JP4095685B2 - トルイレンジイソシアネートの製造方法、トルイレンジアミンと水の特定の混合物、及びトルイレンジイソシアネートを製造するための混合物の使用 - Google Patents
トルイレンジイソシアネートの製造方法、トルイレンジアミンと水の特定の混合物、及びトルイレンジイソシアネートを製造するための混合物の使用 Download PDFInfo
- Publication number
- JP4095685B2 JP4095685B2 JP21690096A JP21690096A JP4095685B2 JP 4095685 B2 JP4095685 B2 JP 4095685B2 JP 21690096 A JP21690096 A JP 21690096A JP 21690096 A JP21690096 A JP 21690096A JP 4095685 B2 JP4095685 B2 JP 4095685B2
- Authority
- JP
- Japan
- Prior art keywords
- water
- tda
- toluylenediamine
- mixture
- produce
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 title claims description 153
- 239000000203 mixture Substances 0.000 title claims description 99
- 238000004519 manufacturing process Methods 0.000 title claims description 48
- JXCHMDATRWUOAP-UHFFFAOYSA-N diisocyanatomethylbenzene Chemical compound O=C=NC(N=C=O)C1=CC=CC=C1 JXCHMDATRWUOAP-UHFFFAOYSA-N 0.000 title claims description 46
- -1 toluylene diamine Chemical class 0.000 title claims description 5
- 238000000034 method Methods 0.000 claims description 53
- DYSXLQBUUOPLBB-UHFFFAOYSA-N 2,3-dinitrotoluene Chemical compound CC1=CC=CC([N+]([O-])=O)=C1[N+]([O-])=O DYSXLQBUUOPLBB-UHFFFAOYSA-N 0.000 claims description 41
- 238000006243 chemical reaction Methods 0.000 claims description 41
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 claims description 39
- 238000004821 distillation Methods 0.000 claims description 38
- 239000012045 crude solution Substances 0.000 claims description 35
- 230000008569 process Effects 0.000 claims description 19
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 claims description 8
- 229910017604 nitric acid Inorganic materials 0.000 claims description 8
- 230000006872 improvement Effects 0.000 claims description 2
- 239000002904 solvent Substances 0.000 description 29
- 239000000047 product Substances 0.000 description 21
- RFFLAFLAYFXFSW-UHFFFAOYSA-N 1,2-dichlorobenzene Chemical compound ClC1=CC=CC=C1Cl RFFLAFLAYFXFSW-UHFFFAOYSA-N 0.000 description 16
- 239000006227 byproduct Substances 0.000 description 14
- 238000007710 freezing Methods 0.000 description 14
- 230000008014 freezing Effects 0.000 description 14
- 239000003085 diluting agent Substances 0.000 description 13
- YGYAWVDWMABLBF-UHFFFAOYSA-N Phosgene Chemical compound ClC(Cl)=O YGYAWVDWMABLBF-UHFFFAOYSA-N 0.000 description 10
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 10
- 239000000243 solution Substances 0.000 description 9
- 238000010992 reflux Methods 0.000 description 8
- 239000007789 gas Substances 0.000 description 7
- 239000001257 hydrogen Substances 0.000 description 7
- 229910052739 hydrogen Inorganic materials 0.000 description 7
- 239000011541 reaction mixture Substances 0.000 description 7
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 239000007788 liquid Substances 0.000 description 6
- 238000003860 storage Methods 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 5
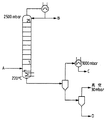
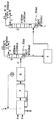
- 238000010586 diagram Methods 0.000 description 5
- 238000010438 heat treatment Methods 0.000 description 5
- 238000005984 hydrogenation reaction Methods 0.000 description 5
- 238000012545 processing Methods 0.000 description 5
- 239000007787 solid Substances 0.000 description 5
- VOZKAJLKRJDJLL-UHFFFAOYSA-N 2,4-diaminotoluene Chemical compound CC1=CC=C(N)C=C1N VOZKAJLKRJDJLL-UHFFFAOYSA-N 0.000 description 4
- RLYCRLGLCUXUPO-UHFFFAOYSA-N 2,6-diaminotoluene Chemical compound CC1=C(N)C=CC=C1N RLYCRLGLCUXUPO-UHFFFAOYSA-N 0.000 description 4
