EP3498295A1 - Anticorps anti-gitr et leurs procédés d'utilisation - Google Patents
Anticorps anti-gitr et leurs procédés d'utilisation Download PDFInfo
- Publication number
- EP3498295A1 EP3498295A1 EP18204948.6A EP18204948A EP3498295A1 EP 3498295 A1 EP3498295 A1 EP 3498295A1 EP 18204948 A EP18204948 A EP 18204948A EP 3498295 A1 EP3498295 A1 EP 3498295A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- antibody
- gitr
- seq
- amino acid
- acid sequence
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000000034 method Methods 0.000 title claims abstract description 71
- 101000801234 Homo sapiens Tumor necrosis factor receptor superfamily member 18 Proteins 0.000 claims abstract description 429
- 102000047758 human TNFRSF18 Human genes 0.000 claims abstract description 426
- 230000000694 effects Effects 0.000 claims abstract description 48
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 31
- 201000011510 cancer Diseases 0.000 claims abstract description 26
- 101710187882 Tumor necrosis factor receptor superfamily member 18 Proteins 0.000 claims description 512
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 338
- 229910052727 yttrium Inorganic materials 0.000 claims description 112
- 108010047041 Complementarity Determining Regions Proteins 0.000 claims description 104
- 210000004027 cell Anatomy 0.000 claims description 99
- 229910052731 fluorine Inorganic materials 0.000 claims description 75
- 229910052739 hydrogen Inorganic materials 0.000 claims description 52
- 229910052717 sulfur Inorganic materials 0.000 claims description 37
- 150000007523 nucleic acids Chemical class 0.000 claims description 31
- 102000039446 nucleic acids Human genes 0.000 claims description 27
- 108020004707 nucleic acids Proteins 0.000 claims description 27
- 239000008194 pharmaceutical composition Substances 0.000 claims description 24
- 239000013598 vector Substances 0.000 claims description 15
- 108060003951 Immunoglobulin Proteins 0.000 claims description 13
- 102000018358 immunoglobulin Human genes 0.000 claims description 13
- 208000036142 Viral infection Diseases 0.000 claims description 7
- 238000011282 treatment Methods 0.000 claims description 7
- 230000009385 viral infection Effects 0.000 claims description 6
- 241001529936 Murinae Species 0.000 claims description 5
- 238000012258 culturing Methods 0.000 claims description 4
- 208000002154 non-small cell lung carcinoma Diseases 0.000 claims description 4
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 claims description 4
- 206010014733 Endometrial cancer Diseases 0.000 claims description 2
- 206010014759 Endometrial neoplasm Diseases 0.000 claims description 2
- 208000000461 Esophageal Neoplasms Diseases 0.000 claims description 2
- 206010030155 Oesophageal carcinoma Diseases 0.000 claims description 2
- 239000003937 drug carrier Substances 0.000 claims description 2
- 201000004101 esophageal cancer Diseases 0.000 claims description 2
- 201000001441 melanoma Diseases 0.000 claims description 2
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 2
- 210000004978 chinese hamster ovary cell Anatomy 0.000 claims 3
- 208000005718 Stomach Neoplasms Diseases 0.000 claims 2
- 206010017758 gastric cancer Diseases 0.000 claims 2
- 201000011549 stomach cancer Diseases 0.000 claims 2
- 201000009030 Carcinoma Diseases 0.000 claims 1
- 102000050627 Glucocorticoid-Induced TNFR-Related Human genes 0.000 claims 1
- 208000006265 Renal cell carcinoma Diseases 0.000 claims 1
- 208000000102 Squamous Cell Carcinoma of Head and Neck Diseases 0.000 claims 1
- 210000003236 esophagogastric junction Anatomy 0.000 claims 1
- 201000000459 head and neck squamous cell carcinoma Diseases 0.000 claims 1
- 208000035473 Communicable disease Diseases 0.000 abstract description 6
- 108060008683 Tumor Necrosis Factor Receptor Proteins 0.000 abstract description 4
- 102000005962 receptors Human genes 0.000 abstract description 4
- 108020003175 receptors Proteins 0.000 abstract description 4
- 102000003298 tumor necrosis factor receptor Human genes 0.000 abstract description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 abstract description 3
- 239000003862 glucocorticoid Substances 0.000 abstract description 3
- 239000000203 mixture Substances 0.000 abstract description 3
- 239000012634 fragment Substances 0.000 description 696
- 230000027455 binding Effects 0.000 description 596
- 239000000427 antigen Substances 0.000 description 521
- 108091007433 antigens Proteins 0.000 description 521
- 102000036639 antigens Human genes 0.000 description 521
- 102100033728 Tumor necrosis factor receptor superfamily member 18 Human genes 0.000 description 514
- 239000011324 bead Substances 0.000 description 229
- 101000597780 Mus musculus Tumor necrosis factor ligand superfamily member 18 Proteins 0.000 description 128
- 229910052721 tungsten Inorganic materials 0.000 description 110
- 229910052799 carbon Inorganic materials 0.000 description 109
- 235000001014 amino acid Nutrition 0.000 description 97
- 241000288906 Primates Species 0.000 description 92
- 210000001744 T-lymphocyte Anatomy 0.000 description 81
- 238000003556 assay Methods 0.000 description 75
- 101000597779 Homo sapiens Tumor necrosis factor ligand superfamily member 18 Proteins 0.000 description 74
- 102000053830 human TNFSF18 Human genes 0.000 description 74
- 229910052740 iodine Inorganic materials 0.000 description 55
- 229910052698 phosphorus Inorganic materials 0.000 description 49
- 239000000725 suspension Substances 0.000 description 43
- 108091008874 T cell receptors Proteins 0.000 description 38
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 38
- 150000001413 amino acids Chemical class 0.000 description 38
- 229940024606 amino acid Drugs 0.000 description 37
- 229910052720 vanadium Inorganic materials 0.000 description 37
- 238000006467 substitution reaction Methods 0.000 description 34
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 30
- 239000004698 Polyethylene Substances 0.000 description 25
- 230000003213 activating effect Effects 0.000 description 21
- 239000005557 antagonist Substances 0.000 description 20
- 230000014509 gene expression Effects 0.000 description 20
- 101710165473 Tumor necrosis factor receptor superfamily member 4 Proteins 0.000 description 18
- 102100022153 Tumor necrosis factor receptor superfamily member 4 Human genes 0.000 description 18
- 125000000539 amino acid group Chemical group 0.000 description 18
- 238000002474 experimental method Methods 0.000 description 18
- PHEDXBVPIONUQT-RGYGYFBISA-N phorbol 13-acetate 12-myristate Chemical compound C([C@]1(O)C(=O)C(C)=C[C@H]1[C@@]1(O)[C@H](C)[C@H]2OC(=O)CCCCCCCCCCCCC)C(CO)=C[C@H]1[C@H]1[C@]2(OC(C)=O)C1(C)C PHEDXBVPIONUQT-RGYGYFBISA-N 0.000 description 18
- 108090000623 proteins and genes Proteins 0.000 description 16
- 230000000638 stimulation Effects 0.000 description 16
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 15
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 15
- 230000001965 increasing effect Effects 0.000 description 14
- 102100029193 Low affinity immunoglobulin gamma Fc region receptor III-A Human genes 0.000 description 13
- 239000000556 agonist Substances 0.000 description 13
- 239000012636 effector Substances 0.000 description 13
- 230000002708 enhancing effect Effects 0.000 description 13
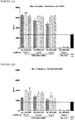
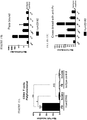
- 238000010186 staining Methods 0.000 description 13
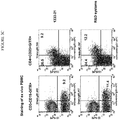
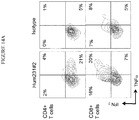
- 238000000684 flow cytometry Methods 0.000 description 12
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 12
- 101000917858 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 description 11
- 108010029485 Protein Isoforms Proteins 0.000 description 11
- 102000001708 Protein Isoforms Human genes 0.000 description 11
- 239000003112 inhibitor Substances 0.000 description 11
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 11
- 238000012360 testing method Methods 0.000 description 11
- 102000004127 Cytokines Human genes 0.000 description 10
- 108090000695 Cytokines Proteins 0.000 description 10
- 108010073807 IgG Receptors Proteins 0.000 description 10
- 102000009490 IgG Receptors Human genes 0.000 description 10
- 239000005089 Luciferase Substances 0.000 description 10
- 210000001266 CD8-positive T-lymphocyte Anatomy 0.000 description 9
- 108010057466 NF-kappa B Proteins 0.000 description 9
- 102000003945 NF-kappa B Human genes 0.000 description 9
- 230000004913 activation Effects 0.000 description 9
- 239000002269 analeptic agent Substances 0.000 description 9
- 238000005516 engineering process Methods 0.000 description 9
- 108090000765 processed proteins & peptides Proteins 0.000 description 9
- 235000018102 proteins Nutrition 0.000 description 9
- 102000004169 proteins and genes Human genes 0.000 description 9
- 230000004936 stimulating effect Effects 0.000 description 9
- 101000917839 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-B Proteins 0.000 description 8
- 108060001084 Luciferase Proteins 0.000 description 8
- 230000001939 inductive effect Effects 0.000 description 8
- 102000004196 processed proteins & peptides Human genes 0.000 description 8
- 210000003289 regulatory T cell Anatomy 0.000 description 8
- 241000282552 Chlorocebus aethiops Species 0.000 description 7
- 230000008878 coupling Effects 0.000 description 7
- 238000010168 coupling process Methods 0.000 description 7
- 238000005859 coupling reaction Methods 0.000 description 7
- 210000003162 effector t lymphocyte Anatomy 0.000 description 7
- 210000004602 germ cell Anatomy 0.000 description 7
- 102000006639 indoleamine 2,3-dioxygenase Human genes 0.000 description 7
- 108020004201 indoleamine 2,3-dioxygenase Proteins 0.000 description 7
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 6
- -1 73K Chemical compound 0.000 description 6
- 239000003795 chemical substances by application Substances 0.000 description 6
- 238000012217 deletion Methods 0.000 description 6
- 230000037430 deletion Effects 0.000 description 6
- 230000006870 function Effects 0.000 description 6
- 238000003780 insertion Methods 0.000 description 6
- 230000037431 insertion Effects 0.000 description 6
- 229910052757 nitrogen Inorganic materials 0.000 description 6
- 229910052700 potassium Inorganic materials 0.000 description 6
- 230000008685 targeting Effects 0.000 description 6
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 5
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 5
- 102220469588 Voltage-dependent L-type calcium channel subunit beta-2_D60A_mutation Human genes 0.000 description 5
- 230000028993 immune response Effects 0.000 description 5
- 238000012004 kinetic exclusion assay Methods 0.000 description 5
- 238000013507 mapping Methods 0.000 description 5
- 229920001184 polypeptide Polymers 0.000 description 5
- 230000035755 proliferation Effects 0.000 description 5
- 102220091023 rs377377296 Human genes 0.000 description 5
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 4
- 102100026122 High affinity immunoglobulin gamma Fc receptor I Human genes 0.000 description 4
- 101000913074 Homo sapiens High affinity immunoglobulin gamma Fc receptor I Proteins 0.000 description 4
- 101000998953 Homo sapiens Immunoglobulin heavy variable 1-2 Proteins 0.000 description 4
- 101000604674 Homo sapiens Immunoglobulin kappa variable 4-1 Proteins 0.000 description 4
- 101000917826 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor II-a Proteins 0.000 description 4
- 102100036887 Immunoglobulin heavy variable 1-2 Human genes 0.000 description 4
- 102100038198 Immunoglobulin kappa variable 4-1 Human genes 0.000 description 4
- 108090000174 Interleukin-10 Proteins 0.000 description 4
- 102000003814 Interleukin-10 Human genes 0.000 description 4
- 102100029204 Low affinity immunoglobulin gamma Fc region receptor II-a Human genes 0.000 description 4
- 241001465754 Metazoa Species 0.000 description 4
- 238000010494 dissociation reaction Methods 0.000 description 4
- 230000005593 dissociations Effects 0.000 description 4
- 230000003993 interaction Effects 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 230000035772 mutation Effects 0.000 description 4
- 238000003127 radioimmunoassay Methods 0.000 description 4
- YPBKTZBXSBLTDK-PKNBQFBNSA-N (3e)-3-[(3-bromo-4-fluoroanilino)-nitrosomethylidene]-4-[2-(sulfamoylamino)ethylamino]-1,2,5-oxadiazole Chemical group NS(=O)(=O)NCCNC1=NON\C1=C(N=O)/NC1=CC=C(F)C(Br)=C1 YPBKTZBXSBLTDK-PKNBQFBNSA-N 0.000 description 3
- 101000998950 Homo sapiens Immunoglobulin heavy variable 1-18 Proteins 0.000 description 3
- 101000998952 Homo sapiens Immunoglobulin heavy variable 1-3 Proteins 0.000 description 3
- 101000998947 Homo sapiens Immunoglobulin heavy variable 1-46 Proteins 0.000 description 3
- 101001037147 Homo sapiens Immunoglobulin heavy variable 1-69 Proteins 0.000 description 3
- 101000989065 Homo sapiens Immunoglobulin heavy variable 7-4-1 Proteins 0.000 description 3
- 101001047520 Homo sapiens Probable non-functional immunoglobulin kappa variable 3-7 Proteins 0.000 description 3
- 102100036884 Immunoglobulin heavy variable 1-18 Human genes 0.000 description 3
- 102100036886 Immunoglobulin heavy variable 1-3 Human genes 0.000 description 3
- 102100036888 Immunoglobulin heavy variable 1-46 Human genes 0.000 description 3
- 102100040232 Immunoglobulin heavy variable 1-69 Human genes 0.000 description 3
- 102100029420 Immunoglobulin heavy variable 7-4-1 Human genes 0.000 description 3
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 3
- 108091028043 Nucleic acid sequence Proteins 0.000 description 3
- 102100022959 Probable non-functional immunoglobulin kappa variable 3-7 Human genes 0.000 description 3
- 241000283984 Rodentia Species 0.000 description 3
- 235000004279 alanine Nutrition 0.000 description 3
- 238000012867 alanine scanning Methods 0.000 description 3
- 239000002246 antineoplastic agent Substances 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 229950006370 epacadostat Drugs 0.000 description 3
- 229940072221 immunoglobulins Drugs 0.000 description 3
- 238000011534 incubation Methods 0.000 description 3
- 230000006698 induction Effects 0.000 description 3
- 208000015181 infectious disease Diseases 0.000 description 3
- 239000003446 ligand Substances 0.000 description 3
- 238000003468 luciferase reporter gene assay Methods 0.000 description 3
- 238000002703 mutagenesis Methods 0.000 description 3
- 231100000350 mutagenesis Toxicity 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 241000894007 species Species 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- ZADWXFSZEAPBJS-SNVBAGLBSA-N (2r)-2-amino-3-(1-methylindol-3-yl)propanoic acid Chemical compound C1=CC=C2N(C)C=C(C[C@@H](N)C(O)=O)C2=C1 ZADWXFSZEAPBJS-SNVBAGLBSA-N 0.000 description 2
- 208000020007 Autosomal agammaglobulinemia Diseases 0.000 description 2
- 241000283690 Bos taurus Species 0.000 description 2
- 229940045513 CTLA4 antagonist Drugs 0.000 description 2
- 241000282472 Canis lupus familiaris Species 0.000 description 2
- 206010009944 Colon cancer Diseases 0.000 description 2
- 108020004635 Complementary DNA Proteins 0.000 description 2
- 241000283073 Equus caballus Species 0.000 description 2
- 206010015866 Extravasation Diseases 0.000 description 2
- 241000282326 Felis catus Species 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 108010004889 Heat-Shock Proteins Proteins 0.000 description 2
- 102000002812 Heat-Shock Proteins Human genes 0.000 description 2
- 241000711549 Hepacivirus C Species 0.000 description 2
- 101100334515 Homo sapiens FCGR3A gene Proteins 0.000 description 2
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 2
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 2
- 241000701044 Human gammaherpesvirus 4 Species 0.000 description 2
- 241000701806 Human papillomavirus Species 0.000 description 2
- 102100027268 Interferon-stimulated gene 20 kDa protein Human genes 0.000 description 2
- 108010002350 Interleukin-2 Proteins 0.000 description 2
- 102000000588 Interleukin-2 Human genes 0.000 description 2
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 2
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 2
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 2
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 2
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 2
- 241000699666 Mus <mouse, genus> Species 0.000 description 2
- YGACXVRLDHEXKY-WXRXAMBDSA-N O[C@H](C[C@H]1c2c(cccc2F)-c2cncn12)[C@H]1CC[C@H](O)CC1 Chemical compound O[C@H](C[C@H]1c2c(cccc2F)-c2cncn12)[C@H]1CC[C@H](O)CC1 YGACXVRLDHEXKY-WXRXAMBDSA-N 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 2
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 2
- 241000282898 Sus scrofa Species 0.000 description 2
- 230000006044 T cell activation Effects 0.000 description 2
- 230000006052 T cell proliferation Effects 0.000 description 2
- 229940126547 T-cell immunoglobulin mucin-3 Drugs 0.000 description 2
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 2
- 239000004473 Threonine Substances 0.000 description 2
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 2
- 101710096997 Tumor necrosis factor ligand superfamily member 18 Proteins 0.000 description 2
- 102100035283 Tumor necrosis factor ligand superfamily member 18 Human genes 0.000 description 2
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 2
- 238000002441 X-ray diffraction Methods 0.000 description 2
- 102220495682 Zinc finger protein 324B_S63G_mutation Human genes 0.000 description 2
- 210000000612 antigen-presenting cell Anatomy 0.000 description 2
- 230000000890 antigenic effect Effects 0.000 description 2
- 230000001363 autoimmune Effects 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 238000010804 cDNA synthesis Methods 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 230000006690 co-activation Effects 0.000 description 2
- 239000002299 complementary DNA Substances 0.000 description 2
- 239000012228 culture supernatant Substances 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 210000004443 dendritic cell Anatomy 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 239000000539 dimer Substances 0.000 description 2
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 2
- 208000035475 disorder Diseases 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 230000002900 effect on cell Effects 0.000 description 2
- 230000036251 extravasation Effects 0.000 description 2
- 208000014829 head and neck neoplasm Diseases 0.000 description 2
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 2
- 229950009034 indoximod Drugs 0.000 description 2
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 2
- 229960000310 isoleucine Drugs 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- 210000002540 macrophage Anatomy 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- MYWUZJCMWCOHBA-VIFPVBQESA-N methamphetamine Chemical compound CN[C@@H](C)CC1=CC=CC=C1 MYWUZJCMWCOHBA-VIFPVBQESA-N 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 210000000822 natural killer cell Anatomy 0.000 description 2
- 210000002501 natural regulatory T cell Anatomy 0.000 description 2
- 239000013642 negative control Substances 0.000 description 2
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 2
- 239000002953 phosphate buffered saline Substances 0.000 description 2
- 102000040430 polynucleotide Human genes 0.000 description 2
- 108091033319 polynucleotide Proteins 0.000 description 2
- 239000002157 polynucleotide Substances 0.000 description 2
- 210000001948 pro-b lymphocyte Anatomy 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 102220003294 rs267606994 Human genes 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 238000004448 titration Methods 0.000 description 2
- 239000013638 trimer Substances 0.000 description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 2
- 229960005486 vaccine Drugs 0.000 description 2
- 239000004474 valine Substances 0.000 description 2
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 1
- ZBMRKNMTMPPMMK-UHFFFAOYSA-N 2-amino-4-[hydroxy(methyl)phosphoryl]butanoic acid;azane Chemical compound [NH4+].CP(O)(=O)CCC(N)C([O-])=O ZBMRKNMTMPPMMK-UHFFFAOYSA-N 0.000 description 1
- 206010061424 Anal cancer Diseases 0.000 description 1
- 208000007860 Anus Neoplasms Diseases 0.000 description 1
- 101000642536 Apis mellifera Venom serine protease 34 Proteins 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 1
- 208000023275 Autoimmune disease Diseases 0.000 description 1
- 108010074708 B7-H1 Antigen Proteins 0.000 description 1
- 102000008096 B7-H1 Antigen Human genes 0.000 description 1
- 241000193830 Bacillus <bacterium> Species 0.000 description 1
- 208000035143 Bacterial infection Diseases 0.000 description 1
- 102220533151 Baculoviral IAP repeat-containing protein 5_C57A_mutation Human genes 0.000 description 1
- 102220533202 Baculoviral IAP repeat-containing protein 5_D71A_mutation Human genes 0.000 description 1
- 102220533147 Baculoviral IAP repeat-containing protein 5_E65A_mutation Human genes 0.000 description 1
- 206010004146 Basal cell carcinoma Diseases 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- 206010006143 Brain stem glioma Diseases 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- 102220586405 CDGSH iron-sulfur domain-containing protein 1_Q76A_mutation Human genes 0.000 description 1
- 102220586401 CDGSH iron-sulfur domain-containing protein 1_S68A_mutation Human genes 0.000 description 1
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 1
- 102000008203 CTLA-4 Antigen Human genes 0.000 description 1
- 102220591067 Cellular tumor antigen p53_T55A_mutation Human genes 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- 102220572027 Claudin-17_R59A_mutation Human genes 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 206010072449 Desmoplastic melanoma Diseases 0.000 description 1
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical group [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 1
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 1
- 238000012286 ELISA Assay Methods 0.000 description 1
- 102220486588 ESF1 homolog_W70A_mutation Human genes 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 102000009109 Fc receptors Human genes 0.000 description 1
- 108010087819 Fc receptors Proteins 0.000 description 1
- 102100027581 Forkhead box protein P3 Human genes 0.000 description 1
- 102220646023 Galectin-10_M73A_mutation Human genes 0.000 description 1
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 description 1
- 208000032612 Glial tumor Diseases 0.000 description 1
- 206010018338 Glioma Diseases 0.000 description 1
- UKKNTTCNGZLJEX-WHFBIAKZSA-N Gln-Ser Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CO)C(O)=O UKKNTTCNGZLJEX-WHFBIAKZSA-N 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 102100034458 Hepatitis A virus cellular receptor 2 Human genes 0.000 description 1
- 101710083479 Hepatitis A virus cellular receptor 2 homolog Proteins 0.000 description 1
- 241000700721 Hepatitis B virus Species 0.000 description 1
- 208000009889 Herpes Simplex Diseases 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101000861452 Homo sapiens Forkhead box protein P3 Proteins 0.000 description 1
- 241000725303 Human immunodeficiency virus Species 0.000 description 1
- 208000005726 Inflammatory Breast Neoplasms Diseases 0.000 description 1
- 206010021980 Inflammatory carcinoma of the breast Diseases 0.000 description 1
- 102000003816 Interleukin-13 Human genes 0.000 description 1
- 108090000176 Interleukin-13 Proteins 0.000 description 1
- 108090000978 Interleukin-4 Proteins 0.000 description 1
- 102000004388 Interleukin-4 Human genes 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- 102000004889 Interleukin-6 Human genes 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- RNKSNIBMTUYWSH-YFKPBYRVSA-N L-prolylglycine Chemical compound [O-]C(=O)CNC(=O)[C@@H]1CCC[NH2+]1 RNKSNIBMTUYWSH-YFKPBYRVSA-N 0.000 description 1
- 102000017578 LAG3 Human genes 0.000 description 1
- 101150030213 Lag3 gene Proteins 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 230000037364 MAPK/ERK pathway Effects 0.000 description 1
- 241000282567 Macaca fascicularis Species 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 241000712079 Measles morbillivirus Species 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 101100407308 Mus musculus Pdcd1lg2 gene Proteins 0.000 description 1
- 101100425749 Mus musculus Tnfrsf18 gene Proteins 0.000 description 1
- 238000005481 NMR spectroscopy Methods 0.000 description 1
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 1
- 108010038807 Oligopeptides Proteins 0.000 description 1
- 102000015636 Oligopeptides Human genes 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 1
- 208000030852 Parasitic disease Diseases 0.000 description 1
- 206010035226 Plasma cell myeloma Diseases 0.000 description 1
- 108700030875 Programmed Cell Death 1 Ligand 2 Proteins 0.000 description 1
- 102100024213 Programmed cell death 1 ligand 2 Human genes 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 102220472212 Protein Wnt-2_C67A_mutation Human genes 0.000 description 1
- 241000589516 Pseudomonas Species 0.000 description 1
- 208000015634 Rectal Neoplasms Diseases 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 description 1
- 206010061934 Salivary gland cancer Diseases 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 108010003723 Single-Domain Antibodies Proteins 0.000 description 1
- 206010041067 Small cell lung cancer Diseases 0.000 description 1
- 241000187747 Streptomyces Species 0.000 description 1
- 102220617491 Syndecan-4_V75A_mutation Human genes 0.000 description 1
- 102000004398 TNF receptor-associated factor 1 Human genes 0.000 description 1
- 108090000920 TNF receptor-associated factor 1 Proteins 0.000 description 1
- 108090000925 TNF receptor-associated factor 2 Proteins 0.000 description 1
- 102000004399 TNF receptor-associated factor 3 Human genes 0.000 description 1
- 108090000922 TNF receptor-associated factor 3 Proteins 0.000 description 1
- 102000003715 TNF receptor-associated factor 4 Human genes 0.000 description 1
- 108090000008 TNF receptor-associated factor 4 Proteins 0.000 description 1
- 102000003718 TNF receptor-associated factor 5 Human genes 0.000 description 1
- 108090000001 TNF receptor-associated factor 5 Proteins 0.000 description 1
- 102100034779 TRAF family member-associated NF-kappa-B activator Human genes 0.000 description 1
- 102220607778 TYRO protein tyrosine kinase-binding protein_E64A_mutation Human genes 0.000 description 1
- 208000024313 Testicular Neoplasms Diseases 0.000 description 1
- 206010057644 Testis cancer Diseases 0.000 description 1
- 208000024770 Thyroid neoplasm Diseases 0.000 description 1
- 102100040247 Tumor necrosis factor Human genes 0.000 description 1
- 102220481607 Tumor necrosis factor receptor superfamily member 14_Y61A_mutation Human genes 0.000 description 1
- 108091005906 Type I transmembrane proteins Proteins 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 102220469585 Voltage-dependent L-type calcium channel subunit beta-2_P62A_mutation Human genes 0.000 description 1
- 102220479829 Voltage-dependent L-type calcium channel subunit beta-2_R56K_mutation Human genes 0.000 description 1
- 206010047741 Vulval cancer Diseases 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 210000005006 adaptive immune system Anatomy 0.000 description 1
- 230000001270 agonistic effect Effects 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 239000003443 antiviral agent Substances 0.000 description 1
- 201000011165 anus cancer Diseases 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 235000009582 asparagine Nutrition 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 230000006472 autoimmune response Effects 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 208000022362 bacterial infectious disease Diseases 0.000 description 1
- 210000003651 basophil Anatomy 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- KQNZDYYTLMIZCT-KQPMLPITSA-N brefeldin A Chemical compound O[C@@H]1\C=C\C(=O)O[C@@H](C)CCC\C=C\[C@@H]2C[C@H](O)C[C@H]21 KQNZDYYTLMIZCT-KQPMLPITSA-N 0.000 description 1
- JUMGSHROWPPKFX-UHFFFAOYSA-N brefeldin-A Natural products CC1CCCC=CC2(C)CC(O)CC2(C)C(O)C=CC(=O)O1 JUMGSHROWPPKFX-UHFFFAOYSA-N 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 210000004970 cd4 cell Anatomy 0.000 description 1
- 230000003915 cell function Effects 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 230000009134 cell regulation Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 230000009137 competitive binding Effects 0.000 description 1
- 239000000306 component Substances 0.000 description 1
- 230000004940 costimulation Effects 0.000 description 1
- 230000000139 costimulatory effect Effects 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 230000016396 cytokine production Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 229910052805 deuterium Inorganic materials 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 238000002050 diffraction method Methods 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 238000002330 electrospray ionisation mass spectrometry Methods 0.000 description 1
- 201000003914 endometrial carcinoma Diseases 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 210000003979 eosinophil Anatomy 0.000 description 1
- 239000013604 expression vector Substances 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 208000005017 glioblastoma Diseases 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 210000003714 granulocyte Anatomy 0.000 description 1
- 210000003128 head Anatomy 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 208000006454 hepatitis Diseases 0.000 description 1
- 231100000283 hepatitis Toxicity 0.000 description 1
- 210000003630 histaminocyte Anatomy 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 201000004653 inflammatory breast carcinoma Diseases 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 230000028709 inflammatory response Effects 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 230000015788 innate immune response Effects 0.000 description 1
- 210000005007 innate immune system Anatomy 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000010212 intracellular staining Methods 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 238000004811 liquid chromatography Methods 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 1
- 208000026037 malignant tumor of neck Diseases 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- 238000012900 molecular simulation Methods 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 201000000050 myeloid neoplasm Diseases 0.000 description 1
- 210000000581 natural killer T-cell Anatomy 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 208000008443 pancreatic carcinoma Diseases 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 210000005134 plasmacytoid dendritic cell Anatomy 0.000 description 1
- 210000004180 plasmocyte Anatomy 0.000 description 1
- 238000011533 pre-incubation Methods 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 108010029020 prolylglycine Proteins 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 206010038038 rectal cancer Diseases 0.000 description 1
- 201000001275 rectum cancer Diseases 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 102220277381 rs1375079282 Human genes 0.000 description 1
- 102200127268 rs137853826 Human genes 0.000 description 1
- 102220341949 rs1555450681 Human genes 0.000 description 1
- 102220077293 rs193922450 Human genes 0.000 description 1
- 102220044576 rs200254470 Human genes 0.000 description 1
- 102200114509 rs201382018 Human genes 0.000 description 1
- 102220133657 rs28909989 Human genes 0.000 description 1
- 102220237139 rs376184349 Human genes 0.000 description 1
- 102220085474 rs72655967 Human genes 0.000 description 1
- 102220099379 rs770702450 Human genes 0.000 description 1
- 102200109183 rs879253818 Human genes 0.000 description 1
- 201000003804 salivary gland carcinoma Diseases 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 238000002864 sequence alignment Methods 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 238000004088 simulation Methods 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 208000000587 small cell lung carcinoma Diseases 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 206010041823 squamous cell carcinoma Diseases 0.000 description 1
- 208000017572 squamous cell neoplasm Diseases 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 201000003120 testicular cancer Diseases 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 208000008732 thymoma Diseases 0.000 description 1
- 201000002510 thyroid cancer Diseases 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 241001529453 unidentified herpesvirus Species 0.000 description 1
- 238000011870 unpaired t-test Methods 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 201000005102 vulva cancer Diseases 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
- 238000002424 x-ray crystallography Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2878—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/14—Blood; Artificial blood
- A61K35/17—Lymphocytes; B-cells; T-cells; Natural killer cells; Interferon-activated or cytokine-activated lymphocytes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/46—Cellular immunotherapy
- A61K39/461—Cellular immunotherapy characterised by the cell type used
- A61K39/4611—T-cells, e.g. tumor infiltrating lymphocytes [TIL], lymphokine-activated killer cells [LAK] or regulatory T cells [Treg]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/46—Cellular immunotherapy
- A61K39/464—Cellular immunotherapy characterised by the antigen targeted or presented
- A61K39/4643—Vertebrate antigens
- A61K39/4644—Cancer antigens
- A61K39/464402—Receptors, cell surface antigens or cell surface determinants
- A61K39/464416—Receptors for cytokines
- A61K39/464417—Receptors for tumor necrosis factors [TNF], e.g. lymphotoxin receptor [LTR], CD30
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/18—Drugs for disorders of the alimentary tract or the digestive system for pancreatic disorders, e.g. pancreatic enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/08—Drugs for disorders of the urinary system of the prostate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/10—Drugs for disorders of the urinary system of the bladder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
- A61P31/22—Antivirals for DNA viruses for herpes viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2809—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against the T-cell receptor (TcR)-CD3 complex
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0636—T lymphocytes
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
- G01N33/5008—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics
- G01N33/502—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics for testing non-proliferative effects
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
- A61K2039/507—Comprising a combination of two or more separate antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K39/46
- A61K2239/31—Indexing codes associated with cellular immunotherapy of group A61K39/46 characterized by the route of administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K39/46
- A61K2239/38—Indexing codes associated with cellular immunotherapy of group A61K39/46 characterised by the dose, timing or administration schedule
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/34—Identification of a linear epitope shorter than 20 amino acid residues or of a conformational epitope defined by amino acid residues
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/75—Agonist effect on antigen
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/20—Cytokines; Chemokines
- C12N2501/25—Tumour necrosing factors [TNF]
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/705—Assays involving receptors, cell surface antigens or cell surface determinants
- G01N2333/70578—NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30 CD40 or CD95
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Definitions
- the present disclosure provides antibodies that specifically bind to human glucocorticoid-induced TNFR family related receptor (GITR) and compositions comprising such antibodies.
- the antibodies specifically bind to human GITR and modulate GITR activity, e.g., enhance, activate or induce GITR activity, utilizing such antibodies.
- the present disclosure also provides methods for treating disorders, such as cancer and infectious diseases, by administering an antibody that specifically binds to human GITR and modulates GITR activity, e.g., enhances, activates or induces GITR activity.
- Glucocorticoid-induced TNFR-related protein a member of the TNFR superfamily, is expressed in many components of the innate and adaptive immune system and stimulates both acquired and innate immunity ( Nocentini G et al., (1994) PNAS 94: 6216-6221 ; Hanabuchi S et al., (2006) Blood 107:3617-3623 ; Nocentini G & Riccardi C (2005) Eur J Immunol 35: 1016-1022 ; Nocentini G et al., (2007) Eur J Immunol 37:1165-1169 ).
- GITR Glucocorticoid-induced TNFR-related protein
- GITRL mainly expressed on Antigen Presenting Cells (APCs), on endothelial cells, and also in tumor cells.
- APCs Antigen Presenting Cells
- the GITR/GITRL system participates in the development of autoimmune/inflammatory responses and potentiates response to infection and tumors.
- treating animals with GITR-Fc fusion protein ameliorates autoimmune/inflammatory diseases while GITR triggering is effective in treating viral, bacterial, and parasitic infections, as well in boosting immune response against tumors ( Nocentini G et al., (2012) Br J Pharmacol 165: 2089-99 ).
- GITR activation increases resistance to tumors and viral infections, is involved in autoimmune/inflammatory processes and regulates leukocyte extravasation (Nocentini G & Riccardi C (2005) supra; Cuzzocrea S et al., (2004) J Leukoc Biol 76: 933-940 ; Shevach EM & Stephens GL (2006) Nat Rev Immunol 6: 613-618 ; Cuzzocrea S et al., (2006) J Immunol 177: 631-641 ; Cuzzocrea S et al., (2007) FASEB J 21: 117-129 ).
- GITR Human GITR is expressed at very low levels in peripheral (non-activated) T cells. After T cell activation, GITR is strongly up-regulated for several days in both CD4 + and CD8 + cells ( Kwon B et al., (1999) J Biol Chem 274: 6056-6061 ; Gurney AL et al., (1999) Curr Biol 9: 215-218 ; Ronchetti S et al., (2004) supra; Shimizu J et al., (2002) supra; Ji HB et al., (2004) supra; Ronchetti S et al., (2002) Blood 100: 350-352 ; Li Z et al., (2003) J Autoimmun 21: 83-92 ), with CD4 + cells having a higher GITR expression than CD8 + cells ( Kober J et al., (2008) Eur J Immunol 38(10): 2678-88 ; Bianchini R et al., (2011) Eur J Immunol 41(8): 2269-
- GITR Given the role of human GITR in modulating immune responses, provided herein are antibodies that specifically bind to GITR and the use of those antibodies to modulate GITR activity.
- an antibody or antigen-binding fragment thereof that specifically binds to GITR partially inhibits GITR ligand (e.g ., human GITRL) from binding to GITR as assessed by a method known to one of skill in the art or described herein (see, e.g., Sections 6.2.5.2 and 6.2.5.4, infra ).
- the antibody or antigen-binding fragment thereof at a concentration of 1000ng/ml inhibits less than 80% of 0.5nM GITRL (e.g ., human GITRL) from binding to GITR coupled to beads (e.g ., human GITR coupled to Luminex® beads) at a concentration of 5pg/ml/bead relative to the binding of 0.5nM GITRL to the GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay.
- 0.5nM GITRL e.g ., human GITRL
- beads e.g ., human GITR coupled to Luminex® beads
- the antibody or antigen-binding fragment thereof inhibits 40% to 70%, 50% to 70%, 50% to 80%, or 40% to 80% of the GITRL (e.g ., human GITRL) from binding to GITR (e.g ., human GITR).
- GITRL e.g ., human GITRL
- GITR e.g ., human GITR
- GITRL e.g ., human GITRL
- the antibody or antigen-binding fragment thereof comprises:
- the antibody or antigen-binding fragment thereof comprises:
- an antibody or antigen-binding fragment thereof that specifically binds to GITR comprising:
- an antibody or antigen-binding fragment thereof that specifically binds to GITR comprising:
- the antibody or antigen-binding fragment thereof comprises a VH CDR1 comprising, consisting of, or consisting essentially of an amino acid sequence selected from the group consisting of SEQ ID NO: 13, 19-23, and 117-119. In certain embodiments, the antibody or antigen-binding fragment thereof comprises a VH CDR1 comprising, consisting of, or consisting essentially of an amino acid sequence selected from the group consisting of 35 and 116. In some embodiments, the antibody or antigen-binding fragment thereof comprises a VH CDR2 comprising, consisting of, or consisting essentially of an amino acid sequence selected from the group consisting of SEQ ID NO: 14, 24-33, and 120-188.
- the antibody or antigen-binding fragment thereof comprises a VH CDR2 comprising, consisting of, or consisting essentially of an amino acid sequence selected from the group consisting of SEQ ID NO: 114, 115, and 194.
- the antibody or antigen-binding fragment thereof comprises a VH CDR3 comprising, consisting of, or consisting essentially of an amino acid sequence selected from the group consisting of SEQ ID NO: 15, 34 and 189.
- the antibody or antigen-binding fragment thereof comprises a VL CDR1 comprising, consisting of, or consisting essentially of an amino acid sequence selected from the group consisting of SEQ ID NO: 16, and 101-104.
- the antibody or antigen-binding fragment thereof comprises a VL CDR2 comprising, consisting of, or consisting essentially of an amino acid sequence selected from the group consisting of SEQ ID NO: 17 and 105.
- the antibody or antigen-binding fragment thereof comprises a VL CDR3 comprising, consisting of, or consisting essentially of an amino acid sequence selected from the group consisting of SEQ ID NO: 18, 106-109, 192, and 193.
- the antibody or antigen-binding fragment thereof comprises the amino acid sequence of the VH CDR1, VH CDR2, and VH CDR3 of an antibody in Table 2.
- the antibody or antigen-binding fragment thereof comprises the amino acid sequence of the VL CDR1, VL CDR2, and VL CDR3 of an antibody in Table 1.
- the antibody or antigen-binding fragment thereof does not prevent GITRL (e.g ., human GITRL) from binding to GITR (e.g ., human GITR) as assessed by a method known to one of skill in the art or described herein (see, e.g., Sections 6.2.5.2 and 6.2.5.4, infra).
- the antibody or antigen-binding fragment thereof at a concentration of 1000ng/ml inhibits less than 80% of 0.5nM GITRL (e.g ., human GITRL) from binding to GITR coupled to beads (e.g ., human GITR coupled to Luminex® beads) at a concentration of 5pg/ml/bead relative to the binding of 0.5nM GITRL to the GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay.
- 0.5nM GITRL e.g ., human GITRL
- beads e.g ., human GITR coupled to Luminex® beads
- the antibody or antigen-binding fragment thereof inhibits 40% to 70%, 50% to 70%, 50% to 80%, or 40% to 80% of the GITRL (e.g ., human GITRL) from binding to GITR ( e.g ., human GITR). In specific embodiments, at least 20% of the amount of GITRL (e.g ., human GITRL) that binds to GITR (e.g.
- human GITR in the absence of the antibody or antigen-binding fragment thereof binds to GITR ( e.g ., human GITR) in the presence of the antibody or antigen-binding fragment thereof in an assay comprising the following steps: (a) coupling GITR ( e.g ., human GITR) to beads at a concentration of 5pg/ml/bead; (b) incubating the GITR ( e.g ., human GITR) coupled beads at a concentration of 40 beads/ ⁇ l with or without the antibody in a well; (c) adding labeled GITRL ( e.g ., labeled human GITRL) to the well to obtain a final concentration of 0.5nM of the GITRL ( e.g ., human GITRL) and 20 beads/ ⁇ l of the GITR coupled beads; and (d) detecting the labeled GITRL ( e.g ., human GITRL) bound to the GITR (e)
- GITRL e.g ., human GITRL
- an antibody or fragment thereof that specifically binds to human GITR comprising: (a) a heavy chain variable region (VH) CDR1 comprising the amino acid sequence of DYAMY (SEQ ID NO: 13); (b) a VH CDR2 comprising the amino acid sequence of VIRTYSGDVTYNQKFKD (SEQ ID NO: 14); (c) a VH CDR3 comprising the amino acid sequence of SGTVRGFAY (SEQ ID NO: 15); (d) a light chain variable region (VL) CDR1 comprising the amino acid sequence of KSSQSLLNSGNQKNYLT (SEQ ID NO: 16); (e) a VL CDR2 comprising the amino acid sequence of WASTRES (SEQ ID NO: 17); and (f) a VL CDR3 comprising the amino acid sequence of QNDYSYPYT (SEQ ID NO: 18).
- an antibody or fragment thereof that specifically binds to human GITR, comprising the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR 3 of an antibody 22 in Table 1 and Table 2.
- the antibody or antigen-binding fragment thereof partially inhibits GITRL (e.g ., human GITRL) from binding to GITR ( e.g. , human GITR) as assessed by a method known to one of skill in the art or described herein (see, e.g., Sections 6.2.5.2 and 6.2.5.4, infra ).
- the antibody or antigen-binding fragment thereof at a concentration of 1000ng/ml inhibits less than 80% of GITR coupled to beads (e.g ., human GITR coupled to Luminex® beads) at a concentration of 5pg/ml/bead relative to the binding of 0.5nM GITRL (e.g ., human GITRL) to the GITR coupled beads (e.g ., human GITR coupled to Luminex® beads) at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay.
- beads e.g ., human GITR coupled to Luminex® beads
- 5pg/ml/bead e.g ., human GITRL
- the antibody or antigen-binding fragment thereof inhibits 50% to 70% of human GITRL binding to human GITR. In specific embodiments, at least 20% of the amount of GITRL (e.g., human GITRL) that binds to GITR (e.g., human GITR) in the absence of the antibody or antigen-binding fragment thereof binds to GITR (e.g., human GITR) in the presence of the antibody or antigen-binding fragment thereof in an assay comprising the following steps: (a) coupling GITR (e.g ., human GITR) to beads at a concentration of 5pg/ml/bead; (b) incubating the GITR ( e.g ., human GITR) coupled beads at a concentration of 40 beads/ ⁇ l with or without the antibody in a well; (c) adding labeled GITRL (e.g ., labeled human GITRL) to the well to obtain a final concentration of 0.5
- 30% to 50% of the amount of human GITRL that binds to human GITR in the absence of the antibody or antigen-binding fragment thereof binds to human GITR in the presence of the antibody or antigen-binding fragment thereof.
- the antibody or antigen-binding fragment thereof is agonistic.
- an antibody or fragment thereof provided herein, which specifically binds to GITR comprises a heavy chain variable region sequence comprising one, two, three or four of the framework regions of the heavy chain variable region sequence of SEQ ID NO: 203.
- an antibody or fragment thereof provided herein, which specifically binds to GITR comprises one, two, three or four framework regions of a heavy chain variable region sequence which is at least 75%, 80%, 85%, 90%, 95% or 100% identical to one, two, three or four of the framework regions of a heavy chain variable region sequence selected from the group consisting of SEQ ID NO: 201, SEQ ID NO: 206, and SEQ ID NOs: 215 to 389.
- an antibody or fragment thereof provided herein, which specifically binds to GITR comprises a heavy chain variable region having human derived framework regions.
- an antibody or antigen-binding fragment thereof provided herein, which specifically binds to GITR comprises a heavy chain variable framework region that is or is derived from an amino acid sequence encoded by a human gene, wherein the amino acid sequence is selected from the group consisting of IGHV1-2*02 (SEQ ID NO: 601), IGHV1-3*01 (SEQ ID NO: 602), IGHV1-46*01 (SEQ ID NO: 603), IGHV1-18*01 (SEQ ID NO: 604), IGHV1-69*01 (SEQ ID NO: 605), and IGHV7-4-1*02 (SEQ ID NO: 606).
- the heavy chain variable framework region that is derived from said amino acid sequence consists of said amino acid sequence with up to 10 amino acid substitutions, deletions, and/or insertions, preferably up to 10 amino acid substitutions.
- the heavy chain variable framework region that is derived from said amino acid sequence consists of said amino acid sequence with 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10 amino acid residues being substituted for an amino acid found in an analogous position in a corresponding non-human heavy chain variable framework region.
- an antibody or fragment thereof provided herein, which specifically binds to GITR comprises a heavy chain variable framework region that is derived from amino acid sequence SEQ ID NO: 601, wherein at least one, two, three, four, or five (in certain embodiments up to 10) amino acids of amino acid sequence SEQ ID NO: 601 is substituted with an amino acid found in an analogous position in a corresponding non-human heavy chain variable framework region.
- the amino acid substitution is at an amino acid position selected from the group consisting of 24, 48, 67, 71, 73, and 94, wherein the amino acid position of each group member is indicated according to the Kabat numbering.
- the amino acid substitution is selected from the group consisting of 24G, 48I, 67A, 71V, 73K, and 94K, wherein the amino acid position of each group member is indicated according to the Kabat numbering
- an antibody or fragment thereof that specifically binds to GITR (e.g ., human GITR) comprising a heavy chain variable region sequence comprising an amino acid sequence selected from the group consisting of SEQ ID NO: 201, 206, and SEQ ID NOS: 215 to 389.
- an antibody or fragment thereof that specifically binds to GITR ( e.g ., human GITR) comprising a heavy chain variable region sequence comprising the amino acid sequence of SEQ ID NO: 203.
- an antibody or fragment thereof that specifically binds to GITR ( e.g ., human GITR) comprising a heavy chain variable region sequence comprising the amino acid sequence of SEQ ID NO: 206.
- an antibody or fragment thereof that specifically binds to GITR comprising a heavy chain sequence comprising an amino acid sequence selected from the group consisting of SEQ ID NOs: 553, 554 and 567 to 570.
- an antibody or fragment thereof that specifically binds to GITR e.g ., human GITR
- a heavy chain sequence comprising an amino acid sequence selected from the group consisting of SEQ ID NOs: 581 and 582.
- an antibody or fragment thereof provided herein, which specifically binds to GITR comprises a light chain variable region sequence comprising one, two, three or four framework regions of the light chain variable region sequence of SEQ ID NO: 204 or SEQ ID NO: 205.
- an antibody or fragment thereof provided herein, which specifically binds to GITR comprises one, two, three or four framework regions of a light chain variable region sequence which is at least 75%, 80%, 85%, 90%, 95%, or 100% identical to one, two, three or four of the framework regions of a light chain variable region sequence selected from the group consisting of SEQ ID NO: 202, SEQ ID NO: 207, SEQ ID NO: 208, and SEQ ID NOs: 400-518.
- an antibody or fragment thereof provided herein, which specifically binds to GITR comprises one, two, three or four framework regions of a light chain variable region sequence which is at least 75%, 80%, 85%, 90%, 95%, or 100% identical to one, two, three or four of the framework regions of the light chain variable region sequence of SEQ ID NO: 519.
- an antibody or fragment thereof provided herein, which specifically binds to GITR comprises a light chain variable sequence having human derived framework regions.
- an antibody or fragment thereof provided herein, which specifically binds to GITR comprises a light chain variable framework region that is or is derived from an amino acid sequence encoded by a human gene, wherein the amino acid sequence is selected from the group consisting of IGKV4-1*01 (SEQ ID NO: 607) and IGKV3-7*02 (SEQ ID NO: 608).
- the light chain variable framework region that is derived from said amino acid sequence consists of said amino acid sequence but for the presence of up to 10 amino acid substitutions, deletions, and/or insertions, preferably up to 10 amino acid substitutions.
- the light chain variable framework region that is derived from said amino acid sequence consists of said amino acid sequence with 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10 amino acid residues being substituted for an amino acid found in an analogous position in a corresponding non-human light chain variable framework region.
- an antibody or fragment thereof provided herein, which specifically binds to GITR comprises a light chain variable framework region that is or is derived from amino acid sequence SEQ ID NO: 607 or SEQ ID NO: 608 wherein at least one, two, three, four, or five (in certain embodiments up to 10) amino acids of amino acid sequence SEQ ID NO: 607 or SEQ ID NO: 608 is substituted with an amino acid found in an analogous position in a corresponding non-human light chain variable framework region.
- the amino acid substitution is at amino acid position 87, wherein the amino acid position is indicated according to the Kabat numbering.
- the amino acid substitution is an amino acid substitution of 87H, wherein the amino acid position is indicated according to the Kabat numbering.
- an antibody or fragment thereof that specifically binds to GITR (e.g ., human GITR) comprising a light chain variable region sequence comprising an amino acid sequence selected from the group consisting of SEQ ID NO: 202, SEQ ID NO: 207, SEQ ID NO: 208 and SEQ ID NOs: 400 to 518.
- an antibody or fragment thereof that specifically binds to GITR (e.g ., human GITR) comprising a light chain variable region sequence comprising the amino acid sequence of SEQ ID NO: 519.
- an antibody or fragment thereof that specifically binds to GITR (e.g ., human GITR) comprising a light chain variable region sequence comprising the amino acid sequence of SEQ ID NO: 204 or SEQ ID NO: 205.
- an antibody or fragment thereof that specifically binds to GITR ( e.g ., human GITR) comprising a light chain variable region sequence comprising the amino acid sequence of SEQ ID NO: 207.
- an antibody or fragment thereof that specifically binds to GITR (e.g ., human GITR) comprising a light chain sequence comprising an amino acid sequence selected from the group consisting of SEQ ID NOs: 555, 556 and 571 to 576.
- an antibody or fragment thereof that specifically binds to GITR (e.g ., human GITR) comprising a heavy chain variable region and a light chain variable region comprising the amino acid sequences of an antibody in the table of Figure 23 or any one of Figures 24A-24C .
- an antibody or fragment thereof that specifically binds to GITR (e.g ., human GITR) comprising a heavy chain variable region and a light chain variable region comprising the amino acid sequences of an antibody in Table 17.
- an antibody or fragment thereof that specifically binds to GITR comprising (a) a heavy chain variable region comprising the amino acid sequence of SEQ ID NO: 206; and (b) a light chain variable region comprising the amino acid sequence of SEQ ID NO: 207.
- an antibody or fragment thereof that specifically binds to GITR comprising (a) a heavy chain variable region comprising the amino acid sequence of SEQ ID NO: 206; and (b) a light chain variable region comprising the amino acid sequence of SEQ ID NO: 208.
- GITR e.g ., human GITR
- VH heavy chain variable region
- VH CDR1 comprises, consists of, or consists essentially of the amino acid sequence of DYAMY (SEQ ID NO: 13)
- VH CDR2 comprises, consists of, or consists essentially of the amino acid sequence of VIRTYSGDVTYNQKFKD (SEQ ID NO: 14)
- VH CDR3 comprises, consists of, or consists essentially of the amino acid sequence of SGTVRGFAY (SEQ ID NO: 15).
- GITR e.g ., human GITR
- VL light chain variable region
- VL CDR1 comprises, consists of, or consists essentially of the amino acid sequence of KSSQSLLNSGNQKNYLT (SEQ ID NO: 16)
- VL CDR2 comprises, consists of, or consists essentially of the amino acid sequence of WASTRES (SEQ ID NO: 17)
- VL CDR3 comprises, consists of, or consists essentially of the amino acid sequence of QNDYSYPYT (SEQ ID NO: 18).
- an antibody or fragment thereof that specifically binds to GITR (e.g ., human GITR) comprising a heavy chain variable region (VH) comprising an amino acid sequence selected from the group consisting of SEQ ID NO: 201, 203, 206 and 215-389.
- VH heavy chain variable region
- VL light chain variable region
- an antibody or fragment thereof that specifically binds to GITR (e.g., human GITR) comprising a light chain variable region (VL) comprising the amino acid sequence of SEQ ID NO: 519.
- the antibody or antigen-binding fragment thereof partially inhibits GITRL (e.g ., human GITRL) from binding to GITR ( e.g ., human GITR) as assessed by a method known to one of skill in the art or described herein (see, e.g., Sections 6.2.5.2 and 6.2.5.4, infra ).
- the antibody or antigen-binding fragment thereof at a concentration of 1000ng/ml inhibits less than 80% of 0.5nM GITRL (e.g ., human GITRL) from binding to GITR coupled to beads (e.g ., human GITR coupled to Luminex® beads) at a concentration of 5pg/ml/bead relative to the binding of 0.5nM GITRL to the GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay.
- 0.5nM GITRL e.g ., human GITRL
- beads e.g ., human GITR coupled to Luminex® beads
- At least 20% of the amount of GITRL (e.g., human GITRL) that binds to GITR (e.g., human GITR) in the absence of the antibody or antigen-binding fragment thereof binds to GITR (e.g., human GITR) in the presence of the antibody or antigen-binding fragment thereof in an assay comprising the following steps: (a) coupling GITR ( e.g ., human GITR) to beads at a concentration of 5pg/ml/bead; (b) incubating the GITR ( e.g ., human GITR) coupled beads at a concentration of 40 beads/ ⁇ l with or without the antibody in a well; (c) adding labeled GITRL ( e.g ., labeled human GITRL) to the well to obtain a final concentration of 0.5nM of the GITRL ( e.g ., human GITRL) and 20 beads/ ⁇ l of the GITR coupled
- GITRL e.g ., human GITRL
- an antibody or fragment thereof that specifically binds to GITR (e.g., human GITR) comprising a heavy chain variable region (VH) and a light chain variable region (VL), wherein the VH and VL comprise the amino acid sequence of an antibody in Figure 23 or any one of Figures 24A-24C .
- an antibody or fragment thereof that specifically binds to GITR (e.g., human GITR) comprising a heavy chain variable region (VH) and a light chain variable region (VL), wherein the VH and VL comprise the amino acid sequence of an antibody in Table 17.
- an antibody provided herein which specifically binds to GITR (e.g ., human GITR), comprises heavy and/or light chain constant regions.
- the heavy chain constant region is selected from the group of human immunoglobulins consisting of IgG 1 , IgG 2 , IgG 3 , IgG 4 , IgA 1 , and IgA 2 .
- the light chain constant region is selected from the group of human immunoglobulins consisting of IgG ⁇ and IgG ⁇ .
- the IgG 1 is non-fucosylated IgG 1 .
- the antibody is an IgG 1 which comprises a N297A or N297Q mutation. In another specific embodiment, the antibody is an IgG 4 which comprises a S228P mutation. In another specific embodiment, the antibody is an IgG2 which comprises a C127S mutation.
- an antibody provided herein, which specifically binds to GITR comprises a heavy chain constant region comprising an amino acid sequence selected from the group consisting of SEQ ID NO: 557-562.
- an antibody provided herein which specifically binds to GITR (e.g ., human GITR), comprises a heavy chain constant region comprising an amino acid sequence selected from the group consisting of SEQ ID NO: 583 and 584.
- an antibody provided herein, which specifically binds to GITR comprises a heavy chain constant region comprising an amino acid sequence selected from the group consisting of SEQ ID NOs: 557-560 with an amino acid substitution of N to A or Q at amino acid position 180.
- an antibody provided herein, which specifically binds to GITR comprises a light chain constant region comprising an amino acid sequence selected from the group consisting of SEQ ID NO: 588-591.
- an antibody provided herein, which specifically binds to GITR comprises a light chain constant region comprising an amino acid sequence selected from the group consisting of SEQ ID NO: 563-566.
- an antibody provided herein which specifically binds to GITR (e.g ., human GITR), comprises (a) a heavy chain comprising an amino acid sequence selected from the group consisting of SEQ ID NO: 553, 554, and 567 to 570; and (b) a light chain comprising an amino acid sequence selected from the group consisting of SEQ ID NO: 555, 556, and 571 to 576.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 553; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 556.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 554; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 556.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 581; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 556.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 582; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 556.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 553; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 555.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 554; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 555.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 567; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 573.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 567; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 576.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 554; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 576.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 581; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 576.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 582; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 576.
- an antibody provided herein which specifically binds to GITR (e.g ., human GITR), comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 553 with an amino acid substitution of N to A or Q at amino acid position 298; and (b) a light chain comprising an amino acid sequence selected from the group consisting of SEQ ID NO: 555, 556, and 571 to 576.
- an antibody provided herein which specifically binds to GITR (e.g ., human GITR), comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 553 with an amino acid substitution of N to A or Q at amino acid position 298; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 556.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 553 with an amino acid substitution of N to A or Q at amino acid position 298; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 555.
- an antibody or fragment thereof that binds to the same epitope of GITR (e.g ., human GITR) as the antibody described herein.
- an isolated antibody that specifically binds to each of i) human GITR, wherein the human GITR comprises residues 26-241 of SEQ ID NO: 701 and ii) a variant of cynomolgus GITR comprising residues 26-234 of SEQ ID NO: 699, wherein the antibody does not specifically bind to cynomolgus GITR comprising residues 26-234 of SEQ ID NO: 704.
- an isolated antibody that specifically binds to each of i) human GITR, wherein the human GITR comprises residues 26-241 of SEQ ID NO: 701 and ii) a variant of cynomolgus GITR comprising residues 26-234 of SEQ ID NO: 699, wherein the antibody does not exhibit substantial binding to cynomolgus GITR comprising residues 26-234 of SEQ ID NO: 704.
- an isolated antibody that specifically binds to human GITR, wherein the human GITR comprises residues 26-241 of SEQ ID NO: 701, wherein the binding between the antibody and a variant GITR is substantially weakened relative to the binding between the antibody and the human GITR, and wherein the variant GITR comprises residues 26-241 of SEQ ID NO: 701 except for an amino acid substitution selected from the group consisting of D60A and G63A.
- the substitution is D60A.
- the substitution is G63A.
- an isolated antibody that specifically binds to human GITR, wherein the human GITR comprises residues 26-241 of SEQ ID NO: 701, and wherein the antibody binds to an epitope comprising residues 60-63 of SEQ ID NO: 701.
- an isolated antibody that specifically binds to human GITR, wherein the human GITR comprises residues 26-241 of SEQ ID NO: 701, and wherein the antibody binds to at least one residue within the amino acid sequence set forth by residues 60-63 of SEQ ID NO: 701.
- the antibody binds to at least one residue selected from the group consisting of residues 60, 62, and 63 of SEQ ID NO: 701.
- the antibody binds to at least one residue selected from the group consisting of residues 62 and 63 of SEQ ID NO: 701. In one embodiment, the antibody activates or enhances an activity of the human GITR. In another embodiment, provided herein is an isolated antibody that specifically binds human GITR, wherein the human GITR comprises residues 26-241 of SEQ ID NO:701, and wherein the antibody binds an epitope of the human GITR comprising at least one of residues 60 or 63 of SEQ ID NO:701.
- an isolated antibody that specifically binds to human GITR, wherein the antibody exhibits, as compared to binding to human GITR, reduced or absent binding to a protein identical to human GITR except for the presence of a D60A or G63A amino acid substitution.
- the antibody induces, activates or enhances an activity of the human GITR.
- an antibody or antigen-binding fragment thereof that competes with an antibody or antigen-binding fragment thereof described herein for binding to GITR ( e.g ., human GITR).
- an antibody or antigen-binding fragment thereof that competes with antibody or antigen-binding fragment thereof described herein for binding to GITR (e.g ., human GITR) to the extent that the antibody or antigen-binding fragment thereof described herein self-competes for binding to GITR (e.g ., human GITR).
- a first antibody or antigen-binding fragment thereof that competes with an antibody or antigen-binding fragment thereof described herein for binding to GITR (e.g ., human GITR), wherein the first antibody or antigen-binding fragment thereof competes for binding in an assay comprising the following steps: (a) incubating GITR-transfected cells with the first antibody or antigen-binding fragment thereof in unlabeled form in a container; (b) adding the antibody or antigen-binding fragment thereof described herein in labeled form to the cells in the container and incubating the cells in the container; and (c) detecting the binding of the antibody or antigen-binding fragment thereof described herein in labeled form to the cells.
- GITR e.g ., human GITR
- a first antibody or antigen-binding fragment thereof competes with an antibody or antigen-binding fragment thereof described herein for binding to GITR (e.g ., human GITR), wherein the competition is exhibited as reduced binding of first antibody or antigen-binding fragment thereof to GITR ( e.g ., human GITR) by more than 80% ( e.g ., 85%, 90%, 95%, or 98%, or between 80% to 85%, 80% to 90%, 85% to 90%, or 85% to 95%).
- an antibody or fragment thereof provided herein, which specifically binds to GITR activates, induces or enhances an activity of GITR ( e.g ., human GITR).
- an antibody provided herein, which specifically binds to GITR is a humanized antibody, murine antibody or chimeric antibody.
- an antibody provided herein, which specifically binds to GITR e.g ., human GITR
- an antibody provided herein, which specifically binds to GITR comprises a detectable label.
- an antibody provided herein is isolated.
- an antibody or fragment described herein which immunospecifically binds to GITR (e.g ., human GITR), induces, activates or enhances an activity of human GITR in a cell independent of TCR triggering.
- the cell is a T cell. In specific embodiments, the cell is not a T cell.
- the cell is selected from the group consisting of a B cell, a plasma cell, a memory cell, a natural killer cell, a granulocyte, a neutrophil, an eosinophil, a basophil, a mast cell, a monocyte, a dendritic cell, a plasmacytoid dendritic cell, an NKT cell, and a macrophage.
- an antibody described herein which immunospecifically binds to GITR (e.g ., human GITR), induces, activates, or enhances an activity of NF- ⁇ B independent of TCR triggering.
- the activity of NF- ⁇ B can be assessed in, e.g., an assay comprising the following steps: (a) incubating T cells (e.g ., Jurkat cells) expressing a NF- ⁇ B-luciferase reporter construct (e.g ., GloResponse NF- ⁇ B-luc2P construct) and GITR ( e.g ., human GITR) with the antibody described herein or an isotype control antibody at an antibody concentration of, e.g., 12.5, 10, 5, 2.5, 1.25, or 0.625 ⁇ g/ml, in the absence of an anti-CD3 antibody; and (b) reading luciferase signal after, e.g., 2, 5, 6, 8 or 18 hours of incubation using, e.g., an EnVision multilabel reader 2100, wherein a positive luciferase signal relative to the isotype control antibody indicates activity of NF- ⁇ B.
- the luciferase reporter construct e.g
- an antibody or fragment described herein, which immunospecifically binds to GITR increases the percentage of polyfunctional (IFN ⁇ + TNF ⁇ +) T cells.
- the increase in the percentage of polyfunctional (IFN ⁇ + TNF ⁇ +) T cells can be assessed in, e.g., an assay comprising the following steps: (a) incubating, e.g., human PBMCs with, e.g., an anti-CD3 antibody at various suboptimal concentrations ( e.g ., 0.3-5 ⁇ g/ml); and, e.g., an antibody described herein, which immunospecifically binds to GITR ( e.g ., human GITR), at, e.g., 5 ⁇ g/ml or an isotype control antibody, for, e.g., 3-4 days at 37°C and 5% CO 2 ; (b) treating cells with, e.g., Brefeld
- the polyfunctional (IFN ⁇ + TNF ⁇ +) T cells are selected from the group consisting of polyfunctional (IFN ⁇ + TNF ⁇ +) CD4+ T cells and polyfunctional (IFN ⁇ + TNF ⁇ +) CD8+ T cells.
