WO2016056228A1 - Car発現ベクター及びcar発現t細胞 - Google Patents
Car発現ベクター及びcar発現t細胞 Download PDFInfo
- Publication number
- WO2016056228A1 WO2016056228A1 PCT/JP2015/005080 JP2015005080W WO2016056228A1 WO 2016056228 A1 WO2016056228 A1 WO 2016056228A1 JP 2015005080 W JP2015005080 W JP 2015005080W WO 2016056228 A1 WO2016056228 A1 WO 2016056228A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- car
- cells
- nucleic acid
- expressing
- ccl19
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/26—Lymph; Lymph nodes; Thymus; Spleen; Splenocytes; Thymocytes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/76—Viruses; Subviral particles; Bacteriophages
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/11—T-cells, e.g. tumour infiltrating lymphocytes [TIL] or regulatory T [Treg] cells; Lymphokine-activated killer [LAK] cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/30—Cellular immunotherapy characterised by the recombinant expression of specific molecules in the cells of the immune system
- A61K40/31—Chimeric antigen receptors [CAR]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4202—Receptors, cell surface antigens or cell surface determinants
- A61K40/4221—CD20
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4231—Cytokines
- A61K40/4234—Interleukins [IL]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4231—Cytokines
- A61K40/4236—Chemokines
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/52—Cytokines; Lymphokines; Interferons
- C07K14/521—Chemokines
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/52—Cytokines; Lymphokines; Interferons
- C07K14/54—Interleukins [IL]
- C07K14/5418—IL-7
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/7051—T-cell receptor (TcR)-CD3 complex
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70517—CD8
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70521—CD28, CD152
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70578—NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2887—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against CD20
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/44—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material not provided for elsewhere, e.g. haptens, metals, DNA, RNA, amino acids
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0636—T lymphocytes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/10—Cells modified by introduction of foreign genetic material
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/31—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterized by the route of administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/38—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the dose, timing or administration schedule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/46—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the cancer treated
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/52—Cytokines; Lymphokines; Interferons
- C07K14/54—Interleukins [IL]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K19/00—Hybrid peptides, i.e. peptides covalently bound to nucleic acids, or non-covalently bound protein-protein complexes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/622—Single chain antibody (scFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/03—Fusion polypeptide containing a localisation/targetting motif containing a transmembrane segment
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/33—Fusion polypeptide fusions for targeting to specific cell types, e.g. tissue specific targeting, targeting of a bacterial subspecies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/70—Fusion polypeptide containing domain for protein-protein interaction
- C07K2319/74—Fusion polypeptide containing domain for protein-protein interaction containing a fusion for binding to a cell surface receptor
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/95—Fusion polypeptide containing a motif/fusion for degradation (ubiquitin fusions, PEST sequence)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/20—Cytokines; Chemokines
- C12N2501/21—Chemokines, e.g. MIP-1, MIP-2, RANTES, MCP, PF-4
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/20—Cytokines; Chemokines
- C12N2501/23—Interleukins [IL]
- C12N2501/2307—Interleukin-7 (IL-7)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2510/00—Genetically modified cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2740/00—Reverse transcribing RNA viruses
- C12N2740/00011—Details
- C12N2740/10011—Retroviridae
- C12N2740/13011—Gammaretrovirus, e.g. murine leukeamia virus
- C12N2740/13041—Use of virus, viral particle or viral elements as a vector
- C12N2740/13043—Use of virus, viral particle or viral elements as a vector viral genome or elements thereof as genetic vector
Definitions
- the present invention relates to a CAR expression vector, a CAR expression T cell into which the CAR expression vector is introduced, and an anticancer agent containing the CAR expression T cell.
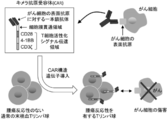
- a chimeric antigen receptor (Chimeric Antigen Receptor: hereinafter also referred to as “CAR”) is a fusion of a single-chain antibody that recognizes cell surface antigens of cancer cells and a signal transduction region that induces activation of T cells. It is an artificial chimeric protein. As shown in FIG. 1, by introducing a gene encoding CAR into normal peripheral blood T cells (peripheral blood T lymphocytes) having no tumor reactivity, CAR-expressing T cells capable of expressing CAR (hereinafter referred to as CAR). (Also simply referred to as “CAR-T cells”) in large quantities. Such CAR-T cells have tumor reactivity and can lead to cancer cell injury without depending on the interaction with the major histocompatibility complex (MHC).
- MHC major histocompatibility complex
- Cancer immunotherapy by administration of CAR-T cells more specifically, a therapy in which T cells are collected from a patient, a gene encoding CAR is introduced into such T cells, amplified, and transferred again to the patient (non- (See Patent Document 1), clinical trials are currently in progress around the world, and results showing effectiveness in hematopoietic malignancies such as leukemia and lymphoma have been obtained.
- a pharmaceutical composition comprising a modified autologous human T cell comprising a nucleic acid encoding a CAR comprising a CD19 antigen-binding region, a transmembrane region, a 4-1BB costimulatory signal region, and a CD3 ⁇ signal region
- a pharmaceutical composition comprising a modified autologous human T cell comprising a nucleic acid encoding a CAR comprising a CD19 antigen-binding region, a transmembrane region, a 4-1BB costimulatory signal region, and a CD3 ⁇ signal region
- a pharmaceutical composition comprising a modified autologous human T cell comprising a nucleic acid encoding a CAR comprising a CD19 antigen-binding region, a transmembrane region, a 4-1BB costimulatory signal region, and a CD3 ⁇ signal region
- a cell containing a nucleic acid encoding a chimeric antigen receptor, including a signal transduction domain see Patent Document 3
- the survival efficiency of CAR-T cells in vivo is low, or there is a problem that the activation of endogenous T cells induced by CAR-T cells and the accumulation in the tumor site are insufficient, or cancer cells have Inhibition of CAR-T cell activity by immunosuppressive signals via the PD-L1 / PD-1 pathway, which is a tumor immune evasion mechanism, and immunosuppressive factors such as TGF- ⁇ and IL-10 secreted in the cancer microenvironment
- the problem has not been solved with conventional technology. Therefore, there are cancer types and cases for which sufficient therapeutic effects are not observed, and there is a demand for the production of more effective CAR-T cells and expression vectors for producing such CAR-T cells. .
- an object of the present invention is to express a T cell immune function promoting factor together with CAR in T cells, and to produce CAR-T cells having high immunity-inducing effect and antitumor activity, and CAR for producing such CAR-T cells. It is to provide an expression vector.
- the inventors of the present invention have tried to improve CAR-T cells for the purpose of achieving a better immunity-inducing effect and antitumor activity in cancer immunotherapy using CAR-T cells.
- CAR-T cells attention was paid to cytokines, chemokines, and signal control proteins, which are factors that promote the immune function of T cells, and a vector that expresses the factors that promote the immune function of T cells together with CAR was constructed.
- cytokines, chemokines, and signal control proteins which are factors that promote the immune function of T cells
- a vector that expresses the factors that promote the immune function of T cells together with CAR was constructed.
- a CAR expression vector which is a nucleic acid encoding a nucleic acid encoding CCL19, a nucleic acid encoding a dominant negative mutant for SHP-1, or a nucleic acid encoding a dominant negative mutant for SHP-2.
- the nucleic acid encoding CAR includes a nucleic acid encoding a polypeptide in the intracellular region of CD28, the intracellular region of 4-1BB, and the intracellular region of CD3 ⁇ , The CAR expression vector according to any one of (6).
- A the CAR expression vector according to any one of (1) to (7) above;
- B a CAR expression vector containing a nucleic acid encoding CAR and a nucleic acid encoding interleukin 7, and a CAR expression vector containing a nucleic acid encoding CAR and a nucleic acid encoding CCL19;
- An anticancer agent comprising the CAR-expressing T cell according to (8) above and a pharmaceutically acceptable additive.
- CAR-T cells having both viability, lymphocyte accumulation ability, and tumor cytotoxic activity, and CAR-T cells having resistance to immunosuppression in a cancer microenvironment are prepared. It becomes possible. If such CAR-T cells are used for immunotherapy of cancer patients, a strong cancer treatment effect is expected, and effective cancer immunotherapy for refractory / progressive cancer is possible. Become.
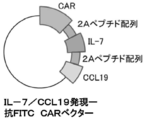
- FIG. 1 It is a figure which shows the structure of CAR, and the basic system of the cancer immunotherapy by a CAR-T cell. It is a figure showing the vector which expresses CAR, interleukin 7 (IL-7), and CCL19. It is a figure which shows the result -1 which confirmed the expression level of CAR in an anti- FITC CAR-IL-7 / CCL19 expression T cell by flow cytometry. The left is no CAR staining and the right is CAR staining. It is a figure which shows the result-2 which confirmed the expression level of CAR in an anti- FITC CAR-IL-7 / CCL19 expression T cell by flow cytometry.
- IL-7 interleukin 7
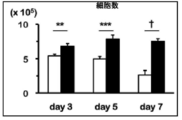
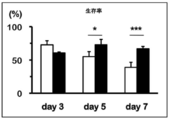
- FIG. 3 is a graph showing the number of cells when anti-FITC CAR-IL-7 / CCL19-expressing T cells were stimulated and cultured for 3 days, 5 days, or 7 days. It is a figure which shows the survival rate when anti-FITC CAR-IL-7 / CCL19 expression T cells are stimulated and cultured for 3 days, 5 days, 7 days.
- FIG. 2 is a graph showing the number of cells when anti-human CD20 CAR-IL-7 / CCL19-expressing T cells were stimulated and cultured for 5 days.
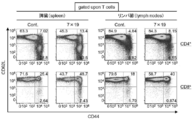
- FIG. 3 shows the results of analysis of CD4, CD8, CD44, and CD62L for leukocyte surface phenotypes by administering anti-human CD20 CAR-IL-7 / CCL19-expressing T cells to mice subcutaneously inoculated with P815-hCD20 and by flow cytometry.
- FIG. It is a figure which shows the result of having investigated the proliferation of the T cell by the flow cytometry after culture
- the CAR expression vector of the present invention is a CAR expression vector containing a nucleic acid encoding a chimeric antigen receptor (CAR) and a nucleic acid encoding a T cell immune function promoting factor, the nucleic acid encoding the immune function promoting factor.
- CAR chimeric antigen receptor
- chimeric antigen receptor is not particularly limited, and is an artificial fusion in which a single-chain antibody that recognizes a cell surface antigen of cancer cells and a signal transduction region that induces activation of T cells are fused via a transmembrane region.
- a typical chimeric protein is an artificial fusion in which a single-chain antibody that recognizes a cell surface antigen of cancer cells and a signal transduction region that induces activation of T cells.
- the nucleic acid encoding CAR is not particularly limited as long as it encodes a polypeptide constituting CAR, and a single-chain antibody that recognizes a cell surface antigen of cancer cells, a transmembrane region, and Nucleic acids encoding a polypeptide of a signal transduction region that induces T cell activation are included.
- the single-chain antibody in CAR consists of a light chain variable region and a heavy chain variable region (scFv) derived from the antigen-binding site of a monoclonal antibody, and a linker peptide is located between the light chain variable region and the heavy chain variable region. Mention may be made of oligos or polypeptides.
- biomolecules specifically expressed in cancer cells and their progenitor cells, and expression is newly recognized by canceration of cells Any biomolecule or a biomolecule whose expression level is increased in cancer cells as compared to normal cells may be used.
- the T cell activation signal transduction region is a region capable of signal transduction into a cell when the single chain antibody recognizes a cell surface antigen of a cancer cell.
- CD28, 4-1BB (CD137) GITR, CD27, OX40, HVEM, CD3 ⁇ , Fc Receptor-associated gamma chain preferably at least one or more selected from polypeptides in the intracellular region, CD28, 4-1BB, and CD3 ⁇ 3 More preferably, it comprises a polypeptide in the intracellular region of the species.
- Each of the intracellular domain polypeptides may be linked via an oligopeptide linker or polypeptide linker consisting of 2 to 10 amino acids, and examples of such a linker sequence include a glycine-serine continuous sequence.
- the transmembrane region in the present invention includes CD8, T cell receptor ⁇ , ⁇ chain, CD28, CD3 ⁇ , CD45, CD4, CD5, CD8, CD9, CD16, CD22, CD33, CD37, CD64, CD80, CD86, CD134. , CD137, CD154, GITR-derived polypeptide in the transmembrane region can be mentioned, and a human CD8 transmembrane region polypeptide can be preferably mentioned.
- the CAR is fixed to the cell membrane of the T cell by the transmembrane region.
- the cell transmembrane region may be composed of an arbitrary oligopeptide or polypeptide, and may contain a hinge region having a length of 1 to 100 amino acids, preferably 10 to 70 amino acids. Examples of the hinge region include the hinge region of human CD8.
- a spacer region composed of any oligopeptide or polypeptide is placed between the single-chain antibody that recognizes the cell surface antigen of cancer cells and the transmembrane region, and between the transmembrane region and the T cell activation signal transduction region. It may be provided.
