WO2015037383A1 - 導電性樹脂組成物及びそのフィルム - Google Patents
導電性樹脂組成物及びそのフィルム Download PDFInfo
- Publication number
- WO2015037383A1 WO2015037383A1 PCT/JP2014/071286 JP2014071286W WO2015037383A1 WO 2015037383 A1 WO2015037383 A1 WO 2015037383A1 JP 2014071286 W JP2014071286 W JP 2014071286W WO 2015037383 A1 WO2015037383 A1 WO 2015037383A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- mass
- film
- component
- resin composition
- parts
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B1/00—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors
- H01B1/20—Conductive material dispersed in non-conductive organic material
- H01B1/24—Conductive material dispersed in non-conductive organic material the conductive material comprising carbon-silicon compounds, carbon or silicon
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/02—Elements
- C08K3/04—Carbon
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/02—Elements
- C08K3/04—Carbon
- C08K3/041—Carbon nanotubes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K7/00—Use of ingredients characterised by shape
- C08K7/22—Expanded, porous or hollow particles
- C08K7/24—Expanded, porous or hollow particles inorganic
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L23/00—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers
- C08L23/02—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers not modified by chemical after-treatment
- C08L23/04—Homopolymers or copolymers of ethene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L23/00—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers
- C08L23/02—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers not modified by chemical after-treatment
- C08L23/16—Ethene-propene or ethene-propene-diene copolymers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L23/00—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers
- C08L23/26—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers modified by chemical after-treatment
- C08L23/28—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers modified by chemical after-treatment by reaction with halogens or halogen-containing compounds
- C08L23/286—Chlorinated polyethene
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/60—Selection of substances as active materials, active masses, active liquids of organic compounds
- H01M4/602—Polymers
- H01M4/604—Polymers containing aliphatic main chain polymers
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/64—Carriers or collectors
- H01M4/66—Selection of materials
- H01M4/668—Composites of electroconductive material and synthetic resins
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M12/00—Hybrid cells; Manufacture thereof
- H01M12/08—Hybrid cells; Manufacture thereof composed of a half-cell of a fuel-cell type and a half-cell of the secondary-cell type
- H01M12/085—Zinc-halogen cells or batteries
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/18—Regenerative fuel cells, e.g. redox flow batteries or secondary fuel cells
- H01M8/184—Regeneration by electrochemical means
- H01M8/188—Regeneration by electrochemical means by recharging of redox couples containing fluids; Redox flow type batteries
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/50—Fuel cells
Definitions
- the present invention relates to a conductive resin composition and a film thereof. More specifically, the present invention relates to a conductive resin composition capable of forming an electrode of a storage battery or a film suitable for covering protection thereof, and a film thereof.
- renewable energies such as solar power generation, wind power generation and wave power generation have attracted attention as new energy sources to replace fossil fuels such as oil and nuclear power.
- these renewable energies are strongly affected by the weather, the output is extremely unstable. Therefore, in order to connect a large amount of these energies to the power grid, for example, it is necessary to level out output fluctuations with a large capacity storage battery.
- a redox flow battery as one of large-capacity storage batteries.
- a redox flow battery performs charge / discharge by separating two types of ion solutions with a cation exchange membrane and simultaneously carrying out an oxidation reaction and a reduction reaction on electrodes provided in both solutions.
- a redox flow battery using a sulfuric acid aqueous solution of vanadium for both electrodes tetravalent vanadium is oxidized to pentavalent at the positive electrode and trivalent vanadium is reduced to divalent at the negative electrode during charging.
- the reverse reaction occurs during discharge.
- the redox flow battery has a feature that the equipment can be easily enlarged.
- Redox flow batteries operate at room temperature, do not use flammable or explosive substances, and do not generate such substances. Compared with sodium-sulfur batteries and lithium ion secondary batteries. And excellent safety.
- the electrode of the redox flow battery is immersed in an electrolyte such as an aqueous sulfuric acid solution, and an oxidation-reduction reaction takes place there. Accordingly, the electrode needs to have high conductivity and chemical resistance, and a carbon fiber aggregate or platinum plating is used as the electrode.
- a carbon fiber aggregate or platinum plating is used as the electrode.
- the carbon fiber aggregate has liquid permeability, there is a disadvantage that the connecting portion between the carbon fiber aggregate and the copper wire is affected by the transported sulfuric acid aqueous solution or the like.
- Platinum plating is a very good conductor and excellent in chemical resistance, but it is a noble metal and has a drawback of being expensive.
- a conductive resin film kneaded with conductive carbon such as ketjen black is used as an electrode (see, for example, Patent Documents 1 to 4), and an electrode such as a carbon fiber aggregate or a copper plate is used as the conductive resin film. It is performed by coating.
- these conductive resin films are kneaded with a large amount of conductive carbon to give sufficiently high conductivity, the tensile elongation, the bending resistance, and the flexibility are quite insufficient. There is an inconvenience that it is easily broken by force. Moreover, if tensile elongation, bending resistance, and flexibility are ensured by reducing the blending amount of the conductive carbon, the volume resistivity exceeds 10 ⁇ ⁇ cm.
- a redox flow battery using such a conductive film as an electrode or its coating is not satisfactory in that the internal resistance is increased.
