JP5159788B2 - 1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物の結晶形 - Google Patents
1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物の結晶形 Download PDFInfo
- Publication number
- JP5159788B2 JP5159788B2 JP2009538905A JP2009538905A JP5159788B2 JP 5159788 B2 JP5159788 B2 JP 5159788B2 JP 2009538905 A JP2009538905 A JP 2009538905A JP 2009538905 A JP2009538905 A JP 2009538905A JP 5159788 B2 JP5159788 B2 JP 5159788B2
- Authority
- JP
- Japan
- Prior art keywords
- methyl
- fluorophenyl
- glucopyranosyl
- thienylmethyl
- hemihydrate
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H7/00—Compounds containing non-saccharide radicals linked to saccharide radicals by a carbon-to-carbon bond
- C07H7/04—Carbocyclic radicals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7028—Compounds having saccharide radicals attached to non-saccharide compounds by glycosidic linkages
- A61K31/7034—Compounds having saccharide radicals attached to non-saccharide compounds by glycosidic linkages attached to a carbocyclic compound, e.g. phloridzin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/12—Drugs for disorders of the metabolism for electrolyte homeostasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/48—Drugs for disorders of the endocrine system of the pancreatic hormones
- A61P5/50—Drugs for disorders of the endocrine system of the pancreatic hormones for increasing or potentiating the activity of insulin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Diabetes (AREA)
- Molecular Biology (AREA)
- Obesity (AREA)
- Hematology (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- Genetics & Genomics (AREA)
- Endocrinology (AREA)
- Epidemiology (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Cardiology (AREA)
- Urology & Nephrology (AREA)
- Heart & Thoracic Surgery (AREA)
- Emergency Medicine (AREA)
- Hospice & Palliative Care (AREA)
- Psychiatry (AREA)
- Dermatology (AREA)
- Child & Adolescent Psychology (AREA)
- Vascular Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Saccharide Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Description
で示される1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼンが記載されている。
1. 1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物の結晶;
2. CuKα放射を用いて測定された粉末X線回折パターンが、以下の2θ値を含む、1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物の結晶:4.36 ± 0.2, 13.54 ± 0.2, 16.00 ± 0.2, 19.32 ± 0.2, 20.80 ± 0.2;
3. X線粉末回折パターンが図1に示されたものと実質的に同一である、1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物の結晶;
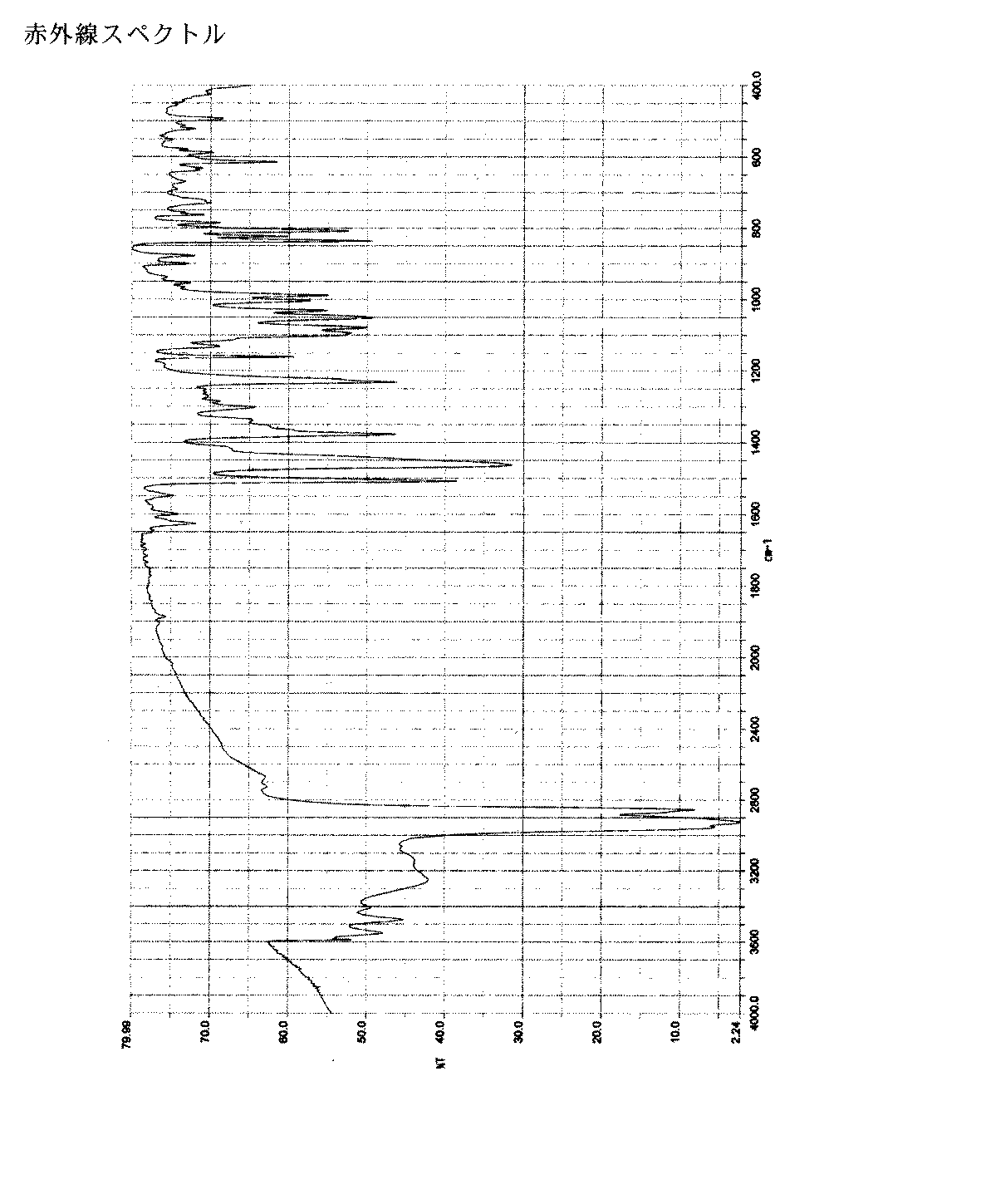
4. IRスペクトルが図2に示されたものと実質的に同一である、1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物の結晶;
5. 1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼンの溶液を形成し、そして該溶液から沈殿または再結晶によりその1/2水和物を結晶化することからなる、1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物の結晶の製造方法;
6. 有効量の1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物の結晶および薬理的に許容しうる担体を含む医薬組成物;
7. 糖尿病、糖尿病性網膜症、糖尿病性神経障害、糖尿病性腎症、遅延創傷治癒、インスリン抵抗性、高血糖症、高インスリン血症、高脂肪酸血症、高グリセロール血症、高脂血症、肥満症、高トリグリセリド血症、X症候群、糖尿病性合併症、アテローム硬化症または高血圧症の処置または進行もしくは発症を遅延させる方法であって、治療有効量の1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物の結晶を投与することを含む方法。
本発明の化合物(I)の結晶形は、X線粉末回折パターンにより特徴付けられる。化合物(I)の1/2水和物の結晶のX線回折パターンは、CuKα放射を用いて測定するX線回折計(RINT-TTR III、リガク、東京、日本)で測定した。X線粉末回折の方法は、以下の通りである:
走査速度: 2.00度/分。
ターゲット: CuKα。
電圧: 50kV。
電流: 300mA。
走査範囲: 3〜40.0度
サンプリング幅: 0.0200度。
鉱物油における本発明の結晶形の赤外線スペクトルは、以下の主なピークを含む:1626,1600,1549,および1507cm−1。
本発明の結晶形は、1/2水和物の形態で存在することが観察された。本発明の結晶形の理論的含水量は、1.98%である。本発明の結晶の熱重量分析は、1.705%の質量損失を示す。
WO2005/012326に記載されたのと同様の方法で、1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼンを調製した。
1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン(1.62g)のアモルファス粉末を、アセトン(15ml)に溶解し、そこにH2O(30ml)および種結晶を加えた。混合物を室温で18時間攪拌し、沈殿物を収集し、アセトン−H2O(1:4、30ml)で洗浄し、減圧下、室温で乾燥させ、1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物(1.52g)を無色の結晶として得た。mp 97-100℃.