- the length of the spacer region can be 1 to 100 amino acids, preferably 10 to 50 amino acids. Examples of such spacer regions include a glycine-serine continuous sequence.
- the nucleic acid encoding a T cell function promoting factor includes a nucleic acid encoding IL-7, a nucleic acid encoding CCL19 (hereinafter also referred to as “the present nucleic acid 1”), and a dominant negative mutant for SHP-1. As long as it is a nucleic acid encoding a dominant negative mutant against SHP-2 (hereinafter also referred to as “Nucleic acid 3”). A plurality of nucleic acids 1 to 3 may be included.
- nucleic acid 1 and the nucleic acid 2 may be a nucleic acid containing the nucleic acid 2 and the present nucleic acid 3.
- nucleic acid encoding IL-7 and the nucleic acid encoding CCL19 in the present nucleic acid 1 only need to contain a nucleic acid encoding IL-7 and a nucleic acid encoding CCL19.
- nucleic acid encoding CCL19 may be arranged upstream or downstream.
- the nucleic acid encoding a dominant negative mutant for SHP1 is not particularly limited as long as it is a nucleic acid encoding a mutant of SHP1 that works predominantly against SHP1 and can inhibit the action of SHP1, and at least 1 in the amino acid sequence of SHP1
- a nucleic acid encoding a variant that consists of an amino acid sequence in which one amino acid is substituted with another amino acid and can inhibit the action of SHP1 can be mentioned.
- nucleic acid encoding a dominant negative mutant for SHP2 is not particularly limited as long as it is a nucleic acid encoding a mutant of SHP2 that works predominantly against SHP2 and can inhibit the action of SHP2, and in the amino acid sequence of SHP2 Mention may be made of a nucleic acid encoding a variant that consists of an amino acid sequence in which at least one amino acid is replaced with another amino acid and can inhibit the action of SHP2.
- each nucleic acid between a nucleic acid encoding a chimeric antigen receptor and a nucleic acid encoding a T cell immune function promoting factor, or a plurality of the nucleic acids 1, 2 and 3 Any nucleic acid may be included between the nucleic acid encoding IL-7 and the nucleic acid encoding CCL19 in the present nucleic acid 1 as long as each nucleic acid can be expressed, but the self-cleaving peptide (2A Peptide), or a sequence encoding IRES (internal ribozyme entry site), preferably a sequence encoding 2A peptide. By ligating using such sequences, each nucleic acid can be efficiently expressed.
- the 2A peptide is a self-cleaving peptide derived from a virus, and has a characteristic that a portion between GP in the amino acid sequence represented by SEQ ID NO: 1 (position of one residue from the C-terminus) is cleaved by the endoplasmic reticulum ( Szymczak et al., Expert Opin. Biol. Ther.5 (5): 627-638 (2005)). Therefore, nucleic acids incorporated before and after the 2A peptide are expressed independently of each other in the cell.
- the 2A peptide is preferably a 2A peptide derived from picornavirus, rotavirus, insect virus, aft virus or trypanosoma virus, and preferably a 2A peptide derived from picornavirus shown in SEQ ID NO: 2 (F2A). More preferred.
- a method of chemically synthesizing a nucleic acid encoding a chimeric antigen receptor based on a base sequence encoding a polypeptide of a single-chain antibody against a cell surface antigen of a cancer cell, a transmembrane region, and a T cell activation signal transduction region Alternatively, it can be prepared by a known technique such as a method of amplification by PCR. It should be noted that the codon selected for encoding the amino acid may be modified to optimize the expression of the nucleic acid in the intended host cell.
- nucleotide sequences encoding polypeptides of single-chain antibodies against cell surface antigens of cancer cells, transmembrane regions, and T cell activation signal transduction regions can be found in known literatures and NCBI (http: //www.ncbi .nlm.nih.gov / guide /) and other databases can be obtained as appropriate.
- information on the base sequence encoding the polypeptide in the transmembrane region of CD28, 4-1BB, and CD3 ⁇ in the T cell activation signal transduction region can be obtained as appropriate by searching a database such as NCBI.
- a database such as NCBI.
- Genbank number: NM_006139.2 update date: May 10, 2014
- human 4-1BB Genbank number: NM_001561.5 (update date: March 16, 2014)
- human CD3 ⁇ May be registered as Genbank number: NM_000734.3 (update date: August 12, 2014).
- nucleotide sequence encoding the polypeptide of human CD8 transmembrane region can be obtained as appropriate by searching a database such as NCBI. Genbank number: NM_001768.6 (updated on May 10, 2014) ) Can be exemplified.
- a monoclonal antibody that recognizes the target cell surface antigen is prepared, and the amino acid sequence of such a monoclonal antibody is determined by a known method such as the Edman method. It can also be obtained based on such amino acid sequences.
- Monoclonal antibody production methods include a method using a hybridoma, a method in which a host is transformed with an expression vector containing an antibody gene by genetic engineering techniques, and a transgenic animal is immunized with a desired antigen. The method of producing can be mentioned.
- the nucleic acid encoding can be prepared by a known technique such as a chemical synthesis method or a PCR amplification method based on each base sequence. It should be noted that the codon selected for encoding the amino acid may be modified to optimize the expression of the nucleic acid in the intended host cell.
- nucleic acids encoding IL-7 and nucleic acids encoding CCL19 can be found in known literatures and NCBI ( http://www.ncbi.nlm.nih.gov/guide/) and other databases can be obtained as appropriate.
- the nucleic acid encoding IL-7 can be appropriately selected depending on the type of cell into which the CAR expression vector of the present invention is introduced.
- Examples include a nucleic acid encoding the amino acid sequence of human IL-7 (SEQ ID NO: 3). As long as it has an effect of enhancing the cell proliferation rate in IL-7, it is 80% or more, preferably 85% or more, more preferably 90% or more, still more preferably 95% or more, most preferably the nucleotide sequence shown in SEQ ID NO: 3.
- a base sequence having 98% or more identity may be used.
- the nucleic acid encoding CCL19 can be appropriately selected according to the type of cell into which the CAR expression vector of the present invention is introduced. Examples thereof include a nucleic acid encoding the amino acid sequence of human CCL19 (SEQ ID NO: 4). As long as it has a T cell migration action, the identity of the base sequence shown in SEQ ID NO: 4 is 80% or more, preferably 85% or more, more preferably 90% or more, still more preferably 95% or more, and most preferably 98% or more. You may use the base sequence which has.
- the nucleic acid encoding the dominant negative mutant for SHP-1 can be appropriately selected according to the type of cell into which the CAR expression vector of the present invention is introduced.
- the amino acid sequence of the dominant negative mutant for human SHP-1 sequence As long as the action of SHP-1 in the dominant negative mutant against SHP-1 can be inhibited, the nucleotide sequence shown in SEQ ID NO: 5 is 80% or more, preferably 85% or more.
- a base sequence having an identity of 90% or more, more preferably 95% or more, and most preferably 98% or more may be used.
- the 453rd serine is the mutation site.
- the nucleic acid encoding the dominant negative mutant for SHP-2 can be appropriately selected according to the type of cell into which the CAR expression vector of the present invention is introduced.
- the amino acid sequence of the dominant negative mutant for human SHP-2 sequence
- the nucleic acid shown in SEQ ID NO: 6 is 80% or more, preferably 85% or more.
- a base sequence having an identity of 90% or more, more preferably 95% or more, and most preferably 98% or more may be used.
- the 459th serine is the mutation site.
- the CAR expression vector of the present invention may be linear or circular, and may be a non-viral vector such as a plasmid, a viral vector, or a transposon vector. Such vectors may also contain control sequences such as promoters and terminators, and selectable marker sequences such as drug resistance genes and reporter genes. By arranging a nucleic acid encoding CAR and a nucleic acid encoding a T cell immune function operably downstream of the promoter sequence, each nucleic acid can be efficiently transcribed. In addition, by including a marker gene, the expression of a nucleic acid encoding a chimeric antigen receptor can be easily confirmed.
- the CAR expression vector of the present invention may contain a nucleic acid encoding a suicide gene, and the position of the suicide gene is not particularly limited, and a nucleic acid encoding IL-7, a nucleic acid encoding CCL19, SHP Of each nucleic acid via a sequence encoding 2A peptide or IRES downstream of a promoter for expressing a nucleic acid encoding a dominant negative mutant for -1, or a nucleic acid encoding a dominant negative mutant for SHP-2 It may be arranged upstream or downstream, and may be arranged downstream of other promoters.
- a drug that activates the function of the suicide gene for example, when the tumor disappears, is administered in vivo according to the course of cancer treatment. It becomes possible to control the number of CAR-expressing T cells.
- suicide gene examples include herpes simplex virus thymidine kinase (HSV-TK) and inducible caspase 9 described in the following documents, and agents that activate the function of the gene: May include AP1903 which is a ganciclovir for the former and a chemical-induction-of-dimerization (CID) for the latter (Cooper LJ., Et. Al. Cytotherapy. 2006; 8 ( 2): 105-17., Jensen M. C. et. Al. Biol Blood Marrow Transplant. 2010 Sep; 16 (9): 1245-56., Jones245BS. Front Pharmacol. 2014 Nov 27; 5: 254., Minagawa K., Pharmaceuticals (Basel). 2015 May 8; 8 (2): 230-49., Bole-Richard E., Front Pharmacol. 2015 Aug 25; 6: 174).
- HSV-TK herpes simplex virus thymidine kinase
- CID chemical-induction-of-dimerization
- the viral vector examples include a retrovirus vector, a lentivirus vector, an adenovirus vector, and an adeno-associated virus vector, preferably a retrovirus vector, and a pMSGV vector (Tamada k et al., Clin Cancer Res 18: 6436-6445 (2002)) and pMSCV vector (manufactured by Takara Bio Inc.) can be mentioned more suitably.
- a retroviral vector is used, the transgene is incorporated into the genome of the host cell and can be stably expressed over a long period of time.
- the CAR-expressing T cell of the present invention contains (a) a T cell obtained by introducing the CAR expression vector of the present invention, and (b) a nucleic acid encoding CAR and a nucleic acid encoding interleukin 7. Obtained by introducing at least two vectors, a CAR expression vector (CAR-IL-7 expression vector) and a CAR expression vector containing a nucleic acid encoding CAR and a nucleic acid encoding CCL19 (CAR-CCL19 expression vector)
- the method is not particularly limited as long as it is a T cell, and the method of introducing the CAR expression vector of the present invention or the CAR-IL-7 expression vector and the CAR-CCL19 expression vector into the T cell is not particularly limited.
- the CAR-IL-7 expression vector only needs to contain a nucleic acid encoding CAR and a nucleic acid encoding interleukin 7, and the CAR-CCL19 expression vector encodes a nucleic acid encoding CAR and CCL19. As long as it can express each nucleic acid, it may contain other nucleic acids such as 2A peptide, IRES, or nucleic acid encoding a suicide gene, as in the case of the CAR expression vector of the present invention. .
- the CAR expression vector and packaging plasmid of the present invention were prepared by using GP2-293 cells (manufactured by Takara Bio), Plat-GP cells (manufactured by Cosmo Bio), PG13 cells (ATCCATCRL-10686), PA317.
- a recombinant virus can be produced by transfecting a packaging cell such as a cell (ATCC CRL-9078), and a T cell can be infected with such a recombinant virus.
- Retrovirus packagin Kit Eco manufactured by Takara Bio Inc. You may carry out using commercially available kits, such as.
- Confirmation of the introduction of the CAR expression vector of the present invention into T cells can be carried out by examining the expression of CAR by PCR, ELISA, Western blotting such as flow cytometry, Northern blotting, Southern blotting, RT-PCR, or inserted into the vector. This can be confirmed by examining the expression of the marker gene.
- T cells examples include human-derived T cells and T cells derived from non-human mammals such as dogs, cats, pigs, and mice.
- T cells are isolated from body fluids such as blood and bone marrow fluid, immune cells that infiltrate tissues such as spleen, thymus, and lymph nodes, or cancer tissues such as primary tumors, metastatic tumors, and cancerous ascites. Can be obtained by purification.
- Examples of such T cells include ⁇ T cells, ⁇ T cells, CD8 + T cells, CD4 + T cells, tumor infiltrating T cells, memory T cells, naive T cells, and NKT cells.
- the single-chain antibody to be expressed is located outside the cell, and by providing such a single-chain antibody, the CAR-expressing T-cell is expressed on the surface of a cancer cell. (TAA) can be recognized.
- a vector containing a nucleic acid encoding a suicide gene may be introduced into the CAR-expressing T cell of the present invention together with the CAR expression vector of the present invention.
- the anticancer agent of the present invention is not particularly limited as long as it contains the CAR-expressing T cell of the present invention and a pharmaceutically acceptable additive.
- the additive include physiological saline and buffered physiological saline. List water, cell culture medium, dextrose, water for injection, glycerol, ethanol and combinations thereof, stabilizers, solubilizers and surfactants, buffers and preservatives, isotonic agents, fillers, and lubricants. Can do.
