KR20120069663A - 생체조직으로부터 단리 할 수 있는 다능성 줄기세포 - Google Patents
생체조직으로부터 단리 할 수 있는 다능성 줄기세포 Download PDFInfo
- Publication number
- KR20120069663A KR20120069663A KR1020127003879A KR20127003879A KR20120069663A KR 20120069663 A KR20120069663 A KR 20120069663A KR 1020127003879 A KR1020127003879 A KR 1020127003879A KR 20127003879 A KR20127003879 A KR 20127003879A KR 20120069663 A KR20120069663 A KR 20120069663A
- Authority
- KR
- South Korea
- Prior art keywords
- cells
- cell
- muse
- pluripotent stem
- derived
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0607—Non-embryonic pluripotent stem cells, e.g. MASC
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/18—Drugs for disorders of the alimentary tract or the digestive system for pancreatic disorders, e.g. pancreatic enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/10—Drugs for disorders of the urinary system of the bladder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0603—Embryonic cells ; Embryoid bodies
- C12N5/0605—Cells from extra-embryonic tissues, e.g. placenta, amnion, yolk sac, Wharton's jelly
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0696—Artificially induced pluripotent stem cells, e.g. iPS
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/28—Bone marrow; Haematopoietic stem cells; Mesenchymal stem cells of any origin, e.g. adipose-derived stem cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/48—Reproductive organs
- A61K35/50—Placenta; Placental stem cells; Amniotic fluid; Amnion; Amniotic stem cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
- C12N15/1138—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing against receptors or cell surface proteins
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2506/00—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells
- C12N2506/13—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells from connective tissue cells, from mesenchymal cells
- C12N2506/1346—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells from connective tissue cells, from mesenchymal cells from mesenchymal stem cells
- C12N2506/1353—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells from connective tissue cells, from mesenchymal cells from mesenchymal stem cells from bone marrow mesenchymal stem cells (BM-MSC)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2509/00—Methods for the dissociation of cells, e.g. specific use of enzymes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0662—Stem cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0662—Stem cells
- C12N5/0663—Bone marrow mesenchymal stem cells (BM-MSC)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0662—Stem cells
- C12N5/0667—Adipose-derived stem cells [ADSC]; Adipose stromal stem cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0662—Stem cells
- C12N5/0668—Mesenchymal stem cells from other natural sources
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Zoology (AREA)
- Biotechnology (AREA)
