EP3111869B1 - System zum versiegeln einer aifblasbaren prothese - Google Patents
System zum versiegeln einer aifblasbaren prothese Download PDFInfo
- Publication number
- EP3111869B1 EP3111869B1 EP16177165.4A EP16177165A EP3111869B1 EP 3111869 B1 EP3111869 B1 EP 3111869B1 EP 16177165 A EP16177165 A EP 16177165A EP 3111869 B1 EP3111869 B1 EP 3111869B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- prosthesis
- tube
- expandable
- optionally
- plug
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000007789 sealing Methods 0.000 title claims description 18
- 239000000560 biocompatible material Substances 0.000 claims description 8
- 239000000126 substance Substances 0.000 claims description 7
- 238000000926 separation method Methods 0.000 claims description 2
- 238000000034 method Methods 0.000 description 82
- 238000002513 implantation Methods 0.000 description 64
- 210000000988 bone and bone Anatomy 0.000 description 56
- 239000000463 material Substances 0.000 description 33
- 208000027418 Wounds and injury Diseases 0.000 description 31
- 230000006378 damage Effects 0.000 description 31
- 208000014674 injury Diseases 0.000 description 31
- 230000033001 locomotion Effects 0.000 description 28
- 206010017076 Fracture Diseases 0.000 description 24
- 210000000513 rotator cuff Anatomy 0.000 description 24
- 239000000945 filler Substances 0.000 description 23
- 239000012634 fragment Substances 0.000 description 23
- 241001260012 Bursa Species 0.000 description 22
- 210000002435 tendon Anatomy 0.000 description 22
- 210000001519 tissue Anatomy 0.000 description 21
- 210000004872 soft tissue Anatomy 0.000 description 20
- 239000008177 pharmaceutical agent Substances 0.000 description 18
- 210000002659 acromion Anatomy 0.000 description 16
- 238000004873 anchoring Methods 0.000 description 15
- 230000000994 depressogenic effect Effects 0.000 description 15
- 239000012530 fluid Substances 0.000 description 15
- 230000008569 process Effects 0.000 description 13
- 208000010392 Bone Fractures Diseases 0.000 description 12
- 230000004054 inflammatory process Effects 0.000 description 9
- 229920000747 poly(lactic acid) Polymers 0.000 description 9
- -1 polyethylene Polymers 0.000 description 9
- 239000004626 polylactic acid Substances 0.000 description 9
- 206010061218 Inflammation Diseases 0.000 description 8
- 208000015181 infectious disease Diseases 0.000 description 8
- 229920001606 poly(lactic acid-co-glycolic acid) Polymers 0.000 description 8
- 229920001610 polycaprolactone Polymers 0.000 description 8
- 102000008186 Collagen Human genes 0.000 description 7
- 108010035532 Collagen Proteins 0.000 description 7
- 239000004568 cement Substances 0.000 description 7
- 229920001436 collagen Polymers 0.000 description 7
- 230000000694 effects Effects 0.000 description 7
- 210000002758 humerus Anatomy 0.000 description 7
- 239000007788 liquid Substances 0.000 description 7
- 229920000117 poly(dioxanone) Polymers 0.000 description 7
- 239000004696 Poly ether ether ketone Substances 0.000 description 6
- 229920000954 Polyglycolide Polymers 0.000 description 6
- 229920000331 Polyhydroxybutyrate Polymers 0.000 description 6
- 239000002639 bone cement Substances 0.000 description 6
- 229920000609 methyl cellulose Polymers 0.000 description 6
- 239000001923 methylcellulose Substances 0.000 description 6
- 235000010981 methylcellulose Nutrition 0.000 description 6
- 239000005015 poly(hydroxybutyrate) Substances 0.000 description 6
- 229920002530 polyetherether ketone Polymers 0.000 description 6
- 229920000096 Plastarch material Polymers 0.000 description 5
- 229920002494 Zein Polymers 0.000 description 5
- JUPQTSLXMOCDHR-UHFFFAOYSA-N benzene-1,4-diol;bis(4-fluorophenyl)methanone Chemical compound OC1=CC=C(O)C=C1.C1=CC(F)=CC=C1C(=O)C1=CC=C(F)C=C1 JUPQTSLXMOCDHR-UHFFFAOYSA-N 0.000 description 5
- 230000035876 healing Effects 0.000 description 5
- 238000001727 in vivo Methods 0.000 description 5
- 230000007246 mechanism Effects 0.000 description 5
- 239000004814 polyurethane Substances 0.000 description 5
- 229920002635 polyurethane Polymers 0.000 description 5
- 230000009443 proangiogenesis Effects 0.000 description 5
- 239000005019 zein Substances 0.000 description 5
- 229940093612 zein Drugs 0.000 description 5
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 4
- 239000004698 Polyethylene Substances 0.000 description 4
- 239000002870 angiogenesis inducing agent Substances 0.000 description 4
- 239000003242 anti bacterial agent Substances 0.000 description 4
- 229940124599 anti-inflammatory drug Drugs 0.000 description 4
- 229940088710 antibiotic agent Drugs 0.000 description 4
- 210000001124 body fluid Anatomy 0.000 description 4
- 210000000281 joint capsule Anatomy 0.000 description 4
- 210000003205 muscle Anatomy 0.000 description 4
- 229920000573 polyethylene Polymers 0.000 description 4
- 239000010703 silicon Substances 0.000 description 4
- 229910052710 silicon Inorganic materials 0.000 description 4
- 229920002994 synthetic fiber Polymers 0.000 description 4
- 229920000271 Kevlar® Polymers 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- 239000007943 implant Substances 0.000 description 3
- 210000003041 ligament Anatomy 0.000 description 3
- 238000002595 magnetic resonance imaging Methods 0.000 description 3
- 238000000465 moulding Methods 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 230000008439 repair process Effects 0.000 description 3
- 239000010935 stainless steel Substances 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- MHSKRLJMQQNJNC-UHFFFAOYSA-N terephthalamide Chemical compound NC(=O)C1=CC=C(C(N)=O)C=C1 MHSKRLJMQQNJNC-UHFFFAOYSA-N 0.000 description 3
- 229920002725 thermoplastic elastomer Polymers 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- 208000034826 Genetic Predisposition to Disease Diseases 0.000 description 2
- 208000001164 Osteoporotic Fractures Diseases 0.000 description 2
- 239000012891 Ringer solution Substances 0.000 description 2
- 208000024288 Rotator Cuff injury Diseases 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 2
- 239000004699 Ultra-high molecular weight polyethylene Substances 0.000 description 2
- 230000002745 absorbent Effects 0.000 description 2
- 239000002250 absorbent Substances 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 210000000459 calcaneus Anatomy 0.000 description 2
- 229910002092 carbon dioxide Inorganic materials 0.000 description 2
- 239000001569 carbon dioxide Substances 0.000 description 2
- 210000000845 cartilage Anatomy 0.000 description 2
- 238000004891 communication Methods 0.000 description 2
- 230000006835 compression Effects 0.000 description 2
- 238000007906 compression Methods 0.000 description 2
- 238000005520 cutting process Methods 0.000 description 2
- 230000007850 degeneration Effects 0.000 description 2
- 230000000881 depressing effect Effects 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 210000003195 fascia Anatomy 0.000 description 2
- 238000002594 fluoroscopy Methods 0.000 description 2
- 210000001624 hip Anatomy 0.000 description 2
- 230000001771 impaired effect Effects 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 230000000399 orthopedic effect Effects 0.000 description 2
- 238000002559 palpation Methods 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 210000001991 scapula Anatomy 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 206010041569 spinal fracture Diseases 0.000 description 2
- 229910001220 stainless steel Inorganic materials 0.000 description 2
- 239000010936 titanium Substances 0.000 description 2
- 229920000785 ultra high molecular weight polyethylene Polymers 0.000 description 2
- 210000000689 upper leg Anatomy 0.000 description 2
- 229920000936 Agarose Polymers 0.000 description 1
- 206010010356 Congenital anomaly Diseases 0.000 description 1
- 206010058314 Dysplasia Diseases 0.000 description 1
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Polymers OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 1
- 229910000861 Mg alloy Inorganic materials 0.000 description 1
- 206010031149 Osteitis Diseases 0.000 description 1
- 206010031252 Osteomyelitis Diseases 0.000 description 1
- 229910001069 Ti alloy Inorganic materials 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 238000011882 arthroplasty Methods 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 230000010256 bone deposition Effects 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 208000003295 carpal tunnel syndrome Diseases 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000002591 computed tomography Methods 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 210000000852 deltoid muscle Anatomy 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 201000010934 exostosis Diseases 0.000 description 1
- 210000000245 forearm Anatomy 0.000 description 1
- 210000004349 growth plate Anatomy 0.000 description 1
- 229920001903 high density polyethylene Polymers 0.000 description 1
- 239000004700 high-density polyethylene Substances 0.000 description 1
- 210000004394 hip joint Anatomy 0.000 description 1
- 210000001503 joint Anatomy 0.000 description 1
- 210000003127 knee Anatomy 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 210000000236 metacarpal bone Anatomy 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 210000002976 pectoralis muscle Anatomy 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 229920002463 poly(p-dioxanone) polymer Polymers 0.000 description 1
- 239000004632 polycaprolactone Substances 0.000 description 1
- 239000000622 polydioxanone Substances 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 210000002320 radius Anatomy 0.000 description 1
- 230000000306 recurrent effect Effects 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 229910001256 stainless steel alloy Inorganic materials 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 210000000623 ulna Anatomy 0.000 description 1
- 238000002604 ultrasonography Methods 0.000 description 1
- 238000011179 visual inspection Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/56—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor
- A61B17/562—Implants for placement in joint gaps without restricting joint motion, e.g. to reduce arthritic pain
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/40—Joints for shoulders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/56—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor
- A61B17/58—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor for osteosynthesis, e.g. bone plates, screws, setting implements or the like
- A61B17/68—Internal fixation devices, including fasteners and spinal fixators, even if a part thereof projects from the skin
- A61B17/70—Spinal positioners or stabilisers ; Bone stabilisers comprising fluid filler in an implant
- A61B17/7061—Spinal positioners or stabilisers ; Bone stabilisers comprising fluid filler in an implant for stabilising vertebrae or discs by improving the condition of their tissues, e.g. using implanted medication or fluid exchange
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/56—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor
- A61B17/58—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor for osteosynthesis, e.g. bone plates, screws, setting implements or the like
- A61B17/68—Internal fixation devices, including fasteners and spinal fixators, even if a part thereof projects from the skin
- A61B17/70—Spinal positioners or stabilisers ; Bone stabilisers comprising fluid filler in an implant
- A61B17/7097—Stabilisers comprising fluid filler in an implant, e.g. balloon; devices for inserting or filling such implants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/56—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor
- A61B17/58—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor for osteosynthesis, e.g. bone plates, screws, setting implements or the like
- A61B17/68—Internal fixation devices, including fasteners and spinal fixators, even if a part thereof projects from the skin
- A61B17/72—Intramedullary pins, nails or other devices
- A61B17/7233—Intramedullary pins, nails or other devices with special means of locking the nail to the bone
- A61B17/7258—Intramedullary pins, nails or other devices with special means of locking the nail to the bone with laterally expanding parts, e.g. for gripping the bone
- A61B17/7275—Intramedullary pins, nails or other devices with special means of locking the nail to the bone with laterally expanding parts, e.g. for gripping the bone with expanding cylindrical parts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/56—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor
- A61B17/58—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor for osteosynthesis, e.g. bone plates, screws, setting implements or the like
- A61B17/88—Osteosynthesis instruments; Methods or means for implanting or extracting internal or external fixation devices
- A61B17/885—Tools for expanding or compacting bones or discs or cavities therein
- A61B17/8852—Tools for expanding or compacting bones or discs or cavities therein capable of being assembled or enlarged, or changing shape, inside the bone or disc
- A61B17/8855—Tools for expanding or compacting bones or discs or cavities therein capable of being assembled or enlarged, or changing shape, inside the bone or disc inflatable, e.g. kyphoplasty balloons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/56—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor
- A61B17/58—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor for osteosynthesis, e.g. bone plates, screws, setting implements or the like
- A61B17/88—Osteosynthesis instruments; Methods or means for implanting or extracting internal or external fixation devices
- A61B17/8802—Equipment for handling bone cement or other fluid fillers
- A61B17/8805—Equipment for handling bone cement or other fluid fillers for introducing fluid filler into bone or extracting it
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B2017/00535—Surgical instruments, devices or methods, e.g. tourniquets pneumatically or hydraulically operated
- A61B2017/00557—Surgical instruments, devices or methods, e.g. tourniquets pneumatically or hydraulically operated inflatable
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0003—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having an inflatable pocket filled with fluid, e.g. liquid or gas
Definitions
- the present inventions relate generally to the field of medical devices and the treatment of human medical conditions using the medical devices. More specifically, the present inventions include expandable prosthetic devices used for treating a variety of conditions, including rotator cuff injuries, broken and/or depressed bone fractures, infection and/or inflammation in the body.
- expandable prosthetic devices used for treating a variety of conditions, including rotator cuff injuries, broken and/or depressed bone fractures, infection and/or inflammation in the body.
- the invention is defined in claim 1.
- An aspect of some embodiments of the invention relates to prostheses adapted to reduce injuries between soft tissues of the body and other tissues.
- soft tissues are for example, tendons and/or ligaments.
- other tissues are, for example, bones.
- the prosthesis is expandable.
- the prosthesis is elastic.
- the prosthesis is rigid.
- the prosthesis is shaped and/or sized to simulate a bursa naturally occurring in the body.
- the bursa simulated is the one expected to be present at the implantation site of the prosthesis in a healthy patient.
- the member is expandable.
- the member is adapted to be at least partially inflated.
- the member is inflated sufficiently to reduce rubbing of the soft tissues against other tissues while permitting at least some movement of the soft tissues relative to the other tissues.
- at least some movement of the soft tissues relative to the other tissues is full movement.
- the member is sponge-like.
- the sponge-like member is provided with a fluid absorbent material which when fluids are absorbed induces expansion of the sponge-like expandable member.
- the prosthesis is constructed of at least one non-biodegradable material.
- the at least one non-biodegradable material is polyethylene, polyurethane, silicon, or poly-paraphenylene terephthalamide.
- the member is contoured to act as a counterpart to natural anatomical features of an implantation site.