- the anticancer agent of the present invention can be administered to a subject in need of cancer treatment using methods known to those skilled in the art, and includes intravenous, intratumoral, intradermal, Mention may be made of subcutaneous, intramuscular, intraperitoneal, intraarterial, intramedullary, intracardiac, intraarticular, intrasynovial, intracranial, intrathecal, and subarachnoid (spinal fluid) injections.
- the amount of the CAR-expressing T cell of the present invention contained in the anticancer agent to be administered can be appropriately adjusted according to the type, location, severity, and age, weight and condition of the subject to be treated, Preferably, 1 ⁇ 10 4 to 1 ⁇ 10 10 , preferably 1 ⁇ 10 5 to 1 ⁇ 10 9 , more preferably 5 ⁇ 10 6 to 5 ⁇ 10 8 can be mentioned in one administration. .
- Anticancer drugs to be administered are 4 times, 3 times, 2 times or 1 day, every other day, every 2 days, every 3 days, every 4 days, every 5 days, once a week, every 7 days, It can be administered independently every 8 days, every 9 days, twice a week, once a month or twice a month.
- Examples of the cancer in the anticancer agent of the present invention and the cancer treatment method described later include adenocarcinoma, squamous cell carcinoma, adenosquamous cell carcinoma, undifferentiated cancer, large cell cancer, and small cell.
- sarcomas such as chondrosarcoma, Ewing sarcoma, malignant hemangioendothelioma, malignant schwannoma, osteosarcoma, soft tissue sarcoma, hepatoblastoma, medulloblastoma, nephroblastoma, neuroblastoma And blastomas such as pancreatic blastoma, pleuropulmonary blastoma and retinoblasto
- the anticancer agent of the present invention can be used in combination with other anticancer agents.
- Other anti-cancer agents include alkylating agents such as cyclophosphamide, bendamustine, iosfamide, dacarbazine, antimetabolites such as pentostatin, fludarabine, cladribine, methotrexate, 5-fluorouracil, 6-mercaptopurine, and enocitabine, Molecular targeting drugs such as rituximab, cetuximab, trastuzumab, kinase inhibitors such as imatinib, getininib, erlotinib, afatinib, dasatinib, sunitinib, trametinib, proteasome inhibitors such as bortezomib, calcidomin inhibitors such as cyclosporine, tacrolimine, Anticancer antibiotics such as C, plant alkaloids such as irinotecan and e
- a method of treating with another anticancer agent and then using the anticancer agent of the present invention is used.
- a method using the anticancer agent of the present invention and another anticancer agent at the same time, a method using the anticancer agent of the present invention, and then using another anticancer agent, A method using treatment with another anticancer agent and then using the anticancer agent of the present invention can be preferably mentioned.
- the anticancer agent of the present invention is used in combination with other anticancer agents, the therapeutic effect of cancer is further improved, and the number of administrations or doses of each anticancer agent is reduced. It becomes possible to reduce the side effects caused by the respective anticancer agents.
- a cancer treatment method comprising administering the CAR-expressing T cell of the present invention to a patient in need of cancer treatment, or 2) an anticancer agent And the use of the CAR-expressing T cell of the present invention and 3) the CAR-expressing T cell of the present invention in the preparation of an anticancer agent.
- kits for preparing CAR-expressing T cells comprising the CAR expression vector of the present invention can be mentioned.
- the CAR expression vector of the present invention is used. It is not particularly limited as long as it is provided, and may contain instructions for preparing CAR-expressing T cells and reagents used for introducing the CAR expression vector of the present invention into T cells.
- T cells expressing IL-7 and CCL19 Selection of T cell immune function promoting factor
- the inventors first selected IL-7 and CCL19 from among a huge number of combinations as regulatory molecules for enhancing further antitumor effects in CAR-T cells based on the knowledge and experience so far, and Two combinations of IL-7 and CCL19 were selected instead of each alone, and a vector expressing both the T cell immune function promoting factor and CAR was prepared.
- the IL-7 is an essential cytokine for T cell survival, and is produced by non-hematopoietic cells such as bone marrow, thymus, and stromal cells of lymphoid organs / tissues. On the other hand, almost no ability to produce T cells is observed.
- the CCL19 is mainly produced from lymph node dendritic cells and macrophages, and has a function of inducing migration of T cells, B cells, and mature dendritic cells via its receptor CCR7.
- Anti-FITC CAR DNA fragment (SEQ ID NO: 7) encoding anti-FITC CAR consisting of anti-FITC scFv, mouse CD8 transmembrane region, mouse CD28-4-1BB-CD3 ⁇ intracellular signal motif, 2A peptide shown in SEQ ID NO: 1 (F2A ), A F2A-MCS DNA fragment (SEQ ID NO: 8) encoding the restriction enzyme site (MCS) following the peptide, mouse IL-7 (no stop codon), followed by IL-7 encoding F2A and mouse CCL19 -F2A-CCL19 DNA fragment (SEQ ID NO: 9) was artificially synthesized.
- 1-819th is anti-FITC scFv
- 829-1074 is mouse CD8 transmembrane region
- 1075-1197 is mouse CD28 intracellular region
- 1198-1332 is 4-1BB intracellular region
- the 1333 to 1674th sequence is a sequence encoding a polypeptide in the intracellular region of CD3 ⁇ .
- the 1st to 462nd sequences encode IL-7
- the 463th to 537th sequences encode F2A
- the 538th to 864th sequences encode CCL19.
- CAR vectors expressing CAR, IL-7 and CCL19 the anti-FITC CAR DNA fragment and the F2A-MCS DNA fragment were ligated to prepare an anti-FITC CAR-F2A-MCS construct.
- the constructed construct was cloned into a pMSGV retrovirus expression vector (Tamada k et al., Clin Cancer Res18: 6436-6445 (2002)) to prepare a pMSGV vector containing anti-FITC CAR-F2A-MCS.
- IL-7 / CCL19 expression-anti-FITC CAR vector was obtained.
- the layout of the obtained vector is shown in FIG.
- an anti-FITC CAR DNA fragment was cloned into a pMSGV retrovirus expression vector to prepare a pMSGV vector (control anti-FITC CAR vector) containing anti-FITC CAR.
- Retroviruses were generated for transduction of mouse T cells. Using Lipofectamine 2000 or 3000 (Life Technologies), the above-mentioned IL-7 / CCL19 expression-anti-FITC CAR vector or control anti-FITC CAR vector and the pCL-Eco plasmid (Imgenex) were added to GP2-293 packaging cells. A retrovirus having IL-7 / CCL19 expression-anti-FITC CAR vector or control anti-FITC CAR vector introduced therein was prepared by transfection into a strain (manufactured by Takara Bio Inc.). The supernatant containing the retrovirus was collected 48 hours after transfection.
- DMEM fetal calf serum
- RPMI-1640 containing 10% FCS, 100 U / ml penicillin, 100 mg / ml streptomycin, 50 mM 2-mercaptoethanol, and 2 mM L-glutamine was added.
- RPMI-1640 containing 10% FCS, 100 U / ml penicillin, 100 mg / ml streptomycin, 50 mM 2-mercaptoethanol, and 2 mM L-glutamine was added.
- mice T cells Transduction of mouse T cells
- 3 ⁇ 10 6 purified mouse T cells derived from the spleen and lymph nodes were mixed with solidified anti-CD3 monoclonal antibody (3 ⁇ g / ml), anti-CD28 monoclonal antibody (1 ⁇ g / ml).
- IL-2 100 IU / ml
- 25 ⁇ g / ml of retronectin registered trademark: manufactured by Takara Bio Inc.
- retronectin registered trademark: manufactured by Takara Bio Inc.
- mice T cells (1 ⁇ 10 6 cells / ml) activated on the plate coated with, and centrifuged at 1500 rpm for 2 hours, and then cultured in the presence of IL-2 (100 IU / ml) for 6 hours. .
- mouse T cells were collected, transferred to a new growth medium (RPMI) containing IL-2 (100 IU / ml), and further cultured for 42 hours to express IL-7 / CCL19 -Mouse T cells introduced with an anti-FITC CAR vector (anti-FITC CAR-IL-7 / CCL19 expressing T cells) or mouse T cells introduced with a control anti-FITC CAR vector (anti-FITC CAR expressing T cells) were obtained.
- RPMI new growth medium
- IL-2 100 IU / ml
- the anti-FITC scFv region sequence contained in the sequence shown in SEQ ID NO: 7 is an anti-human CD20 scFv synthesized by Life Technology Co. based on the sequence of rituximab ( A pMSGV vector (IL-7 / CCL19 expression-anti-human CD20 CAR vector) containing anti-human CD20 CAR-F2A-IL-7-F2A-CCL19 was prepared in the same manner except that the sequence was replaced with the sequence of SEQ ID NO: 10) did.
- control anti-FITC CAR vector in the production of the control anti-FITC CAR vector in the above, the sequence of the anti-FITC scFv region contained in the sequence shown in SEQ ID NO: 7 was replaced with the sequence of the anti-human CD20 scFv (SEQ ID NO: 10).
- a pMSGV vector control anti-human CD20 CAR vector
- Such IL-7 / CCL19 expression-anti-human CD20 CAR vector or control anti-human CD20 CAR vector is introduced into mouse T cells by the same method as described above, and anti-human CD20 CAR-IL-7 / CCL19-expressing T cells or Anti-human CD20 CAR expressing T cells were prepared.
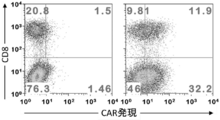
- the expression level of CAR that recognizes human CD20 was also determined by two-color flow cytometry analysis.
- the prepared anti-human CD20 CAR-IL-7 / CCL19-expressing T cells were analyzed using biotin-labeled protein L and APC-conjugated streptavidin.
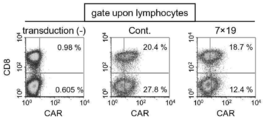
- FIG. 4 shows the results of the expression T cells. In FIG. 4, “transduction ( ⁇ )” indicates a T cell without transduction, “Cont.” Indicates an anti-FITC CAR expression T cell, and “7 ⁇ 19” indicates an anti-FITC CAR-IL- In FIG.
- transduction ( ⁇ ) is a T cell without transduction
- Cont Is an anti-human CD20 CAR-expressing T cell
- 7 ⁇ 19 is an anti-human CD20 CAR.
- -Results for IL-7 / CCL19 expressing T cells Moreover, the numerical value in a figure represents the percentage of each population. As shown in FIGS. 3 to 5, CAR expression was confirmed in anti-FITC CAR-IL-7 / CCL19-expressing T cells and anti-human CD20 CAR-IL-7 / CCL19-expressing T cells.
- IL-7 and CCL19 secretion Measurement of IL-7 and CCL19 concentrations in culture supernatant of anti-FITC CAR-IL-7 / CCL19-expressing T cells-1)
- the prepared anti-FITC CAR-IL-7 / CCL19-expressing T cells or anti-FITC CAR-expressing T cells were stimulated with 1 ⁇ g / ml of solidified FITC-bound trastuzumab and cultured for 3 days, and the supernatant was recovered and IL-
- the concentrations of 7 and CCL19 were measured using a commercially available ELISA kit (manufactured by R & D systems). The results are shown in FIG.
- IL-7 As shown in FIG. 6, in the culture supernatant, IL-7 was detected at 300 pg / ml or more, and CCL19 was detected at 75 pg / ml or more. Therefore, it was confirmed that the anti-FITC CAR-IL-7 / CCL19-expressing T cells express IL-7 and CCL19, and the expressed IL-7 and CCL19 are secreted extracellularly. In the control anti-FITC CAR-expressing T cells, both IL-7 and CCL19 were below the detection limit (Not detected).
- IL-7 and CCL-19 concentrations after 3, 5, and 7 days of culture with and without stimulation with solidified FITC-conjugated trastuzumab or anti-CD3 monoclonal antibody were measured using an ELISA kit. The results are shown in FIG. In FIG. 7, the white column shows no stimulation, the gray column shows stimulation with FITC-conjugated trastuzumab, and the black column shows stimulation with anti-CD3 monoclonal antibody. “Cont.” Indicates an anti-FITC CAR-expressing T cell, and “7 ⁇ 19” indicates an anti-FITC CAR-IL-7 / CCL19-expressing T cell.
- IL-7 and CCL-19 are secreted extracellularly in anti-FITC CAR-IL-7 / CCL19-expressing T cells not only for 3 days but also for 5 days or 7 days. Became clear.
- the white column shows no stimulation
- the shaded column shows stimulation with P815 treated with mitomycin C
- the black column shows stimulation with P815-hCD20
- the gray column shows stimulation with a solidified anti-CD3 monoclonal antibody.
- Cons. Indicates an anti-human CD20 CAR-expressing T cell
- “7 ⁇ 19” indicates an anti-human CD20 CAR-IL-7 / CCL19-expressing T cell.
- the black column indicates anti-FITC CAR-IL-7 / CCL19-expressing T cells
- the white column indicates anti-FITC CAR-expressing T cells
- the horizontal axis indicates the number of culture days.
