114052A1 (en) | 2016-04-28 |
| CN102083461B (zh) | 2014-09-17 |
| US20120226026A1 (en) | 2012-09-06 |
| US9150649B2 (en) | 2015-10-06 |
| UA108598C2 (xx) | 2015-05-25 |
| WO2009134952A2 (en) | 2009-11-05 |
| BRPI0911442A2 (pt) | 2019-03-12 |
| SG189817A1 (en) | 2013-05-31 |
| NZ588851A (en) | 2013-05-31 |
| WO2009134976A1 (en) | 2009-11-05 |
| CA2722109A1 (en) | 2009-11-05 |
| EP2276506A1 (en) | 2011-01-26 |
| JP5980989B2 (ja) | 2016-08-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2487877C2 (ru) | Высокоэффективные конъюгаты и гидрофильные сшивающие агенты (линкеры) | |
| JP2011523628A5 (cg-RX-API-DMAC7.html) | ||
| JP2011519864A5 (cg-RX-API-DMAC7.html) | ||
| JP2021113210A5 (cg-RX-API-DMAC7.html) | ||
| JP6598821B2 (ja) | ペプチドリンカーを有する新規メイタンシノイド誘導体およびその結合体 | |
| JP6462591B2 (ja) | 新規抗体コンジュゲートおよびその使用 | |
| RU2503687C2 (ru) | Сшивающие реагенты и их применение | |
| CY1122302T1 (el) | Νεα anti-cd38 αντισωματα για τη θεραπευτικη αγωγη καρκινου | |
| JP2019532056A5 (cg-RX-API-DMAC7.html) | ||
| BR112021004656A2 (pt) | conjugado fármaco-ligante de análogo exatecano, método para preparar o mesmo e aplicação do mesmo | |
| JP2018523471A5 (cg-RX-API-DMAC7.html) | ||
| JP2012006928A5 (cg-RX-API-DMAC7.html) | ||
| JP7791843B2 (ja) | 抗bcma抗体-薬物コンジュゲート及び使用方法 | |
| CN110831976A (zh) | 用于制备抗体-药物缀合物的双重缀合方法 | |
| JP2018523471A (ja) | システイン置換免疫グロブリン | |
| CA2833477A1 (en) | Novel binder-drug conjugates (adcs) and their use | |
| JP2013506709A (ja) | 有効なコンジュゲートおよび親水性リンカー | |
| RU2016145445A (ru) | Конъюгат антитела и лекарственного средства и его применение для лечения рака | |
| RU2012135395A (ru) | Антитела против рецептора фолиевой кислоты 1, их иммуноконъюгаты и использование | |
| RU2016133846A (ru) | Конъюгат лиганд - цитотоксическое лекарственное средство, способ его получения и его применения | |
| US20190290775A1 (en) | Use of Antibody Drug Conjugates Comprising Tubulin Disrupting Agents to Treat Solid Tumor | |
| JP2013506709A5 (cg-RX-API-DMAC7.html) | ||
| IL278574B1 (en) | Compounds acting on glycans and methods of using them | |
| EA039757B1 (ru) | Конъюгат антитела против erbb2 и лекарственного средства и его композиция, способ его получения и его применение | |
| EA034138B1 (ru) | Цитотоксические бензодиазепиновые производные |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20170501 |