JP5259120B2 - 2成分型ポリウレタン接着剤 - Google Patents
2成分型ポリウレタン接着剤 Download PDFInfo
- Publication number
- JP5259120B2 JP5259120B2 JP2007133249A JP2007133249A JP5259120B2 JP 5259120 B2 JP5259120 B2 JP 5259120B2 JP 2007133249 A JP2007133249 A JP 2007133249A JP 2007133249 A JP2007133249 A JP 2007133249A JP 5259120 B2 JP5259120 B2 JP 5259120B2
- Authority
- JP
- Japan
- Prior art keywords
- adhesive
- polyols
- catalysts
- component
- mixtures
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000000853 adhesive Substances 0.000 title claims abstract description 71
- 230000001070 adhesive effect Effects 0.000 title claims abstract description 71
- 239000004814 polyurethane Substances 0.000 title claims abstract description 13
- 229920002635 polyurethane Polymers 0.000 title claims abstract description 13
- 239000000203 mixture Substances 0.000 claims abstract description 95
- 229920005862 polyol Polymers 0.000 claims abstract description 46
- 150000003077 polyols Chemical class 0.000 claims abstract description 45
- 239000003054 catalyst Substances 0.000 claims abstract description 41
- 239000012948 isocyanate Substances 0.000 claims abstract description 27
- 150000002513 isocyanates Chemical class 0.000 claims abstract description 27
- 239000002981 blocking agent Substances 0.000 claims abstract description 26
- 239000004970 Chain extender Substances 0.000 claims abstract description 13
- 239000000758 substrate Substances 0.000 claims abstract description 13
- MCJGNVYPOGVAJF-UHFFFAOYSA-N quinolin-8-ol Chemical group C1=CN=C2C(O)=CC=CC2=C1 MCJGNVYPOGVAJF-UHFFFAOYSA-N 0.000 claims abstract description 9
- 239000005725 8-Hydroxyquinoline Substances 0.000 claims abstract description 8
- 229960003540 oxyquinoline Drugs 0.000 claims abstract description 8
- 239000000945 filler Substances 0.000 claims abstract description 6
- 150000003512 tertiary amines Chemical class 0.000 claims abstract description 6
- UKLDJPRMSDWDSL-UHFFFAOYSA-L [dibutyl(dodecanoyloxy)stannyl] dodecanoate Chemical compound CCCCCCCCCCCC(=O)O[Sn](CCCC)(CCCC)OC(=O)CCCCCCCCCCC UKLDJPRMSDWDSL-UHFFFAOYSA-L 0.000 claims abstract description 5
- 239000012975 dibutyltin dilaurate Substances 0.000 claims abstract description 5
- 239000004615 ingredient Substances 0.000 claims abstract description 4
- 239000004014 plasticizer Substances 0.000 claims abstract description 4
- 239000012974 tin catalyst Substances 0.000 claims abstract description 4
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 claims description 19
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 claims description 18
- 230000006698 induction Effects 0.000 claims description 16
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 claims description 15
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 claims description 12
- 238000002156 mixing Methods 0.000 claims description 12
- 150000001412 amines Chemical class 0.000 claims description 8
- 235000011187 glycerol Nutrition 0.000 claims description 7
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 7
- 238000000034 method Methods 0.000 claims description 7
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 claims description 6
- WERYXYBDKMZEQL-UHFFFAOYSA-N butane-1,4-diol Chemical compound OCCCCO WERYXYBDKMZEQL-UHFFFAOYSA-N 0.000 claims description 6
- 150000002009 diols Chemical class 0.000 claims description 6
- 229920000570 polyether Polymers 0.000 claims description 6
- SZXQTJUDPRGNJN-UHFFFAOYSA-N dipropylene glycol Chemical compound OCCCOCCCO SZXQTJUDPRGNJN-UHFFFAOYSA-N 0.000 claims description 5
- 229910052739 hydrogen Inorganic materials 0.000 claims description 5
- 239000001257 hydrogen Substances 0.000 claims description 5
- 229920005906 polyester polyol Polymers 0.000 claims description 5
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 claims description 4
- 239000004721 Polyphenylene oxide Substances 0.000 claims description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims description 4
- 125000002524 organometallic group Chemical group 0.000 claims description 4
- 239000004417 polycarbonate Substances 0.000 claims description 4
- 229920000515 polycarbonate Polymers 0.000 claims description 4
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 claims description 3
- 239000002202 Polyethylene glycol Substances 0.000 claims description 3
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 claims description 3
- ZJCCRDAZUWHFQH-UHFFFAOYSA-N Trimethylolpropane Chemical compound CCC(CO)(CO)CO ZJCCRDAZUWHFQH-UHFFFAOYSA-N 0.000 claims description 3
- 125000000217 alkyl group Chemical group 0.000 claims description 3
- RREGISFBPQOLTM-UHFFFAOYSA-N alumane;trihydrate Chemical compound O.O.O.[AlH3] RREGISFBPQOLTM-UHFFFAOYSA-N 0.000 claims description 3
- 229910000019 calcium carbonate Inorganic materials 0.000 claims description 3
- 229910052736 halogen Inorganic materials 0.000 claims description 3
- 150000002367 halogens Chemical class 0.000 claims description 3
- 150000002431 hydrogen Chemical class 0.000 claims description 3
- 229920001223 polyethylene glycol Polymers 0.000 claims description 3
- 239000004114 Ammonium polyphosphate Substances 0.000 claims description 2
- 239000004610 Internal Lubricant Substances 0.000 claims description 2
- 239000006057 Non-nutritive feed additive Substances 0.000 claims description 2
- 229920003171 Poly (ethylene oxide) Polymers 0.000 claims description 2
- NSOXQYCFHDMMGV-UHFFFAOYSA-N Tetrakis(2-hydroxypropyl)ethylenediamine Chemical compound CC(O)CN(CC(C)O)CCN(CC(C)O)CC(C)O NSOXQYCFHDMMGV-UHFFFAOYSA-N 0.000 claims description 2
- 150000001298 alcohols Chemical class 0.000 claims description 2
- 235000019826 ammonium polyphosphate Nutrition 0.000 claims description 2
- 229920001276 ammonium polyphosphate Polymers 0.000 claims description 2
- 239000011324 bead Substances 0.000 claims description 2
- 239000003086 colorant Substances 0.000 claims description 2
- 150000004985 diamines Chemical class 0.000 claims description 2
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 claims description 2
- 239000000835 fiber Substances 0.000 claims description 2
- 239000003063 flame retardant Substances 0.000 claims description 2
- ACCCMOQWYVYDOT-UHFFFAOYSA-N hexane-1,1-diol Chemical compound CCCCCC(O)O ACCCMOQWYVYDOT-UHFFFAOYSA-N 0.000 claims description 2
- 239000011256 inorganic filler Substances 0.000 claims description 2
- 229910003475 inorganic filler Inorganic materials 0.000 claims description 2
- 239000012766 organic filler Substances 0.000 claims description 2
- 239000000049 pigment Substances 0.000 claims description 2
- 229920000768 polyamine Polymers 0.000 claims description 2
- 229920000642 polymer Polymers 0.000 claims description 2
- 229920000098 polyolefin Polymers 0.000 claims description 2
- 239000000377 silicon dioxide Substances 0.000 claims description 2
- 239000000454 talc Substances 0.000 claims description 2
- 229910052623 talc Inorganic materials 0.000 claims description 2
- 239000013008 thixotropic agent Substances 0.000 claims description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 claims 2
- RNFJDJUURJAICM-UHFFFAOYSA-N 2,2,4,4,6,6-hexaphenoxy-1,3,5-triaza-2$l^{5},4$l^{5},6$l^{5}-triphosphacyclohexa-1,3,5-triene Chemical compound N=1P(OC=2C=CC=CC=2)(OC=2C=CC=CC=2)=NP(OC=2C=CC=CC=2)(OC=2C=CC=CC=2)=NP=1(OC=1C=CC=CC=1)OC1=CC=CC=C1 RNFJDJUURJAICM-UHFFFAOYSA-N 0.000 claims 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 claims 1
- 229910052791 calcium Inorganic materials 0.000 claims 1
- 239000011575 calcium Substances 0.000 claims 1
- 239000012784 inorganic fiber Substances 0.000 claims 1
- 238000006243 chemical reaction Methods 0.000 abstract description 14
- 238000009472 formulation Methods 0.000 description 38
- 150000008064 anhydrides Chemical class 0.000 description 21
- 238000012360 testing method Methods 0.000 description 21
- 238000001723 curing Methods 0.000 description 18
- 239000002253 acid Substances 0.000 description 8
- -1 ether glycols Chemical class 0.000 description 8
- IMNIMPAHZVJRPE-UHFFFAOYSA-N triethylenediamine Chemical compound C1CN2CCN1CC2 IMNIMPAHZVJRPE-UHFFFAOYSA-N 0.000 description 7
- WNLRTRBMVRJNCN-UHFFFAOYSA-N adipic acid Chemical compound OC(=O)CCCCC(O)=O WNLRTRBMVRJNCN-UHFFFAOYSA-N 0.000 description 6
- 230000000052 comparative effect Effects 0.000 description 6
- 238000003860 storage Methods 0.000 description 6
- NIMLQBUJDJZYEJ-UHFFFAOYSA-N isophorone diisocyanate Chemical compound CC1(C)CC(N=C=O)CC(C)(CN=C=O)C1 NIMLQBUJDJZYEJ-UHFFFAOYSA-N 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 238000012545 processing Methods 0.000 description 5
- 150000007513 acids Chemical class 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 230000003111 delayed effect Effects 0.000 description 4
- IQPQWNKOIGAROB-UHFFFAOYSA-N isocyanate group Chemical group [N-]=C=O IQPQWNKOIGAROB-UHFFFAOYSA-N 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 229910052757 nitrogen Inorganic materials 0.000 description 4
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- 239000005058 Isophorone diisocyanate Substances 0.000 description 3
- SJRJJKPEHAURKC-UHFFFAOYSA-N N-Methylmorpholine Chemical compound CN1CCOCC1 SJRJJKPEHAURKC-UHFFFAOYSA-N 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 239000001361 adipic acid Substances 0.000 description 3
- 235000011037 adipic acid Nutrition 0.000 description 3
- 229910052797 bismuth Inorganic materials 0.000 description 3
- IISBACLAFKSPIT-UHFFFAOYSA-N bisphenol A Chemical compound C=1C=C(O)C=CC=1C(C)(C)C1=CC=C(O)C=C1 IISBACLAFKSPIT-UHFFFAOYSA-N 0.000 description 3
- 238000003776 cleavage reaction Methods 0.000 description 3
- 239000012973 diazabicyclooctane Substances 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- 150000002334 glycols Chemical class 0.000 description 3
- 229920000728 polyester Polymers 0.000 description 3
- 230000007017 scission Effects 0.000 description 3
- 150000005846 sugar alcohols Polymers 0.000 description 3
- 239000012970 tertiary amine catalyst Substances 0.000 description 3
- JOYRKODLDBILNP-UHFFFAOYSA-N urethane group Chemical group NC(=O)OCC JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 3
- 238000009736 wetting Methods 0.000 description 3
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 2