- anti-FITC CAR-IL-7 / CCL19-expressing T cells have both increased cell proliferation and survival rate, and are produced by anti-FITC CAR-IL-7 / CCL19-expressing T cells. It became clear that IL-7 and CCL19 are exerting biological functions.
- Iso.Cntrl Is rat IgG2a isotype control
- anti-CD127 is anti-CD127 monoclonal neutralizing antibody
- anti-CCR7 is anti-CCR7 monoclonal neutralizing antibody.
- the black column indicates anti-human CD20 CAR-IL-7 / CCL19-expressing T cells
- the white column indicates anti-human CD20 CAR-expressing T cells.
- Each data is shown as the mean ⁇ standard deviation of three wells, *: P ⁇ 0.05, ⁇ : P ⁇ 0.001.
- T cell migration test T cell migration test using anti-FITC CAR-IL-7 / CCL19-expressing T cells
- the effect of CCL19 to induce migration was examined by a cell migration test using a transwell.
- Responsive T cell migration was measured by migration through a polycarbonate filter with a pore size of 5 ⁇ m using a 96-well Transwell® chamber (Cornig Costar).
- anti-FITC CAR-IL-7 / CCL19-expressing T cells or anti-FITC CAR-expressing T cells were stimulated with 1 ⁇ g / ml of solidified FITC-conjugated trastuzumab for 3 days in the lower layer of the chamber.
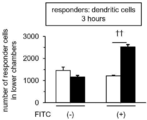
- Responder T cells were prepared from spleen and lymph nodes by negative selection of MACS® (Miltenyi Biotec). Responding T cells were labeled with CytoTell blue (manufactured by AAT Bioquest) and cultured in the upper layer for 3 hours. Migration from the upper layer to the lower layer of the chamber was examined by flow cytometry. The results are shown in FIG. In FIG. 12, the black column indicates anti-FITC CAR-IL-7 / CCL19-expressing T cells, the white column indicates anti-FITC CAR-expressing T cells, and the vertical axis indicates the absolute number of responding T cells that have migrated to the lower chamber. (The same applies to FIGS. 13 and 14 below). Statistical significance was examined by Student's t-test (* p ⁇ 0.05).
- lymphocyte transfer therapy such as CAR-expressing T cells
- lymphocytes having antitumor activity are not simply transferred from the outside, but by some technique, an active interaction between the transferred T cells and the endogenous T cells is induced, and the endogenous T cells are removed.
- anti-FITC CAR-IL-7 / CCL19-expressing T cells have the ability to accumulate endogenous T cells, and thus induce an active interaction between the transferred T cells and the endogenous T cells. It became clear that it was possible to do.
- T cell or dendritic cell migration test using anti-FITC CAR-IL-7 / CCL19-expressing T cells FITC-conjugated trastuzumab or anti-CD3 monoclonal in which anti-FITC CAR-IL-7 / CCL19-expressing T cells or samples containing anti-FITC CAR-expressing T cells (5 ⁇ 10 5 cells) were solidified in the lower chamber of the transwell Stimulated with antibody. On day 3, 4 ⁇ 10 5 T cells stained with CytoTell Blue were placed in the upper layer and incubated for 3 hours or 5 hours.
- each sample was stimulated with solidified FITC-conjugated trastuzumab, and on day 3, 4 ⁇ 10 5 dendritic cells stained with CytoTell Blue were placed in the upper layer and incubated for 3 hours.
- Each responder cell that migrated from the upper layer to the lower layer was analyzed by flow cytometry.
- black columns indicate anti-FITC CAR-IL-7 / CCL19-expressing T cells
- white columns indicate anti-FITC CAR-expressing T cells.
- each data is shown with the average +/- standard deviation of three wells, *: P ⁇ 0.05, **: P ⁇ 0.01, ⁇ : P ⁇ 0.001, ⁇ : P ⁇ 0.00001, ⁇ : P ⁇ 5 ⁇ 10 -5 .
- T cell migration test using anti-human CD20 CAR-IL-7 / CCL19-expressing T cells Samples containing anti-human CD20 CAR-IL-7 / CCL19-expressing T cells (1 ⁇ 10 5 cells) were co-cultured with mitomycin C-treated P815-hCD20 in the lower chamber of the transwell. On day 3, 4 ⁇ 10 5 T cells stained with CytoTell Blue were placed in the upper layer and incubated for 3 hours in the presence of rat IgG2a isotype control, anti-CD127 monoclonal antibody, or anti-CCR7 monoclonal antibody. Each responder T cell that migrated from the upper layer to the lower layer was analyzed by flow cytometry. The results are shown in FIG. In FIG. 15, “Iso. This is the case. In FIG. 15, the black column indicates anti-human CD20 CAR-IL-7 / CCL19-expressing T cells, and the white column indicates anti-human CD20 CAR-expressing T cells.
- the anti-human CD20 CAR-IL-7 / CCL19-expressing T cells have a high ability to accumulate endogenous T cells, and anti-CCR7 suppresses the accumulation of endogenous T cells. From these results, it was revealed that the accumulation of endogenous T cells acts via CCR7, which is a receptor for CCL19.
- CCL19 has an important effect indispensable for immunity induction, such as accumulation of T cells and dendritic cells locally in cancer by CCL19, and it has been clarified to have an excellent immunity induction effect. That is, by expressing two regulatory molecules “IL-7” and “CCL19” in CAR-expressing T cells, it is possible to improve the proliferation ability, survival rate, and immune induction effect of such T cells. Became clear.
- Non-stimulated spleen T cells naive T cells
- activated T cells non-stimulated Dendritic cells in the spleen of the mouse
- anti-FITC CAR-expressing T cells Cont.
- anti-FITC CAR ⁇ produced by activation in the same manner as in “Transduction of mouse T cells” in Example 1 IL-7 / CCL19-expressing T cells (7 ⁇ 19) were analyzed by flow cytometry, and the expression of CD127 or CDR7 was examined.
- T cells are a population of CD3 + CD19 ⁇
- anti-FITC CAR-expressing T cells anti-FITC CAR-IL-7 / CCL19-expressing T cells are positive for FITC-conjugated dextran beads
- dendritic cells are CD11c + The population.
- the result of examining CD127 expression is shown in FIG. 18, and the result of examining CCR7 expression is shown in FIG. In the figure, the numerical values are positive%, “Cont.” Indicates anti-FITC CAR-expressing T cells, and “7 ⁇ 19” indicates anti-FITC CAR-IL-7 / CCL19-expressing T cells.
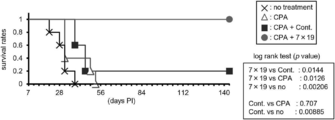
- mice The tumor volume and survival rate of mice were measured twice a week. In tumor volume analysis, standard deviations were calculated in each experimental group. Statistical significance of the three groups was examined by Student's t-tests in tumor volume analysis and log-rank test in survival studies (* P ⁇ 0.05, ** P ⁇ 0.01).
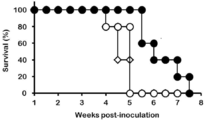
- FIG. 20 shows the result of the change in the tumor volume of the mouse
- FIG. 20 and 21 ⁇ is when anti-human CD20 CAR-expressing T cells are administered
- ⁇ is anti-human CD20 CAR-IL-7 / CCL19-expressing T cells are administered
- ⁇ is CAR-expressing T cells as a non-treatment group
- the horizontal axis in FIG. 20 represents the number of days elapsed after the cells were administered into the mouse vein
- the vertical axis represents the tumor volume (mm 3 )
- the horizontal axis in FIG. 21 represents the elapsed weeks after the cells were administered into the mouse vein.
- the vertical axis represents the survival rate (%).
- mice were inoculated subcutaneously with 5 ⁇ 10 5 P815-hCD20.
- cyclophosphamide (CPA, 100 mg / kg), an anticancer agent, was administered intraperitoneally, and on the 14th day, 1 ⁇ 10 6 anti-human CD20 CAR-IL-7 / CCL19 Expressed T cells or anti-human CD20 CAR expressing T cells were administered intravenously.
- CPA cyclophosphamide
- 1 ⁇ 10 6 anti-human CD20 CAR-IL-7 / CCL19 Expressed T cells or anti-human CD20 CAR expressing T cells were administered intravenously.
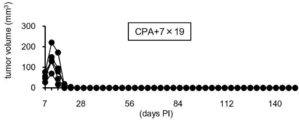
- the results of mouse survival rates are shown in FIG. 22, and the results of tumor volume are shown in FIGS.
- the horizontal axis represents the number of days after subcutaneous inoculation with P815-hCD20 (the day when mice were inoculated with P815-hCD20 subcutaneously was defined as day 0), the vertical axis represents the survival rate (FIG. 22), and tumor volume (tumor volume).
- FIG. 24 is a diagram in which the numerical value on the vertical axis of the CPA + 7 ⁇ 19 graph in FIG. 23 is set to 1/10.
- the minor axis was 4.86 mm to 7.25 mm, and the major axis was 5.92 mm to 8
- the tumor volume was 69.91 mm 3 to 220.50 mm 3 , and the average was 140.02 mm 3 .
- the above results also showed that the tumor that had once proliferated disappeared by treatment with anti-human CD20 CAR-IL-7 / CCL19-expressing T cells.
- the number of lymphocyte cells is first reduced with other anticancer agents, and then anti-human CD20 CAR-IL-7 / CCL19 expression is performed as in the above method. It is preferable to administer T cells in order to enhance the antitumor activity by the CAR-expressing T cells of the present invention. By this method, in vivo homeostasis of CAR-expressing T cells can be enhanced.
- mice were inoculated subcutaneously with 5 ⁇ 10 5 P815-hCD20.
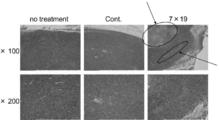
- 1 ⁇ 10 6 anti-human CD20 CAR-IL-7 / CCL19-expressing T cells were administered, and tumor tissue was cut on day 21 after inoculation.
- Each tissue was divided into two. One was stained with hematoxylin and eosin (H & E) and the other was used for immunohistochemical analysis.
- the primary antibody was a combination of anti-CD4 and anti-CD8 monoclonal antibodies, or a combination of anti-CD3 and anti-DEC205 monoclonal antibodies.
- FIGS. 27 (a) and 27 (b) the respective fluorescent staining (CD4 staining (red), CD8 staining (green), CD3 staining (red), DEC205 staining (green), coexistence of CD3 and DEC205 (yellow)
- FIGS. 27 (a) and 27 (b) The results of quantification of the positive region labeled by)) using Hybrid Cell Count program (manufactured by KEYENCE) are shown in FIGS. 27 (a) and 27 (b), respectively. 25 to 27, “no treatment” or “no treat.” Is untreated, “Cont.” Is an anti-human CD20 CAR-expressing T cell, and 7 ⁇ 19 is an anti-human CD20 CAR-IL-7 / CCL19-expressing T cell. This is the group processed by.
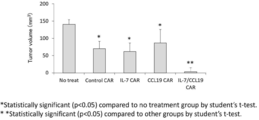
- mice without administration of CAR-expressing T cells that do not express IL-7 and CCL19 were provided.
- the long axis and short axis of the tumor were measured, and the tumor volume (mm 3 ) was calculated in the same manner as described above. The results are shown in FIG. In FIG.
- Anti-human CD20 CAR-IL-7 expressing T cells were prepared as a pMSGV vector (IL-7 expression-anti-human CD20 CAR vector) containing anti-human CD20 CAR-F2A-IL-7. It was obtained by introducing into mouse T cells in the same manner as in “Transduction of T cells”. Similarly, the anti-human CD20 CAR-CCL19-expressing T cells were prepared as a pMSGV vector (CCL19 expression-anti-human CD20 CAR vector) containing anti-human CD20 CAR-F2A-CCL19. It was obtained by introducing into mouse T cells by the same method as described above.
- Each vector was prepared in accordance with the methods of “Preparation of anti-FITC CAR expression vector expressing IL-7 and CCL19” and “Preparation of anti-CD20 CAR expression vector expressing IL-7 and CCL19” in Example 1. I went.
- the sequence encoding IL-7 used was the 1st to 462nd sequence followed by a stop codon sequence in SEQ ID NO: 9, and the sequence encoding CCL19 was the 538th to 864th sequence in SEQ ID NO: 9. The results are shown in FIG.
- T cell cytotoxic activity by 51 Cr release assay-1 selection of T cell immune function promoting factor
- an inhibitory signal is transmitted to immune cells and the antitumor immune response is inhibited, thereby reducing the effect of immunotherapy.
- Inhibitory signals to immune cells are transmitted by SHP-1 and SHP-2. Therefore, in the T cell therapy for cancer, it is possible to enhance the antitumor effect by producing a dominant negative mutant that inhibits the action of SHP-1 or SHP-2 in the T cell itself. Therefore, a vector expressing both CAR and a dominant negative mutant that inhibits the action of SHP-1 and SHP-2 was prepared, and the tumor cytotoxic activity was examined.
