WO2015012283A1 - 非水電解質二次電池用正極活物質とその製造方法、および、非水電解質二次電池 - Google Patents
非水電解質二次電池用正極活物質とその製造方法、および、非水電解質二次電池 Download PDFInfo
- Publication number
- WO2015012283A1 WO2015012283A1 PCT/JP2014/069372 JP2014069372W WO2015012283A1 WO 2015012283 A1 WO2015012283 A1 WO 2015012283A1 JP 2014069372 W JP2014069372 W JP 2014069372W WO 2015012283 A1 WO2015012283 A1 WO 2015012283A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- positive electrode
- active material
- lithium
- electrode active
- particles
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/50—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese
- H01M4/505—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese of mixed oxides or hydroxides containing manganese for inserting or intercalating light metals, e.g. LiMn2O4 or LiMn2OxFy
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01G—COMPOUNDS CONTAINING METALS NOT COVERED BY SUBCLASSES C01D OR C01F
- C01G53/00—Compounds of nickel
- C01G53/40—Complex oxides containing nickel and at least one other metal element
- C01G53/42—Complex oxides containing nickel and at least one other metal element containing alkali metals, e.g. LiNiO2
- C01G53/44—Complex oxides containing nickel and at least one other metal element containing alkali metals, e.g. LiNiO2 containing manganese
- C01G53/50—Complex oxides containing nickel and at least one other metal element containing alkali metals, e.g. LiNiO2 containing manganese of the type (MnO2)n-, e.g. Li(NixMn1-x)O2 or Li(MyNixMn1-x-y)O2
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/362—Composites
- H01M4/366—Composites as layered products
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/52—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of nickel, cobalt or iron
- H01M4/525—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of nickel, cobalt or iron of mixed oxides or hydroxides containing iron, cobalt or nickel for inserting or intercalating light metals, e.g. LiNiO2, LiCoO2 or LiCoOxFy
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2002/00—Crystal-structural characteristics
- C01P2002/50—Solid solutions
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/51—Particles with a specific particle size distribution
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/60—Particles characterised by their size
- C01P2004/64—Nanometer sized, i.e. from 1-100 nanometer
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2006/00—Physical properties of inorganic compounds
- C01P2006/12—Surface area
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
- H01M10/0525—Rocking-chair batteries, i.e. batteries with lithium insertion or intercalation in both electrodes; Lithium-ion batteries
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M2004/026—Electrodes composed of, or comprising, active material characterised by the polarity
- H01M2004/028—Positive electrodes
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Definitions
- the present invention relates to a positive electrode active material for a non-aqueous electrolyte secondary battery, a method for producing the same, and a non-aqueous electrolyte secondary battery using the positive electrode active material as a positive electrode material.
- the lithium ion secondary battery is composed of a negative electrode, a positive electrode, an electrolytic solution and the like, and a material capable of desorbing and inserting lithium is used as an active material used as a material of the negative electrode and the positive electrode.
- lithium ion secondary batteries using a layered or spinel type lithium metal composite oxide as a positive electrode material are Since a high voltage of 4V class can be obtained, practical use is advanced as a battery having a high energy density.
- lithium cobalt complex oxide (LiCoO 2 ) which is relatively easy to synthesize, lithium nickel complex oxide (LiNiO 2 ) using nickel which is cheaper than cobalt, and the like, Lithium-nickel-cobalt-manganese composite oxide (LiNi 1/3 Co 1/3 Mn 1/3 O 2 ), manganese-based lithium-manganese composite oxide (LiMn 2 O 4 ), lithium-nickel-manganese composite oxide (LiNi 0.5 Mn) Lithium composite oxides such as 0.5 O 2 ) have been proposed.
- lithium-nickel-cobalt-manganese composite oxide is attracting attention as a positive electrode material having good cycle characteristics, low resistance, and high output.
- attempts have been made to improve the performance by introducing various additive elements into the lithium-nickel-cobalt-manganese composite oxide.
- the crystallite diameter calculated from the (003) plane by the Scherrer equation and the crystallite diameter calculated from the (110) plane by the Scheller equation are controlled within a specific range.
- a lithium composite oxide has been proposed which achieves both improvement in thermal stability during charging of a lithium ion secondary battery and cycle characteristics.
- the cathode active material has a hollow structure, and the value of FWHM (003) / FWHM (104) is controlled to 0.7 or less, thereby achieving a very low temperature environment of -30 ° C.
- a lithium ion secondary battery capable of exhibiting high output characteristics in a low charge state even under the lower state has been proposed.
- such a positive electrode active material is prepared by mixing a transition metal composite hydroxide crystallized under a predetermined condition with a lithium compound, and the maximum firing temperature is 700 ° C. to 1000 ° C. in an oxidizing atmosphere. It is described that it can be obtained by firing at this temperature for 3 hours to 20 hours.
- the evaluation by half width (FWHM) can not but be a relative evaluation, and the reliability of the crystallinity of the positive electrode active material is high. It is difficult to make an evaluation.
- An object of the present invention is to provide a positive electrode active material for a non-aqueous electrolyte secondary battery capable of improving the output characteristics of a lithium ion secondary battery, in particular, the output characteristics at the time of use in a low temperature environment.
- Another object of the present invention is to provide a method by which such a positive electrode active material can be easily produced in industrial scale production.
- the inventor of the present invention affects the positive electrode resistance of a secondary battery by the crystal structure and powder characteristics of lithium nickel cobalt manganese composite oxide particles that are a positive electrode active material for a non-aqueous electrolyte secondary battery.
- high capacity is obtained when used as a positive electrode of a secondary battery.
- the positive electrode resistance can be reduced while maintaining the at the same time, it has been found that the crystallite diameter can be controlled by the calcination conditions at the time of synthesizing the lithium-nickel-cobalt-manganese composite oxide.
- the present invention has been completed based on these findings.
- the crystallite diameter determined from the half value width of the diffraction peak in the (003) plane is in the range of 80 nm to 140 nm, and the crystallite diameter determined from the half value width of the diffraction peak in the (104) plane is It is preferably in the range of 40 nm to 80 nm.
- the average particle diameter of the secondary particles is preferably 3 ⁇ m to 20 ⁇ m.
- [(d90 ⁇ d10) / average particle size] which is an index indicating the spread of the particle size distribution of the secondary particles, is 0.60 or less.
- a crystallization step to obtain nickel-cobalt-manganese composite hydroxide particles represented by A lithium compound is mixed with the nickel-cobalt-manganese composite hydroxide particles so that the ratio of the number of lithium atoms to the total number of atoms of metal elements other than lithium is 0.95 to 1.20, and lithium A mixing step to obtain a mixture; In the oxidizing atmosphere, the temperature rising time from
- the firing temperature is 0.5 hours to 1.5 hours, and the firing temperature is 850 ° C. to 1000 ° C., and this temperature is 1.0 hour to 5 hours. And firing the lithium mixture under the firing conditions of holding for 0 hours to obtain lithium-nickel-cobalt-manganese composite oxide particles.
- the entire firing time from the start of temperature increase to the end of firing is preferably in the range of 3.0 hours to 9.0 hours.
- Nickel-cobalt-manganese having an average particle size in the range of 3 ⁇ m to 20 ⁇ m in the crystallization step, and [(d90 ⁇ d10) / average particle size], which is an index indicating the spread of particle size distribution, of 0.60 or less It is preferable to obtain composite hydroxide particles.
- the method further comprises a heat treatment step of heat treating the nickel-cobalt-manganese composite hydroxide particles at a temperature in the range of 105 ° C. to 400 ° C. before the mixing step.
- the non-aqueous electrolyte secondary battery of the present invention comprises a positive electrode, a negative electrode, a separator, and a non-aqueous electrolyte, and the positive electrode active material for non-aqueous electrolyte secondary battery of the present invention is used as the positive electrode material of the positive electrode. It is characterized by
- the present invention it is possible to provide a non-aqueous electrolyte secondary battery with high capacity and excellent output characteristics under a low temperature environment. Moreover, according to the present invention, it becomes possible to manufacture the positive electrode active material for a non-aqueous electrolyte secondary battery having such excellent characteristics easily and in large quantities. For this reason, the industrial significance of the present invention is extremely great.
- FIG. 1 is a graph showing the result of particle size distribution measurement of the positive electrode active material of Example 1 obtained by the present invention.
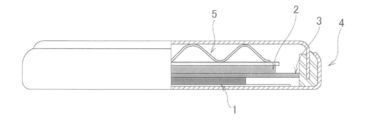
- FIG. 2 is a view schematically showing a cross section of the coin cell used in the battery evaluation of the present invention.
- Lithium-nickel-cobalt-manganese composite oxide particles (hereinafter referred to as “a cathode active material for the non-aqueous electrolyte secondary battery according to the present invention”) It is comprised by "lithium complex oxide particle.”
- the value of s indicating an excess amount of lithium (Li) is -0.05 or more and 0.20 or less, preferably 0 or more and 0.20 or less, more preferably more than 0 and 0. 0. 15 or less. If the value of s is less than ⁇ 0.05, the positive electrode resistance of the non-aqueous electrolyte secondary battery using this positive electrode active material is increased, so the output characteristics can not be improved. On the other hand, when the value of s exceeds 0.20, the initial discharge capacity of the non-aqueous electrolyte secondary battery using this positive electrode active material decreases and the positive electrode resistance increases, so the output characteristics are similarly improved. I can not do it.