- an antibody described herein which immunospecifically binds to GITR (e.g ., human GITR), when bound to activated regulatory T cells, binds to activating Fc gamma receptors selected from the group consisting of CD 16, CD32A and CD64 to a greater extent (e.g ., 1.2 fold, 1.3 fold, 1.4 fold, 1.5 fold, 2 fold, 2.5 fold, 3 fold, 3.5 fold, 4 fold, 4.5 fold, 5 fold, 6 fold, 7 fold, 8 fold, 9 fold, 10 fold, 15 fold, 20 fold, 30 fold, 40 fold, 50 fold, 60 fold, 70 fold, 80 fold, 90 fold, or 100 fold) than the antibody, when bound to activated effector T cells, binds to the activating Fc gamma receptors selected from the group consisting of CD16, CD32A and CD64, as assessed by methods described herein or known to one of skill in the art (e.g ., an Fc gamma receptor IIIA (CD16) reporter
- the activating Fc gamma receptors are expressed on a cell selected from the group consisting of myeloid-derived effector cells and lymphocyte-derived effector cells.
- the activating Fc gamma receptor is CD16.
- an antibody described herein which immunospecifically binds to GITR (e.g ., human GITR), when bound to activated regulatory T cells, causes stronger activation of activating Fc gamma receptors selected from the group consisting of CD16, CD32A and CD64 than the antibody, when bound to activated effector T cells, causes activation of activating Fc gamma receptors selected from the group consisting of CD16, CD32A and CD64.
- GITR e.g ., human GITR
- the activation of the activating Fc gamma receptors when the antibody described herein, which immunospecifically binds to GITR ( e.g ., human GITR), is bound to activated regulatory T cells, is at least about 1.2 fold, 1.3 fold, 1.4 fold, 1.5 fold, 2 fold, 2.5 fold, 3 fold, 3.5 fold, 4 fold, 4.5 fold, 5 fold, 6 fold, 7 fold, 8 fold, 9 fold, 10 fold, 15 fold, 20 fold, 30 fold, 40 fold, 50 fold, 60 fold, 70 fold, 80 fold, 90 fold, or 100 fold stronger than the activation of the activating Fc gamma receptors, when the antibody described herein, which immunospecifically binds to GITR ( e.g ., human GITR), is bound to activated effector T cells, as assessed by methods described herein or known to one of skill in the art (e.g ., an Fc gamma receptor IIIA (CD16) reporter assay or as described in the Examples,
- the activating Fc gamma receptors are expressed on a cell selected from the group consisting of myeloid-derived effector cells and lymphocyte-derived effector cells.
- the activating Fc gamma receptor is CD16.
- an antibody or fragment described herein which immunospecifically binds to GITR (e.g ., human GITR), increases surface expression of OX40 and/or PD-1 in activated T cells by at least about 1.2 fold, 1.3 fold, 1.4 fold, 1.5 fold, 2 fold, 2.5 fold, 3 fold, 3.5 fold, 4 fold, 4.5 fold, 5 fold, 6 fold, 7 fold, 8 fold, 9 fold, 10 fold, 15 fold, 20 fold, 30 fold, 40 fold, 50 fold, 60 fold, 70 fold, 80 fold, 90 fold, 100 fold, 200 fold, 300 fold, 400 fold, 500 fold, 600 fold, 700 fold, 800 fold, 900 fold, or 1000 fold as assessed by methods described herein and/or known to one of skill in the art, relative to surface expression of OX40 and/or PD-1 in activated T cells without the antibody described herein.
- nucleic acid molecules encoding a heavy chain variable region and/or a light chain variable region, or a light chain and/or a heavy chain of an antibody described herein.
- the nucleic acid molecule encodes a heavy chain variable region comprising the nucleic acid sequence of SEQ ID NO: 209.
- the nucleic acid molecule encodes a light chain variable region comprising the nucleic acid sequence of SEQ ID NO: 210 or SEQ ID NO: 211.
- the nucleic acid molecule is isolated.
- a vector (e.g., an isolated vector) comprises a nucleic acid molecule encoding a heavy chain variable region and/or a light chain variable region, or a light chain and/or a heavy chain of an antibody described herein.
- a host cell comprises the nucleic acid molecule or vector. Examples of host cells include E.
- coli Pseudomonas, Bacillus, Streptomyces, yeast, CHO, YB/20, NS0, PER-C6, HEK-293T, NIH-3T3, HeLa, BHK, Hep G2, SP2/0, R1.1, B-W, L-M, COS 1, COS 7, BSC1, BSC40, BMT10 cells, plant cells, insect cells, and human cells in tissue culture.
- GITR e.g ., human GITR
- TCR T cell receptor
- PHA phytohaemagglutinin
- PMA phorbol myristate acetate
- a TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody
- a method for activating T cells comprising incubating ex vivo T cells with an antibody or antigen-binding fragment thereof described herein.
- the method further comprises, prior to, simultaneously with, or subsequent to the incubation with the anti-GITR antibody or antigen-binding fragment thereof, incubating ex vivo the T cells with a TCR complex stimulating agent (e.g ., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody).
- a TCR complex stimulating agent e.g ., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody.
- PHA phytohaemagglutinin
- PMA phorbol myristate acetate
- TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody.
- the T cells were isolated from a subject.
- the stimulated and/or activated T cells are
- a method for preferential expansion of effector T-cells over that of T-regulatory cells in a subject comprising administering to the subject an effective amount of an antibody or antigen-binding fragment thereof described herein.
- the subject is human.
- T cells e.g ., CD4 + and/or CD8 + T cells
- T cell effector function comprising incubating ex vivo the T cells with an antibody or antigen-binding fragment thereof described herein.
- the method further comprises, prior to, simultaneously with or subsequent to incubating the T cells with the antibody or antigen-binding fragment thereof, incubating the T cells with a T cell receptor (TCR) complex stimulating agent (e.g ., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody).
- TCR T cell receptor
- PHA phytohaemagglutinin
- PMA phorbol myristate acetate
- TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody
- the T cells were isolated from a subject. In some embodiments, the method further comprises infusing the T cells after their expansion and/or after their effector function is enhanced into a subject. In certain embodiments, the T cells being infused into the subject are autologous or allogenic. In a specific embodiment, the subject is a human.
- a method for enhancing the expansion of CD8 + T cells comprising incubating ex vivo the T cells with an antibody or antigen-binding fragment thereof described herein.
- the method further comprises, prior to, simultaneously with or subsequent to incubating the T cells with the antibody or antigen-binding fragment thereof, incubating the T cells with a T cell receptor (TCR) complex stimulating agent (e.g ., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody).
- TCR T cell receptor
- PHA phytohaemagglutinin
- PMA phorbol myristate acetate
- a TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody.
- the T cells were isolated from a subject.
- the method further comprises infusing the T cells after their expansion and/or after their effector function is enhanced into a subject.
- the T cells being infused into the subject are autologous or allogenic.
- the subject is a human.
- a method for enhancing the expansion of CD4 + T cells comprising incubating ex vivo the T cells with an antibody or antigen-binding fragment thereof described herein.
- the method further comprises, prior to, simultaneously with or subsequent to incubating the T cells with the antibody or antigen-binding fragment thereof, incubating the T cells with a T cell receptor (TCR) complex stimulating agent (e.g ., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody).
- TCR T cell receptor
- PHA phytohaemagglutinin
- PMA phorbol myristate acetate
- a TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody.
- the T cells were isolated from a subject.
- the method further comprises infusing the T cells after their expansion and/or after their effector function is enhanced into a subject.
- the T cells being infused into the subject are autologous or allogenic.
- the subject is a human.
- a method of activating GITR or activating NF- ⁇ B comprising incubating ex vivo T cells, which have not been stimulated with a T cell receptor (TCR) complex stimulating agent (e.g ., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody), with an antibody or antigen-binding fragment thereof described herein.
- TCR T cell receptor
- the T cells were isolated from a subject.
- the activated T cells are infused into a subject.
- the T cells being infused into the subject are autologous or allogenic.
- the subject is a human.
- provided herein is a method of activating T cells independent of TCR triggering comprising contacting T cells with an antibody or antigen-binding fragment thereof described herein.
- provided herein is a method of inducing, activating or enhancing an activity of NF- ⁇ B independent of TCR triggering comprising contacting T cells with an antibody or antigen-binding fragment thereof described herein.
- provided herein is a method of increasing the percentage of polyfunctional (IFN ⁇ + TNF ⁇ +) T cells comprising contacting T cells with an antibody or antigen-binding fragment thereof described herein.
- provided herein is a method of increasing surface expression of OX40 and/or PD-1 in activated T cells comprising contacting T cells with an antibody or antigen-binding fragment thereof described herein.
- compositions comprising an antibody or antigen-binding fragment thereof, a nucleic acid molecule, a vector, or a host cell described herein, and a pharmaceutically acceptable carrier.
- the pharmaceutical composition can be used to modulate immune response and/or to treat and/or prevent a disorder, such as cancer or an infectious disease.
- a method of modulating an immune response in a subject comprising administering to the subject an effective amount of the pharmaceutical composition described herein.
- the immune response is enhanced or induced.
- provided herein is a method for enhancing the expansion of T cells and/or T cell effector function in a subject, comprising administering to the subject an effective amount of a pharmaceutical composition described herein.
- a method for enhancing the expansion of CD8+ T cells in a subject comprising administering to the subject an effective amount of a pharmaceutical composition described herein.
- the disclosure provides use of an antibody as described herein in the manufacture of a medicament for the treatment of cancer.
- the disclosure provides an antibody as described herein for use in the treatment of cancer.
- the disclosure provides use of a pharmaceutical composition as described herein in the manufacture of a medicament for the treatment of cancer.
- the disclosure provides a pharmaceutical composition as described herein for use in the treatment of cancer.
- a method of treating cancer in a subject comprising administering to the subject an effective amount of the pharmaceutical composition described herein.
- the method of treating cancer further comprises administering an anti-cancer agent to the subject.
- anti-cancer agents that can be administered to a subject in combination with a pharmaceutical composition described herein are described in Section 5.4, infra ( e.g ., Sections 5.4.1 and 5.4.1.1).
- the anti-cancer agent is a vaccine.
- the vaccine comprises a heat shock protein peptide complex (HSPPC), in which the HSPPC comprises a heat shock protein (e.g ., a gp96 protein) complexed with one or more antigenic peptides (e.g ., tumor-associated antigenic peptides).
- HSPPC heat shock protein peptide complex
- the HSPPC comprises a heat shock protein (e.g ., a gp96 protein) complexed with one or more antigenic peptides (e.g ., tumor-associated antigenic peptides).
- the cancer treated is squamous cell cancer, small-cell lung cancer, non-small cell lung cancer, gastrointestinal cancer, Hodgkin's or non-Hodgkin's lymphoma, pancreatic cancer, glioblastoma, glioma, cervical cancer, ovarian cancer, liver cancer, bladder cancer, breast cancer, colon cancer, colorectal cancer, endometrial carcinoma, myeloma, salivary gland carcinoma, kidney cancer, basal cell carcinoma, melanoma, prostate cancer, vulval cancer, thyroid cancer, testicular cancer, esophageal cancer, or a type of head or neck cancer.
- the cancer treated is desmoplastic melanoma, inflammatory breast cancer, thymoma, rectal cancer, anal cancer, or surgically treatable or non-surgically treatable brain stem glioma.
- the subject treated is a human.
- provided herein is a method for activating T cells independent of TCR triggering in a subject, comprising administering to the subject an effective amount of the pharmaceutical composition described herein.
- provided herein is a method for inducing, activating or enhancing an activity of NF- ⁇ B independent of TCR triggering in a subject, comprising administering to the subject an effective amount of the pharmaceutical composition described herein.
- provided herein is a method for increasing the percentage of polyfunctional (IFN ⁇ + TNF ⁇ +) T cells in a subject, comprising administering to the subject an effective amount of the pharmaceutical composition described herein.
- provided herein is a method for increasing surface expression of OX40 and/or PD-1 in activated T cells in a subject, comprising administering to the subject an effective amount of the pharmaceutical composition described herein.
- the antibody as described herein can be used in combination with an IDO inhibitor for treating cancer.
- a method of treating cancer in a subject comprising administering to the subject an effective amount of the pharmaceutical composition described herein, wherein the method further comprises administering to the subject an inhibitor of indoleamine-2,3-dioxygenase (IDO).
- IDO indoleamine-2,3-dioxygenase
- the IDO inhibitor as described herein for use in treating cancer is present in a solid dosage form of a pharmaceutical composition such as a tablet, a pill or a capsule, wherein the pharmaceutical composition includes an IDO inhibitor and a pharmaceutically acceptable excipient.
- the antibody as described herein and the IDO inhibitor as described herein can be administered separately, sequentially or concurrently as separate dosage forms.
- the antibody is administered parenterally and the IDO inhibitor is administered orally.
- the inhibitor is selected from the group consisting of epacadostat (Incyte Corporation), F001287 (Flexus Biosciences), indoximod (NewLink Genetics), and NLG919 (NewLink Genetics).
- Epacadostat has been described in PCT Publication No. WO 2010/005958 , which is incorporated herein by reference in its entirety for all purposes.
- the inhibitor is epacadostat.
- the inhibitor is F001287.
- the inhibitor is indoximod.
- the inhibitor is NLG919.
- the antibody as described herein can be used in combination with a checkpoint targeting agent for treating cancer.
- a method of treating cancer in a subject comprising administering to the subject an effective amount of the pharmaceutical composition described herein, wherein the method further comprises administering to the subject a checkpoint targeting agent.
- the checkpoint targeting agent is selected from the group consisting of an antagonist of PD-1 (e.g ., an antagonist anti-PD-1 antibody), an antagonist of PD-L1 (e.g ., an antagonist anti-PD-Ll antibody), an antagonist of PD-L2 ( e.g ., an antagonist anti-PD-L2 antibody), an antagonist of CTLA-4 ( e.g ., an antagonist anti-CTLA-4 antibody), an antagonist of TIM-3 ( e.g ., an antagonist anti-TIM-3 antibody), an antagonist of LAG-3 ( e.g ., an antagonist anti-LAG-3 antibody), and an agonist of OX40 (e.g ., an agonist anti-OX40 antibody).
- an antagonist of PD-1 e.g ., an antagonist anti-PD-1 antibody
- an antagonist of PD-L1 e.g ., an antagonist anti-PD-Ll antibody
- an antagonist of PD-L2 e.g ., an antagonist anti-PD-L2 antibody
- a checkpoint targeting agent e.g., an antagonist of PD-1 (e.g ., an antagonist anti-PD-1 antibody) or an agonist of OX40 (e.g ., an agonist anti-OX40 antibody) is administered simultaneously with the anti-GITR antibody.
- a checkpoint targeting agent e.g., an antagonist of PD-1 (e.g ., an antagonist anti-PD-1 antibody) or an agonist of OX40 (e.g ., an agonist anti-OX40 antibody) is administered prior to the administration of the anti-GITR antibody.
- a checkpoint targeting agent e.g., an antagonist of PD-1 (e.g ., an antagonist anti-PD-1 antibody) or an agonist of OX40 (e.g ., an agonist anti-OX40 antibody) is administered subsequent to the administration of the anti-GITR antibody.
- the antibody as described herein can be used in combination with an anti-CD25 antibody.
- an anti-CD25 antibody is administered simultaneously with the anti-GITR antibody.
- an anti-CD25 antibody is administered prior to the administration of the anti-GITR antibody.
- an anti-CD25 antibody is administered subsequent to the administration of the anti-GITR antibody.
- a method of treating cancer in a subject comprising administering an antagonist anti-PD-1 antibody to a subject in need thereof who has received an anti-GITR antibody, wherein the PD-1 antibody is administered at a time at which the anti-GITR antibody has increased expression of PD-1 in the subject relative to expression of PD-1 in the subject at the time of the administering.
- a method of treating cancer in a subject comprising administering an agonist anti-OX40 antibody to a subject in need thereof who has received an anti-GITR antibody, wherein the OX40 antibody is administered at a time at which the anti-GITR antibody has increased expression of OX40 in the subject relative to expression of OX40 in the subject at the time of the administering.
- the anti-GITR antibody induces, activates, or enhances an activity of GITR.
- provided herein is a method of treating cancer in a subject comprising administering an anti-GITR antibody to a subject in need thereof, wherein the anti-GITR antibody increases expression of PD-1 in the subject relative to expression of PD-1 in the subject at the time of the administering, and administering an antagonist anti-PD-1 antibody to the subject when expression of PD-1 is increased.
- a method of treating cancer in a subject comprising administering an anti-GITR antibody to a subject in need thereof, wherein the anti-GITR antibody increases expression of OX40 in the subject relative to expression of OX40 in the subject at the time of the administering, and administering an agonist OX40 antibody to the subject when expression of OX40 is increased.
- the anti-GITR antibody induces, activates, or enhances an activity of GITR.
- a method of treating cancer or a viral infection in a subject comprising the steps of: (a) incubating T cells ex vivo with an antibody or antigen-binding fragment thereof described herein; and (b) infusing the T cells into the subject.
- the T cells infused into the subject are autologous or allogenic.
- the T cells were isolated from a subject.
- the T cells are not incubated with a T cell receptor (TCR) complex stimulating agent (e.g ., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody).
- TCR T cell receptor
- PHA phytohaemagglutinin
- PMA phorbol myristate acetate
- a TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody.
- the method further comprises, prior to step (a): (1) assaying the T cells for cell surface expression of GITR; and (2) if step (1) does not result in detection of GITR above a threshold value, inducing expression of GITR on the surface of the T cells by incubating the T cells with a T cell receptor (TCR) complex stimulating agent (e.g ., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody).
- TCR T cell receptor
- PHA phytohaemagglutinin
- PMA phorbol myristate acetate
- a TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody
- the method further comprises, prior to, simultaneously with or subsequent to step (a), incubating the T cells with a T cell receptor (TCR) complex stimulating agent phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody).
- TCR T cell receptor
- PHA phytohaemagglutinin
- PMA phorbol myristate acetate
- a TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody.
- the subject treated is a human.
- provided herein is a method of treating and/or preventing an infectious disease in a subject comprising administering to the subject an effective amount of a pharmaceutical composition described herein. See Section 5.4.1.2 below for examples of infectious diseases.
- a method of treating a viral infection in a subject comprising administering to the subject an effective amount of the pharmaceutical composition described herein.
- the viral infection treated is caused by a human papilloma virus (HPV), a Herpes simplex or other herpes virus, hepatitis B virus (HBV), hepatitis C virus (HCV) or other hepatitis virus, measles virus, HIV or Epstein Barr virus (EBV).
- the method of treating a viral infection further comprises administering an anti-viral agent to the subject.
- the subject treated is a human.
- a method of identifying an anti-GITR antibody that is capable of inducing, activating, or enhancing an activity of GITR in the absence of a TCR agonist comprising contacting a cell expressing GITR with an anti-GITR antibody in the absence of a TCR agonist and measuring GITR activity, wherein increased GITR activity compared to GITR activity in the absence of the anti-GITR antibody indicates the anti-GITR antibody is capable of inducing, activating, or enhancing an activity of GITR in the absence of a TCR agonist.
- the GITR activity is assessed by measuring NF- ⁇ B activity.
- the GITR activity is assessed by measuring activation of TRAF adaptor mediated signaling pathways, wherein the TRAF adaptor is selected from the group consisting of TRAF1, TRAF2, TRAF3, TRAF4, and TRAF5. In certain embodiments, the GITR activity is assessed by measuring activation of the MAPK/ERK pathway.
- the anti-GITR antibody increases the GITR activity at least two-fold compared to GITR activity in the absence of the anti-GITR antibody. In certain embodiments, the anti-GITR antibody increases the GITR activity two-fold to twenty-fold compared to GITR activity in the absence of the anti-GITR antibody.
- the anti-GITR antibody increases the GITR activity two-fold to ten-fold compared to GITR activity in the absence of the anti-GITR antibody.
- the cell is a T cell. In certain embodiments, the cell is not a T cell.
- an anti-GITR antibody that specifically binds to human GITR, wherein said antibody is capable of inducing, activating, or enhancing an activity of GITR in a cell in the absence of TCR triggering.
- an anti-GITR antibody that specifically binds to human GITR, wherein said antibody induces, activates, or enhances an activity of NF- ⁇ B in a cell in the absence of TCR triggering.
- a method of treating cancer comprising administering to a subject in need thereof an anti-GITR antibody that specifically binds to human GITR, wherein said antibody is capable of inducing, activating, or enhancing an activity of GITR and/or NF- ⁇ B in the absence of TCR triggering.
- the binding between a test antibody and a first antigen is "substantially weakened" relative to the binding between the test antibody and a second antigen if the binding between the test antibody and the first antigen is reduced by at least 30%, 40%, 50%, 60%, 70%, or 80% relative to the binding between the test antibody and the second antigen, e.g., in a given experiment, or using mean values from multiple experiments, as assessed by, e.g., an assay comprising the following steps: (a) expressing on the surface of cells (e.g ., 1624-5 cells) the first antigen or the second antigen; (b) staining the cells expressing the first antigen or the second antigen using, e.g., 2 ⁇ g/ml of the test antibody or a polyclonal antibody in a flow cytometry analysis and recording mean fluorescence intensity (MFI) values, e.g., as the mean from more than one measurement, wherein the polyclonal antibody recognizes both the first antigen and
- MFI mean
- an antibody does not exhibit "substantial binding" to an antigen if when measured in a flow cytometry analysis, the mean fluorescence intensity (MFI) value of the antibody to the antigen is not significantly higher than the MFI value of an isotype control antibody to the antigen or the MFI value in the absence of any antibody.
- MFI mean fluorescence intensity
- antibody and “antibodies” are terms of art and can be used interchangeably herein and refer to a molecule with an antigen binding site that specifically binds an antigen.
- Antibodies can include, for example, monoclonal antibodies, recombinantly produced antibodies, monospecific antibodies, multispecific antibodies (including bispecific antibodies), human antibodies, humanized antibodies, chimeric antibodies, immunoglobulins, synthetic antibodies, tetrameric antibodies comprising two heavy chain and two light chain molecules, an antibody light chain monomer, an antibody heavy chain monomer, an antibody light chain dimer, an antibody heavy chain dimer, an antibody light chain- antibody heavy chain pair, intrabodies, heteroconjugate antibodies, single domain antibodies, monovalent antibodies, single chain antibodies or single-chain Fvs (scFv), camelized antibodies, affybodies, Fab fragments, F(ab') 2 fragments, disulfide-linked Fvs (sdFv), anti-idiotypic (anti-Id) antibodies (including, e.g., anti-anti-Id antibodies), and antigen-binding fragments of any of the above.
- antibodies described herein refer to polyclonal antibody populations.
- Antibodies can be of any type (e.g ., IgG, IgE, IgM, IgD, IgA or IgY), any class ( e.g ., IgG 1 , IgG 2 , IgG 3 , IgG 4 , IgA 1 or IgA 2 ), or any subclass ( e.g ., IgG 2a or IgG 2b ) of immunoglobulin molecule.
- antibodies described herein are IgG antibodies, or a class ( e.g ., human IgG 1 or IgG 4 ) or subclass thereof.
- the antibody is a humanized monoclonal antibody. In another specific embodiment, the antibody is a human monoclonal antibody, preferably that is an immunoglobulin. In certain embodiments, an antibody described herein is an IgG 1 , or IgG 4 antibody.
- antigen-binding domain refers to a portion of an antibody molecule which comprises the amino acid residues that confer on the antibody molecule its specificity for the antigen (e.g ., the complementarity determining regions (CDR)).
- the antigen-binding region can be derived from any animal species, such as rodents ( e.g ., mouse, rat or hamster) and humans.
- variable region typically refers to a portion of an antibody, generally, a portion of a light or heavy chain, typically about the amino-terminal 110 to 120 amino acids in the mature heavy chain and about 90 to 115 amino acids in the mature light chain, which differ extensively in sequence among antibodies and are used in the binding and specificity of a particular antibody for its particular antigen.
- CDRs complementarity determining regions
- FR framework regions
- variable region is a human variable region.
- variable region comprises rodent or murine CDRs and human framework regions (FRs).
- FRs human framework regions
- the variable region is a primate ( e.g ., non-human primate) variable region.
- the variable region comprises rodent or murine CDRs and primate (e.g ., non-human primate) framework regions (FRs).
- VL and “VL domain” are used interchangeably to refer to the light chain variable region of an antibody.
- VH and "VH domain” are used interchangeably to refer to the heavy chain variable region of an antibody.
- Kabat numbering and like terms are recognized in the art and refer to a system of numbering amino acid residues in the heavy and light chain variable regions of an antibody, or an antigen-binding portion thereof.
- the CDRs of an antibody can be determined according to the Kabat numbering system (see, e.g., Kabat EA & Wu TT (1971) Ann NY Acad Sci 190: 382-391 and Kabat EA et al., (1991) Sequences of Proteins of Immunological Interest, Fifth Edition, U.S. Department of Health and Human Services, NIH Publication No. 91-3242 ).
- CDRs within an antibody heavy chain molecule are typically present at amino acid positions 31 to 35, which optionally can include one or two additional amino acids, following 35 (referred to in the Kabat numbering scheme as 35A and 35B) (CDR1), amino acid positions 50 to 65 (CDR2), and amino acid positions 95 to 102 (CDR3).
- CDRs within an antibody light chain molecule are typically present at amino acid positions 24 to 34 (CDR1), amino acid positions 50 to 56 (CDR2), and amino acid positions 89 to 97 (CDR3).
- the CDRs of the antibodies described herein have been determined according to the Kabat numbering scheme.
- constant region or “constant domain” are interchangeable and have its meaning common in the art.
- the constant region is an antibody portion, e.g., a carboxyl terminal portion of a light and/or heavy chain which is not directly involved in binding of an antibody to antigen but which can exhibit various effector functions, such as interaction with the Fc receptor.
- the constant region of an immunoglobulin molecule generally has a more conserved amino acid sequence relative to an immunoglobulin variable domain.
- the term "heavy chain” when used in reference to an antibody can refer to any distinct type, e.g., alpha ( ⁇ ), delta ( ⁇ ), epsilon ( ⁇ ), gamma ( ⁇ ) and mu ( ⁇ ), based on the amino acid sequence of the constant domain, which give rise to IgA, IgD, IgE, IgG and IgM classes of antibodies, respectively, including subclasses of IgG, e.g., IgG 1 , IgG 2 , IgG 3 and IgG 4 .
- the term "light chain” when used in reference to an antibody can refer to any distinct type, e.g., kappa ( ⁇ ) or lambda ( ⁇ ) based on the amino acid sequence of the constant domains. Light chain amino acid sequences are well known in the art. In specific embodiments, the light chain is a human light chain.
- Binding affinity generally refers to the strength of the sum total of non-covalent interactions between a single binding site of a molecule (e.g ., an antibody) and its binding partner (e.g ., an antigen). Unless indicated otherwise, as used herein, "binding affinity” refers to intrinsic binding affinity which reflects a 1:1 interaction between members of a binding pair ( e.g ., antibody and antigen).
- the affinity of a molecule X for its partner Y can generally be represented by the dissociation constant (K D ). Affinity can be measured and/or expressed in a number of ways known in the art, including, but not limited to, equilibrium dissociation constant (K D ), and equilibrium association constant (K A ).
- K D is calculated from the quotient of k off /k on
- K A is calculated from the quotient of k on /k off
- k on refers to the association rate constant of, e.g., an antibody to an antigen
- k off refers to the dissociation of, e.g., an antibody to an antigen.
- the k on and k off can be determined by techniques known to one of ordinary skill in the art, such as BIAcore® or KinExA.
- a "conservative amino acid substitution” is one in which the amino acid residue is replaced with an amino acid residue having a similar side chain.
- Families of amino acid residues having side chains have been defined in the art. These families include amino acids with basic side chains (e.g ., lysine, arginine, histidine), acidic side chains (e.g ., aspartic acid, glutamic acid), uncharged polar side chains ( e.g ., glycine, asparagine, glutamine, serine, threonine, tyrosine, cysteine, tryptophan), nonpolar side chains ( e.g ., alanine, valine, leucine, isoleucine, proline, phenylalanine, methionine), beta-branched side chains ( e.g ., threonine, valine, isoleucine) and aromatic side chains ( e.g ., tyrosine, phenyla
- an epitope is a term in the art and refers to a localized region of an antigen to which an antibody can specifically bind.
- An epitope can be, for example, contiguous amino acids of a polypeptide (linear or contiguous epitope) or an epitope can, for example, come together from two or more non-contiguous regions of a polypeptide or polypeptides (conformational, non-linear, discontinuous, or non-contiguous epitope).
- the epitope to which an antibody binds can be determined by, e.g., NMR spectroscopy, X-ray diffraction crystallography studies, ELISA assays, hydrogen/deuterium exchange coupled with mass spectrometry (e.g., liquid chromatography electrospray mass spectrometry), array-based oligo-peptide scanning assays, and/or mutagenesis mapping (e.g ., site-directed mutagenesis mapping).
- NMR spectroscopy e.g., NMR spectroscopy, X-ray diffraction crystallography studies, ELISA assays, hydrogen/deuterium exchange coupled with mass spectrometry (e.g., liquid chromatography electrospray mass spectrometry), array-based oligo-peptide scanning assays, and/or mutagenesis mapping (e.g ., site-directed mutagenesis mapping).
- crystallization may be accomplished using any of the known methods in the art (e.g ., Giegé R et al., (1994) Acta Crystallogr D Biol Crystallogr 50(Pt 4): 339-350 ; McPherson A (1990) Eur J Biochem 189: 1-23 ; Chayen NE (1997) Structure 5: 1269-1274 ; McPherson A (1976) J Biol Chem 251: 6300-6303 ).
- Antibody:antigen crystals may be studied using well known X-ray diffraction techniques and may be refined using computer software such as X-PLOR (Yale University, 1992, distributed by Molecular Simulations, Inc.; see e.g.
- the epitope of an antibody or antigen-binding fragment thereof is determined using alanine scanning mutagenesis studies, such as described in Section 6, infra.
- the terms “immunospecifically binds,” “immunospecifically recognizes,” “specifically binds,” and “specifically recognizes” are analogous terms in the context of antibodies and refer to molecules that bind to an antigen (e.g ., epitope or immune complex) as such binding is understood by one skilled in the art.
- a molecule that specifically binds to an antigen may bind to other peptides or polypeptides, generally with lower affinity as determined by, e.g., immunoassays, BIAcore®, KinExA 3000 instrument (Sapidyne Instruments, Boise, ID), or other assays known in the art.
- molecules that immunospecifically bind to an antigen bind to the antigen with a K A that is at least 2 logs, 2.5 logs, 3 logs, 4 logs or greater than the K A when the molecules bind to another antigen.
- molecules that immunospecifically bind to an antigen do not cross react with other proteins under similar binding conditions. In another specific embodiment, molecules that immunospecifically bind to an antigen do not cross react with other non-GITR proteins. In a specific embodiment, provided herein is an antibody or fragment thereof that binds to GITR with higher affinity than to another unrelated antigen.
- an antibody or fragment thereof that binds to GITR (e.g ., human GITR) with a 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95% or higher affinity than to another, unrelated antigen as measured by, e.g., a radioimmunoassay, surface plasmon resonance, or kinetic exclusion assay.
- the extent of binding of an anti-GITR antibody or antigen-binding fragment thereof described herein to an unrelated, non-GITR protein is less than 10%, 15%, or 20% of the binding of the antibody to GITR protein as measured by, e.g., a radioimmunoassay.
- provided herein is an antibody or fragment thereof that binds to human GITR with higher affinity than to another species of GITR.
- an antibody or fragment thereof described herein, which binds to human GITR will bind to another species of GITR protein with less than 10%, 15%, or 20% of the binding of the antibody or fragment thereof to the human GITR protein as measured by, e.g., a radioimmunoassay, surface plasmon resonance, or kinetic exclusion assay.
- GITR GITR polypeptide
- GITR including, but not limited to, native GITR, an isoform of GITR, or an interspecies GITR homolog of GITR.
- GITR is a 26 kDa type I transmembrane protein.
- GenBankTM accession numbers BC152381 and BC152386 provide exemplary human GITR nucleic acid sequences.
- Swiss-Prot accession number Q9Y5U5-1 (TNR18_HUMAN; SEQ ID NO: 701) and GenBankTM accession number NP_004186 provide exemplary human GITR amino acid sequences for isoform 1.
- Isoform 1 is a type I membrane protein.
- An exemplary mature amino acid sequence of human GITR is provided as SEQ ID NO: 700.
- isoform 2 is a secreted form of human GITR and is approximately 255 amino acids in length.
- Swiss-Prot accession number Q9Y5U5-2 and GenBankTM accession number NP_683699 provide exemplary human GITR amino acid sequences for isoform 2.
- Isoform 3 of human GITR is approximately 234 amino acids in length.
- GITR is human GITR.
- GITR is human GITR isoform 1 (SEQ ID NO: 701).
- the GITR is human isoform 2 (SEQ ID NO: 702) or isoform 3 (SEQ ID NO: 703).
- GITR is also known as tumor necrosis factor receptor superfamily member 18 (TNFRSF18), activation-inducible TNFR family receptor (AITR), GITR-D, and CD357.
- TNFRSF18 tumor necrosis factor receptor superfamily member 18
- AITR activation-inducible TNFR family receptor
- CD357 CD357.
- Human GITR is designated GeneID: 8784 by Entrez Gene.
- amino acid sequence of an immature form of an exemplary GITR protein from cynomolgus monkey is provided in SEQ ID NO: 704.
- the mature form of this exemplary protein is amino acids 26-234 of SEQ ID NO: 704.
- GITRL glucocorticoid-induced TNFR-related protein ligand. GITRL is otherwise known as activation-induced TNF-related ligand (AITRL) and tumor necrosis factor ligand superfamily member 18 (TNFSF18).
- AITRL activation-induced TNF-related ligand
- TNFSF18 tumor necrosis factor ligand superfamily member 18
- GenBankTM accession number AF125303 provides an exemplary human GITRL nucleic acid sequence.
- GenBankTM accession number NP_005083 and Swiss-Prot accession number Q9UNG2 provide exemplary human GITRL amino acid sequences.
- the GITRL is a human GITRL of SEQ ID NO: 716.
- the term "host cell” can be any type of cell, e.g., a primary cell, a cell in culture, or a cell from a cell line.
- the term “host cell” refers to a cell transfected with a nucleic acid molecule and the progeny or potential progeny of such a cell. Progeny of such a cell may not be identical to the parent cell transfected with the nucleic acid molecule, e.g., due to mutations or environmental influences that may occur in succeeding generations or integration of the nucleic acid molecule into the host cell genome.
- the term "effective amount" in the context of the administration of a therapy to a subject refers to the amount of a therapy that achieves a desired prophylactic or therapeutic effect. Examples of effective amounts are provided in Section 5.4.1.3, infra.
- the terms “subject” and “patient” are used interchangeably.
- the subject can be an animal.
- the subject is a mammal such as a non-primate (e.g ., cow, pig, horse, cat, dog, rat etc.) or a primate ( e.g ., monkey or human), most preferably a human.
- a non-human animal e.g ., a non-human animal such as a pig, horse, cow, cat or dog.
- such terms refer to a pet or farm animal.
- such terms refer to a human.
- antibodies e.g ., monoclonal antibodies
- antigen-binding fragments thereof that specifically bind to GITR (e.g ., human GITR) and modulate GITR activity.
- GITR e.g ., human GITR
- an antibody(ies) or fragment(s) thereof that specifically binds to GITR and enhances, induces, or increases one or more GITR activities.
- the antibody(ies) or antigen-binding fragment(s) is isolated.
- nucleic acids such as complementary DNA (cDNA), encoding such antibodies, and antigen-binding fragments thereof.
- vectors e.g ., expression vectors
- cells e.g ., host cells
- nucleic acids polynucleotides
- methods of making such antibodies are also provided.
- compositions e.g ., pharmaceutical compositions
- kits, and detection methods are also provided.
- antibodies e.g ., monoclonal antibodies, such as chimeric or humanized antibodies
- fragments thereof which specifically bind to GITR (e.g ., human GITR).
- an antibody or antigen-binding fragment thereof described herein partially inhibits GITRL (e.g ., human GITRL) from binding to GITR ( e.g ., human GITR).
- an antibody or antigen-binding fragment thereof described herein inhibits binding of GITRL (e.g ., human GITRL) to GITR (e.g ., human GITR) by less than 85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, 20% or 10% as assessed by an assay known to one of skill in the art or described herein.
- GITRL e.g ., human GITRL
- GITR e.g ., human GITR
- an antibody or antigen-binding fragment thereof described herein inhibits binding of GITRL (e.g ., human GITRL) to GITR (e.g ., human GITR) by less than 85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, 20% or 10% as assessed by the assay described in Example 2, infra (e.g ., Sections 6.2.5.2 or 6.2.5.4, infra ).
- GITRL e.g ., human GITRL
- GITR e.g ., human GITR
- human GITR coupled to Luminex® beads at a concentration of 9pg/ml, 8pg/ml, 7pg/ml, 6pg/ml, 5pg/ml, 4pg/ml or 3pg/ml per bead by less than 85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, 20% or 10% relative to the binding of 1.5nM, 1.4nM, 1.3nM, 1.2nM, 1.1nM, 1nM, 0.9nM, 0.8nM, 0.7nM, 0.6nM, 0.5nM, 0.4nM, 0.3nM, 0.2nM or 0.1nM of labeled GITRL to the GITR coupled beads at a concentration of 9pg/ml, 8pg/ml, 7pg/ml, 6pg/ml, 5pg/ml, 4pg/ml or 3pg/ml per bea
- an antibody or antigen-binding fragment thereof at a concentration of 1000ng/ml inhibits less than 80% (in some embodiments, 40% to 70%, 50%, to 80%, or 40% to 80%) of 0.5nM labeled GITRL (e.g ., human GITRL) from binding to GITR coupled to beads (e.g ., human GITR coupled to Luminex® beads) at a concentration of 5pg/ml/bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay.
- 0.5nM labeled GITRL e.g ., human GITRL
- beads e.g ., human GITR coupled to Luminex® beads
- an antibody or antigen-binding fragment thereof described herein at a concentration of 3000ng/ml inhibits binding of 0.5nM GITRL (e.g ., human GITRL) to GITR ( e.g ., human GITR) by less than 85% or less than 80% (in some embodiments, 60% to 85%, 60% to 80%, 70% to 85% or 70% to 80%) when GITR ( e.g ., human GITR) is coupled to beads ( e.g ., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof, in a suspension array assay (e.g ., Luminex® 200 system).
- a suspension array assay e.g ., Luminex® 200 system
- an antibody or antigen-binding fragment thereof described herein at a concentration of 1000ng/ml inhibits binding of 0.5nM GITRL (e.g ., human GITRL) to GITR ( e.g ., human GITR) by less than 85%, less than 80% or less than 75% (in some embodiments, 60% to 85%, 60% to 80%, 70% to 85% or 70% to 80%) when GITR ( e.g ., human GITR) is coupled to beads ( e.g ., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof, in a suspension array assay (e.g ., Luminex® 200 system).
- a suspension array assay e.g ., Luminex® 200 system
- an antibody or antigen-binding fragment thereof described herein at a concentration of 333ng/ml inhibits binding of 0.5nM GITRL (e.g ., human GITRL) to GITR ( e.g ., human GITR) by less than 70% or less than 65% (in some embodiments, 50% to 70%, 55% to 70%, 50% to 65% or 50% to 60%) when GITR ( e.g ., human GITR) is coupled to beads ( e.g ., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof, in a suspension array assay (e.g ., Luminex® 200 system).
- a suspension array assay e.g ., Luminex® 200 system
- an antibody or antigen-binding fragment thereof described herein at a concentration of 111ng/ml inhibits binding of 0.5nM GITRL (e.g ., human GITRL) to GITR ( e.g ., human GITR) by less than 65%, less than 60% or less than 55% (in some embodiments, 40% to 65%, 40% to 60%, 40% to 55% or 30% to 60%) when GITR ( e.g ., human GITR) is coupled to beads ( e.g ., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof, in a suspension array assay (e.g ., Luminex® 200 system).
- a suspension array assay e.g ., Luminex® 200 system
- an antibody or antigen-binding fragment thereof described herein at a concentration of 37ng/ml inhibits binding of 0.5nM GITRL (e.g ., human GITRL) to GITR ( e.g ., human GITR) by less than 40% (in some embodiments, 20% to 40%, 20% to 30%, or 15% to 35%)when GITR ( e.g ., human GITR) is coupled to beads ( e.g ., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof, in a suspension array assay (e.g ., Luminex® 200 system).
- a suspension array assay e.g ., Luminex® 200 system.
- an antibody or antigen-binding fragment thereof described herein at a concentration of 12ng/ml inhibits binding of 0.5nM GITRL (e.g ., human GITRL) to GITR ( e.g ., human GITR) by less than 20% (in some embodiments, 10% to 20%) when GITR (e.g ., human GITR) is coupled to beads (e.g ., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof, in a suspension array assay (e.g ., Luminex® 200 system).
- a suspension array assay e.g ., Luminex® 200 system.
- GITRL e.g ., human GITRL
- GITR e.g ., human GITR
- GITRL e.g ., human GITRL
- GITR e.g ., human GITR
- infra e.g ., Section 6.2.5.2 or 6.2.5.4, infra
- At least 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, or 75% of 1.5nM, 1.4nM, 1.3nM, 1.2nM, 1.1nM, 1nM, 0.9nM, 0.8nM, 0.7nM, 0.6nM, 0.5nM, 0.4nM, 0.3nM, 0.2nM or 0.1nM of labeled GITRL e.g ., labeled human GITRL, such as hGITRL-PE
- GITR coupled to beads e.g ., human GITR coupled to Luminex® beads
- At least 20%, at least 25% or at least 30% of 0.5nM of labeled GITRL binds to GITR coupled to beads (e.g ., human GITR coupled to Luminex® beads) at a concentration of 5pg/ml/bead in the presence of 1000ng/ml of an antibody or antigen-binding fragment thereof relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay.
- labeled GITRL e.g., labeled human GITRL, such as hGITRL-PE
- At least 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, or 75% of 1.5nM, 1.4nM, 1.3nM, 1.2nM, 1.1nM, 1nM, 0.9nM, 0.8nM, 0.7nM, 0.6nM, 0.5nM, 0.4nM, 0.3nM, 0.2nM, 0.1nM of labeled GITRL binds to GITR coupled to beads (e.g., human GITR coupled to Luminex® beads) at a concentration of 9pg/ml, 8pg/ml, 7pg/ml, 6pg/ml, 5pg/ml, 4pg/ml or 3pg/ml per bead in the presence of 3500 ng/ml, 3400 ng/ml, 3300 ng/m
- At least 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, or 75% of 1.5nM, 1.4nM, 1.3nM, 1.2nM, 1.1nM, 1nM, 0.9nM, 0.8nM, 0.7nM, 0.6nM, 0.5nM, 0.4nM, 0.3nM, 0.2nM or 0.1nM of labeled GITRL e.g ., labeled human GITRL, such as hGITRL-PE
- GITR coupled to beads e.g ., human GITR coupled to Luminex® beads
- At least 20%, at least 25% or at least 30% of 0.5nM of labeled GITRL binds to GITR coupled to beads (e.g ., human GITR coupled to Luminex® beads) at a concentration of 5pg/ml/bead in the presence of 3000ng/ml of an antibody or antigen-binding fragment thereof relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay.
- labeled GITRL e.g ., labeled human GITRL, such as hGITRL-PE
- beads e.g ., human GITR coupled to Luminex® beads
- At least 25%, at least 30%, at least 40%, or at least 50% (in some embodiments, 25% to 60%, 40% to 60%, 40% to 70%, or 25% to 50%) of 0.5nM of labeled GITRL binds to GITR coupled to beads (e.g ., human GITR coupled to Luminex® beads) at a concentration of 5pg/ml/bead in the presence of 1000ng/ml of an antibody or antigen-binding fragment thereof relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay.
- labeled GITRL e.g ., labeled human GITRL, such as hGITRL-PE
- beads e.g ., human GITR coupled to Luminex® beads
- At least 30%, at least 40%, at least 50% or at least 60% (in some embodiments, 30% to 60%, 40% to 60%, 40% to 70%, or 30% to 50%) of 0.5nM of labeled GITRL binds to GITR coupled to beads (e.g ., human GITR coupled to Luminex® beads) at a concentration of 5pg/ml/bead in the presence of 333ng/ml of an antibody or antigen-binding fragment thereof relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay.
- labeled GITRL e.g ., labeled human GITRL, such as hGITRL-PE
- beads e.g ., human GITR coupled to Luminex® beads
- At least 40%, at least 50%, at least 60% or at least 65% (in some embodiments, 40% to 70%, 40% to 60%, 40% to 65%, or 40% to 50%) of 0.5nM of labeled GITRL binds to GITR coupled to beads (e.g ., human GITR coupled to Luminex® beads) at a concentration of 5pg/ml/bead in the presence of 11 1ng/ml of an antibody or antigen-binding fragment thereof relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay.
- labeled GITRL e.g ., labeled human GITRL, such as hGITRL-PE
- beads e.g ., human GITR coupled to Luminex® beads
- At least 60%, at least 70% or at least 80% (in some embodiments 60% to 80%, 70% to 80% or 75% to 85%) of 0.5nM of labeled GITRL binds to GITR coupled to beads (e.g ., human GITR coupled to Luminex® beads) at a concentration of 5pg/ml/bead in the presence of 37ng/ml of an antibody or antigen-binding fragment thereof relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay.
- labeled GITRL e.g ., labeled human GITRL, such as hGITRL-PE
- beads e.g ., human GITR coupled to Luminex® beads
- At least 80%, at least 85% or at least 90% (in some embodiments 80% to 90% or 85% to 95%) of 0.5nM of labeled GITRL binds to GITR coupled to beads (e.g.
- human GITR coupled to Luminex® beads at a concentration of 5pg/ml/bead in the presence of 12ng/ml of an antibody or antigen-binding fragment thereof relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay
- an antibody or antigen-binding fragment thereof described herein at a concentration of 3000ng/ml does not inhibit binding of 0.5nM GITRL (e.g ., human GITRL) to GITR ( e.g ., human GITR) by more than 15% or more than 20% when GITR ( e.g ., human GITR) is coupled to beads ( e.g ., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof, in a suspension array assay (e.g ., Luminex® 200 system).
- a suspension array assay e.g ., Luminex® 200 system
- an antibody or antigen-binding fragment thereof described herein at a concentration of 1000ng/ml does not inhibit binding of 0.5nM GITRL (e.g ., human GITRL) to GITR ( e.g ., human GITR) by more than 15%, more than 20% or more than 25% when GITR ( e.g ., human GITR) is coupled to beads ( e.g ., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof, in a suspension array assay (e.g ., Luminex® 200 system).
- a suspension array assay e.g ., Luminex® 200 system
- an antibody or antigen-binding fragment thereof described herein at a concentration of 333ng/ml does not inhibit binding of 0.5nM GITRL (e.g ., human GITRL) to GITR ( e.g ., human GITR) by more than 30% or more than 35% when GITR ( e.g ., human GITR) is coupled to beads ( e.g ., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof, in a suspension array assay (e.g ., Luminex® 200 system).
- a suspension array assay e.g ., Luminex® 200 system
- an antibody or antigen-binding fragment thereof described herein at a concentration of 11 1ng/ml does not inhibit binding of 0.5nM GITRL (e.g ., human GITRL) to GITR ( e.g ., human GITR) by more than 35%, more than 40% or more than 45% when GITR ( e.g ., human GITR) is coupled to beads ( e.g ., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof, in a suspension array assay (e.g ., Luminex® 200 system).
- a suspension array assay e.g ., Luminex® 200 system
- an antibody or antigen-binding fragment thereof described herein at a concentration of 37ng/ml does not inhibit binding of 0.5nM GITRL (e.g ., human GITRL) to GITR ( e.g ., human GITR) by more than 60% when GITR ( e.g ., human GITR) is coupled to beads (e.g ., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof, in a suspension array assay (e.g ., Luminex® 200 system).
- a suspension array assay e.g ., Luminex® 200 system
- an antibody or antigen-binding fragment thereof described herein at a concentration of 12ng/ml does not inhibit binding of 0.5nM GITRL (e.g ., human GITRL) to GITR ( e.g ., human GITR) by more than 80% when GITR ( e.g., human GITR) is coupled to beads (e.g., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof, in a suspension array assay (e.g. , Luminex® 200 system).
- a suspension array assay e.g. , Luminex® 200 system.
- a certain amount of labeled GITRL binds to GITR coupled to beads (e.g., human GITR coupled to Luminex® beads) in the presence of an antibody or antigen-binding fragment thereof described herein in a method comprising: (a) coupling GITR (e.g., human GITR) to beads at a concentration of approximately 9pg/ml, 8pg/ml, 7pg/ml, 6pg/ml, 5pg/ml, 4pg/ml or 3pg/ml per bead; (b) incubating the GITR coupled beads at a concentration of approximately 30 beads/ ⁇ l, 40 beads/ ⁇ l, or 50 beads/ ⁇ l with 3000ng/ml, 2500ng/ml, 2000ng/ml, 1500ng/ml, 1000ng/ml, 750ng/ml, 500ng/ml, 250ng/ml, 100ng/ml
- the amount of the labeled GITRL bound to the GITR coupled beads in the presence of the anti-GITR antibody or antigen-binding fragment thereof is determined relative to the amount of labeled GITRL bound to the GITR coupled beads in the absence of the anti-GITR antibody or antigen-binding fragment thereof.
- the absence of the anti-GITR antibody or antigen-binding fragment thereof means that no antibody or antigen-binding fragment thereof is present in the well.
- the absence of the anti-GITR antibody or antigen-binding fragment thereof means that an isotype control antibody that does not bind to GITR is present in the well.
- the amount of labeled GITRL bound to the GITR coupled beads in the presence of the anti-GITR antibody or antigen-binding fragment thereof is determined to be, in some embodiments, at least 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55% or 60% or 15% to 60%, 20% to 60%, 30% to 70%, or 20% to 50% of the amount of the labeled GITRL bound to the GITR coupled beads in the absence of the anti-GITR antibody or antigen-binding fragment thereof.
- a certain amount of labeled GITRL binds to GITR coupled to beads (e.g., human GITR coupled to Luminex® beads) in the presence of an antibody or antigen-binding fragment thereof described herein in a method comprising: (a) coupling GITR (e.g., human GITR) to beads at a concentration of approximately 5pg/ml per bead; (b) incubating the GITR coupled beads at a concentration of approximately 40 beads/ ⁇ l with 3000ng/ml, 2500ng/ml, 2000ng/ml, 1500ng/ml, 1000ng/ml, 750ng/ml, 500ng/ml, 250ng/ml, 100ng/ml, 50ng/ml or 10ng/ml of an antibody or an antigen-binding fragment thereof described herein in a well for a first period of time (e.g., 30 minutes, 60 minutes, 1.5 hours, 2
- a first period of time e.g., 30 minutes, 60 minutes
- the amount of the labeled GITRL bound to the GITR coupled beads in the presence of the anti-GITR antibody or antigen-binding fragment thereof is determined relative to the amount of labeled GITRL bound to the GITR coupled beads in the absence of the anti-GITR antibody or antigen-binding fragment thereof.
- the absence of the anti-GITR antibody or antigen-binding fragment thereof means that no antibody or antigen-binding fragment thereof is present in the well.
- the absence of the anti-GITR antibody or antigen-binding fragment thereof means that an isotype control antibody that does not bind to GITR is present in the well.
- the amount of labeled GITRL bound to the GITR coupled beads in the presence of the anti-GITR antibody or antigen-binding fragment thereof is determined to be, in some embodiments, at least 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55% or 60% or 20 to 70%, 20% to 60%, 30% to 70%, or 20% to 50% of the amount of the labeled GITRL bound to the GITR coupled beads in the absence of the anti-GITR antibody or antigen-binding fragment thereof.
- GITRL e.g., non-covalently linked trimer of human GIT
- an antibody or antigen-binding fragment thereof described herein at a concentration of 125nM bound to GITR (e.g., human GITR) immobilized on a chip (e.g., CM5 sensor chip) inhibits binding of 125nM of GITRL (e.g., non-covalently linked trimer of human GITRL) to the GITR immobilized on the chip by less than 60%, less than 55%, less than 50%, less than 45%, less than 40%, less than 35%, less than 30%, less than 25%, less than 20% or less than 15%.
- GITRL e.g., non-covalently linked trimer of human GITRL
- an antibody or fragment thereof described herein binds to GITR (e.g., human GITR) with a dissociation rate constant (k off ) of 8.5 x 10 -3 s -1 or less, 3.5 x 10 -3 s -1 or less, 5 x 10 -3 s -1 or less, 2.5 x 10 -3 s -1 or less, 1 x 10 -3 s -1 or less, 8.5 x 10 -4 s -1 or less, 5 x 10 -4 s -1 or less, 3.5 x 10 -4 s -1 or less, 2.5 x 10 -4 s -1 or less, 1 x 10 -4 s -1 or less, 8.5 x 10 -5- s -1 or less, 3.5 x 10 -5- s -1 or less, 5 x 10 -5- s -1 or less, 2.5 x 10 -5- s -1 or less, 1 x 10 -5- s -1 or less, 8.5 x 10 -5-
- an antibody or fragment thereof described herein binds to GITR (e.g., human GITR) with a k off of between 9.5 x 10 -5- s -1 to 1 x 10 -9- s -1 , 8.5 x 10 -5- s -1 to 1 x 10 -9- s -1 , 5 x 10 -5- s -1 to 1 x 10 -9- s -1 , 9.5 x 10 -5- s -1 to 1 x 10 -8- s -1 , 5 x 10 -5 s -1 to 1 x 10 -8- s -1 , 9.5 x 10 -5- s -1 to 1 x 10 -7- s -1 , 5 x 10 -5- s -1 to 1 x 10 -7- s -1 , 9.5 x 10 -5- s -1 to 5 x 10 -6- s -1 , 9.5 x 10 -5- s s -1 to 5
- the k off ⁇ is determined using a monovalent antibody, such as a Fab fragment, as measured by, e.g., BIAcore® surface plasmon resonance technology.
- the k off is determined using a bivalent antibody as measured by, e.g., BIAcore® surface plasmon resonance technology.
- the k off is determined using an assay described in Section 6, infra.
- an antibody or fragment thereof described herein binds to GITR (e.g., human GITR) with an association rate constant (k on ) of at least 10 5 M -1 s -1 , at least 2.5 x 10 5 M -1 s -1 , at least 3.5 x 10 5 M -1 s -1 , at least 5 x 10 5 M -1 s -1 , at least 10 6 M -1 s -1 , at least 2.5 x 10 6 M -1 s -1 , at least 3.5 x 10 6 M -1 s -1 , at least 5 x 10 6 M -1 s -1 , at least 10 7 M -1 s -1 , at least 5 x 10 7 M -1 s -1 , at least 10 8 M -1 s -1 , at least 5 10 8 M -1 s -1 or at least 10 9 M -1 s -1 .
- association rate constant k on
- an antibody or fragment thereof described herein binds to GITR (e.g., human GITR) with a k on of between 1 x 10 5 M -1 s -1 to 5 x 10 5 M -1 s -1 , 1 x 10 5 M -1 s -1 to 1 x 10 6 M -1 s -1 , 3.5 x 10 5 M -1 s -1 to 2.5 x 10 6 M -1 s -1 , 3.5 x 10 5 M -1 s -1 to 3.5 x 10 6 M -1 s -1 , 1 x 10 5 M -1 s -1 to 5 x 10 6 M -1 s -1 , 1 x 10 5 M -1 s - to 1 x 10 7 M -1 s -1 , 1 x 10 5 M -1 s -1 to 5 x 10 7 M -1 s -1 , 1 x 10 5 M -1 s -1 to 10 8 M -1 s -1
- the k on is determined using a monovalent antibody, such as a Fab fragment, as measured by, e.g., BIAcore® surface plasmon resonance technology.
- the k on is determined using a bivalent antibody as measured by, e.g., BIAcore® surface plasmon resonance technology.
- the k on is determined using an assay described in Section 6, infra.
- an antibody or fragment thereof described herein binds to GITR (e.g., human GITR) with a K D of less than 7 nM, 6 nM, 5 nM, 4.5 nM, 4 nM, 3.5 nM, 3 nM, 2.5 nM, 2 nM, 1.5 nM, 1 nM, 0.75 nM, 0.5 nM, 0.25 nM, or 0.1 nM.
- GITR e.g., human GITR
- an antibody or fragment thereof described herein binds to GITR (e.g., human GITR) with a K D of about 7 nM, 6 nM, 5 nM, 4.5 nM, 4 nM, 3.5 nM, 3 nM, 2.5 nM, 2 nM, 1.5 nM, 1 nM, 0.75 nM, 0.5 nM, 0.25 nM, or 0.1 nM.
- GITR e.g., human GITR
- an antibody or fragment thereof described herein binds to GITR (e.g., human GITR) with a K D of 7 nM to 2 nM, 5 nM to 3 nM, 5 nM to 1 nM, 4 nM to 3 nM, 4 nM to 2 nM, 3 nM to 2 nM, 3 nM to 1 nM, 2 nM to 1 nM, 3 nM to 0.1 nM, 2 nM to 0.1 nM, 1 nM to 0.1 nM, or 0.5 nM to 0.1 nM.
- GITR e.g., human GITR
- the K D is calculated as the quotient of k off /k on , and the k on and k off are determined using a monovalent antibody, such as a Fab fragment, as measured by, e.g., BIAcore® surface plasmon resonance technology.
- the K D is calculated as the quotient of k off /k on , and the k on and k off are determined using a bivalent antibody, such as a Fab fragment, as measured by, e.g., BIAcore® surface plasmon resonance technology.
- the K D is determined as set forth in the Examples in Section 6, infra ( e.g., Example 2).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises a light chain variable region (VL) comprising:
- the antibody or antigen-binding fragment thereof comprises one, two, or all three of the VL CDRs above. In certain embodiments, the antibody or antigen-binding fragment thereof comprises the VL CDR1 of one of the antibodies in Table 1. In some embodiments, the antibody or antigen-binding fragment thereof comprises the VL CDR2 of one of the antibodies in Table 1. In certain embodiments, the antibody or antigen-binding fragment thereof comprises the VL CDR3 of one of the antibodies in Table 1.
- the antibody or antigen-binding fragment thereof comprises one, two or all three of the VL CDRs of one of the antibodies in Table 1 (e.g., the VL CDRs in one row of Table 1, for example, all of the VL CDRs are from antibody 231-32-15).
- the antibody or antigen-binding fragment thereof comprises the VL framework regions described herein.
- the antibody or antigen-binding fragment thereof comprises the VL framework regions (FRs) of an antibody set forth in Table 3 (e.g., one, two, three, or four of the framework regions in one row of Table 3).
- an antibody described herein, or an antigen-binding fragment thereof, which specifically binds to GITR comprises a heavy chain variable region (VH) comprising:
- the antibody or antigen-binding fragment thereof comprises one, two or all three of the VH CDRs above. In certain embodiments, the antibody or antigen-binding fragment thereof comprises the VH CDR1 of one of the antibodies in Table 2. In some embodiments, the antibody or antigen-binding fragment thereof comprises the VH CDR2 of one of the antibodies in Table 2. In certain embodiments, the antibody or antigen-binding fragment thereof comprises the VH CDR3 of one of the antibodies in Table 2.
- the antibody or antigen-binding fragment thereof comprises one, two or all three of VH CDRs of one of the antibodies in Table 2 (e.g., the VH CDRs in one row of Table 2, for example, all of the VH CDRs are from the antibody 231-32-15).
- the antibody or antigen-binding fragment thereof comprises the VH frameworks described herein.
- the antibody or antigen-binding fragment thereof comprises the VH framework regions of an antibody set forth in Table 4 (e.g., one, two, three or four of the framework regions in one row of Table 4). Table 1.
- VL CDR Amino Acid Sequences 1 Antibody VL CDR1 (SEQ ID NO:) VL CDR2 (SEQ ID NO:) VL CDR3 (SEQ ID NO:) 231-32-15 KSSQSLLNSGNQKNYLT (16) WASTRES (17) QNDYSYPYT (18) Hum231#1 KSSQSLLNSGNQKNYLT (16) WASTRES (17) QNDYSYPYT (18) Hum231#2 KSSQSLLNSGNQKNYLT (16) WASTRES (17) QNDYSYPYT (18) pab1964 KSSQSLLNSGNQKNYLS (101) WASTRES (105) QNEYSYPYT (106) pab1965 KSSQSLLNSGNQKNYLT (102) WASTRES (105) QNDYSYPYT (107) pab1966 KSSQSLLNSGNQKNYLT (102) WASTRES (105) QNDYSYPYT (107) pab1966 KSSQSLLNS
- VL CDRs are determined by Kabat and the framework regions are the amino acid residues surrounding the CDRs in the variable region in the format FR1, CDR1, FR2, CDR2, FR3, CDR3, and FR4.
- Table 4 VH FR Amino Acid Sequences 4 Antibody VH FR1 (SEQ ID NO:) VH FR2 (SEQ ID NO:) VH FR3 (SEQ ID NO:) VH FR4 (SEQ ID NO:) 231-32-15 WGQGTLVTVSS (668) Hum231#1 WGQGTLVTVSS (668) Hum231#2 WGQGTLVTVSS (668) pab1964 WGQGTLVTVSS (668) pab1965 WGQGTLVTVSS (668) pab1966 WGQGTLVTVSS (668) pab1967 WGQGTLITVSS (667) pab1968 WGQGTL VTVSS (668) pab1969 WGQGTLVTVSS (668) pab19
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises a light chain variable region (VL) comprising:
- the antibody or antigen-binding fragment thereof comprises one, two, or all three of the VL CDRs above. In certain embodiments, the antibody or antigen-binding fragment thereof comprises the VL CDR1 of one of the antibodies in Table 5. In some embodiments, the antibody or antigen-binding fragment thereof comprises the VL CDR2 of one of the antibodies in Table 5. In certain embodiments, the antibody or antigen-binding fragment thereof comprises the VL CDR3 of one of the antibodies in Table 5.
- the antibody or antigen-binding fragment thereof comprises one, two or all three of the VL CDRs of one of the antibodies in Table 5 (e.g., the VL CDRs in one row of Table 5, for example, all of the VL CDRs are from antibody 231-32-15).
- the antibody or antigen-binding fragment thereof comprises the VL framework regions described herein.
- the antibody or antigen-binding fragment thereof comprises the VL framework regions (FRs) of an antibody set forth in Table 7 (e.g., one, two, three, or four of the framework regions in one row of Table 7).
- an antibody described herein, or an antigen-binding fragment thereof, which specifically binds to GITR comprises a heavy chain variable region (VH) comprising:
- the antibody or antigen-binding fragment thereof comprises one, two or all three of the VH CDRs above. In certain embodiments, the antibody or antigen-binding fragment thereof comprises the VH CDR1 of one of the antibodies in Table 6. In some embodiments, the antibody or antigen-binding fragment thereof comprises the VH CDR2 of one of the antibodies in Table 6. In certain embodiments, the antibody or antigen-binding fragment thereof comprises the VH CDR3 of one of the antibodies in Table 6.
- the antibody or antigen-binding fragment thereof comprises one, two or all three of VH CDRs of one of the antibodies in Table 6 (e.g., the VH CDRs in one row of Table 6, for example, all of the VH CDRs are from the antibody 231-32-15).
- the antibody or antigen-binding fragment thereof comprises the VH frameworks described herein.
- the antibody or antigen-binding fragment thereof comprises the VH framework regions of an antibody set forth in Table 8 (e.g., one, two, three or four of the framework regions in one row of Table 8). Table 5.