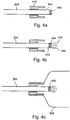
- a system for sealing an inflatable prosthesis comprising: a prosthesis inflation device; a tube operatively connected to the prosthesis near one end and the prosthesis inflation device on the other end; a plug attached to the tube at the prosthesis end of the tube ; and, a rigid ring attached to the prosthesis and slidably attached around the tube between the prosthesis inflation device and the plug; wherein pulling the tube towards the prosthesis inflation device causes plug to lodge in the rigid ring, sealing the prosthesis with the plug.
- the plug is attached to the tube by gripping protrusions.
- tube 204 is provided with gripping protrusions 406 in order to increase the contact surface between tube 204 and plug 402 and therefore the force that may be applied to plug 402 when sealing prosthesis 202.
- plug 402 is ovoid shaped, and/or has a shape such that plug's 402 loose end 408 is larger than the attached end 410 so that, as described in more detail below with respect to Figs. 4A-C , 5 and 7 , plug 402 seals inflatable expandable prosthesis 202 during implantation.
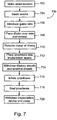
- a guide wire is introduced (706) via the needle into the space between the rotator cuff tendons and the acromion 302 and coracoid process 304, in an exemplary method.
- a dilator is placed (708) over the guide wire and extended into the space.
- a trocar of the dilator is removed (710), leaving a dilator sheath in place.
- Prosthesis deployment in some methods, means no interposition of tendons and/or other soft tissue between the implanted prosthesis and acromion 302 or coracoid process 304 and/or that during movement of the humerus, the prosthesis remains below acromion 302.
- Inflation (716) of prosthesis 202 is performed using prosthesis inflation device 200. It should be understood that only a portion of prosthesis inflation device 200 is shown in Fig. 2 , and that exemplary variations are shown in more detail with respect to Figs. 16-17 .
- Fig. 8 an expandable prosthesis 202 is shown packed for implantation and prior to deployment, in accordance with an exemplary embodiment of the invention. Components of the assembly 800 are enclosed in an external sheath 802 which surrounds at least prosthesis 202, in an exemplary embodiment of the invention.
- External sheath 802 is adapted to maintain prosthesis 202 in a collapsed condition during placing (712) in order to ease insertion of prosthesis 202 into the implantation space or site through the dilator sheath, in an embodiment of the invention. As described above, once prosthesis 202 is in the implantation space, external sheath 802 is removed, enabling prosthesis 202 to be inflated without hindrance apart from the body parts against which prosthesis 202 is pressing.
- tube 204 passes through lumen 804 with lumen 804 providing fluid communication between prosthesis implantation and/or inflation device 200 and an inner space defined by the dimensions of prosthesis 202.
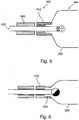
- An attending medical professional performing the implantation procedure holds counterforce ring 506 substantially steady while pulling on tube 204 away from the patient.
- prosthesis inflation device 200 is adapted to perform the steadying of counterforce ring 506 and/or retraction of tube 204 automatically.
- a mechanism is provided to prosthesis inflation device 200 which translates rotational movement to a retracting force on tube 204.
- rotation movement is applied manually.
- tube 204 continues pulling ("retraction" away from patient) of tube 204 causes a portion of plug 402 to break off, the portion of plug 402 lodging itself in lumen 804 of rigid ring 412 thereby sealing prosthesis 202.
- the portion of plug 402 becomes partially deformed as it lodges in lumen 804.
- Prosthesis inflation device 200 now being separated from prosthesis 202 as a result of sealing (718) is withdrawn (720) from the patient and patient is closed.
- a sponge-like expandable prosthesis device is used and therefore, inflation (716) and inflation related actions may not be carried out, for example prosthesis 100 expands rather than inflates.
- the implanted prosthesis is secured, using methods known in the art, to soft tissue and/or bone to prevent the prosthesis from being easily displaced by shoulder movement.
- sutures, clips and/or anchors are used to secure the prosthesis in place.
- an anchoring expandable prosthesis is used.
- Simulating a naturally occurring bursa using a prosthesis is an action taken with respect to method 700.
- simulating is related to inflation (716) in that the prosthesis is inflated to resemble the appropriate size and/or shape and/or characteristics (malleability, compressibility, etc.) of the naturally occurring bursa. Placing the prosthesis at the implantation site and simulating a naturally occurring bursa does not significantly reduce movement of the soft tissues being protected in relation to the other tissues at the implantation site.
- prosthesis 100 or the elastic prosthesis, described above is implanted by inserting the device directly through a small incision, without a cannula, near the implantation site.
- At least part of prosthesis 902 (e . g . tubular member 904) is manufactured, in an embodiment of the invention, by dip molding.
- inflatable tubular member 904 is a seamless balloon made from biocompatible and/or biodegradable synthetic materials such as, but not limited to, PLA, PLGA, PCL, PDO, or any combination and/or families thereof.
- inflatable tubular member 904 is provided with an outer wall thickness adapted to accommodate at least a minimum level of rigidity necessary to maintain the aligned bone fragments during normal activity. For example, forearm bones are normally subjected to forces ranging from a few hundred grams to several kilograms during normal activity.
- metacarpal bones are normally subjected to tens of grams to a few hundred grams of force. It should be noted that these ranges are provided as examples only and that depending on patient and/or the bone fragments being aligned, the wall thickness of inflatable tubular member will be adapted to maintain alignment of the bone fragments in spite of the anticipated stress on prosthesis 902 during normal activity and/or rehabilitation of the patient.
- inflation of prosthesis 902 is performed using a physiologic fluid such as saline, Hartman or Ringer solutions and/or any other biocompatible and/or biodegradable fluid.
- a physiologic fluid such as saline, Hartman or Ringer solutions and/or any other biocompatible and/or biodegradable fluid.
- inflation is performed using a biocompatible and/or biodegradable gel.
- inflation of prosthesis 902 is performed using a gas, for example air and/or carbon dioxide.
- prosthesis 902 is filled with a cement that hardens and/or seals the open end 906 of prosthesis 902. In some methods, the cement is used provide alignment for the fractured bone segments.
- prosthesis 902 (and/or other prostheses described herein) is used with a calibration kit which determines the size of the medullar cavity and/or the proper size inflatable tubular member 904 to use with the medullar cavity.
- the calibration kit is integrated with prosthesis 902.
- the calibration kit is integrated with prosthesis implantation and/or inflation device 900.
- a calibration expandable member is first deployed into the medullar cavity to measure the cavity shape and/or size and then upon deployment of prosthesis 902, its shape and/or size is adapted to match the needs of the measured medullar cavity.
- various sizes of dilators are used in conjunction with the calibration expandable member to assist with determining size.
- alignment of the bone segments is maintained by the rigidity of prosthesis 902.
- the rigidity of prosthesis 902 at least partly depends on the internal pressure of prosthesis 902, the internal pressure being at least partly determined by the filler used and/or the percentage of prosthesis 902 that is filled by the filler.
- an external cast is placed on the area proximal to the fracture.
- Expandable sponge-like device 1200 optionally contains within its cavities at least one biocompatible and/or biodegradable gelling material, such as methyl cellulose, agarose, poly(ethylene-glycol) (“PEG”) gel and/or PLA gel, that expands when it comes into contact with at least one bodily fluid, for example by absorbing water. Such absorption is partly responsible for an expansion of sponge-like device 1200 into its intended deployed position.
- biocompatible and/or biodegradable gelling material such as methyl cellulose, agarose, poly(ethylene-glycol) (“PEG”) gel and/or PLA gel
- device 1200 comprises an inflatable structure.
- inflatable device 1200 is constructed of at least one biocompatible and/or biodegradable material, such as those described herein.
- inflatable device 1200 is spherical or cylindrical, having a diameter of 0.5 cm to 5 cm for a sphere or in the long direction (x-axis) and 0.5 cm to 4 cm in the short direction (y-axis) and a height (z-axis) of 0.5 mm to 20 mm.
- device 1200 is adapted to be inserted deflated into a patient's body through a cannula.
- the cannula is a 5 mm-7 mm cannula.
- device 1200 dimensions are adapted for a particular intended use.
- device 1200 is inflated and/or implanted as described herein with respect to prostheses 100, 202, 902.
- Device 1200 optionally contains pharmaceutical agents, for example anti-inflammatory drugs and/or antibiotics and/or pro-angiogenesis factors to promote healing, which are eluted into the body.
- device 1200 is adapted to elute pharmaceutical agents according to a predefined schedule. Adaptation of device 1200 includes construction of device 1200 using materials or combinations of materials which degrade at a predetermined rate, thereby releasing pharmaceutical agents contained therein at a predetermined rate.
- more than one device 1200 is used for treating inflammation and/or infection:
- each device is adapted to elute pharmaceutical agents in view of an overall plan incorporating a plurality of devices.
- an expandable device such as those described herein, is adapted to be used near an articulation to reinforce the articular capsule.
- the expandable device is introduced in anterior fashion to the shoulder articulation between the articular capsule and the deltoid and pectoralis muscle, in order to prevent recurrent dislocation of the shoulder.
- the expandable device is introduced in front of the hip joint capsule to prevent anterior dislocation of the hip, especially in cases of congenital dysplasia of hip.
- the expandable device consists of in inflatable member made of biocompatible and/or biodegradable material.
- the expandable device has a diameter of 1 cm to 7 cm in the long direction (x-axis) and 1 cm to 5 cm in the short direction (y-axis) with a height (z-axis) of 0.5 mm to 25 mm.
- the device has a height of 3 mm to 15 mm.
- Inner section 1302 is manufactured from materials such as polyurethane, ultra high molecular weight polyethylene ("Spectra®”) and/or Kevlar® and/or any reinforced material that can withstand expected pressures on device 1300 as a result of the intended use.
- inner section 1302 is manufactured from a biocompatible and/or biodegradable substance such as PCL, PGA, PHB, plastarch material, PEEK, zein, PLA, PDO and PLGA, collagen, methyl cellulose, or any combination and/or family members thereof.
- inner section 1302 and outer section 1304 are operatively connected to separate inflation devices.
- only one inflation device is needed, for example if outer section 1304 or internal section 1302 is a sponge-like structure.
- components of device 1300 are removably attached to at least one inflation device such as described elsewhere herein.
- Fig. 14 is a perspective view, with a cutaway side view of two vertebrae 1402, 1404, of a device 1300 for treating depressed fractures of a vertebra in vivo .
- device 1300 is adapted to treat osteoporotic fractures of vertebrae.
- device 1300 is used to deploy a filler, for example cement, to act as a force for restoring the natural shape of the fractured vertebra, thereby relieving pain and restoring at least a modicum of function to the patient.
- a filler for example cement
- Prosthesis inflation and/or implantation device 1600 includes a grip 1602 adapted to be grasped in one hand by a medical professional performing the implantation procedure.
- device 1600 includes a housing 1604 adapted to mount therein a device inflation mechanism, for example a syringe 1606 comprising at least a canister 1608 and a plunger 1610, plunger 1610 adapted to travel within canister 1608 and expel filler out of canister 1608 via an outlet 1612 and into tube 204, described above.
- syringe 1606 is adapted to hold and/or inject 5-20cc of filler.
- syringe 1606 is adapted to hold and/or or inject more or less filler depending on the intended application of syringe 1606 and/or needs of the patient.
- device 1600 includes a compression assembly 1614 adapted to apply force for at least for advancement of plunger 1610 in canister 1608 upon activation of a trigger 1616. Additionally and/or optionally, compression assembly 1614 is adapted to apply force for retraction of plunger 1610.
- device 1600 is used to direct a prosthesis into an implantation site, as the prosthesis is removably connected to device 1600 via tube 204.
- FIG. 17 a cutaway side view of an alternate prosthetic inflation and/or implantation device 1700 is shown.
- device 1700 is adapted to advance and/or retract a canister 1702 portion of a syringe 1704 with a plunger 1706 portion remaining relatively fixed in relation to device 1700.
- Plunger 1706 portion is provided with counterforce, as canister 1702 portion is moved towards a proximal end 1708 of device 1700, by a backstop 1710.
- Backstop 1710 in some examples, is fixed to device 1700. In an example, the placement of the backstop is according to a predetermined level of desired inflation of the prosthesis.
- Safety 1712 is comprised of a ball 1714 and a spring 1716 whereby ball 1714 and backstop 1710 are adapted to be counterparts such that ball 1714 releasably fits into a groove on backstop 1710 shaped to receive ball 1714.
Landscapes
- Health & Medical Sciences (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Life Sciences & Earth Sciences (AREA)
- Surgery (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Engineering & Computer Science (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Medical Informatics (AREA)
- Neurology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Molecular Biology (AREA)
- Pain & Pain Management (AREA)
- Rheumatology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Cardiology (AREA)
- Vascular Medicine (AREA)
- Prostheses (AREA)
- On-Site Construction Work That Accompanies The Preparation And Application Of Concrete (AREA)
- Materials For Medical Uses (AREA)
Claims (14)
- System zum Abdichten einer inflatierbaren Prothese, umfassend:eine inflatierbare Prothese (202, 902),eine Protheseninflatiervorrichtung (200; 900),eine Röhre (204) mit einem ersten Ende und einem zweiten Ende, wobei die Röhre (204) in der Nähe des ersten Endes mit der Prothese (202; 902) und am zweiten Ende mit der Protheseninflatiervorrichtung (200; 900) wirkverbunden ist, undeinen starren Ring (412), der an der Prothese (202; 902) angebracht ist und zwischen der Protheseninflatiervorrichtung (200; 900) und dem ersten Ende der Röhre (204) verschiebbar um die Röhre (204) herum angebracht ist, dadurch gekennzeichnet, dass das System ferner einen am ersten Ende der Röhre (204) an der Röhre (204) angebrachten Stopfen (402) umfasst,wobei Ziehen der Röhre zu der Protheseninflatiervorrichtung (200; 900) hin veranlasst, dass sich der Stopfen im starren Ring (412) festklemmt, wodurch die Prothese (202; 902) mit dem Stopfen (402) abgedichtet wird.
- System nach Anspruch 1, wobei der Stopfen (402) über Greifvorsprünge (406) an der Röhre (204) angebracht ist.
- System nach Anspruch 1, umfassend einen Gegenkraftring (506), der geeignet ist, den starren Ring (412) während des Trennens der Protheseninflatiervorrichtung (200; 900) von der Prothese (202; 902) mit Gegenkraft zu beaufschlagen.
- System nach Anspruch 3, wobei der starre Ring (412) durch Ziehen der Röhre (204) zu der Protheseninflatiervorrichtung (200; 900) hin mit dem Gegenkraftring (506) in Kontakt gebracht wird.
- System nach Anspruch 3 oder Anspruch 4, wobei der Gegenkraftring (506) aus einem biokompatiblen Material konstruiert ist.
- System nach Anspruch 5, wobei das biokompatible Material ungefähr mindestens so hart wie der starre Ring (412) ist.
- System nach Anspruch 1, umfassend eine äußere Hülle (802), die die Prothese (202; 902) umschließt.
- System nach Anspruch 1, wobei der starre Ring (412) ein Lumen definiert und das Abdichten der Prothese (202; 902) mit dem Stopfen (402) umfasst, dass sich der Stopfen im Lumen festklemmt.
- System nach Anspruch 1, wobei die Röhre (204) geeignet ist, den Durchgang einer zum Füllen der Prothese (202; 902) verwendeten Substanz durch sie zu gestatten.
- System nach Anspruch 9, wobei die Röhre geeignet ist, den Durchgang der zum Füllen der Prothese verwendeten Substanz durch sie zu gestatten, indem mindestens eine Öffnung (404) in der Röhre (204) vorgesehen ist.
- System nach Anspruch 1, wobei der Stopfen (402) an dem der Prothese (202; 902) am nächsten liegenden Ende der Röhre (204) angebracht ist.
- System nach Anspruch 1, wobei die Röhre (204) mit Greifvorsprüngen (406) versehen ist, um die Kontaktfläche zwischen der Röhre (204) und dem Stopfen (402) zu vergrößern.
- System nach Anspruch 1, wobei mindestens die Röhre (204) und/oder der Stopfen (402) und/oder der starre Ring (412) aus biologisch abbaubaren und/oder biokompatiblen Materialien hergestellt sind.
- System nach Anspruch 1, wobei der Durchmesser des Stopfens (402) größer ist als der Innendurchmesser des starren Rings (412).
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US91805107P | 2007-03-15 | 2007-03-15 | |
| EP08719972.5A EP2124831B1 (de) | 2007-03-15 | 2008-03-13 | Prothesenvorrichtungen |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP08719972.5A Division EP2124831B1 (de) | 2007-03-15 | 2008-03-13 | Prothesenvorrichtungen |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3111869A1 EP3111869A1 (de) | 2017-01-04 |
| EP3111869B1 true EP3111869B1 (de) | 2017-09-20 |
Family
ID=39760198
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP08719972.5A Active EP2124831B1 (de) | 2007-03-15 | 2008-03-13 | Prothesenvorrichtungen |
| EP16177165.4A Active EP3111869B1 (de) | 2007-03-15 | 2008-03-13 | System zum versiegeln einer aifblasbaren prothese |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP08719972.5A Active EP2124831B1 (de) | 2007-03-15 | 2008-03-13 | Prothesenvorrichtungen |
Country Status (11)
| Country | Link |
|---|---|
| US (3) | US8753390B2 (de) |
| EP (2) | EP2124831B1 (de) |
| JP (2) | JP5271288B2 (de) |
| CN (2) | CN101854886B (de) |
| AU (1) | AU2008224435B2 (de) |
| CA (1) | CA2680812C (de) |
| DK (1) | DK3111869T3 (de) |
| ES (2) | ES2645409T3 (de) |
| IL (1) | IL200939A (de) |
| PL (1) | PL2124831T3 (de) |
| WO (1) | WO2008111073A2 (de) |
Families Citing this family (67)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2005257050A1 (en) | 2004-06-23 | 2006-01-05 | Bioprotect Ltd. | Device system and method for tissue displacement or separation |
| JP2010521216A (ja) * | 2007-03-15 | 2010-06-24 | バイオプロテクト リミテッド | 軟組織固定装置 |
| PL2124831T3 (pl) | 2007-03-15 | 2017-03-31 | Ortho-Space Ltd. | Urządzenia protetyczne |
| CN101743033A (zh) | 2007-05-14 | 2010-06-16 | 生物保护有限公司 | 用于将生物活性剂释放至体内组织的释药装置 |
| US8152846B2 (en) * | 2008-03-06 | 2012-04-10 | Musculoskeletal Transplant Foundation | Instrumentation and method for repair of meniscus tissue |
| AU2009257304A1 (en) | 2008-06-13 | 2009-12-17 | Pivot Medical, Inc. | Methods and apparatus for joint distraction |
| US9808345B2 (en) | 2008-07-24 | 2017-11-07 | Iorthopedics, Inc. | Resilient arthroplasty device |
| US20100191332A1 (en) | 2009-01-08 | 2010-07-29 | Euteneuer Charles L | Implantable Tendon Protection Systems and Related Kits and Methods |
| WO2010100287A1 (en) * | 2009-03-06 | 2010-09-10 | Somatex Medical Technologies Gmbh | Barrier for implantation into bone, in particular for vertebroplasty |
| US12035902B2 (en) | 2009-03-17 | 2024-07-16 | Stryker Corporation | Method and apparatus for distracting a joint |
| US10426453B2 (en) | 2009-03-17 | 2019-10-01 | Pivot Medical, Inc. | Method and apparatus for distracting a joint |
| US9179910B2 (en) | 2009-03-20 | 2015-11-10 | Rotation Medical, Inc. | Medical device delivery system and method |
| EP2437669B1 (de) | 2009-06-04 | 2016-09-07 | Rotation Medical, Inc. | Vorrichtung zur freisetzung von klammern in einem zielgewebe |
| EP3308743A1 (de) | 2009-06-04 | 2018-04-18 | Rotation Medical, Inc. | Verfahren und vorrichtung für die freisetzung von blattförmigen materialien |
| US9278004B2 (en) | 2009-08-27 | 2016-03-08 | Cotera, Inc. | Method and apparatus for altering biomechanics of the articular joints |
| US9861408B2 (en) | 2009-08-27 | 2018-01-09 | The Foundry, Llc | Method and apparatus for treating canine cruciate ligament disease |
| EP2781197B8 (de) | 2009-08-27 | 2018-06-27 | The Foundry, LLC | Vorrichtung zur Kraftumverteilung in beweglichen Gelenken |
| US10349980B2 (en) | 2009-08-27 | 2019-07-16 | The Foundry, Llc | Method and apparatus for altering biomechanics of the shoulder |
| GB2476124A (en) * | 2009-12-08 | 2011-06-15 | R Thomas Grotz | Inflatable arthroplasty implant |
| US10201325B2 (en) | 2010-01-07 | 2019-02-12 | Bioprotect Ltd. | Controlled tissue dissection systems and methods |
| WO2013033447A2 (en) | 2011-09-01 | 2013-03-07 | Grotz R Thomas | Resilient interpositional arthroplasty device |
| WO2011091005A2 (en) | 2010-01-22 | 2011-07-28 | Grotz R Thomas | Resilient knee implant and methods |
| US10307257B2 (en) | 2010-01-22 | 2019-06-04 | Iorthopedics, Inc. | Resilient knee implant and methods |
| US9198750B2 (en) | 2010-03-11 | 2015-12-01 | Rotation Medical, Inc. | Tendon repair implant and method of arthroscopic implantation |
| WO2012017438A1 (en) | 2010-08-04 | 2012-02-09 | Ortho-Space Ltd. | Shoulder implant |
| USD833613S1 (en) | 2011-01-19 | 2018-11-13 | Iorthopedics, Inc. | Resilient knee implant |
| CA2825918C (en) | 2011-02-15 | 2018-08-07 | Rotation Medical, Inc. | Methods and apparatus for delivering and positioning sheet-like materials |
| WO2012145059A1 (en) | 2011-02-15 | 2012-10-26 | Rotation Medical, Inc. | Methods and apparatus for fixing sheet-like materials to a target tissue |
| US10952783B2 (en) | 2011-12-29 | 2021-03-23 | Rotation Medical, Inc. | Guidewire having a distal fixation member for delivering and positioning sheet-like materials in surgery |
| US9289307B2 (en) * | 2011-10-18 | 2016-03-22 | Ortho-Space Ltd. | Prosthetic devices and methods for using same |
| US9271726B2 (en) | 2011-12-19 | 2016-03-01 | Rotation Medical, Inc. | Fasteners and fastener delivery devices for affixing sheet-like materials to bone or tissue |
| US9107661B2 (en) | 2011-12-19 | 2015-08-18 | Rotation Medical, Inc. | Fasteners and fastener delivery devices for affixing sheet-like materials to bone or tissue |
| US9247978B2 (en) | 2011-12-19 | 2016-02-02 | Rotation Medical, Inc. | Apparatus and method for forming pilot holes in bone and delivering fasteners therein for retaining an implant |
| AU2012369140B2 (en) | 2011-12-19 | 2016-11-10 | Rotation Medical, Inc. | Fasteners for affixing sheet -like materials to bone or tissue |
| WO2013101638A1 (en) | 2011-12-29 | 2013-07-04 | Rotation Medical, Inc. | Methods and apparatus for delivering and positioning sheet -like materials in surgery |
| WO2013101641A2 (en) | 2011-12-29 | 2013-07-04 | Rotation Medical, Inc. | Anatomical location markers and methods of use in positioning sheet-like materials during surgery |
| JP5900909B2 (ja) * | 2012-03-27 | 2016-04-06 | 国立大学法人名古屋大学 | ポリヒドロキシアルカノエートを含む材料から作製された3次元構造体、及び骨充填材の調製用キット |
| US9545282B2 (en) * | 2013-03-09 | 2017-01-17 | Ebi, Llc | Bone graft delivery apparatus |
| US9345577B2 (en) * | 2013-03-14 | 2016-05-24 | Microaire Surgical Instruments Llc | Balloon implant device |
| CN105530880A (zh) * | 2014-03-14 | 2016-04-27 | 麦可埃尔外科器械公司 | 气囊植入装置 |
| CA2945821C (en) | 2014-05-09 | 2018-09-04 | Rotation Medical, Inc. | Medical implant delivery system for sheet-like implant |
| US9681960B2 (en) | 2014-05-16 | 2017-06-20 | Howmedica Osteonics Corp. | Guides for fracture system |
| US10575968B2 (en) | 2014-05-16 | 2020-03-03 | Howmedica Osteonics Corp. | Guides for fracture system |
| WO2016073502A1 (en) | 2014-11-04 | 2016-05-12 | Rotation Medical, Inc. | Medical implant delivery system and related methods |
| AU2015343273B2 (en) | 2014-11-04 | 2017-12-14 | Rotation Medical, Inc. | Medical implant delivery system and related methods |
| US10123796B2 (en) | 2014-11-04 | 2018-11-13 | Rotation Medical, Inc. | Medical implant delivery system and related methods |
| BR112017009895A2 (pt) * | 2014-11-13 | 2018-01-16 | sambusseti Antonio | dispositivo para a reconstrução da coifa dos rotadores |
| CA2983341A1 (en) | 2015-05-06 | 2016-11-10 | Rotation Medical, Inc. | Medical implant delivery system and related methods |
| WO2016205186A1 (en) | 2015-06-15 | 2016-12-22 | Rotation Medical, Inc. | Tendon repair implant and method of implantation |
| CN105013072B (zh) * | 2015-07-24 | 2018-08-31 | 严聪颖 | 用于子宫的带药缓释装置 |
| CN104998343A (zh) * | 2015-07-24 | 2015-10-28 | 严聪颖 | 用于子宫的带药缓释装置和植入方法 |
| CN104984468A (zh) * | 2015-07-24 | 2015-10-21 | 陈洁 | 一种治疗肩袖损伤的生物垫片及其使用方法 |
| WO2017046647A1 (en) | 2015-09-18 | 2017-03-23 | Ortho-Space Ltd. | Intramedullary fixated subacromial spacers |
| US10758228B2 (en) | 2015-11-03 | 2020-09-01 | Rotation Medical, Inc. | Fastener delivery system and related methods |
| US9943414B2 (en) * | 2015-12-30 | 2018-04-17 | Wasas, Llc. | System and method for non-binding allograft subtalar joint implant |
| AU2016381936B2 (en) | 2015-12-31 | 2019-02-28 | Rotation Medical, Inc. | Medical implant delivery system and related methods |
| KR101834002B1 (ko) * | 2016-04-08 | 2018-03-14 | (주)올손 | 회전근개 보호용 삽입 보형물 |
| EP3573806A4 (de) | 2017-01-30 | 2019-12-11 | Ortho-Space Ltd. | Bearbeitungsmaschine und verfahren zur verarbeitung von tauchgeformten artikeln |
| WO2018215902A1 (en) | 2017-05-23 | 2018-11-29 | Ortho-Space Ltd. | Bladder rolling machine |
| WO2019113292A1 (en) | 2017-12-07 | 2019-06-13 | Rotation Medical, Inc. | Medical implant delivery system and related methods |
| EP3740165B1 (de) | 2019-02-13 | 2021-06-16 | Joseph A. Abboud | Gelenksabstandshalter |
| CN109758269A (zh) * | 2019-02-27 | 2019-05-17 | 上海微创医疗器械(集团)有限公司 | 用于肩关节的假体以及假体装置 |
| WO2020198651A1 (en) * | 2019-03-27 | 2020-10-01 | Exactech, Inc. | Device for acromion replacement |
| CN110652350A (zh) * | 2019-05-24 | 2020-01-07 | 上海竞捷医疗科技有限公司 | 球囊组件 |
| CN110755179A (zh) * | 2019-10-31 | 2020-02-07 | 上海市第六人民医院 | 用于肩关节的假体 |
| CN113749821B (zh) * | 2021-09-30 | 2024-04-26 | 上海竞捷医疗科技有限公司 | 肩袖假体及其制备方法和肩袖假体装置 |
| CN114767346B (zh) * | 2022-06-21 | 2022-08-30 | 北京天星博迈迪医疗器械有限公司 | 一种植入式关节衬垫 |
Family Cites Families (425)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3384113A (en) | 1965-11-03 | 1968-05-21 | Gen Dynamics Corp | Relief valve |
| US3631854A (en) | 1969-05-19 | 1972-01-04 | Robert Howard Fryer | Inflatable medical assemblies |
| IL34803A (en) | 1969-06-27 | 1975-03-13 | Nyegaard & Co As | N-hydroxyalkyl substituted benzamides and iodo methanesulfonamide and radiological compositions containing them |
| US3800788A (en) | 1972-07-12 | 1974-04-02 | N White | Antral catheter for reduction of fractures |
| US3875595A (en) | 1974-04-15 | 1975-04-08 | Edward C Froning | Intervertebral disc prosthesis and instruments for locating same |
| DE2909439A1 (de) | 1979-03-08 | 1980-09-18 | Schering Ag | Neue nichtionische roentgenkontrastmittel |
| US4364392A (en) * | 1980-12-04 | 1982-12-21 | Wisconsin Alumni Research Foundation | Detachable balloon catheter |
| US4638803A (en) | 1982-09-30 | 1987-01-27 | Rand Robert W | Medical apparatus for inducing scar tissue formation in a body |
| US4513058A (en) * | 1984-04-17 | 1985-04-23 | Wilson Sporting Goods Co. | Impact resistant high air retention bladders |
| US4669478A (en) | 1985-03-21 | 1987-06-02 | Robertson Jack R | Device for diagnosing and relieving female incontinence |
| US4662883A (en) | 1985-07-17 | 1987-05-05 | Mentor Corporation | Self-sealing valve for fluid fillable device |
| US4892550A (en) | 1985-12-30 | 1990-01-09 | Huebsch Donald L | Endoprosthesis device and method |
| US4798205A (en) | 1986-05-08 | 1989-01-17 | Cox-Uphoff International | Method of using a subperiosteal tissue expander |
| US4719918A (en) | 1986-05-08 | 1988-01-19 | Cox-Uphoff Corporation | Subperiosteal tissue expander |
| US5002556A (en) | 1986-11-29 | 1991-03-26 | Terumo Kabushiki Kaisha | Balloon catheter assembly |
| US4819637A (en) | 1987-09-01 | 1989-04-11 | Interventional Therapeutics Corporation | System for artificial vessel embolization and devices for use therewith |
| US4932956A (en) | 1988-05-10 | 1990-06-12 | American Medical Systems, Inc. | Prostate balloon dilator |
| US4932958A (en) | 1988-05-10 | 1990-06-12 | American Medical Systems, Inc. | Prostate balloon dilator |
| US5641505A (en) * | 1988-06-27 | 1997-06-24 | Astra Tech Aktiebolag | Porous flexible sheet for tissue separation |
| AU4191989A (en) | 1988-08-24 | 1990-03-23 | Marvin J. Slepian | Biodegradable polymeric endoluminal sealing |
| US4906244A (en) | 1988-10-04 | 1990-03-06 | Cordis Corporation | Balloons for medical devices and fabrication thereof |
| US4932938A (en) | 1989-05-05 | 1990-06-12 | Medical Engineering Corporation | Urethral indwelling catheter with incontinence control |
| US5021043A (en) | 1989-09-11 | 1991-06-04 | C. R. Bard, Inc. | Method and catheter for dilatation of the lacrimal system |
| DE69108423T2 (de) | 1990-02-08 | 1995-07-27 | Howmedica | Aufblasbarer Dilatator. |
| US5514153A (en) * | 1990-03-02 | 1996-05-07 | General Surgical Innovations, Inc. | Method of dissecting tissue layers |
| US5454365A (en) | 1990-11-05 | 1995-10-03 | Bonutti; Peter M. | Mechanically expandable arthroscopic retractors |
| US5163949A (en) | 1990-03-02 | 1992-11-17 | Bonutti Peter M | Fluid operated retractors |
| US5345927A (en) | 1990-03-02 | 1994-09-13 | Bonutti Peter M | Arthroscopic retractors |
| US5954739A (en) | 1990-03-02 | 1999-09-21 | General Surgical Innovations, Inc. | Method of dissecting tissue layers |
| US5331975A (en) | 1990-03-02 | 1994-07-26 | Bonutti Peter M | Fluid operated retractors |
| US5295994A (en) | 1991-11-15 | 1994-03-22 | Bonutti Peter M | Active cannulas |
| JP2514087Y2 (ja) | 1990-05-25 | 1996-10-16 | 幸三 牧田 | 離脱式両端逆止弁付きバル―ン |
| AU8074591A (en) * | 1990-06-15 | 1992-01-07 | Cortrak Medical, Inc. | Drug delivery apparatus and method |
| US5071429A (en) | 1990-08-24 | 1991-12-10 | Medical Engineering Corporation | Method for inserting a balloon catheter through an endoscope |
| US5163950A (en) | 1990-08-24 | 1992-11-17 | Medical Engineering Corporation | Balloon catheter and endoscope kit |
| US5990382A (en) | 1990-08-29 | 1999-11-23 | Biomedical Enterprises, Inc. | Method and implant for surgical manipulation of bone |
| US5033481A (en) | 1990-10-12 | 1991-07-23 | Inamed Development Company | Intraoperative or interoperative longitudinal tissue expander |
| US5102413A (en) | 1990-11-14 | 1992-04-07 | Poddar Satish B | Inflatable bone fixation device |
| US5046489A (en) * | 1990-12-18 | 1991-09-10 | Greg Gibson | Prosthetic device |
| US5176698A (en) | 1991-01-09 | 1993-01-05 | Scimed Life Systems, Inc. | Vented dilatation cathether and method for venting |
| US5071410A (en) | 1991-03-14 | 1991-12-10 | Pazell John A | Arthroscopic surgery system |
| US5122113A (en) | 1991-03-27 | 1992-06-16 | Hattler Brack G | Inflatable percutaneous oxygenator |
| FR2674743B1 (fr) | 1991-04-03 | 1999-01-15 | Bouvet Jean Claude | Prothese medicale pour articulation. |
| US5728119A (en) | 1991-05-29 | 1998-03-17 | Origin Medsystems, Inc. | Method and inflatable chamber apparatus for separating layers of tissue |
| US5779728A (en) | 1991-05-29 | 1998-07-14 | Origin Medsystems, Inc. | Method and inflatable chamber apparatus for separating layers of tissue |
| US7744617B2 (en) | 1991-05-29 | 2010-06-29 | Covidien Ag | Method and inflatable chamber apparatus for separating layers of tissue |
| US5361752A (en) | 1991-05-29 | 1994-11-08 | Origin Medsystems, Inc. | Retraction apparatus and methods for endoscopic surgery |
| GB9118670D0 (en) | 1991-08-30 | 1991-10-16 | Mcnicholas Thomas A | Surgical devices and uses thereof |
| US5222970A (en) | 1991-09-06 | 1993-06-29 | William A. Cook Australia Pty. Ltd. | Method of and system for mounting a vascular occlusion balloon on a delivery catheter |
| US5524633A (en) | 1991-11-25 | 1996-06-11 | Advanced Surgical, Inc. | Self-deploying isolation bag |
| US5514143A (en) * | 1991-11-27 | 1996-05-07 | Apogee Medical Products, Inc. | Apparatus and method for use during surgery |
| US5344459A (en) | 1991-12-03 | 1994-09-06 | Swartz Stephen J | Arthroscopically implantable prosthesis |
| US5439467A (en) | 1991-12-03 | 1995-08-08 | Vesica Medical, Inc. | Suture passer |
| US5176692A (en) * | 1991-12-09 | 1993-01-05 | Wilk Peter J | Method and surgical instrument for repairing hernia |
| US5336252A (en) * | 1992-06-22 | 1994-08-09 | Cohen Donald M | System and method for implanting cardiac electrical leads |
| US5344451A (en) | 1992-06-24 | 1994-09-06 | Dayton Michael P | Synthetic reconstructive implant device |
| US5823995A (en) | 1992-08-25 | 1998-10-20 | Bard Connaught | Dilatation catheter with stiffening wire anchored in the vicinity of the guide wire port |
| CA2149644A1 (en) * | 1992-11-18 | 1994-05-26 | Terry Russell Knapp | Radiolucent organ displacement device for radiation therapy |
| US5318586A (en) * | 1993-01-19 | 1994-06-07 | Erkan Ereren | Laparoscopic and thoracoscopic expandable instruments |
| US5370691A (en) | 1993-01-26 | 1994-12-06 | Target Therapeutics, Inc. | Intravascular inflatable stent |
| US5453235A (en) | 1993-01-29 | 1995-09-26 | Impra, Inc. | Method of forming dual porosity FTFE tubes by extrusion of concentric preforms |
| NL9300500A (nl) | 1993-03-22 | 1994-10-17 | Industrial Res Bv | Uitzetbare, holle huls voor het plaatselijk ondersteunen en/of versterken van een lichaamsvat, alsmede werkwijze voor het vervaardigen daarvan. |
| US5334210A (en) * | 1993-04-09 | 1994-08-02 | Cook Incorporated | Vascular occlusion assembly |
| EP0696185B1 (de) | 1993-04-28 | 1998-08-12 | Focal, Inc. | Vorrichtung, produkt und verwendung betreffend die intraluminale photothermoformgebung |
| FR2704431B1 (fr) | 1993-04-30 | 1995-07-21 | Sebbin Laboratoires | Utilisation d'hydrogels à base d'acide hyaluronique et/ou de polydésoxyribonucléotides comme matériaux de remplissage de prothèses et prothèses en résultant. |
| US5480400A (en) | 1993-10-01 | 1996-01-02 | Berger; J. Lee | Method and device for internal fixation of bone fractures |
| US5423850A (en) | 1993-10-01 | 1995-06-13 | Berger; J. Lee | Balloon compressor for internal fixation of bone fractures |
| DK129693D0 (da) | 1993-11-17 | 1993-11-17 | Ole Gyring Nieben | Implanterbar stoetteindretning til svage omraader af dyrs eller menneskers kropsvaegge |
| US5458612A (en) * | 1994-01-06 | 1995-10-17 | Origin Medsystems, Inc. | Prostatic ablation method and apparatus for perineal approach |
| JP3403233B2 (ja) | 1994-01-20 | 2003-05-06 | テルモ株式会社 | バルーンカテーテル |
| US7166121B2 (en) | 1994-01-26 | 2007-01-23 | Kyphon Inc. | Systems and methods using expandable bodies to push apart cortical bone surfaces |
| US20030032963A1 (en) | 2001-10-24 | 2003-02-13 | Kyphon Inc. | Devices and methods using an expandable body with internal restraint for compressing cancellous bone |
| DE69534156T2 (de) | 1994-01-26 | 2005-09-29 | Kyphon Inc., Sunnyvale | Verbesserte aufblasbare Einrichtung für eine Verwendung in chirurgischen Protokollen in Bezug auf die Fixierung von Knochen |
| CA2180556C (en) | 1994-01-26 | 2007-08-07 | Mark A. Reiley | Improved inflatable device for use in surgical protocol relating to fixation of bone |
| US20060100635A1 (en) | 1994-01-26 | 2006-05-11 | Kyphon, Inc. | Inflatable device for use in surgical protocol relating to fixation of bone |
| US6248110B1 (en) | 1994-01-26 | 2001-06-19 | Kyphon, Inc. | Systems and methods for treating fractured or diseased bone using expandable bodies |
| US7044954B2 (en) | 1994-01-26 | 2006-05-16 | Kyphon Inc. | Method for treating a vertebral body |
| US20030229372A1 (en) | 1994-01-26 | 2003-12-11 | Kyphon Inc. | Inflatable device for use in surgical protocols relating to treatment of fractured or diseased bone |
| US6716216B1 (en) | 1998-08-14 | 2004-04-06 | Kyphon Inc. | Systems and methods for treating vertebral bodies |
| US5468245A (en) | 1994-02-03 | 1995-11-21 | Vargas, Iii; Joseph H. | Biomedical cement bonding enhancer |
| US6120523A (en) | 1994-02-24 | 2000-09-19 | Radiance Medical Systems, Inc. | Focalized intraluminal balloons |
| US5843116A (en) | 1996-05-02 | 1998-12-01 | Cardiovascular Dynamics, Inc. | Focalized intraluminal balloons |
| US6027486A (en) | 1996-05-02 | 2000-02-22 | Radiance Medical Systems, Inc. | Interactive angioplasty |
| US5645560A (en) | 1995-12-15 | 1997-07-08 | Cardiovascular Dynamics, Inc. | Fixed focal balloon for interactive angioplasty and stent implantation |
| US5516522A (en) * | 1994-03-14 | 1996-05-14 | Board Of Supervisors Of Louisiana State University | Biodegradable porous device for long-term drug delivery with constant rate release and method of making the same |
| US5496203A (en) * | 1994-03-25 | 1996-03-05 | Murray; Robert H. | Balloon valve assembly |
| EP0752836B1 (de) | 1994-03-31 | 2001-07-18 | Ernest C. Manders | Dimensional anpassbares gewebedehngerät |
| US5658324A (en) | 1994-04-14 | 1997-08-19 | Promdx Technology Inc. | System and method for the reduction of secondary trauma |
| US5452732A (en) | 1994-04-26 | 1995-09-26 | Bircoll; Mel | Method of dissecting along connective tissue lines |
| US6248131B1 (en) * | 1994-05-06 | 2001-06-19 | Advanced Bio Surfaces, Inc. | Articulating joint repair |
| US20050043808A1 (en) | 1994-05-06 | 2005-02-24 | Advanced Bio Surfaces, Inc. | Knee joint prosthesis |
| US5571189A (en) | 1994-05-20 | 1996-11-05 | Kuslich; Stephen D. | Expandable fabric implant for stabilizing the spinal motion segment |
| US5906827A (en) | 1994-06-03 | 1999-05-25 | Creative Biomolecules, Inc. | Matrix for the manufacture of autogenous replacement body parts |
| DE69507955T2 (de) | 1994-07-11 | 1999-08-12 | Dacomed Corp., Minneapolis, Minn. | Prosthetische verschlusseinrichtung |
| CN2203592Y (zh) * | 1994-07-16 | 1995-07-19 | 南京大学 | 附有生理盐水注入孔自动闭塞装置的乳房假体硅橡胶皮囊 |
| UA10911C2 (uk) | 1994-08-10 | 1996-12-25 | Мале Впроваджувальне Підприємство "Іhтерфалл" | Біосумісhий гідрогель |
| ES2216021T3 (es) | 1994-10-17 | 2004-10-16 | Raymedica, Inc. | Nucleo del disco espinal protesico. |
| US5507808A (en) * | 1994-10-26 | 1996-04-16 | Becker; Hilton | Filling tube and seal construction |
| US5507770A (en) | 1994-11-23 | 1996-04-16 | Aeroquip Corporation | Intraluminal grafting stent and method for implanting same in a blood vessel |
| US5658329A (en) | 1995-02-14 | 1997-08-19 | Mentor Corporation | Filling material for soft tissue implant prostheses and implants made therewith |
| US5941909A (en) | 1995-02-14 | 1999-08-24 | Mentor Corporation | Filling material for soft tissue implant prostheses and implants made therewith |
| US5749851A (en) | 1995-03-02 | 1998-05-12 | Scimed Life Systems, Inc. | Stent installation method using balloon catheter having stepped compliance curve |
| WO1996032153A1 (en) | 1995-04-14 | 1996-10-17 | Interventional Therapeutics Corporation | Dual valve detachable occlusion balloon and over-the-wire delivery apparatus and method for use therewith |
| US5634937A (en) | 1995-05-19 | 1997-06-03 | General Surgical Innovations, Inc. | Skin seal with inflatable membrane |
| US20040138690A1 (en) | 1995-06-05 | 2004-07-15 | Bonutti Peter M. | Fluid operated retractors |
| US6176240B1 (en) | 1995-06-07 | 2001-01-23 | Conceptus, Inc. | Contraceptive transcervical fallopian tube occlusion devices and their delivery |
| US20050131267A1 (en) | 1995-06-07 | 2005-06-16 | Talmadge Karen D. | System and method for delivering a therapeutic agent for bone disease |
| US5979452A (en) | 1995-06-07 | 1999-11-09 | General Surgical Innovations, Inc. | Endoscopic linton procedure using balloon dissectors and retractors |
| US20050131268A1 (en) | 1995-06-07 | 2005-06-16 | Talmadge Karen D. | System and method for delivering a therapeutic agent for bone disease |
| US6705323B1 (en) | 1995-06-07 | 2004-03-16 | Conceptus, Inc. | Contraceptive transcervical fallopian tube occlusion devices and methods |
| ATE475365T1 (de) | 1995-06-07 | 2010-08-15 | Conceptus Inc | Transzervikal einsetzbare empfängnisverhütungsvorrichtung zum verschluss der eileiter mit mechanischer befestigung am eileiter und einführsystem |
| US20050131269A1 (en) | 1995-06-07 | 2005-06-16 | Talmadge Karen D. | System and method for delivering a therapeutic agent for bone disease |
| US5725568A (en) | 1995-06-27 | 1998-03-10 | Scimed Life Systems, Inc. | Method and device for recanalizing and grafting arteries |
| US5683405A (en) | 1995-08-25 | 1997-11-04 | Research Medical Inc. | Vascular occluder |
| US20020143402A1 (en) | 1995-09-04 | 2002-10-03 | Limber Ltd. | Hip joint prostheses |
| US5632762A (en) | 1995-11-09 | 1997-05-27 | Hemodynamics, Inc. | Ostial stent balloon |
| US5665117A (en) | 1995-11-27 | 1997-09-09 | Rhodes; Valentine J. | Endovascular prosthesis with improved sealing means for aneurysmal arterial disease and method of use |
| US5871537A (en) | 1996-02-13 | 1999-02-16 | Scimed Life Systems, Inc. | Endovascular apparatus |
| WO1997034532A1 (en) | 1996-03-20 | 1997-09-25 | General Surgical Innovations, Inc. | Method and apparatus for combined dissection and retraction |
| US6036640A (en) | 1996-04-29 | 2000-03-14 | Medtronic, Inc. | Device and method for repositioning the heart during surgery |
| US6599275B1 (en) | 1996-06-04 | 2003-07-29 | Cook Incorporated | Implantable medical device |
| US5746762A (en) * | 1996-06-24 | 1998-05-05 | Bass; Lawrence S. | Device and method for surgical flap dissection |
| US5769884A (en) | 1996-06-27 | 1998-06-23 | Cordis Corporation | Controlled porosity endovascular implant |
| US6059750A (en) | 1996-08-01 | 2000-05-09 | Thomas J. Fogarty | Minimally invasive direct cardiac massage device and method |
| US6186978B1 (en) | 1996-08-07 | 2001-02-13 | Target Therapeutics, Inc. | Braid reinforced infusion catheter with inflatable membrane |
| US5941249A (en) | 1996-09-05 | 1999-08-24 | Maynard; Ronald S. | Distributed activator for a two-dimensional shape memory alloy |
| US5968068A (en) | 1996-09-12 | 1999-10-19 | Baxter International Inc. | Endovascular delivery system |
| US5776159A (en) | 1996-10-03 | 1998-07-07 | General Surgical Innovations, Inc. | Combination dissector and expander |
| US7749585B2 (en) | 1996-10-08 | 2010-07-06 | Alan Zamore | Reduced profile medical balloon element |
| JP2002505592A (ja) | 1996-11-15 | 2002-02-19 | アドバンスト バイオ サーフェイシズ,インコーポレイティド | 生体内原位置で組織を修復するのに用いる生体用材料システム |
| US6096052A (en) | 1998-07-08 | 2000-08-01 | Ovion, Inc. | Occluding device and method of use |
| US7073504B2 (en) | 1996-12-18 | 2006-07-11 | Ams Research Corporation | Contraceptive system and method of use |
| US7618451B2 (en) | 2001-05-25 | 2009-11-17 | Conformis, Inc. | Patient selectable joint arthroplasty devices and surgical tools facilitating increased accuracy, speed and simplicity in performing total and partial joint arthroplasty |
| JP2001511686A (ja) | 1997-02-13 | 2001-08-14 | ボストン サイエンティフィック リミテッド | 侵襲性を最小にした骨盤手術のための方法および装置 |
| US5971992A (en) | 1997-03-13 | 1999-10-26 | Solar; Ronald J. | Hydraulic method and apparatus for uniform radial compression and catheter mounting of radially expandable intraluminal stents and stented grafts |
| US5984942A (en) | 1997-04-02 | 1999-11-16 | Femrx, Inc. | Methods and systems for reducing tissue adhesion |
| US6106541A (en) | 1997-05-16 | 2000-08-22 | Hurbis; Charles G. | Surgically implantable nasal dilator |
| ATE291889T1 (de) | 1997-06-05 | 2005-04-15 | Adiana Inc | Vorrichtung zum verschliessen der eileiter |
| US5972015A (en) | 1997-08-15 | 1999-10-26 | Kyphon Inc. | Expandable, asymetric structures for deployment in interior body regions |
| US6045498A (en) | 1997-06-12 | 2000-04-04 | Uromedica, Inc. | Method for adjustably restricting a body lumen |
| EP0890346A1 (de) | 1997-06-13 | 1999-01-13 | Gary J. Becker | Dehnbare intraluminale Endoprothese |
| GB9713624D0 (en) | 1997-06-28 | 1997-09-03 | Anson Medical Ltd | Expandable device |
| WO1999003454A1 (en) | 1997-07-18 | 1999-01-28 | Infimed, Inc. | Biodegradable macromers for the controlled release of biologically active substances |
| WO1999018886A1 (en) | 1997-10-10 | 1999-04-22 | Corbitt John D Jr | Breast implant |
| US7637948B2 (en) | 1997-10-10 | 2009-12-29 | Senorx, Inc. | Tissue marking implant |
| US6638308B2 (en) | 1997-10-10 | 2003-10-28 | John D. Corbitt, Jr. | Bioabsorbable breast implant |
| US8668737B2 (en) | 1997-10-10 | 2014-03-11 | Senorx, Inc. | Tissue marking implant |
| EP1032328A1 (de) | 1997-11-25 | 2000-09-06 | Triad Vascular Systems Inc. | Mehrlagiges endovaskuläres gewebe |
| AU1630599A (en) | 1997-12-08 | 1999-06-28 | Kyphon, Inc. | Systems and methods using expandable bodies to push apart cortical bone surfaces |
| US6395019B2 (en) | 1998-02-09 | 2002-05-28 | Trivascular, Inc. | Endovascular graft |
| US6423032B2 (en) | 1998-03-13 | 2002-07-23 | Arteria Medical Science, Inc. | Apparatus and methods for reducing embolization during treatment of carotid artery disease |
| US6319264B1 (en) | 1998-04-03 | 2001-11-20 | Bionx Implants Oy | Hernia mesh |
| JPH11299725A (ja) | 1998-04-21 | 1999-11-02 | Olympus Optical Co Ltd | 内視鏡用フード |
| US6293960B1 (en) | 1998-05-22 | 2001-09-25 | Micrus Corporation | Catheter with shape memory polymer distal tip for deployment of therapeutic devices |
| US6719773B1 (en) | 1998-06-01 | 2004-04-13 | Kyphon Inc. | Expandable structures for deployment in interior body regions |
| ES2354492T3 (es) | 1998-06-01 | 2011-03-15 | Kyphon Sarl | Estructuras preformadas expandibles para el despliegue en regiones corporales internas. |
| US6074341A (en) | 1998-06-09 | 2000-06-13 | Timm Medical Technologies, Inc. | Vessel occlusive apparatus and method |
| US6591838B2 (en) * | 1998-07-06 | 2003-07-15 | Scimed Life Systems, Inc. | Implant system and method for bulking tissue |
| US6165193A (en) | 1998-07-06 | 2000-12-26 | Microvention, Inc. | Vascular embolization with an expansible implant |
| US6547760B1 (en) * | 1998-08-06 | 2003-04-15 | Cardeon Corporation | Aortic catheter with porous aortic arch balloon and methods for selective aortic perfusion |
| US6206930B1 (en) | 1998-08-10 | 2001-03-27 | Charlotte-Mecklenburg Hospital Authority | Absorbable tissue expander |
| US6019781A (en) * | 1998-10-16 | 2000-02-01 | Worland; Richard L. | Rotator cuff needle |
| US6673290B1 (en) | 1998-11-12 | 2004-01-06 | Scimed Life Systems, Inc. | Electrode structure for heating and ablating tissue and method for making and assembling the same |
| US6238335B1 (en) | 1998-12-11 | 2001-05-29 | Enteric Medical Technologies, Inc. | Method for treating gastroesophageal reflux disease and apparatus for use therewith |
| US6551241B1 (en) | 1999-12-17 | 2003-04-22 | Leonard S. Schultz | Instruments and methods for performing percutaneous surgery |
| US6371904B1 (en) | 1998-12-24 | 2002-04-16 | Vivant Medical, Inc. | Subcutaneous cavity marking device and method |
| US6395208B1 (en) | 1999-01-25 | 2002-05-28 | Atrium Medical Corporation | Method of making an expandable fluoropolymer device |
| ES2632564T3 (es) | 1999-02-01 | 2017-09-14 | Eidgenössische Technische Hochschule Zürich | Bio-materiales formados por reacción de adición nucleófila a grupos insaturados conjugados |
| US6958212B1 (en) | 1999-02-01 | 2005-10-25 | Eidgenossische Technische Hochschule Zurich | Conjugate addition reactions for the controlled delivery of pharmaceutically active compounds |
| US6312457B1 (en) * | 1999-04-01 | 2001-11-06 | Boston Scientific Corporation | Intraluminal lining |
| US6533799B1 (en) | 1999-04-27 | 2003-03-18 | Ams Research Corporation | Cavity measurement device and method of assembly |
| US6860892B1 (en) | 1999-05-28 | 2005-03-01 | General Surgical Innovations, Inc. | Specially shaped balloon device for use in surgery and method of use |
| US6379329B1 (en) | 1999-06-02 | 2002-04-30 | Cordis Neurovascular, Inc. | Detachable balloon embolization device and method |
| US6280457B1 (en) | 1999-06-04 | 2001-08-28 | Scimed Life Systems, Inc. | Polymer covered vaso-occlusive devices and methods of producing such devices |
| CN100391421C (zh) | 1999-08-23 | 2008-06-04 | 孕体股份有限公司 | 避孕输送系统 |
| JP4683804B2 (ja) | 1999-08-23 | 2011-05-18 | コンセプタス インコーポレイテッド | 卵管内避妊のための挿入及び配備カテーテルシステム |
| US6312462B1 (en) | 1999-09-22 | 2001-11-06 | Impra, Inc. | Prosthesis for abdominal aortic aneurysm repair |
| US7060100B2 (en) | 1999-10-08 | 2006-06-13 | Ferree Bret A | Artificial disc and joint replacements with modular cushioning components |
| US7088795B1 (en) | 1999-11-03 | 2006-08-08 | Pulse-Link, Inc. | Ultra wide band base band receiver |
| US6994092B2 (en) | 1999-11-08 | 2006-02-07 | Ev3 Sunnyvale, Inc. | Device for containing embolic material in the LAA having a plurality of tissue retention structures |
| FR2802799B1 (fr) | 1999-12-23 | 2002-08-16 | Depuy France | Ensemble de prothese d'epaule |
| WO2001049193A1 (en) | 2000-01-03 | 2001-07-12 | Orthoscope Ltd. | Intramedullary support strut |
| US7104996B2 (en) * | 2000-01-14 | 2006-09-12 | Marctec. Llc | Method of performing surgery |
| US6391538B1 (en) | 2000-02-09 | 2002-05-21 | The Children's Hospital Of Philadelphia | Stabilization of implantable bioprosthetic tissue |
| US7717958B2 (en) | 2000-02-16 | 2010-05-18 | Trans1, Inc. | Prosthetic nucleus apparatus |
| US6740093B2 (en) * | 2000-02-28 | 2004-05-25 | Stephen Hochschuler | Method and apparatus for treating a vertebral body |
| DE60117524T2 (de) | 2000-04-05 | 2006-08-17 | Kyphon Inc., Sunnyvale | Vorrichtungen zur behandlung von gebrochenen und/oder erkrankten knochen |
| US7815649B2 (en) | 2000-04-07 | 2010-10-19 | Kyphon SÀRL | Insertion devices and method of use |
| US6682473B1 (en) | 2000-04-14 | 2004-01-27 | Solace Therapeutics, Inc. | Devices and methods for attenuation of pressure waves in the body |
| US20030040800A1 (en) | 2000-04-26 | 2003-02-27 | Li Lehmann K. | Apparatus and method for replacing the nucleus pulposus of an intervertebral disc or for replacing an entire intervertebral disc |
| US20030105469A1 (en) * | 2001-05-09 | 2003-06-05 | Regene Ex Ltd. | Bioresorbable inflatable devices, incision tool and methods for tissue expansion and tissue regeneration |
| US8622739B2 (en) | 2001-05-09 | 2014-01-07 | Ben-Zion Karmon | Method for enlarging a jaw bone using a hollow dental implant having a side perforation |
| JP2003534064A (ja) | 2000-05-26 | 2003-11-18 | ヴァルステン・メディカル・エス・アー | バルーンカテーテル |
| US6668836B1 (en) | 2000-06-02 | 2003-12-30 | Reconstructive Technologies, Inc. | Method for tissue expansion using pulsatile motion |
| US6958075B2 (en) | 2001-09-18 | 2005-10-25 | Celsion Corporation | Device and method for treatment of tissue adjacent a bodily conduit by thermocompression |
| US20020068974A1 (en) | 2000-07-21 | 2002-06-06 | Kuslich Stephen D. | Expandable porous mesh bag device and methods of use for reduction, filling, fixation and supporting of bone |
| US20080086133A1 (en) | 2003-05-16 | 2008-04-10 | Spineology | Expandable porous mesh bag device and methods of use for reduction, filling, fixation and supporting of bone |
| CA2420898A1 (en) | 2000-08-28 | 2002-03-07 | Advanced Bio Surfaces, Inc. | Method for mammalian joint resurfacing |
| US7204851B2 (en) | 2000-08-30 | 2007-04-17 | Sdgi Holdings, Inc. | Method and apparatus for delivering an intervertebral disc implant |
| JP4472217B2 (ja) | 2000-10-16 | 2010-06-02 | オリンパス株式会社 | 生体組織のクリップ装置 |
| US6547767B1 (en) | 2000-11-14 | 2003-04-15 | Advanced Cardiovascular Systems, Inc. | Syringe assembly for a catheter |
| CA2365376C (en) | 2000-12-21 | 2006-03-28 | Ethicon, Inc. | Use of reinforced foam implants with enhanced integrity for soft tissue repair and regeneration |
| US6613052B1 (en) | 2000-12-21 | 2003-09-02 | J. Gregory Kinnett | Multi-functional orthopedic surgical instrument and method of using same |