- the shear stress in the defibrating / dispersing step is increased in order to improve the defibrated / dispersed state of the carbon nanotube, the carbon nanotube is broken. Therefore, even if the shear stress is increased in the defibration / dispersion step, it is necessary to add a large amount of carbon nanotubes in order to obtain sufficiently high conductivity.
- conductive films made of a composition obtained by mixing carbon black or carbon nanotubes with a propylene-olefin copolymer wax to form a master batch and mixing with an organic polymer have been proposed (for example, Patent Documents 6 and 7). reference).
- the masterbatch enables high filling of carbon black or carbon nanotubes, but the resulting film is not sufficiently conductive.
- JP-A-1-149370 JP-A-4-259754 Japanese Unexamined Patent Publication No. 7-053813 JP 2001-015144 A JP 2006-111870 A Special table 2012-507586 gazette Special table 2012-507588 gazette
- a further object of the present invention is to provide a film formed from such a conductive resin composition.
- the first form of the present invention is as follows.
- (C) 1 to 60 parts by mass of acetylene black The (A) thermoplastic resin is (A1) 30-80% by mass of chlorinated polyethylene having a chlorine content of 20-45% by mass;
- (A2) comprising 70 to 20% by mass of polyethylene different from the above (A1),
- the sum of (A1) and (A2) is 100 mass%
- the resin composition characterized by the above-mentioned.
- the second aspect of the present invention is as follows.
- (C) 1 to 60 parts by mass of acetylene black The (A) thermoplastic resin is (A3) A resin composition characterized by being a polyethylene satisfying the following characteristics (p) and (q):
- (P) The peak top melting point on the highest temperature side in the DSC melting curve is 120 ° C. or higher; and
- (q) The ratio of the melting enthalpy at a temperature of 110 ° C. or lower to the total melting enthalpy in the DSC melting curve is 50 to 80%. Be.
- the resin composition of the present invention is excellent in film molding processability, and a film formed by molding the resin composition has high conductivity, and is excellent in tensile elongation, bending resistance, and flexibility. Therefore, the film formed from the resin composition is an electrode member of a storage battery, in particular, an electrolyte circulating secondary battery, for example, an electrode in a redox flow battery, a zinc / chlorine battery, a zinc / bromine battery, etc. Can be suitably used.
- thermoplastic resin of the thermoplastic resin component (A) accepts the component (B) which is a carbon filler, ensures film forming processability, and the resulting film has tensile elongation, bending resistance, It also functions to impart mechanical properties such as flexibility.
- the thermoplastic resin of component (A) comprises (A1) 30 to 80% by mass of chlorinated polyethylene having a chlorine content of 20 to 45% by mass, and (A2) the above (A1). It is a thermoplastic resin mixture comprising 70 to 20% by mass of different polyethylene. Here, the sum of (A1) and (A2) is 100% by mass.
- a film of a resin composition having a very good electrolytic solution resistance, in particular, a vanadium sulfate-resistant aqueous solution property can be obtained.
- the blending ratio is preferably 50 to 65% by mass of component (A1) and 50 to 35% by mass of (A2).
- Chlorinated polyethylene having a chlorine content of 20 to 45 mass% in the chlorinated polyethylene component (A1) plays an important role in ensuring the vanadium sulfate aqueous solution resistance of the resulting film.
- the chlorine content needs to be 20 to 45% by mass.
- the chlorine content is 45% by mass or less, it is possible to suppress the possibility of troubles such as burning during molding.
- the chlorine content is 20% by mass or more, the chlorinated polyethylene blending purpose of ensuring the vanadium sulfate-resistant aqueous solution property can be satisfied.
- the chlorine content is preferably 25 to 40% by mass.
- the polyethylene to be chlorinated is not particularly limited.
- low density polyethylene linear low density polyethylene, ultra low density polyethylene, high density polyethylene, ethylene homopolymer, ethylene and ⁇ -olefin (for example, 1 -Butene, 1-hexene, 1-octene and the like) and any mixtures thereof.
- Chlorination may be carried out by any known method as long as it is possible to obtain a chlorine content within the above range.
- an aqueous suspension method can be used.
- Component (A1) reduces the shear stress at the time of molding and prevents troubles such as burning, and from the viewpoint of favorably dispersing component (B) and component (C) to improve initial conductivity, It is preferably non-crystalline.
- the non-crystalline chlorinated polyethylene means that a Diamond DSC type differential scanning calorimeter manufactured by PerkinElmer Japan Co., Ltd. is used and held at 190 ° C. for 5 minutes, and at ⁇ 10 ° C. at 10 ° C./min. Clear melting in the second melting curve (melting curve measured in the last heating process) measured with a program that cools to -10 ° C and holds for 5 minutes and heats up to 190 ° C at 10 ° C / min It is defined that no peak is observed or that a melting peak is present but the heat of fusion ( ⁇ H) is less than 10 J / g.
- the component (A1) reduces the shear stress at the time of molding to prevent troubles such as burning, and the component (B) and the component (C) are well dispersed to improve the initial conductivity. Therefore, those having high fluidity are preferable.
- the component (A1) has a melt flow rate (hereinafter sometimes abbreviated as MFR-A1) measured at 180 ° C. and 211.8 N in accordance with JIS K7210: 1999 of 10 g / 10 min or more. Is preferable, and more preferably 50 g / 10 min or more.