Claims (4)
- X線粉末回折パターンが図1に示されたものと実質的に同一である、1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物の結晶。
- 鉱物油における結晶形の赤外線スペクトルが以下のピークを含む、1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物の結晶:1626,1600,1549,および1507cm −1 。
- IRスペクトルが図2に示されたものと実質的に同一である、1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物の結晶。
- 含水溶媒を用いて1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼンの溶液を形成し、そしてその溶液から沈殿または再結晶により該化合物の1/2水和物を結晶化することからなる、請求項1、2又は3に記載された1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物の結晶の製造方法。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2009538905A JP5159788B2 (ja) | 2006-12-04 | 2007-12-03 | 1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物の結晶形 |
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US86842606P | 2006-12-04 | 2006-12-04 | |
| US60/868,426 | 2006-12-04 | ||
| JP2006327019 | 2006-12-04 | ||
| JP2006327019 | 2006-12-04 | ||
| JP2009538905A JP5159788B2 (ja) | 2006-12-04 | 2007-12-03 | 1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物の結晶形 |
| PCT/JP2007/073729 WO2008069327A1 (en) | 2006-12-04 | 2007-12-03 | CRYSTALLINE FORM OF 1- (β-D-GLUCOPYRANOSYL) -4 -METHYL- 3- [5- (4 -FLUOROPHENYL) -2-THIENYLMETHYL] BENZENE HEMIHYDRATE |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2010511602A JP2010511602A (ja) | 2010-04-15 |
| JP5159788B2 true JP5159788B2 (ja) | 2013-03-13 |
Family
ID=38973167
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009538905A Active JP5159788B2 (ja) | 2006-12-04 | 2007-12-03 | 1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物の結晶形 |
Country Status (34)
| Country | Link |
|---|---|
| US (2) | US7943582B2 (ja) |
| EP (1) | EP2102224B2 (ja) |
| JP (1) | JP5159788B2 (ja) |
| KR (1) | KR101146095B1 (ja) |
| CN (3) | CN102675299A (ja) |
| AR (4) | AR064099A1 (ja) |
| AU (1) | AU2007329895C1 (ja) |
| BR (1) | BRPI0718882B8 (ja) |
| CA (1) | CA2671357C (ja) |
| CL (1) | CL2007003487A1 (ja) |
| CO (1) | CO6210719A2 (ja) |
| CR (1) | CR10861A (ja) |
| DK (1) | DK2102224T4 (ja) |
| EA (1) | EA017103B1 (ja) |
| EC (1) | ECSP099489A (ja) |
| ES (1) | ES2456640T5 (ja) |
| GT (1) | GT200900151A (ja) |
| IL (1) | IL199032A (ja) |
| ME (1) | ME01829B (ja) |
| MX (1) | MX2009005857A (ja) |
| MY (1) | MY153702A (ja) |
| NO (1) | NO344354B1 (ja) |
| NZ (1) | NZ577545A (ja) |
| PA (1) | PA8759401A1 (ja) |
| PE (3) | PE20110841A1 (ja) |
| PL (1) | PL2102224T5 (ja) |
| PT (1) | PT2102224E (ja) |
| RS (1) | RS53274B2 (ja) |
| SI (1) | SI2102224T2 (ja) |
| SV (1) | SV2009003285A (ja) |
| TW (1) | TWI403325B (ja) |
| UY (1) | UY30730A1 (ja) |
| WO (1) | WO2008069327A1 (ja) |
| ZA (1) | ZA200903941B (ja) |
Families Citing this family (110)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| UY30730A1 (es) * | 2006-12-04 | 2008-07-03 | Mitsubishi Tanabe Pharma Corp | Forma cristalina del hemihidrato de 1-(b (beta)-d-glucopiranosil) -4-metil-3-[5-(4-fluorofenil) -2-tienilmetil]benceno |
| NO2200606T3 (ja) | 2007-09-10 | 2018-03-24 | ||
| CN105237502A (zh) | 2008-08-22 | 2016-01-13 | 泰拉科斯萨伯有限责任公司 | 制备sglt2抑制剂的方法 |
| US9056850B2 (en) * | 2008-10-17 | 2015-06-16 | Janssen Pharmaceutica N.V. | Process for the preparation of compounds useful as inhibitors of SGLT |
| US20110046076A1 (en) | 2009-02-13 | 2011-02-24 | Boehringer Ingelheim International Gmbh | Pharmaceutical composition, methods for treating and uses thereof |
| US20110009347A1 (en) * | 2009-07-08 | 2011-01-13 | Yin Liang | Combination therapy for the treatment of diabetes |
| CN102482250B (zh) * | 2009-07-10 | 2014-11-19 | 詹森药业有限公司 | 1-(β-D-吡喃葡糖基)-4-甲基-3-[5-(4-氟苯基)-2-噻吩基甲基]苯的结晶方法 |
| US8869918B2 (en) | 2009-10-07 | 2014-10-28 | Longyear Tm, Inc. | Core drilling tools with external fluid pathways |
| WO2011047113A1 (en) * | 2009-10-14 | 2011-04-21 | Janssen Pharmaceutica Nv | Process for the preparation of compounds useful as inhibitors of sglt2 |
| WO2011070592A2 (en) | 2009-12-09 | 2011-06-16 | Panacea Biotec Ltd. | Novel sugar derivatives |
| CN102115468B (zh) * | 2009-12-31 | 2014-06-11 | 上海特化医药科技有限公司 | 一种2,5-二取代噻吩化合物的合成方法 |
| WO2011107494A1 (de) | 2010-03-03 | 2011-09-09 | Sanofi | Neue aromatische glykosidderivate, diese verbindungen enthaltende arzneimittel und deren verwendung |
| JP2013523681A (ja) | 2010-03-30 | 2013-06-17 | ベーリンガー インゲルハイム インターナショナル ゲゼルシャフト ミット ベシュレンクテル ハフツング | Sglt2インヒビター及びppar−ガンマアゴニストを含む医薬組成物並びにその使用 |
| KR101869110B1 (ko) | 2010-05-11 | 2018-06-19 | 미쓰비시 타나베 파마 코퍼레이션 | 카나글리플로진 함유 정제 |
| JP6227406B2 (ja) | 2010-05-11 | 2017-11-08 | ヤンセン ファーマシューティカ エヌ.ベー. | SGLTの阻害剤としての1−(β−D−グルコピラノシル)−2−チエニル−メチルベンゼン誘導体を含有する医薬製剤 |
| WO2011153712A1 (en) | 2010-06-12 | 2011-12-15 | Theracos, Inc. | Crystalline form of benzylbenzene sglt2 inhibitor |
| US8933024B2 (en) | 2010-06-18 | 2015-01-13 | Sanofi | Azolopyridin-3-one derivatives as inhibitors of lipases and phospholipases |
| US8530413B2 (en) | 2010-06-21 | 2013-09-10 | Sanofi | Heterocyclically substituted methoxyphenyl derivatives with an oxo group, processes for preparation thereof and use thereof as medicaments |
| TW201215388A (en) | 2010-07-05 | 2012-04-16 | Sanofi Sa | (2-aryloxyacetylamino)phenylpropionic acid derivatives, processes for preparation thereof and use thereof as medicaments |
| TW201221505A (en) | 2010-07-05 | 2012-06-01 | Sanofi Sa | Aryloxyalkylene-substituted hydroxyphenylhexynoic acids, process for preparation thereof and use thereof as a medicament |
| TW201215387A (en) | 2010-07-05 | 2012-04-16 | Sanofi Aventis | Spirocyclically substituted 1,3-propane dioxide derivatives, processes for preparation thereof and use thereof as a medicament |
| WO2012006298A2 (en) * | 2010-07-06 | 2012-01-12 | Janssen Pharmaceutica Nv | Formulation for co-therapy treatment of diabetes |
| WO2012041898A1 (en) | 2010-09-29 | 2012-04-05 | Celon Pharma Sp. Z O.O. | Combination of sglt2 inhibitor and a sugar compound for the treatment of diabetes |
| US20120283169A1 (en) | 2010-11-08 | 2012-11-08 | Boehringer Ingelheim International Gmbh | Pharmaceutical composition, methods for treating and uses thereof |
| US20130035281A1 (en) | 2011-02-09 | 2013-02-07 | Boehringer Ingelheim International Gmbh | Pharmaceutical composition, methods for treating and uses thereof |
| WO2012140120A1 (en) * | 2011-04-13 | 2012-10-18 | Janssen Pharmaceutica Nv | Process for the preparation of compounds useful as inhibitors of sglt2 |
| US9035044B2 (en) * | 2011-05-09 | 2015-05-19 | Janssen Pharmaceutica Nv | L-proline and citric acid co-crystals of (2S, 3R, 4R, 5S,6R)-2-(3-((5-(4-fluorophenyl)thiopen-2-yl)methyl)4-methylphenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol |
| WO2012163990A1 (en) | 2011-06-03 | 2012-12-06 | Boehringer Ingelheim International Gmbh | Sglt-2 inhibitors for treating metabolic disorders in patients treated with neuroleptic agents |
| WO2013037390A1 (en) | 2011-09-12 | 2013-03-21 | Sanofi | 6-(4-hydroxy-phenyl)-3-styryl-1h-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| EP2755722B1 (en) | 2011-09-13 | 2017-05-24 | Panacea Biotec Ltd. | Novel sglt inhibitors |
| WO2013045413A1 (en) | 2011-09-27 | 2013-04-04 | Sanofi | 6-(4-hydroxy-phenyl)-3-alkyl-1h-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| BR112014010574A2 (pt) * | 2011-10-31 | 2017-05-02 | Scinopharm Taiwan Ltd | formas cristalinas e não cristalinas de inibidores sglt2 |
| US9193751B2 (en) | 2012-04-10 | 2015-11-24 | Theracos, Inc. | Process for the preparation of benzylbenzene SGLT2 inhibitors |
| CN103965267A (zh) * | 2013-01-24 | 2014-08-06 | 江苏豪森医药集团连云港宏创医药有限公司 | 1-(β-D-吡喃葡糖基)-4-甲基-3-[5-(4-氟苯基)-2-噻吩基甲基]苯与L-苯丙氨酸共晶物及其制备方法 |
| ES2740299T3 (es) | 2013-03-14 | 2020-02-05 | Msd Int Gmbh | Métodos para preparar inhibidores de SGLT2 |
| JP2016512817A (ja) | 2013-03-15 | 2016-05-09 | ヤンセン ファーマシューティカ エヌ.ベー. | 高尿酸血症の治療におけるカナグリフロジンとプロベネシドの組合せ |
| ES2959444T3 (es) | 2013-04-04 | 2024-02-26 | Boehringer Ingelheim Vetmedica Gmbh | Tratamiento de trastornos metabólicos en animales equinos |
| US10323056B2 (en) | 2013-05-08 | 2019-06-18 | Lek Pharmaceuticals D.D. | Crystalline hydrates of 1-(β-D-glucopyranosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene |
| US20160083374A1 (en) | 2013-05-30 | 2016-03-24 | Cadila Healthcare Limited | Amorphous form of canagliflozin and process for preparing thereof |
| CN105611920B (zh) | 2013-10-12 | 2021-07-16 | 泰拉科斯萨普有限责任公司 | 羟基-二苯甲烷衍生物的制备 |
| CN103641822B (zh) * | 2013-10-21 | 2016-06-08 | 江苏奥赛康药业股份有限公司 | 一种卡格列净化合物及其药物组合物 |
| WO2015071761A2 (en) * | 2013-11-11 | 2015-05-21 | Crystal Pharmatech Co., Ltd. | Crystalline forms b, c, and d of canagliflozin |
| CN103588762A (zh) * | 2013-11-27 | 2014-02-19 | 苏州晶云药物科技有限公司 | 坎格列净的新晶型及其制备方法 |
| AU2014364999B2 (en) | 2013-12-17 | 2019-12-12 | Boehringer Ingelheim Vetmedica Gmbh | Treatment of metabolic disorders in feline animals |
| EP3096765B1 (en) * | 2014-01-23 | 2018-12-05 | Boehringer Ingelheim Vetmedica GmbH | Treatment of metabolic disorders in canine animals |
| JP2017504649A (ja) * | 2014-01-31 | 2017-02-09 | ヤンセン ファーマシューティカ エヌ.ベー. | 腎障害及び脂肪性肝障害の治療並びに予防のための方法 |
| CN105121434B (zh) * | 2014-03-19 | 2017-08-29 | 杭州普晒医药科技有限公司 | 坎格列净一水合物及其晶型、它们的制备方法和用途 |
| KR20250096885A (ko) | 2014-04-01 | 2025-06-27 | 베링거잉겔하임베트메디카게엠베하 | 말과 동물에서 대사 장애의 치료 |