- 150000001412 amines Chemical class 0.000 description 4
- 239000003054 catalyst Substances 0.000 description 4
- 238000002844 melting Methods 0.000 description 4
- 230000008018 melting Effects 0.000 description 4
- 239000012071 phase Substances 0.000 description 4
- 238000004062 sedimentation Methods 0.000 description 4
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 238000007796 conventional method Methods 0.000 description 3
- 238000001816 cooling Methods 0.000 description 3
- 238000001035 drying Methods 0.000 description 3
- 238000001914 filtration Methods 0.000 description 3
- 239000012074 organic phase Substances 0.000 description 3
- 238000012856 packing Methods 0.000 description 3
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 3
- 238000000926 separation method Methods 0.000 description 3
- 238000005292 vacuum distillation Methods 0.000 description 3
- 238000004056 waste incineration Methods 0.000 description 3
- FZZMTSNZRBFGGU-UHFFFAOYSA-N 2-chloro-7-fluoroquinazolin-4-amine Chemical compound FC1=CC=C2C(N)=NC(Cl)=NC2=C1 FZZMTSNZRBFGGU-UHFFFAOYSA-N 0.000 description 2
- CZSSKBQAJULWPY-UHFFFAOYSA-N 2-undecylsulfanylacetic acid Chemical compound CCCCCCCCCCCSCC(O)=O CZSSKBQAJULWPY-UHFFFAOYSA-N 0.000 description 2
- CNPURSDMOWDNOQ-UHFFFAOYSA-N 4-methoxy-7h-pyrrolo[2,3-d]pyrimidin-2-amine Chemical compound COC1=NC(N)=NC2=C1C=CN2 CNPURSDMOWDNOQ-UHFFFAOYSA-N 0.000 description 2
- ZPTVNYMJQHSSEA-UHFFFAOYSA-N 4-nitrotoluene Chemical compound CC1=CC=C([N+]([O-])=O)C=C1 ZPTVNYMJQHSSEA-UHFFFAOYSA-N 0.000 description 2
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 2
- 239000007868 Raney catalyst Substances 0.000 description 2
- NPXOKRUENSOPAO-UHFFFAOYSA-N Raney nickel Chemical compound [Al].[Ni] NPXOKRUENSOPAO-UHFFFAOYSA-N 0.000 description 2
- 229910000564 Raney nickel Inorganic materials 0.000 description 2
- 238000009835 boiling Methods 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 238000010924 continuous production Methods 0.000 description 2
- 238000007872 degassing Methods 0.000 description 2
- 239000008367 deionised water Substances 0.000 description 2
- 229910021641 deionized water Inorganic materials 0.000 description 2
- 239000012943 hotmelt Substances 0.000 description 2
- IXCSERBJSXMMFS-UHFFFAOYSA-N hydrogen chloride Substances Cl.Cl IXCSERBJSXMMFS-UHFFFAOYSA-N 0.000 description 2
- 229910000041 hydrogen chloride Inorganic materials 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 239000002699 waste material Substances 0.000 description 2
- AXNUJYHFQHQZBE-UHFFFAOYSA-N 3-methylbenzene-1,2-diamine Chemical compound CC1=CC=CC(N)=C1N AXNUJYHFQHQZBE-UHFFFAOYSA-N 0.000 description 1
- DGRGLKZMKWPMOH-UHFFFAOYSA-N 4-methylbenzene-1,2-diamine Chemical compound CC1=CC=C(N)C(N)=C1 DGRGLKZMKWPMOH-UHFFFAOYSA-N 0.000 description 1
- 0 CCC*1(C)C(C)(C2)C3*2C(C)C1C3 Chemical compound CCC*1(C)C(C)(C2)C3*2C(C)C1C3 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000006286 aqueous extract Substances 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 150000004982 aromatic amines Chemical class 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 238000009903 catalytic hydrogenation reaction Methods 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 239000002826 coolant Substances 0.000 description 1
- 150000004985 diamines Chemical class 0.000 description 1
- 125000005442 diisocyanate group Chemical group 0.000 description 1
- AEQYJEISXUOVLG-UHFFFAOYSA-N dinitromethylbenzene Chemical compound [O-][N+](=O)C([N+]([O-])=O)C1=CC=CC=C1.[O-][N+](=O)C([N+]([O-])=O)C1=CC=CC=C1 AEQYJEISXUOVLG-UHFFFAOYSA-N 0.000 description 1
