JP2012508096A - 低シリカ/アルミナ比を有する菱沸石ゼオライト触媒 - Google Patents
低シリカ/アルミナ比を有する菱沸石ゼオライト触媒 Download PDFInfo
- Publication number
- JP2012508096A JP2012508096A JP2011534913A JP2011534913A JP2012508096A JP 2012508096 A JP2012508096 A JP 2012508096A JP 2011534913 A JP2011534913 A JP 2011534913A JP 2011534913 A JP2011534913 A JP 2011534913A JP 2012508096 A JP2012508096 A JP 2012508096A
- Authority
- JP
- Japan
- Prior art keywords
- zeolite
- catalyst
- catalyst article
- substrate
- less
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000003054 catalyst Substances 0.000 title claims abstract description 158
- 239000010457 zeolite Substances 0.000 title claims abstract description 113
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 title claims abstract description 99
- 229910021536 Zeolite Inorganic materials 0.000 title claims abstract description 96
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 title claims abstract description 51
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 title claims abstract description 29
- 239000000377 silicon dioxide Substances 0.000 title claims abstract description 24
- 229910052676 chabazite Inorganic materials 0.000 title description 82
- UNYSKUBLZGJSLV-UHFFFAOYSA-L calcium;1,3,5,2,4,6$l^{2}-trioxadisilaluminane 2,4-dioxide;dihydroxide;hexahydrate Chemical compound O.O.O.O.O.O.[OH-].[OH-].[Ca+2].O=[Si]1O[Al]O[Si](=O)O1.O=[Si]1O[Al]O[Si](=O)O1 UNYSKUBLZGJSLV-UHFFFAOYSA-L 0.000 title description 81
- 238000000034 method Methods 0.000 claims abstract description 40
- 239000013078 crystal Substances 0.000 claims abstract description 17
- 239000000758 substrate Substances 0.000 claims description 68
- 239000003513 alkali Substances 0.000 claims description 28
- 239000010949 copper Substances 0.000 claims description 25
- 230000008569 process Effects 0.000 claims description 23
- 229910052751 metal Inorganic materials 0.000 claims description 22
- 239000002184 metal Substances 0.000 claims description 22
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 claims description 18
- 229910052802 copper Inorganic materials 0.000 claims description 18
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims description 16
- 238000005342 ion exchange Methods 0.000 claims description 16
- 230000003647 oxidation Effects 0.000 claims description 15
- 238000007254 oxidation reaction Methods 0.000 claims description 15
- 239000000243 solution Substances 0.000 claims description 15
- 238000001354 calcination Methods 0.000 claims description 10
- 239000004071 soot Substances 0.000 claims description 10
- 229910052742 iron Inorganic materials 0.000 claims description 8
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical group [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 claims description 8
- 239000012530 fluid Substances 0.000 claims description 7
- 238000011144 upstream manufacturing Methods 0.000 claims description 7
- 229910052782 aluminium Inorganic materials 0.000 claims description 6
- 150000001768 cations Chemical class 0.000 claims description 6
- 238000010304 firing Methods 0.000 claims description 6
- 238000004519 manufacturing process Methods 0.000 claims description 6
- 229910052700 potassium Inorganic materials 0.000 claims description 6
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 claims description 5
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 5
- 239000011591 potassium Substances 0.000 claims description 5
- 229910052708 sodium Inorganic materials 0.000 claims description 5
- 239000011734 sodium Substances 0.000 claims description 5
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 claims description 4
- 150000003863 ammonium salts Chemical class 0.000 claims description 3
- 238000004891 communication Methods 0.000 claims description 3
- 229910021645 metal ion Inorganic materials 0.000 claims description 2
- 239000012266 salt solution Substances 0.000 claims description 2
- 150000002500 ions Chemical group 0.000 claims 1
- QEMXHQIAXOOASZ-UHFFFAOYSA-N tetramethylammonium Chemical compound C[N+](C)(C)C QEMXHQIAXOOASZ-UHFFFAOYSA-N 0.000 claims 1
- 238000002360 preparation method Methods 0.000 abstract description 6
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 68
- 239000007789 gas Substances 0.000 description 37
- 229910021529 ammonia Inorganic materials 0.000 description 34
- 238000006243 chemical reaction Methods 0.000 description 25
- 239000000203 mixture Substances 0.000 description 25
- 239000002002 slurry Substances 0.000 description 19
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 12
- MWUXSHHQAYIFBG-UHFFFAOYSA-N Nitric oxide Chemical compound O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 description 11
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 10
- 230000003197 catalytic effect Effects 0.000 description 10
- 239000003638 chemical reducing agent Substances 0.000 description 10
- 230000009467 reduction Effects 0.000 description 10
- 238000006722 reduction reaction Methods 0.000 description 10
- 229910018072 Al 2 O 3 Inorganic materials 0.000 description 9
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 9
- 238000002156 mixing Methods 0.000 description 9
- PAWQVTBBRAZDMG-UHFFFAOYSA-N 2-(3-bromo-2-fluorophenyl)acetic acid Chemical compound OC(=O)CC1=CC=CC(Br)=C1F PAWQVTBBRAZDMG-UHFFFAOYSA-N 0.000 description 8
- 229910004298 SiO 2 Inorganic materials 0.000 description 8
- 238000004458 analytical method Methods 0.000 description 7
- 238000011068 loading method Methods 0.000 description 7
- 239000000463 material Substances 0.000 description 7
- 239000002245 particle Substances 0.000 description 7
- 239000002243 precursor Substances 0.000 description 7
- 239000000047 product Substances 0.000 description 7
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 7
- 238000000576 coating method Methods 0.000 description 6
- 229930195733 hydrocarbon Natural products 0.000 description 6
- 150000002430 hydrocarbons Chemical class 0.000 description 6
- 238000001027 hydrothermal synthesis Methods 0.000 description 6
- 229910044991 metal oxide Inorganic materials 0.000 description 6
- 150000004706 metal oxides Chemical class 0.000 description 6
- 229910000510 noble metal Inorganic materials 0.000 description 6
- 239000000843 powder Substances 0.000 description 6
- 239000012265 solid product Substances 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 229910045601 alloy Inorganic materials 0.000 description 5
- 239000000956 alloy Substances 0.000 description 5
- 239000011230 binding agent Substances 0.000 description 5
- 239000008367 deionised water Substances 0.000 description 5
- 229910021641 deionized water Inorganic materials 0.000 description 5
- 239000000706 filtrate Substances 0.000 description 5
- 150000002739 metals Chemical class 0.000 description 5
- 229910052760 oxygen Inorganic materials 0.000 description 5
- 239000003870 refractory metal Substances 0.000 description 5
- -1 silicon alkoxide Chemical class 0.000 description 5