- Nickel (Ni) is an element that contributes to the improvement of the battery capacity.
- the value of x indicating the content of nickel is 0.3 or more and 0.7 or less, preferably 0.4 or more and 0.6 or less. If the value of x is less than 0.3, the battery capacity of the non-aqueous electrolyte secondary battery using this positive electrode active material is reduced. On the other hand, if the value of x exceeds 0.7, the amount of other additive elements decreases, and there is a possibility that the effect of the addition can not be sufficiently obtained.
- Co Co is an element that contributes to the improvement of cycle characteristics.
- the value of y indicating the cobalt content is 0.1 or more and 0.4 or less, preferably 0.2 or more and 0.35 or less.
- the positive electrode active material has good cycle characteristics, that is, high durability. If the value of y is less than 0.1, sufficient cycle characteristics can not be obtained, and the capacity retention rate is lowered. On the other hand, when the value of y exceeds 0.4, the initial discharge capacity is significantly reduced.
- Manganese (Mn) is an element which contributes to the improvement of the thermal stability.
- the value of z indicating the content of manganese is 0.1 or more and 0.4 or less, preferably 0.2 or more and 0.3 or less. If the value of z is less than 0.1, the addition effect can not be obtained sufficiently. On the other hand, if it exceeds 0.4, the battery capacity of the non-aqueous electrolyte secondary battery using this positive electrode active material will be reduced.
- the lithium composite oxide particles can contain an additional element (M).
- M additional element
- additional elements calcium (Ca), magnesium (Mg), aluminum (Al), titanium (Ti), vanadium (V), chromium (Cr), zirconium (Zr), niobium (Nb)
- Mo molybdenum
- Hf hafnium
- Ta tantalum
- W tungsten
- the value of t indicating the content of the additional element (M) is 0 or more and 0.05 or less, preferably 0.0003 or more and 0.05 or less, more preferably 0.001 or more and 0.01 or less.
- the addition of the additive element (M) is optional, when it is added, the value of t is set to 0. from the viewpoint of improving the durability and output characteristics of the non-aqueous electrolyte secondary battery using this positive electrode active material. It is preferable to set it as 0003 or more.
- the value of t exceeds 0.05, the metal element contributing to the Redox reaction decreases, and the battery capacity decreases.
- the additive element (M) is crystallized together with nickel, cobalt and manganese in the crystallization step as described later, and is added to nickel-cobalt-manganese composite hydroxide particles (hereinafter referred to as "composite hydroxide particles").
- composite hydroxide particles nickel-cobalt-manganese composite hydroxide particles
- the particles can be dispersed uniformly, the surface of the composite hydroxide particles may be coated with the additional element (M) after the crystallization step.
- the mixing step it is also possible to mix with the lithium compound together with the composite hydroxide particles, and these methods may be used in combination. Regardless of which method is used, it is necessary to adjust the content so as to obtain the composition of the general formula (A).
- the lithium composite oxide particles constituting the positive electrode active material of the present invention have a half value width (half value width: half value width of a diffraction peak on the (003) plane in Miller index (hkl) in powder X-ray diffraction using CuK ⁇ rays.
- the crystallite diameter (hereinafter referred to as “(104) plane” calculated from the half value width of the diffraction peak on the (104) plane with respect to the crystallite diameter (hereinafter referred to as “(003) plane crystal diameter”) calculated from FWHM)
- the ratio of “crystallite diameter” is more than 0 and less than 0.60, preferably 0.35 or more and 0.55 or less, more preferably 0.35 or more and 0.50 or less, and has a layered structure . That is, the positive electrode active material of the present invention satisfies at least the following formula (1). 0 ⁇ (104) plane crystallite diameter / (003) plane crystallite diameter ⁇ 0.60 (1)
- the crystallite diameter is a scale indicating the average size of single crystals in lithium composite oxide particles, and is an index of crystallinity.
- the crystallite diameter can be calculated by X-ray diffraction measurement (XRD, X-ray diffraction) and calculation using the following Scheller equation.
- XRD X-ray diffraction
- the crystal structure of the lithium composite oxide particles of the present invention is a layered structure and belongs to a hexagonal system.
- lithium ions are occluded in the lithium composite oxide particles and released from the particles in the direction orthogonal to the hexagonal c-axis.
- the (003) plane in the Miller index (hkl) of powder X-ray diffraction using CuK ⁇ ray relates to the c-axis direction of the hexagonal system.
- the (104) plane is in the direction orthogonal to the c-axis of the hexagonal system.
- the (104) plane crystallite diameter ratio is 0.60 or more, crystal growth proceeds in the direction orthogonal to the c-axis and the diffusion distance of lithium ions becomes long, so the output characteristics of the secondary battery Will decrease.
- the difference becomes remarkable, and the output characteristics of the secondary battery are significantly reduced.
- the (104) plane crystal diameter ratio is 0.35 or more in practice, in consideration of manufacturing restrictions.
- the (104) plane crystallite diameter ratio is controlled to the above-described range, and the (104) plane crystallite diameter is 40 nm to 80 nm, and (003) It is preferable to control the plane crystallite diameter to 80 nm to 140 nm.
- the crystallinity of the positive electrode active material can be made favorable, and excellent charge / discharge capacity and cycle characteristics can be realized in a secondary battery using this positive electrode active material as a positive electrode material.
- the (104) plane crystallite diameter is controlled to 40 nm to 75 nm, and more preferably to 40 nm to 70 nm.
- the (003) plane crystallite diameter is preferably controlled to 100 nm to 130 nm, and more preferably 105 nm to 130 nm.
- the positive electrode active material of the present invention is composed of substantially spherical secondary particles formed by aggregation of a plurality of primary particles.
- the shape of the primary particles constituting the secondary particles can be various forms such as plate, needle, cuboid, ellipse, and rhombic shape, and the aggregation state also aggregates in a random direction.
- the shape of the secondary particles is preferably spherical.
- the shapes of the primary and secondary particles and the structure of the secondary particles are, for example, embedded secondary particles in a resin or the like, and after cross-section observation by cross-section polisher, etc., this cross-section is scanned It can confirm by observing with a type electron microscope (SEM).
- SEM type electron microscope
- Such secondary particles sufficiently have an interface or grain boundary through which the electrolytic solution can penetrate between primary particles constituting the secondary particle. Therefore, the electrolytic solution can be efficiently permeated to the surface of the primary particles where lithium ions are released and inserted, and the synergistic effect with the control of the (104) plane crystallite diameter ratio and the crystallite diameter described above By this, it is possible to significantly improve the output characteristics.
- Such secondary particles can be easily obtained by a crystallization process as described later.
- the positive electrode active material of the present invention preferably has an average particle size of 3 ⁇ m to 20 ⁇ m.
- the average particle diameter means a volume-based average particle diameter (MV) obtained by a laser diffraction scattering method.
- the average particle size is less than 3 ⁇ m, the packing density of the lithium composite oxide particles may decrease when the positive electrode is formed, and the battery capacity per volume of the positive electrode may decrease. In addition, excessive reaction with the electrolyte of the battery may occur to reduce safety.
- the average particle size exceeds 20 ⁇ m, the specific surface area of the positive electrode active material is reduced and the interface with the electrolytic solution is reduced, so that the positive electrode resistance may be increased and the output characteristics may be reduced.
- the average particle diameter is more preferably 3 ⁇ m to 10 ⁇ m, and still more preferably 3 ⁇ m to 8 ⁇ m.
- the positive electrode active material has fine particles having a very small particle size with respect to the average particle diameter, and a particle diameter with respect to the average particle diameter. There will be a lot of very large coarse particles.
- the positive electrode is configured using a positive electrode active material in which a large amount of particles are present, not only the secondary battery may generate heat due to the local reaction of the particles, and the safety may also be reduced. May selectively deteriorate and the cycle characteristics may deteriorate.
- the reaction area between the electrolytic solution and the positive electrode active material can not be sufficiently ensured, and the positive electrode resistance increases. May decrease.
- the average particle diameter of the positive electrode active material is small, the ratio of the fine particles and the coarse particles tends to increase, so it is necessary to appropriately control the particle size distribution.
- [(d90 ⁇ d10) / average particle diameter] is preferably 0. It is controlled to be 60 or less, more preferably 0.55 or less, still more preferably 0.30 to 0.45. This makes it possible to suppress the mixing of fine particles and coarse particles, and to ensure the safety and output characteristics of the secondary battery.
- the value of [(d90 ⁇ d10) / average particle diameter] can be determined from the volume integrated diameter (d90, d10) and the volume-based average particle diameter (MV) by the laser diffraction scattering method.
- the specific surface area of the positive electrode active material having a specific surface area of the present invention preferably to 0.3m 2 /g ⁇ 2.5m 2 / g, and 0.5m 2 /g ⁇ 2.0m 2 / g Is more preferred. If the specific surface area is less than 0.3 m 2 / g, the reaction area with the electrolytic solution may not be sufficiently secured in some cases. On the other hand, if it exceeds 2.5 m 2 / g, an excessive reaction between the non-aqueous electrolyte secondary battery and the electrolytic solution may occur to lower the safety.