- the MFR-A1 of the component (A1) is preferably 500 g / 10 min or less.
- chlorinated polyethylenes examples include “Eraslen 303A” (trade name) and “Eraslen 302NA” (trade name) from Showa Denko KK.
- the polyethylene of the polyethylene component (A2) different from (A1) is not particularly limited as long as it is a polyethylene different from (A1).
- the polyethylene of component (A2) is usually not chlorinated.
- the component (A2) polyethylene include low density polyethylene, linear low density polyethylene, ultra-low density polyethylene, high density polyethylene, ethylene homopolymer, ethylene and ⁇ -olefin (eg, 1-butene, 1- One kind of a copolymer with one or two or more of hexene, 1-octene, etc.) can be used alone or as a mixture in which two or more kinds are arbitrarily blended. When used as a mixture, the entire mixture preferably satisfies the following Tm-A2 range or the following MFR-A2 range.
- Component (A2) plays an important role in ensuring film formability. Therefore, those having excellent filler inclusion properties are preferred, and the peak top melting point (hereinafter sometimes abbreviated as Tm-A2) on the DSC melting curve of component (A2) is preferably 110 ° C. or less. . This Tm-A2 is more preferably 105 ° C. or lower. On the other hand, from the viewpoint of suppressing swelling of the component (A2) by the electrolytic solution, Tm-A2 is preferably 60 ° C. or higher.
- the peak top melting point on the highest temperature side in the DSC melting curve is a Diamond DSC type differential scanning calorimeter manufactured by PerkinElmer Japan Co., Ltd., held at 190 ° C. for 5 minutes, and 10 ° C. Second melting curve measured by a program that cools to -10 ° C at / min, holds at -10 ° C for 5 min, and raises the temperature to 190 ° C at 10 ° C / min (melting curve measured in the last temperature raising process) ), The peak top melting point on the highest temperature side.
- the component (A2) preferably has high fluidity in order to reduce shear stress during molding and prevent troubles such as burning.
- the component (A2) has a melt flow rate (hereinafter sometimes abbreviated as MFR-A2) measured at 190 ° C. and 21.18 N in accordance with JIS K7210: 1999 of 1 g / 10 min or more. It is more preferable that it is 5 g / 10 min or more.
- the MFR-A2 of the component (A2) is preferably 100 g / 10 min or less from the viewpoint of fluidity that can ensure sufficient acceptability of the component (B).
- thermoplastic resin of component (A) is (A3) polyethylene satisfying the following characteristics (p) and (q).
- thermoplastic resin of component (A) is (A3) polyethylene satisfying the following characteristics (p) and (q). .
- P) The peak top melting point on the highest temperature side in the DSC melting curve is 120 ° C. or higher.
- Q The ratio of the melting enthalpy at a temperature of 110 ° C. or lower to the total melting enthalpy in the DSC melting curve is 50 to 80%.
- the resin composition obtained by using the component (A3) as the component (A) is particularly excellent in film forming processability, and the film formed by molding the resin composition is particularly excellent in bending resistance and heat resistance. .
- Tm-A3 peak top melting point in the DSC melting curve of the component (A3) is 120 ° C. or higher, that is, when the characteristic (p) is satisfied, A film excellent in curvature and heat resistance can be obtained.
- Tm-A3 is preferably as high as possible. Preferably it is 125 degreeC or more, More preferably, it is 130 degreeC or more. There is no particular upper limit for Tm-A3, but since it is polyethylene, it is at most about 135 ° C. at most.
- This characteristic (q) is an index of polyethylene filler inclusion, and is the ratio of the melting enthalpy at a temperature of 110 ° C. or less to the total melting enthalpy in the DSC second melting curve measured by the above method.
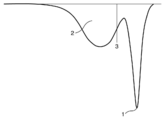
- a conceptual diagram is shown in FIG. In FIG. 1, 1 represents the peak top melting point on the highest temperature side in the DSC second melting curve, 2 represents the integration of melting enthalpies at a temperature of 110 ° C. or less in the DSC second melting curve, and 3 represents The boundary line of the temperature of 110 degreeC in the second melting curve of DSC is shown. Resin composition having excellent moldability when the ratio of the melting enthalpy at a temperature of 110 ° C.
- Xc to the total melting enthalpy in the DSC melting curve of component (A3) is 50% or more. You can get things. Moreover, the film formed from such a resin composition has favorable electrolytic solution resistance. Xc of a component (A3) becomes like this. Preferably it is 60% or more. On the other hand, from the viewpoint of the bending resistance and heat resistance of the film to be formed, Xc of the component (A3) is 80% or less, preferably 70% or less.
- the component (A3) is not particularly limited as long as it is a polyethylene that satisfies the characteristics (p) and (q).
- low density polyethylene linear low density polyethylene, ultra low density polyethylene, high density polyethylene, ethylene homopolymer, ethylene and ⁇ -olefin (for example, 1-butene, 1-hexene, 1-octene, etc. Or a copolymer with two or more).
- these 1 type can be used individually or as a mixture which mix
- the carbon nanotube of component (B) has a diameter of about 1 to 250 nm and a length of 0.1 to about 6-membered ring network (graphene sheet) made of carbon in a single-layer or multi-layer coaxial tubular shape. It is a fibrous substance of about 250 ⁇ m.