- Wood Science & Technology (AREA)
- Genetics & Genomics (AREA)
- Developmental Biology & Embryology (AREA)
- Cell Biology (AREA)
- Microbiology (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- Transplantation (AREA)
- Immunology (AREA)
- Reproductive Health (AREA)
- Urology & Nephrology (AREA)
- Hematology (AREA)
- Physical Education & Sports Medicine (AREA)
- Neurology (AREA)
- Gynecology & Obstetrics (AREA)
- Diabetes (AREA)
- Dermatology (AREA)
- Epidemiology (AREA)
- Virology (AREA)
- Neurosurgery (AREA)
- Ophthalmology & Optometry (AREA)
- Heart & Thoracic Surgery (AREA)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US21378809P | 2009-07-15 | 2009-07-15 | |
| US61/213,788 | 2009-07-15 | ||
| US29015909P | 2009-12-24 | 2009-12-24 | |
| US61/290,159 | 2009-12-24 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167008209A Division KR101764080B1 (ko) | 2009-07-15 | 2010-07-15 | 생체조직으로부터 분리 할 수 있는 만능성 줄기세포 |
| KR1020157013171A Division KR101651795B1 (ko) | 2009-07-15 | 2010-07-15 | 생체조직으로부터 단리 할 수 있는 다능성 줄기세포 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20120069663A true KR20120069663A (ko) | 2012-06-28 |
Family
ID=43449509
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020127003879A Ceased KR20120069663A (ko) | 2009-07-15 | 2010-07-15 | 생체조직으로부터 단리 할 수 있는 다능성 줄기세포 |
| KR1020157013171A Active KR101651795B1 (ko) | 2009-07-15 | 2010-07-15 | 생체조직으로부터 단리 할 수 있는 다능성 줄기세포 |
| KR1020177020765A Active KR101945343B1 (ko) | 2009-07-15 | 2010-07-15 | 생체조직으로부터 분리 할 수 있는 만능성 줄기세포 |
| KR1020167008209A Active KR101764080B1 (ko) | 2009-07-15 | 2010-07-15 | 생체조직으로부터 분리 할 수 있는 만능성 줄기세포 |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020157013171A Active KR101651795B1 (ko) | 2009-07-15 | 2010-07-15 | 생체조직으로부터 단리 할 수 있는 다능성 줄기세포 |
| KR1020177020765A Active KR101945343B1 (ko) | 2009-07-15 | 2010-07-15 | 생체조직으로부터 분리 할 수 있는 만능성 줄기세포 |
| KR1020167008209A Active KR101764080B1 (ko) | 2009-07-15 | 2010-07-15 | 생체조직으로부터 분리 할 수 있는 만능성 줄기세포 |
Country Status (16)
| Country | Link |
|---|---|
| US (1) | US9550975B2 (OSRAM) |
| EP (3) | EP3680323B1 (OSRAM) |
| JP (7) | JP5185443B2 (OSRAM) |
| KR (4) | KR20120069663A (OSRAM) |
| CN (2) | CN107012117B (OSRAM) |
| AU (1) | AU2010271722B8 (OSRAM) |
| CA (1) | CA2768238C (OSRAM) |
| DK (2) | DK2455452T4 (OSRAM) |
| ES (3) | ES2647360T5 (OSRAM) |
| HR (1) | HRP20250245T1 (OSRAM) |
| HU (3) | HUE071170T2 (OSRAM) |
| NO (1) | NO2455452T3 (OSRAM) |
| PL (3) | PL2455452T5 (OSRAM) |
| PT (2) | PT2455452T (OSRAM) |
| SG (1) | SG177705A1 (OSRAM) |
| WO (1) | WO2011007900A1 (OSRAM) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20160056127A (ko) * | 2014-11-11 | 2016-05-19 | 한국원자력의학원 | Cd105를 포함하는 줄기세포능을 가진 섬유아세포 분리용 바이오마커 조성물 및 이를 이용한 줄기세포능을 가진 섬유아세포 분리 방법 |
| KR20180100337A (ko) * | 2016-01-15 | 2018-09-10 | 국립대학법인 도야마 다이가쿠 | 허혈성 뇌경색에의 다능성 줄기 세포의 동원 |
Families Citing this family (89)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9399758B2 (en) | 2009-07-15 | 2016-07-26 | Mari Dezawa | SSEA3(+) pluripotent stem cell that can be isolated from body tissue |
| JP2014132830A (ja) * | 2011-03-30 | 2014-07-24 | Clio Inc | 生体の臍帯又は脂肪組織から単離できる多能性幹細胞 |
| JP2014133703A (ja) * | 2011-03-30 | 2014-07-24 | Clio Inc | 生体組織から単離できるssea−3陽性の多能性幹細胞を含む他家移植用細胞治療用組成物 |
| US9550975B2 (en) | 2009-07-15 | 2017-01-24 | Mari Dezawa | SSEA-3 pluripotent stem cell isolated from body tissue |
| JP2013502220A (ja) | 2009-08-21 | 2013-01-24 | ザ ボード オブ トラスティーズ オブ ザ リーランド スタンフォード ジュニア ユニバーシティ | ヒト体細胞からの誘導多能性幹細胞の生成効率を向上させる方法 |
| US8883210B1 (en) | 2010-05-14 | 2014-11-11 | Musculoskeletal Transplant Foundation | Tissue-derived tissuegenic implants, and methods of fabricating and using same |
| US10130736B1 (en) | 2010-05-14 | 2018-11-20 | Musculoskeletal Transplant Foundation | Tissue-derived tissuegenic implants, and methods of fabricating and using same |
| US9352003B1 (en) | 2010-05-14 | 2016-05-31 | Musculoskeletal Transplant Foundation | Tissue-derived tissuegenic implants, and methods of fabricating and using same |
| SG10201914092XA (en) * | 2011-06-06 | 2020-03-30 | ReGenesys BVBA | Expansion of stem cells in hollow fiber bioreactors |
| US9453840B2 (en) | 2011-07-27 | 2016-09-27 | Kyoto University | Markers for dopaminergic neuron progenitor cells |
| KR101751355B1 (ko) * | 2011-08-24 | 2017-07-04 | (주)아모레퍼시픽 | 인간 피부 진피 유래 성체 줄기세포 |
| US20140248698A1 (en) | 2011-10-21 | 2014-09-04 | Arkray, Inc. | Method for culturing pluripotency-maintained singly dispersed cells by means of laminar flow |
| CN104284976A (zh) * | 2012-03-06 | 2015-01-14 | Sct&B公司 | 胎盘干细胞及其分离方法与应用 |
| AU2013251649B2 (en) * | 2012-04-24 | 2019-04-04 | Vcell Therapeutics, Inc. | Generating pluripotent cells de novo |
| US20140030244A1 (en) * | 2012-07-27 | 2014-01-30 | Bioquark, Inc. | Extracts isolated from electroporated ambhibian oocytes and use thereof in treating diseases and disorders |
| WO2014027474A1 (ja) | 2012-08-17 | 2014-02-20 | 株式会社Clio | 心筋梗塞の修復再生を誘導する多能性幹細胞 |
| TW201441369A (zh) * | 2012-12-21 | 2014-11-01 | Univ Rutgers | 多向分化壓力耐受(muse)細胞分離及擴增 |
| US9709554B2 (en) | 2013-01-31 | 2017-07-18 | Rutgers, The State University Of New Jersey | In vitro model of macrosteatotic (fatty) liver |
| US20160002599A1 (en) | 2013-02-08 | 2016-01-07 | Kyoto University | Production methods for megakaryocytes and platelets |
| JPWO2014133090A1 (ja) * | 2013-02-28 | 2017-02-02 | 国立大学法人浜松医科大学 | 脳腫瘍治療のための多能性幹細胞 |
| US9446033B2 (en) | 2013-03-01 | 2016-09-20 | Clio, Inc. | Pharmaceutical composition including migratory factor for guiding pluripotent stem cells to injury |
| AU2014239954B2 (en) * | 2013-03-15 | 2020-07-16 | The Jackson Laboratory | Isolation of non-embryonic stem cells and uses thereof |
| US10519421B2 (en) | 2013-03-21 | 2019-12-31 | Kyoto University | Induction of motor neurons from pluripotent stem cells |
| JP6473686B2 (ja) | 2013-03-25 | 2019-02-20 | 公益財団法人神戸医療産業都市推進機構 | 細胞の選別方法 |
| JP6461787B2 (ja) | 2013-04-12 | 2019-01-30 | 国立大学法人京都大学 | 肺胞上皮前駆細胞の誘導方法 |
| EP2998391B1 (en) | 2013-05-14 | 2020-02-19 | Kyoto University | Efficient myocardial cell induction method |
| US10131880B2 (en) | 2013-05-22 | 2018-11-20 | The Regents Of The University Of California | Pluripotent human adipose adult stem cells: isolation, characterization and clinical implications |
| AU2014296259B2 (en) | 2013-07-30 | 2017-04-27 | Musculoskeletal Transplant Foundation | Acellular soft tissue-derived matrices and methods for preparing same |
| EP3031905A4 (en) | 2013-08-07 | 2017-04-26 | Kyoto University | Method for producing pancreatic hormone-producing cell |
| US10265347B2 (en) | 2013-08-29 | 2019-04-23 | Norimasa Miura | Biomolecular group related to cell anti-aging |
| WO2015033558A1 (ja) | 2013-09-04 | 2015-03-12 | 株式会社大塚製薬工場 | 多能性幹細胞の調製方法 |
| CN121136933A (zh) | 2013-09-05 | 2025-12-16 | 国立大学法人京都大学 | 由多能干细胞制造产多巴胺神经前体细胞的方法、产多巴胺神经前体细胞、帕金森病治疗剂和试剂盒 |
| EP3064577B1 (en) | 2013-11-01 | 2020-09-09 | Kyoto University | Novel chondrocyte induction method |
| WO2015081094A1 (en) * | 2013-11-27 | 2015-06-04 | University Of Louisville Research Foundation, Inc. | Cardiac progenitor cells and methods of use therefor |
| JP6519038B2 (ja) * | 2014-02-26 | 2019-05-29 | 株式会社生命科学インスティテュート | 脳梗塞治療のための多能性幹細胞 |
| JP2015160820A (ja) * | 2014-02-26 | 2015-09-07 | 株式会社Clio | 慢性腎障害治療のための多能性幹細胞 |
| RU2711942C2 (ru) | 2014-03-19 | 2020-01-23 | Виселл Терапеутикс, Инк. | Способы, относящиеся к плюрипотентным клеткам |
| WO2015178431A1 (ja) | 2014-05-21 | 2015-11-26 | 国立大学法人京都大学 | 膵芽細胞の製造方法および膵芽細胞を含む膵疾患治療剤 |
| KR101592401B1 (ko) * | 2014-06-13 | 2016-02-05 | 주식회사 비비에이치씨 | 지방-유래 중간엽 줄기세포로부터 유도만능 줄기세포를 제조하는 방법 및 그 방법에 의해 제조된 유도만능 줄기세포 |
| JP6391364B2 (ja) * | 2014-08-25 | 2018-09-19 | 国立大学法人 鹿児島大学 | 認知機能障害改善用細胞製剤 |
| JP6452107B2 (ja) * | 2014-09-05 | 2019-01-16 | 国立大学法人 東京大学 | 糖尿病性皮膚潰瘍治療のための多能性幹細胞 |
| WO2016187413A1 (en) | 2015-05-21 | 2016-11-24 | Musculoskeletal Transplant Foundation | Modified demineralized cortical bone fibers |
| JP6169807B2 (ja) * | 2015-05-29 | 2017-07-26 | 日本臓器製薬株式会社 | 多能性幹細胞遊走促進剤 |
| US10912864B2 (en) | 2015-07-24 | 2021-02-09 | Musculoskeletal Transplant Foundation | Acellular soft tissue-derived matrices and methods for preparing same |
| US11052175B2 (en) | 2015-08-19 | 2021-07-06 | Musculoskeletal Transplant Foundation | Cartilage-derived implants and methods of making and using same |
| JP6627027B2 (ja) * | 2015-09-11 | 2020-01-08 | 福美人株式会社 | 皮膚細胞の活性化作用を呈するフラボノイド誘導体 |
| JP6770673B2 (ja) * | 2016-02-26 | 2020-10-21 | 国立大学法人東海国立大学機構 | 急性心筋梗塞の予後マーカー及びその利用 |
| DK3430122T3 (da) * | 2016-03-17 | 2021-08-23 | Synova Life Sciences Inc | Separering, adskillelse og/eller opdeling af celler ved hjælp af mekaniske stød |
| CN109072198B (zh) | 2016-04-22 | 2022-08-26 | 国立大学法人京都大学 | 产多巴胺神经祖细胞的制备方法 |
| JP6994200B2 (ja) * | 2016-05-16 | 2022-02-04 | 国立大学法人東海国立大学機構 | 多能性幹細胞による周産期脳障害の改善及び治療 |
| US20190201455A1 (en) | 2016-07-01 | 2019-07-04 | Tohoku University | Prophylactic or therapeutic agent for organ fibrosis |
| CA3032358A1 (en) | 2016-07-29 | 2018-02-01 | Tohoku University | Prophylactic or therapeutic agent for vascular disorder |
| AU2017305064B2 (en) | 2016-08-03 | 2023-03-16 | Life Science Institute, Inc. | Amelioration and treatment of chronic lung disease using pluripotent stem cells |
| CN109689074A (zh) | 2016-08-03 | 2019-04-26 | 株式会社生命科学研究院 | 采用多能干细胞的缺血再灌注肺损伤的减轻及治疗 |
| WO2018025975A1 (ja) * | 2016-08-03 | 2018-02-08 | 株式会社生命科学インスティテュート | インビトロで多能性幹細胞を分化誘導する方法 |
| WO2018143378A1 (ja) | 2017-02-03 | 2018-08-09 | 国立大学法人九州大学 | 歯髄組織由来細胞から歯髄幹細胞を調製する方法 |
| JP7181534B2 (ja) * | 2017-03-28 | 2022-12-01 | 味の素株式会社 | 未分化維持培地添加剤 |
| JP7712643B2 (ja) * | 2017-06-19 | 2025-07-24 | 国立大学法人東北大学 | 表皮水疱症の治療剤 |
| AU2018289947A1 (en) | 2017-06-20 | 2020-01-30 | Life Science Institute, Inc. | Amelioration and treatment of brain disorder resulting from fetal growth retardation using pluripotent stem cells |
| JP7489016B2 (ja) * | 2017-10-17 | 2024-05-23 | 国立大学法人東北大学 | 骨軟骨修復を誘導する多能性幹細胞 |
| AU2018364852B2 (en) * | 2017-11-10 | 2022-06-30 | Regenesis Science Co., Ltd. | Method for producing cultured cell, and method for producing therapeutic agent for spinal cord injury disease |
| CN111492248B (zh) | 2017-11-28 | 2023-09-12 | 塞尔吐温株式会社 | 通过实时谷胱甘肽测定来测量治疗细胞质量的方法 |
| JP6604492B2 (ja) * | 2018-04-09 | 2019-11-13 | 株式会社生命科学インスティテュート | 脳梗塞治療のための多能性幹細胞 |
| EP3791888A4 (en) | 2018-05-09 | 2022-03-23 | Life Science Institute, Inc. | Therapeutic agent for spinal cord injury |
| JP7072777B2 (ja) * | 2018-07-03 | 2022-05-23 | 株式会社生命科学インスティテュート | 慢性腎障害治療のための多能性幹細胞 |
| JP7285015B2 (ja) | 2018-07-19 | 2023-06-01 | 国立大学法人京都大学 | 多能性幹細胞由来の板状軟骨およびその製造方法 |
| WO2020100788A1 (ja) * | 2018-11-14 | 2020-05-22 | 株式会社片岡製作所 | インスリン産生細胞の製造方法、及び組成物 |
| SG11202105686UA (en) * | 2018-11-30 | 2021-06-29 | Kyoto Prefectural Public Univ Corp | Therapeutic agent of peripheral blood flow disorder |
| US12435311B2 (en) | 2018-12-21 | 2025-10-07 | The University Of Osaka | Lubricin-localized cartilage-like tissue, method for producing same and composition comprising same for treating articular cartilage damage |
| JP2022528965A (ja) * | 2019-04-09 | 2022-06-16 | ラトガース,ザ ステート ユニバーシティ オブ ニュー ジャージー | 細胞集団を濃縮する方法 |
| CA3150769A1 (en) | 2019-08-09 | 2021-02-18 | Tohoku University | Agent for treating or preventing vascular dementia |
| EP4026551A1 (en) | 2019-09-05 | 2022-07-13 | Tohoku University | Therapeutic agent for myocarditis |
| WO2021066076A1 (ja) | 2019-10-01 | 2021-04-08 | 国立大学法人京都大学 | 尿管芽先端部細胞の単離方法 |
| JPWO2021085639A1 (OSRAM) * | 2019-10-31 | 2021-05-06 | ||
| JP7058431B2 (ja) | 2019-12-12 | 2022-04-22 | 国立大学法人千葉大学 | 巨核球および血小板を含む凍結乾燥製剤 |
| WO2021187602A1 (ja) | 2020-03-19 | 2021-09-23 | 国立大学法人京都大学 | 心筋細胞の精製方法 |
| CA3175573A1 (en) | 2020-03-19 | 2021-09-23 | Orizuru Therapeutics, Inc. | Cardiomyocyte purification method |
| CN115843311A (zh) | 2020-04-02 | 2023-03-24 | 国立大学法人东北大学 | 高潜能多能干细胞 |
| US20230256024A1 (en) | 2020-07-13 | 2023-08-17 | Kyoto University | Skeletal muscle precursor cells and method for purifying same, composition for treating myogenic diseases, and method for producing cell group containing skeletal muscle precursor cells |
| US20230270790A1 (en) * | 2020-07-15 | 2023-08-31 | National University Corporation Okayama University | Pluripotent stem cells effective for treatment of motor neuron disease (mnd) |
| JP2021073305A (ja) * | 2021-02-09 | 2021-05-13 | 株式会社生命科学インスティテュート | 慢性腎障害治療のための多能性幹細胞 |
| TWI832070B (zh) * | 2021-07-15 | 2024-02-11 | 國璽幹細胞應用技術股份有限公司 | 幹細胞製劑用於治療關節炎的用途及用於製備幹細胞製劑的方法 |
| JP2023041486A (ja) * | 2021-09-13 | 2023-03-24 | 国立大学法人東北大学 | 毛髪損傷の治療剤 |
| JPWO2023153464A1 (OSRAM) | 2022-02-09 | 2023-08-17 | ||
| EP4530344A1 (en) | 2022-05-23 | 2025-04-02 | Kyoto University | Production method for renal collecting duct cells and pelvic epithelial cells |
| JPWO2024210126A1 (OSRAM) | 2023-04-03 | 2024-10-10 | ||
| WO2024248017A1 (ja) | 2023-05-29 | 2024-12-05 | 国立研究開発法人理化学研究所 | 心筋細胞を成熟させる方法及び成熟した心筋細胞の製造方法 |
| CN117187174B (zh) * | 2023-11-08 | 2024-02-06 | 广州正源生物技术有限公司 | 一种Muse细胞培养基以及一种脂肪Muse细胞的提取方法 |
| JP2025123077A (ja) * | 2024-02-09 | 2025-08-22 | 国立大学法人東北大学 | 脊髄梗塞治療のための多能性幹細胞 |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| HK1049350B (en) | 1999-08-05 | 2012-01-27 | Abt Holding Company | Multipotent adult stem cells and methods for isolation |
| US7575921B2 (en) * | 1999-12-30 | 2009-08-18 | Vbi Technologies, L.L.C. | Spore-like cells and uses thereof |
| WO2002057428A1 (en) * | 2000-10-30 | 2002-07-25 | University Of Massachusetts | Isolation of spore-like cells from tissues exposed to extreme conditions |
| JP4182742B2 (ja) | 2002-12-12 | 2008-11-19 | 日産自動車株式会社 | 自動車の前部車体構造 |
| US20050042595A1 (en) | 2003-08-14 | 2005-02-24 | Martin Haas | Banking of multipotent amniotic fetal stem cells |
| CN1845987A (zh) * | 2003-08-29 | 2006-10-11 | 明尼苏达大学董事会 | 肾来源的干细胞及其分离、分化和使用方法 |
| US20060216821A1 (en) * | 2004-02-26 | 2006-09-28 | Reliance Life Sciences Pvt. Ltd. | Pluripotent embryonic-like stem cells derived from corneal limbus, methods of isolation and uses thereof |
| JP2009517001A (ja) * | 2005-05-17 | 2009-04-30 | リライアンス ライフ サイエンシーズ プライベイト リミテッド | 哺乳動物細胞を使用するヒト胚性幹細胞株の構築 |
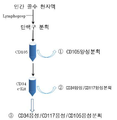
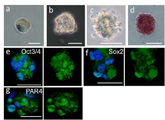
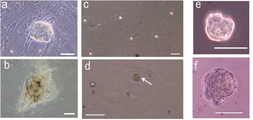
| US9388382B2 (en) * | 2005-10-05 | 2016-07-12 | The Board Of Trustees Of The University Of Illinois | Isolation of CD14 negative, CD45 positive and CD117 positive embryonic-like stem cells free of monocytes from human umbilical cord blood mononuclear cells |
| AU2006304390A1 (en) | 2005-10-17 | 2007-04-26 | Academia Sinica | Pulmonary stem cells, related methods and kits |
| WO2009059032A2 (en) * | 2007-10-30 | 2009-05-07 | University Of Louisville Research Foundation, Inc. | Uses and isolation of very small embryonic-like (vsel) stem cells |
| US8048999B2 (en) | 2005-12-13 | 2011-11-01 | Kyoto University | Nuclear reprogramming factor |
| WO2008080200A1 (en) | 2006-12-29 | 2008-07-10 | Irina Kerkis | Process for obtaining stem cells |
| JP2008307007A (ja) | 2007-06-15 | 2008-12-25 | Bayer Schering Pharma Ag | 出生後のヒト組織由来未分化幹細胞から誘導したヒト多能性幹細胞 |
| US20090172832A1 (en) * | 2007-12-28 | 2009-07-02 | Timothy Caspar | Expression of rubisco enzyme from a non-rubisco locus |
| ITRM20080342A1 (it) * | 2008-06-26 | 2009-12-27 | Univ Degli Studi Udine | Cellule di polpa dentale midollo-simili, metodi per isolamento ed uso. |
| US9550975B2 (en) | 2009-07-15 | 2017-01-24 | Mari Dezawa | SSEA-3 pluripotent stem cell isolated from body tissue |
-
2010
- 2010-07-14 US US12/836,264 patent/US9550975B2/en active Active
- 2010-07-15 PL PL10799956T patent/PL2455452T5/pl unknown
- 2010-07-15 EP EP20160074.9A patent/EP3680323B1/en active Active
- 2010-07-15 ES ES10799956T patent/ES2647360T5/es active Active
- 2010-07-15 EP EP17163323.3A patent/EP3214170B1/en active Active
- 2010-07-15 KR KR1020127003879A patent/KR20120069663A/ko not_active Ceased
- 2010-07-15 ES ES17163323T patent/ES2831030T3/es active Active
- 2010-07-15 CN CN201611060144.1A patent/CN107012117B/zh active Active
- 2010-07-15 JP JP2011522886A patent/JP5185443B2/ja active Active
- 2010-07-15 WO PCT/JP2010/062480 patent/WO2011007900A1/ja not_active Ceased
- 2010-07-15 HU HUE20160074A patent/HUE071170T2/hu unknown
- 2010-07-15 KR KR1020157013171A patent/KR101651795B1/ko active Active
- 2010-07-15 HU HUE17163323A patent/HUE052559T2/hu unknown
- 2010-07-15 SG SG2012003836A patent/SG177705A1/en unknown
- 2010-07-15 KR KR1020177020765A patent/KR101945343B1/ko active Active
- 2010-07-15 KR KR1020167008209A patent/KR101764080B1/ko active Active
- 2010-07-15 DK DK10799956.7T patent/DK2455452T4/da active
- 2010-07-15 EP EP10799956.7A patent/EP2455452B2/en active Active
- 2010-07-15 NO NO10799956A patent/NO2455452T3/no unknown
- 2010-07-15 CA CA2768238A patent/CA2768238C/en active Active
- 2010-07-15 PL PL17163323T patent/PL3214170T3/pl unknown
- 2010-07-15 ES ES20160074T patent/ES3015175T3/es active Active
- 2010-07-15 PT PT107999567T patent/PT2455452T/pt unknown
- 2010-07-15 CN CN201080041908.7A patent/CN102858951B/zh active Active
- 2010-07-15 HU HUE10799956A patent/HUE037448T2/hu unknown
- 2010-07-15 PT PT171633233T patent/PT3214170T/pt unknown
- 2010-07-15 DK DK17163323.3T patent/DK3214170T3/da active
- 2010-07-15 AU AU2010271722A patent/AU2010271722B8/en active Active
- 2010-07-15 PL PL20160074.9T patent/PL3680323T3/pl unknown
- 2010-07-15 HR HRP20250245TT patent/HRP20250245T1/hr unknown
-
2012
- 2012-05-01 JP JP2012104994A patent/JP5852503B2/ja active Active
-
2015
- 2015-12-04 JP JP2015237186A patent/JP6092357B2/ja active Active
-
2017
- 2017-02-08 JP JP2017021036A patent/JP6416298B2/ja active Active
-
2018
- 2018-10-03 JP JP2018188234A patent/JP6641441B2/ja active Active
-
2019
- 2019-12-27 JP JP2019239248A patent/JP6937821B2/ja active Active
-
2021
- 2021-08-31 JP JP2021140656A patent/JP7134317B2/ja active Active
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20160056127A (ko) * | 2014-11-11 | 2016-05-19 | 한국원자력의학원 | Cd105를 포함하는 줄기세포능을 가진 섬유아세포 분리용 바이오마커 조성물 및 이를 이용한 줄기세포능을 가진 섬유아세포 분리 방법 |
| KR20180100337A (ko) * | 2016-01-15 | 2018-09-10 | 국립대학법인 도야마 다이가쿠 | 허혈성 뇌경색에의 다능성 줄기 세포의 동원 |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7134317B2 (ja) | 生体組織から単離できる多能性幹細胞 | |
| US11261426B2 (en) | Pluripotent stem cell that can be isolated from body tissue | |
| WO2012133942A1 (ja) | 生体の臍帯又は脂肪組織から単離できる多能性幹細胞 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination | ||
| PA0105 | International application |
Patent event date: 20120214 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PA0201 | Request for examination | ||
| PG1501 | Laying open of application | ||
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20130930 Patent event code: PE09021S01D |
|
| AMND | Amendment | ||
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20140724 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20130930 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |
|
| AMND | Amendment | ||
| PX0901 | Re-examination |
Patent event code: PX09011S01I Patent event date: 20140724 Comment text: Decision to Refuse Application Patent event code: PX09012R01I Patent event date: 20140221 Comment text: Amendment to Specification, etc. |
|
| E90F | Notification of reason for final refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Final Notice of Reason for Refusal Patent event date: 20141120 Patent event code: PE09021S02D |
|
| A107 | Divisional application of patent | ||
| AMND | Amendment | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20150519 |
|
| E801 | Decision on dismissal of amendment | ||
| PE0801 | Dismissal of amendment |
Patent event code: PE08012E01D Comment text: Decision on Dismissal of Amendment Patent event date: 20151228 Patent event code: PE08011R01I Comment text: Amendment to Specification, etc. Patent event date: 20150519 Patent event code: PE08011R01I Comment text: Amendment to Specification, etc. Patent event date: 20141021 Patent event code: PE08011R01I Comment text: Amendment to Specification, etc. Patent event date: 20140221 |
|
| PX0601 | Decision of rejection after re-examination |
Comment text: Decision to Refuse Application Patent event code: PX06014S01D Patent event date: 20151228 Comment text: Amendment to Specification, etc. Patent event code: PX06012R01I Patent event date: 20150519 Comment text: Final Notice of Reason for Refusal Patent event code: PX06013S02I Patent event date: 20141120 Comment text: Amendment to Specification, etc. Patent event code: PX06012R01I Patent event date: 20141021 Comment text: Decision to Refuse Application Patent event code: PX06011S01I Patent event date: 20140724 Comment text: Amendment to Specification, etc. Patent event code: PX06012R01I Patent event date: 20140221 Comment text: Notification of reason for refusal Patent event code: PX06013S01I Patent event date: 20130930 |