- the specific surface area can be measured by the BET method using nitrogen gas adsorption.
- a lithium hydroxide is added to the composite hydroxide particles and mixed, and a crystallization step of obtaining the composite hydroxide particles,
- the method comprises the steps of: obtaining a lithium mixture; and firing the lithium mixture in an oxidizing atmosphere to obtain lithium composite oxide particles.
- a heat treatment step of heat-treating the composite hydroxide particles or a crushing step of crushing the lithium composite oxide particles obtained in the baking step can be added.
- the crystallization step is not particularly limited, and a known crystallization method can be used, and by controlling the conditions, the particle size, shape, etc. of the composite hydroxide particles obtained can be adjusted. .
- an aqueous solution containing a compound of nickel, cobalt and manganese or a compound of these elements and an additive element (M) in a ratio represented by the general formula (B) may be used as a mixed aqueous solution or these compounds.
- the aqueous solutions contained are separately fed into the reaction vessel.
- an aqueous solution containing an ammonium ion supplier and an aqueous sodium hydroxide solution a reaction aqueous solution is formed in the reaction tank, and its pH value is 10.5-11.6 at a liquid temperature of 25 ° C.
- the composite hydroxide particles generated in the reaction vessel are recovered using a continuous crystallization method or a batch crystallization method. Subsequently, the recovered composite hydroxide particles are washed with water to remove impurities, and dried.
- the composite hydroxide particles obtained by such a crystallization method have the form of spherical secondary particles in which primary particles are aggregated.
- a preferred embodiment of the present invention is a positive electrode active material having an average particle size of 3 ⁇ m to 20 ⁇ m and an index indicating the spread of particle size distribution [(d90 ⁇ d10) / average particle size] of 0.60 or less.
- a batch crystallization method hereinafter referred to as “nucleation separated crystallization method” in which composite hydroxide particles, which are precursors, are clearly separated into a nucleation step and a particle growth step. Is preferably manufactured by
- nucleation and particle growth are clearly separated by individually controlling the pH value of the reaction liquid in the nucleation step and the particle growth step, and the composite having a narrow particle size distribution. It is a method of producing hydroxide particles.
- the pH value of the aqueous solution for nucleation containing a metal compound and an ammonium ion donor is 12.0 to 14.0, preferably 12.0 to 13.0, at a liquid temperature of 25 ° C.
- the aqueous solution for particle growth containing the nucleus formed in this nucleation step has a pH value at a liquid temperature of 25 ° C.
- the nucleation step A composite water having a narrow particle size distribution by growing nuclei (particle growth step) under control of pH value lower than 10.5-12.0, preferably 10.5-11.5. It is a method of producing oxide particles.
- the particle diameter of the composite hydroxide particles obtained by such a method can be controlled by crystallization conditions such as pH value and nucleation amount in the nucleation step and / or reaction time in the particle growth step. Further, by controlling the power for stirring place per unit volume of the reaction liquid, it is possible to control the particle size, for example, it is possible to increase the particle size by reducing the power required for stirring.
- the composite hydroxide particles may be heat treated to form heat treated particles and then mixed with the lithium compound before the mixing step after the crystallization step.
- the composite hydroxide particles from which excess moisture has been removed in the heat treatment step but also nickel-cobalt-manganese composite oxide particles (hereinafter referred to as “complex oxide”) converted to oxides in the heat treatment step Also included are particles) or mixtures of these.
- the heat treatment step is a step of heating the composite hydroxide particles to a temperature of 105 ° C. to 400 ° C. to remove the water contained in the composite hydroxide particles.
- the heating temperature in the heat treatment step is 105 ° C. to 400 ° C., preferably 120 ° C. to 400 ° C. If the heating temperature is less than 105 ° C., excess water in the composite hydroxide particles can not be removed, and the variation may not be sufficiently suppressed. On the other hand, even if the heating temperature exceeds 400 ° C., not only can no further effect be expected, but also the production cost will increase. In addition, the variation can be suppressed by previously determining each metal component contained in the heat treatment particles under the heat treatment condition by analysis and determining the ratio to the lithium compound.
- the atmosphere in the heat treatment step is not particularly limited as long as it is a non-reducing atmosphere, but it is preferable to carry out in an air stream that can be carried out simply.
- the heat treatment time is also not particularly limited, but in some cases, excess water of the composite hydroxide particles can not be sufficiently removed in less than one hour. Therefore, the heat treatment time is preferably 1 hour or more, and more preferably 5 hours to 15 hours.
- the equipment used for such heat treatment is not particularly limited, as long as the composite hydroxide particles can be heated in a non-reducing atmosphere, preferably in an air stream, and an electric furnace without gas generation Are preferably used.
- the composite water hydroxide particles such that Li / Me of the lithium mixture obtained by this mixing step becomes Li / Me of the target positive electrode active material Alternatively, it is necessary to mix the heat-treated particles and the lithium compound.
- the lithium compound to be mixed with the composite hydroxide particles or the heat-treated particles is not particularly limited, but in view of availability, lithium hydroxide, lithium nitrate, lithium carbonate or a mixture thereof is preferably used. can do. In particular, in consideration of ease of handling and stability of quality, it is preferable to use lithium hydroxide or lithium carbonate, and it is more preferable to use lithium carbonate.
- the lithium mixture is preferably sufficiently mixed before firing. If mixing is insufficient, Li / Me may vary among individual particles, and sufficient battery characteristics may not be obtained.
- a common mixer can be used for mixing, for example, a shaker mixer, a V blender, a ribbon mixer, a Julia mixer, a Loedige mixer, etc. can be used.
- the composite hydroxide particles or the heat-treated particles and the lithium compound may be sufficiently mixed to such an extent that the shape of the composite hydroxide particles or the heat-treated particles is not broken.
- the compound of the additive element (M) can also be mixed with the lithium compound.
- the surface of the composite hydroxide particles or the heat-treated particles may be coated with the compound of the additive element (M) and then mixed with the lithium compound.
- these methods may be used in combination. In any case, it is necessary to appropriately adjust the additive element (M) to have the composition of the general formula (A).
- the lithium mixture is preferably 350 ° C. or more and less than 650 ° C., more preferably 450 ° C. or more before the firing step after the mixing step. It is calcined at a temperature of 600 ° C. (calcining temperature). That is, it is preferable to perform calcination at a reaction temperature of lithium hydroxide or lithium carbonate and a nickel-cobalt-manganese composite oxide. Thereby, the diffusion of lithium to the heat-treated particles is promoted, and more uniform lithium composite oxide particles can be obtained.
- the calcination temperature is preferably maintained for 1 hour to 10 hours, more preferably 3 hours to 6 hours. Moreover, it is preferable to set the temperature rising time from the start of heating to reaching the calcination temperature to be 0.8 hours to 5.0 hours, and it is more preferable to set it to 1.0 hours to 4.0 hours. preferable.
- the lithium mixture obtained in the mixing step is fired under predetermined conditions and cooled to room temperature to obtain lithium composite oxide particles.
- the firing temperature is set to 850 ° C. to 1000 ° C.
- the temperature rising time (t 2 ) from 650 ° C. to the firing temperature is set to 0.5 hours to 1.5 hours and at the firing temperature. It is important to set the retention time (t 3 ) to 1 to 5 hours. That is, by firing the lithium mixture under such firing conditions, it is possible to enhance the crystallinity while suppressing the crystal growth in the direction orthogonal to the c-axis, and the (104) plane crystallite diameter ratio is It becomes possible to obtain lithium composite oxide particles controlled to a range of more than 0 and less than 0.60.
- the firing furnace which can be used in the firing step is not particularly limited as long as it can be heated in the atmosphere or in an oxygen stream, but an electric furnace without gas generation is preferable, Either a batch-type electric furnace or a continuous-type electric furnace can be suitably used.
- the firing temperature in the present invention is 850 ° C. to 1000 ° C., preferably 890 ° C. to 1000 ° C., more preferably 890 ° C. to 950 ° C. If the calcination temperature is less than 850 ° C., lithium does not diffuse sufficiently, and surplus lithium and unreacted composite hydroxide particles or composite oxide particles remain, or a lithium composite oxide having high crystallinity is obtained. The problem of not being able to On the other hand, when the firing temperature exceeds 1000 ° C., not only severe sintering occurs between the lithium composite oxide particles, but also abnormal grain growth is caused, and the form of the secondary particles can not be kept spherical.
- [Baking time] a) Temperature rise time to reach 650 ° C from room temperature (t 1 )
- the temperature raising time (t 1 ) until reaching 650 ° C. from room temperature is not particularly limited, but is preferably 0.5 hours to 10 hours, more preferably 0.8 hours to 10 hours, further preferably It shall be 1.0 hour to 8.0 hours. If t 1 is less than 0.5 hours, the reaction between the composite hydroxide particles or the composite oxide particles and lithium in the lithium compound may not proceed sufficiently. On the other hand, if t 1 is greater than 10 hours, productivity is deteriorated.
- room temperature is a temperature in a state where neither heating nor cooling before firing is performed, and although there are fluctuations due to the season, it is usually 10 ° C. to 35 ° C.
