KR20180005263A - 부타디엔을 생합성하기 위한 미생물 및 방법 - Google Patents
부타디엔을 생합성하기 위한 미생물 및 방법 Download PDFInfo
- Publication number
- KR20180005263A KR20180005263A KR1020177037394A KR20177037394A KR20180005263A KR 20180005263 A KR20180005263 A KR 20180005263A KR 1020177037394 A KR1020177037394 A KR 1020177037394A KR 20177037394 A KR20177037394 A KR 20177037394A KR 20180005263 A KR20180005263 A KR 20180005263A
- Authority
- KR
- South Korea
- Prior art keywords
- coa
- butadiene
- reductase
- kinase
- crotyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- KAKZBPTYRLMSJV-UHFFFAOYSA-N Butadiene Chemical compound C=CC=C KAKZBPTYRLMSJV-UHFFFAOYSA-N 0.000 title claims abstract description 1119
- 238000000034 method Methods 0.000 title claims abstract description 238
- 244000005700 microbiome Species 0.000 title claims description 195
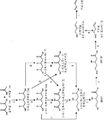
- 230000037361 pathway Effects 0.000 claims abstract description 292
- 230000000813 microbial effect Effects 0.000 claims abstract description 113
- 102000004316 Oxidoreductases Human genes 0.000 claims description 248
- 108090000854 Oxidoreductases Proteins 0.000 claims description 248
- 102000004190 Enzymes Human genes 0.000 claims description 196
- 108090000790 Enzymes Proteins 0.000 claims description 196
- 150000007523 nucleic acids Chemical class 0.000 claims description 148
- 102000039446 nucleic acids Human genes 0.000 claims description 140
- 108020004707 nucleic acids Proteins 0.000 claims description 140
- 102000001253 Protein Kinase Human genes 0.000 claims description 138
- 230000015572 biosynthetic process Effects 0.000 claims description 138
- 108060006633 protein kinase Proteins 0.000 claims description 137
- WCASXYBKJHWFMY-NSCUHMNNSA-N 2-Buten-1-ol Chemical compound C\C=C\CO WCASXYBKJHWFMY-NSCUHMNNSA-N 0.000 claims description 110
- WCASXYBKJHWFMY-UHFFFAOYSA-N gamma-methylallyl alcohol Natural products CC=CCO WCASXYBKJHWFMY-UHFFFAOYSA-N 0.000 claims description 110
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 105
- ZSLZBFCDCINBPY-ZSJPKINUSA-N acetyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 ZSLZBFCDCINBPY-ZSJPKINUSA-N 0.000 claims description 93
- 239000001177 diphosphate Substances 0.000 claims description 91
- 229910019142 PO4 Inorganic materials 0.000 claims description 74
- 239000010452 phosphate Substances 0.000 claims description 74
- MLUCVPSAIODCQM-NSCUHMNNSA-N crotonaldehyde Chemical compound C\C=C\C=O MLUCVPSAIODCQM-NSCUHMNNSA-N 0.000 claims description 72
- MLUCVPSAIODCQM-UHFFFAOYSA-N crotonaldehyde Natural products CC=CC=O MLUCVPSAIODCQM-UHFFFAOYSA-N 0.000 claims description 72
- 108091000080 Phosphotransferase Proteins 0.000 claims description 62
- 102000020233 phosphotransferase Human genes 0.000 claims description 62
- 150000001299 aldehydes Chemical class 0.000 claims description 58
- -1 glutaryl Chemical group 0.000 claims description 53
- 238000004519 manufacturing process Methods 0.000 claims description 48
- 102000004357 Transferases Human genes 0.000 claims description 47
- 108090000992 Transferases Proteins 0.000 claims description 47
- 102100026105 3-ketoacyl-CoA thiolase, mitochondrial Human genes 0.000 claims description 46
- 108010003902 Acetyl-CoA C-acyltransferase Proteins 0.000 claims description 46
- 239000004386 Erythritol Substances 0.000 claims description 42
- 229940009714 erythritol Drugs 0.000 claims description 42
- 102000004157 Hydrolases Human genes 0.000 claims description 37
- 108090000604 Hydrolases Proteins 0.000 claims description 37
- 108030005660 3-hydroxybutyryl-CoA dehydratases Proteins 0.000 claims description 34
- 125000004369 butenyl group Chemical group C(=CCC)* 0.000 claims description 33
- OYCNZOVOKFUQPU-CKRMAKSASA-N 5-[2-[3-[[(2r)-4-[[[(2r,3s,4r,5r)-5-(6-aminopurin-9-yl)-4-hydroxy-3-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]oxy-2-hydroxy-3,3-dimethylbutanoyl]amino]propanoylamino]ethylsulfanyl]-3,5-dioxopentanoic acid Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CC(=O)CC(O)=O)O[C@H]1N1C2=NC=NC(N)=C2N=C1 OYCNZOVOKFUQPU-CKRMAKSASA-N 0.000 claims description 32
- 102000003960 Ligases Human genes 0.000 claims description 32
- 108090000364 Ligases Proteins 0.000 claims description 32
- 102000004195 Isomerases Human genes 0.000 claims description 31
- 108090000769 Isomerases Proteins 0.000 claims description 31
- UHDGCWIWMRVCDJ-UHFFFAOYSA-N 1-beta-D-Xylofuranosyl-NH-Cytosine Natural products O=C1N=C(N)C=CN1C1C(O)C(O)C(CO)O1 UHDGCWIWMRVCDJ-UHFFFAOYSA-N 0.000 claims description 26
- UHDGCWIWMRVCDJ-PSQAKQOGSA-N Cytidine Natural products O=C1N=C(N)C=CN1[C@@H]1[C@@H](O)[C@@H](O)[C@H](CO)O1 UHDGCWIWMRVCDJ-PSQAKQOGSA-N 0.000 claims description 26
- 230000008569 process Effects 0.000 claims description 26
- 230000009467 reduction Effects 0.000 claims description 26
- 108091000039 acetoacetyl-CoA reductase Proteins 0.000 claims description 25
- UHDGCWIWMRVCDJ-ZAKLUEHWSA-N cytidine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O1 UHDGCWIWMRVCDJ-ZAKLUEHWSA-N 0.000 claims description 25
- RGPXHQLKXPEHMD-UHFFFAOYSA-N 3,5-dihydroxypentanoic acid Chemical compound OCCC(O)CC(O)=O RGPXHQLKXPEHMD-UHFFFAOYSA-N 0.000 claims description 24
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 claims description 23
- 108090000489 Carboxy-Lyases Proteins 0.000 claims description 22
- IIYZSYKTQRIPRG-MJBWAWCGSA-N 5-[2-[3-[[(2r)-4-[[[(2r,3s,4r,5r)-5-(6-aminopurin-9-yl)-4-hydroxy-3-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]oxy-2-hydroxy-3,3-dimethylbutanoyl]amino]propanoylamino]ethylsulfanyl]-3-hydroxy-5-oxopentanoic acid Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CC(O)CC(O)=O)O[C@H]1N1C2=NC=NC(N)=C2N=C1 IIYZSYKTQRIPRG-MJBWAWCGSA-N 0.000 claims description 21
- LTYOQGRJFJAKNA-KKIMTKSISA-N Malonyl CoA Natural products S(C(=O)CC(=O)O)CCNC(=O)CCNC(=O)[C@@H](O)C(CO[P@](=O)(O[P@](=O)(OC[C@H]1[C@@H](OP(=O)(O)O)[C@@H](O)[C@@H](n2c3ncnc(N)c3nc2)O1)O)O)(C)C LTYOQGRJFJAKNA-KKIMTKSISA-N 0.000 claims description 21
- LTYOQGRJFJAKNA-DVVLENMVSA-N malonyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CC(O)=O)O[C@H]1N1C2=NC=NC(N)=C2N=C1 LTYOQGRJFJAKNA-DVVLENMVSA-N 0.000 claims description 21
- 108010031139 3-aminobutyryl-CoA deaminase Proteins 0.000 claims description 20
- UNXHWFMMPAWVPI-UHFFFAOYSA-N Erythritol Natural products OCC(O)C(O)CO UNXHWFMMPAWVPI-UHFFFAOYSA-N 0.000 claims description 20
- 235000019414 erythritol Nutrition 0.000 claims description 20
- UNXHWFMMPAWVPI-ZXZARUISSA-N erythritol Chemical compound OC[C@H](O)[C@H](O)CO UNXHWFMMPAWVPI-ZXZARUISSA-N 0.000 claims description 19
- QRDCEYBRRFPBMZ-IUYQGCFVSA-N D-erythritol 4-phosphate Chemical compound OC[C@H](O)[C@H](O)COP(O)(O)=O QRDCEYBRRFPBMZ-IUYQGCFVSA-N 0.000 claims description 16
- ZHBPKXTYCQYISG-UHFFFAOYSA-N 3-hydroxy-5-oxopentanoic acid Chemical compound O=CCC(O)CC(O)=O ZHBPKXTYCQYISG-UHFFFAOYSA-N 0.000 claims description 14
- WIQCZSZRHWCGDK-UHFFFAOYSA-N 3-hydroxy-5-phosphonooxypentanoic acid Chemical compound OC(=O)CC(O)CCOP(O)(O)=O WIQCZSZRHWCGDK-UHFFFAOYSA-N 0.000 claims description 14
- DAZFQYSXXSTLKW-UHFFFAOYSA-N 3,5-dioxopentanoic acid Chemical compound OC(=O)CC(=O)CC=O DAZFQYSXXSTLKW-UHFFFAOYSA-N 0.000 claims description 13
- 108010035023 4-hydroxybutyryl-CoA dehydratase Proteins 0.000 claims description 13
- 108010068207 Glutaconyl-CoA decarboxylase Proteins 0.000 claims description 13
- 108010015451 Glutaryl-CoA Dehydrogenase Proteins 0.000 claims description 13
- 102100028603 Glutaryl-CoA dehydrogenase, mitochondrial Human genes 0.000 claims description 13
- NGHMDNPXVRFFGS-IUYQGCFVSA-N D-erythrose 4-phosphate Chemical compound O=C[C@H](O)[C@H](O)COP(O)(O)=O NGHMDNPXVRFFGS-IUYQGCFVSA-N 0.000 claims description 12
- 108010031246 erythritol kinase Proteins 0.000 claims description 11
- ANTBRXMMNBUYMS-UHFFFAOYSA-N 5-hydroxy-3-oxopentanoic acid Chemical compound OCCC(=O)CC(O)=O ANTBRXMMNBUYMS-UHFFFAOYSA-N 0.000 claims description 9
- 101710088194 Dehydrogenase Proteins 0.000 claims description 9
- 239000001963 growth medium Substances 0.000 claims description 9
- 108090001042 Hydro-Lyases Proteins 0.000 claims description 8
- 102000004867 Hydro-Lyases Human genes 0.000 claims description 8
- CRFNGMNYKDXRTN-CITAKDKDSA-N butyryl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CCC)O[C@H]1N1C2=NC=NC(N)=C2N=C1 CRFNGMNYKDXRTN-CITAKDKDSA-N 0.000 claims description 8
- 238000012258 culturing Methods 0.000 claims description 8
- SYKWLIJQEHRDNH-CKRMAKSASA-N glutaryl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CCCC(O)=O)O[C@H]1N1C2=NC=NC(N)=C2N=C1 SYKWLIJQEHRDNH-CKRMAKSASA-N 0.000 claims description 8
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 7
- 150000002576 ketones Chemical class 0.000 claims description 7
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 claims description 7
- 108010011384 acyl-CoA dehydrogenase (NADP+) Proteins 0.000 claims description 6
- 206010056474 Erythrosis Diseases 0.000 claims description 4
- 102000016912 Aldehyde Reductase Human genes 0.000 claims description 3
- 108010053754 Aldehyde reductase Proteins 0.000 claims description 3
- 150000001298 alcohols Chemical class 0.000 claims description 2
- LDHQCZJRKDOVOX-NSCUHMNNSA-M crotonate Chemical compound C\C=C\C([O-])=O LDHQCZJRKDOVOX-NSCUHMNNSA-M 0.000 claims 9
- QTXZASLUYMRUAN-QLQASOTGSA-N Acetyl coenzyme A (Acetyl-CoA) Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1.O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 QTXZASLUYMRUAN-QLQASOTGSA-N 0.000 claims 1
- 241000195493 Cryptophyta Species 0.000 claims 1
- 108090000913 Nitrate Reductases Proteins 0.000 claims 1
- 229940049920 malate Drugs 0.000 claims 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-L malate(2-) Chemical compound [O-]C(=O)C(O)CC([O-])=O BJEPYKJPYRNKOW-UHFFFAOYSA-L 0.000 claims 1
- 108090000623 proteins and genes Proteins 0.000 description 220
- 102000004169 proteins and genes Human genes 0.000 description 89
- 235000018102 proteins Nutrition 0.000 description 85
- 239000000047 product Substances 0.000 description 80
- 230000000694 effects Effects 0.000 description 59
- 238000006243 chemical reaction Methods 0.000 description 53
- 150000001875 compounds Chemical class 0.000 description 47
- 241000588724 Escherichia coli Species 0.000 description 46
- 206010057190 Respiratory tract infections Diseases 0.000 description 46
- 230000002503 metabolic effect Effects 0.000 description 40
- 230000035772 mutation Effects 0.000 description 40
- 229910052739 hydrogen Inorganic materials 0.000 description 35
- 241000894007 species Species 0.000 description 35
- 229910052698 phosphorus Inorganic materials 0.000 description 31
- 230000014509 gene expression Effects 0.000 description 30
- 108020004414 DNA Proteins 0.000 description 28
- 239000000758 substrate Substances 0.000 description 28
- 238000000855 fermentation Methods 0.000 description 26
- 230000004151 fermentation Effects 0.000 description 26
- 230000006696 biosynthetic metabolic pathway Effects 0.000 description 25
- 229910052700 potassium Inorganic materials 0.000 description 24
- 241000193403 Clostridium Species 0.000 description 23
- 239000012634 fragment Substances 0.000 description 23
- LDHQCZJRKDOVOX-NSCUHMNNSA-N crotonic acid Chemical compound C\C=C\C(O)=O LDHQCZJRKDOVOX-NSCUHMNNSA-N 0.000 description 22
- 229910052757 nitrogen Inorganic materials 0.000 description 21
- 229910052760 oxygen Inorganic materials 0.000 description 20
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 18
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 description 18
- 239000013612 plasmid Substances 0.000 description 17
- 229910052799 carbon Inorganic materials 0.000 description 16
- 210000004027 cell Anatomy 0.000 description 16
- 231100000350 mutagenesis Toxicity 0.000 description 16
- 102000004031 Carboxy-Lyases Human genes 0.000 description 15
- 239000000543 intermediate Substances 0.000 description 15
- 241000193401 Clostridium acetobutylicum Species 0.000 description 14
- 150000001413 amino acids Chemical group 0.000 description 14
- 239000013067 intermediate product Substances 0.000 description 14
- 238000013461 design Methods 0.000 description 13
- 230000006870 function Effects 0.000 description 13
- 238000002703 mutagenesis Methods 0.000 description 13
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 12
- 229910052731 fluorine Inorganic materials 0.000 description 12
- 241000894006 Bacteria Species 0.000 description 11
- 238000012239 gene modification Methods 0.000 description 11
- 230000005017 genetic modification Effects 0.000 description 11
- 235000013617 genetically modified food Nutrition 0.000 description 11
- 230000012010 growth Effects 0.000 description 11
- 238000012216 screening Methods 0.000 description 11
- 238000006467 substitution reaction Methods 0.000 description 11
- 241000589776 Pseudomonas putida Species 0.000 description 10
- 235000001014 amino acid Nutrition 0.000 description 10
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 10
- 125000004356 hydroxy functional group Chemical group O* 0.000 description 10
- 230000001939 inductive effect Effects 0.000 description 10
- 239000001301 oxygen Substances 0.000 description 10
- 229920001791 ((R)-3-Hydroxybutanoyl)(n-2) Polymers 0.000 description 9
- QHHKKMYHDBRONY-RMNRSTNRSA-N 3-hydroxybutanoyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CC(O)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 QHHKKMYHDBRONY-RMNRSTNRSA-N 0.000 description 9
- 239000002028 Biomass Substances 0.000 description 9
- OJFDKHTZOUZBOS-CITAKDKDSA-N acetoacetyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CC(=O)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 OJFDKHTZOUZBOS-CITAKDKDSA-N 0.000 description 9
- 239000002253 acid Substances 0.000 description 9
- 230000001851 biosynthetic effect Effects 0.000 description 9
- KFWWCMJSYSSPSK-PAXLJYGASA-N crotonoyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)/C=C/C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 KFWWCMJSYSSPSK-PAXLJYGASA-N 0.000 description 9
- 239000002609 medium Substances 0.000 description 9
- 230000001105 regulatory effect Effects 0.000 description 9
- 238000000926 separation method Methods 0.000 description 9
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 8
- 241000219195 Arabidopsis thaliana Species 0.000 description 8
- 102000053602 DNA Human genes 0.000 description 8
- 241000282414 Homo sapiens Species 0.000 description 8
- RRHGJUQNOFWUDK-UHFFFAOYSA-N Isoprene Chemical compound CC(=C)C=C RRHGJUQNOFWUDK-UHFFFAOYSA-N 0.000 description 8
- 238000004458 analytical method Methods 0.000 description 8
- ZTQSAGDEMFDKMZ-UHFFFAOYSA-N butyric aldehyde Natural products CCCC=O ZTQSAGDEMFDKMZ-UHFFFAOYSA-N 0.000 description 8
- 238000004364 calculation method Methods 0.000 description 8
- 150000001720 carbohydrates Chemical class 0.000 description 8
- 230000004048 modification Effects 0.000 description 8
- 238000012986 modification Methods 0.000 description 8
- 125000003729 nucleotide group Chemical group 0.000 description 8
- 238000005457 optimization Methods 0.000 description 8
- 238000003752 polymerase chain reaction Methods 0.000 description 8
- 238000004088 simulation Methods 0.000 description 8
- 238000003786 synthesis reaction Methods 0.000 description 8
- 125000004974 2-butenyl group Chemical group C(C=CC)* 0.000 description 7
- 108060002716 Exonuclease Proteins 0.000 description 7
- 102000057621 Glycerol kinases Human genes 0.000 description 7
- 108700016170 Glycerol kinases Proteins 0.000 description 7
- 108091028043 Nucleic acid sequence Proteins 0.000 description 7
- 229940024606 amino acid Drugs 0.000 description 7
- 235000014633 carbohydrates Nutrition 0.000 description 7
- 102000013165 exonuclease Human genes 0.000 description 7
- 239000007789 gas Substances 0.000 description 7
- 238000000126 in silico method Methods 0.000 description 7
- 239000002773 nucleotide Substances 0.000 description 7
- 230000035755 proliferation Effects 0.000 description 7
- CCSDHAPTHIKZLY-RMNRSTNRSA-N 3-aminobutyryl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CC(N)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 CCSDHAPTHIKZLY-RMNRSTNRSA-N 0.000 description 6
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 6
- 108020002908 Epoxide hydrolase Proteins 0.000 description 6
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 6
- 241000589540 Pseudomonas fluorescens Species 0.000 description 6
- 101100297400 Rhizobium meliloti (strain 1021) phaAB gene Proteins 0.000 description 6
- 108020004682 Single-Stranded DNA Proteins 0.000 description 6
- 238000003776 cleavage reaction Methods 0.000 description 6
- 239000013604 expression vector Substances 0.000 description 6
- 230000004907 flux Effects 0.000 description 6
- KWIUHFFTVRNATP-UHFFFAOYSA-N glycine betaine Chemical compound C[N+](C)(C)CC([O-])=O KWIUHFFTVRNATP-UHFFFAOYSA-N 0.000 description 6
- 108010075483 isoprene synthase Proteins 0.000 description 6
- 230000037353 metabolic pathway Effects 0.000 description 6
- 230000004060 metabolic process Effects 0.000 description 6
- 229910052751 metal Inorganic materials 0.000 description 6
- 239000002184 metal Substances 0.000 description 6
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 6
- 239000000203 mixture Substances 0.000 description 6
- 101150110984 phaB gene Proteins 0.000 description 6
- 229920001184 polypeptide Polymers 0.000 description 6
- 239000002243 precursor Substances 0.000 description 6
- 102000004196 processed proteins & peptides Human genes 0.000 description 6
- 108090000765 processed proteins & peptides Proteins 0.000 description 6
- 230000006798 recombination Effects 0.000 description 6
- 238000005215 recombination Methods 0.000 description 6
- 230000007017 scission Effects 0.000 description 6
- 238000012360 testing method Methods 0.000 description 6
- 230000009466 transformation Effects 0.000 description 6
- 238000011282 treatment Methods 0.000 description 6
- BAMBWCGEVIAQBF-CITAKDKDSA-N 4-hydroxybutyryl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CCCO)O[C@H]1N1C2=NC=NC(N)=C2N=C1 BAMBWCGEVIAQBF-CITAKDKDSA-N 0.000 description 5
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 5
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 5
- 102000005486 Epoxide hydrolase Human genes 0.000 description 5
- 241000233866 Fungi Species 0.000 description 5
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 5
- 108700040132 Mevalonate kinases Proteins 0.000 description 5
- 241000193998 Streptococcus pneumoniae Species 0.000 description 5
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 5
- 230000027455 binding Effects 0.000 description 5
- 239000006227 byproduct Substances 0.000 description 5
- 230000000295 complement effect Effects 0.000 description 5
- 230000008878 coupling Effects 0.000 description 5
- 238000010168 coupling process Methods 0.000 description 5
- 238000005859 coupling reaction Methods 0.000 description 5
- 230000001419 dependent effect Effects 0.000 description 5
- 239000008103 glucose Substances 0.000 description 5
- 101150003440 hibch gene Proteins 0.000 description 5
- 238000011534 incubation Methods 0.000 description 5
- 230000003834 intracellular effect Effects 0.000 description 5
- 239000007788 liquid Substances 0.000 description 5
- LTYOQGRJFJAKNA-VFLPNFFSSA-N malonyl-coa Chemical group O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)C(O)C(=O)NCCC(=O)NCCSC(=O)CC(O)=O)O[C@H]1N1C2=NC=NC(N)=C2N=C1 LTYOQGRJFJAKNA-VFLPNFFSSA-N 0.000 description 5
- 239000002357 osmotic agent Substances 0.000 description 5
- 229940031000 streptococcus pneumoniae Drugs 0.000 description 5
- URTLOTISFJPPOU-DEGQQWIJSA-N trans-4-carboxybut-2-enoyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)\C=C\CC(O)=O)O[C@H]1N1C2=NC=NC(N)=C2N=C1 URTLOTISFJPPOU-DEGQQWIJSA-N 0.000 description 5
- TZBGSHAFWLGWBO-ABLWVSNPSA-N (2s)-2-[[4-[(2-amino-4-oxo-5,6,7,8-tetrahydro-1h-pteridin-6-yl)methylamino]benzoyl]amino]-5-methoxy-5-oxopentanoic acid Chemical compound C1=CC(C(=O)N[C@@H](CCC(=O)OC)C(O)=O)=CC=C1NCC1NC(C(=O)NC(N)=N2)=C2NC1 TZBGSHAFWLGWBO-ABLWVSNPSA-N 0.000 description 4
- 108010021809 Alcohol dehydrogenase Proteins 0.000 description 4
- 102000007698 Alcohol dehydrogenase Human genes 0.000 description 4
- 241000193755 Bacillus cereus Species 0.000 description 4
- 244000063299 Bacillus subtilis Species 0.000 description 4
- 235000014469 Bacillus subtilis Nutrition 0.000 description 4
- FERIUCNNQQJTOY-UHFFFAOYSA-M Butyrate Chemical compound CCCC([O-])=O FERIUCNNQQJTOY-UHFFFAOYSA-M 0.000 description 4
- SRBFZHDQGSBBOR-IOVATXLUSA-N D-xylopyranose Chemical compound O[C@@H]1COC(O)[C@H](O)[C@H]1O SRBFZHDQGSBBOR-IOVATXLUSA-N 0.000 description 4
- 241000196324 Embryophyta Species 0.000 description 4
- 108010023922 Enoyl-CoA hydratase Proteins 0.000 description 4
- 102000011426 Enoyl-CoA hydratase Human genes 0.000 description 4
- 108010074122 Ferredoxins Proteins 0.000 description 4
- NBBJYMSMWIIQGU-UHFFFAOYSA-N Propionic aldehyde Chemical compound CCC=O NBBJYMSMWIIQGU-UHFFFAOYSA-N 0.000 description 4
- 241000589516 Pseudomonas Species 0.000 description 4
- 241000191025 Rhodobacter Species 0.000 description 4
- 241000293869 Salmonella enterica subsp. enterica serovar Typhimurium Species 0.000 description 4
- 241000191967 Staphylococcus aureus Species 0.000 description 4
- 101100280476 Streptococcus pneumoniae (strain ATCC BAA-255 / R6) fabM gene Proteins 0.000 description 4
- 241000588902 Zymomonas mobilis Species 0.000 description 4
- 101150079502 acr1 gene Proteins 0.000 description 4
- PYMYPHUHKUWMLA-UHFFFAOYSA-N arabinose Natural products OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 description 4
- SRBFZHDQGSBBOR-UHFFFAOYSA-N beta-D-Pyranose-Lyxose Natural products OC1COC(O)C(O)C1O SRBFZHDQGSBBOR-UHFFFAOYSA-N 0.000 description 4
- 229960003237 betaine Drugs 0.000 description 4
- WERYXYBDKMZEQL-UHFFFAOYSA-N butane-1,4-diol Chemical compound OCCCCO WERYXYBDKMZEQL-UHFFFAOYSA-N 0.000 description 4
- 238000004422 calculation algorithm Methods 0.000 description 4
- 108010031234 carbon monoxide dehydrogenase Proteins 0.000 description 4
- 230000004927 fusion Effects 0.000 description 4
- 230000002068 genetic effect Effects 0.000 description 4
- 108010008386 malonyl-Coa reductase Proteins 0.000 description 4
- 239000000463 material Substances 0.000 description 4
- 238000006241 metabolic reaction Methods 0.000 description 4
- 239000002207 metabolite Substances 0.000 description 4
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 4
- 102000002678 mevalonate kinase Human genes 0.000 description 4
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 4
- 230000007935 neutral effect Effects 0.000 description 4
- LFGREXWGYUGZLY-UHFFFAOYSA-N phosphoryl Chemical group [P]=O LFGREXWGYUGZLY-UHFFFAOYSA-N 0.000 description 4
- 230000037452 priming Effects 0.000 description 4
- 235000000346 sugar Nutrition 0.000 description 4
- 239000013598 vector Substances 0.000 description 4
- VYWBMMZOBUQRRU-UHFFFAOYSA-N 2-hydroxy-3-oxopentanoic acid Chemical compound CCC(=O)C(O)C(O)=O VYWBMMZOBUQRRU-UHFFFAOYSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 108010023941 Acetyl-CoA Hydrolase Proteins 0.000 description 3
- ZKHQWZAMYRWXGA-KQYNXXCUSA-N Adenosine triphosphate Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O ZKHQWZAMYRWXGA-KQYNXXCUSA-N 0.000 description 3
- 102000005369 Aldehyde Dehydrogenase Human genes 0.000 description 3
- 108020002663 Aldehyde Dehydrogenase Proteins 0.000 description 3
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Natural products CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 3
- 241000222122 Candida albicans Species 0.000 description 3
- 241000186226 Corynebacterium glutamicum Species 0.000 description 3
- 241000252867 Cupriavidus metallidurans Species 0.000 description 3
- 102000007528 DNA Polymerase III Human genes 0.000 description 3
- 108010071146 DNA Polymerase III Proteins 0.000 description 3
- 102100029588 Deoxycytidine kinase Human genes 0.000 description 3
- 108010033174 Deoxycytidine kinase Proteins 0.000 description 3
- 241000194032 Enterococcus faecalis Species 0.000 description 3
- 241000206602 Eukaryota Species 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- 108010020056 Hydrogenase Proteins 0.000 description 3
- 240000006024 Lactobacillus plantarum Species 0.000 description 3
- 235000013965 Lactobacillus plantarum Nutrition 0.000 description 3
- 241000699666 Mus <mouse, genus> Species 0.000 description 3
- 108091034117 Oligonucleotide Proteins 0.000 description 3
- 102000016387 Pancreatic elastase Human genes 0.000 description 3
- 108010067372 Pancreatic elastase Proteins 0.000 description 3
- 241000605862 Porphyromonas gingivalis Species 0.000 description 3
- 241000589517 Pseudomonas aeruginosa Species 0.000 description 3
- 241000232299 Ralstonia Species 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 229920002472 Starch Polymers 0.000 description 3
- 241000187432 Streptomyces coelicolor Species 0.000 description 3
- 102100033451 Thyroid hormone receptor beta Human genes 0.000 description 3
- 238000009825 accumulation Methods 0.000 description 3
- 230000003044 adaptive effect Effects 0.000 description 3
- 230000003321 amplification Effects 0.000 description 3
- 230000009286 beneficial effect Effects 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 229940095731 candida albicans Drugs 0.000 description 3
- 239000001569 carbon dioxide Substances 0.000 description 3
- 229910002092 carbon dioxide Inorganic materials 0.000 description 3
- 229910002091 carbon monoxide Inorganic materials 0.000 description 3
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 3
- 150000007942 carboxylates Chemical class 0.000 description 3
- 230000010261 cell growth Effects 0.000 description 3
- 210000000349 chromosome Anatomy 0.000 description 3
- 230000006378 damage Effects 0.000 description 3
- 230000007547 defect Effects 0.000 description 3
- 230000002950 deficient Effects 0.000 description 3
- 235000011180 diphosphates Nutrition 0.000 description 3
- 239000003814 drug Substances 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 229940032049 enterococcus faecalis Drugs 0.000 description 3
- 230000002255 enzymatic effect Effects 0.000 description 3
- 150000002148 esters Chemical group 0.000 description 3
- 101150041588 eutE gene Proteins 0.000 description 3
- 239000012467 final product Substances 0.000 description 3
- 238000002309 gasification Methods 0.000 description 3
- 230000009395 genetic defect Effects 0.000 description 3
- 101150040073 glpK2 gene Proteins 0.000 description 3
- 238000004128 high performance liquid chromatography Methods 0.000 description 3
- 108010071598 homoserine kinase Proteins 0.000 description 3
- 230000001976 improved effect Effects 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 230000001965 increasing effect Effects 0.000 description 3
- 238000003780 insertion Methods 0.000 description 3
- 230000037431 insertion Effects 0.000 description 3
- 229940072205 lactobacillus plantarum Drugs 0.000 description 3
- 239000003550 marker Substances 0.000 description 3
- 230000002438 mitochondrial effect Effects 0.000 description 3
- 231100000219 mutagenic Toxicity 0.000 description 3
- 230000003505 mutagenic effect Effects 0.000 description 3
- 238000003199 nucleic acid amplification method Methods 0.000 description 3
- 230000002018 overexpression Effects 0.000 description 3
- 230000004952 protein activity Effects 0.000 description 3
- 238000002708 random mutagenesis Methods 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 108091008146 restriction endonucleases Proteins 0.000 description 3
- 230000003248 secreting effect Effects 0.000 description 3
- 235000019698 starch Nutrition 0.000 description 3
- 239000008107 starch Substances 0.000 description 3
- 101150031436 sucD gene Proteins 0.000 description 3
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 3
- 230000008685 targeting Effects 0.000 description 3
- KKEYFWRCBNTPAC-UHFFFAOYSA-L terephthalate(2-) Chemical compound [O-]C(=O)C1=CC=C(C([O-])=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-L 0.000 description 3
- 150000003505 terpenes Chemical class 0.000 description 3
- 230000004102 tricarboxylic acid cycle Effects 0.000 description 3
- HGBOYTHUEUWSSQ-UHFFFAOYSA-N valeric aldehyde Natural products CCCCC=O HGBOYTHUEUWSSQ-UHFFFAOYSA-N 0.000 description 3
- KJTLQQUUPVSXIM-ZCFIWIBFSA-M (R)-mevalonate Chemical compound OCC[C@](O)(C)CC([O-])=O KJTLQQUUPVSXIM-ZCFIWIBFSA-M 0.000 description 2
- 108010012839 (deoxy)nucleoside-phosphate kinase Proteins 0.000 description 2
- 102100039358 3-hydroxyacyl-CoA dehydrogenase type-2 Human genes 0.000 description 2
- ALRHLSYJTWAHJZ-UHFFFAOYSA-M 3-hydroxypropionate Chemical compound OCCC([O-])=O ALRHLSYJTWAHJZ-UHFFFAOYSA-M 0.000 description 2
- SJZRECIVHVDYJC-UHFFFAOYSA-M 4-hydroxybutyrate Chemical compound OCCCC([O-])=O SJZRECIVHVDYJC-UHFFFAOYSA-M 0.000 description 2
- 108010093796 4-hydroxybutyrate dehydrogenase Proteins 0.000 description 2
- 125000004203 4-hydroxyphenyl group Chemical group [H]OC1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- 101150103244 ACT1 gene Proteins 0.000 description 2
- 108010049926 Acetate-CoA ligase Proteins 0.000 description 2
- 108010006229 Acetyl-CoA C-acetyltransferase Proteins 0.000 description 2
- 102100035709 Acetyl-coenzyme A synthetase, cytoplasmic Human genes 0.000 description 2
- 241000589291 Acinetobacter Species 0.000 description 2
- HGINCPLSRVDWNT-UHFFFAOYSA-N Acrolein Chemical compound C=CC=O HGINCPLSRVDWNT-UHFFFAOYSA-N 0.000 description 2
- 102100025848 Acyl-coenzyme A thioesterase 8 Human genes 0.000 description 2
- 102100032534 Adenosine kinase Human genes 0.000 description 2
- 102100034044 All-trans-retinol dehydrogenase [NAD(+)] ADH1B Human genes 0.000 description 2
- 101710193111 All-trans-retinol dehydrogenase [NAD(+)] ADH4 Proteins 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 241000193830 Bacillus <bacterium> Species 0.000 description 2
- 101100310362 Bacillus subtilis (strain 168) skfF gene Proteins 0.000 description 2
- 241001453380 Burkholderia Species 0.000 description 2
- 102100031065 Choline kinase alpha Human genes 0.000 description 2
- 241001112696 Clostridia Species 0.000 description 2
- 241000186581 Clostridium novyi Species 0.000 description 2
- RGJOEKWQDUBAIZ-IBOSZNHHSA-N CoASH Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCS)O[C@H]1N1C2=NC=NC(N)=C2N=C1 RGJOEKWQDUBAIZ-IBOSZNHHSA-N 0.000 description 2
- 108091026890 Coding region Proteins 0.000 description 2
- 108020004705 Codon Proteins 0.000 description 2
- 108700010070 Codon Usage Proteins 0.000 description 2
- 102100023044 Cytosolic acyl coenzyme A thioester hydrolase Human genes 0.000 description 2
- 101710152190 Cytosolic acyl coenzyme A thioester hydrolase Proteins 0.000 description 2
- YTBSYETUWUMLBZ-UHFFFAOYSA-N D-Erythrose Natural products OCC(O)C(O)C=O YTBSYETUWUMLBZ-UHFFFAOYSA-N 0.000 description 2
- YTBSYETUWUMLBZ-IUYQGCFVSA-N D-erythrose Chemical compound OC[C@@H](O)[C@@H](O)C=O YTBSYETUWUMLBZ-IUYQGCFVSA-N 0.000 description 2
- WQZGKKKJIJFFOK-QTVWNMPRSA-N D-mannopyranose Chemical compound OC[C@H]1OC(O)[C@@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-QTVWNMPRSA-N 0.000 description 2
- KJTLQQUUPVSXIM-UHFFFAOYSA-N DL-mevalonic acid Natural products OCCC(O)(C)CC(O)=O KJTLQQUUPVSXIM-UHFFFAOYSA-N 0.000 description 2
- 101100243777 Dictyostelium discoideum phbB gene Proteins 0.000 description 2
- 101150051269 ERG10 gene Proteins 0.000 description 2
- 101150071502 ERG12 gene Proteins 0.000 description 2
- 241000194033 Enterococcus Species 0.000 description 2
- 241000190842 Erythrobacter sp. Species 0.000 description 2
- 241000588722 Escherichia Species 0.000 description 2
- 101100172445 Escherichia coli (strain K12) entH gene Proteins 0.000 description 2
- 101100350700 Escherichia coli (strain K12) paaB gene Proteins 0.000 description 2
- 101100350709 Escherichia coli (strain K12) paaG gene Proteins 0.000 description 2
- 101100350711 Escherichia coli (strain K12) paaI gene Proteins 0.000 description 2
- 101100082074 Escherichia coli (strain K12) paaZ gene Proteins 0.000 description 2
- 101100545037 Escherichia coli (strain K12) yuaF gene Proteins 0.000 description 2
- 241001646716 Escherichia coli K-12 Species 0.000 description 2
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 2
- 102100025413 Formyltetrahydrofolate synthetase Human genes 0.000 description 2
- 229930091371 Fructose Natural products 0.000 description 2
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 2
- 239000005715 Fructose Substances 0.000 description 2
- 241000968725 Gammaproteobacteria bacterium Species 0.000 description 2
- 102000030595 Glucokinase Human genes 0.000 description 2
- 108010021582 Glucokinase Proteins 0.000 description 2
- 101100161918 Glycine max SAC1 gene Proteins 0.000 description 2
- 241000590002 Helicobacter pylori Species 0.000 description 2
- 108091027305 Heteroduplex Proteins 0.000 description 2
- 101001035740 Homo sapiens 3-hydroxyacyl-CoA dehydrogenase type-2 Proteins 0.000 description 2
- 229930010555 Inosine Natural products 0.000 description 2
- UGQMRVRMYYASKQ-KQYNXXCUSA-N Inosine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C2=NC=NC(O)=C2N=C1 UGQMRVRMYYASKQ-KQYNXXCUSA-N 0.000 description 2
- 108010066338 Inositol-tetrakisphosphate 1-kinase Proteins 0.000 description 2
- 102100022296 Inositol-tetrakisphosphate 1-kinase Human genes 0.000 description 2
- AMIMRNSIRUDHCM-UHFFFAOYSA-N Isopropylaldehyde Chemical compound CC(C)C=O AMIMRNSIRUDHCM-UHFFFAOYSA-N 0.000 description 2
- 102100023418 Ketohexokinase Human genes 0.000 description 2
- 101100236497 Klebsiella aerogenes maoC gene Proteins 0.000 description 2
- 241000588747 Klebsiella pneumoniae Species 0.000 description 2
- 102000004317 Lyases Human genes 0.000 description 2
- 108090000856 Lyases Proteins 0.000 description 2
- 101710159527 Maturation protein A Proteins 0.000 description 2
- 101710091157 Maturation protein A2 Proteins 0.000 description 2
