JP2015507684A - バイオマスから調製された2,5−フランジカルボン酸系ポリエステル - Google Patents
バイオマスから調製された2,5−フランジカルボン酸系ポリエステル Download PDFInfo
- Publication number
- JP2015507684A JP2015507684A JP2014551279A JP2014551279A JP2015507684A JP 2015507684 A JP2015507684 A JP 2015507684A JP 2014551279 A JP2014551279 A JP 2014551279A JP 2014551279 A JP2014551279 A JP 2014551279A JP 2015507684 A JP2015507684 A JP 2015507684A
- Authority
- JP
- Japan
- Prior art keywords
- diol
- temperature
- copolyester
- isosorbide
- dsc
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- CHTHALBTIRVDBM-UHFFFAOYSA-N furan-2,5-dicarboxylic acid Chemical compound OC(=O)C1=CC=C(C(O)=O)O1 CHTHALBTIRVDBM-UHFFFAOYSA-N 0.000 title claims abstract description 79
- 229920000728 polyester Polymers 0.000 title claims abstract description 50
- 239000002028 Biomass Substances 0.000 title abstract description 15
- KLDXJTOLSGUMSJ-JGWLITMVSA-N Isosorbide Chemical compound O[C@@H]1CO[C@@H]2[C@@H](O)CO[C@@H]21 KLDXJTOLSGUMSJ-JGWLITMVSA-N 0.000 claims abstract description 39
- 229960002479 isosorbide Drugs 0.000 claims abstract description 39
- 239000000178 monomer Substances 0.000 claims abstract description 29
- 229920001634 Copolyester Polymers 0.000 claims abstract description 27
- 150000002009 diols Chemical class 0.000 claims abstract description 18
- 150000002148 esters Chemical class 0.000 claims abstract description 17
- -1 polyethylene Polymers 0.000 claims abstract description 12
- KKEYFWRCBNTPAC-UHFFFAOYSA-N Terephthalic acid Chemical compound OC(=O)C1=CC=C(C(O)=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-N 0.000 claims abstract description 11
- 125000001931 aliphatic group Chemical group 0.000 claims abstract description 9
- 125000005907 alkyl ester group Chemical group 0.000 claims abstract description 8
- PUZXLFQBRGGQEY-UHFFFAOYSA-N 1,3-dioxonane-4,9-dione Chemical compound O=C1CCCCC(=O)OCO1 PUZXLFQBRGGQEY-UHFFFAOYSA-N 0.000 claims abstract description 6
- 239000004698 Polyethylene Substances 0.000 claims abstract description 5
- DNXDYHALMANNEJ-UHFFFAOYSA-N furan-2,3-dicarboxylic acid Chemical compound OC(=O)C=1C=COC=1C(O)=O DNXDYHALMANNEJ-UHFFFAOYSA-N 0.000 claims abstract description 5
- 229920000573 polyethylene Polymers 0.000 claims abstract description 5
- 238000010438 heat treatment Methods 0.000 claims description 41
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 claims description 40
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 claims description 38
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 27
- 238000000034 method Methods 0.000 claims description 24
- 229910052757 nitrogen Inorganic materials 0.000 claims description 20
- WERYXYBDKMZEQL-UHFFFAOYSA-N butane-1,4-diol Chemical compound OCCCCO WERYXYBDKMZEQL-UHFFFAOYSA-N 0.000 claims description 18
- 239000011541 reaction mixture Substances 0.000 claims description 15
- 239000003054 catalyst Substances 0.000 claims description 11
- ADCOVFLJGNWWNZ-UHFFFAOYSA-N antimony trioxide Chemical group O=[Sb]O[Sb]=O ADCOVFLJGNWWNZ-UHFFFAOYSA-N 0.000 claims description 8
- 235000013361 beverage Nutrition 0.000 claims description 5
- 235000013305 food Nutrition 0.000 claims description 5
- 238000004806 packaging method and process Methods 0.000 claims description 3
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 claims description 2
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 claims description 2
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 claims description 2
- 125000002723 alicyclic group Chemical group 0.000 claims description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 2
- 229910052782 aluminium Inorganic materials 0.000 claims description 2
- 229910017052 cobalt Inorganic materials 0.000 claims description 2
- 239000010941 cobalt Substances 0.000 claims description 2
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 claims description 2
- 150000003839 salts Chemical class 0.000 claims description 2
- 229910052710 silicon Inorganic materials 0.000 claims description 2
- 239000010703 silicon Substances 0.000 claims description 2
- 238000003756 stirring Methods 0.000 claims description 2
- 229910052719 titanium Inorganic materials 0.000 claims description 2
- 239000010936 titanium Substances 0.000 claims description 2
- 229910052726 zirconium Inorganic materials 0.000 claims description 2
- 229920000139 polyethylene terephthalate Polymers 0.000 abstract description 5
- 239000005020 polyethylene terephthalate Substances 0.000 abstract description 5
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 69
- 229920000642 polymer Polymers 0.000 description 69
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 66
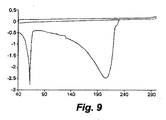
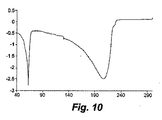
- 238000000113 differential scanning calorimetry Methods 0.000 description 55
- 239000000203 mixture Substances 0.000 description 35
- 239000002904 solvent Substances 0.000 description 34
- 238000005033 Fourier transform infrared spectroscopy Methods 0.000 description 31
- 238000005481 NMR spectroscopy Methods 0.000 description 30
- 230000000875 corresponding effect Effects 0.000 description 30
- YLQBMQCUIZJEEH-UHFFFAOYSA-N Furan Chemical compound C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 29
- 238000006068 polycondensation reaction Methods 0.000 description 22
- 230000015572 biosynthetic process Effects 0.000 description 18
- YQUVCSBJEUQKSH-UHFFFAOYSA-N protochatechuic acid Natural products OC(=O)C1=CC=C(O)C(O)=C1 YQUVCSBJEUQKSH-UHFFFAOYSA-N 0.000 description 18
- WKOLLVMJNQIZCI-UHFFFAOYSA-N vanillic acid Chemical compound COC1=CC(C(O)=O)=CC=C1O WKOLLVMJNQIZCI-UHFFFAOYSA-N 0.000 description 18
- TUUBOHWZSQXCSW-UHFFFAOYSA-N vanillic acid Natural products COC1=CC(O)=CC(C(O)=O)=C1 TUUBOHWZSQXCSW-UHFFFAOYSA-N 0.000 description 18
- 238000001228 spectrum Methods 0.000 description 15
- 238000003786 synthesis reaction Methods 0.000 description 15
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 12
- 238000002441 X-ray diffraction Methods 0.000 description 11
- 238000001816 cooling Methods 0.000 description 11
- 229920005610 lignin Polymers 0.000 description 10
- 239000007788 liquid Substances 0.000 description 10
- 238000002844 melting Methods 0.000 description 10
- 230000008018 melting Effects 0.000 description 10
- 238000002360 preparation method Methods 0.000 description 10
- 239000000243 solution Substances 0.000 description 10
- 239000002253 acid Substances 0.000 description 9
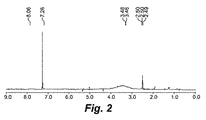
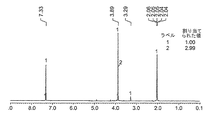
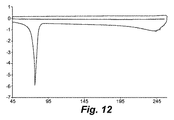
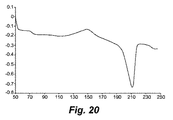
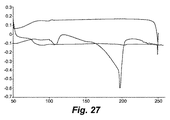
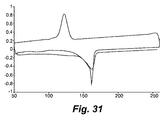
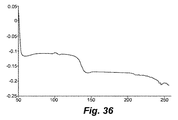
- 238000001938 differential scanning calorimetry curve Methods 0.000 description 9
- 238000000655 nuclear magnetic resonance spectrum Methods 0.000 description 9
- DSLRVRBSNLHVBH-UHFFFAOYSA-N 2,5-furandimethanol Chemical compound OCC1=CC=C(CO)O1 DSLRVRBSNLHVBH-UHFFFAOYSA-N 0.000 description 8
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 8
- 238000005086 pumping Methods 0.000 description 8
- DVVGBNZLQNDSPA-UHFFFAOYSA-N 3,6,11-trioxabicyclo[6.2.1]undeca-1(10),8-diene-2,7-dione Chemical compound O=C1OCCOC(=O)C2=CC=C1O2 DVVGBNZLQNDSPA-UHFFFAOYSA-N 0.000 description 7
- UHVMKWLQGQBLFM-UHFFFAOYSA-N 3,8,13-trioxabicyclo[8.2.1]trideca-1(12),10-diene-2,9-dione Chemical compound O=C1OCCCCOC(=O)C2=CC=C1O2 UHVMKWLQGQBLFM-UHFFFAOYSA-N 0.000 description 7
- NOEGNKMFWQHSLB-UHFFFAOYSA-N 5-hydroxymethylfurfural Chemical compound OCC1=CC=C(C=O)O1 NOEGNKMFWQHSLB-UHFFFAOYSA-N 0.000 description 7
- RJGBSYZFOCAGQY-UHFFFAOYSA-N hydroxymethylfurfural Natural products COC1=CC=C(C=O)O1 RJGBSYZFOCAGQY-UHFFFAOYSA-N 0.000 description 7
- WNLRTRBMVRJNCN-UHFFFAOYSA-N adipic acid Chemical compound OC(=O)CCCCC(O)=O WNLRTRBMVRJNCN-UHFFFAOYSA-N 0.000 description 6
- 238000006243 chemical reaction Methods 0.000 description 6
- 238000002425 crystallisation Methods 0.000 description 6
- 230000008025 crystallization Effects 0.000 description 6
- 238000004090 dissolution Methods 0.000 description 6
- HYBBIBNJHNGZAN-UHFFFAOYSA-N furfural Chemical compound O=CC1=CC=CO1 HYBBIBNJHNGZAN-UHFFFAOYSA-N 0.000 description 6
- 238000000746 purification Methods 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 238000001157 Fourier transform infrared spectrum Methods 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 230000008569 process Effects 0.000 description 5
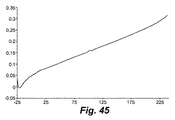
- 238000001757 thermogravimetry curve Methods 0.000 description 5
- 230000005484 gravity Effects 0.000 description 4
- 238000002329 infrared spectrum Methods 0.000 description 4
- QQVIHTHCMHWDBS-UHFFFAOYSA-N isophthalic acid Chemical compound OC(=O)C1=CC=CC(C(O)=O)=C1 QQVIHTHCMHWDBS-UHFFFAOYSA-N 0.000 description 4
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- 239000001361 adipic acid Substances 0.000 description 3
- 235000011037 adipic acid Nutrition 0.000 description 3
- 125000003118 aryl group Chemical group 0.000 description 3
- 150000005690 diesters Chemical class 0.000 description 3
- 238000001704 evaporation Methods 0.000 description 3
- 230000008020 evaporation Effects 0.000 description 3
- 150000002402 hexoses Chemical class 0.000 description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 3
