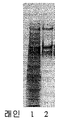
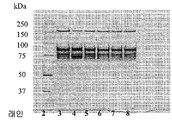
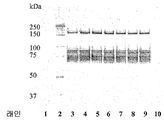
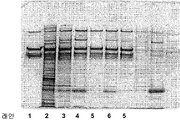
KR20160104740A - 응고 인자 viii을 정제하는 방법 - Google Patents
응고 인자 viii을 정제하는 방법 Download PDFInfo
- Publication number
- KR20160104740A KR20160104740A KR1020167023450A KR20167023450A KR20160104740A KR 20160104740 A KR20160104740 A KR 20160104740A KR 1020167023450 A KR1020167023450 A KR 1020167023450A KR 20167023450 A KR20167023450 A KR 20167023450A KR 20160104740 A KR20160104740 A KR 20160104740A
- Authority
- KR
- South Korea
- Prior art keywords
- fviii
- resin
- buffer
- composition
- chromatography
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/14—Extraction; Separation; Purification
- C07K1/16—Extraction; Separation; Purification by chromatography
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/36—Blood coagulation or fibrinolysis factors
- A61K38/37—Factors VIII
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/55—Protease inhibitors
- A61K38/57—Protease inhibitors from animals; from humans
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D15/00—Separating processes involving the treatment of liquids with solid sorbents; Apparatus therefor
- B01D15/08—Selective adsorption, e.g. chromatography
- B01D15/26—Selective adsorption, e.g. chromatography characterised by the separation mechanism
- B01D15/38—Selective adsorption, e.g. chromatography characterised by the separation mechanism involving specific interaction not covered by one or more of groups B01D15/265 and B01D15/30 - B01D15/36, e.g. affinity, ligand exchange or chiral chromatography
- B01D15/3847—Multimodal interactions
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/14—Extraction; Separation; Purification
- C07K1/36—Extraction; Separation; Purification by a combination of two or more processes of different types
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/745—Blood coagulation or fibrinolysis factors
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/745—Blood coagulation or fibrinolysis factors
- C07K14/755—Factors VIII, e.g. factor VIII C (AHF), factor VIII Ag (VWF)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/48—Hydrolases (3) acting on peptide bonds (3.4)
- C12N9/50—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25)
- C12N9/64—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue
- C12N9/6421—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue from mammals
- C12N9/6424—Serine endopeptidases (3.4.21)
- C12N9/647—Blood coagulation factors not provided for in a preceding group or according to more than one of the proceeding groups
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Zoology (AREA)
- Genetics & Genomics (AREA)
- Engineering & Computer Science (AREA)
- Gastroenterology & Hepatology (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Hematology (AREA)
- Biophysics (AREA)
- Animal Behavior & Ethology (AREA)
- Immunology (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Toxicology (AREA)
- Analytical Chemistry (AREA)
- Biomedical Technology (AREA)
- Wood Science & Technology (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- General Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12940208P | 2008-06-24 | 2008-06-24 | |
| EP08158893.1 | 2008-06-24 | ||
| US61/129,402 | 2008-06-24 | ||
| EP08158893 | 2008-06-24 | ||
| PCT/EP2009/057883 WO2009156430A1 (en) | 2008-06-24 | 2009-06-24 | A process of purifying coagulation factor viii |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020107028866A Division KR101700722B1 (ko) | 2008-06-24 | 2009-06-24 | 응고 인자 viii을 정제하는 방법 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177024076A Division KR101804136B1 (ko) | 2008-06-24 | 2009-06-24 | 응고 인자 viii을 정제하는 방법 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20160104740A true KR20160104740A (ko) | 2016-09-05 |
Family
ID=40076764
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167023450A Abandoned KR20160104740A (ko) | 2008-06-24 | 2009-06-24 | 응고 인자 viii을 정제하는 방법 |
| KR1020177024076A Active KR101804136B1 (ko) | 2008-06-24 | 2009-06-24 | 응고 인자 viii을 정제하는 방법 |
| KR1020107028866A Active KR101700722B1 (ko) | 2008-06-24 | 2009-06-24 | 응고 인자 viii을 정제하는 방법 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177024076A Active KR101804136B1 (ko) | 2008-06-24 | 2009-06-24 | 응고 인자 viii을 정제하는 방법 |
| KR1020107028866A Active KR101700722B1 (ko) | 2008-06-24 | 2009-06-24 | 응고 인자 viii을 정제하는 방법 |
Country Status (19)
| Country | Link |
|---|---|
| US (1) | US8329871B2 (cg-RX-API-DMAC7.html) |
| EP (2) | EP2300497B1 (cg-RX-API-DMAC7.html) |
| JP (1) | JP5619738B2 (cg-RX-API-DMAC7.html) |
| KR (3) | KR20160104740A (cg-RX-API-DMAC7.html) |
| CN (1) | CN102066417B (cg-RX-API-DMAC7.html) |
| AU (1) | AU2009264282B2 (cg-RX-API-DMAC7.html) |
| BR (1) | BRPI0914695B1 (cg-RX-API-DMAC7.html) |
| CA (1) | CA2728047C (cg-RX-API-DMAC7.html) |
| DK (2) | DK2537862T3 (cg-RX-API-DMAC7.html) |
| ES (2) | ES2391613T3 (cg-RX-API-DMAC7.html) |
| IL (3) | IL209758A (cg-RX-API-DMAC7.html) |
| MX (1) | MX2010013908A (cg-RX-API-DMAC7.html) |
| PL (1) | PL2300497T3 (cg-RX-API-DMAC7.html) |
| PT (1) | PT2300497E (cg-RX-API-DMAC7.html) |
| RU (2) | RU2567811C2 (cg-RX-API-DMAC7.html) |
| SI (1) | SI2300497T1 (cg-RX-API-DMAC7.html) |
| UA (1) | UA100901C2 (cg-RX-API-DMAC7.html) |
| WO (1) | WO2009156430A1 (cg-RX-API-DMAC7.html) |
| ZA (1) | ZA201009162B (cg-RX-API-DMAC7.html) |
Families Citing this family (42)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101293504B1 (ko) | 2007-07-11 | 2013-08-07 | 노보 노르디스크 에이/에스 | 혼합형 또는 다중형 수지를 사용하는 인자 vⅰⅰⅰ의 정제 |
| EP2027875A1 (en) * | 2007-08-23 | 2009-02-25 | Octapharma AG | A Process for Isolation and Purification of a Target Protein free of Prion Protein (PrPSC) |
| RU2011110459A (ru) | 2008-08-21 | 2012-09-27 | Октафарма АГ (CH) | Рекомбинантно полученный человеческий фактор viii и ix |
| CN107050437A (zh) * | 2008-09-03 | 2017-08-18 | 奥克塔法马股份有限公司 | 重组制备的因子viii的新型保护组合物 |
| US20110166332A1 (en) * | 2008-09-12 | 2011-07-07 | Ge Healthcare Bio-Sciences Ab | Enhanced antibody aggregate removal with capto adhere in the presence of protein-excluded zwitterions |
| DK3040346T3 (en) * | 2010-03-30 | 2018-06-14 | Octapharma Ag | PROCEDURE FOR CLEANING THE GRANULOCYT COLONY STIMULATING FACTOR, G-CSF |
| ES2740825T3 (es) * | 2010-03-30 | 2020-02-06 | Octapharma Ag | Un procedimiento de purificación de proteínas dependientes de vitamina K |
| KR102049254B1 (ko) | 2010-04-20 | 2019-11-28 | 옥타파마 아게 | 약학 단백질을 위한 신규한 안정화제 |
| WO2012049285A1 (en) | 2010-10-14 | 2012-04-19 | Octapharma Ag | A method for the quantitative glycosylation analysis of proteins |
| MX342235B (es) * | 2010-11-05 | 2016-09-21 | Hoffmann La Roche | Metodo optimizado para captura de anticuerpo por cromatografia de modo mixto. |
| DK3626737T3 (da) * | 2011-05-13 | 2024-02-05 | Octapharma Ag | Fremgangsmåde til at forøge produktiviteten af eukaryote celler i produktionen af rekombinant fviii |
| JP5640189B2 (ja) | 2011-06-17 | 2014-12-17 | 学校法人東日本学園 | ループスアンチコアグラント検出用血液凝固時間の測定方法 |