- VL CDR Amino Acid Sequences 1 Antibody VL CDR1 (SEQ ID NO:) VL CDR2 (SEQ ID NO:) VL CDR3 (SEQ ID NO:) 231-32-15 KSSQSLLNSGNQKNYLT (16) WASTRES (17) QNDYSYPYT (18) Hum231#1 KSSQSLLNSGNQKNYLT (16) WASTRES (17) QNDYSYPYT (18) Hum231#2 KSSQSLLNSGNQKNYLT (16) WASTRES (17) QNDYSYPYT (18) pab1964 KSSQSLLNSGNQKNYLS (101) WASTRES (105) QNEYSYPYT (106) pab1965 KSSQSLLNSGNQKNYLT (102) WASTRES (105) QNDYSYPYT (107) pab1966 KSSQSLLNSGNQKNYLT (102) WASTRES (105) QNDYSYPYT (107) pab1966 KSSQSLLNS
- VL CDRs are determined by Kabat and the framework regions are the amino acid residues surrounding the CDRs in the variable region in the format FR1, CDR1, FR2, CDR2, FR3, CDR3, and FR4.
- Table 8 VH FR Amino Acid Sequences 4 Antibody VH FR1 (SEQ ID NO:) VH FR2 (SEQ ID NO:) VH FR3 (SEQ ID NO:) VH FR4 (SEQ ID NO:) 231-32-15 WGQGTLVTVSS (668) Hum231#1 WGQGTLVTVSS (668) Hum231#2 WGQGTLVTVSS (668) pab1964 WGQGTLVTVSS (668) pab1965 WGQGTLVTVSS (668) pab1966 WGQGTLVTVSS (668) pab1967 WGQGTLITVSS (667) pab1968 WGQGTLVTVSS (668) pab1969 WGQGTLVTVSS (668) pab1970
- an antibody described herein, or an antigen-binding fragment thereof, which specifically binds to GITR comprises:
- the antibody or antigen-binding fragment thereof comprises one, two, three, four, five or all six of the CDRs above.
- the antibody or antigen-binding fragment thereof comprises the VL CDR1 of one of the antibodies in Table 1.
- the antibody or antigen-binding fragment thereof comprises the VL CDR2 of one of the antibodies in Table 1.
- the antibody or antigen-binding fragment thereof comprises the VL CDR3 of one of the antibodies in Table 1.
- the antibody or antigen-binding fragment thereof comprises the VH CDR1 of one of the antibodies in Table 2.
- the antibody or antigen-binding fragment thereof comprises the VH CDR2 of one of the antibodies in Table 2.
- the antibody or antigen-binding fragment thereof comprises the VH CDR3 of one of the antibodies in Table 2. In some embodiments, the antibody or antigen-binding fragment thereof comprises one, two or all three of the VH CDRs of one of the antibodies in Table 2 ( e.g., the VH CDRs in one row of Table 2, for example, all of the VH CDRs are from the antibody 231-32-15). In certain embodiments, the antibody or antigen-binding fragment thereof comprises one, two or all three of the VL CDRs of one of the antibodies in Table 1 ( e.g., the VL CDRs in one row of Table 1, for example, all of the VLCDRs are from the antibody 231-32-15).
- an antibody described herein, or an antigen-binding fragment thereof, which specifically binds to GITR comprises a light chain variable region (VL) and a heavy chain variable region (VH), wherein
- the VL comprises two or all three of the VL CDRs above and/or the VH comprises two or all three of the VH CDRs above.
- the antibody or antigen-binding fragment thereof comprises the VL CDR1 of one of the antibodies in Table 1.
- the antibody or antigen-binding fragment thereof comprises the VL CDR2 of one of the antibodies in Table 1.
- the antibody or antigen-binding fragment thereof comprises the VL CDR3 of one of the antibodies in Table 1.
- the antibody or antigen-binding fragment thereof comprises the VH CDR1 of one of the antibodies in Table 2.
- the antibody or antigen-binding fragment thereof comprises the VH CDR2 of one of the antibodies in Table 2.
- the antibody or antigen-binding fragment thereof comprises the VH CDR3 of one of the antibodies in Table 2. In some embodiments, the antibody or antigen-binding fragment thereof comprises one, two or all three of the VH CDRs of one of the antibodies in Table 2 ( e.g., the VH CDRs in one row of Table 2). In certain embodiments, the antibody or antigen-binding fragment thereof comprises one, two or all three of the VL CDRs of one of the antibodies in Table 1 ( e.g., the VL CDRs in one row in Table 1).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises a light chain variable region (VL) comprising:
- the antibody or antigen-binding fragment thereof comprises one, two, or all three of the VL CDRs above.
- the antibody or antigen-binding fragment thereof comprises the VL CDR1 of one of the antibodies in Table 1.
- the antibody or antigen-binding fragment thereof comprises the VL CDR2 of one of the antibodies in Table 1.
- the antibody or antigen-binding fragment thereof comprises the VL CDR3 of one of the antibodies in Table 1.
- the antibody or antigen-binding fragment thereof comprises one, two or all three of the VL CDRs of one of the antibodies in Table 1 (e.g., the VL CDRs in one row of Table 1).
- the antibody or antigen-binding fragment thereof comprises the VL framework regions described herein.
- the antibody or antigen-binding fragment thereof comprises the VL framework regions (FRs) of an antibody set forth in Table 3 (e.g., one, two, three, or four of the framework regions in one row of Table 3).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises a heavy chain variable region (VH) comprising:
- the antibody or antigen-binding fragment thereof comprises one, two or all three of the VH CDRs above.
- the antibody or antigen-binding fragment thereof comprises the VH CDR1 of one of the antibodies in Table 2.
- the antibody or antigen-binding fragment thereof comprises the VH CDR2 of one of the antibodies in Table 2.
- the antibody or antigen-binding fragment thereof comprises the VH CDR3 of one of the antibodies in Table 2.
- the antibody or antigen-binding fragment thereof comprises one, two or all three of VH CDRs of one of the antibodies in Table 2 (e.g., the VH CDRs in one row in Table 2).
- the antibody or antigen-binding fragment thereof comprises one, two or all three of the VH CDRs of one of the antibodies in Table 2. In some embodiments, the antibody or antigen-binding fragment thereof comprises the VH frameworks described herein. In specific embodiments, the antibody or antigen-binding fragment thereof comprises the VH framework regions of an antibody set forth in Tables 4 ( e.g., one, two, three or four of the framework regions in one row of Table 4).
- an antibody described herein, or an antigen-binding fragment thereof, which specifically binds to GITR comprises:
- the antibody or antigen-binding fragment thereof comprises one, two, three, four, five or all six of the CDRs above.
- the antibody or antigen-binding fragment thereof comprises the VL CDR1 of one of the antibodies in Table 1.
- the antibody or antigen-binding fragment thereof comprises the VL CDR2 of one of the antibodies in Table 1.
- the antibody or antigen-binding fragment thereof comprises the VL CDR3 of one of the antibodies in Table 1.
- the antibody or antigen-binding fragment thereof comprises the VH CDR1 of one of the antibodies in Table 2.
- the antibody or antigen-binding fragment thereof comprises the VH CDR2 of one of the antibodies in Table 2.
- the antibody or antigen-binding fragment thereof comprises the VH CDR3 of one of the antibodies in Table 2. In some embodiments, the antibody or antigen-binding fragment thereof comprises one, two or all three of the VH CDRs of one of the antibodies in Table 2 ( e.g., the VH CDRs in one row in Table 2). In certain embodiments, the antibody or antigen-binding fragment thereof comprises one, two or all three of the VL CDRs of one of the antibodies in Table 1 ( e.g., the VL CDRs in one row in Table 1).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises a light chain variable region (VL) and a heavy chain variable region (VH), wherein
- the VL comprises two or all three of the VL CDRs above and/or the VH comprises two or all three of the VH CDRs above.
- the antibody or antigen-binding fragment thereof comprises the VL CDR1 of one of the antibodies in Table 1.
- the antibody or antigen-binding fragment thereof comprises the VL CDR2 of one of the antibodies in Table 1.
- the antibody or antigen-binding fragment thereof comprises the VL CDR3 of one of the antibodies in Table 1.
- the antibody or antigen-binding fragment thereof comprises the VH CDR1 of one of the antibodies in Table 2.
- the antibody or antigen-binding fragment thereof comprises the VH CDR2 of one of the antibodies in Table 2.
- the antibody or antigen-binding fragment thereof comprises the VH CDR3 of one of the antibodies in Table 2. In some embodiments, the antibody or antigen-binding fragment thereof comprises one, two or all three of the VH CDRs of one of the antibodies in Table 2 ( e.g., the VH CDRs in one row in Table 2). In certain embodiments, the antibody or antigen-binding fragment thereof comprises one, two or all three of the VL CDRs of one of the antibodies in Table 1 ( e.g., the VL CDRs in one row in Table 1).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises a light chain variable region (VL) and a heavy chain variable region (VH), wherein
- an antibody or fragment thereof which specifically binds to GITR (e.g., human GITR) and comprises one, two or three of the light chain variable region (VL) complementarity determining regions (CDRs) of an antibody in Table 1 ( e.g., the VL CDRs in one row of Table 1).
- VL light chain variable region
- CDRs complementarity determining regions
- an antibody or fragment thereof which specifically binds to GITR (e.g., human GITR) and comprises one, two or three of the heavy chain variable region (VH) CDRs of any one of any one of antibodies in Table 2 (e.g., the VH CDRs in one row of Table 2).
- an antibody or fragment thereof which specifically binds to GITR (e.g., human GITR) and comprises a light chain variable region (VL) comprising one, two or all three of the VL CDRs of an antibody in Table 1 ( e.g., the VL CDRs in one row of Table 1).
- the antibody or antigen-binding fragment thereof comprises one, two, three or all four of the VL framework regions described herein.
- the antibody or antigen-binding fragment thereof comprises one, two, three or all four of the VL framework regions (FRs) set forth in Table 3 (e.g., one, two, three or four of the framework regions in one row in Table 3).
- an antibody or fragment thereof which specifically binds to GITR (e.g., human GITR) and comprises a heavy chain variable region (VH) comprising one, two or all three of the VH CDRs of an antibody in Table 2 ( e.g., the VH CDRs in one row of Table 2).
- VH heavy chain variable region
- the antibody or antigen-binding fragment thereof comprises one, two, three or all four of the VH framework regions described herein.
- the antibody or antigen-binding fragment thereof comprises one, two, three or all four of the VH framework regions (FRs) set forth in Table 4 (e.g., one, two, three, or four of the framework regions in one row in Table 4).
- an antibody or fragment thereof which specifically binds to GITR (e.g., human GITR) and comprises light chain variable region (VL) CDRs and heavy chain variable region (VH) CDRs of any one of antibodies Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, Hum231#1, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161, for example as set forth in Tables 1 and 2 (e.g., the VH CDRs and VL CDRs in the same row are all from the same antibody as designated by the name of the antibody in the first column of Tables 1 and 2, for example, the VL CDRs and VH CDRs in the
- the antibody or antigen-binding fragment thereof comprises the VL framework regions and VH frameworks described herein.
- the antibody or antigen-binding fragment thereof comprises VL framework regions (FRs) and VH framework regions set forth in Tables 3 and 4 ( e.g., the VL FRs and VH FRs are all from the same antibody).
- an antibody described herein, or an antigen-binding fragment thereof, which specifically binds to GITR comprises a light chain variable region (VL) comprising VL CDR1, VL CDR2, and VL CDR3 as set forth in Table 1, for example, VL CDR1, VL CDR2, and VL CDR3 of any one of antibodies Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, Hum231#1, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161, (e.g., the VL CDRs are in one row of Table 1).
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 ( e.g., one, two, three, or four of the framework regions in one row in Table 3).
- the antibody or antigen-binding fragment thereof comprises a light chain variable region sequence comprising one, two, three or four of the framework regions of the light chain variable region sequence of SEQ ID NO: 204 or SEQ ID NO: 205.
- the antibody or antigen-binding fragment thereof comprises one, two, three or four of the framework regions of a light chain variable region sequence which is at least 75%, 80%, 85%, 90%, 95%, or 100% identical to one, two, three or four of the framework regions of a light chain variable region sequence selected from the group consisting of SEQ ID NO: 202, SEQ ID NO: 207, SEQ ID NO: 208, and SEQ ID NOs: 400-518.
- the antibody or antigen-binding fragment thereof comprises one, two, three or four of the framework regions of a light chain variable region sequence which is at least 75%, 80%, 85%, 90%, 95%, or 100% identical to one, two, three or four of the framework regions of the light chain variable region sequence of SEQ ID NO: 519.
- an antibody or antigen-binding fragment thereof comprises a light chain variable framework region that is or is derived from an amino acid sequence encoded by a human gene, wherein the amino acid sequence is selected from the group consisting of IGKV4-1*01 (SEQ ID NO: 607) and IGKV3-7*02 (SEQ ID NO: 608).
- the light chain variable framework region that is derived from said amino acid sequence consists of said amino acid sequence but for the presence of up to 10 amino acid substitutions, deletions, and/or insertions, preferably up to 10 amino acid substitutions.
- the light chain variable framework region that is derived from said amino acid sequence consists of said amino acid sequence with 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10 amino acid residues being substituted for an amino acid found in an analogous position in a corresponding non-human light chain variable framework region.
- an antibody or antigen-binding fragment thereof comprises a light chain variable framework region that is derived from amino acid sequence SEQ ID NO: 607 or SEQ ID NO: 608, wherein at least one amino acid in amino acid sequence SEQ ID NO: 607 or SEQ ID NO: 608 is substituted with an amino acid in an analogous position in a corresponding non-human light chain variable framework region.
- the amino acid substitution is at amino acid position 87, wherein the amino acid position is indicated according to the Kabat numbering.
- the amino acid substitution is 87H, wherein the amino acid position is indicated according to the Kabat numbering.
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of Hum231#l, for example, the VL CDR1, VL CDR2, and VL CDR3 of Hum231#1 as set forth in Table 1 (SEQ ID NOS: 16, 17, and 18, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 (e.g., the framework regions of Hum231#1).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of Hum231#2, for example, the VL CDR1, VL CDR2, and VL CDR3 of Hum231#2 as set forth in Table 1 (SEQ ID NOS: 16, 17, and 18, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 (e.g., the framework regions of Hum231#2).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab1964, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab1964 as set forth in Table 1 (SEQ ID NOS: 101, 105, and 106, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 ( e.g., the framework regions of pab1964).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of A pab1965, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab1965 as set forth in Table 1 (SEQ ID NOS: 102, 105, and 107, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 ( e.g., the framework regions of pab1965).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab1966, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab1966 as set forth in Table 1 (SEQ ID NOS: 102, 105, and 107, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 ( e.g., the framework regions of pab1966).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab1967, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab1967 as set forth in Table 1 (SEQ ID NOS: 103, 105, and 108, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 ( e.g., the framework regions of pab1967).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab1968, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab1968 as set forth in Table 1 (SEQ ID NOS: 101, 105, and 107, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 (e.g., the framework regions of pab1968).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab1969, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab1969 as set forth in Table 1 (SEQ ID NOS: 103, 105, and 109, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 ( e.g., the framework regions of pab1969).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab1970, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab1970 as set forth in Table 1 (SEQ ID NOS: 101, 105, and 109, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 (e.g., the framework regions of pab1970).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab1971, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab1971 as set forth in Table 1 (SEQ ID NOS: 103, 105, and 107, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 (e.g., the framework regions of pab1971).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab1972, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab1972 as set forth in Table 1 (SEQ ID NOS: 104, 105, and 107, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 (e.g., the framework regions of pab1972).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab1973, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab1973 as set forth in Table 1 (SEQ ID NOS: 103, 105, and 107, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 (e.g., the framework regions of pab1973).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab1975, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab1975 as set forth in Table 1 (SEQ ID NOS: 102, 105, and 107, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 ( e.g., the framework regions of pab1975).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab1976, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab1976 as set forth in Table 1 (SEQ ID NOS: 101, 105, and 107, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 ( e.g., the framework regions of pab1976).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab1977, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab1977 as set forth in Table 1 (SEQ ID NOS: 103, 105, and 107, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 ( e.g., the framework regions of pab1977).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab1979, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab1979 as set forth in Table 1 (SEQ ID NOS: 102, 105, and 107, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 (e.g., the framework regions of pab1979).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab1980, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab1980 as set forth in Table 1 (SEQ ID NOS: 101, 105, and 107, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 ( e.g., the framework regions of pab1980).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab1981, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab1981 as set forth in Table 1 (SEQ ID NOS: 103, 105, and 107, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 (e.g., the framework regions of pab1981).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab1983, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab1983 as set forth in Table 1 (SEQ ID NOS: 102, 105, and 107, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 (e.g., the framework regions of pab1983).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab2159, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab2159 as set forth in Table 1 (SEQ ID NOS: 102, 105, and 109, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 (e.g., the framework regions of pab2159).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab2160, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab2160 as set forth in Table 1 (SEQ ID NOS: 102, 105, and 107, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody. In specific embodiments, the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily. In some embodiments, the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 (e.g., the framework regions of pab2160).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, and VL CDR3 of pab2161, for example, the VL CDR1, VL CDR2, and VL CDR3 of pab2161 as set forth in Table 1 (SEQ ID NOS: 103, 105, and 109, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from a human light chain variable kappa subfamily.
- the antibody or antigen-binding fragment thereof comprises VL framework regions of an antibody set forth in Table 3 (e.g., the framework regions of pab2161).
- an antibody described herein, or an antigen-binding fragment thereof, which specifically binds to GITR comprises a heavy chain variable region (VH) comprising VH CDR1, VH CDR2, and VH CDR3 as set forth in Table 2, for example, VH CDR1, VH CDR2, and VH CDR3 of any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pabl972, pabl973, pabl975, pabl976, pabl977, pabl979, pabl980, pabl981, pabl983, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161, ( e.g., the VH CDRs in one row in Table 2).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 ( e.g., one, two, three, or four of the framework regions in one row in Table 4). In certain embodiments, the antibody or antigen-binding fragment thereof comprises one, two, three or all four of the framework regions of the heavy chain variable region sequence of SEQ ID NO: 203.
- the antibody or antigen-binding fragment thereof comprises one, two, three, or four of the framework regions of a heavy chain variable region sequence which is at least 75%, 80%, 85%, 90%, 95% or 100% identical to one, two, three or four of the framework regions of a heavy chain variable region sequence selected from the group consisting of SEQ ID NO: 201, SEQ ID NO: 206, and SEQ ID NOS: 215 to 389.
- the antibody or antigen-binding fragment thereof comprises a heavy chain variable framework region that is or is derived from an amino acid sequence encoded by a human gene, wherein the amino acid sequence is selected from the group consisting of IGHV1-2*02 (SEQ ID NO: 601), IGHV1-3*01 (SEQ ID NO: 602), IGHV1-46*01 (SEQ ID NO: 603), IGHV1-18*01 (SEQ ID NO: 604), IGHV1-69*01 (SEQ ID NO: 605), and IGHV7-4-1*02 (SEQ ID NO: 606).
- the amino acid sequence is selected from the group consisting of IGHV1-2*02 (SEQ ID NO: 601), IGHV1-3*01 (SEQ ID NO: 602), IGHV1-46*01 (SEQ ID NO: 603), IGHV1-18*01 (SEQ ID NO: 604), IGHV1-69*01 (SEQ ID NO: 605), and IGHV7-4-1*02 (SEQ ID NO:
- the heavy chain variable framework region that is derived from said amino acid sequence consists of said amino acid sequence but for the presence of up to 10 amino acid substitutions, deletions, and/or insertions, preferably up to 10 amino acid substitutions.
- the heavy chain variable framework region that is derived from said amino acid sequence consists of said amino acid sequence with 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10 amino acid residues being substituted for an amino acid found in an analogous position in a corresponding non-human heavy chain variable framework region.
- the antibody or antigen-binding fragment thereof comprises a heavy chain variable framework region that is derived from amino acid sequence SEQ ID NO: 601, wherein at least one amino acid of amino acid sequence SEQ ID NO: 601 is substituted with an amino acid in an analogous position in a corresponding non-human heavy chain variable framework region.
- the amino acid substitution is at an amino acid position selected from the group consisting of 24, 48, 67, 71, 73, and 94, wherein the amino acid position of each group member is indicated according to the Kabat numbering.
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of Hum231#1, for example, the VH CDR1, VH CDR2, and VH CDR3 of Hum231#1 as set forth in Table 2 (SEQ ID NOS: 13, 14, and 15, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of Hum231#1).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of Hum231#2, for example, the VH CDR1, VH CDR2, and VH CDR3 of Hum231#2 as set forth in Table 2 (SEQ ID NOS: 13, 14, and 15, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of Hum231#2).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab1964, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab1964 as set forth in Table 2 (SEQ ID NOS: 19, 24, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab1964).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab1965, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab1965 as set forth in Table 2 (SEQ ID NOS: 19, 25, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab1965).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab1966, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab1966 as set forth in Table 2 (SEQ ID NOS: 19, 26, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab1966).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab1967, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab1967 as set forth in Table 2 (SEQ ID NOS: 20, 27, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab1967).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab1968, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab1968 as set forth in Table 2 (SEQ ID NOS: 21, 28, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab1968).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab1969, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab1969 as set forth in Table 2 (SEQ ID NOS: 22, 29, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab1969).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab1970, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab1970 as set forth in Table 2 (SEQ ID NOS: 21, 24, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab1970).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab1971, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab1971 as set forth in Table 2 (SEQ ID NOS: 21, 177, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab1971).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab1972, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab1972 as set forth in Table 2 (SEQ ID NOS: 23, 31, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab1972).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab1973, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab1973 as set forth in Table 2 (SEQ ID NOS: 19, 32, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab1973).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab1975, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab1975 as set forth in Table 2 (SEQ ID NOS: 22, 29, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab1975).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab1976, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab1976 as set forth in Table 2 (SEQ ID NOS: 22, 29, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab1976).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab1977, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab1977 as set forth in Table 2 (SEQ ID NOS: 22, 29, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab1977).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab1979, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab1979 as set forth in Table 2 (SEQ ID NOS: 22, 33, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab1979).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab1980, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab1980 as set forth in Table 2 (SEQ ID NOS: 22, 33, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab1980).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab1981, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab1981 as set forth in Table 2 (SEQ ID NOS: 22, 33, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab1981).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab1983, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab1983 as set forth in Table 2 (SEQ ID NOS: 19, 24, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab1983).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab2159, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab2159 as set forth in Table 2 (SEQ ID NOS: 19, 144, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab2159).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab2160, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab2160 as set forth in Table 2 (SEQ ID NOS: 119, 162, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab2160).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VH CDR1, VH CDR2, and VH CDR3 of pab2161, for example, the VH CDR1, VH CDR2, and VH CDR3 of pab2161 as set forth in Table 2 (SEQ ID NOS: 22, 121, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment further comprises one, two, three or all four VH framework regions derived from a human heavy chain variable subfamily (e.g., one of subfamilies 1 to 7).
- the antibody or antigen-binding fragment thereof comprises VH framework regions of an antibody set forth in Table 4 (e.g., the framework regions of pab2161).
- an antibody described herein, or an antigen-binding fragment thereof, which specifically binds to GITR comprises (i) a heavy chain variable region (VH) comprising VH CDR1, VH CDR2, and VH CDR3 as set forth in Table 2, for example, VH CDR1, VH CDR2, and VH CDR3 of any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161, (e.g.
- VL light chain variable region
- VL comprising VL CDR1, VL CDR2, and VL CDR3 as set forth in Table 1, for example, VL CDR1, VL CDR2, and VL CDR3 of any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161, (e.g., the VL CDRs in one row in Table 1).
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively (e.g., the framework regions of a single antibody as designated by its name, for example, all of the FRs are from Hum231#1 or Hum231#2).
- the antibody or antigen-binding fragment thereof described herein comprises one, two, three or four framework regions of a heavy chain variable region sequence which is at least 75%, 80%, 85%, 90%, 95% or 100% identical to one, two, three or four of the framework regions of a heavy chain variable region sequence selected from the group consisting of SEQ ID NO: 201, SEQ ID NO: 206, and SEQ ID NOS: 215 to 389.
- the antibody or antigen-binding fragment thereof described herein comprises a heavy chain variable framework region that is or is derived from an amino acid sequence encoded by a human gene, wherein the amino acid sequence is selected from the group consisting of IGHV1-2*02 (SEQ ID NO: 601), IGHV1-3*01 (SEQ ID NO: 602), IGHV1-46*01 (SEQ ID NO: 603), IGHV1-18*01 (SEQ ID NO: 604), IGHV1-69*01 (SEQ ID NO: 605), and IGHV7-4-1*02 (SEQ ID NO: 606).
- the heavy chain variable framework region that is derived from said amino acid sequence consists of said amino acid sequence but for the presence of up to 10 amino acid substitutions, deletions, and/or insertions, preferably up to 10 amino acid substitutions.
- the heavy chain variable framework region that is derived from said amino acid sequence consists of said amino acid sequence with 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10 amino acid residues being substituted for an amino acid found in an analogous position in a corresponding non-human heavy chain variable framework region.
- the antibody or antigen-binding fragment thereof described herein comprises a heavy chain variable framework region that is derived from amino acid sequence SEQ ID NO: 601, wherein at least one amino acid in amino acid sequence SEQ ID NO: 601 is substituted with an amino acid in an analogous position in a corresponding non-human light chain variable framework region.
- the amino acid substitution is at an amino acid position selected from the group consisting of 24, 48, 67, 71, 73, and 94, wherein the amino acid position of each group member is indicated according to the Kabat numbering.
- the amino acid substitution is selected from the group consisting of 24G, 48I, 67A, 71V, 73K, and 94K, wherein the amino acid position of each group member is indicated according to the Kabat numbering.
- the antibody or antigen-binding fragment thereof described herein comprises VL framework regions of an antibody set forth in Table 3. In certain embodiments, the antibody or antigen-binding fragment thereof described herein comprises a light chain variable region sequence comprising one, two, three or four of the framework regions of the light chain variable region sequence of SEQ ID NO: 204 or SEQ ID NO: 205.
- the antibody or antigen-binding fragment thereof described herein comprises one, two, three or four framework regions of a light chain variable region sequence which is at least 75%, 80%, 85%, 90%, 95%, or 100% identical to one, two, three or four of the framework regions of a light chain variable region sequence selected from the group consisting of SEQ ID NO: 202, SEQ ID NO: 207, SEQ ID NO: 208, and SEQ ID NOs: 400-518.
- the antibody or antigen-binding fragment thereof described herein comprises one, two, three or four framework regions of a light chain variable region sequence which is at least 75%, 80%, 85%, 90%, 95%, or 100% identical to one, two, three or four of the framework regions of the light chain variable region sequence of SEQ ID NO: 519.
- an antibody or antigen-binding fragment thereof described herein comprises a light chain variable framework region that is or is derived from an amino acid sequence encoded by a human gene, wherein the amino acid sequence is selected from the group consisting of IGKV4-1*01 (SEQ ID NO: 607) and IGKV3-7*02 (SEQ ID NO: 608).
- the light chain variable framework region that is derived from said amino acid sequence consists of said amino acid sequence but for the presence of up to 10 amino acid substitutions, deletions, and/or insertions, preferably up to 10 amino acid substitutions.
- the light chain variable framework region that is derived from said amino acid sequence consists of said amino acid sequence with 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10 amino acid residues being substituted for an amino acid found in an analogous position in a corresponding non-human light chain variable framework region.
- an antibody or antigen-binding fragment thereof described herein comprises a light chain variable framework region that is derived from amino acid sequence SEQ ID NO: 607 or SEQ ID NO: 608, wherein at least one amino acid of amino acid sequence SEQ ID NO: 607 or SEQ ID NO: 608 with an amino acid in an analogous position in a corresponding non-human light chain variable framework region.
- the amino acid substitution is at amino acid position 87, wherein the amino acid position is indicated according to the Kabat numbering.
- the amino acid substitution is 87H, wherein the amino acid position is indicated according to the Kabat numbering.
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of Hum231#1, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of Hum231#1 as set forth in Tables 1 and 2 (SEQ ID NOS: 16, 17, 18, 13, 14, and 15, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VH of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of Hum231#1).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of Hum231#2, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of Hum231#2 as set forth in Tables 1 and 2 (SEQ ID NOS: 16, 17, 18, 13, 14, and 15, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the frameworks of a single antibody as designated by its name, for example, the framework regions of Hum231#1 or Hum231#2).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1964, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1964 as set forth in Tables 1 and 2 (SEQ ID NOS: 101, 105, 106, 19, 24, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab1964).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1965, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1965 as set forth in Tables 1 and 2 (SEQ ID NOS: 102, 105, 107, 19, 25, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab1965).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1966, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1966 as set forth in Tables 1 and 2 (SEQ ID NOS: 102, 105, 107, 19, 26, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab1966).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1967, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1967 as set forth in Tables 1 and 2 (SEQ ID NOS: 103, 105, 108, 20, 27, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab1967).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1968, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1968 as set forth in Tables 1 and 2 (SEQ ID NOS: 101, 105, 107, 21, 28, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab1968).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1969, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1969 as set forth in Tables 1 and 2 (SEQ ID NOS: 103, 105, 109, 22, 29, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab1969).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1970, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1970 as set forth in Tables 1 and 2 (SEQ ID NOS: 101, 105, 109, 21, 24, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab1970).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1971, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1971 as set forth in Tables 1 and 2 (SEQ ID NOS: 103, 105, 107, 21, 177, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab1971).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1972, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1972 as set forth in Tables 1 and 2 (SEQ ID NOS: 104, 105, 107, 23, 31, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab1972).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1973, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1973 as set forth in Tables 1 and 2 (SEQ ID NOS: 103, 105, 107, 19, 32, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab1973).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1975, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1975 as set forth in Tables 1 and 2 (SEQ ID NOS: 102, 105, 107, 22, 29, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab1975).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1976, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1976 as set forth in Tables 1 and 2 (SEQ ID NOS: 101, 105, 107, 22, 29, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab1976).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1977, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1977 as set forth in Tables 1 and 2 (SEQ ID NOS: 103, 105, 107, 22, 29, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab1977).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1979, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1979 as set forth in Tables 1 and 2 (SEQ ID NOS: 102, 105, 107, 22, 33, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab1979).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1980, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1980 as set forth in Tables 1 and 2 (SEQ ID NOS: 101, 105, 107, 22, 33, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab1980).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1981, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1981 as set forth in Tables 1 and 2 (SEQ ID NOS: 103, 105, 107, 22, 33, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab1981).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1983, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab1983 as set forth in Tables 1 and 2 (SEQ ID NOS: 102, 105, 107, 19, 24, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab1983).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab2159, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab2159 as set forth in Tables 1 and 2 (SEQ ID NOS: 102, 105, 109, 19, 144, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab2159).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab2160, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab2160 as set forth in Tables 1 and 2 (SEQ ID NOS: 102, 105, 107, 119, 162, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab2160).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab2161, for example, the VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and VH CDR3 of pab2161 as set forth in Tables 1 and 2 (SEQ ID NOS: 103, 105, 109, 22, 121, and 34, respectively).
- the antibody or antigen-binding fragment further comprises one, two, three or all four VL framework regions derived from the VL of a human or primate antibody and one, two, three or all four VH framework regions derived from the VH of a human or primate antibody.
- the antibody or antigen-binding fragment thereof comprises VL framework regions and VH framework regions of an antibody set forth in Tables 3 and 4, respectively ( e.g., the framework regions of pab2161).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising the amino acid sequence of a VL domain of an antibody listed in Figure 23 or any one of Figures 24A-24C ( e.g., the VL domain in one row of Figure 23 or any one of Figures 24A-24C ).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising the amino acid sequence of a VL domain of an antibody listed in Table 17 ( e.g., the VL domain in one row of Table 17).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 207 ( e.g., antibody Hum231#1).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 208 ( e.g., antibody Hum231#2).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 435 ( e.g., antibody pab1964).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 437 ( e.g., antibody pab1965).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 440 ( e.g., antibody pab1966).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 441 ( e.g., antibody pab1967).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 444 ( e.g., antibody pab1968).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 458 ( e.g., antibody pab1969).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 459 ( e.g., antibody pab1970).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 453 ( e.g., antibody pab1971).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 463 ( e.g., antibody pab1972).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 519 ( e.g., antibody pab1973).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 440 ( e.g., antibody pab1975).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 444 ( e.g., antibody pab1976).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 453 ( e.g., antibody pab1977).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 440 ( e.g., antibody pab1979).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 444 ( e.g., antibody pab1980).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 453 ( e.g., antibody pab1981).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 440 ( e.g., antibody pab1983).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 408 ( e.g., antibody pab2159).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 423 ( e.g., antibody pab2160).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 486 ( e.g., antibody pab2161).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of the amino acid sequence of a VL domain of an antibody listed in Figure 23 or any one of Figures 24A-24C (e.g., the VL domain in one row of Figure 23 or any one of Figures 24A-24C ).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of the amino acid sequence of a VL domain of an antibody listed in Table 17 ( e.g., the VL domain in one row of Table 17).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 207 ( e.g., antibody Hum231#1).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 208 ( e.g., antibody Hum23 1#2).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 435 ( e.g., antibody pab1964).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 437 ( e.g., antibody pab1965).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 440 ( e.g., antibody pab1966).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 441 ( e.g., antibody pab1967).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 444 ( e.g., antibody pab1968).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 458 ( e.g., antibody pab1969).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 459 ( e.g., antibody pab1970).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 453 ( e.g., antibody pab1971).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 463 ( e.g., antibody pab1972).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 519 ( e.g., antibody pab1973).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 440 ( e.g., antibody pab1975).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 444 ( e.g., antibody pab1976).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 453 ( e.g., antibody pab1977).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 440 ( e.g., antibody pab1979).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 444 ( e.g., antibody pab1980).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 453 ( e.g., antibody pab1981).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 440 ( e.g., antibody pab1983).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 408 ( e.g., antibody pab2159).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 423 ( e.g., antibody pab2160).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain consisting of or consisting essentially of SEQ ID NO: 486 ( e.g., antibody pab2161).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising the amino acid sequence of a VH domain of an antibody listed in Figure 23 or any one of Figures 24A-24C ( e.g., the VH domain in one row in Figure 23 or any one of Figures 24A-24C ).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising the amino acid sequence of a VH domain of an antibody listed in Table 17 ( e.g., the VH domain in one row in Table 17).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 206 ( e.g., antibody Hum231#1).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 206 ( e.g., antibody Hum231#2).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 249 ( e.g., antibody pab1964).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 251 ( e.g., antibody pab1965).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 254 ( e.g., antibody pab1966).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 255 ( e.g., antibody pab1967).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 259 ( e.g., antibody pab1968).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 276 ( e.g., antibody pab1969).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 277 ( e.g., antibody pab1970).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 280 ( e.g., antibody pab1971).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 284 ( e.g., antibody pab1972).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 304 ( e.g., antibody pab1973).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 276 ( e.g., antibody pab1975).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 276 ( e.g., antibody pab1976).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 276 ( e.g., antibody pab1977).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 345 ( e .g., antibody pab1979).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 345 ( e.g., antibody pab1980).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 345 ( e.g., antibody pab1981).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 249 ( e.g., antibody pab1983).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 224 ( e.g., antibody pab2159).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 237 ( e.g., antibody pab2160).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain comprising SEQ ID NO: 315 ( e.g., antibody pab2161).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of the amino acid sequence of a VH domain of an antibody listed in Figure 23 or any one of Figures 24A-24C ( e.g., the VH domain in one row in Figure 23 or any one of Figures 24A-24C ).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of the amino acid sequence of a VH domain of an antibody listed in Table 17 ( e.g., the VH domain in one row in Table 17).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 206 ( e.g., antibody Hum231#1).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 206 ( e.g., antibody Hum231#2).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 249 ( e.g., antibody pab1964).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 251 ( e.g., antibody pab1965).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 254 ( e.g., antibody pab1966).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 255 ( e.g., antibody pab1967).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 259 ( e.g., antibody pab1968).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 276 ( e.g., antibody pab1969).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 277 ( e.g., antibody pab1970).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 280 ( e.g., antibody pab1971).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 284 ( e.g., antibody pab1972).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 304 ( e.g., antibody pab1973).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 276 ( e.g., antibody pab1975).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 276 ( e.g., antibody pab1976).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 276 ( e.g., antibody pab1977).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 345 ( e.g., antibody pab1979).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 345 ( e.g., antibody pab1980).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 345 ( e.g., antibody pab1981).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 249 ( e.g., antibody pab1983).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 224 ( e.g., antibody pab2159).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 237 ( e.g., antibody pab2160).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain consisting of or consisting essentially of SEQ ID NO: 315 ( e.g., antibody pab2161).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain and a VL domain, wherein the VH domain and the VL domain comprise the amino acid sequence of a VH domain and a VL domain of an antibody listed in Figure 23 or any one of Figures 24A-24C ( e.g., the VH domain and VL domain in one row of Figure 23 or any one of Figures 24A-24C ).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain and a VL domain, wherein the VH domain and the VL domain comprise the amino acid sequence of a VH domain and a VL domain of an antibody listed in Table 17 (e.g., the VH domain and VL domain in one row of Table 17).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 207 and a VH domain comprising SEQ ID NO: 206 ( e.g., antibody Hum231#1).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 208 and a VH domain comprising SEQ ID NO: 206 ( e.g., antibody Hum231#2).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 435 and a VH domain comprising SEQ ID NO: 249 ( e.g., antibody pab1964).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 437 and a VH domain comprising SEQ ID NO: 251 ( e.g., antibody pab1965).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 440 and a VH domain comprising SEQ ID NO: 254 ( e.g., antibody pab1966).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 441 and a VH domain comprising SEQ ID NO: 255 ( e.g., antibody pab1967).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 444 and a VH domain comprising SEQ ID NO: 259 ( e.g., antibody pab1968).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 458 and a VH domain comprising SEQ ID NO: 276 ( e.g., antibody pab1969).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 459 and a VH domain comprising SEQ ID NO: 277 ( e.g., antibody pab1970).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 453 and a VH domain comprising SEQ ID NO: 280 ( e.g., antibody pab1971).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 463 and a VH domain comprising SEQ ID NO: 284 ( e.g., antibody pab1972).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 519 and a VH domain comprising SEQ ID NO: 304 ( e.g., antibody pab1973).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 440 and a VH domain comprising SEQ ID NO: 276 ( e.g., antibody pab1975).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 444 and a VH domain comprising SEQ ID NO: 276 ( e.g., antibody pab1976).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 453 and a VH domain comprising SEQ ID NO: 276 ( e.g., antibody pab1977).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 440 and a VH domain comprising SEQ ID NO: 345 ( e.g., antibody pab1979).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 444 and a VH domain comprising SEQ ID NO: 345 ( e.g., antibody pab1980).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 453 and a VH domain comprising SEQ ID NO: 345 ( e.g., antibody pab1981).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 440 and a VH domain comprising SEQ ID NO: 249 ( e.g., antibody pab1983).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 408 and a VH domain comprising SEQ ID NO: 224 ( e.g., antibody pab2159).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 423 and a VH domain comprising SEQ ID NO: 237 ( e.g., antibody pab2160).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain comprising SEQ ID NO: 486 and a VH domain comprising SEQ ID NO: 315 ( e.g., antibody pab2161).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain and a VL domain, wherein the VH domain and the VL domain consist of or consist essentially of the amino acid sequence of a VH domain and a VL domain of an antibody listed in Figure 23 or any one of Figures 24A-24C ( e.g., the VH domain and VL domain in one row of Figure 23 or any one of Figures 24A-24C ).
- an antibody or fragment thereof that specifically binds to GITR comprises a VH domain and a VL domain, wherein the VH domain and the VL domain consist of or consist essentially of the amino acid sequence of a VH domain and a VL domain of an antibody listed in Table 17 ( e.g., the VH domain and VL domain in one row of Table 17).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 207 and the VH domain consisting of or consisting essentially of SEQ ID NO: 206 ( e.g., antibody Hum231#1).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 208 and the VH domain consists of or consists essentially of SEQ ID NO: 206 ( e.g., antibody Hum231#2).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 435 and the VH domain consists of or consists essentially of SEQ ID NO: 249 ( e.g., antibody pab1964).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 437 and the VH domain consists of or consists essentially of SEQ ID NO: 251 ( e.g., antibody pab1965).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 440 and the VH domain consists of or consists essentially of SEQ ID NO: 254 ( e.g., antibody pab1966).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 441 and the VH domain consists of or consists essentially of SEQ ID NO: 255 ( e.g., antibody pab1967).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 444 and the VH domain consists of or consists essentially of SEQ ID NO: 259 ( e.g., antibody pab1968).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 458 and the VH domain consists of or consists essentially of SEQ ID NO: 276 ( e.g., antibody pab1969).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 459 and the VH domain consists of or consists essentially of SEQ ID NO: 277 ( e.g., antibody pab1970).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 453 and the VH domain consists of or consists essentially of SEQ ID NO: 280 ( e.g., antibody pab1971).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 463 and the VH domain consists of or consists essentially of SEQ ID NO: 284 ( e.g., antibody pab1972).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 519 and the VH domain consists of or consists essentially of SEQ ID NO: 304 ( e.g., antibody pab1973).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 440 and the VH domain consists of or consists essentially of SEQ ID NO: 276 ( e.g., antibody pab1975).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 444 and the VH domain consists of or consists essentially of SEQ ID NO: 276 ( e.g., antibody pab1976).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 453 and the VH domain consists of or consists essentially of SEQ ID NO: 276 ( e.g., antibody pab1977).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 440 and the VH domain consists of or consists essentially of SEQ ID NO: 345 ( e.g., antibody pab1979).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 444 and the VH domain consists of or consists essentially of SEQ ID NO: 345 ( e.g., antibody pab1980).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 453 and the VH domain consists of or consists essentially of SEQ ID NO: 345 ( e.g., antibody pab1981).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 440 and the VH domain consists of or consists essentially of SEQ ID NO: 249 ( e.g., antibody pab 1983).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 408 and the VH domain consists of or consists essentially of SEQ ID NO: 224 ( e.g., antibody pab2159).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 423 and the VH domain consists of or consists essentially of SEQ ID NO: 237 ( e.g., antibody pab2160).
- an antibody or fragment thereof that specifically binds to GITR comprises a VL domain and a VH domain, wherein the VL domain consists of or consists essentially of SEQ ID NO: 486 and the VH domain consists of or consists essentially of SEQ ID NO: 315 ( e.g., antibody pab2161).
- an antibody described herein may be described by its VL domain alone, or its VH domain alone, or by its 3 VL CDRs alone, or its 3 VH CDRs alone. See, for example, Rader C et al., (1998) PNAS 95: 8910-8915 , which is incorporated herein by reference in its entirety, describing the humanization of the mouse anti- ⁇ v ⁇ 3 antibody by identifying a complementing light chain or heavy chain, respectively, from a human light chain or heavy chain library, resulting in humanized antibody variants having affinities as high or higher than the affinity of the original antibody.
- the CDRs of an antibody can be determined according to the Chothia numbering scheme, which refers to the location of immunoglobulin structural loops (see, e.g., Chothia C & Lesk AM, (1987), J Mol Biol 196: 901-917 ; Al-Lazikani B et al., (1997) J Mol Biol 273: 927-948 ; Chothia C et al., (1992) J Mol Biol 227: 799-817 ; Tramontano A et al., (1990) J Mol Biol 215(1): 175-82 ; and U.S. Patent No. 7,709,226 ).
- Chothia numbering scheme refers to the location of immunoglobulin structural loops
- the Chothia CDR-H1 loop is present at heavy chain amino acids 26 to 32, 33, or 34
- the Chothia CDR-H2 loop is present at heavy chain amino acids 52 to 56
- the Chothia CDR-H3 loop is present at heavy chain amino acids 95 to 102
- the Chothia CDR-L1 loop is present at light chain amino acids 24 to 34
- the Chothia CDR-L2 loop is present at light chain amino acids 50 to 56
- the Chothia CDR-L3 loop is present at light chain amino acids 89 to 97.
- the end of the Chothia CDR-HI loop when numbered using the Kabat numbering convention varies between H32 and H34 depending on the length of the loop (this is because the Kabat numbering scheme places the insertions at H35A and H35B; if neither 35A nor 35B is present, the loop ends at 32; if only 35A is present, the loop ends at 33; if both 35A and 35B are present, the loop ends at 34).
- antibodies or fragments thereof that specifically bind to GITR (e.g., human GITR) and comprise one or more Chothia VL CDRs of a VL of any one of the antibodies described herein, (e.g., any one of Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161) and/or one or more Chothia VH CDRs of a VH of any one of the antibodies described herein (e.g., any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967,
- antibodies or fragments thereof that specifically bind to GITR comprise one or more CDRs, in which the Chothia and Kabat CDRs have the same amino acid sequence.
- antibodies or fragments thereof that specifically bind to GITR e.g., human GITR
- antibodies or fragments thereof that specifically bind to GITR (e.g., human GITR) and comprise Chothia CDRs of any of the antibodies described herein (e.g., Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161).
- GITR e.g., human GITR
- Chothia CDRs of any of the antibodies described herein e.g., Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab197
- the CDRs of an antibody can be determined according to the IMGT numbering system as described in Lefranc M-P, (1999) The Immunologist 7: 132-136 and Lefranc M-P et al., (1999) Nucleic Acids Res 27: 209-212 .
- VH-CDR1 is at positions 26 to 35
- VH-CDR2 is at positions 51 to 57
- VH-CDR3 is at positions 93 to 102
- VL-CDR1 is at positions 27 to 32
- VL-CDR2 is at positions 50 to 52
- VL-CDR3 is at positions 89 to 97.
- antibodies or fragments thereof that specifically bind to GITR (e.g., human GITR) and comprise CDRs of any one of the antibodies described herein (e.g., any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161), which are determined by the IMGT numbering system, for example, as described in Lefranc M-P (1999) supra and Lefranc M-P et al., (1999) supra ) .
- the CDRs of an antibody can be determined according to MacCallum RM et al., (1996) J Mol Biol 262: 732-745 . See also, e.g., Martin A. "Protein Sequence and Structure Analysis of Antibody Variable Domains," in Antibody Engineering, Kontermann and Dübel, eds., Chapter 31, pp. 422-439, Springer-Verlag, Berlin (2001 ).
- antibodies or fragments thereof that specifically bind to GITR (e.g., human GITR) and comprise CDRs of any one of the antibodies described herein (e.g., any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161), which are determined by the method in MacCallum RM et al.
- the CDRs of an antibody can be determined according to the AbM numbering scheme, which refers AbM hypervariable regions which represent a compromise between the Kabat CDRs and Chothia structural loops, and are used by Oxford Molecular's AbM antibody modeling software (Oxford Molecular Group, Inc.).
- antibodies or fragments thereof that specifically bind to GITR (e.g., human GITR) and comprise CDRs of any one of the antibodies described herein (e.g., any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161,), which are determined by the AbM numbering scheme.
- the position of one or more CDRs along the VH (e.g., CDR1, CDR2, or CDR3) and/or VL (e.g., CDR1, CDR2, or CDR3) region of an antibody described herein may vary by one, two, three, four, five, or six amino acid positions so long as immunospecific binding to GITR (e.g., human GITR) is maintained (e.g., substantially maintained, for example, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, at least 95%).
- GITR e.g., human GITR
- the length of one or more CDRs along the VH (e.g., CDR1, CDR2, or CDR3) and/or VL (e.g., CDR1, CDR2, or CDR3) region of an antibody described herein may vary ( e.g., be shorter or longer) by one, two, three, four, five, or more amino acids, so long as immunospecific binding to GITR (e.g., human GITR) is maintained ( e.g., substantially maintained, for example, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, at least 95%).
- GITR e.g., human GITR
- a VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and/or VH CDR3 described herein may be one, two, three, four, five or more amino acids shorter than one or more of the CDRs described herein (e.g., SEQ ID NO: 1-34, 101-109, or 114-189 or SEQ ID NO: 35 or 191-194) so long as immunospecific binding to GITR (e.g., human GITR) is maintained (e.g., substantially maintained, for example, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, at least 95%).
- GITR e.g., human GITR
- a VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and/or VH CDR3 described herein may be one, two, three, four, five or more amino acids longer than one or more of the CDRs described herein (e.g., SEQ ID NO: 1-34, 101-109, or 114-189 or SEQ ID NO: 35 or 191-194) so long as immunospecific binding to GITR (e.g., human GITR) is maintained (e.g., substantially maintained, for example, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, at least 95%).
- GITR e.g., human GITR
- the amino terminus of a VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and/or VH CDR3 described herein may be extended by one, two, three, four, five or more amino acids compared to one or more of the CDRs described herein (e.g., SEQ ID NO: 1-34, 101-109, or 114-189 or SEQ ID NO: 35 or 191-194) so long as immunospecific binding to GITR (e.g., human GITR) is maintained (e.g., substantially maintained, for example, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, at least 95%).
- GITR e.g., human GITR
- the carboxy terminus of a VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and/or VH CDR3 described herein may be extended by one, two, three, four, five or more amino acids compared to one or more of the CDRs described herein (e.g., SEQ ID NO: 1-34, 101-109, or 114-189 or SEQ ID NO: 35 or 191-194) so long as immunospecific binding to GITR (e.g., human GITR) is maintained (e.g., substantially maintained, for example, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, at least 95%).
- GITR e.g., human GITR
- the amino terminus of a VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and/or VH CDR3 described herein may be shortened by one, two, three, four, five or more amino acids compared to one or more of the CDRs described herein (e.g., SEQ ID NO: 1-34, 101-109, or 114-189 or SEQ ID NO: 35 or 191-194) so long as immunospecific binding to GITR (e.g., human GITR) is maintained (e.g., substantially maintained, for example, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, at least 95%).
- GITR e.g., human GITR
- the carboxy terminus of a VL CDR1, VL CDR2, VL CDR3, VH CDR1, VH CDR2, and/or VH CDR3 described herein may be shortened by one, two, three, four, five or more amino acids compared to one or more of the CDRs described herein (e.g., SEQ ID NO: 1-34, 101-109, or 114-189 or SEQ ID NO: 35 or 191-194) so long as immunospecific binding to GITR (e.g., human GITR) is maintained (e.g., substantially maintained, for example, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, at least 95%).
- GITR e.g., human GITR
- GITR e.g., human GITR
- Any method known in the art can be used to ascertain whether immunospecific binding to GITR (e.g., human GITR) is maintained, for example, the binding assays and conditions described in the "Examples" section (Section 6) provided herein.
- an antibody comprising an antibody light chain and heavy chain, e.g., a separate light chain and heavy chain.
- the light chain of an antibody described herein is a kappa light chain.
- the light chain of an antibody described herein is a lambda light chain.
- the light chain of an antibody described herein is a human kappa light chain or a human lambda light chain.
- an antibody described herein, which immunospecifically binds to an GITR polypeptide comprises a light chain wherein the amino acid sequence of the VL domain comprises any amino acid sequence described herein ( e.g., SEQ ID NO: 202, 204, 205, 207, 208, or 400-518), and wherein the constant region of the light chain comprises the amino acid sequence of a human kappa light chain constant region.
- an antibody described herein which immunospecifically binds to an GITR polypeptide (e.g., human GITR) comprises a light chain wherein the amino acid sequence of the VL domain comprises any amino acid sequence described herein ( e.g., SEQ ID NO: 519), and wherein the constant region of the light chain comprises the amino acid sequence of a human kappa light chain constant region.
- an antibody described herein which immunospecifically binds an GITR (e.g., human GITR) comprises a light chain wherein the amino acid sequence of the VL domain can comprise any amino acid sequence described herein (e.g., SEQ ID NO: 202, 204, 205, 207, 208, or 400-518), and wherein the constant region of the light chain comprises the amino acid sequence of a human lambda light chain constant region.
- an antibody described herein which immunospecifically binds an GITR (e.g., human GITR) comprises a light chain wherein the amino acid sequence of the VL domain can comprise any amino acid sequence described herein ( e.g., SEQ ID NO: 519), and wherein the constant region of the light chain comprises the amino acid sequence of a human lambda light chain constant region.
- an antibody described herein, which immunospecifically binds to GITR comprises a light chain wherein the amino acid of the VL domain comprises (SEQ ID NOs: 207 or 208) and wherein the constant region of the light chain comprises the amino acid sequence of a human kappa or lambda light chain constant region.
- GITR e.g., human GITR
- the amino acid of the VL domain comprises (SEQ ID NOs: 207 or 208) and wherein the constant region of the light chain comprises the amino acid sequence of a human kappa or lambda light chain constant region.
- Non-limiting examples of human constant region sequences have been described in the art, e.g., see U.S. Patent No. 5,693,780 and Kabat EA et al., (1991) supra.
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises a light chain comprising the amino acid sequence selected from the group consisting of SEQ ID NO: 555, 556, 571-576, and 580.
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises a light chain comprising the amino acid sequence selected from the group consisting of SEQ ID NOs: 571-576.
- the heavy chain of an antibody described herein can be an alpha ( ⁇ ), delta ( ⁇ ), epsilon ( ⁇ ), gamma ( ⁇ ) or mu ( ⁇ ) heavy chain.
- the heavy chain of an antibody described can comprise a human alpha ( ⁇ ), delta ( ⁇ ), epsilon ( ⁇ ), gamma ( ⁇ ) or mu ( ⁇ ) heavy chain.
- an antibody described herein which immunospecifically binds to GITR (e.g., human GITR), comprises a heavy chain wherein the amino acid sequence of the VH domain can comprise any amino acid sequence described herein (e.g., any of SEQ ID NO: 201, 203, 206, or 215-389), and wherein the constant region of the heavy chain comprises the amino acid sequence of a human gamma ( ⁇ ) heavy chain constant region.
- an antibody described herein which specifically binds to GITR (e.g., human GITR), comprises a heavy chain wherein the amino acid sequence of the VH domain comprises an amino acid sequence selected from the group consisting of SEQ ID NOs: 553, 554, 567-570, and 579, and wherein the constant region of the heavy chain comprises the amino acid of a human heavy chain described herein or known in the art.
- an antibody described herein which specifically binds to GITR (e.g., human GITR), comprises a heavy chain wherein the amino acid sequence of the VH domain comprises an amino acid sequence selected from the group consisting of SEQ ID NOs: 581 and 582, and wherein the constant region of the heavy chain comprises the amino acid of a human heavy chain described herein or known in the art.
- GITR e.g., human GITR
- the constant region of the heavy chain comprises the amino acid of a human heavy chain described herein or known in the art.
- Non-limiting examples of human constant region sequences have been described in the art, e.g., see U.S. Patent No. 5,693,780 and Kabat EA et al., (1991) supra.
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises a heavy chain comprising the amino acid sequence selected from the group consisting of SEQ ID NO: 553, 554, 567-570, and 579.
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises a heavy chain comprising the amino acid sequence selected from the group consisting of SEQ ID NO: 581 and 582.
- an antibody or fragment thereof, which binds to GITR comprises a heavy chain comprising the amino acid sequence of SEQ ID NOs: 567-570.
- an antibody described herein, which immunospecifically binds to GITR comprises a VL domain and a VH domain comprising any amino acid sequences described herein, and wherein the constant regions comprise the amino acid sequences of the constant regions of an IgG, IgE, IgM, IgD, IgA or IgY immunoglobulin molecule, or a human IgG, IgE, IgM, IgD, IgA or IgY immunoglobulin molecule.
- an antibody described herein which immunospecifically binds to GITR (e.g., human GITR) comprises a VL domain and a VH domain comprising any amino acid sequences described herein, and wherein the constant regions comprise the amino acid sequences of the constant regions of an IgG, IgE, IgM, IgD, IgA or IgY immunoglobulin molecule, any class ( e.g., IgG 1 , IgG 2 , IgG 3 , IgG 4 , IgA 1 and IgA 2 ), or any subclass ( e.g., IgG 2a and IgG 2b ) of immunoglobulin molecule.
- the constant regions comprise the amino acid sequences of the constant regions of a human IgG, IgE, IgM, IgD, IgA or IgY immunoglobulin molecule, any class ( e.g., IgG 1 , IgG 2 , IgG 3 , IgG 4 , IgA 1 and IgA 2 ), or any subclass (e.g., IgG 2a and IgG 2b ) of immunoglobulin molecule.
- any class e.g., IgG 1 , IgG 2 , IgG 3 , IgG 4 , IgA 1 and IgA 2
- subclass e.g., IgG 2a and IgG 2b
- an antibody described herein which immunospecifically binds to GITR (e.g., human GITR), comprises a VL domain and a VH domain comprising any amino acid sequences described herein, and wherein the constant regions comprise the amino acid sequences of the constant regions of a human IgG 1 ( e.g., allotypes G1m3, G1m17,1 or G1m17,1,2) or human IgG 4 .
- GITR e.g., human GITR
- an antibody described herein which immunospecifically binds to an GITR (e.g., human GITR) comprises a VL domain and a VH domain comprising any amino acid sequences described herein, and wherein the constant regions comprise the amino acid sequences of the constant region of a human IgG 1 (allotype Glm3).
- GITR e.g., human GITR
- the constant regions comprise the amino acid sequences of the constant region of a human IgG 1 (allotype Glm3).
- human constant regions are described in the art, e.g., see Kabat EA et al., (1991) supra.
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises a light chain comprising the amino acid sequence selected from the group consisting of SEQ ID NO: 555, 556, 571-576, and 580 and a heavy chain comprising the amino acid sequence selected from the group consisting of SEQ ID NO: 553, 554, 567-570, and 579.
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises a light chain comprising the amino acid sequence of SEQ ID NO:576 and a heavy chain comprising the amino acid sequence selected from the group consisting of SEQ ID NO: 581 and 582.
- an antibody or fragment thereof, which specifically binds to GITR comprises a light chain comprising the amino acid sequence of SEQ ID NO: 555 or 556 and a heavy chain comprising the amino acid sequence of SEQ ID NO: 554.
- one, two or more mutations are introduced into the Fc region of an antibody described herein or a fragment thereof (e.g., CH2 domain (residues 231-340 of human IgG 1 ) and/or CH3 domain (residues 341-447 of human IgG 1 ) and/or the hinge region, with numbering according to the Kabat numbering system (e.g., the EU index in Kabat)) to alter one or more functional properties of the antibody, such as serum half-life, complement fixation, Fc receptor binding and/or antigen-dependent cellular cytotoxicity.
- a fragment thereof e.g., CH2 domain (residues 231-340 of human IgG 1 ) and/or CH3 domain (residues 341-447 of human IgG 1 ) and/or the hinge region, with numbering according to the Kabat numbering system (e.g., the EU index in Kabat)) to alter one or more functional properties of the antibody, such as serum half-life,
- one, two or more mutations are introduced into the hinge region of the Fc region (CHI domain) such that the number of cysteine residues in the hinge region are altered ( e.g., increased or decreased) as described in, e.g., U.S. Patent No. 5,677,425 .
- the number of cysteine residues in the hinge region of the CH1 domain may be altered to, e.g., facilitate assembly of the light and heavy chains, or to alter ( e.g., increase or decrease) the stability of the antibody.
- one, two or more mutations are introduced into the Fc region of an antibody described herein or a fragment thereof (e.g., CH2 domain (residues 231-340 of human IgG 1 ) and/or CH3 domain (residues 341-447 of human IgG 1 ) and/or the hinge region, with numbering according to the Kabat numbering system (e.g., the EU index in Kabat)) to increase or decrease the affinity of the antibody for an Fc receptor (e.g., an activated Fc receptor) on the surface of an effector cell.
- an Fc receptor e.g., an activated Fc receptor
- Mutations in the Fc region of an antibody or fragment thereof that decrease or increase the affinity of an antibody for an Fc receptor and techniques for introducing such mutations into the Fc receptor or fragment thereof are known to one of skill in the art. Examples of mutations in the Fc receptor of an antibody that can be made to alter the affinity of the antibody for an Fc receptor are described in, e.g., Smith P et al., (2012) PNAS 109: 6181-6186 , U.S. Patent No. 6,737,056 , and International Publication Nos. WO 02/060919 ; WO 98/23289 ; and WO 97/34631 , which are incorporated herein by reference.
- one, two or more amino acid mutations are introduced into an IgG constant domain, or FcRn-binding fragment thereof (preferably an Fc or hinge-Fc domain fragment) to alter ( e.g., decrease or increase) half-life of the antibody in vivo.
- an IgG constant domain, or FcRn-binding fragment thereof preferably an Fc or hinge-Fc domain fragment
- one, two or more amino acid mutations are introduced into an IgG constant domain, or FcRn-binding fragment thereof (preferably an Fc or hinge-Fc domain fragment) to decrease the half-life of the antibody in vivo.
- one, two or more amino acid mutations are introduced into an IgG constant domain, or FcRn-binding fragment thereof (preferably an Fc or hinge-Fc domain fragment) to increase the half-life of the antibody in vivo.
- the antibodies may have one or more amino acid mutations (e.g., substitutions) in the second constant (CH2) domain (residues 231-340 of human IgG 1 ) and/or the third constant (CH3) domain (residues 341-447 of human IgG 1 ), with numbering according to the EU index in Kabat (Kabat EA et al., (1991) supra ) .
- the constant region of the IgG 1 of an antibody or antigen-binding fragment thereof described herein comprises a methionine (M) to tyrosine (Y) substitution in position 252, a serine (S) to threonine (T) substitution in position 254, and a threonine (T) to glutamic acid (E) substitution in position 256, numbered according to the EU index as in Kabat. See U.S. Patent No. 7,658,921 , which is incorporated herein by reference.
- an antibody or antigen-binding fragment thereof comprises an IgG constant domain comprising one, two, three or more amino acid substitutions of amino acid residues at positions 251-257, 285-290, 308-314, 385-389, and 428-436, numbered according to the EU index as in Kabat.
- one, two or more amino acid substitutions are introduced into an IgG constant domain Fc region to alter the effector function(s) of the antibody.
- one or more amino acids selected from amino acid residues 234, 235, 236, 237, 297, 318, 320 and 322, numbered according to the EU index as in Kabat can be replaced with a different amino acid residue such that the antibody has an altered affinity for an effector ligand but retains the antigen-binding ability of the parent antibody.
- the effector ligand to which affinity is altered can be, for example, an Fc receptor or the C1 component of complement. This approach is described in further detail in U.S. Patent Nos. 5,624,821 and 5,648,260 .
- the deletion or inactivation (through point mutations or other means) of a constant region domain may reduce Fc receptor binding of the circulating antibody thereby increasing tumor localization. See, e.g., U.S. Patent Nos. 5,585,097 and 8,591,886 for a description of mutations that delete or inactivate the constant domain and thereby increase tumor localization.
- one or more amino acid substitutions may be introduced into the Fc region of an antibody described herein to remove potential glycosylation sites on Fc region, which may reduce Fc receptor binding ( see, e.g., Shields RL et al., (2001) J Biol Chem 276: 6591-604 ).
- one or more of the following mutations in the constant region of an antibody described herein may be made: an N297A substitution; an N297Q substitution; a L235A substitution and a L237A substitution; a L234A substitution and a L235A substitution; a E233P substitution; a L234V substitution; a L235A substitution; a C236 deletion; a P238A substitution; a D265A substitution; a A327Q substitution; or a P329A substitution, numbered according to the EU index as in Kabat.
- an antibody or antigen-binding fragment thereof described herein comprises the constant domain of an IgG 1 with an N297Q or N297A amino acid substitution.
- one or more amino acids selected from amino acid residues 329, 331 and 322 in the constant region of an antibody described herein, numbered according to the EU index as in Kabat can be replaced with a different amino acid residue such that the antibody has altered C1q binding and/or reduced or abolished complement dependent cytotoxicity (CDC).
