RU2536933C2 - Новое лечение острого лимфобластного лейкоза у детей - Google Patents
Новое лечение острого лимфобластного лейкоза у детей Download PDFInfo
- Publication number
- RU2536933C2 RU2536933C2 RU2011122827/10A RU2011122827A RU2536933C2 RU 2536933 C2 RU2536933 C2 RU 2536933C2 RU 2011122827/10 A RU2011122827/10 A RU 2011122827/10A RU 2011122827 A RU2011122827 A RU 2011122827A RU 2536933 C2 RU2536933 C2 RU 2536933C2
- Authority
- RU
- Russia
- Prior art keywords
- children
- mrd
- acute lymphoblastic
- cells
- treatment
- Prior art date
Links
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 title claims abstract description 353
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 title claims abstract description 301
- 238000011282 treatment Methods 0.000 title claims description 160
- 230000000694 effects Effects 0.000 claims abstract description 35
- 229960003008 blinatumomab Drugs 0.000 claims abstract description 20
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 17
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 claims description 291
- 238000011134 hematopoietic stem cell transplantation Methods 0.000 claims description 101
- 230000000735 allogeneic effect Effects 0.000 claims description 76
- 210000004027 cell Anatomy 0.000 claims description 63
- 208000032839 leukemia Diseases 0.000 claims description 46
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 45
- 201000010099 disease Diseases 0.000 claims description 37
- 210000003719 b-lymphocyte Anatomy 0.000 claims description 35
- 238000001802 infusion Methods 0.000 claims description 32
- 230000008707 rearrangement Effects 0.000 claims description 32
- 238000002054 transplantation Methods 0.000 claims description 29
- 108091008874 T cell receptors Proteins 0.000 claims description 21
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 18
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 claims description 17
- 230000008030 elimination Effects 0.000 claims description 15
- 238000003379 elimination reaction Methods 0.000 claims description 15
- 210000000349 chromosome Anatomy 0.000 claims description 14
- 238000001514 detection method Methods 0.000 claims description 14
- 208000031404 Chromosome Aberrations Diseases 0.000 claims description 13
- 238000003745 diagnosis Methods 0.000 claims description 11
- 230000002489 hematologic effect Effects 0.000 claims description 11
- 210000001948 pro-b lymphocyte Anatomy 0.000 claims description 11
- 210000003958 hematopoietic stem cell Anatomy 0.000 claims description 9
- 210000000130 stem cell Anatomy 0.000 claims description 9
- WEVYNIUIFUYDGI-UHFFFAOYSA-N 3-[6-[4-(trifluoromethoxy)anilino]-4-pyrimidinyl]benzamide Chemical compound NC(=O)C1=CC=CC(C=2N=CN=C(NC=3C=CC(OC(F)(F)F)=CC=3)C=2)=C1 WEVYNIUIFUYDGI-UHFFFAOYSA-N 0.000 claims description 8
- 108700005091 Immunoglobulin Genes Proteins 0.000 claims description 7
- 208000037280 Trisomy Diseases 0.000 claims description 7
- 239000003550 marker Substances 0.000 claims description 6
- 238000001727 in vivo Methods 0.000 claims description 5
- 230000035945 sensitivity Effects 0.000 claims description 5
- 206010033645 Pancreatitis Diseases 0.000 claims 1
- 108700019889 TEL-AML1 fusion Proteins 0.000 claims 1
- 238000002560 therapeutic procedure Methods 0.000 abstract description 35
- 239000003814 drug Substances 0.000 abstract description 30
- 230000000306 recurrent effect Effects 0.000 abstract description 22
- 208000007660 Residual Neoplasm Diseases 0.000 abstract description 6
- 239000000126 substance Substances 0.000 abstract description 2
- 230000001131 transforming effect Effects 0.000 abstract 1
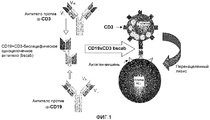
- 102000017420 CD3 protein, epsilon/gamma/delta subunit Human genes 0.000 description 168
- 238000000034 method Methods 0.000 description 89
- 238000002512 chemotherapy Methods 0.000 description 77
- 210000001185 bone marrow Anatomy 0.000 description 37
- 210000001744 T-lymphocyte Anatomy 0.000 description 31
- 210000003969 blast cell Anatomy 0.000 description 26
- 210000003169 central nervous system Anatomy 0.000 description 25
- 238000004458 analytical method Methods 0.000 description 24
- 238000011443 conventional therapy Methods 0.000 description 23
- 238000011476 stem cell transplantation Methods 0.000 description 20
- 230000027455 binding Effects 0.000 description 19
- 210000004369 blood Anatomy 0.000 description 19
- 239000008280 blood Substances 0.000 description 19
- 239000003795 chemical substances by application Substances 0.000 description 19
- 229940079593 drug Drugs 0.000 description 17
- 230000004044 response Effects 0.000 description 17
- 238000013459 approach Methods 0.000 description 16
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 16
- 230000005945 translocation Effects 0.000 description 16
- 239000000427 antigen Substances 0.000 description 15
- 108091007433 antigens Proteins 0.000 description 15
- 102000036639 antigens Human genes 0.000 description 15
