KR100594562B1 - 미세 분말 및 초미세 분말의 제조 방법과 그를 위한이송형 아크 플라스마 시스템 - Google Patents
미세 분말 및 초미세 분말의 제조 방법과 그를 위한이송형 아크 플라스마 시스템 Download PDFInfo
- Publication number
- KR100594562B1 KR100594562B1 KR1020017002087A KR20017002087A KR100594562B1 KR 100594562 B1 KR100594562 B1 KR 100594562B1 KR 1020017002087 A KR1020017002087 A KR 1020017002087A KR 20017002087 A KR20017002087 A KR 20017002087A KR 100594562 B1 KR100594562 B1 KR 100594562B1
- Authority
- KR
- South Korea
- Prior art keywords
- plasma
- powder
- compartment
- vapor
- cooling
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 239000000843 powder Substances 0.000 title claims abstract description 102
- 238000004519 manufacturing process Methods 0.000 title abstract description 24
- 239000002245 particle Substances 0.000 claims abstract description 63
- 239000000463 material Substances 0.000 claims abstract description 61
- 238000000034 method Methods 0.000 claims abstract description 53
- 238000010791 quenching Methods 0.000 claims abstract description 38
- 229910052751 metal Inorganic materials 0.000 claims abstract description 20
- 239000002184 metal Substances 0.000 claims abstract description 20
- 239000000919 ceramic Substances 0.000 claims abstract description 15
- 229910045601 alloy Inorganic materials 0.000 claims abstract description 13
- 239000000956 alloy Substances 0.000 claims abstract description 13
- 239000002131 composite material Substances 0.000 claims abstract description 10
- 238000001816 cooling Methods 0.000 claims description 29
- 239000003085 diluting agent Substances 0.000 claims description 18
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 claims description 14
- 238000010438 heat treatment Methods 0.000 claims description 13
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 12
- 229910002804 graphite Inorganic materials 0.000 claims description 12
- 239000010439 graphite Substances 0.000 claims description 12
- 239000010949 copper Substances 0.000 claims description 10
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 claims description 9
- 229910052802 copper Inorganic materials 0.000 claims description 9
- 230000012010 growth Effects 0.000 claims description 9
- 239000007788 liquid Substances 0.000 claims description 8
- 238000010790 dilution Methods 0.000 claims description 7
- 239000012895 dilution Substances 0.000 claims description 7
- 238000002425 crystallisation Methods 0.000 claims description 6
- 230000008025 crystallization Effects 0.000 claims description 6
- 239000000203 mixture Substances 0.000 claims description 6
- 238000001914 filtration Methods 0.000 claims description 5
- 229910052759 nickel Inorganic materials 0.000 claims description 5
- 230000008569 process Effects 0.000 claims description 5
- 239000007787 solid Substances 0.000 claims description 5
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims description 4
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 claims description 4
- 239000012809 cooling fluid Substances 0.000 claims description 4
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 claims description 4
- 239000000376 reactant Substances 0.000 claims description 4
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 claims description 4
- 229910052721 tungsten Inorganic materials 0.000 claims description 4
- 239000010937 tungsten Substances 0.000 claims description 4
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 claims description 3
- 239000012530 fluid Substances 0.000 claims description 3
- 238000002347 injection Methods 0.000 claims description 3
- 239000007924 injection Substances 0.000 claims description 3
- 229910052750 molybdenum Inorganic materials 0.000 claims description 3
- 239000011733 molybdenum Substances 0.000 claims description 3
- 229910052758 niobium Inorganic materials 0.000 claims description 3
- 239000010955 niobium Substances 0.000 claims description 3
- GUCVJGMIXFAOAE-UHFFFAOYSA-N niobium atom Chemical compound [Nb] GUCVJGMIXFAOAE-UHFFFAOYSA-N 0.000 claims description 3
- 229910052709 silver Inorganic materials 0.000 claims description 3
- 239000004332 silver Substances 0.000 claims description 3
- 229910052715 tantalum Inorganic materials 0.000 claims description 3
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 claims description 3
- 239000012808 vapor phase Substances 0.000 claims description 3
- KJTLSVCANCCWHF-UHFFFAOYSA-N Ruthenium Chemical compound [Ru] KJTLSVCANCCWHF-UHFFFAOYSA-N 0.000 claims description 2
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 claims description 2
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 claims description 2
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 claims description 2
- 229910052793 cadmium Inorganic materials 0.000 claims description 2
- BDOSMKKIYDKNTQ-UHFFFAOYSA-N cadmium atom Chemical compound [Cd] BDOSMKKIYDKNTQ-UHFFFAOYSA-N 0.000 claims description 2
- 229910017052 cobalt Inorganic materials 0.000 claims description 2
- 239000010941 cobalt Substances 0.000 claims description 2
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 claims description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 claims description 2
- 229910052737 gold Inorganic materials 0.000 claims description 2
- 239000010931 gold Substances 0.000 claims description 2
- 229910052742 iron Inorganic materials 0.000 claims description 2
- 150000004767 nitrides Chemical class 0.000 claims description 2
- 229910052763 palladium Inorganic materials 0.000 claims description 2
- 229910052697 platinum Inorganic materials 0.000 claims description 2
- 239000003870 refractory metal Substances 0.000 claims description 2
- 229910052703 rhodium Inorganic materials 0.000 claims description 2
- 239000010948 rhodium Substances 0.000 claims description 2
- MHOVAHRLVXNVSD-UHFFFAOYSA-N rhodium atom Chemical compound [Rh] MHOVAHRLVXNVSD-UHFFFAOYSA-N 0.000 claims description 2
- 229910052707 ruthenium Inorganic materials 0.000 claims description 2
- 229910052719 titanium Inorganic materials 0.000 claims description 2
- 239000010936 titanium Substances 0.000 claims description 2
- 229910052726 zirconium Inorganic materials 0.000 claims description 2
- 238000012546 transfer Methods 0.000 abstract description 25
- 238000009833 condensation Methods 0.000 abstract description 15
- 230000005494 condensation Effects 0.000 abstract description 15
- 238000009826 distribution Methods 0.000 abstract description 15
- 230000008016 vaporization Effects 0.000 abstract description 14
- 150000002739 metals Chemical class 0.000 abstract description 10
- 239000007789 gas Substances 0.000 description 45
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 33
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 16
- 229910052786 argon Inorganic materials 0.000 description 16
- 238000002474 experimental method Methods 0.000 description 14
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 12
- 238000009834 vaporization Methods 0.000 description 11
- 239000001257 hydrogen Substances 0.000 description 10
- 229910052739 hydrogen Inorganic materials 0.000 description 10
- 239000000047 product Substances 0.000 description 10
- 229910052782 aluminium Inorganic materials 0.000 description 9
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 9
- 229910052757 nitrogen Inorganic materials 0.000 description 8
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 8
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 7
- 230000015572 biosynthetic process Effects 0.000 description 7
- PMHQVHHXPFUNSP-UHFFFAOYSA-M copper(1+);methylsulfanylmethane;bromide Chemical compound Br[Cu].CSC PMHQVHHXPFUNSP-UHFFFAOYSA-M 0.000 description 7
- 150000002431 hydrogen Chemical class 0.000 description 7
- 229910021529 ammonia Inorganic materials 0.000 description 6
- 238000006243 chemical reaction Methods 0.000 description 6
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 6
- 239000000112 cooling gas Substances 0.000 description 5
- 238000005272 metallurgy Methods 0.000 description 5
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 4
- 230000006911 nucleation Effects 0.000 description 4
- 238000010899 nucleation Methods 0.000 description 4
- 239000001301 oxygen Substances 0.000 description 4
- 229910052760 oxygen Inorganic materials 0.000 description 4
- 238000005118 spray pyrolysis Methods 0.000 description 4
- 229910001111 Fine metal Inorganic materials 0.000 description 3
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 230000007613 environmental effect Effects 0.000 description 3
- 239000012495 reaction gas Substances 0.000 description 3
- 238000000926 separation method Methods 0.000 description 3
- HBMJWWWQQXIZIP-UHFFFAOYSA-N silicon carbide Chemical compound [Si+]#[C-] HBMJWWWQQXIZIP-UHFFFAOYSA-N 0.000 description 3
- 229910010271 silicon carbide Inorganic materials 0.000 description 3
- 239000000377 silicon dioxide Substances 0.000 description 3
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- CPLXHLVBOLITMK-UHFFFAOYSA-N Magnesium oxide Chemical compound [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 2
- 229910006404 SnO 2 Inorganic materials 0.000 description 2
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 2
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 2
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 2
- 229910002091 carbon monoxide Inorganic materials 0.000 description 2
- 238000010924 continuous production Methods 0.000 description 2
- 239000002826 coolant Substances 0.000 description 2
- 238000000354 decomposition reaction Methods 0.000 description 2
- 239000001307 helium Substances 0.000 description 2
- 229910052734 helium Inorganic materials 0.000 description 2
- SWQJXJOGLNCZEY-UHFFFAOYSA-N helium atom Chemical compound [He] SWQJXJOGLNCZEY-UHFFFAOYSA-N 0.000 description 2
- 239000011344 liquid material Substances 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 239000000523 sample Substances 0.000 description 2
- 229910052710 silicon Inorganic materials 0.000 description 2
- 239000010935 stainless steel Substances 0.000 description 2
- 229910001220 stainless steel Inorganic materials 0.000 description 2
- 229910003468 tantalcarbide Inorganic materials 0.000 description 2
- 229910018072 Al 2 O 3 Inorganic materials 0.000 description 1
- QYEXBYZXHDUPRC-UHFFFAOYSA-N B#[Ti]#B Chemical compound B#[Ti]#B QYEXBYZXHDUPRC-UHFFFAOYSA-N 0.000 description 1
- 229910052582 BN Inorganic materials 0.000 description 1
- PZNSFCLAULLKQX-UHFFFAOYSA-N Boron nitride Chemical compound N#B PZNSFCLAULLKQX-UHFFFAOYSA-N 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- 229910033181 TiB2 Inorganic materials 0.000 description 1
- 229910010413 TiO 2 Inorganic materials 0.000 description 1
- NRTOMJZYCJJWKI-UHFFFAOYSA-N Titanium nitride Chemical compound [Ti]#N NRTOMJZYCJJWKI-UHFFFAOYSA-N 0.000 description 1
- 238000002441 X-ray diffraction Methods 0.000 description 1
- 229910007948 ZrB2 Inorganic materials 0.000 description 1
- 230000003044 adaptive effect Effects 0.000 description 1
- 239000010405 anode material Substances 0.000 description 1
- 238000010923 batch production Methods 0.000 description 1
- JEUVAEBWTRCMTB-UHFFFAOYSA-N boron;tantalum Chemical compound B#[Ta]#B JEUVAEBWTRCMTB-UHFFFAOYSA-N 0.000 description 1
- VWZIXVXBCBBRGP-UHFFFAOYSA-N boron;zirconium Chemical compound B#[Zr]#B VWZIXVXBCBBRGP-UHFFFAOYSA-N 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 239000012159 carrier gas Substances 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 150000001805 chlorine compounds Chemical class 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 239000004020 conductor Substances 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 239000011810 insulating material Substances 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 239000010808 liquid waste Substances 0.000 description 1
- 239000000395 magnesium oxide Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 150000001247 metal acetylides Chemical class 0.000 description 1
- NFFIWVVINABMKP-UHFFFAOYSA-N methylidynetantalum Chemical compound [Ta]#C NFFIWVVINABMKP-UHFFFAOYSA-N 0.000 description 1
- 229910003465 moissanite Inorganic materials 0.000 description 1
- 150000002823 nitrates Chemical class 0.000 description 1
- -1 oxides Chemical class 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 230000000171 quenching effect Effects 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 239000004576 sand Substances 0.000 description 1
- 239000012798 spherical particle Substances 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- MZLGASXMSKOWSE-UHFFFAOYSA-N tantalum nitride Chemical compound [Ta]#N MZLGASXMSKOWSE-UHFFFAOYSA-N 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 108700012359 toxins Proteins 0.000 description 1
- MTPVUVINMAGMJL-UHFFFAOYSA-N trimethyl(1,1,2,2,2-pentafluoroethyl)silane Chemical compound C[Si](C)(C)C(F)(F)C(F)(F)F MTPVUVINMAGMJL-UHFFFAOYSA-N 0.000 description 1
- 239000011364 vaporized material Substances 0.000 description 1
- 231100000925 very toxic Toxicity 0.000 description 1
- ZVWKZXLXHLZXLS-UHFFFAOYSA-N zirconium nitride Chemical compound [Zr]#N ZVWKZXLXHLZXLS-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
- B22F9/02—Making metallic powder or suspensions thereof using physical processes
- B22F9/14—Making metallic powder or suspensions thereof using physical processes using electric discharge
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
- B22F9/02—Making metallic powder or suspensions thereof using physical processes
- B22F9/12—Making metallic powder or suspensions thereof using physical processes starting from gaseous material
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J12/00—Chemical processes in general for reacting gaseous media with gaseous media; Apparatus specially adapted therefor
- B01J12/002—Chemical processes in general for reacting gaseous media with gaseous media; Apparatus specially adapted therefor carried out in the plasma state
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J12/00—Chemical processes in general for reacting gaseous media with gaseous media; Apparatus specially adapted therefor
- B01J12/02—Chemical processes in general for reacting gaseous media with gaseous media; Apparatus specially adapted therefor for obtaining at least one reaction product which, at normal temperature, is in the solid state
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00049—Controlling or regulating processes
- B01J2219/00051—Controlling the temperature
- B01J2219/00121—Controlling the temperature by direct heating or cooling
- B01J2219/00123—Controlling the temperature by direct heating or cooling adding a temperature modifying medium to the reactants
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00049—Controlling or regulating processes
- B01J2219/00051—Controlling the temperature
- B01J2219/0015—Controlling the temperature by thermal insulation means
- B01J2219/00155—Controlling the temperature by thermal insulation means using insulating materials or refractories
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/08—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor
- B01J2219/0803—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor employing electric or magnetic energy
- B01J2219/0805—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor employing electric or magnetic energy giving rise to electric discharges
- B01J2219/0807—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor employing electric or magnetic energy giving rise to electric discharges involving electrodes
- B01J2219/0809—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor employing electric or magnetic energy giving rise to electric discharges involving electrodes employing two or more electrodes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/08—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor
- B01J2219/0803—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor employing electric or magnetic energy
- B01J2219/0805—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor employing electric or magnetic energy giving rise to electric discharges
- B01J2219/0845—Details relating to the type of discharge
- B01J2219/0847—Glow discharge
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/08—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor
- B01J2219/0873—Materials to be treated
- B01J2219/0879—Solid
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/08—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor
- B01J2219/0894—Processes carried out in the presence of a plasma
- B01J2219/0898—Hot plasma
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2999/00—Aspects linked to processes or compositions used in powder metallurgy
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Physics & Mathematics (AREA)
- Engineering & Computer Science (AREA)
- Plasma & Fusion (AREA)
- Manufacture Of Metal Powder And Suspensions Thereof (AREA)
- Physical Or Chemical Processes And Apparatus (AREA)
- Discharge Heating (AREA)
- Powder Metallurgy (AREA)
Abstract
Description
| 운전 변수 | 조건 | 결과 |
| 반응기 | 플라스마 기체 유속= 아르곤 40 ℓ/분, 수소 20 ℓ/분 전력= 24.5 kw 플라스마실 압력= 1.1 atm 희석 기체= 아르곤 85 ℓ/분, 온도>1000K 도가니 소재= 흑연 | 증기화 속도= 1.0kg/h 입자 크기 분포: 90% 이하(d90)=1.77㎛ 50% 이하(d50)=0.78㎛ 10% 이하(d10)=0.21㎛ (도 6a 및 도 7a 참조) 간격=(d90-d10)/d50=2.0 XRD 계수(2θ=43.3o) = 31300 |
| 급냉관 | 제1 구획 길이= 10 cm 내부관 직경= 5 cm 간접 냉각 기체= 아르곤 300 ℓ/분 제2 구획 직접 냉각= 질소 300 ℓ/분 | |
| 수집 | 다공성 금속 필터 |
| 운전 변수 | 조건 | 결과 |
| 반응기 | 플라스마 기체 유속= 아르곤 40 ℓ/분, 수소 20 ℓ/분 전력= 24.5 kw 플라스마실 압력= 1.1 atm 희석기체= 질소 85 ℓ/분, 온도>1000K 도가니 소재= 흑연 | 증기화 속도= 0.9kg/h 입자 크기 분포: 90% 이하(d90)=3.67㎛ 50% 이하(d50)=1.74㎛ 10% 이하(d10)=0.74㎛ (도 6b 참조) 간격=(d90-d10)/d50=1.7 XRD 계수(2θ=43.3o) = 30700 |
| 급냉관 | 제1 구획 길이= 25 cm 내부관 직경= 5 cm 간접 냉각 기체= 아르곤 200 ℓ/분 제2 구획 직접 냉각= 아르곤 300 ℓ/분 | |
| 수집 | 다공성 금속 필터 |
| 운전 변수 | 조건 | 결과 |
| 반응기 | 플라스마 기체 유속= 아르곤 40 ℓ/분, 수소 20 ℓ/분 전력= 24.5 kw 플라스마실 압력= 1.1 atm 희석 기체= 아르곤 20 l/min, 온도>1000K 도가니 소재= 흑연 | 증기화 속도= 0.9kg/h 입자 크기 분포: 90% 이하(d90)=2.91㎛ 50% 이하(d50)=0.81㎛ 10% 이하(d10)=0.25㎛ (도 7b 참조) 간격=(d90-d10)/d50=3.3 XRD 계수(2θ=43.3o) = 35800 |
| 급냉관 | 제1 구획 길이= 10 cm 내부관 직경= 5 cm 간접 냉각 기체= 아르곤 100 ℓ/분 제2 구획 직접 냉각= 질소 300 ℓ/분 | |
| 수집 | 다공성 금속 필터 |
| 운전 변수 | 조건 | 결과 |
| 반응기 | 플라스마 기체 유속= 아르곤 40 ℓ/분, 수소 20 ℓ/분 전력= 28 kw 플라스마실 압력= 1.1 atm 희석 기체= 아르곤 65 ℓ/분, 온도>1000K 도가니 소재= 흑연 | 증기화 속도= 0.5kg/h 입자 크기 분포: 90% 이하(d90)=1.42㎛ 50% 이하(d50)=0.79㎛ 10% 이하(d10)=0.45㎛ 간격=(d90-d10)/d50=1.22 XRD 계수(2θ=44.5o) = 24800 (도 8a 참조) |
| 급냉관 | 제1 구획 길이= 15 cm 내부관 직경= 2.5 cm 간접 냉각 기체= 아르곤 100 ℓ/분 제2 구획 직접 냉각= 아르곤 200 ℓ/분 | |
| 수집 | 다공성 금속 필터 |
| 운전 변수 | 조건 | 결과 |
| 반응기 | 플라스마 기체 유속= 아르곤 40 ℓ/분, 수소 20 ℓ/분 전력= 28 kw 플라스마실 압력= 1.1 atm 희석 기체= 아르곤 65 ℓ/분, 온도>1000K 도가니 소재= 흑연 | 증기화 속도= 0.5kg/h 입자크기 분포: 90% 이하(d90)=1.76㎛ 50% 이하(d50)=0.98㎛ 10% 이하(d10)=0.54㎛ 간격=(d90-d10)/d50=1.24 XRD 계수(2θ=44.5o) = 9300 (도 8b 참조) |
| 급냉관 | 제1 구획 길이= 15 cm 내부관 직경= 2.5 cm 간접 냉각 기체= 아르곤 300 ℓ/분 제2 구획 직접 냉각= 아르곤 200 ℓ/분 | |
| 수집 | 다공성 금속 필터 |
Claims (22)
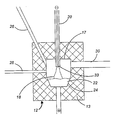
- - 플라스마 반응기 내에 증기화 또는 분해시킬 재료를 제공하는 단계;- 플라스마 토치 공급 기체를 제공하는 단계;- 상기 재료와 전극 사이에 아크를 가하여 상기 재료를 증기화 또는 분해하여 그의 증기를 형성하기에 충분한 높은 온도의 플라스마를 발생시키는 단계;- 1000K 이상의 온도로 가열한 희석 기체를 플라스마 반응기 내에, 상기 플라스마 토치 공급 기체와 물리적으로 분리된 위치에서, 주입하는 단계;- 상기 증기를 플라스마 기체 및 희석 기체에 의해 급냉관(quench tube) 내로 수송하여 상기 증기를 응축시켜서 분말을 형성하는 단계 (여기서, 상기 급냉관은- 그 내부에 존재하는 증기 및 임의의 입자를 간접적으로 냉각 또는 가열하여 입자 성장 및 결정화를 조절하기 위한 제1 구획; 및- 상기 제1 구획에 연결되어 있으며 그 내부에 존재하는 증기 및 임의의 입자를 직접적으로 냉각시키기 위한 제2 구획을 포함한다); 및- 수집 유닛 내에서 분말 입자를 수집하고 임의로 여과하는 단계를 포함하는, 이송형 아크 플라스마 시스템을 이용한 미세 분말 및 초미세 분말의 제조 방법.
- 제1항에 있어서, 상기 급냉관은 그 본체가 관형인 방법.
- 제1항에 있어서, 상기 재료는 금속, 합금, 세라믹 및 복합소재를 포함하는 방법.
- 제1항에 있어서, 상기 증기화 또는 분해할 재료가 애노드이고, 전극이 캐소드이며 비소모성인 방법.
- 제1항에 있어서, 상기 재료를 전기 전도성 도가니 내로 제공하는 방법.
- 제1항에 있어서, 상기 재료를 고체 입자, 와이어, 막대(rod), 액체 또는 이들의 혼합물 형태로 플라스마 반응기 내로 공급하는 방법.
- 제6항에 있어서, 상기 도가니가 흑연, 탄화물, 산화물, 질화물, 붕화물 또는 내화 금속으로 제조된 것인 방법.
- 제2항에 있어서, 상기 간접 냉각 또는 가열을 본체 주위의 채널 내에서 냉각 또는 가열 유체를 순환시킴으로써 수행하는 방법.
- 제1항에 있어서, 반응제를 기체 형태로 1개 이상의 주입구를 통하여 급냉관의 제1 구획으로 주입하는 방법.
- 제1항에 있어서, 상기 직접 냉각을 증기 상으로 냉각 유체를 직접 주입하여 수행하는 방법.
- 삭제
- 제1항에 있어서, 증기화될 재료가 플라스마 반응기 내부에 있는 전기 전도성 도가니에 연속적으로 공급되는 금속이고, 상기 급냉관은- 그 내부에 존재하는 증기 및 임의의 입자를 간접적으로 냉각 또는 가열하여 입자의 성장 및 결정화를 조절하기 위한 관형의 본체를 포함하는 제1 구획 (여기서, 증기는 상기 본체 내부를 통과한다); 및- 상기 제1 구획에 연결되어 있으며 증기 상으로 직접 냉각 유체를 주입하여 그 내부에 존재하는 증기 및 임의의 입자를 직접적으로 냉각시키는 제2 구획을 포함하는 방법.
- 제12항에 있어서, 상기 금속이 은, 금, 카드뮴, 코발트, 구리, 철, 몰리브덴, 니켈, 니오븀, 팔라듐, 백금, 로듐, 루테늄, 탄탈, 티탄, 텅스텐, 지르코늄 및 이들의 합금을 포함하는 방법.
- 삭제
- 삭제
- 삭제
- 삭제
- 삭제
- 삭제
- 삭제
- 삭제
- 삭제
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/136,043 US6379419B1 (en) | 1998-08-18 | 1998-08-18 | Method and transferred arc plasma system for production of fine and ultrafine powders |
| US09/136,043 | 1998-08-18 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20010099622A KR20010099622A (ko) | 2001-11-09 |
| KR100594562B1 true KR100594562B1 (ko) | 2006-06-30 |
Family
ID=22470991
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020017002087A Expired - Lifetime KR100594562B1 (ko) | 1998-08-18 | 1999-08-16 | 미세 분말 및 초미세 분말의 제조 방법과 그를 위한이송형 아크 플라스마 시스템 |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US6379419B1 (ko) |
| EP (1) | EP1115523B1 (ko) |
| JP (3) | JP3541939B2 (ko) |
| KR (1) | KR100594562B1 (ko) |
| AT (1) | ATE240177T1 (ko) |
| AU (1) | AU5275299A (ko) |
| CA (1) | CA2340669C (ko) |
| DE (1) | DE69907933T2 (ko) |
| WO (1) | WO2000010756A1 (ko) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100840229B1 (ko) | 2006-09-08 | 2008-06-23 | 재단법인 포항산업과학연구원 | 초미세 솔더 분말, 초미세 솔더 분말의 제조방법 및 그제조장치 |
| KR101009656B1 (ko) | 2008-09-17 | 2011-01-19 | 희성금속 주식회사 | 초미세 귀금속 분말 제조방법 |
| KR101024971B1 (ko) | 2008-12-12 | 2011-03-25 | 희성금속 주식회사 | 열플라즈마를 이용한 귀금속 분말 및 귀금속 타겟 제조방법 |
| KR101193683B1 (ko) | 2010-03-29 | 2012-10-22 | 현대제철 주식회사 | 지르코늄 코어드 와이어 투입법을 이용한 지르코늄 함유 철계 합금 제조 방법 및 그 제조 장치 |
| KR101408238B1 (ko) * | 2011-12-01 | 2014-06-16 | 소에이 가가쿠 고교 가부시키가이샤 | 금속분말 제조용 플라즈마 장치 |
| KR102465825B1 (ko) * | 2022-09-06 | 2022-11-09 | 이용복 | 열플라즈마를 이용한 금속분말 제조장치 및 그 제조방법 |
Families Citing this family (115)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7576296B2 (en) * | 1995-03-14 | 2009-08-18 | Battelle Energy Alliance, Llc | Thermal synthesis apparatus |
| US6972115B1 (en) | 1999-09-03 | 2005-12-06 | American Inter-Metallics, Inc. | Apparatus and methods for the production of powders |
| KR100743844B1 (ko) * | 1999-12-01 | 2007-08-02 | 도와 마이닝 가부시끼가이샤 | 구리 분말 및 구리 분말의 제조 방법 |
| AU2906401A (en) * | 1999-12-21 | 2001-07-03 | Bechtel Bwxt Idaho, Llc | Hydrogen and elemental carbon production from natural gas and other hydrocarbons |
| WO2001058625A1 (en) * | 2000-02-10 | 2001-08-16 | Tetronics Limited | Plasma arc reactor for the production of fine powders |
| ATE311268T1 (de) * | 2000-02-18 | 2005-12-15 | Canadian Electronic Powders Co | Nickelpulver zur verwendung als elektroden in keramischen mehrschicht-grundmetallelktroden- kondensatoren |
| WO2002005969A2 (en) * | 2000-07-19 | 2002-01-24 | Regents Of The University Of Minnesota | Apparatus and method for synthesizing films and coatings by focused particle beam deposition |
| US7572430B2 (en) * | 2000-11-09 | 2009-08-11 | Cyprus Amax Minerals Company | Method for producing nano-particles |
| US6468497B1 (en) * | 2000-11-09 | 2002-10-22 | Cyprus Amax Minerals Company | Method for producing nano-particles of molybdenum oxide |
| AUPR186200A0 (en) * | 2000-12-04 | 2001-01-04 | Tesla Group Holdings Pty Limited | Plasma reduction processing of materials |
| US7442227B2 (en) | 2001-10-09 | 2008-10-28 | Washington Unniversity | Tightly agglomerated non-oxide particles and method for producing the same |
| CA2463444C (en) * | 2001-11-06 | 2008-02-19 | Cyprus Amax Minerals Company | Apparatus and method for producing pigment nano-particles |
| US6688494B2 (en) * | 2001-12-20 | 2004-02-10 | Cima Nanotech, Inc. | Process for the manufacture of metal nanoparticle |
| US6755886B2 (en) * | 2002-04-18 | 2004-06-29 | The Regents Of The University Of California | Method for producing metallic microparticles |
| US6902601B2 (en) | 2002-09-12 | 2005-06-07 | Millennium Inorganic Chemicals, Inc. | Method of making elemental materials and alloys |
| US6868896B2 (en) * | 2002-09-20 | 2005-03-22 | Edward Scott Jackson | Method and apparatus for melting titanium using a combination of plasma torches and direct arc electrodes |
| RU2238824C1 (ru) * | 2003-08-20 | 2004-10-27 | Открытое акционерное общество "ВНИИЭТО" | Установка для плазмохимического восстановления оксидов металлов |
| RU2238174C1 (ru) * | 2003-09-30 | 2004-10-20 | Военная академия Ракетных войск стратегического назначения им. Петра Великого | Способ получения ультрадисперсного порошка и устройство для его осуществления |
| US7794629B2 (en) * | 2003-11-25 | 2010-09-14 | Qinetiq Limited | Composite materials |
| US7494527B2 (en) * | 2004-01-26 | 2009-02-24 | Tekna Plasma Systems Inc. | Process for plasma synthesis of rhenium nano and micro powders, and for coatings and near net shape deposits thereof and apparatus therefor |
| US7384448B2 (en) * | 2004-02-16 | 2008-06-10 | Climax Engineered Materials, Llc | Method and apparatus for producing nano-particles of silver |
| JP2005289776A (ja) * | 2004-04-05 | 2005-10-20 | Canon Inc | 結晶製造方法および結晶製造装置 |
| JP3938770B2 (ja) * | 2004-04-16 | 2007-06-27 | Tdk株式会社 | ニッケル粉の製造方法とニッケル粉の製造装置とニッケル粉製造用坩堝 |
| EP1743043A4 (en) * | 2004-04-19 | 2008-08-27 | Sdc Materials Llc | MASSIVE DISCOVERY OF MATERIALS BY STEAM PHASE SYNTHESIS |
| CA2512313A1 (en) * | 2004-07-20 | 2006-01-20 | E.I. Dupont De Nemours And Company | Apparatus for making metal oxide nanopowder |
| CA2512317A1 (en) * | 2004-07-20 | 2006-01-20 | E.I. Dupont De Nemours And Company | Process for making metal oxide nanoparticles |
| US7297619B2 (en) * | 2004-08-24 | 2007-11-20 | California Institute Of Technology | System and method for making nanoparticles using atmospheric-pressure plasma microreactor |
| EP1810001A4 (en) | 2004-10-08 | 2008-08-27 | Sdc Materials Llc | APPARATUS AND METHOD FOR SAMPLING AND COLLECTING POWDERS FLOWING IN A GAS STREAM |
| US7476851B2 (en) * | 2004-11-12 | 2009-01-13 | Regents Of The University Of Minnesota | Aerodynamic focusing of nanoparticle or cluster beams |
| US7354561B2 (en) * | 2004-11-17 | 2008-04-08 | Battelle Energy Alliance, Llc | Chemical reactor and method for chemically converting a first material into a second material |
| ATE509693T1 (de) | 2005-01-28 | 2011-06-15 | Tekna Plasma Systems Inc | Induktionsplasmasynthese von nanopulvern |
| US8079838B2 (en) * | 2005-03-16 | 2011-12-20 | Horiba, Ltd. | Pure particle generator |
| US20080277092A1 (en) * | 2005-04-19 | 2008-11-13 | Layman Frederick P | Water cooling system and heat transfer system |
| DE102005028463A1 (de) * | 2005-06-17 | 2006-12-28 | Basf Ag | Verfahren zur Herstellung von nanopartikulären Lanthanoid/Bor-Verbindungen von nanopartikuläre Lanthanoid/Bor-Verbindungen enthaltenden Feststoffgemischen |
| CN100431748C (zh) * | 2005-07-27 | 2008-11-12 | 北京工业大学 | 稀土元素钆的纳米颗粒及纳米晶块体材料的制备方法 |
| US8268405B2 (en) * | 2005-08-23 | 2012-09-18 | Uwm Research Foundation, Inc. | Controlled decoration of carbon nanotubes with aerosol nanoparticles |
| US8240190B2 (en) * | 2005-08-23 | 2012-08-14 | Uwm Research Foundation, Inc. | Ambient-temperature gas sensor |
| CA2581806C (en) * | 2006-03-08 | 2012-06-26 | Tekna Plasma Systems Inc. | Plasma synthesis of nanopowders |
| US7736421B2 (en) * | 2006-10-10 | 2010-06-15 | Aerosol Dynamics Inc. | High saturation ratio water condensation device and method |
| JP5052291B2 (ja) * | 2006-11-02 | 2012-10-17 | 株式会社日清製粉グループ本社 | 合金超微粒子、およびその製造方法 |
| KR100788413B1 (ko) | 2007-03-13 | 2007-12-24 | 호서대학교 산학협력단 | 열플라즈마를 이용한 나노 복합 분말 제조 방법 |
| US9630162B1 (en) * | 2007-10-09 | 2017-04-25 | University Of Louisville Research Foundation, Inc. | Reactor and method for production of nanostructures |
| US8481449B1 (en) | 2007-10-15 | 2013-07-09 | SDCmaterials, Inc. | Method and system for forming plug and play oxide catalysts |
| EP2107862B1 (de) | 2008-04-03 | 2015-09-02 | Maicom Quarz GmbH | Verfahren und Vorrichtung zur Behandlung von Dispersionsmaterialien |
| USD627900S1 (en) | 2008-05-07 | 2010-11-23 | SDCmaterials, Inc. | Glove box |
| CN102196997A (zh) * | 2008-10-27 | 2011-09-21 | 巴斯夫欧洲公司 | 制备纳米颗粒状金属硼化物悬浮液的方法 |
| WO2010108272A1 (en) | 2009-03-24 | 2010-09-30 | Tekna Plasma Systems Inc. | Plasma reactor for the synthesis of nanopowders and materials processing |
| US8591821B2 (en) | 2009-04-23 | 2013-11-26 | Battelle Energy Alliance, Llc | Combustion flame-plasma hybrid reactor systems, and chemical reactant sources |
| KR100943453B1 (ko) * | 2009-08-18 | 2010-02-22 | 이대식 | 초미세 금속분말 제조 장치 및 방법, 이에 사용되는 금속증발장치 |
| KR101134501B1 (ko) * | 2009-12-07 | 2012-04-13 | 주식회사 풍산 | 열플라즈마를 이용한 고순도 구리분말의 제조방법 |
| US8803025B2 (en) | 2009-12-15 | 2014-08-12 | SDCmaterials, Inc. | Non-plugging D.C. plasma gun |
| US8545652B1 (en) | 2009-12-15 | 2013-10-01 | SDCmaterials, Inc. | Impact resistant material |
| US8652992B2 (en) | 2009-12-15 | 2014-02-18 | SDCmaterials, Inc. | Pinning and affixing nano-active material |
| US8470112B1 (en) | 2009-12-15 | 2013-06-25 | SDCmaterials, Inc. | Workflow for novel composite materials |
| US9090475B1 (en) | 2009-12-15 | 2015-07-28 | SDCmaterials, Inc. | In situ oxide removal, dispersal and drying for silicon SiO2 |
| US9126191B2 (en) | 2009-12-15 | 2015-09-08 | SDCmaterials, Inc. | Advanced catalysts for automotive applications |
| US9149797B2 (en) | 2009-12-15 | 2015-10-06 | SDCmaterials, Inc. | Catalyst production method and system |
| US8557727B2 (en) * | 2009-12-15 | 2013-10-15 | SDCmaterials, Inc. | Method of forming a catalyst with inhibited mobility of nano-active material |
| WO2011119494A1 (en) * | 2010-03-22 | 2011-09-29 | The Regents Of The University Of California | Method and device to synthesize boron nitride nanotubes and related nanoparticles |
| KR101175676B1 (ko) * | 2010-03-25 | 2012-08-22 | 희성금속 주식회사 | 폐 루테늄(Ru) 타겟을 이용한 고순도화 및 미세화된 루테늄(Ru)분말 제조법 |
| RU2455119C2 (ru) * | 2010-08-27 | 2012-07-10 | Алексей Александрович Калачев | Способ получения наночастиц |
| CN102166654B (zh) * | 2010-12-30 | 2015-11-25 | 广东高鑫科技股份有限公司 | 高效镍-石墨粉体的制备方法及其专用装置 |
| US8669202B2 (en) | 2011-02-23 | 2014-03-11 | SDCmaterials, Inc. | Wet chemical and plasma methods of forming stable PtPd catalysts |
| KR101285284B1 (ko) * | 2011-04-26 | 2013-07-11 | 희성금속 주식회사 | 폐 루테늄(Ru) 타겟을 이용한 초고순도 루테늄(Ru) 분말 및 타겟의 제조방법 |
| CN102211197B (zh) * | 2011-05-06 | 2014-06-04 | 宁波广博纳米新材料股份有限公司 | 金属蒸发装置及用该装置制备超微细金属粉末的方法 |
| RU2460816C1 (ru) * | 2011-05-20 | 2012-09-10 | Федеральное государственное бюджетное образовательное учреждение высшего профессионального образования "Казанский национальный исследовательский технический университет им. А.Н.Туполева - КАИ" (КНИТУ-КАИ) | Способ получения порошкового материала на основе меди |
| JP5824906B2 (ja) * | 2011-06-24 | 2015-12-02 | 昭栄化学工業株式会社 | 金属粉末製造用プラズマ装置及び金属粉末製造方法 |
| RU2014110365A (ru) | 2011-08-19 | 2015-09-27 | ЭсДиСиМАТИРИАЛЗ, ИНК. | Подложки с покрытием для использования в катализе, каталитические конвертеры и способы покрытия подложек композициями покрытия из оксида |
| CA2855579C (en) | 2011-12-06 | 2019-10-29 | Shoei Chemical Inc. | Plasma device for production of metal powder |
| JP5817636B2 (ja) * | 2012-04-20 | 2015-11-18 | 昭栄化学工業株式会社 | 金属粉末の製造方法 |
| US20150147257A1 (en) * | 2012-06-05 | 2015-05-28 | Dow Corning Corporation | Fluid capture of nanoparticles |
| CN102950293B (zh) * | 2012-10-15 | 2015-01-07 | 宁波广博纳米新材料股份有限公司 | 纳米铝粉的生产方法 |
| CN102950289B (zh) * | 2012-10-15 | 2014-10-15 | 宁波广博纳米新材料股份有限公司 | 纳米级铜锰合金粉的生产方法 |
| CN102950292B (zh) * | 2012-10-15 | 2015-07-08 | 宁波广博纳米新材料股份有限公司 | 亚微米级铜锰镍合金粉的生产方法 |
| CN102950290B (zh) * | 2012-10-15 | 2014-11-26 | 宁波广博纳米新材料股份有限公司 | 纳米级镍锰合金粉的生产方法 |
| CN102950291B (zh) * | 2012-10-15 | 2015-02-11 | 宁波广博纳米新材料股份有限公司 | 亚微米级锡铜合金粉的生产方法 |
| CN102873323B (zh) * | 2012-11-01 | 2014-04-23 | 泰克科技(苏州)有限公司 | 电子钽粉性能改善装置 |
| CN103008673B (zh) * | 2012-11-07 | 2014-08-06 | 宁波广博纳米新材料股份有限公司 | 蒸发冷凝法制备含硫镍粉的方法 |
| JP6089186B2 (ja) * | 2012-11-12 | 2017-03-08 | 国立大学法人弘前大学 | 超微細粉末、高強度鋼焼結体及びそれらの製造方法 |
| US9511352B2 (en) | 2012-11-21 | 2016-12-06 | SDCmaterials, Inc. | Three-way catalytic converter using nanoparticles |
| US9156025B2 (en) | 2012-11-21 | 2015-10-13 | SDCmaterials, Inc. | Three-way catalytic converter using nanoparticles |
| KR20150003580A (ko) * | 2013-07-01 | 2015-01-09 | 희성금속 주식회사 | 루테늄 분말 및 루테늄 타겟의 제조방법 |
| EP3024571B1 (en) | 2013-07-25 | 2020-05-27 | Umicore AG & Co. KG | Washcoats and coated substrates for catalytic converters |
| CN103537703B (zh) * | 2013-09-12 | 2017-04-12 | 江苏博迁新材料股份有限公司 | 一种内回流式除垃圾方法 |
| KR20160074574A (ko) | 2013-10-22 | 2016-06-28 | 에스디씨머티리얼스, 인코포레이티드 | 희박 NOx 트랩의 조성물 |
| CN106061600A (zh) | 2013-10-22 | 2016-10-26 | Sdc材料公司 | 用于重型柴油机的催化剂设计 |
| CN106470752A (zh) | 2014-03-21 | 2017-03-01 | Sdc材料公司 | 用于被动nox吸附(pna)系统的组合物 |
| CN104084595B (zh) * | 2014-07-11 | 2016-08-24 | 湖南娄底华星锑业有限公司 | 一种锑粉生产系统 |
| DE102014220817B4 (de) * | 2014-10-14 | 2021-02-04 | Universität Duisburg-Essen | Lichtbogenreaktor und Verfahren zur Herstellung von Nanopartikeln |
| CN104722764B (zh) * | 2015-03-11 | 2017-01-25 | 江永斌 | 循环冷却的金属粉体蒸发制取装置 |
| CN104690266A (zh) * | 2015-03-18 | 2015-06-10 | 宁波广博纳米新材料股份有限公司 | 用于制备晶片电阻器正面、背面电极的铜锰合金粉 |
| JP5954470B2 (ja) * | 2015-06-26 | 2016-07-20 | 昭栄化学工業株式会社 | 金属粉末製造用プラズマ装置及び金属粉末製造方法 |
| CN108349010B (zh) | 2015-10-19 | 2021-03-09 | 住友金属矿山株式会社 | 镍粉末的制造方法 |
| CN105252012B (zh) * | 2015-11-18 | 2017-02-08 | 长春工业大学 | 一种多电极等离子弧连续制造金属粉末的装置及方法 |
| JP6573563B2 (ja) | 2016-03-18 | 2019-09-11 | 住友金属鉱山株式会社 | ニッケル粉末、ニッケル粉末の製造方法、およびニッケル粉末を用いた内部電極ペーストならびに電子部品 |
| CN105598460B (zh) * | 2016-03-21 | 2018-03-06 | 台州市金博超导纳米材料科技有限公司 | 用于制造微纳米级金属粉末的高温蒸发器 |
| JP6729719B2 (ja) | 2016-12-05 | 2020-07-22 | 住友金属鉱山株式会社 | ニッケル粉末の製造方法 |
| JP6920676B2 (ja) * | 2017-04-19 | 2021-08-18 | パナソニックIpマネジメント株式会社 | 微粒子製造装置および微粒子製造方法 |
| CN107030292A (zh) * | 2017-05-03 | 2017-08-11 | 江苏天楹环保能源成套设备有限公司 | 一种多级冷却制备金属粉末的等离子体雾化装置 |
| JP7194544B2 (ja) * | 2017-10-03 | 2022-12-22 | 三井金属鉱業株式会社 | 粒子の製造方法 |
| CN108130524A (zh) * | 2017-12-22 | 2018-06-08 | 中国科学院电工研究所 | 等离子体射流沉积薄膜装置及浅化表面陷阱能级的方法 |
| US10612111B2 (en) * | 2018-08-21 | 2020-04-07 | Robert Ten | Method and apparatus for extracting high-purity gold from ore |
| KR102141225B1 (ko) * | 2018-08-29 | 2020-08-04 | 한국생산기술연구원 | 온도급감장치를 구비한 열플라즈마 토치 및 이를 이용한 금속 나노 분말 가공장치. |
| CN114206527B (zh) | 2019-07-31 | 2023-05-02 | 住友金属矿山株式会社 | 镍粉末、镍粉末的制造方法 |
| CA3163929C (en) * | 2019-11-07 | 2024-04-09 | Shenzhen Aerospace Science Advanced Materials Co., Ltd | New spherical powder and preparation method therefor |
| JP7557689B2 (ja) * | 2020-03-25 | 2024-09-30 | 国立大学法人弘前大学 | 高強度Zn焼結体の製造方法 |
| RU2746197C1 (ru) * | 2020-05-11 | 2021-04-08 | Федеральное государственное бюджетное образовательное учреждение высшего образования "Пензенский государственный университет" | Способ получения мелкодисперсного порошка тугоплавкого материала |
| RU2746673C1 (ru) * | 2020-10-09 | 2021-04-19 | федеральное государственное автономное образовательное учреждение высшего образования «Национальный исследовательский Томский политехнический университет» | СПОСОБ ПОЛУЧЕНИЯ ПОРОШКА, СОДЕРЖАЩЕГО ОДНОФАЗНЫЙ ВЫСОКОЭНТРОПИЙНЫЙ КАРБИД СОСТАВА Ti-Nb-Zr-Hf-Ta-C С КУБИЧЕСКОЙ РЕШЕТКОЙ |
| CN214260700U (zh) * | 2021-01-08 | 2021-09-24 | 江苏博迁新材料股份有限公司 | 一种使用等离子转移弧加热的高温蒸发器 |
| JP7688809B2 (ja) | 2021-01-25 | 2025-06-05 | 江蘇博遷新材料股▲ふん▼有限公司 | 気相法による微粉末の調製に用いる耐高温の液体還流と排気構造 |
| WO2022156229A1 (zh) * | 2021-01-25 | 2022-07-28 | 钟笔 | 一种用于控制超微粉粒子成型的控制器 |
| CN216421070U (zh) * | 2021-10-19 | 2022-05-03 | 江苏博迁新材料股份有限公司 | 一种物理气相法制备超细粉体材料用的金属蒸气成核装置 |
| KR102572728B1 (ko) * | 2021-11-19 | 2023-08-31 | 한국생산기술연구원 | 금속분말 제조장치 및 이를 이용한 금속분말 제조방법 |
| RU210733U1 (ru) * | 2022-01-28 | 2022-04-28 | Федеральное государственное автономное образовательное учреждение высшего образования "Национальный исследовательский Томский политехнический университет" | Устройство для получения порошка на основе карбида бора |
| JP2023183703A (ja) | 2022-06-16 | 2023-12-28 | 昭栄化学工業株式会社 | 金属粉末の製造方法および金属粉末の製造装置 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH05331508A (ja) * | 1992-05-29 | 1993-12-14 | Murata Mfg Co Ltd | 銅粉末の製造方法 |
| JPH10102109A (ja) * | 1996-09-30 | 1998-04-21 | Tanaka Kikinzoku Kogyo Kk | ニッケル粉末の製造方法 |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE269157C (ko) | 1911-12-12 | |||
| US4076640A (en) * | 1975-02-24 | 1978-02-28 | Xerox Corporation | Preparation of spheroidized particles |
| GB2002208B (en) * | 1977-08-01 | 1982-04-28 | Thermo Electron Corp | Process of and apparatus for producing a particulate product from a feed material |
| US4376470A (en) | 1980-11-06 | 1983-03-15 | Little Giant Industries, Inc. | Fiberglass ladder |
| US4376740A (en) | 1981-01-05 | 1983-03-15 | National Research Institute For Metals | Process for production fine metal particles |
| DD269157A1 (de) * | 1987-12-28 | 1989-06-21 | Akad Wissenschaften Ddr | Plasmareaktor fuer die pyrolyse hochviskoser, teerartiger, kohlenwasserstoffhaltiger produkte |
| US4990179A (en) * | 1990-04-23 | 1991-02-05 | Fmc Corporation | Process for increasing the life of carbon crucibles in plasma furnaces |
| US5147448A (en) * | 1990-10-01 | 1992-09-15 | Nuclear Metals, Inc. | Techniques for producing fine metal powder |
| WO1992014576A1 (en) * | 1991-02-22 | 1992-09-03 | Idaho Research Foundation | Plama production of ultra-fine ceramic carbides |
| US5460701A (en) * | 1993-07-27 | 1995-10-24 | Nanophase Technologies Corporation | Method of making nanostructured materials |
| US5514350A (en) | 1994-04-22 | 1996-05-07 | Rutgers, The State University Of New Jersey | Apparatus for making nanostructured ceramic powders and whiskers |
| US5855642A (en) * | 1996-06-17 | 1999-01-05 | Starmet Corporation | System and method for producing fine metallic and ceramic powders |
| US5782952A (en) * | 1996-08-30 | 1998-07-21 | Massachusetts Institute Of Technology | Method for production of magnesium |
-
1998
- 1998-08-18 US US09/136,043 patent/US6379419B1/en not_active Expired - Lifetime
-
1999
- 1999-08-16 EP EP99938107A patent/EP1115523B1/en not_active Expired - Lifetime
- 1999-08-16 AU AU52752/99A patent/AU5275299A/en not_active Abandoned
- 1999-08-16 WO PCT/CA1999/000759 patent/WO2000010756A1/en active IP Right Grant
- 1999-08-16 AT AT99938107T patent/ATE240177T1/de not_active IP Right Cessation
- 1999-08-16 DE DE69907933T patent/DE69907933T2/de not_active Expired - Lifetime
- 1999-08-16 JP JP2000566062A patent/JP3541939B2/ja not_active Expired - Lifetime
- 1999-08-16 KR KR1020017002087A patent/KR100594562B1/ko not_active Expired - Lifetime
- 1999-08-16 CA CA002340669A patent/CA2340669C/en not_active Expired - Lifetime
-
2003
- 2003-10-27 JP JP2003366114A patent/JP2004036005A/ja active Pending
-
2005
- 2005-02-10 JP JP2005034922A patent/JP2005163188A/ja active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH05331508A (ja) * | 1992-05-29 | 1993-12-14 | Murata Mfg Co Ltd | 銅粉末の製造方法 |
| JPH10102109A (ja) * | 1996-09-30 | 1998-04-21 | Tanaka Kikinzoku Kogyo Kk | ニッケル粉末の製造方法 |
Non-Patent Citations (1)
| Title |
|---|
| Journal of Materials Science, vol.16, no.10, pp2665-2674(1981 ) * |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100840229B1 (ko) | 2006-09-08 | 2008-06-23 | 재단법인 포항산업과학연구원 | 초미세 솔더 분말, 초미세 솔더 분말의 제조방법 및 그제조장치 |
| KR101009656B1 (ko) | 2008-09-17 | 2011-01-19 | 희성금속 주식회사 | 초미세 귀금속 분말 제조방법 |
| KR101024971B1 (ko) | 2008-12-12 | 2011-03-25 | 희성금속 주식회사 | 열플라즈마를 이용한 귀금속 분말 및 귀금속 타겟 제조방법 |
| KR101193683B1 (ko) | 2010-03-29 | 2012-10-22 | 현대제철 주식회사 | 지르코늄 코어드 와이어 투입법을 이용한 지르코늄 함유 철계 합금 제조 방법 및 그 제조 장치 |
| KR101408238B1 (ko) * | 2011-12-01 | 2014-06-16 | 소에이 가가쿠 고교 가부시키가이샤 | 금속분말 제조용 플라즈마 장치 |
| KR102465825B1 (ko) * | 2022-09-06 | 2022-11-09 | 이용복 | 열플라즈마를 이용한 금속분말 제조장치 및 그 제조방법 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1115523B1 (en) | 2003-05-14 |
| JP2004036005A (ja) | 2004-02-05 |
| ATE240177T1 (de) | 2003-05-15 |
| KR20010099622A (ko) | 2001-11-09 |
| JP2005163188A (ja) | 2005-06-23 |
| US6379419B1 (en) | 2002-04-30 |
| JP2002530521A (ja) | 2002-09-17 |
| JP3541939B2 (ja) | 2004-07-14 |
| DE69907933T2 (de) | 2004-04-01 |
| EP1115523A1 (en) | 2001-07-18 |
| CA2340669A1 (en) | 2000-03-02 |
| AU5275299A (en) | 2000-03-14 |
| DE69907933D1 (de) | 2003-06-18 |
| WO2000010756A1 (en) | 2000-03-02 |
| CA2340669C (en) | 2009-04-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR100594562B1 (ko) | 미세 분말 및 초미세 분말의 제조 방법과 그를 위한이송형 아크 플라스마 시스템 | |
| US8859931B2 (en) | Plasma synthesis of nanopowders | |
| EP1843834B1 (en) | Induction plasma synthesis of nanopowders | |
| US9516734B2 (en) | Plasma reactor for the synthesis of nanopowders and materials processing | |
| CA2293547C (en) | Method and device for producing fullerenes | |
| KR100784576B1 (ko) | 미세 분말 제조 방법 및 미세 분말 제조를 위한 프라즈마 아크 반응기 | |
| KR100370184B1 (ko) | 플루오르카본화합물제조방법 | |
| EP1874685B1 (en) | Method and apparatus for the continuous production and functionalization of single-waled carbon nanotubes using a high frequency plasma torch | |
| US8092570B2 (en) | Method for producing titanium metal | |
| US20120027955A1 (en) | Reactor and method for production of nanostructures | |
| CN1189277C (zh) | 常压下制备细粉或超细粉的方法 | |
| KR101143890B1 (ko) | 이송식 또는 비이송식 플라즈마 장치를 이용한 벌크 구리로부터 구리 나노분말의 제조방법 | |
| KR20090026512A (ko) | 아크 플라즈마 장치를 이용한 니켈 나노분말의 제조방법 및장치 | |
| US3404078A (en) | Method of generating a plasma arc with a fluidized bed as one electrode | |
| JPS6330062B2 (ko) | ||
| NO174694B (no) | Apparat og fremgangsmaate for fremstilling av ensartete, fine, borinneholdende, keramiske pulvere | |
| Ryu et al. | Tungsten carbide nanopowder by plasma-assisted chemical vapor synthesis from WCl6–CH4–H2 mixtures | |
| WO1993002787A1 (en) | Process for the production of ultra-fine powdered materials | |
| JP2002180112A (ja) | 高融点金属粉末材料の製造方法 | |
| Juhász et al. | Preparation of Raw Materials for Fine Ceramics in Plasmas |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20010217 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20040804 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20051214 Patent event code: PE09021S01D |
|
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
Patent event code: PE07011S01D Comment text: Decision to Grant Registration Patent event date: 20060605 |
|
| GRNT | Written decision to grant | ||
| PR0701 | Registration of establishment |
Comment text: Registration of Establishment Patent event date: 20060621 Patent event code: PR07011E01D |
|
| PR1002 | Payment of registration fee |
Payment date: 20060622 End annual number: 3 Start annual number: 1 |
|
| PG1601 | Publication of registration | ||
| PR1001 | Payment of annual fee |
Payment date: 20081022 Start annual number: 4 End annual number: 4 |
|
| PR1001 | Payment of annual fee |
Payment date: 20091103 Start annual number: 5 End annual number: 5 |
|
| PR1001 | Payment of annual fee |
Payment date: 20101101 Start annual number: 6 End annual number: 6 |
|
| PR1001 | Payment of annual fee |
Payment date: 20120612 Start annual number: 7 End annual number: 7 |
|
| FPAY | Annual fee payment |
Payment date: 20130528 Year of fee payment: 8 |
|
| PR1001 | Payment of annual fee |
Payment date: 20130528 Start annual number: 8 End annual number: 8 |
|
| FPAY | Annual fee payment |
Payment date: 20140613 Year of fee payment: 9 |
|
| PR1001 | Payment of annual fee |
Payment date: 20140613 Start annual number: 9 End annual number: 9 |
|
| FPAY | Annual fee payment |
Payment date: 20150616 Year of fee payment: 10 |
|
| PR1001 | Payment of annual fee |
Payment date: 20150616 Start annual number: 10 End annual number: 10 |
|
| FPAY | Annual fee payment |
Payment date: 20160609 Year of fee payment: 11 |
|
| PR1001 | Payment of annual fee |
Payment date: 20160609 Start annual number: 11 End annual number: 11 |
|
| FPAY | Annual fee payment |
Payment date: 20170620 Year of fee payment: 12 |
|
| PR1001 | Payment of annual fee |
Payment date: 20170620 Start annual number: 12 End annual number: 12 |
|
| FPAY | Annual fee payment |
Payment date: 20180611 Year of fee payment: 13 |
|
| PR1001 | Payment of annual fee |
Payment date: 20180611 Start annual number: 13 End annual number: 13 |
|
| EXPY | Expiration of term | ||
| PC1801 | Expiration of term |
Termination date: 20200216 Termination category: Expiration of duration |