- the carbon nanotube of the component (B) functions to impart high conductivity to the resin composition and the film as a conductive filler. Therefore, the carbon nanotube of component (B) is preferably one having few lattice defects and high conductivity. Moreover, since the thing with small bulk specific gravity is easy to open, it is preferable.
- Nanosil S.I. A “Nanosil NC7000” (trade name) of the company, “VGCF-X” (trade name) of Showa Denko K.K.
- Component (B) is blended in an amount of 1 part by mass or more, preferably 10 parts by mass or more, more preferably 20 parts by mass or more from the viewpoint of conductivity with respect to 100 parts by mass of component (A). Moreover, from a viewpoint of tensile elongation and bending resistance, it is 60 mass parts or less, Preferably it is 50 mass parts or less, More preferably, it is 45 mass parts or less.
- the acetylene black of the component (C) is a carbon fine particle produced by thermal decomposition of acetylene gas, and is a conductive carbon black having a partially graphitized structure.
- the component (C) acetylene black maintains the processability in the resin composition production (melt-kneading) step and the film-forming step, and helps the component (B) to be defibrated and highly dispersed. And mechanical properties such as tensile elongation and bending resistance can be improved.
- acetylene black of a component (C) itself has electroconductivity, it functions to raise the electroconductivity of a resin composition and its film.
- Examples of commercially available acetylene black include Denka Black (trade name) manufactured by Denki Kagaku Kogyo Co., Ltd.
- Component (C) is blended in an amount of 1 part by mass or more, preferably 6 parts by mass or more, more preferably 100 parts by mass of component (A) from the viewpoint of tensile elongation and bending resistance of the resulting film. It is 10 parts by mass or more.
- the blending amount of component (C) is 60 parts by mass or less, preferably 40 parts by mass or less, more preferably 30 parts by mass, from the viewpoint of the electrolytic solution resistance, tensile elongation, and bending resistance of the resulting film. It is as follows.
- Ketjen black is known as conductive carbon black in addition to acetylene black. Ketjen black has high conductivity, but unlike acetylene black, it has a hollow shell-like structure. Therefore, the conductive filler mainly containing this is kneaded with the component (A) and the component (B). The resin composition thus obtained does not exhibit melt ductility during film formation and is difficult to form.
- additives such as lubricants, antioxidants, anti-aging agents, weathering stabilizers such as light stabilizers and ultraviolet absorbers, heat stabilizers, copper damage inhibitors, mold release agents
- An additive such as an agent and a surfactant can be further contained as long as the object of the present invention is not adversely affected.
- the compounding amount of such an additive may be about 0.001 to 5 parts by mass with respect to 100 parts by mass of the component (A).
- inorganic fillers other than the components (B) and (C) can be further contained as long as they do not contradict the purpose of the present invention.
- examples of such inorganic fillers include light calcium carbonate, heavy calcium carbonate, hydrous magnesium silicate, and talc.
- the compounding amount of the inorganic filler may be about 1 to 20 parts by mass with respect to 100 parts by mass of the component (A).
- the resin composition of the present invention can be obtained by melt kneading the above components (A) to (C) and other optional components used as desired using an arbitrary melt kneader.
- the melt kneader include batch kneaders such as a pressure kneader and a mixer; extrusion kneaders such as a co-rotating twin screw extruder and a different direction rotating twin screw extruder; and a calender roll kneader. These may be used in any combination.
- the obtained resin composition can be formed into a film using, for example, a calendering machine or an extruder and a T-die after being pelletized by an arbitrary method. Pelletization can be performed by methods such as hot cutting, strand cutting, and underwater cutting. Alternatively, the melt-kneaded resin composition may be directly sent to a calendering machine or a T-die to form a film. Any calendar processing machine can be used, and examples thereof include an upright three roll, an upright four roll, an L four roll, an inverted L four roll, and a Z roll. .
- Any extruder can be used, and examples thereof include a single-screw extruder, a same-direction rotating twin-screw extruder, and a different-direction rotating twin-screw extruder.
- Any T die can be used, and examples thereof include a manifold die, a fishtail die, and a coat hanger die.
- the thickness of the film thus obtained is not particularly limited, but may be, for example, 100 to 1000 ⁇ m when used as an electrode member of a redox flow battery (storage battery) using a sulfuric acid aqueous solution of vanadium as both electrodes.
- the film may be crosslinked and cured by a known method, for example, electron beam irradiation, in order to increase its heat resistance and electrolytic solution resistance.
- volume resistivity ⁇ Based on JIS K7194: 1994, the volume resistivity of the film was measured by a four-probe method (probe method). A film that had been conditioned for 24 hours or more in a test room at a temperature of 23 ⁇ 2 ° C. and a relative humidity of 50 ⁇ 5% was cut into a size of 80 mm in the machine direction of the film and 50 mm in the width direction of the film to obtain a test piece. .