- 238000006243 chemical reaction Methods 0.000 description 15
- 108090000765 processed proteins & peptides Proteins 0.000 description 15
- 238000010276 construction Methods 0.000 description 14
- 206010028980 Neoplasm Diseases 0.000 description 13
- 210000000265 leukocyte Anatomy 0.000 description 12
- 229920001184 polypeptide Polymers 0.000 description 12
- 102000004196 processed proteins & peptides Human genes 0.000 description 12
- 150000001413 amino acids Chemical class 0.000 description 11
- 230000002559 cytogenic effect Effects 0.000 description 11
- 239000008177 pharmaceutical agent Substances 0.000 description 11
- 230000001939 inductive effect Effects 0.000 description 10
- 210000004698 lymphocyte Anatomy 0.000 description 10
- 230000000719 anti-leukaemic effect Effects 0.000 description 9
- 238000001990 intravenous administration Methods 0.000 description 9
- 230000001988 toxicity Effects 0.000 description 9
- 231100000419 toxicity Toxicity 0.000 description 9
- 201000011510 cancer Diseases 0.000 description 8
- 229940124597 therapeutic agent Drugs 0.000 description 8
- 108060003951 Immunoglobulin Proteins 0.000 description 7
- 229960000928 clofarabine Drugs 0.000 description 7
- WDDPHFBMKLOVOX-AYQXTPAHSA-N clofarabine Chemical compound C1=NC=2C(N)=NC(Cl)=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@@H]1F WDDPHFBMKLOVOX-AYQXTPAHSA-N 0.000 description 7
- 230000001472 cytotoxic effect Effects 0.000 description 7
- 230000036541 health Effects 0.000 description 7
- 102000018358 immunoglobulin Human genes 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- 206010068051 Chimerism Diseases 0.000 description 6
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 6
- ZBNZXTGUTAYRHI-UHFFFAOYSA-N Dasatinib Chemical compound C=1C(N2CCN(CCO)CC2)=NC(C)=NC=1NC(S1)=NC=C1C(=O)NC1=C(C)C=CC=C1Cl ZBNZXTGUTAYRHI-UHFFFAOYSA-N 0.000 description 6
- 239000002067 L01XE06 - Dasatinib Substances 0.000 description 6
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 6
- 201000002797 childhood leukemia Diseases 0.000 description 6
- 229960000684 cytarabine Drugs 0.000 description 6
- 229960002448 dasatinib Drugs 0.000 description 6
- 230000001976 improved effect Effects 0.000 description 6
- 230000000877 morphologic effect Effects 0.000 description 6
- 230000002085 persistent effect Effects 0.000 description 6
- 238000004393 prognosis Methods 0.000 description 6
- 108090000623 proteins and genes Proteins 0.000 description 6
- 230000001225 therapeutic effect Effects 0.000 description 6
- 108010068250 Herpes Simplex Virus Protein Vmw65 Proteins 0.000 description 5
- 210000002798 bone marrow cell Anatomy 0.000 description 5
- 230000006872 improvement Effects 0.000 description 5
- 238000007913 intrathecal administration Methods 0.000 description 5
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 5
- 230000003389 potentiating effect Effects 0.000 description 5
- 230000004083 survival effect Effects 0.000 description 5
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 4
- 239000005517 L01XE01 - Imatinib Substances 0.000 description 4
- 108091028043 Nucleic acid sequence Proteins 0.000 description 4
- 238000003556 assay Methods 0.000 description 4
- 239000000969 carrier Substances 0.000 description 4
- 229960004397 cyclophosphamide Drugs 0.000 description 4
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical compound NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 4
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 230000018109 developmental process Effects 0.000 description 4
- 238000000684 flow cytometry Methods 0.000 description 4
- 229960002411 imatinib Drugs 0.000 description 4
- KTUFNOKKBVMGRW-UHFFFAOYSA-N imatinib Chemical compound C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 KTUFNOKKBVMGRW-UHFFFAOYSA-N 0.000 description 4
- 230000001900 immune effect Effects 0.000 description 4
- 208000015181 infectious disease Diseases 0.000 description 4
- 231100001231 less toxic Toxicity 0.000 description 4
- 230000010534 mechanism of action Effects 0.000 description 4
- 229960001924 melphalan Drugs 0.000 description 4
- 239000000203 mixture Substances 0.000 description 4
- 150000007523 nucleic acids Chemical group 0.000 description 4
- 239000002773 nucleotide Substances 0.000 description 4
- 125000003729 nucleotide group Chemical group 0.000 description 4
- 210000005259 peripheral blood Anatomy 0.000 description 4
- 239000011886 peripheral blood Substances 0.000 description 4
- 238000010837 poor prognosis Methods 0.000 description 4
- 239000002243 precursor Substances 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 238000011321 prophylaxis Methods 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 125000006850 spacer group Chemical group 0.000 description 4
- 238000011301 standard therapy Methods 0.000 description 4
- 108700028369 Alleles Proteins 0.000 description 3
- 238000002965 ELISA Methods 0.000 description 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 3
