WO2010104013A1 - エチレン-ビニルエステル共重合体加溶媒分解物組成物の製造方法 - Google Patents
エチレン-ビニルエステル共重合体加溶媒分解物組成物の製造方法 Download PDFInfo
- Publication number
- WO2010104013A1 WO2010104013A1 PCT/JP2010/053690 JP2010053690W WO2010104013A1 WO 2010104013 A1 WO2010104013 A1 WO 2010104013A1 JP 2010053690 W JP2010053690 W JP 2010053690W WO 2010104013 A1 WO2010104013 A1 WO 2010104013A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- ethylene
- vinyl ester
- ester copolymer
- solvolysis
- general formula
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L29/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an alcohol, ether, aldehydo, ketonic, acetal or ketal radical; Compositions of hydrolysed polymers of esters of unsaturated alcohols with saturated carboxylic acids; Compositions of derivatives of such polymers
- C08L29/02—Homopolymers or copolymers of unsaturated alcohols
- C08L29/04—Polyvinyl alcohol; Partially hydrolysed homopolymers or copolymers of esters of unsaturated alcohols with saturated carboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F8/00—Chemical modification by after-treatment
- C08F8/12—Hydrolysis
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F2800/00—Copolymer characterised by the proportions of the comonomers expressed
- C08F2800/10—Copolymer characterised by the proportions of the comonomers expressed as molar percentages
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2205/00—Polymer mixtures characterised by other features
- C08L2205/02—Polymer mixtures characterised by other features containing two or more polymers of the same C08L -group
Definitions
- the present invention relates to a method for producing an EVOH resin composition containing a solvolysis product of an ethylene-vinyl ester copolymer (hereinafter sometimes referred to as EVOH resin) and a modified EVOH resin, and in particular, productivity and melt molding. Excellent in properties.
- EVOH resin ethylene-vinyl ester copolymer
- the present invention relates to a method for producing an EVOH resin composition in which the difference between the highest melting peak and the lowest melting peak is particularly small.
- EVOH resin is excellent in transparency, gas barrier properties, fragrance retention, solvent resistance, oil resistance and the like.
- films and sheets of food packaging materials, pharmaceutical packaging materials, industrial chemical packaging materials, agricultural chemical packaging materials, etc., or containers such as tubes, cups, trays, bottles, etc. are used. .
- X is a bond chain and is an arbitrary bond chain excluding an ether bond
- R 1 to R 4 are each independently an arbitrary substituent
- n represents 0 or 1.
- EVOH resin is mainly used for melt molding, it is preferable that it is uniformly melted by heat and solidified uniformly after molding.
- EVOH resins having different ethylene contents, saponification degrees, types of modifying groups, modification amounts, and the like are used. Therefore, in the case of melt molding, since the meltability is not uniform, the compatibility of the composition components becomes insufficient, and because the speed at which each component solidifies tends to cause phase separation, The molded product obtained from the EVOH resin composition has a problem that unevenness in thickness and lines occur.
- a modified EVOH resin in which the ethylene content, saponification degree, and modification degree are adjusted in advance so as to reduce the difference in melting peak of each EVOH resin and a normal EVOH resin are separately manufactured, and then mixed.
- the productivity tends to be impaired.
- the productivity tends to be good and the melting peaks tend to approach each other, but the melting peak difference is still large, and the thickness of the melt molded product There was room for improvement to completely eliminate streaks and streaks.
- An object of the present invention is to provide an EVOH resin composition having excellent resistance.
- ethylene-vinyl ester copolymer (hereinafter, ethylene-vinyl ester copolymer may be abbreviated as EVE resin) (B ′)
- EVE resin ethylene-vinyl ester copolymer
- the blending ratio (B ′) / (A ′) of the ethylene-vinyl ester copolymer (A ′) and the modified ethylene-vinyl ester copolymer (B ′) is 50/50 by weight ratio.
- R 1 , R 2 , and R 3 each independently represent a hydrogen atom or an organic group
- X represents a single bond or a bonded chain
- R 4 , R 5 , and R 6 represent Each independently represents a hydrogen atom or an organic group.
- R 1 , R 2 , and R 3 each independently represent a hydrogen atom or an organic group
- X represents a single bond or a bonded chain
- R 4 , R 5 , and R 6 represent Each independently represents a hydrogen atom or an organic group
- R 7 and R 8 each independently represents a hydrogen atom, a hydrocarbon group or R 9 —CO— (wherein R 9 is an alkyl group); 7 and R 8 may combine to form a 5-membered ring, which is a cyclic carbonate structure or a cyclic acetal structure.
- R 1 , R 2 , and R 3 each independently represent a hydrogen atom or an organic group
- X represents a single bond or a bonded chain
- R 4 , R 5 , and R 6 represent R 7 and R 8 each independently represent a hydrogen atom or R 9 —CO— (wherein R 9 is an alkyl group).
- the structural unit represented by the general formula (1) is a structural unit represented by the following general formula (1a), and the compound represented by the general formula (2) is 3,4-diacyloxy-1-butene.
- the EVE resin (A ′) and the modified EVE resin (B ′) containing a structural unit derived from the compound represented by the general formula (2) are solvolyzed in the same system, and the EVE
- the ratio (B ′) / (A ′) of the ethylene content of the resin (A ′) and the ethylene content of the modified EVE resin (B ′) is 1 or more, and (B ′) / (A ′)
- the blending ratio of 50/50 to 1/99 in weight ratio is particularly unexpected, because the melting peak difference of the obtained EVOH resin composition becomes particularly small, and the productivity and melt moldability are improved. An effect is obtained.
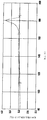
- FIG. 1 shows the melting peak of the EVOH resin composition in Example 1.
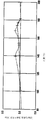
- FIG. 2 shows the melting peak of the EVOH resin composition in Example 2.
- FIG. 3 shows the melting peak of the EVOH resin composition in Comparative Example 1.
- the present invention includes a step of solvolysis of an EVE resin (A ′) and a modified EVE resin (B ′) containing a structural unit derived from the compound represented by the general formula (2) in the same system, and EVOH
- An EVOH resin (A ′) in the step of solvolysis comprising the step of producing an EVOH resin composition containing a resin (A) and a modified EVOH resin (B) having a structural unit represented by the general formula (1)
- the ethylene content ratio (B ′) / (A ′) of the modified EVE resin (B ′) is 1 or more, and the blending ratio of (B ′) / (A ′) is 50 /
- the present invention relates to a method for producing an EVOH resin composition having a ratio of 50 to 1/99.
- R 1 , R 2 , and R 3 each independently represent a hydrogen atom or an organic group
- X represents a single bond or a bonded chain
- R 4 , R 5 , and R 6 represent Each independently represents a hydrogen atom or an organic group.
- R 1 , R 2 , and R 3 each independently represent a hydrogen atom or an organic group
- X represents a single bond or a bonded chain
- R 4 , R 5 , and R 6 represent Each independently represents a hydrogen atom or an organic group
- R 7 and R 8 each independently represents a hydrogen atom, a hydrocarbon group or R 9 —CO— (wherein R 9 is an alkyl group); 7 and R 8 may combine to form a 5-membered ring, which is a cyclic carbonate structure or a cyclic acetal structure.
- the following general formula (3) is an example of a compound in which R 7 and R 8 are bonded to form a 5-membered ring in the general formula (2), and the 5-membered ring has a cyclic carbonate structure.
- the general formula (4) is an example of a compound in which, in the general formula (2), R 7 and R 8 are bonded to form a 5-membered ring, and the 5-membered ring has a cyclic acetal structure.
- R 10 and R 11 each independently represents a hydrogen atom or a hydrocarbon group.
- the EVOH resin (A) is obtained by obtaining an ethylene-vinyl ester copolymer (hereinafter sometimes referred to as EVE resin) (A ′) obtained by copolymerizing ethylene and a vinyl ester monomer. It is a resin obtained by solvolysis of EVE resin (A ′), and is a water-insoluble thermoplastic resin. Generally used for food packaging films.
- EVE resin (A ′) is produced by any known polymerization method, for example, solution polymerization, suspension polymerization, emulsion polymerization and the like.
- vinyl ester monomer vinyl acetate is typically used.
- vinyl formate, vinyl propionate, vinyl valerate, vinyl butyrate, vinyl isobutyrate, vinyl pivalate, vinyl caprate, vinyl laurate, vinyl stearate, vinyl versatate, etc. benzoic acid
- aromatic vinyl esters such as vinyl, and are usually aliphatic vinyl esters having 3 to 20 carbon atoms, preferably 4 to 10 carbon atoms, and particularly preferably 4 to 7 carbon atoms.
- These vinyl ester monomers are usually used alone, but a plurality of them may be used simultaneously if necessary.
- the ethylene-vinyl acetate copolymer is referred to as EVA resin.
- the ethylene content in the EVE resin (A ′) and the EVOH resin (A) does not change before and after the solvolysis, and thus has the same value.
- the ethylene content is a value measured based on ISO 14663, and is usually 20 to 60 mol%, preferably 30 to 50 mol%, particularly preferably 30 to 40 mol%. When the content is too low, the melt moldability tends to be insufficient, and when it is too high, the gas barrier property tends to be insufficient.
- a portion that is not a structural unit derived from ethylene is a structural unit derived from a vinyl ester or another copolymerizable component described later.
- the portion that is not a structural unit derived from ethylene is a vinyl alcohol structural unit obtained by solvolysis of a structural unit derived from vinyl ester, and a structural unit derived from a trace amount of vinyl ester remaining after solvolysis, or It is a structural unit derived from another copolymerizable component described later.
- the content of the vinyl alcohol structural unit in the EVOH resin (A) is usually 40 to 80 mol%, preferably 50 to 70 mol%, particularly preferably 60 to 70 mol%.
- the paste viscosity of the EVE resin (A ′) was measured with a B-type viscometer (rotor No. 2, rotation speed: 10 rpm, paste temperature: 65 ° C.) with a methanol solvent.
- the value is usually 10 1 to 10 5 mPa ⁇ s, preferably 10 2 to 10 4 mPa ⁇ s, particularly preferably 10 2 to 10 3 mPa ⁇ s.
- the paste viscosity at a constant resin content of the EVE resin (A ′) is a numerical value corresponding to the degree of polymerization of the EVE resin (A ′). If the degree of polymerization of the EVE resin (A ′) is high, the viscosity tends to increase, and if it is low, the viscosity tends to decrease.
- R 1 , R 2 , and R 3 each independently represent a hydrogen atom or an organic group
- X represents a single bond or a bonded chain
- R 4 , R 5 , and R 6 represent Each independently represents a hydrogen atom or an organic group.
- Such a resin is usually a resin obtained by solvolysis of a modified EVE resin (B ′) obtained by copolymerization of ethylene, a vinyl ester monomer and a compound represented by the general formula (2).
- R 1 , R 2 , and R 3 each independently represent a hydrogen atom or an organic group
- X represents a single bond or a bonded chain
- R 4 , R 5 , and R 6 represent Each independently represents a hydrogen atom or an organic group
- R 7 and R 8 each independently represents a hydrogen atom, a hydrocarbon group or R 9 —CO— (wherein R 9 is an alkyl group); 7 and R 8 may combine to form a 5-membered ring, which is a cyclic carbonate structure or a cyclic acetal structure.
- the structural unit derived from the vinyl ester monomer is solvolyzed. It is a compound that undergoes solvolysis under the same conditions as the conditions to become a structural unit represented by the above general formula (1).
- Such a resin can be produced, for example, by the method described in Japanese Patent Application Laid-Open No. 2004-359965.
- the following general formula (3) is a compound of the general formula (2) in which R 7 and R 8 are combined to form a 5-membered ring, and the 5-membered ring is a cyclic carbonate structure.
- the following general formula (4) is an example of a compound in which R 7 and R 8 are bonded to form a 5-membered ring, and the 5-membered ring has a cyclic acetal structure in the general formula (2). .
- R 10 and R 11 each independently represent a hydrogen atom or a hydrocarbon group.
- R 7 and R 8 are each independently a hydrogen atom or R 9 —CO— (wherein R 9 is an alkyl group)]
- R 1 to R 6 are the same as those in the general formula (1).
- R 7 and R 8 are each preferably R 9 —CO—.
- R 9 is usually an alkyl group having 1 to 20 carbon atoms, preferably an alkyl group having 1 to 10 carbon atoms, particularly preferably an alkyl group having 1 to 5 carbon atoms, and particularly preferably a methyl group from the viewpoint of industrial productivity. .
- the vinyl ester monomer the same monomers as those used for the EV resin (A ′) and EVOH resin (A) are used, and usually 3 to 20 carbon atoms, preferably 4 to 10 carbon atoms, particularly preferably. Is an aliphatic vinyl ester having 4 to 7 carbon atoms. From the economical viewpoint, vinyl acetate is particularly preferably used. Such vinyl ester monomers are usually used alone, but a plurality of them may be used simultaneously if necessary.
- R 1 to R 6 in the 1,2-diol structural unit represented by the general formula (1) are preferably hydrogen atoms.
- each of R 1 to R 3 is independently usually a carbon atom having 1 to 30 carbon atoms, more preferably 1 to 15 carbon atoms, and still more preferably 1 to 4 carbon atoms. It is a hydrogen group.
- R 4 to R 6 are each independently a hydrocarbon group usually having 1 to 30 carbon atoms, more preferably 1 to 15 carbon atoms, and still more preferably 1 to 4 carbon atoms.
- an alkyl group such as a methyl group, an ethyl group, an n-propyl group, an isopropyl group, an n-butyl group, an isobutyl group and a tert-butyl group, an aromatic hydrocarbon group such as a phenyl group and a benzyl group
- an aromatic hydrocarbon group such as a phenyl group and a benzyl group
- Examples include a halogen atom, a hydroxyl group, an acyloxy group, an alkoxycarbonyl group, a carboxyl group, and a sulfonic acid group.
- R 1 to R 3 are each independently an alkyl group having 1 to 30 carbon atoms or a hydrogen atom, more preferably an alkyl group having 1 to 15 carbon atoms or a hydrogen atom, and further preferably 1 to 4 carbon atoms. And a hydrogen atom is most preferred.
- R 4 to R 6 are each independently an alkyl group having 1 to 30 carbon atoms or a hydrogen atom, more preferably an alkyl group having 1 to 15 carbon atoms or a hydrogen atom, more preferably an alkyl having 1 to 4 carbon atoms.
- a hydrogen atom most preferably a hydrogen atom.
- R 1 to R 6 are all hydrogen atoms.
- provisions on the R 1 ⁇ R 6 are defined also in R 1 ⁇ R 6 in the general formula (2).
- X in the structural unit represented by the general formula (1) preferably has a short bond chain, and most preferably a single bond from the viewpoint of maintaining crystallinity and excellent gas barrier properties.
- a bond chain may be used as long as it does not inhibit the effect of the present invention, and the bond chain is not particularly limited, but hydrocarbon chains such as alkylene, alkenylene, alkynylene, phenylene, naphthylene (these hydrocarbons) May be substituted with halogen such as fluorine, chlorine, bromine, etc.), -O-,-(CH 2 O) m -,-(OCH 2 ) m -,-(CH 2 O) m CH
- a structure containing an ether bond site such as 2 —, a structure containing a carbonyl group such as —CO—, —COCO—, —CO (CH 2 ) m CO—, —CO (C 6 H 4 ) CO—, —S— , -CS-, -SO-
- R is independently an arbitrary substituent, preferably a hydrogen atom or an alkyl group, and m is a natural number, usually 1 to 30, preferably 1 to 15, More preferably, it is 1 to 10.
- —CH 2 OCH 2 — and a hydrocarbon chain having 1 to 10 carbon atoms are preferred, and a hydrocarbon chain having 1 to 6 carbon atoms is more preferred, and carbon atoms having 1 to 6 carbon atoms are particularly preferred. It is a hydrocarbon chain of number 1.
- provisions on the R 1 ⁇ R 6 are defined also in R 1 ⁇ R 6 in the general formula (2).
- the most preferred structure in the 1,2-diol structural unit represented by the general formula (1) is one in which R 1 to R 6 are all hydrogen atoms and X is a single bond. That is, the structural unit represented by the following general formula (1a) is most preferable.
- R 7 and R 8 each independently represent a hydrogen atom, (wherein, R 9 is alkyl group) hydrocarbon group or R 9 -CO- represents, with R 7 R 8 may combine to form a 5-membered ring, which is a cyclic carbonate structure or a cyclic acetal structure.
- R 1 to R 6 are the same as those in the general formula (1).
- [Ii] Structural unit derived from the compound represented by the general formula (3) The structural unit represented by the following general formula (3-1) is a structural unit derived from the compound represented by the general formula (3).
- General formula (3) is an example of a compound in which R 7 and R 8 are bonded to form a 5-membered ring, and the 5-membered ring has a cyclic carbonate structure in general formula (2).
- R 1 to R 6 are the same as those in the general formula (1).
- [Iii] Structural unit derived from compound represented by general formula (4) The structural unit represented by the following general formula (4-1) is a structural unit derived from the compound represented by general formula (4).
- General formula (4) is an example of a compound in which R 7 and R 8 are bonded to form a 5-membered ring, and the 5-membered ring has a cyclic acetal structure in general formula (2).
- R 10 and R 11 each independently represent a hydrogen atom or a hydrocarbon group.
- R 1 to R 6 are the same as those in the general formula (1).
- R 10 and R 11 are usually alkyl groups having 1 to 20 carbon atoms, preferably 1 to 10 alkyl groups, particularly preferably 1 to 5 alkyl groups, particularly preferably from the viewpoint of productivity. It is a methyl group.
- the production method thereof is as follows: [1] As a comonomer, as shown in the general formula (2a), 3,4- Diol-1-butene, 3,4-diacyloxy-1-butene, 3-acyloxy-4-ol-1-butene, 4-acyloxy-3-ol-1-butene, 3,4-diacyloxy-2-methyl- Using 1-butene or the like, these are copolymerized with a vinyl ester monomer and ethylene to obtain a copolymer, which is then subjected to solvolysis, or [2] as a comonomer, represented by the general formula (3) Using vinyl ethylene carbonate, etc., these are copolymerized with vinyl ester monomers and ethylene to obtain copolymers, which are then subjected to solvolysis and decarburization.
- 2,2-dialkyl-4-vinyl-1,3-dioxolane or the like as shown in the general formula (4) is used, and these are co-polymerized with the vinyl ester monomer and ethylene.
- Examples thereof include a method of polymerizing to obtain a copolymer, followed by solvolysis and deacetalization.
- the polymerization proceeds well, and it is easy to introduce the 1,2-diol structural unit uniformly into the main chain of the polymer, and there are few unreacted monomers and the impurities in the product are reduced. Therefore, it is preferable to employ the production method of the above [1], and particularly preferably, 3,4-diasiloxy-1-butene is copolymerized with a vinyl ester monomer and ethylene in terms of excellent copolymerization reactivity. This is a method of solvolysis of the copolymer obtained. Furthermore, it is preferable to use 3,4-diacetoxy-1-butene as 3,4-diasiloxy-1-butene. Moreover, you may use the mixture of the monomer illustrated by the manufacturing method of said [1].
- Cx (vinyl Ethylene carbonate) 0.005 (65 ° C.)
- Cx (2,2-dimethyl-4-vinyl-1,3-dioxolane) in the case of 2,2-dimethyl-4-vinyl-1,3-dioxolane Compared with 0.023 (65 ° C.), it is a hindrance to polymerization and does not easily increase the degree of polymerization or cause a decrease in polymerization rate.
- 3,4-diacetoxy-1-butene is the same as that derived from the vinyl acetate structural unit, which is a main structural unit, as a by-product generated when the copolymer is subjected to solvolysis.
- the fact that there is no need to provide a special apparatus or process is also a great industrial advantage.
- 3,4-diacetoxy-1-butene contains 3,4-diacetoxy-1-butane, 1,4-diacetoxy-1-butene, 1,4-diacetoxy-1-butane, etc. as a small amount of impurities. Also good.
- 3,4-diol-1-butene is available from Eastman Chemical Co., and 3,4-diacetoxy-1-butene is commercially available from Eastman Chemical Co. be able to. Further, 3,4-diacetoxy-1-butene obtained as a by-product during the production process of 1,4-butanediol can also be used.
- the modified EVOH resin having a 1,2-diol structural unit produced by the above production method [2] has a carbonate ring remaining in the side chain when the degree of saponification is low or when decarboxylation is insufficient. The carbonic acid tends to be decarboxylated during melt molding and cause the resin to foam.
- the modified EVOH resin having a 1,2-diol structural unit produced by the production method [3] is the same as the modified EVOH resin having a 1,2-diol structural unit produced by the production method [2].
- the functional group derived from the monomer (acetal ring) remaining in the side chain tends to be detached during melt molding to generate an odor, it must be used with attention to this.
- the content of the structural unit represented by the general formula (1) in the modified EVOH resin (B) is a value measured by the method described in Japanese Patent Application Laid-Open No. 2004-359965 using 1 H-NMR, Usually, it is 0.1 to 30 mol%, preferably 0.5 to 15 mol%, particularly preferably 1 to 8 mol%. When the content is too small, the melt moldability of the EVOH resin composition tends to be poor, and when it is too large, the gas barrier property of the molded product tends to decrease.
- the copolymerization ratio of the compound represented by the general formula (2) corresponds to the content of the structural unit represented by the general formula (1) in the modified EVOH resin (B). Therefore, it is usually 0.1 to 30 mol%, preferably 0.5 to 15 mol%, particularly preferably 1 to 8 mol%. Such content can be adjusted by the amount of monomers charged.
- the ethylene content in the modified EVE resin (B ′) and the modified EVOH resin (B) does not change before and after the solvolysis and is the same value.
- the value measured based on ISO 14663 is usually 20 to 60 mol%, preferably 25 to 50 mol%, particularly preferably 30 to 40 mol%.
- the melt moldability of the EVOH resin tends to be insufficient, and when it is too high, the gas barrier property of the EVOH resin tends to be insufficient.
- the structural unit derived from ethylene and the structural unit derived from the compound represented by the general formula (2) are structural units derived from vinyl ester or other copolymerizable components described later.
- the vinyl ester content in the modified EVE resin (B ′) is usually 10 to 79.9 mol%, preferably 35 to 74.5 mol%, particularly preferably 52 to 69 mol%.
- the portion that is not an ethylene structural unit is a vinyl alcohol structural unit in which a vinyl ester site is solvolyzed, a trace amount of vinyl ester structural unit remaining after solvolysis, or other copolymerization described later.
- the vinyl alcohol content in the EVOH resin (B) is usually from 10 to 79.9 mol%, preferably from 35 to 74.5 mol%, particularly preferably from 52 to 69 mol%.
- the paste viscosity of the modified EVE resin (B ′) was measured with a B-type viscometer (rotor No. 2, rotation speed: 10 rpm, paste temperature: 65 ° C.) with a methanol solvent.
- the measured value is usually 10 1 to 10 5 mPa ⁇ s, preferably 10 2 to 10 4 mPa ⁇ s, particularly preferably 10 2 to 10 3 mPa ⁇ s.
- the paste viscosity at a constant resin content of the EVE resin (B ′) is a numerical value corresponding to the degree of polymerization of the EVE resin (B ′). When the degree of polymerization of the EVE resin (B ′) is high, the viscosity tends to increase, and when it is low, the viscosity tends to decrease.
- the EVOH resin (A) and the modified EVOH resin (B) are, for example, 10% by mol or less of a structural unit derived from a copolymerizable ethylenically unsaturated monomer within a range that does not impair the effects of the present invention. You may have.
- the EVE resin (A ′) and the modified EVE resin (B ′) are also within the range not inhibiting the effects of the present invention, for example, 10 mol of a structural unit derived from a copolymerizable ethylenically unsaturated monomer, for example.
- Examples of such monomers include olefins such as propylene, 1-butene and isobutene, and unsaturated acids such as acrylic acid, methacrylic acid, crotonic acid, (anhydrous) phthalic acid, (anhydrous) maleic acid, and (anhydrous) itaconic acid.
- olefins such as propylene, 1-butene and isobutene
- unsaturated acids such as acrylic acid, methacrylic acid, crotonic acid, (anhydrous) phthalic acid, (anhydrous) maleic acid, and (anhydrous) itaconic acid.
- acrylamides such as acid salts or quaternary salts thereof, methacrylamide, N-alkylmethacrylamide having 1 to 18 carbon atoms, N, N-dimethylmethacrylamide, 2-methacrylamide ⁇ BR> V lopansulfonic acid or a salt thereof , Methacrylamidopropyl Methacrylamides such as methylamine or its acid salts or quaternary salts thereof, N-vinylamides such as N-vinylpyrrolidone, N-vinylformamide, N-vinylacetamide, vinyl cyanides such as acrylonitrile and methacrylonitrile, Vinyl
- Vinyl silanes such as allyl acetate and allyl chloride, allyl alcohols such as dimethoxyallyl alcohol, trimethyl- (3-acrylamido-3-dimethylpropyl) -ammonium Rorido, and the like acrylamido-2-methylpropanesulfonic acid.
- the EVOH resin composition obtained by the production method of the present invention may be subjected to post-modification reaction such as urethanization, acetalization, cyanoethylation, oxyalkylenation and the like within the range not impairing the gist of the present invention.
- the ethylene content of the modified EVOH resin (B) (that is, the modified EVE resin (B ′)) is set to be equal to or higher than the ethylene content of the EVOH resin (A) (that is, the EVE resin (A ′)).
- the blending weight of the resin (B ′) is not more than the same amount as that of the EVE resin (A ′)
- the solvolysis of the EVE resin (A ′) and the modified EVE resin (B ′) in the same system results in a melting peak.
- An EVOH resin composition that appropriately acts on the adjustment of the value and automatically has a particularly small melting peak difference is obtained. Therefore, the production method of the present invention simplifies the production process, and an EVOH resin composition having a particularly small melting peak difference is automatically obtained.
- the ethylene content of the modified EVE resin (B ′) is equal to or higher than the ethylene content of the EVE resin (A ′), and the blending weight of the modified EVE resin (B ′) is the same as that of the EVE resin (A ′).
- the following features are the biggest features.
- the ethylene content ratio (B ′) / (A ′) of the modified EVE resin (B ′) and the EVE resin (A ′) is 1 or more, preferably 1 to 20, more preferably 1 to 10, particularly Preferably, it is more than 1 to 5.
- this ratio is too large, the barrier property tends to decrease, and when it is too small, the melt moldability of the EVOH resin composition tends to be insufficient.
- the difference (B ′) ⁇ (A ′) in the ethylene content is usually 0.1 to 40 mol%, preferably 0.1 to 30 mol%, more preferably 1 to 10 mol%, particularly preferably 2 ⁇ 5 mol%. If this difference is too large, the compatibility tends to be poor. When the difference is within the above range, there is a tendency that the meltability and barrier properties of the EVOH resin composition can be highly compatible while maintaining compatibility.
- the blending ratio of the EVE resin (A ′) and the modified EVE resin (B ′) is 50/50 to 1/99 (weight ratio), preferably 25, in the weight ratio of (B ′) / (A ′). / 75 to 1/99 (weight ratio), particularly preferably 20/80 to 1/99 (weight ratio).
- this ratio is too large, the barrier property after solvolysis tends to decrease, and when it is too small, the melt moldability of the EVOH resin composition obtained after solvolysis tends to be insufficient.
- the viscosity measured with a B-type viscometer (rotor No. 2, rotation speed 10 rpm, paste concentration 40 wt%, methanol solvent, paste temperature 65 ° C.) of EVE resin (A ′) and modified EVE resin (B ′)
- the difference is usually from 0 to 10 5 mPa ⁇ s, preferably from 0 to 10 4 mPa ⁇ s, particularly preferably from 0 to 2000 mPa ⁇ s, from the viewpoint of the compatibility of both resins.
- the smaller the viscosity difference the better the mixing efficiency and the better the compatibility.
- the solvolysis is performed using an alkali catalyst or an acid catalyst in a state where the EVE resin (A ′) and the modified EVE resin (B ′) are dissolved in an alcohol or a water / alcohol mixed solvent.
- the alcohol usually include aliphatic alcohols having 1 to 4 carbon atoms, preferably methanol, ethanol, propanol, and tert-butanol, and particularly preferably methanol from the economical viewpoint.
- the weight ratio is usually 10/90 to 90/10, preferably 20/80 to 80/20, particularly preferably 40/60 to 60/40.
- the concentrations of the EVE resin (A ′) solution and the modified EVE resin (B ′) solution are appropriately selected depending on the viscosity of the system. Usually, it is 10 to 60% by weight (resin content), preferably 25 to 50% by weight (resin content).
- the viscosity can be adjusted by the resin content. When the resin content is large, the viscosity tends to increase, and when it is small, the viscosity tends to decrease.
- a mixed solution of the EVE resin (A ′) and the modified EVE resin (B ′) (1) a method in which both resins are dry blended and dissolved in a common solvent, (2) each resin is dissolved in the solvent. , A method of mixing each resin solution, (3) a method of dissolving one resin in a solvent, adding the other resin to the solution, dissolving and mixing, (4) mixing both resins in a molten state Then, a method of dissolving in a solvent can be mentioned.
- the method (2) is preferable from the viewpoint of productivity, and it is particularly preferable to use the solution after polymerization of each resin as it is.
- Examples of the catalyst used for the solvolysis include an alkali catalyst and an acid catalyst.
- specific examples of the alkali catalyst include alkali metal hydroxides such as sodium hydroxide and potassium hydroxide, alkali metal alkoxides such as sodium methylate, sodium ethylate, potassium methylate and lithium methylate.
- the acid catalyst include inorganic acids such as sulfuric acid, hydrochloric acid, and nitric acid, organic acids such as metasulfonic acid, zeolite, and cation exchange resin. From the viewpoint of handleability and industrial productivity, an alkali catalyst is preferable, and an alkali metal hydroxide is particularly preferable.
- the solvolysis of the present invention is preferably performed under basic conditions, in other words, saponification.
- the amount of the solvolysis catalyst used is appropriately selected depending on the solvolysis method, the target solvolysis degree, and the like. 001-100 millimolar equivalent is suitable.
- the degree of solvolysis is a value measured based on JIS K6726 (however, the EVOH resin composition is a solution uniformly dissolved in water / methanol solvent). In particular, when the solvolysis is saponification, it is called the degree of saponification.
- solvolysis method batch solvolysis, continuous solvolysis on the belt, and continuous solvolysis of the tower type are possible depending on the target degree of solvolysis.
- column type solvolysis under constant pressure is used because the amount of the alkali catalyst can be reduced and the solvolysis reaction easily proceeds with high efficiency.
- the pressure during solvolysis cannot be generally stated depending on the target ethylene content, but is usually selected from the range of 2 to 7 kg / cm 2 , and the temperature at this time is usually 60 to 140 ° C.
- the reaction is carried out for 0.5 to 6.0 hours.
- the methanol solution of the EVOH resin composition solvolyzed as described above is subjected to solid-liquid separation by a known method, for example, a centrifuge or a method of extruding into a coagulating liquid.
- a drying method a known method can be adopted, and fluidized drying performed while being mechanically or stirred and dispersed by hot air, or stationary drying performed without being subjected to dynamic actions such as stirring and dispersion.
- dryers for fluidized drying include cylindrical / grooved agitator dryers, circular tube dryers, rotary dryers, fluidized bed dryers, vibrating fluidized bed dryers, conical rotary dryers, etc.
- a dryer for performing stationary drying a batch box dryer is used as a stationary material type, and a band dryer, tunnel dryer, vertical dryer, etc. are used as a material transfer type. However, it is not limited to these. It is also possible to combine fluidized drying and stationary drying.
- the heating gas used in the drying process is usually air or an inert gas (nitrogen gas, helium gas, argon gas, etc.), and the temperature of the heating gas is not particularly limited and is 40 to 150 ° C. From the viewpoint of the property and prevention of thermal deterioration of the EVOH resin composition.
- the drying treatment time depends on the water content of the EVOH resin composition and its treatment amount, but usually about 15 minutes to 72 hours is preferable from the viewpoint of productivity and prevention of thermal deterioration.
- the water content of the EVOH resin composition after the drying treatment is usually 0.001 to 5% by weight, preferably 0.01 to 2% by weight, particularly preferably 0.00. 1 to 1% by weight.
- the average ethylene content of the EVOH resin composition obtained by the production method of the present invention is a value measured based on ISO 14663, and is usually 20 to 60 mol%, preferably 25 to 50 mol%, particularly preferably 28 to 45 mol. Mol%.
- the average ethylene content is too low, the melt moldability of the EVOH resin composition tends to decrease.
- the average solvolysis degree of the EVOH resin composition obtained by the production method of the present invention is a value measured based on JIS K6726 (however, the EVOH resin composition is a solution uniformly dissolved in water / methanol solvent). In general, it is 90 to 100 mol%, preferably 95 to 100 mol%, particularly preferably 99 to 100 mol%. When the degree of solvolysis is too low, the gas barrier property tends to decrease.
- the value measured based on the method described in JP-A No. 2004-359965 using -NMR is usually 0.1 to 15 mol%, preferably 0.1 to 15 mol%, particularly preferably 0.1 to 15 mol%. 10 mol%, particularly preferably 0.5 to 8 mol%.
- the MFR of the EVOH resin composition obtained by the production method of the present invention is a value measured at 210 ° C. under a load of 2160 g, usually 1 to 120 g / 10 minutes, preferably 1 to 45 g / 10 minutes, particularly preferably. Is 3 to 25 g / 10 min.
- the ratio of the EVOH resin (A) and the modified EVOH resin (B) in the EVOH resin composition obtained by the production method of the present invention is the same as the ratio of the above-described EVE resin (A ′) and modified EVE resin (B ′).
- the blending ratio (A) / (B) and blending ratio (B ′) / (A ′) are usually 50/50 to 1/99 (weight ratio), preferably 25/75 to 1/99 ( (Weight ratio), particularly preferably 20/80 to 1/99 (weight ratio).
- the melting peak value of the resin composition obtained by the production method of the present invention is obtained by measuring a second run in which the melting main peak is measured at a heating rate of 5 ° C./min with a differential scanning calorimeter (DSC). .
- DSC differential scanning calorimeter
- the temperature difference between the highest melting peak and the lowest melting peak is usually 20. C. or lower, preferably 10 ° C. or lower, particularly preferably 0 degrees (that is, only having a single melting peak).
- this value is too large, the compatibility of the EVOH resin (A) and the modified EVOH resin (B) tends to be poor.
- the EVOH resin composition obtained by the production method of the present invention can be blended with a known compounding agent within a range that does not impair the object of the present invention (for example, 10% by weight or less based on the EVOH resin content).
- a known compounding agent include, for example, acids such as acetic acid, phosphoric acid and boric acid as heat stabilizers and water-soluble metal salts thereof such as alkali metals, alkaline earth metals and transition metals; saturated aliphatic amides (such as stearin) as lubricants.
- Acid amides unsaturated fatty acid amides (for example, oleic acid amides), bis fatty acid amides (for example, ethylene bisstearic acid amides), low molecular weight polyolefins (for example, low molecular weight polyethylene having a molecular weight of about 500 to 10,000, or low molecular weight Polypropylene, etc.); aliphatic polyhydric alcohols such as ethylene glycol, glycerin, hexanediol, etc.
- plasticizers as plasticizers; light stabilizers; antioxidants; desiccants; ultraviolet absorbers; coloring agents; antistatic agents; Antibacterial agent; antiblocking agent; insoluble inorganic salt (eg hydrotalcite); filler (eg inorganic Error, etc.); oxygen absorber; EVOH than other resins (e.g. polyolefin, polyamide, etc.) and the like.
- the EVOH resin composition obtained by mixing the EVOH resin (A) and the modified EVOH resin (B) having the structural unit represented by the general formula (1) is a resin before solvolysis.
- the EVE resin (A ′) and the modified EVE resin (B ′) are obtained by a solvolysis method in the same system, the ethylene content of the modified EVE resin (B ′) is changed to the ethylene content of the EVE resin (A ′).
- An EVOH resin composition having a particularly small difference in melting peak can be obtained by blending the modified EVE resin (B ′) with the same amount or less as the EVE resin (A ′).
- each EVE resin was measured according to ISO14663.
- the viscosity of each EVE solution was measured with a B-type viscometer (rotor No. 2, rotation speed 10 rpm, paste temperature 65 ° C.).
- the average degree of solvolysis of each EVOH resin was measured according to JIS K6726 (however, the EVOH resin was a solution uniformly dissolved in water / methanol solvent).
- the content of the side chain 1,2-diol structural unit of the modified EVOH resin (B ′) was measured by the method described in JP-A No. 2004-359965 using 1 H-NMR.
- the melting peak was a second run obtained by measuring the melting peak with a differential scanning calorimeter (DSC) at a heating rate of 5 ° C./min.
- MFR of the obtained EVOH resin composition was measured at 210 ° C. under a load of 2160 g.
- Example 1 As the EVE resin (A ′), an ethylene-vinyl acetate copolymer resin (A1 ′) having an ethylene content of 32 mol% and a resin content of 50% in a methanol solution having a viscosity of 7700 mPa ⁇ s was used, and a modified EVE resin (B ′ ) Modified ethylene-vinyl acetate having a structure unit content of 3,4-di-acetoxy-1-butene of 3 mol%, an ethylene content of 35 mol% and a resin content of 46% in a methanol solution of 4200 mPa ⁇ s. Copolymer resin (B1 ′) was used.
- EVOH resin composition solution of a vinyl acetate copolymer saponified resin (B) was obtained.
- the obtained EVOH resin composition solution was immersed in a coagulation liquid to be deposited, and dried at 120 ° C. for 16 hours to obtain a solid EVOH resin composition.
- the obtained EVOH resin composition had an average degree of solvolysis of 99.8 mol%, MFR of 17.1 g / 10 min, and an average content (mol%) of structural unit (1a) of 1.5 mol%. there were.
- the melting peak of the EVOH resin composition was measured, a single peak was confirmed at 180 ° C.
- FIG. 1 shows the melting peak of the EVOH resin composition.
- Example 2 154 parts by weight of a methanol solution (resin content: 50% by weight) of EVE resin (A1 ′) and 70 parts by weight of a methanol solution (resin content: 46% by weight) of modified EVE resin (B1 ′) were mixed ((B ′) / Solid EVOH in the same manner as in Example 2 except that the weight ratio of (A ′) is 33/77 and the weight ratio of the resin after solvolysis (B) / (A) is 30/70).
- a resin composition was obtained.
- the obtained EVOH resin composition has an average degree of solvolysis of 99.8 mol%, MFR of 15.2 g / 10 min, and the structural unit (1a) average content (mol%) is 1.0 mol%. there were.
- the melting peak of the EVOH resin composition was measured, a single peak was confirmed at 183 ° C.
- FIG. 2 shows the melting peak of the EVOH resin composition.
- Comparative Example 1 22 parts by weight of a methanol solution of EVE resin (A1 ′) (resin content: 50% by weight) and 214 parts by weight of a methanol solution (resin content of 46% by weight) of modified EVE resin (B1 ′) were mixed ((B ′) / (A ′) is 90/10 and the weight ratio of resin after solvolysis is (B) / (A) is 90/10).
- EVOH resin composition was obtained.
- the average degree of solvolysis of the obtained EVOH resin composition was 99.8 mol%
- the MFR was 20.9 g / 10 min
- the average content (mol%) of the structural unit (1a) was 2.7 mol. %Met.
- FIG. 3 shows the melting peak of the EVOH resin composition.
- Table 1 shows conditions and results in Examples and Comparative Examples.
- the melting point peak of the ethylene-vinyl acetate copolymer saponified resin having an ethylene content of 32 mol% and a degree of solvolysis of 99.8 mol% produced by a solvolysis method alone was 183 ° C.
- the melting point peak of the saponified ethylene-vinyl acetate copolymer resin having a content of 38 mol% and a solvolysis degree of 99.8 mol% was 173 ° C.
