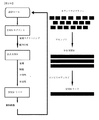
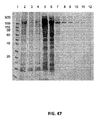
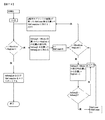
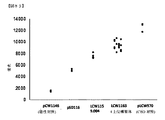
JP6355563B2 - Xten共役組成物およびそれを製造する方法 - Google Patents
Xten共役組成物およびそれを製造する方法 Download PDFInfo
- Publication number
- JP6355563B2 JP6355563B2 JP2014558977A JP2014558977A JP6355563B2 JP 6355563 B2 JP6355563 B2 JP 6355563B2 JP 2014558977 A JP2014558977 A JP 2014558977A JP 2014558977 A JP2014558977 A JP 2014558977A JP 6355563 B2 JP6355563 B2 JP 6355563B2
- Authority
- JP
- Japan
- Prior art keywords
- xten
- sequence
- group
- segment
- composition
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/12—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length by hydrolysis, i.e. solvolysis in general
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/22—Hormones
- A61K38/24—Follicle-stimulating hormone [FSH]; Chorionic gonadotropins, e.g. HCG; Luteinising hormone [LH]; Thyroid-stimulating hormone [TSH]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/64—Drug-peptide, drug-protein or drug-polyamino acid conjugates, i.e. the modifying agent being a peptide, protein or polyamino acid which is covalently bonded or complexed to a therapeutically active agent
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/001—Preparation for luminescence or biological staining
- A61K49/0013—Luminescence
- A61K49/0017—Fluorescence in vivo
- A61K49/0019—Fluorescence in vivo characterised by the fluorescent group, e.g. oligomeric, polymeric or dendritic molecules
- A61K49/0021—Fluorescence in vivo characterised by the fluorescent group, e.g. oligomeric, polymeric or dendritic molecules the fluorescent group being a small organic molecule
- A61K49/0032—Methine dyes, e.g. cyanine dyes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/001—Preparation for luminescence or biological staining
- A61K49/0013—Luminescence
- A61K49/0017—Fluorescence in vivo
- A61K49/005—Fluorescence in vivo characterised by the carrier molecule carrying the fluorescent agent
- A61K49/0052—Small organic molecules
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/001—Preparation for luminescence or biological staining
- A61K49/0013—Luminescence
- A61K49/0017—Fluorescence in vivo
- A61K49/005—Fluorescence in vivo characterised by the carrier molecule carrying the fluorescent agent
- A61K49/0056—Peptides, proteins, polyamino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/14—Extraction; Separation; Purification
- C07K1/16—Extraction; Separation; Purification by chromatography
- C07K1/18—Ion-exchange chromatography
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/02—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing at least one abnormal peptide link
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/02—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing at least one abnormal peptide link
- C07K5/0205—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing at least one abnormal peptide link containing the structure -NH-(X)3-C(=0)-, e.g. statine or derivatives thereof
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pharmacology & Pharmacy (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Epidemiology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Endocrinology (AREA)
- Immunology (AREA)
- Gastroenterology & Hepatology (AREA)
- Zoology (AREA)
- Reproductive Health (AREA)
- Crystallography & Structural Chemistry (AREA)
- Analytical Chemistry (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Pulmonology (AREA)
- Physical Education & Sports Medicine (AREA)
- Urology & Nephrology (AREA)
- Virology (AREA)
- Pain & Pain Management (AREA)
- Diabetes (AREA)
- Tropical Medicine & Parasitology (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261634312P | 2012-02-27 | 2012-02-27 | |
| US61/634,312 | 2012-02-27 | ||
| US201261690187P | 2012-06-18 | 2012-06-18 | |
| US61/690,187 | 2012-06-18 | ||
| US201261709942P | 2012-10-04 | 2012-10-04 | |
| US61/709,942 | 2012-10-04 | ||
| PCT/US2013/028116 WO2013130683A2 (en) | 2012-02-27 | 2013-02-27 | Xten conjugate compositions and methods of making same |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018100513A Division JP2018127496A (ja) | 2012-02-27 | 2018-05-25 | Xten共役組成物およびそれを製造する方法 |
| JP2018100514A Division JP2018130123A (ja) | 2012-02-27 | 2018-05-25 | Xten共役組成物およびそれを製造する方法 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2015514393A JP2015514393A (ja) | 2015-05-21 |
| JP2015514393A5 JP2015514393A5 (enExample) | 2016-04-14 |
| JP6355563B2 true JP6355563B2 (ja) | 2018-07-11 |
Family
ID=49083255
Family Applications (6)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014558977A Active JP6355563B2 (ja) | 2012-02-27 | 2013-02-27 | Xten共役組成物およびそれを製造する方法 |
| JP2018100514A Pending JP2018130123A (ja) | 2012-02-27 | 2018-05-25 | Xten共役組成物およびそれを製造する方法 |
| JP2018100513A Withdrawn JP2018127496A (ja) | 2012-02-27 | 2018-05-25 | Xten共役組成物およびそれを製造する方法 |
| JP2020054163A Withdrawn JP2020114234A (ja) | 2012-02-27 | 2020-03-25 | Xten共役組成物およびそれを製造する方法 |
| JP2022031705A Active JP7564143B2 (ja) | 2012-02-27 | 2022-03-02 | Xten共役組成物およびそれを製造する方法 |
| JP2024166129A Pending JP2024174020A (ja) | 2012-02-27 | 2024-09-25 | Xten共役組成物およびそれを製造する方法 |
Family Applications After (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018100514A Pending JP2018130123A (ja) | 2012-02-27 | 2018-05-25 | Xten共役組成物およびそれを製造する方法 |
| JP2018100513A Withdrawn JP2018127496A (ja) | 2012-02-27 | 2018-05-25 | Xten共役組成物およびそれを製造する方法 |
| JP2020054163A Withdrawn JP2020114234A (ja) | 2012-02-27 | 2020-03-25 | Xten共役組成物およびそれを製造する方法 |
| JP2022031705A Active JP7564143B2 (ja) | 2012-02-27 | 2022-03-02 | Xten共役組成物およびそれを製造する方法 |
| JP2024166129A Pending JP2024174020A (ja) | 2012-02-27 | 2024-09-25 | Xten共役組成物およびそれを製造する方法 |
Country Status (16)
| Country | Link |
|---|---|
| US (4) | US10172953B2 (enExample) |
| EP (2) | EP2819788B1 (enExample) |
| JP (6) | JP6355563B2 (enExample) |
| KR (1) | KR102057356B1 (enExample) |
| CN (3) | CN107496932A (enExample) |
| AU (2) | AU2013226090B2 (enExample) |
| CA (2) | CA2865578C (enExample) |
| CL (1) | CL2014002277A1 (enExample) |
| EA (2) | EA030911B1 (enExample) |
| IL (2) | IL234309B (enExample) |
| MX (2) | MX366864B (enExample) |
| PE (1) | PE20142451A1 (enExample) |
| PH (1) | PH12014501924A1 (enExample) |
| SG (2) | SG10201700340WA (enExample) |
| WO (2) | WO2013130683A2 (enExample) |
| ZA (2) | ZA201406339B (enExample) |
Families Citing this family (131)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3178835B1 (en) | 2009-02-03 | 2019-04-10 | Amunix Pharmaceuticals, Inc. | Extended recombinant polypeptides and compositions comprising same |
| KR102057356B1 (ko) * | 2012-02-27 | 2019-12-18 | 아뮤닉스 파마슈티컬스, 인크. | Xten 콘주게이트 조성물 및 그의 제조 방법 |
| WO2014100913A1 (en) * | 2012-12-24 | 2014-07-03 | Beijing Anxinhuaide Biotech. Co., Ltd | Improving the half life of a therapeutic polypeptide by fusing with a trimeric scaffold protein via a spacer |
| US9289502B2 (en) * | 2013-03-08 | 2016-03-22 | Emerald Therapeutics, Inc. | Preparation of oligo conjugates |
| EP2979702A4 (en) * | 2013-03-25 | 2016-11-16 | Zeria Pharm Co Ltd | GASTROKINETIC POSTPRANDIAL MEDIUM |
| US9322037B2 (en) * | 2013-09-06 | 2016-04-26 | President And Fellows Of Harvard College | Cas9-FokI fusion proteins and uses thereof |
| US9228207B2 (en) | 2013-09-06 | 2016-01-05 | President And Fellows Of Harvard College | Switchable gRNAs comprising aptamers |
| JP6822839B2 (ja) | 2013-09-13 | 2021-01-27 | ザ・スクリップス・リサーチ・インスティテュート | 修飾された治療剤、及びその組成物 |
| CN106456589B (zh) | 2013-12-18 | 2020-07-07 | 斯克利普斯研究所 | 修饰的治疗剂、订合的肽脂质缀合物及其组合物 |
| EP3089745A4 (en) | 2013-12-31 | 2017-05-17 | Placon Therapeutics, Inc. | Compounds, compositions, and methods for the treatment of cancers |
| US9119832B2 (en) | 2014-02-05 | 2015-09-01 | The Regents Of The University Of California | Methods of treating mild brain injury |
| KR101915452B1 (ko) | 2014-06-23 | 2018-11-06 | 플라콘 테라퓨틱스 인코포레이티드 | 백금 화합물, 조성물 및 이의 용도 |
| US10588980B2 (en) | 2014-06-23 | 2020-03-17 | Novartis Ag | Fatty acids and their use in conjugation to biomolecules |
| AU2015284236B2 (en) | 2014-06-30 | 2018-03-08 | Tva (Abc), Llc | Targeted conjugates and particles and formulations thereof |
| US10077453B2 (en) | 2014-07-30 | 2018-09-18 | President And Fellows Of Harvard College | CAS9 proteins including ligand-dependent inteins |
| WO2016028826A1 (en) * | 2014-08-19 | 2016-02-25 | Oxeia Biopharmaceuticals, Inc. | Methods of treating mild brain injury |
| SG11201702122RA (en) * | 2014-09-16 | 2017-04-27 | Univ Western Australia | Treatment of tumours using peptide-protein conjugates |
| WO2016048488A1 (en) * | 2014-09-25 | 2016-03-31 | Oxeia Biopharmaceuticals, Inc. | Methods of treating traumatic brain injury |
| SG11201703803WA (en) * | 2014-11-11 | 2017-06-29 | Amunix Operating Inc | Targeted xten conjugate compositions and methods of making same |
| US11497767B2 (en) | 2015-02-18 | 2022-11-15 | Enlivex Therapeutics R&D Ltd | Combination immune therapy and cytokine control therapy for cancer treatment |
| US11318163B2 (en) | 2015-02-18 | 2022-05-03 | Enlivex Therapeutics Ltd | Combination immune therapy and cytokine control therapy for cancer treatment |
| US11000548B2 (en) | 2015-02-18 | 2021-05-11 | Enlivex Therapeutics Ltd | Combination immune therapy and cytokine control therapy for cancer treatment |
| US11304976B2 (en) | 2015-02-18 | 2022-04-19 | Enlivex Therapeutics Ltd | Combination immune therapy and cytokine control therapy for cancer treatment |
| JP6858128B2 (ja) | 2015-02-18 | 2021-04-14 | エンリヴェックス セラピューティクス リミテッド | 癌治療のための免疫療法とサイトカイン制御療法との組み合わせ |
| US11596652B2 (en) | 2015-02-18 | 2023-03-07 | Enlivex Therapeutics R&D Ltd | Early apoptotic cells for use in treating sepsis |
| EP3875477A1 (en) | 2015-04-17 | 2021-09-08 | Alpine Immune Sciences, Inc. | Immunomodulatory proteins with tunable affinities |
| CN113106053A (zh) | 2015-04-21 | 2021-07-13 | 恩立夫克治疗有限责任公司 | 治疗性汇集的血液凋亡细胞制剂与其用途 |
| CN112649538B (zh) * | 2015-04-28 | 2024-03-29 | 深圳翰宇药业股份有限公司 | 多肽混合物高效液相色谱分析方法 |
| WO2016193380A1 (en) | 2015-06-02 | 2016-12-08 | Novo Nordisk A/S | Insulins with polar recombinant extensions |
| WO2017017109A1 (en) * | 2015-07-27 | 2017-02-02 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Mutated factor x polypeptides and uses thereof for the treatment of haemophilia |
| CN108135881B (zh) * | 2015-08-11 | 2020-11-13 | 同宜医药(苏州)有限公司 | 多配体药物偶联体及其用途 |
| IL319047A (en) | 2015-08-28 | 2025-04-01 | Amunix Operating Inc | Chimeric polypeptide composition and methods for its preparation and use |
| CN117567535A (zh) | 2015-09-10 | 2024-02-20 | 坦伯公司 | 生物正交组合物 |
| MA43348A (fr) * | 2015-10-01 | 2018-08-08 | Novo Nordisk As | Conjugués de protéines |
| WO2017075535A1 (en) | 2015-10-28 | 2017-05-04 | Oxeia Biopharmaceuticals, Inc. | Methods of treating neurodegenerative conditions |
| EP3368546A4 (en) | 2015-10-28 | 2019-06-26 | Tarveda Therapeutics, Inc. | AGAINST SSTR CONJUGATES AND PARTICLES AND FORMULATIONS THEREOF |
| ES2930643T3 (es) | 2015-11-23 | 2022-12-20 | Univ California | Seguimiento y manipulación de ARN celular a través de la administración nuclear de CRISPR/CAS9 |
| AU2016367306B2 (en) * | 2015-12-09 | 2021-04-22 | Medizinische Universität Wien | Monomaleimide-functionalized platinum compounds for cancer therapy |
| CN108473979B (zh) * | 2015-12-28 | 2022-08-19 | 出光兴产株式会社 | 肽标签及包含它的带有标签的蛋白质 |
| CN113546159B (zh) * | 2015-12-29 | 2023-09-08 | 派格生物医药(苏州)股份有限公司 | 包含glp-1受体激动剂和胰高血糖素受体激动剂的组合物及其用途 |
| EP3416661A4 (en) | 2016-02-18 | 2020-03-04 | Enlivex Therapeutics Ltd. | COMBINATION OF IMMUNOTHERAPY AND CYTOKINE CONTROL THERAPY FOR THE TREATMENT OF CANCER |
| AR107560A1 (es) | 2016-02-24 | 2018-05-09 | Lilly Co Eli | Compuesto que disminuye la glucosa en la sangre |
| JP2019513238A (ja) * | 2016-03-30 | 2019-05-23 | クオリザイム・ダイアグノスティクス・ゲゼルシャフト・ミット・ベシュレンクテル・ハフツング・ウント・コムパニー・コマンディットゲゼルシャフトQualizyme Diagnostics Gmbh And Co. Kg | 創傷における微生物感染の検出 |
| CA3019199A1 (en) | 2016-04-15 | 2017-10-19 | Alpine Immune Sciences, Inc. | Icos ligand variant immunomodulatory proteins and uses thereof |
| AU2017248830B2 (en) | 2016-04-15 | 2023-03-09 | Alpine Immune Sciences, Inc. | CD80 variant immunomodulatory proteins and uses thereof |
| WO2017197048A1 (en) * | 2016-05-11 | 2017-11-16 | Amunix Operating Inc. | Albumin binding conjugate compositions and methods of making and using same |
| EP3465221B1 (en) | 2016-05-27 | 2020-07-22 | H. Hoffnabb-La Roche Ag | Bioanalytical method for the characterization of site-specific antibody-drug conjugates |
| CA3029902A1 (en) | 2016-07-07 | 2018-01-11 | The Board Of Trustees Of The Leland Stanford Junior University | Antibody adjuvant conjugates |
| US11834490B2 (en) | 2016-07-28 | 2023-12-05 | Alpine Immune Sciences, Inc. | CD112 variant immunomodulatory proteins and uses thereof |
| US11471488B2 (en) | 2016-07-28 | 2022-10-18 | Alpine Immune Sciences, Inc. | CD155 variant immunomodulatory proteins and uses thereof |
| EP3491013A1 (en) | 2016-07-28 | 2019-06-05 | Alpine Immune Sciences, Inc. | Cd155 variant immunomodulatory proteins and uses thereof |
| US10738338B2 (en) | 2016-10-18 | 2020-08-11 | The Research Foundation for the State University | Method and composition for biocatalytic protein-oligonucleotide conjugation and protein-oligonucleotide conjugate |
| AU2017345764A1 (en) | 2016-10-20 | 2019-05-02 | Alpine Immune Sciences, Inc. | Secretable variant immunomodulatory proteins and engineered cell therapy |
| WO2018165631A1 (en) | 2017-03-09 | 2018-09-13 | President And Fellows Of Harvard College | Cancer vaccine |
| KR20190127797A (ko) | 2017-03-10 | 2019-11-13 | 프레지던트 앤드 펠로우즈 오브 하바드 칼리지 | 시토신에서 구아닌으로의 염기 편집제 |
| IL319966A (en) | 2017-03-16 | 2025-05-01 | Alpine Immune Sciences Inc | CD80 Variant Immune Modulator Proteins and Uses Thereof |
| CA3053812A1 (en) | 2017-03-16 | 2018-09-20 | Alpine Immune Sciences, Inc. | Pd-l2 variant immunomodulatory proteins and uses thereof |
| IL320149A (en) | 2017-03-16 | 2025-06-01 | Alpine Immune Sciences Inc | Pd-l1 variant immunomodulatory proteins and uses thereof |
| EP3606560A2 (en) | 2017-04-05 | 2020-02-12 | Novo Nordisk A/S | Oligomer extended insulin-fc conjugates |
| ES2983582T3 (es) | 2017-04-07 | 2024-10-23 | Tambo Inc | Composiciones bioortogonales |
| WO2018208998A1 (en) | 2017-05-10 | 2018-11-15 | The Regents Of The University Of California | Directed editing of cellular rna via nuclear delivery of crispr/cas9 |
| SG11201912625QA (en) | 2017-06-21 | 2020-01-30 | Jazz Pharmaceuticals Ireland Ltd | Modified l-asparaginase |
| EP3418383A1 (en) | 2017-06-21 | 2018-12-26 | XL-protein GmbH | Modified l-asparaginase |
| AU2018293467B2 (en) * | 2017-06-29 | 2020-11-05 | ImmuPharma Biotech SAS | Pro-drug peptide with improved pharmaceutical properties |
| WO2019023680A1 (en) | 2017-07-28 | 2019-01-31 | President And Fellows Of Harvard College | METHODS AND COMPOSITIONS FOR EVOLUTION OF BASIC EDITORS USING PHAGE-ASSISTED CONTINUOUS EVOLUTION (PACE) |
| JP2019043946A (ja) * | 2017-08-31 | 2019-03-22 | 国立大学法人埼玉大学 | 分子精製用リガンド、分子精製用タグペプチド及びこれらを用いた分子精製方法 |
| KR102813968B1 (ko) | 2017-10-10 | 2025-05-29 | 알파인 이뮨 사이언시즈, 인코포레이티드 | Ctla-4 변이체 면역조절 단백질 및 이의 용도 |
| TW202500579A (zh) | 2017-10-18 | 2025-01-01 | 美商艾爾潘免疫科學有限公司 | 變異型icos 配位體免疫調節蛋白及相關組合物及方法 |
| US11400165B2 (en) * | 2017-11-04 | 2022-08-02 | Advanced Proteome Therapeutics Inc. | Composition and method for modifying polypeptides |
| WO2019118949A1 (en) | 2017-12-15 | 2019-06-20 | The Broad Institute, Inc. | Systems and methods for predicting repair outcomes in genetic engineering |
| AU2018393111B2 (en) | 2017-12-21 | 2025-07-10 | Amunix Pharmaceuticals, Inc. | Release segments and binding compositions comprising same |
| US12297253B2 (en) | 2018-01-03 | 2025-05-13 | Alpine Immune Sciences, Inc. | Multi-domain immunomodulatory proteins and methods of use thereof |