| JP2002196109A (ja) * | 2000-12-25 | 2002-07-10 | Nitto Denko Corp | 光拡散層、光拡散性シート及び光学素子 |
| CA2342641C (en) | 2000-12-29 | 2009-03-03 | Ethicon, Inc. | Hernia repair prosthesis and method |
| US6527693B2 (en) * | 2001-01-30 | 2003-03-04 | Implant Sciences Corporation | Methods and implants for providing radiation to a patient |
| US20020161388A1 (en) | 2001-02-27 | 2002-10-31 | Samuels Sam L. | Elastomeric balloon support fabric |
| US8617048B2 (en) | 2001-03-09 | 2013-12-31 | Boston Scientific Scimed, Inc. | System for implanting an implant and method thereof |
| US20040083002A1 (en) | 2001-04-06 | 2004-04-29 | Belef William Martin | Methods for treating spinal discs |
| US20020147497A1 (en) | 2001-04-06 | 2002-10-10 | Integrated Vascular Systems, Inc. | Methods for treating spinal discs |
| US6632235B2 (en) | 2001-04-19 | 2003-10-14 | Synthes (U.S.A.) | Inflatable device and method for reducing fractures in bone and in treating the spine |
| US6616673B1 (en) | 2001-04-19 | 2003-09-09 | Biomet, Inc. | Segmented joint distractor |
| US20050209629A1 (en) | 2001-04-19 | 2005-09-22 | Kerr Sean H | Resorbable containment device and process for making and using same |
| US7160325B2 (en) | 2001-05-15 | 2007-01-09 | Ams Research Corporation | Implantable medical balloon and valve |
| JP2002360700A (ja) | 2001-06-04 | 2002-12-17 | Japan Lifeline Co Ltd | バルーンカテーテル及びバルーンカテーテル用バルーンの製造方法 |
| EP1399100A1 (de) | 2001-06-27 | 2004-03-24 | Mathys Medizinaltechnik AG | Bandscheibenprothese |
| WO2003002021A2 (en) | 2001-06-29 | 2003-01-09 | The Regents Of The University Of California | Biodegradable/bioactive nucleus pulposus implant and method for treating degenerated intervertebral discs |
| US20090234457A1 (en) | 2001-06-29 | 2009-09-17 | The Regents Of The University Of California | Systems, devices and methods for treatment of intervertebral disorders |
| US6684754B2 (en) | 2001-07-10 | 2004-02-03 | Alan Elbert Comer | Pneumatic muscle analogs for exoskeletal robotic limbs and associated control mechanisms |
| JP4294474B2 (ja) | 2001-07-16 | 2009-07-15 | デピュイ・プロダクツ・インコーポレイテッド | 半月板再生装置 |
| US20040243170A1 (en) | 2001-09-05 | 2004-12-02 | Mitta Suresh | Method and device for percutaneous surgical ventricular repair |
| WO2003049795A2 (en) | 2001-09-28 | 2003-06-19 | Boston Scientific Limited | Medical devices comprising nanocomposites |
| US6666817B2 (en) | 2001-10-05 | 2003-12-23 | Scimed Life Systems, Inc. | Expandable surgical implants and methods of using them |
| US6755861B2 (en) | 2001-10-16 | 2004-06-29 | Granit Medical Innovation, Inc. | Device for providing a portion of an organism with a desired shape |
| US6800082B2 (en) * | 2001-10-19 | 2004-10-05 | Ethicon, Inc. | Absorbable mesh device |
| CN100512777C (zh) | 2001-12-05 | 2009-07-15 | 斯恩蒂斯有限公司 | 椎间盘假体或髓核替代假体 |
| US6746465B2 (en) | 2001-12-14 | 2004-06-08 | The Regents Of The University Of California | Catheter based balloon for therapy modification and positioning of tissue |
| US6790213B2 (en) | 2002-01-07 | 2004-09-14 | C.R. Bard, Inc. | Implantable prosthesis |
| JP4324478B2 (ja) | 2002-01-22 | 2009-09-02 | アドバンスト バイオ サーフェイシズ,インコーポレイティド | 間置関節形成システム |
| JP2003325685A (ja) | 2002-03-06 | 2003-11-18 | Minoru Uematsu | 子宮頚癌腔内放射線照射用バルーン型スペーサー及び子宮頚癌腔内放射線照射用アプリケーター |
| US9155544B2 (en) | 2002-03-20 | 2015-10-13 | P Tech, Llc | Robotic systems and methods |
| US6960215B2 (en) | 2002-05-08 | 2005-11-01 | Boston Scientific Scimed, Inc. | Tactical detachable anatomic containment device and therapeutic treatment system |
| US6837850B2 (en) | 2002-05-14 | 2005-01-04 | Loubert Suddaby | Percutaneous tissue dissection |
| DE10223332A1 (de) | 2002-05-25 | 2003-12-04 | Efmt Entwicklungs Und Forschun | Medizinisches Implantat |
| WO2003105917A2 (en) | 2002-06-12 | 2003-12-24 | Scimed Life Systems, Inc. | Bulking agents |
| US20030236513A1 (en) | 2002-06-19 | 2003-12-25 | Scimed Life Systems, Inc. | Implantable or insertable medical devices for controlled delivery of a therapeutic agent |
| US6932834B2 (en) * | 2002-06-27 | 2005-08-23 | Ethicon, Inc. | Suture anchor |
| US20040038874A1 (en) * | 2002-08-22 | 2004-02-26 | Osemwota Omoigui | Method of treatment of persistent pain |
| AU2003274960C1 (en) * | 2002-09-10 | 2010-04-01 | Cianna Medical, Inc. | Brachytherapy apparatus |
| AU2003270524A1 (en) | 2002-09-10 | 2004-04-30 | Bret A. Ferree | Shock-absorbing joint and spine replacements |
| ES2280484T3 (es) * | 2002-09-27 | 2007-09-16 | Nucletron B.V. | Dispositivo para el tratamiento por radiacion de tejido proliferativo que rodea una cavidad en un cuerpo animal. |
| US7488337B2 (en) | 2002-09-30 | 2009-02-10 | Saab Mark A | Apparatus and methods for bone, tissue and duct dilatation |
| US7300448B2 (en) | 2002-10-04 | 2007-11-27 | Tyco Healthcare Group Lp | Balloon dissector with cannula |
| EP1555962B1 (de) | 2002-10-07 | 2011-02-09 | Conformis, Inc. | Minimal invasives gelenkimplantat mit einer den gelenkflächen angepassten dreidimensionalen geometrie |
| US8027712B2 (en) * | 2002-10-11 | 2011-09-27 | Ion Beam Applications S.A. | Elongated markers for soft tissue volume identification |
| JP2006511255A (ja) | 2002-10-11 | 2006-04-06 | カーティフィシャル・アクティーゼルスカブ | 層状構造を持つ生体適合性ポリマー製品を含む医療機器 |
| US8328710B2 (en) | 2002-11-06 | 2012-12-11 | Senorx, Inc. | Temporary catheter for biopsy site tissue fixation |
| AU2003279506A1 (en) * | 2002-11-12 | 2004-06-03 | Regenex Ltd. | Expandable devices and methods for tissue expansion, regenerationand fixation |
| US6733533B1 (en) | 2002-11-19 | 2004-05-11 | Zimmer Technology, Inc. | Artificial spinal disc |
| AU2003298670A1 (en) | 2002-11-21 | 2004-06-18 | Sdgi Holdings, Inc. | Systems and techniques for intravertebral spinal stabilization with expandable devices |
| US7368124B2 (en) | 2003-03-07 | 2008-05-06 | Depuy Mitek, Inc. | Method of preparation of bioabsorbable porous reinforced tissue implants and implants thereof |
| US8197837B2 (en) | 2003-03-07 | 2012-06-12 | Depuy Mitek, Inc. | Method of preparation of bioabsorbable porous reinforced tissue implants and implants thereof |
| US20040186504A1 (en) | 2003-03-18 | 2004-09-23 | Cagenix, Inc. | Oral tissue contourer |
| US6981980B2 (en) | 2003-03-19 | 2006-01-03 | Phagia Technology | Self-inflating intragastric volume-occupying device |
| US20060058829A1 (en) * | 2003-03-19 | 2006-03-16 | Sampson Douglas C | Intragastric volume-occupying device |
| US7824444B2 (en) | 2003-03-20 | 2010-11-02 | Spineco, Inc. | Expandable spherical spinal implant |
| US7306610B2 (en) * | 2003-03-21 | 2007-12-11 | Cana Lab Corporation | Method and device for forming a hardened cement in a bone cavity |
| WO2004087021A1 (en) | 2003-04-03 | 2004-10-14 | Enztec Limited | Load bearing intervertebral disk |
| ITUD20030094A1 (it) | 2003-04-30 | 2004-11-01 | Lima Lto Spa | Protesi inversa per l'articolazione della spalla. |
| ITUD20030092A1 (it) | 2003-04-30 | 2004-11-01 | Lima Lto Spa | Protesi per l'articolazione della spalla. |
| US7967835B2 (en) | 2003-05-05 | 2011-06-28 | Tyco Healthcare Group Lp | Apparatus for use in fascial cleft surgery for opening an anatomic space |
| CA2525792C (en) * | 2003-05-15 | 2015-10-13 | Biomerix Corporation | Reticulated elastomeric matrices, their manufacture and use in implantable devices |
| US6966931B2 (en) | 2003-05-21 | 2005-11-22 | Tain-Yew Shi | Artificial intervertebral disc with reliable maneuverability |
| US7632291B2 (en) | 2003-06-13 | 2009-12-15 | Trivascular2, Inc. | Inflatable implant |
| US9364330B2 (en) | 2003-06-25 | 2016-06-14 | Biedermann Technologies Gmbh & Co. Kg | Tissue integration design for seamless implant fixation |
| US20040267315A1 (en) * | 2003-06-25 | 2004-12-30 | Wolf Bradley R. | Braided gold suture and method of use |
| US20050015140A1 (en) | 2003-07-14 | 2005-01-20 | Debeer Nicholas | Encapsulation device and methods of use |
| US6958077B2 (en) | 2003-07-29 | 2005-10-25 | Loubert Suddaby | Inflatable nuclear prosthesis |
| GB0320287D0 (en) | 2003-08-29 | 2003-10-01 | Stanmore Implants Worldwide | Shoulder joint prosthetic system |
| US7632294B2 (en) | 2003-09-29 | 2009-12-15 | Promethean Surgical Devices, Llc | Devices and methods for spine repair |
| US7524274B2 (en) * | 2003-11-07 | 2009-04-28 | Cytyc Corporation | Tissue positioning systems and methods for use with radiation therapy |
| EP1682196A2 (de) * | 2003-11-10 | 2006-07-26 | Angiotech International Ag | Medizinische implantate und anti-narben-mittel |
| US20070151570A1 (en) | 2003-11-26 | 2007-07-05 | Farmache Alejandro H | Repairing procedure for the treatment of superficial and/or perforant venous insufficiency of the lower limbs by means of the application of clips, stoppers and/or artificial valves |
| US7316822B2 (en) | 2003-11-26 | 2008-01-08 | Ethicon, Inc. | Conformable tissue repair implant capable of injection delivery |
| US7979150B2 (en) | 2003-12-05 | 2011-07-12 | The Regents Of The University Of Michigan | Biodegradable/bioresorbable tissue augmentation/reconstruction device |
| US20050229433A1 (en) | 2004-03-03 | 2005-10-20 | Cachia Victor V | Catheter deliverable foot implant and method of delivering the same |
| US20050197711A1 (en) | 2004-03-03 | 2005-09-08 | Cachia Victor V. | Catheter deliverable foot implant and method of delivering the same |
| JP4977600B2 (ja) | 2004-04-20 | 2012-07-18 | ジェンザイム・コーポレーション | 外科手術用のメッシュ状インプラント |
| US7654997B2 (en) | 2004-04-21 | 2010-02-02 | Acclarent, Inc. | Devices, systems and methods for diagnosing and treating sinusitus and other disorders of the ears, nose and/or throat |
| US7410480B2 (en) * | 2004-04-21 | 2008-08-12 | Acclarent, Inc. | Devices and methods for delivering therapeutic substances for the treatment of sinusitis and other disorders |
| US20050245938A1 (en) * | 2004-04-28 | 2005-11-03 | Kochan Jeffrey P | Method and apparatus for minimally invasive repair of intervertebral discs and articular joints |
| US20050251245A1 (en) | 2004-05-05 | 2005-11-10 | Karl Sieradzki | Methods and apparatus with porous materials |
| US20070190108A1 (en) | 2004-05-17 | 2007-08-16 | Arindam Datta | High performance reticulated elastomeric matrix preparation, properties, reinforcement, and use in surgical devices, tissue augmentation and/or tissue repair |
| US20050273075A1 (en) * | 2004-06-08 | 2005-12-08 | Peter Krulevitch | Method for delivering drugs to the adventitia using device having microprojections |
| US20050278025A1 (en) | 2004-06-10 | 2005-12-15 | Salumedica Llc | Meniscus prosthesis |
| AU2005257050A1 (en) * | 2004-06-23 | 2006-01-05 | Bioprotect Ltd. | Device system and method for tissue displacement or separation |
| US20060002967A1 (en) * | 2004-07-01 | 2006-01-05 | Smestad Thomas L | Resorbable implant with lubricious coating |
| US8562633B2 (en) | 2004-08-02 | 2013-10-22 | W. L. Gore & Associates, Inc. | Tissue repair device with a bioabsorbable support member |
| US7641688B2 (en) | 2004-09-16 | 2010-01-05 | Evera Medical, Inc. | Tissue augmentation device |
| US20060069403A1 (en) | 2004-09-21 | 2006-03-30 | Shalon Ventures, Inc. | Tissue expansion devices |
| US20060074879A1 (en) * | 2004-09-30 | 2006-04-06 | Microsoft Corporation | Easy-to-use data context filtering |
| US20090088846A1 (en) | 2007-04-17 | 2009-04-02 | David Myung | Hydrogel arthroplasty device |
| US7309324B2 (en) | 2004-10-15 | 2007-12-18 | Futuremed Interventional, Inc. | Non-compliant medical balloon having an integral woven fabric layer |
| US7682335B2 (en) | 2004-10-15 | 2010-03-23 | Futurematrix Interventional, Inc. | Non-compliant medical balloon having an integral non-woven fabric layer |
| US7354419B2 (en) | 2004-10-15 | 2008-04-08 | Futuremed Interventional, Inc. | Medical balloon having strengthening rods |
| US7662082B2 (en) | 2004-11-05 | 2010-02-16 | Theragenics Corporation | Expandable brachytherapy device |
| US7604659B2 (en) * | 2004-11-09 | 2009-10-20 | Lee James M | Method and apparatus for repair of torn rotator cuff tendons |
| AU2005306603B2 (en) | 2004-11-15 | 2011-12-01 | Covidien Lp | Method and apparatus for the repair of a rotator cuff (RTC) tendon or ligament |
| CA2588388A1 (en) * | 2004-12-01 | 2006-06-08 | The Regents Of The University Of California | Systems, devices and methods of treatment of intervertebral disorders |
| US20060173484A1 (en) | 2004-12-22 | 2006-08-03 | Stephen Solomon | Percutaneous breast and buttock modification |
| EP1681077A1 (de) | 2005-01-12 | 2006-07-19 | Acrostak Corp. | Positionierungsvorrichtung und Verfahren zur Resectionskavitätbehandlung |
| US20070078477A1 (en) * | 2005-02-04 | 2007-04-05 | Heneveld Scott H Sr | Anatomical spacer and method to deploy |
| CN101370634A (zh) * | 2005-02-16 | 2009-02-18 | 亚历山大·R·瓦卡若 | 供植入与药物传递的再吸收中空装置 |
| US8828080B2 (en) | 2005-02-22 | 2014-09-09 | Barry M. Fell | Method and system for knee joint repair |
| JP2006247257A (ja) | 2005-03-14 | 2006-09-21 | Medgel Corp | 骨セメント注入装置 |
| CN2776338Y (zh) * | 2005-03-29 | 2006-05-03 | 邹大明 | 注入式硅橡胶面部充填假体 |
| WO2006108067A2 (en) | 2005-04-05 | 2006-10-12 | Triage Medical, Inc. | Tissue dilation systems and related methods |
| US20060233852A1 (en) * | 2005-04-19 | 2006-10-19 | Promethean Surgical Devices | Prosthetic for tissue reinforcement |
| US20060241766A1 (en) * | 2005-04-20 | 2006-10-26 | Sdgi Holdings, Inc. | Method and apparatus for preventing articulation in an artificial joint |
| EP1973582B1 (de) | 2005-05-04 | 2011-09-07 | Synthes GmbH | Langsam kontrahierendes, steifes verbindungselement für medizin und andere verwendungen |
| US20070042326A1 (en) | 2005-06-01 | 2007-02-22 | Osseous Technologies Of America | Collagen antral membrane expander |
| US8292912B2 (en) | 2005-06-10 | 2012-10-23 | Cook Medical Technologies, LLC | Balloon catheter that resists curving |
| US7988735B2 (en) | 2005-06-15 | 2011-08-02 | Matthew Yurek | Mechanical apparatus and method for delivering materials into the inter-vertebral body space for nucleus replacement |
| US20060293709A1 (en) | 2005-06-24 | 2006-12-28 | Bojarski Raymond A | Tissue repair device |
| US20070010846A1 (en) | 2005-07-07 | 2007-01-11 | Leung Andrea Y | Method of manufacturing an expandable member with substantially uniform profile |
| US20070010844A1 (en) | 2005-07-08 | 2007-01-11 | Gorman Gong | Radiopaque expandable body and methods |
| US20070010845A1 (en) | 2005-07-08 | 2007-01-11 | Gorman Gong | Directionally controlled expandable device and methods for use |
| US20070038292A1 (en) * | 2005-08-09 | 2007-02-15 | Moise Danielpour | Bio-absorbable stent |
| TWI273902B (en) | 2005-08-29 | 2007-02-21 | Shr-You Tzuo | Hole-reaming device for orthopedic use |
| US20070050032A1 (en) | 2005-09-01 | 2007-03-01 | Spinal Kinetics, Inc. | Prosthetic intervertebral discs |
| US20070055380A1 (en) | 2005-09-08 | 2007-03-08 | Biomet Manufacturing Corp | Method and apparatus for a glenoid prosthesis |
| ITUD20050185A1 (it) | 2005-11-03 | 2007-05-04 | Lima Lto Spa | Elemento di fissaggio aggiuntivo per una protesi per l' articolazione della spalla |
| JP2009515596A (ja) | 2005-11-10 | 2009-04-16 | ジンマー,インコーポレイティド | 侵襲を最小にする整形外科送出し装置及び器具 |
| WO2007054934A2 (en) | 2005-11-10 | 2007-05-18 | T.A.G. Medical Products A Limited Partnership | Medical implement particularly useful in arthroscopic surgical procedures |
| US20070118218A1 (en) | 2005-11-22 | 2007-05-24 | Hooper David M | Facet joint implant and procedure |
| US20070135921A1 (en) | 2005-12-09 | 2007-06-14 | Park Kee B | Surgical implant |
| WO2007076308A2 (en) | 2005-12-15 | 2007-07-05 | Stout Medical Group, L.P. | Expandable support device and method of use |
| JP2009519770A (ja) | 2005-12-16 | 2009-05-21 | インターフェイス・アソシエイツ・インコーポレーテッド | 医療用の多層バルーン及びその製造方法 |
| US20070150059A1 (en) | 2005-12-22 | 2007-06-28 | Depuy Spine, Inc. | Methods and devices for intervertebral augmentation using injectable formulations and enclosures |
| US7699894B2 (en) | 2005-12-22 | 2010-04-20 | Depuy Spine, Inc. | Nucleus pulposus trial device and technique |
| US20070185231A1 (en) | 2006-01-23 | 2007-08-09 | Liu Y K | Bone cement composite containing particles in a non-uniform spatial distribution and devices for implementation |
| TWI434675B (zh) * | 2006-02-06 | 2014-04-21 | Conformis Inc | 患者可選擇式關節置換術裝置及外科工具 |
| US7850983B2 (en) | 2006-02-07 | 2010-12-14 | Spinalcyte, Llc | Methods and compositions for repair of cartilage using an in vivo bioreactor |
| US20070225810A1 (en) | 2006-03-23 | 2007-09-27 | Dennis Colleran | Flexible cage spinal implant |
| US7993404B2 (en) | 2006-03-29 | 2011-08-09 | Warsaw Orthopedic, Inc. | Transformable spinal implants and methods of use |
| DE202006005868U1 (de) | 2006-04-06 | 2006-06-08 | Aesculap Ag & Co. Kg | Zwischenwirbelimplantat |
| US7520876B2 (en) | 2006-04-21 | 2009-04-21 | Entellus Medical, Inc. | Device and method for treatment of sinusitis |
| WO2007125060A1 (de) | 2006-04-28 | 2007-11-08 | Zimmer Gmbh | Implantat |
| US8092536B2 (en) | 2006-05-24 | 2012-01-10 | Disc Dynamics, Inc. | Retention structure for in situ formation of an intervertebral prosthesis |
| US20080004596A1 (en) | 2006-05-25 | 2008-01-03 | Palo Alto Institute | Delivery of agents by microneedle catheter |
| US8236057B2 (en) | 2006-06-12 | 2012-08-07 | Globus Medical, Inc. | Inflatable multi-chambered devices and methods of treatment using the same |
| FR2902993B1 (fr) | 2006-06-28 | 2008-09-05 | Trois S Ortho Sa | Prothese d'epaule et ensemble d'instruments pour l'implantation de cette prothese |
| JP5484047B2 (ja) | 2006-06-30 | 2014-05-07 | バイオミメティック セラピューティクス, エルエルシー | 回旋筋腱板傷害を処置するためのpdgf−生体マトリックス組成物および方法 |
| US8043362B2 (en) | 2006-08-25 | 2011-10-25 | Kyphon Sarl | Apparatus and methods for use of expandable members in surgical applications |
| US7544213B2 (en) | 2006-09-12 | 2009-06-09 | Adams Jason P | Inflatable hernia patch |
| US20080172126A1 (en) | 2006-10-03 | 2008-07-17 | Reynolds Martin A | Nucleus pulposus injection devices and methods |
| EP2083760B1 (de) | 2006-10-16 | 2014-01-15 | Pioneer Surgical Technology, Inc. | FUSIONSVORRICHTUNG und SYSTEME |
| JP2008131793A (ja) | 2006-11-22 | 2008-06-05 | Sanken Electric Co Ltd | 直流変換装置 |
| ES2869849T3 (es) * | 2006-11-27 | 2021-10-26 | Davol Inc | Un dispositivo especialmente útil para cirugías de reparación de hernias |
| US20080154233A1 (en) | 2006-12-20 | 2008-06-26 | Zimmer Orthobiologics, Inc. | Apparatus for delivering a biocompatible material to a surgical site and method of using same |
| US20080161929A1 (en) | 2006-12-29 | 2008-07-03 | Mccormack Bruce | Cervical distraction device |
| TW200833307A (en) | 2007-02-09 | 2008-08-16 | A Spine Holding Group Corp | Medical implant |
| CN101657164B (zh) | 2007-02-09 | 2013-11-06 | B&D医学发展有限责任公司 | 球囊填塞物 |
| JP4208018B2 (ja) | 2007-02-16 | 2009-01-14 | サンケン電気株式会社 | 直流変換装置 |
| US20080255569A1 (en) | 2007-03-02 | 2008-10-16 | Andrew Kohm | Bone support device, system, and method |
| US8157804B2 (en) | 2007-03-07 | 2012-04-17 | Vertech, Inc. | Expandable blade device for stabilizing long bone fractures |
| US8287442B2 (en) * | 2007-03-12 | 2012-10-16 | Senorx, Inc. | Radiation catheter with multilayered balloon |
| PL2124831T3 (pl) | 2007-03-15 | 2017-03-31 | Ortho-Space Ltd. | Urządzenia protetyczne |
| US20080249529A1 (en) | 2007-03-15 | 2008-10-09 | Depuy Spine, Inc. | Dual incision disc repair device and method |
| JP2010521216A (ja) | 2007-03-15 | 2010-06-24 | バイオプロテクト リミテッド | 軟組織固定装置 |
| US20080243122A1 (en) | 2007-03-29 | 2008-10-02 | Kohm Andrew C | Apparatuses and methods for bone screw augmentation |
| US20080243249A1 (en) | 2007-03-30 | 2008-10-02 | Kohm Andrew C | Devices for multipoint emplacement in a body part and methods of use of such devices |
| US8403937B2 (en) | 2007-03-30 | 2013-03-26 | Kyphon Sarl | Apparatus and method for medical procedures within a spine |
| US9687353B2 (en) | 2007-03-31 | 2017-06-27 | Spinal Kinetics, Inc. | Prosthetic intervertebral discs having balloon-based fillable cores that are implantable by minimally invasive surgical techniques |
| US20080294187A1 (en) | 2007-04-13 | 2008-11-27 | Biomet Microfixation, Llc | Neurosurgical Balloon Retractor |
| DE102007018341A1 (de) | 2007-04-13 | 2008-10-16 | Genrich, Heiner, Dipl.-Ing. (FH) | Implantat als Zwischenlage zwischen artikulierenden Gelenkflächen |
| US20080269746A1 (en) | 2007-04-24 | 2008-10-30 | Osteolign, Inc. | Conformable intramedullary implant with nestable components |
| US20080269897A1 (en) | 2007-04-26 | 2008-10-30 | Abhijeet Joshi | Implantable device and methods for repairing articulating joints for using the same |
| CN101743033A (zh) * | 2007-05-14 | 2010-06-16 | 生物保护有限公司 | 用于将生物活性剂释放至体内组织的释药装置 |
| JP4935499B2 (ja) | 2007-05-18 | 2012-05-23 | サンケン電気株式会社 | 直流変換装置 |
| MX2009012552A (es) | 2007-05-21 | 2010-03-18 | Aoi Medical Inc | Dispositivo de cavitacion de articulacion. |
| US7988696B2 (en) | 2007-06-01 | 2011-08-02 | Joy Medical Devices Corporation | Perforated balloon and method for forming a hardened orthopaedic paste in a bone using same |
| WO2008157727A2 (en) | 2007-06-19 | 2008-12-24 | Calypso Medical Technologies, Inc. | Methods and apparatus for external beam radiation treatments of resection cavities |
| US20110054408A1 (en) | 2007-07-10 | 2011-03-03 | Guobao Wei | Delivery systems, devices, tools, and methods of use |
| WO2009009684A1 (en) | 2007-07-10 | 2009-01-15 | Osteotech, Inc. | Delivery system |
| US20090043344A1 (en) | 2007-08-06 | 2009-02-12 | Zimmer, Inc. | Methods for repairing defects in bone |
| WO2009023250A1 (en) * | 2007-08-13 | 2009-02-19 | Arch Day Design, Llc | Tissue positioning device |
| US20090062872A1 (en) | 2007-08-27 | 2009-03-05 | Singfatt Chin | Balloon cannula system for accessing and visualizing spine and related methods |
| US8328875B2 (en) | 2009-12-30 | 2012-12-11 | Linares Medical Devices, Llc | Combination male/female hip joint and installation kit |
| US20090157084A1 (en) | 2007-09-19 | 2009-06-18 | Arthur Martinus Michael Aalsma | Collapsible and expandable device and methods of using same |
| US20090088788A1 (en) | 2007-09-28 | 2009-04-02 | Steven Mouw | Methods and apparatus having multiple separately actuatable expandable members |
| US20090088789A1 (en) | 2007-09-28 | 2009-04-02 | O'neil Michael J | Balloon With Shape Control For Spinal Procedures |
| JP2011500214A (ja) | 2007-10-19 | 2011-01-06 | ジンテス ゲゼルシャフト ミット ベシュレンクテル ハフツング | 片側プロテーゼ |
| US8043381B2 (en) | 2007-10-29 | 2011-10-25 | Zimmer Spine, Inc. | Minimally invasive interbody device and method |
| US8858563B2 (en) | 2007-10-30 | 2014-10-14 | Hipco, Inc. | Device and method for hip distention and access |
| DE102007051782B4 (de) | 2007-10-30 | 2017-02-02 | Aesculap Ag | Implantat zum Ersetzen einer Facettengelenkfläche |
| US20090177206A1 (en) | 2008-01-08 | 2009-07-09 | Zimmer Spine, Inc. | Instruments, implants, and methods for fixation of vertebral compression fractures |
| US8992620B2 (en) | 2008-12-10 | 2015-03-31 | Coalign Innovations, Inc. | Adjustable distraction cage with linked locking mechanisms |
| US8377135B1 (en) | 2008-03-31 | 2013-02-19 | Nuvasive, Inc. | Textile-based surgical implant and related methods |
| EP2285312A4 (de) | 2008-05-01 | 2014-03-12 | Columna Pty Ltd | Systeme, verfahren und geräte zur bildung und einführung von gewebeprothesen |
| US7976578B2 (en) | 2008-06-04 | 2011-07-12 | James Marvel | Buffer for a human joint and method of arthroscopically inserting |
| EP2361046B1 (de) | 2008-06-06 | 2019-04-24 | Providence Medical Technology, Inc. | Facettengelenkimplantat und einführhilfe |
| AU2009257304A1 (en) * | 2008-06-13 | 2009-12-17 | Pivot Medical, Inc. | Methods and apparatus for joint distraction |
| US9808345B2 (en) | 2008-07-24 | 2017-11-07 | Iorthopedics, Inc. | Resilient arthroplasty device |
| US20100191332A1 (en) * | 2009-01-08 | 2010-07-29 | Euteneuer Charles L | Implantable Tendon Protection Systems and Related Kits and Methods |
| WO2010097724A1 (en) | 2009-02-06 | 2010-09-02 | Ortho-Space Ltd. | Expandable joint implant |
| US20100217399A1 (en) | 2009-02-22 | 2010-08-26 | Groh Gordon I | Base plate system for shoulder arthroplasty and method of using the same |
| US9186181B2 (en) | 2009-03-17 | 2015-11-17 | Pivot Medical, Inc. | Method and apparatus for distracting a joint |
| US8636803B2 (en) | 2009-04-07 | 2014-01-28 | Spinal Stabilization Technologies, Llc | Percutaneous implantable nuclear prosthesis |
| US20110082552A1 (en) | 2009-04-27 | 2011-04-07 | Wistrom Elizabeth V | Prosthetic Intervertebral Discs Implantable By Minimally Invasive Surgical Techniques |
| GB2476124A (en) | 2009-12-08 | 2011-06-15 | R Thomas Grotz | Inflatable arthroplasty implant |
| EP3251631B1 (de) | 2009-12-18 | 2019-05-29 | Airxpanders, Inc. | Gewebeexpander |
| WO2011091005A2 (en) | 2010-01-22 | 2011-07-28 | Grotz R Thomas | Resilient knee implant and methods |
| CN102821715B (zh) | 2010-01-22 | 2015-05-20 | R·托马斯·哥罗兹 | 弹性间置式髋关节成型术装置 |
| WO2013033447A2 (en) | 2011-09-01 | 2013-03-07 | Grotz R Thomas | Resilient interpositional arthroplasty device |
| US20110295370A1 (en) | 2010-06-01 | 2011-12-01 | Sean Suh | Spinal Implants and Methods of Use Thereof |
| WO2012017438A1 (en) * | 2010-08-04 | 2012-02-09 | Ortho-Space Ltd. | Shoulder implant |
| US9179959B2 (en) | 2010-12-22 | 2015-11-10 | Illuminoss Medical, Inc. | Systems and methods for treating conditions and diseases of the spine |
| US8486149B2 (en) | 2011-02-23 | 2013-07-16 | DePuy Synthes Products, LLC | Expandable interbody fusion implant |
| US9289307B2 (en) | 2011-10-18 | 2016-03-22 | Ortho-Space Ltd. | Prosthetic devices and methods for using same |
| US8632593B2 (en) | 2011-11-23 | 2014-01-21 | Globus Medical, Inc. | Stabilizing vertebrae with expandable spacers |
| US20140303730A1 (en) | 2011-11-30 | 2014-10-09 | Beth Israel Deaconess Medical Center | Systems and methods for endoscopic vertebral fusion |
| US8926622B2 (en) | 2012-04-03 | 2015-01-06 | Warsaw Orthopedic, Inc. | Bone delivery systems including holding and filling devices and methods |
| WO2013164830A1 (en) | 2012-05-03 | 2013-11-07 | Ultimate Joint Ltd. | In-situ formation of a joint replacement prosthesis |
| US9044342B2 (en) | 2012-05-30 | 2015-06-02 | Globus Medical, Inc. | Expandable interbody spacer |
| US20140031939A1 (en) | 2012-07-25 | 2014-01-30 | Steve Wolfe | Mesh spacer hybrid |
| WO2014070204A1 (en) | 2012-11-05 | 2014-05-08 | Empire Technology Development Llc | Tumid implant and pump |
| US9345577B2 (en) | 2013-03-14 | 2016-05-24 | Microaire Surgical Instruments Llc | Balloon implant device |
| US9585761B2 (en) | 2013-03-14 | 2017-03-07 | DePuy Synthes Products, Inc. | Angulated rings and bonded foils for use with balloons for fusion and dynamic stabilization |
| US20140277467A1 (en) | 2013-03-14 | 2014-09-18 | Spinal Stabilization Technologies, Llc | Prosthetic Spinal Disk Nucleus |
| US20140378980A1 (en) | 2013-06-24 | 2014-12-25 | Roman Lomeli | Cortical Rim-Supporting Interbody Device |
| US8900304B1 (en) | 2014-06-17 | 2014-12-02 | Abdulrazzaq Alobaid | Kyphoplasty cement encapsulation balloon |
| WO2016073587A1 (en) | 2014-11-04 | 2016-05-12 | Spinal Stabilization Technologies Llc | Percutaneous implantable nuclear prosthesis |
| US10449055B2 (en) | 2015-04-23 | 2019-10-22 | Disc Fix L.L.C. | Systems and methods for treatment of intervertebral disc derangements |
| US10881522B2 (en) | 2017-02-09 | 2021-01-05 | Nadi S Hibri | Radially expandable annulus reinforcement prosthesis |
-
2008
- 2008-03-13 PL PL08719972T patent/PL2124831T3/pl unknown
- 2008-03-13 CN CN200880015430.3A patent/CN101854886B/zh active Active
- 2008-03-13 JP JP2009553278A patent/JP5271288B2/ja not_active Expired - Fee Related
- 2008-03-13 WO PCT/IL2008/000347 patent/WO2008111073A2/en active Application Filing
- 2008-03-13 DK DK16177165.4T patent/DK3111869T3/da active
- 2008-03-13 CA CA2680812A patent/CA2680812C/en not_active Expired - Fee Related
- 2008-03-13 US US12/531,332 patent/US8753390B2/en active Active
- 2008-03-13 ES ES16177165.4T patent/ES2645409T3/es active Active
- 2008-03-13 CN CN201410696820.9A patent/CN104473704B/zh active Active
- 2008-03-13 EP EP08719972.5A patent/EP2124831B1/de active Active
- 2008-03-13 EP EP16177165.4A patent/EP3111869B1/de active Active
- 2008-03-13 ES ES08719972.5T patent/ES2593085T3/es active Active
- 2008-03-13 AU AU2008224435A patent/AU2008224435B2/en not_active Ceased
-
2009
- 2009-09-14 IL IL200939A patent/IL200939A/en not_active IP Right Cessation
-
2013
- 2013-05-10 JP JP2013099793A patent/JP6030993B2/ja active Active
-
2014
- 2014-06-16 US US14/305,311 patent/US11033398B2/en active Active
-
2021
- 2021-05-14 US US17/320,869 patent/US20210338441A1/en active Pending
Non-Patent Citations (1)
| Title |
|---|
| None * |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2008111073A2 (en) | 2008-09-18 |
| IL200939A (en) | 2014-09-30 |
| JP2013208445A (ja) | 2013-10-10 |
| CN101854886B (zh) | 2014-12-24 |
| ES2645409T3 (es) | 2017-12-05 |
| EP2124831B1 (de) | 2016-07-06 |
| ES2593085T3 (es) | 2016-12-05 |
| US20140296987A1 (en) | 2014-10-02 |
| AU2008224435A1 (en) | 2008-09-18 |
| US20210338441A1 (en) | 2021-11-04 |
| JP5271288B2 (ja) | 2013-08-21 |
| CN104473704B (zh) | 2017-06-16 |
| CA2680812A1 (en) | 2008-09-18 |
| US8753390B2 (en) | 2014-06-17 |
| US11033398B2 (en) | 2021-06-15 |
| AU2008224435B2 (en) | 2014-01-09 |
| PL2124831T3 (pl) | 2017-03-31 |
| EP2124831A2 (de) | 2009-12-02 |
| EP2124831A4 (de) | 2013-06-05 |
| DK3111869T3 (da) | 2017-11-20 |
| CA2680812C (en) | 2017-01-24 |
| WO2008111073A3 (en) | 2010-02-18 |
| JP6030993B2 (ja) | 2016-11-24 |
| CN101854886A (zh) | 2010-10-06 |
| US20100023127A1 (en) | 2010-01-28 |
| EP3111869A1 (de) | 2017-01-04 |
| JP2010521214A (ja) | 2010-06-24 |
| IL200939A0 (en) | 2010-05-17 |
| CN104473704A (zh) | 2015-04-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20210338441A1 (en) | Shoulder Implant For Simulating A Bursa | |
| US11826228B2 (en) | Prosthetic devices | |
| US11771485B2 (en) | Device for performing a surgical procedure and method | |
| US20120004728A1 (en) | Bone support device, system and method | |
| US20160302836A1 (en) | Catheter with integrated cement delivery balloon | |
| KR100500868B1 (ko) | 골절되거나 질환이 있는 뼈의 치료와 관련된 외과프로토콜용 개량 팽창 장치 | |
| US20140277211A1 (en) | Device for performing a surgical procedure and method |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 2124831 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT RO SE SI SK TR |
|
| 17P | Request for examination filed |
Effective date: 20170411 |
|
| RBV | Designated contracting states (corrected) |
Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT RO SE SI SK TR |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A61B 17/72 20060101ALI20170512BHEP Ipc: A61B 17/70 20060101ALI20170512BHEP Ipc: A61B 17/56 20060101AFI20170512BHEP Ipc: A61B 17/88 20060101ALI20170512BHEP |
|
| INTG | Intention to grant announced |
Effective date: 20170620 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 2124831 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT RO SE SI SK TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 929539 Country of ref document: AT Kind code of ref document: T Effective date: 20171015 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| GRAT | Correction requested after decision to grant or after decision to maintain patent in amended form |
Free format text: ORIGINAL CODE: EPIDOSNCDEC |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602008052219 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: T3 Effective date: 20171117 |
|
| RAP2 | Party data changed (patent owner data changed or rights of a patent transferred) |
Owner name: ORTHO-SPACE LTD. |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: TRGR Ref country code: ES Ref legal event code: FG2A Ref document number: 2645409 Country of ref document: ES Kind code of ref document: T3 Effective date: 20171205 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: FP |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 1232758 Country of ref document: HK |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171220 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170920 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170920 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170920 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 929539 Country of ref document: AT Kind code of ref document: T Effective date: 20170920 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170920 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171220 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 11 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170920 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170920 |
|
| REG | Reference to a national code |
Ref country code: GR Ref legal event code: EP Ref document number: 20170403138 Country of ref document: GR Effective date: 20180420 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170920 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180120 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170920 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170920 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602008052219 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: GR Ref document number: 1232758 Country of ref document: HK |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20180621 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170920 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180313 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180313 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GR Payment date: 20190327 Year of fee payment: 12 Ref country code: BE Payment date: 20190327 Year of fee payment: 12 Ref country code: DK Payment date: 20190327 Year of fee payment: 12 Ref country code: SE Payment date: 20190326 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20190404 Year of fee payment: 12 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180313 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20200312 Year of fee payment: 13 Ref country code: IT Payment date: 20200221 Year of fee payment: 13 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170920 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170920 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170920 Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20080313 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20200401 Year of fee payment: 13 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: EBP Effective date: 20200331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180313 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20200331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200331 Ref country code: GR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201008 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200331 Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200314 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200331 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732E Free format text: REGISTERED BETWEEN 20210902 AND 20210908 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R081 Ref document number: 602008052219 Country of ref document: DE Owner name: STRYKER EUROPEAN OPERATIONS LIMITED, IE Free format text: FORMER OWNER: ORTHO-SPACE LTD., LOD, IL Ref country code: DE Ref legal event code: R082 Ref document number: 602008052219 Country of ref document: DE Representative=s name: WUESTHOFF & WUESTHOFF, PATENTANWAELTE PARTG MB, DE Ref country code: DE Ref legal event code: R082 Ref document number: 602008052219 Country of ref document: DE Representative=s name: WUESTHOFF & WUESTHOFF PATENTANWAELTE UND RECHT, DE |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MM Effective date: 20210401 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210401 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210313 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20220523 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200313 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210314 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230522 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20231229 Year of fee payment: 17 Ref country code: GB Payment date: 20240108 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20240103 Year of fee payment: 17 |