- Raw materials used Ingredient (A1) (A1-1): Showa Denko Co., Ltd.'s chlorinated polyethylene “Elaslene 303A” (trade name), chlorine content 32 mass%, melt flow rate (180 ° C., 211.8 N) 120 g / 10 min, heat of fusion 2 J / g (A1-2): Chlorinated polyethylene “Elaslene 303B” (trade name), Showa Denko Co., Ltd., chlorine content 32 mass%, melt flow rate (180 ° C., 211.8 N) 25 g / 10 minutes, heat of fusion 50 J / g (A1-3): Chlorinated polyethylene “Elaslene 404B” (trade name), Showa Denko Co., Ltd., chlorine content 40 mass%, melt flow rate (180 ° C., 211.8 N) 25 g / 10 min, heat of fusion 29 J / g
- Ingredient (A2) (A2-1): The Dow Chemical Company polyethylene “engage 8402” (trade name), melt flow rate (190 ° C., 21.18 N) 30 g / 10 min, density 877 Kg / m 3 , Tm-A2: 99 ° C (A2-2): Prime Polymer Co., Ltd. polyethylene “Ultzex 20200J” (trade name), melt flow rate (190 ° C., 21.18 N) 18.5 g / 10 min, density 918 Kg / m 3 , Tm-A2: 125 ° C
- Ingredient (A3) (A3-1): Prime Polymer Co., Ltd. polyethylene “Ultzex 20200J” (trade name), melt flow rate (190 ° C., 21.18 N) 18.5 g / 10 min, density 918 Kg / m 3 , Tm-A3: 125 ° C, Xc 62% (A3-2): Prime Polymer Co., Ltd. polyethylene “Neozex 2024G” (trade name), melt flow rate (190 ° C., 21.18 N) 25 g / 10 min, density 915 Kg / m 3 , Tm-A3: 120 ° C. Xc 75% (A3-3): Prime Polymer Co., Ltd.
- Comparative component (A3 ′) (A3′-1): The Dow Chemical Company polyethylene “D9100.00” (trade name), melt flow rate (190 ° C., 21.18 N) 1 g / 10 min, density 877 Kg / m 3 , Tm— A3: 118 ° C., Xc 29% (A3′-2): The Dow Chemical Company polyethylene “engage 8402” (trade name), melt flow rate (190 ° C., 21.18 N) 30 g / 10 min, density 877 Kg / m 3 , Tm-A3 : 99 ° C, Xc 100% (A3′-3): Prime Polymer Co., Ltd.
- Ingredient (B) (B-1): Nanosil S.I. A. Multi-walled carbon nanotube “Nanosil NC7000” (trade name), average diameter 9.5 nm, average length 1.5 ⁇ m, bulk specific gravity 0.043 g / cm 3 , purity 90% by mass
- Ingredient (C) (C-1): Acetylene black “Denka Black Granule” (trade name) manufactured by Denki Kagaku Kogyo Co., Ltd., average particle diameter of primary particles 35 nm (measured by observation with an electron microscope (TEM)), specific surface area 69 m 2 / g
- Comparative component (C ') (C'-1): Lion Corporation Ketjen Black “KJ300” (trade name)
- Component (D) Optional component (D-1): Stabilizer for chlorinated polyethylene “STANN JF-95B” (trade name) manufactured by Nitto Kasei Kogyo Co., Ltd.
- the film formed from the resin composition of the present invention is resistant to electrolytic solution, volume resistivity, bending resistance, bending resistance after wet heat treatment,
- various properties of tensile elongation were expressed at a high level in a well-balanced manner.
- initial conductivity was also excellent.
- melt flow rates were lower than (A1-1) and crystalline (A1-2) or (A1-3) were used)
- a sufficiently good initial Since conductivity was not obtained, but low volume resistivity was obtained, it can be evaluated that a film sufficient for practical use was obtained from the viewpoint of conductivity in addition to other characteristics.
- Comparative Example 1 had a large amount of component (A2), and the bending resistance after wet heat treatment was insufficient. In Comparative Example 2, the amount of the component (A2) was small and the electrolytic solution resistance was poor. Comparative Examples 3 to 5 are examples in which component (C) is not used. When the component (B) is small, the initial conductivity does not reach that of Examples 1 and 7. When the component (B) is large, is the bending resistance or the bending resistance after wet heat treatment insufficient? It was inferior. Since Comparative Example 6 did not use the component (B), the initial conductivity was inferior and the volume resistivity was large. Moreover, the bending resistance and the bending resistance after wet heat treatment were also inferior.
- Comparative Example 7 is an example using Ketjen black instead of the component (C), it could not be formed into a film. Therefore, tests (i) to (vi) were omitted. Since Comparative Example 8 had a low Tm-A3 and a low Xc, it was inferior in electrolytic solution resistance, and had insufficient bending resistance and bending resistance after wet heat treatment. Since Comparative Example 9 had a low Tm-A3 and a high Xc, the bending resistance and the bending resistance after wet heat treatment were inferior.
- the film formed from the resin composition according to the present invention is an electrode member of a storage battery, particularly an electrode in an electrolyte circulation type secondary battery, such as a redox flow battery, a zinc / chlorine battery, and a zinc / bromine battery, or a coating protection thereof. Can be suitably used.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Electrochemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Dispersion Chemistry (AREA)
- Physics & Mathematics (AREA)
- Nanotechnology (AREA)
- Composite Materials (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Manufacture Of Macromolecular Shaped Articles (AREA)
- Fuel Cell (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Conductive Materials (AREA)
- Inert Electrodes (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/021,188 US9748017B2 (en) | 2013-09-10 | 2014-08-12 | Electrically conductive resin composition, and film produced from same |
| EP17194804.5A EP3284782B1 (en) | 2013-09-10 | 2014-08-12 | Electrically conductive resin composition, and film produced from same |
| EP14844477.1A EP3045497B1 (en) | 2013-09-10 | 2014-08-12 | Electrically conductive resin composition, and film produced from same |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013187524 | 2013-09-10 | ||
| JP2013-187524 | 2013-09-10 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015037383A1 true WO2015037383A1 (ja) | 2015-03-19 |
Family
ID=52665502
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/071286 Ceased WO2015037383A1 (ja) | 2013-09-10 | 2014-08-12 | 導電性樹脂組成物及びそのフィルム |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US9748017B2 (OSRAM) |
| EP (2) | EP3045497B1 (OSRAM) |
| JP (1) | JP6385197B2 (OSRAM) |
| TW (1) | TWI633145B (OSRAM) |
| WO (1) | WO2015037383A1 (OSRAM) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5983850B1 (ja) * | 2015-11-18 | 2016-09-06 | 東洋インキScホールディングス株式会社 | 導電性樹脂組成物、成形体およびその製造方法 |
| JP2017141370A (ja) * | 2016-02-10 | 2017-08-17 | デンカ株式会社 | 導電性高分子材料およびそれを用いた成形品 |
| US9748017B2 (en) | 2013-09-10 | 2017-08-29 | Riken Technos Corporation | Electrically conductive resin composition, and film produced from same |
| JP2017186440A (ja) * | 2016-04-05 | 2017-10-12 | 東洋インキScホールディングス株式会社 | 導電性樹脂組成物および成形体の製造方法 |
| JP2018028031A (ja) * | 2016-08-19 | 2018-02-22 | 東洋インキScホールディングス株式会社 | 導電性樹脂組成物、成形体およびその製造方法 |
| US10767035B2 (en) | 2014-10-09 | 2020-09-08 | Riken Technos Corporation | Method for producing thermoplastic resin composition film |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6810042B2 (ja) * | 2015-08-17 | 2021-01-06 | デンカ株式会社 | カーボンナノファイバー複合体の製造方法、カーボンナノファイバー複合体、残留触媒の除去方法、導電性樹脂組成物、及び分散液、インク又は塗料 |
| CN108155342A (zh) * | 2017-12-28 | 2018-06-12 | 上海君屹工业自动化股份有限公司 | 动力锂电池极耳折弯装置 |
| JP7264453B2 (ja) * | 2019-04-18 | 2023-04-25 | イイダ産業株式会社 | 樹脂系耐火性組成物 |
| JP7736427B2 (ja) * | 2020-08-26 | 2025-09-09 | 松本油脂製薬株式会社 | 導電性ペースト組成物とその利用、及び導電性ペースト組成物に用いられる樹脂粒子 |
Citations (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS62205144A (ja) * | 1986-03-04 | 1987-09-09 | Mitsubishi Plastics Ind Ltd | 導電性シ−ト |
| JPS62256847A (ja) * | 1986-05-01 | 1987-11-09 | Furukawa Electric Co Ltd:The | 導電性組成物 |
| JPS63307605A (ja) * | 1987-06-09 | 1988-12-15 | Fujikura Ltd | 半導電性混和物 |
| JPH01149370A (ja) | 1987-12-04 | 1989-06-12 | Meidensha Corp | プラスチック電極 |
| JPH0329210A (ja) * | 1989-06-26 | 1991-02-07 | Fujikura Ltd | 電力ケーブル |
| JPH04259754A (ja) | 1991-02-15 | 1992-09-16 | Meidensha Corp | カーボンプラスチック電極 |
| JPH0753813A (ja) | 1993-08-10 | 1995-02-28 | Shin Etsu Polymer Co Ltd | 導電性プラスチック組成物 |
| JP2000357419A (ja) * | 1999-05-13 | 2000-12-26 | Union Carbide Chem & Plast Technol Corp | ケーブルの半導電性遮蔽 |
| JP2001015144A (ja) | 1999-06-29 | 2001-01-19 | Sumitomo Electric Ind Ltd | レドックスフロー電池の電池セル用カーボン板および電池セル構造 |
| JP2004207097A (ja) * | 2002-12-26 | 2004-07-22 | Inoac Corp | 導電性樹脂組成物 |
| JP2006111870A (ja) | 2004-09-14 | 2006-04-27 | Showa Denko Kk | 導電性樹脂組成物、その製造方法及び用途 |
| JP2009521535A (ja) * | 2005-08-08 | 2009-06-04 | キャボット コーポレイション | ナノチューブを含むポリマー組成物 |
| JP2012507586A (ja) | 2008-11-06 | 2012-03-29 | クラリアント・ファイナンス・(ビーブイアイ)・リミテッド | プロピレン−プロピレン−コポリマーワックス及びカーボンブラックを含む組成物 |
| JP2012507587A (ja) | 2008-11-06 | 2012-03-29 | クラリアント・ファイナンス・(ビーブイアイ)・リミテッド | プロピレン−オレフィン−コポリマーワックス及びカーボンナノチューブを含む組成物 |
| WO2013153969A1 (ja) * | 2012-04-09 | 2013-10-17 | リケンテクノス株式会社 | 樹脂組成物 |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH08259767A (ja) | 1995-03-20 | 1996-10-08 | Sumitomo Electric Ind Ltd | 導電性プラスチック板およびそれを用いた電池セル |
| JPH09262855A (ja) | 1996-03-29 | 1997-10-07 | Ishikawajima Harima Heavy Ind Co Ltd | カレンダーの温度制御方法及び装置 |
| JPH1067866A (ja) | 1996-08-29 | 1998-03-10 | Shin Etsu Chem Co Ltd | 土木用止水シート及び遮水シート |
| JP3948217B2 (ja) | 2000-06-05 | 2007-07-25 | 昭和電工株式会社 | 導電性硬化性樹脂組成物、その硬化体、及びその成形体 |
| JP2003182763A (ja) | 2001-12-19 | 2003-07-03 | Japan Steel Works Ltd:The | エンボスキャリヤテープおよびその連続製造装置 |
| US20050070658A1 (en) | 2003-09-30 | 2005-03-31 | Soumyadeb Ghosh | Electrically conductive compositions, methods of manufacture thereof and articles derived from such compositions |
| JP2005200620A (ja) | 2003-12-15 | 2005-07-28 | Bridgestone Corp | 熱可塑性樹脂組成物及び熱可塑性樹脂成形品 |
| WO2006106609A1 (en) | 2005-04-04 | 2006-10-12 | Showa Denko K.K. | Electrically conducting curable resin composition, cured product thereof and molded article of the same |
| JP4904526B2 (ja) | 2006-09-07 | 2012-03-28 | 東レペフ加工品株式会社 | 消臭機能を有するポリオレフィン系樹脂積層発泡体、およびそれからなる円筒体または成型体 |
| JP5018460B2 (ja) | 2007-12-26 | 2012-09-05 | 東洋インキScホールディングス株式会社 | カーボンナノチューブ分散体及びそれを用いてなる樹脂組成物ならびに成形体 |
| KR101003345B1 (ko) | 2008-08-19 | 2010-12-22 | 제일모직주식회사 | 전기 전도성, 내마모성 및 내열성이 우수한 열가소성 수지 조성물 |
| CN102227782B (zh) | 2008-09-30 | 2014-03-12 | 保土谷化学工业株式会社 | 含有碳纤维的复合材料 |
| US7939167B2 (en) | 2008-12-30 | 2011-05-10 | Cheil Industries Inc. | Resin composition |
| KR101267272B1 (ko) | 2008-12-30 | 2013-05-23 | 제일모직주식회사 | 수지 조성물 |
| JP2011228059A (ja) * | 2010-04-16 | 2011-11-10 | Sumitomo Electric Ind Ltd | レドックスフロー電池用双極板 |
| TWI405802B (zh) | 2010-06-24 | 2013-08-21 | Nat Univ Tsing Hua | 官能基化石墨烯強化複合材料導電板之製備方法 |
| JP5672124B2 (ja) * | 2011-04-11 | 2015-02-18 | 住友電気工業株式会社 | レドックスフロー電池用双極板及びその製造方法 |
| CN103367761B (zh) | 2011-05-25 | 2015-07-08 | 天津滨海储能技术有限公司 | 复合导电电极及其制造方法 |
| WO2014010708A1 (ja) * | 2012-07-13 | 2014-01-16 | 古河電気工業株式会社 | 集電体、電極、二次電池およびキャパシタ |
| WO2015037383A1 (ja) | 2013-09-10 | 2015-03-19 | リケンテクノス株式会社 | 導電性樹脂組成物及びそのフィルム |
-
2014
- 2014-08-12 WO PCT/JP2014/071286 patent/WO2015037383A1/ja not_active Ceased
- 2014-08-12 US US15/021,188 patent/US9748017B2/en active Active
- 2014-08-12 EP EP14844477.1A patent/EP3045497B1/en active Active
- 2014-08-12 EP EP17194804.5A patent/EP3284782B1/en active Active
- 2014-08-20 JP JP2014167131A patent/JP6385197B2/ja active Active
- 2014-09-05 TW TW103130765A patent/TWI633145B/zh active
Patent Citations (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS62205144A (ja) * | 1986-03-04 | 1987-09-09 | Mitsubishi Plastics Ind Ltd | 導電性シ−ト |
| JPS62256847A (ja) * | 1986-05-01 | 1987-11-09 | Furukawa Electric Co Ltd:The | 導電性組成物 |
| JPS63307605A (ja) * | 1987-06-09 | 1988-12-15 | Fujikura Ltd | 半導電性混和物 |
| JPH01149370A (ja) | 1987-12-04 | 1989-06-12 | Meidensha Corp | プラスチック電極 |
| JPH0329210A (ja) * | 1989-06-26 | 1991-02-07 | Fujikura Ltd | 電力ケーブル |
