KR20200104284A - Hpv-특이적 결합 분자 - Google Patents
Hpv-특이적 결합 분자 Download PDFInfo
- Publication number
- KR20200104284A KR20200104284A KR1020207012701A KR20207012701A KR20200104284A KR 20200104284 A KR20200104284 A KR 20200104284A KR 1020207012701 A KR1020207012701 A KR 1020207012701A KR 20207012701 A KR20207012701 A KR 20207012701A KR 20200104284 A KR20200104284 A KR 20200104284A
- Authority
- KR
- South Korea
- Prior art keywords
- seq
- cdr
- amino acid
- region
- nos
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 230000009870 specific binding Effects 0.000 title description 6
- 230000027455 binding Effects 0.000 claims abstract description 341
- 238000009739 binding Methods 0.000 claims abstract description 341
- 238000000034 method Methods 0.000 claims abstract description 302
- 239000000427 antigen Substances 0.000 claims abstract description 290
- 102000036639 antigens Human genes 0.000 claims abstract description 290
- 108091007433 antigens Proteins 0.000 claims abstract description 290
- 239000012634 fragment Substances 0.000 claims abstract description 266
- 241000701806 Human papillomavirus Species 0.000 claims abstract description 76
- 239000000203 mixture Substances 0.000 claims abstract description 76
- 238000011282 treatment Methods 0.000 claims abstract description 14
- 108010047041 Complementarity Determining Regions Proteins 0.000 claims description 999
- 108091008874 T cell receptors Proteins 0.000 claims description 728
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 claims description 723
- 150000001413 amino acids Chemical group 0.000 claims description 500
- 210000004027 cell Anatomy 0.000 claims description 307
- 108090000623 proteins and genes Proteins 0.000 claims description 154
- 150000007523 nucleic acids Chemical class 0.000 claims description 136
- 239000002773 nucleotide Substances 0.000 claims description 135
- 125000003729 nucleotide group Chemical group 0.000 claims description 135
- 102100029452 T cell receptor alpha chain constant Human genes 0.000 claims description 130
- 229910052717 sulfur Inorganic materials 0.000 claims description 119
- 235000001014 amino acid Nutrition 0.000 claims description 101
- 229910052757 nitrogen Inorganic materials 0.000 claims description 97
- 102000040430 polynucleotide Human genes 0.000 claims description 86
- 108091033319 polynucleotide Proteins 0.000 claims description 86
- 239000002157 polynucleotide Substances 0.000 claims description 86
- 229910052727 yttrium Inorganic materials 0.000 claims description 85
- 229910052720 vanadium Inorganic materials 0.000 claims description 79
- 108091028043 Nucleic acid sequence Proteins 0.000 claims description 78
- 229910052731 fluorine Inorganic materials 0.000 claims description 65
- 101000634853 Homo sapiens T cell receptor alpha chain constant Proteins 0.000 claims description 63
- 102000039446 nucleic acids Human genes 0.000 claims description 61
- 108020004707 nucleic acids Proteins 0.000 claims description 61
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 61
- 108700026244 Open Reading Frames Proteins 0.000 claims description 59
- 229910052698 phosphorus Inorganic materials 0.000 claims description 57
- 230000008685 targeting Effects 0.000 claims description 51
- 108020005004 Guide RNA Proteins 0.000 claims description 44
- 230000036961 partial effect Effects 0.000 claims description 43
- 230000014509 gene expression Effects 0.000 claims description 42
- 239000013598 vector Substances 0.000 claims description 39
- 108091033409 CRISPR Proteins 0.000 claims description 38
- 239000003795 chemical substances by application Substances 0.000 claims description 38
- 230000000295 complement effect Effects 0.000 claims description 38
- 229910052740 iodine Inorganic materials 0.000 claims description 34
- 229910052722 tritium Inorganic materials 0.000 claims description 34
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 33
- 241000282414 Homo sapiens Species 0.000 claims description 30
- 230000034431 double-strand break repair via homologous recombination Effects 0.000 claims description 30
- 210000001744 T-lymphocyte Anatomy 0.000 claims description 29
- 108700019146 Transgenes Proteins 0.000 claims description 28
- 229910052805 deuterium Inorganic materials 0.000 claims description 27
- 229910052739 hydrogen Inorganic materials 0.000 claims description 26
- 210000001266 CD8-positive T-lymphocyte Anatomy 0.000 claims description 25
- 230000004048 modification Effects 0.000 claims description 24
- 238000012986 modification Methods 0.000 claims description 24
- 102100037272 T cell receptor beta constant 1 Human genes 0.000 claims description 21
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 claims description 20
- 235000018102 proteins Nutrition 0.000 claims description 19
- 102000004169 proteins and genes Human genes 0.000 claims description 19
- 108700024394 Exon Proteins 0.000 claims description 17
- 102100037298 T cell receptor beta constant 2 Human genes 0.000 claims description 17
- 201000010099 disease Diseases 0.000 claims description 17
- 235000018417 cysteine Nutrition 0.000 claims description 16
- 208000035475 disorder Diseases 0.000 claims description 16
- 230000010354 integration Effects 0.000 claims description 16
- 101800001494 Protease 2A Proteins 0.000 claims description 14
- 101800001066 Protein 2A Proteins 0.000 claims description 14
- 230000001747 exhibiting effect Effects 0.000 claims description 14
- 206010028980 Neoplasm Diseases 0.000 claims description 12
- 108010076504 Protein Sorting Signals Proteins 0.000 claims description 12
- 102000004389 Ribonucleoproteins Human genes 0.000 claims description 12
- 108010081734 Ribonucleoproteins Proteins 0.000 claims description 12
- 241000193996 Streptococcus pyogenes Species 0.000 claims description 12
- 101710087279 T cell receptor beta constant 1 Proteins 0.000 claims description 12
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 claims description 12
- 230000001105 regulatory effect Effects 0.000 claims description 12
- 238000011144 upstream manufacturing Methods 0.000 claims description 12
- 101710087287 T cell receptor beta constant 2 Proteins 0.000 claims description 11
- 229910052700 potassium Inorganic materials 0.000 claims description 11
- 102000005962 receptors Human genes 0.000 claims description 11
- 108020003175 receptors Proteins 0.000 claims description 11
- 239000013603 viral vector Substances 0.000 claims description 11
- 108091026890 Coding region Proteins 0.000 claims description 10
- 238000012217 deletion Methods 0.000 claims description 10
- 230000037430 deletion Effects 0.000 claims description 10
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 claims description 9
- 238000004519 manufacturing process Methods 0.000 claims description 9
- 229910052709 silver Inorganic materials 0.000 claims description 9
- 239000004332 silver Substances 0.000 claims description 9
- 108020005345 3' Untranslated Regions Proteins 0.000 claims description 8
- 238000003780 insertion Methods 0.000 claims description 8
- 230000037431 insertion Effects 0.000 claims description 8
- 108010074032 HLA-A2 Antigen Proteins 0.000 claims description 7
- 102000025850 HLA-A2 Antigen Human genes 0.000 claims description 7
- 201000011510 cancer Diseases 0.000 claims description 7
- 238000010361 transduction Methods 0.000 claims description 7
- 230000026683 transduction Effects 0.000 claims description 7
- 102000004190 Enzymes Human genes 0.000 claims description 6
- 108090000790 Enzymes Proteins 0.000 claims description 6
- 101710163270 Nuclease Proteins 0.000 claims description 6
- 238000004520 electroporation Methods 0.000 claims description 6
- 230000001293 nucleolytic effect Effects 0.000 claims description 6
- 239000013607 AAV vector Substances 0.000 claims description 4
- 108091035707 Consensus sequence Proteins 0.000 claims description 4
- 241000341655 Human papillomavirus type 16 Species 0.000 claims description 4
- 102000010292 Peptide Elongation Factor 1 Human genes 0.000 claims description 4
- 108010077524 Peptide Elongation Factor 1 Proteins 0.000 claims description 4
- 238000007906 compression Methods 0.000 claims description 4
- 230000006835 compression Effects 0.000 claims description 4
- 239000003623 enhancer Substances 0.000 claims description 4
- 230000004927 fusion Effects 0.000 claims description 4
- 238000000338 in vitro Methods 0.000 claims description 4
- 230000001939 inductive effect Effects 0.000 claims description 4
- 230000035772 mutation Effects 0.000 claims description 4
- 230000008488 polyadenylation Effects 0.000 claims description 4
- 108010017070 Zinc Finger Nucleases Proteins 0.000 claims description 3
- 101710185494 Zinc finger protein Proteins 0.000 claims description 3
- 102100023597 Zinc finger protein 816 Human genes 0.000 claims description 3
- 239000001506 calcium phosphate Substances 0.000 claims description 3
- 229910000389 calcium phosphate Inorganic materials 0.000 claims description 3
- 235000011010 calcium phosphates Nutrition 0.000 claims description 3
- 230000006378 damage Effects 0.000 claims description 3
- 239000003814 drug Substances 0.000 claims description 3
- 230000003834 intracellular effect Effects 0.000 claims description 3
- 239000002245 particle Substances 0.000 claims description 3
- 238000001890 transfection Methods 0.000 claims description 3
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 claims description 3
- 241001655883 Adeno-associated virus - 1 Species 0.000 claims description 2
- 241000702423 Adeno-associated virus - 2 Species 0.000 claims description 2
- 241000202702 Adeno-associated virus - 3 Species 0.000 claims description 2
- 241000580270 Adeno-associated virus - 4 Species 0.000 claims description 2
- 241001634120 Adeno-associated virus - 5 Species 0.000 claims description 2
- 241000972680 Adeno-associated virus - 6 Species 0.000 claims description 2
- 241001164823 Adeno-associated virus - 7 Species 0.000 claims description 2
- 241001164825 Adeno-associated virus - 8 Species 0.000 claims description 2
- 238000010356 CRISPR-Cas9 genome editing Methods 0.000 claims description 2
- 102000052510 DNA-Binding Proteins Human genes 0.000 claims description 2
- 230000004568 DNA-binding Effects 0.000 claims description 2
- 101710096438 DNA-binding protein Proteins 0.000 claims description 2
- 241000713772 Human immunodeficiency virus 1 Species 0.000 claims description 2
- 101150117561 TRBC2 gene Proteins 0.000 claims description 2
- 108010073062 Transcription Activator-Like Effectors Proteins 0.000 claims description 2
- 239000002299 complementary DNA Substances 0.000 claims description 2
- 239000013604 expression vector Substances 0.000 claims description 2
- 108020001507 fusion proteins Proteins 0.000 claims description 2
- 102000037865 fusion proteins Human genes 0.000 claims description 2
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 2
- 230000001177 retroviral effect Effects 0.000 claims description 2
- 108700026220 vif Genes Proteins 0.000 claims description 2
- 101000662909 Homo sapiens T cell receptor beta constant 1 Proteins 0.000 claims 9
- 101000662902 Homo sapiens T cell receptor beta constant 2 Proteins 0.000 claims 9
- 102220278926 rs759871071 Human genes 0.000 claims 4
- 230000002255 enzymatic effect Effects 0.000 claims 3
- 238000010354 CRISPR gene editing Methods 0.000 claims 2
- 108091081024 Start codon Proteins 0.000 claims 2
- 102000006707 alpha-beta T-Cell Antigen Receptors Human genes 0.000 claims 2
- 108010087408 alpha-beta T-Cell Antigen Receptors Proteins 0.000 claims 2
- 108020004999 messenger RNA Proteins 0.000 claims 2
- 241001663880 Gammaretrovirus Species 0.000 claims 1
- 108091092195 Intron Proteins 0.000 claims 1
- 101150053558 TRBC1 gene Proteins 0.000 claims 1
- 230000008878 coupling Effects 0.000 claims 1
- 238000010168 coupling process Methods 0.000 claims 1
- 238000005859 coupling reaction Methods 0.000 claims 1
- 230000013011 mating Effects 0.000 claims 1
- 229920002477 rna polymer Polymers 0.000 claims 1
- 101100540311 Human papillomavirus type 16 E6 gene Proteins 0.000 abstract description 2
- 101000767631 Human papillomavirus type 16 Protein E7 Proteins 0.000 abstract description 2
- 125000003275 alpha amino acid group Chemical group 0.000 description 381
- 229940024606 amino acid Drugs 0.000 description 34
- 102100034922 T-cell surface glycoprotein CD8 alpha chain Human genes 0.000 description 32
- 101000716102 Homo sapiens T-cell surface glycoprotein CD4 Proteins 0.000 description 29
- 102100036011 T-cell surface glycoprotein CD4 Human genes 0.000 description 29
- 108700018351 Major Histocompatibility Complex Proteins 0.000 description 18
- 241000699666 Mus <mouse, genus> Species 0.000 description 18
- 230000020382 suppression by virus of host antigen processing and presentation of peptide antigen via MHC class I Effects 0.000 description 18
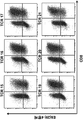
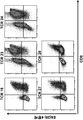
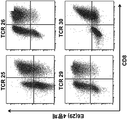
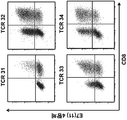
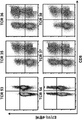
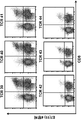
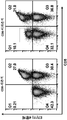
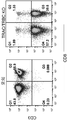
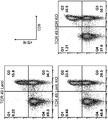
- 238000000684 flow cytometry Methods 0.000 description 15
- 238000006467 substitution reaction Methods 0.000 description 12
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical group NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 11
- 102000004196 processed proteins & peptides Human genes 0.000 description 8
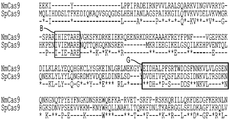
- 101100112922 Candida albicans CDR3 gene Proteins 0.000 description 7
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical group CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 7
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Chemical group CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 7
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 description 7
- 229920001184 polypeptide Polymers 0.000 description 7
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 6
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical group CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 6
- 239000012636 effector Substances 0.000 description 6
- 238000011534 incubation Methods 0.000 description 6
- 230000009261 transgenic effect Effects 0.000 description 6
- 108010019670 Chimeric Antigen Receptors Proteins 0.000 description 5
- 125000000998 L-alanino group Chemical group [H]N([*])[C@](C([H])([H])[H])([H])C(=O)O[H] 0.000 description 5
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical group OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 5
- 125000000174 L-prolyl group Chemical group [H]N1C([H])([H])C([H])([H])C([H])([H])[C@@]1([H])C(*)=O 0.000 description 5
- 125000000510 L-tryptophano group Chemical group [H]C1=C([H])C([H])=C2N([H])C([H])=C(C([H])([H])[C@@]([H])(C(O[H])=O)N([H])[*])C2=C1[H] 0.000 description 5
- 230000001461 cytolytic effect Effects 0.000 description 5
- 238000006471 dimerization reaction Methods 0.000 description 5
- 230000002068 genetic effect Effects 0.000 description 5
- 230000002209 hydrophobic effect Effects 0.000 description 5
- 230000001965 increasing effect Effects 0.000 description 5
- 230000001404 mediated effect Effects 0.000 description 5
- 230000011664 signaling Effects 0.000 description 5
- 108010088729 HLA-A*02:01 antigen Proteins 0.000 description 4
- 108060003951 Immunoglobulin Proteins 0.000 description 4
- 150000001945 cysteines Chemical class 0.000 description 4
- 102000018358 immunoglobulin Human genes 0.000 description 4
- 102220026974 rs111052004 Human genes 0.000 description 4
- 238000002864 sequence alignment Methods 0.000 description 4
- 238000010186 staining Methods 0.000 description 4
- 102100035360 Cerebellar degeneration-related antigen 1 Human genes 0.000 description 3
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 3
- 102000011786 HLA-A Antigens Human genes 0.000 description 3
- 108010075704 HLA-A Antigens Proteins 0.000 description 3
- 101000737793 Homo sapiens Cerebellar degeneration-related antigen 1 Proteins 0.000 description 3
- 241000713666 Lentivirus Species 0.000 description 3
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 3
- 210000005220 cytoplasmic tail Anatomy 0.000 description 3
- 229940088598 enzyme Drugs 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 239000000945 filler Substances 0.000 description 3
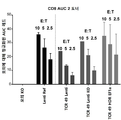
- 230000014828 interferon-gamma production Effects 0.000 description 3
- 239000003550 marker Substances 0.000 description 3
- 239000013642 negative control Substances 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 230000028327 secretion Effects 0.000 description 3
- 102100035361 Cerebellar degeneration-related protein 2 Human genes 0.000 description 2
- 206010008342 Cervix carcinoma Diseases 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- 102000008949 Histocompatibility Antigens Class I Human genes 0.000 description 2
- 108010088652 Histocompatibility Antigens Class I Proteins 0.000 description 2
- 101000737796 Homo sapiens Cerebellar degeneration-related protein 2 Proteins 0.000 description 2
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 2
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 2
- 108010074328 Interferon-gamma Proteins 0.000 description 2
- 102000008070 Interferon-gamma Human genes 0.000 description 2
- 241000588650 Neisseria meningitidis Species 0.000 description 2
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 2
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 2
- 241000700605 Viruses Species 0.000 description 2
- 125000000539 amino acid group Chemical group 0.000 description 2
- 230000000890 antigenic effect Effects 0.000 description 2
- 210000004899 c-terminal region Anatomy 0.000 description 2
- 210000000170 cell membrane Anatomy 0.000 description 2
- 201000010881 cervical cancer Diseases 0.000 description 2
- 108700010039 chimeric receptor Proteins 0.000 description 2
- 230000001086 cytosolic effect Effects 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 238000010348 incorporation Methods 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 229960003130 interferon gamma Drugs 0.000 description 2
- 230000002147 killing effect Effects 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 210000004379 membrane Anatomy 0.000 description 2
- 230000019491 signal transduction Effects 0.000 description 2
- 229910052721 tungsten Inorganic materials 0.000 description 2
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 1
- 208000007860 Anus Neoplasms Diseases 0.000 description 1
- 101100495352 Candida albicans CDR4 gene Proteins 0.000 description 1
- 108090000565 Capsid Proteins Proteins 0.000 description 1
- 102000011727 Caspases Human genes 0.000 description 1
- 108010076667 Caspases Proteins 0.000 description 1
- 102100023321 Ceruloplasmin Human genes 0.000 description 1
- 101150071673 E6 gene Proteins 0.000 description 1
- 101150013359 E7 gene Proteins 0.000 description 1
- 101000946860 Homo sapiens T-cell surface glycoprotein CD3 epsilon chain Proteins 0.000 description 1
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 1
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 1
- 101000954493 Human papillomavirus type 16 Protein E6 Proteins 0.000 description 1
- -1 Jores et al . Proteins 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- 125000000773 L-serino group Chemical group [H]OC(=O)[C@@]([H])(N([H])*)C([H])([H])O[H] 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 201000009906 Meningitis Diseases 0.000 description 1
- 101000686985 Mouse mammary tumor virus (strain C3H) Protein PR73 Proteins 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 108091007491 NSP3 Papain-like protease domains Proteins 0.000 description 1
- 241000588653 Neisseria Species 0.000 description 1
- 102000043276 Oncogene Human genes 0.000 description 1
- 108700020796 Oncogene Proteins 0.000 description 1
- 206010057444 Oropharyngeal neoplasm Diseases 0.000 description 1
- 208000002471 Penile Neoplasms Diseases 0.000 description 1
- 201000000582 Retinoblastoma Diseases 0.000 description 1
- 241000194017 Streptococcus Species 0.000 description 1
- 241000194019 Streptococcus mutans Species 0.000 description 1
- 241000194020 Streptococcus thermophilus Species 0.000 description 1
- 102100035794 T-cell surface glycoprotein CD3 epsilon chain Human genes 0.000 description 1
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 1
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 description 1
- 238000010459 TALEN Methods 0.000 description 1
- 102000001742 Tumor Suppressor Proteins Human genes 0.000 description 1
- 108010040002 Tumor Suppressor Proteins Proteins 0.000 description 1
- 206010047741 Vulval cancer Diseases 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-N acetic acid Substances CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 238000011467 adoptive cell therapy Methods 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 230000036952 cancer formation Effects 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 230000006037 cell lysis Effects 0.000 description 1
- 238000001516 cell proliferation assay Methods 0.000 description 1
- 230000010307 cell transformation Effects 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 230000016396 cytokine production Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 238000002825 functional assay Methods 0.000 description 1
- 238000003209 gene knockout Methods 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 239000000833 heterodimer Substances 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 230000003100 immobilizing effect Effects 0.000 description 1
- 102000027596 immune receptors Human genes 0.000 description 1
- 108091008915 immune receptors Proteins 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 230000002998 immunogenetic effect Effects 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 230000002101 lytic effect Effects 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 210000004986 primary T-cell Anatomy 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 230000022983 regulation of cell cycle Effects 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 231100000617 superantigen Toxicity 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 208000013139 vaginal neoplasm Diseases 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 210000003905 vulva Anatomy 0.000 description 1
- 201000005102 vulva cancer Diseases 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
- C07K16/081—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses from DNA viruses
- C07K16/084—Papovaviridae, e.g. papillomavirus, polyomavirus, SV40, BK virus, JC virus
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/7051—T-cell receptor (TcR)-CD3 complex
