JP5514441B2 - ケラチンバイオセラミック組成物 - Google Patents
ケラチンバイオセラミック組成物 Download PDFInfo
- Publication number
- JP5514441B2 JP5514441B2 JP2008536758A JP2008536758A JP5514441B2 JP 5514441 B2 JP5514441 B2 JP 5514441B2 JP 2008536758 A JP2008536758 A JP 2008536758A JP 2008536758 A JP2008536758 A JP 2008536758A JP 5514441 B2 JP5514441 B2 JP 5514441B2
- Authority
- JP
- Japan
- Prior art keywords
- keratose
- composition
- keratin
- calcium
- weight percent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000000203 mixture Substances 0.000 title claims description 76
- 102000011782 Keratins Human genes 0.000 title description 65
- 108010076876 Keratins Proteins 0.000 title description 65
- 239000003462 bioceramic Substances 0.000 title description 5
- 206010020649 Hyperkeratosis Diseases 0.000 claims description 63
- 210000000988 bone and bone Anatomy 0.000 claims description 35
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 33
- 238000000034 method Methods 0.000 claims description 31
- 230000003115 biocidal effect Effects 0.000 claims description 24
- 239000000945 filler Substances 0.000 claims description 18
- 108090000623 proteins and genes Proteins 0.000 claims description 14
- 102000004169 proteins and genes Human genes 0.000 claims description 13
- 230000000278 osteoconductive effect Effects 0.000 claims description 10
- 229960001139 cefazolin Drugs 0.000 claims description 9
- MLYYVTUWGNIJIB-BXKDBHETSA-N cefazolin Chemical compound S1C(C)=NN=C1SCC1=C(C(O)=O)N2C(=O)[C@@H](NC(=O)CN3N=NN=C3)[C@H]2SC1 MLYYVTUWGNIJIB-BXKDBHETSA-N 0.000 claims description 9
- 108060008539 Transglutaminase Proteins 0.000 claims description 7
- 102000003601 transglutaminase Human genes 0.000 claims description 7
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 claims description 5
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 claims description 5
- 229930182566 Gentamicin Natural products 0.000 claims description 5
- 230000003044 adaptive effect Effects 0.000 claims description 5
- 239000011575 calcium Substances 0.000 claims description 5
- 229910052791 calcium Inorganic materials 0.000 claims description 5
- 239000001506 calcium phosphate Substances 0.000 claims description 5
- 229960002518 gentamicin Drugs 0.000 claims description 5
- 229910052588 hydroxylapatite Inorganic materials 0.000 claims description 5
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 claims description 5
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 claims description 5
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 claims description 4
- ULGZDMOVFRHVEP-RWJQBGPGSA-N Erythromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 ULGZDMOVFRHVEP-RWJQBGPGSA-N 0.000 claims description 4
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 claims description 4
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 claims description 4
- 229910000391 tricalcium phosphate Inorganic materials 0.000 claims description 4
- 235000019731 tricalcium phosphate Nutrition 0.000 claims description 4
- 229940078499 tricalcium phosphate Drugs 0.000 claims description 4
- MYPYJXKWCTUITO-UHFFFAOYSA-N vancomycin Natural products O1C(C(=C2)Cl)=CC=C2C(O)C(C(NC(C2=CC(O)=CC(O)=C2C=2C(O)=CC=C3C=2)C(O)=O)=O)NC(=O)C3NC(=O)C2NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(CC(C)C)NC)C(O)C(C=C3Cl)=CC=C3OC3=CC2=CC1=C3OC1OC(CO)C(O)C(O)C1OC1CC(C)(N)C(O)C(C)O1 MYPYJXKWCTUITO-UHFFFAOYSA-N 0.000 claims description 4
- 108010035532 Collagen Proteins 0.000 claims description 3
- 102000008186 Collagen Human genes 0.000 claims description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 claims description 3
- 108010059993 Vancomycin Proteins 0.000 claims description 3
- 229920001436 collagen Polymers 0.000 claims description 3
- 239000003999 initiator Substances 0.000 claims description 3
- 239000011780 sodium chloride Substances 0.000 claims description 3
- 229960003165 vancomycin Drugs 0.000 claims description 3
- MYPYJXKWCTUITO-LYRMYLQWSA-N vancomycin Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1=C2C=C3C=C1OC1=CC=C(C=C1Cl)[C@@H](O)[C@H](C(N[C@@H](CC(N)=O)C(=O)N[C@H]3C(=O)N[C@H]1C(=O)N[C@H](C(N[C@@H](C3=CC(O)=CC(O)=C3C=3C(O)=CC=C1C=3)C(O)=O)=O)[C@H](O)C1=CC=C(C(=C1)Cl)O2)=O)NC(=O)[C@@H](CC(C)C)NC)[C@H]1C[C@](C)(N)[C@H](O)[C@H](C)O1 MYPYJXKWCTUITO-LYRMYLQWSA-N 0.000 claims description 3
- DHPRQBPJLMKORJ-XRNKAMNCSA-N (4s,4as,5as,6s,12ar)-7-chloro-4-(dimethylamino)-1,6,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-4,4a,5,5a-tetrahydrotetracene-2-carboxamide Chemical compound C1=CC(Cl)=C2[C@](O)(C)[C@H]3C[C@H]4[C@H](N(C)C)C(=O)C(C(N)=O)=C(O)[C@@]4(O)C(=O)C3=C(O)C2=C1O DHPRQBPJLMKORJ-XRNKAMNCSA-N 0.000 claims description 2
- 108010001478 Bacitracin Proteins 0.000 claims description 2
- 229930193140 Neomycin Natural products 0.000 claims description 2
- 229930182555 Penicillin Natural products 0.000 claims description 2
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 claims description 2
- 239000004098 Tetracycline Substances 0.000 claims description 2
- 239000003513 alkali Substances 0.000 claims description 2
- 229960000723 ampicillin Drugs 0.000 claims description 2
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 claims description 2
- 229940073066 azactam Drugs 0.000 claims description 2
- WZPBZJONDBGPKJ-VEHQQRBSSA-N aztreonam Chemical compound O=C1N(S([O-])(=O)=O)[C@@H](C)[C@@H]1NC(=O)C(=N/OC(C)(C)C(O)=O)\C1=CSC([NH3+])=N1 WZPBZJONDBGPKJ-VEHQQRBSSA-N 0.000 claims description 2
- 229960003071 bacitracin Drugs 0.000 claims description 2
- 229930184125 bacitracin Natural products 0.000 claims description 2
- CLKOFPXJLQSYAH-ABRJDSQDSA-N bacitracin A Chemical compound C1SC([C@@H](N)[C@@H](C)CC)=N[C@@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]1C(=O)N[C@H](CCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2N=CNC=2)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)NCCCC1 CLKOFPXJLQSYAH-ABRJDSQDSA-N 0.000 claims description 2
- 229940053013 bio-mycin Drugs 0.000 claims description 2
- 239000000560 biocompatible material Substances 0.000 claims description 2
- 239000005312 bioglass Substances 0.000 claims description 2
- 229910000019 calcium carbonate Inorganic materials 0.000 claims description 2
- WUKWITHWXAAZEY-UHFFFAOYSA-L calcium difluoride Chemical compound [F-].[F-].[Ca+2] WUKWITHWXAAZEY-UHFFFAOYSA-L 0.000 claims description 2
- AXCZMVOFGPJBDE-UHFFFAOYSA-L calcium dihydroxide Chemical compound [OH-].[OH-].[Ca+2] AXCZMVOFGPJBDE-UHFFFAOYSA-L 0.000 claims description 2
- 229910001634 calcium fluoride Inorganic materials 0.000 claims description 2
- 239000000920 calcium hydroxide Substances 0.000 claims description 2
- 229910001861 calcium hydroxide Inorganic materials 0.000 claims description 2
- BRPQOXSCLDDYGP-UHFFFAOYSA-N calcium oxide Chemical compound [O-2].[Ca+2] BRPQOXSCLDDYGP-UHFFFAOYSA-N 0.000 claims description 2
- 239000000292 calcium oxide Substances 0.000 claims description 2
- ODINCKMPIJJUCX-UHFFFAOYSA-N calcium oxide Inorganic materials [Ca]=O ODINCKMPIJJUCX-UHFFFAOYSA-N 0.000 claims description 2
- 239000004068 calcium phosphate ceramic Substances 0.000 claims description 2
- WIIZWVCIJKGZOK-RKDXNWHRSA-N chloramphenicol Chemical compound ClC(Cl)C(=O)N[C@H](CO)[C@H](O)C1=CC=C([N+]([O-])=O)C=C1 WIIZWVCIJKGZOK-RKDXNWHRSA-N 0.000 claims description 2
- 229940097572 chloromycetin Drugs 0.000 claims description 2
- 229960003276 erythromycin Drugs 0.000 claims description 2
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 claims description 2
- 239000001095 magnesium carbonate Substances 0.000 claims description 2
- 229910000021 magnesium carbonate Inorganic materials 0.000 claims description 2
- VTHJTEIRLNZDEV-UHFFFAOYSA-L magnesium dihydroxide Chemical compound [OH-].[OH-].[Mg+2] VTHJTEIRLNZDEV-UHFFFAOYSA-L 0.000 claims description 2
- ORUIBWPALBXDOA-UHFFFAOYSA-L magnesium fluoride Chemical compound [F-].[F-].[Mg+2] ORUIBWPALBXDOA-UHFFFAOYSA-L 0.000 claims description 2
- 229910001635 magnesium fluoride Inorganic materials 0.000 claims description 2
- 239000000347 magnesium hydroxide Substances 0.000 claims description 2
- 229910001862 magnesium hydroxide Inorganic materials 0.000 claims description 2
- 239000000395 magnesium oxide Substances 0.000 claims description 2
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 claims description 2
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical compound [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 claims description 2
- 230000000921 morphogenic effect Effects 0.000 claims description 2
- 229960004927 neomycin Drugs 0.000 claims description 2
- 229940049954 penicillin Drugs 0.000 claims description 2
- WQVJHHACXVLGBL-GOVYWFKWSA-N polymyxin B1 Polymers N1C(=O)[C@H](CCN)NC(=O)[C@@H](NC(=O)[C@H](CCN)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCN)NC(=O)CCCC[C@H](C)CC)CCNC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCN)NC(=O)[C@H](CCN)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]1CC1=CC=CC=C1 WQVJHHACXVLGBL-GOVYWFKWSA-N 0.000 claims description 2
- 229960005322 streptomycin Drugs 0.000 claims description 2
- GBNXLQPMFAUCOI-UHFFFAOYSA-H tetracalcium;oxygen(2-);diphosphate Chemical group [O-2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O GBNXLQPMFAUCOI-UHFFFAOYSA-H 0.000 claims description 2
- 229960002180 tetracycline Drugs 0.000 claims description 2
- 229930101283 tetracycline Natural products 0.000 claims description 2
- 235000019364 tetracycline Nutrition 0.000 claims description 2
- 150000003522 tetracyclines Chemical class 0.000 claims description 2
- 229960000707 tobramycin Drugs 0.000 claims description 2
- NLVFBUXFDBBNBW-PBSUHMDJSA-N tobramycin Chemical compound N[C@@H]1C[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N NLVFBUXFDBBNBW-PBSUHMDJSA-N 0.000 claims description 2
- 229960002227 clindamycin Drugs 0.000 claims 1
- KDLRVYVGXIQJDK-AWPVFWJPSA-N clindamycin Chemical compound CN1C[C@H](CCC)C[C@H]1C(=O)N[C@H]([C@H](C)Cl)[C@@H]1[C@H](O)[C@H](O)[C@@H](O)[C@@H](SC)O1 KDLRVYVGXIQJDK-AWPVFWJPSA-N 0.000 claims 1
- 210000004027 cell Anatomy 0.000 description 34
- 238000009472 formulation Methods 0.000 description 23
- 239000000243 solution Substances 0.000 description 23
- 239000012620 biological material Substances 0.000 description 21
- 210000004209 hair Anatomy 0.000 description 21
- 239000003242 anti bacterial agent Substances 0.000 description 20
- 229940088710 antibiotic agent Drugs 0.000 description 20
- 239000000017 hydrogel Substances 0.000 description 12
- 239000000463 material Substances 0.000 description 12
- 210000000963 osteoblast Anatomy 0.000 description 12
- 235000018102 proteins Nutrition 0.000 description 12
- 239000007983 Tris buffer Substances 0.000 description 11
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 11
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 10
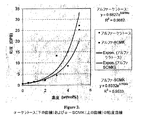
- 206010017076 Fracture Diseases 0.000 description 10
- KFSLWBXXFJQRDL-UHFFFAOYSA-N Peracetic acid Chemical compound CC(=O)OO KFSLWBXXFJQRDL-UHFFFAOYSA-N 0.000 description 10
- 230000007547 defect Effects 0.000 description 10
- 239000000284 extract Substances 0.000 description 10
- 238000000605 extraction Methods 0.000 description 9
- 239000003102 growth factor Substances 0.000 description 9
- 230000002829 reductive effect Effects 0.000 description 9
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 8
- 230000003647 oxidation Effects 0.000 description 8
- 238000007254 oxidation reaction Methods 0.000 description 8
- 208000010392 Bone Fractures Diseases 0.000 description 7
- 230000007062 hydrolysis Effects 0.000 description 7
- 238000006460 hydrolysis reaction Methods 0.000 description 7
- 239000011159 matrix material Substances 0.000 description 7
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 7
- 239000004926 polymethyl methacrylate Substances 0.000 description 7
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 6
- -1 antibiotics Chemical class 0.000 description 6
- 208000014674 injury Diseases 0.000 description 6
- 239000012528 membrane Substances 0.000 description 6
- 210000001519 tissue Anatomy 0.000 description 6
- 230000008733 trauma Effects 0.000 description 6
- 241000283690 Bos taurus Species 0.000 description 5
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 5
- 239000004743 Polypropylene Substances 0.000 description 5
- 238000006243 chemical reaction Methods 0.000 description 5
- 238000004132 cross linking Methods 0.000 description 5
- 239000000499 gel Substances 0.000 description 5
- 239000002609 medium Substances 0.000 description 5
- 230000002138 osteoinductive effect Effects 0.000 description 5
- 229920001155 polypropylene Polymers 0.000 description 5
- 239000011148 porous material Substances 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 238000003756 stirring Methods 0.000 description 5
- 206010019049 Hair texture abnormal Diseases 0.000 description 4
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 4
- 150000001413 amino acids Chemical group 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 239000000919 ceramic Substances 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 230000018109 developmental process Effects 0.000 description 4
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 4
- 239000000835 fiber Substances 0.000 description 4
- 238000011534 incubation Methods 0.000 description 4
- 239000002953 phosphate buffered saline Substances 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- 230000035755 proliferation Effects 0.000 description 4
- 210000002320 radius Anatomy 0.000 description 4
- 238000001356 surgical procedure Methods 0.000 description 4
- CWERGRDVMFNCDR-UHFFFAOYSA-N thioglycolic acid Chemical compound OC(=O)CS CWERGRDVMFNCDR-UHFFFAOYSA-N 0.000 description 4
- 102000016359 Fibronectins Human genes 0.000 description 3
- 108010067306 Fibronectins Proteins 0.000 description 3
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 3
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 239000011324 bead Substances 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 210000002805 bone matrix Anatomy 0.000 description 3
- 239000003599 detergent Substances 0.000 description 3
- 238000000502 dialysis Methods 0.000 description 3
- 238000009792 diffusion process Methods 0.000 description 3
- 239000003814 drug Substances 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 238000001704 evaporation Methods 0.000 description 3
- 230000008020 evaporation Effects 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 210000003780 hair follicle Anatomy 0.000 description 3
- 230000035876 healing Effects 0.000 description 3
- 239000007943 implant Substances 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 208000015181 infectious disease Diseases 0.000 description 3
- 229910052500 inorganic mineral Inorganic materials 0.000 description 3
- 102000006495 integrins Human genes 0.000 description 3
- 108010044426 integrins Proteins 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 230000005012 migration Effects 0.000 description 3
- 238000013508 migration Methods 0.000 description 3
- 239000011707 mineral Substances 0.000 description 3
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 3
- 230000007935 neutral effect Effects 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 238000001226 reprecipitation Methods 0.000 description 3
- 210000000614 rib Anatomy 0.000 description 3
- 238000001338 self-assembly Methods 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 3
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 2
- IYMAXBFPHPZYIK-BQBZGAKWSA-N Arg-Gly-Asp Chemical compound NC(N)=NCCC[C@H](N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(O)=O IYMAXBFPHPZYIK-BQBZGAKWSA-N 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 102000008137 Bone Morphogenetic Protein 4 Human genes 0.000 description 2
- 108010049955 Bone Morphogenetic Protein 4 Proteins 0.000 description 2
- 206010065687 Bone loss Diseases 0.000 description 2
- 108010066259 Collagraft Proteins 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- 102000004127 Cytokines Human genes 0.000 description 2
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 101000844802 Lacticaseibacillus rhamnosus Teichoic acid D-alanyltransferase Proteins 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 description 2
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 2
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 2
- 235000001014 amino acid Nutrition 0.000 description 2
- 239000000908 ammonium hydroxide Substances 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 230000000844 anti-bacterial effect Effects 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 239000004202 carbamide Substances 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 230000004709 cell invasion Effects 0.000 description 2
- 230000012292 cell migration Effects 0.000 description 2
- 230000005859 cell recognition Effects 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 230000035605 chemotaxis Effects 0.000 description 2
- 210000003109 clavicle Anatomy 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 239000000356 contaminant Substances 0.000 description 2
- 238000013270 controlled release Methods 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 239000008367 deionised water Substances 0.000 description 2
- 229910021641 deionized water Inorganic materials 0.000 description 2
- 230000003111 delayed effect Effects 0.000 description 2
- 230000004069 differentiation Effects 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 210000002758 humerus Anatomy 0.000 description 2
- JDNTWHVOXJZDSN-UHFFFAOYSA-N iodoacetic acid Chemical compound OC(=O)CI JDNTWHVOXJZDSN-UHFFFAOYSA-N 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 239000012460 protein solution Substances 0.000 description 2
- 230000008929 regeneration Effects 0.000 description 2
- 238000011069 regeneration method Methods 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 239000012679 serum free medium Substances 0.000 description 2
- 230000002269 spontaneous effect Effects 0.000 description 2
- 210000000130 stem cell Anatomy 0.000 description 2
- 210000001562 sternum Anatomy 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 210000002303 tibia Anatomy 0.000 description 2
- 210000000623 ulna Anatomy 0.000 description 2
- 210000000689 upper leg Anatomy 0.000 description 2
- AZKSAVLVSZKNRD-UHFFFAOYSA-M 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide Chemical compound [Br-].S1C(C)=C(C)N=C1[N+]1=NC(C=2C=CC=CC=2)=NN1C1=CC=CC=C1 AZKSAVLVSZKNRD-UHFFFAOYSA-M 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 238000009631 Broth culture Methods 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 208000003044 Closed Fractures Diseases 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 1
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- SXRSQZLOMIGNAQ-UHFFFAOYSA-N Glutaraldehyde Chemical compound O=CCCCC=O SXRSQZLOMIGNAQ-UHFFFAOYSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 102000011733 Hair-Specific Keratins Human genes 0.000 description 1
- 108010037031 Hair-Specific Keratins Proteins 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 108010025020 Nerve Growth Factor Proteins 0.000 description 1
- 102000015336 Nerve Growth Factor Human genes 0.000 description 1
- 208000002565 Open Fractures Diseases 0.000 description 1
- 206010031256 Osteomyelitis chronic Diseases 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 208000037581 Persistent Infection Diseases 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical class [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 1
- 241000191967 Staphylococcus aureus Species 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 1
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 229910052586 apatite Inorganic materials 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 239000006161 blood agar Substances 0.000 description 1
- 239000002639 bone cement Substances 0.000 description 1
- 230000008468 bone growth Effects 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- 230000010478 bone regeneration Effects 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- BPKIGYQJPYCAOW-FFJTTWKXSA-I calcium;potassium;disodium;(2s)-2-hydroxypropanoate;dichloride;dihydroxide;hydrate Chemical compound O.[OH-].[OH-].[Na+].[Na+].[Cl-].[Cl-].[K+].[Ca+2].C[C@H](O)C([O-])=O BPKIGYQJPYCAOW-FFJTTWKXSA-I 0.000 description 1
- 229960003408 cefazolin sodium Drugs 0.000 description 1
- FLKYBGKDCCEQQM-WYUVZMMLSA-M cefazolin sodium Chemical compound [Na+].S1C(C)=NN=C1SCC1=C(C([O-])=O)N2C(=O)[C@@H](NC(=O)CN3N=NN=C3)[C@H]2SC1 FLKYBGKDCCEQQM-WYUVZMMLSA-M 0.000 description 1
- 230000021164 cell adhesion Effects 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000003399 chemotactic effect Effects 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 230000009918 complex formation Effects 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 230000001054 cortical effect Effects 0.000 description 1
- 239000000287 crude extract Substances 0.000 description 1
- 238000005138 cryopreservation Methods 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 1
- LEVWYRKDKASIDU-IMJSIDKUSA-N cystine group Chemical group C([C@@H](C(=O)O)N)SSC[C@@H](C(=O)O)N LEVWYRKDKASIDU-IMJSIDKUSA-N 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- 210000003275 diaphysis Anatomy 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000008393 encapsulating agent Substances 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 210000001031 ethmoid bone Anatomy 0.000 description 1
- 210000002744 extracellular matrix Anatomy 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 230000004761 fibrosis Effects 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 210000002454 frontal bone Anatomy 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 238000007654 immersion Methods 0.000 description 1
- 238000005470 impregnation Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000010874 in vitro model Methods 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 230000002045 lasting effect Effects 0.000 description 1
- 210000004373 mandible Anatomy 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 210000002050 maxilla Anatomy 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 239000000155 melt Substances 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 210000001872 metatarsal bone Anatomy 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 239000003094 microcapsule Substances 0.000 description 1
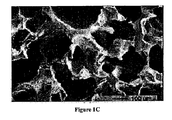
- 238000001000 micrograph Methods 0.000 description 1
- 238000010232 migration assay Methods 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 238000005232 molecular self-assembly Methods 0.000 description 1
- 230000002969 morbid Effects 0.000 description 1
- 230000000877 morphologic effect Effects 0.000 description 1
- VMGAPWLDMVPYIA-HIDZBRGKSA-N n'-amino-n-iminomethanimidamide Chemical compound N\N=C\N=N VMGAPWLDMVPYIA-HIDZBRGKSA-N 0.000 description 1
- 210000000537 nasal bone Anatomy 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 229940053128 nerve growth factor Drugs 0.000 description 1
- 238000010899 nucleation Methods 0.000 description 1
- 210000000103 occipital bone Anatomy 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 230000000399 orthopedic effect Effects 0.000 description 1
- 230000011164 ossification Effects 0.000 description 1
- 230000004820 osteoconduction Effects 0.000 description 1
- 210000005009 osteogenic cell Anatomy 0.000 description 1
- 230000002188 osteogenic effect Effects 0.000 description 1
- 210000003455 parietal bone Anatomy 0.000 description 1
- 210000004417 patella Anatomy 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 210000003049 pelvic bone Anatomy 0.000 description 1
- VSIIXMUUUJUKCM-UHFFFAOYSA-D pentacalcium;fluoride;triphosphate Chemical compound [F-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O VSIIXMUUUJUKCM-UHFFFAOYSA-D 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 239000003495 polar organic solvent Substances 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 230000003449 preventive effect Effects 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 239000012429 reaction media Substances 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- BOLDJAUMGUJJKM-LSDHHAIUSA-N renifolin D Natural products CC(=C)[C@@H]1Cc2c(O)c(O)ccc2[C@H]1CC(=O)c3ccc(O)cc3O BOLDJAUMGUJJKM-LSDHHAIUSA-N 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 238000001878 scanning electron micrograph Methods 0.000 description 1
- 210000001991 scapula Anatomy 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 1
- 210000002832 shoulder Anatomy 0.000 description 1
- 238000007086 side reaction Methods 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000004611 spectroscopical analysis Methods 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 210000001137 tarsal bone Anatomy 0.000 description 1
- 210000004876 tela submucosa Anatomy 0.000 description 1
- 210000003582 temporal bone Anatomy 0.000 description 1
- 238000010257 thawing Methods 0.000 description 1
- 210000000115 thoracic cavity Anatomy 0.000 description 1
- 230000000451 tissue damage Effects 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 230000017423 tissue regeneration Effects 0.000 description 1
- 229940034610 toothpaste Drugs 0.000 description 1
- 239000000606 toothpaste Substances 0.000 description 1
- 238000005292 vacuum distillation Methods 0.000 description 1
- 238000010200 validation analysis Methods 0.000 description 1
- 239000004474 valine Substances 0.000 description 1
- 230000009790 vascular invasion Effects 0.000 description 1
- 238000003260 vortexing Methods 0.000 description 1
- 210000002268 wool Anatomy 0.000 description 1
- 230000037314 wound repair Effects 0.000 description 1
- 210000000216 zygoma Anatomy 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/54—Biologically active materials, e.g. therapeutic substances
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/40—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material
- A61L27/42—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material having an inorganic matrix
- A61L27/425—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material having an inorganic matrix of phosphorus containing material, e.g. apatite
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/58—Materials at least partially resorbable by the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/404—Biocides, antimicrobial agents, antiseptic agents
- A61L2300/406—Antibiotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/02—Materials or treatment for tissue regeneration for reconstruction of bones; weight-bearing implants
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Epidemiology (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Dermatology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Molecular Biology (AREA)
- Inorganic Chemistry (AREA)
- Composite Materials (AREA)
- Materials Engineering (AREA)
- Materials For Medical Uses (AREA)
- Curing Cements, Concrete, And Artificial Stone (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US72897105P | 2005-10-21 | 2005-10-21 | |
| US60/728,971 | 2005-10-21 | ||
| US11/549,748 US8920827B2 (en) | 2005-10-21 | 2006-10-16 | Keratin bioceramic compositions |
| US11/549,748 | 2006-10-16 | ||
| PCT/US2006/040673 WO2007050387A2 (en) | 2005-10-21 | 2006-10-18 | Keratin bioceramic compositions |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2009513190A JP2009513190A (ja) | 2009-04-02 |
| JP2009513190A5 JP2009513190A5 (enExample) | 2013-02-28 |
| JP5514441B2 true JP5514441B2 (ja) | 2014-06-04 |
Family
ID=37968379
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008536758A Active JP5514441B2 (ja) | 2005-10-21 | 2006-10-18 | ケラチンバイオセラミック組成物 |
Country Status (4)
| Country | Link |
|---|---|
| US (2) | US8920827B2 (enExample) |
| EP (1) | EP1945132B1 (enExample) |
| JP (1) | JP5514441B2 (enExample) |
| WO (1) | WO2007050387A2 (enExample) |
Families Citing this family (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7439012B2 (en) | 2004-08-17 | 2008-10-21 | Wake Forest University Health Sciences | Ambient stored blood plasma expanders containing keratose |
| US8920827B2 (en) | 2005-10-21 | 2014-12-30 | Wake Forest University Health Sciences | Keratin bioceramic compositions |
| US7892573B2 (en) * | 2006-02-10 | 2011-02-22 | Wake Forest University Health Sciences | Nerve regeneration employing keratin biomaterials |
| EP1983927B1 (en) * | 2006-02-17 | 2014-11-05 | Wake Forest University Health Sciences | Coatings and biomedical implants formed from keratin biomaterials |
| US8273702B2 (en) * | 2006-02-17 | 2012-09-25 | Wake Forest University Health Sciences | Wound healing compositions containing keratin biomaterials |
| PL227142B1 (pl) * | 2006-11-21 | 2017-11-30 | Inst Medycyny Doświadczalnej I Klinicznej Im M Mossakowskiego Polskiej Akademii Nauk | Mikrostrukturalny preparat białkowy zawierajacy zwiazki biologicznie czynne |
| EP1964583A1 (en) | 2007-02-09 | 2008-09-03 | Royal College of Surgeons in Ireland | Process for producing a collagen/hydroxyapatite composite scaffold |
| US9068162B2 (en) * | 2007-08-17 | 2015-06-30 | Wake Forest University Health Sciences | Keratin biomaterials for cell culture and methods of use |
| US20090047260A1 (en) * | 2007-08-17 | 2009-02-19 | Wake Forest University Health Sciences | Keratin biomaterials for cell culture and methods of use |
| GB2461743A (en) * | 2008-07-11 | 2010-01-20 | Smith & Nephew | Medical device or composition comprising at least two inorganic components |
| US10842645B2 (en) | 2008-08-13 | 2020-11-24 | Smed-Ta/Td, Llc | Orthopaedic implant with porous structural member |
| US9700431B2 (en) | 2008-08-13 | 2017-07-11 | Smed-Ta/Td, Llc | Orthopaedic implant with porous structural member |
| JP5774989B2 (ja) | 2008-08-13 | 2015-09-09 | スメド−ティーエイ/ティーディー・エルエルシー | 整形外科用ねじ |
| US9616205B2 (en) | 2008-08-13 | 2017-04-11 | Smed-Ta/Td, Llc | Drug delivery implants |
| WO2010019781A1 (en) | 2008-08-13 | 2010-02-18 | Smed-Ta/Td, Llc | Drug delivery implants |
| WO2010025386A1 (en) | 2008-08-29 | 2010-03-04 | Smed-Ta/Td, Llc | Orthopaedic implant |
| US8673018B2 (en) * | 2010-02-05 | 2014-03-18 | AMx Tek LLC | Methods of using water-soluble inorganic compounds for implants |
| EP2542564B1 (en) * | 2010-03-05 | 2018-05-09 | Wake Forest University Health Sciences | Controlled delivery system |
| EP2640408B1 (en) | 2010-11-17 | 2016-05-25 | Wake Forest University Health Sciences | Keratin compositions for treatment of bone deficiency or injury |
| US8551525B2 (en) | 2010-12-23 | 2013-10-08 | Biostructures, Llc | Bone graft materials and methods |
| CN102908664B (zh) * | 2011-08-02 | 2016-03-02 | 香港理工大学 | 一种磷灰石/角蛋白复合支架及其制备方法 |
| WO2013025941A1 (en) | 2011-08-17 | 2013-02-21 | Keranetics Llc | Low protein percentage gelling compositions |
| WO2013025928A2 (en) | 2011-08-17 | 2013-02-21 | Keranetics Llc | Methods for extracting keratin proteins |
| US10792239B2 (en) | 2012-11-06 | 2020-10-06 | Kernnetics, Inc. | White keratin compositions |
| US9827245B2 (en) | 2013-03-15 | 2017-11-28 | KeraNetics, LLC | Keratin compositions comprising halofuginone |
| CN103893827B (zh) * | 2014-04-21 | 2016-05-25 | 陕西巨子生物技术有限公司 | 一种增强生物相容性的人工骨支架材料及其制备方法 |
| CN106999546A (zh) * | 2014-05-01 | 2017-08-01 | 弗吉尼亚技术知识资产公司 | 角蛋白纳米材料及其制备方法 |
| US10238507B2 (en) | 2015-01-12 | 2019-03-26 | Surgentec, Llc | Bone graft delivery system and method for using same |
| CN110279891A (zh) * | 2018-03-19 | 2019-09-27 | 凯利得科技股份有限公司 | 由人体毛发制成的医用组合物及其制备方法和应用 |
| US10687828B2 (en) | 2018-04-13 | 2020-06-23 | Surgentec, Llc | Bone graft delivery system and method for using same |
| US11116647B2 (en) | 2018-04-13 | 2021-09-14 | Surgentec, Llc | Bone graft delivery system and method for using same |
| CN114209879B (zh) * | 2021-12-28 | 2022-10-14 | 北京科技大学 | 一种复合骨水泥材料及其制备方法 |
Family Cites Families (120)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE184915C (enExample) | ||||
| DE22643C (de) | J. B. CHARLIER in Nimes, Frankreich | Bremse für Eisenbahnfahrzeuge | ||
| US922692A (en) * | 1907-10-07 | 1909-05-25 | Byron B Goldsmith | Thermoplastic keratin composition. |
| US926999A (en) * | 1907-11-25 | 1909-07-06 | Carl Neuberg | Process of producing digestible substances from keratin. |
| US960914A (en) * | 1909-09-21 | 1910-06-07 | Arthur Heinemann | Pills for the treatment of diabetes mellitus. |
| US1214299A (en) * | 1915-06-30 | 1917-01-30 | William M Grosvenor | Manufacturing colloids. |
| IT377232A (enExample) | 1938-08-26 | 1900-01-01 | ||
| US2434688A (en) * | 1942-11-03 | 1948-01-20 | Ralph L Evans | Regenerated keratin |
| US2447860A (en) * | 1944-11-07 | 1948-08-24 | Us Agriculture | Method of dispersing keratin proteins and the composition resulting therefrom |
| US2517572A (en) * | 1948-11-23 | 1950-08-08 | Chase B Jones | Process of utilizing detergents to solubilize keratin materials |
| US2814851A (en) * | 1953-12-11 | 1957-12-03 | Rubberset Company | Keratin treating process and products thereof |
| US3033755A (en) * | 1961-04-14 | 1962-05-08 | Kolmar Laboratories | Process for removing the water soluble materials from a keratin structure and cosmetic or pharmaceutical product formed therefrom |
| US3655416A (en) * | 1968-12-18 | 1972-04-11 | Lever Brothers Ltd | Lipid-protein membrane and process |
| US3642498A (en) * | 1969-09-18 | 1972-02-15 | Gen Mills Inc | Method of preparing keratin-containing films and coatings |
| US4178361A (en) * | 1973-09-10 | 1979-12-11 | Union Corporation | Sustained release pharmaceutical composition |
| JPS52148581A (en) | 1976-06-04 | 1977-12-09 | Nippon Kagaku Seni Kenkyusho | Process for preparing modified keratinous substance |
| JPS5316091A (en) | 1976-07-30 | 1978-02-14 | Nippon Kagaku Seni Kenkyusho | Process for preparing modified keratinous substance |
| JPS609531B2 (ja) | 1978-04-17 | 1985-03-11 | 積水化学工業株式会社 | 多孔質膜状物の製造方法 |
| JPS5551095A (en) | 1978-10-09 | 1980-04-14 | Seiwa Kasei:Kk | Preparation of keratin hydrolyzate |
| US4285885A (en) | 1979-01-17 | 1981-08-25 | Ciba-Geigy Corporation | Novel distyrylbenzene containing sulfonic acid groups |
| JPS6027680B2 (ja) | 1979-08-22 | 1985-06-29 | 株式会社成和化成 | ケラチン加水分解物の製造方法 |
| JPS5785308A (en) * | 1980-11-17 | 1982-05-28 | Kao Corp | Hair rinse composition |
| JPS5791910A (en) * | 1980-11-28 | 1982-06-08 | Kao Corp | Preshampoo-type hair treatment composition |
| JPS57109797A (en) | 1980-12-26 | 1982-07-08 | Kao Corp | Preparation of low-molecular weight keratinous substance |
| JPS57130910A (en) * | 1981-02-05 | 1982-08-13 | Kao Corp | Hair treatment agent |
| JPS57144213A (en) * | 1981-03-03 | 1982-09-06 | Kao Corp | Hair treatment |
| US4357274A (en) * | 1981-08-06 | 1982-11-02 | Intermedicat Gmbh | Process for the manufacture of sclero protein transplants with increased biological stability |
| US4570629A (en) | 1982-03-17 | 1986-02-18 | University Of Illinois Foundation | Hydrophilic biopolymeric copolyelectrolytes, and biodegradable wound dressing comprising same |
| CA1213520A (en) * | 1982-03-17 | 1986-11-04 | Abe Widra | Hydrophilic biopolymeric copolyelectrolytes, and biodegradable dressings comprising same |
| LU85081A1 (fr) * | 1983-11-09 | 1985-07-17 | Oreal | Medicament et composition medicamenteuse pour le traitement et/ou la prevention des maladies de la peau mettant en jeu un processus inflammatoire |
| CA1260391A (en) * | 1985-03-28 | 1989-09-26 | Karl A. Piez | Xenogeneic collagen/mineral preparations in bone repair |
| US4774227A (en) * | 1986-02-14 | 1988-09-27 | Collagen Corporation | Collagen compositions for bone repair containing autogeneic marrow |
| US4865602A (en) * | 1986-11-06 | 1989-09-12 | Collagen Corporation | Gamma irradiation of collagen/mineral mixtures |
| JP2573007B2 (ja) | 1987-12-28 | 1997-01-16 | 株式会社成和化成 | 修飾された動物毛粉末 |
| US5047249A (en) * | 1988-07-22 | 1991-09-10 | John Morris Co., Inc. | Compositions and methods for treating skin conditions and promoting wound healing |
| JPH0721061B2 (ja) | 1988-08-13 | 1995-03-08 | 株式会社ニッピ | 水溶性ケラチン蛋白質の製造方法 |
| JPH0311099A (ja) | 1989-06-06 | 1991-01-18 | Seiwa Kasei:Kk | ケラチン加水分解物、その製造方法および上記ケラチン加水分解物からなる化粧品基剤 |
| IL95393A0 (en) | 1989-08-18 | 1991-06-30 | John Morris Co | Odor-masked and stabilized compositions for treating keratinous tissue,skin conditions,and promoting wound healing |
| JPH03294578A (ja) | 1989-12-26 | 1991-12-25 | Unitika Ltd | 獣毛繊維の改質方法 |
| FR2661414B1 (fr) | 1990-04-25 | 1995-03-24 | Michel Jean Pierre | Produit a base de keratines modifiees, son procede de preparation et les applications notamment en medecine humaine ou veterinaire. |
| JPH0482561A (ja) | 1990-07-26 | 1992-03-16 | Niigata High Supinaa Kk | 創傷被覆材 |
| JPH0491138A (ja) | 1990-08-06 | 1992-03-24 | Fujimori Kogyo Kk | ケラチンフィルムの製造方法 |
| JP3047923B2 (ja) | 1990-11-16 | 2000-06-05 | 武田薬品工業株式会社 | ケラチン有機溶媒液およびその製造法 |
| GB9123251D0 (en) | 1991-11-01 | 1991-12-18 | Croda Int Plc | Protein-silicone copolymers |
| US5645081A (en) * | 1991-11-14 | 1997-07-08 | Wake Forest University | Method of treating tissue damage and apparatus for same |
| US5636643A (en) * | 1991-11-14 | 1997-06-10 | Wake Forest University | Wound treatment employing reduced pressure |
| DK194091D0 (da) | 1991-11-29 | 1991-11-29 | Metra Aps | Hydrofil og biokompatibel coatede overflader |
| ES2155447T3 (es) | 1992-01-03 | 2001-05-16 | Rhomed Inc | Aplicaciones farmaceuticas de peptidos-iones metalicos. |
| US5281265A (en) | 1992-02-03 | 1994-01-25 | Liu Sung Tsuen | Resorbable surgical cements |
| JP3094181B2 (ja) | 1992-04-09 | 2000-10-03 | 清 山内 | 再生天然ケラチンを壁材とするマイクロカプセル及びその製造方法 |
| JP3094182B2 (ja) | 1992-04-09 | 2000-10-03 | 清 山内 | ケラチンs−スルフォ塩を壁構成原料として用いるマイクロカプセル及びその製造方法 |
| JP3138838B2 (ja) | 1992-04-30 | 2001-02-26 | 東レ・ダウコーニング・シリコーン株式会社 | 混合粉体の製造方法 |
| JPH05320358A (ja) | 1992-05-22 | 1993-12-03 | Ajinomoto Takara Corp:Kk | ケラチン蛋白質高圧成型品 |
| DE69332756T2 (de) * | 1992-05-29 | 2004-02-19 | University Of North Carolina At Chapel Hill | Im immobilisierten zustand getrocknete pharmazeutische verträgliche menschliche blutplättchen |
| JP3283302B2 (ja) | 1992-09-22 | 2002-05-20 | 株式会社成和化成 | 還元ケラチンの製造方法 |
| JPH06116300A (ja) | 1992-10-07 | 1994-04-26 | Seiwa Kasei:Kk | ケラチンフラグメントおよびその製造方法 |
| US5300285A (en) | 1992-10-13 | 1994-04-05 | Dow Corning Corporation | Permanent waving with silicones |
| US5358935A (en) | 1992-11-19 | 1994-10-25 | Robert Allen Smith | Nonantigenic keratinous protein material |
| CN1105029A (zh) * | 1993-05-24 | 1995-07-12 | 花王株式会社 | 制备溶解蛋白质的方法 |
| JP3518691B2 (ja) | 1993-05-25 | 2004-04-12 | 清 山内 | 動物クチクル細胞由来の不溶性還元タンパク質、その製造方法および上記動物クチクル細胞由来の不溶性還元タンパク質を原料として作製された高分子成形品 |
| JP3507959B2 (ja) | 1994-06-21 | 2004-03-15 | 味の素株式会社 | 蒸気貫流式解凍装置用食品収納容器 |
| US5634945A (en) * | 1995-03-02 | 1997-06-03 | Pernia; Luis R. | Biological filler and use of same |
| GB9506926D0 (en) * | 1995-04-04 | 1995-05-24 | Croda Int Plc | Cystine-siicone copolymers and their use for treating keratin substrates |
| DE19521324C1 (de) * | 1995-06-12 | 1996-10-31 | Immuno Ag | Gewebeklebstoff und Verwendung desselben als Hämostyptikum |
| JP3044239B2 (ja) | 1995-06-12 | 2000-05-22 | 農林水産省蚕糸・昆虫農業技術研究所長 | ケラチン蛋白質を担体とする固定化生理活性物質およびその製造方法 |
| JP3209489B2 (ja) | 1995-06-23 | 2001-09-17 | オルガノ株式会社 | イオン交換式純水製造装置の終点検知方法 |
| EP0756336A1 (en) * | 1995-07-28 | 1997-01-29 | American Superconductor Corporation | Cryogenic deformation of high temperature superconductive composite structures |
| JPH09227565A (ja) | 1996-02-23 | 1997-09-02 | Kaken Shiyouyaku Kk | 創傷治療剤 |
| RU2108079C1 (ru) | 1996-08-16 | 1998-04-10 | Василий Александрович Мензул | Способ лечения ожоговых ран |
| RU2106154C1 (ru) | 1996-08-16 | 1998-03-10 | Василий Александрович Мензул | Перевязочный материал |
| US6063061A (en) | 1996-08-27 | 2000-05-16 | Fusion Medical Technologies, Inc. | Fragmented polymeric compositions and methods for their use |
| JP3891508B2 (ja) | 1997-04-18 | 2007-03-14 | 清 山内 | 還元クチクルタンパクまたはその水性媒体分散液およびその製造方法 |
| JP3891509B2 (ja) | 1997-04-18 | 2007-03-14 | 清 山内 | 高等動物体毛由来の還元タンパク質またはその水性媒体分散物およびその製造方法 |
| JPH10337466A (ja) | 1997-06-04 | 1998-12-22 | Akuseenu Kk | ケラチンマイクロカプセル及びその製造方法並びにケラチンマイクロカプセルを含有する化粧料 |
| US6309422B1 (en) * | 1997-06-20 | 2001-10-30 | Alfred Farrington | Bone grafting material |
| US6110487A (en) * | 1997-11-26 | 2000-08-29 | Keraplast Technologies Ltd. | Method of making porous keratin scaffolds and products of same |
| US5932552A (en) | 1997-11-26 | 1999-08-03 | Keraplast Technologies Ltd. | Keratin-based hydrogel for biomedical applications and method of production |
| US5948432A (en) * | 1997-11-26 | 1999-09-07 | Keraplast Technologies Ltd. | Keratin-based sheet material for biomedical applications and method of production |
| US6274163B1 (en) | 1998-04-08 | 2001-08-14 | Keraplast Technologies, Ltd. | Keratinous protein material for wound healing applications and method |
| US6964869B2 (en) * | 1998-07-13 | 2005-11-15 | Wisconsin Alumni Research Foundation | Method and composition for skin grafts |
| CA2342797A1 (en) * | 1998-09-02 | 2000-03-09 | Allergan Sales, Inc. | Preserved cyclodextrin-containing compositions |
| JP2000191792A (ja) | 1998-12-25 | 2000-07-11 | Seiwa Kasei:Kk | ポリシロキサン粒子 |
| US6696073B2 (en) * | 1999-02-23 | 2004-02-24 | Osteotech, Inc. | Shaped load-bearing osteoimplant and methods of making same |
| WO2000054821A1 (en) * | 1999-03-16 | 2000-09-21 | Regeneration Technologies, Inc. | Molded implants for orthopedic applications |
| US6270791B1 (en) * | 1999-06-11 | 2001-08-07 | Keraplast Technologies, Ltd. | Soluble keratin peptide |
| US6544548B1 (en) | 1999-09-13 | 2003-04-08 | Keraplast Technologies, Ltd. | Keratin-based powders and hydrogel for pharmaceutical applications |
| US6316598B1 (en) * | 1999-09-13 | 2001-11-13 | Keraplast Technologies, Ltd. | Water absorbent keratin and gel formed therefrom |
| US6461628B1 (en) * | 1999-09-13 | 2002-10-08 | Keraplast Technologies, Ltd. | Non-woven keratin cell scaffold |
| US6270793B1 (en) * | 1999-09-13 | 2001-08-07 | Keraplast Technologies, Ltd. | Absorbent keratin wound dressing |
| US6371984B1 (en) * | 1999-09-13 | 2002-04-16 | Keraplast Technologies, Ltd. | Implantable prosthetic or tissue expanding device |
| US6783546B2 (en) * | 1999-09-13 | 2004-08-31 | Keraplast Technologies, Ltd. | Implantable prosthetic or tissue expanding device |
| JP2001087754A (ja) | 1999-09-24 | 2001-04-03 | Relief Fund For Oil Pollution Damage In Fishing Ground | 汚染土壌の生物的修復用粉状組成物 |
| KR100312178B1 (ko) | 1999-10-06 | 2001-11-03 | 서경배 | 신규한 아미노산 실리콘 폴리머들, 그의 제조방법, 아미노산 실리콘 폴리머로 표면개질된 화장료용 분체 및 이를 함유하는 색조화장료 조성물 |
| US6649740B1 (en) | 2000-03-01 | 2003-11-18 | Keraplast Technologies, Ltd. | Hydratable form of keratin for use as a soil amendment |
| US6869445B1 (en) * | 2000-05-04 | 2005-03-22 | Phillips Plastics Corp. | Packable ceramic beads for bone repair |
| JP2001329183A (ja) * | 2000-05-22 | 2001-11-27 | Yuichi Mori | ゲル化性組成物 |
| US6320796B1 (en) * | 2000-11-10 | 2001-11-20 | Marvell International, Ltd. | Variable slope charge pump control |
| US6746836B1 (en) * | 2000-12-08 | 2004-06-08 | Abe Widra | Alpha-keratose as a blood plasma expander and use thereof |
| US6825323B2 (en) * | 2001-01-10 | 2004-11-30 | The United States Of America As Represented By The Secretary Of The Army | Compositions for treatment of hemorrhaging with activated factor VIIa in combination with fibrinogen and methods of using same |
| US6833488B2 (en) * | 2001-03-30 | 2004-12-21 | Exotech Bio Solution Ltd. | Biocompatible, biodegradable, water-absorbent material and methods for its preparation |
| TWI267378B (en) * | 2001-06-08 | 2006-12-01 | Wyeth Corp | Calcium phosphate delivery vehicles for osteoinductive proteins |
| CN1298733C (zh) | 2001-07-17 | 2007-02-07 | 凯瑞泰克有限公司 | 制造可溶性角蛋白衍生物 |
| CA2474723A1 (en) * | 2002-01-28 | 2003-08-07 | Keraplast Technologies, Ltd. | Bioactive keratin peptides |
| DE60331638D1 (de) | 2002-04-10 | 2010-04-22 | Keraplast Tech Ltd | Keratin-netzwerke enthaltende wundverbände für gewebedefekte |
| US20040120910A1 (en) * | 2002-04-10 | 2004-06-24 | Dyke Mark Van | Methods for producing, films comprising, and methods for using heterogeneous crosslinked protein networks |
| WO2003094617A2 (en) * | 2002-05-06 | 2003-11-20 | Genentech, Inc. | Use of vegf for treating bone defects |
| EP1534353A4 (en) * | 2002-06-10 | 2010-10-13 | Keratec Ltd | ORTHOPEDIC MATERIALS DERIVED FROM KERATIN |
| US20040062793A1 (en) * | 2002-07-05 | 2004-04-01 | Dyke Mark Van | Tissue defect dressings comprising proteinaceous networks |
| US20040078090A1 (en) | 2002-10-18 | 2004-04-22 | Francois Binette | Biocompatible scaffolds with tissue fragments |
| US7069312B2 (en) * | 2002-12-06 | 2006-06-27 | Microsoft Corporation | Network location signature for disambiguating multicast messages in dual-IP stack and/or multi-homed network environments |
| EP3254710B1 (en) * | 2003-04-11 | 2019-05-22 | Etex Corporation | Osteoinductive bone material |
| US7604617B2 (en) | 2003-04-12 | 2009-10-20 | Medical Research Products-B, Inc. | Percutaneously implantable medical device configured to promote tissue ingrowth |
| US20040225689A1 (en) * | 2003-05-08 | 2004-11-11 | International Business Machines Corporation | Autonomic logging support |
| US8226715B2 (en) * | 2003-06-30 | 2012-07-24 | Depuy Mitek, Inc. | Scaffold for connective tissue repair |
| US7439012B2 (en) * | 2004-08-17 | 2008-10-21 | Wake Forest University Health Sciences | Ambient stored blood plasma expanders containing keratose |
| US8920827B2 (en) | 2005-10-21 | 2014-12-30 | Wake Forest University Health Sciences | Keratin bioceramic compositions |
| US7892573B2 (en) | 2006-02-10 | 2011-02-22 | Wake Forest University Health Sciences | Nerve regeneration employing keratin biomaterials |
| EP1983927B1 (en) | 2006-02-17 | 2014-11-05 | Wake Forest University Health Sciences | Coatings and biomedical implants formed from keratin biomaterials |
| US8273702B2 (en) * | 2006-02-17 | 2012-09-25 | Wake Forest University Health Sciences | Wound healing compositions containing keratin biomaterials |
| US20090047260A1 (en) * | 2007-08-17 | 2009-02-19 | Wake Forest University Health Sciences | Keratin biomaterials for cell culture and methods of use |
-
2006
- 2006-10-16 US US11/549,748 patent/US8920827B2/en active Active
- 2006-10-18 JP JP2008536758A patent/JP5514441B2/ja active Active
- 2006-10-18 WO PCT/US2006/040673 patent/WO2007050387A2/en not_active Ceased
- 2006-10-18 EP EP06836364.7A patent/EP1945132B1/en active Active
-
2014
- 2014-11-19 US US14/547,839 patent/US11173233B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| WO2007050387A2 (en) | 2007-05-03 |
| EP1945132B1 (en) | 2014-12-03 |
| WO2007050387A3 (en) | 2007-12-06 |
| US11173233B2 (en) | 2021-11-16 |
| JP2009513190A (ja) | 2009-04-02 |
| US20150141333A1 (en) | 2015-05-21 |
| US8920827B2 (en) | 2014-12-30 |
| EP1945132A4 (en) | 2011-03-16 |
| EP1945132A2 (en) | 2008-07-23 |
| US20070166348A1 (en) | 2007-07-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5514441B2 (ja) | ケラチンバイオセラミック組成物 | |
| Kenley et al. | Osseous regeneration in the rat calvarium using novel delivery systems for recombinant human bone morphogenetic protein‐2 (rhBMP‐) | |
| JP2009513190A5 (enExample) | ||
| Munhoz et al. | Use of collagen/chitosan sponges mineralized with hydroxyapatite for the repair of cranial defects in rats | |
| US20210386910A1 (en) | Demineralized bone matrix having improved handling characteristics | |
| CA2547461C (en) | Composite structures containing hyaluronic acid the derivatives thereof as new bone substitutes and grafts | |
| JP2010075718A (ja) | 骨の再構成用の再吸収性細胞外マトリックス | |
| US9849215B2 (en) | Implantable compositions and methods for preparing the same | |
| JP2003501217A (ja) | 不溶化デキストラン誘導体および増殖因子に基づく生体材料 | |
| Goh et al. | Fabrication and in vitro biocompatibility of sodium tripolyphosphate-crosslinked chitosan–hydroxyapatite scaffolds for bone regeneration | |
| KR101348335B1 (ko) | 이종골 유래 골이식재 및 그 제조방법 | |
| EP3368095B1 (en) | Bone void filler having sustained therapeutic agent release | |
| US10603409B2 (en) | Bone void filler having calcium coatings | |
| Karalashvili et al. | Decellularized bovine bone graft for zygomatic bone reconstruction | |
| KR100810736B1 (ko) | 다당류-기능화 나노입자 및 수화젤 담체를 포함하는복합체, 이를 포함하는 서방형 약물전달 제제, 뼈충진제 및이들의 제조방법 | |
| Vorrapakdee et al. | Modification of human cancellous bone using Thai silk fibroin and gelatin for enhanced osteoconductive potential | |
| US6884518B2 (en) | Material suitable for an individual's tissue reconstruction | |
| US20250129123A1 (en) | Biomimetic peptides and their use in bone regeneration | |
| Hu et al. | Surface‐modified pliable PDLLA/PCL/β‐TCP scaffolds as a promising delivery system for bone regeneration | |
| Nandi et al. | Application of marine biomaterials in orthopedic and soft tissue surgical challenges | |
| Hu et al. | Surface-modified PCL/β-TCP scaffold as a promising delivery system for rhBMP-2 in bone regeneration. | |
| Shakesheff | Hydrogels derived from demineralized and decellularized bone extracellular matrix | |
| AU2007254593A1 (en) | Resorbable Extracellular Matrix for Reconstruction of Bone |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20091019 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120710 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20121003 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20121011 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20130110 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130621 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130919 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130927 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20131018 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20131025 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20131115 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20131122 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20131224 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20140304 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20140331 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5514441 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |