JP2008508320A - Streptococcusagalactiaeのようなグラム陽性細菌に対する免疫原性組成物 - Google Patents
Streptococcusagalactiaeのようなグラム陽性細菌に対する免疫原性組成物 Download PDFInfo
- Publication number
- JP2008508320A JP2008508320A JP2007523880A JP2007523880A JP2008508320A JP 2008508320 A JP2008508320 A JP 2008508320A JP 2007523880 A JP2007523880 A JP 2007523880A JP 2007523880 A JP2007523880 A JP 2007523880A JP 2008508320 A JP2008508320 A JP 2008508320A
- Authority
- JP
- Japan
- Prior art keywords
- gbs
- gas
- protein
- pneumoniae
- proteins
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 213
- 230000002163 immunogen Effects 0.000 title claims abstract description 158
- 241000192125 Firmicutes Species 0.000 title claims description 58
- 241000193985 Streptococcus agalactiae Species 0.000 title description 7
- 229940030998 streptococcus agalactiae Drugs 0.000 title description 5
- 108010052285 Membrane Proteins Proteins 0.000 claims abstract description 584
- 108010037896 heparin-binding hemagglutinin Proteins 0.000 claims abstract description 212
- 230000001580 bacterial effect Effects 0.000 claims abstract description 102
- 241000193990 Streptococcus sp. 'group B' Species 0.000 claims description 537
- 241001505901 Streptococcus sp. 'group A' Species 0.000 claims description 500
- 241000894006 Bacteria Species 0.000 claims description 80
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 78
- 239000012634 fragment Substances 0.000 claims description 73
- 229920001184 polypeptide Polymers 0.000 claims description 71
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 71
- 239000000427 antigen Substances 0.000 claims description 37
- 108091007433 antigens Proteins 0.000 claims description 36
- 102000036639 antigens Human genes 0.000 claims description 36
- 230000001717 pathogenic effect Effects 0.000 claims description 31
- 238000012258 culturing Methods 0.000 claims description 7
- 238000004519 manufacturing process Methods 0.000 claims description 6
- 241000194017 Streptococcus Species 0.000 claims description 5
- 241000193403 Clostridium Species 0.000 claims description 4
- 241000186216 Corynebacterium Species 0.000 claims description 4
- 241000194033 Enterococcus Species 0.000 claims description 4
- 241000186781 Listeria Species 0.000 claims description 4
- 235000014897 Streptococcus lactis Nutrition 0.000 claims description 4
- 241000191940 Staphylococcus Species 0.000 claims description 3
- 108010037833 Bacterial Adhesins Proteins 0.000 claims 2
- 102400000368 Surface protein Human genes 0.000 claims 2
- 241000194035 Lactococcus lactis Species 0.000 claims 1
- 102000018697 Membrane Proteins Human genes 0.000 abstract description 582
- 108090000623 proteins and genes Proteins 0.000 abstract description 469
- 102000004169 proteins and genes Human genes 0.000 abstract description 434
- 208000015181 infectious disease Diseases 0.000 abstract description 72
- 230000036039 immunity Effects 0.000 abstract description 32
- 230000000069 prophylactic effect Effects 0.000 abstract description 11
- 230000001225 therapeutic effect Effects 0.000 abstract description 11
- 230000001018 virulence Effects 0.000 abstract description 4
- 208000035895 Guillain-Barré syndrome Diseases 0.000 abstract 1
- 235000018102 proteins Nutrition 0.000 description 421
- 108700026244 Open Reading Frames Proteins 0.000 description 157
- 125000003275 alpha amino acid group Chemical group 0.000 description 116
- 230000014509 gene expression Effects 0.000 description 91
- 102000040430 polynucleotide Human genes 0.000 description 91
- 108091033319 polynucleotide Proteins 0.000 description 91
- 239000002157 polynucleotide Substances 0.000 description 91
- 210000002919 epithelial cell Anatomy 0.000 description 85
- 101001053401 Arabidopsis thaliana Acid beta-fructofuranosidase 3, vacuolar Proteins 0.000 description 82
- 230000015572 biosynthetic process Effects 0.000 description 59
- 235000001014 amino acid Nutrition 0.000 description 49
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 47
- 239000004473 Threonine Substances 0.000 description 47
- 235000008521 threonine Nutrition 0.000 description 47
- 102000008186 Collagen Human genes 0.000 description 45
- 108010035532 Collagen Proteins 0.000 description 45
- 229920001436 collagen Polymers 0.000 description 45
- 230000001965 increasing effect Effects 0.000 description 38
- 102100037362 Fibronectin Human genes 0.000 description 37
- 108010067306 Fibronectins Proteins 0.000 description 37
- 239000000758 substrate Substances 0.000 description 37
- 210000002421 cell wall Anatomy 0.000 description 34
- 210000004027 cell Anatomy 0.000 description 33
- 108010049003 Fibrinogen Proteins 0.000 description 32
- 102000008946 Fibrinogen Human genes 0.000 description 32
- 229940012952 fibrinogen Drugs 0.000 description 32
- 239000002243 precursor Substances 0.000 description 31
- 230000028327 secretion Effects 0.000 description 29
- 125000000539 amino acid group Chemical group 0.000 description 27
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 26
- 108091008023 transcriptional regulators Proteins 0.000 description 25
- 230000002103 transcriptional effect Effects 0.000 description 24
- 238000012546 transfer Methods 0.000 description 24
- 108010000916 Fimbriae Proteins Proteins 0.000 description 23
- 101000667110 Homo sapiens Vacuolar protein sorting-associated protein 13B Proteins 0.000 description 23
- 102100039113 Vacuolar protein sorting-associated protein 13B Human genes 0.000 description 23
- 238000000034 method Methods 0.000 description 23
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 22
- 241000193998 Streptococcus pneumoniae Species 0.000 description 22
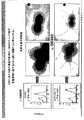
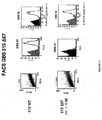
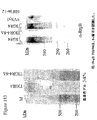
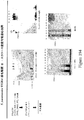
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 22
- 235000004400 serine Nutrition 0.000 description 22
- 241000699670 Mus sp. Species 0.000 description 21
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 20
- 239000004472 Lysine Substances 0.000 description 20
- 239000000284 extract Substances 0.000 description 20
- 238000011282 treatment Methods 0.000 description 19
- 102100020724 Ankyrin repeat, SAM and basic leucine zipper domain-containing protein 1 Human genes 0.000 description 18
- 102100031725 Cortactin-binding protein 2 Human genes 0.000 description 18
- 101000785414 Homo sapiens Ankyrin repeat, SAM and basic leucine zipper domain-containing protein 1 Proteins 0.000 description 18
- 101001055788 Mycolicibacterium smegmatis (strain ATCC 700084 / mc(2)155) Pentapeptide repeat protein MfpA Proteins 0.000 description 18
- 101000936719 Streptococcus gordonii Accessory Sec system protein Asp3 Proteins 0.000 description 18
- 101000936711 Streptococcus gordonii Accessory secretory protein Asp4 Proteins 0.000 description 18
- 230000001886 ciliary effect Effects 0.000 description 18
- 230000006870 function Effects 0.000 description 18
- 239000012528 membrane Substances 0.000 description 18
- 238000012217 deletion Methods 0.000 description 17
- 230000037430 deletion Effects 0.000 description 17
- 101000621943 Acholeplasma phage L2 Probable integrase/recombinase Proteins 0.000 description 16
- 101000768957 Acholeplasma phage L2 Uncharacterized 37.2 kDa protein Proteins 0.000 description 16
- 101000823746 Acidianus ambivalens Uncharacterized 17.7 kDa protein in bps2 3'region Proteins 0.000 description 16
- 101000916369 Acidianus ambivalens Uncharacterized protein in sor 5'region Proteins 0.000 description 16
- 101000769342 Acinetobacter guillouiae Uncharacterized protein in rpoN-murA intergenic region Proteins 0.000 description 16
- 101000823696 Actinobacillus pleuropneumoniae Uncharacterized glycosyltransferase in aroQ 3'region Proteins 0.000 description 16
- 101000786513 Agrobacterium tumefaciens (strain 15955) Uncharacterized protein outside the virF region Proteins 0.000 description 16
- 101000618005 Alkalihalobacillus pseudofirmus (strain ATCC BAA-2126 / JCM 17055 / OF4) Uncharacterized protein BpOF4_00885 Proteins 0.000 description 16
- 101000618348 Allochromatium vinosum (strain ATCC 17899 / DSM 180 / NBRC 103801 / NCIMB 10441 / D) Uncharacterized protein Alvin_0065 Proteins 0.000 description 16
- 101000781117 Autographa californica nuclear polyhedrosis virus Uncharacterized 12.4 kDa protein in CTL-LEF2 intergenic region Proteins 0.000 description 16
- 101000967489 Azorhizobium caulinodans (strain ATCC 43989 / DSM 5975 / JCM 20966 / LMG 6465 / NBRC 14845 / NCIMB 13405 / ORS 571) Uncharacterized protein AZC_3924 Proteins 0.000 description 16
- 101000708323 Azospirillum brasilense Uncharacterized 28.8 kDa protein in nifR3-like 5'region Proteins 0.000 description 16
- 101000770311 Azotobacter chroococcum mcd 1 Uncharacterized 19.8 kDa protein in nifW 5'region Proteins 0.000 description 16
- 101000823761 Bacillus licheniformis Uncharacterized 9.4 kDa protein in flaL 3'region Proteins 0.000 description 16
