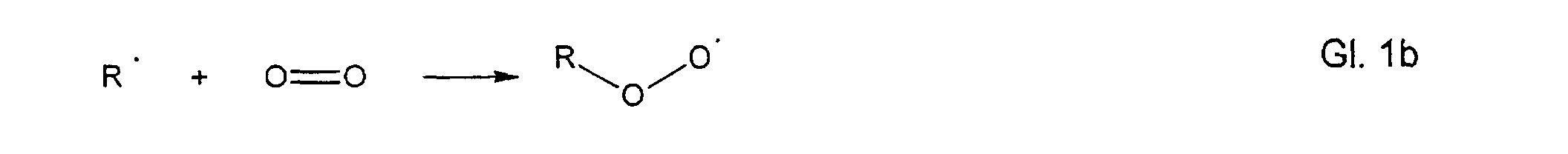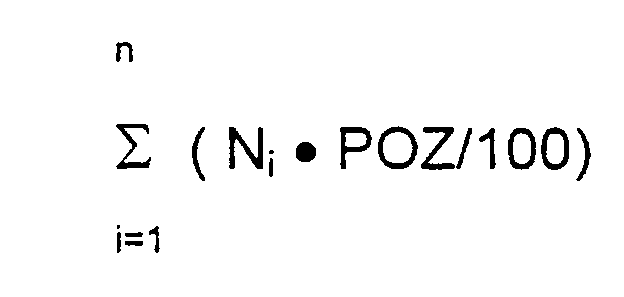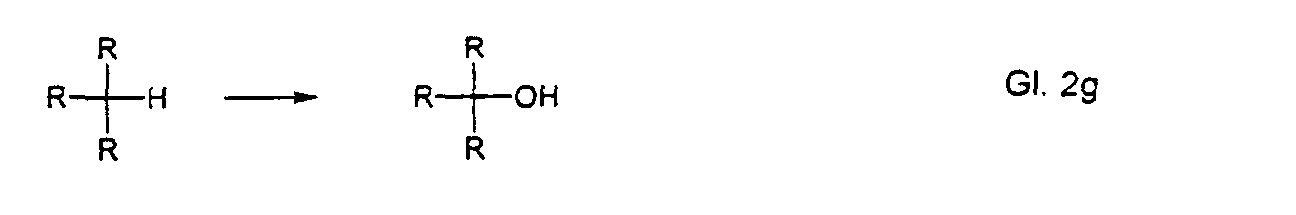EP1233763B1 - Transdermale therapeutische systeme mit verbesserter stabilität und ein verfahren zu ihrer herstellung - Google Patents
Transdermale therapeutische systeme mit verbesserter stabilität und ein verfahren zu ihrer herstellung Download PDFInfo
- Publication number
- EP1233763B1 EP1233763B1 EP00985090A EP00985090A EP1233763B1 EP 1233763 B1 EP1233763 B1 EP 1233763B1 EP 00985090 A EP00985090 A EP 00985090A EP 00985090 A EP00985090 A EP 00985090A EP 1233763 B1 EP1233763 B1 EP 1233763B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- transdermal therapeutic
- therapeutic system
- active substance
- derivatives
- formulation constituents
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 230000001225 therapeutic effect Effects 0.000 title claims description 16
- 238000000034 method Methods 0.000 title claims description 8
- 238000004519 manufacturing process Methods 0.000 title description 5
- 150000002978 peroxides Chemical class 0.000 claims description 39
- 239000011159 matrix material Substances 0.000 claims description 31
- 150000002432 hydroperoxides Chemical class 0.000 claims description 17
- 239000000203 mixture Substances 0.000 claims description 16
- 239000013543 active substance Substances 0.000 claims description 15
- 238000009472 formulation Methods 0.000 claims description 15
- 239000000470 constituent Substances 0.000 claims description 13
- 239000000243 solution Substances 0.000 claims description 13
- RSWGJHLUYNHPMX-UHFFFAOYSA-N Abietic-Saeure Natural products C12CCC(C(C)C)=CC2=CCC2C1(C)CCCC2(C)C(O)=O RSWGJHLUYNHPMX-UHFFFAOYSA-N 0.000 claims description 11
- KHPCPRHQVVSZAH-HUOMCSJISA-N Rosin Natural products O(C/C=C/c1ccccc1)[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 KHPCPRHQVVSZAH-HUOMCSJISA-N 0.000 claims description 11
- DWAQJAXMDSEUJJ-UHFFFAOYSA-M Sodium bisulfite Chemical compound [Na+].OS([O-])=O DWAQJAXMDSEUJJ-UHFFFAOYSA-M 0.000 claims description 11
- 229910052760 oxygen Inorganic materials 0.000 claims description 11
- 239000001301 oxygen Substances 0.000 claims description 11
- 235000010267 sodium hydrogen sulphite Nutrition 0.000 claims description 11
- 239000002904 solvent Substances 0.000 claims description 11
- KHPCPRHQVVSZAH-UHFFFAOYSA-N trans-cinnamyl beta-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OCC=CC1=CC=CC=C1 KHPCPRHQVVSZAH-UHFFFAOYSA-N 0.000 claims description 11
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 10
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 claims description 9
- 239000007857 degradation product Substances 0.000 claims description 8
- 239000007864 aqueous solution Substances 0.000 claims description 6
- 230000015572 biosynthetic process Effects 0.000 claims description 6
- 238000003860 storage Methods 0.000 claims description 6
- 238000010525 oxidative degradation reaction Methods 0.000 claims description 5
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 claims description 4
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 claims description 4
- 239000013032 Hydrocarbon resin Substances 0.000 claims description 4
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 claims description 4
- 239000007795 chemical reaction product Substances 0.000 claims description 4
- 239000003623 enhancer Substances 0.000 claims description 4
- 229920006270 hydrocarbon resin Polymers 0.000 claims description 4
- 230000008569 process Effects 0.000 claims description 4
- 229920005989 resin Polymers 0.000 claims description 4
- 239000011347 resin Substances 0.000 claims description 4
- 150000003505 terpenes Chemical class 0.000 claims description 4
- 235000007586 terpenes Nutrition 0.000 claims description 4
- 150000003097 polyterpenes Chemical class 0.000 claims description 3
- 229920000036 polyvinylpyrrolidone Polymers 0.000 claims description 3
- 239000001267 polyvinylpyrrolidone Substances 0.000 claims description 3
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 claims description 3
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 claims description 2
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 claims description 2
- 239000005977 Ethylene Substances 0.000 claims description 2
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 claims description 2
- XTXRWKRVRITETP-UHFFFAOYSA-N Vinyl acetate Chemical compound CC(=O)OC=C XTXRWKRVRITETP-UHFFFAOYSA-N 0.000 claims description 2
- 150000001252 acrylic acid derivatives Chemical class 0.000 claims description 2
- 150000001253 acrylic acids Chemical class 0.000 claims description 2
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 claims description 2
- 229920001577 copolymer Polymers 0.000 claims description 2
- 238000002425 crystallisation Methods 0.000 claims description 2
- 230000008025 crystallization Effects 0.000 claims description 2
- 229940079826 hydrogen sulfite Drugs 0.000 claims description 2
- 239000003112 inhibitor Substances 0.000 claims description 2
- 239000010410 layer Substances 0.000 claims description 2
- 125000000467 secondary amino group Chemical group [H]N([*:1])[*:2] 0.000 claims description 2
- 229940079827 sodium hydrogen sulfite Drugs 0.000 claims description 2
- 239000007787 solid Substances 0.000 claims description 2
- 125000001302 tertiary amino group Chemical group 0.000 claims description 2
- 235000021122 unsaturated fatty acids Nutrition 0.000 claims description 2
- 150000004670 unsaturated fatty acids Chemical class 0.000 claims description 2
- GEHJYWRUCIMESM-UHFFFAOYSA-L sodium sulfite Chemical compound [Na+].[Na+].[O-]S([O-])=O GEHJYWRUCIMESM-UHFFFAOYSA-L 0.000 claims 2
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 claims 1
- 239000000853 adhesive Substances 0.000 claims 1
- 229910052799 carbon Inorganic materials 0.000 claims 1
- 125000004432 carbon atom Chemical group C* 0.000 claims 1
- 150000002191 fatty alcohols Chemical class 0.000 claims 1
- 239000012669 liquid formulation Substances 0.000 claims 1
- 239000002356 single layer Substances 0.000 claims 1
- 235000010265 sodium sulphite Nutrition 0.000 claims 1
- 239000011877 solvent mixture Substances 0.000 claims 1
- 125000000101 thioether group Chemical group 0.000 claims 1
- 238000006243 chemical reaction Methods 0.000 description 25
- 239000004480 active ingredient Substances 0.000 description 19
- 238000007254 oxidation reaction Methods 0.000 description 11
- 229940079593 drug Drugs 0.000 description 10
- 239000003814 drug Substances 0.000 description 10
- 230000003647 oxidation Effects 0.000 description 10
- -1 peroxy radicals Chemical class 0.000 description 10
- 239000000126 substance Substances 0.000 description 10
- VOXZDWNPVJITMN-ZBRFXRBCSA-N 17β-estradiol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 VOXZDWNPVJITMN-ZBRFXRBCSA-N 0.000 description 8
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 8
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 8
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 8
- CIHOLLKRGTVIJN-UHFFFAOYSA-N tert‐butyl hydroperoxide Chemical compound CC(C)(C)OO CIHOLLKRGTVIJN-UHFFFAOYSA-N 0.000 description 7
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 229920000058 polyacrylate Polymers 0.000 description 6
- 150000003254 radicals Chemical class 0.000 description 6
- 239000002994 raw material Substances 0.000 description 6
- 239000003963 antioxidant agent Substances 0.000 description 5
- 229960005309 estradiol Drugs 0.000 description 5
- 150000002314 glycerols Chemical class 0.000 description 5
- 239000000546 pharmaceutical excipient Substances 0.000 description 5
- 229920000642 polymer Polymers 0.000 description 5
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 4
- 238000006731 degradation reaction Methods 0.000 description 4
- 238000002845 discoloration Methods 0.000 description 4
- 235000011187 glycerol Nutrition 0.000 description 4
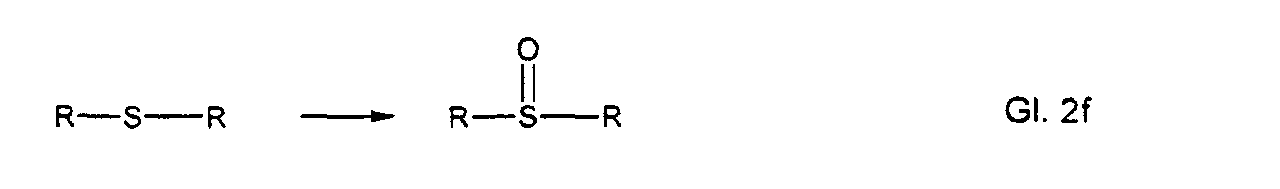
- 150000003462 sulfoxides Chemical class 0.000 description 4
- GUNUOUOMYPMVSK-UHFFFAOYSA-N 7,8-dihydronaphthalen-1-ol Chemical compound C1=CCCC2=C1C=CC=C2O GUNUOUOMYPMVSK-UHFFFAOYSA-N 0.000 description 3
- 150000001204 N-oxides Chemical class 0.000 description 3
- 229920002367 Polyisobutene Polymers 0.000 description 3
- 230000015556 catabolic process Effects 0.000 description 3
- 238000000354 decomposition reaction Methods 0.000 description 3
- 125000004185 ester group Chemical group 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 125000001117 oleyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])/C([H])=C([H])\C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- LSNNMFCWUKXFEE-UHFFFAOYSA-L sulfite Chemical compound [O-]S([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-L 0.000 description 3
- UUOJIACWOAYWEZ-UHFFFAOYSA-N 1-(tert-butylamino)-3-[(2-methyl-1H-indol-4-yl)oxy]propan-2-yl benzoate Chemical compound C1=CC=C2NC(C)=CC2=C1OCC(CNC(C)(C)C)OC(=O)C1=CC=CC=C1 UUOJIACWOAYWEZ-UHFFFAOYSA-N 0.000 description 2
- HBKBEZURJSNABK-MWJPAGEPSA-N 2,3-dihydroxypropyl (1r,4ar,4br,10ar)-1,4a-dimethyl-7-propan-2-yl-2,3,4,4b,5,6,10,10a-octahydrophenanthrene-1-carboxylate Chemical class C([C@@H]12)CC(C(C)C)=CC1=CC[C@@H]1[C@]2(C)CCC[C@@]1(C)C(=O)OCC(O)CO HBKBEZURJSNABK-MWJPAGEPSA-N 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 229940123973 Oxygen scavenger Drugs 0.000 description 2
- UCKMPCXJQFINFW-UHFFFAOYSA-N Sulphide Chemical compound [S-2] UCKMPCXJQFINFW-UHFFFAOYSA-N 0.000 description 2
- 239000012790 adhesive layer Substances 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 238000006701 autoxidation reaction Methods 0.000 description 2
- 125000001743 benzylic group Chemical group 0.000 description 2
- 229960001035 bopindolol Drugs 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- XXJWXESWEXIICW-UHFFFAOYSA-N diethylene glycol monoethyl ether Chemical compound CCOCCOCCO XXJWXESWEXIICW-UHFFFAOYSA-N 0.000 description 2
- 229940075557 diethylene glycol monoethyl ether Drugs 0.000 description 2
- 125000004925 dihydropyridyl group Chemical group N1(CC=CC=C1)* 0.000 description 2
- 150000002148 esters Chemical class 0.000 description 2
- 229930182833 estradiol Natural products 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- 238000004128 high performance liquid chromatography Methods 0.000 description 2
- XMGQYMWWDOXHJM-UHFFFAOYSA-N limonene Chemical compound CC(=C)C1CCC(C)=CC1 XMGQYMWWDOXHJM-UHFFFAOYSA-N 0.000 description 2
- 235000001510 limonene Nutrition 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 229960001597 nifedipine Drugs 0.000 description 2
- HYIMSNHJOBLJNT-UHFFFAOYSA-N nifedipine Chemical compound COC(=O)C1=C(C)NC(C)=C(C(=O)OC)C1C1=CC=CC=C1[N+]([O-])=O HYIMSNHJOBLJNT-UHFFFAOYSA-N 0.000 description 2
- 239000003961 penetration enhancing agent Substances 0.000 description 2
- YYPWGCZOLGTTER-MZMPZRCHSA-N pergolide Chemical compound C1=CC=C2[C@H]3C[C@@H](CSC)CN(CCC)[C@@H]3CC3=CN=C1[C]32 YYPWGCZOLGTTER-MZMPZRCHSA-N 0.000 description 2
- 229960004851 pergolide Drugs 0.000 description 2
- 239000011505 plaster Substances 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 239000002516 radical scavenger Substances 0.000 description 2
- 239000011541 reaction mixture Substances 0.000 description 2
- 238000010992 reflux Methods 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 229940100640 transdermal system Drugs 0.000 description 2
- WTARULDDTDQWMU-RKDXNWHRSA-N (+)-β-pinene Chemical compound C1[C@H]2C(C)(C)[C@@H]1CCC2=C WTARULDDTDQWMU-RKDXNWHRSA-N 0.000 description 1
- ALSTYHKOOCGGFT-KTKRTIGZSA-N (9Z)-octadecen-1-ol Chemical compound CCCCCCCC\C=C/CCCCCCCCO ALSTYHKOOCGGFT-KTKRTIGZSA-N 0.000 description 1
- JAAJQSRLGAYGKZ-UHFFFAOYSA-N 1,2,3,4-tetrahydronaphthalen-1-ol Chemical class C1=CC=C2C(O)CCCC2=C1 JAAJQSRLGAYGKZ-UHFFFAOYSA-N 0.000 description 1
- YNGDWRXWKFWCJY-UHFFFAOYSA-N 1,4-Dihydropyridine Chemical compound C1C=CNC=C1 YNGDWRXWKFWCJY-UHFFFAOYSA-N 0.000 description 1
- AXTGDCSMTYGJND-UHFFFAOYSA-N 1-dodecylazepan-2-one Chemical compound CCCCCCCCCCCCN1CCCCCC1=O AXTGDCSMTYGJND-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 229940127291 Calcium channel antagonist Drugs 0.000 description 1
- 238000006728 Cope elimination reaction Methods 0.000 description 1
- AVXURJPOCDRRFD-UHFFFAOYSA-N Hydroxylamine Chemical compound ON AVXURJPOCDRRFD-UHFFFAOYSA-N 0.000 description 1
- QAQJMLQRFWZOBN-LAUBAEHRSA-N L-ascorbyl-6-palmitate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](O)[C@H]1OC(=O)C(O)=C1O QAQJMLQRFWZOBN-LAUBAEHRSA-N 0.000 description 1
- 239000011786 L-ascorbyl-6-palmitate Substances 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- OUUQCZGPVNCOIJ-UHFFFAOYSA-M Superoxide Chemical compound [O-][O] OUUQCZGPVNCOIJ-UHFFFAOYSA-M 0.000 description 1
- 229960000583 acetic acid Drugs 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000001476 alcoholic effect Effects 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 238000006136 alcoholysis reaction Methods 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 238000005899 aromatization reaction Methods 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 235000010385 ascorbyl palmitate Nutrition 0.000 description 1
- 239000012752 auxiliary agent Substances 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 230000001010 compromised effect Effects 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- GVJHHUAWPYXKBD-UHFFFAOYSA-N d-alpha-tocopherol Natural products OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000013538 functional additive Substances 0.000 description 1
- 239000012362 glacial acetic acid Substances 0.000 description 1
- 229910001385 heavy metal Inorganic materials 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 230000033444 hydroxylation Effects 0.000 description 1
- 238000005805 hydroxylation reaction Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 229940087305 limonene Drugs 0.000 description 1
- 150000002628 limonene derivativess Chemical class 0.000 description 1
- 150000004668 long chain fatty acids Chemical class 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 239000012299 nitrogen atmosphere Substances 0.000 description 1
- 229940055577 oleyl alcohol Drugs 0.000 description 1
- XMLQWXUVTXCDDL-UHFFFAOYSA-N oleyl alcohol Natural products CCCCCCC=CCCCCCCCCCCO XMLQWXUVTXCDDL-UHFFFAOYSA-N 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 150000002926 oxygen Chemical class 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 238000005375 photometry Methods 0.000 description 1
- 239000004014 plasticizer Substances 0.000 description 1
- 229920000191 poly(N-vinyl pyrrolidone) Polymers 0.000 description 1
- 239000004584 polyacrylic acid Substances 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920000193 polymethacrylate Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- ULWHHBHJGPPBCO-UHFFFAOYSA-N propane-1,1-diol Chemical compound CCC(O)O ULWHHBHJGPPBCO-UHFFFAOYSA-N 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 238000004062 sedimentation Methods 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- WBHQBSYUUJJSRZ-UHFFFAOYSA-M sodium bisulfate Chemical compound [Na+].OS([O-])(=O)=O WBHQBSYUUJJSRZ-UHFFFAOYSA-M 0.000 description 1
- 229910000342 sodium bisulfate Inorganic materials 0.000 description 1
- 239000004289 sodium hydrogen sulphite Substances 0.000 description 1
- AKHNMLFCWUSKQB-UHFFFAOYSA-L sodium thiosulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=S AKHNMLFCWUSKQB-UHFFFAOYSA-L 0.000 description 1
- 235000019345 sodium thiosulphate Nutrition 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 125000003375 sulfoxide group Chemical group 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 239000012085 test solution Substances 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 235000010384 tocopherol Nutrition 0.000 description 1
- 229960001295 tocopherol Drugs 0.000 description 1
- 229930003799 tocopherol Natural products 0.000 description 1
- 239000011732 tocopherol Substances 0.000 description 1
- GVJHHUAWPYXKBD-IEOSBIPESA-N α-tocopherol Chemical compound OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-IEOSBIPESA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/70—Web, sheet or filament bases ; Films; Fibres of the matrix type containing drug
- A61K9/7023—Transdermal patches and similar drug-containing composite devices, e.g. cataplasms
- A61K9/703—Transdermal patches and similar drug-containing composite devices, e.g. cataplasms characterised by shape or structure; Details concerning release liner or backing; Refillable patches; User-activated patches
- A61K9/7038—Transdermal patches of the drug-in-adhesive type, i.e. comprising drug in the skin-adhesive layer
- A61K9/7046—Transdermal patches of the drug-in-adhesive type, i.e. comprising drug in the skin-adhesive layer the adhesive comprising macromolecular compounds
- A61K9/7053—Transdermal patches of the drug-in-adhesive type, i.e. comprising drug in the skin-adhesive layer the adhesive comprising macromolecular compounds obtained by reactions only involving carbon to carbon unsaturated bonds, e.g. polyvinyl, polyisobutylene, polystyrene
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/70—Web, sheet or filament bases ; Films; Fibres of the matrix type containing drug
- A61K9/7023—Transdermal patches and similar drug-containing composite devices, e.g. cataplasms
- A61K9/703—Transdermal patches and similar drug-containing composite devices, e.g. cataplasms characterised by shape or structure; Details concerning release liner or backing; Refillable patches; User-activated patches
- A61K9/7038—Transdermal patches of the drug-in-adhesive type, i.e. comprising drug in the skin-adhesive layer
- A61K9/7046—Transdermal patches of the drug-in-adhesive type, i.e. comprising drug in the skin-adhesive layer the adhesive comprising macromolecular compounds
- A61K9/7053—Transdermal patches of the drug-in-adhesive type, i.e. comprising drug in the skin-adhesive layer the adhesive comprising macromolecular compounds obtained by reactions only involving carbon to carbon unsaturated bonds, e.g. polyvinyl, polyisobutylene, polystyrene
- A61K9/7061—Polyacrylates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
Definitions
- Transdermal Therapeutic Systems can be used without neglecting a distinction is made between less common special forms into two basic groups, the so-called matrix systems and the so-called reservoir systems.
- matrix systems in the simplest case, the active ingredient in one self-adhesive layer dissolved or partly only in the form of crystals suspended.
- Reservoir systems provide a sort of bag from an inert backing layer and a drug-permeable membrane, wherein the active ingredient in a liquid preparation is located in this bag.
- the membrane is with a Adhesive layer, which serves to anchor the system on the skin.
- active oxygen is of course the oxygen of the air.
- An effective means to protect the active substance contained in the TTS against this oxygen is to pack the TTS under a nitrogen atmosphere and / or to additionally add antioxidants into the pack.
- hydroperoxides can be formed according to the autoxidation reactions described in the literature according to the following mechanism: R-H ⁇ R ⁇ + H ⁇
- the so-called induction phase free radicals are formed by the action of heat, light with the assistance of heavy metal traces with loss of a hydrogen atom.
- the so-called propagation phase these radicals react with oxygen to form peroxy radicals.
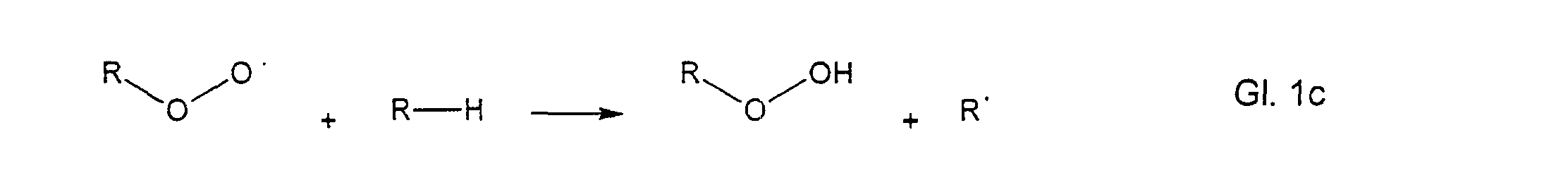
- These peroxy radicals now attack other molecules to form hydroperoxides and a new free radical.
- a chain reaction has been used which continues until this chain breaks off by the reaction of two radicals, as shown in the equation below, for example.
- antioxidants used to protect oxidation-sensitive drugs or Stabilizers can intervene in this reaction chain.
- Antioxidants can a distinction is made in so-called radical scavengers and oxygen scavengers.
- radical scavengers such as. Tocopherol and its derivatives remove or inactivate radicals and thereby interrupt the chain mechanism of autoxidation.
- Oxygen scavengers such as. Ascorbyl palmitate, react directly with the oxidative agent and prevent so the start of the chain reaction.
- antioxidants / stabilizers only makes sense if not already contain the starting materials oxidatively acting hydroperoxides and the Dosage form is protected by the packaging from the ingress of oxygen.
- peroxide number POZ indicates the amount of milliequivalents of active oxygen per kg of substance.
- peroxide number POZ indicates the amount of milliequivalents of active oxygen per kg of substance.
- raw material function POZ Hydrocarbon resin Matrix component 180 Kollidon Matrix component 110 partially hydrogenated glycerol ester of rosin tackifiers 190 hydrogenated glycerol ester of rosin tackifiers 80 Poly- ⁇ -pinene tackifiers 150 Dieethylenglykolmonoethylether Solvent / permeation enhancer 120 oleyl Solvent / permeation enhancer 50 limonene permeation 15
- the object underlying the present invention is a transdermal therapeutic system (TTS), in which the formation of oxidative degradation products of the oxidation sensitive in this TTS containing Active ingredients during storage of the TTS is reduced.
- TTS transdermal therapeutic system
- the solution of this Task is, as already stated above, therein, in the production of a TTS containing one or more oxidation-sensitive agents, only to use such formulation ingredients that are largely free of Hydroperoxides are.
- these are such formulation constituents, which together in the proportions given by the recipe for the TTS one Peroxide number (POZ) of not more than 20, preferably of not more than 10, particularly preferably have a maximum of 5.
- POZ Peroxide number
- formulation constituents is to be understood as meaning all substances of the TTS, apart from the pharmaceutical active substance (s), in which the active ingredient (s) are contained. These include: As building blocks of the single- or multi-layered matrix or the reservoir system: z.
- Hydrocarbons such as polyethylenes, polypropylenes, polyacrylates, polymethacrylates, polyurethanes, polyisobutylenes, polyvinylpyrrolidone; Hydrocarbon resins; silicones; Rubber; Copolymers of vinylpyrrolidone with acrylic acids, acrylic acid derivatives, ethylene and / or vinyl acetate; Resins based on rosin derivatives and / or polyterpenes.
- terpenes or terpene derivatives unsaturated fatty acids or their derivatives, esters of long-chain fatty acids, diethylene glycol or its derivatives; Alkylmethyl sulfoxides, azone and limonenes; Crystallization inhibitors such.
- polyvinylpyrrolidone Polyacrylic acid or cellulose derivatives; Solvents such.
- polyethylene glycol diethylene glycol and / or its derivatives, propanediol or oleyl alcohol.
- the solution of hydrogen sulfite is sufficiently concentrated, the small amount of water introduced can usually be tolerated without any problems. This is especially true if, during coating and drying, the water is removed along with other solvents. Liquid auxiliaries can also be reacted with an aqueous solution of sodium hydrogen sulfite without additional solvent.
- the limit of 10 is shown by the following example calculation on a typical transdermal therapeutic matrix system having a size of 20 cm 2 and a coating weight of 100 g / m 2 and an active ingredient concentration of 10% g / g.
- the system accordingly contains 20 mg or 0.1 mmol of active ingredient and at a peroxide value of 10 a total of 0.2 ⁇ 10 -2 mmol of active oxygen. This means that a maximum of 2% of the drug in the system can be oxidized. Considering that this reaction takes time and slows down in the consumption of active oxygen, with a peroxide value of 10, and possibly an upper limit of 20, there is a good chance that the system will be sufficiently stable over 2 years ,
- the peroxide load is further lowered (preferably to a POZ of 5 or below); by a Treatment of the peroxide-loaded auxiliaries with sulfites according to the described Process or by the choice of excipients that are not used to form peroxides tend.
- Matrix 2a Peroxide Number 38 Styrene / polyisobutylene / styrene block polymer 16% oleyl 10% Hydrocarbon resin 22% Glycerol esters of partially hydrogenated rosin 22% polyisobutylene 7% Paraffin, liquid 3% N-0923 base 20%
- Matrix 2b Peroxide Number 2.6 polyacrylate 60% oleyl 10% N-0923 base 30% Active ingredient content after 3 months based on the initial content of 100% Matrix 2a Matrix 2b 25 ° C 85% 99.5% 40 ° C 44% 89.9%
- Matrix 3a Peroxide Number 35 polyacrylate 16% glycerin 10% Glycerol esters of partially hydrogenated rosin 22% estradiol 20%
- Matrix 3b peroxide number 2 - 3, corresponds to matrix 3a, but use of treated with Na-bisulfite solution glycerol ester of partially hydrogenated rosin
- Matrix Composition bopindolol 15% polyacrylate 65% Glycerol esters of partially hydrogenated rosin 20%
- Matrix 4a prepared with glycerol ester of partially hydrogenated rosin with a POZ of 160
- Matrix 4b prepared with glycerol ester of partially hydrogenated rosin treated with nabisulfite solution Matrix 4a Matrix 4b 30 days at 40 ° C brown discoloration without discoloration
- Reservoir 5a prepared with diethylene glycol monoethyl ether with a POZ of 150,
- Reservoir 5b prepared with diethylene glycol monoethyl ether treated with sodium bisulfite solution 30 days Reservoir 5a Abbauprod. I Reservoir5b Abbauprod. I 25 ° C 1.6% not quantifiable 40 ° C 4.5% 0.3%
- Degradation product II was due to the low concentration in not found in the systems.
- Matrix 6a POZ: approx. 32 pergolide 10% polyacrylate 70% Glycerol esters of partially hydrogenated rosin 20% Matrix 6b, POZ: ca 2-3 pergolide 10% polyacrylate 90% 30 days Matrix 6a sulfoxide Matrix 6b sulfoxide 25 ° C 0.8% not quantifiable 40 ° C 4.2% not quantifiable
- the raw material to be treated is dissolved in a water-miscible solvent, preferably in methanol or ethanol, dissolved and stirred with about 10-30 percent solution of sodium bisulphite (sodium bisulfite).
- sodium bisulphite sodium bisulfite
- the amount Sodium bisulfite solution is such that stoichiometrically all or one sufficient degree all peroxides are destroyed.
- the precipitated reaction product of sodium bisulfite, Sodium hydrogen sulfate may, if desired or necessary, by centrifugation or sedimentation or filtration are separated.
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Dermatology (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Neurosurgery (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Psychology (AREA)
- Endocrinology (AREA)
- Reproductive Health (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Materials For Medical Uses (AREA)
Description
Solch aktiver Sauerstoff ist natürlich der Sauerstoff der Luft. Ein wirksames Mittel, den im TTS enthaltenen Wirkstoff gegen diesen Sauerstoff zu schützen ist es, das TTS unter einer Stickstoffatmosphäre zu verpacken und/oder Antioxidantien zusätzlich in die Packung hineinzugeben.
Im zweiten Schritt, der sogenannten Propagationsphase, reagieren diese Radikale mit Sauerstoff unter Bildung von Peroxyradikalen.
Diese Peroxyradikale greifen nun weitere Moleküle an unter Bildung von Hydroperoxiden und einem neuen freien Radikal. Damit hat eine Kettenreaktion eingesetzt, die solange weiterläuft bis diese Kette durch die Reaktion von zwei Radikalen miteinander, wie z.B. in untenstehender Gleichung dargestellt, abbricht.
Besonders leicht durchzuführen ist ein semiquantitativer Test auf Peroxide mit käuflichen Teststäbchen.
| Rohstoff | Funktion | POZ |
| Kohlenwasserstoffharz | Matrixbestandteil | 180 |
| Kollidon | Matrixbestandteil | 110 |
| partiell hydrierter Glycerolester von Kolophonium | Klebrigmacher | 190 |
| hydrierter Glycerolester von Kolophonium | Klebrigmacher | 80 |
| Poly-β-pinen | Klebrigmacher | 150 |
| Dieethylenglykolmonoethylether | Lösemittel/ Permeationsenhancer | 120 |
| Oleylalkohol | Lösemittel/ Permeationsenhancer | 50 |
| Limonen | Permeationsenhancer | 15 |
Einfacher ist es, die Peroxidbelastung der Einzelstoffe zu messen und die Peroxidzahl des wirkstoffhaltigen Teils der Pflaster nach folgender Formel zu berechnen:
- n :
- Anzahl der Formulierungsbestandteile des wirkstoffhaltigen Teils des Systems
- N :
- prozentualer Gehalt der Formulierungsbestandteile in den wirkstoffhaltigen Bestandteilen des Systems (numerischer Wert)
- POZ :
- Peroxidzahl der Einzelbestandteile des wirkstoffhaltigen Teils des Systems
Experimentell wurde gefunden, daß die in den Rohstoffen enthaltenen
Hydroperoxide auf vielfältige Art und Weise mit dem mit ihnen in Kontakt
kommenden Wirkstoff reagieren können. Als besonders empfindlich haben sich
dabei Wirkstoffe gezeigt, die über eine der folgenden Teilstrukturen verfügen:
- sekundäre oder tertiäre Aminogruppen
- C-C- Doppelbindungen
- C-H-Gruppen in Allylstellung
- benzylische C-H-Gruppen
- tertiäre C-H-Gruppen
- Sulfid oder Sulfoxidgruppen
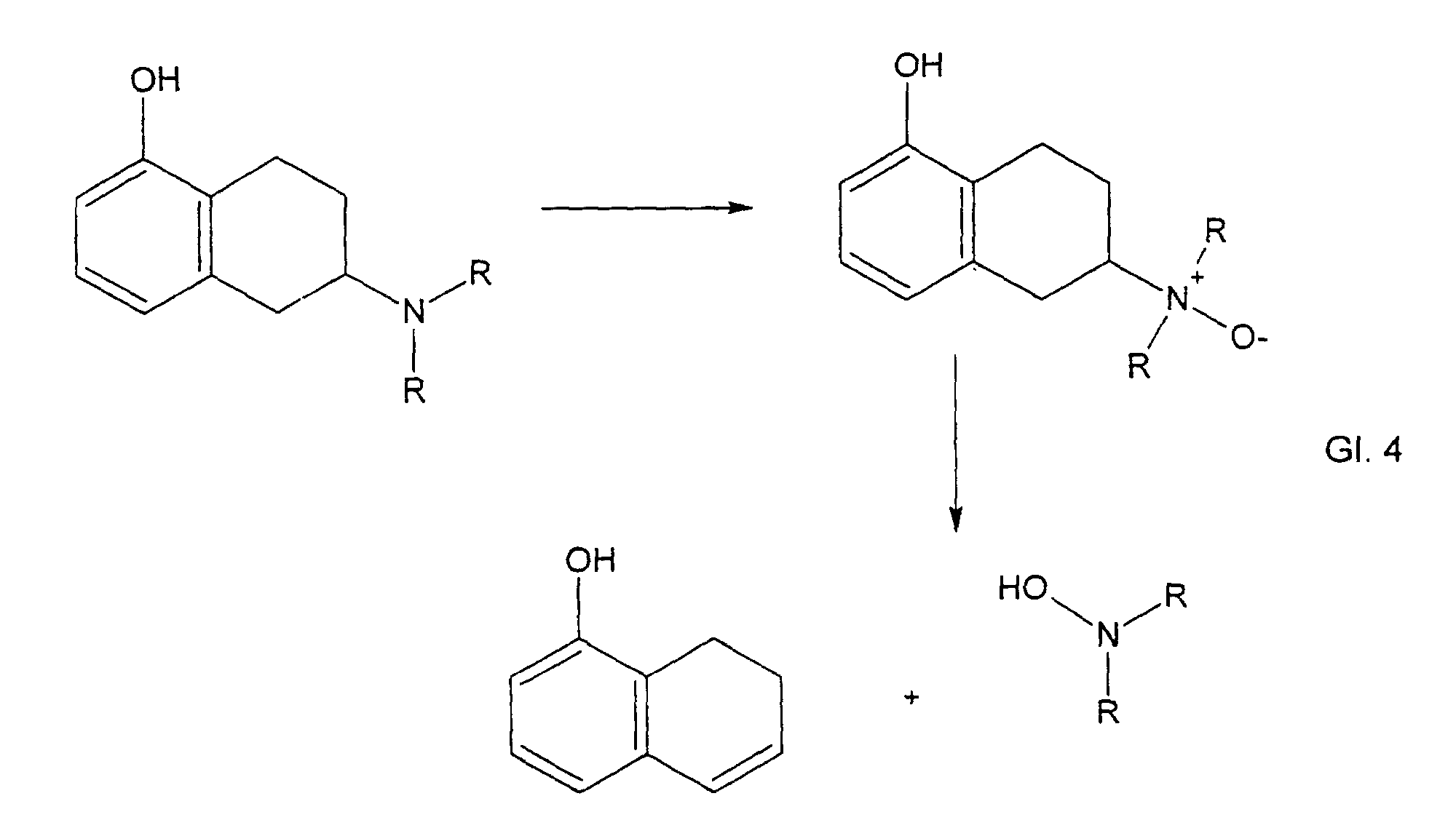
Die Weiterreaktion zum N-Oxid nach erfolgter Oxidation des Dihydropyridinrings wird nur in der Reaktion mit t-Butylhydroperoxid gemäß Gleichung 5b beobachtet. In Pflastersystemen ist die gebildete Menge zu klein, um bei geringen Umsätzen beobachtet zu werden.
Dazu gehören: Als Bausteine der ein- oder mehrschichtigen Matrix bzw. des Reservoirsystems: z. B. Polymere von Kohlenwasserstoffen wie Polyethylene, Polypropylene, Polyacrylate, Polymethacrylate, Polyurethane, Polyisobutylene, Polyvinylpyrolidon; Kohlenwasserstoffharze; Silicone; Kautschuk; Copolymere des Vinylpyrrolidons mit Acrylsäuren, Acrylsäurederivaten, Ethylen und/oder Vinylacetat; Harze auf der Basis von Kolophoniumderivaten und/oder Polyterpenen.
Als funktionale Zusatzstoffe oder Hilfsstoffe: z. B. Weich- bzw. Klebrigmacher wie Kolophoniumester, z. B. hydrierte oder partiell hydrierte Glyzerolester von Kolophonium, Polyterpene; Permeationsenhancer wie z. B. Terpene oder Terpenderivate, ungesättigte Fettsäuren oder deren Derivate, Ester von langkettigen Fettsäuren, Diethylenglykol oder dessen Derivate; Alkylmethylsulfoxide, Azone und Limonene; Kristallisationsinhibitoren wie z. B. Polyvinylpyrrolidon; Polyacrylsäure oder Cellulosederivate; Lösungsmittel wie z. B. Polyethylenglykol, Diethylenglykol und/oder dessen Derivate, Propandiol oder Oleylalkohol.
Flüssige Hilfsstoffe können auch ohne zusätzliches Lösemittel mit einer wässrigen Lösung von Natriumhydrogensulfit umgesetzt werden.
| Matrix 2a: Peroxidzahl 38 | |
| Styrol/Polyisobutylen/Styrol-Blockpolymer | 16 % |
| Oleylalkohol | 10 % |
| Kohlenwasserstoffharz | 22 % |
| Glycerolester von partiell hydriertem | |
| Kolophonium | 22 % |
| Polyisobutylen | 7 % |
| Paraffin, flüssig | 3 % |
| N-0923 Base | 20 % |
| Matrix 2b: Peroxidzahl 2,6 | |
| Polyacrylatkleber | 60 % |
| Oleylalkohol | 10 % |
| N-0923 Base | 30 % |
| Matrix 2a | Matrix 2b | |
| 25 °C | 85 % | 99,5 % |
| 40 °C | 44 % | 89,9 % |
| Matrix 2a | Matrix 2b | |
| 25 °C | 8,1 % | nicht quantifizierbar |
| 40 °C | 34,1 % | 0,4 % |
| Matrix 3a: Peroxidzahl 35 | |
| Polyacrylatkleber | 16 % |
| Glycerin | 10 % |
| Glycerolester von partiell hydriertem | |
| Kolophonium | 22 % |
| Estradiol | 20 % |
enspricht Matrix 3a, aber Einsatz von mit Na-bisulfitlösung behandeltem Glycerolester von partiell hydriertem Kolophonium
| Matrix 3a | Matrix 3b | |
| 25 °C | 0,43 % | nicht nachweisbar |
| 40 °C | 0,75 % | nicht nachweisbar |
| Matrixzusammensetzung: | |
| Bopindolol | 15 % |
| Polyacrylatkleber | 65 % |
| Glycerolester von partiell hydriertem Kolophonium | 20 % |
hergestellt mit Glycerolester von partiell hydriertem Kolophonium mit einer POZ von 160
hergestellt mit Glycerolester von partiell hydriertem Kolophonium, behandelt mit Nabisulfitlösung
| Matrix 4a | Matrix 4b | |
| 30 Tage bei 40 °C | braune Verfärbung | ohne Verfärbung |
| Nifedipine : | 10 % |
| Diethylenglykolmonoethylether | 90 % |
hergestellt mit Diethylenglykolmonoethylether mit einer POZ von 150,
hergestellt mit Diethylenglykolmonoethylether behandelt mit Natriumbisulfitlösung
| 30 Tage | Reservoir 5a Abbauprod. I | Reservoir5b Abbauprod. I |
| 25 °C | 1,6 % | nicht quantifizierbar |
| 40 °C | 4,5 % | 0,3 % |
| Matrix 6a, POZ: ca 32 | |
| Pergolide | 10 % |
| Polyacrylatkleber | 70 % |
| Glycerolester von partiell hydriertem Kolophonium | 20 % |
| Matrix 6b, POZ: ca 2-3 | |
| Pergolide | 10 % |
| Polyacrylatkleber | 90 % |
| 30 Tage | Matrix 6a Sulfoxid |
Matrix 6b Sulfoxid |
| 25 °C | 0,8 % | nicht quantifizierbar |
| 40 °C | 4,2 % | nicht quantifizierbar |
Claims (11)
- Transdermales therapeutisches System (TTS), worin die Bildung von oxidativen Abbauprodukten von mindestens einem, in diesem System enthaltenen und durch Hydroperoxide oxidierbaren Wirkstoff während der Lagerung des TTS verringert ist, dadurch gekennzeichnet, daß die Summe der Peroxidzahlen (POZ) der mit dem oder den Wirkstoff(en) in Kontakt stehenden Formulierungsbestandteilen in der durch die Rezeptur für das TTS gegebenen anteilsmäßigen Gewichtung höchstens 20 beträgt.
- Transdermales therapeutisches System gemäß Anspruch 1, dadurch gekennzeichnet, daß die Summe der Peroxidzahlen höchstens 10, bevorzugt höchstens 5 beträgt.
- Transdermales therapeutisches System gemäß einem oder mehreren der Ansprüche 1-2, dadurch gekennzeichnet, daß der oder die Wirkstoffe jeweils mindestens über eine sekundäre oder tertiäre Aminogruppe, eine Doppelbindung, eine CH-Bindung in Allylstellung, eine C-H-Bindung in Benzylstellung, eine tertiäre CH-Gruppe und/oder eine Sulfidgruppe verfügen.
- Transdermales therapeutisches System gemäß einem oder mehreren der Ansprüche 1-3, dadurch gekennzeichnet, daß es ein ein- oder mehrschichtiges Matrixsystem ist und die den oder die Wirkstoffe enthaltende Matrix Kohlenwasserstoffharze, Polyvinylpyrrolidon oder Copolymere des Vinylpyrrolidons mit Acrylsäuren, Acrylsäurederivaten, Ethylen und/oder Vinylacetat enthält.
- Transdermales therapeutisches System gemäß einem oder mehreren der Ansprüche 1-4, dadurch gekennzeichnet, daß es aus einer oder mehreren selbstklebenden wirkstoffhaltigen Schichten mit klebrigmachenden Harzen auf der Basis von Kolophoniumderivaten und/oder Polyterpenen besteht.
- Transdermales therapeutisches System gemäß einem oder mehreren der Ansprüche 1-5, dadurch gekennzeichnet, daß zu den Formulierungsbestandteilen Permeationsenhancer und/oder Kristallisationsinhibitoren gehören.
- Transdermales therapeutisches System gemäß einem oder mehreren der Ansprüche 1-4 dadurch gekennzeichnet, daß es ein Reservoirsystem ist und der oxidierbare Wirkstoff in einem Lösemittel oder Lösemittelgemisch gelöst ist, das über mindestens einen Ethersauerstoff, ein tertiäres Kohlenstoffatom und/oder eine CH-Gruppe in Allylstellung verfügt.
- Transdermales therapeutisches System gemäß einem oder mehrere der Ansprüche 1-7, dadurch gekennzeichnet, daß als Permeationsenhancer Terpene oder Terpenderivate, ungesättigte Fettsäuren oder deren Derivate, Fettalkohole oder deren Derivate oder Diethylenglykol oder dessen Derivate verwendet werden.
- Verfahren zur Herstellung eines transdermalen therapeutischen Systems gemäß Anspruch 1, dadurch gekennzeichnet, daß entwedera) Formulierungsbestandteile ausgewählt werden, deren durch die Rezeptur für das TTS gegebenen Anteile Peroxidzahlen aufweisen, welche in der Summe eine Zahl von höchstens 20 ergeben, oderb) hydroperoxidhaltige Formulierungsbestandteile in einer niederalkanolischen Lösung mit der wässerigen Lösung eines anorganischen Sulfits oder Hydrogensulfits behandelt werden, gegebenenfalls die ausgefallenen Reaktionsprodukte abgetrennt werden, und aus den Formulierungsbestandteilen gemäß a) oder den gemäß b) behandelten Formulierungsbestandteilen zusammen mit mindestens einem Wirkstoff auf üblichem Wege das transdermale therapeutische System hergestellt wird.
- Verfahren gemäß Anspruch 9, dadurch gekennzeichnet, daß die festen oder flüssigen Formulierungsbestandteile in niederalkanolischer Lösung mit Natriumsulfit oder Natriumhydrogensulfit in wässriger Lösung behandelt werden.
- Verfahren gemäß Anspruch 9 und 10, dadurch gekennzeichnet, daß die Formulierungsbestandteile in methanolischer oder ethanolischer Lösung behandelt werden.
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19957401 | 1999-11-29 | ||
| DE19957401 | 1999-11-29 | ||
| DE10054713A DE10054713C2 (de) | 1999-11-29 | 2000-11-04 | Transdermale Therapeutische Systeme mit verbesserter Stabilität und ein Verfahren zu ihrer Herstellung |
| DE10054713 | 2000-11-04 | ||
| PCT/EP2000/011692 WO2001039753A1 (de) | 1999-11-29 | 2000-11-24 | Transdermale therapeutische systeme mit verbesserter stabilität und ein verfahren zu ihrer herstellung |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP1233763A1 EP1233763A1 (de) | 2002-08-28 |
| EP1233763B1 true EP1233763B1 (de) | 2003-07-30 |
Family
ID=26007565
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP00985090A Expired - Lifetime EP1233763B1 (de) | 1999-11-29 | 2000-11-24 | Transdermale therapeutische systeme mit verbesserter stabilität und ein verfahren zu ihrer herstellung |
Country Status (21)
| Country | Link |
|---|---|
| US (1) | US6699498B1 (de) |
| EP (1) | EP1233763B1 (de) |
| JP (1) | JP5753645B2 (de) |
| CN (1) | CN1195506C (de) |
| AR (1) | AR026621A1 (de) |
| AT (1) | ATE245972T1 (de) |
| AU (1) | AU779523B2 (de) |
| BR (1) | BRPI0015939B8 (de) |
| CA (1) | CA2396686C (de) |
| CZ (1) | CZ295031B6 (de) |
| DK (1) | DK1233763T3 (de) |
| ES (1) | ES2204732T3 (de) |
| HU (1) | HU228822B1 (de) |
| IL (1) | IL149812A0 (de) |
| MX (1) | MXPA02005292A (de) |
| NZ (1) | NZ519069A (de) |
| PL (1) | PL198323B1 (de) |
| PT (1) | PT1233763E (de) |
| RU (1) | RU2266735C2 (de) |
| TW (1) | TWI272108B (de) |
| WO (1) | WO2001039753A1 (de) |
Families Citing this family (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE19814084B4 (de) * | 1998-03-30 | 2005-12-22 | Lts Lohmann Therapie-Systeme Ag | D2-Agonist enthaltendes transdermales therapeutisches System zur Behandlung des Parkinson-Syndroms und Verfahren zu seiner Herstellung |
| DE10053397A1 (de) * | 2000-10-20 | 2002-05-02 | Schering Ag | Verwendung eines dopaminergen Wirkstoffes zur Behandlung von dopaminerg behandelbaren Erkrankungen |
| US20070243240A9 (en) * | 2000-08-24 | 2007-10-18 | Fred Windt-Hanke | Transdermal therapeutic system |
| DE10066158B4 (de) * | 2000-08-24 | 2007-08-09 | Neurobiotec Gmbh | Verwendung eines transdermalen therapeutischen Systems zur Behandlung des Restless-Legs-Syndroms |
| DE10064453A1 (de) * | 2000-12-16 | 2002-07-04 | Schering Ag | Verwendung eines dopaminergen Wirkstoffes zur Behandlung von dopaminerg behandelbaren Erkrankungen |
| US20030027793A1 (en) * | 2001-05-08 | 2003-02-06 | Thomas Lauterback | Transdermal treatment of parkinson's disease |
| US20030026830A1 (en) * | 2001-05-08 | 2003-02-06 | Thomas Lauterback | Transdermal therapeutic system for parkinson's disease inducing high plasma levels of rotigotine |
| US20060263419A1 (en) * | 2002-03-12 | 2006-11-23 | Hans-Michael Wolff | Transdermal therapeutic system for Parkinson's Disease |
| US20060216336A1 (en) * | 2002-05-07 | 2006-09-28 | Hans-Michael Wolff | Transdermal therapeutic system for Parkinson's Disease |
| DE10224612A1 (de) * | 2002-06-04 | 2003-12-24 | Lohmann Therapie Syst Lts | Wirkstoffhaltige filmförmige Zubereitungen mit verbesserter chemischer Stabilität, und Verfahren zu deren Herstellung |
| US8246979B2 (en) | 2002-07-30 | 2012-08-21 | Ucb Pharma Gmbh | Transdermal delivery system for the administration of rotigotine |
| US8211462B2 (en) * | 2002-07-30 | 2012-07-03 | Ucb Pharma Gmbh | Hot-melt TTS for administering rotigotine |
| DE10234673B4 (de) * | 2002-07-30 | 2007-08-16 | Schwarz Pharma Ag | Heißschmelz-TTS zur Verabreichung von Rotigotin und Verfahren zu seiner Herstellung sowie Verwendung von Rotigotin bei der Herstellung eines TTS im Heißschmelzverfahren |
| PT1426049E (pt) * | 2002-12-02 | 2005-09-30 | Sanol Arznei Schwarz Gmbh | Administracao iontoforetica de rotigotina para o tratamento da doenca de parkinson |
| DE10261696A1 (de) * | 2002-12-30 | 2004-07-15 | Schwarz Pharma Ag | Vorrichtung zur transdermalen Verabreichung von Rotigotin-Base |
| TW200514582A (en) * | 2003-10-31 | 2005-05-01 | Hisamitsu Pharmaceutical Co | Transdermal preparation and method for reducing side effect in pergolide therapy |
| US7537774B2 (en) * | 2005-12-23 | 2009-05-26 | Orion Therapeautics, Llc | Therapeutic formulation |
| US8563031B2 (en) * | 2010-05-27 | 2013-10-22 | Absize, Inc. | Piroxicam-containing matrix patches and methods for the topical treatment of acute and chronic pain and inflammation therewith |
| DE102017104026A1 (de) | 2017-02-27 | 2018-08-30 | Lts Lohmann Therapie-Systeme Ag | Nicotin enthaltendes transparentes transdermales therapeutisches System |
| WO2020250840A1 (ja) * | 2019-06-14 | 2020-12-17 | 久光製薬株式会社 | ロチゴチン含有貼付剤 |
Family Cites Families (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS56103117A (en) * | 1980-01-19 | 1981-08-18 | Nitto Electric Ind Co Ltd | Stabilized plaster containing drug |
| JPS60199832A (ja) * | 1984-03-22 | 1985-10-09 | Nitto Electric Ind Co Ltd | 抗酸化性外用製剤 |
| FR2619388A1 (fr) | 1987-08-14 | 1989-02-17 | Rhone Poulenc Chimie | Article stratifie souple pour adhesif de transfert |
| JPH01249885A (ja) * | 1988-03-30 | 1989-10-05 | Narisu Keshohin:Kk | 脂質の酸化防止剤 |
| DE3910578A1 (de) * | 1989-03-29 | 1990-10-04 | Schering Ag | Mittel zur transdermalen applikation enthaltend gestoden |
| JP2788754B2 (ja) * | 1989-05-12 | 1998-08-20 | 日東電工株式会社 | シート状経皮吸収デバイス |
| JPH03232817A (ja) * | 1990-02-07 | 1991-10-16 | Showa Yakuhin Kako Kk | 貼付剤 |
| IE82916B1 (en) * | 1990-11-02 | 2003-06-11 | Elan Corp Plc | Formulations and their use in the treatment of neurological diseases |
| JP2869167B2 (ja) * | 1990-08-09 | 1999-03-10 | 日東電工株式会社 | 徐放性貼付製剤 |
| JP3723229B2 (ja) * | 1991-03-12 | 2005-12-07 | 武田薬品工業株式会社 | 外用貼付剤 |
| JP3034077B2 (ja) * | 1991-04-19 | 2000-04-17 | 久光製薬株式会社 | メラニン抑制貼付剤 |
| DE4210711A1 (de) * | 1991-10-31 | 1993-05-06 | Schering Ag Berlin Und Bergkamen, 1000 Berlin, De | Transdermale therapeutische systeme mit kristallisationsinhibitoren |
| JPH05306227A (ja) * | 1992-04-27 | 1993-11-19 | Lion Corp | 徐放性口腔内疾患治療剤 |
| JPH0624969A (ja) * | 1992-07-09 | 1994-02-01 | Teikoku Seiyaku Co Ltd | ケトプロフェン含有外用貼付剤 |
| DE4224325C1 (de) * | 1992-07-23 | 1994-02-10 | Sanol Arznei Schwarz Gmbh | Wirkstoffpflaster für niedrigschmelzende und/oder flüchtige Wirkstoffe und Verfahren zu seiner Herstellung |
| DE4336557C2 (de) * | 1993-05-06 | 1997-07-17 | Lohmann Therapie Syst Lts | Estradiolhaltiges transdermales therapeutisches System, Verfahren zu seiner Herstellung und seine Verwendung |
| IL109539A0 (en) | 1994-05-03 | 1994-08-26 | Yissum Res Dev Co | Substained-release pharmaceutical system for the delivery of antioxidants |
| JP3157082B2 (ja) * | 1994-09-22 | 2001-04-16 | 昭和薬品化工株式会社 | 医療用パック型製剤用基剤および医療用パック型製剤 |
| JP3117619B2 (ja) * | 1995-06-07 | 2000-12-18 | 花王株式会社 | (メタ)アクリル酸系(共)重合体の製造方法 |
| JP3361674B2 (ja) * | 1995-11-08 | 2003-01-07 | 日本カーバイド工業株式会社 | 医療用粘着剤組成物 |
| FR2744368B1 (fr) * | 1996-02-02 | 1998-04-24 | Fabre Pierre Dermo Cosmetique | Composition dermocosmetique contenant du retinal |
| DE19700913C2 (de) * | 1997-01-14 | 2001-01-04 | Lohmann Therapie Syst Lts | Transdermales therapeutisches System zur Abgabe von Hormonen |
| US6017950A (en) * | 1997-08-05 | 2000-01-25 | Millennium Pharmaceuticals, Inc. | Methods for controlling gram negative bacteria in mammals |
| GB9720470D0 (en) * | 1997-09-25 | 1997-11-26 | Ethical Pharmaceuticals South | Inhibition of crystallization in transdermal devices |
| GB9800526D0 (en) * | 1998-01-12 | 1998-03-11 | Ciba Geigy Ag | Organic compounds |
| DE19814084B4 (de) * | 1998-03-30 | 2005-12-22 | Lts Lohmann Therapie-Systeme Ag | D2-Agonist enthaltendes transdermales therapeutisches System zur Behandlung des Parkinson-Syndroms und Verfahren zu seiner Herstellung |
| JP4275768B2 (ja) * | 1998-06-18 | 2009-06-10 | 久光製薬株式会社 | 水性粘着膏体 |
-
2000
- 2000-11-24 EP EP00985090A patent/EP1233763B1/de not_active Expired - Lifetime
- 2000-11-24 CA CA002396686A patent/CA2396686C/en not_active Expired - Lifetime
- 2000-11-24 NZ NZ519069A patent/NZ519069A/en not_active IP Right Cessation
- 2000-11-24 CZ CZ20021867A patent/CZ295031B6/cs not_active IP Right Cessation
- 2000-11-24 AU AU21623/01A patent/AU779523B2/en not_active Expired
- 2000-11-24 PT PT00985090T patent/PT1233763E/pt unknown
- 2000-11-24 DK DK00985090T patent/DK1233763T3/da active
- 2000-11-24 BR BRPI0015939A patent/BRPI0015939B8/pt not_active IP Right Cessation
- 2000-11-24 IL IL14981200A patent/IL149812A0/xx active IP Right Grant
- 2000-11-24 HU HU0203318A patent/HU228822B1/hu unknown
- 2000-11-24 ES ES00985090T patent/ES2204732T3/es not_active Expired - Lifetime
- 2000-11-24 PL PL355606A patent/PL198323B1/pl unknown
- 2000-11-24 RU RU2002118325/15A patent/RU2266735C2/ru active Protection Beyond IP Right Term
- 2000-11-24 CN CNB008163502A patent/CN1195506C/zh not_active Expired - Lifetime
- 2000-11-24 AT AT00985090T patent/ATE245972T1/de active
- 2000-11-24 MX MXPA02005292A patent/MXPA02005292A/es active IP Right Grant
- 2000-11-24 WO PCT/EP2000/011692 patent/WO2001039753A1/de not_active Ceased
- 2000-11-24 JP JP2001541486A patent/JP5753645B2/ja not_active Expired - Lifetime
- 2000-11-27 TW TW089125152A patent/TWI272108B/zh not_active IP Right Cessation
- 2000-11-27 US US09/723,130 patent/US6699498B1/en not_active Expired - Lifetime
- 2000-11-27 AR ARP000106240A patent/AR026621A1/es active IP Right Grant
Also Published As
| Publication number | Publication date |
|---|---|
| AU779523B2 (en) | 2005-01-27 |
| CA2396686C (en) | 2008-07-08 |
| IL149812A0 (en) | 2002-11-10 |
| NZ519069A (en) | 2004-06-25 |
| RU2002118325A (ru) | 2004-02-20 |
| PL355606A1 (en) | 2004-05-04 |
| CZ295031B6 (cs) | 2005-05-18 |
| BR0015939B1 (pt) | 2013-10-08 |
| RU2266735C2 (ru) | 2005-12-27 |
| CZ20021867A3 (cs) | 2002-11-13 |
| TWI272108B (en) | 2007-02-01 |
| HU228822B1 (en) | 2013-05-28 |
| JP5753645B2 (ja) | 2015-07-22 |
| BRPI0015939B8 (pt) | 2021-05-25 |
| AU2162301A (en) | 2001-06-12 |
| BR0015939A (pt) | 2002-08-20 |
| ES2204732T3 (es) | 2004-05-01 |
| HUP0203318A3 (en) | 2004-06-28 |
| CA2396686A1 (en) | 2001-06-07 |
| CN1195506C (zh) | 2005-04-06 |
| ATE245972T1 (de) | 2003-08-15 |
| WO2001039753A1 (de) | 2001-06-07 |
| DK1233763T3 (da) | 2003-10-20 |
| PL198323B1 (pl) | 2008-06-30 |
| HUP0203318A2 (hu) | 2003-02-28 |
| PT1233763E (pt) | 2003-11-28 |
| CN1402633A (zh) | 2003-03-12 |
| MXPA02005292A (es) | 2002-12-11 |
| US6699498B1 (en) | 2004-03-02 |
| JP2003515554A (ja) | 2003-05-07 |
| AR026621A1 (es) | 2003-02-19 |
| EP1233763A1 (de) | 2002-08-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1233763B1 (de) | Transdermale therapeutische systeme mit verbesserter stabilität und ein verfahren zu ihrer herstellung | |
| EP2515886B1 (de) | Transdermales therapeutisches system zur verabreichung von rivastigmin oder dessen derivaten | |
| EP1509201B1 (de) | Wirkstoffhaltige filmförmige zubereitungen mit verbesserter chemischer stabilität, und verfahren zu deren herstellung | |
| DE69813225T2 (de) | Blockcopolymere | |
| EP1301179B1 (de) | Transdermales therapeutisches system mit hochdispersem siliziumdioxid | |
| DE69101931T2 (de) | Pharmazeutische Zusammensetzung. | |
| DE69505041T2 (de) | Buprenorphine enthaltendes Präparat für perkutäne Absorption | |
| EP0563731B1 (de) | Arzneimittel aus Polyhydroxymethylenderivaten, Verfahren zu deren Herstellung und Verwendung | |
| DE69722247T2 (de) | Pharmazeutische tablette mit verzögerter freisetzung, enthaltend eine matrix aus quervernetzter amylose und hydroxypropyl methylzellulose | |
| EP0934058B1 (de) | Stabile arzneiform mit benzimidazolderivaten als wirkstoff zur oralen verabreichung und verfahren zu ihrer herstellung | |
| EP2468274B1 (de) | Transdermales therapeutisches System zur Verabreichung eines Wirkstoffs | |
| DD243856A5 (de) | Wirkstoffpflaster | |
| EP0794770B1 (de) | Transdermale darreichungsform mit antihistaminisch wirksamen loratidinmetabolit | |
| DE3873581T2 (de) | Nitroglycerinarzneimittelpraeparat fuer perkutane absorption. | |
| DE2732335C2 (de) | Tablette zur enteralen Verabreichung von Indolyloxyalkanolamin-Derivaten | |
| DE10054713C2 (de) | Transdermale Therapeutische Systeme mit verbesserter Stabilität und ein Verfahren zu ihrer Herstellung | |
| DE69830278T2 (de) | Medizinische Selbstklebefolie und Verfahren zu deren Herstellung | |
| EP0227988A2 (de) | Therapeutisches System | |
| CH667013A5 (de) | Pharmazeutisches mittel zur lokalen therapie der psoriasis. | |
| EP2674150A1 (de) | Pharmazeutische Zubereitung mit verbesserter Stabilität des Wirkstoffs | |
| DE102017115701B4 (de) | Fampridin-TTS | |
| DE3642066A1 (de) | Therapeutisches system |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20020515 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE TR |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: GERMAN |
|
| REF | Corresponds to: |
Ref document number: 50003145 Country of ref document: DE Date of ref document: 20030904 Kind code of ref document: P |
|
| REG | Reference to a national code |
Ref country code: GR Ref legal event code: EP Ref document number: 20030403515 Country of ref document: GR |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: TRGR |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20031124 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20031130 |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 20031111 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2204732 Country of ref document: ES Kind code of ref document: T3 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20040504 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 16 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 17 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: LU Payment date: 20191120 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PT Payment date: 20191021 Year of fee payment: 20 Ref country code: IE Payment date: 20191121 Year of fee payment: 20 Ref country code: SE Payment date: 20191121 Year of fee payment: 20 Ref country code: DE Payment date: 20191121 Year of fee payment: 20 Ref country code: FI Payment date: 20191121 Year of fee payment: 20 Ref country code: NL Payment date: 20191120 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20191120 Year of fee payment: 20 Ref country code: ES Payment date: 20191220 Year of fee payment: 20 Ref country code: DK Payment date: 20191122 Year of fee payment: 20 Ref country code: FR Payment date: 20191120 Year of fee payment: 20 Ref country code: GR Payment date: 20191122 Year of fee payment: 20 Ref country code: IT Payment date: 20191128 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20191121 Year of fee payment: 20 Ref country code: TR Payment date: 20191120 Year of fee payment: 20 Ref country code: AT Payment date: 20191121 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20191120 Year of fee payment: 20 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R071 Ref document number: 50003145 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MK Effective date: 20201123 |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: EUP Expiry date: 20201124 Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 Expiry date: 20201123 |
|
| REG | Reference to a national code |
Ref country code: FI Ref legal event code: MAE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MK9A |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK07 Ref document number: 245972 Country of ref document: AT Kind code of ref document: T Effective date: 20201124 Ref country code: BE Ref legal event code: MK Effective date: 20201124 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20201124 Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20201123 Ref country code: PT Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20201207 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20210303 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20201125 |