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2809—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against the T-cell receptor (TcR)-CD3 complex
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/14—Blood; Artificial blood
- A61K35/17—Lymphocytes; B-cells; T-cells; Natural killer cells; Interferon-activated or cytokine-activated lymphocytes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/11—T-cells, e.g. tumour infiltrating lymphocytes [TIL] or regulatory T [Treg] cells; Lymphokine-activated killer [LAK] cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/30—Cellular immunotherapy characterised by the recombinant expression of specific molecules in the cells of the immune system
- A61K40/32—T-cell receptors [TCR]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/46—Viral antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2833—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against MHC-molecules, e.g. HLA-molecules
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/87—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation
- C12N15/90—Stable introduction of foreign DNA into chromosome
- C12N15/902—Stable introduction of foreign DNA into chromosome using homologous recombination
- C12N15/907—Stable introduction of foreign DNA into chromosome using homologous recombination in mammalian cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0636—T lymphocytes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/16—Hydrolases (3) acting on ester bonds (3.1)
- C12N9/22—Ribonucleases [RNase]; Deoxyribonucleases [DNase]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/27—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterized by targeting or presenting multiple antigens
- A61K2239/28—Expressing multiple CARs, TCRs or antigens
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/32—Immunoglobulins specific features characterized by aspects of specificity or valency specific for a neo-epitope on a complex, e.g. antibody-antigen or ligand-receptor
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/03—Fusion polypeptide containing a localisation/targetting motif containing a transmembrane segment
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/20—Type of nucleic acid involving clustered regularly interspaced short palindromic repeats [CRISPR]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2510/00—Genetically modified cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2800/00—Nucleic acids vectors
- C12N2800/80—Vectors containing sites for inducing double-stranded breaks, e.g. meganuclease restriction sites
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Engineering & Computer Science (AREA)
- Zoology (AREA)
- Biomedical Technology (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Wood Science & Technology (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Epidemiology (AREA)
- Cell Biology (AREA)
- Biophysics (AREA)
- General Engineering & Computer Science (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Microbiology (AREA)
- Virology (AREA)
- Hematology (AREA)
- Pharmacology & Pharmacy (AREA)
- Toxicology (AREA)
- Gastroenterology & Hepatology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Physics & Mathematics (AREA)
- Plant Pathology (AREA)
- Developmental Biology & Embryology (AREA)
- Mycology (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762567750P | 2017-10-03 | 2017-10-03 | |
| US62/567,750 | 2017-10-03 | ||
| US201762597411P | 2017-12-11 | 2017-12-11 | |
| US62/597,411 | 2017-12-11 | ||
| US201862653529P | 2018-04-05 | 2018-04-05 | |
| US62/653,529 | 2018-04-05 | ||
| PCT/US2018/053650 WO2019070541A1 (en) | 2017-10-03 | 2018-09-28 | HPV-SPECIFIC BINDING MOLECULES |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20200104284A true KR20200104284A (ko) | 2020-09-03 |
Family
ID=63963486
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207012701A Withdrawn KR20200104284A (ko) | 2017-10-03 | 2018-09-28 | Hpv-특이적 결합 분자 |
Country Status (13)
| Country | Link |
|---|---|
| US (1) | US11952408B2 (enExample) |
| EP (2) | EP4215543A3 (enExample) |
| JP (2) | JP2020537515A (enExample) |
| KR (1) | KR20200104284A (enExample) |
| CN (1) | CN111954679A (enExample) |
| AU (1) | AU2018345539A1 (enExample) |
| BR (1) | BR112020006643A2 (enExample) |
| CA (1) | CA3080546A1 (enExample) |
| IL (1) | IL273631A (enExample) |
| MA (1) | MA50613A (enExample) |
| MX (1) | MX2020003536A (enExample) |
| SG (1) | SG11202002728VA (enExample) |
| WO (1) | WO2019070541A1 (enExample) |
Families Citing this family (47)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10704021B2 (en) | 2012-03-15 | 2020-07-07 | Flodesign Sonics, Inc. | Acoustic perfusion devices |
| WO2015105955A1 (en) | 2014-01-08 | 2015-07-16 | Flodesign Sonics, Inc. | Acoustophoresis device with dual acoustophoretic chamber |
| US11377651B2 (en) | 2016-10-19 | 2022-07-05 | Flodesign Sonics, Inc. | Cell therapy processes utilizing acoustophoresis |
| US11708572B2 (en) | 2015-04-29 | 2023-07-25 | Flodesign Sonics, Inc. | Acoustic cell separation techniques and processes |
| AU2016363025B2 (en) | 2015-12-03 | 2021-04-08 | Juno Therapeutics, Inc. | Modified chimeric receptors and related compositions and methods |
| WO2018067618A1 (en) | 2016-10-03 | 2018-04-12 | Juno Therapeutics, Inc. | Hpv-specific binding molecules |
| IL303806B2 (en) | 2016-12-22 | 2024-05-01 | Cue Biopharma Inc | Multimeric polypeptides modulate T cells and methods for their use |
| WO2018129474A1 (en) | 2017-01-09 | 2018-07-12 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| WO2018170168A1 (en) | 2017-03-15 | 2018-09-20 | Cue Biopharma, Inc. | Methods for modulating an immune response |
| BR112020009889A2 (pt) | 2017-12-14 | 2020-11-03 | Flodesign Sonics, Inc. | acionador e controlador de transdutor acústico |
| BR112020019313A2 (pt) | 2018-04-05 | 2021-01-05 | Juno Therapeutics Inc | Receptores de células t e células modificadas que expressam os mesmos |
| KR20210029707A (ko) * | 2018-04-05 | 2021-03-16 | 주노 쎄러퓨티크스 인코퍼레이티드 | 재조합 수용체를 발현하는 세포의 생산 방법 및 관련 조성물 |
| AU2019362879A1 (en) * | 2018-10-16 | 2021-05-27 | Intellia Therapeutics, Inc. | Compositions and methods for immunotherapy |
| MA55811A (fr) * | 2019-05-01 | 2022-03-09 | Editas Medicine Inc | Cellules exprimant un récepteur recombinant à base d'un locus modifié du tgfbr2, et polynucléotides et méthodes associés |
| PE20220164A1 (es) * | 2019-05-27 | 2022-01-28 | Immatics Us Inc | Vectores viricos y uso de los mismos en terapias celulares adoptivas |
| CN113072635B (zh) * | 2020-01-06 | 2023-08-25 | 香雪生命科学技术(广东)有限公司 | 一种识别hpv抗原的t细胞受体及其编码序列 |
| CN113321726B (zh) * | 2020-02-28 | 2024-05-28 | 香雪生命科学技术(广东)有限公司 | 一种识别hpv的t细胞受体 |
| US20230348558A1 (en) * | 2020-03-23 | 2023-11-02 | The Council Of The Queensland Institute Of Medical Research | Compositions and methods for targeting hpv-infected cells |
| MX2022012062A (es) * | 2020-03-27 | 2023-01-11 | 2Seventy Bio Inc | Receptores de linfocitos t. |
| CN115776991A (zh) * | 2020-05-07 | 2023-03-10 | 华夏英泰(北京)生物技术有限公司 | 改进的t细胞受体-共刺激分子嵌合物 |
| IL296209A (en) | 2020-05-12 | 2022-11-01 | Cue Biopharma Inc | Multimeric t-cell modulatory polypeptides and methods of using them |
| CN113801217B (zh) * | 2020-06-17 | 2025-12-23 | 香雪生命科学技术(广东)有限公司 | 一种识别hpv抗原的高亲和力t细胞受体 |
| JP2023541366A (ja) | 2020-09-09 | 2023-10-02 | キュー バイオファーマ, インコーポレイテッド | 1型真性糖尿病(t1d)を治療するためのmhcクラスii t細胞調節多量体ポリペプチド及びその使用方法 |
| WO2022060904A1 (en) | 2020-09-16 | 2022-03-24 | Obsidian Therapeutics, Inc. | Compositions and methods for expression of t-cell receptors with small molecule-regulated cd40l in t cells |
| WO2022093694A1 (en) * | 2020-10-26 | 2022-05-05 | A2 Biotherapeutics, Inc. | Polypeptides targeting hpv peptide-mhc complexes and methods of use thereof |
| WO2022098850A1 (en) * | 2020-11-05 | 2022-05-12 | Board Of Regents, The University Of Texas System | Engineered t cell receptors targeting egfr antigens and methods of use |
| WO2022099032A1 (en) * | 2020-11-06 | 2022-05-12 | Bioventures, Llc | T cells and bifunctional protein against human papillomavirus |
| CN114539385A (zh) * | 2020-11-26 | 2022-05-27 | 香雪生命科学技术(广东)有限公司 | 一种识别hpv抗原的tcr |
| CA3167637A1 (en) * | 2020-12-21 | 2022-06-30 | Eric Escobar-Cabrera | Stabilized tcr constructs and methods of use |
| EP4301755A1 (en) | 2021-03-03 | 2024-01-10 | Juno Therapeutics, Inc. | Combination of a t cell therapy and a dgk inhibitor |
| EP4334361A1 (en) | 2021-05-05 | 2024-03-13 | Immatics Biotechnologies GmbH | Antigen binding proteins specifically binding prame |
| CN115677846A (zh) * | 2021-07-27 | 2023-02-03 | 香雪生命科学技术(广东)有限公司 | 针对抗原ssx2的高亲和力t细胞受体 |
| US20240424101A1 (en) * | 2021-09-07 | 2024-12-26 | Corregene Biotechnology Co., Ltd. | Antigen binding proteins and uses thereof |
| CN115819555A (zh) * | 2021-09-17 | 2023-03-21 | 香雪生命科学技术(广东)有限公司 | 一种识别ssx2的高亲和力tcr |
| WO2023050063A1 (zh) * | 2021-09-28 | 2023-04-06 | 溧阳瑅赛生物医药有限公司 | 一种识别hla-a*02:01/e629-38的tcr及其应用 |
| CN113789304B (zh) * | 2021-10-14 | 2023-03-31 | 深圳大学总医院 | 高亲和力tcr及其应用 |
| CN113912701B (zh) * | 2021-10-14 | 2024-07-19 | 深圳大学总医院 | Tcr及其在诊断/治疗中的应用 |
| WO2023081900A1 (en) | 2021-11-08 | 2023-05-11 | Juno Therapeutics, Inc. | Engineered t cells expressing a recombinant t cell receptor (tcr) and related systems and methods |
| MX2024005743A (es) * | 2021-11-10 | 2024-05-27 | Tscan Therapeutics Inc | Proteinas de union que reconocen al antigeno hpv16 e7 y usos de las mismas. |
| CN116836261A (zh) * | 2022-03-24 | 2023-10-03 | 香雪生命科学技术(广东)有限公司 | 一种识别mage-a4抗原的高亲和力tcr及其序列和应用 |
| WO2023196884A1 (en) | 2022-04-06 | 2023-10-12 | Juno Therapeutics, Inc. | Detection assay for human papillomavirus (hpv) type 16 (hpv-16) |
| WO2023212551A1 (en) * | 2022-04-28 | 2023-11-02 | Cue Biopharma, Inc. | Modified cytotoxic t cells and methods of use thereof |
| CN120152717A (zh) | 2022-09-08 | 2025-06-13 | 朱诺治疗学股份有限公司 | T细胞疗法和连续或间歇dgk抑制剂给药的组合 |
| CN116589557B (zh) * | 2023-03-15 | 2024-04-02 | 北京大学人民医院 | 一种肿瘤新生抗原特异性tcr及其应用 |
| WO2025137646A1 (en) | 2023-12-22 | 2025-06-26 | Recode Therapeutics, Inc. | Gene editing methods and compositions for treating cystic fibrosis |
| WO2025226774A1 (en) * | 2024-04-24 | 2025-10-30 | Bluesphere Bio, Inc. | T cell receptors targeting minor histocompatibility antigen acc-1 |
| US20250345431A1 (en) | 2024-05-10 | 2025-11-13 | Juno Therapeutics, Inc. | Genetically engineered t cells expressing a cd19 chimeric antigen receptor (car) and uses thereof for allogeneic cell therapy |
Family Cites Families (184)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4235871A (en) | 1978-02-24 | 1980-11-25 | Papahadjopoulos Demetrios P | Method of encapsulating biologically active materials in lipid vesicles |
| US4452773A (en) | 1982-04-05 | 1984-06-05 | Canadian Patents And Development Limited | Magnetic iron-dextran microspheres |
| US4501728A (en) | 1983-01-06 | 1985-02-26 | Technology Unlimited, Inc. | Masking of liposomes from RES recognition |
| US5019369A (en) | 1984-10-22 | 1991-05-28 | Vestar, Inc. | Method of targeting tumors in humans |
| US4690915A (en) | 1985-08-08 | 1987-09-01 | The United States Of America As Represented By The Department Of Health And Human Services | Adoptive immunotherapy as a treatment modality in humans |
| US4795698A (en) | 1985-10-04 | 1989-01-03 | Immunicon Corporation | Magnetic-polymer particles |
| US4777239A (en) | 1986-07-10 | 1988-10-11 | The Board Of Trustees Of The Leland Stanford Junior University | Diagnostic peptides of human papilloma virus |
| IN165717B (enExample) | 1986-08-07 | 1989-12-23 | Battelle Memorial Institute | |
| US4837028A (en) | 1986-12-24 | 1989-06-06 | Liposome Technology, Inc. | Liposomes with enhanced circulation time |
| US5219740A (en) | 1987-02-13 | 1993-06-15 | Fred Hutchinson Cancer Research Center | Retroviral gene transfer into diploid fibroblasts for gene therapy |
| WO1990007380A2 (en) | 1988-12-28 | 1990-07-12 | Stefan Miltenyi | Methods and materials for high gradient magnetic separation of biological materials |
| US5283173A (en) | 1990-01-24 | 1994-02-01 | The Research Foundation Of State University Of New York | System to detect protein-protein interactions |
| US5200084A (en) | 1990-09-26 | 1993-04-06 | Immunicon Corporation | Apparatus and methods for magnetic separation |
| DE69128037T2 (de) | 1990-11-13 | 1998-05-07 | Immunex Corp., Seattle, Wash. | Bifunktionelle wählbare fusionsgene |
| DE4228458A1 (de) | 1992-08-27 | 1994-06-01 | Beiersdorf Ag | Multicistronische Expressionseinheiten und deren Verwendung |
| CA2163427A1 (en) | 1993-05-21 | 1994-12-08 | Stephen D. Lupton | Bifunctional selectable fusion genes based on the cytosine deaminase (cd) gene |
| US6140466A (en) | 1994-01-18 | 2000-10-31 | The Scripps Research Institute | Zinc finger protein derivatives and methods therefor |
| DE69534629D1 (de) | 1994-01-18 | 2005-12-29 | Scripps Research Inst | Derivate von zinkfingerproteinen und methoden |
| GB9824544D0 (en) | 1998-11-09 | 1999-01-06 | Medical Res Council | Screening system |
| DE69535829D1 (de) | 1994-08-20 | 2008-10-16 | Gendaq Ltd | Verbesserung in bezug auf bindungsproteine bei der erkennung von dna |
| US5827642A (en) | 1994-08-31 | 1998-10-27 | Fred Hutchinson Cancer Research Center | Rapid expansion method ("REM") for in vitro propagation of T lymphocytes |
| WO1996013593A2 (en) | 1994-10-26 | 1996-05-09 | Procept, Inc. | Soluble single chain t cell receptors |
| WO1996018105A1 (en) | 1994-12-06 | 1996-06-13 | The President And Fellows Of Harvard College | Single chain t-cell receptor |
| US5789538A (en) | 1995-02-03 | 1998-08-04 | Massachusetts Institute Of Technology | Zinc finger proteins with high affinity new DNA binding specificities |
| GB9603256D0 (en) | 1996-02-16 | 1996-04-17 | Wellcome Found | Antibodies |
| DE19608753C1 (de) | 1996-03-06 | 1997-06-26 | Medigene Gmbh | Transduktionssystem und seine Verwendung |
| US6451995B1 (en) | 1996-03-20 | 2002-09-17 | Sloan-Kettering Institute For Cancer Research | Single chain FV polynucleotide or peptide constructs of anti-ganglioside GD2 antibodies, cells expressing same and related methods |
| US5925523A (en) | 1996-08-23 | 1999-07-20 | President & Fellows Of Harvard College | Intraction trap assay, reagents and uses thereof |
| GB9710809D0 (en) | 1997-05-23 | 1997-07-23 | Medical Res Council | Nucleic acid binding proteins |
| GB9710807D0 (en) | 1997-05-23 | 1997-07-23 | Medical Res Council | Nucleic acid binding proteins |
| ES2244066T3 (es) | 1997-06-24 | 2005-12-01 | Genentech, Inc. | Procedimiento y composiciones de glicoproteinas galactosiladas. |
| ATE533784T1 (de) | 1997-10-02 | 2011-12-15 | Altor Bioscience Corp | Lösliche, einzelkettige proteine des t- zellrezeptors |
| US6183746B1 (en) | 1997-10-09 | 2001-02-06 | Zycos Inc. | Immunogenic peptides from the HPV E7 protein |
| ATE419009T1 (de) | 1997-10-31 | 2009-01-15 | Genentech Inc | Methoden und zusammensetzungen bestehend aus glykoprotein-glykoformen |
| DE69830927T2 (de) | 1997-12-12 | 2006-05-24 | Digene Corp. | Bewertung von humaner papillomvirus verwandter krankheit |
| PT2180007E (pt) | 1998-04-20 | 2013-11-25 | Roche Glycart Ag | Engenharia de glicosilação de anticorpos para melhorar a citotoxicidade celular dependente de anticorpos |
| WO1999060120A2 (en) | 1998-05-19 | 1999-11-25 | Avidex Limited | Soluble t cell receptor |
| WO2000014257A1 (en) | 1998-09-04 | 2000-03-16 | Sloan-Kettering Institute For Cancer Research | Fusion receptors specific for prostate-specific membrane antigen and uses thereof |
| US6140081A (en) | 1998-10-16 | 2000-10-31 | The Scripps Research Institute | Zinc finger binding domains for GNN |
| AU2472400A (en) | 1998-10-20 | 2000-05-08 | City Of Hope | CD20-specific redirected T cells and their use in cellular immunotherapy of CD20+ malignancies |
| US6453242B1 (en) | 1999-01-12 | 2002-09-17 | Sangamo Biosciences, Inc. | Selection of sites for targeting by zinc finger proteins and methods of designing zinc finger proteins to bind to preselected sites |
| US6534261B1 (en) | 1999-01-12 | 2003-03-18 | Sangamo Biosciences, Inc. | Regulation of endogenous gene expression in cells using zinc finger proteins |
| ES2568899T3 (es) | 1999-04-09 | 2016-05-05 | Kyowa Hakko Kirin Co., Ltd. | Procedimiento para controlar la actividad de una molécula inmunofuncional |
| SG143935A1 (en) | 1999-05-06 | 2008-07-29 | Univ Wake Forest | Compositions and methods for identifying antigens which elicit an immune response |
| AU7950400A (en) | 1999-10-19 | 2001-04-30 | Kyowa Hakko Kogyo Co. Ltd. | Process for producing polypeptide |
| US20020061512A1 (en) | 2000-02-18 | 2002-05-23 | Kim Jin-Soo | Zinc finger domains and methods of identifying same |
| WO2001088197A2 (en) | 2000-05-16 | 2001-11-22 | Massachusetts Institute Of Technology | Methods and compositions for interaction trap assays |
| WO2001094944A2 (en) | 2000-06-02 | 2001-12-13 | Memorial Sloan-Kettering Cancer Center | Artificial antigen presenting cells and methods of use thereof |
| JP2002060786A (ja) | 2000-08-23 | 2002-02-26 | Kao Corp | 硬質表面用殺菌防汚剤 |
| US6946292B2 (en) | 2000-10-06 | 2005-09-20 | Kyowa Hakko Kogyo Co., Ltd. | Cells producing antibody compositions with increased antibody dependent cytotoxic activity |
| EP1331266B1 (en) | 2000-10-06 | 2017-01-04 | Kyowa Hakko Kirin Co., Ltd. | Cells producing antibody compositions |
| US7064191B2 (en) | 2000-10-06 | 2006-06-20 | Kyowa Hakko Kogyo Co., Ltd. | Process for purifying antibody |
| JP5312721B2 (ja) | 2000-11-07 | 2013-10-09 | シティ・オブ・ホープ | Cd19特異的再指向免疫細胞 |
| WO2002077012A2 (en) | 2001-03-23 | 2002-10-03 | The Government Of The United States Of America, Represented By The Secretary, Department Of Health And Human Services | Human papilloma virus immunoreative peptides |
| GB0108491D0 (en) | 2001-04-04 | 2001-05-23 | Gendaq Ltd | Engineering zinc fingers |
| US7070995B2 (en) | 2001-04-11 | 2006-07-04 | City Of Hope | CE7-specific redirected immune cells |
| US20090257994A1 (en) | 2001-04-30 | 2009-10-15 | City Of Hope | Chimeric immunoreceptor useful in treating human cancers |
| NZ603111A (en) | 2001-08-03 | 2014-05-30 | Roche Glycart Ag | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| EP1421177A4 (en) | 2001-08-20 | 2006-06-07 | Scripps Research Inst | ZINC FINGER FASTENING DOMAINS FOR CNN |
| DE60203125T2 (de) | 2001-08-31 | 2006-04-06 | Avidex Ltd., Abingdon | Löslicher t zell rezeptor |
| ES2326964T3 (es) | 2001-10-25 | 2009-10-22 | Genentech, Inc. | Composiciones de glicoproteina. |
| US7939059B2 (en) | 2001-12-10 | 2011-05-10 | California Institute Of Technology | Method for the generation of antigen-specific lymphocytes |
| US20040093621A1 (en) | 2001-12-25 | 2004-05-13 | Kyowa Hakko Kogyo Co., Ltd | Antibody composition which specifically binds to CD20 |
| US20030170238A1 (en) | 2002-03-07 | 2003-09-11 | Gruenberg Micheal L. | Re-activated T-cells for adoptive immunotherapy |
| JPWO2003085119A1 (ja) | 2002-04-09 | 2005-08-11 | 協和醗酵工業株式会社 | 抗体組成物のFcγ受容体IIIaに対する結合活性を高める方法 |
| AU2003236020B2 (en) | 2002-04-09 | 2009-03-19 | Kyowa Hakko Kirin Co., Ltd. | Cell with depression or deletion of the activity of protein participating in GDP-fucose transport |
| US20050031613A1 (en) | 2002-04-09 | 2005-02-10 | Kazuyasu Nakamura | Therapeutic agent for patients having human FcgammaRIIIa |
| EP1500400A4 (en) | 2002-04-09 | 2006-10-11 | Kyowa Hakko Kogyo Kk | MEDICAMENT CONTAINING ANTIBODY COMPOSITION |
| JPWO2003085118A1 (ja) | 2002-04-09 | 2005-08-11 | 協和醗酵工業株式会社 | 抗体組成物の製造方法 |
| BR0309145A (pt) | 2002-04-09 | 2005-02-01 | Kyowa Hakko Kogyo Kk | Células das quais o genoma é modificado |
| US7446190B2 (en) | 2002-05-28 | 2008-11-04 | Sloan-Kettering Institute For Cancer Research | Nucleic acids encoding chimeric T cell receptors |
| WO2004033685A1 (en) | 2002-10-09 | 2004-04-22 | Avidex Ltd | Single chain recombinant t cell receptors |
| DE60332957D1 (de) | 2002-12-16 | 2010-07-22 | Genentech Inc | Immunoglobulinvarianten und deren verwendungen |
| US20050129671A1 (en) | 2003-03-11 | 2005-06-16 | City Of Hope | Mammalian antigen-presenting T cells and bi-specific T cells |
| EP1688439A4 (en) | 2003-10-08 | 2007-12-19 | Kyowa Hakko Kogyo Kk | COMPOSITION OF CONDENSED PROTEINS |
| US20070134759A1 (en) | 2003-10-09 | 2007-06-14 | Harue Nishiya | Process for producing antibody composition by using rna inhibiting the function of alpha1,6-fucosyltransferase |
| US7435596B2 (en) | 2004-11-04 | 2008-10-14 | St. Jude Children's Research Hospital, Inc. | Modified cell line and method for expansion of NK cell |
| JP4653109B2 (ja) | 2003-11-05 | 2011-03-16 | ロシュ グリクアート アクチェンゲゼルシャフト | 高められたFcレセプター結合親和性及びエフェクター機能をもつCD20抗体 |
| JPWO2005053742A1 (ja) | 2003-12-04 | 2007-06-28 | 協和醗酵工業株式会社 | 抗体組成物を含有する医薬 |
| AU2004308964B2 (en) | 2003-12-23 | 2010-09-16 | Arbor Vita Corporation | Antibodies for oncogenic strains of HPV and methods of their use |
| DE602005022595D1 (de) | 2004-06-29 | 2010-09-09 | Immunocore Ltd | Einen modifizierten t-zellen-rezeptor exprimierende zellen |
| TR201808537T4 (tr) | 2004-09-23 | 2018-07-23 | Genentech Inc | Sistein değiştirilmiş antikorlar ve konjugatlar. |
| EP1809669A2 (en) | 2004-10-01 | 2007-07-25 | Avidex Ltd | T-cell receptors containing a non-native disulfide interchain bond linked to therapeutic agents |
| JP2008044848A (ja) | 2004-11-30 | 2008-02-28 | Univ Kurume | Hla−a24拘束性腫瘍抗原ペプチド |
| US8252893B2 (en) | 2005-01-31 | 2012-08-28 | Board Of Trustees Of The University Of Arkansas | CD8 T cell epitopes in HPV 16 E6 and E7 proteins and uses thereof |
| US8278056B2 (en) | 2008-06-13 | 2012-10-02 | Oncohealth Corp. | Detection of early stages and late stages HPV infection |
| US8968995B2 (en) | 2008-11-12 | 2015-03-03 | Oncohealth Corp. | Detection, screening, and diagnosis of HPV-associated cancers |
| JP5840837B2 (ja) | 2007-03-30 | 2016-01-06 | メモリアル スローン−ケタリング キャンサー センター | 養子移入tリンパ球における副刺激リガンドの構成性発現 |
| WO2008147187A1 (en) | 2007-05-31 | 2008-12-04 | Academisch Ziekenhuis Leiden H.O.D.N. Lumc | Hpv epitopes targeted by t cells infiltrating cervical malignancies for use in vaccines |
| US20090117140A1 (en) | 2007-09-26 | 2009-05-07 | Mayumi Nakagawa | Human papilloma virus dominant CD4 T cell epitopes and uses thereof |
| EP2433713B1 (en) | 2007-12-07 | 2017-07-26 | Miltenyi Biotec GmbH | Cell processing systems and methods |
| US8479118B2 (en) | 2007-12-10 | 2013-07-02 | Microsoft Corporation | Switching search providers within a browser search box |
| KR20090103571A (ko) | 2008-03-28 | 2009-10-01 | 바이오코아 주식회사 | 면역성 펩타이드 및 그 펩타이드를 포함하는 hpv 관련질환 예방 또는 치료용 조성물 |
| US20120164718A1 (en) | 2008-05-06 | 2012-06-28 | Innovative Micro Technology | Removable/disposable apparatus for MEMS particle sorting device |
| JP5173594B2 (ja) | 2008-05-27 | 2013-04-03 | キヤノン株式会社 | 管理装置、画像形成装置及びそれらの処理方法 |
| KR20090125629A (ko) | 2008-06-02 | 2009-12-07 | 바이오코아 주식회사 | 면역성 펩타이드 및 그 펩타이드를 포함하는 hpv 관련질환 예방 또는 치료용 조성물 |
| KR20090125628A (ko) | 2008-06-02 | 2009-12-07 | 바이오코아 주식회사 | 면역성 펩타이드 및 그 펩타이드를 포함하는 hpv 관련질환 예방 또는 치료용 조성물 |
| US8703489B2 (en) | 2008-08-22 | 2014-04-22 | Sangamo Biosciences, Inc. | Methods and compositions for targeted single-stranded cleavage and targeted integration |
| CN106957361A (zh) | 2009-04-20 | 2017-07-18 | 阿波维塔公司 | Hpv的e6蛋白的特异性抗体及其应用 |
| US10464987B2 (en) | 2009-10-06 | 2019-11-05 | Abbvie Inc. | Human single-chain T cell receptors |
| US9273283B2 (en) | 2009-10-29 | 2016-03-01 | The Trustees Of Dartmouth College | Method of producing T cell receptor-deficient T cells expressing a chimeric receptor |
| HRP20190556T1 (hr) | 2009-11-03 | 2019-06-14 | City Of Hope | SKRAĆENI OBLIK RECEPTORA EPIDERMALNOG FAKTORA RASTA ZA (EGFRt) ODABIR TRANSDUCIRANIH T-STANICA |
| US8956828B2 (en) | 2009-11-10 | 2015-02-17 | Sangamo Biosciences, Inc. | Targeted disruption of T cell receptor genes using engineered zinc finger protein nucleases |
| EP2534163B1 (en) | 2010-02-09 | 2015-11-04 | Sangamo BioSciences, Inc. | Targeted genomic modification with partially single-stranded donor molecules |
| CA2788006C (en) | 2010-02-16 | 2021-08-31 | Oesterreichische Akademie Der Wissenschaften | Anti-hpv e7 antibodies |
| US9228007B1 (en) | 2010-03-11 | 2016-01-05 | The Regents Of The University Of California | Recombinant human progenitor cells, engineered human thymocytes, and engineered human T cells |
| EP2571512B1 (en) | 2010-05-17 | 2017-08-23 | Sangamo BioSciences, Inc. | Novel dna-binding proteins and uses thereof |
| KR101415554B1 (ko) | 2010-09-13 | 2014-07-04 | 바이오코아 주식회사 | 면역성 펩타이드 및 그 펩타이드를 포함하는 hpv 관련 질환 예방 또는 치료용 조성물 |
| EP2625320B1 (en) | 2010-10-08 | 2019-03-27 | President and Fellows of Harvard College | High-throughput single cell barcoding |
| ES2730951T3 (es) | 2010-10-08 | 2019-11-13 | Harvard College | Secuenciación inmune de alto rendimiento |
| PH12013501201A1 (en) | 2010-12-09 | 2013-07-29 | Univ Pennsylvania | Use of chimeric antigen receptor-modified t cells to treat cancer |
| MX359513B (es) | 2011-03-23 | 2018-10-01 | Hutchinson Fred Cancer Res | Metodo y composiciones para inmunoterapia celular. |
| US8398282B2 (en) | 2011-05-12 | 2013-03-19 | Delphi Technologies, Inc. | Vehicle front lighting assembly and systems having a variable tint electrowetting element |
| EA201490364A1 (ru) | 2011-07-29 | 2014-08-29 | Дзе Трастиз Оф Дзе Юниверсити Оф Пенсильвания | Костимулирующие рецепторы-переключатели |
| EP2567972A1 (en) | 2011-09-12 | 2013-03-13 | Adriacell S.p.A. | Antibodies against E7 protein of Human Papilloma Virus (HPV) |
| ES2858978T3 (es) | 2011-10-28 | 2021-09-30 | Regeneron Pharma | Ratones con receptores de linfocitos T genéticamente modificados |
| EP2776451B1 (en) | 2011-11-11 | 2018-07-18 | Fred Hutchinson Cancer Research Center | Cyclin a1-targeted t-cell immunotherapy for cancer |
| WO2013074916A1 (en) | 2011-11-18 | 2013-05-23 | Board Of Regents, The University Of Texas System | Car+ t cells genetically modified to eliminate expression of t- cell receptor and/or hla |
| EP2814846B1 (en) | 2012-02-13 | 2020-01-08 | Seattle Children's Hospital d/b/a Seattle Children's Research Institute | Bispecific chimeric antigen receptors and therapeutic uses thereof |
| WO2013126726A1 (en) | 2012-02-22 | 2013-08-29 | The Trustees Of The University Of Pennsylvania | Double transgenic t cells comprising a car and a tcr and their methods of use |
| CN107557334B (zh) | 2012-05-03 | 2021-06-25 | 弗雷德哈钦森癌症研究中心 | 增强亲和力的t细胞受体及其制备方法 |
| EP2847338B1 (en) | 2012-05-07 | 2018-09-19 | Sangamo Therapeutics, Inc. | Methods and compositions for nuclease-mediated targeted integration of transgenes |
| EP2846816B1 (en) | 2012-05-08 | 2016-09-28 | The Johns Hopkins University | Methods and compositions for infusion of transiently engrafting, selected populations of allogeneic lymphocytes to treat cancer |
| CN111676196A (zh) | 2012-05-25 | 2020-09-18 | 塞勒克提斯公司 | 工程化异体和免疫抑制耐受性t细胞的方法 |
| IL293944A (en) | 2012-08-20 | 2022-08-01 | Hutchinson Fred Cancer Res | Method and preparations for cellular immunotherapy |
| KR102198058B1 (ko) | 2012-10-02 | 2021-01-06 | 메모리얼 슬로안 케터링 캔서 센터 | 면역치료용 조성물 및 방법 |
| WO2014097442A1 (ja) | 2012-12-20 | 2014-06-26 | 三菱電機株式会社 | 車載装置及びプログラム |
| GB201223172D0 (en) | 2012-12-21 | 2013-02-06 | Immunocore Ltd | Method |
| KR102363191B1 (ko) | 2013-02-26 | 2022-02-17 | 메모리얼 슬로안 케터링 캔서 센터 | 면역치료용 조성물 및 방법 |
| JP6346266B2 (ja) | 2013-03-21 | 2018-06-20 | サンガモ セラピューティクス, インコーポレイテッド | 操作されたジンクフィンガータンパク質ヌクレアーゼを使用するt細胞受容体遺伝子の標的化された破壊 |
| AU2014265331B2 (en) | 2013-05-15 | 2019-12-05 | Sangamo Therapeutics, Inc. | Methods and compositions for treatment of a genetic condition |
| EP3309248B1 (en) | 2013-05-29 | 2021-06-09 | Cellectis | Methods for engineering t cells for immunotherapy by using rna-guided cas nuclease system |
| ES3034357T3 (en) | 2013-07-15 | 2025-08-18 | Us Health | Methods of preparing anti-human papillomavirus antigen t cells |
| EP3572423B1 (en) | 2013-07-15 | 2023-12-06 | The United States of America, as represented by the Secretary, Department of Health and Human Services | Anti-human papillomavirus 16 e6 t cell receptors |
| CN103342738B (zh) | 2013-07-24 | 2015-02-18 | 广州恒上医药技术有限公司 | 人乳头瘤病毒e6蛋白可诱发同源蛋白间交叉反应性抗体的精细表位肽 |
| JP5921042B2 (ja) | 2013-07-30 | 2016-05-24 | シャープ株式会社 | 光拡散部材の製造方法 |
| EP3030900A2 (en) | 2013-08-08 | 2016-06-15 | Institut Pasteur | Correlation of disease activity with clonal expansions of human papillomavirus 16-specific cd8+ t-cells in patients with severe erosive oral lichen planus |
| US9108442B2 (en) | 2013-08-20 | 2015-08-18 | Ricoh Company, Ltd. | Image forming apparatus |
| US20150098954A1 (en) | 2013-10-08 | 2015-04-09 | Elwha Llc | Compositions and Methods Related to CRISPR Targeting |
| SG11201603228TA (en) | 2013-10-31 | 2016-05-30 | Hutchinson Fred Cancer Res | Modified hematopoietic stem/progenitor and non-t effector cells, and uses thereof |
| KR102228828B1 (ko) | 2014-03-11 | 2021-03-16 | 셀렉티스 | 동종이형 이식에 양립성인 t-세포들을 만들어내는 방법 |
| CA2943569C (en) | 2014-03-27 | 2021-02-23 | British Columbia Cancer Agency Branch | T-cell epitope identification |
| SG10201809157VA (en) | 2014-04-18 | 2018-11-29 | Editas Medicine Inc | Crispr-cas-related methods, compositions and components for cancer immunotherapy |
| CN106459918A (zh) | 2014-04-24 | 2017-02-22 | 得克萨斯州大学系统董事会 | 施加诱导多能干细胞产生过继细胞疗法产品 |
| WO2015184228A1 (en) * | 2014-05-29 | 2015-12-03 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Anti-human papillomavirus 16 e7 t cell receptors |
| US10204855B2 (en) | 2014-07-11 | 2019-02-12 | Intel Corporation | Bendable and stretchable electronic devices and methods |
| JP7054622B2 (ja) | 2014-07-21 | 2022-04-14 | ノバルティス アーゲー | ヒト化抗bcmaキメラ抗原受容体を使用した癌の処置 |
| RU2017102769A (ru) | 2014-07-29 | 2018-08-28 | Пфайзер Инк. | EGFRvIII СПЕЦИФИЧЕСКИЕ ХИМЕРНЫЕ АНТИГЕННЫЕ РЕЦЕПТОРЫ ДЛЯ ИММУНОТЕРАПИИ РАКА |
| EP3177314B1 (en) | 2014-08-04 | 2020-10-07 | Fred Hutchinson Cancer Research Center | T cell immunotherapy specific for wt-1 |
| NZ730044A (en) | 2014-09-15 | 2024-08-30 | Abvitro Llc | High-throughput nucleotide library sequencing |
| GB201417803D0 (en) | 2014-10-08 | 2014-11-19 | Adaptimmune Ltd | T cell receptors |
| KR20170075013A (ko) | 2014-10-31 | 2017-06-30 | 더 트러스티스 오브 더 유니버시티 오브 펜실바니아 | Cart 세포에서 유전자 발현의 변경 및 그의 용도 |
| ES2790725T3 (es) | 2015-03-16 | 2020-10-29 | Max Delbrueck Centrum Fuer Molekulare Medizin Helmholtz Gemeinschaft | Procedimiento de detección de nuevos epítopos de células T inmunogénicas y aislamiento de nuevos receptores de células T específicas de antígeno mediante una biblioteca de células de MHC |