- 101000819719 Bacillus methanolicus Uncharacterized N-acetyltransferase in lysA 3'region Proteins 0.000 description 16
- 101000789586 Bacillus subtilis (strain 168) UPF0702 transmembrane protein YkjA Proteins 0.000 description 16
- 101000748761 Bacillus subtilis (strain 168) Uncharacterized MFS-type transporter YcxA Proteins 0.000 description 16
- 101000792624 Bacillus subtilis (strain 168) Uncharacterized protein YbxH Proteins 0.000 description 16
- 101000790792 Bacillus subtilis (strain 168) Uncharacterized protein YckC Proteins 0.000 description 16
- 101000765620 Bacillus subtilis (strain 168) Uncharacterized protein YlxP Proteins 0.000 description 16
- 101000819705 Bacillus subtilis (strain 168) Uncharacterized protein YlxR Proteins 0.000 description 16
- 101000916134 Bacillus subtilis (strain 168) Uncharacterized protein YqxJ Proteins 0.000 description 16
- 101000948218 Bacillus subtilis (strain 168) Uncharacterized protein YtxJ Proteins 0.000 description 16
- 101000718627 Bacillus thuringiensis subsp. kurstaki Putative RNA polymerase sigma-G factor Proteins 0.000 description 16
- 101000641200 Bombyx mori densovirus Putative non-structural protein Proteins 0.000 description 16
- 101000754349 Bordetella pertussis (strain Tohama I / ATCC BAA-589 / NCTC 13251) UPF0065 protein BP0148 Proteins 0.000 description 16
- 101000827633 Caldicellulosiruptor sp. (strain Rt8B.4) Uncharacterized 23.9 kDa protein in xynA 3'region Proteins 0.000 description 16
- 101000947628 Claviceps purpurea Uncharacterized 11.8 kDa protein Proteins 0.000 description 16
- 101000947633 Claviceps purpurea Uncharacterized 13.8 kDa protein Proteins 0.000 description 16
- 101000686796 Clostridium perfringens Replication protein Proteins 0.000 description 16
- 101000948901 Enterobacteria phage T4 Uncharacterized 16.0 kDa protein in segB-ipI intergenic region Proteins 0.000 description 16
- 101000805958 Equine herpesvirus 4 (strain 1942) Virion protein US10 homolog Proteins 0.000 description 16
- 101000790442 Escherichia coli Insertion element IS2 uncharacterized 11.1 kDa protein Proteins 0.000 description 16
- 101000788129 Escherichia coli Uncharacterized protein in sul1 3'region Proteins 0.000 description 16
- 101000788370 Escherichia phage P2 Uncharacterized 12.9 kDa protein in GpA 3'region Proteins 0.000 description 16
- 101000788354 Escherichia phage P2 Uncharacterized 8.2 kDa protein in gpA 5'region Proteins 0.000 description 16
- 101000770304 Frankia alni UPF0460 protein in nifX-nifW intergenic region Proteins 0.000 description 16
- 101000797344 Geobacillus stearothermophilus Putative tRNA (cytidine(34)-2'-O)-methyltransferase Proteins 0.000 description 16
- 101000748410 Geobacillus stearothermophilus Uncharacterized protein in fumA 3'region Proteins 0.000 description 16
- 101000787096 Geobacillus stearothermophilus Uncharacterized protein in gldA 3'region Proteins 0.000 description 16
- 101000772675 Haemophilus influenzae (strain ATCC 51907 / DSM 11121 / KW20 / Rd) UPF0438 protein HI_0847 Proteins 0.000 description 16
- 101000631019 Haemophilus influenzae (strain ATCC 51907 / DSM 11121 / KW20 / Rd) Uncharacterized protein HI_0350 Proteins 0.000 description 16
- 101000976889 Haemophilus phage HP1 (strain HP1c1) Uncharacterized 19.2 kDa protein in cox-rep intergenic region Proteins 0.000 description 16
- 101000768938 Haemophilus phage HP1 (strain HP1c1) Uncharacterized 8.9 kDa protein in int-C1 intergenic region Proteins 0.000 description 16
- 101000782488 Junonia coenia densovirus (isolate pBRJ/1990) Putative non-structural protein NS2 Proteins 0.000 description 16
- 101000827627 Klebsiella pneumoniae Putative low molecular weight protein-tyrosine-phosphatase Proteins 0.000 description 16
- 101000811523 Klebsiella pneumoniae Uncharacterized 55.8 kDa protein in cps region Proteins 0.000 description 16
- 101000818409 Lactococcus lactis subsp. lactis Uncharacterized HTH-type transcriptional regulator in lacX 3'region Proteins 0.000 description 16
- 101000878851 Leptolyngbya boryana Putative Fe(2+) transport protein A Proteins 0.000 description 16
- 101000758828 Methanosarcina barkeri (strain Fusaro / DSM 804) Uncharacterized protein Mbar_A1602 Proteins 0.000 description 16
- 101001122401 Middle East respiratory syndrome-related coronavirus (isolate United Kingdom/H123990006/2012) Non-structural protein ORF3 Proteins 0.000 description 16
- 101001130841 Middle East respiratory syndrome-related coronavirus (isolate United Kingdom/H123990006/2012) Non-structural protein ORF5 Proteins 0.000 description 16
- 101710087110 ORF6 protein Proteins 0.000 description 16
- 101000740670 Orgyia pseudotsugata multicapsid polyhedrosis virus Protein C42 Proteins 0.000 description 16
- 101000769182 Photorhabdus luminescens Uncharacterized protein in pnp 3'region Proteins 0.000 description 16
- 101710159752 Poly(3-hydroxyalkanoate) polymerase subunit PhaE Proteins 0.000 description 16
- 101710130262 Probable Vpr-like protein Proteins 0.000 description 16
- 101710197985 Probable protein Rev Proteins 0.000 description 16
- 101000961392 Pseudescherichia vulneris Uncharacterized 29.9 kDa protein in crtE 3'region Proteins 0.000 description 16
- 101000731030 Pseudomonas oleovorans Poly(3-hydroxyalkanoate) polymerase 2 Proteins 0.000 description 16
- 101001065485 Pseudomonas putida Probable fatty acid methyltransferase Proteins 0.000 description 16
- 101000711023 Rhizobium leguminosarum bv. trifolii Uncharacterized protein in tfuA 3'region Proteins 0.000 description 16
- 101000974028 Rhizobium leguminosarum bv. viciae (strain 3841) Putative cystathionine beta-lyase Proteins 0.000 description 16
- 101000756519 Rhodobacter capsulatus (strain ATCC BAA-309 / NBRC 16581 / SB1003) Uncharacterized protein RCAP_rcc00048 Proteins 0.000 description 16
- 101000948219 Rhodococcus erythropolis Uncharacterized 11.5 kDa protein in thcD 3'region Proteins 0.000 description 16
- 101000948156 Rhodococcus erythropolis Uncharacterized 47.3 kDa protein in thcA 5'region Proteins 0.000 description 16
- 101000917565 Rhodococcus fascians Uncharacterized 33.6 kDa protein in fasciation locus Proteins 0.000 description 16
- 101000790284 Saimiriine herpesvirus 2 (strain 488) Uncharacterized 9.5 kDa protein in DHFR 3'region Proteins 0.000 description 16
- 101000929863 Streptomyces cinnamonensis Monensin polyketide synthase putative ketoacyl reductase Proteins 0.000 description 16
- 101000788499 Streptomyces coelicolor Uncharacterized oxidoreductase in mprA 5'region Proteins 0.000 description 16
- 101000788468 Streptomyces coelicolor Uncharacterized protein in mprR 3'region Proteins 0.000 description 16
- 101001102841 Streptomyces griseus Purine nucleoside phosphorylase ORF3 Proteins 0.000 description 16
- 101000708557 Streptomyces lincolnensis Uncharacterized 17.2 kDa protein in melC2-rnhH intergenic region Proteins 0.000 description 16
- 101000845085 Streptomyces violaceoruber Granaticin polyketide synthase putative ketoacyl reductase 1 Proteins 0.000 description 16
- 101000649826 Thermotoga neapolitana Putative anti-sigma factor antagonist TM1081 homolog Proteins 0.000 description 16
- 101000711771 Thiocystis violacea Uncharacterized 76.5 kDa protein in phbC 3'region Proteins 0.000 description 16
- 101710198378 Uncharacterized 10.8 kDa protein in cox-rep intergenic region Proteins 0.000 description 16
- 101710110895 Uncharacterized 7.3 kDa protein in cox-rep intergenic region Proteins 0.000 description 16
- 101710095001 Uncharacterized protein in nifU 5'region Proteins 0.000 description 16
- 101000711318 Vibrio alginolyticus Uncharacterized 11.6 kDa protein in scrR 3'region Proteins 0.000 description 16
- 101000827562 Vibrio alginolyticus Uncharacterized protein in proC 3'region Proteins 0.000 description 16
- 101000778915 Vibrio parahaemolyticus serotype O3:K6 (strain RIMD 2210633) Uncharacterized membrane protein VP2115 Proteins 0.000 description 16
- 229940024606 amino acid Drugs 0.000 description 16
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 15
- 108090000279 Peptidyltransferases Proteins 0.000 description 15
- 108010029485 Protein Isoforms Proteins 0.000 description 15
- 102000001708 Protein Isoforms Human genes 0.000 description 15
- 101100369116 Streptococcus pyogenes serotype M6 (strain ATCC BAA-946 / MGAS10394) tee6 gene Proteins 0.000 description 15
- 150000001413 amino acids Chemical class 0.000 description 15
- ULXTYUPMJXVUHQ-OVTFQNCVSA-N lipid II Chemical compound OC(=O)[C@@H](C)NC(=O)[C@@H](C)NC(=O)[C@H](CCCCN)NC(=O)CC[C@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](C)O[C@@H]1[C@@H](NC(C)=O)[C@@H](OP(O)(=O)OP(O)(=O)OC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(/C)CC\C=C(/C)CCC=C(C)C)O[C@H](CO)[C@H]1O[C@H]1[C@H](NC(C)=O)[C@@H](O)[C@H](O)[C@@H](CO)O1 ULXTYUPMJXVUHQ-OVTFQNCVSA-N 0.000 description 15
- 230000002265 prevention Effects 0.000 description 15
- 238000003786 synthesis reaction Methods 0.000 description 15
- 230000000971 hippocampal effect Effects 0.000 description 14
- 244000057717 Streptococcus lactis Species 0.000 description 13
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 description 13
- 239000013612 plasmid Substances 0.000 description 13
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 12
- 201000010099 disease Diseases 0.000 description 11
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 11
- 239000010410 layer Substances 0.000 description 11
- 239000006228 supernatant Substances 0.000 description 11
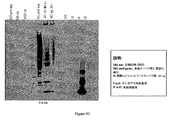
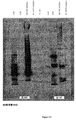
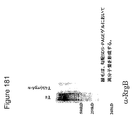
- 238000001262 western blot Methods 0.000 description 11
- 102000036072 fibronectin binding proteins Human genes 0.000 description 10
- 238000003119 immunoblot Methods 0.000 description 10
- 239000000047 product Substances 0.000 description 10
- 125000000341 threoninyl group Chemical class [H]OC([H])(C([H])([H])[H])C([H])(N([H])[H])C(*)=O 0.000 description 10
- 108010087967 type I signal peptidase Proteins 0.000 description 10
- 101710172711 Structural protein Proteins 0.000 description 9
- 238000006243 chemical reaction Methods 0.000 description 9
- 238000010586 diagram Methods 0.000 description 9
- 108091008053 gene clusters Proteins 0.000 description 9
- 229930182470 glycoside Natural products 0.000 description 9
- 150000002338 glycosides Chemical class 0.000 description 9
- 101150030919 srtB gene Proteins 0.000 description 9
- 210000001519 tissue Anatomy 0.000 description 9
- 108091035710 E-box Proteins 0.000 description 8
- 101000815632 Streptococcus suis (strain 05ZYH33) Rqc2 homolog RqcH Proteins 0.000 description 8
- 230000003053 immunization Effects 0.000 description 8
- 238000002649 immunization Methods 0.000 description 8
- 241000894007 species Species 0.000 description 8
- 239000003053 toxin Substances 0.000 description 8
- 231100000765 toxin Toxicity 0.000 description 8
- 108700012359 toxins Proteins 0.000 description 8
- 229960005486 vaccine Drugs 0.000 description 8
- 241001076388 Fimbria Species 0.000 description 7
- 238000000746 purification Methods 0.000 description 7
- 208000035143 Bacterial infection Diseases 0.000 description 6
- 101710085938 Matrix protein Proteins 0.000 description 6
- 101710127721 Membrane protein Proteins 0.000 description 6
- 241000193996 Streptococcus pyogenes Species 0.000 description 6
- 108090000631 Trypsin Proteins 0.000 description 6
- 102000004142 Trypsin Human genes 0.000 description 6
- 230000002238 attenuated effect Effects 0.000 description 6
- 238000001514 detection method Methods 0.000 description 6
- 238000000635 electron micrograph Methods 0.000 description 6
- 150000004676 glycans Chemical class 0.000 description 6
- 230000005745 host immune response Effects 0.000 description 6
- 230000028993 immune response Effects 0.000 description 6
- 238000003018 immunoassay Methods 0.000 description 6
- 238000001727 in vivo Methods 0.000 description 6
- 229920001282 polysaccharide Polymers 0.000 description 6
- 239000005017 polysaccharide Substances 0.000 description 6
- 238000006467 substitution reaction Methods 0.000 description 6
- 239000012588 trypsin Substances 0.000 description 6
- 230000003827 upregulation Effects 0.000 description 6
- -1 Cpa) Proteins 0.000 description 5
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 5
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 5
- 102100027287 Serpin H1 Human genes 0.000 description 5
- 108050008290 Serpin H1 Proteins 0.000 description 5
- 238000004873 anchoring Methods 0.000 description 5
- 239000002775 capsule Substances 0.000 description 5
- 206010013023 diphtheria Diseases 0.000 description 5
- 210000002744 extracellular matrix Anatomy 0.000 description 5
- 230000009545 invasion Effects 0.000 description 5
- 230000002018 overexpression Effects 0.000 description 5
- 230000007918 pathogenicity Effects 0.000 description 5
- 235000004252 protein component Nutrition 0.000 description 5
- 230000001105 regulatory effect Effects 0.000 description 5
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 5
- 210000002966 serum Anatomy 0.000 description 5
- 238000010186 staining Methods 0.000 description 5
- 238000013518 transcription Methods 0.000 description 5
- 230000035897 transcription Effects 0.000 description 5
- 101150047313 52 gene Proteins 0.000 description 4
- 101100002068 Bacillus subtilis (strain 168) araR gene Proteins 0.000 description 4
- 102000014914 Carrier Proteins Human genes 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 4
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 4
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 4
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 4
- 241001135786 Lactobacillus cerevisiae Species 0.000 description 4
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 4
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 4
- 108010076504 Protein Sorting Signals Proteins 0.000 description 4
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 4
- 125000002252 acyl group Chemical group 0.000 description 4
- 235000004279 alanine Nutrition 0.000 description 4
- 101150044616 araC gene Proteins 0.000 description 4
- 208000022362 bacterial infectious disease Diseases 0.000 description 4
- 108091008324 binding proteins Proteins 0.000 description 4
- 210000004899 c-terminal region Anatomy 0.000 description 4
- 239000012228 culture supernatant Substances 0.000 description 4
- 229940088598 enzyme Drugs 0.000 description 4
- 235000013922 glutamic acid Nutrition 0.000 description 4
- 239000004220 glutamic acid Substances 0.000 description 4
- 125000000291 glutamic acid group Chemical group N[C@@H](CCC(O)=O)C(=O)* 0.000 description 4
- 230000013595 glycosylation Effects 0.000 description 4
- 238000006206 glycosylation reaction Methods 0.000 description 4
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 4
- 229960000310 isoleucine Drugs 0.000 description 4
- 235000014705 isoleucine Nutrition 0.000 description 4
- 235000005772 leucine Nutrition 0.000 description 4
- 239000002773 nucleotide Substances 0.000 description 4
- 125000003729 nucleotide group Chemical group 0.000 description 4
- 230000007170 pathology Effects 0.000 description 4
- 239000004474 valine Substances 0.000 description 4
- 235000014393 valine Nutrition 0.000 description 4
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 3
- 101150005896 59 gene Proteins 0.000 description 3
- 241000186227 Corynebacterium diphtheriae Species 0.000 description 3
- 108700011215 E-Box Elements Proteins 0.000 description 3
- 108700007698 Genetic Terminator Regions Proteins 0.000 description 3
- 108091029795 Intergenic region Proteins 0.000 description 3
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 3
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 3
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 3
- 240000006480 Lithocarpus elegans Species 0.000 description 3
- XDMCWZFLLGVIID-SXPRBRBTSA-N O-(3-O-D-galactosyl-N-acetyl-beta-D-galactosaminyl)-L-serine Chemical compound CC(=O)N[C@H]1[C@H](OC[C@H]([NH3+])C([O-])=O)O[C@H](CO)[C@H](O)[C@@H]1OC1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 XDMCWZFLLGVIID-SXPRBRBTSA-N 0.000 description 3
- 206010061372 Streptococcal infection Diseases 0.000 description 3
- 241000194026 Streptococcus gordonii Species 0.000 description 3
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 3
- 125000003277 amino group Chemical group 0.000 description 3
- 244000052616 bacterial pathogen Species 0.000 description 3
- 230000032770 biofilm formation Effects 0.000 description 3
- 150000001720 carbohydrates Chemical class 0.000 description 3
- 235000014633 carbohydrates Nutrition 0.000 description 3
- 210000000170 cell membrane Anatomy 0.000 description 3
- 102000021124 collagen binding proteins Human genes 0.000 description 3
- 108091011142 collagen binding proteins Proteins 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 238000001493 electron microscopy Methods 0.000 description 3
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 3
- 235000004554 glutamine Nutrition 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 229920006158 high molecular weight polymer Polymers 0.000 description 3
- 210000001320 hippocampus Anatomy 0.000 description 3
- 230000002209 hydrophobic effect Effects 0.000 description 3
- 238000002955 isolation Methods 0.000 description 3
- 230000004807 localization Effects 0.000 description 3
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 3
- 230000000269 nucleophilic effect Effects 0.000 description 3
- 244000052769 pathogen Species 0.000 description 3
- 230000037361 pathway Effects 0.000 description 3
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 150000007970 thio esters Chemical class 0.000 description 3
- 101150069666 trmA gene Proteins 0.000 description 3
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 2
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 2
- 241000193163 Clostridioides difficile Species 0.000 description 2
- 108091035707 Consensus sequence Proteins 0.000 description 2
- 241000194032 Enterococcus faecalis Species 0.000 description 2
- 241000194031 Enterococcus faecium Species 0.000 description 2
- 241000588724 Escherichia coli Species 0.000 description 2
- 229920002444 Exopolysaccharide Polymers 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 101000941045 Homo sapiens Cortactin-binding protein 2 Proteins 0.000 description 2
- 206010021531 Impetigo Diseases 0.000 description 2
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 2
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 2
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 2
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 2
- 101100043341 Lactococcus lactis subsp. lactis (strain IL1403) spx2 gene Proteins 0.000 description 2
- 241000186779 Listeria monocytogenes Species 0.000 description 2
- 208000035752 Live birth Diseases 0.000 description 2
- 241000699666 Mus <mouse, genus> Species 0.000 description 2
- 206010028851 Necrosis Diseases 0.000 description 2
- 206010028885 Necrotising fasciitis Diseases 0.000 description 2
- 206010034133 Pathogen resistance Diseases 0.000 description 2
- 201000007100 Pharyngitis Diseases 0.000 description 2
- 241000165031 Pseudomonas lactis Species 0.000 description 2
- 241000194019 Streptococcus mutans Species 0.000 description 2
- 229960005305 adenosine Drugs 0.000 description 2
- 150000003838 adenosines Chemical class 0.000 description 2
- 230000005875 antibody response Effects 0.000 description 2
- 235000009582 asparagine Nutrition 0.000 description 2
- 229960001230 asparagine Drugs 0.000 description 2
- 235000003704 aspartic acid Nutrition 0.000 description 2
- 230000010310 bacterial transformation Effects 0.000 description 2
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 2
- 230000003115 biocidal effect Effects 0.000 description 2
- 239000012472 biological sample Substances 0.000 description 2
- 230000000747 cardiac effect Effects 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 230000003197 catalytic effect Effects 0.000 description 2
- 238000003776 cleavage reaction Methods 0.000 description 2
- 235000018417 cysteine Nutrition 0.000 description 2
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 108091010988 fibronectin binding proteins Proteins 0.000 description 2
- 239000011544 gradient gel Substances 0.000 description 2
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 2
- 230000001900 immune effect Effects 0.000 description 2
- 210000004201 immune sera Anatomy 0.000 description 2
- 229940042743 immune sera Drugs 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 238000007726 management method Methods 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 229930182817 methionine Natural products 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 108010009719 mutanolysin Proteins 0.000 description 2
- 230000017074 necrotic cell death Effects 0.000 description 2
- 201000007970 necrotizing fasciitis Diseases 0.000 description 2
- 238000006384 oligomerization reaction Methods 0.000 description 2
- 230000008520 organization Effects 0.000 description 2
- 210000003800 pharynx Anatomy 0.000 description 2
- 229910052698 phosphorus Inorganic materials 0.000 description 2
- 230000001766 physiological effect Effects 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 238000010926 purge Methods 0.000 description 2
- 102000005962 receptors Human genes 0.000 description 2
- 108020003175 receptors Proteins 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- 230000007017 scission Effects 0.000 description 2
- 239000002356 single layer Substances 0.000 description 2
- 210000003491 skin Anatomy 0.000 description 2
- 238000000527 sonication Methods 0.000 description 2
- 229940031000 streptococcus pneumoniae Drugs 0.000 description 2
- 230000004083 survival effect Effects 0.000 description 2
- 101150025777 tee6 gene Proteins 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- 230000005945 translocation Effects 0.000 description 2
- 235000002374 tyrosine Nutrition 0.000 description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 2
- 229910052720 vanadium Inorganic materials 0.000 description 2
- 210000005166 vasculature Anatomy 0.000 description 2
- 101150037966 67 gene Proteins 0.000 description 1
- 101100437895 Alternaria brassicicola bsc3 gene Proteins 0.000 description 1
- 101100165658 Alternaria brassicicola bsc5 gene Proteins 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 101100032924 Bacillus subtilis (strain 168) radA gene Proteins 0.000 description 1
- 101100478373 Bacillus subtilis (strain 168) srtD gene Proteins 0.000 description 1
- 108091026890 Coding region Proteins 0.000 description 1
- 206010010356 Congenital anomaly Diseases 0.000 description 1
- 101100492392 Didymella fabae pksAC gene Proteins 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 238000003794 Gram staining Methods 0.000 description 1
- 241000588747 Klebsiella pneumoniae Species 0.000 description 1
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- 231100000111 LD50 Toxicity 0.000 description 1
- 206010058780 Meningitis neonatal Diseases 0.000 description 1
- MSFSPUZXLOGKHJ-UHFFFAOYSA-N Muraminsaeure Natural products OC(=O)C(C)OC1C(N)C(O)OC(CO)C1O MSFSPUZXLOGKHJ-UHFFFAOYSA-N 0.000 description 1
- 102100032965 Myomesin-2 Human genes 0.000 description 1
- 208000006816 Neonatal Sepsis Diseases 0.000 description 1
- 206010061308 Neonatal infection Diseases 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 101150073872 ORF3 gene Proteins 0.000 description 1
- 108700022034 Opsonin Proteins Proteins 0.000 description 1
- 108010013639 Peptidoglycan Proteins 0.000 description 1
- 101100226891 Phomopsis amygdali PaP450-1 gene Proteins 0.000 description 1
- 101100226893 Phomopsis amygdali PaP450-2 gene Proteins 0.000 description 1
- 206010036790 Productive cough Diseases 0.000 description 1
- 101150078778 SPB1 gene Proteins 0.000 description 1
- 108010029987 Salivary Proteins and Peptides Proteins 0.000 description 1
- 102000001848 Salivary Proteins and Peptides Human genes 0.000 description 1
- 239000012506 Sephacryl® Substances 0.000 description 1
- 206010040047 Sepsis Diseases 0.000 description 1
- 241000295644 Staphylococcaceae Species 0.000 description 1
- 101000936720 Streptococcus gordonii Accessory secretory protein Asp5 Proteins 0.000 description 1
- 101000912941 Streptococcus gordonii DegV domain-containing protein Proteins 0.000 description 1
- 101000870438 Streptococcus gordonii UDP-N-acetylglucosamine-peptide N-acetylglucosaminyltransferase stabilizing protein GtfB Proteins 0.000 description 1
- 101000834940 Streptococcus pyogenes serotype M6 (strain ATCC BAA-946 / MGAS10394) Trypsin-resistant surface T6 protein Proteins 0.000 description 1
- 101100038645 Streptomyces griseus rppA gene Proteins 0.000 description 1
- 108700027336 Suppressor of Cytokine Signaling 1 Proteins 0.000 description 1
- 102100024779 Suppressor of cytokine signaling 1 Human genes 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 101000645119 Vibrio campbellii (strain ATCC BAA-1116 / BB120) Nucleotide-binding protein VIBHAR_03667 Proteins 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 230000003698 anagen phase Effects 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 230000000890 antigenic effect Effects 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 230000001363 autoimmune Effects 0.000 description 1
- 230000006472 autoimmune response Effects 0.000 description 1
- 230000010065 bacterial adhesion Effects 0.000 description 1
- 230000007921 bacterial pathogenicity Effects 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 239000013553 cell monolayer Substances 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 210000004081 cilia Anatomy 0.000 description 1
- 230000004186 co-expression Effects 0.000 description 1
- 239000000084 colloidal system Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000037029 cross reaction Effects 0.000 description 1
- 230000009260 cross reactivity Effects 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 210000001723 extracellular space Anatomy 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 238000002523 gelfiltration Methods 0.000 description 1
- 108091006104 gene-regulatory proteins Proteins 0.000 description 1
- 102000034356 gene-regulatory proteins Human genes 0.000 description 1
- 125000000404 glutamine group Chemical group N[C@@H](CCC(N)=O)C(=O)* 0.000 description 1
- 125000003147 glycosyl group Chemical group 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 244000052637 human pathogen Species 0.000 description 1
- 230000008105 immune reaction Effects 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 125000000741 isoleucyl group Chemical group [H]N([H])C(C(C([H])([H])[H])C([H])([H])C([H])([H])[H])C(=O)O* 0.000 description 1
- 210000003750 lower gastrointestinal tract Anatomy 0.000 description 1
- 210000005265 lung cell Anatomy 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 238000000386 microscopy Methods 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000001823 molecular biology technique Methods 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 230000008506 pathogenesis Effects 0.000 description 1
- 231100000255 pathogenic effect Toxicity 0.000 description 1
- 230000002028 premature Effects 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 210000002345 respiratory system Anatomy 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 238000002864 sequence alignment Methods 0.000 description 1
- 230000000405 serological effect Effects 0.000 description 1
- 230000009528 severe injury Effects 0.000 description 1
- 230000007480 spreading Effects 0.000 description 1
- 238000003892 spreading Methods 0.000 description 1
- 210000003802 sputum Anatomy 0.000 description 1
- 208000024794 sputum Diseases 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 125000000430 tryptophan group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C2=C([H])C([H])=C([H])C([H])=C12 0.000 description 1
- 210000001635 urinary tract Anatomy 0.000 description 1
- 125000002987 valine group Chemical group [H]N([H])C([H])(C(*)=O)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/09—Lactobacillales, e.g. aerococcus, enterococcus, lactobacillus, lactococcus, streptococcus
- A61K39/092—Streptococcus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/0208—Specific bacteria not otherwise provided for
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/05—Actinobacteria, e.g. Actinomyces, Streptomyces, Nocardia, Bifidobacterium, Gardnerella, Corynebacterium; Propionibacterium
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/08—Clostridium, e.g. Clostridium tetani
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/085—Staphylococcus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/09—Lactobacillales, e.g. aerococcus, enterococcus, lactobacillus, lactococcus, streptococcus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/095—Neisseria
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/12—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria
- C07K16/1267—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria from Gram-positive bacteria
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/52—Bacterial cells; Fungal cells; Protozoal cells
- A61K2039/523—Bacterial cells; Fungal cells; Protozoal cells expressing foreign proteins
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Immunology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Mycology (AREA)
- Microbiology (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Biochemistry (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Peptides Or Proteins (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Applications Claiming Priority (11)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US59280504P | 2004-07-29 | 2004-07-29 | |
| US60983304P | 2004-09-13 | 2004-09-13 | |
| US61683304P | 2004-10-08 | 2004-10-08 | |
| US63341804P | 2004-12-07 | 2004-12-07 | |
| US64006904P | 2004-12-30 | 2004-12-30 | |
| US66032105P | 2005-03-11 | 2005-03-11 | |
| US67375405P | 2005-04-22 | 2005-04-22 | |
| US69300105P | 2005-06-21 | 2005-06-21 | |
| US69545305P | 2005-07-01 | 2005-07-01 | |
| US69764305P | 2005-07-11 | 2005-07-11 | |
| PCT/US2005/027239 WO2006078318A2 (en) | 2004-07-29 | 2005-07-29 | Immunogenic compositions for gram positive bacteria such as streptococcus agalactiae |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011161176A Division JP2011206061A (ja) | 2004-07-29 | 2011-07-22 | Streptococcusagalactiaeのようなグラム陽性細菌に対する免疫原性組成物 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2008508320A true JP2008508320A (ja) | 2008-03-21 |
| JP2008508320A5 JP2008508320A5 (enExample) | 2011-09-08 |
Family
ID=36692677
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007523880A Pending JP2008508320A (ja) | 2004-07-29 | 2005-07-29 | Streptococcusagalactiaeのようなグラム陽性細菌に対する免疫原性組成物 |
| JP2011161176A Pending JP2011206061A (ja) | 2004-07-29 | 2011-07-22 | Streptococcusagalactiaeのようなグラム陽性細菌に対する免疫原性組成物 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011161176A Pending JP2011206061A (ja) | 2004-07-29 | 2011-07-22 | Streptococcusagalactiaeのようなグラム陽性細菌に対する免疫原性組成物 |
Country Status (5)
| Country | Link |
|---|---|
| US (3) | US20060165716A1 (enExample) |
| EP (2) | EP1784211A4 (enExample) |
| JP (2) | JP2008508320A (enExample) |
| CA (1) | CA2575548A1 (enExample) |
| WO (1) | WO2006078318A2 (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012515157A (ja) * | 2009-01-12 | 2012-07-05 | ノバルティス アーゲー | グラム陽性細菌に対するワクチンにおけるCna_Bドメイン抗原 |
| JP2013504556A (ja) * | 2009-09-10 | 2013-02-07 | ノバルティス アーゲー | 気道疾患に対する組み合わせワクチン |
| JPWO2011040133A1 (ja) * | 2009-10-02 | 2013-02-21 | 学校法人東京女子医科大学 | ヒト血清アミロイドa3抗体およびその利用 |
| JP2013520487A (ja) * | 2010-02-26 | 2013-06-06 | ノバルティス アーゲー | 免疫原性タンパク質および組成物 |
Families Citing this family (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7534857B2 (en) * | 1997-12-19 | 2009-05-19 | Centegen, Inc. | Methods and compositions for the treatment and prevention of staphylococcal infections |
| US7323179B2 (en) * | 1997-12-19 | 2008-01-29 | Naomi Balaban | Methods and compositions for the treatment and prevention of Staphylococcus and other bacterial infections |
| US20080219976A1 (en) * | 1997-12-19 | 2008-09-11 | Naomi Balaban | Methods and compositions for treatment and prevention of staphylococcal infections |
| WO2002034771A2 (en) | 2000-10-27 | 2002-05-02 | Chiron Srl | Nucleic acids and proteins from streptococcus groups a & b |
| WO2004018646A2 (en) * | 2002-08-26 | 2004-03-04 | Chiron Corporation | Conserved and specific streptococcal genomes |
| EP1648500B1 (en) | 2003-07-31 | 2014-07-09 | Novartis Vaccines and Diagnostics, Inc. | Immunogenic compositions for streptococcus pyogenes |
| US8945589B2 (en) * | 2003-09-15 | 2015-02-03 | Novartis Vaccines And Diagnostics, Srl | Immunogenic compositions for Streptococcus agalactiae |
| EP1784211A4 (en) | 2004-07-29 | 2010-06-30 | Novartis Vaccines & Diagnostic | IMMUNOGENIC COMPOSITIONS FOR GRAMPOSITIVE BACTERIA SUCH AS STREPTOCOCCUS AGALACTIAE |
| AU2005294275B2 (en) * | 2004-10-08 | 2012-09-13 | Glaxosmithkline Biologicals S.A. | Immunogenic and therapeutic compositions for Streptococcus pyogenes |
| US8168181B2 (en) | 2006-02-13 | 2012-05-01 | Alethia Biotherapeutics, Inc. | Methods of impairing osteoclast differentiation using antibodies that bind siglec-15 |
| CA2822302A1 (en) | 2006-02-13 | 2007-08-23 | Alethia Biotherapeutics Inc. | Methods of impairing osteoclast differentiation |
| MX2008010604A (es) * | 2006-02-17 | 2009-03-05 | Novartis Ag | Purificacion y uso de pilosidades de streptococcus pneumoniae y proteinas pilosas. |
| WO2008020335A2 (en) * | 2006-06-09 | 2008-02-21 | Novartis Ag | Immunogenic compositions for streptococcus agalactiae |
| EP2063911A2 (en) * | 2006-07-26 | 2009-06-03 | Novartis Ag | Immunogenic compositions for gram positive bacteria |
| AU2007348285A1 (en) * | 2006-10-30 | 2008-09-12 | Novartis Ag | Immunogenic and therapeutic compositions for streptococcus pyogenes |
| AU2008299376B2 (en) | 2007-09-12 | 2013-02-28 | Glaxosmithkline Biologicals S.A. | GAS57 mutant antigens and GAS57 antibodies |
| US8953685B2 (en) * | 2007-12-10 | 2015-02-10 | Qualcomm Incorporated | Resource-adaptive video interpolation or extrapolation with motion level analysis |
| NZ586430A (en) | 2007-12-21 | 2012-09-28 | Novartis Ag | Mutant forms of streptolysin o (slo) |
| ITMI20090946A1 (it) | 2009-05-28 | 2010-11-29 | Novartis Ag | Espressione di proteine ricombinanti |
| ES2531115T3 (es) * | 2009-06-01 | 2015-03-10 | Novartis Ag | Combinaciones de clados de RrgB neumocócicos |
| GB201114923D0 (en) * | 2011-08-30 | 2011-10-12 | Novartis Ag | Immunogenic proteins and compositions |
| DE102011121069A1 (de) * | 2011-12-13 | 2013-06-13 | Martin-Luther-Universität Halle-Wittenberg | Vakzinierung mittels rekombinanter Hefe durch Erzeugung einer protektiven humoralen Immunantwort gegen definierte Antigene |
| US9777076B2 (en) | 2012-07-16 | 2017-10-03 | Pfizer Inc. | Saccharides and uses thereof |
| WO2014012165A1 (en) | 2012-07-19 | 2014-01-23 | Alethia Biotherapeutics Inc. | Anti-siglec-15 antibodies |
| US9452205B2 (en) * | 2012-09-24 | 2016-09-27 | Montana State University | Recombinant Lactococcus lactis expressing Escherichia coli colonization factor antigen I (CFA/I) fimbriae and their methods of use |
| BE1022792B1 (fr) * | 2014-08-05 | 2016-09-06 | Glaxosmithkline Biologicals S.A. | Molecule support |
| US10738338B2 (en) | 2016-10-18 | 2020-08-11 | The Research Foundation for the State University | Method and composition for biocatalytic protein-oligonucleotide conjugation and protein-oligonucleotide conjugate |
| TWI598360B (zh) * | 2016-12-19 | 2017-09-11 | 義守大學 | Fsbm重組蛋白及其用途 |
| AU2019364829B2 (en) * | 2018-10-25 | 2025-11-20 | Ajinomoto Co., Inc. | Method for secretory production of protein |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002034771A2 (en) * | 2000-10-27 | 2002-05-02 | Chiron Srl | Nucleic acids and proteins from streptococcus groups a & b |
| WO2004041157A2 (en) * | 2002-09-13 | 2004-05-21 | Chiron Corporation | Group b streptococcus vaccine |
Family Cites Families (171)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SU548947A1 (ru) | 1975-09-05 | 1980-02-05 | Всесоюзный Ордена Ленина Институт Экспериментальной Ветеринарии Васхнил | Вакцина дл специфической профилактики и трерапии трихофитии лошадей |
| NO153458C (no) | 1978-06-07 | 1986-03-26 | Inst Experimentalnoi Veterinar | Fremgangsm¨te for fremstilling av vaksine for profylakse o g behandling av ringorm hos pelsdyr og kaniner for¨rsaket av trichophyton mentagrophytes. |
| US5360897A (en) | 1981-08-31 | 1994-11-01 | The University Of Rochester | Immunogenic conjugates of streptococcus pneumonial capsular polymer and toxin or in toxiad |
| US4454121A (en) * | 1982-07-27 | 1984-06-12 | The University Of Tennessee Research Corporation | Synthetic peptides corresponding to antigenic determinants of the M protein of Streptococcus pyogenes |
| SE8205892D0 (sv) | 1982-10-18 | 1982-10-18 | Bror Morein | Immunogent membranproteinkomplex, sett for framstellning och anvendning derav som immunstimulerande medel och sasom vaccin |
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| FR2550094B1 (fr) * | 1983-08-01 | 1986-03-21 | Anvar | Compositions de vaccins conditionnees pour la vaccination de sujets immunitairement non naifs contre un agent pathogene determine et contenant un haptene comportant lui-meme un site antigenique caracteristique dudit agent pathogene ou un oligomere de cet haptene |
| US5324513A (en) | 1984-03-07 | 1994-06-28 | Institut Pasteur | Composition useful for the fabrication of vaccines |
| US5171568A (en) | 1984-04-06 | 1992-12-15 | Chiron Corporation | Recombinant herpes simplex gb-gd vaccine |
| US5916588A (en) | 1984-04-12 | 1999-06-29 | The Liposome Company, Inc. | Peptide-containing liposomes, immunogenic liposomes and methods of preparation and use |
| US6090406A (en) | 1984-04-12 | 2000-07-18 | The Liposome Company, Inc. | Potentiation of immune responses with liposomal adjuvants |
| US5098704A (en) | 1984-06-18 | 1992-03-24 | Chiron Corporation | Hepatitis surface antigen particle vaccine |
| US4722840A (en) | 1984-09-12 | 1988-02-02 | Chiron Corporation | Hybrid particle immunogens |
| EP0251575B2 (en) | 1986-06-17 | 2002-11-13 | Chiron Corporation | Hepatitis delta diagnostics and vaccines, their preparation and use |
| US5057540A (en) | 1987-05-29 | 1991-10-15 | Cambridge Biotech Corporation | Saponin adjuvant |
| IE62868B1 (en) | 1987-11-18 | 1995-03-08 | Chiron Corp | Hepatitis C virus |
| US5098827A (en) * | 1988-02-26 | 1992-03-24 | The University Of Florida | Novel bacterial markers for pathogenic group B streptococci |
| AU631377B2 (en) | 1988-08-25 | 1992-11-26 | Liposome Company, Inc., The | Affinity associated vaccine |
| US5354846A (en) * | 1988-11-18 | 1994-10-11 | Michael Kehoe | Streptolysin O antigen derivatives, its production and uses |
| GB8827038D0 (en) | 1988-11-18 | 1988-12-21 | Kehoe M | Streptolysin o antigens & uses |
| DE3841091A1 (de) | 1988-12-07 | 1990-06-13 | Behringwerke Ag | Synthetische antigene, verfahren zu ihrer herstellung und ihre verwendung |
| HUT58804A (en) | 1988-12-16 | 1992-03-30 | James Cleland Paton | Process for producing pneumolysine mutants and pneumococcus vaccines |
| GB2233977B (en) | 1989-01-04 | 1993-03-31 | Michael Kehoe | Cytolytic streptolysin o mutants and uses |
| EP0378881B1 (en) | 1989-01-17 | 1993-06-09 | ENIRICERCHE S.p.A. | Synthetic peptides and their use as universal carriers for the preparation of immunogenic conjugates suitable for the development of synthetic vaccines |
| KR0185373B1 (ko) | 1989-03-17 | 1999-05-01 | 로버트 피. 블랙버언 | Hcv 폴리단백질에서 유래되는 hcv 아미노산 서열 부분을 포함하는 폴리펩티드 및 그 사용 |
| CA2011896C (en) | 1989-04-21 | 2001-05-08 | Mark Werner | Ringworm vaccine |
| EP0398748B1 (en) | 1989-05-18 | 2002-01-09 | Chiron Corporation | NANBV diagnostics: polynucleotides useful for screening for hepatitis C virus |
| HU212924B (en) | 1989-05-25 | 1996-12-30 | Chiron Corp | Adjuvant formulation comprising a submicron oil droplet emulsion |
| EP0482068A1 (en) | 1989-07-14 | 1992-04-29 | American Cyanamid Company | Cytokine and hormone carriers for conjugate vaccines |
| IT1237764B (it) | 1989-11-10 | 1993-06-17 | Eniricerche Spa | Peptidi sintetici utili come carriers universali per la preparazione di coniugati immunogenici e loro impiego per lo sviluppo di vaccini sintetici. |
| US6737521B1 (en) * | 1990-05-11 | 2004-05-18 | The Rockefeller University | Delivery and expression of a hybrid surface protein on the surface of gram positive bacteria |
| US5821088A (en) * | 1990-05-11 | 1998-10-13 | Siga Pharmaceuticals, Inc. | Use of gram-positive bacteria to express recombinant proteins |
| GB2276169A (en) | 1990-07-05 | 1994-09-21 | Celltech Ltd | Antibodies specific for carcinoembryonic antigen |
| EP0471177B1 (en) | 1990-08-13 | 1995-10-04 | American Cyanamid Company | Filamentous hemagglutinin of bordetella pertussis as a carrier molecule for conjugate vaccines |
| US5153312A (en) | 1990-09-28 | 1992-10-06 | American Cyanamid Company | Oligosaccharide conjugate vaccines |
| US5284652A (en) | 1990-10-26 | 1994-02-08 | Pier Allan C | Dermatophyte vaccine |
| US5277904A (en) | 1990-10-26 | 1994-01-11 | Pier Allan C | Broad spectrum dermatophyte vaccine |
| US5391712A (en) * | 1991-08-30 | 1995-02-21 | Beckman Instruments, Inc. | Non-hemolytic streptolysin O variants |
| US5378620A (en) * | 1991-08-30 | 1995-01-03 | Beckman Instruments, Inc. | Streptolysin O derivatives |
| WO1993018150A1 (en) | 1992-03-02 | 1993-09-16 | Biocine S.P.A. | Helicobacter pylori proteins useful for vaccines and diagnostics |
| IT1262896B (it) | 1992-03-06 | 1996-07-22 | Composti coniugati formati da proteine heat shock (hsp) e oligo-poli- saccaridi, loro uso per la produzione di vaccini. | |
| EP0761231B1 (en) | 1992-06-25 | 2000-01-12 | SMITHKLINE BEECHAM BIOLOGICALS s.a. | Vaccine composition containing adjuvants |
| IL102687A (en) | 1992-07-30 | 1997-06-10 | Yeda Res & Dev | Conjugates of poorly immunogenic antigens and synthetic pepide carriers and vaccines comprising them |
| DE4240056A1 (de) * | 1992-11-28 | 1994-06-01 | Boehringer Mannheim Gmbh | Streptolysin O Peptidantigene und Verfahren zur Bestimmung von Streptolysin-Antikörper |
| EP0613947B1 (en) | 1993-02-01 | 2004-03-03 | Beckman-Coulter, Inc. | Mitogenic factor, gene thereof and method of microdetection therefor |
| ES2162139T5 (es) | 1993-03-23 | 2008-05-16 | Smithkline Beecham Biologicals S.A. | Composiciones de vacuna que contienen monofosforil-lipido a 3-o-desacilado. |
| JP3644961B2 (ja) * | 1993-06-23 | 2005-05-11 | ベックマン コールター インコーポレイテッド | ストレプトコッカス・ピオゲネスから誘導された組換えdnアーゼb |
| US5585098A (en) * | 1993-11-23 | 1996-12-17 | Ovimmune, Inc. | Oral administration of chicken yolk immunoglobulins to lower somatic cell count in the milk of lactating ruminants |
| GB9326174D0 (en) | 1993-12-22 | 1994-02-23 | Biocine Sclavo | Mucosal adjuvant |
| GB9326253D0 (en) | 1993-12-23 | 1994-02-23 | Smithkline Beecham Biolog | Vaccines |
| US6239116B1 (en) | 1994-07-15 | 2001-05-29 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US6429199B1 (en) | 1994-07-15 | 2002-08-06 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules for activating dendritic cells |
| US6207646B1 (en) | 1994-07-15 | 2001-03-27 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| PT804559E (pt) * | 1994-10-07 | 2005-08-31 | Univ Rockefeller | Enzima para clivagem da regiao ancora de proteinas de superficie de bacterias gram-positivas |
| AUPM873294A0 (en) | 1994-10-12 | 1994-11-03 | Csl Limited | Saponin preparations and use thereof in iscoms |
| AUPM885194A0 (en) * | 1994-10-14 | 1994-11-10 | Council Of The Queensland Institute Of Medical Research, The | Synthetic peptides and vaccines comprising same |
| IL117483A (en) | 1995-03-17 | 2008-03-20 | Bernard Brodeur | MENINGITIDIS NEISSERIA shell protein is resistant to proteinase K. |
| UA56132C2 (uk) | 1995-04-25 | 2003-05-15 | Смітклайн Бічем Байолоджікалс С.А. | Композиція вакцини (варіанти), спосіб стабілізації qs21 відносно гідролізу (варіанти), спосіб приготування композиції вакцини |
| US6284884B1 (en) * | 1995-06-07 | 2001-09-04 | North American Vaccine, Inc. | Antigenic group B streptococcus type II and type III polysaccharide fragments having a 2,5-anhydro-D-mannose terminal structure and conjugate vaccine thereof |
| US6936259B2 (en) * | 1995-06-08 | 2005-08-30 | University Of Saskatchewan | CAMP factor of Streptococcus uberis |
| GB9513261D0 (en) | 1995-06-29 | 1995-09-06 | Smithkline Beecham Biolog | Vaccines |
| US5846547A (en) * | 1996-01-22 | 1998-12-08 | Regents Of The University Of Minnesota | Streptococcal C5a peptidase vaccine |
| US6346392B1 (en) | 1996-11-27 | 2002-02-12 | Smithkline Beecham Corporation | Polynucleotides encoding a novel glutamine transport ATP-binding protein |
| IES960488A2 (en) | 1996-07-03 | 1997-11-19 | Trinity College Dublin | Subunit Vaccine for Streptococcus Equi |
| EP0821070A1 (en) | 1996-07-22 | 1998-01-28 | Carelli, Claude Marcel Henri | Pit-1 gene polymorphism and trait selection in animals |
| DE19630390A1 (de) | 1996-07-26 | 1998-01-29 | Chiron Behring Gmbh & Co | Proteine, insbesondere Membranproteine von Helicobacter pylori, ihre Herstellung und Verwendung |
| CA2639051A1 (en) | 1996-09-04 | 1998-03-12 | Takara Bio Inc. | Fungal antigens and process for producing the same |
| WO1998018931A2 (en) | 1996-10-31 | 1998-05-07 | Human Genome Sciences, Inc. | Streptococcus pneumoniae polynucleotides and sequences |
| JP2001510989A (ja) | 1996-11-01 | 2001-08-07 | スミスクライン・ビーチャム・コーポレイション | 新規コーディング配列 |
| US7033765B1 (en) * | 1997-02-20 | 2006-04-25 | Toronto Research Chemicals, Inc. | Site-specific drug delivery |
| EP1005368B1 (en) | 1997-03-10 | 2009-09-02 | Ottawa Hospital Research Institute | Use of nucleic acids containing unmethylated CpG dinucleotide in combination with alum as adjuvants |
| US6426074B1 (en) * | 1997-03-19 | 2002-07-30 | The Brigham And Women's Hospital Inc. | Group B Streptococcus vaccine |
| US6818222B1 (en) | 1997-03-21 | 2004-11-16 | Chiron Corporation | Detoxified mutants of bacterial ADP-ribosylating toxins as parenteral adjuvants |
| US6299881B1 (en) | 1997-03-24 | 2001-10-09 | Henry M. Jackson Foundation For The Advancement Of Military Medicine | Uronium salts for activating hydroxyls, carboxyls, and polysaccharides, and conjugate vaccines, immunogens, and other useful immunological reagents produced using uronium salts |
| EP0981625A2 (en) * | 1997-05-06 | 2000-03-01 | Human Genome Sciences, Inc. | $i(ENTEROCOCCUS FAECALIS) POLYNUCLEOTIDES AND POLYPEPTIDES |
| US6635623B1 (en) * | 1997-06-13 | 2003-10-21 | Baylor College Of Medicine | Lipoproteins as nucleic acid vectors |
| GB9712347D0 (en) | 1997-06-14 | 1997-08-13 | Smithkline Beecham Biolog | Vaccine |
| GB9713156D0 (en) | 1997-06-20 | 1997-08-27 | Microbiological Res Authority | Vaccines |
| US5948413A (en) | 1997-07-17 | 1999-09-07 | Board Of Trustees Operating Michigan State University | Method and vaccine for treatment of pythiosis insidiosi in humans and lower animals |
| DE69838992T2 (de) | 1997-09-05 | 2008-12-24 | Glaxosmithkline Biologicals S.A., Rixensart | Öl-in-Wasser Emulsionen mit Saponinen |
| ATE349527T1 (de) | 1997-09-12 | 2007-01-15 | Univ Tennessee Res Foundation | Gruppe a streptokokken-impfstoff |
| EP1037997A1 (en) | 1997-09-26 | 2000-09-27 | Medimmune, Inc. | Lmb gene of streptococcus agalactiae |
| US6406883B1 (en) | 1997-09-26 | 2002-06-18 | Luetticken Rudolf | Lmb gene of Streptococcus agalactiae |
| US6756361B1 (en) | 1997-10-14 | 2004-06-29 | Nabi | Enterococcus antigens and vaccines |
| BR9813071A (pt) * | 1997-10-17 | 2000-08-15 | Nestle Sa | Espécie de bactéria láctica |
| ATE476508T1 (de) | 1997-11-06 | 2010-08-15 | Novartis Vaccines & Diagnostic | Neisseriale antigene |
| WO1999026969A1 (en) | 1997-11-21 | 1999-06-03 | University Of Otago | Zoocin a immunity factor |
| BR9814878A (pt) | 1997-11-21 | 2000-10-03 | Genset Sa | Sequência genÈmica e polipeptìdeos de chlamydia pneumoniae, fragmentos dos mesmos e usos dos mesmos, em particular para o diagnóstico, a prevenção e o tratamento de infecção |
| BR9814912A (pt) | 1997-11-28 | 2000-10-03 | Genset Sa | Sequência genÈmica e polipeptìdeos de chlamydia trachomatis, fragmentos dos mesmos e usos dos mesmos, em particular para o diagnóstico, a prevenção e o tratamento de infecção |
| GB9725084D0 (en) | 1997-11-28 | 1998-01-28 | Medeva Europ Ltd | Vaccine compositions |
| EP1035867A1 (en) | 1997-12-02 | 2000-09-20 | Powderject Vaccines, Inc. | Transdermal delivery of particulate vaccine compositions |
| AU1866099A (en) * | 1997-12-31 | 1999-07-26 | Stressgen Biotechnologies Corporation | Streptococcal heat shock proteins of the hsp60 family |
| SG152917A1 (en) | 1998-01-14 | 2009-06-29 | Chiron Srl | Neisseria meningitidis antigens |
| US7041814B1 (en) * | 1998-02-18 | 2006-05-09 | Genome Therapeutics Corporation | Nucleic acid and amino acid sequences relating to Enterobacter cloacae for diagnostics and therapeutics |
| ES2278436T3 (es) | 1998-02-20 | 2007-08-01 | Id Biomedical Corporation | Antigenos de estreptococos del grupo b. |
| GB9807721D0 (en) | 1998-04-08 | 1998-06-10 | Chiron Spa | Antigen |
| WO1999052549A1 (en) | 1998-04-09 | 1999-10-21 | Smithkline Beecham Biologicals S.A. | Adjuvant compositions |
| GB9808327D0 (en) * | 1998-04-20 | 1998-06-17 | Chiron Spa | Antidiotypic compounds |
| EP2261341A3 (en) | 1998-05-01 | 2012-01-04 | Novartis Vaccines and Diagnostics, Inc. | Neisseria meningitidis antigens and compositions |
| US6660520B2 (en) * | 1998-06-05 | 2003-12-09 | Smithkline Beecham Corporation | Nrde |
| US6562798B1 (en) | 1998-06-05 | 2003-05-13 | Dynavax Technologies Corp. | Immunostimulatory oligonucleotides with modified bases and methods of use thereof |
| US7098182B2 (en) * | 1998-07-27 | 2006-08-29 | Microbial Technics Limited | Nucleic acids and proteins from group B streptococcus |
| US6936252B2 (en) * | 1998-07-27 | 2005-08-30 | Microbial Technics Limited | Streptococcus pneumoniae proteins and nucleic acid molecules |
| JP2002531054A (ja) | 1998-07-27 | 2002-09-24 | マイクロビアル テクニクス リミティッド | B群連鎖球菌由来の核酸およびタンパク質 |
| GB9817052D0 (en) | 1998-08-05 | 1998-09-30 | Smithkline Beecham Biolog | Vaccine |
| CN1338005A (zh) | 1998-10-09 | 2002-02-27 | 希龙公司 | 奈瑟球菌基因组序列及其用途 |
| CZ301212B6 (cs) | 1998-10-16 | 2009-12-09 | Smithkline Beecham Biologicals S. A. | Vakcinacní prostredek |
| EP1133572A4 (en) | 1998-11-12 | 2005-06-15 | Univ California | GENOMIC SEQUENCE OF CHLAMYDIA PNEUMONIAE |
| GB9828000D0 (en) | 1998-12-18 | 1999-02-10 | Chiron Spa | Antigens |
| US7128918B1 (en) * | 1998-12-23 | 2006-10-31 | Id Biomedical Corporation | Streptococcus antigens |
| EP1162999B1 (en) | 1999-03-19 | 2006-11-29 | Glaxosmithkline Biologicals S.A. | Vaccine against Streptococcus pneumoniae |
| JP2002541808A (ja) | 1999-04-09 | 2002-12-10 | テクラブ, インコーポレイテッド | ポリサッカリド結合体ワクチンのための組換えトキシンaタンパク質キャリア |
| US7101692B2 (en) * | 1999-04-15 | 2006-09-05 | The Regents Of The University Of California | Identification of sortase gene |
| WO2000067161A2 (en) | 1999-05-04 | 2000-11-09 | Grant Lee H | Method and apparatus for categorizing and retrieving network pages and sites |
| US6833356B1 (en) * | 1999-08-25 | 2004-12-21 | Medimmune, Inc. | Pneumococcal protein homologs and fragments for vaccines |
| KR20020048942A (ko) | 1999-09-24 | 2002-06-24 | 장 스테판느 | 폴리옥시에틸렌 알킬 에테르 또는 에스테르 및 하나이상의 비이온성 계면활성제를 포함하는 애쥬번트 |
| JP2003509473A (ja) | 1999-09-24 | 2003-03-11 | スミスクライン ビーチャム バイオロジカルズ ソシエテ アノニム | ワクチン |
| AU7721500A (en) | 1999-09-29 | 2001-04-30 | Human Genome Sciences, Inc. | Colon and colon cancer associated polynucleotides and polypeptides |
| EP2275129A3 (en) | 2000-01-17 | 2013-11-06 | Novartis Vaccines and Diagnostics S.r.l. | Outer membrane vesicle (OMV) vaccine comprising N. meningitidis serogroup B outer membrane proteins |
| AU3108001A (en) | 2000-01-20 | 2001-12-24 | Coley Pharmaceutical Group, Inc. | Immunostimulatory nucleic acids for inducing a th2 immune response |
| US6777547B1 (en) * | 2000-01-31 | 2004-08-17 | Andreas Podbielski | Collagen-binding proteins from streptococcus pyogenes |
| US6400152B1 (en) * | 2000-01-31 | 2002-06-04 | General Electric Company | Spiral trajectory calculations for MRI |
| EP1268774A2 (en) * | 2000-03-21 | 2003-01-02 | Elitra Pharmaceuticals, Inc. | Identification of essential genes in prokaryotes |
| GB0007432D0 (en) | 2000-03-27 | 2000-05-17 | Microbiological Res Authority | Proteins for use as carriers in conjugate vaccines |
| AUPQ801700A0 (en) * | 2000-06-07 | 2000-06-29 | Peplin Research Pty Ltd | Enzyme and viral activation |
| ATE440861T1 (de) | 2000-07-03 | 2009-09-15 | Novartis Vaccines & Diagnostic | Immunisierung gegen chlamydia pneumoniae |
| AU7227001A (en) * | 2000-07-06 | 2002-01-21 | Shire Biochem Inc | Streptococcus pyogenes antigens |
| EP1810978B1 (en) * | 2000-08-08 | 2013-02-13 | St. Jude Children's Research Hospital | Group B streptococcus polypeptides nucleic acids and therapeutic composition and vaccines thereof |
| AU9475001A (en) | 2000-09-26 | 2002-04-08 | Hybridon Inc | Modulation of immunostimulatory activity of immunostimulatory oligonucleotide analogs by positional chemical changes |
| AU2001294953A1 (en) * | 2000-09-29 | 2002-04-08 | Regents Of The University Of Minnesota | Triterpenes having fungicidal activity against yeast |
| WO2002057315A2 (en) * | 2000-10-10 | 2002-07-25 | University Of Tennessee Research Corporation | Streptococcal streptolysin s vaccines |
| US7160547B2 (en) * | 2000-10-10 | 2007-01-09 | University Of Tennessee Research Corporation | Streptococcal streptolysin S vaccines |
| JP2002135314A (ja) | 2000-10-26 | 2002-05-10 | Sharp Corp | 通信制御方法および通信機器 |
| GB0103424D0 (en) | 2001-02-12 | 2001-03-28 | Chiron Spa | Gonococcus proteins |
| PT1372708E (pt) | 2001-02-13 | 2008-09-29 | Us Gov Sec Army | Vacina para imunização transcutânea |
| JP2004529906A (ja) | 2001-03-19 | 2004-09-30 | イオマイ コーポレイシヨン | 経皮的免疫賦活 |
| GB0107658D0 (en) | 2001-03-27 | 2001-05-16 | Chiron Spa | Streptococcus pneumoniae |
| US20040236072A1 (en) * | 2001-04-13 | 2004-11-25 | Olmsted Stephen Bruce | Surface proteins of streptococcus pyogenes |
| US20070128229A1 (en) * | 2002-04-12 | 2007-06-07 | Wyeth | Surface proteins of Streptococcus pyogenes |
| FR2824074A1 (fr) * | 2001-04-26 | 2002-10-31 | Pasteur Institut | Sequence du genome streptococcus agalactiae, application au developpement de vaccins, d'outils de diagnostic, et a l'identification de cibles therapeutiques |
| US7407664B2 (en) * | 2001-05-18 | 2008-08-05 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services, Centers For Disease Control And Prevention | Peptide vaccines against group A streptococci |
| GB0118249D0 (en) * | 2001-07-26 | 2001-09-19 | Chiron Spa | Histidine vaccines |
| US20060073530A1 (en) * | 2001-08-15 | 2006-04-06 | Olaf Schneewind | Methods and compositions involving sortase B |
| EP1425040A2 (en) | 2001-09-14 | 2004-06-09 | Cytos Biotechnology AG | In vivo activation of antigen presenting cells for enhancement of immune responses induced by virus like particles |
| ATE447967T1 (de) | 2001-09-14 | 2009-11-15 | Cytos Biotechnology Ag | Verpackung von immunstimulierendem cpg in virusähnlichen partikeln: herstellungsverfahren und verwendung |
| GB0123580D0 (en) | 2001-10-01 | 2001-11-21 | Glaxosmithkline Biolog Sa | Vaccine |
| WO2003035836A2 (en) | 2001-10-24 | 2003-05-01 | Hybridon Inc. | Modulation of immunostimulatory properties of oligonucleotide-based compounds by optimal presentation of 5' ends |
| US20040029129A1 (en) * | 2001-10-25 | 2004-02-12 | Liangsu Wang | Identification of essential genes in microorganisms |
| JP2005535287A (ja) | 2002-02-11 | 2005-11-24 | アイディー バイオメディカル コーポレイション | グループb連鎖球菌抗原 |
| GB2385274B (en) * | 2002-02-13 | 2004-04-14 | Ming-Jeng Shue | Vaginal suppository delivery device |
| US20050181388A1 (en) * | 2002-04-02 | 2005-08-18 | Affinium Pharmaceuticals, Inc. | Novel purified polypeptides from bacteria |
| WO2003087353A2 (en) * | 2002-04-08 | 2003-10-23 | Affinium Pharmaceuticals, Inc. | Purified polypeptides involved in membrane biogenesis |
| GB0210128D0 (en) * | 2002-05-02 | 2002-06-12 | Chiron Spa | Nucleic acids and proteins from streptococcus groups A & B |
| WO2004018646A2 (en) * | 2002-08-26 | 2004-03-04 | Chiron Corporation | Conserved and specific streptococcal genomes |
| TW566366U (en) * | 2002-09-27 | 2003-12-11 | Wus Tech Co Ltd | Labor-saving portable battery equipment for power-driven walking assisted scooter |
| US7485710B2 (en) * | 2002-10-15 | 2009-02-03 | Intercell Ag | Nucleic acids coding for adhesion factor of group B streptococcus, adhesion factors of group B streptococcus and further uses thereof |
| DK1601770T3 (da) | 2003-03-04 | 2009-11-02 | Intercell Ag | Streptococcus pyogenes antigener |
| NZ543467A (en) | 2003-04-10 | 2008-07-31 | Novartis Vaccines & Diagnostic | The severe acute respiratory syndrome coronavirus |
| EP1620460A2 (en) * | 2003-05-07 | 2006-02-01 | Intercell AG | Streptococcus agalactiae antigens i + ii |
| JP4896715B2 (ja) | 2003-06-26 | 2012-03-14 | ノバルティス バクシンズ アンド ダイアグノスティックス,インコーポレーテッド | Chlamydiatrachomatisに対する免疫原性組成物 |
| EP1648500B1 (en) * | 2003-07-31 | 2014-07-09 | Novartis Vaccines and Diagnostics, Inc. | Immunogenic compositions for streptococcus pyogenes |
| AU2003904237A0 (en) * | 2003-08-08 | 2003-08-21 | Garvan Institute Of Medical Research | Novel translocation assay |
| US8945589B2 (en) * | 2003-09-15 | 2015-02-03 | Novartis Vaccines And Diagnostics, Srl | Immunogenic compositions for Streptococcus agalactiae |
| EP1721283B1 (en) * | 2004-02-06 | 2022-11-30 | Council of Scientific and Industrial Research | Computational method for identifying adhesin and adhesin-like proteins of therapeutic potential |
| US20060041961A1 (en) * | 2004-03-25 | 2006-02-23 | Abad Mark S | Genes and uses for pant improvement |
| WO2005108419A1 (en) | 2004-05-07 | 2005-11-17 | Lea-Ann Kirkham | Mutant cholesterol-binding cytolysin proteins |
| EP1784211A4 (en) * | 2004-07-29 | 2010-06-30 | Novartis Vaccines & Diagnostic | IMMUNOGENIC COMPOSITIONS FOR GRAMPOSITIVE BACTERIA SUCH AS STREPTOCOCCUS AGALACTIAE |
| AU2005335216A1 (en) | 2004-10-05 | 2007-02-15 | Wyeth | Probe arrays for detecting multiple strains of different species |
| EP1770171A1 (en) | 2005-09-29 | 2007-04-04 | Universität Zu Köln | DNA microarray for rapid identification of Candida albicans in blood cultures. |
| EP2292648A3 (en) | 2006-07-07 | 2011-06-08 | Intercell AG | Small Streptococcus pyogenes antigens and their use |
| AT514720B1 (de) | 2013-08-30 | 2016-03-15 | Blum Gmbh Julius | Stellvorrichtung |
-
2005
- 2005-07-29 EP EP05856900A patent/EP1784211A4/en not_active Withdrawn
- 2005-07-29 CA CA002575548A patent/CA2575548A1/en not_active Abandoned
- 2005-07-29 WO PCT/US2005/027239 patent/WO2006078318A2/en not_active Ceased
- 2005-07-29 US US11/192,046 patent/US20060165716A1/en not_active Abandoned
- 2005-07-29 US US11/658,842 patent/US20090317420A1/en not_active Abandoned
- 2005-07-29 EP EP13159533.2A patent/EP2612679A1/en not_active Withdrawn
- 2005-07-29 JP JP2007523880A patent/JP2008508320A/ja active Pending
-
2010
- 2010-10-18 US US12/906,510 patent/US8778358B2/en not_active Expired - Fee Related
-
2011
- 2011-07-22 JP JP2011161176A patent/JP2011206061A/ja active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002034771A2 (en) * | 2000-10-27 | 2002-05-02 | Chiron Srl | Nucleic acids and proteins from streptococcus groups a & b |
| WO2004041157A2 (en) * | 2002-09-13 | 2004-05-21 | Chiron Corporation | Group b streptococcus vaccine |
Non-Patent Citations (1)
| Title |
|---|
| JPN6011006146; LARSSON, C., et al.: 'Protection against experimental infection with group B streptococcus by immunization with a bivalent' Vaccine Vol.17, 1999, p.454-458 * |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012515157A (ja) * | 2009-01-12 | 2012-07-05 | ノバルティス アーゲー | グラム陽性細菌に対するワクチンにおけるCna_Bドメイン抗原 |
| JP2013504556A (ja) * | 2009-09-10 | 2013-02-07 | ノバルティス アーゲー | 気道疾患に対する組み合わせワクチン |
| JPWO2011040133A1 (ja) * | 2009-10-02 | 2013-02-21 | 学校法人東京女子医科大学 | ヒト血清アミロイドa3抗体およびその利用 |
| JP2013520487A (ja) * | 2010-02-26 | 2013-06-06 | ノバルティス アーゲー | 免疫原性タンパク質および組成物 |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2575548A1 (en) | 2006-07-27 |
| US20110110982A1 (en) | 2011-05-12 |
| JP2011206061A (ja) | 2011-10-20 |
| US20060165716A1 (en) | 2006-07-27 |
| WO2006078318A3 (en) | 2008-10-30 |
| EP1784211A2 (en) | 2007-05-16 |
| EP1784211A4 (en) | 2010-06-30 |
| WO2006078318A2 (en) | 2006-07-27 |
| EP2612679A1 (en) | 2013-07-10 |
| US8778358B2 (en) | 2014-07-15 |
| US20090317420A1 (en) | 2009-12-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8778358B2 (en) | Immunogenic compositions for gram positive bacteria such as Streptococcus agalactiae | |
| JP2008508320A5 (enExample) | ||
| US20100150943A1 (en) | Immunogenic compositions for gram positive bacteria | |
| AU716225B2 (en) | Proteinase K resistant surface protein of neisseria meningitidis | |
| US20100074923A1 (en) | Purification of bacterial antigens | |
| EP2167531B1 (en) | Streptococcus pneumoniae pilus antigens | |
| JP6084631B2 (ja) | Clostridiumdifficile毒素ベースのワクチン | |
| RU2498994C2 (ru) | Мутантные формы стрептолизина о | |
| US9102740B2 (en) | Cna-B domain antigens in vaccines against gram positive bacteria | |
| JP5653215B2 (ja) | Gas57変異体抗原およびgas57抗体 | |
| JP2010538634A5 (enExample) | ||
| US8945589B2 (en) | Immunogenic compositions for Streptococcus agalactiae | |
| JP6401148B2 (ja) | 抗原および抗原の組み合わせ | |
| AU762316B2 (en) | Proteinase K resistant surface protein of neisseria meningitidis | |
| AU2013202316A1 (en) | Compositions comprising Yersinia pestis antigens |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20080620 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090428 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110204 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110502 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110512 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110602 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110609 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110704 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110711 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20110722 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110824 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20111117 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20111125 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120213 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20120213 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20120627 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20121012 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20121029 |
|
| A912 | Re-examination (zenchi) completed and case transferred to appeal board |
Free format text: JAPANESE INTERMEDIATE CODE: A912 Effective date: 20121116 |