WO2014065074A1 - ろう付け用組成物、熱交換器用チューブおよび熱交換器 - Google Patents
ろう付け用組成物、熱交換器用チューブおよび熱交換器 Download PDFInfo
- Publication number
- WO2014065074A1 WO2014065074A1 PCT/JP2013/076005 JP2013076005W WO2014065074A1 WO 2014065074 A1 WO2014065074 A1 WO 2014065074A1 JP 2013076005 W JP2013076005 W JP 2013076005W WO 2014065074 A1 WO2014065074 A1 WO 2014065074A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- less
- powder
- mass
- brazing
- parts
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B23—MACHINE TOOLS; METAL-WORKING NOT OTHERWISE PROVIDED FOR
- B23K—SOLDERING OR UNSOLDERING; WELDING; CLADDING OR PLATING BY SOLDERING OR WELDING; CUTTING BY APPLYING HEAT LOCALLY, e.g. FLAME CUTTING; WORKING BY LASER BEAM
- B23K35/00—Rods, electrodes, materials, or media, for use in soldering, welding, or cutting
- B23K35/02—Rods, electrodes, materials, or media, for use in soldering, welding, or cutting characterised by mechanical features, e.g. shape
- B23K35/0222—Rods, electrodes, materials, or media, for use in soldering, welding, or cutting characterised by mechanical features, e.g. shape for use in soldering, brazing
- B23K35/0244—Powders, particles or spheres; Preforms made therefrom
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B23—MACHINE TOOLS; METAL-WORKING NOT OTHERWISE PROVIDED FOR
- B23K—SOLDERING OR UNSOLDERING; WELDING; CLADDING OR PLATING BY SOLDERING OR WELDING; CUTTING BY APPLYING HEAT LOCALLY, e.g. FLAME CUTTING; WORKING BY LASER BEAM
- B23K35/00—Rods, electrodes, materials, or media, for use in soldering, welding, or cutting
- B23K35/22—Rods, electrodes, materials, or media, for use in soldering, welding, or cutting characterised by the composition or nature of the material
- B23K35/24—Selection of soldering or welding materials proper
- B23K35/28—Selection of soldering or welding materials proper with the principal constituent melting at less than 950 degrees C
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B23—MACHINE TOOLS; METAL-WORKING NOT OTHERWISE PROVIDED FOR
- B23K—SOLDERING OR UNSOLDERING; WELDING; CLADDING OR PLATING BY SOLDERING OR WELDING; CUTTING BY APPLYING HEAT LOCALLY, e.g. FLAME CUTTING; WORKING BY LASER BEAM
- B23K35/00—Rods, electrodes, materials, or media, for use in soldering, welding, or cutting
- B23K35/22—Rods, electrodes, materials, or media, for use in soldering, welding, or cutting characterised by the composition or nature of the material
- B23K35/24—Selection of soldering or welding materials proper
- B23K35/28—Selection of soldering or welding materials proper with the principal constituent melting at less than 950 degrees C
- B23K35/282—Zn as the principal constituent
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B23—MACHINE TOOLS; METAL-WORKING NOT OTHERWISE PROVIDED FOR
- B23K—SOLDERING OR UNSOLDERING; WELDING; CLADDING OR PLATING BY SOLDERING OR WELDING; CUTTING BY APPLYING HEAT LOCALLY, e.g. FLAME CUTTING; WORKING BY LASER BEAM
- B23K35/00—Rods, electrodes, materials, or media, for use in soldering, welding, or cutting
- B23K35/22—Rods, electrodes, materials, or media, for use in soldering, welding, or cutting characterised by the composition or nature of the material
- B23K35/36—Selection of non-metallic compositions, e.g. coatings, fluxes; Selection of soldering or welding materials, conjoint with selection of non-metallic compositions, both selections being of interest
- B23K35/3601—Selection of non-metallic compositions, e.g. coatings, fluxes; Selection of soldering or welding materials, conjoint with selection of non-metallic compositions, both selections being of interest with inorganic compounds as principal constituents
- B23K35/3603—Halide salts
- B23K35/3605—Fluorides
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B23—MACHINE TOOLS; METAL-WORKING NOT OTHERWISE PROVIDED FOR
- B23K—SOLDERING OR UNSOLDERING; WELDING; CLADDING OR PLATING BY SOLDERING OR WELDING; CUTTING BY APPLYING HEAT LOCALLY, e.g. FLAME CUTTING; WORKING BY LASER BEAM
- B23K35/00—Rods, electrodes, materials, or media, for use in soldering, welding, or cutting
- B23K35/22—Rods, electrodes, materials, or media, for use in soldering, welding, or cutting characterised by the composition or nature of the material
- B23K35/36—Selection of non-metallic compositions, e.g. coatings, fluxes; Selection of soldering or welding materials, conjoint with selection of non-metallic compositions, both selections being of interest
- B23K35/3612—Selection of non-metallic compositions, e.g. coatings, fluxes; Selection of soldering or welding materials, conjoint with selection of non-metallic compositions, both selections being of interest with organic compounds as principal constituents
- B23K35/3613—Polymers, e.g. resins
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B23—MACHINE TOOLS; METAL-WORKING NOT OTHERWISE PROVIDED FOR
- B23K—SOLDERING OR UNSOLDERING; WELDING; CLADDING OR PLATING BY SOLDERING OR WELDING; CUTTING BY APPLYING HEAT LOCALLY, e.g. FLAME CUTTING; WORKING BY LASER BEAM
- B23K35/00—Rods, electrodes, materials, or media, for use in soldering, welding, or cutting
- B23K35/22—Rods, electrodes, materials, or media, for use in soldering, welding, or cutting characterised by the composition or nature of the material
- B23K35/36—Selection of non-metallic compositions, e.g. coatings, fluxes; Selection of soldering or welding materials, conjoint with selection of non-metallic compositions, both selections being of interest
- B23K35/362—Selection of compositions of fluxes
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F28—HEAT EXCHANGE IN GENERAL
- F28D—HEAT-EXCHANGE APPARATUS, NOT PROVIDED FOR IN ANOTHER SUBCLASS, IN WHICH THE HEAT-EXCHANGE MEDIA DO NOT COME INTO DIRECT CONTACT
- F28D1/00—Heat-exchange apparatus having stationary conduit assemblies for one heat-exchange medium only, the media being in contact with different sides of the conduit wall, in which the other heat-exchange medium is a large body of fluid, e.g. domestic or motor car radiators
- F28D1/02—Heat-exchange apparatus having stationary conduit assemblies for one heat-exchange medium only, the media being in contact with different sides of the conduit wall, in which the other heat-exchange medium is a large body of fluid, e.g. domestic or motor car radiators with heat-exchange conduits immersed in the body of fluid