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 4
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 4
- 239000010953 base metal Substances 0.000 description 4
- 238000010531 catalytic reduction reaction Methods 0.000 description 4
- 239000011248 coating agent Substances 0.000 description 4
- 239000001301 oxygen Substances 0.000 description 4
- 239000013618 particulate matter Substances 0.000 description 4
- 238000003756 stirring Methods 0.000 description 4
- WGTYBPLFGIVFAS-UHFFFAOYSA-M tetramethylammonium hydroxide Chemical compound [OH-].C[N+](C)(C)C WGTYBPLFGIVFAS-UHFFFAOYSA-M 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 3
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 239000004202 carbamide Substances 0.000 description 3
- 239000000919 ceramic Substances 0.000 description 3
- 239000006260 foam Substances 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 3
- 229910052845 zircon Inorganic materials 0.000 description 3
- GFQYVLUOOAAOGM-UHFFFAOYSA-N zirconium(iv) silicate Chemical compound [Zr+4].[O-][Si]([O-])([O-])[O-] GFQYVLUOOAAOGM-UHFFFAOYSA-N 0.000 description 3
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 2
- JPVYNHNXODAKFH-UHFFFAOYSA-N Cu2+ Chemical compound [Cu+2] JPVYNHNXODAKFH-UHFFFAOYSA-N 0.000 description 2
- CPLXHLVBOLITMK-UHFFFAOYSA-N Magnesium oxide Chemical compound [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 2
- BPQQTUXANYXVAA-UHFFFAOYSA-N Orthosilicate Chemical compound [O-][Si]([O-])([O-])[O-] BPQQTUXANYXVAA-UHFFFAOYSA-N 0.000 description 2
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 2
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 2
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 229910052804 chromium Inorganic materials 0.000 description 2
- 239000011651 chromium Substances 0.000 description 2
- 229910017052 cobalt Inorganic materials 0.000 description 2
- 239000010941 cobalt Substances 0.000 description 2
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 2
- 229910001431 copper ion Inorganic materials 0.000 description 2
- ARUVKPQLZAKDPS-UHFFFAOYSA-L copper(II) sulfate Chemical compound [Cu+2].[O-][S+2]([O-])([O-])[O-] ARUVKPQLZAKDPS-UHFFFAOYSA-L 0.000 description 2
- 229910000366 copper(II) sulfate Inorganic materials 0.000 description 2
- 229910052878 cordierite Inorganic materials 0.000 description 2
- JSKIRARMQDRGJZ-UHFFFAOYSA-N dimagnesium dioxido-bis[(1-oxido-3-oxo-2,4,6,8,9-pentaoxa-1,3-disila-5,7-dialuminabicyclo[3.3.1]nonan-7-yl)oxy]silane Chemical compound [Mg++].[Mg++].[O-][Si]([O-])(O[Al]1O[Al]2O[Si](=O)O[Si]([O-])(O1)O2)O[Al]1O[Al]2O[Si](=O)O[Si]([O-])(O1)O2 JSKIRARMQDRGJZ-UHFFFAOYSA-N 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 229910001092 metal group alloy Inorganic materials 0.000 description 2
- 229910052759 nickel Inorganic materials 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- LYTNHSCLZRMKON-UHFFFAOYSA-L oxygen(2-);zirconium(4+);diacetate Chemical compound [O-2].[Zr+4].CC([O-])=O.CC([O-])=O LYTNHSCLZRMKON-UHFFFAOYSA-L 0.000 description 2
- 239000011541 reaction mixture Substances 0.000 description 2
- 238000003878 thermal aging Methods 0.000 description 2
- 239000010936 titanium Substances 0.000 description 2
- 229910052726 zirconium Inorganic materials 0.000 description 2
- DUFCMRCMPHIFTR-UHFFFAOYSA-N 5-(dimethylsulfamoyl)-2-methylfuran-3-carboxylic acid Chemical compound CN(C)S(=O)(=O)C1=CC(C(O)=O)=C(C)O1 DUFCMRCMPHIFTR-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 229910000505 Al2TiO5 Inorganic materials 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 229910001200 Ferrotitanium Inorganic materials 0.000 description 1
- 229910052581 Si3N4 Inorganic materials 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 238000003915 air pollution Methods 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 229910000272 alkali metal oxide Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- AZDRQVAHHNSJOQ-UHFFFAOYSA-N alumane Chemical class [AlH3] AZDRQVAHHNSJOQ-UHFFFAOYSA-N 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- 229910000323 aluminium silicate Inorganic materials 0.000 description 1
- DIZPMCHEQGEION-UHFFFAOYSA-H aluminium sulfate (anhydrous) Chemical compound [Al+3].[Al+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O DIZPMCHEQGEION-UHFFFAOYSA-H 0.000 description 1
- VXAUWWUXCIMFIM-UHFFFAOYSA-M aluminum;oxygen(2-);hydroxide Chemical compound [OH-].[O-2].[Al+3] VXAUWWUXCIMFIM-UHFFFAOYSA-M 0.000 description 1
- 229910052788 barium Inorganic materials 0.000 description 1
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 229910001423 beryllium ion Inorganic materials 0.000 description 1
- 229910001593 boehmite Inorganic materials 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 230000005465 channeling Effects 0.000 description 1
- 239000008119 colloidal silica Substances 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 150000001879 copper Chemical class 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 239000002283 diesel fuel Substances 0.000 description 1
- KZHJGOXRZJKJNY-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Si]=O.O=[Al]O[Al]=O.O=[Al]O[Al]=O.O=[Al]O[Al]=O KZHJGOXRZJKJNY-UHFFFAOYSA-N 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000003344 environmental pollutant Substances 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 239000010433 feldspar Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 235000013312 flour Nutrition 0.000 description 1
- 239000000446 fuel Substances 0.000 description 1
- 229910021485 fumed silica Inorganic materials 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- FAHBNUUHRFUEAI-UHFFFAOYSA-M hydroxidooxidoaluminium Chemical compound O[Al]=O FAHBNUUHRFUEAI-UHFFFAOYSA-M 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 229910052746 lanthanum Inorganic materials 0.000 description 1
- FZLIPJUXYLNCLC-UHFFFAOYSA-N lanthanum atom Chemical compound [La] FZLIPJUXYLNCLC-UHFFFAOYSA-N 0.000 description 1
- 229910001947 lithium oxide Inorganic materials 0.000 description 1
- HCWCAKKEBCNQJP-UHFFFAOYSA-N magnesium orthosilicate Chemical compound [Mg+2].[Mg+2].[O-][Si]([O-])([O-])[O-] HCWCAKKEBCNQJP-UHFFFAOYSA-N 0.000 description 1
- 239000000395 magnesium oxide Substances 0.000 description 1
- 239000000391 magnesium silicate Substances 0.000 description 1
- 229910052919 magnesium silicate Inorganic materials 0.000 description 1
- 235000019792 magnesium silicate Nutrition 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- 239000007769 metal material Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 229910052863 mullite Inorganic materials 0.000 description 1
- UJVRJBAUJYZFIX-UHFFFAOYSA-N nitric acid;oxozirconium Chemical compound [Zr]=O.O[N+]([O-])=O.O[N+]([O-])=O UJVRJBAUJYZFIX-UHFFFAOYSA-N 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 239000006259 organic additive Substances 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 231100000572 poisoning Toxicity 0.000 description 1