- 230000010354 integration Effects 0.000 description 3
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N phenol group Chemical group C1(=CC=CC=C1)O ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 3
- 238000002411 thermogravimetry Methods 0.000 description 3
- HXRDPPOLNGAQLL-UHFFFAOYSA-N 2,5-dimethyl-3H-furan-2,3-dicarboxylic acid Chemical compound CC1(OC(=CC1C(=O)O)C)C(=O)O HXRDPPOLNGAQLL-UHFFFAOYSA-N 0.000 description 2
- SVTBMSDMJJWYQN-UHFFFAOYSA-N 2-methylpentane-2,4-diol Chemical compound CC(O)CC(C)(C)O SVTBMSDMJJWYQN-UHFFFAOYSA-N 0.000 description 2
- 0 COC(c1ccc(C(O*)=O)[o]1)=O Chemical compound COC(c1ccc(C(O*)=O)[o]1)=O 0.000 description 2
- SRBFZHDQGSBBOR-IOVATXLUSA-N D-xylopyranose Chemical compound O[C@@H]1COC(O)[C@H](O)[C@H]1O SRBFZHDQGSBBOR-IOVATXLUSA-N 0.000 description 2
- 229920002488 Hemicellulose Polymers 0.000 description 2
- 229920006125 amorphous polymer Polymers 0.000 description 2
- 239000006227 byproduct Substances 0.000 description 2
- 125000004432 carbon atom Chemical group C* 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 238000000354 decomposition reaction Methods 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 125000001142 dicarboxylic acid group Chemical group 0.000 description 2
- 239000012634 fragment Substances 0.000 description 2
- 150000004676 glycans Chemical class 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 150000004702 methyl esters Chemical class 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- 239000012299 nitrogen atmosphere Substances 0.000 description 2
- BDJRBEYXGGNYIS-UHFFFAOYSA-N nonanedioic acid Chemical compound OC(=O)CCCCCCCC(O)=O BDJRBEYXGGNYIS-UHFFFAOYSA-N 0.000 description 2
- 150000002972 pentoses Chemical class 0.000 description 2
- XNGIFLGASWRNHJ-UHFFFAOYSA-N phthalic acid Chemical compound OC(=O)C1=CC=CC=C1C(O)=O XNGIFLGASWRNHJ-UHFFFAOYSA-N 0.000 description 2
- 229920001282 polysaccharide Polymers 0.000 description 2
- 239000005017 polysaccharide Substances 0.000 description 2
- 239000002244 precipitate Substances 0.000 description 2
- YPFDHNVEDLHUCE-UHFFFAOYSA-N propane-1,3-diol Chemical compound OCCCO YPFDHNVEDLHUCE-UHFFFAOYSA-N 0.000 description 2
- 239000012429 reaction media Substances 0.000 description 2
- 230000035484 reaction time Effects 0.000 description 2
- CXMXRPHRNRROMY-UHFFFAOYSA-N sebacic acid Chemical compound OC(=O)CCCCCCCCC(O)=O CXMXRPHRNRROMY-UHFFFAOYSA-N 0.000 description 2
- 239000002023 wood Substances 0.000 description 2
- 238000005160 1H NMR spectroscopy Methods 0.000 description 1
- JCTXKRPTIMZBJT-UHFFFAOYSA-N 2,2,4-trimethylpentane-1,3-diol Chemical compound CC(C)C(O)C(C)(C)CO JCTXKRPTIMZBJT-UHFFFAOYSA-N 0.000 description 1
- QHDADHHODABHLK-UHFFFAOYSA-N 2,2-dimethylpentane-1,3-diol Chemical compound CCC(O)C(C)(C)CO QHDADHHODABHLK-UHFFFAOYSA-N 0.000 description 1
- RWLALWYNXFYRGW-UHFFFAOYSA-N 2-Ethyl-1,3-hexanediol Chemical compound CCCC(O)C(CC)CO RWLALWYNXFYRGW-UHFFFAOYSA-N 0.000 description 1
- YQPCHPBGAALCRT-UHFFFAOYSA-N 2-[1-(carboxymethyl)cyclohexyl]acetic acid Chemical compound OC(=O)CC1(CC(O)=O)CCCCC1 YQPCHPBGAALCRT-UHFFFAOYSA-N 0.000 description 1
- WTPYFJNYAMXZJG-UHFFFAOYSA-N 2-[4-(2-hydroxyethoxy)phenoxy]ethanol Chemical compound OCCOC1=CC=C(OCCO)C=C1 WTPYFJNYAMXZJG-UHFFFAOYSA-N 0.000 description 1
- DOPZLYNWNJHAOS-UHFFFAOYSA-N 2-methyl-1,2-butanediol Chemical compound CCC(C)(O)CO DOPZLYNWNJHAOS-UHFFFAOYSA-N 0.000 description 1
- CPHURRLSZSRQFS-UHFFFAOYSA-N 3-[4-[2-[4-(3-hydroxypropoxy)phenyl]propan-2-yl]phenoxy]propan-1-ol Chemical compound C=1C=C(OCCCO)C=CC=1C(C)(C)C1=CC=C(OCCCO)C=C1 CPHURRLSZSRQFS-UHFFFAOYSA-N 0.000 description 1
- RBQLGIKHSXQZTB-UHFFFAOYSA-N 3-methylpentane-2,4-diol Chemical compound CC(O)C(C)C(C)O RBQLGIKHSXQZTB-UHFFFAOYSA-N 0.000 description 1
- OCKGFTQIICXDQW-ZEQRLZLVSA-N 5-[(1r)-1-hydroxy-2-[4-[(2r)-2-hydroxy-2-(4-methyl-1-oxo-3h-2-benzofuran-5-yl)ethyl]piperazin-1-yl]ethyl]-4-methyl-3h-2-benzofuran-1-one Chemical compound C1=C2C(=O)OCC2=C(C)C([C@@H](O)CN2CCN(CC2)C[C@H](O)C2=CC=C3C(=O)OCC3=C2C)=C1 OCKGFTQIICXDQW-ZEQRLZLVSA-N 0.000 description 1
- 238000004483 ATR-FTIR spectroscopy Methods 0.000 description 1
- GSNUFIFRDBKVIE-UHFFFAOYSA-N DMF Natural products CC1=CC=C(C)O1 GSNUFIFRDBKVIE-UHFFFAOYSA-N 0.000 description 1
- ZAFNJMIOTHYJRJ-UHFFFAOYSA-N Diisopropyl ether Chemical compound CC(C)OC(C)C ZAFNJMIOTHYJRJ-UHFFFAOYSA-N 0.000 description 1
- 229930091371 Fructose Natural products 0.000 description 1
- 239000005715 Fructose Substances 0.000 description 1
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- NHTMVDHEPJAVLT-UHFFFAOYSA-N Isooctane Chemical compound CC(C)CC(C)(C)C NHTMVDHEPJAVLT-UHFFFAOYSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- ALQSHHUCVQOPAS-UHFFFAOYSA-N Pentane-1,5-diol Chemical compound OCCCCCO ALQSHHUCVQOPAS-UHFFFAOYSA-N 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- SOGYZZRPOIMNHO-UHFFFAOYSA-N [2-(hydroxymethyl)furan-3-yl]methanol Chemical compound OCC=1C=COC=1CO SOGYZZRPOIMNHO-UHFFFAOYSA-N 0.000 description 1
- YIMQCDZDWXUDCA-UHFFFAOYSA-N [4-(hydroxymethyl)cyclohexyl]methanol Chemical compound OCC1CCC(CO)CC1 YIMQCDZDWXUDCA-UHFFFAOYSA-N 0.000 description 1
- 230000003679 aging effect Effects 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 229910052787 antimony Inorganic materials 0.000 description 1
- WATWJIUSRGPENY-UHFFFAOYSA-N antimony atom Chemical compound [Sb] WATWJIUSRGPENY-UHFFFAOYSA-N 0.000 description 1
- 229910000410 antimony oxide Inorganic materials 0.000 description 1
- PYMYPHUHKUWMLA-UHFFFAOYSA-N arabinose Natural products OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 description 1
- SRBFZHDQGSBBOR-UHFFFAOYSA-N beta-D-Pyranose-Lyxose Natural products OC1COC(O)C(O)C1O SRBFZHDQGSBBOR-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 238000000071 blow moulding Methods 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 239000003610 charcoal Substances 0.000 description 1
- 238000007385 chemical modification Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- PFURGBBHAOXLIO-UHFFFAOYSA-N cyclohexane-1,2-diol Chemical compound OC1CCCCC1O PFURGBBHAOXLIO-UHFFFAOYSA-N 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- FXJUUMGKLWHCNZ-UHFFFAOYSA-N dimethyl furan-2,3-dicarboxylate Chemical compound COC(=O)C=1C=COC=1C(=O)OC FXJUUMGKLWHCNZ-UHFFFAOYSA-N 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000001523 electrospinning Methods 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 125000004185 ester group Chemical group 0.000 description 1
- 230000032050 esterification Effects 0.000 description 1
- 238000005886 esterification reaction Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000004880 explosion Methods 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 238000007765 extrusion coating Methods 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000002803 fossil fuel Substances 0.000 description 1
- 239000000446 fuel Substances 0.000 description 1
- 239000013538 functional additive Substances 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 150000002240 furans Chemical class 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 230000009477 glass transition Effects 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 125000000623 heterocyclic group Chemical class 0.000 description 1
- OHMBHFSEKCCCBW-UHFFFAOYSA-N hexane-2,5-diol Chemical compound CC(O)CCC(C)O OHMBHFSEKCCCBW-UHFFFAOYSA-N 0.000 description 1
- 229920006158 high molecular weight polymer Polymers 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 238000001746 injection moulding Methods 0.000 description 1
- 238000009413 insulation Methods 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 238000003475 lamination Methods 0.000 description 1
- 239000004973 liquid crystal related substance Substances 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 238000003760 magnetic stirring Methods 0.000 description 1
- 125000001160 methoxycarbonyl group Chemical group [H]C([H])([H])OC(*)=O 0.000 description 1
- OJURWUUOVGOHJZ-UHFFFAOYSA-N methyl 2-[(2-acetyloxyphenyl)methyl-[2-[(2-acetyloxyphenyl)methyl-(2-methoxy-2-oxoethyl)amino]ethyl]amino]acetate Chemical compound C=1C=CC=C(OC(C)=O)C=1CN(CC(=O)OC)CCN(CC(=O)OC)CC1=CC=CC=C1OC(C)=O OJURWUUOVGOHJZ-UHFFFAOYSA-N 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- RXOHFPCZGPKIRD-UHFFFAOYSA-N naphthalene-2,6-dicarboxylic acid Chemical compound C1=C(C(O)=O)C=CC2=CC(C(=O)O)=CC=C21 RXOHFPCZGPKIRD-UHFFFAOYSA-N 0.000 description 1
- 229920005615 natural polymer Polymers 0.000 description 1
- SLCVBVWXLSEKPL-UHFFFAOYSA-N neopentyl glycol Chemical compound OCC(C)(C)CO SLCVBVWXLSEKPL-UHFFFAOYSA-N 0.000 description 1
- VTRUBDSFZJNXHI-UHFFFAOYSA-N oxoantimony Chemical compound [Sb]=O VTRUBDSFZJNXHI-UHFFFAOYSA-N 0.000 description 1
- 239000003348 petrochemical agent Substances 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 229920002959 polymer blend Polymers 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 238000000425 proton nuclear magnetic resonance spectrum Methods 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 238000010992 reflux Methods 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 238000007142 ring opening reaction Methods 0.000 description 1
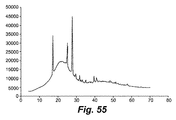
- 238000001542 size-exclusion chromatography Methods 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 238000009987 spinning Methods 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- KKEYFWRCBNTPAC-UHFFFAOYSA-L terephthalate(2-) Chemical compound [O-]C(=O)C1=CC=C(C([O-])=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-L 0.000 description 1
- 238000003878 thermal aging Methods 0.000 description 1
- 238000005979 thermal decomposition reaction Methods 0.000 description 1
- 238000003856 thermoforming Methods 0.000 description 1
- 238000005809 transesterification reaction Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G63/00—Macromolecular compounds obtained by reactions forming a carboxylic ester link in the main chain of the macromolecule
- C08G63/02—Polyesters derived from hydroxycarboxylic acids or from polycarboxylic acids and polyhydroxy compounds
- C08G63/12—Polyesters derived from hydroxycarboxylic acids or from polycarboxylic acids and polyhydroxy compounds derived from polycarboxylic acids and polyhydroxy compounds
- C08G63/16—Dicarboxylic acids and dihydroxy compounds
- C08G63/18—Dicarboxylic acids and dihydroxy compounds the acids or hydroxy compounds containing carbocyclic rings
- C08G63/181—Acids containing aromatic rings
- C08G63/183—Terephthalic acids
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G63/00—Macromolecular compounds obtained by reactions forming a carboxylic ester link in the main chain of the macromolecule
- C08G63/02—Polyesters derived from hydroxycarboxylic acids or from polycarboxylic acids and polyhydroxy compounds
- C08G63/12—Polyesters derived from hydroxycarboxylic acids or from polycarboxylic acids and polyhydroxy compounds derived from polycarboxylic acids and polyhydroxy compounds
- C08G63/16—Dicarboxylic acids and dihydroxy compounds
- C08G63/18—Dicarboxylic acids and dihydroxy compounds the acids or hydroxy compounds containing carbocyclic rings
- C08G63/181—Acids containing aromatic rings
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B1/00—Layered products having a non-planar shape
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G63/00—Macromolecular compounds obtained by reactions forming a carboxylic ester link in the main chain of the macromolecule
- C08G63/66—Polyesters containing oxygen in the form of ether groups
- C08G63/668—Polyesters containing oxygen in the form of ether groups derived from polycarboxylic acids and polyhydroxy compounds
- C08G63/672—Dicarboxylic acids and dihydroxy compounds
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/13—Hollow or container type article [e.g., tube, vase, etc.]
- Y10T428/1352—Polymer or resin containing [i.e., natural or synthetic]
- Y10T428/1397—Single layer [continuous layer]
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Polyesters Or Polycarbonates (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Wrappers (AREA)
- Packages (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Oxygen Or Sulfur (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261582983P | 2012-01-04 | 2012-01-04 | |
| US61/582,983 | 2012-01-04 | ||
| PCT/US2012/071766 WO2013103574A1 (en) | 2012-01-04 | 2012-12-27 | 2,5-furan dicarboxylic acid-based polyesters prepared from biomass |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| JP2015507684A true JP2015507684A (ja) | 2015-03-12 |
Family
ID=47501551
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014551279A Pending JP2015507684A (ja) | 2012-01-04 | 2012-12-27 | バイオマスから調製された2,5−フランジカルボン酸系ポリエステル |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US20130171397A1 (enExample) |
| EP (1) | EP2800771A1 (enExample) |
| JP (1) | JP2015507684A (enExample) |
| CN (1) | CN104379631A (enExample) |
| AU (1) | AU2012363608B2 (enExample) |
| BR (1) | BR112014016453A8 (enExample) |
| CA (1) | CA2859547A1 (enExample) |
| IN (1) | IN2014MN01416A (enExample) |
| MX (1) | MX2014008097A (enExample) |
| RU (1) | RU2606515C2 (enExample) |
| WO (1) | WO2013103574A1 (enExample) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018012572A1 (ja) * | 2016-07-15 | 2018-01-18 | 株式会社クラレ | シーラントフィルム及びその製造方法 |
| KR20190098493A (ko) * | 2018-02-14 | 2019-08-22 | 한국화학연구원 | 폴리헤테로아릴렌에테르 공중합체 및 이의 제조방법 |
| JP2021530374A (ja) * | 2018-07-12 | 2021-11-11 | フラニックス・テクノロジーズ・ベーフェー | 容器の製造方法及びその容器 |
| JP2022144048A (ja) * | 2021-03-18 | 2022-10-03 | 三菱ケミカル株式会社 | ポリエステル樹脂およびそれを用いた成形品 |
| JP2023526036A (ja) * | 2020-05-15 | 2023-06-20 | アーチャー-ダニエルズ-ミッドランド カンパニー | 少なくとも1種のバイオベースのモノマーを含むモノマーの同時製造 |
Families Citing this family (46)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11484627B2 (en) | 2010-10-20 | 2022-11-01 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants, and novel composite structures which may be used for medical and non-medical applications |
| US20120101593A1 (en) | 2010-10-20 | 2012-04-26 | BIOS2 Medical, Inc. | Implantable polymer for bone and vascular lesions |
| US10525169B2 (en) | 2010-10-20 | 2020-01-07 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants, and novel composite structures which may be used for medical and non-medical applications |
| US11207109B2 (en) | 2010-10-20 | 2021-12-28 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants, and novel composite structures which may be used for medical and non-medical applications |
| US11291483B2 (en) | 2010-10-20 | 2022-04-05 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants |
| US11058796B2 (en) | 2010-10-20 | 2021-07-13 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants, and novel composite structures which may be used for medical and non-medical applications |
| US10525168B2 (en) | 2010-10-20 | 2020-01-07 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants, and novel composite structures which may be used for medical and non-medical applications |
| EP2819620A4 (en) | 2012-02-29 | 2015-11-04 | 206 Ortho Inc | METHOD AND APPARATUS FOR TREATING BONE FRACTURES, INCLUDING THE USE OF COMPOSITE IMPLANTS |
| US9725558B2 (en) * | 2013-03-15 | 2017-08-08 | Sulzer Chemtech Ag | Process to prepare a cyclic oligomer and a cyclic oligomer obtainable thereby |
| EP2928942B1 (en) | 2013-03-15 | 2017-03-29 | Sulzer Chemtech AG | A process to prepare a polyester polymer composition comprising a polyester polymer having furanic units and a polyester polymer composition obtainable thereby and the use thereof |
| JP2016525379A (ja) | 2013-05-23 | 2016-08-25 | 206 オーソ,インコーポレーテッド | 複合材インプラントの提供および使用を含む、骨折を治療するための、ならびに/または、骨を補強および/もしくは増強するための方法および装置 |
| DE102013223496A1 (de) * | 2013-11-18 | 2015-05-21 | Tesa Se | Neuartiges Polyester geeignet zur Herstellung von Trägermaterialien für Klebebändern |
| KR101586412B1 (ko) * | 2014-01-28 | 2016-01-18 | 한국기술교육대학교 산학협력단 | 공중합 폴리에스터와 폴리락트산을 포함하는 수지 블렌드 및 그 제조방법 |
| ES2682949T3 (es) * | 2014-03-11 | 2018-09-24 | Synvina C.V. | Procedimiento destinado a la preparación de un poliéster bajo unas condiciones de esterificación específicas |
| US9890242B2 (en) * | 2014-03-11 | 2018-02-13 | Synvina C.V. | Polyester and method for preparing such a polyester |
| US10316140B2 (en) | 2014-03-21 | 2019-06-11 | Furanix Technologies B.V. | Polyesters comprising 2,5-furandicarboxylate and saturated diol units having a high glass transition temperature |
| WO2015166070A1 (en) * | 2014-04-30 | 2015-11-05 | Stichting Dienst Landbouwkundig Onderzoek | Polyisoidide furanoate thermoplastic polyesters and copolyesters and a use thereof in hot fill packaging |
| EP3154444B1 (en) | 2014-05-08 | 2022-02-23 | 206 Ortho, Inc. | Method and apparatus for treating bone fractures, and/or for fortifying and/or augmenting bone, including the provision and use of composite implants, and novel composite structures which may be used for medical and non-medical applications |
| EP3215542B1 (en) | 2014-11-12 | 2021-10-20 | Renmatix, Inc. | Method of coalescing a substance |
| US9321714B1 (en) | 2014-12-05 | 2016-04-26 | Uop Llc | Processes and catalysts for conversion of 2,5-dimethylfuran derivatives to terephthalate |
| US20180265629A1 (en) * | 2015-09-17 | 2018-09-20 | Micromidas, Inc. | Polymers and methods of producing thereof |
| WO2017091435A1 (en) * | 2015-11-24 | 2017-06-01 | Archer Daniels Midland Company | Oligomerizations of fdca and glycols in a one-pot esterification-transesterification process catalyzed by homogeneous organometallic lewis acids |
| CN108473666A (zh) | 2015-12-11 | 2018-08-31 | 埃维昂矿泉水有限公司 | 具有可为生物来源的抗结晶共聚单体的pet聚合物 |
| BR112018014278B1 (pt) | 2016-01-13 | 2022-07-12 | Stora Enso Oyj | Processos para a preparação de ácido 2,5-furandicarboxílico e intermediários e derivados dos mesmos |
| US11111057B2 (en) * | 2016-03-04 | 2021-09-07 | Amisha Patel | Bioplastic collapsible dispensing tube |
| ITUA20162764A1 (it) * | 2016-04-20 | 2017-10-20 | Novamont Spa | Nuovo poliestere e composizioni che lo contengono |
| US20190202977A1 (en) | 2016-09-16 | 2019-07-04 | Micromidas, Inc. | Polymers and methods of producing thereof |
| MX2019003944A (es) | 2016-10-05 | 2019-09-18 | Furanix Technologies Bv | Proceso para la produccion de un polimero de poli(tetrametilen-2,5 -furan dicarboxilato) polimerizado, en estado solido y polimero de este modo producido. |
| US10106564B2 (en) | 2016-12-12 | 2018-10-23 | International Business Machines Corporation | Furan-containing flame retardant molecules |
| US10155907B2 (en) | 2016-12-12 | 2018-12-18 | International Business Machines Corporation | Cross-linkable flame retardant materials |
| US9822208B1 (en) | 2017-01-03 | 2017-11-21 | International Business Machines Corporation | Flame retardant materials derived from furan dicarboxylic methyl ester |
| CN110234677A (zh) | 2017-01-26 | 2019-09-13 | 辛维纳有限合伙公司 | 2,5-呋喃二羧酸类聚酯 |
| BR112020000611B1 (pt) | 2017-07-12 | 2024-03-05 | Stora Enso Oyj | Produtos purificados de via de ácido 2,5-furanoodicarboxílico |
| US20200263053A1 (en) * | 2017-09-19 | 2020-08-20 | Swimc Llc | Coating compositions including a furan-containing polyester, articles, and methods of coating |
| ES2926976T3 (es) | 2017-10-25 | 2022-10-31 | Henkel Ag & Co Kgaa | Adhesivos a base de poliesterpolioles a base de ácido furandicarboxílico obtenido a partir de materias primas renovables |
| WO2019214575A1 (zh) * | 2018-05-10 | 2019-11-14 | 中国科学院长春应用化学研究所 | 一种新型呋喃生物基聚醚酯共聚物及其制备方法 |
| US12246879B2 (en) | 2018-08-12 | 2025-03-11 | Amisha Patel | Environmentally friendly can |
| US11434037B2 (en) * | 2018-08-12 | 2022-09-06 | Amisha Patel | Furan can |
| CN109575257B (zh) * | 2018-11-16 | 2021-09-07 | 中国科学院宁波材料技术与工程研究所 | 聚2,5-呋喃二甲酸-1,4-丁二酸新戊二醇酯及其制备方法、制品 |
| CN110183633B (zh) * | 2019-04-15 | 2021-03-16 | 中国科学院化学研究所 | 1,4;3,6-二缩水己六醇改性的呋喃二甲酸基无规共聚物及其制法与应用 |
| CN110229480B (zh) * | 2019-07-10 | 2021-09-10 | 中国科学院宁波材料技术与工程研究所 | 一种聚呋喃二甲酸丁二醇酯和聚对苯二甲酸丁二醇酯共混物的制备方法 |
| CN110862524B (zh) * | 2019-11-28 | 2021-04-02 | 东华大学 | 一种生物基高透明度高分子薄膜及其制备方法 |
| CN110903469B (zh) * | 2019-11-28 | 2021-04-02 | 东华大学 | 一种低结晶性生物可降解聚酯及其制备方法 |
| TWI868275B (zh) * | 2019-12-20 | 2025-01-01 | 日商東洋紡股份有限公司 | 雙軸配向聚酯膜及其製造方法 |
| CN113493561A (zh) * | 2020-03-20 | 2021-10-12 | 中国科学院大连化学物理研究所 | 一种2,6-萘二甲酸基共聚酯材料及其制备方法 |
| JP2023525479A (ja) * | 2020-04-30 | 2023-06-16 | ペプシコ・インク | 軽量高温充填容器及びそれを作製するための方法 |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH06238747A (ja) * | 1993-02-22 | 1994-08-30 | Toray Ind Inc | ポリエステルフィルム |
| JP2002512268A (ja) * | 1998-04-23 | 2002-04-23 | イー・アイ・デュポン・ドウ・ヌムール・アンド・カンパニー | イソソルビド含有ポリエステルおよびそれの製造方法 |
| JP2006506485A (ja) * | 2002-11-13 | 2006-02-23 | イーストマン ケミカル カンパニー | イソソルビド含有ポリエステルの製造方法 |
| JP2007146153A (ja) * | 2005-11-07 | 2007-06-14 | Canon Inc | 高分子化合物およびその合成方法 |
| JP2008291243A (ja) * | 2007-04-24 | 2008-12-04 | Mitsubishi Chemicals Corp | フラン構造を含む熱可塑性樹脂 |
| US20110282020A1 (en) * | 2008-12-30 | 2011-11-17 | Furanix Technologies B.V. | Process for preparing a polymer having a 2,5-furandicarboxylate moiety within the polymer backbone and such (co)polymers |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2600376A (en) * | 1949-11-26 | 1952-06-17 | Eastman Kodak Co | Polmesters of hydroxybenzoic acids |
| US6352426B1 (en) * | 1998-03-19 | 2002-03-05 | Advanced Plastics Technologies, Ltd. | Mold for injection molding multilayer preforms |
| US5959066A (en) * | 1998-04-23 | 1999-09-28 | Hna Holdings, Inc. | Polyesters including isosorbide as a comonomer and methods for making same |
| US6063465A (en) * | 1998-04-23 | 2000-05-16 | Hna Holdings, Inc. | Polyester container and method for making same |
| US7153587B2 (en) * | 2003-08-12 | 2006-12-26 | Mitsui Chemicals, Inc. | Polyester resin and polyester resin laminate container |
| US20090018264A1 (en) * | 2007-07-12 | 2009-01-15 | Canon Kabushiki Kaisha | Resin composition |
| JP5371259B2 (ja) * | 2008-02-20 | 2013-12-18 | キヤノン株式会社 | ポリエステル樹脂、その製造方法、成形品用組成物及び成形品 |
| CN102190782B (zh) * | 2010-03-17 | 2015-08-19 | 东丽纤维研究所(中国)有限公司 | 一种共聚酯化合物以及制备方法 |
| CN101899145B (zh) * | 2010-07-28 | 2012-07-11 | 江南大学 | 一种2,5-呋喃二甲酸基聚酯的制备方法 |
| CN102276812B (zh) * | 2011-06-29 | 2012-11-21 | 中国科学院长春应用化学研究所 | 一种聚2,5-呋喃二甲酸二醇酯的制备方法 |
| CN102432847B (zh) * | 2011-08-25 | 2013-10-16 | 中国科学院长春应用化学研究所 | 2,5-呋喃二甲酸-对苯二甲酸-脂肪族二元醇共聚酯及其制备方法 |
-
2012
- 2012-12-27 CN CN201280066195.9A patent/CN104379631A/zh active Pending
- 2012-12-27 MX MX2014008097A patent/MX2014008097A/es unknown
- 2012-12-27 JP JP2014551279A patent/JP2015507684A/ja active Pending
- 2012-12-27 EP EP12810023.7A patent/EP2800771A1/en not_active Withdrawn
- 2012-12-27 WO PCT/US2012/071766 patent/WO2013103574A1/en not_active Ceased
- 2012-12-27 RU RU2014132068A patent/RU2606515C2/ru active
- 2012-12-27 BR BR112014016453A patent/BR112014016453A8/pt not_active Application Discontinuation
- 2012-12-27 US US13/728,286 patent/US20130171397A1/en not_active Abandoned
- 2012-12-27 AU AU2012363608A patent/AU2012363608B2/en active Active
- 2012-12-27 CA CA2859547A patent/CA2859547A1/en not_active Abandoned
- 2012-12-27 IN IN1416MUN2014 patent/IN2014MN01416A/en unknown
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH06238747A (ja) * | 1993-02-22 | 1994-08-30 | Toray Ind Inc | ポリエステルフィルム |
| JP2002512268A (ja) * | 1998-04-23 | 2002-04-23 | イー・アイ・デュポン・ドウ・ヌムール・アンド・カンパニー | イソソルビド含有ポリエステルおよびそれの製造方法 |
| JP2006506485A (ja) * | 2002-11-13 | 2006-02-23 | イーストマン ケミカル カンパニー | イソソルビド含有ポリエステルの製造方法 |
| JP2007146153A (ja) * | 2005-11-07 | 2007-06-14 | Canon Inc | 高分子化合物およびその合成方法 |
| JP2008291243A (ja) * | 2007-04-24 | 2008-12-04 | Mitsubishi Chemicals Corp | フラン構造を含む熱可塑性樹脂 |
| US20110282020A1 (en) * | 2008-12-30 | 2011-11-17 | Furanix Technologies B.V. | Process for preparing a polymer having a 2,5-furandicarboxylate moiety within the polymer backbone and such (co)polymers |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018012572A1 (ja) * | 2016-07-15 | 2018-01-18 | 株式会社クラレ | シーラントフィルム及びその製造方法 |
| JPWO2018012572A1 (ja) * | 2016-07-15 | 2019-04-25 | 株式会社クラレ | シーラントフィルム及びその製造方法 |
| US11332574B2 (en) | 2016-07-15 | 2022-05-17 | Kuraray Co., Ltd. | Sealant film and method for producing same |
| KR20190098493A (ko) * | 2018-02-14 | 2019-08-22 | 한국화학연구원 | 폴리헤테로아릴렌에테르 공중합체 및 이의 제조방법 |
| KR102052935B1 (ko) * | 2018-02-14 | 2019-12-06 | 한국화학연구원 | 폴리헤테로아릴렌에테르 공중합체 및 이의 제조방법 |
| JP2021530374A (ja) * | 2018-07-12 | 2021-11-11 | フラニックス・テクノロジーズ・ベーフェー | 容器の製造方法及びその容器 |
| JP7374168B2 (ja) | 2018-07-12 | 2023-11-06 | フラニックス・テクノロジーズ・ベーフェー | 容器の製造方法及びその容器 |
| JP2023526036A (ja) * | 2020-05-15 | 2023-06-20 | アーチャー-ダニエルズ-ミッドランド カンパニー | 少なくとも1種のバイオベースのモノマーを含むモノマーの同時製造 |
| JP7307286B2 (ja) | 2020-05-15 | 2023-07-11 | アーチャー-ダニエルズ-ミッドランド カンパニー | 少なくとも1種のバイオベースのモノマーを含むモノマーの同時製造 |
| JP2022144048A (ja) * | 2021-03-18 | 2022-10-03 | 三菱ケミカル株式会社 | ポリエステル樹脂およびそれを用いた成形品 |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2859547A1 (en) | 2013-07-11 |
| CN104379631A (zh) | 2015-02-25 |
| AU2012363608A1 (en) | 2014-07-03 |
| WO2013103574A1 (en) | 2013-07-11 |
| EP2800771A1 (en) | 2014-11-12 |
| RU2606515C2 (ru) | 2017-01-10 |
| IN2014MN01416A (enExample) | 2015-04-03 |
| RU2014132068A (ru) | 2016-02-27 |
| AU2012363608B2 (en) | 2015-09-24 |
| BR112014016453A8 (pt) | 2017-07-04 |
| BR112014016453A2 (pt) | 2017-06-13 |
| MX2014008097A (es) | 2015-04-13 |
| US20130171397A1 (en) | 2013-07-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2015507684A (ja) | バイオマスから調製された2,5−フランジカルボン酸系ポリエステル | |
| Kainulainen et al. | Utilizing furfural-based bifuran diester as monomer and comonomer for high-performance bioplastics: Properties of poly (butylene furanoate), poly (butylene bifuranoate), and their copolyesters | |
| Gomes et al. | Synthesis and characterization of poly (2, 5‐furan dicarboxylate) s based on a variety of diols | |
| Gopalakrishnan et al. | Synthesis and characterization of bio-based furanic polyesters | |
| Wilsens et al. | Thermotropic polyesters from 2, 5-furandicarboxylic acid and vanillic acid: Synthesis, thermal properties, melt behavior, and mechanical performance | |
| Kasmi et al. | Synthesis and crystallization of new fully renewable resources-based copolyesters: Poly (1, 4-cyclohexanedimethanol-co-isosorbide 2, 5-furandicarboxylate) | |
| Yoon et al. | Advanced polymerization and properties of biobased high T g polyester of isosorbide and 1, 4-cyclohexanedicarboxylic acid through in situ acetylation | |
| CN108659209A (zh) | 一种2,5-呋喃二甲酸共聚酯及其制备方法和应用 | |
| JP2007146153A (ja) | 高分子化合物およびその合成方法 | |
| US11306179B2 (en) | Polyester copolymer | |
| Zhou et al. | Synthesis and properties of long chain polyesters from biobased 1, 5-pentanediol and aliphatic α, ω-diacids with 10-16 carbon atoms | |
| CN105199085A (zh) | 一种二聚酸改性聚丁二酸丁二醇共聚酯及其制备方法 | |
| Goto et al. | Synthesis and characterization of biobased polyesters containing anthraquinones derived from gallic acid | |
| JP2015535023A (ja) | リグニンおよび酢酸から誘導される生物再生可能なポリエチレンテレフタレート模倣体ポリ(ジヒドロフェルラ酸)ならびにそのコポリマー | |
| JP5114993B2 (ja) | ポリエステル樹脂 | |
| CN107245140B (zh) | 高分子量的脂肪-芳香族共聚酯及其制备方法和应用 | |
| Al-Tayyem et al. | Synthesis and characterization of novel bio-based polyesters and poly (ester amide) s based on isosorbide and symmetrical cyclic anhydrides | |
| US11548980B2 (en) | Polyester copolymer | |
| AU2015271988B2 (en) | 2,5-furan dicarboxylic acid-based polyesters prepared from biomass | |
| CN107090078A (zh) | 高分子量高结晶性的聚酯及其制备方法和应用 | |
| Kamran | Towards sustainable engineering plastics: Synthesis and characterisation of semi-aromatic polyamides based on renewable 2, 5-furandicarboxylic acid (FDCA) | |
| JP2022146911A (ja) | ポリエステル及びその製造方法 | |
| JP2004137490A (ja) | グリコール酸共重合体及びその製造方法 | |
| Wilsens | Exploring the application of 2, 5-furandicarboxylic acid as a monomer in high performance polymers: Synthesis, characterization, and properties | |
| Goto et al. | Synthesis and Characterization of Biobased Polyesters with Anthraquinones Derived from α‐Resorcylic Acid |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20150619 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20150623 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150827 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20150924 |