- Temperature rise time (t 2 ) from 650 ° C. to reach the firing temperature is 0.5 hour to 1.5 hours, preferably 0.5 hour to 1.2 hours, more preferably 0 .5 hours to 1.0 hours This makes it possible to control the (104) plane crystallite diameter ratio to the above-described range while suppressing crystal growth in the direction orthogonal to the c-axis.
- the composite hydroxide particles or composite oxide particles can not be sufficiently reacting lithium in the lithium compound. For this reason, it is necessary to react the composite hydroxide particles with lithium after reaching the firing temperature. That is, it is necessary to allow crystallization to proceed while reacting the composite hydroxide particles or composite oxide particles with lithium between reaching the firing temperature and ending the holding at the firing temperature. As a result, there arises a problem that crystal growth of the obtained positive electrode active material proceeds too much.
- t 2 is more than 1.5 hours, crystallization or become uneven lithium composite oxide particles, the secondary particles and primary particles are excessively sintered, high positive electrode resistance of the secondary battery Problems such as
- the holding time (t 3 ) at the baking temperature is 1.0 hour to 5.0 hours, preferably 2.0 hours to 5.0 hours, and more preferably 2.0 hours to 4.0 hours.
- the t 3 is less than 1.0 hours, without crystallization proceeds sufficiently in the lithium composite oxide particles, the crystallinity is lowered.
- t 3 exceeds 5.0 hours, the crystal growth in the direction perpendicular to the c axis progresses, it becomes impossible to control the scope of the present invention (104) plane crystallite diameter ratio.
- Time from 650 ° C. to the end of firing (t 4 )
- the time will be 5.5 hours. In particular, 2.0 hours to 5.5 hours are preferable.
- t 4 is less than 1.5 hours, the composite hydroxide particles or composite oxide particles and the lithium compound do not sufficiently react, and the surplus lithium compound and the unreacted composite hydroxide particles or composite oxide particles remain Or the diffusion of lithium into the composite hydroxide particles or composite oxide particles is insufficient, and the crystal structure is not uniform.
- t 4 exceeds 6.5 hours, in some cases the crystal growth in the direction perpendicular to the c-axis will progress.
- the temperature rising rate in the process of reaching the firing temperature from 650 ° C. does not necessarily have to be constant as long as the temperature rising time during this period is in the above-described range.
- the maximum temperature rising rate during this time is controlled to be preferably 16.0 ° C./min or less, more preferably 12.0 ° C./min or less, and further preferably 10.0 ° C./min or less.
- lithium can be sufficiently diffused in the composite hydroxide particles or the composite oxide particles, and uniform lithium composite oxide particles can be obtained.
- the maximum temperature rising rate exceeds 16 ° C./min, lithium may not be sufficiently diffused, and the obtained lithium composite oxide particles may become nonuniform.
- the average temperature rising rate in the above temperature range is preferably 2.5 ° C./min to 16.0 ° C./min, more preferably 3.0 ° C./min to 12.0 ° C./min, further preferably 5 Control is made to be from 0 ° C./min to 10.0 ° C./min. Moreover, it is preferable to maintain the temperature rising rate during this time at a constant temperature within such a range. Thereby, the above-described effect can be reliably obtained.
- the atmosphere at the time of firing is an oxidizing atmosphere, but it is preferable to carry out in an atmosphere having an oxygen concentration of 18% by volume to 100% by volume, that is, in the air or an oxygen stream. In view of the cost, it is particularly preferable to carry out in an air stream. If the oxygen concentration is less than 18% by volume, the oxidation reaction may not proceed sufficiently, and the crystallinity of the obtained lithium composite oxide particles may not be sufficient.
- the production method of the present invention it is preferable to further include a crushing step of crushing the obtained lithium composite oxide particles after the baking step.
- the lithium composite oxide particles obtained by the firing step may have aggregation or slight sintering.
- the average particle size (MV) of the obtained positive electrode active material is easily adjusted to a preferable range of 3 ⁇ m to 20 ⁇ m by crushing an aggregate or a sintered body of lithium composite oxide particles.
- crushed mechanical energy is injected into an aggregate consisting of a plurality of secondary particles generated by sintering necking between secondary particles at the time of firing, and the secondary particles themselves are hardly destroyed. It is an operation to separate secondary particles and loosen aggregates.
- known means can be used, and for example, a pin mill, a hammer mill or the like can be used. At this time, it is preferable to adjust the crushing power to an appropriate range so as not to break the secondary particles.
- Nonaqueous Electrolyte Secondary Battery of the present invention is composed of the same components as a general nonaqueous electrolyte secondary battery, such as a positive electrode, a negative electrode, a separator, and a nonaqueous electrolytic solution.
- a general nonaqueous electrolyte secondary battery such as a positive electrode, a negative electrode, a separator, and a nonaqueous electrolytic solution.
- the embodiment described below is merely an example, and the non-aqueous electrolyte secondary battery of the present invention is applied to various modifications and improvements based on the embodiment described in the present specification. It is also possible.
- Component material [positive electrode] Using the positive electrode active material of the present invention, for example, a positive electrode of a non-aqueous electrolyte secondary battery is produced as follows.
- a conductive material and a binder are mixed with the powdery positive electrode active material obtained according to the present invention, and if necessary, activated carbon and a solvent for viscosity adjustment are added, and these are kneaded to obtain a positive electrode mixture Make a paste.
- the mixing ratio of each in the positive electrode mixture paste is also an important factor that determines the performance of the non-aqueous electrolyte secondary battery.
- the content of the positive electrode active material is 60 parts by mass to 95 parts by mass as in the case of the positive electrode of a general non-aqueous electrolyte secondary battery. It is desirable that the content be 1 part by mass to 20 parts by mass, and the content of the binder be 1 part by mass to 20 parts by mass.
- the obtained positive electrode mixture paste is applied, for example, on the surface of a current collector made of aluminum foil, and dried to disperse the solvent. If necessary, pressure may be applied by a roll press or the like to increase the electrode density. Thus, a sheet-like positive electrode can be produced.
- the sheet-like positive electrode can be cut into an appropriate size according to the target battery, and can be used for battery production.
- the method of producing the positive electrode is not limited to such a method, and may be another method.
- the conductive material it is possible to use, for example, graphite (natural graphite, artificial graphite, expanded graphite and the like), and carbon black based materials such as acetylene black and ketjen black.
- the binder plays a role of holding the active material particles, and, for example, polyvinylidene fluoride (PVDF), polytetrafluoroethylene (PTFE), fluororubber, ethylene propylene diene rubber, styrene butadiene, cellulose resin and polyacrylic.
- PVDF polyvinylidene fluoride
- PTFE polytetrafluoroethylene
- fluororubber ethylene propylene diene rubber
- styrene butadiene cellulose resin
- cellulose resin polyacrylic.
- An acid can be used.
- a positive electrode active material, a conductive material, and activated carbon can be dispersed, and a solvent that dissolves the binder can be added to the positive electrode mixture.
- a solvent specifically, an organic solvent such as N-methyl-2-pyrrolidone can be used.
- activated carbon can also be added to the positive electrode mixture.
- Electrode In the negative electrode, metal lithium, lithium alloy, etc., or a negative electrode active material capable of absorbing and desorbing lithium ions is mixed with a binder, and an appropriate solvent is added to form a paste-like negative electrode composite material, copper, etc. The solution is applied to the surface of the metal foil current collector, dried, and compressed to form an electrode density as needed.
- the negative electrode active material it is possible to use, for example, natural graphite, organic graphite such as artificial graphite and phenolic resin, and powder of carbon material such as coke.
- a fluorine-containing resin such as PVDF can be used as in the positive electrode
- an organic compound such as N-methyl-2-pyrrolidone A solvent can be used.
- a separator is interposed and disposed between the positive electrode and the negative electrode.
- a separator separates a positive electrode and a negative electrode and holds an electrolyte, and a thin film such as polyethylene or polypropylene having a large number of fine pores can be used.
- Non-aqueous electrolyte The non-aqueous electrolytic solution is obtained by dissolving a lithium salt as a supporting salt in an organic solvent.
- cyclic carbonates such as ethylene carbonate, propylene carbonate, butylene carbonate and trifluoropropylene carbonate
- linear carbonates such as diethyl carbonate, dimethyl carbonate, ethyl methyl carbonate and dipropyl carbonate, and further tetrahydrofuran, 2-
- ether compounds such as methyltetrahydrofuran and dimethoxyethane
- sulfur compounds such as ethyl methyl sulfone and butanesultone
- phosphorus compounds such as triethyl phosphate and trioctyl phosphate be able to.
- LiPF 6 LiBF 4 , LiClO 4 , LiAsF 6 , LiN (CF 3 SO 2 ) 2 , and complex salts thereof can be used.
- non-aqueous electrolyte may contain a radical scavenger, a surfactant, a flame retardant, and the like.
- the non-aqueous electrolyte secondary battery of the present invention which is composed of the positive electrode, the negative electrode, the separator and the non-aqueous electrolyte described above, has various shapes such as cylindrical and laminated. It can be in the form of
- the positive electrode and the negative electrode are stacked via a separator to form an electrode body, and the obtained electrode body is impregnated with a non-aqueous electrolytic solution, and a positive electrode passing the positive electrode current collector and the outside
- the terminal and the negative electrode current collector and the negative electrode terminal leading to the outside are connected using a current collection lead or the like, and sealed in a battery case to complete a non-aqueous electrolyte secondary battery.
- the non-aqueous electrolyte secondary battery using the positive electrode active material of the present invention can improve its output characteristics, in particular, output characteristics when used in a low temperature environment (about 0 ° C. or less).
- the positive electrode resistance at 0 ° C. should be 110 ⁇ or less, preferably 100 ⁇ or less, more preferably 95 ⁇ or less Can. Therefore, the non-aqueous electrolyte secondary battery of the present invention is suitable as a power source for small portable electronic devices that require high output, including use in cold regions, and transport machinery such as electric vehicles.
- the non-aqueous electrolyte secondary battery using the positive electrode active material of the present invention can obtain a high initial discharge capacity of 150 mAh / g or more, preferably 155 mAh / g or more, more preferably 156 mAh / g or more.
- a high capacity retention rate can be obtained even in a long cycle, and it can be said that the capacity is high and the life is long.
- the thermal stability is high and the safety is also excellent.
- the non-aqueous electrolyte secondary battery of the present invention not only has a low positive electrode resistance and a high capacity, but it is easy to ensure safety and simplifies an expensive protection circuit. be able to. For this reason, the non-aqueous electrolyte secondary battery of the present invention is easy to miniaturize, and can be manufactured at low cost.
- the non-aqueous electrolyte secondary battery of the present invention is suitable as a power source for small-sized portable electronic devices and electric vehicles which are restricted by the mounting space.
- the present invention can be used not only as a power source for an electric vehicle driven purely by electric energy, but also as a power source for a so-called hybrid vehicle used in combination with a combustion engine such as a gasoline engine or a diesel engine.
- Example 1 [Crystallization process] First, while the reaction vessel (600 L) was charged with water and stirred, the temperature in the vessel was adjusted to 40 ° C., and nitrogen gas was allowed to flow to create a nitrogen atmosphere. An appropriate amount of 25% by mass sodium hydroxide aqueous solution and 25% by mass ammonia water is added to the water in the reaction tank so that the pH value of the reaction liquid in the tank becomes 12.6 at a liquid temperature of 25 ° C. The solution was adjusted to an aqueous solution before reaction.
- This mixed aqueous solution was added to the pre-reaction aqueous solution in the reaction tank at a rate of 1300 ml / min to obtain a reaction aqueous solution.
- 25% by mass ammonia water and 25% by mass sodium hydroxide aqueous solution are also added to this reaction aqueous solution at a constant rate, and the pH value of the reaction aqueous solution (aqueous solution for nucleation) is 12.6 (nucleation pH value). While controlled, nucleation was performed by crystallization for 2 minutes and 30 seconds.
- this composite hydroxide particle is represented by the general formula: (Ni 0.33 Co 0.33 Mn 0.33 ) 0.993 Zr 0.002 W 0.005 (OH) 2 + ⁇ (0 ⁇ ⁇ ⁇ 0.5) Was confirmed.
- this composite hydroxide The average particle diameter of the particles was 5.1 ⁇ m, and [(d90 ⁇ d10) / average particle diameter] was confirmed to be 0.44.
- the mixing was performed using a shaker mixer device (TURBULA Type T2C, manufactured by Willie et Bachkofen (WAB).
- the lithium mixture obtained in the mixing step was fired at a firing temperature of 950 ° C. in a stream of air (oxygen: 21% by volume). Specifically, the temperature rising time (t 1 ) from room temperature (28 ° C.) to 650 ° C. is controlled to 2.7 hours, and the temperature rising time (t 2 ) from 650 ° C. to 950 ° C. is controlled to 1.3 hours. After raising the temperature at a constant rate, firing was performed at 950 ° C. with a holding time (t 3 ) of 4.5 hours. In the present example, the temperature rising rate (average temperature rising rate) from room temperature to the sintering temperature was 3.8 ° C./min.
- the lithium composite oxide particles thus obtained were cooled to room temperature and then crushed to obtain a positive electrode active material for a non-aqueous electrolyte secondary battery.
- this positive electrode active material is represented by the general formula: Li 1.14 (Ni 0.33 Co 0.33 Mn 0.33 ) 0.993 Zr 0.002 W 0.005 O 2 . Moreover, it was confirmed by observation using a scanning electron microscope (SEM) that the positive electrode active material is composed of secondary particles in which primary particles are aggregated.
- the average particle diameter of this positive electrode active material was 5.0 ⁇ m. [(D90-d10) / average particle size] was confirmed to be 0.41.
- the specific surface area of the positive electrode active material was confirmed to be 1.6 m 2 / g by measurement using a nitrogen adsorption type BET method measuring instrument (manufactured by Yuasa Ionix Co., Ltd., Cantasorb QS-10). .
- the crystal structure of this positive electrode active material is a layered crystal lithium composite of hexagonal crystals. It was confirmed to consist of a single oxide phase.
- the (104) plane of this positive electrode active material is calculated based on Scheller's equation using the half value width (half value width: FWHM) of each diffraction peak from the crystal. And (003) plane crystallite diameter was calculated. As a result, it is confirmed that the (104) plane crystallite diameter of this positive electrode active material is 52.5 nm, the (103) plane crystallite diameter is 105 nm, and the (104) plane crystallite diameter ratio is 0.50.
- a positive electrode (electrode for evaluation) (1) shown in FIG. 2 was produced and dried in a vacuum dryer at 120 ° C. for 12 hours.
- a 2032 type coin battery (B) was produced in a glove box under an Ar atmosphere controlled to have a dew point of -80.degree.
- the 2032-type coin battery (B) was provided with a gasket (4) and a wave washer (5) in addition to the above-described configuration.
- Example 3 In the firing step, the positive electrode activity is the same as in Example 1, except that the firing temperature is 898 ° C., t 1 is 1.8 hours, t 2 is 0.7 hours, and t 3 is 4.0 hours. The material was obtained and evaluated.
- Example 6 In the firing step, the t 1 3.0 hour, t 2 for 1.5 hours, except that the t 3 and 1.0 hours, in the same manner as in Example 1, were evaluated with obtaining a positive electrode active material.
- Example 7 In the firing step, a positive electrode active material was obtained and evaluated in the same manner as in Example 1 except that the firing temperature was changed to 850 ° C.
- Example 8 In the firing step, a positive electrode active material was obtained and evaluated in the same manner as in Example 1 except that the firing temperature was set to 1000 ° C. and t 3 was set to 4.0 hours.
- Example 9 In the firing step, the t 2 0.5 h, (it was t 4 for 1.5 hours) that the t 3 and 1.0 hours except, in the same manner as in Example 1, to obtain a positive electrode active material Evaluated together with
- Example 10 In the firing step, the firing temperature is 950 ° C., t 2 is 1.3 hours, t 3 is 4.0 hours, and the temperature rising rate from 650 ° C. to 810 ° C. is 16.0 ° C./minute.
- a positive electrode active material was obtained and evaluated in the same manner as in Example 1 except that firing was performed with the temperature rising rate as the maximum temperature rising rate.
- Example 11 In the firing step, the firing temperature is 1000 ° C., t 1 is 0.5 hours, t 2 is 0.5 hours, t 3 is 4.0 hours, and the average temperature rising rate is 11.7 ° C. /
- the positive electrode active material was obtained and evaluated in the same manner as in Example 1 except that the amount was divided.
- the positive electrode activity is the same as in Example 1, except that the firing temperature is 800 ° C., t 1 is 3.2 hours, t 2 is 0.8 hours, and t 3 is 4.0 hours. The material was obtained and evaluated.
- the positive electrode activity is the same as in Example 1, except that the firing temperature is 900 ° C., t 1 is 1.1 hours, t 2 is 0.4 hours, and t 3 is 1.0 hour. The material was obtained and evaluated.
- the positive electrode activity is the same as in Example 1, except that the firing temperature is 1050 ° C., t 1 is 2.5 hours, t 2 is 1.5 hours, and t 3 is 4.0 hours. The material was obtained and evaluated.
- the positive electrode active materials of Examples 1 to 11 belonging to the technical scope of the present invention have a (104) plane crystallite diameter ratio of 0.60 or less. Be done.
- the 2032 type coin batteries of Examples 1 to 11 using such a positive electrode active material as a positive electrode material can not only suppress the positive electrode resistance at 0 ° C. to 110 ° C. or less, but also have 150 mAh / g or more It is confirmed that an initial discharge capacity is achievable.
- the (104) plane crystallite diameter ratio exceeds 0.60. That is confirmed.
- the positive electrode resistance at 0 ° C. shows a large value.
Landscapes
- Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Composite Materials (AREA)
- Battery Electrode And Active Subsutance (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Inorganic Compounds Of Heavy Metals (AREA)
- Materials Engineering (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201480040647.5A CN105453312B (zh) | 2013-07-24 | 2014-07-22 | 非水电解质二次电池用正极活性物质及其制造方法以及非水电解质二次电池 |
| EP14830084.1A EP3026740B1 (en) | 2013-07-24 | 2014-07-22 | Non-aqueous electrolyte secondary battery positive electrode active material and method for manufacturing same, and non-aqueous electrolyte secondary battery |
| US14/907,089 US20160172674A1 (en) | 2013-07-24 | 2014-07-22 | Cathode active material for non-aqueous electrolyte secondary battery and manufacturing method thereof, and non-aqueous electrolyte secondary battery |
| KR1020167000140A KR102278009B1 (ko) | 2013-07-24 | 2014-07-22 | 비수 전해질 2차 전지용 정극 활물질과 그의 제조 방법, 및 비수 전해질 2차 전지 |
| US17/539,732 US20220093919A1 (en) | 2013-07-24 | 2021-12-01 | Cathode active material for non-aqueous electrolyte secondary battery and manufacturing method thereof, and non-aqueous electrolyte secondary battery |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013153890A JP6244713B2 (ja) | 2013-07-24 | 2013-07-24 | 非水電解質二次電池用正極活物質の製造方法 |
| JP2013-153890 | 2013-07-24 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/907,089 A-371-Of-International US20160172674A1 (en) | 2013-07-24 | 2014-07-22 | Cathode active material for non-aqueous electrolyte secondary battery and manufacturing method thereof, and non-aqueous electrolyte secondary battery |
| US17/539,732 Division US20220093919A1 (en) | 2013-07-24 | 2021-12-01 | Cathode active material for non-aqueous electrolyte secondary battery and manufacturing method thereof, and non-aqueous electrolyte secondary battery |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015012283A1 true WO2015012283A1 (ja) | 2015-01-29 |
Family
ID=52393317
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/069372 Ceased WO2015012283A1 (ja) | 2013-07-24 | 2014-07-22 | 非水電解質二次電池用正極活物質とその製造方法、および、非水電解質二次電池 |
Country Status (6)
| Country | Link |
|---|---|
| US (2) | US20160172674A1 (enExample) |
| EP (1) | EP3026740B1 (enExample) |
| JP (1) | JP6244713B2 (enExample) |
| KR (1) | KR102278009B1 (enExample) |
| CN (1) | CN105453312B (enExample) |
| WO (1) | WO2015012283A1 (enExample) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104779388A (zh) * | 2015-04-30 | 2015-07-15 | 湖南瑞翔新材料股份有限公司 | 镍钴二元正极材料前驱体制备方法以及该法制得镍钴二元正极材料前驱体 |
| JP2015164119A (ja) * | 2014-01-31 | 2015-09-10 | パナソニック株式会社 | 非水電解質二次電池用正極活物質及び非水電解質二次電池 |
| CN106099051A (zh) * | 2015-04-28 | 2016-11-09 | 日亚化学工业株式会社 | 镍钴复合氢氧化物粒子及其制造方法、非水电解质二次电池用正极活性物质及其制造方法 |
| EP3100981A1 (en) * | 2015-04-28 | 2016-12-07 | Nichia Corporation | Nickel cobalt complex hydroxide particles and method for producing the same, positive electrode active material for non-aqueous electrolyte secondary battery and method for producing the same, and non-aqueous electrolyte secondary battery |
| JP2024102947A (ja) * | 2023-01-20 | 2024-08-01 | プライムプラネットエナジー&ソリューションズ株式会社 | 正極用ncm系活物質、正極および電池 |
| JP2024102945A (ja) * | 2023-01-20 | 2024-08-01 | プライムプラネットエナジー&ソリューションズ株式会社 | 正極用ncm系活物質、正極および電池 |
Families Citing this family (44)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016060105A1 (ja) * | 2014-10-15 | 2016-04-21 | 住友化学株式会社 | リチウム二次電池用正極活物質、リチウム二次電池用正極及びリチウム二次電池 |
| JP7412080B2 (ja) | 2016-04-27 | 2024-01-12 | カムエクス パワー エルエルシー | ナノ結晶を含む多結晶層状金属酸化物 |
| US20190288284A1 (en) * | 2016-07-22 | 2019-09-19 | Umicore | Lithium metal composite oxide powder |
| WO2018021557A1 (ja) * | 2016-07-29 | 2018-02-01 | 住友金属鉱山株式会社 | ニッケルマンガン複合水酸化物とその製造方法、非水系電解質二次電池用正極活物質とその製造方法、および非水系電解質二次電池 |
| CN109803928A (zh) | 2016-07-29 | 2019-05-24 | 住友金属矿山株式会社 | 镍锰复合氢氧化物及其制造方法、非水系电解质二次电池用正极活性物质及其制造方法、以及非水系电解质二次电池 |
| CN109843811B (zh) * | 2016-07-29 | 2022-08-26 | 住友金属矿山株式会社 | 镍锰复合氢氧化物及其制造方法、非水系电解质二次电池用正极活性物质及其制造方法 |
| WO2018020845A1 (ja) | 2016-07-29 | 2018-02-01 | 住友金属鉱山株式会社 | ニッケルマンガン複合水酸化物とその製造方法、非水系電解質二次電池用正極活物質とその製造方法、および非水系電解質二次電池 |
| JP6979460B2 (ja) * | 2016-12-22 | 2021-12-15 | ポスコPosco | 正極活物質、その製造方法、およびこれを含むリチウム二次電池 |
| KR102176633B1 (ko) | 2017-02-28 | 2020-11-09 | 주식회사 엘지화학 | 리튬 이차전지용 양극 활물질, 그 제조방법 및 이를 포함하는 리튬 이차전지 |
| KR102337646B1 (ko) * | 2017-06-23 | 2021-12-10 | 스미토모 긴조쿠 고잔 가부시키가이샤 | 비수계 전해질 이차 전지용 정극 활물질과 그의 제조 방법, 및 비수계 전해질 이차 전지 |
| CN109802132A (zh) * | 2017-11-16 | 2019-05-24 | 中国科学院宁波材料技术与工程研究所 | 具有纳米铆钉结构的正极材料及其制备方法 |
| JP7126173B2 (ja) * | 2017-12-26 | 2022-08-26 | パナソニックIpマネジメント株式会社 | 非水電解質二次電池用正極活物質及び非水電解質二次電池 |
| US11677075B2 (en) * | 2017-12-27 | 2023-06-13 | Proterial, Ltd. | Cathode active material for lithium ion secondary battery, method for manufacturing cathode active material for lithium ion secondary battery, and lithium ion secondary |
| JP7238881B2 (ja) * | 2018-02-22 | 2023-03-14 | 住友金属鉱山株式会社 | 金属複合水酸化物とその製造方法、非水電解質二次電池用正極活物質とその製造方法、および非水電解質二次電池 |
| JP7211137B2 (ja) * | 2018-02-27 | 2023-01-24 | 株式会社Gsユアサ | 非水電解質二次電池用正極活物質、正極活物質の製造に用いる前駆体の製造方法、正極活物質の製造方法、非水電解質二次電池用正極、及び非水電解質二次電池 |
| WO2019185318A1 (en) * | 2018-03-28 | 2019-10-03 | Umicore | Lithium transition metal composite oxide as a positive electrode active material for rechargeable lithium secondary batteries |
| KR102123274B1 (ko) * | 2018-04-25 | 2020-06-17 | 주식회사 에코프로비엠 | 리튬 복합 산화물 |
| CN108878807A (zh) * | 2018-06-04 | 2018-11-23 | 欣旺达电子股份有限公司 | 高镍基四元正极材料及其制备方法 |
| WO2020003642A1 (ja) * | 2018-06-29 | 2020-01-02 | パナソニックIpマネジメント株式会社 | 非水電解質二次電池用正極活物質、及び非水電解質二次電池 |
| JP7316565B2 (ja) * | 2018-11-28 | 2023-07-28 | パナソニックIpマネジメント株式会社 | 非水電解質二次電池用正極活物質、非水電解質二次電池用正極活物質の製造方法及び非水電解質二次電池 |
| CN112909238B (zh) | 2018-12-29 | 2022-04-22 | 宁德时代新能源科技股份有限公司 | 正极活性材料、正极极片及电化学储能装置 |
| KR102568566B1 (ko) * | 2019-02-01 | 2023-08-22 | 주식회사 엘지에너지솔루션 | 이차전지용 양극 활물질 및 이를 포함하는 리튬 이차전지 |
| JP7078141B2 (ja) * | 2019-02-06 | 2022-05-31 | 株式会社村田製作所 | 二次電池用正極活物質および二次電池 |
| CN113454032B (zh) * | 2019-02-22 | 2023-05-30 | 住友金属矿山株式会社 | 锂离子二次电池用正极活性物质、锂离子二次电池用正极活性物质的制造方法、锂离子二次电池 |
| CN113474296A (zh) * | 2019-02-22 | 2021-10-01 | 住友金属矿山株式会社 | 锂离子二次电池用正极活性物质、锂离子二次电池用正极活性物质的制造方法、锂离子二次电池 |
| WO2020175551A1 (ja) * | 2019-02-26 | 2020-09-03 | 住友金属鉱山株式会社 | リチウムイオン二次電池用正極活物質、リチウムイオン二次電池用正極活物質の製造方法、リチウムイオン二次電池 |
| JP7215261B2 (ja) * | 2019-03-15 | 2023-01-31 | 株式会社豊田自動織機 | 層状岩塩構造を示し、リチウム、ニッケル、コバルト、タングステン、ジルコニウム及び酸素を含有する正極活物質、並びに、その製造方法 |
| JP7030738B2 (ja) * | 2019-03-18 | 2022-03-07 | 株式会社東芝 | 電極、非水電解質電池、電池パック及び車両 |
| CN113646929B (zh) * | 2019-04-11 | 2024-10-29 | 杰富意矿物股份有限公司 | 前体、前体的制造方法、正极材料、正极材料的制造方法和锂离子二次电池 |
| CN110518232B (zh) | 2019-04-28 | 2020-12-15 | 宁德时代新能源科技股份有限公司 | 正极活性材料、正极极片及锂离子二次电池 |
| WO2021048399A1 (en) * | 2019-09-13 | 2021-03-18 | Umicore | Process for preparing a positive electrode material for rechargeable lithium ion batteries |
| KR102339985B1 (ko) * | 2019-10-31 | 2021-12-17 | 주식회사 에코프로비엠 | 리튬 복합 산화물 |
| JP7460252B2 (ja) * | 2020-03-20 | 2024-04-02 | エルジー・ケム・リミテッド | リチウム二次電池用正極活物質前駆体の製造方法、正極活物質前駆体、それを用いて製造された正極活物質、正極、およびリチウム二次電池 |
| US12159996B2 (en) | 2020-03-25 | 2024-12-03 | Samsung Sdi Co., Ltd. | Positive electrode active material, positive electrode including the same, and lithium secondary battery employing the positive electrode |
| CN111668475B (zh) * | 2020-05-09 | 2021-10-22 | 万华化学集团股份有限公司 | 五元锂离子电池正极材料、制备方法及用其制成的锂电池 |
| KR102293034B1 (ko) | 2020-06-04 | 2021-08-24 | 에스케이이노베이션 주식회사 | 리튬 이차 전지용 양극 활물질 및 이를 포함하는 리튬 이차 전지 |
| JP7214690B2 (ja) * | 2020-08-31 | 2023-01-30 | プライムプラネットエナジー&ソリューションズ株式会社 | 正極活物質およびリチウムイオン二次電池 |
| KR102879733B1 (ko) * | 2020-09-24 | 2025-10-30 | 에스케이온 주식회사 | 리튬 이차 전지용 양극 활물질 및 이의 제조 방법 |
| CN114447301B (zh) * | 2022-01-21 | 2023-03-10 | 合肥国轩高科动力能源有限公司 | 一种三元正极材料、其制备方法及应用 |
| KR102787045B1 (ko) | 2022-03-22 | 2025-03-25 | 삼성에스디아이 주식회사 | 리튬 이차 전지용 양극 활물질 및 이의 제조 방법과 이를 포함하는 리튬 이차 전지 |
| CN114883556B (zh) * | 2022-06-29 | 2024-06-14 | 蜂巢能源科技股份有限公司 | 一种超高镍正极材料及其制备方法 |
| JP7778114B2 (ja) * | 2023-07-20 | 2025-12-01 | プライムプラネットエナジー&ソリューションズ株式会社 | 正極およびこれを用いた二次電池 |
| KR20250028754A (ko) * | 2023-08-22 | 2025-03-04 | 주식회사 엘지화학 | 양극 활물질의 제조 방법, 양극 활물질 분말, 이를 포함하는 양극 및 리튬 이차전지 |
| US12278363B1 (en) | 2024-04-29 | 2025-04-15 | Camx Power, Llc | Multiple morphology composite cathode materials providing high energy and long cycle life and cells employing the same |
Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH05258751A (ja) | 1991-11-01 | 1993-10-08 | Sanyo Electric Co Ltd | 非水系電池 |
| JPH0855624A (ja) | 1994-03-07 | 1996-02-27 | Tdk Corp | 層状構造酸化物および二次電池 |
| JPH0922693A (ja) | 1995-07-04 | 1997-01-21 | Matsushita Electric Ind Co Ltd | 非水電解液電池およびその正極活物質と正極板の製造法 |
| JPH10308218A (ja) | 1997-03-07 | 1998-11-17 | Nichia Chem Ind Ltd | リチウムイオン二次電池用正極活物質及びその製造方法 |
| JP2000195514A (ja) | 1998-12-24 | 2000-07-14 | Toshiba Corp | 非水溶媒二次電池の製造方法 |
| JP2004335278A (ja) * | 2003-05-08 | 2004-11-25 | Nichia Chem Ind Ltd | 非水電解液二次電池用正極活物質 |
| JP2012018925A (ja) * | 2010-07-06 | 2012-01-26 | Samsung Sdi Co Ltd | リチウム二次電池用正極活物質及びその製造方法、並びにそれを備えるリチウム二次電池 |
| JP2013051772A (ja) | 2011-08-30 | 2013-03-14 | Toyota Motor Corp | 給電コネクタ、車両および車両の制御方法 |
| JP2013051172A (ja) | 2011-08-31 | 2013-03-14 | Toyota Motor Corp | リチウム二次電池 |
| JP2013206552A (ja) * | 2012-03-27 | 2013-10-07 | Tdk Corp | 活物質及びリチウムイオン二次電池 |
| JP2014135273A (ja) * | 2012-12-12 | 2014-07-24 | Samsung Sdi Co Ltd | リチウム二次電池用正極活物質、並びにこれを含むリチウム二次電池用正極及びリチウム二次電池 |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS4915488B1 (enExample) * | 1969-03-25 | 1974-04-15 | ||
| EP0165554A3 (en) * | 1984-06-20 | 1987-04-01 | Bridgestone Corporation | Process for producing a sintered cubic silicon carbide |
| EP2469630B1 (en) * | 2009-08-21 | 2019-04-24 | GS Yuasa International Ltd. | Lithium secondary battery active material, lithium secondary battery electrode and lithium secondary battery |
| JP5638232B2 (ja) * | 2009-12-02 | 2014-12-10 | 住友金属鉱山株式会社 | 非水系電解質二次電池正極活物質用ニッケルコバルトマンガン複合水酸化物粒子とその製造方法、非水系電解質二次電池用正極活物質とその製造方法、および非水系電解質二次電池 |
| WO2011074058A1 (ja) * | 2009-12-14 | 2011-06-23 | トヨタ自動車株式会社 | リチウム二次電池用正極活物質およびその利用 |
| CN103026537B (zh) * | 2010-03-29 | 2016-03-16 | 住友金属矿山株式会社 | 非水类电解质二次电池用正极活性物质及其制造方法、该正极活性物质的前驱体、以及使用了该正极活性物质的非水类电解质二次电池 |
| CN102388490B (zh) * | 2010-06-21 | 2014-11-12 | 丰田自动车株式会社 | 锂二次电池 |
| EP2653447B1 (en) * | 2011-03-28 | 2015-05-20 | Sumitomo Metal Mining Co., Ltd. | Nickel-manganese composite hydroxide particles, method for producing same, positive electrode active material for nonaqueous electrolyte secondary batteries, method for producing positive electrode active material for nonaqueous electrolyte secondary batteries, and nonaqueous electrolyte secondary battery |
| WO2012164752A1 (ja) * | 2011-05-30 | 2012-12-06 | 住友金属鉱山株式会社 | 非水系二次電池用正極活物質とその製造方法、ならびに該正極活物質を用いた非水系電解質二次電池 |
| JP5730676B2 (ja) * | 2011-06-06 | 2015-06-10 | 住友金属鉱山株式会社 | 非水系電解質二次電池用正極活物質とその製造方法、ならびに、ニッケルコバルトマンガン複合水酸化物とその製造方法 |
| KR101696524B1 (ko) * | 2011-06-07 | 2017-01-13 | 스미토모 긴조쿠 고잔 가부시키가이샤 | 니켈 복합 수산화물과 그의 제조 방법, 비수계 전해질 이차 전지용 정극 활물질과 그의 제조 방법, 및 비수계 전해질 이차 전지 |
-
2013
- 2013-07-24 JP JP2013153890A patent/JP6244713B2/ja active Active
-
2014
- 2014-07-22 US US14/907,089 patent/US20160172674A1/en not_active Abandoned
- 2014-07-22 WO PCT/JP2014/069372 patent/WO2015012283A1/ja not_active Ceased
- 2014-07-22 KR KR1020167000140A patent/KR102278009B1/ko active Active
- 2014-07-22 EP EP14830084.1A patent/EP3026740B1/en active Active
- 2014-07-22 CN CN201480040647.5A patent/CN105453312B/zh active Active
-
2021
- 2021-12-01 US US17/539,732 patent/US20220093919A1/en not_active Abandoned
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH05258751A (ja) | 1991-11-01 | 1993-10-08 | Sanyo Electric Co Ltd | 非水系電池 |
| JPH0855624A (ja) | 1994-03-07 | 1996-02-27 | Tdk Corp | 層状構造酸化物および二次電池 |
| JPH0922693A (ja) | 1995-07-04 | 1997-01-21 | Matsushita Electric Ind Co Ltd | 非水電解液電池およびその正極活物質と正極板の製造法 |
| JPH10308218A (ja) | 1997-03-07 | 1998-11-17 | Nichia Chem Ind Ltd | リチウムイオン二次電池用正極活物質及びその製造方法 |
| JP2000195514A (ja) | 1998-12-24 | 2000-07-14 | Toshiba Corp | 非水溶媒二次電池の製造方法 |
| JP2004335278A (ja) * | 2003-05-08 | 2004-11-25 | Nichia Chem Ind Ltd | 非水電解液二次電池用正極活物質 |
| JP2012018925A (ja) * | 2010-07-06 | 2012-01-26 | Samsung Sdi Co Ltd | リチウム二次電池用正極活物質及びその製造方法、並びにそれを備えるリチウム二次電池 |
| JP2013051772A (ja) | 2011-08-30 | 2013-03-14 | Toyota Motor Corp | 給電コネクタ、車両および車両の制御方法 |
| JP2013051172A (ja) | 2011-08-31 | 2013-03-14 | Toyota Motor Corp | リチウム二次電池 |
| JP2013206552A (ja) * | 2012-03-27 | 2013-10-07 | Tdk Corp | 活物質及びリチウムイオン二次電池 |
| JP2014135273A (ja) * | 2012-12-12 | 2014-07-24 | Samsung Sdi Co Ltd | リチウム二次電池用正極活物質、並びにこれを含むリチウム二次電池用正極及びリチウム二次電池 |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015164119A (ja) * | 2014-01-31 | 2015-09-10 | パナソニック株式会社 | 非水電解質二次電池用正極活物質及び非水電解質二次電池 |
| RU2718939C2 (ru) * | 2015-04-28 | 2020-04-15 | Нития Корпорейшн | Частицы сложного гидроксида никеля-кобальта и способ их получения, активный материал положительного электрода для вторичной батареи с безводным электролитом и способ его получения, а также вторичная батарея с безводным электролитом |
| CN106099051A (zh) * | 2015-04-28 | 2016-11-09 | 日亚化学工业株式会社 | 镍钴复合氢氧化物粒子及其制造方法、非水电解质二次电池用正极活性物质及其制造方法 |
| EP3100981A1 (en) * | 2015-04-28 | 2016-12-07 | Nichia Corporation | Nickel cobalt complex hydroxide particles and method for producing the same, positive electrode active material for non-aqueous electrolyte secondary battery and method for producing the same, and non-aqueous electrolyte secondary battery |
| US10336626B2 (en) | 2015-04-28 | 2019-07-02 | Nichia Corporation | Nickel cobalt complex hydroxide particles and method for producing the same, positive electrode active material for non-aqueous electrolyte secondary battery and method for producing the same, and non-aqueous electrolyte secondary battery |
| CN106099051B (zh) * | 2015-04-28 | 2020-08-04 | 日亚化学工业株式会社 | 镍钴复合氢氧化物粒子及其制造方法、非水电解质二次电池用正极活性物质及其制造方法 |
| US11220438B2 (en) | 2015-04-28 | 2022-01-11 | Nichia Corporation | Nickel cobalt complex hydroxide particles and method for producing the same, positive electrode active material for non-aqueous electrolyte secondary battery and method for producing the same, and non-aqueous electrolyte secondary battery |
| US11945729B2 (en) | 2015-04-28 | 2024-04-02 | Nichia Corporation | Positive electrode active material for non-aqueous electrolyte secondary battery comprising lithium transition metal complex oxide, and non-aqueous electrolyte secondary battery |
| CN104779388B (zh) * | 2015-04-30 | 2017-03-01 | 湖南瑞翔新材料股份有限公司 | 镍钴二元正极材料前驱体制备方法以及该法制得镍钴二元正极材料前驱体 |
| CN104779388A (zh) * | 2015-04-30 | 2015-07-15 | 湖南瑞翔新材料股份有限公司 | 镍钴二元正极材料前驱体制备方法以及该法制得镍钴二元正极材料前驱体 |
| JP2024102947A (ja) * | 2023-01-20 | 2024-08-01 | プライムプラネットエナジー&ソリューションズ株式会社 | 正極用ncm系活物質、正極および電池 |
| JP2024102945A (ja) * | 2023-01-20 | 2024-08-01 | プライムプラネットエナジー&ソリューションズ株式会社 | 正極用ncm系活物質、正極および電池 |
| JP7704786B2 (ja) | 2023-01-20 | 2025-07-08 | プライムプラネットエナジー&ソリューションズ株式会社 | 正極用ncm系活物質、正極および電池 |
| JP7759904B2 (ja) | 2023-01-20 | 2025-10-24 | プライムプラネットエナジー&ソリューションズ株式会社 | 正極用ncm系活物質、正極および電池 |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20160037878A (ko) | 2016-04-06 |
| US20160172674A1 (en) | 2016-06-16 |
| EP3026740B1 (en) | 2020-06-17 |
| CN105453312A (zh) | 2016-03-30 |
| CN105453312B (zh) | 2018-02-09 |
| US20220093919A1 (en) | 2022-03-24 |
| JP6244713B2 (ja) | 2017-12-13 |
| EP3026740A4 (en) | 2016-09-21 |
| KR102278009B1 (ko) | 2021-07-15 |
| EP3026740A1 (en) | 2016-06-01 |
| JP2015026454A (ja) | 2015-02-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20220093919A1 (en) | Cathode active material for non-aqueous electrolyte secondary battery and manufacturing method thereof, and non-aqueous electrolyte secondary battery | |
| JP7676851B2 (ja) | 遷移金属複合水酸化物粒子、遷移金属複合水酸化物粒子の製造方法、リチウムイオン二次電池用正極活物質、及びリチウムイオン二次電池 | |
| JP6252010B2 (ja) | 非水電解質二次電池用正極活物質およびその製造方法、並びに、非水電解質二次電池 | |
| US12113213B2 (en) | Cathode active material for non-aqueous electrolyte secondary battery and manufacturing method for same, and non-aqueous electrolyte secondary battery | |
| JP5971109B2 (ja) | ニッケル複合水酸化物とその製造方法、非水系電解質二次電池用正極活物質とその製造方法、および非水系電解質二次電池 | |
| JP6217636B2 (ja) | 非水系電解質二次電池用正極活物質およびその製造方法、ならびに該正極活物質を用いた非水系電解質二次電池 | |
| JP2021103689A (ja) | 非水系電解質二次電池用正極活物質とその製造方法 | |
| JP6062818B2 (ja) | 非水電解質二次電池用正極活物質およびその製造方法、並びに、非水電解質二次電池 | |
| JP7293576B2 (ja) | 金属複合水酸化物とその製造方法、非水電解質二次電池用正極活物質とその製造方法、及び、それを用いた非水電解質二次電池 | |
| JP6347227B2 (ja) | マンガンニッケルチタン複合水酸化物粒子とその製造方法、および、非水系電解質二次電池用正極活物質の製造方法 | |
| WO2012131881A1 (ja) | ニッケルマンガン複合水酸化物粒子とその製造方法、非水系電解質二次電池用正極活物質とその製造方法、および非水系電解質二次電池 | |
| JP6346448B2 (ja) | 非水系電解質二次電池用正極活物質、および、非水系電解質二次電池 | |
| JP6436335B2 (ja) | 遷移金属複合水酸化物粒子とその製造方法、およびそれを用いた非水系電解質二次電池用正極活物質の製造方法 | |
| JP7272134B2 (ja) | リチウムイオン二次電池用正極活物質およびその製造方法、並びに、リチウムイオン二次電池 | |
| JPWO2019163845A1 (ja) | 金属複合水酸化物とその製造方法、非水電解質二次電池用正極活物質とその製造方法、および非水電解質二次電池 | |
| JP7183813B2 (ja) | ニッケルマンガンコバルト含有複合水酸化物およびその製造方法、リチウムイオン二次電池用正極活物質およびその製造方法、並びに、リチウムイオン二次電池 | |
| JP7183815B2 (ja) | ニッケルマンガンコバルト含有複合水酸化物およびその製造方法、リチウムイオン二次電池用正極活物質およびその製造方法、並びに、リチウムイオン二次電池 | |
| JP2023040082A (ja) | 金属複合水酸化物とその製造方法、リチウムイオン二次電池用正極活物質とその製造方法、及び、それを用いたリチウムイオン二次電池 | |
| JPWO2019163846A1 (ja) | 金属複合水酸化物とその製造方法、非水電解質二次電池用正極活物質とその製造方法、および非水電解質二次電池 | |
| JP7415336B2 (ja) | リチウムイオン二次電池用正極活物質前駆体、リチウムイオン二次電池用正極活物質、リチウムイオン二次電池用正極活物質前駆体の製造方法、リチウムイオン二次電池用正極活物質の製造方法、リチウムイオン二次電池 | |
| JP7183812B2 (ja) | ニッケルマンガンコバルト含有複合水酸化物およびその製造方法、リチウムイオン二次電池用正極活物質およびその製造方法、並びに、リチウムイオン二次電池 | |
| JP7464102B2 (ja) | 金属複合水酸化物とその製造方法、非水電解質二次電池用正極活物質とその製造方法、及び、それを用いた非水電解質二次電池 | |
| JP7183814B2 (ja) | ニッケルマンガンコバルト含有複合水酸化物およびその製造方法、リチウムイオン二次電池用正極活物質およびその製造方法、並びに、リチウムイオン二次電池 | |
| JP7206819B2 (ja) | リチウムイオン二次電池用正極活物質とその製造方法、及び、リチウムイオン二次電池 | |
| JP7273260B2 (ja) | リチウムイオン二次電池用正極活物質とその製造方法およびリチウムイオン二次電池 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201480040647.5 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14830084 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20167000140 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14907089 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2014830084 Country of ref document: EP |