- 230000000607 poisoning effect Effects 0.000 description 1
- 231100000719 pollutant Toxicity 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 238000000634 powder X-ray diffraction Methods 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- AABBHSMFGKYLKE-SNAWJCMRSA-N propan-2-yl (e)-but-2-enoate Chemical compound C\C=C\C(=O)OC(C)C AABBHSMFGKYLKE-SNAWJCMRSA-N 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 229910052611 pyroxene Inorganic materials 0.000 description 1
- 229910052761 rare earth metal Inorganic materials 0.000 description 1
- 150000002910 rare earth metals Chemical class 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 239000011214 refractory ceramic Substances 0.000 description 1
- 239000011819 refractory material Substances 0.000 description 1
- 229910052703 rhodium Inorganic materials 0.000 description 1
- 239000010948 rhodium Substances 0.000 description 1
- MHOVAHRLVXNVSD-UHFFFAOYSA-N rhodium atom Chemical compound [Rh] MHOVAHRLVXNVSD-UHFFFAOYSA-N 0.000 description 1
- 239000013049 sediment Substances 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- HBMJWWWQQXIZIP-UHFFFAOYSA-N silicon carbide Chemical compound [Si+]#[C-] HBMJWWWQQXIZIP-UHFFFAOYSA-N 0.000 description 1
- 229910010271 silicon carbide Inorganic materials 0.000 description 1
- HQVNEWCFYHHQES-UHFFFAOYSA-N silicon nitride Chemical compound N12[Si]34N5[Si]62N3[Si]51N64 HQVNEWCFYHHQES-UHFFFAOYSA-N 0.000 description 1
- 238000002791 soaking Methods 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 238000001694 spray drying Methods 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 229910052720 vanadium Inorganic materials 0.000 description 1
- GPPXJZIENCGNKB-UHFFFAOYSA-N vanadium Chemical compound [V]#[V] GPPXJZIENCGNKB-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J29/00—Catalysts comprising molecular sieves
- B01J29/04—Catalysts comprising molecular sieves having base-exchange properties, e.g. crystalline zeolites
- B01J29/06—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof
- B01J29/70—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof of types characterised by their specific structure not provided for in groups B01J29/08 - B01J29/65
- B01J29/7015—CHA-type, e.g. Chabazite, LZ-218
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/34—Chemical or biological purification of waste gases
- B01D53/92—Chemical or biological purification of waste gases of engine exhaust gases
- B01D53/94—Chemical or biological purification of waste gases of engine exhaust gases by catalytic processes
- B01D53/9404—Removing only nitrogen compounds
- B01D53/9409—Nitrogen oxides
- B01D53/9413—Processes characterised by a specific catalyst
- B01D53/9418—Processes characterised by a specific catalyst for removing nitrogen oxides by selective catalytic reduction [SCR] using a reducing agent in a lean exhaust gas
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J29/00—Catalysts comprising molecular sieves
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J29/00—Catalysts comprising molecular sieves
- B01J29/04—Catalysts comprising molecular sieves having base-exchange properties, e.g. crystalline zeolites
- B01J29/06—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof
- B01J29/70—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof of types characterised by their specific structure not provided for in groups B01J29/08 - B01J29/65
- B01J29/72—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof of types characterised by their specific structure not provided for in groups B01J29/08 - B01J29/65 containing iron group metals, noble metals or copper
- B01J29/76—Iron group metals or copper
- B01J29/763—CHA-type, e.g. Chabazite, LZ-218
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/50—Catalysts, in general, characterised by their form or physical properties characterised by their shape or configuration
- B01J35/56—Foraminous structures having flow-through passages or channels, e.g. grids or three-dimensional monoliths
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/02—Impregnation, coating or precipitation
- B01J37/024—Multiple impregnation or coating
- B01J37/0246—Coatings comprising a zeolite
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/08—Heat treatment
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/30—Ion-exchange
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2251/00—Reactants
- B01D2251/20—Reductants
- B01D2251/206—Ammonium compounds
- B01D2251/2062—Ammonia
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/50—Zeolites
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2258/00—Sources of waste gases
- B01D2258/01—Engine exhaust gases
- B01D2258/012—Diesel engines and lean burn gasoline engines
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2258/00—Sources of waste gases
- B01D2258/01—Engine exhaust gases
- B01D2258/014—Stoichiometric gasoline engines
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/34—Chemical or biological purification of waste gases
- B01D53/92—Chemical or biological purification of waste gases of engine exhaust gases
- B01D53/94—Chemical or biological purification of waste gases of engine exhaust gases by catalytic processes
- B01D53/9459—Removing one or more of nitrogen oxides, carbon monoxide, or hydrocarbons by multiple successive catalytic functions; systems with more than one different function, e.g. zone coated catalysts
- B01D53/9477—Removing one or more of nitrogen oxides, carbon monoxide, or hydrocarbons by multiple successive catalytic functions; systems with more than one different function, e.g. zone coated catalysts with catalysts positioned on separate bricks, e.g. exhaust systems
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/0009—Use of binding agents; Moulding; Pressing; Powdering; Granulating; Addition of materials ameliorating the mechanical properties of the product catalyst
- B01J37/0027—Powdering
- B01J37/0036—Grinding
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02T—CLIMATE CHANGE MITIGATION TECHNOLOGIES RELATED TO TRANSPORTATION
- Y02T10/00—Road transport of goods or passengers
- Y02T10/10—Internal combustion engine [ICE] based vehicles
- Y02T10/12—Improving ICE efficiencies
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Materials Engineering (AREA)
- Crystallography & Structural Chemistry (AREA)
- Environmental & Geological Engineering (AREA)
- General Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Analytical Chemistry (AREA)
- Biomedical Technology (AREA)
- Health & Medical Sciences (AREA)
- Combustion & Propulsion (AREA)
- Physics & Mathematics (AREA)
- Thermal Sciences (AREA)
- Catalysts (AREA)
- Exhaust Gas Treatment By Means Of Catalyst (AREA)
- Silicates, Zeolites, And Molecular Sieves (AREA)
- Exhaust Gas After Treatment (AREA)
- Processes For Solid Components From Exhaust (AREA)
Abstract
【選択図】図1
Description
本出願は、2008年11月6日出願の米国仮出願第61/111,960号の利益を主張し、その全体の内容が、参照によりその全体において本明細書に組み込まれる。
ゼオライト
幾つかの本発明の実施形態に関し、CHAゼオライトは自立触媒粒子の形態となり得る。しかし、具体的には、CHAゼオライト触媒は触媒物品を提供するため基材上に配置される。基材は、触媒を調製するために使用されるいかなるものであってもよく、そして通常、セラミックまたは金属のハニカム構造を含む。適切ないかなる基材も使用してよく、例えば基材の入口面または出口面から基材を通して伸びる微細で平行なガス流通路(該ガス流通路は開口しており、流体が通路を流れることができる)を有するモノリス基材(ハニカム流通基材と称される)を使用することができる。その流体入口から流体出口まで本質的に直線である通路は、壁部によって画定されており、この壁部に触媒物品がウォッシュコートとして配置され、これにより通路を流れるガスが触媒物品と接触する。モノリス基材の流体通路は薄壁の流路であり、該流路は、台形、長方形、四角形、正弦曲線、六角形、長円形、円形等の適切ないかなる断面形状および大きさを有していてもよい。このような構造は、断面の1平方インチ当たり、約60〜約400またはそれ以上の入口開口部(すなわちセル)を含んでいてもよい。
ウォッシュコートの調製
本発明の触媒物品は、排気ガス流、特に、ガソリンまたはディーゼルエンジンから発せられる排気ガス流の処理に特定の実用性が見出されている。使用時、排気ガス流は、本発明の実施形態に従って調製された触媒物品と接触する。以下に論じられるように、該触媒物品は、広範な動作温度にわたって卓越してNOx還元を活性化する。そのため、該触媒物品は、SCR触媒として有用である。「SCR」触媒という用語は、還元剤と窒素酸化物の触媒反応が起こり、窒素酸化物を還元する、選択的触媒還元を意味するために、広い意味で本明細書に使用される。また、「還元体」または「還元剤」も、昇温で、NOxを還元するあらゆる化学物質または化合物を意味するために、本明細書では広い意味で使用される。特定の実施形態において、該還元剤は、アンモニア、特にアンモニア前駆体、すなわち、尿素であり、該SCRは、窒素還元体SCRである。しかしながら、本発明の広い範囲では、該還元剤は、燃料、特にディーゼル燃料、およびその留分、および集合的にHC還元体と称されるいかなる炭化水素および酸化炭化水素も含み得る。
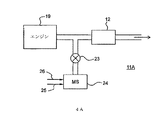
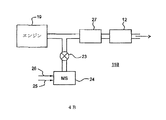
排気ガス処理システム
K−菱沸石は、実施例1に記載されるように、調製された。
Claims (20)
- NOxを低下させるように機能する基材上に配置されるCHA結晶構造を有するゼオライトを含む触媒物品であって、前記ゼオライトは、約15未満のシリカ/アルミナのモル比を有し、約3質量パーセント未満のアルカリ含有量を有する、触媒物品。
- 前記ゼオライトのアルカリ含有量は、約1質量パーセント未満である、請求項1に記載の触媒物品。
- 前記ゼオライトのアルカリ含有量は、約0.5質量パーセント未満である、請求項1に記載の触媒物品。
- 前記ゼオライトは、非合成の、天然に存在するゼオライトを含む、請求項1に記載の触媒物品。
- 前記ゼオライトは、約10未満のシリカ/アルミナのモル比を有する、請求項1に記載の触媒物品。
- 前記ゼオライトは、1個もしくは複数の金属カチオンで修飾される、請求項1に記載の触媒物品。
- 前記金属カチオンは、銅である、請求項6に記載の触媒物品。
- 白金族基の構成成分をさらに含む、請求項1に記載の触媒物品。
- 前記基材は、すすフィルタである、請求項6に記載の触媒物品。
- 前記すすフィルタは、壁流基材を含む、請求項9に記載の触媒物品。
- 前記基材は、ハニカムフロースルー基材を含む、請求項6に記載の触媒物品。
- 前記CHA結晶構造および約15〜約256のシリカ/アルミナのモル比を有する第2のゼオライトをさらに含み、銅/アルミニウムの原子比が、約0.25〜約0.50であり、前記ゼオライトおよび前記第2のゼオライトは混合される、請求項1に記載の触媒物品。
- 前記ゼオライトは、アルミナ源、シリカ源、ならびにナトリウム、カリウム、およびテトラメチルアンモニウム源のうちの1種もしくは複数混合し、水性ゲルを形成することと、加熱することによって前記ゲルを結晶化し、前記ゼオライトを形成することと、を含む、プロセスによって調製される、請求項1に記載の触媒物品。
- 請求項1〜13のうちのいずれかに記載の触媒物品を含む、排気ガス処理システム。
- 前記触媒物品の上流で前記触媒物品と流体連通している酸化触媒およびすすフィルタをさらに含む、請求項14に記載の排気ガス処理システム。
- ガス流中のNOxを低下させる方法であって、請求項6に記載の触媒物品と前記ガス流を接触させることを含む方法。
- 請求項1〜13のうちのいずれかに記載の触媒物品を作製する方法であって、溶液を用いて、初期アルカリ含有量の前記CHA結晶構造を有するゼオライトのアルカリ形態をイオン交換し、前記アルカリ含有量を低下させ、
前記低下したアルカリ含有量の前記イオン交換したゼオライトを焼成し、焼成ゼオライトを得、
次いで、アルカリ含有量をさらに低下させるために、溶液を用いて前記焼成ゼオライトをイオン交換し、約15未満のシリカ/アルミナのモル比および約3質量パーセント未満のアルカリ含有量を有するゼオライトを得ることを特徴とする方法。 - 前記溶液は、アンモニウム塩溶液である、請求項17に記載の方法。
- 前記焼成を、少なくとも約350℃の温度で、少なくとも約1時間行う請求項18に記載の方法。
- 鉄または銅溶液を用いて金属イオン交換を行い、金属促進性のゼオライトを提供することをさらに含む、請求項19に記載の方法。
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11196008P | 2008-11-06 | 2008-11-06 | |
| US61/111,960 | 2008-11-06 | ||
| US12/612,142 US10583424B2 (en) | 2008-11-06 | 2009-11-04 | Chabazite zeolite catalysts having low silica to alumina ratios |
| US12/612,142 | 2009-11-04 | ||
| PCT/US2009/063331 WO2010054034A2 (en) | 2008-11-06 | 2009-11-05 | Chabazite zeolite catalysts having low silica to alumina ratios |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015227283A Division JP6403658B2 (ja) | 2008-11-06 | 2015-11-20 | 低シリカ/アルミナ比を有する菱沸石ゼオライト触媒 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2012508096A true JP2012508096A (ja) | 2012-04-05 |
| JP2012508096A5 JP2012508096A5 (ja) | 2014-04-17 |
Family
ID=42153538
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011534913A Pending JP2012508096A (ja) | 2008-11-06 | 2009-11-05 | 低シリカ/アルミナ比を有する菱沸石ゼオライト触媒 |
| JP2015227283A Active JP6403658B2 (ja) | 2008-11-06 | 2015-11-20 | 低シリカ/アルミナ比を有する菱沸石ゼオライト触媒 |
| JP2018046820A Active JP6480053B2 (ja) | 2008-11-06 | 2018-03-14 | 低シリカ/アルミナ比を有する菱沸石ゼオライト触媒 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015227283A Active JP6403658B2 (ja) | 2008-11-06 | 2015-11-20 | 低シリカ/アルミナ比を有する菱沸石ゼオライト触媒 |
| JP2018046820A Active JP6480053B2 (ja) | 2008-11-06 | 2018-03-14 | 低シリカ/アルミナ比を有する菱沸石ゼオライト触媒 |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US10583424B2 (ja) |
| EP (1) | EP2364213B1 (ja) |
| JP (3) | JP2012508096A (ja) |
| KR (2) | KR101632766B1 (ja) |
| CN (1) | CN102215960B (ja) |
| BR (1) | BRPI0921675B1 (ja) |
| WO (1) | WO2010054034A2 (ja) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2011521871A (ja) * | 2008-05-21 | 2011-07-28 | ビーエーエスエフ ソシエタス・ヨーロピア | CHA構造を有するCu含有ゼオライトの直接合成のための方法 |
| JP2013001637A (ja) * | 2011-06-22 | 2013-01-07 | Mitsubishi Chemicals Corp | アルミノシリケートの製造方法 |
| WO2014054143A1 (ja) * | 2012-10-03 | 2014-04-10 | イビデン株式会社 | ハニカム構造体 |
| JP5740040B1 (ja) * | 2014-07-07 | 2015-06-24 | イビデン株式会社 | ゼオライト、ハニカム触媒及び排ガス浄化装置 |
| JP2015187398A (ja) * | 2014-03-26 | 2015-10-29 | トヨタ自動車株式会社 | 内燃機関の排気浄化装置 |
| EP2966040A1 (en) | 2014-07-07 | 2016-01-13 | Ibiden Co., Ltd. | Cha-zeolite, method for manufacturing this zeolite, honeycomb catalyst, and exhaust gas purifying apparatus |
| US10195596B2 (en) | 2015-05-13 | 2019-02-05 | Ibiden Co., Ltd. | Zeolite, method for producing zeolite, honeycomb catalyst using zeolite, and exhaust gas purifying apparatus |
| US10525411B2 (en) | 2015-05-15 | 2020-01-07 | Ibiden Co., Ltd. | Zeolite, method for producing zeolite, honeycomb catalyst using zeolite, and exhaust gas purifying apparatus |
| WO2023095619A1 (ja) * | 2021-11-29 | 2023-06-01 | 株式会社キャタラー | 排ガス浄化触媒装置 |
Families Citing this family (52)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8207084B2 (en) * | 2009-06-23 | 2012-06-26 | Ford Global Technologies, Llc | Urea-resistant catalytic units and methods of using the same |
| KR20120086711A (ko) | 2009-10-14 | 2012-08-03 | 바스프 코포레이션 | NOx의 선택적 환원을 위한 구리 함유 레빈 분자체 |
| US8409546B2 (en) * | 2009-11-24 | 2013-04-02 | Basf Se | Process for the preparation of zeolites having B-CHA structure |
| US8883119B2 (en) | 2009-11-24 | 2014-11-11 | Basf Se | Process for the preparation of zeolites having CHA structure |
| US9221015B2 (en) | 2010-07-15 | 2015-12-29 | Basf Se | Copper containing ZSM-34, OFF and/or ERI zeolitic material for selective reduction of NOx |
| WO2012007914A2 (en) | 2010-07-15 | 2012-01-19 | Basf Se | Copper containing zsm-34, off and/or eri zeolitic material for selective reduction of nox |
| US9289756B2 (en) | 2010-07-15 | 2016-03-22 | Basf Se | Copper containing ZSM-34, OFF and/or ERI zeolitic material for selective reduction of NOx |
| US8987162B2 (en) * | 2010-08-13 | 2015-03-24 | Ut-Battelle, Llc | Hydrothermally stable, low-temperature NOx reduction NH3-SCR catalyst |
| EP3103979B1 (de) * | 2010-09-13 | 2018-01-03 | Umicore AG & Co. KG | Katalysator zur entfernung von stickoxiden aus dem abgas von dieselmotoren |
| EP2463028A1 (en) | 2010-12-11 | 2012-06-13 | Umicore Ag & Co. Kg | Process for the production of metal doped zeolites and zeotypes and application of same to the catalytic removal of nitrogen oxides |
| JP5895510B2 (ja) * | 2010-12-22 | 2016-03-30 | 東ソー株式会社 | チャバザイト型ゼオライト及びその製造方法、銅が担持されている低シリカゼオライト、及び、そのゼオライトを含む窒素酸化物還元除去触媒、並びに、その触媒を使用する窒素酸化物還元除去方法 |
| WO2012090922A1 (ja) * | 2010-12-27 | 2012-07-05 | 三菱樹脂株式会社 | 窒素酸化物浄化用触媒 |
| EP2783741B1 (en) * | 2011-01-26 | 2021-03-31 | Ford Global Technologies, LLC | SCR and LNT catalysts for combined LNT-SCR applications |