- ALQLPWJFHRMHIU-UHFFFAOYSA-N 1,4-diisocyanatobenzene Chemical compound O=C=NC1=CC=C(N=C=O)C=C1 ALQLPWJFHRMHIU-UHFFFAOYSA-N 0.000 description 2
- UPMLOUAZCHDJJD-UHFFFAOYSA-N 4,4'-Diphenylmethane Diisocyanate Chemical compound C1=CC(N=C=O)=CC=C1CC1=CC=C(N=C=O)C=C1 UPMLOUAZCHDJJD-UHFFFAOYSA-N 0.000 description 2
- 229920000178 Acrylic resin Polymers 0.000 description 2
- 239000004925 Acrylic resin Substances 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- SVYKKECYCPFKGB-UHFFFAOYSA-N N,N-dimethylcyclohexylamine Chemical compound CN(C)C1CCCCC1 SVYKKECYCPFKGB-UHFFFAOYSA-N 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 239000005062 Polybutadiene Chemical class 0.000 description 2
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical class CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 description 2
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 2
- KKEYFWRCBNTPAC-UHFFFAOYSA-N Terephthalic acid Chemical compound OC(=O)C1=CC=C(C(O)=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-N 0.000 description 2
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 2
- 229920000122 acrylonitrile butadiene styrene Polymers 0.000 description 2
- 239000000654 additive Substances 0.000 description 2
- JCXGWMGPZLAOME-UHFFFAOYSA-N bismuth atom Chemical compound [Bi] JCXGWMGPZLAOME-UHFFFAOYSA-N 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 150000001735 carboxylic acids Chemical class 0.000 description 2
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 2
- JGFBRKRYDCGYKD-UHFFFAOYSA-N dibutyl(oxo)tin Chemical class CCCC[Sn](=O)CCCC JGFBRKRYDCGYKD-UHFFFAOYSA-N 0.000 description 2
- AYOHIQLKSOJJQH-UHFFFAOYSA-N dibutyltin Chemical compound CCCC[Sn]CCCC AYOHIQLKSOJJQH-UHFFFAOYSA-N 0.000 description 2
- 125000005442 diisocyanate group Chemical group 0.000 description 2
- XXBDWLFCJWSEKW-UHFFFAOYSA-N dimethylbenzylamine Chemical compound CN(C)CC1=CC=CC=C1 XXBDWLFCJWSEKW-UHFFFAOYSA-N 0.000 description 2
- 150000002170 ethers Chemical class 0.000 description 2
- 229940012017 ethylenediamine Drugs 0.000 description 2
- RRAMGCGOFNQTLD-UHFFFAOYSA-N hexamethylene diisocyanate Chemical compound O=C=NCCCCCCN=C=O RRAMGCGOFNQTLD-UHFFFAOYSA-N 0.000 description 2
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 2
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 2
- 230000000977 initiatory effect Effects 0.000 description 2
- QQVIHTHCMHWDBS-UHFFFAOYSA-N isophthalic acid Chemical compound OC(=O)C1=CC=CC(C(O)=O)=C1 QQVIHTHCMHWDBS-UHFFFAOYSA-N 0.000 description 2
- 238000011068 loading method Methods 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 229920001281 polyalkylene Polymers 0.000 description 2
- 229920002857 polybutadiene Chemical class 0.000 description 2
- 229920001451 polypropylene glycol Polymers 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- CXMXRPHRNRROMY-UHFFFAOYSA-N sebacic acid Chemical compound OC(=O)CCCCCCCCC(O)=O CXMXRPHRNRROMY-UHFFFAOYSA-N 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 238000007655 standard test method Methods 0.000 description 2
- 150000003606 tin compounds Chemical class 0.000 description 2
- RUELTTOHQODFPA-UHFFFAOYSA-N toluene 2,6-diisocyanate Chemical compound CC1=C(N=C=O)C=CC=C1N=C=O RUELTTOHQODFPA-UHFFFAOYSA-N 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- ZIBGPFATKBEMQZ-UHFFFAOYSA-N triethylene glycol Chemical compound OCCOCCOCCO ZIBGPFATKBEMQZ-UHFFFAOYSA-N 0.000 description 2
- 229920006305 unsaturated polyester Polymers 0.000 description 2
- ZQHJVIHCDHJVII-OWOJBTEDSA-N (e)-2-chlorobut-2-enedioic acid Chemical compound OC(=O)\C=C(\Cl)C(O)=O ZQHJVIHCDHJVII-OWOJBTEDSA-N 0.000 description 1
- ZBBLRPRYYSJUCZ-GRHBHMESSA-L (z)-but-2-enedioate;dibutyltin(2+) Chemical compound [O-]C(=O)\C=C/C([O-])=O.CCCC[Sn+2]CCCC ZBBLRPRYYSJUCZ-GRHBHMESSA-L 0.000 description 1
- ZWVMLYRJXORSEP-UHFFFAOYSA-N 1,2,6-Hexanetriol Chemical compound OCCCCC(O)CO ZWVMLYRJXORSEP-UHFFFAOYSA-N 0.000 description 1
- ZTNJGMFHJYGMDR-UHFFFAOYSA-N 1,2-diisocyanatoethane Chemical compound O=C=NCCN=C=O ZTNJGMFHJYGMDR-UHFFFAOYSA-N 0.000 description 1
- 239000005059 1,4-Cyclohexyldiisocyanate Substances 0.000 description 1
- OHLKMGYGBHFODF-UHFFFAOYSA-N 1,4-bis(isocyanatomethyl)benzene Chemical compound O=C=NCC1=CC=C(CN=C=O)C=C1 OHLKMGYGBHFODF-UHFFFAOYSA-N 0.000 description 1
- 229940008841 1,6-hexamethylene diisocyanate Drugs 0.000 description 1
- DTLIXPLJFCRLJY-UHFFFAOYSA-N 1-(1-aminocyclooctyl)cyclooctan-1-amine Chemical compound C1CCCCCCC1(N)C1(N)CCCCCCC1 DTLIXPLJFCRLJY-UHFFFAOYSA-N 0.000 description 1
- ZIXLMPXXNUVJQK-UHFFFAOYSA-N 1-isocyanato-3-(3-isocyanatobutyl)benzene Chemical compound O=C=NC(C)CCC1=CC=CC(N=C=O)=C1 ZIXLMPXXNUVJQK-UHFFFAOYSA-N 0.000 description 1
- JDVGUTQQCHTCMZ-UHFFFAOYSA-N 1-isocyanato-4-(1-isocyanatoethyl)benzene Chemical compound O=C=NC(C)C1=CC=C(N=C=O)C=C1 JDVGUTQQCHTCMZ-UHFFFAOYSA-N 0.000 description 1
- AXFVIWBTKYFOCY-UHFFFAOYSA-N 1-n,1-n,3-n,3-n-tetramethylbutane-1,3-diamine Chemical compound CN(C)C(C)CCN(C)C AXFVIWBTKYFOCY-UHFFFAOYSA-N 0.000 description 1
- GGPLWEZGITVTJX-UHFFFAOYSA-N 2,2,4-trimethyl-1,4,2-oxazasilinane Chemical compound CN1CCO[Si](C)(C)C1 GGPLWEZGITVTJX-UHFFFAOYSA-N 0.000 description 1
- DZDVHNPXFWWDRM-UHFFFAOYSA-N 2,4-diisocyanato-1-methoxybenzene Chemical compound COC1=CC=C(N=C=O)C=C1N=C=O DZDVHNPXFWWDRM-UHFFFAOYSA-N 0.000 description 1
- JAHNSTQSQJOJLO-UHFFFAOYSA-N 2-(3-fluorophenyl)-1h-imidazole Chemical compound FC1=CC=CC(C=2NC=CN=2)=C1 JAHNSTQSQJOJLO-UHFFFAOYSA-N 0.000 description 1
- RWLALWYNXFYRGW-UHFFFAOYSA-N 2-Ethyl-1,3-hexanediol Chemical compound CCCC(O)C(CC)CO RWLALWYNXFYRGW-UHFFFAOYSA-N 0.000 description 1
- WUIXEIPAPIJUGW-UHFFFAOYSA-N 2-[1,1-bis(2-hydroxyphenyl)propyl]phenol Chemical compound C=1C=CC=C(O)C=1C(C=1C(=CC=CC=1)O)(CC)C1=CC=CC=C1O WUIXEIPAPIJUGW-UHFFFAOYSA-N 0.000 description 1
- LXBGSDVWAMZHDD-UHFFFAOYSA-N 2-methyl-1h-imidazole Chemical compound CC1=NC=CN1 LXBGSDVWAMZHDD-UHFFFAOYSA-N 0.000 description 1
- OCXPJMSKLNNYLE-UHFFFAOYSA-N 2-prop-2-enylbutanedioic acid Chemical compound OC(=O)CC(C(O)=O)CC=C OCXPJMSKLNNYLE-UHFFFAOYSA-N 0.000 description 1
- FPRWILGFWFTOHA-UHFFFAOYSA-N 2-sulfanylethyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCCS FPRWILGFWFTOHA-UHFFFAOYSA-N 0.000 description 1
- HVCNXQOWACZAFN-UHFFFAOYSA-N 4-ethylmorpholine Chemical compound CCN1CCOCC1 HVCNXQOWACZAFN-UHFFFAOYSA-N 0.000 description 1
- VNPRJHMMOKDEDZ-UHFFFAOYSA-L 6-methylheptyl 2-[dibutyl-[2-(6-methylheptoxy)-2-oxoethyl]sulfanylstannyl]sulfanylacetate Chemical compound CC(C)CCCCCOC(=O)CS[Sn](CCCC)(CCCC)SCC(=O)OCCCCCC(C)C VNPRJHMMOKDEDZ-UHFFFAOYSA-L 0.000 description 1
- RREANTFLPGEWEN-MBLPBCRHSA-N 7-[4-[[(3z)-3-[4-amino-5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidin-2-yl]imino-5-fluoro-2-oxoindol-1-yl]methyl]piperazin-1-yl]-1-cyclopropyl-6-fluoro-4-oxoquinoline-3-carboxylic acid Chemical compound COC1=C(OC)C(OC)=CC(CC=2C(=NC(\N=C/3C4=CC(F)=CC=C4N(CN4CCN(CC4)C=4C(=CC=5C(=O)C(C(O)=O)=CN(C=5C=4)C4CC4)F)C\3=O)=NC=2)N)=C1 RREANTFLPGEWEN-MBLPBCRHSA-N 0.000 description 1
- 150000004325 8-hydroxyquinolines Chemical class 0.000 description 1
- 229930185605 Bisphenol Natural products 0.000 description 1
- VMNQKNIVLSZXEZ-UHFFFAOYSA-N CCNC(C)[Si](C)(NCC)O[Si](C)(C)C Chemical compound CCNC(C)[Si](C)(NCC)O[Si](C)(C)C VMNQKNIVLSZXEZ-UHFFFAOYSA-N 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 1
- 239000005057 Hexamethylene diisocyanate Substances 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- TXOFSCODFRHERQ-UHFFFAOYSA-N N,N-Dimethylphenethylamine Chemical compound CN(C)CCC1=CC=CC=C1 TXOFSCODFRHERQ-UHFFFAOYSA-N 0.000 description 1
- UEEJHVSXFDXPFK-UHFFFAOYSA-N N-dimethylaminoethanol Chemical compound CN(C)CCO UEEJHVSXFDXPFK-UHFFFAOYSA-N 0.000 description 1
- IGFHQQFPSIBGKE-UHFFFAOYSA-N Nonylphenol Natural products CCCCCCCCCC1=CC=C(O)C=C1 IGFHQQFPSIBGKE-UHFFFAOYSA-N 0.000 description 1
- 229920002176 Pluracol® Polymers 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 229920002367 Polyisobutene Polymers 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 1
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- SLINHMUFWFWBMU-UHFFFAOYSA-N Triisopropanolamine Chemical compound CC(O)CN(CC(C)O)CC(C)O SLINHMUFWFWBMU-UHFFFAOYSA-N 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 description 1
- WXSGUWUJXPVDOQ-UHFFFAOYSA-N [Fe].CCC(=O)C(C)=O Chemical compound [Fe].CCC(=O)C(C)=O WXSGUWUJXPVDOQ-UHFFFAOYSA-N 0.000 description 1
- KXBFLNPZHXDQLV-UHFFFAOYSA-N [cyclohexyl(diisocyanato)methyl]cyclohexane Chemical compound C1CCCCC1C(N=C=O)(N=C=O)C1CCCCC1 KXBFLNPZHXDQLV-UHFFFAOYSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- RGAMPJYGTCSRAG-UHFFFAOYSA-N bis[2-(diethylamino)ethyl] hexanedioate Chemical compound CCN(CC)CCOC(=O)CCCCC(=O)OCCN(CC)CC RGAMPJYGTCSRAG-UHFFFAOYSA-N 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 150000007942 carboxylates Chemical class 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 125000003636 chemical group Chemical group 0.000 description 1
- HNEGQIOMVPPMNR-IHWYPQMZSA-N citraconic acid Chemical compound OC(=O)C(/C)=C\C(O)=O HNEGQIOMVPPMNR-IHWYPQMZSA-N 0.000 description 1
- 229940018557 citraconic acid Drugs 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 238000006482 condensation reaction Methods 0.000 description 1
- 230000001143 conditioned effect Effects 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- VKIRRGRTJUUZHS-UHFFFAOYSA-N cyclohexane-1,4-diamine Chemical compound NC1CCC(N)CC1 VKIRRGRTJUUZHS-UHFFFAOYSA-N 0.000 description 1
- KQWGXHWJMSMDJJ-UHFFFAOYSA-N cyclohexyl isocyanate Chemical compound O=C=NC1CCCCC1 KQWGXHWJMSMDJJ-UHFFFAOYSA-N 0.000 description 1
- GMAYNBHUHYFCPZ-UHFFFAOYSA-N cyclohexyl-(4,4-dimethylcyclohexyl)methanediamine Chemical compound C1CC(C)(C)CCC1C(N)(N)C1CCCCC1 GMAYNBHUHYFCPZ-UHFFFAOYSA-N 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- FFGHLLOLFQHABK-UHFFFAOYSA-L dibutyltin(2+);dodecane-1-thiolate Chemical compound CCCCCCCCCCCCS[Sn](CCCC)(CCCC)SCCCCCCCCCCCC FFGHLLOLFQHABK-UHFFFAOYSA-L 0.000 description 1
- SGIBEPSSERDAHS-UHFFFAOYSA-L dibutyltin(2+);hexanedioate Chemical compound CCCC[Sn+2]CCCC.[O-]C(=O)CCCCC([O-])=O SGIBEPSSERDAHS-UHFFFAOYSA-L 0.000 description 1
- 150000001990 dicarboxylic acid derivatives Chemical class 0.000 description 1
- PKKGKUDPKRTKLJ-UHFFFAOYSA-L dichloro(dimethyl)stannane Chemical compound C[Sn](C)(Cl)Cl PKKGKUDPKRTKLJ-UHFFFAOYSA-L 0.000 description 1
- KORSJDCBLAPZEQ-UHFFFAOYSA-N dicyclohexylmethane-4,4'-diisocyanate Chemical compound C1CC(N=C=O)CCC1CC1CCC(N=C=O)CC1 KORSJDCBLAPZEQ-UHFFFAOYSA-N 0.000 description 1
- 125000005594 diketone group Chemical group 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 1
- 150000004820 halides Chemical class 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- XXMIOPMDWAUFGU-UHFFFAOYSA-N hexane-1,6-diol Chemical compound OCCCCCCO XXMIOPMDWAUFGU-UHFFFAOYSA-N 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- FPYJFEHAWHCUMM-UHFFFAOYSA-N maleic anhydride Chemical compound O=C1OC(=O)C=C1 FPYJFEHAWHCUMM-UHFFFAOYSA-N 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- HNEGQIOMVPPMNR-NSCUHMNNSA-N mesaconic acid Chemical compound OC(=O)C(/C)=C/C(O)=O HNEGQIOMVPPMNR-NSCUHMNNSA-N 0.000 description 1
- LVHBHZANLOWSRM-UHFFFAOYSA-N methylenebutanedioic acid Natural products OC(=O)CC(=C)C(O)=O LVHBHZANLOWSRM-UHFFFAOYSA-N 0.000 description 1
- HNEGQIOMVPPMNR-UHFFFAOYSA-N methylfumaric acid Natural products OC(=O)C(C)=CC(O)=O HNEGQIOMVPPMNR-UHFFFAOYSA-N 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- XFLSMWXCZBIXLV-UHFFFAOYSA-N n,n-dimethyl-2-(4-methylpiperazin-1-yl)ethanamine Chemical compound CN(C)CCN1CCN(C)CC1 XFLSMWXCZBIXLV-UHFFFAOYSA-N 0.000 description 1
- ZWRDBWDXRLPESY-UHFFFAOYSA-N n-benzyl-n-ethylethanamine Chemical compound CCN(CC)CC1=CC=CC=C1 ZWRDBWDXRLPESY-UHFFFAOYSA-N 0.000 description 1
- VXPJBVRYAHYMNY-UHFFFAOYSA-N n-methyl-2-[2-(methylamino)ethoxy]ethanamine Chemical compound CNCCOCCNC VXPJBVRYAHYMNY-UHFFFAOYSA-N 0.000 description 1
- SLCVBVWXLSEKPL-UHFFFAOYSA-N neopentyl glycol Chemical compound OCC(C)(C)CO SLCVBVWXLSEKPL-UHFFFAOYSA-N 0.000 description 1
- SNQQPOLDUKLAAF-UHFFFAOYSA-N nonylphenol Chemical compound CCCCCCCCCC1=CC=CC=C1O SNQQPOLDUKLAAF-UHFFFAOYSA-N 0.000 description 1
- 238000010534 nucleophilic substitution reaction Methods 0.000 description 1
- 150000002902 organometallic compounds Chemical class 0.000 description 1
- 235000011837 pasties Nutrition 0.000 description 1
- WXZMFSXDPGVJKK-UHFFFAOYSA-N pentaerythritol Chemical compound OCC(CO)(CO)CO WXZMFSXDPGVJKK-UHFFFAOYSA-N 0.000 description 1
- UKODFQOELJFMII-UHFFFAOYSA-N pentamethyldiethylenetriamine Chemical compound CN(C)CCN(C)CCN(C)C UKODFQOELJFMII-UHFFFAOYSA-N 0.000 description 1
- 150000002989 phenols Chemical class 0.000 description 1
- XNGIFLGASWRNHJ-UHFFFAOYSA-N phthalic acid Chemical compound OC(=O)C1=CC=CC=C1C(O)=O XNGIFLGASWRNHJ-UHFFFAOYSA-N 0.000 description 1
- 229920001748 polybutylene Polymers 0.000 description 1
- 229920001610 polycaprolactone Polymers 0.000 description 1
- 239000004632 polycaprolactone Substances 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- AOHJOMMDDJHIJH-UHFFFAOYSA-N propylenediamine Chemical compound CC(N)CN AOHJOMMDDJHIJH-UHFFFAOYSA-N 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 238000000518 rheometry Methods 0.000 description 1
- 150000003335 secondary amines Chemical class 0.000 description 1
- 238000007711 solidification Methods 0.000 description 1
- 230000008023 solidification Effects 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 238000009864 tensile test Methods 0.000 description 1
- 150000003568 thioethers Chemical class 0.000 description 1
- IUTCEZPPWBHGIX-UHFFFAOYSA-N tin(2+) Chemical class [Sn+2] IUTCEZPPWBHGIX-UHFFFAOYSA-N 0.000 description 1
- DVKJHBMWWAPEIU-UHFFFAOYSA-N toluene 2,4-diisocyanate Chemical compound CC1=CC=C(N=C=O)C=C1N=C=O DVKJHBMWWAPEIU-UHFFFAOYSA-N 0.000 description 1
- IMFACGCPASFAPR-UHFFFAOYSA-N tributylamine Chemical compound CCCCN(CCCC)CCCC IMFACGCPASFAPR-UHFFFAOYSA-N 0.000 description 1
- QXJQHYBHAIHNGG-UHFFFAOYSA-N trimethylolethane Chemical compound OCC(C)(CO)CO QXJQHYBHAIHNGG-UHFFFAOYSA-N 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 229910052726 zirconium Inorganic materials 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/30—Low-molecular-weight compounds
- C08G18/36—Hydroxylated esters of higher fatty acids
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/08—Processes
- C08G18/16—Catalysts
- C08G18/161—Catalysts containing two or more components to be covered by at least two of the groups C08G18/166, C08G18/18 or C08G18/22
- C08G18/163—Catalysts containing two or more components to be covered by at least two of the groups C08G18/166, C08G18/18 or C08G18/22 covered by C08G18/18 and C08G18/22
- C08G18/165—Catalysts containing two or more components to be covered by at least two of the groups C08G18/166, C08G18/18 or C08G18/22 covered by C08G18/18 and C08G18/22 covered by C08G18/18 and C08G18/24
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/2805—Compounds having only one group containing active hydrogen
- C08G18/2815—Monohydroxy compounds
- C08G18/282—Alkanols, cycloalkanols or arylalkanols including terpenealcohols
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/40—High-molecular-weight compounds
- C08G18/42—Polycondensates having carboxylic or carbonic ester groups in the main chain
- C08G18/4202—Two or more polyesters of different physical or chemical nature
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J175/00—Adhesives based on polyureas or polyurethanes; Adhesives based on derivatives of such polymers
- C09J175/04—Polyurethanes
- C09J175/06—Polyurethanes from polyesters
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Adhesives Or Adhesive Processes (AREA)
- Polyurethanes Or Polyureas (AREA)
Description
本発明は、速い硬化速度及び硬化を開始する前の誘導期間を有する2成分型ポリウレタン接着剤組成物に関する。
2成分型ポリウレタン接着剤組成物は一般に室温で液状又はペースト状である成分を含み、それら成分が共に混合される。組成物の第1の成分はポリオール及び鎖延長剤、触媒ブロッキング剤、所望により他の添加剤などの他の成分を含む。第2の成分はモノマーの、ポリマーの又はプレポリマーのイソシアネートを含む。接着するために、接着剤の2つの成分は十分に混合され、その後組成物が基材に施される。混合された組成物はその後硬化を開始し、固体へと変化しながら接着強度を高めていく。硬化反応はフリーのイソシアネート基とポリオールからの活性水素との間で起こる。主要な硬化反応後に過剰量のフリーのイソシアネート基が存在していると、その過剰量のフリーのイソシアネート基が雰囲気又は基材からの表面水分によって硬化する。
本発明は、1以上のポリオール類の第1の成分及び1以上のイソシアネート類の第2の成分を有する2成分型ポリウレタン接着剤組成物を提供する。この組成物はまた、1以上の触媒及び硬化反応を遅らせるための1以上のブロッキング剤を含む。この組成物は、長いオープン時間、接着される基材に対する改善された濡れ、迅速な硬化速度及び室温及び高温での優れた最終接着性能を与える。充填材、鎖延長剤、可塑剤などの成分が所望により添加されてもよい。
本発明は1以上のポリオール類の第1の成分及び1以上のイソシアネート類の第2の成分を含む2成分型ポリウレタン接着剤組成物に関する。イソシアネート成分及びポリオール成分は、通常、約1:1の当量比で使用される。本発明によれば、1以上のブロッキング剤を1以上の触媒と組み合わせて添加することによって、接着剤の成分の混合時間と硬化の開始時間との間に誘導期間が提供される。この誘導期間は多くの加工及び性能の利点を与える。
を有する。
実施例
2成分型ポリウレタン接着剤の様々な配合物を以下の方法に従って調製した。第1の成分(A成分)を調製するために、ポリオール(単数又は複数)の所望の量が汚れていない反応器へ装填され、組成物が十分に混合された。減圧と加熱が反応器に施された。減圧は反応器の内容物が225oFに達した後1時間続けた。その後減圧を解除し、鎖延長剤、触媒、及び8−ヒドロキシキノリンを、攪拌しながら、所望量で反応器に装填した。材料成分を全て加えた後、混合をさらに20分間続けた。その後、反応器の内容物は、別の汚れていない容器に移され、室温で冷却された。この時点でA成分を2成分型接着剤に使用するための準備が整った。貯蔵の目的のために、その容器は密封される前に乾燥窒素でパージされるべきである。第2の成分(B成分)はウレタンプレポリマーである。これらの実施例の目的のために、イソシアネート含量が17%であるVorite 689 イソシアネート(Caschemから商業的に入手可能)を利用した。
Desmophen S1011-35はBayer Material Sciencesから商業的に入手できる。
Desmophen F-207はBayer Material Scienceから商業的に入手できる。
2つのタイプの試料を引張ラップせん断試験及びクリーベージピール試験のために調製した。基材はABS及びアクリル樹脂の剛性プラスチック(厚さ0.1インチ)であった。基材は引張試験のために1''×4''の寸法に切断され、クリーベージピール試験のために1''×6''の寸法に切断された。
全ての接着試験結果はPSI(pound per square inch)であった。
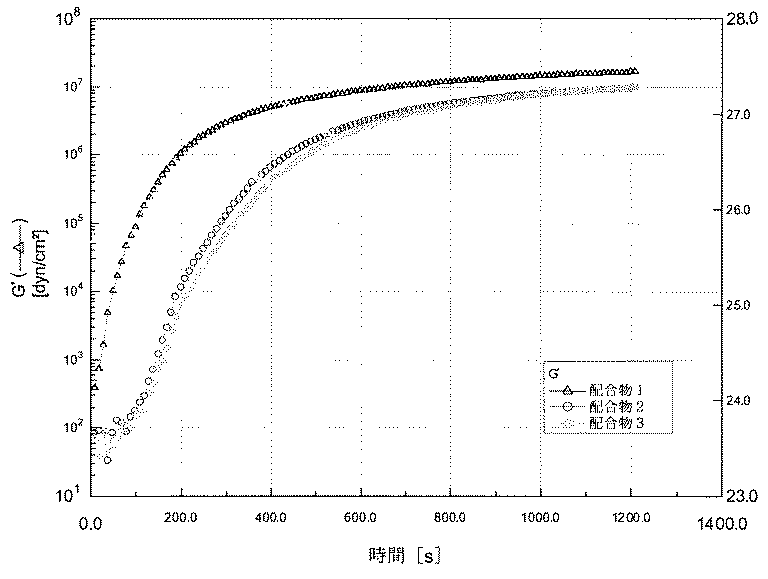
硬化速度試験で不良モードは、アクリル基材に対する接着不良を示すアスタリスクでマークされたもの以外は全て結合力のある不良(cohesive failure)であった。全ての5日の結果における不良モードは、アクリル基材に対する接着不良であった。配合物1の硬化反応速度は多くの加工用途にとっては速すぎるものであった。混合塗布して接合を形成した後10分以内に接着剤は液体状態から固体状態へと変化し、49.7psiのラップせん断強度を生じさせた。配合物1のラップせん断強度はその後の15分間にわたり速やかに増加した。配合物1の非常に早い固化は、接合アセンブリに短いオープン時間を与えるに過ぎない。対照的に、配合物2は強度の増大がよりゆっくりとしている。配合物2及び3のラップせん断強度試験は塗布と硬化の間の誘導期間を明確に示している。配合物2のラップせん断強度は硬化後10分で7.1psiに過ぎず、同じ時間における配合物1の強度よりも有意に低い。15分の時点で、配合物2のラップせん断強度は56.1psiに増加するが、この値さえも同じ時間における配合物1の強度よりも有意に低い。しかし、配合物2の接着強度はその後の5分間にわたり急激に増加する。このラップせん断強度は硬化の20分で149.6psi、25分で184.2psiであったが、これは同じ時間における配合物1のラップせん断強度と同等である。誘導期間を有する遅発硬化プロファイルは、接着剤に対して最大限のオープン時間及び可使時間が要求される多くの用途に好ましい。配合物3において、硬化反応はさらに遅れるが、それはより多くのブロッキング剤が添加されたからである。結局、少量の8−ヒドロキシキノリンをブロッキング剤として添加することによって接着剤の硬化速度及び接着強度の増加が硬化後の初期の段階で効果的に遅れ、その後ブロッキング剤を添加しない配合物に匹敵するレベルまでこれらが急激に増大する。
配合物4、5及び6を配合物1、2及び3と同様の方法に従って調製した。配合物4、5及び6のA成分の組成を表3に示す。
Coscat 83はビスマス触媒である(Caschem Inc.から商業的に入手可能)。
Claims (18)
- ブロッキング剤が8−ヒドロキシキノリンである、請求項1の接着剤。
- ブロッキング剤が接着剤の0.0001〜10重量%の範囲を構成する請求項1の接着剤。
- ブロッキング剤が接着剤の0.01〜0.5重量%の範囲を構成する請求項3の接着剤。
- 触媒がジブチル錫ジラウレートである、請求項1の接着剤。
- 触媒が有機錫触媒及び1以上のアミン触媒を含む、請求項1の接着剤。
- 触媒が接着剤の0.001〜1重量%の範囲を構成する、請求項1の接着剤。
- 触媒が接着剤の0.01〜0.1重量%の範囲を構成する、請求項7の接着剤。
- 1以上のポリオール類が少なくとも2の水酸基を有し、500〜5000の範囲内の分子量を有する、請求項1の接着剤。
- 1以上のポリオール類がポリエーテルポリオール類、ポリエステルポリオール類、ポリカーボネートポリオール類、ポリオレフィンポリオール類及びそれらの混合物からなる群から選ばれる、請求項9の接着剤。
- 1以上のポリオール類が接着剤の25重量%〜75重量%の範囲を構成する、請求項9の接着剤。
- 1以上のイソシアネート類がプレポリマーのイソシアネート、ポリマーのイソシアネート、モノマーのイソシアネート及びそれらの混合物の群から選ばれる、請求項1の接着剤。
- 接着剤が1以上の鎖延長剤をさらに含む、請求項1の接着剤。
- 1以上の鎖延長剤が低分子量ジオール類、ジアミン類及びアミン含有アルコール類、低分子量ポリオール類、ポリアミン類及びアミン含有ポリオール類、エチレングリコール、プロピレングリコール、1,4−ブタンジオール、ジエチレングリコール、ジプロピレングリコール、ヘキサンジオール、トリメチロールプロパン、グリセロール、ヒドロキシル末端ポリエチレンオキサイド(ポリエチレングリコール)、グリセリン、エチレンジアミン、ポリオキシプロピレンジアミン、モノエタノールアミン、ジエタノールアミン、及びトリエタノールアミン、テトラ(2−ヒドロキシプロピル)エチレンジアミン、及びそれらの混合物からなる群から選ばれる、請求項13の接着剤。
- 1以上の鎖延長剤が接着剤の20重量%以下の範囲を構成する、請求項14の接着剤。
- 接着剤が充填材、繊維、可塑剤、顔料、着色剤、難燃剤、加工助剤、チキソトロピー剤、内部潤滑剤、有機又は無機充填材、有機又は無機繊維、タルク、炭酸カルシウム、シリカビーズ、硫酸カルシウム、アルミニウムトリハイドレート、アンモニウムポリフォスフェート、及びそれらの混合物からなる群の1以上をさらに含む、請求項1の接着剤。
- 1以上のポリオール類の第1の成分及び1以上のイソシアネート類の第2の成分を含む2成分型ポリウレタン接着剤組成物の硬化の前に誘導期間を与える方法であって、該第1及び第2の成分のいずれかが更に錫触媒、有機錫触媒、有機金属触媒、アミン触媒、第3アミン触媒及びそれらの混合物を含む群から選ばれる1以上の触媒を含み、該方法が、該接着剤組成物の成分の少なくとも1成分に、下
記構造:
を有する1以上のブロッキング剤を添加し、それら成分を共に混合する工程を含む方法であって、該誘導期間が該成分の混合時間と硬化の開始時間との間の期間である方法。 - 請求項1記載のポリウレタン接着剤組成物の硬化物を有する基材。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/437,926 US7834123B2 (en) | 2006-05-19 | 2006-05-19 | Two component polyurethane adhesive |
| US11/437,926 | 2006-05-19 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2007308705A JP2007308705A (ja) | 2007-11-29 |
| JP5259120B2 true JP5259120B2 (ja) | 2013-08-07 |
Family
ID=38473067
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007133249A Expired - Fee Related JP5259120B2 (ja) | 2006-05-19 | 2007-05-18 | 2成分型ポリウレタン接着剤 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US7834123B2 (ja) |
| EP (1) | EP1857480B1 (ja) |
| JP (1) | JP5259120B2 (ja) |
| CN (1) | CN101307217B (ja) |
| AT (1) | ATE529459T1 (ja) |
| ES (1) | ES2373745T3 (ja) |
| MX (1) | MX2007006037A (ja) |
Families Citing this family (46)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8742050B2 (en) * | 2008-03-28 | 2014-06-03 | Henkel US IP LLC | Two part hybrid adhesive |
| EP2318448B1 (en) * | 2008-08-22 | 2018-08-22 | Dow Global Technologies LLC | Adhesive composition adapted for bonding large mass parts to structures |
| CN101760165B (zh) * | 2008-12-23 | 2013-12-25 | 上海理日化工新材料有限公司 | 一种低熔点、快固化的聚氨酯热熔胶 |
| US9579869B2 (en) * | 2009-02-17 | 2017-02-28 | Henkel IP & Holding GmbH | Liquid moisture curable polyurethane adhesives for lamination and assembly |
| CN101503611B (zh) * | 2009-03-10 | 2012-08-22 | 中山大学 | 复合膜用无溶剂聚氨酯胶粘剂 |
| DE102009045488A1 (de) * | 2009-10-08 | 2011-04-14 | Henkel Ag & Co. Kgaa | 2-Komponenten Klebstoff mit haftungsverbessernden Zusätzen |
| ES2671187T3 (es) | 2009-10-30 | 2018-06-05 | Henkel Ag & Co. Kgaa | Formulaciones que curan con humedad de manera temporalmente modificada |
| ES2594752T3 (es) * | 2010-05-17 | 2016-12-22 | Henkel IP & Holding GmbH | Adhesivo laminado alifático de curado controlado que usa agentes de bloqueo no migratorios para catalizadores organometálicos |
| CN102295906B (zh) * | 2010-06-24 | 2013-09-25 | 上海海鹰粘接科技有限公司 | 一种高强度耐黄变弹性聚氨酯胶及其制备方法和应用 |
| CN101906283B (zh) * | 2010-08-31 | 2012-09-26 | 东莞市普赛达密封粘胶有限公司 | 一种内增塑型单组分聚氨酯密封胶 |
| JP5642047B2 (ja) * | 2010-12-21 | 2014-12-17 | ローム アンド ハース カンパニーRohm And Haas Company | 接着剤組成物 |
| EP2469075A1 (de) * | 2010-12-24 | 2012-06-27 | Sika Technology AG | Klebstoff für das Verfüllen von Fugen und Spalten in Rotorblättern für Windkraftanlagen |
| DE102011002809A1 (de) | 2011-01-18 | 2012-07-19 | Henkel Ag & Co. Kgaa | 2K-PU-Zusammensetzung mit verzögerter Vernetzung |
| DE102011007504A1 (de) | 2011-04-15 | 2012-10-18 | Henkel Ag & Co. Kgaa | PU-Zusammensetzungen mit komplexierten Katalysatoren |
| MX345176B (es) | 2011-05-05 | 2017-01-19 | Adco Products Llc | Adhesivo reactivo de techumbre. |
| KR101619088B1 (ko) * | 2011-05-24 | 2016-05-11 | 단국대학교 산학협력단 | 폴리우레탄 접착제 조성물 및 이의 제조방법 |
| KR101212040B1 (ko) | 2011-07-25 | 2013-01-14 | 주식회사 근원씨앤티 | 2액형 무용제 우레탄 접착제 조성물 제조 방법 및 그에 의해 제조된 조성물 |
| BR112014012528B1 (pt) * | 2011-12-14 | 2020-11-24 | Rohm And Haas Company | composição adesiva ou selante curável bicomponente |
| CN102516918B (zh) * | 2011-12-14 | 2013-10-02 | 湖北回天胶业股份有限公司 | 一种高伸长率的双组分聚氨酯胶粘剂及其制备方法 |
| US9701876B2 (en) * | 2012-11-02 | 2017-07-11 | Adco Products, Llc | Reactive roofing adhesive |
| ES2647620T3 (es) | 2012-12-05 | 2017-12-22 | 3M Innovative Properties Company | Adhesivos basados en poliuretano |
| US10010064B2 (en) | 2013-10-04 | 2018-07-03 | Kerr Corporation | Methods and compositions for treating a hoof of an ungulate animal |
| CN103642449B (zh) * | 2013-12-09 | 2015-09-02 | 山东一诺威聚氨酯股份有限公司 | 用于运动场地的无溶剂不黄变型聚氨酯粘合剂 |
| CN103756623A (zh) * | 2013-12-24 | 2014-04-30 | 上海邦中高分子材料有限公司 | 一种不锈钢纤维增强管道用粘接树脂 |
| CN103740315A (zh) * | 2013-12-24 | 2014-04-23 | 上海邦中高分子材料有限公司 | 一种耐高温反应型热熔胶 |
| CN103665357B (zh) * | 2014-01-07 | 2015-12-09 | 东北林业大学 | 一种含木质素的聚酯多元醇及利用其制备的聚氨酯胶黏剂 |
| DE102014212999A1 (de) * | 2014-07-04 | 2016-01-07 | Henkel Ag & Co. Kgaa | Polyurethan-Kaschierklebstoff enthaltend Füllstoffe |
| JP6766035B2 (ja) * | 2014-09-22 | 2020-10-07 | シーカ・テクノロジー・アーゲー | ガラス上で急速な接着形成を行う貯蔵安定性、湿気硬化ポリウレタン接着剤 |
| CN104371633B (zh) * | 2014-12-05 | 2017-01-11 | 浙江多邦化工有限公司 | 一种双组份无溶剂聚氨酯食品薄膜粘胶剂及其制备方法和使用方法 |
| DE102014226277A1 (de) * | 2014-12-17 | 2016-06-23 | Henkel Ag & Co. Kgaa | Zwei-Komponenten-Polyurethan-Klebstoff zum Verkleben von Faserformteilen |
| WO2016205252A1 (en) * | 2015-06-18 | 2016-12-22 | Dow Global Technologies Llc | Latent two-part polyurethane adhesives curable with infrared radiation |
| CN105153989A (zh) * | 2015-07-09 | 2015-12-16 | 常州市金呈宇五金有限公司 | 一种改性双组份耐磨、耐油性聚氨酯胶黏剂及其制备方法 |
| CN104974708A (zh) * | 2015-07-09 | 2015-10-14 | 常州市金呈宇五金有限公司 | 一种改性聚氨酯胶黏剂及其制备方法 |
| WO2017062252A1 (en) | 2015-10-05 | 2017-04-13 | Dow Global Technologies Llc | Adhesive formulation |
| FR3053972B1 (fr) | 2016-07-12 | 2020-01-24 | Bostik Sa | Composition adhesive bicomposante a base de polyurethane |
| CN106240032B (zh) * | 2016-07-29 | 2018-03-23 | 诚德科技股份有限公司 | 一种pet/pa/cpp复合薄膜及其制备工艺与应用 |
| JP7247115B2 (ja) * | 2017-06-30 | 2023-03-28 | シーカ テクノロジー アクチェンゲゼルシャフト | 調節可能なポットライフを有する2成分ポリウレタン組成物 |
| WO2019014582A1 (en) | 2017-07-13 | 2019-01-17 | Dow Global Technologies Llc | SILYLAMMONIUM SALTS AS LATENT POLYURETHANE CATALYSTS |
| KR20200047444A (ko) | 2017-09-15 | 2020-05-07 | 세키스이가가쿠 고교가부시키가이샤 | 광습기 경화형 수지 조성물, 전자 부품용 접착제 및 표시 소자용 접착제 |
| JP7461297B2 (ja) * | 2018-03-07 | 2024-04-03 | ディディピー スペシャルティ エレクトロニック マテリアルズ ユーエス,エルエルシー | 接着剤組成物 |
| CN109265975B (zh) * | 2018-08-07 | 2020-03-06 | 山东科技大学 | 一种高弹性阻燃双组份壁面材料及制备方法 |
| CN111892902B (zh) * | 2019-05-05 | 2022-10-25 | 郑州大学 | 一种新型中空玻璃用双组份改性聚氨酯密封胶及其制备方法 |
| CN110746925B (zh) * | 2019-06-24 | 2021-12-14 | 南京威邦新材料有限公司 | 一种空气过滤器用双组份快固聚氨酯胶粘剂及其制备方法 |
| CN113583615A (zh) * | 2021-05-31 | 2021-11-02 | 中国人民解放军陆军工程大学 | 高强度弹性密封胶粘材料的制作方法 |
| CN113717680A (zh) * | 2021-09-16 | 2021-11-30 | 山东一诺威聚氨酯股份有限公司 | 低温快速硫化聚氨酯高温粘合剂及其制备方法和应用 |
| CN119955462A (zh) * | 2023-11-09 | 2025-05-09 | 宁德时代新能源科技股份有限公司 | 胶粘剂组合物、其制备方法、电池包和用电装置 |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2866774A (en) * | 1953-09-23 | 1958-12-30 | Univ Notre Dame | Polyether polyurethane rubber |
| DE1229290B (de) | 1965-04-21 | 1966-11-24 | Bayer Ag | Verfahren zur Herstellung von Urethangruppen enthaltenden Schaumstoffen |
| DE1694162A1 (de) | 1967-06-27 | 1971-06-09 | Bayer Ag | Verfahren zur Herstellung von Urethangruppen aufweisenden Schaumstoffen |
| US3635906A (en) * | 1968-11-12 | 1972-01-18 | Du Pont | Preparation of polyurethanes using organo-tin catalyst and time-lapse modifier |
| US3895149A (en) * | 1973-07-05 | 1975-07-15 | Atlantic Richfield Co | Carpet backed with thixotropic polyurethane adhesive |
| DD108103A1 (ja) | 1973-11-29 | 1974-09-05 | ||
| US4322327A (en) | 1979-10-19 | 1982-03-30 | Mitsubishi Petrochemical Co., Ltd. | Slow-curing water-curable urethane prepolymer composition |
| US4395528A (en) | 1981-03-02 | 1983-07-26 | M&T Chemicals Inc. | Catalyst composition and curable polymer compositions containing same |
| DE3213439A1 (de) * | 1982-04-10 | 1983-10-20 | Chemiegesellschaft Gundernhausen mbH, 6101 Roßdorf | Verfahren und vorrichtung zur herstellung von teppichfliesen |
| US4871854A (en) | 1982-06-28 | 1989-10-03 | The United States Of America As Represented By The Secretary Of The Air Force | Cure catalyst for polyurethanes |
| DE3925790A1 (de) * | 1989-08-04 | 1991-02-07 | Bayer Ag | Zweikomponenten-polyurethanklebstoff |
| DE4033221A1 (de) * | 1990-10-19 | 1992-04-23 | Bayer Ag | Zweikomponenten-polyurethanklebstoffe |
| US6001900A (en) | 1993-07-28 | 1999-12-14 | Elf Atochem North America, Inc. | Metal containing e-coat catalysts optionally with tin catalysts |
| AU6801298A (en) * | 1997-06-05 | 1998-12-10 | Rohm And Haas Company | Coating compositions having extended pot life and shortened cure time and combination of chemicals used therein |
| EP1408062A1 (de) | 2002-10-08 | 2004-04-14 | Sika Technology AG | Bismutkatalysierte Polyurethanzusammensetzung |
| JP4012159B2 (ja) * | 2004-01-30 | 2007-11-21 | バンドー化学株式会社 | 電子写真装置用ブレード用接着剤及び電子写真装置用ブレード |
| DE102004043342A1 (de) * | 2004-09-08 | 2006-03-09 | Bayer Materialscience Ag | Blockierte Polyurethan-Prepolymere als Klebstoffe |
-
2006
- 2006-05-19 US US11/437,926 patent/US7834123B2/en not_active Expired - Fee Related
-
2007
- 2007-05-17 CN CN200710103861.2A patent/CN101307217B/zh not_active Expired - Fee Related
- 2007-05-18 EP EP07009958A patent/EP1857480B1/en not_active Not-in-force
- 2007-05-18 JP JP2007133249A patent/JP5259120B2/ja not_active Expired - Fee Related
- 2007-05-18 MX MX2007006037A patent/MX2007006037A/es active IP Right Grant
- 2007-05-18 AT AT07009958T patent/ATE529459T1/de active
- 2007-05-18 ES ES07009958T patent/ES2373745T3/es active Active
Also Published As
| Publication number | Publication date |
|---|---|
| MX2007006037A (es) | 2009-02-16 |
| JP2007308705A (ja) | 2007-11-29 |
| EP1857480A1 (en) | 2007-11-21 |
| CN101307217B (zh) | 2013-04-10 |
| ES2373745T3 (es) | 2012-02-08 |
| US7834123B2 (en) | 2010-11-16 |
| CN101307217A (zh) | 2008-11-19 |
| EP1857480B1 (en) | 2011-10-19 |
| US20070270567A1 (en) | 2007-11-22 |
| ATE529459T1 (de) | 2011-11-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5259120B2 (ja) | 2成分型ポリウレタン接着剤 | |
| US20110003146A1 (en) | Adhesive and its application | |
| JP5530355B2 (ja) | 使用温度範囲を通して実質的に不変の弾性率gを有する2部分型ポリウレタンの硬化性組成物 | |
| JP4986447B2 (ja) | アルコキシシラン官能性組成物 | |
| CN105283291B (zh) | 用于聚氨酯材料的内脱模剂 | |
| EP0344912B1 (en) | Non-hairing moisture curable compositions | |
| CN105026450B (zh) | 单组分湿固化组合物 | |
| BR112019027543A2 (pt) | composição de poliuretano de dois componentes com tempo de vida útil ajustável | |
| JP6178542B2 (ja) | 迅速な走行可能時間を提供する車輌窓装着において有用な接着剤 | |
| JP7337048B2 (ja) | 高強度で長いオープンタイムのポリウレタン反応性ホットメルト | |
| JP2015110788A (ja) | 改良された耐加水分解性を有する反応性ホットメルト接着剤 | |
| JP2020097650A (ja) | 湿気硬化型ポリウレタンホットメルト樹脂組成物、接着剤、及び、物品 | |
| JPH08157801A (ja) | 湿気硬化性ウレタンシーラント組成物 | |
| WO2014196303A1 (ja) | 接着剤組成物 | |
| JP2025515220A (ja) | 二液型ポリウレタン接着剤組成物 | |
| JP2025509994A (ja) | 二液型ポリウレタン系熱伝導性接着剤組成物、ポリウレタン系熱伝導性接着剤およびその物品 | |
| US20170152367A1 (en) | Internal mold release agents for polyurethane materials | |
| JP2020147687A (ja) | ウレタン樹脂組成物、接着剤、及び、床構造体 | |
| JP2021512977A (ja) | 車両窓を設置するために有用な接着剤 | |
| JP4682597B2 (ja) | 湿気硬化性ポリウレタン樹脂系接着剤 | |
| JPH0420582A (ja) | 無溶剤型接着剤 | |
| JP2024178976A (ja) | 1液型接着剤組成物およびそれを用いた接着構造物 | |
| JP2025136941A (ja) | 塩化ビニル樹脂用接着剤及び接着構造体 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100518 |
|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20100730 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20120727 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120731 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20121031 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20121116 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130214 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20130326 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20130424 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20160502 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5259120 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |