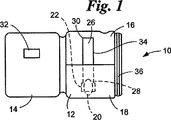
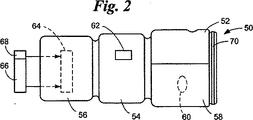
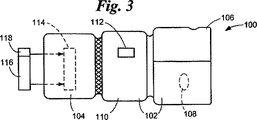
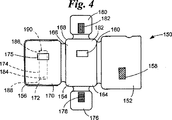
JP4104669B2 - 取り外し可能要素検定装置 - Google Patents
取り外し可能要素検定装置 Download PDFInfo
- Publication number
- JP4104669B2 JP4104669B2 JP54809498A JP54809498A JP4104669B2 JP 4104669 B2 JP4104669 B2 JP 4104669B2 JP 54809498 A JP54809498 A JP 54809498A JP 54809498 A JP54809498 A JP 54809498A JP 4104669 B2 JP4104669 B2 JP 4104669B2
- Authority
- JP
- Japan
- Prior art keywords
- test strip
- sample
- module
- analyte
- reagent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000012360 testing method Methods 0.000 claims abstract description 461
- 238000003556 assay Methods 0.000 claims abstract description 213
- 239000012491 analyte Substances 0.000 claims abstract description 169
- 238000001514 detection method Methods 0.000 claims abstract description 47
- 239000012530 fluid Substances 0.000 claims abstract description 32
- 238000003860 storage Methods 0.000 claims abstract description 8
- 239000003153 chemical reaction reagent Substances 0.000 claims description 171
- 238000006243 chemical reaction Methods 0.000 claims description 152
- 230000009870 specific binding Effects 0.000 claims description 89
- 239000012501 chromatography medium Substances 0.000 claims description 67
- 229940088598 enzyme Drugs 0.000 claims description 36
- 102000004190 Enzymes Human genes 0.000 claims description 34
- 108090000790 Enzymes Proteins 0.000 claims description 34
- 238000000605 extraction Methods 0.000 claims description 31
- 238000000034 method Methods 0.000 claims description 30
- 239000007788 liquid Substances 0.000 claims description 27
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical group [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 claims description 17
- 238000009739 binding Methods 0.000 claims description 15
- 230000027455 binding Effects 0.000 claims description 14
- AZQWKYJCGOJGHM-UHFFFAOYSA-N 1,4-benzoquinone Chemical compound O=C1C=CC(=O)C=C1 AZQWKYJCGOJGHM-UHFFFAOYSA-N 0.000 claims description 12
- 230000008878 coupling Effects 0.000 claims description 12
- 238000010168 coupling process Methods 0.000 claims description 12
- 238000005859 coupling reaction Methods 0.000 claims description 12
- 239000010931 gold Substances 0.000 claims description 12
- 229910052737 gold Inorganic materials 0.000 claims description 11
- 238000003780 insertion Methods 0.000 claims description 11
- 230000037431 insertion Effects 0.000 claims description 11
- IOVCWXUNBOPUCH-UHFFFAOYSA-N Nitrous acid Chemical group ON=O IOVCWXUNBOPUCH-UHFFFAOYSA-N 0.000 claims description 10
- 239000000872 buffer Substances 0.000 claims description 8
- 239000000758 substrate Substances 0.000 claims description 8
- 102000002260 Alkaline Phosphatase Human genes 0.000 claims description 7
- 108020004774 Alkaline Phosphatase Proteins 0.000 claims description 7
- 108010015776 Glucose oxidase Proteins 0.000 claims description 6
- 239000004366 Glucose oxidase Substances 0.000 claims description 6
- 108010001336 Horseradish Peroxidase Proteins 0.000 claims description 6
- 229940116332 glucose oxidase Drugs 0.000 claims description 6
- 235000019420 glucose oxidase Nutrition 0.000 claims description 6
- GGCZERPQGJTIQP-UHFFFAOYSA-N sodium;9,10-dioxoanthracene-2-sulfonic acid Chemical compound [Na+].C1=CC=C2C(=O)C3=CC(S(=O)(=O)O)=CC=C3C(=O)C2=C1 GGCZERPQGJTIQP-UHFFFAOYSA-N 0.000 claims description 6
- 230000008021 deposition Effects 0.000 claims description 5
- 230000004044 response Effects 0.000 claims description 5
- 238000012795 verification Methods 0.000 claims description 5
- 102000005936 beta-Galactosidase Human genes 0.000 claims description 4
- 108010005774 beta-Galactosidase Proteins 0.000 claims description 4
- 239000002609 medium Substances 0.000 claims description 4
- 239000012790 adhesive layer Substances 0.000 claims 9
- 230000003321 amplification Effects 0.000 claims 8
- 238000003199 nucleic acid amplification method Methods 0.000 claims 8
- 239000000284 extract Substances 0.000 claims 4
- 150000003839 salts Chemical class 0.000 claims 4
- 238000011144 upstream manufacturing Methods 0.000 claims 3
- 238000003795 desorption Methods 0.000 claims 1
- 238000003149 assay kit Methods 0.000 abstract description 6
- 238000004519 manufacturing process Methods 0.000 abstract description 5
- 238000010998 test method Methods 0.000 abstract description 5
- 239000000523 sample Substances 0.000 description 130
- 238000002360 preparation method Methods 0.000 description 31
- 239000000427 antigen Substances 0.000 description 28
- 102000036639 antigens Human genes 0.000 description 28
- 108091007433 antigens Proteins 0.000 description 28
- 238000003018 immunoassay Methods 0.000 description 18
- 239000000853 adhesive Substances 0.000 description 16
- 230000001070 adhesive effect Effects 0.000 description 16
- 238000004587 chromatography analysis Methods 0.000 description 13
- 239000000463 material Substances 0.000 description 13
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 12
- 239000008280 blood Substances 0.000 description 9
- 210000004369 blood Anatomy 0.000 description 9
- LPXPTNMVRIOKMN-UHFFFAOYSA-M sodium nitrite Chemical compound [Na+].[O-]N=O LPXPTNMVRIOKMN-UHFFFAOYSA-M 0.000 description 8
- 239000003054 catalyst Substances 0.000 description 7
- 238000002967 competitive immunoassay Methods 0.000 description 7
- 239000004033 plastic Substances 0.000 description 6
- 230000008569 process Effects 0.000 description 6
- 241000224466 Giardia Species 0.000 description 5
- 241000590002 Helicobacter pylori Species 0.000 description 5
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 5
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 5
- 230000001580 bacterial effect Effects 0.000 description 5
- 229940037467 helicobacter pylori Drugs 0.000 description 5
- 239000002184 metal Substances 0.000 description 5
- 229910052751 metal Inorganic materials 0.000 description 5
- 238000004806 packaging method and process Methods 0.000 description 5
- 229910052709 silver Inorganic materials 0.000 description 5
- 239000004332 silver Substances 0.000 description 5
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 4
- 241000725303 Human immunodeficiency virus Species 0.000 description 4
- 230000003416 augmentation Effects 0.000 description 4
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 4
- 238000007813 chromatographic assay Methods 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- 208000006454 hepatitis Diseases 0.000 description 4
- 231100000283 hepatitis Toxicity 0.000 description 4
- 238000003317 immunochromatography Methods 0.000 description 4
- 102000039446 nucleic acids Human genes 0.000 description 4
- 150000007523 nucleic acids Chemical class 0.000 description 4
- 108020004707 nucleic acids Proteins 0.000 description 4
- 239000000123 paper Substances 0.000 description 4
- 238000012545 processing Methods 0.000 description 4
- 235000010288 sodium nitrite Nutrition 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- 102000001554 Hemoglobins Human genes 0.000 description 3
- 108010054147 Hemoglobins Proteins 0.000 description 3
- 241001505901 Streptococcus sp. 'group A' Species 0.000 description 3
- 241000700605 Viruses Species 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 230000004888 barrier function Effects 0.000 description 3
- 229920002678 cellulose Polymers 0.000 description 3
- 239000001913 cellulose Substances 0.000 description 3
- 239000002274 desiccant Substances 0.000 description 3
- 239000003814 drug Substances 0.000 description 3
- 230000006870 function Effects 0.000 description 3
- 238000011534 incubation Methods 0.000 description 3
- 239000013618 particulate matter Substances 0.000 description 3
- 230000000405 serological effect Effects 0.000 description 3
- 241000894007 species Species 0.000 description 3
- 238000012546 transfer Methods 0.000 description 3
- 208000030507 AIDS Diseases 0.000 description 2
- 108090001008 Avidin Proteins 0.000 description 2
- 102000011022 Chorionic Gonadotropin Human genes 0.000 description 2
- 108010062540 Chorionic Gonadotropin Proteins 0.000 description 2
- QIGBRXMKCJKVMJ-UHFFFAOYSA-N Hydroquinone Chemical compound OC1=CC=C(O)C=C1 QIGBRXMKCJKVMJ-UHFFFAOYSA-N 0.000 description 2
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 2
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- 239000004677 Nylon Substances 0.000 description 2
- 229920000297 Rayon Polymers 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 239000002250 absorbent Substances 0.000 description 2
- 230000002745 absorbent Effects 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- 230000003190 augmentative effect Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- 239000011111 cardboard Substances 0.000 description 2
- 238000006555 catalytic reaction Methods 0.000 description 2
- 210000004027 cell Anatomy 0.000 description 2
- 210000002421 cell wall Anatomy 0.000 description 2
- 239000013522 chelant Substances 0.000 description 2
- -1 cholesterol Chemical class 0.000 description 2
- 235000012000 cholesterol Nutrition 0.000 description 2
- ZPUCINDJVBIVPJ-LJISPDSOSA-N cocaine Chemical compound O([C@H]1C[C@@H]2CC[C@@H](N2C)[C@H]1C(=O)OC)C(=O)C1=CC=CC=C1 ZPUCINDJVBIVPJ-LJISPDSOSA-N 0.000 description 2
- 239000005515 coenzyme Substances 0.000 description 2
- 238000012875 competitive assay Methods 0.000 description 2
- 230000002860 competitive effect Effects 0.000 description 2
- 238000011109 contamination Methods 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000035475 disorder Diseases 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 230000002550 fecal effect Effects 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 108091008039 hormone receptors Proteins 0.000 description 2
- 229940084986 human chorionic gonadotropin Drugs 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 230000002452 interceptive effect Effects 0.000 description 2
- 238000002372 labelling Methods 0.000 description 2
- 239000004816 latex Substances 0.000 description 2
- 229920000126 latex Polymers 0.000 description 2
- 239000002523 lectin Substances 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 239000011859 microparticle Substances 0.000 description 2
- 238000012544 monitoring process Methods 0.000 description 2
- 229920001778 nylon Polymers 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 244000052769 pathogen Species 0.000 description 2
- 230000001717 pathogenic effect Effects 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 239000002964 rayon Substances 0.000 description 2
- 238000007789 sealing Methods 0.000 description 2
- 239000000377 silicon dioxide Substances 0.000 description 2
- 239000008279 sol Substances 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 230000003612 virological effect Effects 0.000 description 2
- 239000002699 waste material Substances 0.000 description 2
- 102000004625 Aspartate Aminotransferases Human genes 0.000 description 1
- 108010003415 Aspartate Aminotransferases Proteins 0.000 description 1
- 101000950981 Bacillus subtilis (strain 168) Catabolic NAD-specific glutamate dehydrogenase RocG Proteins 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 229910000906 Bronze Inorganic materials 0.000 description 1
- 244000025254 Cannabis sativa Species 0.000 description 1
- 235000012766 Cannabis sativa ssp. sativa var. sativa Nutrition 0.000 description 1
- 235000012765 Cannabis sativa ssp. sativa var. spontanea Nutrition 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 206010012735 Diarrhoea Diseases 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 208000018522 Gastrointestinal disease Diseases 0.000 description 1
- 102000016901 Glutamate dehydrogenase Human genes 0.000 description 1
- GVGLGOZIDCSQPN-PVHGPHFFSA-N Heroin Chemical compound O([C@H]1[C@H](C=C[C@H]23)OC(C)=O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4OC(C)=O GVGLGOZIDCSQPN-PVHGPHFFSA-N 0.000 description 1
- 102000004157 Hydrolases Human genes 0.000 description 1
- 108090000604 Hydrolases Proteins 0.000 description 1
- 108060003951 Immunoglobulin Proteins 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 208000005016 Intestinal Neoplasms Diseases 0.000 description 1
- 102000003855 L-lactate dehydrogenase Human genes 0.000 description 1
- 108700023483 L-lactate dehydrogenases Proteins 0.000 description 1
- 229920004142 LEXAN™ Polymers 0.000 description 1
- 102000004856 Lectins Human genes 0.000 description 1
- 108090001090 Lectins Proteins 0.000 description 1
- 239000004418 Lexan Substances 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- 206010036790 Productive cough Diseases 0.000 description 1
- 241000194017 Streptococcus Species 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 1
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 1
- 108070000030 Viral receptors Proteins 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 229940125681 anticonvulsant agent Drugs 0.000 description 1
- 239000001961 anticonvulsive agent Substances 0.000 description 1
- 230000000890 antigenic effect Effects 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 1
- 244000052616 bacterial pathogen Species 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 102000023732 binding proteins Human genes 0.000 description 1
- 239000012472 biological sample Substances 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000010974 bronze Substances 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 229960003920 cocaine Drugs 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 239000004020 conductor Substances 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- KUNSUQLRTQLHQQ-UHFFFAOYSA-N copper tin Chemical compound [Cu].[Sn] KUNSUQLRTQLHQQ-UHFFFAOYSA-N 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 229960002069 diamorphine Drugs 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 239000003344 environmental pollutant Substances 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 239000003365 glass fiber Substances 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 230000003301 hydrolyzing effect Effects 0.000 description 1
- 230000003100 immobilizing effect Effects 0.000 description 1
- 238000003365 immunocytochemistry Methods 0.000 description 1
- 102000018358 immunoglobulin Human genes 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 239000002917 insecticide Substances 0.000 description 1
- 201000002313 intestinal cancer Diseases 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 238000005304 joining Methods 0.000 description 1
- 238000012092 latex agglutination test Methods 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 238000011326 mechanical measurement Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 229960001252 methamphetamine Drugs 0.000 description 1
- MYWUZJCMWCOHBA-VIFPVBQESA-N methamphetamine Chemical compound CN[C@@H](C)CC1=CC=CC=C1 MYWUZJCMWCOHBA-VIFPVBQESA-N 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 239000002808 molecular sieve Substances 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 239000005022 packaging material Substances 0.000 description 1
- 239000011087 paperboard Substances 0.000 description 1
- 230000003071 parasitic effect Effects 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 102000054765 polymorphisms of proteins Human genes 0.000 description 1
- 238000012802 pre-warming Methods 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 244000079416 protozoan pathogen Species 0.000 description 1
- 238000004451 qualitative analysis Methods 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 206010039073 rheumatoid arthritis Diseases 0.000 description 1
- 239000012266 salt solution Substances 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 230000001953 sensory effect Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000000741 silica gel Substances 0.000 description 1
- 229910002027 silica gel Inorganic materials 0.000 description 1
- 150000003378 silver Chemical class 0.000 description 1
- LMEWRZSPCQHBOB-UHFFFAOYSA-M silver;2-hydroxypropanoate Chemical group [Ag+].CC(O)C([O-])=O LMEWRZSPCQHBOB-UHFFFAOYSA-M 0.000 description 1
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000004611 spectroscopical analysis Methods 0.000 description 1
- 210000003802 sputum Anatomy 0.000 description 1
- 208000024794 sputum Diseases 0.000 description 1
- 229920002994 synthetic fiber Polymers 0.000 description 1
- 239000012209 synthetic fiber Substances 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 238000004809 thin layer chromatography Methods 0.000 description 1
- 239000011135 tin Substances 0.000 description 1
- 229910052718 tin Inorganic materials 0.000 description 1
- 230000000451 tissue damage Effects 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 231100000167 toxic agent Toxicity 0.000 description 1
- 239000003440 toxic substance Substances 0.000 description 1
- 239000003204 tranquilizing agent Substances 0.000 description 1
- 230000002936 tranquilizing effect Effects 0.000 description 1
- 244000052613 viral pathogen Species 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 238000010792 warming Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G01N33/54366—Apparatus specially adapted for solid-phase testing
- G01N33/54386—Analytical elements
- G01N33/54387—Immunochromatographic test strips
- G01N33/54388—Immunochromatographic test strips based on lateral flow
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S435/00—Chemistry: molecular biology and microbiology
- Y10S435/97—Test strip or test slide
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S435/00—Chemistry: molecular biology and microbiology
- Y10S435/975—Kit
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S436/00—Chemistry: analytical and immunological testing
- Y10S436/807—Apparatus included in process claim, e.g. physical support structures
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S436/00—Chemistry: analytical and immunological testing
- Y10S436/807—Apparatus included in process claim, e.g. physical support structures
- Y10S436/808—Automated or kit
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S436/00—Chemistry: analytical and immunological testing
- Y10S436/825—Pretreatment for removal of interfering factors from sample
Landscapes
- Health & Medical Sciences (AREA)
- Immunology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- Chemical & Material Sciences (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Food Science & Technology (AREA)
- Biochemistry (AREA)
- Cell Biology (AREA)
- Biotechnology (AREA)
- Medicinal Chemistry (AREA)
- Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Microbiology (AREA)
- General Health & Medical Sciences (AREA)
- General Physics & Mathematics (AREA)
- Pathology (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Sampling And Sample Adjustment (AREA)
- Measurement Of The Respiration, Hearing Ability, Form, And Blood Characteristics Of Living Organisms (AREA)
- Investigating Or Analysing Materials By Optical Means (AREA)
- Automatic Analysis And Handling Materials Therefor (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US08/853,760 US5939252A (en) | 1997-05-09 | 1997-05-09 | Detachable-element assay device |
| US08/853,760 | 1997-05-09 | ||
| PCT/US1998/007599 WO1998050778A1 (en) | 1997-05-09 | 1998-04-15 | Detachable-element assay device |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2002503335A JP2002503335A (ja) | 2002-01-29 |
| JP2002503335A5 JP2002503335A5 (enExample) | 2006-01-05 |
| JP4104669B2 true JP4104669B2 (ja) | 2008-06-18 |
Family
ID=25316824
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP54809498A Expired - Fee Related JP4104669B2 (ja) | 1997-05-09 | 1998-04-15 | 取り外し可能要素検定装置 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US5939252A (enExample) |
| EP (1) | EP0980517B1 (enExample) |
| JP (1) | JP4104669B2 (enExample) |
| AT (1) | ATE354082T1 (enExample) |
| AU (1) | AU730243B2 (enExample) |
| CA (1) | CA2286680C (enExample) |
| DE (1) | DE69837087T2 (enExample) |
| WO (1) | WO1998050778A1 (enExample) |
Families Citing this family (63)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TW356712U (en) * | 1998-03-04 | 1999-04-21 | Yung-Shiang Liou | Testing apparatus of immune |
| AUPP915799A0 (en) * | 1999-03-11 | 1999-04-15 | Enterix Inc. | Sample collection and testing system |
| DE10002819B4 (de) * | 2000-01-24 | 2004-07-08 | Sension, Biologische Detektions- Und Schnelltestsysteme Gmbh | Nachweissystem zur Prüfung der Originalität eines Objekts und Testvorrichtung zur Durchführung der Prüfung |
| US6316205B1 (en) | 2000-01-28 | 2001-11-13 | Genelabs Diagnostics Pte Ltd. | Assay devices and methods of analyte detection |
| US6607922B2 (en) | 2000-03-17 | 2003-08-19 | Quantum Design, Inc. | Immunochromatographic assay method and apparatus |
| JP3680090B2 (ja) * | 2000-05-25 | 2005-08-10 | 株式会社ニチレイ | 分析装置 |
| USD468437S1 (en) | 2000-11-21 | 2003-01-07 | Acon Laboratories, Inc. | Test platform |
| US7039033B2 (en) * | 2001-05-07 | 2006-05-02 | Ixi Mobile (Israel) Ltd. | System, device and computer readable medium for providing a managed wireless network using short-range radio signals |
| US7300633B2 (en) | 2001-07-25 | 2007-11-27 | Oakville Hong Kong Company Limited | Specimen collection container |
| US7270959B2 (en) | 2001-07-25 | 2007-09-18 | Oakville Hong Kong Company Limited | Specimen collection container |
| GB0123151D0 (en) * | 2001-09-26 | 2001-11-21 | Biotrace Ltd | Device for use in monitoring swab technique |
| US7560272B2 (en) | 2003-01-04 | 2009-07-14 | Inverness Medical Switzerland Gmbh | Specimen collection and assay container |
| CN1756834B (zh) | 2003-02-24 | 2010-05-26 | 比南股份有限公司 | 干式化学、侧向流动重建流的层析酶驱动测定 |
| US7300802B2 (en) * | 2003-04-25 | 2007-11-27 | Biodigit Laboratories Corp. | Membrane strip biosensor system for point-of-care testing |
| US7517495B2 (en) | 2003-08-25 | 2009-04-14 | Inverness Medical Switzerland Gmbh | Biological specimen collection and analysis system |
| CA2834041C (en) * | 2003-12-31 | 2017-05-16 | President And Fellows Of Harvard College | Assay device and method |
| SE0400191D0 (sv) * | 2004-01-30 | 2004-01-30 | Tendera Ab | A test kit for detecting periodontal disease |
| US7488450B2 (en) * | 2004-03-04 | 2009-02-10 | Beckman Coulter, Inc. | Analyte collection and detection devices |
| US7297530B2 (en) | 2004-04-01 | 2007-11-20 | Biotrace Limited | Device for use in monitoring a swab technique |
| US20050272112A1 (en) * | 2004-04-28 | 2005-12-08 | Imi International Medical Innovations, Inc. | Direct assay of cholesterol in skin removed by tape stripping |
| US8679420B2 (en) * | 2004-07-22 | 2014-03-25 | Immunostics, Inc. | Fecal sampling device and method |
| US20060110285A1 (en) * | 2004-10-06 | 2006-05-25 | Piasio Roger N | Sample receiving device and methods of use thereof |
| US7396689B2 (en) * | 2005-02-04 | 2008-07-08 | Decision Biomarkers Incorporated | Method of adjusting the working range of a multi-analyte assay |
| US8614101B2 (en) * | 2008-05-20 | 2013-12-24 | Rapid Pathogen Screening, Inc. | In situ lysis of cells in lateral flow immunoassays |
| US7189522B2 (en) | 2005-03-11 | 2007-03-13 | Chembio Diagnostic Systems, Inc. | Dual path immunoassay device |
| WO2006098804A2 (en) | 2005-03-11 | 2006-09-21 | Chembio Diagnostic Systems, Inc. | Dual path immunoassay device |
| GB2435510A (en) * | 2006-02-23 | 2007-08-29 | Mologic Ltd | Enzyme detection product and methods |
| GB2435511A (en) | 2006-02-23 | 2007-08-29 | Mologic Ltd | Protease detection |
| GB2435512A (en) | 2006-02-23 | 2007-08-29 | Mologic Ltd | A binding assay and assay device |
| GB2435927A (en) * | 2006-03-03 | 2007-09-12 | Ethicon Inc | Sample collection and testing device with pivot arm |
| US7976792B2 (en) * | 2007-05-30 | 2011-07-12 | Ally Shivji | Biological specimen collection card |
| JP4865664B2 (ja) * | 2007-09-28 | 2012-02-01 | 富士フイルム株式会社 | 多孔性担体内で2以上の液を混合する方法 |
| WO2009091501A1 (en) * | 2008-01-18 | 2009-07-23 | Response Biomedical Corporation | Heated assays for influenza |
| US8962260B2 (en) | 2008-05-20 | 2015-02-24 | Rapid Pathogen Screening, Inc. | Method and device for combined detection of viral and bacterial infections |
| US20130196310A1 (en) | 2008-05-20 | 2013-08-01 | Rapid Pathogen Screening, Inc. | Method and Device for Combined Detection of Viral and Bacterial Infections |
| US9797898B2 (en) | 2008-05-20 | 2017-10-24 | Rapid Pathogen Screening, Inc. | Methods and devices for using mucolytic agents including N-acetyl cysteine (NAC) |
| US8609433B2 (en) * | 2009-12-04 | 2013-12-17 | Rapid Pathogen Screening, Inc. | Multiplanar lateral flow assay with sample compressor |
| US8815609B2 (en) | 2008-05-20 | 2014-08-26 | Rapid Pathogen Screening, Inc. | Multiplanar lateral flow assay with diverting zone |
| JP5117928B2 (ja) | 2008-05-27 | 2013-01-16 | 富士フイルム株式会社 | イムノクロマトグラフデバイス |
| US20100022916A1 (en) | 2008-07-24 | 2010-01-28 | Javanbakhsh Esfandiari | Method and Apparatus for Collecting and Preparing Biological Samples for Testing |
| KR101027036B1 (ko) * | 2009-05-28 | 2011-04-11 | 주식회사 인포피아 | 금 이온의 환원에 의한 측방유동 분석에서의 신호 증폭 방법 및 이를 이용한 측방유동 분석 디바이스 |
| GB2470962A (en) * | 2009-06-12 | 2010-12-15 | Dna Supernova Ltd | Amplification medium for use with a solid-phase substrate |
| EP2496941B1 (en) | 2009-11-04 | 2016-07-13 | Thomas M. Buchanan | Methods and devices to enhance sensitivity and evaluate sample adequacy and reagent reactivity in rapid lateral flow immunoassays |
| US9877672B2 (en) | 2010-01-28 | 2018-01-30 | Ellume Pty Ltd | Sampling and testing device for the human or animal body |
| US8956859B1 (en) | 2010-08-13 | 2015-02-17 | Aviex Technologies Llc | Compositions and methods for determining successful immunization by one or more vaccines |
| JP2012177677A (ja) * | 2011-02-02 | 2012-09-13 | Arkray Inc | 分析装置 |