| JPH04259754A (ja) | 1991-02-15 | 1992-09-16 | Meidensha Corp | カーボンプラスチック電極 |
| JPH0753813A (ja) | 1993-08-10 | 1995-02-28 | Shin Etsu Polymer Co Ltd | 導電性プラスチック組成物 |
| JP2000357419A (ja) * | 1999-05-13 | 2000-12-26 | Union Carbide Chem & Plast Technol Corp | ケーブルの半導電性遮蔽 |
| JP2001015144A (ja) | 1999-06-29 | 2001-01-19 | Sumitomo Electric Ind Ltd | レドックスフロー電池の電池セル用カーボン板および電池セル構造 |
| JP2004207097A (ja) * | 2002-12-26 | 2004-07-22 | Inoac Corp | 導電性樹脂組成物 |
| JP2006111870A (ja) | 2004-09-14 | 2006-04-27 | Showa Denko Kk | 導電性樹脂組成物、その製造方法及び用途 |
| JP2009521535A (ja) * | 2005-08-08 | 2009-06-04 | キャボット コーポレイション | ナノチューブを含むポリマー組成物 |
| JP2012507586A (ja) | 2008-11-06 | 2012-03-29 | クラリアント・ファイナンス・(ビーブイアイ)・リミテッド | プロピレン−プロピレン−コポリマーワックス及びカーボンブラックを含む組成物 |
| JP2012507587A (ja) | 2008-11-06 | 2012-03-29 | クラリアント・ファイナンス・(ビーブイアイ)・リミテッド | プロピレン−オレフィン−コポリマーワックス及びカーボンナノチューブを含む組成物 |
| WO2013153969A1 (ja) * | 2012-04-09 | 2013-10-17 | リケンテクノス株式会社 | 樹脂組成物 |
Non-Patent Citations (2)
| Title |
|---|
| See also references of EP3045497A4 |
| TAKASE HIROFUMI: "Technology of distribution and evaluation method of carbon-nanotubes", JOURNAL OF THE JAPAN SOCIETY OF POLYMER PROCESSING, vol. 18, no. 9, 2006, pages 646 - 652 |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9748017B2 (en) | 2013-09-10 | 2017-08-29 | Riken Technos Corporation | Electrically conductive resin composition, and film produced from same |
| US10767035B2 (en) | 2014-10-09 | 2020-09-08 | Riken Technos Corporation | Method for producing thermoplastic resin composition film |
| JP5983850B1 (ja) * | 2015-11-18 | 2016-09-06 | 東洋インキScホールディングス株式会社 | 導電性樹脂組成物、成形体およびその製造方法 |
| JP2017141370A (ja) * | 2016-02-10 | 2017-08-17 | デンカ株式会社 | 導電性高分子材料およびそれを用いた成形品 |
| JP2017186440A (ja) * | 2016-04-05 | 2017-10-12 | 東洋インキScホールディングス株式会社 | 導電性樹脂組成物および成形体の製造方法 |
| JP2018028031A (ja) * | 2016-08-19 | 2018-02-22 | 東洋インキScホールディングス株式会社 | 導電性樹脂組成物、成形体およびその製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3045497A1 (en) | 2016-07-20 |
| US9748017B2 (en) | 2017-08-29 |
| JP2015078344A (ja) | 2015-04-23 |
| TWI633145B (zh) | 2018-08-21 |
| EP3045497A4 (en) | 2017-07-26 |
| EP3284782B1 (en) | 2022-07-27 |
| EP3284782A1 (en) | 2018-02-21 |
| JP6385197B2 (ja) | 2018-09-05 |
| TW201518372A (zh) | 2015-05-16 |
| EP3045497B1 (en) | 2020-03-25 |
| US20160225484A1 (en) | 2016-08-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6184056B2 (ja) | 樹脂組成物 | |
| JP6385197B2 (ja) | 導電性樹脂組成物、及びそのフィルム | |
| JP6023548B2 (ja) | 導電性連通多孔質フィルムの製造方法 | |
| JP5500300B1 (ja) | シート、電極及び燃料電池 | |
| JPWO2010050219A1 (ja) | 燃料電池用ガス拡散層及びその製造方法、膜電極接合体、並びに燃料電池 | |
| JP6453026B2 (ja) | 熱可塑性樹脂組成物フィルムの製造方法 | |
| JP6780835B2 (ja) | 樹脂組成物、樹脂組成物の製造方法、粉状混合物、レドックスフロー電池用双極板、及び燃料電池用セパレータ | |
| JP6246264B2 (ja) | 樹脂組成物 | |
| KR20230149759A (ko) | 도전재 마스터 배치 및 이를 이용하여 제조된 건식 전극 | |
| JPWO2017150470A1 (ja) | 樹脂多孔質膜及びその製造方法 | |
| CN104245852B (zh) | 树脂组合物及导电性树脂膜 | |
| Bouatia et al. | Development and characterisation of electrically conductive polymeric‐based blends for proton exchange membrane fuel cell bipolar plates | |
| TWI585139B (zh) | 樹脂組成物及導電性樹脂膜 | |
| Naji et al. | Melt processed conductive polycarbonate composites with ternary fillers towards bipolar plate applications | |
| JP4802368B2 (ja) | 高導電性成形品の製造方法 | |
| JP6177011B2 (ja) | 導電性連通多孔質の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14844477 Country of ref document: EP Kind code of ref document: A1 |
|
| REEP | Request for entry into the european phase |
Ref document number: 2014844477 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2014844477 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15021188 Country of ref document: US |