| US20120269719A1 (en) * | 2011-04-18 | 2012-10-25 | Pq Corporation | Large crystal, organic-free chabazite, methods of making and using the same |
| US9527751B2 (en) | 2011-11-11 | 2016-12-27 | Basf Se | Organotemplate-free synthetic process for the production of a zeolitic material of the CHA-type structure |
| PL2776369T3 (pl) * | 2011-11-11 | 2022-06-20 | Basf Se | Sposób syntezy wolny od matrycy organicznej dla wytworzenia materiału zeolitowego o strukturze typu CHA |
| US20140328738A1 (en) * | 2011-12-01 | 2014-11-06 | Johnson Matthey Public Limited Company | Catalyst for Treating Exhaust Gas |
| US9981256B2 (en) | 2011-12-02 | 2018-05-29 | Pq Corporation | Stabilized microporous crystalline material, the method of making the same, and the use for selective catalytic reduction of NOx |
| JP6320298B2 (ja) * | 2011-12-02 | 2018-05-09 | ピーキュー コーポレイション | 安定化された微多孔結晶性物質、その製造方法、およびNOxの選択触媒還元のためのその使用 |
| WO2013155244A1 (en) * | 2012-04-11 | 2013-10-17 | Johnson Matthey Public Limited Company | Zeolite catalyst containing metals |
| CN104918884B (zh) | 2012-09-28 | 2018-01-09 | 太平洋工业发展公司 | 在选择性催化还原反应中用作催化剂的stt‑型沸石的制备方法 |
| US8992869B2 (en) | 2012-12-20 | 2015-03-31 | Caterpillar Inc. | Ammonia oxidation catalyst system |
| KR20140111549A (ko) * | 2013-03-11 | 2014-09-19 | 삼성전자주식회사 | 제올라이트 포함 이산화탄소 흡착제 및 그 제조 방법 |
| DE202013012229U1 (de) * | 2013-04-05 | 2015-10-08 | Umicore Ag & Co. Kg | CuCHA Material für die SCR-Katalyse |
| US20140357474A1 (en) * | 2013-05-30 | 2014-12-04 | Corning Incorporated | Formed ceramic substrate composition for catalyst integration |
| US9999879B2 (en) | 2013-05-30 | 2018-06-19 | Corning Incorporated | Formed ceramic substrate composition for catalyst integration |
| US10456030B2 (en) * | 2013-07-29 | 2019-10-29 | Bioptigen, Inc. | Procedural optical coherence tomography (OCT) for surgery and related methods |
| DE102014112413A1 (de) * | 2013-08-30 | 2015-03-05 | Johnson Matthey Public Limited Company | Zeolithmischkatalysatoren zur behandlung von abgas |
| KR102194141B1 (ko) * | 2013-11-06 | 2020-12-22 | 삼성전자주식회사 | 메조다공성 차바자이트 제올라이트 포함 이산화탄소 흡착제 및 그 제조 방법 |
| GB2522530B (en) * | 2013-12-03 | 2017-02-08 | Johnson Matthey Plc | Copper CHA framework Zeolite catalyst and use thereof |
| GB2530129B (en) * | 2014-05-16 | 2016-10-26 | Johnson Matthey Plc | Catalytic article for treating exhaust gas |
| EP3204157B1 (en) * | 2014-10-07 | 2024-07-03 | Johnson Matthey Public Limited Company | Molecular sieve catalyst for treating exhaust gas |
| JP6546731B2 (ja) * | 2014-10-09 | 2019-07-17 | イビデン株式会社 | ハニカム触媒 |
| US10807082B2 (en) * | 2014-10-13 | 2020-10-20 | Johnson Matthey Public Limited Company | Zeolite catalyst containing metals |
| ES2574500B1 (es) | 2014-12-17 | 2017-03-31 | Consejo Superior De Investigaciones Científicas (Csic) | Síntesis de la zeolita con la estructura cristalina CHA, procedimiento de síntesis y su uso en aplicaciones catalíticas |
| BR112017021069B1 (pt) * | 2015-04-09 | 2022-03-29 | Pq Corporation | Material cristalino microporoso e métodos de produção e de redução catalítica seletiva de óxidos de nitrogênio em gás de exaustão |
| US10377638B2 (en) | 2015-04-09 | 2019-08-13 | Pq Corporation | Stabilized microporous crystalline material, the method of making the same, and the use for selective catalytic reduction of NOx |
| EP3281698A1 (de) * | 2016-08-11 | 2018-02-14 | Umicore AG & Co. KG | Scr-aktives material |
| KR101896334B1 (ko) * | 2016-11-28 | 2018-09-07 | 현대자동차 주식회사 | 배기가스 정화장치 |
| KR101879695B1 (ko) * | 2016-12-02 | 2018-07-18 | 희성촉매 주식회사 | 2가 구리 이온들을 특정비율로 담지한 제올라이트, 이의 제조방법 및 이를 포함하는 촉매조성물 |
| BR112020009175A2 (pt) | 2017-11-10 | 2020-11-03 | Basf Corporation | sistema para tratamento de uma corrente de gás, filtro de fuligem catalisado (csf) e método para reduzir hcs, co e nox |
| US10220376B1 (en) * | 2017-12-05 | 2019-03-05 | James G. Davidson | Catalytic composition and system for exhaust purification |
| US10898889B2 (en) * | 2018-01-23 | 2021-01-26 | Umicore Ag & Co. Kg | SCR catalyst and exhaust gas cleaning system |
| JP7410048B2 (ja) * | 2018-03-21 | 2024-01-09 | ビーエーエスエフ コーポレーション | Chaゼオライト材料および関連する合成方法 |
| CN112020478A (zh) | 2018-05-03 | 2020-12-01 | 沙特基础工业全球技术公司 | 菱沸石(cha)的无sda合成及其用途 |
| WO2020025799A1 (en) * | 2018-08-02 | 2020-02-06 | Basf Se | Process for a continuous synthesis of zeolitic materials using seed crystals loaded with organotemplate |
| US11667536B2 (en) | 2018-08-24 | 2023-06-06 | Umicore Ag & Co. Kg | Method for the preparation of a molecular sieve of the CHA-type |
| CN114555525B (zh) * | 2019-08-02 | 2024-08-13 | 巴斯夫公司 | 包括有机和无机结构导向剂的菱沸石合成方法和具有片状形态的菱沸石沸石 |
| DE102020106882A1 (de) * | 2020-03-13 | 2021-09-16 | Purem GmbH | Abgasbehandlungssystem für eine Abgasanlage einer Brennkraftmaschine und Verfahren zum Betreiben eines derartigen Abgasbehandlungssystems |
| KR20220060315A (ko) * | 2020-11-04 | 2022-05-11 | 현대자동차주식회사 | Scr 촉매의 제조 방법 및 이에 의하여 제조된 scr 촉매 |
| EP4015454A1 (en) * | 2020-12-21 | 2022-06-22 | Tosoh Corporation | Cha-type zeolite and manufacturing method thereof |
| WO2022142836A1 (zh) * | 2020-12-28 | 2022-07-07 | 中化学科学技术研究有限公司 | 一种催化组合物、催化剂层、催化装置和气体处理系统 |
Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5242489A (en) * | 1976-05-20 | 1977-04-02 | Toa Nenryo Kogyo Kk | Nox-reduction catalyst and method of producing thereof |
| US4544538A (en) * | 1982-07-09 | 1985-10-01 | Chevron Research Company | Zeolite SSZ-13 and its method of preparation |
| JPS61168521A (ja) * | 1985-01-09 | 1986-07-30 | アンステイテユ・フランセ・デユ・ペトロール | シリカに富む合成オフレタイトの製造方法 |
| JPS61254256A (ja) * | 1985-04-30 | 1986-11-12 | イ−・アイ・デユポン・ドウ・ヌム−ル・アンド・カンパニ− | メタノ−ル及びアンモニアをジメチルアミンに転換する触媒としての8−環ゼオライト |
| US5173278A (en) * | 1991-03-15 | 1992-12-22 | Mobil Oil Corporation | Denitrification of flue gas from catalytic cracking |
| JPH0780314A (ja) * | 1993-09-16 | 1995-03-28 | Toray Ind Inc | 排ガス浄化触媒、その製造方法および窒素酸化物の浄化方法 |
| JP2004517194A (ja) * | 2000-12-28 | 2004-06-10 | エクソンモービル リサーチ アンド エンジニアリング カンパニー | 高シリカゼオライトを用いるナフサストリームからの硫黄の除去 |
| JP2005502451A (ja) * | 2001-09-07 | 2005-01-27 | エンゲルハード・コーポレーシヨン | NOx還元用の水熱的に安定な金属による助触媒作用を受けているゼオライトベータ |
| US7264789B1 (en) * | 1998-07-29 | 2007-09-04 | Exxonmobil Chemical Patents Inc. | Crystalline molecular sieves |
| JP2008529787A (ja) * | 2005-02-16 | 2008-08-07 | バスフ・カタリスツ・エルエルシー | 石炭燃焼公共施設のためのアンモニア酸化触媒 |
| WO2008106518A2 (en) * | 2007-02-27 | 2008-09-04 | Basf Catalysts Llc | Scr on low thermal mass filter substrates |
| WO2008118434A1 (en) * | 2007-03-26 | 2008-10-02 | Pq Corporation | Novel microporous crystalline material comprising a molecular sieve or zeolite having an 8-ring pore opening structure and methods of making and using same |
| JP2010524677A (ja) * | 2007-04-26 | 2010-07-22 | ジョンソン、マッセイ、パブリック、リミテッド、カンパニー | 遷移金属/ゼオライトscr触媒 |
Family Cites Families (112)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE394541C (de) | 1922-10-24 | 1924-04-22 | Gutberlet & Co A | Seitenziehmarke fuer Druckpressen, Falzmaschinen u. dgl. |
| US3402996A (en) * | 1966-12-19 | 1968-09-24 | Grace W R & Co | Ion exchange of crystalline zeolites |
| US3945943A (en) * | 1971-10-20 | 1976-03-23 | Union Oil Company Of California | Zeolite containing compositions, catalysts and methods of making |
| US4220632A (en) | 1974-09-10 | 1980-09-02 | The United States Of America As Represented By The United States Department Of Energy | Reduction of nitrogen oxides with catalytic acid resistant aluminosilicate molecular sieves and ammonia |
| JPS51147470A (en) * | 1975-06-12 | 1976-12-17 | Toa Nenryo Kogyo Kk | A process for catalytic reduction of nitrogen oxides |
| US4503023A (en) * | 1979-08-14 | 1985-03-05 | Union Carbide Corporation | Silicon substituted zeolite compositions and process for preparing same |
| US4297328A (en) * | 1979-09-28 | 1981-10-27 | Union Carbide Corporation | Three-way catalytic process for gaseous streams |
| US4440871A (en) | 1982-07-26 | 1984-04-03 | Union Carbide Corporation | Crystalline silicoaluminophosphates |
| US4567029A (en) | 1983-07-15 | 1986-01-28 | Union Carbide Corporation | Crystalline metal aluminophosphates |
| US4753927A (en) | 1983-08-12 | 1988-06-28 | Immunetech Pharmaceuticals | Method of blocking immune complex binding to immunoglobulin Fc receptors |
| JPS60125250A (ja) | 1983-12-08 | 1985-07-04 | Shiyuuichi Kagawa | 窒素酸化物の接触分解触媒及びその使用方法 |
| US4735930A (en) | 1986-02-18 | 1988-04-05 | Norton Company | Catalyst for the reduction of oxides of nitrogen |
| JPH0611381B2 (ja) | 1986-10-17 | 1994-02-16 | 株式会社豊田中央研究所 | 排ガス浄化方法 |
| US4861743A (en) | 1987-11-25 | 1989-08-29 | Uop | Process for the production of molecular sieves |
| US4867954A (en) | 1988-04-07 | 1989-09-19 | Uop | Catalytic reduction of nitrogen oxides |
| US4874590A (en) | 1988-04-07 | 1989-10-17 | Uop | Catalytic reduction of nitrogen oxides |
| US5011667A (en) * | 1988-09-08 | 1991-04-30 | Engelhard Corporation | Self-bound sodium chabazite aggregates and methods for preparation thereof |
| JP2557712B2 (ja) | 1988-12-27 | 1996-11-27 | 株式会社豊田中央研究所 | 排気ガス浄化方法 |
| FR2645141B1 (fr) | 1989-03-31 | 1992-05-29 | Elf France | Procede de synthese de precurseurs de tamis moleculaires du type silicoaluminophosphate, precurseurs obtenus et leur application a l'obtention desdits tamis moleculaires |
| US5026532A (en) * | 1989-04-06 | 1991-06-25 | Air Products And Chemicals, Inc. | Process for the preparation of an improved chabazite for the purification of bulk gases |
| US4961917A (en) | 1989-04-20 | 1990-10-09 | Engelhard Corporation | Method for reduction of nitrogen oxides with ammonia using promoted zeolite catalysts |
| US5024981A (en) | 1989-04-20 | 1991-06-18 | Engelhard Corporation | Staged metal-promoted zeolite catalysts and method for catalytic reduction of nitrogen oxides using the same |
| JP2533371B2 (ja) | 1989-05-01 | 1996-09-11 | 株式会社豊田中央研究所 | 排気ガス浄化用触媒 |
| US4925460A (en) * | 1989-07-20 | 1990-05-15 | Air Products And Chemicals, Inc. | Chabazite for gas separation |
| US5477014A (en) | 1989-07-28 | 1995-12-19 | Uop | Muffler device for internal combustion engines |
| US5233117A (en) | 1991-02-28 | 1993-08-03 | Uop | Methanol conversion processes using syocatalysts |
| JPH0557194A (ja) | 1991-07-06 | 1993-03-09 | Toyota Motor Corp | 排気ガス浄化用触媒の製造方法 |
| JP2887984B2 (ja) | 1991-09-20 | 1999-05-10 | トヨタ自動車株式会社 | 内燃機関の排気浄化装置 |
| JPH05147470A (ja) | 1991-11-28 | 1993-06-15 | Mazda Motor Corp | 車両の後部灯火の点灯制御装置 |
| JP3303341B2 (ja) | 1992-07-30 | 2002-07-22 | 三菱化学株式会社 | ベータ型ゼオライトの製造方法 |
| JPH0689300A (ja) | 1992-09-07 | 1994-03-29 | Nagano Japan Radio Co | Ltiシステムのデータ処理方法 |
| US6171556B1 (en) | 1992-11-12 | 2001-01-09 | Engelhard Corporation | Method and apparatus for treating an engine exhaust gas stream |
| CA2146244A1 (en) | 1992-11-19 | 1994-05-26 | Patrick Lee Burk | Method and apparatus for treating an engine exhaust gas stream |
| US6248684B1 (en) | 1992-11-19 | 2001-06-19 | Englehard Corporation | Zeolite-containing oxidation catalyst and method of use |
| EP0624393B1 (en) | 1993-05-10 | 2001-08-16 | Sakai Chemical Industry Co., Ltd., | Catalyst for catalytic reduction of nitrogen oxides |
| US5417949A (en) | 1993-08-25 | 1995-05-23 | Mobil Oil Corporation | NOx abatement process |
| ATE179088T1 (de) | 1993-11-09 | 1999-05-15 | Union Carbide Chem Plastic | Absorption von mercaptanen |
| JPH07155614A (ja) | 1993-12-07 | 1995-06-20 | Toyota Motor Corp | 排気ガス浄化用触媒の製造方法 |
| JPH07232035A (ja) | 1994-02-21 | 1995-09-05 | Toray Ind Inc | 窒素酸化物の浄化方法および浄化装置 |
| US5589147A (en) * | 1994-07-07 | 1996-12-31 | Mobil Oil Corporation | Catalytic system for the reducton of nitrogen oxides |
| JP3375790B2 (ja) * | 1995-06-23 | 2003-02-10 | 日本碍子株式会社 | 排ガス浄化システム及び排ガス浄化方法 |
| JPH0938499A (ja) | 1995-07-28 | 1997-02-10 | Catalysts & Chem Ind Co Ltd | 接触分解触媒 |
| US6133185A (en) | 1995-11-09 | 2000-10-17 | Toyota Jidosha Kabushiki Kaisha | Exhaust gas purifying catalyst |
| JPH10180041A (ja) * | 1996-12-20 | 1998-07-07 | Ngk Insulators Ltd | 排ガス浄化用触媒及び排ガス浄化システム |
| JPH11114413A (ja) | 1997-10-09 | 1999-04-27 | Ngk Insulators Ltd | 排ガス浄化用吸着材 |
| US6162415A (en) | 1997-10-14 | 2000-12-19 | Exxon Chemical Patents Inc. | Synthesis of SAPO-44 |
| JPH11179158A (ja) | 1997-10-15 | 1999-07-06 | Ngk Insulators Ltd | 小細孔多孔体を含む自動車排ガス浄化用の吸着材及び吸着体、これを用いた排ガス浄化システム及び排ガス浄化方法 |
| WO1999056859A1 (en) | 1998-05-07 | 1999-11-11 | Engelhard Corporation | Catalyzed hydrocarbon trap and method using the same |
| JP3580163B2 (ja) | 1998-06-04 | 2004-10-20 | トヨタ自動車株式会社 | 内燃機関の排気浄化装置 |
| US6576203B2 (en) * | 1998-06-29 | 2003-06-10 | Ngk Insulators, Ltd. | Reformer |
| EP1005904A3 (en) | 1998-10-30 | 2000-06-14 | The Boc Group, Inc. | Adsorbents and adsorptive separation process |
| DE19854502A1 (de) | 1998-11-25 | 2000-05-31 | Siemens Ag | Katalysatorkörper und Verfahren zum Abbau von Stickoxiden |
| KR100293531B1 (ko) | 1998-12-24 | 2001-10-26 | 윤덕용 | 이산화탄소로부터탄화수소생성을위한혼성촉매 |
| US6503863B2 (en) | 1999-06-07 | 2003-01-07 | Exxonmobil Chemical Patents, Inc. | Heat treating a molecular sieve and catalyst |
| US6395674B1 (en) | 1999-06-07 | 2002-05-28 | Exxon Mobil Chemical Patents, Inc. | Heat treating a molecular sieve and catalyst |
| US6316683B1 (en) | 1999-06-07 | 2001-11-13 | Exxonmobil Chemical Patents Inc. | Protecting catalytic activity of a SAPO molecular sieve |
| EP1129764B1 (de) | 2000-03-01 | 2005-10-26 | Umicore AG & Co. KG | Katalysator für die Reinigung der Abgase von Dieselmotoren und Verfahren zu seiner Herstellung |
| US6606856B1 (en) | 2000-03-03 | 2003-08-19 | The Lubrizol Corporation | Process for reducing pollutants from the exhaust of a diesel engine |
| US6416732B1 (en) * | 2000-03-23 | 2002-07-09 | Engelhard Corporation | Method of forming aluminosilicate zeolites |
| KR20030015287A (ko) * | 2000-06-22 | 2003-02-20 | 이 아이 듀폰 디 네모아 앤드 캄파니 | 혼합 매트릭스 나노다공성 탄소 멤브레인 |
| US6826906B2 (en) * | 2000-08-15 | 2004-12-07 | Engelhard Corporation | Exhaust system for enhanced reduction of nitrogen oxides and particulates from diesel engines |
| JP3571642B2 (ja) | 2000-11-16 | 2004-09-29 | トヨタ自動車株式会社 | 排気浄化装置用の還元剤 |
| DE10059520A1 (de) | 2000-11-30 | 2001-05-17 | Univ Karlsruhe | Verfahren zur Abtrennung von Zeolith-Kristallen aus Flüssigkeiten |
| US20050096214A1 (en) | 2001-03-01 | 2005-05-05 | Janssen Marcel J. | Silicoaluminophosphate molecular sieve |
| JP5189236B2 (ja) * | 2001-07-25 | 2013-04-24 | 日本碍子株式会社 | 排ガス浄化用ハニカム構造体及び排ガス浄化用ハニカム触媒体 |
| US6709644B2 (en) | 2001-08-30 | 2004-03-23 | Chevron U.S.A. Inc. | Small crystallite zeolite CHA |
| US7014827B2 (en) | 2001-10-23 | 2006-03-21 | Machteld Maria Mertens | Synthesis of silicoaluminophosphates |
| US6696032B2 (en) | 2001-11-29 | 2004-02-24 | Exxonmobil Chemical Patents Inc. | Process for manufacturing a silicoaluminophosphate molecular sieve |
| US6660682B2 (en) * | 2001-11-30 | 2003-12-09 | Exxon Mobil Chemical Patents Inc. | Method of synthesizing molecular sieves |
| US6685905B2 (en) | 2001-12-21 | 2004-02-03 | Exxonmobil Chemical Patents Inc. | Silicoaluminophosphate molecular sieves |
| EP1472202A4 (en) | 2002-01-03 | 2008-01-23 | Exxonmobil Chem Patents Inc | STABILIZATION OF ACIDIC CATALYSTS |
| JP2003290629A (ja) | 2002-04-02 | 2003-10-14 | Nissan Motor Co Ltd | 排ガス浄化システム |
| BR0312496A (pt) * | 2002-07-08 | 2007-06-19 | Engelhard Corp | processo para separar pelo menos um composto de metal e/ou um seu componente de uma mistura, e, processo para realizar uma reação quìmica catalisada |
| JP2005514319A (ja) | 2002-10-24 | 2005-05-19 | エクソンモービル・ケミカル・パテンツ・インク | 酸触媒の安定化 |
| US6928806B2 (en) * | 2002-11-21 | 2005-08-16 | Ford Global Technologies, Llc | Exhaust gas aftertreatment systems |
| JP4264701B2 (ja) | 2002-12-11 | 2009-05-20 | 日産化学工業株式会社 | 低アルカリ金属含有水性シリカゾルの製造方法 |
| US7049261B2 (en) * | 2003-02-27 | 2006-05-23 | General Motors Corporation | Zeolite catalyst and preparation process for NOx reduction |
| JP4413520B2 (ja) | 2003-04-17 | 2010-02-10 | 株式会社アイシーティー | 排ガス浄化用触媒及びその触媒を用いた排ガスの浄化方法 |
| US7157073B2 (en) * | 2003-05-02 | 2007-01-02 | Reading Alloys, Inc. | Production of high-purity niobium monoxide and capacitor production therefrom |
| US7229597B2 (en) | 2003-08-05 | 2007-06-12 | Basfd Catalysts Llc | Catalyzed SCR filter and emission treatment system |
| CN1246223C (zh) | 2003-09-03 | 2006-03-22 | 中国石油化工股份有限公司 | 合成硅磷铝分子筛的方法 |
| JP4842143B2 (ja) | 2003-12-23 | 2011-12-21 | エクソンモービル・ケミカル・パテンツ・インク | カバサイト含有モレキュラーシーブ、その合成及びオイシジェネートからオレフィンへの変換におけるその使用 |
| DE102004013165A1 (de) * | 2004-03-17 | 2005-10-06 | Adam Opel Ag | Verfahren zur Verbesserung der Wirksamkeit der NOx-Reduktion in Kraftfahrzeugen |
| NL1026207C2 (nl) * | 2004-05-17 | 2005-11-21 | Stichting Energie | Werkwijze voor de decompositie van N2O, katalysator daarvoor en bereiding van deze katalysator. |
| US8575054B2 (en) * | 2004-07-15 | 2013-11-05 | Nikki-Universal Co., Ltd. | Catalyst for purifying organic nitrogen compound-containing exhaust gas and method for purifying the exhaust gas |
| US7481983B2 (en) * | 2004-08-23 | 2009-01-27 | Basf Catalysts Llc | Zone coated catalyst to simultaneously reduce NOx and unreacted ammonia |
| JP2006089300A (ja) | 2004-09-21 | 2006-04-06 | Nippon Gas Gosei Kk | Sapo−34の製造方法、および、プロパンを主成分とする液化石油ガスの製造方法 |
| US20060115403A1 (en) * | 2004-11-29 | 2006-06-01 | Chevron U.S.A. Inc. | Reduction of oxides of nitrogen in a gas stream using high-silics molecular sieve CHA |
| EP1837489B9 (en) | 2004-12-17 | 2012-09-12 | Usui Kokusai Sangyo Kaisha Limited | Electric treating method for exhaust gas of diesel engine and its device |
| US8580216B2 (en) * | 2005-02-28 | 2013-11-12 | Ecs Holdings, Inc. | Catalyst and method for reducing nitrogen oxides in exhaust streams with hydrocarbons or alcohols |
| EP1872852A1 (en) | 2005-03-30 | 2008-01-02 | Sued-Chemie Catalysts Japan, Inc. | Ammonia decomposition catalyst and process for decomposition of ammonia using the catalyst |
| BRPI0610326B1 (pt) * | 2005-04-27 | 2015-07-21 | Grace W R & Co | Composições e processos para reduzir emissões de nox durante o craqueamento catalítico de fluído. |
| EP1899059A1 (en) * | 2005-06-27 | 2008-03-19 | ExxonMobil Chemical Patents Inc. | Process for manufacture of silicoaluminophosphate molecular sieves |
| US7879295B2 (en) | 2005-06-30 | 2011-02-01 | General Electric Company | Conversion system for reducing NOx emissions |
| EP1904229A4 (en) | 2005-07-06 | 2014-04-16 | Heesung Catalysts Corp | NH3 OXIDATION CATALYST AND APPARATUS FOR PROCESSING NH3 EMISSION OR RESIDUE |
| US8048402B2 (en) | 2005-08-18 | 2011-11-01 | Exxonmobil Chemical Patents Inc. | Synthesis of molecular sieves having the chabazite framework type and their use in the conversion of oxygenates to olefins |
| RU2008128363A (ru) * | 2005-12-14 | 2010-01-20 | Басф Каталистс Ллк (Us) | ЦЕОЛИТНЫЙ КАТАЛИЗАТОР С УЛУЧШЕННЫМ ВОССТАНОВЛЕНИЕМ NOх В SCR |
| US20070149385A1 (en) | 2005-12-23 | 2007-06-28 | Ke Liu | Catalyst system for reducing nitrogen oxide emissions |
| US7576031B2 (en) * | 2006-06-09 | 2009-08-18 | Basf Catalysts Llc | Pt-Pd diesel oxidation catalyst with CO/HC light-off and HC storage function |
| US8383080B2 (en) | 2006-06-09 | 2013-02-26 | Exxonmobil Chemical Patents Inc. | Treatment of CHA-type molecular sieves and their use in the conversion of oxygenates to olefins |
| ITMO20060202A1 (it) * | 2006-06-21 | 2007-12-22 | Galliano Bentivoglio | Pistola per erogare combustibile liquido |
| US8568678B2 (en) * | 2006-07-08 | 2013-10-29 | Umicore Ag & Co. Kg | Structured SCR catalyst for the reduction of nitrogen oxides in the exhaust gas from lean-burn engines using ammonia as reducing agent |
| CN101121532A (zh) | 2006-08-08 | 2008-02-13 | 中国科学院大连化学物理研究所 | 一种小孔磷硅铝分子筛的金属改性方法 |
| BRPI0808159A2 (pt) * | 2007-01-31 | 2014-07-08 | Basf Catalysts Llc | Artigo de tratamento de gás |
| WO2008106519A1 (en) | 2007-02-27 | 2008-09-04 | Basf Catalysts Llc | Copper cha zeolite catalysts |
| US10384162B2 (en) * | 2007-03-26 | 2019-08-20 | Pq Corporation | High silica chabazite for selective catalytic reduction, methods of making and using same |
| US8209956B2 (en) * | 2007-07-31 | 2012-07-03 | Caterpillar Inc. | SCR emissions control system |
| EP2689846A1 (en) * | 2007-08-13 | 2014-01-29 | PQ Corporation | Selective catalytic reduction of nitrogen oxides in the presence of iron-containing aluminosilicate zeolites |
| US8715618B2 (en) * | 2008-05-21 | 2014-05-06 | Basf Se | Process for the direct synthesis of Cu containing zeolites having CHA structure |
| US8293198B2 (en) | 2009-12-18 | 2012-10-23 | Basf Corporation | Process of direct copper exchange into Na+-form of chabazite molecular sieve, and catalysts, systems and methods |
| US8293199B2 (en) | 2009-12-18 | 2012-10-23 | Basf Corporation | Process for preparation of copper containing molecular sieves with the CHA structure, catalysts, systems and methods |
| WO2016120840A1 (en) * | 2015-01-29 | 2016-08-04 | Johnson Matthey Public Limited Company | Direct incorporation of iron complexes into sapo-34 (cha) type materials |
-
2009
- 2009-11-04 US US12/612,142 patent/US10583424B2/en active Active
- 2009-11-05 WO PCT/US2009/063331 patent/WO2010054034A2/en active Application Filing
- 2009-11-05 KR KR1020117012757A patent/KR101632766B1/ko active IP Right Grant
- 2009-11-05 BR BRPI0921675-8A patent/BRPI0921675B1/pt active IP Right Grant
- 2009-11-05 EP EP09825385.9A patent/EP2364213B1/en active Active
- 2009-11-05 CN CN200980146090.2A patent/CN102215960B/zh active Active
- 2009-11-05 JP JP2011534913A patent/JP2012508096A/ja active Pending
- 2009-11-05 KR KR1020167015795A patent/KR101735255B1/ko active IP Right Grant
-
2015
- 2015-11-20 JP JP2015227283A patent/JP6403658B2/ja active Active
-
2018
- 2018-03-14 JP JP2018046820A patent/JP6480053B2/ja active Active
-
2020
- 2020-02-06 US US16/783,822 patent/US11660585B2/en active Active
Patent Citations (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5242489A (en) * | 1976-05-20 | 1977-04-02 | Toa Nenryo Kogyo Kk | Nox-reduction catalyst and method of producing thereof |
| US4544538A (en) * | 1982-07-09 | 1985-10-01 | Chevron Research Company | Zeolite SSZ-13 and its method of preparation |
| JPS61168521A (ja) * | 1985-01-09 | 1986-07-30 | アンステイテユ・フランセ・デユ・ペトロール | シリカに富む合成オフレタイトの製造方法 |
| JPS61254256A (ja) * | 1985-04-30 | 1986-11-12 | イ−・アイ・デユポン・ドウ・ヌム−ル・アンド・カンパニ− | メタノ−ル及びアンモニアをジメチルアミンに転換する触媒としての8−環ゼオライト |
| US5173278A (en) * | 1991-03-15 | 1992-12-22 | Mobil Oil Corporation | Denitrification of flue gas from catalytic cracking |
| JPH0780314A (ja) * | 1993-09-16 | 1995-03-28 | Toray Ind Inc | 排ガス浄化触媒、その製造方法および窒素酸化物の浄化方法 |
| US7264789B1 (en) * | 1998-07-29 | 2007-09-04 | Exxonmobil Chemical Patents Inc. | Crystalline molecular sieves |
| JP2004517194A (ja) * | 2000-12-28 | 2004-06-10 | エクソンモービル リサーチ アンド エンジニアリング カンパニー | 高シリカゼオライトを用いるナフサストリームからの硫黄の除去 |
| JP2005502451A (ja) * | 2001-09-07 | 2005-01-27 | エンゲルハード・コーポレーシヨン | NOx還元用の水熱的に安定な金属による助触媒作用を受けているゼオライトベータ |
| JP2008529787A (ja) * | 2005-02-16 | 2008-08-07 | バスフ・カタリスツ・エルエルシー | 石炭燃焼公共施設のためのアンモニア酸化触媒 |
| WO2008106518A2 (en) * | 2007-02-27 | 2008-09-04 | Basf Catalysts Llc | Scr on low thermal mass filter substrates |
| JP2010519037A (ja) * | 2007-02-27 | 2010-06-03 | ビーエーエスエフ、カタリスツ、エルエルシー | 低熱容量のフィルター基材上のscr |
| WO2008118434A1 (en) * | 2007-03-26 | 2008-10-02 | Pq Corporation | Novel microporous crystalline material comprising a molecular sieve or zeolite having an 8-ring pore opening structure and methods of making and using same |
| JP2010522688A (ja) * | 2007-03-26 | 2010-07-08 | ピーキュー コーポレイション | 8員環細孔開口構造を有するモレキュラーシーブまたはゼオライトを含んで成る新規マイクロポーラス結晶性物質およびその製法およびその使用 |
| JP2010524677A (ja) * | 2007-04-26 | 2010-07-22 | ジョンソン、マッセイ、パブリック、リミテッド、カンパニー | 遷移金属/ゼオライトscr触媒 |
Non-Patent Citations (2)
| Title |
|---|
| CABSORB-ZS500H, vol. [online], JPN6014041984, 25 September 2014 (2014-09-25), ISSN: 0002910128 * |
| LONG, R.Q. ET AL.: "Selective Catalytic Reduction of NO with Ammonia over Fe3+-Exchanged Mordenite (Fe-MOR): Catalytic P", JOURNAL OF CATALYSIS, vol. 207, no. 2, JPN6012066879, 19 April 2002 (2002-04-19), pages 274 - 285, XP004461232, ISSN: 0002910127, DOI: 10.1006/jcat.2002.3521 * |
Cited By (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9272272B2 (en) | 2008-05-21 | 2016-03-01 | Basf Se | Process for the direct synthesis of Cu containing zeolites having CHA structure |
| US8715618B2 (en) | 2008-05-21 | 2014-05-06 | Basf Se | Process for the direct synthesis of Cu containing zeolites having CHA structure |
| JP2011521871A (ja) * | 2008-05-21 | 2011-07-28 | ビーエーエスエフ ソシエタス・ヨーロピア | CHA構造を有するCu含有ゼオライトの直接合成のための方法 |
| JP2013001637A (ja) * | 2011-06-22 | 2013-01-07 | Mitsubishi Chemicals Corp | アルミノシリケートの製造方法 |
| WO2014054143A1 (ja) * | 2012-10-03 | 2014-04-10 | イビデン株式会社 | ハニカム構造体 |
| JPWO2014054143A1 (ja) * | 2012-10-03 | 2016-08-25 | イビデン株式会社 | ハニカム構造体 |
| JP5873562B2 (ja) * | 2012-10-03 | 2016-03-01 | イビデン株式会社 | ハニカム構造体 |
| JP2015187398A (ja) * | 2014-03-26 | 2015-10-29 | トヨタ自動車株式会社 | 内燃機関の排気浄化装置 |
| JP5740040B1 (ja) * | 2014-07-07 | 2015-06-24 | イビデン株式会社 | ゼオライト、ハニカム触媒及び排ガス浄化装置 |
| EP2966040A1 (en) | 2014-07-07 | 2016-01-13 | Ibiden Co., Ltd. | Cha-zeolite, method for manufacturing this zeolite, honeycomb catalyst, and exhaust gas purifying apparatus |
| EP2966041A1 (en) | 2014-07-07 | 2016-01-13 | Ibiden Co., Ltd. | Cha-zeolite, methods for manufacturing this zeolite, honeycomb catalyst, and exhaust gas purifying apparatus |
| US9656253B2 (en) | 2014-07-07 | 2017-05-23 | Ibiden Co., Ltd. | Zeolite, method for manufacturing zeolite, honeycomb catalyst, and exhaust gas purifying apparatus |
| US9878315B2 (en) | 2014-07-07 | 2018-01-30 | Ibiden Co., Ltd. | Zeolite, method for manufacturing zeolite, honeycomb catalyst, and exhaust gas purifying apparatus |
| US10195596B2 (en) | 2015-05-13 | 2019-02-05 | Ibiden Co., Ltd. | Zeolite, method for producing zeolite, honeycomb catalyst using zeolite, and exhaust gas purifying apparatus |
| US10525411B2 (en) | 2015-05-15 | 2020-01-07 | Ibiden Co., Ltd. | Zeolite, method for producing zeolite, honeycomb catalyst using zeolite, and exhaust gas purifying apparatus |
| WO2023095619A1 (ja) * | 2021-11-29 | 2023-06-01 | 株式会社キャタラー | 排ガス浄化触媒装置 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2364213A4 (en) | 2013-12-04 |
| JP2018134636A (ja) | 2018-08-30 |
| BRPI0921675A2 (pt) | 2016-02-16 |
| KR101632766B1 (ko) | 2016-06-22 |
| WO2010054034A3 (en) | 2010-07-29 |
| KR101735255B1 (ko) | 2017-05-12 |
| WO2010054034A2 (en) | 2010-05-14 |
| US10583424B2 (en) | 2020-03-10 |
| KR20110082603A (ko) | 2011-07-19 |
| BRPI0921675B1 (pt) | 2017-12-05 |
| US20110020204A1 (en) | 2011-01-27 |
| JP2016093809A (ja) | 2016-05-26 |
| US11660585B2 (en) | 2023-05-30 |
| JP6480053B2 (ja) | 2019-03-06 |
| EP2364213B1 (en) | 2018-01-10 |
| EP2364213A2 (en) | 2011-09-14 |
| US20200188894A1 (en) | 2020-06-18 |
| JP6403658B2 (ja) | 2018-10-10 |
| KR20160075818A (ko) | 2016-06-29 |
| CN102215960B (zh) | 2014-09-03 |
| CN102215960A (zh) | 2011-10-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6480053B2 (ja) | 低シリカ/アルミナ比を有する菱沸石ゼオライト触媒 | |
| US11845067B2 (en) | Copper CHA zeolite catalysts | |
| JP5683111B2 (ja) | 銅chaゼオライト触媒 | |
| JP2011510899A (ja) | Cha結晶構造を有する分子篩を含む非沸石系金属を利用する触媒、システム、および方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20121102 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20130710 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130903 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20131118 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20131125 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140131 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140207 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20140228 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20141007 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20141226 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20150205 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150304 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20150721 |