| EP2771358A4 (en) * | 2011-10-26 | 2015-08-26 | Bio Rad Laboratories | ELIMINATION OF VIRAL AGENTS IN MIXED MODE CHROMATOGRAPHY |
| MX379269B (es) | 2012-10-24 | 2025-03-11 | Genzyme Corp | Elucion de biomoleculas de resinas multimodales utilizando mes y mops como modificadores de fase movil. |
| US20140154233A1 (en) * | 2012-12-05 | 2014-06-05 | Csl Limited | Method of purifying therapeutic proteins |
| US10188965B2 (en) | 2012-12-05 | 2019-01-29 | Csl Behring Gmbh | Hydrophobic charge induction chromatographic depletion of a protein from a solution |
| CN103880947B (zh) * | 2012-12-21 | 2016-01-13 | 武汉禾元生物科技股份有限公司 | 一种分离纯化高纯度重组人血清白蛋白的层析方法 |
| EP2964663A2 (en) * | 2013-03-08 | 2016-01-13 | Genzyme Corporation | Continuous purification of therapeutic proteins |
| EP3008085A4 (en) * | 2013-06-13 | 2017-05-10 | Biogen MA Inc. | Anti-factor viii antibodies or uses thereof |
| CA2910770C (en) | 2013-07-12 | 2018-02-27 | Merck Patent Gmbh | Removal of fragments from a sample containing a target protein using activated carbon |
| EP3875106A1 (en) * | 2013-08-08 | 2021-09-08 | Bioverativ Therapeutics Inc. | Purification of chimeric fviii molecules |
| AU2014326257B2 (en) | 2013-09-24 | 2017-08-10 | Pfizer Inc. | Compositions comprising heterogeneous populations of recombinant human clotting factor Xa proteins |
| TWI709569B (zh) | 2014-01-17 | 2020-11-11 | 美商健臻公司 | 無菌層析樹脂及其用於製造方法的用途 |
| TWI709570B (zh) * | 2014-01-17 | 2020-11-11 | 美商健臻公司 | 無菌層析法及製法 |
| CA2934705C (en) * | 2014-01-20 | 2020-03-31 | Octapharma Ag | A process for manufacturing factor viii having an improved ratio of fviii:c/fviii:ag |
| DK3097188T3 (en) * | 2014-01-24 | 2018-10-08 | Am Pharma Bv | DOWNSTREAM PREPARATION OF AN ALKALIC PHOSPHATASE |
| JP6609561B2 (ja) * | 2014-02-04 | 2019-11-20 | バイオジェン・エムエイ・インコーポレイテッド | 翻訳後修飾を濃縮するための、フロースルーモードにおける陽イオン交換クロマトグラフィーの使用 |
| CN104861060A (zh) * | 2014-02-21 | 2015-08-26 | 神州细胞工程有限公司 | 一种纯化凝血因子viii的方法 |
| EP3149023A4 (en) | 2014-05-29 | 2017-11-22 | Agency for Science, Technology and Research | Protein Extraction Methods |
| US10626164B2 (en) * | 2014-07-25 | 2020-04-21 | Csl Limited | Purification of VWF |
| GB201506113D0 (en) | 2015-04-10 | 2015-05-27 | Ge Healthcare Bio Sciences Ab | Method for chromatography |
| GB201506117D0 (en) | 2015-04-10 | 2015-05-27 | Ge Healthcare Bio Sciences Ab | Method for chromatography |
| WO2016207328A1 (en) * | 2015-06-24 | 2016-12-29 | Glycotope Gmbh | PROCESS FOR THE PURIFICATION OF γ-CARBOXYLATED POLYPEPTIDES |
| EP3205665A1 (en) | 2016-02-11 | 2017-08-16 | Octapharma AG | Method of separating factor viii from blood products |
| GB201617240D0 (en) * | 2016-10-11 | 2016-11-23 | Profactor Pharma Ltd | Purification process |
| US11299533B2 (en) | 2017-06-23 | 2022-04-12 | Takeda Pharmaceutical Company Limited | Purification of factor VIII subspecies |
| CN107226859B (zh) * | 2017-08-10 | 2020-11-24 | 博雅生物制药集团股份有限公司 | 一种人凝血因子ⅷ的制备方法 |
| KR102286260B1 (ko) | 2017-08-31 | 2021-08-05 | 주식회사 녹십자 | 설파타아제 단백질을 정제하는 방법 |
| AU2018357917B2 (en) | 2017-10-30 | 2024-08-01 | Takeda Pharmaceutical Company Limited | Environmentally compatible detergents for inactivation of lipid-enveloped viruses |
| BR112021003420A2 (pt) | 2018-08-31 | 2021-05-18 | Genzyme Corporation | resina cromatográfica estéril e uso da mesma em processos de manufatura |
| TWI787710B (zh) * | 2020-01-20 | 2022-12-21 | 中國大陸商上海藥明生物技術有限公司 | 用於親和層析的新型清洗緩衝液 |
| CN118217951B (zh) * | 2024-04-01 | 2025-05-23 | 山东建筑大学 | 一种水中的高纯度磷回收装置和方法 |
Family Cites Families (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4540573A (en) | 1983-07-14 | 1985-09-10 | New York Blood Center, Inc. | Undenatured virus-free biologically active protein derivatives |
| US5605884A (en) * | 1987-10-29 | 1997-02-25 | Rhone-Poulenc Rorer Pharmaceuticals Inc. | Factor VIII formulations in high ionic strength media |
| US5316680A (en) * | 1992-10-21 | 1994-05-31 | Cornell Research Foundation, Inc. | Multimodal chromatographic separation media and process for using same |
| JPH08506710A (ja) | 1993-06-11 | 1996-07-16 | ノーザン・テレコム・リミテッド | ユーザ制御呼管理サービス方法および装置 |
| DE4337573C1 (de) | 1993-11-04 | 1995-05-18 | Octapharma Ag | Verfahren zur Herstellung einer virusinaktivierten Faktor VIII enthaltenden Fraktion mittels chromatographischer Methoden |
| SE9403915D0 (sv) | 1994-11-14 | 1994-11-14 | Annelie Almstedt | Process A |
| AUPR638801A0 (en) * | 2001-07-13 | 2001-08-09 | Life Therapeutics Limited | Factor viii separation |
| NZ548126A (en) | 2004-02-27 | 2009-10-30 | Ge Healthcare Bio Sciences Ab | A process for the purification of antibodies involving addition of a second resin |
| EP1765866B1 (en) * | 2004-06-07 | 2014-01-08 | Therapure Biopharma Inc. | Isolation of plasma or serum proteins |
| EP1707634A1 (en) * | 2005-03-29 | 2006-10-04 | Octapharma AG | Method for isolation of recombinantly produced proteins |
| FR2887883B1 (fr) * | 2005-06-29 | 2007-08-31 | Lab Francais Du Fractionnement | Procede de separation des proteines fibrinogene, facteur xiii et colle biologique d'une fraction plasmatique solubilisee et de preparation de concentres lyophilises desdites proteines |
| EP1739179A1 (en) | 2005-06-30 | 2007-01-03 | Octapharma AG | Serum-free stable transfection and production of recombinant human proteins in human cell lines |
| KR101520105B1 (ko) * | 2006-07-14 | 2015-05-21 | 제넨테크, 인크. | 재조합 단백질의 리폴딩 |
| RU2324495C1 (ru) * | 2006-08-31 | 2008-05-20 | Федеральное государственное унитарное предприятие "Научно-производственное объединение по медицинским иммунобиологическим препаратам "Микроген" Министерства здравоохранения Российской Федерации | Способ получения препарата фактора свертывания viii крови человека |
| WO2008092644A2 (en) * | 2007-02-01 | 2008-08-07 | Baxter International Inc. | Fviii-independent fix-mutant proteins for hemophilia a treatment |
| JP2010529155A (ja) * | 2007-06-13 | 2010-08-26 | ツェー・エス・エル・ベーリング・ゲー・エム・ベー・ハー | 出血性疾患の治療及び予防的処置における血管外投与のためのvwf安定化fviii製剤及びfviiiなしのvwf製剤の使用 |
| KR101293504B1 (ko) | 2007-07-11 | 2013-08-07 | 노보 노르디스크 에이/에스 | 혼합형 또는 다중형 수지를 사용하는 인자 vⅰⅰⅰ의 정제 |
| GB0723712D0 (en) * | 2007-12-04 | 2008-01-16 | Apitope Technology Bristol Ltd | Peptides |
| DE102008032361A1 (de) * | 2008-07-10 | 2010-01-21 | Csl Behring Gmbh | Der Einsatz von Faktor VIII und vWF bzw. vWF-enthaltenden Konzentraten zur Therapie der durch Thrombocyten-Inhibitoren induzierte Koagulopathie |
-
2009
- 2009-06-24 AU AU2009264282A patent/AU2009264282B2/en active Active
- 2009-06-24 KR KR1020167023450A patent/KR20160104740A/ko not_active Abandoned
- 2009-06-24 ES ES09769279T patent/ES2391613T3/es active Active
- 2009-06-24 DK DK12179389.7T patent/DK2537862T3/en active
- 2009-06-24 UA UAA201100699A patent/UA100901C2/ru unknown
- 2009-06-24 JP JP2011515372A patent/JP5619738B2/ja active Active
- 2009-06-24 MX MX2010013908A patent/MX2010013908A/es active IP Right Grant
- 2009-06-24 KR KR1020177024076A patent/KR101804136B1/ko active Active
- 2009-06-24 RU RU2011102437/10A patent/RU2567811C2/ru active
- 2009-06-24 KR KR1020107028866A patent/KR101700722B1/ko active Active
- 2009-06-24 DK DK09769279.2T patent/DK2300497T3/da active
- 2009-06-24 EP EP09769279A patent/EP2300497B1/en active Active
- 2009-06-24 CN CN200980123974.6A patent/CN102066417B/zh active Active
- 2009-06-24 RU RU2015141849A patent/RU2698392C2/ru active
- 2009-06-24 EP EP12179389.7A patent/EP2537862B1/en not_active Revoked
- 2009-06-24 PT PT09769279T patent/PT2300497E/pt unknown
- 2009-06-24 BR BRPI0914695-4A patent/BRPI0914695B1/pt active IP Right Grant
- 2009-06-24 ES ES12179389.7T patent/ES2538706T3/es active Active
- 2009-06-24 PL PL09769279T patent/PL2300497T3/pl unknown
- 2009-06-24 WO PCT/EP2009/057883 patent/WO2009156430A1/en not_active Ceased
- 2009-06-24 SI SI200930362T patent/SI2300497T1/sl unknown
- 2009-06-24 US US12/737,230 patent/US8329871B2/en active Active
- 2009-06-24 CA CA2728047A patent/CA2728047C/en active Active
-
2010
- 2010-12-05 IL IL209758A patent/IL209758A/en active IP Right Grant
- 2010-12-05 IL IL229583A patent/IL229583B/en active IP Right Grant
- 2010-12-21 ZA ZA2010/09162A patent/ZA201009162B/en unknown
-
2013
- 2013-11-24 IL IL229583A patent/IL229583A0/en unknown
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101804136B1 (ko) | 응고 인자 viii을 정제하는 방법 | |
| DK2553095T3 (en) | PROCEDURE FOR CLEANING VITAMIN K-DEPENDENT PROTEINS SUCH AS COAGULATION FACTOR IX | |
| CA3085885A1 (en) | Protein purification and virus inactivation with alkyl glycosides |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| A201 | Request for examination | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20160825 Application number text: 1020107028866 Filing date: 20101222 |
|
| PA0201 | Request for examination | ||
| PG1501 | Laying open of application | ||
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20160928 Patent event code: PE09021S01D |
|
| AMND | Amendment | ||
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20170529 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20160928 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |
|
| A107 | Divisional application of patent | ||
| AMND | Amendment | ||
| J201 | Request for trial against refusal decision | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20170828 Application number text: 1020107028866 Filing date: 20101222 |
|
| PJ0201 | Trial against decision of rejection |
Patent event date: 20170828 Comment text: Request for Trial against Decision on Refusal Patent event code: PJ02012R01D Patent event date: 20170529 Comment text: Decision to Refuse Application Patent event code: PJ02011S01I Appeal kind category: Appeal against decision to decline refusal Decision date: 20171027 Appeal identifier: 2017101004112 Request date: 20170828 |
|
| PB0901 | Examination by re-examination before a trial |
Comment text: Amendment to Specification, etc. Patent event date: 20170828 Patent event code: PB09011R02I Comment text: Request for Trial against Decision on Refusal Patent event date: 20170828 Patent event code: PB09011R01I Comment text: Amendment to Specification, etc. Patent event date: 20161228 Patent event code: PB09011R02I |
|
| B701 | Decision to grant | ||
| PB0701 | Decision of registration after re-examination before a trial |
Patent event date: 20171027 Comment text: Decision to Grant Registration Patent event code: PB07012S01D Patent event date: 20170928 Comment text: Transfer of Trial File for Re-examination before a Trial Patent event code: PB07011S01I |
|
| PC1904 | Unpaid initial registration fee |