| KR102113588B1 (ko) * | 2018-03-26 | 2020-05-22 | (주)터틀바이오 | 히스티딘-프롤린 반복서열을 갖는 올리고펩타이드의 대량생산 시스템 |
| WO2019241758A1 (en) | 2018-06-15 | 2019-12-19 | Alpine Immune Sciences, Inc. | Pd-1 variant immunomodulatory proteins and uses thereof |
| US10953107B2 (en) * | 2018-06-15 | 2021-03-23 | Trustees Of Boston University | Polypeptide compositions and methods for site-specific targeting of therapeutic agents |
| CA3100035A1 (en) | 2018-08-27 | 2020-03-05 | Regeneron Pharmaceuticals, Inc. | Use of raman spectroscopy in downstream purification |
| US20220177587A1 (en) | 2018-09-19 | 2022-06-09 | Alpine Immune Sciences, Inc. | Methods and uses of variant cd80 fusion proteins and related constructs |
| MX2021003543A (es) | 2018-09-27 | 2021-06-23 | Xilio Dev Inc | Polipeptidos de citocinas enmascaradas. |
| US20210324010A1 (en) * | 2018-10-10 | 2021-10-21 | University Of Washington | Fusion products and bioconjugates containing mixed charge peptides |
| US12331320B2 (en) | 2018-10-10 | 2025-06-17 | The Research Foundation For The State University Of New York | Genome edited cancer cell vaccines |
| CA3117409A1 (en) | 2018-10-29 | 2020-05-07 | Biogen Ma Inc. | Humanized and stabilized fc5 variants for enhancement of blood brain barrier transport |
| US12281338B2 (en) | 2018-10-29 | 2025-04-22 | The Broad Institute, Inc. | Nucleobase editors comprising GeoCas9 and uses thereof |
| CN116102621A (zh) * | 2018-11-07 | 2023-05-12 | 浙江道尔生物科技有限公司 | 用于改善活性蛋白或多肽性能的人工重组蛋白及其应用 |
| BR112021008098A2 (pt) | 2018-11-15 | 2021-08-10 | Quantum-Si Incorporated | métodos e composições para o sequenciamento de proteínas |
| US11175228B2 (en) * | 2018-11-28 | 2021-11-16 | Promega Corporation | Reactive peptide labeling |
| WO2020113141A2 (en) | 2018-11-30 | 2020-06-04 | Alpine Immune Sciences, Inc. | Cd86 variant immunomodulatory proteins and uses thereof |
| WO2020154500A1 (en) | 2019-01-23 | 2020-07-30 | The Broad Institute, Inc. | Supernegatively charged proteins and uses thereof |
| JP2022525594A (ja) | 2019-03-15 | 2022-05-18 | ボルト バイオセラピューティクス、インコーポレーテッド | Her2を標的とする免疫結合体 |
| MX2021011426A (es) | 2019-03-19 | 2022-03-11 | Broad Inst Inc | Metodos y composiciones para editar secuencias de nucleótidos. |
| WO2020214867A1 (en) | 2019-04-17 | 2020-10-22 | Alpine Immune Sciences, Inc. | Methods and uses of variant icos ligand (icosl) fusion proteins |
| EP3956349A1 (en) | 2019-04-17 | 2022-02-23 | The Broad Institute, Inc. | Adenine base editors with reduced off-target effects |
| EP3757217A1 (en) * | 2019-06-27 | 2020-12-30 | GlaxoSmithKline Biologicals S.A. | Methods for protein purification |
| AU2020345138A1 (en) * | 2019-09-13 | 2022-04-21 | Biological E Limited | N-terminal extension sequence for expression of recombinant therapeutic peptides |
| US12435330B2 (en) | 2019-10-10 | 2025-10-07 | The Broad Institute, Inc. | Methods and compositions for prime editing RNA |
| WO2021136223A1 (en) | 2019-12-31 | 2021-07-08 | Beijing Ql Biopharmaceutical Co., Ltd. | Fusion proteins of glp-1 and gdf15 and conjugates thereof |
| CN113728013B (zh) | 2020-01-11 | 2022-06-14 | 北京质肽生物医药科技有限公司 | Glp-1和fgf21的融合蛋白的缀合物 |
| WO2021183928A1 (en) * | 2020-03-13 | 2021-09-16 | Mayo Foundation For Medical Education And Research | Assessing and treating acute decompensated heart failure |
| IL297981A (en) | 2020-05-08 | 2023-01-01 | Alpine Immune Sciences Inc | April and baff inhibitory immunomodulatory proteins with and without a t cell inhibitory protein and methods of use thereof |
| MX2022014008A (es) | 2020-05-08 | 2023-02-09 | Broad Inst Inc | Métodos y composiciones para la edición simultánea de ambas cadenas de una secuencia de nucleótidos de doble cadena objetivo. |
| WO2021236983A2 (en) | 2020-05-20 | 2021-11-25 | Quantum-Si Incorporated | Methods and compositions for protein sequencing |
| WO2021262985A1 (en) * | 2020-06-25 | 2021-12-30 | Amunix Pharmaceuticals, Inc. | Cytokine conjugates |
| KR20230061458A (ko) | 2020-09-04 | 2023-05-08 | 에프. 호프만-라 로슈 아게 | Vegf-a 및 ang2에 결합하는 항체 및 사용 방법 |
| TW202228784A (zh) | 2020-09-23 | 2022-08-01 | 奧地利商艾柏力維亞生技有限公司 | 用以於一患者中螯合非預期的抗peg抗體的化合物 |
| KR20230083294A (ko) | 2020-09-30 | 2023-06-09 | 베이징 큐엘 바이오파마슈티컬 컴퍼니 리미티드 | 폴리펩타이드 접합체 및 사용 방법 |
| CN116348598A (zh) * | 2020-10-05 | 2023-06-27 | 赛多利斯比亚分离有限责任公司 | 通过疏水相互作用色谱法改善dna质粒制剂中rna和污染物的去除 |
| CA3195615A1 (en) * | 2020-10-16 | 2022-04-21 | Jonathan Miles Brown | Linker compounds comprising amide bonds |
| TWI815194B (zh) | 2020-10-22 | 2023-09-11 | 美商基利科學股份有限公司 | 介白素2-Fc融合蛋白及使用方法 |
| CN112362797B (zh) * | 2020-10-26 | 2022-05-27 | 浙江国正检测技术有限公司 | 一种饲料中喹诺酮类药物的检测方法 |
| US12404315B2 (en) | 2020-12-21 | 2025-09-02 | Allogene Therapeutics, Inc. | Protease-activating CD45-gate CAR |
| WO2022140783A1 (en) * | 2020-12-23 | 2022-06-30 | Jazz Pharmaceuticals Ireland Ltd. | Methods of purifying charge-shielded fusion proteins |
| CA3216795A1 (en) | 2021-05-07 | 2022-11-10 | Alpine Immune Sciences, Inc. | Methods of dosing and treatment with a taci-fc fusion immunomodulatory protein |
| JP2024522196A (ja) | 2021-06-09 | 2024-06-11 | ザ スクリプス リサーチ インスティテュート | 長時間作用型デュアルgip/glp-1ペプチドコンジュゲートおよび使用方法 |
| CN113671085B (zh) * | 2021-08-30 | 2023-03-03 | 珠海润都制药股份有限公司 | 一种缬沙坦中的2-叠氮基-3-甲基丁酸的检测方法 |
| CN113845585A (zh) * | 2021-11-08 | 2021-12-28 | 浙江毓昌生物技术有限公司 | 使用金属螯合色谱法去除重组人生长激素内毒素的方法 |
| WO2023172883A1 (en) | 2022-03-07 | 2023-09-14 | Alpine Immune Sciences, Inc. | Immunomodulatory proteins of variant cd80 polypeptides, cell therapies thereof and related methods and uses |
| CA3252113A1 (en) | 2022-03-30 | 2023-10-05 | Beijing Ql Biopharmaceutical Co., Ltd. | Liquid pharmaceutical compositions of polypeptide conjugates and their methods of use |
| CN119365485A (zh) | 2022-04-29 | 2025-01-24 | 博闻生物有限责任公司 | 补体因子h相关4特异性抗体及其用途 |
| AU2023259251A1 (en) | 2022-04-29 | 2024-11-14 | Broadwing Bio Llc | Angiopoietin-related protein 7-specific antibodies and uses thereof |
| EP4514845A1 (en) | 2022-04-29 | 2025-03-05 | Broadwing Bio LLC | Bispecific antibodies and methods of treating ocular disease |
| CN116063387B (zh) * | 2022-07-12 | 2023-11-24 | 东北农业大学 | 一种脯氨酸保护型抗酶解抗菌肽及其制备方法和应用 |
| CN115819513B (zh) * | 2022-07-25 | 2023-10-17 | 东北农业大学 | 一种月桂酸修饰抗酶解肽及其制备方法和应用 |
| WO2024077018A2 (en) | 2022-10-04 | 2024-04-11 | Alpine Immune Sciences, Inc. | Methods and uses of taci-fc fusion immunomodulatory protein |
| US12064467B2 (en) | 2022-10-28 | 2024-08-20 | Transdermal Biotechnology, Inc. | Systems and methods for delivery of humanin or other peptides |
| CN115819518B (zh) * | 2022-11-15 | 2025-10-31 | 中国人民解放军军事科学院军事医学研究院 | 一种具有黏附增强作用的阴离子肽 |
| KR20250169617A (ko) | 2023-04-11 | 2025-12-03 | 오츠카 세이야쿠 가부시키가이샤 | 아펠린 수용체 작용제 및 이의 용도 |
| WO2024227154A1 (en) | 2023-04-28 | 2024-10-31 | Broadwing Bio Llc | Complement component 3 (c3)-specific antibodies and uses thereof |
| WO2024236189A1 (en) * | 2023-05-17 | 2024-11-21 | Julius-Maximilians-Universitaet Wuerzburg | Matrix metalloproteinase 13 sensitive peptides and sensors |
| WO2025030053A1 (en) | 2023-08-01 | 2025-02-06 | Insmed Incorporated | Novel igg proteases and methods of use thereof |
| WO2025079347A1 (ja) * | 2023-10-10 | 2025-04-17 | 学校法人東京理科大学 | イノシン塩基の標識方法、イノシン塩基の検出方法、核酸の配列決定方法、イノシン塩基を含む核酸の濃縮方法、イノシン塩基標識剤、及びキット |
| WO2025085553A1 (en) | 2023-10-16 | 2025-04-24 | Paragon Therapeutics, Inc. | Fcrn antagonists with improved half-life and methods of use |
| CN119798359A (zh) * | 2025-01-06 | 2025-04-11 | 兰州大学 | 一种基于巯基-烯烃光点击化学的环肽构建方法及其应用 |
Family Cites Families (116)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ZA811368B (en) | 1980-03-24 | 1982-04-28 | Genentech Inc | Bacterial polypedtide expression employing tryptophan promoter-operator |
| US4398908A (en) | 1980-11-28 | 1983-08-16 | Siposs George G | Insulin delivery system |
| US4435173A (en) | 1982-03-05 | 1984-03-06 | Delta Medical Industries | Variable rate syringe pump for insulin delivery |
| DK27885A (da) | 1985-01-22 | 1986-07-23 | Novo Industri As | Fremstilling af peptider |
| US5118666A (en) | 1986-05-05 | 1992-06-02 | The General Hospital Corporation | Insulinotropic hormone |
| US4933185A (en) | 1986-09-24 | 1990-06-12 | Massachusetts Institute Of Technology | System for controlled release of biologically active compounds |
| US4976696A (en) | 1987-08-10 | 1990-12-11 | Becton, Dickinson And Company | Syringe pump and the like for delivering medication |
| US5041538A (en) | 1987-08-28 | 1991-08-20 | The Salk Institute For Biological Studies | Mammalian follistatin |
| US5182375A (en) | 1987-08-28 | 1993-01-26 | The Salk Institute For Biological Studies | DNA encoding follistatin |
| US5270176A (en) | 1987-11-20 | 1993-12-14 | Hoechst Aktiengesellschaft | Method for the selective cleavage of fusion proteins with lysostaphin |
| US4904584A (en) | 1987-12-23 | 1990-02-27 | Genetics Institute, Inc. | Site-specific homogeneous modification of polypeptides |
| JP2717808B2 (ja) | 1988-08-10 | 1998-02-25 | テルモ株式会社 | シリンジポンプ |
| US6225449B1 (en) | 1991-10-04 | 2001-05-01 | Washington University | Hormone analogs with multiple CTP extensions |
| US5017378A (en) | 1989-05-01 | 1991-05-21 | The University Of Virginia Alumni Patents Foundation | Intraorgan injection of biologically active compounds contained in slow-release microcapsules or microspheres |
| US5599907A (en) | 1989-05-10 | 1997-02-04 | Somatogen, Inc. | Production and use of multimeric hemoglobins |
| US5298022A (en) | 1989-05-29 | 1994-03-29 | Amplifon Spa | Wearable artificial pancreas |
| US5492534A (en) | 1990-04-02 | 1996-02-20 | Pharmetrix Corporation | Controlled release portable pump |
| US5318540A (en) | 1990-04-02 | 1994-06-07 | Pharmetrix Corporation | Controlled release infusion device |
| US5176502A (en) | 1990-04-25 | 1993-01-05 | Becton, Dickinson And Company | Syringe pump and the like for delivering medication |
| US5622929A (en) | 1992-01-23 | 1997-04-22 | Bristol-Myers Squibb Company | Thioether conjugates |
| HUT73494A (en) | 1993-03-29 | 1996-08-28 | Univ Cincinnati | Analogs of peptide yy and uses thereof |
| US5545616A (en) | 1994-09-22 | 1996-08-13 | Genentech, Inc. | Method for predicting and/or preventing preterm labor |
| US5824784A (en) | 1994-10-12 | 1998-10-20 | Amgen Inc. | N-terminally chemically modified protein compositions and methods |
| US5574010A (en) | 1994-11-14 | 1996-11-12 | The Regents Of The University Of California | Treatment of pancreatic tumors with peptide YY and analogs thereof |
| US5532336A (en) | 1995-01-31 | 1996-07-02 | Eli Lilly And Company | Anti-obesity proteins |
| US5521283A (en) | 1995-01-31 | 1996-05-28 | Eli Lilly And Company | Anti-obesity proteins |
| US5789379A (en) | 1995-04-14 | 1998-08-04 | Allelix Biopharmaceutical Inc. | Glucagon-like peptide-2 analogs |
| US6184201B1 (en) | 1995-04-14 | 2001-02-06 | Nps Allelix Corp. | Intestinotrophic glucagon-like peptide-2 analogs |
| US5834428A (en) | 1995-04-14 | 1998-11-10 | 1149336 Ontario Inc. | Glucagon-like peptide-2 and its therapeutic use |
| US5990077A (en) | 1995-04-14 | 1999-11-23 | 1149336 Ontario Inc. | Glucagon-like peptide-2 and its therapeutic use |
| US5830655A (en) | 1995-05-22 | 1998-11-03 | Sri International | Oligonucleotide sizing using cleavable primers |
| SE9503380D0 (sv) | 1995-09-29 | 1995-09-29 | Pharmacia Ab | Protein derivatives |
| CA2237114A1 (en) | 1995-11-07 | 1997-05-15 | Kaneka Corporation | Autoantigens |
| US6936439B2 (en) | 1995-11-22 | 2005-08-30 | Amgen Inc. | OB fusion protein compositions and methods |
| EP0932399B1 (en) | 1996-03-12 | 2006-01-04 | PG-TXL Company, L.P. | Water soluble paclitaxel prodrugs |
| US6441025B2 (en) | 1996-03-12 | 2002-08-27 | Pg-Txl Company, L.P. | Water soluble paclitaxel derivatives |
| US5994500A (en) | 1996-07-19 | 1999-11-30 | 1149336 Ontario Inc. | Antagonists of intestinotrophic GLP-2 peptides |
| US20020025933A1 (en) | 1996-08-30 | 2002-02-28 | Knudsen Liselotte Bjerre | GLP-2 derivatives |
| JP2001524808A (ja) | 1996-12-10 | 2001-12-04 | ジーントレイス・システムズ・インコーポレイテッド | 放出可能な不揮発性の質量標識分子 |
| AT405516B (de) | 1997-02-27 | 1999-09-27 | Immuno Ag | Faktor x-analoge mit modifizierter proteasespaltstelle |
| EP0983303B1 (en) | 1997-05-21 | 2006-03-08 | Biovation Limited | Method for the production of non-immunogenic proteins |
| US6248564B1 (en) | 1997-08-29 | 2001-06-19 | Harvard University | Mutant MHC class I molecules |
| EP1040200A4 (en) * | 1997-11-25 | 2005-04-20 | Gen Hospital Corp | NUCLEOTIDE SEQUENCES ASSOCIATED WITH VIRULENCE AND USES THEREOF |
| DE69838526T2 (de) | 1998-02-05 | 2008-07-03 | Biosense Webster, Inc., Diamond Bar | Gerät zum Freisetzen eines Medikaments im Herzen |
| US6406632B1 (en) | 1998-04-03 | 2002-06-18 | Symyx Technologies, Inc. | Rapid characterization of polymers |
| JP4574007B2 (ja) | 1998-04-28 | 2010-11-04 | メルク・セローノ・ソシエテ・アノニム | ポリオール−ifn−ベータ複体 |
| US7090976B2 (en) | 1999-11-10 | 2006-08-15 | Rigel Pharmaceuticals, Inc. | Methods and compositions comprising Renilla GFP |
| GB9815157D0 (en) | 1998-07-13 | 1998-09-09 | Metron Designs Ltd | High resolution pulse width setting from relatively low frequency clocks |
| US20030190740A1 (en) | 1998-10-13 | 2003-10-09 | The University Of Georgia Research Foundation, Inc | Stabilized bioactive peptides and methods of identification, synthesis, and use |
| ES2278463T3 (es) | 1998-12-08 | 2007-08-01 | Biovation Limited | Metodo para reducir la inmunogenicidad de proteinas. |
| US7666400B2 (en) | 2005-04-06 | 2010-02-23 | Ibc Pharmaceuticals, Inc. | PEGylation by the dock and lock (DNL) technique |
| CN100506844C (zh) | 1999-07-23 | 2009-07-01 | 寒川贤治 | 新的肽 |
| US20030109428A1 (en) | 1999-12-01 | 2003-06-12 | John Bertin | Novel molecules of the card-related protein family and uses thereof |
| US20050287153A1 (en) | 2002-06-28 | 2005-12-29 | Genentech, Inc. | Serum albumin binding peptides for tumor targeting |
| US6967237B2 (en) | 2000-05-30 | 2005-11-22 | Merck & Co., Inc. | Ghrelin analogs |
| US7186683B2 (en) | 2000-09-18 | 2007-03-06 | Sanos Bioscience A/S | Use of GLP for the treatment, prevention, diagnosis, and prognosis of bone-related and nutrition-related disorders |
| US20030228309A1 (en) * | 2000-11-08 | 2003-12-11 | Theodora Salcedo | Antibodies that immunospecifically bind to TRAIL receptors |
| US20030049689A1 (en) | 2000-12-14 | 2003-03-13 | Cynthia Edwards | Multifunctional polypeptides |
| US20020169125A1 (en) | 2001-03-21 | 2002-11-14 | Cell Therapeutics, Inc. | Recombinant production of polyanionic polymers and uses thereof |
| WO2002079415A2 (en) | 2001-03-30 | 2002-10-10 | Lexigen Pharmaceuticals Corp. | Reducing the immunogenicity of fusion proteins |
| KR100407467B1 (ko) | 2001-07-12 | 2003-11-28 | 최수봉 | 리모컨 방식의 인슐린 자동주사기 |
| AU2002322394A1 (en) | 2001-07-30 | 2003-02-17 | Eli Lilly And Company | Method for treating diabetes and obesity |
| US7125843B2 (en) | 2001-10-19 | 2006-10-24 | Neose Technologies, Inc. | Glycoconjugates including more than one peptide |
| WO2005077042A2 (en) | 2004-02-09 | 2005-08-25 | Human Genome Sciences, Inc. | Albumin fusion proteins |
| US20080167238A1 (en) | 2001-12-21 | 2008-07-10 | Human Genome Sciences, Inc. | Albumin Fusion Proteins |
| AU2003261074A1 (en) * | 2002-05-20 | 2003-12-02 | Jason M. Hogan | Protein identification from protein product ion spectra |
| WO2004000802A2 (en) | 2002-06-20 | 2003-12-31 | University Of Maryland Biotechnology Institute | Scaffolded maleimide clusters for multivalent peptide assembly |
| US7294513B2 (en) | 2002-07-24 | 2007-11-13 | Wyatt Technology Corporation | Method and apparatus for characterizing solutions of small particles |
| AU2003297859A1 (en) | 2002-12-13 | 2004-07-09 | The Trustees Of Columbia University In The City Of New York | Biomolecular coupling methods using 1,3-dipolar cycloaddition chemistry |
| US7166575B2 (en) | 2002-12-17 | 2007-01-23 | Nastech Pharmaceutical Company Inc. | Compositions and methods for enhanced mucosal delivery of peptide YY and methods for treating and preventing obesity |
| JP4629046B2 (ja) | 2003-05-14 | 2011-02-09 | ダニスコ・ユーエス・インコーポレーテッド | 反復配列タンパク質ポリマー活性剤結合体、方法および使用 |
| US20070161087A1 (en) | 2003-05-29 | 2007-07-12 | Wolfgang Glaesner | Glp-1 fusion proteins |
| CA2526120A1 (en) | 2003-06-03 | 2005-02-24 | Cell Genesys, Inc. | Compositions and methods for enhanced expression of recombinant polypeptides from a single vector using a peptide cleavage site |
| US20050152979A1 (en) | 2003-09-05 | 2005-07-14 | Cell Therapeutics, Inc. | Hydrophobic drug compositions containing reconstitution enhancer |
| KR101241862B1 (ko) | 2003-09-19 | 2013-03-13 | 노보 노르디스크 에이/에스 | 신규 glp-1 유도체 |
| EP1682106A4 (en) | 2003-10-30 | 2008-06-11 | Univ Emory | MODIFIED FVIII WITH REDUCED IMMUNOGENICITY BY MUTAGENESIS OF A2 AND C2 EPITOPES |
| WO2005069845A2 (en) | 2004-01-14 | 2005-08-04 | Ohio University | Methods of producing peptides/proteins in plants and peptides/proteins produced thereby |
| US8076288B2 (en) | 2004-02-11 | 2011-12-13 | Amylin Pharmaceuticals, Inc. | Hybrid polypeptides having glucose lowering activity |
| EP2314617B1 (en) | 2004-07-23 | 2015-06-24 | Acceleron Pharma Inc. | ActRII receptor polypeptides |
| BRPI0515118A (pt) * | 2004-08-31 | 2008-07-01 | Pharmacia & Upjohn Co Llc | conjugados de hormÈnio do crescimento humano com polietileno glicol |
| MX2007003320A (es) | 2004-09-24 | 2007-05-18 | Amgen Inc | Moleculas fc modificadas. |
| US20060099710A1 (en) * | 2004-11-10 | 2006-05-11 | Donnelly Mark I | Vector for improved in vivo production of proteins |
| JP5735194B2 (ja) | 2005-01-25 | 2015-06-17 | セル セラピューティクス インコーポレーテッド | 改善された生体内半減期を有する生物学的に活性なタンパク質 |
| US8263545B2 (en) | 2005-02-11 | 2012-09-11 | Amylin Pharmaceuticals, Inc. | GIP analog and hybrid polypeptides with selectable properties |
| AU2006242998B2 (en) | 2005-05-04 | 2012-03-22 | Zealand Pharma A/S | Glucagon-like-peptide-2 (GLP-2) analogues |
| US7846445B2 (en) | 2005-09-27 | 2010-12-07 | Amunix Operating, Inc. | Methods for production of unstructured recombinant polymers and uses thereof |
| US7855279B2 (en) | 2005-09-27 | 2010-12-21 | Amunix Operating, Inc. | Unstructured recombinant polymers and uses thereof |
| CA2634034A1 (en) | 2005-12-20 | 2007-06-28 | Duke University | Methods and compositions for delivering active agents with enhanced pharmacological properties |
| SI2402754T2 (sl) | 2006-03-06 | 2023-09-29 | Amunix Operating Inc. | Nestrukturirani rekombinantni polimeri in njihove uporabe |
| MX2008012600A (es) | 2006-03-31 | 2008-12-12 | Baxter Int | Factor viii pegilado. |
| US20090169553A1 (en) * | 2006-05-04 | 2009-07-02 | Molecular Innovations | Novel Protein Fusion/Tag Technology |
| RS54163B1 (sr) * | 2006-05-30 | 2015-12-31 | Genentech Inc. | Anti-cd22 antitela, njihovi imunokonjugati i njihova upotreba |
| WO2008049711A1 (en) | 2006-10-27 | 2008-05-02 | Novo Nordisk A/S | Peptide extended insulins |
| PT2845866T (pt) * | 2006-10-27 | 2017-08-09 | Genentech Inc | Anticorpos e imunoconjugados e utilizações dos mesmos |
| PL2173890T3 (pl) | 2007-06-21 | 2011-07-29 | Univ Muenchen Tech | Białka czynne biologicznie o zwiększonej stabilności in vivo i (lub) in vitro |
| WO2009023270A2 (en) * | 2007-08-15 | 2009-02-19 | Amunix, Inc. | Compositions and methods for modifying properties of biologically active polypeptides |
| EP4074327A1 (en) | 2008-06-27 | 2022-10-19 | Duke University | Therapeutic agents comprising elastin-like peptides |
| US8716448B2 (en) * | 2009-02-03 | 2014-05-06 | Amunix Operating Inc. | Coagulation factor VII compositions and methods of making and using same |
| US8703717B2 (en) * | 2009-02-03 | 2014-04-22 | Amunix Operating Inc. | Growth hormone polypeptides and methods of making and using same |
| EP3178835B1 (en) | 2009-02-03 | 2019-04-10 | Amunix Pharmaceuticals, Inc. | Extended recombinant polypeptides and compositions comprising same |
| US8680050B2 (en) * | 2009-02-03 | 2014-03-25 | Amunix Operating Inc. | Growth hormone polypeptides fused to extended recombinant polypeptides and methods of making and using same |
| CN102481331B (zh) | 2009-06-08 | 2017-09-22 | 阿穆尼克斯运营公司 | 葡萄糖调节多肽及其制备和使用方法 |
| US9849188B2 (en) | 2009-06-08 | 2017-12-26 | Amunix Operating Inc. | Growth hormone polypeptides and methods of making and using same |
| CA2764105A1 (en) | 2009-06-08 | 2010-12-16 | Amunix Operating Inc. | Growth hormone polypeptides and methods of making and using same |
| WO2011028344A2 (en) | 2009-08-25 | 2011-03-10 | Amunix Operating Inc. | Interleukin-1 receptor antagonist compositions and methods of making and using same |
| DK2494075T3 (en) | 2009-10-30 | 2018-07-23 | Univ Northwestern | TABLE-MANAGED NANOCONJUGATES |
| US20110312881A1 (en) | 2009-12-21 | 2011-12-22 | Amunix, Inc. | Bifunctional polypeptide compositions and methods for treatment of metabolic and cardiovascular diseases |
| EP3372617B1 (en) * | 2010-04-02 | 2024-07-24 | Amunix Pharmaceuticals, Inc. | Binding fusion proteins, binding fusion protein-drug conjugates, xten-drug conjugates and methods of making and using same |
| US8557961B2 (en) | 2010-04-02 | 2013-10-15 | Amunix Operating Inc. | Alpha 1-antitrypsin compositions and methods of making and using same |
| DK2563753T6 (en) | 2010-04-27 | 2016-04-04 | Synaffix Bv | Fused cyclooctynforbindelser and their use in metal-free click-reactions |
| AU2011254564B2 (en) * | 2010-05-21 | 2014-03-27 | Xl-Protein Gmbh | Biosynthetic proline/alanine random coil polypeptides and their uses |
| US20130017997A1 (en) | 2010-08-19 | 2013-01-17 | Amunix Operating Inc. | Factor VIII Compositions and Methods of Making and Using Same |
| ES2687651T3 (es) | 2011-09-12 | 2018-10-26 | Amunix Operating Inc. | Composiciones de péptido-2 similar a glucagón y métodos para producir y usar los mismos |
| DK3564260T5 (da) | 2012-02-15 | 2024-09-02 | Bioverativ Therapeutics Inc | Faktor viii-sammensætninger og fremgangsmåder til fremstilling og anvendelse deraf |
| KR102057356B1 (ko) * | 2012-02-27 | 2019-12-18 | 아뮤닉스 파마슈티컬스, 인크. | Xten 콘주게이트 조성물 및 그의 제조 방법 |
| US9601471B2 (en) | 2015-04-23 | 2017-03-21 | Apple Inc. | Three layer stack structure |
-
2013
- 2013-02-27 KR KR1020147026913A patent/KR102057356B1/ko active Active
- 2013-02-27 WO PCT/US2013/028116 patent/WO2013130683A2/en not_active Ceased
- 2013-02-27 US US14/381,199 patent/US10172953B2/en active Active
- 2013-02-27 MX MX2014010348A patent/MX366864B/es active IP Right Grant
- 2013-02-27 CA CA2865578A patent/CA2865578C/en active Active
- 2013-02-27 EP EP13754824.4A patent/EP2819788B1/en active Active
- 2013-02-27 CN CN201611154536.4A patent/CN107496932A/zh active Pending
- 2013-02-27 EP EP18180908.8A patent/EP3406347A3/en not_active Withdrawn
- 2013-02-27 CN CN201380020791.8A patent/CN104302408B/zh active Active
- 2013-02-27 EA EA201491441A patent/EA030911B1/ru unknown
- 2013-02-27 WO PCT/US2013/028117 patent/WO2013130684A1/en not_active Ceased
- 2013-02-27 EA EA201890389A patent/EA037979B1/ru unknown
- 2013-02-27 SG SG10201700340WA patent/SG10201700340WA/en unknown
- 2013-02-27 PE PE2014001324A patent/PE20142451A1/es active IP Right Grant
- 2013-02-27 CA CA3179537A patent/CA3179537A1/en active Pending
- 2013-02-27 JP JP2014558977A patent/JP6355563B2/ja active Active
- 2013-02-27 CN CN202311191058.4A patent/CN117462693A/zh active Pending
- 2013-02-27 AU AU2013226090A patent/AU2013226090B2/en active Active
- 2013-02-27 SG SG11201405276PA patent/SG11201405276PA/en unknown
-
2014
- 2014-08-26 IL IL234309A patent/IL234309B/en active IP Right Grant
- 2014-08-27 MX MX2019008494A patent/MX2019008494A/es unknown
- 2014-08-27 PH PH12014501924A patent/PH12014501924A1/en unknown
- 2014-08-27 CL CL2014002277A patent/CL2014002277A1/es unknown
- 2014-08-28 ZA ZA2014/06339A patent/ZA201406339B/en unknown
-
2018
- 2018-02-23 ZA ZA2018/01246A patent/ZA201801246B/en unknown
- 2018-02-28 AU AU2018201441A patent/AU2018201441B2/en active Active
- 2018-05-25 JP JP2018100514A patent/JP2018130123A/ja active Pending
- 2018-05-25 JP JP2018100513A patent/JP2018127496A/ja not_active Withdrawn
- 2018-09-17 US US16/133,444 patent/US10953073B2/en active Active
-
2019
- 2019-03-13 IL IL265327A patent/IL265327A/en unknown
-
2020
- 2020-03-25 JP JP2020054163A patent/JP2020114234A/ja not_active Withdrawn
- 2020-10-29 US US17/084,082 patent/US20210322518A1/en not_active Abandoned
-
2022
- 2022-03-02 JP JP2022031705A patent/JP7564143B2/ja active Active
-
2023
- 2023-03-13 US US18/182,954 patent/US20240091315A1/en active Pending
-
2024
- 2024-09-25 JP JP2024166129A patent/JP2024174020A/ja active Pending
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7564143B2 (ja) | Xten共役組成物およびそれを製造する方法 | |
| JP7664158B2 (ja) | トランスフェリン受容体標的ペプチド | |
| CN115920077B (zh) | 胰高血糖素/glp-1/gip受体三重激动剂的长效缀合物 | |
| JP5476304B2 (ja) | グルカゴン様ペプチド−1誘導体及びそれらの医薬用途 | |
| KR102092025B1 (ko) | 페길화된 옥신토모둘린 변이체 | |
| JP6426103B2 (ja) | c−Metタンパク質アゴニスト | |
| TW201427993A (zh) | 用於治療肥胖之升糖素與glp-1共促效劑 | |
| Ngambenjawong et al. | Serum stability and affinity optimization of an M2 macrophage-targeting peptide (M2pep) | |
| JP2020143084A (ja) | 治療目的のための抗体−ウレアーゼコンジュゲート | |
| TW201446792A (zh) | Nav1.7之強效及選擇性抑制劑 | |
| JP2023528921A (ja) | Glp1rアゴニスト・nmdarアンタゴニストコンジュゲート | |
| WO2017197048A1 (en) | Albumin binding conjugate compositions and methods of making and using same | |
| BR112014021256B1 (pt) | Composições de conjugado de xten e métodos de produção das mesmas | |
| HK1261502A1 (en) | Xten conjugate compositions and methods of making same | |
| HK1200139B (en) | Xten conjugate compositions and methods of making same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160226 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20160226 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20161212 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20170310 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20170706 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20171006 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20180227 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180525 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20180611 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20180612 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6355563 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| S533 | Written request for registration of change of name |
Free format text: JAPANESE INTERMEDIATE CODE: R313533 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |