WO2012043783A1 - 非水系電解質二次電池用正極活物質とその製造方法、および該正極活物質を用いた非水系電解質二次電池 - Google Patents
非水系電解質二次電池用正極活物質とその製造方法、および該正極活物質を用いた非水系電解質二次電池 Download PDFInfo
- Publication number
- WO2012043783A1 WO2012043783A1 PCT/JP2011/072519 JP2011072519W WO2012043783A1 WO 2012043783 A1 WO2012043783 A1 WO 2012043783A1 JP 2011072519 W JP2011072519 W JP 2011072519W WO 2012043783 A1 WO2012043783 A1 WO 2012043783A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- positive electrode
- active material
- electrode active
- electrolyte secondary
- composite oxide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y30/00—Nanotechnology for materials or surface science, e.g. nanocomposites
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/13—Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof
- H01M4/131—Electrodes based on mixed oxides or hydroxides, or on mixtures of oxides or hydroxides, e.g. LiCoOx
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/52—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of nickel, cobalt or iron
- H01M4/525—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of nickel, cobalt or iron of mixed oxides or hydroxides containing iron, cobalt or nickel for inserting or intercalating light metals, e.g. LiNiO2, LiCoO2 or LiCoOxFy
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01G—COMPOUNDS CONTAINING METALS NOT COVERED BY SUBCLASSES C01D OR C01F
- C01G53/00—Compounds of nickel
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01G—COMPOUNDS CONTAINING METALS NOT COVERED BY SUBCLASSES C01D OR C01F
- C01G53/00—Compounds of nickel
- C01G53/40—Complex oxides containing nickel and at least one other metal element
- C01G53/42—Complex oxides containing nickel and at least one other metal element containing alkali metals, e.g. LiNiO2
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01G—COMPOUNDS CONTAINING METALS NOT COVERED BY SUBCLASSES C01D OR C01F
- C01G53/00—Compounds of nickel
- C01G53/40—Complex oxides containing nickel and at least one other metal element
- C01G53/42—Complex oxides containing nickel and at least one other metal element containing alkali metals, e.g. LiNiO2
- C01G53/44—Complex oxides containing nickel and at least one other metal element containing alkali metals, e.g. LiNiO2 containing manganese
- C01G53/50—Complex oxides containing nickel and at least one other metal element containing alkali metals, e.g. LiNiO2 containing manganese of the type (MnO2)n-, e.g. Li(NixMn1-x)O2 or Li(MyNixMn1-x-y)O2
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/362—Composites
- H01M4/366—Composites as layered products
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/485—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of mixed oxides or hydroxides for inserting or intercalating light metals, e.g. LiTi2O4 or LiTi2OxFy
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/50—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese
- H01M4/505—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese of mixed oxides or hydroxides containing manganese for inserting or intercalating light metals, e.g. LiMn2O4 or LiMn2OxFy
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2002/00—Crystal-structural characteristics
- C01P2002/50—Solid solutions
- C01P2002/52—Solid solutions containing elements as dopants
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2002/00—Crystal-structural characteristics
- C01P2002/50—Solid solutions
- C01P2002/52—Solid solutions containing elements as dopants
- C01P2002/54—Solid solutions containing elements as dopants one element only
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/01—Particle morphology depicted by an image
- C01P2004/03—Particle morphology depicted by an image obtained by SEM
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/30—Particle morphology extending in three dimensions
- C01P2004/45—Aggregated particles or particles with an intergrown morphology
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/60—Particles characterised by their size
- C01P2004/61—Micrometer sized, i.e. from 1-100 micrometer
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/60—Particles characterised by their size
- C01P2004/64—Nanometer sized, i.e. from 1-100 nanometer
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2006/00—Physical properties of inorganic compounds
- C01P2006/12—Surface area
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
- H01M10/0525—Rocking-chair batteries, i.e. batteries with lithium insertion or intercalation in both electrodes; Lithium-ion batteries
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M2004/026—Electrodes composed of, or comprising, active material characterised by the polarity
- H01M2004/028—Positive electrodes
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P70/00—Climate change mitigation technologies in the production process for final industrial or consumer products
- Y02P70/50—Manufacturing or production processes characterised by the final manufactured product
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02T—CLIMATE CHANGE MITIGATION TECHNOLOGIES RELATED TO TRANSPORTATION
- Y02T10/00—Road transport of goods or passengers
- Y02T10/60—Other road transportation technologies with climate change mitigation effect
- Y02T10/70—Energy storage systems for electromobility, e.g. batteries
Definitions
- the present invention relates to a positive electrode active material for a non-aqueous electrolyte secondary battery, a manufacturing method thereof, and a non-aqueous electrolyte secondary battery using the positive electrode active material.
- This lithium ion secondary battery is composed of a negative electrode, a positive electrode, an electrolyte, and the like, and a material capable of desorbing and inserting lithium is used as the active material of the negative electrode and the positive electrode.
- lithium ion secondary battery Such a lithium ion secondary battery is currently being actively researched and developed.
- a lithium ion secondary battery using a layered or spinel type lithium metal composite oxide as a positive electrode material Since a high voltage of 4V class can be obtained, practical use is progressing as a battery having a high energy density.
- the materials mainly proposed so far include lithium cobalt composite oxide (LiCoO 2 ), which is relatively easy to synthesize, lithium nickel composite oxide (LiNiO 2 ) using nickel, which is cheaper than cobalt, and lithium nickel.
- cobalt manganese composite oxide LiNi 1/3 Co 1/3 Mn 1/3 O 2
- lithium manganese composite oxide LiMn 2 O 4
- lithium nickel composite oxide and lithium nickel cobalt manganese composite oxide are attracting attention as materials that have good cycle characteristics and can provide high output with low resistance. In recent years, it is important to reduce resistance required for high output. Is being viewed.
- Patent Document 1 contains at least one element selected from Mo, W, Nb, Ta, and Re in an amount of 0.1 to 5 mol% with respect to the total molar amount of Mn, Ni, and Co.
- Lithium transition metal compound powder for a lithium secondary battery positive electrode material has been proposed, and Mo, W, Nb, Ta, and Li in the surface portion of primary particles and the total of metal elements other than Mo, W, Nb, Ta, and Re
- the total atomic ratio of Re is preferably 5 times or more of the atomic ratio of the entire primary particle. According to this proposal, it is possible to achieve both low cost and high safety of the lithium transition metal compound powder for the lithium secondary battery positive electrode material, high load characteristics, and improved powder handling properties.
- the lithium transition metal compound powder is obtained by pulverizing raw materials in a liquid medium, spray-drying a slurry in which these are uniformly dispersed, and firing the obtained spray-dried body. Therefore, a part of different elements such as Mo, W, Nb, Ta, and Re is replaced with Ni arranged in a layer, and there is a problem that battery characteristics such as battery capacity and cycle characteristics are deteriorated. It was.
- Patent Document 2 discloses a positive electrode active material for a non-aqueous electrolyte secondary battery having at least a layered lithium transition metal composite oxide, the lithium transition metal composite oxide being composed of primary particles and aggregates thereof.
- a positive electrode active material for a secondary battery has been proposed.
- a positive electrode active material for a non-aqueous electrolyte secondary battery having excellent battery characteristics even under a more severe use environment can be obtained, and in particular, the surface of the particle is composed of molybdenum, vanadium, tungsten, boron and fluorine.
- the initial characteristics are improved without impairing the improvement of thermal stability, load characteristics and output characteristics.
- the effect of at least one additive element selected from the group consisting of molybdenum, vanadium, tungsten, boron and fluorine is considered to be an improvement in initial characteristics, that is, initial discharge capacity and initial efficiency, and refers to output characteristics. is not.
- the additive element is mixed with the hydroxide that has been heat-treated at the same time as the lithium compound and fired, a part of the additive element is replaced with nickel arranged in layers. There was a problem that caused the battery characteristics to deteriorate.
- Patent Document 3 discloses a metal including at least one selected from Ti, Al, Sn, Bi, Cu, Si, Ga, W, Zr, B, and Mo around the positive electrode active material and / or a plurality of these.
- a positive electrode active material coated with an intermetallic compound and / or oxide obtained by a combination has been proposed. Such a coating can absorb oxygen gas and ensure safety, but no output characteristics are disclosed.
- the disclosed manufacturing method is a method of coating using a planetary ball mill, and such a coating method causes physical damage to the positive electrode active material, resulting in deterioration of battery characteristics.
- Patent Document 4 a composite oxide particle mainly composed of lithium nickelate is subjected to heat treatment by adhering a tungstic acid compound, and the content of carbonate ions is 0.15% by weight or less.
- a positive electrode active material has been proposed. According to this proposal, there is a tungstic acid compound or a decomposition product of the tungstic acid compound on the surface of the positive electrode active material, and the oxidation activity on the surface of the composite oxide particles in a charged state is suppressed. Although gas generation can be suppressed, the output characteristics are not disclosed at all.
- the disclosed production method preferably comprises a composite oxide particle heated to a boiling point or higher of a solution in which an adherent component is dissolved, and a sulfuric acid compound, a nitric acid compound, a boric acid compound, or a phosphoric acid compound as well as a tungstic acid compound.
- a solution dissolved in a solvent is applied as an adsorbing component. Since the solvent is removed in a short time, the tungsten compound is not sufficiently dispersed on the surface of the composite oxide particles and is uniformly applied. It is difficult.
- an object of the present invention is to provide a positive electrode active material for a non-aqueous electrolyte secondary battery that can provide a high output with a high capacity when used as a positive electrode material.
- the present inventors have conducted intensive research on the powder properties of lithium metal composite oxides used as the positive electrode active material for non-aqueous electrolyte secondary batteries and the effect on the positive electrode resistance of the battery. It has been found that by forming fine particles containing W and Li on the surface of the primary particles constituting the lithium metal composite oxide powder, the positive electrode resistance of the battery can be reduced and the output characteristics can be improved. Furthermore, as a manufacturing method thereof, it is possible to form fine particles containing W and Li on the entire primary particle surface by mixing and heat-treating an alkaline solution containing tungsten and a lithium metal composite oxide solution. The headline and the present invention have been completed.
- the first invention of the present invention is a method for producing a positive electrode active material for a non-aqueous electrolyte secondary battery, the general formula Li z Ni 1-x-y Co x M y O 2 (where 0.10 ⁇ x ⁇ 0.35, 0 ⁇ y ⁇ 0.35, 0.97 ⁇ z ⁇ 1.20, M is at least one element selected from Mn, V, Mg, Mo, Nb, Ti and Al) Lithium metal composite oxide is obtained by adding and mixing an alkaline solution in which a tungsten compound is dissolved in a lithium metal composite oxide powder composed of primary particles and secondary particles formed by aggregation of primary particles.
- the manufacturing method according to the second invention of the present invention further includes a step of washing the lithium metal composite oxide powder with water before the first step in the first invention.
- the amount of tungsten contained in the alkaline solution in which the tungsten compound in the first and second aspects is dissolved is Ni, Co contained in the lithium metal composite oxide powder mixed with the alkaline solution.
- the total number of atoms of M is 0.1 to 3.0 atomic%, and the tungsten concentration in the alkaline solution is 0.05 to 2 mol / l.
- the fourth invention of the present invention is characterized in that the alkaline solution in which tungsten is dissolved in the first to third inventions is a solution in which a tungsten compound is dissolved in an aqueous lithium hydroxide solution.
- the fifth invention of the present invention is a mixture of an alkaline solution in which the tungsten compound is dissolved in the first to fourth inventions and a lithium metal composite oxide powder, wherein the alkaline solution is a liquid and is 50 ° C. or less. It is characterized by being performed at temperature.
- the sixth invention of the present invention is characterized in that the heat treatment of the second step in the first to fifth inventions is performed at 100 to 600 ° C. in an oxygen atmosphere and a vacuum atmosphere.
- the positive electrode active material for a non-aqueous electrolyte secondary battery the general formula Li z Ni 1-x-y Co x M y O 2 ( however, 0.10 ⁇ x ⁇ 0.35, 0 ⁇ y ⁇ 0.35, 0.97 ⁇ z ⁇ 1.20, and M is at least one element selected from Mn, V, Mg, Mo, Nb, Ti and Al)
- the surface of the lithium metal composite oxide powder composed of secondary particles formed by agglomeration of primary particles has fine particles containing W and Li.
- the positive electrode active material for a non-aqueous electrolyte secondary battery the general formula Li z Ni 1-x-y Co x M y O 2 ( however, 0.10 ⁇ x ⁇ 0.35, 0 ⁇ y ⁇ 0.35, 0.97 ⁇ z ⁇ 1.20, and M is at least one element selected from Mn, V, Mg, Mo, Nb, Ti and Al)
- the primary particle surface of the lithium metal composite oxide powder composed of secondary particles formed by aggregation of the primary particles has fine particles containing W and Li.
- the amount of tungsten contained in the fine particles according to the seventh and eighth aspects is W relative to the total number of Ni, Co and M atoms contained in the lithium metal composite oxide powder.
- the number of atoms is 0.1 to 3.0 atomic%, and W and Li in the fine particles are present in the form of lithium tungstate.
- a non-aqueous electrolyte secondary battery has a positive electrode containing the positive electrode active material according to any of the seventh to ninth aspects.
- the positive electrode active material for nonaqueous electrolyte secondary batteries which can implement
- the positive electrode active material for a non-aqueous electrolyte secondary battery of the present invention has a general formula Li z Ni 1-xy Co x M y O 2 (where 0.10 ⁇ x ⁇ 0.35, 0 ⁇ y ⁇ 0.35, 0.97 ⁇ z ⁇ 1.20, and M is at least one element selected from Mn, V, Mg, Mo, Nb, Ti and Al)
- the surface of the lithium metal composite oxide powder comprising secondary particles formed by agglomeration of the primary particles has fine particles containing W and Li.
- the primary particles of the lithium metal composite oxide powder The surface has fine particles containing W and Li.
- the general formula Li z Ni 1-x-y Co x M y O 2 as the base material ((where, 0.10 ⁇ x ⁇ 0.35,0 ⁇ y ⁇ 0.35,0.97 ⁇ z ⁇ 1.20, where M is a lithium metal composite oxide represented by (at least one element selected from Mn, V, Mg, Mo, Nb, Ti, and Al). Furthermore, the output characteristics are improved while maintaining the charge / discharge capacity by the fine particles containing W and Li formed on the surface of the powder made of the lithium metal composite oxide.
- the movement (intercalation) of lithium ions is greatly limited, resulting in a high capacity of the lithium nickel composite oxide.
- the advantages are erased.
- fine particles containing W and Li are formed on the surface of the lithium metal composite oxide powder, but these fine particles have high lithium ion conductivity and have an effect of promoting the movement of lithium ions. .
- a Li conduction path is formed at the interface with the electrolytic solution, thereby reducing the reaction resistance of the active material and improving the output characteristics. Is.
- the specific surface area decreases regardless of the coating thickness, so even if the coating has high lithium ion conductivity, The contact area with the liquid becomes small, which tends to cause a decrease in charge / discharge capacity and an increase in reaction resistance.
- Such fine particles preferably have a particle diameter of 1 to 100 nm. If the particle diameter is less than 1 nm, fine particles may not have sufficient lithium ion conductivity. Further, if the particle diameter exceeds 100 nm, the formation of the coating with the fine particles becomes non-uniform and the effect of reducing the reaction resistance may not be sufficiently obtained.
- the primary particle surface in the present invention refers to the primary particle surface exposed on the outer surface of the secondary particle and the void in the vicinity of the inner surface of the secondary particle through which the electrolyte solution can penetrate through the outer surface of the secondary particle. It includes exposed primary particle surfaces. Furthermore, even a grain boundary between primary particles is included as long as the primary particles are not completely bonded and the electrolyte solution can penetrate.
- This contact with the electrolytic solution occurs not only on the outer surface of the secondary particles constituted by aggregation of primary particles, but also in the vicinity of the surface of the secondary particles and in the internal voids, and also on the incomplete grain boundaries. It is necessary to form fine particles on the surface of the primary particles to promote the movement of lithium ions. Therefore, the reaction resistance of the lithium metal composite oxide particles can be further reduced by forming fine particles on the entire primary particle surface.
- the fine particles do not need to be completely formed on the entire surface of the primary particles, and may be scattered. Even in the scattered state, if fine particles are formed on the primary particle surfaces exposed on the outer surface and the inner voids of the lithium metal composite oxide particles, an effect of reducing the reaction resistance can be obtained.
- the property of the surface of such a lithium metal composite oxide powder can be judged by observing with a field emission scanning electron microscope, for example.
- the lithium metal composite oxide It has been confirmed that fine particles containing W and Li are formed on the surface of the oxide powder.
- fine particles are formed non-uniformly between lithium metal composite oxide powders, the movement of lithium ions between lithium metal composite oxide powders becomes non-uniform. Load is applied, which tends to deteriorate cycle characteristics and increase reaction resistance. Therefore, it is preferable that the fine particles are uniformly formed between the lithium metal composite oxide powders.
- the fine particles of the present invention may contain W and Li, but W and Li are preferably in the form of lithium tungstate. By forming this lithium tungstate, the lithium ion conductivity is further increased, and the effect of reducing the reaction resistance is further increased.
- the amount of tungsten contained in the fine particles is preferably 0.1 to 3.0 atomic% with respect to the total number of Ni, Co and M atoms contained in the lithium metal composite oxide. Thereby, it is possible to achieve both high charge / discharge capacity and output characteristics. If the amount of tungsten is less than 0.1 atomic%, the effect of improving the output characteristics may not be sufficiently obtained. If the amount of tungsten exceeds 3.0 atomic%, the amount of fine particles to be formed increases so that lithium metal is formed. Lithium conduction between the composite oxide and the electrolytic solution may be inhibited, and the charge / discharge capacity may be reduced.
- the amount of lithium contained in the fine particles is not particularly limited, and if contained in the fine particles, an effect of improving lithium ion conductivity can be obtained. Usually, there is surplus lithium on the surface of the lithium metal composite oxide, and the amount of lithium supplied to the fine particles by the surplus lithium when mixed with an alkaline solution may be sufficient, but an amount sufficient to form lithium tungstate It is preferable that The total amount of lithium in the positive electrode active material is such that the ratio “Li / M” of the sum of the number of Ni, Co and Mo atoms (M) in the positive electrode active material to the number of Li atoms is 0.95 to 1. 30 is preferable.
- Li / M When the Li / M is less than 0.95, the reaction resistance of the positive electrode in the non-aqueous electrolyte secondary battery using the obtained positive electrode active material increases, and the output of the battery decreases. On the other hand, if Li / M exceeds 1.30, the initial discharge capacity of the positive electrode active material is reduced and the reaction resistance of the positive electrode is also increased.
- the positive electrode active material of the present invention is a material in which fine particles containing W and Li are formed on the primary particle surface of the lithium metal composite oxide to improve output characteristics.
- the body characteristics may be within the range of a positive electrode active material that is usually used.
- the effect of adhering fine particles containing W and Li to the primary particle surface of the lithium metal composite oxide powder is, for example, lithium cobalt based composite oxide, lithium manganese based composite oxide, lithium nickel cobalt manganese based composite oxide. Furthermore, it can be applied not only to the positive electrode active materials listed in the present invention but also to commonly used positive electrode active materials for lithium secondary batteries.
- the first step is an alkaline solution in which a tungsten compound is dissolved in a lithium metal composite oxide powder composed of primary particles and secondary particles formed by aggregation of primary particles (hereinafter, an alkaline solution in which a tungsten compound is dissolved is alkalinized).
- This is a step of adding and mixing the solution (W).
- W can be disperse
- a tungsten compound is dissolved in an alkaline solution.
- the dissolution method may be a normal powder dissolution method.
- the tungsten compound may be added and dissolved while stirring the solution using a reaction vessel equipped with a stirring device. It is preferable from the uniformity of dispersion that the tungsten compound is completely dissolved in an alkaline solution.
- the tungsten compound is not particularly limited as long as it can be dissolved in an alkaline solution, and it is preferable to use a tungsten compound that is easily soluble in alkali, such as tungsten oxide, lithium tungstate, and ammonium tungstate.
- the amount of tungsten dissolved in the alkaline solution is preferably 0.1 to 3.0 atomic% with respect to the total number of nickel, cobalt and M atoms contained in the mixed lithium metal composite oxide.
- the total amount of tungsten in the alkaline solution (W) is dispersed and adhered to the primary particle surface of the lithium metal composite oxide.
- the amount necessary for forming fine particles on the primary particle surface may be used. However, when the lithium metal composite oxide powder is crushed after the heat treatment, fine particles containing W and Li formed on the surface of the powder may be peeled off, and the tungsten content of the obtained positive electrode active material may be reduced. is there. In such a case, the amount of tungsten to be dissolved in the alkaline solution may be determined in anticipation of the decrease, that is, 5 to 20 atomic% with respect to the added amount of tungsten.
- the tungsten concentration of the alkaline solution (W) is preferably 0.05 to 2 mol / l. If it is less than 0.05 mol / l, a large amount of an alkaline solution to be mixed with a low tungsten concentration is required, so that it is slurried when mixed with the lithium metal composite oxide. By making the slurry, Li contained in the layered lattice of the lithium metal composite oxide is eluted, which leads to a decrease in battery characteristics, which is not preferable. On the other hand, when the tungsten concentration exceeds 2 mol / l, the alkaline solution is small and W may not be uniformly dispersed on the surface of the primary particles.
- the alkali used in the alkaline solution a general alkaline solution that does not contain impurities harmful to the positive electrode active material is used in order to obtain a high charge / discharge capacity.
- Ammonia and lithium hydroxide, which are free of impurities, can be used, but lithium hydroxide is preferably used from the viewpoint of not inhibiting Li intercalation.
- the amount of lithium contained in the positive electrode active material after mixing needs to be within the range of Li / M in the above general formula.
- the atomic ratio is preferably 4.5 to 15.0.
- Li is eluted and supplied from the lithium metal composite oxide. By using lithium hydroxide in this range, an amount of Li sufficient to form lithium tungstate can be supplied.
- the alkaline solution is preferably an aqueous solution.
- a solvent such as alcohol with high volatility
- the alkaline solution becomes secondary.
- the solvent may evaporate and not fully penetrate before it penetrates into the voids inside the particles.
- the pH of the alkaline solution may be any pH that allows the tungsten compound to dissolve, but is preferably 9 to 12.
- the pH is less than 9, the amount of lithium eluted from the lithium metal composite oxide becomes too large, and the battery characteristics may be deteriorated.
- pH exceeds 12 there exists a possibility that the excessive alkali which remains in lithium metal complex oxide may increase too much, and a battery characteristic may deteriorate.
- the composition of the positive electrode active material obtained is only the lithium content that increases as the alkaline solution is added from the lithium metal composite oxide as the base material.
- the viewpoint of high capacity and low reaction resistance generally known type Li z Ni 1-x-y Co x M y O 2 ( however, 0.10 ⁇ x ⁇ 0.35,0 ⁇ y ⁇ 0. 35, 0.97 ⁇ z ⁇ 1.20, and M is at least one element selected from Mn, V, Mg, Mo, Nb, Ti, and Al).
- increasing the contact area with the electrolytic solution is advantageous for improving the output characteristics. Therefore, the primary particles and the secondary particles are formed by agglomeration of the primary particles. It is preferable to use a lithium metal composite oxide powder having voids and grain boundaries that can penetrate.
- the lithium metal composite oxide powder is added to and mixed with the alkaline solution (W) prepared while stirring.
- the mixing is preferably carried out at a temperature of 50 ° C. or lower when the alkaline solution (W) is liquid.
- the alkaline solution (W) needs to penetrate into the voids and grain boundaries of the secondary particles, and needs to be liquid.
- the temperature exceeds 50 ° C. the alkaline solution is dried quickly, and thus there is a possibility that it does not sufficiently penetrate the voids and grain boundaries of the secondary particles. Further, if the drying is fast, elution of Li from the lithium metal composite oxide powder cannot be expected, and in particular, when Li is not contained in the alkaline solution (W), the fine particles formed on the surface may not contain Li.
- the lithium metal composite oxide powder and the alkaline solution (W) are sufficiently mixed in order to make the W dispersion uniform, and a general mixer can be used for the mixing.
- the mixture may be sufficiently mixed with the alkaline solution (W) using a shaker mixer, a Laedige mixer, a Julia mixer, a V blender, or the like so that the shape of the lithium metal composite oxide powder is not destroyed.
- W in the alkaline solution (W) can be uniformly distributed on the primary particle surface of the lithium metal composite oxide.
- the lithium metal composite oxide powder as a base material can be further washed with water before the first step.
- This washing with water may be performed by a known method and conditions as long as lithium is not excessively eluted from the lithium metal composite oxide powder and the battery characteristics are not deteriorated.
- a method of drying and mixing with the alkali solution (W) or a method of mixing with the alkali solution (W) without drying only by solid-liquid separation may be used.
- the water content after mixing with the alkali solution (W) is the maximum water content of the mixture of the dried lithium metal composite oxide powder and the alkali solution (W), that is, the lowest tungsten concentration. It is preferable not to exceed the moisture content when mixed with the alkaline solution (W). When the moisture content increases, lithium eluted from the lithium metal composite oxide becomes excessive, and battery characteristics may be deteriorated.
- the second step is a step of heat-treating the lithium metal composite oxide powder mixed with the alkaline solution (W).
- fine particles containing W and Li are formed from W supplied from the alkaline solution (W) and the alkaline solution (W) or Li supplied by elution of lithium, and the primary particle surface of the lithium metal composite oxide
- a positive electrode active material for a non-aqueous electrolyte secondary battery having fine particles containing W and Li can be obtained.
- the heat treatment method is not particularly limited, but heat treatment is performed at a temperature of 100 to 600 ° C. in an oxygen atmosphere or a vacuum atmosphere in order to prevent deterioration of electrical characteristics when used as a positive electrode active material for a non-aqueous electrolyte secondary battery. Is preferred.
- the heat treatment temperature is less than 100 ° C., the evaporation of moisture is not sufficient and fine particles may not be formed sufficiently.
- the heat treatment temperature exceeds 600 ° C., primary particles of the lithium metal composite oxide are sintered and a part of W is dissolved in the layered structure of the lithium metal composite oxide. The discharge capacity may decrease.
- the atmosphere during the heat treatment is preferably an oxidizing atmosphere such as an oxygen atmosphere or a vacuum atmosphere in order to avoid a reaction with moisture or carbonic acid in the atmosphere.
- the heat treatment time is not particularly limited, but it is preferably 5 to 15 hours in order to sufficiently evaporate the water in the alkaline solution to form fine particles.
- Non-aqueous electrolyte secondary battery of the present invention includes a positive electrode, a negative electrode, a non-aqueous electrolyte solution, and the like, and includes the same components as a general non-aqueous electrolyte secondary battery.
- the embodiment described below is merely an example, and the nonaqueous electrolyte secondary battery of the present invention can be variously modified based on the knowledge of those skilled in the art based on the embodiment described herein. It can be implemented in an improved form. Further, the use of the nonaqueous electrolyte secondary battery of the present invention is not particularly limited.
- a positive electrode of a non-aqueous electrolyte secondary battery is produced as follows. First, a powdered positive electrode active material, a conductive material, and a binder are mixed, and, if necessary, a target solvent such as activated carbon and viscosity adjustment is added and kneaded to prepare a positive electrode mixture paste. Each mixing ratio in the positive electrode mixture paste is also an important factor for determining the performance of the non-aqueous electrolyte secondary battery.
- the content of the positive electrode active material is 60 to 95 parts by mass in the same manner as the positive electrode of a general non-aqueous electrolyte secondary battery, and the conductive material
- the content of is desirably 1 to 20 parts by mass
- the content of the binder is desirably 1 to 20 parts by mass.
- the obtained positive electrode mixture paste is applied to, for example, the surface of a current collector made of aluminum foil and dried to disperse the solvent. If necessary, pressurization may be performed by a roll press or the like to increase the electrode density. In this way, a sheet-like positive electrode can be produced.
- the sheet-like positive electrode can be cut into an appropriate size or the like according to the target battery and used for battery production.
- the manufacturing method of the positive electrode is not limited to the illustrated one, and other methods may be used.
- the conductive agent for example, graphite (natural graphite, artificial graphite, expanded graphite, etc.), carbon black materials such as acetylene black, ketjen black, and the like can be used.
- the binder plays a role of anchoring the active material particles.
- PVDF polyvinylidene fluoride
- PTFE polytetrafluoroethylene
- fluorine rubber ethylene propylene diene rubber
- styrene butadiene cellulose resin
- polyacrylic polyacrylic, and the like.
- An acid or the like can be used.
- a positive electrode active material, a conductive material, and activated carbon are dispersed, and a solvent that dissolves the binder is added to the positive electrode mixture.
- a solvent that dissolves the binder is added to the positive electrode mixture.
- an organic solvent such as N-methyl-2-pyrrolidone can be used as the solvent.
- Activated carbon can be added to the positive electrode mixture in order to increase the electric double layer capacity.
- Negative electrode A negative electrode mixture in which a negative electrode active material capable of occluding and desorbing lithium ions is mixed with a binder and an appropriate solvent is added to the negative electrode. Is applied to the surface of a metal foil current collector such as copper, dried, and compressed to increase the electrode density as necessary.
- the negative electrode active material for example, natural graphite, artificial graphite, a fired organic compound such as phenol resin, or a powdery carbon material such as coke can be used.
- a fluorine-containing resin such as PVDF can be used as the negative electrode binder, as in the case of the positive electrode, and examples of the solvent for dispersing these active materials and the binder include N-methyl-2-pyrrolidone.
- Organic solvents can be used.
- (C) Separator A separator is interposed between the positive electrode and the negative electrode.
- the separator separates the positive electrode and the negative electrode and retains the electrolyte, and a thin film such as polyethylene or polypropylene and a film having many minute holes can be used.
- Non-aqueous electrolyte The non-aqueous electrolyte is obtained by dissolving a lithium salt as a supporting salt in an organic solvent.
- the organic solvent include cyclic carbonates such as ethylene carbonate, propylene carbonate, butylene carbonate, and trifluoropropylene carbonate, chain carbonates such as diethyl carbonate, dimethyl carbonate, ethylmethyl carbonate, and dipropyl carbonate, tetrahydrofuran, 2- One kind selected from ether compounds such as methyltetrahydrofuran and dimethoxyethane, sulfur compounds such as ethylmethylsulfone and butanesultone, phosphorus compounds such as triethyl phosphate and trioctyl phosphate, etc. are used alone or in admixture of two or more. be able to.
- the non-aqueous electrolyte solution may contain a radical scavenger, a surfactant, a flame retardant, and the like.
- the shape of the non-aqueous electrolyte secondary battery of the present invention composed of the positive electrode, negative electrode, separator and non-aqueous electrolyte described above can be various, such as a cylindrical type and a laminated type. Can be.
- the positive electrode and the negative electrode are laminated via a separator to form an electrode body, and the obtained electrode body is impregnated with a non-aqueous electrolyte and communicated with the positive electrode current collector and the outside.
- the positive electrode terminal and the negative electrode current collector and the negative electrode terminal communicating with the outside are connected using a current collecting lead or the like and sealed in a battery case to complete a non-aqueous electrolyte secondary battery. .
- the nonaqueous electrolyte secondary battery using the positive electrode active material of the present invention has a high capacity and a high output.
- the non-aqueous electrolyte secondary battery using the positive electrode active material according to the present invention obtained in a particularly preferred form is, for example, a high initial discharge capacity of 165 mAh / g or more and a low positive electrode when used for the positive electrode of a 2032 type coin battery. Resistance is obtained, and further, high capacity and high output. Moreover, it can be said that it has high thermal stability and is excellent in safety.
- the measuring method of the positive electrode resistance in this invention is illustrated, it will become as follows.
- the frequency dependence of the battery reaction is measured by a general AC impedance method as an electrochemical evaluation method
- the Nyquist diagram based on the solution resistance, the negative electrode resistance and the negative electrode capacity, and the positive electrode resistance and the positive electrode capacity is shown in FIG. Is obtained as follows.
- the battery reaction at the electrode consists of a resistance component accompanying the charge transfer and a capacity component due to the electric double layer. When these are expressed as an electric circuit, it becomes a parallel circuit of resistance and capacity. It is represented by an equivalent circuit in which circuits are connected in series. Fitting calculation is performed on the Nyquist diagram measured using this equivalent circuit, and each resistance component and capacitance component can be estimated.
- the positive electrode resistance is equal to the diameter of the semicircle on the low frequency side of the obtained Nyquist diagram. From the above, the positive electrode resistance can be estimated by performing AC impedance measurement on the manufactured positive electrode and performing fitting calculation on the obtained Nyquist diagram with an equivalent circuit.
- the performance (initial stage discharge capacity, positive electrode resistance) was measured.
- the present invention will be specifically described with reference to examples, but the present invention is not limited to these examples.
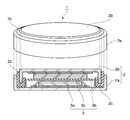
- the coin battery 1 includes a case 2 and an electrode 3 accommodated in the case 2.
- the case 2 has a positive electrode can 2a that is hollow and open at one end, and a negative electrode can 2b that is disposed in the opening of the positive electrode can 2a.
- the negative electrode can 2b is disposed in the opening of the positive electrode can 2a, A space for accommodating the electrode 3 is formed between the negative electrode can 2b and the positive electrode can 2a.
- the electrode 3 includes a positive electrode 3a, a separator 3c, and a negative electrode 3b, which are stacked in this order.
- the positive electrode 3a contacts the inner surface of the positive electrode can 2a
- the negative electrode 3b contacts the inner surface of the negative electrode can 2b.
- the case 2 includes a gasket 2c, and relative movement is fixed by the gasket 2c so as to maintain a non-contact state between the positive electrode can 2a and the negative electrode can 2b.
- the gasket 2c also has a function of sealing the gap between the positive electrode can 2a and the negative electrode can 2b to block the inside and outside of the case 2 in an airtight and liquid tight manner.
- the coin-type battery 1 shown in FIG. 3 was manufactured as follows. First, 52.5 mg of a positive electrode active material for a non-aqueous electrolyte secondary battery, 15 mg of acetylene black, and 7.5 mg of polytetrafluoroethylene resin (PTFE) are mixed, and press-molded to a diameter of 11 mm and a thickness of 100 ⁇ m at a pressure of 100 MPa. A positive electrode 3a was produced. The produced positive electrode 3a was dried at 120 ° C. for 12 hours in a vacuum dryer. Using the positive electrode 3a, the negative electrode 3b, the separator 3c, and the electrolytic solution, the above-described coin-type battery 1 was produced in a glove box in an Ar atmosphere in which the dew point was controlled at ⁇ 80 ° C.
- PTFE polytetrafluoroethylene resin
- the negative electrode 3b a negative electrode sheet in which graphite powder having an average particle diameter of about 20 ⁇ m punched into a disk shape with a diameter of 14 mm and polyvinylidene fluoride were applied to a copper foil was used.
- the separator 3c a polyethylene porous film having a film thickness of 25 ⁇ m was used.
- the electrolytic solution an equivalent mixed solution (manufactured by Toyama Pharmaceutical Co., Ltd.) of ethylene carbonate (EC) and diethyl carbonate (DEC) using 1M LiClO 4 as a supporting electrolyte was used.
- the initial discharge capacity and positive electrode resistance showing the performance of the manufactured coin battery 1 were evaluated as follows.
- the initial discharge capacity is left for about 24 hours after the coin-type battery 1 is manufactured, and after the open circuit voltage OCV (Open Circuit Voltage) is stabilized, the current density with respect to the positive electrode is set to 0.1 mA / cm 2 and the cut-off voltage 4
- OCV Open Circuit Voltage
- the capacity when the battery was charged to 3 V, discharged after a pause of 1 hour to a cutoff voltage of 3.0 V was defined as the initial discharge capacity.
- the positive electrode resistance is determined by charging the coin-type battery 1 at a charging potential of 4.1 V and measuring it by an alternating current impedance method using a frequency response analyzer and a potentiogalvanostat (manufactured by Solartron, 1255B). A plot is obtained. Since this Nyquist plot is expressed as the sum of the characteristic curve indicating the solution resistance, the negative electrode resistance and its capacity, and the positive electrode resistance and its capacity, the fitting calculation was performed using an equivalent circuit based on this Nyquist plot, and the positive resistance The value of was calculated. Note that, in this example, Wako Pure Chemical Industries, Ltd. reagent grade samples were used for the production of composite hydroxide, the production of the positive electrode active material, and the secondary battery.
- a lithium metal composite represented by Li 1.060 Ni 0.76 Co 0.14 Al 0.10 O 2 obtained by a known technique of mixing and baking oxide powder containing Ni as a main component and lithium hydroxide Oxide powder was used as a base material.
- This lithium metal composite oxide powder had an average particle size of 5.0 ⁇ m and a specific surface area of 0.9 m 2 / g.
- the average particle diameter was evaluated using the volume integrated average value in the laser diffraction scattering method, and the specific surface area was evaluated using the BET method based on nitrogen gas adsorption.
- Alkaline solution containing tungsten (W) by adding 0.183 g of tungsten oxide (WO 3 ) and stirring in an aqueous solution in which 0.2 g of lithium hydroxide (LiOH) is dissolved in 3 ml of pure water. Got. Next, an alkaline solution (W) is added to 15 g of the lithium metal composite oxide powder used as a base material, and further sufficiently mixed using a shaker mixer device (TURBULA Type T2C manufactured by Willy et Bacofen (WAB)). Thus, a mixture of the alkaline solution (W) and the lithium metal composite oxide powder was obtained.
- a shaker mixer device TURBULA Type T2C manufactured by Willy et Bacofen (WAB)
- the obtained mixture was put in a magnesia baking vessel, heated to 500 ° C. at a temperature rising rate of 2.8 ° C./min in a 100% oxygen stream, heat-treated for 10 hours, and then cooled to room temperature.
- the tungsten content and Li / M of the lithium metal composite oxide powder after the heat treatment were analyzed by ICP method, the tungsten content was a composition of 0.5 atomic% with respect to the total number of Ni, Co and M atoms. It was confirmed that the Li / M was 1.097. From this, it was confirmed that the mixture of the alkaline solution (W) and the lithium metal composite oxide powder was equivalent to the composition after the heat treatment.
- the mixture was pulverized through a sieve having an aperture of 38 ⁇ m to obtain a positive electrode active material having fine particles containing W and Li on the primary particle surface.
- tungsten content and Li / M of the obtained positive electrode active material were analyzed in the same manner as described above, it was confirmed that the tungsten content was 0.45 atomic% and Li / M was 1.082. These values indicate that excess lithium separated during crushing was removed with a sieve together with tungsten.
- a positive electrode active material for a nonaqueous electrolyte secondary battery was obtained and the battery characteristics were evaluated in the same manner as in Example 1 except that the WO 3 used was 0.549 g. Table 1 shows the results.
- a positive electrode active material for a non-aqueous electrolyte secondary battery was obtained and the battery characteristics were evaluated in the same manner as in Example 1 except that 9 ml of pure water used for preparing the alkaline solution was used. The results are shown in Table 1. .
- Example 1 Except that the heat treatment after mixing was performed in a vacuum atmosphere at 140 ° C. for 14 hours, in the same manner as in Example 1, a positive electrode active material for a non-aqueous electrolyte secondary battery was obtained and the battery characteristics were evaluated. Table 1 shows.
- Lithium metal having a composition represented by Li 1.060 Ni 0.82 Co 0.15 Al 0.03 O 2 as a base material, an average particle size of 13.2 ⁇ m, and a specific surface area of 0.7 m 2 / g A positive electrode active material for a non-aqueous electrolyte secondary battery was obtained in the same manner as in Example 1 except that the composite oxide powder was used, and the battery characteristics were evaluated. The results are shown in Table 1.
- Lithium metal having a composition represented by Li 1.060 Ni 0.34 Co 0.33 Mn 0.33 O 2 as an base material, an average particle diameter of 4.1 ⁇ m, and a specific surface area of 1.0 m 2 / g A positive electrode active material for a non-aqueous electrolyte secondary battery was obtained in the same manner as in Example 1 except that the composite oxide powder was used, and the battery characteristics were evaluated. The results are shown in Table 1.
- a positive electrode active material for a non-aqueous electrolyte secondary battery is obtained in the same manner as in Example 1 except that lithium tungstate (Li 2 WO 4 ) is used as the tungsten compound and the amount dissolved in the alkaline solution is 0.190 g.
- lithium tungstate Li 2 WO 4
- the battery characteristics were evaluated, and the results are shown in Table 1.
- the lithium metal composite oxide as a base material is stirred for 1 minute as a slurry having a concentration of 1.5 g / cc with pure water before mixing with the alkali solution, separated into solid and liquid, then vacuum dried at 200 ° C. and washed with water.
- Example 1 Is the same as in Example 1 to obtain a positive electrode active material for a non-aqueous electrolyte secondary battery and evaluate battery characteristics. Table 1 shows the results.
- a positive electrode active material for a non-aqueous electrolyte secondary battery was obtained and the battery characteristics were evaluated in the same manner as in Example 1 except that the WO 3 used was 1.464 g. Table 1 shows the results.
- a positive electrode active material for a non-aqueous electrolyte secondary battery was obtained and the battery characteristics were evaluated in the same manner as in Example 1 except that 18 ml of pure water used for preparing the alkaline solution was changed, and the results are shown in Table 1. .
- Example 1 Except that the heat treatment after mixing was performed at 700 ° C. for 10 hours, in the same manner as in Example 1, a positive electrode active material for a non-aqueous electrolyte secondary battery was obtained and the battery characteristics were evaluated. Show.
- Comparative Example 1 The lithium metal composite oxide used as the base material in Example 1 was used as Comparative Example 1 without forming fine particles, and the battery characteristics were evaluated. The results are shown in Table 1.
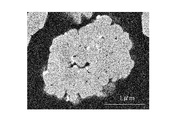
- FIG. 2 shows an example of a cross-sectional SEM observation result of the positive electrode active material obtained in the example of the present invention.
- the obtained positive electrode active material is composed of secondary particles composed of primary particles and primary particles agglomerated. It was confirmed that fine particles containing tungsten and lithium were formed on the primary particle surface.
- Example 9 where the amount of added tungsten is large, the formed fine particles are excessive. For this reason, the initial discharge capacity and the positive electrode resistance are lower than those in Examples 1 to 8.
- Example 10 the amount of pure water used in the preparation of the alkaline solution was large, and it was considered that lithium in the lithium metal composite oxide was eluted, and the initial discharge capacity and positive electrode resistance were lower than those in Examples 1-8.
- Example 11 since the heat treatment temperature after mixing the alkaline solution (W) and the lithium metal composite oxide is as high as 700 ° C., it is considered that tungsten was dissolved in the nickel site of the layered structure of the positive electrode active material, The initial discharge capacity and the positive electrode resistance are lower than those in Examples 1-8.
- Comparative Example 1 since the fine particles containing W and Li according to the present invention are not formed on the surface of the primary particles, the positive electrode resistance is significantly high, and it is difficult to meet the demand for higher output. Since the conventional example was mixed with a solid tungsten compound, the dispersion of W was not sufficient and the supply of Li into the fine particles was not achieved, resulting in a significantly high positive electrode resistance. From the above results, it can be confirmed that the nonaqueous electrolyte secondary battery using the positive electrode active material of the present invention has a high initial discharge capacity and a low positive electrode resistance, and is a battery having excellent characteristics.
- the non-aqueous electrolyte secondary battery of the present invention is suitable for a power source of a small portable electronic device (such as a notebook personal computer or a mobile phone terminal) that always requires a high capacity, and an electric vehicle battery that requires a high output. Also suitable.
- a small portable electronic device such as a notebook personal computer or a mobile phone terminal
- an electric vehicle battery that requires a high output. Also suitable.
- the nonaqueous electrolyte secondary battery of the present invention has excellent safety, and can be downsized and increased in output, so that it is suitable as a power source for electric vehicles subject to restrictions on mounting space.
- the present invention can be used not only as a power source for an electric vehicle driven purely by electric energy but also as a power source for a so-called hybrid vehicle used in combination with a combustion engine such as a gasoline engine or a diesel engine.
Landscapes
- Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Composite Materials (AREA)
- Nanotechnology (AREA)
- Materials Engineering (AREA)
- Physics & Mathematics (AREA)
- General Physics & Mathematics (AREA)
- Crystallography & Structural Chemistry (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- Battery Electrode And Active Subsutance (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020137009483A KR101395478B1 (ko) | 2010-09-30 | 2011-09-30 | 비수계 전해질 2차 전지용 정극 활물질과 그의 제조 방법, 및 상기 정극 활물질을 이용한 비수계 전해질 2차 전지 |
| EP11829336.4A EP2624342B1 (en) | 2010-09-30 | 2011-09-30 | Positive electrode active material for use in nonaqueous electrolyte secondary cells, manufacturing method thereof, and nonaqueous electrolyte secondary cell using said positive electrode active material |
| US13/876,255 US9130212B1 (en) | 2010-09-30 | 2011-09-30 | Positive electrode active material for nonaqueous electrolyte secondary batteries, method for manufacturing the same, and nonaqueous electrolyte secondary battery using said positive electrode active material |
| CN201180047121.6A CN103155240B (zh) | 2010-09-30 | 2011-09-30 | 非水系电解质二次电池用正极活性物质及其制造方法、以及使用该正极活性物质的非水系电解质二次电池 |
| US14/696,745 US9406928B2 (en) | 2010-09-30 | 2015-04-27 | Positive electrode active material for nonaqueous electrolyte secondary batteries, method for manufacturing the same, and nonaqueous electrolyte secondary battery using said positive electrode active material |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010-221587 | 2010-09-30 | ||
| JP2010221587A JP5035712B2 (ja) | 2010-09-30 | 2010-09-30 | 非水系電解質二次電池用正極活物質とその製造方法、および該正極活物質を用いた非水系電解質二次電池 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/876,255 A-371-Of-International US9130212B1 (en) | 2010-09-30 | 2011-09-30 | Positive electrode active material for nonaqueous electrolyte secondary batteries, method for manufacturing the same, and nonaqueous electrolyte secondary battery using said positive electrode active material |
| US14/696,745 Division US9406928B2 (en) | 2010-09-30 | 2015-04-27 | Positive electrode active material for nonaqueous electrolyte secondary batteries, method for manufacturing the same, and nonaqueous electrolyte secondary battery using said positive electrode active material |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012043783A1 true WO2012043783A1 (ja) | 2012-04-05 |
Family
ID=45893220
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/072519 Ceased WO2012043783A1 (ja) | 2010-09-30 | 2011-09-30 | 非水系電解質二次電池用正極活物質とその製造方法、および該正極活物質を用いた非水系電解質二次電池 |
Country Status (6)
| Country | Link |
|---|---|
| US (2) | US9130212B1 (enExample) |
| EP (1) | EP2624342B1 (enExample) |
| JP (1) | JP5035712B2 (enExample) |
| KR (1) | KR101395478B1 (enExample) |
| CN (1) | CN103155240B (enExample) |
| WO (1) | WO2012043783A1 (enExample) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20180337403A1 (en) * | 2015-10-26 | 2018-11-22 | Sumitomo Metal Mining Co., Ltd. | Positive electrode active material for nonaqueous electrolyte secondary battery, and nonaqueous electrolyte secondary battery using the positive electrode active material |
| US10177373B2 (en) * | 2014-04-25 | 2019-01-08 | Sumitomo Metal Mining Co., Ltd. | Positive electrode active material for non-aqueous electrolyte secondary battery and method for producing same, and non-aqueous electrolyte secondary battery manufactured using said positive electrode active material |
| US20190020023A1 (en) * | 2015-10-28 | 2019-01-17 | Sumitomo Metal Mining Co., Ltd. | Positive-electrode active material for non-aqueous electrolyte secondary batteries, production method thereof, and nonaqueous electrolyte secondary battery |
| US11056681B2 (en) * | 2015-04-24 | 2021-07-06 | Sumitomo Metal Mining Co., Ltd. | Positive electrode active material for nonaqueous electrolyte secondary battery, method for producing same, and nonaqueous electrolyte secondary battery using said positive electrode active material |
| US11276856B2 (en) | 2017-01-31 | 2022-03-15 | Panasonic Intellectual Property Management Co., Ltd. | Positive electrode active material for nonaqueous electrolyte secondary batteries and nonaqueous electrolyte secondary battery |
| US11811052B2 (en) | 2018-03-29 | 2023-11-07 | Sumitomo Metal Mining Co., Ltd. | Positive electrode active material for non-aqueous electrolyte secondary battery |
Families Citing this family (49)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5772626B2 (ja) * | 2012-01-25 | 2015-09-02 | 住友金属鉱山株式会社 | 非水系電解質二次電池用正極活物質とその製造方法、および該正極活物質を用いた非水系電解質二次電池 |
| JP5370515B2 (ja) * | 2012-02-22 | 2013-12-18 | 住友金属鉱山株式会社 | 非水系電解質二次電池用正極材料とその製造方法、および該正極材料を用いた非水系電解質二次電池 |
| CA2794290A1 (en) | 2012-10-22 | 2014-04-22 | Hydro-Quebec | Method of producing electrode material for lithium-ion secondary battery and lithium-ion secondary battery using such electrode material |
| KR101613862B1 (ko) | 2013-10-10 | 2016-04-20 | 미쓰이금속광업주식회사 | 리튬 과잉형 층상 리튬 금속 복합 산화물의 제조 방법 |
| TW201613159A (en) | 2014-06-26 | 2016-04-01 | Toda Kogyo Corp | Positive electrode active substance particle powder for non-aqueous electrolyte secondary battery, production method therefor, and non-aqueous electrolyte secondary battery |
| JP6090608B2 (ja) * | 2014-11-28 | 2017-03-08 | 住友金属鉱山株式会社 | 非水系電解質二次電池用正極活物質とその製造方法、および該正極活物質を用いた非水系電解質二次電池 |
| JP6090609B2 (ja) | 2014-11-28 | 2017-03-08 | 住友金属鉱山株式会社 | 非水系電解質二次電池用正極活物質とその製造方法、および該正極活物質を用いた非水系電解質二次電池 |
| JP6128398B2 (ja) * | 2014-12-26 | 2017-05-17 | トヨタ自動車株式会社 | 非水電解質二次電池 |
| JP6210439B2 (ja) * | 2014-12-26 | 2017-10-11 | 住友金属鉱山株式会社 | 非水系電解質二次電池用正極活物質とその製造方法、及び該正極活物質を用いた非水系電解質二次電池 |
| WO2016140207A1 (ja) | 2015-03-03 | 2016-09-09 | 住友金属鉱山株式会社 | 非水系電解質二次電池用正極活物質およびその製造方法 |
| JP6555636B2 (ja) | 2015-03-03 | 2019-08-07 | 住友金属鉱山株式会社 | 非水系電解質二次電池用正極活物質およびその製造方法 |
| JP6544579B2 (ja) * | 2015-03-25 | 2019-07-17 | 住友金属鉱山株式会社 | タングステン酸リチウムの製造方法、およびタングステン酸リチウムを用いた非水系電解質二次電池用正極活物質の製造方法 |
| KR102364783B1 (ko) * | 2015-04-24 | 2022-02-18 | 스미토모 긴조쿠 고잔 가부시키가이샤 | 비수계 전해질 이차 전지용 정극 활물질과 그의 제조 방법, 및 상기 정극 활물질을 이용한 비수계 전해질 이차 전지 |
| JP6569544B2 (ja) * | 2015-05-26 | 2019-09-04 | 住友金属鉱山株式会社 | 非水系電解質二次電池用正極活物質とその製造方法、および該正極活物質を用いた非水系電解質二次電池 |
| CN107851793B (zh) * | 2015-07-30 | 2021-11-09 | 住友金属矿山株式会社 | 非水电解质二次电池用正极活性物质和非水电解质二次电池 |
| US11411214B2 (en) | 2015-10-28 | 2022-08-09 | Sumitomo Metal Mining Co., Ltd. | Positive electrode active material for nonaqueous electrolyte secondary batteries, production method thereof, positive electrode mixture material paste for nonaqueous electrolyte secondary batteries, and nonaqueous electrolyte secondary battery |
| US11121368B2 (en) * | 2015-11-27 | 2021-09-14 | Sumitomo Metal Mining Co., Ltd. | Positive electrode material for nonaqueous electrolyte secondary battery and method for producing the same, and positive electrode composite material paste, and nonaqueous electrolyte secondary battery |
| JP7135269B2 (ja) | 2016-03-24 | 2022-09-13 | 住友金属鉱山株式会社 | 非水系電解質二次電池用正極活物質とその製造方法、非水系電解質二次電池用正極合材ペーストおよび非水系電解質二次電池 |
| KR20170115938A (ko) | 2016-04-08 | 2017-10-18 | 한양대학교 산학협력단 | 양극활물질, 그 제조 방법, 및 이를 포함하는 리튬이차전지 |
| DE102017207683A1 (de) * | 2016-05-09 | 2017-11-09 | Nichia Corporation | Verfahren zur Herstellung eines Nickel-Kobalt-Verbundhydroxids und Verfahren zur Herstellung eines Aktivmaterials einer Positivelektrode für eine wasserfreie Elektrolytsekundärbatterie |
| JP6378246B2 (ja) | 2016-05-09 | 2018-08-22 | トヨタ自動車株式会社 | 正極活物質、及び、当該正極活物質を用いたリチウムイオン二次電池 |
| US10978711B2 (en) * | 2016-08-29 | 2021-04-13 | Sumitomo Metal Mining Co., Ltd. | Positive electrode active material for nonaqueous electrolyte secondary battery, method for producing the same, positive electrode mixture paste for nonaqueous electrolyte secondary battery, and nonaqueous electrolyte secondary battery |
| JP6500001B2 (ja) * | 2016-08-31 | 2019-04-10 | 住友化学株式会社 | リチウム二次電池用正極活物質、リチウム二次電池用正極及びリチウム二次電池 |
| JP7002469B2 (ja) | 2016-12-07 | 2022-01-20 | 住友化学株式会社 | リチウム二次電池用正極活物質の製造方法 |
| JP6343753B2 (ja) * | 2016-12-07 | 2018-06-20 | 住友化学株式会社 | リチウム二次電池用正極活物質、リチウム二次電池用正極及びリチウム二次電池 |
| WO2018160023A1 (ko) | 2017-02-28 | 2018-09-07 | 주식회사 엘지화학 | 리튬 이차전지용 양극 활물질, 그 제조방법 및 이를 포함하는 리튬 이차전지 |
| KR102176633B1 (ko) | 2017-02-28 | 2020-11-09 | 주식회사 엘지화학 | 리튬 이차전지용 양극 활물질, 그 제조방법 및 이를 포함하는 리튬 이차전지 |
| CN108878794B (zh) * | 2017-05-11 | 2021-11-05 | 中国科学院宁波材料技术与工程研究所 | 具有复合包覆层的尖晶石结构锂离子电池正极材料及其制法 |
| US10431820B2 (en) | 2017-07-10 | 2019-10-01 | Uchicago Argonne, Llc | High valent lithiated surface structures for lithium ion battery electrode materials |
| US11637284B2 (en) | 2017-08-29 | 2023-04-25 | Sumitomo Metal Mining Co., Ltd. | Positive electrode active material for non-aqueous electrolyte secondary batteries, production method therefor, and non-aqueous electrolyte secondary batteries using said positive electrode active material |
| KR102420737B1 (ko) | 2017-08-29 | 2022-07-14 | 스미토모 긴조쿠 고잔 가부시키가이샤 | 비수계 전해질 이차 전지용 정극 활물질과 그의 제조 방법, 및 이 정극 활물질을 이용한 비수계 전해질 이차 전지 |
| CN107658439B (zh) * | 2017-08-30 | 2020-05-26 | 格林美(无锡)能源材料有限公司 | 一种钨钛共包覆的锂离子三元正极材料及其制备方法 |
| KR102007565B1 (ko) * | 2017-09-28 | 2019-08-06 | 포항공과대학교 산학협력단 | 리튬 이차 전지용 리튬 니켈 망간 복합 산화물의 제조 방법 |
| KR102498342B1 (ko) * | 2017-09-29 | 2023-02-10 | 주식회사 엘지에너지솔루션 | 리튬 과잉의 리튬 망간계 산화물 및 리튬 과잉의 리튬 망간계 산화물상에 리튬 텅스텐 화합물, 또는 추가적으로 텅스텐 화합물을 더 포함하는 양극 활물질 및 이를 포함하는 리튬 이차전지용 양극 |
| KR102165119B1 (ko) * | 2017-10-20 | 2020-10-14 | 주식회사 엘지화학 | 이차전지용 양극활물질 제조방법 및 이를 이용하는 이차전지 |
| JP7220851B2 (ja) * | 2017-10-31 | 2023-02-13 | 住友金属鉱山株式会社 | 非水系電解質二次電池用正極活物質とその製造方法、及び正極活物質を用いた非水系電解質二次電池 |
| JP6791112B2 (ja) * | 2017-12-25 | 2020-11-25 | 日亜化学工業株式会社 | 非水系二次電池用正極材料の製造方法 |
| CN117185362A (zh) * | 2017-12-28 | 2023-12-08 | 松下知识产权经营株式会社 | 非水电解质二次电池用正极活性物质的制造方法 |
| CN108598379A (zh) * | 2018-02-08 | 2018-09-28 | 中南大学 | 一种钨酸锂包覆镍钴铝酸锂复合材料及其制备方法和应用 |
| JP7232814B2 (ja) | 2018-03-13 | 2023-03-03 | 三洋電機株式会社 | 非水電解質二次電池 |
| JP2019160573A (ja) * | 2018-03-13 | 2019-09-19 | 住友化学株式会社 | リチウム金属複合酸化物粉末、リチウム二次電池用正極活物質、正極、及びリチウム二次電池 |
| JP6962838B2 (ja) * | 2018-03-13 | 2021-11-05 | 住友化学株式会社 | リチウム金属複合酸化物粉末、リチウム二次電池用正極活物質、正極、及びリチウム二次電池 |
| KR102746636B1 (ko) * | 2018-03-21 | 2024-12-24 | 바스프 에스이 | 적어도 부분적으로 코팅된 전극 활물질의 제조 방법 |
| JP7028073B2 (ja) * | 2018-05-31 | 2022-03-02 | 住友金属鉱山株式会社 | 非水系電解質二次電池用正極活物質、非水系電解質二次電池 |
| JP7203360B2 (ja) | 2018-06-28 | 2023-01-13 | パナソニックIpマネジメント株式会社 | 電極構造体及び非水電解質二次電池 |
| CN109879330A (zh) * | 2019-02-26 | 2019-06-14 | 哈尔滨工业大学(深圳) | 一种锂离子电池正极材料及其制作方法 |
| JP7357499B2 (ja) * | 2019-09-26 | 2023-10-06 | パナソニックホールディングス株式会社 | 非水電解質二次電池用正極活物質、及び非水電解質二次電池 |
| US12159996B2 (en) | 2020-03-25 | 2024-12-03 | Samsung Sdi Co., Ltd. | Positive electrode active material, positive electrode including the same, and lithium secondary battery employing the positive electrode |
| WO2024090405A1 (ja) * | 2022-10-26 | 2024-05-02 | 三井金属鉱業株式会社 | タングステン酸リチウム分散液およびその製造方法 |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH1116566A (ja) | 1997-06-20 | 1999-01-22 | Hitachi Ltd | 電 池 |
| JP2000149948A (ja) * | 1998-11-12 | 2000-05-30 | Toshiba Corp | 正極活物質、リチウムイオン二次電池およびその正極活物質の製造方法 |
| JP2001313034A (ja) * | 2000-03-24 | 2001-11-09 | Merck Patent Gmbh | 被覆リチウム複合酸化物粒子およびその製造方法 |
| JP2002075367A (ja) * | 2000-09-04 | 2002-03-15 | Mitsui Chemicals Inc | リチウム電池用正極活物質、その製法およびそれを用いた二次電池 |
| JP2005251716A (ja) | 2004-02-05 | 2005-09-15 | Nichia Chem Ind Ltd | 非水電解質二次電池用正極活物質、非水電解質二次電池用正極合剤および非水電解質二次電池 |
| JP2009289726A (ja) | 2008-05-01 | 2009-12-10 | Mitsubishi Chemicals Corp | リチウム遷移金属系化合物粉体、その製造方法及びその焼成前駆体となる噴霧乾燥体、並びに、それを用いたリチウム二次電池用正極及びリチウム二次電池 |
| JP2010040383A (ja) | 2008-08-06 | 2010-02-18 | Sony Corp | 正極活物質の製造方法および正極活物質 |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001049948A (ja) | 1999-08-11 | 2001-02-20 | Infinix:Kk | 自動ドア装置 |
| US8911903B2 (en) * | 2006-07-03 | 2014-12-16 | Sony Corporation | Cathode active material, its manufacturing method, and non-aqueous electrolyte secondary battery |
| US8962195B2 (en) * | 2007-09-04 | 2015-02-24 | Mitsubishi Chemical Corporation | Lithium transition metal-based compound powder, method for manufacturing the same, spray-dried substance serving as firing precursor thereof, and lithium secondary battery positive electrode and lithium secondary battery using the same |
-
2010
- 2010-09-30 JP JP2010221587A patent/JP5035712B2/ja active Active
-
2011
- 2011-09-30 CN CN201180047121.6A patent/CN103155240B/zh active Active
- 2011-09-30 KR KR1020137009483A patent/KR101395478B1/ko active Active
- 2011-09-30 EP EP11829336.4A patent/EP2624342B1/en active Active
- 2011-09-30 US US13/876,255 patent/US9130212B1/en active Active
- 2011-09-30 WO PCT/JP2011/072519 patent/WO2012043783A1/ja not_active Ceased
-
2015
- 2015-04-27 US US14/696,745 patent/US9406928B2/en active Active
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH1116566A (ja) | 1997-06-20 | 1999-01-22 | Hitachi Ltd | 電 池 |
| JP2000149948A (ja) * | 1998-11-12 | 2000-05-30 | Toshiba Corp | 正極活物質、リチウムイオン二次電池およびその正極活物質の製造方法 |
| JP2001313034A (ja) * | 2000-03-24 | 2001-11-09 | Merck Patent Gmbh | 被覆リチウム複合酸化物粒子およびその製造方法 |
| JP2002075367A (ja) * | 2000-09-04 | 2002-03-15 | Mitsui Chemicals Inc | リチウム電池用正極活物質、その製法およびそれを用いた二次電池 |
| JP2005251716A (ja) | 2004-02-05 | 2005-09-15 | Nichia Chem Ind Ltd | 非水電解質二次電池用正極活物質、非水電解質二次電池用正極合剤および非水電解質二次電池 |
| JP2009289726A (ja) | 2008-05-01 | 2009-12-10 | Mitsubishi Chemicals Corp | リチウム遷移金属系化合物粉体、その製造方法及びその焼成前駆体となる噴霧乾燥体、並びに、それを用いたリチウム二次電池用正極及びリチウム二次電池 |
| JP2010040383A (ja) | 2008-08-06 | 2010-02-18 | Sony Corp | 正極活物質の製造方法および正極活物質 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP2624342A4 * |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10177373B2 (en) * | 2014-04-25 | 2019-01-08 | Sumitomo Metal Mining Co., Ltd. | Positive electrode active material for non-aqueous electrolyte secondary battery and method for producing same, and non-aqueous electrolyte secondary battery manufactured using said positive electrode active material |
| US11056681B2 (en) * | 2015-04-24 | 2021-07-06 | Sumitomo Metal Mining Co., Ltd. | Positive electrode active material for nonaqueous electrolyte secondary battery, method for producing same, and nonaqueous electrolyte secondary battery using said positive electrode active material |
| US20180337403A1 (en) * | 2015-10-26 | 2018-11-22 | Sumitomo Metal Mining Co., Ltd. | Positive electrode active material for nonaqueous electrolyte secondary battery, and nonaqueous electrolyte secondary battery using the positive electrode active material |
| US10950855B2 (en) * | 2015-10-26 | 2021-03-16 | Sumitomo Metal Mining Co., Ltd. | Positive electrode active material for nonaqueous electrolyte secondary battery, and nonaqueous electrolyte secondary battery using the positive electrode active material |
| US20190020023A1 (en) * | 2015-10-28 | 2019-01-17 | Sumitomo Metal Mining Co., Ltd. | Positive-electrode active material for non-aqueous electrolyte secondary batteries, production method thereof, and nonaqueous electrolyte secondary battery |
| US11063257B2 (en) * | 2015-10-28 | 2021-07-13 | Sumitomo Metal Mining Co., Ltd. | Positive electrode active material for nonaqueous electrolyte secondary batteries, production method thereof, and nonaqueous electrolyte secondary battery |
| US11276856B2 (en) | 2017-01-31 | 2022-03-15 | Panasonic Intellectual Property Management Co., Ltd. | Positive electrode active material for nonaqueous electrolyte secondary batteries and nonaqueous electrolyte secondary battery |
| US11811052B2 (en) | 2018-03-29 | 2023-11-07 | Sumitomo Metal Mining Co., Ltd. | Positive electrode active material for non-aqueous electrolyte secondary battery |
Also Published As
| Publication number | Publication date |
|---|---|
| KR101395478B1 (ko) | 2014-05-14 |
| US9406928B2 (en) | 2016-08-02 |
| KR20130051012A (ko) | 2013-05-16 |
| CN103155240A (zh) | 2013-06-12 |
| US9130212B1 (en) | 2015-09-08 |
| JP2012079464A (ja) | 2012-04-19 |
| US20150228974A1 (en) | 2015-08-13 |
| EP2624342B1 (en) | 2016-11-09 |
| CN103155240B (zh) | 2015-08-19 |
| EP2624342A1 (en) | 2013-08-07 |
| JP5035712B2 (ja) | 2012-09-26 |
| EP2624342A4 (en) | 2014-01-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5822708B2 (ja) | 非水系電解質二次電池用正極活物質とその製造方法、および該正極活物質を用いた非水系電解質二次電池 | |
| JP5035712B2 (ja) | 非水系電解質二次電池用正極活物質とその製造方法、および該正極活物質を用いた非水系電解質二次電池 | |
| JP5772626B2 (ja) | 非水系電解質二次電池用正極活物質とその製造方法、および該正極活物質を用いた非水系電解質二次電池 | |
| US11108043B2 (en) | Method for producing positive electrode active material for nonaqueous electrolyte secondary battery | |
| JP5370515B2 (ja) | 非水系電解質二次電池用正極材料とその製造方法、および該正極材料を用いた非水系電解質二次電池 | |
| JP6555636B2 (ja) | 非水系電解質二次電池用正極活物質およびその製造方法 | |
| JP2012079464A5 (enExample) | ||
| JP6818225B2 (ja) | 非水系電解質二次電池用正極活物質の製造方法 | |
| EP3288104B1 (en) | Positive electrode active material for non-aqueous electrolyte secondary battery and method for manufacturing same, and non-aqueous electrolyte secondary battery using said positive electrode active material | |
| JP2013171785A5 (enExample) | ||
| JP2016167439A5 (enExample) | ||
| JP6582750B2 (ja) | 非水系電解質二次電池用正極活物質の製造方法 | |
| JP2016207635A5 (enExample) | ||
| JP7055587B2 (ja) | 非水系電解質二次電池用正極活物質の製造方法 | |
| JP2017117766A (ja) | 非水系電解質二次電池用正極活物質とその製造方法、及び非水系電解質二次電池 | |
| WO2016140207A1 (ja) | 非水系電解質二次電池用正極活物質およびその製造方法 | |
| WO2016104305A1 (ja) | 非水系電解質二次電池用正極活物質とその製造方法、及び該正極活物質を用いた非水系電解質二次電池 | |
| JP6730777B2 (ja) | 非水系電解質二次電池用正極材料とその製造方法、および該正極材料を用いた非水系電解質二次電池 | |
| WO2016171081A1 (ja) | 非水系電解質二次電池用正極活物質とその製造方法、および該正極活物質を用いた非水系電解質二次電池 | |
| JP2018073563A (ja) | 非水系電解質二次電池用正極材料、該正極材料を用いた非水系電解質二次電池、および非水系電解質二次電池用正極材料の製造方法。 | |
| JP6860962B2 (ja) | 非水系電解質二次電池用正極活物質、および該正極活物質を用いた非水系電解質二次電池 | |
| JP2018077938A (ja) | 非水系電解質二次電池用正極材料、該正極材料を用いた非水系電解質二次電池、および非水系電解質二次電池用正極材料の製造方法。 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201180047121.6 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11829336 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13876255 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REEP | Request for entry into the european phase |
Ref document number: 2011829336 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2011829336 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 20137009483 Country of ref document: KR Kind code of ref document: A |