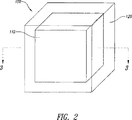
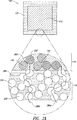
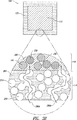
JP5041663B2 - 拡散支配剤形および三次元印刷を含む作製方法 - Google Patents
拡散支配剤形および三次元印刷を含む作製方法 Download PDFInfo
- Publication number
- JP5041663B2 JP5041663B2 JP2004500818A JP2004500818A JP5041663B2 JP 5041663 B2 JP5041663 B2 JP 5041663B2 JP 2004500818 A JP2004500818 A JP 2004500818A JP 2004500818 A JP2004500818 A JP 2004500818A JP 5041663 B2 JP5041663 B2 JP 5041663B2
- Authority
- JP
- Japan
- Prior art keywords
- release
- shell
- polymer
- dosage form
- core
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/13—Amines
- A61K31/135—Amines having aromatic rings, e.g. ketamine, nortriptyline
- A61K31/137—Arylalkylamines, e.g. amphetamine, epinephrine, salbutamol, ephedrine or methadone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/2027—Organic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyvinyl pyrrolidone, poly(meth)acrylates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2072—Pills, tablets, discs, rods characterised by shape, structure or size; Tablets with holes, special break lines or identification marks; Partially coated tablets; Disintegrating flat shaped forms
- A61K9/2077—Tablets comprising drug-containing microparticles in a substantial amount of supporting matrix; Multiparticulate tablets
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2072—Pills, tablets, discs, rods characterised by shape, structure or size; Tablets with holes, special break lines or identification marks; Partially coated tablets; Disintegrating flat shaped forms
- A61K9/2086—Layered tablets, e.g. bilayer tablets; Tablets of the type inert core-active coat
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2095—Tabletting processes; Dosage units made by direct compression of powders or specially processed granules, by eliminating solvents, by melt-extrusion, by injection molding, by 3D printing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/08—Bronchodilators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C64/00—Additive manufacturing, i.e. manufacturing of three-dimensional [3D] objects by additive deposition, additive agglomeration or additive layering, e.g. by 3D printing, stereolithography or selective laser sintering
- B29C64/10—Processes of additive manufacturing
- B29C64/141—Processes of additive manufacturing using only solid materials
- B29C64/153—Processes of additive manufacturing using only solid materials using layers of powder being selectively joined, e.g. by selective laser sintering or melting
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B33—ADDITIVE MANUFACTURING TECHNOLOGY
- B33Y—ADDITIVE MANUFACTURING, i.e. MANUFACTURING OF THREE-DIMENSIONAL [3-D] OBJECTS BY ADDITIVE DEPOSITION, ADDITIVE AGGLOMERATION OR ADDITIVE LAYERING, e.g. BY 3-D PRINTING, STEREOLITHOGRAPHY OR SELECTIVE LASER SINTERING
- B33Y70/00—Materials specially adapted for additive manufacturing
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B33—ADDITIVE MANUFACTURING TECHNOLOGY
- B33Y—ADDITIVE MANUFACTURING, i.e. MANUFACTURING OF THREE-DIMENSIONAL [3-D] OBJECTS BY ADDITIVE DEPOSITION, ADDITIVE AGGLOMERATION OR ADDITIVE LAYERING, e.g. BY 3-D PRINTING, STEREOLITHOGRAPHY OR SELECTIVE LASER SINTERING
- B33Y80/00—Products made by additive manufacturing
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Materials Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Emergency Medicine (AREA)
- Mechanical Engineering (AREA)
- Pulmonology (AREA)
- Oncology (AREA)
- Communicable Diseases (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US37844902P | 2002-05-06 | 2002-05-06 | |
| US60/378,449 | 2002-05-06 | ||
| US42509402P | 2002-11-08 | 2002-11-08 | |
| US60/425,094 | 2002-11-08 | ||
| PCT/US2003/014376 WO2003092633A2 (en) | 2002-05-06 | 2003-05-06 | Diffusion-controlled dosage form and method of fabrication including three dimensional printing |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2005524702A JP2005524702A (ja) | 2005-08-18 |
| JP2005524702A5 JP2005524702A5 (enExample) | 2006-06-08 |
| JP5041663B2 true JP5041663B2 (ja) | 2012-10-03 |
Family
ID=29406838
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2004500818A Expired - Lifetime JP5041663B2 (ja) | 2002-05-06 | 2003-05-06 | 拡散支配剤形および三次元印刷を含む作製方法 |
Country Status (9)
| Country | Link |
|---|---|
| US (2) | US8088415B2 (enExample) |
| EP (1) | EP1503741B1 (enExample) |
| JP (1) | JP5041663B2 (enExample) |
| AT (1) | ATE407665T1 (enExample) |
| AU (1) | AU2003232078A1 (enExample) |
| CA (1) | CA2483028C (enExample) |
| DE (1) | DE60323482D1 (enExample) |
| ES (1) | ES2316791T3 (enExample) |
| WO (1) | WO2003092633A2 (enExample) |
Families Citing this family (70)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2483028C (en) | 2002-05-06 | 2011-04-05 | Massachusetts Institute Of Technology | Diffusion-controlled dosage form and method of fabrication including three dimensional printing |
| AU2004314693B2 (en) * | 2003-09-26 | 2011-04-07 | Alza Corporation | Drug coating providing high drug loading and methods for providing the same |
| US20060002594A1 (en) * | 2004-06-09 | 2006-01-05 | Clarke Allan J | Method for producing a pharmaceutical product |
| US8101244B2 (en) * | 2004-06-09 | 2012-01-24 | Smithkline Beecham Corporation | Apparatus and method for producing or processing a product or sample |
| TWI547431B (zh) * | 2004-06-09 | 2016-09-01 | 史密斯克萊美占公司 | 生產藥物之裝置及方法 |
| JPWO2006004069A1 (ja) * | 2004-07-01 | 2008-04-24 | 日本碍子株式会社 | 微小カプセルおよびその製造方法 |
| EP2131817A2 (en) * | 2007-03-09 | 2009-12-16 | Synthon B.V. | Pharmaceutical composition of quetiapine fumarate |
| WO2009085832A2 (en) * | 2007-12-21 | 2009-07-09 | Mcneil-Ppc, Inc. | Manufacture of tablet |
| CN101716162B (zh) * | 2009-11-26 | 2012-11-14 | 扬州中宝制药有限公司 | 一种氨茶碱缓释胶囊及其制备方法 |
| US8251837B2 (en) * | 2010-08-11 | 2012-08-28 | Nike, Inc. | Floating golf ball |
| AU2011342893A1 (en) | 2010-12-13 | 2013-05-02 | Purdue Pharma L.P. | Controlled release dosage forms |
| US9381154B2 (en) * | 2011-06-09 | 2016-07-05 | Xerox Corporation | Direct inkjet fabrication of drug delivery devices |
| CN105666875B (zh) | 2012-09-05 | 2017-09-12 | 阿普雷奇亚制药公司 | 三维打印系统和设备组件 |
| US8888480B2 (en) | 2012-09-05 | 2014-11-18 | Aprecia Pharmaceuticals Company | Three-dimensional printing system and equipment assembly |
| US20140099351A1 (en) * | 2012-10-04 | 2014-04-10 | Axxia Pharmaceuticals, Llc | Process for making controlled release medical implant products |
| US9339489B2 (en) | 2013-03-15 | 2016-05-17 | Aprecia Pharmaceuticals Company | Rapid disperse dosage form containing levetiracetam |
| HK1216513A1 (zh) | 2013-03-15 | 2016-11-18 | 阿普雷奇亚制药有限责任公司 | 包含左乙拉西坦的快速分散劑型 |
| CN105579161A (zh) | 2013-08-16 | 2016-05-11 | 艾克斯温有限责任公司 | 三维打印的金属铸造模具和其制造方法 |
| EP3057729A4 (en) | 2013-10-17 | 2017-10-18 | The Exone Company | Three-dimensional printed hot isostatic pressing containers and processes for making same |
| EP2875933B1 (de) * | 2013-11-25 | 2019-04-03 | MTU Aero Engines GmbH | Dokumentation generativer Herstellungsverfahren |
| MX2016007786A (es) | 2013-12-16 | 2017-03-03 | Massachusetts Inst Technology | Formulaciones de sal de micronutrientes fortificadas. |
| EP3763383A1 (en) | 2013-12-16 | 2021-01-13 | Massachusetts Institute Of Technology | Micromolded or 3-d printed pulsatile release vaccine formulations |
| WO2015100084A1 (en) | 2013-12-23 | 2015-07-02 | The Exone Company | Method of three-dimensional printing using a multi-component build powder |
| WO2015100086A1 (en) | 2013-12-23 | 2015-07-02 | The Exone Company | Methods and systems for three-dimensional printing utilizing multiple binder fluids |
| IL232696B (en) | 2014-05-19 | 2018-08-30 | Technion Res & Dev Foundation | Compound and method for detecting molecules of interest |
| EP3148730A1 (en) | 2014-05-29 | 2017-04-05 | The Exone Company | Process for making nickel-based superalloy articles by three-dimensional printing |
| EP3169499A2 (en) | 2014-07-17 | 2017-05-24 | The Exone Company | Methods and apparatuses for curing three-dimensional printed articles |
| EP3505163B1 (en) * | 2014-09-08 | 2023-01-18 | University of Central Lancashire | Solid dosage form production |
| US9844930B2 (en) | 2014-11-05 | 2017-12-19 | Xerox Corporation | 3D printing of digestible shells for medicaments |
| WO2016089618A1 (en) | 2014-12-03 | 2016-06-09 | The Exone Company | Process for making densified carbon articles by three dimensional printing |
| CN113133980A (zh) * | 2015-06-03 | 2021-07-20 | 南京三迭纪医药科技有限公司 | 药品剂型及其使用 |
| EP4512394A3 (en) | 2015-06-03 | 2025-10-22 | Triastek, Inc. | Dosage forms and use thereof |
| US20180214383A1 (en) * | 2015-07-16 | 2018-08-02 | National University Of Singapore | Printing drug tablets with fully customizable release profiles for personalized medicine |
| CA2996041A1 (en) | 2015-08-21 | 2017-03-02 | Aprecia Pharmaceuticals LLC | Three-dimensional printing system and equipment assembly |
| GB201519128D0 (en) | 2015-10-29 | 2015-12-16 | Univ Central Lancashire | Solid forms and methods of preparing the same |
| AU2017261372B2 (en) | 2016-05-05 | 2019-02-14 | Triastek, Inc. | Controlled release dosage form |
| US12303603B2 (en) * | 2016-06-20 | 2025-05-20 | Sciperio, Inc | Real-time compounding 3D printer |
| US10765658B2 (en) | 2016-06-22 | 2020-09-08 | Mastix LLC | Oral compositions delivering therapeutically effective amounts of cannabinoids |
| GB201612853D0 (en) | 2016-07-25 | 2016-09-07 | Univ Central Lancashire | Solid dosage form production |
| EP3321002A1 (en) * | 2016-11-15 | 2018-05-16 | Höganäs AB | Feedstock for an additive manufacturing method, additive manufacturing method using the same, and article obtained therefrom |
| DE102017200773A1 (de) | 2017-01-18 | 2018-07-19 | Eos Gmbh Electro Optical Systems | Verfahren zum Nachbehandeln und Nachbehandlungssytem |
| CN110290781A (zh) * | 2017-01-26 | 2019-09-27 | 南京三迭纪医药科技有限公司 | 胃肠特定部位控制释放的剂型 |
| US10369557B2 (en) | 2017-04-12 | 2019-08-06 | International Business Machines Corporation | Three-dimensional printed objects for chemical reaction control |
| US20210213678A1 (en) * | 2017-04-28 | 2021-07-15 | Hewlett-Packard Development Company, L.P. | Producing diffusion-controlled release devices |
| WO2019025869A1 (en) * | 2017-07-31 | 2019-02-07 | Teva Pharmaceutical Industries Limited | GALENIC FORMS WITH CONTROLLED RELEASE MANUFACTURED IN ADDITIVE MANNER |
| US11642841B2 (en) | 2017-10-13 | 2023-05-09 | Ut-Battelle, Llc | Indirect additive manufacturing process |
| CN111698983B (zh) | 2018-01-09 | 2022-10-18 | 南京三迭纪医药科技有限公司 | 一种包含固定剂量adhd非兴奋剂和adhd兴奋剂的复方口服药物剂型 |
| US10350822B1 (en) | 2018-01-09 | 2019-07-16 | Triastek Inc. | Dosage forms with desired release profiles and methods of designing and making thereof |
| ES3040411T3 (en) | 2018-03-22 | 2025-10-30 | Incarda Therapeutics Inc | A novel method to slow ventricular rate |
| DE102018107585B3 (de) * | 2018-03-29 | 2019-03-28 | Universität Rostock | Vorrichtung zur Herstellung von 3D-gedruckten Wirkstofffreisetzungssystemen mit Wirkstoffdepots, sowie Verfahren zur Herstellung von 3D-gedruckten Wirkstofffreisetzungssystemen |
| WO2019199328A1 (en) * | 2018-04-13 | 2019-10-17 | Hewlett-Packard Development Company, L.P. | Three-dimensional printing |
| CN108939162B (zh) * | 2018-07-10 | 2021-11-19 | 上海理工大学 | 一种介孔生物玻璃/海藻酸钠-海藻酸钠分层骨组织工程支架的制备方法 |
| GB201813186D0 (en) | 2018-08-13 | 2018-09-26 | Univ Central Lancashire | Solid dosage from production |
| CN112739331A (zh) * | 2018-09-14 | 2021-04-30 | 默克专利股份有限公司 | 制备包衣的固体药物剂型的方法 |
| ES2930199T3 (es) | 2018-10-15 | 2022-12-07 | Aprecia Pharmaceuticals LLC | Método y sistema para formar una forma farmacéutica dentro de un envase |
| US11254617B2 (en) | 2019-01-09 | 2022-02-22 | Ut-Battelle, Llc | Indirect additive manufacturing process using amine-containing adhesive polymers |
| US12103229B2 (en) * | 2019-03-15 | 2024-10-01 | Ricoh Company, Ltd. | Jettable temporary binders to create removable support materials |
| US11724486B2 (en) | 2019-07-09 | 2023-08-15 | Kyndryl, Inc. | Printing customized medication based on current user data and medical records of the user |
| CN114269545A (zh) * | 2019-08-14 | 2022-04-01 | 默克专利股份有限公司 | 产品的增材制造方法、制造装置和固体药物剂型 |
| GB201913972D0 (en) | 2019-09-27 | 2019-11-13 | Univ Ulster | Pharmaceutical Delivery Device and method of manufacture |
| ES2828509B2 (es) | 2019-11-26 | 2022-03-09 | Fund Idonial | Composicion para la impresion 3d de farmacos semisolidos |
| EP3871859A1 (en) * | 2020-02-26 | 2021-09-01 | Hewlett-Packard Development Company, L.P. | Three-dimensional printing |
| WO2021176360A1 (en) * | 2020-03-02 | 2021-09-10 | Craft Health Pte Ltd | Oral dosage forms for extended drug release |
| AU2021270750B2 (en) | 2020-05-13 | 2024-06-13 | Massachusetts Institute Of Technology | Compositions of polymeric microdevices and their use in cancer immunotherapy |
| JP2023533478A (ja) | 2020-06-26 | 2023-08-03 | アプレシア・ファーマスーティカルズ・エルエルシー | 内部空洞を有する迅速口内分散可能な錠剤 |
| KR20230098628A (ko) | 2020-10-30 | 2023-07-04 | 트리아스텍 인코포레이티드 | 위장관 약학적 제형 |
| US12251208B2 (en) | 2020-11-30 | 2025-03-18 | International Business Machines Corporation | Time controlled medication |
| KR102723494B1 (ko) * | 2020-12-24 | 2024-10-31 | 동아제약 주식회사 | 적층 방식으로 제조된 방출 조절용 약제학적 조성물 |
| CN112940690B (zh) * | 2021-02-05 | 2021-11-02 | 中国科学院苏州纳米技术与纳米仿生研究所南昌研究院 | 一种具有豆荚结构的相变储能材料及其制备方法与应用 |
| EP4618958A1 (en) * | 2022-11-18 | 2025-09-24 | Aprecia Pharmaceuticals LLC | Functionally-dividable orodispersive dosage form |
Family Cites Families (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US948297A (en) * | 1909-07-24 | 1910-02-01 | John A Stone | Corn-husker. |
| US986148A (en) * | 1910-05-09 | 1911-03-07 | Hoechst Ag | Derivative of oxyarylarsinic acids and process of making same. |
| ZA822995B (en) * | 1981-05-21 | 1983-12-28 | Wyeth John & Brother Ltd | Slow release pharmaceutical composition |
| WO1985003436A1 (en) * | 1984-02-10 | 1985-08-15 | A/S Alfred Benzon | Diffusion coated multiple-units dosage form |
| US4810502A (en) * | 1987-02-27 | 1989-03-07 | Alza Corporation | Pseudoephedrine brompheniramine therapy |
| US5133974A (en) * | 1989-05-05 | 1992-07-28 | Kv Pharmaceutical Company | Extended release pharmaceutical formulations |
| US5204055A (en) | 1989-12-08 | 1993-04-20 | Massachusetts Institute Of Technology | Three-dimensional printing techniques |
| NZ242065A (en) * | 1991-03-26 | 1996-06-25 | Csl Ltd | Delayed release implant having a degradable or rupturable polymeric coating |
| US5775402A (en) | 1995-10-31 | 1998-07-07 | Massachusetts Institute Of Technology | Enhancement of thermal properties of tooling made by solid free form fabrication techniques |
| US5490882A (en) | 1992-11-30 | 1996-02-13 | Massachusetts Institute Of Technology | Process for removing loose powder particles from interior passages of a body |
| US6280771B1 (en) | 1997-02-20 | 2001-08-28 | Therics, Inc. | Dosage forms exhibiting multi-phasic release kinetics and methods of manufacture thereof |
| US5490962A (en) | 1993-10-18 | 1996-02-13 | Massachusetts Institute Of Technology | Preparation of medical devices by solid free-form fabrication methods |
| ATE292456T1 (de) * | 1996-09-13 | 2005-04-15 | Shionogi & Co | Zubereitung mit verlängerter freisetzung unter verwendung einer thermischen umwandlung sowie verfahren zu deren herstellung |
| DE69840495D1 (de) | 1997-02-20 | 2009-03-12 | Massachusetts Inst Technology | Darreichungsform, welche rasche dispersionseigenschaften entfaltet, anwendungsverfahren sowie verfahren zur herstellung derselben |
| WO1998043762A2 (en) | 1997-03-31 | 1998-10-08 | Therics, Inc. | Method for dispensing of powders |
| US6213168B1 (en) | 1997-03-31 | 2001-04-10 | Therics, Inc. | Apparatus and method for dispensing of powders |
| US6547994B1 (en) | 1998-11-13 | 2003-04-15 | Therics, Inc. | Rapid prototyping and manufacturing process |
| US6485746B1 (en) * | 2000-08-25 | 2002-11-26 | Neurocrine Biosciences, Inc. | Controlled-release sedative-hypnotic compositions and methods related thereto |
| DE19961897A1 (de) * | 1999-12-20 | 2001-06-28 | Basf Ag | Verwendung eines Filmüberzuges als geschmacksmaskierendes Coating von pharmazeutischen Darreichungsformen |
| DE10015479A1 (de) * | 2000-03-29 | 2001-10-11 | Basf Ag | Feste orale Darreichungsformen mit retardierter Wirkstofffreisetzung und hoher mechanischer Stabilität |
| WO2001087272A2 (en) | 2000-05-18 | 2001-11-22 | Therics, Inc. | Encapsulating a toxic core within a non-toxic region in an oral dosage form |
| WO2002038280A2 (en) * | 2000-11-10 | 2002-05-16 | Therics, Inc. | A wetting-resistant nozzle for dispensing small volumes of liquid and a method for manufacturing a wetting-resistant nozzle |
| CA2483028C (en) | 2002-05-06 | 2011-04-05 | Massachusetts Institute Of Technology | Diffusion-controlled dosage form and method of fabrication including three dimensional printing |
| US20050015728A1 (en) * | 2003-07-17 | 2005-01-20 | International Business Machines Corporation | Method, system, and program product for customizing a user interface |
-
2003
- 2003-05-06 CA CA2483028A patent/CA2483028C/en not_active Expired - Lifetime
- 2003-05-06 AT AT03747680T patent/ATE407665T1/de not_active IP Right Cessation
- 2003-05-06 US US10/431,353 patent/US8088415B2/en active Active
- 2003-05-06 ES ES03747680T patent/ES2316791T3/es not_active Expired - Lifetime
- 2003-05-06 AU AU2003232078A patent/AU2003232078A1/en not_active Abandoned
- 2003-05-06 WO PCT/US2003/014376 patent/WO2003092633A2/en not_active Ceased
- 2003-05-06 JP JP2004500818A patent/JP5041663B2/ja not_active Expired - Lifetime
- 2003-05-06 DE DE60323482T patent/DE60323482D1/de not_active Expired - Lifetime
- 2003-05-06 EP EP03747680A patent/EP1503741B1/en not_active Expired - Lifetime
-
2011
- 2011-11-29 US US13/306,393 patent/US8465777B2/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| DE60323482D1 (de) | 2008-10-23 |
| US8465777B2 (en) | 2013-06-18 |
| ES2316791T3 (es) | 2009-04-16 |
| ATE407665T1 (de) | 2008-09-15 |
| WO2003092633A3 (en) | 2004-04-08 |
| EP1503741B1 (en) | 2008-09-10 |
| CA2483028A1 (en) | 2003-11-13 |
| US20120177696A1 (en) | 2012-07-12 |
| EP1503741A2 (en) | 2005-02-09 |
| CA2483028C (en) | 2011-04-05 |
| US20040005360A1 (en) | 2004-01-08 |
| JP2005524702A (ja) | 2005-08-18 |
| US8088415B2 (en) | 2012-01-03 |
| AU2003232078A1 (en) | 2003-11-17 |
| WO2003092633A2 (en) | 2003-11-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5041663B2 (ja) | 拡散支配剤形および三次元印刷を含む作製方法 | |
| JP4533531B2 (ja) | 制御放出組成物 | |
| JP2701977B2 (ja) | 疎水性媒体を含有する投薬体 | |
| AU2001297011B2 (en) | Methylphenidate modified release formulations | |
| JP6457458B2 (ja) | 時限パルス放出システム | |
| JP2637981B2 (ja) | 吸収制御薬剤組成物 | |
| AU2002320360B2 (en) | Drug delivery system for zero order, zero order-biphasic, ascending or descending drug delivery | |
| EP1267842B1 (en) | Method for producing a controlled-release tablet using a pore forming agent in the coating | |
| HRP960554A2 (en) | Pharmaceutical formulations | |
| KR20040045034A (ko) | 변형된 방출형 제형 | |
| AU2001297011A1 (en) | Methylphenidate modified release formulations | |
| EP2910243A1 (en) | Pharmaceutic osmotic pump preparation | |
| CN1056057C (zh) | 口服延缓释放的曲美他嗪药物组合物 | |
| KR20110025654A (ko) | 제어 방출 골격근 이완제 투약 형태의 제조 | |
| US7858119B1 (en) | Extended release pharmaceuticals | |
| EP0365947A1 (en) | Novel dosage form | |
| WO2007117110A2 (en) | Sustained-release pellets containing tamsulosin hydrochloride and processes for preparing the same | |
| JP2005533084A (ja) | 液体の有効成分の製剤を含んでなりかつ膨張可能な浸透圧組成物によりその放出を制御する経口の投薬形態 | |
| JPH06190267A (ja) | マイクロカプセル | |
| KR20060016382A (ko) | 록사티딘 아세테이트 다층 코팅과립을 이용한약물전달시스템 | |
| Ramadan | Using Ethylcellulose for the Development of 3D Printed Single Layer and Multilayer Controlled-Release Tablets | |
| JP2007500558A (ja) | 液状活性製剤の制御型放出のための透過抵抗性浸透エンジンおよび投薬剤形物 | |
| HK1078002A (en) | A controlled-release preparation having specific pore forming agents in the coating | |
| IE62640B1 (en) | Novel dosage form | |
| HK1082418A (en) | Oral dosage from comprising a liquid active agent formulation and controlling release thereof by an expandable osmotic composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20060417 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20060417 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20091112 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20091218 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20091228 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100512 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110322 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110621 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110628 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110721 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110728 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110819 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110826 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110921 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20120210 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120528 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20120618 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20120705 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20120710 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5041663 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20150720 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| EXPY | Cancellation because of completion of term |