| US8603835B2 (en) | 2011-02-10 | 2013-12-10 | Chembio Diagnostic Systems, Inc. | Reduced step dual path immunoassay device and method |
| US10890590B2 (en) | 2012-09-27 | 2021-01-12 | Ellume Limited | Diagnostic devices and methods |
| US9618428B2 (en) | 2012-11-30 | 2017-04-11 | Ge Healthcare Uk Limited | Biometric device and means for electronic storage and retrieval of biometric data |
| CN103149331B (zh) * | 2013-03-01 | 2015-12-23 | 陈德鑫 | 一种杂草调查测报器 |
| CN105377433A (zh) * | 2013-05-14 | 2016-03-02 | 菲帛罗缇埃克斯有限公司 | 侧流测定装置 |
| BR112016022829B1 (pt) | 2014-04-02 | 2022-03-03 | Chembio Diagnostic Systems, Inc | Dispositivo de teste para determinar a presença de um primeiro ligante em uma amostra líquida, e método para testar uma amostra quanto à presença de um primeiro ligante |
| JP6294155B2 (ja) * | 2014-05-20 | 2018-03-14 | 株式会社ダスキン | アレルゲン検査装置 |
| US20160116466A1 (en) | 2014-10-27 | 2016-04-28 | Chembio Diagnostic Systems, Inc. | Rapid Screening Assay for Qualitative Detection of Multiple Febrile Illnesses |
| US10786229B2 (en) | 2015-01-22 | 2020-09-29 | Ellume Limited | Diagnostic devices and methods for mitigating hook effect and use thereof |
| US10808287B2 (en) | 2015-10-23 | 2020-10-20 | Rapid Pathogen Screening, Inc. | Methods and devices for accurate diagnosis of infections |
| WO2017184665A1 (en) * | 2016-04-19 | 2017-10-26 | Purdue Research Foundation | Temperature controlled valves for paper-based microfluidic systems |
| US20180024073A1 (en) * | 2016-07-21 | 2018-01-25 | Ronald Schornstein | Reach-extended test strip |
| WO2018128749A1 (en) * | 2017-01-06 | 2018-07-12 | Dnt Scientific Research, Llc | Rapid diagnostic test device by driven flow technology |
| WO2024026498A2 (en) * | 2022-07-28 | 2024-02-01 | University Of Houston System | Sample collection apparatus and methods of use |
| CN115980347A (zh) * | 2023-02-09 | 2023-04-18 | 深圳市绿诗源生物技术有限公司 | 用于检测猪轮状病毒的试剂盒及检测方法 |
| GB2630612A (en) * | 2023-05-31 | 2024-12-04 | Eclateral Ltd | Lateral flow test systems and methods |
| CN116858780B (zh) * | 2023-09-05 | 2024-01-09 | 哈尔滨商业大学 | 折叠式检测试纸固定结构及含有该结构的试剂盒 |
Family Cites Families (306)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US35306A (en) * | 1862-05-20 | Improvement in combined cultivator and seeding-machine | ||
| US31006A (en) * | 1861-01-01 | Feeding mechanism for spoke-machines | ||
| US30267A (en) * | 1860-10-02 | Bed-bottom | ||
| NL286850A (enExample) * | 1961-12-18 | 1900-01-01 | ||
| US3511608A (en) * | 1967-12-14 | 1970-05-12 | Harold P Anderson | Multiple layer paper test strip |
| US3720760A (en) * | 1968-09-06 | 1973-03-13 | Pharmacia Ab | Method for determining the presence of reagin-immunoglobulins(reagin-ig)directed against certain allergens,in aqueous samples |
| US3644177A (en) * | 1969-11-12 | 1972-02-22 | Yissum Res Dev Co | Monitoring penicillin in biological substances |
| US3723064A (en) * | 1971-07-26 | 1973-03-27 | L Liotta | Method and device for determining the concentration of a material in a liquid |
| IT1006557B (it) * | 1971-09-08 | 1976-10-20 | Bagshawe Kenneth Dawson | Cella di reazione particolarmente utile in prove di radioimmunita |
| US3785930A (en) * | 1971-12-22 | 1974-01-15 | Eckrich P Sons Inc | Microbiological activity testing device |
| US3798004A (en) * | 1972-04-18 | 1974-03-19 | Ames Yissum Ltd | Test device |
| US4012198A (en) * | 1972-07-25 | 1977-03-15 | Burroughs Wellcome Co. | Immunodiffusion device |
| CA1023251A (en) * | 1973-06-22 | 1977-12-27 | The Standard Oil Company | Method and paper test strip for determining low levels of lead in hydrocarbon fuels |
| US3992158A (en) * | 1973-08-16 | 1976-11-16 | Eastman Kodak Company | Integral analytical element |
| US3901657A (en) * | 1974-04-29 | 1975-08-26 | Sun Scient Inc | Device for testing solutions and body fluids |
| US3915647A (en) * | 1974-08-16 | 1975-10-28 | Polaroid Corp | Device for determining the concentration of a substance in a fluid |
| US3933594A (en) * | 1974-08-16 | 1976-01-20 | Polaroid Corporation | Method and device for determining the concentration of a substance in a fluid |
| US4018662A (en) * | 1975-01-03 | 1977-04-19 | Max-Planck-Gesellschaft Zur Forderung Der Wissenschaften E.V. | Method and apparatus for simultaneous quantitative analysis of several constituents in a sample |
| SE388694B (sv) * | 1975-01-27 | 1976-10-11 | Kabi Ab | Sett att pavisa ett antigen exv i prov av kroppvetskor, med utnyttjande av till porost berarmaterial bundna eller adsorberande antikroppar |
| CA1054034A (en) * | 1975-06-20 | 1979-05-08 | Barbara J. Bruschi | Multilayer analytical element |
| US4042335A (en) * | 1975-07-23 | 1977-08-16 | Eastman Kodak Company | Integral element for analysis of liquids |
| US4642285A (en) * | 1975-09-29 | 1987-02-10 | Diamedix Corporation | Sandwich EIA for antigen |
| US4474878A (en) * | 1975-09-29 | 1984-10-02 | Cordis Laboratories, Inc. | Sandwich EIA for antigen associated with hepatitis |
| US3990850A (en) * | 1976-01-06 | 1976-11-09 | Akzona Incorporated | Diagnostic test card |
| US4094647A (en) * | 1976-07-02 | 1978-06-13 | Thyroid Diagnostics, Inc. | Test device |
| US4055394A (en) * | 1976-10-18 | 1977-10-25 | Akzona Incorporated | Diagnostic test card |
| US4200690A (en) * | 1976-12-16 | 1980-04-29 | Millipore Corporation | Immunoassay with membrane immobilized antibody |
| US4407943A (en) * | 1976-12-16 | 1983-10-04 | Millipore Corporation | Immobilized antibody or antigen for immunoassay |
| CA1095819A (en) * | 1977-01-14 | 1981-02-17 | Eastman Kodak Company | Element for analysis of liquids |
| US4108729A (en) * | 1977-05-16 | 1978-08-22 | U.S. Packaging Corp. | Paper booklet for presumptive diagnosis of Neisseria Gonorrhoeae in the male |
| US4110079A (en) * | 1977-06-09 | 1978-08-29 | Eastman Kodak Company | Analytical element for clinical analysis |
| AT360176B (de) * | 1977-07-01 | 1980-12-29 | Roehm Gmbh | Testkarte fuer den nachweis okkulten bluts im stuhl |
| US4153668A (en) * | 1978-01-03 | 1979-05-08 | Eastman Kodak Company | Multi-zone analytical element and method using same |
| US4160008A (en) * | 1978-01-26 | 1979-07-03 | Miles Laboratories, Inc. | Multilayered test device for determining the presence of a liquid sample component, and method of use |
| AU527489B2 (en) | 1978-03-20 | 1983-03-10 | Abbott Laboratories | Sugar coated reagents for solid phase immunoassay |
| US4631174A (en) * | 1978-05-02 | 1986-12-23 | Fuji Photo Film Co., Ltd. | Multilayer chemical analysis member having an outer waterproof layer |
| US4212742A (en) * | 1978-05-25 | 1980-07-15 | United States Of America | Filtration apparatus for separating blood cell-containing liquid suspensions |
| JPS587332Y2 (ja) * | 1978-06-06 | 1983-02-08 | 富士写真フイルム株式会社 | 多層血液化学分析材料 |
| NL7807532A (nl) * | 1978-07-13 | 1980-01-15 | Akzo Nv | Metaal-immunotest. |
| US4255384A (en) * | 1978-08-14 | 1981-03-10 | Fuji Photo Film Co., Ltd. | Multilayered integral element for the chemical analysis of the blood |
| US4254083A (en) * | 1979-07-23 | 1981-03-03 | Eastman Kodak Company | Structural configuration for transport of a liquid drop through an ingress aperture |
| US4233029A (en) * | 1978-10-25 | 1980-11-11 | Eastman Kodak Company | Liquid transport device and method |
| US4189304A (en) * | 1978-10-27 | 1980-02-19 | Miles Laboratories, Inc. | Device and method for detecting myoglobin |
| US4246339A (en) * | 1978-11-01 | 1981-01-20 | Millipore Corporation | Test device |
| JPH0142041Y2 (enExample) * | 1978-12-11 | 1989-12-11 | ||
| US4258001A (en) * | 1978-12-27 | 1981-03-24 | Eastman Kodak Company | Element, structure and method for the analysis or transport of liquids |
| US4235601A (en) * | 1979-01-12 | 1980-11-25 | Thyroid Diagnostics, Inc. | Test device and method for its use |
| US4361537A (en) * | 1979-01-12 | 1982-11-30 | Thyroid Diagnostics, Inc. | Test device and method for its use |
| US4288228A (en) * | 1979-01-31 | 1981-09-08 | Technicon Instruments Corporation | Whole blood analyses and diffusion apparatus therefor |
| US4301139A (en) * | 1979-06-21 | 1981-11-17 | Ames-Yissum Ltd. | Multilayer column chromatography specific binding assay method, test device and test kit |
| US4271119A (en) * | 1979-07-23 | 1981-06-02 | Eastman Kodak Company | Capillary transport device having connected transport zones |
| JPS5647761A (en) * | 1979-09-27 | 1981-04-30 | Fuji Photo Film Co Ltd | Laminated analyzing piece and immunity analyzing method using the same |
| US4391904A (en) * | 1979-12-26 | 1983-07-05 | Syva Company | Test strip kits in immunoassays and compositions therein |
| US5156953A (en) | 1979-12-26 | 1992-10-20 | Syntex (U.S.A.) Inc. | Simultaneous calibration heterogeneous immunoassay |
| FI61965C (fi) * | 1980-01-17 | 1982-10-11 | Suovaniemi Finnpipette | Foerfarande foer detektering av hemoglobin i avfoering |
| US4323536A (en) * | 1980-02-06 | 1982-04-06 | Eastman Kodak Company | Multi-analyte test device |
| US5009997A (en) | 1980-06-30 | 1991-04-23 | Shah Vipin D | Two site cross-reaction immunometric sandwich assay method |
| US5009996A (en) | 1980-06-30 | 1991-04-23 | International Immunoassay Laboratories, Inc. | Two site cross-reaction immunometric sandwich assay method |
| US4486530A (en) * | 1980-08-04 | 1984-12-04 | Hybritech Incorporated | Immunometric assays using monoclonal antibodies |
| US4376110A (en) * | 1980-08-04 | 1983-03-08 | Hybritech, Incorporated | Immunometric assays using monoclonal antibodies |
| DE3029579C2 (de) * | 1980-08-05 | 1985-12-12 | Boehringer Mannheim Gmbh, 6800 Mannheim | Verfahren und Mittel zur Abtrennung von Plasma oder Serum aus Vollblut |
| US4816224A (en) | 1980-08-05 | 1989-03-28 | Boehringer Mannheim Gmbh | Device for separating plasma or serum from whole blood and analyzing the same |
| US4366241A (en) * | 1980-08-07 | 1982-12-28 | Syva Company | Concentrating zone method in heterogeneous immunoassays |
| JPS5767860A (en) | 1980-10-15 | 1982-04-24 | Fuji Photo Film Co Ltd | Material for multilayer analysis |
| US4668619A (en) * | 1980-10-30 | 1987-05-26 | Miles Laboratories, Inc. | Multilayer homogeneous specific binding assay device |
| JPS5782766A (en) * | 1980-11-11 | 1982-05-24 | Konishiroku Photo Ind Co Ltd | Amynological measuring element |
| DE3044385A1 (de) * | 1980-11-25 | 1982-06-24 | Boehringer Mannheim Gmbh, 6800 Mannheim | Verfahren zur durchfuehrung analytischer bestimmungen und hierfuer geeignetes rotoreinsatzelement |
| US4517288A (en) * | 1981-01-23 | 1985-05-14 | American Hospital Supply Corp. | Solid phase system for ligand assay |
| US4752562A (en) * | 1981-01-23 | 1988-06-21 | Baxter Travenol Laboratories, Inc. | Detection of serum antibody and surface antigen by radial partition immunoassay |
| US4426451A (en) * | 1981-01-28 | 1984-01-17 | Eastman Kodak Company | Multi-zoned reaction vessel having pressure-actuatable control means between zones |
| US4425438A (en) * | 1981-03-13 | 1984-01-10 | Bauman David S | Assay method and device |
| US4442204A (en) * | 1981-04-10 | 1984-04-10 | Miles Laboratories, Inc. | Homogeneous specific binding assay device and preformed complex method |
| US4447526A (en) * | 1981-04-20 | 1984-05-08 | Miles Laboratories, Inc. | Homogeneous specific binding assay with carrier matrix incorporating specific binding partner |
| FI76888C (fi) | 1981-04-29 | 1988-12-12 | Ciba Geigy Ag | Nya medel och foerpackningar foer immunologiska analyser. |
| US4365970A (en) * | 1981-05-01 | 1982-12-28 | Smithkline Instruments, Inc. | Specimen test slide and method for testing occult blood |
| JPS57200862A (en) * | 1981-06-05 | 1982-12-09 | Fuji Photo Film Co Ltd | Mutilayer analysis element utilizing unique binding reaction |
| US4390343A (en) * | 1981-07-06 | 1983-06-28 | Miles Laboratories, Inc. | Multilayer analytical element having an impermeable radiation diffusing and blocking layer |
| DE3265794D1 (en) * | 1981-07-16 | 1985-10-03 | Hoffmann La Roche | Method for the detection of human occult blood in human stool samples |
| JPS5818167A (ja) * | 1981-07-24 | 1983-02-02 | Fuji Photo Film Co Ltd | 分析フイルム及びこれを用いる分析方法 |
| US4363874A (en) * | 1981-08-07 | 1982-12-14 | Miles Laboratories, Inc. | Multilayer analytical element having an impermeable radiation nondiffusing reflecting layer |
| DE3134611A1 (de) | 1981-09-01 | 1983-03-10 | Boehringer Mannheim Gmbh, 6800 Mannheim | Verfahren zur durchfuehrung analytischer bestimmungen und hierfuer geeignetes mittel |
| US4446232A (en) * | 1981-10-13 | 1984-05-01 | Liotta Lance A | Enzyme immunoassay with two-zoned device having bound antigens |
| US4938927A (en) | 1982-01-08 | 1990-07-03 | Environmental Diagnostics, Inc. | Rotary fluid manipulator |
| US5073484A (en) | 1982-03-09 | 1991-12-17 | Bio-Metric Systems, Inc. | Quantitative analysis apparatus and method |
| ZA831268B (en) | 1982-04-30 | 1984-10-31 | Hematec Corp | Home diagnostic aid and method for determining the presence of occult blood for use by the general public |
| US4486536A (en) * | 1982-05-28 | 1984-12-04 | Smithkline Diagnostics, Inc. | Specimen slide for occult blood testing |
| US4435504A (en) * | 1982-07-15 | 1984-03-06 | Syva Company | Immunochromatographic assay with support having bound "MIP" and second enzyme |
| JPS5934154A (ja) * | 1982-08-19 | 1984-02-24 | Konishiroku Photo Ind Co Ltd | 免疫分析素子測定法 |
| US4447546A (en) * | 1982-08-23 | 1984-05-08 | Myron J. Block | Fluorescent immunoassay employing optical fiber in capillary tube |
| US4459358A (en) * | 1982-12-29 | 1984-07-10 | Polaroid Corporation | Multilayer element for analysis |
| AU529210B3 (en) * | 1983-02-02 | 1983-04-14 | Australian Monoclonal Development Pty. Ltd. | Monoclonal antibody in occult blood diagnosis |
| US4578358A (en) * | 1983-05-03 | 1986-03-25 | Warner-Lambert Company | Collection of specimens and detection of occult blood therein |
| AU2706384A (en) | 1983-05-06 | 1984-11-08 | Monoclonal Antibodies Inc. | Colorimetric immunoassay on solid surface |
| DE3323973A1 (de) | 1983-07-02 | 1985-01-03 | Boehringer Mannheim Gmbh, 6800 Mannheim | Erythrozyten-rueckhaltesubstrate |
| US4637978A (en) * | 1983-10-28 | 1987-01-20 | Eastman Kodak Company | Assay for analysis of whole blood |
| US4594327A (en) * | 1983-11-02 | 1986-06-10 | Syntex (U.S.A.) Inc. | Assay method for whole blood samples |
| GB8331514D0 (en) | 1983-11-25 | 1984-01-04 | Janssen Pharmaceutica Nv | Visualization method |
| WO1985002466A1 (en) * | 1983-12-02 | 1985-06-06 | Vertrik Bioteknik Ab | A device for chemical analyses and use thereof |
| EP0149168B1 (en) * | 1983-12-19 | 1991-04-24 | Daiichi Pure Chemicals Co. Ltd. | Immunoassay |
| US4895809A (en) | 1984-01-09 | 1990-01-23 | Varian Associates, Inc. | Immobilized antigen-antibody displacement process |
| US4742002A (en) * | 1984-01-16 | 1988-05-03 | Helena Laboratories Corporation | Test kit and method for determining the presence of blood in a specimen and for testing the effectiveness of peroxidase inactivating solution |
| US5273888A (en) | 1984-01-16 | 1993-12-28 | Helena Laboratories Corporation | Chemical test kit and method for determining the presence of blood in a specimen and for verifying the effectiveness of the chemicals |
| US4676950A (en) * | 1984-02-03 | 1987-06-30 | Foster Research Corporation | Indicator and test device for detecting occult blood |
| US4703017C1 (en) | 1984-02-14 | 2001-12-04 | Becton Dickinson Co | Solid phase assay with visual readout |
| US4757004A (en) | 1984-03-16 | 1988-07-12 | Syntex (U.S.A.) Inc. | Chromatographic devices having modified edges |
| US4743560A (en) * | 1984-03-26 | 1988-05-10 | Becton Dickinson And Company | Solid phase assay |
| US4756828A (en) | 1984-04-12 | 1988-07-12 | Syntex (U.S.A.) Inc. | Chromatographic strip having non-compressed edges |
| US4945205A (en) | 1984-04-12 | 1990-07-31 | Syntex (U.S.A.) Inc. | Chromatographic strip having non-compressed edges |
| US4632901A (en) * | 1984-05-11 | 1986-12-30 | Hybritech Incorporated | Method and apparatus for immunoassays |
| EP0170746A1 (en) | 1984-08-07 | 1986-02-12 | Covalent Technology Corporation | Biologically active material test |
| US4826759A (en) | 1984-10-04 | 1989-05-02 | Bio-Metric Systems, Inc. | Field assay for ligands |
| US4999285A (en) | 1984-11-15 | 1991-03-12 | Syntex (U.S.A.) Inc. | Chromatographic cassette |
| US4624929A (en) * | 1984-12-03 | 1986-11-25 | Syntex (U.S.A.) Inc. | Sample collector and assay device and method for its use |
| DE3445816C1 (de) | 1984-12-15 | 1986-06-12 | Behringwerke Ag, 3550 Marburg | Flaechenfoermiges diagnostisches Mittel |
| US4740468A (en) * | 1985-02-14 | 1988-04-26 | Syntex (U.S.A.) Inc. | Concentrating immunochemical test device and method |
| US4804518A (en) | 1985-02-25 | 1989-02-14 | Levine Robert A | Device for occult blood testing |
| JPS61228351A (ja) | 1985-04-02 | 1986-10-11 | Kyoto Ikagaku Kenkyusho:Kk | 糞便中のヘモグロビンの検出方法 |
| US4678757A (en) * | 1985-04-11 | 1987-07-07 | Smithkline Diagnostics, Inc. | Device and method for whole blood separation and analysis |
| US4623461A (en) * | 1985-05-31 | 1986-11-18 | Murex Corporation | Transverse flow diagnostic device |
| US4693834A (en) * | 1986-05-05 | 1987-09-15 | Murex Corporation | Transverse flow diagnostic kit |
| JPH076984B2 (ja) | 1985-05-31 | 1995-01-30 | 株式会社日立製作所 | 複数項目分析方法 |
| JPS6270763A (ja) | 1985-06-05 | 1987-04-01 | シンビオテイツクス コ−ポレイシヨン | 免疫アツセイインキユベ−シヨン装置 |
| US4931385A (en) | 1985-06-24 | 1990-06-05 | Hygeia Sciences, Incorporated | Enzyme immunoassays and immunologic reagents |
| DE3523439A1 (de) | 1985-06-29 | 1987-01-08 | Boehringer Mannheim Gmbh | Testtraeger |
| US4670381A (en) * | 1985-07-19 | 1987-06-02 | Eastman Kodak Company | Heterogeneous immunoassay utilizing horizontal separation in an analytical element |
| US4806312A (en) | 1985-08-28 | 1989-02-21 | Miles Inc. | Multizone analytical element having detectable signal concentrating zone |
| US4806311A (en) | 1985-08-28 | 1989-02-21 | Miles Inc. | Multizone analytical element having labeled reagent concentration zone |
| DE3530993A1 (de) * | 1985-08-30 | 1987-03-05 | Miles Lab | Teststreifen mit festlegbarer probenaufnahmekapazitaet |
| US4900663A (en) | 1985-09-13 | 1990-02-13 | Environmental Diagnostics, Inc. | Test kit for determining the presence of organic materials and method of utilizing same |
| US4761381A (en) | 1985-09-18 | 1988-08-02 | Miles Inc. | Volume metering capillary gap device for applying a liquid sample onto a reactive surface |
| TW203120B (enExample) | 1985-10-04 | 1993-04-01 | Abbott Lab | |
| GB8526741D0 (en) | 1985-10-30 | 1985-12-04 | Boots Celltech Diagnostics | Binding assay device |
| US4868108A (en) | 1985-12-12 | 1989-09-19 | Hygeia Sciences, Incorporated | Multiple-antibody detection of antigen |
| CA1291031C (en) | 1985-12-23 | 1991-10-22 | Nikolaas C.J. De Jaeger | Method for the detection of specific binding agents and their correspondingbindable substances |
| US4916056A (en) | 1986-02-18 | 1990-04-10 | Abbott Laboratories | Solid-phase analytical device and method for using same |
| US4645743A (en) * | 1986-03-11 | 1987-02-24 | Smithkline Diagnostics, Inc. | Method and device for collecting and testing for fecal occult blood |
| JPH0750114B2 (ja) | 1986-03-17 | 1995-05-31 | 富士写真フイルム株式会社 | 免疫分析器具を用いる分析方法 |
| DE3616105A1 (de) | 1986-05-13 | 1987-11-19 | Merck Patent Gmbh | Teststreifen |
| US4786594A (en) | 1986-05-14 | 1988-11-22 | Syntex (U.S.A.) Inc. | Enzyme immunoassay |
| AU602694B2 (en) | 1986-06-09 | 1990-10-25 | Ortho Diagnostic Systems Inc. | Improved colloidal gold membrane assay |
| US4837145A (en) | 1986-06-09 | 1989-06-06 | Liotta Lance A | Layered immunoassay using antibodies bound to transport particles to determine the presence of an antigen |
| US4959305A (en) | 1986-06-18 | 1990-09-25 | Miles Inc. | Reversible immobilization of assay reagents in a multizone test device |
| US4812293A (en) | 1986-06-30 | 1989-03-14 | Becton, Dickinson And Company | Vacuum actuated assay device and method of using same |
| US5120504A (en) | 1986-07-14 | 1992-06-09 | Hybritech Incorporated | Apparatus for immunoassays with vent chennels in the container side wall |
| CA1288690C (en) | 1986-07-14 | 1991-09-10 | Virginia Petro-Roy | Apparatus and process for immunoassays |
| US4790979A (en) | 1986-08-29 | 1988-12-13 | Technimed Corporation | Test strip and fixture |
| US4959307A (en) | 1986-09-05 | 1990-09-25 | Syntex (U.S.A.) Inc. | Immunoseparating strip |
| US5260194A (en) | 1986-09-05 | 1993-11-09 | Syntex (U.S.A.) Inc. | Immunoseparating strip |
| US5085988A (en) | 1986-09-05 | 1992-02-04 | Syntex (U.S.A.) Inc. | Immunoseparating strip |
| US5085987A (en) | 1986-09-05 | 1992-02-04 | Syntex (U.S.A.) Inc. | Immunoseparating strip |
| US5260193A (en) | 1986-09-05 | 1993-11-09 | Syntex (U.S.A.) Inc. | Immunoseparating strip |
| US4963468A (en) | 1986-09-05 | 1990-10-16 | Syntex (U.S.A.) Inc. | Immunoseparating strip |
| DE3630999A1 (de) | 1986-09-12 | 1988-03-17 | Boehringer Mannheim Gmbh | Mehrschichtiger testtraeger |
| US4960691A (en) | 1986-09-29 | 1990-10-02 | Abbott Laboratories | Chromatographic test strip for determining ligands or receptors |
| US5135719A (en) | 1986-10-29 | 1992-08-04 | Biotrack, Inc. | Blood separation device comprising a filter and a capillary flow pathway exiting the filter |
| US4753776A (en) * | 1986-10-29 | 1988-06-28 | Biotrack, Inc. | Blood separation device comprising a filter and a capillary flow pathway exiting the filter |
| GB8626081D0 (en) | 1986-10-31 | 1986-12-03 | Unilever Plc | Printing processes |
| US5030558A (en) | 1986-11-07 | 1991-07-09 | Syntex (U.S.A.) Inc. | Qualitative immunochromatographic method and device |
| US5232835A (en) | 1986-11-07 | 1993-08-03 | Syntex (U.S.A.) Inc. | Qualitative immunochromatographic method and device |
| US5156952A (en) | 1986-11-07 | 1992-10-20 | Syntex (U.S.A.) Inc. | Qualitative immunochromatographic method and device |
| DE3638654A1 (de) | 1986-11-12 | 1988-05-26 | Boehringer Mannheim Gmbh | Testtraeger zur analytischen bestimmung eines bestandteils einer koerperfluessigkeit |
| DE3787078T2 (de) | 1986-11-24 | 1993-12-09 | Abbott Lab | Proberichtwirkung für analytisches Festphasengerät. |
| US4789629A (en) | 1986-11-24 | 1988-12-06 | Smithkline Beckman Corporation | Method and device for collecting and testing for fecal occult blood |
| US5169789A (en) | 1986-12-03 | 1992-12-08 | New Horizons Diagnostics Corporation | Device and method for self contained solid phase immunodiffusion assay |
| US4770853A (en) | 1986-12-03 | 1988-09-13 | New Horizons Diagnostics Corporation | Device for self contained solid phase immunodiffusion assay |
| US4976926A (en) | 1986-12-15 | 1990-12-11 | Pall Corporation | Vacuum diagnostic device |
| US4789526A (en) | 1986-12-15 | 1988-12-06 | Pall Corporation | Vacuum diagnostic device |
| DE3643516A1 (de) | 1986-12-19 | 1988-06-30 | Boehringer Mannheim Gmbh | Testtraeger fuer die analytische bestimmung von bestandteilen von koerperfluessigkeiten |
| US5079142A (en) | 1987-01-23 | 1992-01-07 | Synbiotics Corporation | Orthogonal flow immunoassays and devices |
| US4797260A (en) | 1987-01-27 | 1989-01-10 | V-Tech, Inc. | Antibody testing system |
| EP0281251A3 (en) | 1987-02-04 | 1988-09-28 | International Immunoassay Laboratories, Inc. | A method for preparing a fecal sample composition for immunoassay testing |
| AU592174B2 (en) | 1987-02-17 | 1990-01-04 | Genesis Labs, Inc. | Immunoassay test strip |
| ES2034186T3 (es) | 1987-02-17 | 1993-04-01 | Cmb Foodcan Plc | Dispositivo de pruebas analiticas. |
| GB8703578D0 (en) | 1987-02-17 | 1987-03-25 | Metal Box Plc | Analytical test strip |
| US4920046A (en) | 1987-02-20 | 1990-04-24 | Becton, Dickinson And Company | Process, test device, and test kit for a rapid assay having a visible readout |
| US4847199A (en) | 1987-02-27 | 1989-07-11 | Eastman Kodak Company | Agglutination immunoassay and kit for determination of a multivalent immune species using a buffered salt wash solution |
| US4918025A (en) | 1987-03-03 | 1990-04-17 | Pb Diagnostic Systems, Inc. | Self contained immunoassay element |
| CA1303983C (en) | 1987-03-27 | 1992-06-23 | Robert W. Rosenstein | Solid phase assay |
| JPH0684970B2 (ja) | 1987-03-31 | 1994-10-26 | 株式会社京都医科学研究所 | 糞便中の潜血検出方法 |
| US5137808A (en) | 1987-04-07 | 1992-08-11 | Syntex (U.S.A.) Inc. | Immunoassay device |
| US4857453A (en) | 1987-04-07 | 1989-08-15 | Syntex (U.S.A.) Inc. | Immunoassay device |
| US4956275A (en) | 1987-04-14 | 1990-09-11 | Molecular Devices Corporation | Migratory detection immunoassay |
| ES2150428T3 (es) | 1987-04-27 | 2000-12-01 | Unilever Nv | Ensayos de union especifica. |
| DE3715485A1 (de) | 1987-05-09 | 1988-11-17 | Boehringer Mannheim Gmbh | Stabilisierung von ss-galactosidase |
| US4855240A (en) | 1987-05-13 | 1989-08-08 | Becton Dickinson And Company | Solid phase assay employing capillary flow |
| US5238847A (en) | 1987-05-20 | 1993-08-24 | Boehringer Mannheim Gmbh | Test kit and process for the determination of an analyte in a pasty sample |
| US4943522A (en) | 1987-06-01 | 1990-07-24 | Quidel | Lateral flow, non-bibulous membrane assay protocols |
| CA1313616C (en) | 1987-06-01 | 1993-02-16 | Robert B. Sargeant | Lateral flow, non-bibulous membrane protocols |
| IL85899A0 (en) | 1987-06-10 | 1988-09-30 | Miles Inc | Method,test device and test kit for separating labeled reagent in an immunometric binding assay |
| US4810470A (en) | 1987-06-19 | 1989-03-07 | Miles Inc. | Volume independent diagnostic device |
| US4959324A (en) | 1989-03-16 | 1990-09-25 | Chemtrak, Inc. | Sample pad assay initiation device and method of making |
| US4973549A (en) | 1987-06-22 | 1990-11-27 | Chemtrak Corporation | Quantitative diagnostic assay employing signal producing agent bound to support and measuring migration distance of detectable signal |
| US4999287A (en) | 1988-05-19 | 1991-03-12 | Chemtrak Corporation | Direct measuring assay strip and method of use thereof |
| JPH0623759B2 (ja) | 1987-07-08 | 1994-03-30 | 富士写真フイルム株式会社 | 全血分析要素 |
| US5120643A (en) | 1987-07-13 | 1992-06-09 | Abbott Laboratories | Process for immunochromatography with colloidal particles |
| US4883764A (en) | 1987-07-20 | 1989-11-28 | Kloepfer Mary A | Blood test strip |
| US4981786A (en) | 1987-09-04 | 1991-01-01 | Syntex (U.S.A.) Inc. | Multiple port assay device |
| EP0306772B1 (en) | 1987-09-11 | 1993-03-10 | Abbott Laboratories | Lateral flow chromatographic binding assay device |
| US4956302A (en) | 1987-09-11 | 1990-09-11 | Abbott Laboratories | Lateral flow chromatographic binding assay device |
| US4912034A (en) | 1987-09-21 | 1990-03-27 | Biogenex Laboratories | Immunoassay test device and method |
| US4853335A (en) | 1987-09-28 | 1989-08-01 | Olsen Duane A | Colloidal gold particle concentration immunoassay |
| DE3733084A1 (de) | 1987-09-30 | 1989-04-13 | Boehringer Mannheim Gmbh | Testtraeger zur analytischen bestimmung eines bestandteils einer koerperfluessigkeit |
| US5006464A (en) | 1987-10-01 | 1991-04-09 | E-Y Laboratories, Inc. | Directed flow diagnostic device and method |
| US4902629A (en) | 1987-10-06 | 1990-02-20 | Personal Diagnostics, Inc. | Apparatus and processes for facilitating reaction between analyte and test reagent system |
| US4859612A (en) | 1987-10-07 | 1989-08-22 | Hygeia Sciences, Inc. | Metal sol capture immunoassay procedure, kit for use therewith and captured metal containing composite |
| US5275785A (en) | 1987-10-30 | 1994-01-04 | Unilever Patent Holdings B.V. | Test device for detecting an analyte in a liquid sample |
| US5256372A (en) | 1987-11-06 | 1993-10-26 | Idexx Corporation | Dipstick test device including a removable filter assembly |
| EP0317001B1 (en) | 1987-11-16 | 1993-02-03 | Janssen Pharmaceutica N.V. | A new universally applicable detection system based 0n ultra small colloidal metal particles |
| DE3740471A1 (de) | 1987-11-28 | 1989-06-08 | Boehringer Mannheim Gmbh | Testtraeger zur analyse einer probenfluessigkeit und verfahren zu seiner herstellung |
| US4818677A (en) | 1987-12-03 | 1989-04-04 | Monoclonal Antibodies, Inc. | Membrane assay using focused sample application |
| US5006474A (en) | 1987-12-16 | 1991-04-09 | Disease Detection International Inc. | Bi-directional lateral chromatographic test device |
| US4988627A (en) | 1987-12-18 | 1991-01-29 | Eastman Kodak Company | Test device with dried reagent drops on inclined wall |
| ES2050697T3 (es) | 1987-12-21 | 1994-06-01 | Abbott Lab | Metodos y dispositivos de ensayo de fijacion cromatografico. |
| US4977078A (en) | 1987-12-22 | 1990-12-11 | Olympus Optical Co., Ltd. | Plate substrate immunoassay device and method for performing a multi-test immunoassay on a specimen |
| US5209904A (en) | 1987-12-23 | 1993-05-11 | Abbott Laboratories | Agglutination reaction device utilizing selectively impregnated porous material |
| US5114673A (en) | 1988-01-21 | 1992-05-19 | Boehringer Mannheim Corporaton | Apparatus for determination of a component in a sample |
| US5206177A (en) | 1988-01-21 | 1993-04-27 | Boehringer Mannheim Corporation | Apparatus for determining an analyte and method therefor |
| US4952517A (en) | 1988-02-08 | 1990-08-28 | Hygeia Sciences, Inc. | Positive step immunoassay |
| US5104793A (en) | 1988-02-16 | 1992-04-14 | Boehringer Mannheim Corporation | Method for determining an analyte in a liquid sample using a zoned test device and an inhibitor for a label used in said method |
| NL8800796A (nl) | 1988-03-29 | 1989-10-16 | X Flow Bv | Werkwijze voor de chemische analyse van bestanddelen van een lichaamsvloeistof, alsmede een testinrichting en testpakket voor een dergelijke analyse. |
| DE3814370A1 (de) | 1988-04-28 | 1989-11-09 | Boehringer Mannheim Gmbh | Testtraeger fuer die analyse einer probenfluessigkeit, verfahren zur durchfuehrung einer solchen analyse und herstellungsverfahren |
| US5145784A (en) | 1988-05-04 | 1992-09-08 | Cambridge Biotech Corporation | Double capture assay method employing a capillary flow device |
| US4963325A (en) | 1988-05-06 | 1990-10-16 | Hygeia Sciences, Inc. | Swab expressor immunoassay device |
| US5137804A (en) | 1988-05-10 | 1992-08-11 | E. I. Du Pont De Nemours And Company | Assay device and immunoassay |
| US5039607A (en) | 1988-05-17 | 1991-08-13 | Syntex (U.S.A.) Inc. | Method for immunochromatographic analysis |
| US5164294A (en) | 1988-05-17 | 1992-11-17 | Syntex (U.S.A.) Inc. | Method for immunochromatographic analysis |
| US5013669A (en) | 1988-06-01 | 1991-05-07 | Smithkline Diagnostics, Inc. | Mass producible biologically active solid phase devices |
| US5051237A (en) | 1988-06-23 | 1991-09-24 | P B Diagnostic Systems, Inc. | Liquid transport system |
| US5096809A (en) | 1988-07-25 | 1992-03-17 | Pacific Biotech, Inc. | Whole blood assays using porous membrane support devices |
| US4877586A (en) | 1988-07-27 | 1989-10-31 | Eastman Kodak Company | Sliding test device for assays |
| DE3826057A1 (de) | 1988-07-30 | 1990-02-01 | Boehringer Mannheim Gmbh | Testtraeger zur analytischen bestimmung eines bestandteils einer fluessigen probe |
| US5338513A (en) | 1988-07-30 | 1994-08-16 | Boehringer Mannheim Gmbh | Test carrier for the analytical determination of a component of a liquid sample |
| US5030555A (en) | 1988-09-12 | 1991-07-09 | University Of Florida | Membrane-strip reagent serodiagnostic apparatus and method |
| US5182191A (en) | 1988-10-14 | 1993-01-26 | Pacific Biotech, Inc. | Occult blood sampling device and assay |
| US5079174A (en) | 1988-12-08 | 1992-01-07 | Boehringer Mannheim Corporation | Apparatus for sequential determination of an analyte in a fluid sample |
| US5106758A (en) | 1988-12-12 | 1992-04-21 | Technicon Instruments Corporation | Analytical test device and the use thereof |
| DE3842702A1 (de) | 1988-12-19 | 1990-06-21 | Boehringer Mannheim Gmbh | Testtraeger zur analytischen untersuchung einer probenfluessigkeit mit hilfe einer spezifischen bindungsreaktion zweier bioaffiner bindungspartner und entsprechendes testverfahren |
| US5202268A (en) | 1988-12-30 | 1993-04-13 | Environmental Diagnostics, Inc. | Multi-layered test card for the determination of substances in liquids |
| US5028535A (en) | 1989-01-10 | 1991-07-02 | Biosite Diagnostics, Inc. | Threshold ligand-receptor assay |
| US5252293A (en) | 1989-01-17 | 1993-10-12 | Vladimir Drbal | Analytical slide with porous filter membrane |
| US5200317A (en) | 1989-02-02 | 1993-04-06 | Abbott Laboratories | Method and device for quantitative chromatography |
| GB8903627D0 (en) | 1989-02-17 | 1989-04-05 | Unilever Plc | Assays |
| US5416000A (en) | 1989-03-16 | 1995-05-16 | Chemtrak, Inc. | Analyte immunoassay in self-contained apparatus |
| US5260221A (en) | 1989-03-16 | 1993-11-09 | Ramel Urs A | Sample pad assay initiation device |
| US5264180A (en) | 1989-03-16 | 1993-11-23 | Chemtrak, Inc. | Mobile reagents in an analyte assay in a self-contained apparatus |
| US4933092A (en) | 1989-04-07 | 1990-06-12 | Abbott Laboratories | Methods and devices for the separation of plasma or serum from whole blood |
| US5064541A (en) | 1989-04-07 | 1991-11-12 | Abbott Laboratories | Devices and methods for the collection of a predetermined volume of plasma or serum |
| NL8901090A (nl) | 1989-04-28 | 1990-11-16 | X Flow Bv | Werkwijze voor het vervaardigen van een microporeus membraan en een dergelijk membraan. |
| US5100619A (en) | 1989-05-09 | 1992-03-31 | Beckman Instruments, Inc. | Device and method for collecting fecal occult blood specimens |
| US5120662A (en) | 1989-05-09 | 1992-06-09 | Abbott Laboratories | Multilayer solid phase immunoassay support and method of use |
| US5234813A (en) | 1989-05-17 | 1993-08-10 | Actimed Laboratories, Inc. | Method and device for metering of fluid samples and detection of analytes therein |
| US5087556A (en) | 1989-05-17 | 1992-02-11 | Actimed Laboratories, Inc. | Method for quantitative analysis of body fluid constituents |
| DE3922960A1 (de) | 1989-07-12 | 1991-01-17 | Boehringer Mannheim Gmbh | Verfahren zur bestimmung eines analyten |
| AU640162B2 (en) | 1989-08-28 | 1993-08-19 | Lifescan, Inc. | Blood separation and analyte detection techniques |
| US5306623A (en) | 1989-08-28 | 1994-04-26 | Lifescan, Inc. | Visual blood glucose concentration test strip |
| US5185127A (en) | 1989-09-21 | 1993-02-09 | Becton, Dickinson And Company | Test device including flow control means |
| CA2025476A1 (en) | 1989-09-27 | 1991-03-28 | Shan F. Ching | Hydrophilic laminated porous membranes and methods of preparing same |
| US5075078A (en) | 1989-10-05 | 1991-12-24 | Abbott Laboratories | Self-performing immunochromatographic device |
| US5064766A (en) | 1989-10-18 | 1991-11-12 | Wardlaw Stephen C | Method for differentiating the source of occult gastrointestinal bleeding |
| US5104812A (en) | 1989-11-27 | 1992-04-14 | Syntex (U.S.A.) Inc. | Device and method for interrupting capillary flow |
| US5135873A (en) | 1989-11-27 | 1992-08-04 | Syntex (U.S.A.) Inc. | Device and method for completing a fluidic circuit which employs a liquid expandable piece of bibulous material |
| US5260222A (en) | 1989-11-27 | 1993-11-09 | Syntex (U.S.A.) Inc. | Device and method for completing a fluidic circuit which employs a liquid expandable piece of bibulous material |
| US5252496A (en) | 1989-12-18 | 1993-10-12 | Princeton Biomeditech Corporation | Carbon black immunochemical label |
| US5435970A (en) | 1989-12-18 | 1995-07-25 | Environmental Diagnostics, Inc. | Device for analysis for constituents in biological fluids |
| JP2618727B2 (ja) | 1990-01-19 | 1997-06-11 | 富士写真フイルム株式会社 | 全血中の被検成分の定量方法 |
| US5141850A (en) | 1990-02-07 | 1992-08-25 | Hygeia Sciences, Inc. | Porous strip form assay device method |
| US5096837A (en) | 1990-02-08 | 1992-03-17 | Pacific Biotech, Inc. | Immunochromatographic assay and method of using same |
| DE4005021A1 (de) | 1990-02-19 | 1991-08-22 | Boehringer Mannheim Gmbh | Testtraeger zur analyse einer probenfluessigkeit |
| ES2081924T3 (es) | 1990-02-22 | 1996-03-16 | Editek Inc | Dispositivo de ensayo de diagnostico de capas multiples para determinar la presencia de sustancias en medios liquidos. |
| JP3070764B2 (ja) | 1990-03-12 | 2000-07-31 | バイオサイト・ダイアグノスティックス・インコーポレイテッド | 生物学的検定装置及びそれを用いた検定方法 |
| JP2576910B2 (ja) | 1990-04-13 | 1997-01-29 | 富士写真フイルム株式会社 | 免疫分析要素および免疫分析方法 |
| US5258163A (en) | 1990-04-14 | 1993-11-02 | Boehringer Mannheim Gmbh | Test carrier for analysis of fluids |
| US5212060A (en) | 1990-04-27 | 1993-05-18 | Genesis Labs, Inc. | Dry test strip comprising a dextran barrier for excluding erythrocytes |
| DE4015378A1 (de) | 1990-05-14 | 1991-11-21 | Boehringer Mannheim Gmbh | Testtraeger fuer die analytische bestimmung eines bestandteils einer probenfluessigkeit |
| DE4015589A1 (de) | 1990-05-15 | 1991-11-21 | Boehringer Mannheim Gmbh | Vorrichtung und deren verwendung zur abtrennung von plasma aus vollblut |
| US5238652A (en) | 1990-06-20 | 1993-08-24 | Drug Screening Systems, Inc. | Analytical test devices for competition assay for drugs of non-protein antigens using immunochromatographic techniques |
| US5158869A (en) | 1990-07-06 | 1992-10-27 | Sangstat Medical Corporation | Analyte determination using comparison of juxtaposed competitive and non-competitive elisa results and device therefore |
| DE4022655A1 (de) | 1990-07-17 | 1992-01-23 | Boehringer Mannheim Gmbh | Testkit zur bestimmung eines analyten in einer pastoesen probe, insbesondere in stuhl |
| US5200321A (en) | 1990-09-07 | 1993-04-06 | The United States Of America As Represented By The Secretary Of The Navy | Microassay on a card |
| US5118428A (en) | 1990-11-13 | 1992-06-02 | Quidel | Method to remove red blood cells from whole blood samples |
| WO1992008977A1 (en) | 1990-11-14 | 1992-05-29 | Southern Research Institute | Rapid diagnostic device and kit |
| US5106582A (en) | 1990-12-18 | 1992-04-21 | Smithkline Diagnostics, Inc. | Specimen test slide and method of testing for fecal occult blood |
| JP2786336B2 (ja) | 1991-03-04 | 1998-08-13 | 富士写真フイルム株式会社 | 免疫分析要素および免疫分析方法 |
| JP3108115B2 (ja) | 1991-03-28 | 2000-11-13 | ロート製薬株式会社 | イムノクロマトグラフ法による物質検出法 |
| US5468648A (en) | 1991-05-29 | 1995-11-21 | Smithkline Diagnostics, Inc. | Interrupted-flow assay device |
| JPH04351962A (ja) | 1991-05-29 | 1992-12-07 | Mochida Pharmaceut Co Ltd | 特異結合分析方法および特異結合分析装置 |
| US5451504A (en) | 1991-07-29 | 1995-09-19 | Serex, Inc. | Method and device for detecting the presence of analyte in a sample |
| US5540888A (en) | 1991-11-11 | 1996-07-30 | British Technology Group Limited | Liquid transfer assay devices |
| JPH05188053A (ja) | 1992-01-10 | 1993-07-27 | Sanwa Kagaku Kenkyusho Co Ltd | 血液から血清又は血漿成分を分離する器具 |
| US5171529A (en) | 1992-01-24 | 1992-12-15 | Robert Schreiber | Occult blood testing device |
| DE4202850A1 (de) | 1992-01-31 | 1993-08-05 | Boehringer Mannheim Gmbh | Analysenelement fuer immunoassays |
| US5314804A (en) | 1992-03-24 | 1994-05-24 | Serim Research Corporation | Test for Helicobacter pylori |
| US5308580A (en) | 1992-05-19 | 1994-05-03 | Millipore Corporation | Sample collection and analytical device |
| US5458852A (en) | 1992-05-21 | 1995-10-17 | Biosite Diagnostics, Inc. | Diagnostic devices for the controlled movement of reagents without membranes |
| US5395754A (en) | 1992-07-31 | 1995-03-07 | Hybritech Incorporated | Membrane-based immunoassay method |
| DE4229591C1 (de) | 1992-09-04 | 1994-03-24 | Draegerwerk Ag | Immunologisches Verfahren zur Bestimmung eines Analyten |
| US5354692A (en) | 1992-09-08 | 1994-10-11 | Pacific Biotech, Inc. | Analyte detection device including a hydrophobic barrier for improved fluid flow |
| US5308775A (en) | 1992-09-15 | 1994-05-03 | Abbott Laboratories | Assay devices for concurrently detecting an analyte and confirming the test result |
| US5500375A (en) | 1993-04-13 | 1996-03-19 | Serex, Inc. | Integrated packaging-holder device for immunochromatographic assays in flow-through or dipstick formats |
| US5397479A (en) | 1993-04-26 | 1995-03-14 | International Remote Imaging Systems, Inc. | Composition and method for enrichment of white blood cells from whole human blood |
| US5441698A (en) | 1993-09-10 | 1995-08-15 | Beckman Instruments, Inc. | Bevel closure and device |
| PT653639E (pt) | 1993-11-12 | 2000-06-30 | Unilever Nv | Equipamentos analiticos e metodos para a sua utilizacao |
| US5491096A (en) | 1993-12-27 | 1996-02-13 | Eli Lilly And Company | Antigen detection with affinity chromatography and parallel processing a control |
| US5521102A (en) | 1994-08-08 | 1996-05-28 | Quidel Corporation | Controlled sensitivity immunochromatographic assay |
| US5569608A (en) | 1995-01-30 | 1996-10-29 | Bayer Corporation | Quantitative detection of analytes on immunochromatographic strips |
| US5766962A (en) | 1995-12-22 | 1998-06-16 | Universal Healthwatch, Inc. | Device for collecting and testing samples |
-
1997
- 1997-05-09 US US08/853,760 patent/US5939252A/en not_active Expired - Fee Related
-
1998
- 1998-04-15 AT AT98918257T patent/ATE354082T1/de not_active IP Right Cessation
- 1998-04-15 DE DE69837087T patent/DE69837087T2/de not_active Expired - Fee Related
- 1998-04-15 AU AU71219/98A patent/AU730243B2/en not_active Ceased
- 1998-04-15 JP JP54809498A patent/JP4104669B2/ja not_active Expired - Fee Related
- 1998-04-15 WO PCT/US1998/007599 patent/WO1998050778A1/en not_active Ceased
- 1998-04-15 EP EP98918257A patent/EP0980517B1/en not_active Expired - Lifetime
- 1998-04-15 CA CA002286680A patent/CA2286680C/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| ATE354082T1 (de) | 2007-03-15 |
| AU7121998A (en) | 1998-11-27 |
| JP2002503335A (ja) | 2002-01-29 |
| WO1998050778A1 (en) | 1998-11-12 |
| EP0980517A1 (en) | 2000-02-23 |
| AU730243B2 (en) | 2001-03-01 |
| CA2286680C (en) | 2007-02-20 |
| EP0980517B1 (en) | 2007-02-14 |
| US5939252A (en) | 1999-08-17 |
| DE69837087T2 (de) | 2007-10-31 |
| DE69837087D1 (de) | 2007-03-29 |
| CA2286680A1 (en) | 1998-11-12 |
| EP0980517A4 (en) | 2003-01-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4104669B2 (ja) | 取り外し可能要素検定装置 | |
| JP3504276B2 (ja) | 競合イムノアッセイ装置 | |
| US5879951A (en) | Opposable-element assay device employing unidirectional flow | |
| EP0733210B1 (en) | Assay device with a barrier for regulating reagent application | |
| JP3506178B2 (ja) | 対置部材クロマトグラフィー検定装置 | |
| JP3585933B2 (ja) | 導伝バリアを用いる対置成分アッセイ装置 | |
| US6316205B1 (en) | Assay devices and methods of analyte detection | |
| US7344893B2 (en) | Immuno-gold lateral flow assay | |
| JP3386122B2 (ja) | 検定装置 | |
| EP0312394A2 (en) | Membrane-supported immunoassays | |
| JPH09506177A (ja) | 流路中断方式による分析装置 | |
| EP1879028B1 (en) | Use of albumin, bovine, p-aminophenyl n-acetyl ß-d glucosaminide as a positive control line in an immunoassay device | |
| JPH05504617A (ja) | 免疫検定診断用キット | |
| WO2014168580A1 (en) | Double-chamber bi-directional reverse flow device |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20050328 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20050629 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20070320 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20070620 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20080304 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20080326 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110404 Year of fee payment: 3 |
|
| LAPS | Cancellation because of no payment of annual fees |