- F28D1/04—Heat-exchange apparatus having stationary conduit assemblies for one heat-exchange medium only, the media being in contact with different sides of the conduit wall, in which the other heat-exchange medium is a large body of fluid, e.g. domestic or motor car radiators with heat-exchange conduits immersed in the body of fluid with tubular conduits
- F28D1/053—Heat-exchange apparatus having stationary conduit assemblies for one heat-exchange medium only, the media being in contact with different sides of the conduit wall, in which the other heat-exchange medium is a large body of fluid, e.g. domestic or motor car radiators with heat-exchange conduits immersed in the body of fluid with tubular conduits the conduits being straight
- F28D1/0535—Heat-exchange apparatus having stationary conduit assemblies for one heat-exchange medium only, the media being in contact with different sides of the conduit wall, in which the other heat-exchange medium is a large body of fluid, e.g. domestic or motor car radiators with heat-exchange conduits immersed in the body of fluid with tubular conduits the conduits being straight the conduits having a non-circular cross-section
- F28D1/05366—Assemblies of conduits connected to common headers, e.g. core type radiators
- F28D1/05383—Assemblies of conduits connected to common headers, e.g. core type radiators with multiple rows of conduits or with multi-channel conduits
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F28—HEAT EXCHANGE IN GENERAL
- F28F—DETAILS OF HEAT-EXCHANGE AND HEAT-TRANSFER APPARATUS, OF GENERAL APPLICATION
- F28F1/00—Tubular elements; Assemblies of tubular elements
- F28F1/10—Tubular elements and assemblies thereof with means for increasing heat-transfer area, e.g. with fins, with projections, with recesses
- F28F1/12—Tubular elements and assemblies thereof with means for increasing heat-transfer area, e.g. with fins, with projections, with recesses the means being only outside the tubular element
- F28F1/126—Tubular elements and assemblies thereof with means for increasing heat-transfer area, e.g. with fins, with projections, with recesses the means being only outside the tubular element consisting of zig-zag shaped fins
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F28—HEAT EXCHANGE IN GENERAL
- F28F—DETAILS OF HEAT-EXCHANGE AND HEAT-TRANSFER APPARATUS, OF GENERAL APPLICATION
- F28F21/00—Constructions of heat-exchange apparatus characterised by the selection of particular materials
- F28F21/08—Constructions of heat-exchange apparatus characterised by the selection of particular materials of metal
- F28F21/081—Heat exchange elements made from metals or metal alloys
- F28F21/084—Heat exchange elements made from metals or metal alloys from aluminium or aluminium alloys
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F28—HEAT EXCHANGE IN GENERAL
- F28F—DETAILS OF HEAT-EXCHANGE AND HEAT-TRANSFER APPARATUS, OF GENERAL APPLICATION
- F28F21/00—Constructions of heat-exchange apparatus characterised by the selection of particular materials
- F28F21/08—Constructions of heat-exchange apparatus characterised by the selection of particular materials of metal
- F28F21/089—Coatings, claddings or bonding layers made from metals or metal alloys
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B23—MACHINE TOOLS; METAL-WORKING NOT OTHERWISE PROVIDED FOR
- B23K—SOLDERING OR UNSOLDERING; WELDING; CLADDING OR PLATING BY SOLDERING OR WELDING; CUTTING BY APPLYING HEAT LOCALLY, e.g. FLAME CUTTING; WORKING BY LASER BEAM
- B23K2101/00—Articles made by soldering, welding or cutting
- B23K2101/04—Tubular or hollow articles
- B23K2101/06—Tubes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B23—MACHINE TOOLS; METAL-WORKING NOT OTHERWISE PROVIDED FOR
- B23K—SOLDERING OR UNSOLDERING; WELDING; CLADDING OR PLATING BY SOLDERING OR WELDING; CUTTING BY APPLYING HEAT LOCALLY, e.g. FLAME CUTTING; WORKING BY LASER BEAM
- B23K2101/00—Articles made by soldering, welding or cutting
- B23K2101/04—Tubular or hollow articles
- B23K2101/14—Heat exchangers
Definitions
- the present invention relates to a brazing composition, a heat exchanger tube, and a heat exchanger. Specifically, the present invention relates to a brazing composition, a heat exchanger tube obtained by using the brazing composition, and a heat exchanger.
- an aluminum heat exchanger used for an automobile or the like for example, a composite member obtained by brazing (joining) fins to a heat exchanger tube made of aluminum or an aluminum alloy is used.
- a composite member used for an aluminum heat exchanger for example, a tube and a fin, a Si powder having a maximum particle size of 30 ⁇ m or less, a fluoride flux such as KAlF 4 , a binder, a solvent, etc.
- a fluoride flux such as KAlF 4
- a binder such as KAlF 4
- a solvent such as a solvent
- an aluminum heat exchanger member obtained by brazing with a coating material containing bismuth (see, for example, Patent Document 1 below).
- brazing properties are ensured by mixing Si powder with the flux.
- an aluminum heat exchanger for example, a base material made of aluminum or an aluminum alloy, and a Zn coating layer (a brazing coating layer) formed on the base material and containing a flux composition such as KAlF 4 and Zn powder. It has been proposed to braze and use a composite material for brazing provided with a membrane (see, for example, Patent Document 2 below).
- a Zn coating layer is formed by mixing Zn powder with the flux composition, thereby improving the corrosion resistance.
- aluminum heat exchangers are required to simultaneously exhibit various effects such as better brazing and corrosion resistance, and adhesion in brazing.
- An object of the present invention is to provide a brazing composition capable of obtaining a well-balanced brazing property, corrosion resistance and adhesion, a heat exchanger tube obtained by using the brazing composition, and a heat exchanger There is to do.
- the brazing composition of the present invention comprises 1 to 10 parts by weight of Zn powder, 1 to 5 parts by weight of Si powder, 3 to 10 parts by weight of a K—Al—F flux.
- the (meth) acrylic resin is contained in an amount of 1 part by mass or more and 3 parts by mass or less, and the mass ratio of Zn powder to Si powder (Zn / Si) is 1 or more and 5 or less.
- the brazing composition of the present invention preferably further contains a polyhydric alcohol having 3 or less carbon atoms bonded by a carbon-carbon bond.
- the heat exchanger tube of the present invention comprises a tube body and a brazing coating film formed on the outer surface of the tube body by the brazing composition described above,
- the total amount of Zn powder, Si powder, K—Al—F-based flux and (meth) acrylic resin is 6 g / m 2 or more and 20 g / m 2 or less.
- the heat exchanger of the present invention is characterized by being obtained using the above-mentioned heat exchanger tube.
- the brazing composition of the present invention contains Zn powder, Si powder, K—Al—F flux and (meth) acrylic resin in a specific ratio, it balances brazing, corrosion resistance and adhesion. Can get well.
- the brazing composition of the present invention is used for the heat exchanger tube of the present invention and the heat exchanger of the present invention, it can be provided with excellent brazing properties, corrosion resistance and adhesion.
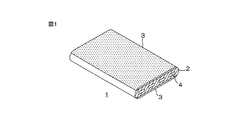
- FIG. 1 is a schematic perspective view showing an embodiment of a heat exchanger tube of the present invention.
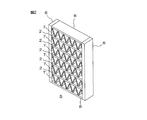
- FIG. 2 is a schematic configuration diagram showing an embodiment of the heat exchanger of the present invention.
- the brazing composition of the present invention contains Zn (zinc) powder, Si (silicon) powder, K—Al—F flux and (meth) acrylic resin.
- the Zn powder is not particularly limited, but the average particle size (measuring device: Laser diffraction / scattering particle size analyzer MT3000II series manufactured by Nikkiso Co., Ltd.) is, for example, 2 ⁇ m or more, preferably 3 ⁇ m or more, for example, 5 ⁇ m.
- the average particle size is, for example, 2 ⁇ m or more, preferably 3 ⁇ m or more, for example, 5 ⁇ m.
- those having a thickness of 4 ⁇ m or less are preferably used.
- Zn By blending Zn powder, Zn can be melted by heating at the time of brazing and a Zn diffusion layer (Zn layer) can be formed, so that corrosion resistance can be improved.
- the content of Zn powder in the brazing composition is 1 part by mass or more, preferably 3 parts by mass or more, more preferably 5 parts by mass or more, 10 parts by mass or less, preferably 9 parts by mass or less, More preferably, it is 8 parts by mass or less.
- the content of the Zn powder is, for example, 5% by mass or more, preferably 10% by mass or more, more preferably 15% by mass or more, for example, 40% by mass with respect to the total amount of the brazing composition. % Or less, preferably 35% by mass or less, more preferably 30% by mass or less.
- the Zn diffusion layer is formed with a uniform and sufficient depth when the brazing composition is applied on an aluminum member at a normal application amount. And excellent corrosion resistance can be ensured.
- the Si powder is not particularly limited, but the average particle size (measuring device: Laser diffraction / scattering particle size analyzer MT3000II series manufactured by Nikkiso Co., Ltd.) is, for example, 1 ⁇ m or more, preferably 2 ⁇ m or more, for example, 30 ⁇ m. Hereinafter, those having a thickness of 8 ⁇ m or less are preferably used.
- an Al—Si alloy molten brazing can be formed by heating during brazing, and brazing properties can be ensured.
- the content of the Si powder in the brazing composition is 1 part by mass or more, preferably 1.2 parts by mass or more, more preferably 1.4 parts by mass or more, and 5 parts by mass or less, preferably 4 It is 3 parts by mass or less, more preferably 3 parts by mass or less.
- the content of the Si powder is, for example, 1% by mass or more, preferably 2% by mass or more, more preferably 3% by mass or more, for example, 20% by mass with respect to the total amount of the brazing composition. % Or less, preferably 19% by mass or less, more preferably 18% by mass or less.
- the content of the Si powder is in the above range, excellent brazing properties can be secured when the brazing composition is applied on an aluminum member at a normal coating amount.
- the mass ratio (Zn / Si) of Zn powder to Si powder is 1 or more, preferably 1.5 or more, more preferably 2 or more, 5 or less, preferably 4.5 or less, more preferably. Is 4 or less.
- the mass ratio of Zn powder to Si powder (Zn / Si) is within the above range, excellent brazing properties are ensured when the above brazing composition is applied on an aluminum member at a normal coating amount.
- the Zn diffusion layer can be formed in a uniform and sufficient depth, and excellent corrosion resistance can be ensured.
- the K-Al-F flux is a fluoride flux containing potassium (K), aluminum (Al) and fluorine (F), and is contained for removing the oxide film.
- K—Al—F flux examples include potassium fluoroaluminate, and specific examples include KAlF 4 and K 3 AlF 6 .
- K-Al-F flux it is also available as a commercial product, and specifically, Nocolok (registered trademark) Flux (potassium fluoroaluminate, manufactured by Solvay) and the like can be mentioned.
- K-Al-F fluxes can be used alone or in combination of two or more.
- the content of the K—Al—F flux in the brazing composition is 3 parts by mass or more, preferably 4 parts by mass or more, more preferably 5 parts by mass or more, preferably 10 parts by mass or less, preferably It is 9 mass parts or less, More preferably, it is 8 mass parts or less.
- the content of the K—Al—F flux is, for example, 5% by mass or more, preferably 10% by mass or more, and more preferably 15% by mass or more with respect to the total amount of the brazing composition. For example, it is 40% by mass or less, preferably 35% by mass or less, and more preferably 30% by mass or less.
- the content of the K—Al—F flux is in the above range, excellent brazing properties can be secured when the brazing composition is applied on an aluminum member at a normal coating amount.
- the core can be prevented from cracking, and excellent clearance, brazing property and corrosion resistance can be ensured.
- (Meth) acrylic resin is a component for uniformly adhering the brazing composition to the joint as a binder, and examples thereof include (meth) acrylic acid ester polymers.
- (meth) acrylic acid ester polymer examples include, for example, (meth) acrylic acid ester homopolymers, (meth) acrylic acid ester copolymers, (meth) acrylic acid esters and hydrophobic monomers, and / or Or the copolymer with a hydrophilic monomer etc. are mentioned.
- (meth) acryl is defined as "acryl and / or methacryl”.
- (meth) acrylic acid esters examples include methyl (meth) acrylate, ethyl (meth) acrylate, propyl (meth) acrylate, butyl (meth) acrylate, 2-ethylhexyl (meth) acrylate, (meth And (meth) acrylic acid C 1 -C 18 alkyl esters such as lauryl acrylate.
- acrylic acid esters can be used alone or in combination of two or more.
- hydrophobic monomer examples include styrenes such as styrene, ⁇ -methylstyrene, vinyltoluene, and p-chlorostyrene.
- hydrophobic monomers can be used alone or in combination of two or more.
- hydrophilic monomers include carboxyl groups such as (meth) acrylic acid, itaconic acid, crotonic acid, (anhydrous) maleic acid, fumaric acid, (anhydrous) citraconic acid, and unsaturated carboxylic acids such as salts thereof.
- examples thereof include monomers containing sulfonic acid groups such as styrene sulfonic acid, vinyl sulfonic acid, allyl sulfonic acid, isoprene sulfonic acid, and unsaturated sulfonic acids such as salts thereof.
- hydrophilic monomer for example, 2-hydroxyethyl (meth) acrylate, hydroxypropyl (meth) acrylate, polypropylene glycol ester (meth) acrylate, polyethylene glycol ester (meth) acrylate, Hydroxyl group content such as (meth) acrylic acid ester (for example, CH 2 ⁇ C (CH 3 ) COO (C 2 H 4 O) n H (n is an integer of 2 to 12) etc.) to which alkylene oxide is added ( And hydroxyl group-containing monomers such as (meth) acrylic acid esters.
- acrylic acid ester for example, CH 2 ⁇ C (CH 3 ) COO (C 2 H 4 O) n H (n is an integer of 2 to 12) etc.
- hydroxyl group-containing monomers such as (meth) acrylic acid esters.
- hydrophilic monomers can be used alone or in combination of two or more.
- the (meth) acrylic acid ester polymer is preferably a homopolymer of (meth) acrylic acid ester or a copolymer of (meth) acrylic acid ester and a hydrophilic monomer.
- the content of the hydroxyl group-containing monomer in all monomers constituting the (meth) acrylic acid ester polymer is, for example, relative to the total amount of monomers 10% by mass or less.
- the monomer constituting the (meth) acrylic acid ester polymer is preferably a methacrylic monomer.
- a methacrylic monomer is used, excellent brazing properties can be ensured as compared with the case of using an acrylic monomer (acrylic acid ester, acrylic acid, etc.).
- Such a (meth) acrylic acid ester polymer is not particularly limited, and can be obtained, for example, by radical polymerization of the above monomers by a known polymerization method such as bulk polymerization, solution polymerization, suspension polymerization or the like.
- the polystyrene-reduced weight average molecular weight of the (meth) acrylic acid ester polymer by gel permeation chromatography is, for example, 10,000 or more, preferably 50,000 or more, for example, 600,000 or less, preferably 50 10,000 or less.
- the weight average molecular weight of the (meth) acrylic acid ester polymer can be appropriately set by adjusting the blending amount of the radical polymerization initiator.
- the acid value of the (meth) acrylic acid ester polymer is, for example, 0 mgKOH / g or more, preferably 15 mgKOH / g or more, for example, 65 mgKOH / g or less, preferably Is 40 mg KOH / g or less.
- the content of the (meth) acrylic resin in the brazing composition is 1 part by mass or more, preferably 1.2 parts by mass or more, more preferably 1.4 parts by mass or more, and preferably 3 parts by mass or less. Is 2.5 parts by mass or less, more preferably 2 parts by mass or less.
- the content of the (meth) acrylic resin is, for example, 1% by mass or more, preferably 2% by mass or more, more preferably 3% by mass or more, based on the total amount of the brazing composition. 15 mass% or less, preferably 10 mass% or less, more preferably 5 mass% or less.
- the brazing composition further contains an organic solvent (excluding polyhydric alcohols described later) from the viewpoint of reducing the surface tension, improving wettability with respect to the application target, and applying uniformly. be able to.
- an organic solvent excluding polyhydric alcohols described later
- the organic solvent is not particularly limited as long as it is an organic solvent usually used in a brazing composition, and examples thereof include monohydric alcohols, ethers, and ketones. These organic solvents can be used alone or in combination of two or more.
- the organic solvent is preferably a monohydric alcohol from the viewpoint of drying properties.
- the monohydric alcohol include, for example, methyl alcohol, ethyl alcohol, n-propyl alcohol, isopropyl alcohol, n-butyl alcohol, isobutyl alcohol, s-butyl alcohol, t-butyl alcohol, 3-methoxy-3- Methyl-1-butanol, ethylene glycol monomethyl ether (methyl cellosolve), ethylene glycol monoethyl ether (ethyl cellosolve), ethylene glycol monobutyl ether (butyl cellosolve), diethylene glycol methyl ether (methyl carbitol), diethylene glycol ethyl ether (ethyl carbitol) , Diethylene glycol butyl ether (butyl carbitol), propylene glycol monomethyl ether, propylene glycol Bruno ethyl ether, propylene glycol monobutyl ether, dipropylene glycol monomethyl ether, dipropylene glycol monoethy
- the content of the organic solvent is, for example, 5 parts by mass or more, preferably 10 parts by mass or more, more preferably 15 parts by mass or more, for example, 45 It is 40 parts by mass or less, more preferably 35 parts by mass or less.
- the content of the organic solvent is, for example, 10% by mass or more, preferably 20% by mass or more, more preferably 30% by mass or more, for example, 70% by mass with respect to the total amount of the brazing composition. % Or less, preferably 60% by mass or less, and more preferably 50% by mass or less.
- the brazing composition preferably further contains an anti-settling agent from the viewpoint of preventing the Zn powder from settling and improving dispersibility.
- anti-settling agents examples include polyhydric alcohols.
- the polyhydric alcohol is a compound containing two or more hydroxyl groups (OH) in the molecule.
- OH hydroxyl groups
- Dihydric alcohols such as glyceryl monoacetate and glyceryl monobutyrate, for example, trihydric alcohols such as glycerin, trimethylolpropane, trimethylolethane and triethylolethane, for example, tetrahydric alcohols such as pentaerythritol and diglycerin, Pentavalent alcohols such as triglycerol, for example
- the above monohydric alcohol can also be used as the anti-settling agent.
- polyhydric alcohols are preferable.
- the polyhydric alcohol is preferably a polyhydric alcohol having 3 or less carbon atoms bonded by a carbon-carbon bond.
- the brazing composition contains a polyhydric alcohol having 3 or less carbon atoms bonded by carbon-carbon bonds, the polyvalent number of carbon atoms bonded by carbon-carbon bonds exceeds 3. Compared with the case of containing alcohol (for example, hexylene glycol), precipitation of Zn powder can be prevented more favorably.
- alcohol for example, hexylene glycol
- polyhydric alcohol specifically, for example, a compound having 3 or less carbon atoms and having two or more hydroxyl groups at the molecular ends, or a hydrocarbon unit having 3 or less carbon atoms is oxygen.
- a compound in which a plurality of atoms are bonded through hetero atoms such as atoms and has two or more hydroxyl groups at the molecular ends can be used.
- More specific examples of the former compound include ethylene glycol, propylene glycol, and glycerin.
- More specific examples of the latter compound include polyols having a plurality of oxyethylene and / or oxypropylene, and more specific examples include diethylene glycol and dipropylene glycol.
- Preferred examples of the polyhydric alcohol include the former compound, that is, a compound having 3 or less carbon atoms and having two or more hydroxyl groups at the molecular ends.
- a polyhydric alcohol having excellent hygroscopicity is preferably selected. If a polyhydric alcohol having excellent hygroscopicity is used, the reaction between Zn powder and water can be suppressed even if water is mixed into the system.
- polyhydric alcohols having 3 or less carbon atoms
- dihydric alcohols having 3 or less carbon atoms
- the content of the anti-settling agent is, for example, 2 parts by mass or more, preferably 3 parts by mass or more, more preferably 4 parts by mass or more. 10 parts by mass or less, preferably 9 parts by mass or less, more preferably 8 parts by mass or less.
- the content of the anti-settling agent is, for example, 5% by mass or more, preferably 8% by mass or more, more preferably 10% by mass or more with respect to the total amount of the brazing composition. % By mass or less, preferably 18% by mass or less, more preferably 15% by mass or less.
- the blending amount of the anti-settling agent is not less than the above lower limit, excellent anti-settling property can be ensured, so that Zn powder can be dispersed well and excellent hygroscopicity can be ensured. Therefore, reaction with Zn powder and water can be suppressed favorably. Moreover, if the compounding quantity of an anti-settling agent is below the said upper limit, the outstanding drying property can be ensured and the outstanding brazing property can be ensured.
- composition for brazing can be obtained by mixing and stirring the above-mentioned respective components in the above-mentioned content ratio by a known method.
- the solid content concentration of the obtained brazing composition is, for example, 15% by mass or more, preferably 30% by mass or more, more preferably 45% by mass or more, for example, 85% by mass or less, preferably 70%. It is 55 mass% or less, More preferably, it is 55 mass% or less.
- the viscosity of the brazing composition at 25 ° C. is, for example, 20 Pa ⁇ s or more, preferably 100 Pa ⁇ s or more. Yes, for example, 1000 Pa ⁇ s or less, preferably 500 Pa ⁇ s or less.
- an antioxidant for example, dibutylhydroxytoluene
- a corrosion inhibitor for example, benzotriazole
- an antifoaming agent for example, silicone oil
- thickeners for example, waxes, hardened oils, fatty acid amides, polyamides, etc.
- colorants and the like can be included as long as the effects of the present invention are not impaired.
- the storage stability, sagging prevention property, brazing property, etc. of the obtained brazing composition can be further improved.
- Such a brazing composition contains Zn powder, Si powder, K—Al—F flux and (meth) acrylic resin in specific ratios, so that brazing, corrosion resistance and adhesion are improved. Can be obtained in a well-balanced manner.
- the brazing composition of the present invention is suitably used in the production of aluminum or aluminum alloy products, such as heat exchanger tubes and heat exchangers.
- FIG. 1 is a schematic perspective view showing one embodiment of a heat exchanger tube of the present invention.
- a heat exchanger tube 1 includes a flat tube 2 as a tube main body and a brazing coating 3 formed on the outer surface of the flat tube 2.
- the flat tube 2 is extruded or drawn into a flat cross section from aluminum or an aluminum alloy, preferably an aluminum alloy JIS 1050.
- the flat tube 2 includes a plurality of heat medium passage holes 4 spaced apart in a direction (width direction) orthogonal to the extending direction of the flat tube 2.
- Each heat medium passage hole 4 has a long cross-sectional shape that is long in the width direction, and is formed so as to penetrate along the direction in which the flat tube 2 extends.
- the brazing coating 3 is a coating formed by applying and drying the brazing composition described above, and is one side and the other side of the flat tube 2, that is, the flat tube 2 having a flat cross section. It is formed on both sides.
- the solid content of each of the above components is such that the Zn powder is 1 g / m 2 or more, preferably 3 g / m 2 or more, more preferably 5 g / m 2 or more, It is 10 g / m 2 or less, preferably 9 g / m 2 or less, more preferably 8 g / m 2 or less.
- the Si powder is 1 g / m 2 or more, preferably 1.2 g / m 2 or more, more preferably 1.4 g / m 2 or more, and 5 g / m 2 or less, preferably 4 g / m 2. Hereinafter, it is more preferably 3 g / m 2 or less.
- the K—Al—F flux is 3 g / m 2 or more, preferably 4 g / m 2 or more, more preferably 5 g / m 2 or more, and 10 g / m 2 or less, preferably 9 g / m. 2 or less, more preferably 8 g / m 2 or less.
- the (meth) acrylic resin is 1 g / m 2 or more, preferably 1.2 g / m 2 or more, more preferably 1.4 g / m 2 or more, and 3 g / m 2 or less, preferably 2 .5g / m 2 or less, more preferably 2 g / m 2 or less.
- the total amount of Zn powder, Si powder, K—Al—F flux and (meth) acrylic resin is 6 g / m 2 or more, preferably 7 g / m 2 or more, more preferably Is 10 g / m 2 or more, for example, 20 g / m 2 or less, preferably 18 g / m 2 or less, more preferably 16 g / m 2 or less.
- the total amount of Zn powder, Si powder, K—Al—F based flux and (meth) acrylic resin is not less than the above lower limit, excellent brazing and corrosion resistance can be ensured. Further, if the total amount of Zn powder, Si powder, K—Al—F flux and (meth) acrylic resin is not more than the above upper limit, excellent brazing property and corrosion resistance can be ensured.
- the thickness of the coating film 3 for brazing is, for example, 5 ⁇ m or more, preferably 10 ⁇ m or more, for example, 25 ⁇ m or less, preferably 20 ⁇ m or less.
- tube 1 for heat exchangers is used suitably in manufacture of the heat exchanger mounted in a motor vehicle etc., for example.
- FIG. 2 is a schematic configuration diagram showing an embodiment of a heat exchanger in which the heat exchanger tube shown in FIG. 1 is used.
- the heat exchanger 5 is spaced from each other in a direction in which the pair of header pipes 6 extend between the pair of header pipes 6 and the pair of header pipes 6 that are opposed to each other with a space therebetween.
- a plurality of flat tubes 2 installed separately from each other and fins 7 provided between the adjacent flat tubes 2 are provided.
- the header pipe 6 is a pipe through which a heat medium is supplied and discharged.
- An inlet pipe (not shown) through which the heat medium flows is connected to one header pipe 6, and the other header pipe 6 has a heat medium.
- a discharge pipe (not shown) for discharging the gas is connected.
- each header pipe 6 has an opening (not shown) on the surface where they face each other.
- the flat tube 2 is the flat tube 2 that constitutes the heat exchanger tube 1 described above, and is erected along the opposing direction of the pair of header pipes 6.
- the flat tube 2 is installed on the header pipe 6 so that the heat medium passage hole 4 and the internal space of the header pipe 6 communicate with each other through an opening formed in the header pipe 6. Yes.
- the heat medium flows into the one header pipe 6 from the inflow pipe (not shown), then passes through the heat medium passage hole 4 of the flat tube 2 and flows into the other header pipe 6 to be discharged. (Not shown) can be discharged.
- the fin 7 is formed in a continuous wave shape by bending a plate material made of aluminum or an aluminum alloy.
- the fins 7 are brazed to the pair of flat tubes 2 at the top and bottom of the corrugation between a pair of flat tubes 2 adjacent to each other.
- the fin 7 is also brazed to the outer surface of the flat tube 2 provided on the outermost side, and a protective plate 8 for protecting the fin 7 is brazed to the outer side of the fin 7. Yes.
- a pair of header pipes 6 are arranged at intervals in parallel to each other.
- both end portions of the plurality of heat exchanger tubes 1 are inserted into openings (not shown) provided on the inner side surfaces of the pair of header pipes 6, and the heat exchanger tubes 1 are inserted into the pair of header pipes 6. Erection.
- the fin 7 is arrange
- the Zn powder in the brazing coating 3 of the heat exchanger tube 1 is melted, and a Zn diffusion layer is formed on the flat tube 2.
- the brazing coating film 3 is melted at, for example, 570 to 600 ° C., and an Al—Si alloy is formed.
- the flat tube 2 and the header pipe 6 are brazed, and the flat tube 2 and the fins 7 are brazed, whereby the heat exchanger 5 is obtained.
- the above brazing composition is used for the heat exchanger tube 1 and the heat exchanger 5 as described above, it can be provided with excellent brazing property, corrosion resistance and adhesion.
- Synthesis Example 1 Synthesis of methacrylic resin After charging 600 parts of 3-methoxy-3-methyl-1-butanol into a reactor equipped with a stirrer, a cooling tube, a dropping funnel and a nitrogen introduction tube, the system temperature was 80 ° C. under a nitrogen stream. The temperature was raised until.
- a mixed solution of 100 parts of methyl methacrylate, 275 parts of isobutyl methacrylate, 25 parts of methacrylic acid and 4 parts of benzoyl peroxide was dropped into the system over about 3 hours, and the polymerization was carried out while maintaining the same temperature for 10 hours. Completed to obtain a methacrylic resin solution.
- the resulting methacrylic resin solution had a solid content concentration of 40% by mass and an acid value when dried of about 40 mgKOH / g.
- Examples 1 to 12 and Comparative Examples 1 to 13 After adding an anti-settling agent to the methacrylic resin solution at the blending ratio shown in Table 1 and Table 2 and stirring sufficiently, Zn powder, Si powder and Nocolok (registered trademark) Flux (K-Al-F flux, Solvay) Prepared) and further stirred and mixed. Thereafter, 3-methoxy-3-methyl-1-butanol was added to prepare a brazing composition having a solid content of 50%.
- the obtained brazing composition was applied to an aluminum plate so that the total amount of the coating film after drying would be the values shown in Table 1 and Table 2, and an aluminum plate was separately formed in an inverted T shape on the application part.
- the brazing test was carried out by placing the sample and heating to 600 ° C. to obtain a joined body sample.
- Coating film adhesion is pencil hardness HB or more
- Coating film adhesion is less than pencil hardness HB, 3B or more
- Coating film adhesion is less than pencil hardness 3B
- Brazing property Brazing properties were evaluated by confirming the presence or absence of discoloration of the joined body samples obtained in the examples and the comparative examples and measuring the fillet length. In addition, there is no discoloration and the longer the fillet length, the better the brazing property.
- the criteria for evaluation are as follows. ⁇ : Undecomposed residue of methacrylic resin and discoloration derived from undissolved residue of Si powder are not observed, and a fillet of 20 mm or more on one side is formed. ⁇ : Blackening due to undecomposed residue of methacrylic resin was partially observed, but a fillet of 20 mm or more on one side was formed. X: Discoloration derived from the undissolved residue of the Si powder is seen in the entire test piece, and a fillet of 20 mm or less on one side is formed.
- ⁇ Zn diffusion depth is 50 ⁇ m or more ⁇ : Zn diffusion depth is less than 50 ⁇ m, 20 ⁇ m or more X: Zn diffusion depth is less than 20 ⁇ m
- the brazing composition of the present invention is suitably used in the production of tubes used in heat exchangers such as aluminum heat exchangers.
- the heat exchanger tube of the present invention is suitably used in the manufacture of a heat exchanger such as an aluminum heat exchanger.
- heat exchanger of the present invention is suitably used as a heat exchanger for automobiles.
Landscapes
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Physics & Mathematics (AREA)
- Thermal Sciences (AREA)
- General Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Geometry (AREA)
- Paints Or Removers (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Laminated Bodies (AREA)
- Nonmetallic Welding Materials (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201380055386.XA CN104755222B (zh) | 2012-10-24 | 2013-09-26 | 钎焊用组合物、热交换器用管及热交换器 |
| KR1020147030573A KR101518193B1 (ko) | 2012-10-24 | 2013-09-26 | 납땜용 조성물, 열교환기용 튜브 및 열교환기 |
| EP13849003.2A EP2913142B1 (en) | 2012-10-24 | 2013-09-26 | Tube for heat exchanger |
| US14/431,840 US10092983B2 (en) | 2012-10-24 | 2013-09-26 | Brazing composition, heat exchanger tube, and heat exchanger |
| BR112015009227-6A BR112015009227B1 (pt) | 2012-10-24 | 2013-09-26 | tubo trocador de calor, trocador de calor e método para produzir um revestimento |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012235159A JP5628266B2 (ja) | 2012-10-24 | 2012-10-24 | 熱交換器用チューブ、熱交換器および塗膜の製造方法 |
| JP2012-235159 | 2012-10-24 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014065074A1 true WO2014065074A1 (ja) | 2014-05-01 |
Family
ID=50544454
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2013/076005 Ceased WO2014065074A1 (ja) | 2012-10-24 | 2013-09-26 | ろう付け用組成物、熱交換器用チューブおよび熱交換器 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US10092983B2 (cg-RX-API-DMAC7.html) |
| EP (1) | EP2913142B1 (cg-RX-API-DMAC7.html) |
| JP (1) | JP5628266B2 (cg-RX-API-DMAC7.html) |
| KR (1) | KR101518193B1 (cg-RX-API-DMAC7.html) |
| CN (1) | CN104755222B (cg-RX-API-DMAC7.html) |
| BR (1) | BR112015009227B1 (cg-RX-API-DMAC7.html) |
| WO (1) | WO2014065074A1 (cg-RX-API-DMAC7.html) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015182318A1 (ja) * | 2014-05-26 | 2015-12-03 | 株式会社Uacj | 熱交換器用チューブ及び熱交換器並びにろう付け用ペースト |
| US20180193962A1 (en) * | 2015-07-06 | 2018-07-12 | Harima Chemicals, Inc. | Composition for brazing, tube for heat exchangers, and heat exchanger |
Families Citing this family (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6090736B2 (ja) * | 2012-10-26 | 2017-03-08 | 株式会社Uacj | アルミニウム合金のろう付方法及びフラックス成分被覆アルミニウム合金部材 |
| JP6106530B2 (ja) * | 2013-06-07 | 2017-04-05 | 株式会社ケーヒン・サーマル・テクノロジー | アルミニウム押出形材製熱交換管外面の防食処理方法および熱交換器の製造方法 |
| CN106661677B (zh) | 2014-07-30 | 2018-09-21 | 株式会社Uacj | 铝合金钎焊板 |
| US10150186B2 (en) | 2014-12-11 | 2018-12-11 | Uacj Corporation | Brazing method |
| JP6521624B2 (ja) * | 2014-12-24 | 2019-05-29 | 三菱アルミニウム株式会社 | 耐食性に優れるプレートフィン型熱交換器に用いる偏平管およびそれを用いた熱交換器 |
| CN106181126A (zh) * | 2015-05-05 | 2016-12-07 | 播磨化成株式会社 | 热交换器用构件、钎焊用组合物及热交换器 |
| JP6186455B2 (ja) | 2016-01-14 | 2017-08-23 | 株式会社Uacj | 熱交換器及びその製造方法 |
| JP6312968B1 (ja) | 2016-11-29 | 2018-04-18 | 株式会社Uacj | ブレージングシート及びその製造方法 |
| JP7053281B2 (ja) | 2017-03-30 | 2022-04-12 | 株式会社Uacj | アルミニウム合金クラッド材及びその製造方法 |
| JP7220146B2 (ja) * | 2017-06-22 | 2023-02-09 | ハリマ化成株式会社 | ろう付け材、ろう付け部材、および、熱交換器 |
| JP6916715B2 (ja) | 2017-11-08 | 2021-08-11 | 株式会社Uacj | ブレージングシート及びその製造方法 |
| JP6770983B2 (ja) * | 2018-01-12 | 2020-10-21 | 三菱マテリアル株式会社 | ろう付け用フラックス組成物と粉末ろう組成物及びアルミニウム合金部材と熱交換器 |
| JP6824208B2 (ja) * | 2018-02-26 | 2021-02-03 | 株式会社タムラ製作所 | フラックス及びソルダペースト |
| WO2020039497A1 (ja) * | 2018-08-21 | 2020-02-27 | ハリマ化成株式会社 | ろう付け材、ろう付け部材、熱交換器、および、ろう付け部材の製造方法 |
| JP7291714B2 (ja) | 2018-09-11 | 2023-06-15 | 株式会社Uacj | ブレージングシートの製造方法 |
| JP7196032B2 (ja) * | 2019-07-24 | 2022-12-26 | Maアルミニウム株式会社 | ろう付け用フラックス組成物と粉末ろう組成物、アルミニウム合金部材と熱交換器及びアルミニウム合金部材と熱交換器の製造方法 |
| WO2021033624A1 (ja) * | 2019-08-20 | 2021-02-25 | ハリマ化成株式会社 | ろう付け材、ろう付け部材および熱交換器 |
| JP7591872B2 (ja) | 2020-04-08 | 2024-11-29 | 株式会社Uacj | ブレージングシートの製造方法 |
| KR102490680B1 (ko) * | 2022-07-04 | 2023-01-26 | 주식회사 삼원 | 열교환기용 알루미늄 압출튜브의 내식성 향상을 위한 페이스트 조성물 및 이를 이용한 열교환기 압출튜브 제조방법 |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11221696A (ja) * | 1998-02-02 | 1999-08-17 | Mitsubishi Alum Co Ltd | ろう付用組成物および該組成物の塗布方法ならびにろう付用品 |
| JP2001293593A (ja) * | 2000-02-17 | 2001-10-23 | Toyo Aluminium Kk | アルミニウムろう付用ペースト状組成物、その塗膜、およびろう付方法 |
| JP2006255755A (ja) * | 2005-03-17 | 2006-09-28 | Mitsubishi Alum Co Ltd | ろう付用アルミニウム合金材およびアルミニウム合金材のろう付方法 |
| JP2009058139A (ja) | 2007-08-30 | 2009-03-19 | Mitsubishi Alum Co Ltd | 耐食性に優れたアルミニウム製熱交換器用部材 |
| JP2010075966A (ja) | 2008-09-25 | 2010-04-08 | Mitsubishi Alum Co Ltd | ろう付用複合材 |
| WO2011148781A1 (ja) * | 2010-05-25 | 2011-12-01 | 住友軽金属工業株式会社 | アルミニウム合金製熱交換器の製造方法 |
| WO2012026823A1 (en) * | 2010-08-23 | 2012-03-01 | Norsk Hydro Asa | Brazing pre-flux coating |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5450666A (en) * | 1994-02-28 | 1995-09-19 | S.A. Day Mfg. Co., Inc. | Flux composition for aluminum brazing |
| US6497770B2 (en) | 2000-02-17 | 2002-12-24 | Toyo Aluminium Kabushiki Kaisha | Flux-containing compositions for brazing aluminum, films and brazing method thereby |
| US8640766B2 (en) | 2003-05-06 | 2014-02-04 | Mitsubishi Aluminum Co., Ltd. | Heat exchanger tube |
| JP5026726B2 (ja) | 2006-04-03 | 2012-09-19 | 東洋アルミニウム株式会社 | アルミニウムろう付用ペースト状組成物、それが塗布されたアルミニウム含有部材、および、それを用いたアルミニウム含有部材のろう付方法 |
| JP5152727B2 (ja) | 2007-12-21 | 2013-02-27 | ハリマ化成株式会社 | アルミニウムろう付け用ペースト組成物 |
-
2012
- 2012-10-24 JP JP2012235159A patent/JP5628266B2/ja active Active
-
2013
- 2013-09-26 WO PCT/JP2013/076005 patent/WO2014065074A1/ja not_active Ceased
- 2013-09-26 CN CN201380055386.XA patent/CN104755222B/zh active Active
- 2013-09-26 KR KR1020147030573A patent/KR101518193B1/ko not_active Expired - Fee Related
- 2013-09-26 US US14/431,840 patent/US10092983B2/en active Active
- 2013-09-26 EP EP13849003.2A patent/EP2913142B1/en active Active
- 2013-09-26 BR BR112015009227-6A patent/BR112015009227B1/pt active IP Right Grant
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11221696A (ja) * | 1998-02-02 | 1999-08-17 | Mitsubishi Alum Co Ltd | ろう付用組成物および該組成物の塗布方法ならびにろう付用品 |
| JP2001293593A (ja) * | 2000-02-17 | 2001-10-23 | Toyo Aluminium Kk | アルミニウムろう付用ペースト状組成物、その塗膜、およびろう付方法 |
| JP2006255755A (ja) * | 2005-03-17 | 2006-09-28 | Mitsubishi Alum Co Ltd | ろう付用アルミニウム合金材およびアルミニウム合金材のろう付方法 |
| JP2009058139A (ja) | 2007-08-30 | 2009-03-19 | Mitsubishi Alum Co Ltd | 耐食性に優れたアルミニウム製熱交換器用部材 |
| JP2010075966A (ja) | 2008-09-25 | 2010-04-08 | Mitsubishi Alum Co Ltd | ろう付用複合材 |
| WO2011148781A1 (ja) * | 2010-05-25 | 2011-12-01 | 住友軽金属工業株式会社 | アルミニウム合金製熱交換器の製造方法 |
| WO2012026823A1 (en) * | 2010-08-23 | 2012-03-01 | Norsk Hydro Asa | Brazing pre-flux coating |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015182318A1 (ja) * | 2014-05-26 | 2015-12-03 | 株式会社Uacj | 熱交換器用チューブ及び熱交換器並びにろう付け用ペースト |
| CN106457483A (zh) * | 2014-05-26 | 2017-02-22 | 株式会社Uacj | 热交换器用管及热交换器以及钎焊用膏 |
| JPWO2015182318A1 (ja) * | 2014-05-26 | 2017-04-20 | 株式会社Uacj | 熱交換器用チューブ及び熱交換器並びにろう付け用ペースト |
| EP3150327A4 (en) * | 2014-05-26 | 2017-05-24 | UACJ Corporation | Heat exchanger tube, heat exchanger, and brazing paste |
| CN106457483B (zh) * | 2014-05-26 | 2019-07-26 | 株式会社Uacj | 热交换器用管及热交换器以及钎焊用膏 |
| US20180193962A1 (en) * | 2015-07-06 | 2018-07-12 | Harima Chemicals, Inc. | Composition for brazing, tube for heat exchangers, and heat exchanger |
Also Published As
| Publication number | Publication date |
|---|---|
| US20150239071A1 (en) | 2015-08-27 |
| US10092983B2 (en) | 2018-10-09 |
| KR101518193B1 (ko) | 2015-05-06 |
| CN104755222A (zh) | 2015-07-01 |
| BR112015009227A2 (pt) | 2017-07-04 |
| EP2913142B1 (en) | 2018-12-19 |
| EP2913142A4 (en) | 2016-07-06 |
| KR20140133960A (ko) | 2014-11-20 |
| BR112015009227B1 (pt) | 2020-11-03 |
| EP2913142A1 (en) | 2015-09-02 |
| JP2014083570A (ja) | 2014-05-12 |
| JP5628266B2 (ja) | 2014-11-19 |
| CN104755222B (zh) | 2016-11-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5628266B2 (ja) | 熱交換器用チューブ、熱交換器および塗膜の製造方法 | |
| JP5152727B2 (ja) | アルミニウムろう付け用ペースト組成物 | |
| WO2003106102A1 (ja) | 水系アルミニウムろう付け用組成物、及びろう付け方法 | |
| JP6153777B2 (ja) | アルミニウムろう付け用ペースト | |
| JP6453721B2 (ja) | ろう付け用組成物、熱交換器用チューブおよび熱交換器 | |
| WO2018235906A1 (ja) | ろう付け材、ろう付け部材、および、熱交換器 | |
| JP5959412B2 (ja) | Al−Cuろう付け用ペーストおよびAl−Cuろう付け方法 | |
| JP5715779B2 (ja) | アルミニウムろう付け用組成物の製造方法及びインナーフィンチューブのろう付け方法 | |
| JP2008207237A (ja) | アルミニウムろう付け用塗料及び当該ろう付け方法 | |
| JP6909746B2 (ja) | ろう材粉末、ろう付け用組成物および接合体 | |
| JP4088887B2 (ja) | 水系アルミニウムろう付け用組成物、及びろう付け方法 | |
| CN106181126A (zh) | 热交换器用构件、钎焊用组合物及热交换器 | |
| JP2000197990A (ja) | ブレ―ジングペ―スト | |
| JP5513876B2 (ja) | アルミニウムろう付け用組成物 | |
| JP7196032B2 (ja) | ろう付け用フラックス組成物と粉末ろう組成物、アルミニウム合金部材と熱交換器及びアルミニウム合金部材と熱交換器の製造方法 | |
| JP2013075305A (ja) | 水系アルミニウムろう付用組成物 | |
| CN105026087A (zh) | 钎焊组合物及其用途 | |
| JP6139932B2 (ja) | ろう付け用組成物およびろう付け方法 | |
| WO2021014607A1 (ja) | ろう付け用フラックス組成物と粉末ろう組成物、アルミニウム合金部材と熱交換器及びアルミニウム合金部材の製造方法と熱交換器の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13849003 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20147030573 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14431840 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2013849003 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112015009227 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112015009227 Country of ref document: BR Kind code of ref document: A2 Effective date: 20150424 |