- 101100456321 Metallosphaera sedula (strain ATCC 51363 / DSM 5348 / JCM 9185 / NBRC 15509 / TH2) Msed_0709 gene Proteins 0.000 description 2
- 241001467552 Mycobacterium bovis BCG Species 0.000 description 2
- 241001607431 Mycobacterium marinum M Species 0.000 description 2
- 241000187479 Mycobacterium tuberculosis Species 0.000 description 2
- 101100445407 Neosartorya fumigata (strain ATCC MYA-4609 / Af293 / CBS 101355 / FGSC A1100) erg10B gene Proteins 0.000 description 2
- 101710163270 Nuclease Proteins 0.000 description 2
- 238000012408 PCR amplification Methods 0.000 description 2
- 241000589597 Paracoccus denitrificans Species 0.000 description 2
- 241000228150 Penicillium chrysogenum Species 0.000 description 2
- 102000035195 Peptidases Human genes 0.000 description 2
- 108091005804 Peptidases Proteins 0.000 description 2
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 2
- 241001600128 Populus tremula x Populus alba Species 0.000 description 2
- 241000986839 Porphyromonas gingivalis W83 Species 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 2
- 101100463818 Pseudomonas oleovorans phaC1 gene Proteins 0.000 description 2
- 241000320117 Pseudomonas putida KT2440 Species 0.000 description 2
- 241000589774 Pseudomonas sp. Species 0.000 description 2
- 241000205156 Pyrococcus furiosus Species 0.000 description 2
- LCTONWCANYUPML-UHFFFAOYSA-M Pyruvate Chemical compound CC(=O)C([O-])=O LCTONWCANYUPML-UHFFFAOYSA-M 0.000 description 2
- 241000700157 Rattus norvegicus Species 0.000 description 2
- 101100332697 Rhodobacter capsulatus (strain ATCC BAA-309 / NBRC 16581 / SB1003) fadB1 gene Proteins 0.000 description 2
- 241000316848 Rhodococcus <scale insect> Species 0.000 description 2
- 241000190950 Rhodopseudomonas palustris Species 0.000 description 2
- 102000012479 Serine Proteases Human genes 0.000 description 2
- 108010022999 Serine Proteases Proteins 0.000 description 2
- 241000187747 Streptomyces Species 0.000 description 2
- 241000187180 Streptomyces sp. Species 0.000 description 2
- 108010075728 Succinate-CoA Ligases Proteins 0.000 description 2
- 102000011929 Succinate-CoA Ligases Human genes 0.000 description 2
- 108010084086 Succinate-Semialdehyde Dehydrogenase Proteins 0.000 description 2
- 102100023673 Succinate-semialdehyde dehydrogenase, mitochondrial Human genes 0.000 description 2
- 241000186339 Thermoanaerobacter Species 0.000 description 2
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical compound O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 description 2
- 241000779671 Yersinia intermedia ATCC 29909 Species 0.000 description 2
- ZPCWGDDUISZRJO-BLPRJPCASA-N [[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(3R)-3-hydroxy-2,2-dimethyl-4-oxo-4-[[3-oxo-3-(2-sulfanylethylamino)propyl]amino]butyl] hydrogen phosphate benzene-1,2-diol Chemical compound OC1=CC=CC=C1O.O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCS)O[C@H]1N1C2=NC=NC(N)=C2N=C1 ZPCWGDDUISZRJO-BLPRJPCASA-N 0.000 description 2
- XJLXINKUBYWONI-DQQFMEOOSA-N [[(2r,3r,4r,5r)-5-(6-aminopurin-9-yl)-3-hydroxy-4-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(2s,3r,4s,5s)-5-(3-carbamoylpyridin-1-ium-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphate Chemical group NC(=O)C1=CC=C[N+]([C@@H]2[C@H]([C@@H](O)[C@H](COP([O-])(=O)OP(O)(=O)OC[C@@H]3[C@H]([C@@H](OP(O)(O)=O)[C@@H](O3)N3C4=NC=NC(N)=C4N=C3)O)O2)O)=C1 XJLXINKUBYWONI-DQQFMEOOSA-N 0.000 description 2
- IKHGUXGNUITLKF-XPULMUKRSA-N acetaldehyde Chemical compound [14CH]([14CH3])=O IKHGUXGNUITLKF-XPULMUKRSA-N 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 101150049512 ald gene Proteins 0.000 description 2
- 108010081577 aldehyde dehydrogenase (NAD(P)+) Proteins 0.000 description 2
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical compound OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 description 2
- 238000000137 annealing Methods 0.000 description 2
- PYMYPHUHKUWMLA-WDCZJNDASA-N arabinose Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)C=O PYMYPHUHKUWMLA-WDCZJNDASA-N 0.000 description 2
- JGGLZQUGOKVDGS-VYTIMWRQSA-N aspartate semialdehyde Chemical compound O[C@@H]1[C@@H](NC(=O)C)CO[C@H](CO)[C@H]1O[C@@H]1[C@@H](NC(C)=O)[C@H](O)[C@H](O[C@@H]2[C@H]([C@@H](O[C@@H]3[C@@H]([C@H](O)[C@@H](O)[C@H](CO)O3)O[C@@H]3[C@@H]([C@H](O)[C@@H](O)[C@H](CO)O3)O[C@@H]3[C@H]([C@H](O)[C@@H](O)[C@H](CO)O3)O)[C@@H](O)[C@H](CO[C@@H]3[C@H]([C@H](O[C@@H]4[C@H]([C@H](O)[C@@H](O)[C@H](CO)O4)O)[C@@H](O)[C@H](CO)O3)O)O2)O)[C@H](CO)O1 JGGLZQUGOKVDGS-VYTIMWRQSA-N 0.000 description 2
- 239000012298 atmosphere Substances 0.000 description 2
- 235000021028 berry Nutrition 0.000 description 2
- 101150008745 bphG gene Proteins 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 238000012219 cassette mutagenesis Methods 0.000 description 2
- YCIMNLLNPGFGHC-UHFFFAOYSA-N catechol Chemical compound OC1=CC=CC=C1O YCIMNLLNPGFGHC-UHFFFAOYSA-N 0.000 description 2
- 238000004113 cell culture Methods 0.000 description 2
- 238000001311 chemical methods and process Methods 0.000 description 2
- 238000010367 cloning Methods 0.000 description 2
- 239000003245 coal Substances 0.000 description 2
- 229960003920 cocaine Drugs 0.000 description 2
- ZPUCINDJVBIVPJ-LJISPDSOSA-N cocaine Natural products O([C@H]1C[C@@H]2CC[C@@H](N2C)[C@H]1C(=O)OC)C(=O)C1=CC=CC=C1 ZPUCINDJVBIVPJ-LJISPDSOSA-N 0.000 description 2
- RGJOEKWQDUBAIZ-UHFFFAOYSA-N coenzime A Natural products OC1C(OP(O)(O)=O)C(COP(O)(=O)OP(O)(=O)OCC(C)(C)C(O)C(=O)NCCC(=O)NCCS)OC1N1C2=NC=NC(N)=C2N=C1 RGJOEKWQDUBAIZ-UHFFFAOYSA-N 0.000 description 2
- 239000005516 coenzyme A Substances 0.000 description 2
- 229940093530 coenzyme a Drugs 0.000 description 2
- 238000000205 computational method Methods 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 2
- 230000007812 deficiency Effects 0.000 description 2
- 238000006297 dehydration reaction Methods 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- KDTSHFARGAKYJN-UHFFFAOYSA-N dephosphocoenzyme A Natural products OC1C(O)C(COP(O)(=O)OP(O)(=O)OCC(C)(C)C(O)C(=O)NCCC(=O)NCCS)OC1N1C2=NC=NC(N)=C2N=C1 KDTSHFARGAKYJN-UHFFFAOYSA-N 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- YQTLUZGXAMXTJV-UHFFFAOYSA-N dicarboxymethyl-oxido-oxophosphanium Chemical compound C(C(=O)O)(C(=O)O)[P+](=O)[O-] YQTLUZGXAMXTJV-UHFFFAOYSA-N 0.000 description 2
- RXKJFZQQPQGTFL-UHFFFAOYSA-N dihydroxyacetone Chemical compound OCC(=O)CO RXKJFZQQPQGTFL-UHFFFAOYSA-N 0.000 description 2
- XPPKVPWEQAFLFU-UHFFFAOYSA-J diphosphate(4-) Chemical compound [O-]P([O-])(=O)OP([O-])([O-])=O XPPKVPWEQAFLFU-UHFFFAOYSA-J 0.000 description 2
- 238000006073 displacement reaction Methods 0.000 description 2
- NOPFSRXAKWQILS-UHFFFAOYSA-N docosan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCCCCCO NOPFSRXAKWQILS-UHFFFAOYSA-N 0.000 description 2
- 230000000925 erythroid effect Effects 0.000 description 2
- 125000004494 ethyl ester group Chemical group 0.000 description 2
- 210000003527 eukaryotic cell Anatomy 0.000 description 2
- 230000001747 exhibiting effect Effects 0.000 description 2
- 101150069125 fadB gene Proteins 0.000 description 2
- 101150092019 fadJ gene Proteins 0.000 description 2
- 238000013467 fragmentation Methods 0.000 description 2
- 238000006062 fragmentation reaction Methods 0.000 description 2
- 229930182830 galactose Natural products 0.000 description 2
- 238000002290 gas chromatography-mass spectrometry Methods 0.000 description 2
- 238000012224 gene deletion Methods 0.000 description 2
- 238000010353 genetic engineering Methods 0.000 description 2
- 101150080232 glpK1 gene Proteins 0.000 description 2
- 229940037467 helicobacter pylori Drugs 0.000 description 2
- NAQMVNRVTILPCV-UHFFFAOYSA-N hexane-1,6-diamine Chemical compound NCCCCCCN NAQMVNRVTILPCV-UHFFFAOYSA-N 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 229960003786 inosine Drugs 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 150000004702 methyl esters Chemical class 0.000 description 2
- GOQYKNQRPGWPLP-UHFFFAOYSA-N n-heptadecyl alcohol Natural products CCCCCCCCCCCCCCCCCO GOQYKNQRPGWPLP-UHFFFAOYSA-N 0.000 description 2
- 239000003345 natural gas Substances 0.000 description 2
- 229930027945 nicotinamide-adenine dinucleotide Natural products 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 230000008723 osmotic stress Effects 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- LPNBBFKOUUSUDB-UHFFFAOYSA-M p-toluate Chemical compound CC1=CC=C(C([O-])=O)C=C1 LPNBBFKOUUSUDB-UHFFFAOYSA-M 0.000 description 2
- 101150037784 paaA gene Proteins 0.000 description 2
- MNBKLUUYKPBKDU-BBECNAHFSA-N palmitoyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CCCCCCCCCCCCCCC)O[C@H]1N1C2=NC=NC(N)=C2N=C1 MNBKLUUYKPBKDU-BBECNAHFSA-N 0.000 description 2
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 2
- 101150046540 phaA gene Proteins 0.000 description 2
- 230000026731 phosphorylation Effects 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- CBIDRCWHNCKSTO-UHFFFAOYSA-N prenyl diphosphate Chemical compound CC(C)=CCO[P@](O)(=O)OP(O)(O)=O CBIDRCWHNCKSTO-UHFFFAOYSA-N 0.000 description 2
- 210000001236 prokaryotic cell Anatomy 0.000 description 2
- QAQREVBBADEHPA-IEXPHMLFSA-N propionyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CC)O[C@H]1N1C2=NC=NC(N)=C2N=C1 QAQREVBBADEHPA-IEXPHMLFSA-N 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 239000000376 reactant Substances 0.000 description 2
- 108091000042 riboflavin kinase Proteins 0.000 description 2
- 238000002864 sequence alignment Methods 0.000 description 2
- 244000209700 shan ge teng Species 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 238000005507 spraying Methods 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 125000001424 substituent group Chemical group 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 101150087812 tesA gene Proteins 0.000 description 2
- 101150026728 tesB gene Proteins 0.000 description 2
- 238000013518 transcription Methods 0.000 description 2
- 230000035897 transcription Effects 0.000 description 2
- 101150102457 ybgC gene Proteins 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- AMBUVNRPXGXPMX-UHFFFAOYSA-N (2-hydroxy-3-methyl-4-oxobutyl) 2-hydroxy-3-phosphonooxypropanoate Chemical compound P(=O)(O)(O)OCC(C(=O)OCC(C(C=O)C)O)O AMBUVNRPXGXPMX-UHFFFAOYSA-N 0.000 description 1
- PYBVXNWDSBYQSA-BYPYZUCNSA-N (2s)-2-aminobutane-1,4-diol Chemical compound OC[C@@H](N)CCO PYBVXNWDSBYQSA-BYPYZUCNSA-N 0.000 description 1
- CABVTRNMFUVUDM-VRHQGPGLSA-N (3S)-3-hydroxy-3-methylglutaryl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)C[C@@](O)(CC(O)=O)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 CABVTRNMFUVUDM-VRHQGPGLSA-N 0.000 description 1
- FQVLRGLGWNWPSS-BXBUPLCLSA-N (4r,7s,10s,13s,16r)-16-acetamido-13-(1h-imidazol-5-ylmethyl)-10-methyl-6,9,12,15-tetraoxo-7-propan-2-yl-1,2-dithia-5,8,11,14-tetrazacycloheptadecane-4-carboxamide Chemical compound N1C(=O)[C@@H](NC(C)=O)CSSC[C@@H](C(N)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](C)NC(=O)[C@@H]1CC1=CN=CN1 FQVLRGLGWNWPSS-BXBUPLCLSA-N 0.000 description 1
- QHHKKMYHDBRONY-WZZMXTMRSA-N (R)-3-hydroxybutanoyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)C[C@H](O)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 QHHKKMYHDBRONY-WZZMXTMRSA-N 0.000 description 1
- PHIQHXFUZVPYII-ZCFIWIBFSA-N (R)-carnitine Chemical compound C[N+](C)(C)C[C@H](O)CC([O-])=O PHIQHXFUZVPYII-ZCFIWIBFSA-N 0.000 description 1
- 108010026367 (deoxy)adenylate kinase Proteins 0.000 description 1
- PSBDWGZCVUAZQS-UHFFFAOYSA-N (dimethylsulfonio)acetate Chemical compound C[S+](C)CC([O-])=O PSBDWGZCVUAZQS-UHFFFAOYSA-N 0.000 description 1
- ODIGIKRIUKFKHP-UHFFFAOYSA-N (n-propan-2-yloxycarbonylanilino) acetate Chemical compound CC(C)OC(=O)N(OC(C)=O)C1=CC=CC=C1 ODIGIKRIUKFKHP-UHFFFAOYSA-N 0.000 description 1
- 102000001556 1-Phosphatidylinositol 4-Kinase Human genes 0.000 description 1
- 108010029190 1-Phosphatidylinositol 4-Kinase Proteins 0.000 description 1
- 102100038028 1-phosphatidylinositol 3-phosphate 5-kinase Human genes 0.000 description 1
- 108030003698 1-phosphatidylinositol-3-phosphate 5-kinases Proteins 0.000 description 1
- 108010052341 1-phosphatidylinositol-4-phosphate 5-kinase Proteins 0.000 description 1
- 108030003684 1-phosphatidylinositol-5-phosphate 4-kinases Proteins 0.000 description 1
- 108010023317 1-phosphofructokinase Proteins 0.000 description 1
- 108030003727 2'-phosphotransferases Proteins 0.000 description 1
- GJDQCBHLUBZQFJ-UHFFFAOYSA-N 2,2-dihydroxypentanoic acid Chemical compound CCCC(O)(O)C(O)=O GJDQCBHLUBZQFJ-UHFFFAOYSA-N 0.000 description 1
- 108010052911 2-dehydro-3-deoxygalactonokinase Proteins 0.000 description 1
- 108030003739 2-dehydro-3-deoxygluconokinases Proteins 0.000 description 1
- JRHWHSJDIILJAT-UHFFFAOYSA-N 2-hydroxypentanoic acid Chemical compound CCCC(O)C(O)=O JRHWHSJDIILJAT-UHFFFAOYSA-N 0.000 description 1
- KPGXRSRHYNQIFN-UHFFFAOYSA-L 2-oxoglutarate(2-) Chemical compound [O-]C(=O)CCC(=O)C([O-])=O KPGXRSRHYNQIFN-UHFFFAOYSA-L 0.000 description 1
- VWFJDQUYCIWHTN-YFVJMOTDSA-N 2-trans,6-trans-farnesyl diphosphate Chemical compound CC(C)=CCC\C(C)=C\CC\C(C)=C\CO[P@](O)(=O)OP(O)(O)=O VWFJDQUYCIWHTN-YFVJMOTDSA-N 0.000 description 1
- GWYFCOCPABKNJV-UHFFFAOYSA-M 3-Methylbutanoic acid Natural products CC(C)CC([O-])=O GWYFCOCPABKNJV-UHFFFAOYSA-M 0.000 description 1
- DXJCKZJNLGWSTI-UHFFFAOYSA-N 3-dimethylsulfonio-2-methylpropanoate Chemical compound [O-]C(=O)C(C)C[S+](C)C DXJCKZJNLGWSTI-UHFFFAOYSA-N 0.000 description 1
- WWEOGFZEFHPUAM-MIZDRFBCSA-N 3-hydroxy-2-methylpropanoyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)C(CO)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 WWEOGFZEFHPUAM-MIZDRFBCSA-N 0.000 description 1
- ZQHYXNSQOIDNTL-UHFFFAOYSA-N 3-hydroxyglutaric acid Chemical compound OC(=O)CC(O)CC(O)=O ZQHYXNSQOIDNTL-UHFFFAOYSA-N 0.000 description 1
- DBXBTMSZEOQQDU-UHFFFAOYSA-N 3-hydroxyisobutyric acid Chemical compound OCC(C)C(O)=O DBXBTMSZEOQQDU-UHFFFAOYSA-N 0.000 description 1
- 108010077268 3-hydroxyisobutyryl-CoA hydrolase Proteins 0.000 description 1
- 102100034767 3-hydroxyisobutyryl-CoA hydrolase, mitochondrial Human genes 0.000 description 1
- REKYPYSUBKSCAT-UHFFFAOYSA-N 3-hydroxypentanoic acid Chemical compound CCC(O)CC(O)=O REKYPYSUBKSCAT-UHFFFAOYSA-N 0.000 description 1
- BERBFZCUSMQABM-IEXPHMLFSA-N 3-hydroxypropanoyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CCO)O[C@H]1N1C2=NC=NC(N)=C2N=C1 BERBFZCUSMQABM-IEXPHMLFSA-N 0.000 description 1
- NMEYBPUHJHMRHU-IEXPHMLFSA-N 3-oxopropanoyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CC=O)O[C@H]1N1C2=NC=NC(N)=C2N=C1 NMEYBPUHJHMRHU-IEXPHMLFSA-N 0.000 description 1
- 108030007077 3-phosphoglyceroyl-phosphate-polyphosphate phosphotransferases Proteins 0.000 description 1
- 108030003683 4-(cytidine 5'-diphospho)-2-C-methyl-D-erythritol kinases Proteins 0.000 description 1
- MEANFMOQMXYMCT-OLZOCXBDSA-N 5,10-methenyltetrahydrofolic acid Chemical compound C([C@H]1CNC2=C([N+]1=C1)C(=O)N=C(N2)N)N1C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C([O-])=O)C=C1 MEANFMOQMXYMCT-OLZOCXBDSA-N 0.000 description 1
- OOXNYFKPOPJIOT-UHFFFAOYSA-N 5-(3-bromophenyl)-7-(6-morpholin-4-ylpyridin-3-yl)pyrido[2,3-d]pyrimidin-4-amine;dihydrochloride Chemical compound Cl.Cl.C=12C(N)=NC=NC2=NC(C=2C=NC(=CC=2)N2CCOCC2)=CC=1C1=CC=CC(Br)=C1 OOXNYFKPOPJIOT-UHFFFAOYSA-N 0.000 description 1
- HVFVETAZTNXXDD-KVMZYSJPSA-N 5-[2-[3-[[(2R)-4-[[[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]oxy-2-hydroxy-3,3-dimethylbutanoyl]amino]propanoylamino]ethylsulfanyl]-3,5-dioxopentanoic acid 3,5-dioxopentanoic acid Chemical compound O=C(CC(=O)O)CC=O.O=C(CC(=O)SCCNC(CCNC([C@@H](C(COP(OP(OC[C@@H]1[C@H]([C@H]([C@@H](O1)N1C=NC=2C(N)=NC=NC12)O)OP(=O)(O)O)(=O)O)(=O)O)(C)C)O)=O)=O)CC(=O)O HVFVETAZTNXXDD-KVMZYSJPSA-N 0.000 description 1
- 108090000713 5-dehydro-2-deoxygluconokinases Proteins 0.000 description 1
- NYYMNZLORMNCKK-UHFFFAOYSA-N 5-hydroxynaphthalene-1-carboxylic acid Chemical compound C1=CC=C2C(C(=O)O)=CC=CC2=C1O NYYMNZLORMNCKK-UHFFFAOYSA-N 0.000 description 1
- 108030007076 5-methyldeoxycytidine-5'-phosphate kinases Proteins 0.000 description 1
- VBKPPDYGFUZOAJ-UHFFFAOYSA-N 5-oxopentanoic acid Chemical compound OC(=O)CCCC=O VBKPPDYGFUZOAJ-UHFFFAOYSA-N 0.000 description 1
- PWVLGFYSTFLJJB-UHFFFAOYSA-N 5-phosphonooxypentanoic acid Chemical compound OC(=O)CCCCOP(O)(O)=O PWVLGFYSTFLJJB-UHFFFAOYSA-N 0.000 description 1
- UBKVUFQGVWHZIR-UHFFFAOYSA-N 8-oxoguanine Chemical compound O=C1NC(N)=NC2=NC(=O)N=C21 UBKVUFQGVWHZIR-UHFFFAOYSA-N 0.000 description 1
- 108010062854 ADP D-fructose-6-phosphate 1-phosphotransferase Proteins 0.000 description 1
- 102100026381 ADP-dependent glucokinase Human genes 0.000 description 1
- 108010058598 ADP-dependent glucokinase Proteins 0.000 description 1
- 108010002988 ADP-thymidine kinase Proteins 0.000 description 1
- 108030003841 AMP-thymidine kinases Proteins 0.000 description 1
- 101710157736 ATP-dependent 6-phosphofructokinase Proteins 0.000 description 1
- 101710200244 ATP-dependent 6-phosphofructokinase isozyme 2 Proteins 0.000 description 1
- IKHGUXGNUITLKF-UHFFFAOYSA-N Acetaldehyde Chemical class CC=O IKHGUXGNUITLKF-UHFFFAOYSA-N 0.000 description 1
- 108010092060 Acetate kinase Proteins 0.000 description 1
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical group CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 1
- 102100037768 Acetyl-CoA acetyltransferase, mitochondrial Human genes 0.000 description 1
- 241000604450 Acidaminococcus fermentans Species 0.000 description 1
- 101100228725 Acidaminococcus fermentans (strain ATCC 25085 / DSM 20731 / CCUG 9996 / CIP 106432 / VR4) gctA gene Proteins 0.000 description 1
- 101100228726 Acidaminococcus fermentans (strain ATCC 25085 / DSM 20731 / CCUG 9996 / CIP 106432 / VR4) gctB gene Proteins 0.000 description 1
- 241000726121 Acidianus Species 0.000 description 1
- 241000588624 Acinetobacter calcoaceticus Species 0.000 description 1
- 108010009924 Aconitate hydratase Proteins 0.000 description 1
- 241000948980 Actinobacillus succinogenes Species 0.000 description 1
- 108010001058 Acyl-CoA Dehydrogenase Proteins 0.000 description 1
- 102000002296 Acyl-CoA Dehydrogenases Human genes 0.000 description 1
- 102100022388 Acylglycerol kinase, mitochondrial Human genes 0.000 description 1
- 108010076278 Adenosine kinase Proteins 0.000 description 1
- 108030003722 Adenosylcobinamide kinases Proteins 0.000 description 1
- 108020000543 Adenylate kinase Proteins 0.000 description 1
- 102100040149 Adenylyl-sulfate kinase Human genes 0.000 description 1
- 108010054404 Adenylyl-sulfate kinase Proteins 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- JPLATTLXZFUKRQ-UHFFFAOYSA-N Agarobiose Natural products OCC1OC(OC2C(O)COC2C(O)C=O)C(O)C(O)C1O JPLATTLXZFUKRQ-UHFFFAOYSA-N 0.000 description 1
- 102100034035 Alcohol dehydrogenase 1A Human genes 0.000 description 1
- 108010061730 Alkylglycerol kinase Proteins 0.000 description 1
- 108030003878 Alkylglycerone kinases Proteins 0.000 description 1
- 108700028369 Alleles Proteins 0.000 description 1
- 241000722954 Anaerobiospirillum succiniciproducens Species 0.000 description 1
- 241001136167 Anaerotignum propionicum Species 0.000 description 1
- 108010006591 Apoenzymes Proteins 0.000 description 1
- 241000207208 Aquifex Species 0.000 description 1
- 241000893512 Aquifex aeolicus Species 0.000 description 1
- 101100388296 Arabidopsis thaliana DTX51 gene Proteins 0.000 description 1
- 241000203069 Archaea Species 0.000 description 1
- 241000205046 Archaeoglobus Species 0.000 description 1
- 241000205042 Archaeoglobus fulgidus Species 0.000 description 1
- 108020004652 Aspartate-Semialdehyde Dehydrogenase Proteins 0.000 description 1
- 241000228212 Aspergillus Species 0.000 description 1
- 241000228245 Aspergillus niger Species 0.000 description 1
- 101100162204 Aspergillus parasiticus (strain ATCC 56775 / NRRL 5862 / SRRC 143 / SU-1) aflH gene Proteins 0.000 description 1
- 241001465318 Aspergillus terreus Species 0.000 description 1
- 241000726110 Azoarcus Species 0.000 description 1
- 101100098786 Bacillus subtilis (strain 168) tapA gene Proteins 0.000 description 1
- 108010015248 Beta-glucoside kinase Proteins 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 241000589562 Brucella Species 0.000 description 1
- 241001646647 Burkholderia ambifaria Species 0.000 description 1
- MGBYLAKBMQZEHC-MRFMBWGRSA-N C(C)(=O)C(C(=O)SCCNC(CCNC([C@@H](C(COP(OP(OC[C@@H]1[C@H]([C@H]([C@@H](O1)N1C=NC=2C(N)=NC=NC12)O)OP(=O)(O)O)(=O)O)(=O)O)(C)C)O)=O)=O)C(CC(=O)O)O Chemical compound C(C)(=O)C(C(=O)SCCNC(CCNC([C@@H](C(COP(OP(OC[C@@H]1[C@H]([C@H]([C@@H](O1)N1C=NC=2C(N)=NC=NC12)O)OP(=O)(O)O)(=O)O)(=O)O)(C)C)O)=O)=O)C(CC(=O)O)O MGBYLAKBMQZEHC-MRFMBWGRSA-N 0.000 description 1
- CJMSKAPBVOCLJT-KVMZYSJPSA-N C(CCCCCCCCC)(=O)OC1=NC=CC=C1O.O=C(CC(=O)SCCNC(CCNC([C@@H](C(COP(OP(OC[C@@H]1[C@H]([C@H]([C@@H](O1)N1C=NC=2C(N)=NC=NC12)O)OP(=O)(O)O)(=O)O)(=O)O)(C)C)O)=O)=O)CC(=O)O Chemical compound C(CCCCCCCCC)(=O)OC1=NC=CC=C1O.O=C(CC(=O)SCCNC(CCNC([C@@H](C(COP(OP(OC[C@@H]1[C@H]([C@H]([C@@H](O1)N1C=NC=2C(N)=NC=NC12)O)OP(=O)(O)O)(=O)O)(=O)O)(C)C)O)=O)=O)CC(=O)O CJMSKAPBVOCLJT-KVMZYSJPSA-N 0.000 description 1
- OLNQPHNMSVWNHS-UHFFFAOYSA-N CC(C(COOP(O)=O)O)C=O Chemical compound CC(C(COOP(O)=O)O)C=O OLNQPHNMSVWNHS-UHFFFAOYSA-N 0.000 description 1
- PTZKWWHWPSNHSD-UHFFFAOYSA-N CCC(C(C(O)=O)=O)=O.OC(CC(O)OP(O)(O)=O)CC(O)=O Chemical compound CCC(C(C(O)=O)=O)=O.OC(CC(O)OP(O)(O)=O)CC(O)=O PTZKWWHWPSNHSD-UHFFFAOYSA-N 0.000 description 1
- 101150097247 CRT1 gene Proteins 0.000 description 1
- 101100110009 Caenorhabditis elegans asd-2 gene Proteins 0.000 description 1
- 241000438254 Caldanaerobacter subterraneus subsp. tengcongensis MB4 Species 0.000 description 1
- 241000589876 Campylobacter Species 0.000 description 1
- 241000589875 Campylobacter jejuni Species 0.000 description 1
- 241000222120 Candida <Saccharomycetales> Species 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- 102100036158 Ceramide kinase Human genes 0.000 description 1
- 108010017573 Ceramide kinase Proteins 0.000 description 1
- 241000192731 Chloroflexus aurantiacus Species 0.000 description 1
- 108010018888 Choline kinase Proteins 0.000 description 1
- 235000007516 Chrysanthemum Nutrition 0.000 description 1
- 240000005250 Chrysanthemum indicum Species 0.000 description 1
- 241000588923 Citrobacter Species 0.000 description 1
- 241000193454 Clostridium beijerinckii Species 0.000 description 1
- 241000193155 Clostridium botulinum Species 0.000 description 1
- 241000441874 Clostridium botulinum C str. Eklund Species 0.000 description 1
- 241000186570 Clostridium kluyveri Species 0.000 description 1
- 241001508458 Clostridium saccharoperbutylacetonicum Species 0.000 description 1
- 241000186216 Corynebacterium Species 0.000 description 1
- 241000366859 Cupriavidus taiwanensis Species 0.000 description 1
- 241000414116 Cyanobium Species 0.000 description 1
- 241001440267 Cyclodes Species 0.000 description 1
- 102100039868 Cytoplasmic aconitate hydratase Human genes 0.000 description 1
- 108030003774 D-arabinokinases Proteins 0.000 description 1
- WUVPHPUDYOVMOE-SCSAIBSYSA-N D-erythrulose 4-phosphate Chemical compound OCC(=O)[C@H](O)COP(O)(O)=O WUVPHPUDYOVMOE-SCSAIBSYSA-N 0.000 description 1
- MNQZXJOMYWMBOU-VKHMYHEASA-N D-glyceraldehyde Chemical compound OC[C@@H](O)C=O MNQZXJOMYWMBOU-VKHMYHEASA-N 0.000 description 1
- RBNPOMFGQQGHHO-UWTATZPHSA-N D-glyceric acid Chemical compound OC[C@@H](O)C(O)=O RBNPOMFGQQGHHO-UWTATZPHSA-N 0.000 description 1
- ZAQJHHRNXZUBTE-UHFFFAOYSA-N D-threo-2-Pentulose Natural products OCC(O)C(O)C(=O)CO ZAQJHHRNXZUBTE-UHFFFAOYSA-N 0.000 description 1
- 238000000018 DNA microarray Methods 0.000 description 1
- 230000004568 DNA-binding Effects 0.000 description 1
- 108030003652 Dehydrogluconokinases Proteins 0.000 description 1
- 108010058222 Deoxyguanosine kinase Proteins 0.000 description 1
- 108030003689 Deoxynucleoside kinases Proteins 0.000 description 1
- 102000007260 Deoxyribonuclease I Human genes 0.000 description 1
- 108010008532 Deoxyribonuclease I Proteins 0.000 description 1
- AHCYMLUZIRLXAA-SHYZEUOFSA-N Deoxyuridine 5'-triphosphate Chemical compound O1[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)C[C@@H]1N1C(=O)NC(=O)C=C1 AHCYMLUZIRLXAA-SHYZEUOFSA-N 0.000 description 1
- 102000011107 Diacylglycerol Kinase Human genes 0.000 description 1
- 108010062677 Diacylglycerol Kinase Proteins 0.000 description 1
- 241000168726 Dictyostelium discoideum Species 0.000 description 1
- 101100269671 Dictyostelium discoideum alrA gene Proteins 0.000 description 1
- 108030003904 Diphosphate-fructose-6-phosphate 1-phosphotransferases Proteins 0.000 description 1
- 108030003830 Diphosphate-glycerol phosphotransferases Proteins 0.000 description 1
- 108030003664 Diphosphate-purine nucleoside kinases Proteins 0.000 description 1
- 108010065057 Diphosphate-serine phosphotransferase Proteins 0.000 description 1
- 102100038390 Diphosphomevalonate decarboxylase Human genes 0.000 description 1
- 101710183613 Diphosphomevalonate decarboxylase Proteins 0.000 description 1
- 241000255581 Drosophila <fruit fly, genus> Species 0.000 description 1
- 108700034280 EC 2.7.1.37 Proteins 0.000 description 1
- 108700034911 EC 3.1.2.- Proteins 0.000 description 1
- 101150045041 ERG8 gene Proteins 0.000 description 1
- WQXNXVUDBPYKBA-UHFFFAOYSA-N Ectoine Natural products CC1=NCCC(C(O)=O)N1 WQXNXVUDBPYKBA-UHFFFAOYSA-N 0.000 description 1
- 241001379910 Ephemera danica Species 0.000 description 1
- 101100350708 Escherichia coli (strain K12) paaF gene Proteins 0.000 description 1
- 101100321116 Escherichia coli (strain K12) yqhD gene Proteins 0.000 description 1
- 108700039887 Essential Genes Proteins 0.000 description 1
- OTMSDBZUPAUEDD-UHFFFAOYSA-N Ethane Chemical compound CC OTMSDBZUPAUEDD-UHFFFAOYSA-N 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- ZFDIRQKJPRINOQ-HWKANZROSA-N Ethyl crotonate Chemical compound CCOC(=O)\C=C\C ZFDIRQKJPRINOQ-HWKANZROSA-N 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- 108091029865 Exogenous DNA Proteins 0.000 description 1
- 102100026859 FAD-AMP lyase (cyclizing) Human genes 0.000 description 1
- VWFJDQUYCIWHTN-FBXUGWQNSA-N Farnesyl diphosphate Natural products CC(C)=CCC\C(C)=C/CC\C(C)=C/COP(O)(=O)OP(O)(O)=O VWFJDQUYCIWHTN-FBXUGWQNSA-N 0.000 description 1
- 108090000698 Formate Dehydrogenases Proteins 0.000 description 1
- 108010080982 Formate-tetrahydrofolate ligase Proteins 0.000 description 1
- 101001076781 Fructilactobacillus sanfranciscensis (strain ATCC 27651 / DSM 20451 / JCM 5668 / CCUG 30143 / KCTC 3205 / NCIMB 702811 / NRRL B-3934 / L-12) Ribose-5-phosphate isomerase A Proteins 0.000 description 1
- 108090000156 Fructokinases Proteins 0.000 description 1
- 102100022633 Fructose-2,6-bisphosphatase Human genes 0.000 description 1
- 108010036781 Fumarate Hydratase Proteins 0.000 description 1
- 102100036160 Fumarate hydratase, mitochondrial Human genes 0.000 description 1
- 241000605986 Fusobacterium nucleatum Species 0.000 description 1
- 241001291923 Fusobacterium nucleatum subsp. nucleatum Species 0.000 description 1
- 102000048120 Galactokinases Human genes 0.000 description 1
- 108700023157 Galactokinases Proteins 0.000 description 1
- 108030003736 Galacturonokinases Proteins 0.000 description 1
- 206010064571 Gene mutation Diseases 0.000 description 1
- 241000626621 Geobacillus Species 0.000 description 1
- 101001110193 Geobacillus kaustophilus (strain HTA426) NADPH-dependent 7-cyano-7-deazaguanine reductase Proteins 0.000 description 1
- 101000892220 Geobacillus thermodenitrificans (strain NG80-2) Long-chain-alcohol dehydrogenase 1 Proteins 0.000 description 1
- 241000589232 Gluconobacter oxydans Species 0.000 description 1
- 108030006685 Glucosamine kinases Proteins 0.000 description 1
- 102100036636 Glucose 1,6-bisphosphate synthase Human genes 0.000 description 1
- 108010038489 Glucose-1,6-bisphosphate synthase Proteins 0.000 description 1
- 108010002196 Glucuronokinase Proteins 0.000 description 1
- 102100025591 Glycerate kinase Human genes 0.000 description 1
- 108010001483 Glycogen Synthase Proteins 0.000 description 1
- 108020004202 Guanylate Kinase Proteins 0.000 description 1
- 241000168517 Haematococcus lacustris Species 0.000 description 1
- 241000606790 Haemophilus Species 0.000 description 1
- 241000606768 Haemophilus influenzae Species 0.000 description 1
- 241000205063 Haloarcula marismortui Species 0.000 description 1
- SQUHHTBVTRBESD-UHFFFAOYSA-N Hexa-Ac-myo-Inositol Natural products CC(=O)OC1C(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C1OC(C)=O SQUHHTBVTRBESD-UHFFFAOYSA-N 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- 102000005548 Hexokinase Human genes 0.000 description 1
- 108700040460 Hexokinases Proteins 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101000780443 Homo sapiens Alcohol dehydrogenase 1A Proteins 0.000 description 1
- 101000716763 Homo sapiens Succinyl-CoA:3-ketoacid coenzyme A transferase 1, mitochondrial Proteins 0.000 description 1
- 241000243251 Hydra Species 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 108010052919 Hydroxyethylthiazole kinase Proteins 0.000 description 1
- 108010003207 Hydroxylysine kinase Proteins 0.000 description 1
- 102100021101 Hydroxylysine kinase Human genes 0.000 description 1
- 102000002284 Hydroxymethylglutaryl-CoA Synthase Human genes 0.000 description 1
- 108010000775 Hydroxymethylglutaryl-CoA synthase Proteins 0.000 description 1
- 108010027436 Hydroxymethylpyrimidine kinase Proteins 0.000 description 1
- 108030003726 Hygromycin B 4-O-kinases Proteins 0.000 description 1
- GRRNUXAQVGOGFE-UHFFFAOYSA-N Hygromycin-B Natural products OC1C(NC)CC(N)C(O)C1OC1C2OC3(C(C(O)C(O)C(C(N)CO)O3)O)OC2C(O)C(CO)O1 GRRNUXAQVGOGFE-UHFFFAOYSA-N 0.000 description 1
- 108060003951 Immunoglobulin Proteins 0.000 description 1
- 108010001139 Inosine kinase Proteins 0.000 description 1
- 108030003815 Inositol 3-kinases Proteins 0.000 description 1
- 102100025479 Inositol polyphosphate multikinase Human genes 0.000 description 1
- 108010012621 Inositol-hexakisphosphate kinase Proteins 0.000 description 1
- 102100031525 Inositol-pentakisphosphate 2-kinase Human genes 0.000 description 1
- 108030003720 Inositol-pentakisphosphate 2-kinases Proteins 0.000 description 1
- 108010071021 Inositol-polyphosphate multikinase Proteins 0.000 description 1
- 108030003665 Inositol-tetrakisphosphate 5-kinases Proteins 0.000 description 1
- 108030003860 Inositol-trisphosphate 3-kinases Proteins 0.000 description 1
- 108090001007 Interleukin-8 Proteins 0.000 description 1
- 108010081409 Iron-Sulfur Proteins Proteins 0.000 description 1
- 102000005298 Iron-Sulfur Proteins Human genes 0.000 description 1
- 241000631636 Ishige Species 0.000 description 1
- 108010075869 Isocitrate Dehydrogenase Proteins 0.000 description 1
- 102000012011 Isocitrate Dehydrogenase Human genes 0.000 description 1
- NUHSROFQTUXZQQ-UHFFFAOYSA-N Isopentenyl diphosphate Natural products CC(=C)CCO[P@](O)(=O)OP(O)(O)=O NUHSROFQTUXZQQ-UHFFFAOYSA-N 0.000 description 1
- 108010025815 Kanamycin Kinase Proteins 0.000 description 1
- 108010062852 Ketohexokinase Proteins 0.000 description 1
- 241000588749 Klebsiella oxytoca Species 0.000 description 1
- 241000235649 Kluyveromyces Species 0.000 description 1
- 235000014663 Kluyveromyces fragilis Nutrition 0.000 description 1
- 241000235058 Komagataella pastoris Species 0.000 description 1
- CZWARROQQFCFJB-UHFFFAOYSA-N L-2-Amino-5-hydroxypentanoic acid Chemical compound OC(=O)C(N)CCCO CZWARROQQFCFJB-UHFFFAOYSA-N 0.000 description 1
- 108030003738 L-arabinokinases Proteins 0.000 description 1
- 102100040648 L-fucose kinase Human genes 0.000 description 1
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 1
- ZAQJHHRNXZUBTE-WVZVXSGGSA-N L-xylulose Chemical compound OC[C@H](O)[C@@H](O)C(=O)CO ZAQJHHRNXZUBTE-WVZVXSGGSA-N 0.000 description 1
- 241000192130 Leuconostoc mesenteroides Species 0.000 description 1
- 102100025357 Lipid-phosphate phosphatase Human genes 0.000 description 1
- 108060001084 Luciferase Proteins 0.000 description 1
- 208000019693 Lung disease Diseases 0.000 description 1
- 240000003293 Magnolia grandiflora Species 0.000 description 1
- 208000002720 Malnutrition Diseases 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 108010015328 Mannokinase Proteins 0.000 description 1
- 102000005741 Metalloproteases Human genes 0.000 description 1
- 108010006035 Metalloproteases Proteins 0.000 description 1
- 241000134732 Metallosphaera Species 0.000 description 1
- 241000157876 Metallosphaera sedula Species 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 241000203407 Methanocaldococcus jannaschii Species 0.000 description 1
- 108010010685 Methenyltetrahydrofolate cyclohydrolase Proteins 0.000 description 1
- 108010030837 Methylenetetrahydrofolate Reductase (NADPH2) Proteins 0.000 description 1
- 102000005954 Methylenetetrahydrofolate Reductase (NADPH2) Human genes 0.000 description 1
- 102100022259 Mevalonate kinase Human genes 0.000 description 1
- 241001182779 Moniliella megachiliensis Species 0.000 description 1
- 241000699660 Mus musculus Species 0.000 description 1
- 241000186359 Mycobacterium Species 0.000 description 1
- 241000186367 Mycobacterium avium Species 0.000 description 1
- 241000052923 Mycobacterium avium subsp. paratuberculosis K-10 Species 0.000 description 1
- 241001025881 Mycobacterium smegmatis str. MC2 155 Species 0.000 description 1
- 101100269700 Mycolicibacterium smegmatis alr gene Proteins 0.000 description 1
- 241000204031 Mycoplasma Species 0.000 description 1
- 241000432072 Mycoplasma pneumoniae M129 Species 0.000 description 1
- 241000204003 Mycoplasmatales Species 0.000 description 1
- 102100035286 N-acetyl-D-glucosamine kinase Human genes 0.000 description 1
- 102100037774 N-acetylgalactosamine kinase Human genes 0.000 description 1
- 108030003719 N-acetylgalactosamine kinases Proteins 0.000 description 1
- 108010032040 N-acetylglucosamine kinase Proteins 0.000 description 1
- 108030003725 N-acetylhexosamine 1-kinases Proteins 0.000 description 1