| CN103980261B (zh) * | 2014-04-01 | 2016-06-29 | 天津大学 | 卡格列净的a晶型及其结晶制备方法 |
| CN103980262B (zh) * | 2014-04-01 | 2016-06-22 | 天津大学 | 卡格列净的b晶型及其结晶制备方法 |
| CN103896930B (zh) * | 2014-04-02 | 2016-08-17 | 安徽联创生物医药股份有限公司 | 卡格列净半水合物药用晶型的制备方法 |
| EP2933255A1 (en) | 2014-04-17 | 2015-10-21 | LEK Pharmaceuticals d.d. | Novel crystalline form of 1-(beta-D-glucopyranosyl)-4- methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene |
| CN103936726B (zh) * | 2014-04-18 | 2016-06-15 | 王军 | 晶体、制备方法及其用途 |
| CN103936800A (zh) * | 2014-05-08 | 2014-07-23 | 安徽联创药物化学有限公司 | 1-(1-甲氧基吡喃葡糖基)-4-甲基-3-[5-(4-氟苯基)-2-噻吩基甲基]苯的制备方法 |
| EP2947077A1 (en) | 2014-05-19 | 2015-11-25 | LEK Pharmaceuticals d.d. | Stereoselective synthesis of intermediates in the preparation of ß-C-arylglucosides |
| CN105330706B (zh) * | 2014-06-05 | 2019-04-16 | 江苏豪森药业集团有限公司 | 卡格列净中间体的制备方法 |
| CN105319294B (zh) * | 2014-06-20 | 2021-03-30 | 重庆医药工业研究院有限责任公司 | 一种分离测定卡格列净及其有关物质的方法 |
| CN104761546A (zh) * | 2014-06-21 | 2015-07-08 | 山东富创医药科技有限公司 | 一种新颖的 (1s)-1,5-脱氢-1-[3-[[5-(4-氟苯基)-2-噻吩基]甲基]-4-甲基苯基]-d-葡萄糖醇晶型及其制备方法 |
| WO2016016774A1 (en) * | 2014-07-31 | 2016-02-04 | Sun Pharmaceutical Industries Limited | Crystalline forms of canagliflozin |
| EP2990029A1 (en) | 2014-08-29 | 2016-03-02 | Sandoz Ag | Pharmaceutical compositions comprising Canagliflozin |
| WO2016035042A1 (en) | 2014-09-05 | 2016-03-10 | Mylan Laboratories Ltd | Process for the preparation of canagliflozin |
| CZ2014634A3 (cs) | 2014-09-16 | 2016-03-23 | Zentiva, K.S. | Komplexy canagliflozinu a cyklodextrinů |
| HUE068152T2 (hu) | 2014-09-25 | 2024-12-28 | Boehringer Ingelheim Vetmedica Gmbh | SGLT2-gátlók és dopamin agonisták kombinációs kezelése anyagcserezavarok megelõzésére lófélékben |
| CN104402946B (zh) * | 2014-11-17 | 2018-01-02 | 连云港恒运药业有限公司 | 卡格列净中间体及其无定形的制备方法 |
| CN105753910A (zh) * | 2014-12-16 | 2016-07-13 | 康普药业股份有限公司 | 一种卡格列净中间体的制备方法 |
| CN104530023A (zh) * | 2014-12-25 | 2015-04-22 | 重庆医药工业研究院有限责任公司 | 一种卡格列净晶型i及其制备方法 |
| CN104945392A (zh) * | 2015-01-27 | 2015-09-30 | 江苏嘉逸医药有限公司 | 结晶型卡格列净一水合物、制备方法及其应用 |
| CN104530024B (zh) * | 2015-02-04 | 2017-08-08 | 上海迪赛诺药业有限公司 | 1‑(β‑D‑吡喃葡糖基)‑4‑甲基‑3‑[5‑(4‑氟苯基)‑2‑噻吩基甲基]苯的晶型及其制备方法 |
| WO2016135747A2 (en) * | 2015-02-27 | 2016-09-01 | Msn Laboratories Private Limited | Process for the preparation of amorphous (1s)-1,5-anhvdro-1-[3-[[5-(4 fluorophennyl)-2-thienyl]methvl]-4-methylphenyl]-d-glucitol and its polymorphs thereof |
| JP2018087140A (ja) * | 2015-02-27 | 2018-06-07 | 田辺三菱製薬株式会社 | 1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼンの新規結晶 |
| WO2016142950A1 (en) * | 2015-03-11 | 2016-09-15 | Harman Finochem Limited | A novel process for preparing (2s,3r,4r,5s,6r)-2-{3-[5-[4-fluoro-phenyl)- thiophen-2-ylmethyl]-4-methyl-phenyl}-6-hydroxymethyl-tetrahydro-pyran-3,4,5- triol and its stable amorphous hemihydrate form |
| EP3298007B1 (en) | 2015-05-22 | 2020-07-22 | Janssen Pharmaceutica NV | Anhydrous crystalline form of (1s)-1,5-anhydro-1-[3-[[5-(4-fluorophenyl)-2-thienyl]methyl]-4-methylphenyl]-d-glucitol |
| CN106279134A (zh) * | 2015-06-23 | 2017-01-04 | 中美华世通生物医药科技(武汉)有限公司 | 卡格列净单晶及其制备方法和用途 |
| CZ2015435A3 (cs) | 2015-06-25 | 2017-01-04 | Zentiva, K.S. | Pevné formy amorfního canagliflozinu |
| US20170071970A1 (en) | 2015-09-15 | 2017-03-16 | Janssen Pharmaceutica Nv | Co-therapy comprising canagliflozin and phentermine for the treatment of obesity and obesity related disorders |
| EP3349762B1 (en) | 2015-09-15 | 2021-08-25 | Laurus Labs Limited | Co-crystals of sglt2 inhibitors, process for their preparation and pharmaceutical compositions thereof |
| WO2017064679A1 (en) * | 2015-10-15 | 2017-04-20 | Lupin Limited | Process for the preparation of amorphous canagliflozin |
| WO2017071813A1 (en) * | 2015-10-30 | 2017-05-04 | Zaklady Farmaceutyczne Polpharma Sa | Process for the preparation of a pharmaceutical agent |
| CZ2015824A3 (cs) | 2015-11-20 | 2017-05-31 | Zentiva, K.S. | Krystalická forma Canagliflozinu a způsob její přípravy |
| KR102742900B1 (ko) | 2015-12-21 | 2024-12-12 | 얀센 파마슈티카 엔브이 | 카나글리플로진 반수화물 결정의 수득을 위한 결정화 절차 |
| CN105541818A (zh) * | 2016-03-04 | 2016-05-04 | 浙江华海药业股份有限公司 | 一种卡格列净水合物新晶型及其制备方法 |
| CN107540706A (zh) * | 2016-06-28 | 2018-01-05 | 山东诚创医药技术开发有限公司 | 伊格列净中间体的制备方法 |
| WO2018020506A1 (en) | 2016-07-25 | 2018-02-01 | Natco Pharma Ltd | Process for the preparation of amorphous form of canagliflozin |
| AU2017344882A1 (en) | 2016-10-19 | 2019-03-28 | Boehringer Ingelheim International Gmbh | Combinations comprising an SSAO/VAP-1 inhibitor and a SGLT2 inhibitor, uses thereof |
| CN108017626A (zh) * | 2016-11-04 | 2018-05-11 | 上海奥博生物医药技术有限公司 | 一种坎格列净半水合物新晶型 |
| CN106588898A (zh) | 2017-02-20 | 2017-04-26 | 浙江华海药业股份有限公司 | 一种卡格列净无定型的制备方法 |
| TWI835735B (zh) | 2017-06-12 | 2024-03-21 | 比利時商健生藥品公司 | 減少或預防第ii型糖尿病患者中心血管事件之方法 |
| CN109553649B (zh) * | 2017-09-26 | 2020-12-04 | 北大方正集团有限公司 | 一种卡格列净中间体的制备方法 |
| ES3035734T3 (en) | 2017-11-30 | 2025-09-08 | Idorsia Pharmaceuticals Ltd | Combination of a 4-pyrimidinesulfamide derivative with an sglt-2 inhibitor for the treatment of endothelin related diseases |
| US20210113561A1 (en) | 2018-04-17 | 2021-04-22 | Boehringer Ingelheim International Gmbh | Pharmaceutical composition, methods for treating and uses thereof |
| WO2020039394A1 (en) | 2018-08-24 | 2020-02-27 | Novartis Ag | New drug combinations |
| EA202191114A1 (ru) | 2018-10-29 | 2021-09-22 | Бёрингер Ингельхайм Интернациональ Гмбх | Производные пиридинилсульфонамида, фармацевтические композиции и их применение |
| CN112955214B (zh) | 2018-10-29 | 2024-05-07 | 勃林格殷格翰国际有限公司 | 吡啶基磺酰胺衍生物、药物组合物及其用途 |
| TW202103709A (zh) | 2019-03-26 | 2021-02-01 | 比利時商健生藥品公司 | 用於治療患有慢性腎臟病之對象的方法 |
| AU2020394498A1 (en) | 2019-11-28 | 2022-06-09 | Boehringer Ingelheim Vetmedica Gmbh | Use of SGLT-2 inhibitors in the drying-off of non-human mammals |
| BR112022016360A2 (pt) | 2020-02-17 | 2022-10-04 | Boehringer Ingelheim Vetmedica Gmbh | Uso de inibidores de sglt-2 para a prevenção e/ou tratamento de doenças cardíacas em felinos |
| EP4138826A1 (en) | 2020-04-22 | 2023-03-01 | Bayer Aktiengesellschaft | Combination of finerenone and a sglt2 inhibitor for the treatment and/or prevention of cardiovascular and/or renal diseases |
| CN113943329A (zh) * | 2020-07-16 | 2022-01-18 | 尚科生物医药(上海)有限公司 | 一种坎格列净中间体的非对映异构体的制备方法 |
| CN116249699A (zh) | 2020-09-30 | 2023-06-09 | 北京睿创康泰医药研究院有限公司 | 一种sglt-2抑制剂·肌氨酸共晶体及其制备方法和应用 |
| CN114478501A (zh) * | 2020-10-28 | 2022-05-13 | 杭州中美华东制药有限公司 | 一种制备稳定的卡格列净半水合物晶型的方法 |
| WO2023006745A1 (en) | 2021-07-28 | 2023-02-02 | Boehringer Ingelheim Vetmedica Gmbh | Use of sglt-2 inhibitors for the prevention and/or treatment of hypertension in non-human mammals |
| WO2023006718A1 (en) | 2021-07-28 | 2023-02-02 | Boehringer Ingelheim Vetmedica Gmbh | Use of sglt-2 inhibitors for the prevention and/or treatment of cardiac diseases in non-human mammals excluding felines, in particular canines |
| JP2024525981A (ja) | 2021-07-28 | 2024-07-12 | ベーリンガー インゲルハイム フェトメディカ ゲーエムベーハー | ヒト以外の哺乳動物における腎疾患の予防及び/又は治療のためのsglt-2阻害剤の使用 |
| EP4456872A1 (en) | 2021-12-30 | 2024-11-06 | NewAmsterdam Pharma B.V. | Obicetrapib and sglt2 inhibitor combination |
| WO2023227492A1 (en) | 2022-05-25 | 2023-11-30 | Boehringer Ingelheim Vetmedica Gmbh | Aqueous pharmaceutical compositions comprising sglt-2 inhibitors |
| EP4676494A1 (en) | 2023-03-06 | 2026-01-14 | Boehringer Ingelheim Vetmedica GmbH | Systems for delivery of liquid pharmaceutical compositions in particular comprising one or more sglt-2 inhibitor(s) |
| KR20250172894A (ko) | 2023-04-24 | 2025-12-09 | 뉴암스테르담 파마 비.브이. | 비정질 오비세트라피브 및 sglt2 억제제 조합 |
| AU2024277852A1 (en) | 2023-05-24 | 2025-10-16 | Boehringer Ingelheim Vetmedica Gmbh | Combination treatment and/or prevention of renal diseases and/or hypertension in non-human mammals comprising one or more sglt-2 inhibitors and telmisartan |
| US20240390317A1 (en) | 2023-05-24 | 2024-11-28 | Boehringer Ingelheim Vetmedica Gmbh | Combination treatment and/or prevention of cardiac diseases in non-human mammals comprising one or more sglt-2 inhibitors and pimobendan and/or telmisartan |
| WO2025224069A1 (en) | 2024-04-23 | 2025-10-30 | Bayer Aktiengesellschaft | Co-crystals of finerenone, pecavaptan, and sglt2 inhibitors |
Family Cites Families (76)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4160861A (en) | 1977-10-03 | 1979-07-10 | Merck & Co., Inc. | Method for the separation of antibiotic macrolides |
| US4584369A (en) | 1981-07-31 | 1986-04-22 | Sloan-Kettering Institute For Cancer Research | Anti-leukemic beta-glycosyl C-nucleosides |
| JP2544609B2 (ja) | 1986-10-07 | 1996-10-16 | 和光純薬工業株式会社 | Tcnq錯体 |
| JP2786896B2 (ja) | 1988-08-19 | 1998-08-13 | ワーナー―ランバート・コンパニー | 置換されたジヒドロイソキノリノンおよび関連化合物 |
| JPH04253974A (ja) | 1991-02-05 | 1992-09-09 | Ishihara Sangyo Kaisha Ltd | スルホニル尿素系化合物、それらの製造方法及びそれらを含有する除草剤 |
| US5149838A (en) | 1991-09-20 | 1992-09-22 | Merck & Co., Inc. | Intermediates for substituted azetidinones useful as anti-inflammatory and antidegenerative agents |
| GB9208161D0 (en) | 1992-04-14 | 1992-05-27 | Pfizer Ltd | Indoles |
| US5334225A (en) | 1992-07-15 | 1994-08-02 | Kao Corporation | Keratinous fiber dye composition containing a 2-substituted amino-5-alkylphenol derivative coupler |
| US5731292A (en) | 1992-11-12 | 1998-03-24 | Tanabe Seiyaku Co., Ltd. | Dihydrochalcone derivatives which are hypoglycemic agents |
| CA2102591C (en) | 1992-11-12 | 2000-12-26 | Kenji Tsujihara | Hypoglycemic agent |
| US6297363B1 (en) | 1993-02-12 | 2001-10-02 | Nomura Co., Ltd. | Glycoside indoles |
| US5830873A (en) | 1994-05-11 | 1998-11-03 | Tanabe Seiyaku Co., Ltd. | Propiophenone derivative and a process for preparing the same |
| US5780483A (en) | 1995-02-17 | 1998-07-14 | Smithkline Beecham Corporation | IL-8 receptor antagonists |
| ATE284690T1 (de) | 1995-10-31 | 2005-01-15 | Lilly Co Eli | Antithrombotische diamine |
| JP3059088B2 (ja) | 1995-11-07 | 2000-07-04 | 田辺製薬株式会社 | プロピオフェノン誘導体およびその製法 |
| JPH09263549A (ja) | 1996-01-25 | 1997-10-07 | Fujisawa Pharmaceut Co Ltd | ベンゼン誘導体の製造法 |
| TW406086B (en) | 1996-12-26 | 2000-09-21 | Tanabe Seiyaku Co | Propiophenone derivatives and process for preparing the same |
| US6153632A (en) | 1997-02-24 | 2000-11-28 | Rieveley; Robert B. | Method and composition for the treatment of diabetes |
| JPH10324632A (ja) | 1997-03-25 | 1998-12-08 | Takeda Chem Ind Ltd | 医薬組成物 |
| JP2000034239A (ja) | 1998-07-16 | 2000-02-02 | Asahi Glass Co Ltd | トリフルオロメチル化芳香族化合物の製造方法 |
| JP3857429B2 (ja) | 1998-07-17 | 2006-12-13 | ポーラ化成工業株式会社 | 含硫黄抗真菌剤 |
| US20020032164A1 (en) | 1998-12-30 | 2002-03-14 | Dale Roderic M. K. | Antimicrobial compounds and methods for their use |
| GB9912961D0 (en) | 1999-06-03 | 1999-08-04 | Pfizer Ltd | Metalloprotease inhibitors |
| PH12000002657B1 (en) | 1999-10-12 | 2006-02-21 | Bristol Myers Squibb Co | C-aryl glucoside SGLT2 inhibitors |
| US6515117B2 (en) | 1999-10-12 | 2003-02-04 | Bristol-Myers Squibb Company | C-aryl glucoside SGLT2 inhibitors and method |
| US6627611B2 (en) | 2000-02-02 | 2003-09-30 | Kotobuki Pharmaceutical Co Ltd | C-glycosides and preparation of thereof as antidiabetic agents |
| JP4456768B2 (ja) | 2000-02-02 | 2010-04-28 | 壽製薬株式会社 | C−配糖体を含有する薬剤 |
| BR0108908A (pt) | 2000-03-03 | 2002-12-24 | Pfizer Prod Inc | Derivados de éteres de pirazol como agentes antiinflamatórios/analgésicos |
| DE60115623T2 (de) | 2000-03-17 | 2006-07-06 | Kissei Pharmaceutical Co., Ltd., Matsumoto | Glucopyranosyloxy-benzylbenzen-derivate, medizinische zusammensetzung und zwischenprodukte für die herstellung der derivate |
| US6555519B2 (en) | 2000-03-30 | 2003-04-29 | Bristol-Myers Squibb Company | O-glucosylated benzamide SGLT2 inhibitors and method |
| US6683056B2 (en) | 2000-03-30 | 2004-01-27 | Bristol-Myers Squibb Company | O-aryl glucoside SGLT2 inhibitors and method |
| EP1338603B1 (en) | 2000-11-02 | 2010-01-20 | Ajinomoto Co., Inc. | Novel pyrazole derivatives and diabetes remedies containing the same |
| US6476352B2 (en) | 2000-12-18 | 2002-11-05 | General Electric Company | Laser beam stop sensor and method for automatically detecting the presence of laser beam stop material using a laser beam stop sensor |
| IL156678A0 (en) | 2000-12-28 | 2004-01-04 | Kissei Pharmaceutical | Glucopyranosyloxypyrazole derivatives and use thereof in medicines |
| CA2438593C (en) | 2001-02-26 | 2010-09-21 | Kissei Pharmaceutical Co., Ltd. | Glucopyranosyloxypyrazole derivatives and medicinal use thereof |
| CA2438595C (en) | 2001-02-27 | 2011-08-09 | Kissei Pharmaceutical Co., Ltd. | Glucopyranosyloxypyrazole derivatives and medicinal use thereof |
| CA2709871C (en) | 2001-03-02 | 2013-08-13 | University Of Western Ontario | Polymer precursors of radiolabeled compounds, and methods of making and using the same |
| US6936590B2 (en) | 2001-03-13 | 2005-08-30 | Bristol Myers Squibb Company | C-aryl glucoside SGLT2 inhibitors and method |
| CA2444481A1 (en) | 2001-04-11 | 2002-10-24 | Bristol-Myers Squibb Company | Amino acid complexes of c-aryl glucosides for treatment of diabetes and method |
| CA2672001A1 (en) | 2001-04-27 | 2002-11-07 | Ajinomoto Co., Inc. | N-substituted pyrazole-o-glycoside derivatives and therapeutic agent for diabetes containing the same |
| GB0112122D0 (en) | 2001-05-18 | 2001-07-11 | Lilly Co Eli | Heteroaryloxy 3-substituted propanamines |
| WO2003000712A1 (en) | 2001-06-20 | 2003-01-03 | Kissei Pharmaceutical Co., Ltd. | Nitrogenous heterocyclic derivative, medicinal composition containing the same, medicinal use thereof, and intermediate therefor |
| JP4115105B2 (ja) | 2001-07-02 | 2008-07-09 | 協和醗酵工業株式会社 | ピラゾール誘導体 |
| JPWO2003011880A1 (ja) | 2001-07-31 | 2004-11-18 | キッセイ薬品工業株式会社 | グルコピラノシルオキシベンジルベンゼン誘導体、それを含有する医薬組成物、その医薬用途及びその製造中間体 |
| EP1432720A1 (en) | 2001-09-05 | 2004-06-30 | Bristol-Myers Squibb Company | O-pyrazole glucoside sglt2 inhibitors and method of use |
| DE60208063T2 (de) | 2001-10-24 | 2006-08-17 | Michael Warrington Burton | Chromogenischen enzymsubstraten und verfahren zum nachweis von beta-d-ribofuranosidase-wirksamkeit |
| CN1589136A (zh) | 2001-11-16 | 2005-03-02 | 古泰尼斯公司 | 含有携带含氧基团的芳族醛的药物与化妆品组合物 |
| US6617313B1 (en) | 2002-03-13 | 2003-09-09 | Council Of Scientific And Industrial Research | Glucopyranoside and process of isolation thereof from pterocarpus marsupium pharmaceutical composition containing the same and use thereof |
| US6562791B1 (en) | 2002-03-29 | 2003-05-13 | Council Of Scientific And Industrial Research | Glucopyranoside, process for isolation thereof, pharmaceutical composition containing same and use thereof |
| JP4523775B2 (ja) | 2002-04-18 | 2010-08-11 | アストラゼネカ・アクチエボラーグ | 複素環化合物 |
| DE10231370B4 (de) | 2002-07-11 | 2006-04-06 | Sanofi-Aventis Deutschland Gmbh | Thiophenglycosidderivate, diese Verbindungen enthaltende Arzneimittel und Verfahren zur Herstellung dieser Arzneimittel |
| TWI254635B (en) | 2002-08-05 | 2006-05-11 | Yamanouchi Pharma Co Ltd | Azulene derivative and salt thereof |
| TW200409778A (en) | 2002-08-09 | 2004-06-16 | Taisho Pharmaceutical Co Ltd | Process for selective production of aryl 5-thio- β-D-aldohexopyranosides |
| US20040102477A1 (en) | 2002-08-23 | 2004-05-27 | Dr. Reddy's Laboratories Limited | Polymorphic forms of (S)-Repaglinide and the processes for preparation thereof |
| JP4606876B2 (ja) | 2002-08-27 | 2011-01-05 | キッセイ薬品工業株式会社 | ピラゾール誘導体、それを含有する医薬組成物及びその医薬用途 |
| DE10258007B4 (de) | 2002-12-12 | 2006-02-09 | Sanofi-Aventis Deutschland Gmbh | Aromatische Fluorglycosidderivate, diese Verbindungen enthaltende Arzneimittel und Verfahren zur Herstellung dieser Arzneimittel |
| DE10258008B4 (de) | 2002-12-12 | 2006-02-02 | Sanofi-Aventis Deutschland Gmbh | Heterocyclische Fluorglycosidderivate, diese Verbindungen enthaltende Arzneimittel und Verfahren zur Herstellung dieser Arzneimittel |
| WO2004063209A2 (en) | 2003-01-03 | 2004-07-29 | Bristol-Myers Squibb Company | Methods of producing c-aryl glucoside sglt2 inhibitors |
| ES2363941T3 (es) | 2003-03-14 | 2011-08-19 | Astellas Pharma Inc. | Derivados de c-glucósido para el tratamiento de diabetes. |
| JP2004300102A (ja) | 2003-03-31 | 2004-10-28 | Kissei Pharmaceut Co Ltd | 縮合複素環誘導体、それを含有する医薬組成物およびその医薬用途 |
| AU2003902263A0 (en) | 2003-05-12 | 2003-05-29 | Fujisawa Pharmaceutical Co., Ltd. | Monosaccharide compounds |
| JP4708187B2 (ja) | 2003-06-20 | 2011-06-22 | キッセイ薬品工業株式会社 | ピラゾール誘導体、それを含有する医薬組成物及びその製造中間体 |
| ES2402098T5 (es) * | 2003-08-01 | 2021-06-09 | Mitsubishi Tanabe Pharma Corp | Compuestos novedosos que tienen actividad inhibidora frente a transportador dependiente de sodio |
| EP1680414A4 (en) | 2003-08-01 | 2009-05-27 | Janssen Pharmaceutica Nv | SUBSTITUTED INDAZOLE-O-GLUCOSIDE |
| WO2005012318A2 (en) | 2003-08-01 | 2005-02-10 | Janssen Pharmaceutica Nv | Substituted fused heterocyclic c-glycosides |
| UA86042C2 (en) | 2003-08-01 | 2009-03-25 | Янссен Фармацевтика Н.В. | Substituted indazole-o-glucosides |
| WO2005012242A2 (en) | 2003-08-01 | 2005-02-10 | Janssen Pharmaceutica Nv | Substituted benzimidazole-, benztriazole-, and benzimidazolone-o-glucosides |
| TW200526678A (en) | 2003-08-01 | 2005-08-16 | Janssen Pharmaceutica Nv | Substituted indole-O-glucosides |
| EP1667524A4 (en) | 2003-09-23 | 2009-01-14 | Merck & Co Inc | NEW CRYSTALLINE FORM OF A PHOSPHORIC ACID SALT OF A DIPEPTIDYLPEPTIDASE IV INHIBITOR |
| ES2338041T3 (es) | 2005-04-15 | 2010-05-03 | Boehringer Ingelheim International Gmbh | Derivados de (heteroariloxi-bencil)-benceno sustituidos con glucopiranosilo en calidad de inhibidores de sglt. |
| US7772191B2 (en) | 2005-05-10 | 2010-08-10 | Boehringer Ingelheim International Gmbh | Processes for preparing of glucopyranosyl-substituted benzyl-benzene derivatives and intermediates therein |
| EP1909776A2 (en) | 2005-07-25 | 2008-04-16 | Merck & Co., Inc. | Dodecylsulfate salt of a dipeptidyl peptidase-iv inhibitor |
| WO2007054978A2 (en) | 2005-11-10 | 2007-05-18 | Jubilant Organosys Limited | Process for preparing paroxetine hydrochloride hemihydrate |
| EP1842850A1 (en) | 2006-03-23 | 2007-10-10 | Sandoz AG | Rosiglitazone hydrochloride hemihydrate |
| UY30730A1 (es) † | 2006-12-04 | 2008-07-03 | Mitsubishi Tanabe Pharma Corp | Forma cristalina del hemihidrato de 1-(b (beta)-d-glucopiranosil) -4-metil-3-[5-(4-fluorofenil) -2-tienilmetil]benceno |
| NO2200606T3 (ja) † | 2007-09-10 | 2018-03-24 |
-
2007
- 2007-11-20 UY UY30730A patent/UY30730A1/es not_active Application Discontinuation
- 2007-12-03 EA EA200970540A patent/EA017103B1/ru active Protection Beyond IP Right Term
- 2007-12-03 WO PCT/JP2007/073729 patent/WO2008069327A1/en not_active Ceased
- 2007-12-03 ZA ZA200903941A patent/ZA200903941B/xx unknown
- 2007-12-03 KR KR1020097013946A patent/KR101146095B1/ko active Active
- 2007-12-03 AU AU2007329895A patent/AU2007329895C1/en active Active
- 2007-12-03 PL PL07850306T patent/PL2102224T5/pl unknown
- 2007-12-03 SI SI200731417T patent/SI2102224T2/sl unknown
- 2007-12-03 ES ES07850306T patent/ES2456640T5/es active Active
- 2007-12-03 PT PT78503067T patent/PT2102224E/pt unknown
- 2007-12-03 BR BRPI0718882A patent/BRPI0718882B8/pt active IP Right Grant
- 2007-12-03 CA CA2671357A patent/CA2671357C/en active Active
- 2007-12-03 EP EP07850306.7A patent/EP2102224B2/en active Active
- 2007-12-03 AR ARP070105383A patent/AR064099A1/es not_active Application Discontinuation
- 2007-12-03 MY MYPI20092200A patent/MY153702A/en unknown
- 2007-12-03 US US11/987,670 patent/US7943582B2/en active Active
- 2007-12-03 MX MX2009005857A patent/MX2009005857A/es active IP Right Grant
- 2007-12-03 CN CN2012100910385A patent/CN102675299A/zh active Pending
- 2007-12-03 PA PA20078759401A patent/PA8759401A1/es unknown
- 2007-12-03 NZ NZ577545A patent/NZ577545A/en unknown
- 2007-12-03 ME MEP-2014-45A patent/ME01829B/me unknown
- 2007-12-03 CN CN2007800431547A patent/CN101573368B/zh active Active
- 2007-12-03 RS RS20140194A patent/RS53274B2/sr unknown
- 2007-12-03 DK DK07850306.7T patent/DK2102224T4/da active
- 2007-12-03 CN CN2012101116538A patent/CN102675380A/zh active Pending
- 2007-12-03 JP JP2009538905A patent/JP5159788B2/ja active Active
- 2007-12-04 PE PE2011001164A patent/PE20110841A1/es not_active Application Discontinuation
- 2007-12-04 CL CL200703487A patent/CL2007003487A1/es unknown
- 2007-12-04 PE PE2007001714A patent/PE20081201A1/es not_active Application Discontinuation
- 2007-12-04 TW TW096146015A patent/TWI403325B/zh active
- 2007-12-04 PE PE2013000220A patent/PE20130591A1/es active IP Right Grant
-
2009
- 2009-05-06 NO NO20091778A patent/NO344354B1/no unknown
- 2009-05-31 IL IL199032A patent/IL199032A/en active IP Right Grant
- 2009-06-04 GT GT200900151A patent/GT200900151A/es unknown
- 2009-06-04 SV SV2009003285A patent/SV2009003285A/es unknown
- 2009-06-11 CR CR10861A patent/CR10861A/es unknown
- 2009-07-02 CO CO09068422A patent/CO6210719A2/es not_active Application Discontinuation
- 2009-07-03 EC EC2009009489A patent/ECSP099489A/es unknown
-
2011
- 2011-05-09 US US13/103,557 patent/US8513202B2/en active Active
-
2017
- 2017-02-01 AR ARP170100253A patent/AR107510A2/es not_active Application Discontinuation
-
2020
- 2020-03-19 AR ARP200100781A patent/AR118450A2/es not_active Application Discontinuation
-
2023
- 2023-07-13 AR ARP230101829A patent/AR129907A2/es not_active Application Discontinuation
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5159788B2 (ja) | 1−(β−D−グルコピラノシル)−4−メチル−3−[5−(4−フルオロフェニル)−2−チエニルメチル]ベンゼン1/2水和物の結晶形 | |
| CN102549005B (zh) | 1-氯-4-(β-D-吡喃葡萄糖-1-基)-2-[4-((S)-四氢呋喃-3-基氧基)-苄基]-苯的晶型的制备方法 | |
| JP6538556B2 (ja) | 1−シアノ−2−(4−シクロプロピル−ベンジル)−4−(β−D−グルコピラノース−1−イル)−ベンゼンの結晶性錯体、その調製方法及び薬物を調製するためのその使用 | |
| KR20160003899A (ko) | 마크롤리드 고체상 형태 | |
| KR20070007196A (ko) | 펙소페나딘 히드로클로라이드의 결정형 및 이의 제조 방법 | |
| WO2008090565A1 (en) | Novel thermodynamically stable polymorphic form-l of letrozole | |
| JP6294665B2 (ja) | スピロケタール誘導体の結晶 | |
| WO2019200502A1 (zh) | 玻玛西尼甲磺酸盐晶型及其制备方法和药物组合物 | |
| TW202016099A (zh) | 一種鴉片類物質受體激動劑的結晶形式及製備方法 | |
| WO2016136830A1 (ja) | 1-(β-D-グルコピラノシル)-4-メチル-3-[5-(4-フルオロフェニル)-2-チエニルメチル]ベンゼンの新規結晶 | |
| WO2021121270A1 (zh) | 一种SGLTs抑制剂的纯化方法及其应用 | |
| KR20140045550A (ko) | 6-(피페리딘-4-일옥시)-2h-이소퀴놀린-1-온 하이드로클로라이드의 결정성 용매화물 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120605 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120615 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120717 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20121120 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20121211 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5159788 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20151221 Year of fee payment: 3 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R153 | Grant of patent term extension |
Free format text: JAPANESE INTERMEDIATE CODE: R153 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R153 | Grant of patent term extension |
Free format text: JAPANESE INTERMEDIATE CODE: R153 |
|
| R153 | Grant of patent term extension |
Free format text: JAPANESE INTERMEDIATE CODE: R153 |