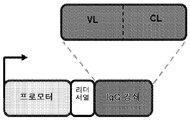
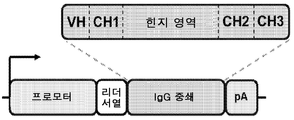
KR20150093834A - Dna 항체 작제물 및 그 이용 방법 - Google Patents
Dna 항체 작제물 및 그 이용 방법 Download PDFInfo
- Publication number
- KR20150093834A KR20150093834A KR1020157018712A KR20157018712A KR20150093834A KR 20150093834 A KR20150093834 A KR 20150093834A KR 1020157018712 A KR1020157018712 A KR 1020157018712A KR 20157018712 A KR20157018712 A KR 20157018712A KR 20150093834 A KR20150093834 A KR 20150093834A
- Authority
- KR
- South Korea
- Prior art keywords
- acid sequence
- ser
- nucleic acid
- val
- gly
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
- C07K16/10—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses from RNA viruses
- C07K16/1081—Togaviridae, e.g. flavivirus, rubella virus, hog cholera virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
- A61K39/21—Retroviridae, e.g. equine infectious anemia virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/42—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum viral
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/30—Cellular immunotherapy characterised by the recombinant expression of specific molecules in the cells of the immune system
- A61K40/33—Antibodies; T-cell engagers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
- C07K16/10—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses from RNA viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
- C07K16/10—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses from RNA viruses
- C07K16/1036—Retroviridae, e.g. leukemia viruses
- C07K16/1045—Lentiviridae, e.g. HIV, FIV, SIV
- C07K16/1063—Lentiviridae, e.g. HIV, FIV, SIV env, e.g. gp41, gp110/120, gp160, V3, PND, CD4 binding site
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/32—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against translation products of oncogenes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N7/00—Viruses; Bacteriophages; Compositions thereof; Preparation or purification thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/53—DNA (RNA) vaccination
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2121/00—Preparations for use in therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/005—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'active' part of the composition delivered, i.e. the nucleic acid delivered
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M37/00—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin
- A61M2037/0007—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin having means for enhancing the permeation of substances through the epidermis, e.g. using suction or depression, electric or magnetic fields, sound waves or chemical agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/10—Immunoglobulins specific features characterized by their source of isolation or production
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/10—Immunoglobulins specific features characterized by their source of isolation or production
- C07K2317/14—Specific host cells or culture conditions, e.g. components, pH or temperature
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/21—Immunoglobulins specific features characterized by taxonomic origin from primates, e.g. man
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/55—Fab or Fab'
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2740/00—Reverse transcribing RNA viruses
- C12N2740/00011—Details
- C12N2740/10011—Retroviridae
- C12N2740/16011—Human Immunodeficiency Virus, HIV
- C12N2740/16034—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2740/00—Reverse transcribing RNA viruses
- C12N2740/00011—Details
- C12N2740/10011—Retroviridae
- C12N2740/16011—Human Immunodeficiency Virus, HIV
- C12N2740/16111—Human Immunodeficiency Virus, HIV concerning HIV env
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2740/00—Reverse transcribing RNA viruses
- C12N2740/00011—Details
- C12N2740/10011—Retroviridae
- C12N2740/16011—Human Immunodeficiency Virus, HIV
- C12N2740/16111—Human Immunodeficiency Virus, HIV concerning HIV env
- C12N2740/16134—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/24011—Flaviviridae
- C12N2770/24111—Flavivirus, e.g. yellow fever virus, dengue, JEV
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/24011—Flaviviridae
- C12N2770/24111—Flavivirus, e.g. yellow fever virus, dengue, JEV
- C12N2770/24134—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/36011—Togaviridae
- C12N2770/36034—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/36011—Togaviridae
- C12N2770/36111—Alphavirus, e.g. Sindbis virus, VEE, EEE, WEE, Semliki
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Virology (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Molecular Biology (AREA)
- Immunology (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Pharmacology & Pharmacy (AREA)
- Oncology (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Communicable Diseases (AREA)
- Microbiology (AREA)
- Hematology (AREA)
- Biotechnology (AREA)
- AIDS & HIV (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Mycology (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Biomedical Technology (AREA)
- General Engineering & Computer Science (AREA)
- Tropical Medicine & Parasitology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020217020972A KR20210088741A (ko) | 2012-12-13 | 2013-12-13 | Dna 항체 작제물 및 그 이용 방법 |
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261737094P | 2012-12-13 | 2012-12-13 | |
| US61/737,094 | 2012-12-13 | ||
| US201361881376P | 2013-09-23 | 2013-09-23 | |
| US61/881,376 | 2013-09-23 | ||
| US201361896646P | 2013-10-28 | 2013-10-28 | |
| US61/896,646 | 2013-10-28 | ||
| PCT/US2013/075137 WO2014093894A2 (en) | 2012-12-13 | 2013-12-13 | Dna antibody constructs and method of using same |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020217020972A Division KR20210088741A (ko) | 2012-12-13 | 2013-12-13 | Dna 항체 작제물 및 그 이용 방법 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20150093834A true KR20150093834A (ko) | 2015-08-18 |
Family
ID=50935092
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020157018712A Ceased KR20150093834A (ko) | 2012-12-13 | 2013-12-13 | Dna 항체 작제물 및 그 이용 방법 |
| KR1020237036307A Ceased KR20230156146A (ko) | 2012-12-13 | 2013-12-13 | Dna 항체 작제물 및 그 이용 방법 |
| KR1020217020972A Ceased KR20210088741A (ko) | 2012-12-13 | 2013-12-13 | Dna 항체 작제물 및 그 이용 방법 |
| KR1020167018560A Active KR102454610B1 (ko) | 2012-12-13 | 2014-12-13 | Dna 항체 작제물 및 그 이용 방법 |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020237036307A Ceased KR20230156146A (ko) | 2012-12-13 | 2013-12-13 | Dna 항체 작제물 및 그 이용 방법 |
| KR1020217020972A Ceased KR20210088741A (ko) | 2012-12-13 | 2013-12-13 | Dna 항체 작제물 및 그 이용 방법 |
| KR1020167018560A Active KR102454610B1 (ko) | 2012-12-13 | 2014-12-13 | Dna 항체 작제물 및 그 이용 방법 |
Country Status (15)
| Country | Link |
|---|---|
| US (4) | US9994629B2 (enExample) |
| EP (1) | EP2931318A4 (enExample) |
| JP (6) | JP6898060B2 (enExample) |
| KR (4) | KR20150093834A (enExample) |
| CN (1) | CN104853782A (enExample) |
| AU (7) | AU2013358944B2 (enExample) |
| BR (2) | BR112015013700A8 (enExample) |
| CA (1) | CA2889723A1 (enExample) |
| EA (1) | EA201591131A1 (enExample) |
| HK (1) | HK1213481A1 (enExample) |
| MX (4) | MX2015007575A (enExample) |
| MY (1) | MY175708A (enExample) |
| SG (1) | SG10202002718RA (enExample) |
| WO (1) | WO2014093894A2 (enExample) |
| ZA (1) | ZA201503583B (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20190033047A (ko) * | 2016-05-05 | 2019-03-28 | 더 트러스티스 오브 더 유니버시티 오브 펜실바니아 | 관문 분자를 표적으로 하는 dna 단클론성 항체 |
Families Citing this family (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2931318A4 (en) * | 2012-12-13 | 2016-07-20 | Univ Pennsylvania | DNA ANTIBODY CONSTRUCTS AND METHODS FOR USE THEREOF |
| SG11201604719WA (en) | 2013-12-13 | 2016-07-28 | Univ Pennsylvania | Dna antibody constructs and method of using same |
| KR102586707B1 (ko) * | 2014-10-01 | 2023-10-11 | 더 트러스티스 오브 더 유니버시티 오브 펜실바니아 | 항원 및 어쥬번트로서 인터류킨-21을 갖는 백신 |
| CA2969214A1 (en) * | 2014-12-01 | 2016-06-09 | The Trustees Of The University Of Pennsylvania | Dna antibody constructs and method of using same |
| ES2894304T3 (es) | 2015-10-25 | 2022-02-14 | Sanofi Sa | Proteínas de unión triespecíficas y/o trivalentes para la prevención o tratamiento de infección por VIH |
| US20180363000A1 (en) * | 2015-12-14 | 2018-12-20 | The Trustees Of The University Of Pennsylvania | Aav-anti pcsk9 antibody constructs and uses thereof |
| KR20230012070A (ko) * | 2016-03-21 | 2023-01-25 | 데이비드 비. 웨이너 | Dna 항체 작제물 및 이의 사용 방법 |
| EP4257193A3 (en) | 2016-04-13 | 2024-01-24 | Sanofi | Trispecific and/or trivalent binding proteins |
| KR20190025826A (ko) * | 2016-05-05 | 2019-03-12 | 더 트러스티스 오브 더 유니버시티 오브 펜실바니아 | 슈도모나스 에루지노사에 대해 사용하기 위한 dna 항체 작제물 |
| MX2018013523A (es) * | 2016-05-05 | 2019-06-10 | Univ Pennsylvania | Anticuerpos monoclonales de adn dirigidos a il-6 y cd126. |
| KR20240155978A (ko) * | 2016-05-05 | 2024-10-29 | 더 트러스티스 오브 더 유니버시티 오브 펜실바니아 | 인플루엔자 바이러스를 표적으로 하는 dna 단일 클론 항체 |
| EP3515482A4 (en) * | 2016-09-19 | 2020-09-30 | The Wistar Institute Of Anatomy And Biology | ASSOCIATION OF NEW ZIKA VIRUS VACCINES AND ANTI-DNA ANTIBODY CONSTRUCTIONS FOR USE AGAINST ZIKA VIRUS |
| TWI778985B (zh) | 2016-10-20 | 2022-10-01 | 法商賽諾菲公司 | 抗chikv抗體及其用途 |
| KR20210117359A (ko) * | 2016-11-07 | 2021-09-28 | 더 위스타 인스티튜트 오브 아나토미 앤드 바이올로지 | 라임병에 사용하기 위한 dna 항체 작제물 |
| US11370830B2 (en) | 2017-04-07 | 2022-06-28 | The Rockefeller University | Neutralizing antibodies that bind to the zika virus domain III envelope region |
| CN111032077A (zh) * | 2017-05-22 | 2020-04-17 | 叶才明 | 抗-登革热病毒抗体、包含该抗体的药学组合物及其用途 |
| US11230592B2 (en) | 2017-09-27 | 2022-01-25 | The Wistar Institute Of Anatomy And Biology | DNA antibody constructs for use against middle east respiratory syndrome coronavirus |
| WO2019152602A1 (en) * | 2018-01-31 | 2019-08-08 | The Wistar Institute Of Anatomy And Biology | Structurally modified flavivirus dmabs |
| US11613576B2 (en) | 2019-04-09 | 2023-03-28 | Sanofi | Trispecific binding proteins, methods, and uses thereof |
| AU2020323601A1 (en) * | 2019-07-31 | 2022-03-03 | The Wistar Institute Of Anatomy And Biology | Multivalent DNA antibody constructs and use thereof |
| CN115066255A (zh) | 2019-09-18 | 2022-09-16 | 星际治疗有限公司 | 合成的dna载体和使用方法 |
| SE544001C2 (en) * | 2020-03-17 | 2021-10-26 | Xbrane Biopharma Ab | Novel combination of tis sequences, signal peptide sequences and nucleic acid sequences encoding heavy and light chains of an antibody |
| SE544000C2 (en) * | 2020-03-17 | 2021-10-26 | Xbrane Biopharma Ab | Novel combination of tis sequence, signal peptide sequence and nucleic acid sequence encoding a recombinant protein |
| WO2021188906A1 (en) * | 2020-03-19 | 2021-09-23 | Nature's Toolbox, Inc. | Novel mrna-based covid-19 multi-valent vaccine and methods of scaled production of the same |
| PE20230349A1 (es) * | 2020-05-14 | 2023-03-02 | Inovio Pharmaceuticals Inc | Vacunas para la papilomatosis respiratoria recurrente y metodos para usar estas |
| EP4255484A4 (en) * | 2020-12-02 | 2025-02-26 | Thomas Jefferson University | DESMOGLEIN-2-TARGETED CHIMERIC ANTIGEN RECEPTOR CONSTRUCTS AND METHODS OF USE |
Family Cites Families (42)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FI46572C (fi) * | 1972-04-07 | 1973-04-10 | Stroemberg Oy Ab | Jännitemuuntaja. |
| US7276488B2 (en) | 1997-06-04 | 2007-10-02 | Oxford Biomedica (Uk) Limited | Vector system |
| US20030039635A1 (en) | 1998-09-30 | 2003-02-27 | Corixa Corporation | Compositions and methods for WT1 specific immunotherapy |
| CN1334343A (zh) * | 2000-07-14 | 2002-02-06 | 中国医学科学院肿瘤医院肿瘤研究所 | 抑制肿瘤生长的新抗体、其衍生物及其应用 |
| US20070037165A1 (en) | 2000-09-08 | 2007-02-15 | Applera Corporation | Polymorphisms in known genes associated with human disease, methods of detection and uses thereof |
| AU2003253447A1 (en) * | 2002-08-02 | 2004-02-23 | Yuhan Corporation | Expression vectors |
| WO2005017149A1 (en) * | 2003-06-03 | 2005-02-24 | Cell Genesys, Inc. | Compositions and methods for enhanced expression of recombinant polypeptides from a single vector using a peptide cleavage site |
| CN1480215A (zh) | 2003-07-07 | 2004-03-10 | 叶新新 | Sars病毒抗原抗体复合疫苗及实验动物模型与方法 |
| KR101282396B1 (ko) * | 2004-05-28 | 2013-07-04 | 어젠시스 인코포레이티드 | Psca 단백질에 결합하는 암 진단용 항체 |
| PL1753871T3 (pl) | 2004-05-28 | 2016-01-29 | Agensys Inc | Przeciwciała i powiązane cząsteczki, które wiążą się z antygenem komórek macierzystych prostaty (PSCA) |
| JP2008506389A (ja) | 2004-07-13 | 2008-03-06 | セル ジェネシス インコーポレイテッド | Aavベクター組成物および免疫グロブリンの発現の増強のための方法ならびにその使用方法 |
| RS53594B1 (sr) | 2004-07-22 | 2015-02-27 | Genentech, Inc. | Preparat her2 antitela |
| JO3000B1 (ar) * | 2004-10-20 | 2016-09-05 | Genentech Inc | مركبات أجسام مضادة . |
| EP1851251A2 (en) | 2005-02-18 | 2007-11-07 | Medarex, Inc. | Monoclonal antibodies against prostate specific membrane antigen (psma) lacking in fucosyl residues |
| US20060216722A1 (en) | 2005-03-25 | 2006-09-28 | Christer Betsholtz | Glomerular expression profiling |
| KR20080031024A (ko) | 2005-07-21 | 2008-04-07 | 아보트 러보러터리즈 | 폴리단백질, 프로단백질 및 단백질분해를 사용한 sORF작제물 포함 다중 유전자 발현 및 방법 |
| US8865875B2 (en) | 2007-08-22 | 2014-10-21 | Medarex, L.L.C. | Site-specific attachment of drugs or other agents to engineered antibodies with C-terminal extensions |
| CA2598966A1 (en) | 2007-09-07 | 2009-03-07 | Institut Pasteur | Anti-chikungunya monoclonal antibodies and uses thereof |
| JP2009171880A (ja) | 2008-01-23 | 2009-08-06 | Yokohama City Univ | アルツハイマー病における次世代遺伝子治療法・免疫治療法の開発 |
| JP5805393B2 (ja) | 2008-03-14 | 2015-11-04 | トランジェーヌ、ソシエテ、アノニムTransgene S.A. | Csf−1rに対する抗体 |
| KR101589511B1 (ko) | 2008-04-04 | 2016-02-01 | 더 트러스티스 오브 더 유니버시티 오브 펜실바니아 | 치쿤구니야 바이러스 단백질의 공통 서열, 이를 인코딩하는 핵산 분자, 조성물 및 이를 이용하는 방법 |
| WO2010043977A2 (en) | 2008-10-13 | 2010-04-22 | Institute For Research In Biomedicine | Dengue virus neutralizing antibodies and uses thereof |
| ES2536996T3 (es) | 2009-07-06 | 2015-06-01 | F. Hoffmann-La Roche Ag | Anticuerpos biespecíficos de unión a digoxigenina |
| MY162326A (en) | 2009-07-13 | 2017-05-31 | Bharat Biotech Int Ltd | A composition useful as rotavirus vaccine and a method therefor |
| US20110045534A1 (en) | 2009-08-20 | 2011-02-24 | Cell Signaling Technology, Inc. | Nucleic Acid Cassette For Producing Recombinant Antibodies |
| EP2480572B1 (en) * | 2009-09-25 | 2019-01-30 | The United States of America, as represented by The Secretary, Department of Health and Human Services | Neutralizing antibodies to hiv-1 and their use |
| JP2013508287A (ja) * | 2009-10-14 | 2013-03-07 | ヤンセン バイオテツク,インコーポレーテツド | 抗体を親和性成熟する方法 |
| US8298820B2 (en) | 2010-01-26 | 2012-10-30 | The Trustees Of The University Of Pennsylvania | Influenza nucleic acid molecules and vaccines made therefrom |
| US20130150563A1 (en) * | 2010-07-09 | 2013-06-13 | Jv Bio Srl | Lipid-conjugated antibodies |
| BR112012031638B1 (pt) | 2010-07-09 | 2021-01-12 | Janssen Vaccines & Prevention B.V. | anticorpo anti-rsv ou fragmento de ligação de antígeno do mesmo, anticorpo multivalente, composição farmacêutica, uso de anticorpo ou fragmento de ligação de antígeno, método de detectar infecção por rsv, e, ácido nucleico isolado |
| US8637035B2 (en) | 2010-07-16 | 2014-01-28 | Academia Sinica | Anti-dengue virus antibodies |
| WO2012065164A2 (en) * | 2010-11-12 | 2012-05-18 | The Trustees Of The University Of Pennsylvania | Consensus prostate antigens nucleic acid molecule encoding the same and vaccine and uses comprising the same |
| EA033467B1 (ru) | 2011-01-31 | 2019-10-31 | Univ Pennsylvania | Молекула нуклеиновой кислоты для индукции иммунного ответа против герпесвируса и ее применение |
| WO2012106578A1 (en) | 2011-02-04 | 2012-08-09 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | HIV NEUTRALIZING ANTIBODIES HAVING MUTATIONS IN CONSTANT DOMAIN (Fc) |
| WO2012115980A1 (en) | 2011-02-22 | 2012-08-30 | California Institute Of Technology | Delivery of proteins using adeno-associated virus (aav) vectors |
| CN102199218A (zh) * | 2011-04-29 | 2011-09-28 | 中国人民解放军军事医学科学院基础医学研究所 | 一种抗Her2抗体-白细胞介素2融合蛋白及其用途 |
| US9540657B2 (en) | 2012-05-25 | 2017-01-10 | California Institute Of Technology | Expression of secreted and cell-surface polypeptides |
| EP2931318A4 (en) | 2012-12-13 | 2016-07-20 | Univ Pennsylvania | DNA ANTIBODY CONSTRUCTS AND METHODS FOR USE THEREOF |
| JP7078350B2 (ja) * | 2013-10-28 | 2022-05-31 | ザ トラスティーズ オブ ザ ユニバーシティ オブ ペンシルバニア | Dna抗体構築物及びその使用方法 |
| MX364732B (es) | 2012-12-13 | 2019-05-06 | Univ Pennsylvania | Vacuna contra el tumor de wilms 1. |
| US20140377269A1 (en) | 2012-12-19 | 2014-12-25 | Adimab, Llc | Multivalent antibody analogs, and methods of their preparation and use |
| SG11201604719WA (en) | 2013-12-13 | 2016-07-28 | Univ Pennsylvania | Dna antibody constructs and method of using same |
-
2013
- 2013-12-13 EP EP13863143.7A patent/EP2931318A4/en active Pending
- 2013-12-13 MX MX2015007575A patent/MX2015007575A/es unknown
- 2013-12-13 KR KR1020157018712A patent/KR20150093834A/ko not_active Ceased
- 2013-12-13 WO PCT/US2013/075137 patent/WO2014093894A2/en not_active Ceased
- 2013-12-13 EA EA201591131A patent/EA201591131A1/ru unknown
- 2013-12-13 HK HK16101459.4A patent/HK1213481A1/zh unknown
- 2013-12-13 KR KR1020237036307A patent/KR20230156146A/ko not_active Ceased
- 2013-12-13 BR BR112015013700A patent/BR112015013700A8/pt not_active Application Discontinuation
- 2013-12-13 US US14/651,740 patent/US9994629B2/en active Active
- 2013-12-13 CN CN201380065674.3A patent/CN104853782A/zh active Pending
- 2013-12-13 JP JP2015548014A patent/JP6898060B2/ja active Active
- 2013-12-13 KR KR1020217020972A patent/KR20210088741A/ko not_active Ceased
- 2013-12-13 AU AU2013358944A patent/AU2013358944B2/en active Active
- 2013-12-13 CA CA2889723A patent/CA2889723A1/en active Pending
-
2014
- 2014-12-13 BR BR112016013493-1A patent/BR112016013493B1/pt not_active IP Right Cessation
- 2014-12-13 SG SG10202002718RA patent/SG10202002718RA/en unknown
- 2014-12-13 KR KR1020167018560A patent/KR102454610B1/ko active Active
- 2014-12-13 MY MYPI2016702163A patent/MY175708A/en unknown
- 2014-12-13 MX MX2016007722A patent/MX388911B/es unknown
- 2014-12-13 AU AU2014361811A patent/AU2014361811B2/en active Active
-
2015
- 2015-05-21 ZA ZA2015/03583A patent/ZA201503583B/en unknown
- 2015-06-12 MX MX2022016002A patent/MX2022016002A/es unknown
-
2016
- 2016-06-13 MX MX2021015971A patent/MX2021015971A/es unknown
- 2016-11-18 AU AU2016259451A patent/AU2016259451A1/en not_active Abandoned
-
2018
- 2018-04-30 AU AU2018202997A patent/AU2018202997A1/en not_active Abandoned
- 2018-06-12 US US16/005,997 patent/US20180346554A1/en not_active Abandoned
- 2018-09-26 US US16/142,457 patent/US11208470B2/en active Active
-
2019
- 2019-03-27 JP JP2019061531A patent/JP2019115355A/ja active Pending
- 2019-04-04 AU AU2019202343A patent/AU2019202343B2/en active Active
-
2020
- 2020-03-02 JP JP2020035240A patent/JP2020108381A/ja active Pending
- 2020-03-13 AU AU2020201853A patent/AU2020201853B2/en active Active
-
2021
- 2021-02-04 JP JP2021016537A patent/JP2021087433A/ja not_active Withdrawn
- 2021-06-03 AU AU2021203643A patent/AU2021203643A1/en not_active Abandoned
- 2021-12-17 US US17/554,642 patent/US20220112273A1/en not_active Abandoned
-
2023
- 2023-03-15 JP JP2023040876A patent/JP2023080085A/ja active Pending
- 2023-06-30 JP JP2023108341A patent/JP2023138998A/ja active Pending
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20190033047A (ko) * | 2016-05-05 | 2019-03-28 | 더 트러스티스 오브 더 유니버시티 오브 펜실바니아 | 관문 분자를 표적으로 하는 dna 단클론성 항체 |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2019202343B2 (en) | DNA antibody constructs and method of using same | |
| US20230023093A1 (en) | Dna antibody constructs and method of using same | |
| JP2021166534A (ja) | Dna抗体構築物及びその使用方法 | |
| JP2017500030A (ja) | Dna抗体構築物及びその使用方法 | |
| HK1247100B (zh) | Dna抗体构建体及其使用方法 | |
| EA042816B1 (ru) | Днк-конструкции антитела и способ их применения |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20150713 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20181203 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20200316 Patent event code: PE09021S01D |
|
| AMND | Amendment | ||
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20210126 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20200316 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |
|
| AMND | Amendment | ||
| PX0901 | Re-examination |
Patent event code: PX09011S01I Patent event date: 20210126 Comment text: Decision to Refuse Application Patent event code: PX09012R01I Patent event date: 20200828 Comment text: Amendment to Specification, etc. |
|
| PX0601 | Decision of rejection after re-examination |
Comment text: Decision to Refuse Application Patent event code: PX06014S01D Patent event date: 20210602 Comment text: Amendment to Specification, etc. Patent event code: PX06012R01I Patent event date: 20210427 Comment text: Decision to Refuse Application Patent event code: PX06011S01I Patent event date: 20210126 Comment text: Amendment to Specification, etc. Patent event code: PX06012R01I Patent event date: 20200828 Comment text: Notification of reason for refusal Patent event code: PX06013S01I Patent event date: 20200316 |
|
| X601 | Decision of rejection after re-examination | ||
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20210705 |