- a DNA fragment encoding a dominant negative mutant (SHP1DN) of mouse SHP1 containing a mutation of the catalytic cysteine residue at position 453 to serine (C453S) was prepared by site-directed mutagenesis by PCR, and position 459
- a DNA fragment encoding a dominant negative mutant (SHP2DN) of mouse SHP2 containing a mutation of a catalytic cysteine residue to serine (C459S) was synthesized by Life Technology.
- the base sequence encoding mouse SHP1DN is shown in SEQ ID NO: 11, and the base sequence encoding mouse SHP2DN is shown in SEQ ID NO: 12.
- the 3 bases from 1357 to 1359 in SEQ ID NO: 11 and 1375 to 1377 in SEQ ID NO: 12 are mutation sites.
- a DNA fragment encoding SHP1DN or SHP2DN was inserted into the MCS of the pMSGG vector containing the anti-human CD20 scFv CAR-F2A-MCS in the IL-7 / CCL19 expression-anti-human CD20 CAR vector preparation process of Example 2, and SHP1DN Expression-anti-human CD20 CAR vector, SHP2DN expression-anti-human CD20 CAR vector were obtained.
- the layout of the resulting vector is shown in FIG.
- the SHP1DN expression-anti-human CD20 CAR vector and SHP2DN expression-anti-human CD20 CAR vector were introduced into mouse T cells in the same manner as in Example 1, and anti-human CD20 CAR-SHP1DN expression T cells and anti-human CD20 CAR were respectively obtained.
- -SHP2DN expressing T cells were obtained.
- the anti-human CD20 CAR-expressing T cell prepared in Example 1 was used.
- Tumor cytotoxic activity by 51 Cr release assay The cytotoxic activity of CAR-expressing T cells against tumors was measured by a standard 4 hour 51 Cr release assay.
- P815 (P815-hCD20) expressing human CD20 was used as a target tumor cell. The tumor cells were collected, cultured for 1 hour at 37 ° C. in the presence of 100 ⁇ Ci Na 2 51 CrO 4 , and then washed three times. Thereafter, as effector T cells, co-cultured with anti-human CD20 CAR-expressing T cells, anti-human CD20 CAR-SHP1DN-expressing T cells, or anti-human CD20 CAR-SHP2DN-expressing T cells.
- the effector / target ratio was set to 0.6, 1.25, 2.5, 5, and 10.
- Maximum release and spontaneous release of the target cells were measured by culturing the cells in a culture solution containing 10% Triton-X (Sigma Aldrich) or a culture solution alone.
- the 51 Cr release in the supernatant was measured with a TopCount scintillation counter (PerkinElmer).
- ⁇ is an anti-human CD20 CAR-expressing T cell
- ⁇ is an anti-human CD20 CAR-SHP1DN-expressing T cell
- ⁇ is an anti-human CD20 CAR-expressing T cell
- Anti-human CD20 CAR-SHP2DN expressing T cells The horizontal axis represents the ratio of effector (T cell) to target (tumor cell) as E / T ratio, and the vertical axis represents specific injury (%).
- Statistical significance was examined by Student's t-test (* p ⁇ 0.05).
- anti-human CD20 CAR-SHP1DN-expressing T cells and anti-human CD20 CAR-SHP2DN-expressing T cells may have significantly higher tumor cytotoxic activity than anti-human CD20 CAR-expressing T cells. It became clear.
- ⁇ indicates that the mixture was mixed with anti-FITC CAR-expressing T cells in the presence of FITC-bound rituximab
- ⁇ indicates that the mixture was mixed with anti-FITC CAR-expressing T cells in the presence of unlabeled rituximab, and FITC binding.
- ⁇ when mixed with anti-FITC CAR-IL-7 / CCL19-expressing T cells in the presence of rituximab, and mixed with anti-FITC CAR-IL-7 / CCL19-expressing T cells in the presence of unlabeled rituximab Indicated by “ ⁇ ”.
- 815-hCD20 (1 ⁇ 10 4 cells / well) is treated with anti-human CD20 CAR so that the effector / target (E / T) ratio is 0.3125, 0.625, 2.5, 5, 10, 20
- the mixture was mixed with the expressed T cells or anti-human CD20 CAR-IL-7 / CCL19-expressing T cells, and the 51 Cr release of the supernatant was measured by the same method as described above, and the ratio of the injury activity was calculated.
- the results are shown in FIG. In FIG. 32, “ ⁇ ” indicates a case where the cells are mixed with anti-human CD20 CAR-IL-7 / CCL19-expressing T cells, and “ ⁇ ” indicates a case where the cells are mixed with anti-human CD20 CAR-expressing T cells.
- anti-FITC CAR-IL-7 / CCL19-expressing T cells maintain the same tumor cytotoxic activity per cell as anti-FITC CAR-expressing T cells, and similarly anti-human CD20 It was revealed that CAR-IL-7 / CCL19-expressing T cells maintained the same level of tumor cytotoxicity per cell as that of anti-human CD20 CAR-expressing T cells.
- mice [Survival of CAR-expressing T cells in vivo and differentiation into memory T cells] (Flow cytometry analysis) DBA / 2 mice were inoculated subcutaneously with 5 ⁇ 10 5 P815-hCD20. On day 10 after inoculation, cyclophosphamide (CPA, 100 mg / kg), an anticancer drug, was administered intraperitoneally, and on day 14 1 ⁇ 10 6 anti-human CD20 CAR-IL-7 / CCL19 Expressed T cells or anti-human CD20 CAR expressing T cells were administered intravenously. On the 21st day after administration of CAR-expressing T cells, leukocytes were isolated from the spleen or tumor-affected lymph nodes (armpit, upper arm, inguinal).
- FIG. 33 shows the results of analyzing CD4, CD8, CD44, and CD62L with respect to the leukocyte surface phenotype by flow cytometry. Further, FIG. 34 shows the results of spleen leukocytes cultured and stimulated with P815-hCD20 treated with mitomycin C for 4 days and examined for T cell proliferation by flow cytometry. CAR expression was confirmed using biotin-labeled protein L and APC-conjugated streptavidin.
- the numbers in FIG. 33 are the gate regions of CD4 + T cells and CD8 + T cells (CD62L + CD44 ⁇ is naive T cells, CD62L + CD44 + is central memory T cells, and CD62L ⁇ CD44 + is effector memory T cells). The numbers in FIG.
- the CAR expression vector of the present invention By using the CAR expression vector of the present invention, it becomes possible to produce CAR-T cells having both viability and lymphocyte accumulation ability, and CAR-T cells having resistance to immunosuppression in a cancer microenvironment. Therefore, it can be used in the field of cancer immunotherapy.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Zoology (AREA)
- Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Immunology (AREA)
- Biomedical Technology (AREA)
- Medicinal Chemistry (AREA)
- Biophysics (AREA)
- Molecular Biology (AREA)
- Biotechnology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Wood Science & Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Cell Biology (AREA)
- Gastroenterology & Hepatology (AREA)
- Toxicology (AREA)
- General Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Pharmacology & Pharmacy (AREA)
- Physics & Mathematics (AREA)
- Plant Pathology (AREA)
- Virology (AREA)
- Hematology (AREA)
- Developmental Biology & Embryology (AREA)
- Mycology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Priority Applications (27)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| SI201530950T SI3205720T1 (sl) | 2014-10-09 | 2015-10-06 | CAR-ekspresijski vektor in CAR-ekspresijske T-celice |
| ES15849416T ES2751945T3 (es) | 2014-10-09 | 2015-10-06 | Vector de expresión de un RAQ y células T que expresan un RAQ |
| BR112017006710A BR112017006710B1 (pt) | 2014-10-09 | 2015-10-06 | vetor de expressão de um receptor de antígeno quimérico e agente anticancerígeno |
| HRP20191839TT HRP20191839T1 (hr) | 2014-10-09 | 2015-10-06 | Car ekspresijski vektor i t stanice koje eksprimiraju car |
| EP15849416.1A EP3205720B1 (en) | 2014-10-09 | 2015-10-06 | Car expression vector and car-expressing t cells |
| SG11201702391RA SG11201702391RA (en) | 2014-10-09 | 2015-10-06 | Car expression vector and car-expressing t cells |
| CN201580053922.1A CN107109421B (zh) | 2014-10-09 | 2015-10-06 | Car表达载体及car表达t细胞 |
| RU2017114545A RU2670147C1 (ru) | 2014-10-09 | 2015-10-06 | Вектор экспрессии car и car-экспрессирующие т-клетки |
| JP2016552830A JP6161098B2 (ja) | 2014-10-09 | 2015-10-06 | Car発現ベクター及びcar発現t細胞 |
| NZ730382A NZ730382A (en) | 2014-10-09 | 2015-10-06 | Car expression vector and car-expressing t cells |
| SM20190663T SMT201900663T1 (it) | 2014-10-09 | 2015-10-06 | Vettore di espressione car e cellule t che esprimono car |
| LT15849416T LT3205720T (lt) | 2014-10-09 | 2015-10-06 | Car raiškos vektorius, ir car ekspresuojančios ląstelės |
| AU2015329444A AU2015329444B2 (en) | 2014-10-09 | 2015-10-06 | CAR expression vector and CAR-expressing T cells |
| EP19196196.0A EP3597742B1 (en) | 2014-10-09 | 2015-10-06 | Car expression vector and car-expressing t cells |
| MX2017004393A MX358472B (es) | 2014-10-09 | 2015-10-06 | Vector de expresion de receptor de antigeno quimerico (car) y celulas t que expresan car. |
| DK15849416.1T DK3205720T3 (da) | 2014-10-09 | 2015-10-06 | Car-eksprimeringsvektor og car-eksprimerende t-celler |
| CA2962375A CA2962375C (en) | 2014-10-09 | 2015-10-06 | Car expression vector and car-expressing t cells |
| PL15849416T PL3205720T3 (pl) | 2014-10-09 | 2015-10-06 | Wektor ekspresyjny CAR i komórki T eksprymujące CAR |
| US15/513,870 US10316102B2 (en) | 2014-10-09 | 2015-10-06 | Car expression vector and car-expressing T cells |
| KR1020177009895A KR101890638B1 (ko) | 2014-10-09 | 2015-10-06 | Car 발현 벡터 및 car 발현 t 세포 |
| RSP20191315 RS59441B1 (sr) | 2014-10-09 | 2015-10-06 | Car ekspresioni vektor i t ćelije koje eksprimiraju car |
| ZA2017/01968A ZA201701968B (en) | 2014-10-09 | 2017-03-22 | Car expression vector and car-expressing t cells |
| PH12017500596A PH12017500596B1 (en) | 2014-10-09 | 2017-03-31 | Car expression vector and car-expressing t cells |
| IL251504A IL251504B (en) | 2014-10-09 | 2017-04-02 | car expression vector and t cells expressing car |
| US16/404,165 US10906984B2 (en) | 2014-10-09 | 2019-05-06 | CAR expression vector and CAR-expressing T cells |
| CY20191101239T CY1122324T1 (el) | 2014-10-09 | 2019-11-26 | Διαβιβαστης εκφρασης car και τ-κυτταρα εκφρασης car |
| US17/133,286 US20210253726A1 (en) | 2014-10-09 | 2020-12-23 | Car expression vector and car-expressing t cells |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014-208200 | 2014-10-09 | ||
| JP2014208200 | 2014-10-09 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US15/513,870 A-371-Of-International US10316102B2 (en) | 2014-10-09 | 2015-10-06 | Car expression vector and car-expressing T cells |
| US16/404,165 Continuation US10906984B2 (en) | 2014-10-09 | 2019-05-06 | CAR expression vector and CAR-expressing T cells |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016056228A1 true WO2016056228A1 (ja) | 2016-04-14 |
Family
ID=55652864
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/005080 Ceased WO2016056228A1 (ja) | 2014-10-09 | 2015-10-06 | Car発現ベクター及びcar発現t細胞 |
Country Status (29)
| Country | Link |
|---|---|
| US (3) | US10316102B2 (enExample) |
| EP (2) | EP3205720B1 (enExample) |
| JP (6) | JP6161098B2 (enExample) |
| KR (1) | KR101890638B1 (enExample) |
| CN (1) | CN107109421B (enExample) |
| AU (1) | AU2015329444B2 (enExample) |
| BR (1) | BR112017006710B1 (enExample) |
| CA (1) | CA2962375C (enExample) |
| CY (1) | CY1122324T1 (enExample) |
| DK (2) | DK3205720T3 (enExample) |
| ES (2) | ES2751945T3 (enExample) |
| HR (2) | HRP20221211T1 (enExample) |
| HU (2) | HUE060047T2 (enExample) |
| IL (1) | IL251504B (enExample) |
| LT (2) | LT3597742T (enExample) |
| MX (1) | MX358472B (enExample) |
| MY (1) | MY167722A (enExample) |
| NZ (1) | NZ730382A (enExample) |
| PH (1) | PH12017500596B1 (enExample) |
| PL (2) | PL3597742T3 (enExample) |
| PT (2) | PT3205720T (enExample) |
| RS (2) | RS63601B1 (enExample) |
| RU (1) | RU2670147C1 (enExample) |
| SG (1) | SG11201702391RA (enExample) |
| SI (2) | SI3205720T1 (enExample) |
| SM (1) | SMT201900663T1 (enExample) |
| TW (1) | TWI688651B (enExample) |
| WO (1) | WO2016056228A1 (enExample) |
| ZA (1) | ZA201701968B (enExample) |
Cited By (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2017153487A (ja) * | 2014-10-09 | 2017-09-07 | 国立大学法人山口大学 | Car発現ベクター及びcar発現t細胞 |
| WO2017159736A1 (ja) * | 2016-03-17 | 2017-09-21 | 国立大学法人山口大学 | 免疫機能制御因子を発現する免疫担当細胞及び発現ベクター |
| JP2018517410A (ja) * | 2015-06-01 | 2018-07-05 | ユーシーエル ビジネス ピーエルシー | 細胞 |
| WO2018131586A1 (ja) | 2017-01-10 | 2018-07-19 | 国立大学法人山口大学 | 抗gpc3抗体 |
| CN108484777A (zh) * | 2017-03-31 | 2018-09-04 | 李吉祐 | 人源化rp215单克隆抗体的嵌合抗原受体构建体及核酸分子与应用 |
| WO2018181207A1 (ja) | 2017-03-27 | 2018-10-04 | ノイルイミューン・バイオテック株式会社 | キメラ抗原受容体 |
| WO2019073973A1 (ja) | 2017-10-10 | 2019-04-18 | 国立大学法人山口大学 | メモリー機能を有するt細胞又はb細胞の増強剤、悪性腫瘍再発抑制剤、及びt細胞又はb細胞にメモリー機能を誘導する誘導剤 |
| WO2019124468A1 (ja) | 2017-12-24 | 2019-06-27 | ノイルイミューン・バイオテック株式会社 | ヒトメソセリンを特異的に認識する細胞表面分子、il-7、及びccl19を発現する免疫担当細胞 |
| WO2020017479A1 (ja) | 2018-07-17 | 2020-01-23 | ノイルイミューン・バイオテック株式会社 | 抗gpc3一本鎖抗体を含むcar |
| WO2020045610A1 (ja) | 2018-08-31 | 2020-03-05 | ノイルイミューン・バイオテック株式会社 | Car発現t細胞及びcar発現ベクター |
| CN112689679A (zh) * | 2019-07-23 | 2021-04-20 | 株式会社东芝 | 产生car-t细胞的方法、核酸引入载体和试剂盒 |
| WO2021085497A1 (ja) | 2019-10-28 | 2021-05-06 | ノイルイミューン・バイオテック株式会社 | がんを治療するための医薬、組み合わせ医薬、医薬組成物、免疫応答性細胞、核酸送達媒体、及び製品 |
| JP2021523743A (ja) * | 2018-05-15 | 2021-09-09 | カースゲン セラピューティクス カンパニー リミテッドCarsgen Therapeutics Co., Ltd. | 遺伝子操作された細胞及びその応用 |
| WO2021256522A1 (ja) | 2020-06-17 | 2021-12-23 | 国立大学法人京都大学 | キメラ抗原受容体発現免疫担当細胞 |
| US11225520B2 (en) | 2016-02-16 | 2022-01-18 | Dana-Farber Cancer Institute, Inc. | Immunotherapy compositions and methods |
| WO2022025638A1 (ko) | 2020-07-29 | 2022-02-03 | (주)티카로스 | 면역시냅스를 안정화시키는 키메라 항원 수용체(car) t 세포 |
| JP2022513076A (ja) * | 2018-11-19 | 2022-02-07 | ボード オブ リージェンツ,ザ ユニバーシティ オブ テキサス システム | Carおよびtcr形質導入用のモジュール式ポリシストロニックベクター |
| JP2022524042A (ja) * | 2019-03-08 | 2022-04-27 | オートラス リミテッド | 操作されたキメラ抗原受容体およびcarの調節因子を含む組成物および方法 |
| JP2022529618A (ja) * | 2019-04-04 | 2022-06-23 | 上海医薬集団股▲フン▼有限公司 | 腫瘍抗原認識受容体を有する免疫細胞とその応用 |
| KR20220156460A (ko) | 2021-05-18 | 2022-11-25 | 주식회사 바이오솔루션 | 세포외소포체 분비능이 증진된 면역세포 및 이를 활용한 면역 항암요법 |
| WO2023007431A1 (en) | 2021-07-29 | 2023-02-02 | Takeda Pharmaceutical Company Limited | Engineered immune cell that specifically targets mesothelin and uses thereof |
| JP2023513752A (ja) * | 2020-02-14 | 2023-04-03 | ベイジン ヨンタイ ルイケ バイオテクノロジー カンパニー リミテッド | 外部から導入された細胞シグナル伝達調節因子を過剰発現する免疫細胞及びその使用 |
| RU2802824C2 (ru) * | 2017-10-10 | 2023-09-04 | Ямагути Университи | Энхансер t-клеток или b-клеток, имеющих функцию памяти, ингибитор рецидива злокачественной опухоли и индуктор для индуцирования функции памяти в t-клетках или b-клетках |
| US12466869B2 (en) | 2018-06-28 | 2025-11-11 | Dana-Farber Cancer Institute, Inc. | Targeting of multiple antigens with multiplex CAR T cells in solid and liquid malignancies |
Families Citing this family (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11447562B2 (en) * | 2017-01-01 | 2022-09-20 | Chi-Yu Gregory Lee | RP215 chimeric antigen receptor construct and methods of making and using same |
| WO2019131770A1 (ja) * | 2017-12-27 | 2019-07-04 | 武田薬品工業株式会社 | 核酸含有脂質ナノ粒子及びその用途 |
| CN108641000A (zh) * | 2018-04-26 | 2018-10-12 | 上海怡豪生物科技有限公司 | 肝癌的双靶点car-t治疗载体及其构建方法和应用 |
| CN108641001A (zh) * | 2018-04-26 | 2018-10-12 | 上海怡豪生物科技有限公司 | 结肠癌的双靶点car-t治疗载体及其构建方法和应用 |
| CN108659133A (zh) * | 2018-04-26 | 2018-10-16 | 上海怡豪生物科技有限公司 | 肺癌的双靶点car-t治疗载体及其构建方法和应用 |
| KR20190141511A (ko) * | 2018-06-14 | 2019-12-24 | 주식회사 녹십자랩셀 | 신규 펩티드, 이를 포함하는 키메라 항원 수용체 및 이를 발현하는 면역 세포 |
| CN108866004A (zh) * | 2018-06-26 | 2018-11-23 | 奥妙生物技术(广州)有限公司 | Shp-1敲除的t细胞及其构建方法 |
| CN109055430A (zh) * | 2018-08-14 | 2018-12-21 | 杭州荣泽生物科技有限公司 | 一种共表达il18和ccl19蛋白及靶向muc1基因car-t细胞的制备方法 |
| EP3848387A4 (en) * | 2018-09-05 | 2022-06-22 | Kong, Seogkyoung | CHIMERIC ANTIGEN RECEPTOR FOR SOLID TUMOR AND T-CELLS EXPRESSING A CHIMERIC ANTIGEN RECEPTOR |
| WO2020057641A1 (zh) * | 2018-09-20 | 2020-03-26 | 科济生物医药(上海)有限公司 | 表达有趋化因子的细胞及用途 |
| US20210338729A1 (en) * | 2018-10-12 | 2021-11-04 | Icell Gene Therapeutics Llc | CHIMERIC ANTIGEN RECEPTORS (CARs) COMPOSITIONS AND METHODS OF USE THEREOF |
| CN109504660B (zh) * | 2018-11-02 | 2021-08-06 | 温州启星生物技术有限公司 | 一种第四代car-t细胞及其构建方法和应用 |
| CN112080526A (zh) * | 2019-06-14 | 2020-12-15 | 南京艾德免疫治疗研究院有限公司 | 表达il-7和car的表达载体及免疫细胞 |
| CN112080525A (zh) * | 2019-06-14 | 2020-12-15 | 南京艾德免疫治疗研究院有限公司 | 一种改良型嵌合抗原受体t细胞的制备 |
| CN110922490B (zh) * | 2019-12-05 | 2023-03-03 | 浙江启新生物技术有限公司 | 分泌白介素7和趋化因子21的car表达载体及应用 |
| JP2021142156A (ja) * | 2020-03-12 | 2021-09-24 | 株式会社三洋物産 | 遊技機 |
| JP2021142158A (ja) * | 2020-03-12 | 2021-09-24 | 株式会社三洋物産 | 遊技機 |
| JP2021142159A (ja) * | 2020-03-12 | 2021-09-24 | 株式会社三洋物産 | 遊技機 |
| CN113528483B (zh) * | 2020-04-22 | 2024-08-16 | 复星凯特生物科技有限公司 | Shp2特异性失活突变蛋白及其在car-t治疗中应用 |
| GB202006820D0 (en) * | 2020-05-07 | 2020-06-24 | Autolus Ltd | Cell |
| EP4261225A4 (en) | 2020-12-10 | 2024-11-13 | Eutilex Co., Ltd. | ANTI-PD-1 ANTIBODY AND ITS USES |
| CN114717203B (zh) * | 2020-12-22 | 2023-06-27 | 广东东阳光药业有限公司 | 一种hIL7/hCCL19双基因重组溶瘤病毒及其制备方法和用途 |
| CN114716548B (zh) | 2021-01-05 | 2024-11-05 | (株)爱恩德生物 | 抗-fgfr3抗体及其用途 |
| CN112961248B (zh) * | 2021-02-22 | 2022-03-18 | 广州百暨基因科技有限公司 | 共表达IL-7和CCR2b的嵌合抗原受体融合蛋白及其应用 |
| CN113913385A (zh) * | 2021-09-01 | 2022-01-11 | 苏州璞惠卓越生物科技有限公司 | 一种抑制蛋白阻断型嵌合抗原受体修饰的免疫细胞及其用途 |
| CN113921082B (zh) * | 2021-10-27 | 2023-04-07 | 云舟生物科技(广州)股份有限公司 | 基因搜索权重调整方法、计算机存储介质及电子设备 |
| WO2023081455A2 (en) * | 2021-11-08 | 2023-05-11 | Memorial Sloan-Kettering Cancer Center | Immune cells expressing glucose transporter 5 (gluts) and compositions and methods including the same |
| JP2025516892A (ja) * | 2022-05-20 | 2025-05-30 | 武田薬品工業株式会社 | 操作された免疫細胞を産生する方法 |
| US12006353B2 (en) | 2022-07-25 | 2024-06-11 | Long Chen | Application of SCFV protein, car gene expression vector, CAR-T cell and application thereof |
| EP4563597A1 (en) | 2022-07-26 | 2025-06-04 | Aimed Bio Inc. | Anti-ror1 antibody and use thereof |
| CN115505601B (zh) * | 2022-10-17 | 2024-05-28 | 深圳市先康达生命科学有限公司 | 一种两级SynNotch逻辑调控结构及其应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013123061A1 (en) * | 2012-02-13 | 2013-08-22 | Seattle Children's Hospital D/B/A Seattle Children's Research Institute | Bispecific chimeric antigen receptors and therapeutic uses thereof |
| WO2013126729A1 (en) * | 2012-02-22 | 2013-08-29 | The Trustees Of The University Of Pennsylvania | Use of the cd2 signaling domain in second-generation chimeric antigen receptors |
| JP2014504294A (ja) * | 2010-12-14 | 2014-02-20 | ユニバーシティ オブ メリーランド,ボルチモア | 汎用の抗タグキメラ抗原受容体発現t細胞及びがんを治療する方法 |
| JP2014507118A (ja) * | 2010-12-09 | 2014-03-27 | ザ トラスティーズ オブ ザ ユニバーシティ オブ ペンシルバニア | 癌を治療するためのキメラ抗原受容体改変t細胞の使用 |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5857318A (ja) * | 1981-10-01 | 1983-04-05 | Nippon Koutai Kenkyusho:Kk | 癌細胞障害性リンパ球の製造法 |
| JP5312721B2 (ja) | 2000-11-07 | 2013-10-09 | シティ・オブ・ホープ | Cd19特異的再指向免疫細胞 |
| KR20050118057A (ko) * | 2004-04-24 | 2005-12-15 | 김상희 | 아세틸디아실글리세롤류의 화합물을 유효성분으로함유하는 항암제 및 건강식품 |
| TWI436775B (zh) * | 2007-08-24 | 2014-05-11 | Oncotherapy Science Inc | 以抗原胜肽合併化療藥劑治療胰臟癌 |
| MX340972B (es) * | 2008-10-08 | 2016-08-02 | Intrexon Corp | Celulas manipuladas por ingenieria que expresan multiples inmunomoduladores, y uso de las mismas. |
| CA2832540C (en) | 2011-04-08 | 2020-09-15 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Anti-epidermal growth factor receptor variant iii chimeric antigen receptors and use of same for the treatment of cancer |
| US20130071414A1 (en) * | 2011-04-27 | 2013-03-21 | Gianpietro Dotti | Engineered cd19-specific t lymphocytes that coexpress il-15 and an inducible caspase-9 based suicide gene for the treatment of b-cell malignancies |
| EP3692794A1 (en) * | 2011-09-16 | 2020-08-12 | Baylor College of Medicine | Targeting the tumor microenvironment using manipulated nkt cells |
| KR101956751B1 (ko) | 2011-10-07 | 2019-03-11 | 고쿠리츠다이가쿠호진 미에다이가쿠 | 키메라 항원 수용체 |
| CN102625373A (zh) | 2012-02-22 | 2012-08-01 | 中兴通讯股份有限公司 | 一种智能载波管理方法及装置 |
| PL3597742T3 (pl) * | 2014-10-09 | 2022-11-14 | Yamaguchi University | Wektor ekspresyjny CAR oraz komórki T wykazujące ekspresję CAR |
-
2015
- 2015-10-06 PL PL19196196.0T patent/PL3597742T3/pl unknown
- 2015-10-06 MX MX2017004393A patent/MX358472B/es active IP Right Grant
- 2015-10-06 SG SG11201702391RA patent/SG11201702391RA/en unknown
- 2015-10-06 RS RS20220895A patent/RS63601B1/sr unknown
- 2015-10-06 HR HRP20221211TT patent/HRP20221211T1/hr unknown
- 2015-10-06 PL PL15849416T patent/PL3205720T3/pl unknown
- 2015-10-06 DK DK15849416.1T patent/DK3205720T3/da active
- 2015-10-06 NZ NZ730382A patent/NZ730382A/en unknown
- 2015-10-06 EP EP15849416.1A patent/EP3205720B1/en active Active
- 2015-10-06 RU RU2017114545A patent/RU2670147C1/ru active
- 2015-10-06 LT LTEP19196196.0T patent/LT3597742T/lt unknown
- 2015-10-06 ES ES15849416T patent/ES2751945T3/es active Active
- 2015-10-06 HU HUE19196196A patent/HUE060047T2/hu unknown
- 2015-10-06 DK DK19196196.0T patent/DK3597742T3/da active
- 2015-10-06 HU HUE15849416A patent/HUE046710T2/hu unknown
- 2015-10-06 US US15/513,870 patent/US10316102B2/en active Active
- 2015-10-06 PT PT158494161T patent/PT3205720T/pt unknown
- 2015-10-06 PT PT191961960T patent/PT3597742T/pt unknown
- 2015-10-06 ES ES19196196T patent/ES2927366T3/es active Active
- 2015-10-06 AU AU2015329444A patent/AU2015329444B2/en active Active
- 2015-10-06 SI SI201530950T patent/SI3205720T1/sl unknown
- 2015-10-06 BR BR112017006710A patent/BR112017006710B1/pt active IP Right Grant
- 2015-10-06 SI SI201531887T patent/SI3597742T1/sl unknown
- 2015-10-06 WO PCT/JP2015/005080 patent/WO2016056228A1/ja not_active Ceased
- 2015-10-06 RS RSP20191315 patent/RS59441B1/sr unknown
- 2015-10-06 LT LT15849416T patent/LT3205720T/lt unknown
- 2015-10-06 KR KR1020177009895A patent/KR101890638B1/ko active Active
- 2015-10-06 CA CA2962375A patent/CA2962375C/en active Active
- 2015-10-06 SM SM20190663T patent/SMT201900663T1/it unknown
- 2015-10-06 JP JP2016552830A patent/JP6161098B2/ja active Active
- 2015-10-06 CN CN201580053922.1A patent/CN107109421B/zh active Active
- 2015-10-06 HR HRP20191839TT patent/HRP20191839T1/hr unknown
- 2015-10-06 EP EP19196196.0A patent/EP3597742B1/en active Active
- 2015-10-06 MY MYPI2017701137A patent/MY167722A/en unknown
- 2015-10-08 TW TW104133272A patent/TWI688651B/zh active
-
2017
- 2017-03-22 ZA ZA2017/01968A patent/ZA201701968B/en unknown
- 2017-03-31 PH PH12017500596A patent/PH12017500596B1/en unknown
- 2017-04-02 IL IL251504A patent/IL251504B/en active IP Right Grant
- 2017-06-06 JP JP2017111736A patent/JP6683990B2/ja active Active
-
2019
- 2019-05-06 US US16/404,165 patent/US10906984B2/en active Active
- 2019-11-26 CY CY20191101239T patent/CY1122324T1/el unknown
-
2020
- 2020-03-19 JP JP2020048623A patent/JP7008350B2/ja active Active
- 2020-12-23 US US17/133,286 patent/US20210253726A1/en not_active Abandoned
-
2021
- 2021-12-28 JP JP2021213604A patent/JP7300763B2/ja active Active
-
2023
- 2023-06-13 JP JP2023097065A patent/JP7527049B2/ja active Active
-
2024
- 2024-07-16 JP JP2024113188A patent/JP7755890B2/ja active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014507118A (ja) * | 2010-12-09 | 2014-03-27 | ザ トラスティーズ オブ ザ ユニバーシティ オブ ペンシルバニア | 癌を治療するためのキメラ抗原受容体改変t細胞の使用 |
| JP2014504294A (ja) * | 2010-12-14 | 2014-02-20 | ユニバーシティ オブ メリーランド,ボルチモア | 汎用の抗タグキメラ抗原受容体発現t細胞及びがんを治療する方法 |
| WO2013123061A1 (en) * | 2012-02-13 | 2013-08-22 | Seattle Children's Hospital D/B/A Seattle Children's Research Institute | Bispecific chimeric antigen receptors and therapeutic uses thereof |
| WO2013126729A1 (en) * | 2012-02-22 | 2013-08-29 | The Trustees Of The University Of Pennsylvania | Use of the cd2 signaling domain in second-generation chimeric antigen receptors |
Non-Patent Citations (7)
| Title |
|---|
| KOJI TAMADA: "Idenshi Kaihen Gijutsu ni yoru Shinki Gan Men'eki Ryoho no Kaihatsu", RESEARCH REPORTS OF UEHARA MEMORIAL FOUNDATION, vol. 28, 5 December 2014 (2014-12-05), XP009501872 * |
| PLAS D. R. ET AL.: "Cutting edge: the tyrosine phosphatase SHP-1 regulates thymocyte positive selection", J. IMMUNOL., vol. 162, no. 10, 1999, pages 5680 - 4, XP055428537 * |
| PLAS D. R. ET AL.: "Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling", SCIENCE, vol. 272, no. 5265, 1996, pages 1173 - 6, XP055428587 * |
| SATO-HASHIMOTO M. ET AL.: "Signal regulatory protein a regulates the homeostasis of T lymphocytes in the spleen", J. IMMUNOL., vol. 187, no. 1, 2011, pages 291 - 7, XP055428502 * |
| SIEGERT S. ET AL.: "Positive and negative regulation of T cell responses by fibroblastic reticular cells within paracortical regions of lymph nodes", FRONT. IMMUNOL., vol. 3, 2012, XP055428532 * |
| TAMADA K. ET AL.: "Redirecting gene -modified T cells toward various cancer types using tagged antibodies", CLIN. CANCER RES., vol. 18, no. 23, 2012, pages 6436 - 45, XP055154500, DOI: doi:10.1158/1078-0432.CCR-12-1449 * |
| YUKIMI SAKODA ET AL.: "Development of cancer immunotherapy by next generation multi- targeting CAR-T cells", THE JAPANESE JOURNAL OF CLINICAL HEMATOLOGY, vol. 55, no. 6, 30 June 2014 (2014-06-30), pages 651 - 656, XP009501876 * |
Cited By (83)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7755890B2 (ja) | 2014-10-09 | 2025-10-17 | 国立大学法人山口大学 | Car発現ベクター及びcar発現t細胞 |
| US10906984B2 (en) | 2014-10-09 | 2021-02-02 | Yamaguchi University | CAR expression vector and CAR-expressing T cells |
| JP2024150546A (ja) * | 2014-10-09 | 2024-10-23 | 国立大学法人山口大学 | Car発現ベクター及びcar発現t細胞 |
| JP2017153487A (ja) * | 2014-10-09 | 2017-09-07 | 国立大学法人山口大学 | Car発現ベクター及びcar発現t細胞 |
| JP2018517410A (ja) * | 2015-06-01 | 2018-07-05 | ユーシーエル ビジネス ピーエルシー | 細胞 |
| US11225520B2 (en) | 2016-02-16 | 2022-01-18 | Dana-Farber Cancer Institute, Inc. | Immunotherapy compositions and methods |
| US11337997B2 (en) | 2016-03-17 | 2022-05-24 | Yamaguchi University | Immunocompetent cell and expression vector expressing regulatory factors of immune function |
| JPWO2017159736A1 (ja) * | 2016-03-17 | 2019-01-31 | 国立大学法人山口大学 | 免疫機能制御因子を発現する免疫担当細胞及び発現ベクター |
| JP2023029553A (ja) * | 2016-03-17 | 2023-03-03 | 国立大学法人山口大学 | 免疫機能制御因子をコードする核酸、及びがん抗原を特異的に認識する細胞表面分子、il-7及びccl19を発現する免疫担当細胞の作製方法 |
| JP2019154455A (ja) * | 2016-03-17 | 2019-09-19 | 国立大学法人山口大学 | 免疫機能制御因子を発現する免疫担当細胞及び発現ベクター |
| JP2021121198A (ja) * | 2016-03-17 | 2021-08-26 | 国立大学法人山口大学 | 免疫機能制御因子を発現する免疫担当細胞 |
| AU2017235116B2 (en) * | 2016-03-17 | 2019-11-07 | Yamaguchi University | Immunocompetent cell and expression vector expressing regulatory factors of immune function |
| WO2017159736A1 (ja) * | 2016-03-17 | 2017-09-21 | 国立大学法人山口大学 | 免疫機能制御因子を発現する免疫担当細胞及び発現ベクター |
| US11931381B2 (en) | 2016-03-17 | 2024-03-19 | Yamaguchi University | Immunocompetent cell and expression vector expressing regulatory factors of immune function |
| JP7479082B2 (ja) | 2016-03-17 | 2024-05-08 | 国立大学法人山口大学 | 免疫機能制御因子をコードする核酸、及びがん抗原を特異的に認識する細胞表面分子、il-7及びccl19を発現する免疫担当細胞の作製方法 |
| JP2024096903A (ja) * | 2016-03-17 | 2024-07-17 | 国立大学法人山口大学 | 免疫機能制御因子をコードする核酸、及びがん抗原を特異的に認識する細胞表面分子、il-7及びccl19を発現する免疫担当細胞の作製方法 |
| US12129291B2 (en) | 2017-01-10 | 2024-10-29 | Yamaguchi University | Anti-GPC3 antibody |
| JP7389424B2 (ja) | 2017-01-10 | 2023-11-30 | 国立大学法人山口大学 | 抗gpc3抗体 |
| JP2020074776A (ja) * | 2017-01-10 | 2020-05-21 | 国立大学法人山口大学 | 抗gpc3抗体 |
| WO2018131586A1 (ja) | 2017-01-10 | 2018-07-19 | 国立大学法人山口大学 | 抗gpc3抗体 |
| US10781249B2 (en) | 2017-01-10 | 2020-09-22 | Yamaguchi University | Anti-GPC3 antibody |
| JP2022191318A (ja) * | 2017-01-10 | 2022-12-27 | 国立大学法人山口大学 | 抗gpc3抗体 |
| JP2019216739A (ja) * | 2017-01-10 | 2019-12-26 | 国立大学法人山口大学 | 抗gpc3抗体 |
| US11718663B2 (en) | 2017-01-10 | 2023-08-08 | Yamaguchi University | Anti-GPC3 antibody |
| JPWO2018131586A1 (ja) * | 2017-01-10 | 2019-11-07 | 国立大学法人山口大学 | 抗gpc3抗体 |
| EP4495141A2 (en) | 2017-03-27 | 2025-01-22 | Noile-Immune Biotech, Inc. | Chimeric antigen receptor |
| KR20190132637A (ko) * | 2017-03-27 | 2019-11-28 | 노일 이뮨 바이오테크 가부시키가이샤 | 키메라 항원 수용체 |
| EP4495141A3 (en) * | 2017-03-27 | 2025-04-23 | Noile-Immune Biotech, Inc. | Chimeric antigen receptor |
| RU2770002C2 (ru) * | 2017-03-27 | 2022-04-14 | Нойл-Иммьюн Байотек, Инк. | Химерный антигенный рецептор |
| WO2018181207A1 (ja) | 2017-03-27 | 2018-10-04 | ノイルイミューン・バイオテック株式会社 | キメラ抗原受容体 |
| KR102333377B1 (ko) | 2017-03-27 | 2021-11-30 | 노일 이뮨 바이오테크 가부시키가이샤 | 키메라 항원 수용체 |
| JPWO2018181207A1 (ja) * | 2017-03-27 | 2020-02-27 | ノイルイミューン・バイオテック株式会社 | キメラ抗原受容体 |
| TWI753141B (zh) * | 2017-03-27 | 2022-01-21 | 日商諾伊爾免疫生物科技股份有限公司 | 嵌合抗原受體 |
| CN108484777B (zh) * | 2017-03-31 | 2022-07-12 | 李吉祐 | 人源化rp215单克隆抗体的嵌合抗原受体构建体及核酸分子与应用 |
| CN108484777A (zh) * | 2017-03-31 | 2018-09-04 | 李吉祐 | 人源化rp215单克隆抗体的嵌合抗原受体构建体及核酸分子与应用 |
| IL273689B2 (en) * | 2017-10-10 | 2024-02-01 | Univ Yamaguchi | Boosts T-cells or B-cells with memory activity, suppresses recurrent malignant growth and boosts memory activity in T-cells or B-cells |
| IL273689B1 (en) * | 2017-10-10 | 2023-10-01 | Univ Yamaguchi | Increases T-cells or B-cells with memory activity, suppresses recurrent malignant growth and increases memory activity in T-cells or B-cells |
| AU2018349889B2 (en) * | 2017-10-10 | 2024-02-08 | National University Corporation Tottori University | Enhancer for T-cells or B-cells having memory function, malignant tumor recurrence inhibitor, and inducer for inducing memory function in T-cells or B-cells |
| WO2019073973A1 (ja) | 2017-10-10 | 2019-04-18 | 国立大学法人山口大学 | メモリー機能を有するt細胞又はb細胞の増強剤、悪性腫瘍再発抑制剤、及びt細胞又はb細胞にメモリー機能を誘導する誘導剤 |
| EP4265634A3 (en) * | 2017-10-10 | 2024-01-24 | National University Corporation Tottori University | Enhancer for t-cells or b-cells having memory function, malignant tumor recurrence inhibitor, and inducer for inducing memory function in t-cells or b-cells |
| AU2024201332B2 (en) * | 2017-10-10 | 2025-05-08 | National University Corporation Tottori University | Enhancer for t-cells or b-cells having memory function, malignant tumor recurrence inhibitor, and inducer for inducing memory function in t-cells or b-cells |
| EP4265634A2 (en) | 2017-10-10 | 2023-10-25 | National University Corporation Tottori University | Enhancer for t-cells or b-cells having memory function, malignant tumor recurrence inhibitor, and inducer for inducing memory function in t-cells or b-cells |
| JPWO2019073973A1 (ja) * | 2017-10-10 | 2020-11-05 | 国立大学法人山口大学 | メモリー機能を有するt細胞又はb細胞の増強剤、悪性腫瘍再発抑制剤、及びt細胞又はb細胞にメモリー機能を誘導する誘導剤 |
| JP7595888B2 (ja) | 2017-10-10 | 2024-12-09 | 国立大学法人山口大学 | メモリー機能を有するt細胞又はb細胞の増強剤、悪性腫瘍再発抑制剤、及びt細胞又はb細胞にメモリー機能を誘導する誘導剤 |
| CN111163758A (zh) * | 2017-10-10 | 2020-05-15 | 国立大学法人山口大学 | 具有记忆功能的t细胞或b细胞的增强剂、恶性肿瘤复发抑制剂、以及对t细胞或b细胞诱导记忆功能的诱导剂 |
| RU2802824C2 (ru) * | 2017-10-10 | 2023-09-04 | Ямагути Университи | Энхансер t-клеток или b-клеток, имеющих функцию памяти, ингибитор рецидива злокачественной опухоли и индуктор для индуцирования функции памяти в t-клетках или b-клетках |
| JP7232447B2 (ja) | 2017-10-10 | 2023-03-03 | 国立大学法人山口大学 | メモリー機能を有するt細胞又はb細胞の増強剤、悪性腫瘍再発抑制剤、及びt細胞又はb細胞にメモリー機能を誘導する誘導剤 |
| JP2023053087A (ja) * | 2017-10-10 | 2023-04-12 | 国立大学法人山口大学 | メモリー機能を有するt細胞又はb細胞の増強剤、悪性腫瘍再発抑制剤、及びt細胞又はb細胞にメモリー機能を誘導する誘導剤 |
| US11617765B2 (en) | 2017-10-10 | 2023-04-04 | Yamaguchi University | Enhancer for T-cells or B-cells having memory function, malignant tumor recurrence inhibitor, and inducer for inducing memory function in T-cells or B-cells |
| JP7475088B2 (ja) | 2017-12-24 | 2024-04-26 | ノイルイミューン・バイオテック株式会社 | ヒトメソセリンを特異的に認識する細胞表面分子、il-7、及びccl19を発現する免疫担当細胞 |
| IL275453B2 (en) * | 2017-12-24 | 2024-06-01 | Noile Immune Biotech Inc | Immunocompetent cell that expresses a cell surface molecule specifically recognizing human mesothelin, il-7 and ccl19 |
| JP7233720B2 (ja) | 2017-12-24 | 2023-03-07 | ノイルイミューン・バイオテック株式会社 | ヒトメソセリンを特異的に認識する細胞表面分子、il-7、及びccl19を発現する免疫担当細胞 |
| JP2023053328A (ja) * | 2017-12-24 | 2023-04-12 | ノイルイミューン・バイオテック株式会社 | ヒトメソセリンを特異的に認識する細胞表面分子、il-7、及びccl19を発現する免疫担当細胞 |
| JP2024086826A (ja) * | 2017-12-24 | 2024-06-28 | ノイルイミューン・バイオテック株式会社 | ヒトメソセリンを特異的に認識する細胞表面分子、il-7、及びccl19を発現する免疫担当細胞 |
| IL275453B1 (en) * | 2017-12-24 | 2024-02-01 | Noile Immune Biotech Inc | An immunocompetent cell expressing a cell surface compound that specifically recognizes human mesothelin, IL-7, and CCL19 |
| KR20200103708A (ko) | 2017-12-24 | 2020-09-02 | 노일 이뮨 바이오테크 가부시키가이샤 | 인간 메소텔린을 특이적으로 인식하는 세포 표면 분자, il-7, 및 ccl19 를 발현하는 면역 담당 세포 |
| WO2019124468A1 (ja) | 2017-12-24 | 2019-06-27 | ノイルイミューン・バイオテック株式会社 | ヒトメソセリンを特異的に認識する細胞表面分子、il-7、及びccl19を発現する免疫担当細胞 |
| JPWO2019124468A1 (ja) * | 2017-12-24 | 2021-01-21 | ノイルイミューン・バイオテック株式会社 | ヒトメソセリンを特異的に認識する細胞表面分子、il−7、及びccl19を発現する免疫担当細胞 |
| JP7696658B2 (ja) | 2017-12-24 | 2025-06-23 | ノイルイミューン・バイオテック株式会社 | ヒトメソセリンを特異的に認識する細胞表面分子、il-7、及びccl19を発現する免疫担当細胞 |
| JP2021523743A (ja) * | 2018-05-15 | 2021-09-09 | カースゲン セラピューティクス カンパニー リミテッドCarsgen Therapeutics Co., Ltd. | 遺伝子操作された細胞及びその応用 |
| US12466869B2 (en) | 2018-06-28 | 2025-11-11 | Dana-Farber Cancer Institute, Inc. | Targeting of multiple antigens with multiplex CAR T cells in solid and liquid malignancies |
| JPWO2020017479A1 (ja) * | 2018-07-17 | 2021-08-02 | ノイルイミューン・バイオテック株式会社 | 抗gpc3一本鎖抗体を含むcar |
| EP3825404A4 (en) * | 2018-07-17 | 2022-04-13 | Noile-Immune Biotech, Inc. | SINGLE-CHAIN ANTI-GPC3 ANTIBODY WITH CAR |
| JP7737740B2 (ja) | 2018-07-17 | 2025-09-11 | ノイルイミューン・バイオテック株式会社 | 抗gpc3一本鎖抗体を含むcar |
| JP2024069500A (ja) * | 2018-07-17 | 2024-05-21 | ノイルイミューン・バイオテック株式会社 | 抗gpc3一本鎖抗体を含むcar |
| US20230071098A1 (en) * | 2018-07-17 | 2023-03-09 | Noile-Immune Biotech, Inc. | Anti-gpc3 single-chain antibody-containing car |
| WO2020017479A1 (ja) | 2018-07-17 | 2020-01-23 | ノイルイミューン・バイオテック株式会社 | 抗gpc3一本鎖抗体を含むcar |
| KR20210053925A (ko) | 2018-08-31 | 2021-05-12 | 노일 이뮨 바이오테크 가부시키가이샤 | Car 발현 t 세포 및 car 발현 벡터 |
| JPWO2020045610A1 (ja) * | 2018-08-31 | 2021-08-26 | ノイルイミューン・バイオテック株式会社 | Car発現t細胞及びcar発現ベクター |
| WO2020045610A1 (ja) | 2018-08-31 | 2020-03-05 | ノイルイミューン・バイオテック株式会社 | Car発現t細胞及びcar発現ベクター |
| JP2022513076A (ja) * | 2018-11-19 | 2022-02-07 | ボード オブ リージェンツ,ザ ユニバーシティ オブ テキサス システム | Carおよびtcr形質導入用のモジュール式ポリシストロニックベクター |
| JP7630832B2 (ja) | 2018-11-19 | 2025-02-18 | ボード オブ リージェンツ,ザ ユニバーシティ オブ テキサス システム | Carおよびtcr形質導入用のモジュール式ポリシストロニックベクター |
| JP7602475B2 (ja) | 2019-03-08 | 2024-12-18 | オートラス リミテッド | 操作されたキメラ抗原受容体およびcarの調節因子を含む組成物および方法 |
| JP2022524042A (ja) * | 2019-03-08 | 2022-04-27 | オートラス リミテッド | 操作されたキメラ抗原受容体およびcarの調節因子を含む組成物および方法 |
| JP7585228B2 (ja) | 2019-04-04 | 2024-11-18 | 上海医薬集団股▲フン▼有限公司 | 腫瘍抗原認識受容体を有する免疫細胞とその応用 |
| JP2022529618A (ja) * | 2019-04-04 | 2022-06-23 | 上海医薬集団股▲フン▼有限公司 | 腫瘍抗原認識受容体を有する免疫細胞とその応用 |
| CN112689679A (zh) * | 2019-07-23 | 2021-04-20 | 株式会社东芝 | 产生car-t细胞的方法、核酸引入载体和试剂盒 |
| WO2021085497A1 (ja) | 2019-10-28 | 2021-05-06 | ノイルイミューン・バイオテック株式会社 | がんを治療するための医薬、組み合わせ医薬、医薬組成物、免疫応答性細胞、核酸送達媒体、及び製品 |
| JP2023513752A (ja) * | 2020-02-14 | 2023-04-03 | ベイジン ヨンタイ ルイケ バイオテクノロジー カンパニー リミテッド | 外部から導入された細胞シグナル伝達調節因子を過剰発現する免疫細胞及びその使用 |
| WO2021256522A1 (ja) | 2020-06-17 | 2021-12-23 | 国立大学法人京都大学 | キメラ抗原受容体発現免疫担当細胞 |
| WO2022025638A1 (ko) | 2020-07-29 | 2022-02-03 | (주)티카로스 | 면역시냅스를 안정화시키는 키메라 항원 수용체(car) t 세포 |
| KR20220156460A (ko) | 2021-05-18 | 2022-11-25 | 주식회사 바이오솔루션 | 세포외소포체 분비능이 증진된 면역세포 및 이를 활용한 면역 항암요법 |
| WO2023007431A1 (en) | 2021-07-29 | 2023-02-02 | Takeda Pharmaceutical Company Limited | Engineered immune cell that specifically targets mesothelin and uses thereof |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7527049B2 (ja) | Car発現ベクター及びcar発現t細胞 | |
| JP6884423B2 (ja) | 免疫機能制御因子を発現する免疫担当細胞及び発現ベクター | |
| JP7233720B2 (ja) | ヒトメソセリンを特異的に認識する細胞表面分子、il-7、及びccl19を発現する免疫担当細胞 | |
| CN119907857A (zh) | 包含嵌合衔接子多肽的组合物和方法 | |
| HK1235426A1 (en) | Car expression vector and car-expressing t cells | |
| HK1235426B (en) | Car expression vector and car-expressing t cells |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15849416 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE2 | Request for preliminary examination filed before expiration of 19th month from priority date (pct application filed from 20040101) | ||
| ENP | Entry into the national phase |
Ref document number: 2016552830 Country of ref document: JP Kind code of ref document: A |
|
| REEP | Request for entry into the european phase |
Ref document number: 2015849416 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2015849416 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2962375 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15513870 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 122019010608 Country of ref document: BR Ref document number: 12017500596 Country of ref document: PH |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 251504 Country of ref document: IL |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2017/004393 Country of ref document: MX |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112017006710 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 20177009895 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2015329444 Country of ref document: AU Date of ref document: 20151006 Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2017114545 Country of ref document: RU Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 112017006710 Country of ref document: BR Kind code of ref document: A2 Effective date: 20170331 |