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 3
- 206010025323 Lymphomas Diseases 0.000 description 3
- 238000010222 PCR analysis Methods 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 206010066901 Treatment failure Diseases 0.000 description 3
- XCPGHVQEEXUHNC-UHFFFAOYSA-N amsacrine Chemical compound COC1=CC(NS(C)(=O)=O)=CC=C1NC1=C(C=CC=C2)C2=NC2=CC=CC=C12 XCPGHVQEEXUHNC-UHFFFAOYSA-N 0.000 description 3
- 229960001220 amsacrine Drugs 0.000 description 3
- 238000009583 bone marrow aspiration Methods 0.000 description 3
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 3
- 230000000973 chemotherapeutic effect Effects 0.000 description 3
- 238000009096 combination chemotherapy Methods 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 238000007596 consolidation process Methods 0.000 description 3
- 230000006378 damage Effects 0.000 description 3
- 239000008121 dextrose Substances 0.000 description 3
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 3
- 229960005420 etoposide Drugs 0.000 description 3
- 230000002349 favourable effect Effects 0.000 description 3
- 230000004927 fusion Effects 0.000 description 3
- 230000002068 genetic effect Effects 0.000 description 3
- 210000003128 head Anatomy 0.000 description 3
- 238000013394 immunophenotyping Methods 0.000 description 3
- 230000000977 initiatory effect Effects 0.000 description 3
- 230000007775 late Effects 0.000 description 3
- 230000007774 longterm Effects 0.000 description 3
- 238000009115 maintenance therapy Methods 0.000 description 3
- 229960004618 prednisone Drugs 0.000 description 3
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 3
- 238000005086 pumping Methods 0.000 description 3
- 108020003175 receptors Proteins 0.000 description 3
- 102000005962 receptors Human genes 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 239000011780 sodium chloride Substances 0.000 description 3
- 230000009870 specific binding Effects 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- 230000001052 transient effect Effects 0.000 description 3
- 238000011269 treatment regimen Methods 0.000 description 3
- 210000004881 tumor cell Anatomy 0.000 description 3
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 2
- 206010003591 Ataxia Diseases 0.000 description 2
- 208000028564 B-cell non-Hodgkin lymphoma Diseases 0.000 description 2
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 description 2
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 2
- 206010056370 Congestive cardiomyopathy Diseases 0.000 description 2
- 201000010046 Dilated cardiomyopathy Diseases 0.000 description 2
- 208000012671 Gastrointestinal haemorrhages Diseases 0.000 description 2
- 101000581981 Homo sapiens Neural cell adhesion molecule 1 Proteins 0.000 description 2
- 206010062016 Immunosuppression Diseases 0.000 description 2
- 239000005536 L01XE08 - Nilotinib Substances 0.000 description 2
- 102100027347 Neural cell adhesion molecule 1 Human genes 0.000 description 2
- 102100024952 Protein CBFA2T1 Human genes 0.000 description 2
- 230000006044 T cell activation Effects 0.000 description 2
- FOCVUCIESVLUNU-UHFFFAOYSA-N Thiotepa Chemical compound C1CN1P(N1CC1)(=S)N1CC1 FOCVUCIESVLUNU-UHFFFAOYSA-N 0.000 description 2
- 230000005856 abnormality Effects 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 238000011316 allogeneic transplantation Methods 0.000 description 2
- 239000002246 antineoplastic agent Substances 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 150000008209 arabinosides Chemical class 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 238000004820 blood count Methods 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 238000010322 bone marrow transplantation Methods 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 238000011260 co-administration Methods 0.000 description 2
- 239000003246 corticosteroid Substances 0.000 description 2
- 229960001334 corticosteroids Drugs 0.000 description 2
- 238000011262 co‐therapy Methods 0.000 description 2
- 229940104302 cytosine Drugs 0.000 description 2
- 231100000433 cytotoxic Toxicity 0.000 description 2
- 229940127089 cytotoxic agent Drugs 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 208000030304 gastrointestinal bleeding Diseases 0.000 description 2
- 230000011132 hemopoiesis Effects 0.000 description 2
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 2
- 229940072221 immunoglobulins Drugs 0.000 description 2
- 230000001506 immunosuppresive effect Effects 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000011368 intensive chemotherapy Methods 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000011459 intrathecal therapy Methods 0.000 description 2
- 230000003902 lesion Effects 0.000 description 2
- 238000002624 low-dose chemotherapy Methods 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 230000003211 malignant effect Effects 0.000 description 2
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 2
- 229960001428 mercaptopurine Drugs 0.000 description 2
- 229960000485 methotrexate Drugs 0.000 description 2
- 210000000822 natural killer cell Anatomy 0.000 description 2
- 229960001346 nilotinib Drugs 0.000 description 2
- HHZIURLSWUIHRB-UHFFFAOYSA-N nilotinib Chemical compound C1=NC(C)=CN1C1=CC(NC(=O)C=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)=CC(C(F)(F)F)=C1 HHZIURLSWUIHRB-UHFFFAOYSA-N 0.000 description 2
- 238000000399 optical microscopy Methods 0.000 description 2
- 238000007911 parenteral administration Methods 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 2
- 229920000053 polysorbate 80 Polymers 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 230000002035 prolonged effect Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 238000010186 staining Methods 0.000 description 2
- 238000010561 standard procedure Methods 0.000 description 2
- 238000011272 standard treatment Methods 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 230000009885 systemic effect Effects 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 231100000331 toxic Toxicity 0.000 description 2
- 230000002588 toxic effect Effects 0.000 description 2
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 2
- 229960004528 vincristine Drugs 0.000 description 2
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- WYWHKKSPHMUBEB-UHFFFAOYSA-N 6-Mercaptoguanine Natural products N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 1
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 1
- 201000004384 Alopecia Diseases 0.000 description 1
- 108020005098 Anticodon Proteins 0.000 description 1
- 206010002961 Aplasia Diseases 0.000 description 1
- 101000719121 Arabidopsis thaliana Protein MEI2-like 1 Proteins 0.000 description 1
- 208000025321 B-lymphoblastic leukemia/lymphoma Diseases 0.000 description 1
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 description 1
- 206010065553 Bone marrow failure Diseases 0.000 description 1
- 206010048610 Cardiotoxicity Diseases 0.000 description 1
- 206010008805 Chromosomal abnormalities Diseases 0.000 description 1
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 206010061819 Disease recurrence Diseases 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- 239000002000 Electrolyte additive Substances 0.000 description 1
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 1
- 206010018910 Haemolysis Diseases 0.000 description 1
- 102100031573 Hematopoietic progenitor cell antigen CD34 Human genes 0.000 description 1
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 description 1
- 101100383038 Homo sapiens CD19 gene Proteins 0.000 description 1
- 101000777663 Homo sapiens Hematopoietic progenitor cell antigen CD34 Proteins 0.000 description 1
- 101000582320 Homo sapiens Neurogenic differentiation factor 6 Proteins 0.000 description 1
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 description 1
- 101000857677 Homo sapiens Runt-related transcription factor 1 Proteins 0.000 description 1
- 201000001431 Hyperuricemia Diseases 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- 208000035967 Long Term Adverse Effects Diseases 0.000 description 1
- 208000028018 Lymphocytic leukaemia Diseases 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 206010028116 Mucosal inflammation Diseases 0.000 description 1
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 description 1
- 102000003896 Myeloperoxidases Human genes 0.000 description 1
- 108090000235 Myeloperoxidases Proteins 0.000 description 1
- 102000003729 Neprilysin Human genes 0.000 description 1
- 108090000028 Neprilysin Proteins 0.000 description 1
- 102100030589 Neurogenic differentiation factor 6 Human genes 0.000 description 1
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 208000037273 Pathologic Processes Diseases 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 241000206607 Porphyra umbilicalis Species 0.000 description 1
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 description 1
- 102100025373 Runt-related transcription factor 1 Human genes 0.000 description 1
- 102000007562 Serum Albumin Human genes 0.000 description 1
- 108010071390 Serum Albumin Proteins 0.000 description 1
- 108700042075 T-Cell Receptor Genes Proteins 0.000 description 1
- 108020004566 Transfer RNA Proteins 0.000 description 1
- 108090000992 Transferases Proteins 0.000 description 1
- 102000004357 Transferases Human genes 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- 206010044565 Tremor Diseases 0.000 description 1
- 206010052428 Wound Diseases 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 238000010817 Wright-Giemsa staining Methods 0.000 description 1
- 239000008351 acetate buffer Substances 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- OFCNXPDARWKPPY-UHFFFAOYSA-N allopurinol Chemical compound OC1=NC=NC2=C1C=NN2 OFCNXPDARWKPPY-UHFFFAOYSA-N 0.000 description 1
- 229960003459 allopurinol Drugs 0.000 description 1
- 231100000360 alopecia Toxicity 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 125000000539 amino acid group Chemical group 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 239000008365 aqueous carrier Substances 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- 210000000227 basophil cell of anterior lobe of hypophysis Anatomy 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 210000000601 blood cell Anatomy 0.000 description 1
- 210000002449 bone cell Anatomy 0.000 description 1
- 208000015322 bone marrow disease Diseases 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 231100000259 cardiotoxicity Toxicity 0.000 description 1
- 210000000748 cardiovascular system Anatomy 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 238000009104 chemotherapy regimen Methods 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- 238000011254 conventional chemotherapy Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000000139 costimulatory effect Effects 0.000 description 1
- 230000000093 cytochemical effect Effects 0.000 description 1
- 230000002380 cytological effect Effects 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 239000000824 cytostatic agent Substances 0.000 description 1
- 230000001085 cytostatic effect Effects 0.000 description 1
- 238000011393 cytotoxic chemotherapy Methods 0.000 description 1
- 231100000050 cytotoxic potential Toxicity 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 238000013480 data collection Methods 0.000 description 1
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 1
- 229960000975 daunorubicin Drugs 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 210000003162 effector t lymphocyte Anatomy 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 235000013373 food additive Nutrition 0.000 description 1
- 239000002778 food additive Substances 0.000 description 1
- 210000004602 germ cell Anatomy 0.000 description 1
- 210000003714 granulocyte Anatomy 0.000 description 1
- 230000003394 haemopoietic effect Effects 0.000 description 1
- 230000035876 healing Effects 0.000 description 1
- 208000014951 hematologic disease Diseases 0.000 description 1
- 229960000890 hydrocortisone Drugs 0.000 description 1
- 230000008105 immune reaction Effects 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 238000010166 immunofluorescence Methods 0.000 description 1
- 230000002163 immunogen Effects 0.000 description 1
- 238000003364 immunohistochemistry Methods 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 239000011261 inert gas Substances 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 230000001678 irradiating effect Effects 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 238000009593 lumbar puncture Methods 0.000 description 1
- 210000001165 lymph node Anatomy 0.000 description 1
- 230000002934 lysing effect Effects 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 238000013507 mapping Methods 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 229940126601 medicinal product Drugs 0.000 description 1
- 238000000386 microscopy Methods 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 230000007823 neuropathy Effects 0.000 description 1
- 201000001119 neuropathy Diseases 0.000 description 1
- 239000012457 nonaqueous media Substances 0.000 description 1
- -1 organic acid esters Chemical class 0.000 description 1
- 230000009054 pathological process Effects 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 208000033808 peripheral neuropathy Diseases 0.000 description 1
- 230000004526 pharmaceutical effect Effects 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 229940127557 pharmaceutical product Drugs 0.000 description 1
- 210000004214 philadelphia chromosome Anatomy 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 239000002953 phosphate buffered saline Substances 0.000 description 1
- 239000011505 plaster Substances 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 238000003752 polymerase chain reaction Methods 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 208000017426 precursor B-cell acute lymphoblastic leukemia Diseases 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 229960004641 rituximab Drugs 0.000 description 1
- 210000004761 scalp Anatomy 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 238000012289 standard assay Methods 0.000 description 1
- 239000008174 sterile solution Substances 0.000 description 1
- 239000011550 stock solution Substances 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 239000008362 succinate buffer Substances 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 230000003319 supportive effect Effects 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 229960001196 thiotepa Drugs 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- MNRILEROXIRVNJ-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=NC=N[C]21 MNRILEROXIRVNJ-UHFFFAOYSA-N 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2809—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against the T-cell receptor (TcR)-CD3 complex
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
- C07K16/3061—Blood cells
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/46—Hybrid immunoglobulins
- C07K16/468—Immunoglobulins having two or more different antigen binding sites, e.g. multifunctional antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Cell Biology (AREA)
- Hematology (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Oncology (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Epidemiology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11232308P | 2008-11-07 | 2008-11-07 | |
| US61/112,323 | 2008-11-07 | ||
| US18329109P | 2009-06-02 | 2009-06-02 | |
| US61/183,291 | 2009-06-02 | ||
| US22126909P | 2009-06-29 | 2009-06-29 | |
| US61/221,269 | 2009-06-29 | ||
| PCT/EP2009/007969 WO2010052013A1 (en) | 2008-11-07 | 2009-11-06 | Treatment of pediatric acute lymphoblastic leukemia |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2014141180A Division RU2677324C2 (ru) | 2008-11-07 | 2014-10-13 | Новое лечение острого лимфобластного лейкоза у детей |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2011122827A RU2011122827A (ru) | 2012-12-20 |
| RU2536933C2 true RU2536933C2 (ru) | 2014-12-27 |
Family
ID=41796119
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2011122827/10A RU2536933C2 (ru) | 2008-11-07 | 2009-11-06 | Новое лечение острого лимфобластного лейкоза у детей |
| RU2014141180A RU2677324C2 (ru) | 2008-11-07 | 2014-10-13 | Новое лечение острого лимфобластного лейкоза у детей |
| RU2018144313A RU2736802C2 (ru) | 2008-11-07 | 2018-12-14 | Новое лечение острого лимфобластного лейкоза у детей |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2014141180A RU2677324C2 (ru) | 2008-11-07 | 2014-10-13 | Новое лечение острого лимфобластного лейкоза у детей |
| RU2018144313A RU2736802C2 (ru) | 2008-11-07 | 2018-12-14 | Новое лечение острого лимфобластного лейкоза у детей |
Country Status (27)
| Country | Link |
|---|---|
| US (2) | US20110262440A1 (enExample) |
| EP (4) | EP2344539B1 (enExample) |
| JP (2) | JP5798036B2 (enExample) |
| KR (1) | KR101695329B1 (enExample) |
| CN (2) | CN102209729B (enExample) |
| AU (1) | AU2009313039B2 (enExample) |
| BR (1) | BRPI0921341A2 (enExample) |
| CA (1) | CA2742242C (enExample) |
| CY (3) | CY1116160T1 (enExample) |
| DK (3) | DK2918604T3 (enExample) |
| ES (3) | ES2748126T3 (enExample) |
| HK (1) | HK1255590B (enExample) |
| HR (3) | HRP20150400T1 (enExample) |
| HU (3) | HUE025452T2 (enExample) |
| IL (1) | IL212651A (enExample) |
| LT (2) | LT2918604T (enExample) |
| MX (1) | MX2011002931A (enExample) |
| NO (1) | NO2918604T3 (enExample) |
| NZ (1) | NZ591312A (enExample) |
| PL (3) | PL2918604T3 (enExample) |
| PT (3) | PT2344539E (enExample) |
| RS (3) | RS53980B1 (enExample) |
| RU (3) | RU2536933C2 (enExample) |
| SG (1) | SG195549A1 (enExample) |
| SI (3) | SI2344539T1 (enExample) |
| SM (3) | SMT201800149T1 (enExample) |
| WO (1) | WO2010052013A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2677324C2 (ru) * | 2008-11-07 | 2019-01-16 | Эмджен Рисерч (Мьюник) Гмбх | Новое лечение острого лимфобластного лейкоза у детей |
Families Citing this family (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6358588A (ja) * | 1986-08-29 | 1988-03-14 | Toshiba Corp | バ−コ−ド読取装置 |
| DE10254601A1 (de) | 2002-11-22 | 2004-06-03 | Ganymed Pharmaceuticals Ag | Differentiell in Tumoren exprimierte Genprodukte und deren Verwendung |
| ES2565439T3 (es) * | 2009-01-09 | 2016-04-04 | Oxford Biomedica (Uk) Ltd | Factores |
| US20150231241A1 (en) * | 2012-08-14 | 2015-08-20 | Ibc Pharmaceuticals, Inc. | Combination therapy for inducing immune response to disease |
| JOP20200236A1 (ar) | 2012-09-21 | 2017-06-16 | Regeneron Pharma | الأجسام المضادة لمضاد cd3 وجزيئات ربط الأنتيجين ثنائية التحديد التي تربط cd3 وcd20 واستخداماتها |
| HK1209434A1 (en) * | 2012-11-13 | 2016-04-01 | Astellas Pharma Inc. | Agents for treatment of claudin expressing cancer diseases |
| TWI635098B (zh) | 2013-02-01 | 2018-09-11 | 再生元醫藥公司 | 含嵌合恆定區之抗體 |
| CN103131777A (zh) * | 2013-02-05 | 2013-06-05 | 南京艾迪康医学检验所有限公司 | 用于检测e2a-pbx融合基因相对表达量的试剂盒 |
| WO2015121383A1 (en) | 2014-02-12 | 2015-08-20 | Michael Uhlin | Bispecific antibodies for use in stem cell transplantation |
| TWI701042B (zh) | 2014-03-19 | 2020-08-11 | 美商再生元醫藥公司 | 用於腫瘤治療之方法及抗體組成物 |
| LT3149480T (lt) | 2014-05-30 | 2019-04-25 | Amgen Research (Munich) Gmbh | B ląstelių pirmtakų ūmia limfoblastine leukemija (all) sergančių pacientų rizikos stratifikacijos būdas |
| EP3175001B1 (en) | 2014-07-30 | 2021-02-10 | Mor Research Applications Ltd. | Prognostic methods and systems of treatment for acute lymphoblastic leukemia |
| WO2016061368A1 (en) * | 2014-10-15 | 2016-04-21 | The Children's Hospital Of Philadelphia | Compositions and methods for treating b-lymphoid malignancies |
| SMT202000438T1 (it) | 2014-11-17 | 2020-09-10 | Regeneron Pharma | Metodi per trattamento di tumore usando l'anticorpo bispecifico cd3xcd20 |
| AU2016215110A1 (en) * | 2015-02-05 | 2017-09-21 | Stc.Unm | Anti-pre-BCR antagonists and methods |
| PL3277725T3 (pl) | 2015-03-30 | 2021-06-14 | Regeneron Pharmaceuticals, Inc. | Regiony stałe łańcucha ciężkiego o zmniejszonym wiązaniu do receptorów Fc gamma |
| PT3280729T (pt) | 2015-04-08 | 2022-08-01 | Novartis Ag | Terapias para cd20, terapias para cd22 e terapias de combinação com uma célula que expressa o recetor de antigénio quimérico (car) para cd19 |
| DK3298042T3 (da) * | 2015-05-20 | 2025-02-24 | Amgen Res Munich Gmbh | B-celledepletering som en diagnostisk markør |
| KR20180087401A (ko) * | 2015-12-22 | 2018-08-01 | 리제너론 파아마슈티컬스, 인크. | 급성 림프아구성 백혈병을 치료하기 위한 이중특이성 항-cd20/항-cd3 항체 |
| GB201602974D0 (en) * | 2016-02-19 | 2016-04-06 | Clube Jasper R | Engineered cells & methods (1) |
| BR112018068628A2 (pt) * | 2016-03-14 | 2019-07-30 | Millennium Pharm Inc | método de prevenção de doença do enxerto contra hospedeiro |
| MA45341A (fr) * | 2016-06-06 | 2019-04-10 | Hutchinson Fred Cancer Res | Procédés de traitement de malignités de lymphocytes b au moyen d'une thérapie cellulaire adoptive |
| JP2020511462A (ja) | 2016-12-03 | 2020-04-16 | ジュノー セラピューティクス インコーポレイテッド | キナーゼ阻害剤との組み合わせで治療用t細胞を使用するための方法および組成物 |
| UY37726A (es) * | 2017-05-05 | 2018-11-30 | Amgen Inc | Composición farmacéutica que comprende constructos de anticuerpos biespecíficos para almacenamiento y administración mejorados |
| MX2019014268A (es) | 2017-06-02 | 2020-08-03 | Juno Therapeutics Inc | Artículos de manufactura y métodos para tratamiento usando terapia celular adoptiva. |
| US12031975B2 (en) | 2017-11-01 | 2024-07-09 | Juno Therapeutics, Inc. | Methods of assessing or monitoring a response to a cell therapy |
| KR102083481B1 (ko) | 2018-03-22 | 2020-03-02 | 강원대학교산학협력단 | 아연-키토산 나노 입자를 포함한 급성 림프구성 백혈병 치료용 약학조성물 |
| LT3844189T (lt) | 2018-08-31 | 2025-02-25 | Regeneron Pharmaceuticals, Inc. | Cd3/cd20 bispecifinių antikūnų dozavimo strategija, mažinanti citokinų išsiskyrimo sindromą |
| BR112021014255A2 (pt) * | 2019-01-30 | 2022-01-18 | Wistar Inst | Molécula de ácido nucleico, composição, e, método de prevenção ou tratamento de uma doença ou distúrbio |
| IL322315A (en) | 2019-05-14 | 2025-09-01 | Provention Bio Inc | Methods and preparations for preventing type 1 diabetes |
| CU20210096A7 (es) | 2019-05-21 | 2022-06-06 | Novartis Ag | Moléculas de unión a cd19 |
| TWI809286B (zh) | 2019-07-05 | 2023-07-21 | 日商小野藥品工業股份有限公司 | 以pd-1/cd3雙特異性蛋白質所進行之血液性癌症治療 |
| MX2022015872A (es) | 2020-06-11 | 2023-05-16 | Provention Bio Inc | Metodos y composiciones para prevenir diabetes tipo 1. |
| IL305188A (en) | 2021-02-16 | 2023-10-01 | Childrens Health Care D/B/A Childrens Minnesota | Methods for treating B-ALL by administering a pre-BCR complex antagonist |
| WO2023062188A1 (en) | 2021-10-15 | 2023-04-20 | Amgen Research (Munich) Gmbh | Subcutaneous administration of cd19-binding t cell engaging antibodies |
| US20250146017A1 (en) | 2023-11-06 | 2025-05-08 | City Of Hope | Methods comprising oncolytic viruses expressing cd19t and bispecific t cell engagers |
| WO2025137344A1 (en) | 2023-12-20 | 2025-06-26 | Bristol-Myers Squibb Company | Antibodies targeting il-18 receptor beta (il-18rβ) and related methods |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007068354A1 (en) * | 2005-12-16 | 2007-06-21 | Micromet Ag | Means and methods for the treatment of tumorous diseases |
| RU2005141512A (ru) * | 2003-05-31 | 2007-07-20 | Микромет Аг (De) | Фармацевтические композиции, включающие биспецифические анти-cd3, анти-cd19 конструкции антител для лечения расстройств, связанных с b-клетками |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1998008875A1 (de) * | 1996-08-28 | 1998-03-05 | Viva Diagnostika Diagnostische Produkte Gmbh | Kombinationspräparationen und ihre verwendung in der immundiagnostik und immuntherapie |
| JP4169478B2 (ja) * | 1998-04-21 | 2008-10-22 | マイクロメット アーゲー | Cd19×cd3特異的ポリペプチドおよびその使用 |
| US8076459B2 (en) * | 2003-10-16 | 2011-12-13 | Micromet Ag | Multispecfic deimmunized CD3-binders |
| ES2402591T3 (es) * | 2006-08-14 | 2013-05-07 | Xencor Inc. | Anticuerpos optimizados que seleccionan como diana CD19 |
| ES2582603T5 (es) * | 2008-10-01 | 2022-12-02 | Amgen Res Munich Gmbh | Anticuerpos biespecíficos de cadena sencilla con especificidad por antígenos diana de alto peso molecular |
| RS53980B1 (sr) * | 2008-11-07 | 2015-10-30 | Amgen Research (Munich) Gmbh | Tretiranje akutne limfoblastne leukemije kod dece |
| PL2511264T3 (pl) * | 2009-01-19 | 2015-08-31 | Abbvie Inc | Środki indukujące apoptozę do leczenia raka oraz chorób immunologicznych i autoimmunologicznych |
| AU2010204555B2 (en) * | 2009-01-19 | 2013-03-07 | Abbvie Inc. | Apoptosis-inducing agents for the treatment of cancer and immune and autoimmune diseases |
| CN102459236B (zh) * | 2009-05-27 | 2014-10-29 | Abbvie公司 | 激酶活性的嘧啶抑制剂 |
| CA2771984A1 (en) * | 2009-09-20 | 2011-03-24 | Abbott Laboratories | Abt-263 crystalline forms and solvates for use in treating bcl-2 protein related diseases |
| US8840888B2 (en) * | 2009-10-27 | 2014-09-23 | Micromet Ag | Dosage regimen for administering a CD19XCD3 bispecific antibody |
-
2009
- 2009-11-06 RS RS20150306A patent/RS53980B1/sr unknown
- 2009-11-06 DK DK15154416.0T patent/DK2918604T3/en active
- 2009-11-06 PT PT97647101T patent/PT2344539E/pt unknown
- 2009-11-06 DK DK09764710.1T patent/DK2344539T3/en active
- 2009-11-06 HU HUE09764710A patent/HUE025452T2/en unknown
- 2009-11-06 WO PCT/EP2009/007969 patent/WO2010052013A1/en not_active Ceased
- 2009-11-06 PL PL15154416T patent/PL2918604T3/pl unknown
- 2009-11-06 BR BRPI0921341A patent/BRPI0921341A2/pt not_active Application Discontinuation
- 2009-11-06 LT LTEP15154416.0T patent/LT2918604T/lt unknown
- 2009-11-06 CA CA2742242A patent/CA2742242C/en active Active
- 2009-11-06 RS RSP20191232 patent/RS59348B1/sr unknown
- 2009-11-06 ES ES17206868T patent/ES2748126T3/es active Active
- 2009-11-06 PL PL09764710T patent/PL2344539T3/pl unknown
- 2009-11-06 RU RU2011122827/10A patent/RU2536933C2/ru active
- 2009-11-06 AU AU2009313039A patent/AU2009313039B2/en active Active
- 2009-11-06 HU HUE15154416A patent/HUE036922T2/hu unknown
- 2009-11-06 LT LTEP17206868.6T patent/LT3330293T/lt unknown
- 2009-11-06 NO NO15154416A patent/NO2918604T3/no unknown
- 2009-11-06 US US13/127,538 patent/US20110262440A1/en not_active Abandoned
- 2009-11-06 SM SM20180149T patent/SMT201800149T1/it unknown
- 2009-11-06 ES ES15154416.0T patent/ES2662929T3/es active Active
- 2009-11-06 SI SI200931185T patent/SI2344539T1/sl unknown
- 2009-11-06 DK DK17206868.6T patent/DK3330293T3/da active
- 2009-11-06 CN CN200980144400.7A patent/CN102209729B/zh active Active
- 2009-11-06 ES ES09764710.1T patent/ES2535257T3/es active Active
- 2009-11-06 CN CN201610281293.4A patent/CN106390114A/zh active Pending
- 2009-11-06 HU HUE17206868A patent/HUE046222T2/hu unknown
- 2009-11-06 EP EP09764710.1A patent/EP2344539B1/en active Active
- 2009-11-06 SI SI200931817T patent/SI2918604T1/en unknown
- 2009-11-06 RS RS20180309A patent/RS56989B1/sr unknown
- 2009-11-06 KR KR1020117010642A patent/KR101695329B1/ko active Active
- 2009-11-06 PL PL17206868T patent/PL3330293T3/pl unknown
- 2009-11-06 SI SI200931995T patent/SI3330293T1/sl unknown
- 2009-11-06 NZ NZ591312A patent/NZ591312A/xx unknown
- 2009-11-06 MX MX2011002931A patent/MX2011002931A/es active IP Right Grant
- 2009-11-06 JP JP2011533632A patent/JP5798036B2/ja active Active
- 2009-11-06 PT PT151544160T patent/PT2918604T/pt unknown
- 2009-11-06 SM SM20190546T patent/SMT201900546T1/it unknown
- 2009-11-06 SG SG2013076310A patent/SG195549A1/en unknown
- 2009-11-06 EP EP19187179.7A patent/EP3594237A1/en active Pending
- 2009-11-06 EP EP15154416.0A patent/EP2918604B1/en active Active
- 2009-11-06 PT PT172068686T patent/PT3330293T/pt unknown
- 2009-11-06 EP EP17206868.6A patent/EP3330293B1/en active Active
- 2009-11-06 HR HRP20150400TT patent/HRP20150400T1/hr unknown
-
2011
- 2011-05-03 IL IL212651A patent/IL212651A/en active IP Right Grant
-
2014
- 2014-10-13 RU RU2014141180A patent/RU2677324C2/ru active
-
2015
- 2015-03-31 CY CY20151100317T patent/CY1116160T1/el unknown
- 2015-04-27 SM SM201500104T patent/SMT201500104B/xx unknown
- 2015-08-20 JP JP2015163116A patent/JP6130451B2/ja active Active
-
2018
- 2018-03-12 HR HRP20180426TT patent/HRP20180426T1/hr unknown
- 2018-03-14 CY CY20181100310T patent/CY1120022T1/el unknown
- 2018-11-16 HK HK18114719.1A patent/HK1255590B/en unknown
- 2018-12-14 RU RU2018144313A patent/RU2736802C2/ru active
-
2019
- 2019-09-20 HR HRP20191715 patent/HRP20191715T1/hr unknown
- 2019-10-03 CY CY20191101035T patent/CY1122428T1/el unknown
-
2022
- 2022-09-16 US US17/932,739 patent/US20230235053A1/en active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2005141512A (ru) * | 2003-05-31 | 2007-07-20 | Микромет Аг (De) | Фармацевтические композиции, включающие биспецифические анти-cd3, анти-cd19 конструкции антител для лечения расстройств, связанных с b-клетками |
| WO2007068354A1 (en) * | 2005-12-16 | 2007-06-21 | Micromet Ag | Means and methods for the treatment of tumorous diseases |
Non-Patent Citations (2)
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2677324C2 (ru) * | 2008-11-07 | 2019-01-16 | Эмджен Рисерч (Мьюник) Гмбх | Новое лечение острого лимфобластного лейкоза у детей |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2536933C2 (ru) | Новое лечение острого лимфобластного лейкоза у детей | |
| US20240109964A1 (en) | Treatment of acute lymphoblastic leukemia | |
| HK1255590A1 (en) | Treatment of pediatric acute lymphoblastic leukemia with bispecific antibodies against cd3xcd19 | |
| HK40022438A (en) | Treatment of pediatric acute lymphoblastic leukemia | |
| HK1215258B (en) | Treatment of pediatric acute lymphoblastic leukemia | |
| HK1158667B (en) | Treatment of pediatric acute lymphoblastic leukemia |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| HZ9A | Changing address for correspondence with an applicant |