- 150000002009 diols Chemical class 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 238000009776 industrial production Methods 0.000 description 1
- 239000013067 intermediate product Substances 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 238000011031 large-scale manufacturing process Methods 0.000 description 1
- 235000021190 leftovers Nutrition 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000003032 molecular docking Methods 0.000 description 1
- 238000006396 nitration reaction Methods 0.000 description 1
- 150000002828 nitro derivatives Chemical class 0.000 description 1
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- 239000011087 paperboard Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000004886 process control Methods 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 239000011949 solid catalyst Substances 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- PJANXHGTPQOBST-VAWYXSNFSA-N trans-stilbene Chemical group C=1C=CC=CC=1/C=C/C1=CC=CC=C1 PJANXHGTPQOBST-VAWYXSNFSA-N 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 239000012808 vapor phase Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C211/00—Compounds containing amino groups bound to a carbon skeleton
- C07C211/43—Compounds containing amino groups bound to a carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton
- C07C211/44—Compounds containing amino groups bound to a carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton having amino groups bound to only one six-membered aromatic ring
- C07C211/49—Compounds containing amino groups bound to a carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton having amino groups bound to only one six-membered aromatic ring having at least two amino groups bound to the carbon skeleton
- C07C211/50—Compounds containing amino groups bound to a carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton having amino groups bound to only one six-membered aromatic ring having at least two amino groups bound to the carbon skeleton with at least two amino groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C209/00—Preparation of compounds containing amino groups bound to a carbon skeleton
- C07C209/30—Preparation of compounds containing amino groups bound to a carbon skeleton by reduction of nitrogen-to-oxygen or nitrogen-to-nitrogen bonds
- C07C209/32—Preparation of compounds containing amino groups bound to a carbon skeleton by reduction of nitrogen-to-oxygen or nitrogen-to-nitrogen bonds by reduction of nitro groups
- C07C209/36—Preparation of compounds containing amino groups bound to a carbon skeleton by reduction of nitrogen-to-oxygen or nitrogen-to-nitrogen bonds by reduction of nitro groups by reduction of nitro groups bound to carbon atoms of six-membered aromatic rings in presence of hydrogen-containing gases and a catalyst
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C209/00—Preparation of compounds containing amino groups bound to a carbon skeleton
- C07C209/82—Purification; Separation; Stabilisation; Use of additives
- C07C209/84—Purification
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C263/00—Preparation of derivatives of isocyanic acid
- C07C263/10—Preparation of derivatives of isocyanic acid by reaction of amines with carbonyl halides, e.g. with phosgene
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19528781A DE19528781A1 (de) | 1995-08-04 | 1995-08-04 | Verfahren zur Herstellung von Toluylendiisocyanat, spezielle Gemische aus Toluylendiamin und Wasser und deren Verwendung zur Herstellung von Toluylendiisocyanat |
| DE19528781.9 | 1995-08-04 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JPH0952873A JPH0952873A (ja) | 1997-02-25 |
| JP4095685B2 true JP4095685B2 (ja) | 2008-06-04 |
Family
ID=7768754
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP21690096A Expired - Lifetime JP4095685B2 (ja) | 1995-08-04 | 1996-07-31 | トルイレンジイソシアネートの製造方法、トルイレンジアミンと水の特定の混合物、及びトルイレンジイソシアネートを製造するための混合物の使用 |
Country Status (15)
| Country | Link |
|---|---|
| US (2) | US5849947A (enExample) |
| EP (2) | EP0757034B1 (enExample) |
| JP (1) | JP4095685B2 (enExample) |
| KR (1) | KR100453862B1 (enExample) |
| CN (2) | CN1070474C (enExample) |
| BR (1) | BR9603278A (enExample) |
| CA (1) | CA2182654A1 (enExample) |
| CZ (1) | CZ292604B6 (enExample) |
| DE (2) | DE19528781A1 (enExample) |
| ES (1) | ES2176380T3 (enExample) |
| PL (1) | PL184186B1 (enExample) |
| PT (1) | PT757034E (enExample) |
| RU (1) | RU2202537C2 (enExample) |
| TW (1) | TW374080B (enExample) |
| UA (1) | UA47399C2 (enExample) |
Families Citing this family (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100311692B1 (ko) * | 1997-12-23 | 2001-11-15 | 신현준 | 활성탄소 섬유의 제조방법 |
| EP1371633A1 (en) * | 2002-06-14 | 2003-12-17 | Bayer Ag | Process for the purification of mixtures of toluenediisocyanate incorporating a dividing-wall distillation column |
| ATE337297T1 (de) * | 2002-10-22 | 2006-09-15 | Bayer Materialscience Ag | Verfahren zur reinigung von diisocyanatotoluol unter verwendung einer destillationskolonne mit trennwand in der endreinigung |
| DE10260093A1 (de) * | 2002-12-19 | 2004-07-01 | Basf Ag | Verfahren zur Abtrennung von Isocyanaten aus einem Reaktionsgemisch |
| JP5175033B2 (ja) * | 2005-03-10 | 2013-04-03 | 三井化学株式会社 | ポリイソシアネートの製造方法およびポリイソシアネートの製造装置 |
| KR101429417B1 (ko) * | 2005-03-10 | 2014-08-13 | 미쓰이 가가쿠 가부시키가이샤 | 폴리이소시아네이트의 제조 방법 및 폴리이소시아네이트의 제조 장치 |
| DE102005032430A1 (de) * | 2005-07-12 | 2007-01-25 | Bayer Materialscience Ag | Verfahren zur Herstellung von Toluylendiamin |
| HUE033435T2 (en) * | 2005-07-12 | 2017-11-28 | Covestro Deutschland Ag | Process for preparing toluenediamine |
| DE102005036870A1 (de) * | 2005-08-02 | 2007-02-08 | Bayer Materialscience Ag | Verfahren zur Gasphasenphosgenierung |
| JP4791783B2 (ja) * | 2005-08-31 | 2011-10-12 | 三井化学株式会社 | ポリイソシアネートの製造方法 |
| DE102006022447A1 (de) * | 2006-05-13 | 2007-11-15 | Bayer Materialscience Ag | Verfahren zur gekoppelten Herstellung von Chlor und Isocyanaten |
| US8030522B2 (en) * | 2006-06-07 | 2011-10-04 | Bayer Materialscience Llc | Process for the production of toluene diisocyanate |
| CN100455559C (zh) * | 2006-11-08 | 2009-01-28 | 大连理工大学 | 芳香族硝基化合物还原制备芳胺的方法 |
| DE102006059678A1 (de) * | 2006-12-18 | 2008-06-19 | Bayer Materialscience Ag | Verfahren zur Herstellung von aromatischen Aminen |
| DE102006060572A1 (de) † | 2006-12-19 | 2008-06-26 | Bayer Materialscience Ag | Verfahren zur Herstellung von Toluylendiaminen durch katalytische Hydrierung von Dinitrotoluolen |
| JP5368997B2 (ja) * | 2007-11-27 | 2013-12-18 | 三井化学株式会社 | トリレンジアミンの脱水方法および脱水装置 |
| KR20110095899A (ko) | 2008-11-19 | 2011-08-25 | 바스프 에스이 | 이소시아네이트의 제조 방법 |
| CN101712621B (zh) * | 2009-09-02 | 2012-10-31 | 甘肃银达化工有限公司 | 连续制备甲苯二胺的方法 |
| CN101671277B (zh) * | 2009-09-18 | 2011-12-28 | 天津大学 | 一种甲苯二异氰酸酯连续生产中热集成的溶剂回收方法 |
| BR112012021120A2 (pt) * | 2010-03-18 | 2016-05-17 | Huntsman Int Llc | processo para a conversão do composto nitro aromático em aminas |
| JP5883661B2 (ja) * | 2012-01-25 | 2016-03-15 | 旭化成ケミカルズ株式会社 | N−置換カルバミン酸エステルの製造方法 |
| IL236155B (en) * | 2014-12-09 | 2021-10-31 | List Holding Ag | Chemical production methods and devices for their execution |
| US11078151B2 (en) | 2017-01-27 | 2021-08-03 | Covestro Llc | Process for the preparation of stable toluene diamine residue/water blends, related compositions, and methods of using such blends as a fuel |
| CN110105248B (zh) * | 2019-05-31 | 2021-07-20 | 上海应用技术大学 | 一种甲苯二异氰酸酯的制备方法 |
| US20230159433A1 (en) * | 2020-05-19 | 2023-05-25 | Covestro Deutschland Ag | Method for preparing toluylene diamine mixtures |
| WO2022248448A1 (en) * | 2021-05-25 | 2022-12-01 | Basf Se | Process for preparing at least one polyisocyanate |
| EP4151619A1 (en) | 2021-09-20 | 2023-03-22 | Covestro Deutschland AG | Method for the removal of water from and transport of aliphatic diamines |
| EP4653418A1 (de) | 2024-05-22 | 2025-11-26 | Covestro Deutschland AG | Verfahren zur herstellung von organischem isocyanat mit verbesserter nachhaltigkeit |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB786407A (en) * | 1956-04-30 | 1957-11-20 | Du Pont | Process for the catalytic hydrogenation of organic nitro compounds |
| US2976320A (en) * | 1957-07-30 | 1961-03-21 | Allied Chem | Catalytic hydrogenation of the dinitro derivatives of toluene |
| GB895197A (en) * | 1960-11-25 | 1962-05-02 | Gen Aniline & Film Corp | Catalytic reduction of aromatic dinitro compounds |
| GB1164519A (en) * | 1966-07-19 | 1969-09-17 | Inst Chemii Ogolnej | Method for preparing Toluylenediisocyanates from Dinitrotoluenes |
| GB1303562A (enExample) * | 1971-03-12 | 1973-01-17 | ||
| DE2456308A1 (de) * | 1974-11-28 | 1976-08-12 | Bayer Ag | Verfahren zur herstellung von aminoverbindungen |
| US4224249A (en) * | 1979-05-14 | 1980-09-23 | Air Products And Chemicals, Inc. | Toluene diamine from non-washed dinitrotoluene |
| US4717774A (en) * | 1985-12-20 | 1988-01-05 | Basf Corporation | Process for the preparation of toluene diamines |
| DE4230098C1 (enExample) * | 1992-09-09 | 1993-06-03 | Bayer Ag, 5090 Leverkusen, De | |
| US5449832A (en) * | 1994-10-05 | 1995-09-12 | Air Products And Chemicals, Inc. | Process for storage and transport of toluenediamine |
| US5714634A (en) * | 1996-06-25 | 1998-02-03 | Air Products And Chemicals, Inc. | Process for storage and transport of toluenediamine |
| US5693862A (en) * | 1996-12-18 | 1997-12-02 | Arco Chemical Technology, L.P. | Process for transport of toluenediamine |
-
1995
- 1995-08-04 DE DE19528781A patent/DE19528781A1/de not_active Withdrawn
-
1996
- 1996-07-23 EP EP96111809A patent/EP0757034B1/de not_active Expired - Lifetime
- 1996-07-23 DE DE59609285T patent/DE59609285D1/de not_active Expired - Lifetime
- 1996-07-23 ES ES96111809T patent/ES2176380T3/es not_active Expired - Lifetime
- 1996-07-23 PT PT96111809T patent/PT757034E/pt unknown
- 1996-07-23 EP EP01100055A patent/EP1090906A1/de not_active Ceased
- 1996-07-31 JP JP21690096A patent/JP4095685B2/ja not_active Expired - Lifetime
- 1996-08-01 US US08/691,222 patent/US5849947A/en not_active Expired - Lifetime
- 1996-08-02 UA UA96083118A patent/UA47399C2/uk unknown
- 1996-08-02 CA CA002182654A patent/CA2182654A1/en not_active Abandoned
- 1996-08-02 PL PL96315490A patent/PL184186B1/pl not_active IP Right Cessation
- 1996-08-02 CN CN96111631A patent/CN1070474C/zh not_active Expired - Lifetime
- 1996-08-02 TW TW85109303A patent/TW374080B/zh not_active IP Right Cessation
- 1996-08-02 CN CNB001317962A patent/CN1163470C/zh not_active Expired - Lifetime
- 1996-08-02 CZ CZ19962299A patent/CZ292604B6/cs not_active IP Right Cessation
- 1996-08-02 RU RU96115368/04A patent/RU2202537C2/ru active
- 1996-08-03 KR KR1019960032490A patent/KR100453862B1/ko not_active Expired - Lifetime
- 1996-08-05 BR BR9603278A patent/BR9603278A/pt not_active Application Discontinuation
-
1998
- 1998-09-08 US US09/149,379 patent/US6472564B1/en not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| CA2182654A1 (en) | 1997-02-05 |
| PT757034E (pt) | 2002-09-30 |
| BR9603278A (pt) | 1998-05-12 |
| EP0757034B1 (de) | 2002-06-05 |
| UA47399C2 (uk) | 2002-07-15 |
| CN1070474C (zh) | 2001-09-05 |
| EP0757034A1 (de) | 1997-02-05 |
| CN1149579A (zh) | 1997-05-14 |
| EP1090906A1 (de) | 2001-04-11 |
| US6472564B1 (en) | 2002-10-29 |
| JPH0952873A (ja) | 1997-02-25 |
| KR100453862B1 (ko) | 2005-04-08 |
| MX9603167A (es) | 1997-07-31 |
| RU2202537C2 (ru) | 2003-04-20 |
| MX195147B (enExample) | 2000-01-31 |
| KR970010735A (ko) | 1997-03-27 |
| DE19528781A1 (de) | 1997-02-06 |
| PL315490A1 (en) | 1997-02-17 |
| ES2176380T3 (es) | 2002-12-01 |
| CZ229996A3 (en) | 1997-02-12 |
| PL184186B1 (pl) | 2002-09-30 |
| CZ292604B6 (cs) | 2003-11-12 |
| CN1332149A (zh) | 2002-01-23 |
| TW374080B (enExample) | 1999-11-11 |
| US5849947A (en) | 1998-12-15 |
| DE59609285D1 (de) | 2002-07-11 |
| CN1163470C (zh) | 2004-08-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4095685B2 (ja) | トルイレンジイソシアネートの製造方法、トルイレンジアミンと水の特定の混合物、及びトルイレンジイソシアネートを製造するための混合物の使用 | |
| JP5449082B2 (ja) | メチレンジアニリンおよびメチレンビス(フェニルイソシアナート)の製造方法 | |
| EP1864969B1 (en) | Process for the production of the toluene diisocyanate | |
| US8026387B2 (en) | Method for producing isocyanates | |
| KR101685699B1 (ko) | 이소시아네이트의 기상 제조 방법 | |
| CN103524356B (zh) | 通过催化氢化二硝基甲苯制备甲苯二胺的方法 | |
| US20080275269A1 (en) | Process for the preparation of 4,4'-diphenylmethane diisocyanate | |
| KR101829481B1 (ko) | 기체 상에서의 이소시아네이트의 제조 방법 | |
| JP4307588B2 (ja) | 脂肪族イソシアネート化合物の製造法 | |
| MXPA96003167A (en) | A process to prepare toluylendiisocyanate, toluylendiamines and water specific mixtures, and mixtures use to prepare toluylendiisocyanate | |
| JP4241968B2 (ja) | ジニトロトルエンの断熱製造法 | |
| EP4405326A1 (en) | Method for the processing and transport of hexane-1,6-diamine or pentane-1,5-diamine | |
| HK1118800A (en) | Process for the preparation of toluenediamines by catalytic hydrogenation of dinitrotoluenes | |
| HU202834B (en) | Process and apparatus for producing aromathic isocyanates |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20061109 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20061124 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20070219 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20070412 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20070705 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20070820 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20080208 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20080310 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110314 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110314 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120314 Year of fee payment: 4 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130314 Year of fee payment: 5 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130314 Year of fee payment: 5 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140314 Year of fee payment: 6 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| EXPY | Cancellation because of completion of term |