| SG11201708674XA (en) | 2015-05-08 | 2017-11-29 | Eureka Therapeutics Inc | Constructs targeting hpv16-e7 peptide/mhc complexes and uses thereof |
| US9790490B2 (en) | 2015-06-18 | 2017-10-17 | The Broad Institute Inc. | CRISPR enzymes and systems |
| AU2016291778B2 (en) | 2015-07-13 | 2021-05-06 | Sangamo Therapeutics, Inc. | Delivery methods and compositions for nuclease-mediated genome engineering |
| US11047011B2 (en) | 2015-09-29 | 2021-06-29 | iRepertoire, Inc. | Immunorepertoire normality assessment method and its use |
| JP6816133B2 (ja) | 2015-10-05 | 2021-01-20 | プレシジョン バイオサイエンシズ,インク. | 改変ヒトt細胞受容体アルファ定常領域遺伝子を含む遺伝子改変細胞 |
| EP3156067A1 (en) | 2015-10-16 | 2017-04-19 | Max-Delbrück-Centrum Für Molekulare Medizin | High avidity hpv t-cell receptors |
| WO2017070429A1 (en) | 2015-10-22 | 2017-04-27 | Regents Of The University Of Minnesota | Methods involving editing polynucleotides that encode t cell receptor |
| JP6777841B2 (ja) | 2015-10-23 | 2020-10-28 | 国立大学法人金沢大学 | 細胞傷害性t細胞の作製方法 |
| SG10202109655VA (en) | 2015-12-04 | 2021-10-28 | Novartis Ag | Compositions and methods for immunooncology |
| WO2017106528A2 (en) | 2015-12-18 | 2017-06-22 | Sangamo Biosciences, Inc. | Targeted disruption of the t cell receptor |
| GB201604494D0 (en) | 2016-03-16 | 2016-04-27 | Immatics Biotechnologies Gmbh | Transfected T-Cells and T-Cell receptors for use in immunotherapy against cancers |
| GB201604953D0 (en) | 2016-03-23 | 2016-05-04 | Immunocore Ltd | T cell receptors |
| MX2018012318A (es) | 2016-04-08 | 2019-07-04 | Immunocore Ltd | Receptores de celulas t. |
| US20190136230A1 (en) | 2016-05-06 | 2019-05-09 | Juno Therapeutics, Inc. | Genetically engineered cells and methods of making the same |
| CN110291402B (zh) | 2016-06-27 | 2023-09-01 | 朱诺治疗学股份有限公司 | 鉴定肽表位的方法、结合此类表位的分子和相关用途 |
| MA45491A (fr) | 2016-06-27 | 2019-05-01 | Juno Therapeutics Inc | Épitopes à restriction cmh-e, molécules de liaison et procédés et utilisations associés |
| CN109996811A (zh) | 2016-08-03 | 2019-07-09 | 约翰·F·迪帕西奥 | 用于用嵌合抗原受体治疗t细胞恶性肿瘤的car-t细胞的基因编辑 |
| US9642906B2 (en) | 2016-09-16 | 2017-05-09 | Baylor College Of Medicine | Generation of HPV-specific T-cells |
| WO2018067618A1 (en) | 2016-10-03 | 2018-04-12 | Juno Therapeutics, Inc. | Hpv-specific binding molecules |
| WO2018073393A2 (en) | 2016-10-19 | 2018-04-26 | Cellectis | Tal-effector nuclease (talen) -modified allogenic cells suitable for therapy |
| EP3546575B1 (en) * | 2016-11-28 | 2024-07-17 | Osaka University | Genome editing method |
| EP4286523A3 (en) * | 2016-12-20 | 2024-03-20 | Bristol-Myers Squibb Company | Methods for increasing the efficiency of homology directed repair (hdr) in the cellular genome |
| ES2979232T3 (es) | 2017-03-31 | 2024-09-25 | Cellectis Sa | Células inmunitarias con receptor de antígeno quimérico anti-CD22 universal |
| JP7170666B2 (ja) | 2017-05-08 | 2022-11-14 | プレシジョン バイオサイエンシズ,インク. | 操作された抗原受容体をコードする核酸分子及び阻害性核酸分子、並びにそれらの使用方法 |
| EP3621981A2 (en) | 2017-05-12 | 2020-03-18 | CRISPR Therapeutics AG | Materials and methods for engineering cells and uses thereof in immuno-oncology |
| CA3066779A1 (en) | 2017-06-28 | 2019-01-03 | Regeneron Pharmaceuticals, Inc. | Anti-human papillomavirus (hpv) antigen-binding proteins and methods of use thereof |
| US11053484B2 (en) | 2017-06-30 | 2021-07-06 | Precision Biosciences, Inc. | Genetically-modified T cells comprising a modified intron in the T cell receptor alpha gene |
| KR20200071079A (ko) * | 2017-09-27 | 2020-06-18 | 유니버시티 오브 써던 캘리포니아 | 공동-자극을 위한 신규한 플랫폼, 신규한 car 설계 및 입양 세포 치료를 위한 다른 향상 |
| CA3095084A1 (en) | 2018-04-05 | 2019-10-10 | Juno Therapeutics, Inc. | T cells expressing a recombinant receptor, related polynucleotides and methods |
| KR20210029707A (ko) | 2018-04-05 | 2021-03-16 | 주노 쎄러퓨티크스 인코퍼레이티드 | 재조합 수용체를 발현하는 세포의 생산 방법 및 관련 조성물 |
| BR112020019313A2 (pt) | 2018-04-05 | 2021-01-05 | Juno Therapeutics Inc | Receptores de células t e células modificadas que expressam os mesmos |
-
2018
- 2018-09-28 US US16/652,379 patent/US11952408B2/en active Active
- 2018-09-28 MA MA050613A patent/MA50613A/fr unknown
- 2018-09-28 AU AU2018345539A patent/AU2018345539A1/en not_active Abandoned
- 2018-09-28 BR BR112020006643-5A patent/BR112020006643A2/pt not_active IP Right Cessation
- 2018-09-28 CA CA3080546A patent/CA3080546A1/en active Pending
- 2018-09-28 EP EP22213635.0A patent/EP4215543A3/en not_active Withdrawn
- 2018-09-28 EP EP18792692.8A patent/EP3692063A1/en not_active Withdrawn
- 2018-09-28 KR KR1020207012701A patent/KR20200104284A/ko not_active Withdrawn
- 2018-09-28 JP JP2020519033A patent/JP2020537515A/ja active Pending
- 2018-09-28 SG SG11202002728VA patent/SG11202002728VA/en unknown
- 2018-09-28 CN CN201880076631.8A patent/CN111954679A/zh active Pending
- 2018-09-28 WO PCT/US2018/053650 patent/WO2019070541A1/en not_active Ceased
- 2018-09-28 MX MX2020003536A patent/MX2020003536A/es unknown
-
2020
- 2020-03-26 IL IL273631A patent/IL273631A/en unknown
-
2023
- 2023-05-01 JP JP2023075371A patent/JP2023099142A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| BR112020006643A2 (pt) | 2020-09-24 |
| JP2023099142A (ja) | 2023-07-11 |
| AU2018345539A1 (en) | 2020-04-16 |
| MX2020003536A (es) | 2020-09-14 |
| IL273631A (en) | 2020-05-31 |
| US20210284709A1 (en) | 2021-09-16 |
| JP2020537515A (ja) | 2020-12-24 |
| US11952408B2 (en) | 2024-04-09 |
| MA50613A (fr) | 2020-08-12 |
| EP4215543A3 (en) | 2023-10-11 |
| SG11202002728VA (en) | 2020-04-29 |
| EP4215543A2 (en) | 2023-07-26 |
| CN111954679A (zh) | 2020-11-17 |
| RU2020115148A (ru) | 2021-11-09 |
| WO2019070541A1 (en) | 2019-04-11 |
| CA3080546A1 (en) | 2019-04-11 |
| EP3692063A1 (en) | 2020-08-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20200104284A (ko) | Hpv-특이적 결합 분자 | |
| JP7589047B2 (ja) | 組換え受容体を発現する細胞の作製方法および関連組成物 | |
| AU2018338647B2 (en) | Novel platforms for co-stimulation, novel car designs and other enhancements for adoptive cellular therapy | |
| JP6970991B2 (ja) | トランスポザーゼポリペプチド及びその使用 | |
| KR102764123B1 (ko) | Τ 세포 수용체 및 이를 발현하는 조작된 세포 | |
| KR20190062505A (ko) | Hpv-특이적 결합 분자 | |
| AU2017250304B2 (en) | Compositions and methods for selective protein expression | |
| AU2025205493A1 (en) | Diverse antigen binding domains, novel platforms and other enhancements for cellular therapy | |
| KR20220016475A (ko) | 변형된 tgfbr2 유전자 좌에서 재조합 수용체를 발현하는 세포, 관련 폴리뉴클레오티드 및 방법 | |
| KR20210020873A (ko) | 재조합 수용체를 발현하는 τ 세포, 관련 폴리뉴클레오티드 및 방법 | |
| TW202237826A (zh) | 基因編輯的自然殺手細胞 | |
| CN109562126A (zh) | 嵌合抗原受体(car)、组合物及其使用方法 | |
| KR20190130608A (ko) | 면역종양학을 위한 조성물 및 방법 | |
| CN116234558A (zh) | 条件性地表达重组受体的工程化t细胞、相关多核苷酸和方法 | |
| KR20230146132A (ko) | 항-bcma 키메라 항원 수용체 | |
| KR20220149606A (ko) | 암 치료를 위한 키메라 항원 수용체 및 관련 방법 및 조성물 | |
| CN114007640A (zh) | 从修饰的cd247基因座表达嵌合受体的细胞、相关多核苷酸和方法 | |
| US20230398148A1 (en) | Cells expressing a chimeric receptor from a modified invariant cd3 immunoglobulin superfamily chain locus and related polynucleotides and methods | |
| RU2804664C2 (ru) | Hpv-специфические связывающие молекулы | |
| RU2835579C2 (ru) | Способы получения клеток, экспрессирующих рекомбинантный рецептор, и родственные композиции |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20200429 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20210928 Comment text: Request for Examination of Application |
|
| PC1202 | Submission of document of withdrawal before decision of registration |
Comment text: [Withdrawal of Procedure relating to Patent, etc.] Withdrawal (Abandonment) Patent event code: PC12021R01D Patent event date: 20240320 |
|
| WITB | Written withdrawal of application |