- the melting point peak of the modified ethylene-vinyl acetate copolymer saponified resin having a structural unit (1a) content of 3 mol%, an ethylene content of 35 mol%, and a solvolysis degree of 99.8 mol% was 150 ° C. .
- the EVE resin (A ′) and the modified EVE resin (B ′) are solvolyzed in the same system, and the ethylene content of the modified EVE resin (B ′) is greater than or equal to the ethylene content of the EVE resin (A ′).
- Comparative Example 1 performed under the condition that the blending weight of the modified EVE resin (B ′) is 9 times the blending amount of the EVE resin (A ′), 139 ° C. (corresponding to the modified EVOH resin (B)) and 168 A melting peak at 0 ° C. (corresponding to EVOH resin (A)) was confirmed, and the difference was 29 ° C.
- Example 1 and Example 2 performed by the production method of the present invention in which the blending weight ratio of the modified EVE resin (B ′) is higher than the content and equal to or less than that of the EVE resin (A ′), An EVOH resin composition having a melting peak difference of 0 ° C. (ie, having only one melting peak) was obtained.
- the ethylene content of the modified EVE resin-modified EVE resin (B ′) is higher than the ethylene content of the EVE resin (A ′). Since the blending weight ratio is equal to or less than the same amount of the EVE resin (A ′), the difference in melting peak is reduced. Since the EVOH resin composition obtained by the production method of the present invention has a small difference in melting peak, the meltability is good during melt molding, the compatibility is improved, and the solidification when solidified from the molten state is uniform. It can be seen that this is an EVOH resin composition that hardly undergoes phase separation. Therefore, when various molded articles are molded, the appearance of the molded article and the physical properties of EVOH can be favorably expressed.
- the present invention relates to an EVOH resin composition
- an EVOH resin composition comprising a modified EVOH resin containing the structural unit represented by the general formula (1) and a different EVOH resin, and an EVOH resin composition having a particularly small melting peak difference in a simple process.
- the EVOH resin composition excellent in productivity and melt moldability of the EVOH resin composition can be provided.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Compositions Of Macromolecular Compounds (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP10750767.5A EP2407491B8 (en) | 2009-03-09 | 2010-03-05 | Process for manufacturing composition of solvolysis product of ethylene-vinyl ester copolymer |
| US13/188,973 US8283416B2 (en) | 2009-03-09 | 2011-07-22 | Process for manufacturing composition of solvolysis product of ethylene-vinyl ester copolymer |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2009054669 | 2009-03-09 | ||
| JP2009-054669 | 2009-03-09 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/188,973 Continuation US8283416B2 (en) | 2009-03-09 | 2011-07-22 | Process for manufacturing composition of solvolysis product of ethylene-vinyl ester copolymer |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2010104013A1 true WO2010104013A1 (ja) | 2010-09-16 |
Family
ID=42728303
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2010/053690 Ceased WO2010104013A1 (ja) | 2009-03-09 | 2010-03-05 | エチレン-ビニルエステル共重合体加溶媒分解物組成物の製造方法 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US8283416B2 (OSRAM) |
| EP (1) | EP2407491B8 (OSRAM) |
| JP (1) | JP4750892B2 (OSRAM) |
| TW (1) | TWI449570B (OSRAM) |
| WO (1) | WO2010104013A1 (OSRAM) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8283416B2 (en) | 2009-03-09 | 2012-10-09 | The Nippon Synthetic Chemical Industry Co., Ltd. | Process for manufacturing composition of solvolysis product of ethylene-vinyl ester copolymer |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2228404A4 (en) * | 2007-12-27 | 2011-08-03 | Nippon Synthetic Chem Ind | METHOD OF PREPARING AN EVOH RESIN COMPOSITION |
| ES2384550T3 (es) * | 2009-04-01 | 2012-07-06 | Kuraray Co., Ltd. | Composición de resina y estructura multicapa que la utiliza |
| TWI593714B (zh) | 2009-10-07 | 2017-08-01 | 日本合成化學工業股份有限公司 | 乙烯-乙烯醇共聚物之製造方法 |
| WO2013146262A1 (ja) * | 2012-03-29 | 2013-10-03 | 東海ゴム工業株式会社 | 導電性組成物および導電膜 |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001018336A (ja) * | 1999-07-05 | 2001-01-23 | Kuraray Co Ltd | 紙基材層を有する多層構造体 |
| JP2004359965A (ja) | 2004-06-10 | 2004-12-24 | Nippon Synthetic Chem Ind Co Ltd:The | エチレン−ビニルアルコール共重合体およびその組成物、その用途および製造方法 |
| JP2006124668A (ja) | 2004-09-28 | 2006-05-18 | Nippon Synthetic Chem Ind Co Ltd:The | エチレン−ビニルアルコール共重合体組成物およびそれを用いた多層構造体 |
| US20070178268A1 (en) | 2004-06-10 | 2007-08-02 | The Nippon Synthetic Chemical Industry Co., Ltd | Ethylene-vinyl alcohol copolymer and molded article thereof |
| US20070196679A1 (en) | 2004-09-28 | 2007-08-23 | Takamasa Moriyama | Ethylene-Vinyl Alcohol Copolymer Composition and Multilayer Structure Using the Same |
| JP2009013368A (ja) * | 2007-07-09 | 2009-01-22 | Nippon Synthetic Chem Ind Co Ltd:The | 光学用ポリビニルアルコール系フィルム、偏光膜、及び偏光板 |
| JP2009024076A (ja) * | 2007-07-19 | 2009-02-05 | Nippon Synthetic Chem Ind Co Ltd:The | 光学用ポリビニルアルコール系フィルム、偏光膜、及び偏光板 |
| JP2009054669A (ja) | 2007-08-24 | 2009-03-12 | Toray Ind Inc | 金属化コンデンサフィルムの製造方法及び金属化フィルム |
| JP2009173903A (ja) * | 2007-12-27 | 2009-08-06 | Nippon Synthetic Chem Ind Co Ltd:The | Evoh樹脂組成物の製造方法 |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5820976B2 (ja) | 1979-12-17 | 1983-04-26 | 日本合成化学工業株式会社 | 溶融混練樹脂組成物 |
| JPS614752A (ja) | 1984-06-19 | 1986-01-10 | Kuraray Co Ltd | 延伸成形性に優れた気体遮断性成形材料 |
| DK730288A (da) * | 1988-01-15 | 1989-07-16 | Kuraray Co | Harpikskompositioner og flerlagede strukturer indeholdende disse |
| US5221566A (en) | 1991-03-29 | 1993-06-22 | Kuraray Co., Ltd. | Multilayered container and package utilizing the same |
| JP5334567B2 (ja) | 2008-01-15 | 2013-11-06 | 日本合成化学工業株式会社 | 樹脂組成物および積層体 |
| JP2009224076A (ja) * | 2008-03-13 | 2009-10-01 | Kuniaki Takahashi | サイドエッジ型バックライト装置の補助導光板及びサイドエッジ型バックライト装置 |
| CN102007179B (zh) | 2008-04-14 | 2012-10-10 | 日本合成化学工业株式会社 | 树脂组合物和使用其的多层结构体 |
| EP2301998B1 (en) | 2008-07-02 | 2014-11-26 | The Nippon Synthetic Chemical Industry Co., Ltd. | Process for producing evoh composite material |
| CN102216392B (zh) | 2008-08-08 | 2014-12-10 | 日本合成化学工业株式会社 | 树脂组合物、熔融成形物、多层结构体及制造树脂组合物的方法 |
| WO2010104013A1 (ja) | 2009-03-09 | 2010-09-16 | 日本合成化学工業株式会社 | エチレン-ビニルエステル共重合体加溶媒分解物組成物の製造方法 |
-
2010
- 2010-03-05 WO PCT/JP2010/053690 patent/WO2010104013A1/ja not_active Ceased
- 2010-03-05 EP EP10750767.5A patent/EP2407491B8/en active Active
- 2010-03-09 JP JP2010051422A patent/JP4750892B2/ja active Active
- 2010-03-09 TW TW099106728A patent/TWI449570B/zh active
-
2011
- 2011-07-22 US US13/188,973 patent/US8283416B2/en active Active
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001018336A (ja) * | 1999-07-05 | 2001-01-23 | Kuraray Co Ltd | 紙基材層を有する多層構造体 |
| JP2004359965A (ja) | 2004-06-10 | 2004-12-24 | Nippon Synthetic Chem Ind Co Ltd:The | エチレン−ビニルアルコール共重合体およびその組成物、その用途および製造方法 |
| US20070178268A1 (en) | 2004-06-10 | 2007-08-02 | The Nippon Synthetic Chemical Industry Co., Ltd | Ethylene-vinyl alcohol copolymer and molded article thereof |
| JP2006124668A (ja) | 2004-09-28 | 2006-05-18 | Nippon Synthetic Chem Ind Co Ltd:The | エチレン−ビニルアルコール共重合体組成物およびそれを用いた多層構造体 |
| US20070196679A1 (en) | 2004-09-28 | 2007-08-23 | Takamasa Moriyama | Ethylene-Vinyl Alcohol Copolymer Composition and Multilayer Structure Using the Same |
| JP2009013368A (ja) * | 2007-07-09 | 2009-01-22 | Nippon Synthetic Chem Ind Co Ltd:The | 光学用ポリビニルアルコール系フィルム、偏光膜、及び偏光板 |
| JP2009024076A (ja) * | 2007-07-19 | 2009-02-05 | Nippon Synthetic Chem Ind Co Ltd:The | 光学用ポリビニルアルコール系フィルム、偏光膜、及び偏光板 |
| JP2009054669A (ja) | 2007-08-24 | 2009-03-12 | Toray Ind Inc | 金属化コンデンサフィルムの製造方法及び金属化フィルム |
| JP2009173903A (ja) * | 2007-12-27 | 2009-08-06 | Nippon Synthetic Chem Ind Co Ltd:The | Evoh樹脂組成物の製造方法 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP2407491A4 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8283416B2 (en) | 2009-03-09 | 2012-10-09 | The Nippon Synthetic Chemical Industry Co., Ltd. | Process for manufacturing composition of solvolysis product of ethylene-vinyl ester copolymer |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2407491A1 (en) | 2012-01-18 |
| TWI449570B (zh) | 2014-08-21 |
| JP2010235932A (ja) | 2010-10-21 |
| JP4750892B2 (ja) | 2011-08-17 |
| EP2407491A4 (en) | 2012-08-15 |
| US8283416B2 (en) | 2012-10-09 |
| EP2407491B8 (en) | 2013-08-21 |
| TW201036695A (en) | 2010-10-16 |
| US20120022217A1 (en) | 2012-01-26 |
| EP2407491B1 (en) | 2013-06-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10414836B2 (en) | Modified polyvinyl alcohol, resin composition, and film | |
| TWI711639B (zh) | 乙烯-乙烯酯系共聚物皂化物丸粒及其製造方法 | |
| JP4217199B2 (ja) | 溶融成形用ポリビニルアルコール系樹脂及びその製造方法、並びにその用途 | |
| JP4750892B2 (ja) | エチレン−ビニルエステル共重合体加溶媒分解物組成物の製造方法 | |
| JP6973084B2 (ja) | エチレン−ビニルアルコール系共重合体ペレット、樹脂組成物および多層構造体 | |
| US20190233600A1 (en) | Resin composition and film | |
| JP6790837B2 (ja) | エチレン−ビニルアルコール系共重合体及び該エチレン−ビニルアルコール系共重合体の製造方法 | |
| JP6274843B2 (ja) | 樹脂組成物及びその樹脂組成物から形成されるフィルム | |
| WO2021210555A1 (ja) | 変性ビニルアルコール系重合体 | |
| JP6770686B2 (ja) | 樹脂組成物及びその製造方法 | |
| JP5590793B2 (ja) | Evoh樹脂組成物の製造方法 | |
| JP6984497B2 (ja) | エチレン−ビニルアルコール系共重合体樹脂組成物および溶融成形用材料ならびに多層構造体 | |
| WO2019103073A1 (ja) | エチレン-ビニルアルコール系共重合体組成物、溶融成形用材料、多層構造体および熱成形容器用材料 | |
| TWI802532B (zh) | 乙烯-乙烯酯系共聚物皂化物組成物及其製造方法 | |
| JP6203038B2 (ja) | 成形品及びその製造方法 | |
| WO2022034876A1 (ja) | ポリビニルアルコール系樹脂組成物及び当該樹脂組成物を用いた溶融成形体 | |
| JP2017082172A (ja) | 成形品 | |
| JP7764702B2 (ja) | 樹脂組成物 | |
| TW202402842A (zh) | 改性乙烯-乙烯醇系共聚物組成物、丸粒、多層結構體、改性乙烯-乙烯醇系共聚物組成物之製造方法及多層結構體之製造方法 | |
| CN119751727A (zh) | 一种乙烯-乙烯醇共聚物树脂组合物及其制备方法和应用 | |
| JP6253389B2 (ja) | 成形品及びその製造方法 | |
| JP2024112308A (ja) | 樹脂組成物、成形品、多層構造体、及び樹脂組成物の製造方法 | |
| JP2016117796A (ja) | エチレン−ビニルエステル系共重合体ケン化物組成物、及びそれを用いた成形物の製造方法 | |
| JP2006124671A (ja) | ラミネート用原反フィルム | |
| JP2002212371A (ja) | ポリビニルアルコール系樹脂組成物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10750767 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2010750767 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |