KR20170001720A - 바이러스 유사 입자 정제 - Google Patents
바이러스 유사 입자 정제 Download PDFInfo
- Publication number
- KR20170001720A KR20170001720A KR1020167036031A KR20167036031A KR20170001720A KR 20170001720 A KR20170001720 A KR 20170001720A KR 1020167036031 A KR1020167036031 A KR 1020167036031A KR 20167036031 A KR20167036031 A KR 20167036031A KR 20170001720 A KR20170001720 A KR 20170001720A
- Authority
- KR
- South Korea
- Prior art keywords
- vlps
- chromatography
- chromatographic
- vlp
- hydroxyapatite
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 239000002245 particle Substances 0.000 title claims description 25
- 238000000746 purification Methods 0.000 title description 71
- 241000700605 Viruses Species 0.000 title description 32
- 238000000034 method Methods 0.000 claims abstract description 121
- 241001263478 Norovirus Species 0.000 claims abstract description 50
- 239000000463 material Substances 0.000 claims description 140
- 238000004587 chromatography analysis Methods 0.000 claims description 83
- 210000004027 cell Anatomy 0.000 claims description 68
- 108090000623 proteins and genes Proteins 0.000 claims description 66
- 229910052588 hydroxylapatite Inorganic materials 0.000 claims description 53
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 claims description 53
- 102000004169 proteins and genes Human genes 0.000 claims description 53
- 238000004191 hydrophobic interaction chromatography Methods 0.000 claims description 44
- 230000008569 process Effects 0.000 claims description 37
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical compound N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 claims description 29
- 229910052921 ammonium sulfate Inorganic materials 0.000 claims description 29
- 235000011130 ammonium sulphate Nutrition 0.000 claims description 29
- 238000010828 elution Methods 0.000 claims description 22
- 239000013592 cell lysate Substances 0.000 claims description 19
- 238000001914 filtration Methods 0.000 claims description 19
- 239000006228 supernatant Substances 0.000 claims description 16
- 238000004255 ion exchange chromatography Methods 0.000 claims description 12
- 241000238631 Hexapoda Species 0.000 claims description 10
- 230000001580 bacterial effect Effects 0.000 claims description 9
- 239000008363 phosphate buffer Substances 0.000 claims description 9
- 210000004962 mammalian cell Anatomy 0.000 claims description 8
- 238000011109 contamination Methods 0.000 claims description 6
- 229960005486 vaccine Drugs 0.000 claims description 6
- 210000005253 yeast cell Anatomy 0.000 claims description 6
- 238000000108 ultra-filtration Methods 0.000 claims description 4
- 238000011026 diafiltration Methods 0.000 claims description 3
- 238000010188 recombinant method Methods 0.000 claims description 3
- BFSVOASYOCHEOV-UHFFFAOYSA-N 2-diethylaminoethanol Chemical compound CCN(CC)CCO BFSVOASYOCHEOV-UHFFFAOYSA-N 0.000 claims 1
- 230000010933 acylation Effects 0.000 claims 1
- 238000005917 acylation reaction Methods 0.000 claims 1
- 241001617329 Norovirus isolates Species 0.000 abstract description 4
- 239000000243 solution Substances 0.000 description 54
- 239000011347 resin Substances 0.000 description 50
- 229920005989 resin Polymers 0.000 description 50
- 239000000872 buffer Substances 0.000 description 45
- 239000000356 contaminant Substances 0.000 description 27
- 229910019142 PO4 Inorganic materials 0.000 description 26
- 235000021317 phosphate Nutrition 0.000 description 26
- 239000012149 elution buffer Substances 0.000 description 25
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 24
- 239000010452 phosphate Substances 0.000 description 24
- 150000003839 salts Chemical class 0.000 description 24
- 239000001488 sodium phosphate Substances 0.000 description 24
- 229910000162 sodium phosphate Inorganic materials 0.000 description 24
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical group [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 24
- 238000007792 addition Methods 0.000 description 21
- 239000000499 gel Substances 0.000 description 21
- 239000000203 mixture Substances 0.000 description 19
- 238000001542 size-exclusion chromatography Methods 0.000 description 17
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 16
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 16
- 238000011210 chromatographic step Methods 0.000 description 16
- 230000002209 hydrophobic effect Effects 0.000 description 16
- 241000056533 Houston virus Species 0.000 description 15
- 239000002202 Polyethylene glycol Substances 0.000 description 15
- 229920001223 polyethylene glycol Polymers 0.000 description 15
- 230000027455 binding Effects 0.000 description 14
- 230000003993 interaction Effects 0.000 description 14
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 14
- 238000010521 absorption reaction Methods 0.000 description 13
- 239000002609 medium Substances 0.000 description 13
- 238000003998 size exclusion chromatography high performance liquid chromatography Methods 0.000 description 13
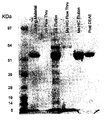
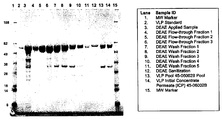
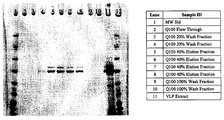
- 238000010186 staining Methods 0.000 description 13
- 238000004458 analytical method Methods 0.000 description 12
- 150000001450 anions Chemical group 0.000 description 12
- 150000001768 cations Chemical group 0.000 description 12
- 239000012501 chromatography medium Substances 0.000 description 12
- 238000009472 formulation Methods 0.000 description 12
- 238000002360 preparation method Methods 0.000 description 12
- 238000001042 affinity chromatography Methods 0.000 description 11
- 230000007717 exclusion Effects 0.000 description 11
- 150000002500 ions Chemical class 0.000 description 11
- 239000000523 sample Substances 0.000 description 11
- 238000000926 separation method Methods 0.000 description 11
- 241000369757 Sapovirus Species 0.000 description 10
- 239000007983 Tris buffer Substances 0.000 description 10
- 239000012530 fluid Substances 0.000 description 10
- 239000012634 fragment Substances 0.000 description 10
- 239000012528 membrane Substances 0.000 description 10
- 239000000725 suspension Substances 0.000 description 10
- 241000282414 Homo sapiens Species 0.000 description 9
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 9
- 238000004128 high performance liquid chromatography Methods 0.000 description 9
- 239000012071 phase Substances 0.000 description 9
- 108090000565 Capsid Proteins Proteins 0.000 description 8
- 102100023321 Ceruloplasmin Human genes 0.000 description 8
- 108020004414 DNA Proteins 0.000 description 8
- 229920005654 Sephadex Polymers 0.000 description 8
- 239000012507 Sephadex™ Substances 0.000 description 8
- 238000005341 cation exchange Methods 0.000 description 8
- 239000012535 impurity Substances 0.000 description 8
- 208000015181 infectious disease Diseases 0.000 description 8
- 102000039446 nucleic acids Human genes 0.000 description 8
- 108020004707 nucleic acids Proteins 0.000 description 8
- 150000007523 nucleic acids Chemical class 0.000 description 8
- 239000008188 pellet Substances 0.000 description 8
- 230000002829 reductive effect Effects 0.000 description 8
- 239000011780 sodium chloride Substances 0.000 description 8
- 238000005199 ultracentrifugation Methods 0.000 description 8
- 101710172711 Structural protein Proteins 0.000 description 7
- 238000005571 anion exchange chromatography Methods 0.000 description 7
- 238000005119 centrifugation Methods 0.000 description 7
- 230000014509 gene expression Effects 0.000 description 7
- 238000004519 manufacturing process Methods 0.000 description 7
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 7
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 6
- 239000011324 bead Substances 0.000 description 6
- 238000004113 cell culture Methods 0.000 description 6
- 238000004440 column chromatography Methods 0.000 description 6
- 239000012228 culture supernatant Substances 0.000 description 6
- 238000011067 equilibration Methods 0.000 description 6
- 239000003446 ligand Substances 0.000 description 6
- 239000003960 organic solvent Substances 0.000 description 6
- 241000894007 species Species 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 6
- 241000701447 unidentified baculovirus Species 0.000 description 6
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 5
- 241000532183 Norovirus GI Species 0.000 description 5
- 241000532184 Norovirus GII Species 0.000 description 5
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 5
- 239000001166 ammonium sulphate Substances 0.000 description 5
- 238000005349 anion exchange Methods 0.000 description 5
- 239000001506 calcium phosphate Substances 0.000 description 5
- 235000011010 calcium phosphates Nutrition 0.000 description 5
- -1 carboxylate salt Chemical class 0.000 description 5
- 238000001142 circular dichroism spectrum Methods 0.000 description 5
- 239000003814 drug Substances 0.000 description 5
- 125000000524 functional group Chemical group 0.000 description 5
- 230000002458 infectious effect Effects 0.000 description 5
- 239000011159 matrix material Substances 0.000 description 5
- 229910052709 silver Inorganic materials 0.000 description 5
- 239000004332 silver Substances 0.000 description 5
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 5
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 5
- MYRTYDVEIRVNKP-UHFFFAOYSA-N 1,2-Divinylbenzene Chemical compound C=CC1=CC=CC=C1C=C MYRTYDVEIRVNKP-UHFFFAOYSA-N 0.000 description 4
- 229920000936 Agarose Polymers 0.000 description 4
- 229920002307 Dextran Polymers 0.000 description 4
- 238000002965 ELISA Methods 0.000 description 4
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 4
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 4
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- 239000011358 absorbing material Substances 0.000 description 4
- 150000001412 amines Chemical group 0.000 description 4
- 229910000389 calcium phosphate Inorganic materials 0.000 description 4
- 150000007942 carboxylates Chemical class 0.000 description 4
- 238000005277 cation exchange chromatography Methods 0.000 description 4
- 239000000919 ceramic Substances 0.000 description 4
- 239000012141 concentrate Substances 0.000 description 4
- 238000000502 dialysis Methods 0.000 description 4
- 239000001963 growth medium Substances 0.000 description 4
- 230000028993 immune response Effects 0.000 description 4
- 238000011068 loading method Methods 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- 239000000178 monomer Substances 0.000 description 4
- 230000007935 neutral effect Effects 0.000 description 4
- 239000011148 porous material Substances 0.000 description 4
- 238000001556 precipitation Methods 0.000 description 4
- 230000002441 reversible effect Effects 0.000 description 4
- 238000009938 salting Methods 0.000 description 4
- 239000012064 sodium phosphate buffer Substances 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- 210000002845 virion Anatomy 0.000 description 4
- 230000003612 virological effect Effects 0.000 description 4
- 238000005406 washing Methods 0.000 description 4
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- HEVGGTGPGPKZHF-UHFFFAOYSA-N Epilaurene Natural products CC1C(=C)CCC1(C)C1=CC=C(C)C=C1 HEVGGTGPGPKZHF-UHFFFAOYSA-N 0.000 description 3
- 208000005577 Gastroenteritis Diseases 0.000 description 3
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 3
- 239000004793 Polystyrene Substances 0.000 description 3
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 125000000217 alkyl group Chemical group 0.000 description 3
- 150000001413 amino acids Chemical group 0.000 description 3
- 238000012870 ammonium sulfate precipitation Methods 0.000 description 3
- 230000005540 biological transmission Effects 0.000 description 3
- 210000000234 capsid Anatomy 0.000 description 3
- 230000001413 cellular effect Effects 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 238000011097 chromatography purification Methods 0.000 description 3
- 239000012539 chromatography resin Substances 0.000 description 3
- 238000004140 cleaning Methods 0.000 description 3
- 239000003636 conditioned culture medium Substances 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 239000000284 extract Substances 0.000 description 3
- 229920000669 heparin Polymers 0.000 description 3
- 229960002897 heparin Drugs 0.000 description 3
- 229910052500 inorganic mineral Inorganic materials 0.000 description 3
- 238000005342 ion exchange Methods 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 229920002521 macromolecule Polymers 0.000 description 3
- 150000002739 metals Chemical class 0.000 description 3
- 239000011707 mineral Substances 0.000 description 3
- 230000000144 pharmacologic effect Effects 0.000 description 3
- 229920002223 polystyrene Polymers 0.000 description 3
- 229910000160 potassium phosphate Inorganic materials 0.000 description 3
- 235000011009 potassium phosphates Nutrition 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 238000001742 protein purification Methods 0.000 description 3
- 125000001453 quaternary ammonium group Chemical group 0.000 description 3
- 238000003259 recombinant expression Methods 0.000 description 3
- 230000001105 regulatory effect Effects 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 2
- ZOOGRGPOEVQQDX-UUOKFMHZSA-N 3',5'-cyclic GMP Chemical compound C([C@H]1O2)OP(O)(=O)O[C@H]1[C@@H](O)[C@@H]2N1C(N=C(NC2=O)N)=C2N=C1 ZOOGRGPOEVQQDX-UUOKFMHZSA-N 0.000 description 2
- 101100282617 Bovine herpesvirus 1.1 (strain Cooper) gC gene Proteins 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 2
- 101710132601 Capsid protein Proteins 0.000 description 2
- 101710197658 Capsid protein VP1 Proteins 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 102000004157 Hydrolases Human genes 0.000 description 2
- 108090000604 Hydrolases Proteins 0.000 description 2
- 241000699670 Mus sp. Species 0.000 description 2
- 101710159752 Poly(3-hydroxyalkanoate) polymerase subunit PhaE Proteins 0.000 description 2
- 229920002873 Polyethylenimine Polymers 0.000 description 2
- 101710130262 Probable Vpr-like protein Proteins 0.000 description 2
- 101710118046 RNA-directed RNA polymerase Proteins 0.000 description 2
- 229920002684 Sepharose Polymers 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 101710108545 Viral protein 1 Proteins 0.000 description 2
- 150000001242 acetic acid derivatives Chemical class 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 125000003277 amino group Chemical group 0.000 description 2
- 239000003957 anion exchange resin Substances 0.000 description 2
- 230000000890 antigenic effect Effects 0.000 description 2
- 125000003118 aryl group Chemical group 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 230000033228 biological regulation Effects 0.000 description 2
- OWMVSZAMULFTJU-UHFFFAOYSA-N bis-tris Chemical compound OCCN(CCO)C(CO)(CO)CO OWMVSZAMULFTJU-UHFFFAOYSA-N 0.000 description 2
- 239000007853 buffer solution Substances 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 150000001734 carboxylic acid salts Chemical class 0.000 description 2
- 230000006037 cell lysis Effects 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 238000012512 characterization method Methods 0.000 description 2
- 229920001429 chelating resin Polymers 0.000 description 2
- 239000007979 citrate buffer Substances 0.000 description 2
- 150000001860 citric acid derivatives Chemical class 0.000 description 2
- 238000010367 cloning Methods 0.000 description 2
- 239000000306 component Substances 0.000 description 2
- 230000007547 defect Effects 0.000 description 2
- 239000008367 deionised water Substances 0.000 description 2
- 229910021641 deionized water Inorganic materials 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 238000010494 dissociation reaction Methods 0.000 description 2
- 230000005593 dissociations Effects 0.000 description 2
- 238000004090 dissolution Methods 0.000 description 2
- 229940116441 divinylbenzene Drugs 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 239000003480 eluent Substances 0.000 description 2
- 239000002158 endotoxin Substances 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- 238000001502 gel electrophoresis Methods 0.000 description 2
- 230000035784 germination Effects 0.000 description 2
- 230000005484 gravity Effects 0.000 description 2
- 238000003306 harvesting Methods 0.000 description 2
- 239000012616 hydrophobic interaction chromatography medium Substances 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 238000007689 inspection Methods 0.000 description 2
- 230000002452 interceptive effect Effects 0.000 description 2
- 238000000464 low-speed centrifugation Methods 0.000 description 2
- 238000000816 matrix-assisted laser desorption--ionisation Methods 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 2
- 238000002264 polyacrylamide gel electrophoresis Methods 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 229920000193 polymethacrylate Polymers 0.000 description 2
- 239000002244 precipitate Substances 0.000 description 2
- 239000002994 raw material Substances 0.000 description 2
- 238000007670 refining Methods 0.000 description 2
- 238000004062 sedimentation Methods 0.000 description 2
- 238000001338 self-assembly Methods 0.000 description 2
- 239000000377 silicon dioxide Substances 0.000 description 2
- 229910052938 sodium sulfate Inorganic materials 0.000 description 2
- 235000011152 sodium sulphate Nutrition 0.000 description 2
- 239000007790 solid phase Substances 0.000 description 2
- 239000002195 soluble material Substances 0.000 description 2
- 238000001179 sorption measurement Methods 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 2
- 230000009885 systemic effect Effects 0.000 description 2
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 238000004627 transmission electron microscopy Methods 0.000 description 2
- 238000001262 western blot Methods 0.000 description 2
- CHRJZRDFSQHIFI-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;styrene Chemical compound C=CC1=CC=CC=C1.C=CC1=CC=CC=C1C=C CHRJZRDFSQHIFI-UHFFFAOYSA-N 0.000 description 1
- 101150029062 15 gene Chemical class 0.000 description 1
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 1
- OTLLEIBWKHEHGU-UHFFFAOYSA-N 2-[5-[[5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy]-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3,5-dihydroxy-4-phosphonooxyhexanedioic acid Chemical compound C1=NC=2C(N)=NC=NC=2N1C(C(C1O)O)OC1COC1C(CO)OC(OC(C(O)C(OP(O)(O)=O)C(O)C(O)=O)C(O)=O)C(O)C1O OTLLEIBWKHEHGU-UHFFFAOYSA-N 0.000 description 1
- GHCZTIFQWKKGSB-UHFFFAOYSA-N 2-hydroxypropane-1,2,3-tricarboxylic acid;phosphoric acid Chemical compound OP(O)(O)=O.OC(=O)CC(O)(C(O)=O)CC(O)=O GHCZTIFQWKKGSB-UHFFFAOYSA-N 0.000 description 1
- 101710170920 62 kDa protein Proteins 0.000 description 1
- 101000768957 Acholeplasma phage L2 Uncharacterized 37.2 kDa protein Proteins 0.000 description 1
- 101000823746 Acidianus ambivalens Uncharacterized 17.7 kDa protein in bps2 3'region Proteins 0.000 description 1
- 101000916369 Acidianus ambivalens Uncharacterized protein in sor 5'region Proteins 0.000 description 1
- 101000769342 Acinetobacter guillouiae Uncharacterized protein in rpoN-murA intergenic region Proteins 0.000 description 1
- 101000823696 Actinobacillus pleuropneumoniae Uncharacterized glycosyltransferase in aroQ 3'region Proteins 0.000 description 1
- 241000256173 Aedes albopictus Species 0.000 description 1
- 101000786513 Agrobacterium tumefaciens (strain 15955) Uncharacterized protein outside the virF region Proteins 0.000 description 1
- 101000618005 Alkalihalobacillus pseudofirmus (strain ATCC BAA-2126 / JCM 17055 / OF4) Uncharacterized protein BpOF4_00885 Proteins 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- 102100020724 Ankyrin repeat, SAM and basic leucine zipper domain-containing protein 1 Human genes 0.000 description 1
- 101000967489 Azorhizobium caulinodans (strain ATCC 43989 / DSM 5975 / JCM 20966 / LMG 6465 / NBRC 14845 / NCIMB 13405 / ORS 571) Uncharacterized protein AZC_3924 Proteins 0.000 description 1
- 101000823761 Bacillus licheniformis Uncharacterized 9.4 kDa protein in flaL 3'region Proteins 0.000 description 1
- 241000194107 Bacillus megaterium Species 0.000 description 1
- 101000819719 Bacillus methanolicus Uncharacterized N-acetyltransferase in lysA 3'region Proteins 0.000 description 1
- 235000014469 Bacillus subtilis Nutrition 0.000 description 1
- 101000789586 Bacillus subtilis (strain 168) UPF0702 transmembrane protein YkjA Proteins 0.000 description 1
- 101000792624 Bacillus subtilis (strain 168) Uncharacterized protein YbxH Proteins 0.000 description 1
- 101000790792 Bacillus subtilis (strain 168) Uncharacterized protein YckC Proteins 0.000 description 1
- 101000819705 Bacillus subtilis (strain 168) Uncharacterized protein YlxR Proteins 0.000 description 1
- 101000948218 Bacillus subtilis (strain 168) Uncharacterized protein YtxJ Proteins 0.000 description 1
- 101000718627 Bacillus thuringiensis subsp. kurstaki Putative RNA polymerase sigma-G factor Proteins 0.000 description 1
- 101000641200 Bombyx mori densovirus Putative non-structural protein Proteins 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 241000149420 Bothrometopus brevis Species 0.000 description 1
- YCSMVPSDJIOXGN-UHFFFAOYSA-N CCCCCCCCCCCC[Na] Chemical class CCCCCCCCCCCC[Na] YCSMVPSDJIOXGN-UHFFFAOYSA-N 0.000 description 1
- 229910014497 Ca10(PO4)6(OH)2 Inorganic materials 0.000 description 1
- BHPQYMZQTOCNFJ-UHFFFAOYSA-N Calcium cation Chemical compound [Ca+2] BHPQYMZQTOCNFJ-UHFFFAOYSA-N 0.000 description 1
- 208000006339 Caliciviridae Infections Diseases 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- 101000947633 Claviceps purpurea Uncharacterized 13.8 kDa protein Proteins 0.000 description 1
- 108700020911 DNA-Binding Proteins Proteins 0.000 description 1
- 102000052510 DNA-Binding Proteins Human genes 0.000 description 1
- 241001466947 Desert Shield virus Species 0.000 description 1
- 238000009007 Diagnostic Kit Methods 0.000 description 1
- 241000255601 Drosophila melanogaster Species 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 238000012286 ELISA Assay Methods 0.000 description 1
- 102000004533 Endonucleases Human genes 0.000 description 1
- 108010042407 Endonucleases Proteins 0.000 description 1
- 101000948901 Enterobacteria phage T4 Uncharacterized 16.0 kDa protein in segB-ipI intergenic region Proteins 0.000 description 1
- 101000805958 Equine herpesvirus 4 (strain 1942) Virion protein US10 homolog Proteins 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 101000790442 Escherichia coli Insertion element IS2 uncharacterized 11.1 kDa protein Proteins 0.000 description 1
- 101000788354 Escherichia phage P2 Uncharacterized 8.2 kDa protein in gpA 5'region Proteins 0.000 description 1
- KRHYYFGTRYWZRS-UHFFFAOYSA-M Fluoride anion Chemical compound [F-] KRHYYFGTRYWZRS-UHFFFAOYSA-M 0.000 description 1
- 101000770304 Frankia alni UPF0460 protein in nifX-nifW intergenic region Proteins 0.000 description 1
- 229930091371 Fructose Natural products 0.000 description 1
- 239000005715 Fructose Substances 0.000 description 1
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 1
- 206010061166 Gastroenteritis bacterial Diseases 0.000 description 1
- 101000797344 Geobacillus stearothermophilus Putative tRNA (cytidine(34)-2'-O)-methyltransferase Proteins 0.000 description 1
- 101000748410 Geobacillus stearothermophilus Uncharacterized protein in fumA 3'region Proteins 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 101000772675 Haemophilus influenzae (strain ATCC 51907 / DSM 11121 / KW20 / Rd) UPF0438 protein HI_0847 Proteins 0.000 description 1
- 101000631019 Haemophilus influenzae (strain ATCC 51907 / DSM 11121 / KW20 / Rd) Uncharacterized protein HI_0350 Proteins 0.000 description 1
- 101000768938 Haemophilus phage HP1 (strain HP1c1) Uncharacterized 8.9 kDa protein in int-C1 intergenic region Proteins 0.000 description 1
- 101000785414 Homo sapiens Ankyrin repeat, SAM and basic leucine zipper domain-containing protein 1 Proteins 0.000 description 1
- 241000725303 Human immunodeficiency virus Species 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 101000782488 Junonia coenia densovirus (isolate pBRJ/1990) Putative non-structural protein NS2 Proteins 0.000 description 1
- 101000811523 Klebsiella pneumoniae Uncharacterized 55.8 kDa protein in cps region Proteins 0.000 description 1
- 101000818409 Lactococcus lactis subsp. lactis Uncharacterized HTH-type transcriptional regulator in lacX 3'region Proteins 0.000 description 1
- 101000878851 Leptolyngbya boryana Putative Fe(2+) transport protein A Proteins 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 241000555303 Mamestra brassicae Species 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 101000758828 Methanosarcina barkeri (strain Fusaro / DSM 804) Uncharacterized protein Mbar_A1602 Proteins 0.000 description 1
- 101001122401 Middle East respiratory syndrome-related coronavirus (isolate United Kingdom/H123990006/2012) Non-structural protein ORF3 Proteins 0.000 description 1
- 102000007474 Multiprotein Complexes Human genes 0.000 description 1
- 108010085220 Multiprotein Complexes Proteins 0.000 description 1
- 101001055788 Mycolicibacterium smegmatis (strain ATCC 700084 / mc(2)155) Pentapeptide repeat protein MfpA Proteins 0.000 description 1
- 241001534230 Nereididae Species 0.000 description 1
- 241000714209 Norwalk virus Species 0.000 description 1
- 108091028043 Nucleic acid sequence Proteins 0.000 description 1
- 108091005461 Nucleic proteins Proteins 0.000 description 1
- 108700026244 Open Reading Frames Proteins 0.000 description 1
- 101000740670 Orgyia pseudotsugata multicapsid polyhedrosis virus Protein C42 Proteins 0.000 description 1
- 108010033276 Peptide Fragments Proteins 0.000 description 1
- 102000007079 Peptide Fragments Human genes 0.000 description 1
- 101000769182 Photorhabdus luminescens Uncharacterized protein in pnp 3'region Proteins 0.000 description 1
- 101000961392 Pseudescherichia vulneris Uncharacterized 29.9 kDa protein in crtE 3'region Proteins 0.000 description 1
- 101000731030 Pseudomonas oleovorans Poly(3-hydroxyalkanoate) polymerase 2 Proteins 0.000 description 1
- 101001065485 Pseudomonas putida Probable fatty acid methyltransferase Proteins 0.000 description 1
- 101000711023 Rhizobium leguminosarum bv. trifolii Uncharacterized protein in tfuA 3'region Proteins 0.000 description 1
- 101000948156 Rhodococcus erythropolis Uncharacterized 47.3 kDa protein in thcA 5'region Proteins 0.000 description 1
- 101000917565 Rhodococcus fascians Uncharacterized 33.6 kDa protein in fasciation locus Proteins 0.000 description 1
- 101000790284 Saimiriine herpesvirus 2 (strain 488) Uncharacterized 9.5 kDa protein in DHFR 3'region Proteins 0.000 description 1
- 241001635203 Sapporo virus-Manchester Species 0.000 description 1
- 101710204410 Scaffold protein Proteins 0.000 description 1
- 101710146343 Scaffold protein D13 Proteins 0.000 description 1
- 241000191940 Staphylococcus Species 0.000 description 1
- 101000936719 Streptococcus gordonii Accessory Sec system protein Asp3 Proteins 0.000 description 1
- 101000788499 Streptomyces coelicolor Uncharacterized oxidoreductase in mprA 5'region Proteins 0.000 description 1
- 101001102841 Streptomyces griseus Purine nucleoside phosphorylase ORF3 Proteins 0.000 description 1
- 101000708557 Streptomyces lincolnensis Uncharacterized 17.2 kDa protein in melC2-rnhH intergenic region Proteins 0.000 description 1
- 241001655322 Streptomycetales Species 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 101000649826 Thermotoga neapolitana Putative anti-sigma factor antagonist TM1081 homolog Proteins 0.000 description 1
- 101000827562 Vibrio alginolyticus Uncharacterized protein in proC 3'region Proteins 0.000 description 1
- 101000778915 Vibrio parahaemolyticus serotype O3:K6 (strain RIMD 2210633) Uncharacterized membrane protein VP2115 Proteins 0.000 description 1
- 108020000999 Viral RNA Proteins 0.000 description 1
- MZVQCMJNVPIDEA-UHFFFAOYSA-N [CH2]CN(CC)CC Chemical group [CH2]CN(CC)CC MZVQCMJNVPIDEA-UHFFFAOYSA-N 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 238000001467 acupuncture Methods 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 208000012873 acute gastroenteritis Diseases 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000000274 adsorptive effect Effects 0.000 description 1
- 239000012615 aggregate Substances 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 125000000539 amino acid group Chemical group 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 238000010923 batch production Methods 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- AXCZMVOFGPJBDE-UHFFFAOYSA-L calcium dihydroxide Chemical compound [OH-].[OH-].[Ca+2] AXCZMVOFGPJBDE-UHFFFAOYSA-L 0.000 description 1
- 239000000920 calcium hydroxide Substances 0.000 description 1
- 229910001861 calcium hydroxide Inorganic materials 0.000 description 1
- 229910001424 calcium ion Inorganic materials 0.000 description 1
- 238000005251 capillar electrophoresis Methods 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 125000002057 carboxymethyl group Chemical group [H]OC(=O)C([H])([H])[*] 0.000 description 1
- 239000003729 cation exchange resin Substances 0.000 description 1
- 229940023913 cation exchange resins Drugs 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 230000034303 cell budding Effects 0.000 description 1
- 239000012930 cell culture fluid Substances 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- 239000002801 charged material Substances 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 238000013375 chromatographic separation Methods 0.000 description 1
- 239000012504 chromatography matrix Substances 0.000 description 1
- 238000000975 co-precipitation Methods 0.000 description 1
- 238000004737 colorimetric analysis Methods 0.000 description 1
- 238000010668 complexation reaction Methods 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 230000001010 compromised effect Effects 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 239000012531 culture fluid Substances 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 238000004925 denaturation Methods 0.000 description 1
- 230000036425 denaturation Effects 0.000 description 1
- 239000000551 dentifrice Substances 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000001212 derivatisation Methods 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 241001493065 dsRNA viruses Species 0.000 description 1
- 238000002296 dynamic light scattering Methods 0.000 description 1
- 238000001493 electron microscopy Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 239000002095 exotoxin Substances 0.000 description 1
- 231100000776 exotoxin Toxicity 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 230000002550 fecal effect Effects 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 102000034238 globular proteins Human genes 0.000 description 1
- 108091005896 globular proteins Proteins 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 230000005745 host immune response Effects 0.000 description 1
- 239000000819 hypertonic solution Substances 0.000 description 1
- 229940021223 hypertonic solution Drugs 0.000 description 1
- 125000001841 imino group Chemical group [H]N=* 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- VMPHSYLJUKZBJJ-UHFFFAOYSA-N lauric acid triglyceride Natural products CCCCCCCCCCCC(=O)OCC(OC(=O)CCCCCCCCCCC)COC(=O)CCCCCCCCCCC VMPHSYLJUKZBJJ-UHFFFAOYSA-N 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 239000012533 medium component Substances 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- MYWUZJCMWCOHBA-VIFPVBQESA-N methamphetamine Chemical compound CN[C@@H](C)CC1=CC=CC=C1 MYWUZJCMWCOHBA-VIFPVBQESA-N 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 238000000386 microscopy Methods 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 230000016379 mucosal immune response Effects 0.000 description 1
- 244000309711 non-enveloped viruses Species 0.000 description 1
- 238000001821 nucleic acid purification Methods 0.000 description 1
- 238000010979 pH adjustment Methods 0.000 description 1
- 239000013618 particulate matter Substances 0.000 description 1
- 238000005192 partition Methods 0.000 description 1
- 230000010412 perfusion Effects 0.000 description 1
- 238000009520 phase I clinical trial Methods 0.000 description 1
- 239000007981 phosphate-citrate buffer Substances 0.000 description 1
- 230000010287 polarization Effects 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 238000003752 polymerase chain reaction Methods 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 238000011027 product recovery Methods 0.000 description 1
- 210000001236 prokaryotic cell Anatomy 0.000 description 1
- 230000000644 propagated effect Effects 0.000 description 1
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 1
- 230000006920 protein precipitation Effects 0.000 description 1
- 239000012521 purified sample Substances 0.000 description 1
- SYUHGPGVQRZVTB-UHFFFAOYSA-N radon atom Chemical compound [Rn] SYUHGPGVQRZVTB-UHFFFAOYSA-N 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 239000012925 reference material Substances 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 239000012266 salt solution Substances 0.000 description 1
- 238000010187 selection method Methods 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 229910001467 sodium calcium phosphate Inorganic materials 0.000 description 1
- 235000011008 sodium phosphates Nutrition 0.000 description 1
- 239000002689 soil Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 238000000527 sonication Methods 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 229910021653 sulphate ion Inorganic materials 0.000 description 1
- 239000013595 supernatant sample Substances 0.000 description 1
- 238000012876 topography Methods 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 239000011534 wash buffer Substances 0.000 description 1
- 239000008215 water for injection Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/14—Extraction; Separation; Purification
- C07K1/16—Extraction; Separation; Purification by chromatography
- C07K1/18—Ion-exchange chromatography
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/005—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from viruses
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N7/00—Viruses; Bacteriophages; Compositions thereof; Preparation or purification thereof
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N7/00—Viruses; Bacteriophages; Compositions thereof; Preparation or purification thereof
- C12N7/02—Recovery or purification
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/16011—Caliciviridae
- C12N2770/16023—Virus like particles [VLP]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/16011—Caliciviridae
- C12N2770/16034—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/16011—Caliciviridae
- C12N2770/16051—Methods of production or purification of viral material
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Immunology (AREA)
- Virology (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- Epidemiology (AREA)
- Biomedical Technology (AREA)
- General Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Mycology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Gastroenterology & Hepatology (AREA)
- Analytical Chemistry (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Peptides Or Proteins (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Separation Using Semi-Permeable Membranes (AREA)
- Treatment Of Liquids With Adsorbents In General (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US90682107P | 2007-03-14 | 2007-03-14 | |
| US60/906,821 | 2007-03-14 | ||
| PCT/US2008/057072 WO2008113011A2 (en) | 2007-03-14 | 2008-03-14 | Virus like particle purification |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020157020558A Division KR20150098681A (ko) | 2007-03-14 | 2008-03-14 | 바이러스 유사 입자 정제 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20170001720A true KR20170001720A (ko) | 2017-01-04 |
Family
ID=39760420
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167036031A Ceased KR20170001720A (ko) | 2007-03-14 | 2008-03-14 | 바이러스 유사 입자 정제 |
| KR1020097021421A Active KR101576219B1 (ko) | 2007-03-14 | 2008-03-14 | 바이러스 유사 입자 정제 |
| KR1020157020558A Ceased KR20150098681A (ko) | 2007-03-14 | 2008-03-14 | 바이러스 유사 입자 정제 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020097021421A Active KR101576219B1 (ko) | 2007-03-14 | 2008-03-14 | 바이러스 유사 입자 정제 |
| KR1020157020558A Ceased KR20150098681A (ko) | 2007-03-14 | 2008-03-14 | 바이러스 유사 입자 정제 |
Country Status (12)
| Country | Link |
|---|---|
| US (4) | US8481693B2 (enExample) |
| EP (1) | EP2134360B1 (enExample) |
| JP (4) | JP5284290B2 (enExample) |
| KR (3) | KR20170001720A (enExample) |
| AU (1) | AU2008224877B2 (enExample) |
| CA (1) | CA2683977C (enExample) |
| DK (1) | DK2134360T3 (enExample) |
| ES (1) | ES2559421T3 (enExample) |
| HU (1) | HUE028605T2 (enExample) |
| PL (1) | PL2134360T3 (enExample) |
| SG (2) | SG10201701988TA (enExample) |
| WO (1) | WO2008113011A2 (enExample) |
Families Citing this family (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2601970B1 (en) | 2006-09-29 | 2016-10-26 | Takeda Vaccines, Inc. | Norovirus vaccine formulations |
| EP2134360B1 (en) | 2007-03-14 | 2015-11-18 | Takeda Vaccines, Inc. | Virus like particle purification |
| US20100266636A1 (en) | 2007-09-18 | 2010-10-21 | Ligocyte Pharmaceuticals, Inc. | Method of conferring a protective immune response to norovirus |
| US10130696B2 (en) | 2007-09-18 | 2018-11-20 | Takeda Vaccines, Inc. | Method of conferring a protective immune response to norovirus |
| EP3067417B1 (en) | 2009-06-16 | 2018-07-25 | Genzyme Corporation | Improved methods for purification of recombinant aav vectors |
| WO2011000058A1 (en) * | 2009-07-03 | 2011-01-06 | The University Of Queensland | A method of preparation of a biological particulate structure |
| US20110142863A1 (en) * | 2009-12-16 | 2011-06-16 | Millipore Corporation | Flow through purification processes for large biomolecules |
| WO2011088225A1 (en) | 2010-01-15 | 2011-07-21 | Bio-Rad Laboratories, Inc. | Surface neutralization of apatite |
| KR101549296B1 (ko) * | 2010-04-14 | 2015-09-01 | 이엠디 밀리포어 코포레이션 | 고역가 고순도 바이러스 스톡의 생산 방법 및 이의 사용 방법 |
| DE102010046817A1 (de) * | 2010-09-28 | 2012-03-29 | Sartorius Stedim Biotech Gmbh | Verfahren zur Abtrennung von Viren aus einem Kontaminanten enthaltenden flüssigen Medium |
| FI122520B (fi) * | 2010-10-15 | 2012-03-15 | Vesna Blazevic | Noroviruksen kapsidi ja rotaviruksen VP6-proteiini käytettäväksi yhdistelmärokotteena |
| WO2012106449A1 (en) | 2011-02-02 | 2012-08-09 | Bio-Rad Laboratories, Inc. | Apatite surface neutralization with alkali solutions |
| BR112014000656A2 (pt) * | 2011-07-11 | 2017-02-14 | Takeda Vaccines Inc | formulações de vacina parenteral contra norovírus |
| EP2554664A1 (de) * | 2011-08-02 | 2013-02-06 | Life Science Inkubator | Verfahren zur Aufreinigung von Virus-ähnlichen Partikeln (VLP) |
| EP2797626B1 (en) * | 2011-12-30 | 2019-07-03 | Deutsches Krebsforschungszentrum | Second generation virus-like particles (vlp) from epstein-barr viruses for vaccination purposes |
| CN104507952B (zh) * | 2012-05-30 | 2018-08-10 | 生物辐射实验室股份有限公司 | 基于磷灰石的色谱树脂的原位恢复 |
| JOP20130186B1 (ar) | 2012-06-22 | 2021-08-17 | Takeda Vaccines Montana Inc | تنقية الجزيئات الشبيهة بالفيروسات |
| SG11201505190VA (en) * | 2013-02-22 | 2015-08-28 | Agency Science Tech & Res | Chromatographic purification of virus preparations with negatively charged particles |
| JP6479305B2 (ja) * | 2013-07-12 | 2019-03-06 | 株式会社Umnファーマ | ウイルス様粒子の精製方法 |
| JP2015015931A (ja) * | 2013-07-12 | 2015-01-29 | 株式会社Umnファーマ | ウイルス様粒子を含む培養物の製造方法 |
| JP2015017065A (ja) * | 2013-07-12 | 2015-01-29 | 株式会社Umnファーマ | ノロウイルスのウイルス様粒子を含む医薬組成物 |
| CN106103720A (zh) | 2013-10-03 | 2016-11-09 | 武田疫苗股份有限公司 | 自细胞系检测和除去弹状病毒的方法 |
| CN105392569B (zh) | 2014-06-23 | 2019-08-20 | 生物辐射实验室股份有限公司 | 磷灰石预处理 |
| WO2015200278A1 (en) | 2014-06-23 | 2015-12-30 | Bio-Rad Laboratories, Inc. | Apatite in-situ restoration |
| CN107603959A (zh) * | 2017-08-30 | 2018-01-19 | 四川大学 | 提高缓冲液盐离子浓度纯化病毒的方法 |
| KR20250077599A (ko) * | 2017-12-07 | 2025-05-30 | 이엠피 바이오테크 게엠베하 | 응용 방사형 기술 크로마토그래피의 시스템 및 방법 |
| JP6704074B2 (ja) * | 2019-02-04 | 2020-06-03 | 株式会社Umnファーマ | ウイルス様粒子の精製方法 |
| CN114008067B (zh) * | 2019-07-04 | 2025-08-05 | 株式会社钟化 | 病毒或病毒样颗粒的纯化方法 |
| WO2022044727A1 (ja) * | 2020-08-28 | 2022-03-03 | 株式会社カネカ | 有用物質の精製方法 |
| CN116348598A (zh) * | 2020-10-05 | 2023-06-27 | 赛多利斯比亚分离有限责任公司 | 通过疏水相互作用色谱法改善dna质粒制剂中rna和污染物的去除 |
| AU2022217587A1 (en) * | 2021-02-02 | 2023-08-17 | C. I. TAKIRON Corporation | Solar power generation system and reflector for solar power generation system |
| WO2022246323A1 (en) | 2021-05-21 | 2022-11-24 | Takeda Vaccines, Inc. | Solid composition, freeze-drying method and glass vial |
| WO2025077807A1 (en) * | 2023-10-12 | 2025-04-17 | Chengdu Kanghua Biological Products Co., Ltd. | Methods for obtaining high-purity norovirus virus-like particles (vlps) |
| WO2025077800A1 (en) * | 2023-10-12 | 2025-04-17 | Chengdu Kanghua Biological Products Co., Ltd. | Purification of norovirus virus-like particles (vlps) |
Family Cites Families (39)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2833106B2 (ja) | 1990-02-21 | 1998-12-09 | 株式会社島津製作所 | 液体クロマトグラフ |
| WO1994026087A2 (en) | 1993-05-14 | 1994-11-24 | Connor Kim C O | Recombinant protein production and insect cell culture and process |
| EP0757717B1 (en) * | 1994-05-16 | 2006-05-31 | Merck & Co. Inc. | Papillomavirus vaccines |
| AR004464A1 (es) | 1994-11-14 | 1998-12-16 | Merck Sharp & Dohme | Un metodo para producir una proteina de capside de papilomavirus |
| JP3316325B2 (ja) | 1994-12-20 | 2002-08-19 | 富士シリシア化学株式会社 | ビール安定化処理用シリカゲル及びその製造方法並びにビールの安定化処理方法 |
| US5641870A (en) | 1995-04-20 | 1997-06-24 | Genentech, Inc. | Low pH hydrophobic interaction chromatography for antibody purification |
| ATE423204T1 (de) | 1996-11-08 | 2009-03-15 | Us Gov Health & Human Serv | Herstellung und reinigung von hepatitis c virus- ähnlichen partikeln |
| WO1999013056A1 (en) * | 1997-09-05 | 1999-03-18 | Medimmune, Inc. | IN VITRO METHOD FOR DISASSEMBLY/REASSEMBLY OF PAPILLOMAVIRUS VIRUS-LIKE PARTICLES (VLPs) |
| DE19823814A1 (de) | 1998-05-27 | 1999-12-02 | Octapharma Ag Ziegelbruecke | Verfahren zur Trennung und/oder Isolierung von Plasmaproteinen mit annularer Chromatographie |
| DK1105466T3 (da) * | 1998-08-14 | 2006-08-14 | Merck & Co Inc | Fremgangsmåde til oprensning af humant papillomvirus-viruslignende partikler |
| US6349843B1 (en) | 1999-04-01 | 2002-02-26 | The Vollrath Company Llc | Pan removal ramp |
| ES2398413T3 (es) * | 1999-06-22 | 2013-03-19 | Japan As Represented By Director-General Of National Institute Of Infectious Diseases | Kit de detección de SRSV |
| DE10012732A1 (de) | 2000-03-18 | 2001-09-20 | Aventis Behring Gmbh | Thrombin-Zubereitungen und Verfahren zu ihrer Herstellung |
| JP2003066021A (ja) | 2001-08-28 | 2003-03-05 | Hiroshi Shionoya | 連続分取液体クロマトグラフィー装置とそれを用いる方法 |
| AU2003213060A1 (en) * | 2002-02-14 | 2003-09-04 | Novavax, Inc. | Optimization of gene sequences of chimeric virus-like particles for expression in insect cells |
| CA2478925C (en) | 2002-04-26 | 2016-06-07 | Robert Lee Fahrner | Non-affinity purification of proteins |
| AU2003268210A1 (en) | 2002-08-28 | 2004-03-19 | Introgen Therapeutics Inc. | Chromatographic methods for adenovirus purification |
| WO2005032457A2 (en) * | 2003-07-21 | 2005-04-14 | Boyce Thompson Institute For Plant Research, Inc. | Vectors and methods for immunization against norwalk virus using transgenic plants |
| US7481997B1 (en) * | 2004-02-12 | 2009-01-27 | Montana State University | Snow mountain virus genome sequence, virus-like particles and methods of use |
| US8277819B2 (en) * | 2005-06-16 | 2012-10-02 | Children's Hospital Medical Center | Norovirus particle for use as an antiviral or vaccine |
| EP1736538A1 (en) * | 2005-06-21 | 2006-12-27 | Cytos Biotechnology AG | Process for the preparative purification of virus-like-particles (VLPs) |
| JP5215865B2 (ja) * | 2005-11-22 | 2013-06-19 | ノバルティス ヴァクシンズ アンド ダイアグノスティクス インコーポレイテッド | ノロウイルス抗原およびサポウイルス抗原 |
| PT3320919T (pt) | 2005-12-29 | 2020-09-03 | Boehringer Ingelheim Animal Health Usa Inc | Composições imunogénicas de pcv2 multivalentes e métodos para produzir composições deste tipo |
| WO2008094197A2 (en) | 2006-07-27 | 2008-08-07 | Ligocyte Pharmaceuticals, Inc. | Chimeric influenza virus-like particles |
| EP2601970B1 (en) | 2006-09-29 | 2016-10-26 | Takeda Vaccines, Inc. | Norovirus vaccine formulations |
| US8202967B2 (en) | 2006-10-27 | 2012-06-19 | Boehringer Ingelheim Vetmedica, Inc. | H5 proteins, nucleic acid molecules and vectors encoding for those, and their medicinal use |
| EP2134360B1 (en) | 2007-03-14 | 2015-11-18 | Takeda Vaccines, Inc. | Virus like particle purification |
| EP2027875A1 (en) | 2007-08-23 | 2009-02-25 | Octapharma AG | A Process for Isolation and Purification of a Target Protein free of Prion Protein (PrPSC) |
| US10130696B2 (en) | 2007-09-18 | 2018-11-20 | Takeda Vaccines, Inc. | Method of conferring a protective immune response to norovirus |
| EP2235165A1 (en) | 2007-12-21 | 2010-10-06 | Cytos Biotechnology AG | Process for clarifying cell homogenates |
| ES2668836T3 (es) * | 2008-08-08 | 2018-05-22 | Takeda Vaccines, Inc. | Partículas similivíricas que comprenden secuencias de aminoácidos de la cápside compuestas para reactividad cruzada potenciada |
| AU2009319979B2 (en) | 2008-11-03 | 2016-10-20 | Takeda Vaccines, Inc. | Improved methods for isolating enveloped virus-based VLPs free of infectious agents |
| US8945895B2 (en) | 2009-07-31 | 2015-02-03 | Baxter International Inc. | Methods of purifying recombinant ADAMTS13 and other proteins and compositions thereof |
| CN102791286B (zh) * | 2010-01-21 | 2017-08-08 | 莱戈赛特医药股份有限公司 | 杯状病毒病毒样颗粒上靶定异源抗原的呈递 |
| JP5903101B2 (ja) | 2010-09-20 | 2016-04-13 | バイオ−ラッド ラボラトリーズ インコーポレーティッド | クロマトグラフィーにおける生成物と複合体形成した夾雑物の分離方法 |
| FI122520B (fi) | 2010-10-15 | 2012-03-15 | Vesna Blazevic | Noroviruksen kapsidi ja rotaviruksen VP6-proteiini käytettäväksi yhdistelmärokotteena |
| CN102260651A (zh) | 2011-06-24 | 2011-11-30 | 华中科技大学同济医学院附属协和医院 | 一种纯化病毒样颗粒蛋白的方法及其应用 |
| JOP20130186B1 (ar) | 2012-06-22 | 2021-08-17 | Takeda Vaccines Montana Inc | تنقية الجزيئات الشبيهة بالفيروسات |
| JP2015015931A (ja) | 2013-07-12 | 2015-01-29 | 株式会社Umnファーマ | ウイルス様粒子を含む培養物の製造方法 |
-
2008
- 2008-03-14 EP EP08782762.2A patent/EP2134360B1/en active Active
- 2008-03-14 KR KR1020167036031A patent/KR20170001720A/ko not_active Ceased
- 2008-03-14 PL PL08782762T patent/PL2134360T3/pl unknown
- 2008-03-14 DK DK08782762.2T patent/DK2134360T3/en active
- 2008-03-14 HU HUE08782762A patent/HUE028605T2/en unknown
- 2008-03-14 JP JP2009553820A patent/JP5284290B2/ja active Active
- 2008-03-14 AU AU2008224877A patent/AU2008224877B2/en active Active
- 2008-03-14 SG SG10201701988TA patent/SG10201701988TA/en unknown
- 2008-03-14 KR KR1020097021421A patent/KR101576219B1/ko active Active
- 2008-03-14 CA CA2683977A patent/CA2683977C/en active Active
- 2008-03-14 SG SG2012018131A patent/SG179488A1/en unknown
- 2008-03-14 WO PCT/US2008/057072 patent/WO2008113011A2/en not_active Ceased
- 2008-03-14 ES ES08782762.2T patent/ES2559421T3/es active Active
- 2008-03-14 US US12/531,248 patent/US8481693B2/en active Active
- 2008-03-14 KR KR1020157020558A patent/KR20150098681A/ko not_active Ceased
-
2013
- 2013-05-29 JP JP2013112580A patent/JP2013176392A/ja active Pending
- 2013-06-10 US US13/914,331 patent/US9359410B2/en active Active
-
2016
- 2016-05-02 US US15/144,265 patent/US10172930B2/en active Active
-
2017
- 2017-02-23 JP JP2017032171A patent/JP2017086090A/ja active Pending
-
2018
- 2018-12-07 US US16/213,297 patent/US10792353B2/en active Active
-
2019
- 2019-01-07 JP JP2019000688A patent/JP2019068846A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| EP2134360A4 (en) | 2011-08-24 |
| SG10201701988TA (en) | 2017-04-27 |
| WO2008113011A2 (en) | 2008-09-18 |
| KR20150098681A (ko) | 2015-08-28 |
| AU2008224877B2 (en) | 2013-07-11 |
| DK2134360T3 (en) | 2016-02-22 |
| CA2683977C (en) | 2017-04-25 |
| US20100150961A1 (en) | 2010-06-17 |
| EP2134360A2 (en) | 2009-12-23 |
| JP2013176392A (ja) | 2013-09-09 |
| ES2559421T3 (es) | 2016-02-12 |
| US10172930B2 (en) | 2019-01-08 |
| KR101576219B1 (ko) | 2015-12-10 |
| US10792353B2 (en) | 2020-10-06 |
| JP2017086090A (ja) | 2017-05-25 |
| HK1138795A1 (zh) | 2010-09-03 |
| HUE028605T2 (en) | 2016-12-28 |
| US20190282686A1 (en) | 2019-09-19 |
| EP2134360B1 (en) | 2015-11-18 |
| JP5284290B2 (ja) | 2013-09-11 |
| US20160317645A1 (en) | 2016-11-03 |
| US9359410B2 (en) | 2016-06-07 |
| US8481693B2 (en) | 2013-07-09 |
| CA2683977A1 (en) | 2008-09-18 |
| US20130344107A1 (en) | 2013-12-26 |
| KR20100015562A (ko) | 2010-02-12 |
| PL2134360T3 (pl) | 2016-05-31 |
| AU2008224877A1 (en) | 2008-09-18 |
| JP2010530734A (ja) | 2010-09-16 |
| JP2019068846A (ja) | 2019-05-09 |
| SG179488A1 (en) | 2012-04-27 |
| WO2008113011A3 (en) | 2008-12-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101576219B1 (ko) | 바이러스 유사 입자 정제 | |
| Li et al. | A hydrophobic interaction chromatography strategy for purification of inactivated foot-and-mouth disease virus | |
| Tseng et al. | A fast and efficient purification platform for cell-based influenza viruses by flow-through chromatography | |
| KR20010072470A (ko) | 사람 유두종바이러스 바이러스-유사 입자의 정제방법 | |
| CN101490248A (zh) | 纯化流感病毒的方法 | |
| US20250034593A1 (en) | Method for Adenovirus Purification | |
| CN107849541A (zh) | 纯化腺病毒载体的方法 | |
| JP2024527602A (ja) | アデノ随伴ウイルスカプシドを分離するための方法、前記方法によって得られる組成物及びその使用 | |
| JP2016514101A (ja) | タンパク質製剤からエンドトキシンを除去するための物質および方法 | |
| CN105018435A (zh) | 一种病毒样颗粒的纯化方法 | |
| AU2013242822B2 (en) | Virus like particle purification | |
| AU2016269506A1 (en) | Virus like particle purification | |
| HK1138795B (en) | Virus like particle purification | |
| JP2024004020A (ja) | A型肝炎ウイルスのクロマトグラフィー精製方法 | |
| Alves | Production, concentration and purification strategies for Bluetongue virus |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20161222 Application number text: 1020157020558 Filing date: 20150728 |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20170117 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20170213 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20181026 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20170213 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |