WO2017170395A1 - 導電性塗料及びそれを用いたシールドパッケージの製造方法 - Google Patents
導電性塗料及びそれを用いたシールドパッケージの製造方法 Download PDFInfo
- Publication number
- WO2017170395A1 WO2017170395A1 PCT/JP2017/012380 JP2017012380W WO2017170395A1 WO 2017170395 A1 WO2017170395 A1 WO 2017170395A1 JP 2017012380 W JP2017012380 W JP 2017012380W WO 2017170395 A1 WO2017170395 A1 WO 2017170395A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- conductive paint
- package
- mass
- parts
- conductive
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/02—Elements
- C08K3/04—Carbon
- C08K3/042—Graphene or derivatives, e.g. graphene oxides
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/02—Elements
- C08K3/08—Metals
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K7/00—Use of ingredients characterised by shape
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D163/00—Coating compositions based on epoxy resins; Coating compositions based on derivatives of epoxy resins
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D5/00—Coating compositions, e.g. paints, varnishes or lacquers, characterised by their physical nature or the effects produced; Filling pastes
- C09D5/24—Electrically-conducting paints
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D7/00—Features of coating compositions, not provided for in group C09D5/00; Processes for incorporating ingredients in coating compositions
- C09D7/20—Diluents or solvents
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D7/00—Features of coating compositions, not provided for in group C09D5/00; Processes for incorporating ingredients in coating compositions
- C09D7/40—Additives
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D7/00—Features of coating compositions, not provided for in group C09D5/00; Processes for incorporating ingredients in coating compositions
- C09D7/40—Additives
- C09D7/60—Additives non-macromolecular
- C09D7/61—Additives non-macromolecular inorganic
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D7/00—Features of coating compositions, not provided for in group C09D5/00; Processes for incorporating ingredients in coating compositions
- C09D7/40—Additives
- C09D7/70—Additives characterised by shape, e.g. fibres, flakes or microspheres
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B1/00—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors
- H01B1/20—Conductive material dispersed in non-conductive organic material
- H01B1/22—Conductive material dispersed in non-conductive organic material the conductive material comprising metals or alloys
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L23/00—Details of semiconductor or other solid state devices
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L23/00—Details of semiconductor or other solid state devices
- H01L23/28—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L23/00—Details of semiconductor or other solid state devices
- H01L23/28—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection
- H01L23/29—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection characterised by the material, e.g. carbon
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L23/00—Details of semiconductor or other solid state devices
- H01L23/28—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection
- H01L23/31—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection characterised by the arrangement or shape
-
- H—ELECTRICITY
- H05—ELECTRIC TECHNIQUES NOT OTHERWISE PROVIDED FOR
- H05K—PRINTED CIRCUITS; CASINGS OR CONSTRUCTIONAL DETAILS OF ELECTRIC APPARATUS; MANUFACTURE OF ASSEMBLAGES OF ELECTRICAL COMPONENTS
- H05K9/00—Screening of apparatus or components against electric or magnetic fields
-
- H—ELECTRICITY
- H05—ELECTRIC TECHNIQUES NOT OTHERWISE PROVIDED FOR
- H05K—PRINTED CIRCUITS; CASINGS OR CONSTRUCTIONAL DETAILS OF ELECTRIC APPARATUS; MANUFACTURE OF ASSEMBLAGES OF ELECTRICAL COMPONENTS
- H05K9/00—Screening of apparatus or components against electric or magnetic fields
- H05K9/0007—Casings
- H05K9/002—Casings with localised screening
- H05K9/0022—Casings with localised screening of components mounted on printed circuit boards [PCB]
- H05K9/0024—Shield cases mounted on a PCB, e.g. cans or caps or conformal shields
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L2924/00—Indexing scheme for arrangements or methods for connecting or disconnecting semiconductor or solid-state bodies as covered by H01L24/00
- H01L2924/30—Technical effects
- H01L2924/301—Electrical effects
- H01L2924/3025—Electromagnetic shielding
Definitions
- the present invention relates to a conductive paint and a method for manufacturing a shield package using the same.
- Patent Document 1 describes that an electromagnetic shielding member having a high shielding effect can be easily obtained by coating a surface of a package by spraying a conductive or semiconductive material.
- a shield layer is formed by spray coating using a solution consisting only of metal particles and a solvent, not only good shielding properties cannot be obtained, but also the adhesion between the shielding layer and the package is poor.
- a conductive paint having a conductive filler such as metal particles, a resin binder and a solvent is sprayed from a nozzle, A conductive coating film is formed on a package or the like.
- solids in the conductive paint that is, resin solids, conductive fillers, etc.
- the conductive coating cannot be normally ejected from the nozzle tip, and thus a conductive coating film having a uniform thickness cannot be formed.
- ⁇ Cleaning the nozzle can eliminate or prevent clogging of the nozzle opening.
- productivity is reduced.
- the present invention has been made in view of the above, and provides a conductive paint having excellent coating stability by spray coating, that is, a conductive paint capable of efficiently forming a conductive coating film having a uniform thickness by spray coating.
- the purpose is to do.
- the conductive paint of the present invention is based on (B) 100 parts by mass of the binder component containing (A) an epoxy resin, (B) 200 to 1800 parts by mass of metal particles, and (C) a curing agent 0.3 To 40 parts by weight, (D) 20 to 600 parts by weight of solvent, and (E) 0.5 to 10 parts by weight of carbon powder.
- the (A) binder component preferably further contains a (meth) acrylate compound.
- the metal particles may have at least one shape selected from the group consisting of flakes, dendrites and fibers.
- the carbon powder may be at least one selected from graphene and graphite.
- the conductive paint preferably has a viscosity of 100 mPa ⁇ s or more measured at 0.5 rpm with a conical plate type rotational viscometer.
- a single cylindrical rotational viscometer is used for rotor No.
- the viscosity measured at 10 rpm using 5 is preferably 30 dPa ⁇ s or less.
- the conductive paint is suitable for shielding electronic component packages.
- the shield package manufacturing method of the present invention is a shield package manufacturing method in which an electronic component is mounted on a substrate and the package in which the electronic component is sealed with a sealing material is covered with a shield layer. Mounting a plurality of electronic components on the substrate, filling the substrate with a sealing material and curing it, sealing the electronic components, and cutting the sealing material between the plurality of electronic components to form a groove , A step of individualizing the package of each electronic component on the substrate by these grooves, a step of spraying the conductive paint of the present invention on the surface of the individualized package, and a package coated with the conductive paint Forming a shield layer by curing the conductive paint, and obtaining a separate shield package by cutting the substrate along the groove It shall have at least a.
- the conductive paint of the present invention has a coating stability improved by containing a predetermined amount of carbon powder, and by using this, a conductive coating film having a uniform thickness can be efficiently formed by a spray method. It becomes possible. Therefore, it is possible to form a shield layer having a uniform thickness with high productivity by spraying this conductive paint on the package surface.
- a shield package excellent in shielding properties and adhesiveness with the package as described above can be efficiently manufactured without using a large-scale apparatus.
- the conductive paint according to the present invention comprises (B) 200 to 1800 parts by mass of metal particles and (C) a curing agent of 0.3 to 40 with respect to 100 parts by mass of the binder component containing (A) an epoxy resin. It contains at least part by mass, (D) 20 to 600 parts by mass of solvent, and (E) 0.5 to 10 parts by mass of carbon powder.
- the use of the conductive paint is not particularly limited, but a shield layer is formed by spraying the spray package or the like on the surface of the package before being singulated or on the surface of the singulated package. It is preferably used to obtain a shield package.
- the binder component in the conductive paint of the present invention contains an epoxy resin as an essential component, and may further contain a (meth) acrylate compound as necessary.
- the epoxy resin is not particularly limited as long as it has one or more epoxy groups in the molecule.
- Examples include bisphenol A type epoxy resins, bisphenol F type epoxy resins, bisphenol S type epoxy resins, bisphenol type epoxy resins, spirocyclic epoxy resins, naphthalene type epoxy resins, biphenyl type epoxy resins, terpene type epoxy resins, tris Glycidyl ether type epoxy resins such as (glycidyloxyphenyl) methane and tetrakis (glycidyloxyphenyl) ethane, glycidylamine type epoxy resins such as tetraglycidyldiaminodiphenylmethane, tetrabromobisphenol A type epoxy resin, cresol novolac type epoxy resin, phenol novolac Type epoxy resin, ⁇ -naphthol novolac type epoxy resin, brominated phenol novolac type epoxy resin, etc. novolac type epoxy Fat, rubber-mod
- an epoxy resin that is liquid at normal temperature and an epoxy resin that is solid at normal temperature as the epoxy resin.
- Solid at room temperature means a state having no fluidity in a solvent-free state at 25 ° C.
- an epoxy resin that is solid at room temperature hereinafter sometimes referred to as “solid epoxy resin”
- the solid epoxy resin can be used by dissolving in a solvent.
- the solvent to be used is not particularly limited, and can be appropriately selected from those described below.
- the content of the solid epoxy resin is preferably in the range of 5 to 30 parts by mass per 100 parts by mass of the binder component.
- the (meth) acrylate compound that can be used in the present invention is an acrylate compound or a methacrylate compound, and is not particularly limited as long as it is a compound having an acryloyl group or a methacryloyl group.
- Examples of (meth) acrylate compounds include isoamyl acrylate, neopentyl glycol diacrylate, trimethylolpropane triacrylate, ditrimethylolpropane tetraacrylate, 2-hydroxy-3-acryloyloxypropyl methacrylate, phenylglycidyl ether acrylate hexamethylene diisocyanate.
- Examples include urethane prepolymers, bisphenol A diglycidyl ether acrylic acid adducts, ethylene glycol dimethacrylate, and diethylene glycol dimethacrylate. These can be used alone or in combination of two or more.
- the content ratio is preferably 5 to 95 parts by mass, more preferably 100 parts by mass of the total amount of the epoxy resin and the (meth) acrylate compound. 20 to 80 parts by mass.
- the (meth) acrylate compound is 5 parts by mass or more, the storage stability of the conductive coating material is excellent, the conductive coating material can be cured more quickly, and the conductivity can be further improved. Furthermore, the dripping of the paint at the time of curing can be prevented.
- a (meth) acrylate compound is 95 mass parts or less, the adhesiveness of a package and a shield layer tends to become favorable.
- an alkyd resin, a melamine resin, a xylene resin or the like can be added to the binder component as a modifier for the purpose of improving the properties of the conductive paint.
- the content ratio when blending a modifier with the binder component is preferably 40 parts by mass or less, more preferably 10 parts by mass in 100 parts by mass of the binder component, from the viewpoint of adhesion between the conductive coating film and the coating object. Part or less.
- a curing agent for curing the binder component is used.
- the curing agent is not particularly limited, and examples thereof include a phenol curing agent, an imidazole curing agent, an amine curing agent, a cationic curing agent, and a radical curing agent. These may be used alone or in combination of two or more.
- phenolic curing agents examples include novolak phenol and naphtholic compounds.
- imidazole curing agents include imidazole, 2-undecylimidazole, 2-heptadecylimidazole, 2-methylimidazole, 2-ethylimidazole, 2-phenylimidazole, 2-ethyl-4-methyl-imidazole, and 1-cyanoethyl.
- imidazole curing agents include imidazole, 2-undecylimidazole, 2-heptadecylimidazole, 2-methylimidazole, 2-ethylimidazole, 2-phenylimidazole, 2-ethyl-4-methyl-imidazole, and 1-cyanoethyl.
- Examples include -2-undecylimidazole and 2-phenylimidazole.
- cationic curing agents include amine salts of boron trifluoride, P-methoxybenzenediazonium hexafluorophosphate, diphenyliodonium hexafluorophosphate, triphenylsulfonium, tetra-n-butylphosphonium tetraphenylborate, tetra- Examples thereof include onium compounds represented by n-butylphosphonium-o, o-diethyl phosphorodithioate and the like.
- radical curing agents examples include di-cumyl peroxide, t-butyl cumyl peroxide, t-butyl hydroperoxide, cumene hydroperoxide, and the like.
- the content of the curing agent is preferably 0.3 to 40 parts by mass, and more preferably 0.5 to 35 parts by mass with respect to 100 parts by mass of the total amount of the binder components.
- the content of the curing agent is 0.3 parts by mass or more, the adhesion between the conductive coating film and the surface of the object to be coated and the conductivity of the conductive coating film become good, and the conductive coating excellent in shielding effect. A film is obtained, and when it is 40 parts by mass or less, the storage stability of the conductive paint is improved.
- the amount is preferably 0.3 to 8 parts by mass with respect to 100 parts by mass of the total amount of binder components.
- the content of the radical curing agent is 0.3 parts by mass or more, the adhesion between the conductive coating film and the surface of the coating object and the conductivity of the conductive coating film become good, and the conductivity is excellent in the shielding effect. If the amount is 8 parts by mass or less, the storage stability of the conductive paint is improved.
- the metal particles that can be used in the present invention are not particularly limited as long as they are conductive particles.
- conductive particles For example, copper particles, silver particles, nickel particles, silver-coated copper particles, gold-coated copper particles, silver-coated nickel particles And gold coated nickel particles.
- the shape of the metal particles include a spherical shape, a flake shape (scale-like shape), a dendritic shape, and a fiber shape, but the application stability of the conductive paint is higher, and the resistance value of the obtained conductive coating film is lower. From the viewpoint of obtaining a conductive coating film with improved shielding properties, it is preferably either flake-like or dendritic.
- These metal particles may be used alone or in combination of two or more.
- the problem of sedimentation is likely to occur in the case of a conductive paint containing spherical metal particles, so that the utility of the present invention is particularly high in terms of improving the coating stability.
- the conductive paint of the present invention preferably contains spherical metal particles.
- the content of the metal particles is preferably 200 to 1800 parts by mass with respect to 100 parts by mass of the binder component.
- the content of the metal particles is 200 parts by mass or more, the conductivity of the conductive coating film is good, and when it is 1800 parts by mass or less, the adhesion between the conductive coating film and the object to be coated, and the conductivity after curing.
- the shield layer is less likely to be broken when cut with a dicing saw described later.
- the average particle size of the metal particles is preferably 1 to 30 ⁇ m, more preferably 1 to 10 ⁇ m.
- the average particle size of the metal particles is 1 ⁇ m or more, there is an advantage that the dispersibility of the metal particles is good, coating stability can be further improved, and oxidation is difficult. Further, when the thickness is 30 ⁇ m or less, the connectivity with the ground circuit of the package is good when the conductive paint is used for package shielding.
- the tap density of the metal particles is preferably 4.0 to 6.5 g / cm 3.
- the conductivity of the obtained conductive coating film is also good.
- the flaky metal particles preferably have an aspect ratio of 2 to 10.
- the aspect ratio is within the above range, the coating stability of the conductive paint becomes better, and the conductivity of the resulting conductive coating film becomes better.
- the conductive paint of the present invention contains carbon powder for improving the coating stability.
- the carbon powder that can be used is not particularly limited.
- amorphous carbon such as carbon black may be used. it can.
- graphene or graphite is preferable from the viewpoint that when the conductive paint is used for package shielding, the connection stability of the shield package is good and there is no problem of safety to living bodies.
- These carbon powders may be used alone or in combination of two or more.
- the carbon powder may contain elements other than carbon within a range not contrary to the object of the present invention, and includes not only inevitable impurities but also additives that are added as necessary in the manufacturing process, for example. Also good.
- Graphite is a mineral formed by laminating hexagonal plate-like crystals of carbon, and graphene is a single layer of one atom by peeling off layered graphite.
- commercially available products can be used without particular limitation.
- carbon black that has been conventionally used as a pigment or a conductive filler can be used without any particular limitation.
- the particle size of the carbon powder used in the present invention is not particularly limited, but it is preferable that the average particle size is 0.5 ⁇ m to 300 ⁇ m from the viewpoint of workability at the time of addition and dispersibility in the conductive paint. More preferably, the thickness is ⁇ 200 ⁇ m.
- the content of carbon powder is preferably 0.5 to 10 parts by mass with respect to 100 parts by mass of the binder component.
- the content of the carbon powder is 0.5 parts by mass or more, a conductive coating film having a uniform thickness can be obtained in order to prevent the metal powder from settling.
- the carbon filler content is 10 parts by mass or less, an appropriate viscosity can be obtained while preventing the metal powder from settling, and nozzle clogging can be suppressed even if the conductive paint is sprayed for a long time.
- the connection stability of the shield layer can be improved without variation.
- the conductive paint of the present invention is preferably applied with a lower viscosity by containing more solvent than a so-called conductive paste in order to uniformly apply the conductive paint by spray spraying.
- the solvent used in the present invention is not particularly limited, and examples thereof include methyl ethyl ketone, acetone, methyl ethyl ketone, acetophenone, methyl cellosolve, methyl cellosolve acetate, methyl carbitol, diethylene glycol dimethyl ether, tetrahydrofuran, dioctel, methyl acetate, and butyl acetate. These may be used alone or in combination of two or more.
- the content of the solvent is preferably adjusted as appropriate according to the use of the conductive paint and the equipment used for application, but is usually preferably 20 to 600 parts by mass with respect to 100 parts by mass of the binder component.
- the content of the solvent is 20 to 600 parts by mass or more, the coating stability by spray coating is excellent, and stable shielding characteristics are easily obtained when a conductive coating is used for package shielding.
- additives such as antifoaming agents, thickeners, pressure-sensitive adhesives, fillers, flame retardants, and colorants may be added to the conductive paint of the present invention within a range that does not impair the purpose of the invention. it can.
- the conductive paint of the present invention measures viscosity with a conical plate type rotational viscometer (so-called cone plate type viscometer) if the viscosity is low, and a single cylindrical rotational viscometer (so-called type B) if the viscosity is high. Or a BH viscometer).
- the viscosity measured at 0.5 rpm using a cone field CP40 (Cone angle: 0.8 °, cone radius: 24 mm) manufactured by Brookfield is 100 mPa. ⁇ It is preferably at least s, more preferably at least 150 mPa ⁇ s.
- the viscosity is 100 mPa ⁇ s or more, it is easy to form a conductive coating film without unevenness by preventing dripping when the coated surface is not horizontal. If the viscosity is measurable with a conical plate type rotational viscometer, there is no problem even if it is high.
- Rotator No. when measuring with a single cylindrical rotational viscometer.
- the viscosity measured at 10 rpm using 5 is preferably 30 dPa ⁇ s or less, and more preferably 25 dPa ⁇ s or less. When it is 30 dPa ⁇ s or less, the spray nozzle is prevented from being clogged, and a conductive coating film is easily formed without unevenness. If the viscosity is measurable with a single cylindrical rotational viscometer, there is no problem even if it is low.
- the method for forming a conductive coating film using the conductive paint of the present invention is not particularly limited, but the following method can be used. That is, it sprays in the shape of a mist with a known spray application device such as an air spray or a spray gun, and is applied evenly on the surface of the application object. At this time, the injection pressure, the injection flow rate, and the distance between the nozzle tip and the application target can be appropriately adjusted as necessary. Next, the object to be coated with the conductive paint is heated as necessary to sufficiently dry the solvent, and further heated to sufficiently cure the binder component in the conductive paint. A coating film is obtained. The heating temperature and heating time at this time can be appropriately adjusted depending on the type of binder component and curing agent.
- the shield layer obtained from the conductive paint has excellent adhesion to a ground circuit formed of copper foil or the like. Specifically, because the adhesion between the copper foil of the ground circuit exposed from a part of the package and the shield layer is good, the package is cut after applying the conductive paint on the package surface to form the shield layer. When separating into individual pieces, it is possible to prevent the shield layer from being peeled off from the ground circuit due to an impact at the time of cutting.
- the adhesive strength between the conductive paint and the copper foil is preferably a shear strength of 3.0 MPa or more measured based on JIS K 6850: 1999. .
- the shear strength is 3.0 MPa or more, there is almost no possibility that the shield layer is peeled off from the ground circuit due to an impact when cutting the package before separation.
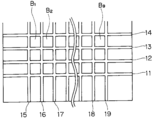
- a plurality of electronic components (IC or the like) 2 are mounted on a substrate 1 and a ground circuit pattern (copper foil) 3 is provided between the plurality of electronic components 2.
- the electronic component 2 is sealed by filling the electronic component 2 and the ground circuit pattern 3 with a sealing material 4 and curing it.
- the sealing material 4 is cut between the plurality of electronic components 2 to form grooves, and the packages of the electronic components on the substrate 1 are individualized by these grooves.
- Reference symbol A indicates an individual package. At least a part of the ground circuit is exposed from the wall surface constituting the groove, and the bottom of the groove does not completely penetrate the substrate.
- the conductive paint is sprayed in a mist form by an arbitrary spray application device, and is applied evenly so that the ground circuit exposed from the package surface and the wall surface is covered with the conductive paint.
- the injection pressure, the injection flow rate, and the distance between the nozzle tip and the surface of the package are adjusted as necessary.
- FIG. 2 is a plan view showing the substrate in this state.
- Reference numerals B1, B2,... B9 denote shield packages before being singulated, and reference numerals 11 to 19 denote grooves between these shield packages, respectively.
- an individual package B is obtained by cutting the substrate with a dicing saw or the like along the bottom of the groove between the packages before the individualization.
- the individual package B thus obtained has a uniform shield layer formed on the package surface (all of the upper surface portion, the side surface portion, and the corner portion of the boundary between the upper surface portion and the side surface portion). Good shielding characteristics can be obtained. Moreover, since the adhesion between the shield layer and the package surface and the ground circuit is excellent, it is possible to prevent the shield layer from being peeled off from the package surface or the ground circuit due to an impact when the package is separated by a dicing saw or the like. .
- Solid epoxy resin Mitsubishi Chemical Co., Ltd., trade name “JER157S70”
- Liquid epoxy resin Glycidylamine epoxy resin: ADEKA Corporation, trade name “EP-3905S”
- Glycidyl ether epoxy resin manufactured by ADEKA Corporation, trade name “EP-4400” (Meth) acrylate resin: 2-hydroxy-3-acryloyloxypropyl methacrylate (manufactured by Kyoeisha Chemical Co., Ltd., trade name “Light Ester G-201P”)
- Carbon powder Graphene: Made by ITEC Co., Ltd., trade name “iGRAFEN- ⁇ s”, average particle size 10 ⁇ m Graphite: manufactured by Nippon Graphite Industry Co., Ltd., product name “CSPE”, average particle size 4.5 ⁇ m
- Curing agent 15 parts by mass of phenol novolak (trade name “Tamanol 758” manufactured by Arakawa Chemical Industries, Ltd.) and 5 parts by mass of 2-methylimidazole (
- the viscosity of the conductive paint (liquid temperature: 25 ° C.) obtained in the above Examples and Comparative Examples was measured with a BH viscometer or a conical plate type viscometer.
- the measurement with a BH type viscometer was performed using a rotor no. 5 was performed at a rotation speed of 10 rpm.
- the measurement with a conical plate type rotational viscometer was performed at 0.5 rpm using a trade name “Programmable Viscometer DV-II + Pro” manufactured by Brookfield, Inc. and a cone spindle CP40.
- the measured viscosity is shown in Table 1.
- the “-” in the viscosity column indicates that measurement cannot be performed with the viscometer.
- Conductivity of conductive coating film As shown in FIG. 3, other than the coated portion on the glass epoxy substrate provided with the copper pad 21 is masked with polyimide tape, and hand spray (product name “Anest Iwata Co., Ltd., trade name“ LPH-101A-144LVG "), apply the conductive paint obtained in each of the examples and comparative examples, preheat at 80 ° C for 60 minutes, and then cure at 160 ° C for 60 minutes to cure the polyimide tape.
- the conductive coating film 22 having a length between the copper pads 21 (x in FIG. 3) of 60 mm, a width (y in FIG. 3) of 5 mm, and a thickness of about 20 ⁇ m was obtained.
- the tensile strength tester manufactured by Shimadzu Corporation, trade name “Autograph AGS-X” was used to pull the bonded surface in parallel, and the maximum load at the time of fracture was divided by the bonded area to obtain shear strength. Was calculated. If the shear strength is 3.0 MPa or more, it can be used without any problem.
- the shear strength of each example was 3.0 MPa or more, and it was confirmed that it could be suitably used as a shield layer.
- the adhesion after solder dipping was evaluated.
- the package is exposed to high temperatures in the solder dip process. For this reason, the adhesion between the shield layer after being exposed to a high temperature, the surface of the package, and the ground circuit is also important. Therefore, in order to measure the adhesion after solder dipping, a conductive paint is applied and bonded to a copper plate in the same manner as described above, heated at 80 ° C. for 60 minutes, and then heated at 160 ° C. for 60 minutes. Cured. Next, the shear strength after heating at 260 ° C. for 30 seconds was measured. The method for measuring the shear strength is the same as described above.
- shear strength after solder dipping is 3.0 MPa or more, it can be used as a shield layer without any problem.
- the shear strength of the conductive paint of each Example was 3.0 MPa or more, and it was confirmed that it could be suitably used as a shield layer.
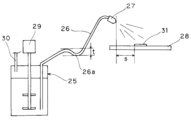
- Coating stability of conductive coating material The conductive coating materials of the above Examples and Comparative Examples are schematically shown in FIG. 4 as a coating device (spraying systems Japan Co., Ltd., spray cart III, spray nozzle: YB1 / 8MVAU-SS + SUMV91-SS) is spray-coated on the square glass epoxy substrate (FR-4, 10cm ⁇ 10cm ⁇ thickness 1mm) shown in FIG. Sex was evaluated.
- reference numeral 25 indicates a tank (paint container), reference numeral 26 indicates a tube, reference numeral 27 indicates a nozzle, reference numeral 28 indicates a turntable, reference numeral 29 indicates a stirring device, and reference numeral 30 indicates a gas introduction.
- Reference numeral 31 denotes a glass epoxy substrate.
- the tank 25 is a container having a substantially cylindrical shape and a capacity of 3 L.
- the tank 25 is provided with a stirring device 29 having stirring blades and introduces a gas such as nitrogen from a gas introduction pipe 30 to pressurize the inside.
- the tube 26 has a length of 3 m and an inner diameter of 4 mm, and connects the tank 25 and the nozzle 27 to each other.
- the tube 26 has a slack (26a) in part, and the height difference (t in FIG. 4) of the slack portion is 3 cm.
- the length of the nozzle 27 is 78 mm, and the injection port diameter is 0.5 mm.
- the distance from the upper surface of the rotating plate 28 to the tip of the nozzle 27 is 8 cm.
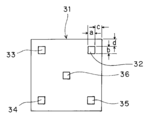
- each polyimide tape 32 to 35 is attached to the glass epoxy substrate 31 in the vicinity of each corner of the glass epoxy substrate 31, and the polyimide tape 36 is attached to the center of the glass epoxy substrate 31.
- the area of each polyimide tape 32 to 36 is 1 cm ⁇ 1 cm (both dimensions a and b in FIG. 5 are 1 cm), and each polyimide tape 32 to 35 is 1 cm inside from each side of the glass epoxy substrate 31 (the dimension in FIG. 5). c and d are both 1 cm), so that the side of the tape is parallel to the side of the substrate.
- This glass epoxy substrate 31 was placed on the center of the upper surface of the turntable 28 of the coating apparatus.
- the horizontal distance from the tip of the nozzle 27 to the glass epoxy substrate 31 was 25 cm.
- 2 kg of the conductive paint is put into the tank 21 and immediately after that, while rotating the rotary table 28 at 160 rpm, spray coating is performed under the following spray conditions, and heated at 80 ° C. for 60 minutes and then at 160 ° C. for 60 minutes.
- a conductive coating film having a thickness of 30 ⁇ m was formed.
- the polyimide tapes 32 to 36 are peeled off after being left at room temperature for 30 minutes.
- the thickness of the peeled portion (arrow X) of the glass epoxy substrate 31 and the peeled portion are removed.
- the thickness of the portion (arrow Y) where the conductive coating film 41 is formed on the glass epoxy substrate 31 adjacent to each other is measured with a micrometer, and the former is subtracted from the latter to obtain five conductive coating films. The thickness of was determined.
- the maximum value and the minimum value of the thickness at five locations were determined.
- a conductive coating film having good conductivity and uniform thickness can be formed by a spray method. Adhesion with was also good. That is, it was confirmed that the dispersion stability of the conductive paint was improved by containing a predetermined amount of carbon powder.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Materials Engineering (AREA)
- Wood Science & Technology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Physics & Mathematics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- General Physics & Mathematics (AREA)
- Computer Hardware Design (AREA)
- Power Engineering (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Inorganic Chemistry (AREA)
- Dispersion Chemistry (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Paints Or Removers (AREA)
- Conductive Materials (AREA)
- Shielding Devices Or Components To Electric Or Magnetic Fields (AREA)
- Manufacturing & Machinery (AREA)
- Structures Or Materials For Encapsulating Or Coating Semiconductor Devices Or Solid State Devices (AREA)
- Encapsulation Of And Coatings For Semiconductor Or Solid State Devices (AREA)
- Dicing (AREA)
- Application Of Or Painting With Fluid Materials (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020187020040A KR102362081B1 (ko) | 2016-03-29 | 2017-03-27 | 도전성 도료 및 그것을 사용한 차폐 패키지의 제조 방법 |
| CN201780016949.2A CN108779363B (zh) | 2016-03-29 | 2017-03-27 | 导电性涂料以及使用了该导电性涂料的屏蔽封装体的制造方法 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016-065478 | 2016-03-29 | ||
| JP2016065478 | 2016-03-29 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017170395A1 true WO2017170395A1 (ja) | 2017-10-05 |
Family
ID=59964507
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2017/012380 WO2017170395A1 (ja) | 2016-03-29 | 2017-03-27 | 導電性塗料及びそれを用いたシールドパッケージの製造方法 |
Country Status (5)
| Country | Link |
|---|---|
| JP (1) | JP6921573B2 (zh) |
| KR (1) | KR102362081B1 (zh) |
| CN (1) | CN108779363B (zh) |
| TW (1) | TWI770013B (zh) |
| WO (1) | WO2017170395A1 (zh) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20200069288A (ko) * | 2017-10-13 | 2020-06-16 | 타츠타 전선 주식회사 | 차폐 패키지 |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7164181B2 (ja) * | 2019-02-27 | 2022-11-01 | ナミックス株式会社 | 電磁波シールド用スプレー塗布剤 |
| JP7185289B2 (ja) * | 2019-03-07 | 2022-12-07 | ナミックス株式会社 | 電磁波シールド用スプレー塗布剤 |
| TWI813872B (zh) | 2019-07-25 | 2023-09-01 | 日商拓自達電線股份有限公司 | 導電性塗料及使用其之屏蔽封裝體之製造方法、以及具有屏蔽層之樹脂成形品之製造方法 |
| CN114830843B (zh) * | 2020-07-31 | 2024-06-18 | 拓自达电线株式会社 | 导电性胶粘剂 |
| KR20230069183A (ko) * | 2020-09-09 | 2023-05-18 | 베스트그래핀(주) | 전자파 차폐용 하이브리드 접착제 조성물, 전자파 차폐용 하이브리드 접착제의 제조방법 및 전자파차폐용 하이브리드 접착필름 |
| CN118541424A (zh) * | 2021-11-16 | 2024-08-23 | 马来西亚国家石油公司 | 石墨烯涂料 |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS58129072A (ja) * | 1982-01-28 | 1983-08-01 | Sutaaraito Kogyo Kk | 導電性プライマ−組成物 |
| JPH11213755A (ja) * | 1998-01-28 | 1999-08-06 | Hitachi Chem Co Ltd | 導電ペースト |
| JP2004047174A (ja) * | 2002-07-09 | 2004-02-12 | Sumitomo Bakelite Co Ltd | 導電性ペースト |
| JP2004055543A (ja) * | 2002-05-31 | 2004-02-19 | Tatsuta Electric Wire & Cable Co Ltd | 導電性ペースト |
| JP2008042152A (ja) * | 2006-08-07 | 2008-02-21 | Taiyo Yuden Co Ltd | 回路モジュールの製造方法及び回路モジュール |
| JP2011151372A (ja) * | 2009-12-25 | 2011-08-04 | Murata Mfg Co Ltd | 電子部品モジュールの製造方法及び電子部品モジュール |
| JP2013149596A (ja) * | 2011-12-21 | 2013-08-01 | Shoei Chem Ind Co | 熱硬化型導電性ペースト |
| JP2015115549A (ja) * | 2013-12-13 | 2015-06-22 | 株式会社東芝 | 半導体装置、および、半導体装置の製造方法 |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6081705A (ja) * | 1983-10-07 | 1985-05-09 | 東洋ゴム工業株式会社 | 広領域周波数帯用電磁波シ−ルド材 |
| JP2003045228A (ja) * | 2001-08-01 | 2003-02-14 | Hitachi Chem Co Ltd | 導電ペースト |
| JP2003258137A (ja) | 2002-02-28 | 2003-09-12 | Mitsubishi Electric Corp | 半導体装置 |
| US7214419B2 (en) * | 2002-05-31 | 2007-05-08 | Tatsuta Electric Wire & Cable Co., Ltd. | Conductive paste multilayered board including the conductive paste and process for producing the same |
| JP3841733B2 (ja) * | 2002-09-06 | 2006-11-01 | 九州耐火煉瓦株式会社 | 導電性組成物、それを含有する導電性塗料、導電性接着剤および電磁波シールド剤 |
| JP5318222B2 (ja) * | 2008-11-25 | 2013-10-16 | ロード コーポレイション | 光硬化性材料でダイ表面を保護する方法 |
| EP2490265A1 (en) * | 2009-10-15 | 2012-08-22 | Hitachi Chemical Company, Ltd. | Conductive adhesive, solar cell, method for manufacturing solar cell, and solar cell module |
| JP6166860B2 (ja) * | 2012-05-16 | 2017-07-19 | 小林 博 | グラフェンの製造方法、該グラフェンを接合したグラフェン接合体の製造方法、および前記グラフェンないしは前記グラフェン接合体を用いた基材ないしは部品の製造方法 |
| CN102876270B (zh) * | 2012-09-20 | 2013-09-25 | 吴江市天源塑胶有限公司 | 一种高粘接强度的环氧树脂导电胶 |
| CN102925100B (zh) * | 2012-11-28 | 2014-07-02 | 上海材料研究所 | 一种高导热性能导电银胶及其制备方法 |
| TWI500737B (zh) * | 2013-05-06 | 2015-09-21 | Chi Mei Corp | 導電性接著劑 |
| CN104178053A (zh) * | 2013-05-28 | 2014-12-03 | 北京中科纳通电子技术有限公司 | 一种石墨烯复合导电胶 |
| JP2015141746A (ja) * | 2014-01-27 | 2015-08-03 | 大阪瓦斯株式会社 | 導電体及びその製造方法 |
| CN104112605A (zh) * | 2014-07-30 | 2014-10-22 | 万裕三信电子(东莞)有限公司 | 电极片及其制备方法、超级电容器及其制备方法 |
| JP6318137B2 (ja) * | 2015-09-30 | 2018-04-25 | Dowaエレクトロニクス株式会社 | 導電性ペースト及び導電膜 |
-
2017
- 2017-03-16 TW TW106108722A patent/TWI770013B/zh active
- 2017-03-23 JP JP2017057907A patent/JP6921573B2/ja active Active
- 2017-03-27 WO PCT/JP2017/012380 patent/WO2017170395A1/ja active Application Filing
- 2017-03-27 CN CN201780016949.2A patent/CN108779363B/zh active Active
- 2017-03-27 KR KR1020187020040A patent/KR102362081B1/ko active IP Right Grant
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS58129072A (ja) * | 1982-01-28 | 1983-08-01 | Sutaaraito Kogyo Kk | 導電性プライマ−組成物 |
| JPH11213755A (ja) * | 1998-01-28 | 1999-08-06 | Hitachi Chem Co Ltd | 導電ペースト |
| JP2004055543A (ja) * | 2002-05-31 | 2004-02-19 | Tatsuta Electric Wire & Cable Co Ltd | 導電性ペースト |
| JP2004047174A (ja) * | 2002-07-09 | 2004-02-12 | Sumitomo Bakelite Co Ltd | 導電性ペースト |
| JP2008042152A (ja) * | 2006-08-07 | 2008-02-21 | Taiyo Yuden Co Ltd | 回路モジュールの製造方法及び回路モジュール |
| JP2011151372A (ja) * | 2009-12-25 | 2011-08-04 | Murata Mfg Co Ltd | 電子部品モジュールの製造方法及び電子部品モジュール |
| JP2013149596A (ja) * | 2011-12-21 | 2013-08-01 | Shoei Chem Ind Co | 熱硬化型導電性ペースト |
| JP2015115549A (ja) * | 2013-12-13 | 2015-06-22 | 株式会社東芝 | 半導体装置、および、半導体装置の製造方法 |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20200069288A (ko) * | 2017-10-13 | 2020-06-16 | 타츠타 전선 주식회사 | 차폐 패키지 |
| JPWO2019073809A1 (ja) * | 2017-10-13 | 2020-12-17 | タツタ電線株式会社 | シールドパッケージ |
| JP6992083B2 (ja) | 2017-10-13 | 2022-01-13 | タツタ電線株式会社 | シールドパッケージ |
| KR102428873B1 (ko) | 2017-10-13 | 2022-08-02 | 타츠타 전선 주식회사 | 차폐 패키지 |
Also Published As
| Publication number | Publication date |
|---|---|
| TW201737368A (zh) | 2017-10-16 |
| JP6921573B2 (ja) | 2021-08-18 |
| KR20180125942A (ko) | 2018-11-26 |
| CN108779363B (zh) | 2021-06-22 |
| CN108779363A (zh) | 2018-11-09 |
| KR102362081B1 (ko) | 2022-02-10 |
| JP2017179360A (ja) | 2017-10-05 |
| TWI770013B (zh) | 2022-07-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2017170395A1 (ja) | 導電性塗料及びそれを用いたシールドパッケージの製造方法 | |
| JP5985785B1 (ja) | 電子部品のパッケージのシールド用導電性塗料及びそれを用いたシールドパッケージの製造方法 | |
| WO2017170398A1 (ja) | 導電性塗料及びそれを用いたシールドパッケージの製造方法 | |
| WO2018012017A1 (ja) | 導電性塗料及びそれを用いたシールドパッケージの製造方法 | |
| JP6831731B2 (ja) | 導電性塗料及びそれを用いたシールドパッケージの製造方法 | |
| TWI778233B (zh) | 導電性塗料及使用該導電性塗料之屏蔽封裝體之製造方法 | |
| WO2021014964A1 (ja) | 導電性塗料、及びそれを用いたシールドパッケージの製造方法、並びにシールド層を有する樹脂成形品の製造方法 | |
| JP2020055977A (ja) | 導電性塗料 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| ENP | Entry into the national phase |
Ref document number: 20187020040 Country of ref document: KR Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17774932 Country of ref document: EP Kind code of ref document: A1 |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 17774932 Country of ref document: EP Kind code of ref document: A1 |