- 108030003884 NADH kinases Proteins 0.000 description 1
- 102100029562 Nicotinamide riboside kinase 1 Human genes 0.000 description 1
- 241000187654 Nocardia Species 0.000 description 1
- 241001037736 Nocardia farcinica IFM 10152 Species 0.000 description 1
- 108020004485 Nonsense Codon Proteins 0.000 description 1
- 238000000636 Northern blotting Methods 0.000 description 1
- 108010011356 Nucleoside phosphotransferase Proteins 0.000 description 1
- 108010044790 Nucleoside-Phosphate Kinase Proteins 0.000 description 1
- 102000005811 Nucleoside-phosphate kinase Human genes 0.000 description 1
- 108010011026 Nucleoside-triphosphate-adenylate kinase Proteins 0.000 description 1
- ZZJCEBOJDGDWPZ-UHFFFAOYSA-N OC(=O)CC(O)(O)CCOP(O)(O)=O Chemical compound OC(=O)CC(O)(O)CCOP(O)(O)=O ZZJCEBOJDGDWPZ-UHFFFAOYSA-N 0.000 description 1
- WRQKSTALTQSVHA-ZCFIWIBFSA-N OCC[C@](C)(CC(O)=O)OP(=O)=O Chemical compound OCC[C@](C)(CC(O)=O)OP(=O)=O WRQKSTALTQSVHA-ZCFIWIBFSA-N 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 108091007960 PI3Ks Proteins 0.000 description 1
- 108010035473 Palmitoyl-CoA Hydrolase Proteins 0.000 description 1
- 102000008172 Palmitoyl-CoA Hydrolase Human genes 0.000 description 1
- 108010021592 Pantothenate kinase Proteins 0.000 description 1
- 102100024122 Pantothenate kinase 1 Human genes 0.000 description 1
- 241001343907 Paraburkholderia phymatum Species 0.000 description 1
- 241000193390 Parageobacillus thermoglucosidasius Species 0.000 description 1
- 208000026681 Paratuberculosis Diseases 0.000 description 1
- 108700023175 Phosphate acetyltransferases Proteins 0.000 description 1
- 102000003993 Phosphatidylinositol 3-kinases Human genes 0.000 description 1
- 108090000430 Phosphatidylinositol 3-kinases Proteins 0.000 description 1
- 102000013576 Phosphatidylinositol-4-Phosphate 3-Kinase Human genes 0.000 description 1
- 108010051404 Phosphatidylinositol-4-Phosphate 3-Kinase Proteins 0.000 description 1
- 108010022684 Phosphofructokinase-1 Proteins 0.000 description 1
- 102000012435 Phosphofructokinase-1 Human genes 0.000 description 1
- 108010022678 Phosphofructokinase-2 Proteins 0.000 description 1
- 102100024279 Phosphomevalonate kinase Human genes 0.000 description 1
- 108090001050 Phosphoric Diester Hydrolases Proteins 0.000 description 1
- 102000004861 Phosphoric Diester Hydrolases Human genes 0.000 description 1
- 240000004713 Pisum sativum Species 0.000 description 1
- 102000013566 Plasminogen Human genes 0.000 description 1
- 108010051456 Plasminogen Proteins 0.000 description 1
- 102000001938 Plasminogen Activators Human genes 0.000 description 1
- 108010001014 Plasminogen Activators Proteins 0.000 description 1
- 241000694540 Pluvialis Species 0.000 description 1
- 108010021757 Polynucleotide 5'-Hydroxyl-Kinase Proteins 0.000 description 1
- 102000008422 Polynucleotide 5'-hydroxyl-kinase Human genes 0.000 description 1
- 229920000388 Polyphosphate Polymers 0.000 description 1
- 108010042149 Polyphosphate-glucose phosphotransferase Proteins 0.000 description 1
- 241000168036 Populus alba Species 0.000 description 1
- 101100287363 Populus alba ISPS gene Proteins 0.000 description 1
- 241000206609 Porphyra Species 0.000 description 1
- 241000605894 Porphyromonas Species 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 102000055027 Protein Methyltransferases Human genes 0.000 description 1
- 108700040121 Protein Methyltransferases Proteins 0.000 description 1
- 241001240958 Pseudomonas aeruginosa PAO1 Species 0.000 description 1
- 241001358835 Pseudomonas fluorescens PF5 Species 0.000 description 1
- 241000922540 Pseudomonas knackmussii Species 0.000 description 1
- 101100350716 Pseudomonas putida paaK gene Proteins 0.000 description 1
- 108010070648 Pyridoxal Kinase Proteins 0.000 description 1
- 102100038517 Pyridoxal kinase Human genes 0.000 description 1
- 241000205226 Pyrobaculum Species 0.000 description 1
- 241000777575 Pyrobaculum aerophilum str. IM2 Species 0.000 description 1
- 102000013009 Pyruvate Kinase Human genes 0.000 description 1
- 108020005115 Pyruvate Kinase Proteins 0.000 description 1
- 101710182361 Pyruvate:ferredoxin oxidoreductase Proteins 0.000 description 1
- 241000481518 Ralstonia eutropha H16 Species 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 108020005091 Replication Origin Proteins 0.000 description 1
- 241000589180 Rhizobium Species 0.000 description 1
- 240000005384 Rhizopus oryzae Species 0.000 description 1
- 235000013752 Rhizopus oryzae Nutrition 0.000 description 1
- 102000048125 Riboflavin kinases Human genes 0.000 description 1
- 108030003763 Riboflavin phosphotransferases Proteins 0.000 description 1
- 102000046755 Ribokinases Human genes 0.000 description 1
- 108030007153 Ribose 1,5-bisphosphate phosphokinases Proteins 0.000 description 1
- 241001451515 Roseburia intestinalis L1-82 Species 0.000 description 1
- 241000750876 Roseburia inulinivorans DSM 16841 Species 0.000 description 1
- 241001049174 Roseburia sp. A2-183 Species 0.000 description 1
- 241000516658 Roseiflexus castenholzii Species 0.000 description 1
- 244000117054 Rungia klossii Species 0.000 description 1
- 235000002492 Rungia klossii Nutrition 0.000 description 1
- 108030003800 S-methyl-5-thioribose kinases Proteins 0.000 description 1
- 241000235070 Saccharomyces Species 0.000 description 1
- 101100215626 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) ADP1 gene Proteins 0.000 description 1
- 244000253911 Saccharomyces fragilis Species 0.000 description 1
- 235000018368 Saccharomyces fragilis Nutrition 0.000 description 1
- 240000000111 Saccharum officinarum Species 0.000 description 1
- 235000007201 Saccharum officinarum Nutrition 0.000 description 1
- 241001138501 Salmonella enterica Species 0.000 description 1
- 241000607356 Salmonella enterica subsp. arizonae Species 0.000 description 1
- 241000235346 Schizosaccharomyces Species 0.000 description 1
- 241000235347 Schizosaccharomyces pombe Species 0.000 description 1
- 238000012300 Sequence Analysis Methods 0.000 description 1
- 241000221095 Simmondsia Species 0.000 description 1
- 244000044822 Simmondsia californica Species 0.000 description 1
- 235000004433 Simmondsia californica Nutrition 0.000 description 1
- 241000589196 Sinorhizobium meliloti Species 0.000 description 1
- 244000057717 Streptococcus lactis Species 0.000 description 1
- 235000014897 Streptococcus lactis Nutrition 0.000 description 1
- 241000187392 Streptomyces griseus Species 0.000 description 1
- 241000958284 Streptomyces griseus subsp. griseus Species 0.000 description 1
- 241001541741 Streptomyces sp. ACT-1 Species 0.000 description 1
- 102000019259 Succinate Dehydrogenase Human genes 0.000 description 1
- 108010012901 Succinate Dehydrogenase Proteins 0.000 description 1
- 102100020868 Succinyl-CoA:3-ketoacid coenzyme A transferase 1, mitochondrial Human genes 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- 241000205101 Sulfolobus Species 0.000 description 1
- 241000205098 Sulfolobus acidocaldarius Species 0.000 description 1
- 241000205095 Sulfolobus shibatae Species 0.000 description 1
- 241000205091 Sulfolobus solfataricus Species 0.000 description 1
- 241000160715 Sulfolobus tokodaii Species 0.000 description 1
- 241000192581 Synechocystis sp. Species 0.000 description 1
- 241000158541 Syntrophus <bacteria> Species 0.000 description 1
- 108010092220 Tetraacyldisaccharide 4'-kinase Proteins 0.000 description 1
- 244000299461 Theobroma cacao Species 0.000 description 1
- 235000009470 Theobroma cacao Nutrition 0.000 description 1
- 241001147775 Thermoanaerobacter brockii Species 0.000 description 1
- 241001313706 Thermosynechococcus Species 0.000 description 1
- 241000204652 Thermotoga Species 0.000 description 1
- 241000589499 Thermus thermophilus Species 0.000 description 1
- 102100037495 Thiamin pyrophosphokinase 1 Human genes 0.000 description 1
- 108030007080 Thiamine-phosphate kinases Proteins 0.000 description 1
- 102000005488 Thioesterase Human genes 0.000 description 1
- 102000006601 Thymidine Kinase Human genes 0.000 description 1
- 108020004440 Thymidine kinase Proteins 0.000 description 1
- 102100037357 Thymidylate kinase Human genes 0.000 description 1
- 102000003978 Tissue Plasminogen Activator Human genes 0.000 description 1
- 108090000373 Tissue Plasminogen Activator Proteins 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- 241000224526 Trichomonas Species 0.000 description 1
- 241000975677 Trichomonas vaginalis G3 Species 0.000 description 1
- 241000908249 Trichosporonoides Species 0.000 description 1
- 108010071199 Triokinase Proteins 0.000 description 1
- 241000223105 Trypanosoma brucei Species 0.000 description 1
- 241001034637 Tsukamurella paurometabola DSM 20162 Species 0.000 description 1
- 108020000553 UMP kinase Proteins 0.000 description 1
- 101710100179 UMP-CMP kinase Proteins 0.000 description 1
- 102100032947 UMP-CMP kinase 2, mitochondrial Human genes 0.000 description 1
- 101710119674 UMP-CMP kinase 2, mitochondrial Proteins 0.000 description 1
- 108700024326 Undecaprenol kinases Proteins 0.000 description 1
- 108010072685 Uracil-DNA Glycosidase Proteins 0.000 description 1
- 102100037111 Uracil-DNA glycosylase Human genes 0.000 description 1
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 1
- 108030003863 Xylitol kinases Proteins 0.000 description 1
- 102100029089 Xylulose kinase Human genes 0.000 description 1
- 241000235013 Yarrowia Species 0.000 description 1
- 241000589153 Zoogloea ramigera Species 0.000 description 1
- 241000235033 Zygosaccharomyces rouxii Species 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- 241000222292 [Candida] magnoliae Species 0.000 description 1
- 241001531188 [Eubacterium] rectale Species 0.000 description 1
- 241000029538 [Mannheimia] succiniciproducens Species 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- 230000021736 acetylation Effects 0.000 description 1
- 238000006640 acetylation reaction Methods 0.000 description 1
- 229920000122 acrylonitrile butadiene styrene Polymers 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 125000002252 acyl group Chemical group 0.000 description 1
- 108010016133 acylglycerol kinase Proteins 0.000 description 1
- 125000004423 acyloxy group Chemical group 0.000 description 1
- 101150024743 adhA gene Proteins 0.000 description 1
- 238000005377 adsorption chromatography Methods 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 239000002154 agricultural waste Substances 0.000 description 1
- 125000003158 alcohol group Chemical group 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- HXXFSFRBOHSIMQ-VFUOTHLCSA-N alpha-D-glucose 1-phosphate Chemical compound OC[C@H]1O[C@H](OP(O)(O)=O)[C@H](O)[C@@H](O)[C@@H]1O HXXFSFRBOHSIMQ-VFUOTHLCSA-N 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- BTFJIXJJCSYFAL-UHFFFAOYSA-N arachidyl alcohol Natural products CCCCCCCCCCCCCCCCCCCCO BTFJIXJJCSYFAL-UHFFFAOYSA-N 0.000 description 1
- 125000001204 arachidyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 210000004507 artificial chromosome Anatomy 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 230000000712 assembly Effects 0.000 description 1
- 238000000429 assembly Methods 0.000 description 1
- 101150006429 atoB gene Proteins 0.000 description 1
- 230000001651 autotrophic effect Effects 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 230000037429 base substitution Effects 0.000 description 1
- 230000003851 biochemical process Effects 0.000 description 1
- 238000005842 biochemical reaction Methods 0.000 description 1
- 238000003766 bioinformatics method Methods 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 125000004063 butyryl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 239000003575 carbonaceous material Substances 0.000 description 1
- 125000001721 carboxyacetyl group Chemical group 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 230000019522 cellular metabolic process Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000012824 chemical production Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 1
- 229960001231 choline Drugs 0.000 description 1
- 230000002759 chromosomal effect Effects 0.000 description 1
- 238000003501 co-culture Methods 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 230000001143 conditioned effect Effects 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 238000010924 continuous production Methods 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 239000012228 culture supernatant Substances 0.000 description 1
- LMGZGXSXHCMSAA-UHFFFAOYSA-N cyclodecane Chemical compound C1CCCCCCCCC1 LMGZGXSXHCMSAA-UHFFFAOYSA-N 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- 230000001461 cytolytic effect Effects 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- 108010000742 dTMP kinase Proteins 0.000 description 1
- NHVNXKFIZYSCEB-XLPZGREQSA-N dTTP Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)C1 NHVNXKFIZYSCEB-XLPZGREQSA-N 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 125000002704 decyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 230000002939 deleterious effect Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 238000004925 denaturation Methods 0.000 description 1
- 230000036425 denaturation Effects 0.000 description 1
- 108010007340 deoxyadenosine kinase Proteins 0.000 description 1
- 230000030609 dephosphorylation Effects 0.000 description 1
- 238000006209 dephosphorylation reaction Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 229940120503 dihydroxyacetone Drugs 0.000 description 1
- XPPKVPWEQAFLFU-UHFFFAOYSA-N diphosphoric acid Chemical compound OP(O)(=O)OP(O)(O)=O XPPKVPWEQAFLFU-UHFFFAOYSA-N 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 229960000735 docosanol Drugs 0.000 description 1
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 238000005553 drilling Methods 0.000 description 1
- WQXNXVUDBPYKBA-YFKPBYRVSA-N ectoine Chemical compound CC1=[NH+][C@H](C([O-])=O)CCN1 WQXNXVUDBPYKBA-YFKPBYRVSA-N 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 238000000909 electrodialysis Methods 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 238000006911 enzymatic reaction Methods 0.000 description 1
- 150000002118 epoxides Chemical class 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 235000020774 essential nutrients Nutrition 0.000 description 1
- 230000032050 esterification Effects 0.000 description 1
- 238000005886 esterification reaction Methods 0.000 description 1
- 108010044215 ethanolamine kinase Proteins 0.000 description 1
- WULRVYVSKOQJJX-UHFFFAOYSA-N ethyl 3,5-dioxopentanoate Chemical compound CCOC(=O)CC(=O)CC=O WULRVYVSKOQJJX-UHFFFAOYSA-N 0.000 description 1
- XVYIDSYAMINCFJ-UHFFFAOYSA-N ethyl 3-hydroxy-5-oxopentanoate Chemical compound CCOC(=O)CC(O)CC=O XVYIDSYAMINCFJ-UHFFFAOYSA-N 0.000 description 1
- FJXPMZXOMBIGQA-UHFFFAOYSA-N ethyl 5-hydroxy-3-oxopentanoate Chemical compound CCOC(=O)CC(=O)CCO FJXPMZXOMBIGQA-UHFFFAOYSA-N 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000002360 explosive Substances 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 108010036889 fatty acid oxidation complex Proteins 0.000 description 1
- 230000004136 fatty acid synthesis Effects 0.000 description 1
- 150000002191 fatty alcohols Chemical class 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000002803 fossil fuel Substances 0.000 description 1
- 238000005194 fractionation Methods 0.000 description 1
- 230000037433 frameshift Effects 0.000 description 1
- 235000011389 fruit/vegetable juice Nutrition 0.000 description 1
- 108010083136 fucokinase Proteins 0.000 description 1
- 239000000446 fuel Substances 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- ZZUFCTLCJUWOSV-UHFFFAOYSA-N furosemide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC(C(O)=O)=C1NCC1=CC=CO1 ZZUFCTLCJUWOSV-UHFFFAOYSA-N 0.000 description 1
- 230000000799 fusogenic effect Effects 0.000 description 1
- ZXQYGBMAQZUVMI-GCMPRSNUSA-N gamma-cyhalothrin Chemical compound CC1(C)[C@@H](\C=C(/Cl)C(F)(F)F)[C@H]1C(=O)O[C@H](C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 ZXQYGBMAQZUVMI-GCMPRSNUSA-N 0.000 description 1
- 238000012246 gene addition Methods 0.000 description 1
- 102000054767 gene variant Human genes 0.000 description 1
- 101150056064 glpK gene Proteins 0.000 description 1
- 230000004190 glucose uptake Effects 0.000 description 1
- 229950010772 glucose-1-phosphate Drugs 0.000 description 1
- 108010087331 glutaconate CoA-transferase Proteins 0.000 description 1
- SYKWLIJQEHRDNH-KRPIADGTSA-N glutaryl-coa Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)C(O)C(=O)NCCC(=O)NCCSC(=O)CCCC(O)=O)O[C@H]1N1C2=NC=NC(N)=C2N=C1 SYKWLIJQEHRDNH-KRPIADGTSA-N 0.000 description 1
- 108010086476 glycerate kinase Proteins 0.000 description 1
- 108010013826 glycerol-3-phosphate - glucose phosphotransferase Proteins 0.000 description 1
- 150000002337 glycosamines Chemical class 0.000 description 1
- 102000006638 guanylate kinase Human genes 0.000 description 1
- 150000003278 haem Chemical class 0.000 description 1
- 229940047650 haemophilus influenzae Drugs 0.000 description 1
- 125000005843 halogen group Chemical group 0.000 description 1
- 239000002920 hazardous waste Substances 0.000 description 1
- 125000000755 henicosyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000005368 heteroarylthio group Chemical group 0.000 description 1
- 108090001018 hexadecanal dehydrogenase (acylating) Proteins 0.000 description 1
- OEXFMSFODMQEPE-HDRQGHTBSA-N hexanoyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CCCCC)O[C@H]1N1C2=NC=NC(N)=C2N=C1 OEXFMSFODMQEPE-HDRQGHTBSA-N 0.000 description 1
- 125000006038 hexenyl group Chemical group 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 230000003301 hydrolyzing effect Effects 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 description 1
- GRRNUXAQVGOGFE-NZSRVPFOSA-N hygromycin B Chemical compound O[C@@H]1[C@@H](NC)C[C@@H](N)[C@H](O)[C@H]1O[C@H]1[C@H]2O[C@@]3([C@@H]([C@@H](O)[C@@H](O)[C@@H](C(N)CO)O3)O)O[C@H]2[C@@H](O)[C@@H](CO)O1 GRRNUXAQVGOGFE-NZSRVPFOSA-N 0.000 description 1
- 229940097277 hygromycin b Drugs 0.000 description 1
- 230000003100 immobilizing effect Effects 0.000 description 1
- 238000003119 immunoblot Methods 0.000 description 1
- 102000018358 immunoglobulin Human genes 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- SEOVTRFCIGRIMH-UHFFFAOYSA-N indole-3-acetic acid Chemical compound C1=CC=C2C(CC(=O)O)=CNC2=C1 SEOVTRFCIGRIMH-UHFFFAOYSA-N 0.000 description 1
- 239000000411 inducer Substances 0.000 description 1
- 206010022000 influenza Diseases 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 229960000367 inositol Drugs 0.000 description 1
- CDAISMWEOUEBRE-GPIVLXJGSA-N inositol Chemical compound O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@H](O)[C@@H]1O CDAISMWEOUEBRE-GPIVLXJGSA-N 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- KQNPFQTWMSNSAP-UHFFFAOYSA-N isobutyric acid Chemical compound CC(C)C(O)=O KQNPFQTWMSNSAP-UHFFFAOYSA-N 0.000 description 1
- QRXWMOHMRWLFEY-UHFFFAOYSA-N isoniazide Chemical compound NNC(=O)C1=CC=NC=C1 QRXWMOHMRWLFEY-UHFFFAOYSA-N 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- GWYFCOCPABKNJV-UHFFFAOYSA-N isovaleric acid Chemical compound CC(C)CC(O)=O GWYFCOCPABKNJV-UHFFFAOYSA-N 0.000 description 1
- 229940031154 kluyveromyces marxianus Drugs 0.000 description 1
- 229920005610 lignin Polymers 0.000 description 1
- 125000005647 linker group Chemical group 0.000 description 1
- 239000003915 liquefied petroleum gas Substances 0.000 description 1
- 238000009630 liquid culture Methods 0.000 description 1
- 238000000622 liquid--liquid extraction Methods 0.000 description 1
- 229960005375 lutein Drugs 0.000 description 1
- KBPHJBAIARWVSC-RGZFRNHPSA-N lutein Chemical compound C([C@H](O)CC=1C)C(C)(C)C=1\C=C\C(\C)=C\C=C\C(\C)=C\C=C\C=C(/C)\C=C\C=C(/C)\C=C\[C@H]1C(C)=C[C@H](O)CC1(C)C KBPHJBAIARWVSC-RGZFRNHPSA-N 0.000 description 1
- 239000001656 lutein Substances 0.000 description 1
- 235000012680 lutein Nutrition 0.000 description 1
- ORAKUVXRZWMARG-WZLJTJAWSA-N lutein Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=C(C)CCCC1(C)C)C=CC=C(/C)C=CC2C(=CC(O)CC2(C)C)C ORAKUVXRZWMARG-WZLJTJAWSA-N 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 125000002960 margaryl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 235000013372 meat Nutrition 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 238000005374 membrane filtration Methods 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- WSFSSNUMVMOOMR-NJFSPNSNSA-N methanone Chemical compound O=[14CH2] WSFSSNUMVMOOMR-NJFSPNSNSA-N 0.000 description 1
- MCVVUJPXSBQTRZ-ONEGZZNKSA-N methyl (e)-but-2-enoate Chemical compound COC(=O)\C=C\C MCVVUJPXSBQTRZ-ONEGZZNKSA-N 0.000 description 1
- NZOZKLSVSHBECX-UHFFFAOYSA-N methyl 3,5-dioxopentanoate Chemical compound COC(=O)CC(=O)CC=O NZOZKLSVSHBECX-UHFFFAOYSA-N 0.000 description 1
- QYSBDCKYMHSZRX-UHFFFAOYSA-N methyl 3-hydroxy-5-oxopentanoate Chemical compound COC(=O)CC(O)CC=O QYSBDCKYMHSZRX-UHFFFAOYSA-N 0.000 description 1
- JBILVOUKVCKKLH-UHFFFAOYSA-N methyl 5-hydroxy-3-oxopentanoate Chemical compound COC(=O)CC(=O)CCO JBILVOUKVCKKLH-UHFFFAOYSA-N 0.000 description 1
- 210000003470 mitochondria Anatomy 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 235000013379 molasses Nutrition 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- VYQNWZOUAUKGHI-UHFFFAOYSA-N monobenzone Chemical compound C1=CC(O)=CC=C1OCC1=CC=CC=C1 VYQNWZOUAUKGHI-UHFFFAOYSA-N 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 239000003471 mutagenic agent Substances 0.000 description 1
- 230000036438 mutation frequency Effects 0.000 description 1
- 230000000869 mutational effect Effects 0.000 description 1
- 125000001421 myristyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- FIPPFBHCBUDBRR-UHFFFAOYSA-N n-Heneikosylalkohol Natural products CCCCCCCCCCCCCCCCCCCCCO FIPPFBHCBUDBRR-UHFFFAOYSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- XGFDHKJUZCCPKQ-UHFFFAOYSA-N n-nonadecyl alcohol Natural products CCCCCCCCCCCCCCCCCCCO XGFDHKJUZCCPKQ-UHFFFAOYSA-N 0.000 description 1
- GLDOVTGHNKAZLK-UHFFFAOYSA-N n-octadecyl alcohol Natural products CCCCCCCCCCCCCCCCCCO GLDOVTGHNKAZLK-UHFFFAOYSA-N 0.000 description 1
- DQCKKXVULJGBQN-XFWGSAIBSA-N naltrexone Chemical compound N1([C@@H]2CC3=CC=C(C=4O[C@@H]5[C@](C3=4)([C@]2(CCC5=O)O)CC1)O)CC1CC1 DQCKKXVULJGBQN-XFWGSAIBSA-N 0.000 description 1
- 229960003086 naltrexone Drugs 0.000 description 1
- 108010021066 nicotinamide riboside kinase Proteins 0.000 description 1
- 125000001196 nonadecyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 230000009972 noncorrosive effect Effects 0.000 description 1
- 125000001400 nonyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000010742 number 1 fuel oil Substances 0.000 description 1
- 235000018343 nutrient deficiency Nutrition 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 239000011368 organic material Substances 0.000 description 1
- 230000000065 osmolyte Effects 0.000 description 1
- 125000002958 pentadecyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 238000005373 pervaporation Methods 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 229930029653 phosphoenolpyruvate Natural products 0.000 description 1
- DTBNBXWJWCWCIK-UHFFFAOYSA-N phosphoenolpyruvic acid Chemical compound OC(=O)C(=C)OP(O)(O)=O DTBNBXWJWCWCIK-UHFFFAOYSA-N 0.000 description 1
- 108010064607 phosphoglucokinase Proteins 0.000 description 1
- 108010006451 phosphomethylpyrimidine kinase Proteins 0.000 description 1
- 108091000116 phosphomevalonate kinase Proteins 0.000 description 1
- 125000005642 phosphothioate group Chemical group 0.000 description 1
- 229940127126 plasminogen activator Drugs 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 239000001205 polyphosphate Substances 0.000 description 1
- 108020000161 polyphosphate kinase Proteins 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 230000001915 proofreading effect Effects 0.000 description 1
- 230000000644 propagated effect Effects 0.000 description 1
- 125000004368 propenyl group Chemical group C(=CC)* 0.000 description 1
- UKUGXTSZVYLLLX-UHFFFAOYSA-N propyl 3,5-dioxopentanoate Chemical compound CCCOC(=O)CC(=O)CC=O UKUGXTSZVYLLLX-UHFFFAOYSA-N 0.000 description 1
- PVMCUVQFSFXKGM-UHFFFAOYSA-N propyl 3-hydroxy-5-oxopentanoate Chemical compound CCCOC(=O)CC(O)CC=O PVMCUVQFSFXKGM-UHFFFAOYSA-N 0.000 description 1
- NJOFVVDVTSWDAX-UHFFFAOYSA-N propyl 5-hydroxy-3-oxopentanoate Chemical compound CCCOC(=O)CC(=O)CCO NJOFVVDVTSWDAX-UHFFFAOYSA-N 0.000 description 1
- 235000019833 protease Nutrition 0.000 description 1
- 235000019419 proteases Nutrition 0.000 description 1
- 239000008262 pumice Substances 0.000 description 1
- 230000008707 rearrangement Effects 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 102000037983 regulatory factors Human genes 0.000 description 1
- 108091008025 regulatory factors Proteins 0.000 description 1
- 238000007634 remodeling Methods 0.000 description 1
- 230000003252 repetitive effect Effects 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- 238000001223 reverse osmosis Methods 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 239000005060 rubber Substances 0.000 description 1
- 238000013341 scale-up Methods 0.000 description 1
- 238000012106 screening analysis Methods 0.000 description 1
- 238000007423 screening assay Methods 0.000 description 1
- CDAISMWEOUEBRE-UHFFFAOYSA-N scyllo-inosotol Natural products OC1C(O)C(O)C(O)C(O)C1O CDAISMWEOUEBRE-UHFFFAOYSA-N 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- CXMXRPHRNRROMY-UHFFFAOYSA-N sebacic acid Chemical compound OC(=O)CCCCCCCCC(O)=O CXMXRPHRNRROMY-UHFFFAOYSA-N 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 238000001542 size-exclusion chromatography Methods 0.000 description 1
- AWUCVROLDVIAJX-GSVOUGTGSA-N sn-glycerol 3-phosphate Chemical compound OC[C@@H](O)COP(O)(O)=O AWUCVROLDVIAJX-GSVOUGTGSA-N 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 238000004230 steam cracking Methods 0.000 description 1
- 125000004079 stearyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000004575 stone Substances 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 108010041757 streptomycin 6-kinase Proteins 0.000 description 1
- IAHFWCOBPZCAEA-UHFFFAOYSA-N succinonitrile Chemical compound N#CCCC#N IAHFWCOBPZCAEA-UHFFFAOYSA-N 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 229920003051 synthetic elastomer Polymers 0.000 description 1
- 239000005061 synthetic rubber Substances 0.000 description 1
- 235000007586 terpenes Nutrition 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 210000001550 testis Anatomy 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 108010073742 thiamin-diphosphate kinase Proteins 0.000 description 1
- 108020000423 thiamine kinase Proteins 0.000 description 1
- 150000007970 thio esters Chemical class 0.000 description 1
- 108020002982 thioesterase Proteins 0.000 description 1
- RYYWUUFWQRZTIU-UHFFFAOYSA-K thiophosphate Chemical compound [O-]P([O-])([O-])=S RYYWUUFWQRZTIU-UHFFFAOYSA-K 0.000 description 1
- 101150072448 thrB gene Proteins 0.000 description 1
- 229960000187 tissue plasminogen activator Drugs 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 108700012359 toxins Proteins 0.000 description 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 1
- KBPHJBAIARWVSC-XQIHNALSSA-N trans-lutein Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=C(C)CC(O)CC1(C)C)C=CC=C(/C)C=CC2C(=CC(O)CC2(C)C)C KBPHJBAIARWVSC-XQIHNALSSA-N 0.000 description 1
- ZFDIRQKJPRINOQ-UHFFFAOYSA-N transbutenic acid ethyl ester Natural products CCOC(=O)C=CC ZFDIRQKJPRINOQ-UHFFFAOYSA-N 0.000 description 1
- 238000010361 transduction Methods 0.000 description 1
- 230000026683 transduction Effects 0.000 description 1
- 238000005809 transesterification reaction Methods 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000000844 transformation Methods 0.000 description 1
- 230000001131 transforming effect Effects 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 125000002889 tridecyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 238000012036 ultra high throughput screening Methods 0.000 description 1
- 238000000108 ultra-filtration Methods 0.000 description 1
- 125000002948 undecyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 229940035893 uracil Drugs 0.000 description 1
- 239000004474 valine Substances 0.000 description 1
- 108700026220 vif Genes Proteins 0.000 description 1
- UATIGEHITDTAGF-CITAKDKDSA-N vinylacetyl-CoA Chemical compound O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CC=C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 UATIGEHITDTAGF-CITAKDKDSA-N 0.000 description 1
- 239000013603 viral vector Substances 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 239000002916 wood waste Substances 0.000 description 1
- FJHBOVDFOQMZRV-XQIHNALSSA-N xanthophyll Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=C(C)CC(O)CC1(C)C)C=CC=C(/C)C=CC2C=C(C)C(O)CC2(C)C FJHBOVDFOQMZRV-XQIHNALSSA-N 0.000 description 1
- 108091022915 xylulokinase Proteins 0.000 description 1
- PIAOXUVIBAKVSP-UHFFFAOYSA-N γ-hydroxybutyraldehyde Chemical compound OCCCC=O PIAOXUVIBAKVSP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N1/00—Microorganisms, e.g. protozoa; Compositions thereof; Processes of propagating, maintaining or preserving microorganisms or compositions thereof; Processes of preparing or isolating a composition containing a microorganism; Culture media therefor
- C12N1/20—Bacteria; Culture media therefor
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C11/00—Aliphatic unsaturated hydrocarbons
- C07C11/12—Alkadienes
- C07C11/16—Alkadienes with four carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F136/00—Homopolymers of compounds having one or more unsaturated aliphatic radicals, at least one having two or more carbon-to-carbon double bonds
- C08F136/02—Homopolymers of compounds having one or more unsaturated aliphatic radicals, at least one having two or more carbon-to-carbon double bonds the radical having only two carbon-to-carbon double bonds
- C08F136/04—Homopolymers of compounds having one or more unsaturated aliphatic radicals, at least one having two or more carbon-to-carbon double bonds the radical having only two carbon-to-carbon double bonds conjugated
- C08F136/06—Butadiene
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N1/00—Microorganisms, e.g. protozoa; Compositions thereof; Processes of propagating, maintaining or preserving microorganisms or compositions thereof; Processes of preparing or isolating a composition containing a microorganism; Culture media therefor
- C12N1/14—Fungi; Culture media therefor
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N1/00—Microorganisms, e.g. protozoa; Compositions thereof; Processes of propagating, maintaining or preserving microorganisms or compositions thereof; Processes of preparing or isolating a composition containing a microorganism; Culture media therefor
- C12N1/14—Fungi; Culture media therefor
- C12N1/16—Yeasts; Culture media therefor
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N1/00—Microorganisms, e.g. protozoa; Compositions thereof; Processes of propagating, maintaining or preserving microorganisms or compositions thereof; Processes of preparing or isolating a composition containing a microorganism; Culture media therefor
- C12N1/14—Fungi; Culture media therefor
- C12N1/16—Yeasts; Culture media therefor
- C12N1/18—Baker's yeast; Brewer's yeast
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/52—Genes encoding for enzymes or proenzymes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P5/00—Preparation of hydrocarbons or halogenated hydrocarbons
- C12P5/02—Preparation of hydrocarbons or halogenated hydrocarbons acyclic
- C12P5/026—Unsaturated compounds, i.e. alkenes, alkynes or allenes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P7/00—Preparation of oxygen-containing organic compounds
- C12P7/02—Preparation of oxygen-containing organic compounds containing a hydroxy group
- C12P7/04—Preparation of oxygen-containing organic compounds containing a hydroxy group acyclic
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Biotechnology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Molecular Biology (AREA)
- Mycology (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Virology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Plant Pathology (AREA)
- Botany (AREA)
- Polymers & Plastics (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Enzymes And Modification Thereof (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US33181210P | 2010-05-05 | 2010-05-05 | |
| US61/331,812 | 2010-05-05 | ||
| PCT/US2011/035105 WO2011140171A2 (en) | 2010-05-05 | 2011-05-04 | Microorganisms and methods for the biosynthesis of butadiene |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020127031134A Division KR101814648B1 (ko) | 2010-05-05 | 2011-05-04 | 부타디엔을 생합성하기 위한 미생물 및 방법 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197001071A Division KR20190006103A (ko) | 2010-05-05 | 2011-05-04 | 부타디엔을 생합성하기 위한 미생물 및 방법 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20180005263A true KR20180005263A (ko) | 2018-01-15 |
Family
ID=45064763
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177037394A Ceased KR20180005263A (ko) | 2010-05-05 | 2011-05-04 | 부타디엔을 생합성하기 위한 미생물 및 방법 |
| KR1020197001071A Ceased KR20190006103A (ko) | 2010-05-05 | 2011-05-04 | 부타디엔을 생합성하기 위한 미생물 및 방법 |
| KR1020127031134A Active KR101814648B1 (ko) | 2010-05-05 | 2011-05-04 | 부타디엔을 생합성하기 위한 미생물 및 방법 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197001071A Ceased KR20190006103A (ko) | 2010-05-05 | 2011-05-04 | 부타디엔을 생합성하기 위한 미생물 및 방법 |
| KR1020127031134A Active KR101814648B1 (ko) | 2010-05-05 | 2011-05-04 | 부타디엔을 생합성하기 위한 미생물 및 방법 |
Country Status (13)
| Country | Link |
|---|---|
| US (5) | US8580543B2 (enExample) |
| EP (1) | EP2566969B1 (enExample) |
| JP (3) | JP5911847B2 (enExample) |
| KR (3) | KR20180005263A (enExample) |
| CN (2) | CN103080324B (enExample) |
| AU (2) | AU2011248182A1 (enExample) |
| BR (1) | BR112012028049A2 (enExample) |
| CA (1) | CA2797409C (enExample) |
| CO (1) | CO6630182A2 (enExample) |
| MX (1) | MX336229B (enExample) |
| SG (2) | SG10201503501TA (enExample) |
| TW (2) | TWI575072B (enExample) |
| WO (1) | WO2011140171A2 (enExample) |
Families Citing this family (66)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8129154B2 (en) | 2008-06-17 | 2012-03-06 | Genomatica, Inc. | Microorganisms and methods for the biosynthesis of fumarate, malate, and acrylate |
| KR20110097951A (ko) | 2008-12-16 | 2011-08-31 | 게노마티카 인코포레이티드 | 합성가스와 다른 탄소원을 유용 제품으로 전환시키기 위한 미생물 및 방법 |
| JP2012529889A (ja) | 2009-06-10 | 2012-11-29 | ゲノマチカ, インク. | Mek及び2−ブタノールの炭素効率のよい生合成のための微生物及び方法 |
| CN109136161A (zh) | 2009-12-10 | 2019-01-04 | 基因组股份公司 | 合成气或其他气态碳源和甲醇转化为1,3-丁二醇的方法和有机体 |
| US8637286B2 (en) | 2010-02-23 | 2014-01-28 | Genomatica, Inc. | Methods for increasing product yields |
| MX336229B (es) * | 2010-05-05 | 2016-01-08 | Genomatica Inc | Microorganismos y metodos para la biosintesis de butadieno. |
| JP2013535203A (ja) | 2010-07-26 | 2013-09-12 | ジェノマティカ・インコーポレイテッド | 芳香族、2,4−ペンタジエノエートおよび1,3−ブタジエンを生合成するための微生物および方法 |
| MX348334B (es) * | 2011-02-02 | 2017-06-07 | Genomatica Inc | Microorganismo y metodos para la biosintesis de butadieno. |
| WO2013188546A2 (en) * | 2012-06-15 | 2013-12-19 | Invista Technologies S.À.R.L. | Methods for biosynthesizing 1,3 butadiene |
| US9422578B2 (en) | 2011-06-17 | 2016-08-23 | Invista North America S.A.R.L. | Methods for biosynthesizing 1,3 butadiene |
| US9663801B2 (en) | 2011-06-17 | 2017-05-30 | Invista North America S.A.R.L. | Methods of producing four carbon molecules |
| US9169486B2 (en) | 2011-06-22 | 2015-10-27 | Genomatica, Inc. | Microorganisms for producing butadiene and methods related thereto |
| SG11201400113XA (en) * | 2011-08-19 | 2014-08-28 | Genomatica Inc | Microorganisms and methods for producing 2,4-pentadienoate, butadiene, propylene, 1,3-butanediol and related alcohols |
| BR112014008061A2 (pt) * | 2011-10-19 | 2017-04-11 | Scientist Of Fortune Sa | método para a produção enzimática de butadieno |
| DK2776571T3 (en) | 2011-11-09 | 2017-06-26 | Amyris Inc | PREPARATION OF ACETYL-COENZYM A-DERIVED ISOPRENOIDS |
| CN104321434A (zh) * | 2011-12-02 | 2015-01-28 | 英威达技术有限责任公司 | 生物合成1,3丁二烯的方法 |
| EP2791342B1 (en) * | 2011-12-16 | 2020-04-29 | Braskem S.A. | Modified microorganisms and methods of making butadiene using same |
| EP2794891A2 (en) * | 2011-12-20 | 2014-10-29 | Scientist of Fortune S.A. | Production of 1,3-dienes by enzymatic conversion of 3-hydroxyalk-4-enoates and/or 3-phosphonoxyalk-4-enoates |
| US20150017698A1 (en) * | 2012-03-02 | 2015-01-15 | Codexis, Inc. a corporation | Recombinant host cells and processes for producing 1,3-butadiene through a 5-hydroxypent-3-enoate intermediate |
| WO2013130481A1 (en) * | 2012-03-02 | 2013-09-06 | Codexis, Inc. | Recombinant host cells and processes for producing 1,3-butadiene through a crotonol intermediate |
| US9803209B2 (en) | 2012-05-18 | 2017-10-31 | Novozymes A/S | Bacterial mutants with improved transformation efficiency |
| US20150152440A1 (en) * | 2012-06-18 | 2015-06-04 | Braskem S.A. | Modified microorganisms and methods of co-producing butadiene with 1-propanol and/or 1,2-propanediol |
| BR112015004488A2 (pt) * | 2012-08-28 | 2018-11-21 | Braskem Sa | método de coprodução de um terpeno e pelo menos um coproduto a partir de uma fonte de carbono fermentável |
| WO2014052630A1 (en) | 2012-09-27 | 2014-04-03 | Novozymes, Inc. | Bacterial mutants with improved transformation efficiency |
| WO2014055649A1 (en) * | 2012-10-02 | 2014-04-10 | Braskem S/A Ap 09 | Modified microorganisms and methods of using same for producing butadiene and succinate |
| WO2014063156A2 (en) * | 2012-10-19 | 2014-04-24 | Braskem S/A Ap 09 | Modified microorganisms and methods of using same for producing butadiene and one or more of 1,3-butanediol, 1,4-butanediol, and/or 1,3-propanediol |
| US11535874B2 (en) * | 2012-10-22 | 2022-12-27 | Genomatica, Inc. | Microorganisms and methods for enhancing the availability of reducing equivalents in the presence of methanol, and for producing succinate related thereto |
| EP2920314A1 (en) * | 2012-11-13 | 2015-09-23 | Global Bioenergies | Process for the enzymatic preparation of isoprene from isoprenol |
| WO2014081973A1 (en) * | 2012-11-21 | 2014-05-30 | The Regents Of The University Of California | Nucleic acids useful for integrating into and gene expression in hyperthermophilic acidophilic archaea |
| CN104903455A (zh) | 2012-11-28 | 2015-09-09 | 英威达技术有限责任公司 | 用于异丁烯生物合成的方法 |
| JP6304924B2 (ja) * | 2012-11-29 | 2018-04-04 | 住友ゴム工業株式会社 | サイドウォール用ゴム組成物、及び空気入りタイヤ |
| WO2014089025A1 (en) * | 2012-12-04 | 2014-06-12 | Genomatica, Inc. | Increased yields of biosynthesized products |
| WO2014106122A1 (en) * | 2012-12-31 | 2014-07-03 | Genomatica, Inc. | Compositions and methods for bio-butadiene production screening |
| JP2014155455A (ja) * | 2013-02-15 | 2014-08-28 | Sekisui Chem Co Ltd | 組換え細胞、並びに、クロトニルCoA又はクロチルアルコールの生産方法 |
| JP2014161252A (ja) * | 2013-02-22 | 2014-09-08 | Sekisui Chem Co Ltd | 組換え細胞 |
| EP2971021A4 (en) | 2013-03-15 | 2016-12-21 | Genomatica Inc | MICROORGANISMS AND METHOD FOR THE PRODUCTION OF BUTADIENE AND RELATED COMPOUNDS BY FORMATASSIMILATION |
| ES2728307T3 (es) | 2013-06-21 | 2019-10-23 | Danisco Us Inc | Composiciones y métodos para la transformación clostridial |
| US10294496B2 (en) | 2013-07-19 | 2019-05-21 | Invista North America S.A.R.L. | Methods for biosynthesizing 1,3 butadiene |
| EP3030668A1 (en) | 2013-08-05 | 2016-06-15 | Invista Technologies S.A R.L. | Methods for biosynthesis of isobutene |
| CN105683385A (zh) | 2013-08-05 | 2016-06-15 | 英威达技术有限责任公司 | 用于生物合成异戊二烯的方法 |
| PL3077501T3 (pl) | 2013-12-03 | 2022-01-31 | Genomatica, Inc. | Mikroorganizmy i sposoby poprawy wydajności produktu na metanolu z użyciem syntezy acetylo-coa |
| WO2015100338A2 (en) | 2013-12-27 | 2015-07-02 | Genomatica, Inc. | Methods and organisms with increased carbon flux efficiencies |
| WO2015147644A1 (en) * | 2014-03-27 | 2015-10-01 | Photanol B.V. | Erythritol production in cyanobacteria |
| CN106795519A (zh) | 2014-06-16 | 2017-05-31 | 英威达技术有限责任公司 | 用于生成戊二酸和戊二酸甲酯的方法 |
| US10487342B2 (en) | 2014-07-11 | 2019-11-26 | Genomatica, Inc. | Microorganisms and methods for the production of butadiene using acetyl-CoA |
| EP3741865B1 (en) | 2014-09-18 | 2024-03-13 | Genomatica, Inc. | Non-natural microbial organisms with improved energetic efficiency |
| CN104298866B (zh) * | 2014-09-30 | 2017-06-06 | 杭州电子科技大学 | 一种克劳斯硫磺回收过程中反应炉动态建模方法 |
| JP6432774B2 (ja) * | 2014-12-25 | 2018-12-05 | 江南化工株式会社 | 細胞賦活剤 |
| MX387737B (es) | 2015-02-27 | 2025-03-18 | White Dog Labs Inc | Método de fermentación mixotrófica para producir acetona, isopropanol, acido butírico y otros bioproductos, y mezclas de los mismos. |
| WO2016160812A1 (en) * | 2015-03-31 | 2016-10-06 | White Dog Labs, Inc. | Method of producing bioproducts |
| KR20170134682A (ko) * | 2015-04-09 | 2017-12-06 | 게노마티카 인코포레이티드 | 향상된 크로틸 알코올 생성을 위한 유전자조작 미생물 및 방법 |
| CN108026214B (zh) | 2015-05-30 | 2021-03-02 | 基因组股份公司 | 乙烯基异构酶-脱水酶、烯醇脱水酶、芳樟醇脱水酶和/或巴豆醇脱水酶及其制备和使用 |
| WO2017064606A1 (en) * | 2015-10-12 | 2017-04-20 | Reliance Industries Limited | Process for preparation of 1,3-butadiene |
| SG11201803164SA (en) | 2015-10-30 | 2018-05-30 | Genomatica Inc | Methanol dehydrogenase fusion proteins |
| GB201605354D0 (en) | 2016-03-30 | 2016-05-11 | Zuvasyntha Ltd | Modified enzyme |
| US10919030B2 (en) | 2016-09-30 | 2021-02-16 | Regents Of The University Of Minnesota | Forming dienes from cyclic ethers and diols, including tetrahydrofuran and 2-methyl-1,4-butanediol |
| JP6317497B2 (ja) * | 2017-03-22 | 2018-04-25 | 住友ゴム工業株式会社 | トレッド用ゴム組成物、及び空気入りタイヤ |
| JP6317498B2 (ja) * | 2017-03-22 | 2018-04-25 | 住友ゴム工業株式会社 | スタッドレスタイヤ用トレッド用ゴム組成物、及びスタッドレスタイヤ |
| JP2020512351A (ja) * | 2017-03-31 | 2020-04-23 | ジェノマティカ, インコーポレイテッド | 発酵ブロスから1,3−ブタンジオールを取得するためのプロセスおよびシステム |
| US11634733B2 (en) | 2017-06-30 | 2023-04-25 | Inv Nylon Chemicals Americas, Llc | Methods, materials, synthetic hosts and reagents for the biosynthesis of hydrocarbons and derivatives thereof |
| WO2019006257A1 (en) | 2017-06-30 | 2019-01-03 | Invista North America .S.A.R.L. | METHODS, SYNTHETIC HOSTS AND REAGENTS FOR HYDROCARBON BIOSYNTHESIS |
| US11505809B2 (en) | 2017-09-28 | 2022-11-22 | Inv Nylon Chemicals Americas Llc | Organisms and biosynthetic processes for hydrocarbon synthesis |
| US20210079334A1 (en) | 2018-01-30 | 2021-03-18 | Genomatica, Inc. | Fermentation systems and methods with substantially uniform volumetric uptake rate of a reactive gaseous component |
| US11541105B2 (en) | 2018-06-01 | 2023-01-03 | The Research Foundation For The State University Of New York | Compositions and methods for disrupting biofilm formation and maintenance |
| EP3814515A2 (en) | 2018-06-26 | 2021-05-05 | Genomatica, Inc. | Engineered microorganisms with g3p---> 3pg enzyme and/or fructose-1,6-bisphosphatase including those having synthetic or enhanced methylotrophy |
| US20220258100A1 (en) * | 2019-07-16 | 2022-08-18 | San Diego State University (SDSU) Foundation, dba San Diego State University Research Foundation | Products of manufacture and methods for methane capturing using biofiltration |
Family Cites Families (158)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4076948A (en) | 1968-10-10 | 1978-02-28 | El Paso Products Company | Process for treatment of adipic acid mother liquor |
| US3513209A (en) | 1968-08-19 | 1970-05-19 | Du Pont | Method of making 1,4-cyclohexadiene |
| GB1230276A (enExample) | 1968-12-09 | 1971-04-28 | ||
| US3965182A (en) | 1969-10-02 | 1976-06-22 | Ethyl Corporation | Preparation of aniline from phenol and ammonia |
| JPS4831084B1 (enExample) | 1970-09-04 | 1973-09-26 | ||
| GB1344557A (en) | 1972-06-23 | 1974-01-23 | Mitsubishi Petrochemical Co | Process for preparing 1,4-butanediol |
| JPS5543759B2 (enExample) | 1972-06-28 | 1980-11-07 | ||
| DE2455617C3 (de) | 1974-11-23 | 1982-03-18 | Basf Ag, 6700 Ludwigshafen | Verfahren zur Herstellung von Butandiol und/oder Tetrahydrofuran über die Zwischenstufe des γ-Butyrolactons |
| DE2501499A1 (de) | 1975-01-16 | 1976-07-22 | Hoechst Ag | Verfahren zur herstellung von butandiol-(1.4) |
| US4190495A (en) | 1976-09-27 | 1980-02-26 | Research Corporation | Modified microorganisms and method of preparing and using same |
| US4301077A (en) | 1980-12-22 | 1981-11-17 | Standard Oil Company | Process for the manufacture of 1-4-butanediol and tetrahydrofuran |
| JPS60114197A (ja) | 1983-11-25 | 1985-06-20 | Agency Of Ind Science & Technol | 微生物によるジカルボン酸の製造法 |
| US4871667A (en) | 1984-11-26 | 1989-10-03 | Agency Of Industrial Science & Technology | Process for preparing muconic acid |
| US4652685A (en) | 1985-11-15 | 1987-03-24 | General Electric Company | Hydrogenation of lactones to glycols |
| US5168055A (en) | 1986-06-11 | 1992-12-01 | Rathin Datta | Fermentation and purification process for succinic acid |
| ES2036188T3 (es) | 1986-06-11 | 1993-05-16 | Michigan Biotechnology Institute | Un procedimiento para la produccion de acido succinico por fermentacion anaerobia. |
| US5143834A (en) | 1986-06-11 | 1992-09-01 | Glassner David A | Process for the production and purification of succinic acid |
| US5182199A (en) | 1987-05-27 | 1993-01-26 | Hartley Brian S | Thermophilic ethanol production in a two-stage closed system |
| JPH01195596A (ja) | 1988-01-30 | 1989-08-07 | Casio Comput Co Ltd | 値札システム |
| US5219757A (en) | 1988-04-27 | 1993-06-15 | Daicel Chemical Industries, Ltd. | Production of optically active 1,3-butanediol by asymmetric assimilation or reduction of 4-hydroxy-2-butanone |
| CA2062753C (en) | 1989-04-27 | 2005-04-26 | N. Robert Ward, Jr. | Precipitate test for microorganisms |
| US5192673A (en) | 1990-04-30 | 1993-03-09 | Michigan Biotechnology Institute | Mutant strain of C. acetobutylicum and process for making butanol |
| US5079143A (en) | 1990-05-02 | 1992-01-07 | The Upjohn Company | Method of indentifying compounds useful as antiparasitic drugs |
| EP0769557B1 (en) | 1990-10-15 | 2000-11-15 | Daicel Chemical Industries, Ltd. | Process for producing optically active 1,3-butanediol |
| US5173429A (en) | 1990-11-09 | 1992-12-22 | The Board Of Trustees Of The University Of Arkansas | Clostridiumm ljungdahlii, an anaerobic ethanol and acetate producing microorganism |
| IL100572A (en) | 1991-01-03 | 1997-01-10 | Lepetit Spa | Amides of antibiotic ge 2270 factors their preparation and pharmaceutical compositions containing them |
| US5416020A (en) | 1992-09-29 | 1995-05-16 | Bio-Technical Resources | Lactobacillus delbrueckii ssp. bulgaricus strain and fermentation process for producing L-(+)-lactic acid |
| US5807722A (en) | 1992-10-30 | 1998-09-15 | Bioengineering Resources, Inc. | Biological production of acetic acid from waste gases with Clostridium ljungdahlii |
| US6136577A (en) | 1992-10-30 | 2000-10-24 | Bioengineering Resources, Inc. | Biological production of ethanol from waste gases with Clostridium ljungdahlii |
| FR2702492B1 (fr) | 1993-03-12 | 1995-05-24 | Rhone Poulenc Chimie | Procédé de production par fermentation d'acide itaconique. |
| US5487987A (en) | 1993-09-16 | 1996-01-30 | Purdue Research Foundation | Synthesis of adipic acid from biomass-derived carbon sources |
| US5521075A (en) | 1994-12-19 | 1996-05-28 | Michigan Biotechnology Institute | Method for making succinic acid, anaerobiospirillum succiniciproducens variants for use in process and methods for obtaining variants |
| US5504004A (en) | 1994-12-20 | 1996-04-02 | Michigan Biotechnology Institute | Process for making succinic acid, microorganisms for use in the process and methods of obtaining the microorganisms |
| US5700934A (en) | 1995-03-01 | 1997-12-23 | Dsm N.V. | Process for the preparation of epsilon-caprolactam and epsilon-caprolactam precursors |
| US5478952A (en) | 1995-03-03 | 1995-12-26 | E. I. Du Pont De Nemours And Company | Ru,Re/carbon catalyst for hydrogenation in aqueous solution |
| US5863782A (en) | 1995-04-19 | 1999-01-26 | Women's And Children's Hospital | Synthetic mammalian sulphamidase and genetic sequences encoding same |
| US5686276A (en) | 1995-05-12 | 1997-11-11 | E. I. Du Pont De Nemours And Company | Bioconversion of a fermentable carbon source to 1,3-propanediol by a single microorganism |
| US5849970A (en) * | 1995-06-23 | 1998-12-15 | The Regents Of The University Of Colorado | Materials and methods for the bacterial production of isoprene |
| FR2736927B1 (fr) | 1995-07-18 | 1997-10-17 | Rhone Poulenc Fibres & Polymer | Enzymes a activite amidase, outils genetiques et microorganismes hotes permettant leur obtention et procede d'hydrolyse mettant en oeuvre lesdites enzymes |
| US5573931A (en) | 1995-08-28 | 1996-11-12 | Michigan Biotechnology Institute | Method for making succinic acid, bacterial variants for use in the process, and methods for obtaining variants |
| US5869301A (en) | 1995-11-02 | 1999-02-09 | Lockhead Martin Energy Research Corporation | Method for the production of dicarboxylic acids |
| US5770435A (en) | 1995-11-02 | 1998-06-23 | University Of Chicago | Mutant E. coli strain with increased succinic acid production |
| JP2000506012A (ja) | 1996-02-27 | 2000-05-23 | ミシガン ステイト ユニヴァーシティー | サーモアンエアロバクター・エタノリカス39eの第二級アルコールデヒドロゲナーゼをコードする遺伝子のクローニング及び発現並びに酵素の生化学的特徴付け |
| US5958745A (en) | 1996-03-13 | 1999-09-28 | Monsanto Company | Methods of optimizing substrate pools and biosynthesis of poly-β-hydroxybutyrate-co-poly-β-hydroxyvalerate in bacteria and plants |
| JP4101295B2 (ja) | 1996-07-01 | 2008-06-18 | バイオエンジニアリング・リソーシズ・インコーポレーテツド | 廃ガスからの酢酸の生物学的生産 |
| KR100459818B1 (ko) | 1996-09-02 | 2004-12-03 | 이. 아이. 두퐁 드 느무르 앤드 컴퍼니 | ε-카프로락탐의 제조방법 |
| US6117658A (en) | 1997-02-13 | 2000-09-12 | James Madison University | Methods of making polyhydroxyalkanoates comprising 4-hydroxybutyrate monomer units |
| KR100516986B1 (ko) | 1997-02-19 | 2005-09-26 | 코닌클리즈케 디에스엠 엔.브이. | 6-아미노카프론산 유도체를 과열 수증기와 접촉시킴으로써 촉매 없이 카프로락탐을 제조하는 방법 |
| US6274790B1 (en) | 1997-04-14 | 2001-08-14 | The University Of British Columbia | Nucleic acids encoding a plant enzyme involved in very long chain fatty acid synthesis |
| KR19990013007A (ko) | 1997-07-31 | 1999-02-25 | 박원훈 | 형질전환된 대장균 ss373(kctc 8818p)과 이를 이용한숙신산의 생산방법 |
| JPH11103863A (ja) | 1997-10-08 | 1999-04-20 | Nippon Shokubai Co Ltd | マレイン酸異性化酵素遺伝子 |
| US6280986B1 (en) | 1997-12-01 | 2001-08-28 | The United States Of America As Represented By The Secretary Of Agriculture | Stabilization of pet operon plasmids and ethanol production in bacterial strains lacking lactate dehydrogenase and pyruvate formate lyase activities |
| US20030087381A1 (en) | 1998-04-13 | 2003-05-08 | University Of Georgia Research Foundation, Inc. | Metabolically engineered organisms for enhanced production of oxaloacetate-derived biochemicals |
| WO1999053035A1 (en) | 1998-04-13 | 1999-10-21 | The University Of Georgia Research Foundation, Inc. | Pyruvate carboxylase overexpression for enhanced production of oxaloacetate-derived biochemicals in microbial cells |
| US6159738A (en) | 1998-04-28 | 2000-12-12 | University Of Chicago | Method for construction of bacterial strains with increased succinic acid production |
| DE19820652A1 (de) | 1998-05-08 | 1999-11-11 | Basf Ag | Kationische Rutheniumkomplexe, Verfahren zu ihrer Herstellung und ihre Verwendung |
| US6432686B1 (en) | 1998-05-12 | 2002-08-13 | E. I. Du Pont De Nemours And Company | Method for the production of 1,3-propanediol by recombinant organisms comprising genes for vitamin B12 transport |
| US6444784B1 (en) | 1998-05-29 | 2002-09-03 | Exxonmobil Research & Engineering Company | Wax crystal modifiers (LAW657) |
| WO2000004163A1 (en) | 1998-07-15 | 2000-01-27 | E.I. Du Pont De Nemours And Company | Tetrahydrofolate metabolism enzymes |
| DE19856136C2 (de) | 1998-12-04 | 2002-10-24 | Pasteur Institut | Verfahren und Vorrichtung zur Selektion beschleunigter Proliferation lebender Zellen in Suspension |
| WO2000046405A2 (en) | 1999-02-02 | 2000-08-10 | Bernhard Palsson | Methods for identifying drug targets based on genomic sequence data |
| US6686310B1 (en) | 1999-02-09 | 2004-02-03 | E. I. Du Pont De Nemours And Company | High surface area sol-gel route prepared hydrogenation catalysts |
| US6365376B1 (en) | 1999-02-19 | 2002-04-02 | E. I. Du Pont De Nemours And Company | Genes and enzymes for the production of adipic acid intermediates |
| US6448473B1 (en) | 1999-03-05 | 2002-09-10 | Monsanto Technology Llc | Multigene expression vectors for the biosynthesis of products via multienzyme biological pathways |
| CA2374482C (en) | 1999-05-21 | 2012-09-18 | Cargill Dow Llc | Methods and materials for the synthesis of organic products |
| US6852517B1 (en) | 1999-08-30 | 2005-02-08 | Wisconsin Alumni Research Foundation | Production of 3-hydroxypropionic acid in recombinant organisms |
| US6660857B2 (en) | 2000-02-03 | 2003-12-09 | Dsm N.V. | Process for the preparation of ε-caprolactam |
| US6878861B2 (en) | 2000-07-21 | 2005-04-12 | Washington State University Research Foundation | Acyl coenzyme A thioesterases |
| NZ523484A (en) | 2000-07-25 | 2005-01-28 | Emmaus Foundation Inc | Methods for increasing the production of ethanol from microbial fermentation |
| JP4490628B2 (ja) | 2000-11-20 | 2010-06-30 | カーギル インコーポレイテッド | 3−ヒドロキシプロピオン酸および他の有機化合物 |
| US7109010B2 (en) | 2000-11-22 | 2006-09-19 | Nature Works Llc | Methods and materials for the synthesis of organic products |
| CN1358841A (zh) | 2000-12-11 | 2002-07-17 | 云南省微生物研究所 | 云南链霉菌 |
| US7501268B2 (en) | 2000-12-28 | 2009-03-10 | Toyota Jidosha Kabushiki Kaisha | Methods of producing prenyl alcohols |
| CA2434224C (en) | 2001-01-10 | 2013-04-02 | The Penn State Research Foundation | Method and system for modeling cellular metabolism |
| US7127379B2 (en) | 2001-01-31 | 2006-10-24 | The Regents Of The University Of California | Method for the evolutionary design of biochemical reaction networks |
| US20030059792A1 (en) | 2001-03-01 | 2003-03-27 | Palsson Bernhard O. | Models and methods for determining systemic properties of regulated reaction networks |
| US6743610B2 (en) | 2001-03-30 | 2004-06-01 | The University Of Chicago | Method to produce succinic acid from raw hydrolysates |
| JP4630486B2 (ja) | 2001-05-28 | 2011-02-09 | ダイセル化学工業株式会社 | 新規な(r)−2,3−ブタンジオール脱水素酵素、その製造方法、及びこれを利用した光学活性アルコールの製造方法 |
| CA2356540A1 (en) | 2001-08-30 | 2003-02-28 | Emory University | Expressed dna sequences involved in mitochondrial functions |
| CA2466133A1 (en) | 2001-11-02 | 2003-05-15 | Rice University | Recycling system for manipulation of intracellular nadh availability |
| DE60327804D1 (de) | 2002-01-18 | 2009-07-09 | Novozymes As | Alanin-2,3-aminomutase |
| WO2003066863A1 (fr) | 2002-02-06 | 2003-08-14 | Showa Denko K.K. | Gene reductase d'un compose carbonyle alpha-substitue-alpha, beta-insature |
| US20030224363A1 (en) | 2002-03-19 | 2003-12-04 | Park Sung M. | Compositions and methods for modeling bacillus subtilis metabolism |
| CA2480216A1 (en) | 2002-03-29 | 2003-10-09 | Genomatica, Inc. | Human metabolic models and methods |
| US20050287655A1 (en) | 2002-05-10 | 2005-12-29 | Kyowa Hakko Kogyo Co., Ltd. | Process for producing mevalonic acid |
| US7856317B2 (en) | 2002-06-14 | 2010-12-21 | Genomatica, Inc. | Systems and methods for constructing genomic-based phenotypic models |
| US7826975B2 (en) | 2002-07-10 | 2010-11-02 | The Penn State Research Foundation | Method for redesign of microbial production systems |
| EP1532516A4 (en) | 2002-07-10 | 2007-05-23 | Penn State Res Found | Method for determining gene knockout strategies |
| WO2004007688A2 (en) | 2002-07-15 | 2004-01-22 | Kosan Biosciences, Inc. | Metabolic pathways for starter units in polyketide biosynthesis |
| AU2003287028B2 (en) | 2002-10-04 | 2008-09-04 | E.I. Du Pont De Nemours And Company | Process for the biological production of 1,3-propanediol with high yield |
| AU2003222214B2 (en) | 2002-10-15 | 2010-08-12 | The Regents Of The University Of California | Methods and systems to identify operational reaction pathways |
| AU2003287625A1 (en) | 2002-11-06 | 2004-06-03 | University Of Florida | Materials and methods for the efficient production of acetate and other products |
| CN1802341A (zh) | 2003-01-13 | 2006-07-12 | 卡吉尔公司 | 制备工业化学品的方法 |
| US7432091B2 (en) | 2003-02-24 | 2008-10-07 | Research Institute Of Innovative Technology For The Earth | Highly efficient hydrogen production method using microorganism |
| RU2268300C2 (ru) | 2003-04-07 | 2006-01-20 | Закрытое акционерное общество "Научно-исследовательский институт Аджиномото-Генетика" | СПОСОБ ПОЛУЧЕНИЯ L-АМИНОКИСЛОТ С ИСПОЛЬЗОВАНИЕМ БАКТЕРИЙ, ОБЛАДАЮЩИХ ПОВЫШЕННОЙ ЭКСПРЕССИЕЙ ГЕНА pckA |
| WO2005010182A1 (ja) | 2003-07-29 | 2005-02-03 | Research Institute Of Innovative Technology For The Earth | コリネ型細菌形質転換体及びそれを用いるジカルボン酸の製造方法 |
| US7927859B2 (en) | 2003-08-22 | 2011-04-19 | Rice University | High molar succinate yield bacteria by increasing the intracellular NADH availability |
| WO2005026349A1 (ja) | 2003-09-17 | 2005-03-24 | Mitsubishi Chemical Corporation | 非アミノ有機酸の製造方法 |
| US7244610B2 (en) | 2003-11-14 | 2007-07-17 | Rice University | Aerobic succinate production in bacteria |
| FR2864967B1 (fr) | 2004-01-12 | 2006-05-19 | Metabolic Explorer Sa | Microorganisme evolue pour la production de 1,2-propanediol |
| CN1926240B (zh) | 2004-01-19 | 2010-06-02 | 帝斯曼知识产权资产管理有限公司 | 6-氨基己酸的生物化学合成 |
| US7608700B2 (en) | 2004-03-08 | 2009-10-27 | North Carolina State University | Lactobacillus acidophilus nucleic acid sequences encoding stress-related proteins and uses therefor |
| DE102004031177A1 (de) | 2004-06-29 | 2006-01-19 | Henkel Kgaa | Neue Geruchsstoffe bildende Genprodukte von Bacillus licheniformis und darauf aufbauende verbesserte biotechnologische Produktionsverfahren |
| US7262046B2 (en) | 2004-08-09 | 2007-08-28 | Rice University | Aerobic succinate production in bacteria |
| JP2008510485A (ja) * | 2004-08-26 | 2008-04-10 | ザ ペン ステイト リサーチ ファウンデーション | 微生物産生系の再設計法 |
| CN101044245B (zh) | 2004-08-27 | 2012-05-02 | 莱斯大学 | 具有增加的琥珀酸产量的突变大肠杆菌菌株 |
| EP2434015B1 (en) | 2004-09-09 | 2013-11-20 | Research Institute Of Innovative Technology For The Earth | DNA fragment having promoter function |
| EP1789569A2 (en) | 2004-09-17 | 2007-05-30 | Rice University | High succinate producing bacteria |
| EP1801227B1 (en) | 2004-10-14 | 2009-04-15 | Sumitomo Chemical Company, Limited | Method for producing 2-hydroxy-4-(methylthio)butanoic acid |
| US7569380B2 (en) | 2004-12-22 | 2009-08-04 | Rice University | Simultaneous anaerobic production of isoamyl acetate and succinic acid |
| DE102005002127A1 (de) * | 2005-01-17 | 2006-07-20 | Basf Ag | Verfahren zur Herstellung von Butadien aus n-Butan |
| JP2006204255A (ja) | 2005-01-31 | 2006-08-10 | Canon Inc | アセチル−CoAアシルトランスフェラーゼ遺伝子破壊ポリヒドロキシアルカノエート生産菌、またこれを利用したポリヒドロキシアルカノエート生産方法 |
| EP1874334A4 (en) | 2005-04-15 | 2011-03-30 | Vascular Biogenics Ltd | COMPOSITIONS WITH BETA 2-GLYCOPROTEIN I-PEPTIDES FOR THE PREVENTION AND / OR TREATMENT OF VASCULAR DISEASES |
| KR100679638B1 (ko) | 2005-08-19 | 2007-02-06 | 한국과학기술원 | 포메이트 디하이드로게나제 d 또는 e를 코딩하는 유전자로 형질전환된 미생물 및 이를 이용한 숙신산의 제조방법 |
| KR100676160B1 (ko) | 2005-08-19 | 2007-02-01 | 한국과학기술원 | 말릭효소를 코딩하는 유전자로 형질전환된 재조합 미생물 및 이를 이용한 숙신산의 제조방법 |
| AU2006287257A1 (en) | 2005-09-09 | 2007-03-15 | Genomatica, Inc. | Methods and organisms for the growth-coupled production of succinate |
| US9297028B2 (en) | 2005-09-29 | 2016-03-29 | Butamax Advanced Biofuels Llc | Fermentive production of four carbon alcohols |
| NZ566406A (en) | 2005-10-26 | 2012-04-27 | Butamax Advanced Biofuels Llc | Fermentive production of four carbon alcohols |
| GB2433260A (en) | 2005-12-16 | 2007-06-20 | Mologic Ltd | A selectable decarboxylase marker |
| US8206970B2 (en) | 2006-05-02 | 2012-06-26 | Butamax(Tm) Advanced Biofuels Llc | Production of 2-butanol and 2-butanone employing aminobutanol phosphate phospholyase |
| DE102006025821A1 (de) | 2006-06-02 | 2007-12-06 | Degussa Gmbh | Ein Enzym zur Herstellung von Mehylmalonatsemialdehyd oder Malonatsemialdehyd |
| US8017364B2 (en) | 2006-12-12 | 2011-09-13 | Butamax(Tm) Advanced Biofuels Llc | Solvent tolerant microorganisms |
| AU2008206403A1 (en) | 2007-01-12 | 2008-07-24 | The Regents Of The University Of Colorado, A Body Corporate | Compositions and methods for enhancing tolerance for the production of organic chemicals produced by microorganisms |
| CA2678946C (en) | 2007-03-16 | 2019-02-12 | Genomatica, Inc. | Compositions and methods for the biosynthesis of 1,4-butanediol and its precursors |
| EP2147111A4 (en) | 2007-04-18 | 2010-06-23 | Gevo Inc | MANIPULATED MICROORGANISMS FOR THE MANUFACTURE OF ISOPROPANOL |
| US20080274522A1 (en) | 2007-05-02 | 2008-11-06 | Bramucci Michael G | Method for the production of 2-butanone |
| WO2008144060A2 (en) | 2007-05-17 | 2008-11-27 | Tetravitae Bioscience, Inc. | Methods and compositions for producing solvents |
| EP2017344A1 (en) | 2007-07-20 | 2009-01-21 | Nederlandse Organisatie voor toegepast- natuurwetenschappelijk onderzoek TNO | Production of itaconic acid |
| CN101970673A (zh) | 2007-07-23 | 2011-02-09 | 帝斯曼知识产权资产管理有限公司 | 真核细胞中的丁醇生产 |
| US7947483B2 (en) | 2007-08-10 | 2011-05-24 | Genomatica, Inc. | Methods and organisms for the growth-coupled production of 1,4-butanediol |
| AU2008310573A1 (en) | 2007-10-12 | 2009-04-16 | The Regents Of The University Of California | Microorganism engineered to produce isopropanol |
| BRPI0823506A8 (pt) * | 2007-12-13 | 2018-05-15 | Danisco Us Inc Genencor Div | composições e métodos para a produção de isoprene |
| US8183028B2 (en) | 2007-12-21 | 2012-05-22 | Ls9, Inc. | Methods and compositions for producing olefins |
| CA2712779C (en) | 2008-01-22 | 2021-03-16 | Genomatica, Inc. | Methods and organisms for utilizing synthesis gas or other gaseous carbon sources and methanol |
| CN102015995B (zh) * | 2008-03-03 | 2014-10-22 | 焦耳无限科技公司 | 产生碳基目的产物的二氧化碳固定工程微生物 |
| SI2265709T1 (en) | 2008-03-27 | 2018-03-30 | Genomatica, Inc. | MICROORGANISMS FOR PREPARATION OF ADYPIC ACID AND OTHER COMPOUNDS |
| JP2011518564A (ja) | 2008-04-23 | 2011-06-30 | ダニスコ・ユーエス・インク | 改良された微生物によるイソプレン産出用のイソプレンシンターゼ変異体 |
| JP2011519561A (ja) | 2008-05-01 | 2011-07-14 | ジェノマティカ, インコーポレイテッド | メタクリル酸の産生のための微生物 |
| US8129154B2 (en) | 2008-06-17 | 2012-03-06 | Genomatica, Inc. | Microorganisms and methods for the biosynthesis of fumarate, malate, and acrylate |
| SG167485A1 (en) | 2008-06-30 | 2011-01-28 | Danisco Us Inc | Polymers of isoprene from renewable resources |
| EP2313491A4 (en) | 2008-07-08 | 2011-12-07 | Opx Biotechnologies Inc | METHODS, COMPOSITIONS AND SYSTEMS FOR BIOSYNTHETIC BIOPRODUCTION OF 1,4-BUTANEDIOL |
| WO2010022763A1 (en) | 2008-08-25 | 2010-03-04 | Metabolic Explorer | Method for the preparation of 2-hydroxy-isobutyrate |
| CA2735883C (en) * | 2008-09-10 | 2020-05-05 | Genomatica, Inc. | Microorganisms for the production of 1,4-butanediol |
| CA2737082A1 (en) * | 2008-09-15 | 2010-03-18 | Danisco Us Inc. | Increased isoprene production using mevalonate kinase and isoprene synthase |
| WO2010031079A1 (en) | 2008-09-15 | 2010-03-18 | Danisco Us Inc. | Systems using cell culture for production of isoprene |
| US8357826B2 (en) | 2008-10-16 | 2013-01-22 | Karl Kharas | Methods and apparatus for synthesis of alcohols from syngas |
| EP3260532A1 (en) | 2008-12-12 | 2017-12-27 | CJ Research Center LLC | Green process and compositions for producing poly(5hv) and 5 carbon chemicals |
| KR20110097951A (ko) | 2008-12-16 | 2011-08-31 | 게노마티카 인코포레이티드 | 합성가스와 다른 탄소원을 유용 제품으로 전환시키기 위한 미생물 및 방법 |
| BRPI1013426A2 (pt) * | 2009-04-30 | 2016-11-08 | Genomatica Inc | organismos para produção de 1,3-butanodiol |
| SI3392340T1 (sl) | 2009-06-04 | 2022-05-31 | Genomatica, Inc. | Mikroorganizmi za proizvodnjo 1,4-BUTANDIOLA in pripadajoči postopki |
| US20120276606A1 (en) | 2009-10-30 | 2012-11-01 | Daicel Corporation | Recombinant microorganisms with 1,3-butanediol-producing function and uses thereof |
| CN109136161A (zh) | 2009-12-10 | 2019-01-04 | 基因组股份公司 | 合成气或其他气态碳源和甲醇转化为1,3-丁二醇的方法和有机体 |
| MX336229B (es) | 2010-05-05 | 2016-01-08 | Genomatica Inc | Microorganismos y metodos para la biosintesis de butadieno. |
| MX348334B (es) * | 2011-02-02 | 2017-06-07 | Genomatica Inc | Microorganismo y metodos para la biosintesis de butadieno. |
| US9169486B2 (en) * | 2011-06-22 | 2015-10-27 | Genomatica, Inc. | Microorganisms for producing butadiene and methods related thereto |
| BR112014008061A2 (pt) * | 2011-10-19 | 2017-04-11 | Scientist Of Fortune Sa | método para a produção enzimática de butadieno |
| US20150050708A1 (en) * | 2013-03-15 | 2015-02-19 | Genomatica, Inc. | Microorganisms and methods for producing butadiene and related compounds by formate assimilation |
| EP2971021A4 (en) * | 2013-03-15 | 2016-12-21 | Genomatica Inc | MICROORGANISMS AND METHOD FOR THE PRODUCTION OF BUTADIENE AND RELATED COMPOUNDS BY FORMATASSIMILATION |
-
2011
- 2011-05-04 MX MX2012012827A patent/MX336229B/es unknown
- 2011-05-04 JP JP2013509203A patent/JP5911847B2/ja active Active
- 2011-05-04 CN CN201180033199.2A patent/CN103080324B/zh active Active
- 2011-05-04 KR KR1020177037394A patent/KR20180005263A/ko not_active Ceased
- 2011-05-04 BR BR112012028049A patent/BR112012028049A2/pt not_active IP Right Cessation
- 2011-05-04 SG SG10201503501TA patent/SG10201503501TA/en unknown
- 2011-05-04 KR KR1020197001071A patent/KR20190006103A/ko not_active Ceased
- 2011-05-04 WO PCT/US2011/035105 patent/WO2011140171A2/en not_active Ceased
- 2011-05-04 KR KR1020127031134A patent/KR101814648B1/ko active Active
- 2011-05-04 SG SG2012081519A patent/SG185432A1/en unknown
- 2011-05-04 CA CA2797409A patent/CA2797409C/en not_active Expired - Fee Related
- 2011-05-04 CN CN201610340556.4A patent/CN106047782A/zh active Pending
- 2011-05-04 AU AU2011248182A patent/AU2011248182A1/en not_active Abandoned
- 2011-05-04 EP EP11778232.6A patent/EP2566969B1/en active Active
- 2011-05-04 US US13/101,046 patent/US8580543B2/en active Active
- 2011-05-05 TW TW100115805A patent/TWI575072B/zh not_active IP Right Cessation
- 2011-05-05 TW TW105130341A patent/TW201720931A/zh unknown
-
2012
- 2012-11-06 CO CO12199796A patent/CO6630182A2/es unknown
-
2013
- 2013-10-21 US US14/059,131 patent/US9732361B2/en not_active Expired - Fee Related
-
2016
- 2016-02-26 AU AU2016201231A patent/AU2016201231A1/en not_active Abandoned
- 2016-03-30 JP JP2016068884A patent/JP2016154551A/ja active Pending
-
2017
- 2017-07-10 US US15/645,880 patent/US10487343B2/en not_active Expired - Fee Related
-
2018
- 2018-08-16 JP JP2018153224A patent/JP2018196387A/ja active Pending
-
2019
- 2019-10-25 US US16/664,648 patent/US20200299736A1/en not_active Abandoned
-
2021
- 2021-03-05 US US17/193,914 patent/US20220025411A1/en not_active Abandoned
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101814648B1 (ko) | 부타디엔을 생합성하기 위한 미생물 및 방법 | |
| US20210147881A1 (en) | Microorganisms and methods for the biosynthesis of butadiene | |
| AU2013203173B2 (en) | Microorganisms and methods for the biosynthesis of butadiene | |
| AU2019204872A1 (en) | Microorganisms and methods for the biosynthesis of butadiene | |
| AU2013202445A1 (en) | Microorganisms and methods for the biosynthesis of butadiene |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20171227 Application number text: 1020127031134 Filing date: 20121128 |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20180126 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20180409 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20181012 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20180409 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |
|
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20190111 Application number text: 1020127031134 Filing date: 20121128 |