EP3265779B1 - Procédé de détection optique d'un mouvement dans un échantillon biologique à expansion spatiale - Google Patents
Procédé de détection optique d'un mouvement dans un échantillon biologique à expansion spatiale Download PDFInfo
- Publication number
- EP3265779B1 EP3265779B1 EP16708082.9A EP16708082A EP3265779B1 EP 3265779 B1 EP3265779 B1 EP 3265779B1 EP 16708082 A EP16708082 A EP 16708082A EP 3265779 B1 EP3265779 B1 EP 3265779B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- sample
- detector
- light
- beam source
- light beam
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 230000033001 locomotion Effects 0.000 title claims description 50
- 238000000034 method Methods 0.000 title claims description 40
- 238000001514 detection method Methods 0.000 title claims description 36
- 230000003287 optical effect Effects 0.000 title claims description 30
- 239000012472 biological sample Substances 0.000 title claims description 23
- 239000000523 sample Substances 0.000 claims description 170
- 230000005855 radiation Effects 0.000 claims description 46
- 238000005259 measurement Methods 0.000 claims description 30
- 238000005286 illumination Methods 0.000 claims description 23
- 230000008859 change Effects 0.000 claims description 20
- 230000003993 interaction Effects 0.000 claims description 17
- 210000004027 cell Anatomy 0.000 claims description 13
- 238000003384 imaging method Methods 0.000 claims description 9
- 230000008602 contraction Effects 0.000 claims description 7
- 230000001427 coherent effect Effects 0.000 claims description 6
- 239000011159 matrix material Substances 0.000 claims description 6
- 238000000338 in vitro Methods 0.000 claims description 5
- 210000001519 tissue Anatomy 0.000 claims description 5
- 229920001222 biopolymer Polymers 0.000 claims description 4
- 210000000663 muscle cell Anatomy 0.000 claims description 4
- 230000000737 periodic effect Effects 0.000 claims description 4
- 230000002123 temporal effect Effects 0.000 claims 2
- 230000010287 polarization Effects 0.000 description 21
- 230000008901 benefit Effects 0.000 description 7
- 210000003205 muscle Anatomy 0.000 description 6
- 230000005540 biological transmission Effects 0.000 description 4
- 238000012544 monitoring process Methods 0.000 description 4
- 230000035945 sensitivity Effects 0.000 description 4
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 3
- 229940072056 alginate Drugs 0.000 description 3
- 235000010443 alginic acid Nutrition 0.000 description 3
- 229920000615 alginic acid Polymers 0.000 description 3
- 238000013461 design Methods 0.000 description 3
- 238000010191 image analysis Methods 0.000 description 3
- 244000005700 microbiome Species 0.000 description 3
- 238000012216 screening Methods 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 238000013459 approach Methods 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 210000004165 myocardium Anatomy 0.000 description 2
- 239000013307 optical fiber Substances 0.000 description 2
- 238000013334 tissue model Methods 0.000 description 2
- 230000000007 visual effect Effects 0.000 description 2
- 230000001464 adherent effect Effects 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 230000022900 cardiac muscle contraction Effects 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000005352 clarification Methods 0.000 description 1
- 239000004020 conductor Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 230000005670 electromagnetic radiation Effects 0.000 description 1
- 210000001671 embryonic stem cell Anatomy 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 230000017525 heat dissipation Effects 0.000 description 1
- 230000020169 heat generation Effects 0.000 description 1
- 238000013537 high throughput screening Methods 0.000 description 1
- 238000002847 impedance measurement Methods 0.000 description 1
- 238000009434 installation Methods 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 230000001795 light effect Effects 0.000 description 1
- 238000000691 measurement method Methods 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 230000004118 muscle contraction Effects 0.000 description 1
- 230000002107 myocardial effect Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 238000009877 rendering Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 230000019100 sperm motility Effects 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 230000009182 swimming Effects 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000820 toxicity test Toxicity 0.000 description 1
- 231100000041 toxicology testing Toxicity 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 238000010865 video microscopy Methods 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01P—MEASURING LINEAR OR ANGULAR SPEED, ACCELERATION, DECELERATION, OR SHOCK; INDICATING PRESENCE, ABSENCE, OR DIRECTION, OF MOVEMENT
- G01P13/00—Indicating or recording presence, absence, or direction, of movement
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N21/00—Investigating or analysing materials by the use of optical means, i.e. using sub-millimetre waves, infrared, visible or ultraviolet light
- G01N21/17—Systems in which incident light is modified in accordance with the properties of the material investigated
- G01N21/21—Polarisation-affecting properties
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N21/00—Investigating or analysing materials by the use of optical means, i.e. using sub-millimetre waves, infrared, visible or ultraviolet light
- G01N21/17—Systems in which incident light is modified in accordance with the properties of the material investigated
- G01N21/47—Scattering, i.e. diffuse reflection
- G01N21/4788—Diffraction
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N21/00—Investigating or analysing materials by the use of optical means, i.e. using sub-millimetre waves, infrared, visible or ultraviolet light
- G01N21/17—Systems in which incident light is modified in accordance with the properties of the material investigated
- G01N21/47—Scattering, i.e. diffuse reflection
- G01N21/49—Scattering, i.e. diffuse reflection within a body or fluid
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N21/00—Investigating or analysing materials by the use of optical means, i.e. using sub-millimetre waves, infrared, visible or ultraviolet light
- G01N21/17—Systems in which incident light is modified in accordance with the properties of the material investigated
- G01N21/47—Scattering, i.e. diffuse reflection
- G01N21/4788—Diffraction
- G01N2021/479—Speckle
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N21/00—Investigating or analysing materials by the use of optical means, i.e. using sub-millimetre waves, infrared, visible or ultraviolet light
- G01N21/17—Systems in which incident light is modified in accordance with the properties of the material investigated
- G01N21/47—Scattering, i.e. diffuse reflection
- G01N2021/4792—Polarisation of scatter light
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/15—Medicinal preparations ; Physical properties thereof, e.g. dissolubility
Definitions
- the invention relates to a method for the optical in-vitro detection of a movement in a biological sample with spatial extension.
- pieces of tissue grown from embryonic stem cells which have differentiated into muscle tissue, are used in toxicity tests to test the harmfulness of a substance to be tested.
- measurement methods are required to detect a movement, e.g. B. a contraction to detect in such a three-dimensional biological sample in the form of a cell cluster.
- Typical diameters of such cell clusters are 100 to 400 ⁇ m, with diameters in the millimeter range also being possible.
- Non-optical methods such as impedance measurements only work when in contact with the sample. However, if the sample shape deviates from the plane (adherent monolayer) or is even three-dimensional, still floating freely in a medium, the aforementioned techniques are not applicable.
- Automated imaging methods have the disadvantage that e.g. B. at a depth of field of 10 microns and the above-mentioned typical size of the cell clusters of 100 to 400 microns 10 to 40 image planes of the sample would have to be measured. With a typical minimum measurement time of approximately 10 seconds to be able to detect a movement and the additional time required for repositioning or focusing, such methods are not suitable for quickly monitoring a large number of samples.
- a serial measurement of large numbers of samples is generally not indicated due to the relatively long observation period due to the time scales of the biological dynamics.
- Imaging methods are not suitable for a parallel measurement of a large number of such samples, since it is difficult to implement, for geometric and installation space reasons, to arrange an imaging optical device in each cavity of the multiwell plate.
- WO 2014/123156 A1 discloses the detection of motion in a sample of muscle cells by calculating the contrast of an imaged speckle pattern.
- JA COLE ET AL "Laser speckle spectroscopy-a new method for using small swimming organisms as biomonitors", BIOIMAGING, Vol. 4, No. 4, December 1996, pages 243-253 discloses a punctiform measurement of a speckle pattern with subsequent Fourier transformation (FFT) to analyze the movement of micro-organisms.
- FFT Fourier transformation
- the object of the invention is in particular to provide a robust, contact-free method for motion detection that does not require any complex image analysis. It is a further object of the invention to provide a method which is suitable for the parallel analysis of a large number of samples in a screening environment.
- the invention is based on the technical knowledge that optical methods are most suitable for contactless monitoring and that since the exact location of a possible movement in the sample is not known in advance, it is necessary to illuminate the entire sample. Since the sample generally has a considerable extension in depth, ie greater than the depth of focus of imaging optics, the interactions of the light with the sample must be measured cumulatively over its entire distance through the sample. Transmission methods are therefore unsuitable, since the non-interacting part of the light would be measured here and the expected variations would be accompanied by high background and noise rendering the technique insensitive.
- the approach described is therefore based on the detection of the scattered, polarized and/or according to the invention the diffraction radiation of the exposed sample, with a search being made for the fluctuations caused by the sample movement in that part of the light which, through interaction with the sample in its beam direction, their state of polarization and/or according to the invention their diffraction pattern has been changed.
- the term “light” is also used instead of the term “radiation”. Both terms are to be regarded as synonymous in the context of the present invention and include electromagnetic radiation in the visible, IR and UV range.
- a method for the optical in-vitro detection of a movement in a biological sample with a spatial extension is thus provided.
- the method according to the invention comprises providing a holder for the sample, a light beam source, an optical system and a detector.
- the optics are designed to illuminate the entire sample in the recording with radiation emanating from the light beam source and to illuminate at least part of the radiation from the light beam source, the diffraction pattern of which is changed at any point within the sample by interaction with the sample to direct a detection surface of the detector, so that the interactions of the radiation emitted by the light beam source with the sample are measured cumulatively over its entire distance through the sample.
- the detector is designed to generate a measurement signal as a function of the detected radiation, the time profile of which indicates a time profile of the intensity of the detected radiation and/or from which the time profile of the intensity of the detected radiation can be derived.
- the method according to the invention also includes illuminating the sample with radiation from the light beam source and detecting a movement in the biological sample as a function of a change in the measurement signal over time.
- a particular advantage of the approach according to the invention is that the sample dynamics can be derived directly from the measured variable of the method, since the fluctuations caused by a sample movement in that part of the light that was changed in the diffraction pattern by interaction with the sample directly as fluctuations in the measurement signal are visible. Complex processing of the measurement data, as is the case with imaging methods, can thus be dispensed with.
- the invention it is possible to detect movement in the sample if a change in the measurement signal exceeds a predetermined threshold value.
- a movement is detected if the change in the measurement signal over time has a periodicity.
- the method can be carried out using comparatively simple optical elements. Optics for focusing on individual image planes and for successive scanning of the sample volume are not necessary.
- the device for carrying out the method can therefore be designed to be inexpensive and structurally compact.
- the method is also suitable for the parallel monitoring of a large number of samples and can be used in a process-efficient manner in screening environments or in automated high-throughput methods, e.g. e.g. high-throughput screening methods.
- the detector has a single-channel design or the detector emits a single-channel measurement signal.
- the measurement signal preferably indicates only the radiation intensity incident on the detector surface per unit of time. A movement in the sample can thus be detected directly based on a change or fluctuation in the signal over time.
- the detector is a non-imaging detector or a detector with a non-spatially resolved measurement signal, e.g. B. a non-spatially resolving photodetector, such as a photodiode.
- a non-imaging detector or a detector with a non-spatially resolved measurement signal e.g. B. a non-spatially resolving photodetector, such as a photodiode.
- Such detectors are compact and inexpensive.
- a spatially expansive biological sample is defined as a three-dimensional biological sample, e.g. B. in the form of a three-dimensional cell and / or tissue culture or a cell cluster understood.
- the diameter of the biological sample can be at least 50 micrometers ( ⁇ m) in at least one spatial direction, more preferably at least 50 micrometers in all spatial directions and is often more than 100 ⁇ m in all spatial directions.
- the diameter of the sample is preferably in the range of 100 ⁇ m to 5 mm, more preferably in the range of 100 ⁇ m up to 1 mm.
- the biological sample is a sample of living cells, ie cells that have active dynamics, ie cells that can trigger movement.
- the detection of a movement in the biological sample should be understood in particular as a movement within the sample or a movement of a sample component of the biological sample. In other words, it should generally be possible to detect dynamic phenomena in or within biological samples with a spatial extent. In the case of cell cultures from muscle cells, such movements can be triggered, for example, by the contraction of individual muscle cells.
- the biological sample cannot be a sample of free-swimming microorganisms, for example sperm.
- the method can be used to detect the movement of the free-swimming microorganisms, for example in the case of sperm to determine sperm motility.
- the optics can include illumination optics arranged on the illumination side, by means of which the radiation from the light beam source is directed onto the entire sample in order to illuminate the sample completely and as uniformly as possible.
- the optics can also include detection optics, by means of which the light emitted by the sample, which is changed in its beam direction, its polarization state and/or its diffraction pattern by interaction with the sample, is directed onto a detection surface of the detector.
- This functional property of the optics can be achieved using one or more appropriately arranged and configured known optical structural elements and components, such as As filters, lenses, diaphragms, refractive elements, etc., can be realized, which is explained below with reference to further exemplary embodiments.
- An advantageous embodiment variant provides that the optics are designed and/or the detector is arranged relative to the illumination beam path and the sample in such a way that there are no beam paths in which radiation from the light beam source transmitted through the sample impinges on the detector and/or in which Light from the light source bypasses the sample and hits the detector.
- a movement in the sample is detected based on a change in the diffraction pattern.
- the light beam source generates coherent light.
- the optics is formed, for. B. by means of a spatial filter, an edge region of a diffraction pattern, which is generated by the sample diffracted light of the light beam source, on the detector. Movement within the sample produces a change in the diffraction pattern, according to the invention a speckle pattern. Investigations within the scope of the invention have shown that the change in the diffraction pattern at its center is difficult to measure since the relative change in the radiation intensity is small. In the edge area, however, the change can be reliably detected and can e.g. B. to a short-term change from a local diffraction maximum to a local diffraction minimum or vice versa in the diffraction pattern. In a diffraction pattern, each point in the pattern contains the diffraction information for the entire sample.
- the optics comprise a pinhole diaphragm, which is arranged between the sample and the detector in such a way that a hole in the pinhole diaphragm is arranged at the edge area of the diffraction pattern generated by the sample.
- a pinhole diaphragm is understood to mean a hole-shaped opening, preferably a small hole-shaped opening and preferably without a lens. Pinholes are used to locally collect light.
- the end faces of optical fibers have long been used for the same purpose, particularly in confocal microscopes.
- All observation angles in which no transmitted light is received should preferably be regarded as the edge area of the diffraction pattern.
- the diffraction pattern is caused by positive and negative interference of light waves that have been diffracted by objects, in this case the sample. Whether there is positive or negative interference depends on the size of the object, the wavelength of the light and the viewing angle. Light that passes through the sample without interacting, ie transmitted light, ballistic photons, has the observation angle 0° and strikes the center of the diffraction pattern.
- the diffraction pattern is outshined by the transmitted light and the S/B (S/B: signal-to-background, signal-background) ratio and the S/N (S/N: signal-to-noise, signal-to-noise) ratio fall drastically. All observation angles in which no transmitted light is received should therefore preferably be regarded as the edge region of the diffraction pattern. Since the entire sample is irradiated here and the illumination is not is collimated, the "reception area" of the transmitted radiation is larger than just one point.
- the pinhole diaphragm can also have a plurality of holes which are arranged relative to the diffraction pattern in such a way that they are located in an edge region of the diffraction pattern.
- the holes are to be arranged in such a way that the superimposition of the diffraction radiation that hits the detector through the holes increases the diffraction effect and does not worsen it.
- the optics can comprise an aperture arranged between the light beam source and the sample, which is designed in such a way that radiation from the light beam source exits through an aperture of the aperture not directly, i. H. bypassing the sample, hits the hole of the pinhole.
- the optics can be a refractive optical element arranged between the light beam source and the sample, i. H. a radiation-refracting element, e.g. a convex lens or a prism, which is designed in such a way that radiation deflected by the refractive element is not emitted directly, ie. H. bypassing the sample, hits the hole of the pinhole.
- a movement in the sample is detected on the basis of a fluctuation in polarized light caused by the movement.
- the optics comprises a first polarization filter and a second polarization filter have different polarization directions, the first polarization filter being arranged between the light beam source and the sample and the second polarizing filter being arranged between the sample and the detector. Movement in the sample leads to a change in the interaction of the polarized light with the sample and a change in polarization states, resulting in a fluctuation in the detector signal.
- the detector and the second polarizing filter can be arranged on the opposite side of the sample with respect to the light beam source, on the side of the sample or on the same side as the light beam source.
- a movement in the sample is detected on the basis of a fluctuation in the light scattered at the sample caused by the movement.
- the detector for detecting scattered light from the sample can be arranged on the same side of the sample (1) as the light beam source.
- the detector for detecting lateral scattered radiation of the sample can be arranged at an angle and/or to the side of the sample in relation to the direction of the illumination beam path.
- the detector for transmitted light detection of scattered light from the sample can be arranged in the transmitted light direction to the sample.
- the optics comprises an aperture arranged between the light beam source and the sample, which is designed to direct light beams in the illumination beam path, which are transmitted through the sample to the detector would hit to block.
- the optics can comprise a refractive optical element arranged between the light beam source and the sample, which is designed to change a direction of the illumination beam path such that light beams transmitted through the sample do not impinge on the detector.
- the optics have a bandpass filter which is arranged in front of the detector and is designed to suppress ambient light. As a result, the sensitivity of the detection can be further increased.
- the optics in particular the illumination optics, comprise an axicon.
- the use of an axicon offers the advantage of more homogeneous illumination of spatially "deep" samples compared to convex lenses, since the focus of an axicon extends along the optical axis - instead of just in one point.
- Another advantage of an axicon is the simple spatial filtering of the incident (unaffected) light, since it is refracted at a constant angle to the optical axis.
- the sample can be located on a carrier matrix.
- the receptacle for the sample can thus include a carrier matrix, preferably a biopolymer.
- the sample can also be in a hanging drop.
- the recording of the sample can thus include a hanging drop.
- the well for the sample can be a well of a multiwell plate (microtiter plate).
- the receptacle can be a cavity of a multiwell plate which is designed to form a hanging drop on the individual cavities (so-called hanging drop multiwell plate).
- hanging drop multiwell plates are available, for example, from Insphero AG, CH-8952 Schlieren under the name “GravityPLUS TM 3D Culture and Assay Platform”.
- the patent EP 2342317 B1 discloses such a panel.
- the method is particularly suitable for the parallel monitoring of a large number of samples, e.g. B. of samples to be examined in the context of automated high-throughput methods.
- the holder for the biological samples is preferably a multiwell plate which has a plurality of wells arranged in rows and columns for holding the samples.
- the detector is designed as a detector array, preferably as a photodiode array, with a grid spacing of the individual detectors corresponding to a grid spacing of the cavities of the multiwell plate.
- the light beam source is designed to illuminate the individual cavities.
- the light beam source for illuminating the samples in the cavities is designed as a laser diode array, with a grid spacing of the individual laser diodes corresponding to the grid spacing of the cavities of the multiwell plate.
- a laser diode array represents a space-saving and energy-efficient lighting source.
- the mount for the laser diode array can be made of a heat-conducting material, preferably aluminum.
- the optics according to this variant, a lens array, z. B. a microlens array include, each lens of the lens array is associated with one of the laser diodes and the lenses direct the light of the laser diodes into the cavities.
- the light beam source can be designed as a conventional light source (laser, arc lamp, etc.), with the light from the light beam source being coupled into the individual cavities containing the samples via a fiber optic bundle to illuminate the samples. Each optical fiber is assigned to a cavity. This offers the advantage that the light source can be operated at a sufficient distance from the sample in order to avoid excessive heat generation in the vicinity of the sample.
- a device for contact-free in vitro detection of motion in a biological sample with spatial extent comprises a receptacle for the biological sample, a light source, a detector and an optical system that is designed to illuminate the entire sample in the receptacle with radiation emanating from the light source and at least part of the radiation from the light source that is emitted at any Place within the sample was changed by an interaction with the sample in its beam direction, its polarization state and / or its diffraction pattern to lead to a detection surface of the detector.
- the detector is designed to generate a measurement signal as a function of the detected radiation, which indicates the time profile of the intensity of the detected radiation and/or from which the time profile of the intensity of the detected radiation can be derived.
- the device can also have an evaluation unit that is set up to detect a movement in the biological sample by displaying and/or evaluating a change over time in the detected radiation.
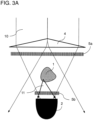
- figure 1 shows a highly schematized representation of a method not according to the invention and a device not according to the invention.
- a device 100 for the optical in-vitro detection of a movement in a biological sample 1 with spatial expansion is provided.
- the device 100 comprises a holder (not shown) for the three-dimensional sample 1, a light beam source 6, an optical system 7, 8 and a detector 2.
- the recording is not limited to a specific type of recordings, but can be appropriate depending on the application and the type of sample, e.g. B. as a support, support plate, as a vessel, as a cavity of a multiwell plate, or as a support matrix in the form of a biopolymer, z. B. alginate on which the sample is grown.
- sample e.g. B. as a support, support plate, as a vessel, as a cavity of a multiwell plate, or as a support matrix in the form of a biopolymer, z. B. alginate on which the sample is grown.
- the light source 6 can, but does not have to be a coherent light source, e.g. B. be a laser. Only the design variants that use a diffraction pattern of the sample for motion detection (cf. Figures 4A to 4C , 5A to 5C ) use coherent radiation.
- This functional property of the optics can be achieved using one or more appropriately arranged and designed known optical devices and components such. As filters, lenses, apertures, refractive elements, etc., can be realized.
- the optics 7, 8 comprise an illumination optics 7 arranged on the illumination side, by means of which the radiation 10 from the light beam source 6 is directed onto the entire sample 1 in order to illuminate the sample completely and as uniformly as possible.
- the illumination optics can comprise suitable optical elements or components for beam shaping, such as diaphragms, lenses and/or filters.
- suitable optical elements or components for beam shaping such as diaphragms, lenses and/or filters.
- the use of an axicon is particularly advantageous.
- the optics 7, 8 also include a detection optics 8, by means of which the light 11 emitted by the sample, ie light from the light beam source 6, which has been changed by interaction with the sample 1 in its beam direction, its polarization state and/or diffraction pattern, on a Detection surface 2a of the detector 2 is passed.
- a detection optics 8 by means of which the light 11 emitted by the sample, ie light from the light beam source 6, which has been changed by interaction with the sample 1 in its beam direction, its polarization state and/or diffraction pattern, on a Detection surface 2a of the detector 2 is passed.
- a detection optics 8 For example, a scattering process is shown at point P1, in which light 11 is scattered in the direction of detector 2 and is imaged onto detector 2 by means of detection optics 8. Since the entire sample 1 is uniformly illuminated, the interaction of the incident light 10 can take place at any point within the sample 1, so that the interactions of the light with the sample are measured cumulatively by the detector
- the detection optics 8 can also contain optical elements that ensure that light whose beam direction, polarization state and/or diffraction pattern has not been changed by interaction with the sample does not strike the detector 2 .
- the detection optics 8 have a bandpass filter placed in front of the detector input and which filters out or suppresses the room light but allows light with a wavelength of the light beam source 6 to pass. This presupposes that a monochromatic light source is used or that a corresponding filter is arranged at the output of the light source in order to illuminate the sample with only a specific wavelength of light.
- the detection optics 8 can use diaphragms, lenses etc. to block beam paths in which the radiation from the light beam source 6 transmitted through the sample 1 would impinge on the detector and/or in which light from the light beam source 6 would impinge on the detector 2 bypassing the sample.
- the detector can also be arranged in such a way that no transmitted light 12 impinges on its detector surface 2a, e.g. B. by lateral arrangement of the detector 2 relative to the illumination beam path 10, as in figure 1 illustrated.
- the detector 2 is designed to generate a measurement signal 9 as a function of the detected radiation 11 , the time profile of which indicates a time profile of an intensity of the detected radiation 11 .
- the detector signal 9 thus corresponds to a volume measurement (full-volume measurement) of the sample.
- the detector 2 is a non-imaging detector or a detector with a non-spatially resolved measurement signal, preferably single-channel, so that only one measurement variable 9 is generated, which corresponds to the fluctuation in the light intensity recorded by the detector per unit of time.
- the detector 2 is z. B. a conventional photodiode 2.
- the sample 1 is illuminated and the corresponding measurement signal 9 is evaluated.
- the light beam source 6 is not shown in FIGS. 2A to 3C, but is located above the optics and detector arrangement shown, which can be seen from the beam path 10 .
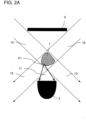
- FIGS 2A to 2E show non-inventive embodiments that use scattered light from the sample for motion detection.
- the detector 2 for transmitted light detection of scattered light 11 of the sample 2 is arranged in the transmitted light direction to the sample 1.
- a diaphragm 3 is arranged, which blocks light beams which would impinge on the detector 2 as beams transmitted through the sample 2 or which could impinge on the detector 2 laterally past the sample.
- the Illumination optics in the form of the diaphragm 3 therefore only allows radiation 12 transmitted through the sample that does not impinge on the detector 12 .
- a smaller aperture 3 which has a refractive optical element, e.g. B. a lens, an axicon, etc. is arranged downstream, which changes the direction of the illumination beam path 10 passed through the aperture 3 in such a way that the beams 12 transmitted through the sample 2 cannot impinge on the detector 2 .
- a refractive optical element e.g. B. a lens, an axicon, etc.
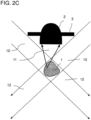
- the detector 2 for epidetection of scattered light in the form of reflection from the sample 1 is arranged on the same side of the sample 1 as the light beam source 6, with the detector surface 2a again facing the sample 2.
- This offers the advantage that no aperture is required to block transmitted light.
- a diaphragm surrounding the detector 2 is provided, which can reduce the influence of disruptive light influences.
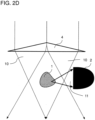
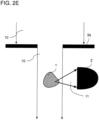
- FIG. 2D The peculiarity of the Figures 2D and 2E illustrated embodiment variants is that lateral scattered light 11 is detected.
- the detector 2 is arranged laterally to the direction of illumination.
- a refractive optical element e.g. B. a lens or prism 4, which changes the direction of the beam path so that light from the illumination beam path 10 cannot hit the detector 2 directly, ie bypassing the sample 1.
- the variant shown differs from the variant in Figure 2E in that instead of the refractive optical element 4 a diaphragm 3a is used, which has an opening for narrowing the beam path 10, so that again light of the illumination beam path 10 cannot hit the detector 2 directly, ie bypassing the sample 1.
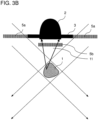
- the illumination optics comprise a first polarization filter 5a, which is arranged between the light beam source and the sample 1.
- the detection optics include a second polarization filter 5b, which has a different polarization direction compared to the first polarization filter 5a and is arranged between sample 1 and detector 2, preferably at the detector entrance.
- the detector 2 and the second polarization filter 5b can be arranged with respect to the light beam source on the opposite side of the sample 1, laterally from the sample 1 or on the same side as the light beam source, which is due to the different variants in the Figures 3A to 3C is shown.
- the polarization filter 5b Due to the different direction of polarization of the two polarization filters 5a and 5b, the polarization filter 5b only lets through light that has been "depolarized” by an interaction with the sample. The arrangement of the two polarization filters 5a and 5b thus ensures that no light transmitted through the sample or light that has bypassed the sample is detected.
- a movement in the sample changes the proportion of depolarized light and leads to a fluctuation in the detector signal, so that a movement in the sample 1 can in turn be recognized directly from the fluctuation in the detector signal.
- a refractive optical element 4 e.g. B. a convex lens or a prism, which focuses the illumination beam path 10 on the sample ( Figures 3A and 3C ).
- the detector 2 for epidetection of scattered light in the form of reflection from the sample 1 is arranged on the same side of the sample 1 as the light beam source 6.
- a screen 3 surrounding the detector 2 can be provided here, which can reduce the influence of disruptive light influences.
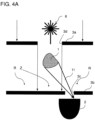
- the Figures 4A to 4C show embodiments of the invention that use a diffraction pattern of the sample in the form of a speckle pattern for motion detection.
- the light source 6 is a coherent light source, e.g. B. a laser diode.
- the light from the light beam source 6 diffracted at the sample produces a diffraction pattern with a center Z of high intensity and an edge region R of low intensity.
- the detection optics include a pinhole 3b arranged in front of the detector 2, which is arranged between the sample 1 and the detector 2 such that a hole 3c of the pinhole 3b is arranged in the edge region R of the diffraction pattern generated by the sample.
- the illumination optics includes an aperture 3a arranged between the light beam source and the sample, which is designed such that radiation from the light beam source 6 exiting through an aperture 3d of the aperture 3a does not strike the hole 3c of the pinhole 3b directly.
- Figures 4B and 4C includes the illumination optics arranged between the light beam source 6 and the sample 1 refractive optical element 4a, z. B. a convex lens, which is designed in such a way that radiation diffracted by the refractive optical element 4a does not hit the hole 3c of the pinhole diaphragm 3b directly, ie bypassing the sample.
- a bandpass filter 13 is arranged between the sample 1 and the pinhole 3b, which filters out room light that does not correspond to the wavelength of the light beam source 6.
- Figure 5A shows an example of a sample 1 in the form of a cell cluster.
- the cell cluster is in a hanging drop from which in Figure 5A only a portion formed in a well of a hanging drop multititer plate can be seen.
- the sample consists of a cardiac muscle tissue model differentiated from stem cells, which adheres to carrier beads 15 in the form of alginate.
- the sample shown has a diameter of approx. 1 millimeter.
- the sample 1 is completely illuminated with a laser diode with light of wavelength 650 nm.
- the on different Sample 1 which is structured on scales, diffracts the coherent light in a variety of ways and generates a complex diffraction pattern (so-called "speckle pattern") in the transmitted light direction.
- speckle pattern complex diffraction pattern
- Figure 5B 17a and 17b show two different states of a speckle pattern at minimum and maximum deflection of the contraction. Images 17a and 17b are for clarity only and are from a different experiment and do not show the speckle pattern of Sample 1 Figure 5A . However, the measuring principle is the same. Via a spatial filter, e.g. B. the pinhole 3b, a point in the edge region R of the diffraction pattern 17a, 17b on the detector 2 is imaged. The change in the pattern 17a, 17b now leads to a fluctuating amount of light that is let through by the pinhole 3b, and thus to a fluctuating detector signal 9, which in Figure 5C is shown. The periodic fluctuation of the signal 9 corresponds to the periodic contractions in muscle tissue. The representation in Figure 5C is only used for clarification, but again does not show a measurement signal that was measured when the sample shown in FIG. 1A was illuminated.
- a spatial filter e.g. B. the pinhole 3b
Landscapes
- Health & Medical Sciences (AREA)
- General Physics & Mathematics (AREA)
- Physics & Mathematics (AREA)
- Life Sciences & Earth Sciences (AREA)
- Immunology (AREA)
- Chemical & Material Sciences (AREA)
- Analytical Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Pathology (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Medicinal Chemistry (AREA)
- Food Science & Technology (AREA)
- Cell Biology (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Investigating Or Analysing Materials By Optical Means (AREA)
- Length Measuring Devices By Optical Means (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biophysics (AREA)
- Pharmacology & Pharmacy (AREA)
Claims (7)
- Procédé de détection optique in vitro d'un mouvement dans un échantillon biologique (1) à expansion spatiale sous la forme d'une culture cellulaire et/ou tissulaire tridimensionnelle ou d'un amas de cellules, dans lequel l'échantillon (1) présente des cellules musculaires vivantes, comprenant les étapes :a) de fourniture d'un logement pour l'échantillon (1), d'une source de rayonnement lumineux (6), d'une optique (7, 8) et d'un détecteur (2),a1) dans lequel la source de rayonnement lumineux (6) génère une lumière cohérente ;a2) dans lequel l'optique (7, 8) est réalisée pour éclairer la totalité de l'échantillon (1) dans le logement avec un rayonnement partant de la source de rayonnement lumineux (6) et pour diriger au moins une partie du rayonnement (11) de la source de rayonnement lumineux (6), qui est modifiée, sur un emplacement quelconque à l'intérieur de l'échantillon (1), du fait d'une interaction avec l'échantillon (1) quant à son motif de diffraction, sur une surface de détection (2a) du détecteur (2), eta3) dans lequel le détecteur (2) est réalisé pour générer, en fonction du rayonnement détecté, un signal de mesure (9), dont l'évolution dans le temps indique une évolution dans le temps d'une intensité du rayonnement (11) détecté et/ou à partir duquel l'évolution dans le temps de l'intensité du rayonnement (11) détecté peut être déduite ;a4) dans lequel le détecteur (2) n'est pas un détecteur à imagerie ou est un détecteur avec un signal de mesure sans résolution spatiale ;a5) dans lequel l'optique (7, 8) est réalisée pour reproduire sur le détecteur (2) une zone de bord d'un motif granulaire, qui est généré par une lumière, diffractée par l'échantillon (1), de la source de rayonnement lumineux (6) ; eta6) dans lequel l'optique (7, 8) comprend un diaphragme à trou (3b) qui est disposé de telle sorte entre l'échantillon (1) et le détecteur (2) qu'un trou (3c) du diaphragme à trou (3b) est disposé dans la zone de bord (R) du motif granulaire généré par l'échantillonb) d'éclairage de l'échantillon (1) avec un rayonnement de la source de rayonnement lumineux (6) ; etc) de détection d'un mouvement dans l'échantillon biologique (1) en fonction d'une modification dans le temps du signal de mesure (9) ;dans lequel un mouvement dans l'échantillon (1) est détecté si la modification dans le temps du signal de mesure (9) présente une périodicité,dans lequel une fluctuation périodique du signal de mesure (9) correspond à des contractions périodiques des cellules musculaires.
- Procédé selon la revendication 1, caractérisé en ce qu'un diamètre de l'échantillon (1)a) est d'au moins 50 micromètres (pm) dans au moins une direction spatiale ; oub) se situe dans la plage de 100 µm à 1 mm.
- Procédé selon l'une quelconque des revendications précédentes, caractérisé en cea) que le détecteur (2) émet un signal de mesure monocanal (9) ; et/oub) que le détecteur (2) est une photodiode.
- Procédé selon l'une quelconque des revendications précédentes, caractérisé en ce que l'optique (7, 8) est réalisée et/ou le détecteur (2) est disposé de telle sorte par rapport au chemin optique d'éclairage (10) et à l'échantillon (1) qu'il n'existe aucun chemin optique, pour lequel un rayonnement (12), transmis par l'échantillon (1), de la source de rayonnement lumineux (6) atteint le détecteur (2) et/ou pour lequel de la lumière de la source de rayonnement lumineux (6) atteint le détecteur en contournant l'échantillon (1).
- Procédé selon l'une quelconque des revendications précédentes,
caractérisé en ce que l'optique (7, 8) comprend :a) un diaphragme (3a) disposé entre la source de rayonnement lumineux (6) et l'échantillon (1), qui est réalisé de telle sorte qu'un rayonnement, sortant par une ouverture de diaphragme (3d) du diaphragme (3a), de la source de rayonnement lumineux (6) n'atteint pas directement le trou (3c) du diaphragme à trou (3b), et/oub) un élément optique réfractif (4a), disposé entre la source de rayonnement lumineux (6) et l'échantillon (1), qui est réalisé de telle sorte qu'un rayonnement diffracté par l'élément réfractif (4a) n'atteint pas directement le trou (3c) du diaphragme à trou (3b). - Procédé selon l'une quelconque des revendications précédentes, caractérisé en ce que l'optique (7, 8) présente un filtre passe-bande (13) disposé devant le détecteur (2), qui est réalisé pour supprimer la lumière ambiante.
- Procédé selon l'une quelconque des revendications précédentes, caractérisé en ce que le logement de l'échantillon comprend une matrice de support, de préférence un biopolymère (15).
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP23182756.9A EP4242637A3 (fr) | 2015-03-06 | 2016-03-02 | Procédé de détection optique d'un mouvement dans un échantillon biologique à expansion spatiale |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE102015003019.1A DE102015003019A1 (de) | 2015-03-06 | 2015-03-06 | Verfahren und Vorrichtung zur optischen Detektion einer Bewegung in einer biologischen Probe mit räumlicher Ausdehnung |
| PCT/EP2016/000377 WO2016142043A1 (fr) | 2015-03-06 | 2016-03-02 | Procédé et dispositif de détection optique d'un mouvement dans un échantillon biologique à expansion spatiale |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP23182756.9A Division EP4242637A3 (fr) | 2015-03-06 | 2016-03-02 | Procédé de détection optique d'un mouvement dans un échantillon biologique à expansion spatiale |
| EP23182756.9A Division-Into EP4242637A3 (fr) | 2015-03-06 | 2016-03-02 | Procédé de détection optique d'un mouvement dans un échantillon biologique à expansion spatiale |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP3265779A1 EP3265779A1 (fr) | 2018-01-10 |
| EP3265779B1 true EP3265779B1 (fr) | 2023-08-09 |
| EP3265779C0 EP3265779C0 (fr) | 2023-08-09 |
Family
ID=55456746
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP23182756.9A Pending EP4242637A3 (fr) | 2015-03-06 | 2016-03-02 | Procédé de détection optique d'un mouvement dans un échantillon biologique à expansion spatiale |
| EP16708082.9A Active EP3265779B1 (fr) | 2015-03-06 | 2016-03-02 | Procédé de détection optique d'un mouvement dans un échantillon biologique à expansion spatiale |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP23182756.9A Pending EP4242637A3 (fr) | 2015-03-06 | 2016-03-02 | Procédé de détection optique d'un mouvement dans un échantillon biologique à expansion spatiale |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US10488400B2 (fr) |
| EP (2) | EP4242637A3 (fr) |
| JP (1) | JP6851328B2 (fr) |
| KR (1) | KR20170140182A (fr) |
| CN (1) | CN107430064B (fr) |
| DE (1) | DE102015003019A1 (fr) |
| ES (1) | ES2961352T3 (fr) |
| WO (1) | WO2016142043A1 (fr) |
Families Citing this family (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10088660B2 (en) | 2017-02-10 | 2018-10-02 | Amgen Inc. | Imaging system for counting and sizing particles in fluid-filled vessels |
| MX2019009440A (es) * | 2017-02-10 | 2019-10-07 | Amgen Inc | Sistema de obtencion de imagenes para contar y dimensionar particulas en recipientes llenos de fluido. |
| BR112020012594A2 (pt) * | 2017-12-27 | 2020-11-24 | Ethicon Llc | imageamento hiperespectral em um ambiente com deficiência de luz |
| DE102018111033A1 (de) * | 2018-05-08 | 2019-11-14 | Byonoy Gmbh | Transmissionsvorrichtung zur Untersuchung von Proben in Kavitäten einer Mikrotiterplatte und Verfahren zum Untersuchen von Proben in Kavitäten einer Mikrotiterplatte mittels Transmission |
| US11141052B2 (en) | 2019-06-20 | 2021-10-12 | Cilag Gmbh International | Image rotation in an endoscopic fluorescence imaging system |
| US11550057B2 (en) | 2019-06-20 | 2023-01-10 | Cilag Gmbh International | Offset illumination of a scene using multiple emitters in a fluorescence imaging system |
| US11589819B2 (en) | 2019-06-20 | 2023-02-28 | Cilag Gmbh International | Offset illumination of a scene using multiple emitters in a laser mapping imaging system |
| US11237270B2 (en) | 2019-06-20 | 2022-02-01 | Cilag Gmbh International | Hyperspectral, fluorescence, and laser mapping imaging with fixed pattern noise cancellation |
| US11671691B2 (en) | 2019-06-20 | 2023-06-06 | Cilag Gmbh International | Image rotation in an endoscopic laser mapping imaging system |
| US11096565B2 (en) | 2019-06-20 | 2021-08-24 | Cilag Gmbh International | Driving light emissions according to a jitter specification in a hyperspectral, fluorescence, and laser mapping imaging system |
| US11389066B2 (en) | 2019-06-20 | 2022-07-19 | Cilag Gmbh International | Noise aware edge enhancement in a pulsed hyperspectral, fluorescence, and laser mapping imaging system |
| US11280737B2 (en) | 2019-06-20 | 2022-03-22 | Cilag Gmbh International | Super resolution and color motion artifact correction in a pulsed fluorescence imaging system |
| US11624830B2 (en) | 2019-06-20 | 2023-04-11 | Cilag Gmbh International | Wide dynamic range using a monochrome image sensor for laser mapping imaging |
| US11924535B2 (en) | 2019-06-20 | 2024-03-05 | Cila GmbH International | Controlling integral energy of a laser pulse in a laser mapping imaging system |
| US11716543B2 (en) | 2019-06-20 | 2023-08-01 | Cilag Gmbh International | Wide dynamic range using a monochrome image sensor for fluorescence imaging |
| US11700995B2 (en) | 2019-06-20 | 2023-07-18 | Cilag Gmbh International | Speckle removal in a pulsed fluorescence imaging system |
| US11793399B2 (en) | 2019-06-20 | 2023-10-24 | Cilag Gmbh International | Super resolution and color motion artifact correction in a pulsed hyperspectral imaging system |
| US11102400B2 (en) | 2019-06-20 | 2021-08-24 | Cilag Gmbh International | Pulsed illumination in a fluorescence imaging system |
| US11012599B2 (en) | 2019-06-20 | 2021-05-18 | Ethicon Llc | Hyperspectral imaging in a light deficient environment |
| US11758256B2 (en) | 2019-06-20 | 2023-09-12 | Cilag Gmbh International | Fluorescence imaging in a light deficient environment |
| US11612309B2 (en) | 2019-06-20 | 2023-03-28 | Cilag Gmbh International | Hyperspectral videostroboscopy of vocal cords |
| US11931009B2 (en) | 2019-06-20 | 2024-03-19 | Cilag Gmbh International | Offset illumination of a scene using multiple emitters in a hyperspectral imaging system |
| US11898909B2 (en) | 2019-06-20 | 2024-02-13 | Cilag Gmbh International | Noise aware edge enhancement in a pulsed fluorescence imaging system |
| US11457154B2 (en) | 2019-06-20 | 2022-09-27 | Cilag Gmbh International | Speckle removal in a pulsed hyperspectral, fluorescence, and laser mapping imaging system |
| US11925328B2 (en) | 2019-06-20 | 2024-03-12 | Cilag Gmbh International | Noise aware edge enhancement in a pulsed hyperspectral imaging system |
| US11674848B2 (en) | 2019-06-20 | 2023-06-13 | Cilag Gmbh International | Wide dynamic range using a monochrome image sensor for hyperspectral imaging |
| US11266304B2 (en) | 2019-06-20 | 2022-03-08 | Cilag Gmbh International | Minimizing image sensor input/output in a pulsed hyperspectral imaging system |
| US12013496B2 (en) | 2019-06-20 | 2024-06-18 | Cilag Gmbh International | Noise aware edge enhancement in a pulsed laser mapping imaging system |
| US11903563B2 (en) | 2019-06-20 | 2024-02-20 | Cilag Gmbh International | Offset illumination of a scene using multiple emitters in a fluorescence imaging system |
| US11134832B2 (en) | 2019-06-20 | 2021-10-05 | Cilag Gmbh International | Image rotation in an endoscopic hyperspectral, fluorescence, and laser mapping imaging system |
| US11622094B2 (en) | 2019-06-20 | 2023-04-04 | Cilag Gmbh International | Wide dynamic range using a monochrome image sensor for fluorescence imaging |
| US11187658B2 (en) | 2019-06-20 | 2021-11-30 | Cilag Gmbh International | Fluorescence imaging with fixed pattern noise cancellation |
| JP7403549B2 (ja) | 2019-09-26 | 2023-12-22 | 京セラ株式会社 | 細胞検出装置および細胞検出方法 |
| WO2021257457A1 (fr) * | 2020-06-15 | 2021-12-23 | Bragg Analytics, Inc. | Système de diagnostic in vitro global basé sur la diffraction |
| EP4165652A4 (fr) | 2020-06-15 | 2024-06-12 | Bragg Analytics, Inc. | Système de diagnostic in vitro global basé sur la diffraction |
| US11607188B2 (en) | 2020-06-15 | 2023-03-21 | Eosdx Inc. | Diffractometer-based global in situ diagnostic system |
| DE102023200837A1 (de) | 2023-02-02 | 2024-08-08 | Carl Zeiss Ag | Probengefäß zur Kultivierung biologischer Proben, Vorrichtung zu dessen Betrieb und Mikroskop |
Family Cites Families (39)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS54109488A (en) * | 1978-02-08 | 1979-08-28 | Fuji Photo Optical Co Ltd | Analyzing method and device of optically scattered image information |
| DE7836339U1 (de) * | 1978-12-07 | 1981-07-16 | Kaufmann, Raimund, Dr., 4005 Meerbusch | Vorrichtung zur bestimmung der geschwindigkeit von in einer fluessigkeit bewegten teilchen |
| JPS6259841A (ja) * | 1985-09-10 | 1987-03-16 | Res Dev Corp Of Japan | 直線偏光を用いる免疫反応の測定方法および装置 |
| JPS62116263A (ja) * | 1985-11-15 | 1987-05-27 | Olympus Optical Co Ltd | 直線偏光の多重散乱を用いる免疫反応の測定方法および装置 |
| JPH0623749B2 (ja) | 1986-03-10 | 1994-03-30 | 新技術事業団 | 毒素、薬剤による細胞損傷の新しい分光測定による検査法 |
| JP2949286B2 (ja) * | 1987-08-26 | 1999-09-13 | 松下電工株式会社 | 減光式二酸化炭素濃度感知器 |
| DE68924749T2 (de) * | 1988-09-15 | 1996-07-04 | The Board Of Trustees Of The University Of Arkansas, Little Rock, Ark. | Kennzeichnung von Teilchen durch modulierte dynamische Lichtstreuung. |
| US5061075A (en) * | 1989-08-07 | 1991-10-29 | Alfano Robert R | Optical method and apparatus for diagnosing human spermatozoa |
| US6671540B1 (en) * | 1990-08-10 | 2003-12-30 | Daryl W. Hochman | Methods and systems for detecting abnormal tissue using spectroscopic techniques |
| DE4215908A1 (de) * | 1992-05-14 | 1993-11-18 | Ubbo Prof Dr Ricklefs | Optische Einrichtung zur Bestimmung der Größe von Partikeln |
| US5627308A (en) * | 1995-05-31 | 1997-05-06 | Dahneke; Barton E. | Method and apparatus for measuring motion of a suspended particle or a suspending fluid |
| US6096510A (en) * | 1995-10-04 | 2000-08-01 | Cytoscan Sciences, Llc | Methods and systems for assessing biological materials using optical detection techniques |
| JP4169827B2 (ja) * | 1997-05-28 | 2008-10-22 | ミクロナス ゲーエムベーハー | 測定装置 |
| US20060105357A1 (en) * | 1998-02-18 | 2006-05-18 | Myomics, Inc. | Tissue sensor and uses thereof |
| DE19836183A1 (de) * | 1998-08-03 | 1999-03-18 | Gimsa Jan Priv Doz Dr | Verfahren und Vorrichtung zur räumlich (nm) und zeitlich (ms) aufgelösten Verfolgung der Bewegung mikroskopischer und submikroskopischer Objekte in mikroskopischen Volumina |
| AU749884B2 (en) * | 1998-08-28 | 2002-07-04 | Febit Ferrarius Biotechnology Gmbh | Support for a method for determining an analyte and a method for producing the support |
| US6100976A (en) * | 1998-09-21 | 2000-08-08 | The Board Of Regents For Oklahoma State University | Method and apparatus for fiber optic multiple scattering suppression |
| GB2361772B (en) * | 2000-04-29 | 2004-05-19 | Malvern Instr Ltd | Mobility and effects arising from surface charge |
| JP4384344B2 (ja) * | 2000-08-09 | 2009-12-16 | 拓之 今野 | レーザ反射光による粒状斑点模様を利用した血液凝固時間測定方法とその装置 |
| DE10041596A1 (de) * | 2000-08-24 | 2002-03-21 | Cellcontrol Biomedical Lab Gmb | Vorrichtung und Verfahren zum ortsaufgelösten Untersuchen vernetzter Zellen und/oder Zellsystemen und Verwendung für die Wirkstoffuntersuchung |
| JP4334899B2 (ja) * | 2003-02-25 | 2009-09-30 | 大塚電子株式会社 | 電気泳動速度測定装置 |
| GB0307756D0 (en) * | 2003-04-03 | 2003-05-07 | Suisse Electronique Microtech | Measuring the concentration and motility of light scattering particles |
| DE102006003877B4 (de) * | 2005-12-09 | 2007-10-31 | Diehl Bgt Defence Gmbh & Co. Kg | Vibrometer |
| EP2079363B1 (fr) * | 2006-10-30 | 2020-06-10 | Elfi-Tech Ltd | Procédé de mesure in vivo de paramètres biologiques |
| JP5024935B2 (ja) | 2007-01-16 | 2012-09-12 | 富士フイルム株式会社 | 光透過性部材の欠陥検出装置及び方法 |
| GB0701201D0 (en) * | 2007-01-22 | 2007-02-28 | Cancer Rec Tech Ltd | Cell mapping and tracking |
| US8821799B2 (en) * | 2007-01-26 | 2014-09-02 | Palo Alto Research Center Incorporated | Method and system implementing spatially modulated excitation or emission for particle characterization with enhanced sensitivity |
| CN102257123B (zh) | 2008-09-22 | 2014-03-26 | 苏黎世大学研究学部 | 悬滴板 |
| CN201311392Y (zh) | 2008-12-11 | 2009-09-16 | 成都恩普生医疗科技有限公司 | 一种基于led冷光源的酶标仪光路检测系统 |
| ES2875831T3 (es) | 2009-01-08 | 2021-11-11 | It Is Int Ltd | Sistema óptico para reacciones químicas y/o bioquímicas |
| US8702942B2 (en) * | 2010-12-17 | 2014-04-22 | Malvern Instruments, Ltd. | Laser doppler electrophoresis using a diffusion barrier |
| JP5883233B2 (ja) * | 2011-03-16 | 2016-03-09 | 英光 古川 | 走査型顕微光散乱測定解析装置および光散乱解析方法 |
| ES2886583T3 (es) * | 2011-05-26 | 2021-12-20 | Massachusetts Gen Hospital | Sistema de tromboelastografía óptica y método para la evaluación de las métricas de coagulación sanguínea |
| DE102011085599B3 (de) | 2011-11-02 | 2012-12-13 | Polytec Gmbh | Vorrichtung und Verfahren zur interferometrischen Vermessung eines Objekts |
| US9411146B2 (en) * | 2011-11-02 | 2016-08-09 | Hamamatsu Photonics K.K. | Observation device |
| DE102012016122A1 (de) * | 2012-08-15 | 2014-02-20 | Westfälische Wilhelms-Universität Münster | Verfahren und Vorrichtung zur Erfassung wenigsten eines Tieres |
| JP6076111B2 (ja) * | 2013-02-07 | 2017-02-08 | 浜松ホトニクス株式会社 | 塊状細胞評価方法および塊状細胞評価装置 |
| DE102013211885A1 (de) | 2013-06-24 | 2014-12-24 | Siemens Aktiengesellschaft | Partikeldetektor und Verfahren zur Detektion von Partikeln |
| DE202014004218U1 (de) * | 2014-05-19 | 2014-05-28 | Particle Metrix Gmbh | Vorrichtung der Partikel Tracking Analyse mit Hilfe von Streulicht (PTA) und zur Erfassung und Charakterisierung von Partikeln in Flüssigkeiten aller Art in der Größenordnung von Nanometern |
-
2015
- 2015-03-06 DE DE102015003019.1A patent/DE102015003019A1/de active Pending
-
2016
- 2016-03-02 US US15/555,773 patent/US10488400B2/en active Active
- 2016-03-02 CN CN201680014161.3A patent/CN107430064B/zh active Active
- 2016-03-02 ES ES16708082T patent/ES2961352T3/es active Active
- 2016-03-02 KR KR1020177027379A patent/KR20170140182A/ko unknown
- 2016-03-02 JP JP2017564793A patent/JP6851328B2/ja active Active
- 2016-03-02 EP EP23182756.9A patent/EP4242637A3/fr active Pending
- 2016-03-02 EP EP16708082.9A patent/EP3265779B1/fr active Active
- 2016-03-02 WO PCT/EP2016/000377 patent/WO2016142043A1/fr active Application Filing
Non-Patent Citations (3)
| Title |
|---|
| KONG FANTING ET AL: "Focusing of light through scattering media", PHOTONS PLUS ULTRASOUND: IMAGING AND SENSING 2011, SPIE, 1000 20TH ST. BELLINGHAM WA 98225-6705 USA, vol. 7899, no. 1, 10 February 2011 (2011-02-10), pages 1 - 6, XP060007440, DOI: 10.1117/12.873836 * |
| LODAHL P ET AL: "Transport of quantum noise through random media", ARXIV.ORG, CORNELL UNIVERSITY LIBRARY, 201 OLIN LIBRARY CORNELL UNIVERSITY ITHACA, NY 14853, 14 October 2004 (2004-10-14), XP080171782, DOI: 10.1103/PHYSREVLETT.94.153905 * |
| TUCHIN V V: "TISSUE OPTICS: TOMOGRAPHY AND TOPOGRAPHY", PROCEEDINGS OF SPIE, IEEE, US, vol. 3726, 6 October 1998 (1998-10-06), pages 168 - 198, XP008003055, ISBN: 978-1-62841-730-2, DOI: 10.1117/12.341389 * |
Also Published As
| Publication number | Publication date |
|---|---|
| EP4242637A2 (fr) | 2023-09-13 |
| WO2016142043A1 (fr) | 2016-09-15 |
| CN107430064A (zh) | 2017-12-01 |
| DE102015003019A1 (de) | 2016-09-08 |
| ES2961352T3 (es) | 2024-03-11 |
| EP3265779A1 (fr) | 2018-01-10 |
| EP4242637A3 (fr) | 2023-10-04 |
| JP2018515787A (ja) | 2018-06-14 |
| US20180038845A1 (en) | 2018-02-08 |
| EP3265779C0 (fr) | 2023-08-09 |
| JP6851328B2 (ja) | 2021-03-31 |
| US10488400B2 (en) | 2019-11-26 |
| CN107430064B (zh) | 2020-10-20 |
| KR20170140182A (ko) | 2017-12-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3265779B1 (fr) | Procédé de détection optique d'un mouvement dans un échantillon biologique à expansion spatiale | |
| DE69828345T2 (de) | Kreuzkorrelationsverfahren und Vorrichtung zur Unterdrückung der Effekte von Mehrfachstreuung | |
| DE19725211C1 (de) | Faserdetektor zur Detektion des Streulichtes oder des Fluoreszenzlichtes einer flüssigen Suspension | |
| DE102010005962B4 (de) | Verfahren zur Bestimmung der statischen und/oder dynamischen Lichtstreuung | |
| DE102009043001A1 (de) | Verfahren zur Bestimmung von Defekten in einem Für elektromagnetische Wellen transparenten Material, insbesonders für optische Zwecke, eine Vorrichtung hierzusowie die Verwendung dieser Materialien | |
| DE2605721A1 (de) | Verfahren und vorrichtung zum automatischen ueberpruefen von eiern auf risse oder bruchstellen in ihrer schale | |
| EP1678547B1 (fr) | Dispositif et procede de mesure des proprietes optiques d'un objet | |
| DE102009036383B3 (de) | Vorrichtung und Verfahren zur winkelaufgelösten Streulichtmessung | |
| EP3374755B1 (fr) | Microscope optique et procédé de détermination d'un indice de réfraction, dépendant de la longeur d'onde, d'un milieu échantillon | |
| DE112020005437T5 (de) | Optisches detektionssystem, detektionsvorrichtung, durchflusszytometer undbildgebungszytometer | |
| EP2255174A1 (fr) | Dispositif et procédé pour déterminer un indice de réfraction d un objet mesuré | |
| EP3368884B1 (fr) | Procédé et dispositif de détection optique d'un mouvement dans un échantillon biologique | |
| EP0695432A1 (fr) | Microscope laser a lumiere diffusee | |
| DE102009013147A1 (de) | Spektroskopie heterogener Proben | |
| DE102019119147A1 (de) | Mikroskop und verfahren zur mikroskopie | |
| DE19939574B4 (de) | Verfahren zur dreidimensionalen Objektabtastung | |
| EP2871463A1 (fr) | Procédé et dispositif destinés à l'examen d'un ou plusieurs objets de phase | |
| DE102005001504B4 (de) | Vorrichtung zur Bestimmung von Eigenschaften von dispersen Bestandteilen in Fluiden | |
| DD265224A1 (de) | Verfahren und anordnung fuer die lichtrastermikroskopie | |
| DE102021102634A1 (de) | Vorrichtung und Verfahren zur photometrischen Massenbestimmung | |
| DE29710396U1 (de) | Faserdetektor und Meßgerät zur Analyse des Streulichtes oder des Fluoreszenzlichtes an flüssigen Suspensionen | |
| DE3233055C2 (de) | Durchflußvorrichtung zur Untersuchung einzelner, in einer Flüssigkeit suspendierter Teilchen | |
| DE102018209448A1 (de) | Vorrichtung zur ortsaufgelösten Detektion und Verwendung einer solchen Vorrichtung | |
| DE102016013095A1 (de) | Hämatologische Diagnose suspendierter Erythrozyten mit Hilfe des rollenden Bewegungsmodus in laminarer Strömung | |
| DE102004031197A1 (de) | Biodetektor |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20170904 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20190515 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| RAP3 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: UNIVERSITAET DES SAARLANDES Owner name: FRAUNHOFER-GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG E.V. |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20230412 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 502016016001 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: GERMAN |
|
| U01 | Request for unitary effect filed |
Effective date: 20230821 |
|
| U07 | Unitary effect registered |
Designated state(s): AT BE BG DE DK EE FI FR IT LT LU LV MT NL PT SE SI Effective date: 20230824 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231110 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231209 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230809 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231109 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231209 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230809 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231110 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230809 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2961352 Country of ref document: ES Kind code of ref document: T3 Effective date: 20240311 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230809 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230809 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230809 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230809 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20240322 Year of fee payment: 9 |
|
| U20 | Renewal fee paid [unitary effect] |
Year of fee payment: 9 Effective date: 20240325 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 502016016001 Country of ref document: DE |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20240513 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20240401 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20240417 Year of fee payment: 9 |