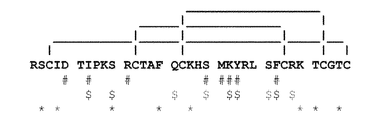
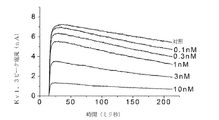
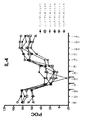
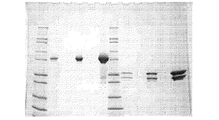
JP2012521360A - Kv1.3の選択的かつ強力なペプチド阻害剤 - Google Patents
Kv1.3の選択的かつ強力なペプチド阻害剤 Download PDFInfo
- Publication number
- JP2012521360A JP2012521360A JP2012501020A JP2012501020A JP2012521360A JP 2012521360 A JP2012521360 A JP 2012521360A JP 2012501020 A JP2012501020 A JP 2012501020A JP 2012501020 A JP2012501020 A JP 2012501020A JP 2012521360 A JP2012521360 A JP 2012521360A
- Authority
- JP
- Japan
- Prior art keywords
- composition
- amino acid
- ala
- lys
- residue
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 108090000765 processed proteins & peptides Proteins 0.000 title claims abstract description 404
- 101000994669 Homo sapiens Potassium voltage-gated channel subfamily A member 3 Proteins 0.000 title description 96
- 102100034355 Potassium voltage-gated channel subfamily A member 3 Human genes 0.000 title description 76
- 239000003112 inhibitor Substances 0.000 title description 36
- 230000003389 potentiating effect Effects 0.000 title description 9
- 239000003053 toxin Substances 0.000 claims abstract description 216
- 231100000765 toxin Toxicity 0.000 claims abstract description 202
- 108700012359 toxins Proteins 0.000 claims abstract description 199
- 239000000203 mixture Substances 0.000 claims abstract description 179
- 238000000034 method Methods 0.000 claims abstract description 69
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 25
- 201000006417 multiple sclerosis Diseases 0.000 claims abstract description 21
- 150000003839 salts Chemical class 0.000 claims abstract description 17
- 208000023275 Autoimmune disease Diseases 0.000 claims abstract description 16
- 239000003937 drug carrier Substances 0.000 claims abstract description 11
- 208000024891 symptom Diseases 0.000 claims abstract description 10
- 229920001223 polyethylene glycol Polymers 0.000 claims description 126
- 125000000539 amino acid group Chemical group 0.000 claims description 106
- 150000001413 amino acids Chemical group 0.000 claims description 73
- 125000005647 linker group Chemical group 0.000 claims description 51
- 241000282414 Homo sapiens Species 0.000 claims description 46
- 108060003951 Immunoglobulin Proteins 0.000 claims description 44
- 102000018358 immunoglobulin Human genes 0.000 claims description 44
- 230000004927 fusion Effects 0.000 claims description 29
- 230000002378 acidificating effect Effects 0.000 claims description 25
- 125000000217 alkyl group Chemical group 0.000 claims description 24
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 claims description 20
- 239000002202 Polyethylene glycol Substances 0.000 claims description 17
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 12
- 208000022559 Inflammatory bowel disease Diseases 0.000 claims description 11
- 108091006905 Human Serum Albumin Proteins 0.000 claims description 9
- 102000008100 Human Serum Albumin Human genes 0.000 claims description 9
- 206010025135 lupus erythematosus Diseases 0.000 claims description 9
- 206010039073 rheumatoid arthritis Diseases 0.000 claims description 9
- 201000004681 Psoriasis Diseases 0.000 claims description 8
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 claims description 8
- 210000004899 c-terminal region Anatomy 0.000 claims description 8
- 230000002757 inflammatory effect Effects 0.000 claims description 8
- 208000006386 Bone Resorption Diseases 0.000 claims description 7
- 206010052779 Transplant rejections Diseases 0.000 claims description 7
- 208000006673 asthma Diseases 0.000 claims description 7
- UCMIRNVEIXFBKS-UHFFFAOYSA-N beta-alanine Chemical compound NCCC(O)=O UCMIRNVEIXFBKS-UHFFFAOYSA-N 0.000 claims description 7
- 230000024279 bone resorption Effects 0.000 claims description 7
- OYIFNHCXNCRBQI-UHFFFAOYSA-N 2-aminoadipic acid Chemical compound OC(=O)C(N)CCCC(O)=O OYIFNHCXNCRBQI-UHFFFAOYSA-N 0.000 claims description 6
- 206010016654 Fibrosis Diseases 0.000 claims description 6
- 206010018364 Glomerulonephritis Diseases 0.000 claims description 6
- 208000009329 Graft vs Host Disease Diseases 0.000 claims description 6
- 206010020751 Hypersensitivity Diseases 0.000 claims description 6
- QUOGESRFPZDMMT-YFKPBYRVSA-N L-homoarginine Chemical compound OC(=O)[C@@H](N)CCCCNC(N)=N QUOGESRFPZDMMT-YFKPBYRVSA-N 0.000 claims description 6
- 108010071690 Prealbumin Proteins 0.000 claims description 6
- 201000001263 Psoriatic Arthritis Diseases 0.000 claims description 6
- 208000036824 Psoriatic arthropathy Diseases 0.000 claims description 6
- 206010039710 Scleroderma Diseases 0.000 claims description 6
- 208000021386 Sjogren Syndrome Diseases 0.000 claims description 6
- 201000009594 Systemic Scleroderma Diseases 0.000 claims description 6
- 206010042953 Systemic sclerosis Diseases 0.000 claims description 6
- 208000026935 allergic disease Diseases 0.000 claims description 6
- 230000007815 allergy Effects 0.000 claims description 6
- 230000004761 fibrosis Effects 0.000 claims description 6
- 208000024908 graft versus host disease Diseases 0.000 claims description 6
- 230000007935 neutral effect Effects 0.000 claims description 6
- 206010012442 Dermatitis contact Diseases 0.000 claims description 5
- QUOGESRFPZDMMT-UHFFFAOYSA-N L-Homoarginine Natural products OC(=O)C(N)CCCCNC(N)=N QUOGESRFPZDMMT-UHFFFAOYSA-N 0.000 claims description 5
- 208000010247 contact dermatitis Diseases 0.000 claims description 5
- AHLPHDHHMVZTML-SCSAIBSYSA-N D-Ornithine Chemical compound NCCC[C@@H](N)C(O)=O AHLPHDHHMVZTML-SCSAIBSYSA-N 0.000 claims description 4
- XIGSAGMEBXLVJJ-YFKPBYRVSA-N L-homocitrulline Chemical compound NC(=O)NCCCC[C@H]([NH3+])C([O-])=O XIGSAGMEBXLVJJ-YFKPBYRVSA-N 0.000 claims description 4
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 4
- CPUSCHYXEUZMSV-UHFFFAOYSA-N 2,6-diamino-2-methylhexanoic acid Chemical compound OC(=O)C(N)(C)CCCCN CPUSCHYXEUZMSV-UHFFFAOYSA-N 0.000 claims description 3
- QNAYBMKLOCPYGJ-UHFFFAOYSA-N D-alpha-Ala Natural products CC([NH3+])C([O-])=O QNAYBMKLOCPYGJ-UHFFFAOYSA-N 0.000 claims description 3
- ONIBWKKTOPOVIA-SCSAIBSYSA-N D-Proline Chemical compound OC(=O)[C@H]1CCCN1 ONIBWKKTOPOVIA-SCSAIBSYSA-N 0.000 claims description 2
- 101100330292 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) cys-12 gene Proteins 0.000 claims description 2
- 101100498071 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) cys-17 gene Proteins 0.000 claims description 2
- 101100386053 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) cys-3 gene Proteins 0.000 claims description 2
- 150000001408 amides Chemical class 0.000 claims description 2
- 102000007584 Prealbumin Human genes 0.000 claims 2
- FXHCFPUEIDRTMR-UHFFFAOYSA-N hydron;1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid;chloride Chemical compound Cl.C1=CC=C2CNC(C(=O)O)CC2=C1 FXHCFPUEIDRTMR-UHFFFAOYSA-N 0.000 claims 1
- 125000003275 alpha amino acid group Chemical group 0.000 abstract description 43
- 108090000623 proteins and genes Proteins 0.000 description 150
- 102000004169 proteins and genes Human genes 0.000 description 132
- 235000018102 proteins Nutrition 0.000 description 129
- 102000004196 processed proteins & peptides Human genes 0.000 description 120
- 235000001014 amino acid Nutrition 0.000 description 90
- 210000004027 cell Anatomy 0.000 description 88
- 229940024606 amino acid Drugs 0.000 description 77
- 239000000047 product Substances 0.000 description 75
- 229920001184 polypeptide Polymers 0.000 description 53
- 230000027455 binding Effects 0.000 description 50
- 102220531375 Uncharacterized protein KIAA2012_Q16K_mutation Human genes 0.000 description 47
- 230000002829 reductive effect Effects 0.000 description 45
- -1 hydrogen halides Chemical class 0.000 description 43
- 210000001744 T-lymphocyte Anatomy 0.000 description 40
- 239000003814 drug Substances 0.000 description 40
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 39
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 38
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 35
- 108091006146 Channels Proteins 0.000 description 33
- 230000000694 effects Effects 0.000 description 33
- 238000006467 substitution reaction Methods 0.000 description 33
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 32
- 238000009472 formulation Methods 0.000 description 32
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 29
- 150000001875 compounds Chemical class 0.000 description 29
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 28
- 239000000178 monomer Substances 0.000 description 27
- 241000700159 Rattus Species 0.000 description 26
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 25
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 24
- 102100034368 Potassium voltage-gated channel subfamily A member 1 Human genes 0.000 description 24
- 239000000427 antigen Substances 0.000 description 24
- 108091007433 antigens Proteins 0.000 description 24
- 102000036639 antigens Human genes 0.000 description 24
- 239000000243 solution Substances 0.000 description 24
- 102000053602 DNA Human genes 0.000 description 23
- 210000004369 blood Anatomy 0.000 description 23
- 239000008280 blood Substances 0.000 description 23
- 150000007523 nucleic acids Chemical group 0.000 description 23
- 229920000642 polymer Polymers 0.000 description 23
- 229940124597 therapeutic agent Drugs 0.000 description 23
- 230000007831 electrophysiology Effects 0.000 description 22
- 238000002001 electrophysiology Methods 0.000 description 22
- 230000001225 therapeutic effect Effects 0.000 description 22
- 108090000862 Ion Channels Proteins 0.000 description 21
- 102000004310 Ion Channels Human genes 0.000 description 21
- NTYJJOPFIAHURM-UHFFFAOYSA-N Histamine Chemical compound NCCC1=CN=CN1 NTYJJOPFIAHURM-UHFFFAOYSA-N 0.000 description 20
- 230000021615 conjugation Effects 0.000 description 20
- 239000012467 final product Substances 0.000 description 20
- 230000004048 modification Effects 0.000 description 20
- 238000012986 modification Methods 0.000 description 20
- 239000011780 sodium chloride Substances 0.000 description 20
- 108020004414 DNA Proteins 0.000 description 19
- 102000039446 nucleic acids Human genes 0.000 description 19
- 108020004707 nucleic acids Proteins 0.000 description 19
- 239000000843 powder Substances 0.000 description 19
- 238000011282 treatment Methods 0.000 description 19
- 241000242759 Actiniaria Species 0.000 description 18
- 108010002350 Interleukin-2 Proteins 0.000 description 18
- 102000000588 Interleukin-2 Human genes 0.000 description 18
- 239000011575 calcium Substances 0.000 description 18
- 238000002474 experimental method Methods 0.000 description 18
- 238000001727 in vivo Methods 0.000 description 18
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 17
- 239000000562 conjugate Substances 0.000 description 17
- 239000012634 fragment Substances 0.000 description 17
- 239000000463 material Substances 0.000 description 17
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 16
- 229940079593 drug Drugs 0.000 description 16
- 235000018977 lysine Nutrition 0.000 description 16
- 239000004472 Lysine Substances 0.000 description 15
- 235000004279 alanine Nutrition 0.000 description 15
- 235000018417 cysteine Nutrition 0.000 description 15
- 125000000151 cysteine group Chemical class N[C@@H](CS)C(=O)* 0.000 description 15
- 238000004519 manufacturing process Methods 0.000 description 15
- 238000007920 subcutaneous administration Methods 0.000 description 15
- 239000000126 substance Substances 0.000 description 15
- AXAVXPMQTGXXJZ-UHFFFAOYSA-N 2-aminoacetic acid;2-amino-2-(hydroxymethyl)propane-1,3-diol Chemical compound NCC(O)=O.OCC(N)(CO)CO AXAVXPMQTGXXJZ-UHFFFAOYSA-N 0.000 description 14
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 14
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 14
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 14
- 210000003719 b-lymphocyte Anatomy 0.000 description 14
- 229910052791 calcium Inorganic materials 0.000 description 14
- 230000001419 dependent effect Effects 0.000 description 14
- 230000014509 gene expression Effects 0.000 description 14
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 14
- 102000005962 receptors Human genes 0.000 description 14
- 108020003175 receptors Proteins 0.000 description 14
- 239000000523 sample Substances 0.000 description 14
- 230000028327 secretion Effects 0.000 description 14
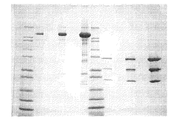
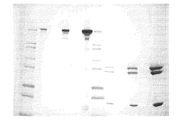
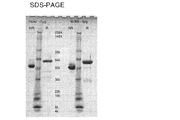
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 14
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 13
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 13
- 238000006243 chemical reaction Methods 0.000 description 13
- NKLPQNGYXWVELD-UHFFFAOYSA-M coomassie brilliant blue Chemical compound [Na+].C1=CC(OCC)=CC=C1NC1=CC=C(C(=C2C=CC(C=C2)=[N+](CC)CC=2C=C(C=CC=2)S([O-])(=O)=O)C=2C=CC(=CC=2)N(CC)CC=2C=C(C=CC=2)S([O-])(=O)=O)C=C1 NKLPQNGYXWVELD-UHFFFAOYSA-M 0.000 description 13
- 239000012636 effector Substances 0.000 description 13
- 230000006870 function Effects 0.000 description 13
- 230000006320 pegylation Effects 0.000 description 13
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 12
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 12
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 12
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 12
- HATRDXDCPOXQJX-UHFFFAOYSA-N Thapsigargin Natural products CCCCCCCC(=O)OC1C(OC(O)C(=C/C)C)C(=C2C3OC(=O)C(C)(O)C3(O)C(CC(C)(OC(=O)C)C12)OC(=O)CCC)C HATRDXDCPOXQJX-UHFFFAOYSA-N 0.000 description 12
- 238000007792 addition Methods 0.000 description 12
- 238000003556 assay Methods 0.000 description 12
- 230000000903 blocking effect Effects 0.000 description 12
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 12
- 210000003630 histaminocyte Anatomy 0.000 description 12
- 239000003446 ligand Substances 0.000 description 12
- 239000011159 matrix material Substances 0.000 description 12
- 238000000816 matrix-assisted laser desorption--ionisation Methods 0.000 description 12
- 239000002773 nucleotide Substances 0.000 description 12
- 125000003729 nucleotide group Chemical group 0.000 description 12
- 239000004094 surface-active agent Substances 0.000 description 12
- IXFPJGBNCFXKPI-FSIHEZPISA-N thapsigargin Chemical compound CCCC(=O)O[C@H]1C[C@](C)(OC(C)=O)[C@H]2[C@H](OC(=O)CCCCCCC)[C@@H](OC(=O)C(\C)=C/C)C(C)=C2[C@@H]2OC(=O)[C@@](C)(O)[C@]21O IXFPJGBNCFXKPI-FSIHEZPISA-N 0.000 description 12
- 239000003643 water by type Substances 0.000 description 12
- PFWOJTLVNOZSGQ-RIMJLNMDSA-N (1R,2aS,4S,7S,10S,13S,19S,22S,25S,28S,31R,36R,39S,45R,48S,51S,54S,57R,60S,63S,66S,69S,72S,75S,78S,81S,84S,87S,90R,93S,96S,99S)-10,51,87-tris(4-aminobutyl)-31-[[(2S)-2-[[(2S)-2-amino-5-carbamimidamidopentanoyl]amino]-3-hydroxypropanoyl]amino]-75-(aminomethyl)-93-(3-amino-3-oxopropyl)-60,96-dibenzyl-19,28-bis[(2S)-butan-2-yl]-4,54,69-tris(3-carbamimidamidopropyl)-25-(carboxymethyl)-2a,22,39,48-tetrakis[(1R)-1-hydroxyethyl]-7,63,81-tris(hydroxymethyl)-72-[(4-hydroxyphenyl)methyl]-84-(1H-imidazol-5-ylmethyl)-99-methyl-66-(2-methylpropyl)-78-(2-methylsulfanylethyl)-1a,3,4a,6,9,12,18,21,24,27,30,38,41,44,47,50,53,56,59,62,65,68,71,74,77,80,83,86,89,92,95,98-dotriacontaoxo-6a,7a,10a,11a,33,34-hexathia-a,2,3a,5,8,11,17,20,23,26,29,37,40,43,46,49,52,55,58,61,64,67,70,73,76,79,82,85,88,91,94,97-dotriacontazatetracyclo[55.47.4.445,90.013,17]dodecahectane-36-carboxylic acid Chemical compound CC[C@H](C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@@H]2CSSC[C@@H]3NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccc(O)cc4)NC(=O)[C@H](CN)NC(=O)[C@H](CCSC)NC(=O)[C@H](CO)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)[C@H](CCCCN)NC3=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC1=O)[C@@H](C)O)[C@@H](C)CC)[C@@H](C)O)[C@@H](C)O)C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CCCNC(N)=N PFWOJTLVNOZSGQ-RIMJLNMDSA-N 0.000 description 11
- 102000004127 Cytokines Human genes 0.000 description 11
- 108090000695 Cytokines Proteins 0.000 description 11
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 11
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 11
- 108091028043 Nucleic acid sequence Proteins 0.000 description 11
- 102000004257 Potassium Channel Human genes 0.000 description 11
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 11
- 230000015572 biosynthetic process Effects 0.000 description 11
- 230000001965 increasing effect Effects 0.000 description 11
- 230000003834 intracellular effect Effects 0.000 description 11
- 108020001213 potassium channel Proteins 0.000 description 11
- 230000009467 reduction Effects 0.000 description 11
- 238000006722 reduction reaction Methods 0.000 description 11
- 210000002966 serum Anatomy 0.000 description 11
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 10
- 108010036949 Cyclosporine Proteins 0.000 description 10
- ODKSFYDXXFIFQN-BYPYZUCNSA-N L-arginine Chemical compound OC(=O)[C@@H](N)CCCN=C(N)N ODKSFYDXXFIFQN-BYPYZUCNSA-N 0.000 description 10
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 10
- 241000282567 Macaca fascicularis Species 0.000 description 10
- 108010076504 Protein Sorting Signals Proteins 0.000 description 10
- 239000002253 acid Substances 0.000 description 10
- 230000004913 activation Effects 0.000 description 10
- 125000003118 aryl group Chemical group 0.000 description 10
- 230000004071 biological effect Effects 0.000 description 10
- 239000003795 chemical substances by application Substances 0.000 description 10
- 239000000539 dimer Substances 0.000 description 10
- 239000003623 enhancer Substances 0.000 description 10
- 125000002485 formyl group Chemical class [H]C(*)=O 0.000 description 10
- 229960001340 histamine Drugs 0.000 description 10
- 229940072221 immunoglobulins Drugs 0.000 description 10
- 210000004379 membrane Anatomy 0.000 description 10
- 239000012528 membrane Substances 0.000 description 10
- 238000001542 size-exclusion chromatography Methods 0.000 description 10
- 150000003384 small molecules Chemical class 0.000 description 10
- 238000012453 sprague-dawley rat model Methods 0.000 description 10
- 239000003826 tablet Substances 0.000 description 10
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 9
- 239000004475 Arginine Substances 0.000 description 9
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 9
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 9
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 9
- 238000004458 analytical method Methods 0.000 description 9
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 9
- 235000009697 arginine Nutrition 0.000 description 9
- 235000009582 asparagine Nutrition 0.000 description 9
- 229960001230 asparagine Drugs 0.000 description 9
- 238000001212 derivatisation Methods 0.000 description 9
- 230000005764 inhibitory process Effects 0.000 description 9
- 238000002347 injection Methods 0.000 description 9
- 239000007924 injection Substances 0.000 description 9
- 239000007788 liquid Substances 0.000 description 9
- 230000037361 pathway Effects 0.000 description 9
- 125000001151 peptidyl group Chemical group 0.000 description 9
- 238000002360 preparation method Methods 0.000 description 9
- 230000002459 sustained effect Effects 0.000 description 9
- 238000003786 synthesis reaction Methods 0.000 description 9
- 108091026890 Coding region Proteins 0.000 description 8
- 239000004471 Glycine Substances 0.000 description 8
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 8
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 8
- 101100242312 Oryza sativa subsp. japonica OSK1 gene Proteins 0.000 description 8
- 101100533605 Oryza sativa subsp. japonica SKP1 gene Proteins 0.000 description 8
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 8
- 241000239226 Scorpiones Species 0.000 description 8
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 8
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Chemical compound CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 8
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 8
- 235000013922 glutamic acid Nutrition 0.000 description 8
- 239000004220 glutamic acid Substances 0.000 description 8
- 125000000291 glutamic acid group Chemical group N[C@@H](CCC(O)=O)C(=O)* 0.000 description 8
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 8
- 235000014304 histidine Nutrition 0.000 description 8
- 238000003780 insertion Methods 0.000 description 8
- 230000037431 insertion Effects 0.000 description 8
- 239000002609 medium Substances 0.000 description 8
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 8
- 239000000863 peptide conjugate Substances 0.000 description 8
- 235000013930 proline Nutrition 0.000 description 8
- 235000004400 serine Nutrition 0.000 description 8
- 238000010183 spectrum analysis Methods 0.000 description 8
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 7
- 108020004705 Codon Proteins 0.000 description 7
- 229930105110 Cyclosporin A Natural products 0.000 description 7
- 239000007995 HEPES buffer Substances 0.000 description 7
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 7
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 7
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 7
- 229920002472 Starch Polymers 0.000 description 7
- 102000025171 antigen binding proteins Human genes 0.000 description 7
- 108091000831 antigen binding proteins Proteins 0.000 description 7
- 230000000975 bioactive effect Effects 0.000 description 7
- 239000000872 buffer Substances 0.000 description 7
- 239000004067 bulking agent Substances 0.000 description 7
- 150000001720 carbohydrates Chemical class 0.000 description 7
- 229910052799 carbon Inorganic materials 0.000 description 7
- 210000000170 cell membrane Anatomy 0.000 description 7
- 229960001265 ciclosporin Drugs 0.000 description 7
- 239000000306 component Substances 0.000 description 7
- 239000003085 diluting agent Substances 0.000 description 7
- 239000007884 disintegrant Substances 0.000 description 7
- 102000037865 fusion proteins Human genes 0.000 description 7
- 108020001507 fusion proteins Proteins 0.000 description 7
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 7
- 235000004554 glutamine Nutrition 0.000 description 7
- 102000046611 human KCNA3 Human genes 0.000 description 7
- 229910052739 hydrogen Inorganic materials 0.000 description 7
- 230000001976 improved effect Effects 0.000 description 7
- 210000003000 inclusion body Anatomy 0.000 description 7
- 230000002401 inhibitory effect Effects 0.000 description 7
- 229930182817 methionine Natural products 0.000 description 7
- 229910052757 nitrogen Inorganic materials 0.000 description 7
- 230000000144 pharmacologic effect Effects 0.000 description 7
- 239000012071 phase Substances 0.000 description 7
- 230000008569 process Effects 0.000 description 7
- 239000002795 scorpion venom Substances 0.000 description 7
- 239000007787 solid Substances 0.000 description 7
- 235000019698 starch Nutrition 0.000 description 7
- 239000008107 starch Substances 0.000 description 7
- 150000003573 thiols Chemical class 0.000 description 7
- PECYZEOJVXMISF-UHFFFAOYSA-N 3-aminoalanine Chemical group [NH3+]CC(N)C([O-])=O PECYZEOJVXMISF-UHFFFAOYSA-N 0.000 description 6
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 6
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 6
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 6
- 229920002307 Dextran Polymers 0.000 description 6
- 206010061218 Inflammation Diseases 0.000 description 6
- LRQKBLKVPFOOQJ-YFKPBYRVSA-N L-norleucine Chemical compound CCCC[C@H]([NH3+])C([O-])=O LRQKBLKVPFOOQJ-YFKPBYRVSA-N 0.000 description 6
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 6
- 229930195725 Mannitol Natural products 0.000 description 6
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 6
- 108020004511 Recombinant DNA Proteins 0.000 description 6
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 6
- 235000003704 aspartic acid Nutrition 0.000 description 6
- 230000008901 benefit Effects 0.000 description 6
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 6
- 229940098773 bovine serum albumin Drugs 0.000 description 6
- 230000028956 calcium-mediated signaling Effects 0.000 description 6
- 238000004422 calculation algorithm Methods 0.000 description 6
- 235000014633 carbohydrates Nutrition 0.000 description 6
- 239000001768 carboxy methyl cellulose Substances 0.000 description 6
- 239000003153 chemical reaction reagent Substances 0.000 description 6
- 230000000295 complement effect Effects 0.000 description 6
- 238000012217 deletion Methods 0.000 description 6
- 230000037430 deletion Effects 0.000 description 6
- 208000035475 disorder Diseases 0.000 description 6
- 239000000796 flavoring agent Substances 0.000 description 6
- 239000000833 heterodimer Substances 0.000 description 6
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 6
- 229960000310 isoleucine Drugs 0.000 description 6
- 239000010410 layer Substances 0.000 description 6
- 210000004072 lung Anatomy 0.000 description 6
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 6
- 239000000594 mannitol Substances 0.000 description 6
- 235000010355 mannitol Nutrition 0.000 description 6
- 239000000816 peptidomimetic Substances 0.000 description 6
- QZAYGJVTTNCVMB-UHFFFAOYSA-N serotonin Chemical compound C1=C(O)C=C2C(CCN)=CNC2=C1 QZAYGJVTTNCVMB-UHFFFAOYSA-N 0.000 description 6
- 238000001228 spectrum Methods 0.000 description 6
- 230000000638 stimulation Effects 0.000 description 6
- 239000000725 suspension Substances 0.000 description 6
- 239000004474 valine Substances 0.000 description 6
- 239000003981 vehicle Substances 0.000 description 6
- 239000002435 venom Substances 0.000 description 6
- 231100000611 venom Toxicity 0.000 description 6
- 210000001048 venom Anatomy 0.000 description 6
- 238000013389 whole blood assay Methods 0.000 description 6
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 5
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 5
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 5
- PEEHTFAAVSWFBL-UHFFFAOYSA-N Maleimide Chemical group O=C1NC(=O)C=C1 PEEHTFAAVSWFBL-UHFFFAOYSA-N 0.000 description 5
- 241000237536 Mytilus edulis Species 0.000 description 5
- 238000005481 NMR spectroscopy Methods 0.000 description 5
- 101001046988 Orthochirus scrobiculosus Potassium channel toxin alpha-KTx 3.7 Proteins 0.000 description 5
- 101001121312 Oryza sativa subsp. japonica Serine/threonine protein kinase OSK1 Proteins 0.000 description 5
- 229930006000 Sucrose Natural products 0.000 description 5
- 108091008874 T cell receptors Proteins 0.000 description 5
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 5
- 230000001363 autoimmune Effects 0.000 description 5
- 230000001580 bacterial effect Effects 0.000 description 5
- 230000001588 bifunctional effect Effects 0.000 description 5
- 229940046731 calcineurin inhibitors Drugs 0.000 description 5
- 229920002678 cellulose Polymers 0.000 description 5
- 239000001913 cellulose Substances 0.000 description 5
- 235000010980 cellulose Nutrition 0.000 description 5
- 238000001311 chemical methods and process Methods 0.000 description 5
- 238000007385 chemical modification Methods 0.000 description 5
- 238000007906 compression Methods 0.000 description 5
- 230000006835 compression Effects 0.000 description 5
- 238000010586 diagram Methods 0.000 description 5
- 238000012377 drug delivery Methods 0.000 description 5
- 150000002148 esters Chemical class 0.000 description 5
- 210000001723 extracellular space Anatomy 0.000 description 5
- 125000000524 functional group Chemical group 0.000 description 5
- 239000000499 gel Substances 0.000 description 5
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Chemical compound NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 5
- 239000000710 homodimer Substances 0.000 description 5
- 208000026278 immune system disease Diseases 0.000 description 5
- 230000004054 inflammatory process Effects 0.000 description 5
- 238000001802 infusion Methods 0.000 description 5
- 239000008101 lactose Substances 0.000 description 5
- 229960001375 lactose Drugs 0.000 description 5
- 239000002502 liposome Substances 0.000 description 5
- 210000003071 memory t lymphocyte Anatomy 0.000 description 5
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 description 5
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 5
- 229960005190 phenylalanine Drugs 0.000 description 5
- 102000040430 polynucleotide Human genes 0.000 description 5
- 108091033319 polynucleotide Proteins 0.000 description 5
- 239000002157 polynucleotide Substances 0.000 description 5
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 5
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 5
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 5
- 239000011591 potassium Substances 0.000 description 5
- 229910052700 potassium Inorganic materials 0.000 description 5
- 235000007686 potassium Nutrition 0.000 description 5
- 229960003975 potassium Drugs 0.000 description 5
- 150000003141 primary amines Chemical class 0.000 description 5
- 230000017854 proteolysis Effects 0.000 description 5
- 238000006268 reductive amination reaction Methods 0.000 description 5
- 230000004044 response Effects 0.000 description 5
- 238000012552 review Methods 0.000 description 5
- 238000010532 solid phase synthesis reaction Methods 0.000 description 5
- 238000010186 staining Methods 0.000 description 5
- 125000001424 substituent group Chemical group 0.000 description 5
- 239000005720 sucrose Substances 0.000 description 5
- 230000008685 targeting Effects 0.000 description 5
- 238000013518 transcription Methods 0.000 description 5
- 230000035897 transcription Effects 0.000 description 5
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 5
- QJYNZEYHSMRWBK-NIKIMHBISA-N 1,2,3,4,6-pentakis-O-galloyl-beta-D-glucose Chemical compound OC1=C(O)C(O)=CC(C(=O)OC[C@@H]2[C@H]([C@H](OC(=O)C=3C=C(O)C(O)=C(O)C=3)[C@@H](OC(=O)C=3C=C(O)C(O)=C(O)C=3)[C@H](OC(=O)C=3C=C(O)C(O)=C(O)C=3)O2)OC(=O)C=2C=C(O)C(O)=C(O)C=2)=C1 QJYNZEYHSMRWBK-NIKIMHBISA-N 0.000 description 4
- FUOOLUPWFVMBKG-UHFFFAOYSA-N 2-Aminoisobutyric acid Chemical compound CC(C)(N)C(O)=O FUOOLUPWFVMBKG-UHFFFAOYSA-N 0.000 description 4
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 4
- 102000009027 Albumins Human genes 0.000 description 4
- 108010088751 Albumins Proteins 0.000 description 4
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 4
- 241000894006 Bacteria Species 0.000 description 4
- 102000004506 Blood Proteins Human genes 0.000 description 4
- 108010017384 Blood Proteins Proteins 0.000 description 4
- 108010029697 CD40 Ligand Proteins 0.000 description 4
- 101150013553 CD40 gene Proteins 0.000 description 4
- 102100032937 CD40 ligand Human genes 0.000 description 4
- 102000004631 Calcineurin Human genes 0.000 description 4
- 108010042955 Calcineurin Proteins 0.000 description 4
- RGHNJXZEOKUKBD-SQOUGZDYSA-N D-gluconic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-SQOUGZDYSA-N 0.000 description 4
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 4
- 241000588724 Escherichia coli Species 0.000 description 4
- 239000001856 Ethyl cellulose Substances 0.000 description 4
- 108010010803 Gelatin Proteins 0.000 description 4
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 4
- 108010051041 HC toxin Proteins 0.000 description 4
- 101150106931 IFNG gene Proteins 0.000 description 4
- 108010074328 Interferon-gamma Proteins 0.000 description 4
- RHGKLRLOHDJJDR-BYPYZUCNSA-N L-citrulline Chemical compound NC(=O)NCCC[C@H]([NH3+])C([O-])=O RHGKLRLOHDJJDR-BYPYZUCNSA-N 0.000 description 4
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 4
- 108090000543 Ligand-Gated Ion Channels Proteins 0.000 description 4
- 102000004086 Ligand-Gated Ion Channels Human genes 0.000 description 4
- 241000699666 Mus <mouse, genus> Species 0.000 description 4
- HSHXDCVZWHOWCS-UHFFFAOYSA-N N'-hexadecylthiophene-2-carbohydrazide Chemical compound CCCCCCCCCCCCCCCCNNC(=O)c1cccs1 HSHXDCVZWHOWCS-UHFFFAOYSA-N 0.000 description 4
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Chemical group NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 4
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Chemical group OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 4
- 241000239299 Orthochirus scrobiculosus Species 0.000 description 4
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 4
- 241000242730 Stichodactyla helianthus Species 0.000 description 4
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 4
- 230000006044 T cell activation Effects 0.000 description 4
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 4
- 239000004473 Threonine Substances 0.000 description 4
- 102000009190 Transthyretin Human genes 0.000 description 4
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 4
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 description 4
- 102000003734 Voltage-Gated Potassium Channels Human genes 0.000 description 4
- 108090000013 Voltage-Gated Potassium Channels Proteins 0.000 description 4
- 150000007513 acids Chemical class 0.000 description 4
- 239000000654 additive Substances 0.000 description 4
- 125000003277 amino group Chemical group 0.000 description 4
- 239000005557 antagonist Substances 0.000 description 4
- 230000006399 behavior Effects 0.000 description 4
- 239000011230 binding agent Substances 0.000 description 4
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 4
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 4
- 239000003086 colorant Substances 0.000 description 4
- 238000004590 computer program Methods 0.000 description 4
- 229920001577 copolymer Polymers 0.000 description 4
- 239000003398 denaturant Substances 0.000 description 4
- 210000004443 dendritic cell Anatomy 0.000 description 4
- 238000011033 desalting Methods 0.000 description 4
- 230000008034 disappearance Effects 0.000 description 4
- 238000005538 encapsulation Methods 0.000 description 4
- 229940088598 enzyme Drugs 0.000 description 4
- 229920001249 ethyl cellulose Polymers 0.000 description 4
- DEFVIWRASFVYLL-UHFFFAOYSA-N ethylene glycol bis(2-aminoethyl)tetraacetic acid Chemical compound OC(=O)CN(CC(O)=O)CCOCCOCCN(CC(O)=O)CC(O)=O DEFVIWRASFVYLL-UHFFFAOYSA-N 0.000 description 4
- 239000013604 expression vector Substances 0.000 description 4
- 235000013355 food flavoring agent Nutrition 0.000 description 4
- 235000019253 formic acid Nutrition 0.000 description 4
- 239000008273 gelatin Substances 0.000 description 4
- 229920000159 gelatin Polymers 0.000 description 4
- 235000019322 gelatine Nutrition 0.000 description 4
- 235000011852 gelatine desserts Nutrition 0.000 description 4
- 239000008103 glucose Substances 0.000 description 4
- 239000008187 granular material Substances 0.000 description 4
- 125000005843 halogen group Chemical group 0.000 description 4
- 239000000017 hydrogel Substances 0.000 description 4
- 230000002209 hydrophobic effect Effects 0.000 description 4
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 4
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 4
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 4
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 4
- 230000005847 immunogenicity Effects 0.000 description 4
- 230000001506 immunosuppresive effect Effects 0.000 description 4
- 238000011534 incubation Methods 0.000 description 4
- 238000001990 intravenous administration Methods 0.000 description 4
- 239000000314 lubricant Substances 0.000 description 4
- 210000004962 mammalian cell Anatomy 0.000 description 4
- 238000001819 mass spectrum Methods 0.000 description 4
- 230000007246 mechanism Effects 0.000 description 4
- 229920000609 methyl cellulose Polymers 0.000 description 4
- 239000001923 methylcellulose Substances 0.000 description 4
- 235000010981 methylcellulose Nutrition 0.000 description 4
- 235000020638 mussel Nutrition 0.000 description 4
- 210000002569 neuron Anatomy 0.000 description 4
- 229960003104 ornithine Drugs 0.000 description 4
- AHLPHDHHMVZTML-BYPYZUCNSA-N ornithyl group Chemical group N[C@@H](CCCN)C(=O)O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 4
- DCWXELXMIBXGTH-UHFFFAOYSA-N phosphotyrosine Chemical compound OC(=O)C(N)CC1=CC=C(OP(O)(O)=O)C=C1 DCWXELXMIBXGTH-UHFFFAOYSA-N 0.000 description 4
- 231100000614 poison Toxicity 0.000 description 4
- 239000002574 poison Substances 0.000 description 4
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 4
- 229920000053 polysorbate 80 Polymers 0.000 description 4
- 239000011148 porous material Substances 0.000 description 4
- 239000002243 precursor Substances 0.000 description 4
- 230000035755 proliferation Effects 0.000 description 4
- 230000002685 pulmonary effect Effects 0.000 description 4
- 230000008707 rearrangement Effects 0.000 description 4
- 230000001105 regulatory effect Effects 0.000 description 4
- 230000027425 release of sequestered calcium ion into cytosol Effects 0.000 description 4
- 238000004007 reversed phase HPLC Methods 0.000 description 4
- 239000002904 solvent Substances 0.000 description 4
- 238000013268 sustained release Methods 0.000 description 4
- 239000012730 sustained-release form Substances 0.000 description 4
- 235000008521 threonine Nutrition 0.000 description 4
- 210000001519 tissue Anatomy 0.000 description 4
- 230000001988 toxicity Effects 0.000 description 4
- 231100000419 toxicity Toxicity 0.000 description 4
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 4
- 125000001493 tyrosinyl group Chemical group [H]OC1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 4
- 238000004704 ultra performance liquid chromatography Methods 0.000 description 4
- NMDDZEVVQDPECF-LURJTMIESA-N (2s)-2,7-diaminoheptanoic acid Chemical compound NCCCCC[C@H](N)C(O)=O NMDDZEVVQDPECF-LURJTMIESA-N 0.000 description 3
- OGNSCSPNOLGXSM-UHFFFAOYSA-N 2,4-diaminobutyric acid Chemical group NCCC(N)C(O)=O OGNSCSPNOLGXSM-UHFFFAOYSA-N 0.000 description 3
- RUVRGYVESPRHSZ-UHFFFAOYSA-N 2-[2-(2-azaniumylethoxy)ethoxy]acetate Chemical compound NCCOCCOCC(O)=O RUVRGYVESPRHSZ-UHFFFAOYSA-N 0.000 description 3
- HXMVNCMPQGPRLN-UHFFFAOYSA-N 2-hydroxyputrescine Chemical compound NCCC(O)CN HXMVNCMPQGPRLN-UHFFFAOYSA-N 0.000 description 3
- BRMWTNUJHUMWMS-UHFFFAOYSA-N 3-Methylhistidine Natural products CN1C=NC(CC(N)C(O)=O)=C1 BRMWTNUJHUMWMS-UHFFFAOYSA-N 0.000 description 3
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 3
- HIYAVKIYRIFSCZ-CYEMHPAKSA-N 5-(methylamino)-2-[[(2S,3R,5R,6S,8R,9R)-3,5,9-trimethyl-2-[(2S)-1-oxo-1-(1H-pyrrol-2-yl)propan-2-yl]-1,7-dioxaspiro[5.5]undecan-8-yl]methyl]-1,3-benzoxazole-4-carboxylic acid Chemical compound O=C([C@@H](C)[C@H]1O[C@@]2([C@@H](C[C@H]1C)C)O[C@@H]([C@@H](CC2)C)CC=1OC2=CC=C(C(=C2N=1)C(O)=O)NC)C1=CC=CN1 HIYAVKIYRIFSCZ-CYEMHPAKSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 241000230610 Anemonia erythraea Species 0.000 description 3
- 101001023095 Anemonia sulcata Delta-actitoxin-Avd1a Proteins 0.000 description 3
- 101000641989 Araneus ventricosus Kunitz-type U1-aranetoxin-Av1a Proteins 0.000 description 3
- 102000003922 Calcium Channels Human genes 0.000 description 3
- 108090000312 Calcium Channels Proteins 0.000 description 3
- 101001028691 Carybdea rastonii Toxin CrTX-A Proteins 0.000 description 3
- 101000685083 Centruroides infamatus Beta-toxin Cii1 Proteins 0.000 description 3
- 101000685085 Centruroides noxius Toxin Cn1 Proteins 0.000 description 3
- 101001028688 Chironex fleckeri Toxin CfTX-1 Proteins 0.000 description 3
- 101000644407 Cyriopagopus schmidti U6-theraphotoxin-Hs1a Proteins 0.000 description 3
- 102100033991 E3 ubiquitin-protein ligase DTX1 Human genes 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 3
- 241001517044 Heteractis magnifica Species 0.000 description 3
- 101001017463 Homo sapiens E3 ubiquitin-protein ligase DTX1 Proteins 0.000 description 3
- 101001026236 Homo sapiens Intermediate conductance calcium-activated potassium channel protein 4 Proteins 0.000 description 3
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 3
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 3
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 3
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 3
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 3
- 102000018706 Kv1.3 Potassium Channel Human genes 0.000 description 3
- 108010027296 Kv1.3 Potassium Channel Proteins 0.000 description 3
- 125000000174 L-prolyl group Chemical group [H]N1C([H])([H])C([H])([H])C([H])([H])[C@@]1([H])C(*)=O 0.000 description 3
- 241000124008 Mammalia Species 0.000 description 3
- 102000006386 Myelin Proteins Human genes 0.000 description 3
- 108010083674 Myelin Proteins Proteins 0.000 description 3
- JDHILDINMRGULE-LURJTMIESA-N N(pros)-methyl-L-histidine Chemical compound CN1C=NC=C1C[C@H](N)C(O)=O JDHILDINMRGULE-LURJTMIESA-N 0.000 description 3
- RHGKLRLOHDJJDR-UHFFFAOYSA-N Ndelta-carbamoyl-DL-ornithine Natural products OC(=O)C(N)CCCNC(N)=O RHGKLRLOHDJJDR-UHFFFAOYSA-N 0.000 description 3
- 102000019315 Nicotinic acetylcholine receptors Human genes 0.000 description 3
- 108050006807 Nicotinic acetylcholine receptors Proteins 0.000 description 3
- 101000679608 Phaeosphaeria nodorum (strain SN15 / ATCC MYA-4574 / FGSC 10173) Cysteine rich necrotrophic effector Tox1 Proteins 0.000 description 3
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 3
- 229940124639 Selective inhibitor Drugs 0.000 description 3
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 description 3
- 101000994422 Tityus discrepans Potassium channel toxin alpha-KTx 15.6 Proteins 0.000 description 3
- MMWCIQZXVOZEGG-HOZKJCLWSA-N [(1S,2R,3S,4S,5R,6S)-2,3,5-trihydroxy-4,6-diphosphonooxycyclohexyl] dihydrogen phosphate Chemical compound O[C@H]1[C@@H](O)[C@H](OP(O)(O)=O)[C@@H](OP(O)(O)=O)[C@H](O)[C@H]1OP(O)(O)=O MMWCIQZXVOZEGG-HOZKJCLWSA-N 0.000 description 3
- 238000002835 absorbance Methods 0.000 description 3
- 230000003213 activating effect Effects 0.000 description 3
- 239000004480 active ingredient Substances 0.000 description 3
- 239000013543 active substance Substances 0.000 description 3
- 239000002671 adjuvant Substances 0.000 description 3
- 235000010443 alginic acid Nutrition 0.000 description 3
- 229920000615 alginic acid Polymers 0.000 description 3
- 239000000783 alginic acid Substances 0.000 description 3
- 229960001126 alginic acid Drugs 0.000 description 3
- 150000004781 alginic acids Chemical class 0.000 description 3
- QWCKQJZIFLGMSD-UHFFFAOYSA-N alpha-aminobutyric acid Chemical compound CCC(N)C(O)=O QWCKQJZIFLGMSD-UHFFFAOYSA-N 0.000 description 3
- 210000000612 antigen-presenting cell Anatomy 0.000 description 3
- 125000000637 arginyl group Chemical group N[C@@H](CCCNC(N)=N)C(=O)* 0.000 description 3
- 125000001584 benzyloxycarbonyl group Chemical group C(=O)(OCC1=CC=CC=C1)* 0.000 description 3
- 238000006664 bond formation reaction Methods 0.000 description 3
- 230000009460 calcium influx Effects 0.000 description 3
- HIYAVKIYRIFSCZ-UHFFFAOYSA-N calcium ionophore A23187 Natural products N=1C2=C(C(O)=O)C(NC)=CC=C2OC=1CC(C(CC1)C)OC1(C(CC1C)C)OC1C(C)C(=O)C1=CC=CN1 HIYAVKIYRIFSCZ-UHFFFAOYSA-N 0.000 description 3
- 239000004202 carbamide Substances 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 230000024245 cell differentiation Effects 0.000 description 3
- 230000001413 cellular effect Effects 0.000 description 3
- 229960002173 citrulline Drugs 0.000 description 3
- 235000013477 citrulline Nutrition 0.000 description 3
- 239000011248 coating agent Substances 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 230000004540 complement-dependent cytotoxicity Effects 0.000 description 3
- 125000004122 cyclic group Chemical group 0.000 description 3
- 230000007423 decrease Effects 0.000 description 3
- YSMODUONRAFBET-UHFFFAOYSA-N delta-DL-hydroxylysine Natural products NCC(O)CCC(N)C(O)=O YSMODUONRAFBET-UHFFFAOYSA-N 0.000 description 3
- 238000010790 dilution Methods 0.000 description 3
- 239000012895 dilution Substances 0.000 description 3
- CLBIEZBAENPDFY-HNXGFDTJSA-N dinophysistoxin 1 Chemical compound C([C@H](O1)[C@H](C)/C=C/[C@H]2CC[C@@]3(CC[C@H]4O[C@@H](C([C@@H](O)[C@@H]4O3)=C)[C@@H](O)C[C@H](C)[C@@H]3[C@@H](CC[C@@]4(O3)[C@@H](CCCO4)C)C)O2)C(C)=C[C@]21O[C@H](C[C@@](C)(O)C(O)=O)CC[C@H]2O CLBIEZBAENPDFY-HNXGFDTJSA-N 0.000 description 3
- AFOSIXZFDONLBT-UHFFFAOYSA-N divinyl sulfone Chemical group C=CS(=O)(=O)C=C AFOSIXZFDONLBT-UHFFFAOYSA-N 0.000 description 3
- 239000002552 dosage form Substances 0.000 description 3
- 231100000673 dose–response relationship Toxicity 0.000 description 3
- 238000004043 dyeing Methods 0.000 description 3
- 239000000839 emulsion Substances 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- YSMODUONRAFBET-UHNVWZDZSA-N erythro-5-hydroxy-L-lysine Chemical compound NC[C@H](O)CC[C@H](N)C(O)=O YSMODUONRAFBET-UHNVWZDZSA-N 0.000 description 3
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 3
- 235000019325 ethyl cellulose Nutrition 0.000 description 3
- LNTHITQWFMADLM-UHFFFAOYSA-N gallic acid Chemical class OC(=O)C1=CC(O)=C(O)C(O)=C1 LNTHITQWFMADLM-UHFFFAOYSA-N 0.000 description 3
- 230000013595 glycosylation Effects 0.000 description 3
- 238000006206 glycosylation reaction Methods 0.000 description 3
- 210000002216 heart Anatomy 0.000 description 3
- 239000001257 hydrogen Substances 0.000 description 3
- 230000016784 immunoglobulin production Effects 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 238000010253 intravenous injection Methods 0.000 description 3
- 208000002551 irritable bowel syndrome Diseases 0.000 description 3
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 3
- 210000004698 lymphocyte Anatomy 0.000 description 3
- 210000002540 macrophage Anatomy 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 108020004999 messenger RNA Proteins 0.000 description 3
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 3
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 3
- 239000004005 microsphere Substances 0.000 description 3
- 210000005012 myelin Anatomy 0.000 description 3
- 210000000822 natural killer cell Anatomy 0.000 description 3
- 230000000269 nucleophilic effect Effects 0.000 description 3
- 230000003204 osmotic effect Effects 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- 238000010647 peptide synthesis reaction Methods 0.000 description 3
- 239000000546 pharmaceutical excipient Substances 0.000 description 3
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 3
- 230000026731 phosphorylation Effects 0.000 description 3
- 238000006366 phosphorylation reaction Methods 0.000 description 3
- 239000006187 pill Substances 0.000 description 3
- 229920001515 polyalkylene glycol Polymers 0.000 description 3
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 3
- 229940068968 polysorbate 80 Drugs 0.000 description 3
- 239000003755 preservative agent Substances 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 239000003380 propellant Substances 0.000 description 3
- 238000005932 reductive alkylation reaction Methods 0.000 description 3
- 229920006395 saturated elastomer Polymers 0.000 description 3
- 150000003335 secondary amines Chemical class 0.000 description 3
- 230000003248 secreting effect Effects 0.000 description 3
- 230000011664 signaling Effects 0.000 description 3
- 239000011734 sodium Substances 0.000 description 3
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 3
- 239000007909 solid dosage form Substances 0.000 description 3
- 239000007790 solid phase Substances 0.000 description 3
- 241000894007 species Species 0.000 description 3
- 238000003860 storage Methods 0.000 description 3
- 235000000346 sugar Nutrition 0.000 description 3
- 239000006188 syrup Substances 0.000 description 3
- 235000020357 syrup Nutrition 0.000 description 3
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 238000002560 therapeutic procedure Methods 0.000 description 3
- 239000002562 thickening agent Substances 0.000 description 3
- 238000013519 translation Methods 0.000 description 3
- 239000013638 trimer Substances 0.000 description 3
- 230000003442 weekly effect Effects 0.000 description 3
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 2
- WMBWREPUVVBILR-WIYYLYMNSA-N (-)-Epigallocatechin-3-o-gallate Chemical compound O([C@@H]1CC2=C(O)C=C(C=C2O[C@@H]1C=1C=C(O)C(O)=C(O)C=1)O)C(=O)C1=CC(O)=C(O)C(O)=C1 WMBWREPUVVBILR-WIYYLYMNSA-N 0.000 description 2
- 125000004765 (C1-C4) haloalkyl group Chemical group 0.000 description 2
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 2
- WNXJIVFYUVYPPR-UHFFFAOYSA-N 1,3-dioxolane Chemical compound C1COCO1 WNXJIVFYUVYPPR-UHFFFAOYSA-N 0.000 description 2
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- UFBJCMHMOXMLKC-UHFFFAOYSA-N 2,4-dinitrophenol Chemical compound OC1=CC=C([N+]([O-])=O)C=C1[N+]([O-])=O UFBJCMHMOXMLKC-UHFFFAOYSA-N 0.000 description 2
- 125000001917 2,4-dinitrophenyl group Chemical group [H]C1=C([H])C(=C([H])C(=C1*)[N+]([O-])=O)[N+]([O-])=O 0.000 description 2
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 2
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 2
- 229940117976 5-hydroxylysine Drugs 0.000 description 2
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 2
- 241000242758 Actinia Species 0.000 description 2
- 241001083548 Anemone Species 0.000 description 2
- 241000242762 Anemonia sulcata Species 0.000 description 2
- 241000239290 Araneae Species 0.000 description 2
- 241000416162 Astragalus gummifer Species 0.000 description 2
- 108091035707 Consensus sequence Proteins 0.000 description 2
- 101150097493 D gene Proteins 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- 150000008574 D-amino acids Chemical class 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- RGHNJXZEOKUKBD-UHFFFAOYSA-N D-gluconic acid Natural products OCC(O)C(O)C(O)C(O)C(O)=O RGHNJXZEOKUKBD-UHFFFAOYSA-N 0.000 description 2
- QSJXEFYPDANLFS-UHFFFAOYSA-N Diacetyl Chemical compound CC(=O)C(C)=O QSJXEFYPDANLFS-UHFFFAOYSA-N 0.000 description 2
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 2
- 241000206602 Eukaryota Species 0.000 description 2
- 108010087819 Fc receptors Proteins 0.000 description 2
- 102000009109 Fc receptors Human genes 0.000 description 2
- 108090000045 G-Protein-Coupled Receptors Proteins 0.000 description 2
- 102000003688 G-Protein-Coupled Receptors Human genes 0.000 description 2
- 241000237858 Gastropoda Species 0.000 description 2
- 241000208818 Helianthus Species 0.000 description 2
- 241000238631 Hexapoda Species 0.000 description 2
- 101100220044 Homo sapiens CD34 gene Proteins 0.000 description 2
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 2
- 239000004354 Hydroxyethyl cellulose Substances 0.000 description 2
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 description 2
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 2
- 241000257303 Hymenoptera Species 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- 108700005091 Immunoglobulin Genes Proteins 0.000 description 2
- 102000013463 Immunoglobulin Light Chains Human genes 0.000 description 2
- 108010065825 Immunoglobulin Light Chains Proteins 0.000 description 2
- 102100037850 Interferon gamma Human genes 0.000 description 2
- 102000008070 Interferon-gamma Human genes 0.000 description 2
- 102000013691 Interleukin-17 Human genes 0.000 description 2
- 102000010789 Interleukin-2 Receptors Human genes 0.000 description 2
- 108010038453 Interleukin-2 Receptors Proteins 0.000 description 2
- FFFHZYDWPBMWHY-VKHMYHEASA-N L-homocysteine Chemical compound OC(=O)[C@@H](N)CCS FFFHZYDWPBMWHY-VKHMYHEASA-N 0.000 description 2
- 125000000510 L-tryptophano group Chemical group [H]C1=C([H])C([H])=C2N([H])C([H])=C(C([H])([H])[C@@]([H])(C(O[H])=O)N([H])[*])C2=C1[H] 0.000 description 2
- FFDGPVCHZBVARC-UHFFFAOYSA-N N,N-dimethylglycine Chemical compound CN(C)CC(O)=O FFDGPVCHZBVARC-UHFFFAOYSA-N 0.000 description 2
- HOKKHZGPKSLGJE-GSVOUGTGSA-N N-Methyl-D-aspartic acid Chemical compound CN[C@@H](C(O)=O)CC(O)=O HOKKHZGPKSLGJE-GSVOUGTGSA-N 0.000 description 2
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 2
- 108091007491 NSP3 Papain-like protease domains Proteins 0.000 description 2
- 239000005642 Oleic acid Substances 0.000 description 2
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 2
- 108010058846 Ovalbumin Proteins 0.000 description 2
- 229920001734 PEG propionaldehyde Polymers 0.000 description 2
- 108091006006 PEGylated Proteins Proteins 0.000 description 2
- 108091005804 Peptidases Proteins 0.000 description 2
- 102000035195 Peptidases Human genes 0.000 description 2
- 229940083963 Peptide antagonist Drugs 0.000 description 2
- 101710124951 Phospholipase C Proteins 0.000 description 2
- 108010039918 Polylysine Proteins 0.000 description 2
- 102100023204 Potassium channel subfamily K member 2 Human genes 0.000 description 2
- 102100034369 Potassium voltage-gated channel subfamily A member 2 Human genes 0.000 description 2
- 241001415846 Procellariidae Species 0.000 description 2
- 239000004365 Protease Substances 0.000 description 2
- 101710137312 Protein orai Proteins 0.000 description 2
- RADKZDMFGJYCBB-UHFFFAOYSA-N Pyridoxal Chemical compound CC1=NC=C(CO)C(C=O)=C1O RADKZDMFGJYCBB-UHFFFAOYSA-N 0.000 description 2
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 2
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 2
- 101001049892 Scorpio palmatus Potassium channel toxin alpha-KTx 6.2 Proteins 0.000 description 2
- 108091060738 Sea anemone family Proteins 0.000 description 2
- 241000270295 Serpentes Species 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 230000005867 T cell response Effects 0.000 description 2
- MUMGGOZAMZWBJJ-DYKIIFRCSA-N Testostosterone Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 MUMGGOZAMZWBJJ-DYKIIFRCSA-N 0.000 description 2
- NYTOUQBROMCLBJ-UHFFFAOYSA-N Tetranitromethane Chemical compound [O-][N+](=O)C([N+]([O-])=O)([N+]([O-])=O)[N+]([O-])=O NYTOUQBROMCLBJ-UHFFFAOYSA-N 0.000 description 2
- 229920001615 Tragacanth Polymers 0.000 description 2
- 108091023040 Transcription factor Proteins 0.000 description 2
- 102000040945 Transcription factor Human genes 0.000 description 2
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 2
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 2
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 2
- YRKCREAYFQTBPV-UHFFFAOYSA-N acetylacetone Chemical compound CC(=O)CC(C)=O YRKCREAYFQTBPV-UHFFFAOYSA-N 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 125000002252 acyl group Chemical group 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 238000012867 alanine scanning Methods 0.000 description 2
- 125000001931 aliphatic group Chemical group 0.000 description 2
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 2
- VREFGVBLTWBCJP-UHFFFAOYSA-N alprazolam Chemical compound C12=CC(Cl)=CC=C2N2C(C)=NN=C2CN=C1C1=CC=CC=C1 VREFGVBLTWBCJP-UHFFFAOYSA-N 0.000 description 2
- 230000009435 amidation Effects 0.000 description 2
- 238000007112 amidation reaction Methods 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 150000003862 amino acid derivatives Chemical class 0.000 description 2
- 229960004977 anhydrous lactose Drugs 0.000 description 2
- 231100000659 animal toxin Toxicity 0.000 description 2
- 239000003963 antioxidant agent Substances 0.000 description 2
- 235000006708 antioxidants Nutrition 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 235000010323 ascorbic acid Nutrition 0.000 description 2
- 239000011668 ascorbic acid Substances 0.000 description 2
- 229960005070 ascorbic acid Drugs 0.000 description 2
- 125000000613 asparagine group Chemical group N[C@@H](CC(N)=O)C(=O)* 0.000 description 2
- 235000019445 benzyl alcohol Nutrition 0.000 description 2
- 125000002619 bicyclic group Chemical group 0.000 description 2
- 239000003833 bile salt Substances 0.000 description 2
- 229940093761 bile salts Drugs 0.000 description 2
- 230000008827 biological function Effects 0.000 description 2
- 230000033228 biological regulation Effects 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- 229910000394 calcium triphosphate Inorganic materials 0.000 description 2
- 238000004364 calculation method Methods 0.000 description 2
- 125000001314 canonical amino-acid group Chemical group 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 150000001718 carbodiimides Chemical class 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 229940125400 channel inhibitor Drugs 0.000 description 2
- 239000007795 chemical reaction product Substances 0.000 description 2
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 2
- 210000000349 chromosome Anatomy 0.000 description 2
- 230000004087 circulation Effects 0.000 description 2
- 238000003776 cleavage reaction Methods 0.000 description 2
- 229920002055 compound 48/80 Polymers 0.000 description 2
- 230000001268 conjugating effect Effects 0.000 description 2
- 108050003126 conotoxin Proteins 0.000 description 2
- 238000013270 controlled release Methods 0.000 description 2
- 239000006071 cream Substances 0.000 description 2
- CVSVTCORWBXHQV-UHFFFAOYSA-N creatine Chemical compound NC(=[NH2+])N(C)CC([O-])=O CVSVTCORWBXHQV-UHFFFAOYSA-N 0.000 description 2
- 238000004132 cross linking Methods 0.000 description 2
- 238000007405 data analysis Methods 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 230000003112 degranulating effect Effects 0.000 description 2
- 239000005547 deoxyribonucleotide Substances 0.000 description 2
- 125000002637 deoxyribonucleotide group Chemical group 0.000 description 2
- 206010012601 diabetes mellitus Diseases 0.000 description 2
- 239000000032 diagnostic agent Substances 0.000 description 2
- 229940039227 diagnostic agent Drugs 0.000 description 2
- 238000000502 dialysis Methods 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- 230000004069 differentiation Effects 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 125000002228 disulfide group Chemical group 0.000 description 2
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 description 2
- 239000003596 drug target Substances 0.000 description 2
- 239000003995 emulsifying agent Substances 0.000 description 2
- 201000002491 encephalomyelitis Diseases 0.000 description 2
- 210000002472 endoplasmic reticulum Anatomy 0.000 description 2
- 125000004185 ester group Chemical group 0.000 description 2
- 238000010228 ex vivo assay Methods 0.000 description 2
- 210000003722 extracellular fluid Anatomy 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 238000000855 fermentation Methods 0.000 description 2
- 230000004151 fermentation Effects 0.000 description 2
- 238000011049 filling Methods 0.000 description 2
- 239000007888 film coating Substances 0.000 description 2
- 238000009501 film coating Methods 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 2
- 235000003599 food sweetener Nutrition 0.000 description 2
- 239000000174 gluconic acid Substances 0.000 description 2
- 235000012208 gluconic acid Nutrition 0.000 description 2
- 150000002334 glycols Chemical class 0.000 description 2
- HHLFWLYXYJOTON-UHFFFAOYSA-N glyoxylic acid Chemical compound OC(=O)C=O HHLFWLYXYJOTON-UHFFFAOYSA-N 0.000 description 2
- 229960000789 guanidine hydrochloride Drugs 0.000 description 2
- PJJJBBJSCAKJQF-UHFFFAOYSA-N guanidinium chloride Chemical compound [Cl-].NC(N)=[NH2+] PJJJBBJSCAKJQF-UHFFFAOYSA-N 0.000 description 2
- 239000007902 hard capsule Substances 0.000 description 2
- 238000004128 high performance liquid chromatography Methods 0.000 description 2
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 description 2
- 229960002591 hydroxyproline Drugs 0.000 description 2
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 2
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 2
- 230000002519 immonomodulatory effect Effects 0.000 description 2
- 210000002865 immune cell Anatomy 0.000 description 2
- 230000001900 immune effect Effects 0.000 description 2
- 230000002163 immunogen Effects 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 229940079322 interferon Drugs 0.000 description 2
- 229960003130 interferon gamma Drugs 0.000 description 2
- 210000000936 intestine Anatomy 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 238000007913 intrathecal administration Methods 0.000 description 2
- PGLTVOMIXTUURA-UHFFFAOYSA-N iodoacetamide Chemical group NC(=O)CI PGLTVOMIXTUURA-UHFFFAOYSA-N 0.000 description 2
- 150000002500 ions Chemical class 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 150000002632 lipids Chemical class 0.000 description 2
- 229920001427 mPEG Polymers 0.000 description 2
- 229920002521 macromolecule Polymers 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 2
- 239000001095 magnesium carbonate Substances 0.000 description 2
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 description 2
- CWWARWOPSKGELM-SARDKLJWSA-N methyl (2s)-2-[[(2s)-2-[[2-[[(2s)-2-[[(2s)-2-[[(2s)-5-amino-2-[[(2s)-5-amino-2-[[(2s)-1-[(2s)-6-amino-2-[[(2s)-1-[(2s)-2-amino-5-(diaminomethylideneamino)pentanoyl]pyrrolidine-2-carbonyl]amino]hexanoyl]pyrrolidine-2-carbonyl]amino]-5-oxopentanoyl]amino]-5 Chemical compound C([C@@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)OC)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCCN)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](N)CCCN=C(N)N)C1=CC=CC=C1 CWWARWOPSKGELM-SARDKLJWSA-N 0.000 description 2
- 239000011859 microparticle Substances 0.000 description 2
- 125000002950 monocyclic group Chemical group 0.000 description 2
- 229940063121 neoral Drugs 0.000 description 2
- 210000000653 nervous system Anatomy 0.000 description 2
- 239000002736 nonionic surfactant Substances 0.000 description 2
- 239000002674 ointment Substances 0.000 description 2
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 2
- 235000021313 oleic acid Nutrition 0.000 description 2
- 239000006186 oral dosage form Substances 0.000 description 2
- 239000003960 organic solvent Substances 0.000 description 2
- 229940092253 ovalbumin Drugs 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 230000035515 penetration Effects 0.000 description 2
- RFWLACFDYFIVMC-UHFFFAOYSA-D pentacalcium;[oxido(phosphonatooxy)phosphoryl] phosphate Chemical compound [Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O.[O-]P([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O RFWLACFDYFIVMC-UHFFFAOYSA-D 0.000 description 2
- WLJVXDMOQOGPHL-UHFFFAOYSA-N phenylacetic acid Chemical compound OC(=O)CC1=CC=CC=C1 WLJVXDMOQOGPHL-UHFFFAOYSA-N 0.000 description 2
- OJUGVDODNPJEEC-UHFFFAOYSA-N phenylglyoxal Chemical compound O=CC(=O)C1=CC=CC=C1 OJUGVDODNPJEEC-UHFFFAOYSA-N 0.000 description 2
- BZQFBWGGLXLEPQ-REOHCLBHSA-N phosphoserine Chemical compound OC(=O)[C@@H](N)COP(O)(O)=O BZQFBWGGLXLEPQ-REOHCLBHSA-N 0.000 description 2
- 230000004962 physiological condition Effects 0.000 description 2
- 229920000656 polylysine Polymers 0.000 description 2
- 229920001282 polysaccharide Polymers 0.000 description 2
- 239000005017 polysaccharide Substances 0.000 description 2
- 150000004804 polysaccharides Chemical class 0.000 description 2
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 2
- 239000004810 polytetrafluoroethylene Substances 0.000 description 2
- 239000013641 positive control Substances 0.000 description 2
- 230000004481 post-translational protein modification Effects 0.000 description 2
- 230000003823 potassium efflux Effects 0.000 description 2
- XOJVVFBFDXDTEG-UHFFFAOYSA-N pristane Chemical compound CC(C)CCCC(C)CCCC(C)CCCC(C)C XOJVVFBFDXDTEG-UHFFFAOYSA-N 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- NGVDGCNFYWLIFO-UHFFFAOYSA-N pyridoxal 5'-phosphate Chemical compound CC1=NC=C(COP(O)(O)=O)C(C=O)=C1O NGVDGCNFYWLIFO-UHFFFAOYSA-N 0.000 description 2
- 238000005215 recombination Methods 0.000 description 2
- 230000006798 recombination Effects 0.000 description 2
- 230000022532 regulation of transcription, DNA-dependent Effects 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 230000007017 scission Effects 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 235000015424 sodium Nutrition 0.000 description 2
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 2
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 2
- HRZFUMHJMZEROT-UHFFFAOYSA-L sodium disulfite Chemical compound [Na+].[Na+].[O-]S(=O)S([O-])(=O)=O HRZFUMHJMZEROT-UHFFFAOYSA-L 0.000 description 2
- 235000010262 sodium metabisulphite Nutrition 0.000 description 2
- 239000007901 soft capsule Substances 0.000 description 2
- 238000005063 solubilization Methods 0.000 description 2
- 230000007928 solubilization Effects 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 239000008174 sterile solution Substances 0.000 description 2
- 150000003431 steroids Chemical class 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- KDYFGRWQOYBRFD-UHFFFAOYSA-N succinic acid Chemical compound OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 2
- KZNICNPSHKQLFF-UHFFFAOYSA-N succinimide Chemical compound O=C1CCC(=O)N1 KZNICNPSHKQLFF-UHFFFAOYSA-N 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 239000000375 suspending agent Substances 0.000 description 2
- 239000003765 sweetening agent Substances 0.000 description 2
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 description 2
- 239000000454 talc Substances 0.000 description 2
- 229910052623 talc Inorganic materials 0.000 description 2
- 235000012222 talc Nutrition 0.000 description 2
- RSPCKAHMRANGJZ-UHFFFAOYSA-N thiohydroxylamine Chemical compound SN RSPCKAHMRANGJZ-UHFFFAOYSA-N 0.000 description 2
- 125000000341 threoninyl group Chemical group [H]OC([H])(C([H])([H])[H])C([H])(N([H])[H])C(*)=O 0.000 description 2
- 231100000331 toxic Toxicity 0.000 description 2
- 230000002588 toxic effect Effects 0.000 description 2
- 235000010487 tragacanth Nutrition 0.000 description 2
- 239000000196 tragacanth Substances 0.000 description 2
- 229940116362 tragacanth Drugs 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- 102000003390 tumor necrosis factor Human genes 0.000 description 2
- 208000027930 type IV hypersensitivity disease Diseases 0.000 description 2
- 108700026220 vif Genes Proteins 0.000 description 2
- 229920003169 water-soluble polymer Polymers 0.000 description 2
- 238000001262 western blot Methods 0.000 description 2
- 239000000811 xylitol Substances 0.000 description 2
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 description 2
- 235000010447 xylitol Nutrition 0.000 description 2
- 229960002675 xylitol Drugs 0.000 description 2
- 229930195727 α-lactose Natural products 0.000 description 2
- IZUAHLHTQJCCLJ-UHFFFAOYSA-N (2-chloro-1,1,2,2-tetrafluoroethyl) hypochlorite Chemical compound FC(F)(Cl)C(F)(F)OCl IZUAHLHTQJCCLJ-UHFFFAOYSA-N 0.000 description 1
- AMHXPZLIADYCSB-ZCFIWIBFSA-N (2R)-2-amino-2-formyl-4-methylsulfanylbutanoic acid Chemical compound CSCC[C@@](N)(C=O)C(O)=O AMHXPZLIADYCSB-ZCFIWIBFSA-N 0.000 description 1
- GMKMEZVLHJARHF-UHFFFAOYSA-N (2R,6R)-form-2.6-Diaminoheptanedioic acid Natural products OC(=O)C(N)CCCC(N)C(O)=O GMKMEZVLHJARHF-UHFFFAOYSA-N 0.000 description 1
- ZPWSPWAOQFKGRC-VIFPVBQESA-N (2S)-2-(N-methyl-4-phosphonoanilino)-3-oxopropanoic acid Chemical compound P(=O)(O)(O)C1=CC=C(C=C1)N([C@@H](C=O)C(=O)O)C ZPWSPWAOQFKGRC-VIFPVBQESA-N 0.000 description 1
- OVJBOPBBHWOWJI-FYNXUGHNSA-N (2S)-2-[[(2S)-1-[(2S)-2-[[(aS,1R,3aS,4S,10S,16S,19R,22S,25S,28S,34S,37S,40R,45R,48S,51S,57S,60S,63S,69S,72S,75S,78S,85R,88S,91R,94S)-40-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-4-amino-2-[[(2S,3S)-2-[[(2S,3S)-2-[[(2S,3R)-2-amino-3-hydroxybutanoyl]amino]-3-methylpentanoyl]amino]-3-methylpentanoyl]amino]-4-oxobutanoyl]amino]-3-methylbutanoyl]amino]hexanoyl]amino]-25,48,78,88,94-pentakis(4-aminobutyl)-a-(2-amino-2-oxoethyl)-22,63,72-tris(3-amino-3-oxopropyl)-69-benzyl-37-[(1R)-1-hydroxyethyl]-34,60-bis(hydroxymethyl)-51,57,75-trimethyl-16-(2-methylpropyl)-3a-(2-methylsulfanylethyl)-2a,3,5a,9,15,18,21,24,27,33,36,39,47,50,53,56,59,62,65,68,71,74,77,80,87,90,93,96,99-nonacosaoxo-7a,8a,42,43,82,83-hexathia-1a,2,4a,8,14,17,20,23,26,32,35,38,46,49,52,55,58,61,64,67,70,73,76,79,86,89,92,95,98-nonacosazahexacyclo[43.35.25.419,91.04,8.010,14.028,32]nonahectane-85-carbonyl]amino]-3-(4-hydroxyphenyl)propanoyl]pyrrolidine-2-carbonyl]amino]-3-(1H-imidazol-5-yl)propanoic acid Chemical compound CC[C@H](C)[C@H](NC(=O)[C@@H](N)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@H]1CSSC[C@@H]2NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]3CSSC[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]4CCCN4C(=O)[C@H](CO)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N3)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCSC)NC2=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O OVJBOPBBHWOWJI-FYNXUGHNSA-N 0.000 description 1
- KIUKXJAPPMFGSW-DNGZLQJQSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-DNGZLQJQSA-N 0.000 description 1
- YJLIKUSWRSEPSM-WGQQHEPDSA-N (2r,3r,4s,5r)-2-[6-amino-8-[(4-phenylphenyl)methylamino]purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol Chemical compound C=1C=C(C=2C=CC=CC=2)C=CC=1CNC1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O YJLIKUSWRSEPSM-WGQQHEPDSA-N 0.000 description 1
- CUUPCSCMBWGJAM-QMMMGPOBSA-N (2s)-2-(difluoromethylamino)-3-(4-phosphonophenyl)propanoic acid Chemical compound FC(F)N[C@H](C(=O)O)CC1=CC=C(P(O)(O)=O)C=C1 CUUPCSCMBWGJAM-QMMMGPOBSA-N 0.000 description 1
- GBNGVAXIQKSKBU-VIFPVBQESA-N (2s)-2-(methylamino)-3-(4-phosphonophenyl)propanoic acid Chemical compound CN[C@H](C(O)=O)CC1=CC=C(P(O)(O)=O)C=C1 GBNGVAXIQKSKBU-VIFPVBQESA-N 0.000 description 1
- VEVRNHHLCPGNDU-MUGJNUQGSA-N (2s)-2-amino-5-[1-[(5s)-5-amino-5-carboxypentyl]-3,5-bis[(3s)-3-amino-3-carboxypropyl]pyridin-1-ium-4-yl]pentanoate Chemical compound OC(=O)[C@@H](N)CCCC[N+]1=CC(CC[C@H](N)C(O)=O)=C(CCC[C@H](N)C([O-])=O)C(CC[C@H](N)C(O)=O)=C1 VEVRNHHLCPGNDU-MUGJNUQGSA-N 0.000 description 1
- GPYTYOMSQHBYTK-LURJTMIESA-N (2s)-2-azaniumyl-2,3-dimethylbutanoate Chemical compound CC(C)[C@](C)([NH3+])C([O-])=O GPYTYOMSQHBYTK-LURJTMIESA-N 0.000 description 1
- RSPOGBIHKNKRFJ-FSPLSTOPSA-N (2s,3s)-2-amino-2,3-dimethylpentanoic acid Chemical compound CC[C@H](C)[C@](C)(N)C(O)=O RSPOGBIHKNKRFJ-FSPLSTOPSA-N 0.000 description 1
- UUDAMDVQRQNNHZ-UHFFFAOYSA-N (S)-AMPA Chemical compound CC=1ONC(=O)C=1CC(N)C(O)=O UUDAMDVQRQNNHZ-UHFFFAOYSA-N 0.000 description 1
- 125000003088 (fluoren-9-ylmethoxy)carbonyl group Chemical group 0.000 description 1
- UKAUYVFTDYCKQA-UHFFFAOYSA-N -2-Amino-4-hydroxybutanoic acid Natural products OC(=O)C(N)CCO UKAUYVFTDYCKQA-UHFFFAOYSA-N 0.000 description 1
- NWUYHJFMYQTDRP-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;1-ethenyl-2-ethylbenzene;styrene Chemical compound C=CC1=CC=CC=C1.CCC1=CC=CC=C1C=C.C=CC1=CC=CC=C1C=C NWUYHJFMYQTDRP-UHFFFAOYSA-N 0.000 description 1
- KILNVBDSWZSGLL-KXQOOQHDSA-N 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCC KILNVBDSWZSGLL-KXQOOQHDSA-N 0.000 description 1
- QWUWMCYKGHVNAV-UHFFFAOYSA-N 1,2-dihydrostilbene Chemical group C=1C=CC=CC=1CCC1=CC=CC=C1 QWUWMCYKGHVNAV-UHFFFAOYSA-N 0.000 description 1
- SNKAWJBJQDLSFF-NVKMUCNASA-N 1,2-dioleoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCC\C=C/CCCCCCCC SNKAWJBJQDLSFF-NVKMUCNASA-N 0.000 description 1
- NRJAVPSFFCBXDT-HUESYALOSA-N 1,2-distearoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCCCC NRJAVPSFFCBXDT-HUESYALOSA-N 0.000 description 1
- WDQFELCEOPFLCZ-UHFFFAOYSA-N 1-(2-hydroxyethyl)pyrrolidin-2-one Chemical compound OCCN1CCCC1=O WDQFELCEOPFLCZ-UHFFFAOYSA-N 0.000 description 1
- LUTLAXLNPLZCOF-UHFFFAOYSA-N 1-Methylhistidine Chemical group OC(=O)C(N)(C)CC1=NC=CN1 LUTLAXLNPLZCOF-UHFFFAOYSA-N 0.000 description 1
- WQUWKZJWBCOHQH-UHFFFAOYSA-N 1-[2-[2-(2-methoxyethoxy)ethoxy]ethyl]pyrrole-2,5-dione Chemical compound COCCOCCOCCN1C(=O)C=CC1=O WQUWKZJWBCOHQH-UHFFFAOYSA-N 0.000 description 1
- PZNPLUBHRSSFHT-RRHRGVEJSA-N 1-hexadecanoyl-2-octadecanoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCCCC(=O)O[C@@H](COP([O-])(=O)OCC[N+](C)(C)C)COC(=O)CCCCCCCCCCCCCCC PZNPLUBHRSSFHT-RRHRGVEJSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- VOXZDWNPVJITMN-ZBRFXRBCSA-N 17β-estradiol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 VOXZDWNPVJITMN-ZBRFXRBCSA-N 0.000 description 1
- 125000003287 1H-imidazol-4-ylmethyl group Chemical group [H]N1C([H])=NC(C([H])([H])[*])=C1[H] 0.000 description 1
- NHJVRSWLHSJWIN-UHFFFAOYSA-N 2,4,6-trinitrobenzenesulfonic acid Chemical compound OS(=O)(=O)C1=C([N+]([O-])=O)C=C([N+]([O-])=O)C=C1[N+]([O-])=O NHJVRSWLHSJWIN-UHFFFAOYSA-N 0.000 description 1
- CAHMGWYMQPWRSF-UHFFFAOYSA-N 2,5-dioxopyrrolidine-1-sulfonic acid Chemical compound OS(=O)(=O)N1C(=O)CCC1=O CAHMGWYMQPWRSF-UHFFFAOYSA-N 0.000 description 1
- 150000003923 2,5-pyrrolediones Chemical class 0.000 description 1
- JECYNCQXXKQDJN-UHFFFAOYSA-N 2-(2-methylhexan-2-yloxymethyl)oxirane Chemical compound CCCCC(C)(C)OCC1CO1 JECYNCQXXKQDJN-UHFFFAOYSA-N 0.000 description 1
- RDFMDVXONNIGBC-UHFFFAOYSA-N 2-aminoheptanoic acid Chemical compound CCCCCC(N)C(O)=O RDFMDVXONNIGBC-UHFFFAOYSA-N 0.000 description 1
- 125000001431 2-aminoisobutyric acid group Chemical group [#6]C([#6])(N*)C(*)=O 0.000 description 1
- SNDPXSYFESPGGJ-UHFFFAOYSA-N 2-aminopentanoic acid Chemical compound CCCC(N)C(O)=O SNDPXSYFESPGGJ-UHFFFAOYSA-N 0.000 description 1
- JUQLUIFNNFIIKC-UHFFFAOYSA-N 2-aminopimelic acid Chemical compound OC(=O)C(N)CCCCC(O)=O JUQLUIFNNFIIKC-UHFFFAOYSA-N 0.000 description 1
- OFYAYGJCPXRNBL-UHFFFAOYSA-N 2-azaniumyl-3-naphthalen-1-ylpropanoate Chemical compound C1=CC=C2C(CC(N)C(O)=O)=CC=CC2=C1 OFYAYGJCPXRNBL-UHFFFAOYSA-N 0.000 description 1
- ZNQVEEAIQZEUHB-UHFFFAOYSA-N 2-ethoxyethanol Chemical compound CCOCCO ZNQVEEAIQZEUHB-UHFFFAOYSA-N 0.000 description 1
- JQPFYXFVUKHERX-UHFFFAOYSA-N 2-hydroxy-2-cyclohexen-1-one Natural products OC1=CCCCC1=O JQPFYXFVUKHERX-UHFFFAOYSA-N 0.000 description 1
- 125000003816 2-hydroxybenzoyl group Chemical group OC1=C(C(=O)*)C=CC=C1 0.000 description 1
- ZOOGRGPOEVQQDX-UUOKFMHZSA-N 3',5'-cyclic GMP Chemical compound C([C@H]1O2)OP(O)(=O)O[C@H]1[C@@H](O)[C@@H]2N1C(N=C(NC2=O)N)=C2N=C1 ZOOGRGPOEVQQDX-UUOKFMHZSA-N 0.000 description 1
- VJINKBZUJYGZGP-UHFFFAOYSA-N 3-(1-aminopropylideneamino)propyl-trimethylazanium Chemical compound CCC(N)=NCCC[N+](C)(C)C VJINKBZUJYGZGP-UHFFFAOYSA-N 0.000 description 1
- BIGBDMFRWJRLGJ-UHFFFAOYSA-N 3-benzyl-1,5-didiazoniopenta-1,4-diene-2,4-diolate Chemical compound [N-]=[N+]=CC(=O)C(C(=O)C=[N+]=[N-])CC1=CC=CC=C1 BIGBDMFRWJRLGJ-UHFFFAOYSA-N 0.000 description 1
- ZHQJIJUMPYNVAZ-UHFFFAOYSA-N 3-hydroxy-1-methylpyrrolidin-2-one Chemical compound CN1CCC(O)C1=O ZHQJIJUMPYNVAZ-UHFFFAOYSA-N 0.000 description 1
- NLPWSMKACWGINL-UHFFFAOYSA-N 4-azido-2-hydroxybenzoic acid Chemical compound OC(=O)C1=CC=C(N=[N+]=[N-])C=C1O NLPWSMKACWGINL-UHFFFAOYSA-N 0.000 description 1
- SLXKOJJOQWFEFD-UHFFFAOYSA-N 6-aminohexanoic acid Chemical compound NCCCCCC(O)=O SLXKOJJOQWFEFD-UHFFFAOYSA-N 0.000 description 1
- 244000215068 Acacia senegal Species 0.000 description 1
- 101150002557 Adnp gene Proteins 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 108700028369 Alleles Proteins 0.000 description 1
- 239000004956 Amodel Substances 0.000 description 1
- 229930183010 Amphotericin Natural products 0.000 description 1
- QGGFZZLFKABGNL-UHFFFAOYSA-N Amphotericin A Natural products OC1C(N)C(O)C(C)OC1OC1C=CC=CC=CC=CCCC=CC=CC(C)C(O)C(C)C(C)OC(=O)CC(O)CC(O)CCC(O)C(O)CC(O)CC(O)(CC(O)C2C(O)=O)OC2C1 QGGFZZLFKABGNL-UHFFFAOYSA-N 0.000 description 1
- 229920000945 Amylopectin Polymers 0.000 description 1
- 241000024188 Andala Species 0.000 description 1
- 108010064733 Angiotensins Proteins 0.000 description 1
- 102000015427 Angiotensins Human genes 0.000 description 1
- 108091006515 Anion channels Proteins 0.000 description 1
- 102000037829 Anion channels Human genes 0.000 description 1
- FTCGGKNCJZOPNB-WHFBIAKZSA-N Asn-Gly-Ser Chemical compound NC(=O)C[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O FTCGGKNCJZOPNB-WHFBIAKZSA-N 0.000 description 1
- 208000032116 Autoimmune Experimental Encephalomyelitis Diseases 0.000 description 1
- 101800004538 Bradykinin Proteins 0.000 description 1
- 102400000967 Bradykinin Human genes 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 1
- 108010063916 CD40 Antigens Proteins 0.000 description 1
- 101100533230 Caenorhabditis elegans ser-2 gene Proteins 0.000 description 1
- 108010045489 Calcium-Activated Potassium Channels Proteins 0.000 description 1
- 102000005702 Calcium-Activated Potassium Channels Human genes 0.000 description 1
- 102000000584 Calmodulin Human genes 0.000 description 1
- 108010041952 Calmodulin Proteins 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- 108010078791 Carrier Proteins Proteins 0.000 description 1
- 102000014914 Carrier Proteins Human genes 0.000 description 1
- 108091005462 Cation channels Proteins 0.000 description 1
- 101000997261 Centruroides margaritatus Potassium channel toxin alpha-KTx 2.2 Proteins 0.000 description 1
- 241000239328 Centruroides noxius Species 0.000 description 1
- 241000239282 Centruroides suffusus Species 0.000 description 1
- 108010023798 Charybdotoxin Proteins 0.000 description 1
- 101000936911 Chionoecetes opilio Sarcoplasmic/endoplasmic reticulum calcium ATPase Proteins 0.000 description 1
- 102000011045 Chloride Channels Human genes 0.000 description 1
- 108010062745 Chloride Channels Proteins 0.000 description 1
- BHYOQNUELFTYRT-UHFFFAOYSA-N Cholesterol sulfate Natural products C1C=C2CC(OS(O)(=O)=O)CCC2(C)C2C1C1CCC(C(C)CCCC(C)C)C1(C)CC2 BHYOQNUELFTYRT-UHFFFAOYSA-N 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 1
- 241000557626 Corvus corax Species 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- ODKSFYDXXFIFQN-SCSAIBSYSA-N D-arginine Chemical compound OC(=O)[C@H](N)CCCNC(N)=N ODKSFYDXXFIFQN-SCSAIBSYSA-N 0.000 description 1
- 229930028154 D-arginine Natural products 0.000 description 1
- 201000004624 Dermatitis Diseases 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 239000004338 Dichlorodifluoromethane Substances 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 102400000686 Endothelin-1 Human genes 0.000 description 1
- 101800004490 Endothelin-1 Proteins 0.000 description 1
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 1
- 108091006020 Fc-tagged proteins Proteins 0.000 description 1
- 102000008946 Fibrinogen Human genes 0.000 description 1
- 108010049003 Fibrinogen Proteins 0.000 description 1
- IECPWNUMDGFDKC-UHFFFAOYSA-N Fusicsaeure Natural products C12C(O)CC3C(=C(CCC=C(C)C)C(O)=O)C(OC(C)=O)CC3(C)C1(C)CCC1C2(C)CCC(O)C1C IECPWNUMDGFDKC-UHFFFAOYSA-N 0.000 description 1
- 108091006027 G proteins Proteins 0.000 description 1
- WMBWREPUVVBILR-UHFFFAOYSA-N GCG Natural products C=1C(O)=C(O)C(O)=CC=1C1OC2=CC(O)=CC(O)=C2CC1OC(=O)C1=CC(O)=C(O)C(O)=C1 WMBWREPUVVBILR-UHFFFAOYSA-N 0.000 description 1
- 102000030782 GTP binding Human genes 0.000 description 1
- 108091000058 GTP-Binding Proteins 0.000 description 1
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 1
- 102000006395 Globulins Human genes 0.000 description 1
- 108010044091 Globulins Proteins 0.000 description 1
- SXRSQZLOMIGNAQ-UHFFFAOYSA-N Glutaraldehyde Chemical compound O=CCCCC=O SXRSQZLOMIGNAQ-UHFFFAOYSA-N 0.000 description 1
- 102000011714 Glycine Receptors Human genes 0.000 description 1
- 108010076533 Glycine Receptors Proteins 0.000 description 1
- AEMRFAOFKBGASW-UHFFFAOYSA-M Glycolate Chemical compound OCC([O-])=O AEMRFAOFKBGASW-UHFFFAOYSA-M 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 229920002683 Glycosaminoglycan Polymers 0.000 description 1
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 1
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 description 1
- 229920000084 Gum arabic Polymers 0.000 description 1
- QXZGBUJJYSLZLT-UHFFFAOYSA-N H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH Natural products NC(N)=NCCCC(N)C(=O)N1CCCC1C(=O)N1C(C(=O)NCC(=O)NC(CC=2C=CC=CC=2)C(=O)NC(CO)C(=O)N2C(CCC2)C(=O)NC(CC=2C=CC=CC=2)C(=O)NC(CCCN=C(N)N)C(O)=O)CCC1 QXZGBUJJYSLZLT-UHFFFAOYSA-N 0.000 description 1
- 102000014702 Haptoglobin Human genes 0.000 description 1
- 108050005077 Haptoglobin Proteins 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101001055314 Homo sapiens Immunoglobulin heavy constant alpha 2 Proteins 0.000 description 1
- 101001055308 Homo sapiens Immunoglobulin heavy constant epsilon Proteins 0.000 description 1
- 101000961156 Homo sapiens Immunoglobulin heavy constant gamma 1 Proteins 0.000 description 1
- 101000961146 Homo sapiens Immunoglobulin heavy constant gamma 2 Proteins 0.000 description 1
- 101000961145 Homo sapiens Immunoglobulin heavy constant gamma 3 Proteins 0.000 description 1
- 101001049841 Homo sapiens Potassium channel subfamily K member 1 Proteins 0.000 description 1
- 101000974742 Homo sapiens Potassium channel subfamily K member 13 Proteins 0.000 description 1
- 101001049834 Homo sapiens Potassium channel subfamily K member 2 Proteins 0.000 description 1
- 101001049835 Homo sapiens Potassium channel subfamily K member 3 Proteins 0.000 description 1
- 102000002265 Human Growth Hormone Human genes 0.000 description 1
- 108010000521 Human Growth Hormone Proteins 0.000 description 1
- 239000000854 Human Growth Hormone Substances 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- LCWXJXMHJVIJFK-UHFFFAOYSA-N Hydroxylysine Natural products NCC(O)CC(N)CC(O)=O LCWXJXMHJVIJFK-UHFFFAOYSA-N 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- 102100026120 IgG receptor FcRn large subunit p51 Human genes 0.000 description 1
- 101710177940 IgG receptor FcRn large subunit p51 Proteins 0.000 description 1
- 102000018071 Immunoglobulin Fc Fragments Human genes 0.000 description 1
- 108010091135 Immunoglobulin Fc Fragments Proteins 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- 102100026216 Immunoglobulin heavy constant alpha 2 Human genes 0.000 description 1
- 102100039345 Immunoglobulin heavy constant gamma 1 Human genes 0.000 description 1
- 102100039346 Immunoglobulin heavy constant gamma 2 Human genes 0.000 description 1
- 102100039348 Immunoglobulin heavy constant gamma 3 Human genes 0.000 description 1
- HCUARRIEZVDMPT-UHFFFAOYSA-N Indole-2-carboxylic acid Chemical compound C1=CC=C2NC(C(=O)O)=CC2=C1 HCUARRIEZVDMPT-UHFFFAOYSA-N 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 108010002386 Interleukin-3 Proteins 0.000 description 1
- 102000000646 Interleukin-3 Human genes 0.000 description 1
- 108090000978 Interleukin-4 Proteins 0.000 description 1
- 102100037441 Intermediate conductance calcium-activated potassium channel protein 4 Human genes 0.000 description 1
- 241000408495 Iton Species 0.000 description 1
- AGPKZVBTJJNPAG-UHNVWZDZSA-N L-allo-Isoleucine Chemical compound CC[C@@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-UHNVWZDZSA-N 0.000 description 1
- 150000008575 L-amino acids Chemical class 0.000 description 1
- 229930064664 L-arginine Natural products 0.000 description 1
- 235000014852 L-arginine Nutrition 0.000 description 1
- LEVWYRKDKASIDU-IMJSIDKUSA-N L-cystine Chemical compound [O-]C(=O)[C@@H]([NH3+])CSSC[C@H]([NH3+])C([O-])=O LEVWYRKDKASIDU-IMJSIDKUSA-N 0.000 description 1
- UKAUYVFTDYCKQA-VKHMYHEASA-N L-homoserine Chemical compound OC(=O)[C@@H](N)CCO UKAUYVFTDYCKQA-VKHMYHEASA-N 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-L L-tartrate(2-) Chemical compound [O-]C(=O)[C@H](O)[C@@H](O)C([O-])=O FEWJPZIEWOKRBE-JCYAYHJZSA-L 0.000 description 1
- 108010000817 Leuprolide Proteins 0.000 description 1
- OYHQOLUKZRVURQ-HZJYTTRNSA-N Linoleic acid Chemical compound CCCCC\C=C/C\C=C/CCCCCCCC(O)=O OYHQOLUKZRVURQ-HZJYTTRNSA-N 0.000 description 1
- NPBGTPKLVJEOBE-IUCAKERBSA-N Lys-Arg Chemical compound NCCCC[C@H](N)C(=O)N[C@H](C(O)=O)CCCNC(N)=N NPBGTPKLVJEOBE-IUCAKERBSA-N 0.000 description 1
- 102000007651 Macrophage Colony-Stimulating Factor Human genes 0.000 description 1
- 108010046938 Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 241001272720 Medialuna californiensis Species 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 1
- 101100118976 Mus musculus Clint1 gene Proteins 0.000 description 1
- 101100328158 Mus musculus Clmp gene Proteins 0.000 description 1
- 101000846105 Mus musculus Short transient receptor potential channel 6 Proteins 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- DTERQYGMUDWYAZ-ZETCQYMHSA-N N(6)-acetyl-L-lysine Chemical compound CC(=O)NCCCC[C@H]([NH3+])C([O-])=O DTERQYGMUDWYAZ-ZETCQYMHSA-N 0.000 description 1
- NTNWOCRCBQPEKQ-YFKPBYRVSA-N N(omega)-methyl-L-arginine Chemical group CN=C(N)NCCC[C@H](N)C(O)=O NTNWOCRCBQPEKQ-YFKPBYRVSA-N 0.000 description 1
- BRMWTNUJHUMWMS-LURJTMIESA-N N(tele)-methyl-L-histidine Chemical group CN1C=NC(C[C@H](N)C(O)=O)=C1 BRMWTNUJHUMWMS-LURJTMIESA-N 0.000 description 1
- NQTADLQHYWFPDB-UHFFFAOYSA-N N-Hydroxysuccinimide Chemical compound ON1C(=O)CCC1=O NQTADLQHYWFPDB-UHFFFAOYSA-N 0.000 description 1
- NTWVQPHTOUKMDI-YFKPBYRVSA-N N-Methyl-arginine Chemical group CN[C@H](C(O)=O)CCCN=C(N)N NTWVQPHTOUKMDI-YFKPBYRVSA-N 0.000 description 1
- JJIHLJJYMXLCOY-BYPYZUCNSA-N N-acetyl-L-serine Chemical compound CC(=O)N[C@@H](CO)C(O)=O JJIHLJJYMXLCOY-BYPYZUCNSA-N 0.000 description 1
- 125000000729 N-terminal amino-acid group Chemical group 0.000 description 1
- 102000003945 NF-kappa B Human genes 0.000 description 1
- 108010057466 NF-kappa B Proteins 0.000 description 1
- 206010029155 Nephropathy toxic Diseases 0.000 description 1
- NCHSYZVVWKVWFQ-BYPYZUCNSA-N Ng-amino-l-arginine Chemical group NNC(N)=NCCC[C@H](N)C(O)=O NCHSYZVVWKVWFQ-BYPYZUCNSA-N 0.000 description 1
- 208000008589 Obesity Diseases 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 102000015636 Oligopeptides Human genes 0.000 description 1
- 108010038807 Oligopeptides Proteins 0.000 description 1
- 101710116435 Outer membrane protein Proteins 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 108090000526 Papain Proteins 0.000 description 1
- 241000237988 Patellidae Species 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 108091093037 Peptide nucleic acid Proteins 0.000 description 1
- 239000004264 Petrolatum Substances 0.000 description 1
- 206010057249 Phagocytosis Diseases 0.000 description 1
- 102000045595 Phosphoprotein Phosphatases Human genes 0.000 description 1
- 108700019535 Phosphoprotein Phosphatases Proteins 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 241001315609 Pittosporum crassifolium Species 0.000 description 1
- 229920001363 Polidocanol Polymers 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 229920000954 Polyglycolide Polymers 0.000 description 1
- 229920002701 Polyoxyl 40 Stearate Polymers 0.000 description 1
- 229920000037 Polyproline Polymers 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 229920001219 Polysorbate 40 Polymers 0.000 description 1
- 229920001214 Polysorbate 60 Polymers 0.000 description 1
- 229920002642 Polysorbate 65 Polymers 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- 102100023242 Potassium channel subfamily K member 1 Human genes 0.000 description 1
- 102100022799 Potassium channel subfamily K member 13 Human genes 0.000 description 1
- 102100023207 Potassium channel subfamily K member 3 Human genes 0.000 description 1
- 102100023205 Potassium channel subfamily K member 4 Human genes 0.000 description 1
- 101710185483 Potassium channel subfamily K member 4 Proteins 0.000 description 1
- NPYPAHLBTDXSSS-UHFFFAOYSA-N Potassium ion Chemical compound [K+] NPYPAHLBTDXSSS-UHFFFAOYSA-N 0.000 description 1
- 101800001357 Potential peptide Proteins 0.000 description 1
- 102400000745 Potential peptide Human genes 0.000 description 1
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical compound CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 230000026279 RNA modification Effects 0.000 description 1
- 241000700157 Rattus norvegicus Species 0.000 description 1
- 241000220010 Rhode Species 0.000 description 1
- 108091028664 Ribonucleotide Proteins 0.000 description 1
- 241000235070 Saccharomyces Species 0.000 description 1
- 102000007355 Sarcoplasmic Reticulum Calcium-Transporting ATPases Human genes 0.000 description 1
- 108010032750 Sarcoplasmic Reticulum Calcium-Transporting ATPases Proteins 0.000 description 1
- 108010077895 Sarcosine Proteins 0.000 description 1
- 238000012300 Sequence Analysis Methods 0.000 description 1
- 102000007562 Serum Albumin Human genes 0.000 description 1
- 108010071390 Serum Albumin Proteins 0.000 description 1
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 1
- 108010052164 Sodium Channels Proteins 0.000 description 1
- 102000018674 Sodium Channels Human genes 0.000 description 1
- 239000004147 Sorbitan trioleate Substances 0.000 description 1
- PRXRUNOAOLTIEF-ADSICKODSA-N Sorbitan trioleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@@H](OC(=O)CCCCCCC\C=C/CCCCCCCC)[C@H]1OC[C@H](O)[C@H]1OC(=O)CCCCCCC\C=C/CCCCCCCC PRXRUNOAOLTIEF-ADSICKODSA-N 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 235000015125 Sterculia urens Nutrition 0.000 description 1
- 240000001058 Sterculia urens Species 0.000 description 1
- 241000242736 Stichodactyla Species 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 229920002253 Tannate Polymers 0.000 description 1
- 241000239292 Theraphosidae Species 0.000 description 1
- 102000002248 Thyroxine-Binding Globulin Human genes 0.000 description 1
- 108010000259 Thyroxine-Binding Globulin Proteins 0.000 description 1
- 102000003929 Transaminases Human genes 0.000 description 1
- 108090000340 Transaminases Proteins 0.000 description 1
- 108010018242 Transcription Factor AP-1 Proteins 0.000 description 1
- 102100023132 Transcription factor Jun Human genes 0.000 description 1
- 102220608299 Transcription factor SOX-2_Y23Q_mutation Human genes 0.000 description 1
- 102000004338 Transferrin Human genes 0.000 description 1
- 108090000901 Transferrin Proteins 0.000 description 1
- DTQVDTLACAAQTR-UHFFFAOYSA-M Trifluoroacetate Chemical compound [O-]C(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-M 0.000 description 1
- 208000025865 Ulcer Diseases 0.000 description 1
- 101150117115 V gene Proteins 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- GELXFVQAWNTGPQ-UHFFFAOYSA-N [N].C1=CNC=N1 Chemical compound [N].C1=CNC=N1 GELXFVQAWNTGPQ-UHFFFAOYSA-N 0.000 description 1
- 235000010489 acacia gum Nutrition 0.000 description 1
- 239000000205 acacia gum Substances 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- 230000021736 acetylation Effects 0.000 description 1
- 238000006640 acetylation reaction Methods 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 230000010933 acylation Effects 0.000 description 1
- 238000005917 acylation reaction Methods 0.000 description 1
- 229940060205 adagen Drugs 0.000 description 1
- 230000033289 adaptive immune response Effects 0.000 description 1
- 230000004721 adaptive immunity Effects 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 229960005305 adenosine Drugs 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 239000012790 adhesive layer Substances 0.000 description 1
- 238000012382 advanced drug delivery Methods 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 235000010419 agar Nutrition 0.000 description 1
- 239000000556 agonist Substances 0.000 description 1
- BNPSSFBOAGDEEL-UHFFFAOYSA-N albuterol sulfate Chemical compound OS(O)(=O)=O.CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1.CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1 BNPSSFBOAGDEEL-UHFFFAOYSA-N 0.000 description 1
- 230000001476 alcoholic effect Effects 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- HAXFWIACAGNFHA-UHFFFAOYSA-N aldrithiol Chemical group C=1C=CC=NC=1SSC1=CC=CC=N1 HAXFWIACAGNFHA-UHFFFAOYSA-N 0.000 description 1
- 150000001345 alkine derivatives Chemical class 0.000 description 1
- 125000003282 alkyl amino group Chemical group 0.000 description 1
- VLSMHEGGTFMBBZ-UHFFFAOYSA-N alpha-Kainic acid Natural products CC(=C)C1CNC(C(O)=O)C1CC(O)=O VLSMHEGGTFMBBZ-UHFFFAOYSA-N 0.000 description 1
- DTOSIQBPPRVQHS-PDBXOOCHSA-N alpha-linolenic acid Chemical compound CC\C=C/C\C=C/C\C=C/CCCCCCCC(O)=O DTOSIQBPPRVQHS-PDBXOOCHSA-N 0.000 description 1
- 235000020661 alpha-linolenic acid Nutrition 0.000 description 1
- HYOWVAAEQCNGLE-JTQLQIEISA-N alpha-methyl-L-phenylalanine Chemical compound OC(=O)[C@](N)(C)CC1=CC=CC=C1 HYOWVAAEQCNGLE-JTQLQIEISA-N 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 230000006229 amino acid addition Effects 0.000 description 1
- 229940124277 aminobutyric acid Drugs 0.000 description 1
- 229960002684 aminocaproic acid Drugs 0.000 description 1
- 125000002344 aminooxy group Chemical group [H]N([H])O[*] 0.000 description 1
- 229940009444 amphotericin Drugs 0.000 description 1
- APKFDSVGJQXUKY-INPOYWNPSA-N amphotericin B Chemical compound O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 APKFDSVGJQXUKY-INPOYWNPSA-N 0.000 description 1
- 208000007502 anemia Diseases 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 239000003945 anionic surfactant Substances 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 230000000692 anti-sense effect Effects 0.000 description 1
- 239000003831 antifriction material Substances 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 206010003246 arthritis Diseases 0.000 description 1
- 229960003272 asparaginase Drugs 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- ICCBZGUDUOMNOF-UHFFFAOYSA-N azidoamine Chemical compound NN=[N+]=[N-] ICCBZGUDUOMNOF-UHFFFAOYSA-N 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 description 1
- 229960001950 benzethonium chloride Drugs 0.000 description 1
- UREZNYTWGJKWBI-UHFFFAOYSA-M benzethonium chloride Chemical compound [Cl-].C1=CC(C(C)(C)CC(C)(C)C)=CC=C1OCCOCC[N+](C)(C)CC1=CC=CC=C1 UREZNYTWGJKWBI-UHFFFAOYSA-M 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- 229940000635 beta-alanine Drugs 0.000 description 1
- 150000001576 beta-amino acids Chemical class 0.000 description 1
- 235000013361 beverage Nutrition 0.000 description 1
- 238000004166 bioassay Methods 0.000 description 1
- 238000002306 biochemical method Methods 0.000 description 1
- 230000008033 biological extinction Effects 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 229940088623 biologically active substance Drugs 0.000 description 1
- 125000004057 biotinyl group Chemical class [H]N1C(=O)N([H])[C@]2([H])[C@@]([H])(SC([H])([H])[C@]12[H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C(*)=O 0.000 description 1
- 239000003114 blood coagulation factor Substances 0.000 description 1
- 239000012503 blood component Substances 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 239000005388 borosilicate glass Substances 0.000 description 1
- QXZGBUJJYSLZLT-FDISYFBBSA-N bradykinin Chemical compound NC(=N)NCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(=O)NCC(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CO)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)CCC1 QXZGBUJJYSLZLT-FDISYFBBSA-N 0.000 description 1
- 125000001246 bromo group Chemical group Br* 0.000 description 1
- 239000007975 buffered saline Substances 0.000 description 1
- 230000001275 ca(2+)-mobilization Effects 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 230000003185 calcium uptake Effects 0.000 description 1
- 150000001721 carbon Chemical group 0.000 description 1
- 150000001244 carboxylic acid anhydrides Chemical class 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 239000003729 cation exchange resin Substances 0.000 description 1
- 229940023913 cation exchange resins Drugs 0.000 description 1
- 239000003093 cationic surfactant Substances 0.000 description 1
- 230000020411 cell activation Effects 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 230000036755 cellular response Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 229920001429 chelating resin Polymers 0.000 description 1
- 125000001309 chloro group Chemical group Cl* 0.000 description 1
- 125000002668 chloroacetyl group Chemical group ClCC(=O)* 0.000 description 1
- 210000003763 chloroplast Anatomy 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- BHYOQNUELFTYRT-DPAQBDIFSA-N cholesterol sulfate Chemical class C1C=C2C[C@@H](OS(O)(=O)=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 BHYOQNUELFTYRT-DPAQBDIFSA-N 0.000 description 1
- 150000001841 cholesterols Chemical class 0.000 description 1
- 210000002825 class switched memory b cell Anatomy 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 230000024203 complement activation Effects 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 239000003636 conditioned culture medium Substances 0.000 description 1
- 239000004020 conductor Substances 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 230000000875 corresponding effect Effects 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 239000007822 coupling agent Substances 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 229960003624 creatine Drugs 0.000 description 1
- 239000006046 creatine Substances 0.000 description 1
- 239000003431 cross linking reagent Substances 0.000 description 1
- CNVQLPPZGABUCM-LIGYZCPXSA-N ctx toxin Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CO)C(=O)N[C@H]1CSSC[C@H]2C(=O)N[C@H](C(N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@H]3CSSC[C@@H](C(N[C@@H](CC=4C5=CC=CC=C5NC=4)C(=O)N[C@@H](CO)C(=O)N[C@H](C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC3=O)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CO)C(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=3NC=NC=3)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N2)C(C)C)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H]([C@@H](C)O)NC1=O)=O)CCSC)C(C)C)[C@@H](C)O)NC(=O)[C@H]1NC(=O)CC1)C1=CC=CC=C1 CNVQLPPZGABUCM-LIGYZCPXSA-N 0.000 description 1
- 125000004093 cyano group Chemical group *C#N 0.000 description 1
- ATDGTVJJHBUTRL-UHFFFAOYSA-N cyanogen bromide Chemical compound BrC#N ATDGTVJJHBUTRL-UHFFFAOYSA-N 0.000 description 1
- OILAIQUEIWYQPH-UHFFFAOYSA-N cyclohexane-1,2-dione Chemical compound O=C1CCCCC1=O OILAIQUEIWYQPH-UHFFFAOYSA-N 0.000 description 1
- UFULAYFCSOUIOV-UHFFFAOYSA-N cysteamine Chemical compound NCCS UFULAYFCSOUIOV-UHFFFAOYSA-N 0.000 description 1
- 229960003067 cystine Drugs 0.000 description 1
- 230000016396 cytokine production Effects 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 230000001461 cytolytic effect Effects 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- 210000004395 cytoplasmic granule Anatomy 0.000 description 1
- 230000001086 cytosolic effect Effects 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 238000013480 data collection Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000007123 defense Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 108010091894 dendrotoxin K Proteins 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000010511 deprotection reaction Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- PXBRQCKWGAHEHS-UHFFFAOYSA-N dichlorodifluoromethane Chemical compound FC(F)(Cl)Cl PXBRQCKWGAHEHS-UHFFFAOYSA-N 0.000 description 1
- 235000019404 dichlorodifluoromethane Nutrition 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 102000038379 digestive enzymes Human genes 0.000 description 1
- 108091007734 digestive enzymes Proteins 0.000 description 1
- 108700003601 dimethylglycine Proteins 0.000 description 1
- YKBZOVFACRVRJN-UHFFFAOYSA-N dinotefuran Chemical compound [O-][N+](=O)\N=C(/NC)NCC1CCOC1 YKBZOVFACRVRJN-UHFFFAOYSA-N 0.000 description 1
- 235000019329 dioctyl sodium sulphosuccinate Nutrition 0.000 description 1
- 150000002009 diols Chemical class 0.000 description 1
- 208000037765 diseases and disorders Diseases 0.000 description 1
- YHAIUSTWZPMYGG-UHFFFAOYSA-L disodium;2,2-dioctyl-3-sulfobutanedioate Chemical compound [Na+].[Na+].CCCCCCCCC(C([O-])=O)(C(C([O-])=O)S(O)(=O)=O)CCCCCCCC YHAIUSTWZPMYGG-UHFFFAOYSA-L 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 125000004119 disulfanediyl group Chemical group *SS* 0.000 description 1
- 230000006334 disulfide bridging Effects 0.000 description 1
- 229940112141 dry powder inhaler Drugs 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 238000011984 electrochemiluminescence immunoassay Methods 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 239000002702 enteric coating Substances 0.000 description 1
- 238000009505 enteric coating Methods 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 239000002532 enzyme inhibitor Substances 0.000 description 1
- 229940030275 epigallocatechin gallate Drugs 0.000 description 1
- 210000005081 epithelial layer Anatomy 0.000 description 1
- 229930182833 estradiol Natural products 0.000 description 1
- 229960005309 estradiol Drugs 0.000 description 1
- 229940011871 estrogen Drugs 0.000 description 1
- 239000000262 estrogen Substances 0.000 description 1
- 238000009164 estrogen replacement therapy Methods 0.000 description 1
- AZHSSKPUVBVXLK-UHFFFAOYSA-N ethane-1,1-diol Chemical compound CC(O)O AZHSSKPUVBVXLK-UHFFFAOYSA-N 0.000 description 1
- 125000004494 ethyl ester group Chemical group 0.000 description 1
- SFNALCNOMXIBKG-UHFFFAOYSA-N ethylene glycol monododecyl ether Chemical compound CCCCCCCCCCCCOCCO SFNALCNOMXIBKG-UHFFFAOYSA-N 0.000 description 1
- 210000003527 eukaryotic cell Anatomy 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 230000028023 exocytosis Effects 0.000 description 1
- 208000012997 experimental autoimmune encephalomyelitis Diseases 0.000 description 1
- 239000000284 extract Substances 0.000 description 1
- 238000009313 farming Methods 0.000 description 1
- 235000019197 fats Nutrition 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 229940012952 fibrinogen Drugs 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000007941 film coated tablet Substances 0.000 description 1
- 238000013100 final test Methods 0.000 description 1
- 235000019634 flavors Nutrition 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- UHCBBWUQDAVSMS-UHFFFAOYSA-N fluoroethane Chemical compound CCF UHCBBWUQDAVSMS-UHFFFAOYSA-N 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 230000033581 fucosylation Effects 0.000 description 1
- 230000005714 functional activity Effects 0.000 description 1
- 238000002825 functional assay Methods 0.000 description 1
- 238000007306 functionalization reaction Methods 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- IECPWNUMDGFDKC-MZJAQBGESA-N fusidic acid Chemical compound O[C@@H]([C@@H]12)C[C@H]3\C(=C(/CCC=C(C)C)C(O)=O)[C@@H](OC(C)=O)C[C@]3(C)[C@@]2(C)CC[C@@H]2[C@]1(C)CC[C@@H](O)[C@H]2C IECPWNUMDGFDKC-MZJAQBGESA-N 0.000 description 1
- 229960004675 fusidic acid Drugs 0.000 description 1
- BTCSSZJGUNDROE-UHFFFAOYSA-N gamma-aminobutyric acid Chemical compound NCCCC(O)=O BTCSSZJGUNDROE-UHFFFAOYSA-N 0.000 description 1
- UHBYWPGGCSDKFX-VKHMYHEASA-N gamma-carboxy-L-glutamic acid Chemical compound OC(=O)[C@@H](N)CC(C(O)=O)C(O)=O UHBYWPGGCSDKFX-VKHMYHEASA-N 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 108091006104 gene-regulatory proteins Proteins 0.000 description 1
- 102000034356 gene-regulatory proteins Human genes 0.000 description 1
- 230000012178 germinal center formation Effects 0.000 description 1
- 208000007565 gingivitis Diseases 0.000 description 1
- 229930195712 glutamate Natural products 0.000 description 1
- 125000000404 glutamine group Chemical group N[C@@H](CCC(N)=O)C(=O)* 0.000 description 1
- YQEMORVAKMFKLG-UHFFFAOYSA-N glycerine monostearate Natural products CCCCCCCCCCCCCCCCCC(=O)OC(CO)CO YQEMORVAKMFKLG-UHFFFAOYSA-N 0.000 description 1
- SVUQHVRAGMNPLW-UHFFFAOYSA-N glycerol monostearate Natural products CCCCCCCCCCCCCCCCC(=O)OCC(O)CO SVUQHVRAGMNPLW-UHFFFAOYSA-N 0.000 description 1
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- ZRALSGWEFCBTJO-UHFFFAOYSA-N guanidine group Chemical group NC(=N)N ZRALSGWEFCBTJO-UHFFFAOYSA-N 0.000 description 1
- 125000001188 haloalkyl group Chemical group 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- BCQZXOMGPXTTIC-UHFFFAOYSA-N halothane Chemical compound FC(F)(F)C(Cl)Br BCQZXOMGPXTTIC-UHFFFAOYSA-N 0.000 description 1
- 229960003132 halothane Drugs 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 210000002443 helper t lymphocyte Anatomy 0.000 description 1
- 108060003552 hemocyanin Proteins 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 125000001072 heteroaryl group Chemical group 0.000 description 1
- 125000003104 hexanoyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 235000009200 high fat diet Nutrition 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 229920002674 hyaluronan Polymers 0.000 description 1
- 229960003160 hyaluronic acid Drugs 0.000 description 1
- 229940042795 hydrazides for tuberculosis treatment Drugs 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 229920001477 hydrophilic polymer Polymers 0.000 description 1
- QJHBJHUKURJDLG-UHFFFAOYSA-N hydroxy-L-lysine Natural products NCCCCC(NO)C(O)=O QJHBJHUKURJDLG-UHFFFAOYSA-N 0.000 description 1
- 230000033444 hydroxylation Effects 0.000 description 1
- 238000005805 hydroxylation reaction Methods 0.000 description 1
- 150000002463 imidates Chemical class 0.000 description 1
- 125000001841 imino group Chemical group [H]N=* 0.000 description 1
- 239000012642 immune effector Substances 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 229940121354 immunomodulator Drugs 0.000 description 1
- 239000003018 immunosuppressive agent Substances 0.000 description 1
- 229940125721 immunosuppressive agent Drugs 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 229940060367 inert ingredients Drugs 0.000 description 1
- 230000006749 inflammatory damage Effects 0.000 description 1
- 230000004941 influx Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 239000007972 injectable composition Substances 0.000 description 1
- 229910001410 inorganic ion Inorganic materials 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 230000014828 interferon-gamma production Effects 0.000 description 1
- 230000004073 interleukin-2 production Effects 0.000 description 1
- 239000000543 intermediate Substances 0.000 description 1
- 102000027411 intracellular receptors Human genes 0.000 description 1
- 108091008582 intracellular receptors Proteins 0.000 description 1
- 229940126181 ion channel inhibitor Drugs 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- 239000002563 ionic surfactant Substances 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- RGXCTRIQQODGIZ-UHFFFAOYSA-O isodesmosine Chemical compound OC(=O)C(N)CCCC[N+]1=CC(CCC(N)C(O)=O)=CC(CCC(N)C(O)=O)=C1CCCC(N)C(O)=O RGXCTRIQQODGIZ-UHFFFAOYSA-O 0.000 description 1
- VLSMHEGGTFMBBZ-OOZYFLPDSA-N kainic acid Chemical compound CC(=C)[C@H]1CN[C@H](C(O)=O)[C@H]1CC(O)=O VLSMHEGGTFMBBZ-OOZYFLPDSA-N 0.000 description 1
- 229950006874 kainic acid Drugs 0.000 description 1
- 125000000468 ketone group Chemical group 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 108010045069 keyhole-limpet hemocyanin Proteins 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 101150036274 kil gene Proteins 0.000 description 1
- 238000004989 laser desorption mass spectroscopy Methods 0.000 description 1
- 229950006462 lauromacrogol 400 Drugs 0.000 description 1
- 238000002386 leaching Methods 0.000 description 1
- 125000001909 leucine group Chemical group [H]N(*)C(C(*)=O)C([H])([H])C(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- GFIJNRVAKGFPGQ-LIJARHBVSA-N leuprolide Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 GFIJNRVAKGFPGQ-LIJARHBVSA-N 0.000 description 1
- 229960004338 leuprorelin Drugs 0.000 description 1
- 238000011694 lewis rat Methods 0.000 description 1
- 235000020778 linoleic acid Nutrition 0.000 description 1
- OYHQOLUKZRVURQ-IXWMQOLASA-N linoleic acid Natural products CCCCC\C=C/C\C=C\CCCCCCCC(O)=O OYHQOLUKZRVURQ-IXWMQOLASA-N 0.000 description 1
- 229960004488 linolenic acid Drugs 0.000 description 1
- KQQKGWQCNNTQJW-UHFFFAOYSA-N linolenic acid Natural products CC=CCCC=CCC=CCCCCCCCC(O)=O KQQKGWQCNNTQJW-UHFFFAOYSA-N 0.000 description 1
- 229940057995 liquid paraffin Drugs 0.000 description 1
- 150000004668 long chain fatty acids Chemical class 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 229940037627 magnesium lauryl sulfate Drugs 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- HBNDBUATLJAUQM-UHFFFAOYSA-L magnesium;dodecyl sulfate Chemical compound [Mg+2].CCCCCCCCCCCCOS([O-])(=O)=O.CCCCCCCCCCCCOS([O-])(=O)=O HBNDBUATLJAUQM-UHFFFAOYSA-L 0.000 description 1
- 150000002688 maleic acid derivatives Chemical class 0.000 description 1
- 238000009364 mariculture Methods 0.000 description 1
- 238000013178 mathematical model Methods 0.000 description 1
- 229940127554 medical product Drugs 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000028161 membrane depolarization Effects 0.000 description 1
- GMKMEZVLHJARHF-SYDPRGILSA-N meso-2,6-diaminopimelic acid Chemical compound [O-]C(=O)[C@@H]([NH3+])CCC[C@@H]([NH3+])C([O-])=O GMKMEZVLHJARHF-SYDPRGILSA-N 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 229940071648 metered dose inhaler Drugs 0.000 description 1
- RMAHPRNLQIRHIJ-UHFFFAOYSA-N methyl carbamimidate Chemical compound COC(N)=N RMAHPRNLQIRHIJ-UHFFFAOYSA-N 0.000 description 1
- NEGQCMNHXHSFGU-UHFFFAOYSA-N methyl pyridine-2-carboximidate Chemical compound COC(=N)C1=CC=CC=N1 NEGQCMNHXHSFGU-UHFFFAOYSA-N 0.000 description 1
- 230000011987 methylation Effects 0.000 description 1
- 238000007069 methylation reaction Methods 0.000 description 1
- 239000000693 micelle Substances 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 239000003094 microcapsule Substances 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 210000001700 mitochondrial membrane Anatomy 0.000 description 1
- 108091005573 modified proteins Proteins 0.000 description 1
- 102000035118 modified proteins Human genes 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 210000000214 mouth Anatomy 0.000 description 1
- 210000002200 mouth mucosa Anatomy 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- 210000004400 mucous membrane Anatomy 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 230000004118 muscle contraction Effects 0.000 description 1
- 229940078490 n,n-dimethylglycine Drugs 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 238000002663 nebulization Methods 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 230000023837 negative regulation of proteolysis Effects 0.000 description 1
- 230000007694 nephrotoxicity Effects 0.000 description 1
- 231100000417 nephrotoxicity Toxicity 0.000 description 1
- 229940071846 neulasta Drugs 0.000 description 1
- FEMOMIGRRWSMCU-UHFFFAOYSA-N ninhydrin Chemical compound C1=CC=C2C(=O)C(O)(O)C(=O)C2=C1 FEMOMIGRRWSMCU-UHFFFAOYSA-N 0.000 description 1
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 1
- 125000001402 nonanoyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000012038 nucleophile Substances 0.000 description 1
- 210000004940 nucleus Anatomy 0.000 description 1
- 235000020824 obesity Nutrition 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- WWZKQHOCKIZLMA-UHFFFAOYSA-M octanoate Chemical compound CCCCCCCC([O-])=O WWZKQHOCKIZLMA-UHFFFAOYSA-M 0.000 description 1
- 125000002801 octanoyl group Chemical group C(CCCCCCC)(=O)* 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 229920001542 oligosaccharide Polymers 0.000 description 1
- 150000002482 oligosaccharides Chemical class 0.000 description 1
- 229940099216 oncaspar Drugs 0.000 description 1
- 238000011275 oncology therapy Methods 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 229940126701 oral medication Drugs 0.000 description 1
- 239000008184 oral solid dosage form Substances 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000008816 organ damage Effects 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 125000000962 organic group Chemical group 0.000 description 1
- 230000000065 osmolyte Effects 0.000 description 1
- 150000003891 oxalate salts Chemical class 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 229940055729 papain Drugs 0.000 description 1
- 235000019834 papain Nutrition 0.000 description 1
- 235000010987 pectin Nutrition 0.000 description 1
- 239000001814 pectin Substances 0.000 description 1
- 229920001277 pectin Polymers 0.000 description 1
- 108010001564 pegaspargase Proteins 0.000 description 1
- 229940002988 pegasys Drugs 0.000 description 1
- 108010044644 pegfilgrastim Proteins 0.000 description 1
- 108010092853 peginterferon alfa-2a Proteins 0.000 description 1
- 108010092851 peginterferon alfa-2b Proteins 0.000 description 1
- 229940106366 pegintron Drugs 0.000 description 1
- WXZMFSXDPGVJKK-UHFFFAOYSA-N pentaerythritol Chemical compound OCC(CO)(CO)CO WXZMFSXDPGVJKK-UHFFFAOYSA-N 0.000 description 1
- MARBWAUIHIOFNA-UHFFFAOYSA-N pentoxy hydrogen carbonate Chemical compound CCCCCOOC(O)=O MARBWAUIHIOFNA-UHFFFAOYSA-N 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 208000028169 periodontal disease Diseases 0.000 description 1
- 210000001322 periplasm Anatomy 0.000 description 1
- 235000019271 petrolatum Nutrition 0.000 description 1
- 229940066842 petrolatum Drugs 0.000 description 1
- 238000002823 phage display Methods 0.000 description 1
- 230000008782 phagocytosis Effects 0.000 description 1
- 230000003285 pharmacodynamic effect Effects 0.000 description 1
- 239000012660 pharmacological inhibitor Substances 0.000 description 1
- UYWQUFXKFGHYNT-UHFFFAOYSA-N phenylmethyl ester of formic acid Natural products O=COCC1=CC=CC=C1 UYWQUFXKFGHYNT-UHFFFAOYSA-N 0.000 description 1
- NMHMNPHRMNGLLB-UHFFFAOYSA-N phloretic acid Chemical compound OC(=O)CCC1=CC=C(O)C=C1 NMHMNPHRMNGLLB-UHFFFAOYSA-N 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 239000002953 phosphate buffered saline Substances 0.000 description 1
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 1
- UEZVMMHDMIWARA-UHFFFAOYSA-M phosphonate Chemical compound [O-]P(=O)=O UEZVMMHDMIWARA-UHFFFAOYSA-M 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- LFGREXWGYUGZLY-UHFFFAOYSA-N phosphoryl Chemical group [P]=O LFGREXWGYUGZLY-UHFFFAOYSA-N 0.000 description 1
- 125000005498 phthalate group Chemical class 0.000 description 1
- 230000001766 physiological effect Effects 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 229920000191 poly(N-vinyl pyrrolidone) Polymers 0.000 description 1
- 108010087782 poly(glycyl-alanyl) Proteins 0.000 description 1
- 229920000747 poly(lactic acid) Polymers 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 230000008488 polyadenylation Effects 0.000 description 1
- 108010054442 polyalanine Proteins 0.000 description 1
- 229940068917 polyethylene glycols Drugs 0.000 description 1
- 108010094020 polyglycine Proteins 0.000 description 1
- 229920000232 polyglycine polymer Polymers 0.000 description 1
- 239000004633 polyglycolic acid Substances 0.000 description 1
- 239000004626 polylactic acid Substances 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 108010055896 polyornithine Proteins 0.000 description 1
- 229920002714 polyornithine Polymers 0.000 description 1
- 235000010483 polyoxyethylene sorbitan monopalmitate Nutrition 0.000 description 1
- 239000000249 polyoxyethylene sorbitan monopalmitate Substances 0.000 description 1
- 235000010989 polyoxyethylene sorbitan monostearate Nutrition 0.000 description 1
- 239000001818 polyoxyethylene sorbitan monostearate Substances 0.000 description 1
- 235000010988 polyoxyethylene sorbitan tristearate Nutrition 0.000 description 1
- 239000001816 polyoxyethylene sorbitan tristearate Substances 0.000 description 1
- 229940099429 polyoxyl 40 stearate Drugs 0.000 description 1
- 229920006324 polyoxymethylene Polymers 0.000 description 1
- 108010026466 polyproline Proteins 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920005606 polypropylene copolymer Polymers 0.000 description 1
- 229920001451 polypropylene glycol Polymers 0.000 description 1
- 108010000222 polyserine Proteins 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 229940101027 polysorbate 40 Drugs 0.000 description 1
- 229940113124 polysorbate 60 Drugs 0.000 description 1
- 229940099511 polysorbate 65 Drugs 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 229920006316 polyvinylpyrrolidine Polymers 0.000 description 1
- 230000031339 positive regulation of inflammatory response Effects 0.000 description 1
- 229940125422 potassium channel blocker Drugs 0.000 description 1
- 239000003450 potassium channel blocker Substances 0.000 description 1
- 229910001414 potassium ion Inorganic materials 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000002953 preparative HPLC Methods 0.000 description 1
- 238000004321 preservation Methods 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 239000013615 primer Substances 0.000 description 1
- 238000007639 printing Methods 0.000 description 1
- 108010040933 progressin Proteins 0.000 description 1
- 230000009465 prokaryotic expression Effects 0.000 description 1
- 108010017378 prolyl aminopeptidase Proteins 0.000 description 1
- 125000001500 prolyl group Chemical group [H]N1C([H])(C(=O)[*])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- FELJDCNGZFDUNR-UHFFFAOYSA-N prolyl-alanine Chemical group OC(=O)C(C)NC(=O)C1CCCN1 FELJDCNGZFDUNR-UHFFFAOYSA-N 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- VZCGEDTVPJTYHY-UHFFFAOYSA-N propanamide Chemical compound CCC(N)=O.CCC(N)=O VZCGEDTVPJTYHY-UHFFFAOYSA-N 0.000 description 1
- SUVIGLJNEAMWEG-UHFFFAOYSA-N propane-1-thiol Chemical group CCCS SUVIGLJNEAMWEG-UHFFFAOYSA-N 0.000 description 1
- 125000001325 propanoyl group Chemical group O=C([*])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- WGYKZJWCGVVSQN-UHFFFAOYSA-N propylamine Chemical group CCCN WGYKZJWCGVVSQN-UHFFFAOYSA-N 0.000 description 1
- 235000013772 propylene glycol Nutrition 0.000 description 1
- 235000019833 protease Nutrition 0.000 description 1
- 125000006239 protecting group Chemical group 0.000 description 1
- 230000004845 protein aggregation Effects 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 230000009145 protein modification Effects 0.000 description 1
- 238000001742 protein purification Methods 0.000 description 1
- 230000029983 protein stabilization Effects 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 230000006337 proteolytic cleavage Effects 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- 239000013014 purified material Substances 0.000 description 1
- 230000001696 purinergic effect Effects 0.000 description 1
- 229960003581 pyridoxal Drugs 0.000 description 1
- 235000008164 pyridoxal Nutrition 0.000 description 1
- 239000011674 pyridoxal Substances 0.000 description 1
- 235000007682 pyridoxal 5'-phosphate Nutrition 0.000 description 1
- 239000011589 pyridoxal 5'-phosphate Substances 0.000 description 1
- 229960001327 pyridoxal phosphate Drugs 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 239000002287 radioligand Substances 0.000 description 1
- 238000011552 rat model Methods 0.000 description 1
- 229920013730 reactive polymer Polymers 0.000 description 1
- 238000003259 recombinant expression Methods 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000007115 recruitment Effects 0.000 description 1
- 239000013643 reference control Substances 0.000 description 1
- 230000013878 renal filtration Effects 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 239000002336 ribonucleotide Substances 0.000 description 1
- 125000002652 ribonucleotide group Chemical group 0.000 description 1
- 238000007151 ring opening polymerisation reaction Methods 0.000 description 1
- XMVJITFPVVRMHC-UHFFFAOYSA-N roxarsone Chemical group OC1=CC=C([As](O)(O)=O)C=C1[N+]([O-])=O XMVJITFPVVRMHC-UHFFFAOYSA-N 0.000 description 1
- 229940063122 sandimmune Drugs 0.000 description 1
- 238000013341 scale-up Methods 0.000 description 1
- 230000000580 secretagogue effect Effects 0.000 description 1
- 210000004739 secretory vesicle Anatomy 0.000 description 1
- 125000001554 selenocysteine group Chemical group [H][Se]C([H])([H])C(N([H])[H])C(=O)O* 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- UQDJGEHQDNVPGU-UHFFFAOYSA-N serine phosphoethanolamine Chemical compound [NH3+]CCOP([O-])(=O)OCC([NH3+])C([O-])=O UQDJGEHQDNVPGU-UHFFFAOYSA-N 0.000 description 1
- 229940076279 serotonin Drugs 0.000 description 1
- 230000009450 sialylation Effects 0.000 description 1
- 238000007086 side reaction Methods 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 235000021309 simple sugar Nutrition 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- APSBXTVYXVQYAB-UHFFFAOYSA-M sodium docusate Chemical group [Na+].CCCCC(CC)COC(=O)CC(S([O-])(=O)=O)C(=O)OCC(CC)CCCC APSBXTVYXVQYAB-UHFFFAOYSA-M 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 235000019337 sorbitan trioleate Nutrition 0.000 description 1
- 229960000391 sorbitan trioleate Drugs 0.000 description 1
- 239000008347 soybean phospholipid Substances 0.000 description 1
- 125000006850 spacer group Chemical group 0.000 description 1
- 230000009870 specific binding Effects 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 229940071117 starch glycolate Drugs 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 239000001384 succinic acid Substances 0.000 description 1
- 229960002317 succinimide Drugs 0.000 description 1
- 229960004793 sucrose Drugs 0.000 description 1
- 229940124530 sulfonamide Drugs 0.000 description 1
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 1
- 150000003457 sulfones Chemical class 0.000 description 1
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 230000008093 supporting effect Effects 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 210000001258 synovial membrane Anatomy 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 239000007885 tablet disintegrant Substances 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 238000011191 terminal modification Methods 0.000 description 1
- 229960003604 testosterone Drugs 0.000 description 1
- 229960000814 tetanus toxoid Drugs 0.000 description 1
- 229940126585 therapeutic drug Drugs 0.000 description 1
- 150000007970 thio esters Chemical class 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- YSMODUONRAFBET-WHFBIAKZSA-N threo-5-hydroxy-L-lysine Chemical compound NC[C@@H](O)CC[C@H](N)C(O)=O YSMODUONRAFBET-WHFBIAKZSA-N 0.000 description 1
- 229940104230 thymidine Drugs 0.000 description 1
- XUIIKFGFIJCVMT-UHFFFAOYSA-N thyroxine-binding globulin Natural products IC1=CC(CC([NH3+])C([O-])=O)=CC(I)=C1OC1=CC(I)=C(O)C(I)=C1 XUIIKFGFIJCVMT-UHFFFAOYSA-N 0.000 description 1
- FGMPLJWBKKVCDB-UHFFFAOYSA-N trans-L-hydroxy-proline Natural products ON1CCCC1C(O)=O FGMPLJWBKKVCDB-UHFFFAOYSA-N 0.000 description 1
- 238000013271 transdermal drug delivery Methods 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 239000012581 transferrin Substances 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- CYRMSUTZVYGINF-UHFFFAOYSA-N trichlorofluoromethane Chemical compound FC(Cl)(Cl)Cl CYRMSUTZVYGINF-UHFFFAOYSA-N 0.000 description 1
- 229940029284 trichlorofluoromethane Drugs 0.000 description 1
- ITMCEJHCFYSIIV-UHFFFAOYSA-M triflate Chemical compound [O-]S(=O)(=O)C(F)(F)F ITMCEJHCFYSIIV-UHFFFAOYSA-M 0.000 description 1
- 230000007306 turnover Effects 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 239000012178 vegetable wax Substances 0.000 description 1
- 229940070384 ventolin Drugs 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 239000004034 viscosity adjusting agent Substances 0.000 description 1
- 239000001993 wax Substances 0.000 description 1
- 230000004584 weight gain Effects 0.000 description 1
- 235000019786 weight gain Nutrition 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 229920001285 xanthan gum Polymers 0.000 description 1
- 235000010493 xanthan gum Nutrition 0.000 description 1
- 239000000230 xanthan gum Substances 0.000 description 1
- 229940082509 xanthan gum Drugs 0.000 description 1
- 108091058551 α-conotoxin Proteins 0.000 description 1
- KNJNGVKTAFTUFL-OCMUWRIYSA-N ω-conotoxin Chemical compound N([C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H]1C(N[C@@H](CSSC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H]1C(N[C@@H](CCCN=C(N)N)C(=O)N[C@H](CO)C(=O)NCC(=O)N[C@H](CCCCN)C(=O)N[C@H](CSSC1)C(N)=O)=O)=O)C(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CCCCN)C(=O)NCC(=O)N[C@H](CCCCN)C(=O)NCC(=O)N[C@H](C)C(=O)N[C@@H](CCCCN)C(=O)N1 KNJNGVKTAFTUFL-OCMUWRIYSA-N 0.000 description 1
- 108091058550 ω-conotoxin Proteins 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/44—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material not provided for elsewhere, e.g. haptens, metals, DNA, RNA, amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39591—Stabilisation, fragmentation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/6811—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug being a protein or peptide, e.g. transferrin or bleomycin
- A61K47/6817—Toxins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6843—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a material from animals or humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/08—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/06—Antimigraine agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
- A61P29/02—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID] without antiinflammatory effect
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/43504—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from invertebrates
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/575—Hormones
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/575—Hormones
- C07K14/605—Glucagons
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/575—Hormones
- C07K14/66—Thymopoietins
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/24—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against cytokines, lymphokines or interferons
- C07K16/244—Interleukins [IL]
- C07K16/248—IL-6
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/21—Immunoglobulins specific features characterized by taxonomic origin from primates, e.g. man
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/94—Stability, e.g. half-life, pH, temperature or enzyme-resistance
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/55—Fusion polypeptide containing a fusion with a toxin, e.g. diphteria toxin
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Immunology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Genetics & Genomics (AREA)
- Zoology (AREA)
- Toxicology (AREA)
- Epidemiology (AREA)
- Gastroenterology & Hepatology (AREA)
- Endocrinology (AREA)
- Rheumatology (AREA)
- Pulmonology (AREA)
- Pain & Pain Management (AREA)
- Biomedical Technology (AREA)
- Physical Education & Sports Medicine (AREA)
- Neurosurgery (AREA)
- Neurology (AREA)
- Diabetes (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Dermatology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Tropical Medicine & Parasitology (AREA)
- Cardiology (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US21059409P | 2009-03-20 | 2009-03-20 | |
| US61/210,594 | 2009-03-20 | ||
| PCT/US2010/028061 WO2010108154A2 (en) | 2009-03-20 | 2010-03-19 | Selective and potent peptide inhibitors of kv1.3 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| JP2012521360A true JP2012521360A (ja) | 2012-09-13 |
Family
ID=42740266
Family Applications (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012501020A Pending JP2012521360A (ja) | 2009-03-20 | 2010-03-19 | Kv1.3の選択的かつ強力なペプチド阻害剤 |
| JP2012501019A Pending JP2012521197A (ja) | 2009-03-20 | 2010-03-19 | 担体免疫グロブリンおよびその使用 |
| JP2015001336A Active JP6173359B2 (ja) | 2009-03-20 | 2015-01-07 | 担体免疫グロブリンおよびその使用 |
| JP2017077334A Active JP6345832B2 (ja) | 2009-03-20 | 2017-04-10 | 担体免疫グロブリンおよびその使用 |
| JP2018097620A Active JP6609347B2 (ja) | 2009-03-20 | 2018-05-22 | 担体免疫グロブリンおよびその使用 |
Family Applications After (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012501019A Pending JP2012521197A (ja) | 2009-03-20 | 2010-03-19 | 担体免疫グロブリンおよびその使用 |
| JP2015001336A Active JP6173359B2 (ja) | 2009-03-20 | 2015-01-07 | 担体免疫グロブリンおよびその使用 |
| JP2017077334A Active JP6345832B2 (ja) | 2009-03-20 | 2017-04-10 | 担体免疫グロブリンおよびその使用 |
| JP2018097620A Active JP6609347B2 (ja) | 2009-03-20 | 2018-05-22 | 担体免疫グロブリンおよびその使用 |
Country Status (7)
| Country | Link |
|---|---|
| US (5) | US20120121591A1 (enExample) |
| EP (3) | EP2432488A4 (enExample) |
| JP (5) | JP2012521360A (enExample) |
| AU (2) | AU2010226391C1 (enExample) |
| CA (4) | CA2755133A1 (enExample) |
| MX (4) | MX2011009797A (enExample) |
| WO (2) | WO2010108153A2 (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016505639A (ja) * | 2013-01-25 | 2016-02-25 | ヤンセン バイオテツク,インコーポレーテツド | Kv1.3アンタゴニスト及び使用方法 |
| WO2018135598A1 (ja) * | 2017-01-19 | 2018-07-26 | 国立大学法人 東京大学 | 新規蛍光標識方法 |
| JP2018521062A (ja) * | 2015-06-29 | 2018-08-02 | ザ・ユニバーシティ・オブ・ブリティッシュ・コロンビア | B1sp融合タンパク質の治療薬、方法、および使用 |
| JP2023019170A (ja) * | 2021-07-28 | 2023-02-09 | 株式会社三協 | 錠剤用崩壊剤及びこれを利用した錠剤 |
Families Citing this family (60)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7833979B2 (en) | 2005-04-22 | 2010-11-16 | Amgen Inc. | Toxin peptide therapeutic agents |
| AU2008262490B2 (en) | 2007-05-22 | 2011-11-17 | Amgen Inc. | Compositions and methods for producing bioactive fusion proteins |
| EA201500156A3 (ru) | 2008-01-03 | 2017-09-29 | Дзе Скриппс Рисерч Инститьют | Доставка антител посредством модульного домена распознавания |
| US8557242B2 (en) | 2008-01-03 | 2013-10-15 | The Scripps Research Institute | ERBB2 antibodies comprising modular recognition domains |
| US8454960B2 (en) | 2008-01-03 | 2013-06-04 | The Scripps Research Institute | Multispecific antibody targeting and multivalency through modular recognition domains |
| US8557243B2 (en) | 2008-01-03 | 2013-10-15 | The Scripps Research Institute | EFGR antibodies comprising modular recognition domains |
| US8574577B2 (en) | 2008-01-03 | 2013-11-05 | The Scripps Research Institute | VEGF antibodies comprising modular recognition domains |
| EP2432488A4 (en) | 2009-03-20 | 2014-01-08 | Amgen Inc | SELECTIVE AND POWERFUL KV1.3 PEPTIDE INHIBITORS |
| TW201129379A (en) | 2009-11-20 | 2011-09-01 | Amgen Inc | Anti-Orai1 antigen binding proteins and uses thereof |
| US20120100166A1 (en) | 2010-07-15 | 2012-04-26 | Zyngenia, Inc. | Ang-2 Binding Complexes and Uses Thereof |
| MX383982B (es) | 2010-09-22 | 2025-03-14 | Amgen Inc | Inmunoglobulinas portadoras y usos de las mismas. |
| UY33959A (es) | 2011-03-16 | 2012-09-28 | Amgen Inc | INHIBIDORES POTENTES Y SELECTIVOS DE NaV1.3 Y NaV1.7 |
| CN102718858B (zh) * | 2011-03-29 | 2014-07-02 | 天津药物研究院 | 胰高血糖素样肽-1类似物单体、二聚体及其制备方法与应用 |
| WO2012162561A2 (en) | 2011-05-24 | 2012-11-29 | Zyngenia, Inc. | Multivalent and monovalent multispecific complexes and their uses |
| SG10201604468QA (en) * | 2011-06-06 | 2016-07-28 | Kineta One Llc | Shk-based pharmaceutical compositions and methods of manufacturing and using the same |
| DK2718320T3 (en) | 2011-06-10 | 2018-03-26 | Medimmune Ltd | ANTI-PSEUDOMONAS-PSL BINDING MOLECULES AND APPLICATIONS THEREOF |
| PE20240686A1 (es) | 2011-06-10 | 2024-04-10 | Hanmi Science Co Ltd | Peptidos derivados de oxintomodulina con actividad sobre el receptor de glucagon y el receptor de peptido similar a glucagon de tipo 1 (glp-1) |
| KR101577734B1 (ko) | 2011-06-17 | 2015-12-29 | 한미사이언스 주식회사 | 옥신토모듈린과 면역글로불린 단편을 포함하는 결합체 및 그의 용도 |
| US10597439B2 (en) | 2011-11-07 | 2020-03-24 | Medimmune Limited | Combination therapies using anti-pseudomonas PSL and PCRV binding molecules |
| KR101895047B1 (ko) * | 2011-12-30 | 2018-09-06 | 한미사이언스 주식회사 | 면역글로불린 단편을 이용한 위치 특이적 글루카곤 유사 펩타이드-2 약물 결합체 |
| MX2014014894A (es) * | 2012-06-04 | 2015-02-20 | Irm Llc | Metodos de marcado especifico del sitio y moleculas producidas mediante los mismos. |
| US9283241B2 (en) * | 2012-07-10 | 2016-03-15 | Clemson University | Treatment to render implants resistant to diabetes |
| KR101968344B1 (ko) | 2012-07-25 | 2019-04-12 | 한미약품 주식회사 | 옥신토모듈린 유도체를 포함하는 고지혈증 치료용 조성물 |
| WO2014058389A1 (en) | 2012-10-12 | 2014-04-17 | Agency For Science, Technology And Research | Optimised heavy chain and light chain signal peptides for the production of recombinant antibody therapeutics |
| KR101993393B1 (ko) | 2012-11-06 | 2019-10-01 | 한미약품 주식회사 | 옥신토모듈린 유도체를 포함하는 당뇨병 또는 비만성 당뇨병 치료용 조성물 |
| PL2916819T3 (pl) | 2012-11-06 | 2020-01-31 | Hanmi Pharm. Co., Ltd. | Płynna formuła koniugatu białka zawierająca oksyntomodulinę i fragment immunoglobulinowy |
| US10344060B2 (en) * | 2013-03-12 | 2019-07-09 | Amgen Inc. | Potent and selective inhibitors of Nav1.7 |
| AR096927A1 (es) | 2013-03-12 | 2016-02-10 | Amgen Inc | INHIBIDORES POTENTES Y SELECTIVOS DE NaV1.7 |
| WO2014144600A2 (en) | 2013-03-15 | 2014-09-18 | Viktor Roschke | Multivalent and monovalent multispecific complexes and their uses |
| AU2013404615B2 (en) | 2013-10-28 | 2018-05-17 | Baylor College Of Medicine | Novel scorpion toxin analogue and method for treating autoimmune diseases |
| CA2934818C (en) * | 2013-12-23 | 2022-05-17 | John Babcook | Antibodies comprising c-terminal light chain polypeptide extensions and conjugates and methods of use thereof |
| EP3152238A4 (en) * | 2014-06-06 | 2018-01-03 | The California Institute for Biomedical Research | Methods of constructing amino terminal immunoglobulin fusion proteins and compositions thereof |
| WO2015191781A2 (en) | 2014-06-10 | 2015-12-17 | Amgen Inc. | Apelin polypeptides |
| US9710451B2 (en) * | 2014-06-30 | 2017-07-18 | International Business Machines Corporation | Natural-language processing based on DNA computing |
| AU2015303825B2 (en) * | 2014-08-15 | 2021-01-14 | Baylor College Of Medicine | Novel potassium channel blockers and use thereof in the treatment of autoimmune diseases |
| TWI802396B (zh) | 2014-09-16 | 2023-05-11 | 南韓商韓美藥品股份有限公司 | 長效glp-1/高血糖素受體雙促效劑治療非酒精性脂肝疾病之用途 |
| WO2016054114A1 (en) | 2014-09-29 | 2016-04-07 | The Regents Of The University Of California | Compositions for expanding regulatory t cells (treg), and treating autoimmune and inflammatory diseases and conditions |
| KR102418477B1 (ko) | 2014-12-30 | 2022-07-08 | 한미약품 주식회사 | 글루카곤 유도체 |
| WO2016112208A2 (en) * | 2015-01-09 | 2016-07-14 | Kineta One, Llp | Topical applications of kv1.3 channel blocking peptides to treat skin inflammation |
| US10787517B2 (en) | 2015-05-18 | 2020-09-29 | Agensys, Inc. | Antibodies that bind to AXL proteins |
| US10787516B2 (en) | 2015-05-18 | 2020-09-29 | Agensys, Inc. | Antibodies that bind to AXL proteins |
| MA48579A (fr) | 2015-09-01 | 2020-03-18 | Agenus Inc | Anticorps anti-pd1 et méthodes d'utilisation de ceux-ci |
| TW201718629A (zh) * | 2015-09-25 | 2017-06-01 | 韓美藥品股份有限公司 | 包含多個生理多肽及免疫球蛋白Fc區之蛋白質接合物 |
| US10388404B2 (en) | 2015-10-27 | 2019-08-20 | International Business Machines Corporation | Using machine-learning to perform linear regression on a DNA-computing platform |
| WO2017083604A1 (en) | 2015-11-12 | 2017-05-18 | Amgen Inc. | Triazine mediated pharmacokinetic enhancement of therapeutics |
| EP4011919A3 (en) | 2015-12-09 | 2022-10-12 | The Scripps Research Institute | Relaxin immunoglobulin fusion proteins and methods of use |
| MA43660A (fr) | 2016-01-22 | 2018-11-28 | Adimab Llc | Anticorps anti-facteur xi de coagulation |
| US12297266B2 (en) | 2016-04-18 | 2025-05-13 | Celldex Therapeutics, Inc. | Agonistic antibodies that bind human CD40 and uses thereof |
| GEAP202114983A (en) | 2016-06-14 | 2021-12-10 | Merck Sharp & Dohme Corp | Anti-coagulation factor xi antibodies |
| WO2018013483A1 (en) * | 2016-07-11 | 2018-01-18 | The California Institute For Biomedical Research | Kv1.3 channel blocking peptides and uses thereof |
| AU2017373945B2 (en) | 2016-12-07 | 2025-01-23 | Agenus Inc. | Antibodies and methods of use thereof |
| MA55504A (fr) | 2019-04-01 | 2022-02-09 | Novo Nordisk As | Anticorps dirigés contre le liraglutide et leur utilisation |
| EP4069291A1 (en) | 2019-12-03 | 2022-10-12 | Evotec International GmbH | Interferon-associated antigen binding proteins and uses thereof |
| US11028132B1 (en) | 2020-04-07 | 2021-06-08 | Yitzhak Rosen | Half-life optimized linker composition |
| WO2025122817A1 (en) * | 2023-12-06 | 2025-06-12 | The General Hospital Corporation | Antagonistic cd40 antibodies |
| WO2025122929A1 (en) * | 2023-12-08 | 2025-06-12 | Janssen Biotech, Inc. | Gprc5d antibodies with enhanced effector function and uses thereof |
| US20250276080A1 (en) | 2024-02-29 | 2025-09-04 | Amgen Inc. | Glucagon receptor agonists and conjugates to glucose-dependent insulinotropic polypeptide antagonists |
| CN120795162A (zh) | 2024-04-02 | 2025-10-17 | 康诺亚生物医药科技(成都)有限公司 | 针对il-13和tslp的双特异性抗体及其用途 |
| WO2025244108A1 (ja) * | 2024-05-23 | 2025-11-27 | 国立大学法人 東京大学 | 新規蛍光標識方法 |
| US12161970B1 (en) | 2024-08-09 | 2024-12-10 | Jiaxing Research Institute, Zhejiang University | CO2 desorption system suitable for limited space in complex sailing region and flexible control method |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008538506A (ja) * | 2005-04-22 | 2008-10-30 | アムジエン・インコーポレーテツド | 延長された血液半減期を有するトキシンペプチド |
Family Cites Families (137)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US30985A (en) | 1860-12-18 | Thomas l | ||
| US3773919A (en) | 1969-10-23 | 1973-11-20 | Du Pont | Polylactide-drug mixtures |
| US4179337A (en) | 1973-07-20 | 1979-12-18 | Davis Frank F | Non-immunogenic polypeptides |
| US3941763A (en) | 1975-03-28 | 1976-03-02 | American Home Products Corporation | PGlu-D-Met-Trp-Ser-Tyr-D-Ala-Leu-Arg-Pro-Gly-NH2 and intermediates |
| JPS6023084B2 (ja) | 1979-07-11 | 1985-06-05 | 味の素株式会社 | 代用血液 |
| JPS5896026A (ja) | 1981-10-30 | 1983-06-07 | Nippon Chemiphar Co Ltd | 新規ウロキナ−ゼ誘導体およびその製造法ならびにそれを含有する血栓溶解剤 |
| US4640835A (en) | 1981-10-30 | 1987-02-03 | Nippon Chemiphar Company, Ltd. | Plasminogen activator derivatives |
| US4609546A (en) | 1982-06-24 | 1986-09-02 | Japan Chemical Research Co., Ltd. | Long-acting composition |
| US4560655A (en) | 1982-12-16 | 1985-12-24 | Immunex Corporation | Serum-free cell culture medium and process for making same |
| US4657866A (en) | 1982-12-21 | 1987-04-14 | Sudhir Kumar | Serum-free, synthetic, completely chemically defined tissue culture media |
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US4767704A (en) | 1983-10-07 | 1988-08-30 | Columbia University In The City Of New York | Protein-free culture medium |
| US4496689A (en) | 1983-12-27 | 1985-01-29 | Miles Laboratories, Inc. | Covalently attached complex of alpha-1-proteinase inhibitor with a water soluble polymer |
| EP0206448B1 (en) | 1985-06-19 | 1990-11-14 | Ajinomoto Co., Inc. | Hemoglobin combined with a poly(alkylene oxide) |
| US4766106A (en) | 1985-06-26 | 1988-08-23 | Cetus Corporation | Solubilization of proteins for pharmaceutical compositions using polymer conjugation |
| GB8516415D0 (en) | 1985-06-28 | 1985-07-31 | Celltech Ltd | Culture of animal cells |
| US4676980A (en) | 1985-09-23 | 1987-06-30 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Target specific cross-linked heteroantibodies |
| JPS63502716A (ja) | 1986-03-07 | 1988-10-13 | マサチューセッツ・インステチュート・オブ・テクノロジー | 糖タンパク安定性の強化方法 |
| US4927762A (en) | 1986-04-01 | 1990-05-22 | Cell Enterprises, Inc. | Cell culture medium with antioxidant |
| US4791192A (en) | 1986-06-26 | 1988-12-13 | Takeda Chemical Industries, Ltd. | Chemically modified protein with polyethyleneglycol |
| WO1988001649A1 (en) | 1986-09-02 | 1988-03-10 | Genex Corporation | Single polypeptide chain binding molecules |
| US5260203A (en) | 1986-09-02 | 1993-11-09 | Enzon, Inc. | Single polypeptide chain binding molecules |
| US4946778A (en) | 1987-09-21 | 1990-08-07 | Genex Corporation | Single polypeptide chain binding molecules |
| US5567610A (en) | 1986-09-04 | 1996-10-22 | Bioinvent International Ab | Method of producing human monoclonal antibodies and kit therefor |
| EP0315456B1 (en) | 1987-11-05 | 1994-06-01 | Hybritech Incorporated | Polysaccharide-modified immunoglobulins having reduced immunogenic potential or improved pharmacokinetics |
| US5283187A (en) | 1987-11-17 | 1994-02-01 | Brown University Research Foundation | Cell culture-containing tubular capsule produced by co-extrusion |
| US4892538A (en) | 1987-11-17 | 1990-01-09 | Brown University Research Foundation | In vivo delivery of neurotransmitters by implanted, encapsulated cells |
| JP3092811B2 (ja) | 1988-07-23 | 2000-09-25 | デルタ バイオテクノロジー リミテッド | ペプチドおよびdna配列 |
| AU632065B2 (en) | 1988-09-23 | 1992-12-17 | Novartis Vaccines And Diagnostics, Inc. | Cell culture medium for enhanced cell growth, culture longevity and product expression |
| GB8823869D0 (en) | 1988-10-12 | 1988-11-16 | Medical Res Council | Production of antibodies |
| US5175384A (en) | 1988-12-05 | 1992-12-29 | Genpharm International | Transgenic mice depleted in mature t-cells and methods for making transgenic mice |
| DE3920358A1 (de) | 1989-06-22 | 1991-01-17 | Behringwerke Ag | Bispezifische und oligospezifische, mono- und oligovalente antikoerperkonstrukte, ihre herstellung und verwendung |
| CA2050918A1 (en) | 1990-01-12 | 1991-07-13 | Raju Kucherlapati | Generation of xenogeneic antibodies |
| WO1996033735A1 (en) | 1995-04-27 | 1996-10-31 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| US5229275A (en) | 1990-04-26 | 1993-07-20 | Akzo N.V. | In-vitro method for producing antigen-specific human monoclonal antibodies |
| US5427908A (en) | 1990-05-01 | 1995-06-27 | Affymax Technologies N.V. | Recombinant library screening methods |
| GB9014932D0 (en) | 1990-07-05 | 1990-08-22 | Celltech Ltd | Recombinant dna product and method |
| GB9015198D0 (en) | 1990-07-10 | 1990-08-29 | Brien Caroline J O | Binding substance |
| US5122469A (en) | 1990-10-03 | 1992-06-16 | Genentech, Inc. | Method for culturing Chinese hamster ovary cells to improve production of recombinant proteins |
| GB9022543D0 (en) | 1990-10-17 | 1990-11-28 | Wellcome Found | Antibody production |
| US5565332A (en) | 1991-09-23 | 1996-10-15 | Medical Research Council | Production of chimeric antibodies - a combinatorial approach |
| EP0617706B1 (en) | 1991-11-25 | 2001-10-17 | Enzon, Inc. | Multivalent antigen-binding proteins |
| FI92507C (fi) | 1991-12-19 | 1994-11-25 | Valto Ilomaeki | Menetelmä ja laite maaperään pakotettavien putkien ohjaamiseksi |
| CA2128862C (en) | 1992-02-11 | 2008-05-20 | Jonathan G. Seidman | Homogenotization of gene-targeting events |
| CA2131151A1 (en) | 1992-03-24 | 1994-09-30 | Kevin S. Johnson | Methods for producing members of specific binding pairs |
| US5573905A (en) | 1992-03-30 | 1996-11-12 | The Scripps Research Institute | Encoded combinatorial chemical libraries |
| WO1993025673A1 (en) | 1992-06-04 | 1993-12-23 | The Regents Of The University Of California | In vivo gene therapy with intron-free sequence of interest |
| SG48760A1 (en) | 1992-07-24 | 2003-03-18 | Abgenix Inc | Generation of xenogenetic antibodies |
| US6534051B1 (en) * | 1992-11-20 | 2003-03-18 | University Of Medicine And Dentistry Of New Jersey | Cell type specific gene transfer using retroviral vectors containing antibody-envelope fusion proteins and wild-type envelope fusion proteins |
| JP3312357B2 (ja) | 1992-12-11 | 2002-08-05 | ザ ダウ ケミカル カンパニー | 多価の一本鎖抗体 |
| US6342225B1 (en) | 1993-08-13 | 2002-01-29 | Deutshces Wollforschungsinstitut | Pharmaceutical active conjugates |
| WO1995015388A1 (en) | 1993-12-03 | 1995-06-08 | Medical Research Council | Recombinant binding proteins and peptides |
| MX9603570A (es) | 1994-02-23 | 1997-03-29 | Chiron Corp | Metodo y composiciones para incrementar la vida media en suero de agentes farmacologicamente activos. |
| US6054287A (en) | 1994-05-27 | 2000-04-25 | Methodist Hospital Of Indiana, Inc. | Cell-type-specific methods and devices for the low temperature preservation of the cells of an animal species |
| US5731168A (en) | 1995-03-01 | 1998-03-24 | Genentech, Inc. | Method for making heteromultimeric polypeptides |
| ATE279517T1 (de) | 1995-03-23 | 2004-10-15 | Immunex Corp | Il-17 receptor |
| US6130364A (en) | 1995-03-29 | 2000-10-10 | Abgenix, Inc. | Production of antibodies using Cre-mediated site-specific recombination |
| US6096871A (en) | 1995-04-14 | 2000-08-01 | Genentech, Inc. | Polypeptides altered to contain an epitope from the Fc region of an IgG molecule for increased half-life |
| US5869451A (en) | 1995-06-07 | 1999-02-09 | Glaxo Group Limited | Peptides and compounds that bind to a receptor |
| US5746516A (en) | 1995-08-11 | 1998-05-05 | Hitachi Powdered Metals Co., Ltd. | Porous bearing system having internal grooves and electric motor provided with the same |
| US5849863A (en) | 1995-09-08 | 1998-12-15 | University Of Colorado | Cytolytic bradykinin antagonists |
| US5834431A (en) | 1995-09-08 | 1998-11-10 | Cortech, Inc. | Des-Arg9 -BK antagonists |
| US5989830A (en) | 1995-10-16 | 1999-11-23 | Unilever Patent Holdings Bv | Bifunctional or bivalent antibody fragment analogue |
| JP4046354B2 (ja) | 1996-03-18 | 2008-02-13 | ボード オブ リージェンツ,ザ ユニバーシティ オブ テキサス システム | 増大した半減期を有する免疫グロブリン様ドメイン |
| GB9712818D0 (en) | 1996-07-08 | 1997-08-20 | Cambridge Antibody Tech | Labelling and selection of specific binding molecules |
| US6077680A (en) | 1996-11-27 | 2000-06-20 | The University Of Florida | ShK toxin compositions and methods of use |
| DK1500329T3 (da) | 1996-12-03 | 2012-07-09 | Amgen Fremont Inc | Humane antistoffer, der specifikt binder TNF-alfa |
| US6689749B1 (en) | 1996-12-19 | 2004-02-10 | Suntory Limited | Neuropeptides originating in scorpion |
| US20020032315A1 (en) | 1997-08-06 | 2002-03-14 | Manuel Baca | Anti-vegf antibodies |
| US6171586B1 (en) | 1997-06-13 | 2001-01-09 | Genentech, Inc. | Antibody formulation |
| US20030044772A1 (en) | 1997-08-04 | 2003-03-06 | Applied Molecular Evolution [Formerly Ixsys] | Methods for identifying ligand specific binding molecules |
| US6022952A (en) | 1998-04-01 | 2000-02-08 | University Of Alberta | Compositions and methods for protein secretion |
| US20020029391A1 (en) | 1998-04-15 | 2002-03-07 | Claude Geoffrey Davis | Epitope-driven human antibody production and gene expression profiling |
| US6660843B1 (en) | 1998-10-23 | 2003-12-09 | Amgen Inc. | Modified peptides as therapeutic agents |
| ES2279649T3 (es) | 1998-10-23 | 2007-08-16 | Amgen Inc. | Compuestos trombopoyeticos. |
| CA2350226C (en) | 1998-11-20 | 2012-04-24 | Fuso Pharmaceutical Industries, Ltd. | Protein expression vector and utilization thereof |
| EP1181390A4 (en) | 1999-03-18 | 2004-08-04 | Human Genome Sciences Inc | 27 HUMAN SECRETED PROTEINS |
| US6887470B1 (en) | 1999-09-10 | 2005-05-03 | Conjuchem, Inc. | Protection of endogenous therapeutic peptides from peptidase activity through conjugation to blood components |
| FR2794461B1 (fr) * | 1999-06-07 | 2004-01-23 | Lab Francais Du Fractionnement | Procede de preparation de nouvelles fractions d'ig humaines possedant une activite immunomodulatrice |
| US6833268B1 (en) | 1999-06-10 | 2004-12-21 | Abgenix, Inc. | Transgenic animals for producing specific isotypes of human antibodies via non-cognate switch regions |
| JO2291B1 (en) | 1999-07-02 | 2005-09-12 | اف . هوفمان لاروش ايه جي | Erythropoietin derivatives |
| US6475220B1 (en) | 1999-10-15 | 2002-11-05 | Whiteside Biomechanics, Inc. | Spinal cable system |
| AU2001239857B9 (en) | 2000-02-25 | 2006-07-27 | Duke University | Anti-EGFRvIII SCFVS with improved cytotoxicity and yield, immunotoxins based thereon, and methods of use thereof |
| WO2001079444A2 (en) | 2000-04-12 | 2001-10-25 | Human Genome Sciences, Inc | Albumin fusion proteins |
| US6756480B2 (en) | 2000-04-27 | 2004-06-29 | Amgen Inc. | Modulators of receptors for parathyroid hormone and parathyroid hormone-related protein |
| US7041870B2 (en) | 2000-11-30 | 2006-05-09 | Medarex, Inc. | Transgenic transchromosomal rodents for making human antibodies |
| US7754208B2 (en) | 2001-01-17 | 2010-07-13 | Trubion Pharmaceuticals, Inc. | Binding domain-immunoglobulin fusion proteins |
| US7829084B2 (en) | 2001-01-17 | 2010-11-09 | Trubion Pharmaceuticals, Inc. | Binding constructs and methods for use thereof |
| US20030133939A1 (en) | 2001-01-17 | 2003-07-17 | Genecraft, Inc. | Binding domain-immunoglobulin fusion proteins |
| EP1377306A1 (en) | 2001-03-09 | 2004-01-07 | Dyax Corp. | Serum albumin binding moieties |
| US20050054051A1 (en) | 2001-04-12 | 2005-03-10 | Human Genome Sciences, Inc. | Albumin fusion proteins |
| US20050089932A1 (en) | 2001-04-26 | 2005-04-28 | Avidia Research Institute | Novel proteins with targeted binding |
| US20060223114A1 (en) | 2001-04-26 | 2006-10-05 | Avidia Research Institute | Protein scaffolds and uses thereof |
| NZ529267A (en) | 2001-05-11 | 2006-05-26 | Amgen Inc | Peptides and related molecules that bind to tall-1 |
| EP1409018B1 (en) | 2001-07-25 | 2010-01-06 | Facet Biotech Corporation | Stable lyophilized pharmaceutical formulation the igg antibody daclizumab |
| JP4213586B2 (ja) | 2001-09-13 | 2009-01-21 | 株式会社抗体研究所 | ラクダ抗体ライブラリーの作製方法 |
| US7138370B2 (en) | 2001-10-11 | 2006-11-21 | Amgen Inc. | Specific binding agents of human angiopoietin-2 |
| US7205275B2 (en) | 2001-10-11 | 2007-04-17 | Amgen Inc. | Methods of treatment using specific binding agents of human angiopoietin-2 |
| US7332474B2 (en) | 2001-10-11 | 2008-02-19 | Amgen Inc. | Peptides and related compounds having thrombopoietic activity |
| JP2005516965A (ja) | 2001-12-28 | 2005-06-09 | アブジェニックス・インコーポレーテッド | 抗muc18抗体を使用する方法 |
| CN1305905C (zh) | 2002-03-22 | 2007-03-21 | Aprogen株式会社 | 人源化抗体及其制备方法 |
| US20030191056A1 (en) | 2002-04-04 | 2003-10-09 | Kenneth Walker | Use of transthyretin peptide/protein fusions to increase the serum half-life of pharmacologically active peptides/proteins |
| US6919426B2 (en) | 2002-09-19 | 2005-07-19 | Amgen Inc. | Peptides and related molecules that modulate nerve growth factor activity |
| ES2639301T3 (es) | 2003-04-30 | 2017-10-26 | Universität Zürich | Procedimientos de tratamiento de cáncer usando una inmunotoxina |
| AR045563A1 (es) | 2003-09-10 | 2005-11-02 | Warner Lambert Co | Anticuerpos dirigidos a m-csf |
| EP2060270A3 (en) * | 2003-10-16 | 2009-12-09 | Imclone LLC | Fibroblast growth factor receptor-1 inhibitors and methods of treatment thereof |
| US7605120B2 (en) | 2003-10-22 | 2009-10-20 | Amgen Inc. | Antagonists of the brandykinin B1 receptor |
| AU2004284090A1 (en) | 2003-10-24 | 2005-05-06 | Avidia, Inc. | LDL receptor class A and EGF domain monomers and multimers |
| NZ548702A (en) | 2004-01-09 | 2009-06-26 | Pfizer | Antibodies to MAdCAM |
| GB0414272D0 (en) | 2004-06-25 | 2004-07-28 | Cellpep Sa | OsK1 derivatives |
| BRPI0516011A (pt) | 2004-09-24 | 2008-08-19 | Amgen Inc | moléculas fc modificadas |
| EP2474316B1 (en) * | 2004-10-07 | 2016-04-06 | The Regents of The University of California | Analogs of shk toxin and their uses in selective inhibition of kv1.3 potassium channels |
| CA2587463A1 (en) | 2004-11-16 | 2006-11-30 | Avidia Research Institute | Protein scaffolds and uses therof |
| US20060234299A1 (en) | 2004-11-16 | 2006-10-19 | Avidia Research Institute | Protein scaffolds and uses thereof |
| DE602005025525D1 (de) * | 2004-11-17 | 2011-02-03 | Amgen Inc | Vollständige humane monoklonale antikörper gegen il-13 |
| CN105085678B (zh) * | 2004-12-21 | 2019-05-07 | 阿斯利康公司 | 血管生成素-2的抗体及其应用 |
| MX2008000230A (es) | 2005-07-13 | 2008-04-07 | Amgen Mountain View Inc | Proteinas de enlace il-6. |
| US8008453B2 (en) | 2005-08-12 | 2011-08-30 | Amgen Inc. | Modified Fc molecules |
| PE20071101A1 (es) | 2005-08-31 | 2007-12-21 | Amgen Inc | Polipeptidos y anticuerpos |
| TW200722436A (en) | 2005-10-21 | 2007-06-16 | Hoffmann La Roche | A peptide-immunoglobulin-conjugate |
| US8168592B2 (en) | 2005-10-21 | 2012-05-01 | Amgen Inc. | CGRP peptide antagonists and conjugates |
| EA015992B1 (ru) | 2006-03-17 | 2012-01-30 | Байоджен Айдек Эмэй Инк. | Стабилизированное антитело и многовалентная антигенсвязывающая молекула на его основе, способы получения и использования вышеназванного стабилизированного антитела |
| TW200813091A (en) * | 2006-04-10 | 2008-03-16 | Amgen Fremont Inc | Targeted binding agents directed to uPAR and uses thereof |
| US7833527B2 (en) | 2006-10-02 | 2010-11-16 | Amgen Inc. | Methods of treating psoriasis using IL-17 Receptor A antibodies |
| PE20081140A1 (es) | 2006-10-25 | 2008-09-22 | Amgen Inc | Agentes terapeuticos a base de peptidos derivados de toxinas |
| ES2465465T3 (es) | 2006-11-01 | 2014-06-05 | Ventana Medical Systems, Inc. | Haptenos, conjugados de haptenos, composiciones de los mismos y método para su preparación y uso |
| EP2118138A1 (en) | 2007-03-12 | 2009-11-18 | Esbatech AG | Sequence based engineering and optimization of single chain antibodies |
| US8793074B2 (en) | 2007-06-21 | 2014-07-29 | Saint Louis University | Sequence covariance networks, methods and uses therefor |
| PL2158315T3 (pl) | 2007-06-25 | 2016-10-31 | Sposoby modyfikowania przeciwciał i zmodyfikowane przeciwciała o ulepszonych właściwościach funkcjonalnych | |
| KR20100058509A (ko) | 2007-07-31 | 2010-06-03 | 메디뮨 엘엘씨 | 다중특이적 에피토프 결합 단백질 및 이의 용도 |
| US20090203038A1 (en) | 2007-12-27 | 2009-08-13 | Abbott Laboratories | Negative Mimic Antibody For Use as a Blocking Reagent In BNP Immunoassays |
| JP2011515376A (ja) | 2008-03-20 | 2011-05-19 | ツェー・エス・エル・ベーリング・ゲー・エム・ベー・ハー | 病的血栓形成に必須であるカルシウムセンサSTIM1及び血小板SOCチャネルOrai1(CRACM1) |
| JP2012508017A (ja) | 2008-11-07 | 2012-04-05 | ファブラス エルエルシー | 抗dll4抗体及びその使用 |
| US8530629B2 (en) | 2009-01-30 | 2013-09-10 | Ab Biosciences, Inc. | Lowered affinity antibodies and uses therefor |
| EP2432488A4 (en) | 2009-03-20 | 2014-01-08 | Amgen Inc | SELECTIVE AND POWERFUL KV1.3 PEPTIDE INHIBITORS |
| CN102971342B (zh) | 2010-01-28 | 2016-08-03 | Ab生物科学公司 | 亲和力降低的新抗体和制备所述抗体的方法 |
| MX383982B (es) | 2010-09-22 | 2025-03-14 | Amgen Inc | Inmunoglobulinas portadoras y usos de las mismas. |
-
2010
- 2010-03-19 EP EP10754211.0A patent/EP2432488A4/en not_active Withdrawn
- 2010-03-19 MX MX2011009797A patent/MX2011009797A/es not_active Application Discontinuation
- 2010-03-19 EP EP18173194.4A patent/EP3385279B1/en active Active
- 2010-03-19 US US13/258,454 patent/US20120121591A1/en not_active Abandoned
- 2010-03-19 US US13/258,668 patent/US8734796B2/en active Active
- 2010-03-19 CA CA2755133A patent/CA2755133A1/en not_active Abandoned
- 2010-03-19 MX MX2014010300A patent/MX347291B/es unknown
- 2010-03-19 JP JP2012501020A patent/JP2012521360A/ja active Pending
- 2010-03-19 CA CA3018235A patent/CA3018235C/en active Active
- 2010-03-19 WO PCT/US2010/028060 patent/WO2010108153A2/en not_active Ceased
- 2010-03-19 JP JP2012501019A patent/JP2012521197A/ja active Pending
- 2010-03-19 MX MX2011009798A patent/MX2011009798A/es active IP Right Grant
- 2010-03-19 CA CA2889453A patent/CA2889453C/en active Active
- 2010-03-19 WO PCT/US2010/028061 patent/WO2010108154A2/en not_active Ceased
- 2010-03-19 EP EP10754210.2A patent/EP2408814B1/en active Active
- 2010-03-19 AU AU2010226391A patent/AU2010226391C1/en active Active
- 2010-03-19 MX MX2014010302A patent/MX347295B/es unknown
- 2010-03-19 CA CA2755336A patent/CA2755336C/en active Active
- 2010-03-19 AU AU2010226392A patent/AU2010226392B9/en not_active Ceased
-
2014
- 2014-05-22 US US14/285,576 patent/US9562107B2/en active Active
- 2014-05-22 US US14/285,579 patent/US9562108B2/en active Active
-
2015
- 2015-01-07 JP JP2015001336A patent/JP6173359B2/ja active Active
-
2017
- 2017-01-12 US US15/405,233 patent/US10189912B2/en active Active
- 2017-04-10 JP JP2017077334A patent/JP6345832B2/ja active Active
-
2018
- 2018-05-22 JP JP2018097620A patent/JP6609347B2/ja active Active
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008538506A (ja) * | 2005-04-22 | 2008-10-30 | アムジエン・インコーポレーテツド | 延長された血液半減期を有するトキシンペプチド |
Non-Patent Citations (1)
| Title |
|---|
| JPN6014011793; GS Gendeh et al: 'A New Potassium Channel Toxin from the Sea Anemone Heteractis magnifica: Isolation, cDNA Cloning, a' Biochemistry Vol.36, 1997, p.11461-11471 * |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016505639A (ja) * | 2013-01-25 | 2016-02-25 | ヤンセン バイオテツク,インコーポレーテツド | Kv1.3アンタゴニスト及び使用方法 |
| US10179808B2 (en) | 2013-01-25 | 2019-01-15 | Janssen Biotech, Inc. | Kv1.3 antagonists and methods of use |
| JP2018521062A (ja) * | 2015-06-29 | 2018-08-02 | ザ・ユニバーシティ・オブ・ブリティッシュ・コロンビア | B1sp融合タンパク質の治療薬、方法、および使用 |
| JP2021180671A (ja) * | 2015-06-29 | 2021-11-25 | ザ・ユニバーシティ・オブ・ブリティッシュ・コロンビア | B1sp融合タンパク質の治療薬、方法、および使用 |
| JP7193590B2 (ja) | 2015-06-29 | 2022-12-20 | ザ・ユニバーシティ・オブ・ブリティッシュ・コロンビア | B1sp融合タンパク質の治療薬、方法、および使用 |
| WO2018135598A1 (ja) * | 2017-01-19 | 2018-07-26 | 国立大学法人 東京大学 | 新規蛍光標識方法 |
| JPWO2018135598A1 (ja) * | 2017-01-19 | 2019-12-12 | 国立大学法人 東京大学 | 新規蛍光標識方法 |
| JP7178701B2 (ja) | 2017-01-19 | 2022-11-28 | 国立大学法人 東京大学 | 新規蛍光標識方法 |
| JP2023019170A (ja) * | 2021-07-28 | 2023-02-09 | 株式会社三協 | 錠剤用崩壊剤及びこれを利用した錠剤 |
| JP7458085B2 (ja) | 2021-07-28 | 2024-03-29 | 株式会社三協 | 錠剤用崩壊剤及びこれを利用した錠剤 |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2010226392B2 (en) | Selective and potent peptide inhibitors of Kv1.3 | |
| US9796766B2 (en) | Potent and selective inhibitors of NAV1.3 and NAV1.7 | |
| US7820623B2 (en) | Conjugated toxin peptide therapeutic agents | |
| JP5220915B2 (ja) | 延長された血液半減期を有するトキシンペプチド | |
| US10344060B2 (en) | Potent and selective inhibitors of Nav1.7 | |
| US9803019B2 (en) | Carrier immunoglobulins and uses thereof | |
| US9636418B2 (en) | Potent and selective inhibitors of NAV1.7 | |
| CN101232903A (zh) | 具有延长的血液半衰期的毒素肽 | |
| NZ615242B2 (en) | Potent and selective inhibitors of nav1.3 and nav1.7 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20130225 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140325 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140620 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140627 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140925 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20150303 |