- CDC complement dependent cytotoxicity
- the Fc region of an antibody described herein is modified to increase the ability of the antibody to mediate antibody dependent cellular cytotoxicity (ADCC) and/or to increase the affinity of the antibody for an Fc ⁇ receptor by mutating one or more amino acids (e.g., introducing amino acid substitutions) at the following positions: 238, 239, 248, 249, 252, 254, 255, 256, 258, 265, 267, 268, 269, 270, 272, 276, 278, 280, 283, 285, 286, 289, 290, 292, 293, 294, 295, 296, 298, 301, 303, 305, 307, 309, 312, 315, 320, 322, 324, 326, 327, 329, 330, 331, 333, 334, 335, 337, 338, 340, 360, 373, 376, 378, 382, 388, 389, 398, 414, 416, 419, 430, 434, 435, 437, 4
- an antibody described herein comprises the constant region of an IgG 4 antibody and the serine at amino acid residue 228 of the heavy chain, numbered according to the EU index as in Kabat, is substituted for proline.
- Antibodies with reduced fucose content have been reported to have an increased affinity for Fc receptors, such as, e.g., FcyRIIIa. Accordingly, in certain embodiments, the antibodies or antigen-binding fragments thereof described herein have reduced fucose content or no fucose content.
- Such antibodies can be produced using techniques known to one skilled in the art. For example, the antibodies can be expressed in cells deficient or lacking the ability of fucosylation. In a specific example, cell lines with a knockout of both alleles of ⁇ 1,6-fucosyltransferase can be used to produce antibodies with reduced fucose content.
- the Potelligent® system (Lonza) is an example of such a system that can be used to produce antibodies with reduced fucose content.
- antibodies or antigen-binding fragments with reduced fucose content or no fucose content can be produced by, e.g.: (i) culturing cells under conditions which prevent or reduce fucosylation; (ii) posttranslational removal of fucose (e.g., with a fucosidase enzyme); (iii) post-translational addition of the desired carbohydrate, e.g., after recombinant expression of a non-glycosylated glycoprotein; or (iv) purification of the glycoprotein so as to select for antibodies or antigen-binding fragments thereof which are not fucsoylated.
- antibodies or antigen-binding fragments thereof described herein have an increased affinity for CD32B (also known as FcyRIIB or FCGR2B), e.g., as compared to an antibody with a wild-type Fc region, e.g., an IgG1 Fc.
- antibodies or antigen-binding fragments thereof described herein have a selectively increased affinity for CD32B (FcyRIIB) over both CD32A (FcyRIIA) and CD16 (FcyRIIIA).
- the antibody or antigen-binding fragment with an increased affinity for CD32B comprises a heavy chain constant region, e.g., an IgG1 constant region, or fragment thereof comprising a mutation selected from the group consisting of: G236D, P238D, S239D, S267E, L328F, L328E, an arginine inserted after position 236, and combinations thereof, numbered according to EU index ( Kabat et al., Sequences of Proteins of Immunological Interest, U.S. Department of Health and Human Services, Bethesda (1991 )).
- EU index Kabat et al., Sequences of Proteins of Immunological Interest, U.S. Department of Health and Human Services, Bethesda (1991 )
- the antibody or antigen-binding fragment with an increased affinity for CD32B comprises a heavy chain constant region, e.g., an IgG1 constant region, or fragment thereof comprising S267E and L328F substitutions. In some embodiments, the antibody or antigen-binding fragment with an increased affinity for CD32B comprises a heavy chain constant region, e.g., an IgG1 constant region, or fragment thereof comprising P238D and L328E substitutions.
- the antibody or antigen-binding fragment with an increased affinity for CD32B comprises a heavy chain constant region, e.g., an IgG1 constant region, or fragment thereof comprising a P238D substitution and substitution selected from the group consisting of E233D, G237D, H268D, P271G, A330R, and combinations thereof.
- the antibody or antigen-binding fragment with an increased affinity for CD32B comprises a heavy chain constant region, e.g., an IgG1 constant region, or fragment thereof comprising P238D, E233D, G237D, H268D, P271G, and A330R substitutions.
- the antibody or antigen-binding fragment with an increased affinity for CD32B comprises a heavy chain constant region, e.g., an IgG1 constant region, or fragment thereof comprising G236D and S267E. In some embodiments, the antibody or antigen-binding fragment with an increased affinity for CD32B comprises a heavy chain constant region, e.g., an IgG1 constant region, or fragment thereof comprising S239D and S267E. In some embodiments, the antibody or antigen-binding fragment with an increased affinity for CD32B comprises a heavy chain constant region, e.g., an IgG1 constant region, or fragment thereof comprising S267E and L328F.
- the antibody or antigen-binding fragment with an increased affinity for CD32B comprises a heavy chain constant region, e.g., an IgG1 constant region, or fragment thereof comprising an arginine inserted after position 236 and L328R.
- a heavy chain constant region e.g., an IgG1 constant region, or fragment thereof comprising an arginine inserted after position 236 and L328R.
- an antibody described herein which immunospecifically binds to GITR (e.g., human GITR), comprises a light chain and a heavy chain, wherein (i) the light chain comprises a VL domain comprising a VL CDR1, VL CDR2, and VL CDR3 having the amino acid sequences of any one of Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, Hum231#1, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161 (e.g., those listed in Table 1); (ii) the heavy chain comprises a VH domain comprising a VH CDR1, VH CDR2, and VH CDR3 having the amino acid sequences
- an antibody described herein which immunospecifically binds to GITR (e.g., human GITR), comprises a light chain and a heavy chain, wherein (i) the light chain comprises a VL domain comprising the amino acid sequence of any one of the antibodies Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, Hum231#1, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161, ( e.g., SEQ ID NO: 202, 204, 205, 207, 208, or 400-518 or SEQ ID NO:519); (ii) the heavy chain comprises a VH domain comprising the amino acid sequence of and one of the antibodies Hum231
- an antibody described herein which immunospecifically binds to GITR (e.g., human GITR), comprises a light chain and a heavy chain, wherein (i) the light chain comprises a VL domain comprising the amino acid sequence of Hum231#1 or Hum231#2 ( e.g., SEQ ID NO: 207 or 208); (ii) the heavy chain comprises a VH domain comprising the amino acid sequence of Hum231#1 or Hum231#2 ( e.g., SEQ ID NO: 206); (iii) the light chain further comprises a constant domain comprising the amino acid sequence of the constant domain of a human kappa light chain; and (iv) the heavy chain further comprises a constant domain comprising the amino acid sequence of the constant domain of a human IgG 1 (optionally IgG 1 (allotype Glm3)) heavy chain.
- the light chain comprises a VL domain comprising the amino acid sequence of Hum231#1 or Hum231#2 (e.g.
- an antibody described herein which immunospecifically binds to GITR (e.g., human GITR), comprises a light chain and a heavy chain, wherein (i) the light chain comprises a VL domain comprising a VL CDR1, VL CDR2, and VL CDR3 having the amino acid sequences of any one of the antibodies described herein, e.g ., Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, Hum231#1, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161 ( e.g., those listed in Table 1); (ii) the heavy chain comprises a VH domain comprising a VH CDR1, VH
- an antibody described herein which immunospecifically binds to GITR (e.g., human GITR), comprises a light chain and a heavy chain, wherein (i) the light chain comprises a VL domain comprising the amino acid sequence of any one of the antibodies described herein, e.g ., Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, Hum231#1, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161 (e.g., SEQ ID NO: 202, 204, 205, 207, 208, or 400-518 or SEQ ID NO: 519); (ii) the heavy chain comprises a VH domain comprising the amino
- an antibody described herein which immunospecifically binds to GITR (e.g., human GITR), comprises a light chain and a heavy chain, wherein (i) the light chain comprises a VL domain comprising the amino acid sequence of either Hum231#1 or Hum231#2 ( e.g., SEQ ID NO: 207 or 208); (ii) the heavy chain comprises a VH domain comprising the amino acid sequence of either Hum231#1 or Hum231#2 ( e.g., SEQ ID NO: 206); (iii) the light chain further comprises a constant domain comprising the amino acid sequence of the constant domain of a human IgG 4 light chain; and (iv) the heavy chain further comprises a constant domain comprising the amino acid sequence of the constant domain of a human IgG 4 heavy chain.
- the light chain comprises a VL domain comprising the amino acid sequence of either Hum231#1 or Hum231#2 (e.g., SEQ ID NO: 207 or 208);
- an antibody provided herein which specifically binds to GITR (e.g., human GITR), comprises (a) a heavy chain comprising an amino acid sequence selected from the group consisting of SEQ ID NO: 553, 554, and 567 to 570; and (b) a light chain comprising an amino acid sequence selected from the group consisting of SEQ ID NO: 555, 556, and 571 to 576.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising an amino acid sequence selected from the group consisting of SEQ ID NO: 581 or 582; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 576.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 553; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 556.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 554; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 556.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 581; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 556.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 582; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 556.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 553; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 555.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 554; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 555.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 567; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 573.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 567; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 576.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 554; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 576.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 581; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 576.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 582; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 576.
- an antibody provided herein which specifically binds to GITR (e.g., human GITR), comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 553 with an amino acid substitution of N to A or Q at amino acid position 298; and (b) a light chain comprising an amino acid sequence selected from the group consisting of SEQ ID NO: 555, 556, and 571 to 576.
- an antibody provided herein which specifically binds to GITR (e.g., human GITR), comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 553 with an amino acid substitution of N to A or Q at amino acid position 298; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 556.
- an antibody provided herein, which specifically binds to GITR comprises (a) a heavy chain comprising the amino acid sequence of SEQ ID NO: 553 with an amino acid substitution of N to A or Q at amino acid position 298; and (b) a light chain comprising the amino acid sequence of SEQ ID NO: 555.
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises one, two, three, or four VL framework regions (FRs) having the amino acid sequence described herein for any one of the antibodies described herein, e.g ., Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161 ( e.g., see Table 3).
- FRs VL framework regions
- an antibody or fragment thereof, which specifically binds to GITR comprises one, two, three, or four VH framework regions (FRs) having the amino acid sequence described herein for any one of the antibodies described herein, e.g ., Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161 ( e.g., see Table 4).
- FRs VH framework regions
- an antibody or fragment thereof, which specifically binds to GITR comprises one, two, three, four, five, six, seven, or eight of the FRs of one of the antibodies described herein (e.g., Hum231#1 Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161).
- GITR e.g., human GITR
- an antibody or fragment thereof described herein which immunospecifically binds to GITR (e.g., human GITR), comprises framework regions ( e.g., framework regions of the VL domain and/or VH domain) that are human framework regions or derived from human framework regions.
- framework regions e.g., framework regions of the VL domain and/or VH domain
- an antibody described herein comprises framework regions ( e.g., framework regions of the VL domain and/or VH domain) that are primate ( e.g., non-human primate) framework regions or derived from primate ( e.g., non-human primate) framework regions.
- CDRs from antigen-specific non-human antibodies are grafted onto homologous human or non-human primate acceptor frameworks.
- the non-human primate acceptor frameworks are from Old World apes.
- the Old World ape acceptor framework is from Pan troglodytes, Pan paniscus or Gorilla gorilla.
- the non-human primate acceptor frameworks are from the chimpanzee Pan troglodytes.
- the non-human primate acceptor frameworks are Old World monkey acceptor frameworks.
- the Old World monkey acceptor frameworks are from the genus Macaca.
- the non-human primate acceptor frameworks are is derived from the cynomolgus monkey Macaca cynomolgus.
- Non-human primate framework sequences are described in U.S. Patent Application Publication No. US 2005/0208625 .
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises one, two or more VL framework regions (FRs) having the amino acid sequences described herein for any one of the antibodies set forth in Table 3, supra.
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises one, two or more VH framework regions (FRs) having the amino acid sequences described herein for any one of the antibodies set forth in Table 4, supra.
- an antibody or fragment thereof described herein which specifically binds to GITR (e.g., human GITR), comprises one, two or more VL framework regions having the amino acid sequences described herein for any one of the antibodies set forth in Table 3, supra, and one, two or more VH framework regions having the amino acid sequences described herein for the antibodies set forth in Table 4, supra.
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the framework regions of VL domain having the amino acid sequence selected from the group consisting of SEQ ID NO: 202, 207, 208, 400-411, 413-416, 418-421, 423-448, 450-452, 454-464, 467-477, 481-486, 488-513, or 515-518 and/or the framework regions of the VH domain having the amino acid sequence selected from the group consisting of SEQ ID NO: 201, 206, 215, 217-234, 236-256, 258, 259, 261-265, 267, 268, 271-273, 276, 277, 280, 281, 283-285, 287, 288, 290, 291, 294, 296-299, 301, 304-306, 308, 313-316, 319, 320, 322-325, 327, 328, 333, 336, 338-340, 342, 34
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises the framework regions of VL domain having the amino acid sequence selected from the group consisting of SEQ ID NO: 202, 207, 208, 400-411, 413-416, 418-421, 423-448, 450-464, 467-477, 481-486, 488-513, or 515-519 and/or the framework regions of the VH domain having the amino acid sequence selected from the group consisting of SEQ ID NO: 201, 206, 215, 217-234, 236-256, 258, 259, 261-265, 267, 268, 270-273, 276, 277, 280, 281, 283-285, 287, 288, 290, 291, 294, 296-299, 301, 304-306, 308, 313-316, 319, 320, 322-325, 327, 328, 333, 336, 338-340, 342, 343, 345,
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises one, two, three or four framework regions of the VL domain having the amino acid sequence of any one of the antibodies described herein, e.g ., Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107 ( e.g., SEQ ID NO: 202, 207, 208, 400-411, 413-416, 418-421, 423-448, 450-452, 454-464, 467-477, 481-486, 488-513, or 515-518) with 1, 2, 3, 4, 5, 6, 7, 8, 9 or more amino acid mutation
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises one, two, three or four framework regions of the VL domain having the amino acid sequence of any one of the antibodies described herein, e.g ., Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161 (e.g., SEQ ID NO: 202, 207, 208, 400-411, 413-416, 418-421, 423-448, 450-464, 467-477, 481-486, 488-513, or 515-519) with 1,
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises one, two, three or four framework regions of the VH domain having the amino acid sequence of any one of the antibodies described herein, e.g ., Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, pab2161 ( e.g., SEQ ID NO: 201, 203, 206, or 215-389) with 1, 2, 3, 4, 5, 6, 7, 8, 9 or more amino acid mutations (e.g., amino acid substitutions, such as conservative amino acid substitutions) and/or the framework
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises one, two, three or four framework regions of the VL domain having the amino acid sequence of any one of the antibodies described herein, e.g ., Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107 ( e.g., SEQ ID NO: 202, 207, 208, 400-411, 413-416, 418-421, 423-448, 450-452, 454-464, 467-477, 481-486, 488-513, or 515-518) with 1, 2, 3, 4, 5, 6, 7, 8, 9 or more amino acid mutation
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises one, two, three or four framework regions of the VL domain having the amino acid sequence of any one of the antibodies described herein, e.g ., Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161 (e.g., SEQ ID NO: 202, 207, 208, 400-411, 413-416, 418-421, 423-448, 450-464, 467-477, 481-486, 488-513, or 515-519) with 1,
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises VL framework regions (FRs) having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the VL framework regions described herein in Table 3, supra.
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises VH framework regions (FRs) having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the VH framework regions described herein Table 4, supra.
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises VH framework regions (FRs) having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the VH framework regions described herein Table 4, supra, and VL framework regions (FRs) having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the VL framework regions described herein Table 3, supra.
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises VL framework regions (FRs) having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the VL framework regions described herein for antibody Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161 ( e.g., as set forth in Table 3).
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises VH framework regions (FRs) having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the VH framework regions described herein for antibody Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161 ( e.g., as set forth in Table 4).
- FRs VH framework regions having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the VH framework regions described herein for antibody Hum231#1, Hum231#2, pab
- an antibody or fragment thereof described herein, which specifically binds to GITR comprises: (i) VL framework regions (FRs) having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the VL framework regions described herein for Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161 ( e.g., as set forth in Table 3); and (ii) VH framework regions (FRs) having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity
- the determination of percent identity between two sequences can also be accomplished using a mathematical algorithm.
- a specific, non-limiting example of a mathematical algorithm utilized for the comparison of two sequences is the algorithm of Karlin S & Altschul SF (1990) PNAS 87: 2264-2268 , modified as in Karlin S & Altschul SF (1993) PNAS 90: 5873-5877 .
- Such an algorithm is incorporated into the NBLAST and XBLAST programs of Altschul SF et al., (1990) J Mol Biol 215: 403 .
- Gapped BLAST can be utilized as described in Altschul SF et al., (1997) Nuc Acids Res 25: 3389 3402 .
- PSI BLAST can be used to perform an iterated search which detects distant relationships between molecules ( Id .) .
- the default parameters of the respective programs e.g., of XBLAST and NBLAST
- NCBI National Center for Biotechnology Information
- Another specific, non limiting example of a mathematical algorithm utilized for the comparison of sequences is the algorithm of Myers and Miller, 1988, CABIOS 4:11 17 .
- Such an algorithm is incorporated in the ALIGN program (version 2.0) which is part of the GCG sequence alignment software package.
- ALIGN program version 2.0
- the percent identity between two sequences can be determined using techniques similar to those described above, with or without allowing gaps. In calculating percent identity, typically only exact matches are counted.
- an antibody or fragment thereof, which immunospecifically binds to GITR comprises a VL domain having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of the VL domain of any one of antibodies Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, Hum231#1, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161 (e.g., SEQ ID NO: 202, 204, 205, 207, 208, or 400-518 or SEQ ID NO:519).
- SEQ ID NO: 202, 204, 205, 207, 208, or 400-518 or SEQ ID NO:519)
- an antibody or fragment thereof which immunospecifically binds to GITR (e.g., human GITR), comprises a VL domain having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of the VL domain of any one of antibodies Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, Hum231#1, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161, (e.g., SEQ ID NO: 202, 204, 205, 207, 208, or 400-518 or SEQ ID NO:519), wherein the antibody or antigen-binding fragment comprises CDRs (
- an antibody or fragment thereof, which immunospecifically binds to GITR comprises a VL domain comprising VL framework regions having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of the framework regions selected from the group consisting of SEQ ID NO: 202, 204, 205, 207, 208, and 400-518.
- an antibody or fragment thereof, which immunospecifically binds to GITR comprises a VL domain comprising VL framework regions having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of SEQ ID NO: 519.
- the antibody comprises VL CDRs that are identical to the VL CDRs of an antibody set forth in Table 1 ( e.g., the VL CDRs in one row in Table 1).
- an antibody or fragment thereof, which immunospecifically binds to GITR comprises a VH domain having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of the VH domain of SEQ ID NO: 201, 203, 206, or 215-389.
- an antibody or fragment thereof which immunospecifically binds to GITR (e.g., human GITR), comprises a VH domain having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of the VH domain of SEQ ID NO: 201, 203, 206, or 215-389, wherein the antibody or antigen-binding fragment comprises CDRs ( e.g., VL CDRs) that are identical to the CDRs ( e.g., VL CDRs) of an antibody set forth in Table 1 and/or Table 2 ( e.g., the CDRs are identical to the CDRs of a particular named antibody referred to by name in Tables 1 and/or 2).
- CDRs e.g., VL CDRs
- an antibody or fragment thereof, which immunospecifically binds to GITR comprises a VH domain comprising VH framework regions having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of the framework regions selected from the group consisting of SEQ ID NO: 201, 203, 206, and 215-389.
- the antibody comprises VH CDRs that are identical to the VH CDRs of an antibody set forth in Table 2 ( e.g., the VH CDRs in one row in Table 2).
- an antibody or fragment thereof, which immunospecifically binds to GITR comprises: (i) a VL domain having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of the VL domain selected from the group consisting of SEQ ID NO: 202, 204, 205, 207, 208, and 400-518; and (ii) a VL domain having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of the VH domain of SEQ ID NO: 201, 203, 206, or 215-389.
- an antibody or fragment thereof, which immunospecifically binds to GITR comprises: (i) a VL domain having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of SEQ ID NO: 519; and (ii) a VL domain having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of the VH domain of SEQ ID NO: 304.
- an antibody or fragment thereof, which immunospecifically binds to GITR comprises: (i) a VL domain having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of the VL domain selected from the group consisting of SEQ ID NO: 202, 204, 205, 207, 208, and 400-518; and (ii) a VH domain having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of the VH domain selected from the group of SEQ ID NO: 201, 203, 206, and 215-389, wherein the antibody or antigen-binding fragment comprises CDRs (e.g., VL CDRs) that are identical to the CDRs ( e.g., VL CDRs) of an antibody set forth in Table 1 and/or Table 2 ( e.g., the
- an antibody or fragment thereof which immunospecifically binds to GITR (e.g., human GITR), comprises: (i) a VL domain having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of SEQ ID NO:519; and (ii) a VH domain having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of SEQ ID NO:304, wherein the antibody or antigen-binding fragment comprises CDRs (e.g., VL CDRs) that are identical to the CDRs ( e.g., VL CDRs) of an antibody set forth in Table 1 and/or Table 2 ( e.g., the CDRs are identical to the CDRs of a particular antibody referred to by name in Tables 1 and/or 2).
- CDRs e.g., VL CDRs
- an antibody or fragment thereof, which immunospecifically binds to GITR comprises: (i) a VL domain comprising VL framework regions having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of the framework regions selected from the group consisting of SEQ ID NO: 202, 204, 205, 207, and 208; and (ii) a VH domain comprising VH framework regions having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of the framework regions selected from the group consisting of SEQ ID NO: 201, 203, 206, and 215-389.
- the antibody comprises VL CDRs that are identical to the VL CDRs of an antibody set forth in Table 3 and/or VH CDRs that are identical to the VH CDRs of an antibody set forth in Table 4.
- an antibody or fragment thereof, which immunospecifically binds to GITR comprises a VH domain having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of the VH domain selected from the group of SEQ ID NO: 201, 203, 206, and 215-389.
- an antibody or fragment thereof which immunospecifically binds to GITR (e.g., human GITR), comprises a VH domain having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of the VH domain selected from the group of SEQ ID NO: 201, 203, 206, and 215-389, wherein the antibody or antigen-binding fragment comprises CDRs (e.g., VL CDRs) that are identical to the CDRs (e.g., VL CDRs) of an antibody set forth in Table 1 and/or Table 2 ( e.g., the CDRs are identical to the CDRs of a particular antibody referred to in Tables 1 and/or 2).
- CDRs e.g., VL CDRs
- an antibody or fragment thereof, which immunospecifically binds to GITR comprises a VH domain comprising VH framework regions having at least 80%, at least 85%, at least 90%, at least 95%, or at least 98% sequence identity to the amino acid sequence of the framework regions selected from the group consisting of SEQ ID NO: 201, 203, 206, and 215-389.
- the antibody comprises VH CDRs that are identical to the VH CDRs of an antibody set forth in Table 2 ( e.g., the VH CDRs in one row in Table 2).
- antibodies that bind the same or an overlapping epitope of GITR (e.g., an epitope of human GITR) as an antibody described herein (e.g., antibody Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107), or antibodies pab2159, pab2160, pab2161, or Hum231#2w.
- an overlapping epitope of GITR e.g., an epitope of human GITR
- an antibody described herein e.g., antibody Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970,
- the epitope of an antibody can be determined by, e.g., NMR spectroscopy, X-ray diffraction crystallography studies, ELISA assays, hydrogen/deuterium exchange coupled with mass spectrometry (e.g., liquid chromatography electrospray mass spectrometry), array-based oligo-peptide scanning assays, and/or mutagenesis mapping (e.g., site-directed mutagenesis mapping).
- crystallization may be accomplished using any of the known methods in the art (e.g., Giegé R et al., (1994) Acta Crystallogr D Biol Crystallogr 50(Pt 4): 339-350 ; McPherson A (1990) Eur J Biochem 189: 1-23 ; Chayen NE (1997) Structure 5: 1269-1274 ; McPherson A (1976) J Biol Chem 251: 6300-6303 ).
- Antibody:antigen crystals may be studied using well known X-ray diffraction techniques and may be refined using computer software such as X-PLOR (Yale University, 1992, distributed by Molecular Simulations, Inc.; see e.g.
- the epitope of an antibody or antigen-binding fragment thereof is determined using alanine scanning mutagenesis studies, such as described in Section 6, infra.
- antibodies that recognize and bind to the same or overlapping epitopes of GITR can be identified using routine techniques such as an immunoassay, for example, by showing the ability of one antibody to block the binding of another antibody to a target antigen, i.e., a competitive binding assay.
- Competition binding assays also can be used to determine whether two antibodies have similar binding specificity for an epitope.
- Competitive binding can be determined in an assay in which the immunoglobulin under test inhibits specific binding of a reference antibody to a common antigen, such as GITR.
- such an assay involves the use of purified antigen (e.g., GITR such as human GITR) bound to a solid surface or cells bearing either of these, an unlabeled test immunoglobulin and a labeled reference immunoglobulin.
- GITR purified antigen
- Competitive inhibition can be measured by determining the amount of label bound to the solid surface or cells in the presence of the test immunoglobulin.
- the test immunoglobulin is present in excess.
- a competing antibody is present in excess, it will inhibit specific binding of a reference antibody to a common antigen by at least 50-55%, 55-60%, 60-65%, 65-70% 70-75% or more.
- a competition binding assay can be configured in a large number of different formats using either labeled antigen or labeled antibody.
- the antigen is immobilized on a 96-well plate.
- the ability of unlabeled antibodies to block the binding of labeled antibodies to the antigen is then measured using radioactive or enzyme labels.
- a competition assay is performed using surface plasmon resonance (BIAcore®), e.g., by an 'in tandem approach' such as that described by Abdiche YN et al., (2009) Analytical Biochem 386: 172-180 , whereby GITR antigen is immobilized on the chip surface, for example, a CM5 sensor chip and the anti-GITR antibodies are then run over the chip.
- GITR antigen is immobilized on the chip surface, for example, a CM5 sensor chip and the anti-GITR antibodies are then run over the chip.
- the anti-GITR antibody is first run over the chip surface to achieve saturation and then the potential, competing antibody is added. Binding of the competing antibody can then be determined and quantified relative to a non-competing control.
- competition binding assays can be used to determine whether an antibody is competitively blocked, e.g., in a dose dependent manner, by another antibody for example, an antibody binds essentially the same epitope, or overlapping epitopes, as a reference antibody, when the two antibodies recognize identical or sterically overlapping epitopes in competition binding assays such as competition ELISA assays, which can be configured in all number of different formats, using either labeled antigen or labeled antibody.
- an antibody can be tested in competition binding assays with an antibody described herein (e.g., antibody Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, pab2161, or Hum231#2w), or a chimeric or Fab antibody thereof, or an antibody comprising VH CDRs and VL CDRs of an antibody described herein ( e.g., Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pa
- antibodies that compete (e.g., in a dose dependent manner) for binding to GITR (e.g., human GITR) with an antibody described herein (e.g., Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107), or antibodies pab2159, pab2160, pab2161, or Hum231#2w, as determined using assays known to one of skill in the art or described herein (e.g., ELISA competitive assays or surface plasmon resonance).
- GITR e.g., human GITR
- an antibody described herein e.g., Hum231#1, Hum231#2, pab1964, pab1965, pab
- antibodies that competitively inhibit (e.g., in a dose dependent manner) an antibody described herein (e.g., Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, pab2161, or Hum231#2w) from binding to GITR (e.g., human GITR), as determined using assays known to one of skill in the art or described herein (e.g., ELISA competitive assays, or suspension array or surface plasmon resonance assay described in Example 6, infra ) .
- GITR e.g., human GITR
- such competitively blocking antibody activates, induces or enhances one or more GITR activities.
- an antibody which competes (e.g., in a dose dependent manner) for specific binding to GITR (e.g., human GITR), with an antibody comprising the amino acid sequences described herein (e.g., VL and/or VH amino acid sequences of antibody Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161, or Hum231#2w), as determined using assays known to one of skill in the art or described herein (e.g., EL
- an antibody that competes with an antibody described herein for binding to GITR (e.g., human GITR) to the same extent that the antibody described herein self-competes for binding to GITR (e.g., human GITR).
- a first antibody that competes with an antibody described herein for binding to GITR (e.g., human GITR), wherein the first antibody competes for binding in an assay comprising the following steps: (a) incubating GITR-transfected cells with the first antibody in unlabeled form in a container; and (b) adding an antibody described herein in labeled form in the container and incubating the cells in the container; and (c) detecting the binding of the antibody described herein in labeled form to the cells.
- GITR e.g., human GITR
- a first antibody that competes with an antibody described herein for binding to GITR (e.g., human GITR), wherein the competition is exhibited as reduced binding of the first antibody to GITR by more than 80% ( e.g., 85%, 90%, 95%, or 98%, or between 80% to 85%, 80% to 90%, 85% to 90%, or 85% to 95%).
- an antibody which competes ( e.g., in a dose dependent manner) for specific binding to GITR (e.g., human GITR), with an antibody comprising a VL domain having the amino acid sequence selected from the group consisting of SEQ ID 202, 207, 208, 400-411, 413-416, 418-421, 423-448, 450-452, 454-464, 467-477, 481-486, 488-513, and 515-518, and a VH domain having the amino acid sequence selected from the group consisting of SEQ ID NO: 201, 206, 215, 217-234, 236-256, 258, 259, 261-265, 267, 268, 271-273, 276, 277, 280, 281, 283-285, 287, 288, 290, 291, 294, 296-299, 301, 304-306, 308, 313-316, 319, 320, 322-325, 327, 328, 333, 336, 3
- an antibody which competes ( e.g., in a dose dependent manner) for specific binding to GITR (e.g., human GITR), with an antibody comprising a VL domain having the amino acid sequence selected from the group consisting of SEQ ID 202, 207, 208, 400-411, 413-416, 418-421, 423-448, 450-452, 454-464, 467-477, 481-486, 488-513, and 515-519, and a VH domain having the amino acid sequence selected from the group consisting of SEQ ID NO: 201, 206, 215, 217-234, 236-256, 258, 259, 261-265, 267, 268, 270-273, 276, 277, 280, 281, 283-285, 287, 288, 290, 291, 294, 296-299, 301, 304-306, 308, 313-316, 319, 320, 322-325, 327, 328, 333, 336,
- an antibody which competes ( e.g., in a dose dependent manner) for specific binding to GITR (e.g., human GITR), with an antibody comprising (i) a VL domain comprising a VL CDR1, VL CDR2, and VL CDR3 having the amino acid sequences of the VL CDRs of an antibody listed in Table 1; and (ii) a VH domain comprising a VH CDR1, VH CDR2, and VH CDR3 having the amino acid sequences of the CDRs of an antibody listed in Table 2 (e.g., the VH CDRs of a particular antibody referred to by name in Table 1, such as 231-32-15, Hum231#1, or Hum231#2).
- an antibody that competes (e.g., in a dose-dependent manner), for specific binding to GITR (e.g., human GITR), with an antibody comprising the VH and VL CDRs of 231-32-15 (SEQ ID NO: 201 and 202).
- an antibody described herein is one that is competitively blocked ( e.g., in a dose dependent manner) by an antibody comprising a VL domain having the amino acid sequence selected from the group consisting of SEQ ID NO: 202, 207, 208, 400-411, 413-416, 418-421, 423-448, 450-452, 454-464, 467-477, 481-486, 488-513, and 515-518 and a VH domain having the amino acid sequence selected from the group consisting of SEQ ID NO: 201, 206, 215, 217-234, 236-256, 258, 259, 261-265, 267, 268, 271-273, 276, 277, 280, 281, 283-285, 287, 288, 290, 291, 294, 296-299, 301, 304-306, 308, 313-316, 319, 320, 322-325, 327, 328, 333, 336, 338-340, 342, 343, 345, 350,
- an antibody described herein is one that is competitively blocked ( e.g., in a dose dependent manner) by an antibody comprising a VL domain having the amino acid sequence selected from the group consisting of SEQ ID NO: 202, 207, 208, 400-411, 413-416, 418-421, 423-448, 450-464, 467-477, 481-486, 488-513, and 515-519 and a VH domain having the amino acid sequence selected from the group consisting of SEQ ID NO: 201, 206, 215, 217-234, 236-256, 258, 259, 261-265, 267, 268, 270-273, 276, 277, 280, 281, 283-285, 287, 288, 290, 291, 294, 296-299, 301, 304-306, 308, 313-316, 319, 320, 322-325, 327, 328, 333, 336, 338-340, 342, 343, 345, 350, 354-356
- an antibody described herein is one that is competitively blocked by an antibody comprising a VL domain having the amino acid sequence of SEQ ID NO: 207 or 208 and a VH domain having the amino acid sequence of SEQ ID NO: 206 for specific binding to GITR (e.g., human GITR).
- GITR e.g., human GITR
- an antibody described herein is one that is competitively blocked (e.g., in a dose dependent manner) by an antibody comprising (i) a VL domain comprising a VL CDR1, VL CDR2, and VL CDR3 having the amino acid sequences of the CDRs of antibody listed in Table 1 (e.g., the VL CDRs of a particular antibody referred by name in Table 1); and (ii) a VH domain comprising a VH CDR1, VH CDR2, and VH CDR3 having the amino acid sequences of the CDRs of antibody listed in Table 2.
- an antibody or an antigen-binding fragment thereof, which immunospecifically binds to the same epitope as that of an antibody (e.g., any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, pab2161, or Hum231#2w) comprising the amino acid sequences described herein (see, e.g., Tables 1-4) for specific binding to GITR (e.g., human GITR). Assays known to one of skill in the art or described herein (e.g., X-ray crystallography, ELISA assays, etc.)
- an antibody or an antigen-binding fragment thereof described herein immunospecifically binds to the same epitope as that of an antibody (e.g., any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107) comprising a VL domain having an amino acid sequence selected from the group consisting of SEQ ID NO: 202, 207, 208, 400-411, 413-416, 418-421, 423-448, 450-452, 454-464, 467-477, 481-486, 488-513, and 515-518, and a VH domain having an amino acid sequence selected from the group consisting of SEQ ID NO: 201,
- an antibody or an antigen-binding fragment thereof described herein immunospecifically binds to the same epitope as that of an antibody (e.g., any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, pab2161, or Hum231#2w) comprising a VL domain having an amino acid sequence selected from the group consisting of SEQ ID NO: 202, 207, 208, 400-411, 413-416, 418-421, 423-448, 450-464, 467-477, 481-486, 488-513, and 515-519, and a VH domain having an antibody (e.g.
- an antibody or an antigen-binding fragment thereof described herein immunospecifically binds to the same epitope as that bound by an antibody comprising the VH domain and VL domain of antibody Hum231#1 or Hum231#2 (SEQ ID NOs: 206 and 207 or SEQ ID NOs: 206 and 208, respectively), or an epitope that overlaps the epitope of antibody comprising the VH domain and VL domain of antibody Hum231#1 or Hum231#2 (SEQ ID NOs: 206 and 207 or SEQ ID NOs: 206 and 208, respectively).
- an antibody or an antigen-binding fragment thereof described herein immunospecifically binds to the same epitope as that of an antibody comprising (i) a VL domain comprising a VL CDR1, VL CDR2, and VL CDR3 having the amino acid sequences of the CDRs of antibody listed in Table 1 (e.g., the VL CDRs of a particular antibody referred to by name in Table 1) and (ii) a VH domain comprising a VH CDR1, VH CDR2, and VH CDR3 having the amino acid sequences of the CDRs of antibody listed in Table 2 (e.g., the VH CDRs of a particular antibody referred to by name in Table 2).
- an antibody described herein or an antigen-binding fragment thereof which specifically binds to GITR (e.g., human GITR) and competitively blocks ( e.g., in a dose dependent manner) antibody 231-32-15, Hum231#1, Hum231#2 or Hum231#2w from binding to GITR (e.g., human GITR), comprises a light chain variable region (VL) and a heavy chain variable region (VH), wherein
- an antibody described herein, or an antigen-binding fragment thereof which specifically binds to GITR (e.g., human GITR) and competitively blocks ( e.g., in a dose dependent manner) antibody 231-32-15, Hum231#1, Hum231#2 or Hum231#2w from binding to GITR (e.g., human GITR), comprises a light chain variable region (VL) and a heavy chain variable region (VH), wherein
- an antibody that competes for binding with an antibody described herein for binding GITR e.g., human GITR
- GITR e.g., human GITR
- binds to the same or an overlapping epitope of an antibody described herein e.g., Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, pab2161, or Hum231#2w) prevents binding of GITRL (e.g., human GITRL) to GITR (e.g., human GITR) by less than 85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%
- an antibody or antigen-binding fragment thereof which competes with an antibody described herein for binding to GITR (e.g., human GITR) or binds to the same epitope or overlapping epitope of an antibody described herein, inhibits binding of GITRL (e.g., human GITRL) to GITR ( e.g., human GITR) by less than 85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, 20% or 10% as assessed by the assay described in Example 2, infra ( e.g., Section 6.2.5.2 or 6.2.5.4, infra ).
- GITRL e.g., human GITRL
- GITR e.g., human GITR
- an antibody or antigen-binding fragment thereof which competes with an antibody described herein for binding to GITR (e.g ., human GITR) or binds to the same epitope or overlapping epitope of an antibody described herein, at a concentration of 1000 ng/ml, 950 ng/ml, 900 ng/ml, 850 ng/ml, 800 ng/ml, 750 ng/ml, 700 ng/ml, 650 ng/ml, 600 ng/ml, 550 ng/ml, 500 ng/ml, 450 ng/ml, 400 ng/ml, 350 ng/ml, 333 ng/ml, 300 ng/ml, 250 ng/ml, 200 ng/ml, 100 ng/ml, 50 ng/ml or 10 ng/ml inhibits binding of 1.5nM, 1.4nM, 1.3nM, 1.2nM, 1.1nM, 1
- an antibody or antigen-binding fragment thereof which competes with an antibody described herein for binding to GITR (e.g ., human GITR) or binds to the same epitope or overlapping epitope of an antibody described herein, at concentration of 1000 ng/ml to 750 ng/ml, 1000 ng/ml to500 ng/ml, 850 ng/ml to 500 ng/ml, 750 ng/ml to 500 ng/ml, 600 ng/ml to 500 ng/ml, 500 ng/ml to 400 ng/ml, 400 ng/ml to 300 ng/ml, or 300 ng/ml to 200 ng/ml inhibits binding of 1.5nM, 1.4nM, 1.3nM, 1.2nM, 1.1nM, 1nM, 0.9nM, 0.8nM, 0.7nM, 0.6nM, 0.5nM, 0.4nM
- an antibody or antigen-binding fragment thereof which competes with an antibody described herein for binding to GITR (e.g ., human GITR) or binds to the same epitope or overlapping epitope of an antibody described herein, at concentration of 3500 ng/ml, 3400 ng/ml, 3300 ng/ml, 3200 ng/ml, 3100 ng/ml, 3000 ng/ml, 2900 ng/ml, 2800 ng/ml, 2700 ng/ml, 2600 ng/ml, 2500 ng/ml, 2400 ng/ml, 2300 ng/ml, 2200 ng/ml, 2100 ng/ml, 2000 ng/ml, 1900 ng/ml, 1800 ng/ml, 1700 ng/ml, 1600 ng/ml, 1500 ng/ml, 1400 ng/ml, 1300 ng/ml, 1200 ng/m
- an antibody or antigen-binding fragment thereof which competes with an antibody described herein for binding to GITR (e.g ., human GITR) or binds to the same epitope or overlapping epitope of an antibody described herein, at concentration of 3500 ng/ml to 3200 ng/ml, 3500 ng/ml to 3000ng/ml, 3200 ng/ml to 2500 ng/ml, 3000 to 2200 ng/ml, 2500 ng/ml to 1800 ng/ml, 2000 ng/ml to 1500 ng/ml, 1700 ng/ml to 1200 ng/ml, or 1500 ng/ml to 1000 ng/ml inhibits binding of 1.5nM, 1.4nM, 1.3nM, 1.2nM, 1.1nM, 1nM, 0.9nM, 0.8nM, 0.7nM, 0.6nM, 0.5nM, 0.4nM, 0.3nM,
- an antibody which competes for binding with an antibody described herein for binding GITR (e.g ., human GITR) or binds to the same or an overlapping epitope of an antibody described herein, at a concentration of 3000ng/ml prevents binding of 0.5nM GITRL (e.g., human GITRL) to GITR ( e.g., human GITR) by less than 85% or less than 80% when GITR ( e.g., human GITR) is coupled to beads ( e.g., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay (e.g ., Luminex® 200 system).
- beads e.g., Luminex® beads
- an antibody which competes for binding with an antibody described herein for binding GITR (e.g ., human GITR) or binds to the same or an overlapping epitope of an antibody described herein, at a concentration of 1000ng/ml prevents binding of 0.5nM GITRL (e.g., human GITRL) to GITR ( e.g., human GITR) by less than 85%, less than 80% or less than 75% when GITR ( e.g., human GITR) is coupled to beads ( e.g., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay (e.g ., Luminex® 200 system).
- beads e.g., Luminex® beads
- an antibody which competes for binding with an antibody described herein for binding GITR (e.g ., human GITR) or binds to the same or an overlapping epitope of an antibody described herein, at a concentration of 333ng/ml prevents binding of 0.5nM GITRL (e.g., human GITRL) to GITR ( e.g., human GITR) by less than 70% or less than 65% when GITR ( e.g., human GITR) is coupled to beads ( e.g., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay (e.g ., Luminex® 200 system).
- a suspension array assay e.g ., Luminex® 200 system
- an antibody which competes for binding with an antibody described herein for binding GITR (e.g ., human GITR) or binds to the same or an overlapping epitope of an antibody described herein, at a concentration of 111ng/ml prevents binding of 0.5nM GITRL (e.g ., human GITRL) to GITR ( e.g ., human GITR) by less than 65%, less than 60% or less than 55% when GITR ( e.g., human GITR) is coupled to beads ( e.g., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay (e.g ., Luminex® 200 system).
- beads e.g., Luminex® beads
- an antibody which competes for binding with an antibody described herein for binding GITR (e.g ., human GITR) or binds to the same or an overlapping epitope of an antibody described herein, at a concentration of 37ng/ml prevents binding of 0.5nM GITRL (e.g ., human GITRL) to GITR ( e.g., human GITR) by less than 40% when GITR ( e.g., human GITR) is coupled to beads ( e.g ., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay (e.g ., Luminex® 200 system).
- beads e.g ., Luminex® beads
- an antibody which competes for binding with an antibody described herein for binding GITR (e.g ., human GITR) or binds to the same or an overlapping epitope of an antibody described herein, at a concentration of 12ng/ml prevents binding of 0.5nM GITRL (e.g., human GITRL) to GITR ( e.g., human GITR) by less than 20% when GITR ( e.g., human GITR) is coupled to beads ( e.g., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay (e.g ., Luminex® 200 system).
- beads e.g., Luminex® beads
- a certain amount of labeled GITRL binds to GITR coupled to beads (e.g., human GITR coupled to Luminex® beads) in the presence of an antibody, which competes for binding with an antibody described herein for binding to GITR or binds to the same or an overlapping epitope of an antibody described herein, in a method comprising: (a) coupling GITR (e.g., human GITR) to beads (e.g., human GITR coupled to Luminex® beads) at a concentration of 5pg/ml per bead; (b) incubating the GITR coupled beads at a concentration of 40 beads/ ⁇ l with 3000ng/ml, 2500ng/ml, 2000ng/ml, 1500ng/ml, 1000ng/ml, 750ng/ml, 500ng/ml, 250ng/ml, 100ng/ml, 50ng/ml or 10ng
- the amount of the labeled GITRL bound to the GITR coupled beads in the presence of the competing antibody or the antibody that binds to the same or overlapping epitope is determined relative to the amount of labeled GITRL bound to the GITR coupled beads in the absence of the competing antibody or the antibody that binds to the same or overlapping epitope.
- the absence of the competing antibody or the antibody that binds to the same or overlapping epitope means that no antibody or antigen-binding fragment thereof is present in the well.
- the absence of the competing antibody or the antibody that binds to the same or overlapping epitope means that an isotype control antibody that does not bind to GITR is present in the well.
- the amount of labeled GITRL bound to the GITR coupled beads in the presence of the competing antibody or the antibody that binds to the same or overlapping epitope is determined to be, in some embodiments, at least 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55% or 60%, or 20% to 60%, 30% to 50%, or 20% to 70% of the amount of the labeled GITRL bound to the GITR coupled beads in the absence of the competing antibody or the antibody that binds to the same or overlapping epitope.
- GITRL e.g., human GITRL
- GITR e.g., human GITR
- an antibody or antigen-binding fragment thereof which competes with an antibody described herein for binding to GITR (e.g., human GITR) or binds to the same epitope or overlapping epitope of an antibody described herein, assessed by an assay known to one of skill in the art or described herein.
- GITRL e.g., human GITRL
- GITR e.g., human GITR
- an antibody or antigen-binding fragment thereof which competes with an antibody described herein for binding to GITR ( e.g., human GITR) or binds to the same epitope or overlapping epitope of an antibody described herein, as assessed by the assay described in Example 2, infra ( e.g., Sections 6.2.5.2 or 6.2.5.4, infra ) .
- At least 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, or 75% of 1.5nM, 1.4nM, 1.3nM, 1.2nM, 1.1nM, 1nM, 0.9nM, 0.8nM, 0.7nM, 0.6nM, 0.5nM, 0.4nM, 0.3nM, 0.2nM or 0.1nM of labeled GITRL e.g., labeled human GITRL, such as hGITRL-PE
- GITR coupled to beads e.g., human GITR coupled to Luminex® beads
- At least 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, or 75% of 1.5nM, 1.4nM, 1.3nM, 1.2nM, 1.1nM, 1nM, 0.9nM, 0.8nM, 0.7nM, 0.6nM, 0.5nM, 0.4nM, 0.3nM, 0.2nM or 0.1nM of labeled GITRL e.g., labeled human GITRL, such as hGITRL-PE
- GITR coupled to beads e.g., human GITR coupled to Luminex® beads
- an antibody which competes for binding with an antibody described herein for binding GITR (e.g., human GITR) or binds to the same or an overlapping epitope of an antibody described herein, at a concentration of 3000ng/ml does not prevent binding of 0.5nM GITRL (e.g., human GITRL) to GITR ( e.g., human GITR) by more than 15% or more than 20% when GITR ( e.g., human GITR) is coupled to beads (e.g., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay (e.g., Luminex® 200 system).
- beads e.g., Luminex® beads
- an antibody which competes for binding with an antibody described herein for binding GITR (e.g., human GITR) or binds to the same or an overlapping epitope of an antibody described herein, at a concentration of 1000ng/ml does not prevent binding of 0.5nM GITRL (e.g., human GITRL) to GITR ( e.g., human GITR) by more than 15%, more than 20% or more than 25% when GITR ( e.g., human GITR) is coupled to beads (e.g., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay (e.g., Luminex® 200 system).
- beads e.g., Luminex® beads
- an antibody which competes for binding with an antibody described herein for binding GITR (e.g., human GITR) or binds to the same or an overlapping epitope of an antibody described herein, at a concentration of 333ng/ml does not prevent binding of 0.5nM GITRL (e.g., human GITRL) to GITR ( e.g., human GITR) by more than 30% or more than 35% when GITR ( e.g., human GITR) is coupled to beads (e.g., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay (e.g., Luminex® 200 system).
- beads e.g., Luminex® beads
- an antibody which competes for binding with an antibody described herein for binding GITR (e.g., human GITR) or binds to the same or an overlapping epitope of an antibody described herein, at a concentration of 111ng/ml does not prevent binding of 0.5nM GITRL (e.g., human GITRL) to GITR ( e.g., human GITR) by more than 35%, more than 40% or more than 45% when GITR ( e.g., human GITR) is coupled to beads (e.g., Luminex® beads) at a concentration of 5pg/ml per bead relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof in a suspension array assay (e.g., Luminex® 200 system).
- beads e.g., Luminex® beads
- an antibody which competes for binding with an antibody described herein for binding GITR (e.g., human GITR) or binds to the same or an overlapping epitope of an antibody described herein, at a concentration of 37ng/ml does not prevent binding of 0.5nM GITRL (e.g., human GITRL) to GITR (e.g., human GITR) by more than 60% when GITR ( e.g., human GITR) is coupled to beads ( e.g., Luminex® beads) at a concentration of 5pg/ml relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof per bead in a suspension array assay (e.g., Luminex® 200 system).
- beads e.g., Luminex® beads
- an antibody which competes for binding with an antibody described herein for binding GITR (e.g., human GITR) or binds to the same or an overlapping epitope of an antibody described herein, at a concentration of 12ng/ml does not prevent binding of 0.5nM GITRL (e.g., human GITRL) to GITR ( e.g., human GITR) by more than 85% when GITR ( e.g., human GITR) is coupled to beads (e.g., Luminex® beads) at a concentration of 5pg/ml relative to the binding of 0.5nM of labeled GITRL to GITR coupled beads at a concentration of 5pg/ml/bead in the absence of the anti-GITR antibody or antigen-binding fragment thereof per bead in a suspension array assay (e.g., Luminex® 200 system).
- beads e.g., Luminex® beads
- an antibody which competes for binding with an antibody described herein for binding GITR (e.g., human GITR) or binds to the same or an overlapping epitope of an antibody described herein, at a concentration of 150nM, 145nM, 140nM, 135nM, 130nM, 125nM, 120nM, 115nM, 110nM, 105nM or 100nM bound to GITR (e.g., human GITR) immobilized on a chip ( e.g., CM5 sensor chip) inhibits binding of 150nM, 145nM, 140nM, 135nM, 130nM, 125nM, 120nM, 115nM, 110nM, 105nM or 100nM of GITRL (e.g., non-covalently linked trimer of human GITRL) to the GITR immobilized on the chip by less than 60%, less than 55%, less than 50%, less than
- an antibody which competes for binding with an antibody described herein for binding GITR (e.g., human GITR) or binds to the same or an overlapping epitope of an antibody described herein, wherein the competing antibody or the antibody that binds to the same or overlapping epitope at a concentration of 125nM bound to GITR (e.g., human GITR) immobilized on a chip (e.g., CM5 sensor chip) inhibits binding of 125nM of GITRL (e.g., non-covalently linked trimer of human GITRL) to the GITR immobilized on the chip by less than 60%, less than 55%, less than 50%, less than 45%, less than 40%, less than 35%, less than 30%, less than 25%, less than 20% or less than 15%.
- a chip e.g., CM5 sensor chip
- an antibody or fragment thereof which competes with an antibody described herein for binding to GITR (e.g., human GITR) or binds to the same epitope or overlapping epitope of an antibody described herein, binds to GITR (e.g., human GITR) with a dissociation rate constant (k off ) of 8.5 x 10 -3 s -1 or less, 3.5 x 10 -3 s -1 or less, 5 x 10 -3 s -1 or less, 2.5 x 10 -3 s -1 or less, 1 x 10 -3 s -1 or less, 8.5 x 10 -4 s -1 or less, 5 x 10 -4 s -1 or less, 3.5 x 10 -4 s -1 or less, 2.5 x 10 -4 s -1 or less, 1 x 10 -4 s -1 or less, 8.5 x 10 -5- s -1 or less, 3.5 x 10 -5- s -1 -1 -1
- an antibody or fragment thereof which competes with an antibody described herein for binding to GITR (e.g., human GITR) or binds to the same epitope or overlapping epitope of an antibody described herein, binds to GITR (e.g., human GITR) with a k off of between 9.5 x 10 -5- s -1 to 1 x 10 -9- s -1 , 8.5 x 10 -5- s -1 to 1 x 10 -9- s -1 , 5 x 10 -5- s -1 to 1 x 10 -9- s -1 , 9.5 x 10 -5- s -1 to 1 x 10 -8- s -1 , 5 x 10 -5 s -1 to 1 x 10 -8- s -1 , 9.5 x 10 -5- s -1 to 1 x 10 -7- s -1 , 5 x 10 -5- s -1 to 1 x 10 -7- s
- the k off is determined using a monovalent antibody, such as a Fab fragment, as measured by, e.g., BIAcore® surface plasmon resonance technology.
- the k off is determined using a bivalent antibody as measured by, e.g., BIAcore® surface plasmon resonance technology.
- the k off is determined using an assay described in Section 6, infra.
- an antibody or fragment thereof which competes with an antibody described herein for binding to GITR (e.g., human GITR) or binds to the same epitope or overlapping epitope of an antibody described herein, binds to GITR (e.g., human GITR) with an association rate constant (k on ) of at least 10 5 M -1 s -1 , at least 2.5 x 10 5 M -1 s -1 , at least 3.5 x 10 5 M -1 s -1 , at least 5 x 10 5 M -1 s -1 , at least 10 6 M -1 s -1 , at least 2.5 x 10 6 M -1 s -1 , at least 3.5 x 10 6 M -1 s -1 , at least 5 x 10 6 M -1 s -1 , at least 10 7 M -1 s -1 , at least 5 x 10 7 M -1 s -1 , at least 10 8 M -1 s -1 ,
- an antibody or fragment thereof which competes with an antibody described herein for binding to GITR (e.g., human GITR) or binds to the same epitope or overlapping epitope of an antibody described herein, binds to GITR (e.g., human GITR) with a k on of between 1 x 10 5 M -1 s -1 to 5 x 10 5 M -1 s -1 , 1 x 10 5 M -1 s -1 to 1 x 10 6 M -1 s -1 , 3.5 x 10 5 M -1 s -1 to 2.5 x 10 6 M -1 s -1 , 3.5 x 10 5 M -1 s -1 to 3.5 x 10 6 M -1 s -1 , 1 x 10 5 M -1 s -1 to 5 x 10 6 M -1 s -1 , 1 x 10 5 M -1 s - to 1 x 10 7 M -1 s -1 , 1 x 10 5 M -1
- the k on is determined using a monovalent antibody, such as a Fab fragment, as measured by, e.g., BIAcore® surface plasmon resonance technology.
- the k on is determined using a bivalent antibody as measured by, e.g., BIAcore® surface plasmon resonance technology.
- the k on is determined using an assay described in Section 6, infra.
- an antibody or fragment thereof which competes with an antibody described herein for binding to GITR (e.g., human GITR) or binds to the same epitope or overlapping epitope of an antibody described herein, binds to GITR (e.g., human GITR) with a K D of less than 7 nM, 6nM, 5 nM, 4.5 nM, 4 nM, 3.5 nM, 3 nM, 2.5 nM, 2 nM, 1.5 nM, 1 nM, 0.75 nM, 0.5 nM, 0.25 nM, or 0.1 nM.
- an antibody or fragment thereof which competes with an antibody described herein for binding to GITR (e.g., human GITR) or binds to the same epitope or overlapping epitope of an antibody described herein, binds to GITR (e.g., human GITR) with a K D of about 7nM, 6nM, 5 nM, 4.5 nM, 4 nM, 3.5 nM, 3 nM, 2.5 nM, 2 nM, 1.5 nM, 1 nM, 0.75 nM, 0.5 nM, 0.25 nM, or 0.1 nM.
- an antibody or fragment thereof described herein which competes with an antibody described herein for binding to GITR (e.g., human GITR) or binds to the same epitope or overlapping epitope of an antibody described herein, binds to GITR (e.g., human GITR) with a K D of 7 nM to 4 nM, 7 nM to 5 nM, 6 nM to 4 nM, 5 nM to 3 nM, 5 nM to 1 nM, 5 nM to 0.5 nM, 4 nM to 3 nM, 4 nM to 2 nM, 4 nM to 1 nM, 4 nM to 0.5 nM, 3 nM to 2 nM, 3 nM to 1 nM, 3 nM to 0.5 nM, 2 nM to 1 nM, 2 nM to 0.5 nM, 3 nM to 0.1 nM, 2
- the K D is calculated as the quotient of k off /k on , and the k on and k off are determined using a monovalent antibody, such as a Fab fragment, as measured by, e.g., BIAcore® surface plasmon resonance technology.
- the K D is calculated as the quotient of k off /k on , and the k on and k off are determined using a bivalent antibody, such as a Fab fragment, as measured by, e.g., BIAcore® surface plasmon resonance technology.
- the K D is determined as set forth in the Examples in Section 6, infra ( e.g., Example 2).
- the epitope of an antibody described herein is used as an immunogen to produce antibodies. See, e.g., Section 5.2 infra for methods for producing antibodies.
- an antibody or fragment thereof described which specifically binds to GITR (e.g., human GITR), does not inhibit ( e.g., in a dose dependent manner) the binding of the murine antibody 6C8 to GITR (e.g., human GITR) in an assay known to one of skill in the art or described herein. See, e.g., U.S. Patent No. 7,812,135 for a description of the murine antibody 6C8.
- GITR e.g., human GITR
- the murine antibody 6C8 binds to GITR (e.g., human GITR) in the presence of an antibody or fragment thereof described, which specifically binds to GITR ( e.g., human GITR), as assessed in an assay known to one of skill in the art or described herein.
- an antibody or fragment thereof described which specifically binds to GITR (e.g., human GITR), does not inhibit ( e.g., in a dose dependent manner) the binding of the murine antibody 6C8 to GITR (e.g., human GITR) as assessed in the assay described in Example 6, infra.
- At least 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95% or more of the murine antibody 6C8 binds to GITR (e.g., human GITR) in the presence of an antibody or fragment thereof described, which specifically binds to GITR ( e.g., human GITR), as assessed in the assay described in Example 6, infra.
- anti-GITR antibodies described herein may be multispecific antibodies, e.g., bispecific antibodies.
- an anti-GITR antibody described herein is a bispecific antibody, wherein the antibody has specificities for at least two different, typically non-overlapping epitopes.
- a bispecific antibody comprises one arm comprising of an antibody described herein with specificity for GITR (e.g., human GITR), and a second arm comprising an antibody with specificity for a different epitope on GITR (e.g., human GITR) or an epitope on a different molecule, e.g., PD-1, PD-L1, PD-L2, CTLA-4, TIM-3, LAG-3 or OX40.
- the bispecific antibody may comprise one arm comprising an antibody described herein with specificity for GITR (e.g., human GITR) and a second arm comprising an antibody with specificity for CTLA-4, such as tremelimumab (Pfizer), ipilimumab (Yervoy®, Bristol-Meyers Squibb), an antibody that binds to the same epitope as tremelimumab or an overlapping epitope thereto, or an antibody that binds to the same epitope as ipilimumab or an overlapping epitope thereto.
- tremelimumab Pfizer
- ipilimumab Yervoy®, Bristol-Meyers Squibb
- an antibody that binds to the same epitope as ipilimumab or an overlapping epitope thereto or an antibody that binds to the same epitope as ipilimumab or an overlapping epitope thereto.
- an antibody or fragment thereof described herein which immunospecifically binds to GITR (e.g., human GITR), functions as an agonist.
- an antibody or fragment thereof described herein which immunospecifically binds to GITR (e.g., human GITR), increases GITR (e.g., human GITR) activity by at least about 1.2 fold, 1.3 fold, 1.4 fold, 1.5 fold, 2 fold, 2.5 fold, 3 fold, 3.5 fold, 4 fold, 4.5 fold, 5 fold, 6 fold, 7 fold, 8 fold, 9 fold, 10 fold, 15 fold, 20 fold, 30 fold, 40 fold, 50 fold, 60 fold, 70 fold, 80 fold, 90 fold, or 100 fold as assessed by methods described herein and/or known to one of skill in the art, relative to GITR (e.g., human GITR) activity in the presence or absence of GITRL (e.g., human GITRL) stimulation without any antibody or with an unrelated antibody (e.g., an antibody that does not immunospecifically bind to GITR).
- GITRL e.g., human GITRL
- an antibody or fragment thereof described herein which immunospecifically binds to GITR (e.g., human GITR), increases GITR (e.g., human GITR) activity by at least 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 98%, or 99% as assessed by methods described herein and/or known to one of skill in the art, relative to GITR (e.g., human GITR) activity in the presence or absence of GITRL ( e.g., human GITRL) stimulation without any antibody or with an unrelated antibody (e.g., an antibody that does not immunospecifically bind to GITR).
- GITRL e.g., human GITRL
- Non-limiting examples of GITR (e.g., human GITR) activity can include cell proliferation, GITR (e.g., human GITR) signaling, cell survival, and cytokine production ( e.g., IL-2, IL-6, IL-10, TNF- ⁇ , and IFN- ⁇ ).
- GITR e.g., human GITR
- an antibody or fragment thereof described herein, which immunospecifically binds to GITR e.g., human GITR
- an antibody or fragment thereof described herein which immunospecifically binds to GITR (e.g., human GITR), induces, enhances, or increases a GITR (e.g., human GITR) activity in the absence of GITRL ( e.g., human GITRL).
- the antibody or antibody-binding fragment induces, enhances, or increases a GITR activity and does not inhibit ( e.g., does not completely inhibit or only partially inhibits) GITRL from binding to GITR.
- an increase in a GITR activity is assessed as described in the Examples, infra.
- an antibody or fragment thereof described herein which immunospecifically binds to GITR (e.g., human GITR), induces, enhances, or increases the cellular proliferation of cells that express GITR and that respond to GITR signaling (e.g., cells that proliferate in response to GITR stimulation and GITR signaling, such as T cells).
- GITR e.g., human GITR
- Cell proliferation assays are described in the art, such as a 3 H-thymidine incorporation assay, BrdU incorporation assay or CFSE assay, such as described in Example 3, and can be readily carried out by one of skill in the art.
- T cells e.g., CD4 + or CD8 + effector T cells
- a T cell mitogen or T cell receptor complex stimulating agent e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody
- GITR e.g., human GITR
- PHA phytohaemagglutinin
- PMA phorbol myristate acetate
- TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody
- CD8 + T cells stimulated with a T cell mitogen or T cell receptor complex stimulating agent e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody
- a T cell mitogen or T cell receptor complex stimulating agent e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA)
- a TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody
- CD4 + T cells stimulated with a T cell mitogen or T cell receptor complex stimulating agent e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody
- a T cell mitogen or T cell receptor complex stimulating agent e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA)
- a TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody
- CD4 + T cells and CD8 + T cells stimulated with a T cell mitogen or T cell receptor complex stimulating agent e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody
- a T cell mitogen or T cell receptor complex stimulating agent e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA)
- a TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody
- T cells that have not been stimulated with a T cell mitogen or T cell receptor complex stimulating agent e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody
- a T cell mitogen or T cell receptor complex stimulating agent e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody
- GITR e.g., human GITR
- GITR e.g., human GITR
- GITR e.g., human GITR
- an antibody or fragment thereof described herein which immunospecifically binds to GITR (e.g., human GITR), increases cell proliferation (e.g., T cells, such as CD4 and CD8 effector T cells) by at least about 1.2 fold, 1.3 fold, 1.4 fold, 1.5 fold, 2 fold, 2.5 fold, 3 fold, 3.5 fold, 4 fold, 4.5 fold, 5 fold, 6 fold, 7 fold, 8 fold, 9 fold, 10 fold, 15 fold, 20 fold, 30 fold, 40 fold, 50 fold, 60 fold, 70 fold, 80 fold, 90 fold, or 100 fold, as assessed by methods described herein or known to one of skill in the art (e.g., 3 H-thymidine incorporation assay, BrdU incorporation assay or CFSE assay, such as described in Example 3, infra), relative to GITR (e.g., human GITR) activity in the presence or absence of GITRL (e.g., human GITRL) stimulation without any antibody or with
- an antibody or fragment thereof described herein which immunospecifically binds to GITR (e.g., human GITR), increases cell proliferation (e.g., T cells, such as CD4 and CD8 effector T cells) by at least at least about 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 98%, or 99%, as assessed by methods described herein or known to one of skill in the art (e.g., 3 H-thymidine incorporation assay, BrdU incorporation assay or CFSE assay, such as described in Example 3, infra), relative to GITR (e.g., human GITR) activity in the presence or absence of GITRL (e.g., human GITRL) stimulation without any antibody or with an unrelated antibody (e.g., an antibody that does not immunospecifically bind to GITR).
- T cells such as CD4 and CD8 effector
- T cells e.g., CD4 + or CD8 + effector T cells
- a T cell mitogen e.g., an anti-CD3 antibody or phorbol ester
- GITR e.g., human GITR
- T cells e.g., CD4 + or CD8 + effector T cells
- a T cell mitogen or T cell receptor complex stimulating agent e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody
- GITR e.g., human GITR
- PHA phytohaemagglutinin
- PMA phorbol myristate acetate
- a TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody
- GITR e.g., human GITR
- an antibody or fragment thereof described herein which immunospecifically binds to GITR (e.g., human GITR), increases the survival of cells (e.g., T cells, such as CD4 and CD8 effector T cells).
- T cells e.g., CD4 + or CD8 + effector T cells
- a T cell mitogen or T cell receptor complex stimulating agent e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody
- PHA phytohaemagglutinin
- PMA phorbol myristate acetate
- TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody
- an antibody or fragment thereof described herein which immunospecifically binds to GITR (e.g., human GITR), increases cell survival (e.g., T cells, such as CD4 and CD8 effector T cells) by at least about 1.2 fold, 1.3 fold, 1.4 fold, 1.5 fold, 2 fold, 2.5 fold, 3 fold, 3.5 fold, 4 fold, 4.5 fold, 5 fold, 6 fold, 7 fold, 8 fold, 9 fold, 10 fold, 15 fold, 20 fold, 30 fold, 40 fold, 50 fold, 60 fold, 70 fold, 80 fold, 90 fold, or 100 fold, as assessed by methods described herein or known to one of skill in the art (e.g., a trypan blue exclusion assay), relative to cell survival in the presence or absence of GITRL (e.g., human GITRL) stimulation without any antibody or with an unrelated antibody (e.g., an antibody that does not immunospecifically bind to GITR).
- GITRL e.g., human GITRL
- an antibody or fragment thereof described herein which immunospecifically binds to GITR (e.g., human GITR), increases cell survival (e.g., T cells, such as CD4 and CD8 effector T cells) by at least about 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 98%, or 99%, as assessed by methods described herein or known to one of skill in the art (e.g., a trypan blue exclusion assay), relative to cell survival in the presence or absence of GITRL (e.g., human GITRL) stimulation without any antibody or with an unrelated antibody (e .g., an antibody that does not immunospecifically bind to GITR).
- GITRL e.g., human GITRL
- T cells e.g., CD4 + or CD8 + effector T cells
- a T cell mitogen e.g., an anti-CD3 antibody or phorbol ester
- an antibody or fragment thereof described herein, which immunospecifically binds to GITR e.g., human GITR
- PHA phytohaemagglutinin
- T cells e.g., CD4 + or CD8 + effector T cells
- a T cell mitogen or T cell receptor complex stimulating agent e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody
- PHA phytohaemagglutinin
- PMA phorbol myristate acetate
- TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody
- GITR e.g., human GITR
- an antibody or fragment thereof described herein which immunospecifically binds to GITR (e.g., human GITR), protects effector T cells (e.g., CD4 + and CD8 + effector T cells) from activation-induced cell death.
- an antibody or fragment thereof described herein, which immunospecifically binds to GITR e.g., human GITR
- induces resistance of effector T cells e.g., CD4 + and CD8 + effector T cells
- an antibody or fragment thereof described herein which immunospecifically binds to GITR (e.g., human GITR), induces, enhances, or increases cytokine production (e.g., IL-2, IL-6, IL-10, TNF- ⁇ , and IFN- ⁇ ) by at least about 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 98%, or 99%, as assessed by methods described herein ( see the Examples, infra, such as Example 3) or known to one of skill in the art, relative to cytokine production in the presence or absence of GITRL (e.g., human GITRL) stimulation without any antibody or with an unrelated antibody (e.g., an antibody that does not immunospecifically bind to GITR).
- GITRL e.g., human GITRL
- an antibody or fragment thereof described herein which immunospecifically binds to GITR (e.g., human GITR), induces or enhances cytokine production (e.g., IL-2, IL-6, IL-10, TNF- ⁇ , and IFN- ⁇ ) by at least about 1.2 fold, 1.3 fold, 1.4 fold, 1.5 fold, 2 fold, 2.5 fold, 3 fold, 3.5 fold, 4 fold, 4.5 fold, 5 fold, 6 fold, 7 fold, 8 fold, 9 fold, 10 fold, 15 fold, 20 fold, 30 fold, 40 fold, 50 fold, 60 fold, 70 fold, 80 fold, 90 fold, or 100 fold, as assessed by methods described herein ( see the Examples, infra, such as Example 3) or known to one of skill in the art, relative to cytokine production in the presence or absence of GITRL (e.g., human GITRL) stimulation without any antibody or with an unrelated antibody (e.g., an antibody that does not immunospecifically bind to GITR
- T cells e.g., CD4 + or CD8 + effector T cells
- a T cell mitogen or T cell receptor complex stimulating agent e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody
- PHA phytohaemagglutinin
- PMA phorbol myristate acetate
- TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody
- GITR e.g., human GITR
- cytokine production e.g., IL-2, IL-6, IL-10, TNF- ⁇ , and IFN- ⁇
- cytokine production e.g., IL-2, IL-6, IL-10, TNF- ⁇ , and IFN- ⁇
- cytokine production e.g., IL-2, IL-6, IL-10, TNF- ⁇ ,
- T cells e.g., CD4 + or CD8 + effector T cells
- a T cell mitogen or T cell receptor complex stimulating agent e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody
- PHA phytohaemagglutinin
- PMA phorbol myristate acetate
- TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody
- GITR e.g., human GITR
- cytokine production e.g., IL-2, IL-6, IL-10, TNF- ⁇ , and IFN- ⁇
- cytokine production e.g., IL-2, IL-6, IL-10, TNF- ⁇ , and IFN- ⁇
- cytokine production e.g., IL-2, IL-6, IL-10, TNF- ⁇ ,
- an anti-GITR antibody or antigen binding fragment thereof induces, enhances or activates an activity of GITR, in the absence of a TCR agonist (e.g., an anti-CD3 antibody).
- GITR activity can be assessed by measuring activation of canonical and non-canonical NF- ⁇ B pathways.
- GITR activity can be assessed by measuring activation of TRAF adapter mediated signaling pathways.
- the TRAF adapter is selected from the group consisting of TRAF1, TRAF2, TRAF3, TRAF4, and TRAF5.
- GITR activity can be assessed by measuring activation of MAPK/ERK pathway (also called the Ras-Raf-MEK-ERK pathway).
- TCR agonist examples include, but are not limited to, antibodies targeting the T cell receptor complex (e.g., an anti-CD3 antibody) and peptides bound to human leukocyte antigens, e.g., MHC class I and MHC class II, wherein the peptides are derived from self, mutated self, or pathogen associated proteins (e.g., viral or bacterial).
- T cell receptor complex e.g., an anti-CD3 antibody
- peptides bound to human leukocyte antigens e.g., MHC class I and MHC class II, wherein the peptides are derived from self, mutated self, or pathogen associated proteins (e.g., viral or bacterial).
- An anti-GITR antibody or antigen-binding fragment thereof can be fused or conjugated ( e.g., covalently or noncovalently linked) to a detectable label or substance.
- detectable labels or substances include enzyme labels, such as, glucose oxidase; radioisotopes, such as iodine ( 125 I, 121 I), carbon ( 14 C), sulfur ( 35 S), tritium ( 3 H), indium ( 121 In), and technetium ( 99 Tc); luminescent labels, such as luminol; and fluorescent labels, such as fluorescein and rhodamine, and biotin.
- detectable labels or substances include enzyme labels, such as, glucose oxidase; radioisotopes, such as iodine ( 125 I, 121 I), carbon ( 14 C), sulfur ( 35 S), tritium ( 3 H), indium ( 121 In), and technetium ( 99 Tc); luminescent labels, such as luminol; and fluorescent labels, such as
- Antibodies or fragments thereof that immunospecifically bind to GITR can be produced by any method known in the art for the synthesis of antibodies, for example, by chemical synthesis or by recombinant expression techniques.
- the methods described herein employs, unless otherwise indicated, conventional techniques in molecular biology, microbiology, genetic analysis, recombinant DNA, organic chemistry, biochemistry, PCR, oligonucleotide synthesis and modification, nucleic acid hybridization, and related fields within the skill of the art. These techniques are described, for example, in the references cited herein and are fully explained in the literature.
- an antibody described herein is an antibody (e.g., recombinant antibody) prepared, expressed, created or isolated by any means that involves creation, e.g., via synthesis, genetic engineering of DNA sequences.
- an antibody comprises sequences (e.g., DNA sequences or amino acid sequences) that do not naturally exist within the antibody germline repertoire of an animal or mammal ( e.g., human) in vivo.
- provided herein is a method of making an antibody or an antigen-binding fragment thereof which immunospecifically binds to GITR (e.g., human GITR) comprising culturing a cell or host cell described herein.
- a method of making an antibody or an antigen-binding fragment thereof which immunospecifically binds to GITR comprising expressing ( e.g., recombinantly expressing) the antibody or antigen-binding fragment thereof using a cell or host cell described herein (e.g., a cell or a host cell comprising polynucleotides encoding an antibody described herein).
- the cell is an isolated cell.
- the exogenous polynucleotides have been introduced into the cell.
- the method further comprises the step of purifying the antibody or antigen-binding fragment thereof obtained from the cell or host cell.
- Monoclonal antibodies can be prepared using a wide variety of techniques known in the art including the use of hybridoma, recombinant, and phage display technologies, or a combination thereof.
- monoclonal antibodies can be produced using hybridoma techniques including those known in the art and taught, for example, in Harlow E & Lane D, Antibodies: A Laboratory Manual, (Cold Spring Harbor Laboratory Press, 2nd ed. 1988 ); Hammerling GJ et al., in: Monoclonal Antibodies and T-Cell Hybridomas 563 681 (Elsevier, N.Y., 1981 ).
- the term "monoclonal antibody” as used herein is not limited to antibodies produced through hybridoma technology.
- monoclonal antibodies can be produced recombinantly from host cells exogenously expressing an antibody described herein or a fragment thereof, for example, light chain and/or heavy chain of such antibody.
- a "monoclonal antibody,” as used herein, is an antibody produced by a single cell (e.g., hybridoma or host cell producing a recombinant antibody), wherein the antibody immunospecifically binds to GITR (e.g., human GITR) as determined, e.g., by ELISA or other antigen-binding or competitive binding assay known in the art or in the Examples provided herein.
- GITR e.g., human GITR
- a monoclonal antibody can be a chimeric antibody or a humanized antibody.
- a monoclonal antibody is a monovalent antibody or multivalent (e.g., bivalent) antibody.
- a monoclonal antibody is a monospecific or multispecific antibody (e.g., bispecific antibody).
- Monoclonal antibodies described herein can, for example, be made by the hybridoma method as described in Kohler G & Milstein C (1975) Nature 256: 495 or can, e.g., be isolated from phage libraries using the techniques as described herein, for example. Other methods for the preparation of clonal cell lines and of monoclonal antibodies expressed thereby are well known in the art (see, for example, Chapter 11 in: Short Protocols in Molecular Biology, (2002) 5th Ed., Ausubel FM et al., supra ).
- a mouse or other appropriate host animal such as a sheep, goat, rabbit, rat, hamster or macaque monkey
- lymphocytes that produce or are capable of producing antibodies that will specifically bind to the protein (e.g., GITR (e.g., human GITR)) used for immunization.
- lymphocytes may be immunized in vitro.
- Lymphocytes then are fused with myeloma cells using a suitable fusing agent, such as polyethylene glycol, to form a hybridoma cell ( Goding JW (Ed), Monoclonal Antibodies: Principles and Practice, pp. 59-103 (Academic Press, 1986 )). Additionally, a RIMMS (repetitive immunization multiple sites) technique can be used to immunize an animal ( Kilpatrick KE et al., (1997) Hybridoma 16:381-9 , incorporated by reference in its entirety).
- a suitable fusing agent such as polyethylene glycol
- mice can be immunized with an antigen (e.g., GITR (e.g., human GITR)) and once an immune response is detected, e.g., antibodies specific for the antigen are detected in the mouse serum, the mouse spleen is harvested and splenocytes isolated. The splenocytes are then fused by well known techniques to any suitable myeloma cells, for example cells from cell line SP20 available from the American Type Culture Collection (ATCC®) (Manassas, VA), to form hybridomas. Hybridomas are selected and cloned by limited dilution.
- lymph nodes of the immunized mice are harvested and fused with NS0 myeloma cells.
- the hybridoma cells thus prepared are seeded and grown in a suitable culture medium that preferably contains one or more substances that inhibit the growth or survival of the unfused, parental myeloma cells.
- a suitable culture medium that preferably contains one or more substances that inhibit the growth or survival of the unfused, parental myeloma cells.
- the culture medium for the hybridomas typically will include hypoxanthine, aminopterin, and thymidine (HAT medium), which substances prevent the growth of HGPRT-deficient cells.
- myeloma cells that fuse efficiently, support stable high-level production of antibody by the selected antibody-producing cells, and are sensitive to a medium such as HAT medium.
- myeloma cell lines are murine myeloma lines, such as NS0 cell line or those derived from MOPC-21 and MPC-11 mouse tumors available from the Salk Institute Cell Distribution Center, San Diego, CA, USA, and SP-2 or X63-Ag8.653 cells available from the American Type Culture Collection, Rockville, MD, USA.
- Culture medium in which hybridoma cells are growing is assayed for production of monoclonal antibodies directed against GITR (e.g., human GITR).
- GITR e.g., human GITR
- the binding specificity of monoclonal antibodies produced by hybridoma cells is determined by methods known in the art, for example, immunoprecipitation or by an in vitro binding assay, such as radioimmunoassay (RIA) or enzyme-linked immunoabsorbent assay (ELISA).
- RIA radioimmunoassay
- ELISA enzyme-linked immunoabsorbent assay
- the clones may be subcloned by limiting dilution procedures and grown by standard methods ( Goding JW (Ed), Monoclonal Antibodies: Principles and Practice , supra ). Suitable culture media for this purpose include, for example, D-MEM or RPMI 1640 medium.
- the hybridoma cells may be grown in vivo as ascites tumors in an animal.
- the monoclonal antibodies secreted by the subclones are suitably separated from the culture medium, ascites fluid, or serum by conventional immunoglobulin purification procedures such as, for example, protein A-Sepharose, hydroxylapatite chromatography, gel electrophoresis, dialysis, or affinity chromatography.
- Antibodies described herein include antibody fragments which recognize specific GITR (e.g., human GITR) and can be generated by any technique known to those of skill in the art.
- Fab and F(ab') 2 fragments described herein can be produced by proteolytic cleavage of immunoglobulin molecules, using enzymes such as papain (to produce Fab fragments) or pepsin (to produce F(ab') 2 fragments).
- a Fab fragment corresponds to one of the two identical arms of an antibody molecule and contains the complete light chain paired with the VH and CH1 domains of the heavy chain.
- a F(ab') 2 fragment contains the two antigen-binding arms of an antibody molecule linked by disulfide bonds in the hinge region.
- the antibodies described herein or antigen-binding fragments thereof can also be generated using various phage display methods known in the art.
- phage display methods functional antibody domains are displayed on the surface of phage particles which carry the polynucleotide sequences encoding them.
- DNA sequences encoding VH and VL domains are amplified from animal cDNA libraries ( e.g., human or murine cDNA libraries of affected tissues).
- the DNA encoding the VH and VL domains are recombined together with a scFv linker by PCR and cloned into a phagemid vector.
- the vector is electroporated in E. coli and the E. coli is infected with helper phage.
- Phage used in these methods are typically filamentous phage including fd and M13, and the VH and VL domains are usually recombinantly fused to either the phage gene III or gene VIII.
- Phage expressing an antigen binding domain that binds to a particular antigen can be selected or identified with antigen, e.g., using labeled antigen or antigen bound or captured to a solid surface or bead.
- phage display methods that can be used to make the antibodies described herein include those disclosed in Brinkman U et al., (1995) J Immunol Methods 182: 41-50 ; Ames RS et al., (1995) J Immunol Methods 184: 177-186 ; Kettleborough CA et al., (1994) Eur J Immunol 24: 952-958 ; Persic L et al., (1997) Gene 187: 9-18 ; Burton DR & Barbas CF (1994) Advan Immunol 57: 191-280 ; PCT Application No. PCT/GB91/001134 ; International Publication Nos.
- WO 90/02809 WO 91/10737 , WO 92/01047 , WO 92/18619 , WO 93/1 1236 , WO 95/15982 , WO 95/20401 , and WO 97/13844 ; and U.S. Patent Nos. 5,698,426 , 5,223,409 , 5,403,484 , 5,580,717 , 5,427,908 , 5,750,753 , 5,821,047 , 5,571,698 , 5,427,908 , 5,516,637 , 5,780,225 , 5,658,727 , 5,733,743 and 5,969,108 .
- the antibody coding regions from the phage can be isolated and used to generate whole antibodies, including human antibodies, or any other desired antigen binding fragment, and expressed in any desired host, including mammalian cells, insect cells, plant cells, yeast, and bacteria, e.g., as described below.
- Techniques to recombinantly produce antibody fragments such as Fab, Fab' and F(ab') 2 fragments can also be employed using methods known in the art such as those disclosed in PCT publication No.
- PCR primers including VH or VL nucleotide sequences, a restriction site, and a flanking sequence to protect the restriction site can be used to amplify the VH or VL sequences from a template, e.g., scFv clones.
- a template e.g., scFv clones.
- the PCR amplified VH domains can be cloned into vectors expressing a VH constant region
- the PCR amplified VL domains can be cloned into vectors expressing a VL constant region, e.g., human kappa or lambda constant regions.
- VH and VL domains can also be cloned into one vector expressing the necessary constant regions.
- the heavy chain conversion vectors and light chain conversion vectors are then co-transfected into cell lines to generate stable or transient cell lines that express full-length antibodies, e.g., IgG, using techniques known to those of skill in the art.
- a chimeric antibody is a molecule in which different portions of the antibody are derived from different immunoglobulin molecules.
- a chimeric antibody can contain a variable region of a mouse or rat monoclonal antibody fused to a constant region of a human antibody.
- Methods for producing chimeric antibodies are known in the art. See, e.g., Morrison SL (1985) Science 229: 1202-7 ; Oi VT & Morrison SL (1986) BioTechniques 4: 214-221 ; Gillies SD et al., (1989) J Immunol Methods 125: 191-202 ; and U.S. Patent Nos. 5,807,715 , 4,816,567 , 4,816,397 , and 6,331,415 .
- a humanized antibody is capable of binding to a predetermined antigen and which comprises a framework region having substantially the amino acid sequence of a human immunoglobulin and CDRs having substantially the amino acid sequence of a non-human immunoglobulin (e.g., a murine immunoglobulin).
- a humanized antibody also comprises at least a portion of an immunoglobulin constant region (Fc), typically that of a human immunoglobulin.
- the antibody also can include the CH1, hinge, CH2, CH3, and CH4 regions of the heavy chain.
- a humanized antibody can be selected from any class of immunoglobulins, including IgM, IgG, IgD, IgA and IgE, and any isotype, including IgG 1 , IgG 2 , IgG 3 and IgG 4 .
- Humanized antibodies can be produced using a variety of techniques known in the art, including but not limited to, CDR-grafting (European Patent No. EP 239400 ; International Publication No. WO 91/09967 ; and U.S. Patent Nos. 5,225,539 , 5,530,101 , and 5,585,089 ), veneering or resurfacing (European Patent Nos.
- Single domain antibodies for example, antibodies lacking the light chains, can be produced by methods well known in the art. See Riechmann L & Muyldermans S (1999) J Immunol 231: 25-38 ; Nuttall SD et al., (2000) Curr Pharm Biotechnol 1(3): 253-263 ; Muyldermans S, (2001) J Biotechnol 74(4): 277-302 ; U.S. Patent No. 6,005,079 ; and International Publication Nos. WO 94/04678 , WO 94/25591 and WO 01/44301 .
- antibodies that immunospecifically bind to a GITR antigen can, in turn, be utilized to generate anti-idiotype antibodies that "mimic" an antigen using techniques well known to those skilled in the art. (See, e.g., Greenspan NS & Bona CA (1989) FASEB J 7(5): 437-444 ; and Nissinoff A (1991) J Immunol 147(8): 2429-2438 ).
- an antibody described herein which binds to the same epitope of GITR (e.g., human GITR) as an anti-GITR antibody described herein, is a human antibody or an antigen-binding fragment thereof.
- an antibody described herein which competitively blocks ( e.g., in a dose-dependent manner) any one of the antibodies described herein, ( e.g., Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, pab2161, or Hum231#2w) from binding to GITR (e.g., human GITR), is a human antibody
- Human antibodies can be produced using any method known in the art.
- transgenic mice which are incapable of expressing functional endogenous immunoglobulins, but which can express human immunoglobulin genes, can be used.
- the human heavy and light chain immunoglobulin gene complexes can be introduced randomly or by homologous recombination into mouse embryonic stem cells.
- the human variable region, constant region, and diversity region can be introduced into mouse embryonic stem cells in addition to the human heavy and light chain genes.
- the mouse heavy and light chain immunoglobulin genes can be rendered non-functional separately or simultaneously with the introduction of human immunoglobulin loci by homologous recombination.
- homozygous deletion of the J H region prevents endogenous antibody production.
- the modified embryonic stem cells are expanded and microinjected into blastocysts to produce chimeric mice.
- the chimeric mice are then bred to produce homozygous offspring which express human antibodies.
- the transgenic mice are immunized in the normal fashion with a selected antigen, e.g., all or a portion of an antigen (e.g., GITR).
- Monoclonal antibodies directed against the antigen can be obtained from the immunized, transgenic mice using conventional hybridoma technology.
- the human immunoglobulin transgenes harbored by the transgenic mice rearrange during B cell differentiation, and subsequently undergo class switching and somatic mutation.
- mice capable of producing human antibodies include the XenomouseTM (Abgenix, Inc.; U.S. Patent Nos. 6,075,181 and 6,150,184 ), the HuAb-MouseTM (Mederex, Inc./Gen Pharm; U.S. Patent Nos. 5,545,806 and 5,569, 825 ), the Trans Chromo MouseTM (Kirin) and the KM MouseTM (Medarex/Kirin).
- Human antibodies which specifically bind to GITR can be made by a variety of methods known in the art including phage display methods described above using antibody libraries derived from human immunoglobulin sequences. See also U.S. Patent Nos. 4,444,887 , 4,716,111 , and 5,885,793 ; and International Publication Nos. WO 98/46645 , WO 98/50433 , WO 98/24893 , WO 98/16654 , WO 96/34096 , WO 96/33735 , and WO 91/10741 .
- human antibodies can be produced using mouse-human hybridomas.
- human peripheral blood lymphocytes transformed with Epstein-Barr virus (EBV) can be fused with mouse myeloma cells to produce mouse-human hybridomas secreting human monoclonal antibodies, and these mouse-human hybridomas can be screened to determine ones which secrete human monoclonal antibodies that immunospecifically bind to a target antigen (e.g., GITR ( e.g., human GITR)).
- GITR e.g., human GITR
- polynucleotides comprising a nucleotide sequence encoding an antibody described herein or a fragment thereof (e.g., a variable light chain region and/or variable heavy chain region) that immunospecifically binds to a GITR (e.g., human GITR) antigen, and vectors, e.g., vectors comprising such polynucleotides for recombinant expression in host cells (e.g., E. coli and mammalian cells).
- GITR e.g., human GITR
- vectors e.g., vectors comprising such polynucleotides for recombinant expression in host cells (e.g., E. coli and mammalian cells).
- polynucleotides comprising nucleotide sequences encoding any of the antibodies provided herein, as well as vectors comprising such polynucleotide sequences, e.g., expression vectors for their efficient expression in host cells, e.g., mammalian cells.
- an "isolated" polynucleotide or nucleic acid molecule is one which is separated from other nucleic acid molecules which are present in the natural source (e.g., in a mouse or a human) of the nucleic acid molecule.
- an "isolated" nucleic acid molecule such as a cDNA molecule, can be substantially free of other cellular material, or culture medium when produced by recombinant techniques, or substantially free of chemical precursors or other chemicals when chemically synthesized.
- the language "substantially free” includes preparations of polynucleotide or nucleic acid molecule having less than about 15%, 10%, 5%, 2%, 1%, 0.5%, or 0.1% (in particular less than about 10%) of other material, e.g., cellular material, culture medium, other nucleic acid molecules, chemical precursors and/or other chemicals.
- a nucleic acid molecule(s) encoding an antibody described herein is isolated or purified.
- polynucleotides comprising nucleotide sequences encoding antibodies or antigen-binding fragments thereof, which immunospecifically bind to a GITR polypeptide (e.g., human GITR) and comprises an amino acid sequence as described herein, as well as antibodies which compete with such antibodies for binding to a GITR polypeptide ( e.g., in a dose-dependent manner), or which binds to the same epitope as that of such antibodies.
- a GITR polypeptide e.g., human GITR
- antibodies which compete with such antibodies for binding to a GITR polypeptide (e.g., in a dose-dependent manner), or which binds to the same epitope as that of such antibodies.
- polynucleotides comprising a nucleotide sequence encoding the light chain or heavy chain of an antibody described herein.
- the polynucleotides can comprise nucleotide sequences encoding a light chain comprising the VL FRs and CDRs of antibodies described herein (see, e.g., Tables 1 and 3).
- the polynucleotides can comprise nucleotide sequences encoding a heavy chain comprising the VH FRs and CDRs of antibodies described herein (see, e.g., Tables 2 and 4).
- a polynucleotide described herein encodes a VL domain comprising an amino acid sequence selected from the group consisting of SEQ ID NO: 202, 204, 205, 207, 208, and 400-518. In specific embodiments, a polynucleotide described herein encodes a VL domain comprising the amino acid sequence of SEQ ID NO: 519. In specific embodiments, a polynucleotide described herein encodes a VH domain comprising an amino acid sequence selected from the group consisting of SEQ ID NOs: 201, 203, 206, and 215-389.
- a polynucleotide described herein encodes a VL domain comprising the amino acid sequence of any one of antibodies 231-32-15, Hum231#1 or Hum231#2 ( e.g., SEQ ID NOs: 202, 207 or 208).
- a polynucleotide described herein encodes a VH domain comprising the amino acid sequence of any one of antibodies 231-32-15, Hum231#1 or Hum231#2 (e.g., SEQ ID NOs: 201 or 206).
- a polynucleotide described herein encodes a VL domain and a VH domain comprising the amino acid sequence of any one of antibodies 231-32-15, Hum231#1 or Hum231#2 ( e.g., SEQ ID NOs: 201-202 and/or 206-208).
- polynucleotides comprising a nucleotide sequence encoding an anti-GITR antibody comprising three VL chain CDRs, e.g., containing VL CDR1, VL CDR2, and VL CDR3 of any one of antibodies described herein (e.g., see Table 1, for example, the VL CDRs in one row in Table 1).
- polynucleotides comprising three VH chain CDRs, e.g., containing VH CDR1, VH CDR2, and VH CDR3 of any one of antibodies described herein (e.g., see Table 2, for example, the VH CDRs in one row in Table 2).
- polynucleotides comprising a nucleotide sequence encoding an anti-GITR antibody comprising three VH chain CDRs, e.g., containing VL CDR1, VL CDR2, and VL CDR3 of any one of antibodies described herein (e.g., see Table 1, e.g., the VL CDRs in one row in Table 1) and three VH chain CDRs, e.g., containing VH CDR1, VH CDR2, and VH CDR3 of any one of antibodies described herein (e.g., see Table 2, e.g., the VH CDRs in one row in Table 2).
- a polynucleotide described herein encodes the VL CDRs of any one of antibodies 231-32-15, Hum231#1 or Hum231#2 (e.g., SEQ ID NOs: 16, 17, or 18). In specific embodiments, a polynucleotide described herein encodes the VH CDRs of any one of antibodies 231-32-15, Hum231#1 or Hum231#2 ( e.g., SEQ ID NOs: 13, 14, or 15).
- a polynucleotide described herein encodes VL CDRs and VH CDRs of any one of antibodies 231-32-15, Hum231#1 or Hum231#2 ( e.g., SEQ ID NOs: 13-18).
- polynucleotides comprising a nucleotide sequence encoding an anti-GITR antibody comprising a VL domain, e.g., containing FR1-CDR1-FR2-CDR2-FR3-CDR3-FR4, comprising an amino acid sequence described herein (e.g., see Tables 1 and 3, e.g., the VL CDRs and VLFRs of a particular antibody identified by name in the tables).
- polynucleotides comprising a nucleotide sequence encoding an anti-GITR antibody comprising a VH domain, e.g., containing FR1-CDR1-FR2-CDR2-FR3-CDR3-FR4, comprising an amino acid sequence described herein (e.g., see Tables 2 and 4, e.g., the VH CDRs and VH FRs of a particular antibody identified by name in the Tables).
- a polynucleotide described herein comprises a nucleotide sequence encoding an antibody provided herein comprising a light chain variable region comprising an amino acid sequence described herein (e.g., SEQ ID NOs: 202, 204, 205, 207, 208, and 400-518 or SEQ ID NO:519), wherein the antibody immunospecifically binds to GITR (e.g., human GITR).
- GITR e.g., human GITR
- a polynucleotide described herein comprises a nucleotide sequence encoding antibodies Hum231#1 or Hum231#2 or Hum231#2w provided herein comprising a light chain variable region comprising an amino acid sequence described herein (e.g., SEQ ID NOs: 207 or 208).
- a polynucleotide described herein comprises a nucleotide sequence encoding an antibody provided herein comprising a heavy chain variable region comprising an amino acid sequence described herein (e.g., SEQ ID NO: 201, 203, 206, and 215-389), wherein the antibody immunospecifically binds to GITR ( e.g., human GITR).
- a polynucleotide described herein comprises a nucleotide sequence encoding antibodies Hum231#1, Hum231#2 or Hum231#2w provided herein comprising a heavy chain variable region comprising an amino acid sequence described herein (e.g., SEQ ID NO: 206).
- a polynucleotide comprises a nucleotide sequence encoding an antibody described herein comprising a VL domain comprising one or more VL FRs having the amino acid sequence described herein (e.g., see Table 3, e.g., the framework regions in one row of the table), wherein the antibody immunospecifically binds to GITR (e.g., human GITR).
- GITR e.g., human GITR
- a polynucleotide comprises a nucleotide sequence encoding an antibody described herein comprising a VH domain comprising one or more VH FRs having the amino acid sequence described herein (e.g., see Table 4, e.g., the framework regions in one row of the table), wherein the antibody immunospecifically binds to GITR (e.g., human GITR).
- GITR e.g., human GITR
- a polynucleotide provided herein comprises a nucleotide sequence encoding an antibody described herein comprising: framework regions ( e.g., framework regions of the VL domain and VH domain) that are human framework regions, wherein the antibody immunospecifically binds GITR ( e.g., human GITR).
- a polynucleotide provided herein comprises a nucleotide sequence encoding an antibody or fragment thereof (e.g., CDRs or variable domain) described in Section 5.1 above.
- a polynucleotide comprising a nucleotide sequence encoding an antibody comprising a light chain and a heavy chain, e.g., a separate light chain and heavy chain.
- a polynucleotide provided herein comprises a nucleotide sequence encoding a kappa light chain.
- a polynucleotide provided herein comprises a nucleotide sequence encoding a lambda light chain.
- a polynucleotide provided herein comprises a nucleotide sequence encoding an antibody described herein comprising a human kappa light chain or a human lambda light chain.
- a polynucleotide provided herein comprises a nucleotide sequence encoding an antibody described herein, which immunospecifically binds to GITR (e.g., human GITR), wherein the antibody comprises a light chain, and wherein the amino acid sequence of the VL domain can comprise any amino acid sequence described herein ( e.g., SEQ ID NO: 202, 204, 205, 207, 208 and 400-518 or SEQ ID NO:519), and wherein the constant region of the light chain comprises the amino acid sequence of a human kappa light chain constant region.
- a polynucleotide provided herein comprises a nucleotide sequence encoding an antibody described herein, which immunospecifically binds to GITR (e.g., human GITR), and comprises a light chain, wherein the amino acid sequence of the VL domain can comprises any amino acid sequence described herein (e.g., SEQ ID NO: 202, 204, 205, 207, 208 and 400-518 or SEQ ID NO:519), and wherein the constant region of the light chain comprises the amino acid sequence of a human lambda light chain constant region.
- human constant region sequences can be those described in U.S. Patent No. 5,693,780.
- a polynucleotide provided herein comprises a nucleotide sequence encoding an antibody described herein, which immunospecifically binds to GITR (e.g., human GITR), wherein the antibody comprises a heavy chain, wherein the amino acid sequence of the VH domain can comprise any amino acid sequence described herein ( e.g., SEQ ID NO: 201, 203, 206 and 215-389), and wherein the constant region of the heavy chain comprises the amino acid sequence of a human gamma ( ⁇ ) heavy chain constant region.
- GITR e.g., human GITR
- the amino acid sequence of the VH domain can comprise any amino acid sequence described herein (e.g., SEQ ID NO: 201, 203, 206 and 215-389)
- the constant region of the heavy chain comprises the amino acid sequence of a human gamma ( ⁇ ) heavy chain constant region.
- a polynucleotide provided herein comprises a nucleotide sequence(s) encoding a VH domain and/or a VL domain of an antibody described herein (e.g., Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107 such as SEQ ID NO: 209 or 800-974 for the VH domain or SEQ ID NO: 210, 211 or 1001-1126 for the VL domain), which immunospecifically binds to GITR (e.g., human GITR).
- GITR e.g., human GITR
- a polynucleotide provided herein comprises a nucleotide sequence(s) encoding a VH domain and/or a VL domain of an antibody described herein (e.g., Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161 such as SEQ ID NO: 209 or 800-974 for the VH domain or SEQ ID NO: 210, 211 or 1000-1118 for the VL domain), which immunospecifically binds to GITR (e.g., human GITR).
- GITR e.g., human GITR
- a polynucleotide provided herein comprises a nucleotide sequence(s) encoding a VH domain and/or a VL domain of antibody Hum231#1 or Hum231# ( e.g., SEQ ID NOs: 209-211).
- a polynucleotide provided herein comprises a nucleotide sequence encoding an antibody described herein (or an antigen-binding fragment thereof), which immunospecifically binds GITR (e.g., human GITR), wherein the antibody comprises a VL domain and a VH domain comprising any amino acid sequences described herein, and wherein the constant regions comprise the amino acid sequences of the constant regions of a human IgG 1 ( e.g., allotype 1, 17, or 3) or human IgG 4 .
- GITR e.g., human GITR
- polynucleotides comprising a nucleotide sequence encoding an anti-GITR antibody, or an antigen-binding fragment or domain thereof, designated herein, see, e.g., Tables 1-4, for example antibody Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15, or antibodies 1-107, or antibodies pab2159, pab2160, pab2161, or Hum231#2w.
- polynucleotides encoding an anti-GITR antibody or a fragment thereof that are optimized, e.g., by codon/RNA optimization, replacement with heterologous signal sequences, and elimination of mRNA instability elements.
- Methods to generate optimized nucleic acids encoding an anti-GITR antibody or a fragment thereof (e.g., light chain, heavy chain, VH domain, or VL domain) for recombinant expression by introducing codon changes and/or eliminating inhibitory regions in the mRNA can be carried out by adapting the optimization methods described in, e.g., U.S. Patent Nos.
- RNA potential splice sites and instability elements (e.g., A/T or A/U rich elements) within the RNA can be mutated without altering the amino acids encoded by the nucleic acid sequences to increase stability of the RNA for recombinant expression.
- the alterations utilize the degeneracy of the genetic code, e.g., using an alternative codon for an identical amino acid.
- Such methods can increase expression of an anti-GITR antibody or fragment thereof by at least 1 fold, 2 fold, 3 fold, 4 fold, 5 fold, 10 fold, 20 fold, 30 fold, 40 fold, 50 fold, 60 fold, 70 fold, 80 fold, 90 fold, or 100 fold or more relative to the expression of an anti-GITR antibody encoded by polynucleotides that have not been optimized.
- an optimized polynucleotide sequence encoding an anti-GITR antibody described herein or a fragment thereof can hybridize to an antisense (e.g., complementary) polynucleotide of an unoptimized polynucleotide sequence encoding an anti-GITR antibody described herein or a fragment thereof ( e.g., VL domain and/or VH domain).
- an optimized nucleotide sequence encoding an anti-GITR antibody described herein or a fragment hybridizes under high stringency conditions to antisense polynucleotide of an unoptimized polynucleotide sequence encoding an anti-GITR antibody described herein or a fragment thereof.
- an optimized nucleotide sequence encoding an anti-GITR antibody described herein or a fragment thereof hybridizes under high stringency, intermediate or lower stringency hybridization conditions to an antisense polynucleotide of an unoptimized nucleotide sequence encoding an anti-GITR antibody described herein or a fragment thereof.
- Information regarding hybridization conditions has been described, see, e.g., U.S. Patent Application Publication No. US 2005/0048549 ( e.g., paragraphs 72-73), which is incorporated herein by reference.
- the polynucleotides can be obtained, and the nucleotide sequence of the polynucleotides determined, by any method known in the art.
- Nucleotide sequences encoding antibodies described herein, e.g., antibodies described in Tables 1-4, and modified versions of these antibodies can be determined using methods well known in the art, i.e., nucleotide codons known to encode particular amino acids are assembled in such a way to generate a nucleic acid that encodes the antibody.
- Such a polynucleotide encoding the antibody can be assembled from chemically synthesized oligonucleotides (e.g., as described in Kutmeier G et al., (1994), BioTechniques 17: 242-6 ), which, briefly, involves the synthesis of overlapping oligonucleotides containing portions of the sequence encoding the antibody, annealing and ligating of those oligonucleotides, and then amplification of the ligated oligonucleotides by PCR.
- chemically synthesized oligonucleotides e.g., as described in Kutmeier G et al., (1994), BioTechniques 17: 242-6 ), which, briefly, involves the synthesis of overlapping oligonucleotides containing portions of the sequence encoding the antibody, annealing and ligating of those oligonucleotides, and then amplification of the ligated oligonucleot
- a polynucleotide encoding an antibody described herein can be generated from nucleic acid from a suitable source (e.g., a hybridoma) using methods well known in the art (e.g., PCR and other molecular cloning methods). For example, PCR amplification using synthetic primers hybridizable to the 3' and 5' ends of a known sequence can be performed using genomic DNA obtained from hybridoma cells producing the antibody of interest. Such PCR amplification methods can be used to obtain nucleic acids comprising the sequence encoding the light chain and/or heavy chain of an antibody.
- Such PCR amplification methods can be used to obtain nucleic acids comprising the sequence encoding the variable light chain region and/or the variable heavy chain region of an antibody.
- the amplified nucleic acids can be cloned into vectors for expression in host cells and for further cloning, for example, to generate chimeric and humanized antibodies.
- a nucleic acid encoding the immunoglobulin can be chemically synthesized or obtained from a suitable source (e.g., an antibody cDNA library or a cDNA library generated from, or nucleic acid, preferably poly A+ RNA, isolated from, any tissue or cells expressing the antibody, such as hybridoma cells selected to express an antibody described herein) by PCR amplification using synthetic primers hybridizable to the 3' and 5' ends of the sequence or by cloning using an oligonucleotide probe specific for the particular gene sequence to identify, e.g., a cDNA clone from a cDNA library that encodes the antibody. Amplified nucleic acids generated by PCR can then be cloned into replicable cloning vectors using any method well known in the art.
- a suitable source e.g., an antibody cDNA library or a cDNA library generated from, or nucleic acid, preferably poly A+ RNA, isolated from,
- DNA encoding anti-GITR antibodies described herein can be readily isolated and sequenced using conventional procedures (e.g., by using oligonucleotide probes that are capable of binding specifically to genes encoding the heavy and light chains of the anti-GITR antibodies).
- Hybridoma cells can serve as a source of such DNA. Once isolated, the DNA can be placed into expression vectors, which are then transfected into host cells such as E.
- coli cells simian COS cells, Chinese hamster ovary (CHO) cells (e.g., CHO cells from the CHO GS SystemTM (Lonza)), or myeloma cells that do not otherwise produce immunoglobulin protein, to obtain the synthesis of anti-GITR antibodies in the recombinant host cells.
- CHO Chinese hamster ovary
- PCR primers including VH or VL nucleotide sequences, a restriction site, and a flanking sequence to protect the restriction site can be used to amplify the VH or VL sequences in scFv clones.
- the PCR amplified VH domains can be cloned into vectors expressing a heavy chain constant region, e.g., the human gamma 4 constant region, and the PCR amplified VL domains can be cloned into vectors expressing a light chain constant region, e.g., human kappa or lambda constant regions.
- the vectors for expressing the VH or VL domains comprise an EF-1 ⁇ promoter, a secretion signal, a cloning site for the variable domain, constant domains, and a selection marker such as neomycin.
- the VH and VL domains can also be cloned into one vector expressing the necessary constant regions.
- the heavy chain conversion vectors and light chain conversion vectors are then co-transfected into cell lines to generate stable or transient cell lines that express full-length antibodies, e.g., IgG, using techniques known to those of skill in the art.
- the DNA also can be modified, for example, by substituting the coding sequence for human heavy and light chain constant domains in place of the murine sequences, or by covalently joining to the immunoglobulin coding sequence all or part of the coding sequence for a non-immunoglobulin polypeptide.
- polynucleotides that hybridize under high stringency, intermediate or lower stringency hybridization conditions to polynucleotides that encode an antibody described herein.
- polynucleotides described herein hybridize under high stringency, intermediate or lower stringency hybridization conditions to polynucleotides encoding a VH domain (e.g., SEQ ID NO: 201, 203, 206, and 215-389) and/or VL domain ( e.g., 202, 204, 205, 207, 208, and 400-518 or SEQ ID NO: 519) provided herein.
- VH domain e.g., SEQ ID NO: 201, 203, 206, and 215-389
- VL domain e.g., 202, 204, 205, 207, 208, and 400-518 or SEQ ID NO: 519) provided herein.
- Hybridization conditions have been described in the art and are known to one of skill in the art.
- hybridization under stringent conditions can involve hybridization to filter-bound DNA in 6x sodium chloride/sodium citrate (SSC) at about 45° C followed by one or more washes in 0.2xSSC/0.1% SDS at about 50-65° C;
- hybridization under highly stringent conditions can involve hybridization to filter-bound nucleic acid in 6xSSC at about 45° C followed by one or more washes in 0.1xSSC/0.2% SDS at about 68° C.
- Hybridization under other stringent hybridization conditions are known to those of skill in the art and have been described, see, for example, Ausubel FM et al., eds., (1989) Current Protocols in Molecular Biology, Vol. I, Green Publishing Associates, Inc. and John Wiley & Sons, Inc., New York at pages 6.3.1-6.3.6 and 2.10.3 .
- cells e.g., host cells
- cells expressing (e.g., recombinantly) antibodies described herein (or an antigen-binding fragment thereof) which specifically bind to GITR (e.g., human GITR) and related polynucleotides and expression vectors.
- vectors e.g., expression vectors
- polynucleotides comprising nucleotide sequences encoding anti-GITR antibodies or a fragment for recombinant expression in host cells, preferably in mammalian cells.
- host cells comprising such vectors for recombinantly expressing anti-GITR antibodies described herein (e.g., human or humanized antibody).
- methods for producing an antibody described herein, comprising expressing such antibody from a host cell.
- Recombinant expression of an antibody described herein e.g., a full-length antibody, heavy and/or light chain of an antibody, or a single chain antibody described herein
- GITR e.g., human GITR
- Recombinant expression of an antibody described herein involves construction of an expression vector containing a polynucleotide that encodes the antibody.
- a polynucleotide encoding an antibody molecule, heavy and/or light chain of an antibody, or a fragment thereof (e.g., heavy and/or light chain variable domains) described herein has been obtained, the vector for the production of the antibody molecule can be produced by recombinant DNA technology using techniques well known in the art.
- replicable vectors comprising a nucleotide sequence encoding an antibody molecule described herein, a heavy or light chain of an antibody, a heavy or light chain variable domain of an antibody or a fragment thereof, or a heavy or light chain CDR, operably linked to a promoter.
- Such vectors can, for example, include the nucleotide sequence encoding the constant region of the antibody molecule (see, e.g., International Publication Nos. WO 86/05807 and WO 89/01036 ; and U.S. Patent No. 5,122,464 ) and variable domains of the antibody can be cloned into such a vector for expression of the entire heavy, the entire light chain, or both the entire heavy and light chains.
- An expression vector can be transferred to a cell (e.g., host cell) by conventional techniques and the resulting cells can then be cultured by conventional techniques to produce an antibody described herein (e.g., an antibody comprising the CDRs of any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161) or a fragment thereof.
- an antibody described herein e.g., an antibody comprising the CDRs of any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab
- host cells containing a polynucleotide encoding an antibody described herein or fragments thereof, or a heavy or light chain thereof, or fragment thereof, or a single chain antibody described herein (e.g., an antibody comprising the CDRs of any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161), operably linked to a promoter for expression of such sequences in the host cell.
- a promoter for expression of such sequences in the host cell operably linked to a promoter for expression of such sequences in the host cell.
- a host cell contains a vector comprising a polynucleotide encoding both the heavy chain and light chain of an antibody described herein (e.g., an antibody comprising the CDRs of any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161), or a fragment thereof.
- a host cell contains two different vectors, a first vector comprising a polynucleotide encoding a heavy chain or a heavy chain variable region of an antibody described herein (e.g., an antibody comprising the CDRs of any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161), or a fragment thereof, and a second vector comprising a polynucleotide encoding a light chain or a light chain variable region of an antibody described herein (e.g., an antibody comprising the CDRs of any one of antibodies Hum231#1, Hum2
- a first host cell comprises a first vector comprising a polynucleotide encoding a heavy chain or a heavy chain variable region of an antibody described herein (e.g., an antibody comprising the CDRs of any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161), or a fragment thereof
- a second host cell comprises a second vector comprising a polynucleotide encoding a light chain or a light chain variable region of an antibody described herein (e.g., an antibody comprising the CDRs of any one of antibodies Hum231#1,
- a heavy chain/heavy chain variable region expressed by a first cell associated with a light chain/light chain variable region of a second cell to form an anti-GITR antibody described herein e.g., antibody comprising the CDRs of any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161) or an antigen-binding fragment thereof.
- a population of host cells comprising such first host cell and such second host cell.
- a population of vectors comprising a first vector comprising a polynucleotide encoding a light chain/light chain variable region of an anti-GITR antibody described herein (e.g., antibody comprising the CDRs of any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161), and a second vector comprising a polynucleotide encoding a heavy chain/heavy chain variable region of an anti-GITR antibody described herein (e.g., antibody comprising the CDRs of any one of antibodies Hum231#1, Hum2
- a variety of host-expression vector systems can be utilized to express antibody molecules described herein (e.g., an antibody comprising the CDRs of any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161 (see, e.g., U.S. Patent No. 5,807,715 ).
- Such host-expression systems represent vehicles by which the coding sequences of interest can be produced and subsequently purified, but also represent cells which can, when transformed or transfected with the appropriate nucleotide coding sequences, express an antibody molecule described herein in situ.
- These include but are not limited to microorganisms such as bacteria ( e.g., E. coli and B.
- subtilis transformed with recombinant bacteriophage DNA, plasmid DNA or cosmid DNA expression vectors containing antibody coding sequences; yeast ( e.g., Saccharomyces Pichia ) transformed with recombinant yeast expression vectors containing antibody coding sequences; insect cell systems infected with recombinant virus expression vectors ( e.g., baculovirus) containing antibody coding sequences; plant cell systems ( e .
- green algae such as Chlamydomonas reinhardtii
- recombinant virus expression vectors e.g., cauliflower mosaic virus, CaMV; tobacco mosaic virus, TMV
- recombinant plasmid expression vectors e.g., Ti plasmid
- mammalian cell systems e.g., COS (e.g., COS1 or COS), CHO, BHK, MDCK, HEK 293, NS0, PER.C6, VERO, CRL7O3O, HsS78Bst, HeLa, and NIH 3T3, HEK-293T, HepG2, SP210, R1.1, B-W, L-M, BSC1, BSC40, YB/20 and BMT10 cells) harboring recombinant expression constructs containing promoters derived from the genome of mammalian cells (e.g., metallothione
- cells for expressing antibodies described herein are CHO cells, for example CHO cells from the CHO GS SystemTM (Lonza).
- cells for expressing antibodies described herein are human cells, e.g., human cell lines.
- a mammalian expression vector is pOptiVECTM or pcDNA3.3.
- bacterial cells such as Escherichia coli, or eukaryotic cells ( e.g., mammalian cells), especially for the expression of whole recombinant antibody molecule, are used for the expression of a recombinant antibody molecule.
- mammalian cells such as Chinese hamster ovary (CHO) cells
- a vector such as the major intermediate early gene promoter element from human cytomegalovirus
- antibodies described herein are produced by CHO cells or NS0 cells.
- the expression of nucleotide sequences encoding antibodies described herein which immunospecifically bind GITR is regulated by a constitutive promoter, inducible promoter or tissue specific promoter.
- a number of expression vectors can be advantageously selected depending upon the use intended for the antibody molecule being expressed. For example, when a large quantity of such an antibody is to be produced, for the generation of pharmaceutical compositions of an antibody molecule, vectors which direct the expression of high levels of fusion protein products that are readily purified can be desirable. Such vectors include, but are not limited to, the E.
- coli expression vector pUR278 Ruether U & Mueller-Hill B (1983) EMBO J 2: 1791-1794 ), in which the antibody coding sequence can be ligated individually into the vector in frame with the lac Z coding region so that a fusion protein is produced; pIN vectors ( Inouye S & Inouye M (1985) Nuc Acids Res 13: 3101-3109 ; Van Heeke G & Schuster SM (1989) J Biol Chem 24: 5503-5509 ); and the like.
- pGEX vectors can also be used to express foreign polypeptides as fusion proteins with glutathione 5-transferase (GST).
- fusion proteins are soluble and can easily be purified from lysed cells by adsorption and binding to matrix glutathione agarose beads followed by elution in the presence of free glutathione.
- the pGEX vectors are designed to include thrombin or factor Xa protease cleavage sites so that the cloned target gene product can be released from the GST moiety.
- Autographa californica nuclear polyhedrosis virus (AcNPV), for example, can be used as a vector to express foreign genes.
- the virus grows in Spodoptera frugiperda cells.
- the antibody coding sequence can be cloned individually into non-essential regions (for example the polyhedrin gene) of the virus and placed under control of an AcNPV promoter (for example the polyhedrin promoter).
- a number of viral-based expression systems can be utilized.
- the antibody coding sequence of interest can be ligated to an adenovirus transcription/translation control complex, e.g., the late promoter and tripartite leader sequence. This chimeric gene can then be inserted in the adenovirus genome by in vitro or in vivo recombination.
- Insertion in a non-essential region of the viral genome will result in a recombinant virus that is viable and capable of expressing the antibody molecule in infected hosts (e.g., see Logan J & Shenk T (1984) PNAS 81(12): 3655-9 ).
- Specific initiation signals can also be required for efficient translation of inserted antibody coding sequences. These signals include the ATG initiation codon and adjacent sequences. Furthermore, the initiation codon must be in phase with the reading frame of the desired coding sequence to ensure translation of the entire insert.
- These exogenous translational control signals and initiation codons can be of a variety of origins, both natural and synthetic. The efficiency of expression can be enhanced by the inclusion of appropriate transcription enhancer elements, transcription terminators, etc. (see, e.g., Bitter G et al., (1987) Methods Enzymol. 153: 516-544 ).
- a host cell strain can be chosen which modulates the expression of the inserted sequences, or modifies and processes the gene product in the specific fashion desired. Such modifications (e.g., glycosylation) and processing (e.g., cleavage) of protein products can be important for the function of the protein.
- Different host cells have characteristic and specific mechanisms for the post-translational processing and modification of proteins and gene products. Appropriate cell lines or host systems can be chosen to ensure the correct modification and processing of the foreign protein expressed.
- eukaryotic host cells which possess the cellular machinery for proper processing of the primary transcript, glycosylation, and phosphorylation of the gene product can be used.
- Such mammalian host cells include but are not limited to CHO, VERO, BHK, Hela, MDCK, HEK 293, NIH 3T3, W138, BT483, Hs578T, HTB2, BT2O and T47D, NS0 (a murine myeloma cell line that does not endogenously produce any immunoglobulin chains), CRL7O3O, COS (e.g., COS1 or COS), PER.C6, VERO, HsS78Bst, HEK-293T, HepG2, SP210, R1.1, B-W, L-M, BSC1, BSC40, YB/20, BMT10 and HsS78Bst cells.
- COS e.g., COS1 or COS
- PER.C6 VERO, HsS78Bst, HEK-293T
- HepG2 SP210, R1.1, B-W, L-M, BSC1, BSC40, YB
- anti-GITR antibodies described herein e.g., an antibody comprising the CDRs of any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161) are produced in mammalian cells, such as CHO cells.
- the antibodies described herein or antigen-binding fragments thereof have reduced fucose content or no fucose content.
- Such antibodies can be produced using techniques known one skilled in the art.
- the antibodies can be expressed in cells deficient or lacking the ability of to fucosylate.
- cell lines with a knockout of both alleles of ⁇ 1,6-fucosyltransferase can be used to produce antibodies or antigen-binding fragments thereof with reduced fucose content.
- the Potelligent® system (Lonza) is an example of such a system that can be used to produce antibodies or antigen-binding fragments thereof with reduced fucose content.
- stable expression cells can be generated.
- a cell provided herein stably expresses a light chain/light chain variable domain and a heavy chain/heavy chain variable domain which associate to form an antibody described herein (e.g., an antibody comprising the CDRs of any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab1973, pab1975, pab1976, pab1977, pab1979, pab1980, pab1981, pab1983, 231-32-15 or antibodies 1-107, or antibodies pab2159, pab2160, or pab2161) or an antigen-binding fragment thereof.
- an antibody comprising the CDRs of any one of antibodies Hum231#1, Hum231#2, pab1964, pab1965, pab1966, pab1967, pab1968, pab1969, pab1970, pab1971, pab1972, pab
- host cells can be transformed with DNA controlled by appropriate expression control elements (e.g., promoter, enhancer, sequences, transcription terminators, polyadenylation sites, etc .), and a selectable marker.
- appropriate expression control elements e.g., promoter, enhancer, sequences, transcription terminators, polyadenylation sites, etc .
- engineered cells can be allowed to grow for 1-2 days in an enriched media, and then are switched to a selective media.
- the selectable marker in the recombinant plasmid confers resistance to the selection and allows cells to stably integrate the plasmid into their chromosomes and grow to form foci which in turn can be cloned and expanded into cell lines.
- This method can advantageously be used to engineer cell lines which express an anti-GITR antibody described herein or a fragment thereof.
- Such engineered cell lines can be particularly useful in screening and evaluation of compositions that interact directly or indirectly with the antibody molecule.
- a number of selection systems can be used, including but not limited to, the herpes simplex virus thymidine kinase ( Wigler M et al., (1977) Cell 11(1): 223-32 ), hypoxanthineguanine phosphoribosyltransferase ( Szybalska EH & Szybalski W (1962) PNAS 48(12): 2026-2034 ) and adenine phosphoribosyltransferase ( Lowy I et al., (1980) Cell 22(3): 817-23 ) genes can be employed in tk-, hgprt- or aprt-cells, respectively.
- antimetabolite resistance can be used as the basis of selection for the following genes: dhfr, which confers resistance to methotrexate ( Wigler M et al., (1980) PNAS 77(6): 3567-70 ; O'Hare K et al., (1981) PNAS 78: 1527-31 ); gpt, which confers resistance to mycophenolic acid ( Mulligan RC & Berg P (1981) PNAS 78(4): 2072-6 ); neo, which confers resistance to the aminoglycoside G-418 ( Wu GY & Wu CH (1991) Biotherapy 3: 87-95 ; Tolstoshev P (1993) Ann Rev Pharmacol Toxicol 32: 573-596 ; Mulligan RC (1993) Science 260: 926-932 ; and Morgan RA & Anderson WF (1993) Ann Rev Biochem 62: 191-217 ; Nabel GJ & Felgner PL (1993) Trends Biotechnol 11(5)
- the expression levels of an antibody molecule can be increased by vector amplification (for a review, see Bebbington CR & Hentschel CCG, The use of vectors based on gene amplification for the expression of cloned genes in mammalian cells in DNA cloning, Vol. 3 (Academic Press, New York, 1987 )).
- vector amplification for a review, see Bebbington CR & Hentschel CCG, The use of vectors based on gene amplification for the expression of cloned genes in mammalian cells in DNA cloning, Vol. 3 (Academic Press, New York, 1987 )).
- a marker in the vector system expressing antibody is amplifiable
- increase in the level of inhibitor present in culture of host cell will increase the number of copies of the marker gene. Since the amplified region is associated with the antibody gene, production of the antibody will also increase ( Crouse GF et al., (1983) Mol Cell Biol 3: 257-66
- the host cell can be co-transfected with two or more expression vectors described herein, the first vector encoding a heavy chain derived polypeptide and the second vector encoding a light chain derived polypeptide.
- the two vectors can contain identical selectable markers which enable equal expression of heavy and light chain polypeptides.
- the host cells can be co-transfected with different amounts of the two or more expression vectors. For example, host cells can be transfected with any one of the following ratios of a first expression vector and a second expression vector: 1:1, 1:2, 1:3, 1:4, 1:5, 1:6, 1:7, 1:8, 1:9, 1:10, 1:12, 1:15, 1:20, 1:25, 1:30, 1:35, 1:40, 1:45, or 1:50.
- a single vector can be used which encodes, and is capable of expressing, both heavy and light chain polypeptides.
- the light chain should be placed before the heavy chain to avoid an excess of toxic free heavy chain ( Proudfoot NJ (1986) Nature 322: 562-565 ; and Köhler G (1980) PNAS 77: 2197-2199 ).
- the coding sequences for the heavy and light chains can comprise cDNA or genomic DNA.
- the expression vector can be monocistronic or multicistronic.
- a multicistronic nucleic acid construct can encode 2, 3, 4, 5, 6, 7, 8, 9, 10 or more, or in the range of 2-5, 5-10 or 10-20 genes/nucleotide sequences.
- a bicistronic nucleic acid construct can comprise in the following order a promoter, a first gene (e.g., heavy chain of an antibody described herein), and a second gene and ( e.g., light chain of an antibody described herein).
- a promoter e.g., a promoter
- a first gene e.g., heavy chain of an antibody described herein
- a second gene and e.g., light chain of an antibody described herein.
- the transcription of both genes can be driven by the promoter, whereas the translation of the mRNA from the first gene can be by a cap-dependent scanning mechanism and the translation of the mRNA from the second gene can be by a cap-independent mechanism, e.g., by an IRES.
- an antibody molecule described herein can be purified by any method known in the art for purification of an immunoglobulin molecule, for example, by chromatography (e.g., ion exchange, affinity, particularly by affinity for the specific antigen after Protein A, and sizing column chromatography), centrifugation, differential solubility, or by any other standard technique for the purification of proteins.
- chromatography e.g., ion exchange, affinity, particularly by affinity for the specific antigen after Protein A, and sizing column chromatography
- centrifugation e.g., ion exchange, affinity, particularly by affinity for the specific antigen after Protein A, and sizing column chromatography
- differential solubility e.g., differential solubility, or by any other standard technique for the purification of proteins.
- the antibodies described herein can be fused to heterologous polypeptide sequences described herein or otherwise known in the art to facilitate purification.
- an antibody or an antigen-binding fragment thereof described herein is isolated or purified.
- an isolated antibody is one that is substantially free of other antibodies with different antigenic specificities than the isolated antibody.
- a preparation of an antibody described herein is substantially free of cellular material and/or chemical precursors. The language "substantially free of cellular material” includes preparations of an antibody in which the antibody is separated from cellular components of the cells from which it is isolated or recombinantly produced.
- an antibody that is substantially free of cellular material includes preparations of antibody having less than about 30%, 20%, 10%, 5%, 2%, 1%, 0.5%, or 0.1% (by dry weight) of heterologous protein (also referred to herein as a "contaminating protein") and/or variants of an antibody, for example, different post-translational modified forms of an antibody or other different versions of an antibody (e.g., antibody fragments).
- heterologous protein also referred to herein as a "contaminating protein”
- variants of an antibody for example, different post-translational modified forms of an antibody or other different versions of an antibody (e.g., antibody fragments).
- the antibody is recombinantly produced, it is also generally substantially free of culture medium, i.e., culture medium represents less than about 20%, 10%, 2%, 1%, 0.5%, or 0.1% of the volume of the protein preparation.
- the antibody When the antibody is produced by chemical synthesis, it is generally substantially free of chemical precursors or other chemicals, i.e ., it is separated from chemical precursors or other chemicals which are involved in the synthesis of the protein. Accordingly, such preparations of the antibody have less than about 30%, 20%, 10%, or 5% (by dry weight) of chemical precursors or compounds other than the antibody of interest.
- antibodies described herein are isolated or purified.
- compositions comprising an antibody or antigen-binding fragment thereof described herein having the desired degree of purity in a physiologically acceptable carrier, excipient or stabilizer
- Acceptable carriers, excipients, or stabilizers are nontoxic to recipients at the dosages and concentrations employed, and include buffers such as phosphate, citrate, and other organic acids; antioxidants including ascorbic acid and methionine; preservatives (such as octadecyldimethylbenzyl ammonium chloride; hexamethonium chloride; benzalkonium chloride, benzethonium chloride; phenol, butyl or benzyl alcohol; alkyl parabens such as methyl or propyl paraben; catechol; resorcinol; cyclohexanol; 3-pentanol; and m-cresol); low molecular weight (less than about 10 residues)
- compositions comprise an antibody or antigen-binding fragment thereof described herein, and optionally one or more additional prophylactic or therapeutic agents, in a pharmaceutically acceptable carrier.
- pharmaceutical compositions comprise an effective amount of an antibody or antigen-binding fragment thereof described herein, and optionally one or more additional prophylactic of therapeutic agents, in a pharmaceutically acceptable carrier. See Section 5.4, infra, for examples of prophylactic or therapeutic agents.
- the antibody is the only active ingredient included in the pharmaceutical composition.
- Pharmaceutical compositions described herein can be useful in enhancing, inducing or activating a GITR activity and treating a condition, such as cancer and an infectious disease.
- Pharmaceutically acceptable carriers used in parenteral preparations include aqueous vehicles, nonaqueous vehicles, antimicrobial agents, isotonic agents, buffers, antioxidants, local anesthetics, suspending and dispersing agents, emulsifying agents, sequestering or chelating agents and other pharmaceutically acceptable substances.
- aqueous vehicles include Sodium Chloride Injection, Ringers Injection, Isotonic Dextrose Injection, Sterile Water Injection, Dextrose and Lactated Ringers Injection.
- Nonaqueous parenteral vehicles include fixed oils of vegetable origin, cottonseed oil, corn oil, sesame oil and peanut oil.
- Antimicrobial agents in bacteriostatic or fungistatic concentrations can be added to parenteral preparations packaged in multiple-dose containers which include phenols or cresols, mercurials, benzyl alcohol, chlorobutanol, methyl and propyl p-hydroxybenzoic acid esters, thimerosal, benzalkonium chloride and benzethonium chloride.
- Isotonic agents include sodium chloride and dextrose.
- Buffers include phosphate and citrate.
- Antioxidants include sodium bisulfate.
- Local anesthetics include procaine hydrochloride.
- Suspending and dispersing agents include sodium carboxymethylcelluose, hydroxypropyl methylcellulose and polyvinylpyrrolidone.
- Emulsifying agents include Polysorbate 80 (TWEEN® 80).
- a sequestering or chelating agent of metal ions includes EDTA.
- Pharmaceutical carriers also include ethyl alcohol, polyethylene glycol and propylene glycol for water miscible vehicles; and sodium hydroxide, hydrochloric acid, citric acid or lactic acid for pH adjustment.
- a pharmaceutical composition may be formulated for any route of administration to a subject.
- routes of administration include intranasal, oral, pulmonary, transdermal, intradermal, and parenteral.
- Parenteral administration characterized by either subcutaneous, intramuscular or intravenous injection, is also contemplated herein.
- injectables can be prepared in conventional forms, either as liquid solutions or suspensions, solid forms suitable for solution or suspension in liquid prior to injection, or as emulsions.
- the injectables, solutions and emulsions also contain one or more excipients. Suitable excipients are, for example, water, saline, dextrose, glycerol or ethanol.
- compositions to be administered can also contain minor amounts of non-toxic auxiliary substances such as wetting or emulsifying agents, pH buffering agents, stabilizers, solubility enhancers, and other such agents, such as for example, sodium acetate, sorbitan monolaurate, triethanolamine oleate and cyclodextrins.
- auxiliary substances such as wetting or emulsifying agents, pH buffering agents, stabilizers, solubility enhancers, and other such agents, such as for example, sodium acetate, sorbitan monolaurate, triethanolamine oleate and cyclodextrins.
- Preparations for parenteral administration of an antibody include sterile solutions ready for injection, sterile dry soluble products, such as lyophilized powders, ready to be combined with a solvent just prior to use, including hypodermic tablets, sterile suspensions ready for injection, sterile dry insoluble products ready to be combined with a vehicle just prior to use and sterile emulsions.
- the solutions may be either aqueous or nonaqueous.
- suitable carriers include physiological saline or phosphate buffered saline (PBS), and solutions containing thickening and solubilizing agents, such as glucose, polyethylene glycol, and polypropylene glycol and mixtures thereof.
- PBS physiological saline or phosphate buffered saline
- thickening and solubilizing agents such as glucose, polyethylene glycol, and polypropylene glycol and mixtures thereof.
- Topical mixtures comprising an antibody are prepared as described for the local and systemic administration.
- the resulting mixture can be a solution, suspension, emulsions or the like and can be formulated as creams, gels, ointments, emulsions, solutions, elixirs, lotions, suspensions, tinctures, pastes, foams, aerosols, irrigations, sprays, suppositories, bandages, dermal patches or any other formulations suitable for topical administration.
- An antibody or antigen-binding fragment thereof described herein can be formulated as an aerosol for topical application, such as by inhalation (see, e.g., U.S. Patent Nos. 4,044,126 , 4,414,209 and 4,364,923 , which describe aerosols for delivery of a steroid useful for treatment of inflammatory diseases, particularly asthma).
- These formulations for administration to the respiratory tract can be in the form of an aerosol or solution for a nebulizer, or as a micro fine powder for insufflations, alone or in combination with an inert carrier such as lactose.
- the particles of the formulation will, in one embodiment, have diameters of less than 50 microns, in one embodiment less than 10 microns.
- An antibody or antigen-binding fragment thereof described herein can be formulated for local or topical application, such as for topical application to the skin and mucous membranes, such as in the eye, in the form of gels, creams, and lotions and for application to the eye or for intracisternal or intraspinal application.
- Topical administration is contemplated for transdermal delivery and also for administration to the eyes or mucosa, or for inhalation therapies.
- Nasal solutions of the antibody alone or in combination with other pharmaceutically acceptable excipients can also be administered.
- Transdermal patches including iontophoretic and electrophoretic devices, are well known to those of skill in the art, and can be used to administer an antibody.
- patches are disclosed in U.S. Patent Nos. 6,267,983 , 6,261,595 , 6,256,533 , 6,167,301 , 6,024,975 , 6,010715 , 5,985,317 , 5,983,134 , 5,948,433 , and 5,860,957 .
- a pharmaceutical composition comprising an antibody or antigen-binding fragment thereof described herein is a lyophilized powder, which can be reconstituted for administration as solutions, emulsions and other mixtures. It may also be reconstituted and formulated as solids or gels.
- the lyophilized powder is prepared by dissolving an antibody or antigen-binding fragment thereof described herein, or a pharmaceutically acceptable derivative thereof, in a suitable solvent.
- the lyophilized powder is sterile.
- the solvent may contain an excipient which improves the stability or other pharmacological component of the powder or reconstituted solution, prepared from the powder.
- Excipients that may be used include, but are not limited to, dextrose, sorbitol, fructose, corn syrup, xylitol, glycerin, glucose, sucrose or other suitable agent.
- the solvent may also contain a buffer, such as citrate, sodium or potassium phosphate or other such buffer known to those of skill in the art at, in one embodiment, about neutral pH.
- sterile filtration of the solution followed by lyophilization under standard conditions known to those of skill in the art provides the desired formulation.
- the resulting solution will be apportioned into vials for lyophilization. Each vial will contain a single dosage or multiple dosages of the compound.
- the lyophilized powder can be stored under appropriate conditions, such as at about 4°C to room temperature.
- Reconstitution of this lyophilized powder with water for injection provides a formulation for use in parenteral administration.
- the lyophilized powder is added to sterile water or other suitable carrier. The precise amount depends upon the selected compound. Such amount can be empirically determined.
- the antibodies or antigen-binding fragments thereof described herein and other compositions provided herein can also be formulated to be targeted to a particular tissue, receptor, or other area of the body of the subject to be treated. Many such targeting methods are well known to those of skill in the art. All such targeting methods are contemplated herein for use in the instant compositions. For non-limiting examples of targeting methods, see, e.g., U.S. Patent Nos.
- an antibody or antigen-binding fragment thereof described herein is targeted to a tumor.
- compositions to be used for in vivo administration can be sterile. This is readily accomplished by filtration through, e.g., sterile filtration membranes.
- presented herein are methods for modulating one or more immune functions or responses in a subject, comprising to a subject in need thereof administering an anti-GITR antibody or antigen-binding fragment thereof described herein, or a composition thereof.
- methods for activating, enhancing or inducing one or more immune functions or responses in a subject comprising to a subject in need thereof administering an anti-GITR antibody or antigen-binding fragment thereof, or a composition thereof.
- presented herein are methods for preventing and/or treating diseases in which it is desirable to activate or enhance one or more immune functions or responses, comprising administering to a subject in need thereof an anti-GITR antibody or antigen-binding fragment thereof described herein or a composition thereof.
- the method comprises combination therapy, wherein the anti-GITR antibody or antigen-binding fragment thereof is administered to a subject in combination with another therapy, such as those described below, to activate or enhance one or more immune functions or responses.
- the anti-GITR antibody or antigen-binding fragment thereof is administered as an adjuvant in combination with an antigenic composition.
- the antigenic composition comprises a cancer or tumor antigen (e.g., the bcr/abl antigen in leukemia, HPVE6 and E7 antigens of the oncogenic virus associated with cervical cancer, the MAGE1 and MZ2-E antigens in or associated with melanoma, or the MVC-1 and HER-2 antigens in or associated with breast cancer).
- the antigenic composition comprises an antigen derived from a pathogen (e.g., a viral antigen, parasitic antigen, bacterial antigen or fungal antigen).
- viral antigens examples include the nucleoprotein (NP) of influenza virus, HIV antigens (e.g., gag proteins of HIV, HIV env protein ( e.g., gp120 and/or gp41), HIV Nef protein, HIV Pol proteins, HIV reverse transcriptase, or HIV protease), Ebola virus (EBOV) antigens (e.g., EBOV NP or glycoprotein), small pox antigens, hepatitis A, B or C virus antigens, human rhinovirus antigens, Herpes simplex virus antigens, poliovirus antigens, foot-and-mouth disease virus (FMDV) antigens, rabies virus antigens, rotavirus antigens, coxsackie virus antigens, and human papilloma virus (HPV) antigens.
- NP nucleoprotein
- HIV antigens e.g., gag proteins of HIV, HIV env protein ( e.g., gp120 and
- bacterial antigens examples include Bordetella pertussis (e.g., P69 protein and filamentous haemagglutinin (FHA) antigens), Vibrio cholerae, Bacillus anthracis, and E. coli antigens such as E. coli heat Labile toxin B subunit (LT-B), E. coli K88 antigens, and enterotoxigenic E. coli antigens.
- Bordetella pertussis e.g., P69 protein and filamentous haemagglutinin (FHA) antigens
- Vibrio cholerae Vibrio cholerae
- Bacillus anthracis Bacillus anthracis
- E. coli antigens such as E. coli heat Labile toxin B subunit (LT-B), E. coli K88 antigens, and enterotoxigenic E. coli antigens.
- the term “in combination” refers to the use of more than one therapy (e.g., one or more prophylactic and/or therapeutic agents).
- the use of the term “in combination” does not restrict the order in which therapies are administered to a subject with a disease or disorder, or the route of administration.
- a first therapy e.g., a prophylactic or therapeutic agent
- a first therapy can be administered prior to (e.g., 5 minutes, 15 minutes, 30 minutes, 45 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 12 hours, 24 hours, 48 hours, 72 hours, 96 hours, 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks, 6 weeks, 8 weeks, or 12 weeks before), concomitantly with, or subsequent to ( e.g., 5 minutes, 15 minutes, 30 minutes, 45 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 12 hours, 24 hours, 48 hours, 72 hours, 96 hours, 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks, 6 weeks, 8 weeks, or 12 weeks after) the administration of a second therapy (e.g., a prophylactic or therapeutic agent) to a subject with a disease or disorder or a symptom thereof.
- a second therapy e.g., a prophylactic or therapeutic agent
- a therapy e.g., an agent administered in combination with an anti-GITR antibody or antigen-binding fragment thereof to a subject is administered in the same composition (e.g., pharmaceutical composition).
- a therapy e.g., an agent administered in combination with an anti-GITR antibody or antigen-binding fragment thereof is administered to a subject in a different composition (e.g ., two or more pharmaceutical compositions).
- the two compositions may be administered at the same or different times and/or by the same or different routes of administration.
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered to a subject in combination with a vaccine composition to induce, activate or enhance the immune response elicited by the vaccine composition.
- the vaccine composition is a cancer vaccine.
- a cancer vaccine is an agent, molecule, or immunogen which stimulates or elicits an endogenous immune response in an individual or subject against one or more cancer antigens.
- the cancer antigen can be a tumor associated peptide, or protein that induces or enhances immune response and is derived from tumor associated genes and encoded proteins including, for example, MAGE-A1, MAGE-A2, MAGE-A3, MAGE-A4, MAGE-A5, MAGE-A6, MAGE-A7, MAGE-A8, MAGE-A9, MAGE-A10, MAGE-A11, MAGE-A12, MAGE-A13, GAGE-1, GAGE-2, GAGE-3, GAGE-4, GAGE-5, GAGE-6, GAGE-7, GAGE-8, BAGE-1, RAGE-1, LB33/MUM-1, PRAME, NAG, MAGE-Xp2 (MAGE-B2), MAGEXp3 (MAGE-B3), MAGE-Xp4 (AGE-B4), tyrosinase, brain glycogen phosphorylase, Melan-A, MAGE-C1, MAGE-C2, NY-ESO-1, LAGE-1, SSX-1, SSX-2
- Cancer vaccines are useful to either increase recognition of cancer cells by the immune system or enhance the anti-tumor response through lymphocyte activation.
- Effector T cells have been successfully generated by immunization with intact tumor cells or extract, purified antigens, use of peptides optimized for binding to both MHC and TcR, immune dominant peptides, DNA encoding tumor antigens, recombinant viruses encoding tumor antigens or antigen pulsed antigen-presenting cells.
- enhancement of immune recognition and cell expansion may be improved by the use of costimulators and cytokines, injection of vectors to express cytokines, in vitro antigen-pulsed and activated autologous dendritic cells and by blocking negative modulators (e.g., using immune checkpoint targeting agents) and by depleting T-regulatory cells.
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered to a subject in combination with a heat shock protein based tumor vaccine or a heat shock protein based pathogen vaccine. See Sections 5.4.1.1 and 5.4.1.2 below regarding heat shock protein based tumor vaccines or heat shock protein based pathogen vaccines for use in combination with an anti-GITR antibody or antigen-binding fragment thereof described herein.
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered in combination with an adjuvant to induce, activate or enhance the agonistic effects of the anti-GITR antibody.
- an adjuvant can be used depending on the treatment context.
- Non-limiting examples of appropriate adjuvants include, for example, Complete Freund's Adjuvant (CFA), Incomplete Freund's Adjuvant (IFA), motanide ISA (incomplete seppic adjuvant), the Ribi adjuvant system (RAS), Titer Max, muramyl peptides, Syntex Adjuvant Formulation (SAF), alum (aluminum hydroxide and/or aluminum phosphate), aluminum salt adjuvants, Gerbu® adjuvants, nitrocellulose absorbed antigen, encapsulated or entrapped antigen, immuno-stimulating complexes such as saponins, Quil A, QS-21 and others.
- CFA Complete Freund's Adjuvant
- IFA Incomplete Freund's Adjuvant
- motanide ISA incomplete seppic adjuvant
- Ribi adjuvant system Ribi adjuvant system
- Titer Max muramyl peptides
- SAF Syntex Adjuvant Formulation
- alum aluminum hydro
- adjuvants include CpG oligonucleotides and double stranded RNA molecules, such as poly(A), poly(U). Combinations of the above adjuvants may also be used.
- one or more adjuvants are a saponin, such as QS-21, QS-21 and 3 De-O-acylated monophosphoryl lipid A (3 D-MPL), and immunostimulatory oligonucleotides and saponin adjuvants as disclosed in U.S. Patent Nos. 6,645,495 ; 7,029,678 and 7,858,589 , respectively.
- GITR-responsive cells e.g., T cells, such as effector T-cells
- methods for enhancing the stimulation of GITR-responsive cells comprising incubating ex vivo the GITR-responsive cells (e.g., T cells) with an antibody or antigen-binding fragment thereof described herein.
- the GITR-responsive cells are incubated with a stimulating agent (e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody) prior to, simultaneously with or subsequent to the incubation with the anti-GITR antibody or antigen-binding fragment thereof.
- a stimulating agent e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA)
- a TCR complex stimulating antibody such as an anti-CD3 antibody and anti-CD28 antibody
- the GITR-responsive cells e.g., T cells
- the GITR-responsive cells following stimulation with the anti-GITR antibody or antigen binding fragment thereof are administered to a subject ( e.g., a human).
- the GITR-response cells e.g., T cells
- GITR-responsive cells e.g., T cells
- methods for activating GITR-responsive cells comprising incubating the GITR-responsive cells (e.g., T cells) with an antibody or antigen-binding fragment thereof described herein.
- the GITR-responsive cells are incubated with a stimulating agent (e.g., a T cell receptor complex stimulating agent such as, e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody) prior to, simultaneously with or subsequent to the incubation with the anti-GITR antibody or antigen-binding fragment thereof.
- a stimulating agent e.g., a T cell receptor complex stimulating agent such as, e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an
- the GITR-responsive cells (e.g., T cells) were isolated from a subject ( e.g., a human). In certain embodiments, the GITR-responsive cells following activation with the anti-GITR antibody or antigen-binding fragment thereof are administered to a subject ( e.g., a human).
- the GITR-responsive cells (e.g., T cells) may be administered to the same or a different subject than the cells were originally isolated from.
- cells responsive to GITR are incubated in cell culture with an anti-GITR antibody or antigen-binding fragment thereof described herein, and administered to a subject to enhance immune function (e.g., to enhance the expansion/proliferation of GITR-responsive cells, such as T cells, and/or enhance T cell effector function) and/or to treat cancer and/or prevent or treat an infectious disease.
- enhance immune function e.g., to enhance the expansion/proliferation of GITR-responsive cells, such as T cells, and/or enhance T cell effector function
- cancers and infectious diseases are provided herein. See, e.g., Example 7 below for exemplary methods.
- the GITR-responsive cells are effector T cells (e.g., CD4 + and CD8 + ).
- the GITR-responsive cells are isolated from a subject. In some embodiments, the GITR-responsive cells are assessed for GITR expression prior to incubation with an anti-GITR antibody or antigen-binding fragment thereof described herein. In certain embodiments, the GITR-responsive cells are incubated with a mitogen (e.g., a T cell mitogen, such as, e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA), or a TCR complex stimulating antibody, such as an anti-CD3 antibody and anti-CD28 antibody) prior to, simultaneously with or subsequent to the incubation with an anti-GITR antibody or antigen-binding fragment thereof described herein.
- a mitogen e.g., a T cell mitogen, such as, e.g., phytohaemagglutinin (PHA) and/or phorbol myristate acetate (PMA)
- a TCR complex stimulating antibody such as an anti
- the GITR responsive cells may be incubated with an anti-GITR antibody or antigen-binding fragment thereof described herein for, e.g., 5 minutes, 10 minutes, 15 minutes, 30 minutes, 45 minutes, 1 hour, 2 hours, 3 hours, 4 hours, 5 hours, 6 hours, 8 hours, 12 hours, 18 hours, 24 hours or more.
- the GITR-responsive cells which are administered to a subject were derived from the subject (i.e., the GITR-responsive cells are autologous).
- the GITR-responsive cells which are administered to a subject were derived from a different subject.
- GITR-responsive cells following incubation with anti-GITR antibody or antigen-binding fragment thereof may be administered locally or systemically to a subject via any route known to one of skill in the art (e.g., parenteral administration, such as subcutaneous, intravenous, or intramuscular administration, or intratumoral administration).
- a suitable dose of GITR-responsive cells following incubation with anti-GITR antibody or an antigen-binding fragment thereof administered to subject may be at least 100, 200, 300, 400, 500, 700, 1,000, 5,000, 10,000, 25,000, 50,000, 100,000, 1 x10 6 , 1 x10 7 , or 1 x10 8 cells.
- the GITR-responsive cells following incubation with anti-GITR antibody or antigen-binding fragment thereof may be administered 1, 2, 3, 4, 5, 6, 7, 8 or more times.
- the frequency and dose of GITR-responsive cells following incubation with anti-GITR antibody or antigen-binding fragment thereof which are administered to a subject will vary depending on several factors, including, e.g., the condition of the patient.
- a method for enhancing the expansion of T cells e.g., CD4 + and/or CD8 + T cells
- provided herein is a method for enhancing the expansion of CD8 + T cells in a subject, comprising administering to the subject an effective amount of an antibody or antigen-binding fragment thereof described herein, or a pharmaceutical composition described herein.
- a method for enhancing the expansion of CD4 + T cells in a subject comprising administering to the subject an effective amount of an antibody or antigen-binding fragment thereof described herein, or a pharmaceutical composition described herein.
- the subject is human.
- provided herein is a method for enhancing the expansion of T cells (e.g., CD4 + and/or CD8 + T cells) and/or T cell effector function in a subject, comprising administering to the subject an effective amount of an antibody or antigen-binding fragment thereof described herein, or a pharmaceutical composition described herein.
- a method for enhancing the expansion of CD8 + T cells and/or T cell effector function in a subject comprising administering to the subject an effective amount of an antibody or antigen-binding fragment thereof described herein, or a pharmaceutical composition described herein.
- provided herein is a method for enhancing the expansion of CD4 + T cells and/or T cell effector function in a subject, comprising administering to the subject an effective amount of an antibody or antigen-binding fragment thereof described herein, or a pharmaceutical composition described herein.
- the subject is human.
- a method for preferential expansion of effector T-cells over the expansion of T-regulatory cells comprising incubating ex vivo T-cells with an antibody or antigen-binding fragment thereof described herein.
- the anti-GITR antibody or antigen-binding fragment thereof expands effector T-cells over T-regulatory cells by 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80% or greater.
- the anti-GITR antibody or antigen-binding fragment thereof expands effector T-cells over T-regulatory cells by 10% to 20%, 15% to 25%, 25% to 50%, 30% to 60%, 50% to 75% or 65% to 85%.
- the effector T-cells and T-regulatory cells can be distinguished from each other by cell surface markers, such as those disclosed in the examples infra.
- the T-cells were isolated from a subject ( e.g., a human).
- the T-cells after expansion are administered to a subject ( e.g., a human).
- a method for preferential expansion of effector T-cells over the expansion of T-regulatory cells in a subject comprising administering to the subject an effective amount of an antibody or antigen-binding fragment thereof described herein, or a composition thereof.
- the anti-GITR antibody or antigen-binding fragment thereof expands effector T-cells over T-regulatory cells by 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80% or greater.
- the anti-GITR antibody or antigen-binding fragment thereof expands effector T-cells over T-regulatory cells by 10% to 20%, 15% to 25%, 25% to 50%, 30% to 60%, 50% to 75% or 65% to 85%.
- the effector T-cells and T-regulatory cells can be distinguished from each other by cell surface markers and/or intracellular markers, such as those disclosed in the examples infra.
- the subject is human.
- treatment of a subject with an anti-GITR antibody or antigen-binding fragment described herein, or a composition thereof achieves one, two, three, four or more of the following effects: (i) reduction or amelioration of the severity of a disease or symptom associated therewith; (ii) reduction in the duration of a symptom associated with a disease; (iii) inhibition of the progression of a disease or symptom associated therewith; (iv) regression of a disease or symptom associated therewith; (v) prevention of the development or onset of a symptom associated with a disease that is present in the patient; (vi) inhibition of the recurrence of a symptom associated with a disease; (vii) reduction in the hospitalization of a subject; (viii) reduction in the hospitalization length; (ix) an increase in the survival of a subject with a disease; (x) a reduction in the number of symptoms associated with a disease; and (xi) an enhancement, improvement, supplementation, complementation or augmentation of the therapeutic effect(
- an anti-GITR antibody or antigen-binding fragment thereof, or a composition thereof is administered to a subject in combination with an immunotherapeutic agent.
- Immunotherapeutic agents for use in the combination therapies disclosed herein include, but are not limited to, a Her2/neu receptor antibody such as trastuzumab (marketed as Herceptin®), an anti-CD52 antibody such as alemtuzumab (marketed as Campath®.
- an anti-CD33 antibody such as gemtuzumab linked to calicheamicin (marketed as Mylotarg®), an anti-CD20 antibody such as rituximab (marketed as Rituxan® and MabThera®), Ibritumomab tiuxetan (marketed as Zevalin®), anti-TNFa antibodies such as infliximab (marketed as Remicade®) or adalimmumab (marketed as Humira®), a soluble TNFR2 molecule such as etanercept (marketed as Enbrel®), an antibody to the CD25 chain of the IL-2 receptor such as basiliximab (marketed as Simulect®), an anti-CD40/CD40L antibody such as humanized IgG 1 anti-human CD40 antibody (SGN-40), Toll-like receptor agonists such as monophosphoril lipid A (MPL®), CpG, single-stranded RNA, nucleotides
- an anti-GITR antibody or antigen-binding fragment thereof described herein can be used to treat a condition associated with cancer or a condition resulting from the administration of an anti-cancer therapy (such as, e.g., chemotherapy or radiation).
- an anti-GITR antibody or antigen-binding fragment thereof can be used to treat or manage lymphocytopenia.
- an anti-GITR antibody or antigen-binding fragment thereof is administered to a patient diagnosed with cancer to increase the proliferation and/or effector function of one or more immune cell populations (e.g., T cell effector cells, such as CD4 + and CD8 + T cells) in the patient.
- one or more immune cell populations e.g., T cell effector cells, such as CD4 + and CD8 + T cells
- an anti-GITR antibody or antigen-binding fragment thereof described herein activates or enhances or induces one or more immune functions or responses in a subject by at least 99%, at least 98%, at least 95%, at least 90%, at least 85%, at least 80%, at least 75%, at least 70%, at least 60%, at least 50%, at least 45%, at least 40%, at least 45%, at least 35%, at least 30%, at least 25%, at least 20%, or at least 10%, or in the range of between 10% to 25%, 25% to 50%, 50% to 75%, or 75% to 95% relative to the immune function in a subject not administered the anti-GITR antibody or antigen-binding fragment thereof described herein using assays well known in the art, e.g., ELISPOT, ELISA, and cell proliferation assays.
- assays well known in the art, e.g., ELISPOT, ELISA, and cell proliferation assays.
- the immune function is cytokine production (e.g., interferon-gamma, IL-2, IL-5, IL-10, IL-12, or transforming growth factor (TGF) -alpha production).
- the immune function is T cell proliferation/expansion, which can be assayed, e.g., by flow cytometry to detect the number of cells expressing markers of T cells ( e.g., CD3, CD4, or CD8).
- the immune function is antibody production, which can be assayed, e.g., by ELISA.
- the immune function is effector function, which can be assayed, e.g., by a cytotoxicity assay or other assays well known in the art.
- the immune function is a Th1 response.
- the immune function is a Th2 response.
- the immune function is a memory response.
- non-limiting examples of immune functions that may be enhanced or induced by an anti-GITR antibody or antigen-binding fragment thereof are proliferation/expansion of effector lymphocytes (e.g., increase in the number of effector T lymphocytes), inhibition of apoptosis of effector lymphocytes (e.g., effector T lymphocytes), and suppression of Tregs.
- an immune function enhanced or induced by an anti-GITR antibody or antigen-binding fragment thereof described herein is proliferation/expansion in the number of or activation of CD4 + T cells (e.g., Th1 and Th2 helper T cells), CD8 + T cells (e.g., cytotoxic T lymphocytes, alpha/beta T cells, and gamma/delta T cells), B cells ( e.g., plasma cells), memory T cells, memory B cells, tumor-resident T cells, CD122 + T cells, natural killer (NK) cells), macrophages, monocytes, dendritic cells, mast cells, eosinophils, basophils or polymorphonucleated leukocytes.
- CD4 + T cells e.g., Th1 and Th2 helper T cells
- CD8 + T cells e.g., cytotoxic T lymphocytes, alpha/beta T cells, and gamma/delta T cells
- B cells e.g., plasma cells
- an anti-GITR antibody or antigen-binding fragment thereof described herein activates or enhances the proliferation/expansion or number of lymphocyte progenitors.
- an anti-GITR antibody or antigen-binding fragment thereof described herein increases the number of CD4 + T cells (e.g., Th1 and Th2 helper T cells), CD8 + T cells (e.g., cytotoxic T lymphocytes, alpha/beta T cells, and gamma/delta T cells), B cells (e.g., plasma cells), memory T cells, memory B cells, tumor-resident T cells, CD122 + T cells, natural killer cells (NK cells), macrophages, monocytes, dendritic cells, mast cells, eosinophils, basophils or polymorphonucleated leukocytes by approximately at least 99%, at least 98%, at least 95%, at least 90%, at least 85%, at least 80%, at least 75%, at least 70%, at least 60%, at least
- an antibody described herein which immunospecifically binds to GITR (e.g., human GITR), induces, activates or enhances an activity of human GITR independent of TCR triggering.
- an antibody described herein, which immunospecifically binds to GITR induces, activates or enhances an activity of NF- ⁇ B independent of TCR triggering.
- the activity of NF- ⁇ B can be assessed in, e.g., an assay comprising the following steps: (a) incubating T cells (e.g., Jurkat cells) expressing a NF- ⁇ B-luciferase reporter construct (e.g., GloResponse NF- ⁇ B-luc2P construct) and GITR ( e.g., human GITR) with the antibody described herein or an isotype control antibody at an antibody concentration of, e.g., 12.5, 10, 5, 2.5, 1.25, or 0.625 ⁇ g/ml, in the absence of an anti-CD3 antibody; and (b) reading luciferase signal after, e.g., 2, 5, 6, 8 or 18 hours of incubation using, e.g., an EnVision multilabel reader 2100, wherein a positive luciferase signal relative to the isotype control antibody indicates the activity of NF- ⁇ B.
- the luciferase reporter construct e.g.,
- provided herein is a method of activating T cells independent of TCR triggering comprising contacting T cells with an antibody or antigen-binding fragment thereof described herein.
- a method of inducing, activating or enhancing an activity of NF- ⁇ B independent of TCR triggering comprising contacting T cells with an antibody or antigen-binding fragment thereof described herein.
- the activity of NF- ⁇ B can be assessed in, e.g., an assay comprising the following steps: (a) incubating T cells (e.g., Jurkat cells) expressing a NF- ⁇ B-luciferase reporter construct (e.g., GloResponse NF- ⁇ B-luc2P construct) and GITR ( e.g., human GITR) with the antibody described herein or an isotype control antibody at an antibody concentration of, e.g., 12.5, 10, 5, 2.5, 1.25, or 0.625 ⁇ g/ml, in the absence of an anti-CD3 antibody; and (b) reading luciferase signal after, e.g., 2, 5, 6, 8 or 18 hours of incubation using, e.g., an EnVision multilabel reader 2100, wherein a positive luciferase signal relative to the isotype control antibody indicates the activity of NF- ⁇ B.
- the luciferase reporter construct e.g.,
- a method of increasing the percentage of polyfunctional (IFN ⁇ + TNF ⁇ +) T cells comprising contacting T cells with an antibody or antigen-binding fragment thereof described herein.
- An increase in the percentage of polyfunctional (IFN ⁇ + TNF ⁇ +) T cells can be assessed in, e.g., an assay comprising the following steps: (a) incubating, e.g., human PBMCs with, e.g., an anti-CD3 antibody at various suboptimal concentrations ( e.g., 0.3-5 ⁇ g/ml); and, e.g., an antibody described herein, which immunospecifically binds to GITR ( e.g., human GITR), at, e.g., 5 ⁇ g/ml or an isotype control antibody, for, e.g., 3-4 days at 37°C and 5% CO 2 ; (b) treating cells with, e.g., Brefeldin A for, e.g., 6
- the polyfunctional (IFN ⁇ + TNF ⁇ +) T cells are selected from the group consisting of polyfunctional (IFN ⁇ + TNF ⁇ +) CD4 + T cells and polyfunctional (IFN ⁇ + TNF ⁇ +) CD8 + T cells.
- a method of increasing surface expression of OX40 and PD-1 in activated T cells comprising contacting T cells with an antibody or antigen-binding fragment thereof described herein.
- the surface expression of OX40 and PD-1 in activated T cells can be increased by at least about 1.2 fold, 1.3 fold, 1.4 fold, 1.5 fold, 2 fold, 2.5 fold, 3 fold, 3.5 fold, 4 fold, 4.5 fold, 5 fold, 6 fold, 7 fold, 8 fold, 9 fold, 10 fold, 15 fold, 20 fold, 30 fold, 40 fold, 50 fold, 60 fold, 70 fold, 80 fold, 90 fold, 100 fold, 200 fold, 300 fold, 400 fold, 500 fold, 600 fold, 700 fold, 800 fold, 900 fold, or 1000 fold as assessed by methods described herein and/or known to one of skill in the art, relative to surface expression of OX40 and PD-1 in activated T cells without the antibody described herein.
- an anti-GITR antibody or antigen-binding fragment thereof described herein or a composition thereof is the only active agent administered to a subject.
- an anti-GITR antibody or antigen-binding fragment thereof described herein on proliferation of cancer cells can be detected by routine assays, such as by assays that measure the uptake of radiolabeled thymidine.
- cell viability can be measured by assays that measure lactate dehydrogenase (LDH), a stable cytosolic enzyme that is released upon cell lysis, or by the release of [ 51 Cr] upon cell lysis.
- LDH lactate dehydrogenase
- the cells are incubated in media containing the dye, the cells are washed, and the remaining dye, reflecting cellular uptake of the dye, is measured spectrophotometrically.
- the dye is sulforhodamine B (SRB), whose binding to proteins can be used as a measure of cytotoxicity ( Skehan P et al., (1990) J Nat Cancer Inst 82: 1107-12 ).
- SRB sulforhodamine B
- a tetrazolium salt such as MTT, is used in a quantitative colorimetric assay for mammalian cell survival and proliferation by detecting living, but not dead, cells (see, e.g., Mosmann T (1983) J Immunol Methods 65: 55-63 ).
- apoptotic cells are measured in both the attached and "floating" compartments of the cultures. Both compartments are collected by removing the supernatant, trypsinizing the attached cells, and combining both preparations following a centrifugation wash step (10 minutes, 2000 rpm).
- the protocol for treating tumor cell cultures with sulindac and related compounds to obtain a significant amount of apoptosis has been described in the literature (see, e.g., Piazza GA et al., (1995) Cancer Res 55: 3110-6 ).
- Features of this method include collecting both floating and attached cells, identification of the optimal treatment times and dose range for observing apoptosis, and identification of optimal cell culture conditions.
- apoptosis is quantitated by measuring DNA fragmentation.
- Commercial photometric methods for the quantitative in vitro determination of DNA fragmentation are available. Examples of such assays, including TUNEL (which detects incorporation of labeled nucleotides in fragmented DNA) and ELISA-based assays, are described in Biochemica, (1999) 2: 34 37 (Roche Molecular Biochemicals ).
- apoptosis can be observed morphologically.
- Cancer cell lines on which such assays can be performed are well known to those of skill in the art. Apoptosis, necrosis and proliferation assays can also be performed on primary cells, e.g., a tissue explant.
- the administration of an anti-GITR antibody or antigen-binding fragment thereof described herein or a composition thereof to a subject with cancer achieves at least one, two, three, four or more of the following effects: (i) the reduction or amelioration of the severity of one or more symptoms of cancer; (ii) the reduction in the duration of one or more symptoms associated with cancer; (iii) the prevention in the recurrence of a symptom associated with cancer; (iv) the reduction in hospitalization of a subject; (v) a reduction in hospitalization length; (vi) the increase in the survival of a subject; (vii) the enhancement or improvement of the therapeutic effect of another therapy; (viii) the inhibition of the development or onset of one or more symptoms associated with cancer; (ix) the reduction in the number of symptoms associated with cancer; (x) improvement in quality of life as assessed by methods well known in the art; (x) inhibition of the recurrence of a tumor; (xi) the regression of
- two or more different anti-GITR antibodies or antigen-binding fragments thereof described herein are administered to a subject.
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered to a subject in combination with one or more other therapies, e.g., anti-cancer agents, cytokines, cellular vaccines or anti-hormonal agents, to treat cancer.
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered in combination with radiation therapy comprising, e.g., the use of x-rays, gamma rays and other sources of radiation to destroy the cancer cells.
- the radiation treatment is administered as external beam radiation or teletherapy wherein the radiation is directed from a remote source.
- the radiation treatment is administered as internal therapy or brachytherapy wherein a radioactive source is placed inside the body close to cancer cells or a tumor mass.
- an anti-GITR antibody or antigen-binding fragment thereof described herein can activate or enhance the immune function or response in a cancer patient with a compromised immune system due to anti-cancer therapy.
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered to a subject in combination with chemotherapy.
- an anti-GITR antibody or antigen-binding fragment thereof described herein can be used before, during or after radiation therapy or chemotherapy.
- chemotherapeutic agents include cyclophosphamide, methotrexate, cyclosporin A, leflunomide, cisplatin, ifosfamide, taxanes such as taxol and paclitaxol, topoisomerase I inhibitors (e.g., CPT 11, topotecan, 9 AC, and GG 211), gemcitabine, vinorelbine, oxaliplatin, 5-fluorouracil (5-FU), leucovorin, vinorelbine, temodal, cytochalasin B, gramicidin D, emetine, mitomycin, etoposide, tenoposide, vincristine, vinblastine, colchicin, doxorubicin, daunorubicin, dihydroxy anthracin dione, mitoxantrone, mithramycin, actinomycin D, 1 dehydrotestosterone, glucocorticoids, procaine, tetracaine
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered to a subject in combination with cyclophosphamide, e.g., a low dose of cyclophosphamide.
- Cyclophosphamide (Elostan, Cytoxan), is a chemotherapeutic agent that has an immunomodulatory function when used at low doses ( e.g., up to 300 mg/m 2 , 300 mg/m 2 or about 300 mg/m 2 when administered intravenously).
- low doses of cyclophosphamide can reduce the number and proliferative capacity of regulatory T cells (Treg) (e.g., CD4+CD25+FoxP3+ cells or, alternatively, CD45+CD3+CD4+CD8-FOXP3+CD25hiCD127low cells) and modulate immune-suppressive networks.
- Treg regulatory T cells
- the dosage of a cyclophosphamide administration is about 50 mg/m 2 , 100 mg/m 2 , 200 mg/m 2 , 300 mg/m 2 , 500 mg/m 2 , or more.
- the dosage of a cyclophosphamide administration is in a range of 10 mg/m 2 to 100 mg/m 2 , 50 mg/m 2 to 200 mg/m 2 , 50 mg/m 2 to 300 mg/m 2 , 50 mg/m 2 to 500 mg/m 2 .
- cyclophosphamide is administered to the subject within 1 hour, 2 hours, 3 hours, 4 hours, 8 hours, 12 hours, 1 day, 5 days or more prior to or following an initial administration of an anti-GITR antibody or antigen-binding fragment thereof described herein.
- anti-GITR antibody or antigen-binding fragment thereof described herein is used in combination with cyclophosphamide, e.g., low dose cyclophosphamide, for the treatment of metastatic renal cell carcinoma (RCC).
- cyclophosphamide e.g., low dose cyclophosphamide
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered to a subject in combination with a Treg-inhibitory agent.
- Treg-inhibitory agents include Zenapax® (daclizumab) (Roche), which is a human anti-CD25 monoclonal antibody used, e.g., for inducing immune suppression in organ transplantation. Daclizumab blocks IL-2 binding to CD25, which is also a signal for the maintenance of Tregs.
- Another agent that inhibits Tregs is Sutent® (Sunitinib) (Pfizer) which is a small molecule, multi-targeted tyrosine kinase inhibitor approved for the treatment of renal cell carcinoma (RCC) and other cancers.
- IDO indoleamine 2,3-dioxygenase
- IDO is an immunosuppressive agent expressed in certain normal and neoplastic cells and may be associated with an increase in Tregs in cancer patients.
- Additional Treg inhibitors include agents that block trafficking of Tregs to the tumor microenvironment.
- agents may include antibodies against certain chemokines and chemokine receptors such as CCL17, CCL22 and CCR4.
- Treg-inhibitory agents that may be used in accordance with methods described herein are disclosed in the following patent applications, which are incorporated herein by reference in their entirety for all purposes: U.S. Patent Publication Nos. US 2009/0214533 , US 2012/0142750 , US 2011/0305713 , US 2009/0004213 , US 2012/0219559 , US 2010/0278844 , US 2013/0323283 and US 2008/0152665 .
- an anti-GITR antibody or antigen-binding fragment thereof described herein can be administered to a subject before, during or after surgery.
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered to a subject in combination with an immune modulator or antibody.
- Immune modulators or antibodies may be, but are not limited to, adjuvants, antigens, anti-CD3 (e.g., OKT3), checkpoint targeting agents or modulators, or interleukins.
- checkpoint targeting agent or “checkpoint modulator” can be used interchangeably and refer to an agent that selectively modulates expression or activity of a regulatory molecule (e.g., a co-inhibitory checkpoint molecule (e.g., protein) or a costimulatory checkpoint molecule (e.g., protein), which may be, e.g., a receptor or a ligand) of an immune system checkpoint.
- a regulatory molecule e.g., a co-inhibitory checkpoint molecule (e.g., protein) or a costimulatory checkpoint molecule (e.g., protein)
- a costimulatory checkpoint molecule e.g., protein
- Checkpoint targeting agents can be selected from the group consisting of an agonist of a checkpoint molecule, an antagonist of a checkpoint molecule, a polypeptide (e.g., a peptide ligand, an antibody, an antibody fragment) that selectively targets a checkpoint molecule; a small molecule that selectively targets a checkpoint molecule; and a regulatory nucleic acid (e.g., an siRNA, miRNA) that selectively modulates expression or activity of a checkpoint molecule.
- a polypeptide e.g., a peptide ligand, an antibody, an antibody fragment
- a small molecule that selectively targets a checkpoint molecule
- a regulatory nucleic acid e.g., an siRNA, miRNA
- a checkpoint targeting agent can be selected from the group consisting of an antagonist of PD-1, an antagonist of PD-L1, an antagonist of PD-L2, an antagonist of CTLA-4, an antagonist of TIM-3, an antagonist of LAG-3, an agonist of GITR, and an agonist of OX40.
- an anti-GITR agonistic antibody e.g., Hum231#l, Hum231#2 or Hum231#2w
- antigen-binding fragment thereof may be administered in combination with, e.g., an anti-CTLA-4 antagonist antibody or antigen-binding fragment thereof, or another checkpoint targeting agent, either in a single pharmaceutical composition, or in separate pharmaceutical compositions administered together or separately.
- provided herein are methods for treating cancer in a subject comprising administering to the subject an anti-GITR antibody or antigen-binding fragment thereof and an agonist of OX40 and/or an antagonist(s) of LAG-3, TIM-3, PD-1 and/or CTLA-4.
- the cancer is selected from glioblastoma multiforme, metastatic melanoma, resistant metastatic melanoma, metastatic ovarian cancer, metastatic renal cell carcinoma, head and neck cancer, gastric cancer, esophageal cancer, non-small cell lung cancer, pediatric brain tumors, low-grade asctrocytoma, ependymoma, and meduloblastoma.
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered to a subject in combination with an agent that inhibits (partially or completely) CTLA-4 signal transduction, such as an antibody that specifically binds to human CTLA-4 (e.g., tremelimumab (Pfizer); ipilimumab (Yervoy®, Bristol-Myers Squibb)) or a CTLA-4-Ig fusion protein.
- an anti-GITR antibody or antigen-binding fragment thereof is used in combination with an antagonist of CTLA-4 (e.g., tremelimumab or ipilimumab) for the treatment of metastatic ovarian cancer.
- an anti-GITR antibody or antigen-binding fragment thereof is used in combination with an antagonist of CTLA-4 (e.g., tremelimumab or ipilimumab) for the treatment of metastatic ovarian cancer that is resistant to an antagonist of CTLA-4 (e.g., tremelimumab or ipilimumab).
- an anti-GITR antibody or antigen-binding fragment thereof and an antagonist of CTLA-4 are used for the treatment of glioblastoma multiforme.
- the glioblastoma multiforme is recurrent.
- the glioblastoma multiforme is newly diagnosed. In some embodiments, the glioblastoma multiforme is in a subject having non -methylated MGMT promoters. In some embodiments, the glioblastoma multiforme is refractory to Bevacizumab therapy. In some embodiments, the glioblastoma multiforme is in a subject that has not received Bevacizumab therapy. Examples of anti-CTLA-4 antibodies that may be used in treatment methods disclosed herein are disclosed in the following patents and patent applications, which are incorporated herein by reference in their entirety for all purposes: International Publication Nos.
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered in combination with an agent that inhibits (partially or completely) LAG-3 signal transduction, such as an antibody that specifically binds to human LAG-3.
- an agent that inhibits (partially or completely) LAG-3 signal transduction such as an antibody that specifically binds to human LAG-3.
- anti-LAG-3 antibodies or antibody fragments thereof that may be used in treatment methods described herein are disclosed in the following patents and patent applications, which are incorporated herein by reference in their entirety for all purposes: U.S. Patent Nos. 6,143,273 and 6,197,524 ; U.S. Patent Publication Nos. US 2011/0150892 , US 2010/0233183 and US 2010/196394 .
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered in combination with an agent that inhibits (partially or completely) TIM-3 signal transduction, such as an antibody that specifically binds to human TIM-3.
- an agent that inhibits (partially or completely) TIM-3 signal transduction such as an antibody that specifically binds to human TIM-3.
- anti-TIM-3 antibodies or antibody fragments thereof that may be used in treatment methods described herein are disclosed in the following patents and patent applications, which are incorporated herein by reference in their entireties for all purposes: U.S. Patent Nos. 7,470,428 and 8,101,176 ; U.S. Publication Nos. US 2013/0022623 , US 2010/0100131 , US 2010/0100131 and US 2010/061992 .
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered in combination with an agent that inhibits (partially or completely) PD-1 signal transduction, such as an antibody that specifically binds to human PD-1.
- an anti-PD-1 antibody or antibody fragment thereof is administered to a subject as described herein.
- the anti-PD-1 antibody is Nivolumab (BMS-936558 or MDX1106) or Lambrolizumab (MK-3475) or Pidilizumab (CT-011).
- CT-011 Pidilizumab
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered in combination with an agent that inhibits (partially or completely) PD-L1 activity, such as an antibody that specifically binds to human PD-L1.
- the anti-PD-Ll antibody is BMS-936559, MPDL3280A, MEDI4736 or MSB0010718C.
- Further non-limiting examples of anti-PD-L1 antibodies that may be used in treatment methods disclosed herein are disclosed in the following patents and patent applications, which are incorporated herein by reference in their entireties for all purposes: U.S. Patent No. 8,168,179 and U.S. Publication Nos. US 2010/0203056 and US 2003/0232323 .
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered in combination with an agent that inhibits (partially or completely) PD-L2 activity, such as an antibody that specifically binds to human PD-L2.
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered in combination with an agent that activates or enhances OX-40 signal transduction, such as an antibody that specifically binds to human OX-40.
- an agent that activates or enhances OX-40 signal transduction such as an antibody that specifically binds to human OX-40.
- anti-OX40 antibodies or antibody fragments thereof that may be used in treatment methods described herein are disclosed in the following patents and patent applications, which are incorporated herein by reference in their entireties for all purposes: U.S. Patent No. 7,550,140 ; 7,807,156 ; 8,283,450 ; 8,614,295 and 7,531,170 ; U.S. Publication No. US 2010/0196359 , US 2010/0136030 and US 2013/0183315 .
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered to a subject in combination with a vaccine, such as described herein, including Section 5.4.1 above.
- a vaccine such as described herein, including Section 5.4.1 above.
- an anti-GITR antibody or antigen-binding fragment thereof is administered to a subject in combination with a heat shock protein based tumor-vaccine.
- Heat shock proteins are a family of highly conserved proteins found ubiquitously across all species. Their expression can be powerfully induced to much higher levels as a result of heat shock or other forms of stress, including exposure to toxins, oxidative stress or glucose deprivation. Five families have been classified according to molecular weight: HSP-110, -90, -70, -60 and -28.
- HSPs deliver immunogenic peptides through the cross-presentation pathway in antigen presenting cells (APCs) such as macrophages and dendritic cells (DCs), leading to T cell activation.
- APCs antigen presenting cells
- DCs dendritic cells
- HSPs function as chaperone carriers of tumor-associated antigenic peptides forming complexes able to induce tumor-specific immunity.
- the HSP-antigen complexes Upon release from dying tumor cells, the HSP-antigen complexes are taken up by antigen-presenting cells (APCs) wherein the antigens are processed into peptides that bind MHC class I and class II molecules leading to the activation of anti-tumor CD8 + and CD4 + T cells.
- the immunity elicited by HSP complexes derived from tumor preparations is specifically directed against the unique antigenic peptide repertoire expressed by the cancer of each subject.
- a heat shock protein peptide complex is a protein peptide complex consisting of a heat shock protein non-covalently complexed with antigenic peptides. HSPPCs elicit both innate and adaptive immune responses. In a specific embodiment, the antigenic peptide(s) displays antigenicity for the cancer being treated. HSPPCs are efficiently seized by APCs via membrane receptors (mainly CD91) or by binding to Toll-like receptors. HSPPC internalization results in functional maturation of the APCs with chemokine and cytokine production leading to activation of natural killer cells (NK), monocytes and Th1 and Th-2-mediated immune responses.
- NK natural killer cells
- HSPPCs used in methods disclosed herein comprise one or more heat shock proteins from the hsp60, hsp70, or hsp90 family of stress proteins complexed with antigenic peptides.
- HSPPCs comprise hsc70, hsp70, hsp90, hsp110, grp170, gp96, calreticulin, or combinations of two or more thereof.
- an anti-GITR antibody or antigen-binding fragment thereof is administered to a subject in combination with a heat shock protein peptide complex (HSPPC), e.g., heat shock protein peptide complex-96 (HSPPC-96), to treat cancer, e.g., glioblastoma multiforme.
- HSPPC-96 comprises a 96 kDa heat shock protein (Hsp), gp96, complexed to antigenic peptides.
- HSPPC-96 is a cancer immunotherapy manufactured from a subject's tumor and contains the cancer's antigenic "fingerprint.”
- this fingerprint contains unique antigens that are present only in that particular subject's specific cancer cells and injection of the vaccine is intended to stimulate the subject's immune system to recognize and attack any cells with the specific cancer fingerprint.
- the HSPPC e.g., HSPPC-96
- the HSPPC is produced from the tumor tissue of a subject.
- the HSPPC e.g., HSPPC-96
- the HSPPC is produced from tumor of the type of cancer or metastatis thereof being treated.
- the HSPPC e.g., HSPPC-96
- the tumor tissue is non-necrotic tumor tissue.
- at least 1 gram e.g., at least 1, at least 2, at least 3, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, or at least 10 grams
- at least 1 gram e.g., at least 1, at least 2, at least 3, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, or at least 10 grams
- non-necrotic tumor tissue is frozen prior to use in vaccine preparation.
- the HSPPC e.g., HSPPC-96
- the HSPPC is isolated from the tumor tissue by purification techniques, filtered and prepared for an injectable vaccine.
- a subject is administered 6-12 doses of the HSPPC, e.g., HSPCC-96.
- the HSPPC, e.g., HSPPC-96, doses may be administered weekly for the first 4 doses and then biweekly for the 2-8 additional doses.
- an anti-GITR antibody or antigen-binding fragment thereof is used in combination with HSPPC-96 for the treatment of metastatic melanoma (e.g., resistant metastatic melanoma), metastatic ovarian cancer, or metastatic renal cell carcinoma.
- an anti-GITR antibody or antigen-binding fragment thereof is used in combination with HSPPC-96 for the treatment of glioblastoma multiforme.
- an anti-GITR antibody or antigen-binding fragment thereof and an antagonist of CTLA-4 are used in combination with HSPPC-96 for the treatment of glioblastoma multiforme.
- the subject to be treated is immunosuppressed (e.g., due to infection, e.g., HIV, or by reason of having undergone anti-cancer therapy (e.g., chemotherapy radiation) prior to administration of the HSPPC.
- immunosuppressed e.g., due to infection, e.g., HIV, or by reason of having undergone anti-cancer therapy (e.g., chemotherapy radiation) prior to administration of the HSPPC.
- HSPPCs that may be used in accordance with the methods described herein are disclosed in the following patents and patent applications, which are incorporated herein by reference in their entireties for all purposes, U.S. Patent Nos. 6,391,306 , 6,383,492 , 6,403,095 , 6,410,026 , 6,436,404 , 6,447,780 , 6,447,781 and 6,610,659 .
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered to a subject in combination with a compound that targets an immunomodulatory enzyme(s) such as IDO (indoleamine-(2,3)-dioxygenase) and TDO (tryptophan 2,3-dioxygenase).
- an immunomodulatory enzyme(s) such as IDO (indoleamine-(2,3)-dioxygenase) and TDO (tryptophan 2,3-dioxygenase).
- such compound is selected from the group consisting of epacadostat (Incyte Corporation), F001287 (Flexus Biosciences), indoximod (NewLink Genetics), and NLG919 (NewLink Genetics).
- the compound is epacadostat.
- the compound is F001287.
- the compound is indoximod.
- the compound is NLG919.
- Elevated numbers of CD4 + CD25 + Tregs in cancer patients prevent the host from mounting an effective anti-tumor immune response. Additionally, high Treg frequencies are associated with reduced patient survival ( Curiel TJ et al., (2004) Nat Medicine 10(9): 942-9 ; Woo EY et al., (2002) J Immunol 168: 4272-6 ). However, an enhancement of immune activity in cancer patients' PBMC was observed after depletion of regulatory T cells ( Dannull J et al., (2005) J Clin Invest 115(12): 3623-33 ).
- an immune-based strategy may incorporate ex-vivo or in-vivo Treg depletion with an anti-GITR antibody or antigen-binding fragment thereof causing depletion of GITR-positive Treg cells.
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered to a subject in combination with an oncolytic virus such as Talimogene laherparepvec (OncoVEX GM-CSF) and CGTG-102 (Ad5/3-D24-GMCSF).
- an oncolytic virus such as Talimogene laherparepvec (OncoVEX GM-CSF) and CGTG-102 (Ad5/3-D24-GMCSF).
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered to a subject in combination with a cytokine(s) that is effective in inhibiting tumor growth/metastasis.
- cytokines, lymphokines, or other hematopoietic factors include, but are not limited to, M-CSF, GM-CSF, TNF, IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-11, IL-12, IL-13, IL-14, IL-15, IL-16, IL-17, IL-18, IFN, TNF ⁇ , TNF1, TNF2, G-CSF, Meg-CSF, GM-CSF, thrombopoietin, stem cell factor, and erythropoietin.
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered to a subject in combination with a receptor tyrosine kinase inhibitor(s) such as imatinib mesylate (marketed as Gleevec® or Glivac®), erlotinib (an EGF receptor inhibitor) now marketed as Tarveca, or sunitinib (marketed as Sutent®).
- a receptor tyrosine kinase inhibitor(s) such as imatinib mesylate (marketed as Gleevec® or Glivac®), erlotinib (an EGF receptor inhibitor) now marketed as Tarveca, or sunitinib (marketed as Sutent®).
- an anti-GITR antibody or antigen-binding fragment thereof described herein is administered to a subject in combination with an antagonistic TGF-beta antibody, such as Fresolimumab® (GC1008), an antibody targeting and inhibiting TGF-beta 1, 2 or 3 isoforms.
- an antagonistic TGF-beta antibody such as Fresolimumab® (GC1008), an antibody targeting and inhibiting TGF-beta 1, 2 or 3 isoforms.
- an anti-GITR antibody or antigen-binding fragment thereof described herein or a composition thereof is administered to a subject suffering from or diagnosed with cancer.
- an anti-GITR antibody or composition thereof is administered to a subject suffering from or diagnosed with a glioblastoma multiforme.
- the glioblastoma multiforme is recurrent.
- the glioblastoma multiforme is newly diagnosed.
- the glioblastoma multiforme is in a subject having non -methylated MGMT promoters.
- the glioblastoma multiforme is recurrent.
- the glioblastoma multiforme is newly diagnosed. In some embodiments, the glioblastoma multiforme is in a subject having non -methylated MGMT promoters. In some embodiments, the glioblastoma multiforme is refractory to Bevacizumab therapy. In some embodiments, the glioblastoma multiforme is in a subject that has not received Bevacizumab therapy.
- the patients being treated in accordance with the methods described herein are patients already being treated with antibiotics, anti-cancer agents, or other biological therapy/immunotherapy.
- these patients are refractory patients, patients who are too young for conventional therapies, and patients with reoccurring viral infections despite management or treatment with existing therapies.
- the subject being administered an anti-GITR antibody or antigen-binding fragment thereof described herein or a composition thereof has not received a therapy prior to the administration of the antibody or composition thereof.
- an anti-GITR antibody or antigen-binding fragment thereof described herein or a composition thereof is administered to a subject who has received a therapy prior to administration of the antibody or composition thereof.
- an anti-GITR antibody or antigen-binding fragment thereof, or composition thereof is administered to a subject receiving or recovering from immunosuppressive therapy.
- an anti-GITR antibody or composition thereof is administered to a subject with cancer and the cancer cells express GITRL.
- the subject to be treated in accordance with the methods disclosed herein is immunosuppressed (e.g., due to infection, e.g., HIV, or by reason of having undergone anti-cancer therapy (e.g., chemotherapy radiation).
- anti-GITR antibodies affect the surface expression of proteins including OX40, CD25, and PD-1. Accordingly, in certain embodiments, an anti-GITR antibody or antigen-binding fragment thereof or composition thereof is administered prior to the administration of an antibody or antigen-binding fragment thereof that binds to OX40, CD25, or PD-1.
- An antagonist PD-1 antibody or antigen-binding fragment thereof can be administered at a time at which the agonist GITR antibody or antigen-binding fragment thereof has increased surface expression of PD-1 in a subject relative to expression of PD-1 in the subject at the time of the administering.
- an antagonist PD-1 antibody or antigen-binding fragment thereof can be administered at least 12 hours, at least 1 day, at least 2 days, at least 3 days, at least 4 days, at least 5 days, at least 6 days, or at least 7 days after administration of the agonist GITR antibody.
- An antagonist PD-1 antibody or antigen-binding fragment thereof can be administered from 12 hours to two weeks, from 1 day to two weeks, from 2 days to two weeks, or from 3 days to two weeks after administration of the agonist GITR antibody or antigen-binding fragment thereof.
- An antagonist PD-1 antibody or antigen-binding fragment thereof can be administered from 12 hours to one week, from 1 day to one week, from 2 days to one week, or from 3 days to one week after administration of the agonist GITR antibody or antigen-binding fragment thereof.
- An agonist antibody or antigen-binding fragment thereof can be administered at a time at which the agonist GITR antibody or antigen-binding fragment thereof has increased surface expression of in a subject relative to expression of in the subject at the time of the administering.
- an agonist antibody or antigen-binding fragment thereof can be administered at least 12 hours, at least 1 day, at least 2 days, at least 3 days, at least 4 days, at least 5 days, at least 6 days, or at least 7 days after administration of the agonist GITR antibody.
- An agonist antibody or antigen-binding fragment thereof can be administered from 12 hours to two weeks, from 1 day to two weeks, from 2 days to two weeks, or from 3 days to two weeks after administration of the agonist GITR antibody or antigen-binding fragment thereof.
- An agonist antibody or antigen-binding fragment thereof can be administered from 12 hours to one week, from 1 day to one week, from 2 days to one week, or from 3 days to one week after administration of the agonist GITR antibody or antigen-binding fragment
- An anti-CD25 antibody or antigen-binding fragment thereof can be administered at a time at which the agonist GITR antibody or antigen-binding fragment thereof has increased surface expression of CD25 in a subject relative to expression of CD25 in the subject at the time of the administering.
- an anti-CD25 antibody or antigen-binding fragment thereof can be administered at least 12 hours, at least 1 day, at least 2 days, at least 3 days, at least 4 days, at least 5 days, at least 6 days, or at least 7 days after administration of the agonist GITR antibody.
- An anti-CD25 antibody or antigen-binding fragment thereof can be administered from 12 hours to two weeks, from 1 day to two weeks, from 2 days to two weeks, or from 3 days to two weeks after administration of the agonist GITR antibody or antigen-binding fragment thereof.
- An anti-CD25 antibody or antigen-binding fragment thereof can be administered from 12 hours to one week, from 1 day to one week, from 2 days to one week, or from 3 days to one week after administration of the agonist GITR antibody or antigen-binding fragment thereof.
- An antagonist CTLA-4 antibody or antigen-binding fragment thereof can be administered after administration of an agonist GITR antibody or antigen-binding fragment.
- an antagonist CTLA-4 antibody or antigen-binding fragment thereof can be administered at least 12 hours, at least 1 day, at least 2 days, at least 3 days, at least 4 days, at least 5 days, at least 6 days, or at least 7 days after administration of the agonist GITR antibody.
- An antagonist CTLA-4 antibody or antigen-binding fragment thereof can be administered from 12 hours to two weeks, from 1 day to two weeks, from 2 days to two weeks, or from 3 days to two weeks after administration of the agonist GITR antibody or antigen-binding fragment thereof.
- An antagonist CTLA-4 antibody or antigen-binding fragment thereof can be administered from 12 hours to one week, from 1 day to one week, from 2 days to one week, or from 3 days to one week after administration of the agonist GITR antibody or antigen-binding fragment thereof.
- cancer examples include, but are not limited to, B cell lymphomas (e.g., B cell chronic lymphocytic leukemia, B cell non-Hodgkin lymphoma, cutaneous B cell lymphoma, diffuse large B cell lymphoma), basal cell carcinoma, bladder cancer, blastoma, brain metastasis, breast cancer, Burkitt lymphoma, carcinoma (e.g., adenocarcinoma ( e.g., of the gastroesophageal junction)), cervical cancer, colon cancer, colorectal cancer (colon cancer and rectal cancer), endometrial carcinoma, esophageal cancer, Ewing sarcoma, follicular lymphoma, gastric cancer, gastroesophageal junction carcinoma, gastrointestinal cancer, glioblastoma (e.g., glioblastoma multiforme, e.g., newly diagnosed or recurrent), glioma,
- B cell lymphomas e.g., B cell chronic
- the cancer treated in accordance with the methods described herein is a human sarcoma or carcinoma, e.g., fibrosarcoma, myxosarcoma, liposarcoma, chondrosarcoma, osteogenic sarcoma, chordoma, angiosarcoma, endothelio sarcoma, lymphangiosarcoma, lymphangioendothelio sarcoma, synovioma, mesothelioma, Ewing's tumor, leiomyosarcoma, rhabdomyosarcoma, colon carcinoma, pancreatic cancer, breast cancer, ovarian cancer, prostate cancer, squamous cell carcinoma, basal cell carcinoma, adenocarcinoma, sweat gland carcinoma, sebaceous gland carcinoma, papillary carcinoma, papillary adenocarcinomas, cystadenocarcinoma, medullary carcinoma, bronchogenic carcinoma, renal cell carcinoma (e.g., fibros
- the cancer treated in accordance with the methods described herein is an acute lymphocytic leukemia or acute myelocytic leukemia (e.g., myeloblastic, promyelocytic, myelomonocytic, monocytic and erythroleukemia); chronic leukemia (chronic myelocytic (granulocytic) leukemia or chronic lymphocytic leukemia); Hodgkin's disease; non-Hodgkin's disease; acute myeloid leukemia; B-cell lymphoma; T-cell lymphoma; anaplastic large cell lymphoma; intraocular lymphoma; follicular lymphoma; small intestine lymphoma; orsplenic marginal zone lymphoma.
- acute lymphocytic leukemia or acute myelocytic leukemia e.g., myeloblastic, promyelocytic, myelomonocytic, monocytic and erythro
- the cancer treated in accordance with the methods described herein is multiple myeloma, Waldenstrom's macroglobulinemia, heavy chain disease, gastrointestinal stromal tumors, head and/or neck cancer (e.g., squamous cell carcinoma of the hypopharynx, squamous cell carcinoma of the larynx, cell carcinoma of the oropharynx, or verrucous carcinoma of the larynx), endometrial stromal sarcoma, mast cell sarcoma, adult soft tissue sarcoma, uterine sarcoma, merkel cell carcinoma, urothelial carcinoma, melanoma with brain metastases, uveal melanoma, uveal melanoma with liver metastases, non-small cell lung cancer, rectal cancer, or myelodysplastic syndrome,
- the cancer treated in accordance with the methods is metastatic.
- the cancer treated in accordance with the methods described herein includes prostate cancer, breast cancer, lung cancer, colorectal cancer, melanoma, bronchial cancer, bladder cancer, brain or central nervous system cancer, peripheral nervous system cancer, uterine or endometrial cancer, cancer of the oral cavity or pharynx, non-Hodgkin's lymphoma, thyroid cancer, kidney cancer, biliary tract cancer, small bowel or appendix cancer, salivary gland cancer, thyroid gland cancer, adrenal gland cancer, squamous cell cancer, mesothelioma, osteocarcinoma, thyoma/thymic carcinoma, glioblastoma, myelodysplastic syndrome, soft tissue sarcoma, DIPG, adenocarcinoma, osteosarcoma, chondrosarcoma, leukemia, or pancreatic cancer.
- the cancer treated in accordance with the methods described herein includes a carcinoma (e.g., an adenocarcinoma), lymphoma, blastoma, melanoma, sarcoma or leukemia.
- a carcinoma e.g., an adenocarcinoma
- lymphoma e.g., blastoma, melanoma, sarcoma or leukemia.
- the cancer treated in accordance with the methods described herein includes squamous cell cancer, small-cell lung cancer, non-small cell lung cancer, gastrointestinal cancer, Hodgkin's lymphoma, non-Hodgkin's lymphoma, pancreatic cancer, glioblastoma, glioma, cervical cancer, ovarian cancer, liver cancer ( e.g., hepatic carcinoma and hepatoma), bladder cancer, breast cancer, inflammatory breast cancer, Merkel cell carcinoma, colon cancer, colorectal cancer, stomach cancer, urinary bladder cancer, endometrial carcinoma, myeloma ( e.g., multiple myeloma), salivary gland, carcinoma, kidney cancer ( e.g., renal cell carcinoma and Wilms' tumors), basal cell carcinoma, melanoma, prostate cancer, vulval cancer, thyroid cancer, testicular cancer, esophageal cancer, serous adenocarcinoma or various types of head and neck cancer.
- liver cancer
- the cancer treated in accordance with the methods described herein includes desmoplastic melanoma, inflammatory breast cancer, thymoma, rectal cancer, anal cancer, or surgically treatable or non-surgically treatable brain stem glioma.
- the cancer is a solid tumor.
- the cancer is glioblastoma multiforme.
- the glioblastoma multiforme is recurrent.
- the glioblastoma multiforme is newly diagnosed.
- the glioblastoma multiforme is in a subject having non -methylated MGMT promoters.
- the glioblastoma multiforme is refractory to Bevacizumab therapy.
- the glioblastoma multiforme is in a subject that has not received Bevacizumab therapy.
- the cancer treated in accordance with the methods described herein is metastatic melanoma (e.g., resistant metastatic melanoma), metastatic ovarian cancer, or metastatic renal cell carcinoma.
- the cancer treated in accordance with the methods described herein is melanoma that is resistant to ipilimumab.
- the cancer treated in accordance with the methods described herein is melanoma that is resistant to nivolumab.
- the cancer treated in accordance with the methods described herein is melanoma that is resistant to ipilimumab and nivolumab.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Immunology (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Virology (AREA)
- Molecular Biology (AREA)
- Oncology (AREA)
- Biomedical Technology (AREA)
- Biochemistry (AREA)
- Cell Biology (AREA)
- Communicable Diseases (AREA)
- Genetics & Genomics (AREA)
- Biotechnology (AREA)
- Urology & Nephrology (AREA)
- Hematology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Microbiology (AREA)
- Epidemiology (AREA)
- Zoology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Mycology (AREA)
- Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Toxicology (AREA)
- Developmental Biology & Embryology (AREA)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201462004071P | 2014-05-28 | 2014-05-28 | |
| US201562161250P | 2015-05-13 | 2015-05-13 | |
| EP15729987.6A EP3148579B1 (fr) | 2014-05-28 | 2015-05-28 | Anticorps anti-gitr et leurs procédés d'utilisation |
| PCT/US2015/032895 WO2015184099A1 (fr) | 2014-05-28 | 2015-05-28 | Anticorps anti-gitr et leurs procédés d'utilisation |
Related Parent Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP15729987.6A Division EP3148579B1 (fr) | 2014-05-28 | 2015-05-28 | Anticorps anti-gitr et leurs procédés d'utilisation |
| EP15729987.6A Division-Into EP3148579B1 (fr) | 2014-05-28 | 2015-05-28 | Anticorps anti-gitr et leurs procédés d'utilisation |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| EP3498295A1 true EP3498295A1 (fr) | 2019-06-19 |
Family
ID=53433276
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP18204948.6A Pending EP3498295A1 (fr) | 2014-05-28 | 2015-05-28 | Anticorps anti-gitr et leurs procédés d'utilisation |
| EP15729987.6A Active EP3148579B1 (fr) | 2014-05-28 | 2015-05-28 | Anticorps anti-gitr et leurs procédés d'utilisation |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP15729987.6A Active EP3148579B1 (fr) | 2014-05-28 | 2015-05-28 | Anticorps anti-gitr et leurs procédés d'utilisation |
Country Status (26)
| Country | Link |
|---|---|
| US (9) | US10155818B2 (fr) |
| EP (2) | EP3498295A1 (fr) |
| JP (3) | JP6847666B2 (fr) |
| KR (2) | KR102433464B1 (fr) |
| CN (2) | CN108064242B (fr) |
| AU (3) | AU2015266958A1 (fr) |
| CA (1) | CA2949998A1 (fr) |
| CY (1) | CY1124787T1 (fr) |
| DK (1) | DK3148579T3 (fr) |
| EA (1) | EA201692458A1 (fr) |
| ES (1) | ES2860751T3 (fr) |
| HR (1) | HRP20210364T1 (fr) |
| HU (1) | HUE053857T2 (fr) |
| IL (3) | IL293212B2 (fr) |
| LT (1) | LT3148579T (fr) |
| MA (2) | MA40041B1 (fr) |
| MX (2) | MX2016015614A (fr) |
| NZ (1) | NZ726513A (fr) |
| PH (1) | PH12016502345A1 (fr) |
| PL (1) | PL3148579T3 (fr) |
| PT (1) | PT3148579T (fr) |
| RS (1) | RS61678B1 (fr) |
| SG (2) | SG10201912986PA (fr) |
| SI (1) | SI3148579T1 (fr) |
| TW (2) | TW202132337A (fr) |
| WO (1) | WO2015184099A1 (fr) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10829559B2 (en) | 2014-05-28 | 2020-11-10 | Agenus Inc. | Anti-GITR antibodies and methods of use thereof |
| US10836830B2 (en) | 2015-12-02 | 2020-11-17 | Agenus Inc. | Antibodies and methods of use thereof |
| US11359028B2 (en) | 2016-11-09 | 2022-06-14 | Agenus Inc. | Anti-OX40 antibodies and anti-GITR antibodies |
Families Citing this family (134)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20140212398A1 (en) | 2011-09-08 | 2014-07-31 | Yeda Research And Development Co. Ltd. | Anti third party central memory t cells, methods of producing same and use of same in transplantation and disease treatment |
| KR101566539B1 (ko) | 2012-06-08 | 2015-11-05 | 국립암센터 | 신규한 Th2 세포 전환용 에피토프 및 이의 용도 |
| CN106459203B (zh) | 2014-06-06 | 2021-08-03 | 百时美施贵宝公司 | 抗糖皮质激素诱导肿瘤坏死因子受体(gitr)的抗体及其用途 |
| EP3215141A4 (fr) | 2014-11-05 | 2018-06-06 | Flexus Biosciences, Inc. | Agents immunorégulateurs |
| UY36390A (es) * | 2014-11-05 | 2016-06-01 | Flexus Biosciences Inc | Compuestos moduladores de la enzima indolamina 2,3-dioxigenasa (ido), sus métodos de síntesis y composiciones farmacéuticas que los contienen |
| DK3218005T5 (da) | 2014-11-12 | 2024-10-14 | Seagen Inc | Glycan-interagerende forbindelser og anvendelsesfremgangsmåder |
| SG11201708223QA (en) | 2015-04-17 | 2017-11-29 | Bristol Myers Squibb Co | Compositions comprising a combination of an anti-pd-1 antibody and another antibody |
| SG10202002131PA (en) | 2015-05-21 | 2020-05-28 | Harpoon Therapeutics Inc | Trispecific binding proteins and methods of use |
| CN107743586B (zh) | 2015-06-03 | 2021-07-02 | 百时美施贵宝公司 | 用于癌症诊断的抗gitr抗体 |
| ES2963038T3 (es) | 2015-07-16 | 2024-03-25 | Yeda Res & Dev | Uso de células T de memoria central antitercero |
| MX2018000948A (es) | 2015-07-23 | 2018-09-27 | Inhibrx Inc | Proteinas de fusion que se unen a gitir multivalentes y multiespecificas. |
| CN108473579B (zh) * | 2015-10-22 | 2022-03-01 | 埃博灵克斯股份有限公司 | Gitr激动剂 |
| IL302822A (en) | 2015-11-12 | 2023-07-01 | Seagen Inc | Compounds interacting with glycans and methods of use |
| JP6983776B2 (ja) | 2015-11-19 | 2021-12-17 | ブリストル−マイヤーズ スクイブ カンパニーBristol−Myers Squibb Company | グルココルチコイド誘発腫瘍壊死因子受容体(gitr)に対する抗体およびその使用 |
| WO2017096276A1 (fr) * | 2015-12-02 | 2017-06-08 | Agenus Inc. | Anticorps anti-gitr et procédés d'utilisation associés |
| US20200123265A1 (en) * | 2015-12-02 | 2020-04-23 | Agenus Inc. | Anti-gitr antibodies and methods of use thereof |
| UA126115C2 (uk) | 2016-03-08 | 2022-08-17 | Янссен Байотек, Інк. | Антитіло до gitr |
| TWI822521B (zh) | 2016-05-13 | 2023-11-11 | 美商再生元醫藥公司 | 藉由投予pd-1抑制劑治療皮膚癌之方法 |
| ES2905823T3 (es) | 2016-05-20 | 2022-04-12 | Biohaven Therapeutics Ltd | Uso de riluzol, profármacos de riluzol o análogos de riluzol con inmunoterapias para tratar cánceres |
| US11623958B2 (en) | 2016-05-20 | 2023-04-11 | Harpoon Therapeutics, Inc. | Single chain variable fragment CD3 binding proteins |
| NZ749355A (en) | 2016-05-27 | 2023-04-28 | Agenus Inc | Anti-tim-3 antibodies and methods of use thereof |
| US11090344B2 (en) | 2016-05-27 | 2021-08-17 | Dnatrix, Inc. | Adenovirus and immunomodulator combination therapy |
| WO2017211321A1 (fr) * | 2016-06-08 | 2017-12-14 | 上海交通大学医学院 | Séquence de région constante à chaîne lourde d'anticorps pour améliorer l'activité d'anticorps agonistes |
| SG11201810525XA (en) | 2016-06-10 | 2018-12-28 | Regeneron Pharma | Anti-gitr antibodies and uses thereof |
| WO2017220990A1 (fr) | 2016-06-20 | 2017-12-28 | Kymab Limited | Anticorps anti-pd-l1 |
| JP2019519536A (ja) | 2016-07-01 | 2019-07-11 | ファイブ プライム セラピューティクス, インコーポレイテッド | Gitrアゴニストおよびcpgを用いた組み合わせ抗腫瘍療法 |
| CA3030765A1 (fr) | 2016-07-14 | 2018-01-18 | Bristol-Myers Squibb Company | Anticorps anti-tim3 et leurs utilisations |
| CA3030659A1 (fr) * | 2016-07-20 | 2018-01-25 | Igm Biosciences, Inc. | Molecules de fixation de gitr multimeriques |
| CN109689689B (zh) * | 2016-07-22 | 2022-12-06 | 丹娜法伯癌症研究所公司 | 糖皮质激素诱导肿瘤坏死因子受体(gitr)抗体及其使用方法 |
| EP3541421A4 (fr) * | 2016-11-17 | 2020-07-01 | Seattle Genetics, Inc. | Compositions et méthodes pour le traitement du cancer |
| EP3541847A4 (fr) | 2016-11-17 | 2020-07-08 | Seattle Genetics, Inc. | Composés interagissant avec le glycane et méthodes d'utilisation |
| JOP20190100A1 (ar) | 2016-11-19 | 2019-05-01 | Potenza Therapeutics Inc | بروتينات ربط مولد ضد مضاد لـ gitr وطرق استخدامها |
| EP3571295A1 (fr) | 2017-01-18 | 2019-11-27 | Yeda Research and Development Co. Ltd | Cellules veto génétiquement modifiées et leur utilisation en immunothérapie |
| US10751368B2 (en) | 2017-01-18 | 2020-08-25 | Yeda Research And Development Co. Ltd. | Methods of transplantation and disease treatment |
| MX2019008905A (es) * | 2017-01-27 | 2019-11-18 | Ultrahuman One Ltd | Agentes de union. |
| MX2019009619A (es) | 2017-02-10 | 2019-12-18 | Eutilex Co Ltd | Anticuerpo anti-cancer convertible de celula t regulatoria inducible por ifn-gamma (irtca) y usos del mismo. |
| CA3054289A1 (fr) | 2017-02-21 | 2018-08-30 | Regeneron Pharmaceuticals, Inc. | Anticorps anti-pd-1 pour le traitement du cancer du poumon |
| KR20240044544A (ko) | 2017-03-03 | 2024-04-04 | 씨젠 인크. | 글리칸-상호작용 화합물 및 사용 방법 |
| JP7458188B2 (ja) | 2017-03-31 | 2024-03-29 | ブリストル-マイヤーズ スクイブ カンパニー | 腫瘍を処置する方法 |
| WO2018185618A1 (fr) | 2017-04-03 | 2018-10-11 | Novartis Ag | Conjugués de médicament-anticorps anti-cdh6 et combinaisons d'anticorps anti-gitr et méthodes de traitement |
| AR111651A1 (es) | 2017-04-28 | 2019-08-07 | Novartis Ag | Conjugados de anticuerpos que comprenden agonistas del receptor de tipo toll y terapias de combinación |
| WO2018209298A1 (fr) | 2017-05-12 | 2018-11-15 | Harpoon Therapeutics, Inc. | Protéines de liaison à la mésothéline |
| EP3625260A1 (fr) | 2017-05-16 | 2020-03-25 | Bristol-Myers Squibb Company | Traitement du cancer avec des anticorps agonistes anti-gitr |
| WO2018229715A1 (fr) | 2017-06-16 | 2018-12-20 | Novartis Ag | Compositions comprenant des anticorps anti-cd32b et procédés d'utilisation correspondants |
| CN109136275B (zh) | 2017-06-19 | 2021-03-16 | 百奥赛图(北京)医药科技股份有限公司 | 人源化gitr基因改造动物模型的制备方法及应用 |
| MA49457A (fr) | 2017-06-22 | 2020-04-29 | Novartis Ag | Molécules d'anticorps se liant à cd73 et leurs utilisations |
| WO2018237173A1 (fr) | 2017-06-22 | 2018-12-27 | Novartis Ag | Molécules d'anticorps dirigées contre cd73 et utilisations correspondantes |
| JP2020524694A (ja) | 2017-06-22 | 2020-08-20 | ノバルティス アーゲー | がんの処置における使用のためのIL−1β結合性抗体 |
| WO2018235056A1 (fr) | 2017-06-22 | 2018-12-27 | Novartis Ag | Anticorps se liant à il-1beta destinés à être utilisés dans le traitement du cancer |
| WO2019006007A1 (fr) | 2017-06-27 | 2019-01-03 | Novartis Ag | Régimes posologiques pour anticorps anti-tim3 et leurs utilisations |
| SG11201911936YA (en) | 2017-06-30 | 2020-01-30 | Bristol Myers Squibb Co | Substituted quinolinycyclohexylpropanamide compounds and improved methods for their preparation |
| JP2020532279A (ja) * | 2017-06-30 | 2020-11-12 | 江▲蘇▼恒瑞医▲薬▼股▲フン▼有限公司Jiangsu Hengrui Medicine Co., Ltd. | 抗gitr抗体、その抗原結合性断片、およびその医薬用途 |
| AU2018302283A1 (en) | 2017-07-20 | 2020-02-06 | Novartis Ag | Dosage regimens of anti-LAG-3 antibodies and uses thereof |
| WO2019046338A1 (fr) * | 2017-08-28 | 2019-03-07 | Angiex, Inc. | Anticorps anti-tm4sf1 et leurs procédés d'utilisation |
| AU2018346955B2 (en) | 2017-10-13 | 2024-08-29 | Harpoon Therapeutics, Inc. | B cell maturation antigen binding proteins |
| CN111247169A (zh) | 2017-10-15 | 2020-06-05 | 百时美施贵宝公司 | 治疗肿瘤的方法 |
| US20210040205A1 (en) | 2017-10-25 | 2021-02-11 | Novartis Ag | Antibodies targeting cd32b and methods of use thereof |
| KR20200089286A (ko) | 2017-11-16 | 2020-07-24 | 노파르티스 아게 | 조합 요법 |
| WO2019140229A1 (fr) | 2018-01-12 | 2019-07-18 | Bristol-Myers Squibb Company | Anticorps dirigés contre tim3 et leurs utilisations |
| CN111836644B (zh) * | 2018-03-08 | 2024-07-23 | 凡恩世制药(北京)有限公司 | 抗密蛋白18.2抗体及其用途 |
| EP3768716A1 (fr) | 2018-03-21 | 2021-01-27 | Five Prime Therapeutics, Inc. | Anticorps se liant à vista à ph acide |
| US11242393B2 (en) | 2018-03-23 | 2022-02-08 | Bristol-Myers Squibb Company | Antibodies against MICA and/or MICB and uses thereof |
| US20210024873A1 (en) | 2018-03-27 | 2021-01-28 | Bristol-Myers Squibb Company | Real-time monitoring of protein concentration using ultraviolet signal |
| EP3774911A1 (fr) | 2018-03-30 | 2021-02-17 | Bristol-Myers Squibb Company | Méthodes de traitement de tumeur |
| US20210147547A1 (en) | 2018-04-13 | 2021-05-20 | Novartis Ag | Dosage Regimens For Anti-Pd-L1 Antibodies And Uses Thereof |
| AR126019A1 (es) | 2018-05-30 | 2023-09-06 | Novartis Ag | Anticuerpos frente a entpd2, terapias de combinación y métodos de uso de los anticuerpos y las terapias de combinación |
| WO2019232244A2 (fr) | 2018-05-31 | 2019-12-05 | Novartis Ag | Molécules d'anticorps anti-cd73 et leurs utilisations |
| WO2019241730A2 (fr) | 2018-06-15 | 2019-12-19 | Flagship Pioneering Innovations V, Inc. | Augmentation de l'activité immunitaire par modulation de facteurs de signalisation post-cellulaires |
| JP7411627B2 (ja) | 2018-07-09 | 2024-01-11 | ファイヴ プライム セラピューティクス インク | Ilt4と結合する抗体 |
| AR116109A1 (es) | 2018-07-10 | 2021-03-31 | Novartis Ag | Derivados de 3-(5-amino-1-oxoisoindolin-2-il)piperidina-2,6-diona y usos de los mismos |
| JOP20210001A1 (ar) | 2018-07-10 | 2021-01-05 | Novartis Ag | مشتقات 3-(5- هيدروكسي -1- أوكسو أيزو إندولين -2- يل) بيبريدين -2، 6- دايون واستخدامها لمعالجة أمراض مرتبطة ببروتين ذات أصبع الزنك من عائلة (ikaros 2 (ikzf2 |
| SG11202100102VA (en) | 2018-07-11 | 2021-02-25 | Five Prime Therapeutics Inc | Antibodies binding to vista at acidic ph |
| WO2020021465A1 (fr) | 2018-07-25 | 2020-01-30 | Advanced Accelerator Applications (Italy) S.R.L. | Procédé de traitement de tumeurs neuroendocrines |
| CA3108796A1 (fr) * | 2018-08-08 | 2020-02-13 | Cedars-Sinai Medical Center | Compositions et methodes de traitement du cancer et de maladies auto-immunes |
| IL281683B2 (en) | 2018-09-25 | 2023-04-01 | Harpoon Therapeutics Inc | dll3 binding proteins and methods of use |
| US20230053449A1 (en) | 2018-10-31 | 2023-02-23 | Novartis Ag | Dc-sign antibody drug conjugates |
| JP2022514315A (ja) | 2018-12-20 | 2022-02-10 | ノバルティス アーゲー | 3-(1-オキソイソインドリン-2-イル)ピペリジン-2,6-ジオン誘導体を含む投与計画及び薬剤組み合わせ |
| WO2020128620A1 (fr) | 2018-12-21 | 2020-06-25 | Novartis Ag | Utilisation d'anticorps se liant à il-1bêta |
| CA3119584A1 (fr) | 2018-12-21 | 2020-06-25 | Novartis Ag | Utilisation d'anticorps de liaison a il-1s dans le traitement ou la prevention du syndrome myelodysplasique |
| EP3897613A1 (fr) | 2018-12-21 | 2021-10-27 | Novartis AG | Utilisation d'anticorps de liaison à il-1bêta |
| WO2020128637A1 (fr) | 2018-12-21 | 2020-06-25 | Novartis Ag | UTILISATION D'ANTICORPS DE LIAISON À IL-1β DANS LE TRAITEMENT D'UN CANCER MSI-H |
| JP7483732B2 (ja) | 2019-02-15 | 2024-05-15 | ノバルティス アーゲー | 3-(1-オキソ-5-(ピペリジン-4-イル)イソインドリン-2-イル)ピペリジン-2,6-ジオン誘導体及びその使用 |
| JP7488826B2 (ja) | 2019-02-15 | 2024-05-22 | ノバルティス アーゲー | 置換3-(1-オキソイソインドリン-2-イル)ピペリジン-2,6-ジオン誘導体及びその使用 |
| WO2020172658A1 (fr) | 2019-02-24 | 2020-08-27 | Bristol-Myers Squibb Company | Procédés d'isolement d'une protéine |
| RU2734432C1 (ru) * | 2019-04-23 | 2020-10-16 | Закрытое Акционерное Общество "Биокад" | Моноклональное антитело, которое специфически связывается с GITR |
| MA55805A (fr) | 2019-05-03 | 2022-03-09 | Flagship Pioneering Innovations V Inc | Métodes de modulation de l'activité immunitaire |
| WO2020237221A1 (fr) | 2019-05-23 | 2020-11-26 | Bristol-Myers Squibb Company | Méthodes de surveillance de milieu |
| EP3976831A1 (fr) | 2019-05-30 | 2022-04-06 | Bristol-Myers Squibb Company | Signatures géniques multi-tumorales destinées à être adaptées à une thérapie immuno-oncologique |
| JP2022534981A (ja) | 2019-05-30 | 2022-08-04 | ブリストル-マイヤーズ スクイブ カンパニー | 細胞局在化シグネチャーおよび組み合わせ治療 |
| KR20220016155A (ko) | 2019-05-30 | 2022-02-08 | 브리스톨-마이어스 스큅 컴퍼니 | 면역-종양학 (i-o) 요법에 적합한 대상체를 확인하는 방법 |
| CA3150273A1 (fr) * | 2019-08-08 | 2021-02-11 | Cedars-Sinai Medical Center | Procede de generation de lymphocytes t actives pour une therapie anticancereuse |
| JP2022548881A (ja) | 2019-09-18 | 2022-11-22 | ノバルティス アーゲー | Entpd2抗体、組合せ療法並びに抗体及び組合せ療法を使用する方法 |
| WO2021055698A1 (fr) | 2019-09-19 | 2021-03-25 | Bristol-Myers Squibb Company | Anticorps se liant à vista à un ph acide |
| AU2020370832A1 (en) | 2019-10-21 | 2022-05-19 | Novartis Ag | TIM-3 inhibitors and uses thereof |
| IL292347A (en) | 2019-10-21 | 2022-06-01 | Novartis Ag | Combination treatments with ventoclax and tim-3 inhibitors |
| EP4076434A1 (fr) | 2019-12-17 | 2022-10-26 | Flagship Pioneering Innovations V, Inc. | Polythérapies anticancéreuses ayant des inducteurs de désassemblage cellulaire dépendant du fer |
| CA3165399A1 (fr) | 2019-12-20 | 2021-06-24 | Novartis Ag | Utilisations d'anticorps anti-tgf-betas et inhibiteurs de point de controle pour le traitement des maladies proliferatives |
| US20230058489A1 (en) | 2020-01-17 | 2023-02-23 | Novartis Ag | Combination comprising a tim-3 inhibitor and a hypomethylating agent for use in treating myelodysplastic syndrome or chronic myelomonocytic leukemia |
| CA3168173A1 (fr) | 2020-03-06 | 2021-09-10 | Robert Babb | Anticorps anti-gitr et leurs utilisations |
| JPWO2021225159A1 (fr) * | 2020-05-08 | 2021-11-11 | ||
| EP4165169A1 (fr) | 2020-06-11 | 2023-04-19 | Novartis AG | Inhibiteurs de zbtb32 et leurs utilisations |
| US20230321067A1 (en) | 2020-06-23 | 2023-10-12 | Novartis Ag | Dosing regimen comprising 3-(1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives |
| US20230355804A1 (en) | 2020-06-29 | 2023-11-09 | Flagship Pioneering Innovations V, Inc. | Viruses engineered to promote thanotransmission and their use in treating cancer |
| JP2023536164A (ja) | 2020-08-03 | 2023-08-23 | ノバルティス アーゲー | ヘテロアリール置換3-(1-オキソイソインドリン-2-イル)ピペリジン-2,6-ジオン誘導体及びその使用 |
| IL300916A (en) | 2020-08-31 | 2023-04-01 | Bristol Myers Squibb Co | Cell localization signature and immunotherapy |
| WO2022043557A1 (fr) | 2020-08-31 | 2022-03-03 | Advanced Accelerator Applications International Sa | Méthode de traitement de cancers exprimant le psma |
| US20230338587A1 (en) | 2020-08-31 | 2023-10-26 | Advanced Accelerator Applications International Sa | Method of treating psma-expressing cancers |
| US20230374064A1 (en) | 2020-10-05 | 2023-11-23 | Bristol-Myers Squibb Company | Methods for concentrating proteins |
| JP2024508207A (ja) | 2020-12-02 | 2024-02-26 | ブイアイビー ブイゼットダブリュ | がんに対する組み合わせ治療におけるltbrアゴニスト |
| WO2022120179A1 (fr) | 2020-12-03 | 2022-06-09 | Bristol-Myers Squibb Company | Signatures géniques multi-tumorales et leurs utilisations |
| CA3196999A1 (fr) | 2020-12-28 | 2022-07-07 | Masano HUANG | Methodes de traitement des tumeurs |
| IL303648A (en) | 2020-12-28 | 2023-08-01 | Bristol Myers Squibb Co | Antibody preparations and methods of using them |
| TW202241952A (zh) | 2020-12-31 | 2022-11-01 | 法商英耐特醫藥公司 | 結合至NKp46及CD123之多功能自然殺手(NK)細胞接合體 |
| CA3206018A1 (fr) * | 2021-01-21 | 2022-07-28 | Twist Bioscience Corporation | Methodes et compositions se rapportant a des recepteurs d'adenosine |
| TW202246334A (zh) | 2021-02-02 | 2022-12-01 | 美商美國禮來大藥廠 | Gitr拮抗劑及其使用方法 |
| WO2022212784A1 (fr) | 2021-03-31 | 2022-10-06 | Flagship Pioneering Innovations V, Inc. | Polypeptides de thanotransmission et leur utilisation dans le traitement du cancer |
| CN113035298B (zh) * | 2021-04-02 | 2023-06-20 | 南京信息工程大学 | 递归生成大阶数行限制覆盖阵列的药物临床试验设计方法 |
| EP4314068A1 (fr) | 2021-04-02 | 2024-02-07 | The Regents Of The University Of California | Anticorps dirigés contre un cdcp1 clivé et leurs utilisations |
| TW202304979A (zh) | 2021-04-07 | 2023-02-01 | 瑞士商諾華公司 | 抗TGFβ抗體及其他治療劑用於治療增殖性疾病之用途 |
| AU2022272117A1 (en) * | 2021-05-10 | 2024-01-04 | Medimabbio Inc. | Anti-gitr antibodies and uses thereof |
| AR125874A1 (es) | 2021-05-18 | 2023-08-23 | Novartis Ag | Terapias de combinación |
| CA3224374A1 (fr) | 2021-06-29 | 2023-01-05 | Flagship Pioneering Innovations V, Inc. | Cellules immunitaires modifiees pour favoriser la thanotransmission de phenylethanolamines et leurs utilisations |
| WO2023173011A1 (fr) | 2022-03-09 | 2023-09-14 | Bristol-Myers Squibb Company | Expression transitoire de protéines thérapeutiques |
| KR20240144422A (ko) | 2022-03-15 | 2024-10-02 | 컴퓨젠 엘티디. | 암 치료의 단독치료법 및 병용치료법에서 il-18bp 길항제 항체 및 이의 용도 |
| IL314907A (en) | 2022-03-15 | 2024-10-01 | Yeda Res & Dev | Antibodies against GITR and their uses |
| WO2023178329A1 (fr) | 2022-03-18 | 2023-09-21 | Bristol-Myers Squibb Company | Procédés d'isolement de polypeptides |
| WO2023235847A1 (fr) | 2022-06-02 | 2023-12-07 | Bristol-Myers Squibb Company | Compositions d'anticorps et leurs procédés d'utilisation |
| EP4429067A1 (fr) | 2022-09-15 | 2024-09-11 | LG Energy Solution, Ltd. | Système de gestion de batterie, bloc-batterie, véhicule électrique et procédé de prédiction de temps de charge de batterie |
| WO2024077191A1 (fr) | 2022-10-05 | 2024-04-11 | Flagship Pioneering Innovations V, Inc. | Molécules d'acide nucléique codant pour des trif et des polypeptides supplémentaires et leur utilisation dans le traitement du cancer |
| WO2024097328A1 (fr) * | 2022-11-03 | 2024-05-10 | Incyte Corporation | Polythérapies comprenant un anticorps anti-gitr pour le traitement de cancers |
| WO2024151687A1 (fr) | 2023-01-09 | 2024-07-18 | Flagship Pioneering Innovations V, Inc. | Commutateurs génétiques et leur utilisation dans le traitement du cancer |
| WO2024194605A1 (fr) | 2023-03-17 | 2024-09-26 | Quell Therapeutics Limited | Thérapie treg |
| CN117777306B (zh) * | 2023-07-04 | 2024-08-20 | 深圳豪石生物科技有限公司 | 一种靶向cldn18.2的增强型嵌合抗原受体及其用途 |
| CN117777307B (zh) * | 2023-09-26 | 2024-08-20 | 深圳豪石生物科技有限公司 | 一种cldn18.2特异性嵌合t细胞受体、嵌合t细胞受体免疫细胞及其应用 |
Citations (174)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4044126A (en) | 1972-04-20 | 1977-08-23 | Allen & Hanburys Limited | Steroidal aerosol compositions and process for the preparation thereof |
| US4364923A (en) | 1972-04-20 | 1982-12-21 | Allen & Hanburs Limited | Chemical compounds |
| US4444887A (en) | 1979-12-10 | 1984-04-24 | Sloan-Kettering Institute | Process for making human antibody producing B-lymphocytes |
| WO1986005807A1 (fr) | 1985-04-01 | 1986-10-09 | Celltech Limited | Lignee cellulaire de myelomes transformee et procede d'expression d'un gene codant un polypeptide eucaryotque employant cette lignee |
| EP0239400A2 (fr) | 1986-03-27 | 1987-09-30 | Medical Research Council | Anticorps recombinants et leurs procédés de production |
| US4716111A (en) | 1982-08-11 | 1987-12-29 | Trustees Of Boston University | Process for producing human antibodies |
| WO1989001036A1 (fr) | 1987-07-23 | 1989-02-09 | Celltech Limited | Vecteurs d'expression a base d'adn recombinant |
| US4816397A (en) | 1983-03-25 | 1989-03-28 | Celltech, Limited | Multichain polypeptides or proteins and processes for their production |
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| WO1990002809A1 (fr) | 1988-09-02 | 1990-03-22 | Protein Engineering Corporation | Production et selection de proteines de liaison diversifiees de recombinaison |
| WO1991009967A1 (fr) | 1989-12-21 | 1991-07-11 | Celltech Limited | Anticorps humanises |
| WO1991010737A1 (fr) | 1990-01-11 | 1991-07-25 | Molecular Affinities Corporation | Production d'anticorps utilisant des librairies de genes |
| WO1991010741A1 (fr) | 1990-01-12 | 1991-07-25 | Cell Genesys, Inc. | Generation d'anticorps xenogeniques |
| WO1992001047A1 (fr) | 1990-07-10 | 1992-01-23 | Cambridge Antibody Technology Limited | Procede de production de chainon de paires a liaison specifique |
| US5122464A (en) | 1986-01-23 | 1992-06-16 | Celltech Limited, A British Company | Method for dominant selection in eucaryotic cells |
| WO1992018619A1 (fr) | 1991-04-10 | 1992-10-29 | The Scripps Research Institute | Banques de recepteurs heterodimeres utilisant des phagemides |
| EP0519596A1 (fr) | 1991-05-17 | 1992-12-23 | Merck & Co. Inc. | Procédé pour réduire l'immunogénécité des domaines variables d'anticorps |
| WO1992022324A1 (fr) | 1991-06-14 | 1992-12-23 | Xoma Corporation | Fragments d'anticorps produits par des microbes et leurs conjugues |
| WO1993011236A1 (fr) | 1991-12-02 | 1993-06-10 | Medical Research Council | Production d'anticorps anti-auto-antigenes a partir de repertoires de segments d'anticorps affiches sur phage |
| US5223409A (en) | 1988-09-02 | 1993-06-29 | Protein Engineering Corp. | Directed evolution of novel binding proteins |
| US5225539A (en) | 1986-03-27 | 1993-07-06 | Medical Research Council | Recombinant altered antibodies and methods of making altered antibodies |
| WO1993017105A1 (fr) | 1992-02-19 | 1993-09-02 | Scotgen Limited | Anticorps modifies, produits et procedes s'y rapportant |
| WO1994004678A1 (fr) | 1992-08-21 | 1994-03-03 | Casterman Cecile | Immunoglobulines exemptes de chaines legeres |
| EP0592106A1 (fr) | 1992-09-09 | 1994-04-13 | Immunogen Inc | Remodelage d'anticorps des rongeurs |
| WO1994025591A1 (fr) | 1993-04-29 | 1994-11-10 | Unilever N.V. | PRODUCTION D'ANTICORPS OU DE FRAGMENTS FONCTIONNALISES D'ANTICORPS, DERIVES DES IMMUNOGLOBULINES A CHAINE LOURDE DE $i(CAMELIDAE) |
| WO1994029351A2 (fr) | 1993-06-16 | 1994-12-22 | Celltech Limited | Anticorps |
| WO1995001997A1 (fr) | 1993-07-09 | 1995-01-19 | Smithkline Beecham Corporation | ANTICORPS RECOMBINANTS ET HUMANISES, DIRIGES CONTRE L'IL-1β ET DESTINES AU TRAITEMENT DE TROUBLES INFLAMMATOIRES INDUITS PAR IL-1 CHEZ L'HOMME |
| US5413923A (en) | 1989-07-25 | 1995-05-09 | Cell Genesys, Inc. | Homologous recombination for universal donor cells and chimeric mammalian hosts |
| WO1995015982A2 (fr) | 1993-12-08 | 1995-06-15 | Genzyme Corporation | Procede de generation d'anticorps specifiques |
| US5427908A (en) | 1990-05-01 | 1995-06-27 | Affymax Technologies N.V. | Recombinant library screening methods |
| WO1995020401A1 (fr) | 1994-01-31 | 1995-08-03 | Trustees Of Boston University | Banques d'anticorps polyclonaux |
| US5516637A (en) | 1994-06-10 | 1996-05-14 | Dade International Inc. | Method involving display of protein binding pairs on the surface of bacterial pili and bacteriophage |
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5545806A (en) | 1990-08-29 | 1996-08-13 | Genpharm International, Inc. | Ransgenic non-human animals for producing heterologous antibodies |
| US5565332A (en) | 1991-09-23 | 1996-10-15 | Medical Research Council | Production of chimeric antibodies - a combinatorial approach |
| US5569825A (en) | 1990-08-29 | 1996-10-29 | Genpharm International | Transgenic non-human animals capable of producing heterologous antibodies of various isotypes |
| WO1996033735A1 (fr) | 1995-04-27 | 1996-10-31 | Abgenix, Inc. | Anticorps humains derives d'une xenosouris immunisee |
| WO1996034096A1 (fr) | 1995-04-28 | 1996-10-31 | Abgenix, Inc. | Anticorps humains derives de xeno-souris immunisees |
| US5585097A (en) | 1992-03-24 | 1996-12-17 | British Technology Group Limited | Humanized anti-CD3 specific antibodies |
| WO1997013844A1 (fr) | 1995-10-06 | 1997-04-17 | Cambridge Antibody Technology Limited | Elements de fixation specifiques destines au facteur beta humain de croissance transformant, materiaux et procedes associes |
| US5624821A (en) | 1987-03-18 | 1997-04-29 | Scotgen Biopharmaceuticals Incorporated | Antibodies with altered effector functions |
| US5625126A (en) | 1990-08-29 | 1997-04-29 | Genpharm International, Inc. | Transgenic non-human animals for producing heterologous antibodies |
| US5633425A (en) | 1990-08-29 | 1997-05-27 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| US5661016A (en) | 1990-08-29 | 1997-08-26 | Genpharm International Inc. | Transgenic non-human animals capable of producing heterologous antibodies of various isotypes |
| WO1997034631A1 (fr) | 1996-03-18 | 1997-09-25 | Board Of Regents, The University Of Texas System | Domaines analogues a l'immunoglobuline a demi-vies prolongees |
| US5677425A (en) | 1987-09-04 | 1997-10-14 | Celltech Therapeutics Limited | Recombinant antibody |
| US5693780A (en) | 1991-07-25 | 1997-12-02 | Idec Pharmaceuticals Corporation | Recombinant antibodies for human therapy |
| US5698426A (en) | 1990-09-28 | 1997-12-16 | Ixsys, Incorporated | Surface expression libraries of heteromeric receptors |
| US5709874A (en) | 1993-04-14 | 1998-01-20 | Emory University | Device for local drug delivery and methods for using the same |
| US5733743A (en) | 1992-03-24 | 1998-03-31 | Cambridge Antibody Technology Limited | Methods for producing members of specific binding pairs |
| WO1998016654A1 (fr) | 1996-10-11 | 1998-04-23 | Japan Tobacco, Inc. | Production de proteine multimere par procede de fusion cellulaire |
| US5750753A (en) | 1996-01-24 | 1998-05-12 | Chisso Corporation | Method for manufacturing acryloxypropysilane |
| US5759542A (en) | 1994-08-05 | 1998-06-02 | New England Deaconess Hospital Corporation | Compositions and methods for the delivery of drugs by platelets for the treatment of cardiovascular and other diseases |
| WO1998023289A1 (fr) | 1996-11-27 | 1998-06-04 | The General Hospital Corporation | Modulation de la fixation de l'igg au fcrn |
| WO1998024893A2 (fr) | 1996-12-03 | 1998-06-11 | Abgenix, Inc. | MAMMIFERES TRANSGENIQUES POSSEDANT DES LOCI DE GENES D'IMMUNOGLOBULINE D'ORIGINE HUMAINE, DOTES DE REGIONS VH ET Vλ, ET ANTICORPS PRODUITS A PARTIR DE TELS MAMMIFERES |
| US5766886A (en) | 1991-12-13 | 1998-06-16 | Xoma Corporation | Modified antibody variable domains |
| US5780225A (en) | 1990-01-12 | 1998-07-14 | Stratagene | Method for generating libaries of antibody genes comprising amplification of diverse antibody DNAs and methods for using these libraries for the production of diverse antigen combining molecules |
| US5807715A (en) | 1984-08-27 | 1998-09-15 | The Board Of Trustees Of The Leland Stanford Junior University | Methods and transformed mammalian lymphocyte cells for producing functional antigen-binding protein including chimeric immunoglobulin |
| US5814318A (en) | 1990-08-29 | 1998-09-29 | Genpharm International Inc. | Transgenic non-human animals for producing heterologous antibodies |
| US5821047A (en) | 1990-12-03 | 1998-10-13 | Genentech, Inc. | Monovalent phage display |
| WO1998046645A2 (fr) | 1997-04-14 | 1998-10-22 | Micromet Gesellschaft Für Biomedizinische Forschung Mbh | Nouveau procede de production de recepteurs d'anti-antigenes humains et leur utilisation |
| WO1998050433A2 (fr) | 1997-05-05 | 1998-11-12 | Abgenix, Inc. | Anticorps monoclonaux humains contre le recepteur du facteur de croissance epidermique |
| US5837242A (en) | 1992-12-04 | 1998-11-17 | Medical Research Council | Multivalent and multispecific binding proteins, their manufacture and use |
| US5840674A (en) | 1990-11-01 | 1998-11-24 | Oregon Health Sciences University | Covalent microparticle-drug conjugates for biological targeting |
| US5860957A (en) | 1997-02-07 | 1999-01-19 | Sarcos, Inc. | Multipathway electronically-controlled drug delivery system |
| US5869620A (en) | 1986-09-02 | 1999-02-09 | Enzon, Inc. | Multivalent antigen-binding proteins |
| US5869046A (en) | 1995-04-14 | 1999-02-09 | Genentech, Inc. | Altered polypeptides with increased half-life |
| US5900252A (en) | 1990-04-17 | 1999-05-04 | Eurand International S.P.A. | Method for targeted and controlled release of drugs in the intestinal tract and more particularly in the colon |
| US5948433A (en) | 1997-08-21 | 1999-09-07 | Bertek, Inc. | Transdermal patch |
| US5965726A (en) | 1992-03-27 | 1999-10-12 | The United States Of America As Represented By The Department Of Health And Human Services | Method of eliminating inhibitory/ instability regions of mRNA |
| US5972366A (en) | 1994-11-28 | 1999-10-26 | The Unites States Of America As Represented By The Secretary Of The Army | Drug releasing surgical implant or dressing material |
| US5983134A (en) | 1995-04-23 | 1999-11-09 | Electromagnetic Bracing Systems Inc. | Electrophoretic cuff apparatus drug delivery system |
| US5985317A (en) | 1996-09-06 | 1999-11-16 | Theratech, Inc. | Pressure sensitive adhesive matrix patches for transdermal delivery of salts of pharmaceutical agents |
| US5985307A (en) | 1993-04-14 | 1999-11-16 | Emory University | Device and method for non-occlusive localized drug delivery |
| US5989830A (en) | 1995-10-16 | 1999-11-23 | Unilever Patent Holdings Bv | Bifunctional or bivalent antibody fragment analogue |
| US6004534A (en) | 1993-07-23 | 1999-12-21 | Massachusetts Institute Of Technology | Targeted polymerized liposomes for improved drug delivery |
| US6005079A (en) | 1992-08-21 | 1999-12-21 | Vrije Universiteit Brussels | Immunoglobulins devoid of light chains |
| US6010715A (en) | 1992-04-01 | 2000-01-04 | Bertek, Inc. | Transdermal patch incorporating a polymer film incorporated with an active agent |
| US6024975A (en) | 1992-04-08 | 2000-02-15 | Americare International Diagnostics, Inc. | Method of transdermally administering high molecular weight drugs with a polymer skin enhancer |
| US6039975A (en) | 1995-10-17 | 2000-03-21 | Hoffman-La Roche Inc. | Colon targeted delivery system |
| US6048736A (en) | 1998-04-29 | 2000-04-11 | Kosak; Kenneth M. | Cyclodextrin polymers for carrying and releasing drugs |
| US6060082A (en) | 1997-04-18 | 2000-05-09 | Massachusetts Institute Of Technology | Polymerized liposomes targeted to M cells and useful for oral or mucosal drug delivery |
| US6071495A (en) | 1989-12-22 | 2000-06-06 | Imarx Pharmaceutical Corp. | Targeted gas and gaseous precursor-filled liposomes |
| US6075181A (en) | 1990-01-12 | 2000-06-13 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| WO2000037504A2 (fr) | 1998-12-23 | 2000-06-29 | Pfizer Inc. | Anticorps monoclonaux humains diriges contre l'antigene ctla-4 |
| WO2000042072A2 (fr) | 1999-01-15 | 2000-07-20 | Genentech, Inc. | Variants polypeptidiques ayant une fonction effectrice alteree |
| US6120751A (en) | 1997-03-21 | 2000-09-19 | Imarx Pharmaceutical Corp. | Charged lipids and uses for the same |
| US6121022A (en) | 1995-04-14 | 2000-09-19 | Genentech, Inc. | Altered polypeptides with increased half-life |
| US6131570A (en) | 1998-06-30 | 2000-10-17 | Aradigm Corporation | Temperature controlling device for aerosol drug delivery |
| US6132992A (en) | 1993-02-01 | 2000-10-17 | Bristol-Myers Squibb Co. | Expression vectors encoding bispecific fusion proteins and methods of producing biologically active bispecific fusion proteins in a mammalian cell |
| US6139865A (en) | 1996-10-01 | 2000-10-31 | Eurand America, Inc. | Taste-masked microcapsule compositions and methods of manufacture |
| US6143273A (en) | 1994-05-06 | 2000-11-07 | Institut Gustave Roussy | Therapeutic composition containing antibodies to soluble polypeptide fractions of LAG-3 protein |
| US6150184A (en) | 1996-08-20 | 2000-11-21 | Ramtron International Corporation | Method of fabricating partially or completely encapsulated top electrode of a ferroelectric capacitor |
| US6167301A (en) | 1995-08-29 | 2000-12-26 | Flower; Ronald J. | Iontophoretic drug delivery device having high-efficiency DC-to-DC energy conversion circuit |
| US6165745A (en) | 1992-04-24 | 2000-12-26 | Board Of Regents, The University Of Texas System | Recombinant production of immunoglobulin-like domains in prokaryotic cells |
| US6194551B1 (en) | 1998-04-02 | 2001-02-27 | Genentech, Inc. | Polypeptide variants |
| WO2001014424A2 (fr) | 1999-08-24 | 2001-03-01 | Medarex, Inc. | Anticorps contre l'antigene ctla-4 humain et utilisation |
| US6197524B1 (en) | 1995-07-21 | 2001-03-06 | Institute National De La Sante Et De La Recherche Medicale | Methods for detecting, identifying, isolating, and selectively labelling and targeting TH1 lymphocyte by means of the LAG-3 protein |
| US6207156B1 (en) | 1997-03-21 | 2001-03-27 | Brigham And Women's Hospital, Inc. | Specific antibodies and antibody fragments |
| WO2001044301A1 (fr) | 1999-11-29 | 2001-06-21 | Unilever Plc | Immobilisation de molecules de liaison d'antigene a domaine unique |
| US6253872B1 (en) | 1996-05-29 | 2001-07-03 | Gmundner Fertigteile Gesellschaft M.B.H & Co., Kg | Track soundproofing arrangement |
| US6256533B1 (en) | 1999-06-09 | 2001-07-03 | The Procter & Gamble Company | Apparatus and method for using an intracutaneous microneedle array |
| US6261595B1 (en) | 2000-02-29 | 2001-07-17 | Zars, Inc. | Transdermal drug patch with attached pocket for controlled heating device |
| US6267983B1 (en) | 1997-10-28 | 2001-07-31 | Bando Chemical Industries, Ltd. | Dermatological patch and process for producing thereof |
| US6271359B1 (en) | 1999-04-14 | 2001-08-07 | Musc Foundation For Research Development | Tissue-specific and pathogen-specific toxic agents and ribozymes |
| US6274552B1 (en) | 1993-03-18 | 2001-08-14 | Cytimmune Sciences, Inc. | Composition and method for delivery of biologically-active factors |
| US6277375B1 (en) | 1997-03-03 | 2001-08-21 | Board Of Regents, The University Of Texas System | Immunoglobulin-like domains with increased half-lives |
| US6316652B1 (en) | 1995-06-06 | 2001-11-13 | Kosta Steliou | Drug mitochondrial targeting agents |
| US6383492B1 (en) | 1997-02-07 | 2002-05-07 | Fordham University | Treatment of infectious diseases with gp96-peptide complexes |
| US6407213B1 (en) | 1991-06-14 | 2002-06-18 | Genentech, Inc. | Method for making humanized antibodies |
| US6410026B1 (en) | 1997-12-11 | 2002-06-25 | Fordham University | Methods for preparation of vaccines against cancer |
| WO2002060919A2 (fr) | 2000-12-12 | 2002-08-08 | Medimmune, Inc. | Molecules a demi-vies longues, compositions et utilisations de celles-ci |
| US6447781B1 (en) | 1995-09-13 | 2002-09-10 | Fordham University | Therapeutic and prophylactic methods using heat shock proteins |
| US20030086930A1 (en) | 2001-05-23 | 2003-05-08 | Mueller Eileen E. | Uses of anti-CTLA-4 antibodies |
| US6610659B1 (en) | 1994-01-13 | 2003-08-26 | Mount Sinai School Of Medicine Of New York University | Use of heat shock protein 70 preparations in vaccination against cancer and infectious disease |
| US6645495B1 (en) | 1997-08-29 | 2003-11-11 | Antigenics, Inc. | Compositions of saponin adjuvants and excipients |
| US20030232323A1 (en) | 2001-11-13 | 2003-12-18 | Wyeth | Agents that modulate immune cell activation and methods of use thereof |
| US20040014194A1 (en) | 2002-03-27 | 2004-01-22 | Schering Corporation | Beta-secretase crystals and methods for preparing and using the same |
| US6737056B1 (en) | 1999-01-15 | 2004-05-18 | Genentech, Inc. | Polypeptide variants with altered effector function |
| US6808710B1 (en) | 1999-08-23 | 2004-10-26 | Genetics Institute, Inc. | Downmodulating an immune response with multivalent antibodies to PD-1 |
| US20050042664A1 (en) | 2003-08-22 | 2005-02-24 | Medimmune, Inc. | Humanization of antibodies |
| US20050048549A1 (en) | 2003-01-21 | 2005-03-03 | Liangxian Cao | Methods and agents for screening for compounds capable of modulating gene expression |
| US20050208625A1 (en) | 1998-04-28 | 2005-09-22 | Taylor Alexander H | Monoclonal antibodies with reduced immunogenicity |
| US20050226875A1 (en) | 2004-03-26 | 2005-10-13 | Pfizer Inc | Uses of anti-CTLA-4 antibodies |
| US7029678B2 (en) | 1993-12-23 | 2006-04-18 | Smithkline Beecham Biologicals (S.A.) | Vaccines |
| US7034121B2 (en) | 2000-01-27 | 2006-04-25 | Genetics Institue, Llc | Antibodies against CTLA4 |
| WO2006105021A2 (fr) | 2005-03-25 | 2006-10-05 | Tolerrx, Inc. | Molecules de liaison gitr et leurs utilisations |
| US7183076B2 (en) | 1997-05-02 | 2007-02-27 | Genentech, Inc. | Method for making multispecific antibodies having heteromultimeric and common components |
| US20070243184A1 (en) | 2005-11-08 | 2007-10-18 | Steven Fischkoff | Prophylaxis and treatment of enterocolitis associated with anti-ctla-4 antibody therapy |
| US20080152665A1 (en) | 2006-09-01 | 2008-06-26 | Claude Leclerc | Therapy of cancer based on targeting adaptive, innate and/or regulatory component of the immune response |
| US7465446B2 (en) | 2003-05-30 | 2008-12-16 | Medarex, Inc. | Surrogate therapeutic endpoint for anti-CTLA4-based immunotherapy of disease |
| US7470428B2 (en) | 2002-01-30 | 2008-12-30 | The Brigham And Women's Hospital, Inc. | Compositions and methods related to TIM-3, a Th1-specific cell surface molecule |
| US20090004213A1 (en) | 2007-03-26 | 2009-01-01 | Immatics Biotechnologies Gmbh | Combination therapy using active immunotherapy |
| US7488802B2 (en) | 2002-12-23 | 2009-02-10 | Wyeth | Antibodies against PD-1 |
| US7531170B1 (en) | 2002-09-11 | 2009-05-12 | La Jolla Institute For Allergy And Immunology | Methods of treating OX40 mediated recall immune responses and agents useful for identifying same |
| US20090123477A1 (en) | 2004-12-23 | 2009-05-14 | Thomas Hanke | Antibodies |
| US7550140B2 (en) | 2002-06-13 | 2009-06-23 | Crucell Holland B.V. | Antibody to the human OX40 receptor |
| WO2009100140A1 (fr) | 2008-02-04 | 2009-08-13 | Medarex, Inc. | Anticorps anti-clta-4 avec blocage réduit de la liaison de ctla-4 à b7 et leurs utilisations |
| US20090214533A1 (en) | 2006-08-17 | 2009-08-27 | The Trustees Of Columbia University In The City Of New York | Methods for converting or inducing protective immunity |
| WO2010005958A2 (fr) | 2008-07-08 | 2010-01-14 | Incyte Corporation | 1,2,5-oxadiazoles constituant des inhibiteurs de l'indoleamine 2,3-dioxygénase |
| US7658921B2 (en) | 2000-12-12 | 2010-02-09 | Medimmune, Llc | Molecules with extended half-lives, compositions and uses thereof |
| US20100061992A1 (en) | 2006-11-15 | 2010-03-11 | The Brigham And Women's Hospital, Inc. | Therapeutic uses of tim-3 modulators |
| US20100100131A1 (en) | 2008-10-21 | 2010-04-22 | K2M, Inc. | Spinal buttress plate |
| US7709226B2 (en) | 2001-07-12 | 2010-05-04 | Arrowsmith Technology Licensing Llc | Method of humanizing antibodies by matching canonical structure types CDRs |
| US20100136030A1 (en) | 2007-02-27 | 2010-06-03 | Lamhamedi-Cherradi Salah-Eddine | Antagonist ox40 antibodies and their use in the treatment of inflammatory and autoimmune diseases |
| US20100196359A1 (en) | 2005-11-25 | 2010-08-05 | Shinichiro Kato | Human Monoclonal Antibody Human CD134 (Ox40) and Methods of Making and Using Same |
| US20100196394A1 (en) | 2003-02-28 | 2010-08-05 | The Johns Hopkins University | Anti-cancer vaccine composition |
| US20100203056A1 (en) | 2008-12-09 | 2010-08-12 | Genentech, Inc. | Anti-pd-l1 antibodies and their use to enhance t-cell function |
| US20100233183A1 (en) | 2007-04-30 | 2010-09-16 | Frederic Triebel | Cytotoxic anti-lag-3 monoclonal antibody and its use in the treatment or prevention of organ transplant rejection and autoimmune disease |
| US20100278844A1 (en) | 2007-09-18 | 2010-11-04 | Frances Balkwill | Cancer Marker and Therapeutic Target |
| US7858589B2 (en) | 1998-08-10 | 2010-12-28 | Antigenics Inc. | Compositions of CpG and saponin adjuvants and uses thereof |
| US20110044953A1 (en) | 2006-03-30 | 2011-02-24 | The Regents Of The University Of California | Methods and Compositions for Localized Secretion of Anti-CTLA-4 Antibodies |
| US7951917B1 (en) | 1997-05-02 | 2011-05-31 | Genentech, Inc. | Method for making multispecific antibodies having heteromultimeric and common components |
| US20110150892A1 (en) | 2008-08-11 | 2011-06-23 | Medarex, Inc. | Human antibodies that bind lymphocyte activation gene-3 (lag-3) and uses thereof |
| US8008449B2 (en) | 2005-05-09 | 2011-08-30 | Medarex, Inc. | Human monoclonal antibodies to programmed death 1 (PD-1) and methods for treating cancer using anti-PD-1 antibodies alone or in combination with other immunotherapeutics |
| US20110305713A1 (en) | 2005-10-21 | 2011-12-15 | Medical College Of Georgia Research Institute, Inc | Methods and compositions to enhance vaccine efficacy by reprogramming regulatory t cells |
| US8114845B2 (en) | 2008-08-25 | 2012-02-14 | Amplimmune, Inc. | Compositions of PD-1 antagonists and methods of use |
| US8142778B2 (en) | 2002-04-12 | 2012-03-27 | Medarex, Inc. | Methods of treatment using CTLA-4 antibodies |
| US8168179B2 (en) | 2002-07-03 | 2012-05-01 | Ono Pharmaceutical Co., Ltd. | Treatment method using anti-PD-L1 antibody |
| US8168757B2 (en) | 2008-03-12 | 2012-05-01 | Merck Sharp & Dohme Corp. | PD-1 binding proteins |
| US20120142750A1 (en) | 2005-10-21 | 2012-06-07 | Regents Of The University Of Minnesota | Indoleamine 2,3-dioxygenase pathways in the generation of regulatory t cells |
| US8227577B2 (en) | 2007-12-21 | 2012-07-24 | Hoffman-La Roche Inc. | Bivalent, bispecific antibodies |
| US8226946B2 (en) | 2008-08-06 | 2012-07-24 | Light Sciences Oncology, Inc. | Enhancement of light activated therapy by immune augmentation using anti-CTLA-4 antibody |
| US20120219559A1 (en) | 2009-08-13 | 2012-08-30 | The Johns Hopkins University | Methods of modulating immune function |
| US20130022623A1 (en) | 2011-07-01 | 2013-01-24 | Cellerant Therapeutics, Inc. | Antibodies that specifically bind to tim3 |
| WO2013033091A1 (fr) | 2011-09-01 | 2013-03-07 | Halo Maritime Defense Systems | Barrage marin et porte marine |
| WO2013039954A1 (fr) * | 2011-09-14 | 2013-03-21 | Sanofi | Anticorps anti-gitr |
| US20130183315A1 (en) | 2011-07-11 | 2013-07-18 | Glenmark Pharmaceuticals S.A. | Antibodies that bind to OX40 and their uses |
| US20130202623A1 (en) | 2010-02-16 | 2013-08-08 | Nicolas Chomont | Pd-1 modulation and uses thereof for modulating hiv replication |
| US8541002B2 (en) | 2003-09-12 | 2013-09-24 | Agenus Inc. | Vaccine for treatment and prevention of herpes simplex virus infection |
| US8586713B2 (en) | 2009-06-26 | 2013-11-19 | Regeneron Pharmaceuticals, Inc. | Readily isolated bispecific antibodies with native immunoglobulin format |
| US8591886B2 (en) | 2007-07-12 | 2013-11-26 | Gitr, Inc. | Combination therapies employing GITR binding molecules |
| US20130323283A1 (en) | 2010-12-01 | 2013-12-05 | The Children's Hospital Of Philadelphia | Compositions and methods for treating foxp3+ treg related diseases |
| US8614295B2 (en) | 2009-02-17 | 2013-12-24 | Ucb Pharma S.A. | Antibody molecules having specificity for human OX40 |
Family Cites Families (65)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6111090A (en) | 1996-08-16 | 2000-08-29 | Schering Corporation | Mammalian cell surface antigens; related reagents |
| US6503184B1 (en) | 1997-10-21 | 2003-01-07 | Human Genome Sciences, Inc. | Human tumor necrosis factor receptor-like proteins TR11, TR11SV1 and TR11SV2 |
| AU1102399A (en) | 1997-10-21 | 1999-05-10 | Human Genome Sciences, Inc. | Human tumor necrosis factor receptor-like proteins tr11, tr11sv1, and tr11sv2 |
| EP1053321A1 (fr) | 1998-02-09 | 2000-11-22 | Genentech, Inc. | Nouveaux homologues recepteurs du facteur necrosant des tumeurs et acides nucleiques codant ceux-ci |
| DK2857516T3 (en) | 2000-04-11 | 2017-08-07 | Genentech Inc | Multivalent antibodies and uses thereof |
| US20030133936A1 (en) | 2001-07-12 | 2003-07-17 | Byrne Michael Chapman | CD25markers and uses thereof |
| AUPS054702A0 (en) | 2002-02-14 | 2002-03-07 | Immunaid Pty Ltd | Cancer therapy |
| WO2004107618A2 (fr) | 2003-05-23 | 2004-12-09 | Wyeth | Ligand du gitr et molecules et anticorps lies au ligand du gitr et leurs utilisations |
| US20050048054A1 (en) | 2003-07-11 | 2005-03-03 | Shino Hanabuchi | Lymphocytes; methods |
| EP2270511A3 (fr) | 2003-10-24 | 2011-08-24 | Immunaid Pty Ltd | Procédé de thérapie |
| CN1980957A (zh) | 2004-03-23 | 2007-06-13 | 比奥根艾迪克Ma公司 | 受体偶联剂及其治疗用途 |
| TW200732350A (en) | 2005-10-21 | 2007-09-01 | Amgen Inc | Methods for generating monovalent IgG |
| EP1955708A4 (fr) | 2005-11-24 | 2013-02-06 | Dainippon Sumitomo Pharma Co | Nouveau potentialisateur d'induction de lymphocyte t cytotoxique a memoire |
| EP1981969A4 (fr) | 2006-01-19 | 2009-06-03 | Genzyme Corp | Anticorps anti-gitr destines au traitement du cancer |
| GB0603081D0 (en) | 2006-02-15 | 2006-03-29 | Dynal Biotech Asa Oslo | Method |
| KR101571027B1 (ko) | 2006-06-12 | 2015-11-23 | 이머전트 프로덕트 디벨롭먼트 시애틀, 엘엘씨 | 효과기 기능을 갖는 단일쇄 다가 결합 단백질 |
| JP2008278814A (ja) * | 2007-05-11 | 2008-11-20 | Igaku Seibutsugaku Kenkyusho:Kk | アゴニスティック抗ヒトgitr抗体による免疫制御の解除とその応用 |
| WO2009045443A2 (fr) | 2007-10-02 | 2009-04-09 | The University Of Rochester | Méthodes et compositions associées aux réponses synergiques aux mutations oncogènes |
| CN104558179A (zh) | 2009-04-27 | 2015-04-29 | 协和发酵麒麟株式会社 | 用于治疗血液肿瘤的抗IL-3Rα抗体 |
| EP2436397B1 (fr) | 2009-05-29 | 2017-05-10 | Chugai Seiyaku Kabushiki Kaisha | Composition pharmaceutique contenant un antagoniste d'un ligand de la famille de l'egf en tant que composant |
| SG178991A1 (en) | 2009-09-03 | 2012-04-27 | Schering Corp | Anti-gitr antibodies |
| BR112012013736A2 (pt) | 2009-12-07 | 2018-08-14 | Univ Leland Stanford Junior | processo para intesificação de terapia com anticorpos antitumor |
| JP6066732B2 (ja) | 2010-03-05 | 2017-01-25 | ザ・ジョンズ・ホプキンス・ユニバーシティー | 標的免疫調節抗体および融合タンパク質に基づく組成物および方法 |
| US20120014947A1 (en) | 2010-07-16 | 2012-01-19 | The University Of Chicago | Methods and compositions to reduce liver damage associated with conditions or therapies that affect the immune system |
| WO2012064760A2 (fr) | 2010-11-08 | 2012-05-18 | Biovest International, Inc. | Matériels et méthodes pour l'orientation d'une réponse immunitaire vers un épitope |
| JP6400470B2 (ja) | 2011-05-16 | 2018-10-03 | ジェネロン(シャンハイ)コーポレイション リミテッド | 多重特異性Fab融合タンパク質および使用法 |
| CA2837169C (fr) | 2011-05-24 | 2021-11-09 | Zyngenia, Inc. | Complexes multispecifiques contenant un peptide de liaison de l'angiopoietin-2 et utilisations connexes |
| US9382319B2 (en) | 2011-09-26 | 2016-07-05 | Jn Biosciences Llc | Hybrid constant regions |
| WO2013049307A2 (fr) | 2011-09-30 | 2013-04-04 | University Of Miami | Développement d'une mémoire immunitaire améliorée par une inhibition de mtor des lymphocytes t ciblée par un aptamère |
| AU2013203422A1 (en) | 2012-01-06 | 2013-07-18 | Bioalliance C.V. | Anti-transferrin receptor antibodies and methods using same |
| WO2013126809A1 (fr) | 2012-02-23 | 2013-08-29 | Sloan-Kettering Institute For Cancer Research | Prédiction de la sensibilité à un traitement par des produits thérapeutiques d'immunomodulation et procédé de surveillance des effets ascopaux au cours d'un tel traitement |
| WO2013142255A2 (fr) | 2012-03-22 | 2013-09-26 | University Of Miami | Agents de liaison multi-spécifiques |
| KR101566539B1 (ko) | 2012-06-08 | 2015-11-05 | 국립암센터 | 신규한 Th2 세포 전환용 에피토프 및 이의 용도 |
| CN103566377A (zh) | 2012-07-18 | 2014-02-12 | 上海博笛生物科技有限公司 | 癌症的靶向免疫治疗 |
| JP2015524821A (ja) | 2012-08-02 | 2015-08-27 | ジェイエヌ バイオサイエンシーズ エルエルシー | システイン変異及びμ尾部を介して多量体化した抗体又は融合タンパク質 |
| EP3508215A3 (fr) | 2012-12-03 | 2019-10-02 | Bristol-Myers Squibb Company | Amélioration de l'activité anticancéreuse de protéines de fusion fc immunomodulatrices |
| US9834610B2 (en) | 2013-01-31 | 2017-12-05 | Thomas Jefferson University | Fusion proteins for modulating regulatory and effector T cells |
| WO2014121099A1 (fr) | 2013-01-31 | 2014-08-07 | Thomas Jefferson University | Protéine de fusion agoniste des cd40 ox40 et ses utilisations |
| ES2699599T3 (es) | 2013-03-15 | 2019-02-11 | Abbvie Biotherapeutics Inc | Variantes de Fc |
| EP2970486B1 (fr) | 2013-03-15 | 2018-05-16 | Xencor, Inc. | Modulation de cellules t avec des anticorps bispecifiques et des fusions fc |
| FR3008408B1 (fr) | 2013-07-11 | 2018-03-09 | Mc Saf | Nouveaux conjugues anticorps-medicament et leur utilisation en therapie |
| JP6636920B2 (ja) | 2013-07-16 | 2020-01-29 | ジェネンテック, インコーポレイテッド | Pd−1軸結合アンタゴニスト及びtigit阻害剤を使用するがんの治療方法 |
| US20150038682A1 (en) | 2013-08-02 | 2015-02-05 | Jn Biosciences Llc | Antibodies or fusion proteins multimerized via homomultimerizing peptide |
| AR097306A1 (es) | 2013-08-20 | 2016-03-02 | Merck Sharp & Dohme | Modulación de la inmunidad tumoral |
| TW201605896A (zh) | 2013-08-30 | 2016-02-16 | 安美基股份有限公司 | Gitr抗原結合蛋白 |
| EP3082853A2 (fr) | 2013-12-20 | 2016-10-26 | The Broad Institute, Inc. | Polythérapie comprenant un vaccin à base de néoantigènes |
| CN107172880B (zh) | 2014-03-24 | 2021-09-28 | 癌症研究技术有限公司 | 含有修饰IgG2结构域的引起激动或拮抗特性的修饰抗体及其用途 |
| US20150307620A1 (en) | 2014-04-16 | 2015-10-29 | University Of Connecticut | Linked immunotherapeutic agonists that costimulate multiple pathways |
| KR102433464B1 (ko) | 2014-05-28 | 2022-08-17 | 아게누스 인코포레이티드 | 항-gitr 항체 및 이의 사용 방법 |
| CN106459203B (zh) | 2014-06-06 | 2021-08-03 | 百时美施贵宝公司 | 抗糖皮质激素诱导肿瘤坏死因子受体(gitr)的抗体及其用途 |
| AU2015305754B2 (en) | 2014-08-19 | 2018-10-25 | Merck Sharp & Dohme Llc | Anti-tigit antibodies |
| JO3663B1 (ar) | 2014-08-19 | 2020-08-27 | Merck Sharp & Dohme | الأجسام المضادة لمضاد lag3 وأجزاء ربط الأنتيجين |
| RU2017115315A (ru) | 2014-10-03 | 2018-11-08 | Дана-Фарбер Кэнсер Инститьют, Инк. | Антитела к рецептору глюкокортикоид-индуцированного фактора некроза опухоли (gitr) и способы их применения |
| MA41044A (fr) | 2014-10-08 | 2017-08-15 | Novartis Ag | Compositions et procédés d'utilisation pour une réponse immunitaire accrue et traitement contre le cancer |
| CN107250157B (zh) | 2014-11-21 | 2021-06-29 | 百时美施贵宝公司 | 包含修饰的重链恒定区的抗体 |
| JP2018505911A (ja) | 2014-12-05 | 2018-03-01 | イミュネクスト,インコーポレーテッド | 推定上のvista受容体としてのvsig8の同定と、vista/vsig8調節剤を産生するためのその使用 |
| TWI595006B (zh) | 2014-12-09 | 2017-08-11 | 禮納特神經系統科學公司 | 抗pd-1抗體類和使用彼等之方法 |
| IL252295B2 (en) | 2014-12-19 | 2023-10-01 | Dana Farber Cancer Inst Inc | Chimeric antigen receptors and methods of using them |
| GB201500319D0 (en) | 2015-01-09 | 2015-02-25 | Agency Science Tech & Res | Anti-PD-L1 antibodies |
| CA2979712C (fr) | 2015-03-25 | 2024-01-23 | The Regents Of The University Of Michigan | Compositions nanoparticulaires servant a distribuer des biomacromolecules |
| SG11201708223QA (en) | 2015-04-17 | 2017-11-29 | Bristol Myers Squibb Co | Compositions comprising a combination of an anti-pd-1 antibody and another antibody |
| US20200123265A1 (en) | 2015-12-02 | 2020-04-23 | Agenus Inc. | Anti-gitr antibodies and methods of use thereof |
| US11447557B2 (en) | 2015-12-02 | 2022-09-20 | Agenus Inc. | Antibodies and methods of use thereof |
| WO2017096276A1 (fr) | 2015-12-02 | 2017-06-08 | Agenus Inc. | Anticorps anti-gitr et procédés d'utilisation associés |
| MA46770A (fr) | 2016-11-09 | 2019-09-18 | Agenus Inc | Anticorps anti-ox40, anticorps anti-gitr, et leurs procédés d'utilisation |
-
2015
- 2015-05-28 KR KR1020167036746A patent/KR102433464B1/ko active IP Right Grant
- 2015-05-28 CN CN201580041149.7A patent/CN108064242B/zh active Active
- 2015-05-28 PL PL15729987T patent/PL3148579T3/pl unknown
- 2015-05-28 JP JP2016569715A patent/JP6847666B2/ja active Active
- 2015-05-28 MA MA40041A patent/MA40041B1/fr unknown
- 2015-05-28 NZ NZ726513A patent/NZ726513A/en unknown
- 2015-05-28 IL IL293212A patent/IL293212B2/en unknown
- 2015-05-28 TW TW109136463A patent/TW202132337A/zh unknown
- 2015-05-28 HU HUE15729987A patent/HUE053857T2/hu unknown
- 2015-05-28 CN CN202211180038.2A patent/CN115925946A/zh active Pending
- 2015-05-28 DK DK15729987.6T patent/DK3148579T3/da active
- 2015-05-28 SG SG10201912986PA patent/SG10201912986PA/en unknown
- 2015-05-28 SI SI201531531T patent/SI3148579T1/sl unknown
- 2015-05-28 WO PCT/US2015/032895 patent/WO2015184099A1/fr active Application Filing
- 2015-05-28 US US14/724,452 patent/US10155818B2/en active Active
- 2015-05-28 RS RS20210303A patent/RS61678B1/sr unknown
- 2015-05-28 ES ES15729987T patent/ES2860751T3/es active Active
- 2015-05-28 AU AU2015266958A patent/AU2015266958A1/en not_active Abandoned
- 2015-05-28 MX MX2016015614A patent/MX2016015614A/es unknown
- 2015-05-28 CA CA2949998A patent/CA2949998A1/fr active Pending
- 2015-05-28 MA MA047849A patent/MA47849A/fr unknown
- 2015-05-28 KR KR1020227027870A patent/KR20220116578A/ko active IP Right Grant
- 2015-05-28 EP EP18204948.6A patent/EP3498295A1/fr active Pending
- 2015-05-28 EA EA201692458A patent/EA201692458A1/ru unknown
- 2015-05-28 EP EP15729987.6A patent/EP3148579B1/fr active Active
- 2015-05-28 PT PT157299876T patent/PT3148579T/pt unknown
- 2015-05-28 TW TW104117180A patent/TWI709573B/zh active
- 2015-05-28 SG SG11201609721WA patent/SG11201609721WA/en unknown
- 2015-05-28 LT LTEP15729987.6T patent/LT3148579T/lt unknown
- 2015-05-28 IL IL249092A patent/IL249092B/en unknown
-
2016
- 2016-11-25 PH PH12016502345A patent/PH12016502345A1/en unknown
- 2016-11-28 MX MX2020013062A patent/MX2020013062A/es unknown
-
2018
- 2018-04-25 US US15/962,667 patent/US20180244793A1/en not_active Abandoned
- 2018-04-25 US US15/962,673 patent/US10577426B2/en active Active
- 2018-04-25 US US15/962,752 patent/US10800849B2/en active Active
- 2018-10-30 US US16/175,453 patent/US10280226B2/en active Active
-
2019
- 2019-03-15 US US16/355,268 patent/US10829559B2/en active Active
- 2019-12-27 JP JP2019238784A patent/JP2020048584A/ja active Pending
-
2020
- 2020-01-15 US US16/744,163 patent/US11401335B2/en active Active
- 2020-08-21 US US16/999,374 patent/US11897962B2/en active Active
-
2021
- 2021-01-29 AU AU2021200582A patent/AU2021200582B2/en active Active
- 2021-03-03 HR HRP20210364TT patent/HRP20210364T1/hr unknown
- 2021-03-12 CY CY20211100215T patent/CY1124787T1/el unknown
-
2022
- 2022-06-22 US US17/808,248 patent/US20230039577A1/en active Pending
- 2022-08-12 AU AU2022215304A patent/AU2022215304A1/en active Pending
- 2022-12-19 JP JP2022202168A patent/JP2023021446A/ja active Pending
-
2023
- 2023-07-02 IL IL304178A patent/IL304178A/en unknown
Patent Citations (198)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4044126A (en) | 1972-04-20 | 1977-08-23 | Allen & Hanburys Limited | Steroidal aerosol compositions and process for the preparation thereof |
| US4364923A (en) | 1972-04-20 | 1982-12-21 | Allen & Hanburs Limited | Chemical compounds |
| US4414209A (en) | 1972-04-20 | 1983-11-08 | Allen & Hanburys Limited | Micronized aerosol steroids |
| US4444887A (en) | 1979-12-10 | 1984-04-24 | Sloan-Kettering Institute | Process for making human antibody producing B-lymphocytes |
| US4716111A (en) | 1982-08-11 | 1987-12-29 | Trustees Of Boston University | Process for producing human antibodies |
| US4816397A (en) | 1983-03-25 | 1989-03-28 | Celltech, Limited | Multichain polypeptides or proteins and processes for their production |
| US6331415B1 (en) | 1983-04-08 | 2001-12-18 | Genentech, Inc. | Methods of producing immunoglobulins, vectors and transformed host cells for use therein |
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US5807715A (en) | 1984-08-27 | 1998-09-15 | The Board Of Trustees Of The Leland Stanford Junior University | Methods and transformed mammalian lymphocyte cells for producing functional antigen-binding protein including chimeric immunoglobulin |
| WO1986005807A1 (fr) | 1985-04-01 | 1986-10-09 | Celltech Limited | Lignee cellulaire de myelomes transformee et procede d'expression d'un gene codant un polypeptide eucaryotque employant cette lignee |
| US5122464A (en) | 1986-01-23 | 1992-06-16 | Celltech Limited, A British Company | Method for dominant selection in eucaryotic cells |
| US5225539A (en) | 1986-03-27 | 1993-07-06 | Medical Research Council | Recombinant altered antibodies and methods of making altered antibodies |
| EP0239400A2 (fr) | 1986-03-27 | 1987-09-30 | Medical Research Council | Anticorps recombinants et leurs procédés de production |
| US5869620A (en) | 1986-09-02 | 1999-02-09 | Enzon, Inc. | Multivalent antigen-binding proteins |
| US5624821A (en) | 1987-03-18 | 1997-04-29 | Scotgen Biopharmaceuticals Incorporated | Antibodies with altered effector functions |
| US5648260A (en) | 1987-03-18 | 1997-07-15 | Scotgen Biopharmaceuticals Incorporated | DNA encoding antibodies with altered effector functions |
| WO1989001036A1 (fr) | 1987-07-23 | 1989-02-09 | Celltech Limited | Vecteurs d'expression a base d'adn recombinant |
| US5677425A (en) | 1987-09-04 | 1997-10-14 | Celltech Therapeutics Limited | Recombinant antibody |
| US5223409A (en) | 1988-09-02 | 1993-06-29 | Protein Engineering Corp. | Directed evolution of novel binding proteins |
| WO1990002809A1 (fr) | 1988-09-02 | 1990-03-22 | Protein Engineering Corporation | Production et selection de proteines de liaison diversifiees de recombinaison |
| US5403484A (en) | 1988-09-02 | 1995-04-04 | Protein Engineering Corporation | Viruses expressing chimeric binding proteins |
| US5571698A (en) | 1988-09-02 | 1996-11-05 | Protein Engineering Corporation | Directed evolution of novel binding proteins |
| US5585089A (en) | 1988-12-28 | 1996-12-17 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5413923A (en) | 1989-07-25 | 1995-05-09 | Cell Genesys, Inc. | Homologous recombination for universal donor cells and chimeric mammalian hosts |
| WO1991009967A1 (fr) | 1989-12-21 | 1991-07-11 | Celltech Limited | Anticorps humanises |
| US6071495A (en) | 1989-12-22 | 2000-06-06 | Imarx Pharmaceutical Corp. | Targeted gas and gaseous precursor-filled liposomes |
| WO1991010737A1 (fr) | 1990-01-11 | 1991-07-25 | Molecular Affinities Corporation | Production d'anticorps utilisant des librairies de genes |
| US5780225A (en) | 1990-01-12 | 1998-07-14 | Stratagene | Method for generating libaries of antibody genes comprising amplification of diverse antibody DNAs and methods for using these libraries for the production of diverse antigen combining molecules |
| US5939598A (en) | 1990-01-12 | 1999-08-17 | Abgenix, Inc. | Method of making transgenic mice lacking endogenous heavy chains |
| WO1991010741A1 (fr) | 1990-01-12 | 1991-07-25 | Cell Genesys, Inc. | Generation d'anticorps xenogeniques |
| US6075181A (en) | 1990-01-12 | 2000-06-13 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| US5900252A (en) | 1990-04-17 | 1999-05-04 | Eurand International S.P.A. | Method for targeted and controlled release of drugs in the intestinal tract and more particularly in the colon |
| US5427908A (en) | 1990-05-01 | 1995-06-27 | Affymax Technologies N.V. | Recombinant library screening methods |
| US5580717A (en) | 1990-05-01 | 1996-12-03 | Affymax Technologies N.V. | Recombinant library screening methods |
| US5969108A (en) | 1990-07-10 | 1999-10-19 | Medical Research Council | Methods for producing members of specific binding pairs |
| WO1992001047A1 (fr) | 1990-07-10 | 1992-01-23 | Cambridge Antibody Technology Limited | Procede de production de chainon de paires a liaison specifique |
| US5545806A (en) | 1990-08-29 | 1996-08-13 | Genpharm International, Inc. | Ransgenic non-human animals for producing heterologous antibodies |
| US5569825A (en) | 1990-08-29 | 1996-10-29 | Genpharm International | Transgenic non-human animals capable of producing heterologous antibodies of various isotypes |
| US5661016A (en) | 1990-08-29 | 1997-08-26 | Genpharm International Inc. | Transgenic non-human animals capable of producing heterologous antibodies of various isotypes |
| US5625126A (en) | 1990-08-29 | 1997-04-29 | Genpharm International, Inc. | Transgenic non-human animals for producing heterologous antibodies |
| US5814318A (en) | 1990-08-29 | 1998-09-29 | Genpharm International Inc. | Transgenic non-human animals for producing heterologous antibodies |
| US5633425A (en) | 1990-08-29 | 1997-05-27 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| US5698426A (en) | 1990-09-28 | 1997-12-16 | Ixsys, Incorporated | Surface expression libraries of heteromeric receptors |
| US5840674A (en) | 1990-11-01 | 1998-11-24 | Oregon Health Sciences University | Covalent microparticle-drug conjugates for biological targeting |
| US5821047A (en) | 1990-12-03 | 1998-10-13 | Genentech, Inc. | Monovalent phage display |
| WO1992018619A1 (fr) | 1991-04-10 | 1992-10-29 | The Scripps Research Institute | Banques de recepteurs heterodimeres utilisant des phagemides |
| US5658727A (en) | 1991-04-10 | 1997-08-19 | The Scripps Research Institute | Heterodimeric receptor libraries using phagemids |
| EP0519596A1 (fr) | 1991-05-17 | 1992-12-23 | Merck & Co. Inc. | Procédé pour réduire l'immunogénécité des domaines variables d'anticorps |
| WO1992022324A1 (fr) | 1991-06-14 | 1992-12-23 | Xoma Corporation | Fragments d'anticorps produits par des microbes et leurs conjugues |
| US6407213B1 (en) | 1991-06-14 | 2002-06-18 | Genentech, Inc. | Method for making humanized antibodies |
| US5693780A (en) | 1991-07-25 | 1997-12-02 | Idec Pharmaceuticals Corporation | Recombinant antibodies for human therapy |
| US5565332A (en) | 1991-09-23 | 1996-10-15 | Medical Research Council | Production of chimeric antibodies - a combinatorial approach |
| WO1993011236A1 (fr) | 1991-12-02 | 1993-06-10 | Medical Research Council | Production d'anticorps anti-auto-antigenes a partir de repertoires de segments d'anticorps affiches sur phage |
| US5885793A (en) | 1991-12-02 | 1999-03-23 | Medical Research Council | Production of anti-self antibodies from antibody segment repertoires and displayed on phage |
| US5766886A (en) | 1991-12-13 | 1998-06-16 | Xoma Corporation | Modified antibody variable domains |
| WO1993017105A1 (fr) | 1992-02-19 | 1993-09-02 | Scotgen Limited | Anticorps modifies, produits et procedes s'y rapportant |
| US5733743A (en) | 1992-03-24 | 1998-03-31 | Cambridge Antibody Technology Limited | Methods for producing members of specific binding pairs |
| US5585097A (en) | 1992-03-24 | 1996-12-17 | British Technology Group Limited | Humanized anti-CD3 specific antibodies |
| US6174666B1 (en) | 1992-03-27 | 2001-01-16 | The United States Of America As Represented By The Department Of Health And Human Services | Method of eliminating inhibitory/instability regions from mRNA |
| US6794498B2 (en) | 1992-03-27 | 2004-09-21 | The United States Of America As Represented By The Department Of Health And Human Services | Method of eliminating inhibitory/instability regions of mRNA |
| US6414132B1 (en) | 1992-03-27 | 2002-07-02 | The United States Of America As Represented By The Department Of Health And Human Services | Method of eliminating inhibitory/instability regions of mRNA |
| US6291664B1 (en) | 1992-03-27 | 2001-09-18 | The United States Of America As Represented By The Department Of Health And Human Services | Method of eliminating inhibitory/instability regions of mRNA |
| US5965726A (en) | 1992-03-27 | 1999-10-12 | The United States Of America As Represented By The Department Of Health And Human Services | Method of eliminating inhibitory/ instability regions of mRNA |
| US6010715A (en) | 1992-04-01 | 2000-01-04 | Bertek, Inc. | Transdermal patch incorporating a polymer film incorporated with an active agent |
| US6024975A (en) | 1992-04-08 | 2000-02-15 | Americare International Diagnostics, Inc. | Method of transdermally administering high molecular weight drugs with a polymer skin enhancer |
| US6165745A (en) | 1992-04-24 | 2000-12-26 | Board Of Regents, The University Of Texas System | Recombinant production of immunoglobulin-like domains in prokaryotic cells |
| WO1994004678A1 (fr) | 1992-08-21 | 1994-03-03 | Casterman Cecile | Immunoglobulines exemptes de chaines legeres |
| US6005079A (en) | 1992-08-21 | 1999-12-21 | Vrije Universiteit Brussels | Immunoglobulins devoid of light chains |
| EP0592106A1 (fr) | 1992-09-09 | 1994-04-13 | Immunogen Inc | Remodelage d'anticorps des rongeurs |
| US5837242A (en) | 1992-12-04 | 1998-11-17 | Medical Research Council | Multivalent and multispecific binding proteins, their manufacture and use |
| US6132992A (en) | 1993-02-01 | 2000-10-17 | Bristol-Myers Squibb Co. | Expression vectors encoding bispecific fusion proteins and methods of producing biologically active bispecific fusion proteins in a mammalian cell |
| US6274552B1 (en) | 1993-03-18 | 2001-08-14 | Cytimmune Sciences, Inc. | Composition and method for delivery of biologically-active factors |
| US5709874A (en) | 1993-04-14 | 1998-01-20 | Emory University | Device for local drug delivery and methods for using the same |
| US5985307A (en) | 1993-04-14 | 1999-11-16 | Emory University | Device and method for non-occlusive localized drug delivery |
| WO1994025591A1 (fr) | 1993-04-29 | 1994-11-10 | Unilever N.V. | PRODUCTION D'ANTICORPS OU DE FRAGMENTS FONCTIONNALISES D'ANTICORPS, DERIVES DES IMMUNOGLOBULINES A CHAINE LOURDE DE $i(CAMELIDAE) |
| WO1994029351A2 (fr) | 1993-06-16 | 1994-12-22 | Celltech Limited | Anticorps |
| WO1995001997A1 (fr) | 1993-07-09 | 1995-01-19 | Smithkline Beecham Corporation | ANTICORPS RECOMBINANTS ET HUMANISES, DIRIGES CONTRE L'IL-1β ET DESTINES AU TRAITEMENT DE TROUBLES INFLAMMATOIRES INDUITS PAR IL-1 CHEZ L'HOMME |
| US6004534A (en) | 1993-07-23 | 1999-12-21 | Massachusetts Institute Of Technology | Targeted polymerized liposomes for improved drug delivery |
| WO1995015982A2 (fr) | 1993-12-08 | 1995-06-15 | Genzyme Corporation | Procede de generation d'anticorps specifiques |
| US7029678B2 (en) | 1993-12-23 | 2006-04-18 | Smithkline Beecham Biologicals (S.A.) | Vaccines |
| US6610659B1 (en) | 1994-01-13 | 2003-08-26 | Mount Sinai School Of Medicine Of New York University | Use of heat shock protein 70 preparations in vaccination against cancer and infectious disease |
| WO1995020401A1 (fr) | 1994-01-31 | 1995-08-03 | Trustees Of Boston University | Banques d'anticorps polyclonaux |
| US6143273A (en) | 1994-05-06 | 2000-11-07 | Institut Gustave Roussy | Therapeutic composition containing antibodies to soluble polypeptide fractions of LAG-3 protein |
| US5516637A (en) | 1994-06-10 | 1996-05-14 | Dade International Inc. | Method involving display of protein binding pairs on the surface of bacterial pili and bacteriophage |
| US5759542A (en) | 1994-08-05 | 1998-06-02 | New England Deaconess Hospital Corporation | Compositions and methods for the delivery of drugs by platelets for the treatment of cardiovascular and other diseases |
| US5972366A (en) | 1994-11-28 | 1999-10-26 | The Unites States Of America As Represented By The Secretary Of The Army | Drug releasing surgical implant or dressing material |
| US6121022A (en) | 1995-04-14 | 2000-09-19 | Genentech, Inc. | Altered polypeptides with increased half-life |
| US5869046A (en) | 1995-04-14 | 1999-02-09 | Genentech, Inc. | Altered polypeptides with increased half-life |
| US5983134A (en) | 1995-04-23 | 1999-11-09 | Electromagnetic Bracing Systems Inc. | Electrophoretic cuff apparatus drug delivery system |
| WO1996033735A1 (fr) | 1995-04-27 | 1996-10-31 | Abgenix, Inc. | Anticorps humains derives d'une xenosouris immunisee |
| WO1996034096A1 (fr) | 1995-04-28 | 1996-10-31 | Abgenix, Inc. | Anticorps humains derives de xeno-souris immunisees |
| US6316652B1 (en) | 1995-06-06 | 2001-11-13 | Kosta Steliou | Drug mitochondrial targeting agents |
| US6197524B1 (en) | 1995-07-21 | 2001-03-06 | Institute National De La Sante Et De La Recherche Medicale | Methods for detecting, identifying, isolating, and selectively labelling and targeting TH1 lymphocyte by means of the LAG-3 protein |
| US6167301A (en) | 1995-08-29 | 2000-12-26 | Flower; Ronald J. | Iontophoretic drug delivery device having high-efficiency DC-to-DC energy conversion circuit |
| US6447781B1 (en) | 1995-09-13 | 2002-09-10 | Fordham University | Therapeutic and prophylactic methods using heat shock proteins |
| WO1997013844A1 (fr) | 1995-10-06 | 1997-04-17 | Cambridge Antibody Technology Limited | Elements de fixation specifiques destines au facteur beta humain de croissance transformant, materiaux et procedes associes |
| US5989830A (en) | 1995-10-16 | 1999-11-23 | Unilever Patent Holdings Bv | Bifunctional or bivalent antibody fragment analogue |
| US6039975A (en) | 1995-10-17 | 2000-03-21 | Hoffman-La Roche Inc. | Colon targeted delivery system |
| US5750753A (en) | 1996-01-24 | 1998-05-12 | Chisso Corporation | Method for manufacturing acryloxypropysilane |
| WO1997034631A1 (fr) | 1996-03-18 | 1997-09-25 | Board Of Regents, The University Of Texas System | Domaines analogues a l'immunoglobuline a demi-vies prolongees |
| US6253872B1 (en) | 1996-05-29 | 2001-07-03 | Gmundner Fertigteile Gesellschaft M.B.H & Co., Kg | Track soundproofing arrangement |
| US6150184A (en) | 1996-08-20 | 2000-11-21 | Ramtron International Corporation | Method of fabricating partially or completely encapsulated top electrode of a ferroelectric capacitor |
| US5985317A (en) | 1996-09-06 | 1999-11-16 | Theratech, Inc. | Pressure sensitive adhesive matrix patches for transdermal delivery of salts of pharmaceutical agents |
| US6139865A (en) | 1996-10-01 | 2000-10-31 | Eurand America, Inc. | Taste-masked microcapsule compositions and methods of manufacture |
| WO1998016654A1 (fr) | 1996-10-11 | 1998-04-23 | Japan Tobacco, Inc. | Production de proteine multimere par procede de fusion cellulaire |
| WO1998023289A1 (fr) | 1996-11-27 | 1998-06-04 | The General Hospital Corporation | Modulation de la fixation de l'igg au fcrn |
| WO1998024893A2 (fr) | 1996-12-03 | 1998-06-11 | Abgenix, Inc. | MAMMIFERES TRANSGENIQUES POSSEDANT DES LOCI DE GENES D'IMMUNOGLOBULINE D'ORIGINE HUMAINE, DOTES DE REGIONS VH ET Vλ, ET ANTICORPS PRODUITS A PARTIR DE TELS MAMMIFERES |
| US6436404B1 (en) | 1997-02-07 | 2002-08-20 | Fordham University | Prevention of primary and metastatic neoplastic diseases with GP96-peptide complexes |
| US6391306B1 (en) | 1997-02-07 | 2002-05-21 | Fordham University | Treatment of infectious diseases with hsp90-peptide complexes |
| US6447780B1 (en) | 1997-02-07 | 2002-09-10 | Fordham University | Prevention of primary and metastatic neoplastic diseases with hsp90-peptide complexes |
| US5860957A (en) | 1997-02-07 | 1999-01-19 | Sarcos, Inc. | Multipathway electronically-controlled drug delivery system |
| US6383492B1 (en) | 1997-02-07 | 2002-05-07 | Fordham University | Treatment of infectious diseases with gp96-peptide complexes |
| US6403095B1 (en) | 1997-02-07 | 2002-06-11 | Fordham University | Treatment of primary and metastatic neoplastic diseases with HSP70-peptide complexes |
| US6277375B1 (en) | 1997-03-03 | 2001-08-21 | Board Of Regents, The University Of Texas System | Immunoglobulin-like domains with increased half-lives |
| US6120751A (en) | 1997-03-21 | 2000-09-19 | Imarx Pharmaceutical Corp. | Charged lipids and uses for the same |
| US6207156B1 (en) | 1997-03-21 | 2001-03-27 | Brigham And Women's Hospital, Inc. | Specific antibodies and antibody fragments |
| WO1998046645A2 (fr) | 1997-04-14 | 1998-10-22 | Micromet Gesellschaft Für Biomedizinische Forschung Mbh | Nouveau procede de production de recepteurs d'anti-antigenes humains et leur utilisation |
| US6060082A (en) | 1997-04-18 | 2000-05-09 | Massachusetts Institute Of Technology | Polymerized liposomes targeted to M cells and useful for oral or mucosal drug delivery |
| US7183076B2 (en) | 1997-05-02 | 2007-02-27 | Genentech, Inc. | Method for making multispecific antibodies having heteromultimeric and common components |
| US7951917B1 (en) | 1997-05-02 | 2011-05-31 | Genentech, Inc. | Method for making multispecific antibodies having heteromultimeric and common components |
| WO1998050433A2 (fr) | 1997-05-05 | 1998-11-12 | Abgenix, Inc. | Anticorps monoclonaux humains contre le recepteur du facteur de croissance epidermique |
| US5948433A (en) | 1997-08-21 | 1999-09-07 | Bertek, Inc. | Transdermal patch |
| US6645495B1 (en) | 1997-08-29 | 2003-11-11 | Antigenics, Inc. | Compositions of saponin adjuvants and excipients |
| US6267983B1 (en) | 1997-10-28 | 2001-07-31 | Bando Chemical Industries, Ltd. | Dermatological patch and process for producing thereof |
| US6410026B1 (en) | 1997-12-11 | 2002-06-25 | Fordham University | Methods for preparation of vaccines against cancer |
| US6194551B1 (en) | 1998-04-02 | 2001-02-27 | Genentech, Inc. | Polypeptide variants |
| US20050208625A1 (en) | 1998-04-28 | 2005-09-22 | Taylor Alexander H | Monoclonal antibodies with reduced immunogenicity |
| US6048736A (en) | 1998-04-29 | 2000-04-11 | Kosak; Kenneth M. | Cyclodextrin polymers for carrying and releasing drugs |
| US6131570A (en) | 1998-06-30 | 2000-10-17 | Aradigm Corporation | Temperature controlling device for aerosol drug delivery |
| US7858589B2 (en) | 1998-08-10 | 2010-12-28 | Antigenics Inc. | Compositions of CpG and saponin adjuvants and uses thereof |
| WO2000037504A2 (fr) | 1998-12-23 | 2000-06-29 | Pfizer Inc. | Anticorps monoclonaux humains diriges contre l'antigene ctla-4 |
| US6737056B1 (en) | 1999-01-15 | 2004-05-18 | Genentech, Inc. | Polypeptide variants with altered effector function |
| WO2000042072A2 (fr) | 1999-01-15 | 2000-07-20 | Genentech, Inc. | Variants polypeptidiques ayant une fonction effectrice alteree |
| US6271359B1 (en) | 1999-04-14 | 2001-08-07 | Musc Foundation For Research Development | Tissue-specific and pathogen-specific toxic agents and ribozymes |
| US6256533B1 (en) | 1999-06-09 | 2001-07-03 | The Procter & Gamble Company | Apparatus and method for using an intracutaneous microneedle array |
| US6808710B1 (en) | 1999-08-23 | 2004-10-26 | Genetics Institute, Inc. | Downmodulating an immune response with multivalent antibodies to PD-1 |
| WO2001014424A2 (fr) | 1999-08-24 | 2001-03-01 | Medarex, Inc. | Anticorps contre l'antigene ctla-4 humain et utilisation |
| WO2001044301A1 (fr) | 1999-11-29 | 2001-06-21 | Unilever Plc | Immobilisation de molecules de liaison d'antigene a domaine unique |
| US7034121B2 (en) | 2000-01-27 | 2006-04-25 | Genetics Institue, Llc | Antibodies against CTLA4 |
| US6261595B1 (en) | 2000-02-29 | 2001-07-17 | Zars, Inc. | Transdermal drug patch with attached pocket for controlled heating device |
| US7658921B2 (en) | 2000-12-12 | 2010-02-09 | Medimmune, Llc | Molecules with extended half-lives, compositions and uses thereof |
| WO2002060919A2 (fr) | 2000-12-12 | 2002-08-08 | Medimmune, Inc. | Molecules a demi-vies longues, compositions et utilisations de celles-ci |
| US20030086930A1 (en) | 2001-05-23 | 2003-05-08 | Mueller Eileen E. | Uses of anti-CTLA-4 antibodies |
| US7709226B2 (en) | 2001-07-12 | 2010-05-04 | Arrowsmith Technology Licensing Llc | Method of humanizing antibodies by matching canonical structure types CDRs |
| US20030232323A1 (en) | 2001-11-13 | 2003-12-18 | Wyeth | Agents that modulate immune cell activation and methods of use thereof |
| US8101176B2 (en) | 2002-01-30 | 2012-01-24 | The Brigham And Women's Hospital, Inc. | Compositions and methods related to TIM-3, a TH1-specific cell surface molecule |
| US7470428B2 (en) | 2002-01-30 | 2008-12-30 | The Brigham And Women's Hospital, Inc. | Compositions and methods related to TIM-3, a Th1-specific cell surface molecule |
| US20040014194A1 (en) | 2002-03-27 | 2004-01-22 | Schering Corporation | Beta-secretase crystals and methods for preparing and using the same |
| US8142778B2 (en) | 2002-04-12 | 2012-03-27 | Medarex, Inc. | Methods of treatment using CTLA-4 antibodies |
| US7550140B2 (en) | 2002-06-13 | 2009-06-23 | Crucell Holland B.V. | Antibody to the human OX40 receptor |
| US8168179B2 (en) | 2002-07-03 | 2012-05-01 | Ono Pharmaceutical Co., Ltd. | Treatment method using anti-PD-L1 antibody |
| US7807156B1 (en) | 2002-09-11 | 2010-10-05 | La Jolla Institute For Allergy And Immunology | Methods of treating OX40 mediated recall immune responses |
| US7531170B1 (en) | 2002-09-11 | 2009-05-12 | La Jolla Institute For Allergy And Immunology | Methods of treating OX40 mediated recall immune responses and agents useful for identifying same |
| US7488802B2 (en) | 2002-12-23 | 2009-02-10 | Wyeth | Antibodies against PD-1 |
| US20050048549A1 (en) | 2003-01-21 | 2005-03-03 | Liangxian Cao | Methods and agents for screening for compounds capable of modulating gene expression |
| US20100196394A1 (en) | 2003-02-28 | 2010-08-05 | The Johns Hopkins University | Anti-cancer vaccine composition |
| US7465446B2 (en) | 2003-05-30 | 2008-12-16 | Medarex, Inc. | Surrogate therapeutic endpoint for anti-CTLA4-based immunotherapy of disease |
| US20050042664A1 (en) | 2003-08-22 | 2005-02-24 | Medimmune, Inc. | Humanization of antibodies |
| US8541002B2 (en) | 2003-09-12 | 2013-09-24 | Agenus Inc. | Vaccine for treatment and prevention of herpes simplex virus infection |
| US20050226875A1 (en) | 2004-03-26 | 2005-10-13 | Pfizer Inc | Uses of anti-CTLA-4 antibodies |
| US20090123477A1 (en) | 2004-12-23 | 2009-05-14 | Thomas Hanke | Antibodies |
| WO2006105021A2 (fr) | 2005-03-25 | 2006-10-05 | Tolerrx, Inc. | Molecules de liaison gitr et leurs utilisations |
| US7812135B2 (en) | 2005-03-25 | 2010-10-12 | Tolerrx, Inc. | GITR-binding antibodies |
| US8008449B2 (en) | 2005-05-09 | 2011-08-30 | Medarex, Inc. | Human monoclonal antibodies to programmed death 1 (PD-1) and methods for treating cancer using anti-PD-1 antibodies alone or in combination with other immunotherapeutics |
| US20110305713A1 (en) | 2005-10-21 | 2011-12-15 | Medical College Of Georgia Research Institute, Inc | Methods and compositions to enhance vaccine efficacy by reprogramming regulatory t cells |
| US20120142750A1 (en) | 2005-10-21 | 2012-06-07 | Regents Of The University Of Minnesota | Indoleamine 2,3-dioxygenase pathways in the generation of regulatory t cells |
| US20070243184A1 (en) | 2005-11-08 | 2007-10-18 | Steven Fischkoff | Prophylaxis and treatment of enterocolitis associated with anti-ctla-4 antibody therapy |
| US20100196359A1 (en) | 2005-11-25 | 2010-08-05 | Shinichiro Kato | Human Monoclonal Antibody Human CD134 (Ox40) and Methods of Making and Using Same |
| US8283450B2 (en) | 2005-11-25 | 2012-10-09 | Kyowa Hakko Kirin Co., Ltd. | Human monoclonal antibody human CD134 (OX40) and methods of making and using same |
| US20110044953A1 (en) | 2006-03-30 | 2011-02-24 | The Regents Of The University Of California | Methods and Compositions for Localized Secretion of Anti-CTLA-4 Antibodies |
| US20090214533A1 (en) | 2006-08-17 | 2009-08-27 | The Trustees Of Columbia University In The City Of New York | Methods for converting or inducing protective immunity |
| US20080152665A1 (en) | 2006-09-01 | 2008-06-26 | Claude Leclerc | Therapy of cancer based on targeting adaptive, innate and/or regulatory component of the immune response |
| US20100061992A1 (en) | 2006-11-15 | 2010-03-11 | The Brigham And Women's Hospital, Inc. | Therapeutic uses of tim-3 modulators |
| US20100136030A1 (en) | 2007-02-27 | 2010-06-03 | Lamhamedi-Cherradi Salah-Eddine | Antagonist ox40 antibodies and their use in the treatment of inflammatory and autoimmune diseases |
| US20090004213A1 (en) | 2007-03-26 | 2009-01-01 | Immatics Biotechnologies Gmbh | Combination therapy using active immunotherapy |
| US20100233183A1 (en) | 2007-04-30 | 2010-09-16 | Frederic Triebel | Cytotoxic anti-lag-3 monoclonal antibody and its use in the treatment or prevention of organ transplant rejection and autoimmune disease |
| US8591886B2 (en) | 2007-07-12 | 2013-11-26 | Gitr, Inc. | Combination therapies employing GITR binding molecules |
| US20100278844A1 (en) | 2007-09-18 | 2010-11-04 | Frances Balkwill | Cancer Marker and Therapeutic Target |
| US8227577B2 (en) | 2007-12-21 | 2012-07-24 | Hoffman-La Roche Inc. | Bivalent, bispecific antibodies |
| US8263073B2 (en) | 2008-02-04 | 2012-09-11 | Medarex, Inc. | Anti-CTLA-4 antibodies with reduced blocking of binding of CTLA-4 to B7 and uses thereof |
| WO2009100140A1 (fr) | 2008-02-04 | 2009-08-13 | Medarex, Inc. | Anticorps anti-clta-4 avec blocage réduit de la liaison de ctla-4 à b7 et leurs utilisations |
| US8168757B2 (en) | 2008-03-12 | 2012-05-01 | Merck Sharp & Dohme Corp. | PD-1 binding proteins |
| WO2010005958A2 (fr) | 2008-07-08 | 2010-01-14 | Incyte Corporation | 1,2,5-oxadiazoles constituant des inhibiteurs de l'indoleamine 2,3-dioxygénase |
| US8226946B2 (en) | 2008-08-06 | 2012-07-24 | Light Sciences Oncology, Inc. | Enhancement of light activated therapy by immune augmentation using anti-CTLA-4 antibody |
| US20110150892A1 (en) | 2008-08-11 | 2011-06-23 | Medarex, Inc. | Human antibodies that bind lymphocyte activation gene-3 (lag-3) and uses thereof |
| US8114845B2 (en) | 2008-08-25 | 2012-02-14 | Amplimmune, Inc. | Compositions of PD-1 antagonists and methods of use |
| US20100100131A1 (en) | 2008-10-21 | 2010-04-22 | K2M, Inc. | Spinal buttress plate |
| US20100203056A1 (en) | 2008-12-09 | 2010-08-12 | Genentech, Inc. | Anti-pd-l1 antibodies and their use to enhance t-cell function |
| US8614295B2 (en) | 2009-02-17 | 2013-12-24 | Ucb Pharma S.A. | Antibody molecules having specificity for human OX40 |
| US8586713B2 (en) | 2009-06-26 | 2013-11-19 | Regeneron Pharmaceuticals, Inc. | Readily isolated bispecific antibodies with native immunoglobulin format |
| US20120219559A1 (en) | 2009-08-13 | 2012-08-30 | The Johns Hopkins University | Methods of modulating immune function |
| US20130202623A1 (en) | 2010-02-16 | 2013-08-08 | Nicolas Chomont | Pd-1 modulation and uses thereof for modulating hiv replication |
| US20130323283A1 (en) | 2010-12-01 | 2013-12-05 | The Children's Hospital Of Philadelphia | Compositions and methods for treating foxp3+ treg related diseases |
| US20130022623A1 (en) | 2011-07-01 | 2013-01-24 | Cellerant Therapeutics, Inc. | Antibodies that specifically bind to tim3 |
| US20130183315A1 (en) | 2011-07-11 | 2013-07-18 | Glenmark Pharmaceuticals S.A. | Antibodies that bind to OX40 and their uses |
| WO2013033091A1 (fr) | 2011-09-01 | 2013-03-07 | Halo Maritime Defense Systems | Barrage marin et porte marine |
| WO2013039954A1 (fr) * | 2011-09-14 | 2013-03-21 | Sanofi | Anticorps anti-gitr |
Non-Patent Citations (184)
| Title |
|---|
| "Antibody:antigen crystals may be studied using well known X-ray diffraction techniques and may be refined using computer software such as X-PLOR", 1992, MOLECULAR SIMULATIONS, INC. |
| "Antibody:antigen crystals may be studied using well known X-ray diffraction techniques and may be refined using computer software such as X-PLOR", YALE UNIVERSITY, 1992 |
| "Biochemica", ROCHE MOLECULAR BIOCHEMICALS, vol. 2, 1999, pages 34 37 |
| "Current Protocols in Human Genetics", 1994, JOHN WILEY & SONS |
| "Current Protocols in Immunology", 1987, JOHN WILEY & SONS |
| "Current Protocols in Molecular Biology", 1993, JOHN WILEY & SONS |
| "Current Protocols in Molecular Biology", vol. I, 1989, GREEN PUBLISHING ASSOCIATES, INC. AND JOHN WILEY & SONS, INC., pages: 6.3.1 - 6.3.6,2.10.3 |
| "GenBank", Database accession no. 683700 |
| "GenBank", Database accession no. AF125303 |
| "GenBank", Database accession no. BC152381 |
| "GENBANK", Database accession no. BC152386 |
| "GenBank", Database accession no. NP 00418 |
| "GenBank", Database accession no. NP 005083 |
| "GenBank", Database accession no. NP 683699 |
| "Remington's Pharmaceutical Sciences", 1990, MACK PUBLISHING CO. |
| ABDICHE YN ET AL., ANALYTICAL BIOCHEM, vol. 386, 2009, pages 172 - 180 |
| ABDICHE YN ET AL., ANALYTICAL BIOCHEMISTRY, vol. 386, 2009, pages 172 - 180 |
| AL-LAZIKANI B ET AL., J MOL BIOL, vol. 273, 1997, pages 927 - 948 |
| ALTSCHUL SF ET AL., J MOL BIOL, vol. 215, 1990, pages 403 |
| ALTSCHUL SF ET AL., NUC ACIDS RES, vol. 25, 1997, pages 3389 3402 |
| AMES RS ET AL., J IMMUNOL METHODS, vol. 184, 1995, pages 177 - 186 |
| AUSUBEL FM ET AL.,: "Short Protocols in Molecular Biology 5th Ed.", 2002, JOHN WILEY AND SONS, article "Chapter 11" |
| AUSUBEL FM ET AL.: "Current Protocols in Molecular Biology", 1987, JOHN WILEY & SONS |
| BACA M ET AL., J BIOL CHEM, vol. 272, no. 16, 1997, pages 10678 - 84 |
| BEBBINGTON CR; HENTSCHEL CCG: "The use of vectors based on gene amplification for the expression of cloned genes in mammalian cells in DNA cloning", vol. 3, 1987, ACADEMIC PRESS |
| BETTER M ET AL., SCIENCE, vol. 240, 1988, pages 1041 - 1043 |
| BIANCHINI R ET AL., EUR J IMMUNOL, vol. 41, no. 8, 2011, pages 2269 - 78 |
| BIRREN B ET AL.,: "Genome Analysis: A Laboratory Manual", 1999, COLD SPRING HARBOR LABORATORY PRESS |
| BITTER G ET AL., METHODS ENZYMOL., vol. 153, 1987, pages 516 - 544 |
| BRICOGNE G, ACTA CRYSTALLOGR D BIOL CRYSTALLOGR, vol. 49, 1993, pages 37 - 60 |
| BRICOGNE G, ACTA CRYSTALLOGR D BIOL CRYSTALLOGR, vol. 49, no. 1, 1993, pages 37 - 60 |
| BRICOGNE G: "Meth Enzymol", vol. 276A, 1997, pages: 361 - 423 |
| BRINKMAN U ET AL., J IMMUNOL METHODS, vol. 182, 1995, pages 41 - 50 |
| BRODEUR ET AL.: "Monoclonal Antibody Production Techniques and Applications", 1987, MARCEL DEKKER, INC., pages: 51 - 63 |
| BULLIARD Y ET AL., J EXP MED, vol. 210, 2013, pages 1685 - 1693 |
| BURTON DR; BARBAS CF, ADVAN IMMUNOL, vol. 57, 1994, pages 191 - 280 |
| CALDAS C ET AL., PROTEIN ENG., vol. 13, no. 5, 2000, pages 353 - 60 |
| CHAMPE M ET AL., J BIOL CHEM, vol. 270, 1995, pages 1388 - 1394 |
| CHAYEN NE, STRUCTURE, vol. 5, 1997, pages 1269 - 1274 |
| CHEUNG RC ET AL., VIROLOGY, vol. 176, 1990, pages 546 - 52 |
| CHOTHIA C ET AL., J MOL BIOL, vol. 227, 1992, pages 799 - 817 |
| CHOTHIA C; LESK AM, J MOL BIOL, vol. 196, 1987, pages 901 - 917 |
| CHU ET AL., MOLECULAR IMMUNOLOGY, vol. 45, 2008, pages 3926 - 3933 |
| CLACKSON T ET AL., NATURE, vol. 352, 1991, pages 624 - 628 |
| COCKETT MI ET AL., BIOTECHNOLOGY, vol. 8, no. 7, 1990, pages 662 - 7 |
| COE D ET AL., CANCER IMMUNOL IMMUNOTHER, vol. 59, 2010, pages 1367 - 77 |
| COLBERE-GARAPIN F ET AL., J MOL BIOL, vol. 150, 1981, pages 1 - 14 |
| COUTO JR ET AL., CANCER RES, vol. 55, no. 8, 1995, pages 1717 - 22 |
| COUTO JR ET AL., CANCER RES., vol. 55, no. 23, 1995, pages 5973s - 5977s |
| CROUSE GF ET AL., MOL CELL BIOL, vol. 3, 1983, pages 257 - 66 |
| CUNNINGHAM BC; WELLS JA, SCIENCE, vol. 244, 1989, pages 1081 - 1085 |
| CURIEL TJ ET AL., NAT MEDICINE, vol. 10, no. 9, 2004, pages 942 - 9 |
| CUZZOCREA S ET AL., FASEB J, vol. 21, 2007, pages 117 - 129 |
| CUZZOCREA S ET AL., J IMMUNOL, vol. 177, 2006, pages 631 - 641 |
| CUZZOCREA S ET AL., J LEUKOC BIOL, vol. 76, 2004, pages 933 - 940 |
| DALL'ACQUA WF ET AL., J BIOL CHEM, vol. 281, 2006, pages 23514 - 24 |
| DANNULL J ET AL., J CLIN INVEST, vol. 115, no. 12, 2005, pages 3623 - 33 |
| ECKSTEIN: "Oligonucleotides and Analogues: A Practical Approach", 1991, IRL PRESS |
| ED HARLOW E & LANE D: "Antibodies: A Laboratory Manual", pages: 386 - 389 |
| FOECKING MK; HOFSTETTER H, GENE, vol. 45, 1986, pages 101 - 5 |
| GAIT: "Oligonucleotide Synthesis: A Practical Approach", 1984, IRL PRESS |
| GIEGE R ET AL., ACTA CRYSTALLOGR D BIOL CRYSTALLOGR, vol. 5, 1994, pages 339 - 350 |
| GIEGE R ET AL., ACTA CRYSTALLOGR D BIOL CRYSTALLOGR, vol. 50, 1994, pages 339 - 350 |
| GILLIES SD ET AL., J IMMUNOL METHODS, vol. 125, 1989, pages 191 - 202 |
| GODING JW: "Monoclonal Antibodies: Principles and Practice", 1986, ACADEMIC PRESS, pages: 59 - 103 |
| GREENSPAN NS; BONA CA, FASEB J, vol. 7, no. 5, 1989, pages 437 - 444 |
| GURNEY AL ET AL., CURR BIOL, vol. 9, 1999, pages 215 - 218 |
| HAMMERLING GJ ET AL.: "Monoclonal Antibodies and T-Cell Hybridomas", 1981, ELSEVIER, pages: 563 681 |
| HANABUCHI S ET AL., BLOOD, vol. 107, 2006, pages 3617 - 3623 |
| HARLOW E; LANE D: "Antibodies: A Laboratory Manual", 1988, COLD SPRING HARBOR PRESS |
| HARLOW E; LANE D: "Antibodies: A Laboratory Manual, 2nd ed.", 1988, COLD SPRING HARBOR LABORATORY PRESS |
| IMAI-NISHIYA H ET AL., BMC BIOTECHNOL., vol. 7, 2007, pages 84 |
| INOUYE S; INOUYE M, NUC ACIDS RES, vol. 13, 1985, pages 3101 - 3109 |
| JOHNSON G ET AL., NUCLEIC ACIDS RES, vol. 28, 2000, pages 214 - 218 |
| KAAS Q ET AL., BRIEFINGS IN FUNCTIONAL GENOMICS & PROTEOMICS, vol. 6, no. 4, 2007, pages 253 - 64 |
| KABAT EA ET AL.: "Sequences of Proteins of Immunological Interest, Fifth Edition,", 1991, U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES, NIH |
| KABAT EA; WU TT, ANN NY ACAD SCI, vol. 190, 1971, pages 382 - 391 |
| KABAT ET AL.: "Sequences of Proteins of Immunological Interest", 1991, U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES |
| KARLIN S; ALTSCHUL SF, PNAS, vol. 87, 1990, pages 2264 - 2268 |
| KARLIN S; ALTSCHUL SF, PNAS, vol. 90, 1993, pages 5873 - 5877 |
| KETTLEBOROUGH CA ET AL., EUR J IMMUNOL, vol. 24, 1994, pages 952 - 958 |
| KILPATRICK KE ET AL., HYBRIDOMA, vol. 16, 1997, pages 381 - 9 |
| KIM SJ; HONG HJ, J MICROBIOL, vol. 45, 2007, pages 572 - 577 |
| KIRKLAND TN ET AL., J IMMUNOL, vol. 137, 1986, pages 3614 - 9 |
| KOBER J ET AL., EUR J IMMUNOL, vol. 38, no. 10, 2008, pages 2678 - 88 |
| KOHLER G, PNAS, vol. 77, 1980, pages 2197 - 2199 |
| KOHLER G; MILSTEIN C, NATURE, vol. 256, 1975, pages 495 |
| KOZBOR D, J IMMUNOL, vol. 133, 1984, pages 3001 - 5 |
| KRIEGLER M: "Gene Transfer and Expression, A Laboratory Manual", 1990, STOCKTON PRESS |
| KUROKI M ET AL., CANCER RES, vol. 50, 1990, pages 4872 - 4879 |
| KUROKI M ET AL., HYBRIDOMA, vol. 11, 1992, pages 391 - 407 |
| KUROKI M ET AL., IMMUNOL INVEST, vol. 21, 1992, pages 523 - 538 |
| KUTMEIER G ET AL., BIOTECHNIQUES, vol. 17, 1994, pages 242 - 6 |
| KWON B ET AL., J BIOL CHEM, vol. 274, 1999, pages 6056 - 6061 |
| LAEMMLI UK, NATURE, vol. 227, no. 5259, 1970, pages 680 - 5 |
| LEFRANC MP ET AL., DEV COMPO IMMUNOL, vol. 29, no. 3, 2005, pages 185 - 203 |
| LEFRANC M-P ET AL., NUCLEIC ACIDS RES, vol. 27, 1999, pages 209 - 212 |
| LEFRANC MP ET AL., NUCLEIC ACIDS RES, vol. 27, no. 1, 1999, pages 209 - 12 |
| LEFRANC MP, NUCLEIC ACIDS RES, vol. 29, no. 1, 2001, pages 207 - 9 |
| LEFRANC MP, NUCLEIC ACIDS RES, vol. 31, no. 1, 2003, pages 307 - 10 |
| LEFRANC M-P, THE IMMUNOLOGIST, vol. 7, 1999, pages 132 - 136 |
| LI Z ET AL., J AUTOIMMUN, vol. 21, 2003, pages 83 - 92 |
| LOGAN J; SHENK T, PNAS, vol. 81, no. 12, 1984, pages 3655 - 9 |
| LONBERG N; HUSZAR D, INT REV IMMUNOL, vol. 13, 1995, pages 65 - 93 |
| LONGMORE GD; SCHACHTER H, CARBOHYDR RES, vol. 100, 1982, pages 365 - 92 |
| LOWY I ET AL., CELL, vol. 22, no. 3, 1980, pages 817 - 23 |
| MACCALLUM RM ET AL.: "J Mol Biol", vol. 262, 1996, pages: 732 - 745 |
| MANIATIS T ET AL.: "Molecular Cloning: A Laboratory Manual", 1982, COLD SPRING HARBOR LABORATORY PRESS |
| MARTIN A.: "Antibody Engineering", 2001, SPRINGER-VERLAG, article "Protein Sequence and Structure Analysis of Antibody Variable Domains", pages: 422 - 439 |
| MCHUGH RS ET AL., IMMUNITY, vol. 16, 2002, pages 311 - 323 |
| MCPHERSON A, EUR J BIOCHEM, vol. 189, 1990, pages 1 - 23 |
| MCPHERSON A, J BIOL CHEM, vol. 251, 1976, pages 6300 - 6303 |
| METH ENZYMOL, vol. 114 & 11, 1985 |
| MIMOTO ET AL., PROTEIN ENGINEERING, DESIGN & SELECTION, vol. 10, 2013, pages 589 - 598 |
| MO A. ET AL., VACCINE, vol. 29, 2011, pages 8530 - 8541 |
| MOLDENHAUER G ET AL., SCAND J IMMUNOL, vol. 32, 1990, pages 77 - 82 |
| MOREA V ET AL., METHODS, vol. 20, no. 3, 2000, pages 267 - 79 |
| MOREL GA ET AL., MOL IMMUNOL, vol. 25, no. 1, 1988, pages 7 - 15 |
| MORGAN RA; ANDERSON WF, ANN REV BIOCHEM, vol. 62, 1993, pages 191 - 217 |
| MORRISON SL, SCIENCE, vol. 229, 1985, pages 1202 - 7 |
| MOSMANN T, J IMMUNOL METHODS, vol. 65, 1983, pages 55 - 63 |
| MULLIGAN RC, SCIENCE, vol. 260, 1993, pages 926 - 932 |
| MULLIGAN RC; BERG P, PNAS, vol. 78, no. 4, 1981, pages 2072 - 6 |
| MULLINAX RL ET AL., BIOTECHNIQUES, vol. 12, no. 6, 1992, pages 864 - 9 |
| MUYLDERMANS S, J BIOTECHNOL, vol. 74, no. 4, 2001, pages 277 - 302 |
| MYERS; MILLER, CABIOS, vol. 4, 1988, pages 11 17 |
| NABEL GJ; FEIGNER PL, TRENDS BIOTECHNOL, vol. 11, no. 5, 1993, pages 211 - 5 |
| NAGANAWA Y ET AL., HUMAN ANTIBODIES, vol. 14, 2005, pages 27 - 31 |
| NISSINOFF A, J IMMUNOL, vol. 147, no. 8, 1991, pages 2429 - 2438 |
| NOCENTINI G ET AL., BR J PHARMACOL, vol. 165, 2012, pages 2089 - 99 |
| NOCENTINI G ET AL., EUR J IMMUNOL, vol. 37, 2007, pages 1165 - 1169 |
| NOCENTINI G ET AL., PNAS, vol. 94, 1994, pages 6216 - 6221 |
| NOCENTINI G; RICCARDI C, EUR J IMMUNOL, vol. 35, 2005, pages 1016 - 1022 |
| NUTTALL SD ET AL., CURR PHARM BIOTECHNOL, vol. 1, no. 3, 2000, pages 253 - 263 |
| O'HARE K ET AL., PNAS, vol. 78, 1981, pages 1527 - 31 |
| OI VT; MORRISON SL, BIOTECHNIQUES, vol. 4, 1986, pages 214 - 221 |
| PADLAN EA, MOL IMMUNOL, vol. 28, no. 4/5, 1991, pages 489 - 498 |
| PAGE B ET AL., INTL J ONCOLOGY, vol. 3, 1993, pages 473 - 6 |
| PEDERSEN JT ET AL., J MOL BIOL, vol. 235, no. 3, 1994, pages 959 - 73 |
| PERSIC L ET AL., GENE, vol. 187, 1997, pages 9 - 18 |
| PIAZZA GA ET AL., CANCER RES, vol. 55, 1995, pages 3110 - 6 |
| PROUDFOOT NJ, NATURE, vol. 322, 1986, pages 562 - 565 |
| QUAH BJ ET AL., NAT PROTOC, vol. 2, no. 9, 2007, pages 2049 - 56 |
| RADER C ET AL., PNAS, vol. 95, 1998, pages 8910 - 8915 |
| RETTER I ET AL., NUCLEIC ACIDS RES, vol. 33, 2005, pages D671 - D674 |
| RIECHMANN L; MUYLDERMANS S, J IMMUNOL, vol. 231, 1999, pages 25 - 38 |
| ROGUSKA MA ET AL., PNAS, vol. 91, 1994, pages 969 - 973 |
| ROGUSKA MA ET AL., PROTEIN ENG, vol. 9, no. 10, 1996, pages 895 904 |
| RONCHETI S ET AL., EUR J IMMUNOL, vol. 34, 2004, pages 613 - 622 |
| RONCHETTI S ET AL., BLOOD, vol. 100, 2002, pages 350 - 352 |
| ROVERSI P ET AL., ACTA CRYSTALLOGR D BIOL CRYSTALLOGR, vol. 56, 2000, pages 1316 - 1323 |
| RUETHER U; MUELLER-HILL B, EMBO J, vol. 2, 1983, pages 1791 - 1794 |
| RUIZ M ET AL., NUCLEIC ACIDS RES, vol. 28, no. 1, 2000, pages 219 - 21 |
| SAMBROOK J ET AL.: "Molecular Cloning: A Laboratory Manual", 2001, COLD SPRING HARBOR LABORATORY PRESS |
| SAMBROOK J ET AL.: "Molecular Cloning: A Laboratory Manual, Second Edition,", 1989, COLD SPRING HARBOR LABORATORY PRESS |
| SANDHU JS, GENE, vol. 150, no. 2, 1994, pages 409 - 10 |
| SANTERRE RF ET AL., GENE, vol. 30, no. 1-3, 1984, pages 147 - 56 |
| SAWAI H ET AL., AM J REPROD IMMUNOL, vol. 34, 1995, pages 26 - 34 |
| SHEVACH EM; STEPHENS GL, NAT REV IMMUNOL, vol. 6, 2006, pages 613 - 618 |
| SHIELDS RL ET AL., J BIOL CHEM, vol. 276, 2001, pages 6591 - 604 |
| SHIMIZU J ET AL., NAT IMMUNOL, vol. 3, 2002, pages 135 - 142 |
| SHINMOTO H ET AL., CYTOTECHNOLOGY, vol. 46, 2004, pages 19 - 23 |
| SKEHAN P ET AL., J NAT CANCER INST, vol. 82, 1990, pages 1107 - 12 |
| SMITH P ET AL., PNAS, vol. 109, 2012, pages 6181 - 6186 |
| SNELL LM ET AL., J IMMUNOL, vol. 185, 2010, pages 7223 - 7234 |
| STAHLI C ET AL., METHODS ENZYMOL, vol. 9, 1983, pages 242 - 253 |
| STROHL, CURRENT OPINION IN BIOLOGY, vol. 20, 2009, pages 685 - 691 |
| STUDNICKA GM ET AL., PROT ENGINEERING, vol. 7, no. 6, 1994, pages 805 - 814 |
| SYSTEMS R & D: "Human GITR/TNFRSF18 antibody", INTERNET CITATION, 10 June 2010 (2010-06-10), pages 1, XP002691136, Retrieved from the Internet <URL:http://www.rndsystems.com/pdf/mab689.pdf> [retrieved on 20130128] * |
| SZYBALSKA EH; SZYBALSKI W, PNAS, vol. 48, no. 12, 1962, pages 2026 - 2034 |
| TAN P ET AL., J IMMUNOL, vol. 169, 2002, pages 1119 - 25 |
| TIAN J ET AL., PLOS ONE, vol. 7, no. 10, 2012, pages e46936 |
| TOLSTOSHEV P, ANN REV PHARMACOL TOXICOL, vol. 32, 1993, pages 573 - 596 |
| TONE M ET AL., PNAS, vol. 100, 2003, pages 15059 - 15064 |
| TRAMONTANO A ET AL., J MOL BIOL, vol. 215, no. 1, 1990, pages 175 - 82 |
| VAN HEEKE G; SCHUSTER SM, J BIOL CHEM, vol. 24, 1989, pages 5503 - 5509 |
| WAGENER C ET AL., J IMMUNOL METHODS, vol. 68, 1984, pages 269 - 274 |
| WAGENER C ET AL., J IMMUNOL, vol. 130, 1983, pages 2308 - 2315 |
| WIGLER M ET AL., CELL, vol. 11, no. 1, 1977, pages 223 - 32 |
| WIGLER M ET AL., PNAS, vol. 77, no. 6, 1980, pages 3567 - 70 |
| WOO EY ET AL., J IMMUNOL, vol. 168, 2002, pages 4272 - 6 |
| WU GY; WU CH, BIOTHERAPY, vol. 3, 1991, pages 87 - 95 |
| WYCKOFF HW ET AL., METH. ENZYMOL, vol. 114,115, 1985 |
| YU KY ET AL., BIOCHEM BIOPHYS RES COMMUN, vol. 310, 2003, pages 433 - 438 |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10829559B2 (en) | 2014-05-28 | 2020-11-10 | Agenus Inc. | Anti-GITR antibodies and methods of use thereof |
| US11401335B2 (en) | 2014-05-28 | 2022-08-02 | Agenus Inc. | Anti-GITR antibodies and methods of use thereof |
| US11897962B2 (en) | 2014-05-28 | 2024-02-13 | Agenus Inc. | Anti-GITR antibodies and methods of use thereof |
| US10836830B2 (en) | 2015-12-02 | 2020-11-17 | Agenus Inc. | Antibodies and methods of use thereof |
| US11447557B2 (en) | 2015-12-02 | 2022-09-20 | Agenus Inc. | Antibodies and methods of use thereof |
| US11359028B2 (en) | 2016-11-09 | 2022-06-14 | Agenus Inc. | Anti-OX40 antibodies and anti-GITR antibodies |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11897962B2 (en) | Anti-GITR antibodies and methods of use thereof | |
| US20190135919A1 (en) | Anti-ctla-4 antibodies and methods of use thereof | |
| JP2020500538A (ja) | 抗ctla−4抗体およびそれらの使用方法 | |
| EA040077B1 (ru) | Анти-gitr антитела и способы их применения |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION HAS BEEN PUBLISHED |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 3148579 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: WOLCHOK, JEDD DAVID Inventor name: GONZALEZ, ANA M. Inventor name: MERGHOUB, TAHA Inventor name: TSUJI, TAKEMASA Inventor name: LEGER, OLIVIER Inventor name: RITTER, GERD Inventor name: UNDERWOOD, DENNIS J. Inventor name: ZAPPASODI, ROBERTA Inventor name: WILSON, NICHOLAS S. Inventor name: SEIBERT, VOLKER Inventor name: SCHAER, DAVID Inventor name: VAN DIJK, MARC |
|
| 17P | Request for examination filed |
Effective date: 20191219 |
|
| RAV | Requested validation state of the european patent: fee paid |
Extension state: MA Effective date: 20191219 |
|
| RAX | Requested extension states of the european patent have changed |
Extension state: ME Payment date: 20191219 Extension state: BA Payment date: 20191219 |
|
| RBV | Designated contracting states (corrected) |
Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20200326 |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 40010126 Country of ref document: HK |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230413 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20240416 |